Abstract
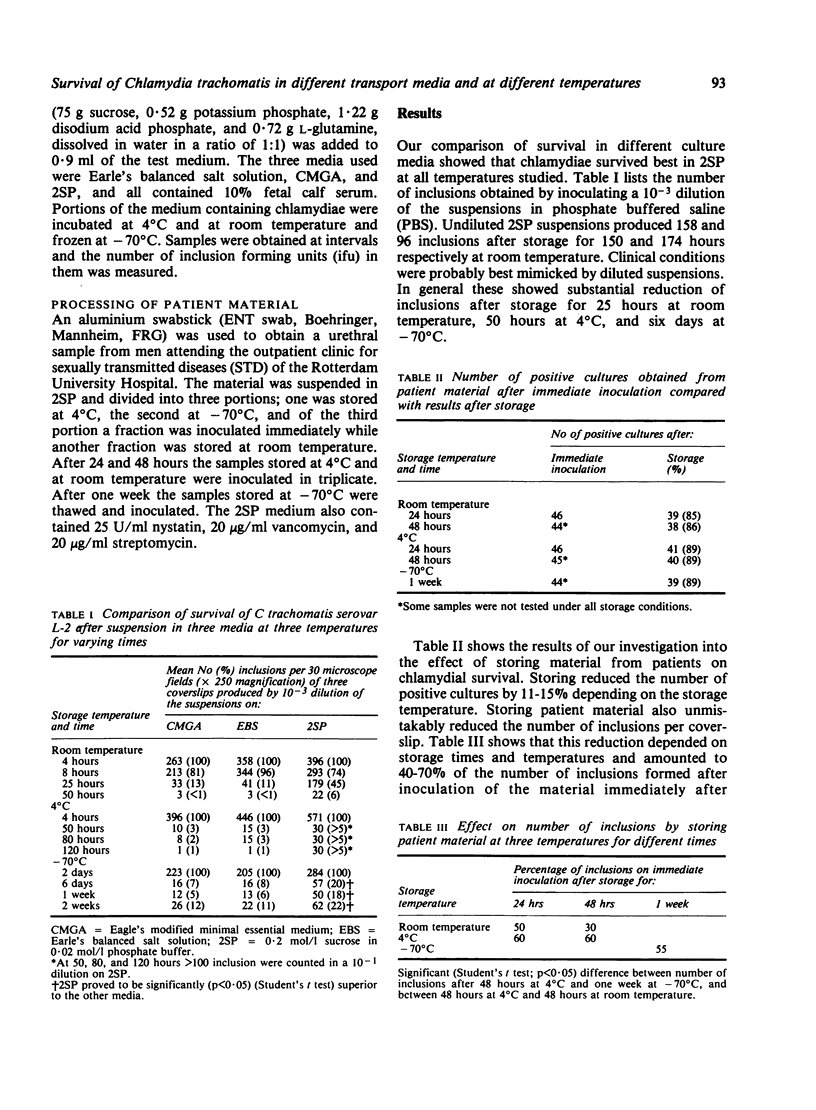
We compared the survival of a laboratory strain of Chlamydia trachomatis serovar L-2 in different media and at different temperatures (room temperature, 4 degrees C, and -70 degrees C). At these temperatures the best storage medium was 2SP (0.2 mol/l sucrose in 0.02 mol/l phosphate buffer supplemented with 10% fetal calf serum). We used material obtained from patients to study the sensitivity of the culture method as a function of sample storage time and temperature. Compared with results on direct inoculation, material stored in 2SP for 48 hours gave 11% fewer positive cultures at 4 degrees C and 14% fewer at room temperature. Of samples which gave negative results on direct inoculation, 4% were positive after storage at 4 degrees C for 48 hours and 2% after storage at -70 degrees C for a week. As expected, the number of inclusion forming units in the original material proved to be important for the percentage of positive cultures among the stored samples.
Full text
PDF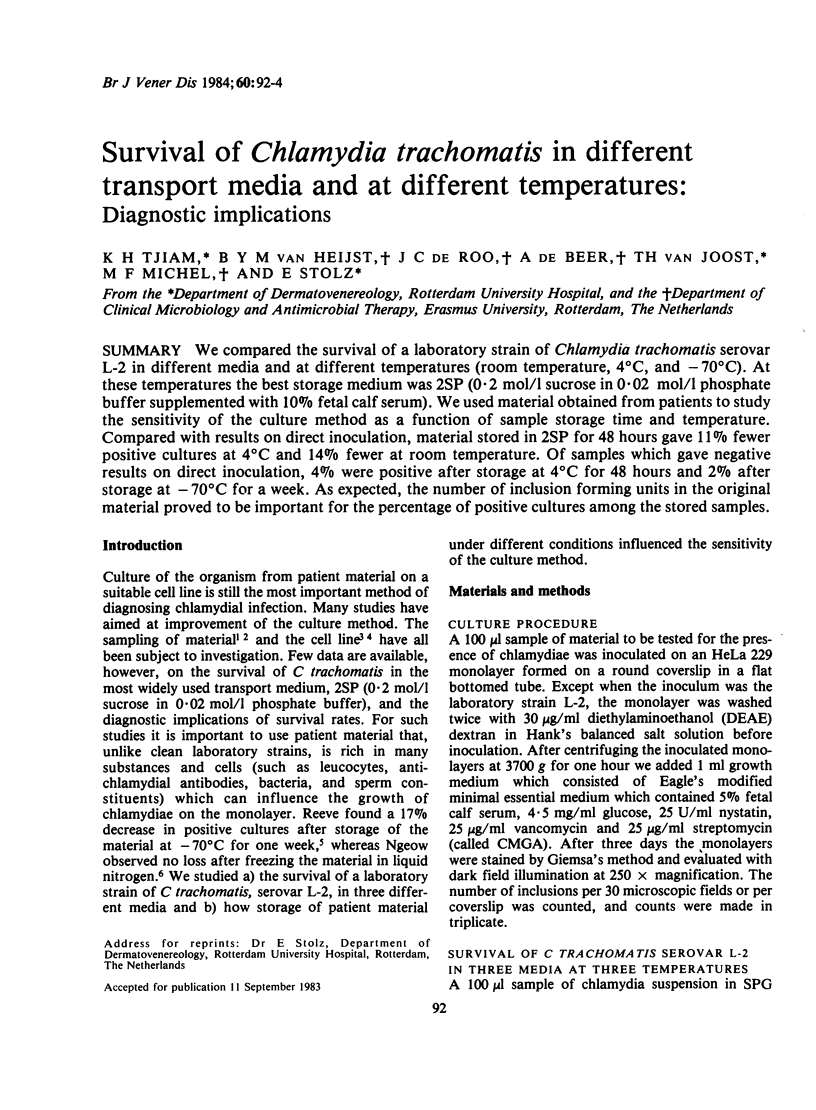

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dunlop E. M., Vaughan-Jackson J. D., Darougar S. Chlamydial infection. Improved methods of collection of material for culture from the urogenital tract and rectum. Br J Vener Dis. 1972 Dec;48(6):421–424. doi: 10.1136/sti.48.6.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. T., Taylor-Robinson D. Comparison of various McCoy cell treatment procedures used for detection of Chlamydia trachomatis. J Clin Microbiol. 1979 Aug;10(2):198–201. doi: 10.1128/jcm.10.2.198-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A., Zeeberg B. Toxic effect of sampling swabs and transportation test tubes on the formation of intracytoplasmic inclusions of Chlamydia trachomatis in McCoy cell cultures. Br J Vener Dis. 1981 Aug;57(4):268–272. doi: 10.1136/sti.57.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngeow Y. F., Munday P. E., Evans R. T., Taylor-Robinson D. Taking cell cultures to the patient in an attempt to improve chlamydial isolation. Br J Vener Dis. 1981 Feb;57(1):44–46. doi: 10.1136/sti.57.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice M. J., Farrant J. Survival of chlamydiae after cooling to -196 degrees C. J Clin Microbiol. 1977 Jul;6(1):4–9. doi: 10.1128/jcm.6.1.4-9.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve P., Owen J., Oriel J. D. Laboratory procedures for the isolation of chlamydia trachomatis from the human genital tract. J Clin Pathol. 1975 Nov;28(11):910–914. doi: 10.1136/jcp.28.11.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway G. L., Moss V., Mumtaz G., Atia W., Emmerson A. M., Oriel J. D. Provision of a chlamydial culture service to a sexually transmitted diseases clinic. Br J Vener Dis. 1982 Aug;58(4):236–238. doi: 10.1136/sti.58.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]


