Abstract
Objective
To systematically assess credibility and certainty of associations between cannabis, cannabinoids, and cannabis based medicines and human health, from observational studies and randomised controlled trials (RCTs).
Design
Umbrella review.
Data sources
PubMed, PsychInfo, Embase, up to 9 February 2022.
Eligibility criteria for selecting studies
Systematic reviews with meta-analyses of observational studies and RCTs that have reported on the efficacy and safety of cannabis, cannabinoids, or cannabis based medicines were included. Credibility was graded according to convincing, highly suggestive, suggestive, weak, or not significant (observational evidence), and by GRADE (Grading of Recommendations, Assessment, Development and Evaluations) (RCTs). Quality was assessed with AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews 2). Sensitivity analyses were conducted.
Results
101 meta-analyses were included (observational=50, RCTs=51) (AMSTAR 2 high 33, moderate 31, low 32, or critically low 5). From RCTs supported by high to moderate certainty, cannabis based medicines increased adverse events related to the central nervous system (equivalent odds ratio 2.84 (95% confidence interval 2.16 to 3.73)), psychological effects (3.07 (1.79 to 5.26)), and vision (3.00 (1.79 to 5.03)) in people with mixed conditions (GRADE=high), improved nausea/vomit, pain, spasticity, but increased psychiatric, gastrointestinal adverse events, and somnolence among others (GRADE=moderate). Cannabidiol improved 50% reduction of seizures (0.59 (0.38 to 0.92)) and seizure events (0.59 (0.36 to 0.96)) (GRADE=high), but increased pneumonia, gastrointestinal adverse events, and somnolence (GRADE=moderate). For chronic pain, cannabis based medicines or cannabinoids reduced pain by 30% (0.59 (0.37 to 0.93), GRADE=high), across different conditions (n=7), but increased psychological distress. For epilepsy, cannabidiol increased risk of diarrhoea (2.25 (1.33 to 3.81)), had no effect on sleep disruption (GRADE=high), reduced seizures across different populations and measures (n=7), improved global impression (n=2), quality of life, and increased risk of somnolence (GRADE=moderate). In the general population, cannabis worsened positive psychotic symptoms (5.21 (3.36 to 8.01)) and total psychiatric symptoms (7.49 (5.31 to 10.42)) (GRADE=high), negative psychotic symptoms, and cognition (n=11) (GRADE=moderate). In healthy people, cannabinoids improved pain threshold (0.74 (0.59 to 0.91)), unpleasantness (0.60 (0.41 to 0.88)) (GRADE=high). For inflammatory bowel disease, cannabinoids improved quality of life (0.34 (0.22 to 0.53) (GRADE=high). For multiple sclerosis, cannabinoids improved spasticity, pain, but increased risk of dizziness, dry mouth, nausea, somnolence (GRADE=moderate). For cancer, cannabinoids improved sleep disruption, but had gastrointestinal adverse events (n=2) (GRADE=moderate). Cannabis based medicines, cannabis, and cannabinoids resulted in poor tolerability across various conditions (GRADE=moderate). Evidence was convincing from observational studies (main and sensitivity analyses) in pregnant women, small for gestational age (1.61 (1.41 to 1.83)), low birth weight (1.43 (1.27 to 1.62)); in drivers, car crash (1.27 (1.21 to 1.34)); and in the general population, psychosis (1.71 (1.47 to 2.00)). Harmful effects were noted for additional neonatal outcomes, outcomes related to car crash, outcomes in the general population including psychotic symptoms, suicide attempt, depression, and mania, and impaired cognition in healthy cannabis users (all suggestive to highly suggestive).
Conclusions
Convincing or converging evidence supports avoidance of cannabis during adolescence and early adulthood, in people prone to or with mental health disorders, in pregnancy and before and while driving. Cannabidiol is effective in people with epilepsy. Cannabis based medicines are effective in people with multiple sclerosis, chronic pain, inflammatory bowel disease, and in palliative medicine but not without adverse events.
Study registration
PROSPERO CRD42018093045.
Funding
None.
Introduction
Cannabis contains over 100 cannabinoids, of which Δ9-tetrahydrocannabinol and cannabidiol are the most clinically relevant. Tetrahydrocannabinol is a partial agonist at CB1 and binds CB2 receptors. CB1 is widely expressed by central and peripheral neurones but also by immune cells and other type of cells in the brain and in the periphery, and when it binds with tetrahydrocannabinol, a so-called high is induced, which is responsible for potential misuse. CB2 receptors are also expressed by neurons, but less than CB1, and are most abundantly expressed in immune cells.1 2 3 Cannabidiol, however, does not produce the high and thus does not carry the same potential for substance misuse.4 Furthermore, cannabidiol does not seem to promote psychosis inducing effects.5 Cannabis use can evolve into cannabis use disorder, broadly defined as an inability to quit cannabis use, continuous use despite harmful consequences (eg, cannabinoid hyperemesis syndrome6), or functional impairment.7 8
According to the Global Burden of Disease 2019 study, more than 23.8 million people have cannabis use disorder globally,9 and cannabis use ranks third worldwide among consumed substances of misuse, after alcohol and tobacco.10 11 12 13 Cannabis use disorder is more common in men and high income countries. The prevalence of cannabis use disorder in the USA has been estimated to be around 6.3% in a lifetime and 2.5% for 12 months, and in Europe, around 15% of people aged 15-35 years reported cannabis use in the previous year.14 Of those using cannabis, one in three developed problems related to cannabis use that impaired functioning,13 and 10% used cannabis on a daily basis.15 Cannabis use disorder can affect up to 50% of people who use cannabis daily.16
In Europe, over the past decade, self-reported use of cannabis within the past month has increased by almost 25% in people aged 15-34 years, and more than 80% in people who are 55-64 years.17 Cannabis or products containing tetrahydrocannabinol (cannabinoids) are widely available and have increasingly high tetrahydrocannabinol content.18 For instance, in Europe, tetrahydrocannabinol content increased from 6.9% to 10.6% from 2010 to 2019.17 Evidence has suggested that cannabis may be harmful, for mental19 20 and physical health,21 as well as driving safety,22 across observational studies but also in experimental settings.23 Conversely, more than a decade ago, cannabidiol was proposed as a candidate drug for the treatment of neurological disorders such as treatment-resistant childhood epilepsy. Furthermore, it has been proposed that this substance might be useful for anxiety and sleep disorders, and even as an adjuvant treatment for psychosis.24 Moreover, cannabis based medications (ie, medications that contain cannabis components) have been investigated as putative treatments for several different conditions and symptoms.23
The multifarious nature of cannabis’s main active components, contrasting evidence from observational studies reporting detrimental effects of cannabis, and therapeutic findings of cannabis based medicines from interventional studies, is reflected in different legislative approaches. Thus, in most countries cannabis use is illegal, but in a small and growing number of countries and states cannabis is legally sold without the need for a medical prescription.25 26 27
Publication of meta-analyses investigating the effects of cannabinoids on health and other outcomes have substantially increased. However, most meta-analytical findings synthesised data from observational studies and are prone to several sources of bias.28 29 To date, no umbrella review has systematically evaluated the evidence around cannabis, cannabinoids and cannabis nased medicines and health outcomes in humans from meta-analyses encompassing both observational studies and randomised controlled trials. Thus, this work aimed to systematically evaluate the breadth, quality, credibility, and certainty of associations between cannabis, cannabinoids, cannabis based medicines, and human health. We aimed to use established quantitative criteria, account for several sources of bias,30 31 32 and identify converging findings from different study designs.
Methods
Searches and inclusion criteria
We conducted an umbrella review of meta-analyses of observational studies(ie, case-control and cohort studies) and randomised controlled trials that reported on any outcome associated with cannabis and cannabinoids use in humans. We followed an a-priori protocol (PROSPERO CRD42018093045). We adhered to PRIOR and PRISMA 2020 guidelines (adapting PRISMA to the abstract of an umbrella review; supplementary tables 1-2).33 34 Two of the authors independently screened literature that was retrieved systematically by searching PubMed, Embase, and PsycINFO from database inception up to 9 February 2022, without language restrictions, and extracted data into a spreadsheet. The search key is available in the supplementary methods. We also manually searched the Cochrane Library. When two or more meta-analyses examined the same association, we selected only the one that included the largest number of studies. We excluded systematic reviews without a meta-analysis, meta-analyses of risk factors for cannabinoids use, meta-analyses of cross-sectional studies only, pooled analyses of studies identified without a systematic search, and individual studies.
Outcomes
The co-primary outcomes were the efficacy and safety of cannabinoids on target symptoms (eg seizures in epilepsy) in meta-analyses of randomised controlled trials. The secondary outcomes were any outcome reported in the meta-analyses of observational studies.
Data extraction and quality assessment
Extracted information from meta-analyses and individual studies included in meta-analyses were the bibliographic identifiers of the publication (ie, PubMed-Indexed for Medline or the digital object identifier), first author, year of publication, design of included studies (ie, cohort, case-control, randomised controlled trial), number of included studies in the meta-analysis, specific population under investigation (ie, general population, pregnant women, or people with medical disorders), the exposure and comparison definitions (eg author defined marijuana use v no use or heavy use of cannabis v no use), the outcomes, and their effect size and dispersion measure (when adjusted and unadjusted effect sizes were reported, we selected the adjusted ones). We also extracted what factors analyses were adjusted for. The methodological quality of each included meta-analysis was assessed by two independent investigators using A Measurement Tool to Assess Systematic Reviews version 2 (AMSTAR 2).35
Data analysis
For each association from observational studies (ie, between exposure to cannabis or cannabinoids and outcomes), we extracted the effect sizes of individual studies reported in each meta-analysis, recalculating the pooled effect sizes and 95% confidence intervals, using random effects models. Specifically, we re-analysed each eligible association under the random effects model with DerSimonian and Laird method if included studies were equal or more than 10,36 and Hartung, Knapp, Sidik, and Jonkman if less than 10.37 We transformed the initial effect sizes or modified the direction of associations presented by the original authors to present comparable estimates (ie, equivalent odds ratio; supplementary methods).38 Heterogeneity was tested with the I2 and Tau statistics.39 I2 measures the proportion of the total variability due to heterogeneity, Tau measures true heterogeneity as an absolute measure of heterogeneity, instead. Moreover, 95% prediction intervals for the summary random effect sizes were computed to estimate the possible range in which the effect sizes of future studies were anticipated to fall.40 We calculated prediction intervals using both the estimated between-study heterogeneity variance given from tau2 as well as the standard error of the pooled effect. We then examined small study effect bias (ie, whether smaller studies generated larger effect sizes compared with larger studies).38 41 42 43 44 45 46 Small study effect was deemed present when both the Egger regression asymmetry test indicated publication bias (P value ≤0.10), and the random effects summary effect size was larger than the effect size of the largest study contributing to that association.42 44 45 46 Finally, we evaluated significance bias using an updated method to detect the publication selection of statistically significant findings based on observable excess statistical significance.47 48 We computed the test of excess statistical significance and the proportion of statistical significance, which have adequate control for type I errors and high statistical power. The presence of excess significance bias for individual meta-analyses was considered if either excess statistical significance or proportion of statistical significance were greater than 1.645.47
All analyses were conducted in Stata/SE, version 17.0.
Assessment of the credibility of evidence
In accordance with previous umbrella reviews,49 50 51 52 eligible associations from observational studies were classified into five levels according to the strength of the evidence of potential environmental risk or protective factors: convincing (class I), highly suggestive (class II), suggestive (class III), weak (class IV), and not significant. Briefly, credibility of evidence from observational studies is rated on the basis of the number of events developing the outcome of interest, P value of the association, small study effect, excess of significance bias, prediction intervals, statistical significance of the largest study, and heterogeneity. The specific criteria are exhaustively reported in the supplementary methods. We used sensitivity analyses on all levels of evidence, removing the criterion of more than 1000 cases, and on adjusted estimates and cohort studies on class I and II evidence only (supplementary methods).
We classified evidence from meta-analyses of randomised controlled trials, updating a previously proposed framework, classifying certainty of evidence as high, moderate, low, or very low,53 based on GRADE (Grading of Recommendations, Assessment, Development and Evaluations).32 GRADE is a transparent framework that is widely used to develop and present evidence synthesis, providing a set of explicit criteria across different domains to assess level of evidence, and making clinical practice recommendations. As recommended by GRADE, the level of evidence was determined by risk of bias, inconsistency, indirectness, imprecision, and publication bias (supplementary methods).
Patient and public involvement
This study was author funded and we did not involve patients and the public in this work, but we will apply for funding to involve them in the knowledge translation of present findings. Knowledge translation activities will include, but will not be limited to, dissemination of findings via personal and institutional social media, education of health professional trainees, and continuous medical education activities for health professionals. We will involve patient and public representatives in creating a plain language summary of findings to be distributed to the clinical population with mental health disorders, and pregnant women, informing policy makers across different countries with written communications.
Results
Literature search
Starting from 6657 records after duplicate removal, we excluded 5941 studies at title and abstract screening stage, and 599 at full-text level, resulting in 101 publications included. Studies identified by manual search had already been identified from the systematic search. The list of studies excluded after full-text assessment, with reason for exclusion, is reported in supplementary table 3, and the article selection flow is reported in figure 1.33 Of the 101 articles, 50 were meta-analyses of observational studies (215 meta-analytical associations),21 22 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 and 51 were meta-analyses of randomised controlled trials23 74 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 (364 meta-analytical associations) Of note, one meta-analysis reported on both observational and randomised controlled trials (table 1, table 2, supplementary material 2).74
Fig 1.
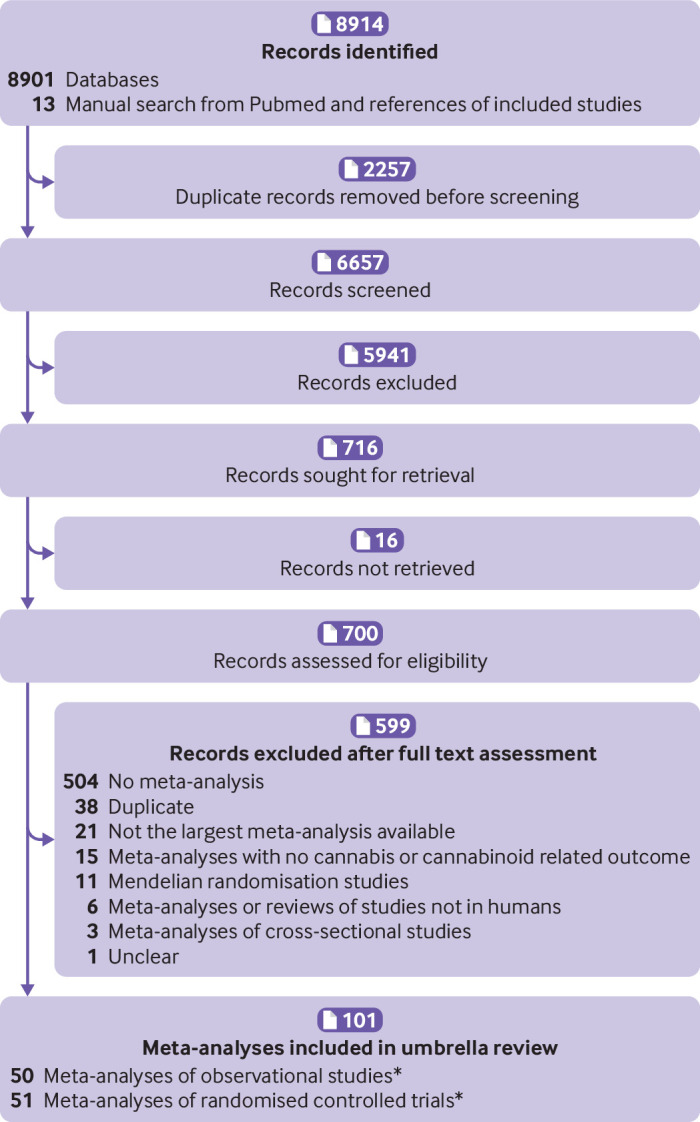
Study selection flow. References of excluded studies after full text assessment available in supplementary table 3. *One meta-analysis included both observational and randomised controlled trials
Table 1.
Characteristics of included meta-analyses of observational studies, or non-randomised studies
| Author, year | k | Population (age) | Type of cannabinoid exposure* | Outcomes | Quality |
|---|---|---|---|---|---|
| Asbridge, 201222 | 9 | General population (adolescents, adults) | THC | Car crash death or injuries | Moderate |
| Bhagavan, 202064 | 5 | Insomnia (adults) | CBM | Sleep quality or quantity | High |
| Blest-Hopley, 201975 | 12 | General population (adolescents, adults) | Regular cannabis use | Brain executive and default mode network | Moderate |
| Bogaty, 201886 | 14 | Psychosis (adolescents, adults) | Cannabis current use | Cognition | Moderate |
| Borges, 201697 | 16 | General population (adolescents, adults) | Cannabis use | Suicide ideation, attempt, and suicide | Moderate |
| Burns, 201298 | 9 | First episode psychosis (adolescents, adults) | Cannabis use | Duration of untreated psychosis | Low |
| Chisini, 201999 | 4 | General population (adolescents, adults) | Cannabis use | Periodontitis | Low |
| Conner, 2016100 | 31 | Pregnant women (adult) | Marijuana use | Low birth weight | Moderate |
| De Carvalho, 2015101 | 6 | General population (adolescents, adults) | Marijuana use | Head and neck cancer | Moderate |
| Escelsior, 202154 | 16 | General population (adolescents, adults) | Cannabinoids | Self-injurious behaviour | Low |
| Farooqui, 201955 | 9 | HCV+NAFDL (adult) | Marijuana use | Liver fibrosis | Moderate |
| Foglia, 201756 | 15 | Psychosis (adolescents, adults) | Cannabis use (current, past) | Antipsychotics adherence | High |
| Ghasemiesfe, 201921 | 25 | General population (adults) | Marijuana ever use, use >10 year | Cancer (lung, head and neck, oral, testicular) | High |
| Ghasemiesfe, 201857 | 22 | General population (adolescents, adults) | Marijuana use | Sputum production and cough | High |
| Gibbs, 201558 | 6 | General population (adult) | Cannabis use | Mania symptoms | Moderate |
| Goldenberg, 201759 | 20 | Mixed medical conditions† (adult) | Cannabis, cannabinoids | Health related quality of life | Moderate |
| Grant, 200260 | 11 | General population (adult) | Cannabis use | Cognition | Moderate |
| Gunn, 201661 | 6 | Pregnant women (adult) | Cannabis use | Maternal anaemia | Moderate |
| Gurney, 201562 | 3 | General population (adults <50) | Cannabis use, current | Testicular cancer, non-seminoma | Moderate |
| Hostiuc, 201863 | 24 | General population (adolescents, adults) | Cannabis use | Car events | Moderate |
| Johnson, 201765 | 13 | General population (adolescents, young adults) | Cannabis use | Physical dating violence | High |
| Kamp, 201866 | 5 | General population (adults) | Cannabis use | Dopamine receptors, transporter and synthesis | Moderate |
| Kiburi, 202167 | 18 | General population (adolescent) | Cannabis use | Psychosis | High |
| Kraan, 201668 | 7 | Ultra-high risk of psychosis (adolescents, adults) | Cannabis use | Psychosis (transition to) | High |
| Lev-Ran, 201469 | 14 | General population (adolescents, adults) | Cannabis use (normal, heavy) | Depression | High |
| Lorenzetti, 201970 | 30 | General population (adolescents, adults) | Regular cannabis use | Brain volume | High |
| Marchand, 202271 | 16 | Pregnant women (adults) | Marijuana use | Low birth weight, small for gestational age, preterm delivery, NICU, Apgar, head circumference | Low |
| Moore, 200772 | 35 | General population (adolescents, adults) | Cannabis use | Depression, psychosis symptoms and suicidal ideation | Moderate |
| Myles, 201273 | 38 | General population (adolescents, adults) | Cannabis use | Age at onset of schizophrenia and other psychoses | Moderate |
| Noori, 202174* | 12 | Chronic pain on opioids | Cannabis use | Opioid use | Low |
| Power, 202176 | 7 | General population (adolescents, young adults) | Cannabis use, frequent/dependent | IQ, verbal IQ | High |
| Rabin, 201177 | 8 | Schizophrenia (adults) | Cannabis use | Cognition | Low |
| Rocchetti, 201378 | 14 | General population (adolescents, adults) | Cannabis use | Hippocampal volume | Moderate |
| Rodriguez-Almaraz, 202079 | 5 | Malignant CNS tumours (adults) | Cannabis use | Survival | Low |
| Rogeberg, 201980 | 12 | Drivers (adult) | Cannabis, THC positive | Car crush and car crush culpability | Low |
| Ruisch, 201881 | 36 | Pregnant women (adult) | Cannabis use | Offspring conduct problems | Moderate |
| Ruiz-Veguilla, 201282 | 5 | Psychosis (adolescents, adults) | Cannabis use | Neurological soft signs | Moderate |
| Sabe, 202083 | 20 | Schizophrenia (adults) | Cannabis use | Negative symptoms | High |
| Sánchez-Gutiérrez, 202084 | 7 | First-episode psychosis (adolescents, adults) | Cannabis use | Cognition | Low |
| Schoeler, 201685 | 24 | Psychosis | Cannabis use (continued, past) | Psychosis, relapse | Moderate |
| Schoeler, 201687 | 88 | Healthy subjects, psychosis (adult) | Cannabis use | Cognition | Low |
| Schreiner, 201288 | 33 | General population (adolescents, adults) | Cannabis use (past) | Cognition | Critically low |
| Schumacher, 201889 | 11 | General population (adolescent, adult) | Cannabis | Condom use | Moderate |
| Scott, 201890 | 69 | General population (adolescent, adult) | Cannabis (current, past) | Cognition | High |
| Smith, 201491 | 11 | General population (adolescents, adults) | Cannabis (heavy use) | Behavioural inhibition | Moderate |
| Sultan, 201892 | 13 | General population (not reported) | THC | Heart rate change | Moderate |
| Szoke, 201493 | 29 | General population (adult) | Cannabis use | Schizotypy | Moderate |
| Wijarnpreecha, 201894 | 3 | HCV (adult) | Cannabis use | Advanced liver fibrosis | High |
| Xue, 202195 | 10 | General population (adolescents, adults) | Cannabis use | Anxiety | Low |
| Zhang, 201596 | 6 | General population (adult) | Cannabis use | Lung cancer | Low |
CBM=cannabis based medications; CNS=central nervous system; HCV=hepatitis C virus; IQ=intelligent quotient; k=number of studies included in the overall eligible systematic review with meta-analysis; NAFLD=non-alcoholic fatty liver disease; NICU=admission to neonatal intensive care unit; THC=tetrahydrocannabinol.
Included both observational studies and randomised controlled trials.
Fibromyalgia, HIV, inflammatory bowel disease, and neuropatic pain.
Table 2.
Characteristics of included meta-analyses of randomised controlled trials
| Author, year | k | Population (age) | Type of cannabinoid/genetic exposure* | Outcomes | Quality |
|---|---|---|---|---|---|
| Allan, 2018112 | 22 | Mixed conditions (adults) | CBM | Pain, spasticity, nausea and vomiting, adverse events | High |
| Allende-Salazar, 2017123 | 32 | Mixed conditions (chronic non-cancer pain)(adults) | CBM | Pain | Low |
| Amato, 2017134 | 41 | Mixed conditions(adults) | CBM | Nausea, adverse events | High |
| Andreae, 2015145 | 5 | Mixed conditions (chronic neuropathic pain) (adults) | Cannabis (inhaled) | Pain | High |
| Aviram, 2017148 | 23 | Mixed conditions(adults) | CBM | Pain, adverse events | Low |
| Bahji, 2020149 | 9 | Dementia (older people) | Cannabinoids | Psychiatric symptoms | Low |
| Bahji, 2020150 | 14 | Anxiety (adults) | Cannabinoids | Anxiety, acceptability | High |
| Bajtel, 2022151 | 16 | Mixed conditions (adults) | CBM | Drowsiness, fatigue, headache, nausea | Moderate |
| Black, 2019102 | 86 | Psychiatric disorders (adults) | THC/cannabidiol | Psychiatric symptoms | High |
| Chesney, 2020103 | 12 | Mixed conditions (children, adults) | Cannabinoids | Adverse events | High |
| Couch, 2018104 | 53 | Crohn’s (adults) | THC/cannabidiol | Disease activity index | Moderate |
| Da Rovare, 2017105 | 16 | Multiple sclerosis/paraplegia (spasticity, adults) | CBM | Dizziness, dry mouth, nausea, somnolence | Moderate |
| De Carvalho, 2020106 | 4 | Treatment-resistant epilepsy (children, adults) | Cannabis, cannabidiol | Seizures, adverse events, pain | Low |
| De Vita, 2018107 | 18 | Healthy subjects experimental pain (adults) | Cannabinoids | Pain | High |
| Doeve, 2020108 | 4 | Inflammatory bowel disease (adults) | Cannabis | Remission, biomarkers, symptoms, quality of life | Critically low |
| Elliott, 2018109 | 23 | Epilepsy (children) | Cannabidiol | Seizures, response, quality of life, sleep, vomit, diarrhoea | Moderate |
| Fu, 2018110 | 23 | Multiple sclerosis (adult) | Cannabinoids | Spasticity, adverse events | Critically low |
| Gazendam, 2020111 | 6 | Surgery (adult) | CBM | Pain | Low |
| Hauser, 2019113 | 4 | Cancer (adult) | Nabiximol, THC | Pain, maintenance of opioid dosage, daily breakthrough opioid dosage | High |
| Hindley, 2020118 | 15 | General population (adult) | THC/cannabidiol | Psychiatric symptoms | High |
| Kopelli, 2020114 | 3 | Schizophrenia (adult) | Cannabidiol | Total symptoms, cognition | Low |
| Lattanzi, 2020115 | 3 | Dravet syndrome (children) | Cannabidiol | Seizure, acceptability, adverse events | Critically low |
| Lattanzi, 2020116 | 4 | Dravet syndrome, Lennox-Gastaut (children) | Cannabidiol | Seizure | Critically low |
| Lattanzi, 2018117 | 2 | Lennox-gastaut syndrome (children) | Cannabidiol | Seizure, tolerability, adverse events | High |
| Lattanzi, 2018119 | 4 | Treatment-resistant Dravet syndrome, Lennox-Gastaut (children) | Cannabidiol | Seizure, acceptability, tolerability, adverse events | Moderate |
| Lobos Urbina, 2016120 | 29 | Cancer (adults) | Cannabinoids | Pain, quality of life, adverse events | Low |
| McKee, 2021121 | 31 | Opioid use disorder and Cannabis use disorder (adults) | CBM | Opioid use and cannabis use | Low |
| McCartney, 2021122 | 80 | General population (adults) | THC | Driving impairment, cognitive impairment | Low |
| Meza, 2017124 | 7 | Multiple sclerosis (adults) | Cannabinoids | Spasticity, pain, adverse events | Low |
| Morales, 2017125 | 4 | Cancer (chemotherapy, adults) | Cannabinoids | Nausea, vomit, adverse events | Low |
| Mucke, 2018126 | 16 | Mixed conditions (chronic neuropathic pain, adults) | CBM | Pain, psychological distress, sleep problems | High |
| Mucke, 2018127 | 8 | Cancer, HIV (adults) | CBM | Weight gain | High |
| Noori, 202174* | 5 | Cancer pain on opioids | CBM | Constipation, nausea, opioid use, pain, sleep, vomit | Low |
| Rodríguez, 2018128 | 9 | Cannabis use disorder (adults) | CBM | Abstinence, craving symptoms, adverse events | Low |
| Ruthirakuhan, 2019129 | 6 | Alzheimer’s disease (elderlies) | CBM | Agitation, cognition, neuropsychiatric symptoms, BMI, adverse events, tolerability | High |
| Sainsbury, 2021130 | 17 | Mixed conditions (chonic, neuropathic pain, adults) | CBM, THC/cannabidiol | Pain | Low |
| Spanagel, 2021131 | 26 | Mixed conditions (chonic, neuropathic pain, adults) | CBM, THC/cannabidiol | Appetite, sleep | High |
| Simon, 2022132 | 4 | Cancer with cachexia (adults) | Cannabinoids | Appetite | Low |
| Smith, 2015133 | 23 | Cancer (chemotherapy, adults) | CBM | Nausea vomit, dysphoria, euphoria, sedation, dizziness, discontinuation due to adverse events, participant preference | High |
| Stockings, 2018135 | 91 | Mixed conditions (chronic pain, non-cancer, adults) | CBM | Pain | High |
| Stockings, 2018136 | 36 | Epilepsy (any age) | CBM | Seizure, quality of life, adverse events, tolerability | Moderate |
| Sultan, 2017137 | 25 | General population (adults) | CBM | Heart rate, blood pressure, blood flow | Moderate |
| Thanabalasingam, 2021138 | 3 | Parkinson’s disease (adults) | Cannabinoids | Motor symptoms | Low |
| Torres-Moreno, 2018139 | 17 | Multiple sclerosis (adults) | CBM | Pain, spasticity, bladder disfunction | High |
| Treves, 2021140 | 8 | Mixed conditions (children) | CBM, THC/cannabidiol | Appetite, gastrointestinal adverse events, serious adverse events, seizures, | High |
| Velayudan, 2021141 | 46 | Mixed conditions (adults) | CBM | Adverse events, acceptability, tolerability | Low |
| Wang, 2008142 | 31 | Mixed conditions (adults) | Cannabis | Adverse events | Low |
| Wang, 2021143 | 32 | Mixed conditions (chronic pain, adults) | CBM or cannabinoids | Pain | High |
| Watanabe, 2021144 | 47 | Mixed conditions | CBM | Hypothension, orthostatic hypothension | Low |
| Whiting, 201523 | 79 | Mixed conditions (not reported) | CBM, THC/cannabidiol | Adverse events | High |
| Wong, 2020146 | 43 | Mixed conditions (chronic pain, non-cancer, adults) | Cannabinoids | Pain | Low |
| Zhang, 2021147 | 2 | Schizophrenia (adults) | Cannabinoids | Psychotic symptoms | Low |
CBM=cannabis-based medications; THC=tetrahydrocannabinol. For full details of the populations see the supplementary appendix 2, supplementary table of characteristics of included meta-analyses.
Specific single nucleotide polymorphisms are reported in supplementary table 8.
Meta-analyses of randomised controlled trials
The eligible meta-analyses of randomised controlled trials were published between 2008 and 2022. The quality of included meta-analyses according to AMSTAR 2 was high in 20 meta-analyses, moderate in seven, low in 21, and critically low in four (table 2). The median number of studies included in meta-analyses was five (interquartile range 3-9, range 2-42) and the median number of participants was 540 (251-1276, 37-4243).
Cannabidiol was specifically evaluated in seven meta-analyses, while others considered different combinations of cannabis, cannabinoids, tetrahydrocannabinol, and cannabis-based medicines including nabiximols, dronabinol, nabilone, levonantradol, and CT3. Overall, 364 unique meta-analytical associations were identified reporting on acceptability or tolerability of physical adverse events (n=213), psychiatric or psychological related outcomes (n=54), pain related outcomes (n=39), cognitive related (n=20), euphoria or feeling high (n=5), quality of life (n=5), and other various outcomes (n=28). Supplementary table 4 (associations with low or very low certainty) shows the summary effects of the unique meta-analyses or associations for randomised controlled trials.
Summary of associations
Based on the GRADE approach, 14 statistically significant meta-analytical associations (3.8%) met the high certainty criteria, 92 (25.3%) moderate certainty, 200 associations (55.0%) met the low certainty, and 58 associations (16.9%) met the very low certainty. The table detailing the classification of the level of evidence is presented in the supplementary material (supplementary table 4). In the following sections, we principally described the associations with high and moderate GRADE by subgroup of populations.
GRADE of evidence of cannabinoids and outcomes
Mixed chronic pain conditions
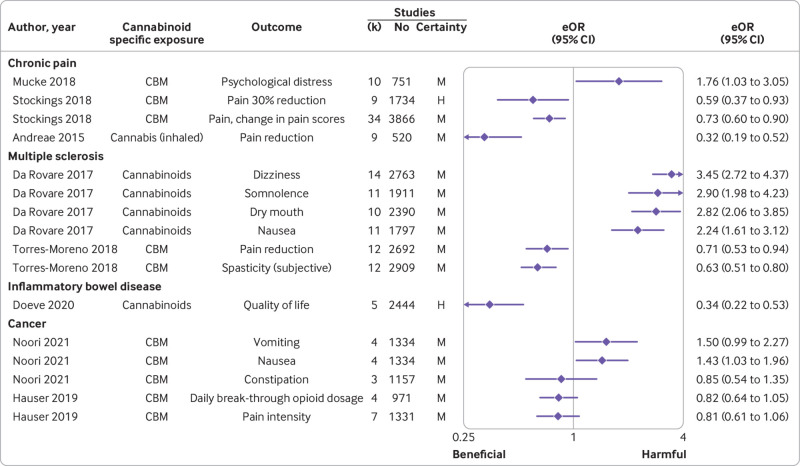
Among the 34 associations in this population, cannabis-based medicines or cannabinoids reduced pain by 30% (equivalent odds ratio 0.59 (95% confidence interval 0.37 to 0.93)), but for pain relief no effect emerged (equivalent odds ratio not calculable, mean difference −0.09 (95% confidence interval −0.30 to 0.10)) with high certainty. An additional seven beneficial effects were supported by moderate certainty, including analgesic efficacy (n=5), pain reduction (n=1), and change in pain scores (n=1), yet no effect emerged on patient global impression much or very much improved (n=1), and 50% pain reduction (n=1) (fig 2 supplementary table 4). Two other associations with harmful effects were supported by moderate certainty, including psychological distress (n=1) and withdrawals due to adverse events (n=1). Low (n=17) or very low (n=3) certainty were found for the remaining associations (supplementary table 5).
Fig 2.
Moderate and high certainty evidence according to Grading of Recommendations, Assessment, Development and Evaluations (GRADE), from randomised controlled trials on outcomes of cannabis based medications in people with chronic pain, multiple sclerosis, inflammatory bowel disease, and cancer. Only associations for which an eOR was available are displayed. Results are displayed in descending order of level of evidence and effect size. CBM=cannabis based medications eOR=equivalent odds ratio; H=high; M=moderate
Multiple sclerosis and paraplegia
None of the 18 associations in this population was supported by high certainty. Two beneficial effects of cannabis based medicines were supported by moderate certainty, including pain reduction (n=1), and spasticity (subjective; n=1) (fig 2, supplementary table 4). An additional four harmful effects were supported by moderate certainty, including dizziness (n=1), dry mouth (n=1), nausea (n=1) and somnolence (n=1) (fig 2, supplementary table 4). Low (n=10) or very low (n=2) certainty were found for the remaining associations (supplementary table 4).
Inflammatory bowel or Crohn’s disease
Among the three associations in this population one between cannabinoids and better quality of life (fig 2, supplementary table 4) presented high certainty (equivalent odds ratio 0.34 (95% confidence interval 0.22 to 0.53)). Low (n=1) or very low (n=1) certainty were found for the remaining associations (supplementary table 5).
Cancer
None of the 60 associations in this population was supported by high certainty. A beneficial effect emerged on sleep disturbances (n=1), as well as an increased risk of adverse events of gastrointestinal disorders (n=1), nervous system disorders (n=1), serious adverse events (n=1), tolerability (n=1), nausea (n=1), and no effect on daily breakthrough opioid dosage (n=1), constipation (n=1), pain (n=4), risk of psychiatric disorder (n=1), vomiting (n=1), or withdrawal due to adverse events (n=1), with moderate certainty (fig 2, supplementary table 4).
Epilepsy
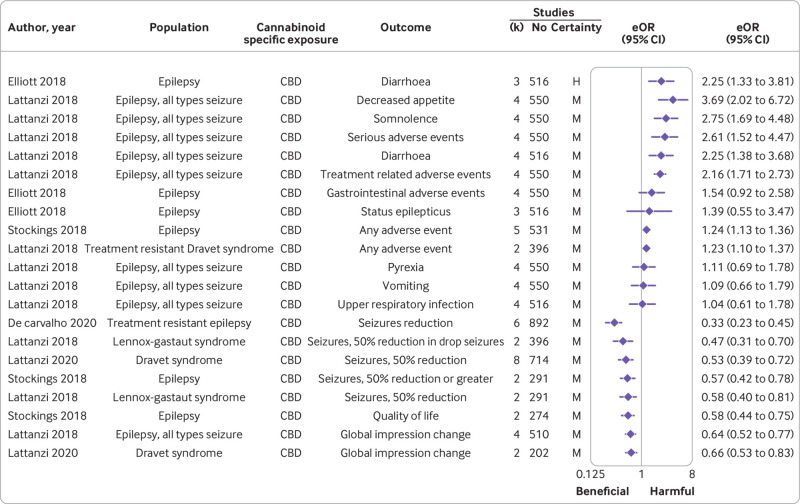
Among the 46 associations in this population one between cannabidiol and diarrhoea presented high certainty with harmful effects (equivalent odds ratio 2.25 (95% confidence interval 1.33 to 3.81)), and no effect on sleep disruption (equivalent odds ratio not calculable, mean difference −0.29 (95% confidence interval −0.88 to 0.30)). Moderate certainty emerged for seven harmful effects, namely any adverse event (n=2), decreased appetite (n=1), diarrhoea (n=1), serious adverse events (n=1), somnolence (n=1), treatment related adverse events (n=1), as well as for 10 beneficial effects: seizures reduction (n=7), global impression improvement (n=2), and quality of life (n=1). No effect was noted, with moderate certainty, for gastrointestinal side effects (n=1), quality of life in children (n=1), status epilepticus (n=1), upper respiratory infection (n=1), vomiting (n=1), pyrexia (n=1). Low (n=16) or very low (n=3) certainty were reported for the remaining associations (fig 3, supplementary table 4).
Fig 3.
Moderate and high certainty evidence according to Grading of Recommendations, Assessment, Development and Evaluations (GRADE), from randomised controlled trials on outcomes of cannabis based medications (CBD) in people with epilepsy. Results are displayed in descending order of level of evidence and effect size; only associations for which an eOR was available are displayed. eOR=equivalent odds ratio; H=high; M=moderate
Mixed conditions
Among the 140 associations in this population three between cannabis-based medicines and various adverse events (supplementary figure 1, supplementary table 4) presented high certainty with harmful effects (equivalent odds ratio 2.84 (95% confidence interval 2.16 to 3.73) for central nervous system adverse events; 3.07 (1.79 to 5.26) for psychological adverse events, and 3.00 (1.79 to 5.03) for vision related adverse events). Moderate certainty supported a beneficial effect on nausea or vomit reduction (n=1), pain reduction (n=3), spasticity reduction (global impression of change) (n=1), an increased risk of feeling high (n=1), gastrointestinal adverse events (n=2), gastrointestinal disorder (non-serious; n=1), emerging psychiatric disorder (n=1), somnolence (n=1), and withdrawal due to adverse events (n=1), while no associations were reported with application site discomfort (n=1), cardiac adverse events (n=1), headache (n=1), musculoskeletal and connective disorder (n=1) and musculoskeletal adverse events (n=1), quality of sleep (n=1), renal urinary disorder (n=1), respiratory disorder (n=1), spasticity reduction (n=1), or weakness (n=1).
Two other beneficial effects of cannabidiol were noted with high certainty, on seizures (equivalent odds ratio 0.59 (95% confidence interval 0.38 to 0.92) for 50% seizure reduction and 0.59 (0.36 to 0.96) for seizure events; supplementary figure 1, supplementary table 4), but moderate evidence supported an increased risk of pneumonia (n=1), somnolence (n=1), gastrointestinal hyperactivity events (n=1), and withdrawal due to adverse events (n=1) (supplementary figure 1, supplementary table 4).
Low (cannabis-based medicines, n=63; cannabis, n=28) or very low (cannabis based medicines, n=9; cannabidiol, n=12) certainty were found for the remaining associations (supplementary table 5).
General population
Among the 23 associations in this population, two between cannabis and emerging psychiatric symptoms presented at high certainty with harmful effects (positive psychotic symptom severity, equivalent odds ratio 5.21 (3.36 to 8.01) and total psychiatric symptoms, equivalent odds ratio 7.49 (5.31 to 10.42)). An additional 12 harmful effects were supported by moderate certainty, including negative symptom severity (n=1), and cognitive outcomes (n=11) (supplementary figure 2, supplementary table 5). Low (n=7) or very low (n=2) certainty were found for the remaining associations (supplementary table 4).
Healthy people
Among the three associations in this population two between cannabinoids and pain outcomes presented high certainty with beneficial effects (equivalent odds ratio 0.74 (95% confidence interval 0.59 to 0.91) for pain threshold and 0.60 (0.41 to 0.88) for pain unpleasantness). Low (n=1) certainty was noted for the remaining association (supplementary table 4).
Mental health disorders, dementia, Alzheimer’s, and Parkinson’s disease
None of the 37 associations in various neuropsychiatric populations (ie, psychiatric disorders, dementia, Alzheimer’s disease, Parkinson’s disease, opioid use disorder, and cannabis use disorder) was supported by either high or moderate certainty. Low (n=26) or very low (n=11) certainty was found for all the associations (supplementary table 4).
Meta-analyses of observational studies
The eligible meta-analyses of observational studies were published between 2002 and 2022. The quality of included meta-analyses according to AMSTAR 2 was high in 13 meta-analyses, moderate in 24, low in 12, and critically low in one (table 1). The median number of individual studies included in the meta-analyses was 6 (interquartile range 4-13, range 2-69), the median number of participants was 1063 (526-4414, 44-5 962 412), and the median number of cases was 814 (447-2078, 126-8060).
The meta-analyses of observational studies reported a wide range of meta-analytical associations between cannabinoids and related health outcomes (supplementary table 5): cognitive, neuropsychological (n=81), brain function, volume (n=38), maternal and neonatal (n=12), psychosis symptoms and relapse (n=15), cancer (n=14), motor vehicle accidents (n=7), suicide (n=6), depression (n=4), behavioural inhibition (n=5), adherence to antipsychotic treatment (n=4), liver fibrosis (n=3), physical dating violence (n=2), and others (n=24). The 215 meta-analytical associations included 878 individual estimates from individual studies: 375 were derived from cohort studies, 493 from case-control studies, and 10 from mixed study designs.
Summary of associations
Of the 215 examined meta-analytical associations, 109 (51%) had a nominally statistically significant effect (P≤0.05) under the random-effects models, but only 14 of those (7%) reached a P value of 10−6 or less. Only 15 meta-analytical associations (7%) had more than 1000 cases and none had more than 20 000 participants for continuous outcomes. Sixty-eight meta-analytical associations (32%) exhibited large heterogeneity (I2 >50%), and only 12 of them (6%) had a 95% prediction interval that excluded the null value. Additionally, small study effects were found for 13 meta-analytical associations (6%) and excess significance bias was found for 15 (7%).
Only two associations (1%) showed a convincing level of evidence (class I), and one (<1%) showed highly suggestive evidence (class II). Of the remaining associations, four (2%) showed suggestive evidence (class III), 102 (47%) weak evidence (class IV), and 106 (49%) had no evidence (not significant). The table detailing the classification of the level of evidence is presented in the supplementary material (supplementary table 5). In the following sections, we principally described the associations with the highest classes (I-convincing, II-highly suggestive, III-suggestive) of the evidence in the main and general sensitivity analysis by subgroup of populations.
Credibility of evidence of associations between cannabinoids and outcomes
Pregnant women
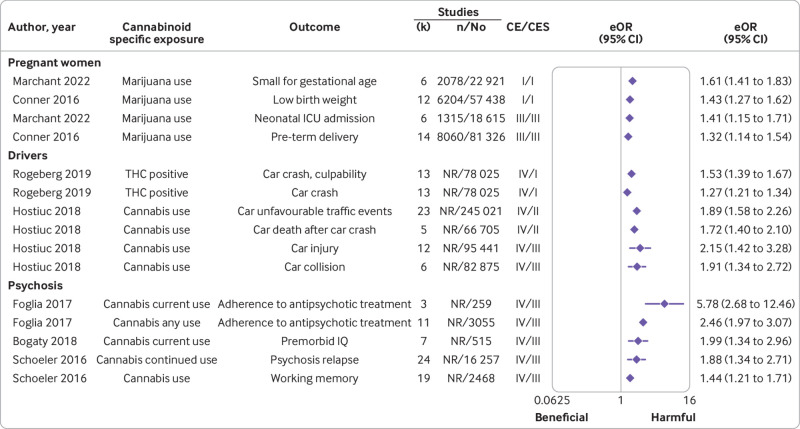
Among the 19 associations in this population only two outcomes (fig 4, supplementary table 5) presented convincing evidence with harmful effects of cannabinoids (marijuana use and low birth weight, equivalent odds ratio 1.43 (95% confidence interval 1.27 to 1.62)) and marijuana and small for gestational age (1.61 (1.41 to 1.83); both unadjusted estimates). Class III evidence emerged for two other associations with harmful effects: one between marijuana use and preterm delivery (1.32 (1.14 to 1.54)) and one between marijuana and neonatal intensive care unit admission (1.41 (1.15 to 1.71); both unadjusted estimates). After removing the criterion of number of cases of more than 1000 in the sensitivity analysis, no change was reported in the level of class I and III evidence, however, one additional association was upgraded from weak (class IV) to suggestive evidence (class III; mean birth weight, unadjusted; supplementary table 5). No evidence was found for the remaining associations (supplementary table 5).
Fig 4.
Observational meta-analytical associations between cannabis and outcomes in pregnant women, drivers, and people with psychosis supported by convincing, highly suggestive, or suggestive evidence in main or sensitivity analysis. Results are displayed in descending order of level of evidence and effect size; only associations for which an eOR was available are displayed. n=cases; N=population; CE=class of evidence (convincing (I), highly suggestive (II), suggestive (III), weak (IV)); CES=class of evidence after removing the n>1000 cases criterion; eOR=equivalent odds ratio; NR=not reported
The association between marijuana use and low birth weight was downgraded to no evidence using only adjusted estimates or cohort studies. The association between marijuana and small for gestational age remained at the same level (ie. convincing) using only cohort studies (adjusted sensitivity analysis not possible). The association with preterm delivery remained suggestive in analyses of only cohort studies, but the level was downgraded to no evidence with use of only adjusted estimates (supplementary table 5).
Drivers
None of the seven associations in this population was supported by convincing or highly suggestive evidence (class I and II) (fig 4, supplementary table 5). Evidence was weak (class IV) for the seven associations between cannabis use and driving outcomes with harmful effects (supplementary table 4). In the sensitivity analysis, after removing the criterion of number of studies as more than 1000, two associations were upgraded from weak (class IV) to convincing evidence (class I) for tetrahydrocannabinol and harmful effects of car crash and culpability (adjusted estimates). Two other associations between cannabis use and car death after car crash (unadjusted) and unfavourable traffic events related to cars (unadjusted) were upgraded from weak (class IV) to highly suggestive evidence (class II). Two additional associations between cannabis use and car collision and car injury (both unadjusted estimates) were upgraded from weak (class IV) to suggestive evidence (class III) (fig 4, supplementary table 5).
Psychosis
None of the 50 associations in this population was supported by convincing or highly suggestive evidence (class I and II) (fig 4, supplementary table 5). Weak evidence (class IV) was available for 13 associations with harmful effects, whereas no evidence was found for the remaining associations (supplementary table 5). After removing the criterion of more than 1000 cases in the sensitivity analysis, five associations of cannabinoids with harmful effects (ie, working memory, psychosis relapse, premorbid IQ (unadjusted), poor adherence to antipsychotics (two associations adjusted)) were upgraded from weak (class IV) to suggestive evidence (class III).
General population
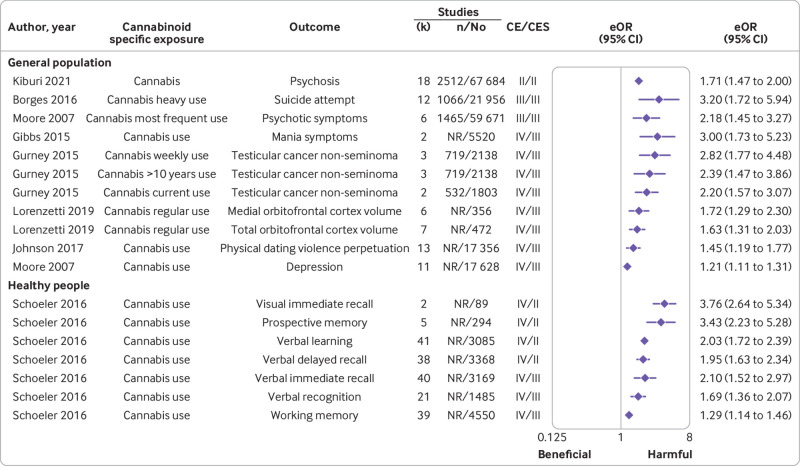
Among the 119 associations in this population, only one between cannabis and psychosis (fig 5, supplementary table 5) presented highly suggestive evidence with harmful effects of cannabinoids in adolescents (equivalent odds ratio 1.71 (95% confidence interval 1.47 to 2.00); no information on adjustments). Evidence was suggestive (class III) for two other associations with harmful effects: one between heavy use of cannabis and suicide attempt (3.20 (1.72 to 5.94)) and one between most frequent use of cannabis and psychotic symptoms (2.18 (1.45 to 3.27); both adjusted estimates). Weak or no evidence were found for the remaining associations (supplementary table 5). After removing the criterion of more than 1000 cases in the sensitivity analysis, the level of class II and III evidence did not change. However, one additional association with harmful effects between tetrahydrocannabinol and increased heart rate (unadjusted) was upgraded from weak (class IV) to highly suggestive evidence (class II). Additionally, eight associations with harmful effects were upgraded from weak (class IV) to suggestive evidence (class III) including mania symptoms (adjusted), depression (adjusted), testicular cancer (three associations), orbitofrontal cortex volume (medial, total), and physical dating violence (supplementary table 5). The association with cannabis and psychosis also remained highly suggestive (table 2), but the level of evidence was upgraded to convincing when only cohort studies were included (adjusted sensitivity analysis not possible).
Fig 5.
Observational meta-analytical associations between cannabis and outcomes in the general population and healthy people supported by convincing, highly suggestive, or suggestive evidence in main or sensitivity analysis excluding 1000 cases criterion. Results are displayed in descending order of level of evidence and effect size; only associations for which an eOR was available are displayed. n=cases; N=population; CE=class of evidence (convincing (I), highly suggestive (II), suggestive (III), weak (IV)); CES=class of evidence after removing the n>1000 cases criterion; eOR=equivalent odds ratio; NR=not reported
Healthy people who use cannabis
None of the eight associations in healthy people who use cannabis was supported by convincing or highly suggestive evidence (class I and II) (fig 5, supplementary table 5). Only weak evidence (class IV) was noted for eight associations between cannabis use and cognitive outcomes with harmful effects (supplementary table 5). After removing the criterion of more than 1000 cases in the sensitivity analysis, four associations with harmful effects (ie, visual immediate recall, prospective memory, verbal learning, and verbal delayed recall) were upgraded from weak (class IV) to highly suggestive evidence (class II). Additionally, three associations (ie, verbal immediate recall, verbal recognition, and working memory) were upgraded from weak (class IV) to suggestive evidence (class III) (fig 5, supplementary table 5).
Other populations
Across people with cannabis use disorder, insomnia, chronic pain, mixed conditions, hepatitis C virus or non-alcoholic fatty liver disease, and central nervous system malignant disease, none of the 12 associations was supported by convincing or highly suggestive evidence (class I and II, supplementary table 5). Weak evidence (class IV) was noted for five associations between cannabis use with harmful effects, whereas no evidence was found for the remaining associations (supplementary table 5). After removing criterion of more than 1000 cases in the sensitivity analysis, three associations of cannabinoids with beneficial effects (namely sleep quality or quantity improvement, pain relief, and hepatic steatosis (all unadjusted)) were upgraded from weak (class IV) to suggestive evidence (class III).
Other details of cannabis use, adjustment of analyses, and quality of individual studies
Details on type of cannabis, route of administration, use, variables that analyses were adjusted for, and quality or risk of bias of individual studies included in eligible meta-analyses are reported in supplementary material 3.
Of the 512 individual studies included in the eligible meta-analyses, 325 were observational studies and 187 were randomised controlled trials. Among the 325 observational studies (cohort n=160, cross-sectional n=97, case-control n=68), 211 reported on cannabis, 108 on marijuana, two on dronabinol, two on nabilone, one on cannabidiol, and one on tetrahydrocannabinol and cannabidiol. Of these, 312 focused on recreational use of cannabinoids, 12 on medical use, and one on both; 292 studies did not report the route of administration, which was inhaled in 28 studies and oral in five studies. Overall analyses were unadjusted in 79 studies and adjusted or matched in the remaining studies. The median Newcastle-Ottawa score of case-control and cohort studies was 7 (interquartile range 7-9).
Among the 187 randomised controlled trials, 64 reported on tetrahydrocannabinol, 32 on nabilone, 26 on nabiximols, 22 on cannabis, 18 on cannabidiol, and the remaining on various combinations of cannabis-based medicines, or other individual cannabis based medicines. Of these, 186 focused on medical use of cannabinoids, and one on recreational use; the route of administration was oral in 121, oral spray in 29, inhaled in 21, intravenous in six, intramuscular in four, oral and inhaled in three, and transdermal in two studies. The risk of bias was high in 79 randomised controlled trials, unclear in 55, low in 48, and moderate in five.
Discussion
Principal findings
This umbrella review grades the credibility and certainty of evidence on the effect of cannabinoid use, encompassing observational and interventional evidence.
Regarding harmful outcomes, among all meta-analytical associations supported by at least suggestive evidence in observational studies and moderate certainty in randomised controlled trials, converging evidence supports an increased risk of psychosis associated with cannabinoids in the general population. Specifically, cannabis use was associated with psychosis in adolescents (highly suggestive credibility, convincing certainty in main sensitivity analyses) and adults (suggestive credibility, suggestive certainty), and with psychosis relapse in people with a psychotic disorder (weak credibility, suggestive certainty). Use of cannabinoids in adult non-clinical and clinical populations was associated with positive (high certainty) and negative (moderate certainty) psychotic symptoms in randomised controlled trials.
Evidence from observational studies (weak credibility, suggestive certainty) and randomised controlled trials (high credibility, moderate certainty) show an association between cannabis and general psychiatric symptoms, including depression and mania, as well as detrimental effects on prospective memory, verbal delayed recall, verbal learning, and visual immediate recall (weak credibility, highly suggestive in observational evidence, moderate certainty in randomised controlled trials). Across different clinical and non-clinical populations, observational evidence suggests an association between cannabis use and motor vehicle accidents (weak credibility, convincing certainty). Additionally, evidence from randomised controlled trials shows an association with somnolence (cannabinoids (moderate certainty) and cannabidiol (high certainty)),103 and cannabis based medicines and visual impairment (high certainty), disorientation, dizziness, sedation, and vertigo (moderate certainty), among others.
These associations are of particular concern given the epidemiology and age pattern of cannabis use disorders, and the population attributable fraction of cannabis for schizophrenia, which is almost 10%.152 According to the Global Burden of Disease 2019, cannabis use disorders are associated with 690 000 (95% uncertainty interval 421 000-1 080 000) disability adjusted life years per 100 000 individuals globally.9 Prevalence and disability related to cannabis start to be measurable at ages 10-14 years (11 900 disability adjusted life years), peak at ages 20-24 years (163 000 disability adjusted life years), then gradually decrease.9 12 153 The age pattern of cannabis use disorders coincide with the peak age at onset of mental health disorders. According to the largest meta-analysis on the age at onset of mental disorders published to date, which pooled 192 studies and 708 561 individuals, around 34.6% of mental health disorders have onset by age 14 years, 48.4% by 18 years, and 62.5% by 25 years; the age that any mental health disorder onset peaks is at 14.5 years.154 For cannabis use disorders, 66% of people will have onset by age 25 years, with age of peak onset 20.5 years. Of note, age at peak onset of schizophrenia spectrum disorders is also in the early 20s, with a slightly lower proportion of people with onset by 25 years (47.8%). In addition to the association between cannabis and psychosis, cannabis is also associated with a worse outcome after onset, including poorer cognition,87 lower adherence to antipsychotics,56 and higher risk of relapse.85 In other words, use of cannabis when no psychotic disorder has already occurred increases the risk of its onset, and using cannabis after its onset, worsens clinical outcomes. Mood disorders also have their peak of onset close to that for cannabis use, which is of concern given the associations shown in this work between cannabis and depression, mania, and suicide attempt. Moreover, high tetrahydrocannabinol content cannabis could serve as a so-called gateway to other substances, particularly in younger people: this effect has been shown in humans155 and animal models,156 157 strengthening the recommendation to avoid cannabis use in adolescents and young adulthood.
Evidence suggests detrimental effects on cognition, an association with motor vehicle accidents, together with the age pattern of cannabis use (disorder), and related burden, which raise two additional matters. Firstly, given the adverse effects of cannabis on verbal delayed recall, verbal learning, visual immediate recall, and mental health, negative effects on scholastic or academic performance are reasonably expected, particularly in people who heavily use. Secondly, psychiatric symptoms such as suicide ideation and attempt, mania, and poor cognition, among other adverse events (eg, somnolence, disorientation, dizziness, sedation, vertigo, and visual impairment) might mediate the association between cannabis and increased risk of motor vehicle accidents. According to the DRUID project (driving under the influence of drugs, alcohol, and medicines in Europe), tetrahydrocannabinol ((0.5-2.2), measured as tetrahydrocannabinol or carboxy-tetrahydrocannabinol, in oral fluid or blood) is the second most frequent compound detected in seriously injured drivers, after alcohol (14.1-30.2%), then cocaine and amphetamines.158
Numerous observational associations indicated harmful outcomes, but they were either isolated without converging evidence from different study designs, supported by weak evidence only, or downgraded to not significant. Downgrading applied to the association between cannabis and low birth weight, and preterm delivery,100 which might be mediated by smoking.
Regarding the therapeutic potential of cannabis-based medicines, cannabidiol was beneficial in reducing seizures in certain forms of epilepsy in children and adults, including Lennox-Gastaut syndrome, Dravet syndrome, or other types of epilepsy. Cannabis based medicines were beneficial for pain and spasticity in multiple sclerosis, as well as for chronic pain in various conditions, and in palliative care, yet not without adverse events. However, cannabidiol and other cannabis-based medicines were associated with lower acceptability and tolerability than placebo in children and adults, and cannabis based medicines were also associated with psychiatric adverse events, as stated previously. These findings must be put into a clinical perspective to be fully appreciated and compared with available alternatives. Regarding epilepsy, established anticonvulsants are not free from adverse events, including sedation, weight gain, cognitive impairment, and psychiatric symptoms.159 160 161 Regarding chronic pain, excessive use of prescribed opioid medications has contributed to the opioid crisis, indicating the need for novel pharmacological and non-pharmacological treatment options for chronic pain162 to reduce prescribed opioid medications abuse. Regarding multiple sclerosis, botulinum toxin seems to be the only pharmacological alternative to cannabis based medicines for spasticity.110 163 Finally, the clinical populations included in eligible meta-analyses had treatment resistant or chronic conditions or were being treated in the context of palliative care and ongoing chemotherapy, and other treatment options had not proven effective. Thus, cannabis-based medicines could be reasonable options for chronic pain in different conditions, muscle spasticity in multiple sclerosis, and for nausea and vomiting in mixed clinical populations, and for sleep in people with cancer. Importantly, in patients with chronic pain, evaluation of the clinical effects considering the whole clinical presentation (several of the included reviews question the clinical value), the effects of prolonged use of cannabinoids still needs to be tested because current findings only come from short term randomised controlled trials. Also, active comparisons between cannabidiol and available options for epilepsy, as well as between cannabis-based medicines and other pain medications, other treatments for muscle spasticity in multiple sclerosis, or treatments for sleep in persons with cancer are needed, with a focus on both efficacy and safety, to inform future guidelines.
Overall, a mismatch is manifest between the legislation ruling cannabinoids versus alcohol use, considering both the well-known harms of alcohol on physical and mental health, in any age group,164 and the epidemiological figures. According to Global Burden of Disease 2019, alcohol use disorders were associated with 17 000 000 (95% uncertainty interval 13 500 000-21 500 000) disability adjusted life years per 100 000 individuals,9 roughly 25 times higher than for cannabis. Also, disability related to cannabis was largely limited to individuals aged 10-24 years, whereas alcohol is associated with disability from early stages of life, increasing continuously to 2 120 000 disability adjusted life years at age 35-39 years, and very slowly decreasing to less than 200 000 disability adjusted life years only after age 80 years.9 If cannabis use prevalence increased in the younger portion of the population due to large scale legalisation, whether the gap described previously would diminish is unclear. Moreover, to the best of our knowledge alcohol has no role as a medical treatment, whereas our research shows that cannabinoids can have beneficial effects in specific clinical conditions. The (scientific) reasoning behind extreme or ideological legislative approaches, namely complete legalisation and commercialization of cannabis even in young adults versus complete prohibition, and the different legislative requirements between cannabis and alcohol in disclosing to consumers the associated risks remains unclear.9
Strengths and limitations
The main strength of this work is that we pool evidence from different sources of evidence and deliberately consider convergence of results from different study designs. Also, this umbrella review is the first to pool observational and interventional studies on the effects of cannabinoids on humans.
Our results should be interpreted with caution. Firstly, the evidence from observational studies has ecological validity with regards to the type of cannabis available in the legal or illegal market on a large scale. However, tetrahydrocannabinol content and other cannabinoids on which no meta-analytical evidence was included can vary considerably among legal and illegally sold products. At the individual level, this variation can mean the difference between harmful or neutral or beneficial effects. Moreover, evidence from more than a decade ago, might not be representative of the cannabis that can be purchased nowadays illegally and legally, which is rich in tetrahydrocannabinol. This means that findings of this work might be underestimating harmful effects of cannabis. Also, the clinical effect of tetrahydrocannabinol on GABA and glutamate signalling via partial agonism on CB1 receptors depends on the concentration and distribution of CB1 receptors in the brain of each individual.10 As such, not all individuals will experience the same effects of cannabis on their mental health and cognition. Nonetheless, a crossover trial of 64 volunteers found that short term detrimental effects of 10 mg of inhaled tetrahydrocannabinol on psychological measures and cognition was not influenced by the co-administration of up to 30 mg of cannabidiol, potentially mitigating potential concerns with a role of tetrahydrocannabinol or cannabidiol ratio as a confounder of findings of this work.165 However, this trial was limited by a very short follow-up (90 min) and high loss to follow-up (28%). Furthermore, cannabidiol products that contain either no tetrahydrocannabinol, or subclinical amounts, are unlikely to result in psychological or cognitive impairment. Similarly, cannabis use disorder seems to have similar rates of people who use recreationally versus medically.166 Secondly, another reason to be cautious is that umbrella reviews neglect evidence from individual cohort studies or randomised controlled trials that have not been previously pooled in meta-analyses. However, individual studies need replication, are frequently exploratory, and need to be pooled in systematic reviews (and ideally meta-analyses) so that a comprehensive understanding of a given association or intervention can be appraised. Hence, any evidence that was not included in this umbrella review, even if potentially relevant, could be exploratory or preliminary. Thirdly, confounding factors could drive associations in observational evidence. However, we have applied stringent criteria, as confirmed by downgrading convincing evidence to non-significant on the association between cannabis and pregnancy outcomes. The quantitative criteria we applied to grade evidence from observational evidence accounted for selection and publication bias, excess of significance driven by small studies with larger effect sizes than the largest study in the meta-analysis, or marginal statistical significance driven by large sample sizes. Also, we have discussed findings from observational evidence in the context of converging evidence from different sources of evidence. For instance, observational studies might be affected by confounding factors, whereas randomised controlled trials might not be representative of the real-world population and affected by selection bias instead. We believe that converging evidence from these two study designs strengthens the ecological and methodological credibility of our findings. Fourthly, excess of significance bias testing might have been underpowered in meta-analyses with few studies, which could arguably apply to all meta-analyses included in this umbrella review, yet, a specific threshold of number of studies to set adequate power of excess of significance bias has not been established. Fifthly, we could have included meta-analyses based on their quality instead of the number of studies. However, that would have introduced a selection bias, leaving out a large portion of evidence. Sixthly, to harmonise effect sizes, we calculated the equivalent odds ratio as a measure of strength of the association. Yet, the harmonisation comes at the cost of losing information on time-to-event analyses and of course any association should be considered more in depth considering the frequency of each outcome, and the follow-up duration and time to event occurrence in each of the included studies. Additionally, the number of cases over the overall population of included studies does not reflect the prevalence of outcomes of interest. For instance, the global prevalence of preterm birth is 11.3%167 versus 9.9% reported in this work and varies across regions and countries’ income levels. The global prevalence of small for gestational age is 27%167 versus 9.1% reported here, with large variations across regions. The prevalence of testicular cancer is 0.04% versus 29.5% reported in the included meta-analysis of case-control studies. Lastly, and most importantly, the results of this work aim to inform future guidelines. These guidelines should account for additional aspects such as cost-effectiveness considerations, clinical relevancies (eg, numbers needed to treat for benefit), long term effects of cannabinoids on which evidence is lacking, and stakeholders, including patients and family members perspectives.
Future research assessing use of cannabis should clearly report what type of cannabis that patients used, how cannabis was administered, the content of tetrahydrocannabinol and cannabidiol, and the amount of cannabis consumed. The dose of exposure to different cannabinoids is needed to infer any causal relation between cannabis and outcomes.
Conclusions
Convincing or converging evidence supports that cannabis use is associated with poor mental health and cognition, increased the risk of car crashes, and can have detrimental effects on offspring if used during pregnancy. Cannabis use should be avoided in adolescents and young adults (when neurodevelopment is still occurring), when most mental health disorders have onset and cognition is paramount for optimising academic performance and learning, as well as in pregnant women and drivers. Conversely, cannabidiol could be considered a potential beneficial treatment option in epilepsy across age groups to reduce seizures. Cannabis based medicines could also be considered for chronic pain across different conditions, such as multiple sclerosis, spasticity in multiple sclerosis, for nausea and vomiting in people with mixed conditions and for sleep in cancer. However, clinical relevance must be considered before a possible incorporation into clinical guidelines; for example, including numbers needed to treat for benefit, risk to benefit ratios, comparative efficacy and safety with existing treatment options, and development of patient information concerning potential adverse events. Cannabidiol appears to be safe regarding psychiatric symptoms, but more research needs to be conducted before this drug can be recommended for the treatment of any psychiatric disorder. The remaining associations between cannabis and health outcomes are not supported by converging or convincing evidence.
Law and public health policy makers and researchers should consider this evidence synthesis when making policy decisions on cannabinoids use regulation, and when planning a future epidemiological or experimental research agenda, with particular attention to the tetrahydrocannabinol content of cannabinoids. Future guidelines are needed to translate current findings into clinical practice, while involving stakeholders.
What is already known on this topic
Observational evidence reported that cannabinoids were associated with numerous health outcomes and have been tested for several conditions in randomised controlled trials
Credibility and coherence of findings from different sources of evidence on the same outcomes have not been assessed to date
What this study adds
Most outcomes associated with cannabinoids are supported by weak evidence (observational studies), low to very low certainty (randomised controlled trials), or are not significant (observational studies, randomised controlled trials)
Convincing or converging evidence recommends avoiding cannabis during adolescence and early adulthood in people prone to have or have mental health disorder, who are pregnant, and while driving
Cannabidiol is effective for epilepsy, notably in children, while other cannabinoids can be effective in use for multiple sclerosis, chronic pain, inflammatory bowel disease, and palliative care
Web extra.
Extra material supplied by authors
Web appendix: Online appendix 1
Web appendix: Online appendix 2: table of characteristics of included meta-analyses
Web appendix: Online appendix 3: table of characteristics of individual studies, adjustment type, and type of cannabis
Contributors: MS, MDT, and JYK are joint first authors. JIS and MS contributed equally as corresponding authors. ED, MS, and JIS designed and supervised the study. FB, LB, MJC, GC, MDT, ED, JYK, SM, AM, FM, and MS screened the literature and extracted the data. MDT, ED, and MS drafted the manuscript. ED conducted the analyses, and MS had full access to data. All authors approved the project design, and critically reviewed, contributed to the final version of this work, and approve it. ED, MS, and MDT are guarantors of this work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: None.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: MS received honoraria/has been a consultant for AbbVie, Angelini, Lundbeck, Otsuka. DC has received grant monies for research from Eli Lilly, Janssen Cilag, Roche, Allergen, Bristol-Myers Squibb, Pfizer, Lundbeck, Astra Zeneca, Hospira; Travel Support and Honoraria for Talks and Consultancy from Eli Lilly, Bristol-Myers Squibb, Astra Zeneca, Lundbeck, Janssen Cilag, Pfizer, Organon, Sanofi-Aventis, Wyeth, Hospira, Servier, Seqirus; and is a current or past Advisory Board Member for Lu AA21004: Lundbeck; Varenicline: Pfizer; Asenapine: Lundbeck; Aripiprazole LAI: Lundbeck; Lisdexamfetamine: Shire; Lurasidone: Servier; Brexpiprazole: Lundbeck; Treatment Resistant Depression: LivaNova; Cariprazine: Seqirus. He is founder of the Optimal Health Program, currently operating as Optimal Health Australia; and is part owner of Clarity Healthcare. He is on the scientific advisory of The Mental Health Foundation of Australia. He does not knowingly have stocks or shares in any pharmaceutical company. EV has received grants and served as consultant, advisor or CME speaker for the following entities: AB-Biotics, AbbVie, Angelini, Biogen, Boehringer-Ingelheim, Celon Pharma, Dainippon Sumitomo Pharma, Ferrer, Gedeon Richter, GH Research, Glaxo-Smith Kline, Janssen, Lundbeck, Novartis, Orion Corporation, Organon, Otsuka, Sage, Sanofi-Aventis, Sunovion, Takeda, and Viatris, outside of the submitted work. CUC has been a consultant or advisor to or have received honoraria from: AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Boehringer-Ingelheim, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Janssen/Johnson & Johnson, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Newron, Noven, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Seqirus, SK Life Science, Sunovion, Sun Pharma, Supernus, Takeda, Teva, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and is also a stock option holder of Cardio Diagnostics, Mindpax, and LB Pharma.
All authors declare manuscript is an honest, accurate, and transparent account of the study being reported; no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained in published protocol.
Dissemination to participants and related patient and public communities: Our dissemination of findings will be shared via personal and institutional social media, the education of health professional trainees, continuous medical education activities for health professionals, and co-produced plain language summary of findings to be distributed to the clinical population with mental health disorders and to pregnant women. We will inform policy makers across different countries with written communications.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Ethical approval for this study was not required.
Data availability statement
The whole dataset is available from authors on request.
References
- 1. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 2008;153:199-215. 10.1038/sj.bjp.0707442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharkey KA, Wiley JW. The role of the endocannabinoid system in the brain-gut axis. Gastroenterology 2016;151:252-66. 10.1053/j.gastro.2016.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci 2018;19:833. 10.3390/ijms19030833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ElSohly MA, Slade D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. In: Life Sciences. Life Sci, 2005: 539-48. [DOI] [PubMed] [Google Scholar]
- 5. Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite effects of δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 2010;35:764-74. 10.1038/npp.2009.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sorensen CJ, DeSanto K, Borgelt L, Phillips KT, Monte AA. Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatment-a systematic review. J Med Toxicol 2017;13:71-87. 10.1007/s13181-016-0595-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V). Washington: American Psychiatric Association; 2013. [Google Scholar]
- 8. WHO . International Classification of Diseases - 11 (ICD-11). Version: 2020. World Health Organization, 2020. [Google Scholar]
- 9. Vos T, Lim SS, Abbafati C, et al. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204-22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Connor JP, Stjepanović D, Le Foll B, Hoch E, Budney AJ, Hall WD. Cannabis use and cannabis use disorder. Nat Rev Dis Primers 2021;7:16. 10.1038/s41572-021-00247-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peacock A, Leung J, Larney S, et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018;113:1905-26. 10.1111/add.14234 [DOI] [PubMed] [Google Scholar]
- 12.United Nations. World Drug Report 2020. 2020 (United Nations publication, Sales No. E.20.XI.6). [Google Scholar]
- 13. Hasin DS, Kerridge BT, Saha TD, et al. Prevalence and correlates of DSM-5 cannabis use disorder, 2012-2013: findings from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Am J Psychiatry 2016;173:588-99. 10.1176/appi.ajp.2015.15070907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Monitoring Centre for Drugs and Drug Addiction . European drug report 2018: Trends and developments. https://www.emcdda.europa.eu/publications/edr/trends-developments/2018_en 2018.
- 15. Hasin DS. US Epidemiology of cannabis use and associated problems. Neuropsychopharmacology 2018;43:195-212. 10.1038/npp.2017.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med 2014;370:2219-27. 10.1056/NEJMra1402309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Manthey J, Freeman TP, Kilian C, López-Pelayo H, Rehm J. Public health monitoring of cannabis use in Europe: prevalence of use, cannabis potency, and treatment rates. Lancet Reg Health Eur 2021;10:100227. 10.1016/j.lanepe.2021.100227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slade D, Mehmedic Z, Chandra S, ElSohly M. Is cannabis becoming more potent? In: Castle D, D’Souza DC, Murray RM, eds. Marijuana and Madness. 2nd ed. Cambridge University Press, 2011: 35-54 10.1017/CBO9780511706080.005 . [DOI] [Google Scholar]
- 19. Gobbi G, Atkin T, Zytynski T, et al. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: a systematic review and meta-analysis. JAMA Psychiatry 2019;76:426-34. 10.1001/jamapsychiatry.2018.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E. Meta-analysis of the association between the level of cannabis use and risk of psychosis. Schizophr Bull 2016;42:1262-9. 10.1093/schbul/sbw003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghasemiesfe M, Barrow B, Leonard S, Keyhani S, Korenstein D. Association between marijuana use and risk of cancer: a systematic review and meta-analysis. JAMA Network Open 2019;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ 2012;344:e536. 10.1136/bmj.e536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015;313:2456-73. 10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- 24. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (cannabidiol) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry 2018;175:225-31. 10.1176/appi.ajp.2017.17030325 [DOI] [PubMed] [Google Scholar]
- 25. Myran DT, Staykov E, Cantor N, et al. How has access to legal cannabis changed over time? An analysis of the cannabis retail market in Canada 2 years following the legalisation of recreational cannabis. Drug Alcohol Rev 2022;41:377-85. 10.1111/dar.13351 [DOI] [PubMed] [Google Scholar]
- 26. Myran DT, Pugliese M, Tanuseputro P, Cantor N, Rhodes E, Taljaard M. The association between recreational cannabis legalization, commercialization and cannabis-attributable emergency department visits in Ontario, Canada: an interrupted time-series analysis. Addiction 2022;117:1952-60. 10.1111/add.15834 [DOI] [PubMed] [Google Scholar]
- 27. Myran DT, Imtiaz S, Konikoff L, Douglas L, Elton-Marshall T. Changes in health harms due to cannabis following legalisation of non-medical cannabis in Canada in context of cannabis commercialisation: A scoping review. Drug Alcohol Rev 2023;42:277-98. 10.1111/dar.13546 [DOI] [PubMed] [Google Scholar]
- 28. Ioannidis JPA, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials 2007;4:245-53. 10.1177/1740774507079441 [DOI] [PubMed] [Google Scholar]
- 29. Siontis KC, Ioannidis JPA. Replication, duplication, and waste in a quarter million systematic reviews and meta-analyses. Circ Cardiovasc Qual Outcomes 2018;11:e005212. 10.1161/CIRCOUTCOMES.118.005212 [DOI] [PubMed] [Google Scholar]
- 30. Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health 2018;21:95-100. 10.1136/ebmental-2018-300014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Solmi M, Köhler CA, Stubbs B, et al. Environmental risk factors and nonpharmacological and nonsurgical interventions for obesity: An umbrella review of meta-analyses of cohort studies and randomized controlled trials. Eur J Clin Invest 2018;48:e12982. 10.1111/eci.12982 [DOI] [PubMed] [Google Scholar]
- 32. Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gates M, Gates A, Pieper D, et al. Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement. BMJ 2022;378:e070849. 10.1136/bmj-2022-070849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 37. IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 2014;14:25. 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Radua J, Ramella-Cravaro V, Ioannidis JPA, et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry 2018;17:49-66. 10.1002/wps.20490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 41. Kim JY, Son MJ, Son CY, et al. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiatry 2019;6:590-600. 10.1016/S2215-0366(19)30181-6 [DOI] [PubMed] [Google Scholar]
- 42. Dragioti E, Solmi M, Favaro A, et al. Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiatry 2019;76:1241-55. 10.1001/jamapsychiatry.2019.2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dragioti E, Karathanos V, Gerdle B, Evangelou E. Does psychotherapy work? An umbrella review of meta-analyses of randomized controlled trials. Acta Psychiatr Scand 2017;136:236-46. 10.1111/acps.12713 [DOI] [PubMed] [Google Scholar]
- 44. Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: An umbrella review of systematic reviews and meta-analyses. Alzheimers Dement 2017;13:406-18. 10.1016/j.jalz.2016.07.152 [DOI] [PubMed] [Google Scholar]
- 45. Belbasis L, Bellou V, Evangelou E, Ioannidis JPA, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol 2015;14:263-73. 10.1016/S1474-4422(14)70267-4 [DOI] [PubMed] [Google Scholar]
- 46. Dragioti E, Evangelou E, Larsson B, Gerdle B. Effectiveness of multidisciplinary programmes for clinical pain conditions: an umbrella review. J Rehabil Med 2018;50:779-91. 10.2340/16501977-2377 [DOI] [PubMed] [Google Scholar]
- 47. Stanley TD, Doucouliagos H, Ioannidis JPA, Carter EC. Detecting publication selection bias through excess statistical significance. Res Synth Methods 2021;12:776-95. 10.1002/jrsm.1512 [DOI] [PubMed] [Google Scholar]
- 48. Ioannidis JPA, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials 2007;4:245-53. 10.1177/1740774507079441 [DOI] [PubMed] [Google Scholar]
- 49. Köhler CA, Evangelou E, Stubbs B, et al. Mapping risk factors for depression across the lifespan: an umbrella review of evidence from meta-analyses and Mendelian randomization studies. J Psychiatr Res 2018;103:189-207. 10.1016/j.jpsychires.2018.05.020 [DOI] [PubMed] [Google Scholar]
- 50. Papola D, Ostuzzi G, Gastaldon C, et al. Antipsychotic use and risk of life-threatening medical events: umbrella review of observational studies. Acta Psychiatr Scand 2019;140:227-43. 10.1111/acps.13066 [DOI] [PubMed] [Google Scholar]
- 51. Bortolato B, Köhler CA, Evangelou E, et al. Systematic assessment of environmental risk factors for bipolar disorder: an umbrella review of systematic reviews and meta-analyses. Bipolar Disord 2017;19:84-96. 10.1111/bdi.12490 [DOI] [PubMed] [Google Scholar]
- 52. Solmi M, Köhler CA, Stubbs B, et al. Environmental risk factors and nonpharmacological and nonsurgical interventions for obesity: An umbrella review of meta-analyses of cohort studies and randomized controlled trials. Eur J Clin Invest 2018;48:e12982. 10.1111/eci.12982 [DOI] [PubMed] [Google Scholar]
- 53. Pollock A, Farmer SE, Brady MC, et al. An algorithm was developed to assign GRADE levels of evidence to comparisons within systematic reviews. J Clin Epidemiol 2016;70:106-10. 10.1016/j.jclinepi.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Escelsior A, Belvederi Murri M, Corsini GP, et al. Cannabinoid use and self-injurious behaviours: A systematic review and meta-analysis. J Affect Disord 2021;278:85-98. 10.1016/j.jad.2020.09.020 [DOI] [PubMed] [Google Scholar]
- 55. Farooqui MT, Khan MA, Cholankeril G, et al. Marijuana is not associated with progression of hepatic fibrosis in liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2019;31:149-56. 10.1097/MEG.0000000000001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Foglia E, Schoeler T, Klamerus E, Morgan K, Bhattacharyya S. Cannabis use and adherence to antipsychotic medication: a systematic review and meta-analysis. Psychol Med 2017;47:1691-705. 10.1017/S0033291717000046 [DOI] [PubMed] [Google Scholar]
- 57. Ghasemiesfe M, Ravi D, Vali M, et al. Marijuana use, respiratory symptoms, and pulmonary function: a systematic review and meta-analysis. Ann Intern Med 2018;169:106-15. 10.7326/M18-0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gibbs M, Winsper C, Marwaha S, Gilbert E, Broome M, Singh SP. Cannabis use and mania symptoms: a systematic review and meta-analysis. J Affect Disord 2015;171:39-47. 10.1016/j.jad.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 59. Goldenberg M, Reid MW, IsHak WW, Danovitch I. The impact of cannabis and cannabinoids for medical conditions on health-related quality of life: a systematic review and meta-analysis. Drug Alcohol Depend 2017;174:80-90. 10.1016/j.drugalcdep.2016.12.030 [DOI] [PubMed] [Google Scholar]
- 60. Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc 2003;9:679-89. 10.1017/S1355617703950016 [DOI] [PubMed] [Google Scholar]
- 61. Gunn JKL, Rosales CB, Center KE, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open 2016;6:e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gurney J, Shaw C, Stanley J, Signal V, Sarfati D. Cannabis exposure and risk of testicular cancer: a systematic review and meta-analysis. BMC Cancer 2015;15:897. 10.1186/s12885-015-1905-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hostiuc S, Moldoveanu A, Negoi I, Drima E. The association of unfavorable traffic events and cannabis usage: a meta-analysis. Front Pharmacol 2018;9:99. 10.3389/fphar.2018.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bhagavan C, Kung S, Doppen M, et al. Cannabinoids in the treatment of insomnia disorder: a systematic review and meta-analysis. CNS Drugs 2020;34:1217-28. 10.1007/s40263-020-00773-x [DOI] [PubMed] [Google Scholar]
- 65. Johnson RM, LaValley M, Schneider KE, Musci RJ, Pettoruto K, Rothman EF. Marijuana use and physical dating violence among adolescents and emerging adults: A systematic review and meta-analysis. Drug Alcohol Depend 2017;174:47-57. 10.1016/j.drugalcdep.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kamp F, Proebstl L, Penzel N, et al. Effects of sedative drug use on the dopamine system: a systematic review and meta-analysis of in vivo neuroimaging studies. Neuropsychopharmacology 2019;44:660-7. 10.1038/s41386-018-0191-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kiburi SK, Molebatsi K, Ntlantsana V, Lynskey MT. Cannabis use in adolescence and risk of psychosis: are there factors that moderate this relationship? A systematic review and meta-analysis. Substance abuse. New York, Kluwer Academic-Plenum-Human Sciences Press, 2021: 1-25. [DOI] [PubMed] [Google Scholar]
- 68. Kraan T, Velthorst E, Koenders L, et al. Cannabis use and transition to psychosis in individuals at ultra-high risk: review and meta-analysis. Psychol Med 2016;46:673-81. 10.1017/S0033291715002329 [DOI] [PubMed] [Google Scholar]
- 69. Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med 2014;44:797-810. 10.1017/S0033291713001438 [DOI] [PubMed] [Google Scholar]
- 70. Lorenzetti V, Chye Y, Silva P, Solowij N, Roberts CA. Does regular cannabis use affect neuroanatomy? An updated systematic review and meta-analysis of structural neuroimaging studies. Eur Arch Psychiatry Clin Neurosci 2019;269:59-71. 10.1007/s00406-019-00979-1 [DOI] [PubMed] [Google Scholar]
- 71. Marchand G, Masoud AT, Govindan M, et al. Birth outcomes of neonates exposed to marijuana in utero: a systematic review and meta-analysis. JAMA Netw Open 2022;5:e2145653-2145653. 10.1001/jamanetworkopen.2021.45653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007;370:319-28. 10.1016/S0140-6736(07)61162-3 [DOI] [PubMed] [Google Scholar]
- 73. Myles N, Newall H, Nielssen O, Large M. The association between cannabis use and earlier age at onset of schizophrenia and other psychoses: meta-analysis of possible confounding factors. Curr Pharm Des 2012;18:5055-69. 10.2174/138161212802884816 [DOI] [PubMed] [Google Scholar]
- 74. Noori A, Miroshnychenko A, Shergill Y, et al. Opioid-sparing effects of medical cannabis or cannabinoids for chronic pain: a systematic review and meta-analysis of randomised and observational studies. BMJ Open 2021;11:e047717-047717. 10.1136/bmjopen-2020-047717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Blest-Hopley G, Giampietro V, Bhattacharyya S. Residual effects of cannabis use in adolescent and adult brains - A meta-analysis of fMRI studies. Neurosci Biobehav Rev 2018;88:26-41. 10.1016/j.neubiorev.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 76. Power E, Sabherwal S, Healy C, O’ Neill A, Cotter D, Cannon M. Intelligence quotient decline following frequent or dependent cannabis use in youth: a systematic review and meta-analysis of longitudinal studies. Psychol Med 2021;51:194-200. 10.1017/S0033291720005036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rabin RA, Zakzanis KK, George TP. The effects of cannabis use on neurocognition in schizophrenia: a meta-analysis. Schizophr Res 2011;128:111-6. 10.1016/j.schres.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 78. Rocchetti M, Crescini A, Borgwardt S, et al. Is cannabis neurotoxic for the healthy brain? A meta-analytical review of structural brain alterations in non-psychotic users. Psychiatry Clin Neurosci 2013;67:483-92. 10.1111/pcn.12085 [DOI] [PubMed] [Google Scholar]
- 79. Rodriguez-Almaraz J-E, Chang S, Clarke J, et al. A systematic review and meta-analysis examining the effects of cannabis and its derivatives in adults with malignant CNS tumors. Neurooncol Pract 2020;7:376-83. 10.1093/nop/npaa013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rogeberg O. A meta-analysis of the crash risk of cannabis-positive drivers in culpability studies-avoiding interpretational bias. Accid Anal Prev 2019;123:69-78. 10.1016/j.aap.2018.11.011 [DOI] [PubMed] [Google Scholar]
- 81. Ruisch IH, Dietrich A, Glennon JC, Buitelaar JK, Hoekstra PJ. Maternal substance use during pregnancy and offspring conduct problems: A meta-analysis. Neurosci Biobehav Rev 2018;84:325-36. 10.1016/j.neubiorev.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 82. Ruiz-Veguilla M, Callado LF, Ferrin M. Neurological soft signs in patients with psychosis and cannabis abuse: a systematic review and meta-analysis of paradox. Curr Pharm Des 2012;18:5156-64. 10.2174/138161212802884753 [DOI] [PubMed] [Google Scholar]
- 83. Sabe M, Zhao N, Kaiser S. Cannabis, nicotine and the negative symptoms of schizophrenia: Systematic review and meta-analysis of observational studies. Neurosci Biobehav Rev 2020;116:415-25. 10.1016/j.neubiorev.2020.07.007 [DOI] [PubMed] [Google Scholar]
- 84. Sánchez-Gutiérrez T, Fernandez-Castilla B, Barbeito S, González-Pinto A, Becerra-García JA, Calvo A. Cannabis use and nonuse in patients with first-episode psychosis: A systematic review and meta-analysis of studies comparing neurocognitive functioning. Eur Psychiatry 2020;63:e6. 10.1192/j.eurpsy.2019.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schoeler T, Monk A, Sami MB, et al. Continued versus discontinued cannabis use in patients with psychosis: a systematic review and meta-analysis. Lancet Psychiatry 2016;3:215-25. 10.1016/S2215-0366(15)00363-6 [DOI] [PubMed] [Google Scholar]
- 86. Bogaty SER, Lee RSC, Hickie IB, Hermens DF. Meta-analysis of neurocognition in young psychosis patients with current cannabis use. J Psychiatr Res 2018;99:22-32. 10.1016/j.jpsychires.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 87. Schoeler T, Kambeitz J, Behlke I, Murray R, Bhattacharyya S. The effects of cannabis on memory function in users with and without a psychotic disorder: findings from a combined meta-analysis. Psychol Med 2016;46:177-88. 10.1017/S0033291715001646 [DOI] [PubMed] [Google Scholar]
- 88. Schreiner AM, Dunn ME. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. Exp Clin Psychopharmacol 2012;20:420-9. 10.1037/a0029117 [DOI] [PubMed] [Google Scholar]
- 89. Schumacher A, Marzell M, Toepp AJ, Schweizer ML. Association between marijuana use and condom use: A meta-analysis of between-subject event-based studies. J Stud Alcohol Drugs 2018;79:361-9. 10.15288/jsad.2018.79.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Scott JC, Slomiak ST, Jones JD, Rosen AFG, Moore TM, Gur RC. Association of Cannabis With Cognitive Functioning in Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018;75:585-95. 10.1001/jamapsychiatry.2018.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Smith JL, Mattick RP, Jamadar SD, Iredale JM. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend 2014;145:1-33. 10.1016/j.drugalcdep.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 92. Sultan SR, Millar SA, O’Sullivan SE, England TJ. A systematic review and meta-analysis of the in vivo haemodynamic effects of Δ9-tetrahydrocannabinol. Vol 11. Pharmaceuticals. MDPI AG, 2018: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Szoke A, Galliot A-M, Richard J-R, et al. Association between cannabis use and schizotypal dimensions—a meta-analysis of cross-sectional studies. Psychiatry Res 2014;219:58-66. 10.1016/j.psychres.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 94. Wijarnpreecha K, Panjawatanan P, Ungprasert P. Use of cannabis and risk of advanced liver fibrosis in patients with chronic hepatitis C virus infection: A systematic review and meta-analysis. J Evid Based Med 2018;11:272-7. 10.1111/jebm.12317 [DOI] [PubMed] [Google Scholar]
- 95. Xue S, Husain MI, Zhao H, Ravindran AV. Cannabis Use and Prospective Long-Term Association with Anxiety: A Systematic Review and Meta-Analysis of Longitudinal Studies: Usage du cannabis et association prospective à long terme avec l’anxiété: une revue systématique et une méta-analyse d’études longitudinales. Can J Psychiatry 2021;66:126-38. 10.1177/0706743720952251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang LR, Morgenstern H, Greenland S, et al. Cannabis and Respiratory Disease Research Group of New Zealand . Cannabis smoking and lung cancer risk: Pooled analysis in the International Lung Cancer Consortium. Int J Cancer 2015;136:894-903. 10.1002/ijc.29036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Borges G, Bagge CL, Orozco R. A literature review and meta-analyses of cannabis use and suicidality. J Affect Disord 2016;195:63-74. 10.1016/j.jad.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 98. Burns JK. Cannabis use and duration of untreated psychosis: a systematic review and meta-analysis. Curr Pharm Des 2012;18:5093-104. 10.2174/138161212802884672 [DOI] [PubMed] [Google Scholar]
- 99. Chisini LA, Cademartori MG, Francia A, et al. Is the use of Cannabis associated with periodontitis? A systematic review and meta-analysis. J Periodontal Res 2019;54:311-7. 10.1111/jre.12639 [DOI] [PubMed] [Google Scholar]
- 100. Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal marijuana use and adverse neonatal outcomes: a systematic review and meta-analysis. Obstetrics Gynecol 2016;128:713-23. [DOI] [PubMed] [Google Scholar]
- 101. de Carvalho MFF, Dourado MR, Fernandes IB, Araújo CTP, Mesquita AT, Ramos-Jorge ML. Head and neck cancer among marijuana users: a meta-analysis of matched case-control studies. Arch Oral Biol 2015;60:1750-5. 10.1016/j.archoralbio.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 102. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry 2019;6:995-1010. 10.1016/S2215-0366(19)30401-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chesney E, Oliver D, Green A, et al. Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology 2020;45:1799-806. 10.1038/s41386-020-0667-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Couch DG, Maudslay H, Doleman B, Lund JN, O’Sullivan SE. The Use of Cannabinoids in Colitis: A Systematic Review and Meta-Analysis. Inflamm Bowel Dis 2018;24:680-97. 10.1093/ibd/izy014 [DOI] [PubMed] [Google Scholar]
- 105. da Rovare VP, Magalhães GPA, Jardini GDA, et al. Cannabinoids for spasticity due to multiple sclerosis or paraplegia: a systematic review and meta-analysis of randomized clinical trials. Complement Ther Med;2017;34:170-85. [DOI] [PubMed] [Google Scholar]
- 106. de Carvalho Reis R, Almeida KJ, da Silva Lopes L, de Melo Mendes CM, Bor-Seng-Shu E. Efficacy and adverse event profile of cannabidiol and medicinal cannabis for treatment-resistant epilepsy: systematic review and meta-analysis. Epilepsy Behav 2020;102:106635. 10.1016/j.yebeh.2019.106635 [DOI] [PubMed] [Google Scholar]
- 107. De Vita MJ, Moskal D, Maisto SA, Ansell EB. Association of cannabinoid administration with experimental pain in healthy adults: a systematic review and meta-analysis. JAMA Psychiatry 2018;75:1118-27. 10.1001/jamapsychiatry.2018.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Doeve BH, van de Meeberg MM, van Schaik FDM, Fidder HH. A systematic review with meta-analysis of the efficacy of cannabis and cannabinoids for inflammatory bowel disease: what can we learn from randomized and nonrandomized studies? J Clin Gastroenterol 2021;55:798-809. [DOI] [PubMed] [Google Scholar]
- 109. Elliott J, DeJean D, Clifford T, et al. Cannabis-based products for pediatric epilepsy: A systematic review. Epilepsia 2019;60:6-19. 10.1111/epi.14608 [DOI] [PubMed] [Google Scholar]
- 110. Fu X, Wang Y, Wang C, et al. A mixed treatment comparison on efficacy and safety of treatments for spasticity caused by multiple sclerosis: a systematic review and network meta-analysis. Clin Rehabil 2018;32:713-21. 10.1177/0269215517745348 [DOI] [PubMed] [Google Scholar]
- 111. Gazendam A, Nucci N, Gouveia K, Abdel Khalik H, Rubinger L, Johal H. Cannabinoids in the Management of Acute Pain: A Systematic Review and Meta-analysis. Cannabis Cannabinoid Res 2020;5:290-7. 10.1089/can.2019.0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Allan GM, Finley CR, Ton J, et al. Systematic review of systematic reviews for medical cannabinoids: Pain, nausea and vomiting, spasticity, and harms. Can Fam Physician 2018;64:e78-94. [PMC free article] [PubMed] [Google Scholar]
- 113.Häuser W, Welsch P, Klose P, Radbruch L, Fitzcharles MA. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: A systematic review with meta-analysis of randomised controlled trials. Vol 33, Schmerz. Springer Verlag; 2019. 424-36. [DOI] [PubMed] [Google Scholar]
- 114. Kopelli E, Samara M, Siargkas A, Goulas A, Papazisis G, Chourdakis M. The role of cannabidiol oil in schizophrenia treatment. a systematic review and meta-analysis. Psychiatry Res 2020;291:113246. 10.1016/j.psychres.2020.113246 [DOI] [PubMed] [Google Scholar]
- 115. Lattanzi S, Brigo F, Trinka E, et al. Adjunctive cannabidiol in patients with dravet syndrome: a systematic review and meta-analysis of efficacy and safety. CNS Drugs 2020;34:229-41. 10.1007/s40263-020-00708-6 [DOI] [PubMed] [Google Scholar]
- 116. Lattanzi S, Trinka E, Striano P, et al. Cannabidiol efficacy and clobazam status: A systematic review and meta-analysis. Epilepsia 2020;61:1090-8. 10.1111/epi.16546 [DOI] [PubMed] [Google Scholar]
- 117. Lattanzi S, Brigo F, Cagnetti C, Trinka E, Silvestrini M. Efficacy and safety of adjunctive cannabidiol in patients with Lennox–Gastaut syndrome: a systematic review and meta-analysis. CNS Drugs 2018;32:905-16. 10.1007/s40263-018-0558-9 [DOI] [PubMed] [Google Scholar]
- 118. Hindley G, Beck K, Borgan F, et al. Psychiatric symptoms caused by cannabis constituents: a systematic review and meta-analysis. Lancet Psychiatry 2020;7:344-53. 10.1016/S2215-0366(20)30074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lattanzi S, Brigo F, Trinka E, et al. Efficacy and Safety of Cannabidiol in Epilepsy: A Systematic Review and Meta-Analysis. Drugs 2018;78:1791-804. 10.1007/s40265-018-0992-5 [DOI] [PubMed] [Google Scholar]
- 120. Lobos Urbina D, Peña Durán J. Are cannabinoids effective for treatment of pain in patients with active cancer? Medwave 2016;16(Suppl 3):e6539. 10.5867/medwave.2016.6539 [DOI] [PubMed] [Google Scholar]
- 121. McKee KA, Hmidan A, Crocker CE, et al. Potential therapeutic benefits of cannabinoid products in adult psychiatric disorders: A systematic review and meta-analysis of randomised controlled trials. J Psychiatr Res 2021;140:267-81. 10.1016/j.jpsychires.2021.05.044 [DOI] [PubMed] [Google Scholar]
- 122. McCartney D, Arkell TR, Irwin C, McGregor IS. Determining the magnitude and duration of acute Δ9-tetrahydrocannabinol (Δ9-tetrahydrocannabinol)-induced driving and cognitive impairment: A systematic and meta-analytic review. Neurosci Biobehav Rev 2021;126:175-93. 10.1016/j.neubiorev.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 123. Allende-Salazar RF, Rada G. Are cannabinoids an effective treatment for chronic non-cancer pain? Medwave 2017;17(Suppl2):e6972. 10.5867/medwave.2017.6972 [DOI] [PubMed] [Google Scholar]
- 124. Meza R, Peña J, García K, Corsi O, Rada G. Are cannabinoids effective in multiple sclerosis? Medwave 2017;17(Suppl1):e6865. 10.5867/medwave.2017.6865 [DOI] [PubMed] [Google Scholar]
- 125. Morales M, Corsi O, Peña J. Are cannabinoids effective for the management of chemotherapy induced nausea and vomiting? Medwave 2017;17:e7119. 10.5867/medwave.2017.09.7119 [DOI] [PubMed] [Google Scholar]
- 126. Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018;3:CD012182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mücke M, Weier M, Carter C, et al. Systematic review and meta-analysis of cannabinoids in palliative medicine. J Cachexia Sarcopenia Muscle 2018;9:220-34. 10.1002/jcsm.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Rodríguez A, Zavala C. Cannabinoids for the treatment of cannabis abuse disorder. Medwave 2018;18:e7287. 10.5867/medwave.2018.06.7286 [DOI] [PubMed] [Google Scholar]
- 129. Ruthirakuhan M, Lanctôt KL, Vieira D, Herrmann N. Natural and synthetic cannabinoids for agitation and aggression in alzheimer’s disease: a meta-analysis. J Clin Psychiatry 2019;80:18r12617. 10.4088/JCP.18r12617 [DOI] [PubMed] [Google Scholar]
- 130. Sainsbury B, Bloxham J, Pour MH, Padilla M, Enciso R. Efficacy of cannabis-based medications compared to placebo for the treatment of chronic neuropathic pain: a systematic review with meta-analysis. J Dent Anesth Pain Med 2021;21:479-506. 10.17245/jdapm.2021.21.6.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Spanagel R, Bilbao A. Approved cannabinoids for medical purposes - Comparative systematic review and meta-analysis for sleep and appetite. Neuropharmacology 2021;196:108680. 10.1016/j.neuropharm.2021.108680 [DOI] [PubMed] [Google Scholar]
- 132. Simon L, Baldwin C, Kalea AZ, Slee A. Cannabinoid interventions for improving cachexia outcomes in cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2022;13:23-41. 10.1002/jcsm.12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev 2015;2015:CD009464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Amato L, Minozzi S, Mitrova Z, et al. Revisione sistematica sull’efficacia terapeutica e la sicurezza della cannabis per i pazienti affetti da sclerosi multipla, dolore neuropatico cronico e pazienti oncologici che assumono chemioterapie. Epidemiol Prev 2017;41. [DOI] [PubMed] [Google Scholar]
- 135. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain 2018;159:1932-54. 10.1097/j.pain.0000000000001293 [DOI] [PubMed] [Google Scholar]
- 136. Stockings E, Zagic D, Campbell G, et al. Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. J Neurol Neurosurg Psychiatry 2018;89:741-53. 10.1136/jnnp-2017-317168 [DOI] [PubMed] [Google Scholar]
- 137. Sultan SR, Millar SA, England TJ, O’Sullivan SE. A Systematic Review and Meta-Analysis of the Haemodynamic Effects of Cannabidiol. Front Pharmacol 2017;8:81. 10.3389/fphar.2017.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Thanabalasingam SJ, Ranjith B, Jackson R, Wijeratne DT. Cannabis and its derivatives for the use of motor symptoms in Parkinson’s disease: a systematic review and meta-analysis. Ther Adv Neurol Disord 2021;14:17562864211018561. 10.1177/17562864211018561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Torres-Moreno MC, Papaseit E, Torrens M, Farré M. Assessment of efficacy and tolerability of medicinal cannabinoids in patients with multiple sclerosis: a systematic review and meta-analysis. JAMA Netw Open 2018;1:e183485. 10.1001/jamanetworkopen.2018.3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Treves N, Mor N, Allegaert K, et al. Efficacy and safety of medical cannabinoids in children: a systematic review and meta-analysis. Sci Rep 2021;11:23462. 10.1038/s41598-021-02770-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Velayudhan L, McGoohan K, Bhattacharyya S. Safety and tolerability of natural and synthetic cannabinoids in adults aged over 50 years: A systematic review and meta-analysis. PLoS Med 2021;18:e1003524. 10.1371/journal.pmed.1003524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. CMAJ 2008;178:1669-78. 10.1503/cmaj.071178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wang L, Hong PJ, May C, et al. Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: a systematic review and meta-analysis of randomised clinical trials. BMJ 2021;374:n1034. 10.1136/bmj.n1034 [DOI] [PubMed] [Google Scholar]
- 144. Watanabe AH, Navaravong L, Sirilak T, et al. A systematic review and meta-analysis of randomized controlled trials of cardiovascular toxicity of medical cannabinoids. J Am Pharm Assoc (2003) 2021;61:e1-13. 10.1016/j.japh.2021.03.013 [DOI] [PubMed] [Google Scholar]
- 145. Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain 2015;16:1221-32. 10.1016/j.jpain.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wong SSC, Chan WS, Cheung CW. Analgesic effects of cannabinoids for chronic non-cancer pain: a systematic review and meta-analysis with meta-regression. J Neuroimmune Pharmacol 2020;15:801-29. 10.1007/s11481-020-09905-y [DOI] [PubMed] [Google Scholar]
- 147. Zhang S, Li M, Guo Z. Effect of cannabidiol on schizophrenia based on randomized controlled trials: A meta-analysis. Ann Médico-psychologiques, Rev Psychiatr, 2021. [Google Scholar]
- 148. Aviram J, Samuelly-Leichtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician 2017;20:E755-96. 10.36076/ppj.20.5.E755 [DOI] [PubMed] [Google Scholar]
- 149. Bahji A, Meyyappan AC, Hawken ER. Cannabinoids for the neuropsychiatric symptoms of dementia: a systematic review and meta-analysis. Can J Psychiatry 2020;65:365-76. 10.1177/0706743719892717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bahji A, Meyyappan AC, Hawken ER. Efficacy and acceptability of cannabinoids for anxiety disorders in adults: a systematic review & meta-analysis. J Psychiatr Res 2020;129:257-64. 10.1016/j.jpsychires.2020.07.030 [DOI] [PubMed] [Google Scholar]
- 151. Bajtel Á, Kiss T, Tóth B, et al. The Safety of Dronabinol and Nabilone: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmaceuticals (Basel) 2022;15:100. 10.3390/ph15010100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Dragioti E, Radua J, Solmi M, et al. Global population attributable fraction of potentially modifiable risk factors for mental disorders: a meta-umbrella systematic review. Mol Psychiatry 2022;27:3510-9. 10.1038/s41380-022-01586-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Substance Abuse and Mental Health Services Administration. (2020). Key substance use and mental health indicators in the United States: results from the 2019 National Survey on Drug Use and Health. (HHS Publication No. PEP20-07-01-001, NSDUH Series H-55).
- 154. Solmi M, Radua J, Olivola M, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry 2022;27:281-95. 10.1038/s41380-021-01161-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lynskey MT, Heath AC, Bucholz KK, et al. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA 2003;289:427-33. 10.1001/jama.289.4.427 [DOI] [PubMed] [Google Scholar]
- 156. Friedman AL, Meurice C, Jutkiewicz EM. Effects of adolescent Δ9-tetrahydrocannabinol exposure on the behavioral effects of cocaine in adult Sprague-Dawley rats. Exp Clin Psychopharmacol 2019;27:326-37. 10.1037/pha0000276 [DOI] [PubMed] [Google Scholar]
- 157. Lecca D, Scifo A, Pisanu A, et al. Adolescent cannabis exposure increases heroin reinforcement in rats genetically vulnerable to addiction. Neuropharmacology 2020;166:107974. 10.1016/j.neuropharm.2020.107974 [DOI] [PubMed] [Google Scholar]
- 158. European Monitoring Centre for Drugs and Drug Addiction. Driving under the influence of drugs, alcohol and medicines in Europe—findings from the DRUID project. 2012. https://www.emcdda.europa.eu/publications/thematic-papers/druid_en [Google Scholar]
- 159. Yang C, Peng Y, Zhang L, Zhao L. Safety and tolerability of lacosamide in patients with epilepsy: a systematic review and meta-analysis. Front Pharmacol 2021;12:694381. 10.3389/fphar.2021.694381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zaccara G, Giovannelli F, Maratea D, Fadda V, Verrotti A. Neurological adverse events of new generation sodium blocker antiepileptic drugs. Meta-analysis of randomized, double-blinded studies with eslicarbazepine acetate, lacosamide and oxcarbazepine. Seizure 2013;22:528-36. 10.1016/j.seizure.2013.03.016 [DOI] [PubMed] [Google Scholar]
- 161. Josephson CB, Engbers JDT, Jette N, et al. Prediction tools for psychiatric adverse effects after levetiracetam prescription. JAMA Neurol 2019;76:440-6. 10.1001/jamaneurol.2018.4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Vyas MB, LeBaron VT, Gilson AM. The use of cannabis in response to the opioid crisis: A review of the literature. Nurs Outlook 2018;66:56-65. 10.1016/j.outlook.2017.08.012 [DOI] [PubMed] [Google Scholar]
- 163. Shakespeare DT, Boggild M, Young C. Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst Rev 2003;(4):CD001332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Bonomo Y, Norman A, Biondo S, et al. The Australian drug harms ranking study. J Psychopharmacol 2019;33:759-68. 10.1177/0269881119841569 [DOI] [PubMed] [Google Scholar]
- 165. Englund A, Oliver D, Chesney E, et al. Does cannabidiol make cannabis safer? A randomised, double-blind, cross-over trial of cannabis with four different:THC ratios. Neuropsychopharmacol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Mills L, Lintzeris N, O’Malley M, Arnold JC, McGregor IS. Prevalence and correlates of cannabis use disorder among Australians using cannabis products to treat a medical condition. Drug Alcohol Rev 2022;41:1095-108. 10.1111/dar.13444 [DOI] [PubMed] [Google Scholar]
- 167. Lee ACC, Katz J, Blencowe H, et al. CHERG SGA-Preterm Birth Working Group . National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health 2013;1:e26-36. 10.1016/S2214-109X(13)70006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Online appendix 1
Web appendix: Online appendix 2: table of characteristics of included meta-analyses
Web appendix: Online appendix 3: table of characteristics of individual studies, adjustment type, and type of cannabis
Data Availability Statement
The whole dataset is available from authors on request.