Abstract
The presence of spiral bacteria in the feline stomach has been recognized for over a century, but the identities and degrees of prevalence of such organisms in privately owned cats are still poorly documented. The aims of this study were (i) to adapt different diagnostic tools and evaluate their practicality for diagnosing feline gastric Helicobacter colonization, (ii) to determine the prevalence of gastric Helicobacter-like organisms in pet cats, (iii) to identify the feline species, and (iv) to correlate the presence of a Helicobacter infection with gastritis. Biopsy samples were taken gastroscopically from the antra and the corpora of clinically healthy pet cats. Helicobacter-like organisms were detected by Gram staining, Warthin-Starry staining, and rapid urease testing in biopsy specimens and by [13C]urea breath testing in 79, 77, 78, and 85% of cases, respectively. PCR analysis revealed that 78% of the cats (38 of 49) were infected by Helicobacter heilmannii; however, none of them was harboring Helicobacter pylori or Helicobacter felis. Culture was positive for one cat; the organism was identified as Helicobacter pametensis by dot blot DNA hybridization. By a combination of the detection methods, 91% of the pet cats were found to be Helicobacter positive. For 46 cats (79%) diagnostic tests were concordant. All cats showed mild to moderate gastritis in either the antrum or the corpus, regardless of the presence or density of gastric bacteria. In summary, pet cats are frequently colonized by H. heilmannii without a significant correlation between infection and degree of gastritis.
Helicobacters colonize the stomachs and intestines of humans and several animal species, such as cats, dogs, ferrets, pigs, cheetahs, and monkeys. In humans, Helicobacter pylori is the major agent of chronic diffuse superficial gastritis, plays a causative role in peptic ulcers, and is considered a cofactor in the development of gastric malignancies. H. pylori has also been found in cats (7), and it can promote gastritis when introduced into specific-pathogen-free cats (4). The significance of this infection as a cause of gastritis in pet cats is nevertheless unclear. The main gastric Helicobacter species in cats are primarily Helicobacter heilmannii (formerly “Gastrospirillum hominis”) and Helicobacter felis. These two species are collectively referred to as gastric Helicobacter-like organisms (GHLO) because they cannot be distinguished by light microscopy (11). The prevalence of GHLO in cats has been reported to be between 57 and 100% (5). So far, H. heilmannii has not been reliably cultured in vitro (1). However, the two organisms can be identified by electron microscopy or by comparison of their 16S rRNA and urease gene sequences (15, 16).
The purposes of the present study were to assess the presence of gastric Helicobacter species by noninvasive and invasive diagnostic methods and to test whether Helicobacter infections are associated with gastritis.
MATERIALS AND METHODS
Cats and endoscopy.
Fifty-eight privately owned, healthy cats (22 male and 36 female), between 4 months and 13 years old, undergoing routine surgical procedures were studied. Most cats had access to the outdoors. Owner consent was obtained for all cats.
Fasting cats were anesthetized with diazepam (0.2 mg/kg of body weight) and ketamine (3 to 5 mg/kg given until effective); 20 cats were intubated, and anesthesia was maintained with halothane in oxygen. During gastroscopy the macroscopic appearance of the mucosa was recorded and biopsy samples were taken. Endoscope and biopsy forceps were disinfected with 4% Sekusept Plus solution (Henkel, Muttenz, Switzerland) for 30 min and thoroughly flushed with tap water.
Tests based on urease activity.
Biopsy samples from the antra and corpora were placed in a rapid urease test kit (CUTest; Temmler Pharma, Marburg, Germany) and analyzed.
Endotracheal-tube breath samples from 17 cats were injected into a Vacutainer (Europa Scientific, Crewe, England). Baseline values were recorded; then, 2 mg of [13C]urea per kg in 1 ml of distilled water was placed in the stomach via the endoscope working channel. Breath samples were obtained 15, 30, and 45 min later. For 35 cats [13C]urea was given orally rather than intragastrically and breath test sampling was performed on conscious animals with a face mask attached via a double valve directly to a Vacutainer. Ratios of 13CO2 to 12CO2 were measured in a stable isotope ratio mass spectrometer (Europa Scientific). Differences between the ratios before and after [13C]urea administration were expressed as Δ13C. Cats were considered breath test positive if their value was above a threshold calculated as the mean plus 1 standard deviation of the values obtained for all cats found to be negative by morphological assays and rapid urease testing.
Histopathology.
Impression smears from the antrum were Gram stained. Sections of antrum and corpus were stained with hematoxylin and eosin and with Warthin-Starry stain. For the histopathological assessment the presence of lymphocyte aggregates, the number of leukocytes, and the degree of GHLO colonization were recorded. Grading of leukocytic infiltration was as follows: for inflammatory cells (mean of three fields at ×400), absent, mild (<10), moderate (10 to 50), or severe (>50); for the presence of GHLO (×500), absent, 1 to 50, or >50.
Gastritis was defined as follows: “no gastritis,” no lymphocyte aggregates or leukocytes; “mild gastritis,” no lymphocyte aggregates and <10 leukocytes per field; “moderate gastritis,” lymphocyte aggregates present and/or 10 to 50 leukocytes per field; “severe gastritis,” lymphocyte aggregates present and >50 leukocytes per field.
Microbiology and strain identification.
Microaerobic culture of one antral biopsy specimen was done as previously described (17). Isolates with characteristics of helicobacters were identified by dot blot DNA hybridization with probes for species of gastric helicobacters (2). The reference DNA (that of Helicobacter pametensis) was extracted from the type strain, CCUG 29255.
DNA extraction and PCR amplification.
Antral biopsy samples were ground with an automatic minigrinder using sterile disposable pestles (Pellet Pestle-motor; Polylabo, Geneva, Switzerland), and 200 μl of 10 mM Tris-HCl (pH 7.4) containing 100 μg of proteinase K per ml was added. After incubation at 55°C for 3 h and boiling at 98°C for 10 min, proteins were removed by standard phenol-CHCl3 extraction, and the DNA was precipitated with ethanol and resuspended in 50 μl of distilled water.
The Helicobacter urease B gene was amplified from 1 to 2 μl of each sample in a final reaction volume of 50 μl containing 1× PCR buffer (Pharmacia Biotech, Uppsala, Sweden), 200 μM deoxyribonucleoside triphosphates (Pharmacia), 100 pmol of primers (Microsynth GmbH, Balgach, Switzerland) and 2.5 U of Taq DNA polymerase (Pharmacia). Controls with distilled water were inserted after every six samples.
PCRs were performed as follows: one cycle of 94°C for 3 min, 57°C for 2 min, and 72°C for 3 min followed by 31 cycles of 94°C for 30 s, 57°C for 30 s, and 72°C for 1 min and a final extension at 72°C for 5 min. PCR products were analyzed by electrophoresis on a 1% agarose gel. Primers were derived from the urease B sequence of each Helicobacter sp. For sequences, EMBL accession numbers, and product lengths, see Table 1.
TABLE 1.
Primers used for H. heilmannii, H. pylori, and H. felis urease B gene amplification
| Species | Primer direction a and sequence | EMBL accession no. | Product size (bp) |
|---|---|---|---|
| H. heilmannii | F, 5′-GGGCGATAAAGTGCGCTTG-3′ | L25079 | 580 |
| R, 5′-CTGGTCAATGAGAGCAGG-3′ | |||
| H. pylori | F, 5′-GGAATTCCAGATCTATGAAAAAGATTAGCAGAAAAG-3′ | M60398 | 1,707 |
| R, 5′-GGAATTCGTCGACCTAGAAAATGCTAAAGAGTTG-3′ | |||
| H. felis | F, 5′-ATGAAACTAACGCCTAAAGAACTAG-3′ | X69080 | 1,150 |
| R, 5′-GGAGAGATAAAGTGAATATGCGT-3′ |
F, forward; R, reverse.
Statistical analysis.
A cat was considered infected if one or more test results were positive. The association between gastritis score and GHLO colonization was evaluated by Fisher’s exact test (P ≤ 0.05).
RESULTS
Endoscopic appearance of the gastric mucosa.
In most cats, the endoscopic appearance of the stomach was normal; only two cats (ages 1 and 5 years) showed mucosal erosions.
Detection of urease activity by rapid urease testing and the [13C]urea-breath test.
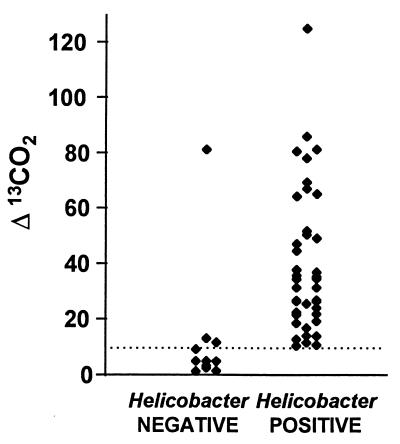
Rapid urease testing revealed urease-positive organisms in 45 of 58 cats (78%). The [13C]urea breath test showed a peak Δ13C value after 30 minutes. All but one animal found to be negative by morphological assays and rapid urease test had very low [13C]urea breath test values (Fig. 1). Excluding the outlier, the mean plus standard deviation obtained for the negative cats was 6 + 4. Forty-four of 52 cats tested (85%) were positive.
FIG. 1.
[13C]urea breath test results of Δ values for noninfected (negative) and infected (positive) cats. A diagnosis of “no infection” was made when all results of morphological and rapid urease testing were negative; infection was assumed to be present when one or more tests were positive. Each point represents one cat. The dotted line shows the cutoff.
Visualization of bacteria and histopathology.
Gram staining of tissue smears and Warthin-Starry staining were positive for 46 and 45 cats of 58, respectively (Table 2). Only large spiral organisms, morphologically resembling H. felis or H. heilmannii but not H. pylori, were seen.
TABLE 2.
Evaluation of Helicobacter infection status by different tests
| Morphologya
|
Urease activityb
|
Genetic PCR result | No. of cats with pattern | ||
|---|---|---|---|---|---|
| GS | WSS | RUT | BT | ||
| + | + | + | + | + | 29 |
| + | + | + | NDc | + | 3 |
| + | + | + | + | ND | 9 |
| + | + | + | + | − | 2 |
| + | + | + | ND | − | 2 |
| + | − | − | + | + | 1 |
| − | − | − | + | + | 1 |
| − | − | − | − | + | 4 |
| − | − | − | + | − | 2 |
| − | − | − | ND | − | 1 |
| − | − | − | − | − | 4 |
GS, Gram staining; WSS, Warthin-Starry staining.
RUT, rapid urease test; BT, [13C]urea breath test.
ND, not done.
No correlation was found between the degree of Helicobacter colonization and the gastritis score (Table 3). Mild gastritis was associated with diffuse infiltration mainly of few leukocytes, while moderate gastritis was usually based on the presence of lymphocyte aggregates. A gastric lymphoma was diagnosed in the antrum of an 8-month-old H. heilmannii-infected cat.
TABLE 3.
Gastritis results for non-Helicobacter-infected and Helicobacter-infected cats
| Degree of gastritis | No. of cats with result
|
|||
|---|---|---|---|---|
| Noninfected (n = 5)
|
Infected (n = 51)
|
|||
| Antrum | Corpus | Antrum | Corpus | |
| None | 0 | 0 | 0 | 7 |
| Mild | 3 | 5 | 28 | 40 |
| Moderate | 2 | 0 | 23 | 4 |
| Severe | 0 | 0 | 0 | 0 |
Microbiology.
Although culture of the spiral bacteria present in the gastric biopsies was initiated for all cats, only one isolate was obtained, and it was identified as H. pametensis.
PCR.
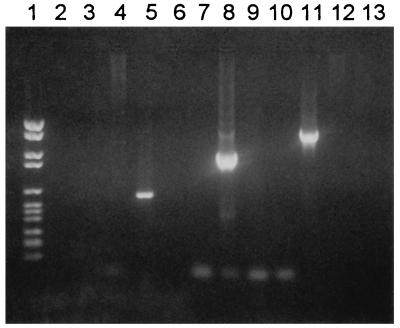
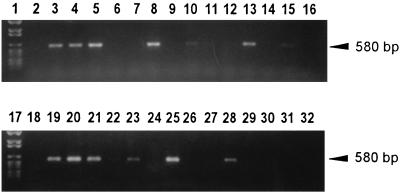
Each set of primers was shown to amplify the gene from which it was derived, without cross-hybridizing with the corresponding gene of the two other species (Fig. 2). No amplification products corresponding to either H. felis or H. pylori were detected, but 38 of 49 biopsy specimens tested (78%) were positive for H. heilmannii (Fig. 3).
FIG. 2.
PCR with specific primers from the urease B gene of H. heilmannii (lanes 2 through 5), H. felis (lanes 6 through 9), and H. pylori (lanes 10 through 13). PCR products were separated by gel electrophoresis. Lanes: 1, molecular weight VI ladder (Boehringer); 3, 7, and 11, H. pylori DNA; 4, 8, and 12, H. felis DNA; 5, 9, and 13, H. heilmannii DNA; 2, 6, and 10, negative controls (water).
FIG. 3.
Products from PCR, performed with specific primers for H. heilmannii ureB, were resolved by electrophoresis. Lanes: 1 and 17, molecular weight VI ladder (Boehringer); 3, H. heilmannii DNA; 4 through 8, 10 through 15, 18 through 23, and 25 through 30, DNA extracted from gastric biopsy samples from cats 11 through 33; 32, H. felis DNA; 2, 9, 16, 24, and 31, negative controls. Lanes 3 through 5, 8, 10, 13, 15, 19 through 21, 23, 25, and 28 are positive (10 and 15 with faint bands).
Evaluation of different detection methods (Table 2).
Forty-one of 58 cats (71%) were positive and 5 cats (9%) were negative for all tests performed. Morphological tests, such as Gram and Warthin-Starry staining, were concordant in all cats but one. In assays relying on measuring the urease activity, the breath test detected the presence of urease-positive bacteria in four more animals than the rapid urease test. PCR identified the presence of H. heilmannii in four cats considered negative by all other criteria and did not amplify the bacterial DNA of four cats with positive results in the other tests.
DISCUSSION
This study shows a prevalence of gastric Helicobacter spp. in >90% of healthy pet cats. A high prevalence of GHLO was reported not only for cats with gastrointestinal symptoms (5, 10) but also for clinically healthy cats (9, 19). While earlier reports (14, 19) showed a correlation between age and the presence of GHLO, we and others (5) did not find more spiral organisms in older cats. Indeed, two out of four cats over 5 years old were Helicobacter negative.
PCR is a specific method to distinguish between different Helicobacter spp. It is also sensitive for the diagnosis of H. pylori in humans and cats (12). Our primers had no cross-reactivity between the different Helicobacter species. PCR showed that 38 of 49 cats were colonized by H. heilmannii, while none was infected by H. felis or H. pylori.
Cats can be infected with different Helicobacter species, and mixed infections occur (11). Furthermore, H. pylori has been detected in several feline stomachs (8), but the public health implication of this observation is unclear (7). While some human epidemiological studies showed contact with cats to be a risk factor in the acquisition of H. pylori (13), other reports could not confirm this (18).
H. felis is characterized by the presence of periplasmic fibrils (11), while H. heilmannii is not. Species identification based upon such criteria must be made cautiously, since the stability of this in vivo characteristic has not been established. Recently, the in vitro loss of periplasmic fibrils by H. felis was described (3). Unfortunately, H. heilmannii cannot be grown in vivo (15). Similarly, biopsy samples from 53 cats infected with GHLO, only one was positive in culture for H. pametensis.
For four cats negative by all other diagnostic assays, PCR results were positive. Contamination during DNA extraction or PCR preparation seems unlikely. These positive PCR results suggest that the number of helicobacters might be too low to be detected by morphological or urease-based tests. Alternatively, contamination could occur with nondisposable biopsy forceps during biopsy sampling (12). For four cats positive by all other diagnostic assays, PCR results were negative. Possibly these negative results were due to yet another Helicobacter sp. with a urease that our primers were unable to amplify.
The urea breath test is the most reliable noninvasive test for an H. pylori infection in humans and has been used in natural and experimental animal Helicobacter infections (6). We were able to establish the [13C]urea breath test as a noninvasive assay to diagnose cats naturally colonized with Helicobacter spp. The maximum change of 13C over baseline values was found 30 min postdosing. The discrepancy between the measurements of urease activity by rapid urease testing and by urea breath testing in four cats could be due to a patchy Helicobacter colonization, with the risk of missing colonized spots by biopsy sampling.
We and others (19) found no correlation between the severity of mucosal lesions of noninfected cats and that of infected cats. Cats not infected with GHLO had histopathological changes of the mucosa that were similar to but milder than those of infected cats (9, 10). Other authors showed an association of GHLO with mild to moderate gastritis (14). In some studies (9, 14) large mucosal specimens were obtained at necropsy, while we evaluated only small biopsy samples taken during gastroscopy, which might partly explain the discrepancy. While from our endoscopic biopsy samples the diagnosis of a GHLO colonization was made for 85% of infected cats, the assessment of inflammation was less clear.
In the present study, concordant results among the different diagnostic tests were reached for 79% of the cats evaluated. Biopsy-based assays showed a high degree of agreement, with only one discordant result between the Gram and Warthin-Starry staining methods.
In summary, cats are frequently colonized by H. heilmannii. No correlation between infection and gastritis score was found. The [13C]urea breath test can be used reliably as a noninvasive diagnosis method, while a quick urease assay or Gram staining of a tissue smear can be used on gastric biopsy material. The combination of a morphological and a urease-based test maximizes the chance of an accurate diagnosis. PCR with specific primers can differentiate reliably between Helicobacter species.
ACKNOWLEDGMENTS
R.N. is supported in part by grants from Sandoz and Roche Research Foundation. I.C.-T. is a recipient of a Swiss Confederation Grant for Academic Scientists. Part of this work was supported by grants from the Swiss National Foundation (31-46858.96, 31-43240.95, and 32-40901.94).
REFERENCES
- 1.Andersen L P, Norgaard A, Holck S, Blom J, Elsborg L. Isolation of a “Helicobacter heilmannii”-like organism from the human stomach. Eur J Clin Microbiol Infect Dis. 1996;15:95–96. doi: 10.1007/BF01586196. [DOI] [PubMed] [Google Scholar]
- 2.Burnens A P, Stanley J, Schaad U B, Nicolet J. Novel Campylobacter-like organisms resembling Helicobacter fennelliae isolated from a boy with gastroenteritis and from dogs. J Clin Microbiol. 1993;31:1916–1917. doi: 10.1128/jcm.31.7.1916-1917.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton K A, Dewhirst F E, Paster B J, Tzellas N, Coleman B E, Paola J, Sherding R. Prevalence and varieties of Helicobacter species in dogs from random sources and pet dogs: animal and public health implications. J Clin Microbiol. 1996;34:3165–3170. doi: 10.1128/jcm.34.12.3165-3170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox J G, Batchelder M, Marini R P, Yan L, Handt L K, Li X, Shames B, Hayward A, Campbell J, Murphy J C. Helicobacter pylori-induced gastritis in the domestic cat. Infect Immun. 1995;63:2674–2681. doi: 10.1128/iai.63.7.2674-2681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geyer C, Colbatzky F, Lechner J, Hermanns W. Occurrence of spiral-shaped bacteria in gastric biopsies of dogs and cats. Vet Rec. 1993;133:18–19. doi: 10.1136/vr.133.1.18. [DOI] [PubMed] [Google Scholar]
- 6.Glauser M, Michetti P, Blum A L, Corthésy-Theulaz I. Carbon-14-urea breath test as a noninvasive method to monitor Helicobacter felis colonization in mice. Digestion. 1996;57:30–34. doi: 10.1159/000201309. [DOI] [PubMed] [Google Scholar]
- 7.Handt L K, Fox J G, Dewhirst F E, Fraser G J, Paster B J, Yan L L, Rozmiarek H, Rufo R, Stalis I H. Helicobacter pylori isolated from the domestic cat: public health implications. Infect Immun. 1994;62:2367–2374. doi: 10.1128/iai.62.6.2367-2374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handt L K, Fox J G, Stalis I H, Rufo R, Lee G, Linn J, Li X, Kleanthous H. Characterization of feline Helicobacter pylori strains and associated gastritis in a colony of domestic cats. J Clin Microbiol. 1995;33:2280–2289. doi: 10.1128/jcm.33.9.2280-2289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Happonen I, Saari S, Castren L, Tyni O, Hänninen M-L, Westermarck E. Occurrence and topographical mapping of gastric Helicobacter-like organisms and their association with histological changes in apparently healthy dogs and cats. J Vet Med Ser A. 1996;43:305–315. doi: 10.1111/j.1439-0442.1996.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 10.Hermanns W, Kregel K, Breuer W, Lechner J. Helicobacter-like organisms: histopathological examination of gastric biopsies from dogs and cats. J Comp Pathol. 1995;112:307–318. doi: 10.1016/s0021-9975(05)80083-0. [DOI] [PubMed] [Google Scholar]
- 11.Lee A, Hazell S L, O’Rourke J L, Kouprach S. Isolation of a spiral-shaped bacterium from the cat stomach. Infect Immun. 1988;56:2843–2850. doi: 10.1128/iai.56.11.2843-2850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mapsone N P, Lynch D A F, Lewis F A, Axon A T R, Tompkins D S, Dixon M F, Quirke P. Identification of Helicobacter pylori DNA in the mouth and stomach of patients with gastritis using PCR. J Clin Pathol. 1993;46:540–543. doi: 10.1136/jcp.46.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris A, Nicholson G, Lloyd G. Seroepidemiology of Campylobacter pyloridis. N Z Med J. 1986;99:657–659. [PubMed] [Google Scholar]
- 14.Otto G, Hazell S L, Fox J G, Howlett C R, Murphy J C, O’Rourke J L, Lee A. Animal and public health implications of gastric colonization of cats by Helicobacter-like organisms. J Clin Microbiol. 1994;32:1043–1049. doi: 10.1128/jcm.32.4.1043-1049.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solnick J, O’Rourke J L, Lee A, Paster B J, Dewhirst F E, Tompkins L S. An uncultured gastric spiral organism is a newly identified Helicobacter in humans. J Infect Dis. 1993;168:379–385. doi: 10.1093/infdis/168.2.379. [DOI] [PubMed] [Google Scholar]
- 16.Solnick J, O’Rourke J L, Lee A, Tompkins L S. Molecular analysis of urease genes from a newly identified uncultured species of Helicobacter. Infect Immun. 1994;62:1631–1638. doi: 10.1128/iai.62.5.1631-1638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stucki U, Frey J, Nicolet J, Burnens A P. Identification of Campylobacter jejuni on the basis of a species-specific gene that encodes a membrane protein. J Clin Microbiol. 1995;33:855–859. doi: 10.1128/jcm.33.4.855-859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb P M, Knight T, Elder J B, Newell D G, Forman D. Is Helicobacter pylori transmitted from cats to humans? Helicobacter. 1996;2:79–81. doi: 10.1111/j.1523-5378.1996.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 19.Weber A F, Hasa O, Sautter J H. Some observations concerning the presence of spirilla in the fundic glands of dogs and cats. Am J Vet Res. 1958;19:677–680. [PubMed] [Google Scholar]