Abstract
The classical paradigm of G protein-coupled receptor (GPCR) signaling via G proteins is grounded in a view that downstream responses are relatively transient and confined to the cell surface, but this notion has been revised in recent years following the identification of several receptors that engage in sustained signaling responses from subcellular compartments following internalization of the ligand–receptor complex. This phenomenon was initially discovered for the parathyroid hormone (PTH) type 1 receptor (PTH1R), a vital GPCR for maintaining normal calcium and phosphate levels in the body with the paradoxical ability to build or break down bone in response to PTH binding. The diverse biological processes regulated by this receptor are thought to depend on its capacity to mediate diverse modes of cyclic adenosine monophosphate (cAMP) signaling. These include transient signaling at the plasma membrane and sustained signaling from internalized PTH1R within early endosomes mediated by PTH. Here we discuss recent structural, cell signaling, and in vivo studies that unveil potential pharmacological outputs of the spatial versus temporal dimension of PTH1R signaling via cAMP. Notably, the combination of molecular dynamics simulations and elastic network model–based methods revealed how precise modulation of PTH signaling responses is achieved through structure-encoded allosteric coupling within the receptor and between the peptide hormone binding site and the G protein coupling interface. The implications of recent findings are now being explored for addressing key questions on how location bias in receptor signaling contributes to pharmacological functions, and how to drug a difficult target such as the PTH1R toward discovering nonpeptidic small molecule candidates for the treatment of metabolic bone and mineral diseases.
Keywords: arrestins, endosomal signaling, G protein–coupled receptor (GPCR), location bias, parathyroid hormone, PTH receptor, receptor signaling
Graphical Abstract
Essential Points.
Molecular dynamics (MD) simulations linked to the anisotropic network model (ANM)–based method address the key question of how to identify druggable allosteric sites in the parathyroid hormone (PTH) type 1 receptor (PTH1R) and small molecules that can execute allosteric modulation of receptor signaling and function
Structure-encoded allosteric coupling between PTH1R and PTH residues helps design synthetic PTH analogs with the desired bias to demonstrate that physiological responses to PTH depend on the subcellular location of receptor signaling via cyclic adenosine monophosphate (cAMP).
PTH1R-generated endosomal cAMP signaling is thought to ensure vitamin D and bone homeostasis whereas the PTHR-generated plasma membrane cAMP maintains phosphate ion homeostasis.
The Role of PTH1R in Human Health
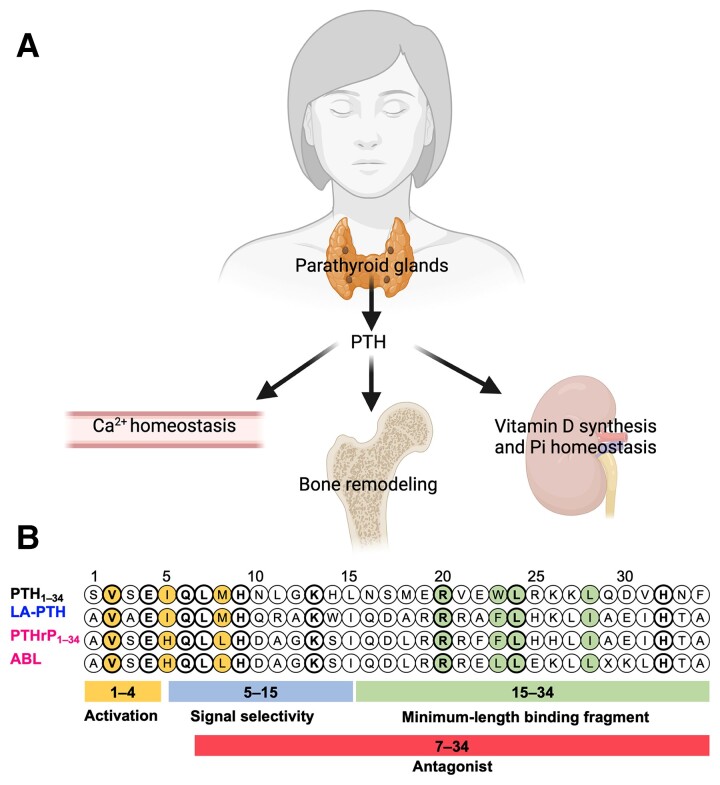
The parathyroid hormone (PTH) type 1 receptor (hereafter referred to as PTH1R) is a G protein–coupled receptor (GPCR) and the canonical cell surface receptor for PTH and PTH-related protein (PTHrP) (1). Secreted as an 84 amino acid peptide, PTH is the quintessential regulator of ionized serum calcium (Ca2+) and phosphate (PO43–) levels in the body (Fig. 1A). In response to hypocalcemic conditions when the Ca2+ concentration fall below the homeostatic set point in blood, or approximately 1.2 ± 0.1 mM, PTH is secreted from the parathyroid glands and acts in an endocrine fashion by exerting effects on specific bone and kidney cells to adjust the balance of calcium and phosphate ions, and regulate bone remodeling (2, 3). In bone, PTH-mediated activation of the PTH1R expressed by osteoblasts and osteocytes ultimately promotes bone matrix resorption and liberation of Ca2+ into the systemic circulation (4–7). In the kidneys, PTH increases Ca2+ reabsorption by distal tubule cells by modulating expression and activity of Ca2+ transport proteins (8, 9). Concomitant with this effect of PTH is a decrease in inorganic phosphate reabsorption in proximal tubule cells via reduction of cell surface sodium–dependent phosphate transporters (10–12), which ensures that Ca2+ remains in the ionized state. PTH also acts on renal proximal tubule cells to increase circulating levels of active vitamin D3 (VitD) via upregulation of the 25-hydroxyvitamin D3 1α-hydroxylase, and active VitD subsequently serves to increase absorption of Ca2+ from the gastrointestinal tract. PTHrP is secreted as a 139, 141, or 171 amino acid protein that is capable of inducing analogous effects on bone and kidney cells observed for PTH, which is not surprising given the shared amino acid sequence homology within the N-terminal region of these peptides that has been shown to be critical for activity (13–15). While it was originally associated as a factor responsible for the hypercalcemia frequently observed in cancer patients, PTHrP is now recognized to play a role in the growth and development of a variety of tissues, including bone (16), mammary glands (17), and teeth (18). PTHrP mostly acts in a paracrine manner on numerous tissues, including bone, growth plate, and mammary gland during development (15, 19). While native PTH is constituted of 84 amino acids and PTHrP consists of splice variants of 139, 141, and 173 amino acids, synthetic or recombinant N-terminal fragments PTH1-34 (also known as teriparatide) and PTHrP1-36 maintain full activity and are primarily used in research studies (14, 20–22) (Fig. 1B).
Figure 1.
Functions and sequence of PTH. (A) Main PTH functions in the body. (B) Sequence alignment of PTH1R peptide ligands. Residues conserved among all the listed PTH1R peptide ligands are in bold. Residues critical for receptor activation and residues critical for receptor binding are colored. X is α-methylalanine in ABL.
Abnormalities in PTH1R signaling cause a variety of diseases, previously reviewed in detail (23). In brief, the lack of a functional PTH1R, such as through expression of homozygous inactivating PTH1R mutants, causes fetal mortality in Blomstrand chondrodysplasia (24, 25). Heterozygous expression of inactive PTH1R can cause enchondromatosis/Ollier disease, in which patients develop benign cartilaginous tumors (26), or primary failure of tooth eruption (27, 28). Conversely, heterozygous constitutively active PTH1R variants with a single mutation in the transmembrane (TM) helices 2 (H223R), 6 (T410P), or 7 (I458R) cause Jansen metaphyseal chondrodysplasia, a very rare disease characterized by short-limbed dwarfism and hypercalcemia (29–31).
Anomalous hormone levels also trigger severe diseases associated with PTH1R functions. PTHrP hypersecretion from tumoral cells induces excessive PTH1R activity, leading to humoral hypercalcemia of malignancy encountered in ≈ 25% of cancer patients (32). Oversecretion or undersecretion of PTH causes hyperparathyroidism and hypoparathyroidism, respectively. Hyperparathyroidism treatment typically involves surgical removal of the parathyroid glands (33) or pharmaceutical therapy by calcimimetics (allosteric activators of the calcium-sensing receptor, a class C GPCR) to reduce PTH secretion by parathyroid glands. Patient intolerance and the risk of hypocalcemia associated with parathyroidectomy, however, limit these approaches. Current treatment of hypoparathyroidism includes oral calcium, active VitD, and daily subcutaneous administration of recombinant human PTH1-34 or PTH (34).
While continuous infusion of PTH was found to promote net bone catabolism (2, 35), intermittent PTH1-34 injections promote net bone anabolism and are used to treat osteoporosis (36–38). A synthetic analog of PTHrP1-34, abaloparatide (ABL) (Fig. 1B) was recently developed to treat postmenopausal women with osteoporosis (39). Recent studies have reported that ABL can be more effective than teriparatide at promoting bone formation while reducing the hypercalcemic effect (39–42). The molecular basis for the differing capacity of these ligands to engage a calcemic response in human is unknown but may be related to differences in their binding mode to the PTH1R. Studies in cultured cells expressing the recombinant PTH1R show that PTH1-34 and ABL stabilize different PTH1R conformations (21, 43). This ligand-dependent PTH1R conformation selectivity is thought to cause distinct residence time (1/koff where koff is the dissociation rate constant) of ligands to the receptor (prolonged for PTH1-34) (21), which in turn alters the spatiotemporal mode of PTH1R signaling, an emerging paradigm discussed in the following paragraphs.
The PTHR Signaling Paradigm
GPCR Signaling
All GPCRs share a common structural architecture: a hallmark transmembrane domain (TMD) consisting of 7 membrane spanning α-helices connected by 3 extracellular loops and 3 intracellular loops, N-terminal (extracellular) and C-terminal (cytoplasmic) portions that differ among GPCR families. Based on sequence and functional analyses, human GPCRs are classified into 5 families: glutamate, rhodopsin, adhesion, frizzled, and secretin families, as defined by the International Union of Basic and Clinical Pharmacology (IUPHAR) “GRAFS” classification (44, 45).
Most prominent members of the glutamate receptor family (formerly class C) include metabotropic glutamate receptors, Ca2+-sensing receptor, γ-aminobutyric acid B receptors, and type I taste receptors. The N-terminus of these GPCRs is large and contains the ligand recognition domain composed of 2 lobes that form a cavity in which ligands bind, altogether reminiscent of the Venus flytrap and therefore often referred to as a Venus flytrap domain. The Venus flytrap domain may also accommodate allosteric binding sites and is connected to the TMD through a cysteine-rich domain, which also plays a role in receptor activation.
The rhodopsin family (also known as class A GPCRs) has the greatest number of members, which typically exhibit small N-terminal regions (46, 47). It comprises more than 700 receptors that share high sequence similarity (48) and several local structural characteristics with rhodopsin, including an NPXXY motif on TM helix 7 (TM7) and a DRY motif between TM3 and intracellular loop 2 (ICL2) (49). Ligand binding for receptors in this class typically takes place within a cavity formed between TM helices. Notable GPCRs in this class that hold significant physiological relevance include muscarinic acetylcholine receptors, dopamine receptors, adrenergic receptors, opioid receptors, adenosine receptors, and histamine receptors.
The adhesion family has recently attracted much attention due to several unique features. With a sole exception of ADGRA1 (GPR123), these receptors typically possess extremely large extracellular N-terminal domains that contain a highly conserved GPCR autoproteolysis-inducing domain, within which the conserved GPCR proteolysis site is located. Autoproteolysis typically takes place in endoplasmic reticulum and generates 2 fragments, N-terminal fragment and C-terminal fragment, that remain connected via noncovalent interactions. For most adhesion GPCRs, the N-terminal portion of the C-terminal fragment, spanning about 15 amino acid residues, comprises a tethered agonist peptide termed Stachel sequence. In addition to these characteristic adhesion GPCR features, the N-termini of some of these receptors also contain an abundance of serine and threonine residues that constitute sites for O-glycosylation, which act as mucin-like domains to create rigid structures that protrude from the cell surface and are important in cell–cell adhesion.
The frizzled/taste 2 receptor family is far less understood than other classes, and their similarity is largely confined to results from phylogenetic analysis. Only recently have frizzled receptors been established as true GPCRs following reports that binding of Wnt ligand can induce G protein coupling. Frizzled receptors are characterized by long N-termini with conserved cysteine residues that are considered critical for Wnt binding.
The PTH1R belongs to the secretin family (or class B GPCR) characterized by a long N-terminus (or extracellular domain, ECD) that adopts a conserved fold composed of 2 major elements—an N-terminal α-helix and 2 antiparallel β-sheets—that are connected by several loops and stabilized by 3 disulfide bridges. Class B GPCRs bind relatively large peptide hormone ligands that act most often in an endocrine manner rather than a paracrine fashion, with PTHrP as an exception. There are currently 15 identified members of this family in humans (50).
In the conventional paradigm for understanding GPCR signaling, hormones transmit signals into cells through a series of biophysical and biochemical events that are initiated at the plasma membrane by ligand binding to a receptor (51, 52). This first event stabilizes the active receptor conformation that couples to and activates heterotrimeric G proteins. G protein activation involves release of the guanosine-5′-diphosphate (GDP) molecule bound to the Gα subunit of the G protein and the binding of a guanosine-5′-triphosphate (GTP); in this way, the activated GPCR acts as a guanine exchange factor. Since the concentration of GTP is several fold higher than that of GDP in cells, GTP readily binds to nucleotide-free Gα (53). GTP binding triggers the dissociation or rearrangement of Gα from the Gβγ heterodimer thus permitting Gα-GTP and Gβγ to independently regulate activities of membrane-bound enzymes and ion channels, respectively. There are 4 main classes of Gα subunits that have been identified based on sequence similarity (54): Gs, which stimulates adenylyl cyclase activity to increase intracellular cyclic adenosine monophosphate (cAMP) levels; Gi/o, which inhibits adenylyl cyclase activity; Gq/11, which leads to an increase in intracellular Ca2+ levels via activation of phospholipase C (PLC) β; and G12/13, which regulates Rho guanine nucleotide exchange factors.
Each G protein subunit contains distinct domains that play specific roles during GPCR signaling. The Gα subunit consists of GTPase and flexible α-helical domains (55). In the presence of nucleotides, the GTPase domain binds and hydrolyses GTP to GDP, while the helical domain forms a lid over the nucleotide binding site (53, 56). There are 5 subtypes of Gβ and 12 subtypes of Gγ subunits expressed in humans (57). The Gβ subunit adopts a β-propeller structure with 7 blades along with a long α-helical N-terminus (55), whereas Gγ consists of 2 α-helical segments, which strongly bind to the N-terminal helix and to the blades 1, 4-7 of Gβ. While the specific actions of each subtype combination are under investigation, the Gβ1γ2 heterodimer is ubiquitously expressed and regulates many effectors, including several adenylyl cyclase subtypes, ion channels, and phosphoinositide 3-kinase gamma (PI3Kγ) (57, 58). Within seconds after agonist binding, the activated receptor interacts with GPCR kinases that initiate receptor desensitization by (1) preventing G protein coupling (59), and (2) receptor phosphorylation that engages or stabilizes receptor association with β-arrestins (β-arrestin 1 and β-arrestin 2) to promote internalization of the receptor–arrestin complex into endosomes (60–63). Such receptor desensitization leads to the termination of G protein–mediated downstream signaling and reassociation of subunits into the heterotrimeric complex (64). Following receptor internalization to endosomes, the agonist dissociation ensues, and the receptor can be either targeted to lysosomes for degradation (referred to as receptor downregulation) or dephosphorylated and recycled back to the plasma membrane for a new cycle of activation and signaling (referred to as receptor resensitization) (65).
Paradigm Shift in PTH1R Signaling via G Proteins
The PTH1R predominantly activates Gs and Gq/11 in response to PTH or PTHrP binding. Gs activates adenylyl cyclases resulting in the production of cAMP and protein kinase A (PKA) activation (1, 66), whereas the Gq/11 (Gαq, Gα11) subfamily activates phospholipase Cβ (PLCβ), which then cleaves phosphatidylinositol (4, 5)-bisphosphate to generate diacylglycerol and inositol (1,4,5)-trisphosphate (IP3). IP3 activates calcium channels at the endoplasmic reticulum, releasing stored Ca2+ into the cytosol, while diacylglycerol activates protein kinase C) The PTH1R can also activate the G12/13 subfamily (Gα12, Gα13), which engages the phospholipase D/RhoA signaling pathway (67, 68), and Gi (69).
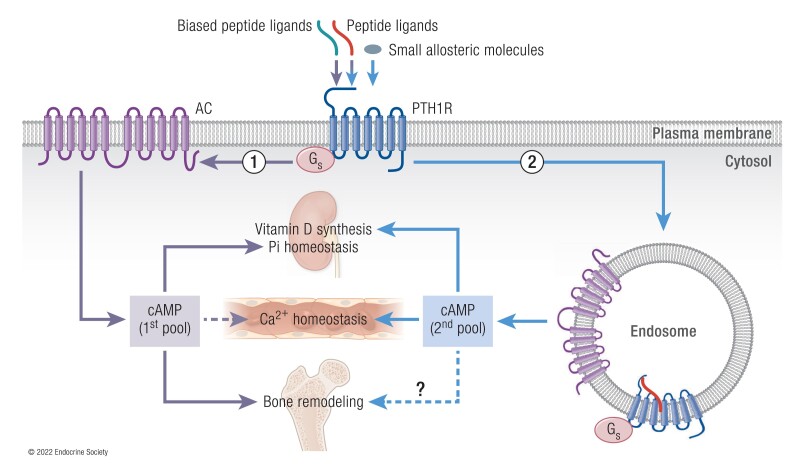
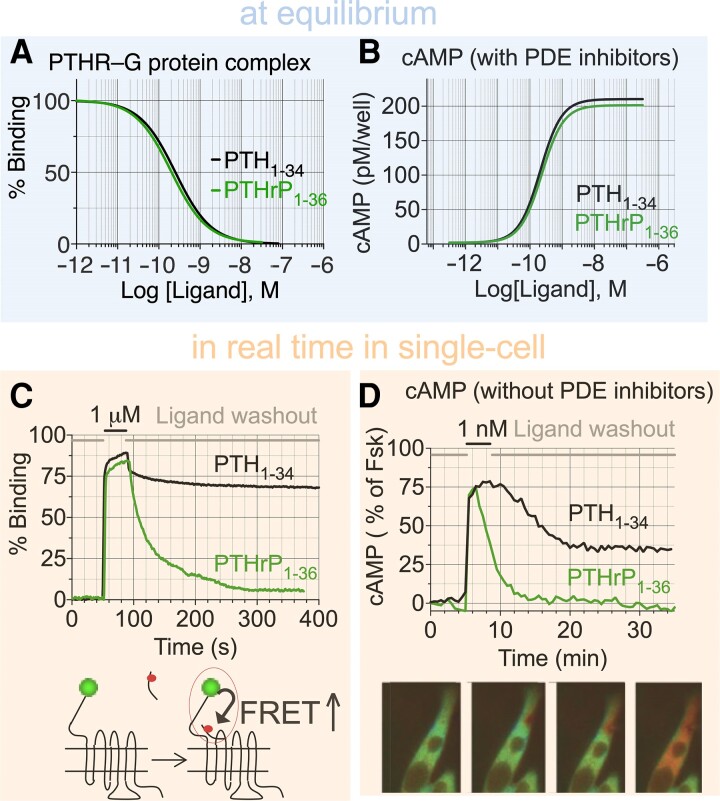
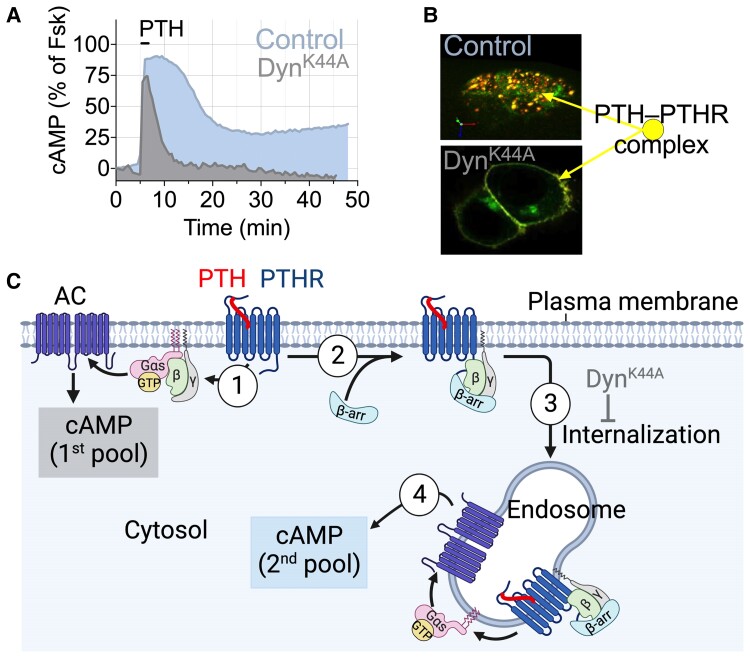
Back in the 2000s, it was well established that PTH1-34 infusion stimulates significant increases in serum Ca2+, serum vitamin D in its active form (ie, 1,25-dihydroxy vitamin D3), urinary phosphate ion (PO43–), and bone resorption. However, it was unclear why these physiological parameters were not increased at a comparable dose of PTHrP1-36 infusion given that PTH and PTHrP share similar pharmacological properties as they relate to binding affinity and stimulation of cAMP production (Fig. 2A and 2B) (70).The development and application of quantitative Förster resonance energy transfer (FRET)-based approaches to determine kinetics and rate-limiting reactions for each step along the PTH1R signaling cascade at the single-cell level (reviewed in (71)) unveiled signaling differences between PTH1-34 and PTHrP1-36 and a new mode of GPCR signaling via cAMP. Ligand–PTH1R interaction and cAMP production measured by FRET showed that PTHrP1-36 binding is fully reversible and induces transient cAMP after ligand washout, whereas PTH1-34 remains bound and induces sustained cAMP generation (Fig. 2C). Knowing that PTH induces rapid PTH1R internalization (72–74), the protracted cAMP response is inconsistent with the conventional model of GPCR desensitization as G protein-dependent signaling by activated receptor is quickly terminated following receptor phosphorylation by GPCR kinases and the recruitment of β-arrestins, which both sterically occlude G protein binding and promote endocytosis of ligand-bound GPCRs. Thus, signaling responses by GPCRs were initially considered transient in nature and confined to the plasma membrane. However, this model has been revised in recent years after the observation that blocking PTH1R internalization prevents the sustained cAMP response (75, 76) (Fig. 3A and 3B) and that PTH mediates sustained cAMP signaling from early endosomes following internalization of a ternary PTH1R–Gβγ−β-arrestin complex (Fig. 3C) (77). This unexpected aspect of PTH1R signaling is due to the apparent ligand-dependent nature of endosomal signaling by this receptor and will be discussed in the next section. Notably, the production of 2 distinct pools of cAMP has been verified for several other GPCRs in class A and class B (76, 78, 79) and reviewed in (80) and is now considered an integral part of GPCR signaling (81).
Figure 2.
Pharmacological properties of PTH1R. (A, B) Comparison between the competitive binding isotherms (A) and concentration–effect curves for cAMP accumulation in HEK293 cells expressing the recombinant human PTH1R. (C) Recording real-time binding of peptide ligands to PTH1R in a single cell. Ligand and receptor interaction is measured by FRET between green fluorescent protein (GFP)-tagged PTH1R and tetramethylrhodamine (TMR)-labeled peptide ligands. Changes of GFP emission due to a FRET increase in response to rapid superfusion of ligand–TMR (horizontal black bar) are shown. (D) cAMP production over 35 minutes measured by FRET changes from a single HEK293 cells stably expressing PTH1R and transiently expressing the cytoplasmic cAMP FRET sensor epac1-CFP/YFP and in the absence of phosphodiesterase (PDE) inhibitors. Cells were continuously perfused with control buffer or peptide ligands (horizontal black bar). Images show the propagation of the cAMP production represented as pseudocolored FRET ratio before (green) and after (red) stimulation of a single cell with PTH1-34.
Figure 3.
Modes of PTH1R signaling via cAMP. (A) Time courses of cAMP production induced by PTH1-34 in single cell (here a mouse osteoblast) without (control) or with expression of the recombinant dominant-negative dynamin mutant (DynK44A) that blocks receptor internalization. (B) Confocal images representing a 3D view of HEK-293 cells expressing PTH1R N-terminally tagged with GFP (PTHRGFP) with TMR-labeled PTH1-34 (PTHTMR). In control cells, PTH1RGFP and PTHTMR colocalized (colored spots) in endosomes; in cells coexpressing Dyn-K44A, the ligand–receptor complex remains at the cell surface. (C) The 1st pool of cAMP production takes place at the cell membrane following PTH binding to PTH1R (step 1). This response is short-lived due to rapid receptor desensitization via PTHR phosphorylation by GPCR kinases, followed by recruitment of β-arrestins (β-arrs) (step 2) driving receptor internalization and its redistribution in early endosomes (step 3). PTH interacts tightly with PTH1R in a conformation-dependent fashion and induces the 2nd pool cAMP production from endosomes (step 4). Endosomal cAMP production is prolonged until endosomal acidification induces the release of PTH from the receptor. Created with BioRender.
Mechanisms of Endosomal PTH1R Signaling via cAMP and its Regulation
Two distinct active PTH1R conformations are thought to be responsible for the distinct duration of cAMP production (20, 71). The binding affinity of 1 of the conformations, referred to as the R0 conformation, is independent of G protein coupling. The R0 conformation is preferentially stabilized by PTH and maintains cAMP production when the receptor internalizes in early endosomes. The second conformation, referred to as the RG conformation, corresponds to the classical G protein–dependent high-affinity receptor, which is indistinguishably stabilized by PTH or PTHrP1-36. Accordingly, whereas the RG conformation stabilized by PTHrP1-36 is G protein dependent and is associated with transient cAMP responses from the plasma membrane, the R0 state is not altered by G protein coupling but nevertheless permits sustained cAMP generation following internalization of the receptor (20, 76, 82, 83). The C-terminal portion of PTH (ie, residues 15-34) plays a critical role in receptor selectivity and affinity by binding to the PTH1R extracellular domain (PTH1RECD) (84, 85). Structural studies revealed a perfect alignment for peptides containing the same C-terminal 15-34 amino acid residues, namely, CPTH-type including PTH and ePTH (a mimetic PTH agonist where PTH amino acid residues 1,3,10,11,12,14,18,34 are replaced with aminocyclopentane-1-carboxylic acid, α-aminoisobutyric acid, Gln, homoarginine, Ala, Trp, norleucine, and Tyr, respectively), or CPTHrP-type including PTHrP, but C-terminal tips of the CPTH-type and CPTHrP-type peptides diverge to opposite sides of PTHRECD (indicated by arrows in Fig. 4A). Such differential binding mode at PTH1RECD likely translates to differential overall binding to PTH1R and results in distinct signaling modes. The N-terminal portion, especially the most N-terminal residues, is critical for PTH1R signaling, as deletion of PTH Ser1 and Val2 significantly reduces cAMP production (86, 87). Also, PTH7-34 acts as a PTH1R antagonist (88). From sequence analyses, PTH1-34 shares several residues and chemical moieties that are not present in PTHrP1-34 or ABL, which may be critical for stabilizing the R0 conformation and inducing sustained cAMP signaling in endosomes; these include Ala1, Ala3, Ile5, Met8, the amide group on residue 10 (Fig. 1B). Additionally, Arg11, Ala12, and Trp14 were initially identified in an alanine scanning to increase activity of a short N-terminal PTH fragment that was referred to as modified-PTH1-14 (M-PTH1-14) (89–92). Further optimization of the signaling properties of M-PTH1-14 resulted in the chimeric peptide M-PTH1-34, which binds the the R0 conformation of PTH1R with high affinity and trigger prolonged elevation of serum Ca2+ (sCa) when injected subcutaneously into mice (82). Additional structure–activity studies to improve the solubility of this peptide by substitution of C-terminal hydrophobic PTHrP residues Leu18, Phe22, and His26 by Ala, Ala, and Lys, respectively, resulted in the [Ala18,22, Lys26]-M-PTH1-14/PTHrP15-36 peptide referred to as LA-PTH, which maintains a higher affinity binding to the PTHR R0 conformation than PTH, and sustains endosomal cAMP signaling at time points by which PTH has dissociated from the receptor (93, 94). Given the high affinity and residence time of LA-PTH (43, 83, 95) for PTH1R, this ligand was used to solve high-resolution cryogenic electron microscopy (cryo-EM) structures of PTH1R in complex with GS. This part is discussed under “Structural Characterization of PTH1R.”
Figure 4.
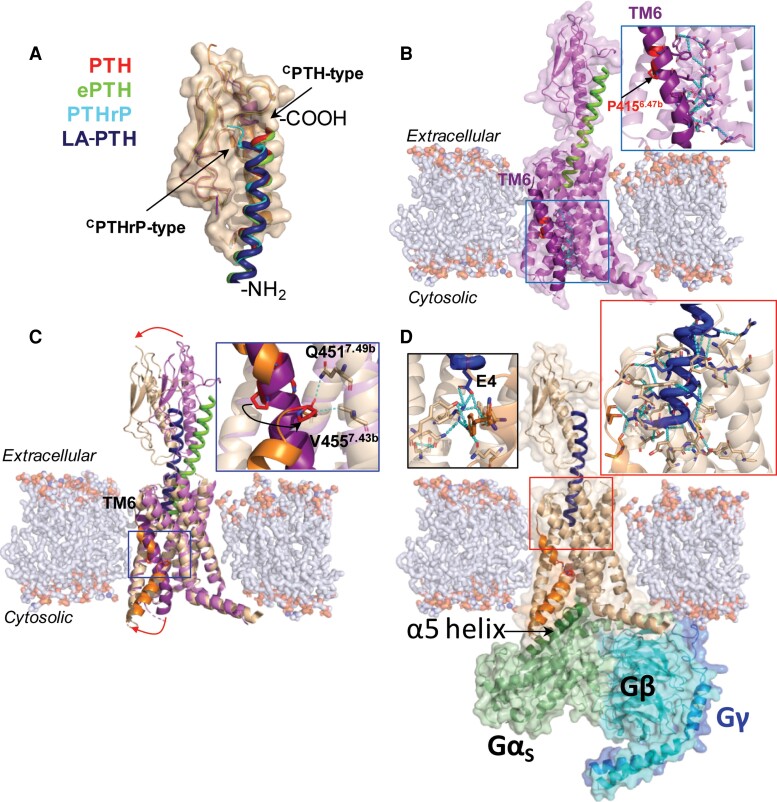
Structural characterization of PTH1R activation. (A) Overlaid structures of PTH1RECD in complex with distinct peptides, aligned by PTH1RECD. PTH, red coil (PDB 3C4M); PTHrP, cyan coil, (PDB 3H3G); ePTH, green coil (PDB 6FJ3); LA-PTH, dark blue coil (PDB 6NBF). The N- and C-termini of the peptides are denoted by -NH2 and -COOH, respectively. (B) Crystal structure of inactive state PTHR bound to ePTH (green coil, PDB 6FJ3). (C) Overlay of inactive (receptor in purple, ePTH in green, PDB 6FJ3) and fully activated (receptor in wheat, LA-PTH in dark blue, PDB 6NBF) PTH1R structures, aligned by their TMDs. TM6 is in deep purple or orange in inactive and fully active states, respectively. (D) Cryo-EM structure of fully activated LA-PTH-bound (dark blue coil) PTH1R (state 1) coupled to Gs heterotrimer (PDB 6NBF).
In the case of PTH1R, a ternary complex composed of PTH-bound PTH1R, β-arrestin, and Gβγ is required for endosomal cAMP production. The mechanism by which this ternary complex maintains production and elevated levels of cAMP was studied in cells expressing recombinant or native PTH1R and proceeds as follows (Fig. 5A-5C). First, it promotes the activation cycle of Gs from endosomes (77). Second, it activates extracellular signal–regulated protein kinase 1/2 (ERK1/2) via β-arrestins, thus reducing the activity of the cAMP-specific phosphodiesterase PDE4 (96). Third, it stimulates the conditional activation of adenylate cyclase type 2 via Gβγ (94). Following the recognition of these functional roles, White et al (97) have demonstrated that Gq activation by PTH is determinant in the formation of the ternary PTH1R–Gβγ−arrestin complex. Gβγ released upon Gq activation promotes the conversion of PI(4,5)P2 to PI(34,5)P3 by phosphoinositide 3-kinase β (PI3Kβ), which in turn promotes β-arrestin recruitment to PTH1R and formation of the ternary receptor complex (Fig. 5B, steps 3 and 4).
Figure 5.
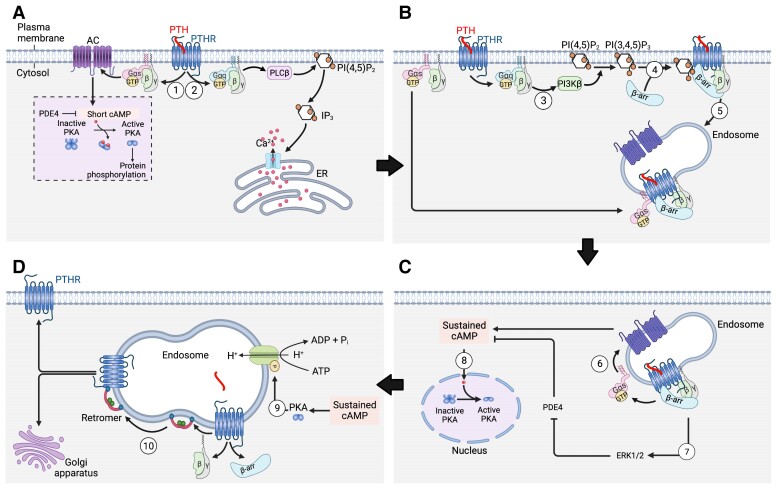
Mechanism of endosomal PTH1R signaling via cAMP. (A) PTH1R activates heterotrimeric Gs and Gq proteins at the plasma membrane in response to PTH (steps 1 and 2). Gs activates adenylate cyclases (AC) leading to cAMP production and activation of cytosolic protein kinase A (PKA). The 1st pool of cAMP production is short due to the action of phosphodiesterases (PDE). Gq activates phospholipase C (PLCβ) that cleaves phosphatidylinositol (4,5)-bisphosphate (PI(4,5)P2) into inositol (14,5)-trisphosphate (IP3). Free diffusing IP3 binds and activates IP3-gated Ca2+ channels, which in turn release stored Ca2+ into the cytosol. (B) Following Gq activation, Gβγ promotes PI3Kβ-dependent generation of PI(34,5)P3 (step 3) at the plasma membrane that triggers β-arrestin (β-arr) recruitment to the PTHR (step 4) and the formation and internalization of the ternary PTH1R–β-arr–Gβγ complex. Reassembly of the ternary PTH1R complex with Gαs is thought to be dependent on GαS diffusion. (C) PTH triggers sustained cAMP production after receptor internalization to endosomes via a β-arrestins (β-arr) and clathrin-coated pit-dependent pathway (2nd pool) (step 6). The sustained phase of endosomal cAMP production is due to the inhibitory action of extracellular signal-regulated protein kinase 1/2 (ERK1/2) on PDE4 (step 7). Endosome-generated cAMP can efficiently diffuse into the nucleus to activate nuclear PKA (step 8). (D) Endosomal cAMP production is prolonged until endosomal acidification caused by v-ATPase activity induces the release of PTH from the receptor (step 9) and assembly of the PTH1R-retromer complex (step 10) allowing receptor transfer to the Golgi apparatus and receptor recycling. (Created with BioRender after adaptation from Sutkeviciute and Vilardaga (80).
Termination of the sustained cAMP response by the PTH1R is predominantly controlled by mechanisms instilled within the endosomal trafficking pathway. Previous work has highlighted that the release of β-arrestins from the PTH1R localized in endosomes is concomitant with association of the retromer complex with the receptor (96) (Fig. 5D). The retromer complex is comprised of 2 membrane-bound sorting nexins (SNX1 and SNX2) as well as a heterotrimer consisting of vesicle protein sorting (Vps) 26, 29, and 35, which collectively serve to regulate the sorting of cargo proteins from early endosomes to the trans-Golgi network (98, 99). Examination of this complex in the context of PTH1R-mediated cAMP showed that overexpression of Vps subunits reduces the duration of cAMP generation, whereas siRNA targeting of the subunits prolonged cAMP signaling time courses (96). This process was recently shown to be dependent upon a negative feedback loop in which activated PKA increases the proton pump activity of endosomal membrane-bound v-ATPase, resulting in endosomal acidification (100). The decrease in endosomal pH then leads to the disassembly of the signaling PTH1R–Gβγ–arrestin complex and concomitant assembly of inactive PTH1R with the retromer, forming a complex that traffics to the Golgi apparatus (96, 100) and/or engages receptor recycling back to the plasma membrane (Fig. 5D) (100).
Structural Characterization of PTH1R
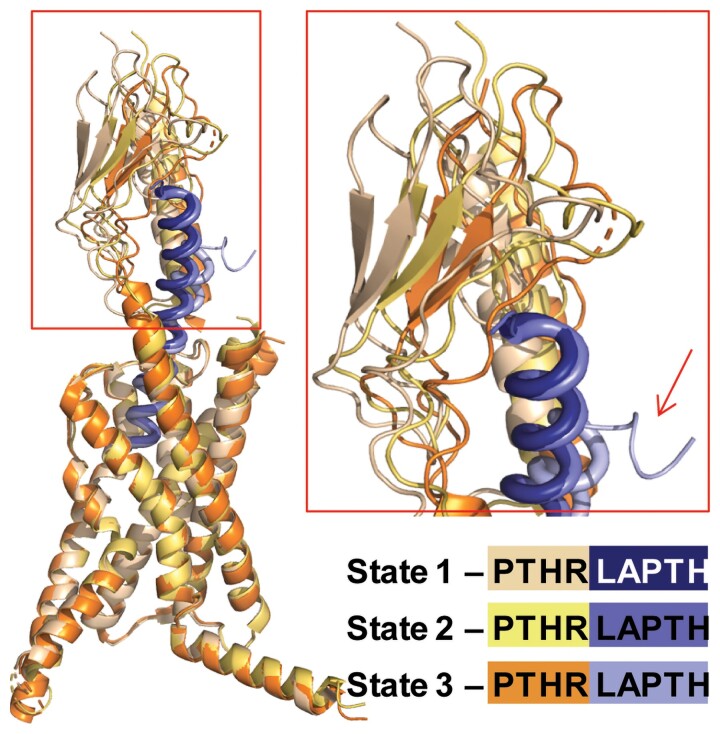
As for many class B GPCRs, the crystal structure of the isolated PTH1RECD was first resolved either in the absence or presence of ligands (84, 85, 101). These structures demonstrate direct interaction between the C-terminal portion of peptide ligands and the receptor ECD and provide snapshots of the first step in peptide ligand binding to its receptor (Fig. 4A). High resolution structures of PTH1R were then solved by different approaches concomitantly. An inactive signaling conformation of PTH1R was crystallized with a mimetic PTH agonist ePTH (102) (Fig. 4B). The receptor is maintained in an inactive state via a dense interaction network (cyan dashes in Fig. 4B) sealing TM6 to the core of the receptor (see detailed in a close-up view, where interacting residues are shown in stick representation). The conserved PxxG motive in the middle of TM6 (highlighted in red with P4156.42b, using the class B GPCR numbering in superscript, in Fig. 4B) faces outward and away from inactive-state stabilizing network; however, these observations need confirmation given that stabilizing mutations and fusion protein inserted in ICL3 of the receptor construct used for crystallization likely contribute to the inactive receptor state. Three fully active conformations (referred to as states 1-3) of LA-PTH-bound PTH1R in complex with Gs were determined by cryo-EM (87) (Fig. 6). LA-PTH interactions with PTH1R involve a continuous α-helix connecting the PTH1RECD and the TMD (PTH1RTMD), as seen in other published structures of class B GPCR–Gs complexes (103–106). Specifically, the C-terminal residues 18-34 of LA-PTH are dissociated from the PTH1RECD in the state 3 structure (Fig. 6), suggesting that residues at the C-terminus of the peptide can undergo many dissociation–rebinding cycles while in complex with PTH1R. Contacts between peptide residues 1-14 and the receptor TMD are likely critical for maintaining active peptide–receptor complex during dissociation and possibly reassociation events of the C-terminal peptide part.
Figure 6.
Structural dynamics of LA-PTH–PTH1R complex. Overlaid 3 distinct conformational states of LA-PTH–PTH1R complex obtained from cryo-EM data analysis, aligned by TMDs. PTH1R and LA-PTH are shown as different shades of purple and orange, respectively. The oscillation of the PTH1RECD is evident (close-up view), while the TMD core shows higher degree of stability, likely due to tight binding of NLA-PTH. Notably, state 3 captures the event of partial dissociation of CLA-PTH from PTH1RECD (arrow).
From these data, a hypothetical model for sustained endosomal signaling by LA-PTH can be proposed in which the large number of contacts between LA-PTH and PTH1RTMD (shown as cyan dashed lines in Fig. 4D, left close-up view) reduce ligand dissociation at endosomal pH, despite dissociation of the peptide C-terminal part. In contrast, the destabilization of PTH–PTH1R at endosomal pH leads to the release of PTH from the receptor and termination of signaling (100) (step 9 in Fig. 5D).
To date, structures of nearly all class B GPCRs in active Gs-coupled states have been solved (107–117) and display a common structural feature—a sharp outward kink in the middle region of TM6, which opens the receptor cytosolic interface for coupling to G proteins or β-arrestins. Such hallmark feature is also present in active state PTH1R structures (87) (Fig. 4C and 4D) and is the most pronounced structural rearrangement occurring when the receptor switches from the inactive to its fully active state as highlighted in Fig. 4C. In particular, the P4156.42b of the conserved class B GPCR PxxG motif faces away from TMD core in an inactive state but is twisted inward in the active state and engages in interactions with Q4517.45b and V4557.49b on TM7 (see close-up view in Fig. 4C), overall resulting in partial unwinding of a portion of TM6 above (C-terminal to) the PxxG motif and a large outward movement of the lower part of TM6 N-terminal to the PxxG motif (red continuous arrow in Fig. 4C) (112). This results in formation of a sharp kink in the middle of TM6 of fully active PTH1R and a large opening of the receptor's cytosolic core for G protein binding. In addition, PTH1RECD may also undergo rearrangement of its position with respect to TMD to adapt the peptide ligand into TMD for receptor activation (top red arrow in Fig. 4C); the type, extent, and stability of such motion might be dependent on the type of peptide ligand agonist.
Other structural rearrangements upon receptor activation also include minor reorganization in the extracellular portions of TM1 and TM7 move toward TM6 in the active state receptor. The other TM helices also exhibit shifts, mostly outward from the receptor core upon activation. These structural rearrangements may contribute to the outward TM6 kink (112). Comparing active state cryo-EM structures with the inactive state crystal structure shows that the first 3 residues of LA-PTH push against TM6, and ligand residue Glu4 stabilizes an extensive polar core network within the receptor (Fig. 4D, left close-up), both of which promote the outward TM6 kink characteristic of receptor activation. The large outward movement of TM6 is required and presumably induced and stabilized by GαS subunit binding via interactions of its C-terminal α5-helix (highlighted green in Fig. 4D) with the receptor's cytosolic core. Such profound movement of TM6 to open cytosolic core of class B GPCRs likely is not induced by agonist peptide binding alone. Recent cryo-EM studies of calcitonin gene–related peptide receptor in apo and CGRP-bound states revealed that CGRP-bound and apo receptor structures are highly similar (118). Agonist binding increases structural dynamics at lower halves of TM5 and TM6 in CGRP-bound receptor indicating that agonist binding destabilizes inactive-state interaction networks, and this is required for effective G protein coupling eventually leading to a sharp kink formation in TM6 receptors and full opening of the cytosolic core. Such activation mechanism is likely common among all class B GPCRs.
PTH Binding to PTHR: A Structure-Encoded Allosteric Mechanism
The binding of PTH to PTH1R proceeds via a 2-step association mechanism (119). The molecular model of this 2-step reaction is displayed in Fig. 7. In the first step, the C-terminal part of the ligand (residues 16-34) rapidly binds to the receptor N-extracellular domain (PTH1RECD) following bimolecular reaction kinetics, defined by kobs = koff + kon[L], where kobs is the measured rate constant (s–1), and kon and koff are the association and dissociation rate constants, respectively, and [L] is the ligand concentration. In the second and slower step, the N-terminal part of the ligand (residues 1-15) binds to the receptor TMD (PTH1RTMD) via a complex reaction involving a bimolecular interaction coupled to conformational changes in both peptide hormone and receptor. This reaction follows a hyperbolic dependence on hormone concentration.
Figure 7.
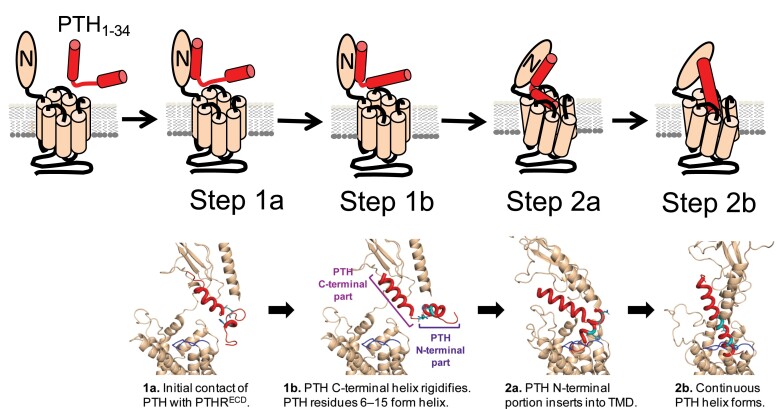
Expanded 2-step model of PTH binding to PTH1R. Schematic representation and structural model where PTH1R is colored wheat, with ECL2 highlighted in dark blue. PTH is colored red, with residues His9, Asn16, and Ser17 displayed as cyan sticks. In the fast first step (step 1), binding of the C-terminal part of PTH to PTH1RECD (step 1a) rigidifies the C-terminal helix as well as residues 6-15 within the N-terminal part of PTH (step 1b). These conformational changes may expand the small N-terminal helix from residues 6-9 to 6-15. In the slow second step (step 2), the N-terminal part of PTH is inserted into PTH1RTMD (step 2a). Interactions between the N-terminal portion of PTH and PTH1RTMD and ECL2 residues, promote the formation of a continuous PTH helix (step 2b). Adapted from Fig. 2 in Clark et al (120).
NMR experiments titrating unlabeled purified PTH1RECD into a sample of 15N-labeled PTH1-34 provided further insights on the molecular mechanism of this complex reaction (120). In brief, the binding of the PTH C-terminal part to PTH1RECD triggers a distinct and more structured conformation in the N-terminal region of PTH1-34 independent of the interaction with PTH1RTMD, which in turn primes this N-PTH region for interaction with PTH1RTMD and resulting in a continuous PTH helix conformation. Such placement of the PTH N-terminal part, especially of residues 1-4, stabilizes the active receptor conformation, given that N-terminal PTH residues 1-4 are essential to trigger activation of Gs proteins and cAMP production (87).
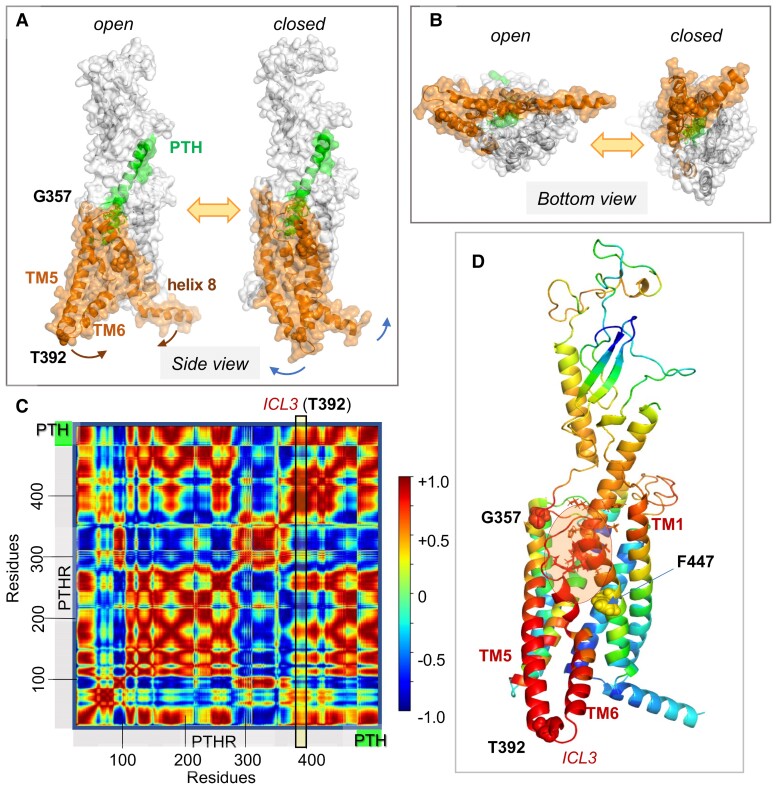
Toward elucidating the allosteric changes in PTH1R structure triggered by PTH binding, we examined the spectrum of conformational motions, or thermal fluctuations (also called Brownian motions), accessible to the PTH–PTH1R complex embedded in a lipid bilayer (120). The spectrum comprises a wide range of relaxational motions, from low-frequency modes that cooperatively engage the entire structure to those in the high-frequency regime that are highly localized (121). Several studies have demonstrated that the low-frequency modes of motions, robustly defined by the overall architecture, represent cooperative changes in structure that are often required for biological function, including allosteric activation (122). The mode spectrum can be uniquely determined for each structure by normal mode analysis. We analyzed that of the PTH–PTH1R complex using an extension of the anisotropic network model (123) (ANM) that takes into account the constraints exerted by the lipid bilayer implemented in the DynOmics server (124), and we focused on the most cooperative movements predicted for the complex. Our analysis revealed the predisposition of the complex to a cooperative motion, which enables the “opening” of the intracellularly exposed ends of TM5 and TM6 and the connecting intracellular loop ICL3 (Fig. 8A and 8B), reminiscent of the activated state of class B GPCRs (see Fig. 4D), suggesting that the conformational transition triggered by ligand binding can readily induce a structural change at the cytoplasmic ends of TM5 and TM6.
Figure 8.
PTH1R collective dynamics, inter-residue cross-correlations, and allosteric communication. (A, B) Collective motions accessible to PTH-bound PTH1R in the presence of lipid bilayer (not shown). A and B show the respective side and bottom views of 2 conformers visited during fluctuations of the complex along ANM mode 14 that cooperatively engages the entire complex (126). Here, PTH is green, PTH1R residues G357-L481, orange. Note the large swinging movements at the cytoplasmic ends of TM5-TM6 with respect to helix 8, facilitated by a disruption at TM6. Corresponding animation are in ref. (126). (C) Cross-correlation map between residue motions presented in A and B. The color code refers to the correlation cosines between the directions of motion of all residue pairs (scale bar on the right). Red and blue regions indicate the regions engaged in strongly correlated (coupled, same direction) and anticorrelated (coupled, opposite direction) movements. (D) PTH1R–PTH complex color coded by the type and strength of correlations of all movements of the residues with the TM5-TM6 cytoplasmic end (derived from the column corresponding to T392 at the ICL3 in C; see the vertical box). Red and blue residues undergo coupled same and opposite direction fluctuations with respect to T392 (or ICL3). G357, T392, and F447 are displayed in space filling. PTH V1-H14 (enclosed in a semitransparent orange ellipse, residues shown in sticks) is strongly correlated with PTHR G357-F447 (red) and the N-terminal (extracellularly exposed) part of TM1, and anticorrelated with F447-L481 (blue). Adapted from Clark et al (120).
To visualize more clearly the coupling between the PTH binding site and the cytoplasmic regions that undergo large rearrangements, we analyzed the cross-correlations between (PTH and PTH1R) residue motions in this mode. The results are presented as a heat map in Fig. 8C. The red blocks indicate the structural elements that undergo highly correlated movements, and the blue blocks indicate the pairs of structural elements that undergo anticorrelated (opposite direction) fluctuations. We focused on the type and strength of coupling between the PTH N-terminal residues S1-H14 inserted into the PTH1RTMD (Fig. 8D; orange ellipse) and the ICL3 connecting TM5 and TM6, using T392 located at the cytosolic tip of TM6, as a reference point for probing conformational rearrangements associated with receptor activation. The heat map clearly showed that PTH V1-H14 was strongly correlated with TM5-ICL3-TM6, as well as the extracellularly exposed region of TM1. Furthermore, examination of the correlation between T392/ICL3 and all PTH1R residues (vertical rectangular box in Fig. 8C and ribbon diagram in Fig. 8D color-coded by these correlations) showed that T392 belongs to a highly coherent block encompassing the substructure from approximately the mid-part of TM5 all the way to its cytoplasmic end, the entire TM6 and the N-terminal half of TM7 (up to F447), in strong support of the coupling of this entire substructure to PTH S1-H14. This highly coupled region is naturally expected to allosterically transmit the perturbations at the PTH binding site to the TM5-ICL3-TM6 cytoplasmic region.
PTH1R Druggability
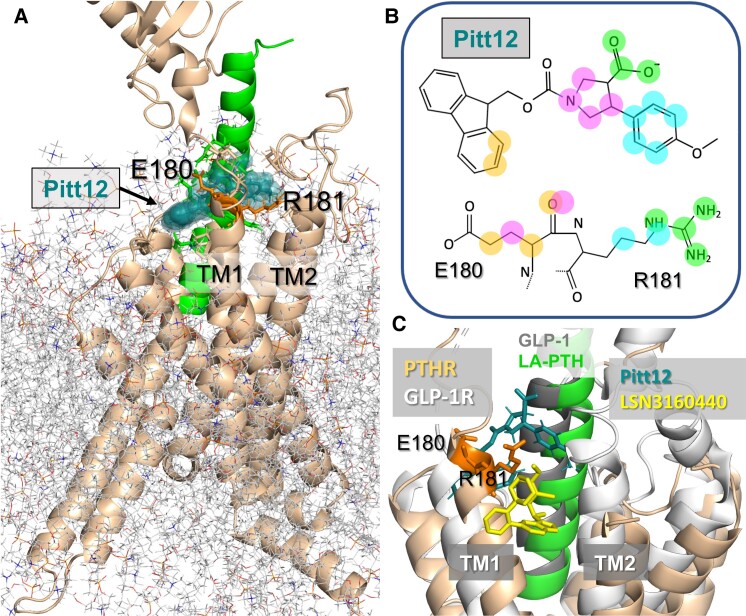
Overall, ANM analysis (or its extension ESSA (125) implemented in the interface ProDy) unveils the structural elements that mediate the allosteric signaling properties of target proteins. Recent application to PTH1R, in conjunction with druggability simulations, pointed to an allosteric and druggable region in the vicinity of the extracellular ends of TM1 and TM2 (126). In particular, the TM1 residues E180 and R181 were distinguished by their high affinity for binding drug-like probe molecules. Pharmacophore modeling using snapshots from druggability simulations, followed by virtual screening of libraries of small molecules led to the identification of a series of small molecules. One of these small molecules, Pitt12, was experimentally verified to act as a negative allosteric modulator of PTH1R (126). The binding pose of Pitt12 onto LA-PTH-bound PTH1R observed in molecular dynamics (MD) simulations, as illustrated in Fig. 9A, displays the particular atoms engaged in highly stabilizing interactions with selected E180 and R181 atoms (Fig. 9B). Notably, a recently resolved positive allosteric modulator of the glucagon-like peptide 1 (GLP-1) receptor (GLP1R), another Class B GPCR mentioned above, also binds the same site, but this modulator (LSN3160440) inserts slightly deeper into the TMD. The structural alignment of the allosteric modulator binding sites for Pitt12-bound PTH1R and LSN3160440-bound GLP1R (Fig. 9C) supports the view that the interfacial region between the N-terminal region of TM1 and the bound peptide or substrate (here LA-PTH and GLP-1) harbors a site with a high propensity to bind allosteric small molecules that might be a common allosteric druggable site among all class B GPCRs. The allosteric effect of ligand binding to this site is consistent with the highly cooperative interaction between the PTH N-terminal residues, the cytoplasm-exposed halves of TM5 and TM6 and connecting loop ICL3, and the N-terminal extracellularly exposed end of TM1 noted in Fig. 8D. Although Pitt12 and LSN3160440 share a similar binding site and pose in their respective receptors, these small allosteric molecules produce opposite modulation of receptor signaling via GS/cAMP. In the case of LSN3160440, it acts as a positive allosteric modulator by stabilizing the interaction of GLP-19-36 for the active GLP1R conformation (127). In the case of Pitt12, MD simulations predict that it operates by shifting the position of the PTH1-34 peptide's N-terminal tip resulting in an outward displacement of TM5 and TM6 helices; this conformational rearrangement would be consistent with the negative allosteric modulator activity of Pitt12 in reducing PTH1R coupling to G protein. Another nonpeptidic molecule, PCO371, acting as agonist for the PTH1R with a µM potency for the stimulation of cAMP in cells expressing the recombinant at PTH1R has been previously discovered (127). The binding site is not resolved and initial phase 1 clinical trials to test this molecule as a potential orally available drug candidate for the treatment of hypoparathyroidism (hypocalcemia) have been terminated (128, 129). Acting as a negative allosteric modulator, Pitt 12 is under optimization for improving its efficacy and potency as a potential candidate for future treatment of bone and mineral diseases linked to PTH1R hyperactivity due to hypersecretion of PTH or PTHrP encountered in hyperparathyroidism or in hypercalcemia of malignancy, respectively.
Figure 9.
Interaction of a negative allosteric modulator with PTH1R. (A) Results from MD simulations of the binding of an allosteric modulator, named Pitt12, to LA-PTH–bound PTH1R (PDB 6NBF) (87). The binding pose and structure of Pitt12 were deduced from druggability simulations followed by pharmacophore modeling using Pharmmaker and virtual screening of libraries of small molecules (126). PTH1R is shown in the wheat ribbon diagram and LA-PTH in green. E180 and R181 (orange sticks) were identified as 2 critical residues that coordinate the small molecule. Pitt12 is shown in teal space filling. (B) 2D representations of Pitt12. Atoms highlighted in colored spheres (at the top) interact with the E180 and R181 atoms shown by the same color in the diagram at the bottom. (C) Comparison of the computationally predicted binding pose of Pitt12 onto PTH1R, and experimentally resolved (127) binding pose of a positive allosteric modulator, LSN3160440, onto GLP-1R (PDB 6VCB) (127). GLP-1R is shown in light gray, GLP-1 (structurally aligned against LA-PTH) is in dark gray, and LSN3160440 is in yellow. A and B are adapted from Sutkeviciute et al (126).
Location Bias in PTH1R Signaling and Pharmacological Implications
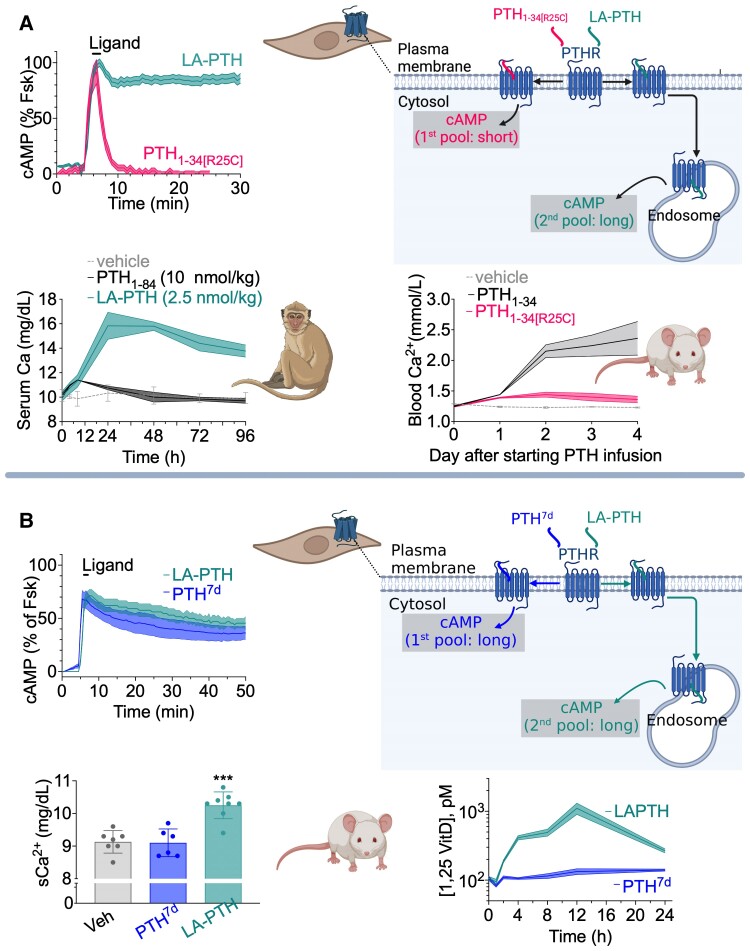
Sustained endosomal cAMP production has likely important pharmacological and physiological implications, as injection of LA-PTH into mice and monkeys increases serum calcium and decreases serum phosphate more efficiently than does PTH1-34 (83, 95). Conversely, a PTH mutant (PTH-R25C) found in patients with a severe form of chronic hypocalcemia cannot stimulate endosomal PTH1R signaling via cAMP in cells and fails to elevate blood Ca2+ elevation in animals (21, 130) (Fig. 10A). The recent (2021) design of a novel PTH1-34 variant contributed to better understand the functional role of location bias (plasma membrane vs endosomes) in PTH1R signaling independently of the duration of cAMP. The ANM analysis of inter-residues cross-correlations in the PTH-bound receptor (Fig. 8C) let to conceive a PTH analog via epimerization of Leu 7 (PTH7d) with the desired bias property to sustain cAMP production exclusively from the plasma membrane (131). Findings from studies in cells and mice comparing the effects of the 2 biased PTH agonists, PTH7d and LA-PTH, which induce a similar elevation and duration of cAMP production but generated in separate cell locations—plasma membrane (for PTH7d) vs endosomes (for LA-PTH)—helped examine the role of location bias in cAMP production for prime pharmacological functions of the PTH1R (131) (Fig. 10B). In mice, LA-PTH and PTH7d injections led to a similar reduction in serum phosphate ion due to inhibition of renal phosphate reabsorption (a major physiological role of PTH acting via PTH1R to maintain plasma PO43– level constant); however, LA-PTH induces significantly more elevation of serum vitamin D and Ca2+ than PTH7d (Fig. 9B). In polarized Mardin–Darby canine kidney cells, the LA-PTH–generated endosomal cAMP pool ensures nuclear cAMP accumulation and nuclear PKA activity leading to production of the 25-hydroxyvitamin D 1-α-hydroxylase (1α[OH]ase), the rate-limiting enzyme catalyzing the formation of active vitamin D. Conversely, the PTH7d-generated plasma membrane cAMP pool does not lead to nuclear PKA activity and elevation of the 1α[OH]ase. These observations support possible functional implications of distinct locations of cAMP generation in the control of mineral-ion balance and vitamin D levels.
Figure 10.
Pharmacological actions of biased PTH ligands in mice. (A) Pharmacological actions of LA-PTH and PTH-R25C in cell, mice and monkeys from studies reported in (95, 131). (B) LA-PTH and PTH7d are biased PTH agonists inducing a similar sustained cAMP production that originates from distinct cell locations: plasma membrane for PTH7d and endosomes for LA-PTH. When injected into mice, both ligands induce a similar reduction in serum phosphate ion; however, LA-PTH mediates a remarkable increase in serum levels of calcium ion and vitamin D.
PTH1R Coupling to β-Arrestins
Binding Mode of Arrestin to GPCRs
Arrestins engage with activated GPCR at 2 main sites: (1) a positively charged groove located in the N-terminal domain of arrestin binds to the phosphorylated C-terminal tail (C-tail) of the receptor; (2) several regions, including a finger loop, interact with the receptor core (132, 133). Binding of phosphorylated receptor C-tail to arrestin displaces the arrestin C-tail, disrupting a 3-element interaction and a polar core that stabilize the inactive arrestin conformation (134). These changes promote an active conformation in which the N- and C-terminal domains are rotated by 20°. The arrestin finger loop adopts a helical conformation upon engagement with the receptor core (135). Residues in the finger loop, lariat loop, and elsewhere on arrestin form hydrophobic and polar interactions with the receptor core. In comparison with an arrestin structure bound to a synthetic receptor phosphopeptide, the structure of rhodopsin (the light-sensing GPCR)-bound arrestin (135) exhibits unique loop conformations and a small change in the interdomain twist. While binding to the receptor C-tail was previously considered a prerequisite for engagement with the receptor core (132), recent research suggests that arrestin can also engage with the receptor core independent of receptor C-tail engagement (136, 137). Extensive MD simulations of rhodopsin–arrestin-1 complex determined that interactions between arrestin loops, not including the finger loop, with receptor intracellular loops (ICLs) 2 and 3 are the main mediators of arrestin activation via the receptor core. Furthermore, both receptor core and phosphorylated C-tail binding trigger arrestin activation by promoting distinct conformations of arrestin C-loop and lariat loop. While the molecular mechanism of arrestin C-tail displacement is not clear, the displaced C-tail binds clathrin and clathrin adaptor AP2, permitting receptor internalization (62, 137, 138).
PTH1R–Arrestin Interaction
Part of the contact residues between PTH-activated PTH1R and β-arrestin-1 (βarr1) have been identified using photo-crosslinking coupled to mass spectrometry experiments (120, 139). Crosslinks were identified between F75 of βarr1 and PTH1R residues V3845.64b and T3925.71b, located at the cytoplasmic end of TM5 (120). These photo-crosslinks support a conformation in which the βarr1 finger loop is engaging with the receptor cytosolic core as observed in the crystal structure of rhodopsin bound to the murine visual arrestin (140).
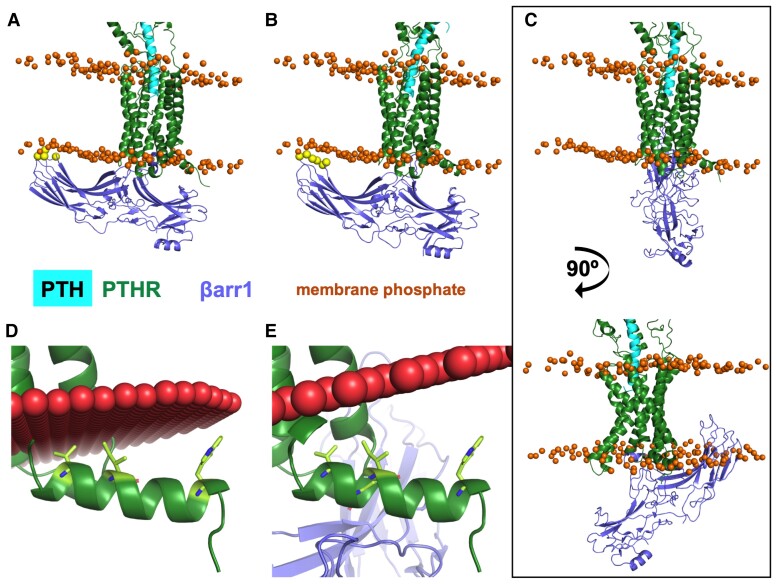
To visualize these photo-crosslinks, models of PTH1R bound to βarr1 were generated using MD snapshots of PTH1R (120) and 3 structures of GPCR–arrestin complexes: rhodopsin–visual arrestin (PDB 5W0P, model 1) (135), M2 muscarinic receptor–βarr1 (PDB 6U1N, model 2) (141), and neurotensin receptor 1–βarr1 (PDB 6UP7, model 3) (142) (Fig. 11). PyMOL was used to perform structural, sequence-independent alignment of the TMD of the GPCR–βarr1 template structure to the PTH1RTMD (residues 180–460) from each snapshot. In the model of PTH1R–βarr1 complex generated using rhodopsin–visual-arr (model 1) (135), C-edge loops are slightly embedded in the membrane (Fig. 11A), as observed in other GPCR–arrestin structures (135, 141–143). The βarr1 in model 3 clashes significantly with the membrane (Fig. 11C), and interactions between PTH1R helix 8 residues and the membrane are eliminated (Fig. 11D and E). Model 1 satisfies distance restraints between βarr1 F75 and PTHR V3845.64b/T3925.7b that are well within photo-crosslinking restraints (Fig. 11A). The inward movement of receptor TM6 relative to the Gs-bound conformation has been reported essential for βarr coupling (144). The PTH1R–βarr1 model permits this TM6 inward movement without clashing with βarr1. Interface in this PTH1R–βarr1 complex (model 1) includes many contacts with ICL1 and ICL2, more so than in model 2 (Fig. 11B).
Figure 11.
Position of β-arrestin 1 relative to the membrane in PTH1R–βarr1 models. In all panels, PTH is cyan, PTH1R is dark green, and β-arrestin 1 is slate blue. The orange spheres represent membrane phosphates after 200 ns MD simulation. In A and B, yellow spheres are β-arrestin 1 C-edge loop residues that contact the membrane. (A) Model 1: PTH1R–βarr1 model generated using the rhodopsin–arrestin structure (PDB 5W0P) as a template. (B) Model 2: PTH1R–βarr1 model with M2R–βarr1 template structure (PDB 6U1N). (C-E) Model 3: PTH1R–βarr1 model with NTSR1–βarr1 template structure (PDB 6UP7). (C) Top. Model 3 with same orientation as A and B. Bottom. Model 3 rotated 90° to highlight significant embedment of β-arr1 C-edge in the membrane. In D and E, PTH1R alone and model 3 positioned in membrane (red spheres) by the OPM server. Helix 8 residues from PTH1R alone interacting with the membrane in panel D are represented as lime sticks. Such interactions are eliminated in model 3.
Next Questions to Address
Ongoing basic research is providing important insights into structural mechanisms of PTH1R signaling in response to PTH and PTHrP, including ligand binding, receptor activation, and coupling to Gs and β-arrestins. Since its initial discovery, significant advancements have been made toward elucidating molecular and cellular determinants that underlie PTH1R endosomal cAMP. Even so, several outstanding questions remain. Although we made models of PTH1R–βarr based on available structures, conformational heterogeneity in GPCR–arrestin complexes has been observed (142). Cryo-EM analysis of 2D class averages from purified PTH1R–βarr could reveal possible conformations of this complex. Importantly, it is not clear what are the structural differences between PTH1R–arrestin complexes at the plasma membrane versus those in endosomes (ie, supercomplex with both β-arrestin and Gs bound). What structural features contribute to PTH1R coupling to Gq and G12/13? Also, how does PTHrP/ABL stabilize unique conformations of PTH1R that lead to transient cAMP production at the plasma membrane? Recent studies addressed in part this question by showing that PTHrP1-36 does not mimic signaling mediated by the native PTHrP1-141, which induces sustained cAMP at the plasma membrane due to impaired β-arrestin coupling (145, 146). These data motivate a reinterpretation of our prior understanding on how native PTHrP acts on the PTH1R. Additional structural and cellular studies will also provide a better understanding of the differences in signaling observed between PTH1R ligands. Do different ligands trigger distinctive membrane lipids dynamics that result in either receptor internalization or retention of receptors at the plasma membrane? Does the ternary PTH1R–Gβγ–arrestin complex internalize into endosomes whose lipid composition differs when PTH1R is activated by either PTH1-34 or LA-PTH? If there is such a difference, does it contribute to the prolonged PTH1R–LA-PTH interaction that engages sustained cAMP? Furthermore, research is needed to determine whether endosomal cAMP production directly regulates bone turnover. Answering these questions will help explain the differential effects of PTH1R ligands on bone turnover, calcium levels, and vitamin D homeostasis and could serve as a foundation for the development of clinically relevant PTH analogs tailored to target specific aspects of PTH1R signaling involved in bone and mineral diseases.
Abbreviations
- ABL
abaloparatide
- cAMP
cyclic adenosine monophosphate
- ECD
extracellular domain
- ENM
elastic network model
- FRET
Förster resonance energy transfer
- GDP
guanosine-5′-diphosphate
- GLP-1
glucagon-like peptide 1
- GLP1R
GLP-1 receptor
- GPCR
G protein–coupled receptor
- GTP
guanosine-5′-triphosphate
- ICL
intracellular loop
- IP3
inositol (1,4,5)-trisphosphate
- MD
molecular dynamics
- PI3K
phosphoinositide 3-kinase
- PLC
phospholipase C
- PTH
parathyroid hormone
- PTH1R
PTH type 1 receptor
- PTHR
PTH receptor
- PTHrP
PTH-related protein
- TM
transmembrane
- TMD
transmembrane domain
- VitD
vitamin D3
Contributor Information
Jean-Pierre Vilardaga, Laboratory for GPCR Biology, Department of Pharmacology and Chemical Biology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261, USA.
Lisa J Clark, Laboratory for GPCR Biology, Department of Pharmacology and Chemical Biology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261, USA.
Alex D White, Laboratory for GPCR Biology, Department of Pharmacology and Chemical Biology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261, USA.
Ieva Sutkeviciute, Laboratory for GPCR Biology, Department of Pharmacology and Chemical Biology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261, USA.
Ji Young Lee, Department of Computational and Systems Biology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261, USA.
Ivet Bahar, Department of Computational and Systems Biology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261, USA.
Funding
Redaction of this chapter was supported by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), the National Institute of Dental and Craniofacial Research (NIDCR), and the National Institute for General Medical Sciences (NIGMS) of the US National Institutes of Health (NIH) under grant Awards Numbers R01DK116780, R01DK122259, and R21DE032478 (to J.-P.V.), and R01GM139297-01A1 (to I.B.)
Disclosure
I.S., J.Y.L., I.B. and J.-P.V. acknowledge potential competing financial interests for Small molecule allosteric modulators of class B GPCR and method to identify them.
References
- 1. Jüppner H, Abou-Samra AB, Freeman M, et al. . A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254(5034):1024‐1026. [DOI] [PubMed] [Google Scholar]
- 2. Horwitz MJ, Tedesco MB, Sereika SM, et al. . Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D. J Bone Miner Res. 2005;20(10):1792‐1803. [DOI] [PubMed] [Google Scholar]
- 3. Miao D, He B, Lanske B, et al. . Skeletal abnormalities in PTH-null mice are influenced by dietary calcium. Endocrinology. 2004;145(4):2046‐2053. [DOI] [PubMed] [Google Scholar]
- 4. Silva BC, Costa AG, Cusano NE, Kousteni S, Bilezikian JP. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J Endocrinol Invest. 2011;34(10):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyce BF, Rosenberg E, de Papp AE, Duong LT. The osteoclast, bone remodelling and treatment of metabolic bone disease. Eur J Clin Invest. 2012;42(12):1332‐1341. [DOI] [PubMed] [Google Scholar]
- 6. O'Brien CA, Nakashima T, Takayanagi H. Osteocyte control of osteoclastogenesis. Bone. 2013;54(2):258‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saini V, Marengi DA, Barry KJ, et al. . Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J Biol Chem. 2013;288(28):20122‐20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Abel M, Hoenderop JG, van der Kemp AW, Friedlaender MM, van Leeuwen JP, Bindels RJ. Coordinated control of renal Ca(2+) transport proteins by parathyroid hormone. Kidney Int. 2005;68(4):1708‐1721. [DOI] [PubMed] [Google Scholar]
- 9. de Groot T, Lee K, Langeslag M, et al. . Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol. 2009;20(8):1693‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biber J, Hernando N, Forster I, Murer H. Regulation of phosphate transport in proximal tubules. Pflugers Archiv. 2009;458(1):39‐52. [DOI] [PubMed] [Google Scholar]
- 11. Picard N, Capuano P, Stange G, et al. . Acute parathyroid hormone differentially regulates renal brush border membrane phosphate cotransporters. Pflugers Arch. 2010;460(3):677‐687. [DOI] [PubMed] [Google Scholar]
- 12. Nagai S, Okazaki M, Segawa H, et al. . Acute down-regulation of sodium-dependent phosphate transporter NPT2a involves predominantly the cAMP/PKA pathway as revealed by signaling-selective parathyroid hormone analogs. J Biol Chem. 2011;286(2):1618‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suva LJ, Winslow GA, Wettenhall RE, et al. . A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science. 1987;237(4817):893‐896. [DOI] [PubMed] [Google Scholar]
- 14. Nissenson RA, Diep D, Strewler GJ. Synthetic peptides comprising the amino-terminal sequence of a parathyroid hormone-like protein from human malignancies. Binding to parathyroid hormone receptors and activation of adenylate cyclase in bone cells and kidney. J Biol Chem. 1988;263(26):12866‐12871. [PubMed] [Google Scholar]
- 15. McCauley LK, Martin TJ. Twenty-five years of PTHrP progress: from cancer hormone to multifunctional cytokine. J Bone Miner Res. 2012;27(6):1231‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karaplis AC, Luz A, Glowacki J, et al. . Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8(3):277‐289. [DOI] [PubMed] [Google Scholar]
- 17. Wysolmerski JJ, Stewart AF. The physiology of parathyroid hormone-related protein: an emerging role as a developmental factor. Annu Rev Physiol. 1998;60(1):431‐460. [DOI] [PubMed] [Google Scholar]
- 18. Philbrick WM, Dreyer BE, Nakchbandi IA, Karaplis AC. Parathyroid hormone-related protein is required for tooth eruption. Proc Natl Acad Sci USA. 1998;95(20):11846‐11851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Philbrick WM, Wysolmerski JJ, Galbraith S, et al. . Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev. 1996;76(1):127‐173. [DOI] [PubMed] [Google Scholar]
- 20. Dean T, Vilardaga JP, Potts JT Jr, Gardella TJ. Altered selectivity of parathyroid hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol Endocrinol. 2008;22(1):156‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. White AD, Fang F, Jean-Alphonse FG, et al. . Ca2+ allostery in PTH-receptor signaling. Proc Natl Acad Sci USA. 2019;116(8):3294‐3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Potts JT J, Tregear GW, Keutmann HT, et al. . Synthesis of a biologically active N-terminal tetratriacontapeptide of parathyroid hormone. Proc Natl Acad Sci USA. 1971;68(1):63‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheloha RW, Gellman SH, Vilardaga JP, Gardella TJ. PTH receptor-1 signalling-mechanistic insights and therapeutic prospects. Nat Rev Endocrinol. 2015;11(12):712‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jobert AS, Zhang P, Couvineau A, et al. . Absence of functional receptors for parathyroid hormone and parathyroid hormone-related peptide in Blomstrand chondrodysplasia. J Clin Invest. 1998;102(1):34‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoogendam J, Farih-Sips H, Wÿnaendts LC, Löwik CW, Wit JM, Karperien M. Novel mutations in the parathyroid hormone (PTH)/PTH-related peptide receptor type 1 causing Blomstrand osteochondrodysplasia types I and II. J Clin Endocrinol Metab. 2007;92(3):1088‐1095. [DOI] [PubMed] [Google Scholar]
- 26. Pansuriya TC, Kroon HM, Bovee JV. Enchondromatosis: insights on the different subtypes. Int J Clin Exp Pathol. 2010;3(6):557‐569. [PMC free article] [PubMed] [Google Scholar]
- 27. Hanisch M, Hanisch L, Kleinheinz J, Jung S. Primary failure of eruption (PFE): a systematic review. Head Face Med. 2018;14(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tokavanich N, Gupta A, Nagata M, et al. . A three-dimensional analysis of primary failure of eruption in humans and mice. Oral Dis. 2020;26(2):391‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schipani E, Kruse K, Jüppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995:268(5207):98‐100. [DOI] [PubMed] [Google Scholar]
- 30. Schipani E, Langman C, Hunzelman J, et al. . A novel parathyroid hormone (PTH)/PTH-related peptide receptor mutation in Jansen's Metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 1999;84(9):3052‐3057. [DOI] [PubMed] [Google Scholar]
- 31. Schipani E, Langman CB, Parfitt AM, et al. . Constitutively activated receptors for parathyroid hormone and parathyroid hormone-related peptide in Jansen's Metaphyseal chondrodysplasia. N Engl J Med. 1996;335(10):708‐714. [DOI] [PubMed] [Google Scholar]
- 32. Feldenzer KL, Sarno J. Hypercalcemia of malignancy. J Adv Pract Oncol. 2018;9(5):496‐504. [PMC free article] [PubMed] [Google Scholar]
- 33. Giovanella L, Bacigalupo L, Treglia G, Piccardo A. Will 18F-fluorocholine PET/CT replace other methods of preoperative parathyroid imaging? Endocrine. 2020;71(2):285‐297. [DOI] [PubMed] [Google Scholar]
- 34. Rubin MR. Recent advances in understanding and managing hypoparathyroidism. F1000Res. 2020;9:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357(9):905‐916. [DOI] [PubMed] [Google Scholar]
- 36. Cohen A, Shiau S, Nair N, et al. . Effect of teriparatide on bone remodeling and density in premenopausal idiopathic osteoporosis: A phase II trial. J Clin Endocrinol Metab. 2020;105(10):e3540‐e3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ono K, Ohashi S, Oka H, et al. . Evaluations of daily teriparatide using finite-element analysis over 12 months in rheumatoid arthritis patients. J Bone Miner Metab. 2020;39(2):270‐277. [DOI] [PubMed] [Google Scholar]
- 38. Neer RM, Arnaud CD, Zanchetta JR, et al. . Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434‐1441. [DOI] [PubMed] [Google Scholar]
- 39. Leder BZ, O'Dea LS, Zanchetta JR, et al. . Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2015;100(2):697‐706. [DOI] [PubMed] [Google Scholar]
- 40. Sleeman A, Clements JN. Abaloparatide: A new pharmacological option for osteoporosis. Am J Health Syst Pharm. 2019;76(3):130‐135. [DOI] [PubMed] [Google Scholar]
- 41. Miller PD, Hattersley G, Lau E, et al. . Bone mineral density response rates are greater in patients treated with abaloparatide compared with those treated with placebo or teriparatide: results from the ACTIVE phase 3 trial. Bone. 2019;120:137‐140. [DOI] [PubMed] [Google Scholar]
- 42. Arlt H, Mullarkey T, Hu D, et al. . Effects of abaloparatide and teriparatide on bone resorption and bone formation in female mice. Bone Rep. 2020;13:100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hattersley G, Dean T, Corbin BA, Bahar H, Gardella TJ. Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology. 2016;157(1):141‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schiöth HB, Fredriksson R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen Comp Endocrinol. 2005;142(1-2):94‐101. [DOI] [PubMed] [Google Scholar]
- 45. Alexander SP, Davenport AP, Kelly E, et al. . The concise guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. Br J Pharmacol. 2015;172(24):5744‐5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pándy-Szekeres G, Munk C, Tsonkov TM, et al. . GPCRdb in 2018: adding GPCR structure models and ligands. Nucleic Acids Res. 2017;46(D1):D440‐D446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Congreve M, de Graaf C, Swain NA, Tate CG. Impact of GPCR structures on drug discovery. Cell. 2020;181(1):81‐91. [DOI] [PubMed] [Google Scholar]
- 48. Pal K, Melcher K, Xu HE. Structure and mechanism for recognition of peptide hormones by class B G-protein-coupled receptors. Acta Pharmacol Sin. 2012;33(3):300‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Palczewski K, Kumasaka T, Hori T, et al. . Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000:289(5480):739‐745. [DOI] [PubMed] [Google Scholar]
- 50. de Graaf C, Song G, Cao C, et al. . Extending the structural view of class B GPCRs. Trends Biochem Sci. 2017;42(12):946‐960. [DOI] [PubMed] [Google Scholar]
- 51. Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf). 2007;190(1):9‐19. [DOI] [PubMed] [Google Scholar]
- 52. Lefkowitz RJ. Seven transmembrane receptors: a brief personal retrospective. Biochim Biophys Acta. 2007;1768(4):748‐755. [DOI] [PubMed] [Google Scholar]
- 53. Oldham WM, Hamm HE. Structural basis of function in heterotrimeric G proteins. Q Rev Biophys. 2006;39(2):117‐166. [DOI] [PubMed] [Google Scholar]
- 54. Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296(5573):1636‐1639. [DOI] [PubMed] [Google Scholar]
- 55. Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9(1):60‐71. [DOI] [PubMed] [Google Scholar]
- 56. Rasmussen SG, DeVree BT, Zou Y, et al. . Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011;477(7366):549‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dupré DJ, Robitaille M, Rebois RV, Hébert TE. The role of Gβγ subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol. 2009;49(1):31‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vadas O, Dbouk HA, Shymanets A, et al. . Molecular determinants of PI3Kγ-mediated activation downstream of G-protein-coupled receptors (GPCRs). Proc Natl Acad Sci USA. 2013;110(47):18862‐18867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dicker F, Quitterer U, Winstel R, Honold K, Lohse MJ. Phosphorylation-independent inhibition of parathyroid hormone receptor signaling by G protein-coupled receptor kinases. Proc Natl Acad Sci USA. 1999;96(10):5476‐5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lohse MJ, Andexinger S, Pitcher J, et al. . Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of beta-arrestin and arrestin in the beta 2-adrenergic receptor and rhodopsin systems. J Biol Chem. 1992; 267(12):8558‐8564. [PubMed] [Google Scholar]
- 61. Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99(6):570‐582. [DOI] [PubMed] [Google Scholar]
- 62. Goodman OB, Krupnick JG, Santini F, et al. . Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996:383(6599):447‐450. [DOI] [PubMed] [Google Scholar]
- 63. Lin FT, Krueger KM, Kendall HE, et al. . Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem. 1997;272(49):31051‐31057. [DOI] [PubMed] [Google Scholar]
- 64. Hamm HE. How activated receptors couple to G proteins. Proc Natl Acad Sci USA. 2001;98(9):4819‐4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RJ. The role of sequestration in G protein-coupled receptor resensitization. Regulation of beta2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem. 1997; 272(1):5‐8. [DOI] [PubMed] [Google Scholar]
- 66. Abou-Samra AB, Jüppner H, Force T, et al. . Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA. 1992;89(7):2732‐2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Singh AT, Gilchrist A, Voyno-Yasenetskaya T, Radeff-Huang JM, Stern PH. G alpha12/G alpha13 subunits of heterotrimeric G proteins mediate parathyroid hormone activation of phospholipase D in UMR-106 osteoblastic cells. Endocrinology. 2005;146(5):2171‐2175. [DOI] [PubMed] [Google Scholar]
- 68. Singh AT, Frohman MA, Stern PH. Parathyroid hormone stimulates phosphatidylethanolamine hydrolysis by phospholipase D in osteoblastic cells. Lipids. 2005;40(11):1135‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Avet C, Mancini A, Breton B, et al. . Effector membrane translocation biosensors reveal G protein and βarrestin coupling profiles of 100 therapeutically relevant GPCRs. Elife. 2022;11:e74101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fraher LJ, Hodsman AB, Jonas K, et al. . A comparison of the in vivo biochemical responses to exogenous parathyroid hormone-(1-34) [PTH-(1-34)] and PTH-related peptide-(1-34) in man. J Clin Endocrinol Metab. 1992;75(2):417‐423. [DOI] [PubMed] [Google Scholar]
- 71. Vilardaga JP, Gardella TJ, Wehbi VL, Feinstein TN. Non-canonical signaling of the PTH receptor. Trends Pharmacol Sci. 2012;33(8):423‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Malecz N, Bambino T, Bencsik M, Nissenson RA. Identification of phosphorylation sites in the G protein-coupled receptor for parathyroid hormone. Receptor phosphorylation is not required for agonist-induced internalization. Mol Endocrinol. 1998;12(12):1846‐1856. [DOI] [PubMed] [Google Scholar]
- 73. Castro M, Dicker F, Vilardaga JP, Krasel C, Bernhardt M, Lohse MJ. Dual regulation of the parathyroid hormone (PTH)/PTH-related peptide receptor signaling by protein kinase C and beta-arrestins. Endocrinology. 2002;143(10):3854‐3865. [DOI] [PubMed] [Google Scholar]
- 74. Vilardaga JP, Krasel C, Chauvin S, Bambino T, Lohse MJ, Nissenson RA. Internalization determinants of the parathyroid hormone receptor differentially regulate beta-arrestin/receptor association. J Biol Chem. 2002;277(10):8121‐8129. [DOI] [PubMed] [Google Scholar]
- 75. Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5(6):428‐434. [DOI] [PubMed] [Google Scholar]
- 76. Ferrandon S, Feinstein TN, Castro M, et al. . Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5(10):734‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wehbi VL, Stevenson HP, Feinstein TN, Calero G, Romero G, Vilardaga JP. Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gβγ complex. Proc Natl Acad Sci USA. 2013;110(4):1530‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Calebiro D, Nikolaev VO, Gagliani MC, et al. . Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7(8):e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Feinstein TN, Yui N, Webber MJ, et al. . Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J Biol Chem. 2013;288(39):27849‐27860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sutkeviciute I, Vilardaga JP. Structural insights into emergent signaling modes of G protein-coupled receptors. J Biol Chem. 2020;295(33):11626‐11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Crilly SE, Puthenveedu MA. Compartmentalized GPCR signaling from intracellular membranes. J Membr Biol. 2021;254(3):259‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Okazaki M, Ferrandon S, Vilardaga JP, Bouxsein ML, Potts JT Jr, Gardella TJ. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc Natl Acad Sci USA. 2008;105(43):16525‐16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Maeda A, Okazaki M, Baron DM, et al. . Critical role of parathyroid hormone (PTH) receptor-1 phosphorylation in regulating acute responses to PTH. Proc Natl Acad Sci USA. 2013;110(15):5864‐5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pioszak AA, Xu HE. Molecular recognition of parathyroid hormone by its G protein-coupled receptor. Proc Natl Acad Sci USA. 2008;105(13):5034‐5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pioszak AA, Parker NR, Gardella TJ, Xu HE. Structural basis for parathyroid hormone-related protein binding to the parathyroid hormone receptor and design of conformation-selective peptides. J Biol Chem. 2009;284(41):28382‐28391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Goltzman D, Peytremann A, Callahan E, Tregear GW, Potts JT Jr. Analysis of the requirements for parathyroid hormone action in renal membranes with the use of inhibiting analogues. J Biol Chem. 1975;250(8):3199‐3203. [PubMed] [Google Scholar]
- 87. Zhao LH, Ma S, Sutkeviciute I, et al. . Structure and dynamics of the active human parathyroid hormone receptor-1. Science. 2019;364(6436):148‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Horiuchi N, Holick MF, Potts JT Jr, Rosenblatt M. A parathyroid hormone inhibitor in vivo: design and biological evaluation of a hormone analog. Science. 1983;220(4601):1053‐1055. [DOI] [PubMed] [Google Scholar]
- 89. Shimizu M, Carter PH, Gardella TJ. Autoactivation of type-1 parathyroid hormone receptors containing a tethered ligand. J Biol Chem. 2000;275(26):19456‐19460. [DOI] [PubMed] [Google Scholar]
- 90. Shimizu M, Potts JT Jr, Gardella TJ. Minimization of parathyroid hormone. Novel amino-terminal parathyroid hormone fragments with enhanced potency in activating the type-1 parathyroid hormone receptor. J Biol Chem. 2000;275(29):21836‐21843. [DOI] [PubMed] [Google Scholar]
- 91. Shimizu N, Guo J, Gardella TJ. Parathyroid hormone (PTH)-(1-14) and -(1-11) analogs conformationally constrained by alpha-aminoisobutyric acid mediate full agonist responses via the juxtamembrane region of the PTH-1 receptor. J Biol Chem. 2001;276(52):49003‐49012. [DOI] [PubMed] [Google Scholar]
- 92. Shimizu M, Carter PH, Khatri A, Potts JT Jr, Gardella TJ. Enhanced activity in parathyroid hormone-(1-14) and -(1-11): novel peptides for probing ligand-receptor interactions. Endocrinology. 2001;142(7):3068‐3074. [DOI] [PubMed] [Google Scholar]
- 93. Noda H, Okazaki M, Joyashiki E, et al. . Optimization of PTH/PTHrP hybrid peptides to derive a long-acting PTH analog (LA-PTH). JBMR Plus. 2020;4(7):e10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jean-Alphonse FG, Wehbi VL, Chen J, et al. β2-adrenergic receptor control of endosomal PTH receptor signaling via Gβγ. Nat Chem Biol. 2017;13(3):259‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shimizu M, Joyashiki E, Noda H, et al. . Pharmacodynamic actions of a long-acting PTH analog (LA-PTH) in thyroparathyroidectomized (TPTX) rats and normal monkeys. J Bone Miner Res. 2016;31(7):1405‐1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Feinstein TN, Wehbi VL, Ardura JA, et al. . Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol. 2011;7(5):278‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. White AD, Jean-Alphonse FG, Fang F, et al. . Gq/11-dependent regulation of endosomal cAMP generation by parathyroid hormone class B GPCR. Proc Natl Acad Sci USA. 2020;117(13):7455‐7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Collins BM, Norwood SJ, Kerr MC, et al. . Structure of Vps26B and mapping of its interaction with the retromer protein complex. Traffic. 2008;9(3):366‐379. [DOI] [PubMed] [Google Scholar]
- 99. Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7(8):568‐579. [DOI] [PubMed] [Google Scholar]
- 100. Gidon A, Al-Bataineh MM, Jean-Alphonse FG, et al. . Endosomal GPCR signaling turned off by negative feedback actions of PKA and v-ATPase. Nat Chem Biol. 2014;10(9):707‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pioszak AA, Harikumar KG, Parker NR, Miller LJ, Xu HE. Dimeric arrangement of the parathyroid hormone receptor and a structural mechanism for ligand-induced dissociation. J Biol Chem. 2010;285(16):12435‐12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ehrenmann J, Schöppe J, Klenk C, et al. . High-resolution crystal structure of parathyroid hormone 1 receptor in complex with a peptide agonist. Nat Struct Mol Biol. 2018;25(12):1086‐1092. [DOI] [PubMed] [Google Scholar]
- 103. Ma S, Shen Q, Zhao LH, et al. . Molecular basis for hormone recognition and activation of corticotropin-releasing factor receptors. Mol Cell. 2020;77(3):669‐680.e664. [DOI] [PubMed] [Google Scholar]
- 104. Qiao A, Han S, Li X, et al. . Structural basis of Gs and Gi recognition by the human glucagon receptor. Science. 2020;367(6484):1346‐1352. [DOI] [PubMed] [Google Scholar]
- 105. Kobayashi K, Shihoya W, Nishizawa T, et al. . Cryo-EM structure of the human PAC1 receptor coupled to an engineered heterotrimeric G protein. Nat Struct Mol Biol. 2020;27(3):274‐280. [DOI] [PubMed] [Google Scholar]
- 106. Liang YL, Belousoff MJ, Zhao P, et al. . Toward a structural understanding of class B GPCR peptide binding and activation. Mol Cell. 2020;77(3):656‐668.e655. [DOI] [PubMed] [Google Scholar]
- 107. dal Maso E, Glukhova A, Zhu Y, et al. . The molecular control of calcitonin receptor signaling. ACS Pharmacol Transl Sci. 2019;2(1):31‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Liang YL, Khoshouei M, Deganutti G, et al. . Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor. Nature. 2018;561(7724):492‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liang YL, Khoshouei M, Glukhova A, et al. . Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor-Gs complex. Nature. 2018;555(7694):121‐125. [DOI] [PubMed] [Google Scholar]
- 110. Zhang Y, Sun B, Feng D, et al. . Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature. 2017;546(7657):248‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Song G, Yang D, Wang Y, et al. . Human GLP-1 receptor transmembrane domain structure in complex with allosteric modulators. Nature. 2017;546(7657):312‐315. [DOI] [PubMed] [Google Scholar]
- 112. Hilger D, Kumar KK, Hu H, et al. . Structural insights into differences in G protein activation by family A and family B GPCRs. Science. 2020;369(6503): eaba3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dong M, Deganutti G, Piper SJ, et al. . Structure and dynamics of the active Gs-coupled human secretin receptor. Nat Commun. 2020;11(1):4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Duan J, Shen DD, Zhou XE, et al. . Cryo-EM structure of an activated VIP1 receptor-G protein complex revealed by a NanoBiT tethering strategy. Nat Commun. 2020;11(1):4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sun W, Chen LN, Zhou Q, et al. . A unique hormonal recognition feature of the human glucagon-like peptide-2 receptor. Cell Res. 2020;30(12):1098‐1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhao F, Zhang C, Zhou Q, et al. . Structural insights into hormone recognition by the human glucose-dependent insulinotropic polypeptide receptor. Elife. 2021;10:e68719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wang X, Cheng X, Zhao L, et al. . Molecular insights into differentiated ligand recognition of the human parathyroid hormone receptor 2. Proc Natl Acad Sci USA. 2021;118(32):e2101279118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Josephs TM, Belousoff MJ, Liang YL, et al. . Structure and dynamics of the CGRP receptor in apo and peptide-bound forms. Science. 2021;372(6538):eabf7258. [DOI] [PubMed] [Google Scholar]
- 119. Castro M, Nikolaev VO, Palm D, Lohse MJ, Vilardaga JP. Turn-on switch in parathyroid hormone receptor by a two-step parathyroid hormone binding mechanism. Proc Natl Acad Sci USA. 2005;102(44):16084‐16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Clark LJ, Krieger J, White AD, et al. . Allosteric interactions in the parathyroid hormone GPCR-arrestin complex formation. Nat Chem Biol. 2020;16(10):1096‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bahar I, Lezon TR, Bakan A, Shrivastava IH. Normal Mode analysis of biomolecular structures: functional mechanisms of membrane proteins. Chem Rev. 2010;110(3):1463‐1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zhang Y, Doruker P, Kaynak B, et al. . Intrinsic dynamics is evolutionarily optimized to enable allosteric behavior. Curr Opin Struct Biol. 2020;62:14‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lezon TR, Bahar I. Constraints imposed by the membrane selectively guide the alternating access dynamics of the glutamate transporter GltPh. Biophys J. 2012;102(6):1331‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Li H, Chang YY, Lee JY, Bahar I, Yang LW. Dynomics: dynamics of structural proteome and beyond. Nucleic Acids Res. 2017;45(W1):W374‐W380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kaynak BT, Bahar I, Doruker P. Essential site scanning analysis: A new approach for detecting sites that modulate the dispersion of protein global motions. Comput Struct Biotechnol J. 2020;18:1577‐1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sutkeviciute I, Lee JY, White AD, et al. . Precise druggability of the PTH type 1 receptor. Nat Chem Biol. 2022;18(3):272‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bueno AB, Sun B, Willard FS, et al. . Structural insights into probe-dependent positive allosterism of the GLP-1 receptor. Nat Chem Biol. 2020;16(10):1105‐1110. [DOI] [PubMed] [Google Scholar]
- 128. Tamura T, Noda H, Joyashiki E, et al. . Identification of an orally active small-molecule PTHR1 agonist for the treatment of hypoparathyroidism. Nat Commun. 2016;7(1):13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Nishimura Y, Esaki T, Isshiki Y, et al. . Lead optimization and avoidance of reactive metabolite leading to PCO371, a potent, selective, and orally available human parathyroid hormone receptor 1 (hPTHR1) agonist. J Med Chem. 2020; 63(10):5089‐5099. [DOI] [PubMed] [Google Scholar]
- 130. Lee S, Mannstadt M, Guo J, et al. . A homozygous [Cys25]PTH(1-84) mutation that impairs PTH/PTHrP receptor activation defines a novel form of hypoparathyroidism. J Bone Miner Res. 2015;30(10):1803‐1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. White AD, Peña KA, Clark LJ, et al. . Decoding location bias information of cAMP signaling in a class B GPCR. Sci Signal. 2021;14(703):eabc5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Shukla AK, Manglik A, Kruse AC, et al. . Structure of active beta-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497(7447):137‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chen Q, Iverson TM, Gurevich VV. Structural basis of arrestin-dependent signal transduction. Trends Biochem Sci. 2018;43(6):412‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C. Crystal structure of beta-arrestin at 1.9 A: possible mechanism of receptor binding and membrane translocation. Structure. 2001;9(9):869‐880. [DOI] [PubMed] [Google Scholar]
- 135. Zhou XE, He Y, de Waal PW, et al. . Identification of phosphorylation codes for arrestin recruitment by G protein-coupled receptors. Cell. 2017;170(3):457‐469.e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Eichel K, Jullie D, Barsi-Rhyne B, et al. . Catalytic activation of beta-arrestin by GPCRs. Nature. 2018;557(7705):381‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Latorraca NR, Wang JK, Bauer B, et al. . Molecular mechanism of GPCR-mediated arrestin activation. Nature. 2018;557(7705):452‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Laporte SA, Miller WE, Kim KM. Caron MG. beta-arrestin/AP-2 interaction in G protein-coupled receptor internalization: identification of a beta-arrestin binging site in beta 2-adaptin. J Biol Chem. 2002;277(11):9247‐9254. [DOI] [PubMed] [Google Scholar]
- 139. Bottke T, Ernicke S, Serfling R, etal. Exploring GPCR-arrestin interfaces with genetically encoded crosslinkers. EMBO Rep. 2020;21(11):e15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kang Y, Zhou XE, Gao X, et al. . Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature. 2015;523(7562):561‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Staus DP, Hu H, Robertson MJ, et al. . Structure of the M2 muscarinic receptor-beta-arrestin complex in a lipid nanodisc. Nature. 2020;579(7798):297‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Huang W, Masureel M, Qu Q, et al. . Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature. 2020;579(7798):303‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yin W, Li Z, Jin M, et al. . A complex structure of arrestin-2 bound to a G protein-coupled receptor. Cell Res. 2019;29(12):971‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Shiraishi Y, Natsume M, Kofuku Y, et al. . Phosphorylation-induced conformation of β(2)-adrenoceptor related to arrestin recruitment revealed by NMR. Nat Commun. 2018;9(1):194‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ho PWM, Chan AS, Pavlos NJ, Sims NA, Martin TJ. Brief exposure to full length parathyroid hormone-related protein (PTHrP) causes persistent generation of cyclic AMP through an endocytosis-dependent mechanism. Biochem Pharmacol. 2019;169:113627. [DOI] [PubMed] [Google Scholar]
- 146. Peña KA, White AD, Savransky S, et al. . Biased GPCR signaling by the native parathyroid hormone-related protein 1 to 141 relative to its N-terminal fragment 1 to 36. J Biol Chem. 2022;298(9):102332. [DOI] [PMC free article] [PubMed] [Google Scholar]