Abstract
Long interspersed element 1 (L1) are a family of autonomous, actively mobile transposons that occupy ~17% of the human genome. The pleiotropic effects L1 induces in host cells (promoting genome instability, inflammation, or cellular senescence) are established, and L1’s associations with aging and aging diseases are widely recognized. However, because of the cell type-specific nature of transposon control, the catalogue of L1 regulators remains incomplete. Here, we employ an eQTL approach leveraging transcriptomic and genomic data from the GEUVADIS and 1000Genomes projects to computationally identify new candidate regulators of L1 expression in lymphoblastoid cell lines. To cement the role of candidate genes in L1 regulation, we experimentally modulate the levels of top candidates in vitro, including IL16, STARD5, HSDB17B12, and RNF5, and assess changes in TE family expression by Gene Set Enrichment Analysis (GSEA). Remarkably, we observe subtle but widespread upregulation of TE family expression following IL16 and STARD5 overexpression. Moreover, a short-term 24-hour exposure to recombinant human IL16 was sufficient to transiently induce subtle but widespread upregulation of L1 subfamilies. Finally, we find that many L1 expression-associated genetic variants are co-associated with aging traits across genome-wide association study databases. Our results expand the catalogue of genes implicated in L1 transcriptional control and further suggest that L1 contributes to aging processes. Given the ever-increasing availability of paired genomic and transcriptomic data, we anticipate this new approach to be a starting point for more comprehensive computational scans for transposon transcriptional regulators.
Introduction
Transposable elements (TEs) constitute ~45% of the human genome 1. Among these, the long interspersed element-1 (LINE-1 or L1) family of transposons is the most abundant, accounting for ~16–17% 1,2, and remains autonomously mobile, with humans harboring an estimated 80–100 retrotransposition-competent L1 copies 3. These retrotransposition competent L1s belong to evolutionarily younger L1PA and L1Hs subfamilies, are ~6 kilobases long, carry an internal promoter in their 5’-untranslated region (UTR), and encode two proteins — L1ORF1p and L1ORF2p — that are necessary for transposition 4. The remaining ~500,000 copies are non-autonomous or immobile because of the presence of inactivating mutations or truncations 1 and include L1 subfamilies of all evolutionary ages, including the evolutionarily older L1P and L1M subfamilies. Though not all copies are transposition competent, L1s can nevertheless contribute to aspects of aging 5,6 and aging-associated diseases 7–10.
Though mechanistic studies characterizing the role of L1 in aging and aging-conditions are limited, its effects are clearly pleiotropic. L1 can contribute to genome instability via insertional mutagenesis, leading to an expansion of copy number with organismal aging 11 and during cellular senescence 12. L1 can also play a contributing role in shaping inflammatory and cellular senescence phenotypes. The secretion of a panoply of pro-inflammatory factors is a hallmark of cell senescence, called the senescence associated secretory phenotype (SASP) 13. Importantly, the SASP is believed to stimulate the innate immune system and contribute to chronic, low-grade, sterile inflammation with age, a phenomenon referred to as “inflamm-aging” 13,14. During deep senescence, L1 are transcriptionally de-repressed and consequently generate cytosolic DNA that initiates an immune response consisting of the production and secretion of pro-inflammatory interferons 15. Finally, L1 is causally implicated in aging-associated diseases, including cancer. L1 may contribute to cancer by (i) serving as a source for chromosomal rearrangements that can lead to tumor-suppressor genes deletion 16 or (ii) introducing its active promoter next to normallycsilenced oncogenes 17. Thus, because of the pathological effects L1 can have on hosts, it is critical that hosts maintain precise control over L1 activity.
Eukaryotic hosts have evolved several pre- and post-transcriptional mechanisms for regulating TEs 18,19. Nevertheless, our knowledge of regulatory genes remains incomplete because of cell type-specific regulation and the complexity of methods required to identify regulators. Indeed, one clustered regularly interspaced short palindromic repeats (CRISPR) screen in two cancer cell lines for regulators of L1 transposition identified >150 genes involved in diverse biological functions 20 (e.g. chromatin regulation, DNA replication, and DNA repair). However, only about ~36% of the genes identified in the primary screen exerted the same effects in both cell lines 20, highlighting the potentially cell type-specific nature of L1 control. Moreover, given the complexities of in vitro screens, especially in non-standard cell lines or primary cells, in silico screens for L1 regulators may facilitate the task of identifying and cataloguing candidate regulators across cell and tissue types. One such attempt was made by generating gene-TE co-expression networks from RNA sequencing (RNA-seq) data generated from multiple tissue types of cancerous origin 21. Although co-expression modules with known TE regulatory functions, such as interferon signaling, were correlated with TE modules, it is unclear whether other modules may harbor as of now uncharacterized TE-regulating properties, since no validation experiments were carried out. Additionally, this co-expression approach is limited, as no mechanistic directionality can be assigned between associated gene and TE clusters, complicating the prioritization of candidate regulatory genes for validation. Thus, there is a need for the incorporation of novel “omic” approaches to tackle this problem. Deciphering the machinery that controls TE activity in healthy somatic cells will be crucial, in order to identify checkpoints lost in diseased cells.
The 1000Genomes Project and GEUVADIS Consortium provide a rich set of genomic resources to explore the mechanisms of human TE regulation in silico. Indeed, the 1000Genomes project generated a huge collection of genomic data from thousands of human subjects across the world, including single nucleotide variant (SNV) and structural variant (SV) data 22,23. To accomplish this, the project relied on lymphoblastoid cell lines (LCLs), which are generated by infecting resting B-cells in peripheral blood with Epstein-Barr virus (EBV). Several properties make them advantageous for use in large-scale projects (e.g. they can be generated relatively uninvasively, provide a means of obtaining an unlimited amount of a subject’s DNA and other biomolecules, and can serve as an in vitro model for studying the effects of genetic variation with phenotypes of interest) 24,25. Indeed, the GEUVADIS Consortium generated transcriptomic data for a subset of subjects sampled by the 1000Genomes Project, and used their genomic data to define the effects genetic variation on gene expression 26. Together, these resources provide a useful toolkit for investigating the genetic regulation of TEs, generally, and L1, specifically.
In this study, we (i) develop a pipeline to identify novel candidate regulators of L1 expression in lymphoblastoid cell lines, (ii) provide experimental evidence for the involvement of top candidates in L1 expression control, and (iii) expand and reinforce the catalogue of diseases linked to L1.
Results
In silico scanning for L1 subfamily candidate regulators by eQTL analysis
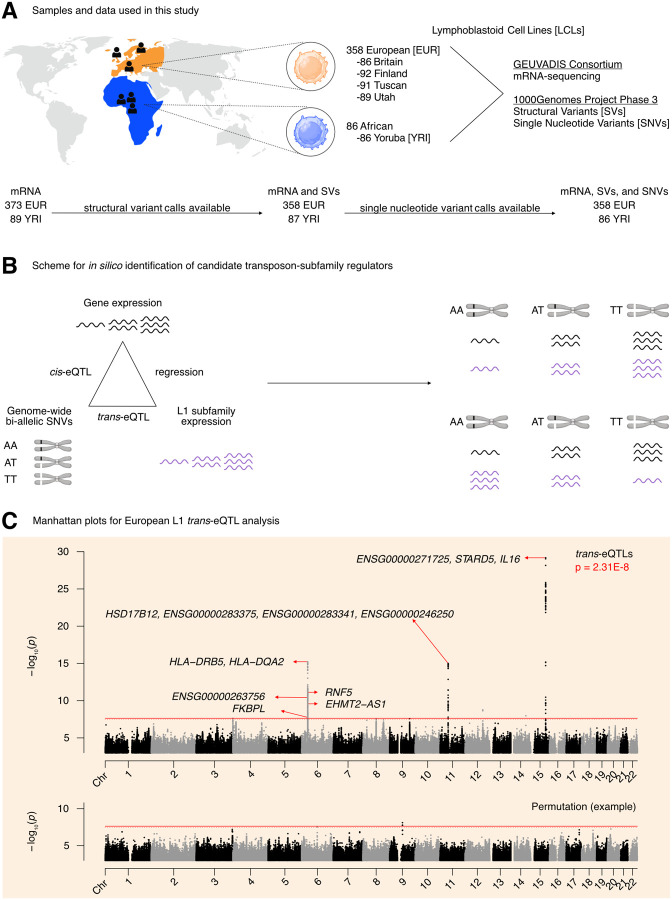
To identify new candidate regulators of L1 transcription, we decided to leverage publicly available human “omic” datasets with both genetic and transcriptomic information. For this analysis, we focused on samples for which the following data was available: (i) mRNA-seq data from the GEUVADIS project, (ii) SNVs called from whole-genome sequencing data overlayed on the hg38 human reference genome made available by the 1000Genomes project, and (iii) repeat structural variation data made available by the 1000Genomes project. This yielded samples from 358 European and 86 Yoruban individuals, all of whom declared themselves to be healthy at the time of sample collection (Figure 1A). Using the GEUVADIS data, we obtained gene and TE subfamily expression counts using TEtranscripts 27. As a quality control step, we checked whether mapping rates segregated with ancestry groups, which may bias results. However, the samples appeared to cluster by laboratory rather than by ancestry (Figure S1A). As additional quality control metrics, we also checked whether the SNV and SV data segregated by ancestry following principal component analysis (PCA). These analyses demonstrated that the top two and the top three principal components from the SNV and SV data, respectively, segregated ancestry groups (Figure S1B, Figure S1C).
Figure 1. Overview of the pipeline developed to scan for L1 transcriptional regulators in silico.
(A) An illustration of the samples and “omic” data used in this study. Of the 358 European individuals, 187 were female and 171 were male. Of the 86 African individuals, 49 were female and 37 were male. (Note that Utah subjects are of Northern European ancestry, and thus part of the European cohort for analytical purposes). (B) A schematic illustrating how genetic variants, gene expression, and TE expression can be integrated to identify highly correlated SNV-Gene-TE trios. (C) A Manhattan plot for the L1 subfamily trans-eQTL analysis in the European cohort. The genes that passed our three-part integration approach are listed next to the most significant trans-eQTL SNV they were associated with in cis. The dashed line at p = 3.44E-8 corresponds to an average empirical FDR < 0.05, based on 20 random permutations. One such permutation is illustrated in the bottom panel. The solid line at p = 2.31E-8 corresponds to a Benjamini-Hochberg FDR < 0.05. The stricter of the two thresholds, p = 2.31E-8, was used to define significant trans-eQTLs. FDR: False Discovery Rate. Some panels were created with BioRender.com.
We then chose to do a three-part integration of the available “omic” data (Figure 1B). Since TEtranscripts quantifies TE expression aggregated at the TE subfamily level and discards TE position information, we chose to carry out a trans-eQTL analysis against global expression of each L1 subfamily. We reasoned that there would have to be factors (i.e., miRNAs, proteins, non-coding RNAs) mediating the effects of SNVs on L1 subfamily expression. Thus, to identify candidate genic mediators, we searched for genes with cis-eQTLs that overlapped with L1 trans-eQTLs. As a final filter, we reasoned that for a subset of regulators, L1 subfamily expression would respond to changes in the expression of those regulators. Consequently, we chose to quantify the association between L1 subfamily expression and candidate gene expression by linear regression. We hypothesized that this three-part integration would result in combinations of significantly correlated SNVs, genes, and L1 subfamilies (Figure 1B).
The trans-eQTL analysis against every expressed L1 subfamily led to the identification of 499 trans-eQTLs distributed across chromosomes 6, 11, 12, 14, and 15 that passed genome-wide significance (Figure 1C, Supplementary Table S1A). The cis-eQTL analysis led to the identification of 845,260 cis-eQTLs that passed genome-wide significance (Supplementary Figure S2, Supplementary Table S1B). After integrating the identified cis- and trans-eQTLs and running linear regression, we identified 1,272 SNV-Gene-L1 trios that fulfilled our three-part integration approach (Supplementary Table S1C). Among this pool of trios, we identified 7 unique protein-coding genes including IL16, STARD5, HLA-DRB5, HLA-DQA2, HSD17B12, RNF5, and FKBPL (Figure 1C). We note that although EHMT2 did not pass out screening approach, it does overlap EHMT2-AS1, which did pass our screening thresholds (Figure 1C). We also note that several other unique non-coding genes, often overlapping the protein-coding genes listed, were also identified (Figure 1C). For simplicity of interpretation, we focused on protein-coding genes during downstream experimental validation.
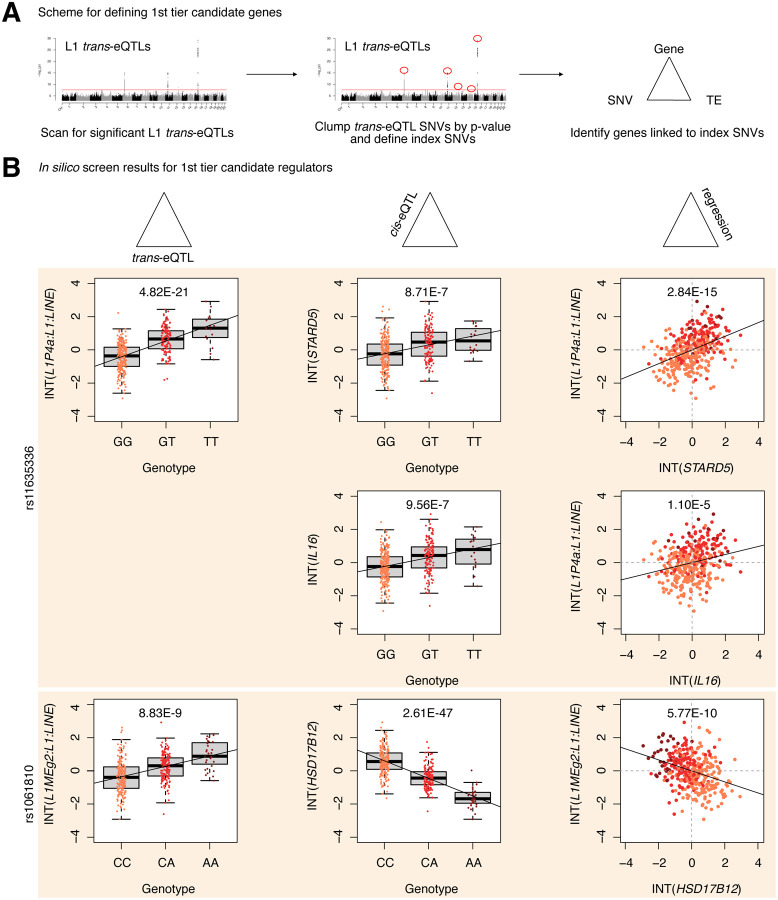
Next, to define first and second tier candidate regulators, we clumped SNVs in linkage disequilibrium (LD) by L1 trans-eQTL p-value to identify the most strongly associated genetic variant in each genomic region (Figure 2A, Supplementary Figure S3A). LD-clumping identified the following index SNVs (i.e. the most strongly associated SNVs in a given region): rs11635336 on chromosome 15, rs9271894 on chromosome 6, rs1061810 on chromosome 11, rs112581165 on chromosome 12, and rs72691418 on chromosome 14 (Supplementary Table S1D). Genes linked to these SNVs were considered first tier candidate regulators and included IL16, STARD5, HLA-DRB5, HLA-DQA2, and HSD17B12 (Figure 2B, Supplementary Table S1E). The remaining genes were linked to clumped, non-index SNVs and were consequently considered second tier candidates and included RNF5, EHMT2-AS1, and FKBPL (Supplementary Figure S3B). Additionally, for simplicity of interpretation, we considered only non-HLA genes during downstream experimental validation, since validation could be complicated by the highly polymorphic nature of HLA loci 28 and their involvement in multi-protein complexes.
Figure 2. Identification of 1st tier candidate L1 expression regulators in the European cohort.
(A) A schematic for how 1st tier candidate genes were defined. In short, these were genes in trios with index SNVs that were at the top of their respective peak. (B) The three-part integration results for three protein-coding genes—STARD5, IL16, HSD17B12—that we considered first tier candidates for functional, in vitro testing. In the left column are the trans-eQTLs, in the middle column are the cis-eQTLs, and in the right column are the linear regressions for gene expression against L1 subfamily expression. Expression values following an inverse normal transform (INT) are shown. The FDR for each analysis is listed at the top of each plot. FDR: False Discovery Rate.
Finally, to computationally determine whether candidate genes may causally influence L1 subfamily expression, we carried out mediation analysis on all SNV-gene-L1 trios (Supplementary Figure S4A). Interestingly, 868 out of the 1,272 (68.2%) trios exhibited significant (FDR < 0.05) mediation effects (Supplementary Table S1F). Among the 1st tier candidate regulators, significant, partial, and consistent mediation effects could be attributed to STARD5, IL16, HSD17B12, and HLA-DRB5 (Supplementary Figure S4B, Supplementary Table S1F). To note, while significant mediation could be attributed to the index SNV for STARD5, significant mediation could only be attributed to clumped SNVs for IL16 and HSD17B12. Given that STARD5 and IL16 share cis-eQTL SNVs, this suggests that STARD5 may be the more potent mediator. Among the 2nd tier candidate regulators, significant, partial, and consistent mediation effects could be attributed to RNF5, EHMT2-AS1, and FKBPL (Supplementary Figure S4C, Supplementary Table S1F). These results suggest that candidate genes may mediate the effects between linked SNVs and L1 subfamilies.
In silico scanning for L1 subfamily candidate regulators in an African population
We next sought to assess the cross-ancestry regulatory properties of candidate genes by repeating our scan using the Yoruban samples as a smaller but independent replication cohort. Here, rather than conduct a genome-wide scan for cis- and trans-associated factors, we opted for a targeted approach focusing only on gene cis-eQTLs and L1 subfamily trans-eQTLs that were significant in the analysis with European samples (Supplementary Figure S5A). The targeted trans-eQTL analysis led to the identification of 227 significant (FDR < 0.05) trans-eQTLs distributed across chromosomes 6 and 11 (Supplementary Table S2A). The targeted cis-eQTL analysis led to the identification of 1,248 significant (FDR < 0.05) cis-eQTLs (Supplementary Table S2B). After integrating the identified cis- and trans-eQTLs and running linear regression, we identified 393 SNV-Gene-L1 trios that fulfilled our three-part integration approach (Supplementary Table S2C). Among this pool of trios, we identified 2 unique protein-coding genes—HSD17B12 and HLA-DRB6—as well as several unique non-coding genes (Supplementary Table S2C). Again, we clumped SNVs in linkage disequilibrium (LD) by L1 trans-eQTL p-value. LD-clumping identified the following index SNVs: rs2176598 on chromosome 11 and rs9271379 on chromosome 6 (Supplementary Table S2D). Genes linked to these SNVs were considered first tier candidate regulators and included both HSD17B12 and HLA-DRB6 (Supplementary Figure S5B, Supplementary Table S2E). Finally, we carried out mediation analysis on all SNV-gene-L1 trios; however, no significant (FDR < 0.05) mediation was observed (Supplementary Table S2F). These results implicate HSD17B12 and the HLA loci as candidate, cross-ancestry L1 expression regulators.
To assess why some candidate genes did not replicate in the Yoruba cohort, we manually inspected cis- and trans-eQTL results for trios with those genes (Supplementary Figure S6A). Interestingly, we identified rs9270493 and rs9272222 as significant (FDR < 0.05) trans-eQTLs for L1MEb expression. However, those SNVs were not significant cis-eQTLs for RNF5 and FKBPL expression, respectively. For trios involving STARD5, IL16, and EHMT2-AS1, neither the cis-eQTL nor the trans-eQTL were significant. We note that for most of these comparisons, although the two genotypes with the largest sample sizes were sufficient to establish a trending change in cis or trans expression, this trend was often broken by the third genotype with spurious sample sizes. This suggests that replication in the Yoruba cohort may be limited by the small cohort sample size in the GEUVADIS project.
TE families and known TE-associated pathways are differentially regulated across L1 trans-eQTL variants
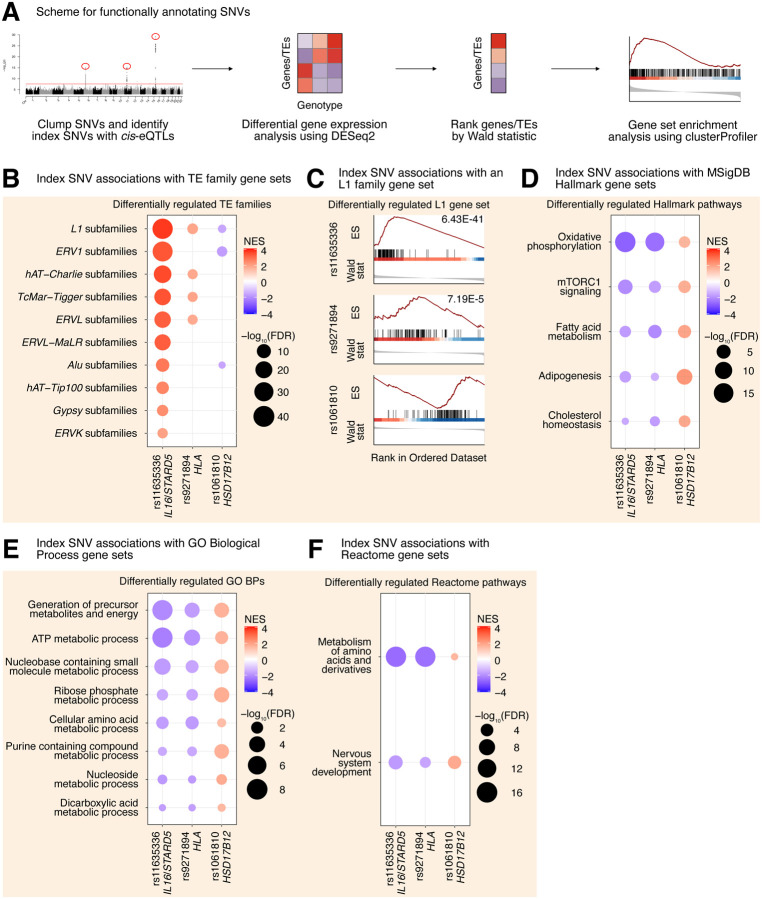
Though our eQTL analysis identified genetic variants associated with the expression of specific, evolutionarily older L1 subfamilies, we reasoned that there may be more global but subtle differences in TE expression profiles among genotype groups, given that TE expression is highly correlated 21. Thus, for each gene-associated index SNV identified in the European eQTL analysis, we carried out differential expression analysis for all expressed genes and TEs (Supplementary Table S3A–S3C; Figure 3A). At the individual gene level, we detected few significant (FDR < 0.05) changes: 4 genes/TEs varied with rs11635336 genotype (IL16/STARD5), 4 genes/TEs varied with rs9271894 genotype (HLA), and 5 gene/TEs varied with rs1061810 genotype (HSD17B12) (Supplementary Table S3A–S3C). Importantly, however, these genes/TEs overlapped the genes/TEs identified in the cis- and trans-eQTL analyses, providing an algorithmically independent link among candidate SNV-gene-TE trios.
Figure 3. L1 trans-eQTLs are associated with subtle, widespread differences in TE families and known TE-associated pathways.
(A) Scheme for functionally annotating gene-linked index SNVs by GSEA. (B) GSEA analysis for shared, significantly regulated TE family gene sets across genotypes for rs11635336 (IL16/STARD5), rs9271894 (HLA), and rs1061810 (HSD17B12). (C) GSEA plots for the L1 family gene set results summarized in (B). For these plots, the FDR value is listed. GSEA analysis for top, shared, concomitantly regulated (D) MSigDB Hallmark pathway, (E) GO Biological Process, and (F) Reactome pathway gene sets across genotypes for rs11635336 (IL16/STARD5), rs9271894 (HLA), and rs1061810 (HSD17B12). Shared gene sets were ranked by combining p-values from each individual SNV analysis using Fisher’s method. In each bubble plot, the size of the dot represents the −log10(FDR) and the color reflects the normalized enrichment score. FDR: False Discovery Rate.
In contrast to gene-level analyses, Gene Set Enrichment Analysis (GSEA) provides increased sensitivity to subtle but consistent/widespread transcriptomic changes at the level of gene sets (e.g. TE families, biological pathways, etc.). Thus, we leveraged our differential expression analysis in combination with GSEA to identify repeat family and biological pathway gene sets impacted by SNV genotype in the GEUVADIS dataset (Supplementary Table S3D–S3O; Figure 3A). Interestingly, changes in the genotype of rs11635336 (IL16/STARD5), rs9271894 (HLA), and rs1061810 (HSD17B12) were associated with an upregulation, upregulation, and downregulation, respectively, of multiple TE family gene sets (Figure 3B, Supplementary Table S3P). Differentially regulated TE family gene sets included DNA transposons, such as the hAT-Charlie family, and long terminal repeat (LTR) transposons, such as the endogenous retrovirus-1 (ERV1) family (Figure 3B, Supplementary Table S3P). Noteworthy, the L1 family gene set was the only TE gene set whose expression level was significantly altered across all three SNV analyses (Figure 3B, Supplementary Table S3P). Consistent with their relative significance in the L1 trans-eQTL analysis, the L1 family gene set was most strongly upregulated by alternating the IL16/STARD5 SNV (NES = 3.74, FDR = 6.43E-41), intermediately upregulated by alternating the HLA SNV (NES = 1.90, FDR = 7.19E-5), and least strongly changed by alternating the HSD17B12 SNV (NES = −1.57, FDR = 2.11E-2) (Figure 3C). We briefly note here that rs9270493, a clumped SNV linked to RNF5, was also linked to upregulation of the L1 family gene set (Supplementary Table S3Q–S3R). These results suggest that TE subfamily trans-eQTLs are associated with subtle but global differences in TE expression beyond a lone TE subfamily.
Next, we asked if other biological pathways were regulated concomitantly with TE gene sets in response to gene-linked index SNVs, reasoning that such pathways would act either upstream (as regulatory pathways) or downstream (as response pathways) of TE alterations. GSEA with the MSigDB Hallmark pathway gene sets 29,30 identified 5 gene sets fitting this criterion, including “oxidative phosphorylation”, “mTORC1 signaling”, “fatty acid metabolism”, “adipogenesis”, and “cholesterol homeostasis” (Figure 3D, Supplementary Table S3S). Interestingly, several of these pathways or genes in these pathways have been implicated in TE regulation before. Rapamycin, which acts through mTORC1, has been shown to alter the expression of L1 and other repeats 31,32. Estrogens, which are involved in cholesterol and lipid metabolism, have been found to drive changes in repeat expression, and the receptors for both estrogens and androgens are believed to bind repeat DNA 32,33. Pharmacological inhibition of the mitochondrial respiratory chain and pharmacological reduction of endogenous cholesterol synthesis have also been shown to induce changes in L1 protein levels or repeat expression more broadly 34,35. GSEA with the GO Biological Process gene sets (Figure 3E, Supplementary Table S3T) and the Reactome gene sets (Figure 3F, Supplementary Table S3U) also identified several metabolism-related pathways including “ATP metabolic process”, “Generation of precursor metabolites and energy”, and “metabolism of amino acids and derivatives”. These results add to the catalogue of pathways associated with differences in L1 expression.
In our eQTL analysis, we also identified two orphan index SNVs, rs112581165 and rs72691418, to which we could not attribute a protein-coding gene mediator. To determine whether these SNVs also regulate any transposon families or biological pathways, we repeated the differential expression analysis (with all expressed genes and TEs) (Supplementary Table S4A–S4B) and the GSEA (Supplementary Table S4C–S4J) with these SNVs (Supplementary Figure S7A). At the individual gene level, we detected 3193 genes/TEs that varied significantly (FDR < 0.05) with rs112581165 genotype and 1229 genes/TEs that varied significantly with rs72691418 genotype (Supplementary Table S4A–S4B). Similar to above, we next carried out GSEA to identify changes in functionally relevant gene sets. Like the gene-linked index SNVs, changes in the genotype of rs112581165 and rs72691418 were both associated with a downregulation and upregulation, respectively, of 10 TE families (Supplementary Figure S7B, Supplementary Table S4K). Noteworthy, the L1 family gene set was among the most strongly dysregulated TE family gene sets for both rs112581165 (NES = −4.32, FDR = 5.18E-89) and rs72691418 (NES = 4.01, FDR = 5.38E-79) (Supplementary Figure S7C). These results suggest that TE subfamily trans-eQTLs are associated with subtle differences in TE expression beyond the lone TE subfamily, even in the absence of a protein-coding gene cis-eQTL.
Like before, we asked if other biological pathways were regulated concomitantly with TE gene sets in response to orphan index SNVs. The top 10 Hallmark pathway gene sets identified by GSEA included gene sets that were previously identified (“oxidative phosphorylation”, “fatty acid metabolism”, and “mTORC1 signaling”), as well as several new pathways (Supplementary Figure S7D, Supplementary Table S4L). Among the new pathways, “DNA repair” 20 and the “P53 pathway” 36,37 have also been linked to L1 control, and proteins in the “Myc targets v1” gene set interact with L1 ORF1p 38. GSEA with the GO Biological Process gene sets (Supplementary Figure S7E, Supplementary Table S4M) and the Reactome gene sets (Supplementary Figure S7F, Supplementary Table S4N) identified several metabolism-related pathways and several translation-related pathways, such as “cytoplasmic translation”, “eukaryotic translation initiation”, and “eukaryotic translation elongation”. Importantly, proteins involved in various aspects of proteostasis have been shown to be enriched among L1 ORF1p-interacting proteins 38. Again, these results add to the catalogue of pathways associated with differences in TE expression, even in the absence of a candidate cis mediator.
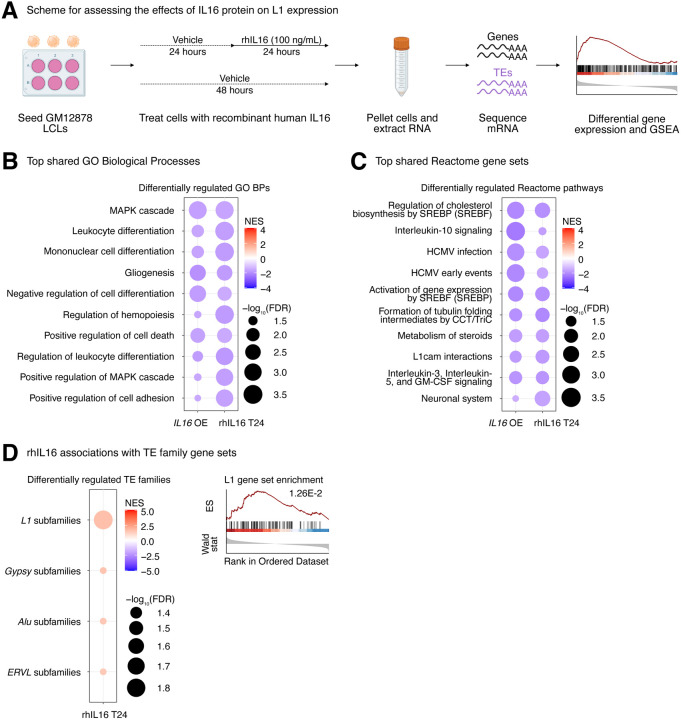
Modulation of top candidate gene activity in a lymphoblastoid cell line induces small but widespread TE expression changes
We decided to validate the L1 regulatory properties of top candidate genes associated with L1 trans-eQTLs. For experimental purposes, we selected the GM12878 lymphoblastoid cell line, because (i) it is of the same cell type as the transcriptomic data used here for our eQTL analysis, and (ii) its epigenomic landscape and culture conditions have been well well-characterized as part of the ENCODE project 39,40. For validation purposes, we selected IL16, STARD5, HSD17B12, and RNF5 out of the 7 protein-coding gene candidates. We chose these genes for validation because the first 3 are associated with top trans-eQTL SNVs and the fourth one had very strong predicted mediation effects. To note, although GM12878 was part of the 1000Genomes Project, it was not included in the GEUVADIS dataset. However, based on its genotype, we can predict the relative expression of candidate regulators (Supplementary Figure S8A), which suggest that GM12878 may be most sensitive to modulations in IL16 and STARD5 expression, given their relatively low endogenous expression. Interestingly, examination of the ENCODE epigenomic data in GM12878 cells 39 demonstrated that the region near the IL16/STARD5-linked index SNV (rs11635336) was marked with H3K4Me1 and H3K27Ac, regulatory signatures of enhancers (Supplementary Figure S8C). Similarly, the region near the HLA-linked index SNV (rs9271894) was marked with H3K4Me1, marked with H3K27Ac, and accessible by DNase, suggesting regulatory properties of the region as an active enhancer (Supplementary Figure S8C). These results further highlight the regulatory potential of the IL16-, STARD5-, and HLA-linked SNVs.
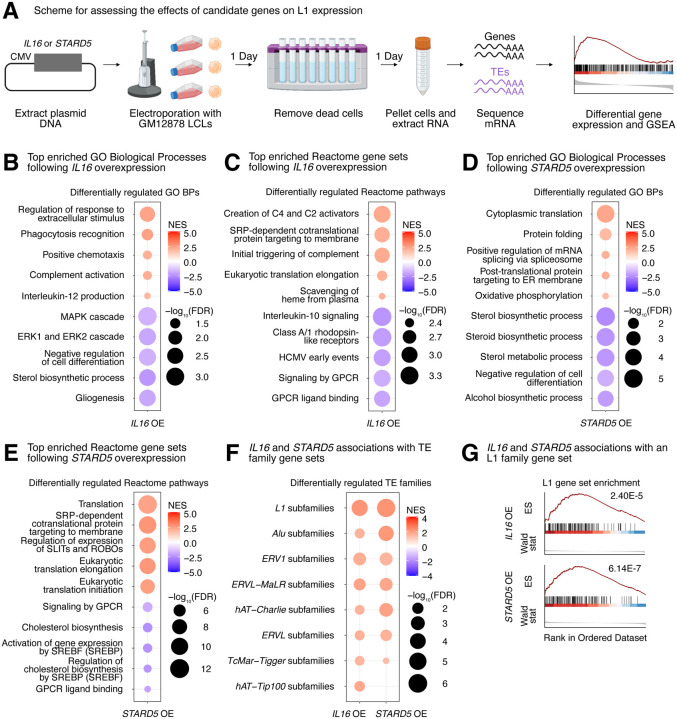
First, we decided to test the transcriptomic impact of overexpressing our top candidates in GM12878 LCLs. Cells were electroporated with overexpression plasmids (or corresponding empty vector), and RNA was isolated after 48h (Figure 4A, Supplementary Figure S9A). Differential expression analysis comparing control and overexpression samples confirmed the overexpression of candidate genes (Supplementary Figure S9B, Supplementary Table S5A–S5D). Intriguingly, we observed that IL16 was significantly upregulated following STARD5 overexpression (Supplementary Figure S9C, Supplementary Table S5B), although the inverse was not observed (Supplementary Figure S9C, Supplementary Table S5A), suggesting that IL16 may act downstream of STARD5. We note here that, consistent with the use of a high expression vector, the IL16 upregulation elicited by STARD5 overexpression (log2 fold change = 0.45) was weaker than the upregulation from the IL16 overexpression (log2 fold change = 1.89) (Supplementary Table S5A–S5B).
Figure 4. Impact of IL16 and STARD5 overexpression on LCL gene and TE expression landscapes.
IL16 and STARD5 overexpression induce changes consistent with their known biology, as well as subtle but widespread upregulation of TE families. (A) Scheme for experimentally validating the roles of IL16 and STARD5 in L1 regulation. GSEA analysis for top, differentially regulated (B) GO Biological Process and (C) Reactome pathway gene sets following IL16 overexpression. GSEA analysis for top, differentially regulated (D) GO Biological Process and (E) Reactome pathway gene sets following STARD5 overexpression. (F) GSEA analysis for shared, significantly regulated TE family gene sets following IL16 and STARD5 overexpression. (G) GSEA plots for the L1 family gene set results summarized in (F). For these plots, the FDR value is listed. In each bubble plot, the size of the dot represents the −log10(FDR) and the color reflects the normalized enrichment score. FDR: False Discovery Rate. Some panels were created with BioRender.com.
To further assess the biological relevance of each overexpression, we carried out GSEA using the GO Biological Process, Reactome pathway, and Hallmark pathway gene sets (Supplementary Table S5E–S5P). Importantly, GSEA using GO Biological Process and Reactome pathway gene sets highlighted differences that were consistent with the known biology of our candidate genes. Firstly, IL16 is involved in regulating T-cell activation, B-cell differentiation, and functions as a chemoattractant 41–46. Moreover, it modulates macrophage polarization by regulating IL-10 expression 47. IL16 overexpressing cells showed upregulation for “phagocytosis recognition” and “positive chemotaxis”, downregulation for “negative regulation of cell differentiation”, and downregulation for “Interleukin 10 signaling” (Figure 4B–4C). Secondly, STARD5 encodes a cholesterol transporter and is upregulated in response to endoplasmic reticulum (ER) stress 48–50. STARD5 overexpressing cells showed downregulation of various cholesterol-related gene sets such as “sterol biosynthetic process”, “sterol metabolic process”, and “regulation of cholesterol biosynthesis by SREBP (SREBF)” (Figure 4D–4E). Thirdly, HSD17B12 encodes a steroid dehydrogenase involved in converting estrone into estradiol and is essential for proper lipid homeostasis 51–53. HSD17B12 overexpressing cells showed downregulation of cholesterol-related gene sets, including “sterol biosynthetic process” and “regulation of cholesterol biosynthesis by SREBF (SREBP)” (Supplementary Figure S9D–S9E). Finally, RNF5 encodes an ER and mitochondrial-bound E3 ubiquitin-protein ligase that ubiquitin-tags proteins for degradation 54–57. RNF5 overexpressing cells demonstrated alterations in gene sets involved in proteostasis and ER biology, including upregulation of “ERAD pathway”, “response to endoplasmic reticulum stress”, and “intra-Golgi and retrograde Golgi-to-ER traffic” (Supplementary Figure S9F–S9G). These results suggest that our approach leads to biological changes consistent with the known biological impact of the genes being overexpressed.
Next, we sought to determine whether modulation of candidate genes had any impact on TE expression in general, and L1 in particular. Although there were no significant changes for individual TE subfamilies following IL16 and STARD5 overexpression (Supplementary Table S5A–S5B), we identified subtle but widespread upregulation of various TE families across both conditions by GSEA (Figure 4F, Supplementary Table S5Q–S5R). Interestingly, 7 families, including L1, ERV1, ERVL-MaLR, Alu, ERVL, TcMar-Tigger, and hAT-Charlie families, were commonly upregulated under both conditions (Figure 4F). In contrast, cells overexpressing HSD17B12 or RNF5 did not drive widespread changes in L1 family expression, as assessed by GSEA (Supplementary Table S5S–S5T). Noteworthy, the L1 family gene set was more strongly upregulated following STARD5 overexpression (NES = 2.25, FDR = 6.14E-7) compared to IL16 overexpression (NES = 2.24, FDR = 2.40E-5) (Figure 4G, Supplementary Table S5Q–S5R). Since IL16 is upregulated in response to STARD5 overexpression, this suggests that STARD5 may synergize with IL16 for the regulation of L1 transcription.
Then, we decided to further characterize the impact of IL16 activity on TEs, since (i) its overexpression led to a global upregulation of TE transcription, and (ii) it was itself upregulated in response to STARD5 overexpression, which also led to increased TE expression. Thus, since IL16 is a soluble cytokine, we independently assessed its regulatory properties by exposing GM12878 cells to recombinant human IL16 peptide [rhIL16] for 24 hours (Figure 5A, Supplementary Figure S10A). Differential gene expression analysis (Supplementary Table S6A) and comparison with the IL16 overexpression results demonstrated that differentially expressed genes were weakly but significantly correlated (Supplementary Figure S10B). Additionally, we carried out GSEA using the GO Biological Process, Reactome pathway, and Hallmark pathway gene sets (Supplementary Table S6B–S6E) and compared those results with the GSEA from the IL16 overexpression (Supplementary Table S6F–S6H). Consistent with the known biology of IL16, GSEA highlighted a downregulation of many immune cell-related gene sets, including “leukocyte differentiation”, “mononuclear cell differentiation”, and “Interleukin-10 signaling” (Figure 5B–5C, Supplementary Table S6F–S6H). Like the overexpression results, exposure of GM12878 to rhIL16 for 24 hours led to an upregulation of an L1 family gene set by GSEA, although the effect was less pronounced than with the overexpression (Figure 5D). Even though treatment of GM12878 with rhIL16 for 48 hours exhibited known features of IL16 biology (Supplementary Figure S10B–S10D, Supplementary Table S6J–S6Q), the L1 upregulation was no longer detectable, though other TEs remained upregulated (Supplementary Figure S10E, Supplementary Table S6Q). These results further support the notion that IL16 acts as a modulator of L1 expression.
Figure 5. rhIL16 treatment is sufficient to transiently upregulate an L1 family gene set.
(A) Scheme for experimentally validating the role of rhIL16 in L1 regulation. GSEA analysis for top, shared, concomitantly regulated (B) GO Biological Process and (C) Reactome pathway gene sets following IL16 overexpression and rhIL16 exposure for 24 hours. Shared gene sets were ranked by combining p-values from each individual treatment analysis using Fisher’s method. (D) GSEA analysis for top, differentially regulated TE family gene sets following rhIL16 exposure for 24 hours. The GSEA plot for the L1 family gene set result summarized in the bubble plot is also shown. For this plot, the FDR value is listed. In each bubble plot, the size of the dot represents the −log10(FDR) and the color reflects the normalized enrichment score. FDR: False Discovery Rate. Some panels were created with BioRender.com.
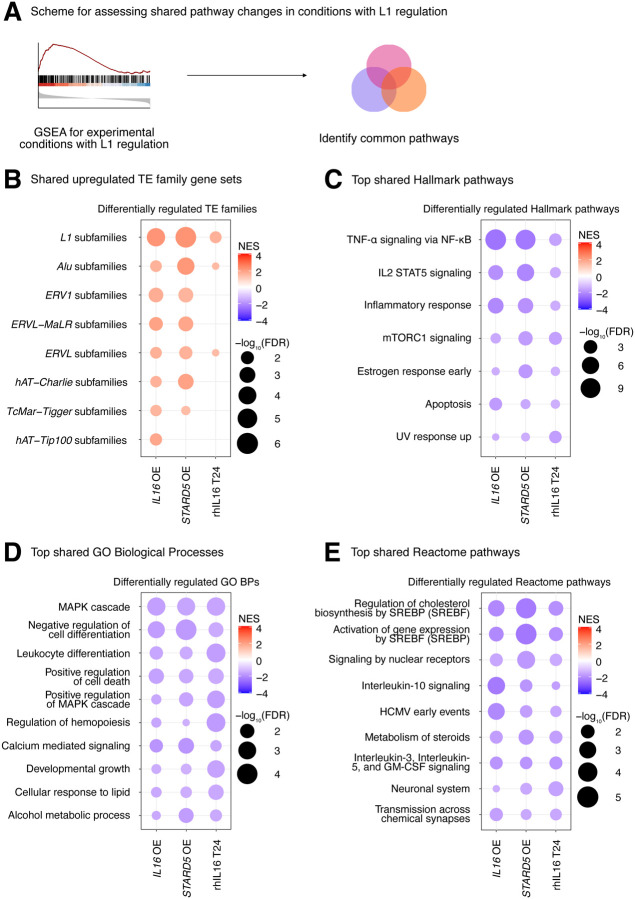
Finally, we sought to define the biological pathways regulated concomitantly with the L1 family gene set under all experimental conditions where it was upregulated (i.e., IL16 overexpression, STARD5 overexpression, and 24 hours of rhIL16 exposure) (Figure 6A, Figure 6B, Supplementary Table S7A). Again, we reasoned that such pathways would act either upstream (as regulatory pathways) or downstream (as response pathways) of TE alterations. GSEA with the Hallmark pathway gene sets identified 7 gene sets fitting this criterion, including “TNFα signaling via NF-KB”, “IL2 STAT5 signaling”, “inflammatory response”, “mTORC1 signaling”, “estrogen response early”, “apoptosis”, and “UV response up” (Figure 6C, Supplementary Table S7B). GSEA with the GO Biological Process gene sets (Figure 6D, Supplementary Table S7C) and the Reactome pathway gene sets (Figure 6E, Supplementary Table S7D) also identified MAPK signaling, virus-related pathways like “HCMV early events”, pathways involved in cell differentiation, and pathways involved in cholesterol and steroid metabolism like “signaling by nuclear receptors”. These results further cement the catalogue of pathways associated with differences in TE expression.
Figure 6. Consistent cellular responses to IL16 overexpression, STARD5 overexpression, and rhIL16 exposure for 24 hours.
IL16 overexpression, STARD5 overexpression, and rhIL16 exposure for 24 hours are associated with subtle but widespread differences in TE families and known TE-associated pathways. (A) Scheme for assessing concordantly regulated TE family and pathway gene sets across conditions where an L1 gene set is upregulated. GSEA analysis for top, shared, concomitantly regulated (B) TE family, (C) MSigDB Hallmark pathway, (D) GO Biological Process, and (E) Reactome pathway gene sets following IL16 overexpression, STARD5 overexpression, and rhIL16 exposure for 24 hours. Shared gene sets were ranked by combining p-values from each individual treatment analysis using Fisher’s method. In each bubble plot, the size of the dot represents the −log10(FDR) and the color reflects the normalized enrichment score. FDR: False Discovery Rate.
L1 trans-eQTLs are co-associated with aging traits in GWAS databases.
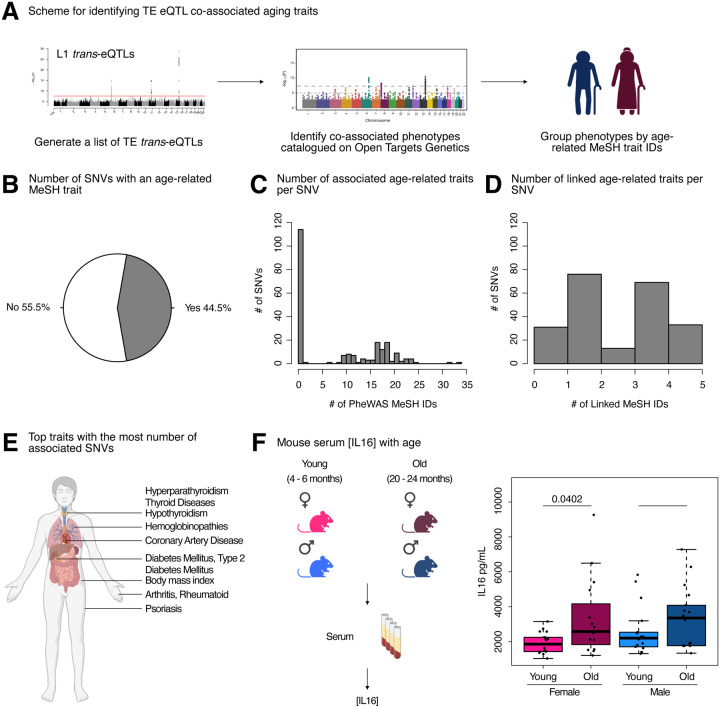
Although TE de-repression has been observed broadly with aging and age-related disease 5,58, whether this de-repression acts as a causal driver, or a downstream consequence, of aging phenotypes remains unknown. We reasoned that if increased TE expression at least partially drives aging phenotypes, L1 trans-eQTLs should be enriched for associations to aging traits in genome-wide association studies [GWAS] or phenome-wide association studies [PheWAS].
To test our hypothesis, we queried the Open Targets Genetics platform with our 499 trans-eQTL SNVs, mapped traits to standardized MeSH IDs, and then manually curated MeSH IDs related to aging-related traits (Figure 7A). Consistent with our hypothesis, a large proportion of L1 trans-eQTL SNVs (222/499 or 44.5%) were either (i) associated with an aging MeSH trait by PheWAS or (ii) LD-linked to a lead variant associated with an aging MeSH trait (Figure 7B). Moreover, among the 222 SNVs with significant aging-trait associations, we observed frequent mapping to more than a single age-related trait by PheWAS, with many SNVs associated with 10–25 traits (Figure 7C, Supplementary Table S8A). Additionally, many of the 222 SNVs mapped to 1–5 aging traits through a proxy lead variant (Figure 7D, Supplementary Table S8A). Among the most frequently associated or linked traits, we identified type 2 diabetes mellitus, hyperparathyroidism, thyroid diseases, coronary artery disease, hypothyroidism, and psoriasis, among many others (Figure 7E, Supplementary Table S8B).
Figure 7. L1 trans-eQTLs are co-associated with aging traits in GWAS databases.
(A) Scheme for obtaining trans-eQTL SNV-associated aging phenotypes from the Open Targets Genetics platform. (B) A pie chart representing the number of SNVs (222/499) associated with an aging-related MeSH trait, either by PheWAS or indirectly linked to the phenotype through a proxy lead SNP in LD with the SNV. (C) Histogram depicting the distribution of number of aging MeSH traits associated with the 222/499 SNVs by PheWAS. (D) Histogram depicting the distribution of number of aging MeSH traits linked with the 222/499 SNVs through a proxy lead SNP in LD with the SNVs. (E) A diagram highlighting the organ targets of the top 10 most frequently associated aging traits. (F) The concentrations of circulating IL16 in aging mice of both sexes was assessed by ELISA. Significance across age in each sex was assessed using a Wilcoxon test. The p-values from each sex (females in pink and males in blue) were combined by meta-analysis using Fisher’s method. Any p-value < 0.05 was considered significant. Some panels were created with BioRender.com.
As a parallel approach, we queried the Open Targets Genetics platform with our L1 trans-eQTL SNVs, as well as 500 combinations of random SNVs sampled from all SNVs used in the eQTL analyses. We then leveraged broader phenotype categories annotated by the platform, including 14 disease categories that we considered aging-related, to determine whether L1 eQTL associations were enriched for any disease categories (Supplementary Figure S11A). L1 eQTL associations were significantly enriched (FDR < 0.05 and ES > 1) for 13 out of 14 disease categories, including cell proliferation disorders, immune system diseases, and musculoskeletal diseases (Supplementary Figure S11B–N). The cardiovascular diseases category was the only disease category for which we did not observe a significant enrichment (Supplementary Figure S11O). The enrichment for cell proliferation disorders is consistent with the associations of L1 activity with cellular senescence 12,15 and cancer 59,60. The enrichment for immune system diseases is consistent with the role of L1 as a stimulator of the interferon pathway, inflammation, and senescence 15, as well as the more general notion that transposons can mimic viruses and stimulate immune responses from their hosts 61. The enrichment for musculoskeletal diseases is consistent with an increase in L1 expression and copy number with age in muscle tissue from aging mice 11. These results reinforce the notion that L1 activity is strongly and non-randomly associated with an assortment of age-related diseases.
Intriguingly, a large fraction of co-associated SNVs were on chromosome 6 near the HLA locus, which has previously been shown to be a hotspot of age-related disease traits 62. Despite its association to our strongest L1 trans-eQTL SNV, little is known about the regulation and impact of IL16 during aging. One study, however, found that IL16 expression increases with age in ovarian tissue, and the frequency of IL16 expressing cells is significantly higher in ovarian tissue from women at early and late menopause, compared to premenopausal women 63. Given these findings, and since L1 expression levels and copy number have been found to increase with age 5, we asked whether circulating IL16 levels may also change with age, using C57BL/6JNia mice as a model (Figure 7F, Supplementary Table S8C). Consistent with the notion that increased IL16 levels may, at least partially, drive age-related TE de-repression, we observed a significant increase in circulating IL16 levels in female mice with age, and a trending increase with age in male mice (although the levels showed more animal-to-animal variability). By meta-analysis, circulating IL16 levels changed significantly with age across sexes (Figure 7F). These results further support the hypothesis that IL16 is involved in L1 biology and may modulate L1 age-related changes. In sum, our results provide one of the first pieces of evidence of a causal link between L1 expression levels and age-related decline.
Discussion
A new approach to identify regulators of TE expression
In this work, we developed a pipeline to computationally identify candidate L1 transcriptional regulators by eQTL analysis. We provide experimental evidence for the involvement of top candidates in regulating L1 expression, demonstrating as a proof-of-principle that this approach can be broadly used on other large “omic”-characterized cohorts with human (i.e. GTEx 64,65 or HipSci 66) or mouse (i.e. DO mice 67) subjects to identify other regulators of L1 activity. These datasets, combined with our approach, could be utilized to rigorously characterize conserved or group-specific TE regulatory mechanisms on multiple layers, such as across TE families (like Alu or ERVs), across cell or tissue types, across ancestry groups, and across species. This approach, which leverages existing datasets to perform in silico screening, could be a powerful method to expand our knowledge of TE regulation in non-diseased cells and tissues.
Though our initial scan identified genetic variants associated with expression differences in specific L1 subfamilies, secondary analyses by GSEA suggest that genetic variants are associated with subtle but global differences in the expression of many TE families. Our pipeline identified candidate genes, including HSD17B12 and HLA genes, that likely play a conserved role in L1 regulation across human populations of different ancestries. Though some top candidates from the European cohort scan, such as IL16, STARD5, and RNF5, were not significant in the African cohort analysis, it is likely that some of these genes would appear in cross-ancestry scans with larger samples sizes. We detected subtle but global differences in L1 family expression following IL16 overexpression, STARD5 overexpression, and rhIL16 treatment for 24 hours, further suggesting that our top candidates have regulatory potential.
New candidate L1 regulators are involved in viral response
As another, theoretical line of evidence for the potential involvement of our top candidate genes in L1 regulation, we highlight known interactions between tested candidate genes and viral infections, which may be relevant under conditions where transposons are recognized as viral mimics 61. Indeed, IL16 has been extensively studied for its ability to inhibit human immunodeficiency virus (HIV) replication, partly by suppressing mRNA expression 68–70. Additionally, but in contrast to its HIV-suppressive properties, IL16 can enhance the replication of influenza A virus (IAV) and facilitate its infection of hosts, potentially through its repression of type I interferon beta and interferon-stimulated genes 71. IL16 can also contribute to the establishment of lifelong gamma herpesvirus infection 72. STARD5 is another candidate implicated in the influenza virus replication cycle 73. HSD17B12 promotes the replication of hepatitis C virus via the very-long-chain fatty acid (VLCFA) synthesis pathway and the production of lipid droplets important for virus assembly 74,75. Additionally, HSD17B12 has been found interacting with the coronavirus disease 2019 (COVID-19) protein nonstructural protein 13 (NSP13), which is thought to antagonize interferon signaling 76. Finally, RNF5 has been implicated in both promoting and antagonizing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by either stabilizing the interactions of membrane protein (M) 77 or inducing degradation of structural protein envelope (E) 78, respectively. Fundamentally, RNF5 regulates virus-triggered interferon signaling by targeting the stimulator of interferon genes (STING) or mitochondrial antiviral signaling protein (MAVS) for ubiquitin-mediated protein degradation 56,57. These studies reinforce the roles of tested candidate regulators in virus-associated processes, including interferon-mediated signaling.
Future considerations for the use of trans-eQTL analysis in identification of L1 regulators
While we believe our approach can readily be applied to other datasets, we would like to note potential further considerations with the approach we implemented, some of which were simply beyond the scope of this paper. Firstly, though it is common to use probabilistic estimation of expression residuals (PEER) 79 to enhance detection of cis-eQTLs, PEER was not implemented in our analysis as a precautionary measure, in order to avoid potentially blurring global TE signals, which likely led to a more conservative list of candidate cis gene mediators. Second, given the technical complexity in generating the vast amount of mRNA-seq data used for the eQTL analysis, it is possible that technical covariates introduced non-linear effects that would not be easily removed by approaches like PEER or SVA 80. For that reason, we opted to supplement our computational predictions with experimental data. Third, the L1 trans-eQTLs identified were specific to older L1 subfamilies (L1P and L1M) and were not shared across subfamilies. One factor that may partially explain this is the heightened difficulty of quantifying the expression of evolutionarily younger L1 subfamilies using short-read sequencing 81.
More generally, significant single gene differences are often difficult to reproduce across studies, and it is for this reason that methods like GSEA were developed, to robustly identify broader changes in sets of genes 29. Consistently, GSEA suggests that many TE families, beyond the single L1 subfamilies identified in the eQTL analysis, are differentially regulated among samples with different genotypes for trans-eQTL SNVs and among samples where IL16/IL16 and STARD5 were manipulated. We note that although HLA and HSD17B12 loci were significant in both the European and African cohorts, we were not able to independently identify all of the same candidate regulators. This is likely due to a combination of small sample size for the African cohort and the existence of population-specific L1 regulation. Future studies with larger sample sizes may be useful for expanding the catalogue of loci that are biologically meaningful for L1 expression across more than one population. Importantly, our computational scan is limited to loci exhibiting genomic variation among tested individuals. This will vary with factors like the ancestry groups of the populations being studied. Moreover, variants that confer extreme fitness defects may not exist at a sufficiently high level in a population to allow for an assessment of their involvement as eQTLs.
Finally, although we focused on protein-coding candidate regulators, it is possible that the non-coding genes identified in our scan may also causally drive differences in L1 expression. Though not explored here, other regulatory factors like small RNAs may also act as partial mediators. Since the GEUVADIS Consortium generated small RNA data in parallel to the mRNA data used in this study 26, a future application of our pipeline could be to scan for cis small RNA mediators in the same biological samples. These unexplored factors may explain the associations between orphan SNV genotypes and TE family gene set changes.
L1 trans-eQTLs are enriched for genetic variants linked to aging and age-related disease
Consistent with the notion that L1 is associated with aging and aging phenotypes 5,58, we observed that L1 trans-eQTL SNVs were associated with aging phenotypes in GWAS/PheWAS databases. This is very surprising, but interesting, given that all 1000Genomes Project participants declared themselves to be healthy at the time of sample collection. Assuming this to be true, our results suggest that L1 expression differences exist in natural, healthy human populations, and these expression differences precede onset of aging diseases. Though it is often unclear whether L1 mis-regulation is a consequence or driver of aging phenotypes, our results suggest that L1 levels may drive aging phenotypes. As we continue to expand the catalogue of L1 regulators, especially in healthy cells and tissues, the L1 regulatory processes that are disrupted over the course of aging will become increasingly clear. To that end, this work may serve as a guide for conducting more comprehensive scans for candidate TE regulators.
In summary, we developed an eQTL-based pipeline that leverages genomic and transcriptomic data to scan the human genome for novel candidate regulators of L1 subfamily expression. Though the initial scan identified genetic variants associated with expression differences in specific L1 subfamilies, secondary analyses by GSEA suggest that genetic variants are associated with subtle but global differences in the expression of many TE families. Our pipeline identified candidate genes, including HSD17B12 and HLA genes, that likely play a conserved role in L1 regulation across human populations of different ancestries. Though some top candidates from the European cohort scan, such as IL16, STARD5, and RNF5, were not significant in the African cohort analysis, it is likely that some of these genes would appear in cross-ancestry scans with larger samples sizes. We detected subtle but global differences in L1 family expression following IL16 overexpression, STARD5 overexpression, and rhIL16 treatment for 24 hours, further suggesting that some candidate genes have regulatory potential. We generate a list of pathways, such as mTORC1 signaling and cholesterol metabolism, that may act upstream of L1 expression. Finally, the co-association of some genetic variants with both L1 expression differences and various age-related diseases suggests that L1 differences may precede and contribute to the onset of disease. Our results expand the potential mechanisms by which L1 expression is regulated and by which L1 may influence aging-related phenotypes.
Material and Methods
Publicly available data acquisition
The eQTL analysis was carried out on 358 European (EUR) individuals and 86 Yoruban (YRI) individuals for which paired single nucleotide variant, structural variant, and transcriptomic data were available from Phase 3 of the 1000 Genomes Project 22,23 and from the GEUVADIS consortium 26. Specifically, Phase 3 autosomal SNVs called on the GRCh38 reference genome were obtained from The International Genome Sample Resource (IGSR) FTP site (http://ftp.1000genomes.ebi.ac.uk/vol1/ftp/data_collections/1000_genomes_project/release/20190312_biallelic_SNV_and_INDEL/). Structural variants were also obtained from the IGSR FTP site (http://ftp.1000genomes.ebi.ac.uk/vol1/ftp/phase3/integrated_sv_map/). mRNA-sequencing fastq files generated by the GEUVADIS consortium were obtained from ArrayExpress under accession E-GEUV-1.
Aggregating and pre-processing genotype data for eQTL analyses
To prepare SNVs for association analyses, all SNVs were first annotated with rsIDs from dbSNP build 155 using BCFtools v1.10.2 82. VCFtools v0.1.17 83 was then used to remove indels and keep variants with the following properties in each of the two populations: possessed a minimum and maximum of two alleles, possessed a minor allele frequency (MAF) of at least 1%, passed Hardy-Weinberg equilibrium thresholding at p < 1e-6, with no missing samples, and located on an autosome. We note here that sex chromosomes were not included in the analysis since (i) Y chromosome SNVs were not available and (ii) analyses with X chromosome SNVs require unique algorithms and cannot simply be incorporated into traditional association pipelines 84,85. VCF files containing these filtered SNVs were then converted to PLINK BED format using PLINK v1.90b6.17 86, keeping the allele order. PLINK BED files were subsequently used to generate preliminary 0/1/2 genotype matrices using the ‘--recodeA’ flag in PLINK. These preliminary matrices were manipulated in terminal, using the gcut v9.0 function to remove unnecessary columns and datamash v1.7 to transpose the data, to generate the final 0/1/2 matrices used for the eQTL analyses. Finally, PLINK was used to prune the list of filtered SNVs, using the “--indep-pairwise 50 10 0.1” flag, and to generate principal components (PCs) from the pruned genotypes.
To control for inter-individual differences in genomic transposon copy number load, we applied 1 of 2 approaches, depending on the analysis. For approach 1, the net number of L1 and Alu insertions was quantified across the 444 samples. We chose to aggregate the L1 and Alu copy numbers, since Alu relies on L1 machinery for mobilization 87, and so the aggregate number may provide a finer view of L1-associated copy number load. Briefly, VCFTools was used to extract autosomal structural variants from the 1000Genomes structural variant calls. L1 and Alu insertions and deletions were then extracted with BCFtools by keeping entries with the following expressions: ‘SVTYPE=”LINE1”’, ‘SVTYPE=”ALU”’, ‘SVTYPE=”DEL_LINE1”’, and ‘SVTYPE=”DEL_ALU”’. The resulting VCF files were then transformed to 0/1/2 matrices in the same manner as the SNVs. A net copy number score was obtained for each sample by adding the values for the L1 and Alu insertions and subtracting the values for the L1 and Alu deletions. For approach 2, the complete structural variant matrix was filtered with VCFtools using the same parameters as with the SNV matrices. The filtered structural variant matrix was then pruned with PLINK, and these pruned structural variant genotypes were used to generate principal components, in the same fashion as with the SNV matrix. The net copy number score or the structural variant principal components, depending on the analysis, were included as covariates.
mRNA-seq read trimming, mapping, and quantification
Fastq files were first trimmed using fastp v0.20.1 88 with the following parameters: detect_adapter_for_pe, disable_quality_filtering, trim_front1 17, trim_front2 17, cut_front, cut_front_window_size 1, cut_front_mean_quality 20, cut_tail, cut_tail_window_size 1, cut_tail_mean_quality 20, cut_right, cut_right_window_size 5, cut_right_mean_quality 20, and length_required 36. Read quality was then inspected using fastqc v0.11.9.
Next, the GRCh38 primary human genome assembly and comprehensive gene annotation were obtained from GENCODE release 33 89. Since LCLs are generated by infecting B-cells with Epstein-Barr virus, the EBV genome (GenBank ID V01555.2) was included as an additional contig in the human reference genome. The trimmed reads were aligned to this modified reference genome using STAR v2.7.3a 90 with the following parameters: outFilterMultimapNmax 100, winAnchorMultimapNmax 100, and outFilterMismatchNoverLmax 0.04. Finally, the TEcount function in the TEtranscripts v2.1.4 27 package was employed to obtain gene and TE counts, using the GENCODE annotations to define gene boundaries and a repeat GTF file provided on the Hammell lab website (downloaded on February 19 2020 from https://labshare.cshl.edu/shares/mhammelllab/www-data/TEtranscripts/TE_GTF/GRCh38_GENCODE_rmsk_TE.gtf.gz) to define repeat boundaries.
Gene cis-eQTL and L1 trans-eQTL analyses
Gene and TE count files were loaded into R v4.2.1. Lowly expressed genes were first filtered out if 323/358 European samples and 78/86 Yoruban samples did not have over 0.44 counts per million (cpm) or 0.43 cpm, respectively. These fractions were selected because they corresponded to expression in ~90% of samples and thus helped maintain maximal statistical power by focusing on genes ubiquitously expressed across each entire population. The cpm thresholds were selected because they corresponded to 10 reads in the median-length library within each set of samples.
Then, counts underwent a variance stabilizing transformation (vst) using DESeq2 v1.36.0 91. The following covariates were regressed out from vst normalized expression data using the ‘removeBatchEffect’ function in Limma v3.52.2 92: lab, population category, principal components 1–2 of the pruned SNVs, biological sex, net L1/Alu copy number, and EBV expression levels. Since the Yoruban samples were all from the same population, the population variable was omitted in their batch correction. Here, we note several things. First, EBV expression was included as a covariate because heightened TE expression is often a feature of viral infections 93. Secondly, although PEER 79 is often used to remove technical variation for cis-eQTL analysis, this can come at the expense of correcting out genome-wide biological effects. This can be problematic in some settings, such as trans-eQTL analysis. Thus, PEER factors were not included. The batch-corrected data underwent a final inverse normal transformation (INT), using the RankNorm function in the R package RNOmni v1.0.1, to obtain normally distributed gene expression values.
The INT expression matrices were split into genes and L1 subfamilies, which were used to identify gene cis-eQTLs and L1 subfamily trans-eQTLs in the European superpopulation using MatrixEQTL v2.3 94. For gene cis-eQTLs, SNVs were tested for association with expressed genes within 1 million base pairs. We opted to use a trans-eQTL approach using aggregate subfamily-level TE expression since the trans approach should allow us to identify regulators of many elements rather than one. The Benjamini-Hochberg false discovery rate (FDR) was calculated in each analysis, and we used the p-value corresponding to an FDR of < 5% as the threshold for eQTL significance. In addition, the cis-eQTL and trans-eQTL analyses were also repeated using 20 permuted expression datasets in which the sample names were scrambled, and the p-value corresponding to an average empirical FDR of < 5% was used as a secondary threshold. To note, we calculated the average empirical FDR at a given p-value pi by (i) counting the total number of null points with p ≤ pi, (ii) dividing by the number of permutations, to obtain an average number of null points with p ≤ pi, and (iii) dividing the average number of null points with p ≤ pi by the number of real points with p ≤ pi. eQTLs were called as significant if they passed the stricter of the two thresholds. SNV-gene and SNV-L1 associations that were significant in the European superpopulation were then targeted and tested in the Yoruban population using R’s built-in linear modelling functions. In this case, only the Benjamini-Hochberg FDR was calculated, and significant eQTLs were called if they possessed an FDR < 5%.
Defining SNV-gene-L1 trios and mediation analysis
For each population, the cis- and trans-eQTL results were integrated to identify SNVs associated with both gene and L1 subfamily expression. We reasoned that L1 expression would respond to differences in expression of bona fide regulators. Consequently, gene expression and L1 subfamily expression associations were assessed by linear regression, and the p-values from this analysis were Benjamini-Hochberg FDR-corrected. Candidate SNV-gene-L1 trios were defined as those with cis-eQTL, trans-eQTL, and expression regression FDRs < 5%. To identify top, index SNVs in regions of linkage disequilibrium (LD), SNVs within 500 kilobases of each other with an R2> 0.10 were clumped together by trans-eQTL p-value using PLINK v1.90b6.17. Mediation analysis was carried out using the ‘gmap.gpd’ function in eQTLMAPT v0.1.0 95 on all candidate SNV-gene-L1 trios. Empirical p-values were calculated using 30,000 permutations, and Benjamini-Hochberg FDR values were calculated from empirical p-values. Mediation effects were considered significant for trios with FDR < 5%.
Differential expression analysis across trans-eQTL SNV genotypes
Transcriptomic changes associated with alternating the allele of each SNV of interest were evaluated using DESeq2 v1.36.0. Using the same filtered counts prepared for the eQTL analysis, a linear model was constructed with the following covariates for each SNV: SNV genotype in 0/1/2 format, biological sex, lab, population category, principal components 1–2 of the pruned SNVs, and principal components 1–3 of the pruned SVs (to account for structural variant population structure). As before, the population label was omitted from the Yoruban population analysis. Significant genes and TEs were those with an FDR < 5%.
Functional enrichment analyses
We used the Gene Set Enrichment Analysis (GSEA) paradigm as implemented in the R package clusterProfiler v4.4.4 96. Gene Ontology, Reactome, and Hallmark pathway gene sets were obtained from the R package msigdbr v7.5.1, an Ensembl ID-mapped collection of gene sets from the Molecular Signature Database 29,30. Additionally, TE subfamilies were aggregated into TE family gene sets using the TE family designations specified in the TE GTF file (downloaded on February 19 2020 from https://labshare.cshl.edu/shares/mhammelllab/www-data/TEtranscripts/TE_GTF/GRCh38_GENCODE_rmsk_TE.gtf.gz) used during the RNA-seq quantification step. The DESeq2 v1.36.0 Wald-statistic was used to generate a combined ranked list of genes and TEs for functional enrichment analysis. All gene sets with an FDR < 5% were considered significant. For plots with a single analysis, the top 5 downregulated and top 5 upregulated gene sets were plotted, at most. For plots with multiple analyses, shared gene sets with the desired expression patterns in each individual analysis were first identified. Then, the p-values for shared gene sets were combined using Fisher’s method, and this meta-analysis p-value was used to rank shared gene sets. Finally, the top 5 gene sets with one expression pattern and the top 5 gene sets with the opposite expression pattern were plotted. If there were less than 5 gene sets in either group, those were replaced with gene sets exhibiting the opposite regulation, in order to plot 10 shared gene sets whenever possible.
Cell lines and cell culture conditions.
GM12878 (RRID: CVCL_7526) lymphoblastoid cells were purchased from the Coriell Institute. We opted to use GM12878 as a well-characterized representative cell line for candidate validation, given that (i) it is of the same cell type as the transcriptomic data used here for our eQTL analysis, and (ii) its epigenomic landscape and culture conditions are well-characterized as part of the ENCODE project 39,40.
GM12878 cells were maintained in RPMI (Corning cat. 15–040-CV) containing 15% FBS and 1X Penicillin-Streptomycin-Glutamine (Corning cat. 30–009-CI). Cells were cultured in a humidified incubator at 37°C and 5% CO2, subculturing cells 1:5 once cells reached a density of ~106 mL−1. All cells used were maintained below passage 30 and routinely tested for mycoplasma contamination using the PlasmoTest Mycoplasma Detection Kit (InvivoGen).
Plasmids
The empty pcDNA3.1(+) backbone (Invitrogen cat. V79020) was a kind gift from the lab of Dr. Changhan David Lee at the University of Southern California Leonard Davis School of Gerontology. Overexpression vectors for IL16 (CloneID OHu48263C), STARD5-FLAG (CloneID OHu07617D), HSD17B12-FLAG (CloneID OHu29918D), and RNF5-FLAG (CloneID OHu14875D) on a pcDNA3.1 backbone were purchased from GenScript. Plasmid sequences were verified for accuracy using Plasmidsaurus’s whole plasmid sequencing service.
Transfections
Escherichia coli were cultured in LB Broth (ThermoFischer Scientific) supplemented with 50 μg/mL carbenicillin to an optical density 600 (OD600) of 2 – 4. Plasmid extractions were carried out using the Nucleobond Xtra Midi Plus EF kit (Macherey-Nagel) following manufacturer recommendations. Plasmids were aliquoted and stored at −20°C until the time of transfection. On the day of transfection, GM12878 cells were collected in conical tubes, spun down (100xG, 5 minutes, room temperature), resuspended in fresh media, and counted by trypan blue staining using a Countess II FL automated cell counter (Thermo Fisher). The number of cells necessary for the experiment were then aliquoted, spun down, and washed with Dulbecco’s phosphate-buffered saline (DPBS)(Corning, cat. #21–031-CV).
GM12878 cells were transfected by electroporation using the Neon Transfection System (Invitrogen) with the following parameters: 1200 V, 20 ms, and 3 pulses for GM12878 cells in Buffer R. Per reaction, we maintained a plasmid mass:cell number ratio of 10 μg : 2*106 cells. For mRNA-sequencing, 8*106 GM12878 cells were independently transfected for each biological replicate, with 4 replicates per overexpression condition, and cultured in a T25 flask. Immediately after transfection, cells were cultured in Penicillin-Streptomycin-free media for ~24 hours.
Afterwards, to promote selection of viable and healthy transfected GM12878 cells, we enriched for viable cells using the EasySep Dead Cell Removal (Annexin V) Kit (STEMCELL Technologies) before seeding 2*106 live cells in the same media used for cell maintenance. After another 24 hours, cell viability was measured by trypan blue staining on a Countess automated cell counter and cells were spun down (100xG, 5 min, room temperature) and lysed in TRIzol Reagent (Invitrogen) for downstream total RNA isolation (see below).
Recombinant human IL16 (rhIL16) peptide treatment
Human rIL16 was obtained from PeproTech (cat. #200–16) and resuspended in 0.1% bovine serum albumin (BSA) solution (Akron, cat. #AK8917–0100). GM12878 cells were seeded at a concentration of 500,000 live cells per mL of media on 6-well suspension plates with 3 independent replicates per condition. Cells were exposed to 0, 24, or 48 hours of 100 ng mL−1 of rhIL16. To replace or exchange media 24 hours after seeding, cells were transferred to conical tubes, spun down (100xG, 5 min, room temperature), resuspended in 5 mL of the appropriate media, and transferred back to 6-well suspension plates. After 48 hours, cell viability was measured by trypan blue staining and cells were spun down (100xG, 5 min, room temperature) and lysed in TRIzol Reagent (Invitrogen).
RNA extractions and mRNA sequencing
RNA was extracted using the Direct-zol RNA Miniprep kit (Zymo Research) following manufacturer recommendations. The integrity of RNA samples was evaluated using an Agilent High Sensitivity RNA ScreenTape assay (Agilent Technologies), ensuring that all samples had a minimum eRIN score of 8 before downstream processing. We then submitted total RNA samples to Novogene (Sacramento, California) for mRNA library preparation and sequencing on the NovaSeq 6000 platform as paired-end 150 bp reads.
Analysis of overexpression and rhIL16 exposure mRNA-seq
mRNA-seq reads were trimmed, mapped, and quantified like for the eQTL analysis, except for the overexpression sample data. For this data, one modification was made: the EBV-inclusive reference genome was further modified to include the pcDNA3.1 sequence as an additional contig. Lowly expressed genes were filtered using a cpm threshold as in the eQTL processing, but that cpm threshold had to be satisfied by as many samples as the size of the smallest biological group. For the overexpression data, surrogate variables were estimated with the ‘svaseq’ function 80 in the R package ‘sva’ v3.44.9, and they were regressed out from the raw read counts using the ‘removeBatchEffect’ function in the R package Limma v3.52.2. DESeq2 was used to identify significantly (FDR < 5%) differentially expressed genes and TEs between groups. Functional enrichment analysis was carried out as previously described.
PheWAS analysis
To gather the known associated traits for the 499 TE-related SNVs, we used Open Targets Genetics (https://genetics.opentargets.org/), a database of GWAS summary statistics 97. First, we queried the database using the 499 TE-related SNVs and collected traits that were directly associated (with P < 5×10−8) with the SNVs, as well as traits associated with lead variants that were in linkage disequilibrium (LD) with the queried SNPs (with R2 > 0.6). For age-related traits (ARTs), we used the comprehensive list of 365 Medical Subject Headings (MeSH) terms reported by 98 (downloaded from https://github.com/kisudsoe/Age-related-traits). To identify known age-related traits, the known associated traits were translated into the equivalent MeSH terms using the method described by 98. Then, the MeSH-translated known associated traits for the 499 TE-related SNVs were filtered by the MeSH terms for age-related traits.
As a parallel approach, we mapped the RsIDs for all SNVs used during the eQTL analyses to their corresponding bi-allelic Open Targets variant IDs, when available. The variant IDs corresponding to L1 trans-eQTL SNVs were extracted, and 500 different equal-length combinations of random SNVs were generated. Next, we queried the Open Targets database using the lists of L1-associated and random SNVs and collected the associated traits (with P < 5×10−8). Importantly, the database assigns traits to broader categories, including 14 disease categories that we considered age-related. We counted the number of L1-associated or random SNVs mapping to each category, and we used the random SNV counts to generate an empirical cumulative distribution function (ecdf) for each category. We calculated enrichment p-values using the formula p = 1− ecdf(mapped eQTLs) and then Benjamini-Hochberg FDR-corrected all p-values. An enrichment score (ES) was also calculated for each category using the formula ES = number of mapped L1 eQTLs / median number of randomly mapping SNVs. Categories with an ES > 1 and FDR < 0.05 were considered significantly enriched.
Mouse husbandry
All animals were treated and housed in accordance with the Guide for Care and Use of Laboratory Animals. All experimental procedures were approved by the University of Southern California’s Institutional Animal Care and Use Committee (IACUC) and are in accordance with institutional and national guidelines. Samples were derived from animals on approved IACUC protocol #20770.
Quantification of mouse serum IL16 by ELISA
Serum was collected from male and female C57BL/6JNia mice (4–6 and 20–24 months old) obtained from the National Institute on Aging (NIA) colony at Charles Rivers. All animals were euthanized between 8–11 am in a “snaking order” across all groups to minimize batch-processing confounds due to circadian processes. All animals were euthanized by CO2 asphyxiation followed by cervical dislocation. Circulating IL16 levels were quantitatively evaluated from mouse serum by enzyme-linked immunosorbent assay (ELISA). Serum was diluted 1/10 before quantifying IL16 concentrations using Abcam’s Mouse IL-16 ELISA Kit (ab201282) in accordance with manufacturer instructions. Technical replicates from the same sample were averaged to one value before statistical analysis and plotting. P-values across age within each sex were calculated using a non-parametric 2-sided Wilcoxon test, and p-values from each sex-specific analysis were combined using Fisher’s method.
Supplementary Material
Acknowledgements
Some panels were created with BioRender.com. We would like to thank Prof. Rachel Brem for her feedback and insights on the eQTL analyses. We would also like to thank Dr. Minhoo Kim for her feedback on the manuscript.
This work was supported by NSF Graduate Research Fellowship Program (NSF GRFP) DGE-1842487 (J.I.B.), NIA T32 AG052374 (J.I.B.), the University of Southern California with a Provost Fellowship (J.I.B.), NIA R25 AG076400 (C.R.M.), and NIGMS R35 GM142395 (to B.A.B).
Footnotes
Competing interest statement
The authors have no conflict of interest.
Data availability
New sequencing data generated in this study is accessible through the Sequence Read Archive (SRA) under BioProject PRJNA937306. All code is available on the Benayoun lab GitHub (https://github.com/BenayounLaboratory/TE-eQTL_LCLs). Analyses were conducted using R version 4.2.1. Code was re-run independently on R version 4.3.0 to check for reproducibility.
References
- 1.Lander E.S., Linton L.M., Birren B., Nusbaum C., Zody M.C., Baldwin J., Devon K., Dewar K., Doyle M., FitzHugh W., et al. (2001). Initial sequencing and analysis of the human genome. Nature 409, 860–921. 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 2.Venter J.C., Adams M.D., Myers E.W., Li P.W., Mural R.J., Sutton G.G., Smith H.O., Yandell M., Evans C.A., Holt R.A., et al. (2001). The Sequence of the Human Genome. Science 291, 1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 3.Brouha B., Schustak J., Badge R.M., Lutz-Prigge S., Farley A.H., Moran J.V., and Kazazian H.H. (2003). Hot L1s account for the bulk of retrotransposition in the human population. Proceedings of the National Academy of Sciences 100, 5280–5285. doi: 10.1073/pnas.0831042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moran J.V., Holmes S.E., Naas T.P., DeBerardinis R.J., Boeke J.D., and Kazazian H.H. Jr. (1996). High Frequency Retrotransposition in Cultured Mammalian Cells. Cell 87, 917–927. 10.1016/S0092-8674(00)81998-4. [DOI] [PubMed] [Google Scholar]
- 5.Bravo J.I., Nozownik S., Danthi P.S., and Benayoun B.A. (2020). Transposable elements, circular RNAs and mitochondrial transcription in age-related genomic regulation. Development 147. 10.1242/dev.175786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Della Valle F., Reddy P., Yamamoto M., Liu P., Saera-Vila A., Bensaddek D., Zhang H., Prieto Martinez J., Abassi L., Celii M., et al. (2022). LINE-1 RNA causes heterochromatin erosion and is a target for amelioration of senescent phenotypes in progeroid syndromes. Science Translational Medicine 14, eabl6057. doi: 10.1126/scitranslmed.abl6057. [DOI] [PubMed] [Google Scholar]
- 7.Simon M., Van Meter M., Ablaeva J., Ke Z., Gonzalez R.S., Taguchi T., De Cecco M., Leonova K.I., Kogan V., Helfand S.L., et al. (2019). LINE1 Derepression in Aged Wild-Type and SIRT6-Deficient Mice Drives Inflammation. Cell Metabolism 29, 871–885.e875. 10.1016/j.cmet.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y., Oreskovic E., Zhang Q., Lu Q., Gilman A., Lin Y.S., He J., Zheng Z., Lu J.Y., Lee J., et al. (2021). Transposon-triggered innate immune response confers cancer resistance to the blind mole rat. Nature Immunology 22, 1219–1230. 10.1038/s41590-021-01027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao H., Ji Q., Wu Z., Wang S., Ren J., Yan K., Wang Z., Hu J., Chu Q., Hu H., et al. (2022). Destabilizing heterochromatin by APOE mediates senescence. Nature Aging 2, 303–316. 10.1038/s43587-022-00186-z. [DOI] [PubMed] [Google Scholar]
- 10.Liu E.Y., Russ J., Cali C.P., Phan J.M., Amlie-Wolf A., and Lee E.B. (2019). Loss of Nuclear TDP-43 Is Associated with Decondensation of LINE Retrotransposons. Cell Reports 27, 1409–1421.e1406. 10.1016/j.celrep.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Cecco M., Criscione S.W., Peterson A.L., Neretti N., Sedivy J.M., and Kreiling J.A. (2013). Transposable elements become active and mobile in the genomes of aging mammalian somatic tissues. Aging (Albany NY) 5, 867–883. 10.18632/aging.100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Cecco M., Criscione S.W., Peckham E.J., Hillenmeyer S., Hamm E.A., Manivannan J., Peterson A.L., Kreiling J.A., Neretti N., and Sedivy J.M. (2013). Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell 12, 247–256. 10.1111/acel.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campisi J. (2013). Aging, Cellular Senescence, and Cancer. Annual Review of Physiology 75, 685–705. 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franceschi C., Garagnani P., Parini P., Giuliani C., and Santoro A. (2018). Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology 14, 576–590. 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 15.De Cecco M., Ito T., Petrashen A.P., Elias A.E., Skvir N.J., Criscione S.W., Caligiana A., Brocculi G., Adney E.M., Boeke J.D., et al. (2019). L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566, 73–78. 10.1038/s41586-018-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Martin B., Alvarez E.G., Baez-Ortega A., Zamora J., Supek F., Demeulemeester J., Santamarina M., Ju Y.S., Temes J., Garcia-Souto D., et al. (2020). Pan-cancer analysis of whole genomes identifies driver rearrangements promoted by LINE-1 retrotransposition. Nature Genetics 52, 306–319. 10.1038/s41588-019-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flasch D.A., Chen X., Ju B., Li X., Dalton J., Mulder H.L., Easton J., Wang L., Baker S.J., Chiang J., and Zhang J. (2022). Somatic LINE-1 promoter acquisition drives oncogenic FOXR2 activation in pediatric brain tumor. Acta Neuropathologica 143, 605–607. 10.1007/s00401-022-02420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin H.L., and Moran J.V. (2011). Dynamic interactions between transposable elements and their hosts. Nature Reviews Genetics 12, 615–627. 10.1038/nrg3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rebollo R., Romanish M.T., and Mager D.L. (2012). Transposable Elements: An Abundant and Natural Source of Regulatory Sequences for Host Genes. Annual Review of Genetics 46, 21–42. 10.1146/annurev-genet-110711-155621. [DOI] [PubMed] [Google Scholar]
- 20.Liu N., Lee C.H., Swigut T., Grow E., Gu B., Bassik M.C., and Wysocka J. (2018). Selective silencing of euchromatic L1s revealed by genome-wide screens for L1 regulators. Nature 553, 228–232. 10.1038/nature25179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung N., Jonaid G.M., Quinton S., Ross A., Sexton C.E., Alberto A., Clymer C., Churchill D., Navarro Leija O., and Han M.V. (2019). Transcriptome analyses of tumor-adjacent somatic tissues reveal genes co-expressed with transposable elements. Mobile DNA 10, 39. 10.1186/s13100-019-0180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auton A., Abecasis G.R., Altshuler D.M., Durbin R.M., Abecasis G.R., Bentley D.R., Chakravarti A., Clark A.G., Donnelly P., Eichler E.E., et al. (2015). A global reference for human genetic variation. Nature 526, 68–74. 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sudmant P.H., Rausch T., Gardner E.J., Handsaker R.E., Abyzov A., Huddleston J., Zhang Y., Ye K., Jun G., Hsi-Yang Fritz M., et al. (2015). An integrated map of structural variation in 2,504 human genomes. Nature 526, 75–81. 10.1038/nature15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sie L., Loong S., and Tan E.K. (2009). Utility of lymphoblastoid cell lines. Journal of Neuroscience Research 87, 1953–1959. 10.1002/jnr.22000. [DOI] [PubMed] [Google Scholar]
- 25.Hussain T., and Mulherkar R. (2012). Lymphoblastoid Cell lines: a Continuous in Vitro Source of Cells to Study Carcinogen Sensitivity and DNA Repair. Int J Mol Cell Med 1, 75–87. [PMC free article] [PubMed] [Google Scholar]
- 26.Lappalainen T., Sammeth M., Friedländer M.R., ‘t Hoen P.A.C., Monlong J., Rivas M.A., Gonzàlez-Porta M., Kurbatova N., Griebel T., Ferreira P.G., et al. (2013). Transcriptome and genome sequencing uncovers functional variation in humans. Nature 501, 506–511. 10.1038/nature12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Y., Tam O.H., Paniagua E., and Hammell M. (2015). TEtranscripts: a package for including transposable elements in differential expression analysis of RNA-seq datasets. Bioinformatics 31, 3593–3599. 10.1093/bioinformatics/btv422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams T.M. (2001). Human Leukocyte Antigen Gene Polymorphism and the Histocompatibility Laboratory. The Journal of Molecular Diagnostics 3, 98–104. 10.1016/S1525-1578(10)60658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., and Mesirov J.P. (2005). Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences 102, 15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liberzon A., Birger C., Thorvaldsdóttir H., Ghandi M., Mesirov J.P., and Tamayo P. (2015). The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 1, 417–425. 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marasca F., Sinha S., Vadalà R., Polimeni B., Ranzani V., Paraboschi E.M., Burattin F.V., Ghilotti M., Crosti M., Negri M.L., et al. (2022). LINE1 are spliced in non-canonical transcript variants to regulate T cell quiescence and exhaustion. Nature Genetics 54, 180–193. 10.1038/s41588-021-00989-7. [DOI] [PubMed] [Google Scholar]
- 32.Wahl D., Cavalier A.N., Smith M., Seals D.R., and LaRocca T.J. (2020). Healthy Aging Interventions Reduce Repetitive Element Transcripts. The Journals of Gerontology: Series A 76, 805–810. 10.1093/gerona/glaa302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampathkumar N.K., Bravo J.I., Chen Y., Danthi P.S., Donahue E.K., Lai R.W., Lu R., Randall L.T., Vinson N., and Benayoun B.A. (2020). Widespread sex dimorphism in aging and age-related diseases. Hum Genet 139, 333–356. 10.1007/s00439-019-02082-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baeken M.W., Moosmann B., and Hajieva P. (2020). Retrotransposon activation by distressed mitochondria in neurons. Biochemical and Biophysical Research Communications 525, 570–575. 10.1016/j.bbrc.2020.02.106. [DOI] [PubMed] [Google Scholar]
- 35.Valdebenito-Maturana B., Valdebenito-Maturana F., Carrasco M., Tapia J.C., and Maureira A. (2023). Activation of Transposable Elements in Human Skeletal Muscle Fibers upon Statin Treatment. International Journal of Molecular Sciences 24, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiwari B., Jones A.E., Caillet C.J., Das S., Royer S.K., and Abrams J.M. (2020). p53 directly represses human LINE1 transposons. Genes & Development 34, 1439–1451. 10.1101/gad.343186.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ardeljan D., Steranka J.P., Liu C., Li Z., Taylor M.S., Taylor M.S., Payer L.M., Gorbounov M., Sarnecki J.S., Deshpande V., et al. (2020). Cell fitness screens reveal a conflict between LINE-1 retrotransposition and DNA replication. Nat Struct Mol Biol 27, 168–178. 10.1038/s41594-020-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luqman-Fatah A., Watanabe Y., Uno K., Ishikawa F., Moran J.V., and Miyoshi T. (2023). The interferon stimulated gene-encoded protein HELZ2 inhibits human LINE-1 retrotransposition and LINE-1 RNA-mediated type I interferon induction. Nature Communications 14, 203. 10.1038/s41467-022-35757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The E.P.C. (2011). A User's Guide to the Encyclopedia of DNA Elements (ENCODE). PLOS Biology 9, e1001046. 10.1371/journal.pbio.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The E.P.C. (2012). An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74. 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Center D.M., and Cruikshank W. (1982). Modulation of lymphocyte migration by human lymphokines. I. Identification and characterization of chemoattractant activity for lymphocytes from mitogen-stimulated mononuclear cells. J Immunol 128, 2563–2568. [PubMed] [Google Scholar]
- 42.Cruikshank W.W., Center D.M., Nisar N., Wu M., Natke B., Theodore A.C., and Kornfeld H. (1994). Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression. Proc Natl Acad Sci U S A 91, 5109–5113. 10.1073/pnas.91.11.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theodore A.C., Center D.M., Nicoll J., Fine G., Kornfeld H., and Cruikshank W.W. (1996). CD4 ligand IL-16 inhibits the mixed lymphocyte reaction. J Immunol 157, 1958–1964. [PubMed] [Google Scholar]
- 44.Cruikshank W.W., Lim K., Theodore A.C., Cook J., Fine G., Weller P.F., and Center D.M. (1996). IL-16 inhibition of CD3-dependent lymphocyte activation and proliferation. J Immunol 157, 5240–5248. [PubMed] [Google Scholar]
- 45.Center D.M., Kornfeld H., and Cruikshank W.W. (1996). Interleukin 16 and its function as a CD4 ligand. Immunol Today 17, 476–481. 10.1016/0167-5699(96)10052-i. [DOI] [PubMed] [Google Scholar]
- 46.Wilson K.C., Center D.M., and Cruikshank W.W. (2004). Mini ReviewThe Effect of Interleukin-16 and its Precursor on T Lymphocyte Activation and Growth. Growth Factors 22, 97–104. 10.1080/08977190410001704679. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y., Du K.L., Guo P.Y., Zhao R.M., Wang B., Zhao X.L., and Zhang C.Q. (2019). IL-16 regulates macrophage polarization as a target gene of mir-145–3p. Mol Immunol 107, 1–9. 10.1016/j.molimm.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Agudo D., Malacrida L., Kakiyama G., Sparrer T., Fortes C., Maceyka M., Subler M.A., Windle J.J., Gratton E., Pandak W.M., and Gil G. (2019). StarD5: an ER stress protein regulates plasma membrane and intracellular cholesterol homeostasis. Journal of Lipid Research 60, 1087–1098. 10.1194/jlr.M091967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez-Agudo D., Calderon-Dominguez M., Medina M.A., Ren S., Gil G., and Pandak W.M. (2012). ER stress increases StarD5 expression by stabilizing its mRNA and leads to relocalization of its protein from the nucleus to the membranes. J Lipid Res 53, 2708–2715. 10.1194/jlr.M031997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soccio R.E., Adams R.M., Maxwell K.N., and Breslow J.L. (2005). Differential Gene Regulation of StarD4 and StarD5 Cholesterol Transfer Proteins: ACTIVATION OF StarD4 BY STEROL REGULATORY ELEMENT-BINDING PROTEIN-2 AND StarD5 BY ENDOPLASMIC RETICULUM STRESS *. Journal of Biological Chemistry 280, 19410–19418. 10.1074/jbc.M501778200. [DOI] [PubMed] [Google Scholar]
- 51.Luu-The V., Tremblay P., and Labrie F. (2006). Characterization of type 12 17beta-hydroxysteroid dehydrogenase, an isoform of type 3 17beta-hydroxysteroid dehydrogenase responsible for estradiol formation in women. Mol Endocrinol 20, 437–443. 10.1210/me.2005-0058. [DOI] [PubMed] [Google Scholar]
- 52.Heikelä H., Ruohonen S.T., Adam M., Viitanen R., Liljenbäck H., Eskola O., Gabriel M., Mairinoja L., Pessia A., Velagapudi V., et al. (2020). Hydroxysteroid (17β) dehydrogenase 12 is essential for metabolic homeostasis in adult mice. Am J Physiol Endocrinol Metab 319, E494–e508. 10.1152/ajpendo.00042.2020. [DOI] [PubMed] [Google Scholar]
- 53.Nagasaki S., Miki Y., Akahira J., Suzuki T., and Sasano H. (2009). Transcriptional regulation of 17beta-hydroxysteroid dehydrogenase type 12 by SREBP-1. Mol Cell Endocrinol 307, 163–168. 10.1016/j.mce.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Didier C., Broday L., Bhoumik A., Israeli S., Takahashi S., Nakayama K., Thomas S.M., Turner C.E., Henderson S., Sabe H., and Ronai Z.e. (2003). RNF5, a RING Finger Protein That Regulates Cell Motility by Targeting Paxillin Ubiquitination and Altered Localization. Molecular and Cellular Biology 23, 5331–5345. 10.1128/MCB.23.15.5331-5345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tcherpakov M., Delaunay A., Toth J., Kadoya T., Petroski M.D., and Ronai Z.e.A. (2009). Regulation of Endoplasmic Reticulum-associated Degradation by RNF5-dependent Ubiquitination of JNK-associated Membrane Protein (JAMP) *. Journal of Biological Chemistry 284, 12099–12109. 10.1074/jbc.M808222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong B., Zhang Y., Tan B., Liu T.T., Wang Y.Y., and Shu H.B. (2010). The E3 ubiquitin ligase RNF5 targets virus-induced signaling adaptor for ubiquitination and degradation. J Immunol 184, 6249–6255. 10.4049/jimmunol.0903748. [DOI] [PubMed] [Google Scholar]
- 57.Zhong B., Zhang L., Lei C., Li Y., Mao A.-P., Yang Y., Wang Y.-Y., Zhang X.-L., and Shu H.-B. (2009). The Ubiquitin Ligase RNF5 Regulates Antiviral Responses by Mediating Degradation of the Adaptor Protein MITA. Immunity 30, 397–407. 10.1016/j.immuni.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 58.Lai R.W., Lu R., Danthi P.S., Bravo J.I., Goumba A., Sampathkumar N.K., and Benayoun B.A. (2019). Multi-level remodeling of transcriptional landscapes in aging and longevity. BMB Rep 52, 86–108. 10.5483/BMBRep.2019.52.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato S., Gillette M., de Santiago P.R., Kuhn E., Burgess M., Doucette K., Feng Y., Mendez-Dorantes C., Ippoliti P.J., Hobday S., et al. (2023). LINE-1 ORF1p as a candidate biomarker in high grade serous ovarian carcinoma. Scientific Reports 13, 1537. 10.1038/s41598-023-28840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodić N., Sharma R., Sharma R., Zampella J., Dai L., Taylor M.S., Hruban R.H., Iacobuzio-Donahue C.A., Maitra A., Torbenson M.S., et al. (2014). Long Interspersed Element-1 Protein Expression Is a Hallmark of Many Human Cancers. The American Journal of Pathology 184, 1280–1286. 10.1016/j.ajpath.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindholm H.T., Chen R., and De Carvalho D.D. (2023). Endogenous retroelements as alarms for disruptions to cellular homeostasis. Trends in Cancer 9, 55–68. 10.1016/j.trecan.2022.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Jeck W.R., Siebold A.P., and Sharpless N.E. (2012). Review: a meta-analysis of GWAS and age-associated diseases. Aging Cell 11, 727–731. 10.1111/j.1474-9726.2012.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramirez J., Bitterman P., Basu S., and Barua A. (2022). Changes in IL-16 Expression in the Ovary during Aging and Its Potential Consequences to Ovarian Pathology. J Immunol Res 2022, 2870389. 10.1155/2022/2870389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lonsdale J., Thomas J., Salvatore M., Phillips R., Lo E., Shad S., Hasz R., Walters G., Garcia F., Young N., et al. (2013). The Genotype-Tissue Expression (GTEx) project. Nature Genetics 45, 580–585. 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carithers L.J., Ardlie K., Barcus M., Branton P.A., Britton A., Buia S.A., Compton C.C., DeLuca D.S., Peter-Demchok J., Gelfand E.T., et al. (2015). A Novel Approach to High-Quality Postmortem Tissue Procurement: The GTEx Project. Biopreservation and Biobanking 13, 311–319. 10.1089/bio.2015.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Streeter I., Harrison P.W., Faulconbridge A., The HipSci Consortium, Flicek P., Parkinson H., and Clarke L. (2016). The human-induced pluripotent stem cell initiative—data resources for cellular genetics. Nucleic Acids Research 45, D691–D697. 10.1093/nar/gkw928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chick J.M., Munger S.C., Simecek P., Huttlin E.L., Choi K., Gatti D.M., Raghupathy N., Svenson K.L., Churchill G.A., and Gygi S.P. (2016). Defining the consequences of genetic variation on a proteome-wide scale. Nature 534, 500–505. 10.1038/nature18270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baier M., Werner A., Bannert N., Metzner K., and Kurth R. (1995). HIV suppression by interleukin-16. Nature 378, 563. 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 69.Zhou P., Goldstein S., Devadas K., Tewari D., and Notkins A.L. (1997). Human CD4+ cells transfected with IL-16 cDNA are resistant to HIV-1 infection: inhibition of mRNA expression. Nat Med 3, 659–664. 10.1038/nm0697-659. [DOI] [PubMed] [Google Scholar]
- 70.Idziorek T., Khalife J., Billaut-Mulot O., Hermann E., Aumercier M., Mouton Y., Capron A., and Bahr G.M. (1998). Recombinant human IL-16 inhibits HIV-1 replication and protects against activation-induced cell death (AICD). Clin Exp Immunol 112, 84–91. 10.1046/j.1365-2249.1998.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jia R., Jiang C., Li L., Huang C., Lu L., Xu M., Xu J., and Liang X. (2021). Interleukin 16 Enhances the Host Susceptibility to Influenza A Virus Infection. Frontiers in Microbiology 12. 10.3389/fmicb.2021.736449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu S., Lei Z., Li J., Wang L., Jia R., Liu Z., Jiang C., Gao Y., Liu M., Kuang L., et al. (2020). Interleukin 16 contributes to gammaherpesvirus pathogenesis by inhibiting viral reactivation. PLoS Pathog 16, e1008701. 10.1371/journal.ppat.1008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watanabe T., Watanabe S., and Kawaoka Y. (2010). Cellular Networks Involved in the Influenza Virus Life Cycle. Cell Host & Microbe 7, 427–439. 10.1016/j.chom.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Germain M.A., Chatel-Chaix L., Gagné B., Bonneil É., Thibault P., Pradezynski F., de Chassey B., Meyniel-Schicklin L., Lotteau V., Baril M., and Lamarre D. (2014). Elucidating novel hepatitis C virus-host interactions using combined mass spectrometry and functional genomics approaches. Mol Cell Proteomics 13, 184–203. 10.1074/mcp.M113.030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohamed B., Mazeaud C., Baril M., Poirier D., Sow A.A., Chatel-Chaix L., Titorenko V., and Lamarre D. (2020). Very-long-chain fatty acid metabolic capacity of 17-beta-hydroxysteroid dehydrogenase type 12 (HSD17B12) promotes replication of hepatitis C virus and related flaviviruses. Scientific Reports 10, 4040. 10.1038/s41598-020-61051-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feng K., Min Y.-Q., Sun X., Deng F., Li P., Wang H., and Ning Y.-J. (2021). Interactome profiling reveals interaction of SARS-CoV-2 NSP13 with host factor STAT1 to suppress interferon signaling. Journal of Molecular Cell Biology 13, 760–762. 10.1093/jmcb/mjab068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan Z., Hu B., Xiao H., Tan X., Li Y., Tang K., Zhang Y., Cai K., and Ding B. (2022). The E3 Ubiquitin Ligase RNF5 Facilitates SARS-CoV-2 Membrane Protein-Mediated Virion Release. mBio 13, e03168–03121. doi: 10.1128/mbio.03168-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Z., Hao P., Zhao Z., Gao W., Huan C., Li L., Chen X., Wang H., Jin N., Luo Z.-Q., et al. (2023). The E3 ligase RNF5 restricts SARS-CoV-2 replication by targeting its envelope protein for degradation. Signal Transduction and Targeted Therapy 8, 53. 10.1038/s41392-023-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stegle O., Parts L., Piipari M., Winn J., and Durbin R. (2012). Using probabilistic estimation of expression residuals (PEER) to obtain increased power and interpretability of gene expression analyses. Nat Protoc 7, 500–507. 10.1038/nprot.2011.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leek J.T. (2014). svaseq: removing batch effects and other unwanted noise from sequencing data. Nucleic Acids Research 42, e161–e161. 10.1093/nar/gku864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Savytska N., Heutink P., and Bansal V. (2022). Transcription start site signal profiling improves transposable element RNA expression analysis at locus-level. Frontiers in Genetics 13. 10.3389/fgene.2022.1026847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Danecek P., Bonfield J.K., Liddle J., Marshall J., Ohan V., Pollard M.O., Whitwham A., Keane T., McCarthy S.A., Davies R.M., and Li H. (2021). Twelve years of SAMtools and BCFtools. GigaScience 10. 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Danecek P., Auton A., Abecasis G., Albers C.A., Banks E., DePristo M.A., Handsaker R.E., Lunter G., Marth G.T., Sherry S.T., et al. (2011). The variant call format and VCFtools. Bioinformatics 27, 2156–2158. 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao F., Chang D., Biddanda A., Ma L., Guo Y., Zhou Z., and Keinan A. (2015). XWAS: A Software Toolset for Genetic Data Analysis and Association Studies of the X Chromosome. J Hered 106, 666–671. 10.1093/jhered/esv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keur N., Ricaño-Ponce I., Kumar V., and Matzaraki V. (2022). A systematic review of analytical methods used in genetic association analysis of the X-chromosome. Briefings in Bioinformatics 23. 10.1093/bib/bbac287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A.R., Bender D., Maller J., Sklar P., de Bakker P.I.W., Daly M.J., and Sham P.C. (2007). PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. The American Journal of Human Genetics 81, 559–575. 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahl V., Keller H., Schmidt S., and Weichenrieder O. (2015). Retrotransposition and Crystal Structure of an Alu RNP in the Ribosome-Stalling Conformation. Molecular Cell 60, 715–727. 10.1016/j.molcel.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 88.Chen S., Zhou Y., Chen Y., and Gu J. (2018). fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890. 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frankish A., Diekhans M., Ferreira A.-M., Johnson R., Jungreis I., Loveland J., Mudge J.M., Sisu C., Wright J., Armstrong J., et al. (2018). GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Research 47, D766–D773. 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., and Gingeras T.R. (2012). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Love M.I., Huber W., and Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15, 550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., and Smyth G.K. (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research 43, e47–e47. 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Macchietto M.G., Langlois R.A., and Shen S.S. (2020). Virus-induced transposable element expression up-regulation in human and mouse host cells. Life Sci Alliance 3. 10.26508/lsa.201900536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shabalin A.A. (2012). Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics 28, 1353–1358. 10.1093/bioinformatics/bts163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang T., Peng Q., Liu B., Liu X., Liu Y., Peng J., and Wang Y. (2020). eQTLMAPT: Fast and Accurate eQTL Mediation Analysis With Efficient Permutation Testing Approaches. Frontiers in Genetics 10. 10.3389/fgene.2019.01309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu T., Hu E., Xu S., Chen M., Guo P., Dai Z., Feng T., Zhou L., Tang W., Zhan L., et al. (2021). clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. The Innovation 2, 100141. 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghoussaini M., Mountjoy E., Carmona M., Peat G., Schmidt E.M., Hercules A., Fumis L., Miranda A., Carvalho-Silva D., Buniello A., et al. (2021). Open Targets Genetics: systematic identification of trait-associated genes using large-scale genetics and functional genomics. Nucleic Acids Res 49, D1311–d1320. 10.1093/nar/gkaa840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim S.-S., Hudgins A.D., Gonzalez B., Milman S., Barzilai N., Vijg J., Tu Z., and Suh Y. (2021). A Compendium of Age-Related PheWAS and GWAS Traits for Human Genetic Association Studies, Their Networks and Genetic Correlations. Frontiers in Genetics 12. 10.3389/fgene.2021.680560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
New sequencing data generated in this study is accessible through the Sequence Read Archive (SRA) under BioProject PRJNA937306. All code is available on the Benayoun lab GitHub (https://github.com/BenayounLaboratory/TE-eQTL_LCLs). Analyses were conducted using R version 4.2.1. Code was re-run independently on R version 4.3.0 to check for reproducibility.