Abstract
People who live or work in moldy buildings often complain of “brain fog” that interferes with cognitive performance. Until recently, there was no published research on the effects of controlled exposure to mold stimuli on cognitive function or an obvious mechanism of action, fueling controversy over these claims. The constellation of health problems reported by mold-exposed individuals (respiratory issues, fatigue, pain, anxiety, depression, and cognitive deficits) correspond to those caused by innate immune activation following exposure to bacterial or viral stimuli. To determine if mold-induced innate immune activation might cause cognitive issues, we quantified the effects of both toxic and nontoxic mold on brain immune activation and spatial memory in the Morris water maze. We intranasally administered either 1) intact, toxic Stachybotrys chartarum spores; 2) ethanol-extracted, nontoxic Stachybotrys chartarum spores; or 3) control saline vehicle to mice. Inhalation of nontoxic spores caused significant deficits in the test of long-term memory of platform location, while not affecting short-term memory. Inhalation of toxic spores increased motivation to reach the platform. Interestingly, in both groups of mold-exposed males, numbers of interleukin-1β-immunoreactive cells in many areas of the hippocampus significantly correlated with latency to find the platform, path length, and swimming speed during training, but not during testing for long-term memory. These data add to our prior evidence that mold inhalation can interfere with cognitive processing in different ways depending on the task, and that brain inflammation is significantly correlated with changes in behavior.
Keywords: spatial memory, anxiety, brain fog, IL-1β, neuroinflammation, innate immune activation
1. Introduction
The ability of mold inhalation to cause respiratory problems has been well documented and widely accepted [1]. However, people exposed to moldy buildings complain of a plethora of other problems, including painful muscles and joints, chronic fatigue, balance problems, anxiety, depression, and cognitive issues often described as “brain fog” [2–5]. The ability of mold exposure to cause such serious health concerns has been the subject of much litigation, but there is relatively little scientific research on this topic, and it is still hotly disputed [6–9]. Among other issues, early mold studies were criticized for their small sample sizes, use of patient self reports, poorly-documented mold exposures, correlational designs, and the lack of any documented physiological mechanism that could cause such disparate effects. However, there is now compelling evidence that mold exposure causes serious multi-system health problems in humans including peripheral immune activation and behavioral dysregulation that implies central effects [5, 10–12]. In particular, Kilburn’s study [11] quantified wide-ranging neural problems in mold-exposed individuals including increased reaction times, visual abnormalities, decreased grip strengths, balance problems, peripheral neuropathies, as well as diverse, significant cognitive and emotional problems. Shoemaker et al. [10] found that changes in blood inflammatory markers in mold-exposed patients were correlated with significant structural differences in six of eleven brain areas studied. Nevertheless, specific mechanisms by which mold exposure caused neurological or behavioral problems were still not identified.
Given the lack of published research on the effects of controlled doses of characterized mold stimuli on both brain and behavior, we proposed a plausible physiological mechanism to explain how mold exposure alters brain function leading to changes in both emotional and cognitive behavior and developed a mouse model to test our hypothesis [13]. Our model is based on decades of research on bacterial and viral innate immune activation and its pervasive effects on behavior [14–16]. Exposure to bacteria, or merely to a component of bacterial cell walls, quickly results in innate immune activation with cytokine secretion at the site of exposure. Innate immune activation in the periphery is rapidly communicated to the brain where it activates microglia, the brain’s most numerous resident immune cells, to secrete additional cytokines. These cytokines alter neural function, causing fever, pain, changes in motivation, and social withdrawal. Innate immune activation of the brain also results in well-documented cognitive effects [14, 15]. Behaviors supported by the hippocampus seem particularly vulnerable. This is not surprising, given that the hippocampus contains a greater density of microglia than other brain regions and is particularly vulnerable to inflammation [17]. Typically, performance on cognitive tasks dependent on hippocampal function, such as contextual fear conditioning or the Morris water maze (MWM), is adversely affected by innate immune activation [16, 18]. We hypothesized that mold exposure would cause similar changes in brain immune activation accompanied by similar changes in behavior, since the innate immune system has pattern recognition receptors (PRRs) that recognize structural elements of mold and/or its RNA/DNA just as it has PRRs for bacteria and viruses [19]. Previous studies [20–22]} showed that mold exposure caused immune activation and inflammation throughout the respiratory system. We were interested in determining if the peripheral inflammatory response initiated by mold inhalation would spread to the brain with concomitant effects on behavior.
In addition to determining if mold stimuli could cause innate immune activation in the brain and affect cognitive and emotional behavior, we were interested in the relative effects of nontoxic versus toxic mold stimuli. Much of the research on mold attributed adverse health effects to the production of mycotoxins, poisonous secondary metabolites that defend mold colonies against competitors [2, 4, 23–26]. Attributing adverse effects to mycotoxins had the advantage of providing a clear mechanism of action through the documented abilities of toxins to interfere with cellular function or even kill cells [23, 27]. However, animal studies showed that exposure to nontoxic mold stimuli [21,22], or just to a component of fungal cell walls [28] was sufficient to cause innate immune activation in the lungs. Innate immune activation provides a mechanism for both toxic and nontoxic mold stimuli to affect multiple physiological systems affecting both brain structure/function and behavior. We expected nontoxic stimuli to cause less immune activation and behavioral change than toxic molds since they provide fewer constituents that trigger immune responses.
The current study examined the effects of mold exposure in adult mice on a hippocampal-dependent behavioral task: spatial navigation in the MWM. Animals exposed to bacterial challenge often show clear deficits on this task, though such deficits are sometimes indicative of performance factors rather than problems in learning and memory [18]. We quantified the effects of treatment with 1) toxic Stachybotrys spores or 2) Stachybotrys spores rendered nontoxic by ethanol extraction compared to 3) the control saline vehicle on hippocampal inflammation and spatial navigation. Since task-induced anxiety is often listed as a confounding variable in interpreting MWM data, we compared the animals’ behavior on the elevated plus maze (EPM) to that on the MWM.
2. Materials and Methods
2.1. Experimental animals
All treatments, behavioral tests, and data analyses were done blind to the animals’ treatment groups. All animal methodology was approved by the Institutional Animal Care and Use Committees of Hunter College and Queens College, CUNY and met all local and federal guidelines for animal research.
C57BI/6 mice were chosen because they are a robust strain, with good hippocampal function, and show superior performance on the MWM [29]. C57BI/6 mice also clear mold spores from the lungs faster than other strains [30, 31], making them a more conservative model system. Thirteen-week-old male C57BI/6 mice (Jackson Laboratories, Bar Harbor, ME) were run in 3 cohorts of 12 males allowing all behavioral testing to be done during the morning with mold spore instillations in the late afternoon. Data from the 3 cohorts were combined for analyses. Because group housing can cause confounding effects in mice experiencing innate immune activation [32], mice were housed individually in filter-topped shoebox cages with paper bedding, a piece of PVC piping, and a nestlet (Ancare, Bellmore, NY) on a 12:12 light:dark cycle at 22.2 + 1.4° C. Mice were habituated to their housing at Hunter College, handling, transport to behavioral testing rooms, and testing for at least 4 weeks prior to instillations.
2.2. Mold exposure
All spore handling, instillations, and cage changes were done in a Class II biosafety cabinet (ThermoFisher Model 1365). Each cohort was divided into 3 treatment groups using a stratified randomized block design controlling for body weight and instilled with either: 1) intact Stachybotrys chartarum spores (TX), 2) S. chartarum spores extracted twice with ethanol to remove toxins and denature proteins (NTX), or 3) the sterile non-pyrogenic saline vehicle containing 0.1% TWEEN 20 (VEH). The spores, strain JS58-17 originally isolated from a home in Cleveland, Ohio [22], were grown and prepared at Hunter College from stock provided by Dr. Dorr Dearborn, Case Western Reserve University, according to his protocols. Spore dose was determined using a hemocytometer to count spores to an accuracy of ± 4%.
Mice were briefly anesthetized with isoflurane and nasally instilled [33, 34] 3 times/week with 15,000 spores in 0.25 μl vehicle/g body weight or vehicle alone. The mouse’s external nares were gently cleaned with 70% ethanol and half the day’s dose was instilled in each nostril using a Rainin 0.5-10 μl pipette. Mice were held in a vertical position for 2 min post instillation to maximize spore inhalation. Mice did not show obvious signs of discomfort that are often reported following high doses of lipopolysaccharide (LPS, e.g., disheveled fur, squinting, aberrant movement).
2.3. Behavioral testing
After several weeks of handling and acclimation to the laboratory, mice were trained and tested on the rotorod, conditioned fear task, and MWM (MWM1, Fig. 1). They then received 3 weeks of instillations without behavioral testing. Beginning in week 4, they were tested on the elevated plus maze, a second time on MWM (MWM2), and then an additional two times on the conditioned fear task and rotorod. Mice were then tested a third and final time on MWM (MWM3, Fig. 1 summarizes the timeline for MWM and EPM tests). Fewer than half the mice learned the task on MWM1 prior to treatment, so those results are not presented. Unfortunately, the MWM2 data from Cohort 3 were lost due to a camera malfunction. Thus, we only present the results of MWM3 and the EPM test. The results of other tests are reported elsewhere [13]. Instillations always occurred the day before MWM Day 1 training and again after all tests were completed on Day 2. All testing orders were counterbalanced across mice and treatment groups. All tests were run blind to the animals’ treatments.
Fig. 1.
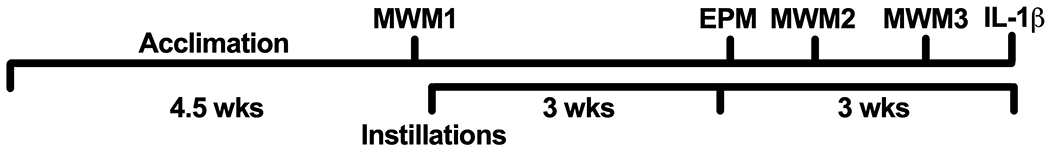
Following arrival in the lab, mice were acclimated to their new surroundings, handling, and transport to the behavioral testing room for 4.5 weeks. They received their first Morris water maze test before beginning instillations. After three weeks of instillations 3 times per week without testing, they resumed behavioral testing. Triweekly instillations continued to the end of the experiment. Instilled mice were first tested on elevated plus maze (EPM), and later given their second and third MWM tests. At the end of the experiment, the density of cells in the hippocampus expressing interleukin-1β immunoreactivity (IL-1β-ir) was quantified.
2.3.1. Morris water maze
Overview:
For each of the 3 tests, mice were trained with 4 visible platform trials at 30-min intervals on Day 1. The following day, they were given 3 trials at 30-min intervals with the platform in the same location but hidden underwater. This 2-day procedure was conducted once prior to mold treatment (MWM1, platform in north quadrant) and twice during mold treatments (MWM2, platform in south quadrant; and MWM3, platform in the same position as MWM1).
Apparatus:
We used a circular polypropylene pool with a diameter of 120cm and height of 74cm. It was filled to a depth of 66 cm, and heaters were used to maintain the water temperature at 25-27°C. This is warmer than commonly used, but closer to mice’s preferred temperature of 31 °C [35]. Water temperature is critical to performance on the MWM. Immune-activated mice show deficits in MWM performance when tested in 23°C but not 18°C water [36]. Nontoxic tempera paint was added to make the water opaque. Visual cues, including bright posters, were placed around the room to help the mice orient spatially. The cues were left in place for the entire experiment.
Procedure:
Mice were trained and tested on the MWM using a 2-day protocol adapted from Gulinello et al. [37]. This protocol incorporates two features shown to increase learning in mice [38]. Mice were tested on visible trials (Day 1) before nonvisible trials (Day 2), which eliminates many off-task behaviors. On both days, trials were spaced at 30-min intervals, rather than being massed, decreasing stress [38]. In brief on Day 1, mice received 4 visible trials with the platform (7.6 cm diameter) covered with textured green plastic shelf liner (Easy Liner, select grip, Amazon) to highlight its location above water in the north quadrant of the pool and provide traction to help the mice climb onto the platform. On day 2, beginning 24 hours after each mouse’s first training trial, mice were tested 3 times with the platform in the same location but covered in white plastic Easy Liner and hidden underwater. On all trials, mice were given 3 min to find the platform and allowed to sit on it for 10 sec before being removed. If they did not find the platform, they were gently guided to it and allowed to sit on the platform for 10 sec before being returned to their home cages. One end of the cage was placed under a heat lamp, so the mouse could warm itself if desired. For each trial, all mice were released from the same location and retrieved from the same location, but the locations changed randomly from trial to trial.
We had not initially planned to analyze the third cohort of mice together with the first two, but 4/24 mice in the first two cohorts never met our criterion of finding the visible platform in under 40 sec and were eliminated from further analyses. We thus decided to add Cohort 3’s data to the analysis to bring the minimum group size back to our original target of eight. There were differences between the first two cohorts and Cohort 3 in additional behavioral tests given, the timing of the various tests, and in the number of release points in the MWM. However, these changes in methodology did not significantly alter the behavior of Cohort 3 compared to the two earlier cohorts, so data from the three cohorts were combined. Mice in Cohorts 1 and 2 were released into either the east or west quadrant of the pool facing the pool wall, so they could not initiate their search by pushing off the wall. However, Garthe and Kempermann [39] suggested that increasing the difficulty of the task was more likely to reveal deficits linked to hippocampal function. Thus, we increased the difficulty of the task for Cohort 3 by increasing the number of release and retrieval points. The pool wall was labeled like a clock face for the experimenter (The mice could not see these markings). The pool was labeled so that 12 was centered in the middle of what had previously been the outside edge of the north quadrant. The platform was placed so that an imaginary radius coming out from 12 would bisect the platform. Mice were placed in the pool facing either 3, 5, 7, or 9. They were removed by an experimenter approaching from 3, 6, 9, or 12.
Evaluation:
All tests were recorded by a SONY Handycam suspended from the ceiling. The resulting videos were digitized and analyzed by Topscan (Clever Sys, Inc., Reston, VA). For each trial, Clever Sys calculated 1) latency to reach the platform, 2) path length to the platform, 3) time spent in thigmotaxis, swimming within 8cm of the pool wall, and 4) velocity. Mice that did not escape to the platform in under 40 sec on at least one visible trial were eliminated from analyses. Mice that did not find the platform on nonvisible trials were assigned a latency of 180 sec.
2.3.2. Elevated plus maze (EPM)
EPM testing was administered to all mice as their first behavioral test following 3 weeks of mold or VEH exposure. Mice were placed in the center (11.4 X 11.4 cm) of a black plexiglass Kinder Scientific EPM (closed arms 10 cm × 55.9 cm, open arms 11.4 cm × 55.9 cm, 91.4 cm above the floor) and their entries into the open and closed arms, time spent in the open and closed arms, and distance traveled in the open and closed arms were automatically quantified by Kinder Motor Monitor software (Build #08356-14). Open arm measures are presented corrected for total entries, the total duration spent, or the total distance traveled in both arms respectively, to control for possible differences in total activity [40]. The apparatus was cleaned with Conflikt (Fisher Scientific), followed by 70% ethanol, and then water after each mouse. These EPM data from the 3 cohorts of mice tested on MWM are a subset of data reported previously [13].
2.4. Histochemical analysis
The methodology for tissue preparation, immunohistochemistry for the inflammatory cytokine interleukin-1β (IL-1β), and quantification of labeled cells are presented in [13]. We present IL-1β-immunoreactive (IL-1β-ir) expression as number of positive cells per area of hippocampus sampled. We only have these data for Cohorts 1 and 2.
2.5. Statistical analysis
Data were analyzed in GraphPad Prism (Version 9 for Mac). All tests were two tailed. For the Gulinello et al. water maze protocol [37], the critical comparisons are the relative performance of the three groups on 1) the last visible test on Day 1 (D1V4) which measures the mouse’s motivation to escape to the platform, 2) the first nonvisible test on Day 2 (D2NV1) which provides a measure of the mouse’s longterm (24 hr) memory of platform location, and 3) the second and third nonvisible tests on Day 2 which evaluate problems with short-term memory. The data sets for the three treatment groups for MWM3 day 2 nonvisible test 1 (D2NV1) path length, latency to reach the platform, and time in thigmotaxis had significantly unequal variances so these three data sets were analyzed with Welch’s ANOVAs followed by Dunnett’s T3 multiple comparisons tests comparing spore-treated groups to VEH controls. The EPM and IL-1β data did not violate the assumptions underlying standard ANOVAs and so were analyzed by oneway ANOVAs followed by the two-stage, linear step-up procedure of Benjamini, Krieger, and Yakutieli comparing the three groups while controlling for the false discovery rate (FDR). The combined IL-1β data from spore-treated mice (NTX + TX = SP) were compared to VEH data using unpaired t tests with Welch’s correction. Pearson correlation coefficients were used to calculate relationships between variables. When multiple comparisons were made across correlations, the Benjamini-Hochberg procedure [41] was used to control for FDR and determine the appropriate alpha. FDR was set to 0.05. The data of one NTX mouse was excluded because it was identified as an outlier on both behavioral tests and cellular measures using Rout with a conservative Q=0.2%.
3. RESULTS
3.1. Effects of mold inhalation on MWM performance
Mice were given 3 widely-spaced MWM sessions, each consisting of 4 visible platform trials at 30-min intervals on day 1. On day 2, they were given 3 trials testing their ability to find the now hidden platform. Mice that did not reach the visible platform in 40 seconds or less by the fourth visible trial of each training were classified as nonlearners. On the first MWM session prior to experimental treatment, only 47% of mice met criterion. By the third MWM session reported here, 83% learned the task. To our surprise, VEH mice had the largest proportion of nonlearners, 4/12 mice. Two of 11 NTX mice did not reach criterion, while all 12 TX mice reached criterion. Data from mice that did not meet criterion were excluded from further analysis.
All mice that learned the task, regardless of treatment group, showed equivalent performance by the fourth visible platform trial on day 1 (D1V4) of MWM3 (Fig. 2) in terms of A) velocity, B) path length, C) latency to reach the platform, and D) time spent in thigmotaxis. However, there were significant differences between the 3 groups on the first non-visible trial 24 hours later (day 2 nonvisible test 1, D2NV1). When mice had to use long-term memory (24 hr) to find the now-hidden platform, NTX mice showed significant performance deficits as measured by both F) path length from release point to platform (Welch’s ANOVA W=6.05 [2, 13.4], P=0.013) and G) their latency to reach the platform (Welch’s ANOVA, W=5.67 [2, 13.3], P=0.016). The performance of VEH males on the first nonvisible trial on Day 2 was very close to their performance on the last visible trial on Day 1 (Fig. 2. Compare VEH A-D to VEH E-H). All VEH males that learned the task during Day 1‘s visible trials found the now hidden platform under the original criterion of 40 sec, while 67% of NTX and 27% of TX mice took longer. Two NTX and 1 TX male did not find the platform in the allotted 180 sec test, though they had easily accomplished the task on Day 1 when the platform was visible. D1V4 and D2NV1 path lengths and latencies to reach the platform were highly correlated in VEH mice (Pearson r=0.72. P=0.046; r=0.80, P=0.018 respectively). The performance of NTX and TX mice on the first nonvisible trial was not predicted by their performance on the last visible training trial (NTX path lengths r=0.23, P=0.56, latencies r=0.43, P=0.24, TX path lengths r=0.14, P=0.67, latencies r=0.08, P=0.81).
Fig. 2.
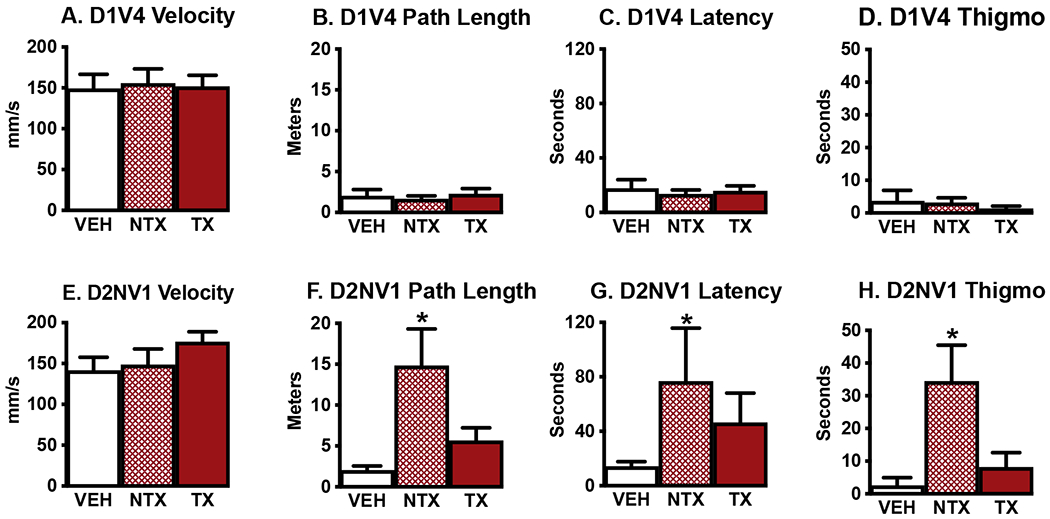
A.-D. By the final visible training trial (day 1, trial 4, D1V4) there were no differences in performance between groups. E.-H. However, 24 hr later, mice treated with NTX spores showed significantly impaired performance compared to VEH males in finding the hidden platform on their first nonvisible trial on day 2 (D2NV1). E. Velocity did not differ across groups. F. NTX males took longer paths to find the platform, G. took longer latencies to find the platform, and H. spent more time swimming within 8 cm of the pool wall. Thigmo = thigmotaxis. Means + SEM. *Significantly different from VEH, P < 0.05 Ns: VEH=8, NTX=9, TX=12. Ns differed across groups because 4 VEH and 2 NTX males did not learn the task, while all TX mice met criterion during visible platform training.
NTX mice appeared to be using a different strategy than VEH mice to find the hidden platform on D2NV1. NTX mice spent significantly more time in thigmotaxis, swimming within 8 cm of the pool wall, than VEH mice (Fig. 2H. Welch’s ANOVA W=4.2 [2, 14.7], P=0.036). The performance deficits of NTX males in finding the hidden platform appeared specific to long-term memory. On the next two nonvisible trials 30 and 60 min later (D2NV2, D2NV3) with the platform still hidden, NTX latencies, path lengths, and time in thigmotaxis did not differ from those of VEH or TX males (e.g., mean latency to platform D2NV3, VEH=36±15, NTX=33±7, TX=35±12 sec). Velocities did not differ significantly across groups on any trial. The slower latencies of both NTX and TX males to reach the platform on D2NV1 compared to D1V4 were not a function of slower swimming speeds but were highly correlated with their increased path lengths (NTX: Pearson’s r= 0.94, P=0.0002, N=9; TX: r=0.82, P=0.001, N=12). TX males actually increased their velocities from D1V4 to D2NV1 (Fig. 2A vs 2E, Paired t test, t=3.0, df=11, P=0.012), while the swimming speeds of VEH and NTX males did not change.
3.2. Effects of mold inhalation on anxiety-like behavior and relation to MWM performance
Mice treated with NTX spores showed increased anxiety-like behavior on the EPM compared to both VEH and TX males. NTX males made fewer entries into the open arms compared to all arms (Fig. 3A, ANOVA F=4.88, P=0.01), spent shorter durations in the open arms compared to all arms (Fig. 3B, ANOVA F=6.44, P=0.004), and traveled shorter distances in the open arms compared to all arms (Fig. 3C, ANOVA F=6.84, P=0.003). Although NTX mice showed significantly higher levels of anxiety-like behavior on EPM and greater amounts of time in thigmotaxis when they could not see the platform on the D2NV1 MWM test, none of the three measures of EPM performance correlated with a measure of MWM performance. Additionally, no correlations between responses on these two tests were found in the other two groups or across all mice.
Fig. 3.
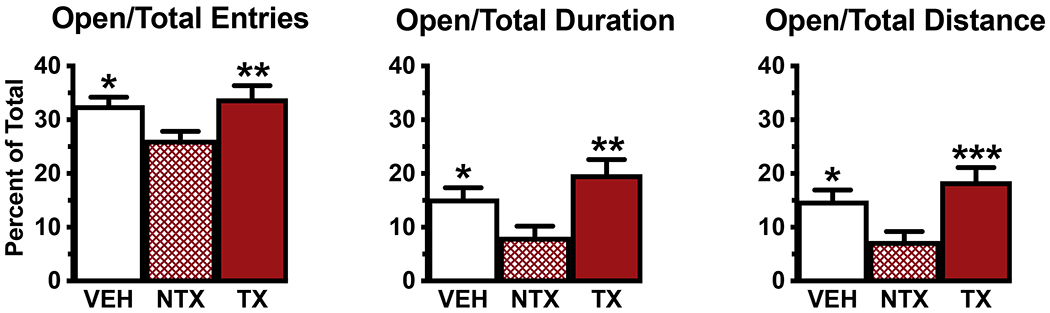
NTX mice showed increased anxiety-like behavior on the EPM compared to both VEH and TX groups. A. NTX mice entered the open arms less often and B. spent less time in the open arms compared to entries and duration in all arms. C. NTX mice traveled less distance in the open arms compared to distance traveled in all arms. Means + SEM, *significantly different from NTX, P < 0.05. **P < 0.01, ***P < 0.001. Ns: VEH=12, NTX=11, TX=12.
3.3. Relationship between IL-1β levels in hippocampus and MWM performance
TX treatment resulted in significantly more IL-1β-ir cells in both dorsomedial CA1 and dorsomedial CA1 + DG per mm2 than found in those areas in VEH mice (Fig. 4, data from [13]). NTX treatment resulted in more IL-1β-ir cells than found in VEH males but fewer than found in TX males. In the dorsomedial CA1 + DG, TX males had significantly more IL-1β-ir cells per mm2 than NTX mice. In both dorsomedial CA1 and CA1 + DG, spore-treated mice (TX + NTX) had significantly more IL-1β-ir cells per unit area than VEH mice (Unpaired t tests with Welch’s correction, t=3.24, P=0.004; t=3.04, P=0.007, respectively). Density of IL-1β-ir cells in the dorsomedial dentate gyrus as a whole or in its granular and molecular layers quantified separately did not differ across groups.
Fig. 4.
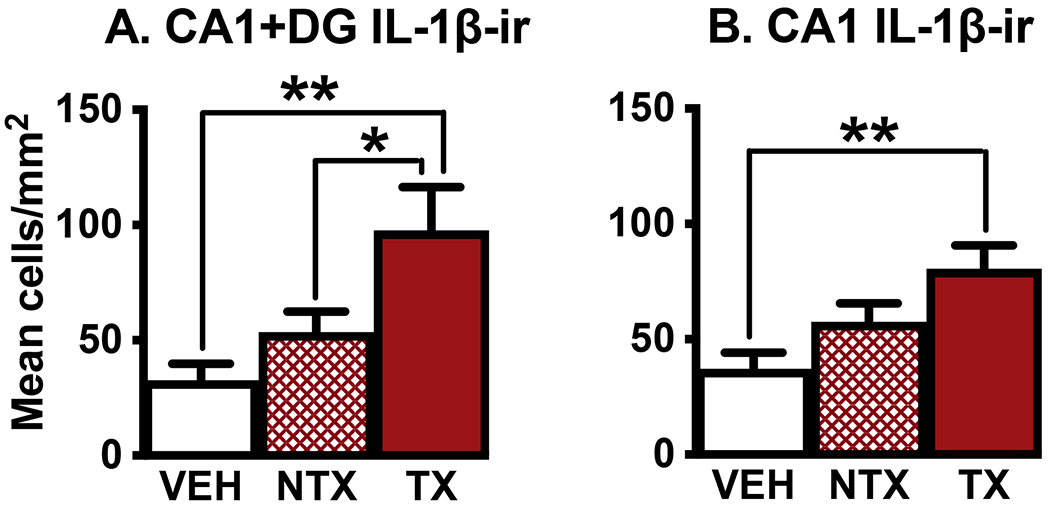
Compared to VEH treatment, TX spore exposure significantly increased numbers of cells expressing the inflammatory cytokine IL-1β-ir in the dorsomedial hippocampal subareas CA1+DG combined and in the CA1 subdivision itself. In dorsomedial CA1+DG, TX mice also had significantly more IL-1β expressing cells than mice exposed to NTX spores. CA1=cornu ammonis 1. DG=dentate gyrus. Ns=7,8,6 for CA+DG and 8,8,8 for CA1, respectively. *P < 0.05, * *P < 0.01
There were no significant correlations between numbers of IL-1β-ir cells and latency to find the hidden platform or path length on D2NV1 or time swimming in thigmotaxis on either D2NV1 or D1V4. However, performance during the last visible training trial on Day 1 was correlated with numbers of IL-1β-ir cells in various areas of the dorsomedial hippocampus in spore-treated mice (TX + NTX, see Table 1). Correlation coefficients were strongest in TX-treated males in dorsomedial CA1 + DG and CA1. In TX males, the higher the density of IL-1β-ir cells in these two areas, the longer it took mice to reach the platform and the longer their paths. NTX males showed much lower correlations between hippocampal inflammation and MWM performance. However, when data from the two spore-treated groups were combined, numbers of IL-1β-ir cells in all five hippocampal areas were significantly positively correlated with latency to reach the platform. Only numbers of IL-1β-ir cells in dorsomedial CA1 + DG and CA1 itself were significantly positively correlated with path length in spore-treated males. Correlation coefficients between densities of IL-1β-ir cells and MWM performance were very low for VEH males and often in the opposite direction. However, the correlations between numbers of IL-1β-ir cells in dorsomedial CA1 + DG and D1V4 latencies and path lengths in spore-treated males were sufficiently strong to result in weaker, but still significant correlations on these measures when the data from all males (i.e., TX + NTX + VEH) was considered. Numbers of IL-1β-ir cells in CA1 + DG and CA1 alone were negatively correlated with swimming speed in spore-treated males on this trial. The higher the IL-1β levels, the slower the animals swam.
Table 1.
Higher numbers of IL-1β-ir cells in the dorsomedial hippocampus were correlated with poorer MWM performance during the final training trial (D1V4) in spore-treated males. Pearson correlation coefficients between numbers of IL-1β-ir cells in five hippocampal areas and A. latency to find the visible platform, B. path length, and C. velocity, corresponding P values, and Ns. All = all mice, SP = spore-treated (i.e,, NTX + TX). Significant correlations are bolded. IL-1β-ir cell data were only available for the first two cohorts of mice. CA1 = cornu ammonis 1; DG = dentate gyrus as a whole; Granular = within the granular cell layer of the dentate gyrus; Molecular = within the molecular layer of the dentate gyrus.
| CA1 + DG | CA1 | DG | DG Granular | DG Molecular | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. D1V4 Latency | |||||||||||||||
| ALL | 0.54, | 0.017, | 19 | 0.45, | 0.054, | 19 | 0.41, | 0.09, | 18 | 0.46, | 0.056, | 18 | 0.43 | 0.07, | 18 |
| VEH | −0.04, | 0.93, | 6 | 0.11, | 0.84, | 6 | −0.23, | 0.65, | 6 | 0.007, | 0.99, | 6 | −0.18, | 0.73, | 6 |
| NTX | 0.42, | 0.48, | 5 | 0.41, | 0.49, | 5 | 0.52, | 0.37, | 5 | 0.70, | 0.19, | 5 | 0.57, | 0.33, | 5 |
| TX | 0.90, | 0.002, | 8 | 0.80, | 0.017, | 8 | 0.74, | 0.057, | 8 | 0.68, | 0.09, | 8 | 0.73, | 0.06, | 8 |
| SP | 0.74, | 0.004, | 13 | 0.64, | 0.018, | 13 | 0.67, | 0,016, | 13 | 0.67, | 0.017, | 13 | 0.68, | 0.015, | 13 |
| B. D1V4 Path Length | |||||||||||||||
| ALL | 0.52, | 0.02, | 19 | 0.42, | 0.08, | 19 | 0.25 | 0.30, | 19 | 0.19, | 0.44, | 19 | 0.24, | 0.31, | 19 |
| VEH | −0.09, | 0.86, | 6 | 0.08, | 0.89, | 6 | −0.29, | 0.57, | 6 | −0.03 | 0.95, | 6 | −0.21, | 0.69, | 6 |
| NTX | 0.17, | 0.78, | 5 | 0.12, | 0.85, | 5 | 0.06, | 0.92, | 5 | −0.06, | 0.92, | 5 | 0.02, | 0.97, | 5 |
| TX | 0.87, | 0.005, | 8 | 0.78, | 0.02, | 8 | 0.49, | 0.22, | 8 | 0.29, | 0.49, | 8 | 0.44, | 0.27, | 8 |
| SP | 0.70, | 0.008, | 13 | 0.56, | 0.04, | 13 | 0.40, | 0.17, | 13 | 0,22, | 0.45, | 13 | 0.36, | 0.22, | 13 |
| C. D1V4 Velocity | |||||||||||||||
| ALL | −0.41, | 0.08, | 19 | −0.41, | 0.08, | 19 | −0.05 | 0.82, | 19 | −0.11, | 0.64, | 19 | −0.08, | 0.75, | 19 |
| VEH | 0.29, | 0.57, | 6 | 0.13, | 0.80, | 6 | 0.41, | 0.42, | 6 | 0.05 | 0.92, | 6 | 0.30, | 0.57, | 6 |
| NTX | −0.68, | 0.20, | 5 | −0.53, | 0.36, | 5 | −0.69, | 0.20, | 5 | −0.85, | 0.07, | 5 | −0.81, | 0.10, | 5 |
| TX | −0.65, | 0.08, | 8 | −0.64, | 0.08, | 8 | −0.10, | 0.82, | 8 | 0.00 | 0.99, | 8 | −0.06, | 0.88, | 8 |
| SP | −0.59, | 0.03, | 13 | −0.60, | 0.03, | 13 | −0.19, | 0.54, | 13 | −0,12, | 0.69, | 13 | −0.17, | 0.58, | 13 |
4. Discussion
Our results provide additional evidence that inhalation of NTX mold affects cognitive processing while inhalation of TX mold affects motivation, and these effects are related to brain inflammation. Mold exposure affected both the motivation and the ability to find the hidden platform in the MWM task in unexpected ways. We had expected both TX and NTX spores to interfere with learning the task, with TX spores having greater effects because of their greater inflammatory potential [13, 22]. TX spores present not only a variety of toxins, but also enzymes and volatile organic compounds that interfere with basic cell functions [22, 23]. However, mice exposed to toxic spores appeared the most motivated to quickly reach the platform as measured by 1) percent males learning the task [ as in 42] and 2) increased swimming speeds [as in 38], While some TX males had difficulty finding the platform once it was hidden, only NTX spore exposure significantly interfered with this task.
Evaluation of performance on the MWM following immune challenge poses some difficulties since a variety of interacting factors impact performance, including animal strain, degree of immune activation, timing of immune activation relative to task training and testing, pool size, water temperature, and the precise protocol used [18, 36]. However, the advantages of using the MWM to evaluate cognitive difficulties are thought to outweigh the disadvantages [38]. We attempted to optimize the task to decrease stress and increase the probability of revealing differences between experimental treatments. We used a two-day version of the task originated by Gulinello et al. [37] that begins on the first day by assessing the mouse’s motivation to swim to a visible platform. The mouse’s memory of platform location is tested on the second day with the platform in the same location but hidden underwater. This paradigm has the advantage of allowing subjects to habituate to the task, while providing individual performance baselines and reducing confounding variables like anxiety and stress [37]. Performing visible platform trials first is particularly important in testing mice [38]. Trials were spaced at 30-min intervals in 25-27°C water, which also decreases stress [36, 38].
After three widely-spaced trainings [i.e., 1) before mold exposure began, 2) after three weeks of treatment and 3) again after roughly 5 weeks of treatment], 17% of mice did not swim to the visible platform in under 40 sec, and their data were not included in further analyses. The high percentage of nonlearners was probably related to the warm water temperature used. Warm temperatures are less stressful, but also less motivating. Mice have difficulty maintaining core body temperature when tested in cold water, motivating them to escape to the platform quickly [36, 43]. We used a warm temperature range because past research found that immune-activated mice were more likely to show performance deficits when tested in warmer water. For example, mice treated with 100 ng IL-1β ip showed performance deficits on MWM when tested in 23°C but not 18°C water [36]. Contrary to expectation, VEH mice were the least likely to meet criterion (4/12 failed), while 2/11 NTX males failed to escape to the visible platform under 40 sec, and all TX males mastered the task. Previous research [36] found that while moderate doses of IL-1β (e.g., 100ng ip) interfered with performance of male mice on MWM, a high dose (1,000 ng ip) significantly improved performance. Our TX males had significantly more IL-1β-ir cells per unit area in dorsomedial CA1 + DG than VEH or NTX males [13]. Numbers of IL-1β-ir cells in NTX mice fell between those in VEH and TX groups [13]. The fact that all TX males were motivated to reach the platform quickly may have been related to the effects of IL-1β on thermoregulation. IL-1β is a well-documented pyrogen [44]. Heat-seeking behavior is a standard symptom of brain immune activation [45]. Mice showing fevers following LPS treatment prefer ambient temperatures several degrees C higher than normal [35]. Although we used warmer water than most studies, if the higher numbers of IL-1β-ir cells in TX males resulted in increased body temperatures, TX males may have perceived the water as cooler than the other groups, motivating them to escape to the platform. Barring motor issues, swimming speed is used as a measure of motivation [38]. Another indication that TX males were more motivated to escape were their significantly increased velocities on D2NV1 when the platform was no longer visible compared to their speeds on the last visible training trial. The swimming speeds of the other two groups did not change. Immune activation is usually associated with reduced activity and energy conservation to support immune function. Spore-treated mice showed this more typical effect of immune activation on D1V4 when higher levels of immune activation were associated with slower swim speeds. It therefore seemed somewhat contradictory that TX mice showed increased energy outlay to reach the platform on D2NV1. However, other research has shown that depending on the symptoms of sickness behavior induced, immune-activated subjects may invest more energy than controls to complete a task [46]. The need of immune-activated TX males to seek a warmer environment seems to have overridden the general need to conserve energy.
By the last training trial (D1V4), learners in all three groups showed equivalent performance on all measures (i.e., velocities, path lengths, latencies, and time in thigmotaxis). The ability of VEH males to find the hidden platform on the first trial on Day 2 (D2NV1) was almost identical to their performance on their last visible platform training trial 24 hours earlier. In contrast, in both TX and NTX males, D2NV1 performance was not correlated with D1V4 performance. Overall, NTX males showed significantly increased latencies, increased path lengths, and increased time in thigmotaxis on D2NV1 compared to VEH males. However, the performance of NTX males did not differ from that of VEH males on the two later hidden platform trials on D2, suggesting that short-term memory was not affected. Similar results were found in rats [47]. Male rats were trained and tested for two days, 3 trials per day, with the platform hidden. The performance of males injected with 100 ng of IL-1β icv 60 min prior to training on Day 1 improved over trials on Day 1 just like that of controls, but that improvement did not carry over to Day 2. Just like our mice, these rats showed impaired performance only on the first trial on Day 2 and did not differ from controls on later trials.
On D2NV1, individual NTX and TX males varied in their responses to spore treatment. A few males maintained the level of performance shown on the last visible trial while others struggled to find the platform. All VEH males reached the platform in under 40 sec on D2NV1, but only 27% of NTX and 67% of TX males met the original learning criterion on their first nonvisible trial. Two NTX and one TX male did not locate the platform by the end of the trial (i.e., 180 sec). While TX males performed better than NTX on D2NV1, they experienced some level of difficulty--67% of TX males’ latencies and path lengths were longer on D2NV1 than they had been on D1V4, and their mean values on both measures were over 2.5 times longer than those of the VEH mice.
In many cases when animals experiencing immune activation show increased latencies to complete a task such as MWM, the increased latencies are explained by the resulting sickness behavior which results in decreased speed of locomotion [18]. However, at no point in our experiment did velocities differ significantly between the three experimental groups. The increases in latencies to reach the platform on D2NV1 in NTX and TX males were highly correlated with their increased path lengths, not decreased velocities. As noted previously, TX males actually increased their velocities on D2NV1. The impaired performance of spore-treated males on D2NV1 did not result from deficits in motor performance.
Increased thigmotaxis in the MWM is often attributed to increased anxiety, allowing animals to search for the platform while staying in less anxiety-inducing, less stressful areas of the pool [18]. Some studies found that performance on MWM correlated with that on EPM. Animals rated less anxious on EPM were less likely to engage in thigmotactic behavior in the MWM [36, 48]. Our NTX males were rated as significantly more anxious on EPM than either VEH or TX males, making a smaller percentage of their entries into the open arms, spending a smaller percentage of time in the open arms, and traveling a smaller percentage of their total distance in the open arms. However, while NTX males were rated as more anxious on EPM and spent significantly more time in thigmotaxis on D2NV1, their EPM scores were not correlated with the time they spent in thigmotaxis. This may be related to the fact that MWM testing occurred at least 10 days after EPM testing. If thigmotactic behavior is considered a measure of anxiety, it is interesting to note that NTX males only spent more time in thigmotaxis on D2NV1, not in the four earlier visible trials or the two later nonvisible trials. Thus, swimming in the pool per se did not elicit thigmotactic behavior. NTX males only showed this behavior when the trial procedure had changed, and they could not see the platform.
As Cunningham and Sanderson [18] pointed out, there are several possible causes for deficits like those shown by NTX males on D2NV1: 1) There might be difficulty getting platform location into long-term memory. Moderate increases in IL-1β during learning can interfere with long-term potentiation and memory storage [16]. NTX males had 64% more IL-1β-ir cells in the dorsomedial hippocampus than VEH males suggesting a moderate increase in IL-1β. 2) The fact that mice were instilled only on the day prior to MWM training leaves open the possibility that their internal states varied sufficiently between training and testing days to make it more difficult to retrieve the memory of platform location on D2NV1. 3) An increase in anxiety when the platform was hidden on D2NV1 might have caused NTX mice to engage in a thigmotactic strategy. We cannot determine which of these issues might be responsible for the difficulties NTX mice had in locating the hidden platform, but clearly NTX mold exposure interfered with their cognitive processing.
IL-1β is critical in activating and coordinating the full spectrum of pathogen-induced sickness behaviors [49]. Rapid production and release of IL-1β is a critical step in the initiation of the innate-immune response to mold [50]. IL-1β levels in the hippocampus modulate hippocampal-dependent learning and memory. IL-1β dosage effects appear to follow a U-shaped curve rather than a straight line. Small increases in IL-1β are crucial for establishing long-term potentiation and enhancing memory consolidation, while moderate increases can be disruptive [16]. Exposure to even higher levels can facilitate learning [16, 36]. We were therefore interested in whether performance on this hippocampal-dependent version of the MWM (e.g., random start locations [36], no spatial cues inside the maze [51]) would be related to IL-1β levels in the hippocampus. TX males had significantly higher numbers of IL-1β-ir cells per unit area in CA1 than VEH males and more in dorsomedial CA1 + DG than either VEH or NTX males [13]. There were no significant differences in numbers of IL-1β-ir cells in the DG across groups. We previously found that numbers of IL-1β-ir cells in different areas of the hippocampus in both NTX and TX males were highly correlated with increased fear of the auditory cue over time in a conditioned fear task [13]. Given these data and IL-1β’s documented role in memory consolidation, it was surprising to find that there were no significant correlations between IL-1β-ir cells and performance during memory recall on D2NV1. However, there were many significant correlations between numbers of IL-1β-ir cells and performance on the final visible training trial. Across all groups, the higher the number of IL-1β-ir cells in dorsomedial CA1 + DG, the longer the latencies and path lengths to reach the platform on D1V4. These positive correlations were primarily due to very high correlation coefficients in TX males. Correlation coefficients between these variables were lower in NTX males, but sufficient that when the two spore-treated groups were combined, the correlations were significant. There were also significant correlations in the combined spore-treated group between numbers of IL-1β-ir cells in the DG and its two subregions and latency to reach the platform on D1V4. Interestingly, although mold exposure did not lead to a decrease in speed compared to controls as is often seen in immune-activated animals, increased numbers of IL-1β-ir cells in the dorsomedial CA1+DG and CA1 alone in spore-treated mice (NTX + TX) were correlated with slower swimming speeds on D1V4. It should be noted that several days elapsed between MWM3 testing and sampling tissue to quantify IL-1β-ir cells. The many significant correlations between D1V4 performance and numbers of IL-1β-ir cells suggest that recurring mold exposure leads to fairly stable numbers of IL-1β-ir cells in the hippocampus.
To control for possible circadian effects, we ran this experiment using three cohorts of mice, so that all behavioral testing could be done in the morning, and all treatments in a small window in the late afternoon. Data from the three cohorts were combined for analyses. We attempted to control as many variables as possible across cohorts, but there were some differences. All cohorts were run in the summer, but in two different years. Different individuals were involved in handling, treating, and testing the three cohorts. Experimenter sex significantly affects behavioral test results in mice [52, 53]. All mice in this study were handled by both sexes, but the sex and identity of individuals performing specific tasks varied cohort to cohort, test to test. In addition, the MWM task was made more difficult for the third cohort of mice by adding additional release points. The consistency of results across cohorts despite these differences demonstrates the robust nature of these effects.
The current data provide additional documentation that mold exposure affects cognitive processing, that exposure to NTX mold has adverse consequences, and that mold’s effects are correlated with brain immune activation. We were unable to accurately predict whether TX or NTX spores would have more serious effects on a particular behavioral measure. Across all our experiments (current + [13], exposure to TX mold resulted in higher levels of brain inflammation and stronger correlations between measures of brain inflammation and behavior, but exposure to NTX spores caused deficits in contextual fear, increased sensitivity to pain, and enhanced response to the auditory cue in a conditioned fear paradigm that did not differ significantly from the responses of TX males. Both NTX and TX spore inhalation decreased hippocampal neurogenesis, but they affected different stages of the process, with NTX spores decreasing numbers of immature adult-born neurons while TX spores inhibited neuron maturation. Only NTX spore treatment resulted in increased anxiety-like behavior on EPM and impaired cognitive processing in the MWM.
The effects of immune activation depend on the integration of the actions of all activated PRRs [54]. There are at least 19 PRRs known to recognize components of fungal cell walls and/or fungal DNA/RNA [19]. Given their many additional chemical components, it is probable that TX stimuli activate more PRRs than NTX stimuli, leading to differential effects between the two groups. Activation of additional PRRs in TX males may have inhibited anxiety-like behavior and provided additional motivation to learn the MWM task. It is also possible that the extraction step used to remove toxins and denature proteins in TX spores to create the NTX stimuli altered antigen availability in NTX spores resulting in differential PRR activation.
In total, our studies clearly document the adverse effects of repeated exposure to nontoxic, as well as toxic, mold spores on brain and emotional and cognitive function. However, our carefully prepared mold stimuli were not representative of typical human mold exposure. Water-damaged buildings typically develop complex mixtures of molds, bacteria, and particulates. These mixtures activate stronger, synergistic immune responses than predicted from the effects of the individual stimuli [1, 4, 12]. Reported human exposures are substantially lower than the spore doses we used. However, attempts to quantify mold contamination generally underestimate true values [55, 56]. Common methodology involves very brief air or dust sampling in limited areas, missing large spatial and temporal variations [8]. Results expressed in terms of numbers of viable spores vary greatly depending on the culture medium used. Many molds do not even reproduce under laboratory conditions [56], and viable spores often represent a very small proportion of total spores [55]. Mold concentrations determined by qPCR typically are several orders of magnitude higher than those identified by culturing spores, but qPCR techniques introduce a different set of biases [56]. Crucially, most mold exposure is to small particles, not spores. Small particles, either mold fragments or particles from moldy surfaces, are more important sources of toxin exposure than mold spores [23]. Nanoparticles are often hundreds to as much as a million times more numerous and penetrate deeper into the lungs than whole spores [1, 56, 57]. Nanoparticles are more likely to cause adverse health outcomes [58, 59]. Spore counts do not predict fragment numbers, and fragments are rarely counted [58]. When mold nanoparticles are inhaled, there are the additional risks of direct access to the brain via olfactory pathways as well as access to the general circulation [60–62]. For example, inhalation of a mycotoxin produced by Stachybotrys caused innate immune activation in nasal and brain tissue, apoptosis of olfactory sensory neurons, atrophy of the olfactory nerve, and brain inflammation [26].
Molds also produce a wide variety of bioactive metabolites—as many as 90 from a single species [63]. Secondary metabolites of molds have been identified in the dust, respirable airborne particles, carpeting, wallpaper, heating, ventilation and air-conditioning systems of water-damaged buildings [64]. Despite the major health threats they may present, these metabolites are rarely quantified [55]. In searching for fungal compounds causing health issues in the wake of Hurricane Katrina, Inamdar and coworkers [65] identified a volatile organic metabolite emitted by molds that provides much of the distinctive musty odor. Exposure to as little as 0.5 ppm of this fungal metabolite caused degeneration of dopamine neurons and resulted in Parkinson’s-like symptoms.
Even if mold exposure is terminated quickly, the effects of the brain’s initial inflammatory response often persist beyond its resolution [66]. This microglial priming is thought to occur through epigenetic modification of homeostatic setpoints [67]. For example, initial exposure to moldy workplaces changed the immune response to later mold exposure. Peripheral blood mononuclear cells from mold-exposed people produced significantly different patterns of cytokine and chemokine secretion than those of unexposed controls [68]. Additionally, roughly 25% of Americans carry major histocompatibility complex gene variants thought to make them vulnerable to prolonged inflammation when exposed to mold. Autoimmune problems and changes in brain structure/function are common in these individuals following mold exposure (see Table 3 in [69]). Kraft and coworkers [70] pointed out that mold exposure can be particularly detrimental in people already suffering from immune dysregulation, such as those with chronic inflammatory disorders or HIV, because mold exacerbates existing pathologies.
People living or working in water-damaged buildings are often exposed to molds for extended periods resulting in prolonged innate immune activation of the type already demonstrated to be neurotoxic in a variety of neural diseases [71, 72]. Patients subjected to chronic mold exposure develop a wide range of immune abnormalities, including systemic and neural inflammation, neural autoantibodies, mitochondrial damage, and compromised energy production [12, 73, 74]. They also show diverse neurological abnormalities including movement disorders, pain syndromes, impaired balance, and cognitive issues [11, 12]. Mold exposure, like that to bacteria and viruses, has been hypothesized to contribute to the pathogenesis of a variety of neurodegenerative diseases. The strongest evidence is for Alzheimer’s disease [75–77]. A variety of fungal species have been identified in the brains of Alzheimer’s disease patients, and the order in which brain areas were affected led researchers to suggest that olfactory inhalation was the most likely route of exposure [78]. Researchers have also suggested that fungal exposure contributes to the etiology of Parkinson’s disease [65, 79, 80], and a plethora of autoimmune disorders including multiple sclerosis [81], amyotrophic lateral sclerosis [82], and chronic fatigue syndrome [12, 64].
Mold-damaged buildings are clearly a problem. Floods, building and plumbing leaks result in widespread mold contamination. Overall estimates of water-damaged buildings with possible mold contamination in North America ranged from 27-36% in questionnaire studies to 42-56% when air was actually sampled [58]. Remediation is problematic. Improper remediation techniques cause high, often continuing, levels of exposure. Some remediation methods actually increase toxin production, and no method tested resulted in total elimination of viable mold [63, 83].
The extent of the contribution of mold exposure to neural and behavioral dysfunction in humans under ecologically-relevant conditions remains to be determined. Our data document for the first time that exposure to known quantities of characterized mold stimuli, both toxic and nontoxic, affected spatial learning. Performance of mold-exposed subjects was correlated with levels of hippocampal inflammation. These data further support our contention that exposure to both toxic and nontoxic molds results in a diverse array of cognitive and emotional problems through innate immune activation. Just the presence of mold, like that of bacteria or viruses, can trigger PRRs causing innate immune activation. Active infection is not needed. Toxins are not needed. Mold exposure, both toxic and nontoxic, must be considered a risk factor, like organic chemical exposure or air pollution, that can increase an individual’s inflammatory burden with possible serious consequences for health and behavior.
Highlights.
Inhalation of mold spores affected Morris water maze performance.
Nontoxic mold inhalation interfered with long-term memory in the Morris water maze.
Toxic mold inhalation appeared to increase motivation to reach the escape platform.
Performance during training was correlated with levels of brain inflammation.
These data support our hypothesis that innate immune activation is one mechanism through which both toxic and nontoxic mold stimuli affect brain and behavior.
Acknowledgments:
We thank Dr. Dorr Dearborn, Case Western Reserve University for providing mold spores and his protocols for spore handling. We thank Drs. Dearborn and J. David Miller for helpful discussions in framing this research. We thank Nial Adams, Gina Manes, and David Morris for assistance with animal handling, treatments and testing, and Edna Normand and Shana Uvaydov for assisting with immunohistochemical analyses.
Funding:
This research was supported by Grant 1SC2 MH085472 from NIMH/NIGMS to CFH, CUNY CIRG 1937 to CFH & CLP, PSC CUNY Grants 69172-0037, 61214-0039, 65379-0043 to CFH and TRADA-43-687, TRADA-44-632, TRADA-46-257 to CLP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authorship contribution statement
CFH designed the experiments, obtained funding, oversaw treatments and behavioral testing, analyzed data, and wrote the first draft. DL prepared treatments, assisted with treatments, testing, and final analyses. RP and RDS coordinated treatments, behavioral testing, and data acquisition, KGP and LAS did the IL-1β analyses, TR assisted in treatments, testing, and data analyses. JCF grew spore stock, assisted in treatments, and behavioral testing. KDG assisted in software management and analysis. CLP obtained funding, supervised tissue analysis, helped interpret data, and write the manuscript. All authors read and helped to revise the manuscript.
The authors have no conflicts to report.
References
- [1].Heseltine E, Rosen JB, WHO guidelines for indoor air quality: Dampness and mould, (2009) 228pp. [PubMed] [Google Scholar]
- [2].Empting L, Neurologic and neuropsychiatric syndrome features of mold and mycotoxin exposure, Toxicol. Ind. Health 25(9-10) (2009) 577–581. [DOI] [PubMed] [Google Scholar]
- [3].Rea WJ, A large case-series of successful treatment of patients exposed to mold and mycotoxin, Clin Ther. 40(6) (2018) 889–893. [DOI] [PubMed] [Google Scholar]
- [4].Ratnaseelan AM, Tsilioni I, Theoharides TC, Effects of mycotoxins on neuropsychiatric symptoms and immune processes, Clin. Ther 40(6) (2018) 903–917. [DOI] [PubMed] [Google Scholar]
- [5].Hyvönen S, Lohi J, Tuuminen T, Moist and mold exposure is associated with high prevalence of neurological symptoms and MCS in a Finnish hospital workers cohort, Saf. Health Work 11(2) (2020) 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pettigrew HD, Selmi CF, Teuber SS, Gershwin ME, Mold and human health: Separating the wheat from the chaff, Clin. Rev. Allergy Immunol 38(2-3) (2010) 148–155. [DOI] [PubMed] [Google Scholar]
- [7].Hurraß J, Heinzow B, Aurbach U, Bergmann KC, Bufe A, Buzina W, Cornely OA, Engelhart S, Fischer G, Gabrio T, Heinz W, Herr CEW, Kleine-Tebbe J, Klimek L, Köberle M, Lichtnecker H, Lob-Corzilius T, Merget R, Mülleneisen N, Nowak D, Rabe U, Raulf M, Seidl HP, Steiß JO, Szewszyk R, Thomas P, Valtanen K, Wiesmüller GA, Medical diagnostics for indoor mold exposure, Int. J. Hyg. Environ. Health 220(2 Pt B) (2017) 305–328. [DOI] [PubMed] [Google Scholar]
- [8].Borchers AT, Chang C, Gershwin ME, Mold and human health: A reality check, Clin. Rev. Allergy Immunol 52(3) (2017) 305–322. [DOI] [PubMed] [Google Scholar]
- [9].Nordin S, Mechanisms underlying nontoxic indoor air health problems: A review, Int. J. Hyg. Environ. Health 226 (2020) 113489. [DOI] [PubMed] [Google Scholar]
- [10].Shoemaker RC, House D, Ryan JC, Structural brain abnormalities in patients with inflammatory illness acquired following exposure to water-damaged buildings: A volumetric MRI study using Neuroquant(r), Neurotoxicol. Teratol 45 (2014) 18–26. [DOI] [PubMed] [Google Scholar]
- [11].Kilburn KH, Neurobehavioral and pulmonary impairment in 105 adults with indoor exposure to molds compared to 100 exposed to chemicals, Toxicol. Ind. Health 25(9-10) (2009) 681–692. [DOI] [PubMed] [Google Scholar]
- [12].Morris G, Berk M, Walder K, Maes M, The putative role of viruses, bacteria, and chronic fungal biotoxin exposure in the genesis of intractable fatigue accompanied by cognitive and physical disability, Mol. Neurobiol 53(4) (2016) 2550–2571. [DOI] [PubMed] [Google Scholar]
- [13].Harding CF, Pytte CL, Page KG, Ryberg KJ, Normand E, Remigio GJ, DeStefano RA, Morris DB, Voronina J, Lopez A, Stalbow LA, Williams EP, Abreu N, Mold inhalation causes innate immune activation, neural, cognitive and emotional dysfunction, Brain Behav. Immun 87 (2020) 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dantzer R, Kelley KW, Twenty years of research on cytokine-induced sickness behavior, Brain Behav. Immun 21(2) (2007) 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McCusker RH, Kelley KW, Immune-neural connections: How the immune system’s response to infectious agents influences behavior, J. Exp. Biol 216(Pt 1) (2013) 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yirmiya R, Goshen I, Immune modulation of learning, memory, neural plasticity and neurogenesis, Brain Behav. Immun 25 (2011) 181–213. [DOI] [PubMed] [Google Scholar]
- [17].Lawson LJ, Perry VH, Dri P, Gordon S, Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain, Neuroscience. 39(1) (1990) 151–179. [DOI] [PubMed] [Google Scholar]
- [18].Cunningham C, Sanderson DJ, Malaise in the water maze: Untangling the effects of LPS and IL-1beta on learning and memory, Brain Behav. Immun 22 (2008) 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dambuza M, Levitz SM, Netea MG, Brown GD, Fungal recognition and host defense mechanisms, Microbiology Spectrum. 5(4) (2017) doi: 10.1128/microbiolspec.FUNK-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Beijer L, Thorn J, Rylander R, Effects after inhalation of (1-->3)-beta-D-glucan and relation to mould exposure in the home., Mediators Inflamm. 11(3) (2002) 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Leino M, Makela M, Reijula K, Haahtela T, Mussalo-Rauhamaa H, Tuomi T, Hintikka EL, Alenius H, Intranasal exposure to a damp building mould, Stachybotrys chartarum, induces lung inflammation in mice by satratoxin-independent mechanisms, Clin. Exp. Allergy 33(11) (2003) 1603–1610. [DOI] [PubMed] [Google Scholar]
- [22].Yike I, Rand TG, Dearborn DG, Acute inflammatory responses to Stachybotrys chartarum in the lungs of infant rats: Time course and possible mechanisms., Toxicol Sci. 84(2) (2005) 408–417. [DOI] [PubMed] [Google Scholar]
- [23].Ammann HM, Inhalation exposure and toxic effects of mycotoxins, in: LI DW (Ed.), Biology of Microfungi: Fungal Biology. Springer International, New York, 2016, pp. 495–523. [Google Scholar]
- [24].Gordon WA, Cantor JB, Johanning E, Charatz HJ, Ashman TA, Breeze JL, Haddad L, Abramowitz S, Cognitive impairment associated with toxigenic fungal exposure: A replication and extension of previous findings, Appl. Neuropsychol 11(2) (2004) 65–74. [DOI] [PubMed] [Google Scholar]
- [25].Hossain MA, Ahmed MS, Ghannoum MA, Attributes of Stachybotrys chartarum and its association with human disease, J. Allergy Clin. Immunol 113(2) (2004) 200–209. [DOI] [PubMed] [Google Scholar]
- [26].Islam Z, Harkema JR, Pestka JJ, Satratoxin G from the black mold Stachybotrys chartarum evokes olfactory sensory neuron loss and inflammation in the murine nose and brain, Environ. Health Perspect 114(7) (2006) 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kankkunen P, Rintahaka J, Aalto A, Leino M, Majuri ML, Alenius H, Wolff H, Matikainen S, Trichothecene mycotoxins activate inflammatory response in human macrophages., J Immunol. 182(10) (2009) 6418–6425. [DOI] [PubMed] [Google Scholar]
- [28].Rand TG, Sun M, Gilyan A, Downey J, Miller JD, Dectin-1 and inflammation-associated gene transcription and expression in mouse lungs by a toxic (1,3)-beta-D: glucan, Arch. Toxicol 84 (2010) 205–220. [DOI] [PubMed] [Google Scholar]
- [29].Crawley JN, What’s wrong with my mouse?: Behavioral phenotyping of transgenic and knockout mice, (2007) 544pp. [Google Scholar]
- [30].Rosenblum Lichtenstein JH, Molina RM, Donaghey TC, Brain JD, Strain differences influence murine pulmonary responses to Stachybotrys chartarum, Am. J. Respir. Cell. Mol. Biol 35(4) (2006) 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rosenblum Lichtenstein JH, Molina RM, Donaghey TC, Amuzie CJ, Pestka JJ, Coull BA, Brain JD, Pulmonary responses to Stachybotrys chartarum and its toxins: Mouse strain affects clearance and macrophage cytotoxicity, Toxicol. Sci 116(1) (2010) 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lyte M, Opitz N, Goehler LE, Gaykema RP, Overmeier JB, Recommended housing conditions and test procedures can interact to obscure a significant experimental effect, Behav. Res. Methods 37 (2005) 651–656. [DOI] [PubMed] [Google Scholar]
- [33].Amuzie CJ, Islam Z, Kim JK, Seo JH, Pestka JJ, Kinetics of satratoxin G tissue distribution and excretion following intranasal exposure in the mouse, Toxicol. Sci 116(2) (2010) 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cooley JD, Wong WC, Jumper CA, Hutson JC, Williams HJ, Schwab CJ, Straus DC, An animal model for allergic penicilliosis induced by the intranasal instillation of viable Penicillium chrysogenum conidia, Thorax. 55 (2000) 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gordon CJ, The mouse thermoregulatory system: Its impact on translating biomedical data to humans, Physiol. Behav 179 (2017) 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gibertini M, Cytokines and cognitive behavior, NeuroImmunomodulat. 5 (1998) 160–165. [DOI] [PubMed] [Google Scholar]
- [37].Gulinello M, Gertner M, Mendoza G, Schoenfeld BP, Oddo S, LaFerla F, Choi CH, McBride SM, Faber DS, Validation of a 2-day water maze protocol in mice, Behav. Brain Res 196(2) (2009) 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vorhees CV, Williams MT, Assessing spatial learning and memory in rodents, ILAR J. 55(2) (2014) 310–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Garthe A, Kempermann G, An old test for new neurons: Refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis, Front. Neurosci 7 (2013) 63(1-11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].File SE, Factors controlling measures of anxiety and responses to novelty in the mouse, Behav. Brain Res. 25 (2001) 131–157. [DOI] [PubMed] [Google Scholar]
- [41].McDonald JH, Handbook of Biological Statistics, (2014) [Google Scholar]
- [42].Gallagher M, Burwell R, Burchinal M, Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze., Behav Neurosci. 129(4) (2015) 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Iivonen H, Nurminen L, Harri M, Tanila H, Puolivali J, Hypothermia in mice tested in Morris water maze, Behav. Brain Res 141 (2003) 207–213. [DOI] [PubMed] [Google Scholar]
- [44].Garlanda C, Dinarello CA, Mantovani A, The interleukin-1 family: Back to the future., Immunity. 39(6) (2013) 1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Osterhout JA, Kapoor V, Eichhorn SW, Vaughn E, Moore JD, Liu D, Lee D, DeNardo LA, Luo L, Zhuang X, Dulac C, A preoptic neuronal population controls fever and appetite during sickness., Nature. 606(7916) (2022) 937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lasselin J, Treadway MT, Lacourt TE, Soop A, Olsson MJ, Karshikoff B, Paues-Göranson S, Axelsson J, Dantzer R, Lekander M, Lipopolysaccharide alters motivated behavior in a monetary reward task: A randomized trial., Neuropsychopharmacology. 42(4) (2017) 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Oitzl MS, van Oers H, Schobitz B, de Kloet ER, Interleukin-1β, but not interleukin-6, impairs spatial navigation learning, Brain Res. 613 (1993) 160–163. [DOI] [PubMed] [Google Scholar]
- [48].Herrero AI, Sandi C, Venero C, Individual differences in anxiety trait are related to spatial learning abilities and hippocampal expression of mineralocorticoid receptors, Neurobiol. Learn. Mem 86(2) (2006) 150–159. [DOI] [PubMed] [Google Scholar]
- [49].Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, From inflammation to sickness and depression: When the immune system subjugates the brain., Nat Rev Neurosci. 9(1) (2008) 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Drummond RA, Gaffen SL, Hise AG, Brown GD, Innate defense against fungal pathogens, Cold Spring Harb. Perspect. Med 5(6) (2015) a019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rice JP, Wallace DG, Hamilton DA, Lesions of the hippocampus or dorsolateral striatum disrupt distinct aspects of spatial navigation strategies based on proximal and distal information in a cued variant of the Morris water task, Behav. Brain Res 289 (2015) 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bohlen M, Hayes ER, Bohlen B, Bailoo JD, Crabbe JC, Wahlsten D, Experimenter effects on behavioral test scores of eight inbred mouse strains under the influence of ethanol, Behav. Brain Res 272 (2014) 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS, Olfactory exposure to males, including men, causes stress and related analgesia in rodents, Nat. Methods 11(6) (2014) 629–632. [DOI] [PubMed] [Google Scholar]
- [54].Rosenberger K, Derkow K, Dembny P, Krüger C, Schott E, Lehnardt S, The impact of single and pairwise toll-like receptor activation on neuroinflammation and neurodegeneration, J. Neuroinflammation 11 (2014) 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Eduard W, Fungal spores: A critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting, Crit. Rev. Toxicol 39(10) (2009) 799–864. [DOI] [PubMed] [Google Scholar]
- [56].Nevalainen A, Täubel M, Hyvärinen A, Indoor fungi: Companions and contaminants, Indoor Air. 25(2) (2015) 125–156. [DOI] [PubMed] [Google Scholar]
- [57].Miller JD, McMullin DR, Fungal secondary metabolites as harmful indoor air contaminants: 10 years on, Appl. Microbiol. Biotechnol 98(24) (2014) 9953–9966. [DOI] [PubMed] [Google Scholar]
- [58].Gorny RL, Reponen T, Willeke K, Schmechel D/, Robine E, Boissier M, Grinshpun SA, Fungal fragments as indoor air biocontaminants, Appl. Environ. Microbiol 68(7) (2002) 3522–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Adhikari A, Reponen T, Rylander R, Airborne fungal cell fragments in homes in relation to total fungal biomass, Indoor Air. 23(2) (2013) 142–147. [DOI] [PubMed] [Google Scholar]
- [60].Tonelli LH, Postolache TT, Airborne inflammatory factors: “from the nose to the brain”, Front. Biosci 2 (2010) 135–152. [DOI] [PubMed] [Google Scholar]
- [61].Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roqué PJ, Neurotoxicity of traffic-related air pollution, Neurotoxicology. 59 (2017) 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jayaraj RL, Rodriguez EA, Wang Y, Block ML, Outdoor ambient air pollution and neurodegenerative diseases: The neuroinflammation hypothesis, Curr. Environ. Health Rep 4(2) (2017) 166–179. [DOI] [PubMed] [Google Scholar]
- [63].Peitzsch M, Bloom E, Haase R, Must A, Larsson L, Remediation of mould damaged building materials--efficiency of a broad spectrum of treatments, J. Environ. Monit 14(3) (2012) 908–915. [DOI] [PubMed] [Google Scholar]
- [64].Brewer JH, Thrasher JD, Straus DC, Madison RA, Hooper D, Detection of mycotoxins in patients with chronic fatigue syndrome, Toxins (Basel). 5(4) (2013) 605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Inamdar AA, Hossain MM, Bernstein AI, Miller GW, Richardson JR, Bennett JW, Fungal-derived semiochemical 1-octen-3-ol disrupts dopamine packaging and causes neurodegeneration, Proc. Natl. Acad. Sci. USA 110(48) (2013) 19561–19566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bilbo SD, Frank a. Beach award: Programming of neuroendocrine function by early-life experience: A critical role for the immune system, Horm. Behav 63(5) (2013) 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tchessalova D, Posillico CK, Tronson NC, Neuroimmune activation drives multiple brain states, Front. Syst. Neurosci 12(39) (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rosenblum Lichtenstein JH, Hsu YH, Gavin IM, Donaghey TC, Molina RM, Thompson KJ, Chi CL, Gillis BS, Brain JD, Environmental mold and mycotoxin exposures elicit specific cytokine and chemokine responses, PLoS One. 10(5) (2015) e0126926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Shoemaker RC, Heyman A, Mancia A, Ryan JC, Inflammation induced chronic fatiguing illnesses: A steady march towards understanding mechanisms and identifying new biomarkers and therapies, Internal Med. Rev 3(10) (2017) 1–29. [Google Scholar]
- [70].Kraft S, Buchenauer L, Polte T, Mold, mycotoxins and a dysregulated immune system: A combination of concern, Int. J.Mol. Sci 22(12269) (2021) 12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sankowski R, Mader S, Valdés-Ferrer SI, Systemic inflammation and the brain: Novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration, Front. Cell. Neurosci 9(28) (2015) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ransohoff RM, How neuroinflammation contributes to neurodegeneration, Science. 353(6301) (2016) 777–783. [DOI] [PubMed] [Google Scholar]
- [73].Abou-Donia MB, Lieberman A, Curtis L, Neural autoantibodies in patients with neurological symptoms and histories of chemical/mold exposures, Toxicol. Ind. Health 34(1) (2018) 44–53. [DOI] [PubMed] [Google Scholar]
- [74].Lieberman A, Curtis L, Mold exposure and mitochondrial antibodies, Altern. Ther. Health Med 26(6) (2020) 44–47. [PubMed] [Google Scholar]
- [75].Pisa D, Alonso R, Rábano A, Rodal I, Carrasco L, Different brain regions are infected with fungi in Alzheimer’s disease, Sci. Rep 5(15015) (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bredesen DE, Inhalational Alzheimer’s disease: An unrecognized—and treatable—epidemic, Aging Dis. 8(2) (2016) 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pisa D, Alonso R, Rábano A, Carrasco L, Corpora amylacea of brain tissue from neurodegenerative diseases are stained with specific antifungal antibodies., Front. Neurosci 10(86) (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Alonso R, Pisa D, Aguado B, Carrasco L, Identification of fungal species in brain tissue from Alzheimer’s disease by next-generation sequencing., J.Alzheimers Dis 58(1) (2017) 55–67. [DOI] [PubMed] [Google Scholar]
- [79].Fernández-Espejo E, Microorganisms that are related with increased risk for Parkinson’s disease, Neurologia (Engl Ed). (2020) S0213-4853(20)30301. [DOI] [PubMed] [Google Scholar]
- [80].Arce-López B, Alvarez-Erviti L, De Santis B, Izco M, López-Calvo S, Marzo-Sola ME, Debegnach F, Lizarraga E, López de Cerain A, González-Peñas E, Vettorazzi A, Biomonitoring of mycotoxins in plasma of patients with Alzheimer’s and Parkinson’s disease, Toxins (Basel). 13(477) (2021) toxins13070477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Dehhaghi M, Kazemi Shariat Panahi H, Guillemin GJ, Microorganisms’ footprint in neurodegenerative diseases., Front. Cell. Neurosci 12(466) (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].French PW, Ludowyke R, Guillemin GJ, Fungal neurotoxins and sporadic amyotrophic lateral sclerosis, Neurotox. Res 35(4) (2019) 969–980. [DOI] [PubMed] [Google Scholar]
- [83].Tuuminen T, Lohi J, Immunological and toxicological effects of bad indoor airto cause dampness and mold hypersensitivity syndrome, AIMS Allergy and Immunology. 2(4) (2019) 190–204. [Google Scholar]


