Abstract
Autosomal recessive limb-girdle muscular dystrophy 21 (LGMDR21) is caused by pathogenic variants in protein O-glucosyltransferase 1 (POGLUT1), which is responsible for O-glucosylation of specific epidermal growth factor (EGF) repeats found in ∼50 mammalian proteins, including Notch receptors. Previous data from patient biopsies indicated that impaired Notch signaling, reduction of muscle stem cells, and accelerated differentiation are probably involved in disease etiopathology. Using patient induced pluripotent stem cells (iPSCs), their corrected isotypes, and control iPSCs, gene expression profiling indicated dysregulation of POGLUT1, NOTCH, muscle development, extracellular matrix (ECM), cell adhesion, and migration as involved pathways. They also exhibited reduced in vitro POGLUT1 enzymatic activity and NOTCH signaling as well as defective myogenesis, proliferation, migration and differentiation. Furthermore, in vivo studies demonstrated significant reductions in engraftment, muscle stem cell formation, PAX7 expression, and maintenance, along with an increased percentage of mislocalized PAX7+ cells in the interstitial space. Gene correction in patient iPSCs using CRISPR-Cas9 nickase led to the rescue of the main in vitro and in vivo phenotypes. These results demonstrate the efficacy of iPSCs and gene correction in disease modeling and rescue of the phenotypes and provide evidence of the involvement of muscle stem cell niche localization, PAX7 expression, and cell migration as possible mechanisms in LGMDR21.
Keywords: MT: RNA/DNA Editing, POGLUT1, LGMDR21, iPSCs, gene correction, CRISPR-Cas9, skeletal muscle, muscle stem cells
Graphical abstract

Darabi and colleagues used iPSCs to study a new type of limb girdle muscular dystrophy (LGMDR21) for disease modeling and gene correction using CRIPSR-Cas9n. This study highlights the suitability of iPSCs to identify disease mechanisms and the efficacy of gene correction for reversing affected pathogenic mechanisms.
Introduction
Limb-girdle muscular dystrophies (LGMDs) are a diverse group of rare genetic muscle disorders characterized by involvement of proximal muscles.1,2,3 So far, more than 30 subtypes and many genes have been identified that, when mutated, can cause LGMD in a dominant (LGMDD) or recessive (LGMDR) pattern.4,5,6,7 Disease phenotype can vary, based on the involved gene and mutation type, from mild forms with late onset to severe forms with early onset and rapid progression of skeletal, cardiac, and respiratory muscles failure.1,2 Diagnosis of LGMD is based on clinical, imaging (such as MRI and muscle ultrasound), and muscle biopsy findings along with molecular genetic testing.8,9,10 In recent years, the availability of next-generation sequencing (NGS) techniques, such as whole-exome sequencing (WES) and whole-genome sequencing (WGS) has transformed the diagnostic capability for LGMDs by allowing unbiased detection of gene variations at a single-base resolution.11,12,13,14,15,16,17 Nevertheless, there is still no definitive cure for LGMDs despite significant progress in gene discovery.1 Therapeutic options for LGMDs have been mostly limited to physical and occupational therapies and symptomatic and supportive care.10,18 Fortunately, with the advancements in gene and personalized stem cell therapies, such as induced pluripotent stem cells (iPSCs), there is new hope on the horizon.2,19,20,21
Autosomal recessive LGMD 21 (LGMDR21) is a recently reported recessive LGMD caused by mutations in the protein O-glucosyltransferase 1 gene (POGLUT1), leading to its reduced enzymatic activity.22,23 POGLUT1 is a conserved endoplasmic reticulum (ER)-localized glycosyltransferase enzyme responsible for O-glucosylation of the epidermal growth factor (EGF) repeats at a specific consensus sequence [CXSX(P/A)C] found in ∼50 mammalian proteins, including the Notch receptors.24,25,26 O-glucose glycans are required for proper folding and trafficking of Notch as well as ligand binding, its cleavage, and activation.24,27,28 The functional importance of O-glucosylation in Notch signaling was first discovered in Drosophila, where loss-of-function mutations in Poglut1 mimic the loss-of-function mutations of Notch.24,27 Similar studies in mice also indicated embryonic lethality and several defects similar to Notch1 loss-of-function phenotypes, such as impaired somitogenesis, cardiogenesis, and vascular remodeling.25,29 Therefore, Notch signaling is considered the major evolutionarily conserved target of this enzyme.29,30
LGMDR21 was originally reported in four siblings of a consanguineous family from southern Spain who harbored a biallelic (homozygous) missense mutation of c.699T>G transversion in the POGLUT1 gene, leading to p.D233E missense substitution (aspartic to glutamic). The mutated aspartic acid residue is located in the evolutionarily conserved CAP10 domain and near the catalytic motif of the enzyme, leading to significant reduction of enzyme activity by 80%–85% without changing its expression at the mRNA or protein level or its subcellular localization at the ER.22 Heterozygous carriers of the POGLUT1 D233E mutation stay healthy, indicating that 50%–60% enzyme activity is enough to preserve normal enzyme function in skeletal muscle. Later, more LGMDR21 patients in unrelated families from different countries were identified, carrying other biallelic novel mutations in POGLUT1.23 All of these patients suffered from clinical LGMD phenotypes.23 In vitro evaluation of the patient biopsies and cell assays indicated a significant reduction in POGLUT1 enzymatic activity and protein stability, leading to defective Notch signaling, reduced NOTCH1 intracellular domain (N1ICD) and its target gene (HES1), and significant reduction of the satellite cells as one of the potential pathogenic mechanisms in this type of LGMD.22,23
Altogether, these findings suggest that LGMDR21 is possibly caused by muscle stem cell dysfunction because of the mutations in a Notch pathway modifier, POGLUT1. This feature of LGMDR21 provides an excellent opportunity to be used for mechanistic studies and to develop new therapies. We have recently generated and fully characterized integration-free iPSCs from LGMDR21 patients with the homozygous missense mutation (c.699T>G).31 Here, using a directed myogenic differentiation method developed by our lab,32,33 we demonstrated the important role of POGLUT1 in skeletal muscle differentiation of iPSCs spanning from the initial stages of myogenic lineage commitment to their subsequent expansion and terminal differentiation. In vitro data also identified significant dysregulation of genes associated with POGLUT1 and NOTCH, muscle development, extracellular matrix (ECM) formation, cell adhesion, and migration. We also developed a CRISPR-Cas9 nickase gene editing method for correction of the mutation, leading to rescue of defective phenotypes. Finally, engraftment studies in this report provide fresh insights into the possible role of POGLUT1 in the formation, maintenance, and homing potential of PAX7+ muscle stem cells in vivo.
Results
CRISPR-Cas9 nickase (CRISPR-Cas9n)-mediated gene correction of LGMGR21 iPSCs
To correct the c.699T>G point mutation in LGMADR21 iPSCs, we used a Cas9-mediated homology-directed repair (HDR) strategy, as demonstrated in Figures 1A and 1B. To reduce the off-target mutagenesis risk, a Cas9n strategy was designed to induce targeted double-stranded DNA nicking near the mutation site, facilitating HDR while significantly enhancing target site specificity and reducing off targets.34 Although initially single-stranded DNA oligodeoxynucleotides (ssODNs) were used for HDR,35,36 we were not able to detect any corrected clones, possibly because of its overall low efficiency and lack of selection. Therefore, a conventional HDR targeting vector with positive and negative selection cassettes was generated (Figure 1B). After screening the 100-bp flanking regions of the mutation site, four pairs of potential sgRNAs were selected and cloned into a Cas9n vector. A surveyor assay on HEK293T cells confirmed the appropriate cutting efficiency of the selected pairs (Figure 1C). After electroporation of LGMDR21 iPSCs with Cas9n vectors and linearized correcting HDR vector, targeted iPSC clones were selected by puromycin and used for sequencing. Next, corrected clones with mutation reversal (one-copy correction, G>T) were identified, based on PCR and sequencing results (Figures 1D–1G). Finally, the selection cassette was removed by short-term transfection with a Cre recombinase vector. and the selected clones were evaluated for karyotype and pluripotency markers, which were normal.
Figure 1.
Gene correction strategy of the iPSCs
(A) Analyzed sequence near the mutation site and selected gRNAs and their cutting sites. Red and blue sequences mark the PAM and target sites. Red arrows mark the cutting sites. (B) Targeting strategy using an HDR vector containing homology arms, positive and negative selection cassettes, and final removal of the selection cassette. Primer sites and PCR products for wild-type (1.2 kb), targeted alleles (1.7 kb), and after Cre removal (0.7 and 0.9 kb) are marked. (C) Surveyor assay confirming the cutting efficiency of the selected Cas9n pairs. The 1+4 pair demonstrated the best in vitro targeting efficiency and was used for gene correction experiments. (D) PCR image of wild-type and targeted alleles demonstrates the presence of targeted iPSCs among screened single-cell clones. (E) PCR for confirmation of removal of the selection cassette among screened single-cell clones. (F) Bright-field image of a single-copy-corrected iPSC. (G) Sequencing results of healthy control (CTL), patient (PT), and patient-corrected (PTC) iPSCs confirm correct replacement of the mutated codon.
Evaluation of the effect of POGLUT1 in skeletal muscle differentiation in vitro
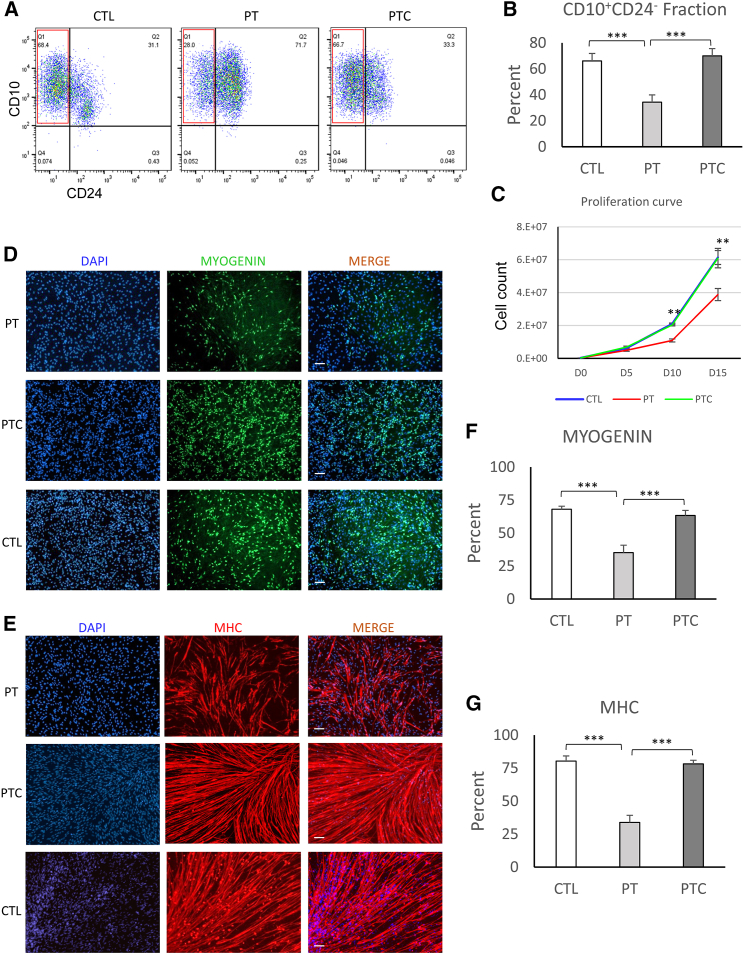
To determine the role of POGLUT1 in skeletal myogenesis in vitro, we studied the time course of differentiation of iPSCs into skeletal myogenic progenitors. We used an established directed differentiation approach developed by our lab to study the temporal differentiation of iPSCs into presomitic mesoderm (PSM) and, subsequently, somite/myogenic progenitors.32,33 Healthy control (CTL), uncorrected LDMGR21 patient (PT) iPSCs, and their corrected isogenic (PTC) iPSCs were initially differentiated into PSM by short-term induction of WNT (using CHIR99021 [CHIR]) for 5 days, followed by 10-day differentiation into somite/skeletal muscle progenitors by BMP inhibition and addition of important cytokines. Next, the myogenic induction efficiency was evaluated by surface marker analysis using flow cytometry for the percentage of CD10+CD24− cells, which represent PAX7−MYF5+ skeletal myogenic progenitors (Figures S1A and S1B).32,33 The skeletal myogenic fraction (CD10+CD24−) among differentiated cells on day 15 demonstrated a significantly lower percentage (p < 0.001) in PT -derived differentiated iPSCs compared with healthy CTL iPSCs (Figures 2A and 2B), indicating lower myogenic potential in LGMDR21 PT iPSCs. PTC iPSCs exhibited a significant increase in the myogenic cell fraction (CD10+CD24−) (p < 0.001), comparable with the levels obtained from healthy iPSCs. Evaluation of the proliferation rate of the cells during the myogenic induction phase (days 0–15) also revealed significant differences among the studied iPSC-derived myogenic progenitors (Figure 2C). PT iPSCs demonstrated a significantly reduced proliferation rate compared with PTC or healthy iPSCs (p < 0.01 by days 10 and 15 of differentiation). PTC iPSCs demonstrated a significantly improved proliferation rate, similar to healthy iPSCs.
Figure 2.
Defective in vitro myogenesis of PT iPSCs and their rescue after gene correction
(A) A representative flow cytometric analysis of the myogenic fraction (CD10+CD24−) on day 15 of differentiation of iPSCs demonstrates a significant reduction of the myogenic fraction in PT vs. healthy CTL and its rescue in PTC iPSCs. (B) Quantitative analysis of the myogenic fraction in studied iPSCs demonstrates a significant reduction of the myogenic fraction in PT cells compared with healthy CTL iPSCs and its rescue after gene correction. Data are mean +SEM from 5 independent experiments. (C) Proliferation curve of studied cells during the myogenic induction phase (days 0–15) indicates significant reduction of the proliferation rate in PT cells and its rescue after correction. Data are mean +SEM from 5 independent experiments. (D and E) Immunofluorescence (IF) staining of myogenic cells after differentiation into myotubes for MYOGENIN and myosin heavy chain (MHC). (F and G) Quantification of the IF images indicates a significant reduction of differentiation in PT cells compared with CTL cells and their rescue after single-copy correction. Data are mean +SEM from 5 independent experiments (5 representative image/marker/experiment). ∗∗p < 0.01, ∗∗∗p < 0.001.
To determine the differentiation potential of the myogenic progenitors, committed myogenic cells (day 15 sorted CD10+CD24− cells) were differentiated into myotubes by switching to differentiation medium and were evaluated for expression of MYOGENIN and myosin heavy chain (MHC) (Figures 2D–2G). Quantification of the positive cell fractions for these markers also indicated significant differences among uncorrected cells compared with the corrected and healthy CTL (p < 0.0001). Both terminal myogenic markers (MYOGENIN and MHC) demonstrated significantly lower expression in PT cells (p < 0.001). PTC cells demonstrated significantly improved differentiation comparable with healthy CTL cells (p < 0.001). Another noteworthy observation during the expansion and differentiation stages was premature and spontaneous myotube differentiation of uncorrected cells after sorting and their subsequent detachment from the culture plate prior to terminal differentiation conditions. This finding indicated their accelerated differentiation potential, which coincides with findings from PT sample biopsies.22 Taken together, the in vitro results support a possible role of POGLUT1 in initial induction of myogenic differentiation in iPSCs as well as its importance for myogenic cell expansion (proliferation rate) and differentiation potential. In addition, rescue of in vitro phenotypes in corrected iPSCs further supports the etiologic role of POGLUT1 mutation in the observed defects in myogenesis.
Gene expression profiling of iPSCs and myogenic progenitors
To determine the impact of LGMDR21 mutation on gene regulation in the context of myogenic differentiation, we performed RNA sequencing (RNA-seq) on PT iPSCs carrying the homozygous c.699T>G variant in the POGLUT1 gene and the corresponding isogenic cell line with a copy of the variant corrected. In addition, to screen out gene expression impacts that are artifacts of the gene correction technology (e.g., Cas9 expression, potential off-target insertion or deletion [indel]), we profiled transcript levels from an iPSC line derived from a healthy individual who does not carry the variant (healthy CTL iPSCs). Cells were differentiated using our established myogenic differentiation method, and samples were harvested at different time points (day 0, undifferentiated iPSCs; day 5, early-differentiated PSM cells; day 15, sorted myogenic progenitors; day 20, differentiated myotubes), as described previously.32,33 For each cell line, we performed the myogenic differentiation time-course and RNA-seq experiment twice, which resulted in a total of 24 RNA-seq datasets (3 cell lines × 4 time points × 2 replicates = 24 libraries). On average, we collected 25.5 million uniquely mapped RNA-seq reads from each sample with a minimum coverage of 20.8 million reads. This sequencing coverage allowed us to quantitate 21,946 genes. Using principal-component analysis, we found that cell differentiation stages account for the majority of variance in data, with principal component 1 (PC1) (33% variance) clearly separating day 0, day 5, day 15, and day 20 in accordance with chronological order and PC2 (21% variance) separating day 5 from the rest (Figure 3A). Our variations of interest (i.e., donor effect and mutation effect) are separated mainly along the axes of PC4 (5.5% variance) and PC6 (3.2% variance), respectively (Figure 3B), indicating closer association of corrected cells’ gene profiles to CTL iPSCs.
Figure 3.
Gene expression profiling of PT, PTC, and CTL iPSCs during the myogenic differentiation time course
(A) Major variation in transcript level reflects differentiation stage differences. A scatterplot shows RNA-seq data projected onto the first two PCs; each data point represents a sample: PC1 (33% variance) and PC2 (21% variance). (B) A scatterplot shows RNA-seq data projected onto the fourth and sixth PCs to visualize the donor effects and variant correction effects: PC4 (5.5% variance) and PC6 (3.2% variance). (C) Heatmap representation of results from a targeted search for gene set enrichment. The color code reflects the significance level (i.e., −log10 p value) of enrichment for each differentiation stage by gene set combination. MC.Notch, a manually curated list of Notch-related genes (Table S1). (D) Heatmap representations of gene set enrichment analysis results from a systematic search across the GO database. Shown are the top 5 categories identified for day 15 variant correction effect genes. The color key reflects the significance level (i.e., −log10 p value) of enrichment. (E) A scatterplot visualizes the between-differentiation stage log2 fold change in gene expression level comparing PT samples with and without variant correction for day 15 vs. day 20. Red data points represent day 15 vs. day 20 LGMDR21-impacted differentiation genes that passed the 1% FWER significance cutoff, and the corresponding effect size is proportional to the shortest distance to the black diagonal line from the center of each data point. (F) Network representation of STRING connections for day 15 vs. day 20 LGMDR21-impacted differentiation genes. Each node represents a gene, and edges represent connections between genes that passed a STRING score cutoff of 0.9. Nodes are color coded by gene set annotations.
Results from our principal-component analysis also indicated a substantially larger donor effect than mutation effect. Consequently, a joint analysis using PT and CTL data to identify mutation effects was likely to suffer from power loss. Therefore, we first focused our analysis on PT samples (corrected vs. uncorrected) to identify the significant effect of mutation correction on gene expression. Significant findings were then tested in a PT-vs.-CTL comparison for replication. The idea behind this approach is that genes that are replicated in the PT-vs.-CTL comparison, which we termed consensus genes, are more likely to be real effects from the mutant allele (i.e., less likely to be an artifact resulting from the gene editing procedure). At 1% false discovery rate (FDR), we found 2,989 differentially expressed genes for correction effect on day 0, 677 on day 5, 3,839 on day 15, and 783 on day 20. Of note, the mutation correction effect on day 5 appears highly asymmetric, with 595 genes showing a significant increase in transcript level vs. 82 genes showing a significant decrease in transcript level in response to mutation correction. Of the significant mutation effects identified in PT samples, using a 10% FDR in replication tests for identifying consensus genes, we found 959 genes (32%) replicated in PT-CTL comparison for day 0, 342 genes (51%) replicated for day 5, 2,162 genes (56%) replicated for day 15, and 660 genes (84%) replicated for day 20. A lower rate of replication was observed for day 0, which could potentially indicate a higher impact from gene editing side effects at the initial time point. With these four sets of consensus genes, we next tested for gene set enrichment to identify biological processes or molecular functions impacted by the POGLUT1 mutation. We first zoomed in on gene sets we found to be clearly relevant to our study, such as myogenesis-related gene sets, POGLUT1 targets, and the Notch signaling pathway. We found significant enrichment of consensus genes in myogenesis-related gene sets and POGLUT1 targets in myogenic progenitors and myotubes (i.e., day 15 and day 20; Figure 3C), which is consistent with the idea that the impact of this POGLUT1 mutation on the myogenesis process mainly manifests in cells committed to the myogenic cell fate. Next, we focused on the Notch pathway as one of the main targets of POGLUT1.24,25,26 When using the Kyoto Encyclopedia of Genes and Genomes (KEGG) Notch signaling pathway gene set (hsa04330), we initially found no significant enrichment of consensus genes from any of the four differentiation stages. This might be due to the fact that POGLUT1-mediated Notch glycosylation impacts the expression of a subset of the Notch signaling pathway genes. To investigate this, we used a broader Notch gene set from the Gene Ontology database (GO:0007219), which includes 286 Notch signaling pathway-related genes (163 quantitated in our dataset). We found significant enrichment of consensus genes in the GO:0007219 Notch gene set (Figure 3C), especially in myogenic progenitors (p = 4.09e−08) and in myotubes (p = 3.45e−05), indicating the impact of c.699T>G mutation on the transcript level of Notch signaling pathway genes. It is noteworthy that the apparently conflicting results between the KEGG Notch signaling pathway and GO Notch gene set is not simply reflecting a power increase from using a larger gene set because we found a larger effect size (i.e., odds ratio) in consensus gene enrichment in the GO Notch gene set compared with the KEGG Notch gene set (Figure S2A). We observed an increase in odds ratio from 1.73 to 3.01 for myogenic progenitors (day 15) and from 2.37 to 3.57 for myotubes (day 20). Consistently, using a manually curated Notch gene list of 30 genes (Table S1), which includes only Notch ligands, receptors, and downstream effectors, we found significant enrichment of consensus genes in myogenic progenitors (p = 7.65e−05) and, to a lesser extent, in myotubes (p = 1.19e−02).
We next systematically searched for GO enrichment using GOseq. GOseq accounts for the gene length bias commonly observed in differential expression analyses results.37 We found strong enrichment of ECM-related GO terms and terms related to signal transduction, especially at the later time points (Figures 3D and S2B). For example, for day 15 consensus genes, the top 5 molecular function GO terms are (1) ECM structural constituent (GO:0005201, p = 3.48e−15), (2) signaling receptor binding (GO:0005102, p = 2.93e−12), (3) calcium ion binding (GO:0005509, p = 1.21e−11), (4) heparin binding (a group of glycosaminoglycans found mainly as an intracellular component of mast cells, GO:0008201, p = 5.19e−10), and (5) glycosaminoglycan binding (GO:0005539, p = 1.32e−9). These GO terms, albeit generic, are highly relevant to the possible impact of LGMDR21 mutation on ECM components and cell adhesion, skeletal muscle development, and glycosylation of proteins involved in signaling pathways.
Next, among the consensus genes, we sought to identify genes that were differentially impacted by the mutation correction effect between time points. In other words, for each consensus gene, we tested for differences in gene expression profile changes between differentiation stages that are dependent on the mutation status. We termed these genes LGMDR21-impacted differentiation genes. Using a multiple regression model including a time point by mutation effect interaction term (materials and methods), at a stringent 1% family-wise error rate (FWER), we identified 323 LGMDR21-impacted differentiation genes for comparison between day 0 and day 5 (Figure S2C), 379 genes between day 5 and day 15 (Figure S2D), and 189 genes between day 15 and day 20 (Figure 3E). To provide independent support for our findings, we searched the STRING network database for known connections between LGMDR21-impacted differentiation genes. The STRING database curates multiple sources of gene connections, including experimental evidence of physical interaction, co-expression, and co-occurrence, in the literature and provides a combined score indicating the strength of connection.38 Using a stringent STRING score cutoff of 0.9, against the whole genome background, we found significantly more network connections between LGMDR21-impacted differentiation genes than expected by chance for all three pairs of time points (day 0–5: 137 observed edges vs. 59 expected, p < 1e−16; day 5–15: 199 observed edges vs. 62 expected, p < 1e−16; day 15–20: 56 observed edges vs. 14 expected, p = 1.11e−16). Of note, the day 5–15 comparison results need to be interpreted with caution because the day 15 data were collected from sorted myogenic cells (CD10+CD24−), while the day 5 data were collected from cells without sorting; in other words, the differentiation stage effect and the sorting effect are confounded. For the myotube differentiation process (i.e., day 15–20), we found that, of the total of 184 nodes, FN1 (fibronectin, 19 connections), DCN (decorin, 7 connections), LAMC1 (laminin subunit gamma 1, 5 connections), COL3A1 (collagen type III alpha 1 chain, 5 connections), and MET (MET proto-oncogene, receptor tyrosine kinase, 5 connections) were the most highly connected nodes in the network (Figure 3F), indicating the possibility of c.699T>G mutation in POGLUT1 impacting glycosylation of ECM glycoproteins and the components of the MET signaling pathway (important for cell survival, proliferation, myocyte fusion, and migration39) during differentiation as a part of the etiology of LGMD21. Consistent with the notion of alteration in the ECM, we found premature myotube differentiation and detachment of cells from the tissue culture plate in PT -derived myogenic cells (as pointed out in the in vitro differentiation results above). The impact on MET signaling and the ECM is also observed in stem cell-to-mesoderm differentiation (i.e., day 0–5) with MMP2 (matrix metallopeptidase 2, 9 connections), LAMA5 (laminin subunit alpha 5, 9 connections), LAMC2 (9 connections), LAMA3 (8 connections), CDH1 (cadherin 1, 8 connections), COL1A2 (8 connections), ITGA6 (integrin subunit alpha 6, 8 connections), PXN (paxillin, 8 connections), and MET (7 connections) being the most highly connected nodes among the 309 nodes in the network (Figure S2E). These observations point to the possible role of POGLUT1 in regulation of the muscle progenitor niche and their adhesion and migration through glycosylation of ECM components during myogenesis as a part of the LGMDR21 etiology.
In vitro evaluation of the effect of gene correction on enzyme activity, myogenic progenitor cell adhesion, and their migration potential
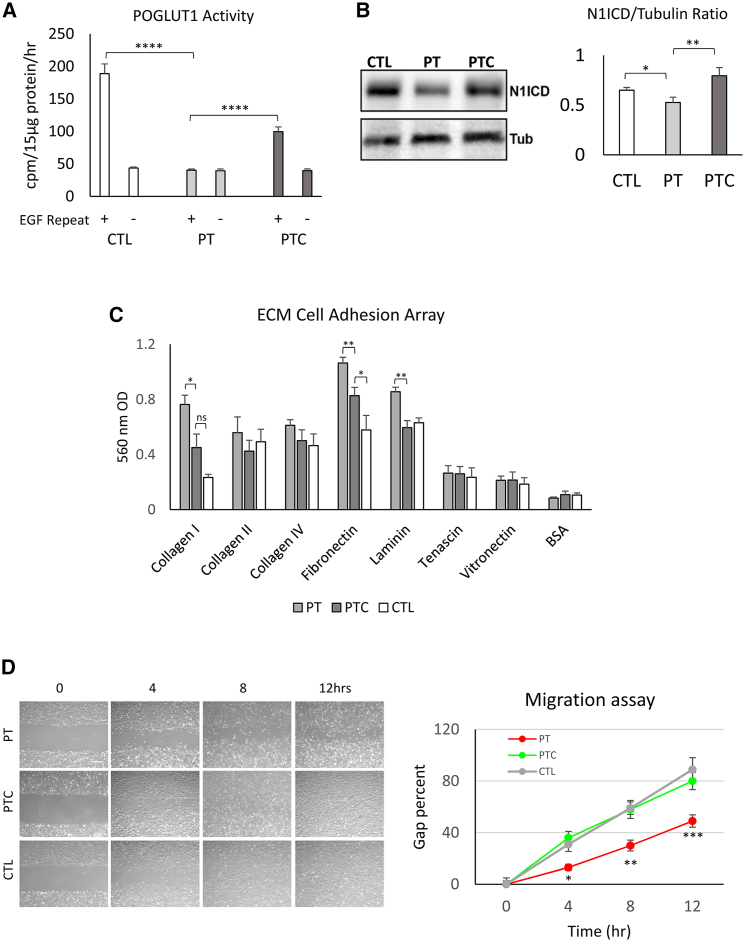
To determine the effect of one-copy gene correction on POGLUT1 enzyme activity, samples from CTL, PT, and PTC iPSCs were differentiated into myogenic progenitors and used for an enzymatic assay. Cell lysates (5 biological replicates) containing 15 μg of protein were incubated for 1 h with the substrate (human factor IX EGF repeat), and the rate of incorporation of [6-3H] glucose into the human factor IX EGF repeat was determined by scintillation counting of the eluate. Reactions without factor IX EGF repeat were used as background CTLs.40 As shown in Figure 4A, the PT samples did not show any significant enzymatic activity in the presence of the substrate. We note that, given the low endogenous levels of POGLUT1, this assay is not sensitive enough to detect the residual enzymatic activity of the endogenous POGLUT1 D233E protein expressed in PT cells. In contrast, corrected samples demonstrated significantly higher enzymatic activity (p < 0.0001) compared with uncorrected samples. Compared with healthy CTL samples with 2 wild-type (healthy) alleles, the level of enzyme activity in corrected samples was around 50% of the normal level, which agrees with one-copy correction of POGLUT1 in PTC samples. As mentioned before, heterozygous carriers of the POGLUT1 D233E mutation stay healthy, indicating that 50%–60% enzyme activity is enough to preserve normal enzyme function in skeletal muscle.22 Therefore, these one-copy-corrected cells are an appropriate tool to study the relevant pathways affected in PT cells.
Figure 4.
Evaluation of enzyme activity, N1ICD, cell adhesion, and migration in iPSC-derived myogenic progenitors from PT, PTC, and CTL cells
(A) Measurement of POGLUT1 activity in the absence or presence of factor IX EGF repeats indicate a significant reduction of enzyme activity in PT cells compared with the CTL and its partial rescue after one-copy gene correction in PTC cells. Data are mean +SEM from five experimental replicates for each cell line. (B) A representative WB gel image of N1ICD demonstrates its reduction in PT cells compared with the CTL and its rescue after gene correction. The bar graph represents the N1ICD/tubulin ratio from five independent experimental replicates. (C) Bar graph representation of the ECM cell adhesion potential of the iPSC-derived myogenic progenitors from the study samples. Values are the mean +SEM from 4 independent experimental replicates. (D) The migration time course of the studied cells using a scratch wound healing assay demonstrates a migration deficiency of PT cells compared with the CTL and its rescue after gene correction. The graph on the right demonstrates quantitative evaluation of the migration potential at each time point. Data are mean ± SEM from 6 experimental replicates. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
To evaluate the effect of gene correction on Notch signaling, we performed western blot quantification of N1ICD with an antibody that only detects the γ-secretase-cleaved version of NOTCH1 and can therefore be used as a direct readout for NOTCH1 activation.41 Samples from PT, PTC, and CTL iPSC myogenic progenitors were harvested (5 experimental replicates), and the level of N1ICD was determined using western blotting (WB). As demonstrated in Figures 4B and S3A, while PT cells demonstrated a reduction in N1ICD level compared with CTL cells, PTC cells demonstrated higher levels of the N1ICD, comparable with CTL cells, suggesting a rescue effect of gene correction on NOTCH1 activation.
Because RNA-seq data analysis also demonstrated significant dysregulation in genes related to the ECM, cell adhesion, and MET signaling (important for migration), we performed in vitro assays for cell adhesion and migration to evaluate the potential effect of the POGLUT1 mutation and its correction on these aspects of cell function. To evaluate the cell adhesion properties of iPSC-derived myogenic progenitors with ECM, we used a colorimetric ECM cell adhesion array system containing major ECM components (seven ECM proteins: collagens I, II, and IV; fibronectin, laminin, tenascin, and vitronectin and BSA as a negative CTL). Experimental replicates (n = 4) from each iPSC-derived myogenic progenitor cell group (CTL, PT, and PTC) were incubated with the abovementioned ECM proteins on coated plates, and the rates of cell adhesion were determined. As demonstrated in Figure 4C, while there was no significant difference in BSA CTL substrate adhesion, PT samples demonstrated statistically significant higher levels of adhesion to collagen I, fibronectin, and laminin (p < 0.05, 0.01, and 0.01 respectively). Corrected iPSC-derived myogenic (PTC) cells demonstrated reduced potential to adhere to these ECM substrates, similar to CTL iPSC-derived myogenic cells. Because ECM interaction enables many important cellular functions, such as cell communication, signal transduction, and migration, the aberrant adhesion potential of LGMDR21 myogenic cells might be a factor contributing to the pathogenic mechanism of LGMDR21.
Because the alteration in ECM interaction might also affect the cell migration potential, and because the RNA-seq data also demonstrated dysregulation of MET signaling, we studied the in vitro migration potential of the cells using a scratch wound healing assay. Experimental replicates (n = 6) from each myogenic progenitor cell group (CTL, PT, and PTC) were plated on coated wells in myogenic expansion medium (containing muscle growth factors, including HGF, which is the ligand for the MET receptor),32,33,42 and after reaching confluency, a surface scratch wound was created using a glass pipette. Cells were studied for migration into the wound area, and images were taken at 4-h intervals for 12 h. Percent coverage of the wound was quantified at each time point using surface area measurement by ImageJ software. As shown in Figure 4D, PT samples showed a significant reduction of migration potential into the wound area compared with healthy CTL cells (p < 0.05 at the 4 h, p < 0.01 at the 8 h, and p < 0.001 at the 12 h time point) and were not able to completely cover the wound surface 12 h later. PTC cells demonstrated restored migration potential comparable with healthy CTL cells and were able to cover the wound surface at much earlier time points. These data supported the RNA-seq findings regarding potential involvement of cell migration as a mechanism for disease pathology. One of the components of the HGF/MET pathway, hepatocyte growth factor activator (HGFA; official name HGFAC) harbors two predicted POGLUT1 target O-glucosylation.26 This might explain dysfunction of MET signaling and cell migration in LGMDR21 PT cells in addition to dysregulation of ECM components, glycoproteins, and adhesion molecules (shown by RNA-seq data), which are also equally important for cell adhesion and migration. Furthermore, MET signaling controls several important aspects of cell function, such as proliferation, survival, apoptosis, adhesion, and migration, through different downstream signaling interactions with other pathways (such as ERK/mitogen-activated protein kinase [MAPK] and mTOR).39 These might create a compounding effect on different aspects of cellular function and disease pathology in the case of POGLUT1 mutation.
In vivo evaluation of the effect of gene correction on the engraftment potential of myogenic progenitors differentiated from LGMDR21 iPSCs
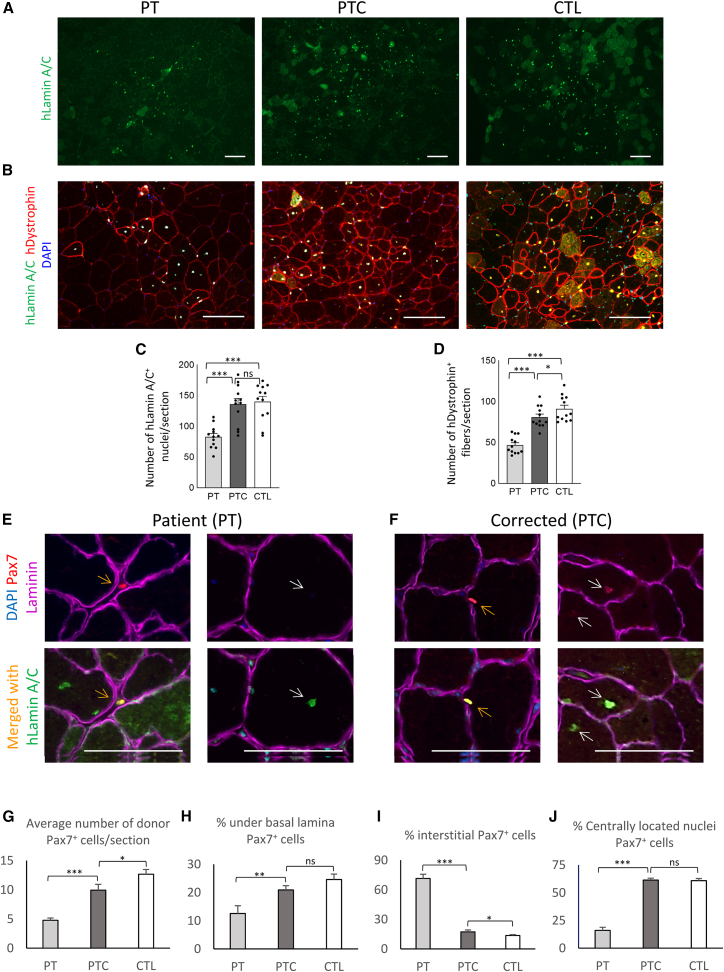
To study the effect of gene correction on myogenic progenitor cell function in vivo, we performed engraftment studies in mice to evaluate the rate of donor cell survival, myofiber engraftment, and muscle stem cell formation by PT vs. PTC and healthy CTL iPSC-derived myogenic progenitors. To facilitate donor cell engraftment, an acute muscle injury model was used. The tibialis anterior (TA) muscles of adult immunodeficient NSG mice were injured using cardiotoxin injection, and CD10+CD24− sorted myogenic progenitors (PAX7−MYF5+) from each cell group (PTC, PTC, and CTL) were injected into the injury site 48 h later (n = 6 mouse per group). Muscles were harvested 6 weeks later and sectioned for immunohistological evaluation of human donor cell engraftment into the recipient mouse muscles as myofibers or muscle stem cells. Staining with a donor-specific nuclear marker (human lamin A/C) indicated the presence of significantly fewer numbers of donor-derived cells in the engrafted regions of the PT group compared with the CTL (p < 0.001) (Figures 5A, 5C, and S3B). On the other hand, the rate of donor cell presence for gene-corrected (PTC) cells was significantly higher compared with the PT group (p < 0.001) and comparable with CTL cells (Figure 5A and 5C). To quantify the rate of cell engraftment into myofibers, muscle sections were stained for donor-cell specific markers (human lamin A/C and human dystrophin) to identify human donor cells engrafted into myofibers (Figures 5B and 5D). The PT group demonstrated significantly lower levels of donor cell engraftment into myofibers expressing human dystrophin (p < 0.001), while PTC cells demonstrated significantly improved levels of engraftment, approaching the level of CTL cells. These data are in agreement with the in vitro data regarding improved myogenic potential of the cells after gene correction, possibly because of improved cell proliferation and terminal differentiation.
Figure 5.
In vivo transplantation study of iPSC-derived myogenic progenitors from PT, PTC, and CTL cells in a muscle injury model in NSG mice
(A) Representative low-magnification images demonstrate donor cell presence in host myofibers using immunostaining for a human donor cell marker (human lamin A/C). (B) Representative higher-magnification images demonstrate donor cell engraftment into host myofibers expressing human dystrophin and human lamin A/C. (C and D) Quantitative evaluation of donor cells demonstrates significant reduction of donor cell presence and myofiber engraftment in PT cells compared with the CTL and its rescue after gene correction. (E) A representative image of PT -derived cell engraftment. Left images demonstrate a PT -derived cell (expressing PAX7 and hLamin A/C, orange arrow) residing in the interstitial space between the myofibers. The right image shows a PT -derived cell (expressing hLamin A/C, white arrow) in a regenerating fiber and negative for PAX7. (F) A representative image of a one-copy gene-corrected PT derived cell. The left images demonstrate a PTC cell (expressing PAX7 and hLamin A/C, orange arrow) under the basal lamina. The right image shows two PTC cells (expressing PAX7 and hLamin A/C, white arrows) in a regenerating fiber. (G–J) Quantification of the average number of donor PAX7+ cells, based on their localization to myofibers. Data are mean +SEM (n = 6 mouse/cell group). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Because previous studies of PT muscle biopsy samples suggested reduced numbers of PAX7+ cells and satellite stem cell pools as a possible pathologic feature of LGMDR21,22,23 we studied the potential of donor cells for formation of PAX7+ cells. Muscle sections were stained for laminin, Pax7, and human-specific lamin A/C to identify donor cells positive for Pax7 (PAX7+hLamin A/C+) as well as their precise location related to myofibers by staining for laminin. PT cells demonstrated overall significantly lower rates of Pax7 expression in total (Figure 5G, bar graph; p < 0.001) as well as reduced numbers of PAX7+ cells under basal lamina (muscle stem cell localization) (Figure 5H, bar graph; p < 0.01) and reduced PAX7+ cells within regenerating fibers (centrally located nuclei, Figures 5E, white arrows, and 5J; bar graph; p < 0.001). In addition, further evaluation of the sections indicated a higher frequency of the presence of PAX7+ PT donor cells (PAX7+hLamin A/C+) in interstitial spaces between myofibers (Figures 5E, orange arrows, and 5I, bar graph; p < 0.001) as opposed to the normal location of the muscle stem cells (i.e., under the basal lamina). Evaluation of the corrected samples (PTC cells), however, indicated a significantly higher percentage of Pax7+ cells (PAX7+hLamin A/C+) under the basal lamina (Figures 5F, orange arrows, and 5H, bar graph; p < 0.01). In addition, PTC samples demonstrated a significantly lower percentage of Pax7+ cells in the myofiber interstitial space (Figure 5I, bar graph; p < 0.001) as well as a higher percentage of Pax7 expression (PAX7+hLamin A/C+) in regenerating myofibers (centrally located nuclei; Figures 5F,white arrows, and 5J, bar graph; p < 0.001), comparable with the CTL cell group. We did not detect any significant difference in the number of host-derived satellite cells in the studied sections (Figure S3C).
Overall evaluation of these in vivo data indicates a lower efficiency of the PT cells to express Pax7 and seed muscle stem cells, as well as their abnormal niche localization (i.e., residing in the interstitial space). Because proper muscle stem cell localization under the myofiber basal lamina is contingent upon proper expression of the proteins related to satellite cell basal lamina assembly and its adhesion markers, we interrogated our transcriptomic data for expression of these genes. Closer evaluation of RNA-seq data revealed significant (>2-fold, p < 0.001, FDR < 0.01) dysregulation of many of these genes in PT myogenic cells (such as genes related to basal lamina assembly [COL18A1, ITGA71, SGCA, DAG1, CD82, and COL4A2] and genes related to satellite cell function and adhesion [MEGF10, GPC1, MCAM1, and TSPAN7]). Notably, gene correction in PTC cells restored proper expression of these markers in consensus with CTL cells. Because niche localization of muscle stem cells is also dependent on proper Notch signaling,43 these findings support the notion that abnormal muscle stem cell niche localization because of a deficit in basal lamina assembly and impaired adhesion to myofibers in emerging muscle stem cells can be considered a potential disease pathology mechanism in LGMDR21 PTs.
Discussion
LGMDs are a diverse and heterogeneous group of Mendelian muscular dystrophies that lead to weakness and wasting of the limb girdle muscles. With the advancement of new genome sequencing methods, so far more than 30 different autosomal loci have been identified as causative gene defects in LGMDs.6 These pathogenic gene variants often lead to abnormal expression and dysfunction of a diverse cluster of genes important for mechano-signaling, nuclear and mitochondrial function or sarcolemma, formation of the dystroglycan complex, and ECM.44 While many of the causative mutations and pathogenic defects in major types of LGMDs have been studied and identified, there are still many unsolved or novel types of LGMD subtypes that warrant further investigation.2 LGMDR21 is one of the recently identified subtypes caused by recessive mutation in POGLUT1.22,23 In the current study, we took advantage of LGMDR21 PT-derived iPSCs to replicate myogenesis in vitro and study the time course development of skeletal myogenic progenitors. This method allowed access to abundant PT iPSC-derived myogenic progenitors at various differential stages for disease modeling and screening of pathogenic mechanisms. This also allowed development of a platform for site-specific gene correction using the CRISPR-Cas9n system. Another advantage of this iPSC-based model system is the ability to evaluate the effect of gene correction on pathology reversal and phenotypic rescue in vitro and in vivo. Alternatively, iPSC-derived myogenic cells also can be considered another relevant target for gene correction and disease modeling; however, this might be a more difficult alternative because of lower targeting efficiency and clonal expansion potential.
Using well-characterized and integration-free iPSCs from LGMDR21 patients, we designed a CRISPR-Cas9n strategy along with an HDR template (including positive and negative selection markers) for targeting the mutated region in exon 7. Compared with the double-strand-cleaving Cas9 system, paired nickases have significantly lower off-target risks, which makes them a more favorable choice for gene editing.34,45,46 In addition, generation of long overhangs on each side of the cleaved ends by the nickase pairs provides more precise control and efficiency for gene insertion and integration.34,47 Of note, we also tried to correct the mutation using ssODNs;48 however, we were not able to obtain any corrected clones after several attempts. This was likely due to the low frequency of correction and lack of a positive selection strategy. This points to the importance of inclusion of the positive and negative selection cassettes in HDR gene correction vectors to improve selection specificity and efficiency in the corrected clones. Using the HDR method, we were able to obtain several single-copy-corrected clones; however, both-copy-corrected clones were not detectable among screened clones. Nevertheless, because one healthy POGLUT1 allele is enough to preserve normal muscle phenotype in heterozygous carriers, one-copy-corrected iPSCs were studied for the effect of gene correction in this study.
From a biological standpoint, one important finding of the current study is the reduced myogenic differentiation potential of LGMDR21 PT iPSCs compared with CTL cells. Temporal differentiation of iPSCs toward the PSM, myotomal cells, and purification of myogenic progenitors using surface markers32,33 clearly demonstrated a reduced potential for myogenesis in PT cells. This reduced myogenic potential was accompanied by a reduced proliferation rate and premature differentiation and detachment, leading to loss of myotubes prior to the terminal differentiation stage. These defects can be attributed to altered expression of important pathways and gene groups, as described by the RNA-seq data. Indeed, significant dysregulation of genes related to POGLUT1, NOTCH, MAPKs, muscle development, ECM, and glycoproteins is likely to be a major contributing factor in defects related to myogenic induction, proliferation, and differentiation of the PT iPSCs. One-copy correction of the POGLUT1 mutation in PTC iPSCs was enough to reverse the observed pathologies in myogenic induction efficiency, percentage of myogenic cell fraction, proliferation rate, and differentiation potential of the cells in a trend comparable with CTL iPSCs.
Another noteworthy finding of the current study is the possible mechanistic involvement of the defects in cell adhesion and migration potential of myogenic cells in myogenic phenotypes caused by POGLUT1 mutations. Importantly, in line with GO and STRING analyses of gene expression data, which revealed significant alterations of ECM components and glycoproteins and possible involvement of MET signaling, in vitro ECM adhesion and migration assays showed altered adhesion and migration deficiency in PT myogenic cells and their rescue after single-copy correction of the POGLUT1 gene. As mentioned before, in addition to dysregulated expression of the MET signaling components, the presence of 2 predicated POGLUT1 O-glucosylation targets on HGFA with potential functional importance might be an important contributing factor. Furthermore, downstream cross-talk between MET and other important signaling pathways, such as ERK/MAPK and mTOR,49 which regulate important cell functions such as survival, proliferation, and adhesion, might also contribute to disease pathology in LGMDR21.
Finally, to determine the effect of POGLUT1 dysfunction and its rescue after gene correction on in vivo muscle progenitor cell function, engraftment studies in a muscle injury model in NSG mice were performed. Considering the importance of Notch signaling in muscle stem cell maintenance and its self-renewal and the role of POGLUT1 as a Notch modifier,24,25,43,50,51 we evaluated the rate of donor cell engraftment in myofibers as well as their potential for seeding newly formed muscle stem cells. In vivo data demonstrated a significantly lower level of myofiber engraftment and a reduced percentage of donor-derived PAX7+ cells in regenerating fibers, as well as in the normal stem cell compartment (under basal lamina) in muscles treated with PT iPSC-derived myogenic cells. Moreover, another finding of the current study is an increased presence of PT donor-derived PAX7+ cells in the interstitial space of myofibers, as opposed to the normal location of muscle stem cells (under the basal lamina). This finding, along with abnormal expression of the markers associated with basal lamina assembly components and adhesion molecules in PT myogenic cells (as mentioned for the in vivo results) supports a possible deficiency of LGMDR21 PT iPSC-derived myogenic cells to properly recapitulate and assemble the natural muscle stem cell niche components needed for their proper homing under the basal lamina. Considering the proven role of Notch signaling in homing of muscle progenitors in the satellite cell niche,43,50 this phenotype similarity supports the notion of potential Notch dysregulation in LGMDR21 PT cells. One-copy correction of POGLUT1 in PTC iPSCs was enough to rescue the observed in vivo phenotypes (engraftment efficiency and muscle stem cell seeding) in PTC iPSC-treated muscles.
Another notable in vivo finding was the lower potential of PT cells to maintain PAX7 expression in regenerating myofiber nuclei. This is particularly important because longer Pax7 retention leads to increased proliferation and expansion of early myogenic progenitor pools, important for muscle regeneration. This finding is also in harmony with the in vitro data regarding the lower rate of proliferation and accelerated maturation of myotubes in PT cells, possibly because of the deficiency in Pax7 expression and retention as a mechanism of defective myogenesis in LGMDR21 PTs. Considering the role of Notch in sustaining Pax7 expression in muscle stem cells and progenitors, this finding also supports a role of POGLUT1 as a Notch modifier in maintaining Pax7 expression in muscle. Involvement of the Notch pathway in muscular dystrophies has been reported in at least two other types of muscular dystrophies associated with pathogenic variants in JAG2 and MEGF10.52,53,54 Clinically, these three dystrophies share many common phenotypes, such as a variable age of onset based on pathogenic variants and similar CK levels and muscle MRI patterns. Shared disease mechanisms, such as satellite cell dysfunction secondary to Notch impairment, are also common features of this group of dystrophies. Furthermore, our finding of dysregulated NOTCH and MEGF10, which are involved in intracellular cross-talk and have important roles in satellite cell function and myogenesis;43,55,56 impairment of cell adhesion, proliferation, and migration in LGMDR21 iPSC-derived myogenic cells; and mislocalization of PAX7+ cells further support commonality in pathogenic mechanisms in this group of dystrophies.
Taken together, the current study describes a powerful approach of using gene correction strategies in iPSCs for genetic disorders such as LGMDs to model the disease and evaluate the effect of gene correction in rescuing disease phenotypes. For LGMDR21, this study provides fresh mechanistic insights into the involvement of cell function defects (such as reduced expansion and differentiation potential, defective ECM adhesion and migration, NOTCH signaling defects, and possible involvement of the c-Met HGF axis) using in vitro modeling and rescue experiments. In addition, in vivo studies confirm reduced expression and maintenance of Pax7, along with impaired homing efficiency of the PAX7+ muscle progenitors into the muscle stem cell compartment, because of defective niche assembly, a phenotype akin to Notch deficiency. These factors, taken together, are likely the major contributors in LGMDR21 pathology. Last, site-specific gene correction rescue of the phenotypes in LGMDR21 iPSCs underlines the significance and importance of gene correction approaches in developing individualized gene and stem cell therapies for LGMD PTs.
Materials and methods
iPSC culture and differentiation
Human iPSCs were maintained and expanded on Matrigel-coated (BD Biosciences) flasks using mTeSR medium (STEMCELL Technologies) as described previously.32,33 For skeletal muscle lineage differentiation of iPSCs, we used a differentiation protocol developed previously by our lab. Briefly, cells were differentiated into PSM by 5-day induction of WNT (using CHIR, Selleck Chemical) and inhibition of transforming growth factor β (TGF-β; SB431542, Selleck Chemical), followed by BMP (using LDN193189, Stemgent) and TGF-β inhibition, combined with addition of other growth factors to induce formation of skeletal muscle progenitors.32,33 Skeletal myogenic progenitors were purified using surface markers (CD10+CD24−) and expanded for two passages before in vivo application or in vitro differentiation into myotubes. Terminal differentiation into myotubes was performed by switching the medium to IMDM (Invitrogen) supplemented with 15% knockout serum replacement (Invitrogen) for a few days until myotubes were formed.32 The step-by-step differentiation protocol can be found in our recent article.33
Gene correction method
After the Surveyor assay and validation of nickase pairs, Cas9n gRNA constructs were delivered via electroporation along with a targeting construct containing two homology arms for POGLUT1 flanking an EF1-RFP-T2A-PURO selection cassette to generate a double-strand break (DSB) and facilitate HDR. This targeting construct contained loxP sites for removal of the selection cassette following selection and validation. After selection of single-cell derived iPSC clones for 10 days using puromycin, corrected clones were verified by PCR analysis and DNA sequencing. Next, the selection cassette was removed by transient expression of Cre, followed by PCR confirmation and sequencing. Corrected clones were further evaluated for normal karyotype, pluripotency, and expression of iPSC markers using the Stemlight iPSC Reprogramming Antibody Kit (Stemlight, catalog number 9092S) according to the kit instructions.
RNA-seq method
Human iPSCs (PT, PTC, and healthy CTL) were differentiated into skeletal myogenic cells as described above, and samples were harvested at four different time points: undifferentiated iPSCs on day 0, early PSM differentiated cells on day 5, sorted myogenic progenitors on day 15, and terminally differentiated myotubes on day 20. For each time point, two independent experimental replicates were used for sequencing and analyses. RNA was extracted from each sample (1 × 106 cells) using the miRNeasy RNA Isolation Kit (217004, QIAGEN) according to the manufacturer’s protocol. cDNAs were made using oligo(dT) primers and used for generation of indexed libraries by the Illumina kit. Pooled libraries were sequenced using an Illumina HiSeq 4000 for a 50-cycle single end run.
RNA-seq data processing and data transformation
RNA-seq reads were mapped to the human transcriptome (GENCODE release 37) and human genome (GRCh38) using the TopHat2 (v.2.1.1)57 default setting, searching only for splice junctions indicated by GENCODE GTF (v.37). The mapping procedure allowed a maximum of 2 mismatches. Only uniquely mapped reads were retained. To quantify the expression level for each gene, the numbers of sequencing reads aligned to each gene (based on GENCODE annotation) for each sample were tabulated using the featureCounts function from the Subread package (2.0.1).58 Before normalizing the data, we first removed genes that were not sufficiently quantified in our dataset. For a gene to be included in the downstream analysis, we required at least one sequencing read aligned to the gene in more than 18 of the 24 samples. After filtering out genes that were not sufficiently quantified, the gene level count data were transformed to log2 counts per million (CPM) and TMM normalized using the edgeR package.59
Analyses
Statistical analyses were performed in the R statistical computing environment (v.4.1).
Principal-component analyses
Singular value decomposition was performed on centered and scaled RNA-seq count data using the prcomp function in the stats package.
Differential expression tests
Differential expression tests were computed using the limma Bioconductor package.60,61
To test variant correction-induced transcript level changes, we fitted log2-transformed, TMM-normalized RNA-seq data to a fixed effects model using variant correction status as the predictor. For each gene, the regression coefficient for the correction term is tested against the null hypothesis that the coefficient is equal to zero using empirical Bayes moderated t statistics.
To identify significant differences in gene expression profile changes between differentiation stages that are dependent on the mutation status, we used a multiple regression model with a correction status by differentiation stage interaction term. More specifically, for each gene, let Ei be expression level quantification from individual i, Si the indicator variable for differentiation stage, and Ci the indicator variable for variant correction status. We fitted the following model:
For each gene, we used empirical Bayes moderated t statistics to test against the null hypothesis that the regression coefficient for the interaction term (i.e., β3) is zero.
To account for multiple testing, nominal p values were adjusted using the q value method62 or the Benjamini-Hochberg procedure to calculate FDRs.
GO enrichment analyses
To identify pathways or GO terms enriched with significant variant correction effects (i.e., differentially expressed genes between variant correction status), we used Fisher’s exact test for a targeted search in a list of a handful of gene sets, and for a systematic search we used the GOseq package (v.1.28.0).37 GOseq accounts for potential biases introduced by gene length with a built-in gene length database to compute a probability weighting function. We used the default Wallenius method and searched across the GO database and KEGG pathways to test for enrichment. Genes that had no GO term associations were excluded from the analyses.
STRING network analyses
To identify known connections between LGMDR21-impacted-differentiation genes, we searched the STRING network against the whole-genome background using default settings (Homo sapiens) on the browser interface (https://string-db.org/), except the STRING score cutoff used. Instead of the default value of 0.4 we used a stringent score cutoff of 0.9. In the figures, we excluded genes without STRING network connections.
WB for NOTCH components
Sorted myogenic progenitors from PT, PTC, and CTL iPSCs from five biological replicates were collected and quantified for N1ICD protein expression using WB. Briefly, cells were harvested in RIPA buffer supplemented with protease inhibitors (Promega). The Pierce BCA Protein Assay (Bio-Rad) was used to quantify total protein levels, using bovine serum albumin as a standard. Protein extracts (25 μg per well) were separated on a 4%–15% SDS-PAGE Mini-PROTEAN TGX Precast Gradient Gel (Bio-Rad) and transferred onto polyvinylidene difluoride membranes (Bio-Rad). Membranes were blocked in 5% skim milk powder dissolved in 0.1% TBS-T at room temperature for 1 h. The membrane was incubated with primary antibodies (mouse anti-tubulin, Santa Cruz Biotechnology, sc-8035, 1:1,000; rabbit anti-cleaved NOTCH1, Cell Signaling Technology, 4147, 1:1,000) overnight at 4°C. The following secondary antibodies were used: horseradish peroxidase (HRP)-linked anti-mouse (Jackson ImmunoResearch, 115-035-003, 1:10,000) and anti-rabbit (Jackson ImmunoResearch, 111-035-003, 1:10,000), followed by enhanced chemiluminescence (ECL) detection. Captured blot images were used for band intensity quantification using ImageJ software.
POGLUT1 enzyme activity assay
Cells from five biological replicates per group (3 × 106 cells per sample) were lysed in Tris-buffered saline (TBS; pH 8), 1% NP40, and cOmplete ULTRA (EDTA free) on ice for 1 h. After centrifugation, the supernatants were collected and used for POGLUT1 assays. POGLUT1 assays were modified from a method described previously method.40 A 10-μL reaction mixture contained 50 mM HEPES (pH 7.0), 10 mM MnCl2, 10 μM human factor IX EGF repeat, 0.17 μM (0.01 mCi/mL) UDP-[6-3H]glucose (American Radiolabeled Chemicals), and cell lysate containing 15 μg of protein. The reaction was incubated at 37°C for 1 h and stopped by adding 900 μL of 100 mM EDTA (pH 8.0). The sample was loaded onto a C18 cartridge (100 mg, Agilent) and washed with 5 mL of H2O, and the human factor IX EGF repeat was eluted with 0.9 mL of 80% methanol. Incorporation of [6-3H] glucose into the human factor IX EGF repeat was determined by scintillation counting of the eluate. Reactions without the factor IX EGF repeat were used as background CTLs.
Cell adhesion assay
To measure adhesion potential of the myogenic progenitors into different ECM components, we used a colorimetric ECM adhesion array kit (ECM540, Millipore Sigma) containing 7 different human ECM protein-coated wells (collagen I, collagen II, collagen IV, fibronectin, laminin, tenascin, and vitronectin) and one BSA-coated well (negative CTL). Experimental replicates (n = 4) from each myogenic progenitor cell group (CTL, PT, and PTC) were harvested using EDTA and seeded onto the coated substrates for 2 h, where adherent cells were captured. Subsequently, unbound cells were washed away, and the adherent cells were fixed and stained. After stain extraction, the relative cell attachment was determined using absorbance readings (optical density 560 [OG560]) according to the manual.
Cell migration assay
The in vitro migration potential of the cells was quantified using a scratch wound healing assay. Biological replicates (n = 4) from each myogenic progenitor cell group (CTL, PT, and PTC) were plated on Matrigel-coated 6-well plates at a density of 6 × 105 cells/well in myogenic expansion medium (containing muscle growth factors including HGF), and after reaching confluency, a surface scratch wound was created using a glass pipette. Cells were studied for migration into the wound area, and images were taken at 4-h intervals for 12 h. Percent coverage of the wound was quantified at each time point using surface area measurement by ImageJ software.
In vivo transplantation studies
Mouse experiments and transplantation assays were performed according to protocols approved by the University of Texas Health Medical Center at Houston and institutional animal care and use committee (IACUC), which comply with the requirements of the guidelines of US National Institutes of Health (NIH) for the humane care of animals. To evaluate the engraftment potential of the cells, iPSC-derived myogenic progenitors were injected into non-irradiated TA muscles of 6- to 8-week-old immunodeficient NSG mice (equal sex ratio, randomly divided into experimental groups, n = 6/per experimental group) as described before.32 TA muscles were injured by cardiotoxin injection 2 days before, followed by cell injection (3 × 105/10 μL PBS).
Histology and quantification
For in vitro studies, cells were fixed with 4% paraformaldehyde (PFA) and stained for the differentiation markers myogenin and MHC as described before.32,63 For quantification, five low-magnification images for each set of experiments (five experimental replicates) were used to calculate the number of cells positive for each marker. For in vivo experiments (n = 6 mouse/group), muscles were harvested weeks later, OCT embedded, frozen, sectioned at 20-μm intervals and stained for different markers to identify donor cells (human lamin A/C, human dystrophin, laminin, PAX7, and DAPI) as described before.32,63 For engraftment quantification, serial cross-sections of the injection sites at 20-μm intervals were used (20 sections/slide and 10 slides/muscle) to count the number of donor nuclei (hLamin A/C+) and fibers (hDystrophin+) as well as nuclei positive for PAX7. Identification of host (mouse)-derived vs. donor (human)-derived PAX7+ cells was done using staining for PAX7 (from DHSB, non-specific), human lamin A/C, and laminin (i.e., mouse PAX7+ cells are PAX7+/hLamin A/C−, and human-derived PAX7+ cells are PAX7+/hLamin A/C+).
Statistical analysis
Difference among groups was analyzed using ANOVA and unpaired two-tailed Student’s t test. p < 0.05 was considered significant.
Data and code availability
The accession number for the RNA-seq data reported in this paper is GEO: GSE225148.
Acknowledgments
This research was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the NIH under award 1R01AR076770 to R.D. and H.J.-N. C.L. and R.D. were also partially supported by Department of Defense award MD200001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author contributions
R.D. conceived the project, designed and supervised the experiments, and wrote the manuscript. J.L.O.-V., J.W., and N.X. performed the primary in vitro and in vivo experiments. N.N. performed WB. M.T. performed enzyme activity assay measurement. A.W.S. and S.H.W. analyzed gene expression data. C.P., C.L., H.J.-N., R.S.H., S.H.W., and R.D. contributed to experimental design, data interpretation, and editing the manuscript. J.L.O.-V., J.W., H.J.-N., and S.H.W. contributed to drafting of the final manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2023.07.037.
Supplemental information
References
- 1.Murphy A.P., Straub V. The Classification, Natural History and Treatment of the Limb Girdle Muscular Dystrophies. J. Neuromuscul. Dis. 2015;2:S7–S19. doi: 10.3233/JND-150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vissing J. Limb girdle muscular dystrophies: classification, clinical spectrum and emerging therapies. Curr. Opin. Neurol. 2016;29:635–641. doi: 10.1097/WCO.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 3.Narayanaswami P., Carter G., David W., Weiss M., Amato A.A. Evidence-based guideline summary: Diagnosis and treatment of limb-girdle and distal dystrophies: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Issues Review Panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology. 2015;84:1720–1721. [PubMed] [Google Scholar]
- 4.Magri F., Brajkovic S., Govoni A., Brusa R., Comi G.P. Revised Genetic Classification of Limb Girdle Muscular Dystrophies. Curr. Mol. Med. 2014;14:934–943. doi: 10.2174/1566524014666141010130244. [DOI] [PubMed] [Google Scholar]
- 5.Nigro V., Savarese M. Genetic basis of limb-girdle muscular dystrophies: the 2014 update. Acta Myol. 2014;33:1–12. [PMC free article] [PubMed] [Google Scholar]
- 6.Taghizadeh E., Rezaee M., Barreto G.E., Sahebkar A. Prevalence, pathological mechanisms, and genetic basis of limb-girdle muscular dystrophies: A review. J. Cell. Physiol. 2019;234:7874–7884. doi: 10.1002/jcp.27907. [DOI] [PubMed] [Google Scholar]
- 7.Cohen E., Bonne G., Rivier F., Hamroun D. The 2022 version of the gene table of neuromuscular disorders (nuclear genome) Neuromuscul. Disord. 2021;31:1313–1357. doi: 10.1016/j.nmd.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Angelini C., Giaretta L., Marozzo R. An update on diagnostic options and considerations in limb-girdle dystrophies. Expert Rev. Neurother. 2018;18:693–703. doi: 10.1080/14737175.2018.1508997. [DOI] [PubMed] [Google Scholar]
- 9.Witherick J., Brady S. Update on muscle disease. J. Neurol. 2018;265:1717–1725. doi: 10.1007/s00415-018-8856-1. [DOI] [PubMed] [Google Scholar]
- 10.Mitsuhashi S., Kang P.B. Update on the genetics of limb girdle muscular dystrophy. Semin. Pediatr. Neurol. 2012;19:211–218. doi: 10.1016/j.spen.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Ghaoui R., Cooper S.T., Lek M., Jones K., Corbett A., Reddel S.W., Needham M., Liang C., Waddell L.B., Nicholson G., et al. Use of Whole-Exome Sequencing for Diagnosis of Limb-Girdle Muscular Dystrophy: Outcomes and Lessons Learned. JAMA Neurol. 2015;72:1424–1432. doi: 10.1001/jamaneurol.2015.2274. [DOI] [PubMed] [Google Scholar]
- 12.Biancalana V., Laporte J. Diagnostic use of Massively Parallel Sequencing in Neuromuscular Diseases: Towards an Integrated Diagnosis. J. Neuromuscul. Dis. 2015;2:193–203. doi: 10.3233/JND-150092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chae J.H., Vasta V., Cho A., Lim B.C., Zhang Q., Eun S.H., Hahn S.H. Utility of next generation sequencing in genetic diagnosis of early onset neuromuscular disorders. J. Med. Genet. 2015;52:208–216. doi: 10.1136/jmedgenet-2014-102819. [DOI] [PubMed] [Google Scholar]
- 14.Lek M., MacArthur D. The Challenge of Next Generation Sequencing in the Context of Neuromuscular Diseases. J. Neuromuscul. Dis. 2014;1:135–149. [PubMed] [Google Scholar]
- 15.Reddy H.M., Cho K.A., Lek M., Estrella E., Valkanas E., Jones M.D., Mitsuhashi S., Darras B.T., Amato A.A., Lidov H.G., et al. The sensitivity of exome sequencing in identifying pathogenic mutations for LGMD in the United States. J. Hum. Genet. 2017;62:243–252. doi: 10.1038/jhg.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Özyilmaz B., Kirbiyik Ö., Özdemir T.R., Kaya Özer Ö., Kutbay Y.B., Erdogan K.M., Güvenç M.S., Kale M.Y., Gazeteci H., Kiliç B., et al. Impact of next-generation sequencing panels in the evaluation of limb-girdle muscular dystrophies. Ann. Hum. Genet. 2019;83:331–347. doi: 10.1111/ahg.12319. [DOI] [PubMed] [Google Scholar]
- 17.Ankala A., da Silva C., Gualandi F., Ferlini A., Bean L.J.H., Collins C., Tanner A.K., Hegde M.R. A comprehensive genomic approach for neuromuscular diseases gives a high diagnostic yield. Ann. Neurol. 2015;77:206–214. doi: 10.1002/ana.24303. [DOI] [PubMed] [Google Scholar]
- 18.Siciliano G., Simoncini C., Giannotti S., Zampa V., Angelini C., Ricci G. Muscle exercise in limb girdle muscular dystrophies: pitfall and advantages. Acta Myol. 2015;34:3–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Chu M.L., Moran E. The Limb-Girdle Muscular Dystrophies: Is Treatment on the Horizon? Neurotherapeutics. 2018;15:849–862. doi: 10.1007/s13311-018-0648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taheri F., Taghizadeh E., Pour M.J.R., Rostami D., Renani P.G., Rastgar-Moghadam A., Hayat S.M.G. Limb-girdle Muscular Dystrophy and Therapy: Insights into Cell and Gene-based Approaches. Curr. Gene Ther. 2020;19:386–394. doi: 10.2174/1566523220666200218113526. [DOI] [PubMed] [Google Scholar]
- 21.Bengtsson N.E., Seto J.T., Hall J.K., Chamberlain J.S., Odom G.L. Progress and prospects of gene therapy clinical trials for the muscular dystrophies. Hum. Mol. Genet. 2016;25:R9–R17. doi: 10.1093/hmg/ddv420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Servián-Morilla E., Takeuchi H., Lee T.V., Clarimon J., Mavillard F., Area-Gómez E., Rivas E., Nieto-González J.L., Rivero M.C., Cabrera-Serrano M., et al. A POGLUT1 mutation causes a muscular dystrophy with reduced Notch signaling and satellite cell loss. EMBO Mol. Med. 2016;8:1289–1309. doi: 10.15252/emmm.201505815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Servián-Morilla E., Cabrera-Serrano M., Johnson K., Pandey A., Ito A., Rivas E., Chamova T., Muelas N., Mongini T., Nafissi S., et al. POGLUT1 biallelic mutations cause myopathy with reduced satellite cells, alpha-dystroglycan hypoglycosylation and a distinctive radiological pattern. Acta Neuropathol. 2020;139:565–582. doi: 10.1007/s00401-019-02117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acar M., Jafar-Nejad H., Takeuchi H., Rajan A., Ibrani D., Rana N.A., Pan H., Haltiwanger R.S., Bellen H.J. Rumi is a CAP10 domain glycosyltransferase that modifies Notch and is required for Notch signaling. Cell. 2008;132:247–258. doi: 10.1016/j.cell.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Valdivia R., Takeuchi H., Samarghandi A., Lopez M., Leonardi J., Haltiwanger R.S., Jafar-Nejad H. Regulation of mammalian Notch signaling and embryonic development by the protein O-glucosyltransferase Rumi. Development. 2011;138:1925–1934. doi: 10.1242/dev.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rana N.A., Nita-Lazar A., Takeuchi H., Kakuda S., Luther K.B., Haltiwanger R.S. O-glucose trisaccharide is present at high but variable stoichiometry at multiple sites on mouse Notch1. J. Biol. Chem. 2011;286:31623–31637. doi: 10.1074/jbc.M111.268243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonardi J., Fernandez-Valdivia R., Li Y.D., Simcox A.A., Jafar-Nejad H. Multiple O-glucosylation sites on Notch function as a buffer against temperature-dependent loss of signaling. Development. 2011;138:3569–3578. doi: 10.1242/dev.068361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeuchi H., Yu H., Hao H., Takeuchi M., Ito A., Li H., Haltiwanger R.S. O-Glycosylation modulates the stability of epidermal growth factor-like repeats and thereby regulates Notch trafficking. J. Biol. Chem. 2017;292:15964–15973. doi: 10.1074/jbc.M117.800102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramkumar N., Harvey B.M., Lee J.D., Alcorn H.L., Silva-Gagliardi N.F., McGlade C.J., Bestor T.H., Wijnholds J., Haltiwanger R.S., Anderson K.V. Protein O-Glucosyltransferase 1 (POGLUT1) Promotes Mouse Gastrulation through Modification of the Apical Polarity Protein CRUMBS2. PLoS Genet. 2015;11:e1005551. doi: 10.1371/journal.pgen.1005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haltom A.R., Lee T.V., Harvey B.M., Leonardi J., Chen Y.J., Hong Y., Haltiwanger R.S., Jafar-Nejad H. The protein O-glucosyltransferase Rumi modifies eyes shut to promote rhabdomere separation in Drosophila. PLoS Genet. 2014;10:e1004795. doi: 10.1371/journal.pgen.1004795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J., Hunt S.D., Matthias N., Servián-Morilla E., Lo J., Jafar-Nejad H., Paradas C., Darabi R. Generation of an induced pluripotent stem cell line (CSCRMi001-A) from a patient with a new type of limb-girdle muscular dystrophy (LGMD) due to a missense mutation in POGLUT1 (Rumi) Stem Cell Res. 2017;24:102–105. doi: 10.1016/j.scr.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J., Matthias N., Lo J., Ortiz-Vitali J.L., Shieh A.W., Wang S.H., Darabi R. A Myogenic Double-Reporter Human Pluripotent Stem Cell Line Allows Prospective Isolation of Skeletal Muscle Progenitors. Cell Rep. 2018;25:1966–1981.e4. doi: 10.1016/j.celrep.2018.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu N., Wu J., Ortiz-Vitali J.L., Li Y., Darabi R. Directed Differentiation of Human Pluripotent Stem Cells toward Skeletal Myogenic Progenitors and Their Purification Using Surface Markers. Cells. 2021;10 doi: 10.3390/cells10102746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ran F.A., Hsu P.D., Lin C.Y., Gootenberg J.S., Konermann S., Trevino A.E., Scott D.A., Inoue A., Matoba S., Zhang Y., Zhang F. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen F., Pruett-Miller S.M., Huang Y., Gjoka M., Duda K., Taunton J., Collingwood T.N., Frodin M., Davis G.D. High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nat. Methods. 2011;8:753–755. doi: 10.1038/nmeth.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young M.D., Wakefield M.J., Smyth G.K., Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Organ S.L., Tsao M.S. An overview of the c-MET signaling pathway. Ther. Adv. Med. Oncol. 2011;3:S7–S19. doi: 10.1177/1758834011422556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao L., Luo Y., Moloney D.J., Haltiwanger R. O-glycosylation of EGF repeats: identification and initial characterization of a UDP-glucose: protein O-glucosyltransferase. Glycobiology. 2002;12:763–770. doi: 10.1093/glycob/cwf085. [DOI] [PubMed] [Google Scholar]
- 41.Huppert S.S., Ilagan M.X.G., De Strooper B., Kopan R. Analysis of Notch function in presomitic mesoderm suggests a gamma-secretase-independent role for presenilins in somite differentiation. Dev. Cell. 2005;8:677–688. doi: 10.1016/j.devcel.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Wu W., Zhang X., Chen Y., Wang B., Wu J., Xiong Y., Jia B., Wang J., Xia J., Pu Y., et al. Evaluation of the Therapeutic Potential of Human iPSCs in a Murine Model of VML. Mol. Ther. 2021;31:121–127. doi: 10.1016/j.ymthe.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bröhl D., Vasyutina E., Czajkowski M.T., Griger J., Rassek C., Rahn H.P., Purfürst B., Wende H., Birchmeier C. Colonization of the satellite cell niche by skeletal muscle progenitor cells depends on Notch signals. Dev. Cell. 2012;23:469–481. doi: 10.1016/j.devcel.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Barton E.R., Pacak C.A., Stoppel W.L., Kang P.B. The ties that bind: functional clusters in limb-girdle muscular dystrophy. Skeletal Muscle. 2020;10:22. doi: 10.1186/s13395-020-00240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen B., Zhang W., Zhang J., Zhou J., Wang J., Chen L., Wang L., Hodgkins A., Iyer V., Huang X., Skarnes W.C. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat. Methods. 2014;11:399–402. doi: 10.1038/nmeth.2857. [DOI] [PubMed] [Google Scholar]
- 46.Mali P., Aach J., Stranges P.B., Esvelt K.M., Moosburner M., Kosuri S., Yang L., Church G.M. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 2013;31:833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mali P., Esvelt K.M., Church G.M. Cas9 as a versatile tool for engineering biology. Nat. Methods. 2013;10:957–963. doi: 10.1038/nmeth.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen F., Pruett-Miller S.M., Davis G.D. Gene editing using ssODNs with engineered endonucleases. Methods Mol. Biol. 2015;1239:251–265. doi: 10.1007/978-1-4939-1862-1_14. [DOI] [PubMed] [Google Scholar]
- 49.Baldanzi G., Graziani A. Physiological Signaling and Structure of the HGF Receptor MET. Biomedicines. 2014;3:1–31. doi: 10.3390/biomedicines3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wen Y., Bi P., Liu W., Asakura A., Keller C., Kuang S. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol. Cell Biol. 2012;32:2300–2311. doi: 10.1128/MCB.06753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang C., Wen Y., Kuroda K., Hannon K., Rudnicki M.A., Kuang S. Notch signaling deficiency underlies age-dependent depletion of satellite cells in muscular dystrophy. Dis. Model. Mech. 2014;7:997–1004. doi: 10.1242/dmm.015917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coppens S., Barnard A.M., Puusepp S., Pajusalu S., Õunap K., Vargas-Franco D., Bruels C.C., Donkervoort S., Pais L., Chao K.R., et al. A form of muscular dystrophy associated with pathogenic variants in JAG2. Am. J. Hum. Genet. 2021;108:1164. doi: 10.1016/j.ajhg.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyden S.E., Mahoney L.J., Kawahara G., Myers J.A., Mitsuhashi S., Estrella E.A., Duncan A.R., Dey F., DeChene E.T., Blasko-Goehringer J.M., et al. Mutations in the satellite cell gene MEGF10 cause a recessive congenital myopathy with minicores. Neurogenetics. 2012;13:115–124. doi: 10.1007/s10048-012-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Logan C.V., Lucke B., Pottinger C., Abdelhamed Z.A., Parry D.A., Szymanska K., Diggle C.P., van Riesen A., Morgan J.E., Markham G., et al. Mutations in MEGF10, a regulator of satellite cell myogenesis, cause early onset myopathy, areflexia, respiratory distress and dysphagia (EMARDD) Nat. Genet. 2011;43:1189–1192. doi: 10.1038/ng.995. [DOI] [PubMed] [Google Scholar]
- 55.Holterman C.E., Le Grand F., Kuang S., Seale P., Rudnicki M.A. Megf10 regulates the progression of the satellite cell myogenic program. J. Cell Biol. 2007;179:911–922. doi: 10.1083/jcb.200709083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saha M., Mitsuhashi S., Jones M.D., Manko K., Reddy H.M., Bruels C.C., Cho K.A., Pacak C.A., Draper I., Kang P.B. Consequences of MEGF10 deficiency on myoblast function and Notch1 interactions. Hum. Mol. Genet. 2017;26:2984–3000. doi: 10.1093/hmg/ddx189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S.L. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao Y., Smyth G.K., Shi W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013;41:e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smyth G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 62.Storey J.D., Tibshirani R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Darabi R., Arpke R.W., Irion S., Dimos J.T., Grskovic M., Kyba M., Perlingeiro R.C.R. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10:610–619. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the RNA-seq data reported in this paper is GEO: GSE225148.