Abstract
The continuity of a lumen within an epithelial tubule is critical for its function. We previously found that the F-actin binding protein Afadin is required for timely lumen formation and continuity in renal tubules formed from the nephrogenic mesenchyme in mice. Afadin is known effector and interactor of the small GTPase Rap1, and in the current study, we examine the role of Rap1 in nephron tubulogenesis. Here, we demonstrate that Rap1 is required for nascent lumen formation and continuity in cultured 3D epithelial spheroids and in vivo in murine renal epithelial tubules derived from the nephrogenic mesenchyme, where its absence ultimately leads to severe morphogenetic defects in the tubules. By contrast, Rap1 is not required for lumen continuity or morphogenesis in renal tubules derived from the ureteric epithelium, which differ in that they form by extension from a pre-existing tubule. We further demonstrate that Rap1 is required for correct localization of Afadin to adherens junctions both in vitro and in vivo. Together, these results suggest a model in which Rap1 localizes Afadin to junctional complexes, which in turn regulates nascent lumen formation and positioning to ensure continuous tubulogenesis.
Keywords: Kidney development, lumen, tubulogenesis, Rap1, Afadin, polarity, nephron, epithelial cells, tubules
SUMMARY STATEMENT
We find that Rap1 is required for central lumen formation in renal epithelia and that this is mediated via correct localization of the F-actin binding protein Afadin.
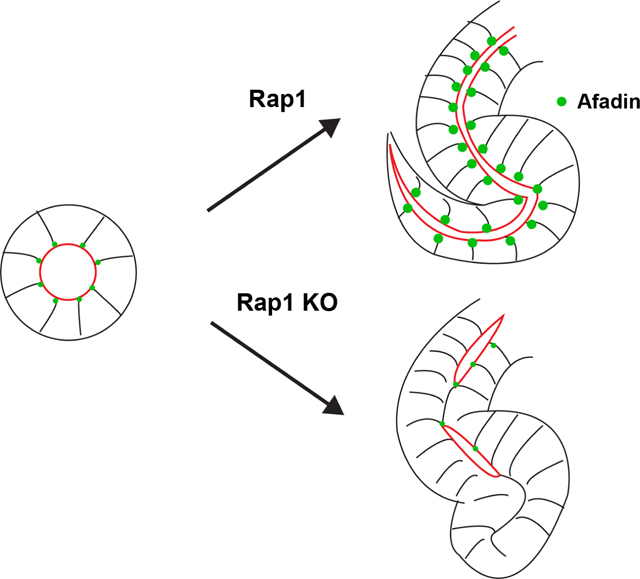
Graphical Abstract
INTRODUCTION
In the kidney, a mature nephron contains a polarized epithelial tubule comprised of multiple functionally and structurally distinct segments. During kidney development, nephron tubule formation occurs when the nephrogenic cap mesenchyme undergoes a mesenchyme-to-epithelia transition, forming an epithelial sphere that subsequently elongates into an s-shaped tubule (1, 2). The s-shaped tubule then fuses with a tubule formed by the ureteric bud epithelia. Together, the joined tubules elongate and differentiate to form the entire tubular portion of the nephron.
Lumen continuity in nephron tubules is critical for the excretory function of the kidney. Indeed, disruption of the polarization process and lumen formation leads to renal dysplasia and cystogenesis in mice (3), linking cell polarity and renal disease. However, many of the cellular mechanisms and proteins that help establish apical-basal polarity to create a single continuous lumen remain elusive. Understanding underlying in vivo mechanisms is critical for understanding how congenital and acquired defects of tubulogenesis lead to disease.
The assembly and apical positioning of adherens junctions that link neighboring cells to each other and to the actin cytoskeleton is an early step in establishing apical-basal polarity (4). Afadin, an F-actin binding protein and nectin adaptor, has emerged as a key regulator of the adherens junction (5). In the developing kidney of mice, Afadin is required for both timely formation and positioning of the lumen in tubules, thereby establishing apical-basal polarity and lumen continuity during kidney tubulogenesis (6). It has a similar role in lumen formation and tubulogenesis in the developing pancreas (7). Afadin also controls apical-basal spindle orientation (8–10), a role that is conserved in several cell types and likely participates in generating a central, continuous lumen.
Afadin interacts with many proteins. Specifically, it has been shown to act as an effector to the small GTPases Rap1a and 1b, which bind to the N-terminal Ras-association (RA) domains of Afadin (11, 12). Rap1a and Rap1b are separate genes (13) that encode for proteins differing in only 9 amino acids and are thought to be functionally redundant. Single cell RNA-seq datasets (14) have revealed that both genes are expressed in renal epithelia, and henceforth, Rap1a and Rap1b will collectively be referred to as Rap1. Rap1 cycles between an inactive GDP-bound state mediated by many specific GTPase-activating proteins (GAPs) and an active GTP-bound state mediated by several specific guanine nucleotide exchange factors (GEFs) (15). In addition to Afadin, activated Rap1 binds to many downstream effectors and is known to play critical roles in various cellular processes such as cell-cell/cell-matrix adhesion, morphogenesis and regulation of polarity (15–18). In Drosophila, Rap1 correctly positions Cno, the Afadin homolog, and cadherin-catenin complexes at apical junctions (19–21). In mammalian tissue, loss of Rap1 results in impaired adhesive interactions and polarity in ocular epithelium, resulting in defective lens morphogenesis (22). Rap1 is also required for junctional integrity and the early morphogenesis of endothelial tubes (23, 24).
To understand the role of Rap1 in nephron tubulogenesis and determine if it participates in an Afadin-signaling module, we examined Rap1 function in the developing mouse kidney and in 3D epithelial spheroid culture. Our data show that Rap1 is required in vivo for timely lumen formation and continuity in renal epithelial tubules derived from the nephrogenic cap mesenchyme and in 3D epithelial spheroids. These results underscore the role of Rap1 in nascent lumen formation. The Rap1 phenotype is highly similar to that of loss of Afadin (6), and we find that Rap1 is required for correct localization of Afadin to adherens junctions. These results suggest that Rap1 localizes Afadin to adherens junctions in renal tubules, thereby ensuring timely lumen formation and continuity.
MATERIALS AND METHODS
We crossed Rap1af/f;Rap1bf/f females to Rap1af/+; Rap1bf/+; HoxB7-cre males to obtain Rap1af/f; Rap1bf/f; HoxB7 cre mutant mice (25, 26). Similarly crosses were performed with Six2-cre (27). Mice were maintained on mixed genetic backgrounds and genotyped by standard PCR. Neonatal kidneys were harvested and fixed for 2 hours in 4% paraformaldehyde (PFA) in PBS. Six-week-old mice were perfused with 4% PFA and their isolated kidneys fixed for 4 hours with 4% PFA. Procedures were performed according to UTSW-IACUC-approved guidelines.
MDCK culture, shRNA-mediated gene silencing and CRISPR gene editing
MDCK type II cells were grown and imaged as previously described (29). Transfection of MDCK cells with mcherry-Rap1 (a gift of Mark Philips, NYU) was performed with Lipofectamine 2000 (Invitrogen) and stable lines were selected. The shRNA oligos were designed against Rap1a and Rap1b using the iRNAi program (http://www.mekentosj.com/science/irnai) using guidelines from the Addgene pLKO.1 protocol (www.addgene.org). The selected shRNA oligos were as follows: Rap1a shRNA1 : CCGGTATGACCCAACGATAGAAGCTCGAGCTTCTATCGTTGGGTCATATTTTTG ; Rap1a shRNA2 : CCGGGATTCCTACAGAAAGCAAGCTCGAGCTTGCTTTCTGTA-GGAATCTTTTTG; Rap1b shRNA1 : CCGGGGGCTTGCATTATAATTACCTCGAGGTAAT-TATAATGCAAGCCCTTTTTG; Rap1b shRNA2 : CCGGCAGGGAGCCACAGTATTTAC-TCGAGTAAATACTGTGGCTCCCTGTTTTTG.
Rap1a/1b shRNAs were cloned into pLKO.1 puromycin and viral transduction of MDCK cells was performed as previously described (30). For CRISPR knockout lines, sgRNA oligonucleotides were designed using the CRISPR Genome Engineering Resources (http://crispr.mit.edu/). The sgRNAs used for this study were as follows: Rap1a sgRNA1 : CACCgCTGGAAGATGAGCGAGTAGT; Rap1a sgRNA2: CACCgCTAGACAGTTCAGTTTGTTC; Rap1b sgRNA1: CACCgGTTCTTG-GCTCAGGAGGCGT; Rap1b sgRNA2: CACCgCTTGAAATCTTGGATACTGC. Double knockout (KO) clones were generated and selected according to Cong and Zhang (31). Cells were verified for loss of Rap1 by western blot.
Immunofluorescence
Kidney sections were permeabilized with 0.3% Triton X-100/PBS (PBST) and blocked with 10% donkey sera/PBST. Antigen retrieval was performed with Trilogy (Cell Marque). MDCK cysts were fixed in 4% PFA for 10 min and then permeabilized with 0.3% PBST and blocked with 0.7% fish skin gelatin in TBS with 0.025% saponin (PFS). Samples were incubated with primary antibodies overnight (4°C), then with fluorophore-conjugated secondary antibodies. Kidney sections were mounted with Prolong Gold (Invitrogen). Confocal imaging was performed on a Zeiss LSM880 confocal microscope. Images were minimally processed and re-sampled to 300 dpi using Adobe Photoshop.
Western blot
MDCK cysts were lysed in ice-cold buffer containing 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 20 mM sodium phosphate ( pH 7.2), 100 mM NaCl, 50 mM Tris-HCl, 50 mM NaF, 4 mM Na3VO4, 1 mM PMSF and a protease inhibitor cocktail (Roche). Sample buffer (4X) was added to lysates and they were boiled for 5 min. These lysates were then centrifuged at 17,000g for 15 min at 4°C to remove insoluble aggregates, and SDS-PAGE and western blotting were performed.
Antibodies
For immunostaining, primary antibodies were used at 1:100 unless stated otherwise: Afadin (Sigma, A0224), Par6b (SCBT, sc-67392), Podocalyxin (Developmental Studies Hybridoma Bank (DSHB), 3F2/D8), ZO1 (Millipore, MABT11), Cadherin 6 (a gift of G. Dressler, University of Michigan, MI, USA), Phalloidin 647 (Invitrogen, 42008A), NCAM (DSHB, 5B8); Ecad (Thermo,13–1900), Muc-1(Thermo, MA5–11202). For western blots, we used Rap1 (1:1000, BD, 610195), Afadin (1:1000, Sigma, A0224) and β-actin (1:5000, Abcam, ab8227) antibodies with HRP-conjugated secondary antibodies (Jackson Immunoresearch).
Statistics
In vitro experiments were performed in triplicate and are representative of at least two similar experiments. All data shown are mean ± s.d. Statistical significance was performed using an unpaired, two-tailed Student’s t-test unless stated otherwise.
RESULTS
Localization of mCherry-Rap1 during lumen formation
To assess if Rap1 has a role in lumenogenesis, we first examined Rap1 localization during lumen formation in early stage spheroids of Madin-Darby Canine Kidney (MDCK) cells. We generated stable MDCK cell lines with mCherry-Rap1a (32) since our tests of several Rap1 antibodies in control and Rap1-depleted spheroids did not validate any antibodies for immunostaining.
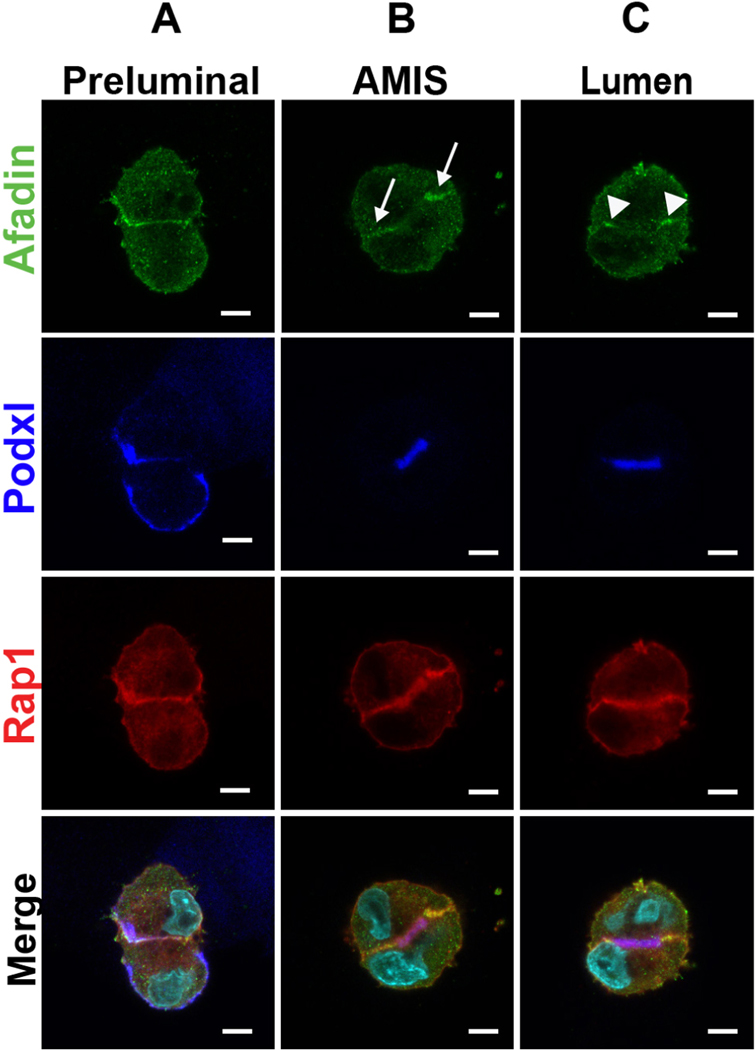
In two-cell spheroids, sequential stages of lumen formation can be delineated by the localization of Podocalyxin (Podxl) and Afadin (33). Prior to lumen initiation (Fig. 1A), Podxl localizes to the peripheral surface of the cells and Afadin localizes to the cell-cell interface. As previously observed, Podxl is then internalized in vesicles by each cell (not shown, see ref (30)) which then fuse to form the apical membrane initiation site (AMIS) (Fig. 1B). At the AMIS stage, Afadin becomes restricted to the entire lateral cell-cell junctions (arrows) (Fig. 1B). As the AMIS matures and opens a lumen (Fig. 1C), Podxl remains at the apical surface while Afadin becomes further restricted to apical cell-cell junctions (arrowheads). The mCherry-Rap1a remains distributed to both apical surfaces and apical junctions throughout lumen formation (Fig. 1A–C).
Figure 1. mCherry-Rap1a and Afadin localization during stages of lumen formation.
A. Immunofluorescence of two-cell stage MDCK spheroids with Afadin (green), mCherry-Rap1a (red), and podxl (blue) prior to lumen formation.
B. Immunofluorescence as in A at the apical membrane initiation site (AMIS) stage.
C. Immunofluorescence at the lumen stage.
Arrows depict Afadin at lateral membranes and arrowheads show Afadin at apical-lateral junctions. Results are representative of at least 2 independent experiments. Scale bars: 5 μm.
Rap1 is required for normal lumen formation in vitro and recruits Afadin to cell-cell junctions
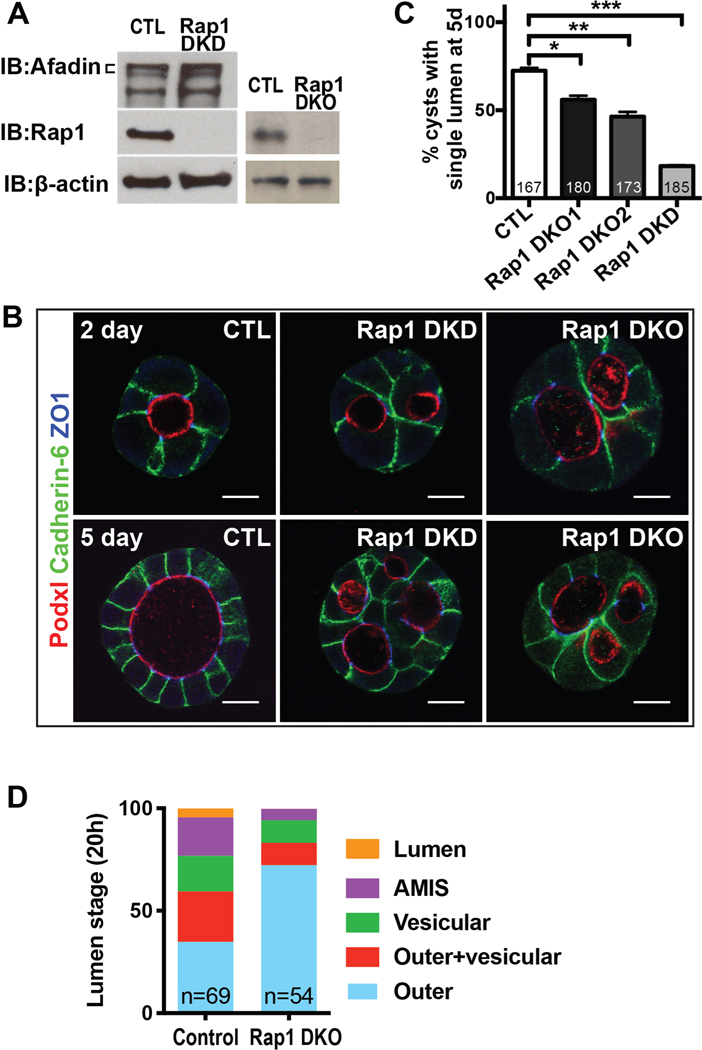
To assess the role of Rap1 in lumen formation, we generated stable double knockdown (DKD) cells and double knockout (DKO) cell lines for Rap1a and Rap1b, which are 95% identical at the amino acid level. We confirmed that these cell lines were depleted or devoid of Rap1a/b (Fig. 2A). The Rap1a/b deficient spheroids (denoted Rap1 DKD and DKO) formed multiple lumens (Fig. 2B,C), similar to the phenotype of spheroids lacking Afadin (33). They also exhibited a delay in initial lumen formation in 2-cell stage spheroids (Fig. 2D), which is similar to spheroids lacking Afadin.
Figure 2. Rap1a/b is required for timely and normal lumen formation in spheroids.
A. Western blots of Rap1a/b and Afadin in Rap1a/b double knockdown (Rap1 DKD) and double knockout (Rap1 DKO) cells.
B. Immunofluorescence of Podxl1, cadherin-6, and ZO1 in 2-day and 5-day spheroids with Rap1 DKD and DKO. The Rap1 DKD/DKO spheroids have multiple lumens. Scale bars: 10 μm.
C. Quantification of percentage of spheroids with a single lumen at 5 days. Rap1a/b DKD and DKO spheroids have a lower percentage with a single lumen. Results from two double DKO clonal lines are shown.
D. Quantification of the percentage of two cell spheroids at the indicated lumen stages at 20h. See Fig 1A and text for details. Rap1a/b DKO spheroids have fewer in the AMIS and lumen stages, indicating delay in lumen formation.
Results are representative of at least 2 independent experiments.
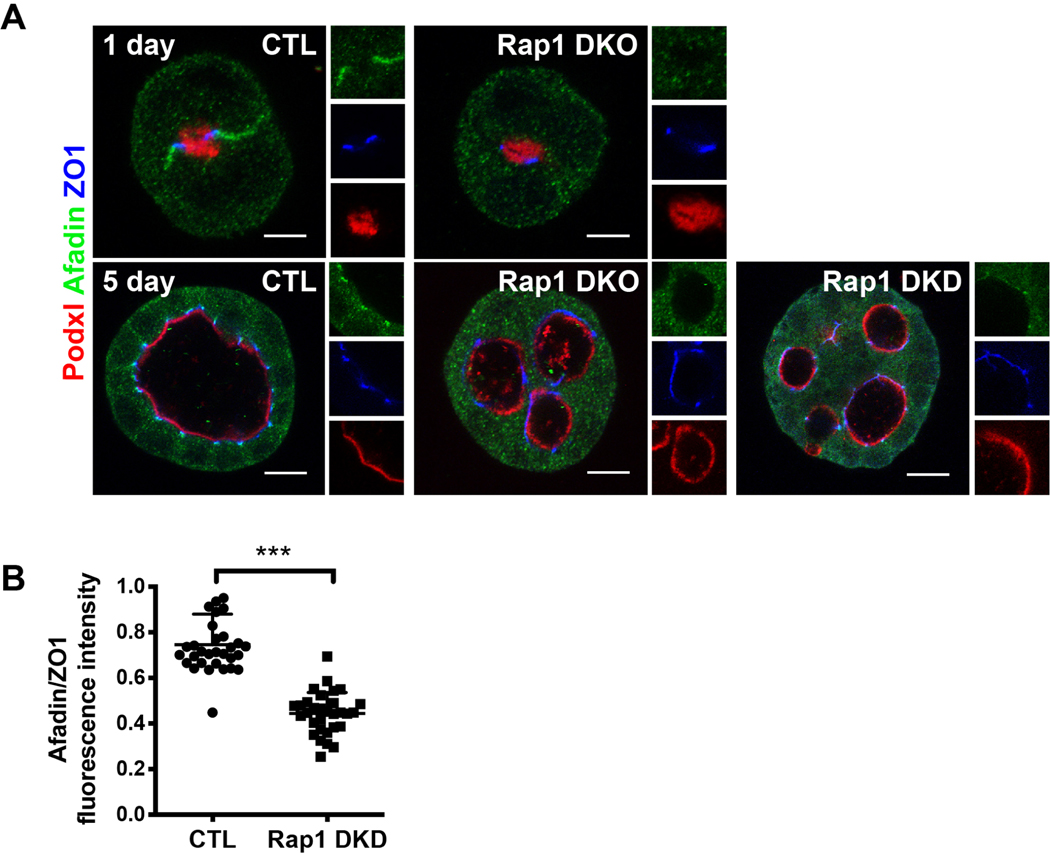
In Rap1 DKD/DKO spheroids, Afadin localization was diffuse in both early (1-day) and 5-day spheroids (Fig. 3A). There was a noticeable loss of Afadin at apical-lateral cell junctions, compared with controls. We quantified this result in 5-day spheroids by assessing the ratio of Afadin to ZO1 intensity at apical-lateral junctions (Fig. 3B). Since total Afadin levels were unchanged in the Rap1 DKD/DKO cells (Fig. 2A), we concluded that Rap1 regulates Afadin localization, but not levels, in renal epithelia in vitro.
Figure 3. Rap1a/b is required for recruitment of Afadin to cellular junctions in spheroids.
A. Immunofluorescence with Podxl1, Afadin, and ZO1 in 1-day (20 hours) and 5-day spheroids. Rap1a/b DKD and DKO spheroids had loss of Afadin localization to the lateral cell junctions at day 1 and apical-lateral junctions at day 5. Scale bars: 5 μm (upper panels), 10 μm (lower panels).
B. Quantification of the Afadin/ZO1 ratios at apical-lateral junctions by fluorescence intensity (***p<0.001, 95% CI −0.36 to −0.24).
Results are representative of at least 2 independent experiments.
Rap1a/b is required for normal lumen formation in the developing nephron, but not ureteric-bud derived epithelial tubules
During kidney development, epithelial tubules of the kidney arise from two embryonic sources, the cap mesenchyme and the ureteric bud, which give rise to nephron tubules and collecting ducts, respectively. These two tubules form by different mechanisms. The cap mesenchyme undergo a mesenchymal-to-epithelial transition to form an epithelial vesicle, with de novo formation of a central lumen called the renal vesicle. The renal vesicle subsequently elongates to form an s-shaped tubule called the s-shaped body. By contrast, the ureteric bud tubule forms as a branch (or extension) from a pre-existing tubule, the nephric duct. Thus, its lumen is an extension of the preexisting lumen of the nephric duct. The lumen from the s-shaped body ultimately connects with the ureteric bud lumen via anastomosis (6).
We first examined the role of Rap1 in ureteric bud tubulogenesis using conditional deletion of Rap1a/b with the HoxB7-cre mouse line, which recombines in the ureteric bud epithelia and its derivatives. At E14.5, mutant kidneys had normal sized kidneys with normal appearance (Fig. 4A). Immunofluorescent staining for the lumenal protein mucin-1 (Muc1), which labels the ureteric bud lumen (as well as distal nephron), revealed that double mutants (Rap1af/f; Rap1bf/f; HoxB7-cre) had normal continuous ureteric lumens enriched in apical Muc1, similar to controls (Fig. 4B).
Figure 4. Absence of Rap1 causes lumenal and morphogenetic defects in nascent lumen formation of developing nephron tubules but not ureteric-bud tubules.
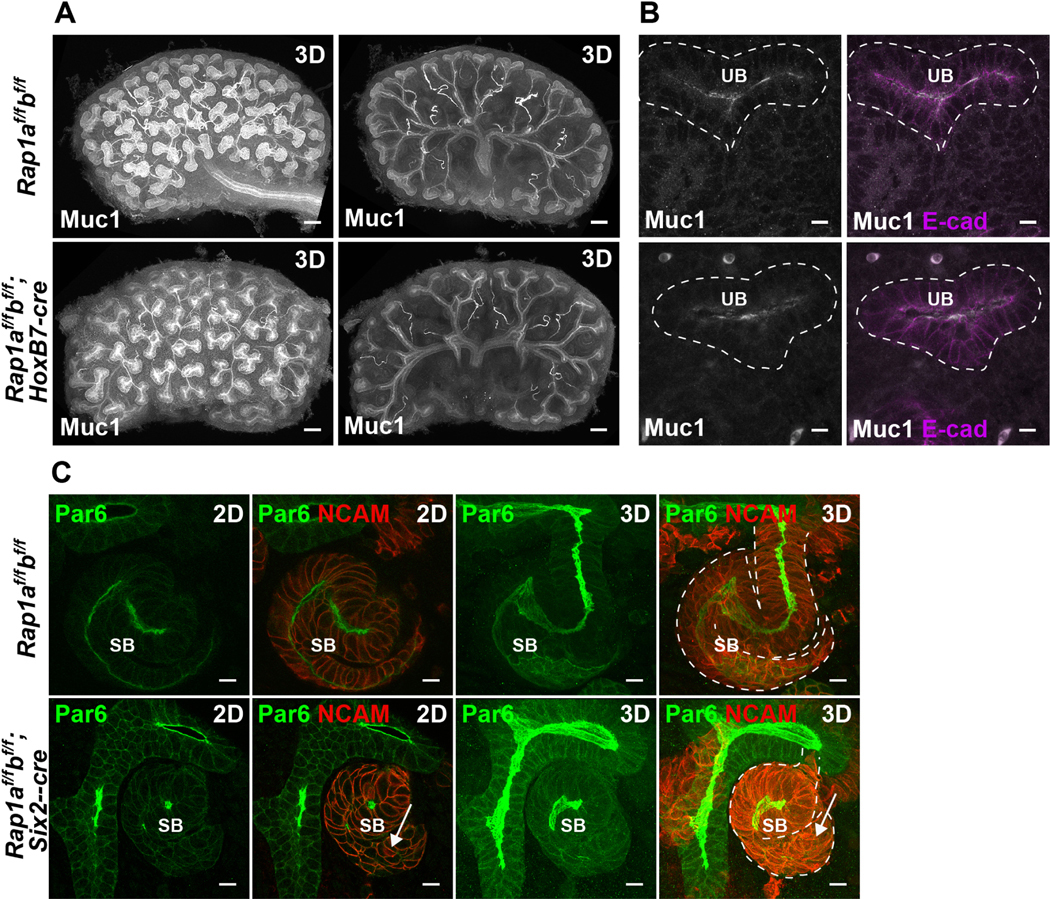
A. E14.5 control (Rap1af/f; Rap1bf/f) and mutant (Rap1af/f; Rap1bf/f; Hoxb7-cre) kidneys were immunostained with Muc1 (white) which labels ureteric bud lumens and distal tubule lumens. Left images are 3D projections from a confocal z-stack (z is 390 μm). Right images are 3D projections from the central images (130 μm), which allows better visualization of the ureteric bud and stalk lumens. Scale: 100 μm.
B. High magnification images of immunofluorescent staining with Muc 1 (white) and E-cadherin (magenta) depicting the ureteric bud (UB) epithelia and lumens. Scale: 10 μm.
C. E14.5 control (Rap1af/f; Rap1bf/f) and mutant kidneys lacking Rap1a/b from metanephric mesenchyme and its derivatives (Rap1af/f; Rap1bf/f; Six2-cre) were immunostained with Par6 (green) and NCAM (red). Par6 marks the lumens and NCAM delineates the metanephric mesenchyme and s-shaped bodies (SB). Single images and their projected z-stacks (6 μm) are indicated as 2D and 3D, respectively. Note the abnormal shape of the s-shaped body and lack of a continuous lumen in the mutant (arrows). Scale: 10 μm.
Next, we investigated its role in nephron tubulogenesis using the Six2-cre, which recombines in the cap mesenchyme (27). Mice with conditional deletion of Rap1a/b (Rap1af/f; Rap1bf/f; Six2-cre) displayed defects in lumen formation, with marked discontinuous lumens evident in s-shaped bodies (Fig. 4C, Fig. S1, Movies 1a, 1b). The lumens were demarcated by Par6 (Pard6b) at the apical surface and NCAM at lateral cell junctions. This phenotype is similar to what is observed with conditional deletion of Afadin with Six2-cre (6). Some of the nephron tubules in Rap1af/f; Rap1bf/f; Six2-cre mutant mice also had abnormal morphogenesis, as clearly depicted in Fig. 4C, which displays an s-shaped body with abnormal shape, particularly at its tail (Fig. 4C, arrow).
We performed Chi-square analysis at the neonatal stage and at 4 weeks-of-age for litters born from a cross of Rap1af/f; Rap1bf/f and Rap1af/+; Rap1bf/+; Six2-cre mice. From 17 litters of neonatal mice, there were 12 mutants of 87 total mice, which was not a deviation from the expected Mendelian frequencies (X2 = 2.034, 3 degrees of freedom, N = 87, p = 0.565). In contrast, the analysis of 17 litters at 4 weeks-of-age showed a clear deviation from expected Mendelian frequencies (X2 = 17.61, 3 degrees of freedom, N = 99, p < 0.005): there was only 1 mutant of 99 total mice. We observed that most mutant mice die between the ages of P1 and P5. Thus, because of their early demise, we were unable to perform histological and phenotypic analyses on adult mice.
Given the similar phenotype to Afadin mutants, prior data showing that Afadin is an effector of Rap1, and our results in the MDCK spheroids, we next examined Afadin localization in both Six-cre and HoxB7 mutants. Absence of Rap1 from the nephron tubules (Rap1af/f; Rap1bf/f; Six2-cre) caused a reduction in apical lateral Afadin (Fig. 5A). These results underscore the role of Rap1 in nascent lumen formation during nephron formation and suggest that this is mediated through its effect on Afadin localization. Interestingly, even though deletion of Rap1 from the ureteric tubules (Rap1af/f; Rap1bf/f; HoxB7-cre) did not cause lumenal defects, Afadin was also clearly reduced at the apical lateral junctions of ureteric epithelia (Fig. 5B).
Figure 5. Absence of Rap1 leads to mislocalization of Afadin in vivo in nephron and ureteric bud epithelia.
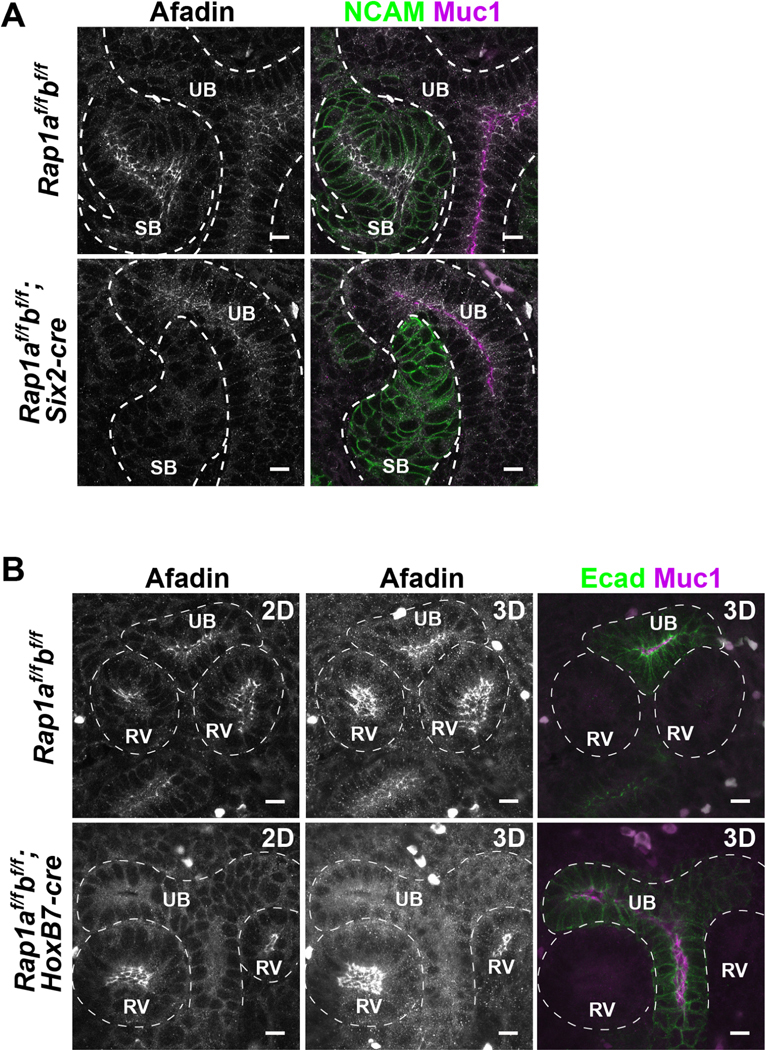
A. E14.5 control (Rap1af/f; Rap1bf/f) and mutant (Rap1af/f; Rap1bf/f; Six2-cre) kidneys lacking Rap1a/b from metanephric mesenchyme were immunostained with Afadin (white), NCAM (green) and Muc1 (magenta). Scale: 10 μm.
B. E14.5 control (Rap1af/f; Rap1bf/f) and mutant (Rap1af/f; Rap1bf/f; HoxB7-cre) kidneys lacking Rap1a/b from metanephric mesenchyme were immunostained with Afadin (white), E-cadherin (green) and Muc1 (magenta). Single images and projected z-stacks (6 μm) are indicated as 2D and 3D, respectively. Scale: 10 μm.
Results are representative of n = 3 for each genotype.
DISCUSSION
Rap1 and its effector proteins are known to regulate interactions between AJs, polarity determinants, and the cytoskeleton during epithelial cell polarity establishment and maintenance (15–18). However, its role in organ development and tissue architecture, particularly in mammals, is still not well explored. In the current study, we performed in vitro and in vivo experiments to examine the role of Rap1 in renal epithelia during kidney development. Our results demonstrate that Rap1 is required for normal lumen continuity in vitro and in nephron epithelia in vivo, but not in ureteric epithelia. These findings provide novel insights into how GTPase signaling drives kidney morphogenesis.
Given its critical role in regulating junctions (34), it is perhaps not surprising that Rap1 would be important during tissue formation. Dynamic remodeling of adherens and tight junctions between cells occurs as cells change shape, proliferate and migrate, and progressively assemble into tissues during organogenesis. Rap1 is intimately involved in these cellular processes. In Drosophila, activity of Rap1 modulates α-catenin-dependent coupling of junctions and the actin cytoskeleton during epithelial invagination that occurs at gastrulation (35). Only a few groups have examined Rap1 functions in forming mammalian embryonic tissues. One study in mice demonstrated that global loss of Rap1 resulted in early lethality of embryos at E10.5, whereas conditional deletion in blood vessels resulted in severe vascular defects and hemorrhage in a portion of the mutant embryos due to loss of cell-cell junction integrity (23). Along those lines, Rap1 was also shown to enhance barrier function in small blood vessels by potentiating vascular endothelial (VE) cadherin junctions (36). We find our work concurs with these previous studies in that Rap1 is required for tissue morphogenesis and regulates localization of the critical cell adhesion adaptor Afadin, which is required for proper lumen formation in the nephron tubules.
In previous work we showed that, like Rap1, Afadin is essential for the proper morphogenesis of a variety of epithelial tissues. Using genetic deletion of Afadin from the nephron epithelium, we showed it is essential for initial polarity establishment and subsequent lumen placement in the renal vesicle and s-shaped body (33). In a separate study, we found that Afadin, which is expressed in nascent lumens of the pancreatic epithelium, is similarly required for continuity of ductal lumens in that organ (7). In both the kidney and pancreas Afadin knockout organs, there is striking mislocalization of the lumens and their associated adherens and tight junctions. These studies demonstrate that Afadin is essential for de novo lumen formation in these tissues, and suggest the lumenal defects may be due to junction mislocalization.
Both Rap1 and Afadin have therefore both been shown to be crucial for correct placement of lumens and their junctions. Because Afadin is a known interactor and effector of Rap1 (11), it suggests that the role of Rap1 in lumen continuity is mediated at least in part through Afadin localization. In Drosophila, Rap1 and the Afadin orthologue Canoe are both essential for positioning of adherens junctions and cell polarity (19), and Rap1 regulates Afadin localization (19, 21). Our results therefore extend observations in invertebrates to mammalian epithelial tubules. Given that Rap1 and Afadin localize to adherens junctions in a wide range of organisms from flies to mammals, it is likely that these factors represent evolutionarily conserved and essential upstream regulators of cell-cell junctions and the morphogenetic processes that depend upon their interactions.
An interesting aspect of Rap1 function in the developing kidney is its differential requirement in nephron versus ureteric epithelium. We theorize that the differences in the nature of lumen formation in these tubules underlies the phenotypic differences. The MDCK cells utilized in our in vitro experiments are thought to be derived from cells of the distal nephron and more closely approximate nephron epithelia than ureteric epithelia (37). Prior studies have demonstrated that apical exocytosis of intracellular vesicles carrying components of the apical membrane act to initiate and expand the lumenal (apical) surface of MDCK spheroids (30). A similar mechanism has been proposed for lumen initiation in s-shaped body lumen generation in the nephron epithelia (38), which similarly undergoes polarization, apical membrane biogenesis, and nascent lumen formation. Furthermore, in the nephron epithelia, multiple tiny lumens must then interconnect to form a continuous lumen (6).
In contrast to this, the ureteric bud epithelium is already polarized and has a pre-existing lumen such that the ureteric tubule and its lumen extend by addition of new cells through cell division. Thus, even though single cell RNA-seq datasets (14) demonstrate that Rap1a and Rap1b expression (as well as Rap2a, Rap2b, and Rap2c) are similar in both epithelial tubule types, our study suggests that the nature of generating epithelial polarity de novo (as occurs in the nephron) and the connection of discrete small lumens requires Rap1. It is currently unknown which Rap1 GEFs, and GAPs are present in these cell types, and it is quite likely that differences in these proteins underlie phenotypic differences.
Although the phenotypes are similar to that of Afadin mutants, they are not identical. In addition to polarity and lumenal defects in developing nephrons, there are significant morphogenetic defects in Rap1 nephron-specific mutants. In Rap1 mutants, the s-shaped body is smaller with a thickened, lumenless section at its proximal end. These defects are qualitatively more severe than those observed in Afadin mutants. Additionally, Rap1 mutant cells have an abnormal shape, appearing more rounded than wild type nephron epithelia. Consistent with the additional phenotypes, Rap1 pups die in the neonatal stage, which does not occur with Afadin mutants. Our results are consistent with recent work demonstrating that Rap has a more profound and early effect on the morphogenesis of germband extension in the Drosophila embryo compared to loss of Afadin/Cno (39).
The underlying cause of these phenotypic differences remains unclear; however, one important observation relating to the more severe phenotype is that the membrane localization of Rap1 in vitro during lumen formation is broader than that of Afadin, similar to observations in Drosophila epithelia (19). Taken together, these findings strongly suggest that the cell shape and morphogenetic defects of Rap1 mutants are not solely mediated by Afadin, and that additional (non-Afadin) effectors play a critical role. Indeed, Rap1 has been shown to play a role in regulating cell shape in a manner independent of Afadin (19, 40). This role may be due to known Rap1 effectors such as RIAM, which enables talin and integrin activation and is known to induce changes in cell shape (41, 42).
In summary, we have identified that Rap1 is essential for de novo lumen formation and tubule morphogenesis in the mammalian kidney that is partially mediated via a Rap1-Afadin axis. Future experiments to elucidate the role of Rap1 regulators such as Rap1 GEFs and GAPs and additional Rap1 effectors are likely to provide new insight into lumen formation in the kidney.
Supplementary Material
Highlights.
Rap1 is required for lumen continuity in vitro and in mouse nephron tubules.
Rap1 also mediates normal morphogenesis of nephron tubules.
Rap1 is dispensable for lumen continuity and morphogenesis in ureteric tubules.
Rap1 correctly localizes its effector, Afadin, in kidney tubules.
Rap1 functions in nephron tubules via both Afadin and additional Rap1 effectors.
ACKNOWLEDGEMENTS
We would like to thank M. Philips (NYU) for the Rap1-mCherry plasmid, G. Dressler (U. Michigan) for Cadherin-6 antibody, and Z. Yang for help with preliminary experiments.
FUNDING
This work was supported by NIH R01s DK099478 (DKM and OC), DK116622 (DKM) and DK118032 (DKM), the Carolyn R. Bacon Distinguished Professorship in Medical Science and Education (DKM), and NIH P30DK079328 (UTSW O’Brien Kidney Center Research Core).
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grobstein C Inductive epithlio-mesenchymal interaction in cultured organ rudiments of the mouse metanephros. Science. 1953;118:52–5. [DOI] [PubMed] [Google Scholar]
- 2.Marciano DK. A holey pursuit: lumen formation in the developing kidney. Pediatr Nephrol. 2017;32(1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weide T, Vollenbroker B, Schulze U, Djuric I, Edeling M, Bonse J, et al. Pals1 Haploinsufficiency Results in Proteinuria and Cyst Formation. J Am Soc Nephrol. 2017;28(7):2093–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11(7):502–14. [DOI] [PubMed] [Google Scholar]
- 5.Mandai K, Rikitake Y, Shimono Y, Takai Y. Afadin/AF-6 and canoe: roles in cell adhesion and beyond. Prog Mol Biol Transl Sci. 2013;116:433–54. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Zimmerman S, Brakeman PR, Beaudoin GM, 3rd, Reichardt LF, Marciano DK. De novo lumen formation and elongation in the developing nephron: a central role for afadin in apical polarity. Development. 2013;140(8):1774–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azizoglu DB, Braitsch C, Marciano DK, Cleaver O. Afadin and RhoA control pancreatic endocrine mass via lumen morphogenesis. Genes Dev. 2017;31(23–24):2376–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmena A, Makarova A, Speicher S. The Rap1-Rgl-Ral signaling network regulates neuroblast cortical polarity and spindle orientation. J Cell Biol. 2011;195(4):553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wee B, Johnston CA, Prehoda KE, Doe CQ. Canoe binds RanGTP to promote Pins(TPR)/Mud-mediated spindle orientation. J Cell Biol. 2011;195(3):369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakotomamonjy J, Brunner M, Juschke C, Zang K, Huang EJ, Reichardt LF, et al. Afadin controls cell polarization and mitotic spindle orientation in developing cortical radial glia. Neural Dev. 2017;12(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boettner B, Harjes P, Ishimaru S, Heke M, Fan HQ, Qin Y, et al. The AF-6 homolog canoe acts as a Rap1 effector during dorsal closure of the Drosophila embryo. Genetics. 2003;165(1):159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linnemann T, Geyer M, Jaitner BK, Block C, Kalbitzer HR, Wittinghofer A, et al. Thermodynamic and kinetic characterization of the interaction between the Ras binding domain of AF6 and members of the Ras subfamily. The Journal of biological chemistry. 1999;274(19):13556–62. [DOI] [PubMed] [Google Scholar]
- 13.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56(1):77–84. [DOI] [PubMed] [Google Scholar]
- 14.Combes AN, Phipson B, Lawlor KT, Dorison A, Patrick R, Zappia L, et al. Single cell analysis of the developing mouse kidney provides deeper insight into marker gene expression and ligand-receptor crosstalk. Development. 2019;146(12). [DOI] [PubMed] [Google Scholar]
- 15.Boettner B, Van Aelst L. Control of cell adhesion dynamics by Rap1 signaling. Curr Opin Cell Biol. 2009;21(5):684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol. 2005;17(2):123–8. [DOI] [PubMed] [Google Scholar]
- 17.Frische EW, Zwartkruis FJ. Rap1, a mercenary among the Ras-like GTPases. Dev Biol. 2010;340(1):1–9. [DOI] [PubMed] [Google Scholar]
- 18.Jaskiewicz A, Pajak B, Orzechowski A. The Many Faces of Rap1 GTPase. Int J Mol Sci. 2018;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi W, Harris NJ, Sumigray KD, Peifer M. Rap1 and Canoe/afadin are essential for establishment of apical-basal polarity in the Drosophila embryo. Mol Biol Cell. 2013;24(7):945–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawyer JK, Harris NJ, Slep KC, Gaul U, Peifer M. The Drosophila afadin homologue Canoe regulates linkage of the actin cytoskeleton to adherens junctions during apical constriction. J Cell Biol. 2009;186(1):57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonello TT, Perez-Vale KZ, Sumigray KD, Peifer M. Rap1 acts via multiple mechanisms to position Canoe and adherens junctions and mediate apical-basal polarity establishment. Development. 2018;145(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddala R, Nagendran T, Lang RA, Morozov A, Rao PV. Rap1 GTPase is required for mouse lens epithelial maintenance and morphogenesis. Dev Biol. 2015;406(1):74–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chrzanowska-Wodnicka M, White GC, 2nd, Quilliam LA, Whitehead KJ. Small GTPase Rap1 Is Essential for Mouse Development and Formation of Functional Vasculature. PLoS One. 2015;10(12):e0145689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu JJ, Stockton RA, Gingras AR, Ablooglu AJ, Han J, Bobkov AA, et al. A mechanism of Rap1-induced stabilization of endothelial cell--cell junctions. Mol Biol Cell. 2011;22(14):2509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan BX, Vautier F, Ito W, Bolshakov VY, Morozov A. Enhanced cortico-amygdala efficacy and suppressed fear in absence of Rap1. J Neurosci. 2008;28(9):2089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129(22):5301–12. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3(2):169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewandoski M, Meyers EN, Martin GR. Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb Symp Quant Biol. 1997;62:159–68. [PubMed] [Google Scholar]
- 29.Marciano DK, Brakeman PR, Lee CZ, Spivak N, Eastburn DJ, Bryant DM, et al. p120 catenin is required for normal renal tubulogenesis and glomerulogenesis. Development. 2011;138(10):2099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12(11):1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cong L, Zhang F. Genome engineering using CRISPR-Cas9 system. Methods Mol Biol. 2015;1239:197–217. [DOI] [PubMed] [Google Scholar]
- 32.Bivona TG, Wiener HH, Ahearn IM, Silletti J, Chiu VK, Philips MR. Rap1 up-regulation and activation on plasma membrane regulates T cell adhesion. J Cell Biol. 2004;164(3):461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao L, Yang Z, Hiremath C, Zimmerman SE, Long B, Brakeman PR, et al. Afadin orients cell division to position the tubule lumen in developing renal tubules. Development. 2017;144(19):3511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007;120(Pt 1):17–22. [DOI] [PubMed] [Google Scholar]
- 35.Wang YC, Khan Z, Wieschaus EF. Distinct Rap1 activity states control the extent of epithelial invagination via alpha-catenin. Dev Cell. 2013;25(3):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto K, Takagi Y, Ando K, Fukuhara S. Rap1 Small GTPase Regulates Vascular Endothelial-Cadherin-Mediated Endothelial Cell-Cell Junctions and Vascular Permeability. Biol Pharm Bull. 2021;44(10):1371–9. [DOI] [PubMed] [Google Scholar]
- 37.Herzlinger DA, Easton TG, Ojakian GK. The MDCK epithelial cell line expresses a cell surface antigen of the kidney distal tubule. J Cell Biol. 1982;93(2):269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Zimmerman SE, Tsunezumi J, Braitsch C, Trent C, Bryant DM, et al. Role of CD34 family members in lumen formation in the developing kidney. Dev Biol. 2016;418(1):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez-Vale KZ, Yow KD, Gurley NJ, Greene M, Peifer M. Rap1 regulates apical contractility to allow embryonic morphogenesis without tissue disruption and acts in part via Canoe-independent mechanisms. Mol Biol Cell. 2023;34(1):ar7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boettner B, Van Aelst L. The Rap GTPase activator Drosophila PDZ-GEF regulates cell shape in epithelial migration and morphogenesis. Mol Cell Biol. 2007;27(22):7966–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, et al. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol. 2006;16(18):1796–806. [DOI] [PubMed] [Google Scholar]
- 42.Lafuente EM, van Puijenbroek AA, Krause M, Carman CV, Freeman GJ, Berezovskaya A, et al. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004;7(4):585–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.