Abstract
Extremophiles possess unique cellular and molecular mechanisms to assist, tolerate, and sustain their lives in extreme habitats. These habitats are dominated by one or more extreme physical or chemical parameters that shape existing microbial communities and their cellular and genomic features. The diversity of extremophiles reflects a long list of adaptations over millions of years. Growing research on extremophiles has considerably uncovered and increased our understanding of life and its limits on our planet. Many extremophiles have been greatly explored for their application in various industrial processes. In this review, we focused on the characteristics that microorganisms have acquired to optimally thrive in extreme environments. We have discussed cellular and molecular mechanisms involved in stability at respective extreme conditions like thermophiles, psychrophiles, acidophiles, barophiles, etc., which highlight evolutionary aspects and the significance of extremophiles for the benefit of mankind.
Keywords: Extreme environments, Extremophiles, Thermophiles, Extremozymes, Halophiles, Acidophiles, Barophiles, Psychrophiles, Polyextremophiles
Introduction
Microorganisms are likely to live in moderate conditions, i.e., 37 °C temperature, pH 7.4, salinity up to 3%, and pressure of 1 atm. These conditions are considered favorable for colonization (Merino et al. 2019), and therefore, these conditions are known as “physiologic” conditions. Over the last half-century, many researchers searched for microorganisms that can survive under extreme conditions (Yuan et al. 2009; Narsing Rao et al. 2021). With the advancements in knowledge, technology, and new cultural techniques, it is possible to isolate species from extreme environments that were earlier considered to be inhabitable (Yuan et al. 2009; Kumar et al. 2021; Narsing Rao et al. 2021). Numerous scientists have demonstrated that microorganisms exist in all sorts of environments, but it is necessary to find suitable means to isolate them from understudied sites (Jacob et al. 2017; Wang et al. 2022). For example, the Dead Sea was previously considered to be devoid of life, but today it is well understood that it contains living organisms (Nissenbaum 1975; Jacob et al. 2017). The microorganisms surviving in extreme conditions are called extremophiles, and there is a broad range of different extremophiles able to thrive under distinct conditions: high or low temperature, high or low pressure, high or low pH, radiation, elevated salty or ionic conditions, low water activity, and the presence of chelating ions or metals (Baker-Austin and Dopson 2007; Singh et al. 2019; Wang et al. 2013; Junge et al. 2011; Etten et al. 2022).
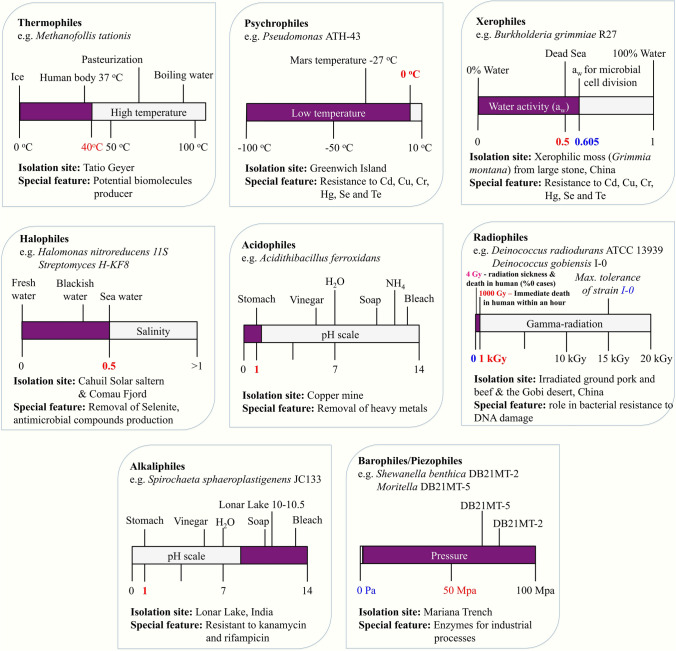
MacElroy (1974) coined the term extremophile, which includes bacteria, archaea, fungi, some algae, viruses, and ciliated protozoans (Xu 2014). A majority of extremophiles are prokaryotes (Zgonik et al. 2021). Their great genome flexibility, probably allowed them to rapidly adapt to a wide spectrum of extreme environments, and pushing up the limits of life suggests the potential for extremophiles to be able to become inhabitants of, a priori, hostile environments like Mars, Europa (a moon of Jupiter), and Enceladus (Martin and McMinn 2018). This leads us to consider the expanding field of extraterrestrial life and astrobiology studies (von Hegner 2020). The extremophiles including thermophiles, psychrophiles, acidophiles, alkaliphiles, halophiles, barophiles, xerophiles, and radiophiles, require extreme features to overcome to thrive in their typical environments (Pikuta et al. 2007; Rotter et al. 2021; Marsh et al. 2021; Narsing Rao et al. 2021). Besides, more than a single extreme feature can be needed to survive and grow in determined environments, and the microorganisms in these sites have been described as polyextremophiles. A description of various extreme environments is shown in Fig. 1.
Fig. 1.
Graphical representation of extreme habitats and some examples of extremophilic prokaryotes with their ability growth requirement or special feature
(Modified from Kato et al. 1998; Orellana et al. 2018; MicroSoc 2022; NIH 2022)
This review highlights the importance of extremophiles. The aim of discussing these organisms is to gain a better understanding of the diversity of life on Earth as well as the limits of biological processes. By looking at the characteristics and mechanisms lying behind the adaptations of extremophiles, we can figure out what adaptations have helped them live in harsh environments. This knowledge can be implemented in various practical applications, such as in biotechnology, medicine, and astrobiology. Moreover, studying extremophiles can provide insight into the origins of life on Earth and the potential for life beyond our planet. Furthermore, exploring extremophiles can expand our knowledge of the diversity of life and the potential for biological processes in challenging environments.
Significance of extremophiles
The interest in studying extremophiles arises as a consequence of a broad range of biotechnological applications. The success of thermophilic polymerases is reflected by extremely thermostable Pfu from Pyrococcus furiosus (Lundberg et al. 1991; Zeng et al. 2021), Vent from Thermococcus litoralis (Cariello et al. 1991; Marathe et al. 2021) and Taq DNA polymerases from Thermus aquaticus (Mroczkowski et al. 1994; Turvey et al. 2022). Thermostable DNA polymerases lead to a market of over $ US 2 billion. Similarly, extremophiles have also been exploited by industrial sectors dealing with biohydrogen production (Saha et al. 2022), biobutanol production (Kochhar et al. 2022), biomining (Coker 2016; 2019; Santomartino et al. 2022), microbial carotenoid production (Tapia et al. 2021), proteases (Perfumo et al. 2020), lipases (Verma et al. 2021), glycosyl hydrolases (Liew et al. 2022), sugar phosphates, phospholipids (Ramos 2021) and for biomedical applications like antimicrobials and antitumor molecules (Zgonik et al. 2021; Zhang et al. 2021).
Geothermal areas and their biological productivity
Geothermal areas are widely spread on Earth's surface and are generally related to points of geological movement of Earth's tectonic plates (Khalil et al. 2011; Mohammad et al. 2017). The geothermal areas can be differentiated into terrestrial (i.e., hot springs) and marine (i.e., hydrothermal vents) (Martins et al. 2013; Leston et al. 2014). Ding et al. (2017) suggest hydrothermal vent habitats are some of the most biologically productive places on Earth. Chemoautotrophs that live in these regions are important primary producers, transferring energy from the geothermal source to higher trophic levels via a variety of key microbial chemosynthetic processes such as sulfur-oxidation, nitrification, carbon monoxide utilization, H2 consumption, metal redox bioprocesses and methanogenesis (Sievert and Vetriani 2012). There are a number of areas around the world where volcanic activity is a major environmental factor (Saghatelyan et al. 2021; Gunda et al. 2021; Yaroshenko et al. 2022). The most general characteristics of the heat zone consist of sulfur, clay, and fumaroles (Tetzner et al. 2021; Cardona et al. 2021). Iceland is another hotspot area for geothermal activity. The heat from Iceland is harnessed in many ways, like for house heating, power generation, industrial purposes, and greenhouse farming. The geothermal area is most widely used for energy production, but it also has disadvantages like the release of toxic gases, i.e., methane, hydrogen sulfide, and carbon dioxide, which are very harmful to the living organisms (https://www.nationalgeographic.org/encyclopedia/geothermal-energy/).
Thermophiles—high-temperature loving microorganisms
Thermophilic microorganisms are thought to live mostly in geothermal areas like hot springs and geothermally heated soil (Saghatelyan et al. 2021; Salano et al. 2017; Crowe et al. 2014). Hot springs are areas beneath the Earth’s surface where warm or hot groundwater erupts and this water in general contains an abundance of dissolved solids and minerals, which are essential for the progression of life and living forms (Narsing Rao et al. 2018; 2021). These types of environments are regarded to be the origin of life on Earth (Narsing Rao et al. 2018, 2021). Hot springs and geothermal waters were considered sterile environments for a long time. Thomas Brock's discovery of Thermus aquaticus in Yellowstone National Park transformed our understanding of hot spring microbial diversity (Brock 1997). Geothermal heat in the Earth’s mantle and heated water regulate species abundance and distribution in hot springs. Selective pressures on microbial species have resulted in the evolution of certain prokaryotic phyla capable of high-temperature growth and the production of economically relevant thermostable bioactive compounds (Rekadwad and Pathak 2016; Narsing Rao et al. 2018). Bacteria and Archaea are the two phylogenetic domains that are thermophiles (Stetter 1999) and are classified into different categories based on their temperature tolerance and growth, such as thermotolerant (can survive above 45 °C), moderate thermophile (grow optimally between 45 and 65 °C), strict thermophile (grow optimally from 65 to 80 °C), and hyperthermophile (grow above 80 °C).
In the hydrothermal environment, bacteria can exist at temperatures above 50 °C (Zeldes et al. 2015), whereas the temperature range of 60–68 °C is favorable for flourishing bacterial growth, but above 90 °C, archaea become dominant (Li et al. 2015). For example, Pyrolobus fumarii can survive from 106 to 113 °C (Blöchl et al. 1997), and Methanopyrus kandleri strain 116 can grow up to 122 °C (Takai et al. 2008). Thermophilic microbial communities can also be seen in the soil and hot compost of wastes, where the degradation of wastes leads to an increase in temperature from 65 to 80 °C, which leads to the transition of microbial communities from the mesophilic to the thermophilic temperature range (Hirota et al. 2019). In the hot spring areas, unique thermophilic microbial communities were found merging lithotrophic and heterotrophic microorganisms. The thermophilic microbial communities change their compositions at high temperatures, but above 70–75 °C, photosynthesis stops (Wang et al. 2013; Cuecas et al. 2014). To counteract these consequences, chemotrophic bacteria use the Calvin cycle or reverse tricarboxylic acid cycle to convert carbon dioxide into biomass, therefore serving as primary producers (Kajla et al. 2022).
Thermophiles have evolved unique adaptations and mechanisms that allow them to survive and thrive in high-temperature environments. One of the most important adaptations is the production of specialized proteins that remain stable at high temperatures, such as heat shock proteins, chaperones, and enzymes (Narsing Rao et al. 2022). These proteins are essential for maintaining the structural integrity of the cell, preventing denaturation and aggregation of cellular components, and facilitating the proper folding and assembly of proteins (Zhu et al. 2020). Thermophiles have also developed unique lipid membranes that can withstand high temperatures, which are composed of ether-linked phospholipids and are more resistant to oxidative damage than the more common ester-linked phospholipids found in most organisms. Additionally, some thermophiles are capable of forming biofilms, which protect against heat stress and other environmental challenges (Straub et al. 2018). The evolutionary history of thermophiles is not well understood, but it is thought that they may have originated in deep-sea hydrothermal vents or other extreme environments, and subsequently adapted to life in terrestrial hot springs and geothermal vents (Holm et al. 1992). The study of thermophiles provides valuable insights into the evolution of life on Earth and the mechanisms by which organisms adapt to extreme conditions. It also has potential applications in biotechnology, such as in the development of heat-resistant enzymes for industrial processes and the production of biofuels (Saxena et al. 2017; Damer and Deamer 2020; Lv et al. 2022).
Mechanism for thermal stability and evolution
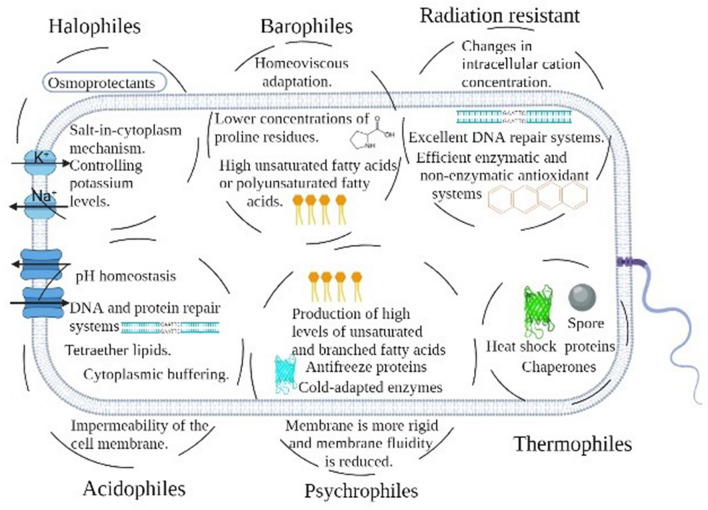
The thermal stability of microorganisms and their biomolecules depends on various factors. Both the microorganism`s optimal growth temperature and their cellular adaptations have an impact on how they react to temperature (Figs. 1 and 4). For instance, Carnobacterium spp., growing at low temperatures, grew as individual cells, while at high temperatures they grew as long chains of cells. Pleomorphism, cell length, and size increased with the temperature rise (Novitsky et al. 1974; Räsänen et al. 2001). Cells grown at low temperatures contained more teichoic acid (by weight) in the cell wall when compared to cells grown at high temperatures (Novitsky et al. 1974). When cell walls were grown at a low temperature, the ratio of ester alanine to phosphate was much higher than when they were grown at a high temperature. Novitsky et al., (1974) found that cells grown at high temperatures had less autolysis, cell walls that held more Mg2+, and less peptide cross-bridging in their peptidoglycan layer.
Fig. 4.
Molecules conferring stability and involved evolutionary mechanisms among six important extremophiles
Spores have been recognized as the hardest known form of life on Earth, and in their dormant state, they undergo no detectable metabolism and exhibit a higher degree of resistance to wet and dry heat (Nicholson et al. 2000). A large number of factors are involved in spore resistance to wet heat, including DNA saturation with alpha- and beta-type small acid-soluble spore proteins, dipicolinic acid, divalent metal ion content and identity, growth temperature optimum of the strain, sporulation temperature, solid versus liquid sporulation medium, core water content, and additives present in the solution, in particular the pH, during wet heat treatment (Setlow 2014). The amount of water in the spore core has largely influenced spore resistance to wet heat (Setlow 2006). The major factor that determines spore wet heat resistance is the core water content. Spores formed at higher temperatures generally have lower core water contents (Setlow 2006). Lower core water content generally gives more wet, heat-resistant spores. Although the core water content is the major factor determining the level of spore wet heat resistance, the sporulation temperature, spore core mineralization, and alpha- and beta-type small acid-soluble spore proteins also contribute to spore wet heat resistance (Gerhardt and Marquis 1989; Nicholson et al. 2000; Setlow 2000, 2006).
DPA may play an indirect role in spore resistance to wet heat; when DPA is not accumulated in the spore core, the water content of the core does not decrease as much as it does in DPA-replete spores. Because core water content is such an important element in spore-wet heat resistance, everything that increases spore core water content reduces spore-wet heat resistance (Driks and Eichenberger 2016). Spores with high Ca2+ levels are the most wet heat resistant; those with high levels of Mg2+ or Mn2+ are less heat resistant; and those in which K+ or Na+ have been substituted for Ca2+ are the least wet heat resistant (Gerhardt and Marquis 1989; Bender and Marquis 1985; Setlow 2006). The alpha- and beta-type SASP are also important in spore resistance to dry heat (Setlow 2006). Analysis of biofilm suggests that heat causes changes in the bacterial community members in biofilm and the composition of EPS. At higher temperatures, there was a greater protein to polysaccharide ratio in the EPS, as well as decreased extracellular sugar synthesis (Pinel et al. 2021). Thermophiles have a range of cellular adaptations to resist denaturation and degradation. They have more saturated and straight-chain fatty acids in their membrane lipids than mesophiles (Ulrih et al. 2009). Thermophilic proteins appear to be smaller and more basic in some situations, which may lead to greater stability. Chaperones, which aid in the refolding of denatured proteins, are an additional approach utilized to increase protein stability (Jaenicke et al. 1996; Rampelotto 2010). Monovalent and divalent salts enhance the stability of nucleic acids because these salts screen the negative charges of the phosphate groups, and KCl and MgCl2 protect the DNA from depurination and hydrolysis. Another way to stabilize DNA is through the employment of DNA-binding proteins and the compaction of the genome into chromatin (Marguet and Forterre 1998; Rampelotto 2010).
Bacteria and archaea can change how much lipid is in their cytoplasmic membranes in response to changes in temperature. Bacteria may accomplish this at higher temperatures by increasing the chain length of the lipid acyl chains, the ratio of iso/anteiso branching, and/or the degree of saturation of the acyl chain (Gaughran 1947; Albers et al. 2000). At high temperatures, the degree of cyclization of the C40 isopranoid in the tetraether lipids of some archaea increases. This makes the lipids pack together more tightly, which makes it harder for the lipids to move and keeps the membrane from becoming too fluid. The permeability of the cytoplasmic membrane is an important part of controlling growth, and it has been suggested (Kates et al. 1993; Albers et al. 2000) that the membrane's ion-permeability goes up with temperature. Membrane proton and sodium permeabilities increase with temperature. Thermophilic archaea are capable of adjusting the lipid content of their membranes such that proton permeability stays constant at the corresponding growth temperature (Konings et al. 2022). Thermophilic bacteria, on the other hand, are an exception. They rely on the less permeable sodium ions to generate a sodium motive force, which is subsequently used to drive energy-requiring membrane-bound processes. According to Nikaido (2003), primary ATP-driven transport systems or secondary proton- or sodium-motive-force-driven transport systems were primarily responsible for mediating solute transport across bacterial and archaeal membranes. Hyperthermophilic bacteria and archaea, in contrast to most bacteria, prefer primary uptake systems. Several high-affinity ATP-binding cassette (ABC) transporters for sugars from hyperthermophiles have been identified and characterized. The actions of these ABC transporters enable these organisms to flourish in nutrient-deficient settings (Albers et al. 2001; Gunde-Cimerman et al. 2018).
The main reason that thermophilic bacteria can live at high temperatures is that their enzymes and proteins are stable at high temperatures. There is only a slight difference in the protein sequence of aminoalkanoic acid in comparison to mesophilic microorganisms. The secretion of di-inositol phosphate, diglycerol phosphate, and manosylglycerate by thermophilic microorganisms makes proteins stable (Penhallurick et al. 2021; Vavitsas et al. 2022). Thermophiles have firmed cell membranes due to the presence of saturated fatty acids. The hyperthermophiles have different bacterial cell membranes due to the presence of isoprene. Isoprene is the unit that has ether linkages as compared to ester linkages present in bacterial and eukaryotic cell membranes. The hyperthermophile cells have very large macromolecules forcing their cellular envelopes, which lack heat insulation (Vieille and Zeikus 2001). For example, the members of the order Thermotogales (hyper-thermophiles) harbor tetra ether, tetra ester, and mixed ester/ether in their plasma membranes, contributing to core lipids (Sahonero-Canavesi et al. 2022). There is a major difference between bacteria and archaea, which is due to the different composition of the archaeal cell membrane (protein-based cell walls and ether links in their cell membranes). That is the reason why bacteria and archaea are apart from each other (Madigan et al. 2020; Subedi et al. 2021).
There is not much known about what happens to the many thermolabile biomolecules that are needed for important cellular processes in all living things. Examples are: NADP, ATP, pyridoxal phosphate, etc. Recently, Cuecas et al. (2016) showed the ability of thermophiles to maintain relatively high intracellular viscosity, which preserved NADH thermal stability up to around 80 °C. Hyperthermophiles, who live optimally at higher temperatures, would require additional mechanisms to thrive at those temperatures (Daniel and Cowan 2000; Cuecas et al. 2016). Gonzalez (2018) has shown the first evidence of the requirement for well-structured and dimensional molecular tunnels in hyperthermophiles, which suggest a critical role for molecular channeling in biomolecular stability at high temperatures. Many investigations showed that the thermophilic microorganisms can show maximum growth at 110 °C (Vieille et al. 1996; Vieille and Zeikus 2001), whereas Pyrococcus kukulkanii NCB100 and Methanopyrus kandleri growth can also occur at 112 and 122 °C, respectively (Callac et al. 2016; NPA 2022). Nowadays, the detection of thermophilic microorganisms has increased over the last few decades due to advancements in molecular methods, which allow for the easy detection of the DNA of many organisms at one time (Zeldes et al. 2015). Culture-independent molecular methods have made it possible to find microorganisms that cannot be grown in a laboratory (Hugenholtz et al. 2021), but scientists still need prokaryotic cultures to confirm physiological traits and complete genomic features that could explain their phenotypic abilities and molecular responses (Franco-Duarte et al. 2019). Also, physical culture is needed to study the different molecules involved in thermostability, which could be useful in the future.
Molecular cloning of thermophilic genes into mesophilic hosts
Genetic and protein engineering are advanced techniques used for the commercial production of enzymes with enhanced stability at high temperatures and high pH. With the advancement in biology tools, and genetic and gene transfer analysis it is possible to produce thermostable recombinant enzymes. The recombinant technique like cloning and gene expression in mesophilic bacteria, e.g., E. coli, leads to enhance the production of thermostable enzymes. The thermostable enzymes can be produced by expressing the genes in E. coli, e.g., α-amylase gene of Pyrococus furiosus was cloned in E. coli and B. subtilis which worked actively at 100 °C (Kengen 2017; Ajeje et al. 2021).
Psychrophiles—cold-loving microorganisms
Eurypsychrophile (psychrotolerant) and stenopsychrophile (psychrophile)
A psychrophile is a type of microorganism that can live in cold places (Morita 1975) and is different from a mesophile and a thermophile (Figs. 1 and 4). A psychrophilic microorganism can grow optimally at 10 °C and shows some growth at 0 °C or below (Kashyap et al. 2022). Psychrotolerant microorganisms are those microorganisms that can grow at 0 °C, whereas optimal growth is generally in the range of 20–40 °C (Bölter 2004). The biggest difference between psychrotolerants and psychrophiles is that psychrotolerants can grow at higher temperatures, between 20 and 40 °C, while psychrophiles grow optimally at or below 20 °C. At present, those terms have been updated, and instead of psychrophile and psychrotolerant, the novel terms eurypsyhrophile (cold lovers able to grow well in the mesophile range too) and stenopsycrophile (strictly cold lovers) have been proposed (Yang et al. 2021) (Fig. 2). In the last 20 years, scientists have conducted numerous investigations in glacial ice cores, and the results have confirmed a great microbial diversity, showing viable cells in ice cores for over 120,000 years (Tendulkar et al. 2021).
Fig. 2.
Mini-metagenome analysis of psychrophilic microbial biofilm (Permission for utilization of copyright material—Yang et al. 2021—has been obtained from CCC Rightslink from in journal/magazine)
Phylogenetic diversity of psychrophiles—sea ice microbial communities (SIMCOs)
Microbial diversity blooms during the spring and summer seasons in the sea ice. Sea ice microbial communities (SIMCOs) during these seasons showed a dominance of diatoms, resulting in the ice appearing brown-green. During these seasons, a number of characteristic microbial activities have been observed in sea ice, including metabolite production, microbial trophic interactions, and the assemblage of diverse phylogenetic bacterial communities (Garrison et al. 1986). Research expedition studies revealed the presence of novel new members of existing phyla in this region (Gosink et al. 1997; Bowman et al. 1998). Many researchers have observed that the diversity in Arctic and Antarctic sea ice belongs to stenopsycrophile bacteria (Junge et al. 2004). Culturable and molecular techniques showed that beta-proteobacteria, a type of bacteria that was thought to only live in freshwater, was also be found inside the ice (Achberger et al. 2016; Brinkmeyer et al. 2004). Along with this, some purple sulfur-producing anaerobic phototrophic bacteria were also present in the Baltic ice (Tank et al. 2011). Other studies on Baltic ice showed that de-nitrification occurs in these zones, which leads to the accumulation of nitrate. It has been found that the Arctic Sea consists of a large number of anaerobic nitrate and ammonium oxidizing bacteria (Junge et al. 2011). Recently, some studies have been conducted in the winter season to study the compositions of communities in the Arctic region, and surprisingly, they found bacteria and archaea, which resemble the underwater communities (Gao et al. 2021). The study of bacterial communities in the wintertime showed the presence of gamma-proteobacteria, whereas in summer and spring bacterial communities, no gamma-proteobacteria were found. This showed that mortality during the seasons plays a major role in microbial succession that results in a difference between seasonal bacterial communities (Collins et al. 2010; Junge et al. 2011; Cono et al. 2022).
Discovery of gas vacuolated sea ice bacteria
Earlier, it was hypothesized that the gas vesicles, which are intracellular structures found in bacteria and archaea, help cells with mobility and buoyancy (Walsby 1994). In the sea habitat, the gas vesicles are also present in the cyanobacteria, which help them in floating over the surface of the water where they get abundant sunlight for photosynthesis (Walsby et al. 1997). In the hypolimnion region of the lake (Steenbergen et al. 1987), some anoxygenic photosynthetic bacteria are present, which also consist of gas vacuoles (Clark and Kolb 2020). This was initially reported by Irgens et al. in (1989) in their preliminary description of gas-vacuolated marine bacteria from the Antarctic Peninsula, which are typically indigenous to SIMCO (Irgens et al. 1989). Those bacteria possesses gas-vacuoles were isolated from upper depths of ice column and near the ice edge (Irgens et al. 1989). The taxonomy of the gas vacuolated bacteria describes that the vacuolated bacterium belongs to Polaromonas vacuolata gen. nov., sp. nov., which is a member of the class betaproteobacteria (Irgens et al. 1996). However, diverse varieties of vacuolated bacteria were isolated from the SIMCO layer, showing that the gas-vacuolated bacteria represented consist of two major phylogenetic groups, i.e., proteobacteria and bacteroidetes (Junge et al. 2011).
Other mechanisms of adaptation to psychrophile
A few psychrophilic bacteria like lactic acide bacteria (LAB) can maintain their membrane fluidity alteration in their membrane permiliabilty (Huang et al. 2016; Gao et al. 2022). By reducing membrane fluidity while crystallizing at low temperatures, LAB cells become more stiff, which raises the concentration of intracellular components (Papadimitriou et al. 2016). L. lactis has the highest intracellular viscosities when it is growing at 15–20 °C, and it is able to decrease its intracellular viscosity at lower or higher temperatures. This allows these cells to grow and thrive at low temperatures by counteracting the effect of temperature that would cause viscosities that are too high to allow the machinery of the cell to work properly and allow biomolecules to move around inside the cell (Cuecas et al. 2016).
Acidophiles: low pH loving microorganisms
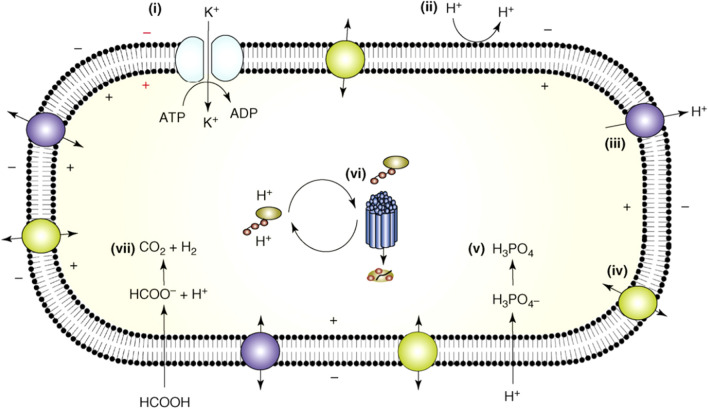
Those microorganisms that can thrive in acidic conditions are known as acidophiles. The acidophiles generally grow optimally at pH below 3. Acidophiles are classified into three domains, i.e., bacteria (Acidithiobacillus, Acidiphilum, and Alicyclobacillus), archaea (Crenarchaeota, Acidianus, Desulphurolobus, Metalolosphaera, Stygiolobus, Sulfolobus, Thermoplasma, and Picrophilus) (Sharma et al. 2012), and eukaryotes (Vorticella, Euglena, Rhodotorula, Cryptococcus, Acontium, Trichosporuon, Klebsormidium, Zygnema, Dunaliella, and Cyanidium) (Aguilera 2013). Several areas around the world possess natural or man-made acid-containing places, including hot springs, acid mines, etc., (Rekadwad and Gonzalez 2019). The presence of a protective capsule in bacteria and mechanisms to maintain the pH of the intracellular environment have been reported as key factors in acidophiles (Figs. 1 and 4) that allow them to grow in extremely acidic conditions. Some of their most characteristic strategies are represented in Fig. 3 (Baker-Austin and Dopson 2007).
Fig. 3.
Various processes of pH homeostasis in acidophiles. (i) Reversal of the Dc in acidolphiles to partially refract incoming protons flow. (ii) Impermeable membrane to retard protons influx. (iii) Maintenance of pH gradient via active proton export (indicated in blue color). (iv) Secondary transporters (indicated in green color). (v) Sequestering protons for maintaining pH homeostasis (vi) DNA and protein repair systems (vii) Degradation of organic acids (Permission to reuse in journal/magazine has been obtained through Copyright Clearance Center’s RightsLink® service from article Baker-Austin and Dopson 2007, 15:165–171, TRENDS in Microbiology, https://doi.org/10.1016/j.tim.2007.02.005)
pH homeostasis in acidophiles
Biodiversity and nature of acidophilic microorganisms
Environments showing extreme acidic conditions can be a result of nature or induced by human activities (Hedrich and Schippers 2021). Today’s prokaryotic acidophiles are in focus due to their applications in the biotechnology field (i.e., bio-mining) and ecological contamination in areas suffering from acid mine drainage (Bellenberg et al. 2021; Hedrich and Schippers 2021). In these environments, the oxidation of metallic sulfides produces highly acidic conditions (due to sulfate ions), which generates the required scenario for the solubilization of metals such as iron, copper, etc. (Amils et al. 2014; Siliakus et al. 2017; Amin et al. 2021).
Aerobic alkaliphiles: high-pH and di-oxygen-tolerant microorganisms
Those microorganisms that require pH 9 or above for growth and development are known as alkaliphiles, and generally, if the pH reduces to 6.5, the growth is inhibited (Horikoshi et al. 1999; Bouacem et al. 2022; Muntyan et al. 2022). Sodium carbonate is largely used to adjust the pH around 10 (Horikoshi 2004). To culture these microorganisms, saline medium containing soluble carbonate (soluble carbonate alkalinity around 0.9–5.0) should be utilized (Sorokin et al. 2019). Alkaliphilic microorganisms are present in soils, representing roughly 1/10–1/100 of the total number of microorganisms (Horikoshi 1991; 1999). Further examination revealed the occurrence in the Mariana Trench at a depth of 10,898 m (Takami et al. 1997; Pikuta et al. 2007) of some alkaliphilic microorganisms, i.e., Micrococcus, Bacillus, Pseudomonas, and eukaryotes like yeasts (Newsome and Falagán, 2021; Macedo et al. 2021).
Anaerobic alkaliphiles: high-pH and di-oxygen-intolerant microorganisms
Extremophile Clostridium thermohydrosulfuricum, which was initially reported from the soil samples collected from sewage plant in Georgia and from hot springs of from hot springs in Utah and majority hot springs in Wyoming, capable of growth at a pH range of 5–9 and can tolerate temperature up to 78 °C (Wiegel et al. 1979). Similarly, Anaerobranca horikoshii JW/YL-138T and other eight similar strains can survive at 60 °C and pH ranged from 6.9 to 10.3 (optimum growth at pH 8.5) (Horikoshi 1999). This examination observed that C. paradoxum has novel thermophilic properties, which can maintain the homeostasis inside the cell and can thrive well in media with a pH of 7.6–9.8 (Cook et al. 1996; Horikoshi 1999). Similarly, Amphibacillus xylanus can be used to ammoniate leucine and glucose and act as an essential amine (Niimura et al. 1990). Most of the scientists believed that under normal soil condition alkaliphiles can be isolated but at high pH their number is high (Horikoshi 1999), but a study at Volcano Island, Italy, showed that Thermococcus alcaliphilus can exist in the shallow marine zone at pH 6.5–10.5 and temperatures of 56–90 °C (Dirmeier et al. 1998). These anaerobic alkaliphiles have polysulfides, which are the essential component used to diminish hydrogen sulfide from the environment (Horikoshi 1999). This examination has been done by using the DNA-DNA hybridization and 16S rRNA amplicon sequencing techniques, which can be further used to identify additional alkaliphilic species like Thermococcus acidaminovorans (Dirmeier et al. 1998).
Haloalkaliphiles: microorganisms survive at high salt concentration and high pH
Haloalkaliphiles are the conditions where hypersalinization situations occur. This type of environment is mostly found in soft drink deserts and soft drink lakes. These types of lakes are found, for instance, in Kenya (Magadi Lake) and Egypt (Wadi Natrun). It was observed that the pH condition of these lakes lies between 10.5 and 12.0. In these lakes, some microorganisms required antacids, which could be done by adding more salts to the lake; these types of microorganisms are known as haloalkaliphiles (Salano et al. 2017). These microorganisms show numerous gas vacuoles and are highly pigmented. These microorganisms contain 62.7 mol% of GC. Based on their phylogenetic tree relationship with Archaea, most haloalkaliphiles are classified under the Natronobacterium genus. The characteristics of this genus are gram-positive bacteria, obligate anaerobes, alkaliphilic bacteria, and fermentative bacteria. Xin et al. (2001) found novel strains in the Tibet Soft Drink Lake, and the characteristics of these strains were gram-negative bacteria, non-motile, and rigorously growing in saline conditions. A new genus and species, Tindallia magadii (Kevbrin et al. 1998), were proposed.
Methanogens
Halophilic methanogens were taken from distinct hypersaline conditions. These straisalts ranging fromns can be easily grown in a medium having a pH of 7 and containing salt 0.5–2.5 M (Ventosa et al. 1998). Another type of methylotrophic methanogen has been found at Magadi Lake, and it has been classified under the Methanohalophilus (Methanosalsus zhilinaeae) (Koga et al. 1998; Sorokin et al. 2017; Bueno de Mesquita et al. 2021). Besides, the methanogens are often able to grow over a broad range of saline concentrations, leading to physiological and morphological differential responses (Galagan et al. 2002).
Cyanobacteria
Alkaliphilic cyanobacteria have been found in 16 genera and 34 species in a variety of environments and environmental conditions (Oren 2015; Namsaraev and Zavarzin 2016; Gupta and Khanna 2019). It has been found that these species carry electrogenic antiporters, as is the case with Synechocystis sp., (Nobel 2014; Nagao and Kojima 2017), an alkaliphile cyanobacterium has also been used to obtain urease (Vicente et al. 2008).
Halophiles: salt-loving microorganisms
Salt is an important part of all living things. It is needed to keep the osmotic pressure between cell compartments and between the inside and outside of the cell (Figs. 1 and 4). Halophiles require a high concentration of salt for their survival, i.e., 0.2 M NaCl. Halophiles are a group of microorganisms that includes eukaryotes and prokaryotes (Singh et al. 2019). There are different types of halophiles depending on their preferred saline concentration, such as slightly halophiles (bear salt concentrations of 0.2–0.85 M), moderately halophiles (tolerate salt concentrations of 0.85–3.4 M), and true halophiles (tolerate salt concentrations between 3.4 and 5.1 M) (Dutta and Bandopadhyay 2022). The most extreme halophiles are reported to belong generally to the Archaea. Hence, halophiles are organisms that can be found everywhere around the world where saline conditions prevail, like in beachfront areas, salt mine areas, and solar salterns (Mani et al. 2020). In earlier civilizations, halophilic microorganisms were used as biomarkers for salt creation in old societies like China and the Middle East. Even today, halophilic organisms are used for gem creation, like the red–purple shading that builds in salts due to sun radiation. They were also utilized for making cheese, restoring meat, making nutrient supplements, etc. These halophiles are now used as biomarkers, biofuel drugs, and medical care businesses, which are probably going to be accessible (Rotter et al. 2021) and gain importance for exploration of other planets like Mars (Oren 2014) in the near future.
Barophiles (piezophiles): pressure tolerating microorganisms
Barophillic microorganisms, also called piezophiles, are tiny entities that can live in very deep parts of the ocean floor. Pressure increases with depth due to the weight of the water column above. Thus, values above 1000 atm can be reached at the deepest zones of the oceans, such as the Mariana Trench in the Western Pacific Ocean, with a depth of up to 11,000 m (Kato 1999). Barophiles are mostly found on the seafloor, where pressure regularly surpasses 380 atm (38 MPa). Some of the barophiles are found in the lower part of the Pacific Ocean, where extreme pressure exists, i.e., 117 MPa (www.bionity.com), at a depth of 1.5–2.4 km (Fang et al. 2017). The high-pressure factors experienced by these life forms can make the ordinarily liquid cell film waxy and impermeable to supplements. These novel living beings have adjusted in novel ways to get lenient under such pressure factors where microbes colonize in remote ocean living spaces. One model, xenophyophores, has been found in the most profound sea channel, 6.6 miles (10,541 m) underneath the surface (Kato et al. 1998).
Barophiles are microorganisms that can endure high pressure and require very specific and minimal growth conditions (Figs. 1 and 4). Halomonas species, for instance, need a pressure of 1000 atm (100 MPa) and a temperature of 3 °C. Most piezophiles are hazy and UV-sensitive (Ichiye 2018; Marino et al. 2019). Among the Archaea, different species have been reported as barophiles. Examples are the anaerobic hyperthermophiles within the order Thermococcales: Thermococcus peptonophilus (Canganella et al. 1997; Canganella and Wiegel 2014), Pyrococcus horikoshii (Gonzalez et al. 1998), and Pyrococcus abyssi (Erauso et al. 1993) that show enhanced growth under high pressure. Another example is the case of Methanopyrus kandleri strain 116, which can grow up to 122 °C under increased pressure (Takai et al. 2008) so that the water can be maintained as a liquid at such high temperatures.
Barophiles: microorganisms growing under high-pressure
The remote ocean climate is described by high pressing factors and low temperature, yet nearby aqueous vent locales of amazingly high temperature exist. Remote ocean microorganisms have extraordinarily adapted features that empower them to live and thrive in this limited climate (Verde et al. 2016). Ongoing examination of the physiology and atomic science of remote ocean barophilic microorganisms has recognized pressing factor-managed operons and shown that microbial development is impacted by the connection among temperature and pressing factors in the remote ocean climate (Horikoshi 1999; Kato 1999; Pham et al. 2019).
Kato and his colleagues thought about living things in the Mariana Trench, which is the deepest place in the ocean on Earth. It is about 11,000 m down. Genuine sea residue was obtained by the automated Japanese submersible Kaiko (Kato et al. 1998). These researchers deciphered a fraction of these microbial communities, concluding several features. The microbial species detected belong mostly to the genera Shewanella DB21MT-2 and Moritella DB21MT-5 (Nogi et al. 1998; Kato et al. 1998). The least pressing factor for development was 50 MPa, and development up to 70–80 MPa was noted. Microbes at this depth additionally have a psychrophilic character given the low water temperatures. The researcher’s expanded unsaturated fat structure was recognized by gas-fluid chromatography and mass spectrometry in the two species. The most well-known unsaturated fats in the barophiles were a) hexadecenoic acid (16:1), b) eicosapentaenoic acid (EPA) (20:5), and c) docosahexaenoic acid (DHA) (22:6) (Kato et al. 1998). This investigation demonstrated the occurrence of microbial activity and singular responses at the deepest ocean floors.
Xerophiles and xerotolerant microorganisms: survivors of dry places
Numerous microorganisms have adapted to survive in environments in which water is scarce; these organisms are termed xerotolerant bacteria (Fig. 1) (Suzina et al. 2022). Burkholderia grimmiae R27, isolated from xerophilous moss (Grimmia montana) grown on a large stone in China, is capable of growth under conditions of minimum life-supporting water activity. It is resistant to toxic metals such as cadmium (Cd), chromium (Cr), copper (Cu), selenium (Se), mercury (Hg), and tellurium (Te) (Tian et al. 2013). It is environmental, physiological, and molecular adaptations that enable xerotolerant bacteria to survive in different environments. Bacterial cells that are getting too dry lose cytoplasmic volume because their surface area and capsular layer shrink (Suzina et al. 2022). This loss of water inside the cell lowers the turgor pressure, which increases the number of metabolites and ions inside the cell, concentrates macromolecules, and makes the cell less fluid (Filipović et al. 2020). When there are more van der Waals interactions between phospholipids, the membrane becomes less stable. Most of the time, water molecules attached to phosphate head groups weaken these interactions. Changes in protein function, biosynthesis, transport, and repair result in the formation of free radicals such as O-2 (Alberts et al. 2002; Bogdanov et al. 2008; Cooper and McNeil 2015).
Changes in the gel-to-liquid transition temperature that are not the same everywhere lead to membrane fusion and rupture, changes in the structure of the lamellae, protein clumping, and large molecules leaking out. Therefore, dehydration causes DNA damage and activates the DNA repair and protection pathways. When reactive oxygen species build up inside a cell, it leads to oxidative stress and cell death (Lebre et al. 2017). To avoid cell dysfunction and cell death, xerophilic bacteria possess molecular mechanisms to protect their cells and macromolecules. Protection of cells and macromolecules was ensured by intracellular accumulation of late embryogenesis abundant (LEA) proteins that perform pleiotropic roles (Liu et al. 2021).
Radiophiles: radiation-resistant bacteria
In inhospitable environments, a group of microorganisms thrive in the presence of radiation (ultraviolet light, gamma rays, and X-rays) and huge oxidative stress, termed radiophiles or radiation-resistant extremophiles (Figs. 1 and 4). Radiophiles produce primary and secondary metabolic products that can protect the DNA of an organism (Sysoev et al. 2021; Basu 2022). Therefore, extremolytes (electrolytes either produced or contained in extremophile cells) of these microorganisms can be used in the formulation of anticancer drugs, antioxidant-containing products, and commercial skin protectants (sunscreens), such as mycosporin-like amino acids from red algal species Porphyra rosengurttii. Methylobacterium terricola 17Sr1-39 (Kim et al. 2020), Hymenobacter armeniacus BT189, Hymenobacter montanus BT664 (Park et al. 2022), and Sphingomonas radiodurans S9-5 (Liu et al. 2022) were recently found in gamma-irradiated soil from the Republic of Korea and Mount Everest. Moreover, deinoxanthin isolated from Deinococcus radiodurans and bacterioruberin from Rubrobacter spp. and Halobacterium spp., etc., are potential drugs that can be used to treat cancer (Sysoev et al. 2021). Deinococcus radiodurans ATCC 13939 and Deinococcus gobiensis I-0 were reported as the most radiation-tolerant species that can grow in the presence of 15 kGy and 14 kGy gamma rays. These microbial species have a role in DNA damage repair (Brooks and Murray 1981; Yuan et al. 2009).
Polyextremophiles
Microorganisms in their characteristic living environments are thought to face a number of limiting factors and pressures during their life cycle. Numerous extremophiles possess conditions with more than one limit boundary (Mesbah and Wiegel 2012), for instance, extremophiles that flourish in the profundity of the seas or near underground aquifers (Kato and Takai 2000). In the primary circumstance, if the extremophiles are found in deep-sea mud, they could be piezophiles and psychrophiles. They could be piezophiles and thermophiles if they inhabit a deep-sea hydrothermal vent (Poli et al. 2017; Merlino et al. 2018). With an end goal to give a complete gander at the limits of temperature and pH, researchers arranged more than 200 extremophile species found in the writing. They are called thermoacidophiles, thermoalkaliphiles, psychroacidophiles, and psychroalkaliphiles. Since film smoothness diminishes at low temperatures, the lower penetrability to protons (H+) benefits the growth of acidophiles and alkaliphiles (Dalmaso et al. 2015; Varrella et al. 2020).
Important molecules from extremophilic prokaryotes
Biomolecules of different types or functions are looked for in extremophilic microorganisms (Fig. 4), in addition to the large number of enzymes that have a wide range of functions and unique properties.
Retinal proteins
Halophiles like Halobacterium are a major source for bacteriorhodopsins. It has been observed that an haloarchaeon contains rhodopsins (Fendrihan et al. 2006). Bacteriorhodopsins are simple natural transducers that capture sunlight, forming electrochemical potential. This process was first investigated in the 1970s (Saeedi et al. 2012). Bacteriorhodopsins are used in a number of applications because of their chemical properties, such as their stability at a broad range of temperatures and pH levels, for example in bio-hybrid electronic devices (Li et al. 2018). These characteristics showed the various applications of the bacteriorhodopsins, like how they could be used for nonlinear optics, as sensors, and to handle optical information (Li et al. 2018). NASA also used a bacteriorhodopsin film to illuminate the distance of gas spill locations (https://astrobiology.nasa.gov/news/terrestrial-powerhouses/). So all these applications showed that bacteriorhodopsins are being used in modern robots.
Compatible solutes
Halophiles can retain water inside the cell by producing osmolytes to maintain osmolality. Most of the halophilic microbes usually present high concentrations of osmolytes in the cytoplasm (Roberts 2005). The osmolytes maintain the pH of molecules. However, in order to comprehend a fundamental mechanism like cell size regulation, the electrical impacts of ion fluxes need to become an integral component of cell biology (Kay 2017).The osmolytes are generally composed of amino acids and polyols. The halophilic and halotolerant species are zwitterionic. Halophiles have been used for cosmetics, and the solutes have been proposed as stabilizers during DNA amplification routines by PCR (Harding et al. 2016). The biosynthetic pathway in Ectothiorhodospira halochloris has been transformed into Escherichia coli to produce betaine and enhance salt-tolerant efficiency (León et al. 2018). Similarly, halophilic Dunaliella salina is now used as a wellspring of glycerol, as a conditioner, as a defensive material (Monte et al. 2020), and as a functional nutrition supplement, e.g., lithium (I) bio-fortified Dunaliella salina (Yüce et al. 2021).
Hereditary transfer of salt tolerance and resistance
The salt tainting can be seen in farming areas, which cover up to ¼ of the 33% of land used for farming due to excessive use of water systems. Most of the plants are highly sensitive to salt, which leads to changes in osmotic pressure and a decrease in the plant's capacity to absorb water (Shrivastava and Kumar 2015). The sodium proton antiporter characteristics are inherited by plants, resulting in a salt-sensitive response by the plant. Salt resistance in plants at levels similar to the sea (530 mM) could be enhanced by importing systems that have evolved in halophilic microorganisms, which could really improve the agricultural use of increased salt in extensive arable lands (Rust and Ekmekcioglu 2016).
Potential applications of extremophiles and extremozymes
Thermostable enzymes vs. cold-active enzymes
The need for thermostable enzymes has skyrocketed as a result of increasing industrialization. The rise in demand can be attributed to the thermostability and practicality of enzymes that can withstand high temperatures during the bioprocessing process in industries (Gavande et al. 2021). Some of the industrially important enzymes are mentioned below (Table 1).
Table 1.
Important examples of extremophilic bacteria and archaea with adopted mechanisms and potential applications
| Industry | Extremozyme | Brand name/Company | Specific uses | |
|---|---|---|---|---|
| Detergents | Amylases | Purafect® OxAm (Genencor), Stainzyme® Plus, Preferenz™ S100 (DuPont) | Degradation of starch-based stains | Sarmiento et al. (2015) |
| Cellulases | Rocksoft™ Antartic, Antartic LTC (Dyadic), UTA-88 and UTA-90 (Hunan Youtell Biochemical), Retrocell Recop and Retrocell ZircoN (EpyGen Biotech), Celluzyme®, Celluclean® (Novozymes) | Cotton fabric washing and cleaning cotton cloths | ||
| Lipases | Lipoclean®, Lipex®, Lipolase® Ultra, Kannase, Liquanase®, Polarzyme® (Novozymes) | Degradation of lipid-based stains | ||
| Mannanases | Mannaway® (Novozymes), Effectenz™ (DuPont) | Mannan-gum degradation and removal | ||
| Pectate lyases | XPect® (Novozymes) | Removel of pectin-containing stains | ||
| Proteases | Purafect® Prima, Properase®, Excellase (Genencor) | Degradation protein-based strains | ||
| Textile | Amylases | Optisize®, COOL, Optisize® NEXT (Genencor/DuPont) | Desizing of woven fabrics | |
| Cellulases | Primafast® GOLD HSL, IndiAge® NeutraFlex, PrimaGreen®, EcoLight 1, EcoFade LT100 (Genencor/DuPont) | Biofinishing of cellulosic fibers may combined with dyeing | ||
| Molecular Biology | Alkaline phosphatases | Antartic phosphatases (New England Biolabs Inc) | Dephosphorylation of 5’ end of a linearized DNA fragment | |
| Uracil-DNA-N-Glycosylases | Uracil-DNA-N-Glycosylases (UNG), (ArticZymes), Antartic Thermolabile UDG (New England Biolabs Inc.) | Release of free-form of Uracil from Uracil containing DNA | ||
| Food & beverages | Pectinases | Novoshape® (Novozymes), Pectinase 62L (Biocatalysts), Lallzyme® (Lallemand) | Uses in brewery, wine, bread-making and fruit juice processing | |
| Textile, Research and cosmetics | Catalase | Catalase (CAT) (Swissaustral) | Fiber treatment and beauty products | |
|
Sequencing & Next-generation sequencing |
DNA Polymerase | Q5® High-Fidelity DNA Polymerases (New England Biolabs), MightyAmp, Takara bio (Tokyo), KOD FX, Toyobo (Tokyo), BIOTAQ (Bioline), Taq DNA polymerase (Go Taq Flexi, Promega), Hemo KlenTaq (New England Biolabs), KAPA Blood (KAPA Biosystems), Phusion Blood II (Thermo Fisher Scientific) | PCR, Highest fidelity amplification | Miura et al. (2013) |
Some examples of commercially available thermostable extremozymes
Taq DNA polymerases
In the past few decades, there have been major advancements in the discovery and use of DNA polymerases for DNA amplification reactions. Among them, the best known is the polymerase chain reaction (PCR). In a commercial PCR kit, Taq DNA polymerase is the primary thermostable enzyme. The Taq polymerase shows exonuclease activity in the 5’ to 3’ direction, whereas no activity was shown at 3’ to 5’, leading to a relatively low fidelity insertion rate that carries a measurable error rate. Although PCR has developed rapidly, the problem of increasing fidelity has been of interest (Wang et al. 2022). For example, among other alternatives, the use of Pfu DNA polymerase (an enzyme obtained from the hyperthermophile Pyrococcus furiosus) greatly increases fidelity during amplification by PCR (Kim et al. 2008).
Thermophilic proteases
Proteases are the enzymes that are widely used to decompose other proteins, classified as exopeptidases and endopeptidases (Walter et al 1980). The protease enzymes constitute 20% of worldwide industrial enzyme production (Razzaq et al. 2019). These enzymes are primarily used in the food, pharmaceutical, textile, and leather industries to meet the need for thermostable proteases that can withstand harsh conditions during biotechnological processes (Šnajder et al. 2019). Therefore, there is a broad market and demand for highly thermostable proteases.
Starch-degrading enzymes
The hydrolysis and modification of the commercial staple by the starch industry requires 30% amylolytic enzymes (van der Maarel et al. 2002; Park et al. 2017). The process of starch hydrolysis involves glucoamylases (Prajapati et al. 2014), isoamylases (Rahman et al. 1998), and alpha-amylases (El-Fallal et al. 2012). The amylolytic enzymes are classified as endo- and exoacting. Endo-acting enzymes hydrolyze glycosidic linkages and result in the formation of oligosaccharides, while exo-acting enzymes lead to the formation of oligo- and monosaccharides. For the economy and efficacy of processes, the enzymatic conversion of starch can be done by gelatinization and saccharification by using the alpha-amylases at high temperatures of 100–110 °C (gelatinization) and 80–90 °C (liquefaction) (de Souza and de Oliveira Magalhães 2010; Mehta and Satyanarayana 2016). Therefore, the latest technology is required for enzymatic operation in the scientific and industrial fields.
Cellulose degrading enzymes
Cellulose is one of the most abundant organic compounds on the planet and is often used as a raw material source in the production of biofuels, feed, and as a building-block chemical. Cellulose has more hydrolyzing properties as compared to starch due to the difference in crystalline form. The enzymes that are mostly used for cellulose hydrolysis are exoglucanases and endoglucanases (Wu et al. 2018; Escuder-Rodríguez et al. 2018). These enzymes have many applications in the industry, like being used as a component in detergents, coloring materials, enhancing the nutritional quality of materials, washing jeans, etc.
Xylan degrading enzymes
Xylan is a hemicellulosic compound that has great applications in the paper and pulp industries (Nechita et al. 2021). The xylanases used in the paper and pulp industries should be thermostable in order to tolerate the high temperature and high pH during biomass processing (Verma 2021). The paper and pulp industries are growing at a fast pace, so we have to focus on the high yield production of xylanases to meet the demand of these industries (Bhardwaj et al. 2021; Joshi et al. 2022). To meet this demand nowadays, metagenomic analysis is focused on isolating non-cultivable microorganisms from man-made and natural habitats (Rabee et al. 2022; Wei et al. 2022).
Lipid degrading enzymes
Lipases are versatile and can lead to bioconversion reactions in many ways, like hydrolysis, inter-esterification, acidolysis, alcoholysis, and amylolysis. Thermophilic lipases should exhibit distinctive qualities such as stereo-specificity, substrate specificity, and heterogeneous reaction catalysis (Royter et al. 2009). Nonetheless, more study is urgently required to properly comprehend each of these elements. The generated esters are used to give flavor and aroma to food in the food industry (Kumar Verma et al. 2022). Along with this, the ester’s long chains are used as fuel, lubricants, and additives in cosmetics. There is another important application of ester like pitch removal from the pulp in paper industries, impurities removal in non-cellulosic substrates, fats hydrolysis in dairy industries, and synthesis of novel drugs in pharmaceutical industries (Guo et al. 2021; Yıldırım and Avci 2022). For economic stability, the thermostable lipase enzymes are used so that they can work at 50 °C. Although many researchers have found some lipases that can operate at 100 °C, generally they have a short life span at high temperatures (Borrelli and Trono 2015; Chandra et al. 2020).
Conclusion
Extremophiles, the organisms that are capable of surviving and thriving under extreme environmental conditions, have become an exciting field of research for scientists. Extremophiles are capable of meeting novel standards required for sustainability through their applications and goods, but they are generally difficult to be cultured. The study of these organisms has revealed several unique mechanisms, e.g., production of specialized enzymes that can function in extreme temperatures, pH levels, and pressures, production of protective proteins that help the organism resist harsh conditions, formation of biofilms that help the organism resist desiccation and other extreme conditions, ability to adjust membrane fluidity in response to changes in temperature and pressure, production of pigments that protect against UV radiation, development of heat shock proteins that help the organism recover from exposure to high temperatures, utilization of alternative metabolic pathways that allow the organism to function in the absence of oxygen, production of compatible solutes that help the organism maintain water balance in high-salt environments, utilization of unique lipids in their cell membranes that maintain fluidity in extreme conditions and use of molecular chaperones that help proteins maintain their proper shape in extreme environments, that they employ to survive in harsh environments. One of the most significant mechanisms found in extremophiles is the production of specialized enzymes that can function at extreme temperatures, pH levels, and pressures. These enzymes have found applications in several industrial processes, including bioremediation and bioprocessing. Other mechanisms found in extremophiles include the production of protective proteins and the formation of biofilms that help them resist desiccation and other harsh conditions. These mechanisms have significant potential for developing new biotechnological and medical applications. Extremophiles have also provided important insights into the evolution of life on Earth. By studying the genetic material of these organisms, scientists have been able to understand the adaptive strategies they use to survive in different environments. The applications of extremophiles are numerous, and they are increasingly being used in several areas of research. For example, the study of extremophiles has led to the development of new technologies for treating wastewater, producing biofuels, and manufacturing pharmaceuticals. In conclusion, the study of extremophiles has provided unique insights into the mechanisms they use to survive and adapt to harsh environments. These mechanisms have found applications in several industrial processes and are increasingly being studied for medical applications. This review aims to provide a better understanding of the features of extremophiles and their potential biotechnological and medicinal applications resulting from this knowledge. Furthermore, the study of extremophiles has provided important insights into the evolution of life on Earth. As research in this area continues, we can expect to see further applications and benefits from the study of these remarkable organisms. Thus, use of “multi-omics” approaches help in a better ways to know functions of genes, characteristics, and to find newer applications.
Acknowledgements
This research was supported by Key-Area Research and Development Program of Guangdong Province (2022B0202110001), National Natural Science Foundation of China (Nos: 31972856 and 32061143043) and Yenepoya (Deemed to be University), India (No. YU/SeedGrant/104-2021). All authors duly acknowledge Dr. Manik Prabhu Narsing Rao (former affiliation: Sun Yat-Sen University, Guangzhou 510275, PR China) for his advice during preparation of the manuscript.
Data Availability
Data availability is not applicable.
Declarations
Conflict of interest
The authors declare that they had no conflict of interest.
Ethical approval
This article does not contain any studies related to human participants or animals.
Contributor Information
Bhagwan Narayan Rekadwad, Email: rekadwad@gmail.com, Email: microbeai3.0@gmail.com.
Wen-Jun Li, Email: liwenjun3@mail.sysu.edu.cn.
Juan M. Gonzalez, Email: jmgrau@irnase.csic.es
References
- Achberger AM, Christner BC, Michaud AB, et al. Microbial Community Structure of Subglacial Lake Whillans. West Antarctica Front Microbiol. 2016;22(7):1457. doi: 10.3389/fmicb.2016.01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera A. Eukaryotic organisms in extreme acidic environments the Rio Tinto Case. Life. 2013;3:363–374. doi: 10.3390/life3030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajeje SB, Hu Y, Song G, et al. Thermostable cellulases/xylanases from thermophilic and hyperthermophilic microorganisms: current perspective. Front Bioeng Biotechnol. 2021;9:794304. doi: 10.3389/fbioe.2021.794304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers SV, van de Vossenberg JL, Driessen AJ, Konings WN. Adaptations of the archaeal cell membrane to heat stress. Front Biosci. 2000;5:D813–D820. doi: 10.2741/albers. [DOI] [PubMed] [Google Scholar]
- Albers SV, van de Vossenberg JL, Driessen AJ, Konings WN. Bioenergetics and solute uptake under extreme conditions. Extremophiles. 2001;5:285–294. doi: 10.1007/s007920100214. [DOI] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, et al. Molecular biology of the cell. 4. New York: Garland Science; 2002. [Google Scholar]
- Amils R, Fernandez-Remolar D, The IPBSL Team Rio Tinto: a geochemical and mineralogical terrestrial analogue of Mars. Life. 2014;4:511–534. doi: 10.3390/life4030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin K, Tranchimand S, Benvegnu T, et al. Glycoside hydrolases and glycosyltransferases from hyperthermophilic Archaea: insights on their characteristics and applications in biotechnology. Biomolecules. 2021;11:1557. doi: 10.3390/biom11111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Austin C, Dopson M. Life in acid: pH homeostasis in acidophiles. Trends Microbiol. 2007;15:165–171. doi: 10.1016/j.tim.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Basu B. The radiophiles of Deinococcaceae family: resourceful microbes for innovative biotechnological applications. Curr Res Microb Sci. 2022;3:100153. doi: 10.1016/j.crmicr.2022.100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellenberg S, Salas B, Ganji S, et al. Diffusible signal factor signaling controls bioleaching activity and niche protection in the acidophilic mineral-oxidizing leptospirilli. Sci Rep. 2021;11:16275. doi: 10.1038/s41598-021-95324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender GR, Marquis RE. Spore heat resistance and specific mineralization. Appl Environ Microbiol. 1985;50:1415–1421. doi: 10.1128/aem.50.6.1414-1421.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj N, Kumar B, Agrawal K, Verma P. Current perspective on production and applications of microbial cellulases: a review. Bioresour Bioprocess. 2021;8:95. doi: 10.1186/s40643-021-00447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blöchl E, Rachel R, Burggraf S, et al. Pyrolobus fumarii gen, and sp, nov, represents a novel group of Archaea extending the upper temperature limit for life to 113 degrees C. Extremophiles. 1997;1:14–21. doi: 10.1007/s007920050010. [DOI] [PubMed] [Google Scholar]
- Bogdanov M, Mileykovskaya E, Dowhan W. Lipids in the assembly of membrane proteins and organization of protein supercomplexes: implications for lipid-linked disorders. Subcell Biochem. 2008;49:197–239. doi: 10.1007/978-1-4020-8831-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölter M. Ecophysiology of psychrophilic and psychrotolerant microorganisms. Cell Mol Biol (Noisy-le-grand) 2004;50:563–573. [PubMed] [Google Scholar]
- Borrelli GM, Trono D. Recombinant lipases and phospholipases and their use as biocatalysts for industrial applications. Int J Mol Sci. 2015;16:20774–20840. doi: 10.3390/ijms160920774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouacem K, Amziane-Touazi M, Ben Hania W, et al. Isolation and characterization of moderately thermophilic aerobic cultivable bacteria from Hammam Righa Hot Spring (Algeria): description of their hydrolytic capacities. Algerian J Environ Sci Technol. 2022;8:2524–2536. [Google Scholar]
- Bowman JP, McCammon SA, Lewis T et al (1998) Psychroflexus torquis gen. nov., sp. nov., a psychrophilic species from Antarctic sea ice, and reclassification of Flavobacterium gondwanense (Dobson et al. 1993) as Psychroflexus gondwanense gen. nov., comb. nov. Microbiology (Reading) 144: 1601–1609. [DOI] [PubMed]
- Brinkmeyer R, Glöckner F-O, Helmke E. Predominance of β-proteobacteria in summer melt pools on Arctic pack ice. Limnol Oceanogr. 2004;49:1013–1021. doi: 10.4319/lo.2004.49.4.1013. [DOI] [Google Scholar]
- Brock TD. The value of basic research: discovery of Thermus aquaticus and other extreme thermophiles. Genetics. 1997;146:1207–1210. doi: 10.1093/genetics/146.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BW, Murray RGE. Nomenclature for "Micrococcus radiodurans" and other radiation-resistant cocci: Deinococcaceae fam, nov., and Deinococcus gen, nov., including five species. Int J Syst Bacteriol. 1981;31:353–360. doi: 10.1099/00207713-31-3-353. [DOI] [Google Scholar]
- Bueno de Mesquita CP, Zhou J, Theroux SM, Tringe SG. Methanogenesis and salt tolerance genes of a novel halophilic Methanosarcinaceae metagenome-assembled genome from a former solar saltern. Genes. 2021;12:1609. doi: 10.3390/genes12101609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callac N, Oger P, Lesongeur F, et al. Pyrococcus kukulkanii sp, nov, a hyperthermophilic piezophilic archaeon isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2016;66:3142–3149. doi: 10.1099/ijsem.0.001160. [DOI] [PubMed] [Google Scholar]
- Canganella F, Wiegel J. Anaerobic Thermophiles. Life. 2014;4:77–104. doi: 10.3390/life4010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canganella F, Gonzalez JM, Yanagibayashim M, et al. Pressure and temperature effects on growth and viability of the hyperthermophilic archaeon Thermococcus peptonophilus. Arch Microbiol. 1997;168:1–7. doi: 10.1007/s002030050462. [DOI] [PubMed] [Google Scholar]
- Cardona C, Gil-Cruz F, Franco-Marín L, et al. Volcanic activity accompanying the emplacement of dacitic lava domes and effusion of lava flows at Nevados de Chillán Volcanic Complex – Chilean Andes (2012) to (2020) J Volcanol Geother Res. 2021;420:107409. doi: 10.1016/j.jvolgeores.2021.107409. [DOI] [Google Scholar]
- Cariello NF, Swenberg JA, Skopek TR. Fidelity of Thermococcus litoralis DNA polymerase (Vent) in PCR determined by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1991;19:4193–4198. doi: 10.1093/nar/19.15.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra P, Enespa Singh R, Arora PK. Microbial lipases and their industrial applications: a comprehensive review. Microb Cell Fact. 2020;19:169. doi: 10.1186/s12934-020-01428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BC, Kolb VM. Macrobiont: cradle for the origin of life and creation of a biosphere. Life. 2020;10:278. doi: 10.3390/life10110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker JA. Extremophiles and biotechnology: current uses and prospects. F1000Res. 2016;5:396. doi: 10.12688/f1000research.7432.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker JA (2019) Recent advances in understanding extremophiles. F1000Res 8:F1000 Faculty Rev-1917
- Collins RE, Rocap G, Deming JW. Persistence of bacterial and archaeal communities in sea ice through an Arctic winter. Environ Microbiol. 2010;12:1828–1841. doi: 10.1111/j.1462-2920.2010.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cono V, Smedile F, Crisafi F, et al. Wintertime simulations induce changes in the structure diversity and function of antarctic sea ice-associated microbial communities. Microorganisms. 2022;10:623. doi: 10.3390/microorganisms10030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook GM, Russell JB, Reichert A, Wiegel J. The Intracellular pH of Clostridium paradoxum, an anaerobic, alkaliphilic, and thermophilic bacterium. Appl Environ Microbiol. 1996;62(12):4576–4579. doi: 10.1128/aem.62.12.4576-4579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ST, McNeil PL. Membrane repair: mechanisms and pathophysiology. Physiol Rev. 2015;95:1205–1240. doi: 10.1152/physrev.00037.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuecas A, Cruces J, Galisteo-Lopex JF, et al. Cellular viscosity in prokaryotes and thermal stability of low molecular weight biomolecules. Biophy J. 2016;111:875–882. doi: 10.1016/j.bpj.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe MA, Power JF, Morgan XC, et al. Pyrinomonas methylaliphatogenes gen. nov., sp. nov., a novel group 4 thermophilic member of the phylum Acidobacteria from geothermal soils. Int J Syst Evol Microbiol. 2014;64:220–227. doi: 10.1099/ijs.0.055079-0. [DOI] [PubMed] [Google Scholar]
- Cuecas A, Portillo MC, Kanoksilapatham W, Gonzalez JM. Bacterial distribution along a 50 ºC temperature gradient reveals a parceled out hot spring environment. Microb Ecol. 2014;68:729–739. doi: 10.1007/s00248-014-0437-y. [DOI] [PubMed] [Google Scholar]
- Dalmaso GZ, Ferreira D, Vermelho AB. Marine extremophiles: a source of hydrolases for biotechnological applications. Mar Drugs. 2015;13:1925–1965. doi: 10.3390/md13041925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damer B, Deamer D. The hot spring hypothesis for an origin of life. Astrobiology. 2020;20:429–452. doi: 10.1089/ast.2019.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel RM, Cowan DA. Biomolecular stability and life at high temperatures. Cell Mol Life Sci. 2000;57:250–264. doi: 10.1007/PL00000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza PM, de Oliveira MP. Application of microbial α-amylase in industry—a review. Braz J Microbiol. 2010;41:850–861. doi: 10.1590/S1517-83822010000400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Zhang Y, Wang H, et al. Microbial community structure of deep-sea hydrothermal vents on the ultraslow spreading southwest Indian ridge. Front Microbiol. 2017;8:1012. doi: 10.3389/fmicb.2017.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirmeier R, Keller M, Hafenbradl D, Braun FJ, Rachel R, Burggraf S, Stetter KO. Thermococcus acidaminovorans sp. nov., a new hyperthermophilic alkalophilic archaeon growing on amino acids. Extremophiles. 1998;2:109–114. doi: 10.1007/s007920050049. [DOI] [PubMed] [Google Scholar]
- Driks A, Eichenberger P. The bacterial spore: from molecules to systems. Washington, DC: Wiley; 2016. pp. 1–236. [Google Scholar]
- Dutta B, Bandopadhyay R. Biotechnological potentials of halophilic microorganisms and their impact on mankind. Beni-Suef Univ J Basic Appl Sci. 2022;11:75. doi: 10.1186/s43088-022-00252-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Fallal A, Dobara MA, El-Sayed A, and Omar N (2012) Starch and microbial α-amylases: from concepts to biotechnological applications. In: (Ed.) Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology. IntechOpen. 10.5772/51571
- Erauso G, Reysenbach AL, Godfroy A, et al. Pyrococcus abyssi sp. nov., a new hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Arch Microbiol. 1993;160:338–349. doi: 10.1007/BF00252219. [DOI] [Google Scholar]
- Escuder-Rodríguez JJ, DeCastro ME, Cerdán ME, et al. Cellulases from thermophiles found by metagenomics. Microorganisms. 2018;6:66. doi: 10.3390/microorganisms6030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etten JV, Cho CH, Joon HS, Bhattacharya D. Extremophilic red algae as models for understanding adaptation to hostile environments and the evolution of eukaryotic life on the early earth. Semin Cell Dev Biol. 2022 doi: 10.1016/jsemcdb(2022)03007. [DOI] [PubMed] [Google Scholar]
- Fang J, Kato C, Runko GM, et al. Predominance of viable spore-forming piezophilic bacteria in high-pressure enrichment cultures from ~15 to 24 km-deep coal-bearing sediments below the ocean floor. Front Microbiol. 2017;8:137. doi: 10.3389/fmicb.2017.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrihan S, Legat A, Pfaffenhuemer M, et al. Extremely halophilic archaea and the issue of long-term microbial survival. Rev Environ Sci Biotech. 2006;5:203–218. doi: 10.1007/s11157-006-0007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipović A (2020) Water plant and soil relation under stress situations. In: Meena RS, Datta R (eds) Soil moisture importance. IntechOpen 10.5772/intechopen93528
- Franco-Duarte R, Černáková L, Kadam S, et al. Advances in chemical and biological methods to identify microorganisms-from past to present. Microorganisms. 2019;7:130. doi: 10.3390/microorganisms7050130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galagan JE, Nusbaum C, Roy A, et al. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 2002;12:532–542. doi: 10.1101/gr.223902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Qu L, Du G. Bacterial and archaeal communities in deep sea waters near the Ninetyeast Ridge in Indian Ocean. J Oceanol Limnol. 2021;39:582–597. doi: 10.1007/s00343-020-9343-y. [DOI] [Google Scholar]
- Gao X, Kong JE, Zhu H, Mao B, Cui S, Zhao J. Lactobacillus, Bifidobacterium and Lactococcus response to environmental stress: mechanisms and application of cross-protection to improve resistance against freeze-drying. J Appl Microbiol. 2022;132(2):802–821. doi: 10.1111/jam.15251. [DOI] [PubMed] [Google Scholar]
- Garrison DL, Sullivan CW, Ackley SF. Sea Ice microbial communities in Antarctica. BioSci. 1986;36:243–250. doi: 10.2307/1310214. [DOI] [Google Scholar]
- Gaughran ERL. The saturation of bacterial lipids as a function of temperature. J Bacteriol. 1947;53:506–509. [PubMed] [Google Scholar]
- Gavande PV, Basak A, Sen S, et al. Functional characterization of thermotolerant microbial consortium for lignocellulolytic enzymes with central role of Firmicutes in rice straw depolymerisation. Sci Rep. 2021;11:3032. doi: 10.1038/s41598-021-82163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt P, Marquis RE (1989) Spore thermoresistance mechanisms In: Smith I, Slepecky RA, Setlow P (eds) Regulation of Prokaryotic Development. American Society for Microbiology Washington DC, pp43–63
- Gonzalez JM. Molecular tunnels in enzymes and thermophily: a case study on the relationship to growth temperature. Microorganisms. 2018;6:1–10. doi: 10.3390/microorganisms6040109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Masuchi Y, Robb FT, et al. Pyrococcus horikoshii sp. nov., a hyperthermophilic archaeon isolated from a hydrothermal vent at the Okinawa Trough. Extremophiles. 1998;2:123–130. doi: 10.1007/s007920050051. [DOI] [PubMed] [Google Scholar]
- Gosink JJ, Herwig RP, Staley JT. Octadecabacter arcticus gen. nov., sp. nov., and O. antarcticus, sp. nov., Nonpigmented, Psychrophilic Gas Vacuolate Bacteria from Polar Sea Ice and Water. Syst Appl Microbiol. 1997;20:356–365. doi: 10.1016/S0723-2020(97)80003-3. [DOI] [Google Scholar]
- Gunda GKT, Champatiray PK, Chauhan M. Modelling of volcanic ash with HYSPLIT and satellite observations: a case study of the (2018) Barren Island volcano eruption event Andaman Territory India. Curr Sci. 2021;121:529–538. doi: 10.18520/cs/v121/i4/529-538. [DOI] [Google Scholar]
- Gunde-Cimerman N, Plemenitaš A, Oren A. Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol Rev. 2018;42:353–375. doi: 10.1093/femsre/fuy009. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wang RY, Kang JX. Efficient synthesis of primary and secondary amides via reacting esters with alkali metal amidoboranes. Nat Commun. 2021;12:5964. doi: 10.1038/s41467-021-25836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Khanna S. Alkaliphilic cyanobacteria: a review on diversity, ecology and applications. J Appl Phycol. 2019;31(3):1353–1368. [Google Scholar]
- Harding T, Brown MW, Simpson AG, Roger AJ. Osmoadaptative strategy and its molecular signature in obligately halophilic heterotrophic protists. Genome Biol Evol. 2016;8:2241–2258. doi: 10.1093/gbe/evw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich S, Schippers A. Distribution of acidophilic microorganisms in natural and man-made acidic environments. Curr Issues Mol Biol. 2021;40:25–48. doi: 10.21775/cimb.040.025. [DOI] [PubMed] [Google Scholar]
- Hirota K, Miura C, Motomura N. Isolation and identification of bacteria from high-temperature compost at temperatures exceeding 90 ºC. Afr J Microbiol Res. 2019;13:134–144. doi: 10.5897/AJMR2018.9022. [DOI] [Google Scholar]
- Holm NG, Cairns-Smith AG, Daniel RM, et al. Marine hydrothermal systems and the origin of life: future research. Orig Life Evol Biosph. 1992;22(1–4):181–242. doi: 10.1007/BF01808024. [DOI] [PubMed] [Google Scholar]
- Horikoshi K. Microorganisms in alkaline environments. Tokyo: Kodansha-VCH; 1991. [Google Scholar]
- Horikoshi K. Alkaliphiles: some applications of their products for biotechnology. Microbiol Mol Biol Rev. 1999;63:735–750. doi: 10.1128/MMBR.63.4.735-750.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi K. Alkaliphiles. Proc Jpn Acad Ser B Phys Biol Sci. 2004;80(4):166–178. doi: 10.2183/pjab.80.166. [DOI] [Google Scholar]
- Huang R, Pan M, Wan C, Shah NP, Tao X, Wei H. Physiological and transcriptional responses and cross protection of Lactobacillus plantarum ZDY2013 under acid stress. J Dairy Sci. 2016;99:1002–1010. doi: 10.3168/jds.2015-9993. [DOI] [PubMed] [Google Scholar]
- Hugenholtz P, Chuvochina M, Oren A. Prokaryotic taxonomy and nomenclature in the age of big sequence data. ISME J. 2021;15:1879–1892. doi: 10.1038/s41396-021-00941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiye T. Enzymes from piezophiles. Semin Cell Dev Biol. 2018;84:138–146. doi: 10.1016/j.semcdb.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irgens RL, Suzuki I, Staley JT. Gas vacuolate bacteria obtained from marine waters of Antarctica. Curr Microbiol. 1989;18:261–265. doi: 10.1007/BF01570303. [DOI] [Google Scholar]
- Irgens RL, Gosink JJ, and Staley JT (1996) Polaromonas vacuolata gen. nov., sp. nov., a psychrophilic, marine, gas vacuolate bacterium from Antarctica. Int J Syst Bacteriol 46(3): 822–826 [DOI] [PubMed]
- Jacob JH, Hussein EI, Shakhatreh MAK, Cornelison CT. Microbial community analysis of the hypersaline water of the Dead Sea using high-throughput amplicon sequencing. Microbiol Open. 2017;6:e00500. doi: 10.1002/mbo3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R. Stability and folding of ultrastable proteins: eye lens crystallins and enzymes from thermophiles. FASEB J. 1996;10:84–92. doi: 10.1096/fasebj.10.1.8566552. [DOI] [PubMed] [Google Scholar]
- Joshi JB, Priyadharshini R, Uthandi S. Glycosyl hydrolase 11 (xynA) gene with xylanase activity from thermophilic bacteria isolated from thermal springs. Microb Cell Fact. 2022;21:62. doi: 10.1186/s12934-022-01788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge K, Eicken H, Deming JW. Bacterial Activity at − 2 to − 20 degrees C in Arctic wintertime sea ice. Appl Environ Microbiol. 2004;70:550–557. doi: 10.1128/AEM.70.1.550-557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge K, Christner B, Staley JT (2011) Diversity of Psychrophilic Bacteria from Sea Ice—and Glacial Ice Communities In: Horikoshi K (eds) Extremophiles Handbook. Springer, Tokyo. 10.1007/978-4-431-53898-1_39
- Kajla S, Kumari R, Nagi GK. Microbial CO2 fixation and biotechnology in reducing industrial CO2 emissions. Arch Microbiol. 2022;204:149. doi: 10.1007/s00203-021-02677-w. [DOI] [PubMed] [Google Scholar]
- Kashyap AK, Dubey SK. Jain BP (2022) 1-Cyanobacterial diversity concerning the extreme environment and their bioprospecting. In: Singh P, Fillat M, Kumar A, editors. Cyanobacterial lifestyle and its applications in biotechnology. Academic Press; 2022. pp. 1–22. [Google Scholar]
- Kates MM, Moldoveanu NM, Stewart LC. On the revised structure of the major phospholipid of Halobacterium salinarium. BiochimBiophysActa. 1993;1169:46–53. doi: 10.1016/0005-2760(93)90080-s. [DOI] [PubMed] [Google Scholar]
- Kato C (1999) Barophiles (Piezophiles) In: Horikoshi K, Tsujii K (eds) Extremophiles in deep-sea. Environments. Springer, Tokyo. 10.1007/978-4-431-67925-7_5
- Kato C, Takai (2000) Microbial diversity of deep-sea extremophiles--Piezophiles Hyperthermophiles and subsurface microorganisms. Biol Sci Space 14: 341–52 [DOI] [PubMed]
- Kato C, Li L, Nogi Y, et al. Extremely barophilic bacteria isolated from the Mariana Trench Challenger Deep at a depth of 11000 meters. Appl Environ Microbiol. 1998;64:1510–1513. doi: 10.1128/AEM.64.4.1510-1513.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR. How cells can control their size by pumping ions. Front Cell Dev Biol. 2017;5:41. doi: 10.3389/fcell.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kengen S. Pyrococcus furiosus 30 years on. Micro Biotechnol. 2017;10:1441–1444. doi: 10.1111/1751-7915.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevbrin VV, Zhilina TN, Rainey FA, Zavarzin GA. Tindallia magadii gen gen. nov. sp. Novnov.: an alkaliphilic anaerobic ammonifier from soda lake deposits. Curr Microbiol. 1998;37:94–100. doi: 10.1007/s002849900345. [DOI] [PubMed] [Google Scholar]
- Khalil A. Screening and characterization of thermophilic bacteria (lipase cellulase and amylase producers) from hot springs in Saudi Arabia. J Food Agri Environ. 2011;9:672–675. [Google Scholar]
- Kim SW, Kim DU, Kim JK, Kang LW, Cho HS. Crystal structure of Pfu, the high fidelity DNA polymerase from Pyrococcus furiosus. Int J Biol Macromol. 2008;42(4):356–361. doi: 10.1016/j.ijbiomac.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Kim J, Chhetri G, Kim I, et al. Methylobacterium terricola sp. nov., a gamma radiation-resistant bacterium isolated from gamma ray-irradiated soil. Int J Syst Evol Microbiol. 2020;70:2449–2456. doi: 10.1099/ijsem.0.004054. [DOI] [PubMed] [Google Scholar]
- Kochhar N, Kavya IK, Shrivastava S, et al. Perspectives on the microorganism of extreme environments and their applications. Curr Res Microb Sci. 2022;3:100134. doi: 10.1016/j.crmicr.2022.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga Y, Morii H, Akagawa-Matsushita M, Ohga M. Correlation of polar lipid composition with 16S rRNA phylogeny in methanogens. Further analysis of lipid component parts. Biosci Biotechnol Biochem. 1998;62:230–236. doi: 10.1271/bbb.62.230. [DOI] [PubMed] [Google Scholar]
- Konings WN, Albers SV, Koning S, et al. The cell membrane plays a crucial role in survival of bacteria and archaea in extreme environments. Antonie Van Leeuwenhoek. 2022;81(1–4):61–72. doi: 10.1023/a:1020573408652. [DOI] [PubMed] [Google Scholar]
- Kumar R, Sood U, Kaur J, et al. The rising dominance of microbiology: what to expect in the next 15 years? Microb Biotechnol. 2021;15:110–128. doi: 10.1111/1751-7915.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Verma D, Gddoa T, Al-Sahlany S, et al. Recent trends in microbial flavour Compounds: A review on Chemistry synthesis mechanism and their application in food. Saudi J Biol Sci. 2022;29:1565–1576. doi: 10.1016/j.sjbs.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebre P, De Maayer P, Cowan D. Xerotolerant bacteria: surviving through a dry spell. Nat Rev Microbiol. 2017;15:285–296. doi: 10.1038/nrmicro.2017.16. [DOI] [PubMed] [Google Scholar]
- León MJ, Hoffmann T, Sánchez-Porro C, et al. Compatible solute synthesis and import by the moderate halophile Spiribacter salinus: physiology and genomics. Front Microbiol. 2018;9:108. doi: 10.3389/fmicb.2018.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leston S, Nunes M, Rosa J, et al. Prospection, collection, and preservation of marine samples. In: Rocha-Santos T, Duarte AC, et al., editors. Analysis of marine samples in search of bioactive compounds. Elsevier; 2014. pp. 15–34. [Google Scholar]
- Li H, Yang Q, Li J, et al. The impact of temperature on microbial diversity and AOA activity in the Tengchong Geothermal Field. China Sci Rep. 2015;5:17056. doi: 10.1038/srep17056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YT, Tian Y, Tian H, et al. A review on bacteriorhodopsin-based bioelectronic devices. Sensors. 2018;18:1368. doi: 10.3390/s18051368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew KJ, Liang CH, Lau YT, et al. Thermophiles and carbohydrate–active enzymes (CAZymes) in bioflm microbial consortia that decompose lignocellulosic plant litters at high temperatures. Sci Rep. 2022;12:2850. doi: 10.1038/s41598-022-06943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang C, Wang Z, et al. Pleiotropic roles of late embryogenesis abundant proteins of Deinococcus radiodurans against oxidation and desiccation. Comput Struct Biotechnol J. 2021;19:3407–3415. doi: 10.1016/j.csbj.2021.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen T, Cui X, et al. Sphingomonas radiodurans sp, nov., a novel radiation-resistant bacterium isolated from the north slope of Mount Everest. Int J Syst Evol Microbiol. 2022;72:005312. doi: 10.1099/ijsem.0.005312. [DOI] [PubMed] [Google Scholar]
- Lundberg KS, Shoemaker DD, Adams MWW, et al. High-fidelity amplification using a thermostable DNA polymerase isolated from Pyrococcus furiosus. Gene. 1991;108:1–6. doi: 10.1016/0378-1119(91)90480-Y. [DOI] [PubMed] [Google Scholar]
- Lv Z, Ding J, Wang H, Wan J, Chen Y, Liang L, Yu T, Wang Y, Wang F. Isolation of a Novel Thermophilic Methanogen and the Evolutionary History of the Class Methanobacteria. Biol. 2022;11(10):1514. doi: 10.3390/biology11101514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo MWFS, Cunha N, Carneiro JA. Marine organisms as a rich source of biologically active peptides. Front Mar Sci. 2021;8:667764. doi: 10.3389/fmars.2021.667764. [DOI] [Google Scholar]
- MacElroy RD. Some comments on the evolution of extremophiles. Biosystems. 1974;6:74–75. doi: 10.1016/0303-2647(74)90026-4. [DOI] [Google Scholar]
- Madigan MM, Bender KS, Buckley DH (2020) Brock biology of microorganisms, 16th edn. Pearson
- Mani K, Taib N, Hugoni M. Transient dynamics of Archaea and Bacteria in sediments and brine across a salinity gradient in a Solar Saltern of Goa. India Front Microbiol. 2020;11:1891. doi: 10.3389/fmicb.2020.01891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marathe IA, Lai SM, Zahurancik WJ. Protein cofactors and substrate influence Mg2+-dependent structural changes in the catalytic RNA of archaeal RNase P. Nucleic Acids Res. 2021;49:9444–9458. doi: 10.1093/nar/gkab655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguet E, Forterre P. Protection of DNA by salts against thermodegradation at temperatures typical for hyperthermophiles. Extremophiles. 1998;2:115–122. doi: 10.1007/s007920050050. [DOI] [PubMed] [Google Scholar]
- Marsh WS, Heise BW, Krzmarzick MJ. Isolation and characterization of a halophilic Modicisalibacter sp. strain Wilcox from produced water. Sci Rep. 2021;11:6943. doi: 10.1038/s41598-021-86196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, McMinn A. Sea ice, extremophiles and life on extra-terrestrial ocean worlds. Int J Astrobiol. 2018;17(1):1–16. doi: 10.1017/S1473550416000483. [DOI] [Google Scholar]
- Martins A, Tenreiro T, Andrade G, et al. Photoprotective bioactivity present in a unique marine bacteria collection from portuguese deep sea hydrothermal vents. Mar Drugs. 2013;11:1506–1523. doi: 10.3390/md11051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Satyanarayana T. Bacterial and archaeal α-amylases: diversity and amelioration of the desirable characteristics for industrial applications. Front Microbiol. 2016;7:1129. doi: 10.3389/fmicb.2016.01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino N, Aronson HS, Bojanova DP. Living at the extremes: extremophiles and the limits of life in a planetary context. Front Microbiol. 2019;10:780. doi: 10.3389/fmicb.2019.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlino G, Barozzi A, Michoud G. Microbial ecology of deep-sea hypersaline anoxic basins. FEMS Microbiol Ecol. 2018;94:fiy085. doi: 10.1093/femsec/fiy085. [DOI] [PubMed] [Google Scholar]
- Mesbah NM, Wiegel J. Life under multiple extreme conditions: diversity and physiology of the halophilic alkalithermophiles. Appl Environ Microbiol. 2012;78:4074–4082. doi: 10.1128/AEM.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MicroSoc (2022) Microbiology Society. https://www.microbiologyresearchorg/ assessed on (2022)-07–23 at 11:32:04
- Miura M, Tanigawa C, Fujii Y, Kaneko S. Comparison of six commercially-available DNA polymerases for direct PCR. Rev Inst Med Trop Sao Paulo. 2013;55:401–106. doi: 10.1590/S0036-46652013000600005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad BT, Al Daghistani HI, Jaouani A, et al. Isolation and characterization of thermophilic bacteria from Jordanian hot springs: Bacillus licheniformis and Thermomonas hydrothermalis isolates as potential producers of thermostable enzymes. Int J Microbiol. 2017;2017:6943952. doi: 10.1155/2017/6943952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte J, Ribeiro C, Parreira C, et al. Biorefinery of Dunaliella salina: sustainable recovery of carotenoids polar lipids and glycerol. Biores Technol. 2020;297:122509. doi: 10.1016/j.biortech.2019.122509. [DOI] [PubMed] [Google Scholar]
- Morita RY. Psychrophilic bacteria. Bacteriol Rev. 1975;39:144–167. doi: 10.1128/br.39.2.144-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczkowski BS, Huvar A, Lernhardt W, et al. Secretion of thermostable DNA polymerase using a novel baculovirus vector. J Biol Chem. 1994;269:13522–13528. doi: 10.1016/S0021-9258(17)36862-X. [DOI] [PubMed] [Google Scholar]
- Muntyan MS, Viryasov MB, Sorokin DY, Skulachev VP. Sodium energetic cycle in the natronophilic bacterium Thioalkalivibrio versutus. Int J Mol Sci. 2022;23:1965. doi: 10.3390/ijms23041965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao R, Kojima K. Ion transporters in the thylakoid membrane of cyanobacteria–an unexpected diversity of candidates for studies on photosynthesis. Photosynth Res. 2017;133(1–3):119–134. [Google Scholar]
- Namsaraev XB, Zavarzin GA. Alkaliphilic cyanobacteria: diversity, ecology and applications. Microbiol. 2016;85(1):1–17. [Google Scholar]
- Narsing Rao MP, Liu L, Jiao JY et al (2018) Hot springs of India: occurrence and microbial diversity. In: Egamberdieva D, Birkeland, NK, Panosyan H, Li WJ (eds) Microorganisms for sustainability. Singapore, Springer, vol 8, pp 29–55
- Narsing Rao MP, Dong ZY, Luo ZH, et al. Physicochemical and microbial diversity analyses of Indian hot springs. Front Microbiol. 2021;12:627200. doi: 10.3389/fmicb.2021.627200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsing Rao MP, Luo ZH, Dong ZY, et al. Metagenomic analysis further extends the role of Chloroflexi in fundamental biogeochemical cycles. Environ Res. 2022;209:112888. doi: 10.1016/j.envres.2022.112888. [DOI] [PubMed] [Google Scholar]
- Nechita P, Mirela R, Ciolacu F. Renewable material with potential properties for food packaging applications. Sustainability. 2021;13:13504. doi: 10.3390/su132413504. [DOI] [Google Scholar]
- Newsome L, Falagán C. The Microbiology of metal mine waste: bioremediation applications and implications for planetary health. Geohealth. 2021;5:e(2020)GH000380. doi: 10.1029/2020GH000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson WL, Munakata N, Horneck G, et al. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev. 2000;64:548–572. doi: 10.1128/MMBR.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH (2022) National Institutes of Health. https://www.nihgov/ assessed on (2022)-07-23 at 11:33:50
- Niimura Y, Koh E, Yanagida F, et al. Amphibacillus xylanus gen. nov., sp. nov., a facultatively anaerobic spore forming Xylan-digesting bacterium which lacks cytochrome quinone and catalase. Int J Syst Bacteriol. 1990;40:297–301. doi: 10.1099/00207713-40-3-297. [DOI] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissenbaum A. The microbiology and biogeochemistry of the Dead Sea. Microbial Ecol. 1975;2:139–161. doi: 10.1007/BF02010435. [DOI] [PubMed] [Google Scholar]
- Nobel PS (2014) Achievable bioproducts from cyanobacteria: their use in agriculture, industry and medicine. In: Microalgae-based biofuels and bioproducts, pp. 213–234. Woodhead Publishing. 10.1533/9780857097410.2.213
- Nogi Y, Kato C, Horikoshi K. Moritella japonica sp novsp. nov., a novel barophilic bacterium isolated from a Japan Trench sediment. J Gen Appl Microbiol. 1998;44:289–295. doi: 10.2323/jgam.44.289. [DOI] [PubMed] [Google Scholar]
- Novitsky TJ, Chan M, Himes RH, Akagi JM. Effect of temperature on the growth and cell wall chemistry of a facultative thermophilic Bacillus. J Bacteriol. 1974;117:858–865. doi: 10.1128/jb.117.2.858-865.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NPA (2022) National Park Arkansas https://www.npsgov/hosp/learn/thermophileshtm assessed (2022)-07-02 10:41:23
- Orellana R, Macaya C, Bravo G, et al. Living at the frontiers of life: extremophiles in Chile and their potential for bioremediation. Front Microbiol. 2018;9:2309. doi: 10.3389/fmicb.2018.02309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A. Halophilic archaea on Earth and in space: growth and survival under extreme conditions. Phil Trans R Soc A. 2014;372:20140194. doi: 10.1098/rsta.2014.0194. [DOI] [PubMed] [Google Scholar]
- Oren A (2015) Cyanobacterial diversity in extreme environments. In: Ecology of Cyanobacteria II, pp. 591–611. Springer, Dordrecht. 10.1007/978-94-017-7412-8_21
- Papadimitriou K, Alegría Á, Bron PA, de Angelis M, Gobbetti M, Kleerebezem M, Lemos JA, Linares DM, Ross P, Stanton C, Turroni F, van Sinderen D, Varmanen P, Ventura M, Zúñiga M, Tsakalidou E, Kok J. Stress physiology of lactic acid bacteria. Microbiol Mol Biol Rev. 2016;80(3):837–890. doi: 10.1128/MMBR.00076-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Na Y, Kim J, et al. Properties and applications of starch modifying enzymes for use in the baking industry. Food Sci Biotechnol. 2017;27:299–312. doi: 10.1007/s10068-017-0261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Chang Y, Kim MK (2022) Hymenobacter armeniacus sp. nov. and Hymenobacter montanus sp. nov. two radiation-resistant bacteria from soil. Int J Syst Evol Microbiol 72:005267. [DOI] [PubMed]
- Penhallurick RW, Durnal MD, Harold A, Ichiye T. Adaptations for pressure and temperature in dihydrofolate reductases. Microorganisms. 2021;9:1706. doi: 10.3390/microorganisms9081706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfumo A, Freiherr von Sass GJ, Nordmann EL, et al. Discovery and characterization of a new cold-active protease from an extremophilic bacterium via comparative genome analysis and in vitro expression. Front Microbiol. 2020;11:881. doi: 10.3389/fmicb.2020.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham JV, Yilma MA, Feliz A, et al. A review of the microbial production of bioactive natural products and biologics. Front Microbiol. 2019;10:1404. doi: 10.3389/fmicb.2019.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikuta EV, Hoover RB, Tang J. Microbial extremophiles at the limits of life. Crit Rev Microbiol. 2007;33:183–209. doi: 10.1080/10408410701451948. [DOI] [PubMed] [Google Scholar]
- Pinel I, Biškauskaitė R, Pal'ová E, et al. Assessment of the impact of temperature on biofilm composition with a laboratory heat exchanger module. Microorganisms. 2021;9:1185. doi: 10.3390/microorganisms9061185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli A, Finore I, Romano I, et al. Microbial diversity in extreme marine habitats and their biomolecules. Microorganisms. 2017;5:25. doi: 10.3390/microorganisms5020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapati VS, Trivedi UB, Patel KC. Kinetic and thermodynamic characterization of glucoamylase from Colletotrichum sp KCP1. Indian J Microbiol. 2014;54:87–93. doi: 10.1007/s12088-013-0413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabee AE, Sayed Alahl A, Lamara M, Ishaq SL. Fibrolytic rumen bacteria of camel and sheep and their applications in the bioconversion of barley straw to soluble sugars for biofuel production. PLoS ONE. 2022;17:e0262304. doi: 10.1371/journal.pone.0262304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Wong KS, Jane J, et al. Characterization of SU1 isoamylase a determinant of storage starch structure in maize. Plant Physiol. 1998;117:425–435. doi: 10.1104/pp.117.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JL. Extremophile enzymes for food additives and fertilizers. Microb Biotechnol. 2021 doi: 10.1111/1751-791513944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampelotto PH. Resistance of microorganisms to extreme environmental conditions and its contribution to astrobiology. Sustainability. 2010;2:1602–1623. doi: 10.3390/su2061602. [DOI] [Google Scholar]
- Räsänen LA, Elväng AM, Jansson J, Lindström K. Effect of heat stress on cell activity and cell morphology of the tropical rhizobium Sinorhizobium arboris. FEMS Microbiol Ecol. 2001;34:267–278. doi: 10.1111/j.1574-6941.2001.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Razzaq A, Shamsi S, Ali A, et al. Microbial proteases applications. Front Bioeng Biotechnol. 2019;7:110. doi: 10.3389/fbioe.2019.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekadwad B, Gonzalez JM. Multidisciplinary involvement and potential of thermophiles. Folia Microbiol. 2019;64:389–406. doi: 10.1007/s12223-018-0662-8. [DOI] [PubMed] [Google Scholar]
- Rekadwad B, Pathak A. First report on revelatory prokaryotic diversity of Unkeshwar hot spring (India) having biotechnological potential. Indian J Biotechnol. 2016;15:195–200. [Google Scholar]
- Roberts MF. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst. 2005;1:5. doi: 10.1186/1746-1448-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter A, Barbier M, Bertoni F, et al. The essentials of marine biotechnology. Front Marine Sci. 2021;8:629629. doi: 10.3389/fmars.2021.629629. [DOI] [Google Scholar]
- Royter M, Schmidt M, Elend C. Thermostable lipases from the extreme thermophilic anaerobic bacteria Thermoanaerobacter thermohydrosulfuricus SOL1 and Caldanaerobacter subterraneus subsp tengcongensis. Extremophiles. 2009;13:769–783. doi: 10.1007/s00792-009-0265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust P, Ekmekcioglu C (2016) Impact of salt intake on the pathogenesis and treatment of hypertension. In: Islam MS (eds) Hypertension: from basic research to clinical practice. Advances in Experimental Medicine and Biology Vol 956, Springer, Cham. 10.1007/5584_(2016)_147 [DOI] [PubMed]
- Saeedi P, Moosaabadi JM, Sebtahmadi SS, et al. Potential applications of bacteriorhodopsin mutants. Bioengineered. 2012;3:326–328. doi: 10.4161/bioe.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghatelyan A, Margaryan A, Panosyan H, Birkeland NK. Microbial diversity of terrestrial geothermal springs in Armenia and Nagorno-Karabakh: a review. Microorganisms. 2021;9:1473. doi: 10.3390/microorganisms9071473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha R, Bhattacharya D, Mukhopadhyay M. Enhanced production of biohydrogen from lignocellulosic feedstocks using microorganisms: a comprehensive review. Energy Convers Manag X. 2022;13:100153. [Google Scholar]
- Sahonero-Canavesi DX, Villanueva L, Bale NJ, et al. Changes in the distribution of membrane lipids during growth of Thermotoga maritima at different temperatures: indications for the potential mechanism of biosynthesis of ether-bound diabolic acid (membrane-spanning) lipids. Appl Environ Microbiol. 2022;88:e0176321. doi: 10.1128/AEM.01763-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salano OA, Makonde HM, Kasili RW, et al. Diversity and distribution of fungal communities within the hot springs of soda lakes in the Kenyan rift valley. Afri J Microbiol Res. 2017;11:764–775. [Google Scholar]
- Santomartino R, Zea L, Cockell CS. The smallest space miners: principles of space biomining. Extremophiles. 2022;26:7. doi: 10.1007/s00792-021-01253-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento F, Peralta R, Blamey JM. Cold and hot extremozymes: industrial relevance and current trends. Front Bioeng Biotechnol. 2015;3:148. doi: 10.3389/fbioe.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Dhakan DB, Mittal P, Waiker P, Chowdhury A, Ghatak A, Sharma VK. Metagenomic analysis of hot Springs in Central India reveals hydrocarbon degrading thermophiles and pathways essential for survival in extreme environments. Front Microbiol. 2017;7:2123. doi: 10.3389/fmicb.2016.02123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P (2000) Resistance of bacterial spores. In: Storz G, Hengge-Aronis R (eds) Bacterial Stress Responses American Society for Microbiology, Washington DC, pp 217–230
- Setlow P. Spores of Bacillus subtilis: their resistance to and killing by radiation heat and chemicals. J Appl Microbiol. 2006;101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- Setlow P. Spore resistance properties. Microbiol Spectr. 2014;2:5. doi: 10.1128/microbiolspec.TBS-0003-2012. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kawarabayashi Y, Satyanarayana T. Acidophilic bacteria and archaea: acid stable biocatalysts and their potential applications. Extremophiles. 2012;16:1–19. doi: 10.1007/s00792-011-0402-3. [DOI] [PubMed] [Google Scholar]
- Shrivastava P, Kumar R. Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci. 2015;22:123–131. doi: 10.1016/j.sjbs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert SM, Vetriani C. Chemoautotrophy at deep-sea vents: past present and future. Oceanography. 2012;25:218–233. doi: 10.5670/oceanog.2012.21. [DOI] [Google Scholar]
- Siliakus MF, van der Oost J, Kengen SWM. Adaptations of archaeal and bacterial membranes to variations in temperature pH and pressure. Extremophiles. 2017;21:651–67099. doi: 10.1007/s00792-017-0939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Jain K, Desai C et al (2019) Chapter 18—microbial community dynamics of extremophiles/extreme environment. In: Microbial Diversity in the Genomic Era, pp 323–332. 10.1016/B978-0-12-814849-500018-6
- Šnajder M, Carrillo Rincón AF, Magdevska V. Extracellular production of the engineered thermostable protease pernisine from Aeropyrum pernix K1 in Streptomyces rimosus. Microb Cell Fact. 2019;18:196. doi: 10.1186/s12934-019-1245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin DY, Makarova KS, Abbas B, et al. Discovery of extremely halophilic methyl-reducing euryarchaea provides insights into the evolutionary origin of methanogenesis. Nat Microbiol. 2017;2:17081. doi: 10.1038/nmicrobiol.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin DY, Yakimov M, Messina E, Merkel AY, Bale NJ, Sinninghe Damste JS. Natronolimnobius sulfurireducens sp. nov. and Halalkaliarchaeum desulfuricum gen. nov., sp. nov., the first sulfur-respiring alkaliphilic haloarchaea from hypersaline alkaline lakes. Int J Syst Evol Biol. 2019;69:2662–2673. doi: 10.1099/ijsem.0.003506. [DOI] [PubMed] [Google Scholar]
- Steenbergen CLM, Korthals HJ, van Nes M. Ecological observations on phototrophic sulfur bacteria and the role of these bacteria in the sulfur cycle of monomictic Lake Vechten (The Netherlands) Acta Academiae Aboensis. 1987;47:97–115. [Google Scholar]
- Stetter KO. Extremophiles and their adaptation to hot environments. FEBS Lett. 1999;452(1–2):22–25. doi: 10.1016/S0014-5793(99)00663-8. [DOI] [PubMed] [Google Scholar]
- Straub CT, Counts JA, Nguyen DMN, Wu CH, Zeldes BM, Crosby JR, Conway JM, Otten JK, Lipscomb GL, Schut GJ, Adams MWW, Kelly RM. Biotechnology of extremely thermophilic archaea. FEMS Microbiol Rev. 2018;42(5):543–578. doi: 10.1093/femsre/fuy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi BP, Martin WF, Carbone V, et al. Archaeal pseudomurein and bacterial murein cell wall biosynthesis share a common evolutionary ancestry. FEMS Microbes. 2021;2:xtab012. doi: 10.1093/femsmc/xtab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzina NE, Sorokin VV, Polivtseva VN, et al. From rest to growth: life collisions of Gordonia polyisoprenivorans 135. Microorganisms. 2022;10:465. doi: 10.3390/microorganisms10020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sysoev M, Grötzinger SW, Renn D, et al. Bioprospecting of novel extremozymes from Prokaryotes—the advent of culture-independent methods. Front Microbiol. 2021;12:630013. doi: 10.3389/fmicb.2021.630013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai K, Nakamura K, Toki T, et al. Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci USA. 2008;105:10949–10954. doi: 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami H, Inoue A, Fuji F, Horikoshi K. Microbial flora in the deepest sea mud of the Mariana Trench. FEMS Microbiol Lett. 1997;152:279–285. doi: 10.1111/j.1574-6968.1997.tb10440.x. [DOI] [PubMed] [Google Scholar]
- Tank M, Blümel M, Imhoff JF. Communities of purple sulfur bacteria in a Baltic Sea coastal lagoon analyzed by puf LM gene libraries and the impact of temperature and NaCl concentration in experimental enrichment cultures. FEMS Microbiol Ecol. 2011;78:428–438. doi: 10.1111/j.1574-6941.2011.01175.x. [DOI] [PubMed] [Google Scholar]
- Tapia C, López B, Astuya A, et al. Antiproliferative activity of carotenoid pigments produced by extremophile bacteria. Nat Prod Res. 2021;35:4638–4642. doi: 10.1080/14786419.2019.1698574. [DOI] [PubMed] [Google Scholar]
- Tendulkar S, Hattiholi A, Chavadar M, Dodamani S. Psychrophiles: a journey of hope. J Biosci. 2021;46:64. doi: 10.1007/s12038-021-00180-4. [DOI] [PubMed] [Google Scholar]
- Tetzner DR, Thomas ER, Allen CS, Piermattei A. Evidence of recent active volcanism in the Balleny Islands (Antarctica) from ice core records. J Geophy Res Atmos. 2021;126:e2021JD035095. doi: 10.1029/2021JD035095. [DOI] [Google Scholar]
- Tian Y, Kong BH, Liu SL. Burkholderia grimmiae sp novsp. nov., isolated from a xerophilous moss (Grimmia montana) Int J Syst Evol Microbiol. 2013;63:2108–2113. doi: 10.1099/ijs.0.045492-0. [DOI] [PubMed] [Google Scholar]
- Turvey MW, Gabriel KN, Lee W. Single-molecule Taq DNA polymerase dynamics. Sci Adv. 2022;8:eabl3522. doi: 10.1126/sciadv.abl3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrih NP, Gmajner D, Raspor P. Structural and physicochemical properties of polar lipids from thermophilic archaea. Appl Microbiol Biotechnol. 2009;84:249–260. doi: 10.1007/s00253-009-2102-9. [DOI] [PubMed] [Google Scholar]
- van der Maarel MJ, van der Veen B, Uitdehaag JC. Properties and applications of starch-converting enzymes of the alpha-amylase family. J Biotechnol. 2002;94:137–155. doi: 10.1016/S0168-1656(01)00407-2. [DOI] [PubMed] [Google Scholar]
- Varrella S, Tangherlini M, Corinaldesi C. Deep Hypersaline Anoxic Basins as untapped reservoir of polyextremophilic prokaryotes of biotechnological interest. Mar Drugs. 2020;18:91. doi: 10.3390/md18020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavitsas K, Glekas PD, Hatzinikolaou DG. Synthetic biology of thermophiles: taking bioengineering to the extremes? Appl Microbiol. 2022;2:165–174. doi: 10.3390/applmicrobiol2010011. [DOI] [Google Scholar]
- Ventosa A, Nieto JJ, Oren A. Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev. 1998;62:504–544. doi: 10.1128/MMBR.62.2.504-544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde C, Giordano D, Bellas CM, et al. Polar marine microorganisms and climate change. Adv Microb Physiol. 2016;69:187–215. doi: 10.1016/bs.ampbs.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Verma D. Extremophilic prokaryotic endoxylanases: diversity applicability and molecular insights. Front Microbiol. 2021;12:728475. doi: 10.3389/fmicb.2021.728475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V, Meghawanshi GK, Kumar R. Current perspectives for microbial lipases from extremophiles and metagenomics. Biochimie. 2021;182:23–36. doi: 10.1016/j.biochi.2020.12.027. [DOI] [PubMed] [Google Scholar]
- Vicente JB, Frazão C, Lamosa P, Santos H (2008) Calcium and urea as modulators of Synechocystis sp. PCC 6803 urease activity. Appl. Environ. Microbiol. 74(1):221–228
- Vieille C, Zeikus GJ. Hyperthermophilic enzymes: sources uses and molecular mechanisms for thermostability. Microbiol Mol Biol Rev. 2001;65:1–43. doi: 10.1128/MMBR.65.1.1-43.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieille C, Burdette DS, Zeikus JG. Thermozymes. Biotechnol Annu Rev. 1996;2:1–83. doi: 10.1016/S1387-2656(08)70006-1. [DOI] [PubMed] [Google Scholar]
- von Hegner I. Extremophiles: a special or general case in the search for extra-terrestrial life? Extremophiles. 2020;24:167–175. doi: 10.1007/s00792-019-01144-1. [DOI] [PubMed] [Google Scholar]
- Walsby AE. Gas vesicles. Microbiol Rev. 1994;58:94–144. doi: 10.1128/mr.58.1.94-144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby AE, Hayes PK, Boje R, et al. The selective advantage of buoyancy provided by gas vesicles for planktonic cyanobacteria in the Baltic Sea. New Phytol. 1997;136:407–417. doi: 10.1046/j.1469-8137.1997.00754.x. [DOI] [PubMed] [Google Scholar]
- Walter R, Simmons WH, Yoshimoto T. Proline specific endo- and exopeptidases. Mol Cell Biochem. 1980;30:111–127. doi: 10.1007/BF00227927. [DOI] [PubMed] [Google Scholar]
- Wang S, Hou W, Dong H, et al. Control of temperature on microbial community structure in hot springs of the Tibetan Plateau. PLoS ONE. 2013;8:e62901. doi: 10.1371/journal.pone.0062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu Y, Liu H, et al. Recent advances and application of whole genome amplification in molecular diagnosis and medicine. Med Comm. 2022;3:e116. doi: 10.1002/mco2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Dragone NB, Avard G, Hynek BM. Microbial survival in an extreme martian analog ecosystem: Poás Volcano Costa Rica. Front Astron Space Sci. 2022;9:817900. doi: 10.3389/fspas.2022.817900. [DOI] [Google Scholar]
- Wei C, Sun D, Yuan W, et al. Metagenomics revealing molecular profiling of microbial community structure and metabolic capacity in Bamucuo Tibet. bioRxiv. 2022 doi: 10.1101/(2022)0118476867. [DOI] [PubMed] [Google Scholar]
- Wiegel J, Ljungdahl LG, Rawson JR. Isolation from soil and properties of the extreme thermophile Clostridium thermohydrosulfuricum. J Bacteriol. 1979;139(3):800–810. doi: 10.1128/jb.139.3.800-810.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Zheng S, Pedroso MM, et al. Processivity and enzymatic mechanism of a multifunctional family 5 endoglucanase from Bacillus subtilis BS-5 with potential applications in the saccharification of cellulosic substrates. Biotechnol Biofuels. 2018;11:20. doi: 10.1186/s13068-018-1022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Itoh T, Zhou P, et al. Natronobacterium nitratireducens sp novsp. nov., a aloalkaliphilic archaeon isolated from a soda lake in China. Int J Syst Evol Microbiol. 2001;51:1825–1829. doi: 10.1099/00207713-51-5-1825. [DOI] [PubMed] [Google Scholar]
- Xu X. Ciliates in extreme environments. J Eukary Microbiol. 2014;61:410–418. doi: 10.1111/jeu.12120. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fang A, Feng K, et al. Mini-metagenome analysis of psychrophilic electroactive biofilms based on single cell sorting. Sci Total Environ. 2021;762:144328. doi: 10.1016/j.scitotenv.2020.144328. [DOI] [PubMed] [Google Scholar]
- Yaroshenko G, Koulakov I, Al-Arifi N, et al. Structure of the magma plumbing system beneath Semisopochnoi Island (Aleutian Arc) inferred from seismic tomography. Sci Rep. 2022;12:10771. doi: 10.1038/s41598-022-14794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yıldırım A, Avci C. A simple and efficient approach for the synthesis of cholesterol esters of long-chain saturated fatty acids by using Ph3P·SO3 as a versatile organocatalyst. Steroids. 2022;183:109011. doi: 10.1016/j.steroids.2022.109011. [DOI] [PubMed] [Google Scholar]
- Yuan M, Zhang W, Dai S, et al. Deinococcus gobiensis sp novsp. nov., an extremely radiation-resistant bacterium. Int J Syst Evol Microbiol. 2009;59:1513–1517. doi: 10.1099/ijs.0.004523-0. [DOI] [PubMed] [Google Scholar]
- Yüce GH, Karatay SE, Aksu Z, Dönmez G. Lithium (I) biofortified Dunaliella salina as a potential functional nutrition supplement. Algal Res. 2021;56:102257. doi: 10.1016/j.algal.2021.102257. [DOI] [Google Scholar]
- Zeldes BM, Keller MW, Loder AJ, et al. Extremely thermophilic microorganisms as metabolic engineering platforms for production of fuels and industrial chemicals. Front Microbiol. 2015;6:1209. doi: 10.3389/fmicb.2015.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Alain K, Shao Z. Microorganisms from deep–sea hydrothermal vents. Mar Life Sci Technol. 2021;3:204–230. doi: 10.1007/s42995-020-00086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgonik V, Mulec J, Eleršek T, et al. Extremophilic Microorganisms in Central Europe. Microorganisms. 2021;9:2326. doi: 10.3390/microorganisms9112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zeng R, Labes A. Editorial: marine microbial-derived molecules and their potential medical and cosmetic applications. Front Microbiol. 2021;12:706152. doi: 10.3389/fmicb.2021.706152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Adebisi WA, Ahmad F, Sethupathy S, Danso B, Sun J. Recent Development of Extremophilic Bacteria and Their Application in Biorefinery. Front Bioeng Biotechnol. 2020;8:483. doi: 10.3389/fbioe.2020.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is not applicable.