Abstract
The gut microbiota consists of a set of microorganisms that colonizes the intestine and ferment fibers, among other nutrients, from the host’s diet. A healthy gut microbiota, colonized mainly by beneficial microorganisms, has a positive effect on digestion and plays a role in disease prevention. However, dysregulation of the gut microbiota can contribute to various diseases. The nutrition of the host plays an important role in determining the composition of the gut microbiota. A healthy diet, rich in fiber, can beneficially modulate the gut microbiota. In this sense, oats are a source of both soluble and insoluble fiber. Oats are considered a functional ingredient with prebiotic potential and contain plant proteins, unsaturated fats, and antioxidant compounds. The impact of oat consumption on the gut microbiota is still emerging. Associations between oat consumption and the abundance of Akkermansia muciniphila, Roseburia, Lactobacillus, Bifidobacterium, and Faecalibacterium prausnitzii have already been observed. Therefore, this integrative review summarizes the findings from studies on the relationship between oat consumption, the gut microbiota, and the metabolites, mainly short-chain fatty acids, it produces.
Keywords: functional food, prebiotic, diet, β-glucan, metabolites, microbiome
1. Introduction
Oats (Avena sativa L.) contain soluble fibers such as β-glucan but also provide insoluble fibers such as lignin and cellulose [1]. Oats can be considered a functional food with prebiotic potential due to their fiber, lipid, and phenolic compound content [2]. Specifically, the prebiotic potential of β-glucan has been evaluated in several studies [3,4,5,6]. Of note, β-glucan has been associated with hypoglycemic and cholesterol-lowering effects [7]. More recently, it has been reported that β-glucan is fermented by the human gut microbiota, potentially impacting the composition of the microbiota while producing short-chain fatty acids (SCFAs), acetate, propionate, and butyrate [8]. Furthermore, oats have unique phenolic compounds, including avenacolysates, avenacins, and avenanthramides [9]. Some studies suggest that these phenolics compounds possess antioxidant and anti-inflammatory properties, and may provide protection against coronary heart disease, colon cancer, and skin irritation [10]. In addition, oats also provide proteins, including globulins such as avenalins, in addition to prolamins (avenins) [11].
On their own, oats do not contain gluten and can be consumed by patients with celiac disease [11]. Gluten is a protein found in some grains, such as wheat, barley, and rye [12]. Celiac disease is a systemic gastrointestinal disorder mediated by the immune system and induced by gluten ingestion [13]. While wheat and barley contain gliadin, oat changes this prolamin’s form to avenin, which has a molecular structure homologous to gliadin but is not identical [11]. While oats themselves are gluten-free, they are often cross-contaminated with other grains during processing [14]. Thus, gluten-free oats require controlled, contamination-free cultivation, processing, preparation, and storage [13]. The “Codex Alimentarius” of 2008 established 20 mg/kg as the maximum limit for the presence of gluten in “gluten-free” foods [15]. Most patients with celiac disease tolerate a minimum daily amount of 10 mg gluten, but it depends on the sensitivity of each individual [16]. Different reviews and meta-analyses concluded that a moderate consumption of oats is not associated with symptoms and immunological profile characteristics of patients with celiac disease [17,18,19]. However, the consumption of oats is only recommended for patients with celiac disease in remission with monitoring and caution [20]. Of note, although pure oats are safe for most people with celiac disease, adverse immunological reactions may occur [20,21,22].
Diet is one factor most strongly associated with gut microbiota modulation, if not the most associated. The gut microbiota is involved in several bodily functions, including nutrient digestion, metabolism, and fermentation, as well as endocrine and immunomodulatory functions [23,24,25]. The gut microbiota supports barrier function, improves intestinal permeability, and promotes mucous layer integrity [23]. Further, through dietary fiber fermentation, the gut microbiota produces gases (hydrogen and carbon dioxide), SCFAs, branched-chain fatty acids, and some organic acids and alcohols [24].
Due to its complexity and interindividual variation, there is a lack of definition of a “healthy gut microbiota” [25]. Further, the taxonomic composition of gut microbes among healthy individuals is diverse. Thus, identifying a specific set of microbes comprising universally “healthy” microbiota is unlikely. However, it is logical to conclude that a “healthy” gut microbiota would support the absence of any overt disease within the individual. Thus, a microbiome that resists stress and perturbation and can recover to an optimal functional profile is a more promising functional way to define a “healthy” gut microbiome [26]. Nonetheless, some situations can impair the microbiota and generate dysbiosis, which has been associated with several disease states, including inflammatory bowel disease (IBD), obesity, diabetes, allergies, and immune disease [27,28]. From a taxonomic composition standpoint, IBD has been associated with a reduction of Bacteroidetes and Firmicutes phyla, as well as an increase in Proteobacteria and Escherichia coli [27,29,30,31]. The transplantation of gut microbiota from obese mice to lean, germ-free mice increased intestinal permeability, lipogenesis, and adipogenesis [23,32]. In fatty liver diseases, low levels of Firmicutes and Bacteroides have been reported, in addition to high E. coli levels [28]. In individuals with Type 2 diabetes, a decrease in the phylum Firmicutes and Clostridia class was observed compared to healthy individuals [33]. Additionally, Bacteroides fragilis, Enterococcus faecalis, Helicobacter hepaticus, and Fusobacterium nucleatum have been associated with intestinal carcinogenesis [34,35,36], while an increased abundance of Akkermansia muciniphila has been observed in cancer patients who responded well to treatment [37].
The emerging connection of the gut to several other body systems (the gut–brain, gut–lung, and gut–skin axis) reveals a potential impact on health and quality of life. The gut microbiota connection axes have been investigated in several disease states, such as tuberculosis, psoriasis, and Parkinson’s disease [27]. As for the gut–lung axis, there is evidence that individuals with asthma have a higher expression of bacterial histamine decarboxylase in the gut, as well as increased abundance of the histamine-secreting species, Morganella morganii [27]. Gut–brain axis communication is proposed to occur through the enteric nervous system, including the sympathetic and parasympathetic systems [27]. Emerging data in cross-sectional clinical trials suggest the gut microbiota may be associated with anxiety, stress, depression, and cognitive learning behaviors [38].
The gut microbiota is impacted by age, genetics, exercise, antibiotics, smoking, and, especially, diet [28]. Dietary nutrients interact directly with the gut microbiota, affecting its composition and growth kinetics [39]. Since certain fibers can act as prebiotics or substrates that are selectively utilized by host microorganisms conferring a health benefit, examining the impact of including fiber-containing foods, such as oats, barley, chia, flaxseed, and soy, in the diet to modulate the gut microbiota has been a topic of continued research interest [40]. The fiber content of the grains varies according to the species, part of the plant, location, and characteristics of cultivation and processing [41]. Barley and oats have a high fiber content, around 10 to 15% [42]. A high-fiber diet is associated with greater microbial diversity [43]. However, a low-fiber diet was associated with an increase in enteric pathogenic microorganisms and a reduction in beneficial Firmicutes, which are responsible for metabolizing plant-derived polysaccharides into SCFAs [43]. As an example of a substrate for gut microbiota fermentation, soluble fiber from oats has been shown to be an energy source for butyrate-producing bacteria [44].
Although most of the scientific literature has focused on the effects of oat consumption on cardiovascular health, the present work proposes to focus on the benefits of oat consumption for promoting gut health, proper balance of the colon microbiota, and production of SCFAs by this complex community.
2. Methodology
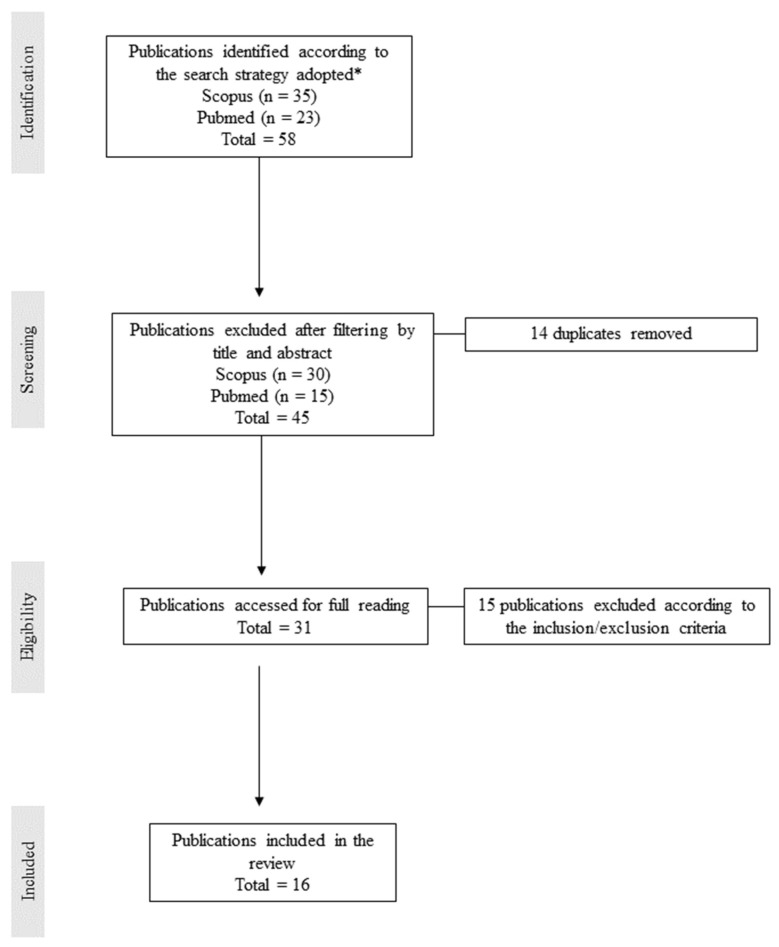
The methodological approach utilized in this work was based on Kutcher and LeBaron’s guide on completing an integrative review [45]. The criteria for inclusion were the selection of original articles (clinical trials, in vitro, and in vivo trials) from 2012 to 2023. All relevant papers on the modulation of the gut microbiota with oat products or supplements were downloaded from Scopus and PubMed. Terms identified comprised three cores: the food matrix “oat,” the main interest “microbiota,” and the metabolites that represent an important relationship between oats and the gut microbiota, “SCFA—short-chain fatty acids.” Using the identified terms, an advanced search procedure was performed as follows: “oat” AND “microbiota” AND “short-chain fatty acids” AND “SCFA” on October and November 2022. This procedure was repeated in April 2023. From this advanced search, 58 studies were identified, including 35 from Scopus and 23 from PubMed (Figure 1).
Figure 1.
Methodology diagram of integrative review. * Advanced search by keywords “oat” and “microbiota” and “short-chain fatty acids” and “SCFA” (from 2012 to 2023).
Screening of publications was performed by reviewing the title, abstract, and keywords to determine potentially relevant studies. After screening, 45 articles were identified and duplicates were removed, leaving 31 publications for full text review. During full-text review, 15 publications were excluded because they were either reviews, not specific to oats, or the study was in a diseased population. Following full text review, 16 publications were included in the review.
Table 1 includes in vivo studies using animals, Table 2 includes in vitro studies, and Table 3 includes clinical studies in humans.
Table 1.
Scientific articles in vivo (animals) reporting the relation between oat consumption and microbiota modulation.
| Authors (Year) | Country | Title | Journal | Food Matrix or Supplement | Aim | Methodology | Outcomes of Interest |
|---|---|---|---|---|---|---|---|
| Wang, Qi, Guo, Song, Pang, Fang, and Peng (2022) [46] | China | Effects of Oats, Tartary Buckwheat, and Foxtail Millet Supplementation on Lipid Metabolism, Oxide-Inflammatory Responses, Gut Microbiota, and Colonic SCFA Composition in High-Fat-Diet-Fed Rats | Nutrients | Cooked oats, tartaric buckwheat, and foxtail millet | Investigate the effect of cooked oats, tartaric buckwheat, and foxtail millet action on lipid levels, oxide-inflammatory responses, intestinal microbiota, and SCFA in rats. | Sixty male Sprague Dawley rats (n = 12 per group) were fed a basal diet, a high-fat diet (HFD), HFD with 22% cooked oats, HFD with 22% buckwheat, and HFD with 22% millet for 12 weeks. |
|
| Han, Gao, Song, Zhang, Li, and Zhang (2021) [47] | China | Oat Fiber Modulates Hepatic Circadian Clock via Promoting Gut Microbiota-Derived Short-Chain Fatty Acids | Journal of Agricultural and Food Chemistry | Oat Fiber | Evaluate the action of SCFAs produced by gut microbiota on circadian rhythm. | Seventy-two male C57BL/6 mice (24 per group) were fed a control diet, HFD, or HFD with 0.8% oat for 21 weeks. |
|
| Ji, Ma, Zhang, Wang, Tao, Pei, and Hu (2021) [48] | China | Dietary Intake of Mixture Coarse Cereals Prevents Obesity by Altering the Gut Microbiota in High-Fat-Diet-Fed Mice | Food and Chemical Toxicology | Mix with millet, maize, oat, soybean, and purple potato | Evaluate the consumption of mixture coarse cereals on obesity prevention and gut microbiota in HFD-fed mice. | Forty-eight male C57BL/6 mice (n = 8 per group) were fed a chow diet (10% calories from fat) containing either 0%, 20%, or 40% mixture coarse cereals; or a HFD (45% calories from fat) containing either 0%, 20%, or 40% mixture coarse cereals for 8 weeks. |
|
| Kundi, Lee, Pihlajamaki, Chan, Leung, Yu So, Nordlund, Kolehmainen, and El-Nezami (2020) [49] | China | Dietary Fiber from Oat and Rye Brans Ameliorate Western Diet–Induced Body Weight Gain and Hepatic Inflammation by the Modulation of Short-Chain Fatty Acids, Bile Acids, and Tryptophan Metabolism | Molecular Nutrition and Food Research | Oat bran | Elucidate the protective mechanisms conferred by oat and rye fibers in a Western diet. | Forty-eight male C57BL/6N mice (n = 12 per group) were fed a chow diet, a Western diet (WD), or a WD with 10% oat or rye bran, for 17 weeks. |
|
| Gao, Song, Li, Zhang, Wan, Wang, Zhang, and Han (2020) [50] | China | Effects of Oat Fiber Intervention on Cognitive Behavior in LDLR−/− Mice Modeling Atherosclerosis by Targeting the Microbiome-Gut-Brain Axis | Journal of Agricultural and Food Chemistry | Oat fiber | Elucidate the oat fiber action on cognitive behavior through neuroinflammatory signals and the gut–brain axis. | Twenty male LDLR−/− mice (n = 10 per group) were fed a high-fat-and-cholesterol (HFC) diet (46% kcal from fat) with or without 0.8% oat fiber for 14 weeks. Ten male wild-type mice who were fed chow were used as controls. |
|
| Huang, Yu, Li, Guan, Liu, Song, Liu, and Duan (2020) [51] * | China | Effect of Embryo-Remaining Oat Rice on the Lipid Profile and Intestinal Microbiota in High-Fat-Diet-Fed Rats | Food Research International | Embryo-remaining oat rice (EROR) | Investigate the effects of Embryo-remaining oat rice (EROR) on lipid profile, cecal SCFAs, and intestinal microbiota in HFD-fed rats. | Twenty-four male SD rats (n = 6 per group) were fed a normal diet, HFD, HFD with 10% EROR, HFD with 50% EROR for 4 weeks. |
|
| Sun, Tong, Liang, Wang, Liu, Zhou, and Zhou (2019) [52] | China | Effect of Oat and Tartary Buckwheat-based Food on Cholesterol-Lowering and Gut Microbiota in Hypercholesterolemic Hamsters | Journal of Oleo science | Oat (65%) and tartary buckwheat (25%) blend | Investigate the effects of oat-based food on cholesterol and the composition of the gut microbiota. | Thirty male golden hamsters (n = 10 per group) were fed a control diet, HFD, and HFD with 10% oat/buckwheat blend for 30 days. |
|
| Dong, Zhu, Ma, Xiang, Shen, and Liu (2016) [53] | China | Oat Products Modulate the Gut Microbiota and Produce Anti-Obesity Effects in Obese Rats | Journal of Functional Foods | Oat meal: OM; oat flour: OF; and oat bran: OB | Compare the actions of oat meal, oat flour, and high-fiber oat bran on lipid metabolism, as well as gut microbiota of HFD-fed rats. | Eighty male SD (n = 10 per group) rats were fed either a chow-control diet, an HFD, an HFD with oat meal, an HFD with oat flour, or an HFD with high-fiber oat bran for 8 weeks. |
|
| Wilczak, Blaszczyk, Kamola, Gajewska, Harasym, Jatosinska, Gudej, Suchecka, Oczkowski, Gromadzka–Ostrowska (2015) [54] | Poland | The Effect of Low- or High-Molecular-Weight Oat β-glucans on the Inflammatory and Oxidative Stress Status in the Colon of Rats with LPS-Indices Enteritis | Food and Function | Low- and high-molecular-weight oat β-glucans | Investigate the protective effect of low- and high-molecular-weight β-glucans in gut immune markers, microbiota changes and SCFAs. | Seventy-two male SD rats (n = 12 per group) were fed a control diet, low-molecular-weight β-glucan (1%) diet, or high-molecular-weight β-glucan (1%) diet for 6 weeks; half were administered LPS to induce enteritis. |
|
* The study used in vivo and in vitro experiments. Therefore, it is mentioned in this table and Table 2.
Table 2.
Scientific articles in vitro reporting the relation between oat consumption and microbiota modulation.
| Authors (Year) | Country | Title | Journal | Food Matrix or Supplement | The Aim | Methodology | Outcomes of Interest |
|---|---|---|---|---|---|---|---|
| Bai, Zhang, Zhang, Zhang, Huo, Guo (2022) [55] | China | In Vitro Fermentation of Pretreated Oat Bran by Human Fecal Inoculum and Impact on Microbiota | Journal of Functional Foods | Oat bran pretreated | Determine the prebiotic effects of different pretreatments of oat bran. | Oat bran was steamed, microwaved, or dryed with hot air. The samples were exposed to in vitro simulated digestion and added to human fecal inoculum to investigate fermentation metabolites and impacts on microbiota. |
|
| Liang, xie, Evivie, Zhao, Chen, Xu, Liu, Li, Huo (2021) [56] | China | Study on Supplementary Food with Beneficial Effects on the Gut Microbiota of Infants | Food Bioscience | Ready-to-use supplementary food with oat, corn, and millet | Investigate the impact of oat-, corn-, and millet-supplementary foods on gut microbiota and SCFA production. | Oat-, corn-, and millet-supplementary foods and an oligosaccharide control were added to human fecal inoculum from 6 infant donors (age 6–12 months) to investigate fermentation metabolites and impacts on microbiota. |
|
| Glei, Zetzmann, Lorkowschi, Dawczynski, and Scholormann (2020) [57] | Germany | Chemo-Preventive Effects of Raw and Roasted Oat Flakes After In Vitro Fermentation With Human Fecal Microbiota | International Journal of Food Sciences and Nutrition | Oat flakes | Analyzed the chemo-preventive effects of raw and roasted oat flakes, evaluating the processing in colon adenoma cells. | The oat flakes were roasted at 140 °C–160 °C for 20 min. The raw and roasted oat flakes were exposed to an in vitro simulated digestion and fermentation with human microbiota. Then, the fermentation supernatants (FS) obtained were characterized, and chemo-preventive effects were analyzed in LT97 colon adenoma cells. |
|
| Huang, Yu, Li, Guan, Liu, Song, Liu, and Duan (2020) [51] * | China | Effect of Embryo-Remaining Oat Rice on the Lipid Profile and Intestinal Microbiota in High-Fat-Diet-Fed Rats | Food Research International | Embryo-remaining oat rice (EROR) | Investigate the effects of an extract of EROR on lipid accumulation. | Water and lipid extracts of EROR were incubated with HepG2 cells to evaluate lipid accumulation in vitro. |
|
| Kristek, Wiese, Heuer, Kosik, Schar, Soycan, Alsharif, Kuhnle, Walton, and Spencer (2019) [58] | United Kingdom and Denmark | Oat Bran, But Not Its Isolated Bioactive β-glucans or Polyphenols, Has a Bifidogenic Effect in an In Vitro Fermentation Model of the Gut Microbiota | British Journal of Nutrition | Oat bran, β-glucan extract, oat polyphenols | Evaluate and compare the effects of an oatpolyphenol mix (avenanthramide, hydroxycinnamic acids, and benzoic acid derivatives), β-glucan extract (BG), and oat bran on gut microbiota. | The oat bran was digested in vitro, and the polyphenols were extracted from undigested (raw) and digested (after in vitro digestion) oat bran. Two different doses (1 and 3% (w/v)) of oat bran and matched concentrations of β-glucan extract or polyphenol mix were added to anaerobic fecal batch cultures. |
|
| Carlson, Erickson, Hess, Gould and Slavin (2017) [4] | United States | Prebiotic Dietary Fiber and Gut Health: Comparing the In Vitro Fermentations of Beta-Glucan, Inulin, and Xylooligosaccharide | Nutrients | β-glucan and Oatwell (oat bran with 22% β-glucan) | Compare the fermentation effects of prebiotics (inulin, xylooligosaccharides and β-glucan based products) in the production of SCFA. | Five common prebiotic dietary fibers, OatWell, WholeFiber (dried chicory root blend with inulin, pectin, hemi and cellulose), xylooligosaccharide, pure inulin, and pure β-glucan, were incubated in fecal inoculum from healthy adults to measure microbiota, SCFA, and gas production. |
|
* The study used in vivo and in vitro experiments. Therefore, it is mentioned in Table 1 and this table.
Table 3.
Clinical studies reporting the relation between oat consumption and microbiota modulation.
| Authors (Year) | Country | Title | Journal | Food Matrix or Supplement | The Aim | Methodology | Outcomes of Interest |
|---|---|---|---|---|---|---|---|
| Xu, Feng, Chu, Wang, Shete, Tuohy, Liu, Zhou, Kamil, Pan, Liu, Yang, Yang, Zhu, Lv, Xiong, Wang, Sun, Sun, and Yang (2021) [59] | China | The Prebiotic Effects of Oats on Blood Lipids, Gut Microbiota, and Short-Chain Fatty Acids in Mildly Hypercholesterolemic Subjects Compared with Rice: A Randomized, Controlled Trial | Frontiers in Immunology | Oat | Evaluate the relationship of blood lipids, intestinal microbiota, and SCFAs in a Chinese population with mild hypercholesterolemia. | In a randomized parallel design, 210 mildly hypercholesterolemic males and females from Beijing, Nanjing, and Shanghai were assigned to a diet containing 80 g/d of oats or rice (control) for 45 days, along with their habitual diet. |
|
| Valeur, Puaschitz, Midtvedt, and Berstad (2016) [60] | Norway | Oatmeal Porridge: Impact on Microflora-Associated Characteristics in Healthy Subjects | British Journal of Nutrition | Oatmeal porridge | Evaluate the effect of oatmeal porridge consumption every day for one week on fecal SCFAs, gas production, and inflammatory markers. | Ten healthy males and females consumed a daily portion of 60 g oatmeal porridge for 7 days. Lactulose-induced intestinal gas production, fecal excretion of SCFA, fecal levels of β-galactosidase and urease, and PGE2 levels were analyzed. |
|
3. Oat as a Functional Food
According to the Food and Nutrition Board of the Institute of Medicine, the adequate intake (AI) for fiber is 14 g of total fiber per 1000 kcal, or 25 g for adult women and 38 g for adult men [61]. The AI for fiber represents the median fiber intake level observed to achieve the lowest risk of coronary heart disease [61]. While there is no tolerable upper-intake level for fiber, an excessive intake of dietary fiber can cause flatulence, bloating, and diarrhea. Therefore, consuming dietary fiber in adequate amounts can be beneficial to health, reducing the risk of several chronic diseases and supporting digestive health through modulation of laxation, fermentation, and the gut microbiota [62]. In this sense, oat intake can play a role in achieving the AI for fiber. The nutritional composition of oats includes carbohydrates, soluble fiber, proteins, lipids (such as unsaturated fatty acids), vitamins, minerals, and phenolic compounds. The International Food Information Council (IFIC) defines the term “functional foods” as foods or food components that provide a health benefit beyond basic nutrition [63]. Oats could be considered a functional food because of the presence of β-glucan, a soluble fiber shown to reduce cholesterol [63,64]. While there is no regulatory definition of functional food, the US Food and Drug Administration (FDA) authorized the use of a health claim regarding soluble oat fiber and the reduction in coronary heart disease risk based primarily on its cholesterol-lowering abilities [65,66]. The soluble fiber in oats may also influence gut microbiota.
Oats also have high protein levels compared to other cereals [67]. The proteins present in oats are mainly avenins and avenalins [11]. Oat proteins are of a higher quality than many other cereal grains since they have higher levels of essential amino acids, such as lysine [67]. Thus, oats may be a complementary alternative to animal protein due to the current market demand for non-animal protein substitutes. Bioactive peptides from oat protein have been studied and identified as compounds potentially beneficial to health in in vitro and animal studies [67]. Some of the benefits under investigation include reducing blood pressure and cholesterol, immunomodulation, and blood glucose control [67]. However, more clinical studies in humans are needed, as well as a better understanding of effective doses and potential mechanisms of action specific to oat protein.
Other bioactive compounds from oat include Vitamin E, flavonoids, phenolics, phytosterols, and avenanthramides [68]. These metabolites act as defense mechanisms during plant growth and have antioxidant functions [68,69]. Vitamin E, or tocopherol isomers, has a high antioxidant capacity and may also have anti-inflammatory properties, which could impact the risk of cancers and cardiovascular disease [70]. Phenolic compounds found in oats include caffeic acid, coumaric acid, phytic acid, gallic acid, and vanillic acid [68]. Avenanthramides (AVAs) are the most abundant phenolic alkaloids unique to oats [69,71]. There are more than 25 forms of AVAs described in oats, which have an antioxidant activity 30 times greater than other phenolic compounds [71,72]. AVAs have also been investigated for their anti-inflammatory, antiproliferative, pro-apoptotic and antiatherogenic actions [68,69,71].
3.1. Anticholesterolemic, Hypoglycemic, and Antihypertensive Properties
There is robust evidence that the consumption of oats or oat-containing foods lowers total cholesterol and low-density lipoprotein (LDL) cholesterol in healthy adults who are overweight or obese, as well as individuals with Type 2 diabetes [73,74,75]. A dose-response meta-analysis concluded that 3 g of β-glucan per day were able to effectively reduce total cholesterol [76]. One potential mechanism by which β-glucan modulates cholesterol metabolism is by increasing viscosity in the intestine, thereby influencing bile acid metabolism [64]. Increasing the viscosity of the intestinal contents limits bile acid reabsorption in the terminal ileum and increases their fecal excretion. In the absence of bile acid recycling, de novo synthesis of bile acids is required, resulting in the utilization of cholesterol [64,77]. Animal and human studies have demonstrated fecal excretion of bile acids and an elevation of bile acid synthesis after consumption of oats or isolated β-glucan [78,79]. Oats and isolated β-glucan may also modulate gut microbiota composition and function to influence cholesterol metabolism. Further, bile acids can be deconjugated by certain microbiota, which inhibits their reabsorption [67]. These microbes include certain Bifidobacterium, Bacteroides, and Lactobacillus species that have high activities of bile salt hydrolase, the enzyme responsible for the deconjugation of bile acids [64,80]. β-glucan has been shown to increase these microbes in animal and in vitro studies [64]. In addition, the proteins and lipids present in oats may also contribute to cholesterol control [81]. Higher concentrations of proteins and lipids in oats were responsible for lower serum levels of total cholesterol and LDL-cholesterol in rats fed with a hypocholesterolemic diet [81]. Health benefits are also attributed to phytochemicals present in oats. Antioxidant compounds, plant sterols, Vitamin E, and polyunsaturated acids are associated with the prevention of cardiovascular disease [82].
The viscosity created in the intestine by oat β-glucan may also contribute to the reduction of blood glucose [82]. The viscous nature of the bolus slows transit and makes it difficult for digestive enzymes to access carbohydrate molecules [83]. It also limits the access of monosaccharides to the luminal surface to be absorbed. Oat β-glucan has also been shown to promote satiety, possibly contributing to weight control [53,71]. A randomized controlled trial in adults with hypertension demonstrated a reduction in blood pressure in participants following a DASH diet with oat bran supplementation [84]. The participants were also able to reduce hypertension medications and experienced changes in the gut microbiota (increased abundance of Bifidobacterium and Spirillum populations) [84]. While further research is needed, there is emerging data that SCFAs, produced by the fermentation of fiber by gut microbiota, may activate receptors in the kidneys and blood vessels to inhibit renin release and decrease blood pressure [85]. Further, in animal and in vitro models, oat β-glucan has demonstrated immunomodulatory, anti-inflammatory, and antioxidant effects, but these need further investigation in human clinical trials [54,86].
3.2. Prebiotic Potential
The International Scientific Association for Probiotics and Prebiotics (ISAPP) defines a “prebiotic” as a substrate that is selectively used by host microorganisms and confers health benefits [40]. Prebiotics are fermented by the gut microbiota and selectively favor the growth of beneficial microorganisms [87,88]. While the ISAPP definition is the most widely used in scientific literature, few global regulatory agencies have defined prebiotics for use in foods or for claims. The FDA has not established a regulatory definition for prebiotics, but they are regulated similar to other food ingredients, such as food additives, or generally recognized as safe (GRAS) ingredients [89]. The European Food Safety Authority (EFSA) uses the FAO 2008 definition, which describes prebiotics as “non-viable food components that confer a health benefit to the host associated with modulation of the microbiota” [89,90]. Importantly, EFSA has noted that changes in microbiota alone are not a benefit, but the change in microbiota must result in a health benefit to the host [91,92]. Japan does not have a regulatory definition for prebiotic but does consider oligosaccharides and dietary fiber as foods that modify gastrointestinal conditions; it can be used for health-promotion purposes [93]. The Canada Food Inspection Agency considers the use of the prebiotic term on food labels to be an implied health claim, and a health claim can only be used when a physiological benefit is demonstrated in humans [94]. Thus, all definitions of a prebiotic include the mention of health promotion of the host through modulation of the gut microbiota [91]. Specific prebiotic benefits have not been defined but could include promoting the integrity of the intestinal mucosal barrier, improving nutrient digestion and absorption, increasing immunity, reducing pH and production of SCFAs, as well as inhibiting pathogenic microorganisms [43]. However, the challenge in meeting some regulatory definitions of prebiotics is proving these benefits result from the changes in the gut microbiome populations or function.
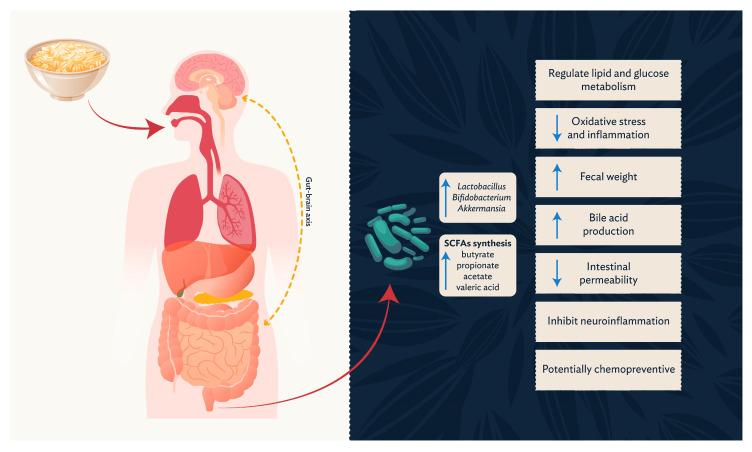
Figure 2 represents a summary of findings from the articles selected for this review. Therefore, the figure shows the performance of oats as a functional food, the modulation of the gut microbiota, and the production of SCFAs. Oat β-glucan has demonstrated potential prebiotic properties through its fermentation to SCFAs, but additional research is needed to demonstrate a health benefit of SCFAs. β-glucan has also been shown in human and animal studies to promote the growth of certain beneficial gut microbes. A randomized controlled trial reported a significant increase in Bifidobacterium and Akkermansia, as well as a reduction in the Sutterellaceae family when 80 g/d of oats were consumed as part of a normal diet for 45 days [59]. Other animal and in vitro studies reported an increase in Bifidobacterium, as well as other beneficial bacteria such as Lactobacillus, the Firmicutes phylum, and the Eubacteriaceae family [46,48,51,52,53,56]. Kristek et al. (2019) concluded that oat bran had a greater impact on the intestinal microbiota than supplementation with β-glucan or isolated polyphenols, suggesting the importance of food form [58]. Oat bran increased the proliferation of Bifidobacterium adolescentis after in vitro fermentation, but β-glucan alone did not [58]. Another in vitro study evaluated different ways of preparing oat bran, such as steaming, microwaving, and drying with hot air [55]. Preparation methods did not change the amount of dietary fiber or β-glucan, and all oat bran treatments were able to decrease the abundance of Escherichia–Shigella and increase Faecalibacterium prausnitzii in the gut microbiota [55]. However, steamed oat bran produced more SCFAs (acetic, propanoic, and butyric acid) than microwaved or hot air-dried oat bran [55]. This suggests heat treatment of oat or oat bran may increase its fermentability [95].
Figure 2.
Effects of oat ingestion on gut modulation, including the increase of Lactobacillus, Bifidobacterium and Akkermansia. Further, oat intake results in increased butyrate, propionate, acetate, and valeric acid production. These microbes and metabolites are responsible for health benefits, such as regulation of lipid and glucose metabolism, decreases in oxidative stress and inflammation, increases in fecal weight, increased bile acid production, decreased intestinal permeability, inhibition of neuroinflammation, and potentially chemo-preventive properties. These systemic effects are possibly due to interactions within the gut–brain axis (dotted yellow arrow). The red arrows indicate the way of oat ingestion and digestion, and the blue arrows indicate an increase or decrease in markers of interest.
3.3. Short-Chain Fatty Acids and Related Metabolites
The gut microbiome has expansive metabolic capacity, and many different metabolites are produced as the microbes ferment undigested food. Short-chain fatty acids (SCFAs) are the most prevalent and well-researched metabolites produced during bacterial fermentation. A decrease in the abundance of SCFA-producing bacteria has been associated with chronic conditions, such as diabetes, inflammatory bowel disease, and atherosclerosis [96]. However, more clinical trials are needed to further elucidate the health effects of SCFAs and most plausible mechanisms for potential benefits.
Acetate, propionate, butyrate, isobutyrate, valerate, isovalerate, and hexanoate are the main SCFAs produced from dietary fibers in the human colon [23]. Acetate is produced at the greatest concentration by the intestinal microbiota [96]. Butyrate is produced at lower concentrations and is generated primarily by Firmicutes [97]. Propionate is produced by some Firmicutes, Bacteroidetes, and Verrucomicrobia, especially Akkermansia muciniphila [98,99,100]. Most acetate and propionate are absorbed into circulation, while butyrate is the primary energy source for intestinal epithelial cells [101,102].
SCFA-sensitive receptors are expressed in many cell types throughout the body, including immune cells, adipose tissue, cardiac tissue, skeletal muscle, and neurons [98,103]. Thus, SCFAs, particularly propionate and acetate, have the potential for broad action in the host, including metabolism, cell differentiation and proliferation, gene regulation, protein methylation, and phosphorylation [96,100]. In vitro and animal studies suggest SCFA production is associated with the synthesis and secretion of intestinal hormones, such as GLP-1 and PYY, which play a role in satiety, intestinal transit, and homeostasis [104,105]. In addition to being an energy source for epithelial cells, butyrate may also suppress cancer cell expansion [96]. Butyrate has also been considered as a therapeutic target for the treatment of Type 2 diabetes [106].
SCFAs have also been purported to have anti-inflammatory and immunomodulatory effects [107]. SCFAs have been shown to inhibit the expression of adhesion molecules, suppress macrophage and neutrophil recruitment, and decrease LPS-induced TNFα [108,109]. Butyrate has been shown in vitro to reduce intestinal permeability and stimulate mucus production to strengthen the epithelial barrier [96]. Butyrate can also modulate antimicrobial peptide secretion in the intestinal epithelium [96].
Oats may contribute to the production of SCFAs due to their fiber content, particularly β-glucan. Table 1 includes studies on oat and gut microbiota in animal models. In a study with rats fed a high-fat diet (HFD), oat supplementation increased butyrate concentrations in the intestine, which was associated with improved lipid metabolism, reduced oxidative stress, and attenuated inflammatory responses [46]. In a similar study, oat fiber helped attenuate the development of obesity and dyslipidemia caused by HFD in mice [47]. The authors suggested that the benefit of oat fiber may be due to SCFA production that could impact lipid and glucose metabolism [47]. This is supported by another study that reported a significant increase in intestinal production of acetate, propionate, and butyrate in hamsters fed HFD for 30 days [52]. The hamsters also experienced a decrease in total plasma and hepatic cholesterol, LDL-cholesterol, triglycerides, and an increase in fecal weight and bile acids [52]. In a study with oat flour and oat bran supplemented in HFD-fed rats, both products altered the gut microbiota composition and increased SCFAs concentrations, which was associated with decreased body weight, inflammation, and lipid levels [53]. Oat bran had promising effects on body weight and inflammation, significantly reducing adipocyte size and inflammatory TNF-α mRNA expression levels [53]. Finally, a diet containing a mixture of coarse cereals, including millet, maize, oat, soybean, and purple potato, reduced body weight gain and fat accumulation, improved glucose tolerance and serum lipid levels, and reduced systemic inflammation in mice treated with HFD [48]. These effects were associated with an increase in SCFA synthesis and an increase in the relative abundance of Lactobacillus and Bifidobacterium [48]. Intestinal integrity markers, including mucin and tight junction proteins, have also been associated with SCFA production in mice consuming oat bran [49]. A study investigating the gut–brain axis in an animal model of atherosclerosis reported 0.8% oat fiber supplementation for 14 weeks, which delayed the progression of cognitive impairment and increased expression of SCFA receptors and intestinal microbiota diversity [50]. The neuroprotective potential was suggested to result from the production of anti-inflammatory metabolites triggered by the fermentation of oat fiber to SCFAs [50].
In vitro studies have frequently been used to evaluate the impact of different dietary fibers and fiber treatments on the gut microbiota and subsequent metabolites. The findings of in vitro studies on oats are presented in Table 2. In one study evaluating different ways to process oat bran, steamed oat bran increased SCFA production more than microwave and hot-air oven processing [55]. When compared with other grains and fibers, such as corn, millet, and oligosaccharides in infant fecal inoculum, oat seems to preferentially increase Lactobacillus and promote butyrate production, even though overall SCFA production is similar among the grains [56]. However, other studies with adult fecal inoculum have reported increases in acetate and propionate and a decrease in butyrate with oat bran treatment, as well as increases in Bifidobacterium [103].
Table 3 details the two clinical trials reviewed on oat consumption and microbiota modulation. Valeur et al. (2016) evaluated 10 healthy subjects who ingested 60 g of oat porridge daily for one week [60]. Although the production of intestinal gas and excretion of SCFA did not change significantly, fecal levels of β-galactosidase and urease decreased after ingestion [60]. β-galactosidase is a microbial enzyme with activity similar to human lactase [60]. Thus, the authors hypothesized that the decrease in fecal levels of β-galactosidase reflects an adaptation of the microbiota not needing this enzyme for the digestion of oat porridge [60]. Furthermore, bacterial urease is an enzyme responsible for the hydrolysis of urea into ammonia [110]. In certain cases, high levels of ammonia are associated with toxicity, which configures an unfavorable microbiota [60,110]. Therefore, the reduction of urease from the consumption of oat porridge demonstrated an interesting prebiotic potential [60]. A more recent study with 210 mildly hypercholesterolemic individuals in China demonstrated a reduction in total and non-high-density lipoprotein levels after consuming 80 g of oats daily for 45 days [59]. Oat consumption also resulted in increased production of acetic acid and propionic acid when compared to the baseline, but not compared to the rice treatment (control) [59].
4. Conclusions and Future Perspectives
Current evidence suggests that oats can beneficially alter the composition of the gut microbiota and promote the synthesis of SCFAs. At present, most research has been conducted in animals and in vitro. These efforts suggest plausible mechanisms of action that demonstrate the need for future work in this area. Future clinical trials are needed to demonstrate the impact of oats on the human gut microbiota, with consideration for internal and external influences on the microbiota and high interindividual variability.
Author Contributions
Conceptualization, G.A.F. and A.E.C.A.; methodology, G.A.F.; writing—original draft preparation, G.A.F.; writing—review and editing, G.A.F., A.E.C.A. and L.M.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Author LMS is employed by company PepsiCo, Inc. The views expressed in this manuscript are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
Funding Statement
G.A.F received a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Brazil—financial code 001, and this research was funded by PepsiCo, Inc. (Sorocaba/São Paulo, 18087-101). We thank the National Council for Scientific and Technological Development (CNPq) (Grants: 303772/2022-0).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Butt M.S., Tahir-Nadeem M., Khan M.K.I., Shabir R., Butt M.S. Oat: Unique among the Cereals. Eur. J. Nutr. 2008;47:68–79. doi: 10.1007/s00394-008-0698-7. [DOI] [PubMed] [Google Scholar]
- 2.Rose D.J. Impact of Whole Grains on the Gut Microbiota: The next Frontier for Oats? Br. J. Nutr. 2014;112:S44–S49. doi: 10.1017/S0007114514002244. [DOI] [PubMed] [Google Scholar]
- 3.Chávez de la Vega M.I., Alatorre-Santamaría S., Gómez-Ruiz L., García-Garibay M., Guzmán-Rodríguez F., González-Olivares L.G., Cruz-Guerrero A.E., Rodríguez-Serrano G.M. Influence of Oat β-Glucan on the Survival and Proteolytic Activity of Lactobacillus Rhamnosus GG in Milk Fermentation: Optimization by Response Surface. Fermentation. 2021;7:210. doi: 10.3390/fermentation7040210. [DOI] [Google Scholar]
- 4.Carlson J., Erickson J., Hess J., Gould T., Slavin J. Prebiotic Dietary Fiber and Gut Health: Comparing the in Vitro Fermentations of Beta-Glucan, Inulin and Xylooligosaccharide. Nutrients. 2017;9:1361. doi: 10.3390/nu9121361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fehlbaum S., Prudence K., Kieboom J., Heerikhuisen M., van den Broek T., Schuren F., Steinert R., Raederstorff D. In Vitro Fermentation of Selected Prebiotics and Their Effects on the Composition and Activity of the Adult Gut Microbiota. Int. J. Mol. Sci. 2018;19:3097. doi: 10.3390/ijms19103097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EFSA Panel on Dietic Products, Nutrition and Allergies (NDA) Scientific Opinion on the Substantiation of a Health Claim Related to Oat Beta Glucan and Lowering Blood Cholesterol and Reduced Risk of (Coronary) Heart Disease Pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2010;8:1885. doi: 10.2903/j.efsa.2010.1885. [DOI] [Google Scholar]
- 7.Gangopadhyay N., Hossain M., Rai D., Brunton N. A Review of Extraction and Analysis of Bioactives in Oat and Barley and Scope for Use of Novel Food Processing Technologies. Molecules. 2015;20:10884–10909. doi: 10.3390/molecules200610884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C., Huang X., Wang H., Geng F., Nie S. Effect of β-Glucan on Metabolic Diseases: A Review from the Gut Microbiota Perspective. Curr. Opin. Food Sci. 2022;47:100907. doi: 10.1016/j.cofs.2022.100907. [DOI] [Google Scholar]
- 9.Boz H. Phenolic Amides (Avenanthramides) in Oats—A Review. Czech J. Food Sci. 2015;33:399–404. doi: 10.17221/696/2014-CJFS. [DOI] [Google Scholar]
- 10.Meydani M. Potential Health Benefits of Avenanthramides of Oats. Nutr. Rev. 2009;67:731–735. doi: 10.1111/j.1753-4887.2009.00256.x. [DOI] [PubMed] [Google Scholar]
- 11.Garsed K., Scott B.B. Can Oats Be Taken in a Gluten-Free Diet? A Systematic Review. Scand. J. Gastroenterol. 2007;42:171–178. doi: 10.1080/00365520600863944. [DOI] [PubMed] [Google Scholar]
- 12.Balakireva A., Zamyatnin A. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxification Capabilities. Nutrients. 2016;8:644. doi: 10.3390/nu8100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caio G., Volta U., Sapone A., Leffler D.A., De Giorgio R., Catassi C., Fasano A. Celiac Disease: A Comprehensive Current Review. BMC Med. 2019;17:142. doi: 10.1186/s12916-019-1380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smulders M.J.M., van de Wiel C.C.M., van den Broeck H.C., van der Meer I.M., Israel-Hoevelaken T.P.M., Timmer R.D., van Dinter B.-J., Braun S., Gilissen L.J.W.J. Oats in Healthy Gluten-Free and Regular Diets: A Perspective. Food Res. Int. 2018;110:3–10. doi: 10.1016/j.foodres.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Food and Agriculture Organization of the United States (FAO) WHO CODEX ALIMENTARIUS: International Food Standards. Standard for Foods for Special Dietary Use for Persons Intolerant to Gluten: CXS 118-1979. [(accessed on 23 February 2023)]. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B118-1979%252FCXS_118e_2015.pdf.
- 16.Wieser H., Segura V., Ruiz-Carnicer Á., Sousa C., Comino I. Food Safety and Cross-Contamination of Gluten-Free Products: A Narrative Review. Nutrients. 2021;13:2244. doi: 10.3390/nu13072244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poley J.R. The Gluten-Free Diet: Can Oats and Wheat Starch Be Part of It? J. Am. Coll. Nutr. 2017;36:1–8. doi: 10.1080/07315724.2015.1085815. [DOI] [PubMed] [Google Scholar]
- 18.Pinto-Sánchez M.I., Causada-Calo N., Bercik P., Ford A.C., Murray J.A., Armstrong D., Semrad C., Kupfer S.S., Alaedini A., Moayyedi P., et al. Safety of Adding Oats to a Gluten-Free Diet for Patients With Celiac Disease: Systematic Review and Meta-Analysis of Clinical and Observational Studies. Gastroenterology. 2017;153:395–409.e3. doi: 10.1053/j.gastro.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Kosová K., Leišová-Svobodová L., Dvořáček V. Oats as a Safe Alternative to Triticeae Cereals for People Suffering from Celiac Disease? A Review. Plant Foods Hum. Nutr. 2020;75:131–141. doi: 10.1007/s11130-020-00800-8. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmanová I., Sánchez D., Szczepanková A., Tlaskalová-Hogenová H. The Pros and Cons of Using Oat in a Gluten-Free Diet for Celiac Patients. Nutrients. 2019;11:2345. doi: 10.3390/nu11102345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilissen L., van der Meer I., Smulders M. Why Oats Are Safe and Healthy for Celiac Disease Patients. Med. Sci. 2016;4:21. doi: 10.3390/medsci4040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Vieille S., Pulido O.M., Abbott M., Koerner T.B., Godefroy S. Celiac Disease and Gluten-Free Oats: A Canadian Position Based on a Literature Review. Can. J. Gastroenterol. Hepatol. 2016;2016:1870305. doi: 10.1155/2016/1870305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adak A., Khan M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019;76:473–493. doi: 10.1007/s00018-018-2943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson J.W., Baird P., Davis R.H., Jr., Ferreri S., Knudtson M., Koraym A., Waters V., Williams C.L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009;67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 25.McBurney M.I., Davis C., Fraser C.M., Schneeman B.O., Huttenhower C., Verbeke K., Walter J., Latulippe M.E. Establishing What Constitutes a Healthy Human Gut Microbiome: State of the Science, Regulatory Considerations, and Future Directions. J. Nutr. 2019;149:1882–1895. doi: 10.1093/jn/nxz154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd-Price J., Abu-Ali G., Huttenhower C. The Healthy Human Microbiome. Genome Med. 2016;8:51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manos J. The Human Microbiome in Disease and Pathology. APMIS. 2022;130:690–705. doi: 10.1111/apm.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomaa E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek. 2020;113:2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Medina M., Garcia-Gil L.J. Escherichia Coli in Chronic Inflammatory Bowel Diseases: An Update on Adherent Invasive Escherichia coli Pathogenicity. World J. Gastrointest. Pathophysiol. 2014;5:213. doi: 10.4291/wjgp.v5.i3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lane E.R., Zisman T., Suskind D. The Microbiota in Inflammatory Bowel Disease: Current and Therapeutic Insights. J. Inflamm. Res. 2017;10:63–73. doi: 10.2147/JIR.S116088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu W., Winter M.G., Byndloss M.X., Spiga L., Duerkop B.A., Hughes E.R., Büttner L., de Lima Romão E., Behrendt C.L., Lopez C.A., et al. Precision Editing of the Gut Microbiota Ameliorates Colitis. Nature. 2018;553:208–211. doi: 10.1038/nature25172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollander D. Intestinal Permeability, Leaky Gut, and Intestinal Disorders. Curr. Gastroenterol. Rep. 1999;1:410–416. doi: 10.1007/s11894-999-0023-5. [DOI] [PubMed] [Google Scholar]
- 33.Larsen N., Vogensen F.K., van den Berg F.W.J., Nielsen D.S., Andreasen A.S., Pedersen B.K., Al-Soud W.A., Sørensen S.J., Hansen L.H., Jakobsen M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baliou S., Adamaki M., Spandidos A.D., Kyriakopoulos M.A., Christodoulou L., Zoumpourlis V. The Microbiome, Its Molecular Mechanisms and Its Potential as a Therapeutic Strategy against Colorectal Carcinogenesis (Review) World Acad. Sci. J. 2019;1:3–19. [Google Scholar]
- 35.Kostic A.D., Gevers D., Pedamallu C.S., Michaud M., Duke F., Earl A.M., Ojesina A.I., Jung J., Bass A.J., Tabernero J., et al. Genomic Analysis Identifies Association of Fusobacterium with Colorectal Carcinoma. Genome Res. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lv G., Cheng N., Wang H. The Gut Microbiota, Tumorigenesis, and Liver Diseases. Engineering. 2017;3:110–114. doi: 10.1016/J.ENG.2017.01.017. [DOI] [Google Scholar]
- 37.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., et al. Gut Microbiome Influences Efficacy of PD-1–Based Immunotherapy against Epithelial Tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 38.Cryan J.F., O’Riordan K.J., Sandhu K., Peterson V., Dinan T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020;19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 39.Korem T., Zeevi D., Suez J., Weinberger A., Avnit-Sagi T., Pompan-Lotan M., Matot E., Jona G., Harmelin A., Cohen N., et al. Growth Dynamics of Gut Microbiota in Health and Disease Inferred from Single Metagenomic Samples. Science. 2015;349:1101–1106. doi: 10.1126/science.aac4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 41.Biel W., Kazimierska K., Bashutska U. Nutritional Value of Wheat, Triticale, Barley and Oat Grains. Acta Sci. Pol. Zootech. 2020;19:19–28. doi: 10.21005/asp.2020.19.2.03. [DOI] [Google Scholar]
- 42.Guo H., Wu H., Sajid A., Li Z. Whole Grain Cereals: The Potential Roles of Functional Components in Human Health. Crit. Rev. Food Sci. Nutr. 2022;62:8388–8402. doi: 10.1080/10408398.2021.1928596. [DOI] [PubMed] [Google Scholar]
- 43.Simpson H.L., Campbell B.J. Review Article: Dietary Fibre-Microbiota Interactions. Aliment. Pharmacol. Ther. 2015;42:158–179. doi: 10.1111/apt.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chudan S., Ishibashi R., Nishikawa M., Tabuchi Y., Nagai Y., Ikushiro S., Furusawa Y. Effect of Soluble Oat Fiber on Intestinal Microenvironment and TNBS-Induced Colitis. Food Funct. 2023;14:2188–2199. doi: 10.1039/D2FO03396H. [DOI] [PubMed] [Google Scholar]
- 45.Kutcher A.M., LeBaron V.T. A Simple Guide for Completing an Integrative Review Using an Example Article. J. Prof. Nurs. 2022;40:13–19. doi: 10.1016/j.profnurs.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Qi W., Guo X., Song G., Pang S., Fang W., Peng Z. Effects of Oats, Tartary Buckwheat, and Foxtail Millet Supplementation on Lipid Metabolism, Oxido-Inflammatory Responses, Gut Microbiota, and Colonic SCFA Composition in High-Fat Diet Fed Rats. Nutrients. 2022;14:2760. doi: 10.3390/nu14132760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han S., Gao H., Song R., Zhang W., Li Y., Zhang J. Oat Fiber Modulates Hepatic Circadian Clock via Promoting Gut Microbiota-Derived Short Chain Fatty Acids. J. Agric. Food Chem. 2021;69:15624–15635. doi: 10.1021/acs.jafc.1c06130. [DOI] [PubMed] [Google Scholar]
- 48.Ji Y., Ma N., Zhang J., Wang H., Tao T., Pei F., Hu Q. Dietary Intake of Mixture Coarse Cereals Prevents Obesity by Altering the Gut Microbiota in High-Fat Diet Fed Mice. Food Chem. Toxicol. 2021;147:111901. doi: 10.1016/j.fct.2020.111901. [DOI] [PubMed] [Google Scholar]
- 49.Kundi Z.M., Lee J.C., Pihlajamäki J., Chan C.B., Leung K.S., So S.S.Y., Nordlund E., Kolehmainen M., El-Nezami H. Dietary Fiber from Oat and Rye Brans Ameliorate Western Diet–Induced Body Weight Gain and Hepatic Inflammation by the Modulation of Short-Chain Fatty Acids, Bile Acids, and Tryptophan Metabolism. Mol. Nutr. Food Res. 2021;65:1900580. doi: 10.1002/mnfr.201900580. [DOI] [PubMed] [Google Scholar]
- 50.Gao H., Song R., Li Y., Zhang W., Wan Z., Wang Y., Zhang H., Han S. Effects of Oat Fiber Intervention on Cognitive Behavior in LDLR–/– Mice Modeling Atherosclerosis by Targeting the Microbiome–Gut–Brain Axis. J. Agric. Food Chem. 2020;68:14480–14491. doi: 10.1021/acs.jafc.0c05677. [DOI] [PubMed] [Google Scholar]
- 51.Huang K., Yu W., Li S., Guan X., Liu J., Song H., Liu D., Duan R. Effect of Embryo-Remaining Oat Rice on the Lipid Profile and Intestinal Microbiota in High-Fat Diet Fed Rats. Food Res. Int. 2020;129:108816. doi: 10.1016/j.foodres.2019.108816. [DOI] [PubMed] [Google Scholar]
- 52.Sun N.-X., Tong L.-T., Liang T.-T., Wang L.-L., Liu L.-Y., Zhou X.-R., Zhou S.-M. Effect of Oat and Tartary Buckwheat—Based Food on Cholesterol—Lowering and Gut Microbiota in Hypercholesterolemic Hamsters. J. Oleo Sci. 2019;68:251–259. doi: 10.5650/jos.ess18221. [DOI] [PubMed] [Google Scholar]
- 53.Dong J., Zhu Y., Ma Y., Xiang Q., Shen R., Liu Y. Oat Products Modulate the Gut Microbiota and Produce Anti-Obesity Effects in Obese Rats. J. Funct. Foods. 2016;25:408–420. doi: 10.1016/j.jff.2016.06.025. [DOI] [Google Scholar]
- 54.Wilczak J., Błaszczyk K., Kamola D., Gajewska M., Harasym J.P., Jałosińska M., Gudej S., Suchecka D., Oczkowski M., Gromadzka-Ostrowska J. The Effect of Low or High Molecular Weight Oat Beta-Glucans on the Inflammatory and Oxidative Stress Status in the Colon of Rats with LPS-Induced Enteritis. Food Funct. 2015;6:590–603. doi: 10.1039/C4FO00638K. [DOI] [PubMed] [Google Scholar]
- 55.Bai X., Zhang M., Zhang Y., Zhang Y., Huo R., Guo X. In Vitro Fermentation of Pretreated Oat Bran by Human Fecal Inoculum and Impact on Microbiota. J. Funct. Foods. 2022;98:105278. doi: 10.1016/j.jff.2022.105278. [DOI] [Google Scholar]
- 56.Liang S., Xie Q., Evivie S.E., Zhao L., Chen Q., Xu B., Liu F., Li B., Huo G. Study on Supplementary Food with Beneficial Effects on the Gut Microbiota of Infants. Food Biosci. 2021;43:101291. doi: 10.1016/j.fbio.2021.101291. [DOI] [Google Scholar]
- 57.Glei M., Zetzmann S., Lorkowski S., Dawczynski C., Schlörmann W. Chemopreventive effects of raw and roasted oat flakes after in vitro fermentation with human faecal microbiota. Int. J. of Food Sci. Nutr. 2021;72:57–69. doi: 10.1080/09637486.2020.1772205. [DOI] [PubMed] [Google Scholar]
- 58.Kristek A., Wiese M., Heuer P., Kosik O., Schär M.Y., Soycan G., Alsharif S., Kuhnle G.G.C., Walton G., Spencer J.P.E. Oat Bran, but Not Its Isolated Bioactive β-Glucans or Polyphenols, Have a Bifidogenic Effect in an in Vitro Fermentation Model of the Gut Microbiota. Br. J. Nutr. 2019;121:549–559. doi: 10.1017/S0007114518003501. [DOI] [PubMed] [Google Scholar]
- 59.Xu D., Feng M., Chu Y., Wang S., Shete V., Tuohy K.M., Liu F., Zhou X., Kamil A., Pan D., et al. The Prebiotic Effects of Oats on Blood Lipids, Gut Microbiota, and Short-Chain Fatty Acids in Mildly Hypercholesterolemic Subjects Compared with Rice: A Randomized, Controlled Trial. Front. Immunol. 2021;12:787797. doi: 10.3389/fimmu.2021.787797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valeur J., Puaschitz N.G., Midtvedt T., Berstad A. Oatmeal Porridge: Impact on Microflora-Associated Characteristics in Healthy Subjects. Br. J. Nutr. 2016;115:62–67. doi: 10.1017/S0007114515004213. [DOI] [PubMed] [Google Scholar]
- 61.Trumbo P., Schlicker S., Yates A.A., Poos M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002;102:1621–1630. doi: 10.1016/S0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 62.Dahl W.J., Stewart M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J. Acad. Nutr. Diet. 2015;115:1861–1870. doi: 10.1016/j.jand.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 63.Henry C.J. Functional Foods. Eur. J. Clin. Nutr. 2010;64:657–659. doi: 10.1038/ejcn.2010.101. [DOI] [PubMed] [Google Scholar]
- 64.Joyce S.A., Kamil A., Fleige L., Gahan C.G.M. The Cholesterol-Lowering Effect of Oats and Oat Beta Glucan: Modes of Action and Potential Role of Bile Acids and the Microbiome. Front. Nutr. 2019;6:171. doi: 10.3389/fnut.2019.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ross S. Functional Foods: The Food and Drug Administration Perspective. Am. J. Clin. Nutr. 2000;71:1735S–1738S. doi: 10.1093/ajcn/71.6.1735S. [DOI] [PubMed] [Google Scholar]
- 66.US Department of Health and Human Services. Food and Drug Administration . Food Labeling: Health Claims; Oats and Coronaty Heart Disease. Final Rule. Vol. 62. Federal Register; Washington, DC, USA: 1997. [(accessed on 7 April 2023)]. pp. 3584–3601. Available online: https://www.govinfo.gov/content/pkg/FR-1997-01-23/pdf/97-1598.pdf. [Google Scholar]
- 67.Rafique H., Dong R., Wang X., Alim A., Aadil R.M., Li L., Zou L., Hu X. Dietary-Nutraceutical Properties of Oat Protein and Peptides. Front. Nutr. 2022;9:950400. doi: 10.3389/fnut.2022.950400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raguindin P.F., Adam Itodo O., Stoyanov J., Dejanovic G.M., Gamba M., Asllanaj E., Minder B., Bussler W., Metzger B., Muka T., et al. A Systematic Review of Phytochemicals in Oat and Buckwheat. Food. Chem. 2021;338:127982. doi: 10.1016/j.foodchem.2020.127982. [DOI] [PubMed] [Google Scholar]
- 69.Kim I.-S., Hwang C.-W., Yang W.-S., Kim C.-H. Multiple Antioxidative and Bioactive Molecules of Oats (Avena sativa L.) in Human Health. Antioxidants. 2021;10:1454. doi: 10.3390/antiox10091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aggarwal B.B., Sundaram C., Prasad S., Kannappan R. Tocotrienols, the Vitamin E of the 21st Century: Its Potential against Cancer and Other Chronic Diseases. Biochem. Pharmacol. 2010;80:1613–1631. doi: 10.1016/j.bcp.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paudel D., Dhungana B., Caffe M., Krishnan P. A Review of Health-Beneficial Properties of Oats. Foods. 2021;10:2591. doi: 10.3390/foods10112591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hernandez-Hernandez O., Pereira-Caro G., Borges G., Crozier A., Olsson O. Characterization and Antioxidant Activity of Avenanthramides from Selected Oat Lines Developed by Mutagenesis Technique. Food Chem. 2021;343:128408. doi: 10.1016/j.foodchem.2020.128408. [DOI] [PubMed] [Google Scholar]
- 73.Wolever T.M., Tosh S.M., Gibbs A.L., Brand-Miller J., Duncan A.M., Hart V., Lamarche B., Thomson B.A., Duss R., Wood P.J. Physicochemical Properties of Oat β-Glucan Influence Its Ability to Reduce Serum LDL Cholesterol in Humans: A Randomized Clinical Trial. Am. J. Clin. Nutr. 2010;92:723–732. doi: 10.3945/ajcn.2010.29174. [DOI] [PubMed] [Google Scholar]
- 74.Whitehead A., Beck E.J., Tosh S., Wolever T.M. Cholesterol-Lowering Effects of Oat β-Glucan: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2014;100:1413–1421. doi: 10.3945/ajcn.114.086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ho H.V.T., Sievenpiper J.L., Zurbau A., Blanco Mejia S., Jovanovski E., Au-Yeung F., Jenkins A.L., Vuksan V. The Effect of Oat β -Glucan on LDL-Cholesterol, Non-HDL-Cholesterol and ApoB for CVD Risk Reduction: A Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Br. J. Nutr. 2016;116:1369–1382. doi: 10.1017/S000711451600341X. [DOI] [PubMed] [Google Scholar]
- 76.Tiwari U., Cummins E. Meta-Analysis of the Effect of β-Glucan Intake on Blood Cholesterol and Glucose Levels. Nutrition. 2011;27:1008–1016. doi: 10.1016/j.nut.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 77.McRorie J.W., McKeown N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017;117:251–264. doi: 10.1016/j.jand.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 78.Andersson M., Ellegård L., Andersson H. Oat Bran Stimulates Bile Acid Synthesis within 8 h as Measured by 7α-Hydroxy-4-Cholesten-3-One. Am. J. Clin. Nutr. 2002;76:1111–1116. doi: 10.1093/ajcn/76.5.1111. [DOI] [PubMed] [Google Scholar]
- 79.Tong L.-T., Guo L., Zhou X., Qiu J., Liu L., Zhong K., Zhou S. Effects of Dietary Oat Proteins on Cholesterol Metabolism of Hypercholesterolaemic Hamsters. J. Sci. Food Agric. 2016;96:1396–1401. doi: 10.1002/jsfa.7236. [DOI] [PubMed] [Google Scholar]
- 80.Jones B.V., Begley M., Hill C., Gahan C.G.M., Marchesi J.R. Functional and Comparative Metagenomic Analysis of Bile Salt Hydrolase Activity in the Human Gut Microbiome. Proc. Natl. Acad. Sci. USA. 2008;105:13580–13585. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo L., Tong L.-T., Liu L., Zhong K., Qiu J., Zhou S. The Cholesterol-Lowering Effects of Oat Varieties Based on Their Difference in the Composition of Proteins and Lipids. Lipids Health Dis. 2014;13:182. doi: 10.1186/1476-511X-13-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ryan D., Kendall M., Robards K. Bioactivity of Oats as It Relates to Cardiovascular Disease. Nutr. Res. Rev. 2007;20:147–162. doi: 10.1017/S0954422407782884. [DOI] [PubMed] [Google Scholar]
- 83.Panahi S., Ezatagha A., Temelli F., Vasanthan T., Vuksan V. β-Glucan from Two Sources of Oat Concentrates Affect Postprandial Glycemia in Relation to the Level of Viscosity. J. Am. Coll. Nutr. 2007;26:639–644. doi: 10.1080/07315724.2007.10719641. [DOI] [PubMed] [Google Scholar]
- 84.Xue Y., Cui L., Qi J., Ojo O., Du X., Liu Y., Wang X. The Effect of Dietary Fiber (Oat Bran) Supplement on Blood Pressure in Patients with Essential Hypertension: A Randomized Controlled Trial. Nutr. Metab. Cardiovasc. Dis. 2021;31:2458–2470. doi: 10.1016/j.numecd.2021.04.013. [DOI] [PubMed] [Google Scholar]
- 85.Miyamoto J., Kasubuchi M., Nakajima A., Irie J., Itoh H., Kimura I. The Role of Short-Chain Fatty Acid on Blood Pressure Regulation. Curr. Opin. Nephrol. Hypertens. 2016;25:379–383. doi: 10.1097/MNH.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y., Zhang H., Wang L., Qian H., Qi X., Ding X., Hu B., Li J. The Effect of Oat β -Glucan on in Vitro Glucose Diffusion and Glucose Transport in Rat Small Intestine. J. Sci. Food Agric. 2016;96:484–491. doi: 10.1002/jsfa.7114. [DOI] [PubMed] [Google Scholar]
- 87.Quraishi M.N., Sergeant M., Kay G., Iqbal T., Chan J., Constantinidou C., Trivedi P., Ferguson J., Adams D.H., Pallen M., et al. The Gut-Adherent Microbiota of PSC–IBD Is Distinct to That of IBD. Gut. 2017;66:386–388. doi: 10.1136/gutjnl-2016-311915. [DOI] [PubMed] [Google Scholar]
- 88.Kataoka K. The Intestinal Microbiota and Its Role in Human Health and Disease. J. Med. Investig. 2016;63:27–37. doi: 10.2152/jmi.63.27. [DOI] [PubMed] [Google Scholar]
- 89.Hutkins R.W., Krumbeck J.A., Bindels L.B., Cani P.D., Fahey G., Goh Y.J., Hamaker B., Martens E.C., Mills D.A., Rastal R.A., et al. Prebiotics: Why Definitions Matter. Curr. Opin. Biotechnol. 2016;37:1–7. doi: 10.1016/j.copbio.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pineiro M., Asp N.-G., Reid G., Macfarlane S., Morelli L., Brunser O., Tuohy K. FAO Technical Meeting on Prebiotics. J. Clin. Gastroenterol. 2008;42((Suppl. 3)):S156–S159. doi: 10.1097/MCG.0b013e31817f184e. [DOI] [PubMed] [Google Scholar]
- 91.van Loveren H., Sanz Y., Salminen S. Health Claims in Europe: Probiotics and Prebiotics as Case Examples. Annu. Rev. Food Sci. Technol. 2012;3:247–261. doi: 10.1146/annurev-food-022811-101206. [DOI] [PubMed] [Google Scholar]
- 92.EFSA Panel on Dietic Products, Nutrition and Allergies (NDA) Scientific Opinion on the Substantiation of Health Claims Related to Various Food(s)/Food Constituents(s) and Increasing Numbers of Gastro-Intestinal Microorganisms (ID 760, 761, 779, 780, 779, 1905), and Decreasing Potentially Pathogenic Gastro-Intestina. EFSA J. 2010;8:1809. doi: 10.2903/j.efsa.2010.1809. [DOI] [Google Scholar]
- 93.Saito M. Role of FOSHU (Food for Specified Health Uses) for Healthier Life. YAKUGAKU ZASSHI. 2007;127:407–416. doi: 10.1248/yakushi.127.407. [DOI] [PubMed] [Google Scholar]
- 94.Canadian Food Inspection Agency . Health Claims on Food Labels. Canadian Food Inspection Agency; Ottawa, ON, Canada: 2019. Use of the Term “Prebiotic(s)”. [Google Scholar]
- 95.Lin B., Gong J., Wang Q., Cui S., Yu H., Huang B. In-Vitro Assessment of the Effects of Dietary Fibers on Microbial Fermentation and Communities from Large Intestinal Digesta of Pigs. Food Hydrocoll. 2011;25:180–188. doi: 10.1016/j.foodhyd.2010.02.006. [DOI] [Google Scholar]
- 96.Martin-Gallausiaux C., Marinelli L., Blottière H.M., Larraufie P., Lapaque N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021;80:37–49. doi: 10.1017/S0029665120006916. [DOI] [PubMed] [Google Scholar]
- 97.Belzer C., Chia L.W., Aalvink S., Chamlagain B., Piironen V., Knol J., de Vos W.M. Microbial Metabolic Networks at the Mucus Layer Lead to Diet-Independent Butyrate and Vitamin B12 Production by Intestinal Symbionts. mBio. 2017;8:e00770-17. doi: 10.1128/mBio.00770-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flint H.J. Gut Microbial Metabolites in Health and Disease. Gut Microbes. 2016;7:187–188. doi: 10.1080/19490976.2016.1182295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Louis P., Hold G.L., Flint H.J. The Gut Microbiota, Bacterial Metabolites and Colorectal Cancer. Nat. Rev. Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 100.Morrison D.J., Preston T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boets E., Gomand S.V., Deroover L., Preston T., Vermeulen K., De Preter V., Hamer H.M., Van den Mooter G., De Vuyst L., Courtin C.M., et al. Systemic Availability and Metabolism of Colonic-Derived Short-Chain Fatty Acids in Healthy Subjects: A Stable Isotope Study. J. Physiol. 2017;595:541–555. doi: 10.1113/JP272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Verhaar B.J.H., Prodan A., Nieuwdorp M., Muller M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients. 2020;12:2982. doi: 10.3390/nu12102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 104.Tazoe H., Otomo Y., Karaki S., Kato I., Fukami Y., Terasaki M., Kuwahara A. Expression of Short-Chain Fatty Acid Receptor GPR41 in the Human Colon. Biomed. Res. 2009;30:149–156. doi: 10.2220/biomedres.30.149. [DOI] [PubMed] [Google Scholar]
- 105.Larraufie P., Martin-Gallausiaux C., Lapaque N., Dore J., Gribble F.M., Reimann F., Blottiere H.M. SCFAs Strongly Stimulate PYY Production in Human Enteroendocrine Cells. Sci. Rep. 2018;8:74. doi: 10.1038/s41598-017-18259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arora T., Tremaroli V. Therapeutic Potential of Butyrate for Treatment of Type 2 Diabetes. Front. Endocrinol. 2021;12:761834. doi: 10.3389/fendo.2021.761834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maslowski K.M., Vieira A.T., Ng A., Kranich J., Sierro F., Yu D., Schilter H.C., Rolph M.S., Mackay F., Artis D., et al. Regulation of Inflammatory Responses by Gut Microbiota and Chemoattractant Receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li M., van Esch B.C.A.M., Wagenaar G.T.M., Garssen J., Folkerts G., Henricks P.A.J. Pro- and Anti-Inflammatory Effects of Short Chain Fatty Acids on Immune and Endothelial Cells. Eur. J. Pharmacol. 2018;831:52–59. doi: 10.1016/j.ejphar.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 109.Vinolo M.A.R., Rodrigues H.G., Nachbar R.T., Curi R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shen T.-C.D., Albenberg L., Bittinger K., Chehoud C., Chen Y.-Y., Judge C.A., Chau L., Ni J., Sheng M., Lin A., et al. Engineering the Gut Microbiota to Treat Hyperammonemia. J. Clin. Investig. 2015;125:2841–2850. doi: 10.1172/JCI79214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.