Abstract
The cyclooxygenase (COX)/prostaglandin E2 (PGE2) signaling pathway has emerged as a critical target for anti-inflammatory therapeutic development in neurological diseases. However, medical use of COX inhibitors in the treatment of various neurological disorders has been limited due to well-documented cardiovascular and cerebrovascular complications. It has been widely proposed that modulation of downstream microsomal prostaglandin E synthase-1 (mPGES-1) enzyme may provide more specificity for inhibiting PGE2-elicited neuroinflammation. Heightened levels of mPGES-1 have been detected in a variety of brain diseases such as epilepsy, stroke, glioma, and neurodegenerative diseases. Subsequently, elevated levels of PGE2, the enzymatic product of mPGES-1, have been demonstrated to modulate a multitude of deleterious effects. In epilepsy, PGE2 participates in retrograde signaling to augment glutamate release at the synapse leading to neuronal death. The excitotoxic demise of neurons incites the activation of microglia, which can become overactive upon further stimulation by PGE2. A selective mPGES-1 inhibitor was able to reduce gliosis and the expression of proinflammatory cytokines in the hippocampus following status epilepticus. A similar mechanism has also been observed in stroke, where the overactivation of microglia by PGE2 upregulated the expression and secretion of proinflammatory cytokines. This intense activation of neuroinflammatory processes triggered the secondary injury commonly observed in stroke, and blockade of mPGES-1 reduced infarction size and edema, suppressed induction of proinflammatory cytokines, and improved post-stroke well-being and cognition. Furthermore, elevated levels of PGE2 have been shown to intensify the proliferation of glioma cells, mediate P-glycoprotein expression at the blood-brain barrier (BBB) and facilitate breakdown of the BBB. For these reasons, targeting mPGES-1, the central and inducible enzyme of the COX cascade, may provide a more specific therapeutic strategy for treating neuroinflammatory diseases.
Keywords: Blood-brain barrier (BBB), cyclooxygenase (COX), epilepsy, glioma, ischemic stroke, neurodegenerative diseases, neuroinflammation, prostaglandin E synthase (PGES), seizures, status epilepticus
Impact statement
Neuroinflammation often proceeds or exacerbates the progression of neurological diseases and as such presents a promising therapeutic strategy in the treatment of these disorders. The COX/PGE2 signaling pathway has shown therapeutic potential in this context; however, inhibition of the upstream COX enzymes has deleterious cardiovascular and cerebrovascular effects. The inducible downstream target mPGES-1, the main producer of PGE2 during neuroinflammation, may thus represent a more specific therapeutic strategy for managing PGE2 expression. mPGES-1 has seldom been explored in the context of neurological diseases, despite being a unique target for the treatment of neuroinflammation. We explore, for the first time, the effects of selective inhibition of mPGES-1 in the context of epilepsy, stroke, glioma, and other neurodegenerative diseases. By examining the role of mPGES-1 in these diseases and identifying the shortcomings of current therapeutics, we hope to foster development of novel mPGES-1 inhibitors and encourage exploration in these devastating diseases.
Introduction
The inflammatory response is a dynamic process involving the activation of both the innate and adaptive immune systems. 1 With the infiltration of white blood cells and secretion of various cytokines and chemokines, inflammation aims to defend and facilitate recovery under various deleterious conditions such as infections, organ malfunctions, or tissue injuries caused by aseptic factors. 2 Neuroinflammation, a term used to describe the inflammatory response within the central nervous system (CNS), is a unique process involving immune cells in the brain, particularly microglia. 3 Since neuroinflammation has been the hallmark of many neurological disorders, it is not surprising that cyclooxygenase (COX), a critical inflammatory executor, has received much attention for its therapeutic potential in this context over the years.
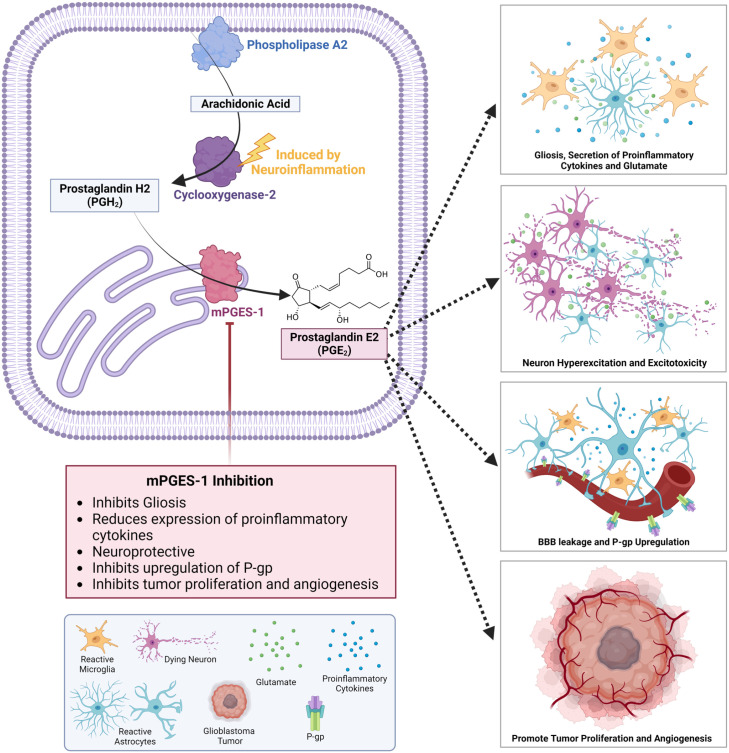
Among prostanoids, cytokines, and chemokines, prostaglandin E2 (PGE2) is widely considered a key component of proinflammatory signaling in the central and peripheral nervous systems.4,5 The biological synthesis of PGE2 is catalyzed by two critical enzymes, namely, COX and prostaglandin E synthase (PGES). In brief, the formation of PGE2 is first initiated by the release of arachidonic acid (AA) from the cell membrane by phospholipase A2. AA is then converted to prostaglandin H2 (PGH2) by either the constitutively expressed COX-1 or COX-2, which is induced in response to noxious stimuli. PGH2 is a short-lived intermediary metabolite, which is then converted to PGE2 by one of three terminal PGESs: microsomal prostaglandin E synthases-1 (mPGES-1), microsomal prostaglandin E synthases-2 (mPGES-2), or cytosolic prostaglandin E synthase (cPGES) (Figure 1). 6 In the periphery, cPGES and mPGES-2 are constitutively expressed, coupled with COX-1, and essential for the homeostatic maintenance of PGE2. However, in the brain, baseline expression of COX-2/mPGES-1, but not cPGES or mPGES-2, has been detected in postsynaptic dendritic spines, 7 suggesting that COX-2/mPGES-1 may play a crucial role in maintaining homeostatic PGE2 signaling within the brain. Recently, we reported that the upregulation of mPGES-1 led to excess PGE2 production in stroke and epilepsy models, which might perpetuate secondary injuries associated with neuroinflammation 8 and excitotoxicity, 9 respectively. Moreover, upregulated mPGES-1 expression has been found in a wide range of brain diseases such as epilepsy,10–12 glioma, 13 stroke, 14 Alzheimer’s disease,15–17 and Parkinson’s disease,18,19 highlighting the therapeutic potential of its modulation in treating these devastating disorders.
Figure 1.
The mPGES-1/PGE2 axis in inflammation-associated neurological conditions. Phospholipase A2 catalyzes the initial release of arachidonic acid from the phospholipid membrane, which is converted into prostaglandin H2 (PGH2) by the inducible cyclooxygenase-2 (COX-2) enzyme. Next, PGH2 is converted by microsomal prostaglandin E synthase-1 (mPGES-1), an integral endoplasmic reticulum (ER) membrane protein, to prostaglandin E2 (PGE2). From there, PGE2 is then released from the cell. It can mediate a wide variety of processes such as reactive gliosis, the secretion of proinflammatory cytokines from microglia, the release of excess glutamate from astrocytes, neuronal hyperexcitation and excitotoxicity, the upregulation of P-glycoprotein (P-gp), blood-brain barrier (BBB) leakage and infiltration of peripheral immune cells. PGE2 also can promote tumor proliferation, angiogenesis, and support an immunosuppressive microenvironment.
The central role of mPGES-1 within the COX/PGE2 cascade and its elevated expression in various brain diseases make it an attractive therapeutic target. 20 In addition, PGE2 produced by mPGES-1 mediates a multitude of deleterious effects including activation of glial cells, secretion of proinflammatory cytokines and chemokines, enhanced glutaminergic signaling leading to excitotoxicity, dysregulation of the blood-brain barrier (BBB), and enriched tumor-promoting effects (Figure 1). Therefore, targeting mPGES-1 may provide an appealing therapeutic strategy to overcome the unfavorable effects of excess PGE2 in neurological diseases.
In this review, we analyze the published studies targeting mPGES-1 in the exploration of therapeutic alternatives for different neurological disorders including epilepsy, brain malignancies, stroke, and neurodegenerative diseases. The evidence gathered here provides a general scope that may facilitate the development of novel mPGES-1 inhibitors and foster innovative approaches for the treatment of these inflammation-associated neurological disorders.
Epilepsy
Nearly 10% of people worldwide will experience at least one seizure during their life, which increases their risk of developing chronic epilepsy. Characterized by highly synchronized, uncontrollable brain activity and spontaneous recurrent seizures, epilepsy is one of the most common neurological conditions, afflicting nearly 65 million people worldwide. 21 Although nearly 40 US Food and Drug Administration (FDA)-approved antiseizure drugs (ASDs) are currently available, none have been shown to prevent the development of epilepsy after precipitating events or modify disease progression. These medications are also well known for their notoriously debilitating side effects including cognitive impairments, dizziness, fatigue, visual and mood disturbances, and incoordination. 21 Moreover, up to 30–40% of epilepsy patients acquire resistance to current therapies and thus are unable to control their seizures even with adequate trials of two tolerated, appropriately selected ASDs. 22 There is an urgent need for novel treatment options for seizures and epilepsy due to the significant limitations of current antiseizure medications.23–25
PGE2 has long been implicated in facilitating the deleterious effects of seizures.26–29 However, the effects of mPGES-1 inhibition on seizures and epilepsy have seldom been explored since most efforts targeting seizure-induced PGE2 have been focused on COX-2, the enzyme mediating the first step of PGE2 biosynthesis.30,31 Upregulation of mPGES-1 in the brain has been detected in animals exposed to various types of epileptogenic stimuli (chemical, electrical, etc.).10,32 In addition, excess PGE2 was detected in the hippocampus of mice following pentylenetetrazol (PTZ) kindling and mPGES-1 expression was found to be highly correlated with PGE2 production, thereby highlighting the fact that excess PGE2 is mainly derived from mPGES-1. 33 Interestingly, PGE2 has been proposed to mediate the release of glutamate from the presynaptic neuron via its retroactive activation of the EP2 receptor. This activates AMPA/NMDA receptors on the postsynaptic neuron, causing the neuron to fire without intense stimulation, thus decreasing the seizure threshold. Subsequently, large amounts of Ca2+ enter the postsynaptic neuron, which in turn activates calcium-related signaling pathways and upregulates the expression of COX-2/mPGES-1. Increased levels of mPGES-1 further enhance PGE2 production and release from the postsynaptic neuron, which further promotes glutamate release from the presynaptic neuron, 34 suggesting a critical role of the COX-2/mPGES-1/PGE2 axis in promoting the neuronal hyperexcitability and lowering the seizure threshold.
Using mPGES-1 knockout (KO) mice, it was demonstrated that repeated exposure to PTZ in animals lacking mPGES-1 showed a decline in seizure severity and a diminished propensity to develop spontaneous recurrent seizures when compared with wildtype (WT) animals. 10 Furthermore, kainic acid (KA)-induced seizures have been shown to evoke mPGES-1 expression within endothelial cells of the BBB. Endothelial cells then secrete PGE2, which binds to its downstream receptor EP3 found on astrocytic end-feet of the BBB. This then signals the uptake of Ca2+ within astrocytes and allows glutamate to be released from astrocytes into the synaptic cleft. 11 Similarly to the actions of PGE2 at the pre-postsynaptic interface, excess glutamate released from astrocytes at the neuron-glia junction allows excess Ca2+ to enter post-synaptic neurons, again resulting in excitotoxicity. However, mPGES-1 KO mice showed lower glutamate release and subsequently less neuronal death when compared with WT mice, thus emphasizing the role of PGE2 in regulating excitotoxicity. Furthermore, this notion was recapitulated in rats following microinjection of KA in the hippocampus, where they observed late-stage induction of mPGES-1 in brain venous endothelial cells. Delayed induction of mPGES-1 may imply that KA does not directly act upon endothelial cells and may proceed through an indirect mechanism. Nevertheless, increased PGE2 levels and aggravated neuronal death following mPGES-1 induction in the hippocampal CA3 region were observed in WT but not in mPGES-1 KO mice. 12 Thus, it is imperative that future studies investigate the effects of mPGES-1 inhibition by small-molecule inhibitors on neurodegeneration following prolonged seizures.
It is well understood that the BBB is the main barrier defending the brain against foreign intruders, xenobiotics, and peripheral immune cells. However, in the CNS diseases such as epilepsy, the integrity of BBB becomes compromised, and the tight junction proteins ubiquitously expressed in the BBB are lost. Simultaneously, the BBB begins to activate and upregulate efflux transporters such as P-glycoprotein (P-gp) in endothelial cells and astrocytic end-feet to serve as a secondary line of defense to protect the brain from drug toxicity. 35 Several studies have implicated the role of COX-2/mPGES-1/PGE2 pathway in upregulating the expression of P-gp following seizures induced by pilocarpine36,37 and KA. 32 It was uncovered that P-gp upregulation induced by PGE2 is mediated through a glutamate/NMDA-dependent pathway and that mPGES-1 inhibition in the presence of glutamate was able to prevent the upregulation of P-gp in endothelial cells ex vivo. 32 Moreover, the upregulation of P-gp has been shown to decrease the concentration of ASDs within the brain, and may be one of the driving forces behind ASD resistance. 38 Therefore, identifying the role of mPGES-1/PGE2 in perpetuating the expression of P-gp following seizures may represent a novel therapeutic mechanism to potentially resensitize resistant patients to currently available ASDs.
Experimental data from our recent study using an mPGES-1 inhibitor, N-phenyl-N’-(4-benzyloxyphenoxycarbonyl)-4-chlorophenylsulfonyl hydrazide (PBCH, also known as 7d and MPO-0063),39,40 has provided the very first evidence in WT animals indicating that pharmacological inhibition of mPGES-1 has promising therapeutic effects. In this study, mice underwent status epilepticus (SE) induced by pilocarpine for 1 h, after which seizure activity was terminated with diazepam. Animals then received PBCH (10 mg/kg, i.p.) at 2, 8, and 20 h following SE, aiming to suppress mPGES-1 activity in its early elevation peaks after SE onset by the first two doses and during the receding phase of its induction by the third dose.41,42 Results showed that animals treated with PBCH had diminished levels of PGE2 in the brain following seizure induction, accompanied by reductions in SE-provoked proinflammatory cytokines, reactive gliosis, and neuronal death in the hippocampus. 43 While mPGES-1 inhibition in the context of neuroinflammation has yet to be fully explored, the aforementioned results from this proof-of-concept study implicate that mPGES-1 may serve as a feasible target for the treatment of neuroinflammation following seizure.
Malignant glioma
Glioblastoma multiform (GBM) is the most aggressive primary brain tumor and arises from aberrant glial cells. It is the most frequent and destructive tumor in the CNS, with an incidence rate of approximately 1 in 30,000, and a medium survival, even with aggressive treatment, of only about 15 months.44,45 This debilitating tumor promotes a number of neurological related symptoms, including seizures, headaches, visual disturbances, memory and cognitive deficits, and changes in personality. Despite years of intensive research, there is still no cure for GBM with an abysmal 2 year survival of <25%. 44 Thus, there is an urgent need to develop novel treatments for patients with GBM.
The tumor-promoting effects of mPGES-1/PGE2 in multiple tumor types have been previously established;46–51 however, their role in malignant glioma remains obscure. It has been reported that in recurrent grade II gliomas that required additional surgical resection, expression levels of mPGES-1 was higher than in tumors that only needed a single surgery, suggesting a positive correlation between mPGES-1 expression and glioma grade.13,52 In addition, pharmacological inhibition and genetic deletion of mPGES-1 sufficiently inhibited PGE2 production, and thus hindered glioblastoma cell growth. 53 Moreover, mPGES-1/PGE2 signaling plays a critical role in tumor angiogenesis, and a recent study found that inhibition of mPGES-1 by isoliquiritigenin normalized glioma vasculature and potentiated the therapeutic efficacy of temozolomide in a rat C6 glioma model. 54 Importantly, this antiangiogenic effect through blocking mPGES-1 could be explained by the downregulation of p-Akt, FGF-2, TGF-β, and VEGF, and could be reversed by Akt overexpression in rat C6 and human U87 glioma cells, 54 indicating that mPGES-1 induced proangiogenic actions are mediated, at least in part, via the PGE2-Akt signaling pathway. Therefore, targeting mPGES-1 may provide a novel therapeutic strategy for human glioblastoma by inhibiting tumor angiogenesis. Furthermore, mPGES-1 expression in microglia co-cultured with glioma cells or primed with glioma medium extensively increased the production of PGE2 by microglia. 55 Enhancement of PGE2 production by microglia led to a decrease in the expression of the proinflammatory cytokine, tumor necrosis factor-alpha (TNF-α), which fostered an immunosuppressive state within the brain and subsequently allowed tumor cells to continue to grow in the absence of immunodetection of the host. 55 Therefore, pharmacological inhibition of mPGES-1 may provide a unique strategy to simultaneously inhibit tumor growth and elevate the levels of TNF-α to sensitize the immune response and allowing for the elimination of tumor cells.
In contrast, another group reported that higher expression of mPGES-1 was positively correlated with more prolonged survival in GBM patients. 56 In this study, it was observed that the overexpression of mPGES-1 augmented the sensitivity of primary cultures of GBM to apoptosis, whereas the knockdown of mPGES-1 decreased the apoptotic threshold in vitro and promoted tumor growth in xenograft mice. Intriguingly, mPGES-1 induced Bax-dependent apoptosis in GBM cells. This effect can be recapitulated by intracellular injection of PGE2 rather than exogenous PGE2 being added directly to the culture medium, 56 highlighting that the mPGES-1-mediated apoptotic effect is independent of cytoplasmic membrane-bound PGE2 receptors. These findings raise the concern that suppressing mPGES-1 may compromise the antitumor effects in controlling GBM cell growth. Nonetheless, these studies together uncover the multifaceted roles of mPGES-1/PGE2 in the development of gliomas. Advancing our understanding of their actions in the development and progression of glioblastoma could help us develop more efficacious treatment options for patients with GBM.
Stroke
Stroke is a group of acute cerebrovascular events characterized by its high morbidity, mortality, adult disability rates, and ischemic stroke accounts for more than 80% of all stroke cases. The current therapy approved by the US FDA to treat ischemic stroke is the recombinant tissue-type plasminogen activator-based intravenous thrombolysis. This treatment unfortunately has a very limited therapeutic window and several potential risks. Alternatively, there is an endovascular therapy through intra-arterial mechanical thrombectomy which has a slightly extended intervention window; however, it is only applicable to those patients with large artery occlusions. 57 Therefore, novel therapeutic innovation is desperately needed for the large proportion of ischemic stroke patients who are unable to obtain current remedies.
The neurological impairment by ischemic stroke results from primary ischemic brain injuries and secondary neuronal damage when delayed reperfusion occurs. Among the pathophysiological components following ischemic stroke, immune activation and neuroinflammation is a late-onset, infarct core-derived process that is associated with non-necrotic neuronal death within the neighboring penumbra and contributes to secondary brain injuries. 58 Therefore, ischemic stroke is reasonably considered a neuroinflammatory condition, and it has been suggested that targeting pivotal inflammatory pathways such as COX-2/mPGES-1/PGE2 after ischemic stroke may provide neuroprotection.59–61
As the terminal enzyme in the biosynthesis of PGE2, mPGES-1 is inducible across different cell types, such as neurons, microglia, and endothelial cells in the cerebral cortex after transient focal ischemia. 14 Our recent study of ischemic stroke in mice demonstrated that mPGES-1 was the most prominently induced enzyme three days following ischemic injury, in comparison with the other four pertinent enzymes responsible for PGE2 biosynthesis, including COX-1, COX-2, mPGES-2, and cPGES. 8 In a cerebral ischemia/reperfusion study in rats, enzymes responsible for PGE2 synthesis including COX-1, COX-2, mPGES-1, and mPGES-2 were upregulated, while the main PGE2 degradation enzyme (15-hydroxyprostaglandin dehydrogenase) 15-PGDH was downregulated after reperfusion for 24 h. Upregulation of enzymes responsible for the PGE2 synthesis caused its excessive production within ischemic tissues, which in turn led to the subsequent upregulation of IL-1β and TNF-α 24 h post stroke, further emphasizing the notion that PGE2 signaling may exacerbate the secondary injuries through a neuroinflammation-based mechanism. 62 In addition, a positive correlation of COX-2/mPGES-1/PGE2 induction was observed following ischemic and hemorrhagic strokes in patients with moyamoya disease, a condition that causes the arteries of the brain to narrow or close. 63 Similar responses were observed in several studies of human patients following stroke, thus confirming the prevalence of COX-2/mPGES-1/PGE2 in this context. Importantly, these results emphasize the therapeutic potential of targeting mPGES-1 following ischemic stroke and may provide a novel mechanism through which the therapeutic time window of currently available therapies could be extended.
Considering the subtype diversity and functional divergence of PGE2 receptors, the pathophysiological role of induced mPGES-1 in ischemic stroke remains to be fully understood. A negative feedback role of the COX-2/mPGES-1/PGE2 pathway has been noted in mitigating the post-stroke oxidative stress-induced ferroptosis through downstream signaling. 64 These results support a favorable role of inducible mPGES-1 specifically in terms of curbing oxidative stress-related programmed neuron death. However, in regard to assessment of overall outcomes, it has been speculated that mPGES-1 may act as more of a mediator of detrimental post-ischemic neuroinflammation and secondary brain injuries.
It has been suggested that mPGES-1 deficiency may be essential to achieve favorable post-stroke outcomes, as it was found that infarction size, edema, and cell apoptosis in the ipsilateral cortex were consistently reduced in mice lacking mPGES-1 in comparison with WT littermates. These results were largely driven by the absence of mPGES-1-derived PGE2, thus implicating the deleterious role of mPGES-1 in the context of stroke. 14 It was also demonstrated that mPGES-1 and COX-2 are co-induced by excessive glutamate production after the onset of brain ischemia. These enzymes are co-localized in the infarct region and act together to coordinately worsen ischemic injury. Given that the role of PGE2 activity appears to be controversial and intensively depends on its specific receptor subtype, Ikeda-Matsuo et al. 65 have provided further evidence demonstrating that the activity of mPGES-1 aggravated post-stroke outcomes through EP3 receptors and the activation of Rho kinase and/or G protein αi. In addition, we demonstrated that inhibition of the EP2 receptor also provided therapeutic effects following stroke by mitigating excitotoxicity, decreasing neurological deficits and infarct volumes in addition to downregulating proinflammatory cytokines (IL-1β, IL-6, and TNFα). 66 Taken together, these results highlight the multifaceted deleterious roles PGE2 can play by interacting with its downstream receptors, and thus implicate that mPGES-1 may represent a more specific therapeutic target in the treatment of stroke.
Experimental data from our recent study using the mPGES-1 inhibitor PBCH (or MPO-0063) has provided the very first pharmacological evidence indicating that selectively targeting mPGES-1 has therapeutic potential as a subacute adjunct treatment of ischemic stroke, along with the first-line recanalization strategy. 8 Systemic administration with the mPGES-1 inhibitor in mice after transient middle cerebral artery occlusion (MCAO) improved post-stroke well-being, decreased infarction size and edema, depressed induction of brain proinflammatory cytokines, alleviated locomotor dysfunction and anxiety-like behavior, and reduced the long-term cognitive impairments. In addition, mPGES-1 inhibition by PBCH had no impact on the count of plasma immune cells, addressing the concern that systemic mPGES-1 inhibition might escalate post-stroke peripheral immunosuppression-related infections. 8
Overall, more preclinical evidence is required to support a clinical trial that targets mPGES-1 specifically for ischemic stroke. Nevertheless, current literature suggests that the role of mPGES-1/PGE2 may represent a viable target to extend the narrow therapeutic window for currently available therapies. Moreover, it has been noted that large artery occlusion is substantially permanent for many patients. As such, it would be advantageous for future research to evaluate the efficacy of mPGES-1 inhibition to bolster confidence in targeting mPGES-1 in a condition more applicable to the human patients.67,68
Neurodegenerative diseases
As the average life expectancy continues to increase, there has been a steady growth in the global burden of neurological disorders. 69 Despite decades of research, there is still no cure for many neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS). 70 However, emerging evidence from recent studies suggest several neuroinflammatory pathways, particularly mPGES-1/PGE2 signaling, might provide viable targets for new treatment of these devastating conditions.
Initially reported at the onset of AD symptoms, patients displayed elevated levels of PGE2 in their cerebrospinal fluid (CSF), suggesting the involvement of mPGES-1 in the development of AD. 70 Furthermore, it was demonstrated that elevated levels of mPGES-1 were consistently found in neurons, microglia, astrocytes, and endothelial cells in postmortem brain tissue of AD patients. 16 More importantly, only mPGES-1 was induced among the three PGES isoenzymes (cPGES, mPGES-1, and mPGES-2) in AD patients. These results suggest that neuroinflammation driving AD development could be largely mediated by mPGES-1/PGE2 signaling. 71
One of the distinctive pathological features of AD is the abnormal buildup of two proteins: phosphorylated tau and amyloid-β (Aβ) peptides. Mechanisms triggering tau phosphorylation are still rather obscure; however, it has been demonstrated that the accumulation of intracellular calcium (Ca2+) is one of the major drivers of this process. Recent evidence has revealed that Ca2+ stimulated activation of mPGES-1 and tau hyperphosphorylation occurred through a PGE2-mediated manner. Furthermore, knockdown of mPGES-1 significantly inhibited tau phosphorylation in APP/PS1 transgenic mice. 17 In vitro data showed that treatment with Aβ led to the upregulation of mPGES-1 and PGE2 production, followed by apoptotic cell death in WT neuronal cells but not in mPGES-1 deficient cells. 15 Similarly, deletion of mPGES-1 reduced the accumulation of microglia around the aggregated amyloid in Tg2576 AD mice, further suppressing neuroinflammation and disease progression. 72 Taken together, these studies emphasize the role of mPGES-1 in the development and progression of AD and may present a valuable therapeutic target.
Interestingly, mPGES-1 is also involved in the pathogenesis of PD, which is characterized by the loss of dopaminergic neurons in the substantia nigra of the striatum. Increased expression of mPGES-1 in dopaminergic neurons has been reported in both human and animal models of PD,18,19 and mPGES-1 inhibition led to a reduction in PGE2 secreted by neurons that were stimulated with 6-OHDA. 73 In vitro data revealed that mPGES-1 promotes dopaminergic neuronal death through excessive production of PGE2. In animal models, genetic deletion of mPGES-1 attenuated the impairment of striatal dopamine content induced by 6-OHDA administration. 19 Consistent with this finding, a selective inhibitor of mPGES-1 significantly increased cell survival after 6-OHDA treatment in vitro and attenuated motor impairments and dopaminergic neuronal damage in vivo. 18 These findings suggest that mPGES-1 is a critical mediator in the pathology of PD.
MS is a neurodegenerative disease characterized by the demyelination of neurons. Given the inflammatory nature of this disease, the mPGES-1 signaling pathway is closely involved with its development and progression. Overt expression of mPGES-1 in microglia and macrophages has been observed in patients with MS. 74 Furthermore, studies of experimental autoimmune encephalomyelitis (EAE), an animal model for MS, have demonstrated that significant induction of mPGES-1 was detected in microglia and endothelial cells. Consequently, excessive PGE2 led to more production of proinflammatory cytokines including IFN-γ, TNF-α, IL-6, and IL-17, and exacerbated the symptoms of EAE through upregulated downstream receptors EP1, EP2, and EP4. However, these detrimental effects were abolished in mPGES-1 KO mice. Overall, these animals showed a reduction in EAE score and improvement of locomotor activity,74,75 implicating a crucial role of mPGES-1/PGE2 in the pathogenesis of MS. Future studies should be directed to evaluate the therapeutic potential of this context. Therapeutic strategies selectively targeting mPGES-1 with small-molecule inhibitors could potentially lead to new pharmacotherapies for eliminating toxic senile plaques and interrupting neurodegeneration.
Conclusions
As the pivotal catalyzing enzyme within the COX/PGE2 signaling cascade, mPGES-1 plays versatile roles in mediating the neuroinflammatory response. Induction of mPGES-1 triggers excess production of PGE2, leading to enhanced secretion of proinflammatory mediators by various cell types, therefore worsening the prognosis of multiple neurological disorders. Based on the promising preclinical data, compared with conventional NSAIDs and COXIBs, mPGES-1 inhibitors have demonstrated an overall beneficial effect with regard to efficacy, potency, and safety concerns. 76 The translation of these preclinical results to humans, however, is complicated by genetic differences between human and murine PTGES genes. Thus far, only two mPGES-1 inhibitors have entered clinical trials and neither have reached the drug market. 77 Most classical human mPGES-1 inhibitors discovered since 2001 have failed to show adequate inhibition of their murine counterpart due to the inter-species amino acid differences between human and murine mPGES-1 proteins. 78 Recent development of novel cross-species small-molecule inhibitors have started to fill the vacancy of drug-like mPGES-1 inhibitors applicable in murine disease models. These compounds are characteristic of their compatibility in selectively and potently inhibiting both human and murine mPGES-1 without intervening in the activities of COX enzymes.8,39,79,80 Our recent publication highlighted the enhanced dual-species activity of our lead compound UT-11, over its predecessor C3 at inhibiting mPGES-1 mediated inflammation both in vitro and in vivo. Compounds were screen in vitro and tested in vivo for their efficacy at suppressing mPGES-1 mediated inflammation, following stimulation with lipopolysaccharide (LPS). Results from this proof-of-concept study demonstrate the feasibility of using LPS induction as an appropriate translational model to screen and test inhibitors, and future studies should utilize this method to streamline inhibitor development.81,82 As more studies investigate this critical inflammatory protein, we foresee an increase in the development of novel inhibitors of mPGES-1 with improved species selectivity in the near future.
Footnotes
Authors’ Contributions: MNS, QL, NY, YC, LL, and RH contributed equally to the writing of the manuscript. MNS generated Figure 1 using BioRender and researching references. YY, CYY, BM, and JJ reviewed and edited the manuscript. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS) grants R01NS100947 (JJ), R21NS109687 (JJ), and R61NS124923 (JJ), and the National Heart, Lung, and Blood Institute (NHLBI) grant R01HL141432 (CYY). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iDs: Madison N Sluter  https://orcid.org/0000-0002-0183-3392
https://orcid.org/0000-0002-0183-3392
Qianqian Li  https://orcid.org/0000-0001-7721-9834
https://orcid.org/0000-0001-7721-9834
Jianxiong Jiang  https://orcid.org/0000-0003-3955-8928
https://orcid.org/0000-0003-3955-8928
References
- 1. Dinarello CA. Anti-inflammatory agents: present and future. Cell 2010;140:935–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454:428–35 [DOI] [PubMed] [Google Scholar]
- 3. Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res 2014;79:1–12 [DOI] [PubMed] [Google Scholar]
- 4. Andreasson K. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins Other Lipid Mediat 2010;91:104–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y. Prostaglandin E2-induced inflammation: relevance of prostaglandin E receptors. Biochim Biophys Acta 2015;1851:414–21 [DOI] [PubMed] [Google Scholar]
- 6. Sluter MN, Hou R, Li L, Yasmen N, Yu Y, Liu J, Jiang J. EP2 antagonists (2011-2021): a decade’s journey from discovery to therapeutics. J Med Chem 2021;64:11816–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C. Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci 2005;25:9858–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li L, Yasmen N, Hou R, Yang S, Lee JY, Hao J, Yu Y, Jiang J. Inducible prostaglandin E synthase as a pharmacological target for ischemic stroke. Neurotherapeutics 2022;19:366–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagib MM, Yu Y, Jiang J. Targeting prostaglandin receptor EP2 for adjunctive treatment of status epilepticus. Pharmacol Ther 2020;209:107504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimada T, Takemiya T, Sugiura H, Yamagata K. Role of inflammatory mediators in the pathogenesis of epilepsy. Mediators Inflamm 2014;2014:901902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takemiya T, Matsumura K, Sugiura H, Yasuda S, Uematsu S, Akira S, Yamagata K. Endothelial microsomal prostaglandin E synthase-1 facilitates neurotoxicity by elevating astrocytic Ca2+ levels. Neurochem Int 2011;58:489–96 [DOI] [PubMed] [Google Scholar]
- 12. Takemiya T, Matsumura K, Sugiura H, Maehara M, Yasuda S, Uematsu S, Akira S, Yamagata K. Endothelial microsomal prostaglandin E synthase-1 exacerbates neuronal loss induced by kainate. J Neurosci Res 2010;88:381–90 [DOI] [PubMed] [Google Scholar]
- 13. Mattila S, Tuominen H, Koivukangas J, Stenbäck F. The terminal prostaglandin synthases mPGES-1, mPGES-2, and cPGES are all overexpressed in human gliomas. Neuropathology 2009;29:156–65 [DOI] [PubMed] [Google Scholar]
- 14. Ikeda-Matsuo Y, Ota A, Fukada T, Uematsu S, Akira S, Sasaki Y. Microsomal prostaglandin E synthase-1 is a critical factor of stroke-reperfusion injury. Proc Natl Acad Sci U S A 2006;103:11790–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuroki Y, Sasaki Y, Kamei D, Akitake Y, Takahashi M, Uematsu S, Akira S, Nakatani Y, Kudo I, Hara S. Deletion of microsomal prostaglandin E synthase-1 protects neuronal cells from cytotoxic effects of beta-amyloid peptide fragment 31-35. Biochem Biophys Res Commun 2012;424:409–13 [DOI] [PubMed] [Google Scholar]
- 16. Chaudhry UA, Zhuang H, Crain BJ, Doré S. Elevated microsomal prostaglandin-E synthase-1 in Alzheimer’s disease. Alzheimers Dement 2008;4:6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao LL, Guan PP, Liang YY, Huang XS, Wang P. Calcium ions stimulate the hyperphosphorylation of tau by activating microsomal prostaglandin E synthase 1. Front Aging Neurosci 2019;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang S, Huh E, Moon GH, Ahn J, Woo J, Han HS, Lee HH, Chung KS, Lee KT, Oh MS, Lee JY. In vitro and in vivo neuroprotective effect of novel mPGES-1 inhibitor in animal model of Parkinson’s disease. Bioorg Med Chem Lett 2022;74:128920. [DOI] [PubMed] [Google Scholar]
- 19. Ikeda-Matsuo Y, Miyata H, Mizoguchi T, Ohama E, Naito Y, Uematsu S, Akira S, Sasaki Y, Tanabe M. Microsomal prostaglandin E synthase-1 is a critical factor in dopaminergic neurodegeneration in Parkinson’s disease. Neurobiol Dis 2019;124:81–92 [DOI] [PubMed] [Google Scholar]
- 20. Ikeda-Matsuo Y. The role of mPGES-1 in inflammatory brain diseases. Biol Pharm Bull 2017;40:557–63 [DOI] [PubMed] [Google Scholar]
- 21. Devinsky O, Vezzani A, O’Brien TJ, Jette N, Scheffer IE, de Curtis M, Perucca P. Epilepsy. Nat Rev Dis Primers 2018;4:18024. [DOI] [PubMed] [Google Scholar]
- 22. Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069–77 [DOI] [PubMed] [Google Scholar]
- 23. Varvel NH, Jiang J, Dingledine R. Candidate drug targets for prevention or modification of epilepsy. Ann Rev Pharmacol Toxicol 2015;55:229–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loscher W, Klein P. The feast and famine: epilepsy treatment and treatment gaps in early 21st century. Neuropharmacology 2020;170:108055. [DOI] [PubMed] [Google Scholar]
- 25. Jiang J, Santhakumar V, Zhu X. Editorial: neuroinflammation in acquired epilepsy. Front Cell Dev Biol 2022;10:1074537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Du Y, Kemper T, Qiu J, Jiang J. Defining the therapeutic time window for suppressing the inflammatory prostaglandin E2 signaling after status epilepticus. Expert Rev Neurother 2016;16:123–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang J, Yu Y, Kinjo ER, Du Y, Nguyen HP, Dingledine R. Suppressing pro-inflammatory prostaglandin signaling attenuates excitotoxicity-associated neuronal inflammation and injury. Neuropharmacology 2019;149:149–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu Y, Nguyen DT, Jiang J. G protein-coupled receptors in acquired epilepsy: druggability and translatability. Prog Neurobiol 2019;183:101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Y, Nagib MM, Yasmen N, Sluter MN, Littlejohn TL, Yu Y, Jiang J. Neuroinflammatory mediators in acquired epilepsy: an update. Inflamm Res 2023;72:683–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rojas A, Jiang J, Ganesh T, Yang MS, Lelutiu N, Gueorguieva P, Dingledine R. Cyclooxygenase-2 in epilepsy. Epilepsia 2014;55:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dhir A. An update of cyclooxygenase (COX)-inhibitors in epilepsy disorders. Expert Opin Investig Drugs 2019;28:191–205 [DOI] [PubMed] [Google Scholar]
- 32. Soldner ELB, Hartz AMS, Akanuma SI, Pekcec A, Doods H, Kryscio RJ, Hosoya KI, Bauer B. Inhibition of human microsomal PGE2 synthase-1 reduces seizure-induced increases of P-glycoprotein expression and activity at the blood-brain barrier. FASEB J 2019;33:13966–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu X, Yao Y, Yang J, Zhengxie J, Li X, Hu S, Zhang A, Dong J, Zhang C, Gan G. COX-2-PGE(2) signaling pathway contributes to hippocampal neuronal injury and cognitive impairment in PTZ-kindled epilepsy mice. Int Immunopharmacol 2020;87:106801. [DOI] [PubMed] [Google Scholar]
- 34. López DE, Ballaz SJ. The role of brain cyclooxygenase-2 (Cox-2) beyond neuroinflammation: neuronal homeostasis in memory and anxiety. Mol Neurobiol 2020;57:5167–76 [DOI] [PubMed] [Google Scholar]
- 35. Loscher W. Epilepsy and alterations of the blood-brain barrier: cause or consequence of epileptic seizures or both? Handb Exp Pharmacol 2022;273:331–50 [DOI] [PubMed] [Google Scholar]
- 36. Zibell G, Unkrüer B, Pekcec A, Hartz AM, Bauer B, Miller DS, Potschka H. Prevention of seizure-induced up-regulation of endothelial P-glycoprotein by COX-2 inhibition. Neuropharmacology 2009;56:849–55 [DOI] [PubMed] [Google Scholar]
- 37. Pekcec A, Unkrüer B, Schlichtiger J, Soerensen J, Hartz AM, Bauer B, van Vliet EA, Gorter JA, Potschka H. Targeting prostaglandin E2 EP1 receptors prevents seizure-associated P-glycoprotein up-regulation. J Pharmacol Exp Ther 2009;330:939–47 [DOI] [PubMed] [Google Scholar]
- 38. Löscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol 2005;76:22–76 [DOI] [PubMed] [Google Scholar]
- 39. Park EB, Kim KJ, Jeong HR, Lee JK, Kim HJ, Lee HH, Lim JW, Shin JS, Koeberle A, Werz O, Lee KT, Lee JY. Synthesis, structure determination, and biological evaluation of phenylsulfonyl hydrazide derivatives as potential anti-inflammatory agents. Bioorg Med Chem Lett 2016;26:5193–7 [DOI] [PubMed] [Google Scholar]
- 40. Lee HH, Moon Y, Shin JS, Lee JH, Kim TW, Jang C, Park C, Lee J, Kim Y, Kim Y, Werz O, Park BY, Lee JY, Lee KT. A novel mPGES-1 inhibitor alleviates inflammatory responses by downregulating PGE(2) in experimental models. Prostaglandins Other Lipid Mediat 2019;144:106347. [DOI] [PubMed] [Google Scholar]
- 41. Yu Y, Jiang J. COX-2/PGE(2) axis regulates hippocampal BDNF/TrkB signaling via EP2 receptor after prolonged seizures. Epilepsia Open 2020;5:418–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang J, Yang MS, Quan Y, Gueorguieva P, Ganesh T, Dingledine R. Therapeutic window for cyclooxygenase-2 related anti-inflammatory therapy after status epilepticus. Neurobiol Dis 2015;76:126–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yasmen N, Sluter MN, Li L, Yu Y, Jiang J. Transient inhibition of microsomal prostaglandin E synthase-1 after status epilepticus blunts brain inflammation and is neuroprotective. Mol Brain 2023;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qiu J, Shi Z, Jiang J. Cyclooxygenase-2 in glioblastoma multiforme. Drug Discov Today 2017;22:148–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol 2013;15:ii1–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer 2010;10:181–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiang J, Dingledine R. Role of prostaglandin receptor EP2 in the regulations of cancer cell proliferation, invasion, and inflammation. J Pharmacol Exp Ther 2013;344:360–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hou R, Yu Y, Sluter MN, Li L, Hao J, Fang J, Yang J, Jiang J. Targeting EP2 receptor with multifaceted mechanisms for high-risk neuroblastoma. Cell Rep 2022;39:111000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hou R, Yu Y, Jiang J. Prostaglandin E2 in neuroblastoma: targeting synthesis or signaling? Biomed Pharmacother 2022;156:113966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang Q, Morris RJ, Bode AM, Zhang T. Prostaglandin pathways: opportunities for cancer prevention and therapy. Cancer Res 2022;82:949–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thumkeo D, Punyawatthananukool S, Prasongtanakij S, Matsuura R, Arima K, Nie H, Yamamoto R, Aoyama N, Hamaguchi H, Sugahara S, Takeda S, Charoensawan V, Tanaka A, Sakaguchi S, Narumiya S. PGE2-EP2/EP4 signaling elicits immunosuppression by driving the mregDC-Treg axis in inflammatory tumor microenvironment. Cell Rep 2022;39:110914. [DOI] [PubMed] [Google Scholar]
- 52. Jiang J, Qiu J, Li Q, Shi Z. Prostaglandin E2 signaling: alternative target for glioblastoma? Trends Cancer 2017;3:75–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Payner T, Leaver HA, Knapp B, Whittle IR, Trifan OC, Miller S, Rizzo MT. Microsomal prostaglandin E synthase-1 regulates human glioma cell growth via prostaglandin E(2)-dependent activation of type II protein kinase A. Mol Cancer Ther 2006;5:1817–26 [DOI] [PubMed] [Google Scholar]
- 54. Wang C, Chen Y, Wang Y, Liu X, Liu Y, Li Y, Chen H, Fan C, Wu D, Yang J. Inhibition of COX-2, mPGES-1 and CYP4A by isoliquiritigenin blocks the angiogenic Akt signaling in glioma through ceRNA effect of miR-194-5p and lncRNA NEAT1. J Exp Clin Cancer Res 2019;38:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nakano Y, Kuroda E, Kito T, Uematsu S, Akira S, Yokota A, Nishizawa S, Yamashita U. Induction of prostaglandin E2 synthesis and microsomal prostaglandin E synthase-1 expression in murine microglia by glioma-derived soluble factors. Laboratory investigation. J Neurosurg 2008;108:311–9 [DOI] [PubMed] [Google Scholar]
- 56. Lalier L, Cartron PF, Pedelaborde F, Olivier C, Loussouarn D, Martin SA, Meflah K, Menanteau J, Vallette FM. Increase in PGE2 biosynthesis induces a Bax dependent apoptosis correlated to patients’ survival in glioblastoma multiforme. Oncogene 2007;26:4999–5009 [DOI] [PubMed] [Google Scholar]
- 57. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50:e344–418 [DOI] [PubMed] [Google Scholar]
- 58. Kim E, Cho S. CNS and peripheral immunity in cerebral ischemia: partition and interaction. Exp Neurol 2021;335:113508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smith CJ, Denes A, Tyrrell PJ, Di Napoli M. Phase II anti-inflammatory and immune-modulating drugs for acute ischaemic stroke. Expert Opin Investig Drugs 2015;24:623–43 [DOI] [PubMed] [Google Scholar]
- 60. Jiang J, Yu Y. Small molecules targeting cyclooxygenase/prostanoid cascade in experimental brain ischemia: do they translate? Med Res Rev 2021;41:828–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li L, Sluter MN, Yu Y, Jiang J. Prostaglandin E receptors as targets for ischemic stroke: novel evidence and molecular mechanisms of efficacy. Pharmacol Res 2021;163:105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xu Y, Liu Y, Li K, Miao S, Lv C, Wang C, Zhao J. Regulation of PGE(2) pathway during cerebral ischemia reperfusion injury in rat. Cell Mol Neurobiol 2021;41:1483–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang J, Xiong Z, Wang S, He Y, Sun S, Wu X, Wang L, Zhang H, You C, Wang Y, Chen J. Cyclooxygenase-2 and prostaglandin E2 are associated with middle cerebral artery occlusion and hemorrhage in patients with moyamoya disease. Curr Neurovasc Res 2016;13:68–74 [DOI] [PubMed] [Google Scholar]
- 64. Xu Y, Liu Y, Li K, Yuan D, Yang S, Zhou L, Zhao Y, Miao S, Lv C, Zhao J. COX-2/PGE2 pathway inhibits the ferroptosis induced by cerebral ischemia reperfusion. Mol Neurobiol 2022;59:1619–31 [DOI] [PubMed] [Google Scholar]
- 65. Ikeda-Matsuo Y, Tanji H, Ota A, Hirayama Y, Uematsu S, Akira S, Sasaki Y. Microsomal prostaglandin E synthase-1 contributes to ischaemic excitotoxicity through prostaglandin E2 EP3 receptors. Br J Pharmacol 2010;160:847–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li L, Yu Y, Hou R, Hao J, Jiang J. Inhibiting the PGE2 receptor EP2 mitigates excitotoxicity and ischemic injury. ACS Pharmacol Transl Sci 2020;3:635–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McBride DW, Zhang JH. Precision stroke animal models: the permanent MCAO model should be the primary model, not transient MCAO. Transl Stroke Res. Epub ahead of print 17 July 2017. DOI: 10.1007/s12975-017-0554-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lyden P, Buchan A, Boltze J, Fisher M; STAIR XI Consortium. Top priorities for cerebroprotective studies-a paradigm shift: report from STAIR XI. Stroke 2021;52:3063–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:459–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Combrinck M, Williams J, De Berardinis MA, Warden D, Puopolo M, Smith AD, Minghetti L. Levels of CSF prostaglandin E2, cognitive decline, and survival in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2006;77:85–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Woodling NS, Andreasson KI. Untangling the Web: toxic and protective effects of neuroinflammation and PGE2 signaling in Alzheimer’s disease. ACS Chem Neurosci 2016;7:454–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Akitake Y, Nakatani Y, Kamei D, Hosokawa M, Akatsu H, Uematsu S, Akira S, Kudo I, Hara S, Takahashi M. Microsomal prostaglandin E synthase-1 is induced in Alzheimer’s disease and its deletion mitigates Alzheimer’s disease-like pathology in a mouse model. J Neurosci Res 2013;91:909–19 [DOI] [PubMed] [Google Scholar]
- 73. Kang X, Qiu J, Li Q, Bell KA, Du Y, Jung DW, Lee JY, Hao J, Jiang J. Cyclooxygenase-2 contributes to oxidopamine-mediated neuronal inflammation and injury via the prostaglandin E2 receptor EP2 subtype. Sci Rep 2017;7:9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kihara Y, Matsushita T, Kita Y, Uematsu S, Akira S, Kira J, Ishii S, Shimizu T. Targeted lipidomics reveals mPGES-1-PGE2 as a therapeutic target for multiple sclerosis. Proc Natl Acad Sci U S A 2009;106:21807–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Takeuchi C, Matsumoto Y, Kohyama K, Uematsu S, Akira S, Yamagata K, Takemiya T. Microsomal prostaglandin E synthase-1 aggravates inflammation and demyelination in a mouse model of multiple sclerosis. Neurochem Int 2013;62:271–80 [DOI] [PubMed] [Google Scholar]
- 76. Bahia MS, Katare YK, Silakari O, Vyas B, Silakari P. Inhibitors of microsomal prostaglandin E2 synthase-1 enzyme as emerging anti-inflammatory candidates. Med Res Rev 2014;34:825–55 [DOI] [PubMed] [Google Scholar]
- 77. Psarra A, Nikolaou A, Kokotou MG, Limnios D, Kokotos G. Microsomal prostaglandin E(2) synthase-1 inhibitors: a patent review. Expert Opin Ther Pat 2017;27:1047–59 [DOI] [PubMed] [Google Scholar]
- 78. Bergqvist F, Morgenstern R, Jakobsson PJ. A review on mPGES-1 inhibitors: from preclinical studies to clinical applications. Prostaglandins Other Lipid Mediat 2020;147:106383. [DOI] [PubMed] [Google Scholar]
- 79. Park SJ, Han SG, Ahsan HM, Lee K, Lee JY, Shin JS, Lee KT, Kang NS, Yu YG. Identification of novel mPGES-1 inhibitors through screening of a chemical library. Bioorg Med Chem Lett 2012;22:7335–9 [DOI] [PubMed] [Google Scholar]
- 80. Kim M, Lee S, Park EB, Kim KJ, Lee HH, Shin JS, Fischer K, Koeberle A, Werz O, Lee KT, Lee JY. Hit-to-lead optimization of phenylsulfonyl hydrazides for a potent suppressor of PGE2 production: synthesis, biological activity, and molecular docking study. Bioorg Med Chem Lett 2016;26:94–9 [DOI] [PubMed] [Google Scholar]
- 81. Sluter MN, Bhuniya R, Yuan X, Ramaraju A, Chen Y, Yu Y, Parmar KR, Temrikar ZH, Srivastava A, Meibohm B, Jiang J, Yang C-Y. Novel, brain-permeable, cross-species benzothiazole inhibitors of microsomal prostaglandin E synthase-1 (MPGES-1) dampen neuroinflammation in vitro and in vivo. ACS Pharmacol Transl Sci 2023;6:587–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ikeda-Matsuo Y, Ikegaya Y, Matsuki N, Uematsu S, Akira S, Sasaki Y. Microglia-specific expression of microsomal prostaglandin E2 synthase-1 contributes to lipopolysaccharide-induced prostaglandin E2 production. J Neurochem 2005;94:1546–58 [DOI] [PubMed] [Google Scholar]