Abstract
Mucosal-associated invariant T (MAIT) cells are innate-like T cells that recognize microbial metabolites through a semi-invariant T cell receptor (TCR). Major questions remain regarding the extent of human MAIT cell functional and clonal diversity. To address these, we analyzed the single-cell transcriptome and TCR repertoire of blood and liver MAIT cells and developed functional RNA-sequencing, a method to integrate function and TCR clonotype at single-cell resolution. MAIT cell clonal diversity was comparable to conventional memory T cells, with private TCR repertoires shared across matched tissues. Baseline functional diversity was low and largely related to tissue site. MAIT cells showed stimulus-specific transcriptional responses in vitro, with cells positioned along gradients of activation. Clonal identity influenced resting and activated transcriptional profiles but intriguingly was not associated with the capacity to produce IL-17. Overall, MAIT cells show phenotypic and functional diversity according to tissue localization, stimulation environment and clonotype.
Subject terms: T cells, Adaptive immunity, T-cell receptor
Garner et al. analyzed the single-cell transcriptome and TCR repertoire of matched blood and liver, and resting and activated, human MAIT cells. They identify donor-specific TCR repertoires shared across tissues and a transcriptome that is largely homogeneous at rest, but highly adaptive to different tissue and stimulation environments.
Main
Mucosal-associated invariant T (MAIT) cells are innate-like T cells, abundant in human blood and tissues, particularly the liver and mucosa1. MAIT cells express semi-invariant Vα7.2-Jα33/12/20 (TRAV1-2-TRAJ33/12/20) T cell receptors (TCRs) specific for microbial riboflavin metabolites presented by MR1 (ref. 1). They can also be activated independent of their TCR by cytokines such as IL-12 and IL-18 (ref. 2). Upon activation, MAIT cells secrete type 1/17 cytokines and exhibit cytotoxic activity1.
A major outstanding question is whether human MAIT cells comprise transcriptionally and functionally distinct subsets. Alterations in frequency, phenotype and function occur in numerous human diseases, and mouse models indicate protective and pathogenic roles3. Understanding the characteristics of the MAIT cell population in health could aid the development of therapeutics targeting specific subsets or functions in disease.
In human blood, MAIT cells are relatively homogeneous, exhibiting a predominantly CD8+ effector-memory phenotype, and characteristic expression of surface molecules (for example, CD161) and transcription factors (for example, PLZF and RORγt)1. However, there is some variability, for example, between CD8+, CD4−CD8− (DN) and CD4+ cells4–6, and in the expression of innate immune receptors6,7. Despite universal RORγt expression, <5% of human MAIT cells produce IL-17 ex vivo8,9. This could reflect a committed type 17 subset, as in mice10–12. Alternatively, all human MAIT cells may have the capacity to produce IL-17 under appropriate conditions. Conclusive data addressing these competing hypotheses are lacking.
MAIT cell function is altered by tissue localization and stimulation. Compared with blood, gut and liver MAIT cells display an activated, tissue-resident transcriptome12–14. Genital tract15 and oral mucosal16 cells show type 17 skewing. MAIT cells exhibit distinct transcriptional responses to TCR and cytokine stimulation17,18 and produce increased IL-17 upon sustained stimulation9. Whether functional diversity indicates the presence of multiple subsets or environment-driven plasticity remains unknown.
In addition, questions remain regarding MAIT cell TCR repertoires, including variability across tissues and donors, and the relationship between TCR usage and function. Studies variably demonstrate similar TCR repertoires across tissues19,20 or differences in TRAJ/TRBV usage16,21,22. Diverse chain usage could have functional implications. For example, MAIT cell clonal distribution changes during human Salmonella Paratyphi A infection—cells transduced with TCRβ chains from expanded and contracted clonotypes show greater and lesser responses to TCR stimulation, respectively23. In vitro studies show differential activation potential dependent on clonotype24 or TCRβ usage7,25. Thus, a relationship between TCR architecture and function is suggested but has not been studied systematically.
Overall, human MAIT cells show variation in phenotype, function and TCR repertoire. However, it is unknown whether they comprise multiple functionally distinct subsets, and how phenotype and function relate to TCR usage. To investigate this, we analyzed the single-cell transcriptome and TCR repertoire of human MAIT cells from matched blood and liver, as well as blood cells at rest and following TCR, cytokine or dual TCR+cytokine, stimulation. Our findings revealed a largely homogenous transcriptional program at rest, with variation linked to tissue localization. Activation triggered a plastic, stimulus-specific, effector program. The TCR repertoire was surprisingly diverse, and clonal identity influenced the transcriptome of resting and activated MAIT cells. Following dual stimulation, we identified an IL-17-expressing cluster. IL-17+ cells expressed other effector molecules, such as IFNG, and showed similar TCR usage to IL-17− cells, suggesting they reflect an activation state rather than a bona fide MAIT17 lineage.
Results
MAIT cells show tissue-specific transcription and regulation
To investigate heterogeneity using an unbiased genome-wide approach, we performed single-cell RNA-sequencing (scRNA-seq) and single-cell TCR-sequencing (scTCR-seq) of sorted MAIT cells (CD3+MR1/5-OP-RU+) from matched human blood and liver (Supplementary Table 1 and Supplementary Fig. 1). Findings from an initial experiment (Exp 1; n = 3 blood, 4 liver) were validated in a second experiment (Exp 2; n = 8 blood, 3 liver). Conventional memory T (Tmem; CD3+MR1/5-OP-RU−CCR7−) cells were analyzed in some donors (Exp 1: n = 2 blood, 2 liver; Exp 2: all samples). Exp 2 included 130 oligo-conjugated antibodies for single-cell surface protein analysis.
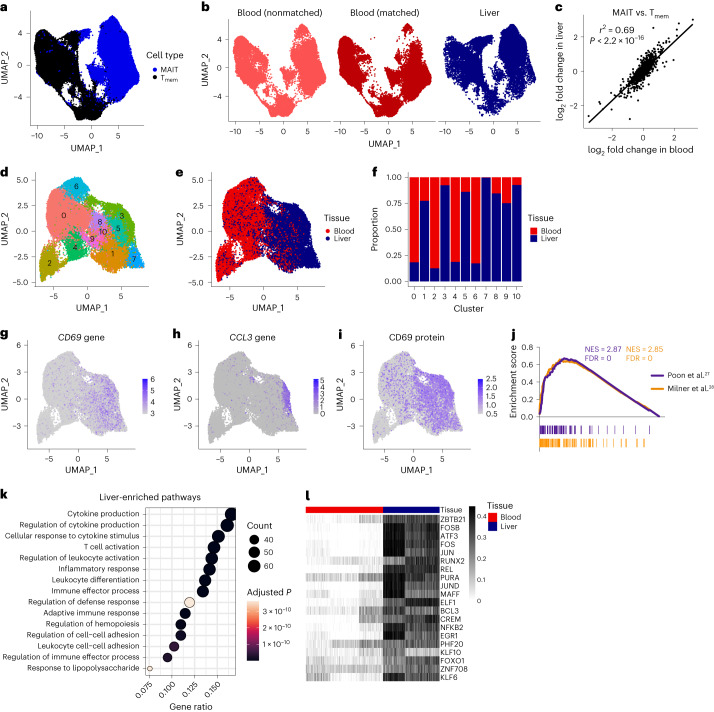
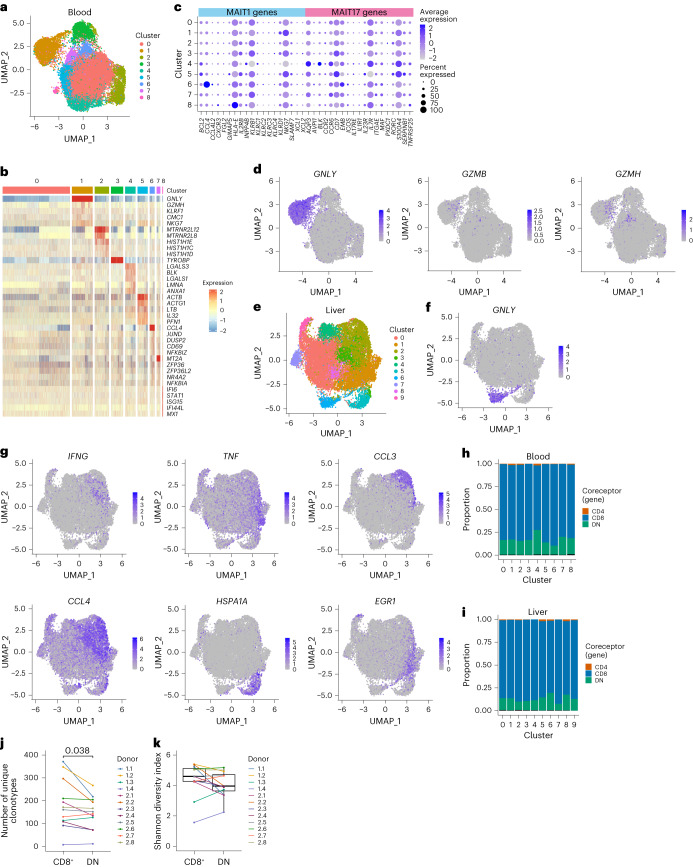
After filtering, Exp 1 and 2 comprised 89,456 cells. MAIT and Tmem cells, and blood and liver cells, were transcriptionally distinct (Fig. 1a,b). Blood T cells from liver donors (who underwent surgery for removal of benign or malignant lesions) were comparable to T cells from healthy donors (Fig. 1b), suggesting our data are not reflective of disease. CD4+ Tmem cells localized to distinct clusters, while rare CD4+ MAIT cells were distributed throughout the UMAP (Extended Data Fig. 1a,b). MAIT and Tmem cells differentially expressed 532 and 558 genes, and 37 and 33 proteins, in blood and liver, respectively (Supplementary Table 2a–d). Differences in gene and protein expression were highly correlated between tissues (Fig. 1c and Extended Data Fig. 1c–g). We defined core MAIT cell signatures of 167 genes (including KLRB1 and SLC4A10) and 11 proteins (including Vα7.2 TCRα and CD161; Supplementary Table 2e).
Fig. 1. Liver MAIT cells exhibit an activated, tissue-resident transcriptional and regulatory profile.
a, UMAP of blood and liver MAIT cells and conventional memory T (Tmem) cells colored by cell type. n = 89,456 cells from 12 donors. b, UMAP split by sample type, namely blood from healthy donors (nonmatched), blood from liver donors (matched) and liver. c, Pearson’s correlation between the log2 fold change in gene expression between MAIT and Tmem cells in the blood, and MAIT and Tmem cells in the liver. d,e, UMAP of matched blood and liver MAIT cells (n = 35,407 cells from six donors) colored by the 11 identified clusters (d) or by tissue (e). f, Proportion of cells in each cluster from the blood and liver. g–i, UMAPs colored by expression of CD69 (g) and CCL3 (h) genes or CD69 protein (i). j, Gene set enrichment analysis of liver compared with blood MAIT cells using published human and mouse tissue-resident memory T cell gene signatures. NES, normalized enrichment score. k, Over-representation analysis on the genes significantly upregulated in liver MAIT cells compared with blood MAIT cells. Top 15 gene ontology terms and associated Benjamini–Hochberg adjusted P values are shown. l, Heatmap showing activity (row-scaled AUCell scores) of the 20 most differentially active regulons (largest difference in average AUCell score) between matched blood and liver MAIT cells in Exp 1. n = 3 donors.
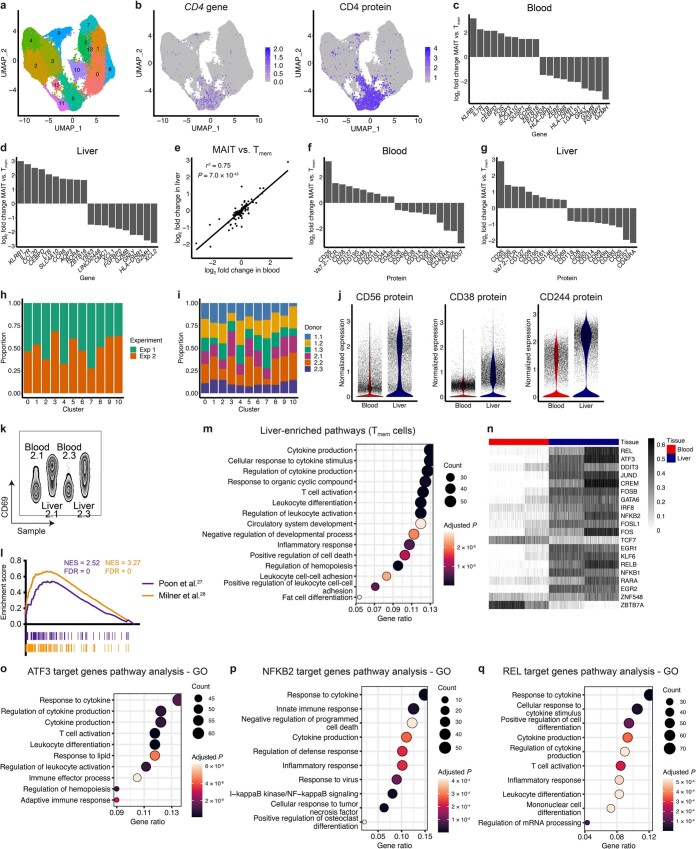
Extended Data Fig. 1. MAIT and Tmem cells, and blood and liver MAIT cells, exhibit distinct phenotypes and transcriptomes.
a, UMAP of blood and liver MAIT cells and conventional memory T (Tmem) cells colored by the 14 identified clusters. n = 89,456 cells from 12 donors (11 blood, 7 liver). b, UMAPs of blood and liver MAIT and Tmem cells colored by CD4 gene (left) and protein (right) expression. c,d, Bar plots showing the log2 fold change in expression of the top ten genes upregulated in MAIT cells compared with Tmem cells, and vice versa, in the blood (c) and liver (d). e, Pearson’s correlation between the log2 fold change in protein expression between MAIT and Tmem cells in the blood, and MAIT and Tmem cells in the liver. f,g, Bar plots showing the log2 fold change in expression of the top ten proteins upregulated in MAIT cells compared with Tmem cells, and vice versa, in the blood (f) and liver (g). h,i, Proportion of cells in each cluster (analysis of MAIT cells from six matched blood-liver donors) from Exp 1 and Exp 2 (h) and from each donor (i). j, Expression of CD56, CD38 and CD244 (2B4) proteins in blood and liver MAIT cells. k, Flow cytometry plot showing CD69 expression on blood and liver MAIT cells from two representative donors. l, Gene set enrichment analysis of liver compared with blood Tmem cells using published human and mouse tissue-resident memory T cell gene signatures. NES, normalized enrichment score. m, Over-representation analysis on the genes significantly upregulated in liver Tmem cells compared with blood Tmem cells. Top 15 gene ontology (GO) terms and associated Benjamini–Hochberg adjusted P values are shown. n, Heatmap showing activity (row-scaled AUCell scores) of the 20 most differentially active regulons (largest difference in average AUCell score) between matched blood and liver MAIT cells in Exp 2. n = 3 donors. o-q, Over-representation analysis on predicted ATF3 (o), NFKB2 (p) and REL (q) target genes. Top ten GO terms and associated Benjamini–Hochberg adjusted P values are shown.
MAIT cells from six matched blood-liver pairs (35,407 cells) comprised 11 clusters (Fig. 1d). Clusters largely contained cells from one tissue, but multiple experiments and donors (Fig. 1e,f and Extended Data Fig. 1h,i). Blood and liver cells differentially expressed 566 genes (most upregulated in the liver) and 24 proteins (Supplementary Table 3a,b). Liver-enriched genes encoded tissue-residency markers (for example, ITGAE), TCR-induced transcription factors (for example, EGR1), effector cytokines (for example, IFNG) and chemokines/chemokine receptors (for example, CXCR6). Some genes showed uniformly higher expression in the liver compared with the blood (for example, CD69; Fig. 1g); others were enriched in specific clusters (for example, CCL3; Fig. 1h). Interestingly, 84/167 core MAIT cell genes were upregulated in the liver (for example, RORA and IL23R). Liver-upregulated proteins included CD69 and CD244 (2B4), markers of tissue residency and cell activation (Fig. 1i and Extended Data Fig. 1j,k). Tmem cells showed similar tissue imprinting (Supplementary Table 3c,d). We defined core liver signatures of 300 genes and eight proteins (Supplementary Table 3e). Core liver proteins included canonical markers of tissue-resident memory T (TRM) cells26 (for example, CD69 and CD103) and ICAM1, required for MAIT1 retention in mouse liver12. Using gene set enrichment analysis, we demonstrated enrichment of human and mouse TRM cell gene signatures27,28 in liver MAIT (Fig. 1j) and Tmem (Extended Data Fig. 1l) cells. Other liver-enriched pathways related to cell activation, cell adhesion and inflammation (Fig. 1k and Extended Data Fig. 1m).
SCENIC29 was used to discover tissue-specific MAIT cell transcription factor regulons—modules of genes predicted to be regulated by a given transcription factor. Due to batch effects, cells from Exp 1 and Exp 2 were analyzed separately. Compared with blood, liver MAIT cells showed increased activity of AP-1 (for example, FOS and JUN) and NF-κB (for example, NFKB1 and NFKB2) transcription factors, and the TCR-induced transcription factor EGR1 (Fig. 1l, Extended Data Fig. 1n and Supplementary Table 4a,b). RUNX3 (regulates CD8+ TRM cell differentiation28) activity was also increased. AP-1- and NF-κB-regulated genes were enriched for pathways associated with T cell activation, inflammation and cytokine production (Extended Data Fig. 1o–q).
In summary, MAIT cells in blood and liver are transcriptionally distinct. MAIT and Tmem cells show similar adaptation to the liver environment and MAIT cell signature genes are consistent across tissues. We identify AP-1 and NF-κB transcription factors as central regulators of MAIT cell liver-specific gene expression.
MAIT cells have a limited TCRα but diverse TCRβ repertoire
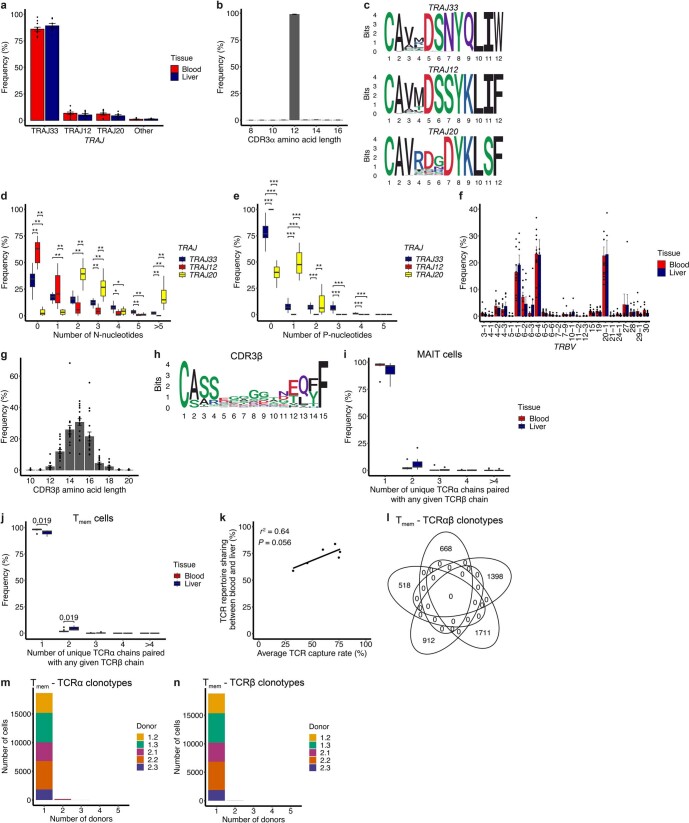
We next investigated whether TCR repertoires were tissue-specific. Previous analyses were limited by scale or depth20,21,30. Our dataset of >30,000 paired TCRs from 12 donors and matched tissues provided a unique opportunity to examine TCR repertoire characteristics and diversity.
Broad characteristics of the TCR repertoire were comparable in blood and liver. TRAJ33, TRAJ12 and TRAJ20 were used by 87%, 6% and 6% of TCRs, respectively (Extended Data Fig. 2a). The CDR3α region was highly restricted in length and sequence and included the canonical Tyr95α residue31,32 (Extended Data Fig. 2b,c). CDR3α sequence, and the number of N-nucleotides and P-nucleotides, varied with TRAJ gene usage (Extended Data Fig. 2c–e). TRBV expression was diverse but biased toward TRBV6-1, TRBV6-4 and TRBV20-1 (Extended Data Fig. 2f). CDR3β length and sequence were highly variable (Extended Data Fig. 2g,h).
Extended Data Fig. 2. MAIT cells have a restricted TCRα but diverse TCRβ chain, resulting in private TCRαβ repertoires.
a, Proportion of blood and liver cells expressing TRAJ33, TRAJ12, TRAJ20 and other TRAJ gene segments. b, Distribution of CDR3α amino acid lengths. c, Sequence logos generated from all TRAJ33, TRAJ12 or TRAJ20 CDR3α amino acid sequences of length 12 (n = 26,529, 1,852 and 1,451 sequences, respectively). d,e, Frequency of N-nucleotides (d) and P-nucleotides (e) in TRAJ33, TRAJ12 and TRAJ20 TCRs from 12 donors. f, Proportion of blood and liver MAIT cells expressing different TRBV gene segments. Plot includes TRBV gene segments with a frequency >1% in at least one sample. g, Distribution of CDR3β amino acid lengths. h, Sequence logo generated from all MAIT cell CDR3β amino acid sequences of length 15 (n = 9,300 sequences). i,j, TCR chain pairing at the population level. Number of unique TCRα chains paired with any given TCRβ chain in blood and liver MAIT (i; n = 11 blood, 7 liver samples) or Tmem (j; n = 10 blood, 5 liver samples) cells. k, Pearson’s correlation between the average TCR capture rate (percentage of cells with a paired TRAV1-2 TCR) for a donor (n = 6) and percentage MAIT cell TCR repertoire sharing between matched blood and liver. l, Venn diagram showing the number of TCRαβ clonotypes shared between the five Tmem cell donors. m,n, Number of Tmem cells from each donor belonging to TCRα clonotypes (m) or TCRβ clonotypes (n) found in 1, 2, 3, 4, or 5 (all) donors. a-c and f-i show data from n = 18 samples (11 blood, 7 liver), 12 donors. Data in a, b, f, g are presented as mean ± s.e.m. In d, e, i, j, boxes span the 25th–75th percentiles, the midline denotes the median and whiskers extend to ± 1.5 × IQR. Points in i and j indicate outliers. Two-sided Wilcoxon rank-sum test (a, f, i, j) and two-sided Wilcoxon signed-rank test (d, e) for all pairwise comparisons. Benjamini-Hochberg adjusted P values are shown (nonsignificant results omitted). *P < 0.05, **P < 0.01, ***P < 0.001.
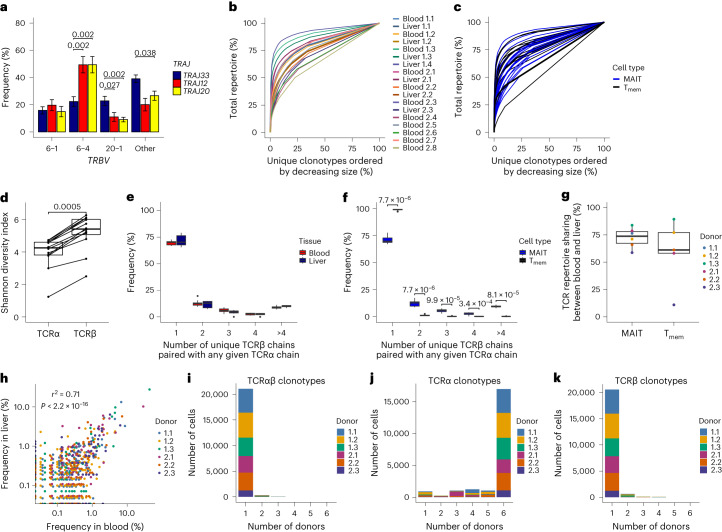
Studies of small numbers of TCR sequences suggest TRAJ usage could influence TCRαβ pairing21,30. Our data revealed increased pairing of TRAJ12 and TRAJ20 TCRα chains with TRBV6-4 TCRβ chains compared with TRAJ33, while TRAJ33 TCRα chains more frequently paired with TRBV20-1 TCRβ chains (Fig. 2a).
Fig. 2. MAIT cells have a restricted TCRα but diverse TCRβ chain, resulting in private TCRαβ repertoires.
a, Percentage of TRAJ33, TRAJ12 and TRAJ20 TCRα chains paired with TRBV6-1, TRBV6-4, TRBV20-1 or other TCRβ chains. Mean ± s.e.m. is shown. b, Line plot colored by sample demonstrating the clonality of the TCRαβ repertoire. c, Line plot comparing MAIT (blue) and Tmem (black) cell TCRαβ clonality. d, Shannon diversity index for TCRα clonotypes and TCRβ clonotypes for each donor. e,f, TCR chain pairing at the population level. Number of unique TCRβ chains paired with any given TCRα chain in blood and liver MAIT cells (e) or MAIT and Tmem cells (f; blood and liver cells combined). g, Percentage of MAIT and Tmem cells belonging to a TCRαβ clonotype shared between matched blood and liver. h, Pearson’s correlation between clonotype frequency in matched blood and liver. i–k, Number of cells from each donor belonging to TCRαβ (i), TCRα (j) or TCRβ (k) functional clonotypes found in 1, 2, 3, 4, 5 or 6 (all) donors. Plots show TCR data for MAIT cells (or MAIT and Tmem cells in c, f and g) from all donors (a–f; n = 12) or matched blood-liver donors (g–k; n = 6). In d–g, boxes span the 25th–75th percentiles, the midline denotes the median and whiskers extend to ±1.5 × IQR. Points in e and f indicate outliers. Two-sided Wilcoxon signed-rank test (a and d) and two-sided Wilcoxon rank-sum test (e–g) for all pairwise comparisons. Benjamini–Hochberg adjusted P values are shown (nonsignificant results omitted).
TCR clonotypes were defined as cells with identical TCR gene segment usage and CDR3 nucleotide sequences (TRAV1-2 TCRα required for MAIT cells). MAIT cells were oligoclonal, with oligoclonality comparable across donors and tissues (Fig. 2b) and with Tmem cells (Fig. 2c). MAIT cell clonotypes defined using only the TCRα chain (TCRα clonotypes) were more oligoclonal than those defined using only the TCRβ chain (TCRβ clonotypes; Fig. 2d). At the population level, MAIT cell TCRα chain pairing was promiscuous, with ~30% of TCRα chains paired with >1 unique TCRβ—generating multiple clones with identical TCRα chains (Fig. 2e). Conversely, most TCRβ chains paired with a single TCRα (Extended Data Fig. 2i). Tmem cell TCRαβ pairings were essentially unique (Fig. 2f and Extended Data Fig. 2j).
Overall, the MAIT cell TCRα repertoire is highly restricted, while the TCRβ repertoire is considerably more diverse. TCR repertoire characteristics were similar in blood and liver, and we identified a preference for TRAJ12/20-TRBV6-4 and TRAJ33-TRBV20-1 pairings.
TCR repertoires are donor-specific but shared across tissues
As TCR usage was similar across tissues, we hypothesized that blood and liver MAIT cells might show clonal overlap. On average, 72% of MAIT cells belonged to a TCR clonotype present in matched blood and liver (Fig. 2g) and clonotype frequency correlated between tissues (Fig. 2h). Clonal sharing was similarly high for Tmem cells (Fig. 2g). The extent of MAIT cell TCR repertoire overlap between tissues correlated with TCR capture rate, suggesting our data underestimates blood-liver sharing (Extended Data Fig. 2k).
We next examined repertoire overlap between donors, with clonotypes defined using CDR3 amino acid sequences (functional clonotypes). Despite their semi-invariant TCR, 98% of MAIT cells belonged to a donor-specific clonotype, with no clonotypes shared between all six donors (Fig. 2i). Tmem cell functional clonotypes showed no overlap between donors (Extended Data Fig. 2l).
Given the restricted MAIT cell TCRα repertoire, we reasoned that functional clonotypes defined using the TCRα chain only (functional TCRα clonotypes) may show high overlap between donors. Supporting this hypothesis, the six donors shared 27 functional TCRα clonotypes comprising 79% (16,973/21,421) of MAIT cells (Fig. 2j). In contrast, 1.1% (196/18,564) and <0.1% of Tmem cells belonged to a functional TCRα clonotype found in two and three donors, respectively (Extended Data Fig. 2m). Functional TCRβ clonotypes were largely donor-specific for MAIT and Tmem cells (Fig. 2k and Extended Data Fig. 2n).
Thus, distinct from Tmem cells, the MAIT cell TCRα repertoire is public. In contrast, the TCRβ chain is markedly more private and is what governs the uniqueness of individual MAIT cell TCR repertoires.
Within-tissue transcriptional heterogeneity is limited
We next explored within-tissue heterogeneity.
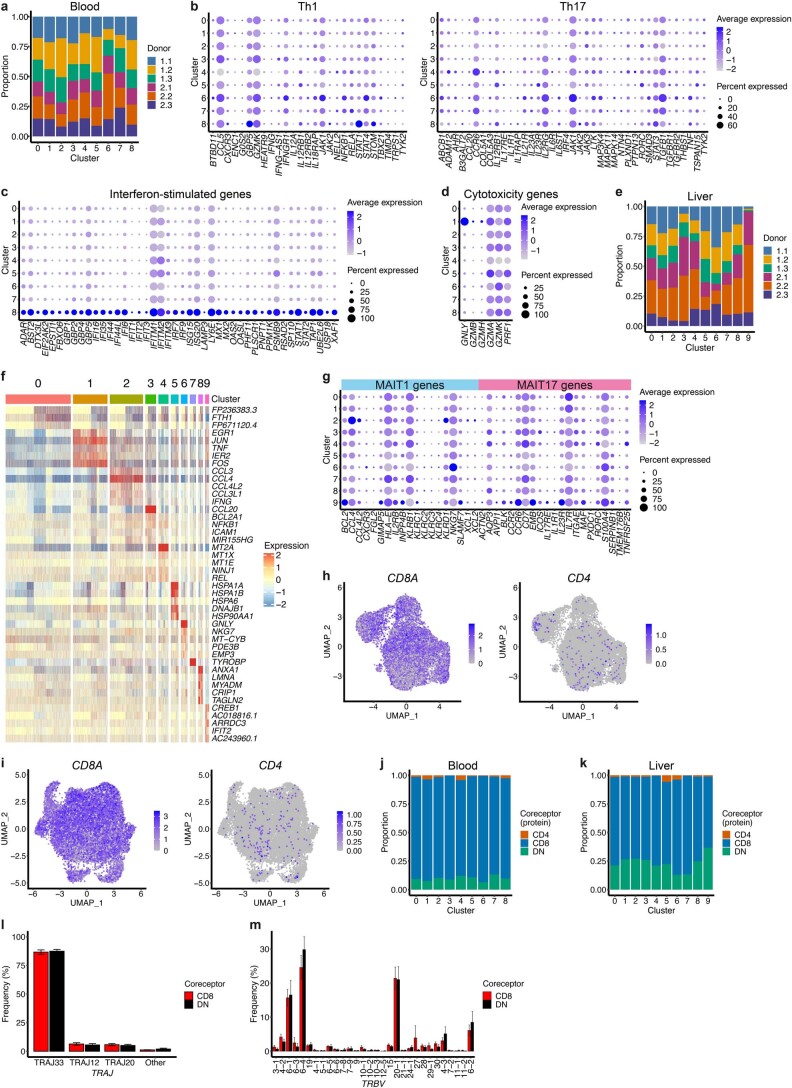
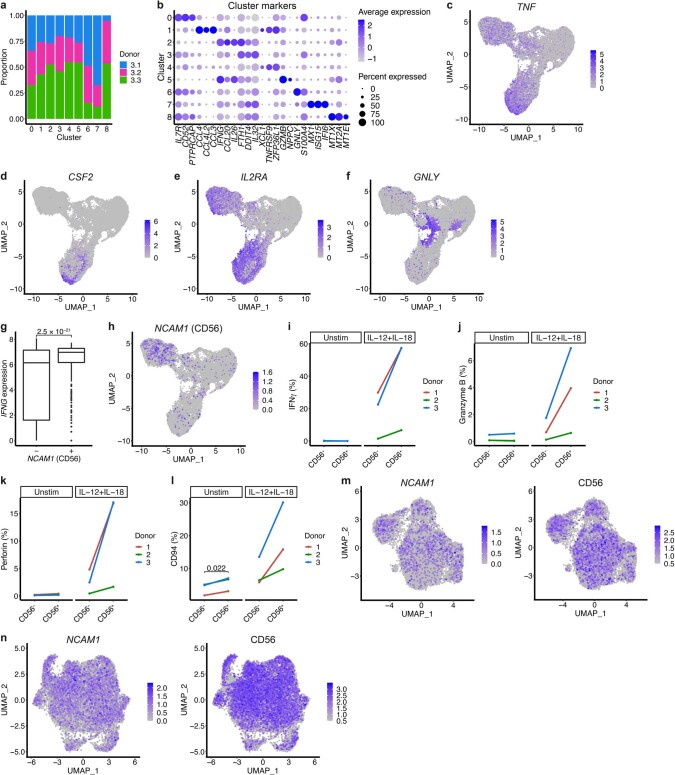
Blood MAIT cells comprised nine clusters (Fig. 3a and Extended Data Fig. 3a). Transcriptional diversity between clusters was low, with few genes displaying cluster-specific expression (Fig. 3b and Supplementary Table 5a). Apart from the three clusters discussed below, cluster markers were not indicative of specific functions or known T cell differentiation states. Using mouse MAIT1 and MAIT17 (refs. 33,34) or human Th1 and Th17 (ref. 35) gene signatures, we were unable to identify human MAIT1 and MAIT17 subsets (Fig. 3c and Extended Data Fig. 3b). CCL4 was upregulated in cluster 6 and interferon-stimulated genes in cluster 8 (Fig. 3b and Extended Data Fig. 3c), perhaps indicating some degree of basal cell activation. Cells in cluster 2 appeared primed for cytotoxicity with increased expression of granulysin and granzymes (Fig. 3d and Extended Data Fig. 3d). This cluster did not simply indicate cell activation, as GZMB and GZMH (lowly expressed in resting MAIT cells36) were only expressed by a small percentage of cells.
Fig. 3. MAIT cells within the blood and liver show minimal transcriptional heterogeneity.
a, UMAP of blood MAIT cells from matched blood-liver donors (n = 6) colored by the nine identified clusters. b, Heatmap showing row-scaled log-transformed normalized expression of the top five or all (if <5) marker genes for each blood MAIT cell cluster. c, Expression of MAIT1 and MAIT17 genes in blood MAIT cell clusters. Dot color indicates the level of gene expression and dot size indicates the percentage of cells expressing the gene. d, UMAPs of blood MAIT cells colored by expression of GNLY, GZMB and GZMH. e, UMAP of liver MAIT cells from matched blood-liver donors (n = 6) colored by the ten identified clusters. f, UMAP of liver MAIT cells colored by expression of GNLY. g, UMAPs of liver MAIT cells colored by expression of IFNG, TNF, CCL3, CCL4, HSPA1A and EGR1. h,i, Proportion of CD4+, CD8+ and DN cells in each blood (h) and liver (i) cluster. Coreceptor identity defined by the expression of CD4, CD8A and CD8B genes (Methods). j, Number of unique clonotypes in CD8+ and DN MAIT cells from each donor (n = 12; CD8+ cell number within each donor downsampled to match the number of DN cells). k, Shannon diversity index for TCRαβ clonotypes in CD8+ and DN MAIT cells from each donor (n = 12). Boxes span the 25th–75th percentiles, the midline denotes the median and whiskers extend to ±1.5 × IQR. Two-sided Wilcoxon signed-rank test in j and k (nonsignificant results omitted).
Extended Data Fig. 3. MAIT cells within the blood and liver show minimal transcriptional heterogeneity.
a, Proportion of cells in each blood MAIT cell cluster from each donor (n = 6). b-d, Expression of Th1 and Th17 (b), interferon-stimulated (c) and cytotoxicity (d) genes in blood MAIT cell clusters. Dot color indicates the level of gene expression and dot size indicates the percentage of cells expressing the gene. e, Proportion of cells in each liver MAIT cell cluster from each donor (n = 6). f, Heatmap showing row-scaled log-transformed normalized expression of the top five or all (if <5) marker genes for each liver MAIT cell cluster. g, Expression of MAIT1 and MAIT17 genes in liver MAIT cell clusters. Dot color indicates the level of gene expression and dot size indicates the percentage of cells expressing the gene. h,i, UMAPs of blood (h) and liver (i) MAIT cells colored by expression of CD8A (left) and CD4 (right). CD4+ cells positioned in front of CD4− cells to allow better visibility. j,k, Proportion of CD4+, CD8+ and DN cells in each blood (j) and liver (k) cluster. Coreceptor identity defined based on the expression of CD4 and CD8 proteins (Methods). l,m, Frequency of CD8+ and DN MAIT cells expressing the indicated TRAJ (l) and TRBV (m) genes. n = 12 donors. TRBV genes expressed in >1% of CD8+ or DN cells from any donor are included. Mean ± s.e.m. is shown. Two-sample Wilcoxon signed-rank test for all pairwise comparisons (nonsignificant results omitted).
Liver MAIT cells comprised ten clusters (Fig. 3e and Extended Data Fig. 3e) with modest transcriptional differences (Extended Data Fig. 3f and Supplementary Table 5b). Clusters did not correspond to known differentiation states such as MAIT1 and MAIT17 (Extended Data Fig. 3g). As in blood, there was a GNLY-expressing cluster (cluster 6; Fig. 3f), while remaining clusters expressed different activation- or stress-induced molecules (Fig. 3g). Most genes showed a gradient of expression across clusters.
Despite reported phenotypic, functional and/or transcriptional differences4–6, CD8+, DN and CD4+ MAIT cells did not comprise separate clusters in blood or liver (Fig. 3h,i and Extended Data Fig. 3h–k), and differentially expressed few genes and proteins (Supplementary Table 6a–h). Previous bulk RNA-seq data indicated higher TCR repertoire diversity in CD8+ relative to DN MAIT cells5. After downsampling to equalize numbers of CD8+ and DN cells per donor, we identified a small increase in the frequency of unique clonotypes among CD8+ MAIT cells (Fig. 3j), but no difference in TRAJ/TRBV chain usage (Extended Data Fig. 3l,m), and an equivalent Shannon diversity index (Fig. 3k). Therefore, consistent with minor transcriptional differences, CD8+ and DN MAIT cells show similar TCR usage.
Overall, contrasting with mice, human MAIT cells show limited transcriptional heterogeneity within tissues and do not comprise distinct MAIT1 and MAIT17 subsets, or subsets defined by coreceptor expression.
TCR clonotypes show variable bias in cluster localization
We next examined whether the limited transcriptional heterogeneity within tissues correlated with clonal identity. Given the donor-specific private TCR repertoire, we separately clustered each sample, then used the exact multinomial test to determine whether clonotypes were nonrandomly distributed across clusters.
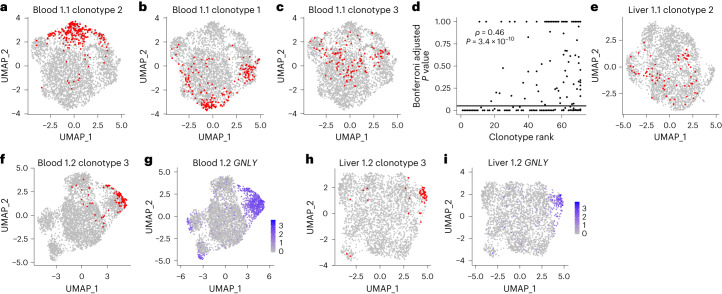
There was a range of associations between clonotype and cluster, both within and between donors. Some clonotypes predominantly localized in a single cluster (Fig. 4a and Extended Data Fig. 4a). Some showed a subtle but still significant bias in cluster localization (Fig. 4b and Extended Data Fig. 4a). Others were randomly distributed (Fig. 4c and Extended Data Fig. 4a). Bias in cluster distribution was more frequently significant for larger clonotypes (Fig. 4d), suggesting nonsignificant results for some smaller clonotypes may reflect a lack of statistical power.
Fig. 4. TCRαβ clonotypes are variably associated with transcriptional clusters.
a–c, UMAPs of blood MAIT cells from donor 1.1 showing in red cells from a single TCRαβ clonotype. Plots show clonotype 2 (a), clonotype 1 (b) and clonotype 3 (c). Clonotype 1 is the largest clonotype from a donor, clonotype 2 is the second largest and so on. d, Spearman’s rank correlation between clonotype size (rank) and the Bonferroni adjusted P value for association between clonotype and cluster (exact multinomial test, performed for clonotypes from all donors (n = 12) with ≥20 cells). e, UMAP of liver MAIT cells from donor 1.1 with cells from clonotype 2 indicated in red (the same clonotype as in a). f,g, UMAPs of blood MAIT cells from donor 1.2 with cells from clonotype 3 indicated in red (f) or expression of GNLY shown in blue (g). h,i, UMAPs of liver MAIT cells from donor 1.2 with cells from clonotype 3 indicated in red (h) or expression of GNLY shown in blue (i).
Extended Data Fig. 4. Clusters identified in individual blood or liver samples.
a-d, Individual sample UMAPs colored by the identified clusters—blood 1.1 (a), liver 1.1 (b), blood 1.2 (c), liver 1.2 (d).
For a given clonotype, the extent of bias in cluster localization was not necessarily concordant in blood and liver (Fig. 4a,e and Extended Data Fig. 4a,b). This may be due in part to different clonotype sizes in the two tissues. Some clonotypes had a stable transcriptional phenotype across tissues. For example, a TRAV1-2/TRAJ12 clonotype from donor 1.2 preferentially localized to the GNLY-expressing cluster in blood (Fig. 4f,g and Extended Data Fig. 4c) and liver (Fig. 4h,i and Extended Data Fig. 4d). Therefore, the transcriptional profile of resting MAIT cells is influenced by their clonal identity.
MAIT cell functional diversity is stimulus-specific
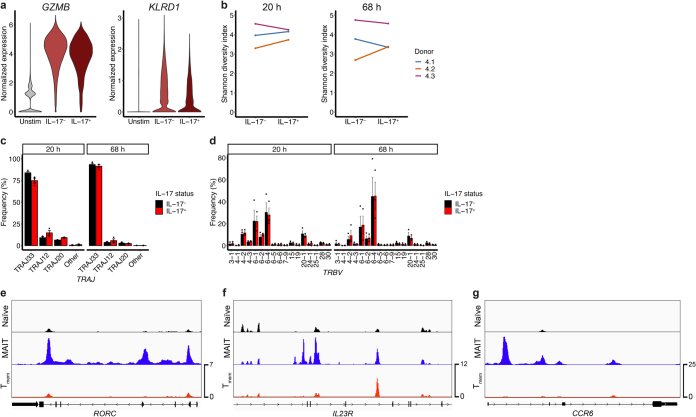
Since we did not identify subsets of resting MAIT cells, we investigated whether functional subsets were present following activation. CD8+ T cells were left unstimulated or stimulated with MR1/5-OP-RU (TCR) or IL-12 + IL-18 (cytokine). After 20 h, CD8+ MAIT cells were sorted (CD26+CD161hiVα7.2+ for unstimulated and cytokine-stimulated, and CD26+CD161hi for TCR-stimulated due to TCR downregulation) for scRNA-seq and scTCR-seq (Exp 3; Supplementary Fig. 2), an approach we termed functional RNA-sequencing (fRNA-seq).
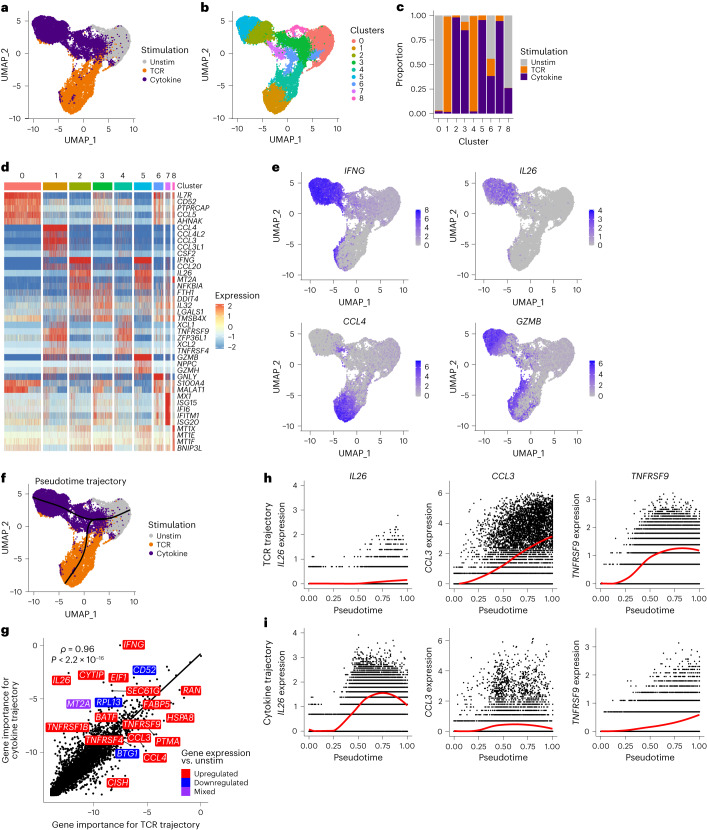
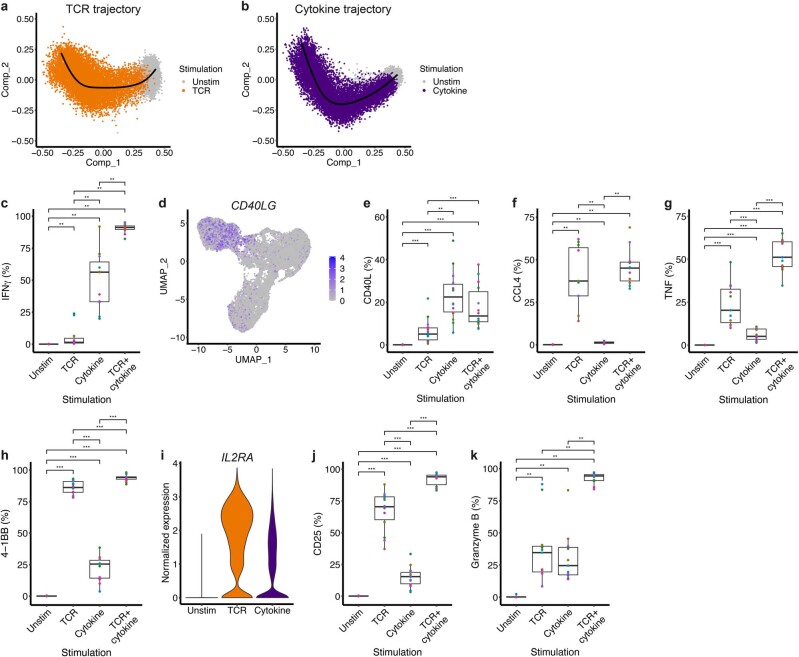
fRNA-seq revealed stimulus-specific transcriptional responses (Fig. 5a and Supplementary Table 7a,b). MAIT cells (27,305 cells) comprised nine clusters—these were present in all donors but largely stimulus-specific (Fig. 5b,c and Extended Data Fig. 5a). Consistent with their homogeneous resting transcriptome, unstimulated cells predominantly localized in one cluster. TCR-stimulated cells localized in clusters 1 and 4. Cells in cluster 1 were more activated than those in cluster 4, displaying increased expression of chemokines and cytokines including CCL4, TNF and CSF2 (Fig. 5d,e, Extended Data Fig. 5b–d and Supplementary Table 8). Clusters 2, 3 and 5 largely comprised cytokine-stimulated cells and appeared to indicate different degrees of cell activation. Expression of activation markers (for example, IL2RA) and effector molecules (for example, GZMB) was low in cluster 3, but high in cluster 5 (Fig. 5d,e, Extended Data Fig. 5b,e and Supplementary Table 8). Cells in cluster 2 expressed high levels of IFNG but less GZMB than cells in cluster 5. Interferon-stimulated genes were uniquely expressed in cluster 7 (mostly cytokine-stimulated; Fig. 5d and Extended Data Fig. 5b).
Fig. 5. TCR- and cytokine-activated MAIT cells follow distinct linear trajectories.
a,b, UMAPs of MAIT cells from all donors colored by stimulation condition (a) or the nine identified clusters (b). n = 27,305 cells from three donors. c, Proportion of cells in each cluster from the three stimulation conditions. d, Heatmap showing row-scaled log-transformed normalized expression of the top five marker genes for each cluster. e, UMAPs colored by expression of IFNG, IL26, CCL4 and GZMB. f, UMAP of MAIT cells from all donors with the branching pseudotime trajectory identified using Slingshot shown in black. g, Spearman’s rank correlation between gene importance (log2 1/gene importance rank) on SCORPIUS TCR and cytokine trajectories. Labels indicate the most differentially important genes, ten with higher importance on the TCR trajectory and ten with higher importance on the cytokine trajectory. Colors indicate whether gene expression was upregulated (red), downregulated (blue) or mixed (purple; upregulated in TCR and downregulated in cytokine or vice versa) relative to unstimulated cells. h,i, Expression of IL26, CCL3 and TNFRSF9 along SCORPIUS TCR (h) and cytokine (i) trajectories.
Extended Data Fig. 5. Gene and protein expression by TCR- and cytokine-stimulated MAIT cells.
a, Proportion of cells from each donor (n = 3) in each cluster (unstimulated, TCR-stimulated and cytokine-stimulated MAIT cells combined). b, Expression of the top three marker genes per cluster. Dot color indicates the level of gene expression and dot size indicates the percentage of cells expressing the gene. c-f, UMAPs colored by expression of TNF (c), CSF2 (d), IL2RA (e) and GNLY (f). g, Expression of IFNG in cytokine-stimulated MAIT cells negative (n = 11,122 cells) and positive (n = 387 cells; log-transformed normalized expression >0) for the expression of NCAM1 (CD56). Boxes span the 25th–75th percentiles, the midline denotes the median and whiskers extend to ±1.5 × IQR. Points indicate outliers. Two-sample Wilcoxon rank-sum test. h, UMAP colored by expression of NCAM1 (CD56). i-l, Percentage of sorted CD56− and CD56+ MAIT cells expressing IFNγ (i), granzyme B (j), perforin (k) and CD94 (l) when left unstimulated or stimulated with IL-12 + IL-18 for 20 h (as measured by flow cytometry). n = 3 donors. Two-sided paired t-test between CD56- and CD56+ cells in both conditions (nonsignificant results omitted). m,n, CD56 gene (left) and protein (right) expression in blood (m) and liver (n) MAIT cells. Protein expression measured in Exp 2 only.
As in Exp 1 and 2, we identified a GNLY-expressing cluster (cluster 6; Fig. 5d and Extended Data Fig. 5f) that contained cells from all three conditions (Fig. 5c). The cells did not express other cytotoxic molecules (for example, GZMB) or markers of activation, inconsistent with our initial hypothesis that GNLY-expressing cells at rest are primed for cytotoxicity.
It was suggested that CD56 expression identifies a MAIT cell subset with enhanced cytokine responsiveness7. Following cytokine stimulation, CD56 (NCAM1)-expressing MAIT cells showed increased IFNG production relative to their nonexpressing counterparts (Extended Data Fig. 5g). However, as CD56 expression was qualitatively increased following cytokine stimulation (Extended Data Fig. 5h), we could not establish the usefulness of CD56 as a baseline indicator of functional potential. To address this, we stimulated sorted CD56− and CD56+ MAIT cells with IL-12 + IL-18 for 20 h, and measured IFNγ, granzyme B, perforin and CD94 expression by flow cytometry. There was a trend toward increased expression of all tested markers in activated CD56+ cells relative to CD56− cells (Extended Data Fig. 5i–l). Thus, CD56 expression correlates with MAIT cells primed for cytokine responsiveness. However, CD56+ cells did not comprise a transcriptionally distinct cluster of MAIT cells in resting blood or liver (Extended Data Fig. 5m,n). Further experiments are necessary to understand the overall impact of CD56 expression on MAIT cell biology.
Pseudotime analysis reveals linear activation trajectories
As MAIT cell clusters captured cells at different stages of activation, we further explored transcriptional responses to stimulation using pseudotime analysis. The Slingshot37 algorithm identified a branching trajectory with a single branch point close to unstimulated cells (Fig. 5f), suggesting MAIT cells become transcriptionally distinct early following TCR and cytokine stimulation. Results were validated using SCORPIUS38 (Extended Data Fig. 6a,b).
Extended Data Fig. 6. MAIT cell activation trajectories and validation of stimulus-specific and shared activation markers and cytokines.
a,b, Multidimensional scaling plots of unstimulated and TCR-stimulated MAIT cells (a), or unstimulated and cytokine-stimulated MAIT cells (b). SCORPIUS TCR (a) and cytokine (b) trajectories are shown in black. n = 3 donors. c,e-h,j,k, Percentage of MAIT cells expressing IFNγ (c), CD40L (e), CCL4 (f), TNF (g), 4-1BB (h), CD25 (j) and granzyme B (k) when left unstimulated or stimulated with plate-bound MR1/5-OP-RU (TCR), IL-12 + IL-18 (cytokine) or both (TCR+cytokine) for 20 h. Protein expression was measured by flow cytometry on all MAIT cells from 11 donors in two independent experiments (c, f, g, k) or CD4− MAIT cells from 14 donors in three independent experiments (e, h, j). Boxes span the 25th–75th percentiles, the midline denotes the median and whiskers extend to ±1.5 × IQR. Two-sample Wilcoxon signed-rank test. Benjamini-Hochberg adjusted P values are shown (nonsignificant results omitted). **P < 0.01, ***P < 0.001. d, UMAP of unstimulated, TCR-stimulated and cytokine-stimulated MAIT cells colored by expression of CD40LG. i, Violin plot showing expression of IL2RA by unstimulated, TCR-stimulated and cytokine-stimulated MAIT cells.
Through random forest regression, we identified the genes most important for predicting cell pseudotime on the TCR and cytokine trajectories (Supplementary Table 9). Gene importance was highly correlated between the two trajectories (Fig. 5g), with nine of the top 20 genes overlapping, including IL2RA (CD25) and TNFRSF18 (GITR; both upregulated) and IL7R (downregulated). However, several notable genes were important primarily for one trajectory (Fig. 5g–i). IFNG and IL26 were specific to the cytokine trajectory, while CCL3, CCL4 and TNFRSF9 (4-1BB) showed greater importance for the TCR trajectory. Protein expression, as measured by flow cytometry, was consistent with gene expression (Extended Data Fig. 6c–k)—IFNγ and CD40L were more strongly induced by cytokine stimulation and CCL4, TNF, 4-1BB and CD25 by TCR stimulation. Granzyme B was similarly induced by both stimuli.
Regulation of TCR- and cytokine-induced transcription
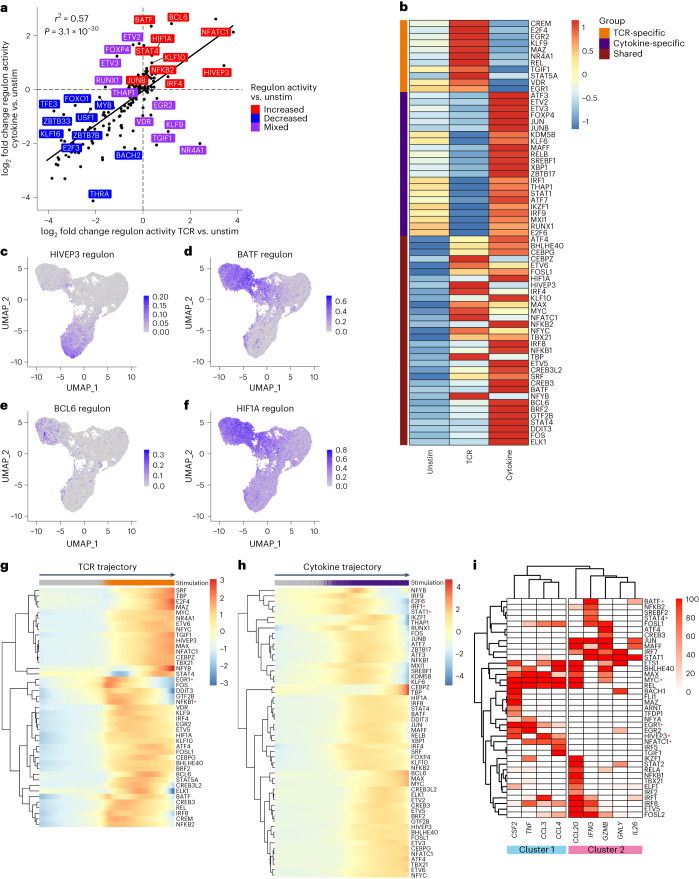
Using SCENIC29, we identified 159 high-confidence regulons regulating shared and stimulus-specific gene expression. Although global changes in transcription factor activity relative to unstimulated cells were highly correlated for TCR- and cytokine-stimulated cells (Fig. 6a), some regulons showed markedly different activity between conditions.
Fig. 6. Transcriptional regulation of TCR- and cytokine-stimulated MAIT cells exhibits shared and distinct properties.
a, Pearson’s correlation between the log2 fold change in regulon activity (AUCell scores) between TCR-stimulated and unstimulated MAIT cells, and cytokine-stimulated and unstimulated MAIT cells. Labels show the regulons with the largest difference in log2 fold change relative to unstimulated cells between the TCR and cytokine trajectory, ten with increased (red), ten with decreased (blue) and ten with mixed (purple; increased in TCR and decreased in cytokine or vice versa) activity following stimulation. b, Heatmap showing the activity (row-scaled average AUCell scores) of TCR-specific (orange), cytokine-specific (purple) and shared (maroon) upregulated regulons in each stimulation condition. c–f, UMAPs colored by the activity of HIVEP3 (c), BATF (d), BCL6 (e) and HIF1A (f) regulons. g, Heatmap showing regulon activity (smoothed AUCell scores) over pseudotime on the SCORPIUS TCR trajectory for regulons upregulated upon TCR stimulation. Gray, unstimulated cells; orange, TCR-stimulated cells. h, Heatmap showing regulon activity (smoothed AUCell scores) over pseudotime on the SCORPIUS cytokine trajectory for regulons upregulated upon cytokine stimulation. Gray, unstimulated cells; purple, cytokine-stimulated cells. i, Regulation of select MAIT cell effector genes. Heatmap is colored by the percent occurrence of each gene within each transcription factor regulon. High-confidence regulons predicted to regulate at least one of the genes in >50% of pySCENIC runs are included. Red asterisks in g–i indicate regulons mentioned in the text.
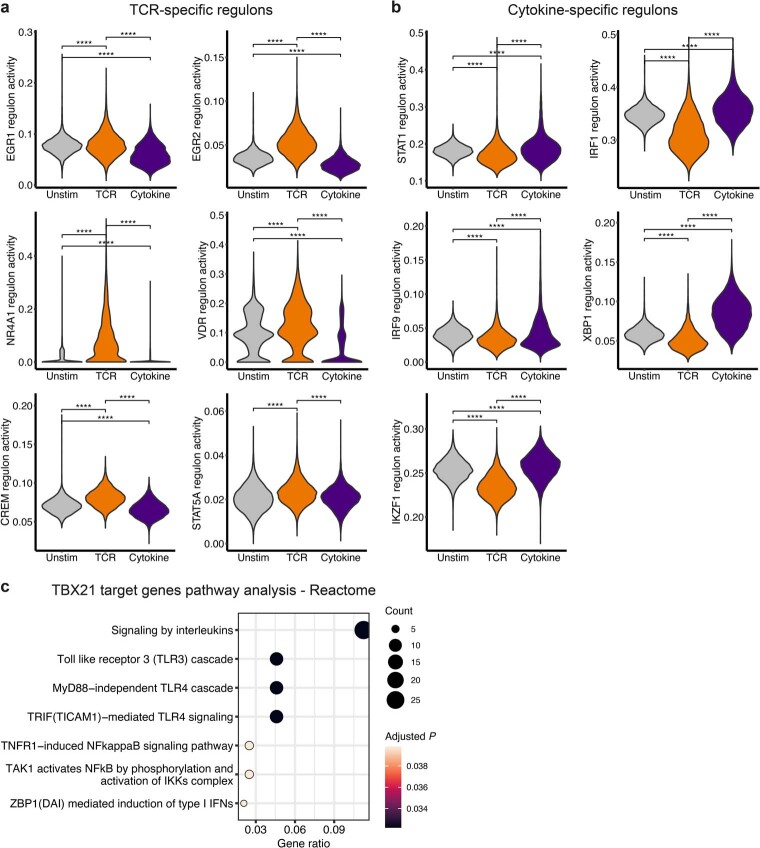
Relative to unstimulated cells, 65 regulons had increased activity: 11 were TCR-specific, 22 were cytokine-specific and 32 were shared (Fig. 6b). TCR-specific regulons included TCR-induced transcription factors (EGR1, EGR2, NR4A1), and VDR, CREM and STAT5A, which have varied roles in regulating Th17 differentiation and IL-17 production39–41 (Fig. 6b and Extended Data Fig. 7a). Cytokine-specific regulons included STAT1, interferon regulatory factors, XBP1 and IKZF1 (Ikaros; Fig. 6b and Extended Data Fig. 7b). Ikaros regulates activated conventional CD8+ T cell responsiveness to IL-12 (ref. 42).
Extended Data Fig. 7. Transcriptional regulation of TCR- and cytokine-stimulated MAIT cells exhibits shared and distinct properties.
a,b, Violin plots showing the activity (AUCell scores) of selected TCR-specific (a) and cytokine-specific (b) transcription factor regulons in unstimulated, TCR-stimulated and cytokine-stimulated MAIT cells. n = 3 donors. Differential activity analysis was performed for all pairwise comparisons using MAST. Bonferroni adjusted P values are shown (nonsignificant results omitted). ****P < 0.0001. c, Over-representation analysis on predicted T-bet (TBX21) target genes. Significant Reactome pathways and associated Benjamini-Hochberg adjusted P values are shown.
The 32 regulons with increased activity upon TCR and cytokine stimulation were also all differentially active between the two conditions. TBX21 activity was most similar between TCR- and cytokine-stimulated cells—its target genes were enriched for interleukin, Toll-like receptor and NF-κB signaling pathways (Extended Data Fig. 7c). As expected, NFATC1 and STAT4 showed increased activity in TCR- and cytokine-stimulated cells, respectively. We identified new candidate regulators of stimulus-specific MAIT cell functions, namely HIVEP3 for TCR-stimulated cells, and BATF, BCL6 and HIF1A for cytokine-stimulated cells (Fig. 6c–f). HIVEP3 is essential for the development of innate-like T cells including MAIT cells43. Among its many roles, BATF promotes effector CD8+ T cell differentiation through the upregulation of key transcription factors, cytokine receptors and signaling molecules44.
As with upregulated genes, regulon activity varied across stimulated cells. Most regulons progressively increased in activity over pseudotime (Fig. 6g,h). However, activity of some regulons peaked early and subsequently declined, for example, EGR1 and NFKB1 on the TCR trajectory, and STAT1 and IRF1 on the cytokine trajectory. Several early-activated regulons regulated later-activated transcription factors. STAT1 was a predicted regulator of TBX21. HIVEP3 was a predicted target of EGR1, supporting its TCR-specific activation and function.
To identify candidate regulators of MAIT cell function, we examined effector gene localization within SCENIC regulons. Unsupervised hierarchical clustering identified two clusters of effector genes (Fig. 6i). Cluster 1 comprised genes preferentially induced by TCR signaling, namely CSF2, TNF, CCL3 and CCL4, suggesting similar regulation. Genes in cluster 1 were regulated by EGR1, NFATC1 and MYC. In addition, HIVEP3 was a predicted regulator of CSF2, CCL3 and CCL4. Cluster 2 comprised both cytokine-specific genes (for example, IL26) and genes induced by both stimuli (for example, GZMB). As expected, IFNG was regulated by STAT4, but was also in the BATF regulon, suggesting BATF could contribute to enhanced IFNG production in cytokine-stimulated MAIT cells compared with TCR-stimulated MAIT cells.
Overall, TCR- and cytokine-stimulated MAIT cells exhibit shared and stimulus-specific regulation. Our data reveal new candidate regulators of TCR- and cytokine-specific responses and their predicted target genes.
Clonal identity influences MAIT cell activation potential
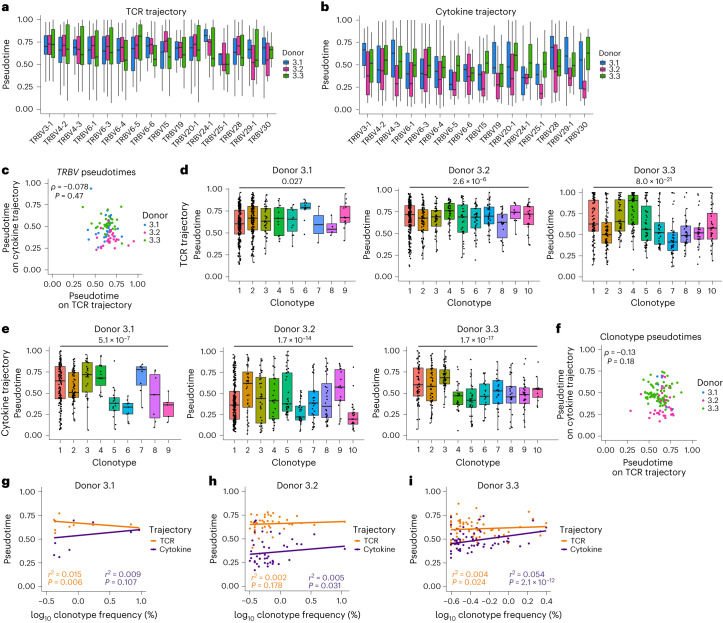
TCR clonotypes showed varied associations with resting transcriptional clusters (Fig. 4) and published data suggest functional differences linked to TCRβ usage7,23,25. Using fRNA-seq, we investigated whether MAIT cell activation potential (pseudotime position) correlated with TCRβ usage or clonal identity. Within donors, activation capacity was significantly associated with TRBV usage, but there was considerable variability among cells with the same TRBV gene (Fig. 7a,b and Extended Data Fig. 8a,b). TRBV pseudotimes did not correlate between donors (Fig. 7a,b and Extended Data Fig. 8c,d) or between TCR and cytokine trajectories (Fig. 7c), indicating no intrinsic difference in the activation potential of TRBV genes.
Fig. 7. Clonal identity influences MAIT cell activation potential.
a,b, Box plots split by donor (n = 3) showing pseudotime values on SCORPIUS TCR (a) and cytokine (b) trajectories for MAIT cells expressing different TRBV gene segments. Kruskal–Wallis test P values for a are 8.5 × 10−21, 2.2 × 10−9 and 1.3 × 10−15, and for b are 0.045, 0.045 and 2.2 × 10−20 for donors 3.1, 3.2 and 3.3, respectively. c, Spearman’s rank correlation between average TRBV pseudotimes on SCORPIUS TCR and cytokine trajectories. d,e, Pseudotime values for cells from the largest ten clonotypes in each donor or all clonotypes containing ≥20 cells (n = 9, 10 and 10 clonotypes for donors 3.1, 3.2 and 3.3, respectively) on SCORPIUS TCR (d) and cytokine (e) trajectories. Kruskal–Wallis test was performed for all clonotypes containing ≥20 cells. f, Spearman’s rank correlation between average clonotype pseudotimes on SCORPIUS TCR and cytokine trajectories. g–i, Pearson’s correlation between log10 clonotype frequency and pseudotime on SCORPIUS TCR and cytokine trajectories for donors 3.1 (g), 3.2 (h) and 3.3 (i). Plots show stimulated cells only (a–i), TRBV gene segments with a frequency of >1% in any donor (a–c) and clonotypes containing ≥20 cells (d–i). In a, b, d and e, boxes span the 25th–75th percentiles, the midline denotes the median and whiskers extend to ±1.5 × IQR.
Extended Data Fig. 8. Influence of TCR usage on MAIT cell activation potential.
a,b, Spearman’s rank correlation between average TRBV pseudotimes on SCORPIUS and Slingshot (two trajectory analysis methods) TCR (a) and cytokine (b) trajectories. Pseudotime values scaled between 0 and 100. c,d, Spearman’s rank correlation between average TRBV pseudotimes for pairs of donors on SCORPIUS TCR (c) and cytokine (d) trajectories. e,f, Spearman’s rank correlation between average clonotype pseudotimes on SCORPIUS and Slingshot TCR (e) and cytokine (f) trajectories. Pseudotime values scaled between 0 and 100. Plots show stimulated cells only, TRBV gene segments with a frequency >1% in any donor (a-d) and clonotypes containing ≥20 cells (e, f).
Therefore, we hypothesized that observed variation could reflect clonal differences. Consistent with this, activation capacity differed between clonotypes on the TCR and cytokine trajectory (Fig. 7d,e and Extended Data Fig. 8e,f). High variability within clonotypes (Fig. 7d,e) suggested additional major influences on MAIT cell activation capacity. Clonotype pseudotimes were not correlated in response to TCR and cytokine stimulation (Fig. 7f), and there was no consistent association between clonotype size and responsiveness to stimulation (Fig. 7g–i). Therefore, larger clones are not intrinsically more functional than smaller clones. Given that variation between clonotypes was observed on the cytokine trajectory as well as the TCR trajectory, differences in clonotype functionality may not solely be associated with the strength of TCR-ligand binding.
IL-17− and IL-17+ cells overlap in function and TCR usage
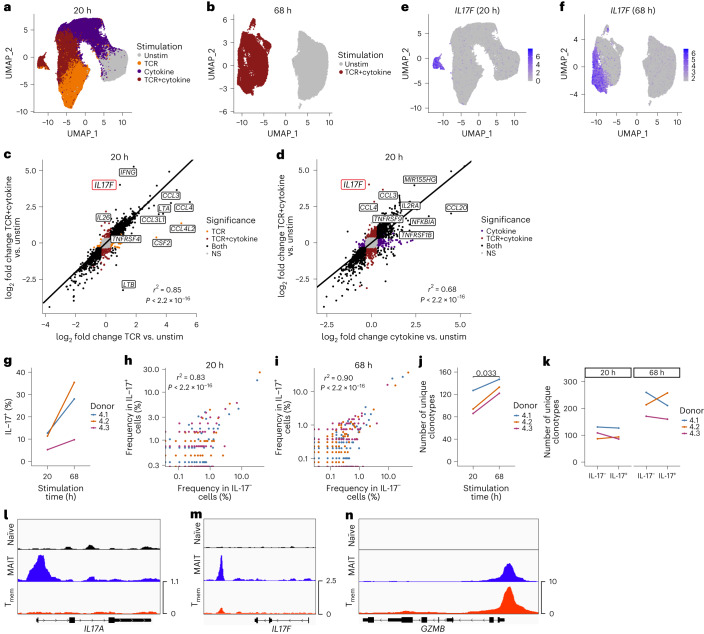
MAIT1 and MAIT17 subsets were not detected in blood or liver. However, due to minimal IL-17 production, our stimulation experiment (Exp 3) did not allow us to address the source and functionality of IL-17-producing human MAIT cells. Therefore, our second stimulation experiment (Exp 4) included a dual TCR+cytokine condition that induces enhanced IL-17 production9. Isolated T cells were left unstimulated or stimulated for 20 h with MR1/5-OP-RU (TCR), IL-12 + IL-18 (cytokine) or both, before MAIT cell sorting (Supplementary Fig. 3). For the TCR+cytokine condition, we performed an additional 3 d (68 h) stimulation, previously shown to increase IL-17 production9.
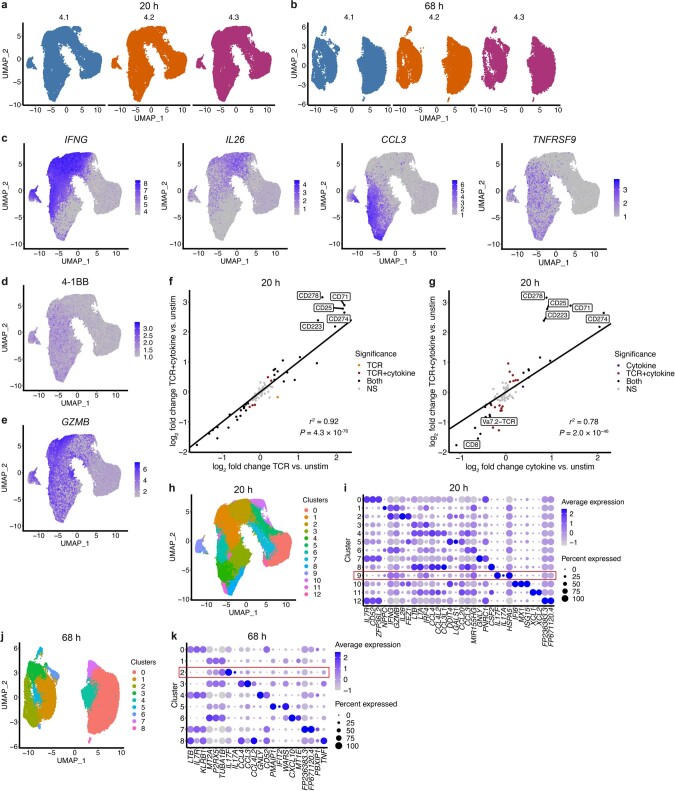
We analyzed 96,867 and 42,765 MAIT cells from three donors at 20 h and 68 h, respectively. After 20 h, unstimulated and stimulated cells were phenotypically and transcriptionally distinct (Supplementary Table 10a–f), with TCR+cytokine-stimulated cells localizing between TCR and cytokine single-stimulated cells on the UMAP (Fig. 8a and Extended Data Fig. 9a). Likewise, TCR+cytokine-stimulated cells were distinct from unstimulated cells at 68 h (Fig. 8b, Extended Data Fig. 9b and Supplementary Table 10g,h).
Fig. 8. IL-17− and IL-17+ MAIT cells are functionally and clonally related.
a, UMAP of 20 h-stimulated MAIT cells colored by stimulation condition. n = 96,867 cells from three donors. b, UMAP of 68 h-stimulated MAIT cells colored by stimulation condition. n = 42,765 cells from three donors. c, Pearson’s correlation between the log2 fold change in gene expression between TCR-stimulated and unstimulated MAIT cells, and TCR+cytokine-stimulated and unstimulated MAIT cells (20 h stimulation). Labels highlight selected genes that were differentially regulated by the two stimuli. Point colors indicate whether the gene was significantly differentially expressed in response to TCR stimulation only (orange), TCR+cytokine stimulation only (maroon), both (black) or neither (NS; gray). d, Pearson’s correlation between the log2 fold change in gene expression between cytokine-stimulated and unstimulated MAIT cells, and TCR+cytokine-stimulated and unstimulated MAIT cells (20 h stimulation). Labels highlight selected genes that were differentially regulated by the two stimuli. Point colors indicate whether the gene was significantly differentially expressed in response to cytokine stimulation only (purple), TCR+cytokine stimulation only (maroon), both (black) or neither (NS; gray). e,f, UMAPs of 20 h-stimulated (e) and 68 h-stimulated (f) MAIT cells colored by expression of IL17F. g, Percentage of cells within the IL-17-expressing cluster following 20 h or 68 h stimulation. h,i, Pearson’s correlation between clonotype frequency in IL-17− (cells within all other clusters) and IL-17+ (cells within the IL-17+ cluster) TCR+cytokine-stimulated MAIT cells at 20 h (h) and 68 h (i). j, Number of unique clonotypes detected within the IL-17+ cluster following 20 h or 68 h stimulation. Cell numbers for each donor were downsampled to ensure equal numbers of TCR+cytokine-stimulated cells at the two timepoints. k, Number of unique clonotypes within IL-17− (cells within all other clusters) and IL-17+ (cells within the IL-17+ cluster) TCR+cytokine-stimulated MAIT cells following 20 h or 68 h stimulation. Cell numbers for each donor were downsampled to ensure equal numbers of IL-17− and IL-17+ cells at a given timepoint. l–n, Representative ATAC-seq tracks showing IL17A (l), IL17F (m) and GZMB (n) gene loci in naïve T (black), MAIT (blue) and Tmem (red) cells. n = 3 donors in a–k. Two-sided paired t-test was performed in g, j and k (nonsignificant results omitted).
Extended Data Fig. 9. TCR-, cytokine- and TCR+cytokine-stimulated MAIT cells.
a,b, UMAPs of 20 h- (a) and 68 h- (b) stimulated MAIT cells split and colored by donor. c, UMAPs of 20 h-stimulated MAIT cells colored by expression of IFNG, IL26, CCL3 and TNFRSF9. d, UMAP of 20 h-stimulated MAIT cells colored by expression of 4-1BB protein. e, UMAP of 20 h-stimulated MAIT cells colored by expression of GZMB. f, Pearson’s correlation between the log2 fold change in protein expression between TCR-stimulated and unstimulated MAIT cells, and TCR+cytokine-stimulated and unstimulated MAIT cells. Labels highlight selected genes that were differentially regulated by the two stimuli. Point colors indicate whether the protein was significantly differentially expressed in response to TCR stimulation only (orange), TCR+cytokine stimulation only (maroon), both (black) or neither (NS; gray). g, Pearson’s correlation between the log2 fold change in protein expression between cytokine-stimulated and unstimulated MAIT cells, and TCR+cytokine-stimulated and unstimulated MAIT cells. Labels highlight selected genes that were differentially regulated by the two stimuli. Point colors indicate whether the protein was significantly differentially expressed in response to cytokine stimulation only (purple), TCR+cytokine stimulation only (maroon), both (black) or neither (NS; gray). h, UMAP of 20 h-stimulated MAIT cells colored by the 13 identified clusters. i, Dot plot showing the top three marker genes per cluster in h. Red box indicates the IL-17-expressing cluster. Dot color indicates the level of gene expression and dot size indicates the percentage of cells expressing the gene. j, UMAP of 68 h-stimulated MAIT cells colored by the nine identified clusters. k, Dot plot showing the top three marker genes per cluster in j. Red box indicates the IL-17-expressing cluster. Dot color indicates the level of gene expression and dot size indicates the percentage of cells expressing the gene.
At 20 h, IFNG and IL26 were primarily upregulated following cytokine stimulation; CCL3, CCL4 and TNFRSF9 (and the corresponding 4-1BB protein) were specific to the TCR condition; and GZMB was similarly upregulated by both stimuli, consistent with Exp 3 (Extended Data Fig. 9c–e and Supplementary Table 10a,c). TCR+cytokine stimulation upregulated both TCR- and cytokine-specific genes (Supplementary Table 10e,g). In general, gene and protein expression were highly correlated in the single and dual stimulation conditions (Fig. 8c,d and Extended Data Fig. 9f,g). Notably, the expression of IL17F was significantly increased following dual relative to single stimulation (Fig. 8c,d). IL-17-expressing cells comprised a distinct cluster at both timepoints (Fig. 8e,f and Extended Data Fig. 9h–k). Three-day stimulation induced a higher fraction of IL-17+ MAIT cells compared with 1 d stimulation (Fig. 8g). IL17F was expressed by all cells in the cluster, while a small percentage produced IL17A (Extended Data Fig. 9i,k).
To investigate whether IL-17-expressing MAIT cells comprise a distinct subset, we examined transcriptional differences between IL-17− and IL-17+ MAIT cells following dual stimulation. At 20 h, IL-17− and IL-17+ cells differentially expressed 23 genes (Supplementary Table 11a). Along with IL17A and IL17F, IL-17-expressing cells showed increased expression of CCR6 and CCL20 but reduced GZMB and KLRD1. Nevertheless, IL-17+ cells expressed high levels of GZMB and KLRD1 compared with unstimulated cells (Extended Data Fig. 10a). Expression of IFNG, IL26, CCL3 and other effector molecules was comparable in IL-17− and IL-17+ cells (Extended Data Fig. 9c). At 68 h, IL-17− and IL-17+ cells differentially expressed 28 genes (Supplementary Table 11c), but again differences in effector gene expression were small and only five differentially expressed genes overlapped at the two timepoints. Protein analysis revealed similar findings—IL-17− and IL-17+ cells differentially expressed three and six proteins at 20 h and 68 h, respectively (Supplementary Table 11b,d).
Extended Data Fig. 10. IL-17-expressing MAIT cells and type 17 gene loci.
a, Violin plots showing GZMB (left) and KLRD1 (CD94; right) expression by unstimulated MAIT cells, and IL-17− and IL-17+ TCR+cytokine-stimulated MAIT cells at 20 h. b, Shannon diversity index for TCRαβ clonotypes in IL-17− and IL-17+ MAIT cells from the TCR+cytokine stimulation condition at 20 h (left) and 68 h (right). Cell numbers for each donor were downsampled to ensure equal numbers of IL-17− and IL-17+ cells at a given timepoint. c,d, Percentage of IL-17− and IL-17+ TCR+cytokine-stimulated MAIT cells expressing different TRAJ (c) and TRBV (d) gene segments. n = 3 donors. TRBV genes expressed in >1% of IL-17− or IL-17+ cells from any donor are included. Mean ± s.e.m. is shown. Two-sample Wilcoxon signed-rank test for all pairwise comparisons (nonsignificant results omitted). e-g, Representative ATAC-seq tracks showing RORC (e), IL23R (f) and CCR6 (g) gene loci in naïve T (black), MAIT (blue) and Tmem (red) cells.
The similar transcriptional profiles of IL-17− and IL-17+ cells, and the increased frequency of IL-17-expressing cells at 68 h relative to 20 h, suggested that IL-17+ cells may represent a functional state obtainable by all MAIT cells under appropriate stimulation conditions. To investigate this, we compared the TCR repertoire of IL-17− and IL-17+ cells. Clonotype abundance strongly correlated between the two groups at both timepoints (Fig. 8h,i). The number of unique clonotypes among IL-17-expressing cells was increased at 68 h compared with 20 h (Fig. 8j), indicating that new cells become IL-17+. However, there was no difference in the number of unique clonotypes among IL-17− and IL-17+ cells (Fig. 8k) or in the Shannon diversity index (Extended Data Fig. 10b) (data downsampled to ensure equivalent numbers of IL-17− and IL-17+ cells within each donor at a given timepoint). Moreover, TRAJ and TRBV usage was comparable (Extended Data Fig. 10c,d).
To examine the regulation of IL-17 gene expression in MAIT cells, we generated a bulk ATAC-seq dataset (n = 3 donors) comprising three blood CD8+ T cell subsets: naïve T cells, MAIT cells and Tmem cells. As expected given their expression by resting MAIT cells1, the type 17-associated genes RORC, IL23R and CCR6 showed increased accessibility in MAIT cells compared with Tmem and naïve T cells (Extended Data Fig. 10e–g). In addition, MAIT cells showed increased accessibility of peaks associated with IL17A and IL17F (Fig. 8l,m). The two differentially accessible IL17A peaks were located at the promoter and in the upstream intergenic region, while the two IL17F peaks were in the downstream intergenic region. Compared with the GZMB promoter peak (Fig. 8n), the IL17A promoter peak was of lower magnitude, while IL17F lacked a peak at the promoter. Reduced accessibility of IL17A and IL17F promoters may explain delayed IL-17 secretion relative to rapid granzyme B upregulation following MAIT cell activation.
Our data suggest that increased numbers of MAIT cells acquire the capacity to produce IL-17 over time, perhaps due to a requirement for chromatin remodeling, and that aside from IL-17 production, IL-17− and IL-17+ MAIT cells show similar transcriptional and functional profiles. This is in stark contrast to resting mouse MAIT cells that comprise distinct MAIT1 and MAIT17 subsets10–12,33,34.
Discussion
Our single-cell data from blood and liver, and TCR- and/or cytokine-stimulated MAIT cells, suggest that human MAIT cells comprise a single, highly adaptable, cell population. Transcriptional plasticity is governed by tissue localization, clonal identity and activation state (influenced by type and duration of stimulation). Despite their semi-invariant TCR and shared antigen specificity, diverse TCRβ usage results in private MAIT cell TCR repertoires. This may have important functional consequences, as the clonal identity of an individual MAIT cell influenced its resting and activated transcriptional profile.
Liver MAIT cells were transcriptionally distinct from blood, expressing genes and proteins associated with activation and tissue residency. Basal activation could reflect responses to microbial ligands transported from the gut to the liver via the hepatic portal vein45. Liver residency is consistent with bulk RNA-seq analysis of human liver MAIT cells and with mouse parabiosis experiments12. However, MAIT cell frequency and TCR usage are similar in human thoracic duct and matched blood19, and TCR repertoires overlapped in blood and liver. In human intestinal and uterine transplantation, tissue MAIT cells are largely recipient-derived at >1-year posttransplantation46,47. Therefore, the extent of human MAIT cell tissue residency requires further examination. A small fraction of liver MAIT cells may be circulating cells in transit through the liver. However, this is unlikely to have meaningfully impacted our conclusions.
Mouse MAIT cells comprise developmentally, transcriptionally and functionally distinct MAIT1 and MAIT17 subsets10–12,33,34. In contrast, human MAIT cells displayed low baseline transcriptional heterogeneity in blood and liver, and clusters were not indicative of MAIT1 and MAIT17 cells or other T cell polarization states. Resting MAIT cell clusters did not clearly associate with the clusters identified following activation. Thus, fRNA-seq adds an important dimension to the analysis of T cell biology. TCR+cytokine stimulation induced IL-17 in a fraction of MAIT cells, but IL-17− and IL-17+ cells similarly expressed other effector molecules and had overlapping TCR repertoires. Therefore, we hypothesize that all human MAIT cells have the capacity to produce IL-17 under appropriate conditions.
TCR and cytokine stimulation induced distinct responses, underpinned by altered regulatory networks. Activated MAIT cells did not comprise discrete functional lineages but were distributed along stimulus-specific activation trajectories. HIVEP3, a TCR-specific transcription factor (regulating CCL3 and CCL4), and BATF, a cytokine-specific transcription factor (regulating IFNG and IL26), were new predicted regulators of MAIT cell function. A limitation of this analysis is that high-confidence regulons were not identified for RORγt and PLZF, perhaps due to relatively poor gene detection.
Basic TCR repertoire characteristics were consistent with prior studies21,30. Surprisingly, the extent of MAIT cell clonality was comparable with Tmem cells and individuals displayed largely private TCRαβ repertoires. This challenges the paradigm of MAIT cells as a clonally-restricted population with large numbers of public TCRs and validates previous studies with small cell numbers or bulk TCR repertoire data20,48. However, the TCRα chain, key for ligand recognition31,32, was highly shared between individuals. TCRαβ clonotypes overlapped considerably in blood and liver. While consistent with shared TCRβ usage in matched blood and lymph19, differential TRAJ/TRBV usage was identified in studies without matched blood and tissue (breast22, kidney and intestine21). Identification of private TCR repertoires highlights the importance of matched samples for accurately comparing TCR usage across tissues.
We identified an association between the clonal identity and transcriptome of individual MAIT cells. We hypothesize that the clonotype-cluster association in resting blood and liver reflects differences in the basal activation of clones. Differential activation capacity dependent on TCR clonotype is consistent with altered clonal distribution following Salmonella infection23 and increased clonality with age20. However, activation capacity was not correlated with clonotype size. This appears to contrast with the superior proliferation of MAIT cells expressing the most abundant Vβ segments upon in vitro Escherichia coli stimulation7. Discordant results could reflect differences in experimental approach or the absence of a direct correlation between activation kinetics and proliferative potential. Further study is necessary to understand the driving factors and functional consequences of clonal differences in activation capacity.
A recent paper49 and a preprint50 present human MAIT cell scRNA-seq data that are relevant to our findings. However, our study is unique in several key regards, namely the inclusion of TCR data—allowing clonotype to be linked to function—and detailed characterization of responses to multiple stimuli and at multiple timepoints. This is critical for understanding the diversity of MAIT cell functions, including IL-17 production. Consistent with our study, Chandra et al.50 reported a tissue residency signature in lung MAIT cells and failed to identify a MAIT17 subset. Vorkas et al.49 analyzed blood MAIT cells following 15 h and 7 d TCR stimulation (direct ex vivo analysis or following cytokine stimulation was not performed). Based on the identification of 12 clusters and gene expression differences between CD4- and CD8-expressing cells (although these did not form separate clusters), the authors concluded that human MAIT cells comprise multiple subsets. However, our trajectory and clonality analysis (based on TCR-confirmed MAIT cells) suggest a continuum of response to stimulation by a single population.
In conclusion, we present a genome-wide single-cell characterization of the transcriptome and TCR repertoire of blood and liver, and resting and activated, human MAIT cells. Our data indicate largely private TCR repertoires, highly shared between matched blood and liver. MAIT cells showed stimulus-specific transcriptional responses, and we identified candidate regulators of the TCR- and cytokine-specific response. While human MAIT cells produce IL-17 following TCR+cytokine stimulation, IL-17+ cells have a similar TCR repertoire and effector profile to IL-17− cells, suggesting they do not comprise a bona fide MAIT17 subset. CD4/CD8 coreceptor expression was not associated with distinct transcriptional states. At rest and following activation, MAIT cell clones show subtle differences in transcriptional profile and functional capacity, which may have important biological consequences, particularly in the context of suboptimal stimulation. Our data provide new insights into human MAIT cell biology, relevant to related innate-like subsets, and a comprehensive resource for further MAIT cell studies in health and disease.
Methods
Data generation
Liver tissue collection and processing (Exp 1 and 2)
Liver tissue (n = 7) and matched blood (n = 6) were obtained from patients undergoing liver resection at the Churchill Hospital, Oxford, UK and the University Hospital Basel, Basel, Switzerland (Supplementary Table 1). Patients had no chronic liver disease, active excess alcohol consumption (>14 g per day), infection, immunosuppression or family history of liver disease.
Disease-free liver tissue was collected from the resection margin, cut into small pieces with a scalpel and ground through a 70 μm cell strainer. Cells were washed with R10 (RPMI-1640 (Sigma-Aldrich), 10% FBS (Sigma-Aldrich), 1% penicillin–streptomycin (Thermo Fisher Scientific); 931g, 10 min, 4 °C) and mononuclear cells isolated by density gradient centrifugation on a discontinuous 35%/70% Percoll (GE Healthcare) gradient (931g, 20 min, 21 °C, no brake). Mononuclear cells were collected from the interface and washed with R10 (596g, 10 min, 4 °C). Residual red blood cells were lysed with ACK for 3–5 min. Cells were washed twice (596g, 10 min, 4 °C) and cryopreserved (90% FBS, 10% DMSO (Sigma-Aldrich)) in liquid nitrogen.
Ethics statement
Samples were obtained with written informed consent through the Oxford Gastrointestinal Illnesses Biobank (REC ref. 16/YH/0247) or under Ethikkommission Nordwest- und Zentralschweiz (EKNZ) numbers EKNZ-2014-362, EKNZ-2016-01188 and EKNZ-2019-02118.
Peripheral blood mononuclear cell (PBMC) isolation
PBMCs were isolated from fresh whole blood by density gradient centrifugation (Lymphoprep, Axis-Shield) at 931g for 30 min with no brake. Cells were cryopreserved in liquid nitrogen and thawed in complete medium (R10, 1X nonessential amino acids (Thermo Fisher Scientific), 1 mM sodium pyruvate (Thermo Fisher Scientific), 10 mM HEPES (pH 7.0–7.5; Thermo Fisher Scientific), 50 μM β-mercaptoethanol (Thermo Fisher Scientific)) on the day of use.
Stimulation of isolated CD8+/CD3+ T cells for scRNA-seq and scTCR-seq (Exp 3 and 4) or activation marker/cytokine validation
Pierce streptavidin-coated high-capacity flat-bottom 96-well plates (Thermo Fisher Scientific) were coated with 50 μl biotinylated MR1/5-OP-RU monomer (NIH Tetramer Core Facility) at 10 μg per ml in PBS (Sigma-Aldrich) overnight at 4 °C. Cryopreserved PBMCs were thawed in complete medium. CD8+ T cells were isolated using CD8 MicroBeads (Exp 3; Miltenyi Biotec) and CD3+ T cells using the REAlease CD3 MicroBead Kit (Exp 4 and validation experiments; Miltenyi Biotec) following the manufacturer’s instructions. Isolated CD8+/CD3+ T cells were washed in complete medium and resuspended at 1 × 107 cells per ml. One million (20 h stimulation) or 500,000 (68 h stimulation) cells were added per well to the appropriate 96-well plates (MR1/5-OP-RU-coated plate for TCR and TCR+cytokine stimulation, round-bottom plate for unstimulated and cytokine stimulation). IL-12 (50 ng ml−1; R&D Systems) and IL-18 (50 ng ml−1; R&D Systems) were added for cytokine stimulation; αCD28 (1 μg ml−1; clone: CD28.2; BioLegend) for TCR stimulation; IL-12, IL-18 and αCD28 for TCR+cytokine stimulation; and complete medium for unstimulated cells (final volume 200 μl per well). Cells were incubated for 20 h or 68 h at 37 °C, 5% CO2. For intracellular cytokine staining, brefeldin A (BioLegend) and monensin (BioLegend) were added for the final 4 h.
Tetramer staining (Exp 1 and 2)
Biotinylated human MR1/5-OP-RU and MR1/6-FP monomers were provided by the NIH Tetramer Core Facility. Tetramers were generated using streptavidin-PE (high concentration) or streptavidin-BV421 (both BioLegend) following the NIH Tetramer Core Facility protocol. Tetramer staining was performed for 40 min at 21 °C in FACS buffer (PBS, 0.5% BSA (Sigma-Aldrich), 1 mM EDTA (Sigma-Aldrich)).
Surface staining and cell sorting for scRNA-seq and scTCR-seq (Exp 1–4)
TotalSeq-C hashtag antibodies (BioLegend) were used in Exp 2 and 4. Hashtag antibody dilutions were prepared according to the manufacturer’s instructions. Namely, antibody vials were centrifuged at 10,000g, 30 s, 4 °C, before antibody dilution in FACS buffer. Diluted hashtags were centrifuged at 14,000g, 10 min, 4 °C. Cells were incubated in Human TruStain FcX (BioLegend) for 10 min at 4 °C before the addition of diluted hashtag antibodies (0.2 μg per well) for 10 min at 4 °C. Surface fluorochrome-conjugated antibodies were added without washing off the hashtag antibodies. Surface staining was performed in Brilliant Stain Buffer Plus (BD Biosciences) for 30 min at 4 °C. Cells were washed twice in PBS with 0.5% BSA, resuspended in presort buffer (PBS, 1% BSA, 25 mM HEPES) containing 3–5 nM SYTOX Green Nucleic Acid Stain (Thermo Fisher Scientific) and incubated for 20 min at 4 °C. Cells were sorted on a BD FACSAria III with an 85 μm nozzle. Sorted cells were collected in RPMI-1640, 10% FBS, 25 mM HEPES, or HBSS (Thermo Fisher Scientific), 50% FBS, 25 mM HEPES. Sort purity was >99%. For Exp 2 and 4, sorted cells were stained with the TotalSeq-C Human Universal Cocktail V1.0 (BioLegend) according to the manufacturer’s instructions. Staining reagents are listed in Supplementary Table 12.
Stimulation of CD56− and CD56+ MAIT cells
CD3+ T cells were isolated using the REAlease CD3 MicroBead Kit following the manufacturer’s instructions. Surface antibody and live/dead (SYTOX Green Nuclear Acid Stain) staining was performed as above, then CD56− and CD56+ MAIT cells (Vα7.2+CD161hi) were sorted on a BD FACSAria III with an 85 μm nozzle. Sorted cells were collected in HBSS, 50% FBS, 25 mM HEPES, then centrifuged at 400g, 5 min, 21 °C and incubated overnight at 37 °C, 5% CO2. Rested cells were washed in complete medium, plated in a 96-well round-bottom plate and stimulated with IL-12 (50 ng ml−1) and IL-18 (50 ng ml−1) at 37 °C, 5% CO2 for 20 h, with the addition of brefeldin A and monensin for the final 4 h.
Surface marker and intracellular cytokine staining for flow cytometry
Surface staining was performed in Brilliant Stain Buffer Plus for 30 min at 4 °C. Stained cells were washed twice in FACS buffer. For intracellular cytokine staining, cells were fixed in Cytofix/Cytoperm (BD Biosciences) for 20 min at 4 °C, then washed twice in 1X Perm/Wash (BD Biosciences). Intracellular staining was performed in 1X Perm/Wash for 30 min at 4 °C. Cells were acquired on a BD LSR II flow cytometer with BD FACSDiva Software (v8.0.1). Staining reagents are listed in Supplementary Table 12.
10x Genomics library generation and sequencing
Sequencing libraries were generated using 10x Genomics Chromium Single Cell V(D)J Reagent Kits (v1.0 Chemistry; Exp 1 and 3) or 10x Genomics Chromium Next GEM Single Cell 5′ Reagent Kits v2 (Dual Index; Exp 2 and 4) following the manufacturer’s instructions. For Exp 1 and 3, cells were loaded onto the Chromium Controller (10x Genomics) at a concentration of ~1 × 106 cells per ml, with 6,000–8,000 cells loaded per channel. For Exp 2 and 4, 17,750–30,000 cells were loaded per channel. Library quality and concentration were assessed using a TapeStation (Agilent) and Qubit 2.0–4 Fluorometer (Thermo Fisher Scientific), respectively. Library generation for Exp 1 and 3 was performed at the Oxford Genomics Centre (Wellcome Centre for Human Genetics, University of Oxford), and for Exp 2 and 4 was performed in-house. Libraries were sequenced on an Illumina HiSeq 4000 (Exp 1) or Illumina NovaSeq 6000 (Exp 2–4) at the Oxford Genomics Centre. Sequencing depths were as follows: Exp 1—39,013–46,998 reads per cell for scRNA-seq, 10,323–30,883 reads per cell for scTCR-seq; Exp 2—76,710–89,378 reads per cell for scRNA-seq, 19,673–41,612 reads per cell for TotalSeq-C feature barcoding antibodies, 5,192–7,065 reads per cell for scTCR-seq; Exp 3—75,638–88,871 reads per cell for scRNA-seq, 12,517–27,197 reads per cell for scTCR-seq; Exp 4—53,120–102,777 reads per cell for scRNA-seq, 9,333–17,717 reads per cell for TotalSeq-C feature barcoding antibodies, 2,904–14,636 reads per cell for scTCR-seq.
ATAC-seq library generation and sequencing
Naïve T cells (CD8+CD45RO−CCR7+), MAIT cells (CD8+CCR7−MR1/5-OP-RU+) and Tmem cells (CD8+CCR7−MR1/5-OP-RU−) were sorted from CD8-enriched (CD8 MicroBeads) PBMCs (n = 3 donors, 50,000 cells per population). ATAC-seq was performed as previously described51. Briefly, cells were pelleted at 500g for 10 min at 4 °C and the supernatant was removed. Cells were resuspended in 50 μl cold lysis and transposition mix (25 μl TD buffer, 2.5 μl TDE1 (Illumina, FC-121-1030; product discontinued), 22 μl nuclease-free H2O (Thermo Fisher Scientific), 0.5 μl 1% digitonin (Promega)) and incubated for 30 min at 37 °C with agitation at 300 rpm (Thermo-Shaker TS-100, Biosan). Transposed DNA was purified using the Qiagen MinElute Reaction Cleanup Kit and eluted in 13 μl elution buffer (10 mM Tris–HCl, pH 8). Purified library fragments were PCR amplified for 11 cycles with barcoded primers using NEBNext High-Fidelity 2X PCR Master Mix (New England Biolabs). Amplified DNA was purified using the Qiagen MinElute PCR Purification Kit (23 μl elution volume) and PCR primer contamination was removed using SPRI beads (5 min dry time, 15 μl elution volume; Agencourt AMPure XP PCR Purification, Beckman Coulter). Fragment size distribution was analyzed using a 2100 Bioanalyzer (Agilent) with the High Sensitivity DNA Kit. Libraries were quantified using the KAPA Library Quantification Kit (Roche). Paired-end sequencing (40 bp) was performed on an Illumina NextSeq 500 using the High Output v2 Kit (75 cycles). Libraries were sequenced to a depth of 215–271 million paired-end reads per sample.
Data analysis
10x Genomics raw data processing
FASTQ files were generated from BCL files using Illumina bcl2fastq. For Exp 1 and 3, FASTQ files for gene expression and TCR data were processed using Cell Ranger (v3.0.1–3.0.2; https://support.10xgenomics.com/single-cell-gene-expression/software/pipelines/latest/what-is-cell-ranger) count and vdj pipelines, respectively. For Exp 2 and 4, FASTQ files for all modalities were processed using the Cell Ranger (v7.0.1) multi pipeline. For TCR analysis, the filtered_contig_annotations.csv file was filtered to retain only high-confidence, full-length, productive contigs corresponding to TCRα or TCRβ chains.
Hashtag demultiplexing
Hashtag demultiplexing (Exp 2 and 4) was performed using the consensus calling approach from cellhashR52 (v1.0.3) with the following methods: BFFcluster52, BFFraw52, GMM-Demux53, MULTI-seq54, Seurat HTODemux55 and DropletUtils hashedDrops55.
Quality control
Quality control was performed separately for cells from each channel of the Chromium Controller. Filtered feature-barcode matrices from Cell Ranger count/multi were imported into R using Seurat (v4.0.3–4.3.0)56. Cells with low unique molecular identifier counts, low gene counts and/or a high percentage of mitochondrial reads, were removed. For Exp 1 and 2, cells labeled as empty droplets or damaged cells by DropletQC57 (v0.0.0.9000) were removed (damaged cells in Exp 4 were also removed). For Exp 2 and 4, only cells called as consensus singlets by hashtag demultiplexing were retained. Cells with two TCRα and two TCRβ chains, or more than two TCRα and/or TCRβ chains, were assumed to be doublets and discarded. TCR and BCR genes were removed to ensure downstream clustering analysis was not influenced by TCR or BCR chain usage.
Normalization, integration, dimensionality reduction and clustering (Exp 1 and 2)
For combined analysis of Exp 1 and 2, data from each donor were normalized separately using sctransform58 (v0.3.5). Highly variable genes (HVGs) were defined as the 3,000 genes with the largest residual variance following variance stabilizing transformation. Cells from different donors were integrated using Seurat56. Integration features (n = 3,000) were selected using matched blood and liver samples, with STACAS59 (v2.0.1) blacklisted genes subsequently removed. For anchor finding, dimensionality reduction was performed using canonical correlation analysis (MAIT cells only) or reciprocal principal component analysis (PCA; MAIT and Tmem cells combined). The number of dimensions used for identifying and weighting anchors was selected empirically by performing integration with multiple input dimensions and evaluating downstream clustering results. Following integration, dimensionality reduction was performed using PCA. Scree plots were used to determine how many PCs to use for UMAP generation and clustering. Cell clusters were identified using Seurat’s graph-based clustering approach. Briefly, a shared nearest neighbor graph was constructed using dimensionally-reduced data, and then clusters were determined by optimizing the standard modularity function (Louvain algorithm).
Normalization, dimensionality reduction, batch correction and clustering (Exp 3 and 4)
Per experiment and timepoint, data from all donors combined were normalized using sctransform58 (v0.3.2–0.3.5). HVGs were defined as the 3,000 genes with the largest residual variance following variance stabilizing transformation (in Exp 4, STACAS59 blacklisted genes were removed from HVGs). Dimensionality reduction was performed using PCA. Batch correction for donor was performed using Harmony60 (v0.1.1) with 50 input PCs (θ = 2, λ = 1). Scree plots were used to determine how many PCs to use for UMAP generation and clustering. Cell clusters were identified using Seurat’s graph-based clustering approach.
Differential gene expression analysis
Differential gene expression analysis between clusters was performed using MAST61 (v1.18.0–1.24.1; FindMarkers function from Seurat) with cellular detection rate as a covariate. Cluster markers were defined as genes with significantly increased expression in one cluster relative to the average of all other clusters (fold change > 1.25 and adjusted P < 0.05 based on Bonferroni correction using all genes in the dataset). Differential gene expression analysis between conditions (for example, tissues, coreceptors and stimuli) was performed using MAST61 with cellular detection rate and donor as covariates. Genes with a fold change > 1.25 and a Bonferroni adjusted P < 0.05 were defined as significantly differentially expressed. Input data were log-transformed normalized counts generated by global-scaling normalization (NormalizeData function from Seurat).
Coreceptor assignment (Exp 1 and 2)
Cells were defined as CD8+, DN or CD4+ based on normalized coreceptor gene or protein (measured using TotalSeq-C antibodies) expression. For assignment based on gene expression, cells were defined as CD8+ if CD8A > 0 and/or CD8B > 0, CD4+ if CD4 > 0, and DN if CD8A, CD8B and CD4 were undetected. For assignment based on protein expression (Exp 2 only), cells were defined as CD8+ if CD8 > 0.3, CD4+ if CD4 > 2.5 and CD8 < 0.2, and DN if neither CD8+ nor CD4+. Thresholds were selected empirically by examining histograms of normalized count data.
Pseudotime analysis
Pseudotime analysis was performed using Slingshot37 (v2.0.0) and SCORPIUS38 (v1.0.8). UMAP coordinates and Seurat cluster labels (0.1 resolution) were provided as input to Slingshot, with the main unstimulated cluster specified as the start of the trajectory. Normalized expression values (sctransform) were provided as input to SCORPIUS. Two separate SCORPIUS trajectories were generated from unstimulated and TCR-stimulated cells, and unstimulated and cytokine-stimulated cells. Gene importance along SCORPIUS trajectories was determined using random forest regression (gene_importances function, num_permutations = 10). Differential gene importance was calculated by taking the ratio of gene importance ranks on the TCR and cytokine trajectories (higher rank as numerator). Genes defined as differentially important had an importance false discovery rate (FDR) < 0.05 and were within the top 150 most highly ranked genes for either the TCR or cytokine trajectory.
Transcription factor regulon analysis
Transcription factor regulons were identified using SCENIC29,62 (pySCENIC v0.11.2–0.12.1). Briefly, the raw expression matrix was filtered to retain genes expressed in >1% of cells and with a count >3 × 0.01 × number of cells. Modules comprising transcription factors and coexpressed genes were generated using GRNBoost2, then pruned to remove indirect targets lacking enrichment for the corresponding transcription factor motif (cisTarget). This resulted in a set of transcription factor regulons. Due to stochasticity in gene regulatory network inference using GRNBoost2, each pySCENIC run can identify a different number of regulons, as well as different target genes for each transcription factor. Thus, pySCENIC was run 100 times. High-confidence regulons were defined as regulons that occurred in >80% of runs and that contained at least five high-confidence target genes. High-confidence target genes were those found within a regulon in >80% of runs. Cells were scored for the activity of each high-confidence regulon (including only high-confidence target genes) using AUCell (v1.16.0–1.20.1). Regulons differentially active between tissues or stimulation conditions were determined using MAST61 with donor as a covariate. Regulons with a Bonferroni adjusted P < 0.01 were defined as differentially active. Smoothed regulon activity scores (AUCell scores) over SCORPIUS trajectories were generated by loess regression (loess function from the stats R package).
Gene set enrichment analysis (GSEA) and over-representation analysis
GSEA63 (v4.3.2) for published mouse and human TRM cell gene signatures27,28 was performed using pseudobulk gene counts (normalized gene counts summed for all cells within a sample) with 1,000 gene permutations. Over-representation analysis for gene ontology (GO) terms and Reactome pathways was performed using clusterProfiler64 (v4.7.1) and ReactomePA65 (v1.36.0), respectively. Gene symbols were converted to Entrez IDs using the Bioconductor org.Hs.eg.db annotation package (v3.13.0–3.16.0). Background genes were defined as genes expressed (count >0) in ≥1% of cells (or for Exp 3, genes expressed in at least five cells). Redundant enriched GO terms were removed using the simplify function from clusterProfiler.
Gene lists
MAIT1 and MAIT17 gene signatures were generated by overlapping MAIT1 and MAIT17 genes from two published scRNA-seq datasets33,34. Mouse gene symbols were converted to human gene symbols using the biomaRt R package (getLDS function; v2.54.1) with the ENSEMBL_MART_ENSEMBL BioMart database and the hsapiens_gene_ensembl and mmusculus_gene_ensembl datasets. Human Th1 and Th17 gene signatures were generated by combining genes from the NanoString nCounter Human Immunology V2 Panel Gene List (https://nanostring.com/support-documents/ncounter-human-immunology-v2-panel-gene-list) and a meta-analysis published by Radens et al.35. Interferon-stimulated genes were obtained from Schoggins and Rice66.
Nucleotide and functional TCR clonotypes
Tables of TCRα and TCRβ usage for each cell in a sample were combined to generate one table per donor for nucleotide clonotype calling, and one table for all donors for functional clonotype calling. MAIT cells were required to have a TRAV1-2 TCRα chain and at least one TCRβ chain. Tmem cells were required to have at least one TCRα chain and at least one TCRβ chain. Cells expressing TRAV1-2 paired with TRAJ33, TRAJ12 or TRAJ20 and with a 12 amino acid CDR3α region were assumed to be contaminating MAIT cells and were removed before Tmem cell clonotype calling.
Nucleotide clonotypes (TCRαβ) were defined as cells with identical TCR gene segment usage, and CDR3α and CDR3β nucleotide sequences. TCRα clonotypes and TCRβ clonotypes were defined as cells with identical TCRα segment usage and CDR3α sequences, or identical TCRβ segment usage and CDR3β sequences, respectively. TCRαβ clonotypes were numbered according to size, with clonotype 1 being the largest, clonotype 2 being the second largest and so on. Clonotypes of identical size were randomly ordered for numbering. TCRαβ clonotypes were assigned ranks in a similar manner, but clonotypes of identical size were given the same rank.
Functional clonotypes (TCRαβ) were defined as cells with at least one identical TCRα and TCRβ chain amino acid sequence (gene segment usage and CDR3 sequences). Functional TCRα and functional TCRβ clonotypes were defined as cells with at least one matching TCRα or TCRβ chain amino acid sequence, respectively. Given the presence of TCR dropout, functional clonotypes were permitted to contain a mixture of cells with one or two TCRα or TCRβ chains, providing all detected chains matched those within the clonotype.
TCR analyses
TCR analyses were performed only for cells with a defined TCR clonotype. The Shannon diversity index was calculated using the diversity function from the vegan R package (v2.6.4). To test for an association between clonotype (clonotypes from n = 12 donors with ≥20 cells) and cluster, a multinomial test was performed using the EMT R package (v1.3)—MonteCarlo = FALSE when the number of distinct possible outcomes (events) < 1 × 106, else MonteCarlo = TRUE with ntrial = 10 × events or 1 × 108 (whichever smaller). P values were Bonferroni adjusted for the number of clonotypes tested per donor. Sequence logos were generated using ggseqlogo (v0.1). The overall height of the stacked letters at each position indicates the sequence conservation, while the relative abundance of each amino acid is indicated by the height of individual letters within the stack. Acidic bases are shown in red, basic residues in blue, hydrophobic in black and polar in green. The number of N-nucleotides and P-nucleotides in 36-nucleotide CDR3α sequences was determined using IMGT/JunctionAnalysis67 (v2.3.0).
ATAC-seq
Read quality was checked using FastQC (v0.11.5; Babraham Bioinformatics) and adapter sequences were removed using Trimmomatic68 (v0.36). Reads were mapped to hg38 using Bowtie 2 (ref. 69; --very-sensitive -X 2000 --no-mixed --no-discordant --no-unal; v2.3.4.1). BAM files from multiple sequencing lanes were merged using samtools70 (v1.6) and duplicate reads were removed using Picard MarkDuplicates (v2.15.0). BAM files were filtered to remove reads mapping to the mitochondrial genome (samtools) and blacklisted regions (bedtools71 v2.26.0). Blacklisted regions comprised the ENCODE blacklist72 and a custom ATAC-seq blacklist generated by J. Buenrostro73. Read start sites were adjusted to correspond to the center of the transposase binding site—reads on the forward strand were offset by +5 bp and reads on the reverse strand were offset by −4 bp using deepTools74 alignmentSieve (v3.1.0). Cut sites were identified as the 5′ ends of forward and reverse reads. Peak calling was performed with MACS2 (ref. 75; --nomodel -p 0.1 -f BAMPE --call-summits; v2.1.1) and an optimal peak list defined using the irreproducibility discovery rate framework76. Cut sites within peaks were quantified using featureCounts77 (v1.6.0). Peaks were annotated to genes using HOMER78 annotatePeaks.pl (v4.8). Differentially accessible peaks (fold change > 2, FDR < 0.05) were identified using edgeR79 (v3.24.3). BAM files were converted into BigWig files using deepTools74 bamCoverage (--normalizeUsing CPM --ignoreForNormalization chrM chrX chrY --binSize 1; v3.5.1).
Statistics and reproducibility
Statistical tests are listed in the relevant sections of the Methods and/or in the figure legends. Standard statistical tests were performed in R (v4.1.1–4.2.0) using the stats or rstatix (v0.7.2) packages. All tests were two-sided. Initial blood-liver (Exp 1) and stimulation (Exp 3) scRNA-seq experiments were followed by validation experiments (Exp 2 and 4)—the findings of these were highly concordant. Genes of interest from Exp 1 and 3 were validated at the protein level by CITE-seq (Exp 2 and 4) and flow cytometry. No statistical method was used to predetermine sample size. No samples were excluded from the analyses. The experiments were not randomized. The investigators were not blinded to allocation during experiments and outcome assessment.
Plots
Most plots were generated using the ggplot2 R package (v3.3.4–3.4.2). FACS plots were generated in FlowJo (v10.8.1; BD Biosciences). Heatmaps were generated using pheatmap (v1.0.12) or ComplexHeatmap (v2.14.0). GSEA plots were generated in GraphPad Prism (v9.5.1; GraphPad Software, LLC). ATAC-seq traces were generated using Integrative Genomics Viewer (v2.16.0).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41590-023-01575-1.
Supplementary information
Supplementary Figs. 1–3.
Characteristics of liver tissue donors.
Supplementary Tables 2–12.
Acknowledgements
We thank biobankers of the Translational Gastroenterology Unit and surgeons of Oxford University Hospitals NHS Foundation Trust for collecting patient samples, especially M. Silva for facilitating this; S. Slevin for liver processing; K. Lynch for liver patient identification and sample processing; M. Salio for the plate-bound MR1 stimulation protocol; H. Ferry for sorting; D. Sims and D. Agarwal for discussions regarding computational analysis; and the NIH Tetramer Core Facility for providing MR1 monomers. We thank the Oxford Genomics Centre at the Wellcome Centre for Human Genetics (funded by Wellcome grant ref. 203141/A/16/Z) for the generation and initial processing of the sequencing data. Analysis was performed using computer systems at the MRC WIMM Centre for Computational Biology. L.C.G. and A.A. are supported by Wellcome (109028/Z/15/Z and 216417/Z/19/Z, respectively). M.E.B.F. is supported by Beyond Celiac and the Academy of Medical Sciences. M.F.S. is supported by the Swiss National Science Foundation (PZ00P3_167828 and PZ00P3_189490), Goldschmidt-Jacobson Stiftung and Uniscientia Stiftung. N.M.P. is supported by an Oxford-UCB Postdoctoral Fellowship. P.K. is supported by Wellcome (222426/Z/21/Z), the NIH (U19 I082360), the NIHR Oxford Biomedical Research Centre and an NIHR Senior Fellowship.
Extended data
Source data
Statistical source data.
Author contributions
L.C.G., N.M.P. and P.K. designed the project and the experiments. L.C.G., A.A., M.E.B.F., M.J.L., G.F.H., M.F.S., N.M.P. and P.K. recruited patients and/or performed liver tissue processing. L.C.G. performed all other experiments and all data analysis. L.C.G. wrote the manuscript. All authors critically reviewed and approved the manuscript.
Peer review
Peer review information
Nature Immunology thanks Johan Sandberg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: L.A. Dempsey, in collaboration with the Nature Immunology team.
Data availability
Sequencing data generated in this study have been deposited in NCBI’s Gene Expression Omnibus (GEO) and are accessible through GEO SuperSeries accession number GSE194189. The Bioconductor org.Hs.eg.db annotation package and ENSEMBL_MART_ENSEMBL BioMart database are publicly available. Source data are provided with this paper.
Code availability
No custom software was generated for this manuscript. The software used for all analyses is listed in the relevant sections of the Methods.
Competing interests
P.K. has acted as a consultant to UCB, Biomunex, AstraZeneca and Infinitopes. N.M.P. has acted as a consultant to Infinitopes. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nicholas M. Provine, Paul Klenerman.
Contributor Information
Lucy C. Garner, Email: lucy.garner@ndm.ox.ac.uk
Paul Klenerman, Email: paul.klenerman@ndm.ox.ac.uk.
Extended data
is available for this paper at 10.1038/s41590-023-01575-1.
Supplementary information
The online version contains supplementary material available at 10.1038/s41590-023-01575-1.
References
- 1.Garner LC, Klenerman P, Provine NM. Insights into mucosal-associated invariant T cell biology from studies of invariant natural killer T cells. Front. Immunol. 2018;9:1478. doi: 10.3389/fimmu.2018.01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ussher JE, et al. CD161++CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur. J. Immunol. 2014;44:195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godfrey DI, Koay HF, McCluskey J, Gherardin NA. The biology and functional importance of MAIT cells. Nat. Immunol. 2019;20:1110–1128. doi: 10.1038/s41590-019-0444-8. [DOI] [PubMed] [Google Scholar]
- 4.Kurioka A, et al. Shared and distinct phenotypes and functions of human CD161++ Vα7.2+ T cell subsets. Front. Immunol. 2017;8:1031. doi: 10.3389/fimmu.2017.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dias J, et al. The CD4−CD8− MAIT cell subpopulation is a functionally distinct subset developmentally related to the main CD8+ MAIT cell pool. Proc. Natl Acad. Sci. USA. 2018;115:E11513–E11522. doi: 10.1073/pnas.1812273115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gherardin NA, et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol. Cell Biol. 2018;96:507–525. doi: 10.1111/imcb.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dias J, Leeansyah E, Sandberg JK. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc. Natl Acad. Sci. USA. 2017;114:E5434–E5443. doi: 10.1073/pnas.1705759114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dusseaux M, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 9.Cole S, et al. Interleukin (IL)-12 and IL-18 synergize to promote MAIT cell IL-17A and IL-17F production independently of IL-23 signaling. Front. Immunol. 2020;11:585134. doi: 10.3389/fimmu.2020.585134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahimpour A, et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 2015;212:1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koay HF, et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat. Immunol. 2016;17:1300–1311. doi: 10.1038/ni.3565. [DOI] [PubMed] [Google Scholar]
- 12.Salou M, et al. A common transcriptomic program acquired in the thymus defines tissue residency of MAIT and NKT subsets. J. Exp. Med. 2018;216:133–151. doi: 10.1084/jem.20181483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slichter CK, et al. Distinct activation thresholds of human conventional and innate-like memory T cells. JCI Insight. 2016;1:e86292. doi: 10.1172/jci.insight.86292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamichhane R, et al. Human liver‐derived MAIT cells differ from blood MAIT cells in their metabolism and response to TCR‐independent activation. Eur. J. Immunol. 2021;51:879–892. doi: 10.1002/eji.202048830. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs A, et al. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 2016;10:35–45. doi: 10.1038/mi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobkowiak MJ, et al. Tissue-resident MAIT cell populations in human oral mucosa exhibit an activated profile and produce IL-17. Eur. J. Immunol. 2018;49:133–143. doi: 10.1002/eji.201847759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leng T, et al. TCR and inflammatory signals tune human MAIT cells to exert specific tissue repair and effector functions. Cell Rep. 2019;28:3077–3091. doi: 10.1016/j.celrep.2019.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamichhane R, et al. TCR- or cytokine-activated CD8+ mucosal-associated invariant T cells are rapid polyfunctional effectors that can coordinate immune responses. Cell Rep. 2019;28:3061–3076. doi: 10.1016/j.celrep.2019.08.054. [DOI] [PubMed] [Google Scholar]
- 19.Voillet V, et al. Human MAIT cells exit peripheral tissues and recirculate via lymph in steady state conditions. JCI Insight. 2018;3:e98487. doi: 10.1172/jci.insight.98487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loh L, et al. Human mucosal-associated invariant T cells in older individuals display expanded TCRαβ clonotypes with potent antimicrobial responses. J. Immunol. 2020;204:1119–1133. doi: 10.4049/jimmunol.1900774. [DOI] [PubMed] [Google Scholar]
- 21.Lepore M, et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat. Commun. 2014;5:3866. doi: 10.1038/ncomms4866. [DOI] [PubMed] [Google Scholar]
- 22.Zumwalde NA, Haag JD, Gould MN, Gumperz JE. Mucosal associated invariant T cells from human breast ducts mediate a Th17-skewed response to bacterially exposed breast carcinoma cells. Breast Cancer Res. 2018;20:111. doi: 10.1186/s13058-018-1036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howson LJ, et al. MAIT cell clonal expansion and TCR repertoire shaping in human volunteers challenged with Salmonella Paratyphi A. Nat. Commun. 2018;9:253. doi: 10.1038/s41467-017-02540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gold MC, et al. MR1-restricted MAIT cells display ligand discrimination and pathogen selectivity through distinct T cell receptor usage. J. Exp. Med. 2014;211:1601–1610. doi: 10.1084/jem.20140507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckle SBG, et al. A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. J. Exp. Med. 2014;211:1585–1600. doi: 10.1084/jem.20140484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar BV, et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 2017;20:2921–2934. doi: 10.1016/j.celrep.2017.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poon MML, et al. Tissue adaptation and clonal segregation of human memory T cells in barrier sites. Nat. Immunol. 2023;24:309–319. doi: 10.1038/s41590-022-01395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milner JJ, et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature. 2017;552:253–257. doi: 10.1038/nature24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aibar S, et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods. 2017;14:1083–1086. doi: 10.1038/nmeth.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reantragoon R, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J. Exp. Med. 2013;210:2305–2320. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reantragoon R, et al. Structural insight into MR1-mediated recognition of the mucosal associated invariant T cell receptor. J. Exp. Med. 2012;209:761–774. doi: 10.1084/jem.20112095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel O, et al. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat. Commun. 2013;4:2142. doi: 10.1038/ncomms3142. [DOI] [PubMed] [Google Scholar]
- 33.Koay HF, et al. A divergent transcriptional landscape underpins the development and functional branching of MAIT cells. Sci. Immunol. 2019;4:eaay6039. doi: 10.1126/sciimmunol.aay6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legoux F, et al. Molecular mechanisms of lineage decisions in metabolite-specific T cells. Nat. Immunol. 2019;20:1244–1255. doi: 10.1038/s41590-019-0465-3. [DOI] [PubMed] [Google Scholar]
- 35.Radens CM, Blake D, Jewell P, Barash Y, Lynch KW. Meta-analysis of transcriptomic variation in T-cell populations reveals both variable and consistent signatures of gene expression and splicing. RNA. 2020;26:1320–1333. doi: 10.1261/rna.075929.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurioka A, et al. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015;8:429–440. doi: 10.1038/mi.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Street K, et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genom. 2018;19:477. doi: 10.1186/s12864-018-4772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cannoodt, R. et al. SCORPIUS improves trajectory inference and identifies novel modules in dendritic cell development. Preprint at bioRxiv10.1101/079509 (2016).
- 39.Rauen T, Hedrich CM, Tenbrock K, Tsokos GC. cAMP responsive element modulator: a critical regulator of cytokine production. Trends Mol. Med. 2013;19:262–269. doi: 10.1016/j.molmed.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kongsbak M, Levring TB, Geisler C, von Essen MR. The vitamin D receptor and T cell function. Front. Immunol. 2013;4:148. doi: 10.3389/fimmu.2013.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Clambey ET, et al. The Ikaros transcription factor regulates responsiveness to IL-12 and expression of IL-2 receptor alpha in mature, activated CD8 T cells. PLoS ONE. 2013;8:e57435. doi: 10.1371/journal.pone.0057435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krovi SH, et al. Thymic iNKT single cell analyses unmask the common developmental program of mouse innate T cells. Nat. Commun. 2020;11:6238. doi: 10.1038/s41467-020-20073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurachi M, et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat. Immunol. 2014;15:373–383. doi: 10.1038/ni.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lett MJ, et al. Stimulatory MAIT cell antigens reach the circulation and are efficiently metabolised and presented by human liver cells. Gut. 2022;71:2526–2538. doi: 10.1136/gutjnl-2021-324478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.FitzPatrick MEB, et al. Human intestinal tissue-resident memory T cells comprise transcriptionally and functionally distinct subsets. Cell Rep. 2021;34:108661. doi: 10.1016/j.celrep.2020.108661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bister J, et al. Human endometrial MAIT cells are transiently tissue resident and respond to Neisseria gonorrhoeae. Mucosal Immunol. 2020;14:357–365. doi: 10.1038/s41385-020-0331-5. [DOI] [PubMed] [Google Scholar]
- 48.Youssef GB, et al. Ontogeny of human mucosal-associated invariant T cells and related T cell subsets. J. Exp. Med. 2018;215:459–479. doi: 10.1084/jem.20171739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vorkas CK, et al. Single-cell transcriptional profiling reveals signatures of helper, effector, and regulatory MAIT cells during homeostasis and activation. J. Immunol. 2022;208:1042–1056. doi: 10.4049/jimmunol.2100522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandra, S. et al. Transcriptomes and metabolism define mouse and human MAIT cell heterogeneity. Preprint at bioRxiv10.1101/2021.12.20.473182 (2021).
- 51.Corces MR, et al. Lineage-specific and single-cell chromatin accessibility charts human hematopoiesis and leukemia evolution. Nat. Genet. 2016;48:1193–1203. doi: 10.1038/ng.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boggy GJ, et al. BFF and cellhashR: analysis tools for accurate demultiplexing of cell hashing data. Bioinformatics. 2022;38:2791–2801. doi: 10.1093/bioinformatics/btac213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xin H, et al. GMM-Demux: sample demultiplexing, multiplet detection, experiment planning, and novel cell-type verification in single cell sequencing. Genome Biol. 2020;21:188. doi: 10.1186/s13059-020-02084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGinnis CS, et al. MULTI-seq: sample multiplexing for single-cell RNA sequencing using lipid-tagged indices. Nat. Methods. 2019;16:619–626. doi: 10.1038/s41592-019-0433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoeckius M, et al. Cell hashing with barcoded antibodies enables multiplexing and doublet detection for single cell genomics. Genome Biol. 2018;19:224. doi: 10.1186/s13059-018-1603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hao Y, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muskovic W, Powell JE. DropletQC: improved identification of empty droplets and damaged cells in single-cell RNA-seq data. Genome Biol. 2021;22:329. doi: 10.1186/s13059-021-02547-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hafemeister C, Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20:296. doi: 10.1186/s13059-019-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andreatta M, Carmona SJ. STACAS: Sub-type anchor correction for alignment in Seurat to integrate single-cell RNA-seq data. Bioinformatics. 2021;37:882–884. doi: 10.1093/bioinformatics/btaa755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korsunsky I, et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods. 2019;16:1289–1296. doi: 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Finak G, et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 2015;16:278. doi: 10.1186/s13059-015-0844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van de Sande B, et al. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nat. Protoc. 2020;15:2247–2276. doi: 10.1038/s41596-020-0336-2. [DOI] [PubMed] [Google Scholar]
- 63.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu T, et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation (Camb.) 2021;2:100141. doi: 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu G, He Q-Y. ReactomePA: an R/Bioconductor package for reactome pathway analysis and visualization. Mol. Biosyst. 2016;12:477–479. doi: 10.1039/C5MB00663E. [DOI] [PubMed] [Google Scholar]
- 66.Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions. Curr. Opin. Virol. 2011;1:519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giudicelli V, Lefranc M-P. IMGT/JunctionAnalysis: IMGT standardized analysis of the V-J and V-D-J junctions of the rearranged immunoglobulins (IG) and T cell receptors (TR) Cold Spring Harb. Protoc. 2011;2011:716–725. doi: 10.1101/pdb.prot5634. [DOI] [PubMed] [Google Scholar]
- 68.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buenrostro JD, et al. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature. 2015;523:486–490. doi: 10.1038/nature14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramírez F, et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016;44:W160–W165. doi: 10.1093/nar/gkw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, et al. Model-based analysis of ChIP-seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Q, Brown JB, Huang H, Bickel PJ. Measuring reproducibility of high-throughput experiments. Ann. Appl. Stat. 2011;5:1752–1779. doi: 10.1214/11-AOAS466. [DOI] [Google Scholar]
- 77.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 78.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–3.
Characteristics of liver tissue donors.
Supplementary Tables 2–12.
Data Availability Statement
Sequencing data generated in this study have been deposited in NCBI’s Gene Expression Omnibus (GEO) and are accessible through GEO SuperSeries accession number GSE194189. The Bioconductor org.Hs.eg.db annotation package and ENSEMBL_MART_ENSEMBL BioMart database are publicly available. Source data are provided with this paper.
No custom software was generated for this manuscript. The software used for all analyses is listed in the relevant sections of the Methods.