Abstract
Purpose
To determine whether surgeon volume affects revision rate following primary anterior cruciate ligament reconstruction (ACLR) with allograft and to determine whether surgeon volume impacts allograft tissue type used.
Methods
All patients aged 14 years or older who underwent primary allograft ACLR at a large hospital system between January 2015 to December 2019 with minimum 2-year follow-up were included. Patients with double-bundle ACLR, multiligament reconstruction, and absent allograft type data were excluded. Surgeon volume was categorized as 35 or more ACLR/year for high-volume surgeons and less than 35 ACLR/year for low-volume surgeons. Revision was defined as subsequent ipsilateral ACLR. Patient characteristics, operative details, allograft type, and revision ACLR rates were retrospectively collected. Revision rate and allograft type were analyzed based on surgeon volume.
Results
A total of 457 primary allograft ACLR cases (mean age: 38.8 ± 12.3 years) were included. Low-volume surgeons experienced greater revision rates (10% vs 5%, P = .04) and used allograft in a younger population (37.6 vs 40.0 years old, P = .03) than high-volume surgeons. Subgroup analysis of the total cohort identified a significantly increased failure rate in patients <25 years old compared with ≥25 years old (30% vs 4%, P < .001). Allograft type selection varied significantly between surgeon volume groups, with low-volume surgeons using more bone–patellar tendon–bone (P < .001) and less semitendinosus allograft (P = .01) than high-volume surgeons. No differences in revision rate were observed based on allograft type (P = .71).
Conclusions
There was a greater revision rate following primary allograft ACLR among low-volume surgeons compared with high-volume surgeons. Low-volume surgeons also used allograft in a younger population than did high-volume surgeons.
Level of Evidence
Level III, retrospective comparative prognostic trial.
Graft selection in primary anterior cruciate ligament reconstruction (ACLR) remains a controversial topic. Allograft is generally recognized as inferior to autograft, as numerous studies have demonstrated increased failure rates in young patients with the use of allograft tissue.1, 2, 3, 4 Despite this, registry and survey-based epidemiologic studies have reported the steady use of allograft over the last 2 decades.5,6 This may be attributed to the benefits of allograft, which include avoidance of donor-site morbidity, preservation of autogenous tissue, decreased surgical times, and improved cost-effectiveness compared with autograft.7,8 Furthermore, the use of nonirradiated allograft has demonstrated similar failure rates and patient-reported outcomes compared with autograft in nonadolescent patients.9,10
The importance of strict indications with allograft use has been elucidated over the past few decades, although indications vary by surgeon.11 Reported rates of allograft use in primary ACLR vary between 1%5 and 42%12 and are influenced by numerous factors, including patient age, body mass index (BMI), sex,13 the country in which surgery is performed,14 and surgeon volume and fellowship-training status.13 The influence of surgeon characteristics on graft choice is particularly relevant when considering failure of ACLR, as increasing evidence highlights low surgeon ACLR volume as a risk factor for graft failure and subsequent ipsilateral knee surgery.15,16
The reasons for the increased ACLR failure rate in low-volume surgeons remain incompletely understood. Technical error, which is one of the most common causes of failure of ACLR, likely contributes.17,18 Femoral tunnel malposition in particular has been cited as the most frequent error18,19 and has been demonstrated to be more prevalent in low-volume compared with high-volume surgeons.19 Another possible explanation is the increased use of allograft tissue, which has been shown to be more widespread in low-volume surgeons.13 The influence of surgeon volume on failure rates in patients undergoing allograft ACLR is poorly understood. Allograft is an important variable because of the strict indications for its use, particularly older patients with lower demands who undergo ACLR, that plausibly could vary by low- versus high-volume ACLR surgeons.20, 21, 22
The purposes of this study were to determine whether surgeon volume affects revision rate following primary ACLR with allograft and to determine whether surgeon volume impacts allograft tissue type used. The hypotheses were that low-volume surgeons would experience increased revision rates following primary ACLR with allograft compared with high-volume surgeons and that surgeon volume would not impact allograft type selection for primary ACLR.
Methods
This retrospective study was approved by the institutional review board (study ID: #19030196) and included data on patients who received primary allograft ACLR performed at a large hospital network from January 2015 through December 2019 with minimum 2-year follow-up. The following exclusion criteria were used for our data set: patients younger than 14 years old, double-bundle ACLR, revision ACLR as the first recorded procedure over that timeframe, multiligament knee reconstruction, and missing allograft type data.
Data on patient characteristics (age, sex, BMI, laterality), intraoperative details (allograft type, procedure time), and ipsilateral revision ACLR were collected from the chart. Revision ACLR was used as a proxy for failure. Although failure may also be defined through patient-reported outcomes, the primary concern with allograft ACLR has generally been an increased graft re-rupture and thus revision rate.7 Allograft tissue type was categorized into Achilles, bone–patellar tendon–bone (BPTB), tibialis (combined tibialis anterior and posterior), and semitendinosus. Soft tissue allografts of unknown exact tissue type, generally pre-sutured allografts, were excluded. The primary outcome was ipsilateral revision ACLR. Revision rate was determined for high- and low-volume surgeons separately. Surgeon volume was categorized as ≥35 ACLR per year for high-volume surgeons and <35 ACLR per year for low-volume surgeons, based on previous literature demonstrating an increased risk for subsequent knee surgery among surgeons performing <35 ACLR/year.16
Statistical Analyses
Categorical variables were compared between groups with the χ2 or Fisher exact test. Two group comparisons of continuous variables employed the independent samples t-test, and multigroup comparisons of continuous variables used the one-way analysis of variance F-test. Post-hoc comparisons were adjusted for multiplicity with the Benjamini–Hochberg method. Dichotomous variables were presented by using proportion (%), whereas the mean and standard deviation were used to present continuous and ordinal data. A logistic regression of revision on continuous age and dichotomous surgeon volume was performed. All tests were 2-sided and were performed in SAS, version 9.4 (SAS Institute, Cary, NC).
Results
A total of 3,735 ACLRs were screened for inclusion, of which 896 cases used allograft. After excluding patients younger than 14 years old (n = 19), revision ACLRs (n = 174), multiligament reconstructions (n = 112), and absent allograft type data (n = 134), 457 patients were included for analysis. There were 228 allograft ACLR completed by 7 high-volume surgeons and 229 allograft ACLR completed by 14 low-volume surgeons. High-volume surgeons performed an average of 54 ACLR per year, of which allograft accounted for 17% of total cases, and low-volume surgeons performed an average of 13 ACLR per year, of which allograft accounted for 36% of total cases (Fig 1).
Fig 1.
Flowchart of included patients. (ACLR, anterior cruciate ligament reconstruction.)
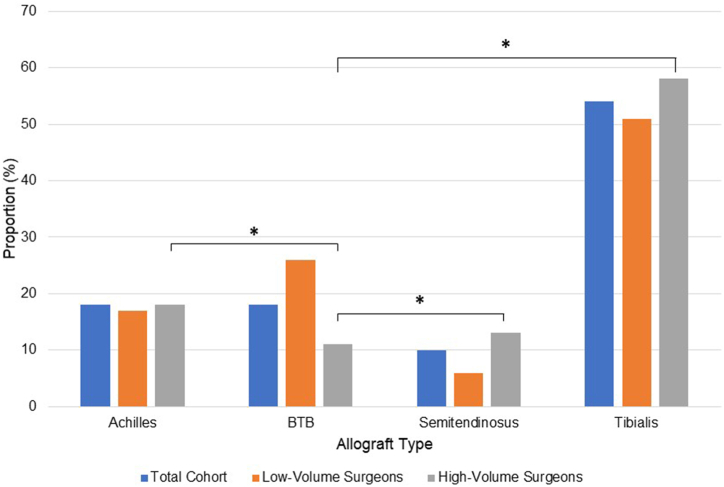
The mean age of the cohort was 38.8 ± 12.3 years, and 47% of the study population was female. The average BMI among the 431 patients with available data was 29.8 ± 6.3. In the total cohort, tibialis was the most commonly used allograft (54%), followed by Achilles (18%), BPTB (18%), and semitendinosus (10%). Allograft selection was significantly impacted by surgeon volume, with high-volume surgeons using less BPTB allograft compared with Achilles (P = .01), hamstring (P < .0006), and tibialis allografts (P = .001) (Table 1 and Fig 2). Achilles and tibialis allograft use was not significantly different between low- and high-volume surgeons (P > .05) (Table 1). No significant difference in revision rate was found between the different allograft tissue types (P > .05) (Table 1).
Table 1.
Comparison of Demographic Characteristics and Revision Rate Based on Allograft Tissue Type
| Allograft Type |
|||||
|---|---|---|---|---|---|
| Achilles (n = 81) | BPTB (n = 84) | SemiT (n = 44) | Tibialis (n = 248) | P | |
| Age, y, mean ± SD | 40.4 ± 12.2 | 41.6 ± 11.2 | 39.2 ± 13.9 | 37.3 ± 12.2 | .02 |
| Sex, male, % | 54 | 61 | 50 | 50 | .34 |
| BMI, mean ± SD | 30.5 ± 7.7 (n = 79) | 29.7 ± 5.9 (n = 83) | 29.8 ± 5.0 (n = 41) | 29.7 ± 6.2 (n = 228) | .76 |
| Laterality, right side, % | 47 (n = 79) | 46 (n = 83) | 43 (n = 41) | 46 (n = 228) | .98 |
| Procedure time, min,∗ mean ± SD | 85.7 ± 39.8 (n = 80) | 98.2 ± 44.6 (n = 82) | 95.1 ± 24.8 (n = 43) | 81.3 ± 33.3 (n = 247) | .001 |
| Graft use by high-volume surgeons† (%) | 52 | 30 | 68 | 53 | <.001 |
| Revision, % | 5 | 10 | 9 | 8 | .71 |
Bold indicates statistical significance at P < .05.
BMI, body mass index; BPTB, bone–patellar tendon–bone; SD, standard deviation; SemiT, semitendinosus, Tibialis, tibialis anterior or posterior.
Benjamini-Hochberg adjusted P values for procedure time: Achilles vs BPTB (.12); Achilles vs semitendinosus (.16); Achilles vs tibialis (.45); BPTB vs semitendinosus (.63); BPTB vs tibialis (.01); semitendinosus vs tibialis (.01).
Benjamini–Hochberg adjusted P values for graft utilization by high-volume surgeons: Achilles vs BPTB (.01); Achilles vs semitendinosus (.09); Achilles vs tibialis (.88); BPTB vs semitendinosus (<.001); BPTB vs tibialis (.001); semitendinosus vs tibialis (.09).
Fig 2.
Allograft type use across total cohort and based on surgeon volume. ∗Indicates statistical significance at P < .05. Post-hoc comparisons for allograft type: Achilles (0.70), BPTB (<0.001), semitendinosus (0.01), tibialis (0.17). (BPTB, bone–patellar tendon–bone.)
Revision rate following primary allograft ACLR differed significantly between low-volume and high-volume surgeons (10% vs 5%, respectively; P = .04). Further, the mean age of patients in the low-volume group was lower than patients in the high-volume group (37.6 years vs 40.0 years, respectively; P = .03), and low-volume surgeons had shorter procedure times than high-volume surgeons (81.7 vs 91.1 minutes; P = .01) (Table 2). Patients who subsequently underwent a revision ACLR were found to be younger (27.5 years vs 39.8 years, P < .001) and have longer primary ACLR procedure times (116.8 vs 84.0 minutes, P < .001) than patients who did not require a revision ACLR (Table 3). These findings were also apparent when analyzing the high-volume and low-volume cohorts separately (Tables 4 and 5). Subgroup analysis of the total cohort additionally identified a 30% failure rate following primary allograft ACLR in patients <25 years old, compared with a 4% failure rate in patients ≥25 years old (P < .001).
Table 2.
Comparison of Demographic Characteristics and Revision Rate Based on Surgeon Volume
| Surgeon Volume | |||
|---|---|---|---|
| Low-Volume (n = 229) | High-Volume (n = 228) | P | |
| Age, y, mean ± SD | 37.6 ± 13.1 | 40.0 ± 11.3 | .03 |
| Sex, male, % | 54 | 51 | .48 |
| BMI, mean ± SD | 30.3 ± 6.5 (n = 204) | 29.4 ± 6.2 (n = 227) | .11 |
| Laterality, right, % | 47 | 45 | .60 |
| Procedure time, min, mean ± SD | 81.7 ± 36.9 (n=224) | 91.1 ± 35.9 | .01 |
| Revision, % | 10 | 5 | .04∗ |
Bold indicates statistical significance at P < .05.
BMI, body mass index; SD, standard deviation; Tibialis, tibialis anterior or posterior.
The adjusted P value is .22 after controlling for age in a logistic regression.
Table 3.
Comparison Of Demographic Characteristics Between Patients With Nonrevision and Revision
| Revision | |||
|---|---|---|---|
| Nonrevision (n = 421) | Revision (n = 36) | P | |
| Age, y, mean ± SD | 39.8 ± 11.8 | 27.5 ± 12.1 | <.001 |
| Sex, male, % | 52 | 64 | .15 |
| BMI, mean ± SD | 30.0 ± 6.4 n = 404) | 27.9 ± 5.2 (n = 27) | .10 |
| Laterality, right, % | 45 | 53 | .39 |
| Procedure time, min, mean ± SD | 84.0 ± 35.1 (n = 419) | 116.8 ± 42.2 (n = 33) | <.0001 |
Bold indicates statistical significance at P < .05.
BMI, body mass index; SD, standard deviation.
Table 4.
Comparison of Nonrevision Versus Revision Patients Among Those Patients Who Were Operated on by a Low-Volume Surgeon
| Revision | |||
|---|---|---|---|
| Nonrevision (n = 205) | Revision (n = 24) | P | |
| Age, y, mean ± SD | 38.9 ± 12.8 | 26.5 ± 11.0 | <.001 |
| Sex, male, % | 54 | 58 | .66 |
| BMI, mean ± SD | 30.6 ± 6.5 (n = 189) | 27.6 ± 5.3 (n = 15) | .09 |
| Laterality, right, (%) | 46 | 58 | .25 |
| Procedure time, min, mean ± SD | 78.3 ± 34.5 (n = 203) | 114.1 ± 43.9 (n = 21) | <.0001 |
| Graft type (soft-tissue), (%)∗ | 57 | 63 | .58 |
Bold indicates statistical significance at P < .05.
BMI, body mass index; BPTB, bone–patellar tendon–bone; SD, standard deviation.
Soft-tissue = semitendinosus and tibialis anterior; non-soft tissue = BPTB and Achilles.
Table 5.
Comparison of Nonrevision Versus Revision Patients Among Those Patients Who Were Operated on by a High-Volume Surgeon
| Revision | |||
|---|---|---|---|
| Non-Revision (n = 216) | Revision (n = 12) | P | |
| Age, y, mean ± SD | 40.6 ± 10.9 | 29.6 ± 14.2 | <.001 |
| Sex, male, % | 50 | 75 | .09 |
| BMI, mean ± SD | 29.4 ± 6.2 (n = 215) | 28.3 ± 5.3 | .53 |
| Laterality, right, % | 45 | 42 | .83 |
| Procedure time, min, mean ± SD | 89.4 ± 34.9 | 121.4 ± 40.6 | .002 |
| Graft type (soft-tissue), %∗ | 70 | 75 | 1.00 |
Bold indicates statistical significance at P < .05.
BMI, body mass index; BPTB, bone–patellar tendon–bone; SD, standard deviation.
Soft-tissue = semitendinosus and tibialis anterior; non-soft tissue = BPTB and Achilles.
No significant differences in revision rate were observed between male and female patients (9.6% vs 6.0%, respectively; P = .15) in the total cohort. However, among female patients, low-volume surgeons had a greater failure rate compared with high-volume surgeons (10% vs 3%, P = .03), whereas no difference in failure rate was observed among male patients (11% vs 8%, P = .35).
Discussion
The most important finding of this study is the significantly greater revision rate following primary ACLR with allograft observed in low-volume surgeons compared with high-volume surgeons. This finding is consistent with previous literature showing that patients have a decreased rate of subsequent ipsilateral knee surgery after undergoing primary ACLR by high-volume compared with low-volume surgeons.15,16
There are several possible explanations for this, with a primary candidate being age-based indications for allograft use in primary ACLR. Low-volume surgeons used allograft in a significantly younger population than high-volume surgeons, and revised patients were substantially younger than nonrevised patients. Further, when we controlled for the impact of age, the difference in revision rate in our study became nonsignificant, indicating age-related indications for allograft use may play an important role in our findings. Relatively strict indications for allograft use, especially based on patient age and activity level, should be considered by all surgeons. It is well-documented that younger patients experience greater failure rates with allograft ACLR compared with autograft. Several studies have reported failure rates up to 40% for allograft use in younger patients, and a 3-fold increase in failure rates when comparing allograft with autograft in this population.4,23 Conversely, allograft is better suited for patients aged 35 to 40 years or older, with recent studies demonstrating failure rates under 10% in this population with acceptable patient-reported outcome scores.21,24 Allograft use for primary ACLR may be an appealing choice due to the avoidance of donor site morbidity and decreased procedure times,25 yet these benefits must be weighed against the greater failure rates observed in some populations in the literature. In our study, high-volume surgeons demonstrated allograft use in a lower percentage of their ACLRs, suggesting high-volume surgeons have stricter indications for allograft use (i.e., in older and lower-demand patients). These stricter indications may have contributed to the lower revision rate among high-volume surgeons, especially when considering the significant finding of greater allograft failure rates in patients <25 years old compared to patients ≥25 years old found in our study.
The increased revision rate among low-volume surgeons may also be explained by placement and position of the femoral and/or tibial tunnels. Tunnel malposition is often cited as the most common cause for ACLR failure,18 and studies have shown that low-volume surgeons place their femoral tunnels more anterior and proximal, and their tibial tunnels more posterior, than high-volume surgeons.19 Nonanatomic femoral tunnel placement has been identified as a risk factor for revision ACLR, and anatomic tunnel placement has important implications in reducing failure rate and improving rotatory knee stability after ACLR.26, 27, 28 Although our study did not examine tunnel placement postoperatively, tunnel malposition is an important possible explanation for the increased failure rate observed among low-volume surgeons.
The differences observed in allograft selection based on surgeon volume may also play a role in the increased revision rate among low-volume surgeons. Although previous studies have demonstrated greater rates of failure in young patients following allograft versus autograft ACLR,2,3 the effect of allograft type on revision rate is less well-defined. Limited literature has indicated BPTB allograft as a risk factor for revision ACLR compared with soft-tissue allografts.29 Our study showed a significant difference in graft choice between high-volume surgeons and low-volume surgeons, with low-volume surgeons using significantly more BPTB allograft and less semitendinosus allograft compared with high-volume surgeons. Although nonsignificant, perhaps due to insufficient sample size, our study similarly demonstrated the highest failure rate occurring with BTB allograft. It is therefore possible that the more frequent use of BPTB allograft among low-volume surgeons plays a role in the increased revision rate observed in this group.
Interestingly, in our study, high-volume surgeons demonstrated greater procedure times relative to low-volume surgeons for primary ACLR with allograft. This contradicts previous literature showing that low-volume surgeons experience greater procedure times for primary ACLR.30 However, the authors of this study indicated that the most time-consuming step for low-volume surgeons is with tendon harvesting, which is not applicable for allograft ACLR. There are several possible explanations for this finding in our study. It is possible that high-volume surgeons are operating on more complex patients or spending more time repairing concomitant meniscal tears with meniscus repair versus meniscectomy, as literature has shown greater rates of meniscal repair among high-volume surgeons and institutions.31 High-volume surgeons also may be more likely to operate in teaching environments and have trainees such as residents assisting on procedures, which may delay procedural times. Finally, high-volume surgeons may spend more time on achieving anatomic and posterior femoral tunnel placement, as previous literature shows that high-volume surgeons place more anatomic femoral tunnels than low-volume surgeons.19 As discussed, anatomic tunnel placement has important implications in postoperative outcomes and knee stability after ACLR26, 27, 28 and presents a possible explanation for both the longer procedure times and lower revision rate observed among high-volume surgeons in our study.
Limitations
Our study has several limitations. As a retrospective study, causality cannot be inferred. The selection bias present in this retrospective study introduces surgeon indications as a variable, which is in fact a benefit to exploring differences between high- and low-volume surgeons. However, the study was likely underpowered to detect differences in revision rate by allograft type. As mentioned previously, tunnel malposition is the most common cause of ACLR failure, and our study did not analyze postoperative tunnel position with respect to surgeon volume and failure rates. We were therefore unable to determine the relative effect of tunnel position versus allograft choice and patient selection in contributing to the increased rate of revision ACLR seen with low-volume surgeons. Patients may have sought care elsewhere for a revision ACLR, which may lead to the observed revision rates being an underestimate of the true revision rates. Nevertheless, this study was performed at a very large hospital and insurance network, increasing the likelihood that patients sought their follow-up care within the system. Finally, this study did not collect patient-reported outcomes and return to baseline activity, which are important aspects of postoperative care following primary ACLR. The focus on revision rate was intentional because failure and revision, as opposed to subjective outcomes, have generally been the primary concern with allograft ACLR.7
Conclusions
There was a greater revision rate following primary allograft ACLR among low-volume surgeons compared with high-volume surgeons. Low-volume surgeons also used allograft in a younger population than did high-volume surgeons.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: VM reports educational grants, consulting fees and speaking fees from Smith & Nephew plc, educational grants from Arthrex, is a board member of the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS). In addition, VM is the deputy editor-in-chief of Knee Surgery, Sports Traumatology, Arthroscopy (KSSTA) and has a patent Quantified injury diagnostics—U.S. Patent No. 9,949,684, Issued on 24 April 2018, issued to University of Pittsburgh. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
References
- 1.Maletis G.B., Inacio M.C., Desmond J.L., Funahashi T.T. Reconstruction of the anterior cruciate ligament: Association of graft choice with increased risk of early revision. Bone Joint J. 2013;95-B:623–628. doi: 10.1302/0301-620X.95B5.30872. [DOI] [PubMed] [Google Scholar]
- 2.Kaeding C.C., Aros B., Pedroza A., et al. Allograft versus autograft anterior cruciate ligament reconstruction: Predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3:73–81. doi: 10.1177/1941738110386185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraeutler M.J., Bravman J.T., McCarty E.C. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: A meta-analysis of 5182 patients. Am J Sports Med. 2013;41:2439–2448. doi: 10.1177/0363546513484127. [DOI] [PubMed] [Google Scholar]
- 4.Cruz A.I., Jr., Beck J.J., Ellington M.D., et al. Failure rates of autograft and allograft ACL reconstruction in patients 19 years of age and younger: A systematic review and meta-analysis. JB JS Open Access. 2020;5 doi: 10.2106/JBJS.OA.20.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold M.P., Calcei J.G., Vogel N., et al. ACL Study Group survey reveals the evolution of anterior cruciate ligament reconstruction graft choice over the past three decades. Knee Surg Sports Traumatol Arthrosc. 2021;29:3871–3876. doi: 10.1007/s00167-021-06443-9. [DOI] [PubMed] [Google Scholar]
- 6.Tibor L., Chan P.H., Funahashi T.T., Wyatt R., Maletis G.B., Inacio M.C. Surgical technique trends in primary ACL reconstruction from 2007 to 2014. J Bone Joint Surg Am. 2016;98:1079–1089. doi: 10.2106/JBJS.15.00881. [DOI] [PubMed] [Google Scholar]
- 7.Hulet C., Sonnery-Cottet B., Stevenson C., et al. The use of allograft tendons in primary ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27:1754–1770. doi: 10.1007/s00167-019-05440-3. [DOI] [PubMed] [Google Scholar]
- 8.Cole D.W., Ginn T.A., Chen G.J., et al. Cost comparison of anterior cruciate ligament reconstruction: Autograft versus allograft. Arthroscopy. 2005;21:786–790. doi: 10.1016/j.arthro.2005.04.102. [DOI] [PubMed] [Google Scholar]
- 9.Dhillon J., Kraeutler M.J., Belk J.W., McCarty E.C., McCulloch P.C., Scillia A.J. Autograft and nonirradiated allograft for anterior cruciate ligament reconstruction demonstrate similar clinical outcomes and graft failure rates: An updated systematic review. Arthrosc Sports Med Rehabil. 2022;4:e1513–e1521. doi: 10.1016/j.asmr.2022.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariscalco M.W., Magnussen R.A., Mehta D., Hewett T.E., Flanigan D.C., Kaeding C.C. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: A systematic review. Am J Sports Med. 2014;42:492–499. doi: 10.1177/0363546513497566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vyas D., Rabuck S.J., Harner C.D. Allograft anterior cruciate ligament reconstruction: Indications, techniques, and outcomes. J Orthop Sports Phys Ther. 2012;42:196–207. doi: 10.2519/jospt.2012.4083. [DOI] [PubMed] [Google Scholar]
- 12.Maletis G.B., Inacio M.C., Funahashi T.T. Analysis of 16,192 anterior cruciate ligament reconstructions from a community-based registry. Am J Sports Med. 2013;41:2090–2098. doi: 10.1177/0363546513493589. [DOI] [PubMed] [Google Scholar]
- 13.Inacio M.C., Paxton E.W., Maletis G.B., et al. Patient and surgeon characteristics associated with primary anterior cruciate ligament reconstruction graft selection. Am J Sports Med. 2012;40:339–345. doi: 10.1177/0363546511424130. [DOI] [PubMed] [Google Scholar]
- 14.Duchman K.R., Lynch T.S., Spindler K.P. Graft selection in anterior cruciate ligament surgery: Who gets what and why? Clin Sports Med. 2017;36:25–33. doi: 10.1016/j.csm.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Lyman S., Koulouvaris P., Sherman S., Do H., Mandl L.A., Marx R.G. Epidemiology of anterior cruciate ligament reconstruction: Trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91:2321–2328. doi: 10.2106/JBJS.H.00539. [DOI] [PubMed] [Google Scholar]
- 16.Schairer W.W., Marx R.G., Dempsey B., Ge Y., Lyman S. The relation between volume of ACL reconstruction and future knee surgery. Orthop J Sports Med. 2017;5 2325967117S00298. [Google Scholar]
- 17.Kaeding C.C., Pedroza A.D., Reinke E.K., Huston L.J., Consortium M., Spindler K.P. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: Prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. Am J Sports Med. 2015;43:1583–1590. doi: 10.1177/0363546515578836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan J.A., Dahm D., Levy B., Stuart M.J., Group M.S. Femoral tunnel malposition in ACL revision reconstruction. J Knee Surg. 2012;25:361–368. doi: 10.1055/s-0031-1299662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes J.D., Gibbs C.M., Almast A., et al. More anatomic tunnel placement for anterior cruciate ligament reconstruction by surgeons with high volume compared to low volume. Knee Surg Sports Traumatol Arthrosc. 2022;30:2014–2019. doi: 10.1007/s00167-022-06875-x. [DOI] [PubMed] [Google Scholar]
- 20.Sylvia S.M., Gill T.J., Engler I.D., Carroll K.M., Salzler M.J. Anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft in patients aged 50 and older leads to improved activity levels and acceptable patient-reported outcomes. Arthrosc Sports Med Rehabil. 2021;3:e1961–e1965. doi: 10.1016/j.asmr.2021.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sylvia S.M., Perrone G.S., Stone J.A., et al. The majority of patients aged 40 and older having allograft anterior cruciate ligament reconstruction achieve a patient acceptable symptomatic state. Arthroscopy. 2022;38:1537–1543. doi: 10.1016/j.arthro.2021.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Bowman E.N., Limpisvasti O., Cole B.J., ElAttrache N.S. Anterior cruciate ligament reconstruction graft preference most dependent on patient age: a survey of United States surgeons. Arthroscopy. 2021;37:1559–1566. doi: 10.1016/j.arthro.2021.01.042. [DOI] [PubMed] [Google Scholar]
- 23.Bottoni C.R., Smith E.L., Shaha J., et al. Autograft versus allograft anterior cruciate ligament reconstruction: A prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43:2501–2509. doi: 10.1177/0363546515596406. [DOI] [PubMed] [Google Scholar]
- 24.Musahl V., Engler I.D., Nazzal E.M., et al. Current trends in the anterior cruciate ligament part II: Evaluation, surgical technique, prevention, and rehabilitation. Knee Surg Sports Traumatol Arthrosc. 2022;30:34–51. doi: 10.1007/s00167-021-06825-z. [DOI] [PubMed] [Google Scholar]
- 25.Marrale J., Morrissey M.C., Haddad F.S. A literature review of autograft and allograft anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15:690–704. doi: 10.1007/s00167-006-0236-1. [DOI] [PubMed] [Google Scholar]
- 26.Byrne K.J., Hughes J.D., Gibbs C., et al. Non-anatomic tunnel position increases the risk of revision anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2022;30:1388–1395. doi: 10.1007/s00167-021-06607-7. [DOI] [PubMed] [Google Scholar]
- 27.Jaecker V., Zapf T., Naendrup J.H., et al. High non-anatomic tunnel position rates in ACL reconstruction failure using both transtibial and anteromedial tunnel drilling techniques. Arch Orthop Trauma Surg. 2017;137:1293–1299. doi: 10.1007/s00402-017-2738-3. [DOI] [PubMed] [Google Scholar]
- 28.Zantop T., Diermann N., Schumacher T., Schanz S., Fu F.H., Petersen W. Anatomical and nonanatomical double-bundle anterior cruciate ligament reconstruction: Importance of femoral tunnel location on knee kinematics. Am J Sports Med. 2008;36:678–685. doi: 10.1177/0363546508314414. [DOI] [PubMed] [Google Scholar]
- 29.Tejwani S.G., Chen J., Funahashi T.T., Love R., Maletis G.B. Revision risk after allograft anterior cruciate ligament reconstruction: Association with graft processing techniques, patient characteristics, and graft type. Am J Sports Med. 2015;43:2696–2705. doi: 10.1177/0363546515589168. [DOI] [PubMed] [Google Scholar]
- 30.Harato K., Kobayashi S., Toyoda T., Hasegawa T., Tsukimura Y., Niki Y. Technical obstacles for low-volume surgeons in primary anterior cruciate ligament reconstruction. J Knee Surg. 2020;33:1238–1242. doi: 10.1055/s-0039-1692674. [DOI] [PubMed] [Google Scholar]
- 31.Suchman K.I., Behery O.A., Mai D.H., Anil U., Bosco J.A. The demographic and geographic trends of meniscal procedures in new york state: An analysis of 649,470 patients over 13 years. J Bone Joint Surg Am. 2018;100:1581–1588. doi: 10.2106/JBJS.17.01341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.