Abstract
Introduction
Dyslipidemia is a major cardiovascular disease risk factor associated with increased mortality. The intake of plant food-derived bioactive compounds is associated with beneficial cardiovascular effects, including decreased blood lipid levels and cardiovascular risk. We aimed to evaluate the effects of anthocyanin intake on blood lipid levels by analyzing relevant randomized controlled trials.
Methods
We searched the PubMed and Embase databases using the “Patient/Population, Intervention, Comparison, and Outcomes” format to determine whether anthocyanin supplementation intervention affected blood lipid levels compared with placebo supplementation in human participants.
Results
A total of 41 studies with 2,788 participants were included in the meta-analysis. Anthocyanin supplementation significantly reduced triglyceride [standardized mean difference (SMD) = −0.10; 95% confidence interval [CI], −0.18, −0.01) and low-density lipoprotein-cholesterol (SMD = −0.16; 95% CI −0.26, −0.07) levels and increased high-density lipoprotein-cholesterol levels (SMD = 0.42; 95% CI 0.20, 0.65).
Discussion
Anthocyanin supplementation significantly improved blood lipid component levels in the included studies. Larger, well-designed clinical trials are needed to further investigate the effects of anthocyanin intake on blood lipid levels and the safety of anthocyanin supplementation for treating dyslipidemia.
Systematic review registration
https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021257087, identifier: CRD42021257087.
Keywords: metabolic syndrome, dyslipidemia, food supplementation, triglyceride, HDL cholesterol
1. Introduction
Dyslipidemia, one of the key components of metabolic syndrome, is a major risk factor for cardiovascular disease (CVD) and increased mortality (1, 2). Globally, CVD is a leading cause of morbidity and mortality; hence, the priority of developing effective means to reduce CVD risk cannot be overstated. Lifestyle changes, such as eating a healthy diet and increasing physical activity, have been shown to reduce CVD risk. Various bioactive compounds derived from plant foods are also associated with beneficial cardiovascular effects and CVD risk reduction.
Water-soluble anthocyanin pigment supplementation, which is a secondary metabolite belonging to the flavonoids family, has antioxidant and antiinflammatory properties and many health benefits including protection against carcinoma and diabetes. The positive effects of anthocyanin supplementation on dyslipidemia have been reported in several clinical trials. However, although meta-analyses of randomized controlled trials have been conducted, the relationship between anthocyanin supplementation and dyslipidemia has not been consistently reviewed to date (3–5).
Cyanidin, a type of anthocyanin, is a pigment mainly found in red-skinned or red-fleshed fruits, including apples, hawthorn berries, bilberries, cranberries, chokeberries or aronia berries, and lingonberries (6). Numerous in vitro and animal studies have reported that cyanidin may modulate the lipid metabolism (7–9); however, systematic reviews and meta-analyses on the lipid profile improvement effect of cyanidin in human are lacking.
In the present study, we aimed to analyze the effects of anthocyanin supplementation on blood lipid levels by conducting a systematic review and meta-analysis of relevant randomized control trials.
2. Materials and methods
This systematic review and meta-analysis were reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Appendix 1) (10). The protocol was registered in PROSPERO (CRD42021257087).
2.1. Search strategy
The PubMed and Embase databases were searched from their inception to June 2023 using the search terms “Anthocyanin OR Cyanidin” and “Black food” to identify relevant articles. Only studies conducted in clinical settings and published in the English language were considered. Additional articles were identified via a manual search of the reference lists of the original articles, reviews, and meta-analyses. The duplicate results were eliminated using EndNote software, and the titles and abstracts were screened by two authors (H-HJ and Y-ML) using Rayyan QCRI online software (https://www.rayyan.ai). The relevant studies then underwent dual full-text screening.
2.2. Inclusion criteria
The meta-analysis was performed using the “Patient/Population, Intervention, Comparison, and Outcomes” format to determine whether an intervention with anthocyanin supplementation (I) had any effect on blood lipids (O) compared with placebo supplementation (C) among participants (P). The outcomes of interest were levels of triglyceride (TG), total cholesterol, low-density lipoprotein (LDL)-cholesterol, and high-density lipoprotein (HDL)-cholesterol. Parallel or crossover randomized control trials were included, whereas observational studies and review articles were excluded. The intervention duration and dose were at least 2 weeks and 10 mg/day, respectively. Two authors (H-HJ and Y-ML) independently reviewed data from all the studies that fulfilled the inclusion criteria, and any conflicts were consensually resolved.
2.3. Data extraction and risk of bias assessment
Two reviewers (H-HJ and Y-ML) independently extracted the following data from the included studies: authors, year of publication, study design, place, study population (age, number, proportion of women, and health status), and intervention (sources, dose, type, duration, and concentration of anthocyanin and cyanidin) (Table 1). Two reviewers (H-HJ and Y-ML) independently assessed the risk of bias using the Jadad scale (52). This scale considers randomization, blinding, and accountability of all patients. If all these items are regarded as appropriate, a score of 5 is assigned. The Jadad scale scores of ≥3 and <3 were considered to have a low and high risk of bias, respectively. Any disagreements were consensually resolved.
Table 1.
Characteristics and findings of the studies included in the meta-analysis.
| Study (Ref.) | Design | Place | Participants (% of women) | Age (in years) | Duration (weeks) | Healthy status | Food sources | Intervention types | Anthocyanin (mg/day) | Cyanidin (mg/day) | Jadad scale |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Murkovic et al. (11) | PA | Austria | 34 (41%) | 29 | 2 | Healthy | Elderberry | Capsule | 120 | 120 | 3 |
| Hansen et al. (12) | PA | Denmark | 50 (56%) | 53 | 4 | Healthy | Red grape | Tablets | 28.8 or 57.5 | NI | 4 |
| Zern et al. (13) | CO | USA | 44 (100%) | 48 | 4 | Healthy | Grape | Lyophilized powder | 27.7 | NI | 1 |
| Cerda et al. (14) | PA | Spain | 30 (0%) | 62 | 5 | Patients with stable COPD | Pomegranates | Juice | 190 | NI | 3 |
| Karlsen et al. (15) | PA | USA | 118 (51%) | 61 | 3 | Healthy | Bilberry and blackcurrant | Capsule | 300 | NI | 1 |
| Erlund et al. (16) | PA | Finland | 71 (65%) | 58 | 8 | With CVD risk | Bilberry, lingonberry, blackcurrant, and strawberry | Mixed types (crushed, purée, and juice) | 515 | 515 | 2 |
| Curtis et al. (17) | PA | UK | 50 (100%) | 58 | 12 | Healthy | Elderberry | Capsule | 500 | 500 | 4 |
| Qin et al. (18) | PA | China | 120 (65%) | 55 | 12 | Healthy | Bilberry and blackcurrant | Capsule | 320 | 105.6 | 4 |
| Basu et al. (19) | PA | USA | 48 (97%) | 50 | 8 | MetS | Blueberry | Lyophilized powder | 742 | NI | 2 |
| Stull et al. (20) | PA | USA | 32 (84%) | 51 | 6 | Obese | Blueberry | Lyophilized powder | 668 | NI | 4 |
| Basu et al. (21) | PA | USA | 36 (100%) | 52 | 8 | MetS | Cranberry | Juice | 24.8 | 12.6 | 3 |
| Dohadwala et al. (22) | CO | USA | 44 (32%) | 62 | 4 | CAD | Cranberry | Juice | 94 | NI | 4 |
| Zhu et al. (23) | PA | China | 146 (58%) | 40–65 | 12 | Dyslipidemia | Bilberry and blackcurrant | Capsule | 320 | 105.6 | 3 |
| Hassellund et al. (24) | CO | Norway | 27 (0%) | 41 | 4 | Pre-hypertensive | Bilberry and blackcurrant | Capsule | 640 | 211.2 | 5 |
| Riso et al. (25) | CO | USA | 18 (0%) | 48 | 6 | Healthy | Wild blueberry | Lyophilized powder | 375 | NI | 5 |
| Flammer et al. (26) | PA | USA | 69 (45%) | 50 | 16 | With CVD risk | Cranberry | Juice | 69.5 | NI | 3 |
| Wright et al. (27) | PA | Australia | 16 (0%) | 53 | 4 | Overweight and Obese | Purple carrot | Dried | 118.5 | 118.5 | 4 |
| Zhu et al. (28) | PA | China | 146 (58%) | 56 | 24 | Dyslipidemia | Bilberry and blackcurrant | Capsule | 320 | 105.6 | 4 |
| Basu et al. (29) | PA | USA | 60 (92%) | 49 | 12 | MetS | Strawberry | Freeze-dried | 78 or 155 | 78 or 155 | 2 |
| Lynn et al. (30) | PA | UK | 43 (63%) | 38 | 6 | Healthy | Tart cherry | Juice | 273.5 | NI | 3 |
| Soltani et al. (31) | PA | Iran | 50 (50%) | 47 | 4 | Dyslipidemia | Whortleberry | Capsule | 90 | 90 | 5 |
| Li et al. (32) | PA | China | 58 (41%) | 58 | 24 | T2D | Bilberry and blackcurrant | Capsule | 320 | 105.6 | 3 |
| Novotny et al. (33) | PA | USA | 56 (54%) | 51 | 8 | Healthy | Cranberry | Juice | 20.6 | NI | 5 |
| Soltani et al. (34) | PA | Iran | 60 (35%) | 50 | 6 | T2D | Cornelian cherry | Capsule | 600 | NI | 4 |
| Stull et al. (35) | PA | USA | 44 (64%) | 57 | 6 | MetS | Blueberry | Lyophilized powder | 580.6 | NI | 5 |
| Zhang et al. (36) | PA | China | 74 (47%) | 46 | 12 | NAFLD | Bilberry and blackcurrant | Capsule | 320 | 105.6 | 5 |
| Lee et al. (37) | PA | Korea | 63 (38%) | 31 | 8 | Overweight and Obese | Black soybean | Capsule | 31.5 | 21.5 | 4 |
| Zhang et al. (38) | PA | China | 146 (58%) | 56 | 24 | Dyslipidemia | NI | Capsule (Polyphenols AS) | 320 | NI | 3 |
| Xie et al. (39) | PA | China | 160 (66%) | 61 | 12 | Pre-diabetes | Bilberry and blackcurrant | Capsule | 320 | 105.6 | 5 |
| Yang et al. (40) | PA | USA | 49 (51%) | 35 | 12 | Healthy | Aronia | Capsule | 45.1 | 45.1 | 5 |
| Hollands et al. (41) | CO | UK | 38 (51%) | 52 | 4 | Healthy | Blood orange | Juice | 50 | NI | 3 |
| Kim et al. (42) | PA | USA | 37 (70%) | 44 | 12 | MetS | Açaí berry | Juice | 99.8 | 99.8 | 4 |
| Bakuradze et al. (43) | PA | Germany | 57 (0%) | 24 | 24 | Healthy | Red grape, lingonberry, blueberry, and aronia berry | Juice | 205.9 | 75.4 | 2 |
| Curtis et al. (44) | PA | UK | 115 (32%) | 63 | 9 | MetS | Blueberry | Lyophilized powder | 182 or 364 | NI | 5 |
| Guo et al. (45) | PA | China | 107 (67%) | 25 | 2 | Healthy | Bilberry and blackcurrant | capsule | 20, 40, 80, 160, 320 | 6.6, 13.2, 26.4, 52.8, 105.6 | 5 |
| Stote et al. (46) | PA | USA | 52 (0%) | 67 | 8 | T2D | Blueberry | Lyophilized powder | 261.8 | NI | 4 |
| Chan et al. (47) | CO | China | 20 (55%) | 56 | 4 | T2D | Bilberry | capsule | >350 | NI | 5 |
| Sekikawa et al. (48) | PA | Japan | 32 (50%) | 37 | 6 | Healthy | Bilberry | Capsule | 43.2 | NI | 5 |
| Xu et al. (49) | PA | China | 176 (74%) | 57 | 12 | Dyslipidemia | Bilberry and blackcurrant | Capsule | 40, 80, 320 | 13.2, 26.4, 105.6 | 5 |
| Yang et al. (50) | PA | China | 140 (67%) | 61 | 12 | prediabetes | Bilberry and black currant | Capsule (Biolink AS) | 320 | NI | 5 |
| Aboufarrang et al. (51) | CO | UK | 52 (54%) | 63 | 4 | Dyslipidemia | Bilberry or black rice | Capsule | 320 | 54 or 297.8 | 5 |
PA, parallel-arm; CO, crossover; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; NI, no information; CAD, coronary artery disease; T2D, type 2 diabetes; NAFLD, non-alcoholic fatty liver disease; MetS, metabolic syndrome.
2.4. Publication bias
We used Egger's regression test for funnel plot asymmetry to assess the potential publication bias of the included studies (53). P-values of <0.05 were considered significant.
2.5. Statistical analysis
We used the standardized mean difference (SMD) with the 95% confidence interval (CI) as effect size measures. In studies where the mean difference was not reported, the mean differences were calculated by subtracting the baseline mean from the post-intervention mean; the standard deviation (SD) differences were estimated using the following formula:
where SDB is the baseline SD and SDF is the SD of the final measures in the study (54).
The correlation value was conservatively set at 0.5 to calculate the change in SD (55). Owing to the clinical heterogeneity of the studies, including differences in study design, doses, and intervention, a random-effects model was used for the meta-analysis of quantitative data. A forest plot was mapped to indicate the pooled SMD and 95% CI. Between-study heterogeneity was assessed by forest plot visualization. Subsequently, the Q-test and I2 statistic were used to quantitatively evaluate the statistical heterogeneity. In general, a P-value of <0.1 for Q statistics and an I2 > 50% indicate considerable heterogeneity (54). Sensitivity analysis was conducted to investigate the effect of each study on the pooled effect size if the I2 values were >50%. Furthermore, a subgroup analysis was performed to explore the possible source of heterogeneity for the following subsets: participants (number and healthy status), studies (design and area), or interventions (duration and dosage). All analyses were performed using R statistical software (Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Identification and selection of studies
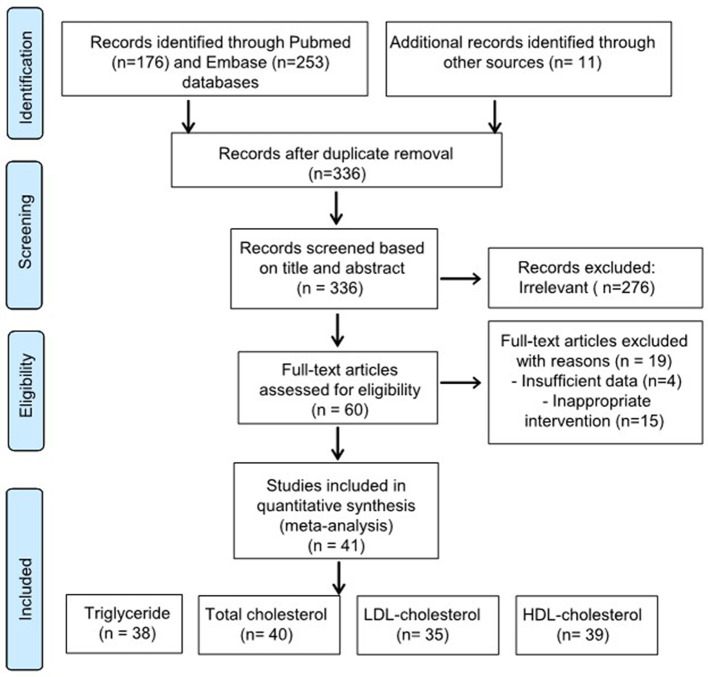
A total of 440 studies were initially identified: 429 from the database search and 11 from the manual search. A total of 104 duplicate studies were removed, and the titles and abstracts of 336 studies were screened by two authors. Subsequently, 276 studies were excluded. The remaining 60 studies underwent double full-text review. Subsequently, 19 studies were excluded: 4 were excluded because of insufficient data presentation (56–59) and 15 were excluded because of inappropriate interventions (60–74). Finally, 41 studies were included in the systematic review. The PRISMA flowchart for the selection process is presented in Figure 1.
Figure 1.
Preferred reporting items for systematic review and meta-analyses flowchart.
3.2. Description of included studies
A total of 41 studies that enrolled 2,788 participants were included in the systematic review and meta-analysis. The characteristics of the included studies are summarized in Table 1. Among the 41 studies, 34 were parallel-arm studies (11, 12, 14–21, 23, 26–40, 42–46, 48–50), whereas 7 were crossover studies (13, 22, 24, 25, 41, 47, 51). Most studies included both sexes, three studies included only females (13, 17, 21) and six included only males (14, 24, 25, 27, 43, 46). All studies were conducted on adults, and the average age of the participants ranged from 24 to 67 years. Among the 41 studies, 14 were conducted in the United States (13, 15, 19–22, 25, 26, 29, 33, 35, 40, 42, 46), 11 in China (18, 23, 28, 32, 36, 38, 39, 45, 47, 49, 50), 5 in the UK (17, 30, 41, 44, 51), 2 in Iran (31, 34), and 1 each in Austria (11), Denmark (12), Spain (14), Finland (16), Norway (24), Australia (27), Korea (37), Germany (43), and Japan (48). Twenty-six studies were conducted in individuals with diseases such as chronic obstructive pulmonary disease (14), dyslipidemia (23, 28, 31, 38, 49), hypertension (24), obesity (20, 27, 37), type 2 diabetes mellitus (32, 34, 39, 46, 47, 50), CVDs (16, 22, 26), non-alcoholic fatty liver disease (36), and metabolic syndrome (19, 21, 29, 35, 42, 44), and 15 studies were conducted in healthy individuals (11–13, 15, 17, 18, 25, 30, 33, 40, 41, 43, 45, 48, 51). In total, 15 studies focused on an intervention with bilberry and black currant (15, 16, 18, 23, 24, 28, 32, 36, 39, 45, 47–51); 15 on elderberry (11, 17), blueberry (19, 20, 25, 35, 43, 46), cranberry (21, 22, 26, 33), whortleberry (31), açaí berry (42), or mixed fruits (43) supplementation; and 10 on grapes (12, 13), pomegranates (14), purple carrot (27), tart cherry (30), cornelian cherry (34), black soybean (37), aronia berries (40), strawberry (29), blood orange (41), and black rice (51) supplementation. One study (38) that reported on anthocyanin supplementation did not mention the food source. The duration of the interventions in the included studies varied (2–24 weeks). Among the 41 studies, 22 used supplements of concentrated anthocyanins in capsule (11, 15, 17, 18, 23, 24, 28, 31, 32, 34, 36–40, 45, 47–51) or tablet (12) form, 9 studies (14, 21, 22, 26, 30, 33, 41–43) used juice form supplements, and 9 other studies (13, 19, 20, 25, 27, 29, 35, 44, 46) used dried powder form supplements. One study (16) used mixed-type supplements, including juice and puree forms. The anthocyanin concentration ranged from a minimum of 20 mg/day to a maximum of 742 mg/day, with an average of 238.5 mg/day. Among the 41 studies, 21 presented intake levels of cyanidin with total anthocyanins. The total anthocyanin intake was 215.3 mg/day in 21 studies, which was marginally lower than the intake of 238.5 mg/day in all 41 studies. The cyanidin concentration of supplements ranged from a minimum of 6.6 mg/day to a maximum of 515 mg/day, with an average of 116.5 mg/day.
3.3. Potential sources of bias
The risk of bias assessments for individual studies are presented in Supplementary Table 1. Thirty-five of the included studies had a low risk of bias (Jadad score ≥ 3) and six had a high risk of bias (Jadad score <3). All 41 studies were randomized; however, only 20 appropriately described the method of randomization. Of the 33 studies that mentioned blinding, only 26 described the method of blinding. In 36 of the included 41 studies, the reasons for withdrawal or dropout of participants were described.
3.4. Outcomes
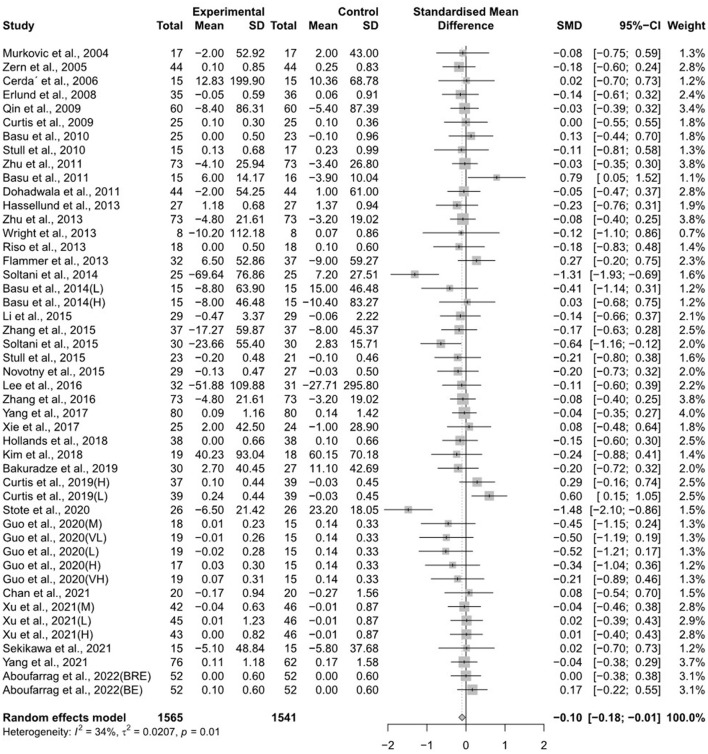
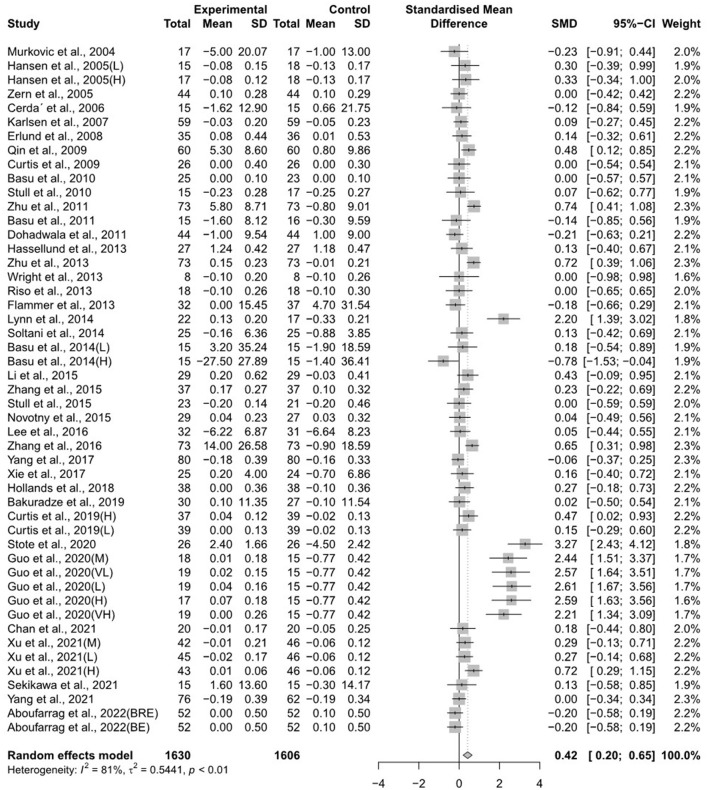
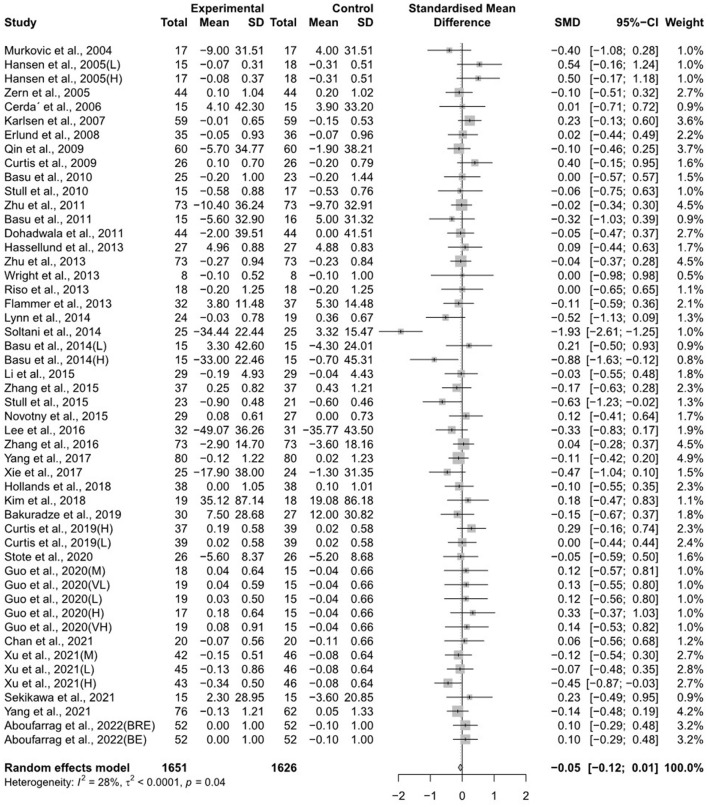
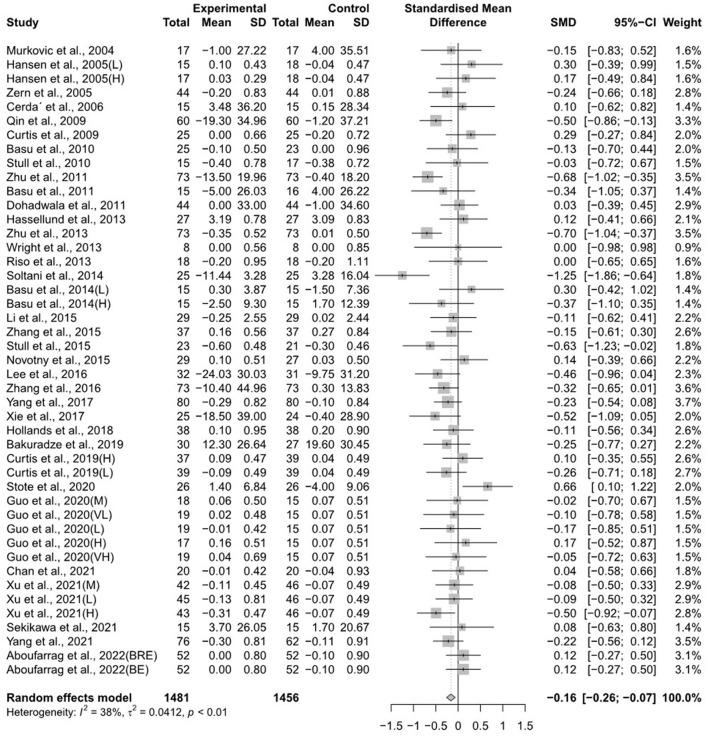
The forest plots for the overall random effects of anthocyanin-rich food supplementation on the levels of TG, total cholesterol, LDL-cholesterol, and HDL-cholesterol are illustrated in Figures 2–5. The overall pooled statistics revealed that the TG levels of participants (47 results from 38 studies) were significantly reduced (SMD = −0.10; 95% CI −0.18, −0.01), and a small degree of heterogeneity was observed in the analysis of TG (I2 = 34% and P = 0.01) (Figure 2). A total of 40 studies that included 50 effect sizes investigated the impact of anthocyanin supplementation on total cholesterol levels (Figure 3). The pooled statistics revealed that the SMD of total cholesterol was −0.05 (95% CI −0.12, 0.01), and a small degree of between-study heterogeneity was observed in the analysis (I2 = 28% and P = 0.04). Overall, 44 effect sizes from 35 studies were included in the analysis of the effect of anthocyanin supplements on LDL-cholesterol levels and revealed a significant effect (SMD = −0.16; 95% CI −0.26, −0.07) (Figure 4). The forest plot for the overall effect of anthocyanin supplements on HDL-cholesterol levels, which included the results of 39 studies, is presented in Figure 5. The SMD of the overall pooled HDL-cholesterol levels was 0.42 (95% CI 0.20, 0.65), which was a considerable increase. Statistical heterogeneity was observed in the analysis of LDL-cholesterol (I2 = 38% and P = 0.01) and HDL-cholesterol (I2 = 81% and P < 0.01) levels. Sensitivity analysis of the effect of anthocyanin supplementation on HDL-cholesterol levels revealed that the removal of any study did not alter the significance of the pooled effect size (Supplementary Figure 1). Note that, for the results of the meta-analysis of the 21 studies that included cyanidin intake, the effect on blood lipids had a greater impact than the results of all 41 studies (Supplementary Figures 2–5); moreover, cyanidin significantly reduced TG (SMD = −0.10; 95% CI −0.19, −0.01) and LDL-cholesterol (SMD = −0.23; 95% CI −0.37, −0.10) levels and increased HDL-cholesterol (SMD = 0.50; 95% CI 0.18, 0.82) levels.
Figure 2.
A forest plot of the change in the standardized mean differences (with 95% confidence intervals) of triglycerides in participants administered anthocyanin supplements compared with the control.
Figure 5.
A forest plot of the change in the standardized mean differences (with 95% confidence intervals) of high-density lipoprotein-cholesterol in participants administered anthocyanin supplements compared with the control.
Figure 3.
A forest plot of the change in the standardized mean differences (with 95% confidence intervals) of total cholesterol in participants administered anthocyanin supplements compared with the control.
Figure 4.
A forest plot of the change in the standardized mean differences (with 95% confidence intervals) of low-density lipoprotein-cholesterol in participants administered anthocyanin supplements compared with the control.
3.5. Subgroup analyses
Furthermore, we performed subgroup analyses to explore the possible source of heterogeneity among the studies (Table 2). The effect of anthocyanin supplementation on HDL-cholesterol was significantly greater in the subgroup with a normal cholesterol level <200 mg/dL than in the subgroup with a higher cholesterol level ≥ 200 mg/dL at the baseline (P = 0.020). However, heterogeneity (I2) was 51% in hypercholesterolemic participants (total cholesterol ≥ 200) and lower than 91% in normal-level participants. The effect on HDL-cholesterol exhibited a higher increase in the subgroup with an average age <40 years (SMD = 1.30; 95% CI 0.54, 2.05) than in the subgroup with an average age ≥40 years (SMD = 0.21; 95% CI 0.07, 0.35). In particular, the effect of anthocyanin supplementation on HDL-cholesterol was significantly higher in studies with a low risk of bias than in the groups with a high risk of bias (P = 0.002). Subgroup analysis among study design, anthocyanin dosage, and risk of bias indicated decreased heterogeneity within subgroups. However, substantial unexplained heterogeneity was observed between these subgroups.
Table 2.
Subgroup analysis for the effect of anthocyanin on high-density lipoprotein-cholesterol.
| Subgroup | Effect size | I2 2 (%) | P-value3 | |
|---|---|---|---|---|
| K 1 | (95% CI) | |||
| Overall | 49 | 0.42 (0.20, 0.65) | 81 | |
| Study design | ||||
| Parallel study | 41 | 0.52 (0.25, 0.79) | 83 | <0.001 |
| Crossover study | 8 | −0.04 (−0.20, 0.12) | 0 | |
| Study duration | ||||
| <8 weeks | 24 | 0.59 (0.18, 0.99) | 85 | 0.220 |
| ≥8 weeks | 25 | 0.30 (0.08, 0.51) | 75 | |
| Cholesterol level 4 | ||||
| Normal (<200 mg/dL) | 18 | 1.00 (0.42, 1.57) | 91 | 0.020 |
| Higher (≥ 200 mg/dL) | 30 | 0.19 (0.06, 0.31) | 51 | |
| Study area | ||||
| East | 20 | 0.80 (0.41, 1.19) | 85 | 0.007 |
| West | 29 | 0.17 (−0.06, 0.40) | 72 | |
| Participant's age | ||||
| <40 years old | 11 | 1.30 (0.54, 2.05) | 91 | 0.007 |
| ≥40 years old | 38 | 0.21 (0.07, 0.35) | 68 | |
| No. of participants | ||||
| <50 | 19 | 0.77 (0.27, 1.27) | 86 | 0.052 |
| ≥50 | 30 | 0.24 (0.06, 0.42) | 75 | |
| Anthocyanin dosage | ||||
| <50 mg/day | 10 | 0.54 (−0.06, 1.14) | 83 | 0.099 |
| ≥50 and <350 mg/day | 30 | 0.50 (0.18, 0.81) | 85 | |
| ≥350 mg/day | 9 | 0.14 (−0.05, 0.32) | 0 | |
| Formula | ||||
| Low processing | 20 | 0.15 (0.02, 0.27) | 79 | 0.194 |
| High processing | 29 | 0.54 (0.26, 0.83) | 81 | |
| Risk of bias | ||||
| Low risk | 42 | 0.51 (0.25, 0.77) | 83 | 0.002 |
| High risk | 7 | 0.01(−0.17, 0.20) | 0 | |
1Number of studies combined. 2Overall test for heterogeneity within subgroups by the random effects model. 3Test for subgroup difference by random effect model. 4Excluding one study in which total cholesterol level was not presented at the baseline.
In addition, the difference in the effect of anthocyanin supplementation on lipid improvement was compared by dividing healthy participants and participants with dyslipidemia into subgroups (Supplementary Table 2). As a result of performing subgroup analysis according to dyslipidemia status, there was no significant difference between the two groups in TG and TC levels. However, the effect of anthocyanin supplementation on LDL-cholesterol (P = 0.027) and HDL-cholesterol (P = 0.020) was significantly different between the two groups. In particular, LDL-cholesterol showed a significant reduction only in dyslipidemia participants.
3.6. Publication bias
Publication bias was observed according to the results of Egger's test and demonstrated significant bias for LDL-cholesterol (P = 0.0253) and HDL-cholesterol (P = 0.0087) levels. Despite signs of publication bias using Egger's test, the Duval and Tweedie trim and fill method (75) revealed that the adjusted estimate remained significant (Supplementary Table 3 and Supplementary Figure 6). LDL-cholesterol (SMD = −0.13; 95% CI −0.21, −0.05) on anthocyanin supplementation still showed a significant reduction effect with zero heterogeneity (I2 = 0%) except for four outlier studies (23, 28, 31, 46). Excluding 10 outlier studies (29, 30, 45, 46, 51), the effect size of HDL-cholesterol decreased compared with the overall study results but showed low heterogeneity (I2= 31%) and a significantly increasing effect (SMD = 0.20; 95% CI 0.10, 0.30). There was no demonstrable publication bias for TG (P = 0.0697) and total cholesterol (P = 0.5943).
3.7. Adverse events
Among the 41 studies, 37 studies for anthocyanin supplements reported no serious adverse events leading to withdrawal. In four studies (19, 24, 40, 44), adverse events leading to withdrawal were reported in the anthocyanin supplement groups. In total, 6 studies (24, 37, 40, 47, 49, 50) reported mild adverse events, including dark stools, headache, insomnia, and diarrhea, and 17 studies (12–16, 20–22, 25–27, 33, 35, 39, 42, 43, 51) did not report any adverse events. The distribution of adverse events in the treatment groups and placebo is presented in Supplementary Table 4.
4. Discussion
The present study aimed to investigate the effects of anthocyanin supplements on blood lipid levels by focusing on the results of randomized controlled trials. A total of 41 studies that enrolled 2,788 participants were included in the meta-analysis, which revealed that anthocyanin supplements had significantly improved blood lipid levels; they reduced TG and LDL-cholesterol levels and increased HDL-cholesterol levels.
A meta-analysis of 12–13 randomized controlled trials reported that anthocyanin supplements did not significantly improve blood lipid levels; however, in the subgroup analyses, decreased total cholesterol and LDL-cholesterol levels were observed when anthocyanin supplementation exceeded 300 mg/day (3). Another meta-analysis of 27 trials indicated that anthocyanin supplements were associated with decreased total cholesterol and LDL-cholesterol levels and marginally increased HDL-cholesterol levels in both healthy subjects and in those with cardiometabolic disease; however, no significant effects were observed on TG levels (4). Shah and Shah (5) reported that anthocyanin supplements significantly reduced TG and LDL-cholesterol levels and increased HDL-cholesterol levels in both the healthy and patient populations, with no significant effect on total cholesterol levels through a meta-analysis of 9–13 randomized controlled trials. The present meta-analysis included 41 studies (2,788 participants) with inconsiderable heterogeneity and publication bias. Therefore, our results are expected to provide more reliable and integrative insight into the effect of anthocyanin supplements on blood lipid levels in both healthy and patient populations.
Several studies have elucidated the mechanisms responsible for the beneficial effects of anthocyanins on blood lipid levels. Anthocyanin reportedly increases cholesterol efflux from macrophages, contributing to reverse cholesterol transport (18, 76, 77). Moreover, it decreases the mass and activity of plasma cholesteryl ester transfer protein, which is associated with increased efficiency of reverse cholesterol transport (18). Anthocyanins can activate AMP-activated protein kinase, which inhibits cholesterol and TG synthesis by HMG-CoA and acetyl-CoA carboxylase inhibition, respectively (78). Furthermore, anthocyanin dose-dependently reduces the micellar solubility of cholesterol and exhibits a significant reduction in cholesterol uptake in Caco-2 cells (79–81). It reportedly increased the excretion of fecal neutral and acidic sterols in experimental animals fed a cholesterol-enriched diet (82, 83).
In the present study, anthocyanin supplements did not have a significant effect on total cholesterol levels. This was consistent with the report of Shah and Shah (5). However, anthocyanin supplementation led to significant improvements in the lipid profiles (total cholesterol, TG, HDL-cholesterol, and LDL-cholesterol) of patients with dyslipidemia (84). In addition, Daneshzad (3) showed that anthocyanin supplementation had significant effects on total cholesterol for more than 300 mg/day for more than 12 weeks. However, our subgroup analysis did not indicate significant differences between subgroups according to cholesterol levels (≥ 200 mg/dL or <200 mg/dL) or dosage or duration (data not shown). Meanwhile, the total cholesterol level did not predict the risk of CVD and coronary heart disease compared with TG, HDL-cholesterol, and lipid ratios (85, 86). Further studies are needed to identify the protective effects of anthocyanins on increased morbidity or mortality using lipid ratios such as the total cholesterol:HDL-cholesterol and TG:HDL-cholesterol ratio.
CVD can be prevented by appropriately addressing the major risk factors such as dyslipidemia, hypertension, oxidative stress, and inflammatory stress (87). The present study revealed that anthocyanins could help reduce CVD risk by decreasing blood TG and LDL-cholesterol levels and increasing HDL-cholesterol levels. Several studies have reported that anthocyanins may have a significant blood-pressure-lowering activity (88). Furthermore, anthocyanins have potent antioxidant and antiinflammatory effects (89). In addition, anthocyanin supplementation improved vascular function, which is a strong predictor for CVD (90). Thus, increased intake of anthocyanin-rich foods may effectively reduce CVD risk.
Among the included studies, 21 presented intake levels of cyanidin. The average of total anthocyanin intake was 215.3 mg/day in those studies, and the cyanidin concentration was 116.5 mg/day (6.6~515.0 mg/day). The effect of cyanidin on blood lipids had a greater impact than the results of total anthocyanin. One recent study (51) failed to compare the effect of the two anthocyanin types (cyanidin-type vs. delphinidin-type) on blood lipid levels. Further study is needed to identify and clarify the mechanism of anthocyanin's structure on each bioactivity.
The strengths of the present study are the inclusion of all clinical trials that investigated the effects of anthocyanin on blood lipid levels. However, this study has some limitations. First, only articles in the English language were included in the meta-analysis, which raised concerns regarding the identification and selection of relevant studies. Second, significant between-study heterogeneity unexplained by differences in the methods of anthocyanin intervention, study design, and study population was observed.
In conclusion, we evaluated the effects of anthocyanin supplementation on blood lipid levels via a systematic review and meta-analysis of randomized controlled trials. Our results revealed that anthocyanin supplementation had a significant effect on TG, LDL-cholesterol, and HDL-cholesterol levels. Larger, well-designed clinical trials are needed to investigate the efficacy and safety of anthocyanin supplementation for the treatment of dyslipidemia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
H-HJ and Y-ML: conceptualization, data curation, and writing—original draft preparation. Y-ML and I-GH: writing—reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding Statement
This research was supported by the research program for Agricultural Science & Technology Development under the National Institute of Agricultural Science (NAS)-Rural Development Administration (RDA), South Korea, under grant numbers PJ01420101 and PJ01703102.
Abbreviations
CI, Confidence interval; CVD, Cardiovascular disease; HDL, High-density lipoprotein; MetS, Metabolic syndrome; MI, Myocardial Infraction; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RDA, Rural Development Administration; SD, Standard deviation; SMD, Standardized mean difference.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1207751/full#supplementary-material
References
- 1.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. (2005) 28:1769–78. 10.2337/diacare.28.7.1769 [DOI] [PubMed] [Google Scholar]
- 2.Cornier M-A, Dabelea d, hernandez tl, lindstrom rc, steig aj, stob nr, et al. the metabolic Syndrome. Endocr Rev. (2008) 29:777–822. 10.1210/er.2008-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daneshzad E, Shab-Bidar S, Mohammadpour Z, Djafarian K. Effect of anthocyanin supplementation on cardio-metabolic biomarkers: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. (2019) 38:1153–65. 10.1016/j.clnu.2018.06.979 [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Ling W, Du Z, Chen Y, Li D, Deng S, et al. Effects of anthocyanins on cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. (2017) 8:684–93. 10.3945/an.116.014852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah K, Shah P. Effect of anthocyanin supplementations on lipid profile and inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Cholesterol. (2018) 2018:93. 10.1155/2018/8450793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stintzing FC, Stintzing AS, Carle R, Frei B, Wrolstad RE. Color and antioxidant properties of cyanidin-based anthocyanin pigments. J Agric Food Chem. (2002) 50:6172–81. 10.1021/jf0204811 [DOI] [PubMed] [Google Scholar]
- 7.Jia Y, Hoang MH, Jun H-J, Lee JH, Lee S-J. Cyanidin, a natural flavonoid, is an agonistic ligand for liver × receptor alpha and beta and reduces cellular lipid accumulation in macrophages and hepatocytes. Bioorg Med Chem Lett. (2013) 23:4185–90. 10.1016/j.bmcl.2013.05.030 [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Zhang M, Wang Z, Guo Z, Wang Z, Chen Q. Cyanidin-3-O-glucoside attenuates endothelial cell dysfunction by modulating Mir-204-5p/Sirt1-mediated inflammation and apoptosis. Biofactors. (2020) 46:803–12. 10.1002/biof.1660 [DOI] [PubMed] [Google Scholar]
- 9.Li X, Shi Z, Zhu Y, Shen T, Wang H, Shui G, et al. Cyanidin-3-O-glucoside improves non-alcoholic fatty liver disease by promoting pink1-mediated mitophagy in mice. Br J Pharmacol. (2020) 177:3591–607. 10.1111/bph.15083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murkovic M, Abuja PM, Bergmann AR, Zirngast A, Adam U, Winklhofer-Roob BM, et al. Effects of elderberry juice on fasting and postprandial serum lipids and low-density lipoprotein oxidation in healthy volunteers: a randomized, double-blind, placebo-controlled study. Eur J Clin Nutr. (2004) 58:244–9. 10.1038/sj.ejcn.1601773 [DOI] [PubMed] [Google Scholar]
- 12.Hansen AS, Marckmann P, Dragsted LO, Finne Nielsen IL, Nielsen SE, Gronbaek M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease. Eur J Clin Nutr. (2005) 59:449–55. 10.1038/sj.ejcn.1602107 [DOI] [PubMed] [Google Scholar]
- 13.Zern TL, Wood RJ, Greene C, West KL, Liu Y, Aggarwal D, et al. Grape polyphenols exert a cardioprotective effect in pre-and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr. (2005) 135:1911–7. 10.1093/jn/135.8.1911 [DOI] [PubMed] [Google Scholar]
- 14.Cerda B, Soto C, Albaladejo MD, Martinez P, Sanchez-Gascon F, Tomas-Barberan F, et al. Pomegranate juice supplementation in chronic obstructive pulmonary disease: a 5-week randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. (2006) 60:245–53. 10.1038/sj.ejcn.1602309 [DOI] [PubMed] [Google Scholar]
- 15.Karlsen A, Retterstøl L, Laake P, Paur I, Kjølsrud-Bøhn S, Sandvik L, et al. Anthocyanins inhibit nuclear factor-κB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr. (2007) 137:1951–4. 10.1093/jn/137.8.1951 [DOI] [PubMed] [Google Scholar]
- 16.Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, et al. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr. (2008) 87:323–31. 10.1093/ajcn/87.2.323 [DOI] [PubMed] [Google Scholar]
- 17.Curtis PJ, Kroon PA, Hollands WJ, Walls R, Jenkins G, Kay CD, et al. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J Nutr. (2009) 139:2266–71. 10.3945/jn.109.113126 [DOI] [PubMed] [Google Scholar]
- 18.Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, et al. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. (2009) 90:485–92. 10.3945/ajcn.2009.27814 [DOI] [PubMed] [Google Scholar]
- 19.Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. (2010) 140:1582–7. 10.3945/jn.110.124701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. (2010) 140:1764–8. 10.3945/jn.110.125336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu A, Betts NM, Ortiz J, Simmons B, Wu M, Lyons TJ. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutrition Research. (2011) 31:190–6. 10.1016/j.nutres.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dohadwala MM, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. (2011) 93:934–40. 10.3945/ajcn.110.004242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Y, Xia M, Yang Y, Liu F, Li Z, Hao Y, et al. Purified anthocyanin supplementation improves endothelial function via no-CGMP activation in hypercholesterolemic individuals. Clin Chem. (2011) 57:1524–33. 10.1373/clinchem.2011.167361 [DOI] [PubMed] [Google Scholar]
- 24.Hassellund SS, Flaa A, Kjeldsen SE, Seljeflot I, Karlsen A, Erlund I, et al. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: a double-blind randomized placebo-controlled crossover study. J Hum Hypertens. (2013) 27:100–6. 10.1038/jhh.2012.4 [DOI] [PubMed] [Google Scholar]
- 25.Riso P, Klimis-Zacas D, Del Bo C, Martini D, Campolo J, Vendrame S, et al. Effect of a wild blueberry (vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur J Nutr. (2013) 52:949–61. 10.1007/s00394-012-0402-9 [DOI] [PubMed] [Google Scholar]
- 26.Flammer AJ, Martin EA, Gössl M, Widmer RJ, Lennon RJ, Sexton JA, et al. Polyphenol-rich cranberry juice has a neutral effect on endothelial function but decreases the fraction of osteocalcin-expressing endothelial progenitor cells. Eur J Nutr. (2013) 52:289–96. 10.1007/s00394-012-0334-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright OR, Netzel GA, Sakzewski AR. A randomized, double-blind, placebo-controlled trial of the effect of dried purple carrot on body mass, lipids, blood pressure, body composition, and inflammatory markers in overweight and obese adults: the quench trial. Can J Physiol Pharmacol. (2013) 91:480–8. 10.1139/cjpp-2012-0349 [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Ling W, Guo H, Song F, Ye Q, Zou T, et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis. (2013) 23:843–9. 10.1016/j.numecd.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 29.Basu A, Betts NM, Nguyen A, Newman ED, Fu D, Lyons TJ. Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J Nutr. (2014) 144:830–7. 10.3945/jn.113.188169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynn A, Mathew S, Moore CT, Russell J, Robinson E, Soumpasi V, et al. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: a randomised controlled trial. Plant Foods Hum Nutr. (2014) 69:122–7. 10.1007/s11130-014-0409-x [DOI] [PubMed] [Google Scholar]
- 31.Soltani R, Hakimi M, Asgary S, Ghanadian SM, Keshvari M, Sarrafzadegan N. Evaluation of the effects of vaccinium arctostaphylos L. Fruit extract on serum lipids and hs-crp levels and oxidative stress in adult patients with hyperlipidemia: a randomized, double-blind, placebo-controlled clinical trial evid based complement. Alternat Med. (2014) 2014:217451. 10.1155/2014/217451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D, Zhang Y, Liu Y, Sun R, Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr. (2015) 145:742–8. 10.3945/jn.114.205674 [DOI] [PubMed] [Google Scholar]
- 33.Novotny JA, Baer DJ, Khoo C, Gebauer SK, Charron CS. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating c-reactive protein, triglyceride, and glucose concentrations in adults. J Nutr. (2015) 145:1185–93. 10.3945/jn.114.203190 [DOI] [PubMed] [Google Scholar]
- 34.Soltani R, Gorji A, Asgary S, Sarrafzadegan N, Siavash M. Evaluation of the effects of Cornus mas L. fruit extract on glycemic control and insulin level in type 2 diabetic adult patients: a randomized double-blind placebo-controlled clinical trial. Evid Based Complement Alternat Med. (2015) 2015:740954. 10.1155/2015/740954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stull AJ, Cash KC, Champagne CM, Gupta AK, Boston R, Beyl RA, et al. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. Nutrients. (2015) 7:4107–23. 10.3390/nu7064107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang PW, Chen FX Li D, Ling WH, Guo HH. A consort-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine. (2015) 94:e758. 10.1097/MD.0000000000000758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee M, Sorn SR, Park Y, Park HK. Anthocyanin rich-black soybean testa improved visceral fat and plasma lipid profiles in overweight/obese Korean adults: a randomized controlled trial. J Med Food. (2016) 19:995–1003. 10.1089/jmf.2016.3762 [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Zhu Y, Song F, Yao Y, Ya F, Li D, et al. Effects of purified anthocyanin supplementation on platelet chemokines in hypocholesterolemic individuals: a randomized controlled trial. Nutr Metab. (2016) 13:86. 10.1186/s12986-016-0146-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie L, Vance T, Kim B, Lee SG, Caceres C, Wang Y, et al. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: a randomized controlled trial. Nutr Res. (2017) 37:67–77. 10.1016/j.nutres.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 40.Yang L, Ling W, Yang Y, Chen Y, Tian Z, Du Z, et al. Role of purified anthocyanins in improving cardiometabolic risk factors in chinese men and women with prediabetes or early untreated diabetes-a randomized controlled trial. Nutrients. (2017) 9:1104. 10.3390/nu9101104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hollands WJ, Armah CN, Doleman JF, Perez-Moral N, Winterbone MS, Kroon PA. 4-Week consumption of anthocyanin-rich blood orange juice does not affect ldl-cholesterol or other biomarkers of cvd risk and glycaemia compared with standard orange juice: a randomised controlled trial. Br J Nutr. (2018) 119:415–21. 10.1017/S0007114517003865 [DOI] [PubMed] [Google Scholar]
- 42.Kim H, Simbo SY, Fang C, McAlister L, Roque A, Banerjee N. et al. Acai (euterpe oleracea mart) beverage consumption improves biomarkers for inflammation but not glucose- or lipid-metabolism in individuals with metabolic syndrome in a randomized, double-blinded, placebo-controlled. Clin Trial Food Funct. (2018) 9:3097–103. 10.1039/C8FO00595H [DOI] [PubMed] [Google Scholar]
- 43.Bakuradze T, Tausend A, Galan J, Groh IAM, Berry D, Tur JA, et al. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radic Res. (2019) 53:1045–55. 10.1080/10715762.2019.1618851 [DOI] [PubMed] [Google Scholar]
- 44.Curtis PJ, van der Velpen V, Berends L, Jennings A, Feelisch M, Umpleby AM, et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am J Clin Nutr. (2019) 109:1535–45. 10.1093/ajcn/nqy380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo Y, Zhang P, Liu Y, Zha L, Ling W, Guo H, et al. Dose-response evaluation of purified anthocyanins on inflammatory and oxidative biomarkers and metabolic risk factors in healthy young adults: a randomized controlled trial. Nutrition. (2020) 74:110745. 10.1016/j.nut.2020.110745 [DOI] [PubMed] [Google Scholar]
- 46.Stote KS, Wilson MM, Hallenbeck D, Thomas K, Rourke JM, Sweeney MI, et al. Effect of blueberry consumption on cardiometabolic health parameters in men with type 2 diabetes: an 8-week, double-blind, randomized, placebo-controlled trial. Curr Dev Nutr. (2020) 4:nzaa030. 10.1093/cdn/nzaa030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan SW, Chu TTW, Choi SW, Benzie IFF, Tomlinson B. Impact of short-term bilberry supplementation on glycemic control, cardiovascular disease risk factors, and antioxidant status in chinese patients with type 2 diabetes. Phytother Res. (2021) 35:3236–45. 10.1002/ptr.7038 [DOI] [PubMed] [Google Scholar]
- 48.Sekikawa T, Kizawa Y, Takeoka A, Sakiyama T, Li Y, Yamada T. The effect of consuming an anthocyanin-containing supplement derived from bilberry (vaccinium myrtillus) on eye function: a randomized, double-blind, placebo-controlled parallel study. Funct Foods Health Dis. (2021) 11:782. 10.31989/ffhd.v11i3.782 [DOI] [Google Scholar]
- 49.Xu Z, Xie J, Zhang H, Pang J, Li Q, Wang X, et al. Anthocyanin supplementation at different doses improves cholesterol efflux capacity in subjects with dyslipidemia-a randomized controlled trial. Eur J Clin Nutr. (2021) 75:345–54. 10.1038/s41430-020-0609-4 [DOI] [PubMed] [Google Scholar]
- 50.Yang L, Qiu Y, Ling W, Liu Z, Yang L, Wang C, et al. Anthocyanins regulate serum adipsin and visfatin in patients with prediabetes or newly diagnosed diabetes: a randomized controlled trial. Eur J Nutr. (2021) 60:1935–44. 10.1007/s00394-020-02379-x [DOI] [PubMed] [Google Scholar]
- 51.Aboufarrag H, Hollands WJ, Percival J, Philo M, Savva GM, Kroon PA. No effect of isolated anthocyanins from bilberry fruit and black rice on ldl cholesterol or other biomarkers of cardiovascular disease in adults with elevated cholesterol: a randomized, placebo-controlled, cross-over trial. Mol Nutr Food Res. (2022) 66:2101157. 10.1002/mnfr.202101157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 53.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. London: John Wiley & Sons; (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukuta H, Goto T, Wakami K, Ohte N. Effects of drug and exercise intervention on functional capacity and quality of life in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Eur J Prev Cardiol. (2016) 23:78–85. 10.1177/2047487314564729 [DOI] [PubMed] [Google Scholar]
- 56.de Mello VD, Lankinen MA, Lindström J, Puupponen-Pimiä R, Laaksonen DE, Pihlajamäki J, et al. Fasting serum hippuric acid is elevated after bilberry (vaccinium myrtillus) consumption and associates with improvement of fasting glucose levels and insulin secretion in persons at high risk of developing type 2 diabetes. Mol Nutr Food Res. (2017) 61:1700019. 10.1002/mnfr.201700019 [DOI] [PubMed] [Google Scholar]
- 57.Davinelli S, Bertoglio JC, Zarrelli A, Pina R, Scapagnini G. A randomized clinical trial evaluating the efficacy of an anthocyanin–maqui berry extract (Delphinol®) on oxidative stress biomarkers. J Am Coll Nutr. (2015) 34:28–33. 10.1080/07315724.2015.1080108 [DOI] [PubMed] [Google Scholar]
- 58.Emamat H, Zahedmehr A, Asadian S, Nasrollahzadeh J. The effect of barberry (berberis integerrima) on lipid profile and systemic inflammation in subjects with cardiovascular risk factors: a randomized controlled trial. BMC Compl Med Therap. (2022) 22:59. 10.1186/s12906-022-03539-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung AJ, Sharma A, Lee S-H, Lee S-J, Kim J-H, Lee H-J. Efficacy of black rice extract on obesity in obese postmenopausal women: a 12-week randomized, double-blind, placebo-controlled preliminary clinical trial. Menopause. (2021) 28:1391–9. 10.1097/GME.0000000000001862 [DOI] [PubMed] [Google Scholar]
- 60.Vidlar A, Vostalova J, Ulrichova J, Student V, Stejskal D, Reichenbach R, et al. The effectiveness of dried cranberries ( Vaccinium Macrocarpon) in men with lower urinary tract symptoms. Br J Nutr. (2010) 104:1181–9. 10.1017/S0007114510002059 [DOI] [PubMed] [Google Scholar]
- 61.Kianbakht S, Abasi B, Hashem Dabaghian F. Improved lipid profile in hyperlipidemic patients taking vaccinium arctostaphylos fruit hydroalcoholic extract: a randomized double-blind placebo-controlled clinical trial. Phytother Res. (2014) 28:432–6. 10.1002/ptr.5011 [DOI] [PubMed] [Google Scholar]
- 62.Gamel TH, Abdel-Aal EM, Tucker AJ, Pare SM, Faughnan K, O'Brien CD, et al. Consumption of whole purple and regular wheat modestly improves metabolic markers in adults with elevated high-sensitivity c-reactive protein: a randomised, single-blind parallel-arm study. Br J Nutr. (2020) 124:1179–89. 10.1017/S0007114520002275 [DOI] [PubMed] [Google Scholar]
- 63.Duthie SJ, Jenkinson AM, Crozier A, Mullen W, Pirie L, Kyle J, et al. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur J Nutr. (2006) 45:113–22. 10.1007/s00394-005-0572-9 [DOI] [PubMed] [Google Scholar]
- 64.Edirisinghe I, Banaszewski K, Cappozzo J, Sandhya K, Ellis CL, Tadapaneni R, et al. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. (2011) 106:913–22. 10.1017/S0007114511001176 [DOI] [PubMed] [Google Scholar]
- 65.Cook MD, Myers SD, Gault ML, Edwards VC, Willems MET. Dose effects of new zealand blackcurrant on substrate oxidation and physiological responses during prolonged cycling. Eur J Appl Physiol. (2017) 117:1207–16. 10.1007/s00421-017-3607-z [DOI] [PubMed] [Google Scholar]
- 66.Jokioja J, Linderborg KM, Kortesniemi M, Nuora A, Heinonen J, Sainio T, et al. Anthocyanin-rich extract from purple potatoes decreases postprandial glycemic response and affects inflammation markers in healthy men. Food Chem. (2020) 310:125797. 10.1016/j.foodchem.2019.125797 [DOI] [PubMed] [Google Scholar]
- 67.Herrera-Arellano A, Flores-Romero S, Chavez-Soto M, Tortoriello J. Effectiveness and tolerability of a standardized extract from hibiscus sabdariffa in patients with mild to moderate hypertension: a controlled and randomized clinical trial. Phytomedicine. (2004) 11:375–82. 10.1016/j.phymed.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 68.Dallas C, Gerbi A, Tenca G, Juchaux F, Bernard F-X. Lipolytic Effect of a polyphenolic citrus dry extract of red orange, grapefruit, orange (Sinetrol) in human body fat adipocytes. Mechanism of action by inhibition of camp-phosphodiesterase (PDE). Phytomedicine. (2008) 15:783–92. 10.1016/j.phymed.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 69.de Liz S, Cardoso AL, Copetti CLK, de Fragas Hinnig P, Vieira FGK, da Silva EL. et al. Açaí (euterpe oleracea mart) and juçara (euterpe edulis mart) juices improved hdl-c levels and antioxidant defense of healthy adults in a 4-week randomized cross-over study. Clin Nutr. (2020) 39:3629–36. 10.1016/j.clnu.2020.04.007 [DOI] [PubMed] [Google Scholar]
- 70.Naruszewicz M, Łaniewska I, Millo B, Dłuzniewski M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (Mi). Atherosclerosis. (2007) 194:e179–e84. 10.1016/j.atherosclerosis.2006.12.032 [DOI] [PubMed] [Google Scholar]
- 71.Gurrola-Díaz CM, García-López PM, Sánchez-Enríquez S, Troyo-Sanromán R, Andrade-González I, Gómez-Leyva J. Effects of hibiscus sabdariffa extract powder and preventive treatment (Diet) on the lipid profiles of patients with metabolic syndrome (Mesy). Phytomedicine. (2010) 17:500–5. 10.1016/j.phymed.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 72.Asgary S, Sahebkar A, Afshani MR, Keshvari M, Haghjooyjavanmard S, Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytotherapy Res. (2014) 28:193–9. 10.1002/ptr.4977 [DOI] [PubMed] [Google Scholar]
- 73.Cardoso AL, Teixeira LL, Hassimotto NMA, Baptista SL, Copetti CLK, Rieger DK, et al. Kinetic profile of urine metabolites after acute intake of a phenolic compounds-rich juice of Juçara (euterpe edulis mart) and antioxidant capacity in serum and erythrocytes: a human study. Int J Mol Sci. (2023) 24:9555. 10.3390/ijms24119555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hunt JE, Coelho MO, Buxton S, Butcher R, Foran D, Rowland D, et al. Consumption of New Zealand blackcurrant extract improves recovery from exercise-induced muscle damage in non-resistance trained men and women: a double-blind randomised trial. Nutrients. (2021) 13:2875. 10.3390/nu13082875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 76.Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, et al. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. (2012) 111:967–81. 10.1161/CIRCRESAHA.112.266502 [DOI] [PubMed] [Google Scholar]
- 77.Xia M, Hou M, Zhu H, Ma J, Tang Z, Wang Q, et al. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proliferator-activated receptor Γ-Liver × receptor α-Abca1 pathway. J Biol Chem. (2005) 280:36792–801. 10.1074/jbc.M505047200 [DOI] [PubMed] [Google Scholar]
- 78.Wallace TC, Slavin M, Frankenfeld CL. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients. (2016) 8:32. 10.3390/nu8010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yao S-L, Xu Y, Zhang Y-Y, Lu Y-H. Black rice and anthocyanins induce inhibition of cholesterol absorption in vitro. Food Funct. (2013) 4:1602–8. 10.1039/c3fo60196j [DOI] [PubMed] [Google Scholar]
- 80.Thilavech T, Adisakwattana S. Cyanidin-3-rutinoside acts as a natural inhibitor of intestinal lipid digestion and absorption. BMC Complement Altern Med. (2019) 19:1–10. 10.1186/s12906-019-2664-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chamnansilpa N, Aksornchu P, Adisakwattana S, Thilavech T, Mäkynen K, Dahlan W, et al. Anthocyanin-rich fraction from thai berries interferes with the key steps of lipid digestion and cholesterol absorption. Heliyon. (2020) 6:e05408. 10.1016/j.heliyon.2020.e05408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang Y, Chen J, Zuo Y, Ma KY, Jiang Y, Huang Y, et al. Blueberry anthocyanins at doses of 05 and 1% lowered plasma cholesterol by increasing fecal excretion of acidic and neutral sterols in hamsters fed a cholesterol-enriched diet. Eur J Nutr. (2013) 52:869–75. 10.1007/s00394-012-0393-6 [DOI] [PubMed] [Google Scholar]
- 83.Wang L, Zhu H, Zhao Y, Jiao R, Lei L, Chen J, et al. Cranberry anthocyanin as an herbal medicine lowers plasma cholesterol by increasing excretion of fecal sterols. Phytomedicine. (2018) 38:98–106. 10.1016/j.phymed.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 84.Liu C, Sun J, Lu Y, Bo Y. Effects of anthocyanin on serum lipids in dyslipidemia patients: a systematic review and meta-analysis. PLoS ONE. (2016) 11:e0162089. 10.1371/journal.pone.0162089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Collaboration APCS. A comparison of lipid variables as predictors of cardiovascular disease in the asia pacific region. Ann Epidemiol. (2005) 15:405–13. 10.1016/j.annepidem.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 86.Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. (2007) 298:776–85. 10.1001/jama.298.7.776 [DOI] [PubMed] [Google Scholar]
- 87.WHO. Cardiovascular Diseases (Cvds). (2016). Available online at: http://www.who.int/mediacentre/factsheets/fs317/en (accessed April 1, 2021).
- 88.Vendrame S, Klimis-Zacas D. Potential factors influencing the effects of anthocyanins on blood pressure regulation in humans: a review. Nutrients. (2019) 11:1431. 10.3390/nu11061431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miguel MG. Anthocyanins: antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. (2011) 5:7–15. 10.3390/molecules15129252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fairlie-Jones L, Davison K, Fromentin E, Hill AM. The effect of anthocyanin-rich foods or extracts on vascular function in adults: a systematic review and meta-analysis of randomised controlled trials. Nutrients. (2017) 9:908. 10.3390/nu9080908 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.