Abstract
The vacuolating cytotoxin and the cytotoxin-associated protein, encoded by vacA and cagA, respectively, are important virulence determinants of Helicobacter pylori. Sixty-five H. pylori strains were isolated from dyspeptic patients (19 with peptic ulcer disease, 43 with chronic gastritis, and 3 with gastric cancer) and studied for differences in the vacA and cagA genes and their relationship to VacA and CagA expression, cytotoxin activity, and the clinical outcome of infection. By PCR, fifty-four (83.1%) of 65 strains had the vacA signal sequence genotype s1 and only 10 (15.4%) had the type s2. After primer modification, the vacA middle-region types m1 and m2 were detected in 24 (36.9%) and 41 (63.1%) strains, respectively. The combinations s1-m2 (31 [47.7%]) and s1-m1 (23 [35.4%]) occurred more frequently than s2-m2 (10 [15.4%]) (P = 0.01). No strain with the combination s2-m1 was found. All 19 patients with peptic ulcers harbored type s1 strains, in contrast to 32 (74.4%) of 43 patients with gastritis (P = 0.02). The vacA genotype s1 was associated with the presence of cagA (P < 0.0001), VacA expression (P < 0.0001), and cytotoxin activity (P = 0.003). The cagA gene was detectable in 48 (73.8%) of 65 isolates and present in 16 (84.2%) of 19 ulcer patients and 29 (67.4%) of 43 patients with gastritis (P = 0.17). The vacA genotypes of German H. pylori isolates are identical to those previously reported. H. pylori strains of vacA type s1 are associated with the occurrence of peptic ulceration and the presence of cagA, cytotoxin activity, and VacA expression.
Helicobacter pylori, a spiral-shaped, microaerophilic bacterium that colonizes the stomach in humans has been identified as the cause of chronic gastritis, peptic ulcer disease (PUD), gastric cancer, and mucosa-associated lymphoid tissue lymphoma (15, 18, 25, 27). Once established, it may reside in the gastric mucosa for years, possibly for the life of the host (21), because the immunological defense mechanisms of the host fail to eliminate it.
There are specific virulence determinants in H. pylori strains, apart from immunological factors in the host, that influence the clinical outcome of infection. Virulence factors found in a subset of clinical isolates, such as the vacuolating cytotoxin (VacA) and the cytotoxin-associated protein (CagA), have been recently identified (4, 6, 11, 12, 16).
Only 50 to 65% of strains produce an 87-kDa cytotoxin that induces vacuolation of HeLa or primary gastric epithelial cells in vitro (4, 8, 12, 14, 16, 20). Infection with VacA-producing strains is associated with the presence of PUD (5, 12, 13). Recently, specific vacA genotypes, which are characterized by differences in the signal sequence and middle-region of the gene, have been identified in isolates obtained from U.S. subjects (1, 2). The vacA signal sequence type s1, but not type s2, was closely associated with in vitro cytotoxin activity, PUD, and the presence of the cagA gene. It is unknown whether H. pylori isolates from Europe have identical vacA genotypes or carry vacA alleles different from strains isolated in the United States.
The cagA gene is present in about 60 to 70% of H. pylori strains and encodes a high-molecular-weight protein (120 to 140 kDa) (4, 22). CagA-producing H. pylori strains have been detected in patients with PUD more frequently than in patients with chronic gastritis alone (4, 6, 10, 29). Sequencing of the cagA gene in H. pylori revealed a region of internal duplications which may be responsible for CagA size heterogeneity (4). However, no information is available on the influence on the clinical outcome with respect to diversity at the cagA gene level.
The objectives of this study were (i) to characterize the vacA alleles and analyze differences in the cagA gene that are present in German H. pylori strains and (ii) to correlate differences found within these genes with VacA and CagA protein expression, in vitro cytotoxicity, and clinical outcome.
MATERIALS AND METHODS
Patients, biopsy sampling, and cultivation of H. pylori strains.
Sixty-five H. pylori isolates from a consecutive series of patients with H. pylori infection undergoing upper gastrointestinal endoscopy and two reference strains, H. pylori 60190 (ATCC 49503) (cytotoxin producing, cagA+) and Tx30a (non-cytotoxin-producing, cagA negative) were used in this study (1, 16). Endoscopic and histological diagnoses were recorded for all patients. An ulcer was defined as an excavated mucosal break with a diameter of >5 mm. All patients gave informed consent to biopsy sampling. The study was approved by the Ethics Committee of the University of Heidelberg. Gastric biopsy specimens obtained from the antrum of all 65 patients and additionally from the corpus of 6 patients were homogenized, inoculated onto Columbia agar with 10% human blood and 10% horse serum, and grown under microaerophilic conditions at 37°C for 3 days. Multiple bacterial colonies were harvested from agar plates and frozen in Brucella broth containing 30% glycerine at −70°C. Subsequent analyses were performed on strains derived from the frozen stocks. All H. pylori isolates were positive for oxidase, catalase, and urease. The strains were numbered, and all analyses were performed without prior knowledge of the clinical diagnosis. Moreover, each analysis was performed by different investigators who were unaware of the other results.
Preparation of samples for PCR amplification.
Genomic DNA was initially isolated from H. pylori cultures by phenol-chloroform-isoamylalcohol extraction and ethanol precipitation according to standard protocols. Identical PCR results were obtained when supernatants from bacterial suspensions in sterile water were directly amplified. Therefore, further analyses were performed without the DNA isolation step.
PCR amplification and detection of amplified DNA products.
For vacA, primers vac1F and vac1R were used to amplify the signal sequence region (Table 1) (1). Amplification fragments of 201 and 228 bp were expected from genotype s1 and s2, respectively. The middle region of the vacA gene was analyzed with primers vac3F and vac3R (for m1) and vac4F and vac4R (for m2), which amplified 388-bp fragments for m1 and 346-bp fragments for m2.
TABLE 1.
Oligonucleotide primers used for typing of H. pylori vacA and cagA genes
| Gene | Region amplified | Primer designation | Primer sequence | PCR product size (location) |
|---|---|---|---|---|
| vacA | s1a | vac1F | 5′ GAAATACAACAAACACACCGC 3′ | 201 (800–1000b) |
| vac1R | 5′ GGCTTGTTTGAGCCCCCAG 3′ | |||
| s2a | vac1F | 5′ GAAATACAACAAACACACCGC 3′ | 228 (349–576c) | |
| vac1R | 5′ GGCTTGTTTGAGCCCCCAG 3′ | |||
| m1 | vac3F | 5′ GGTCAAAATGCGGTCATGG 3′ | 388 (2741–3128b) | |
| vac3R | 5′ CATCAGTATTTCGCACCACA 3′ | |||
| m2 | vac4F | 5′ CCAGGAAACATTGCCGGCAAA 3′ | 346 (2290–2635c) | |
| vac4R | 5′ CATAACTAGCGCCTTGCAC 3′ | |||
| cagA | Hydrophilic region | cag1 | 5′ GGAATTGTCTGATAAACTTG 3′ | 615 (2577–3191d) |
| cag3 | 5′ CCATTATTGTTATTGTTATTG 3′ | 612 (3114–3725e) | ||
| Region of internal duplications | cag2 | 5′ GGAACCCTAGTCGGTAATG 3′ | 450 (3070–3519d) | |
| cag4 | 5′ ATCTTTGAGCTTGTCTATCG 3′ | 558 (3604–4161e) |
PCR products of regions s1 and s2 were differentiated on the basis of molecular size and restriction endonuclease digestion with NlaIII.
Location in published vacA sequence of strain 60190 (8).
Location in strain Tx30a (GenBank accession no. U29401).
Location in published cagA sequence of strain CCUG 17874 (4).
Location in published cagA sequence of strain ATCC 53726 (22).
For the amplification of cagA sequences, two primer sets were used. The first primer set, cag1 and cag3, amplified a fragment of 612 to 615 bp from the hydrophilic region of cagA (Table 1) (4, 22). Primers cag2 and cag4 were derived from a region of internal duplications (4) and amplified DNA with expected fragment lengths of 450 and 558 bp (4, 22).
PCR amplification was performed as previously described (17), under the following conditions: initial denaturation at 95°C for 3 min followed by 35 cycles of denaturation at 95°C for 50 s, annealing and extension for 160 s, and final extension at 72°C for 2 min. Annealing temperatures were set at 55°C for vac1F-vac1R, at 60°C for vac3F-vac3R and vac4F-vac4R, and at 50°C for primers cag1-cag3 and cag2-cag4. Negative and positive controls (DNA of strain H. pylori 60190) were assayed in each run. Thirty microliters of each PCR mixture was subjected to gel electrophoresis on 5% polyacrylamide gels. Aliquots of the PCR products obtained with primers vac1F-vac1R and cag2-cag4 underwent restriction endonuclease digestion with NlaIII (New England Biolabs, Beverly, Mass.) for 2 h at 37°C prior to electrophoresis.
The PCR products obtained from amplification with primers cag2 and cag4 were hybridized with the probe hyb1 (5′ GATAAAGTTGATGATCTCAG 3′), which identified the complete amplification product in undigested samples and fragments of 102 and 143 bp in NlaIII-treated samples. Hybridization was performed as previously described (17), except that hybridization and washing temperatures were set at 56°C.
SDS-PAGE and Western blot analysis.
Gel electrophoresis and Western blotting were performed as previously described (19). In brief, supernatants of whole-cell sonicates, containing 10 μg of protein, from each strain were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After transfer to a nitrocellulose membrane, the membrane was blocked with low-fat skim milk and fetal calf serum in phosphate-buffered saline and incubated with a 1:10,000 dilution of rabbit antisera to VacA and to CagA (kindly provided by T. L. Cover, Nashville, Tenn., and by A. Covacci, Siena, Italy). Antiserum to VacA had been produced by immunizing rabbits with native VacA enriched in supernatant preparations from H. pylori 60190 (6). Rabbit antiserum to CagA was derived from a recombinant fusion protein obtained by subcloning a DNA fragment of cagA into the expression vector pEx34b (28). The membrane was incubated with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G antibody (dilution, 1:10,000) and then with enhanced chemiluminescence detection reagents (Western Light Chemilumiscent Detection System; Tropix, Bedford, Mass.) and exposed to highly sensitive X-ray films for 30 min.
Cytotoxicity assay on HeLa cells.
HeLa cells were cultured in plastic flasks containing Dulbecco’s modified Eagle’s medium with 25 mM HEPES buffer (pH 7.2), 5% fetal calf serum, and 2 mM glutamine at 37°C in a 5% CO2 atmosphere. Cells were seeded in 12-well plates at a concentration of 105 cells per well. H. pylori was cultured for 3 days at 37°C in 20% brain heart infusion, 70% RPMI, and 10% fetal calf serum in a gyratory water bath set under a microaerophilic atmosphere. Supernatants (final optical density at 550 nm, 0.6) were prepared by centrifugation (16,000 × g at 4°C) and filtration through a 0.2-μm-pore-size cellulose filter (12). The culture supernatants were diluted 1:1 with medium and incubated with HeLa cells for 24 h. Culture medium was supplemented with 10 mM ammonium chloride to potentiate cytotoxin activity (9). Vacuolation was assessed by light microscopy after staining with neutral red (7). Cytotoxicity was defined as positive if vacuolation was observed in more than 20% of cells.
Statistical methods.
Analysis of data was performed by using the χ2 test with Yates’ correction and Fisher’s exact test. Probability levels (P) of <0.05 were considered statistically significant.
RESULTS
Strain collection.
Sixty-five strains were isolated from H. pylori-positive patients with dyspeptic symptoms. Endoscopic and histological examination revealed gastritis in 43 patients, PUD in 19 patients (9 with gastric ulcers of the antrum and 10 with duodenal ulcers), and gastric adenocarcinoma in 3 patients. In all patients with gastric ulcers, moderate to severe antral predominant chronic gastritis was diagnosed histologically. Patients with duodenal ulcers presented with duodenitis and, in some cases, with chronic antral gastritis. In six patients, two isolates were obtained from the antrum and the corpus. For each of these patients, both isolates showed identical features by PCR, Southern blotting, and Western blot analysis.
Determination of vacA genotypes and relationship to gastrointestinal disease.
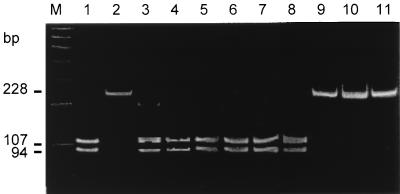
The vacA gene was detectable in all H. pylori isolates. By using the primers vac1F and vac1R to amplify the vacA signal sequences, the predicted PCR products of either 201 or 228 bp were obtained from 64 (98.5%) of 65 isolates. Fifty-four (83.1%) of 65 strains yielded the smaller product, representing genotype s1, and 10 (15.4%) yielded the larger product, representing genotype s2. None of the strains yielded a PCR product of any other size. All amplicons from genotype s1 but none of the amplicons from genotype s2 were cut by NlaIII into fragments of a predicted size of 94 and 107 bp (Fig. 1).
FIG. 1.
PAGE after PCR amplification of H. pylori DNA using primers vac1F-vac1R and NlaIII digestion. Only DNA amplicons obtained with vacA genotype s1 are cut by NlaIII into fragments of 107 and 94 bp. Lanes: M, 100-bp DNA marker; 1, strain H. pylori 60190 (vacA genotype s1); 2, Tx30a (vacA genotype s2); 3 to 8, type s1 isolates; 9 to 11, type s2 isolates. (The photographs in this and subsequent figures were scanned with a Hewlett-Packard Scan Jet 4c using the PaperPort Software Version 3.0.1 for Windows [Visioneer Communications, Inc.])
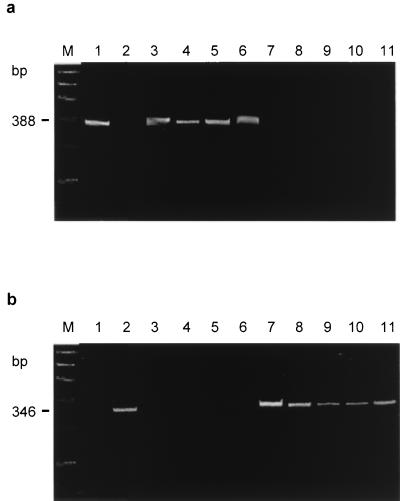
All 65 H. pylori strains and the two reference strains contained DNA which was amplified either by the primers vac3F and vac3R, representing type m1, or by the primers vac4F and vac4R, specific for type m2 (Fig. 2). Twenty-four (36.9%) isolates were classified as type m1, and 41 (63.1%) were classified as type m2. None had DNA amplified by both primer sets or gave PCR products of sizes other than those predicted. Among the 65 isolates studied, vacA homologs containing three of the four possible combinations of signal sequence and middle-region types were identified. The s1-m1 and s1-m2 combinations were found in 23 (35.4%) and 31 (47.7%) strains, respectively, and the s2-m2 combination was identified in 10 (15.4%) of 65 isolates. The s2-m1 combination was not detectable (P = 0.01).
FIG. 2.
PAGE after PCR amplification of H. pylori DNA using primers vac3F-vac3R (a) and vac4F-vac4R (b). Lanes: M, 100-bp DNA marker; 1 to 11, H. pylori strains as described in the legend to Fig. 1.
Infection with a type s1 strain was found in all 19 (100%) patients with PUD, compared with 32 (74.4%) of 43 subjects with gastritis (P = 0.02). All type s2 strains were isolated from patients with gastritis. The distribution of type s1-m1 and type s1-m2 strains was similar in patients with PUD (s1-m1, 8; s1-m2, 11) and gastritis (s1-m1, 13; s1-m2, 19), indicating that the vacA middle region types were not independently associated with the occurrence of PUD.
Prevalence and size variation of the cagA gene in H. pylori isolates.
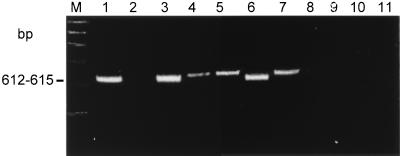
Forty-eight (73.8%) of 65 strains were cagA+. In 47 of 48 isolates and in reference strain H. pylori 60190, PCR products were obtained with both cagA primer sets (Fig. 3). One strain showed an amplicon only with the primer set cag2-cag4 but not with the primer set cag1-cag3. Sixteen (84.2%) of 19 strains isolated from patients with PUD but only 29 (67.4%) of 43 isolates obtained from patients with gastritis carried the cagA gene (P = 0.17). All three isolates from patients with gastric cancer were cagA+.
FIG. 3.
PAGE after PCR amplification of H. pylori DNA using primers cag1-cag3. Lanes: M, 100-bp DNA marker; 1 to 11, H. pylori strains as described in the legend to Fig. 1.
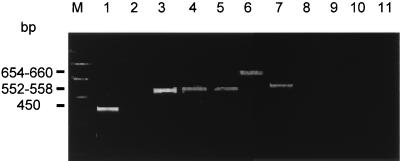
The PCR products of cagA+ strains obtained with primer set cag2-cag4 differed in size, varying from 450 bp in 8 isolates, including H. pylori 60190, to 552 to 558 bp in 22 isolates and 654 to 660 bp in 16 isolates (Fig. 4). NlaIII digestion of the 450-bp product resulted in DNA fragments of 307 to 313 bp and 143 bp. In addition, a fragment of 102 bp was obtained with PCR products of 552 to 558 bp and 654 to 660 bp after NlaIII digestion. Southern blotting with the probe hyb1 identified all fragments of 143 and 102 bp, if present. cagA PCR fragments of 450 bp or 552 to 558 bp were found more frequently in H. pylori strains isolated from patients with PUD (14 of 16 [87.5%]) than in those from patients with only gastritis (15 of 29 [51.7%]; P = 0.012).
FIG. 4.
PAGE after PCR amplification of H. pylori DNA using primers cag2-cag4. Lanes: M, 100-bp DNA marker; 1 to 11, H. pylori strains as described in the legend to Fig. 1. Note the size variation of DNA amplicons of different H. pylori strains.
Relationship between vacA genotypes and presence of cagA gene.
Forty-seven (87.0%) of 54 vacA type s1 strains were cagA+ (P < 0.0001). Of the 48 cagA+ strains, 47 (97.9%) were found to have the vacA genotype s1. The signal sequence type could not be determined in one cagA+ strain. None of the 10 type s2 strains carried the cagA gene. No association was found between vacA middle-region type and cagA status.
Relationship between vacA genotype or cagA gene and protein expression and cytotoxin activity.
By SDS-PAGE, supernatants of whole-cell sonicates showed the CagA protein band, varying between 120 and 140 kDa, which was recognized by immunoblotting with CagA antiserum in 35 (85.4%) of 41 cagA+ H. pylori strains. In cagA mutant strains, only 1 (5.9%) of 17 isolates was positive by immunoblotting (P < 0.0001). VacA protein was detected by VacA antiserum in 44 (75.9%) of 58 H. pylori isolates. Forty-one (87.2%) of 47 strains with the genotype s1 were detected by VacA antiserum. In contrast, only 3 (30%) of 10 strains with vacA type s2 showed a weak protein band by Western blotting (P < 0.0001).
Of the 58 H. pylori strains tested, supernatants from 34 (58.6%) induced vacuolation of HeLa cells (Tox+). Seventeen (81.0%) of 21 strains with the vacA genotype s1-m1 and 16 (61.9%) of 26 strains with s1-m2 genotype were Tox+. In contrast, only 1 (10%) of 10 strains with the genotype s2-m2 induced a weak vacuolation in HeLa cells. Thus, strains possessing the vacA type s1 (33 of 47 [70.2%]) were more frequently associated with vacuolating cytotoxicity than s2 type strains (P = 0.001). Although H. pylori strains isolated from patients with PUD were more likely to be Tox+ (13 of 17 [76.5%]) than those obtained from patients with gastritis only (21 of 37 [56.8%]), the differences were not significant (P = 0.16). Twenty-seven (67.5%) of 40 cagA+ strains and 8 (47.1%) of 17 cagA-negative strains induced vacuolation in HeLa cells (P = 0.147), demonstrating that cagA is not associated with cytotoxin activity.
DISCUSSION
In this study, new oligonucleotide primers were established to characterize the vacA genotypes in a consecutive series of isolated H. pylori strains. Using these primers, the vacA genotypes s1 and s2 were identified in all but one of the H. pylori strains. In addition, restriction endonuclease digestion with NlaIII allowed definitive differentiation of genotypes s1 and s2 by cutting only the PCR product of type s1. For determination of the vacA middle-region genotypes, the published oligonucleotide primers vac3R and vac4F were modified, because computer-assisted alignment of the various vacA sequences of European H. pylori strains deposited in the GenBank database revealed single base exchanges at the locations of the original primers (1). Therefore, primer sequences with complete identity next to these locations were selected. By using these primers for PCR, reliable classification of the vacA middle-region genotype was possible in all H. pylori strains. In contrast, use of the originally described primers (1) allowed characterization of middle-region types in only 70% of the H. pylori strains isolated from German patients (18a). This suggests that the mosaicism of the vacA gene described for H. pylori strains from the United States is also found in German H. pylori isolates. However, regional variation in the vacA gene may occur due to the enormous heterogeneity of H. pylori, which would thus require modification of the oligonucleotide primers to characterize the vacA alleles in European H. pylori strains.
H. pylori strains with the vacA signal sequence type s1 were predominant in the series of German H. pylori isolates, whereas strains with type s2 rarely occurred. However, the percentage of strains with the vacA genotype s2 may have been underestimated, since the strains investigated in this study were obtained from dyspeptic patients. Cytotoxic H. pylori strains with vacA genotype s1 may occur more frequently in such patients. vacA genotype s1 was strongly associated with the presence of cagA and was more frequent in patients with PUD (1, 2). The difference between vacA type s2 and type s1 leader sequences is generated by small inserts totaling 27 bp (1). It has been hypothesized that strains with vacA genotype s2 lack effective secretion of VacA through the cytoplasmic membrane of the bacteria (1, 20). Alternatively, differences in the N-terminal residues of the secreted VacA protein, arising from different signal sequence cleavage sites in VacA for type s1 and type s2, may be responsible for differences in protein function (1). In contrast to strains with vacA type s1, type s2 isolates express the VacA protein only in small amounts (1). This explains why almost all isolates with the vacA type s1 but only 3 of 10 type s2 strains had VacA detectable by immunoblot. Furthermore, cytotoxin activity was observed almost exclusively in type s1 strains. Only one type s2 strain showed weak vacuolation of HeLa cells. However, some strains with genotype s1 and lower cytotoxin activity may not have been identified because of the use of nonconcentrated broth culture supernatants for vacuolation testing (12).
Subgroup analysis demonstrated that the vacA middle-region type was not independently associated with the clinical outcome of H. pylori infection, the presence of cagA, or detectable cytotoxin activity. Almost half of the strains contained the combination s1-m2, a finding which is different from the results found in the United States (1), where the genotypes s1-m1 and s2-m2 are more common. The vacA genotype s2-m1 was not found, suggesting that strains with this genotype suffer from a selective disadvantage or are not viable.
About 74% of all isolates were cagA+ by PCR, which is in agreement with the results of other studies from Europe (10, 26, 28, 29). The majority of the patients with PUD (84%) were infected with cagA+ strains in contrast to strains isolated from patients with gastritis only, in whom 67% of the H. pylori strains were cagA+ (11, 26, 28). By PCR, size variations of the cagA gene were found within the various H. pylori strains. This may account for the size heterogeneity of CagA detected by immunoblot in recent studies (4, 26, 29). Restriction endonuclease analysis and Southern blotting suggested that these size variations may have been generated by a variable number of internal repeat units located downstream of nucleotide 3406 of the gene (4). Unexpectedly, the fragment length of this region of cagA after PCR was found to be shorter in strains isolated from patients with PUD than in those from patients with gastritis alone. It is unclear whether these differences in the cagA gene actually effect the clinical outcome of H. pylori infection or whether these results are incidental. The exact role of the cagA gene, which codes for the highly immunogenic CagA protein, is not known. The cagA gene represents a putative virulence marker and is included in the cag pathogenicity island, a 40-kb segment with several genes, including picA and picB, involved in cytokine induction (3, 24). It is conceivable that size variation of CagA could affect its antigenic properties and thereby alter the host’s immune system’s ability to recognize the antigen.
The presence of the cagA gene was correlated with the expression of CagA protein as determined by immunoblotting (29). However, cagA was not associated with cytotoxicity in HeLa cells and only 68% of cagA+ strains were cytotoxin positive. These results are consistent with other reports that cytotoxin production is derived from the vacA gene but not from the cagA gene (23, 26).
In conclusion, the vacA genotypes in German H. pylori strains were identical to those previously reported and could be characterized by PCR after modification of the reported oligonucleotide primers. H. pylori strains with the vacA signal sequence type s1 and middle-region allele m2 were predominant in German H. pylori isolates. All combinations of these vacA alleles occurred, with the exception of s2-m1. Type s1 strains were associated with peptic ulceration, presence of the cagA gene, VacA expression, and cytotoxin activity. Size variations in a distinct region of cagA are common, but their significance for clinical outcome needs further evaluation. The findings suggest that cagA+ H. pylori strains with the vacA genotype s1 increase the risk for PUD. Thus, vacA genotyping may allow identification of infected subjects at different risk levels.
ACKNOWLEDGMENTS
We thank J. Mohr for her excellent technical help and I. Zuna for performing the statistical analysis.
This study was supported by ASTRA GmbH, Wedel, Germany.
REFERENCES
- 1.Atherton J C, Cao P, Peek R M, Tummuru M K R, Blaser M J, Cover T L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 2.Atherton J C, Peek R M, Tham K T, Cover T L, Blaser M J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 3.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, Rappuoli R I. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cover T L, Cao P, Lind C D, Tham K T, Blaser M J. Correlation between vacuolating cytotoxin production by Helicobacter pylori isolates in vitro and in vivo. Infect Immun. 1993;61:5008–5012. doi: 10.1128/iai.61.12.5008-5012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cover T L, Dooley C P, Blaser M J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990;58:603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cover T L, Puryear W, Perez-Perez G I, Blaser M J. Effect of urease on HeLa cell vacuolation induced by Helicobacter pylori cytotoxin. Infect Immun. 1991;59:1264–1270. doi: 10.1128/iai.59.4.1264-1270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cover T L, Tummuru M K R, Cao P, Thompson S A, Blaser M J. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994;269:10566–10573. [PubMed] [Google Scholar]
- 9.Cover T L, Vaughn S G, Cao P, Blaser M J. Potentiation of Helicobacter pylori vacuolating cytotoxin activity by nicotine and other weak bases. J Infect Dis. 1992;166:1073–1078. doi: 10.1093/infdis/166.5.1073. [DOI] [PubMed] [Google Scholar]
- 10.Crabtree J E, Figura N, Taylor J D, Bugnoli M, Armellini D, Tompkins D S. Expression of 120 kilodalton protein and cytotoxicity in Helicobacter pylori. J Clin Pathol. 1992;45:733–734. doi: 10.1136/jcp.45.8.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabtree J E, Taylor J D, Wyatt J I, Heatley R V, Shallcross T M, Tompkins D S, Rathbone B J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration and gastric pathology. Lancet. 1991;59:1264–1270. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 12.Figura N, Guglielmetti P, Rossolini A, Barberi A, Cusi G, Musmanno R A, Russi M, Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989;27:225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goosens H, Glupczynski Y, Burette A, Lambert J-P, Vlaes L, Butzler J-P. Role of the vacuolating toxin from Helicobacter pylori in the pathogenesis of duodenal and gastric ulcer. Med Microbiol Lett. 1992;1:153–159. [Google Scholar]
- 14.Harris R P, Cover T L, Crowe D R, Orenstein J M, Graham M F, Blaser M J, Smith P D. Helicobacter pylori cytotoxin induces vacuolation of primary human mucosal epithelial cells. Infect Immun. 1996;64:4867–4871. doi: 10.1128/iai.64.11.4867-4871.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hentschel I, Brandstätter G, Dragosics B, Hirschl A M, Nemec H, Schütze K, Taufer M, Wurzer H. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori in the pathogenensis of duodenal and gastric ulcer. N Engl J Med. 1993;328:308–312. doi: 10.1056/NEJM199302043280503. [DOI] [PubMed] [Google Scholar]
- 16.Leunk R D, Johnson P T, David B C, Kraft W G, Morgan D R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- 17.Maiwald M, Ditton H-J, Sonntag H-G, von Knebel Doeberitz M. Characterization of contaminating DNA in Taq polymerase which occurs during amplification with a primer set for Legionella 5S ribosomal RNA. Mol Cell Probes. 1994;8:11–14. doi: 10.1006/mcpr.1994.1002. [DOI] [PubMed] [Google Scholar]
- 18.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orenteich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1231. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 18a.Rudi, J. Unpublished observation.
- 19.Rudi J, Kolb C, Maiwald M, Zuna I, Galle P R, Stremmel W. Serum antibodies against the Helicobacter pylori proteins CagA and VacA are associated with an increased risk for gastric adenocarcinoma. Dig Dis Sci. 1997;42:1652–1659. doi: 10.1023/a:1018849112533. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt W, Haas R. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: structural similarities with the IgA protease type of exported protein. Mol Microbiol. 1994;12:307–319. doi: 10.1111/j.1365-2958.1994.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 21.Taylor D N, Blaser M J. The epidemiology of Helicobacter pylori infection. Epidemiol Rev. 1991;13:42–59. doi: 10.1093/oxfordjournals.epirev.a036078. [DOI] [PubMed] [Google Scholar]
- 22.Tummuru M K R, Cover T L, Blaser M J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tummuru M K R, Cover T L, Blaser M J. Mutation of the cytotoxin-associated cagA gene does not affect the vacuolating cytotoxin activity of Helicobacter pylori. Infect Immun. 1994;62:2609–2613. doi: 10.1128/iai.62.6.2609-2613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tummuru M K, Sharma S A, Blaser M J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 25.Warren J R, Marshall B J. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1274. [PubMed] [Google Scholar]
- 26.Weel J F L, van der Hulst R W M, Gerrits Y, Roorde P, Feller M, Dankert J, Tytgat G N J, van der Ende A. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996;173:1171–1175. doi: 10.1093/infdis/173.5.1171. [DOI] [PubMed] [Google Scholar]
- 27.Wotherspoon A C, Ortiz-Hidalgo C, Falzon M R, Isaacson P G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 28.Xiang Z, Bugnoli M, Ponzetto A, Morgando A, Figura N, Covacci A, Petracca R, Pennatini C, Censini S, Armellini D, Rappuoli R. Detection in an enzyme immunoassay of an immune response to a recombinant fragment of the 128-kilodalton protein (CagA) of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1993;12:739–745. doi: 10.1007/BF02098460. [DOI] [PubMed] [Google Scholar]
- 29.Xiang Z, Censini S, Bayelli P F, Telford J L, Figura N, Rappuoli R, Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995;63:94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]