Abstract
Objective
Type 2 diabetes (T2D) is linked to metabolic, mitochondrial and inflammatory alterations, atherosclerosis development and cardiovascular diseases (CVDs). The aim was to investigate the potential therapeutic benefits of GLP-1 receptor agonists (GLP-1 RA) on oxidative stress, mitochondrial respiration, leukocyte-endothelial interactions, inflammation and carotid intima–media thickness (CIMT) in T2D patients.
Research design and methods
Type 2 diabetic patients (255) and control subjects (175) were recruited, paired by age and sex, and separated into two groups: without GLP-1 RA treatment (196) and treated with GLP-1 RA (59). Peripheral blood polymorphonuclear leukocytes (PMNs) were isolated to measure reactive oxygen species (ROS) production by flow cytometry and oxygen consumption with a Clark electrode. PMNs were also used to assess leukocyte-endothelial interactions. Circulating levels of adhesion molecules and inflammatory markers were quantified by Luminex's technology, and CIMT was measured as surrogate marker of atherosclerosis.
Results
Treatment with GLP-1 RA reduced ROS production and recovered mitochondrial membrane potential, oxygen consumption and MPO levels. The velocity of leukocytes rolling over endothelial cells increased in PMNs from GLP-1 RA-treated patients, whereas rolling and adhesion were diminished. ICAM-1, VCAM-1, IL-6, TNFα and IL-12 protein levels also decreased in the GLP-1 RA-treated group, while IL-10 increased. CIMT was lower in GLP-1 RA-treated T2D patients than in T2D patients without GLP-1 RA treatment.
Conclusions
GLP-1 RA treatment improves the redox state and mitochondrial respiration, and reduces leukocyte-endothelial interactions, inflammation and CIMT in T2D patients, thereby potentially diminishing the risk of atherosclerosis and CVDs.
Keywords: Type 2 diabetes, GLP-1 RA, Mitochondrial dysfunction, Leukocytes, Oxidative stress, Atherogenesis/atherosclerosis
Graphical abstract
Highlights
-
•
Risk of atherosclerosis and cardiovascular diseases is higher in type 2 diabetes.
-
•
The question was to investigate GLP-1 RA benefits on diabetes-related factors.
-
•
GLP-1 RA improved the redox state and mitochondrial respiration of leukocytes.
-
•
GLP-1 RA diminished leukocyte-endothelial interactions, inflammation and CIMT.
-
•
The risk of cardiovascular diseases could be reduced with GLP-1 RA treatment.
Abbreviations
- BMI
body-mass index
- CIMT
carotid intima-media thickness
- CVD
cardiovascular disease
- DCFH
2′,7′-Dichlorodihydrofluorescein diacetate
- GLP-1
glucagon-like peptide-1
- GLP-1 R
glucagon-like peptide-1 receptor
- GLP-1 RA
glucagon-like peptide-1 receptor agonist
- HE
Hydroethidine
- MPO
myeloperoxidase
- mtROS
mitochondrial ROS
- NLRP
Nod-Like Receptor Protein
- PMN
polymorphonuclear leukocyte
- ROS
reactive oxygen species
- SGLT2
sodium-glucose transporter 2
- T2D
type 2 diabetes
- TMRM
tetramethylrhodamine methyl ester perchlorate
- TNFα
Tumor necrosis factor alpha
1. Introduction
Type 2 diabetes (T2D) is a metabolic disorder that constitutes a predominant health problem worldwide. Its representative metabolic disturbances are insulin resistance, hyperglycaemia and altered lipid metabolism, all of them related to chronic inflammation and impaired vasculature, leading to complications that affect both macro- and microvessels [1,2]. These alterations can cause a change in the balance of antioxidants and oxidants in favor of the latter [3].
The unbalanced oxidative environment in T2D can contribute to an increase in advanced glycation end products, thus triggering glycation of haemoglobin and metabolic disturbances that promote a systemic proinflammatory state [4]. This proinflammatory state enhances the release of proinflammatory cytokines such as TNFα and IL-6, as well as reactive oxygen species (ROS) [5]. Together, sustained ROS production and increased inflammation promote the development of diabetic atherosclerosis [6,7]. Leukocytes are activated in T2D and release cytokines and adhesion molecules [8], interacting with the vessel wall, penetrating the inner layers of arteries and escalating the progression of carotid atherosclerosis and cardiovascular events [9,10].
Intensive glucose-lowering treatments have been in part unsuccessful to noticeably decrease cardiovascular morbidity and mortality in T2D patients with high risk of developing cardiovascular conditions [11]. Optimal CVD care is supported by evidence-based research on preventative medications. In this sense, several large cardiovascular outcome trials conducted with novel hypoglycaemic medications, such as glucagon-like peptide-1 receptor agonists (GLP-1 RA), have recently demonstrated significant reductions of major adverse cardiovascular events [12]. GLP-1 is a gut incretin hormone released from intestinal L cells that regulates blood glucose levels via the GLP-1 receptor (GLP-1R) [13]. GLP-1 RA have multiple biological effects, including glycaemic control, improved lipid metabolism, blood pressure reduction and weight loss [14]. GLP-1 RA can also exert mitochondrial and anti-apoptotic effects in various cell types, including pancreatic β cells and cardiomyocytes [15]. However, the mechanisms responsible for these effects are unknown.
Mitochondrial dysfunction is an initial event in the sequence of alterations that occur during T2D in endothelial cells and leukocytes [16]. Mitochondrial fragmentation, ROS overproduction and accumulation of malfunctioning mitochondria are key features of T2D [17]. Taking this into account, we have carried out the present pilot study to investigate the potential therapeutic benefits of GLP-1 RA (administered for at least 1 year) on mitochondrial function, leukocyte–endothelial interactions, adhesion molecules, proinflammatory markers and carotid intima–media thickness (CIMT), all of which are involved in the development of atherosclerosis and CVDs.
2. Material and methods
2.1. Subjects and sample collection
Patients with T2D (255) and age and sex-paired control subjects (175) were recruited at the Endocrinology and Nutrition Service of the Dr. Peset Hospital (Valencia, Spain). T2D patients were diagnosed following the guidelines established by the American Diabetes Association, and the presence of morbid obesity or any autoimmune, malignant, organic, hematological, inflammatory or infectious disease accounted for the exclusion criteria. T2D patients were separated into those without GLP-1 RA treatment (n = 196) and those treated with GLP-1 RA for one year (n = 59). Participants were informed and gave their written consent. The protocol was approved by the Clinical Research Ethics Committee of the University Hospital Dr. Peset (ID: 100.22). The study was conducted following the ethical principles of the Helsinki Declaration.
After 12 h of fasting, anthropometric parameters were assessed and blood was drawn from the median cubital vein. Biochemical determinations were measured as previously described [7] in our hospital's Clinical Analysis Service.
2.2. Isolation of polymorphonuclear leukocytes (PMNs)
PMNs were obtained from whole blood collected in EDTA-coated tubes from all subjects. Isolation was performed with a MACSxpress® Whole Blood Starting Kit (Miltenyi Biotec) following the manufacturer’s guidelines.
2.3. Flow cytometry
Whole blood samples (500uL) were stained for flow cytometry, as previously described [7].
2.4. O2 consumption
Oxygen consumption by PMNs was measured with a Clark electrode (Rank Brothers), as detailed before [16].
2.5. Leukocyte-endothelial cell interaction
Human umbilical vein endothelial cells (HUVEC/TERT2, ATCC) were cultured to form a monolayer). PMNs were resuspended (106 cells/mL) in RPMI media supplemented with 10% v/v FBS (Biowest) and subsequently perfused over the HUVEC monolayer for 5 min at a speed of 0.3 mL/min using a dynamic adhesion system with a parallel flow chamber following a previous method [7].
2.6. Evaluation of adhesion and inflammatory molecules
Plasma was obtained by centrifuging blood, collected in EDTA-coated tubes [18] 1500g, 10 min, 4 °C to analyze serum levels of the adhesion molecules ICAM-1, P-selectin, VCAM-1, myeloperoxidase (MPO) and cytokines IL6, TNFα, IL12 and IL10, in a Luminex® 200 (Luminex Corporation), following the MILLIPLEX® Kit manufacturer’s procedures (Millipore Corporation).
2.7. Carotid Intima–Media thickness measurement (CIMT)
A week after the blood extraction, the CIMT of T2D patients and healthy subjects was evaluated at the Cardiology Service of the Dr. Peset Hospital following the American Echocardiography Association’s guidelines [19].
2.8. Statistical analysis
After normal distribution analysis of the data, the three groups were compared with one-way ANOVA and Tukey post-hoc tests, using SPSS 27.0 (SPSS Statistics Inc.) and GraphPad (www.graphpad.com). P values < 0.05 were considered statistically significant.
3. Results
3.1. Anthropometrical and biochemical parameters
Anthropometrical measurements are displayed in Table 1 and reflect the metabolic alterations characteristic of T2D. T2D patients displayed higher levels of glucose, HbA1c, HOMA-IR index and insulin than controls. Furthermore, lipid metabolism alterations were observed, even though some of these were attributed to statin treatment. We confirmed that body-mass index (BMI) was an independent factor that did not influence the rest of differences. There were no statistical differences between T2D that had received GLP-1 RA treatment and those that had not. Other treatments are detailed in Table 1.
Table 1.
Summary of the main anthropological, clinical and biochemical parameters of the population of study and the treatments received.
| Characteristics | Control | Type 2 diabetes without GLP-1RA | Type 2 diabetes with GLP-1RA | BMI corrected p-value |
|---|---|---|---|---|
| N | 175 | 196 | 59 | |
| Sex (% women) | 46% | 45% | 37% | ns |
| Age (years) | 54.9 ± 13.5 | 59.9 ± 10.5 | 56.5 ± 9.9 | ns |
| Duration of diabetes (years) | – | 10.4 ± 8.1 | 13.8 ± 8.7 | ns |
| Weight (kg) | 71.2 ± 15.2 | 89 ± 13.7*** | 95.5 ± 17.6*** | *** |
| Waist circumference (cm) | 78.5 ± 12.5 | 111.4 ± 20.1*** | 112.9 ± 12.3*** | *** |
| BMI (kg/m2) | 24.9 ± 14.5 | 32.2 ± 5.6*** | 33.3 ± 4.9*** | *** |
| SBP (mm Hg) | 117.4 ± 23.1 | 136.9 ± 27.6*** | 134 ± 14*** | *** |
| DBP (mm Hg) | 71.9 ± 17.7 | 76.6 ± 22.2 | 73.5 ± 20.9 | ns |
| Glucose (mg/dL) | 87.6 ± 10.3 | 136.7 ± 46.4*** | 138.7 ± 54.9*** | *** |
| HbA1c-DCCT (%) | 5.2 ± 0.5 | 6.8 ± 1.5*** | 7.1 ± 1.4*** | *** |
| Hb1Ac (mmol/mol) | 32.9 ± 4.5 | 51.5 ± 13.6*** | 57 ± 13.3*** | *** |
| Total cholesterol (mg/dL) | 184.6 ± 35.7 | 147.4 ± 38.2*** | 151.5 ± 34.7*** | *** |
| HDL-c (mg/dL) | 57.8 ± 14.6 | 45.7 ± 12.4*** | 43.5 ± 10.4*** | *** |
| LDL-c (mg/dL) | 108.2 ± 30.7 | 77.8 ± 14.6*** | 74.5 ± 33*** | *** |
| Triglycerides (mg/dL) | 66 (54–99) | 113 (83–161) *** | 134 (93–198) *** | *** |
| HOMA-IR | 1.9 ± 1.5 | 5.2 ± 2.6*** | 6 ± 3.8*** | *** |
| Insulin (μUI/mL) | 7.6 ± 5.3 | 14.7 ± 7.1** | 14.6 ± 14.3** | ** |
| Treatment | ||||
| Antidiabetic drugs | ||||
| Insulin | – | 28,8% | 39% | |
| Insulin + Metformin | – | 13,1% | 26,8% | |
| Insulin + DPP4 inhibitors | – | 7,6% | 0% | |
| Insulin + SGLT2 inhibitors | – | 5,1% | 19,5% | |
| Metformin | – | 62,6% | 78% | |
| Metformin + SGLT2 inhibitors | – | 6,6% | 17,1% | |
| Metformin + DPP4 inhibitors | – | 25,3% | 0% | |
| SGLT2 inhibitors | – | 11,6% | 29,3% | |
| Lipid-lowering medication | – | 74,2% | 85,4% | |
| Statins | – | 56,6% | 78% | |
| Statins + Fibrates | – | 6,6% | 7,3% | |
| Statins + Ezetimibe | – | 3% | 4,9% | |
| Antihypertensive medication | – | 46,5% | 48,8% | |
For normally distributed data, mean ± standard deviations are shown, while the median (25th and 75th quartiles) are displayed for non-normally distributed data. Statistical analysis was made with ANOVA and the Tukey post-hoc test. Kolmogorov-Smirnov test was employed for non-normally distributed data. Proportions were compared with a Chi-square test. The influence of BMI was tested and corrected with the univariate linear model. **p<0.01 and ***p < 0.001 vs control. In the summary of the treatments of T2D patients, results expressed as percentage (%) of patients with each treatment.
3.2. GLP-1 RA ameliorates oxidative stress and mitochondrial function
Total ROS (Fig. 1A, p < 0.001; representative plots in Supplementary Fig. 1), mtROS and superoxide production (Fig. 1B and C, both p < 0.01, representative plots in Supplementary Fig. 1) were higher in our T2D patients compared to the control group. T2D patients treated with GLP-1 RA produced lower levels of total and mtROS than those that were not treated (Fig. 1A–B, p < 0.05) and displayed reduced levels of superoxide production (Fig. 1C). Mitochondrial membrane potential (representative plots in Supplementary Fig. 1) and O2 consumption decreased in the T2D group (p < 0.01, p < 0.05, respectively) but were restored in the GLP-1 RA treated group (p < 0.01 and p < 0.05) (Fig. 1D and E). Additionally, myeloperoxidase (MPO) levels in serum were higher in T2D patients, which were reversed by GLP-1 RA treatment (Fig. 1F, both p < 0.01).
Fig. 1.
Oxidative stress parameters in PMNs from control subjects and T2D patients with or without GLP-1 RA treatment. ROS production (A), mitochondrial ROS (B) and whole-cell superoxide production (C) mitochondrial membrane potential (D) were measured by flow cytometry. Oxygen consumption was evaluated by a Clark electrode (E), and MPO protein levels (F) in serum samples from the three groups of subjects were analyzed with a Luminex® 200 analyzer system. Data are represented as mean ± SD. Comparisons were made with one –way ANOVA. *p < 0.05, **p < 0.01 and ***p < 0.001 vs Control; #p < 0.05, ##p < 0.01 vs T2D.
3.3. Leukocyte-endothelial interaction and adhesion molecules are decreased with GLP-1 RA treatment
In the leukocyte-endothelial interaction assays (see supplementary representative videos), the rolling velocity of PMNs from T2D over the HUVEC monolayer was reduced, while their rolling flux was increased with respect to the control group (Fig. 2A and B, both p < 0.001, representative videos in supplementary files). The T2D group also displayed a higher number of adhered cells (Fig. 2C, p < 0.001, representative plots in supplementary files). These interactions were restored in the PMNs from GLP-1 RA-treated patients, in which higher rolling velocity and lower rolling flux and adhesion were observed with respect to the non-treated patients (Fig. 2A–C, all of them p < 0.05).
Fig. 2.
Evaluation of leukocyte-endothelial interaction and adhesion molecules in T2D patients (treated or not with GLP-1 RA) and control subjects. Rolling velocity (A, μm/s), rolling flux (B, leukocytes/min) and adhesion (C, leukocytes/mm2). Adhesion molecules: ICAM-1 (D), VCAM-1 (E) and P-selectin (F) were measured using a Luminex® 200 analyzer system. Data are represented as mean ± SD. Results were compared between groups with one –way ANOVA. *p < 0.05 and ***p < 0.001 vs Control; #p < 0.05 vs T2D.
These results were supported by the quantification of adhesion molecules in serum from patients. ICAM-1, VCAM-1 and P-selectin proteins were elevated in T2D patients compared to the control group (Fig. 2D–F, p < 0.05). Treatment with GLP-1 RA lowered ICAM-1 and VCAM-1 levels in the treated T2D patients (Fig. 2D and E, p < 0.05), while it did not affect P-selectin levels (Fig. 2F).
3.4. GLP-1 RA modulates serum inflammation molecules in T2D patients
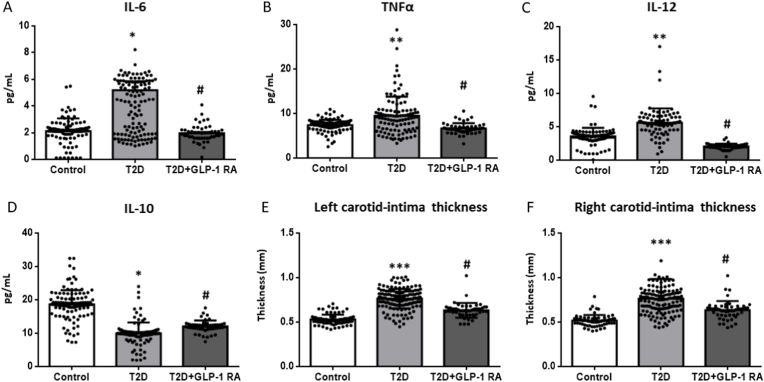
Our analysis of the inflammatory state revealed that levels of IL-6 (p < 0.05), TNFα and IL-12 (both p < 0.01) were higher in the serum of T2D patients, while those of IL-10 were lower (p < 0.05). IL-6 and TNFα were reverted to control levels in T2D patients treated with GLP-1 RA (both p < 0.05) whereas IL-12 was further decreased compared to the non-treated and control groups (p < 0.05). Conversely, GLP-1 RA treatment increased IL-10 (p < 0.05), though not to control levels.
3.5. CIMT is affected by T2D and GLP-1 RA treatment
CIMT can be related to vascular complications. Measurements of both the right and left CIMT followed the same trend (Fig. 3E and F); thickness was increased in T2D patients compared to the control group (p < 0.001) and was reduced in the GLP-1 RA-treated patients (p < 0.05).
Fig. 3.
Serum levels of cytokines and measurement of carotid intima–media thickness (CIMT). Differences between control and T2D groups (with or without GLP-1 RA treatment) are shown in the graphs. IL-6 (A), TNFα (B), IL-12 (C) and IL-10 (D) levels were measured with a Luminex® 200 analyzer system. Comparisons were made with ANOVA and a Tukey post-hoc test. *p < 0.05 and **p < 0.01 vs Control; #p < 0.05 vs T2D. Left carotid (E) and right carotid (F) were analyzed. Data are represented as mean ± SD. Statistical analysis was performed using ANOVA with a Tukey post-test. ***p < 0.001 vs Control; #p < 0.05 vs T2D.
A scheme summarizing the findings of the present study has been shown in Fig. 4.
Fig. 4.
Summary of the main findings of the study. GLP-1 RA treatment in T2D patients decreases CIMT and the concentration of pro-inflammatory and adhesion molecules in circulation. GLP-1 RA also increases PMN rolling velocity, and reduces PMN rolling flux and adhesion. PMNs of patients treated with GLP-1 RA display reduced levels of ROS and improved mitochondrial membrane potential and O2 consumption, compared to patients not treated with GLP-1 RA. ΔΨm: mitochondrial membrane potential, CIMT: carotid intima–media thickness, PMN: polymorphonuclear leukocytes, ROS: reactive oxygen species. Partially created with Biorender.
4. Discussion and conclusion
The present study shows the beneficial effects of GLP-1 RA on different hallmarks of T2D. We have evaluated endocrine and anthropometric parameters, mitochondrial and oxidative stress parameters, adhesion molecules, cytokines, leukocyte–endothelium interactions and CIMT in controls and GLP-1 RA-treated and non-treated T2D patients.
During T2D, leukocytes are closely associated to ROS production and are also highly susceptible to their oxidative damage [20,21]. Our results show an increase in total and mitochondrial ROS, superoxide and MPO levels and a decline in O2 consumption and mitochondrial membrane potential in the leukocytes of T2D patients not treated with GLP-1 RA, suggesting that leukocyte mitochondrial function is compromised under chronic hyperglycaemia. These findings are in agreement with those of previous studies demonstrating elevated mtROS production in T2D patients that were associated to the development of silent myocardial ischaemia [3,7].
In our group of T2D patients treated with GLP-1 RA, oxidative stress-related alterations were reversed in part, thus confirming its beneficial effects by which it preserves mitochondrial function. In line with this, GLP-1 RA have been shown to activate cytoprotective pathways [22,23], protect neurons against oxidative stress [24], and exert anti-apoptotic effects in different cell types, including pancreatic β cells [15,23], cardiomyocytes [23,25] and nerve cells [24]. However, the underlying mechanisms promoting these effects are not yet fully elucidated.
Metabolic syndrome underlies pathological processes including atherosclerosis, that is characterized by enhanced recruitment of leukocytes to the endothelium. A pro-inflammatory state promotes enhanced leukocyte–endothelial cell interactions and favors the early onset of atherosclerosis [26]. Increased leukocyte–endothelium interactions and/or impairment of leukocyte function have been related to oxidative stress in subjects with insulin resistance [27] and animal models of T1D and T2D [28]. In the present study, we have used an ex vivo model in which human PMNs are perfused over a monolayer of human endothelial cells mimicking the shear stress to that observed in vivo [[29], [30]]. We observed that T2D increased rolling flux and adhesion and decreased rolling velocity of PMN in T2D patients that were not treated with GLP-1 RA. In the GLP-1 RA-treated T2D group, we observed a similar degree of leukocyte-endothelial interactions to the control group, with reduced rolling flux and adhesion and increased rolling velocity with respect to the non-treated group, suggesting a beneficial action that slows down the development of atherosclerosis. A study in patients with chronic heart failure showed no major improvements in left ventricular ejection fraction [31]. However, Lopez et al. (2022) demonstrated that the addition of GLP-1 RA to SGLT2 inhibitors may reduce the risk of adverse events in patients with heart failure with reduced ejection fraction who have T2D and atherosclerotic CVD, but it does not affect the risk of heart failure hospitalization [32]. Furthermore, in a network meta-analysis, GLP-1 RA was reported to reduce cardiovascular mortality, non-fatal myocardial infarction, and non-fatal stroke [33]. These outcomes suggest that GLP-1 RA can exert cardiovascular protection through its anti-atherosclerotic properties.
In the present study, we have observed an increase in ICAM-1, VCAM-1 and P-selectin in T2D, which is in accordance to previous research [34] and matches the observed enhanced leukocyte–endothelium interactions. We observed increased IL-6, TNFα and IL-12 levels and decreased IL-10 levels in T2D patients, all of which represents an inflammatory state. High levels of TNFα that arise as a consequence of ROS-induced oxidative stress in activated leukocytes, are thought to inhibit insulin signaling and impair glucose uptake [35]. In addition, IL-6 plays an important role in atherosclerosis in T2D [36]. GLP-1 RA treatment in T2D patients reversed these effects by decreasing IL-6, TNFα and IL-12 levels and increasing IL-10 levels, thus demonstrating an anti-inflammatory effect. These results are in agreement with clinical data obtained in patients treated with GLP-1 RA, whose isolated leukocytes exhibited a decreased production of TNFα and IL-1 [37]. GLP-1 RA have been shown to decrease systemic inflammation, as measured by CRP levels in T2D patients [38].
Finally, we evaluated CIMT, which could be related in some way with early stages of atherosclerosis [39]. T2D promotes CIMT thickening [40], and one of the main consequences of this alteration is cerebrovascular events such as stroke. Left and right CIMT were increased in the non-treated T2D group, while it decreased in both carotids of the GLP-1 RA-treated group, suggesting a slowing down of the atherosclerotic process. In accordance, a recent study by Marx et al. [13] have reported that GLP-1 RAs help to reduce atherosclerotic cardiovascular risk in patients with T2D. By stimulating GLP-1R in hypothalamic neurons, GLP-1 RAs increase satiety, which leads to weight loss. In contrast to the evidence suggesting an improved vascular health by GLP-1 RA treatment, a study performed in obese subjects with impaired glucose tolerance found that exenatide was not more effective than metformin in ameliorating endothelial function, measured by digital reactive hyperemia [41]. However, the duration of the treatment period in this study, 3 months, may have been insufficient to produce greater effects on endothelial function. In addition, instead of T2D patients, the non-diabetic population included in the study may not have developed endothelial dysfunction to an extent sensitive enough to observe improvements by GLP-1 RA treatment. A recent randomized controlled trial using exenatide in T2D patients concluded that GLP-1 RA treatment for 18 months did not improve carotid plaque volume or composition [42]. A limitation of our study is the cross-sectional design, which may explain, at least partially, the differences between our findings and those from Koska et al. In addition, some differences in the study populations are striking. For example, the longer duration of the disease in our cohort (13.8 ± 8.7 years in the GLP-1 RA treated patients vs 6 ± 4 years in Koska’s study), that may have promoted a greater atherosclerosis progression in our subjects, allowing more noticeable anti-atherosclerotic effects of GLP-1 RAs. Another disparity is that almost all subjects in Koska’s study are men (men proportion of >95% vs 60% in our study population), which raises the possibility that men may have limited benefit from the use of these drugs to prevent the onset or slow progression of atherosclerosis, although this requires further research. Data from preclinical models of atherosclerosis have shown that GLP-1 RA reduces the development of the atherosclerotic lesion and its progression by contributing to less vulnerable and more stabilized plaques [43], most likely through anti-inflammatory and antiatherogenic effects in macrophages, monocytes, endothelial cells and vascular smooth muscle cells, which express GLP-1R [44]. Indeed, the expression levels of genes related to the pathogenesis of atherosclerosis, such as those involved in leukocyte recruitment, leukocyte rolling and adhesion/extravasation, showed altered expression in mouse models of atherosclerosis that were reversed when mice were treated with GLP-1 RA [45]. Interestingly, evidence supports that GLP-1 RA may act through inducing autophagy [46,47], a key energy homeostasis response associated to therapeutic effects of weight loss in obese patients [18]. Our findings suggest that, in addition to these mechanisms, GLP-1 RA ameliorate leukocyte’s redox state, mitochondrial function and reduce their interaction with the endothelium, ultimately contributing to a lower cardiovascular risk. Since both GLP-1 RA-treated and non-treated patients displayed similar anthropometric and glucose-related parameter levels, while the former showed an overall improved oxidative stress and inflammatory profile, it seems that GLP-1 RA exerts direct effects on leukocyte mitochondria, redox signaling and/or inflammatory-adhesion molecular pathways.
In summary, our findings highlight the beneficial effects of GLP-1 RA in the context of T2D, in which it improves mitochondrial dysfunction, decreases inflammatory mediators and leukocyte-endothelium interactions, and reduces CIMT. Future research into these regards will undoubtedly aid to clarify the molecular mechanisms involved in these effects of GLP-1 RA and to prevent the atherosclerotic process in T2D.
Funding information
This research was supported by the European Regional Development Fund (ERDF ‘‘A way to build Europe’’); FISABIO (Foundation for the Promotion of Health and Biomedical Research in the Valencian Region, grants UGP-21-236 and UGP15-220); Generalitat Valenciana, Conselleria de Innovación, Universidades, Ciencia y Sociedad Digital (grants PROMETEO CIPROM/2022/32, CIGE/2022/122, CIGRIS/2021/112), Spanish Ministry of Science and Innovation (grants CIBERehd CB06/04/0071, PI22/00424 and PI22/1009 from Carlos III Health Institute), and “Ayuda SED a Proyectos de Investigación en Diabetes dirigidos por Jóvenes Investigadores 2023", of the “Sociedad Española de Diabetes”). S.R-L is recipient of a Maria Zambrano fellowship [ZA21-049] from the requalification of the Spanish University System (Ministry of Universities, Government of Spain, European Union, NextGeneration EU). De Marañón A.M received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 945425”
Declaration of competing interest
None.
There's no financial/personal interest or belief that could affect the objectivity.
Acknowledgments
The authors thank Brian Normanly (University of Valencia-CIBERehd) for his editorial assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102849.
Contributor Information
Clara Luna-Marco, Email: clara.luna@fisabio.es.
Arantxa M. de Marañon, Email: amardema@alumni.uv.es.
Alberto Hermo-Argibay, Email: heraral@alumni.uv.es.
Yohaly Rodriguez-Hernandez, Email: roheryo@alumni.uv.es.
Jonathan Hermenejildo, Email: jonathanhbello@gmail.com.
Meylin Fernandez-Reyes, Email: meylin.fernandezreyes@gmail.com.
Nadezda Apostolova, Email: nadezda.apostolova@uv.es.
Jose Vila, Email: jose.m.salinas@uv.es.
Eva Sola, Email: eva.sola@uv.es.
Carlos Morillas, Email: carlos.morillas@uv.es.
Susana Rovira-Llopis, Email: susana.rovira@uv.es.
Milagros Rocha, Email: milagros.rocha@uv.es.
Victor M. Victor, Email: victor.victor@uv.es.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Representative examples of the original plots from flow cytometry for each fluorochrome and group (corresponds to data shown in Fig. 1).
Data availability
Data will be made available on request.
References
- 1.Orasanu G., Plutzky J. The Pathologic continuum of diabetic vascular disease. J. Am. Coll. Cardiol. 2009;53(5, Supplement):S35–S42. doi: 10.1016/j.jacc.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glovaci D., Fan W., Wong N.D. Epidemiology of diabetes mellitus and cardiovascular disease. Curr. Cardiol. Rep. 2019;21(4):21. doi: 10.1007/s11886-019-1107-y. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Mijares A., Rocha M., Rovira-Llopis S., Bañuls C., Bellod L., de Pablo C., et al. Human leukocyte/endothelial cell interactions and mitochondrial dysfunction in type 2 diabetic Patients and their association with silent myocardial ischemia. Diabetes Care. 2013;36(6):1695–1702. doi: 10.2337/dc12-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 5.Donath M.Y., Dinarello C.A., Mandrup-Poulsen T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat. Rev. Immunol. 2019;19(12):734–746. doi: 10.1038/s41577-019-0213-9. [DOI] [PubMed] [Google Scholar]
- 6.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122(6):877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Marañón A.M., Díaz-Pozo P., Canet F., Díaz-Morales N., Abad-Jiménez Z., López-Domènech S., et al. Metformin modulates mitochondrial function and mitophagy in peripheral blood mononuclear cells from type 2 diabetic patients. Redox Biol. 2022;53 doi: 10.1016/j.redox.2022.102342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouedraogo R., Gong Y., Berzins B., Wu X., Mahadev K., Hough K., et al. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J. Clin. Invest. 2007;117(6):1718–1726. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau K.K., Wong Y.K., Chan Y.H., Yiu K.H., Teo K.C., Li L.S.W., et al. Prognostic implications of surrogate markers of atherosclerosis in low to intermediate risk patients with Type 2 Diabetes. Cardiovasc. Diabetol. 2012;11:101. doi: 10.1186/1475-2840-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sardu C., De Lucia C., Wallner M., Santulli G. Diabetes mellitus and its cardiovascular complications: new insights into an old disease. J. Diabetes Res. 2019 May 19;2019 doi: 10.1155/2019/1905194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long-term effects of intensive glucose lowering on cardiovascular outcomes. N. Engl. J. Med. 2011;364(9):818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jojima T., Uchida K., Akimoto K., Tomotsune T., Yanagi K., Iijima T., et al. Liraglutide, a GLP-1 receptor agonist, inhibits vascular smooth muscle cell proliferation by enhancing AMP-activated protein kinase and cell cycle regulation, and delays atherosclerosis in ApoE deficient mice. Atherosclerosis. 2017;261:44–51. doi: 10.1016/j.atherosclerosis.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Marx N., Husain M., Lehrke M., Verma S., Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in Patients with type 2 diabetes. Circulation. 2022;146(24):1882–1894. doi: 10.1161/CIRCULATIONAHA.122.059595. [DOI] [PubMed] [Google Scholar]
- 14.Muscogiuri G., DeFronzo R.A., Gastaldelli A., Holst J.J. Glucagon-like Peptide-1 and the central/Peripheral nervous system: crosstalk in diabetes. Trends Endocrinol. Metabol. 2017;28(2):88–103. doi: 10.1016/j.tem.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Ding M., Fang Q.H., Cui Y.T., Shen Q.L., Liu Q., Wang P.H., et al. Liraglutide prevents β-cell apoptosis via inactivation of NOX2 and its related signaling pathway. J. Diabet. Complicat. 2019;33(4):267–277. doi: 10.1016/j.jdiacomp.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Mijares A., Rocha M., Apostolova N., Borras C., Jover A., Bañuls C., et al. Mitochondrial complex I impairment in leukocytes from type 2 diabetic patients. Free Radic. Biol. Med. 2011;50(10):1215–1221. doi: 10.1016/j.freeradbiomed.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Zhan M., Usman I.M., Sun L., Kanwar Y.S. Disruption of renal tubular mitochondrial quality control by myo-inositol oxygenase in diabetic kidney disease. J. Am. Soc. Nephrol. 2015;26(6):1304. doi: 10.1681/ASN.2014050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abad-Jiménez Z., López-Domènech S., García-Gargallo C., Vezza T., Gómez-Abril S.Á., Morillas C., et al. Roux-en-Y gastric bypass modulates AMPK, autophagy and inflammatory response in leukocytes of obese Patients. Biomedicines. 2022;10(2):430. doi: 10.3390/biomedicines10020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Marañón A.M., Iannantuoni F., Abad-Jiménez Z., Canet F., Díaz-Pozo P., López-Domènech S., et al. Association between Proinflammatory markers, leukocyte–endothelium interactions, and carotid intima–media thickness in type 2 diabetes: role of glycemic control. J. Clin. Med. 2020;9(8):2522. doi: 10.3390/jcm9082522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupré-Crochet S., Erard M., Nüβe O. ROS production in phagocytes: why, when, and where. J. Leukoc. Biol. 2013;94(4):657–670. doi: 10.1189/jlb.1012544. [DOI] [PubMed] [Google Scholar]
- 21.Rubattu S., Forte M., Raffa S. Circulating leukocytes and oxidative stress in cardiovascular diseases: a state of the art. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/2650429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noyan-Ashraf M.H., Momen M.A., Ban K., Sadi A.M., Zhou Y.Q., Riazi A.M., et al. GLP-1R agonist liraglutide activates cytoprotective Pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58(4):975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y., Wang S., Chen X., Wang Z., Wang X., Zhou Q., et al. Liraglutide prevents high glucose induced HUVECs dysfunction via inhibition of PINK1/Parkin-dependent mitophagy. Mol. Cell. Endocrinol. 2022;545 doi: 10.1016/j.mce.2022.111560. [DOI] [PubMed] [Google Scholar]
- 24.Mohiuddin M.S., Himeno T., Inoue R., Miura-Yura E., Yamada Y., Nakai-Shimoda H., et al. Glucagon-like Peptide-1 receptor agonist Protects dorsal root ganglion neurons against oxidative insult. J. Diabetes Res. 2019;2019 doi: 10.1155/2019/9426014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palazzuoli A., Ceccarelli E., Ruocco G., Nuti R. Clinical impact of oral antidiabetic medications in heart failure patients. Heart Fail. Rev. 2018;23(3):325–335. doi: 10.1007/s10741-018-9669-0. [DOI] [PubMed] [Google Scholar]
- 26.Okazaki S., Sakaguchi M., Miwa K., Furukado S., Yamagami H., Yagita Y., et al. Association of interleukin-6 with the Progression of carotid atherosclerosis. Stroke. 2014;45(10):2924–2929. doi: 10.1161/STROKEAHA.114.005991. [DOI] [PubMed] [Google Scholar]
- 27.Victor VM, Rocha M, Bañuls C, Alvarez A, de Pablo C, Sanchez-Serrano M, et al. Induction of oxidative stress and human leukocyte/endothelial cell interactions in polycystic ovary syndrome Patients with insulin resistance. J. Clin. Endocrinol. Metab.. 2011 Oct 1;96(10):3115–3122. [DOI] [PubMed]
- 28.Pettersson U.S., Christoffersson G., Massena S., Ahl D., Jansson L., Henriksnäs J., et al. Increased recruitment but impaired function of leukocytes during inflammation in mouse models of type 1 and type 2 diabetes. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Marañon A.M., Iannantuoni F., Abad-Jiménez Z., Canet F., Díaz-Pozo P., López-Domènech S., et al. Relationship between PMN-endothelium interactions, ROS production and Beclin-1 in type 2 diabetes. Redox Biol. 2020;34 doi: 10.1016/j.redox.2020.101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao R.M., Yang L., Garcia-Cardena G., Luscinskas F.W. Endothelial-Dependent mechanisms of leukocyte recruitment to the vascular wall. Circ. Res. 2007;101(3):234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 31.Lepore J.J., Olson E., Demopoulos L., Haws T., Fang Z., Barbour A.M., et al. Effects of the novel long-acting GLP-1 agonist, albiglutide, on cardiac function, cardiac metabolism, and exercise capacity in Patients with chronic heart failure and reduced ejection fraction. JACC Heart Fail. 2016;4(7):559–566. doi: 10.1016/j.jchf.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Lopez P.D., Bhatia K., Bohra C., Mahmood K., Baruch L., Eng C. Benefits of adding glucagon-like Peptide 1 receptor agonists to sodium-glucose Co-transporter 2 inhibitors in diabetic Patients with atherosclerotic disease and heart failure. Am. J. Cardiol. 2022;181:87–93. doi: 10.1016/j.amjcard.2022.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Palmer S.C., Tendal B., Mustafa R.A., Vandvik P.O., Li S., Hao Q., et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. doi: 10.1136/bmj.m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wasserman D.H., Wang T.J., Brown N.J. The vasculature in prediabetes. Circ. Res. 2018;122(8):1135–1150. doi: 10.1161/CIRCRESAHA.118.311912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bajpai A., Tilley D.G. The role of leukocytes in diabetic cardiomyopathy. Front. Physiol. 2018 doi: 10.3389/fphys.2018.01547. https://www.frontiersin.org/articles/10.3389/fphys.2018.01547 [Internet] [cited 2023 Jun 5];9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golden S.H., Folsom A.R., Coresh J., Sharrett A.R., Szklo M., Brancati F. Risk factor groupings related to insulin resistance and their synergistic effects on subclinical atherosclerosis: the atherosclerosis risk in communities study. Diabetes. 2002;51(10):3069–3076. doi: 10.2337/diabetes.51.10.3069. [DOI] [PubMed] [Google Scholar]
- 37.Hogan A.E., Gaoatswe G., Lynch L., Corrigan M.A., Woods C., O'Connell J., et al. Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia. 2014;57(4):781–784. doi: 10.1007/s00125-013-3145-0. [DOI] [PubMed] [Google Scholar]
- 38.Aroda V.R., Rosenstock J., Terauchi Y., Altuntas Y., Lalic N.M., Morales Villegas E.C., et al. Pioneer 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in Patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724–1732. doi: 10.2337/dc19-0749. [DOI] [PubMed] [Google Scholar]
- 39.Nezu T., Hosomi N., Aoki S., Matsumoto M. Carotid intima-media thickness for atherosclerosis. J. Atherosclerosis Thromb. 2016;23(1):18–31. doi: 10.5551/jat.31989. [DOI] [PubMed] [Google Scholar]
- 40.Zhao B., Liu Y., Zhang Y., Chen Y., Yang Z., Zhu Y., et al. Gender difference in carotid intima-media thickness in type 2 diabetic patients: a 4-year follow-up study. Cardiovasc. Diabetol. 2012;11(1):1–6. doi: 10.1186/1475-2840-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly A.S., Bergenstal R.M., Gonzalez-Campoy J.M., Katz H., Bank A.J. Effects of Exenatide vs. Metformin on endothelial function in obese patients with pre-diabetes: a randomized trial. Cardiovasc. Diabetol. 2012;11:64. doi: 10.1186/1475-2840-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koska J., Migrino R.Q., Chan K.C., Cooper-Cox K., Reaven P.D. The effect of exenatide once weekly on carotid atherosclerosis in individuals with type 2 diabetes: an 18-month randomized placebo-controlled study. Diabetes Care. 2021;44(6):1385–1392. doi: 10.2337/dc20-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arakawa M., Mita T., Azuma K., Ebato C., Goto H., Nomiyama T., et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like Peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59(4):1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLean B.A., Wong C.K., Campbell J.E., Hodson D.J., Trapp S., Drucker D.J. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation. Endocr. Rev. 2021;42(2):101–132. doi: 10.1210/endrev/bnaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rakipovski G., Rolin B., Nøhr J., Klewe I., Frederiksen K.S., Augustin R., et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE−/− and LDLr−/− mice by a mechanism that includes inflammatory Pathways. JACC Basic Transl. Sci. 2018;3(6):844–857. doi: 10.1016/j.jacbts.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng R.H., Zhang W.W., Ji Y.N., Bai X.J., Yan C.P., Wang J., et al. Exogenous supplement of glucagon like peptide-1 protects the heart against aortic banding induced myocardial fibrosis and dysfunction through inhibiting mTOR/p70S6K signaling and promoting autophagy. Eur. J. Pharmacol. 2020;883 doi: 10.1016/j.ejphar.2020.173318. [DOI] [PubMed] [Google Scholar]
- 47.Tian X., Gao Y., Kong M., Zhao L., Xing E., Sun Q., et al. GLP-1 receptor agonist protects palmitate-induced insulin resistance in skeletal muscle cells by up-regulating sestrin2 to promote autophagy. Sci. Rep. 2023;13(1):9446. doi: 10.1038/s41598-023-36602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.