Abstract
Although thaumatin-like proteins (TLPs) are involved in resistance to a variety of fungal diseases, whether the TLP5 and TLP6 genes in tomato plants (Solanum lycopersicum) confer resistance to the pathogenesis of soil-borne diseases has not been demonstrated. In this study, five soil-borne diseases (fungal pathogens: Fusarium solani, Fusarium oxysporum, and Verticillium dahliae; bacterial pathogens: Clavibacter michiganense subsp. michiganense and Ralstonia solanacearum) were used to infect susceptible “No. 5” and disease-resistant “S-55” tomato cultivars. We found that SlTLP5 and SlTLP6 transcript levels were higher in susceptible cultivars treated with the three fungal pathogens than in those treated with the two bacterial pathogens and that transcript levels varied depending on the pathogen. Moreover, the SlTLP5 and SlTLP6 transcript levels were much higher in disease-resistant cultivars than in disease-susceptible cultivars, and the SlTLP5 and SlTLP6 transcript levels were higher in cultivars treated with the same fungal pathogen than in those treated with bacterial pathogens. SlTLP6 transcript levels were higher than SlTLP5. SlTLP5 and SlTLP6 overexpression and gene-edited transgenic mutants were generated in both susceptible and resistant cultivars. Overexpression and knockout increased and decreased resistance to the five diseases, respectively. Transgenic plants overexpressing SlTLP5 and SlTLP6 inhibited the activities of peroxidase (POD), superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT) after inoculation with fungal pathogens, and the activities of POD, SOD, and APX were similar to those of fungi after infection with bacterial pathogens. The activities of CAT were increased, and the activity of β-1,3-glucanase was increased in both the fungal and bacterial treatments. Overexpressed plants were more resistant than the control plants. After SlTLP5 and SlTLP6 knockout plants were inoculated, POD, SOD, and APX had no significant changes, but CAT activity increased and decreased significantly after the fungal and bacterial treatments, contrary to overexpression. The activity of β-1,3-glucanase decreased in the treatment of the five pathogens, and the knocked-out plants were more susceptible to disease than the control. In summary, this study contributes to the further understanding of TLP disease resistance mechanisms in tomato plants.
Keywords: bacterial pathogens, disease resistance, fungal pathogens, soil-borne disease, thaumatin-like protein
1. Introduction
Plants have evolved a range of components to fight pathogens, including the expression of pathogenesis-related (PR) proteins in response to complex and diverse environments. Currently, at least 17 PR proteins are known to be induced by oomycetes, fungi, bacteria, nematodes, viruses, and viroids, as well as insect bites [1,2]. As a PR plant disease-resistance protein, the PR protein has been used widely in crop protection against fungi [3]. The anti-fungal activity of thaumatin-like protein (TLP) has been studied intensively. Plant TLP mainly inhibits pathogenic and non-pathogenic fungi by lysing fungal spores, inhibiting spore germination, and reducing the vitality of young mycelium [4,5]. Recently, TLP genes were found to confer resistance to pathogenic fungi, including sclerotia, powdery mildew, and Pseudomonas syringae [6,7]. TLP transgenic plants can delay the development of a variety of fungal diseases and enhance plant resistance to pathogenic fungi [8,9]. Furthermore, TLP genes can be induced by a variety of biotic and abiotic stresses [10,11,12,13,14,15].
Fungicides are applied to control plant diseases in traditional agriculture. However, the widespread use of pesticides may result in serious environmental pollution and food safety problems. TLP genes have been successfully expressed in a variety of plants, and indica rice cultivars overexpressing TLPs enhance resistance to Rhizoctonia solani, a pathogen of rice sheath blight disease, and Sarocladium oryzae, a pathogen of rice sheath rot [16]. Activity studies of recombinant Solanum nigrum TLPs in vitro have demonstrated that they are equally sensitive to Fusarium solani f. sp. glycines, Colletotrichum spp., Macrophomina phaseolina, and Phytophthora nicotianae var. parasitica [17] and exhibited resistance to a variety of pathogenic fungi in transgenic plants. Given the broad resistance of TLPs, breeding TLP transgenic plants is an effective approach to achieving plant disease resistance.
A disease-resistance gene cluster was identified from chromosome 8 of the inbred line CLN2037E by our project team. The cluster contained six genes, five of which were TLP genes, including Solyc08g080660 and Solyc08g080670, referred to as SlTLP5 and SlTLP6, respectively. The overexpression and knockout of SlTLP5 and SlTLP6 corresponded to an increase and decrease in resistance to late blight, respectively [18]. The role of SlTLP5 and SlTLP6 in the defense signal transduction of tomato plants after infection with five soil-borne diseases was illustrated in our study. Five soil-borne diseases were used to infect resistant “S-55” and susceptible “No. 5” tomato cultivars, and the transcription levels of SlTLP5 and SlTLP6 in resistant and susceptible cultivars were determined via quantitative real-time PCR (RT-qPCR). Transgenic plants overexpressing SlTLP5 and SlTLP6 and gene-edited transgenic plants were generated in susceptible and resistant cultivars, respectively, to identify the disease resistance of transgenic plants.
2. Materials and Methods
2.1. Experimental Material and Bacterial Infection
The susceptible “No. 5” and resistant “S-55” inbred tomato cultivars were developed and preserved by our research group, and the plants were cultivated in growth chambers with light for 16 h (28 °C) and without light for 8 h (20 °C). The strains Fusarium solani, Fusarium oxysporum, Verticillium dahliae, Clavibacter michiganense subsp. michiganense, and Ralstonia solanacearum were obtained from the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences (Kunming, Yunnan Province, China). The strains R. solanacearum and C. michiganense subsp. michiganense were cultured in nutrient broth at 28 °C with a rotational speed of 150 rpm. After 48 h, they were washed three times via centrifugation with sterile water, and R. solanacearum and F. oxysporum suspensions were resuspended in deionized water and adjusted to OD600 = 0.1 (108 cfu·mL−1) using a spectrophotometer (Shimadzu UV-2600, Kyoto, Japan). The pathogens F. oxysporum, V. dahliae, and F. solani were cultured in PDA (Oxoid, Basingstoke, UK) medium for 4–6 days at 28 °C under an oscillation of 200 rpm. The mycelium was filtered off with four layers of gauze; the suspension was diluted with sterile distilled water to a suitable concentration (calculated using a hemocytometer plate-counting method). The inoculated F. oxysporum and V. dahliae bacterial solutions had a concentration of 1 × 109 cfu·mL−1, and the inoculated F. solani bacterial solution had a concentration of 1 × 108 cfu·mL−1. Then, 50 mL of each of the five pathogen suspensions was poured onto seven leaves of tomato seedlings in each pot, and those at d 0 were used as the control group. The inoculated seedlings were placed in a plant growth chamber and preserved at 100% relative humidity (RH) and 20 ± 1 °C dark conditions for 24 h. Subsequently, RH was lowered to 60% from 80% under light for 14 h/d [19]. The tomato leaves were frozen in liquid nitrogen after treatment for 0 (control), 3, 5, 7, and 9 days, and then stored at −80°C.
2.2. Identification of Disease Resistance in Tomato Leaves via RT-qPCR
Total RNA was extracted from tomato leaves of the disease-susceptible and disease-resistant inbred lines “No. 5” and “S-55” after isolation and treatment using the Huayuoyang Rapid Universal Plant RNA Extraction Kit (Huayuoyang Biotechnology Co., Ltd., Beijing, China), and the quality and quantity of extracted RNA were confirmed using 1.5% (w/v) agar gel electrophoresis and NanoDrop 1000 spectrophotometry (Thermo Fisher Scientific, New York, NY, USA). Two micrograms of total RNA were used to synthesize cDNA using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen, Beijing, China) according to the manufacturer’s protocol. The primers were designed using the Primer Premier 6 software (Premier, San Francisco, CA, USA) and synthesized by Tsingke Biotechnology (Qingke Biotechnology Co., Ltd., Beijing, China). RT-qPCR was performed with a CFX96 PCR machine (Bio-Rad, Hercules, CA, USA) using a SYBR Green PCR Master Mix (TransGen). The 2−ΔΔCT method [20] was used to calculate relative mRNA abundance. The tomato housekeeping gene ribosomal protein L2 [20,21] was used as an internal control, and each value represented the mean of three biological replicates.
2.3. Generation of Overexpressing Transgenic Tomato Plants
The function of SlTLP5 and SlTLP6 was verified using an overexpression technique. The “No. 5” tomato inbred line was used for overexpression experiments.
Amplification primers for the CDS region of SlTLP5 and SlTLP6 were designed using the Primer6 software, and BamHI and SacI restriction sites were introduced to each primer, respectively. The SlTLP5 and SlTLP6 genes were ligated into the binary vector pBI121. Leaf disc transformation was used for transformation [22], and explants were obtained from the cotyledons of one-week-old seedlings. The kanamycin and RT-PCR methods were used to screen transgenic plant strains. The plants overexpressing SlTLP5 and SlTLP6 were validated using two pairs of specific primers and universal primers. The expression levels of SlTLP5 and SlTLP6 in transgenic lines were detected using RT-qPCR.
2.4. Generation of Gene-Edited Tomato Plants
The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 system was used to generate SlTLP5 and SlTLP6 knockout strains in the resistant tomato cultivar “S-55.” Two adjacent sgRNA target sites were selected within the open reading frames of SlTLP5 and SlTLP6, and PCR was used to introduce the target sequence downstream of the promoter and upstream of the sgRNA sequence. The sgRNA expression cassette was assembled into the gene-editing binary vector pYLCRISPR/Cas9P35S [23] using multiple rounds of overlapping PCR and introduced into the “S-55” plants using Agrobacterium tumefaciens-mediated transformation. PCR amplifications were performed using the DNA of positive tomato plants that were preliminarily selected through kanamycin screening. The mutation site and mutation type were analyzed by comparison with the original sequence. T0 mutant plants with early gene expression termination were selected for inbreeding. The pure strains obtained via screening and sequencing were infected with pathogens to identify the disease resistance of gene-edited tomato plants. The above primers can be found in Table S1.
2.5. Disease Resistance of Transgenic Plants
Overexpressing and gene-edited transgenic plants were infected with the five pathogens, as described above (R. solanacearum and C. michiganense subsp. michiganense as noted previously) [24,25]. Infected plants were preserved in a growth chamber at 27 °C and wilting or symptoms were recorded on day 7. Wilting symptoms were scored using a grade of 0 to 4: (0) healthy plants without wilting; (1) plants with 25% withered leaves; (2) plants with 50% withered leaves; (3) plants with 75% withered leaves; and (4) plants with completely withered leaves. The disease index was calculated by averaging the disease scores for each plant in the experiment.
Fungal pathogens F. oxysporum [26,27], V. dahliae, and F. solani were quantified based on disease severity [28] and classified into five levels according to observations during pathogen invasion: (0) healthy plants (without obvious wilting or yellowing symptoms); (1) Cotyledon wilt or fall off; (2) plants with 30–50% true leaves withered or shed; (3) plants with 50–80% true leaves withered or shed; and (4) plants with all leaves shed or dead. The disease classification was scored 14 days after pathogen infection, using five individual plants for each treatment.
2.6. Determination of ROS Antioxidant Physiological Indexes in Transgenic Plants
The transgenic plants treated for 7 days were stained with diaminobenzidine (DAB)/nitroblue tetrazolium (NBT) to observe H2O2 (hydrogen peroxide) and O2– accumulation in the leaves of the whole infected plant. The plants transfected with the pBI121 empty vector were used as a control for overexpressing plants, and off-target effector plants were used as a control for gene-edited plants [29]. Peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) activities were determined as reported previously [30].
2.7. Determination of β-1,3-GA Activity
In infected plants, β-1,3-GA can catalyze the hydrolysis of β-1,3-glucosidic bond, thus destroying the fungal cell wall. Therefore, the determination of β-1,3-GA activity is widely used in plant pathological studies. The activity of β-1,3-GA was determined as described by Zong et al. [31], and crude enzyme solution (0.03 mL) was pipetted into 0.07 mL of kelp toxin and incubated for 40 min at 37 °C. Then, 1.5 mL of 3,5-dinitrosalicylic acid reagent was added to a 100 °C water bath for 5 min, and the absorbance was measured at 540 nm. The glucose (mg) produced per hour by the breakdown of kelp toxin through the enzymatic reaction system was used as the enzyme activity unit.
2.8. Data Statistics
Each experiment was performed at least three times. The IBM SPSS Statistics 24 statistical software (IBM, Chicago, IL, USA) was used for data processing, and one-way analysis of variance was adopted for data analysis. Duncan’s test was used for post hoc analysis, and the difference at the 95% level was considered significant. Normality, homogeneity of variance, and data independence were assessed before all analyses, and the results are expressed as the mean ± standard deviation.
3. Results
3.1. Response of the SlTLP5 and SlTLP6 Genes in Five Soil-Borne Diseases
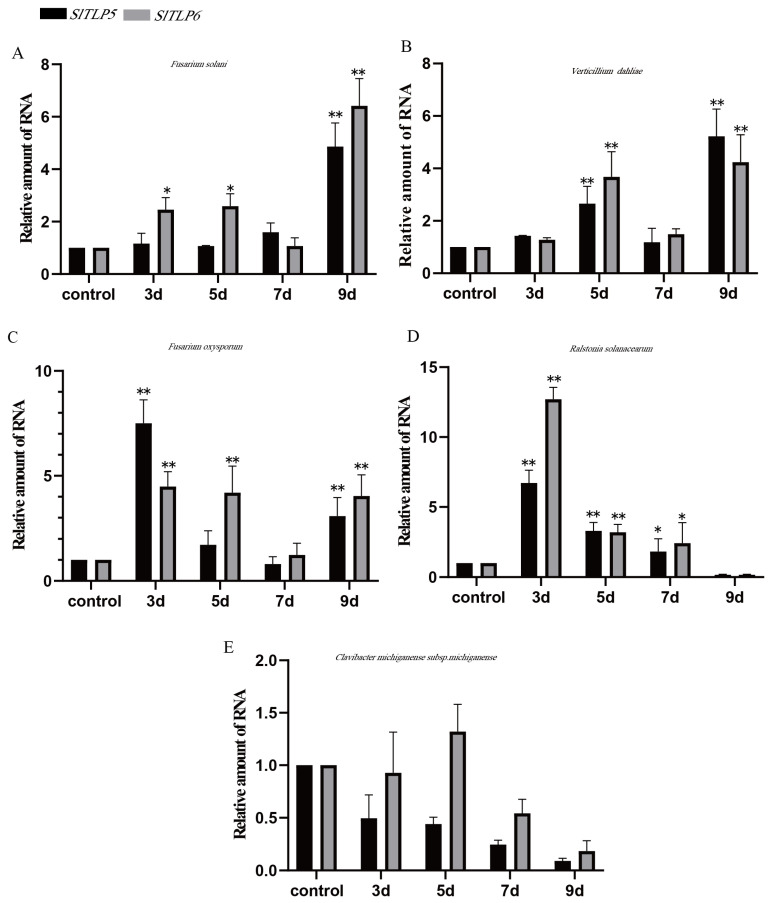
After infection with five pathogens, the expression patterns of SlTLP5 and SlTLP6 were detected using RT-qPCR on the “No. 5” and “S-55” inbred lines on day 0 (control) and days 3, 5, 7, and 9. In the “No. 5” susceptible cultivar, the transcript levels of SlTLP5 and SlTLP6 increased in those plants treated with three fungal pathogens and were very similar in those treated with the same pathogen. As for the bacterial pathogen treatment, plants treated with R. solanacearum exhibited a stronger response than those treated with C. michiganense subsp. michiganense, and SlTLP5 and SlTLP6 expression reached significant levels on day 3 after the R. solanacearum treatment, with very similar SlTLP5 and SlTLP6 transcript levels. The SlTLP6 increased but did not reach significant levels after the C. michiganense subsp. michiganense treatment, and SlTLP5 showed a decreasing trend (Figure 1A–E).
Figure 1.
Response of SlTLP5 and SlTLP6 genes in susceptible cultivar “No. 5”. Total RNA was extracted from seedlings at four infection stages, days 0, 3, 5, 7, and 9, and expression of the genes was assessed using RT-qPCR. (A–E) represent F. solani, V. dahliae, F. oxysporum, R. solanacearum, and C. michiganense subsp. michiganense treatments, respectively. Note: The asterisk indicates a significant difference compared to the control group (* p < 0.05 and ** p < 0.01). Error bars indicate standard deviations (SDs) for three replicates.
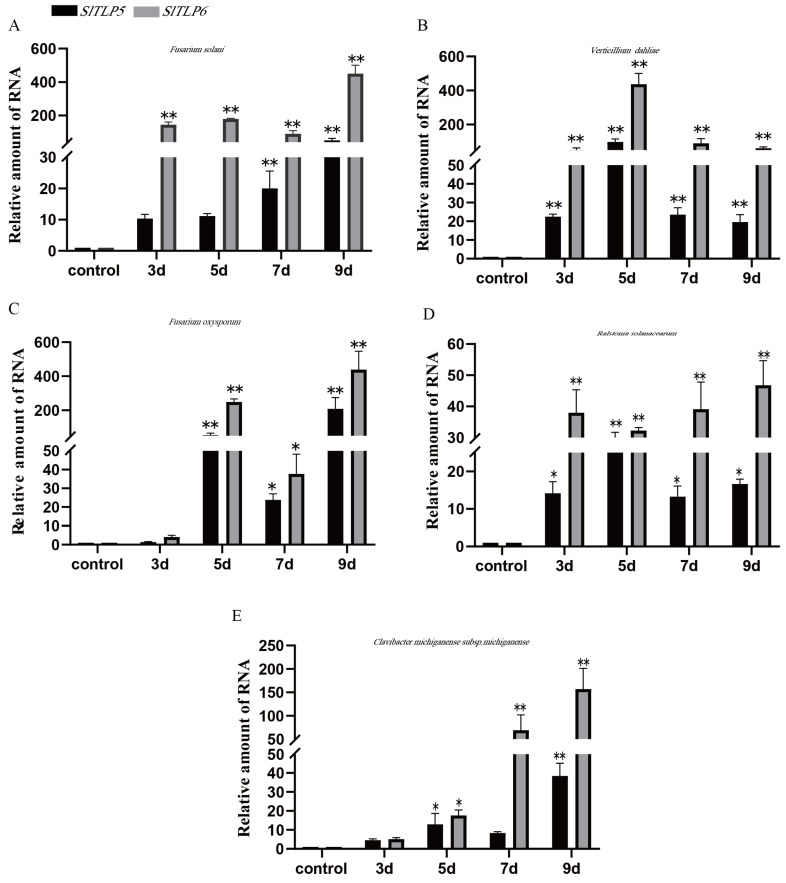
In the “S-55” disease-resistant cultivar, the transcript levels of the SlTLP5 and SlTLP6 genes increased after treatment with the three fungal pathogens, and the transcript levels were very similar in plants treated with the same pathogen. The transcript levels of the SlTLP5 and SlTLP6 genes both increased and reached significant levels with the bacterial pathogen treatment (Figure 2A–E).
Figure 2.
Response of SlTLP5 and SlTLP6 in the resistant cultivar “S-55”. Total RNA was extracted from seedlings at four infection stages, days 0, 3, 5, 7, and 9, and expression of genes was assessed using RT-qPCR. (A–E) represent F. solani, V. dahliae, F. oxysporum, R. solanacearum, and C. michiganense subsp. michiganense treatments, respectively. Note: The asterisk indicates a significant difference compared to the control group (* p < 0.05 and ** p < 0.01). Error bars indicate SDs for three replicates.
After infection with the five pathogens, the transcription levels of the SlTLP5 and SlTLP6 genes in the disease-resistant cultivars were higher than those in the susceptible cultivars, and the transcription level of SlTLP6 was slightly greater than that of SlTLP5.
3.2. Overexpression of TLP Conferred Increased Disease Resistance to Tomato Plants
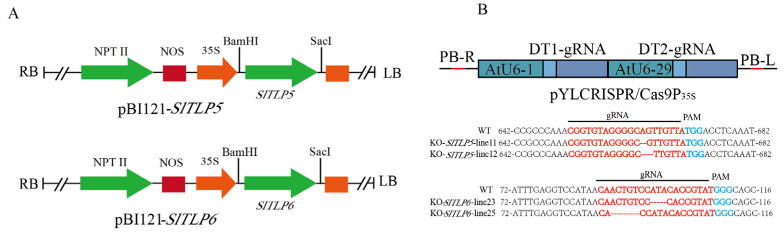
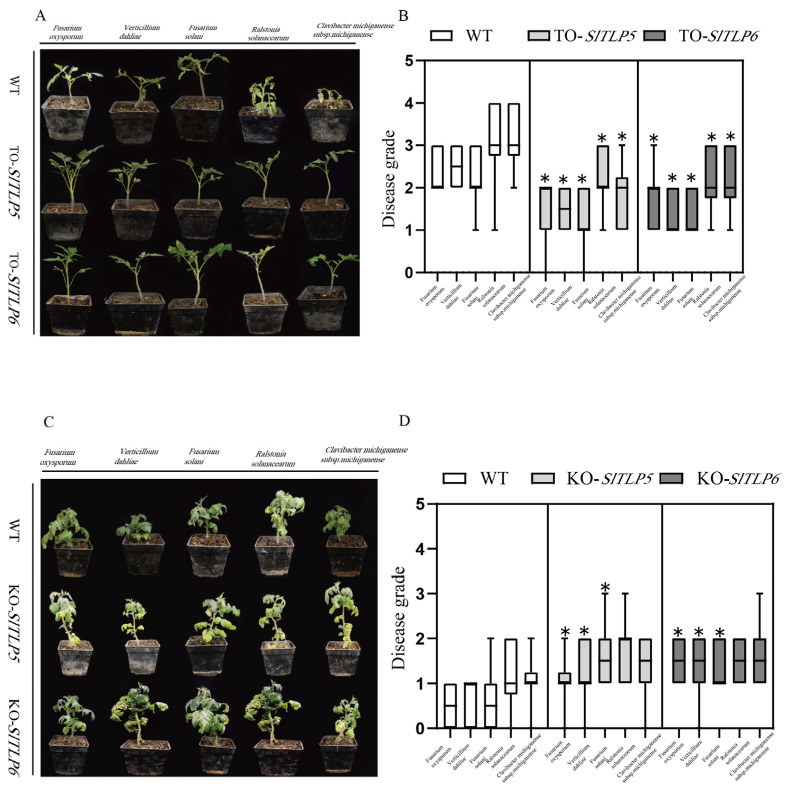
Recombinant binary vectors pBI121-SlTLP5 and pBI121-SlTLP6 were constructed and used to transfect transgenic plants and an empty plasmid frame control (Figure 3A). The positive plants overexpressing SlTLP5 and SlTLP6 were detected using RT-qPCR. To identify disease resistance in the transgenic plants, transgenic tomato seedlings were inoculated with the five pathogens, as described in the Materials and Methods. We found that both transgenic lines exhibited increased resistance to all five diseases compared with the control plants, and they reached a significant level (Figure 4A,B). The SlTLP5- and SlTLP6-overexpressing lines demonstrated a stronger resistance to fungal diseases than bacterial diseases. After treatment with the bacterial pathogens, the control group withered severely, with disease indices of 3.1 and 3.2 for R. solanacearum and C. michiganense subsp. michiganense, respectively. The plants overexpressing SlTLP5 and SlTLP6 were less injured than the control group (Supplementary Table S2), with disease indices of only 1.8 and 2.2, respectively, and the lines overexpressing SlTLP5 were slightly more resistant than those overexpressing SlTLP6.
Figure 3.
Generation of transgenic plants. (A) SlTLP5 and SlTLP6 overexpression vector backbone construction. (B) Schematic representation of CRISPR/Cas9 cassettes used for SlTLP5 and SlTLP6 mutant vectors. The plant genome sequences of SlTLP5 and SlTLP6 were aligned via CLUSTALX nucleic acid sequence alignment, and gRNA sequences are highlighted in red.
Figure 4.
Gene function validation via overexpression and CRISPR/Cas9 gene editing. Plants treated with bacterial and fungal pathogens were photographed on days 7 and 14 after infection for wilting disease and disease resistance identification. (A) Plants overexpressing SlTLP5 and SlTLP6 demonstrated increased disease resistance. (B) Disease grades (* indicates p < 0.05) for To-TLP5, To-TLP6, and empty plasmid transgenic plants after infection with five pathogens. (C) SlTLP5 and SlTLP6 knockouts decreased plant disease resistance. (D) Disease grading for KO-SlTLP5, KO-SlTLP6, and off-target transgenic plants after infection with the five pathogens. The error bar is the standard deviation of three biological replicates: Student’s t-test; asterisks (*) indicate p < 0.05.
3.3. TLP Knockout Reduces Disease Resistance in Tomato Plants
We assumed that SlTLP5 and SlTLP6 are required in multiple disease resistance. CRISPR/Cas9 SlTLP5 and SlTLP6 loss-of-function alleles were obtained in the resistant “S-55” variety. Twenty-eight transgenic plants were identified in the SlTLP5 knockout, carrying 1- and 2-nucleotide deletions (referred to as KO-SlTLP5-Line11 and KO-SlTLP5-Line12, respectively), and thirty-one transgenic plants were identified in the SlTLP6 knockout, carrying 3- and 5-nucleotide deletions (referred to as KO-SlTLP6-Line23 and KO-SlTLP6-Line25, respectively) (Figure 3B). Under normal growth conditions, the phenotypes of all the gene-edited plants were indistinguishable from those of the control plants.
The KO-SlTLP5-Line, KO-SlTLP6-Line, and off-target control plants were infected with the five pathogens. We found that SlTLP5-knockout- and SlTLP6-knockout line plants withered severely compared with the control plants (Figure 4C,D). The data revealed that both the TLP5 and TLP6 knockout plants had significantly higher disease severity than the control plants among those treated with the three fungal pathogens (Supplementary Table S2). Both the SlTLP5 and SlTLP6 knockout plants exhibited increased disease severity than the control plants among those treated with the bacterial pathogens, but significant levels were not reached, indicating that SlTLP5 and SlTLP6 are more sensitive to fungal pathogens.
3.4. Physiological Changes in Resistance of Overexpressing Plants of Susceptible Cultivars
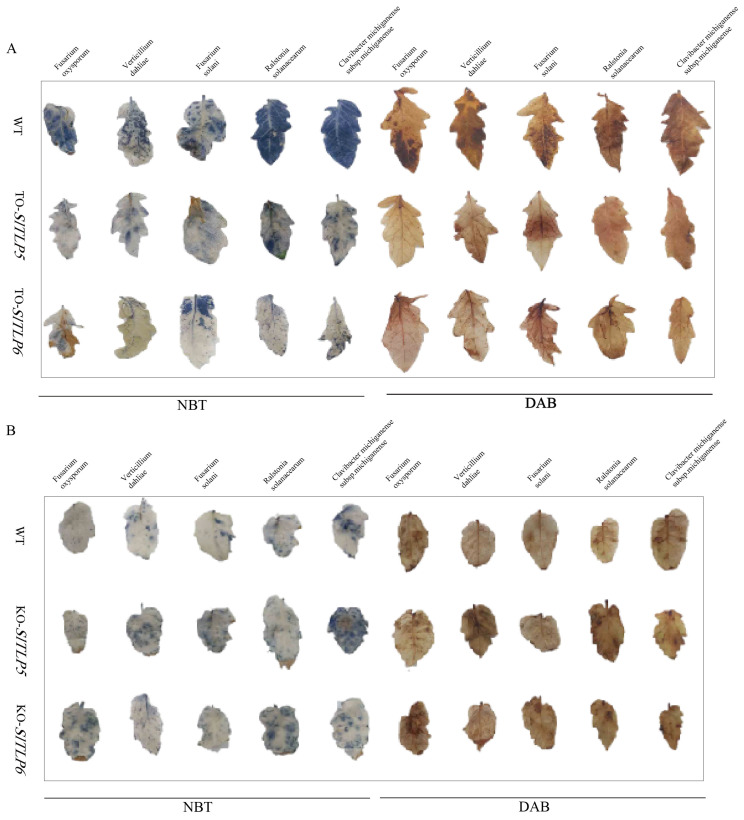
After treatment with the five pathogens for 7 days, DAB and NBT staining revealed that there were fewer spots on the leaves of plants overexpressing SlTLP5 and SlTLP6 and more spots on the control plants (Figure 5A).
Figure 5.
H2O2 and O2− accumulation in leaves. (A) H2O2 and O2− accumulation in leaves reduces in overexpressing transgenic plants. (B) H2O2 and O2− accumulation increases in the leaves of knockout plants.
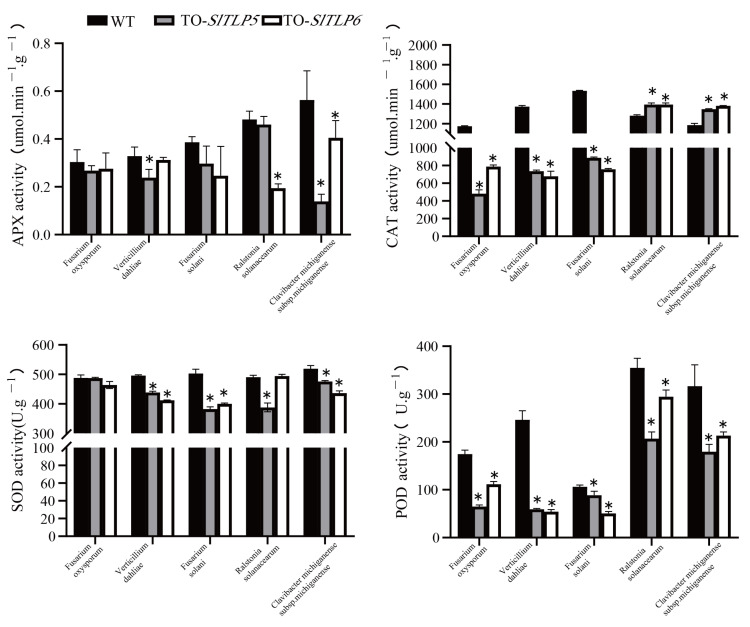
The enzyme activity of the antioxidant system was determined through its ROS levels. The transgenic lines overexpressing SlTLP5 and SlTLP6 showed the same trend under the fungal pathogen treatment. Compared with the control group, in transgenic lines overexpressing SlTLP5 and SlTLP6, the activities of POD, SOD, APX, and CAT antioxidant enzymes did not increase but instead decreased, with the most significant decrease in CAT. However, the activities of APX, SOD, and POD decreased under the bacterial pathogen treatment (Supplementary Table S3), whereas CAT activity in response to bacterial pathogens was the opposite compared with that of the fungal pathogen treatment, and it increased significantly after CAT overexpression (Figure 6).
Figure 6.
Changes in physiological parameters of overexpressing transgenic plants infected with pathogens. The error bar is the standard deviation of three biological replicates: Student’s t-test; asterisks (*) indicate p < 0.05.
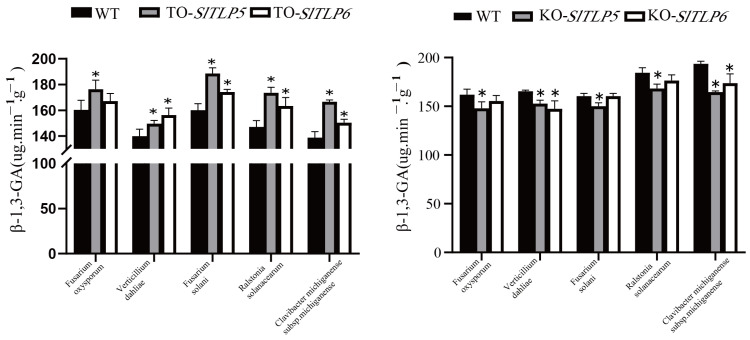
β-1,3-GA activity was determined in transgenic plants overexpressing SlTLP5 and SlTLP6 after the pathogen treatment. The β-1,3-GA activity in all the transgenic lines overexpressing SlTLP5 and SlTLP6 treated with the five pathogens significantly increased (Figure 7 and Figure 8) and was significantly higher than that of the control group. Notably, the transgenic lines overexpressing SlTLP5 and SlTLP6 had increased resistance to diseases, and this was probably due to increased β-1,3-GA activity.
Figure 7.
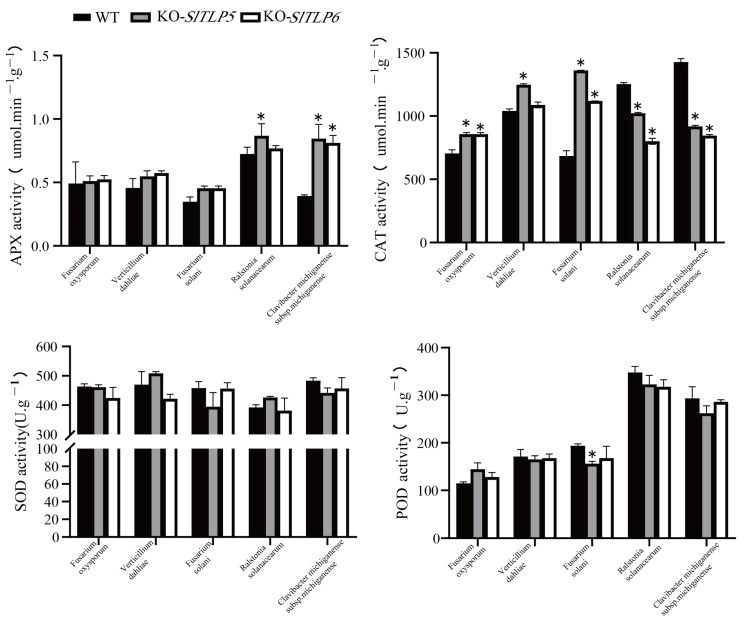
Changes in physiological parameters of knockout transgenic plants infected with pathogens. The error bar is the standard deviation of three biological replicates: Student’s t-test; asterisks (*) indicate p < 0.05.
Figure 8.
Changes in β-1,3-glucanase activity in overexpressing and knockout transgenic plants. The error bar is the standard deviation of three biological replicates: Student’s t-test; asterisks (*) indicate p < 0.05.
3.5. Physiological Changes in Resistance of Knockout Plants of Disease-Resistant Cultivars
Seven days after infection, DAB and NBT staining revealed that there were more spots on the leaves of the positive SlTLP5 and SlTLP6 knockout plants, indicating that H2O2 and O2 − accumulation caused more damage to them (Figure 5B). The enzyme activity of the antioxidant system was determined using ROS. We found that the changes in ROS enzyme activity in the SlTLP5 and SlTLP6 knockout lines were similar. In response to the three fungal pathogens, we found that there were no significant changes in POD, SOD, APX, or CAT activity after the knockout of SlTLP5 and SlTLP6. In response to the two bacterial pathogens, there was no significant change in POD or SOD (Supplementary Table S3), and the activities of APX and CAT increased and decreased, respectively, after the knockout of SlTLP5 and SlTLP6, the results of which were similar to those after fungal infection (Figure 7).
In the SlTLP5 and SlTLP6 lines, the activity of β-1,3-Ga decreased after treatment with the five pathogens; in particular, β-1,3-Ga activity in the TLP5 lines was significantly lower than in the control and TLP6 lines (Figure 8), indicating that the activity of β-1,3-Ga was affected by the knockout of the TLP5 and TLP6 lines, and thus, the plant resistance was reduced.
4. Discussion
It is important to understand the defense mechanism of plants to induce basic resistance under different stress conditions. TLP, a member of the PR-5 family, includes TLP, Osmotin, and Zeamatin [1]. The PR-5 family is associated with responses to biotic stress. TLPs [32] have been identified in more than 180 plants, including dicots, monocots, gymnosperms, bryophytes, and algae, and some TLPs demonstrate broad-spectrum resistance to a variety of pathogens. Their overexpression can enhance resistance to pathogens, including Alternaria solani, Sclerotinia sclerotiorum, P. syringae pv. DC3000, Puccinia triticina, and Colletotrichum gloeosporioides f. sp. manitiss [7,8,33,34]. In addition, TLPs can also be induced by bacterial pathogens, abiotic stresses (e.g., wounding, drought, osmotic stress, low temperature, high salt, and UV radiation), and phytohormones [35,36,37]. In this study, the susceptible tomato cultivar “No. 5” and the resistant tomato cultivar “S-55” were infected with five soil-borne diseases. According to the RT-qPCR results, SlTLP5 and SlTLP6 were expressed to different degrees in “No. 5”, and the cultivars that were treated with the three fungi had higher SlTLP5 and SlTLP6 levels than those infected with C. michiganense subsp. michiganense and R. solanacearum (Figure 1). SlTLP5 and SlTLP6 were significantly increased in the disease-resistant tomato cultivar “S-55”. Similarly, in the disease-resistant plants, those treated with three different pathogenic fungi had higher SlTLP5 and SlTLP6 levels than those infected with C. michiganense subsp. michiganense, or R. solanacearum. TLP was more sensitive to fungal diseases, and SlTLP6 transcript levels were higher than those of SlTLP5 (Figure 2).
The anti-fungal effect of TLP has been applied to the breeding of a variety of crops. For example, CkTLP significantly enhanced resistance to V. dahliae in Arabidopsis [38]. Cold-induced taTLP accumulated in apoplasts contributes to the resistance of winter wheat to Microdochium nivale [39]. Additionally, the antibacterial activity of TLP was also found in rice [40] and potatoes [41]. Overexpression lines and knockout lines were generated in “No. 5” and “S-55” (Figure 7). The overexpression lines had enhanced disease resistance after treatment with the five pathogens, and the knockout lines had reduced disease resistance (Figure 8) and stronger resistance to fungal diseases than to bacteria.
Fungal-mediated biotic stress activates the plant immune system by sensing pathogen-associated molecular patterns and molecular receptors [42]. Subsequently, ROS are formed to induce abscisic acid, salicylic acid, and jasmonic acid signals and upregulate PR genes [43]. Tobacco TLPs determine the morphology of cell death through a critical role in the RAS2/cAMP-mediated regulation of intracellular ROS accumulation [44]. It was hypothesized that ROS in tomato plants are involved in the disease resistance mechanism of SlTLP5 and SlTLP6, and the data revealed that the overexpression of SlTLP5 and SlTLP6 in the “No. 5” cultivar treated with the fungal pathogens decreased APX, CAT, POD, and SOD activities toward ROS. There was a slight difference between those treated with bacterial pathogens and those treated with fungi, in that CAT increased in those treated with the bacterial pathogens (Figure 6). SlTLP5 and SlTLP6 knockout experiments in the resistant “S-55” cultivar revealed that there was little difference in APX, POD, and SOD activities toward ROS (Figure 7), and CAT activity increased and decreased in fungal and bacterial diseases, respectively, indicating that ROS possessed different regulatory pathways in the TLP disease resistance mechanism. Generally, APX, POD, and SOD activities were negatively correlated with SlTLP5 and SlTLP6 in the five soil-borne diseases, and CAT was positively and negatively correlated in the bacterial and fungal diseases, respectively.
Plant TLPs inhibit pathogenic and non-pathogenic fungi mainly by lysing fungal spores and inhibiting spore germination and growth patterns [45,46]. This probably occurs because of intra-β-1,3-GA activity in the acidic domain space of TLPs [47] and their role in inducing mechanisms involved in pathogen defense, including generating phenylpropanoid and phytoalexin [48,49]. Chitinase, β-1,3-GA, and miRNAs are involved in the positive regulation (miR164a, miR168a, and miR393) and negative regulation (miR394) of Fusarium resistance in Allium plants [50,51,52,53]. After Elsinoe ampelina inoculation, the TLP gene exhibited increased expression, as did chitinase and antibacterial protein genes such as β-1,3GA [54,55], PR1/PR1a [56], polygalacturonase inhibitor protein [57], and lipid transfer protein [58]. Likewise, β-1,3-GA activity was determined and we found that it significantly increased in both SlTLP5- and SlTLP6-overexpressing transgenic plants treated with all five pathogens, regardless of pathogen species, and it was stronger in SlTLP5-overexpressing plants than in plants overexpressing SlTLP6 (Figure 8). β-1,3-GA activity decreased in the SlTLP5 and SlTLP6 knockout transgenic lines and decreased significantly in the SlTLP5 knockout plants treated with the five pathogens, suggesting that the expression of TLP is associated with β-1,3-GA activity and that it positively regulated β-1,3-GA activity to confer resistance to the five tomato pathogens.
Although the TLP family has been extensively studied, the core mechanism of resistance, particularly upstream regulation, remains unclear. However, the mechanism of SlTLP5 and SlTLP6 disease resistance can provide a reference for subsequent studies. In other words, the ROS pathway feedback was different under fungal and bacterial infections. The expression of TLP inhibited POD, SOD, APX, and CAT activities under fungal infection. POD, SOD, and APX activities were similar to those during bacterial infection, whereas CAT activity increased. Although the RT-qPCR results indicated that SITLP6 transcript levels were higher than those of SITLP5, SITLP5 provided slightly more resistance than SITLP6 according to the symptom score, which is consistent with previous studies [18]. This may be due to higher β-1,3-GA activity in plants overexpressing SITLP5 than in those overexpressing SITLP6. In conclusion, TLP confers plant resistance through β-1,3-GA activity under pathogen infection. The resistance conferred by SITLP5 and SITLP6 to the five pathogens makes them ideal candidates for plant transformations aimed at producing resistant crops.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14081622/s1, Table S1. Primers used in the article; Table S2: Disease resistance of transgenic plants; Table S3: Determination of physiological indexes of transgenic plants.
Author Contributions
Conceptualization, X.L. and B.X.; Data curation, Z.L., J.X. and Z.Y.; Formal analysis, X.L., C.J. and Y.Z.; Funding acquisition, K.Z.; Software, J.L. and M.D.; Writing—original draft, X.L.; Writing—review and editing, X.L. and K.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the National Natural Science Foundation of China (32160715); Yunnan Fundamental Research Projects (202301AS070078); the Key Project of the Basic Research Program of Yunnan Province (202201AS070309); the Research and Integrated Applications of Key Technology in the Standardized Production of Facility Vegetables (202102AE090005); and the Innovation Guidance and Technology-Based Enterprise Cultivation Program (202204BI090006).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Van Loon L., Rep M., Pieterse C. Significance of Inducible Defense-related Proteins in Infected Plants. Annu. Rev. Phytopathol. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- 2.Narusaka Y., Narusaka M., Abe H., Hosaka N., Kobayashi M., Shiraishi T., Iwabuchi M. High-throughput screening for plant defense activators using a β-glucuronidase-reporter gene assay in Arabidopsis thaliana. Plant Biotechnol. 2009;26:345–349. doi: 10.5511/plantbiotechnology.26.345. [DOI] [Google Scholar]
- 3.Zhang C., Liu L., Zheng Z., Sun Y., Zhou L., Yang Y., Cheng F., Zhang Z., Wang X., Huang S., et al. Fine mapping of the Ph-3 gene conferring resistance to late blight (Phytophthora infestans) in tomato. Theor. Appl. Genet. 2013;126:2643–2653. doi: 10.1007/s00122-013-2162-1. [DOI] [PubMed] [Google Scholar]
- 4.Rajam M.V., Chandola N., Saiprasad Goud P., Singh D., Kashyap V., Choudhary M.L., Sihachakr D. Thaumatin gene confers resistance to fungal pathogens as well as tolerance to abiotic stresses in transgenic tobacco plants. Biol. Plant. 2007;51:135–141. doi: 10.1007/s10535-007-0026-8. [DOI] [Google Scholar]
- 5.Abad L.R., D’Urzo M.P., Liu D., Narasimhan M.L., Reuveni M., Zhu J.K., Niu X., Singh N.K., Hasegawa P.M., Bressan R.A. Antifungal activity of tobacco osmotin has specificity and involves plasma membrane permeabilization. Plant Sci. 1996;118:11–23. doi: 10.1016/0168-9452(96)04420-2. [DOI] [Google Scholar]
- 6.Radhajeyalakshmi R., Velazhahan R., Balasubramanian P., Doraiswamy S. Overexpression of thaumatin-like protein in transgenic tomato plants confers enhanced resistance to Alternaria solani. Arch. Phytopathol. Plant Prot. 2005;38:257–265. doi: 10.1080/03235400400027382. [DOI] [Google Scholar]
- 7.Aghazadeh R., Zamani M., Motallebi M., Moradyar M. Agrobacterium-Mediated Transformation of the Oryza sativa Thaumatin-Like Protein to Canola (R Line Hyola308) for Enhancing Resistance to Sclerotinia sclerotiorum. Iran. J. Biotechnol. 2017;15:201–207. doi: 10.15171/ijb.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan X., Qiao H., Zhang X., Guo C., Wang M., Wang Y., Wang X. Analysis of the grape (Vitis vinifera L.) thaumatin-like protein (TLP) gene family and demonstration that TLP29 contributes to disease resistance. Sci. Rep. 2017;7:4269. doi: 10.1038/s41598-017-04105-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhury S., Basu A., Kundu S. Overexpression of a New Osmotin-Like Protein Gene (SindOLP) Confers Tolerance against Biotic and Abiotic Stresses in Sesame. Front. Plant Sci. 2017;8:410. doi: 10.3389/fpls.2017.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J.-J., Sturrock R., Ekramoddoullah A.K.M. The superfamily of thaumatin-like proteins: Its origin, evolution, and expression towards biological function. Plant Cell Rep. 2010;29:419–436. doi: 10.1007/s00299-010-0826-8. [DOI] [PubMed] [Google Scholar]
- 11.Wang X., Tang C., Deng L., Cai G., Liu X., Liu B., Han Q., Buchenauer H., Wei G., Han D., et al. Characterization of a pathogenesis-related thaumatin-like protein gene TaPR5 from wheat induced by stripe rust fungus. Physiol. Plant. 2010;139:27–38. doi: 10.1111/j.1399-3054.2009.01338.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim B.-G., Fukumoto T., Tatano S., Gomi K., Ohtani K., Tada Y., Akimitsu K. Molecular cloning and characterization of a thaumatin-like protein-encoding cDNA from rough lemon. Physiol. Mol. Plant Pathol. 2009;74:3–10. doi: 10.1016/j.pmpp.2009.07.001. [DOI] [Google Scholar]
- 13.Kim D.H., Noh M.Y., Park K.B., Jo Y.H. Expression profiles of two thaumatin-like protein (TmTLP) genes in responses to various micro-organisms from Tenebrio molitor. Entomol. Res. 2017;47:35–40. doi: 10.1111/1748-5967.12197. [DOI] [Google Scholar]
- 14.Beatrice C., Linthorst J.M.H., Cinzia F., Luca R. Enhancement of PR1 and PR5 gene expressions by chitosan treatment in kiwifruit plants inoculated with Pseudomonas syringae pv. actinidiae. Eur. J. Plant Pathol. 2016;148:163–179. doi: 10.1007/s10658-016-1080-x. [DOI] [Google Scholar]
- 15.Rout E., Nanda S., Joshi R.K. Molecular characterization and heterologous expression of a pathogen induced PR5 gene from garlic (Allium sativum L.) conferring enhanced resistance to necrotrophic fungi. Eur. J. Plant Pathol. 2016;144:345–360. doi: 10.1007/s10658-015-0772-y. [DOI] [Google Scholar]
- 16.Kalpana K., Maruthasalam S., Rajesh T., Poovannan K., Kumar K.K., Kokiladevi E., Raja J.A., Sudhakar D., Velazhahan R., Samiyappan R., et al. Engineering sheath blight resistance in elite indica rice cultivars using genes encoding defense proteins. Plant Sci. 2006;170:203–215. doi: 10.1016/j.plantsci.2005.08.002. [DOI] [Google Scholar]
- 17.De A Campos M., Silva M.S., Magalhães C.P., Ribeiro S.G., Sarto R.P., Vieira E.A., de Sá M.F.G. Expression in Escherichia coli, purification, refolding and antifungal activity of an osmotin from Solanum nigrum. Microb. Cell Factories. 2008;7:7. doi: 10.1186/1475-2859-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu H., Deng M., Yang Z., Mao L., Jiang S., Yue Y., Zhao K. Two Tomato (Solanum lycopersicum) Thaumatin-Like Protein Genes Confer Enhanced Resistance to Late Blight (Phytophthora infestans) Phytopathology. 2021;111:1790–1799. doi: 10.1094/PHYTO-06-20-0237-R. [DOI] [PubMed] [Google Scholar]
- 19.Jiang N., Cui J., Meng J., Luan Y. A Tomato Nucleotide Binding Sites—Leucine-Rich Repeat Gene Is Positively Involved in Plant Resistance to Phytophthora infestans. Phytopathology. 2018;108:980–987. doi: 10.1094/PHYTO-12-17-0389-R. [DOI] [PubMed] [Google Scholar]
- 20.Zhao K., Shen X., Yuan H., Liu Y., Liao X., Wang Q., Liu L., Li F., Li T. Isolation and Characterization of Dehydration-Responsive Element-Binding Factor 2C (MsDREB2C) from Malus sieversii Roem. Plant Cell Physiol. 2013;54:1415–1430. doi: 10.1093/pcp/pct087. [DOI] [PubMed] [Google Scholar]
- 21.Løvdal T., Lillo C. Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal. Biochem. 2009;387:238–242. doi: 10.1016/j.ab.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Hong Y., Cui J., Liu Z., Luan Y. SpWRKY6 acts as a positive regulator during tomato resistance to Phytophthora infestans infection. Biochem. Biophys. Res. Commun. 2018;506:787–792. doi: 10.1016/j.bbrc.2018.10.155. [DOI] [PubMed] [Google Scholar]
- 23.Ma X.L., Zhang Q.Y., Zhu Q.L., Liu W., Chen Y., Qiu R., Wang B., Yang Z.F., Li H., Lin Y.R., et al. A Robust CRISPR/Cas9 System for Convenient, High-Efficiency Multiplex Genome Editing in Monocot and Dicot Plants. Mol. Plant. 2015;8:1274–1284. doi: 10.1016/j.molp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Álvarez B., Vasse J., Le-Courtois V., Trigalet-Démery D., López M.M., Trigalet A. Comparative Behavior of Ralstonia solanacearum Biovar 2 in Diverse Plant Species. Phytopathology. 2008;98:59–68. doi: 10.1094/PHYTO-98-1-0059. [DOI] [PubMed] [Google Scholar]
- 25.Sebastià P., de Pedro-Jové R., Daubech B., Kashyap A., Coll N.S., Valls M. The Bacterial Wilt Reservoir Host Solanum dulcamara Shows Resistance to Ralstonia solanacearum Infection. Front. Plant Sci. 2021;12:755708. doi: 10.3389/fpls.2021.755708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji H., Mao H., Li S., Feng T., Zhang Z., Cheng L., Luo S., Borkovich K.A., Ouyang S. Fol-milR1, a pathogenicity factor of Fusarium oxysporum, confers tomato wilt disease resistance by impairing host immune responses. New Phytol. 2021;232:705–718. doi: 10.1111/nph.17436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Validov S.Z., Kamilova F.D., Lugtenberg B.J.J. Monitoring of pathogenic and non-pathogenic Fusarium oxysporum strains during tomato plant infection. Microb. Biotechnol. 2011;4:82–88. doi: 10.1111/j.1751-7915.2010.00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Çakır B., Gül A., Yolageldi L., Özaktan H. Response to Fusarium oxysporum f.sp. radicis-lycopersici in tomato roots involves regulation of SA- and ET-responsive gene expressions. Eur. J. Plant Pathol. 2014;139:379–391. doi: 10.1007/s10658-014-0394-9. [DOI] [Google Scholar]
- 29.Cui J., Luan Y., Jiang N., Bao H., Meng J. Comparative transcriptome analysis between resistant and susceptible tomato allows the identification of lncRNA16397 conferring resistance to Phytophthora infestans by co-expressing glutaredoxin. Plant J. 2017;89:577–589. doi: 10.1111/tpj.13408. [DOI] [PubMed] [Google Scholar]
- 30.Cui J., Jiang N., Zhou X., Hou X., Yang G., Meng J., Luan Y. Tomato MYB49 enhances resistance to Phytophthora infestans and tolerance to water deficit and salt stress. Planta. 2018;248:1487–1503. doi: 10.1007/s00425-018-2987-6. [DOI] [PubMed] [Google Scholar]
- 31.Zong Y., Liu J., Li B., Qin G., Tian S. Effects of yeast antagonists in combination with hot water treatment on postharvest diseases of tomato fruit. Biol. Control. 2010;54:316–321. doi: 10.1016/j.biocontrol.2010.06.003. [DOI] [Google Scholar]
- 32.De Jesús-Pires C., Ferreira-Neto J.R., Pacifico Bezerra-Neto J., Kido E.A., de Oliveira Silva R.L., Pandolfi V., Wanderley-Nogueira A.C., Binneck E., da Costa A.F., Pio-Ribeiro G., et al. Plant Thaumatin-like Proteins: Function, Evolution and Biotechnological Applications. Curr. Protein Pept. Sci. 2020;21:36–51. doi: 10.2174/1389203720666190318164905. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Yan H., Wei X., Zhang J., Wang H., Liu D. Expression analysis and functional characterization of a pathogen-induced thaumatin-like gene in wheat conferring enhanced resistance to Puccinia triticina. J. Plant Interact. 2017;12:332–339. doi: 10.1080/17429145.2017.1367042. [DOI] [Google Scholar]
- 34.Ojola P.O., Nyaboga E.N., Njiru P.N., Orinda G. Overexpression of rice thaumatin-like protein (Ostlp) gene in transgenic cassava results in enhanced tolerance to Colletotrichum gloeosporioides f. sp. manihotis. J. Genet. Eng. Biotechnol. 2018;16:125–131. doi: 10.1016/j.jgeb.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodrigo I., Vera P., Frank R., Conejero V. Identification of the viroid-induced tomato pathogenesis-related (PR) protein P23 as the thaumatin-like tomato protein NP24 associated with osmotic stress. Plant Mol. Biol. 1991;16:931–934. doi: 10.1007/BF00015088. [DOI] [PubMed] [Google Scholar]
- 36.He L., Li L., Zhu Y., Pan Y., Zhang X., Han X., Li M., Chen C., Li H., Wang C. BolTLP1, a Thaumatin-like Protein Gene, Confers Tolerance to Salt and Drought Stresses in Broccoli (Brassica oleracea L. var. Italica). Int. J. Mol. Sci. 2021;22:11132. doi: 10.3390/ijms222011132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Futamura N., Tani N., Tsumura Y., Nakajima N., Sakaguchi M., Shinohara K. Characterization of genes for novel thaumatin-like proteins in Cryptomeria japonica. Tree Physiol. 2006;26:51–62. doi: 10.1093/treephys/26.1.51. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q., Li F., Zhang X., Zhang Y., Hou Y., Zhang S., Wu Z. Purification and Characterization of a CkTLP Protein from Cynanchum komarovii Seeds that Confers Antifungal Activity. PLoS ONE. 2011;6:e16930. doi: 10.1371/journal.pone.0016930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuwabara C., Takezawa D., Shimada T., Hamada T., Fujikawa S., Arakawa K. Abscisic acid- and cold-induced thaumatin-like protein in winter wheat has an antifungal activity against snow mold, Microdochium nivale. Physiol. Plant. 2002;115:101–110. doi: 10.1034/j.1399-3054.2002.1150112.x. [DOI] [PubMed] [Google Scholar]
- 40.Chen W.P., Chen P.D., Liu D.J., Kynast R., Friebe B., Velazhahan R., Muthukrishnan S., Gill B.S. Development of wheat scab symptoms is delayed in transgenic wheat plants that constitutively express a rice thaumatin-like protein gene. Theor. Appl. Genet. 1999;99:755–760. doi: 10.1007/s001220051294. [DOI] [Google Scholar]
- 41.Liu D., Raghothama K.G., Hasegawa P.M., Bressan R.A. Osmotin overexpression in potato delays development of disease symptoms. Proc. Natl. Acad. Sci. USA. 1994;91:1888–1892. doi: 10.1073/pnas.91.5.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zipfel C., Felix G. Plants and animals: A different taste for microbes? Curr. Opin. Plant Biol. 2005;8:353–360. doi: 10.1016/j.pbi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Akbudak M.A., Yildiz S., Filiz E. Pathogenesis related protein-1 (PR-1) genes in tomato (Solanum lycopersicum L.): Bioinformatics analyses and expression profiles in response to drought stress. Genomics. 2020;112:4089–4099. doi: 10.1016/j.ygeno.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Narasimhan M.L., Damsz B., Coca M.A., Ibeas J.I., Yun D.-J., Pardo J.M., Hasegawa P.M., Bressan R.A. A Plant Defense Response Effector Induces Microbial Apoptosis. Mol. Cell. 2001;8:921–930. doi: 10.1016/S1097-2765(01)00365-3. Erratum in Mol. Cell 2001, 8, 1153. [DOI] [PubMed] [Google Scholar]
- 45.Anand A., Lei Z., Sumner L.W., Mysore K.S., Arakane Y., Bockus W.W., Muthukrishnan S. Apoplastic extracts from a transgenic wheat line exhibiting lesion-mimic phenotype have multiple pathogenesis-related proteins that are antifungal. Mol. Plant-Microbe Interact. 2004;17:1306–1317. doi: 10.1094/MPMI.2004.17.12.1306. [DOI] [PubMed] [Google Scholar]
- 46.Grenier J., Potvin C., Asselin A. Barley Pathogenesis-Related Proteins with Fungal Cell Wall Lytic Activity Inhibit the Growth of Yeasts. Plant Physiol. 1993;103:1277–1283. doi: 10.1104/pp.103.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osmond R.I., Hrmova M., Fontaine F., Imberty A., Fincher G.B. Binding interactions between barley thaumatin-like proteins and (1,3)-β-D-glucans: Kinetics, specificity, structural analysis and biological implications. Eur. J. Biochem. 2001;268:4190–4199. doi: 10.1046/j.1432-1327.2001.02331.x. [DOI] [PubMed] [Google Scholar]
- 48.El-Kereamy A., El-Sharkawy I., Ramamoorthy R., Taheri A., Errampalli D., Kumar P., Jayasankar S. Prunus domestica Pathogenesis-Related Protein-5 Activates the Defense Response Pathway and Enhances the Resistance to Fungal Infection. PLoS ONE. 2011;6:e17973. doi: 10.1371/journal.pone.0017973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Theis T., Stahl U. Antifungal proteins: Targets, mechanisms and prospective applications. Cell. Mol. Life Sci. 2004;61:437–455. doi: 10.1007/s00018-003-3231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chand S.K., Nanda S., Mishra R., Joshi R.K. Multiple garlic (Allium sativum L.) microRNAs regulate the immunity against the basal rot fungus Fusarium oxysporum f. sp. Cepae. Plant Sci. 2017;257:9–21. doi: 10.1016/j.plantsci.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Anisimova O.K., Shchennikova A.V., Kochieva E.Z., Filyushin M.A. Pathogenesis-Related Genes of PR1, PR2, PR4, and PR5 Families Are Involved in the Response to Fusarium Infection in Garlic (Allium sativum L.) Int. J. Mol. Sci. 2021;22:6688. doi: 10.3390/ijms22136688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Filyushin M.A., Anisimova O.K., Kochieva E.Z., Shchennikova A.V. Genome-Wide Identification and Expression of Chitinase Class I Genes in Garlic (Allium sativum L.) Cultivars Resistant and Susceptible to Fusarium proliferatum. Plants. 2021;10:720. doi: 10.3390/plants10040720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X., Wang H., Zhu W., Li W., Wang F. Transcriptome Analysis Reveals the Effects of Chinese Chive (Allium tuberosum R.) Extract on Fusarium oxysporum f. sp. radicis-lycopersici Spore Germination. Curr. Microbiol. 2020;77:855–864. doi: 10.1007/s00284-020-01875-x. [DOI] [PubMed] [Google Scholar]
- 54.Jayasankar S., Li Z., Gray D.J. In-vitro selection of Vitis vinifera ‘Chardonnay’ with Elsinoe ampelina culture filtrate is accompanied by fungal resistance and enhanced secretion of chitinase. Planta. 2000;211:200–208. doi: 10.1007/s004250000285. [DOI] [PubMed] [Google Scholar]
- 55.Schickler H., Chet I. Heterologous chitinase gene expression to improve plant defense against phytopathogenic fungi. J. Ind. Microbiol. Biotechnol. 1997;19:196–201. doi: 10.1038/sj.jim.2900447. [DOI] [Google Scholar]
- 56.Singh N.K., Kumar K.R.R., Kumar D., Shukla P., Kirti P.B. Characterization of a Pathogen Induced Thaumatin-Like Protein Gene AdTLP from Arachis diogoi, a Wild Peanut. PLoS ONE. 2013;8:e83963. doi: 10.1371/journal.pone.0083963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Matteo A., Federici L., Mattei B., Salvi G., Johnson K.A., Savino C., De Lorenzo G., Tsernoglou D., Cervone F. The crystal structure of polygalacturonase-inhibiting protein (PGIP), a leucine-rich repeat protein involved in plant defense. Proc. Natl. Acad. Sci. USA. 2003;100:10124–10128. doi: 10.1073/pnas.1733690100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blein J.-P., Coutos-Thévenot P., Marion D., Ponchet M. From elicitins to lipid-transfer proteins: A new insight in cell signalling involved in plant defence mechanisms. Trends Plant Sci. 2002;7:293–296. doi: 10.1016/S1360-1385(02)02284-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.