ABSTRACT
Introduction:
The successful restoration of damaged tissue requires a complicated, dynamic process called wound healing, which is supported by a wide range of cellular activities. Natural materials generated from medicinal plants have been identified, and their therapeutic potential evaluation has resulted in the development of novel, affordable medicines that can be used to treat a variety of illnesses, including chronic wounds, with limited side effects.
Aims and Objectives:
This study aimed to assess the wound-healing property of Glycyrrhiza Glabra (Athimathuram) plant extracts by using an in vitro scratch assay test, as well as to evaluate their cellular toxicity.
Materials and Methods:
Using the Soxhlet device, ethanolic extraction of the plant material was done, and the cytotoxicity of the extract on the Vero cell line was examined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. One of the most often utilized mammalian in vitro cell lines in research was Vero cells. To assess the wound healing properties of G. glabra plant extract, an in vitro scratch assay was used, and their potential mechanisms of action were examined.
Results:
Even at higher concentrations, the MTT assay showed that G. Glabra plant extracts had no cytotoxic effects on the cells. In vitro scratch assay showed that the healing process of the cell line was increased by 23.33% when compared with the controlled cell lines.
Conclusion:
Our research demonstrated that G. glabra has in vitro wound healing capabilities. As a result, G. glabra can be suggested as a possible source of compounds that treat wounds.
KEYWORDS: Athimathuram, glycyrrhiza glabra, herbal medicine, licorice, VERO cell line, wound healing
INTRODUCTION
The term “wound” refers to damage to living tissue that causes disturbance of the normal anatomical structure and function.[1] They develop due to tissue damage caused by physical, chemical, thermal, microbiological, or immunological factors. Wounds have a substantial social and economic impact on patients and their families. They induce excruciating pain, physical disabilities like immobility and loss of function, loss of self-esteem, despair, anxiety, and early mortality.[2]
Cells such as fibroblasts, keratinocytes, endothelial cells, macrophages, and other immune cells rapidly proliferate and migrate to the site upon tissue damage and begin the intricate healing process. Consequently, one of the crucial stages of the healing process for wounds is cell migration toward the wound, which is generally controlled by a variety of stimuli in the tissue microenvironment.[3]
The ability to measure a cell’s propensity to migrate under well-regulated experimental settings makes in vitro assays essential to the study of cell migration.[4]
The wound-healing process is delicate due to repeated disruptions and failures. Various local and systemic factors can interfere with one or more phases of wound healing, as a result of which the process is hampered. Age, stress, and chronic illnesses like diabetes mellitus, hepatic, renal failure, obesity, alcoholism, medicine, and immunocompromising disorders are all systemic contributors, while factors like infection, oxygenation, venous insufficiency, and the presence of foreign material in the wound would be the local factors.[3]
The conventional methods of the management of the wounds include debridement of the necrotic tissue with antimicrobials, followed by the application of topical agents as the first line of treatment. Recent and advanced techniques include the administration of hyperbaric oxygen, growth factors, negative pressure wound therapy, and skin grafts.[5]
Herbal treatments and medications have played an important role in the treatment of various illnesses since ancient times. Despite an extensive amount of literature available on the medicinal benefits, there are no established methods to assess the efficacy of plant materials with regard to their phytochemical, pharmacological, and therapeutic activity.[6]
Plant-based medicines have been used in human healthcare for thousands of years. Drugs made from plants were widely used in China and India.[7]
Numerous in vitro and animal models are available to test whether novel therapeutic compounds derived from these medicinal plants have the ability to cure wounds. One of them was the in vitro cell-based scratch assay test, which is an affordable and well established model that helps with the early comprehension of how effectively novel therapeutic agents help in the healing of wounds. Using this test, researchers may examine cell migration and intercellular communication. Because a scratch is made on a cell monolayer and time-lapse photos are taken at regular intervals, this procedure is also known as a scratch assay.
The little perennial plant Glycyrrhiza glabra (G. Glabra) often referred to as Athimathurum, licorice, sweet wood, or mulaithi, is native to Eurasia, northern Africa, and western Asia. It belongs to the Fabaceae family. The Glycyrrhiza genus, which has more than 30 species, is extensively scattered around the world.[8]
The most significant medicinal components of G. Glabra are its rhizomes and roots, which have been used either alone or in combination with other herbs to treat a variety of conditions, including epilepsy, fever, sexual debility, digestive system disorders, paralysis, leukorrhea, psoriasis, prostate cancer, malaria, hemorrhagic diseases, and jaundice. Additionally, it may be added to tobacco products to flavor them and be utilized as a flavoring ingredient in foods and beverages.[9]
Vero cells are widely used mammalian continuous cell lines in research; they were isolated from the kidney of an African green monkey in the 1960s. The multiplication and analysis of intracellular bacteria and parasites, as well as the evaluation of the molecular effects of drugs, poisons, and other substances on mammalian cells, are just a few of the numerous uses for this cell line.[10]
In the current study, an in vitro scratch assay test was performed on the Vero cell lines and was used to assess the wound-healing effectiveness of the ethanolic extract of the stem of G. Glabra.
MATERIALS AND METHODS
Collection of the test plant
G. Glabra was commercially available in shops; their plant stem and root were collected from Annai Aravindh Herbals, Maduravoyal, Chennai-95.
Preparation of the test sample
The G. glabra stem is properly washed with clean water. The stem and root were placed over a piece of clean newspaper on top of a woven basket and allowed to air dry. After drying for more than a few days, a substantial weight and volume loss is apparent. To accelerate the process, the stem and the root were broken up into tiny pieces and dried. Before being ground into a fine powder and prepared for extraction, the dried stem and root were checked for the presence of any dust or other foreign materials.
Extract preparation
According to several early experiments, ethanol solvent is the most effective in producing chemicals from G. glabra. At 78.37°C, condensation is used to extract the test plant powder using ethanol as the solvent.
Cell line and culture
The Vero cell lines were obtained from the National Center for Cell Sciences, Pune, and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Himedia Laboratories), which was combined with 10% Fetal Bovine Serum (Cistron Laboratories) and antibiotics (streptomycin 100 μg/ml and penicillin 100 μg/mL). The cells were passaged when they reached 80% confluency and were kept at 37°C with 50 μg/ml CO2 in a humidified incubator. To run the experiments, the viability was estimated and used to seed the cells at the proper densities.
Cytotoxicity activity: (MTT assay- Mosmann, 1983)
The cytotoxicity of G. Glabra extracts on Vero cell lines was evaluated by MTT assay. Cells (1 × 105/well) were seeded in 100 μl of DMEM in 96-well plates (Costar Corning, Rochester, NY) and incubated at 37°C with 5% CO2 condition. After the cell reaches confluence, the various concentrations (7.8, 15.6, 31.2, 62.5, 125, 250, 500, and 1000 μg/ml) of the samples were added and incubated for 24 hours. After incubation, the sample was removed from the well and washed with phosphate-buffered saline (pH 7.4) or DMEM without serum. About 100 μl/well (5 mg/ml) of 0.5% 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-tetrazolium bromide (MTT) was added and incubated for 4 hours. After incubation, the formazan crystals were solubilized with 1 ml of dimethyl sulfoxide (DMSO, Sigma) in all the wells. The absorbance at 570 nm was measured with UV-spectrophotometer using DMSO as the blank. Measurements were performed, and the concentration required for a 50% inhibition of viability (IC50) was determined graphically.[11]
Wound healing (scratch) assay
This assay is done to check cell migration. Protocols will vary according to the cell type being studied. However, there are some basic, fundamental steps that are applicable for almost all cell types: (i) cell culture preparation; (ii) scratch-making; (iii) data acquisition; and (iv) data analysis.
PROCEDURE:
The Vero cell line was used for the wound healing assay.
Cells were seeded into the six-well plate and incubated for 24 hours.
After incubation, the cells were observed for growth, and the assay was preceded.
The medium was discarded, and the plate was kept under a microscope for examination.
A sterile 200 μl tip was used, the wound was created, and the wells were washed with sterile Phosphate Buffered saline (PBS) to ensure the detached cells were removed [Refer Figure 1].
Around 1 ml of the test sample (G. Glabra extract) was added to the well and incubated.
Control well (without the test sample) was also maintained.
After 24 hours of incubation, the plate was observed for the growth and migration of the cells.
Figure 1.
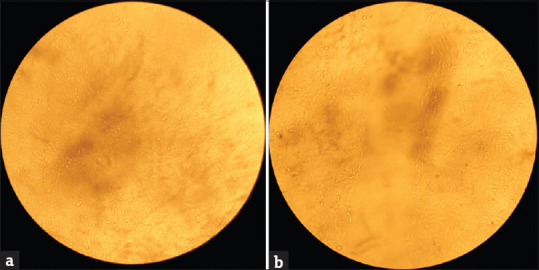
Microscopical image of VERO cell line and artificial creation of a wound. (a) Normal VERO Cell line (b) Wound Created (0th-hour measurement:- 0.6)
METRICS TO QUANTIFY CELL MIGRATION
The rate of cell migration can be quantified using a single metric or a combination of metrics. The following are the most commonly used metrics:
By manually tracing the cell-free region in collected photos using the Image J public domain software, the wound area may be determined (NIH, Bethesda, MD). In a typical scenario, the wound area will shrink over time. The change in the wound area over time can be used to indicate the migration rate. In 2016, Rötzer et al. demonstrated how the scratch wound assay may be used to evaluate the ability of keratinocytes to migrate under various experimental circumstances.[12] The percentage of area decrease or wound closure is another way to represent the migration rate. As cells move over time, the closure percentage will rise:

RESULTS
The % cell viability was calculated using the following formula:
% Cell viability = A570 of treated cells/A570 of control cells × 100
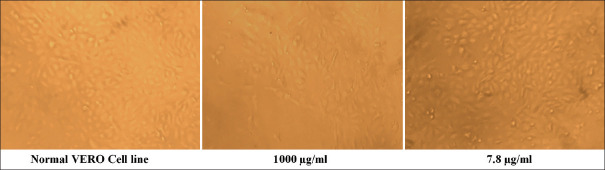
The cytotoxicity of the sample is tested using the Vero cell line; the samples were serially diluted to attain the desired concentrations and tested against the cells; the viability of the cells is calculated using the absorbance of treated and untreated cells; the viability of the cells seems to be increasing with decrease in concentration; the viability attained at the highest concentration is about 80% and increases as shown in the graph; this indicates that the sample is non-cytotoxic toward Vero cells [refer Figure 2 and Table 1].
Figure 2.
Microscopical image of VERO cell line at various concentrations of G. Glabra extracts. Normal VERO Cell line 1000 μg/ml 7.8 μg/ml
Table 1.
Cell viability of G. Glabra extracts on VERO cell lines
| Concentration (µg/ml) | Dilutions | Absorbance (O.D) | Cell Viability (%) |
|---|---|---|---|
| 1000 | Neat | 0.566 | 79.16 |
| 500 | 1:1 | 0.594 | 83.08 |
| 250 | 1:2 | 0.631 | 88.25 |
| 125 | 1:4 | 0.659 | 92.17 |
| 62.5 | 1:8 | 0.687 | 96.08 |
| 31.2 | 1:16 | 0.723 | 101.12 |
| 15.6 | 1:32 | 0.753 | 105.31 |
| 7.8 | 1:64 | 0.794 | 111.05 |
| cell control | - | 0.715 | 100.00 |
Graphs are plotted using the % of cell viability at the Y-axis and the concentration of the sample in X-axis. Cell control and sample control is included in each assay to compare the full cell viability assessments [Refer to Figure 3].
Figure 3.
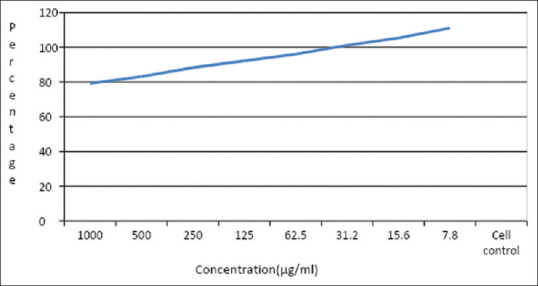
Graphical illustration of the cell viability assessments of control and test sample
Wound healing assay
Wound healing is done to estimate the cell migration by using the same cell line. The cells are seeded in a six-well plate and incubated in a CO2 incubator until a monolayer of a cell is formed. A wound is created on all the wells using a sterile tip, and the sample of the desired concentration is loaded and incubated in a CO2 incubator. After 24 hours, the wound is observed under an inverted microscope for movement, the distance between the wounds is measured, and photographs are taken. The distance between the separation seems to be reduced when compared to the initial wound created, indicating the wound is closing. The cell migrated in the sample is higher when compared to control cells, showing that the sample has wound-healing properties [Refer Figure 4].
Figure 4.
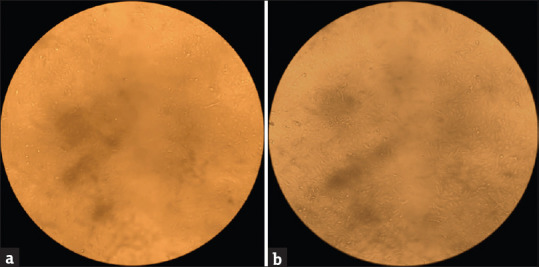
Microscopical image of the wound closure in VERO cell line after 24 hours of incubation of the G. Glabra extracts and control sample. (a)Control (24th hour measurement:- 0.49) (b) Sample (24th hour measurement:- 0.35)
METRICS TO QUANTIFY CELL MIGRATION
Cell migration for the control and test samples at 0 and 24 hrs was 0.60,0.49 and 0.60,0.35 respectively. Wound closure for the control and test sample was found to be 18.33% and 41.66% respectively using the formula mentioned above. It shows that the healing process of the cell line is increased in the sample as compared to the control. The calculation reveals that the healing process of the cell line increased by 23.33%.
DISCUSSION
Wound healing is a dynamic, orchestrated process that restores the cellular structure of injured tissues to their original healthy state. Since the beginning of human agriculture, plants have been one of the most significant sources of medicine.
G. Glabra, or Licorice, a native of south-east Europe and south-west Asia, was one of the widely used herbal medicine and a traditionally used flavoring/aromatic and calming plant that belongs to the family Papilionaceae/Fabaceae.[13] G. Glabra is a herbaceous perennial that may reach a height of 1 m. It has pinnate leaves that are 715 cm long and have 9-17 leaflets.
The most significant medicinal components of G. Glabra are its rhizomes and roots, which have been used either alone or in combination with other herbs to treat a variety of conditions, including epilepsy, fever, sexual debility, digestive system disorders, paralysis, leukorrhea, psoriasis, prostate cancer, malaria, hemorrhagic diseases, and jaundice.[9]
In ancient times, G. Glabra was used to treat bacterial and fungal infections, but so far only limited studies are available in the literature that report the wound-healing activity of this plant. Studies have shown that G. Glabra can have a role in the wound healing of gastric, oral, and colitis mucosal ulcers and in burning wound healing.[14-16] Considering the aforementioned facts, in this study, an in vitro scratch assay test was performed on the Vero cell lines using ethanolic extracts to check the effectiveness of this plant on wound healing.
The MTT assay was used to investigate the cytotoxic effects of the ethanolic extract of G. Glabra on the Vero cell line. Cells that had not been treated had a 100% viability rate. UV-spectrophotometer was used at 570 nm to determine the viable cells. At the highest concentrations of G. Glabra 1000 μg/mL and 500 μg/mL, the cells’ percentage of viability was found to be 80% and 83%, respectively, indicating no cytotoxic effect. Similar results were shown in the study conducted by Metar Siriwattanasatorn et al. in 2020. In their study, the authors found accelerated in vitro wound healing ability of the G. Glabra extracts.[17]
Cell migration is a key factor in the process of wound healing. Vero cell lines were used in our study. In both the sample and control cell lines, a wound of 0.6 mm was produced using Vero cell lines. It was then observed at 0 hours and 24 hours. After 24 hours of creating a wound in the cell line, a microscopic inspection was performed to ascertain the wound healing activity. Cell migration was measured. G. Glabra extract showed improved wound healing rates by around 24% when compared to the control. Our research demonstrated the efficiency of G. Glabra in the healing of wounds in the Vero cell lines.
Hanafi et al. 2018 in their studies found that G. Glabra creams were effective in acute dermal wound healing in a Guinea pig animal model. In their study, they found the neovascularization, epidermal formation, and collagen deposition were significantly improved by the application of G. Glabra extracts at different concentrations in the wounds when compared to the controls.[13]
In order to understand the wound-healing capacity of G. Glabra and extend the positive impacts of this plant to humans, further research was necessary to demonstrate the wound-healing characteristics of this plant in vivo.
CONCLUSION
Even today, effective management of wounds remains challenging in clinical settings. Wound care has been the subject of many studies, with a focus on novel therapeutic approaches and the development of plant-based products for the management of a variety of wounds. Researchers are looking at new formulae, dressings, and medicinal plant components in an effort to create a delivery system for the care and treatment of wounds that is affordable, effective, efficient, stable, and sustainable. The study has looked at G. Glabra’s capacity to heal wounds. In Vero cell lines, it was discovered that the ethanolic extract of G. Glabra improved wound closure. Furthermore, it was found that the extract had no cytotoxic effects. These findings imply that G. Glabra may possess wound-healing abilities and may represent a viable source for the extraction of natural chemicals with wound-healing capabilities. The G. Glabra’s full potential can be realized with more study of the plant.
Financial support and sponsorship
Self funded.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tyavambiza C, Dube P, Goboza M, Meyer S, Madiehe AM, Meyer M. Wound healing activities and potential of selected african medicinal plants and their synthesized biogenic nanoparticles. Plants (Basel) 2021;10:2635. doi: 10.3390/plants10122635. https://doi.org/10.3390/ plants10122635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, et al. Prevalence and incidence of chronic wounds and related complications:A protocol for a systematic review. Syst Rev. 2017;6:1–7. doi: 10.1186/s13643-016-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–29. doi: 10.1177/0022034509359125. doi:10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobadilla AVP, Arévalo J, Sarró E, Byrne HM, Maini PK, Carraro T, et al. In vitro cell migration quantification method for scratch assays. J R Soc Interface. 2019;16:20180709. doi: 10.1098/rsif.2018.0709. http://dx.doi.org/10.1098/rsif. 2018 0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han G, Ceilley R. Chronic wound healing:A review of current management and treatments. Adv Ther. 2017;34:599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma A, Khanna S, Kaur G, Singh I. Medicinal plants and their components for wound healing applications. Futur J Pharm Sci. 2021;7:53. https://doi.org/10.1 186/s43094-021-00202-w. [Google Scholar]
- 7.Alam G, Singh MP, Singh A. Wound healing potential of some medicinal plants. Int J Phar Sci Rev Res. 2011;9:136–45. [Google Scholar]
- 8.El-Saber Batiha G, Beshbishy AM, El-Mleeh A, Abdel-Daim MM, Devkota HP. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of glycyrrhiza glabra L (Fabaceae) Biomolecules. 2020;10 doi: 10.3390/biom10030352. https://doi.org/10.3390/biom10030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MBPP. Liquorice (Glycyrrhiza glabra):A phytochemical and pharmacological review. Phytother Res. 2018;32:2323–39. doi: 10.1002/ptr.6178. doi:10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ammerman NC, Beier-Sexton M, Azad AF. Growth and maintenance of Vero cell lines. Curr Protoc Microbiol. 2008 doi: 10.1002/9780471729259.mca04es11. Appendix 4:Appendix 4E. doi:10.1002/9780471729259.mca04es11. PMID:19016439;PMCID:PMC2657228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosmann T. Rapid colorimetric assay for cellular growth and survival:Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. doi:10.1016/0022-1759 (83) 90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Rötzer V, Hartlieb E, Winkler J, Walter E, Schlipp A, Sardy M, et al. Desmoglein 3-dependent signaling regulates keratinocyte migration and wound healing. J Invest Dermatol. 2016;136:301–10. doi: 10.1038/JID.2015.380. doi:10.1038/JID.2015.380. [DOI] [PubMed] [Google Scholar]
- 13.Hanafi N, Amiri FT, Shahani S, Enayatifard R, Ghasemi M, Karimpour AA. Licorice cream promotes full-thickness wound healing in guinea pigs. Marmara Pharm J. 2018;22:411–21. [Google Scholar]
- 14.Memariani Z, Hajimahmoodi M, Minaee B, Khodagholi F, Yans A, Rahimi R, et al. Protective effect of a polyherbal traditional formula consisting of Rosa damascena Mill., Glycyrrhiza glabra L. and Nardostachys jatamansi DC., against ethanol-induced gastric ulcer. Iran J Pharm Res. 2017;16:694–707. [PMC free article] [PubMed] [Google Scholar]
- 15.Najeeb VD, Al-Refai AS. Antibacterial effect and healing potential of topically applied licorice root extract on experimentally induced oral wounds in rabbits. Saudi J Oral Sci. 2015;2:10–3. [Google Scholar]
- 16.Chen X, Fang D, Li L, Chen L, Li Q, Gong F, et al. Glycyrrhizin ameliorates experimental colitis through attenuating interleukin-17-producing T cell responses via regulating antigen-presenting cells. Immunol Res. 2017;65:666–80. doi: 10.1007/s12026-017-8894-2. [DOI] [PubMed] [Google Scholar]
- 17.Siriwattanasatorn M, Itharat A, Thongdeeying P, Ooraikul B. In Vitro wound healing activities of three most commonly used thai medicinal plants and their three markers. Evid Based Complement Alternat Med. 2020;2020:6795383. doi: 10.1155/2020/6795383. https://doi.org/10.1155/2020/6795383. [DOI] [PMC free article] [PubMed] [Google Scholar]