Significance
The controlling germline stem cell (GSC) niche is known to control stem cell self-renewal, but it remains poorly understood how the differentiation niche signals directly to stem cell progeny to control their differentiation. In the Drosophila ovary, inner germarial sheath cells form the differentiation niche for GSC progeny differentiation. Gap junctions transport the secondary messenger cAMP from the differentiation niche, which activates PKA signaling in early stem cell progeny to control their stepwise differentiation, including cyst formation, meiotic DNA break formation, and oocyte specification. This study represents a significant advance in our understanding of the mechanisms underlying niche-mediated direct control of stem cell progeny differentiation and has significant implications for our understanding of stem cell biology and regenerative medicine.
Keywords: niche, stem cell, differentiation, gap junction, cAMP
Abstract
The niche has been shown to control stem cell self-renewal in different tissue types and organisms. Recently, a separate niche has been proposed to control stem cell progeny differentiation, called the differentiation niche. However, it remains poorly understood whether and how the differentiation niche directly signals to stem cell progeny to control their differentiation. In the Drosophila ovary, inner germarial sheath (IGS) cells contribute to two separate niche compartments for controlling both germline stem cell (GSC) self-renewal and progeny differentiation. In this study, we show that IGS cells express Inx2 protein, which forms gap junctions (GJs) with germline-specific Zpg protein to control stepwise GSC lineage development, including GSC self-renewal, germline cyst formation, meiotic double-strand DNA break formation, and oocyte specification. Germline-specific Zpg and IGS-specific Inx2 knockdowns cause similar defects in stepwise GSC development. Additionally, secondary messenger cAMP is transported from IGS cells to GSCs and their progeny via GJs to activate PKA signaling for controlling stepwise GSC development. Therefore, this study demonstrates that the niche directly controls GSC progeny differentiation via the GJ-cAMP-PKA signaling axis, which provides important insights into niche control of stem cell differentiation and highlights the importance of GJ-transported cAMP in tissue regeneration. This may represent a general strategy for the niche to control adult stem cell development in various tissue types and organisms since GJs and cAMP are widely distributed.
Stem cells exist in adult tissues to maintain homeostasis through continuous self-renewal and differentiation in organisms ranging from Drosophila to humans. Stem cells have also been proposed to treat various human diseases, including diabetes and Parkinson’s and Alzheimer’s diseases (1). The niche has been demonstrated to control stem cell self-renewal in various systems (2, 3). The differentiation of stem cell progeny has recently been proposed to be also controlled by another niche, the differentiation niche, first in the Drosophila ovary and then in the mouse testis (4–6). One of the major obstacles for clinical applications of stem cells in treating human diseases is the lack of a comprehensive understanding of how stem cells are regulated by the niche, particularly their lineage specification. In this study, we have shown that cAMP can be transported from the differentiation niche through gap junctions (GJs) to stem cell progeny to directly control multistep differentiation processes.
The Drosophila ovary is an effective model for studying stem cell–niche interactions in the regulation of germline stem cell (GSC) self-renewal and differentiation because of simple anatomy, well-defined cell types, and available versatile genetic tools (7, 8). Every female has a pair of ovaries, each containing 12 to 16 ovarioles. The germarium, a tubular structure located at the apical tip of each ovariole, contains 2 to 3 GSCs and early GSC progeny, including mitotically active cystoblasts (CBs)/cysts (2, 4, 8-cell cysts), newly produced ball-like 16-cell cysts, and oval-shaped 16-cell cysts, which are located at regions 1, 2a, and 2b of the germarium, respectively (9) (Fig. 1 A and B). The newly formed ball-like 16-cell cysts enter the prophase of meiosis to initiate chromosomal pairing and meiotic recombination (10, 11), whereas those oval-shaped 16-cell cysts begin to choose one permanent oocyte from two phenotypically similar pro-oocytes and then proceed to be covered up by the follicle epithelium. GSCs anteriorly contact cap cells and are wrapped around by cellular processes of laterally localized inner germarial sheath (IGS) cells (aka escort cells), while IGSs localized posterior to those contacting GSCs are situated at the germarial surface to encase CBs and mitotic cysts in region 2a, newly formed ball-like 16 cysts in region 2a, and oval-shaped 16-cell cysts in region 2b. Cap cells function as the niche to control GSC self-renewal through BMP signaling and E-cadherin-mediated cell adhesion (12–14), whereas those GSC-contacting IGS cells contribute to the niche for controlling GSC self-renewal (4, 5, 15). The remaining IGS cells directly contact early GSC progeny to form the differentiation niche for regulating their differentiation (4, 5). The mechanisms underlying niche regulation of GSC progeny differentiation have just begun to be elucidated.
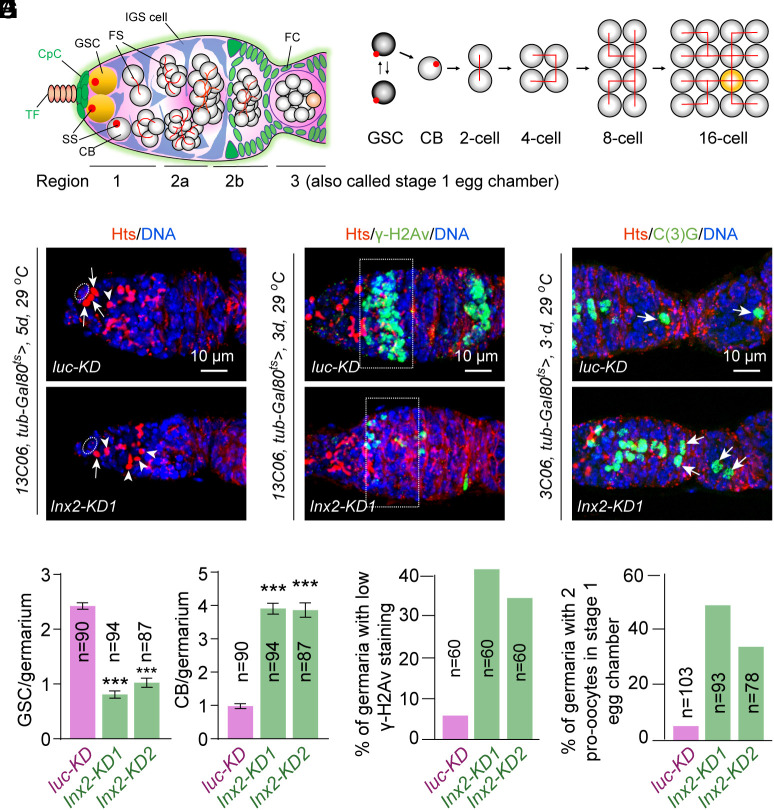
Fig. 1.
IGS-expressed Inx2 controls stepwise GSC development. (A) A schematic diagram illustrating the Drosophila germarium, which is divided into three regions based on germ cell developmental stages. Region 1 contains mitotic germ cells, including germline stem cells (GSCs), cystoblasts (CBs), and mitotic cysts (two-cell, four-cell, and eight-cell cysts). Region 2 contains ball-like 16-cell cysts enveloped by IGS cells (2a) and lens-shaped 16-cell cysts surrounded by follicle cells (2b). Region 3 contains a stage 1 egg chamber. Abbreviations: TF, terminal filament; CPC, cap cell; IGS, inner germarial sheath; CB, cystoblast; SS, spectrosome; FS, fusome; and FC, follicle cells. (B) A GSC undergoes self-renewing division to generate a GSC and a CB. The CB divides four times with incomplete cytokinesis to form a 16-cell cyst. Different developmental stages of cysts, including 2-cell, 4-cell, 8-cell, and 16-cell cysts, can be easily identified by their distinct branched fusome patterns. (C and D) IGS-specific Inx2KD significantly decreases GSCs and increases CB-like cells compared with the control. Cap cells, GSCs, and CBs are highlighted by ovals, arrows, and arrowheads, respectively. D: quantification results. n = number of germaria. (Scale bars, 10 µm.) Student’s t test: ***P ≤ 0.001. (E–H) IGS-specific Inx2KD decreases γ-H2Av levels (E: region 2a by broken rectangle) and increases the incidence of two oocytes (G: arrowheads) in 16-cell cysts compared with the control (with one oocyte). (F and H) quantification results. n = number of germaria.
One of the mechanisms for IGS cells to promote CB differentiation into cysts is to antagonize Bone Morphogenetic Protein (BMP) signaling. Hedgehog (Hh), Wnt, Epidermal Growth Factor Receptor (EGFR), and Jak–Stat signaling pathways function in IGS cells to prevent BMP signaling from interfering with GSC progeny differentiation (15–24). Recent single-cell analysis has demonstrated the existence of four IGS populations, IGS1-4: IGS1, IGS2, IGS3, and IGS4 represent those directly interacting with GSCs, CBs/mitotic cysts in region 1a, early meiotic 16-cell cyst in region 2a, and oocyte-forming 16-cells in region 2b, respectively (25, 26). In addition, IGS1, IGS2, IGS3, and IGS4 control GSC self-renewal, CB differentiation into cysts, meiotic DSB formation, and timely oocyte determination, respectively (25). Within mammalian ovaries, somatic cells are also known to play critical roles in controlling the differentiation of germ cells, as well as various stages of meiosis, and oocyte maturation (27, 28). However, it remains unclear whether and how these different niche compartments directly control the differentiations steps. This study demonstrates that GJ-mediated direct signaling from the IGS controls GSC maintenance, cyst formation, meiotic DSB formation, and oocyte determination.
GJs are the intercellular channels formed by two hexameric hemichannels on two adjacent cells to exchange ions and small molecules (29). GJs can be either homotypic (two identical hexamers) or heterotypic (two different hexamers). Among the eight Drosophila innexin proteins, Inx2 has been shown to be expressed and required in IGS cells for germline cyst formation, whereas Zpg protein (also known as Inx4) is expressed and required in the germline for controlling CB-to-cyst differentiation (30–32). However, it remains to be determined whether Inx2 and Zpg form GJs to mediate direct signaling from the IGS to GSC progeny. This study shows that Inx2 on the IGS and Zpg on the germline form GJs to regulate GSC maintenance, cyst formation, meiotic DSB formation, and oocyte determination by transporting the secondary messenger cAMP. Therefore, this study has uncovered a direct signaling mechanism used by the niche to control stepwise GSC lineage development.
Results
Inx2 Functions in the IGS to Control GSC Maintenance, CB Differentiation, Meiotic DSB Formation, and Oocyte Determination.
Although Inx2 knockdown in IGS cells throughout their development disrupts cyst formation (32), it remains unknown whether it is also required in adult IGS cells to control CB differentiation into cysts. Our RNA fluorescent in situ hybridization (FISH) results have further confirmed that Inx2 mRNA is primarily expressed in somatic cells of the adult ovary, including cap cells, IGS cells, and follicle cells (SI Appendix, Fig. S1A). To determine whether Inx2 is required in adult IGS cells to control GSC maintenance and progeny differentiation, we used 13C06-Gal4 (in combination with tub-Gal80 ts: 13C06 ts) to knock down Inx2 on adult IGS cells for 5 d and then quantified GSCs and CBs. 13C06-Gal4 drives expression of Gal4 protein in IGS cells and follicle progenitors, and also occasionally in one to two cap cells (33). At 25 °C, which allows the temperature-sensitive Gal80ts mutant protein to repress Gal4-mediated shRNA expression, GSCs and their progeny in the females carrying 13C06-Gal4; tub-Gal80 ts and UAS-Inx2 shRNA develop normally (SI Appendix, Fig. S1 B and C). Compared to the control, Inx2 knockdown in adult IGS cells causes the significant GSC loss and the accumulation of significantly more CB-like cells 5 d after 29 °C, which activates Inx2 shRNA expression by inactivating Gal80ts, indicating that Inx2 functions in adult IGS cells to control GSC maintenance and CB differentiation (Fig. 1 C and D and SI Appendix, Fig. S1 D and E). Inx2 knockdown by IGS-specific c587-Gal4 leads to similar mutant phenotypes (SI Appendix, Fig. S1 F and G). After 2 wk at 29 °C, Inx2 knockdown in adult IGS cells causes the accumulation of even more CBs and disrupts egg chamber numbers compared to the control (SI Appendix, Fig. S1 H–J). Our results demonstrate that Inx2 is required in adult IGS cells to maintain GSCs and promote CB differentiation into germline cysts.
To determine whether Inx2 is required in adult IGS cells to control meiotic double-strand DNA break (DSB) formation and oocyte determination, we proceeded to knock down Inx2 on adult IGS cells for 3 d, which causes no obvious CB differentiation defects since CB differentiation defects could influence later meiotic entry and oocyte development. Those early meiotic16-cell cysts in region 2a generate DSBs for meiotic recombination, which can be recognized by an antibody specific to the phosphorylated histone 2A variant (γ-H2Av) (10, 11, 34, 35). Subsequently, those meiotic 16-cell cysts in region 2b proceed to choose one permanent oocyte from the two pro-oocytes, which can be identified by C(3)G protein expression (10, 11, 34). About 90% of the control germaria contain the high- γ-H2Av meiotic 16-cell cysts with the remaining 10% carrying the low- γ-H2Av meiotic 16-cell cysts, which is still at the earlier phases of the meiosis. Interestingly, knocking down Inx2 on adult IGS cells for 3 d leads to an increase of low γ-H2Av expression in early 16-cell cysts and an increase in stage-1 egg chambers with two pro-oocytes compared to the control (Fig. 1 E–H). These results indicate that Inx2 is required in adult IGS cells to control GSC maintenance, CB-to-cyst formation, meiotic DSB formation, and timely oocyte determination. It is worth noting that the 3-d Inx2 knockdown does not result in any obvious IGS cell loss, which helps rule out the IGS cell loss as the cause of those GSC progeny differentiation defects (SI Appendix, Fig. S1 K and L)
Zpg on Germ Cells and Inx2 on IGS Cells form GJs to Control GSC Maintenance, CB-to-cyst Differentiation, Meiotic DSB Formation, and Oocyte Determination.
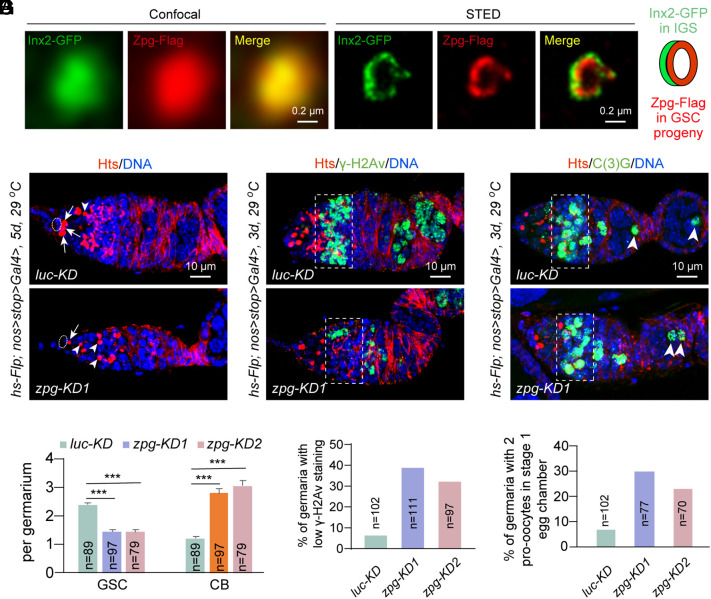
GJ protein Zpg is known to be specifically expressed in germ cells, including GSCs and their early progeny, and its function is important for CB differentiation (30, 31). To visualize endogenous Inx2 and Zpg protein expression in the ovary, we generated Bac transgenes expressing the C-terminally GFP-tagged Inx2 protein (Inx2-GFP) or C-terminally Flag-tagged Zpg protein (Zpg-Flag) under the control of their endogenous promoters to examine their subcellular localization. Inx2-GFP is primarily expressed in somatic cells in the ovarioles, including IGS cells and follicle cells, while Zpg-Flag is enriched in germ cells (SI Appendix, Fig. S2 A and B). Interestingly, some of Inx2-GFP and Zpg-Flag proteins are also colocalized at the cellular junctions between IGS cells and GSCs/GSC progeny. Our FISH results have further confirmed the expression of zpg mRNA primarily in germ cells labeled by Vasa protein (SI Appendix, Fig. S3A). Then, we used the stimulated emission depletion (STED) technique, which can achieve the resolution of 50 nm, to show that Inx2-GFP and Zpg-Flag are indeed colocalized to form heterologous GJ channels at the interface between IGS cells and GSC progeny (Fig. 2A). These results raise the tantalizing possibility that Inx2 on IGS cells and Zpg on germ cells form heterologous GJ channels for mediating direct IGS-to-GSC/progeny communication and thus regulate GSC lineage development.
Fig. 2.
GJ protein Zpg intrinsically controls GSC maintenance and early GSC progeny differentiation. (A) Confocal images show Inx2 and Zpg colocalization. STED images indicate that Inx2 on IGS cells and Zpg on GSC progeny form a heterologous GJ channel. (Scale bars, 0.2 µm.) (B and C) Germline-specific zpgKD decreases GSC and increases CB-like cells compared to the control. Cap cells, GSCs, and CBs are highlighted by ovals, arrows, and arrowheads, respectively. (C) quantification results. n = number of germaria. (Scale bars, 10 µm.) Student’s t test: ***P ≤ 0.001. (D and E) Germline-specific zpgKD decreases γ-H2Av expression (broken rectangle) in meiotic 16-cell cysts in region 2a compared to the control. (E) quantification results. n = number of germaria. (Scale bars, 10 µm.) (F and G) Germline-specific zpgKD increases the incidence of two pro-oocytes (arrowheads) cysts in stage-1 egg chambers. (G) quantification results. n = number of germaria. (Scale bars, 10 µm.)
To test the possibility, we used hsFlp; nos>stop>Gal4 and two independent zpg RNAi lines to knock down zpg specifically in adult germline. In the absence of a heat-shock treatment, the presence of the transcription stop cassette effectively prevents the Gal4-mediated activation of shRNA expression so the non-heat-shocked females exhibit normal GSC lineage development (SI Appendix, Fig. S3 B and C). Following a heat-shock treatment at 37 °C, Flipase (FLP)-mediated FRT recombination helps remove the FRT-franked stop cassette (36), which leads to nanos (nos)-driven expression of Gal4 specifically in germ cells to activate the shRNA-mediated zpg knockdown (SI Appendix, Fig. S3 D and E). As in the previous study (30), 5-d-long zpg knockdown blocks normal CB differentiation, causing the accumulation of CB-like cells (Fig. 2 B and C). In addition, its knockdown also causes premature GSC loss, indicating that Zpg also intrinsically maintain GSCs (Fig. 2 B and C). Moreover, a 2-wk zpg knockdown causes more severe germline development defects than 1-wk knockdown (SI Appendix, Fig. S3 F–H). In addition, 3-d germline-specific zpg knockdown results in the decreased γ-H2Av expression in early 16-cell cysts and the increased frequency of stage-1 egg chambers with two pro-oocytes compared to the control, indicating that Zpg also intrinsically regulates meiotic DSB formation and timely oocyte determination (Fig. 2 D–G). Since the GSC developmental defects caused by zpg knockdown in germ cells are reminiscent of those caused by Inx2 knockdown in IGS cells, we propose that Inx2 on IGS cells and Zpg on germ cells form GJ channel connections for directly controlling GSC maintenance and stepwise GSC progeny differentiation.
Inx2-Zpg GJs Transport cAMP from IGS Cells to GSCs and their Progeny.
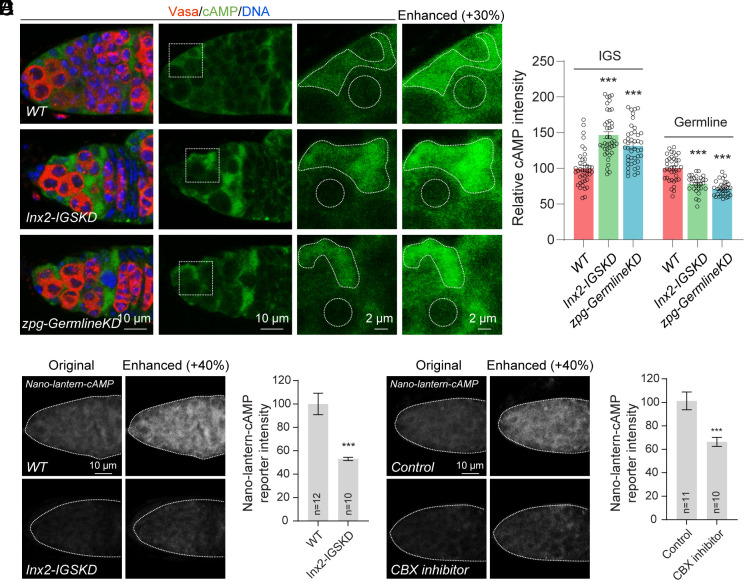
Connexin 43-formed GJs can transport cAMP in bone cells to mediate PKA signaling and regulate gene expression (37). First, we investigate the possibility that the cAMP in IGS cells can be transported to GSCs and their early progeny through GJs. Here, we used a commercial rabbit anti-cAMP monoclonal antibody, which specificity is also confirmed by knocking down or overexpressing cAMP synthesis enzyme adenyl cyclase Rutabaga (Rut) in the germarium (SI Appendix, Fig. S4 A and B), to monitor cAMP levels in IGS cells and germ cells using immunofluorescent staining. In the control germarium, IGS cells produce higher levels of cAMP than cap cells, GSCs and their early progeny, suggesting that IGS cells are likely the major source for cAMP (Fig. 3A). Interestingly, either IGS-specific Inx2KD or germline-specific zpgKD can significantly increase cAMP levels in IGS cells and decrease cAMP levels in GSCs and their early GSC progeny (Fig. 3 A and B). This result supports the idea that cAMP is indeed transported from IGS cells to GSCs and their early GSC progeny through GJs.
Fig. 3.
IGS-expressed cAMP is transported to GSCs and their early progeny via GJs. (A and B) Single section confocal images showing that cAMP is highly enriched in IGS cells and weakly expressed in GSCs and their early progeny. 13C06ts-driven Inx2 knockdown and nos-Geneswitch-Gal4-driven germline zpg knockdown increase cAMP levels in IGS cells and decrease cAMP levels in GSCs and their early progeny. With signal enhancement, cAMP expression can still be detected in GSCs and their progeny. (B) quantification results. (Scale bars, 10 µm, 2 µm.) Student’s t test: ***P ≤ 0.001. (C and D) Live imaging of freshly dissected germaria expressing the nos-Nano-lantern-cAMP reporter. 13C06ts-driven Inx2 knockdown decreases the cAMP reporter activity in germ cells. (D) quantification results. (Scale bars, 10 µm.) Student’s t test: ***P ≤ 0.001. (E and F) Live imaging of freshly dissected germaria expressing the cAMP reporter with or without the treatment of the CBX inhibitor. After a 15-min treatment, cAMP levels are significantly reduced in GSCs and their progeny. (F) quantification results. (Scale bars, 10 µm.) Student’s t test: ***P ≤ 0.001.
To further confirm cAMP transport from IGS cells to GSC progeny, we next constructed a transgenic strain carrying an engineered bright luminescent protein Nano-lantern fused with the cAMP-binding motif of human EPAC1 under the control of germline-specific nanos promoter, nos-Nano-lantern-cAMP, which is confirmed to be specifically and highly expressed in germ cells by immunostaining (SI Appendix, Fig. S4C). Nano-lantern is a chimeric protein of an engineered Renilla luciferase and GFP variant Venus for producing high bioluminescence, which allows the Nano-lantern-cAMP reporter to perform real-time living imaging for monitoring cAMP levels (38). Based on live imaging on freshly dissected germaria, GSCs and their early progeny generally response to cAMP throughout the germline (Fig. 3C). Consistently, IGS-specific Inx2KD can significantly reduce cAMP responses in GSCs and their progeny (Fig. 3 C and D). Carbenoxolone (CBX) is an effective and specific GJ inhibitor (39). After 15-min incubation of freshly dissected germaria with CBX, cAMP responses are significantly down-regulated in GSCs and their progeny compared to the control (Fig. 3 E and F). In addition, Inx2 knockdown in IGS cells and the CBX application do not change the expression levels of the Nano-lantern protein in germ cells, suggesting that the observed cAMP changes are not due to the changed reporter expression (SI Appendix, Fig. S4 C–F). Furthermore, we have also used the recently developed genetically encoded cAMP reporter G-Flamp1, which can monitor cellular cAMP levels in vivo (40), to confirm that IGS cells express much higher levels of cAMP than germ cells (SI Appendix, Fig. S4 G–J). Taken together, our results strongly argue that cAMP in IGS cells is transported to GSCs and their progeny via GJs.
Niche-Initiated cAMP Signaling Is Required for Controlling GSC Maintenance and Stepwise Early Progeny Differentiation.
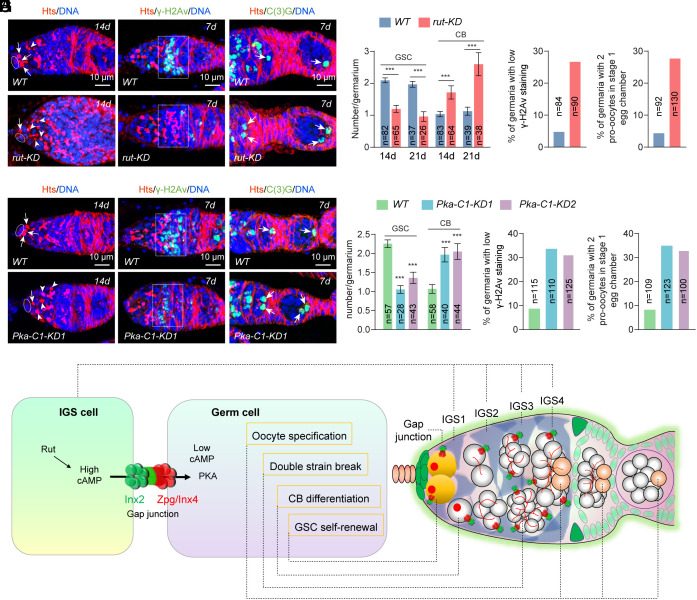
Based on the Drosophila single-nucleus transcriptomic atlas, rut is highly expressed in the somatic cells but not the germ cells of the adult ovary (41). Our mRNA FISH result has further confirmed that rut mRNAs are expressed in IGS cells at higher levels than in germ cells (SI Appendix, Fig. S5A). Consistent with the expression patterns of rut, IGS cells accumulate cAMP at much higher levels than germ cells, including GSCs and their early progeny (Fig. 3A). Knocking down rut expression in IGS cells drastically decreases cAMP levels, whereas overexpressing rut in those cells dramatically increases cAMP levels, indicating that Rut is the major adenyl cyclase responsible for cAMP production in IGS cells (SI Appendix, Fig. S4 A and B). Excitingly, IGS-specific rut knockdown disrupts GSC self-renewal and CB differentiation into cysts, meiotic DSB formation, and oocyte determination, indicating that Rut is required in IGS cells for producing cAMP to regulate GSC lineage development (SI Appendix, Fig. S5 B–E and Fig. 4 A–D). Therefore, our findings indicate that IGS-produced cAMP is critical for GSC maintenance and stepwise GSC progeny differentiation.
Fig. 4.
Rut and Pka-C1 function in the niche and germline, respectively, to control GSC maintenance, CB differentiation, meiotic DSB formation, and pre-oocyte elimination. (A) IGS-specific rutKD significantly decreases GSCs and increases CB-like cells compared with the control. Cap cells, GSCs, and CBs are highlighted by ovals, arrows, and arrowheads, respectively. (B and C) IGS-specific rutKD decreases γ-H2Av levels in 16-cell cysts (region 2a by broken rectangle) and increases the incidence of 2 oocytes (arrowheads) in 16-cell cysts compared with the control (with one oocyte). (Scale bars, 10 µm.) (D) Quantification results. n = number of germaria. Student’s t test: ***P ≤ 0.001. (E) Germline-specific Pka-C1-KD significantly decreases GSCs and increases CB-like cells compared with the control. Cap cells, GSCs and CBs are highlighted by ovals, arrows, and arrowheads, respectively. (Scale bars, 10 µm.) (F and G) IGS-specific Pka-C1-KD decreases γ-H2Av levels in 16-cell cysts (region 2a by broken rectangle) and increases the incidence of 2 oocytes (arrowheads) in 16-cell cysts compared with the control (with one oocyte). (Scale bars, 10 µm.) (H) Quantification results. n = number of germaria. Student’s t test: ***P ≤ 0.001. (I) A working model explaining how GJ-mediated intercellular cAMP/PKA signaling regulates stepwise GSC progeny differentiation.
One of the major cAMP effectors is protein kinase A (PKA), which is composed of two regulatory subunits and two catalytic subunits (42). cAMP binding to the regulatory subunits causes their dissociation from the catalytic subunits, thus releasing active PKA for carrying out the biological functions via phosphorylating target proteins. In Drosophila, there are three catalytic subunits, Pka-C1, Pka-C2, and Pka-C3. Based on the Drosophila single-nucleus expression atlas, only Pka-C1, but not Pka-C2 and Pka-C3, is highly expressed in the germ cells of the adult ovary, although three of them of are expressed in the somatic cells of the germarium (41). To facilitate the efficient knockdown of Pka-C1 in germ cells, including GSCs and their early progeny at the adult stage, we generated nos-GeneSwitch-Gal4 (nos-GS), which RU486-dependent GeneSwitch-Gal4 is under the control of the germline-specific nos promoter. GeneSwitch-Gal4 is the fusion of yeast transcriptional activator Gal4 with human progesterone receptor, which only enters the nucleus and binds to the UAS-containing promoter to activate the expression of a target gene in the presence of RU486 (mifepristone) (43). Excitingly, 2 wk after feeding adult females with RU486, germline-specific knockdown of Pka-C1 in the adult ovary significantly decreases GSCs, increases CB accumulation, and causes defective DSB formation and oocyte determination (SI Appendix, Fig. S5 F–H and Fig. 4 E–H). These results demonstrate that cAMP can activate PKA in the germline to control GSC maintenance, CB differentiation into cysts, meiotic DSB formation, and timely oocyte determination.
Discussion
The Drosophila ovary is an effective system for studying stem cell–niche interactions at the molecular and cellular level (7, 8). In the Drosophila ovary, cap cells and anterior-row IGS cells function as the niche for controlling GSC self-renewal by activating BMP signaling and promoting E-cadherin-mediated cell adhesion (7, 44, 45), whereas posterior IGS cells promote CB differentiation into cysts partly by preventing BMP signaling (4, 5, 17, 20). Our recent used single-cell RNA analysis demonstrates that IGS cells form four linearly arranged niche compartments (IGS1–IGS4) for controlling GSC self-renewal, CB differentiation, meiotic DSB formation, and timely oocyte determination (25). However, it remains unclear whether posterior IGS cells control stepwise GSC progeny differentiation via direct signaling. In this study, we show that Inx2 on IGS cells and Zpg on GSCs and their progeny form GJs for transporting cAMP from the niche to directly control GSC maintenance, CB differentiation, meiotic DSB formation, and timely oocyte determination. IGS-specific Inx2 knockdown and germline-specific zpg knockdown disrupt GSC maintenance, CB differentiation, meiotic DSB formation, and timely oocyte specification. In addition, cAMP synthesized by Rut in IGS cells are transported to GSCs and their early progeny in an Inx2/Zpg-dependent manner. Furthermore, IGS-specific rut knockdown and germline-specific Pka-C1 knockdown lead to similar GSC developmental defects caused by genetic ablation of GJs. Taken together, our findings suggest that GJ-mediated direct signaling from the niche to GSCs and their early progeny controls stepwise GSC development (Fig. 4I). Since GJs and the cAMP/PKA pathway are functionally conserved and widely expressed in various stem cell systems, the regulatory strategy gained from this study could also be applicable to other stem cell systems.
IGS Cells Control GSC Maintenance, CB Formation, Meiotic DSB Formation, and Oocyte Determination Via GJ-Mediated Signaling.
Inx2 has previously been shown to be expressed and required in IGS cells for controlling cyst formation (32). In this study, we have used two independent RNAi lines to knock down Inx2 on adult IGS cells to demonstrate that Inx2 functions in the IGS to control GSC maintenance, CB differentiation, meiotic DSB formation, and timely oocyte determination. Similarly, Zpg is known to be expressed and required in germ cells to promote CB differentiation (30). Interestingly, we used two independent RNAi lines to knock down Zpg in GSCs and their progeny to demonstrate that Zpg functions intrinsically to control GSC maintenance, CB differentiation, meiotic DSB formation, and timely oocyte determination. Excitingly, our super-resolution imaging has demonstrated that Inx2 on IGS cells and Zpg on germ cells are colocalized on their juxtaposed membranes to form GJ channels. These findings have led us to propose that the IGS controls stepwise GSC development via Inx2/Zpg-mediated signaling (Fig. 4I). In the future, it will be critical to understand how GJs are dismantled and reassembled dynamically when IGS cells disengage a cyst and engage a new cyst. Although Cx43-formed GJs in the niches of hematopoietic stem cells, neural stem cells, and skin cells control stem cell maintenance (46), it remains unclear whether GJs in the niche can also control their progeny differentiation as in the Drosophila ovary.
Inx2/Zpg Heterologous GJs Transport cAMP from the Niche to Control Stepwise GSC Development.
GJs are known to transport small molecules such as small RNAs, peptides, secondary messengers, and ions (29). For example, connexin 43-formed GJs are capable of transporting cAMP between bone cells (37). In this study, we demonstrate that Inx2/Zpg heterologous GJs are capable of transporting cAMP from the niche to control GSC maintenance, CB differentiation, meiotic DSB formation, and timely oocyte determination. First, cAMP produced in IGS cells by adenyl cyclase Rut influences its levels in germ cells. We have used an anti-cAMP antibody and immunofluorescent staining to show that cAMP accumulates in IGS cells at higher levels than in GSCs and their early progeny, which is consistent with the expression patterns of adenyl cyclase Rut. IGS-specific rut knockdown significantly and dramatically decreases cAMP levels in IGS cells, GSCs and their early progeny, whereas IGS-specific rut overexpression increases cAMP levels in both IGS cells, GSCs and their early progeny. These results indicate that Rut is the key adenyl cyclase in IGS cells for synthesizing cAMP to maintain cAMP levels in GSCs and their early progeny. Second, cAMP is transported from IGS cells to GSCs and their early progeny. Knocking down Inx2 on IGS cells and Zpg on germ cells can decrease cAMP levels in GSCs and their early progeny using the germline-specifically expressed Nano-lantern-cAMP reporter. In addition, blocking the function of GJs by the specific inhibitor CBX can also disrupt cAMP transport from IGS cells to GSCs and their early progeny. Corroborating with the results of IGS-specific rut knockdown and overexpression, these findings demonstrate that the niche directly provides cAMP for GSCs and their early progeny via GJs. Third, cAMP production in the niche is critical for controlling multiple steps GSCs and their progeny development. We have used IGS-specific rut knockdown to demonstrate that cAMP in IGS cells is critical for controlling GSC self-renewal, CB differentiation, meiotic DSB formation, and timely oocyte determination. Finally, PKA is the major effector of the cAMP pathway in the germline for controlling GSC maintenance and early progeny differentiation. cAMP binds to the regulatory subunits of the PKA kinase complex, which leads to the release active catalytic kinases for carrying out the biological functions (42). Germline-specific knockdown of Pka-C1, which is one of the catalytic subunits in Drosophila, retards GSC maintenance, CB differentiation, meiotic DSB formation, and timely oocyte determination. Therefore, our study has demonstrated that niche-produced cAMP directly regulates GSC maintenance, CB differentiation, meiotic DSB formation, and timely oocyte determination via GJ-mediated transport and has also reported the existence of direct signaling from the niche for controlling stepwise GSC progeny differentiation (Fig. 4I). In the future, we will investigate the detailed mechanisms underlying how PKA signaling controls stepwise GSC development by identifying its protein targets.
GJs have been implicated in soma-germline and stem cell-niche interactions in various organisms. In Drosophila, cAMP/PKA signaling has been shown to regulate germline development by follicle cells during late oogenesis (47), whereas cAMP has also been implicated in the regulation of oocyte maturation by somatic cumulus cells in mammals (48). Since GJs exist between somatic cells and germ cells in the mammalian testis and ovary, they might be responsible for transporting cAMP from the soma to the germline. Cx43-formed GJs in stem cell-niche junctions also regulate stem cell functions in hematopoietic and nervous systems, but the GJ-transmitted signals are still missing (46). cAMP-PKA signaling is known to regulate hematopoietic stem cells (49) and neural stem cells (50). These raise the interesting possibility that the GJ-transported cAMP from the niche also regulates stem cell functions. Therefore, it will be of great interest to investigate whether GJs also transport cAMP from the soma to regulate late germ cell development and from the niche to regulate hematopoietic and neural stem cell functions in mammals.
Materials and Methods
Drosophila Culture.
The following Drosophila stocks used in this study are described in FlyBase, unless specified: c587-Gal4, 13C06-Gal4, nos-Gal4, hsFlp, nos>stop>Gal4, tubulin-gal80ts, Inx2 RNAi (BL29306 and BL80409), zpg RNAi (BL35607 and BL77147), rut RNAi (BL80468), Pka-C1 RNAi (BL35169 and BL57743), and UASt-G-Flamp1 (a kind gift from Yulong Li). Flies were maintained and crossed at room temperature on standard cornmeal/molasses/agar media unless specified. For maximizing the effect of RNAi-mediated knockdown or gene overexpression, newly eclosed flies were shifted to 29 °C for specified days before the analysis of ovarian phenotypes.
Construction of Transgenic Drosophila Stains.
The bacterial recombineering technology was used to introduce C-terminal insertion of Flag tag into zpg in the BAC clone CH321-75N13 and C-terminal insertion of GFP tag into Inx2 in the BAC clone CH322-22F14, respectively. The clones were integrated on the second chromosome. To generate the nos-GeneSwitch-Gal4 transgenic strain, the DNA fragments expressing GAL4-DBD, human progesterone receptor-ligand-binding domain, and P65-AD were fused together in the same reading frame and were then cloned into the Pnos-5′UTR-eGFP-3′UTR vector (51). To generate the nos-Nano-lantern-cAMP and nos-G-Flamp1 transgenes, the DNA fragment containing Nano-lantern-cAMP1.6 or G-Flamp1 were cloned into the Pnos-5′UTR-eGFP-3′UTR vector to replace the eGFP coding sequence. The DNA constructs were integrated into the attp40 site on the second chromosome or into the attp2 site on the third chromosome via PhiC31-mediated transgenesis, respectively.
Immunostaining and Confocal Imaging.
Immunostaining was performed according to our previous procedures (52, 53). The following antibodies were used: mouse monoclonal anti-Hts antibody (1:50, 1B1, DSHB), mouse monoclonal anti-Vasa antibody (1:50, anti-vasa, DSHB), rabbit polyclonal anti-pS137 γ-H2Av antibody (1:1,000, Rockland, #600-401-914), chicken polyclonal anti-GFP antibody (1:500, Invitrogen, #A10262), goat polyclonal anti-GFP antibody (1:500, Rockland, #600-101-215), rabbit polyclonal anti-C3G (1:10,000, gift from Lilly, NICHD/DIR), and rabbit monoclonal anti-cAMP antibody (1:200, Abcam ab134901). For rabbit polyclonal anti-Piwi antibodies, a peptide (MADDQGRGRRRPLNC) from Drosophila Piwi was synthesized and injected into the rabbit by GeneScript USA Inc., and the Protein A/G purified IgG was used for staining (1:1,000).
All the images except STED were taken with a Leica TCS SP5 or SP8 or Nikon Ti2 confocal microscope. STED images were acquired on a Leica SP8 Gated STED Microscopy with 100×, 1.4 oil N.A. objective. Green channel (Inx2-GFP, labeled with Abberior STAR 635) was excited with a pulsed white light (80 MHz) tuned to 635 nm; red channel (Zpg-Flag, labeled with Alexa Fluor 594) was imaged with the same white laser but tuned to 594 nm. Both channels were depleted with a pulsed 775 nm laser at 80 to 90% maximum output. All STED images were acquired in 2D mode to maximize lateral resolution, and each image was averaged eight times in the line average mode. Emission photons were collected with internal Leica HyD hybrid detectors with a time gate setting at 1 to 6 ns. Raw STED images were further deconvolved in Huygens Professional software (version 14.10; Scientific Volume Imaging), and a theoretical estimated point spread function was used for deconvolution. Other paraments were using the system default value except that the background was measured from raw image and signal to noise was set in the range of 15 to 20.
FISH.
Hybridization chain reaction (HCR) was used to achieve high-sensitivity FISH for Inx2, zpg, or rut mRNA. Probe sets against Inx2 (LOT: PRC145), zpg (LOT: PRD632), vasa (LOT: PRG380), or rut (LOT: PRR469) mRNAs were ordered from Molecular Instruments, Inc. Immunostaining with anti-Vas, including primary antibody incubation, secondary antibody incubation, postfixation, and dehydration of ovaries, was performed according to the previous publication prior to in situ hybridization. Then, standard steps following the HCR v3.0 protocol for whole-mount fruit fly embryos were as we did previously (25).
GJ Inhibitor CBX Treatment and Live Imaging.
CBX, which was used at a 2 × 10−4 g/mL concentration in the Grace medium, was added to newly dissected ovaries for 15 min to maximize blockage efficiency. Control groups were added to pure Grace Medium for the same amount of time. After treatment, ovaries were mounted in PBS and imaged within 10 min.
Quantification and Statistical Analysis.
GSCs and CBs were quantified according to our previous studies (52, 53). Briefly, the spectrosome-containing single germ cells attached to cap cells were counted as GSCs, whereas those spectrosome-containing single germ cells, which are not attached to cap cells, were counted as CBs under the fluorescent microscope. The statistical analysis was done using Microsoft Excel or GraphPad Prism 7 with Student’s t test method. P values are indicated in figure legends, and the results are presented as mean or mean ± SEM (***P ≤ 0.001; **P ≤ 0.01; *P ≤ 0.05; n.s., no significance).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We would like to thank Xie laboratory members for advice and discussion, Bloomington Drosophila Stock Center, the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947), Developmental Studies Hybridoma Bank, HKUST BioCRF Facility, and M. Lilly and Y. Li for reagents. This work was supported early by Stowers Institute for Medical Research (T.X.) and a grant from the NIH (R01HD097664 to T.X.) and later by the grants from Hong Kong Research Grants Council (TRS_T13-602/21-N and GRF_16104621 to T.X., GRF_16103822 to R.T.).
Author contributions
R.T., X.A.T., R.X., Z.P., Z.Y., and T.X. designed research; R.T., X.A.T., R.X., Z.P., and Z.Y. performed research; R.T., X.A.T., R.X., Z.P., Z.Y., and T.X. analyzed data; and R.T. and T.X. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Renjun Tu, Email: renjuntu@ust.hk.
Ting Xie, Email: tgx@ust.hk.
Data, Materials, and Software Availability
Original data underlying this manuscript can be accessed from the HKUST’s public depository at https://dataspace.hkust.edu.hk/bib/V12G0P (54). For the reagents, please contact Ting Xie (tgx@ust.hk) or Renjun Tu (renjuntu@ust.hk).
Supporting Information
References
- 1.Mayhall E. A., Paffett-Lugassy N., Zon L. I., The clinical potential of stem cells. Curr. Opin. Cell Biol. 16, 713–720 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Morrison S. J., Spradling A. C., Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L., Xie T., Stem cell niche: Structure and function. Annu. Rev. Cell. Dev. Biol. 21, 605–631 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Kirilly D., Wang S., Xie T., Self-maintained escort cells form a germline stem cell differentiation niche. Development 138, 5087–509 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., et al. , Histone H3K9 trimethylase Eggless controls germline stem cell maintenance and differentiation. PLoS Genet. 7, e1002426 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFalco T., et al. , Macrophages contribute to the spermatogonial niche in the adult testis. Cell Rep. 12, 1107–1119 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie T., Control of germline stem cell self-renewal and differentiation in the Drosophila ovary: Concerted actions of niche signals and intrinsic factors. WIREs Dev. Biol. 2, 261–273 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Spradling A., Fuller M. T., Braun R. E., Yoshida S., Germline stem cells. Cold Spring. Harb. Perspect. Biol. 3, a002642 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spradling A. C., “Developmental genetics of oogenesis” in The Development of Drosophila Melanogaster, Bate M., Martinez Arias A., Eds. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, ed. 1, 1993), vol. I, pp. 1–71. [Google Scholar]
- 10.Hughes S. E., Miller D. E., Miller A. L., Hawley R. S., Female meiosis: Synapsis, recombination, and segregation in drosophila melanogaster. Genetics 208, 875–908 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lake C. M., Hawley R. S., The molecular control of meiotic chromosomal behavior: Events in early meiotic prophase in Drosophila oocytes. Annu. Rev. Physiol. 74, 425–451 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Xie T., Spradling A. C., decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94, 251–260 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Song X., et al. , Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 131, 1353–1364 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Song X., Xie T., DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc. Natl. Acad. Sci. U.S.A. 99, 14813–14818 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., Page-McCaw A., Wnt6 maintains anterior escort cells as an integral component of the germline stem cell niche. Development 145, dev158527 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng C. Y., et al. , Smad-independent BMP signaling in somatic cells limits the size of the germline stem cell pool. Stem Cell Rep. 11, 811–827 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu T., et al. , COP9-Hedgehog axis regulates the function of the germline stem cell progeny differentiation niche in the Drosophila ovary. Development 142, 4242–4252 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Huang J., Reilein A., Kalderon D., Yorkie and Hedgehog independently restrict BMP production in escort cells to permit germline differentiation in the Drosophila ovary. Development 144, 2584–2594 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mottier-Pavie V. I., Palacios V., Eliazer S., Scoggin S., Buszczak M., The Wnt pathway limits BMP signaling outside of the germline stem cell niche in Drosophila ovaries. Dev. Biol. 417, 50–62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S., et al. , Wnt signaling-mediated redox regulation maintains the germ line stem cell differentiation niche. Elife 4, e08174 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo L., Wang H., Fan C., Liu S., Cai Y., Wnt ligands regulate Tkv expression to constrain Dpp activity in the Drosophila ovarian stem cell niche. J. Cell Biol. 209, 595–608 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamada-Kawaguchi N., Nore B. F., Kuwada Y., Smith C. I., Yamamoto D., Btk29A promotes Wnt4 signaling in the niche to terminate germ cell proliferation in Drosophila. Science 343, 294–297 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Upadhyay M., et al. , Transposon dysregulation modulates dWnt4 signaling to control germline stem cell differentiation in drosophila. PLoS Genet. 12, e1005918 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maimon I., Popliker M., Gilboa L., Without children is required for Stat-mediated zfh1 transcription and for germline stem cell differentiation. Development 141, 2602–2610 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu R., et al. , Multiple niche compartments orchestrate stepwise germline stem cell progeny differentiation. Curr. Biol. 31, 827–839.e823 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi J., et al. , A progressive somatic cell niche regulates germline cyst differentiation in the drosophila ovary. Curr. Biol. 31, 840–852.e845 (2021). [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell L., Smith L. B., Rebourcet D., Sertoli cells as key drivers of testis function. Semin. Cell Dev. Biol. 121, 2–9 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Clarke H. J., Regulation of germ cell development by intercellular signaling in the mammalian ovarian follicle. Wiley Interdiscip. Rev. Dev. Biol. 7, wdev.294 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodenough D. A., Paul D. L., Gap junctions. Cold Spring. Harb. Perspect. Biol. 1, a002576 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilboa L., Forbes A., Tazuke S. I., Fuller M. T., Lehmann R., Germ line stem cell differentiation in Drosophila requires gap junctions and proceeds via an intermediate state. Development 130, 6625–6634 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Tazuke S. I., et al. , A germline-specific gap junction protein required for survival of differentiating early germ cells. Development 129, 2529–2539 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Mukai M., et al. , Innexin2 gap junctions in somatic support cells are required for cyst formation and for egg chamber formation in Drosophila. Mech. Dev. 128, 510–523 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Sahai-Hernandez P., Nystul T. G., A dynamic population of stromal cells contributes to the follicle stem cell niche in the Drosophila ovary. Development 140, 4490–4498 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimura I., Lilly M. A., Bruno inhibits the expression of mitotic cyclins during the prophase I meiotic arrest of Drosophila oocytes. Dev. Cell 10, 127–135 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Lake C. M., Holsclaw J. K., Bellendir S. P., Sekelsky J., Hawley R. S., The development of a monoclonal antibody recognizing the Drosophila melanogaster phosphorylated histone H2A variant (gamma-H2AV). G3(Bethesda) 3, 1539–1543 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X., et al. , Piwi is required in multiple cell types to control germline stem cell lineage development in the Drosophila ovary. PLoS One 9, e90267 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta A., et al. , Communication of cAMP by connexin43 gap junctions regulates osteoblast signaling and gene expression. Cell. Signal. 28, 1048–1057 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito K., et al. , Luminescent proteins for high-speed single-cell and whole-body imaging. Nat. Commun. 3, 1262 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manjarrez-Marmolejo J., Franco-Perez J., Gap junction blockers: An overview of their effects on induced seizures in animal models. Curr. Neuropharmacol. 14, 759–771 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L., et al. , A high-performance genetically encoded fluorescent indicator for in vivo cAMP imaging. Nat. Commun. 13, 5363 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H., et al. , Fly cell atlas: A single-nucleus transcriptomic atlas of the adult fruit fly. Science 375, eabk2432 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sassone-Corsi P., The cyclic AMP pathway. Cold Spring. Harb. Perspect. Biol. 4, a011148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osterwalder T., Yoon K. S., White B. H., Keshishian H., A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. U.S.A. 98, 12596–12601 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song X., Zhu C. H., Doan C., Xie T., Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296, 1855–1857 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Xie T., Spradling A. C., A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328–330 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Genet N., Bhatt N., Bourdieu A., Hirschi K. K., Multifaceted roles of connexin 43 in stem cell niches. Curr. Stem. Cell Rep. 4, 1–12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson S. M., Blochlinger K., cut interacts with Notch and protein kinase A to regulate egg chamber formation and to maintain germline cyst integrity during Drosophila oogenesis. Development 124, 3663–3672 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Gilchrist R. B., et al. , Oocyte maturation and quality: Role of cyclic nucleotides. Reproduction 152, R143–R157 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Yang Y., et al. , Involvement of cAMP-PKA pathway in adenosine A1 and A2A receptor-mediated regulation of acetaldehyde-induced activation of HSCs. Biochimie 115, 59–70 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Zyuz’kov G. N., et al. , Participation of cAMP/PKA-mediated signaling pathways in functional activity of regeneration-competent cells in the nervous tissue under conditions of ethanol-induced neurodegeneration. Bull. Exp. Biol. Med. 167, 723–727 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Forrest K. M., Clark I. E., Jain R. A., Gavis E. R., Temporal complexity within a translational control element in the nanos mRNA. Development 131, 5849–5857 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Zou F., et al. , Drosophila YBX1 homolog YPS promotes ovarian germ line stem cell development by preferentially recognizing 5-methylcytosine RNAs. Proc. Natl. Acad. Sci. U.S.A. 117, 3603–3609 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tu R., Duan B., Song X., Xie T., Dlp-mediated Hh and Wnt signaling interdependence is critical in the niche for germline stem cell progeny differentiation. Sci. Adv. 6, eaaz0480 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tu R., Original Data Regarding “Gap Junction-transported cAMP from the Niche Controls Stem Cell Progeny Differentiation”. DataSpace@HKUST. https://dataspace.hkust.edu.hk/bib/V12G0P. Deposited 4 July 2023. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Original data underlying this manuscript can be accessed from the HKUST’s public depository at https://dataspace.hkust.edu.hk/bib/V12G0P (54). For the reagents, please contact Ting Xie (tgx@ust.hk) or Renjun Tu (renjuntu@ust.hk).