Abstract
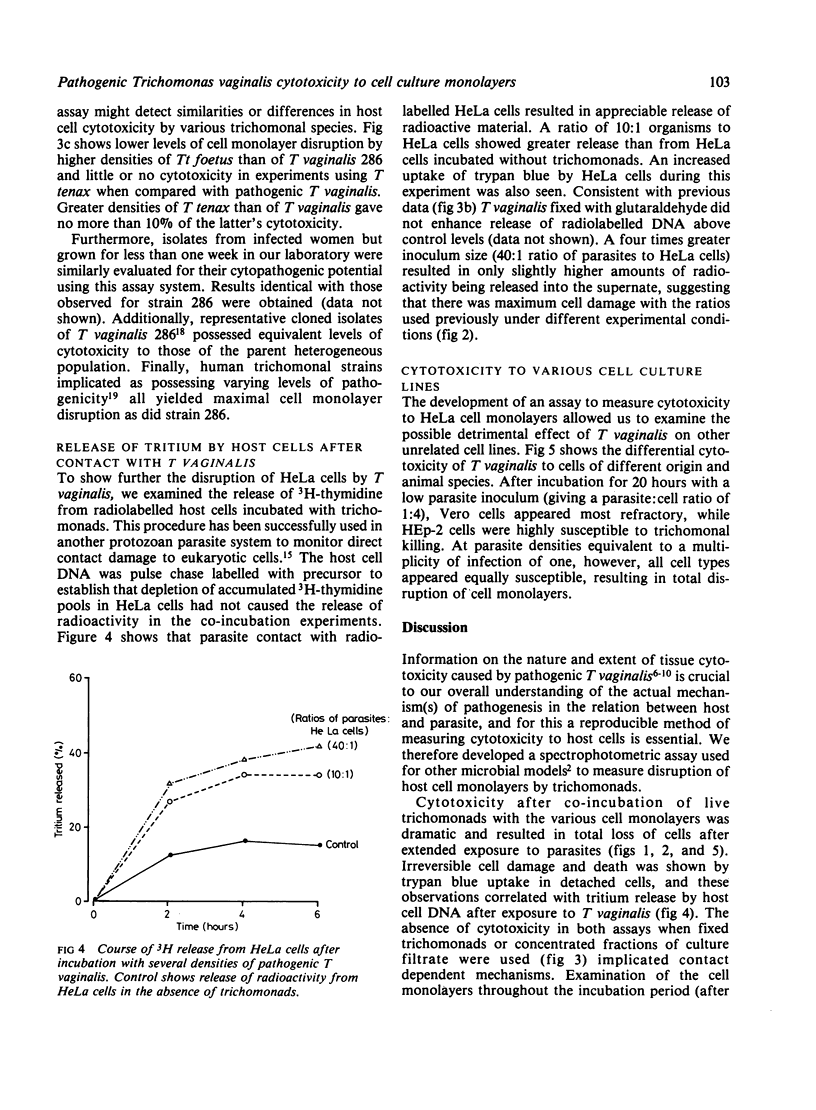
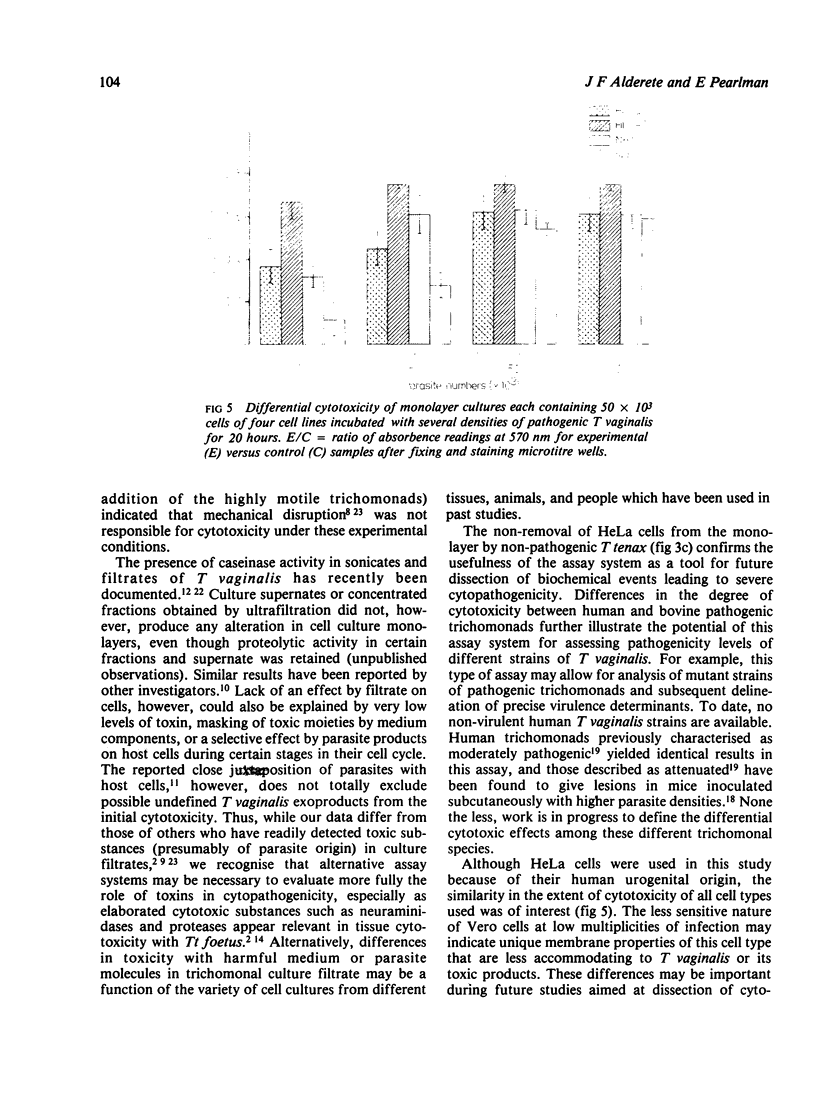
Exposure of monolayer cultures of human urogenital and vaginal (HeLa), human epithelial (HEp-2), normal baboon testicular (NBT), and monkey kidney (Vero) cells to live pathogenic Trichomonas vaginalis resulted in extensive disruption of monolayers. Trypan blue was taken up by all host cells released from cell monolayers, which indicated irreversible damage of these cell types by trichomonads. Time and dose related data on cytotoxicity kinetics were obtained using increasing ratios of parasites to cells. All cell types were most sensitive to trichomonads at a multiplicity of infection of one. Release of tritiated thymidine (3H-thymidine) of the deoxyribonucleic acid (DNA) of prelabelled host cells after incubation with T vaginalis corroborated that extensive cytotoxicity was caused by pathogenic trichomonads in man. Only living parasites were cytotoxic, and no trichomonal toxic products were implicated in disruption of the cell monolayer cultures. A pathogenic bovine trichomonad, Tritrichomonas foetus KV-1, produced half as much cell damage as did T vaginalis. Trichomonas tenax, a non-pathogenic member of the normal flora of the oral cavity in man, produced no measurable cytotoxicity to HeLa cells when compared with the pathogenic human trichomonads.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F. Antigen analysis of several pathogenic strains of Trichomonas vaginalis. Infect Immun. 1983 Mar;39(3):1041–1047. doi: 10.1128/iai.39.3.1041-1047.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIAN R. T., MILLER N. F., LUDOVICI P. P., RILEY G. M. A study of Trichomonas vaginalis in human cell culture. Am J Obstet Gynecol. 1963 Apr 1;85:947–954. doi: 10.1016/s0002-9378(16)35599-5. [DOI] [PubMed] [Google Scholar]
- Coombs G. H. Proteinases of Leishmania mexicana and other flagellate protozoa. Parasitology. 1982 Feb;84(1):149–155. doi: 10.1017/s003118200005174x. [DOI] [PubMed] [Google Scholar]
- Diamond L. S. Techniques of axenic cultivation of Entamoeba histolytica Schaudinn, 1903 and E. histolytica-like amebae. J Parasitol. 1968 Oct;54(5):1047–1056. [PubMed] [Google Scholar]
- FROST J. K. Trichomonas vaginalis and cervical epithelial changes. Ann N Y Acad Sci. 1962 Sep 29;97:792–799. doi: 10.1111/j.1749-6632.1962.tb34689.x. [DOI] [PubMed] [Google Scholar]
- Gentry M. K., Dalrymple J. M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980 Sep;12(3):361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath J. P. Behaviour and pathogenicity of Trichomonas vaginalis in epithelial cell cultures: a study by light and scanning electron microscopy. Br J Vener Dis. 1981 Apr;57(2):106–117. doi: 10.1136/sti.57.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg B. M., Livingston M. C., Frost J. K. Pathogenicity of fresh isolates of Trichomonas vaginalis: "the mouse assay" versus clinical and pathologic findings. Acta Cytol. 1966 Sep-Oct;10(5):353–361. [PubMed] [Google Scholar]
- Krieger J. N. Urologic aspects of trichomoniasis. Invest Urol. 1981 May;18(8):411–417. [PubMed] [Google Scholar]
- Kulda J., Honigberg B. M. Behavior and pathogenicity of Tritrichomonas foetus in chick liver cell cultures. J Protozool. 1969 Aug;16(3):479–495. doi: 10.1111/j.1550-7408.1969.tb02304.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Faubert G. Partial purification and some properties of a neutral sulfhydryl and an acid proteinase from Entamoeba histolytica. Can J Microbiol. 1977 Apr;23(4):420–425. doi: 10.1139/m77-062. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Müller M. Purification and characterization of a low molecular weight thiol proteinase from the flagellate protozoon Tritrichomonas foetus. J Biol Chem. 1979 Mar 10;254(5):1526–1533. [PubMed] [Google Scholar]
- Müller M., Meingassner J. G., Miller W. A., Ledger W. J. Three metronidazole-resistant strains of Trichomonas vaginalis from the United States. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 1):808–812. doi: 10.1016/s0002-9378(16)32741-7. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Acquisition of alpha 1-Antitrypsin by a pathogenic strain of Trichomonas vaginalis. Infect Immun. 1983 May;40(2):640–646. doi: 10.1128/iai.40.2.640-646.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect Immun. 1982 Aug;37(2):755–762. doi: 10.1128/iai.37.2.755-762.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., Croft B. Y., Guerrant R. L. Cytopathogenic mechanisms of Entamoeba histolytica. J Exp Med. 1980 Aug 1;152(2):377–390. doi: 10.1084/jem.152.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein M. F., Chapel T. A. Trichomoniasis, candidiasis, and the minor venereal diseases. Clin Obstet Gynecol. 1975 Mar;18(1):73–88. doi: 10.1097/00003081-197503000-00008. [DOI] [PubMed] [Google Scholar]
- Spence M. R., Hollander D. H., Smith J., McCaig L., Sewell D., Brockman M. The clinical and laboratory diagnosis of Trichomonas vaginalis infection. Sex Transm Dis. 1980 Oct-Dec;7(4):168–171. doi: 10.1097/00007435-198010000-00004. [DOI] [PubMed] [Google Scholar]