Supplemental Digital Content is available in the text.
Keywords: ileal pouch-anal anastomosis, ulcerative colitis, pouch failure
Abstract
Objective:
This systematic review aims to assess the incidence of pouch failure and the correlation between ileal pouch-anal anastomosis (IPAA)-related complications and pouch failure.
Background:
Previous studies demonstrated wide variation in postoperative complication rates following IPAA.
Methods:
A systematic review was performed by searching the MEDLINE, EMBASE, and Cochrane Library databases for studies reporting on pouch failure published from January 1, 2010, to May 6, 2020. A meta-analysis was performed using a random-effects model, and the relationship between pouch-related complications and pouch failure was assessed using Spearman’s correlations.
Results:
Thirty studies comprising 22,978 patients were included. Included studies contained heterogenic patient populations, different procedural stages, varying definitions for IPAA-related complications, and different follow-up periods. The pooled pouch failure rate was 7.7% (95% confidence intervals: 5.56–10.59) and 10.3% (95% confidence intervals: 7.24–14.30) for studies with a median follow-up of ≥5 and ≥10 years, respectively. Observed IPAA-related complications were anastomotic leakage (1–17%), pelvic sepsis (2–18%), fistula (1–30%), stricture (1–34%), pouchitis (11–61%), and Crohn’s disease of the pouch (0–18%). Pelvic sepsis (r = 0.51, P < 0.05) and fistula (r = 0.63, P < 0.01) were correlated with pouch failure. A sensitivity analysis including studies with a median follow-up of ≥5 years indicated that only fistula was significantly correlated with pouch failure (r = 0.77, P < 0.01).
Conclusions:
The single long-term determinant of pouch failure was pouch fistula, which is a manifestation of a chronic leak. Therefore, all effort should be taken to prevent an acute leak from becoming a chronic leak by early diagnosis and proactive management of the leak.
Mini abstract:
This systematic review aims to assess the incidence of pouch failure and the correlation between IPAA-related complications and pouch failure. Long-term pouch failure was correlated with fistula, suggesting that early septic complications may result in fistula formation during long-term follow-up, leading to an increased risk of pouch failure.
The ileal pouch-anal anastomosis (IPAA), introduced in 1978 by Parks and Nicholls,1 has evolved as the gold standard to restore intestinal continuity in patients with ulcerative colitis (UC), familial adenomatous polyposis (FAP), and in selected patients with Crohn’s disease (CD) after proctocolectomy. Although IPAA is associated with low mortality2 and good patient satisfaction,3 long-term pouch failure occurs in 5–15% of cases.4 Various IPAA-related complications (ie, anastomotic leakage, pelvic sepsis, fistula, stricture, pouchitis, CD of the pouch) are associated with pouch failure, of which pelvic sepsis appears to be the most important risk factor.5–8 To reduce the risk of pelvic sepsis, the focus has been on optimization of preoperative performance status, staged procedures,9–11 minimally invasive techniques,12 diversion of the pouch,13 and adequate postoperative management14 (ie, early detection and active treatment of anastomotic leaks). The optimal timing of IPAA creation after colectomy9–11 and the role of routine fecal diversion to reduce pelvic sepsis13,15,16 are still debated topics.
A large number of observational studies, mostly from specialized centers, have reported postoperative outcomes following IPAA surgery.16–24 The rate of IPAA-related complications varies widely in the literature and may have increased in the era of biologics.25 However, ambiguous definitions for anastomotic complications, differences in postoperative assessment, and duration of follow-up make a comparison of outcomes following IPAA challenging. To improve the current understanding of outcomes following IPAA, this systematic review aimed to compile the literature to date and determine the incidence of pouch failure and pouch-related complications. In addition, we examined the correlation between pouch-related complications and pouch failure.
METHODS
This systematic review and meta-analysis were performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)26 statement and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.27 This review has been registered on the PROSPERO Registry [ID=CRD42020221518].
Eligibility Criteria
Retrospective and prospective observational studies containing original data on pouch failure after primary IPAA in patients aged ≥18 years were included. Studies published from 2010 onwards were selected for the current meta-analysis, as studies published between 2000 and 2009 were included in a previous meta-analysis.2 Studies, including patients with UC only or UC and a combination of patients with CD, indeterminate colitis, FAP, and colorectal cancer, were eligible. Studies in which pouch failure was solely defined as pouch excision were excluded. Studies with a small sample size (<100 patients) and therefore potentially carrying an increased risk of sampling bias were excluded. Studies without a retrievable full text (ie, abstracts, nonpublished data) or English version were also excluded. To avoid repeated use of data from 1 patient due to multiple publications from the same institution, only the most recent or largest series per institution was included.
Literature Search Strategy
A comprehensive database search was performed using the MEDLINE (PubMed), EMBASE (Ovid), and Cochrane Library databases, focusing on studies evaluating pouch failure following IPAA published between January 1, 2010, and May 6, 2020. The search contained both MeSH and free-text terms and was composed in consultation with a clinical librarian (FE). The following search terms were used: (“restorative proctocolectomy,” “ileal pouch anal anastomosis,” “IPAA,” “ileal pouch”) AND (“postoperative complications,” “anastomotic leak,” “anastomotic complication,” “pelvic sepsis,” “pouch failure,” “pouch function,” “pouch fistula,” “long term outcome,” “long term complication”). Reference mining of the included studies was conducted to find any additional articles. The full literature search is shown in Table, Supplemental Digital Content 1, http://links.lww.com/AOSO/A42.
Study Selection
Study selection was performed in 3 phases according to the PRISMA statement (Figure, Supplemental Digital Content 1, http://links.lww.com/AOSO/A41). All identified studies were independently screened based on their title and abstract by 2 reviewers (L.H. and K.W.) using Rayan online software.28 Subsequently, the 2 independent researchers (L.H. and K.W.) screened the full texts and selected studies for inclusion in the systematic review and meta-analysis. Any discrepancies between the researches were resolved through a joint discussion that included a third researcher (W.B.).
Data Collection
Data collection included study characteristics (author, publication year, study design, inclusion period, number of included patients, country, follow-up time), patient characteristics (age, gender, diagnosis), surgical characteristics (number of stages of IPAA, percentage of diverted pouches), and postoperative outcome (pouch failure, anastomotic leak, pelvic sepsis, pouch-related fistula, stricture, CD after IPAA creation, pouchitis). A one-stage procedure was defined as a proctocolectomy with IPAA creation. In a 2-stage procedure, the proctocolectomy with IPAA creation and defunctioning ileostomy was followed by reversal at the second stage. A modified 2-stage was defined as a subtotal colectomy with defunctioning ileostomy, followed by completion proctectomy with IPAA and ileostomy reversal at the second stage. In a 3-stage procedure, a subtotal colectomy with end ileostomy was performed, followed by completion proctectomy with IPAA and defunctioning ileostomy, and ileostomy reversal at the third stage. Data and definitions of postoperative outcomes were extracted from included published reports. Study authors were not contacted for additional data.
Risk of Bias Assessment
The risk of bias was assessed by 2 independent researchers (L.H. and K.W.) using the Joanna Briggs Institute (JBI) checklist for case series.29 The predefined criteria for each of the 10 questions in the JBI checklist to assign low, unclear or high risk of bias are presented in Table, Supplemental Digital Content 2, http://links.lww.com/AOSO/A42. The risk of bias across studies was assessed and included the risk of publication, detection, and reporting bias.
Statistical Analysis
Categorical data are presented as numbers and percentages, and continuous data are presented as mean and SD or median and interquartile range as appropriate according to the variable’s distribution. Postoperative complications were pooled. The meta-analysis was performed by inverse variance weighting with a random-effects model in the “meta” package using R statistical software (R Development Core Team, version 3.6.1).30 Results were presented in forest plots giving an estimate of the mean proportion with a 95% confidence interval (CI). We assessed heterogeneity using I2, where I2 ≥ 50% was considered to represent significant heterogeneity and resulted in the use of a random effect model. For pouch failure, a sensitivity analysis was performed including only studies with a median follow-up of ≥5 and ≥10 years. A Spearman’s correlation between pouch-related complications and pouch failure was performed using IBM SPSS statistics for Windows version 26 (IBM Corp., Armong, NY). A sensitivity analysis was performed to identify factors correlated with long-term pouch failure in studies with a median follow-up of ≥5 years. A 2-sided P value of less than 0.05 was considered statistically significant.
RESULTS
Search Results
A total of 1947 studies were identified through the database search: 803 studies via PubMed, 1064 via EMBASE, and 80 via the Cochrane Library. After removal of duplicates, 1330 records were screened for eligibility based on their title and abstract. This resulted in the screening of 191 full-text articles and the inclusion of 30 studies (Table 1). Hand screening of reference lists yielded no additional articles eligible for inclusion. The PRISMA study selection flow chart is shown in Figure, Supplemental Digital Content 1, http://links.lww.com/AOSO/A41.
TABLE 1.
Study and Patient Characteristics of 30 Included Studies Reporting on Pouch Failure Following Primary IPAA Published Since 2010
| Author | Year | Country | Study Period | Study Type | N | Disease UC/IC/CD/FAP/ Other or Unspecified | Age | Gender (% Female) | Laparoscopic Approach (%) | Stapled Anastomosis (%) | Primary Diverting Ileostomy (%) | Median Follow-up Time (yrs) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carcamo et al31 | 2020 | Chile | 1984–2017 | R, single center | 116 | 116 | 35 | 67 (57.8) | 11 (9.48) | – | 116 (100) | 20 |
| Cataneo et al32 | 2019 | USA | 2004–2017 | R, single center | 176 | – | – | – | 79 (44.9) | – | 176 (100) | – |
| Dafnis33 | 2016 | Sweden | 1993–2012 | R, single center | 124 | 112/0/11/0/1 | 44 | 53 (42.7) | – | 115 (92.7) | 123 (99.2) | 11.2 |
| Die et al34 | 2020 | Spain | 1983–2015 | R, single center | 139 | 139 | – | 60 (43.2) | – | 85 (61.2) | 108 (77.7) | 12 * |
| Feinberg et al20 | 2020 | USA | 1986–2016 | R, single center | 3468 | 3300/168/0/0/0 | 39 | 1512 (43.6) | – | – | 3143 (90.7) | 7.9 |
| Hashimoto et al35 | 2013 | Japan | 2000–2012 | R, single center | 119 | 119 | 35/39 | 42 (38.2) n = 110 | 33 (30.0) | 67 (60.9) | – | 5.5 |
| Helavirta et al36 | 2016 | Finland | 1985–2009 | R, single center | 352 | 352 | 36 | 149 (42.3) | – | 69 (19.6) | 133 (37.8) | 5 |
| Ikeuchi et al37 | 2010 | Japan | 1983–2007 | R, single center | 944 | 944 | – | – | 1 (0.1) | 21 (2.2) | 686 (72.6) | – |
| Karjalainen et al23,38 | 2019 | Finland | 2005–2016 | R, single center | 510 | 510 | 39 | 198 (38.8) | 0 (0.0) | 52 (10.2) | 119 (23.3) | 6.9 |
| Karlbom et al39 | 2011 | Sweden | 1985–1996 | R, single center | 188 | 188 | 32 | 72 (38.3) | – | – | 154 (81.9) | 12.5 |
| Kayal et al40 | 2019 | USA | 2008–2017 | R, single center | 386 | 363/23 | 37 | 140 (46.6) | 281 (72.8) | 275 (71.2) | 311 (80.6) | 2 |
| Landerholm et al18 | 2017 | Sweden | 1964–2010 | R, population based | 1720 | 1720 | 36.8 | 643 (37.4) | – | – | – | 12.6 |
| Lee et al41 | 2019 | USA | 2000–2015 | R, single center | 212 | 212 | 36 | 87 (41.0) | 45 (21.2) | 212 (100) | 212 (100) | 4.7* |
| Leowardi et al42 | 2010 | Germany | 1988–1996 | R, single center | 294 | 294 | 33 | 133 (45.2) | – | 0 (0) | 294 (100) | 11.5 |
| Lightner et al19Farouk et al43 | 2017 | USA | 1981–2015 | R, single center | 1875 | 1875 | 34 | 855 (45.4) | – | – | 1855 (98.4) | – |
| Lorenzo et al44 | 2016 | Italy | 1985–2014 | R, single center | 185 | 185 | 33 | 61 (33.0) | – | 128 (69.1) | 128 (69.2) | 11.3 |
| Mark-Christensen et al22,45 | 2017 | Denmark | 1980–2013 | R, population based | 1991 | 1991 | 33 | 954 (47.8) | 165 (8.3) | – | 1849 (92.9) | 11.4 |
| McCombie et al46 | 2016 | New Zealand | 1984–2013 | R, population based | 136 | 104/4/15/12/1 | 39 | 61 (44.9) | – | – | – | 12 |
| Mege et al10 | 2016 | France | 2000–2015 | R, single center | 185 | 164/1/20/0/0 | 39/43 | 78 (42.2) | 185 (100) | 180 (97.3) | 185 (100) | 3 |
| Mennigen et al47 | 2011 | Germany | 1997–2009 | R, single center | 122 | 122 | 34/38 | 44 (36.1) | – | 71 (58.2) | 89 (73.0) | 3.3 |
| Rokke et al48 | 2011 | Norway | 1988–2002 | R, single center | 134 | 134 | 43 | 57 (42.5) | – | 134 (100) | 54 (43.9) | 11 |
| Sahami et al16 | 2016 | NL, Belgium, USA | 1990–2014 | R, multicenter | 621 | 545/59/17/0/0 | 38 | 270 (43.6) | 289 (46.5) | 601 (96.8) | 305 (49.1) | 3.3 |
| Sampietro et al49 | 2018 | Italy | 2007–2016 | R, single center | 150 | 143/3/4/0/0 | 43/51 | 58 (38.6) | 150 (100) | 143 (95.3) | 138 (92.0) | 5.1 |
| Tan et al50 | 2014 | Australië | 1999–2011 | R, single center | 142 | 142 | 43 | 60 (42.3) | – | 141 (99.3) | 142 (100) | 3 |
| Uchino et al24 | 2017 | Japan | 2005–2014 | R, multicenter | 2376 | 2376 | 40 | 963 (40.5) | – | 1078 (45.4) | 1664 (70.0) | 6.7 |
| Wasmuth et al51,52 | 2010 | Norway | 1984–2007 | R, single center | 315 | 287/7/5/11/5 | 32/36 | 119 (37.8) | – | 187 (59.4) | 256 (84.2) | 13.3 |
| Wibmer et al53 | 2010 | Germany | 1997–2008 | R, single center | 185 | 141/0/0/44/0 | 37 | 79 (42.7) | 66 (35.7) | 0 (0) | 163 (88.1) | 3.9* |
| Worley et al21 | 2017 | UK, Belgium, Denmark, NL, Spain | 1976–2017 | R, multicenter | 5083 | 3603/162/29/449/840 | 30–39 | 2230 (44.0) | – | – | 2554 (81.4) | – |
| Zaghiyan et al54 | 2016 | USA | 1997–2007 | R, single center | 334 | 237/97/0/0/0 | 38 | 148 (44.3) | – | – | 334 (100) | 5.8 |
| Bertucci Zoccali et al55 | 2019 | USA | 2000–2010 | R, single center | 411 | 411 | 36 | 171 (41.6) | 151 (36.7) | 256 (62.3) | 359 (87.3) | 5.2 |
Dashes indicate not specified.
*Mean follow-up.
CD indicates Crohn’s disease; FAP, familial adenomatous polyposis; IC, indeterminate colitis; IPAA, ileal pouch-anal anastomosis; NL, Netherlands; R, retrospective; UC, ulcerative colitis; UK, United Kingdom; USA, United States of America.
Study Characteristics
This systematic review includes 30 studies with a total of 22,978 patients, consisting of 20,839 patients with UC, 524 with indeterminate colitis, 101 with CD, 516 with FAP, and 1023 with other or unspecified diagnosis. Six of the 30 studies were multicenter studies. Three studies originated from Asian countries, whereas 27 studies originated from Western countries. The majority of studies (19/30) had a median follow-up of at least 5 years and 4 studies did not report the follow-up time (Table 1).
Surgical Characteristics
A laparoscopic approach was used in 24% of cases, which was reported in 13 studies. Nineteen studies reported on whether a 1-, 2-, modified 2-, or 3-stage approach was used. The majority of pouches were done using a 2-stage approach (42.2%), while 19.7% used a 1-stage approach, 9.0% used a modified 2-stage, and 29.1% used a 3-stage approach (Table, Supplemental Digital Content 3, http://links.lww.com/AOSO/A42). In total, 83.7% of the pouches were primary diverted (range of 23.3–100%), which was reported in 27 studies. Eighteen studies reported the pouch type, and the majority used a J-pouch (86.2%). A stapled anastomosis was used in 48.3% of the procedures, which was reported in 20 studies.
Definitions and Diagnostic Criteria Used for IPAA-related Complications
Pouch failure was defined as the need for a permanent ileostomy with or without pouch excision in 26 of the studies10,18,21–24,31–37,39,40,42,44,47,48,50,51,53,55; 3 studies additionally included pouch revision.16,20,41 Pouch failure was undefined in 4 studies.19,46,49,54 Anastomotic leak was defined as any defect at the anastomotic site confirmed by imaging or during surgical reintervention in 7 studies16,20,23,24,55,56 and was undefined in 9 studies.21,32,33,36,41,48,49,52,53 Pelvic sepsis included pelvic abscesses with or without anastomotic leak20,23,31,32,36,39,41,43,46,52 in 10 studies; included anastomotic leaks, pelvic abscesses, and fistulas in 2 studies21,45; and was undefined in 4 studies.34,37,47,53 Fistula was defined as a fistula originating from anywhere in the pouch in 10 studies16,20,23,31,33,34,39,44,50,53; originating from the anastomosis only in 1 study48; and was undefined in 7 studies.19,21,36,41,46,47,55 Stricture was defined as narrowing at the anastomotic site requiring dilation in 8 studies16,33,36,39,44,46,48,50 and was undefined in 7 studies.10,19,34,41,47,53,55 A diagnosis of CD was based on clinical and pathological findings in 5 studies.24,37,42,44,55 In 2 other studies, the diagnosis was only based on clinical findings including severe inflammation of the pouch and proximal small bowel and strictures of the proximal small bowel and fistulae formation more than 3 months54 or more than 6 months after surgery.40 Four studies lacked a clear description of the diagnostic criteria used for a change in diagnosis from UC to CD after IPAA creation.19,31,34,39 A diagnosis of pouchitis was based on clinical and endoscopic findings in 13 studies23,33,35,39–41,44,46,49–51,54,55 and was mainly based on clinical symptoms in 2 studies.19,36 Seven studies lacked a clear description of the diagnostic criteria used for pouchitis.10,16,31,34,47,48,53
Critical Appraisal and Risk of Bias
The overall risk of bias for each item of the JBI checklist across all included studies is presented in Figure, Supplemental Digital Content 2, http://links.lww.com/AOSO/A41. The study-level risk of bias for each individual study is presented in Figure, Supplemental Digital Content 3, http://links.lww.com/AOSO/A41. A funnel plot for pouch failure is presented in Figure, Supplemental Digital Content 4, http://links.lww.com/AOSO/A41. All studies were retrospective, although 13 studies prospectively collected their data.10,19,20,31,33,34,37,41,47,49,51,54,55 One study collected their data in a partially prospective manner.22 The inclusion of consecutive patients was not described in 27% (8/30) of the included studies, which suggests a risk of selection bias. Definitions for pouch-related complications and methods used for the assessment of one or more of these complications were lacking in 63% (19/30) of the studies. These findings suggest a moderate to high risk of detection bias.
Pouch Failure and IPAA-related Complications
The incidence of complications following IPAA is displayed in Tables 2 and 3. The overall pooled incidence of pouch failure was 6.7% (95% CI: 5.28–8.44, Figure 1). The sensitivity analysis revealed a pouch failure rate of 7.7% (95% CI: 5.56–10.59) for studies with a median follow-up of ≥5 years and 10.3% (95% CI: 7.24–14.30) for studies with a median follow-up of ≥10 years. Five studies reported 20-year cumulative pouch failure rates with a range of 6.7–18.2%18,19,22,31,37 (Table 4). Pouch failure rates increased with follow-up time, although at 1 center, the pouch failure rate only marginally increased from 5.3% at 5-year follow-up to 6.7% at 30-year follow-up.19 The majority of studies with long-term outcomes demonstrated a comparable pouch failure rate after 5 years (range 4.0–9.1%; Table 4), while the incidence of pouch-related complications varied widely among studies (Tables 2 and 3). In the 2 studies with the highest anastomotic leak rate (>14%), no pouch failure rate increase could be demonstrated (5.4% and 6.8%).10,16 In some studies, the fistula rate was higher compared with the anastomotic leak rate21,23,53 or pelvic sepsis rate.19,31,34,46 In the majority (6/7) of the included studies that reported on both pouch-related fistula and a diagnosis of CD after IPAA creation, the fistula rate was substantially higher compared with rate of CD, which is a different entity.16,19,31,34,39,44
TABLE 2.
Complications Following IPAA Procedures
| N | Pouch Failuren (%) | Anastomotic Leakn (%) | Pelvic Sepsisn (%) | Pouch-related Fistulan (%) | Stricturen (%) | Crohn’s Disease de novon (%) | Pouchitisn (%) | |
|---|---|---|---|---|---|---|---|---|
| Carcamo | 116 | 9 (7.5) | – | 10 (8.6) | 22 (19.0) | – | 9 (7.8) | 27 (23.3) |
| Cataneo | 176 | 5 (4.2) n = 120 | 6 (3.4) | 15 (8.5) | – | – | – | – |
| Dafnis | 124 | 3 (2.4) | 1 (0.8) | – | 1 (0.8) | 1 (0.8) | – | 37 (33.0) |
| Die | 139 | 42 (30.2) | – | 17 (12.2) | 41 (29.5) | 14 (10.1) | 7 (5.0) | 30 (21.6) |
| Feinberg | 3468 | 161 (4.6) | 122 (3.5) | 188 (5.4) | 87 (2.5) | – | – | – |
| Hashimoto | 119 | 9 (7.6) | – | – | – | – | – | 26 (23.6) n = 110 |
| Helavirta | 352 | 42 (11.9) | 44 (12.5) | 61 (17.3) | 42 (11.9) | 49 (13.9) | – | 134 (38.1) |
| Ikeuchi | 944 | 28 (3.0) | – | 21 (2.2) | – | – | 12 (1.3) | – |
| Karjalainen | 510 | 13 (2.5) | 28 (5.5) | 37 (7.3) | 36 (7.1) | – | – | 240 (47.1) |
| Karlbom | 188 | 16 (8.5) | 12 (6.4) | 25 (13.3) n | 11 (5.6) | 32 (17.0) | 2 (1.1) | 44 (23.4) |
| Kayal | 386 | 26 (6.7) | – | – | – | – | 46 (11.9) | 205 (53.1) |
| Landerholm | 1720 | 103 (6.0) | – | – | – | – | – | – |
| Lee | 212 | 10 (4.7) | 15 (7.1) | 37 (17.5) | 20 (9.4) | 18 (8.5) | – | 75 (35.4) |
| Leowardi | 294 | 37 (12.6) | – | – | – | – | 0 (0.0) | – |
| Lightner/Farouk | 1875 | 99 (5.3) | – | 73 (4.8) n = 1508 | 205 (11.1) n = 1840 | 618 (33.6) n = 1840 | 46 (2.5) n = 1840 | 1130 (61.4) n = 1840 |
| Lorenzo | 185 | 20 (10.8) | – | – | 32 (17.3) | 24 (13.0) | 13 (7.0) | 53 (28.7) |
| Mark-Christensen | 1991 | 295 (14.8) | – | 244 (16.8) n = 1456 | – | – | – | – |
| McCombie | 121 | 14 (13.0) n = 108 | – | 9 (7.4) | 30 (26.8) n = 112 | 17(15.2) n = 112 | – | 60 (55.6) n = 108 |
| Mege | 185 | 10 (5.4) | 26 (14.1) | – | – | 18 (9.7) | – | 33 (17.8) |
| Mennigen | 122 | 4 (3.3) | – | 11 (9.0) | 6 (4.9) | 24 (19.7) | – | 31 (25.4) |
| Rokke | 134 | 13 (9.7) | 6 (4.5) | – | 6 (4.5) | 6 (4.5) | – | 35 (26.3) |
| Sahami | 621 | 42 (6.8) | 105 (16.9) | – | 44 (7.1) | 47 (7.6) | 21 (3.4) | 68 (11.0) |
| Sampietro | 150 | 7 (5.1) n = 137 | 8 (5.3) | – | – | – | – | 35 (23.3) |
| Tan | 142 | 4 (2.8) | – | – | 9 (6.3) | 3 (2.1) | – | 42 (29.6) |
| Uchino | 2376 | 61 (2.6) n = 2349 | 176 (7.4) | – | – | – | 16 (0.7) | – |
| Wasmuth | 315 | 23 (7.3) | 29 (9.5) n = 304 | 39 (12.8) n = 304 | – | – | – | 112 (35.6) |
| Wibmer | 185 | 23 (12.4) | 4 (2.2) | 17 (9.2) | 12 (6.5) | 11 (6.0) | – | 31 (16.8) |
| Worley | 5083 | 239 (4.7) | 168 (3.3) | 478 (9.4) | 239 (4.7) | – | – | – |
| Zaghiyan | 334 | 13 (3.9) | – | – | – | – | 42(17.8) n = 236 | 60 (18.0) |
| Zoccali | 411 | 42 (10.2) | 38 (9.3) | – | 36 (8.8) | 63 (15.3) | 60 (14.6) | 166 (40.4) |
Dashes indicate not specified.
IPAA indicates ileal pouch-anal anastomosis.
TABLE 3.
Pooled Incidence of Complications Following IPAA With 95% CI
| Number of Studies | Number of Patients | Pooled Incidence (%) (95% CI) | Range (%) | |
|---|---|---|---|---|
| Pouch failure | 30 | 22,869 | 6.7 (5.28–8.44) | 2.4–30.0 |
| Pouch failure ≥5 yrs FU | 19 | 12,994 | 7.7 (5.56–10.59) | 2.4–30.0 |
| Pouch failure ≥10 yrs FU | 11 | 5314 | 10.3 (7.24–14.30) | 2.4–30.0 |
| Anastomotic leakage | 16 | 14,479 | 6.3 (4.50–8.83) | 0.8–16.9 |
| Pelvic sepsis | 16 | 14,884 | 9.2 (7.05–11.87) | 2.2–17.5 |
| Fistula | 18 | 13,944 | 8.6 (6.07–11.94) | 0.8–29.5 |
| Stricture | 15 | 4952 | 10.3 (6.63–15.60) | 0.8–33.6 |
| Crohn’s disease de novo | 11 | 7115 | 4.0 (1.93–8.04) | 0.0–17.8 |
| Pouchitis | 22 | 6856 | 30.0 (23.41–37.62) | 11.0–61.4 |
CI indicates confidence interval; FU, follow-up; IPAA, ileal pouch-anal anastomosis.
FIGURE 1.
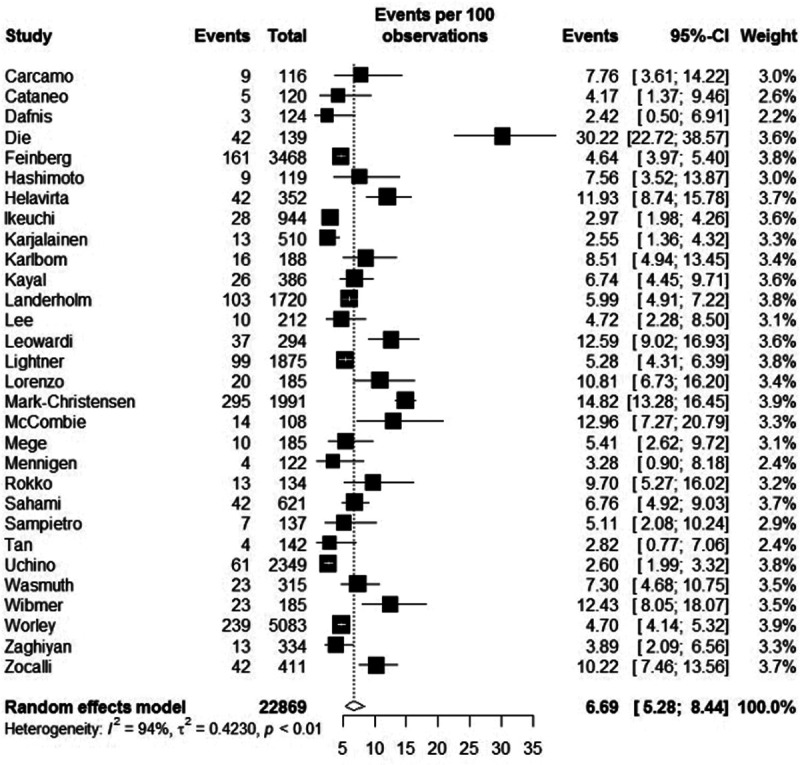
Plot of the individual studies presenting pouch failure rates with 95% CI and the overall incidence of pouch failure with 95% CI. CI indicates confidence intervals.
TABLE 4.
Overview of Studies Reporting on Cumulative Long-term Pouch Failure Rates
| N | 5 yr | 10 yr | 15 yr | 20 yr | 30 yr | |
|---|---|---|---|---|---|---|
| Carcamo | 116 | – | 3.5% | – | 6.9% | – |
| Feinberg | 3468 | – | 6.0% | – | – | – |
| Ikeuchi | 944 | – | 3.0% | – | 11.0% | – |
| Karlbom | 188 | 5.4% | 6.9% | – | – | – |
| Landerholm | 1720 | 4.0% | 6.0% | – | 8.0% | – |
| Leowardi | 294 | 7.7% | 11.3% | 15.5% | – | – |
| Lightner | 1875 | 5.3% | 6.3% | 6.5% | 6.7% | 6.7% |
| Mark-Christensen | 1991 | 9.1% | 12.1% | – | 18.2% | – |
| Sahami | 621 | 6.0% | 11.5% | – | – | – |
| Sampietro | 150 | 7.0% | – | – | – | – |
| Uchino | 2376 | – | 4.2% | – | – | – |
Dashes indicate not specified.
Predictors of Pouch Failure
Correlation analysis of the relationship between pouch-related complications and pouch failure of the included studies indicated that pelvic sepsis (r = 51, P < 0.05) and fistula (r = 0.63, P < 0.01) were significantly correlated with pouch failure (Table 5 and Figure 2). In addition, we performed a sensitivity analysis on the studies with a median follow-up of ≥5 years to identify which factor was the best predictor of long-term pouch failure. The fistula rate was the only factor that remained significantly correlated with pouch failure (r = 0.77, P < 0.01). There was no correlation between CD and fistula (r = 0.13, P = 0.65).
TABLE 5.
Correlations Between IPAA-related Complications and Pouch Failure
| Spearman’s correlation | Spearman’s correlation* | |
|---|---|---|
| Anastomotic leakage | r = 0.32, P = 0.22 | r = 0.60, P = 0.07 |
| Pelvic Sepsis | r = 0.51, P < 0.05 | r = 0.53, P = 0.14 |
| Fistula | r = 0.63, P < 0.01 | r = 0.77, P < 0.01 |
| Stricture | r = 0.21, P = 0.45 | r = 0.12, P = 0.78 |
| Crohn’s disease de novo | r = 0.04, P = 0.90 | r = 0.24, P = 0.57 |
| Pouchitis | r = 0.08, P = 0.71 | r = 0.13, P = 0.65 |
Bold font indicates statistical significance.
*Sensitivity analysis included studies with a median follow-up of ≥5 years.
FIGURE 2.
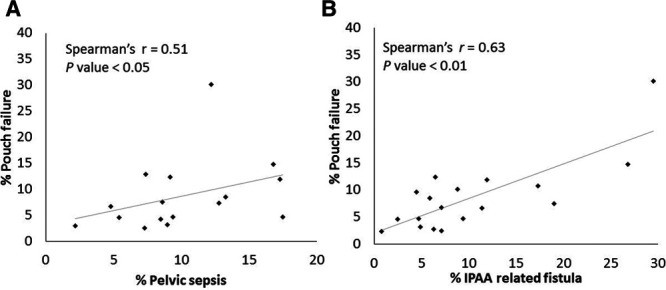
Correlation between pelvic sepsis and pouch failure (A), and IPAA-related fistula and pouch failure (B).
DISCUSSION
In this systematic review which included 30 studies comprising 22,978 patients, we observed a pooled pouch failure rate of 7.8% and 10.3% after a median follow-up of ≥5 and ≥10 years following IPAA, respectively. The definitions used for IPAA-related complications and reported outcomes following IPAA were highly variable. High volume expert centers19–21 demonstrated favorable outcomes over population-based data.22,46 Pouch failure was correlated with pelvic sepsis and pouch-related fistula. However, long-term pouch failure (≥5 years) was only correlated with pouch-related fistula. Our results suggest that only leaks without healing of the anastomosis resulting in fistula formation or chronic sinus are responsible for the failure rate. Not the leaks that are completely healed. This outcome is in line with a large cohort study including 3468 patients, which showed that fistula (hazard ratio, 2.2; 95% CI: 1.2–4.0) was significantly associated with pouch failure, while anastomotic leak was not (hazard ratio, 1.5; 95% CI: 0.75–3.0).20 Data of the current review underlines the necessity to improve the management of anastomotic leaks, to prevent leaks from becoming a chronic anastomotic problem.
Over the last decade, pouch surgery has evolved through centralization and the incorporation of new techniques, including double stapled anastomosis and minimal invasive surgery. Still, pouch failure and pouch-related complication rates did not exhibit an improvement over results from a previously published systematic review, which reported pooled pouch failure rates (follow-up ≥5 years) of 4.7% (studies published between 2000 and 2009) and 8.5% (studies published before 2000).2 Therefore, knowledge on IPAA-related outcomes should be improved (eg, using uniform definitions and data acquisition methods). Ultimately, this could lead to the identification of best practices and an improvement of outcomes.
This review demonstrated a lack of uniformity in the reporting of IPAA-related complications. The different definitions and diagnostic criteria used between studies for IPAA-related complications and time spans have complicated comparisons. For example, differences were found for the terms “short term” (ie, in hospital, <30 days, <90 days, before ileostomy closure) and “long term” (ie, after hospital discharge, ≥30 days, ≥90 days, after ileostomy closure). In addition, differences in clinical manifestations of the disease play a role. For example, an anastomotic defect can have different manifestations at various stages in the perioperative period such as abdominal sepsis, contained pelvic abscess, presacral sinus, anastomotic stricture, or pouch fistula. Therefore, it is necessary to obtain clear, detailed, and uniform data on how complications were scored. Study comparison could be improved if predefined definitions and diagnostic criteria for IPAA-related complications are used, as previously described by Fazio et al.57
Differences in timing and methods used for the assessment of anastomotic integrity may have contributed to the varying anastomotic leak rate ranging from 0.8% to 16.9%. In patients without primary defunctioning (only 16% in this study population), symptomatic leaks resulting in pelvic sepsis are usually diagnosed within 1 week following IPAA.58 However, asymptomatic leaks in a defunctioned anastomosis will only become apparent in the majority of patients after assessment of the anastomosis through pouchoscopy, a pouchogram, CT imaging with transanal contrast, or MR.59 The diagnostic accuracy of pouchoscopy or pouchogram to detect peripouch infection is lower compared with CT imaging.60–62 When no cross-sectional imaging is performed, especially in diverted patients, there is a potential risk for a delay of diagnosis or misdiagnosis of the anastomotic leakage. This might lead to an underestimation of the actual leak rate. In some patients, an insufficiently treated chronic anastomotic leak can mimic chronic pouchitis or CD of the pouch.63
The diagnosis of CD of the pouch in UC patients remains challenging because there are no uniform diagnostic criteria. In the majority of the studies included, a diagnosis of CD was based solely on clinical findings (ie, stricturing or fistulizing disease) without pathologic confirmation. Sossenheimer et al assessed the relationship between abnormal pouchography and long-term pouch complications. The authors suggest that CD of the pouch might be overly assigned, as all patients with contrast extravasation at the initial pouchogram who lost their pouch were at some point labeled as having CD based on clinical findings.59 Lightner et al. analyzed 35 patients with UC who underwent pouch excision for CD, of which 16 patients had fistulizing disease.64 When analyzing the patients with fistulizing disease, the anastomotic leak rate was 0% (0/4) in the group with a pathologic diagnosis of CD versus 91.6% (11/12) in the group without a pathologic diagnosis of CD.64 Differentiation between fistulizing disease caused by technical complications or CD is important, as the former can be managed surgically and does not require immunosuppressive treatment. Non-CD-related fistulas should be considered a late manifestation of anastomotic leaks due to delayed diagnosis or unsuccessful treatment.
Data of the current review suggest that there is room for improvement in the management of anastomotic leaks, trying to avoid the occurrence of chronic leaks and fistula formation. One way of doing that is through early identification of leaks using close observation with C-reactive protein and computed tomography with anal contrast. Routine cross-sectional imaging should be considered, especially in diverted patients with potential silent leaks. Proactive management of an anastomotic leak can be achieved with conventional techniques as transanal or CT-guided drainage65 and more modern techniques as endoluminal vacuum-assisted closure.14 Endoluminal vacuum-assisted closure has been shown to effectively salvage the anastomosis at an early stage, preventing pouch failure due to chronic leaks/anastomotic fistula.14 Unfortunately, this technique is not available in all countries.
The diversion rate of the included studies ranged from 23.3% to 100%, which reflects the lack of consensus regarding routine fecal diversion. Primary diverting ileostomy was performed least frequently in Finland.23,36 The main reason to divert is to mitigate the consequence of anastomotic leakage and improve pouch survival. A systematic review including 1486 patients showed that nondiversion was associated with an increased risk of anastomotic leak, while long-term outcomes of pouch survival were similar to those of diverted patients.66 However, a nationwide cohort study from Denmark showed that primarily nondiverted pouches had a significantly higher risk of pouch failure.22 In contrast, several other studies showed no relation between a protective ileostomy and pouch failure.13,15,24,48,52,67
This review has several limitations. As discussed before, heterogeneity across the included studies impairs the objective comparison of study outcomes. Many studies combined UC and other diagnoses and did not separate outcomes based on diagnosis. In addition, incomplete data registration has led to a large number of unspecified diagnosis, which might be related to the voluntary nature of some registries. Due to the lack of individual patient data, the effect of patient (ie, preoperative status, medication use) and surgical characteristics (ie, procedural stages, hand-sewn versus stapled anastomosis, laparoscopic versus open approach) on postoperative outcomes could not be assessed. Furthermore, besides detection and reporting bias, it is likely that publication bias exists because centers with poor outcomes may be less eager to publish their results. The strength of this systematic review is that it comprised multiple large cohort series with a considerable long-term follow-up, providing a comprehensive overview of the currently available long-term outcomes following IPAA.
In conclusion, the pouch failure rate did not improve over time when compared with prior analysis.2 Anastomotic leaks and long-term sequela are still a major problem. The key finding of this systematic review is that the long-term pouch failure rate was neither correlated with pelvic sepsis nor anastomotic leakage but only with pouch fistula. Anastomotic fistula, presacral sinus, and chronic leak are all indicators of a chronic anastomotic problem. All efforts should therefore be taken (ie, proactive diagnosis and management) to avoid acute leaks from becoming a chronic leak. To facilitate further comparative studies and identify best practices, a prospective registration of patients undergoing IPAA, with predefined standardized outcomes and standardized assessment of anastomotic integrity is necessary. For this reason, the MIRACLE project was initiated, a prospective European multicenter study, which determines long-term anastomotic integrity in patients who underwent a restorative proctocolectomy for UC (Trial NL 9083). The ECCO UR-CARE registry will be used to accommodate prospective registration of patient, surgical, and medical treatment characteristics, postoperative management, and outcomes. This registration system will provide a better understanding of changes in practice over time and their effects on outcomes. Variation in practices might help to identify best practices, resulting in the optimization of long-term pouch preservation.
ACKNOWLEDGMENTS
We thank Faridi van Etten (clinical librarian) for help with the literature search.
Supplementary Material
Footnotes
Disclosure: The authors declare that they have nothing to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. 1978; 2:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Zeeuw S, Ahmed Ali U, Ali UA, et al. Update of complications and functional outcome of the ileo-pouch anal anastomosis: overview of evidence and meta-analysis of 96 observational studies. Int J Colorectal Dis. 2012; 27:843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heikens JT, de Vries J, Goos MR, et al. Quality of life and health status before and after ileal pouch-anal anastomosis for ulcerative colitis. Br J Surg. 2012; 99:263–269. [DOI] [PubMed] [Google Scholar]
- 4.Hueting WE, Buskens E, van der Tweel I, et al. Results and complications after ileal pouch anal anastomosis: a meta-analysis of 43 observational studies comprising 9,317 patients. Dig Surg. 2005; 22:69–79. [DOI] [PubMed] [Google Scholar]
- 5.Fazio VW, Tekkis PP, Remzi F, et al. Quantification of risk for pouch failure after ileal pouch anal anastomosis surgery. Ann Surg. 2003; 238:605–614; discussion 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forbes SS, O’Connor BI, Victor JC, et al. Sepsis is a major predictor of failure after ileal pouch-anal anastomosis. Dis Colon Rectum. 2009; 52:1975–1981. [DOI] [PubMed] [Google Scholar]
- 7.Prudhomme M, Dehni N, Dozois RR, et al. Causes and outcomes of pouch excision after restorative proctocolectomy. Br J Surg. 2006; 93:82–86. [DOI] [PubMed] [Google Scholar]
- 8.Lightner AL, Dattani S, Dozois EJ, et al. Pouch excision: indications and outcomes. Colorectal Dis. 2017; 19:912–916. [DOI] [PubMed] [Google Scholar]
- 9.Zittan E, Wong-Chong N, Ma GW, et al. Modified two-stage ileal pouch-anal anastomosis results in lower rate of anastomotic leak compared with traditional two-stage surgery for ulcerative colitis. J Crohns Colitis. 2016; 10:766–772. [DOI] [PubMed] [Google Scholar]
- 10.Mège D, Figueiredo MN, Manceau G, et al. Three-stage laparoscopic ileal pouch-anal anastomosis is the best approach for high-risk patients with inflammatory bowel disease: an analysis of 185 consecutive patients. J Crohns Colitis. 2016; 10:898–904. [DOI] [PubMed] [Google Scholar]
- 11.Samples J, Evans K, Chaumont N, et al. Variant two-stage ileal pouch-anal anastomosis: an innovative and effective alternative to standard resection in ulcerative colitis. J Am Coll Surg. 2017; 224:557–563. [DOI] [PubMed] [Google Scholar]
- 12.Chandrasinghe P, Carvello M, Wasmann K, et al. Transanal ileal pouch-anal anastomosis for ulcerative colitis has comparable long-term functional outcomes to transabdominal approach: a multicentre comparative study. J Crohns Colitis. 2019; 22:726–733. [DOI] [PubMed] [Google Scholar]
- 13.Lovegrove RE, Tilney HS, Remzi FH, et al. To divert or not to divert: a retrospective analysis of variables that influence ileostomy omission in ileal pouch surgery. Arch Surg. 2011; 146:82–88. [DOI] [PubMed] [Google Scholar]
- 14.Wasmann KA, Reijntjes MA, Stellingwerf ME, et al. Endo-sponge assisted early surgical closure of ileal pouch-anal anastomotic leakage preserves long-term function: a cohort study. J Crohns Colitis. 2019; 13:1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grobler SP, Hosie KB, Keighley MR. Randomized trial of loop ileostomy in restorative proctocolectomy. Br J Surg. 1992; 79:903–906. [DOI] [PubMed] [Google Scholar]
- 16.Sahami S, Buskens CJ, Fadok TY, et al. Defunctioning ileostomy is not associated with reduced leakage in proctocolectomy and ileal pouch anastomosis surgeries for IBD. J Crohns Colitis. 2016; 10:779–785. [DOI] [PubMed] [Google Scholar]
- 17.Widmar M, Munger JA, Mui A, et al. Diverted versus undiverted restorative proctocolectomy for chronic ulcerative colitis: an analysis of long-term outcomes after pouch leak short title: outcomes after pouch leak. Int J Colorectal Dis. 2019; 34:691–697. [DOI] [PubMed] [Google Scholar]
- 18.Landerholm K, Abdalla M, Myrelid P, et al. Survival of ileal pouch anal anastomosis constructed after colectomy or secondary to a previous ileorectal anastomosis in ulcerative colitis patients: a population-based cohort study. Scand J Gastroenterol. 2017; 52:531–535. [DOI] [PubMed] [Google Scholar]
- 19.Lightner AL, Mathis KL, Dozois EJ, et al. Results at up to 30 years after ileal pouch-anal anastomosis for chronic ulcerative colitis. Inflamm Bowel Dis. 2017; 23:781–790. [DOI] [PubMed] [Google Scholar]
- 20.Feinberg AE, Lavryk O, Aiello A, et al. Conditional survival after IPAA for ulcerative and indeterminate colitis: does long-term pouch survival improve or worsen with time? Dis Colon Rectum. 2020; 63:927–933. [DOI] [PubMed] [Google Scholar]
- 21.Worley GHT, Fearnhead NS, Brown SR, et al. ; Association of Coloproctology of Great Britain and Ireland (ACPGBI) Inflammatory Bowel Disease Clinical Advisory Group, commentators in the 2017 ACPGBI Ileoanal Pouch Report, and Ileoanal Pouch Registry contributors. Review of current practice and outcomes following ileoanal pouch surgery: lessons learned from the Ileoanal Pouch Registry and the 2017 Ileoanal Pouch Report. Colorectal Dis. 2018; 20:913–922. [DOI] [PubMed] [Google Scholar]
- 22.Mark-Christensen A, Erichsen R, Brandsborg S, et al. Pouch failures following ileal pouch-anal anastomosis for ulcerative colitis. Colorectal Dis. 2018; 20:44–52. [DOI] [PubMed] [Google Scholar]
- 23.Karjalainen EK, Renkonen-Sinisalo L, Mustonen HK, et al. Morbidity related to diverting ileostomy after restorative proctocolectomy in patients with ulcerative colitis. Colorectal Dis. 2019; 21:671–678. [DOI] [PubMed] [Google Scholar]
- 24.Uchino M, Ikeuchi H, Sugita A, et al. ; a research grant on intractable disease affiliated with the Japan Ministry of Health Labor Welfare. Pouch functional outcomes after restorative proctocolectomy with ileal-pouch reconstruction in patients with ulcerative colitis: Japanese multi-center nationwide cohort study. J Gastroenterol. 2018; 53:642–651. [DOI] [PubMed] [Google Scholar]
- 25.Peyrin-Biroulet L, Germain A, Patel AS, et al. Systematic review: outcomes and post-operative complications following colectomy for ulcerative colitis. Aliment Pharmacol Ther. 2016; 44:807–816. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 28.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016; 5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2019; 18:2127–2133. [DOI] [PubMed] [Google Scholar]
- 30.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019; 22:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carcamo L, Miranda P, Zúñiga A, et al. Ileal pouch-anal anastomosis in ulcerative colitis: outcomes, functional results, and quality of life in patients with more than 10-year follow-up. Int J Colorectal Dis. 2020; 35:747–753. [DOI] [PubMed] [Google Scholar]
- 32.Cataneo J, Mowschenson P, Cataldo TE, et al. Rectal eversion: safe and effective way to achieve low transaction in minimally invasive Ileal pouch-anal anastomosis surgery, short- and long-term outcomes. Surg Endosc. 2020; 34:1290–1293. [DOI] [PubMed] [Google Scholar]
- 33.Dafnis G. Early and late surgical outcomes of ileal pouch-anal anastomosis within a defined population in Sweden. Eur J Gastroenterol Hepatol. 2016; 28:842–849. [DOI] [PubMed] [Google Scholar]
- 34.Die J, Ocaña J, Abadía P, et al. Experience, complications and prognostic factors of the ileoanal pouch in ulcerative colitis: an observational study. Cir Esp (Engl Ed). 2020; 98:64–71. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto T, Itabashi M, Ogawa S, et al. Treatment strategy for preventing pouchitis as a postoperative complication of ulcerative colitis: the significance of the management of cuffitis. Surg Today. 2014; 44:1730–1734. [DOI] [PubMed] [Google Scholar]
- 36.Helavirta I, Huhtala H, Hyöty M, et al. Restorative proctocolectomy for ulcerative colitis in 1985-2009. Scand J Surg. 2016; 105:73–77. [DOI] [PubMed] [Google Scholar]
- 37.Ikeuchi H, Uchino M, Matsuoka H, et al. Surgery for ulcerative colitis in 1,000 patients. Int J Colorectal Dis. 2010; 25:959–965. [DOI] [PubMed] [Google Scholar]
- 38.Karjalainen EK, Renkonen-Sinisalo L, Mustonen HK, Farkkila M, Lepisto AH. Restorative proctocolectomy in ulcerative colitis: effect of preoperative immunomodulatory therapy on postoperative complications and pouch failure. Scandi J Surg. 2021; 110:51–58. [DOI] [PubMed] [Google Scholar]
- 39.Karlbom U, Lindfors A, Påhlman L. Long-term functional outcome after restorative proctocolectomy in patients with ulcerative colitis. Colorectal Dis. 2012; 14:977–984. [DOI] [PubMed] [Google Scholar]
- 40.Kayal M, Plietz M, Rizvi A, et al. Inflammatory pouch conditions are common after ileal pouch anal anastomosis in ulcerative colitis patients. Inflamm Bowel Dis. 2019; 6:1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee GC, Deery SE, Kunitake H, et al. Comparable perioperative outcomes, long-term outcomes, and quality of life in a retrospective analysis of ulcerative colitis patients following 2-stage versus 3-stage proctocolectomy with ileal pouch-anal anastomosis. Int J Colorectal Dis. 2019; 34:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leowardi C, Hinz U, Tariverdian M, et al. Long-term outcome 10 years or more after restorative proctocolectomy and ileal pouch-anal anastomosis in patients with ulcerative colitis. Langenbecks Arch Surg. 2010; 395:49–56. [DOI] [PubMed] [Google Scholar]
- 43.Farouk R, Dozois RR, Pemberton JH, et al. Incidence and subsequent impact of pelvic abscess after ileal pouch-anal anastomosis for chronic ulcerative colitis. Dis Colon Rectum. 1998; 41:1239–1243. [DOI] [PubMed] [Google Scholar]
- 44.Lorenzo G, Maurizio C, Maria LP, et al. Ileal pouch-anal anastomosis 20 years later: is it still a good surgical option for patients with ulcerative colitis? Int J Colorectal Dis. 2016; 31:1835–1843. [DOI] [PubMed] [Google Scholar]
- 45.Mark-Christensen A, Kjær MD, Ganesalingam S, et al. Increasing incidence of pelvic sepsis following ileal pouch-anal anastomosis for ulcerative colitis in Denmark: a Nationwide Cohort Study. Dis Colon Rectum. 2019; 62:965–971. [DOI] [PubMed] [Google Scholar]
- 46.McCombie A, Lee Y, Vanamala R, et al. Early postoperative complications have long-term impact on quality of life after restorative proctocolectomy. Medicine (Baltimore). 2016; 95:e3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mennigen R, Senninger N, Bruwer M, et al. Impact of defunctioning loop ileostomy on outcome after restorative proctocolectomy for ulcerative colitis. Int J Colorectal Dis. 2011; 26:627–633. [DOI] [PubMed] [Google Scholar]
- 48.Røkke O, Iversen K, Olsen T, et al. Long-term followup with evaluation of the surgical and functional results of the ileal pouch reservoir in restorative proctocolectomy for ulcerative colitis. ISRN Gastroenterol. 2011; 2011:625842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sampietro GM, Colombo F, Frontali A, et al. Totally laparoscopic, multi-stage, restorative proctocolectomy for inflammatory bowel diseases. A prospective study on safety, efficacy and long-term results. Dig Liver Dis. 2018; 50:1283–1291. [DOI] [PubMed] [Google Scholar]
- 50.Tan KK, Ravindran P, Young CJ, et al. The extent of inflammation is a predictor for pouch-related complications in ileal pouches in patients with ulcerative or indeterminate colitis. Colorectal Dis. 2014; 16:620–625. [DOI] [PubMed] [Google Scholar]
- 51.Wasmuth HH, Tranø G, Midtgård TM, et al. Long-term function after ileal pouch-anal anastomosis—function does not deteriorate with time. Colorectal Dis. 2010; 12(10 Online):e283–e290. [DOI] [PubMed] [Google Scholar]
- 52.Wasmuth HH, Tranø G, Endreseth B, et al. Long-term surgical load in patients with ileal pouch-anal anastomosis. Colorectal Dis. 2009; 11:711–718. [DOI] [PubMed] [Google Scholar]
- 53.Wibmer AG, Kroesen AJ, Gröne J, et al. Predictors of permanent ileostomy after restorative proctocolectomy. Br J Surg. 2010; 97:1561–1566. [DOI] [PubMed] [Google Scholar]
- 54.Zaghiyan K, Kamiński JP, Barmparas G, et al. De novo Crohn’s disease after ileal pouch-anal anastomosis for ulcerative colitis and inflammatory bowel disease unclassified: long-term follow-up of a prospective inflammatory bowel disease Registry. Am Surg. 2016; 82:977–981. [PubMed] [Google Scholar]
- 55.Bertucci Zoccali M, Hyman NH, Skowron KB, et al. Exposure to anti-tumor necrosis factor medications increases the incidence of pouchitis after restorative proctocolectomy in patients with ulcerative colitis. Dis Colon Rectum. 2019; 62:1344–1351. [DOI] [PubMed] [Google Scholar]
- 56.Karlbom U, Raab Y, Ejerblad S, et al. Factors influencing the functional outcome of restorative proctocolectomy in ulcerative colitis. Br J Surg. 2000; 87:1401–1408. [DOI] [PubMed] [Google Scholar]
- 57.Fazio VW, Kiran RP, Remzi FH, et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013; 257:679–685. [DOI] [PubMed] [Google Scholar]
- 58.Gorgun E, Remzi FH. Complications of ileoanal pouches. Clin Colon Rectal Surg. 2004; 17:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sossenheimer PH, Glick LR, Dachman AH, et al. Abnormal pouchogram predicts pouch failure even in asymptomatic patients. Dis Colon Rectum. 2019; 62:463–469. [DOI] [PubMed] [Google Scholar]
- 60.Thoeni RF, Fell SC, Engelstad B, et al. Ileoanal pouches: comparison of CT, scintigraphy, and contrast enemas for diagnosing postsurgical complications. AJR Am J Roentgenol. 1990; 154:73–78. [DOI] [PubMed] [Google Scholar]
- 61.Crema MD, Richarme D, Azizi L, et al. Pouchography, CT, and MRI features of ileal J pouch-anal anastomosis. AJR Am J Roentgenol. 2006; 187:W594–W603. [DOI] [PubMed] [Google Scholar]
- 62.Santorelli C, Hollingshead J, Clark SK. Clinical value of pouchogram prior to ileostomy closure after ileal pouch anal anastomosis. Tech Coloproctol. 2018; 22:541–544. [DOI] [PubMed] [Google Scholar]
- 63.van der Ploeg VA, Maeda Y, Faiz OD, et al. The prevalence of chronic peri-pouch sepsis in patients treated for antibiotic-dependent or refractory primary idiopathic pouchitis. Colorectal Dis. 2017; 19:827–831. [DOI] [PubMed] [Google Scholar]
- 64.Lightner AL, Fletcher JG, Pemberton JH, et al. Crohn’s disease of the pouch: a true diagnosis or an oversubscribed diagnosis of exclusion? Dis Colon Rectum. 2017; 60:1201–1208. [DOI] [PubMed] [Google Scholar]
- 65.Kirat HT, Remzi FH, Shen B, et al. Pelvic abscess associated with anastomotic leak in patients with ileal pouch-anal anastomosis (IPAA): transanastomotic or CT-guided drainage? Int J Colorectal Dis. 2011; 26:1469–1474. [DOI] [PubMed] [Google Scholar]
- 66.Weston-Petrides GK, Lovegrove RE, Tilney HS, et al. Comparison of outcomes after restorative proctocolectomy with or without defunctioning ileostomy. Arch Surg. 2008; 143:406–412. [DOI] [PubMed] [Google Scholar]
- 67.Sahami S, Bartels SA, D’Hoore A, et al. A multicentre evaluation of risk factors for anastomotic leakage after restorative proctocolectomy with ileal pouch-anal anastomosis for inflammatory bowel disease. J Crohns Colitis. 2016; 10:773–778. [DOI] [PubMed] [Google Scholar]


