Abstract
Background:
Despite the promising implications for novel immune therapeutics, few clinical trials have tested these therapies to date. An understanding of how immune pharmacotherapies influence complex alcohol use disorder (AUD) profiles, including subjective response to alcohol is very limited. Initial findings show that ibudilast, a neuroimmune modulator, reduces rates of heavy drinking and measures of alcohol craving.
Methods:
This study serves as a secondary analysis of a two-week clinical trial of ibudilast that enrolled a non-treatment seeking sample with AUD. Eligible participants (N = 52) were randomized to ibudilast or matched placebo and completed daily diary assessments (DDAs) during the two-week period. Each morning, participants retrospectively reported on their mood and craving levels both before and during the previous day’s drinking episode, as well as stimulation and sedation levels during the previous day’s drinking episode. Multilevel models compared the effects of ibudilast and placebo on subjective alcohol response. Exploratory analyses tested whether ibudilast moderated the relationship between daily stimulation/ sedation and alcohol intake and whether withdrawal-related dysphoria moderated ibudilast’s effects on subjective response.
Results:
Ibudilast did not significantly alter mean levels of stimulation or sedation (p’s > .05). It did, however, moderate the effect of daily stimulation on drinking (p = .045). Ibudilast attenuated alcohol-induced increases in craving compared with placebo (p = .047), but not other subjective response measures. Only among individuals without withdrawal-related dysphoria did ibudilast significantly temper daily alcohol-induced changes in urge to drink and positive mood.
Conclusions:
Ibudilast’s effects on subjective alcohol responses appear to be nuanced and perhaps most salient for individuals drinking for positive reinforcement versus to feel normal. Consistent with previous findings, reductions in alcohol craving may represent a primary mechanism of ibudilast. The ecologically valid nature of DDAs provide a clinically useful window into how individuals experience alcohol’s effects while taking ibudilast.
Keywords: subjective response, alcohol use disorder, immune, treatment, pharmacotherapy
INTRODUCTION
Harmful use of alcohol is the leading risk factor for premature disability and mortality globally among individuals aged 15 to 49 years (2018). Excessive drinking, along with biological and environmental risk factors, can progress to alcohol use disorder (AUD), which is characterized by repeated alcohol use despite negative consequences (Gilpin and Koob, 2008; Kranzler and Soyka, 2018). Notwithstanding the wide range of health and psychological consequences associated with AUD, a large treatment gap remains, with less than 8% of persons with past-year AUD receiving alcohol care and even fewer receiving evidence-based care (SAMSHA, 2019). Multi-system strategies are needed to advance treatments and increase utilization rates among the diverse set of people with AUD. Development of novel, effective pharmacotherapies is one approach likely to help (Litten et al., 2012). To support people in recovery, medications must target factors sustaining drinking. Identifying mechanisms of action, such as through randomized controlled trials (RCTs), human laboratory paradigms, and collection of real-world data represents a vital step in medications development (Carpenter et al., 2020).
Behavioral pharmacology has established subjective response to alcohol as a reliable, multi-faceted phenotype serving as a central biobehavioral marker of positive and negative reinforcement from alcohol (Bujarski and Ray, 2014). Individual variability in alcohol’s acute subjective effects, specifically greater stimulation and reward and lower sedation, predict liability for AUD, including escalation of alcohol use and AUD symptomatology (King et al., 2021; Quinn and Fromme, 2011; Schuckit et al., 2007). Positive mood, negative mood, and craving are acutely modulated by alcohol use, such that individuals typically experience an increase in positive mood and craving and decrease in negative mood along rising breath alcohol concentrations (BrACs), serving as reinforcers of alcohol intake (Bujarski and Ray, 2014). Thus, researchers routinely assess whether pharmacotherapies can effectively modulate subjective response to alcohol through experimental human laboratory paradigms (Bujarski and Ray, 2016; Ray et al., 2016). Importantly, a recent meta-analysis has shown that medication effects on subjective responses to alcohol in the laboratory predict their efficacy in clinical trials for AUD (Ray et al., 2021). In an initial safety and efficacy trial, our laboratory used an intravenous alcohol administration paradigm to test whether the novel pharmacotherapy, ibudilast, modulated subjective response in a clinical sample of AUD (Ray et al., 2017). While ibudilast did not significantly alter subjective response, subjective effects of mood were dependent on participant’s degree of depression symptomatology.
Novel designs testing alcohol’s subjective effects are emerging, such as daily diary methods and ecological momentary assessment (EMA) in which participants report on their drinking experiences in real-world settings (Miranda et al., 2014; Trela et al., 2016). For instance, using EMA in an RCT that enrolled adolescents with problematic drinking, Miranda Jr. et al. (2014) found that naltrexone attenuated alcohol-induced increases in stimulation and enhanced alcohol-induced sedation, as compared to placebo. These naturalistic reports are consistent with findings on naltrexone’s subjective effects in human laboratory settings (Ray et al., 2019). Although less temporally precise than EMA, daily diary methods, which typically include data collection once daily, have lower participant burden and can enhance compliance. While assessment of medication effects on acute subjective response to alcohol via daily diary assessments (DDAs) is limited, past work has utilized these designs to assess daily relationships among urge, mood, and drinking (Helstrom et al., 2016; Kranzler et al., 2013). In a trial of naltrexone for heavy drinking among young adults, morning DDAs revealed that higher daily urge was associated with a greater likelihood of taking the medication, which in return, predicted a lower likelihood of same-day intoxication among the treatment group (Bold et al., 2016). The current study consists of a secondary analysis of a two-week experimental medication RCT of ibudilast, which demonstrated treatment-related reductions in rates of heavy drinking, as reported through daily diary methods, and reduced neural alcohol cue-reactivity (Grodin et al., 2021). This study seeks to further test ibudilast’s effects on subjective response to alcohol in the natural environment via DDA. When comparing participant report of drink quantity between these two methods (i.e., EMA vs. DDA), estimates of alcohol consumption are largely consistent, such that 75% of reports fell within 1 standard drink (Stevens et al., 2020). Similarly, research from affective science suggests that DDA versus EMA do not meaningfully alter estimates of emotion variability in the real-world nor their associations with health outcomes (Schneider et al., 2020). In sum, micro-longitudinal, naturalistic daily reporting is a valuable and highly complementary method to clinical trials, as they can increase power and ecological validity, reduce recall error, and result in more cost-effective RCTs (Carpenter et al., 2020).
Despite a mounting body of work connecting the immune system with the development and maintenance of AUD (Crews et al., 2019) and the important implications for the development of these novel therapeutics (Meredith et al., 2021a), few RCTs have tested immunotherapies in the context of AUD to date. Thus, our understanding of how these medications influence complex AUD profiles, including subjective response, is limited (Ray et al., 2017). Alcohol is believed to alter immune signaling and contribute to neuroinflammation indirectly through systemic inflammation and directly via events in the brain that stimulate release of inflammatory molecules, induce neural damage, and alter neural signaling (Crews and Vetreno, 2016). In preclinical models, an inflammatory state alters ethanol intake, preference, and behavioral responses to ethanol (Blednov et al., 2014; Blednov et al., 2018; Liu et al., 2011; Northcutt et al., 2015). In human AUD samples, peripheral proinflammatory markers are consistently elevated and correlate with alcohol use (Adams et al., 2020; Crews et al., 2017). As such, considerable interest exists for novel treatments that can restore healthy levels of inflammation and immune signaling to promote recovery from AUD (Erickson et al., 2019; Meredith et al., 2021a).
Phosphodiesterase (PDE) inhibitors are a class of immune therapies tested extensively in preclinical models of AUD (Blednov et al., 2018; Franklin et al., 2015; Hu et al., 2011; Logrip et al., 2014; Ozburn et al., 2020). PDEs are enzymes that play a central role in the regulation of intracellular levels of cyclic adenosine monophosphate (cAMP), along with its downstream signal transductions (Wen et al., 2018). Acute alcohol exposure activates cAMP signal transduction, while chronic exposure to alcohol attenuates cAMP signaling pathways in specific brain regions (Logrip, 2015). PDE4 isoforms are expressed in neuronal and glial cells in brain regions implicated in the rewarding and reinforcing effects of alcohol, such as the nucleus accumbens and amygdala (Pérez-Torres et al., 2000). Ibudilast is a selective PDE inhibitor and macrophage migration inhibitory factor (MIF) inhibitor that crosses the blood-brain barrier (Gibson et al., 2006), attenuates astrocyte and microglial activation, and increases anti-inflammatory cytokine expression (Mizuno et al., 2004). Notably, preclinical work demonstrated that ibudilast reduced voluntary ethanol intake in three different rodent models of AUD (Bell et al., 2015). Thus, ibudilast represents a promising pharmacotherapy for AUD, but its mechanisms of action remain largely unknown in clinical samples.
To date, our laboratory has tested ibudilast in two clinical samples with AUD. In an initial safety and efficacy trial, ibudilast improved mood resilience following stress exposure and reduced tonic levels of craving (Ray et al., 2017). Mood resilience was defined as a faster recovery of positive mood to baseline levels in the treatment condition following exposure to a stressful personal narrative. However, as noted above, ibudilast did not robustly alter subjective response during an alcohol administration paradigm. Yet, this study had a relatively small sample size (N = 24), and findings could not be extended to subjective effects of alcohol in real-world settings, as participants were required to maintain abstinence during the trial for safety reasons. Extending medications development to naturalistic settings, particularly for novel pharmacotherapies like ibudilast, is needed, as it enables researchers to assess medication effects with far greater ecological validity and to examine dynamic within-person processes through repeated assessments. Electronic real-world data capture is a cost-effective way to collect numerous occasions of alcohol self-administration and related subjective effects in participants’ natural environment (Carpenter et al., 2020). As such, work testing ibudilast’s ability to modulate subjective response in naturalistic drinking settings has the potential to further our understanding of its biobehavioral mechanisms, particularly in the context of powerful natural reinforcers and cues. For this reason, the present study will extend findings published from a two-week clinical trial of ibudilast in our laboratory, which utilized daily diary methods (Grodin et al., 2021). DDAs of subjective alcohol response were collected during this trial to identify biobehavioral mechanisms of ibudilast, but had yet to be analyzed.
The present study sought to test the effect of neuroimmune modulation by ibudilast on subjective response to alcohol in the naturalistic environment. This secondary analysis leveraged DDAs from a two-week experimental medication RCT of ibudilast, stratified on sex and withdrawal-related dysphoria, that enrolled non-treatment seeking participants with AUD. The DDAs included reports of alcohol use and subjective response measures of stimulation, sedation, mood, and craving. Each morning, participants retrospectively reported on their mood and craving levels both before and during the previous day’s drinking episodes, as well as stimulation and sedation levels during the previous day’s drinking episodes. We hypothesized that ibudilast would significantly reduce average levels of alcohol-related stimulation and increase average levels of alcohol-related sedation compared with placebo during participant naturalistic drinking episodes. Second, we hypothesized that ibudilast would significantly attenuate daily alcohol-induced changes (i.e., from before to during drinking timepoints) in craving and mood compared with placebo. Two sets of exploratory analyses were also undertaken in which we tested (1) if ibudilast moderated the effect of alcohol-related stimulation and sedation on same-day number of drinks consumed and (2) if the presence of withdrawal-related dysphoria moderated ibudilast’s effects on daily alcohol-induced changes in mood and craving.
MATERIALS AND METHODS
Trial Design
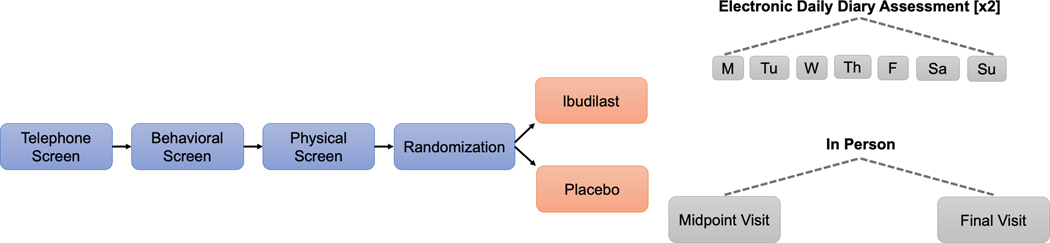
The current study is a secondary analysis of data collected during a two-week clinical trial of ibudilast for heavy drinking reduction and negative mood improvement in a sample of non-treatment seeking individuals with AUD (ClinicalTrials.gov identifier: NCT03489850). Primary trial findings and full study procedures were previously published (see (Grodin et al., 2021)). Fifty-two eligible participants were randomized to either ibudilast (50 mg BID) or matched placebo. Randomized participants were asked to attend in-person study visits on Day 1 (randomization), Day 8 (midpoint), and Day 15 (final assessment), and complete electronic DDAs to report on previous day craving, mood, and alcohol and cigarette use. When participants endorsed previous day alcohol consumption, they also reported on levels of stimulation and sedation. Participants completed a neuroimaging scan at study midpoint. The clinical trial was approved by the University of California, Los Angeles Institutional Review Board [UCLA IRB#17–001741]. Prior to completing study procedures, all participants provided written informed consent after receiving a full study explanation.
Participants
A community-based sample of individuals with current DSM-5 AUD was recruited for the trial through social media and mass transit advertisements in the greater Los Angeles area. Study inclusion criteria were: (a) between 21 and 50 years of age; (b) meet current DSM-5 diagnostic criteria for mild-to-severe AUD (First et al., 2015); and (c) report heavy drinking levels 30 days prior to their screening visit, as defined by the National Institute on Alcohol Abuse and Alcoholism as >14 drinks per week for men and >7 drinks per week for women. Exclusion criteria were: (a) currently receiving or seeking treatment for AUD; (b) current DSM-5 diagnosis of another substance use disorder (excluding alcohol or nicotine); (c) lifetime DSM-5 diagnosis of bipolar disorder or any psychotic disorder; (d) current use of psychoactive drugs, other than cannabis, as verified by a urine toxicology screen; (e) if female: pregnancy, nursing, or decision to not use a reliable method of birth control; (f) presence of nonremovable ferromagnetic objects, claustrophobia, serious head injury, or prolonged period of unconsciousness (>30 min; due to neuroimaging protocol); (g) medical condition that could interfere with safe study participation; and (h) recent use of medications contraindicated with ibudilast treatment (e.g., alpha or beta agonists, theophylline, or other sympathomimetics). Participants were also required to have reliable internet access to complete electronic DDAs.
A total of 190 individuals consented to participate in the initial in-person screening visit. Of those, 81 individuals were deemed clinically eligible and were invited to complete a physical screening to determine medical eligibility. A total of 52 participants were randomized to study medication (n = 24) or placebo (n = 28). Included in the present analyses are 50 participants (n = 23 ibudilast; n = 27 placebo) who completed at least one daily diary report after randomization. Participants were compensated up to $250 for their participation in the study and received an additional $100 bonus if all study visits and ≥80% of DDAs were completed.
Screening and Trial Procedures
The clinical trial was conducted at an outpatient research clinic in an academic medical center. Interested individuals completed an initial telephone-screening interview and eligible callers were then invited to the laboratory for an in-person behavioral screening visit. At the start of all in-person visits, participants were required to have a BrAC of 0.00g/dl and a urine toxicology test negative for all drugs excluding cannabis. Eligible participants were asked to complete an in-person physical screening visit consisting of laboratory tests and physical exam by a study physician. Participants meeting all study eligibility criteria who attended the in-person randomization visit were randomly assigned to receive either 50 mg BID of ibudilast or matched placebo. Randomization was stratified by sex and participant report of experiences with withdrawal-related dysphoria. This a-priori stratification variable was intended to capture the “dark side of addiction” (Koob and Mason, 2016), whereby individuals reporting withdrawal-related dysphoria were estimated to experience greater dysfunction of the immune system. MediciNova, Inc. (La Jolla, CA, USA) supplied ibudilast and placebo for the trial but did not provide any financial support for the study. The UCLA Research pharmacy prepared and dispensed all study medication in blister packs. Research staff, participants, and providers remained blind to medication condition during the trial. Participants were titrated on ibudilast as follows: 20 mg BID during days 1–2 and 50 mg BID during days 3–14. Target medication dose was selected based on safety considerations as well as preclinical and clinical data (Beardsley et al., 2010; Cho et al., 2010; Hutchinson et al., 2009; Worley et al., 2016). Medication compliance was monitored through pill counts and self-report via DDA. Side effects were closely monitored and reviewed by study physicians.
Screening Assessments
During the in-person screening visit, participants completed a set of assessments for individual differences and eligibility screening. Assessments included collection of demographic information (e.g., age, sex, race, income), substance use characteristics and history, and psychological functioning and diagnoses. Surveys used to characterize the sample (see Table 1) included the Beck Depression Inventory (Beck et al., 1996) (BDI-II) to assess levels of depression symptomatology, the Snaith-Hamilton Pleasure Scale (SHAPS) to measure anhedonia (Snaith et al., 1995), the Alcohol Use Disorders Identification Test (Saunders et al., 1993) (AUDIT) to capture alcohol problem severity, Penn Alcohol Craving Scale (Flannery et al., 1999) (PACS) to measure tonic craving levels, and the Reasons for Heavy Drinking Questionnaire (Adams et al., 2016) (RHDQ) to capture one’s motivations for heavy drinking. In addition, the RHDQ determined the presence of withdrawal-related dysphoria for randomization stratification as follows: raw scores ranging from 0 – 10 on the RHDQ question #6: “I drink because when I stop, I feel bad (I am nervous, irritable, and I sleep poorly)”, were dichotomized into yes /no, based on a cut-off of 6+ points. Interviews used to determine eligibility criteria and determine baseline quantity and frequency of alcohol use were administered by clinical graduate students or trained research staff and included the Timeline FollowBack (TLFB) measuring alcohol, cigarette, and cannabis use over the previous 30 days (Sobell and Sobell, 1992; Sobell et al., 1986), the Clinical Institute Withdrawal Assessment for Alcohol Scale - Revised (Sullivan et al., 1989) (CIWA-Ar) assessing clinically significant alcohol withdrawal, and the Structured Clinical Interview for DSM-5 (First et al., 2002) (SCID-5) to determine current AUD diagnosis and severity and to screen for exclusionary psychiatric diagnoses.
Table 1.
Participant characteristics by treatment condition
| Variable | Combined (N = 50) | Ibudilast (n = 23) | Placebo (n = 27) |
|---|---|---|---|
| Demographic | |||
| Age (Years) | 32.66 (8.52) | 34.13 (9.30) | 31.41 (7.75) |
| Sex (No., %) | |||
| Male | 33 (66.0%) | 16 (69.6%) | 17 (63.0%) |
| Female | 17 (34.0%) | 7 (30.4%) | 10 (37.0%) |
| Education (Years) | 15.16 (2.11) | 15.13 (2.49) | 15.18 (1.78) |
| Race (No., %) | |||
| White | 28 (56.0%) | 16 (69.6%) | 12 (44.4%) |
| Black or African American | 7 (14.0%) | 5 (21.7%) | 2 (7.4%) |
| Asian | 4 (8.0%) | 0 (0.0%) | 4 (14.8%) |
| Pacific Islander | 1 (2.0%) | 0 (0.0%) | 1 (3.7%) |
| Mixed Race | 6 (12.0%) | 1 (4.4%) | 5 (18.5%) |
| Another Race | 4 (8.0%) | 1 (4.4%) | 3 (11.1%) |
| Ethnicity (No., %) | |||
| Not Hispanic/ Latinx | 38 (76.0%) | 18 (78.3%) | 20 (74.1%) |
| Hispanic/ Latinx | 12 (24.0%) | 5 (21.7%) | 7 (26.0%) |
| Annual Household Income (No., %) | |||
| $0 – $29,999 | 19 (38.0%) | 10 (43.4%) | 9 (33.3%) |
| $30,000 – $59,999 | 15 (30.0%) | 5 (21.7%) | 10 (37.0%) |
| $60,000 – $89,999 | 9 (18.0%) | 4 (17.4%) | 5 (18.5%) |
| $90,000 – $119,999 | 2 (4.0%) | 1 (4.3%) | 1 (3.7%) |
| > $120,000 | 5 (10.0%) | 3 (13.0%) | 2 (7.4%) |
| Substance Use | |||
| Drinks per drinking daya | 5.61 (3.26) | 5.83 (2.61) | 5.42 (3.76) |
| % heavy drinking daysa | 45.92 (31.22) | 48.08 (29.68) | 44.09 (32.93) |
| Positive THC screen (No., %) | 15 (30.0%) | 7 (30.4%) | 8 (29.6%) |
| Smokes cigarettes (No., %) | 27 (54.0%) | 16 (59.3%) | 11 (47.8%) |
| PACS Total | 12.38 (6.29) | 12.87 (5.24) | 11.96 (7.13) |
| SCID AUD symptom count | 5.02 (2.33) | 5.30 (2.42) | 4.78 (2.26) |
| AUDIT total | 16.42 (6.02) | 16.26 (6.00) | 16.56 (6.48) |
| Withdrawal-related dysphoria (No., %) | 19 (38.0%) | 8 (34.8%) | 11 (36.7%) |
| Mental Health | |||
| BDI-II Total | 10.74 (8.23) | 12.83 (8.42) | 8.96 (7.78) |
| SHAPS Total | 1.66 (2.92) | 1.87 (1.84) | 1.48 (3.63) |
Note.
determined by Timeline FollowBack collected on the 30 days prior to baseline visit; PACS = Penn Alcohol Craving Scale; SCID = Structured Clinical Interview for the DSM-5; AUD = alcohol use disorder; AUDIT = Alcohol Use Disorders Identification Test; BDI-II = Beck Depression Inventory II; SHAPS = Snaith-Hamilton Pleasure Scale.
Electronic Daily Diary Assessments
Each morning throughout the two-week trial, participants were asked to retrospectively report on their previous day experiences by completing an electronic DDA survey (see Appendix). Study staff provided instructions on DDA completion and participants practiced filling out the survey at their randomization visit. Daily text messages or emails containing links to DDAs were sent to participants at 8am each morning during their 14-day medication period. Additional telephone or text reminders were sent by study staff as needed. At the start of each daily survey, participants were asked, “Did you drink any alcohol yesterday?” If participants endorsed alcohol use the previous day, they reported on drink type and quantity, and then completed two sets of items: 1) ratings of mood, craving, and urge before drinking, and 2) ratings of mood, craving, urge, stimulation, and sedation while drinking. For example, participants were asked: “Before you drank, how strong was your urge to drink alcohol yesterday?” and “While drinking, how strong was your urge to drink alcohol yesterday?” The current analyses focus primarily on drinking days, given our interest in medication-related changes in subjective response to alcohol.
Mood states were assessed via the short form of the Profile of Mood States (POMS-SF) survey (Curran et al., 1995; McNair et al., 1971). POMS-SF is a standard, validated psychological rating scale that measures dimensions of transient mood states by asking subjects to indicate how well each item describes their mood on a 5-point Likert scale (Not at All = 0 to Extremely = 4). To keep the survey brief and thus reduce the burden on participants, only select items from POMS-SF were chosen for DDAs (see Statistical Analyses). Reports of stimulation and sedation were assessed via the validated Brief Biphasic Alcohol Effects Scale (Rueger et al., 2009) (B-BAES). The B-BAES is a six-item measure on the acute stimulant and sedative effects of alcohol on an 11-point scale (Not at All = 0 to Extremely = 10 points). Urge to drink was captured via the item, “How strong was your urge to drink alcohol yesterday” (No Urge = 0 to Strongest Ever = 10), in line with previously published reports (Ray et al., 2007; Ray et al., 2010). Phasic craving was assessed using the first and last items from the validated Alcohol Urge Questionnaire (Bohn et al., 1995; MacKillop, 2006) (AUQ) on a 7-point Likert scale (Strongly Disagree = 0 to Strongly Agree = 6). Participants reported the quantity of standard alcoholic drinks consumed according to established guidelines and provided details about non-standard drinks (e.g., malt liquor, sake, hard seltzers, etc.). Drink entries were reviewed and verified by study staff.
Statistical Analyses
DDA Item Scoring
All descriptive and statistical analyses were completed in SAS Version 9.4 on the sample of participants who completed at least one DDA. Select items from the POMS-SF tension (i.e., Anxious, Uneasy) and depression subscales (i.e., Downhearted, Discouraged), were summed to form a negative mood state score (range 0 – 16) and select items from the vigor subscale (i.e., Joyful, Cheerful, Energetic, Lively) were summed to form a positive mood state score (range from 0 – 16) for each timepoint, consistent with previous reports (Bujarski et al., 2015; Sheets et al., 2015). The two AUQ items were summed to form a craving score (range 0 – 12). Stimulation and sedation subscales from B-BAES were calculated using standard methods (range 0 – 30).
Multilevel Models
Models were fit in SAS using the MIXED procedure and a multilevel framework, unstructured covariance matrix, residual maximum likelihood (REML) estimation, and random intercepts with observations nested within subjects to account for clustering and to preserve suitable Type-1 error rates (Raudenbush and Bryk, 2002). Kenward-Rogers degrees of freedom were chosen to reduce bias and obtain more accurate p-value estimates. Main and simple effects were probed by recentering dichotomous variables (e.g., medication condition, time) and using the simple slopes approach. Daily alcohol use quantity, mood states, craving, and urge data from non-drinking days were treated as missing. Comparable three-level models were fit for variables having both before and during drinking observations (i.e., positive mood, negative mood, urge, and craving), such that these observations were nested within day and days were nested within subjects. All models were tested with the following level-2 covariates: sex, AUD severity (DSM-5 symptom count), and baseline drinks per drinking day (DPDD). In addition, daily number of drinks consumed during the trial was included as a predictor with random effect in all subjective response models to account and control for potential day-level drink quantity effects on subjective response. To examine both between- and within-subject effects and interactions, covariates were centered at the grand mean (CGM) and focal within-subject variables were centered within cluster (CWC) (Enders and Tofighi, 2007).
Specifically, to assess the effect of medication on the acute stimulant and sedative effects of alcohol, one model for each B-BAES subscale was estimated in which stimulation or sedation served as the outcome and medication condition (ibudilast vs. placebo) served as the focal predictor. To assess the effect of medication on alcohol-induced changes in mood and craving, three-level models were run for each positive mood, negative mood, craving, and urge scores, as predicted by medication condition, time (before vs. during drinking) and a medication time interaction.
Two sets of exploratory analyses were conducted. First, to explore how medication effects might impact drinking outcomes, we tested whether ibudilast moderated the effect of stimulation/ sedation on same-day drinking during the trial, given support for these variables as strong predictors of alcohol use (King et al., 2021; Schuckit et al., 2007). As such, a within-subject cross-level interaction of medication stimulation or sedation was added with random slopes, and same-day number of drinks served as the outcome. In a similar fashion, we also tested whether ibudilast moderated the effect on stimulation/ sedation on next-day drinking (yes/ no) using cross-lagged logistic models; this analysis served to test whether subjective response predicted future drinking behaviors. Second, given the trial’s a priori interest in a withdrawal-related dysphoria characteristic, we tested whether dysphoria would moderate ibudilast’s effects on alcohol-induced changes in mood and craving. A three-way interaction was added to models estimating the outcomes- positive mood, negative mood, urge, and craving (i.e., withdrawal-related dysphoria medication time). Stimulation and sedation variables were limited to a single timepoint and were thus excluded from analyses testing before to during drinking changes.
RESULTS
Participant Characteristics
The final sample of randomized participants who completed at least one DDA, consisted of 50 non-treatment seeking individuals with current AUD (n = 23 ibudilast; n = 27 placebo). Overall, 66% of the sample reported their sex as male, 68% reported an annual household income < $60,000, and the average age was 32.7 years (see Table 1). Regarding race, participants most frequently identified as White (56%), followed by 14% Black or African American, and 12% mixed race. In addition, 24% of the sample identified as Hispanic/Latinx. Participants had an average of 5.6 DPDD in the month prior to their baseline visit. Medication adherence was high, as both medication groups exceeded 97% adherence rates. Adverse events by symptom category did not significantly differ between medication groups.
Daily Diary Assessments
In total, 653 DDAs were completed (92.6% completion rate) with participants missing between 0 to 4 days of reports during the two-week trial. Participants completed an average of 13.06 (SD = 1.14) DDAs, comprised of 7.92 (SD = 3.49) drinking days on average (range 1–14 days; total n = 396) and 5.14 (SD = 3.62) non-drinking days on average (range 0–12; total n = 257). Interclass correlations (ICCs) from unconditional subjective response models support the use of multilevel nested models. For instance, ICCs from stimulation and sedation models respectively, were .548 and .538, indicating that approximately 54% of the total variability is attributable to clustering observations within subjects. ICCs from three-level models on positive and negative mood, urge and craving accounting for day- and subject-level clustering ranged from .594 to .776 (i.e., clustering accounted for 59% to 78% of total variability). See Table 2 for estimated marginal means of subjective response and DPDD variables during the two-week trial by medication condition.
Table 2.
Estimated marginal means for subjective response variables by medication condition
| Variable | Ibudilast (n = 23) | Placebo (n = 27) | ||
|---|---|---|---|---|
| Before Drinking Mean (SE) | During Drinking Mean (SE) | Before Drinking Mean (SE) | During Drinking Mean (SE) | |
| Positive mooda | 5.85 (0.63) | 7.05 (0.63) | 6.41 (0.58) | 7.57 (0.58) |
| Negative mooda | 3.05 (0.44) | 2.15 (0.44) | 2.56 (0.41) | 1.71 (0.41) |
| Urgeb | 3.41 (0.41) | 3.87 (0.41) | 3.95 (0.38) | 4.67 (0.38) |
| Cravingc | 4.46 (0.49) | 4.70 (0.49) | 4.93 (0.45) | 5.63 (0.45) |
| Stimulationd | - | 13.30 (1.30) | - | 16.23 (1.20) |
| Sedationd | - | 5.81 (1.05) | - | 8.22 (0.97) |
| Daily number of drinks per drinking daye | - | 5.42 (0.41) | - | 6.08 (0.38) |
Note: Estimated marginal means drawn from subjective response models
subscale drawn from the Profile of Mood States- Short Form, possible range: 0 – 16
single-item Urge rating, possible range 0 – 10
subscale drawn from the Alcohol Urge Questionnaire, possible range 0 – 12
subscales from Brief Biphasic Alcohol Effects Scale, possible range 0 – 30
raw average number of drinks per drinking day during the trial according to participant report on daily diaries.
Effect of Ibudilast on Stimulation and Sedation
Stimulation.
Only daily number of drinks was a significant covariate of stimulation (p < .001), such that greater alcohol intake was associated with higher daily stimulation. After accounting for covariates, medication condition did not significantly predict average levels of stimulation (b = −2.93, p = .108).
Sedation.
Only AUD severity was a significant covariate of sedation, such that greater AUD severity was associated with higher mean sedation (p = .035). After accounting for covariates, medication condition did not significantly predict mean sedation during the trial (b = −2.41, p = .103).
Effect of Ibudilast and Subjective Response on Drinking Outcomes
Ibudilast Stimulation.
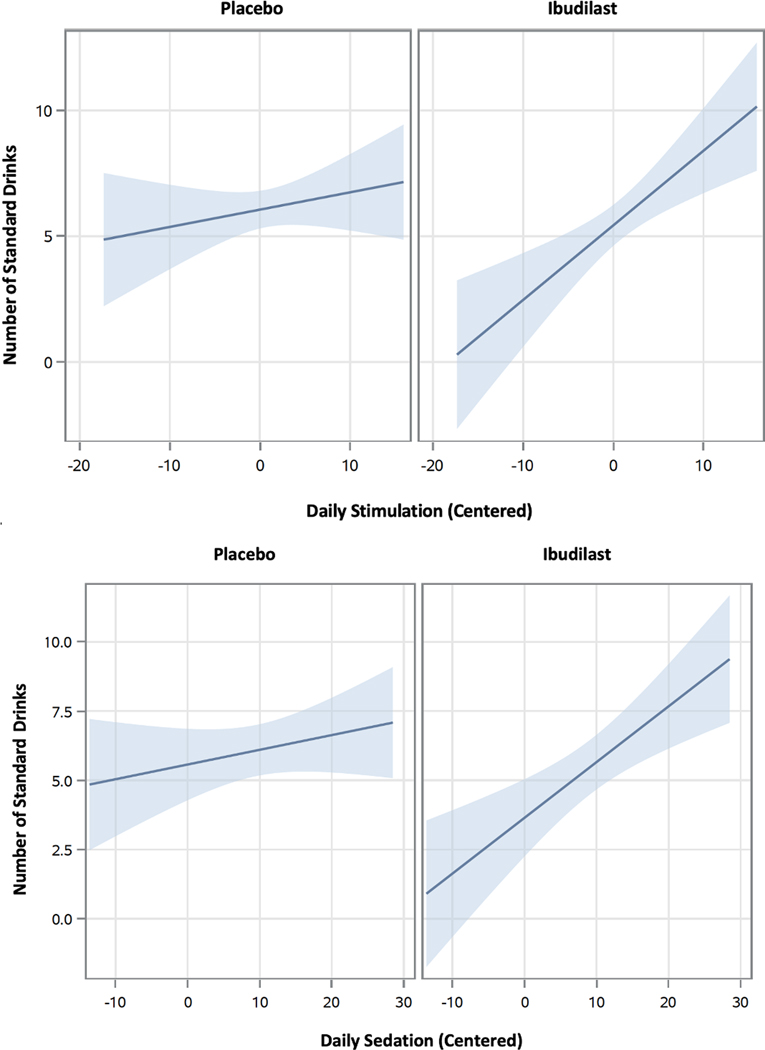
When testing whether medication condition moderated the effect of stimulation on same-day drinking during the trial, two covariates were significant: DPDD at baseline (p < .0001) and sex (p = .023), where baseline drinking and male sex were associated with greater mean DPDD during the trial. While medication condition was not a significant predictor of mean trial DPDD in this full model (b = −0.67, p = .232), a significant cross-level medication stimulation interaction was detected (b = 0.23, p = .045). As such, medication moderated the effect of daily stimulation on same-day number of drinks consumed. When probing for simple effects, results showed that only participants taking ibudilast (b = 0.30, p = .001), but not placebo (b = 0.07, p = .350), reported a significant, positive relationship between daily stimulation and same-day alcohol use (see Figure 2). This suggests that for a day with a 1-point higher score on stimulation, we would expect a 0.30-point relative within-person increase in number of drinks among those taking ibudilast. However, for the logistic model, a cross-level medication daily stimulation interaction did not significantly predict likelihood of next-day drinking (p = .646), nor did medication condition predict likelihood of next-day drinking (p = .721).
Figure 2.
Moderating effect of ibudilast treatment on daily stimulation/ sedation and number of drinks. Note: Visual shows that participants on ibudilast (<i>p</i>‘s ≤ .001), but not placebo (<i>p</i>‘s > .250), reported a significant positive relationship between daily stimulation/ sedation and same-day alcohol use.
Ibudilast Sedation.
When testing whether medication condition moderated the effect of sedation on same-day drinking during the trial, two covariates were significant: DPDD at baseline (p < .0001) and sex (p = .035), where baseline drinking and male sex were associated with greater mean DPDD during the trial. In this full model, medication condition (b = −1.93, p = .054) and the cross-level medication sedation interaction (b = 0.14, p = .059) were only marginally significant. When probing simple effects, results again showed that participants on ibudilast (b = 0.20, p < .001), but not placebo (b = 0.05, p = .278), reported a significant positive relationship between daily sedation and same-day alcohol use (see Figure 2). This suggests that for a day with a 1-point higher score on sedation, we would expect a 0.20-point relative within-person increase in number of drinks among those taking ibudilast. However, for the logistic model, a cross-level medication daily sedation interaction did not significantly predict likelihood of next-day drinking (p = .858), nor did medication condition predict likelihood of next-day drinking (p = .730).
Effect of Ibudilast on Alcohol-Induced Changes in Mood and Craving
Positive and Negative Mood.
In testing the impact of medication on alcohol-induced changes in mood, daily number of drinks during the trial was a significant within-subject predictor of positive mood, where greater daily drinking predicted higher same-day positive mood (p = .004). No other covariates were significant. Neither the medication time interaction (b = 0.04, p = .895), nor medication condition significantly predicted same-day positive mood (b = −0.54, p = .545). Participant report of positive mood was significantly greater at the during drinking timepoint (b = 1.20, p < .0001). Similarly, neither the medication time interaction (b = −0.05, p = .817), nor medication condition significantly predicted same-day negative mood (b = 0.55, p = .429). As expected, participant report of negative mood was significantly lower at the during drinking timepoint (b = −0.88, p < .0001).
Urge and Craving.
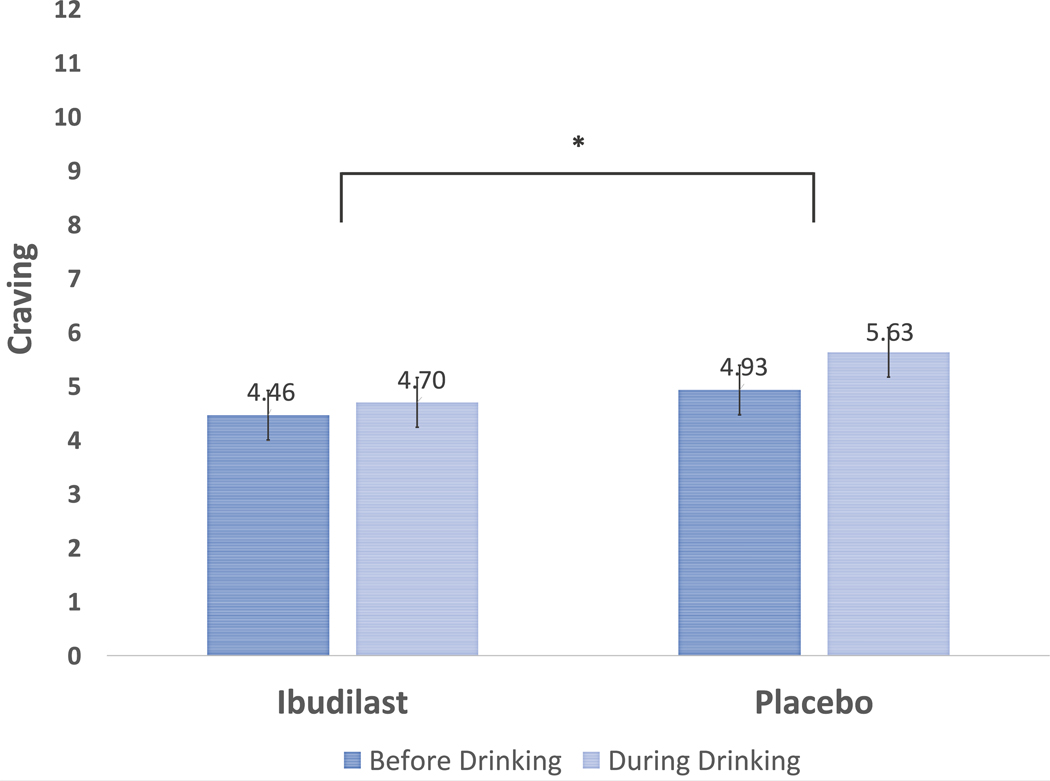
In testing the impact of medication on alcohol-induced changes in urge and craving, baseline DPDD and daily number of drinks consumed during the trial were significant predictors of urge and craving (p’s < .005); AUD severity was also a significant predictor of craving (p = .037). For these significant covariates, higher scores were associated with greater urge and craving. Neither the medication time interaction (b = −0.27, p = .173), nor medication condition (b = −0.27, p = .667) significantly predicted daily urge. Participant report of urge was significantly greater at the during drinking timepoint (b = 0.59, p < .0001). While medication condition was not significantly associated with average daily craving, the cross-level medication time interaction (b = 0.46, p = .047) did significantly predict craving. When probing simple effects by recentering, results show that only for the placebo condition (b = 0.69, p < .0001), but not ibudilast (b = 0.24, p = .167), did same-day craving significantly increase during alcohol intake (see Figure 3), showing that ibudilast attenuated within-subject alcohol-induced increases in craving.
Figure 3.
Ibudilast attenuates alcohol-induced increases in craving compared to placebo.Note: * indicates a significant (<i>p</i> = .047) medication (ibudilast vs. placebo) x time (before vs. during drinking) interaction for alcohol craving reported on daily diary assessments.
Moderating Role of Withdrawal-Related Dysphoria
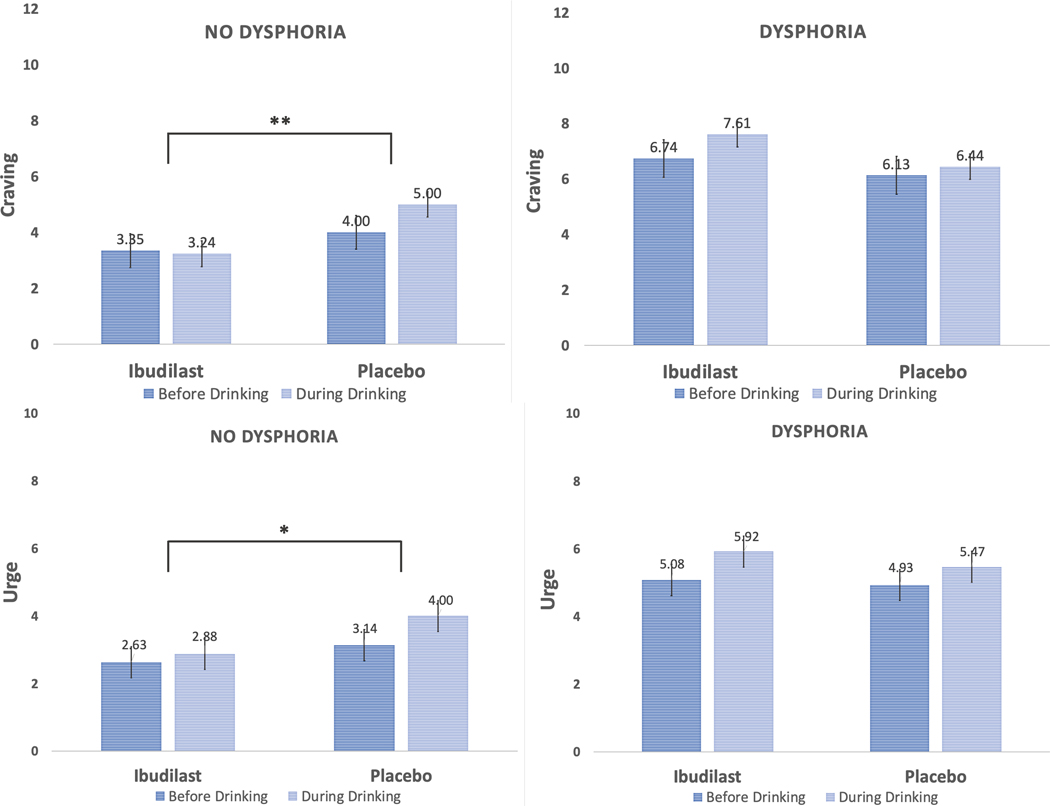
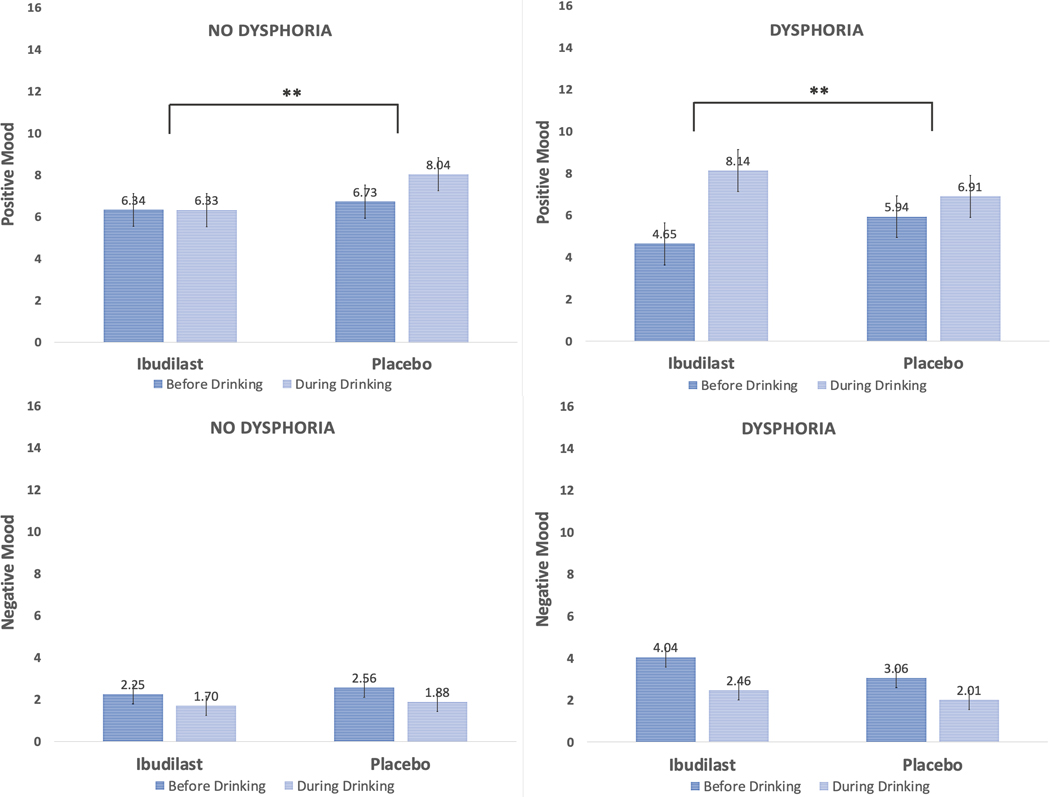
In total, 19 participants were categorized as having withdrawal-related dysphoria (n = 8 ibudilast; n = 11 placebo). Given the modestly sized subgroupings, this set of analyses are exploratory and should be interpreted as such. In testing our exploratory models on whether the presence of withdrawal-related dysphoria moderated the effect of ibudilast on same-day alcohol-induced changes in mood, craving, and urge, two significant covariates emerged. For models predicting craving and urge, baseline DPDD (p’s < .005) and daily number of drinks during the trial (p’s < .0005), were positively associated with craving and urge; daily number of drinks was also positively associated with same-day positive mood (p = .004). Several three-way interactions were detected, such that for daily changes in craving (b = −1.64, p = .0004; see Figure 4), urge (b = −0.88, p = .028; see Figure 4), and positive mood (b = −3.40, p < .0001; see Figure 5), a significant dysphoria medication time interaction emerged after accounting for relevant covariates. Yet, this three-way interaction term was not significant for the model predicting negative mood (p = .300; see Figure 5). A consistent pattern emerged when probing these interactions, such that among participants without reported withdrawal-related dysphoria, the medication time interactions were significant for craving (p = .0002), urge (p = .021), and positive mood (p = .001). Participants without withdrawal dysphoria and randomized to ibudilast reported smaller and non-significant increases in subjective responses (p’s range from .190 to .952) compared with placebo (p’s <.0001). For instance, while taking placebo, participants had an average daily alcohol-induced increase in positive mood of 1.31 points, while those on ibudilast displayed an average daily alcohol-induced decrease in positive mood by 0.02 points. For those reporting presence of withdrawal-related dysphoria, no significant medication time interactions were detected for craving (p = .110) nor urge (p = .301), but the interaction was significant for positive mood (p < .0001). Unexpectedly, participants with withdrawal dysphoria on ibudilast reported greater same-day alcohol-induced increases in positive mood (p < .0001) than placebo (p = .005).
Figure 4.
Alcohol-induced changes in craving and urge by medication condition and presence of withdrawal-related dysphoria. Note: Three-way interactions (medication x time x withdrawal-related dysphoria) were detected for daily changes in craving (<i>p</i> = .0004) and urge (<i>p</i> = .028); ** indicates a significant (<i>p</i> < .001) medication (ibudilast vs. placebo) x time (before vs. during drinking) interaction for alcohol craving among those without reported withdrawal-related dysphoria; * indicates a significant (<i>p</i> = .021) medication x time interaction for alcohol urge among those without reported withdrawal-related dysphoria.
Figure 5.
Alcohol-induced changes in mood by medication condition and presence of withdrawal-related dysphoria. Note: Three-way interaction (medication x time x withdrawal-related dysphoria) was detected for daily changes in positive mood (<i>p </i>< .001); ** indicates a significant (<i>p</i> < .01) medication (ibudilast vs. placebo) x time (before vs. during drinking) interaction for positive mood, whereby the ibudilast group showed smaller alcohol-related changes in mood among those without withdrawal-related dysphoria, yet greater changes in mood among those with withdrawal-related dysphoria.
DISCUSSION
In this secondary analysis, we tested biobehavioral mechanisms of ibudilast, a neuroimmune modulator, through naturalistic daily reporting of subjective response to alcohol collected during a two-week RCT enrolling 50 non-treatment seeking participants with AUD. Electronic DDAs were administered each morning to participants to capture their previous day drinking behaviors and subjective alcohol response measures. First, we were interested in understanding whether ibudilast altered average levels of stimulation and sedation during drinking episodes. Results showed that ibudilast treatment did not significantly change average levels of stimulation nor sedation during the trial compared with placebo. These findings are consistent with an initial safety trial in which ibudilast did not significantly affect any subjective response variables during an experimentally controlled alcohol infusion in the laboratory (Ray et al., 2017). Relatedly, a trial combining laboratory and EMA methods showed that topiramate reduced drinking-related craving but not the stimulant or sedative effects of alcohol (Miranda et al., 2016). However, animal literature shows that apremilast, another PDE inhibitor, did alter a wide range of ethanol-induced effects in mice, such as reducing acute functional tolerance and increasing the sedative, intoxicating effects, and aversive properties of ethanol (Blednov et al., 2018). Perhaps unlike certain pharmacotherapies for AUD such as naltrexone, neuroimmune modulators, like ibudilast may not reduce drinking by robustly suppressing alcohol’s stimulant properties or amplifying its sedative effects. Rather, ibudilast may more directly alter other central mechanisms like alcohol craving or may exert a wider range of effects on multiple mechanisms that cumulatively impact drinking outcomes.
Second, we tested a related exploratory aim examining the moderating effect of ibudilast on alcohol-related stimulation and sedation and same-day number of drinks consumed. Participants on ibudilast reported a significant, positive relationship between their stimulation and sedation ratings and same-day drinking levels, neither of which was observed in the placebo condition. This suggests that participants randomized to ibudilast consumed more alcohol on days when they retrospectively reported feeling more stimulated (or sedated) during a drinking episode than on days when they felt less stimulated (or sedated). Yet for those on placebo, we did not detect a significant relationship between one’s feelings of stimulation or sedation and alcohol use. These findings are consistent with EMA data showing that naltrexone potentiated participant’s subjective “high” across rising levels of estimated BrAC (Miranda et al., 2014). Similarly, topiramate was shown to strengthen the association between mean positive affect and frequency of cannabis use (Emery et al., 2021). These results are also in line with a secondary analysis of our lab’s initial efficacy trial, whereby ibudilast potentiated the association between mood states and one’s craving for alcohol following a stress exposure paradigm compared with placebo (Meredith et al., 2021b). Mechanistically, PDE4 inhibitors attenuate alcohol-induced neuroimmune activation and dysregulation of GABAergic signaling (Avila et al., 2017; Blednov et al., 2018; Carlson et al., 2016). These important processes are connected to behavioral responses to ethanol (Crews et al., 2017; Liang and Olsen, 2014). Thus, micro-longitudinal reports collected during the current trial helped to elucidate dynamic, day-to-day associations between within-person subjective effects and drinking, such that ibudilast seemed to moderate these relationships for a given individual, rather than by altering average subjective response levels across participants.
For our second primary aim, we assessed whether ibudilast, compared with placebo, attenuated daily alcohol-induced changes in positive mood, negative mood, urge, and craving (i.e., from pre-drinking to during drinking levels). Among the full sample, we found that ibudilast significantly dampened within-person alcohol-induced increases in craving seen under the placebo condition, but not other subjective response indicators. This suggests that one of the mechanisms by which ibudilast exerts its effects on drinking outcomes, such as reductions in heavy drinking (see (Grodin et al., 2021)), may be by diminishing one’s desire to continue drinking during an episode. Considering its immunomodulatory actions, ibudilast may reduce the acute and chronic proinflammatory effects of alcohol, either indirectly through suppression of peripheral inflammation or directly by altering cAMP signaling pathways and suppressing cytokine expression and in the brain (e.g., rewards regions relevant to craving) (Avila et al., 2017). In return, acute alcohol-induced increases in craving are blunted. Supporting these findings is research on methamphetamine use disorder (MUD). An RCT for inpatients with MUD showed that ibudilast (50 mg BID) significantly blunted the rewarding effects of methamphetamine during an infusion in the laboratory (Worley et al., 2016) and similarly diminished drug-induced increases in proinflammatory levels during infusion (Li et al., 2020). Continuing, previous results from our group implicate ibudilast in the reduction of tonic craving (Ray et al., 2017) and neural alcohol-cue reactivity, as evidenced by attenuation of cue-elicited activation in the ventral striatum compared with placebo (Grodin et al., 2021). It is thus plausible that reductions in alcohol craving and reward, across these contexts, represent a primary mechanism of action of ibudilast for AUD. Craving likely represents a more proximal determinant of alcohol use than stimulation and sedation, which are shown to indirectly influence alcohol self-administration through craving (Green et al., 2019; Wardell et al., 2015).
An additional exploratory aim was to test whether a characteristic of AUD severity, withdrawal-related dysphoria, moderated ibudilast’s effects on daily alcohol-induced changes in mood and craving. Notably, we found that individuals without a reported history of withdrawal-related dysphoria who were treated with ibudilast showed attenuation of alcohol-induced changes in craving, urge, and positive mood when compared to placebo. This tempering of alcohol’s effects may reflect ibudilast’s enhancement of anti-inflammatory and neurotrophic factors suspected to impact dopaminergic signaling in rewards regions, such as the nucleus accumbens, where PDE4 and PDE10 are highly expressed (Bland et al., 2009; Ramirez and Smith, 2014). However, individuals who endorsed this withdrawal-dysphoric profile did not appear to benefit from treatment via this mechanism, such that ibudilast did not significantly blunt acute rewarding and reinforcing effects of alcohol. Although intriguing, these moderation findings should be interpreted with caution given the limited sample size, particularly the subgroup of individuals reporting experiences with withdrawal-related dysphoria (n = 19). Despite these findings, preliminary analyses from this two-week RCT show that withdrawal dysphoria did not moderate clinical response to ibudilast regarding rates of heavy drinking or drinks per drinking day. Notably, these subjective response results are somewhat in contrast to what might be expected for individuals with a history of withdrawal and experiencing the “dark side of addiction”, such that these individuals may potentially show greater dysfunction of the immune system and thus may be predicted to have better response to an anti-inflammatory treatment, such as ibudilast. However, it is suspected that other mechanisms may be central to the maintenance of AUD among individuals with withdrawal dysphoria, beyond the enhancing effects of alcohol. Namely, these individuals may primarily drink to feel ‘normal’ and alleviate physiological or psychological distress, particularly during early abstinence (Adams et al., 2016; Koob and Mason, 2016), which was not the focus on the current study. The present findings also differ somewhat from our laboratory’s initial efficacy trial of ibudilast, in which individuals with higher levels of depression (e.g., experiencing the “dark side of addiction”) showed attenuation of alcohol-induced increases in positive mood and ‘wanting’ during intravenous alcohol administration (Ray et al., 2017). A relevant difference between these studies is that participants enrolled in the efficacy trial were likely in a state of early abstinence, as they were asked to refrain from drinking for safety reasons; yet those enrolled in the present trial were not asked to change their drinking behaviors and consumed alcohol on roughly 60% of trial days and around 6 DPDD on average. In preclinical models, withdrawal increases the expression of innate immune markers in brain regions regulating autonomic and emotional states (Freeman et al., 2012) and while speculative, may thus represent a unique condition with the potential to impact ibudilast’s therapeutic effects. For instance, ibudilast reduced opioid withdrawal symptoms among individuals with heroin dependence (Cooper et al., 2016) and another PDE4 inhibitor, rolipram, diminished withdrawal-induced behaviors indicative of negative affect in rodents (Gong et al., 2017). Future research evaluating the impact of withdrawal states on immune signaling in larger clinical samples is needed to advance understanding of these complex processes and immune intervention.
These findings should be considered in the context of the study’s strengths and limitations. One limitation is that DDAs were reported retrospectively once daily, which is less temporally accurate than EMA designs. As such, items on subjective response and drinking were reported by participants concurrently the morning following a drinking episode and did not capture one’s subjective response level at a specific BrAC or blood alcohol curve limb. As such, this weakens our ability to draw a causal link between the effect of subjective response on alcohol intake and may introduce recall bias. Next, participants with more non-drinking days and incomplete DDAs during the trial are suspected to have greater error variance in their data given the lower number of observations with subjective response data. The lack of daily pre-drinking data on stimulation and sedation prevented us from examining daily changes in these variables, such that we could not account for pre-drinking levels. The sample was comprised of non-treatment seeking individuals with moderate AUD on average and the majority (62%) did not fall in the withdrawal-related dysphoria category. Future work with ibudilast in more diverse and treatment-seeking samples with more significant experiences of withdrawal-related dysphoria is needed. This study’s strengths include a clinical AUD sample enrolled in a rigorous double-blind RCT testing a promising novel pharmacotherapy. This trial displayed strong medication adherence rates and tolerability. Further, DDAs had high completion rates and the data comprise a substantial number of drinking episodes (e.g., ~400 DDAs). Morning reports are also less likely to be affected by the intoxicating effects of alcohol that may lend to reporting errors, as could be seen with EMA or nightly reports. Finally, to our knowledge, this is the first study on the effect of immune modulation on subjective alcohol response in the natural environment.
In closing, this daily diary study complements findings from our previous reports of ibudilast treatment for AUD by examining medication effects on subjective response during real-world drinking episodes. The nuanced nature of the findings, including the distinction among those with and without withdrawal-related dysphoria and within vs. between person subjective response effects, speak to the heterogeneity of AUD and dynamic mechanisms maintaining alcohol use. Ibudilast’s effects on subjective alcohol responses, such as positive mood and craving, appear to be nuanced and perhaps most salient for individuals drinking for positive reinforcement as opposed to normalizing. Treatment with ibudilast potentiated the within-person relations between stimulation/ sedation and alcohol intake in this trial, such that an individual’s quantity of consumption on a given day appears to be more tightly connected to subjective response. The ecologically valid nature of these DDA, through retrospective reports of past day drinking and subjective responses to alcohol, provide a clinically useful window into how individuals experience and recall alcohol’s effects while taking ibudilast, compared to placebo. Novel medications and novel biological targets call for careful assessment of mechanisms beyond the “usual suspects”, such as changes in mean levels of subjective response and alcohol craving. Ultimately, the combination of multiple scientific approaches, including human laboratory, DDAs, neuroimaging, and biomarker assessment, offer complementary and clinically useful findings that can inform the development of ibudilast, and immune treatments for AUD more broadly.
Supplementary Material
Figure 1.
Study design for two-week randomized controlled trial of ibudilast for alcohol use disorder with daily diary assessments.
Funding:
This research was supported by the National Institute on Drug Abuse grant P50 DA005010-33 (Pilot Project PI: LAR), the National Institute on Alcohol Abuse and Alcoholism grants K24AA025704 (LAR) and F32AA027699 (ENG), and a UCLA Graduate Division Fellowship (LRM). Study medication was provided by MediciNova, Inc. Funding sources had no role other than financial support.
Footnotes
Conflicts of Interest: LAR has received study medication from Pfizer and MediciNova and has consulted for GSK and Guidepoint. Authors report no other conflicts of interest.
References
- 2018. Global status report on alchol and health 2018, (Geneva: ). [Google Scholar]
- Adams C, Conigrave JH, Lewohl J, Haber P. & Morley KC 2020. Alcohol use disorder and circulating cytokines: A systematic review and meta-analysis. Brain Behav Immun, 89(501–512. [DOI] [PubMed] [Google Scholar]
- Adams ZW, Schacht JP, Randall P. & Anton RF 2016. The Reasons for Heavy Drinking Questionnaire: Factor Structure and Validity in Alcohol-Dependent Adults Involved in Clinical Trials. J Stud Alcohol Drugs, 77(2), pp 354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila DV, Myers SA, Zhang J, Kharebava G, McClain CJ, Kim HY, Whittemore SR, Gobejishvili L. & Barve S. 2017. Phosphodiesterase 4b expression plays a major role in alcohol-induced neuro-inflammation. Neuropharmacology, 125(376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Shelton KL, Hendrick E. & Johnson KW 2010. The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur J Pharmacol, 637(1–3), pp 102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA & Brown GK 1996. Manual for the Beck Depression Inventory-II, San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bell RL, Lopez MF, Cui C, Egli M, Johnson KW, Franklin KM & Becker HC 2015. Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addict Biol, 20(1, pp 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Hutchinson MR, Maier SF, Watkins LR & Johnson KW 2009. The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain, Behavior, and Immunity, 23(4, pp 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M. & Harris RA 2014. Inhibition of phosphodiesterase 4 reduces ethanol intake and preference in C57BL/6J mice. Front Neurosci, 8(129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Da Costa AJ, Harris RA & Messing RO 2018. Apremilast Alters Behavioral Responses to Ethanol in Mice: II. Increased Sedation, Intoxication, and Reduced Acute Functional Tolerance. Alcohol Clin Exp Res, 42(5, pp 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD & Staehler BA 1995. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res, 19(3, pp 600–6. [DOI] [PubMed] [Google Scholar]
- Bold KW, Fucito LM, Corbin WR, DeMartini KS, Leeman RF, Kranzler HR & O’Malley SS 2016. Daily relations among affect, urge, targeted naltrexone, and alcohol use in young adults. Exp Clin Psychopharmacol, 24(5, pp 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S. & Ray LA 2014. Subjective response to alcohol and associated craving in heavy drinkers vs. alcohol dependents: an examination of Koob’s allostatic model in humans. Drug Alcohol Depend, 140(161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S. & Ray LA 2016. Experimental psychopathology paradigms for alcohol use disorders: Applications for translational research. Behav Res Ther, 86(11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Roche DJ, Sheets ES, Krull JL, Guzman I. & Ray LA 2015. Modeling naturalistic craving, withdrawal, and affect during early nicotine abstinence: A pilot ecological momentary assessment study. Exp Clin Psychopharmacol, 23(2, pp 81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Bohnsack JP & Morrow AL 2016. Ethanol Regulation of Synaptic GABAA alpha4 Receptors Is Prevented by Protein Kinase A Activation. J Pharmacol Exp Ther, 357(1, pp 10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RW, Squeglia LM, Emery NN, McClure EA, Gray KM, Miranda R Jr. & Tomko RL 2020. Making pharmacotherapy trials for substance use disorder more efficient: Leveraging real-world data capture to maximize power and expedite the medication development pipeline. Drug Alcohol Depend, 209(107897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Crichlow GV, Vermeire JJ, Leng L, Du X, Hodsdon ME, Bucala R, Cappello M, Gross M, Gaeta F, Johnson K. & Lolis EJ 2010. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc Natl Acad Sci U S A, 107(25, pp 11313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Johnson KW, Pavlicova M, Glass A, Vosburg SK, Sullivan MA, Manubay JM, Martinez DM, Jones JD, Saccone PA & Comer SD 2016. The effects of ibudilast, a glial activation inhibitor, on opioid withdrawal symptoms in opioid-dependent volunteers. Addict Biol, 21(4, pp 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ & Coleman LG Jr. 2017. The role of neuroimmune signaling in alcoholism. Neuropharmacology, 122(56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, Rodd ZA, Spear LP, Swartzwelder HS & Vetreno RP 2019. Mechanisms of Persistent Neurobiological Changes Following Adolescent Alcohol Exposure: NADIA Consortium Findings. Alcohol Clin Exp Res, 43(9, pp 1806–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT & Vetreno RP 2016. Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl), 233(9, pp 1543–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran SL, Andrykowski MA & Studts JL 1995. Short Form of the Profile of Mood States (POMS-SF): Psychometric information. Psychological Assessment, 7(1, pp 80–83. [Google Scholar]
- Emery NN, Carpenter RW, Meisel SN & Miranda R Jr. 2021. Effects of topiramate on the association between affect, cannabis craving, and cannabis use in the daily life of youth during a randomized clinical trial. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK & Tofighi D. 2007. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods, 12(2, pp 121–38. [DOI] [PubMed] [Google Scholar]
- Erickson EK, Grantham EK, Warden AS & Harris RA 2019. Neuroimmune signaling in alcohol use disorder. Pharmacol Biochem Behav, 177(34–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M. & Williams JBW 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. (SCID-I/P), New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- First MB, Williams JBW, Karg RS & Spitzer RL 2015. Structured Clinical Interview for DSM-5-Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV), Arlington, VA: American Psychiatric Association. [Google Scholar]
- Flannery BA, Volpicelli JR & Pettinati HM 1999. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res, 23(8, pp 1289–95. [PubMed] [Google Scholar]
- Franklin KM, Hauser SR, Lasek AW, McClintick J, Ding Z-M, McBride WJ & Bell RL 2015. Reduction of alcohol drinking of alcohol-preferring (P) and high-alcohol drinking (HAD1) rats by targeting phosphodiesterase-4 (PDE4). Psychopharmacology, 232(13, pp 2251–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman K, Brureau A, Vadigepalli R, Staehle MM, Brureau MM, Gonye GE, Hoek JB, Hooper DC & Schwaber JS 2012. Temporal changes in innate immune signals in a rat model of alcohol withdrawal in emotional and cardiorespiratory homeostatic nuclei. Journal of Neuroinflammation, 9(1, pp 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson LC, Hastings SF, McPhee I, Clayton RA, Darroch CE, Mackenzie A, MacKenzie FL, Nagasawa M, Stevens PA & MacKenzie SJ 2006. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. European journal of pharmacology, 538(1–3, pp 39–42. [DOI] [PubMed] [Google Scholar]
- Gilpin NW & Koob GF 2008. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res Health, 31(3, pp 185–95. [PMC free article] [PubMed] [Google Scholar]
- Gong M-F, Wen R-T, Xu Y, Pan J-C, Fei N, Zhou Y-M, Xu J-P, Liang J-H & Zhang H-T 2017. Attenuation of ethanol abstinence-induced anxiety- and depressive-like behavior by the phosphodiesterase-4 inhibitor rolipram in rodents. Psychopharmacology, 234(20, pp 3143–3151. [DOI] [PubMed] [Google Scholar]
- Green R, Grodin E, Lim AC, Venegas A, Bujarski S, Krull J. & Ray LA 2019. The Interplay Between Subjective Response to Alcohol, Craving, and Alcohol Self-Administration in the Human Laboratory. Alcohol Clin Exp Res, 43(5, pp 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Bujarski S, Towns B, Burnette E, Nieto S, Lim A, Lin J, Miotto K, Gillis A, Irwin MR, Evans C. & Ray LA 2021. Ibudilast, a neuroimmune modulator, reduces heavy drinking and alcohol cue-elicited neural activation: a randomized trial. Transl Psychiatry, 11(1, pp 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helstrom AW, Blow FC, Slaymaker V, Kranzler HR, Leong S. & Oslin D. 2016. Reductions in Alcohol Craving Following Naltrexone Treatment for Heavy Drinking. Alcohol Alcohol, 51(5, pp 562–6. [DOI] [PubMed] [Google Scholar]
- Hu W, Lu T, Chen A, Huang Y, Hansen R, Chandler LJ & Zhang H-T 2011. Inhibition of phosphodiesterase-4 decreases ethanol intake in mice. Psychopharmacology, 218(2, pp 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR & Johnson KW 2009. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav Immun, 23(2, pp 240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Vena A, Hasin DS, deWit H, O’Connor SJ & Cao D. 2021. Subjective Responses to Alcohol in the Development and Maintenance of Alcohol Use Disorder. Am J Psychiatry, 178(6, pp 560–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF & Mason BJ 2016. Existing and Future Drugs for the Treatment of the Dark Side of Addiction. Annu Rev Pharmacol Toxicol, 56(299–322. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Covault J. & Tennen H. 2013. Variation in OPRM1 moderates the effect of desire to drink on subsequent drinking and its attenuation by naltrexone treatment. Addict Biol, 18(1, pp 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR & Soyka M. 2018. Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA, 320(8, pp 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MJ, Briones MS, Heinzerling KG, Kalmin MM & Shoptaw SJ 2020. Ibudilast attenuates peripheral inflammatory effects of methamphetamine in patients with methamphetamine use disorder. Drug Alcohol Depend, 206(107776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J. & Olsen RW 2014. Alcohol use disorders and current pharmacological therapies: the role of GABA(A) receptors. Acta Pharmacol Sin, 35(8, pp 981–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R. & Noronha A. 2012. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol, 17(3, pp 513–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL Jr., Elnabawi A, Merchenthaler I, Sieghart W, June HL Sr. & Aurelian L. 2011. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci U S A, 108(11, pp 4465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML 2015. Phosphodiesterase regulation of alcohol drinking in rodents. Alcohol, 49(8, pp 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Vendruscolo LF, Schlosburg JE, Koob GF & Zorrilla EP 2014. Phosphodiesterase 10A Regulates Alcohol and Saccharin Self-Administration in Rats. Neuropsychopharmacology, 39(7, pp 1722–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J. 2006. Factor structure of the alcohol urge questionnaire under neutral conditions and during a cue-elicited urge state. Alcohol Clin Exp Res, 30(8, pp 1315–21. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M. & Droppleman LF 1971. Manual for the Profile of Mood States, San Diego: Educational and Industrial Testing Service. [Google Scholar]
- Meredith LR, Burnette EM, Grodin EN, Irwin MR & Ray LA 2021a. Immune treatments for alcohol use disorder: A translational framework. Brain Behav Immun, 97(349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith LR, Green R, Grodin EN, Chorpita M, Miotto K. & Ray LA 2021b. Ibudilast moderates the effect of mood and alcohol craving during stress exposure. Exp Clin Psychopharmacol, Advanced online publication; ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R Jr., MacKillop J, Treloar H, Blanchard A, Tidey JW, Swift RM, Chun T, Rohsenow DJ & Monti PM 2016. Biobehavioral mechanisms of topiramate’s effects on alcohol use: an investigation pairing laboratory and ecological momentary assessments. Addict Biol, 21(1, pp 171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Ray L, Blanchard A, Reynolds EK, Monti PM, Chun T, Justus A, Swift RM, Tidey J, Gwaltney CJ & Ramirez J. 2014. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addict Biol, 19(5, pp 941–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Kurotani T, Komatsu Y, Kawanokuchi J, Kato H, Mitsuma N. & Suzumura A. 2004. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology, 46(3, pp 404–11. [DOI] [PubMed] [Google Scholar]
- Northcutt AL, Hutchinson MR, Wang X, Baratta MV, Hiranita T, Cochran TA, Pomrenze MB, Galer EL, Kopajtic TA, Li CM, Amat J, Larson G, Cooper DC, Huang Y, O’Neill CE, Yin H, Zahniser NR, Katz JL, Rice KC, Maier SF, Bachtell RK & Watkins LR 2015. DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry, 20(12, pp 1525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Metten P, Potretzke S, Townsley KG, Blednov YA & Crabbe JC 2020. Effects of Pharmacologically Targeting Neuroimmune Pathways on Alcohol Drinking in Mice Selectively Bred to Drink to Intoxication. Alcoholism: Clinical and Experimental Research, 44(2, pp 553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Torres S, Miró X, Palacios JM, Cortés R, Puigdoménech P. & Mengod G. 2000. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and [3H] rolipram binding autoradiography: comparison with monkey and rat brain. Journal of chemical neuroanatomy, 20(3–4, pp 349–374. [DOI] [PubMed] [Google Scholar]
- Quinn PD & Fromme K. 2011. Subjective response to alcohol challenge: a quantitative review. Alcohol Clin Exp Res, 35(10, pp 1759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez AD & Smith SM 2014. Regulation of dopamine signaling in the striatum by phosphodiesterase inhibitors: novel therapeutics to treat neurological and psychiatric disorders. Cent Nerv Syst Agents Med Chem, 14(2, pp 72–82. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW & Bryk AS 2002. Hierarchical linear models: Applications and data analysis methods, Newbury Park, CA: Sage. [Google Scholar]
- Ray LA, Bujarski S. & Roche DJ 2016. Subjective Response to Alcohol as a Research Domain Criterion. Alcohol Clin Exp Res, 40(1, pp 6–17. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Shoptaw S, Roche DJ, Heinzerling K. & Miotto K. 2017. Development of the Neuroimmune Modulator Ibudilast for the Treatment of Alcoholism: A Randomized, Placebo-Controlled, Human Laboratory Trial. Neuropsychopharmacology, 42(9, pp 1776–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Du H, Green R, Roche DJO & Bujarski S. 2021. Do behavioral pharmacology findings predict clinical trial outcomes? A proof-of-concept in medication development for alcohol use disorder. Neuropsychopharmacology, 46(3, pp 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Green R, Roche DJO, Magill M. & Bujarski S. 2019. Naltrexone effects on subjective responses to alcohol in the human laboratory: A systematic review and meta-analysis. Addict Biol, 24(6, pp 1138–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Miranda R Jr., Kahler CW, Leventhal AM, Monti PM, Swift R. & Hutchison KE 2007. Pharmacological effects of naltrexone and intravenous alcohol on craving for cigarettes among light smokers: a pilot study. Psychopharmacology (Berl), 193(4, pp 449–56. [DOI] [PubMed] [Google Scholar]
- Ray LA, Miranda R Jr., Tidey JW, McGeary JE, MacKillop J, Gwaltney CJ, Rohsenow DJ, Swift RM & Monti PM 2010. Polymorphisms of the mu-opioid receptor and dopamine D4 receptor genes and subjective responses to alcohol in the natural environment. J Abnorm Psychol, 119(1, pp 115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueger SY, McNamara PJ & King AC 2009. Expanding the utility of the Biphasic Alcohol Effects Scale (BAES) and initial psychometric support for the Brief-BAES (B-BAES). Alcohol Clin Exp Res, 33(5, pp 916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMSHA. 2019. Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19–5068, NSDUH Series H-54), (Rockville, MD: Center for Behavioral Health Statistics and Quality; ). [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR & Grant M. 1993. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction, 88(6, pp 791–804. [DOI] [PubMed] [Google Scholar]
- Schneider S, Junghaenel DU, Gutsche T, Mak HW & Stone AA 2020. Comparability of Emotion Dynamics Derived From Ecological Momentary Assessments, Daily Diaries, and the Day Reconstruction Method: Observational Study. J Med Internet Res, 22(9, pp e19201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Pierson J, Hesselbrock V, Bucholz KK, Kramer J, Kuperman S, Dietiker C, Brandon R. & Chan G. 2007. The ability of the Self-Rating of the Effects of Alcohol (SRE) Scale to predict alcohol-related outcomes five years later. J Stud Alcohol Drugs, 68(3, pp 371–8. [DOI] [PubMed] [Google Scholar]
- Sheets ES, Bujarski S, Leventhal AM & Ray LA 2015. Emotion differentiation and intensity during acute tobacco abstinence: A comparison of heavy and light smokers. Addict Behav, 47(70–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D. & Trigwell P. 1995. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry, 167(1, pp 99–103. [DOI] [PubMed] [Google Scholar]
- Sobell LC & Sobell MB 1992. Timeline Follow-Back. In: Litten & Allen JP (eds.) Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press. [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D. & Basian E. 1986. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav, 11(2, pp 149–61. [DOI] [PubMed] [Google Scholar]
- Stevens AK, Sokolovsky AW, Treloar Padovano H, White HR & Jackson KM 2020. Heaviness of Alcohol Use, Alcohol Problems, and Subjective Intoxication Predict Discrepant Drinking Reports in Daily Life. Alcohol Clin Exp Res, 44(7, pp 1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA & Sellers EM 1989. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict, 84(11, pp 1353–7. [DOI] [PubMed] [Google Scholar]
- Trela CJ, Piasecki TM, Bartholow BD, Heath AC & Sher KJ 2016. The natural expression of individual differences in self-reported level of response to alcohol during ecologically assessed drinking episodes. Psychopharmacology (Berl), 233(11, pp 2185–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell JD, Ramchandani VA & Hendershot CS 2015. A multilevel structural equation model of within- and between-person associations among subjective responses to alcohol, craving, and laboratory alcohol self-administration. J Abnorm Psychol, 124(4, pp 1050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen RT, Zhang FF & Zhang HT 2018. Cyclic nucleotide phosphodiesterases: potential therapeutic targets for alcohol use disorder. Psychopharmacology (Berl), 235(6, pp 1793–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley MJ, Swanson AN, Heinzerling KG, Roche DJ & Shoptaw S. 2016. Ibudilast attenuates subjective effects of methamphetamine in a placebo-controlled inpatient study. Drug Alcohol Depend, 162:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.