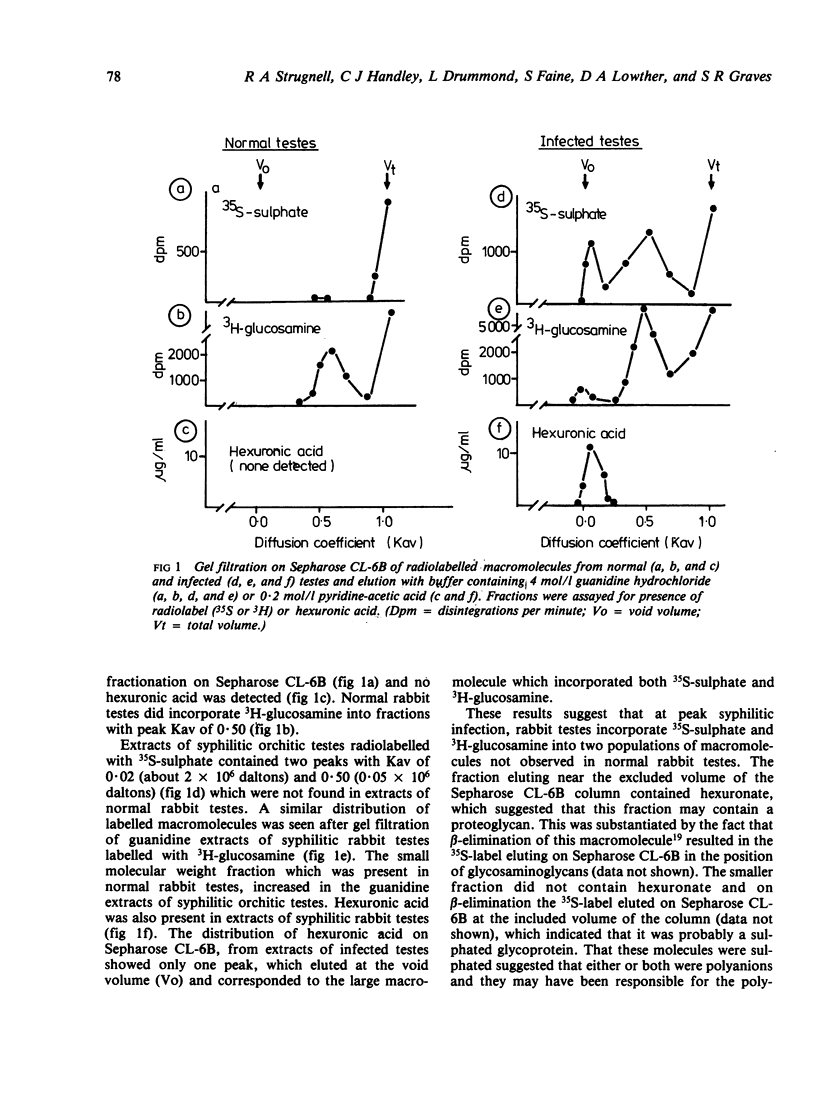
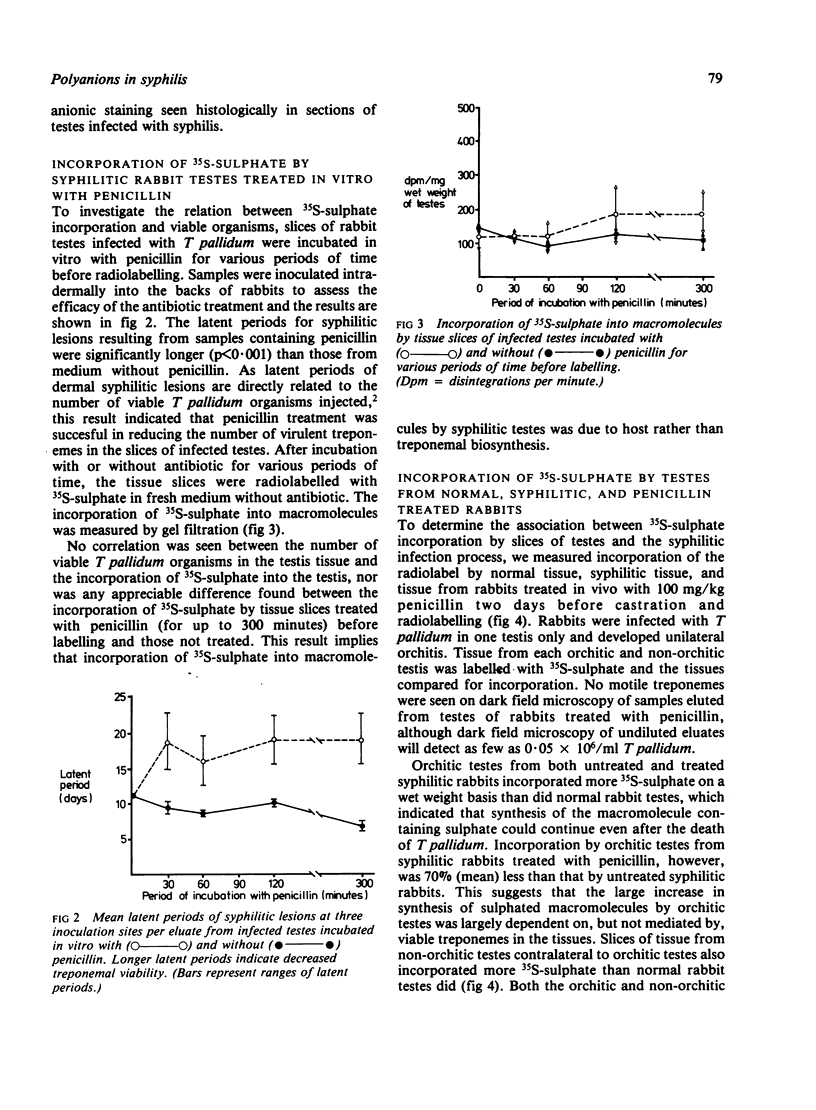
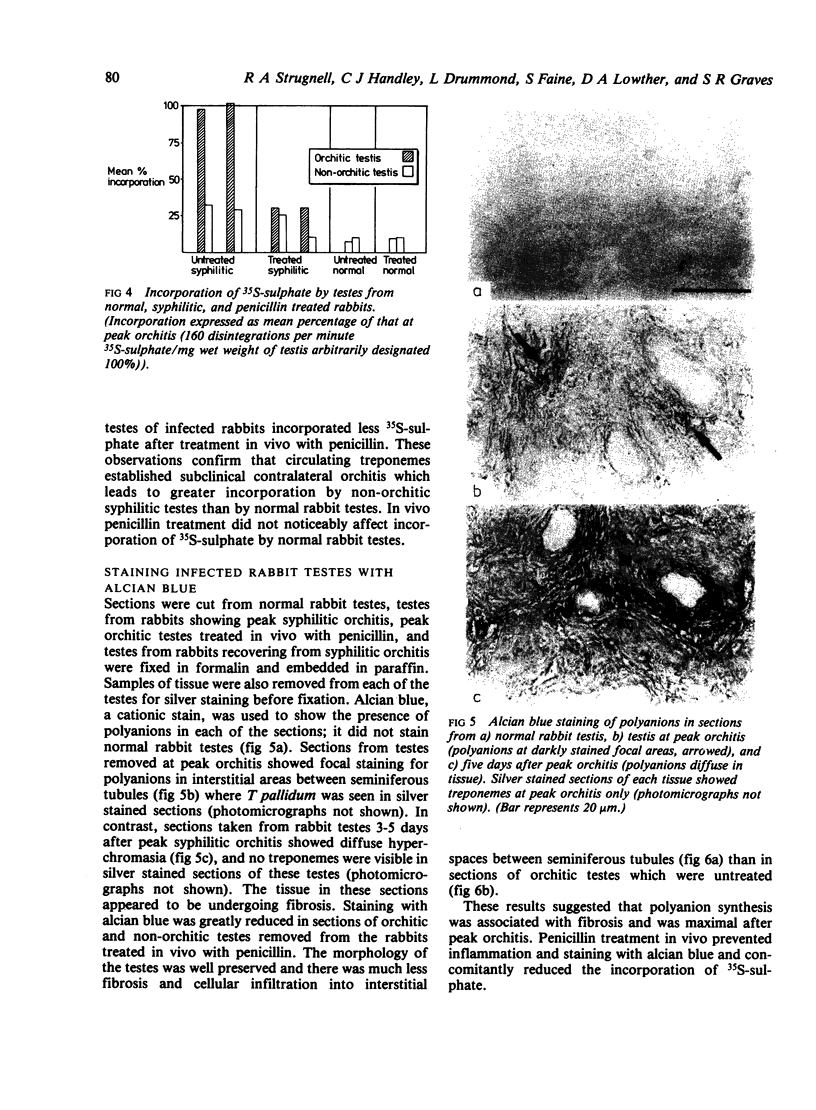
Abstract
We investigated by means of radiolabelled precursors the source and nature of the polyanionic macromolecules present in rabbit tissues during active syphilis infection. Previous studies indicated that Treponema pallidum itself does not synthesise glycosaminoglycans, at least in vitro. In replicate experiments on unilaterally infected rabbits, tissue from the orchitic testis incorporated two to three times more 35S-sulphate and 3H-glucosamine (on a wet weight basis) than tissue from the non-orchitic contralateral testis. Incorporation of 35S-sulphate was independent of the number of viable T pallidum organisms present in the infested tissue, which suggested that incorporation represented biosynthesis by the host and not the treponeme. Testes from syphilitic rabbits two days after treatment with high doses (100 mg/kg) of penicillin incorporated less 35S-sulphate than untreated infected testes, but more than normal uninfected rabbit testes. This suggests that active syphilitic infection was necessary for maximum biosynthesis of the macromolecule(s) by host tissue. Hydrodynamic profiles showed incorporation of radiolabelled precursors into two distinct fractions of different sizes, which may represent a proteoglycan and a sulphated glycoprotein. Alcian blue staining of syphilitic testes at or after peak orchitis showed focal deposition of newly synthesised polyanionic components during peak orchitis and a more generalised fibrosis in testes after peak orchitis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M. Reappraisal of lymphocyte responsiveness to concanavalin A during experimental syphilis: evidence that glycosaminoglycans in the sera and tissues interfere ith active binding sites on the lectin and not with the lymphocytes. Infect Immun. 1982 Feb;35(2):552–559. doi: 10.1128/iai.35.2.552-559.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey R. F., Johnson R. C., Fitzgerald T. J. Suppression of lymphocyte response to concanavalin A by mucopolysaccharide material from Treponema pallidum-infected rabbits. Infect Immun. 1979 Oct;26(1):64–69. doi: 10.1128/iai.26.1.64-69.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Cleveland P., Johnson R. C., Miller J. N., Sykes J. A. Scanning electron microscopy of Treponema pallidum (Nichols strain) attached to cultured mammalian cells. J Bacteriol. 1977 Jun;130(3):1333–1344. doi: 10.1128/jb.130.3.1333-1344.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Influence of testicular fluid infected with Treponema pallidum on intradermal lesions. Br J Vener Dis. 1980 Jun;56(3):125–128. doi: 10.1136/sti.56.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Wolff E. T. Mucopolysaccharide material resulting from the interaction of Treponema pallidum (Nichols strain) with cultured mammalian cells. Infect Immun. 1978 Nov;22(2):575–584. doi: 10.1128/iai.22.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J. Pathogenesis and immunology of Treponema pallidum. Annu Rev Microbiol. 1981;35:29–54. doi: 10.1146/annurev.mi.35.100181.000333. [DOI] [PubMed] [Google Scholar]
- Oakes S. G., Repesh L. A., Pozos R. S., Fitzgerald T. J. Electrophysiological dysfunction and cellular disruption of sensory neurones during incubation with Treponema pallidum. Br J Vener Dis. 1982 Aug;58(4):220–227. doi: 10.1136/sti.58.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repesh L. A., Fitzgerald T. J., Oakes S. G., Pozos R. S. Scanning electron microscopy of the attachment of Treponema pallidum to nerve cells in vitro. Br J Vener Dis. 1982 Aug;58(4):211–219. doi: 10.1136/sti.58.4.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT V., DAMMIN G. J. Hyaluronidase and experimental syphilis. III. Metachromasia in syphilitic orchitis and its relationship to hyaluronic acid. Am J Syph Gonorrhea Vener Dis. 1950 Nov;34(6):501–514. [PubMed] [Google Scholar]
- SCOTT V., DAMMIN G. J. Morphologic and histochemical sequences in syphilitic and in tuberculous orchitis in the rabbit. Am J Syph Gonorrhea Vener Dis. 1954 May;38(3):189–202. [PubMed] [Google Scholar]
- Shoshan S. Wound healing. Int Rev Connect Tissue Res. 1981;9:1–26. doi: 10.1016/b978-0-12-363709-3.50007-3. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Nissley S. P., Kimura J. H., Rechler M. M., Caplan A. I., Hascall V. C. Effects of insulin and multiplication-stimulating activity on proteoglycan biosynthesis in chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981 Feb 25;256(4):2045–2052. [PubMed] [Google Scholar]
- Strugnell R. A., Handley C. J., Lowther D. A., Faine S., Graves S. R. Treponema pallidum does not synthesise in vitro a capsule containing glycosaminoglycans or proteoglycans. Br J Vener Dis. 1984 Feb;60(1):8–13. doi: 10.1136/sti.60.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Steiner B. M., Graves S. Inhibition of macromolecular synthesis in cultured rabbit cells by Treponema pallidum (Nichols). Infect Immun. 1983 Aug;41(2):636–643. doi: 10.1128/iai.41.2.636-643.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler J. A., Jones A. M., Jones R. H., Kubica K. M. Demonstration of extracellular material at the surface of pathogenic T. pallidum cells. Br J Vener Dis. 1976 Feb;52(1):1–8. doi: 10.1136/sti.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]