Abstract
INTRODUCTION:
Drug induced acute pancreatitis is a difficult diagnosis for clinicians. We previously published an “Evidence-Based Classification System” on Drug-Induced Acute Pancreatitis widely used by clinicians to assist in the identification of drugs. Unfortunately, this prior analysis based only on published case reports has been misunderstood. The prior review did not include studies with higher evidentiary value, such as randomized trials, case-control studies, and/or pharmacoepidemiologic studies. The use of the prior classification system has led to many patients being inappropriately labeled as having drug-induced acute pancreatitis. We now propose a “Revised” Evidence- Based Classification System for the purpose of determining which drugs cause acute pancreatitis based on the Grading of Recommendations, Development, and Evaluation criteria.
METHODS:
A search of the English Language literature was performed to identify all case reports with medication and/or drug induced acute pancreatitis. We divided the drugs implicated as causing acute pancreatitis into four groups based on the quality of evidence as defined by GRADE quality parameters.
RESULTS:
Although 141 drugs were identified in the literature as causing acute pancreatitis, only 106 drugs published in the literature as causing acute pancreatitis were high quality case reports. Only 3 drugs had evidence as causing acute pancreatitis from randomized controlled clinical trials, including 6-mercaptopurine and azathioprine.
DISCUSSION:
The vast majority of drugs implicated as causing acute pancreatitis in the literature have low or very low quality of evidence supporting those claims.
KEYWORDS: acute pancreatitis, drug-induced, medication-induced
INTRODUCTION
Acute pancreatitis is the most common gastrointestinal disease leading to hospitalization in the United States (1). A careful history, laboratory testing, and imaging reveals the etiology in most patients. The purpose of identifying the etiology is to prevent recurrent disease, including the possibility of more severe disease (2). However, in as many as a third of patients with acute pancreatitis, the etiology remains elusive. Idiopathic acute pancreatitis is defined as pancreatitis with no etiology established after initial laboratory (including triglyceride level) and imaging (transabdominal ultrasound, MRI, and/or computed tomography scan) tests (3). There have been hundreds of claims of possible causes of acute pancreatitis in these patients, including almost 200 medications (4). The claims of causation are based almost exclusively as case reports or case series with limited evidentiary value.
We previously published an “Evidence-Based Classification System” on Drug-Induced Acute Pancreatitis widely used by clinicians (5). Unfortunately, this prior analysis based only on published case reports has been misunderstood and often wrongly applied (6). For that analysis, we did not include studies with higher evidentiary value, such as randomized trials, case-control studies, and/or pharmacoepidemiologic studies. Despite the extensive list of drugs included in the prior classification, the classes were defined ONLY by the quantity and quality of published case reports and the demonstration of rechallenges and/or consistent latency among the case reports.
The general scientific method is the methodology that must be used in making causal claims about human health and disease (7). Epidemiological methods involving human subjects (study participants) are the most important means for identifying and testing hypotheses involving human disease causation. According to the United States Preventative Services Task Force, these studies are prioritized from strongest to weakest: randomized controlled trials (RCTs), controlled trials without randomization, cohort studies, case-control studies, case reports, and case series (8). Published case reports and case series are plagued by reporting bias and are considered the lowest quality of evidence.
As the evidentiary value of case reports to clinicians and applicability to patients with unexplained acute pancreatitis is profoundly limited, the importance of an evidence-based approach including an extensive review of RCT, cohort studies, and pharmacoepidemiologic analyses was needed. On the basis of this review, we now propose a “Revised Evidence-Based Classification System” for the purpose of determining which drugs cause acute pancreatitis based on the Grading of Recommendations, Development, and Evaluation criteria (9).
METHODS
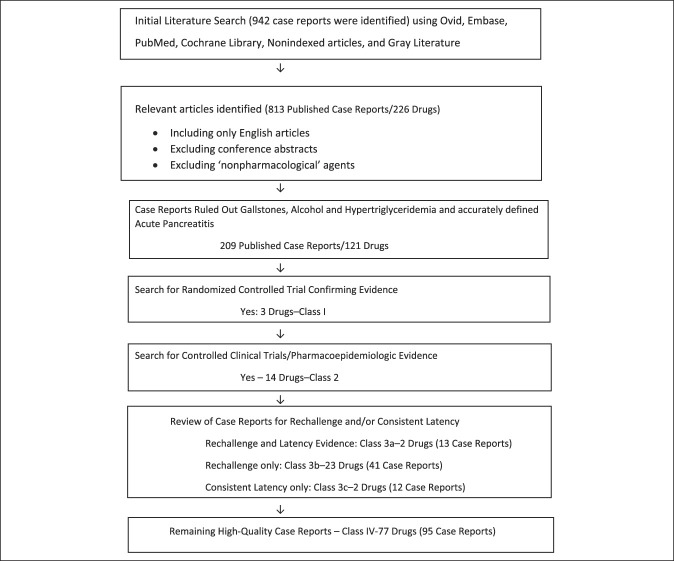
A Medical Literature Analysis and Retrieval System Online search of the English language literature was performed to identify all case reports with medication and/or drug-induced acute pancreatitis using Medical Search Headings terms: drug induced, medication induced, drug associated, medication associated, and chemical induced with acute pancreatitis. Due to biased overreporting, pharmaceutical data, including the Physician Desk Reference and the US Food and Drug Administration (FDA) Adverse Reporting System, were not used in this analysis. Any drug or medication identified in case reports as having an association with acute pancreatitis led to a search of randomized controlled clinical trials and case-control trials, which listed adverse events, effects, side effects of the drug compared with those of placebo and/or other studied drugs to determine whether acute pancreatitis was increasingly associated with the studied drug (Figure 1). To be included, the diagnosis of acute pancreatitis needed to be established by The American College of Gastroenterology Guidelines: 2 of 3 criteria: (i) abdominal pain consistent with the disease, (ii) serum amylase and/or lipase greater than 3 times normal, and/or (iii) imaging (computed tomography or MRI) consistent with the diagnosis of acute pancreatitis (10).
Figure 1.
Method of evaluation of case reports and published studies.
The case reports were then separated into those case reports that were of high quality and those deemed not worthy of consideration. High-quality case reports were those that ruled out the more common causes of acute pancreatitis: gallstones, alcohol, and hypertriglyceridemia (see Supplementary Digital Content, http://links.lww.com/CTG/A981).
If ultrasound and/or MRI were not performed or gallstones were not described as being ruled out, the case report was deemed not worthy of consideration. A comment or laboratory parameter of the triglyceride level must have been reported for the case report to be included. In addition, a comment to the absence of alcohol abuse must have been included.
We divided the drugs implicated as causing acute pancreatitis into 4 groups based on the quality of evidence as defined by Grading of Recommendations, Development, and Evaluation quality parameters (Table 1). RCTs have the most evidence (class 1), while case-control and pharmacoepidemiologic studies come in a close second in evidentiary value (class 2). High-quality case reports were then divided into 2 classes. The first class (class 3) included high-quality case reports that had a rechallenge and/or a consistent latency. Case reports that had a rechallenge, where a drug is stopped and then restarted with another attack of acute pancreatitis, were considered better evidence. In addition, those drugs with 3 or more case reports with a consistent latency were also considered higher quality of evidence. If there were more than 3 case reports, three-fourths of the case reports need to have a consistent latency to be included. Consistent latency was defined as per our previous classification system. Latencies were defined as early (less than 24 hours), intermediate (1–30 days), and long (more than 30 days). A class 3a drug had both case reports with a rechallenge and a consistent latency. Class 3b and class 3c were case reports that had either a rechallenge or consistent latency. Last, if a drug had high-quality case reports but no case report had a rechallenge nor a consistent latency, due to the limited evidentiary value of case reports, they were considered class 4. All other case reports were deemed low quality and not included in the analysis. The authors felt that any case report that did not rule out the more common causes of acute pancreatitis, alcohol, gallstones, and/or hypertriglyceridemia, was not worthy of inclusion.
Table 1.
Evidence-based classification for drug-induced acute pancreatitis–revised
| Class 1. High Quality of Evidence for causation of acute pancreatitis: Randomized Controlled Clinical Trials. |
| Class 2. Moderate quality of evidence for causation of acute pancreatitis: Case-control studies and/or pharmacoepidemiology studies. |
| Class 3. Low-quality evidence for causation of acute pancreatitis: High-quality case reports. |
| Class 3a: Case reports showing “rechallenge and consistent latency” |
| Class 3b: Case report showing rechallenge only |
| Class 3c Case report showing consistent latency only. |
| Class 4. Very low-quality evidence: high-quality case reports but no rechallenge nor consistent latency. |
RESULTS
In our initial review, 226 drugs were identified in the literature as causing acute pancreatitis (Figure 1). One hundred five drugs were eliminated simply as poor quality, as previously reported. In the final analysis, we identified only 209 published case reports and 121 drugs published in the literature as causing acute pancreatitis with high-quality case reports. Table 2 summarizes which drugs have the highest quality of evidence as causing acute pancreatitis based on our further analysis. Only 3 drugs had evidence as causing acute pancreatitis from randomized controlled clinical trials, including 6-mercaptopurine and azathioprine (class 1). Only 14 drugs were shown from case-control trials or pharmacoepidemiologic studies as causing acute pancreatitis (class 2). Of published case reports, only 104 drugs had well-written case reports that excluded alcohol, gallstones, and hypertriglyceridemia and had a rechallenge or consistent latency among the reports. The cases were divided into 4 groups. We found 2 drugs that had evidence of both a rechallenge and consistent latency (class 3a), 23 drugs that had only a rechallenge (class 3b), and only 2 drugs with a consistent latency and lack of rechallenge among the high-quality case reports (class 3c). The last group of 95 remaining high-quality case reports yielded 77 drugs, which were considered the lowest quality of evidence of causation, class 4.
Table 2.
Evidence-based list of drugs that cause acute pancreatitis
| Class 1. Didanosine (18–20), azathioprine (21,22), 6-mercaptopurine (23) |
| Class 2. Acetaminophena (24), ACE inhibitors (25,26), Typical Antipsychotics (not atypical) (27), Benzodiazepinesa (28), DPP4 inhibitors (29), GLP1 agonists (30,31), Immune Checkpoint Inhibitors (32,33), Codeine (34), Methimazole (35,36), Metronidazole (37,38), Peg/L Asparaginase (39,40), Protease Inhibitors (41), SSRIs (42–44), Valproic Acid (45) |
| Class 3. |
| Class 3a: 5-ASA (46–54) Sulindac (55–58) |
| Class 3b: Acetaminophen-Codeine (59), Bezafibrate (60), Bortezomib (61,62), Cannabis (63–67), Capecitabine (68,69), Carbimazole (70,71), Estrogen (72), Fenofibrate (73), Isoniazid (74–76), Methyldopa (77), Nelfinavir (78), Nitrofurantoin (79,80), Pravastatin (81–83), Paclitaxel (84,85), Procainamide (86), Pyritinol (87), Rosuvastatin (88,89), simvastatin (90), Sulfasalazine (91,92), Thalidomide (93), Tetracycline (94–96) Trimethoprim-Sulfamethoxazole (97,98), Vemurafenib (99) |
| Class 3c: Doxycycline (100–104), Interferon alpha 2b and Ribavirin (105–111) |
| Class 4. Adefovir (112), Amiodarone (113), Arginine (114,115), Atorvastatin (116), Axitinib (117), Betamethasone/Roxithromycin (118), Canagliflozin (119), Carbamazepine intoxication (120), Candesartan (121), Celecoxib (122), Clozapine (123–125), Clomipramineb (126),Cyclosporin (127), Danazol (128), Dapsone (129), Dexamethasone (130,131), Docetaxel (132), Doxorubicin and ifosfamide (133), Diclofenac (134), Dimethyl Fumarate (135), Eluxadoline (136,137), Erythromycin (138,139), Erythromycin/Lovastatin (140), Etoposide and Lobaplatin (141), Fluvastatin (142), Flurbiprofen (143), Furosemide (144), Growth Hormone (145), Hydrochlorothiazide/Irbesartan (146), Ibuprofen (147,148),b Indomethacin (149,150), Ifosfamide (151), Interferon alpha 2a (152), Irbesartan (153), Itraconazole (154), Ketoprofen (155), Lamotrigine (156), Lanreotide (157), Loperamide (158), Mefenamic acid (159). Metformin (160,161), Metolazone (162), Methandrostenolone (163), Methylprednisolone (164), Methylprednisone/prednisoneb (165), Micafungin (166), Mirtazapine (167,168), Mycophenolate mofetil (169), Naproxen (170,171), Nicotine gum (172), Ocrelizumab (173), Octreotide (174,175), Ofloxacin-ornidazole (176), Omeprazole (177), Olanzapine (178), Pantoprazole (179), Penicillin (180), Phenolphthalein (181), Pentamidine (182), Posaconazole (183), Propofol (184–188), Ranitidine (189), Regorafenib (190), Riluzole (191), Risperidone (192), Rivaroxaban (193), Salicylazosulfapyridine (194), Secnidazole (195), Simvastatin/Salicylate (196), Sorafenib (197–199), sunitinib (197), Sunitinib/Axitinib (200),Tadalafil (201), Theophylline overdose (202), Tigecycline (203,204), Vedolizumab (205), Vismodegib (206) |
At toxic doses.
At high dose.
DISCUSSION
While the World Health Organization database lists more than 500 drugs as causing acute pancreatitis, there is a paucity of evidence that most of these drugs is the etiology of acute pancreatitis (4). Similarly, the US FDA adverse event database for “Medwatch” reports lists hundreds of medications as “possibly” causing acute pancreatitis with minimal evidence. The US FDA and others have concluded that these case reports have limited scientific “causal value” (11). The limitations of these spontaneous reporting systems include subjective and imprecise reporting practices, overreporting biases, and poor quality.
Clinicians may choose to stop a medication on a particular patient due to individual suspicion; however, blaming the drug as causing acute pancreatitis merely due to a prior published case report or cases series must be recognized as of limited scientific value. Case reports are simply too biased to rely on for causation. The fundamental problem with causal inference is the primary reason why RCTs are considered the gold standard of scientific research in therapeutic and etiologic research. Randomized trials provide the best approximation to solving this problem by assuming that individuals who do not receive the medication are similar as possible to the individuals who do not receive the medication (control group).
Although we used randomized trials for the best evidence of a drug causing or not causing acute pancreatitis, there are limitations to this approach. The trials may not have been designed in a method to identify cases of acute pancreatitis between the studied and control groups. Thus, adverse reporting for acute pancreatitis by research staff may have been underreported. In addition, the number of persons enrolled in the trials may have been too small to detect differences in the number of patients who develop acute pancreatitis. While these limitations are real, we believe that randomized trials still provide the best evidence for causation. The RCT is considered the most rigorous and robust research method of determining whether a cause-effect relation exists.
While epidemiologic studies, such as prospective cohort studies and case-control studies, do not have randomly identified controls, the careful selection of controls allows for another solution to the fundamental problem of bias. Case reports about disease causation that lack controls are of questionable validity and reliability. Although all adverse events begin as case reports (even if not actually reported in the scientific literature), it is important to emphasize that case reports only provide clues to etiology (12). If a clinician uses these case reports as evidence of causation, they are likely to be led astray as toward the best scientific explanation of the available evidence (13). At best, case reports and case series generate (rather than test) causal hypotheses about any relationship between medications and adverse events (14).
Largely due to tradition in the literature and the volume of publications, we chose to include case reports and to separate them into 4 classes and subclasses (classes 3a, 3b, 3c and class 4). However, we included only those case reports that had sufficient quality as defined by the Atlanta criteria and having ruled out other more common causes of acute pancreatitis. As other authors had appreciated the increased quality of evidence in case reports that had a rechallenge and/or a consistent latency, we considered these high-quality case reports separately as a class within class 3 (4,5,15). While a consistent latency provides some evidence of an underlying common mechanism, no biologic mechanism for any medication causing acute pancreatitis has been established (16). Four decades ago, Mallory and Kern (1980) included “rechallenge” as higher quality of evidence than a simple case report. However, few of the published case reports with rechallenge occur after a sufficient period after the attack of acute pancreatitis. In addition, the dosage, timing, and outcome are often missing. Clinicians should be aware that acute pancreatitis will recur in a third of patients within a year and as many as 10% within the first month of an attack of acute pancreatitis (3). Which is more likely, the drug was the cause in the rechallenge, or the acute pancreatitis would have recurred regardless of the drug?
In pharmacovigilance, the strongest evidence of causation of a drug are results from analytical studies that select appropriate control populations and provide evidence revealing a relative risk sufficiently strong to withstand the impact of bias and confounding. The only medications that have this level of evidence as causing acute pancreatitis are 6-MP and its metabolite, azathioprine. Didanosine also has similar clinical trial evidence as causing acute pancreatitis. The evidence is reproducible in multiple studies, including multiple RCTs. The analogy between the strength of evidence and metabolic similarity between 6-MP and azathioprine provides further evidence for these 2 drugs. The lack of a dose-response relationship and the inability to explain a biologic (or psychosocial) mechanism does not limit the evidence that these drugs cause acute pancreatitis (17).
In conclusion, most drugs implicated as causing acute pancreatitis in the literature have low or very low quality of evidence supporting those claims. Most drugs have been implicated as causing acute pancreatitis based on low-quality case reports rather than high-quality randomized trials, cohort studies, or pharmacoepidemiologic studies. In patients with acute pancreatitis who are taking 6-MP, azathioprine, or didanosine, the medication should be stopped because there is sufficient evidence that these drugs cause acute pancreatitis. However, stopping all the other medications in patients with idiopathic pancreatitis is unwarranted based on the evidence in the literature. While clinicians may stop a medication based on the fear that the drug may be the cause in a particular patient, relying on case reports for causation is inappropriate. Further study may identify idiosyncratic reasons that a particular person is susceptible to drug-induced acute pancreatitis. However, there is little scientific evidence that most medications believed to cause acute pancreatitis are the true etiology.
CONFLICTS OF INTEREST
Guarantor of the article: Scott Tenner, MD.
Specific author contributions: J.S. and S.T.: manuscript was written. M.V.: proof-reading, verification of references was performed. J.S. and N.B.: data collection and analysis of case reports were performed. D.M. and S.T.: data collection and analysis of randomized controlled trials and large pharmacoepidemiologic databases were performed.
Financial support: None to report.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Drugs have been implicated as causing acute pancreatitis.
✓ The best quality of evidence that a drug causes acute pancreatitis is from randomized controlled clinical trials and pharmacoepidemiologic databases.
✓ Well written case reports with a rechallenge and/or consistent latency can provide some evidence of causation. However, this is the weakest evidence of a drug causing acute pancreatitis.
WHAT IS NEW HERE
✓ Despite claims that numerous drugs cause acute pancreatitis, the evidence that a particular drug causes acute pancreatitis is quite limited.
✓ A Novel Classification System is shown based on the GRADE Criteria and will help guide clinicians in considering the evidence that a particular drug causes acute pancreatitis.
ACKNOWLEDGMENTS
We are deeply grateful to the following individuals who assisted in the literature search: Larry J. Prokop, MLIS. Librarian, Mayo Clinic, Rochester, MN; Shubham Kamal, MD, Resident, Department of Psychiatry, Carilion Clinic, VA; and Prakash Gupta, MD, Research Fellow, Department of Neurosurgery, Washington University in St Louis, MO.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A981
Contributor Information
Jasmine Saini, Email: jasmine.saini64@gmail.com.
Daniel Marino, Email: dmarino2019@gmail.com.
Nison Badalov, Email: nisonbadalov@yahoo.com.
Melanie Vugelman, Email: melanievugelman@gmail.com.
REFERENCES
- 1.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143(5):1179–87.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology guideline: Management of acute pancreatitis. Am J Gastroenterol 2013;108(9):1400–15; 1416. [DOI] [PubMed] [Google Scholar]
- 3.Guda NM, Muddana V, Whitcomb DC, et al. Recurrent acute pancreatitis: International state-of-the-science conference with recommendations. Pancreas 2018;47(6):653–66. [DOI] [PubMed] [Google Scholar]
- 4.Simons-Linares CR, Elkhouly MA, Salazar MJ. Drug-induced acute pancreatitis in adults: An update. Pancreas 2019;48(10):1263–73. [DOI] [PubMed] [Google Scholar]
- 5.Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: An evidence-based review. Clin Gastroenterol Hepatol 2007;5(6):648–61.e3; quiz 644. [DOI] [PubMed] [Google Scholar]
- 6.Tenner S. Drug induced acute pancreatitis: Does it exist? World J Gastroenterol 2014;20(44):16529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weed DL. Interpreting epidemiological evidence: How meta-analysis and causal inference methods are related. Int J Epidemiol 2000;29(3):387–90. [PubMed] [Google Scholar]
- 8.Agency for Healthcare Research and Quality (2008). U.S. Preventive Services Task Force Procedure Manual. AHRQ Publication No. 08-05118-EF. AHRQ: Washington, DC, 2008. [Google Scholar]
- 9.Guyatt GH, Oxman AD, Vist GE, et al. Grade: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013;62(1):102–11. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy DLGS, Lillie RB. In: Strom BL. (ed). Spontaneous reporting in the United States Chapter 10, 3rd edn. John Wiley and Sons: Hoboken, NJ, 2000. [Google Scholar]
- 12.Vandenbroucke JP. Case reports in an evidence-based world. J R Soc Med 1999;92(4):159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancashire RJ, Cheng K, Langman MJ. Discrepancies between population-based data and adverse reaction reports in assessing drugs as causes of acute pancreatitis. Aliment Pharmacol Ther 2003;17(7):887–93. [DOI] [PubMed] [Google Scholar]
- 14.Goldman SA. Limitations and strengths of spontaneous reports data. Clin Ther 1998;20(Suppl C):C40–4. [DOI] [PubMed] [Google Scholar]
- 15.McArthur KE. Review article: Drug-induced pancreatitis. Aliment Pharmacol Ther 1996;10(1):23–38. [DOI] [PubMed] [Google Scholar]
- 16.Tenner S. Molecular biology, epidemiology, and the elusive nature of pancreatitis. Clin translational Gastroenterol 2015;6(3):e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stricker BH, Psaty BM. Detection, verification, and quantification of adverse drug reactions. BMJ 2004;329(7456):44–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe SH, Wensing AMJ, Hassink EAM, et al. Comparison of two once-daily regimens with a regimen consisting of nelfinavir, didanosine, and stavudine in antiretroviral therapy-naïve adults: 48-week results from the antiretroviral regimen evaluation study (ARES). HIV Clin Trials 2005;6(5):235–45. [DOI] [PubMed] [Google Scholar]
- 19.Phidisa II Writing Team for Project Phidisa, Ratsela A, Polis M, Dhlomo S, et al. A randomized factorial trial comparing 4 treatment regimens in treatment-naive HIV-infected persons with AIDS and/or a CD4 cell count <200 cells/μL in South Africa. J Infect Dis 2010;202(10):1529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swindells S, Cohen CJ, Berger DS, et al. Abacavir, efavirenz, didanosine, with or without hydroxyurea, in HIV-infected adults failing initial nucleoside/protease inhibitor-containing regimens. BMC Infect Dis 2005;5(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosnes J, Bourrier A, Laharie D, et al. Early administration of azathioprine vs conventional management of Crohn's disease: A randomized controlled trial. Gastroenterology 2013;145(4):758–65.e2; quiz e14-5. [DOI] [PubMed] [Google Scholar]
- 22.Mañosa M, Cabré E, Bernal I, et al. Addition of metronidazole to azathioprine for the prevention of postoperative recurrence of Crohn's disease: A randomized, double-blind, placebo-controlled trial. Inflamm Bowel Dis 2013;19(9):1889–95. [DOI] [PubMed] [Google Scholar]
- 23.Present DH, Korelitz BI, Wisch N, et al. Treatment of Crohn's disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med 1980;302(18):981–7. [DOI] [PubMed] [Google Scholar]
- 24.Chen S-J, Lin C-S, Hsu C-W, et al. Acetaminophen poisoning and risk of acute pancreatitis: A population-based cohort study. Medicine 2015;94(29):e1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eland IA, Sundström A, Velo GP, et al. Antihypertensive medication and the risk of acute pancreatitis: The European case-control study on drug-induced acute pancreatitis (EDIP). Scand J Gastroenterol 2006;41(12):1484–90. [DOI] [PubMed] [Google Scholar]
- 26.Kuoppala J, Enlund H, Pulkkinen J, et al. ACE inhibitors and the risk of acute pancreatitis-a population-based case-control study. Pharmacoepidemiol Drug Saf 2017;26(7):853–7. [DOI] [PubMed] [Google Scholar]
- 27.Gasse C, Jacobsen J, Pedersen L, et al. Risk of hospitalization for acute pancreatitis associated with conventional and atypical antipsychotics: A population-based case-control study. Pharmacotherapy 2008;28(1):27–34. [DOI] [PubMed] [Google Scholar]
- 28.Liaw G-W, Hung D-Z, Chen W-K, et al. Relationship between acute benzodiazepine poisoning and acute pancreatitis risk: A population-based cohort study. Medicine 2015;94(52):e2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faillie JL, Babai S, Crépin S, et al. Pancreatitis associated with the use of GLP-1 analogs and DPP-4 inhibitors: A case/non-case study from the French pharmacovigilance database. Acta Diabetol 2014;51(3):491–7. [DOI] [PubMed] [Google Scholar]
- 30.Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011;141(1):150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monami M, Nreu B, Scatena A, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): Data from randomized controlled trials. Diabetes Obes Metab 2017;19(9):1233–41. [DOI] [PubMed] [Google Scholar]
- 32.Abu-Sbeih H, Tang T, Lu Y, et al. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. J Immunother Cancer 2019;7(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman CF, Clark V, Raikhel AV, et al. Thinking critically about classifying adverse events: Incidence of pancreatitis in patients treated with nivolumab + ipilimumab. J Natl Cancer Inst 2016;109(4):djw260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Tabner AJ, Johnson GD, et al. Increased risk of acute pancreatitis with codeine use in patients with a history of cholecystectomy. Dig Dis Sci 2020;65(1):292–300. [DOI] [PubMed] [Google Scholar]
- 35.Brix TH, Lund LC, Henriksen DP, et al. Methimazole and risk of acute pancreatitis. Lancet Diabetes Endocrinol 2020;8(3):187–9. [DOI] [PubMed] [Google Scholar]
- 36.Pecere A, Caputo M, Sarro A, et al. Methimazole treatment and risk of acute pancreatitis: A population-based cohort study. J Clin Endocrinol Metab 2020;105(12):e4527–30. [DOI] [PubMed] [Google Scholar]
- 37.Barbulescu A, Oskarsson V, Lindblad M, et al. Oral metronidazole use and risk of acute pancreatitis: A population-based case-control study. Clin Epidemiol 2018;10:1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nørgaard M, Ratanajamit C, Jacobsen J, et al. Metronidazole and risk of acute pancreatitis: A population-based case-control study. Aliment Pharmacol Ther 2005;21(4):415–20. [DOI] [PubMed] [Google Scholar]
- 39.Christ TN, Stock W, Knoebel RW. Incidence of asparaginase-related hepatotoxicity, pancreatitis, and thrombotic events in adults with acute lymphoblastic leukemia treated with a pediatric-inspired regimen. J Oncol Pharm Pract 2018;24(4):299–308. [DOI] [PubMed] [Google Scholar]
- 40.Lebovic R, Pearce N, Lacey L, et al. Adverse effects of pegaspargase in pediatric patients receiving doses greater than 3,750 IU. Pediatr Blood Cancer 2017;64(10):e26555. [DOI] [PubMed] [Google Scholar]
- 41.Qin W, Zhao B, Shang Y, et al. Clinical profile of acute pancreatitis following treatment with protease inhibitors: A real-world analysis of post-marketing surveillance data. Expert Opin Drug Saf 2021;20(9):1109–15. [DOI] [PubMed] [Google Scholar]
- 42.Lin HF, Liao KF, Chang CM, et al. Association of use of selective serotonin reuptake inhibitors with risk of acute pancreatitis: A case-control study in Taiwan. Eur J Clin Pharmacol 2017;73(12):1615–21. [DOI] [PubMed] [Google Scholar]
- 43.Ljung R, Rück C, Mattsson F, et al. Selective serotonin reuptake inhibitors and the risk of acute pancreatitis: A Swedish population-based case-control study. J Clin Psychopharmacol 2012;32(3):336–40. [DOI] [PubMed] [Google Scholar]
- 44.Nørgaard M, Jacobsen J, Gasse C, et al. Selective serotonin reuptake inhibitors and risk of acute pancreatitis: A population-based case-control study. J Clin Psychopharmacol 2007;27(3):259–62. [DOI] [PubMed] [Google Scholar]
- 45.Nørgaard M, Jacobsen J, Ratanajamit C, et al. Valproic acid and risk of acute pancreatitis: A population-based case-control study. Am J Ther 2006;13(2):113–7. [DOI] [PubMed] [Google Scholar]
- 46.Meczker Á, Mikó A, Hegyi P. 5-ASA induces mild acute pancreatitis. Case report and review of the literature. J Gastrointestin Liver Dis 2018;27(2):189–94. [DOI] [PubMed] [Google Scholar]
- 47.Correia JP, Ponte AI, Silva JC, et al. Mesalazine-induced acute pancreatitis: A rare adverse reaction but with important therapeutic implications in ulcerative colitis. Eur J Gastroenterol Hepatol 2021;33(4):595. [DOI] [PubMed] [Google Scholar]
- 48.Adachi E, Okazaki K, Matsushima Y, et al. Acute pancreatitis secondary to 5-aminosalicylic acid therapy in a patient with uicerative colitis. Int J Pancreatol 1999;25(3):217–21. [DOI] [PubMed] [Google Scholar]
- 49.Fiorentini MT, Fracchia M, Galatola G, et al. Acute pancreatitis during oral 5-aminosalicylic acid therapy. Dig Dis Sci 1990;35(9):1180–2. [DOI] [PubMed] [Google Scholar]
- 50.Fernández J, Sala M, Panés J, et al. Acute pancreatitis after long-term 5-aminosalicylic acid therapy. Am J Gastroenterol 1997;92(12):2302–3. [PubMed] [Google Scholar]
- 51.Erdkamp F, Houben M, Ackerman E, et al. Pancreatitis induced by mesalamine. Neth J Med 1992;41(1-2):71–3. [PubMed] [Google Scholar]
- 52.Radke M, Bartolomaeus G, Muller M, et al. Acute pancreatitis in Crohn's disease due to 5-ASA therapy. J Pediatr Gastroenterol Nutr 1993;16(3):337–9. [DOI] [PubMed] [Google Scholar]
- 53.Daniel F, Seksik P, Cacheux W, et al. Tolerance of 4-aminosalicylic acid enemas in patients with inflammatory bowel disease and 5-aminosalicylic-induced acute pancreatitis. Inflamm Bowel Dis 2004;10(3):258–60. [DOI] [PubMed] [Google Scholar]
- 54.Abdullah AM, Scott RB, Martin SR. Acute pancreatitis secondary to 5-aminosalicylic acid in a child with ulcerative colitis. J Pediatr Gastroenterol Nutr 1993;17:441–4. [DOI] [PubMed] [Google Scholar]
- 55.Siefkin AD. Sulindac and pancreatitis. Ann Intern Med 1980;93(6):932–3. [DOI] [PubMed] [Google Scholar]
- 56.Zygmunt DJ, Williams HJ, Bienz SR. Acute pancreatitis associated with long-term sulindac therapy. West J Med 1986;144(4):461–2. [PMC free article] [PubMed] [Google Scholar]
- 57.Klein S, Khan M. Hepatitis, toxic epidermal necrolysis and pancreatitis in association with sulindac therapy. J Rheumatol 1983;10(3):512–3. [PubMed] [Google Scholar]
- 58.Memon A. Pancreatitis and sulindac. Ann Intern Med 1982;97(1):139. [DOI] [PubMed] [Google Scholar]
- 59.Hastier P, Demarquay J-F, Maes B, et al. Acute pancreatitis induced by codeine-acetaminophen association: A case report with positive rechallenge. Pancreas 1996;13(3):324–6. [DOI] [PubMed] [Google Scholar]
- 60.Gang N, Langevitz P, Livneh A. Relapsing acute pancreatitis induced by re-exposure to the cholesterol lowering agent bezafibrate. Am J Gastroenterol 1999;94(12):3626–8. [DOI] [PubMed] [Google Scholar]
- 61.Elouni B, Ben Salem C, Zamy M, et al. Bortezomib-induced acute pancreatitis. JOP 2010;11(3):275–6. [PubMed] [Google Scholar]
- 62.Talamo G, Sivik J, Pandey MK, et al. Bortezomib-induced acute pancreatitis: Case report and review of the literature. J Oncol Pharm Pract 2014;22(2):332–4. [DOI] [PubMed] [Google Scholar]
- 63.Grant P, Gandhi P. A case of cannabis-induced pancreatitis. JOP 2004;5(1):41–3. [PubMed] [Google Scholar]
- 64.Fatma H, Mouna B, Leila M, et al. Cannabis: A rare cause of acute pancreatitis. Clin Res Hepatol Gastroenterol 2013;37(1):e24–5. [DOI] [PubMed] [Google Scholar]
- 65.Howaizi M, Chahine M, Haydar F, et al. Cannabis-induced recurrent acute pancreatitis. Acta Gastroenterol Belg 2012;75(4):446–7. [PubMed] [Google Scholar]
- 66.Wargo KA, Geveden BN, McConnell VJ. Cannabinoid-induced pancreatitis: A case series. JOP 2007;8(5):579–83. [PubMed] [Google Scholar]
- 67.Singh R, Torre K, Saba M, et al. Cannabis-induced pancreatitis: A new clinical entity. Pancreas 2020;49(7):e66–e67. [DOI] [PubMed] [Google Scholar]
- 68.Yucel H, Warmerdam LV. Capecitabine-induced pancreatitis. J Oncol Pharm Pract 2010;16(2):133–4. [DOI] [PubMed] [Google Scholar]
- 69.Jones KL, Valero V. Capecitabine-induced pancreatitis. Pharmacother J Hum Pharmacol Drug Ther 2003;23(8):1076–8. [DOI] [PubMed] [Google Scholar]
- 70.Marazuela M, Paco GSd, Jiménez I, et al. Acute pancreatitis, hepatic cholestasis, and erythema nodosum induced by carbimazole treatment for Graves' disease. Endocr J 2002;49(3):315–8. [DOI] [PubMed] [Google Scholar]
- 71.Chng CL, Kek PC, Khoo DH, et al. Carbimazole-induced acute pancreatitis and cholestatic hepatitis. Endocr Pract 2011;17(6):960–2. [PubMed] [Google Scholar]
- 72.Blake WED, Pitcher ME. Estrogen-related pancreatitis in the setting of normal plasma lipids: Case report. Menopause 2003;10(1):99–101. [DOI] [PubMed] [Google Scholar]
- 73.Kassim T, Hermes J-M, Abdussalam A, et al. Fenofibrate: A nonlithogenic means of recurrent drug-induced pancreatitis. Case Rep Gastrointest Med 2018;2018:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pandey AS, Surana A. Isoniazid-induced recurrent acute pancreatitis. Trop Doctor 2011;41(4):249–50. [DOI] [PubMed] [Google Scholar]
- 75.Mattioni S, Zamy M, Mechai F, et al. Isoniazid-induced recurrent pancreatitis. JOP 2012;13(3):314–6. [PubMed] [Google Scholar]
- 76.Kharibam P, Jithesh G, Ronanki K, et al. A rare case of isoniazid induced recurrent acute pancreatitis. J Cardiovasc Dis Res 2021;12(6):1121–4. [Google Scholar]
- 77.Rominger JM, Gutierrez JG, Curtis D, et al. Methyldopa-induced pancreatitis. Am J Dig Dis 1978;23(8):756–8. [DOI] [PubMed] [Google Scholar]
- 78.Di Martino V, Ezenfis J, Benhamou Y, et al. Severe acute pancreatitis related to the use of nelfinavir in HIV infection: Report of a case with positive rechallenge. AIDS 1999;13(11):1421–3. [DOI] [PubMed] [Google Scholar]
- 79.Christophe JL. Pancreatitis induced by nitrofurantoin. Gut 1994;35(5):712–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mouallem M, Sirotin T, Farfel Z. Nitrofurantoin-induced pancreatitis. Isr Med Assoc J 2003;5(10):754–5. [PubMed] [Google Scholar]
- 81.Tarar ZI, Zafar MU, Ghous G, et al. Pravastatin-induced acute pancreatitis: A case report and literature review. J Invest Med High Impact Case Rep 2021;9:232470962110283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsigrelis C, Pitchumoni CS. Pravastatin: A potential cause for acute pancreatitis. World J Gastroenterol 2006;12(43):7055–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anagnostopoulos GK, Tsiakos S, Margantinis G, et al. Acute pancreatitis due to pravastatin therapy. JOP 2003;4(3):129–32. [PubMed] [Google Scholar]
- 84.Hoff PM, Valero V, Holmes FA, et al. Paclitaxel-induced pancreatitis: A case report. J Natl Cancer Inst 1997;89(1):91–3. [DOI] [PubMed] [Google Scholar]
- 85.Raiss H, Amarti LE, Tigaud JD, et al. Probable paclitaxel-induced pancreatitis: Uncommon case report and literature review. J Gastrointest Oncol 2017;8(6):E80–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Falko JM, Thomas FB. Acute pancreatitis due to procainamide-induced lupus erythematosus. Ann Intern Med 1975;83(6):832–3. [DOI] [PubMed] [Google Scholar]
- 87.Straumann A, Bauer M, Pichler WJ, et al. Acute pancreatitis due to pyritinol: An immune-mediated phenomenon. Gastroenterology 1998;115(2):452–4. [DOI] [PubMed] [Google Scholar]
- 88.Chintanaboina J, Gopavaram D. Recurrent acute pancreatitis probably induced by rosuvastatin therapy: A case report. Case Rep Med 2012;2012:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh S, Nautiyal A, Dolan JG. Recurrent acute pancreatitis possibly induced by atorvastatin and rosuvastatin. Is statin induced pancreatitis a class effect? JOP 2004;5(6):502–4. [PubMed] [Google Scholar]
- 90.Pezzilli R, Barakat B, Ceciliato R, et al. Acute pancreatitis due to simvastatin therapy: Increased severity after rechallenge. Dig Liver Dis 2004;36(9):639–40. [DOI] [PubMed] [Google Scholar]
- 91.Brazer SR, Medoff JR. Sulfonamide-induced pancreatitis. Pancreas 1988;3(5):583–6. [DOI] [PubMed] [Google Scholar]
- 92.Mehershahi S, Haider A, Shaikh D, et al. Drug-induced acute pancreatitis after long-term sulfasalazine therapy. Cureus 2020;12(9):e10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chung LW, Yeh S-P, Hsieh C-Y, et al. Life-threatening acute pancreatitis due to thalidomide therapy for chronic graft-versus-host disease. Ann Hematol 2008;87(5):421–3. [DOI] [PubMed] [Google Scholar]
- 94.Torosis J, Vender R. Tetracycline-induced pancreatitis. J Clin Gastroenterol 1987;9(5):580–1. [DOI] [PubMed] [Google Scholar]
- 95.Nicolau DP, Mengedoht DE, Kline JJ. Tetracycline-induced pancreatitis. Am J Gastroenterol 1991;86(11):1669–71. [PubMed] [Google Scholar]
- 96.Elmore MF, Rogge JD. Tetracycline-induced pancreatitis. Gastroenterology 1981;81:1134–6. [PubMed] [Google Scholar]
- 97.Versleijen MW, Naber AH, Riksen NP, et al. Recurrent pancreatitis after trimethoprim-sulfamethoxazole rechallenge. Neth J Med 2005;63:275–7. [PubMed] [Google Scholar]
- 98.Brett AS, Shaw SV. Simultaneous pancreatitis and hepatitis associated with trimethoprim-sulfamethoxazole. Am J Gastroenterol 1999;94(1):267–8. [DOI] [PubMed] [Google Scholar]
- 99.Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: Case report. Pharmacother J Hum Pharmacol Drug Ther 2013;33(4):e43–4. [DOI] [PubMed] [Google Scholar]
- 100.Inayat F, Virk HH, Yoon DJ, et al. Drug-induced pancreatitis: A rare manifestation of doxycycline administration. N Am J Med Sci 2016;8(2):117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moy BT, Kapila N. Probable doxycycline-induced acute pancreatitis. Am J Health System Pharm 2016;73(5):286–91. [DOI] [PubMed] [Google Scholar]
- 102.Shah N, Razzano A, Grendell J. Doxycycline induced severe acute pancreatitis: A rare finding to A common medication. BMJ Case Rep 2021;14(2):e239640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rawla P, Raj JP. Doxycycline-induced acute pancreatitis: A rare adverse event. Gastroenterol Res 2017;10(4):244–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ocal S, Selçuk H, Korkmaz M, et al. Acute pancreatitis following doxycycline and ornidazole coadministration. JOP 2010;11:614–6. [PubMed] [Google Scholar]
- 105.Ozdogan O, Tahan V, Cincin A, et al. Acute pancreatitis associated with the use of peginterferon. Pancreas 2007;34(4):485–7. [DOI] [PubMed] [Google Scholar]
- 106.Kim SR, Imoto S, Mita K, et al. Pegylated interferon plus ribavirin combination therapy for chronic hepatitis C with high viral load of serum hepatitis C virus RNA, genotype 1b, discontinued on attaining sustained virological response at week 16 after onset of acute pancreatitis. Digestion 2009;79(1):36–9. [DOI] [PubMed] [Google Scholar]
- 107.Chaudhari S, Park J, Anand BS, et al. Acute pancreatitis associated with interferon and ribavirin therapy in patients with chronic hepatitis C. Dig Dis Sci 2004;49(6):1000–6. [DOI] [PubMed] [Google Scholar]
- 108.Ando K, Kim SR, Imoto S, et al. Acute pancreatitis associated with pegylated interferon and ribavirin treatment of chronic hepatitis C, genotype 1b with high viral load. Case Rep Gastroenterol 2009;3:372–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Silva Jd, Giroldi SB, Basso FdO, et al. Acute pancreatitis during interferon-alpha and ribavirin treatment for hepatitis C. BMJ Case Rep 2009;2009(1):bcr0920080998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tahan V, Tahan G, Dane F, et al. Acute pancreatitis attributed to the use of pegylated interferon in a patient with chronic hepatitis C. J Gastrointestin Liver Dis 2007;16(2):224–5. [PubMed] [Google Scholar]
- 111.Choi JW, Lee JS, Paik WH, et al. Acute pancreatitis associated with pegylated interferon-alpha-2a therapy in chronic hepatitis C. Clin Mol Hepatol 2016;22(1):168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weber A, Carbonnel F, Simon N, et al. Severe acute pancreatitis related to the use of adefovir in a liver transplant recipient. Gastroenterol Clin Biol 2008;32(3):247–9. [DOI] [PubMed] [Google Scholar]
- 113.Chen YY, Chen CY, Leung KK. Acute pancreatitis and amiodarone: A case report. World J Gastroenterol 2007;13(6):975–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saka M, Tüzün A, Ateş Y, et al. Acute pancreatitis possibly due to arginine use: A case report. Turk J Gastroenterol 2004;15(1):56–8. [PubMed] [Google Scholar]
- 115.Binet Q, Dufour I, Agneessens E, et al. The second case of a young man with L-arginine-induced acute pancreatitis. Clin J Gastroenterol 2018;11(5):424–7. [DOI] [PubMed] [Google Scholar]
- 116.Prajapati S, Shah S, Desai C, et al. Atorvastatin-induced pancreatitis. Indian J Pharmacol 2010;42:324–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Péron J, Khenifer S, Potier V, et al. Axitinib-induced acute pancreatitis: A case report. Anticancer Drugs 2014;25(4):478–9. [DOI] [PubMed] [Google Scholar]
- 118.Renkes P, Petitpain N, Cosserat F, et al. Can roxithromycin and betamethasone induce acute pancreatitis? JOP 2003;4(5):184–6. [PubMed] [Google Scholar]
- 119.Cheungpasitporn W, Srivali N, Thongprayoon C, et al. Acute pancreatitis in the use of canagliflozin: A rare side-effect of the novel therapy for type 2 diabetes mellitus. J Basic Clin Pharm 2015;6(3):101–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsao CY, Wright FS. Acute chemical pancreatitis associated with carbamazepine intoxication. Epilepsia 1993;34(1):174–6. [DOI] [PubMed] [Google Scholar]
- 121.Gill CJ, Jennings AE, Newton JB, et al. Fatal acute pancreatitis in a patient chronically treated with candesartan. J Pharm Technology 2005;21(2):79–82. [Google Scholar]
- 122.Godino J, Butani RC, Wong PWK, et al. Acute drug-induced pancreatitis associated with celecoxib [5]. J Clin Rheumatol 1999;5(5):305–7. [PubMed] [Google Scholar]
- 123.Yildiz MI, Karacam Dogan M, Mutlu E, et al. All in one: Clozapine-associated acute pancreatitis with multiple organ involvement. J Clin Psychopharmacol 2021;41(2):214–6. [DOI] [PubMed] [Google Scholar]
- 124.Bayard JM, Descamps OS, Evrard S, et al. Case report: Acute pancreatitis induced by clozapine. Acta Gastroenterol Belg 2005;68(1):92–4. [PubMed] [Google Scholar]
- 125.Raja M, Azzoni A. A case of clozapine-associated pancreatitis. Open Neuropsychopharmacol J 2011;4(1):5–7. [Google Scholar]
- 126.Roberge RJ, Martin TG, Hodgman M, et al. Acute chemical pancreatitis associated with a tricyclic antidepressant (clomipramine) overdose. J Toxicol Clin Toxicol 1994;32(4):425–9. [DOI] [PubMed] [Google Scholar]
- 127.Branquinho D, Ramos-Andrade D, Elvas L, et al. Drug-induced acute pancreatitis and pseudoaneurysms: An ominous combination. GE Port J Gastroenterol 2016;23(6):309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Balasch J, Martinez-Román S, Carreras J, et al. Acute pancreatitis associated with danazol treatment for endometriosis. Hum Reprod 1994;9(6):1163–5. [DOI] [PubMed] [Google Scholar]
- 129.Jha SH, Reddy JA, Dave JK. Dapsone-induced acute pancreatitis. Ann Pharmacother 2003;37(10):1438–40. [DOI] [PubMed] [Google Scholar]
- 130.Vara-Luiz F, Pe D'Arca Barbosa F, Antunes Albuquerque A, et al. An uncommon cause of acute pancreatitis in a patient with COVID-19. Cureus 2022;14(8):e27910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yanar F, Agcaoglu O, Sarici IS, et al. Clinical challenges in drug induced pancreatitis: Presentation of two cases and review of the literature. Int J Surg Case Rep 2013;4(8):708–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ben Abdallah I, Berrazega Y, Rachdi H, et al. Docetaxel monotherapy induces necrotic acute pancreatitis. J Oncol Pharm Pract 2022;28(6):1446–9. [DOI] [PubMed] [Google Scholar]
- 133.Ben Kridis W, Khanfir A, Frikha M. Acute pancreatitis induced by anticancer chemotherapy. Acta Clinica Belgica 2013;68(4):309–10. [DOI] [PubMed] [Google Scholar]
- 134.Khan IH, Edward N. Pancreatitis associated with diclofenac. Postgrad Med J 1993;69(812):486–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Luo H, Bhatt H, Mohamad S, et al. Acute pancreatitis: Possible association of dimethyl fumarate for the treatment of relapsing-remitting multiple sclerosis. J Neurol 2015;262(3):779–80. [DOI] [PubMed] [Google Scholar]
- 136.Khetpal N, Yadav L, Khalid S, et al. Eluxadoline-induced recurrent pancreatitis in a young female without a gallbladder: A case report and literature review. Cureus 2018;10(12):e3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chhaparia A, Hammami MB, Vareedayah A, et al. Eluxadoline-associated pancreatitis in a post-cholecystectomy patient: A case report. Del Med J 2017;89(3):90–2. [PubMed] [Google Scholar]
- 138.Berger TM, Cook WJ, O'Marcaigh AS, et al. Acute pancreatitis in a 12-year-old girl after an erythromycin overdose. Pediatrics 1992;90(4):624–6. [PubMed] [Google Scholar]
- 139.Fang C-C, Wang H-P, Lin J-T. Erythromycin-induced acute pancreatitis. J Toxicol Clin Toxicol 1996;34(1):93–5. [DOI] [PubMed] [Google Scholar]
- 140.Wong PW, Dillard TA, Kroenke K. Multiple organ toxicity from addition of erythromycin to long-term lovastatin therapy. South Med J 1998;91(2):202–5. [DOI] [PubMed] [Google Scholar]
- 141.Cao C-L, Duan P-Y, Zhang W-J, et al. Acute pancreatitis induced by etoposide–lobaplatin combination chemotherapy used for the treatment of lung cancer: A case report and literature review. Medicine 2017;96(29):e7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tysk C, Al-Eryani AY, Shawabkeh AA. Acute pancreatitis induced by fluvastatin therapy. J Clin Gastroenterol 2002;35(5):406–8. [DOI] [PubMed] [Google Scholar]
- 143.Famularo G, De Simone C, Minisola G, et al. Cross-reaction allergic pancreatitis with ketoprofen and flurbiprofen. Pancreas 2007;35(2):187–8. [DOI] [PubMed] [Google Scholar]
- 144.Chao C-T, Chao J-Y. Case report: Furosemide and pancreatitis: Importance of dose and latency period before reaction. Can Fam Physician 2013;59(1):43–5. [PMC free article] [PubMed] [Google Scholar]
- 145.Rutten J-P, Poeze M, Dejong CHC. Acute pancreatitis caused by excessive use of growth hormone in a 40-year-old man. Pancreas 2008;36(2):217. [DOI] [PubMed] [Google Scholar]
- 146.Fisher AA, Bassett ML. Acute pancreatitis associated with angiotensin II receptor antagonists. Ann Pharmacother 2002;36(12):1883–6. [DOI] [PubMed] [Google Scholar]
- 147.Moslim MA, Sodeman TC, Nawras AT. A case of suggested ibuprofen-induced acute pancreatitis. Am J Ther 2016;23(6):e1918–e1921. [DOI] [PubMed] [Google Scholar]
- 148.Magill P, Ridgway PF, Conlon KC, et al. A case of probable ibuprofen-induced acute pancreatitis. JOP 2006;7(3):311–4. [PubMed] [Google Scholar]
- 149.Memis D, Akalin E, Yucel T. Indomethacin-induced pancreatitis: A case report. JOP 2005;6(4):344–7. [PubMed] [Google Scholar]
- 150.Guerra M. Toxicity of indomethacin: Report of a case of acute pancreatitis. JAMA 1967;200(6):552–3. [PubMed] [Google Scholar]
- 151.Hung M-C, Hung G-Y, Lin P-C, et al. Acute pancreatitis associated with ifosfamide. J Chin Med Assoc 2007;70(4):176–9. [DOI] [PubMed] [Google Scholar]
- 152.Vignon RK, Seddik H, Rouibaa F, et al. Acute pancreatitis during pegylated interferon therapy in a patient with chronic hepatitis B. J Gastrointestin Liver Dis 2009;18(4):512. [PubMed] [Google Scholar]
- 153.Famularo G, Minisola G, Nicotra GC, et al. Acute pancreatitis associated with irbesartan therapy. Pancreas 2005;31(3):294–5. [DOI] [PubMed] [Google Scholar]
- 154.Passier JL, van Puijenbroek EP, Jonkers GJ, et al. Pancreatitis associated with the use of itraconazole. Neth J Med 2010;68(6):285–9. [PubMed] [Google Scholar]
- 155.Cobb TK, Pierce JR. Acute pancreatitis associated with ketoprofen. South Med J 1992;85(4):430–1. [DOI] [PubMed] [Google Scholar]
- 156.Elmusa E, Raza MW, Muneeb A, et al. Lamotrigine-induced acute pancreatitis. Cureus 2022;14(12):e33135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sequeira Lopes da Silva JT, González Casas O, Bejarano Moguel V, et al. Lanreotide autogel-induced acute pancreatitis in a patient with acromegaly. Gastroenterol Hepatol 2013;36(1):21–5. [DOI] [PubMed] [Google Scholar]
- 158.Labgaa I, Uldry E, Doerig C, et al. Loperamide-induced recurrent acute pancreatitis. Clin Res Hepatol Gastroenterol 2016;40(1):e13–4. [DOI] [PubMed] [Google Scholar]
- 159.Wurm S, Schreiber F, Spindelboeck W. Mefenamic acid: A possible cause of drug-induced acute pancreatitis. Pancreatology 2015;15(5):570–2. [DOI] [PubMed] [Google Scholar]
- 160.Alsubaie S, Almalki MH. Metformin induced acute pancreatitis. Dermatoendocrinol 2013;5(2):317–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fimognari FL, Corsonello A, Pastorell R, et al. Metformin-induced pancreatitis: A possible adverse drug effect during acute renal failure. Diabetes Care 2006;29(5):1183. [DOI] [PubMed] [Google Scholar]
- 162.Fuchs JE, Keith MR, Galanos AN. Probable raetolazone-induced pancreatitis. DICP 1989;23(9):711. [DOI] [PubMed] [Google Scholar]
- 163.Rosenfeld GA, Chang A, Poulin M, et al. Cholestatic jaundice, acute kidney injury and acute pancreatitis secondary to the recreational use of methandrostenolone: A case report. J Med Case Rep 2011;5(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sabre A, Guthrie MM, Maleknia R. Acute necrotising pancreatitis derived from low-dose corticosteroid use: An important reminder of clinical management. BMJ Case Rep 2015;2015:bcr2015209325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Iqbal K, Rathore SS, Hanyalu Shankar V, et al. A case of acute pancreatitis in a patient receiving high-dose steroids for optic neuritis. Cureus 2021;13(10):e19132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sato K, Hayashi M, Utsugi M, et al. Acute pancreatitis in a patient treated with micafungin. Clin Ther 2007;29(7):1468–73. [DOI] [PubMed] [Google Scholar]
- 167.Lankisch PG, Werner H-M. Mirtazapine: Another drug responsible for drug-induced acute pancreatitis? A letter of warning. Pancreas 2003;26(2):211. [DOI] [PubMed] [Google Scholar]
- 168.He S, Ikner TP, Taylor BV, et al. Mirtazapine-associated acute pancreatitis in a patient with insomnia and co-occurring psychiatric disorders. J Natl Med Assoc 2022;114(6):617–20. [DOI] [PubMed] [Google Scholar]
- 169.Einollahi B, Dolatimehr F. Acute pancreatitis induced by mycophenolate mofetil in a kidney transplant patient. J Nephropharmacol 2015;4(2):72–4. [PMC free article] [PubMed] [Google Scholar]
- 170.Aygencel G, Akbuga B, Keles A. Acute pancreatitis following naproxen intake. Eur J Emerg Med 2006;13(6):372. [DOI] [PubMed] [Google Scholar]
- 171.Castiella A, Lopez P, Bujanda L, et al. Possible association of acute pancreatitis with naproxen. J Clin Gastroenterol 1995;21(3):258. [DOI] [PubMed] [Google Scholar]
- 172.Nicholson JA, Smith D, Scott MH. Nicotine gum causing pancreatitis: A case report. Pancreas 2010;39(1):116. [DOI] [PubMed] [Google Scholar]
- 173.Adamec I, Reiner Z, Pecin I, et al. Acute pancreatitis after ocrelizumab treatment for relapsing remitting multiple sclerosis. Mult Scler Relat Disord 2020;45:102381. [DOI] [PubMed] [Google Scholar]
- 174.Gradon JD, Schulman RH, Chapnick EK, et al. Octreotide-induced acute pancreatitis in a patient with acquired immunodeficiency syndrome. South Med J 1991;84(11):1410–1. [DOI] [PubMed] [Google Scholar]
- 175.Fredenrich A, Sosset C, Bernard JL, et al. Acute pancreatitis after short-term octreotide. Lancet 1991;338(8758):52–3. [DOI] [PubMed] [Google Scholar]
- 176.Bush N, Sharma V, Chandrahasan K, et al. Ofloxacin-ornidazole fixed-dose combination medication-induced pancreatitis with positive rechallenge. J Fam Med Prim Care 2020;9(6):3157–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kathi P, Smith S, Rabadi R, et al. Omeprazole-associated necrotizing pancreatitis. Am J Ther 2020;27(4):e411–2. [DOI] [PubMed] [Google Scholar]
- 178.Baysal B, Kayar Y, Özmen A, et al. Olanzapine-induced acute pancreatitis. Turk J Gastroenterol 2015;26(3):289–90. [DOI] [PubMed] [Google Scholar]
- 179.Das S, Ganguly A, Ghosh A, et al. Oral pantoprazole-induced acute pancreatitis in an 11-year-old child. Ther Drug Monit 2012;34(3):242–4. [DOI] [PubMed] [Google Scholar]
- 180.Sammett D, Greben C, Sayeed-Shah U. Acute pancreatitis caused by penicillin. Dig Dis Sci 1998;43(8):1778–83. [DOI] [PubMed] [Google Scholar]
- 181.Lambrianides A, Rosin R. Acute pancreatitis complicating excessive intake of phenolphthalein. Postgrad Med J 1984;60(705):491–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pauwels A, Eliaszewicz M, Larrey D, et al. Pentamidine-induced acute pancreatitis in a patient with AIDS. J Clin Gastroenterol 1990;12(4):457–9. [DOI] [PubMed] [Google Scholar]
- 183.Pilmis B, Coignard-Biehler H, Rouzaud C, et al. Acute reversible pancreatitis induced by posaconazole. J Antimicrob Chemother 2017;72(2):628–30. [DOI] [PubMed] [Google Scholar]
- 184.Akazawa Y, Ohtani M, Namikawa S, et al. Severe necrotizing pancreatitis immediately after non-abdominal surgery under general anesthesia with propofol. Clin J Gastroenterol 2021;14(6):1798–803. [DOI] [PubMed] [Google Scholar]
- 185.Jawaid Q, Presti M, Neuschwander-Tetri B, et al. Acute pancreatitis after single-dose exposure to propofol: A case report and review of literature. Dig Dis Sci 2002;47(3):614–8. [DOI] [PubMed] [Google Scholar]
- 186.Parekh A, Zhang H. Propofol-induced severe necrotizing pancreatitis. ACG Case Rep J 2021;8(1):e00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Muniraj T, Aslanian HR. Hypertriglyceridemia independent propofol-induced pancreatitis. JOP 2012;13(4):451–3. [DOI] [PubMed] [Google Scholar]
- 188.Betrosian AP, Balla M, Papanikolaou M, et al. Post-operative pancreatitis after propofol administration. Acta Anaesthesiologica Scand 2001;45(8):1052. [DOI] [PubMed] [Google Scholar]
- 189.Herrmann R, Shaw RG, Fone DJ. Ranitidine-associated recurrent acute pancreatitis. Aust New Zealand J Med 1990;20(3):243–4. [DOI] [PubMed] [Google Scholar]
- 190.Pereira M, Prakash A, Puranik AD, et al. Regorafenib-associated acute pancreatitis diagnosed on 18F-FDG PET/CT. Clin Nucl Med 2021;46(5):e256–7. [DOI] [PubMed] [Google Scholar]
- 191.Rodrigo L, Moreno M, Calleja S, et al. Riluzole-induced acute pancreatitis. Am J Gastroenterol 2001;96(7):2268–9. [DOI] [PubMed] [Google Scholar]
- 192.Cordeiro QJ, Elkis H. Pancreatitis and cholestatic hepatitis induced by risperidone. J Clin Psychopharmacol 2001;21(5):529–30. [DOI] [PubMed] [Google Scholar]
- 193.Si̇rkeci̇ Ö, Erkuş Si̇rkeci̇ E, Tasel B. Rivaroxaban-induced acute pancreatitis. Eur Res J 2019;5(3):569–71. [Google Scholar]
- 194.Block MB, Genant HK, Kirsner JB. Pancreatitis as an adverse reaction to salicylazosulfapyridine. N Engl J Med 1970;282(7):380–2. [DOI] [PubMed] [Google Scholar]
- 195.Slim R, Ben Salem C, Zamy M, et al. Secnidazole-induced acute pancreatitis: A new side-effect for an old drug? JOP 2010;11(1):85–6. [PubMed] [Google Scholar]
- 196.Antonopoulos S, Mikros S, Kokkoris S, et al. A case of acute pancreatitis possibly associated with combined salicylate and simvastatin treatment. JOP 2005;6(3):264–8. [PubMed] [Google Scholar]
- 197.Sevin A, Chen A, Atkinson B. Tyrosine kinase inhibitor induced pancreatitis. J Oncol Pharm Pract 2012;19(3):257–60. [DOI] [PubMed] [Google Scholar]
- 198.Kattah Martinez L, Marín Carrillo L, Rojas Melo L. Sorafenib-induced acute pancreatitis in a patient with differentiated thyroid cancer. Eur Thyroid J 2018;7(3):145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Amar S, Wu KJ, Tan WW. Sorafenib-induced pancreatitis. Mayo Clinic Proc 2007;82(4):521. [DOI] [PubMed] [Google Scholar]
- 200.Oflazoglu U, Varol U, Alacacioglu A, et al. Case report of a renal cell carcinoma patient with acute pancreatitis under both sunitinib and axitinib treatment. J Oncological Sci 2016;2(2-3):63–5. [Google Scholar]
- 201.Navabi SJ, Khosravifar M, Navabi SM, et al. Acute pancreatitis induced by tadalafil: A case report. Clin J Gastroenterol 2020;13(3):459–64. [DOI] [PubMed] [Google Scholar]
- 202.Liu P-H, Lee B-J, Wang C-Y, et al. Acute pancreatitis after severe theophylline overdose. Clin Toxicol 2008;46(10):1103. [DOI] [PubMed] [Google Scholar]
- 203.Gilson M, Moachon L, Jeanne L, et al. Acute pancreatitis related to tigecycline: Case report and review of the literature. Scand J Infect Dis 2008;40(8):681–3. [DOI] [PubMed] [Google Scholar]
- 204.Lipshitz J, Kruh J, Cheung P, et al. Tigecycline-induced pancreatitis. J Clin Gastroenterol 2009;43(1):93. [DOI] [PubMed] [Google Scholar]
- 205.Picardo S, So K, Venugopal K, et al. Vedolizumab-induced acute pancreatitis: The first reported clinical case. BMJ Case Rep 2018;2018:bcr2017222554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Velter C, Blanc J, Robert C. Acute pancreatitis after vismodegib for basal cell carcinoma: A causal relation? Eur J Cancer 2019;118:67–9. [DOI] [PubMed] [Google Scholar]