Abstract
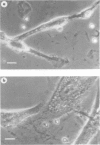
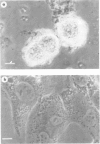
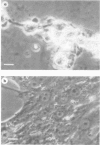
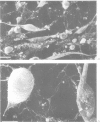
The incubation of Treponema pallidum with rabbit testicular cells, HEP-2 cells, human foreskin cells, rat cardiac cells, and rat skeletal muscle cells caused morphological disruption of these cultured cells. Control preparations of heat-inactivated treponemes, a high-speed supernatant in which treponemes had been pelleted, and culture medium failed to damage the tissue cells, as did viable treponemes when the cells were incubated in inverted Sykes-Moore chambers. Thus, cellular disruption is not associated with soluble treponemal, soluble inflammatory, or soluble testicular constituents but is mediated by the specific attachment of T pallidum. This organism apparently elaborates some type of toxic activity that lyses membranes: this may explain some of the histopathology of syphilitic disease.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azar H. A., Pham T. D., Kurban A. K. An electron microscopic study of a syphilitic chancre. Engulfment of Treponema pallidum by plasma cells. Arch Pathol. 1970 Aug;90(2):143–150. [PubMed] [Google Scholar]
- Brown J. E., Rothman S. W., Doctor B. P. Inhibition of protein synthesis in intact HeLa cells by Shigella dysenteriae 1 toxin. Infect Immun. 1980 Jul;29(1):98–107. doi: 10.1128/iai.29.1.98-107.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev Biol. 1972 Jun;28(2):407–429. doi: 10.1016/0012-1606(72)90023-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Miller J. N., Sykes J. A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977 Nov;18(2):467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Miller J. N., Sykes J. A. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect Immun. 1975 May;11(5):1133–1140. doi: 10.1128/iai.11.5.1133-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J. Pathogenesis and immunology of Treponema pallidum. Annu Rev Microbiol. 1981;35:29–54. doi: 10.1146/annurev.mi.35.100181.000333. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Brunton L. L., Schnaitman T. C., Rebhun L. I., Gilman A. G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun. 1974 Aug;10(2):320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Graham D. J., Nell E. E., Dannenberg A. M., Jr Macrophages in immunity to syphilis: suppressive effect of concurrent infection with Mycobacterium bovis BCG on the development of syphilitic lesions and growth of Treponema pallidum in tuberculin-positive rabbits. Infect Immun. 1979 Nov;26(2):751–763. doi: 10.1128/iai.26.2.751-763.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T. Pathogenesis of Shigella diarrhea. 3. Effects of shigella enterotoxin in cell culture. Trans N Y Acad Sci. 1973 Jan;35(1):51–58. doi: 10.1111/j.2164-0947.1973.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Lauderdale V., Goldman J. N. Serial ultrathin sectioning demonstrating the intracellularity of T. Pallidum. An electron microscopic study. Br J Vener Dis. 1972 Apr;48(2):87–96. doi: 10.1136/sti.48.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin W. J., Jr, Robinson R. B., Hermsmeyer K. Correlation of function and morphology of neonatal rat and embryonic chick cultured cardiac and vascular muscle cells. Circ Res. 1979 Oct;45(4):528–540. doi: 10.1161/01.res.45.4.528. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Eiklid K. Isolation and characterization of Shigella shigae cytotoxin. J Biol Chem. 1980 Jan 10;255(1):284–289. [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYKES J. A., MOORE E. B. A simple tissue culture chamber. Tex Rep Biol Med. 1960;18:288–297. [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N. Intracellular location of Treponema pallidum (Nichols strain) in the rabbit testis. Infect Immun. 1971 Sep;4(3):307–314. doi: 10.1128/iai.4.3.307-314.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N., Kalan A. J. Treponema pallidum within cells of a primary chancre from a human female. Br J Vener Dis. 1974 Feb;50(1):40–44. doi: 10.1136/sti.50.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VICARI G., OLITZKI A. L., OLITZKI Z. The action of the thermolabile toxin of Shigella dysenteriae on cells cultivated in vitro. Br J Exp Pathol. 1960 Apr;41:179–189. [PMC free article] [PubMed] [Google Scholar]