Summary
Background
Case reports suggest that SARS-CoV-2 infection could lead to immune dysregulation and trigger autoimmunity while COVID-19 vaccination is effective against severe COVID-19 outcomes. We aim to examine the association between COVID-19 and development of autoimmune diseases (ADs), and the potential protective effect of COVID-19 vaccination on such an association.
Methods
A retrospective cohort study was conducted in Hong Kong between 1 April 2020 and 15 November 2022. COVID-19 was confirmed by positive polymerase chain reaction or rapid antigen test. Cox proportional hazard regression with inverse probability of treatment weighting was applied to estimate the risk of incident ADs following COVID-19. COVID-19 vaccinated population was compared against COVID-19 unvaccinated population to examine the protective effect of COVID-19 vaccination on new ADs.
Findings
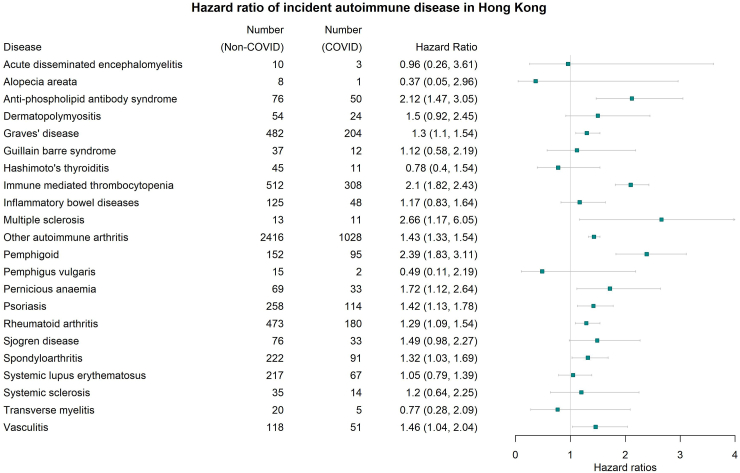
The study included 1,028,721 COVID-19 and 3,168,467 non-COVID individuals. Compared with non-COVID controls, patients with COVID-19 presented an increased risk of developing pernicious anaemia [adjusted Hazard Ratio (aHR): 1.72; 95% Confidence Interval (CI): 1.12–2.64]; spondyloarthritis [aHR: 1.32 (95% CI: 1.03–1.69)]; rheumatoid arthritis [aHR: 1.29 (95% CI: 1.09–1.54)]; other autoimmune arthritis [aHR: 1.43 (95% CI: 1.33–1.54)]; psoriasis [aHR: 1.42 (95% CI: 1.13–1.78)]; pemphigoid [aHR: 2.39 (95% CI: 1.83–3.11)]; Graves' disease [aHR: 1.30 (95% CI: 1.10–1.54)]; anti-phospholipid antibody syndrome [aHR: 2.12 (95% CI: 1.47–3.05)]; immune mediated thrombocytopenia [aHR: 2.1 (95% CI: 1.82–2.43)]; multiple sclerosis [aHR: 2.66 (95% CI: 1.17–6.05)]; vasculitis [aHR: 1.46 (95% CI: 1.04–2.04)]. Among COVID-19 patients, completion of two doses of COVID-19 vaccine shows a decreased risk of pemphigoid, Graves' disease, anti-phospholipid antibody syndrome, immune-mediated thrombocytopenia, systemic lupus erythematosus and other autoimmune arthritis.
Interpretation
Our findings suggested that COVID-19 is associated with an increased risk of developing various ADs and the risk could be attenuated by COVID-19 vaccination. Future studies investigating pathology and mechanisms would be valuable to interpreting our findings.
Funding
Supported by RGC Collaborative Research Fund (C7154-20GF).
Keywords: Autoimmune disorders, COVID-19 vaccine, SARS-CoV-2
Research in context.
Evidence before this study
We searched PubMed for publications with language restriction to English from December 2019 (when SARS-CoV-2 was first reported) to 4 February 2023 using search terms “SARS-CoV-2” or “COVID-19” and “Inflammatory bowel diseases” or “Spondyloarthritis” or “Psoriasis” or “Hashimoto's thyroiditis” or “Graves' disease” or “Alopecia areata” or “Pemphigus vulgaris” or “Pernicious anaemia” or “Pemphigoid” or “Guillain barre syndrome” or “Vasculitis” or “Acute disseminated encephalomyelitis” or “Transverse myelitis” or “Systemic lupus erythematosus” or “Rheumatoid arthritis” or “Sjogren disease” or “Systemic sclerosis” or “Polymyositis” or “Dermatomyositis” or “Multiple sclerosis” or “Anti-phospholipid antibody syndrome” or “Immune mediated thrombocytopenia” or “Acute aseptic arthritis” or “Reactive arthritis” with search terms found in abstract and title. We found ample clinical case reports covering a wide spectrum of autoimmune disorders following COVID-19 and two epidemiological studies' attempts to depict associations between COVID-19 and autoimmune phenomenon. One study using a US electronic medical database identified an increased risk for a number of autoimmune diseases among patients with COVID-19; another study published using the same database found a decreased risk of incident inflammatory bowel diseases following COVID-19. No study assessed the effect of COVID-19 vaccine on the occurrence of autoimmunity.
Added value of this study
We analysed territory-wide electronic medical records with vaccine-record linked cohorts (1,028,721, COVID-19 and 3,168,467 non-COVID) to assess the association between COVID-19 and 22 types of autoimmune diseases. We found COVID-19 to be linked with an increased risk of pernicious anaemia, spondyloarthritis, rheumatoid arthritis, other autoimmune arthritis, psoriasis, pemphigoid, Graves' disease, anti-phospholipid antibody syndrome, immune mediated thrombocytopenia, multiple sclerosis, and vasculitis compared to patients with similar baseline characteristics without COVID-19. Multivariable regression show that COVID-19 vaccination likely prevents development of COVID-19 induced autoimmune diseases.
Implications of all the available evidence
As with the US-based epidemiological study, we verified the increased risk of autoimmune diseases associated with COVID-19 but with a more conservative hazard ratio estimation. Our observation supports the belief that protection is conferred on several autoimmune sequelae by completing a minimum of two doses of COVID-19 vaccination. In view of the available evidence, COVID-19 could potentially trigger an autoimmune disorder, and COVID-19 vaccination could attenuate the effects. The findings could be used to encourage vaccine uptake and assist with early diagnosis and optimisation of clinical management of post-COVID-19 syndrome.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an RNA virus that causes COVID-19 (coronavirus disease 2019). There were more than 768 million confirmed COVID-19 cases and 6.9 million recorded deaths globally at the time of writing.1 Alongside the ongoing pandemic, there are a growing number of case reports on new-onset autoimmune diseases (ADs) following COVID-19 diagnosis on the nervous system [Acute disseminated encephalomyelitis, transverse myelitis, and Guillain-Barré syndrome (GBS)], musculoskeletal system (Spondyloarthritis, rheumatoid arthritis, and dermatopolymyositis), endocrine system (Graves' disease, Hashimoto thyroiditis) and other sites.2, 3, 4 Viral infection is believed to modify the induction and development of autoimmune diseases such as influenza A virus (IAV), Hepatitis C virus (HCV), Parvovirus B19, Epstein-Barr-virus (EBV). Current evidence suggest that viral-induced autoimmunity can be triggered by various mechanisms including molecular mimicry, bystander activation, and immortalisation of infected B cells.4,5 Multiple autoantibodies have been detected among SARS-CoV-2 infected patients including anti-CCP antibodies (biomarkers for psoriasis/inflammatory arthritis/Grave's disease), anti-nuclear antibodies (Biomarker for GBS).6 In addition, cytokine release syndrome describes uncontrolled secretion of pro-inflammatory cytokines induced by SARS-CoV-2 infection, majorly interleukin, tumour necrosis factor-α,7,8 which disrupts human innate and acquired immune responses leading to intolerance to self-antigens.9 SARS-CoV-2 infection was also associated with diminished and dysfunctional T-regulatory cells that suppress autoimmune phenomena in patients with severe COVID-19.10
Despite numerous clinical case reports and speculated pathology pathway, analytic epidemiological studies designed to illustrate the relationship between COVID-19 and new onset ADs remain limited. Chang et al. reported significantly increased risk of developing a series of ADs following COVID-19 among the US COVID-19 cohort.11 The study excludes cohorts with COVID-19 vaccination completely to avoid potential interference from vaccination. This captures the absolute risk associated with COVID-19, however, it may influence the representativeness of the study and introduce selection bias. In addition, the study focused primarily on rheumatic ADs but omitted other frequently reported autoimmune disorders. Hadi et al.12 identified a decreased incidence ratio of inflammatory bowel disease (IBD) among the US COVID-19 cohort, contrary to the increased risk of IBD observed in Chang's study. We recognised an existing research gap for current epidemiological evidence on the Asian population, disease spectrum, and protective effect from COVID-19 vaccination. To address this knowledge gap, we investigated the effect of SARS-CoV-2 infection and COVID-19 vaccination on the occurrence of newly diagnosed ADs in this territory-wide retrospective cohort study.
Method
Data source
Territory-wide electronic medical records (EMRs) were retrieved from the Hong Kong Hospital Authority (HA). HA manages all public hospitals, specialist and general outpatient clinics in Hong Kong (HK). It provides free public healthcare services to all HK residents (>7.3 million), covering approximately 80% of all routine hospital admissions.13 The anonymised EMRs dataset contains information about demographics, death, diagnoses, and hospitalisation. Two COVID-19 vaccine platforms [mRNA (Pfizer-BNT162b2) and inactivated virus vaccines (Sinovac-CoronaVac)] have been approved in Hong Kong for emergency use, vaccination date, dose, and technology information were provided by the Department of Health (DH) of the Hong Kong Government and further linked with the EMR database. Diagnostic COVID-19 test results were sourced from DH and Centre for Health Protection in HK. The record-linked EMR database has been broadly used for investigating SAR-CoV-2 vaccine safety and effectiveness, denoting good population representativeness and data accuracy.14, 15, 16
Cohort identification
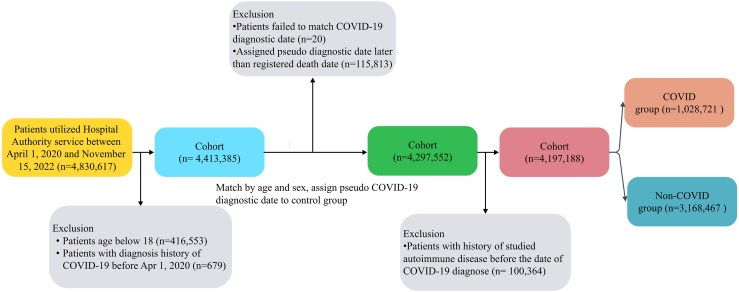
We classified the cohort into a COVID-19 group and non-COVID group (without recorded diagnostic test) according to the diagnostic COVID-19 test results between 1 April 2020 and 15 November 2022. COVID-19 patients were confirmed by positive polymerase chain reaction (PCR) or positive rapid antigen test (RAT). COVID-19 diagnostic date was selected as index date for further cohort selection and individual follow-up. Individuals in the COVID-19 group were matched with individuals in the non-COVID group by age and sex using maximum ratio matching to assign pseudo-index dates for the non-COVID group to facilitate follow-up. Individuals aged below 18 years old, or with clinical history of interested AD outcomes before the index date were excluded. All individuals were followed up from the index date until the date of death, the occurrence of incident ADs, or study end date, whichever occurred earlier.
Ethical statement
Ethical approval for this study was granted by the Institutional Review Board of the University of HK/HA HK West Cluster (UW20-556 and UW21-149) and Department of Health, HK (L/M21/2021 and L/M175/2022). The informed consent from participants were waived as patients' confidentiality was well maintained in this retrospective cohort study.
Outcomes of clinical diagnosis
Study outcomes are incident ADs diagnosis in inpatient and outpatient settings. Determination of ADs list was per discussion with local clinicians across subspecialties and literature review of clinical case reports. Selected ADs including inflammatory bowel diseases, pernicious anaemia, spondyloarthritis, rheumatoid arthritis, dermatopolymyositis, other autoimmune arthritis, psoriasis, alopecia areata, pemphigus vulgaris, pemphigoid, vasculitis, Hashimoto's thyroiditis, Graves' disease, anti-phospholipid antibody syndrome, immune-mediated thrombocytopenia, GBS, acute disseminated encephalomyelitis, transverse myelitis, systemic lupus erythematosus (SLE), Sjogren disease, systemic sclerosis, multiple sclerosis. Outcomes were identified based on the International Classification of Diseases, Ninth Revision, clinical modification (ICD-9-CM) (Supplementary Table S1).
Statistical analysis
We conducted Inverse Probability of Treatment Weighting (IPTW) on age, sex, dose of COVID-19 vaccination, Charlson comorbidity index (CCI) to eliminate the effect of these confounding variables between groups. Variables with standardised mean difference (SMD) < 0.1 were regarded as well-balanced. The crude incidence rate (per 10,000 person-years) of each outcome in COVID-19 patients and matched controls were estimated. The adjusted hazard ratio (aHR) and 95% confidence interval (CI) of each outcome were estimated using Cox proportional hazard regressions. The proportional hazard assumption was tested using Schoenfeld residuals, a flexible parametric survival model17 that was adapted to deal with study outcomes violated the proportional hazard assumption. Subgroup analyses were performed by 1) sex; 2) severe COVID-19 (Defined as hospitalisation within 14 days following COVID-19 diagnoses; 3) Age group (18–40, 41–64, ≥65 years). In the sensitivity analysis, we applied 28-days washout period focused only on outcomes occurring 28 days following COVID-19 diagnoses.
To further explore the potentially protective effect from COVID-19 vaccination on COVID-19-associated ADs, in the additional analysis, we classified patients into four categories COVID -unvaccinated, COVID -vaccinated, non- COVID -vaccinated, non- COVID -unvaccinated. We then applied Cox proportional hazard regressions adjusting for age, sex, CCI, and categorised variables indicating patients' COVID-19 vaccination status (at least two dose vaccination) and COVID-19 diagnosis status.
As we tested 22 outcomes in our analysis, studied autoimmune diseases were further grouped into organ-specific autoimmune disease and systemic autoimmune disease (Supplementary Table S2) to address the issue of multiple comparison.
STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement checklists were followed to guide transparent reporting of the cohort study. Two-tailed P-value less than 0.05 was considered statistically significant. All statistical analyses were performed using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). The analyses were conducted independently by three co-authors for quality control (KP, DLY and JYZ).
Role of the funding source
KP, XL, DLY, JYZ, EYFW, CSLC, FTTL, CKHW, EWYC, ICKW claim full access to all the data in the study. XL and ICKW are responsible for the decision to submit for publication. The funding body of this study is research grants committee (RGC) from Hong Kong Special Administrative Region, it plays no role in study design, data collection, analysis, and interpretation, decision to submit the paper for publication, or writing of the report.
Results
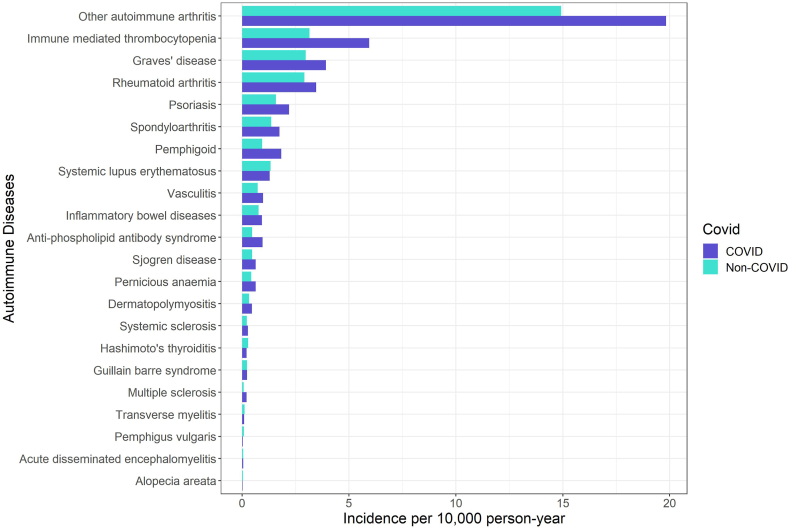
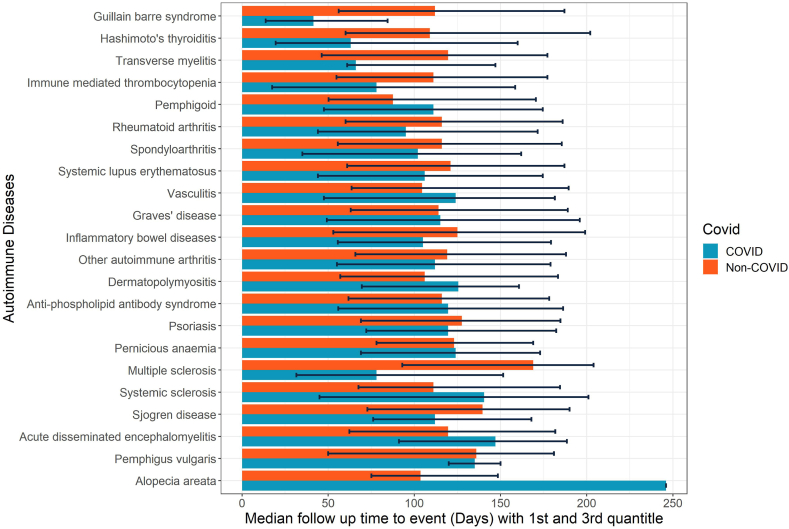
We identified 1,028,721 COVID-19 individuals, 3,168,467 non-COVID individuals from the database (Fig. 1). After IPTW, we obtained a well-balanced cohort with all SMD <0.1 (Table 1). Diabetes, cerebrovascular disease, and malignancy attributed to the top three comorbidities. The crude incidence rate of ADs ranged from other autoimmune arthritis [19.83 (COVID-19) versus 14.92 (Control) per 10,000-person year] to alopecia areata [0.02 (COVID-19) versus 0.05 (Control) per 10,000-person year], in general, the COVID-19 group had a higher crude incidence rate compared with non-COVID group (Fig. 2). Among the COVID-19 group, the median time to the occurrence of ADs ranged from GBS [41.5 (13.75, 84.5)] to alopecia areata [246 (246, 246)] (Fig. 3).
Fig. 1.
Patient identification flow.
Table 1.
Baseline characteristics of COVID-19 patients and matched controls in Hong Kong.
| Baseline characteristics | Controls (N = 3,168,467) | COVID-19 patients (N = 1,028,721) | SMD | Controls (N = 3,168,467) | COVID-19 patients (N = 3,168,131) | SMD |
|---|---|---|---|---|---|---|
| Age, years | 54.09 (18.08) | 52.68 (18.12) | 0.077 | 54.09 (18.08) | 54.25 (18.33) | 0.009 |
| Sex | 0.014 | 0.003 | ||||
| Male | 1,391,897 (43.9) | 458,872 (44.6) | 1,391,897 (43.9) | 1,395,733 (44.1) | ||
| Female | 1,776,570 (56.1) | 569,849 (55.4) | 1,776,570 (56.1) | 1,772,398 (55.9) | ||
| Charlson Comorbidity Index | 0.31 (0.84) | 0.34 (0.93) | 0.040 | 0.31 (0.84) | 0.32 (0.84) | 0.015 |
| Pre-existing comorbidities | ||||||
| Myocardial infarction | 20,735 (0.7) | 7938 (0.8) | 0.014 | 20,735 (0.7) | 24,840 (0.8) | 0.015 |
| Congestive Heart Failure | 27,066 (0.9) | 11,959 (1.2) | 0.031 | 27,066 (0.9) | 39,530 (1.2) | 0.039 |
| Peripheral vascular disease | 7702 (0.2) | 2804 (0.3) | 0.006 | 7702 (0.2) | 8777 (0.3) | 0.007 |
| Cerebrovascular disease | 107,212 (3.4) | 37,577 (3.7) | 0.015 | 107,212 (3.4) | 121,227 (3.8) | 0.024 |
| Chronic obstructive pulmonary disease | 56,414 (1.8) | 22,388 (2.2) | 0.028 | 56,414 (1.8) | 69,173 (2.2) | 0.029 |
| Dementia | 8719 (0.3) | 4983 (0.5) | 0.034 | 8719 (0.3) | 17,918 (0.6) | 0.045 |
| Paralysis | 6257 (0.2) | 2336 (0.2) | 0.006 | 6257 (0.2) | 7217 (0.2) | 0.007 |
| Chronic renal failure | 33,670 (1.1) | 13,672 (1.3) | 0.025 | 33,670 (1.1) | 39,118 (1.2) | 0.016 |
| Mild liver disease | 4049 (0.1) | 1531 (0.1) | 0.006 | 4049 (0.1) | 4271 (0.1) | 0.002 |
| Moderate-severe liver disease | 2644 (0.1) | 1073 (0.1) | 0.007 | 2644 (0.1) | 2674 (0.1) | <0.001 |
| Ulcers | 29,593 (0.9) | 10,640 (1.0) | 0.010 | 29,593 (0.9) | 31,796 (1.0) | 0.007 |
| Inflammatory polyarthropathies other than rheumatoid arthritis | 276 (0.0) | 99 (0.0) | 0.001 | 276 (0.0) | 305 (0.0) | 0.001 |
| Acquired Immune Deficiency Syndrome | 4 (0.0) | 0 (0.0) | 0.002 | 4.0 (0.0) | 0.0 (0.0) | 0.002 |
| Malignancy | 87,658 (2.8) | 31,196 (3.0) | 0.016 | 87,658 (2.8) | 82,897 (2.6) | 0.009 |
| Metastatic solid tumour | 13,071 (0.4) | 5284 (0.5) | 0.015 | 13,071 (0.4) | 9483.4 (0.3) | 0.019 |
| Diabetes without chronic complication | 345,420 (10.9) | 114,733 (11.2) | 0.008 | 345,420 (10.9) | 348,693 (11.0) | 0.003 |
| Diabetes with chronic complication | 22,405 (0.7) | 8213 (0.8) | 0.011 | 22,405 (0.7) | 22,979 (0.7) | 0.002 |
| Doses of COVID-19 vaccines received | 0.154 | 0.002 | ||||
| 0 | 478,424 (15.1) | 105,195 (10.2) | 478,424 (15.1) | 480,183 (15.2) | ||
| 1 | 209,584 (6.6) | 81,059 (7.9) | 209,584 (6.6) | 209,879 (6.6) | ||
| 2 | 1,028,818 (32.5) | 363,420 (35.3) | 1,028,818 (32.5) | 1,028,472 (32.5) | ||
| 3/4 | 1,451,641 (45.8) | 479,047 (46.6) | 1,451,641 (45.8) | 1,449,597 (45.8) |
SMD: Standard mean difference.
Fig. 2.
Crude incidence of each autoimmune disease by COVID-19 and non-COVID groups.
Fig. 3.
Median follow-up time from COVID-19 diagnosis to new onset of autoimmune diseases. The error bars denote the 1st and 3rd quantile of the respective median follow up time.
As illustrated in Fig. 4, we found infection with COVID-19 was associated with an increased risk of new onset pernicious anaemia [adjusted Hazard Ratio (aHR): 1.72; 95% Confidence Interval (CI): 1.12–2.64]; Spondyloarthritis [aHR: 1.32 (95% CI: 1.03–1.69)]; Rheumatoid arthritis [aHR: 1.29 (95% CI: 1.09–1.54)]; Other autoimmune arthritis [aHR: 1.43 (95% CI: 1.33–1.54)]; Psoriasis [aHR: 1.42 (95% CI: 1.13–1.78)]; Pemphigoid [aHR: 2.39 (95% CI: 1.83–3.11)]; Graves' disease [aHR: 1.30 (95% CI: 1.10–1.54)]; Anti-phospholipid antibody syndrome [aHR: 2.12 (95% CI: 1.47–3.05)]; Immune mediated thrombocytopenia [aHR: 2.1 (95% CI: 1.82–2.43)]; Multiple sclerosis [aHR: 2.66 (95% CI: 1.17–6.05)]; Vasculitis [aHR: 1.46 (95% CI: 1.04–2.04)] compared to those without a history of COVID-19 infection. According to Schoenfeld residuals test, nine out of twenty-two outcomes violated the proportional hazard assumption and was refitted with the flexible parametric survival model, which is free from proportional assumption. Flexible parametric survival model generated a similar hazard ratio compared with the Cox regression model with a narrow confidence interval (Supplementary Table S3).
Fig. 4.
Effect of COVID-19 on the risk of newly diagnosed autoimmune diseases (COVID versus non-COVID). Hazard Ratio (HR) and 95% Confidence Interval (95% CI) were estimated by Cox regression, HR > 1 (or <1) indicates patients with COVID-19 had higher (lower) risk of newly diagnosed autoimmune diseases compared to the non-COVID-19 control cohort. The error bars denote the 95% CI of the respective HR.
Similar associations were identified in subgroup analysis stratified by sex (Supplementary Figure S1), the risk of COVID-19-asocciated ADs did not differ across sex, although diseases like spondyloarthritis were only statistically significant for males and Grave's disease only statistically significant for females. The risk of ADs was highest among the older age group (≥65 years) for other autoimmune arthritis, pemphigoid, anti-phospholipid antibody syndrome. The risk was highest among the 41–64 year old age group for spondyloarthritis, rheumatoid arthritis, psoriasis, multiple sclerosis; Graves' disease presented an increased risk only among the 18–40 year old age group. Particularly, COVID-19 showed an increased risk of Sjogren disease in the ≥65 year old age group [aHR: 1.99 (95% CI: 1.04–3.80)]; but not for all cohorts (Supplementary Figure S2). Among individuals with severe COVID-19 requiring hospitalisation, incident transverse myelitis [aHR: 7.8 (95% CI: 1.39–43.85)] and inflammatory bowel diseases [aHR: 4.73 (95% CI: 1.28–17.49)] were higher, while such an association was not detected among the general COVID-19 infection group (Supplementary Figure S3). Severe COVID-19 also exhibited an augmented HR compared with the general cohort. We identified a similar association in line with the main analysis focused only on ADs occurring 28 days following COVID-19 diagnosis, except that COVID-19 no longer presented a statistically increased risk of developing spondyloarthritis, multiple sclerosis and vasculitis (Supplementary Figure S4).
In the additional analysis, COVID-vaccinated patients had an associated reduced risk of developing other autoimmune arthritis [aHR: 0.74 (95% CI: 0.65–0.84)], pemphigoid [aHR: 0.45 (95% CI: 0.29–0.70)], immune mediated thrombocytopenia [aHR: 0.41 (95% CI: 0.33–0.52)], Graves' disease [aHR: 0.58 (95% CI: 0.43–0.77)], Anti-phospholipid antibody syndrome [aHR: 0.55 (95% CI: 0.31–0.99)], and SLE [aHR: 0.29 (95% CI: 0.18–0.47)] compared with COVID-unvaccinated patients. An overall decreasing trend of risk was observed among all studied ADs (Supplementary Figure S5).
Stratified by disease site, increased trends were observed in organ-specific autoimmune disease [aHR: 1.41 (95% CI: 1.28–1.55)] and systemic autoimmune disease [aHR: 1.49 (95% CI: 1.41–1.58)] (Supplementary Table S2).
Discussion
This territory-wide cohort study reported increased risk of incident autoimmune disorder among patients with COVID-19 infection, which indicates the viral infection to be a risk factor for the development of autoimmunity. Various ADs associated with COVID-19 have been reported in line with our study findings; spondyloarthritis,18 rheumatoid arthritis,19 other autoimmune arthritis,20 pemphigoid,21 Graves' disease,22 anti-phospholipid antibody syndrome,23 immune mediated thrombocytopenia,24 multiple sclerosis,25 and vasculitis.26 In the post-pandemic era, healthcare providers should be alert to the autoimmune sequelae associated with COVID-19 for long-term and optimised clinical management of patients with a history of COVID-19.
Human papillomavirus, hepatitis B and influenza have been reported to contribute to onset or exacerbation of autoimmune disorders via molecular mimicry,27,28 defined as the similarity between certain vaccine components and self-peptides resulting in cross-reactivation of the immune system against pathogenic antigens and mistakenly attacking similar proteins. COVID-19 vaccination also activates the by-production of autoantibodies, speculated to be the cause of ADs (e.g., platelet factor 4 antibody-mediated platelet may lead to immune mediated thrombocytopenia). Vaccine adjuvants used in some vaccines work to strengthen the immune response through activation of the NLR pyrin domain containing 3 (NLRP3) inflammasome, which essentially functions as innate and adaptive immune system and is linked to a range of autoimmunity.29 Following the launch of COVID-19 vaccination programmes, case reports have highlighted newly diagnosed autoimmune disorders, including autoimmune liver diseases, GBS, IgA nephropathy, as potential rare side effects of COVID-19 vaccination.30 On the other hand, population-based surveillance study suggests that COVID-19 vaccine is safe in the immune-compromised population without increased risk of acute adverse event,31 however, whether the association between COVID-19 vaccine and new onset autoimmune manifestations is coincidental or causal remains to be elucidated. A descriptive cohort study in Hong Kong found no increased risk of incident ADs among the vaccination group versus non-vaccination group within 28 days following the first or second dose of COVID-19 vaccination.32
In this study we compared individuals with full vaccination (at least two dose vaccination) versus partially/non vaccinated individuals (1/0 dose vaccination) among COVID-19 patients, consistent with previous studies, we did not identify any increased risk of ADs associated with COVID-19 vaccination. Rather, COVID-19 vaccination reduced the risk of COVID-19-associated pemphigoid, immune mediated thrombocytopenia, Graves' disease, anti-phospholipid antibody syndrome, SLE and other autoimmune arthritis. In view of the possibility that COVID-19 infection could activate an ADs response, it is not unexpected that COVID-19 vaccination could confer protection from infection-induced autoimmunity by reducing the severity of COVID-19.33 Our findings give reassurance to a sound vaccination safety profile and suggest additional benefits of COVID-19 vaccination on COVID-19-associated ADs.
Recently, a retrospective cohort study based on the US population found increased risk of newly diagnosed ADs among COVID-19 patients.11 The list of ADs overlapped with our disease selection and we found statistically significant increased risk in both studies for rheumatoid arthritis [aHR: 1.29, 95% CI: 1.09–1.54 (Hong Kong) versus aHR: 2.98, 95% CI: 2.78–3.20 (US)]; spondyloarthritis [aHR: 1.32, 95% CI: 1.03–1.69 (Hong Kong) versus aHR: 3.21, 95% CI: 2.50–4.13 (US)]; psoriasis [aHR: 1.42, 95% CI: 1.13–1.78 (Hong Kong) versus aHR: 2.91, 95% CI: 2.67–3.17 (US)]. Increased risk was identified as statistically significant in US but not in Hong Kong including SLE [aHR: 1.05, 95% CI: 0.79–1.39 (Hong Kong) versus aHR: 2.99, 95% CI: 2.68–3.34 (US)]; dermatopolymyositis [aHR: 1.50, 95% CI: 0.92–2.45 (Hong Kong) versus aHR: 1.96, 95% CI: 1.47–2.61 (US)]; Systemic sclerosis [aHR: 1.2, 95% CI: 0.64–2.25 (Hong Kong) versus aHR: 2.58, 95% CI: 2.02–3.28 (US)]; Sjögren's syndrome [aHR: 1.49, 95% CI: 0.98–2.27 (Hong Kong) versus aHR: 2.62, 95% CI: 2.29–3.00 (US)]; inflammatory bowel disease [aHR: 1.17, 95% CI: 0.83–1.64 (Hong Kong) versus aHR: 1.78, 95% CI: 1.72–1.84 (US)].
In general, the reported aHR in Hong Kong was lower than the aHR reported in the US regardless of the type of ADs. Several structural differences in study design could serve to explain this phenomenon. First, individuals with COVID-19 vaccination were removed completely from the US cohort to exclude interference from vaccination. Given the above-mentioned hypothesis that COVID-19 vaccine confers protective effect on COVID-19-indcuded ADs, the level of COVID-19 related risk could be attenuated by the high vaccination rate among COVID-19 infected patients in our study cohort (Table 1, 81.9% completed at least two dose vaccination). This assumption is further verified by another US-based cohort study12 investigating a major autoimmune outcome—IBD, using multi-institutional research network TriNetX. Within a propensity score matched cohort with COVID-19 vaccination incorporated, the incidence rate ratio among COVID-19 positive group was significantly lower than the COVID-19 negative group [Relative risk: 0.64 (95% CI: 0.54–0.65)] with hypothesised protective effect from COVID-19 vaccination. Second, Omicron is the dominant SAR-CoV-2 variant in the Hong Kong cohort, which might render a milder disease severity and probably a lower risk of autoimmune sequelae associated with COVID-19.34,35
Our study had several merits. First, we covered the clinical spectrum of autoimmune manifestations in various body systems of COVID-19 patients to bridge the current research gap on a possible link between COVID-19 and autoimmunity. Second, we incorporated COVID-19 vaccination records to extend the vaccine benefits, complementing the earlier retrospective cohort study where vaccinated individuals were excluded completely. Third, current case reports focus only on the acute phase of COVID-19 or within one month after recovery, while long-term outcomes were difficult to link with COVID-19. By using a high quality electronic medical database, we were able to explore the long-term clinical events beyond the case report. Fourth, we conducted multivariable regression as well as subgroup analyses to identify risk factors other than COVID-19 to define vulnerable subgroup populations. Our findings could serve to optimise current disease surveillance, management, diagnosis, and prognosis procedures for vulnerable subpopulations, such as females and the elderly without completed full doses of COVID-19 vaccine.
We admit several limitations in the study. First, COVID-19 cases could be under-reported in our study. It is optional to upload the positive COVID-19 test results to governmental system in Hong Kong.36 The current non-COVID group (without history of COVID-19 positive test from PCR or RAT) may be contaminated by individuals who did not report their positive condition, therefore might not be purely COVID-19 free. Consequently, the study is likely suffered from misclassification and the degree of increased risk among COVID-19 patients might be underestimated. Second, the diagnosis of ADs was based on ICD-9-CM codes from the clinical setting. Albeit the likelihood of misdiagnosis; this should occur evenly in both COVID-19 and non-COVID-19 groups with minimal impact on hazard ratio estimation. Of note, joint pain was commonly reported in patients with COVID-19.37 However, as a common flaw of the structure of EMR-based retrospective studies, our study was unable to capture all symptom-related outcomes that were possibly recorded in the clinical notes but not the ICD-9-CM based diagnosis. Third, we exclude all patients diagnosed with studied autoimmune disorder before the index date, the generalisability of our conclusions was therefore limited to autoimmune naïve individuals. Fourth, patients diagnosed with COVID-19 are likely associated with more frequent healthcare seeking behaviour in the short term, thus increasing the chance of being diagnosed with ADs compared with the non-COVID population. We therefore applied a 28-day washout period to address the potential bias induced by different levels of health seeking behaviour among the COVID-19 and non-COVID groups. Fifth, given the broader spectrum of autoimmune diseases, we only selected a subset of autoimmune diseases per discussion among local physicians across subspecialties and world-wide clinical case report, which covers different body systems. Sixth, studied COVID-19 vaccine comprised of mRNA (Pfizer-BNT162b2) and inactivated virus vaccines (Sinovac-CoronaVac) only, caution should be applied when generalising the study outcome to other vaccines with different technologies and brands. Last, social economic confounders like incomes, access to healthcare, and education were not assessed in the absence of corresponding information in EMR database.
This territory-wide retrospective cohort study suggests COVID-19 is associated with increased risk of developing various autoimmune disorders and the risk is attenuated by full COVID-19 vaccination. Physicians should be aware of the possible connections between SARS-CoV-2 and autoimmune manifestations to optimise disease management procedures, early detection and intervention in light of the ongoing pandemic. Future studies investigating pathology and mechanisms would be valuable to interpreting our findings.
Contributors
KP, XL, ICKW were responsible for study concept and design; XL, EYFW, CSLC, FTTL, CKHW, EWYC, ICKW were responsible for data acquisition; KP, JYZ, DLY were responsible for data extractions, cleaning, analysis, verification, and quality control; KP and XL were responsible for manuscript preparation; SCWC, WKL, CSL were responsible for clinical inputs and advice; All authors contributed equally to data interpretation and critical revision of the manuscript of significant intellectual contribution; ICKW was responsible for funding acquisition; ICKW and XL were responsible for the study supervision and the decision to submit for publication; KP, XL, DLY, JYZ, EYFW, CSLC, FTTL, CKHW, EWYC, ICKW claims full access to all the data in the study.
Data sharing statement
The Hospital Authority electronic medical record data underlying the results presented in the study are available from Administrative Assessment of External Data Requests, Hong Kong Hospital Authority Head Office (contact via hacpaaedr@ha.org.hk).
Declaration of interests
XL received research grants from the Research Fund Secretariat of the Food and Health Bureau (HMRF, HKSAR), Research Grants Council Early Career Scheme (RGC/ECS, HKSAR), Research Grants Council Research Impact Fund (RGC/RIF, HKSAR), Janssen and Pfizer; internal funding from the University of Hong Kong; consultancy fee from Merck Sharp & Dohme and Pfizer, unrelated to this work. SCWC has received traveling support from Novartis and GSK. EYFW has received research grants from the Food and Health Bureau of the Government of the Hong Kong SAR, National Natural Science Foundation of China, and the Hong Kong Research Grants Council, outside the submitted work. CSLC has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen; personal fees from Primevigilance Ltd.; outside the submitted work. FTTL has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from Food and Health Bureau of the Government of the Hong Kong SAR, outside the submitted work. CKHW reports receipt of research funding from the EuroQoL Group Research Foundation, the Hong Kong Research Grants Council, the Hong Kong Health and Medical Research Fund, AstraZeneca, and Boehringer Ingelheim; all of which are outside this work. EWC reports grants from the Food and Health Bureau (Hong Kong), the Research Grants Council (RGC, Hong Kong), National Natural Science Fund of China, Bayer, AstraZeneca, Novartis, RGA Reinsurance Company, Pfizer, Narcotics Division of the Security Bureau of HKSAR; Consulting fee from Pfizer, Novartis, and AstraZeneca; honorarium from Hospital Authority (Hong Kong), Pfizer, Novartis, and AstraZeneca, outside the submitted work. WKL received speaker fees from Ferring, Janssen, Takeda and Daiichi Sankyo. He has also participated on the Data Safety Advisory Board of AstraZeneca and Pfizer. All outside the submitted work. CSL reports payment of speaker fees from AbbVie, AstraZeneca, GSK, Janssen, Pfizer and Roche. He has also participated on the AstraZeneca Data Safety Advisory Board. All outside the submitted work. ICKW receives research funding outside the submitted work from Amgen, Bristol Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, Takeda, the Hong Kong Research Grants Council, the Health Bureau of the Government of the Hong Kong Special Administrative Region, National Institute for Health Research in England, European Commission, and the National Health and Medical Research Council in Australia; has received consultancy fee from the World Health Organization and IQVIA, and is a non-executive director of Jacobson Medical in Hong Kong. All other authors report no conflicts of interest.
Acknowledgements
This work was supported by RGC Collaborative Research Fund (C7154-20GF). We appreciate Ms Lisa Lam for proofreading the manuscript.
Footnotes
Translation: For the Chinese translation of the abstract see Supplementary Materials section.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102154.
Contributor Information
Xue Li, Email: sxueli@hku.hk.
Ian C.K. Wong, Email: wongick@hku.hk.
Appendix A. Supplementary data
References
- 1.World Health Organization . 2023. WHO coronavirus (COVID-19) dashboard.https://covid19.who.int/ [Google Scholar]
- 2.Tutal E., Ozaras R., Leblebicioglu H. Systematic review of COVID-19 and autoimmune thyroiditis. Travel Med Infect Dis. 2022;47 doi: 10.1016/j.tmaid.2022.102314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariño H., Heartshorne R., Michael B.D., et al. Neuroimmune disorders in COVID-19. J Neurol. 2022;269(6):2827–2839. doi: 10.1007/s00415-022-11050-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gracia-Ramos A.E., Martin-Nares E., Hernandez-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10(12):3592. doi: 10.3390/cells10123592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smatti M.K., Cyprian F.S., Nasrallah G.K., Al Thani A.A., Almishal R.O., Yassine H.M. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11(8):762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halpert G., Shoenfeld Y. SARS-CoV-2, the autoimmune virus. Autoimmun Rev. 2020;19(12) doi: 10.1016/j.autrev.2020.102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montazersaheb S., Hosseiniyan Khatibi S.M., Hejazi M.S., et al. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J. 2022;19(1):92. doi: 10.1186/s12985-022-01814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Najafi S., Rajaei E., Moallemian R., Nokhostin F. The potential similarities of COVID-19 and autoimmune disease pathogenesis and therapeutic options: new insights approach. Clin Rheumatol. 2020;39(11):3223–3235. doi: 10.1007/s10067-020-05376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mobasheri L., Nasirpour M.H., Masoumi E., Azarnaminy A.F., Jafari M., Esmaeili S.-A. SARS-CoV-2 triggering autoimmune diseases. Cytokine. 2022;154 doi: 10.1016/j.cyto.2022.155873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang R., Yen-Ting Chen T., Wang S.-I., Hung Y.-M., Chen H.-Y., Wei C.-C.J. Risk of autoimmune diseases in patients with COVID-19: a retrospective cohort study. eClinicalMedicine. 2023;56 doi: 10.1016/j.eclinm.2022.101783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadi Y., Dulai P.S., Kupec J., et al. Incidence, outcomes, and impact of COVID-19 on inflammatory bowel disease: propensity matched research network analysis. Aliment Pharmacol Ther. 2022;55(2):191–200. doi: 10.1111/apt.16730. [DOI] [PubMed] [Google Scholar]
- 13.Chan E.W., Lau W.C., Leung W.K., et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology. 2015;149(3):586–595.e583. doi: 10.1053/j.gastro.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Li X., Tong X., Yeung W.W.Y., et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2022;81(4):564–568. doi: 10.1136/annrheumdis-2021-221571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai F.T.T., Li X., Peng K., et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine: a case-control study. Ann Intern Med. 2022;175(3):362–370. doi: 10.7326/M21-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X., Peng K., Cheng F.W.T., et al. Tuberculosis following two-dose SARS-CoV-2 vaccination with messenger RNA vaccine (BNT162b2) and inactivated virus vaccine (CoronaVac) J Infect. 2022;86(3):256–308. doi: 10.1016/j.jinf.2022.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X.R., Pawitan Y., Clements M.S. Generalized survival models for correlated time-to-event data. Stat Med. 2017;36(29):4743–4762. doi: 10.1002/sim.7451. [DOI] [PubMed] [Google Scholar]
- 18.El Hasbani G., Jawad A., Uthman I. Axial and peripheral spondyloarthritis triggered by sars-cov-2 infection: a report of two cases. Reumatismo. 2021;73(1):59–63. doi: 10.4081/reumatismo.2021.1374. [DOI] [PubMed] [Google Scholar]
- 19.Derksen V.F.A.M., Kissel T., Lamers-Karnebeek F.B.G., et al. Onset of rheumatoid arthritis after COVID-19: coincidence or connected? Ann Rheum Dis. 2021;80(8):1096. doi: 10.1136/annrheumdis-2021-219859. [DOI] [PubMed] [Google Scholar]
- 20.Kocyigit B.F., Akyol A. Reactive arthritis after COVID-19: a case-based review. Rheumatol Int. 2021;41(11):2031–2039. doi: 10.1007/s00296-021-04998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson N., Eckhardt D., Delano A. New-onset bullous pemphigoid in a COVID-19 patient. Case Rep Dermatol Med. 2021;2021 doi: 10.1155/2021/5575111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mateu-Salat M., Urgell E., Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves' disease after COVID-19. J Endocrinol Invest. 2020;43(10):1527–1528. doi: 10.1007/s40618-020-01366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahramnezhad F., Ghorbani B., Ghaedrahamt M., Jamaati H. Coronavirus-disease-2019-induced antiphospholipid-like syndrome: a case report. J Med Case Rep. 2021;15(1):408. doi: 10.1186/s13256-021-02966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deruelle E., Ben Hadj Salem O., Sep Hieng S., Pichereau C., Outin H., Jamme M. Immune thrombocytopenia in a patient with COVID-19. Int J Hematol. 2020;112(6):883–888. doi: 10.1007/s12185-020-02943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palao M., Fernández-Díaz E., Gracia-Gil J., Romero-Sánchez C.M., Díaz-Maroto I., Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. 2020;45 doi: 10.1016/j.msard.2020.102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiff D.D., Meyer C.G., Marlin B., Mannion M.L. New onset ANCA-associated vasculitis in an adolescent during an acute COVID-19 infection: a case report. BMC Pediatr. 2021;21(1):333. doi: 10.1186/s12887-021-02812-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segal Y., Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15(6):586–594. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wraith D.C., Goldman M., Lambert P.-H. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362(9396):1659–1666. doi: 10.1016/S0140-6736(03)14802-7. [DOI] [PubMed] [Google Scholar]
- 29.Li Z., Guo J., Bi L. Role of the NLRP3 inflammasome in autoimmune diseases. Biomed Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110542. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y., Xu Z., Wang P., et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165(4):386–401. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 31.Gil-Vila A., Ravichandran N., Selva-O'Callaghan A., et al. COVID-19 Vaccination in Autoimmune Diseases (COVAD) study: vaccine safety in idiopathic inflammatory myopathies. Muscle Nerve. 2022;66(4):426–437. doi: 10.1002/mus.27681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X., Gao L., Tong X., et al. Autoimmune conditions following mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccination: a descriptive cohort study among 1.1 million vaccinated people in Hong Kong. J Autoimmun. 2022;130 doi: 10.1016/j.jaut.2022.102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMenamin M.E., Nealon J., Lin Y., et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22(10):1435–1443. doi: 10.1016/S1473-3099(22)00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyberg T., Ferguson N.M., Nash S.G., et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolter N., Jassat W., Walaza S., et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.RTHK . 2022. Survey finds 12% of over 60s didn't report infections.https://news.rthk.hk/rthk/en/component/k2/1665512-20220904.htm [Google Scholar]
- 37.Tamariz L., Bast E., Abad M., Klimas N., Caralis P., Palacio A. Post COVID-19 joint pain: preliminary report of the relationship with antinuclear antibodies and inflammation. J Med Virol. 2022;94(8):3479–3481. doi: 10.1002/jmv.27753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.