Abstract
Biofilm formation on indwelling medical devices represents an exclusive evasion mechanism for many pathogenic bacteria to establish chronic infections. Staphylococcus aureus is one of the major bacterial pathogens that are able to induce both animal and human infections. The continued emergence of multiple drug-resistant S. aureus, especially methicillin-resistant S. aureus, is problematic due to limited treatment options. Biofilm formation by S. aureus complicates the treatment of methicillin-resistant S. aureus infections. Therefore, elucidating the mechanisms of biofilm formation in this pathogen is important for the development of alternative therapeutic strategies. Various environmental and genetic factors contribute to biofilm formation. In this review, we address the environmental factors and discuss how they affect biofilm formation by S. aureus.
Keywords: Staphylococcus aureus, MRSA, biofilm, antibiotics, environmental conditions
Significance and formation of biofilms by Staphylococcus aureus
Biofilm formation of pathogenic bacteria in medical devices plays an important role in the development of chronic infections. The increased use of medical implants in health care is strongly associated with elevated biofilm-related infections. S. aureus is one of the most frequent agents causing hospital-acquired catheter-related bloodstream infections (CRBSI) associated with biofilm formation. 1 In 2017, approximately 120,000 cases of S. aureus bloodstream infections occurred and led to approximately 20,000-associated deaths in the United States. 2
Multiple drug-resistant bacterial infections present a rapidly growing threat to public health worldwide. In the United States, hospital-acquired methicillin-resistant S. aureus (HA-MRSA) isolates have been responsible for 40%–80% of all hospital-acquired S. aureus infections, causing approximately 11,000 deaths in 2011. 3 Community-acquired-MRSA (CA-MRSA), a more virulent form, has spread worldwide, causing severe skin and soft tissue infections. 4 S. aureus is also one of the main pathogens that cause bovine mastitis, incurring an estimated US$2 billion annual loss to the US dairy industry. 5 Livestock-associated-MRSA (LA-MRSA) was identified in food animals 6 and in retail meats worldwide. 7 The emergence of MRSA and vancomycin-resistant S. aureus (VRSA) increases treatment complications of CRBSI-associated infections. Public health concern stems from limited available options for treating MRSA infections. Beta-lactam class antibiotics, such as methicillin, have been successfully used in the treatment of S. aureus infections. However, numerous isolates of this pathogen have acquired resistance to these drugs soon after their introduction for treatment. Although the Food and Drug Administration’s (FDA) Guidelines require more restricted and judicious use of medically important antibiotics in livestock to curtail the emergence of more resistant strains, this is clearly not sufficient to prevent the evolution of new and more virulent progeny.
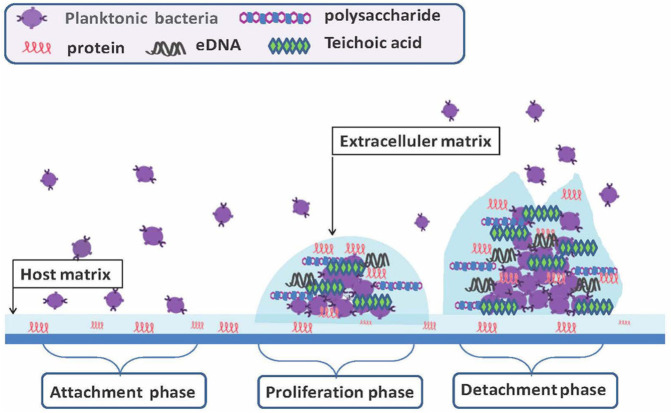
The formation of bacterial biofilm involves multiple steps, including attachment, proliferation, and detachment during infection (Figure 1). The initial attachment most likely occurs via binding of bacterial surface proteins or receptors to host matrix proteins, such as fibrinogen, fibronectin, and vitronectin. During the proliferation phase, biofilm-forming cells embed within a protective extracellular matrix composed of mainly extracellular polysaccharide intercellular adhesion (PIA), teichoic acids, protein, and DNA. The matrix components form a barrier to protect bacteria from being killed by antibiotics or clearance by host defense mechanisms. 8 The detachment process of staphylococcal cells from the biofilm body remains elusive, although some evidence suggests that production of the aliphatic delta-toxin complex might be involved in the release of staphylococcal cells from biofilms. 9
Figure 1.
Schematic representation of biofilm formation during infection.
In order to adapt to variable habitats, S. aureus has evolved a series of two-component signal regulators that enable the bacteria to sense its immediate surroundings and to modulate bacterial cellular responses and the expression of virulence genes. In many cases, the response regulators modulate target gene expression directly as a transcriptional activator and/or repressor. S. aureus is able to protect itself in response to stressed conditions by switching to alternative lifestyles, such as in a biofilm.10–13 These conditions are complicated, and some environmental factors associated with biofilm formation are discussed below.
Effect of environmental conditions on biofilm formation
Several environmental factors have been demonstrated to affect the ability of S. aureus to form biofilm as follows:
Composition of culture media
A variable phenotype of biofilm formation can be observed in different culture media. 14 It has been reported that the Luria-Bertani broth (LB) medium was the optimum medium for the biofilm formation compared to tryptic soy broth (TSB) supplemented with 2% glucose (TSBglu) and brain heart infusion (BHI). 15 However, Singh and co-workers showed that BHI broth was more effective for biofilm formation than TSB. 16 Supplementation with glucose, sucrose, and sodium chloride could significantly increase biofilm formation. 16
Arce Miranda and co-workers found that biofilm formation by S. aureus was improved under an aerobic condition in thioglycolate medium compared to TSB medium, with lower reactive oxygen species (ROS) and nitric oxide (NO) production. 17 This indicates that ROS, reactive nitrogen intermediates (RNI) and its downstream derivatives may affect biofilm development. 17 It was suggested that the formation of biofilm may induce cellular stress, which in turn affects bacterial growth under different conditions and generates ROS and RNI, and consequently decreases the extracellular matrix under unfavorable conditions. 17
Ions in medium can influence biofilm formation by S. aureus since the staphylococcal cells exhibit a moderate hydrophobic property. The ability of S. aureus to initially adhere to polystyrene was reported to be associated with the ionic strength of the culture medium. 18 It was found that the depletion of iron in the culture medium enhanced biofilm formation, while the addition of iron repressed the development of biofilm in a Fur-independent mechanism. 19 Low iron conditions induced biofilm formation independently of the overproduction of PNAG polysaccharide. 19 Furthermore, staphylococcal Emp and Eap proteins regulated by Fur, were found to be important for biofilm formation by S. aureus Newman strain in low iron conditions. 20 Other regulators, including Sae, Agr, and SarA, also play critical roles in biofilm formation under iron-restricted conditions. 20 Although it has been demonstrated that the expression of the ica operon is required for biofilm formation in iron-depleted conditions, 20 it was shown that the depletion of iron decreased biofilm formation and PIA production in S. aureus strain 113. 21 Moreover, iron chloride (FeCl3.6H2O) was able to enhance the biofilm formation by S. aureus Xen 31 strain in a dose-dependent manner. 22 This suggested the importance of iron for some S. aureus isolates to develop a biofilm. 22 Salicylic acid (SAL) is a major metabolite of aspirin that is able to bind iron and form a complex. It was reported that SAL was able to repress the production of CodY and then turn on the expression of the ica operon, which could profoundly affect polysaccharide composition in the biofilms of S. aureus. 23 Together, these findings indicate that the impact of iron on biofilm formation is dependent on the genetic background of S. aureus isolates.
Calcium impacts the biofilm architecture of S. aureus. Altering Ca2+ concentrations affected the thickness and topography of biofilm. 24 The role of calcium in biofilm formation may differ between different S. aureus isolates. 25 The Newman strain of S. aureus had a limited ability to form biofilms when cultured in the presence of calcium chelators. In contrast, S. aureus strain 10883 could generate robust biofilms in the presence of calcium chelators. 25 It seems that calcium may mediate biofilm formation through interactions with a surface adhesin clumping factor B (ClfB). In strain 10833 with a null mutation of clfB, the addition of calcium chelators in the culture medium abolished the phenomenon of elevated biofilm formations. 25
Our laboratory has also observed the strain-dependent effect on biofilm formation by S. aureus in the same culture medium (Figure 2, unpublished data). Different S. aureus strains showed variable capacities to form biofilms in the TSB medium supplemented with 3% NaCl and 0.5% glucose, indicating the role of genetic background in biofilm formation.
Figure 2.
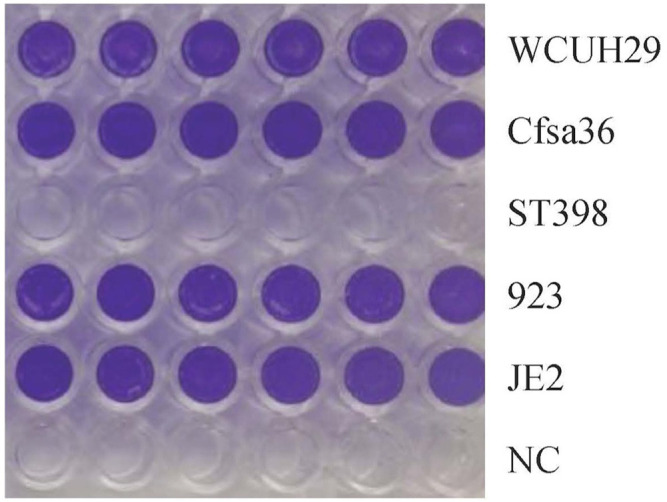
Impact of different S. aureus isolates on biofilm formation in 96-well culture plate (Ji’s lab, unpublished data). S. aureus was incubated in TSB overnight at 37°C with shaking, then diluted at 1:200 in TSB supplemented with 3% NaCl and 0.5% glucose. The diluted bacterial cultures were transferred into the wells of human plasma-coated plate and incubated 24 h without shaking. The bacterial cultures were carefully removed, and each well was gently washed three times with PBS. The biofilm was fixed with ethanol and air dry, then stained with crystal violet solution, and washed three times with PBS. WCUH29: HA-MRSA, CFsa36: a clinical S. aureus isolate from a patient with cystic fibrosis, ST398: a LA-MRSA from a swine, 923: a CA-MRSA, JE2: a CA-MRSA. NC: no bacterial cell (negative control).
Osmotic pressure
Changing osmotic pressure in the culture medium has been reported to affect the ability of S. aureus to form biofilms. This phenomenon is associated with changes in the expression of genes contributing to the modulation of biofilm formation, including icaA, sarA, rbf, and sigma B.26–28 The addition of NaCl enhanced aggregation, biofilm stability, and strength in a dose-dependent manner,12,29–31 with an increased expression of the ica operon at 4% and 6% NaCl. 32 It was found that the addition of 7% NaCl could increase biofilm formation by a foodborne S. aureus strain harboring icaA, and could also induce the biofilm development in an icaA negative strain. 33 It was suggested that NaCl-enhanced biofilm formation is more prevalent among methicillin-sensitive S. aureus (MSSA) isolates than MRSA isolates. 34 It was also reported that the addition of 4% NaCl in the BHI medium didn’t affect the capacity of biofilm formation for some ica-positive MRSA. 35 NaCl could decrease biofilm formation for some S. aureus at 37°C, but not at 25°C.18,36 It was postulated that NaCl may repress the biofilm formation for some S. aureus isolates directly or indirectly by inducing overproduction of the Sigma B factor. 37 These findings indicate that a more complicated regulatory network is involved in the response to osmolality stress during biofilm formation. Contrary to these findings, Singh and co-workers reported no significant effect of NaCl on biofilm formation by S. aureus isolated from clinical sources. 16 However, when the NaCl was combined with glucose, and sucrose, a significant increase of biofilm formation was noted. 16
The presence of glucose in the culture medium was found to promote biofilm formation in a dose-dependent manner.18,38,39 When combined with NaCl in the TSB medium, glucose was able to enhance biofilm formation. 12 The reason why the combination of glucose and sodium chloride can increase biofilm formation has been hypothesized to be that glucose might be phosphorylated by glucokinase to form glucose-6-phosphate, which subsequently enters the anabolic phase and favors biofilm formation. 40
Biofilm culture plates
Pretreatment of wells in culture plates or flow chamber with human plasma was found to affect the biofilm formation by some S. aureus isolates.41,42 The presence of human plasma in the BHI improved the reproducibility and capacity of S. aureus to form biofilms in both flow chambers under controlled shear flow and in static well plates. 43 The biofilms of most tested S. aureus isolates exhibited a thicker biomass in the presence of plasma and glucose in the culture medium. 44 A fibrous-like structure and fibrin could be observed in the biofilm matrix formed by S. aureus in the presence of plasma.44–46S. aureus produces coagulase that converts plasma fibrinogen into an insoluble fibrin clot, which in turn may promote the capacity of S. aureus to adhere to various surfaces.47,48 Adhesion is the first step in biofilm formation. Relevant factors, including extracellular matrix proteins that bind to bacterial adhesins can thus affect the ability of S. aureus to attach to the surface in the presence of plasma during culture (Figure 1). 49 It was found that the coagulase was required for biofilm formation of S. aureus during skin infection. 46 Therefore, it was postulated that the bacterial driven deposition of fibrin may play a key role in biofilm formation during bacterial growth with plasma. 44
It has been shown that S. aureus RN4220 was able to form biofilm on a hydrophilic, negatively charged polystyrene surface when cultured in both TSB and BHI media. 31 When changing the culture surface to untreated, more hydrophobic polystyrene material, the same organism was unable to form biofilms under the same culture conditions. 31 S. aureus had a higher capacity to develop biofilms on hydrophilic surfaces, such as glass and stainless steel, compared to hydrophobic surfaces, such as polypropylene and polystyrene. 38 It was suggested that the hydrophobicity profoundly affected bacterial attachment or detachment to the surface during biofilm formation.38,50 Contrarily, it was reported that S. aureus ATCC 12600, a slime producing strain, was able to develop a lower density of biofilms on hydrophilic surfaces compared to hydrophobic surfaces. 51
The smoothness of material surfaces influences biofilm development. S. aureus was able to generate early-stage biofilm colonies after 7 days and form mature biofilms after 14 days on a smooth poly(dimethyl siloxane) elastomer (PDMSe) surface. 52 However, the organism could not develop early biofilm colonies until day 21 on the topographical surface (Sharklet AF surface), which is an engineered surface microtopography based on the skin of sharks. 52 It was found that more S. aureus isolates were able to form biofilm on the polystyrene surface than on stainless steel and rubber surfaces, whereas none of the tested strains were able to generate biofilms on silicone. 53 Interestingly, under the condition of a sub-lethal dose of vancomycin, VRSA could form a thicker biofilm layer on hydrophobic silicon and nylon surfaces, which contained unsaturated, saturated, and cyclic fatty acids, as compared to hydrophilic glass surfaces that only contained straight-chain saturated fatty acids. 54 It is possible that the exposure to vancomycin may influence cell wall structures, which consequently affects the ability of VRSA to interact with extracellular fatty acids on different hydrophobic surfaces.
Aerobic conditions
It was observed that MRSA strains generated weaker biofilms during aerobic growth compared to those grown in CO2-rich conditions. 55 Oxygen regulated cid expression spatially and temporally. 56 Cid, in coordination with lrg, mediated bacterial cell death in biofilm development of S. aureus. 56 A hypoxic condition could elevate cell lysis and biofilm formation by increasing expression of autolysin, AtlA, and by inhibiting teichoic acid production. 56 Autolysin-induced lysis enabled bacterial cells to release DNA, a critical component of the biofilm matrix, to promote biofilm formation. 56 It was found that the srrAB two-component system regulated bacterial cell lysis and biofilm formation in response to the accumulation of reduced menaquinone. 57 A sub-atmospheric condition not only inhibited bacterial growth, biofilm development, and wood biofilm, it also decreased the production of virulence factors and biofilm components, such as α-hemolysin, PIA, and extracellular adhesins and DNA.58,59
The role of NO in the biofilm formation of S. aureus relies on its levels. A high concentration of NO inhibited biofilm development, whereas a lower or sub-physiological concentration of NO stimulated biofilm formation. 60 In a thioglycolate medium, microaerobic conditions of growth led to less biofilm formation than in aerobic growth. 17 The sustained exposure of NO-releasing nanoparticles alleviated biofilm thicknesses and decreased bacterial viability both in vitro and in vivo in a rat central venous catheter model of infection. 61
Acidity and alkalinity of the culture medium
The formation of biofilms requires appropriate levels of acidity in a bacterial culture medium. Neither a highly acidic (pH 3) nor a strong alkaline (pH 12) condition could benefit biofilm development of S. aureus. 39 Weak acidic pH conditions promoted biofilm formation compared to a basic pH. However, S. aureus produced more stable biofilms at the physiological related pH environments. 17 This supported the finding that the addition of extra glucose in the medium could enhance the biofilm formation of MRSA. 62
The citrate exhibited two distinct functions on bacterial growth and biofilm formation.62–64 The first was that addition of a low concentration of sodium citrate increased biofilm formation in an ica independent manner.62,63 Citrate was able to stimulate the expression of FnbA and FnbB, two major fibronectin-binding proteins important for biofilm formation of CA-MRSA.62,63 Several other intermediates of tricarboxylic acid cycle promoted biofilm formation under low sodium citrate conditions. 64 The graRS two-component system is required for citrate-induced cell-to-cell interactions. 64 The second finding was that a high citrate condition was bactericidal to the organism. 64
Physical culture conditions
Alteration of shaking magnitude and fluctuation levels may influence the biomass, density, and thickness of biofilms when cultured. It has been reported that increasing of sheer magnitude and fluctuation levels promoted the biofilm tolerance to vancomycin and ciprofloxacin. 65
Physiologically relevant and low-fluid-shear environments may benefit an attachment-independent biofilm development. Castro and co-workers found that bacterial cells also exhibited a slow growth rate, produced fewer virulence factors, and initiated alternative metabolic pathways during down-regulating RNA chaperone Hfq. 66 Static culture conditions enabled SCCmec IV MRSA strains to generate more biomass than those SCCmec I/III MRSA isolates, and the biomass of SCCmec II MRSA biofilms was remarkably less than that of SCCmec I/III MRSA biofilms. 67 However, the opposite phenomena were observed under dynamic conditions for these MRSA isolates. 67 It was suggested that the flow conditions could be beneficial for bacterial growth and the formation of the biofilm matrix. 68 It is possible that the dynamic culture environments such as shear forces facilitate bacterial cell-surface and cell–cell interactions and the utilization of nutrients, and then promote biofilm formation.
Antibiotics-induced stress
Exposure to sub-lethal levels of β-lactam antibiotics was found to cause bacterial aggregation and biofilm formation by some S. aureus strains through autolysin-induced bacterial lysis and release of DNA to form a fundamental matrix. 69 The presence of a sub-inhibitory concentration of oxacillin was also reported to induce the icaA-dependent PIA, promoting the biofilm formation of most MRSA isolates. 54 Moreover, low levels of clindamycin induced a stress response at a transcriptional level through sigma B and up-regulated major biofilm-associated genes, such as atlA, lrgA, agrA, psm, fnbA, and fnbB, and consequently enhanced the capacity of S. aureus to develop biofilms. 70 VRSA and vancomycin-intermediate resistant S. aureus (VIRS) biofilm formation was remarkably elevated after exposure to vancomycin.71,72 After exposure to sub-inhibitory vancomycin, VRSA was capable of developing biofilms on nylon and silicon indwellings. 54 Vancomycin-enhanced biofilm formation was attributed to the cidA-mediated autolysis and release of extracellular DNA, and PIA production. 71 Chang and co-workers have reported that vancomycin enhanced VRSA biofilm formation via up-regulation of atlA and sarA genes. 73 In contrast to VRSA and VIRS biofilms, vancomycin impeded autolysis of vancomycin-sensitive S. aureus (VSSA) during biofilm formation. 74
Other factors
Alcohol is another factor that could support biofilm formation by S. aureus.75,76S. aureus cells within alcohol-induced-biofilms were found to maintain viability, but at reduced rates. 77 Alcohol-enhanced biofilm formation probably resulted from its induction of icaA and icaD expression. 76 However, the combination of alcohol and ethylenediaminetetraacetic acid was able to alleviate biofilm production with unknown mechanisms. 39 Some other factors that affect bacterial biofilm formation include culture temperature and duration.17,78
Conclusion
S. aureus is an important pathogen that causes a variety of infections. The formation of biofilms on indwelling medical devices enables S. aureus to evade host immune responses and establish chronic infections. The ability to develop biofilms varies among different S. aureus isolates. Multiple environmental factors, including nutrients, antibacterial agents, pH, shearing force, temperature, and so on are able to induce stress responses and can profoundly affect the life cycle stages of biofilm formation, including initial attachment, maturation, and detachment.
Acknowledgments
The authors thank Ms. Michelle Ji and Nansea Ji for their careful editing of the manuscript and for providing constructive suggestions.
Author biographies
Ying Liu is an Associate Professor at the Shanghai Agriculture and Forestry Vocational and Technical College, China. Dr. Liu obtained her Ph.D. degree from the college of life science at East China Normal University and was a Research Scholar at the University of Minnesota.
Jiang Zhang is an Associate Professor and a Department Chair of Agricultural Biological Technology at the Shanghai Agriculture and Forestry Vocational and Technical College.
Yinduo Ji is a Professor of microbiology at the University of Minnesota, USA. Dr. Ji's laboratory is interested in the molecular and cellular pathogenesis of S. aureus, functional genomics, and antibacterial drug discovery.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by award MIN-63-113 from the General Agricultural Research fund for the EZD Signature Program Funding in the College of Veterinary Medicine at the University of Minnesota.
ORCID iD: Yinduo Ji  https://orcid.org/0000-0002-0199-0195
https://orcid.org/0000-0002-0199-0195
References
- 1.O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep 2002; 51(RR-10): 1–29. [PubMed] [Google Scholar]
- 2.Kourtis AP, Hatfield K, Baggs J, et al. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible staphylococcus aureus bloodstream infections—United States. MMWR Morb Mortal Wkly Rep 2019; 68(9): 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klevens RM, Edwards JR, Tenover FC, et al. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin Infect Dis 2006; 42(3): 389–391. [DOI] [PubMed] [Google Scholar]
- 4.Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 2005; 352(5): 468–475. [DOI] [PubMed] [Google Scholar]
- 5.Petrovski KR, Trajcev M, Buneski G. A review of the factors affecting the costs of bovine mastitis. J S Afr Vet Assoc 2006; 77(2): 52–60. [DOI] [PubMed] [Google Scholar]
- 6.Harper AL, Ferguson DD, Leedom Larson KR, et al. An overview of livestock-associated MRSA in agriculture. J Agromedicine 2010; 15(2): 101–104. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien AM, Hanson BM, Farina SA, et al. MRSA in conventional and alternative retail pork products. PLoS ONE 2012; 7(1): e30092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mack D, Davies AP, Harris LG, et al. Microbial interactions in Staphylococcus epidermidis biofilms. Anal Bioanal Chem 2007; 387(2): 399–408. [DOI] [PubMed] [Google Scholar]
- 9.Vuong C, Gerke C, Somerville GA, et al. Quorum-sensing control of biofilm factors in Staphylococcus epidermidis. J Infect Dis 2003; 188(5): 706–718. [DOI] [PubMed] [Google Scholar]
- 10.Cramton SE, Ulrich M, Gotz F, et al. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect Immun 2001; 69(6): 4079–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pamp SJ, Frees D, Engelmann S, et al. Spx is a global effector impacting stress tolerance and biofilm formation in Staphylococcus aureus. J Bacteriol 2006; 188(13): 4861–4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rode TM, Langsrud S, Holck A, et al. Different patterns of biofilm formation in Staphylococcus aureus under food-related stress conditions. Int J Food Microbiol 2007; 116(3): 372–383. [DOI] [PubMed] [Google Scholar]
- 13.Agostinho A, James G, Wazni O, et al. Inhibition of Staphylococcus aureus biofilms by a novel antibacterial envelope for use with implantable cardiac devices. Clin Transl Sci 2009; 2(3): 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valle J, Toledo-Arana A, Berasain C, et al. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol Microbiol 2003; 48: 1075–1087. [DOI] [PubMed] [Google Scholar]
- 15.Pietruczuk-Padzik A, Stefanska J, Semczuk K, et al. Evaluation of biofilm formation by Staphylococcus aureus isolated from sputum of cystic fibrosis patients. Med Dosw Mikrobiol 2010; 62(1): 1–8. [PubMed] [Google Scholar]
- 16.Singh AK, Prakash P, Achra A, et al. Standardization and classification of In vitro biofilm formation by clinical isolates of staphylococcus aureus. J Glob Infect Dis 2017; 9(3): 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arce Miranda JE, Sotomayor CE, Albesa I, et al. Oxidative and nitrosative stress in Staphylococcus aureus biofilm. FEMS Microbiol Lett 2011; 315(1): 23–29. [DOI] [PubMed] [Google Scholar]
- 18.Vazquez-Sanchez D, Habimana O, Holck A. Impact of food-related environmental factors on the adherence and biofilm formation of natural staphylococcus aureus isolates. Curr Microbiol 2013; 66(2): 110–121. [DOI] [PubMed] [Google Scholar]
- 19.Johnson M, Cockayne A, Williams PH, et al. Iron-responsive regulation of biofilm formation in staphylococcus aureus involves fur-dependent and fur-independent mechanisms. J Bacteriol 2005; 187(23): 8211–8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson M, Cockayne A, Morrissey JA. Iron-regulated biofilm formation in staphylococcus aureus newman requires ica and the secreted protein emp. Infect Immun 2008; 76(4): 1756–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin MH, Shu JC, Huang HY, et al. Involvement of iron in biofilm formation by staphylococcus aureus. PLoS ONE 2012; 7(3): e34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahire JJ, Dicks LM. Nisin incorporated with 2,3-dihydroxybenzoic acid in nanofibers inhibits biofilm formation by a methicillin-resistant strain of staphylococcus aureus. Probiotics Antimicrob Proteins 2015; 7(1): 52–59. [DOI] [PubMed] [Google Scholar]
- 23.Dotto C, Lombarte Serrat A, Cattelan N, et al. The active component of aspirin, salicylic acid, promotes staphylococcus aureus biofilm formation in a PIA-dependent manner. Front Microbiol 2017; 8(null): 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shukla SK, Rao TS. Effect of calcium on Staphylococcus aureus biofilm architecture: a confocal laser scanning microscopic study. Colloids Surf B Biointerfaces 2013; 103(null): 448–454. [DOI] [PubMed] [Google Scholar]
- 25.Abraham NM, Lamlertthon S, Fowler VG, et al. Chelating agents exert distinct effects on biofilm formation in Staphylococcus aureus depending on strain background: role for clumping factor B. J Med Microbiol 2012; 61(Pt. 8): 1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cramton SE, Gerke C, Schnell NF, et al. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 1999; 67(10): 5427–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerca N, Brooks JL, Jefferson KK. Regulation of the intercellular adhesin locus regulator (icaR) by SarA, σB, and icaR in Staphylococcus aureus. J Bacteriol 2008; 190: 6530–6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cue D, Lei MG, Luong TT, et al. Rbf promotes biofilm formation by Staphylococcus aureus via repression of icaR, a negative regulator of icaADBC. J Bacteriol 2009; 191(20): 6363–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moretro T, Hermansen L, Holck AL, et al. Biofilm formation and the presence of the intercellular adhesion locus ica among staphylococci from food and food-processing environments. Appl Environ Microbiol 2003; 69(9): 5648–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rachid S, Ohlsen K, Wallner U, et al. Alternative transcription factor sigma B is involved in regulation of biofilm expression in a staphylococcus aureus mucosal isolate. J Bacteriol 2000; 182(23): 6824–6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy CA, O’Gara JP. Contribution of culture media and chemical properties of polystyrene tissue culture plates to biofilm development by Staphylococcus aureus. J Med Microbiol 2004; 53(Pt11): 1171–1173. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Choi KH, Yoon Y. Effect of NaCl on biofilm formation of the isolate from staphylococcus aureus outbreak linked to ham. Korean J Food Sci Anim Resour 2014; 34(2): 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirani ZA, Khan MN, Aziz M, et al. Effect of stress on Biofilm formation by icaA positive and negative strains of methicillin resistant staphylococcus aureus. J Coll Physicians Surg Pak 2012; 22(1): 10–14. [PubMed] [Google Scholar]
- 34.O’Neill E, Pozzi C, Houston P, et al. Association between methicillin susceptibility and biofilm regulation in staphylococcus aureus isolates from device-related infections. J Clin Microbiol 2007; 45(5): 1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzpatrick F, Humphreys H, O’Gara JP. Evidence for icaADBC-independent biofilm development mechanism in methicillin-resistant Staphylococcus aureus clinical isolates. J Clin Microbiol 2005; 43(4): 1973–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu H, Zou Y, Lee HY, et al. Effect of NaCl on the biofilm formation by foodborne pathogens. J Food Sci 2010; 75(9): M580–M585. [DOI] [PubMed] [Google Scholar]
- 37.Lim Y, Jana M, Luong TT, et al. Control of glucose- and NaCl-induced biofilm formation by rbf in Staphylococcus aureus. J Bacteriol 2004; 186(3): 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JS, Bae YM, Lee SY, et al. Biofilm formation of staphylococcus aureus on various surfaces and their resistance to chlorine sanitizer. J Food Sci 2015; 80(10): M2279–M2286. [DOI] [PubMed] [Google Scholar]
- 39.Khalil MA, Sonbol FI. Investigation of biofilm formation on contact eye lenses caused by methicillin resistant Staphylococcus aureus. Niger J Clin Pract 2014; 17(6): 776–784. [DOI] [PubMed] [Google Scholar]
- 40.Vasu D, Kumar PS, Prasad UV, et al. Phosphorylation of staphylococcus aureus protein-tyrosine kinase affects the function of glucokinase and biofilm formation. Iran Biomed J 2017; 21(2): 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beenken KE, Blevins JS, Smeltzer MS. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect Immun 2003; 71(7): 4206–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim MK, Drescher K, Pak OS, et al. Filaments in curved streamlines: rapid formation of Staphylococcus aureus biofilm streamers. New J Phys 2014; 16(6): 065024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen P, Abercrombie JJ, Jeffrey NR, et al. An improved medium for growing Staphylococcus aureus biofilm. J Microbiol Methods 2012; 90(2): 115–118. [DOI] [PubMed] [Google Scholar]
- 44.Kwiecinski J, Kahlmeter G, Jin T. Biofilm formation by staphylococcus aureus isolates from skin and soft tissue infections. Curr Microbiol 2015; 70(5): 698–703. [DOI] [PubMed] [Google Scholar]
- 45.Akiyama H, Huh WK, Fujii K, et al. Confocal laser microscopic observation of glycocalyx production by Staphylococcus aureus in vitro. J Dermatol Sci 2002; 29(1): 54–61. [DOI] [PubMed] [Google Scholar]
- 46.Akiyama H, Ueda M, Kanzaki H, et al. Biofilm formation of Staphylococcus aureus strains isolated from impetigo and furuncle: role of fibrinogen and fibrin. J Dermatol Sci 1997; 16(1): 2–10. [DOI] [PubMed] [Google Scholar]
- 47.Vanassche T, Kauskot A, Verhaegen J, et al. Fibrin formation by staphylothrombin facilitates Staphylococcus aureus-induced platelet aggregation. Thromb Haemost 2012; 107(6): 1107–1121. [DOI] [PubMed] [Google Scholar]
- 48.Vanassche T, Peetermans M, Van Aelst LN, et al. The role of staphylothrombin-mediated fibrin deposition in catheter-related staphylococcus aureus infections. J Infect Dis 2013; 208(1): 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar A, Ting YP. Presence of pseudomonas aeruginosa influences biofilm formation and surface protein expression of staphylococcus aureus. Environ Microbiol 2015; 17(11): 4459–4468. [DOI] [PubMed] [Google Scholar]
- 50.Van Houdt R, Michiels CW. Biofilm formation and the food industry, a focus on the bacterial outer surface. J Appl Microbiol 2010; 109(4): 1117–1131. [DOI] [PubMed] [Google Scholar]
- 51.Gu J, Valdevit A, Chou TM, et al. Substrate effects on cell-envelope deformation during early-stage Staphylococcus aureus biofilm formation. Soft Matter 2017; 13(16): 2967–2976. [DOI] [PubMed] [Google Scholar]
- 52.Chung KK, Schumacher JF, Sampson EM, et al. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases 2007; 2(2): 89–94. [DOI] [PubMed] [Google Scholar]
- 53.Lee SH, Mangolin BL, Goncalves JL, et al. Biofilm-producing ability of Staphylococcus aureus isolates from Brazilian dairy farms. J Dairy Sci 2014; 97(3): 1812–1816. [DOI] [PubMed] [Google Scholar]
- 54.Mirani ZA, Jamil N. Role of extra-cellular fatty acids in vancomycin induced biofilm, formation by vancomycin resistant Staphylococcus aureus. Pak J Pharm Sci 2013; 26(2): 383–389. [PubMed] [Google Scholar]
- 55.Ursic V, Tomic V, Kosnik M. Effect of different incubation atmospheres on the production of biofilm in methicillin-resistant staphylococcus aureus(MRSA) grown in nutrient-limited medium. Curr Microbiol 2008; 57(4): 386–390. [DOI] [PubMed] [Google Scholar]
- 56.Moormeier DE, Endres JL, Mann EE, et al. Use of microfluidic technology to analyze gene expression during staphylococcus aureus biofilm formation reveals distinct physiological niches. Appl Environ Microbiol 2013; 79(11): 3413–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mashruwala AA, Guchte AV, Boyd JM. Impaired respiration elicits SrrAB-dependent programmed cell lysis and biofilm formation in Staphylococcus aureus. Elife 2017; 6(null): piie23845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li T, Wang G, Yin P, et al. Effect of negative pressure on growth, secretion and biofilm formation of Staphylococcus aureus. Antonie Van Leeuwenhoek 2015; 108(4): 907–917. [DOI] [PubMed] [Google Scholar]
- 59.Li T, Zhang L, Han LI, et al. Early application of negative pressure wound therapy to acute wounds contaminated with Staphylococcus aureus: an effective approach to preventing biofilm formation. Exp Ther Med 2016; 11(3): 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jardeleza C, Foreman A, Baker L, et al. The effects of nitric oxide on staphylococcus aureus biofilm growth and its implications in chronic rhinosinusitis. Int Forum Allergy Rhinol 2011; 1(6): 438–444. [DOI] [PubMed] [Google Scholar]
- 61.Mihu MR, Cabral V, Pattabhi R, et al. Sustained nitric oxide-releasing nanoparticles interfere with methicillin-resistant staphylococcus aureus adhesion and biofilm formation in a rat central venous catheter model. Antimicrob Agents Chemother 2016; 61: e02020-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Neill E, Pozzi C, Houston P, et al. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J Bacteriol 2008; 190(11): 3835–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCourt J, O’Halloran DP, McCarthy H, et al. Fibronectin-binding proteins are required for biofilm formation by community-associated methicillin-resistant Staphylococcus aureus strain LAC. FEMS Microbiol Lett 2014; 353(2): 157–164. [DOI] [PubMed] [Google Scholar]
- 64.Shanks RM, Meehl MA, Brothers KM, et al. Genetic evidence for an alternative citrate-dependent biofilm formation pathway in Staphylococcus aureus that is dependent on fibronectin binding proteins and the GraRS two-component regulatory system. Infect Immun 2008; 76(6): 2469–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kostenko V, Salek MM, Sattari P, et al. Staphylococcus aureus biofilm formation and tolerance to antibiotics in response to oscillatory shear stresses of physiological levels. FEMS Immunol Med Microbiol 2010; 59(3): 421–431. [DOI] [PubMed] [Google Scholar]
- 66.Castro SL, Nelman-Gonzalez M, Nickerson CA, et al. Induction of attachment-independent biofilm formation and repression of Hfq expression by low-fluid-shear culture of staphylococcus aureus. Appl Environ Microbiol 2011; 77(18): 6368–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanhommerig E, Moons P, Pirici D, et al. Comparison of biofilm formation between major clonal lineages of methicillin resistant staphylococcus aureus. PLoS ONE 2014; 9(8): e104561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berlutti F, Frioni A, Natalizi T, et al. Influence of sub-inhibitory antibiotics and flow condition on Staphylococcus aureus ATCC 6538 biofilm development and biofilm growth rate: bioTimer assay as a study model. J Antibiot 2014; 67(11): 763–769. [DOI] [PubMed] [Google Scholar]
- 69.Kaplan JB, Izano EA, Gopal P, et al. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation in staphylococcus aureus. MBio 2012; 3(4): e00198-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schilcher K, Andreoni F, Dengler Haunreiter V, et al. Modulation of Staphylococcus aureus biofilm matrix by subinhibitory concentrations of clindamycin. Antimicrob Agents Chemother 2016; 60(10): 5957–5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsu CY, Lin MH, Chen CC, et al. Vancomycin promotes the bacterial autolysis, release of extracellular DNA, and biofilm formation in vancomycin-non-susceptible Staphylococcus aureus. FEMS Immunol Med Microbiol 2011; 63(2): 236–247. [DOI] [PubMed] [Google Scholar]
- 72.He X, Yuan F, Lu F, et al. Vancomycin-induced biofilm formation by methicillin-resistant, Staphylococcus aureus, is associated with the secretion of membrane vesicles. Microb Pathog 2017; 110(null): 225–231. [DOI] [PubMed] [Google Scholar]
- 73.Chang W, Ding D, Zhang S, et al. Methicillin-resistant staphylococcus aureus grown on vancomycin-supplemented screening agar displays enhanced biofilm formation. Antimicrob Agents Chemother 2015; 59(12): 7906–7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh R, Ray P. Vancomycin modulated autolysis in Staphylococcus aureus: does it vary with the susceptibility and planktonic or biofilm phenotype? FEMS Immunol Med Microbiol 2012; 65: 1–4. [DOI] [PubMed] [Google Scholar]
- 75.Redelman CV, Maduakolam C, Anderson GG. Alcohol treatment enhances Staphylococcus aureus biofilm development. FEMS Immunol Med Microbiol 2012; 66(3): 411–418. [DOI] [PubMed] [Google Scholar]
- 76.Cincarova L, Polansky O, Babak V, et al. Changes in the Expression of Biofilm-Associated Surface Proteins in Staphylococcus aureus Food-Environmental Isolates Subjected to Sublethal Concentrations of Disinfectants. Biomed Res Int 2016; 2016(null): 4034517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luther MK, Bilida S, Mermel LA, et al. Ethanol and isopropyl alcohol exposure increases biofilm formation in staphylococcus aureus, and staphylococcus epidermidis. Infect Dis Ther 2015; 4(2): 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oliveira M, Nunes SF, Carneiro C, et al. Time course of biofilm formation by Staphylococcus aureus and Staphylococcus epidermidis mastitis isolates. Vet Microbiol 2007; 124(1–2): 187–191. [DOI] [PubMed] [Google Scholar]