Abstract
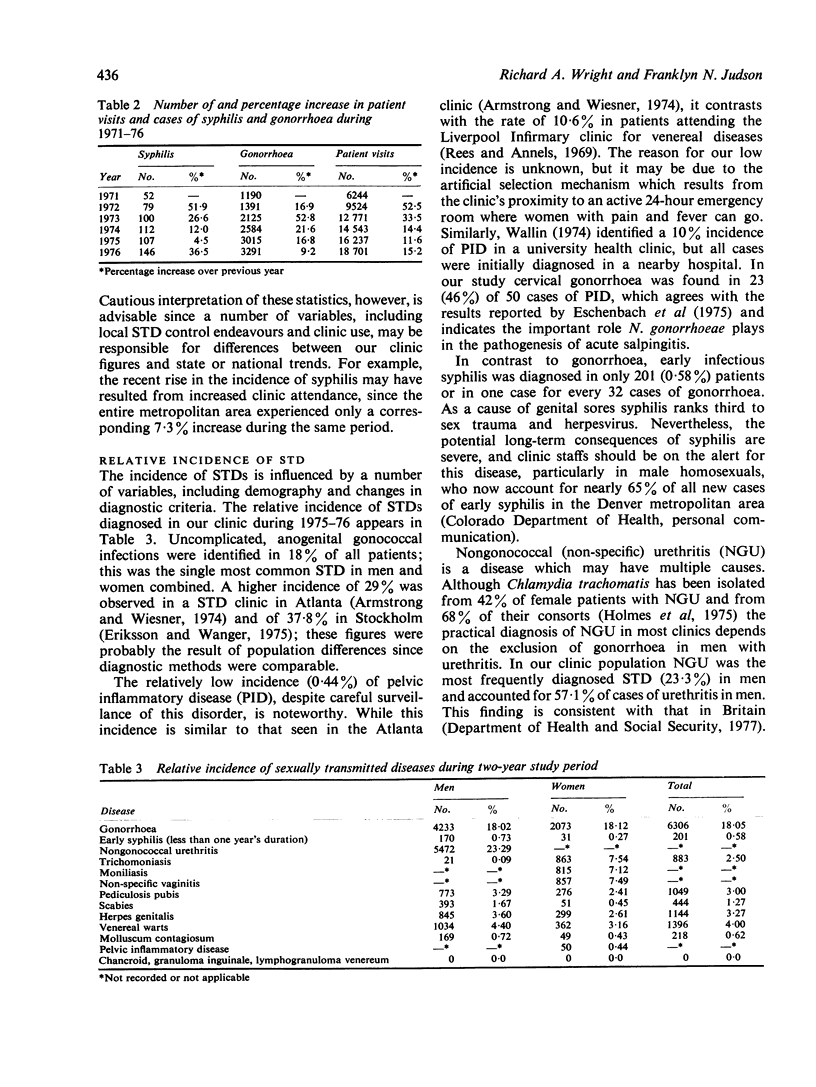
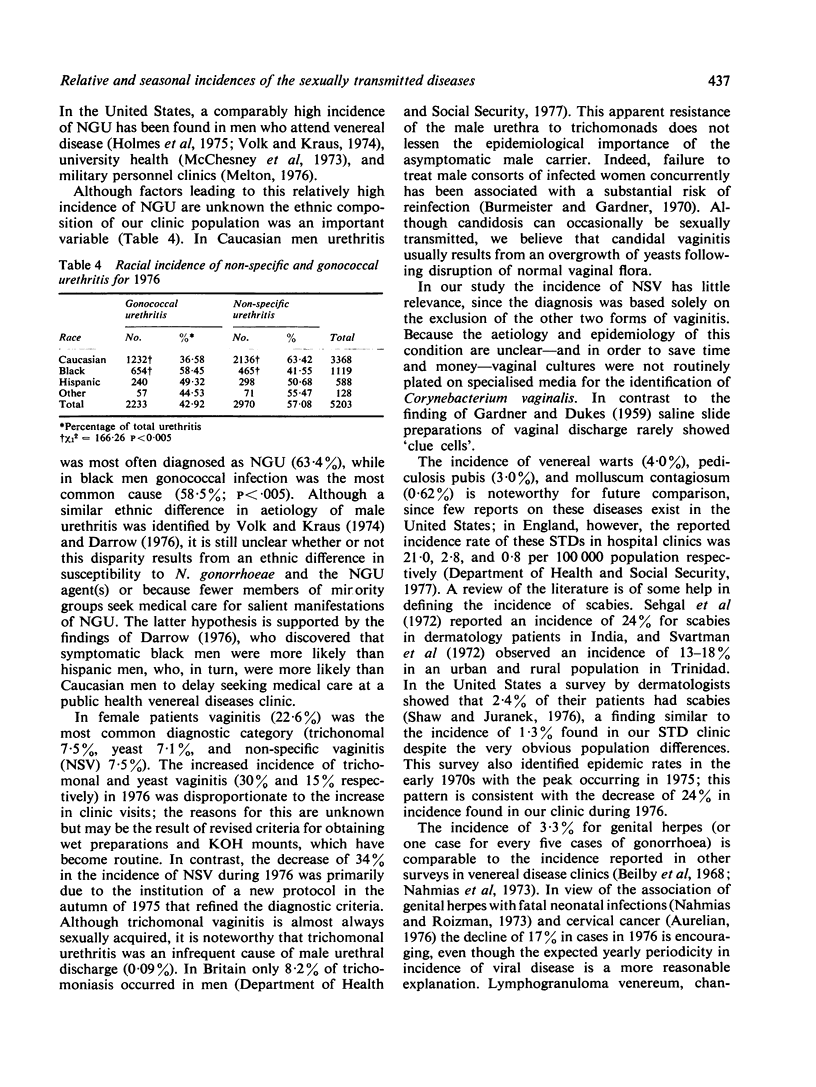
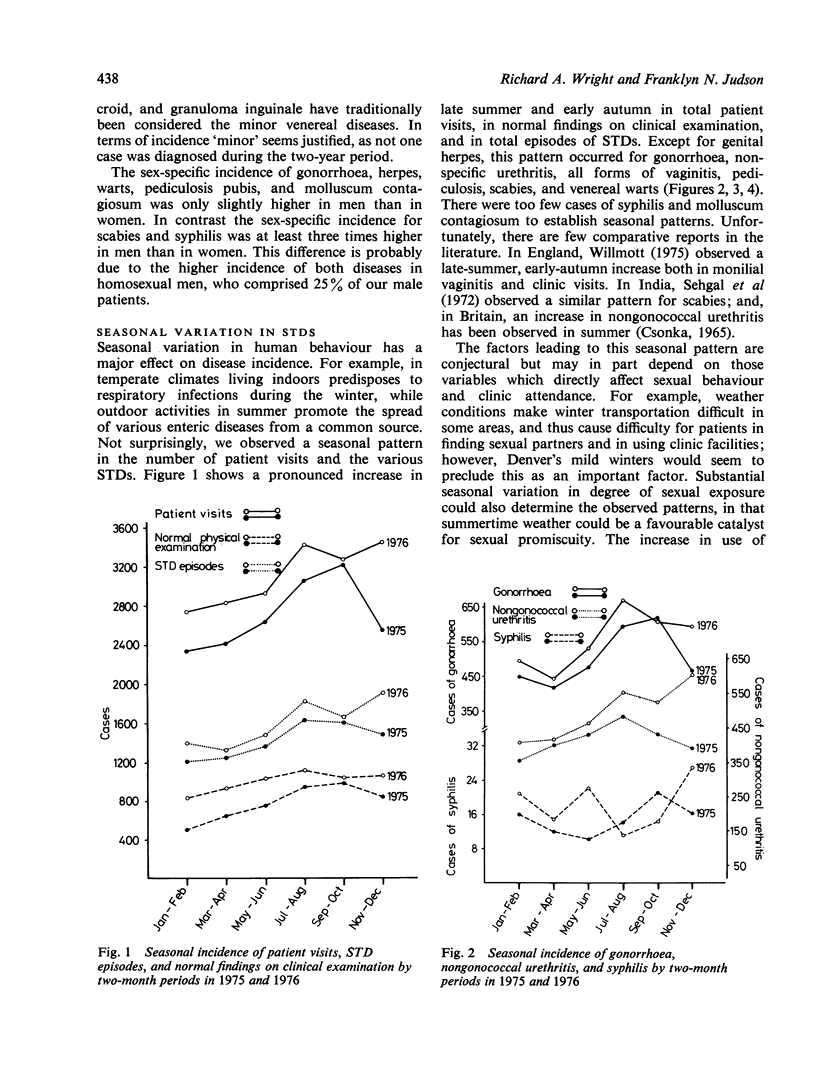
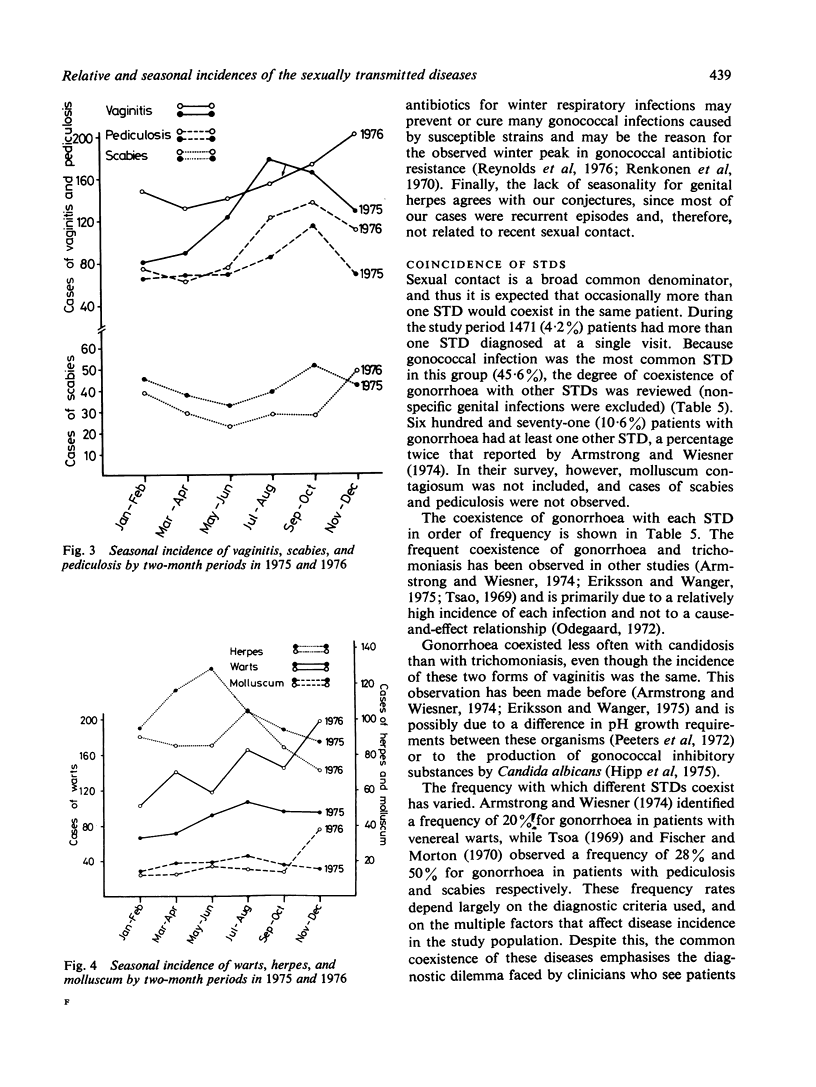
In the United States statistics on sexually transmitted diseases (STDs), other than gonorrhoea and syphilis, are meagre. In this study the relative and seasonal incidences of most STDs in an American clinic where 34,938 patient visits were recorded over a two-year period (1975-76) are assessed. Gonorrhoea was the most common STD in male and female patients combined (18%), while nongonococcal urethritis (NGU) was most common in men (23%), and vaginitis (trichomonal 7.5%, yeast 7.1%, and non-specific 7.1%) was the most common in women. A significantly higher incidence of NGU occurred in Caucasian (63%) than in black (42%) men (P less than 0.005). No other STD was diagnosed in more than 5% of patients, and 31% had normal findings on clinical examination and investigation, and could be described as the 'worried well'. Two or more STDs co-existed in 4.2% of patients. In 1976 the incidence of genital herpes and scabies decreased in contrast to other STDs and total patient visits, which increased. A seasonal peak in late summer and early autumn was observed for most STDs. These observations indicate the importance of a comprehensive approach when attempting to compile accurate statistics on selected epidemiological aspects of sexually transmitted diseases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. H. The need for problem-oriented venereal disease clinics. J Am Vener Dis Assoc. 1974 Sep;1(1):23–28. [PubMed] [Google Scholar]
- Aurelian L. Sexually transmitted cancers? The case for genital herpes. J Am Vener Dis Assoc. 1976 Mar;2(3):10–20. [PubMed] [Google Scholar]
- Beilby J. O., Cameron C. H., Catterall R. D., Davidson D. Herpesvirus hominis infection of the cervix associated with gonorrhoea. Lancet. 1968 May 18;1(7551):1065–1066. doi: 10.1016/s0140-6736(68)91414-1. [DOI] [PubMed] [Google Scholar]
- Burmeister R. E., Gardner H. L. Vaginitis: diagnosis and treatment. Postgrad Med. 1970 Aug;48(2):159–163. doi: 10.1080/00325481.1970.11693525. [DOI] [PubMed] [Google Scholar]
- CSONKA G. W. NON-GONOCOCCAL URETHRITIS. Br J Vener Dis. 1965 Mar;41:1–8. doi: 10.1136/sti.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow W. W. Veneral infections in three ethnic groups in Sacramento. Am J Public Health. 1976 May;66(5):446–450. doi: 10.2105/ajph.66.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson G., Wanger L. Frequency of N. gonorrhoeae, T. vaginalis, and C. albicans in female venereological patients. A one-year study. Br J Vener Dis. 1975 Jun;51(3):192–197. doi: 10.1136/sti.51.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenbach D. A., Buchanan T. M., Pollock H. M., Forsyth P. S., Alexander E. R., Lin J. S., Wang S. P., Wentworth B. B., MacCormack W. M., Holmes K. K. Polymicrobial etiology of acute pelvic inflammatory disease. N Engl J Med. 1975 Jul 24;293(4):166–171. doi: 10.1056/NEJM197507242930403. [DOI] [PubMed] [Google Scholar]
- Fisher I., Morton R. S. Phthirus pubis infestation. Br J Vener Dis. 1970 Aug;46(4):326–329. doi: 10.1136/sti.46.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDNER H. L., DUKES C. D. Hemophilus vaginalis vaginitis. Ann N Y Acad Sci. 1959 Nov 18;83:280–289. doi: 10.1111/j.1749-6632.1960.tb40901.x. [DOI] [PubMed] [Google Scholar]
- Hipp S. S., Lawton W. D., Savage M., Gaafar H. A. Selective interaction of Neisseria gonorrhoeae and Candida albicans and its possible role in clinical specimens. J Clin Microbiol. 1975 May;1(5):476–477. doi: 10.1128/jcm.1.5.476-477.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. K., Handsfield H. H., Wang S. P., Wentworth B. B., Turck M., Anderson J. B., Alexander E. R. Etiology of nongonococcal urethritis. N Engl J Med. 1975 Jun 5;292(23):1199–1205. doi: 10.1056/NEJM197506052922301. [DOI] [PubMed] [Google Scholar]
- Jaffe H. W., Zaidi A. A., Thornsberry C., Reynolds G. H., Wiesner P. J. Trends and seasonality of antibiotic resistance of Neisseria gonorrhoeae. J Infect Dis. 1977 Nov;136(5):684–688. doi: 10.1093/infdis/136.5.684. [DOI] [PubMed] [Google Scholar]
- McChesney J. A., Zedd A., King H., Russell C. M., Hendley J. O. Acute urethritis in male college students. JAMA. 1973 Oct 1;226(1):37–39. [PubMed] [Google Scholar]
- Melton L. J., 3rd Comparative incidence of gonorrhea and nongonococcal urethritis in the United States Navy. Am J Epidemiol. 1976 Nov;104(5):535–542. doi: 10.1093/oxfordjournals.aje.a112327. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. 3. N Engl J Med. 1973 Oct 11;289(15):781–789. doi: 10.1056/NEJM197310112891505. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Von Reyn C. F., Josey W. E., Naib Z. M., Hutton R. D. Genital herpes simplex virus infection and gonorrhoea. Association and analogies. Br J Vener Dis. 1973 Jun;49(3):306–309. doi: 10.1136/sti.49.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard K. Trichomonas vaginalis infection and rectal gonorrhoea in women. Acta Derm Venereol. 1972;52(4):326–328. [PubMed] [Google Scholar]
- Peeters F., Snauwaert R., Segers J., Amery W., van Cutsem J. Observations on candidal vaginitis. Vaginal pH, microbiology, and cytology. Am J Obstet Gynecol. 1972 Jan 1;112(1):80–86. doi: 10.1016/0002-9378(72)90533-9. [DOI] [PubMed] [Google Scholar]
- Rees E., Annels E. H. Gonococcal salpingitis. Br J Vener Dis. 1969 Sep;45(3):205–215. doi: 10.1136/sti.45.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkonen O. V., Sivonen A., Lassus A., Salo O. P. Current status of the in vitro sensitivity of gonococci to penicillin in Finland. Acta Derm Venereol. 1970;50(2):151–153. [PubMed] [Google Scholar]
- Sehgal V. N., Rao T. L., Rege V. L., Vadiraj S. N. Scabies: a study of incidence and a treatment method. Int J Dermatol. 1972 Apr-Jun;11(2):106–111. doi: 10.1111/j.1365-4362.1972.tb01733.x. [DOI] [PubMed] [Google Scholar]
- Shaw P. K., Juranek D. D. Recent trends in scabies in the United States. J Infect Dis. 1976 Oct;134(4):414–416. doi: 10.1093/infdis/134.4.414. [DOI] [PubMed] [Google Scholar]
- Svartman M., Finklea J. F., Earle D. P., Potter E. V., Poon-King T. Epidemic scabies and acute glomerulonephritis in Trinidad. Lancet. 1972 Jan 29;1(7744):249–251. doi: 10.1016/s0140-6736(72)90634-4. [DOI] [PubMed] [Google Scholar]
- Tsao W. Trichomoniasis and gonorrhoea. Br Med J. 1969 Mar 8;1(5644):642–643. doi: 10.1136/bmj.1.5644.642-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk J., Kraus S. J. Nongonococcal urethritis. A venereal disease as prevalent as epidemic gonorrhea. Arch Intern Med. 1974 Sep;134(3):511–514. doi: 10.1001/archinte.134.3.511. [DOI] [PubMed] [Google Scholar]
- Wallin J. Gonorrhoea in 1972. A 1-year study of patients attending the VD Unit in Uppsala. Br J Vener Dis. 1975 Feb;51(1):41–47. doi: 10.1136/sti.51.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmott F. E. Genital yeasts in female patients attending a VD clinic. Br J Vener Dis. 1975 Apr;51(2):119–122. doi: 10.1136/sti.51.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]


