Abstract
Background
Fatigue is a common and debilitating symptom in people receiving dialysis that is associated with an increased risk of death, cardiovascular disease and depression. Fatigue can also impair quality of life (QoL) and the ability to participate in daily activities. Fatigue has been established by patients, caregivers and health professionals as a core outcome for haemodialysis (HD).
Objectives
We aimed to evaluate the effects of pharmacological and non‐pharmacological interventions on fatigue in people with kidney failure receiving dialysis, including HD and peritoneal dialysis (PD), including any setting and frequency of the dialysis treatment.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 18 October 2022 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Registry Platform (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
Studies evaluating pharmacological and non‐pharmacological interventions affecting levels of fatigue or fatigue‐related outcomes in people receiving dialysis were included. Studies were eligible if fatigue or fatigue‐related outcomes were reported as a primary or secondary outcome. Any mode, frequency, prescription, and duration of therapy were considered.
Data collection and analysis
Three authors independently extracted data and assessed the risk of bias. Treatment estimates were summarised using random effects meta‐analysis and expressed as a risk ratio (RR) or mean difference (MD), with a corresponding 95% confidence interval (CI) or standardised MD (SMD) if different scales were used. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
Ninety‐four studies involving 8191 randomised participants were eligible. Pharmacological and non‐pharmacological interventions were compared either to placebo or control, or to another pharmacological or non‐pharmacological intervention. In the majority of domains, risks of bias in the included studies were unclear or high.
In low certainty evidence, when compared to control, exercise may improve fatigue (4 studies, 217 participants (Iowa Fatigue Scale, Modified Fatigue Impact Scale, Piper Fatigue Scale (PFS), or Haemodialysis‐Related Fatigue scale score): SMD ‐1.18, 95% CI ‐2.04 to ‐0.31; I2 = 87%) in HD.
In low certainty evidence, when compared to placebo or standard care, aromatherapy may improve fatigue (7 studies, 542 participants (Fatigue Severity Scale (FSS), Rhoten Fatigue Scale (RFS), PFS or Brief Fatigue Inventory score): SMD ‐1.23, 95% CI ‐1.96 to ‐0.50; I2 = 93%) in HD.
In low certainty evidence, when compared to no intervention, massage may improve fatigue (7 studies, 657 participants (FSS, RFS, PFS or Visual Analogue Scale (VAS) score): SMD ‐1.06, 95% CI ‐1.47, ‐0.65; I2 = 81%) and increase energy (2 studies, 152 participants (VAS score): MD 4.87, 95% CI 1.69 to 8.06, I2 = 59%) in HD.
In low certainty evidence, when compared to placebo or control, acupressure may reduce fatigue (6 studies, 459 participants (PFS score, revised PFS, or Fatigue Index): SMD ‐0.64, 95% CI ‐1.03 to ‐0.25; I2 = 75%) in HD.
A wide range of heterogenous interventions and fatigue‐related outcomes were reported for exercise, aromatherapy, massage and acupressure, preventing our capability to pool and analyse the data.
Due to the paucity of studies, the effects of pharmacological and other non‐pharmacological interventions on fatigue or fatigue‐related outcomes, including non‐physiological neutral amino acid, relaxation with or without music therapy, meditation, exercise with nandrolone, nutritional supplementation, cognitive‐behavioural therapy, ESAs, frequent HD sections, home blood pressure monitoring, blood flow rate reduction, serotonin reuptake inhibitor, beta‐blockers, anabolic steroids, glucose‐enriched dialysate, or light therapy, were very uncertain.
The effects of pharmacological and non‐pharmacological treatments on death, cardiovascular diseases, vascular access, QoL, depression, anxiety, hypertension or diabetes were sparse. No studies assessed tiredness, exhaustion or asthenia. Adverse events were rarely and inconsistently reported.
Authors' conclusions
Exercise, aromatherapy, massage and acupressure may improve fatigue compared to placebo, standard care or no intervention. Pharmacological and other non‐pharmacological interventions had uncertain effects on fatigue or fatigue‐related outcomes in people receiving dialysis. Future adequately powered, high‐quality studies are likely to change the estimated effects of interventions for fatigue and fatigue‐related outcomes in people receiving dialysis.
Keywords: Humans, Cardiovascular Diseases, Fatigue, Fatigue/etiology, Fatigue/therapy, Kidney, Quality of Life, Randomized Controlled Trials as Topic, Renal Dialysis, Renal Insufficiency
Plain language summary
Are interventions for fatigue effective among people with kidney failure requiring dialysis?
What is the issue?
Fatigue is a frequent and debilitating symptom that can limit life participation in people receiving dialysis. Fatigue is linked to impaired quality of life, cardiovascular disease, death and depression in people on dialysis. Several potential interventions, including drugs or other non‐pharmacological treatments (e.g. exercise, diet, massage, aromatherapy, acupressure), have been evaluated for their effect on fatigue in people on dialysis.
What did we do?
We evaluated whether drugs or other non‐pharmacological interventions are beneficial for adults and children receiving haemodialysis or peritoneal dialysis to manage fatigue. We evaluated all clinical studies available and summarised the results. We evaluated how certain we could be about the evidence related to interventions for fatigue using a system called "GRADE".
What did we find?
Ninety‐four studies involving 8191 randomised participants were available. Patients in the studies were given a drug, non‐pharmacological intervention, standard care or a sugar pill (placebo). The treatment they received was decided by random chance. The studies were generally short‐term (over a few months). There were no studies in children. Exercise, aromatherapy, massage and acupressure improve fatigue compared to placebo or standard care. Drugs or other non‐pharmacological interventions have uncertain effects on fatigue in people on dialysis.
Conclusions
Exercise, aromatherapy, massage and acupressure improve fatigue compared to placebo or standard care. It remains uncertain whether drugs or other non‐pharmacological interventions have any impact on fatigue in people on dialysis when compared to a sugar pill, standard care or other treatments for fatigue.
Summary of findings
Summary of findings 1. Exercise versus control for people receiving dialysis.
| Exercise versus control for people receiving dialysis | ||||||
|
Patient or population: people receiving dialysis Settings: multinational Intervention: exercise Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Exercise | |||||
|
Fatigue (IFS, MFI, PIPER, or HD‐related fatigue scale) median follow‐up: 2.7 months |
The mean score for fatigue ranged across control groups from 29.75 to 81.17 (IFS, MFI, PFS, or HD related fatigue scale) | The standardised mean of fatigue in the intervention group was 1.18 lower than the control group (95% CI 2.04 lower to 0.31lower) | ‐‐ | 217 (4) | ⊕⊕⊝⊝ low1,2,3 | Exercise may improve fatigue compared to control in people undergoing HD |
| Weakness | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Energy | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Tiredness | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Exhaustion | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Asthenia | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; IFS: Iowa Fatigue Scale; MFI: Multidimensional Fatigue Inventory; PFS: Piper Fatigue Scale; HD: haemodialysis. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence certainty was downgraded by one level due to study limitations
2 Evidence certainty was downgraded by one level due to imprecision (Optimal Information Size (OIS)) not met and indirectness in outcome measure
3 Evidence certainty was downgraded by one level due to inconsistency
Summary of findings 2. Aromatherapy versus placebo or standard care for people receiving dialysis.
| Aromatherapy versus placebo or standard care for people receiving dialysis | ||||||
|
Patient or population: people receiving dialysis Settings: multinational Intervention: aromatherapy Comparison: placebo or standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or standard care | Aromatherapy | |||||
|
Fatigue (PIPER, BFI, FSS, RFS) median follow‐up: 0.9 months |
The mean score for fatigue ranged across control groups from 6.21 to 45.1 (PFS, BFI, FSS, RFS) | The mean fatigue in the intervention group was 1.23 lower than the control group (95% CI 1.96 lower to 0.50 lower) | ‐‐ | 542 (7) | ⊕⊕⊝⊝ low1,2,3 | Aromatherapy may improve fatigue compared to placebo or standard care in people undergoing HD |
| Weakness | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Energy | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Tiredness | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Exhaustion | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Asthenia | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; PFS: Piper Fatigue Scale; BFI: Brief Fatigue Inventory; FSS: Fatigue Severity Scale; RFS: Rhoten fatigue scale; HD: haemodialysis. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence certainty was downgraded by one level due to study limitations
2 Evidence certainty was downgraded by one level due to imprecision (Optimal Information Size (OIS) not met and indirectness in outcome measure
3 Evidence certainty was downgraded by one level due to inconsistency
Summary of findings 3. Massage versus no intervention for people receiving dialysis.
| Massage versus no intervention for people receiving dialysis | ||||||
|
Patient or population: people receiving dialysis Settings: multinational Intervention: massage Comparison: no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Massage | |||||
|
Fatigue (PFS, FSS, VAS) median follow‐up: 0.9 months |
The mean score for fatigue ranged across control groups from 5.17 to 80.74 (PFS, FSS, or VAS scores) | The mean fatigue in the intervention group was 1.06 lower than the control group (95% CI 1.47 lower to 0.65 lower) | ‐‐ | 657 (7) | ⊕⊕⊝⊝ low1,2,3 | Massage may improve fatigue compared to not intervention in people undergoing HD |
| Weakness | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
|
Energy (VAS) median follow‐up: 0.9 months |
The mean score for energy ranged across control groups from 18.93 to 21.97 (VAS) | The mean energy in the intervention group was 4.87 more than the control group (95% CI 1.69 more to 8.06more) | ‐‐ | 152 (2) | ⊕⊕⊝⊝ low1,3 | Massage may increase energy compared to not intervention in people undergoing HD |
| Tiredness | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Exhaustion | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Asthenia | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; PFS: Piper Fatigue Scale; FSS: Fatigue Severity Scale; VAS: Visual Analogue Scale; HD: haemodialysis. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence certainty was downgraded by one level due to study limitations
2 Evidence certainty was downgraded by one level due to imprecision (Optimal Information Size (OIS)) not met and indirectness in outcome measure
3 Evidence certainty was downgraded by one level due to inconsistency
Summary of findings 4. Acupressure versus placebo or control for people receiving dialysis.
| Acupressure versus placebo or control for people receiving dialysis | ||||||
|
Patient or population: people receiving dialysis Settings: multinational Intervention: acupressure Comparison: placebo or control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or control | Acupressure | |||||
|
Fatigue [PFS, revised PFS, FI] median follow‐up: 1 month |
The mean score for fatigue ranged across control groups from 4.7 to 125.1 (PFS, revised PFS, FI) | The standardised mean of fatigue in the intervention group was 0.64 lower than the control group (95% CI 1.03 lower to 0.25 lower) | ‐‐ | 459 (6) | ⊕⊕⊝⊝ low1,2,3 | Acupressure may reduce fatigue compared to placebo or control in people undergoing HD |
| Weakness | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Energy | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Tiredness | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Exhaustion | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| Asthenia | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ | No studies reported this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; PFS: Piper Fatigue Scale; revised PFS: revised Piper Fatigue Scale; FI: Fatigue Index; HD: haemodialysis. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence certainty was downgraded by one level due to study limitations
2 Evidence certainty was downgraded by one level due to imprecision (Optimal Information Size (OIS)) not met and indirectness in outcome measure
3 Evidence certainty was downgraded by one level due to inconsistency
Background
Description of the condition
Fatigue is common in people on dialysis, and it is associated with an increased risk of death, cardiovascular disease (CVD), depression and impaired quality of life (QoL) (Chiaranai 2016; Evangelidis 2017; Jhamb 2008; Ju 2021; Manera 2019). The prevalence of fatigue is estimated to range from 42% to 89% in adult patients on haemodialysis (HD) and peritoneal dialysis (PD) (Chang 2001; Jhamb 2008; Maruyama 2021; Picariello 2017a; Yngman‐Uhlin 2010).
Fatigue is defined as a continuum sense of tiredness or exhaustion that can prevent patients from being able to do their usual activities (Jhamb 2008; Ju 2018b; Lee 1991).
The causes of fatigue are complex and multifactorial and may be related to uremia, anaemia, inflammation, fluid shifts and metabolic processes (Ju 2018a). For patients receiving HD, physiological factors, such as anaemia, have been shown to be associated with fatigue, and studies suggest that the use of erythropoietin stimulating agents (ESAs) to treat anaemia improves QoL, fatigue and energy levels in patients on HD (Johansen 2012; Ross 2003). Cytokines may contribute to fatigue in people on HD as elevated levels of pro‐inflammatory cytokines are seen in kidney failure requiring kidney replacement therapy (KRT) (Artom 2014; Bergstrom 2000; Rao 2007; van Sandwijk 2019). Treatment‐related factors such as dialysis frequency or modality have also been shown to affect fatigue (Jhamb 2008; Picariello 2017a). Post‐dialysis fatigue is an intense fatigue experienced by patients after an HD session (Bossola 2020). Patients who received daily HD have reported less post‐dialysis fatigue than those who had more days off between dialysis sessions, suggesting that this symptom may be related to treatment frequency. Modalities, such as nocturnal dialysis, may help patients recover from post‐dialysis fatigue faster (Liangos 2010). Psychosocial and lifestyle factors correlated with fatigue in HD include depression, physical inactivity, and poor sleep quality (Jhamb 2008; Maruyama 2021).
In the PD population, clinical factors associated with fatigue scores include cholesterol, weekly creatinine clearance, transferrin, alkaline phosphatase, and serum intact parathyroid hormone (Chang 2001; Tian 2020).
Fatigue can be extremely debilitating (Chiaranai 2016; Debnath 2021; Horigan 2013; Yngman‐Uhlin 2010), and patients experience a limitation in freedom, a loss of sense of self and social connectedness (Davey 2019; Monaro 2014). Fatigue has recently been established by patients and health professionals as a core outcome to be reported in all trials in people receiving HD (Evangelidis 2017; Tong 2017).
Description of the intervention
As the causes of fatigue are uncertain and likely to be multifactorial, a range of pharmacological (including ESAs), novel anaemia therapies or levocarnitine) and non‐pharmacological interventions (such as diet, massage, aromatherapy, meditation, cognitive behavioural therapy (CBT) or frequency of dialysis treatments) were considered.
How the intervention might work
Both pharmacological and non‐pharmacological interventions may improve fatigue. For example, ESAs or other interventions to achieve higher haemoglobin (Hb) targets and levocarnitine to modify the effects of defective fatty‐acid metabolism have been shown to improve symptoms of fatigue (Foley 2009; Johansen 2012; Ossareh 2003; Schreiber 2005). Recently, hypoxia‐inducible factors (HIF), a new class of drugs to treat anaemia, might be effective in the treatment of fatigue, but data are still sparse (Chertow 2021). Non‐pharmacological interventions that focus on psychosocial and lifestyle aspects, including diet, exercise, sleep, foot reflexology, aromatherapy and yoga, may also help to improve fatigue (Eglence 2013; Habibzadeh 2020; Karadag 2019; Salehi 2020; Yurtkuran 2007). Physical activity may improve fatigue through indirect effects on cytokine levels or by increasing muscle strength (Jhamb 2008). CBT has also demonstrated improvement in sleep and fatigue in this population (Chen 2008; Chen 2011a; Unruh 2020). Frequent and longer dialysis treatment may reduce post‐dialysis fatigue and improve general well‐being (Bossola 2020). However, the exact causal mechanism of improvements seen in these studies remains unknown.
Why it is important to do this review
It is widely known that fatigue is one of the most common and debilitating symptoms experienced by people on dialysis. In the HD population, fatigue has been consistently identified as the most critically important outcome and a high research priority in people on HD (Evangelidis 2017; Ju 2018a; Urquhart‐Secord 2016). The last decade has seen a growing number of studies on pharmacological and lifestyle interventions to improve fatigue. There have been systematic reviews focusing on one particular type of pharmacological intervention, such as levocarnitine (Schreiber 2005) or ESAs (Johansen 2012). Few systematic reviews have been published on non‐pharmacological interventions for fatigue (Astroth 2013; Bouya 2018; Melo 2020; Song 2018). Furthermore, it is unclear how the efficacy of these interventions compares to pharmacological interventions.
In this review, we summarised and synthesised all current evidence of the benefits and harms of interventions that have been evaluated for their impact on fatigue in people on dialysis. The definition of fatigue and fatigue‐related outcomes were reported according to the definition provided by the authors. We considered all pharmacological and non‐pharmacological interventions as the potential causes of fatigue are diverse and likely to be multifactorial. In doing so, this review may shed light on any existing evidence for an intervention that effectively reduces or manages fatigue. Information on the efficacy of different interventions and other factors that facilitate or challenge the improvement of fatigue will allow clinicians to provide effective care for their patients' experience of this debilitating symptom. Furthermore, as fatigue is associated with other outcomes such as death, cardiovascular diseases and broader QoL, improvement in this symptom may translate into better patient outcomes overall.
Objectives
We aimed to evaluate the effects of any pharmacological and non‐pharmacological interventions on fatigue in people with kidney failure requiring dialysis, such as HD and PD, including any setting (e.g. dialysis performed in the clinic or at home) and frequency.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth, or other predictable methods) of interventions whereby fatigue or fatigue‐related outcomes were reported as either primary or secondary outcome.
Types of participants
Inclusion criteria
Patients of any age with kidney failure on any form of dialysis. The dialysis treatment could be performed both in the clinic and at home. Any frequency of the dialysis treatment was included.
Exclusion criteria
None.
Types of interventions
We considered any intervention affecting levels of self‐reported fatigue in patients on dialysis. Studies were included if fatigue was reported as an outcome.
Pharmacological treatment (including but not limited to): psychostimulants (amphetamines, modafinil, armodafinil, methylphenidate, pemoline), amantadine, corticosteroids (dexamethasone, prednisone, methylprednisolone), donepezil, antidepressants (selective serotonin reuptake inhibitors, paroxetine), anxiolytics, ESAs, HIF, human growth hormone, tumour necrosis factor (TNF) inhibitor, acetylsalicylic acid, megestrol acetate, alfacalcidol and intravenous (IV) levocarnitine
Non‐pharmacological treatment (including but not limited to): nutrition (albumin, diet), therapeutic exercise (e.g. inspiratory muscle training exercise, aerobic exercise), alternative and complementary medicine (acupressure, Chinese herbal medicine and acupuncture), psychosocial (psychotherapy, psycho‐education such as cognitive restructuring, coping strategies, stress management), educational (goal‐setting, providing information/advice on symptom management/nutrition).
Any mode, frequency, prescription, and duration of therapy were considered. The intervention may be administered at any time or day (i.e. dialysis or non‐dialysis days) and in clinical or non‐clinical settings.
Types of outcome measures
We used time points of measurements as reported by investigators, as well as assessing the outcome measures at the end of the treatment.
Primary outcomes
Fatigue and fatigue‐related outcomes such as tiredness, exhaustion, weakness, energy/vitality and asthenia that have been assessed through any self‐report measure (open‐ended questionnaires such as fatigue diary, fatigue‐specific scales (e.g. Functional Assessment of Chronic Illness Therapy Fatigue subscale (FACIT‐F), Chalder Fatigue Scale (CFS)), or fatigue sub‐scale as part of a measure assessing a broader construct (e.g. Short Form‐36 (SF‐36), or visual analogue scale (VAS)). We considered all patient‐reported outcome measures for fatigue, given the lack of validation work conducted in the dialysis population. To avoid misinterpretation of the data, definitions of fatigue and fatigue‐related outcomes were reported according to the definitions provided by the authors. Fatigue and fatigue‐related outcomes (including tiredness, exhaustion, weakness, energy/vitality and asthenia) were assessed separately.
Secondary outcomes
QoL, depression, anxiety, death (any cause and cardiovascular), vascular access, CVD, hypertension, diabetes, sleep and mood.
Search methods for identification of studies
No restrictions based on the date of the study publications, language, or publication were applied when searching and selecting studies for inclusion. The search was conducted with the Cochrane Kidney and Transplant Information Specialist using search terms relevant to this review.
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 18 October 2022 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these searches, as well as a list of hand‐searched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Grey literature sources (e.g. abstracts, dissertations, and theses), in addition to those already included in the Cochrane Kidney and Transplant Register of Studies, were also searched.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. The titles and abstracts were screened independently by three authors (PN, AJ, VS). Three authors (PN, AJ, VS) independently assessed retrieved abstracts and, if necessary, the full text of these studies to determine which studies satisfy the inclusion criteria.
Data extraction and management
Data relating to study design (RCT, quasi‐RCT), participant characteristics (e.g. age, gender, dialysis vintage, comorbidity), interventions (pharmacological, non‐pharmacological) and outcomes (as described above) were extracted. Three authors (PN, AJ, VS) independently carried out data extraction using a standard data extraction form. Studies reported in non‐English languages were translated before assessment. Where more than one publication of a study exists, the publications were grouped together, and the report with the most complete data was included in the meta‐analyses. Where relevant outcomes are only published in earlier versions, these data were used. Any discrepancies between published versions were highlighted. Any further information required from the original author was requested by written correspondence, and any relevant information obtained in this manner was included in the review. Disagreements were resolved by consensus in consultation with another author (AJ).
Assessment of risk of bias in included studies
The following items were independently assessed by two authors (PN, VS) using the risk of bias assessment tool (Higgins 2022) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. adverse events, cardiovascular events, death), results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement are used to assess the effects of treatment (e.g. depression, fatigue), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales were used.
Unit of analysis issues
Cluster‐randomised studies
We anticipated that studies using clustered randomisation had controlled for clustering effects. In case of doubt, we contacted the first authors to ask for individual participant data to calculate an estimate of the intracluster correlation coefficient (ICC). If this was not possible, we obtained external estimates of the ICC from a similar study or from a study of a similar population as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). When the ICC was established, we used it to re‐analyse the study data. If ICCs from other sources were used, we reported this and conducted sensitivity analyses to investigate the effect of variations in the ICC.
Cross‐over studies
We included all randomised cross‐over studies in the systematic review if they report a paired (comparison within the patient) analysis using all periods. If not, we only used the data from the first period.
Studies with more than two treatment arms
If more than one of the interventions is a fatigue intervention, and there is sufficient information in the study to assess the similarity of the interventions, we combined similar interventions to allow for a single pair‐wise comparison.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing the corresponding author), and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population were carefully performed. Attrition rates, for example, drop‐outs, losses to follow‐up and withdrawals, were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2022).
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We quantified statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I2 values was as follows:
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P value from the Chi2 test or a CI for I2) (Higgins 2022).
Assessment of reporting biases
If possible, funnel plots were used to assess for the potential existence of small study bias (Higgins 2022). There were insufficient studies per comparision to do this.
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
We reported the results of our findings separately, focusing on fatigue, as reported by the authors. Adverse effects were tabulated and assessed with descriptive techniques, as they were likely to be different for the various interventions used. Where possible, the risk differences with 95% CI were calculated for each adverse effect, either compared to no treatment or to another agent.
Based on available data, we planned to perform the following subgroup analyses.
Age: < 18 years versus ≥ 18 years; and < 64 years versus ≥ 64 years
Gender: female versus male
Risk of bias: high versus low (versus unclear) (allocation concealment, blinding of outcome assessors, incomplete outcome data)
Indication: studies targeting fatigue versus reporting fatigue
Intervention type: pharmacological versus non‐pharmacological
Presence of comorbidities: CVD (yes versus no), diabetes (yes versus no), hypertension (yes versus no), depression (clinical diagnosis versus none)
Fatigue outcome measures used: validation data available versus de novo
Dialysis type: PD versus HD
Dialysis vintage: < 5 years versus ≥ 5 years
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size:
Repeating the analysis excluding abstract‐only publication
Repeating the analysis excluding industry‐funded studies
Repeating the analysis, taking account of the risk of bias (allocation concealment)
Repeating the analysis, excluding any very long or large studies, to establish how much they dominate the results.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in 'Summary of findings' tables. These tables presented key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2022a). The 'Summary of findings' tables also included an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2022b). We presented the following outcomes in the 'Summary of findings' tables:
Fatigue
Weakness
Energy
Tiredness
Exhaustion
Asthenia
Results
Description of studies
The following section contains broad descriptions of the studies considered in this review. For further details on each individual study, please see Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
After searching the Specialised Register, a total of 311 records were identified. After screening titles, abstracts, and full‐text review, 94 studies (249 reports) were included, and 16 studies (43 reports) were excluded. Sixteen ongoing studies were identified. One study states recruitment was completed in 2010 (NCT00440869); however, no results have been identified. These 17 studies will be assessed in a future update of this review (Figure 1).
1.
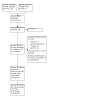
Flow diagram of study selection
Included studies
The Characteristics of included studies tables reported the characteristics of the participants and the interventions in the included studies. A total of 94 studies (8191 randomised participants) were included in this review.
Study design, setting and characteristics
Four studies had a quasi‐randomised design, two studies had a cluster‐randomisation design, 13 studies had a cross‐over design, and the remaining studies were RCTs. Studies were conducted from 1979 to 2022 in Australia (two studies), Brazil (three studies), Canada (seven studies), China (one study), Denmark (one study), Egypt (two studies), Germany (one study), Greece (four studies), Hong Kong (one study), India (two studies), Iran (22 studies), Italy (one study), Japan (four studies), Switzerland (one study), Taiwan (11 studies), Turkey (nine studies), the UK (three studies), the USA (15 studies), were performed in multinational setting (two studies) or did not report information about the country (two studies). Study follow‐up ranged from one week (four studies) to 21.8 months (one study), with a median of 1.8 months. Fourteen studies received funding from pharmaceutical companies. Six studies were available only as conference abstracts.
Study participants
Three studies were conducted in people with PD, five studies in people with both HD and PD, one study was performed in people with either HD or haemodiafiltration (HDF), one study did not specify the type of dialysis, whilst all other studies were performed in people receiving HD. The mean dialysis vintage ranged from 0.3 to 12.7 years, with a median of 4.1 years. The sample size varied from five to 596 participants, with a median of 61 participants. The mean study age ranged from 38 years to 69 years, with a median of 56 years. No studies evaluated treatment in children.
Thirteen studies included people with and without cardiovascular comorbidities at baseline; one study excluded people with CVD, while one study included only patients with previous CVD. Forty‐seven studies included people with and without diabetes. Of these studies, only one study reported subgroup analyses for people with and without diabetes. Two studies did not include people with diabetes, while one study was performed in people with diabetes. Thirty‐three studies were performed in people with and without hypertension; one study did not include people with hypertension, while one study was focused only on people with hypertension. Clinical diagnosis of depression was rarely reported: two studies excluded people with depression, two studies included only people with depression at baseline, and one study included people with and without depression.
The definition of fatigue and fatigue‐related outcomes were reported according to the definition provided by the authors. Fatigue was assessed using different tools (see Appendix 3).
Kidney Disease Questionnaire (KDQ) (Brass 2001; Canadian EPO 1990)
Piper Fatigue Scale (PFS) (Amini 2016; Bicer 2022; Eroglu 2022; Kaplin Serin 2020; Mohamed 2014; Muz 2017; Roshanravan 2016; Ozdemir 2013; Sabouhi 2013; Tsay 2004a; Tsay 2004b)
Revised PFS (Cho 2004)
36‐item Short‐Form Health Survey (SF‐36) (ASCEND 2016; Fatigue‐HD 2019; Johansen 2006)
Kidney Disease Quality of Life‐Short Form (KDQOL‐SF) (Fukuda 2015; PEDAL 2020)
Fatigue Severity Scale (FSS) (Ahmady 2019; Bagheri‐Nesami 2016; Chen 2008a; Chen 2011a; Fatigue‐HD 2019; Habibzadeh 2020; Karadag 2019; Lazarus 2020; Mohajeranirad 2021; Mohammadpourhodki 2021; Shahdadi 2016)
Multidimensional Fatigue Inventory (MFI‐20) (Balouchi 2016; Biniaz 2015; Salehi 2020)
VAS for Fatigue (VAS‐F) (Bicer 2022; Cecen 2021; Schardong 2021; Unal 2016; Yurtkuran 2007)
FACIT‐F (Parfrey 2005)
Profile of Mood States Fatigue subscale (POMS‐F) (Johansen 1999; Johansen 2006)
Fatigue Index (FI) (Su 2009)
Rhoten fatigue scale (RFS) (Varaei 2020)
Brief Fatigue Inventory (BFI) (Hadadian 2018; Hassanzadeh 2018; Lin 2011)
CFS (fatigue severity) and Work and Social Adjustment Scale (fatigue‐related functional impairment) (Picariello 2018)
Standardized Outcomes in Nephrology ‐ Haemodialysis (SONG‐HD) Fatigue score (SWIFT 2020)
Modified Fatigue Impact Scale (MFIS) (Fatigue‐HD 2019)
Hemodialysis‐Related Fatigue Scale (HFS) (Huang 2021)
KDQ (Semeniuk 2000)
Iowa Fatigue Scale (IFS) (Soliman 2015)
The name of the tool used for assessing fatigue was not clearly stated (Babamohammadi 2006; Grigoriou 2021; Krase 2022)
Seven studies reported fatigue only as an adverse event.
Interventions
A broad range of interventions have been reported in the included studies.
Non‐physiological neutral amino acids versus placebo
L‐threo‐3,4‐dihydroxyphenilserine (L‐DOPS) (Akizawa 2002)
L‐carnitine (Bellinghieri 1983; Brass 2001; Fatouros 2010; Semeniuk 2000)
Serotonin reuptake inhibitor versus placebo
Sertraline (ASSertID 2015)
Beta‐blockers versus angiotensin‐converting enzyme inhibitors
Atenolol versus lisinopril (HDPAL 2014)
Anabolic steroids versus placebo
Nandolone decanoate (Johansen 1999; Johansen 2006)
Anabolic steroids versus exercise
Nandrolone decanoate (Johansen 2006)
Anabolic steroids alone versus anabolic steroids plus exercise
Nandrolone decanoate (Johansen 2006)
Anabolic steroids plus exercise versus exercise alone
Nandrolone decanoate (Johansen 2006)
Anabolic steroids plus exercise versus placebo
Nandrolone decanoate (Johansen 2006)
Iron replacement product versus placebo
Ferumoxytol versus saline sterile injection (Singh 2008a)
Continuous erythropoietin receptor activation (C.E.R.A)
C.E.R.A once/week versus C.E.R.A once every two weeks, both groups using EPO alpha (BA16285 2007)
Erythropoietin stimulating agents versus placebo
Erythropoietin (EPO) alpha to achieve a Hb target of 9.5 to 11.0 g/dL (low‐target group) or 11.5 to 13.0 g/dL or 13.5 to 16 g/dL (high‐target group) (Canadian EPO 1990; Lillevang 1990)
Haemoglobin targets
EPO alpha to achieve a Hb target of 9.5 to 10.5 g/dL (normal‐target group) or 13.0 to 14.0 g/dL (high‐target group) (Foley 2000; Parfrey 2005)
EPO alpha to achieve normal HB target group to subnormal HB target group with or without ESA (Linde 2001)
Nutritional supplementation versus placebo
Nutritional drink supplementation (containing vitamin B1, vitamin B2, niacin, vitamin B6, vitamin B12, folic acid, vitamin C, carnitine, coenzyme Q10, naive galacto‐oligosaccharide, and zinc) (Fukuda 2015)
Ascorbic acid (vitamin C) (Biniaz 2015; Singer 2010)
Helichrysum Psudoplicatum supplementation (Mohajeranirad 2021)
Dialysate sodium concentration
Dialysate sodium versus another concentration of dialysate sodium in general (Barre 1988; Mohamed 2013)
Steady dialysate sodium versus linear sodium ramping (Sang 1997)
Steady dialysate sodium versus stepwise sodium ramping (Sang 1997)
Linear sodium ramping versus stepwise sodium ramping (Sang 1997)
Glucose‐enriched dialysate
Dialysis sessions with dialysate containing glucose 400 mg/100 mL to dialysis sessions with dialysate of the same composition but without glucose (Leski 1979)
Glucose‐enriched dialysate (200 mg/100 mL) to dialysate without glucose (Raimann 2010)
Cold versus warm dialysis
Cold temperature dialysis (35.5°C) to warm temperature dialysis (37°C) (Sajadi 2016)
Citrate versus standard care
Citrate dialysate to standard dialysate (Schmitz 2016)
Cuprophan versus polysulfone
Cuprophan low flux dialyser membranes to polysulfone low flux dialyser membranes (Singh 2003)
Cuprophane versus polymethyl‐methacrylate
Cuprophan low flux dialyser membranes to polymethyl‐methacrylate (PMMA) low flux dialyser membranes (Sklar 1998)
Frequent versus conventional haemodialysis
Frequent HD (six times/week) to conventional HD (three times/week) (FHN DAILY 2007; FHN NOCTURNAL 2007)
Haemodialysis with sodium bath versus isolated ultrafiltration
Hypernatric HD with 150 to 155 mEq/L sodium bath (two cycles) to isolated ultrafiltration (two cycles) (Sklar 1999)
Haemodialysis with sodium bath versus isolated diffusion
Hypernatric HD with 150 to 155 mEq/L sodium bath (two cycles) to isolated diffusion (two cycles) (Sklar 1999)
Haemodialysis with sodium bath versus sham procedures with or without recirculation
Hypernatric HD with 150 to 155 mEq/L sodium bath (two cycles) to sham procedures with isolated membrane (two cycles) or sham procedures without recirculation exposure to a dialysis membrane (two cycles) (Sklar 1999)
Isolated ultrafiltration versus isolated diffusion
Isolated ultrafiltration (two cycles) to isolated diffusion (two cycles) (Sklar 1999)
Isolated ultrafiltration versus sham procedures with or without recirculation
Isolated ultrafiltration (two cycles) to sham procedures with isolated membrane (two cycles) or sham procedures without recirculation exposure to a dialysis membrane (two cycles) (Sklar 1999)
Isolated diffusion versus sham procedures with or without recirculation
Isolated diffusion (two cycles) to sham procedures with isolated membrane (two cycles) or sham procedures without recirculation exposure to a dialysis membrane (two cycles) (Sklar 1999)
Blood flow rate reduction versus standard care
Blood flow rate reduction of 100 mL/min to a minimum of 300 mL/min (Duggal 2019)
Self‐blood pressure monitoring versus ambulatory blood pressure monitoring
Home blood pressure (BP) monitoring to predialysis BP monitoring (BOLD 2020)
Relaxation versus no intervention
Progressive muscle relaxation or relaxation exercise (Amini 2016; Hadadian 2018; Kaplin Serin 2020)
No specified relaxation technique (Hassanzadeh 2018)
Relaxation versus aromatherapy
Benson relaxation technique to inhalation of lavender essential oil (Hassanzadeh 2018)
Relaxation versus exercise
Progressive muscle relaxation to aerobic exercise (Amini 2016)
Relaxation plus music therapy versus no intervention
Benson technique plus music therapy (Eroglu 2022)
Meditation versus no intervention
Brief mindfulness meditation (Thomas 2017)
Yoga (Reilly‐Spong 2015; Yurtkuran 2007)
Exercise versus placebo or control
Aerobic exercise (Amini 2016; Figueiredo 2018; Krase 2022; PEDAL 2020)
Leg ergometry exercise (Chang 2010; Konstadinidou‐ND 2002; Salehi 2020)
Muscle function (Johansen 2006)
Personal Energy Planning (PEP) programme (Fatigue‐HD 2019)
Hybrid exercise (Grigoriou 2021)
Breathing‐based leg exercises (Huang 2021)
Range of motion (ROM) exercise (Soliman 2015)
Inspiratory muscle training (Figueiredo 2018; Pellizzaro 2013)
Electrical muscle stimulation (Suzuki 2018)
Peripheral muscle training (Pellizzaro 2013)
Exercise versus another exercise
Inspiratory muscle training versus aerobic exercise (Figueiredo 2018)
Respiratory muscle training versus peripheral muscle training (Pellizzaro 2013)
Aromatherapy versus standard care
Lavender essence (Ahmady 2019; Bagheri‐Nesami 2016; Karadag 2019; Mohammadpourhodki 2021; Varaei 2020)
Sweet orange oil and lavender oil (Muz 2017)
Orange essence (Ahmady 2019; Mohammadpourhodki 2021; Varaei 2020)
Not specified aromatherapy (Hassanzadeh 2018)
Aromatherapy versus another type of aromatherapy
Lavander extract versus orange extract (Ahmady 2019; Balouchi 2016; Jalalian 2015; Mohammadpourhodki 2021; Varaei 2020)
Massage versus no intervention
Slow‐stroke back massage (Hasankhani 2013; Shahdadi 2016)
Foot reflexology (Cecen 2021; Ozdemir 2013; Roshanravan 2016)
-
Slow‐stroke back massage or foot reflexology (Unal 2016)
NOTE: outcome data provided were not extracted for slow‐stroke back massage since two different massages were compared with the control
Hand massage (Cecen 2021)
Olive oil massage (Lazarus 2020)
Chamomile, almond or no oils (Habibzadeh 2020)
Massage versus another type of massage
Foot reflexology versus back massage (Unal 2016)
Hand massage versus foot massage (Cecen 2021)
Chamomile or almond versus no oils (Habibzadeh 2020)
Massage versus sham massage
Foot reflexology (Roshanravan 2016)
Sham massage versus no intervention
Foot reflexology without pressing certain parts of the foot (Roshanravan 2016)
Acupressure versus placebo or control
Transcutaneous electrical acupoint stimulation (TEAS) versus no intervention (Vishnevskii 2014)
Far‐infrared (FIR) rays on each acupoint versus no intervention (Lin 2011)
FIR rays versus heath pad therapy (Su 2009)
Acupressure versus routine unit care or no intervention (Cho 2004; Sabouhi 2013; Tsay 2004a)
-
Acupressure or TEAS versus control (Tsay 2004b)
NOTE: outcome data were not extracted for TEAS since two different acupressure techniques were compared to the control
Acupressure versus placebo (Bicer 2022)
Acupressure versus another type of acupressure
Acupressure versus TEAS (Tsay 2004b)
Acupressure versus sham acupressure
Acupressure (Sabouhi 2013; Tsay 2004a)
Herbal acupoint therapy (Tsai 2016)
TEAS versus TEAS‐sham group (Hadadian 2016)
Sham acupressure versus standard care
Sham acupressure performed away from the actual intervention site with or without usual care (Sabouhi 2013; Tsay 2004a)
Cognitive‐behavioural therapy versus no intervention
CBT for fatigue (BReF intervention) versus waiting‐list control (Picariello 2018)
Cognitive‐behavioural therapy versus education
CBT versus sleep hygiene education (Chen 2008a; Chen 2011a)
Cognitive‐behavioural therapy versus serotonin reuptake inhibitor
CBT versus sertraline (ASCEND 2016)
Education versus control
Nurse‐led case management programme (Chow 2010; Li 2014b; Mohamed 2014)
Pharmacist‐led pharmaceutical care plus routine care (Dashti‐Khavidaki 2013)
Physical education program (Motedayen 2014)
Home‐care educational program (Babamohammadi 2006)
Usual care (SOCIABLE 2017; SWIFT 2020)
Anti‐thrombotic polymethyl‐methacrylate versus placebo
Anti‐thrombotic polymethyl‐methacrylate membrane (VENOUS 2020)
Light versus no intervention
Photobiomodulation therapy (Schardong 2021)
Excluded studies
Thirty‐three studies were excluded. The reasons for exclusion were:
Not randomised (Eglence 2013; Laupacis 1992)
Wrong population (TREAT 2005)
Fatigue was not reported as either a primary or secondary outcome (13 studies: CHAIR 2015; Churchill 1987; Dashti‐Khavidaki 2011; Gram 1998; Heshmatifar 2015; Heshmati Far 2015; Macagnan 2019; Nakamoto 2008; Sharp 2005; Shimizu 1983; Siami 1991; Tawney 2000; Tsai 2015).
Studies awaiting classification
One study stated recruitment was completed in 2010; however, no published results have been identified (NCT00440869).
Ongoing studies
We identified 16 ongoing studies.
Intradialytic yoga versus usual care (ACTRN12617000420347)
Intradialytic yoga versus educational program (NCT02361268)
Home‐based physical training versus non‐training group (ACTRN12618000724279)
Intradialytic exercise versus not intervention (Cardoso 2019; CTRI/2018/02/012021)
Walking, resistance training or combination training (ACTRN12620000408987)
Virtual reality versus standard care (Burrai 2019a)
High‐dose HDF continuation versus conventional high‐flux HD (CONVINCE 2020)
Self‐management strategies versus dietary information (NCT01620580)
Individual face‐to‐face educational intervention session versus usual care (Sharma 2022)
Motor cortex, dorsolateral prefrontal cortex or sham treatments (Quintiliano 2019)
Psychosocial counselling sessions led by a social worker versus usual care (van der Borg 2016)
CBT (TĀCcare or technology‐delivered health education) versus no treatment (TACcare 2018)
Plantar electrical nerve stimulation versus non‐functional device (Hamad 2021)
CBT versus trazodone versus placebo (SLEEP‐HD 2021)
Intradialytic creatine supplementation creatine supplementation (0.5, 1.0, 1.5 or 2.0 mM) versus placebo (van der Veen 2021)
Risk of bias in included studies
The risk of bias for studies overall are summarised in Figure 2 and the risk of bias in each study is described in Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Forty‐one studies were judged to be low risk for adequately providing methods used for random sequence generation. Fifty‐one studies were judged to be unclear risk as they stated to be randomised but provided no further details on how this was undertaken. Two studies were judged to be high risk.
Allocation concealment
Allocation concealment was assessed as adequate in 18 studies, high risk in three studies, and unclear risk in 73 studies.
Blinding
Performance bias
Eight studies were blinded and considered to be at low risk of bias for performance bias, and 73 studies were not blinded and were considered at high risk of performance bias. Thirteen studies were assessed as unclear risk of bias.
Detection bias
Blinding of outcome assessment was judged to be at low risk in seven studies, and 87 studies were considered at high risk of detection bias.
Incomplete outcome data
Data follow‐up was complete in 13 studies, incomplete in 63 studies, whilst 18 studies were assessed as unclear risk of bias.
Selective reporting
Eight studies reported expected and clinically‐relevant outcomes and were deemed to be at low risk of bias, and 86 studies did not report key patient‐centred outcomes, including fatigue, cardiovascular disease, death and vascular access.
Other potential sources of bias
Forty‐five studies appeared to be free from other sources of bias, 15 studies reported other sources of bias (including the role of funding source and/or imbalance in baseline treatment groups). It was unclear risk whether 34 studies had other sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Non‐physiological neutral amino acids versus placebo
Three studies (Akizawa 2002; Bellinghieri 1983; Brass 2001) compared non‐physiological neutral amino acids, including L‐DOPS (Akizawa 2002) and L‐carnitine (Bellinghieri 1983; Brass 2001) to placebo in people receiving HD, during a median follow‐up of 1.8 months. The certainty of the evidence was very low.
Fatigue
Compared to placebo, non‐physiological neutral amino acids had uncertain effects on fatigue (Analysis 1.1 (1 study, 180 participants): KDQ score; MD ‐0.05, 95% CI ‐0.44 to 0.34; very low certainty evidence).
1.1. Analysis.

Comparison 1: Non‐physiological neutral amino acid versus placebo, Outcome 1: Fatigue
Change in fatigue
Compared to placebo, non‐physiological neutral amino acids had uncertain effects on the change in fatigue (Analysis 1.2 (1 study, 180 participants): KDQ score; MD 0.20, 95% CI ‐0.08 to 0.48; very low certainty evidence).
1.2. Analysis.

Comparison 1: Non‐physiological neutral amino acid versus placebo, Outcome 2: Change in fatigue
Number of participants with improvement in fatigue
Compared to placebo, non‐physiological neutral amino acids had uncertain effects on the improvement in fatigue (Analysis 1.3 (1 study, 121 participants): RR 1.25, 95% CI 0.80 to 1.95; very low certainty evidence).
1.3. Analysis.

Comparison 1: Non‐physiological neutral amino acid versus placebo, Outcome 3: Number with improvement of fatigue
Number of participants with aggravation of fatigue
Compared to placebo, non‐physiological neutral amino acids may reduce the number of participants with aggravation of fatigue, but the evidence is very uncertain (Analysis 1.4 (1 study, 121 participants): RR 0.18, 95% CI 0.06 to 0.52; very low certainty evidence).
1.4. Analysis.

Comparison 1: Non‐physiological neutral amino acid versus placebo, Outcome 4: Number with aggravation of fatigue
Death (any cause)
Compared to placebo, non‐physiological neutral amino acids had uncertain effects on death (any cause) (Analysis 1.5: 3 studies, 356 participants), as no events were reported in the eligible studies.
1.5. Analysis.

Comparison 1: Non‐physiological neutral amino acid versus placebo, Outcome 5: Death (any cause)
Cardiovascular death
Compared to placebo, non‐physiological neutral amino acids had uncertain effects on cardiovascular death (Analysis 1.6: 2 studies, 163 participants), as no events were reported in the eligible studies.
1.6. Analysis.

Comparison 1: Non‐physiological neutral amino acid versus placebo, Outcome 6: Cardiovascular death
Quality of life (overall)
Compared to placebo, non‐physiological neutral amino acids had uncertain effects on the overall QoL (Analysis 1.7 (1 study, 180 participants): KDQ score; MD ‐0.02, 95% CI ‐0.35 to 0.31; very low certainty evidence).
1.7. Analysis.

Comparison 1: Non‐physiological neutral amino acid versus placebo, Outcome 7: Quality of life (overall)
Change in quality of life (overall)
Compared to placebo, non‐physiological neutral amino acids had uncertain effects on the change in overall QoL (Analysis 1.8 (1 study, 180 participants): KDQ score; MD 0.15, 95% CI ‐0.08 to 0.38; very low certainty evidence).
1.8. Analysis.

Comparison 1: Non‐physiological neutral amino acid versus placebo, Outcome 8: Change in quality of life
Depression
Compared to placebo, non‐physiological neutral amino acids had uncertain effects on depression (Analysis 1.9 (1 study, 180 participants): KDQ score; MD ‐0.17, 95% CI ‐0.59 to 0.25; very low certainty evidence).
1.9. Analysis.

Comparison 1: Non‐physiological neutral amino acid versus placebo, Outcome 9: Depresssion
Change in depression
Compared to placebo, non‐physiological neutral amino acids had uncertain effects on change in depression (Analysis 1.10 (1 study, 180 participants): KDQ score; MD 0.13, 95% CI ‐0.21 to 0.47; very low certainty evidence).
1.10. Analysis.

Comparison 1: Non‐physiological neutral amino acid versus placebo, Outcome 10: Change in depression
Hypertension
Compared to placebo, non‐physiological neutral amino acids had uncertain effects on hypertension (Analysis 1.11 (1 study, 193 participants): RR 1.47, 95% CI 0.06 to 35.48; very low certainty evidence).
1.11. Analysis.

Comparison 1: Non‐physiological neutral amino acid versus placebo, Outcome 11: Hypertension
No other primary or secondary outcomes were reported.
Relaxation versus no intervention
Three studies (Amini 2016; Hassanzadeh 2018; Kaplin Serin 2020) compared progressive muscle relaxation (Amini 2016; Kaplin Serin 2020) or Benson muscle relaxation techniques (Hassanzadeh 2018) to no intervention in people receiving HD during a median follow‐up of 1.4 months. The certainty of the evidence was very low.
Fatigue
Compared to no intervention, relaxation may improve fatigue, but the evidence is very uncertain (Analysis 2.1 (3 studies, 234 participants): PFS or BFI score; SMD ‐1.51, 95% CI ‐2.28 to ‐0.73; I2 = 85%; very low certainty evidence). Substantial heterogeneity was observed between the studies.
2.1. Analysis.

Comparison 2: Relaxation versus no intervention, Outcome 1: Fatigue
Death (any cause)
Compared to no intervention, relaxation had uncertain effects on death (any cause) (Analysis 2.2: 1 study, 96 participants), as no events were reported.
2.2. Analysis.

Comparison 2: Relaxation versus no intervention, Outcome 2: Death (any cause)
Cardiovascular death
Compared to no intervention, relaxation had uncertain effects on cardiovascular death (Analysis 2.3: 1 study, 96 participants), as no events were reported.
2.3. Analysis.

Comparison 2: Relaxation versus no intervention, Outcome 3: Cardiovascular death
Anxiety
Compared to no intervention, relaxation had uncertain effects on anxiety (Analysis 2.4 (1 study, 68 participants): Beck Anxiety Index (BAI) score; MD ‐1.40, 95% CI ‐4.55 to 1.75; very low certainty evidence).
2.4. Analysis.

Comparison 2: Relaxation versus no intervention, Outcome 4: Anxiety
Sleep quality
Compared to no intervention, relaxation may improve sleep quality, but the evidence is very uncertain (Analysis 2.5 (1 study, 68 participants): Pittsburgh Sleep Quality Index (PSQI) score; MD ‐6.52, 95% CI ‐7.60 to ‐5.44; very low certainty evidence).
2.5. Analysis.

Comparison 2: Relaxation versus no intervention, Outcome 5: Sleep quality
No other primary or secondary outcomes were reported.
Relaxation versus exercise
Amini 2016 compared progressive muscle relaxation versus aerobic exercise in people receiving HD, during a follow‐up of 1.8 months. The certainty of the evidence was very low.
Fatigue
Compared to exercise, relaxation may reduce fatigue, but the evidence is very uncertain (Analysis 3.1 (1 study, 65 participants): PFS score; MD ‐17.66, 95% CI ‐30.32 to ‐5.00; very low certainty evidence).
3.1. Analysis.

Comparison 3: Relaxation versus exercise, Outcome 1: Fatigue
Anxiety
Compared to exercise, relaxation had uncertain effects on anxiety (Analysis 3.2 (1 study, 65 participants): BAI score; MD ‐1.52, 95% CI ‐6.46 to 3.42; very low certainty evidence).
3.2. Analysis.

Comparison 3: Relaxation versus exercise, Outcome 2: Anxiety
Sleep quality
Compared to exercise, relaxation had uncertain effects on sleep quality (Analysis 3.3 (1 study, 65 participants): PSQI score; MD 0.31, 95% CI ‐0.51 to 1.13; very low certainty evidence).
3.3. Analysis.

Comparison 3: Relaxation versus exercise, Outcome 3: Sleep quality
No other primary or secondary outcomes were reported.
Relaxation plus music therapy versus no intervention
Eroglu 2022 compared relaxation plus music therapy to no intervention in people receiving HD during a follow‐up of 2.3 months. The certainty of the evidence was very low.
Death (any cause)
Compared to no intervention, relaxation plus music therapy had uncertain effects on death (any cause) (Analysis 4.1: 1 study, 62 participants), as no events were reported.
4.1. Analysis.

Comparison 4: Relaxation + music versus no intervention, Outcome 1: Death (any cause)
Cardiovascular death
Compared to exercise, relaxation plus music therapy had uncertain effects on cardiovascular death (Analysis 4.2: 1 study, 62 participants), as no events were reported.
4.2. Analysis.

Comparison 4: Relaxation + music versus no intervention, Outcome 2: Cardiovascular death
No other primary or secondary outcomes were reported.
Meditation versus no intervention
Two studies (Thomas 2017; Yurtkuran 2007) compared meditation, including brief mindfulness meditation (Thomas 2017) or yoga (Yurtkuran 2007), to no intervention in people receiving HD during a median follow‐up of 2.4 months. The certainty of the evidence was very low.
Fatigue
Compared to no intervention, meditation may reduce fatigue, but the evidence is very uncertain (Analysis 5.1 (1 study, 37 participants): VAS score; MD ‐3.60, 95% CI ‐6.99 to ‐0.21; very low certainty evidence).
5.1. Analysis.

Comparison 5: Meditation versus no intervention, Outcome 1: Fatigue
Death (any cause)
Compared to no intervention, meditation had uncertain effects on death (any cause) (Analysis 5.2: 2 studies, 81 participants), as no events were reported in the eligible studies.
5.2. Analysis.

Comparison 5: Meditation versus no intervention, Outcome 2: Death (any cause)
Cardiovascular death
Compared to no intervention, meditation had uncertain effects on cardiovascular death (Analysis 5.3: 2 studies, 81 participants), as no events were reported in the eligible studies.
5.3. Analysis.

Comparison 5: Meditation versus no intervention, Outcome 3: Cardiovascular death
Depression
Compared to no intervention, meditation had uncertain effects on depression (Analysis 5.4 (1 study, 32 participants): Patient Health Questionnaire (PHQ score); MD 2.00, 95% CI ‐1.90 to 5.90; very low certainty evidence).
5.4. Analysis.

Comparison 5: Meditation versus no intervention, Outcome 4: Depression
Change in depression
Compared to no intervention, meditation had uncertain effects on change in depression (Analysis 5.5 (1 study, 32 participants): PHQ score; MD ‐1.00, 95% CI ‐4.02 to 2.02; very low certainty evidence).
5.5. Analysis.

Comparison 5: Meditation versus no intervention, Outcome 5: Change in depression
Anxiety
Compared to no intervention, meditation had uncertain effects on anxiety (Analysis 5.6 (1 study, 32 participants): Generalized Anxiety Disorder (GAD) score; MD 1.90, 95% CI ‐1.31 to 5.11; very low certainty evidence).
5.6. Analysis.

Comparison 5: Meditation versus no intervention, Outcome 6: Anxiety
Change in anxiety
Compared to no intervention, meditation had uncertain effects on change in anxiety (Analysis 5.7 (1 study, 32 participants): GAD score; MD ‐0.10, 95% CI ‐3.37 to 3.17; very low certainty evidence).
5.7. Analysis.

Comparison 5: Meditation versus no intervention, Outcome 7: Change in anxiety
Sleep disturbance
Compared to no intervention, meditation had uncertain effects on sleep disturbance (Analysis 5.8 (1 study, 37 participants): VAS score; MD ‐0.90, 95% CI ‐5.35 to 3.55; very low certainty evidence).
5.8. Analysis.

Comparison 5: Meditation versus no intervention, Outcome 8: Sleep disturbance
No other primary or secondary outcomes were reported.
Exercise versus control
Nine studies (Amini 2016; Chang 2010; Huang 2021; Krase 2022; Konstadinidou‐ND 2002; PEDAL 2020; Salehi 2020; Soliman 2015; Suzuki 2018) compared to exercise, including aerobic exercise (Amini 2016; Krase 2022; PEDAL 2020), leg ergometry exercise (Chang 2010; Konstadinidou‐ND 2002; Salehi 2020), breathing exercise (Huang 2021), range of motion exercise (Soliman 2015), and electrical muscle stimulation (Suzuki 2018), to control in people receiving HD, during a median follow‐up of 2.7 months. Control included different types of intervention, according to the authors' definition (e.g. no intervention, standard care, education, a combination of two different types of exercise programmes). The certainty of the evidence was low to very low (Table 1).
Fatigue
Compared to control, exercise may improve fatigue (Analysis 6.1 (4 studies, 217 participants): IFS, MFIS, PFS, or HFS score; SMD ‐1.18, 95% CI ‐2.04 to ‐0.31; I2 = 87%, low certainty evidence). Substantial heterogeneity was observed between the studies.
6.1. Analysis.

Comparison 6: Exercise versus control, Outcome 1: Fatigue
Number of participants reporting fatigue
Compared to control, exercise had uncertain effects on the number of participants reporting fatigue (Analysis 6.2 (1 study, 58 participants): RR 5.17, 95% CI 0.32 to 84.13; very low certainty evidence).
6.2. Analysis.

Comparison 6: Exercise versus control, Outcome 2: Number reporting fatigue
Change in fatigue
Compared to control, exercise may improve change in fatigue, but the evidence is very uncertain (Analysis 6.3 (1 study, 67 participants): SF‐36 score; MD ‐21.25, 95% CI ‐35.96 to ‐6.54; very low certainty evidence).
6.3. Analysis.

Comparison 6: Exercise versus control, Outcome 3: Change in fatigue
General fatigue
Compared to control, exercise may improve general fatigue, but the evidence is very uncertain (Analysis 6.4 (1 study, 37 participants): MFIS score; MD ‐3.36, 95% CI ‐5.68 to ‐1.04; very low certainty evidence).
6.4. Analysis.

Comparison 6: Exercise versus control, Outcome 4: General fatigue
Physical fatigue
Compared to control, exercise may reduce physical fatigue, but the evidence is very uncertain (Analysis 6.5 (1 study, 37 participants): MFIS score; MD ‐2.97, 95% CI ‐5.04 to ‐0.90; very low certainty evidence).
6.5. Analysis.

Comparison 6: Exercise versus control, Outcome 5: Physical fatigue
Mental fatigue
Compared to control, exercise may reduce mental fatigue, but the evidence is very uncertain (Analysis 6.6 (1 study, 37 participants): MFIS score; MD ‐3.62, 95% CI ‐5.65 to ‐1.59; very low certainty evidence), compared to control.
6.6. Analysis.

Comparison 6: Exercise versus control, Outcome 6: Mental fatigue
Number of participants with moderate fatigue
Compared to control, exercise had uncertain effects on the number of participants with moderate fatigue (Analysis 6.7 (1 study, 30 participants): RR 0.05, 95% CI 0.00 to 0.86; very low certainty evidence).
6.7. Analysis.

Comparison 6: Exercise versus control, Outcome 7: Number with moderate fatigue
Number of participants with severe fatigue
Compared to control, exercise had uncertain effects on the number of participants with severe fatigue (Analysis 6.8: 1 study, 30 participants), as no events were reported in the eligible study.
6.8. Analysis.

Comparison 6: Exercise versus control, Outcome 8: Number with severe fatigue
Vitality
Compared to control, exercise had uncertain effects on vitality (Analysis 6.9 (1 study, 26 participants): SF‐8 score; MD 1.70, 95% CI ‐2.89 to 6.29; very low certainty evidence).
6.9. Analysis.

Comparison 6: Exercise versus control, Outcome 9: Vitality
Energy/fatigue
Compared to control, exercise had uncertain effects on energy/fatigue (Analysis 6.10 (1 study, 236 participants): KDQOL‐SF score; MD 0.00, 95% CI ‐6.56 to 6.56; very low certainty evidence).
6.10. Analysis.

Comparison 6: Exercise versus control, Outcome 10: Energy/fatigue
Death (any cause)
Compared to control, exercise may result in little to no difference in death (any cause) (Analysis 6.11 (8 studies, 739 participants): RR 0.87, 95% CI 0.43 to 1.76; I2 = 0%, low certainty evidence).
6.11. Analysis.

Comparison 6: Exercise versus control, Outcome 11: Death (any cause)
Cardiovascular death
Compared to control, exercise had uncertain effects on cardiovascular death (Analysis 6.12 (5 studies, 587 participants): RR 0.61, 95% CI 0.10 to 3.62; very low certainty evidence).
6.12. Analysis.

Comparison 6: Exercise versus control, Outcome 12: Cardiovascular death
Quality of life (overall)
Compared to control, exercise had uncertain effects on the overall QoL (Analysis 6.13 (1 study, 232 participants): KDQOL score; MD 4.40, 95% CI ‐0.77 to 9.57; very low certainty evidence).
6.13. Analysis.

Comparison 6: Exercise versus control, Outcome 13: Quality of life (overall)
General health
Compared to control, exercise may improve general health, but the evidence is very uncertain (Analysis 6.14 (1 study, 26 participants): SF‐8 score; MD 5.30, 95% CI 1.09 to 9.51; very low certainty evidence).
6.14. Analysis.

Comparison 6: Exercise versus control, Outcome 14: General health
Anxiety
Compared to control, exercise had uncertain effects on anxiety (Analysis 6.15 (1 study, 67 participants): KDQ score; MD 0.12, 95% CI ‐5.09 to 5.33; very low certainty evidence).
6.15. Analysis.

Comparison 6: Exercise versus control, Outcome 15: Anxiety
Cardiovascular events
Compared to control, exercise had uncertain effects on cardiovascular events (Analysis 6.16: 1 study, 58 participants), as no events were reported.
6.16. Analysis.

Comparison 6: Exercise versus control, Outcome 16: Cardiovascular events
No other primary or secondary outcomes were reported.
Exercise plus nandrolone versus no intervention plus nandrolone placebo
Johansen 2006 compared exercise plus nandrolone to the group that did not perform exercise plus nandrolone placebo in people receiving HD during a follow‐up of 2.7 months. The certainty of the evidence was very low.
Fatigue
Compared to no exercise and nandrolone placebo, exercise plus nandrolone had uncertain effects on fatigue (Analysis 7.1 (1 study, 36 participants): SF‐36 score; MD 0.60, 95% CI ‐2.08 to 3.28; very low certainty evidence).
7.1. Analysis.

Comparison 7: Exercise with nandrolone versus control with nandrolone placebo, Outcome 1: Fatigue
Change in fatigue
Compared to no exercise and nandrolone placebo, exercise plus nandrolone had uncertain effects on change in fatigue (Analysis 7.2 (1 study, 36 participants): SF‐36 score; MD ‐2.30, 95% CI ‐6.46 to 1.86; very low certainty evidence).
7.2. Analysis.

Comparison 7: Exercise with nandrolone versus control with nandrolone placebo, Outcome 2: Change in fatigue
Death (any cause)
Compared to no exercise and nandrolone placebo, exercise plus nandrolone had uncertain effects on death (any cause) (Analysis 7.3 (1 study, 40 participants): RR 0.33, 95% CI 0.01 to 7.72; very low certainty evidence).
7.3. Analysis.

Comparison 7: Exercise with nandrolone versus control with nandrolone placebo, Outcome 3: Death (any cause)
No other primary or secondary outcomes were reported.
Exercise versus exercise
Figueiredo 2018 compared inspiratory muscle training to aerobic training in people receiving HD during a follow‐up of 3.7 months. The certainty of the evidence was very low.
Death (any cause)
Compared to aerobic training, inspiratory muscle training had uncertain effects on death (any cause) (Analysis 8.1 (1 study, 24 participants): RR 0.39, 95% CI 0.02 to 8.69; very low certainty evidence).
8.1. Analysis.

Comparison 8: Exercise (inspiratory muscle training) versus exercise (aerobic training), Outcome 1: Death (any cause)
No other primary or secondary outcomes were reported.
Single exercise versus combined exercises
Figueiredo 2018 compared a single exercise (inspiratory muscle training or aerobic training) to combined exercises in people undergoing HD during a follow‐up of 3.7 months. The certainty of the evidence was very low.
Death (any cause)
Compared to combined exercises, inspiratory muscle training or aerobic training had uncertain effects on death (any cause) (Analysis 9.1 (1 study, 37 participants): RR 0.54, 95% CI 0.04 to 7.97; very low certainty evidence).
9.1. Analysis.

Comparison 9: Single versus combined exercise, Outcome 1: Death (any cause)
No other primary or secondary outcomes were reported.
Education versus control
Eight studies (Babamohammadi 2006; Chow 2010; Dashti‐Khavidaki 2013; Fatigue‐HD 2019; Li 2014b; Mohamed 2014; Motedayen 2014; SOCIABLE 2017) compared education, including nurse‐led case management programmes (Chow 2010; Li 2014b; Mohamed 2014), pharmacist‐led pharmaceutical care plus routine care (Dashti‐Khavidaki 2013), physical education programme (Motedayen 2014), personal energy planning programme (Fatigue‐HD 2019), home‐care educational programme (Babamohammadi 2006), and SOCIABLE (Seniors Optimizing Community Integration to Advance Better Living with End‐stage kidney disease) services (SOCIABLE 2017) to control in people receiving HD or PD, during a median follow‐up of 2.7 months. Control included different types of intervention, according to the authors' definition (e.g. not intervention, standard care, routine hospital discharge service, standard nursing instruction and routine hospital care). The certainty of the evidence was low or very low.
Fatigue
Compared to control, education had uncertain effects on fatigue (Analysis 10.1 (2 studies, 177 participants): PFS score; SMD ‐0.23, 95% CI ‐0.97 to 0.52; I2 = 72%; very low certainty evidence). Moderate heterogeneity was observed between the studies. Note: the name of the questionnaire for fatigue was not clearly stated in Babamohammadi 2006.
10.1. Analysis.

Comparison 10: Education versus control, Outcome 1: Fatigue
Remission to fatigue
Compared to control, education had uncertain effects on remission to fatigue (Analysis 10.2 (1 study, 66 participants): RR 9.00, 95% CI 0.50 to 160.78; very low certainty evidence) in people receiving HD.
10.2. Analysis.

Comparison 10: Education versus control, Outcome 2: Remission of fatigue symptoms
Medium fatigue symptoms
Compared to control, education had uncertain effects on medium fatigue symptoms (Analysis 10.3 (1 study, 66 participants): RR 1.50, 95% CI 1.00 to 2.26; very low certainty evidence) in people receiving HD.
10.3. Analysis.

Comparison 10: Education versus control, Outcome 3: Medium fatigue symptoms
Severe fatigue symptoms
Compared to control, education may decrease severe fatigue symptoms, but the evidence is very uncertain (Analysis 10.4 (1 study, 66 participants): RR 0.29, 95% CI 0.12 to 0.70; very low certainty evidence) in people receiving HD.
10.4. Analysis.

Comparison 10: Education versus control, Outcome 4: Severe fatigue symptoms
Weakness
Compared to control, education may slightly decrease weakness, but the evidence is very uncertain (Analysis 10.5 (1 study, 37 participants): fatigue questionnaire score; MD 0.91, 95% CI 0.07 to 1.75; very low certainty evidence) in people receiving HD. Note: the name of the questionnaire for fatigue was not clearly stated in Babamohammadi 2006.
10.5. Analysis.

Comparison 10: Education versus control, Outcome 5: Weakness
Energy/fatigue
Compared to control, education had uncertain effects on energy/fatigue (Analysis 10.6 (2 studies, 220 participants): KDQOL score; MD 4.50, 95% CI ‐0.55 to 9.54; I2 = 0%, low certainty evidence) in people receiving PD.
10.6. Analysis.

Comparison 10: Education versus control, Outcome 6: Energy/fatigue
Death (any cause)
Compared to control, education had uncertain effects on death (any cause) (Analysis 10.7 (5 studies, 314 participants): RR 0.94, 95% CI 0.25 to 3.57; I2 = 22%, low certainty evidence) in people receiving HD or PD.
10.7. Analysis.

Comparison 10: Education versus control, Outcome 7: Death (any cause)
Cardiovascular death
Compared to control, education had uncertain effects on cardiovascular death (Analysis 10.8: 2 studies, 110 participants), as no events were reported in the eligible studies in people receiving HD.
10.8. Analysis.

Comparison 10: Education versus control, Outcome 8: Cardiovascular death
Quality of life (overall)
Compared to control, education had uncertain effects on the overall QoL (Analysis 10.9 (2 studies, 220 participants): KDQOL score; MD 1.86, 95% CI ‐2.96 to 6.69; I2 = 0%, low certainty evidence) in people receiving PD. Data for QoL were assessed as QoL and overall health.
10.9. Analysis.

Comparison 10: Education versus control, Outcome 9: Quality of life (overall)
Sleep (overall)
Compared to control, education may improve sleep (overall) (Analysis 10.10 (2 studies, 220 participants): KDQOL score; MD 7.46, 95% CI 2.04, 12.87; I2 = 0%, low certainty evidence) in people receiving PD.
10.10. Analysis.

Comparison 10: Education versus control, Outcome 10: Sleep (overall)
No other primary or secondary outcomes were reported.
Nutritional supplementation versus placebo
Three studies (Biniaz 2015; Fukuda 2015; Singer 2010) compared nutritional supplementation, including nutritional drink supplementation (Fukuda 2015) or vitamin C supplementation (Biniaz 2015; Singer 2010), to placebo in people receiving HD or PD during a median follow‐up of 2.7 months. The certainty of the evidence was very low.
Fatigue
Compared to placebo, nutritional supplementation had uncertain effects on fatigue (Analysis 11.1 (2 studies, 230 participants): VAS or MFIS score; SMD ‐0.33, 95% CI ‐1.16 to 0.50; I2 = 86%; very low certainty evidence) in people receiving HD. Substantial heterogeneity was observed between the studies.
11.1. Analysis.

Comparison 11: Nutritional supplements versus placebo, Outcome 1: Fatigue
Vitality
Compared to placebo, nutritional supplementation had uncertain effects on vitality (Analysis 11.2 (1 study, 173 participants): KDQOL‐SF score; MD 3.70, 95% CI ‐2.70 to 10.10; very low certainty evidence) in people receiving HD.
11.2. Analysis.

Comparison 11: Nutritional supplements versus placebo, Outcome 2: Vitality
General health
Compared to placebo, nutritional supplementation had uncertain effects on general health (Analysis 11.3 (1 study, 173 participants): KDQOL‐SF score; MD 4.70, 95% CI ‐0.94 to 10.34; very low certainty evidence) in people receiving HD.
11.3. Analysis.

Comparison 11: Nutritional supplements versus placebo, Outcome 3: General health
Death (any cause)
Compared to placebo, nutritional supplementation had uncertain effects on death (any cause) (Analysis 11.4: 1 study, 75 participants), as no events were reported in people receiving HD or PD.
11.4. Analysis.

Comparison 11: Nutritional supplements versus placebo, Outcome 4: Death (any cause)
Cardiovascular death
Compared to placebo, nutritional supplementation had uncertain effects on cardiovascular death (Analysis 11.5: 1 study, 75 participants), as no events were reported in people receiving HD or PD.
11.5. Analysis.

Comparison 11: Nutritional supplements versus placebo, Outcome 5: Cardiovascular death
Sleep problems
Compared to placebo, nutritional supplementation had uncertain effects on sleep problems (Analysis 11.6 (1 study, 173 participants): KDQOL‐SF score; MD ‐0.24, 95% CI ‐1.41 to 0.93; very low certainty evidence) in people receiving HD.
11.6. Analysis.

Comparison 11: Nutritional supplements versus placebo, Outcome 6: Sleep problems
No other primary or secondary outcomes were reported.
Cognitive‐behavioural therapy versus no intervention
Picariello 2018 compared CBT to no intervention (waiting‐list control) in people receiving HD during a follow‐up of three months. The certainty of the evidence was very low.
Fatigue
Compared to no intervention, CBT had uncertain effects on fatigue (Analysis 12.1 (1 study, 18 participants): CFS score; MD ‐3.67, 95% CI ‐9.55 to 2.21; very low certainty evidence).
12.1. Analysis.

Comparison 12: Cognitive behavioural therapy versus no intervention, Outcome 1: Fatigue
Death (any cause)
Compared to no intervention, CBT had uncertain effects on death (any cause) (Analysis 12.2: 1 study, 24 participants), as no events were reported.
12.2. Analysis.

Comparison 12: Cognitive behavioural therapy versus no intervention, Outcome 2: Death (any cause)
Cardiovascular death
Compared to no intervention, CBT had uncertain effects on cardiovascular death (Analysis 12.3: 1 study, 24 participants), as no events were reported.
12.3. Analysis.

Comparison 12: Cognitive behavioural therapy versus no intervention, Outcome 3: Cardiovascular death
Depression
Compared to no intervention, CBT had uncertain effects on depression (Analysis 12.4 (1 study, 18 participants): PHQ score; MD ‐1.86, 95% CI ‐8.29 to 4.57; very low certainty evidence).
12.4. Analysis.

Comparison 12: Cognitive behavioural therapy versus no intervention, Outcome 4: Depression
Anxiety
Compared to no intervention, CBT had uncertain effects on anxiety (Analysis 12.5 (1 study, 16 participants): GAD score; MD ‐0.01, 95% CI ‐4.83 to 4.81; very low certainty evidence).
12.5. Analysis.

Comparison 12: Cognitive behavioural therapy versus no intervention, Outcome 5: Anxiety
Sleep quality
Compared to no intervention, CBT had uncertain effects on sleep quality (Analysis 12.6 (1 study, 16 participants): PSQI score; MD 1.39, 95% CI ‐1.54 to 4.32; very low certainty evidence).
12.6. Analysis.

Comparison 12: Cognitive behavioural therapy versus no intervention, Outcome 6: Sleep quality
No other primary or secondary outcomes were reported.
Cognitive‐behavioural therapy versus education
Two studies (Chen 2008a; Chen 2011a) compared CBT to education in people receiving HD during a median follow‐up of 1.2 months. The certainty of the evidence was very low.
Fatigue
Compared to education, CBT had uncertain effects on fatigue (Analysis 13.1 (1 study, 72 participants): FSS score; MD ‐0.30, 95% CI ‐1.07 to 0.47; very low certainty evidence).
13.1. Analysis.

Comparison 13: Cognitive behavioural therapy versus education, Outcome 1: Fatigue
Number of participants with a decline in fatigue
Compared to education, CBT had uncertain effects on the number of participants with a decline in fatigue (Analysis 13.2 (1 study, 72 participants): RR 1.61, 95% CI 1.10 to 2.36; very low certainty evidence).
13.2. Analysis.

Comparison 13: Cognitive behavioural therapy versus education, Outcome 2: Number with decline in fatigue
Death (any cause)
Compared to education, CBT had uncertain effects on death (any cause) (Analysis 13.3: 2 studies, 106 participants), as no events were reported in the eligible studies.
13.3. Analysis.

Comparison 13: Cognitive behavioural therapy versus education, Outcome 3: Death (any cause)
Cardiovascular death
Compared to education, CBT had uncertain effects on cardiovascular death (Analysis 13.4: 2 studies, 106 participants), as no events were reported in the eligible studies.
13.4. Analysis.

Comparison 13: Cognitive behavioural therapy versus education, Outcome 4: Cardiovascular death
Depression
Compared to education, CBT had uncertain effects on depression (Analysis 13.5 (1 study, 72 participants): Beck Depression Inventory (BDI) score; MD ‐2.30, 95% CI ‐8.29 to 3.69; very low certainty evidence).
13.5. Analysis.

Comparison 13: Cognitive behavioural therapy versus education, Outcome 5: Depression
Number of participants with a decline in depression
Compared to education, CBT had uncertain effects on the number of participants with a decline in depression (Analysis 13.6 (1 study, 72 participants): RR 1.64, 95% CI 1.06 to 2.54; very low certainty evidence).
13.6. Analysis.

Comparison 13: Cognitive behavioural therapy versus education, Outcome 6: Number with decline in depression
Anxiety
Compared to education, CBT had uncertain effects on anxiety (Analysis 13.7 (1 study, 72 participants): BAI score; MD ‐3.10, 95% CI ‐8.81 to 2.61; very low certainty evidence).
13.7. Analysis.

Comparison 13: Cognitive behavioural therapy versus education, Outcome 7: Anxiety
Number of participants with a decline in anxiety
Compared to education, CBT had uncertain effects on the number of participants with a decline in anxiety (Analysis 13.8 (1 study, 72 participants): RR 1.45, 95% CI 0.92 to 2.29; very low certainty evidence).
13.8. Analysis.

Comparison 13: Cognitive behavioural therapy versus education, Outcome 8: Number with decline in anxiety
Sleep (overall)
Compared to education, CBT may improve sleep (overall), but the evidence is very uncertain (Analysis 13.9 (1 study, 72 participants): PSQI score; MD ‐1.70, 95% CI ‐3.39 to ‐0.01; very low certainty evidence), compared to education.
13.9. Analysis.

Comparison 13: Cognitive behavioural therapy versus education, Outcome 9: Sleep (overall)
No other primary or secondary outcomes were reported.
Cognitive‐behavioural therapy versus serotonin reuptake inhibitor
ASCEND 2016 compared CBT to serotonin reuptake inhibitor (sertraline) in people receiving HD during a follow‐up of 2.7 months. The certainty of the evidence was very low.
Death (any cause)
Compared to serotonin reuptake inhibitor, CBT had uncertain effects on death (any cause) (Analysis 14.1 (1 study, 120 participants): RR 5.00, 95% CI 0.25 to 102.00; very low certainty evidence).
14.1. Analysis.

Comparison 14: Cognitive behavioural therapy versus serotonin reuptake inhibitor, Outcome 1: Death (any cause)
No other primary or secondary outcomes were reported.
Aromatherapy versus placebo or standard care
Seven studies (Ahmady 2019; Bagheri‐Nesami 2016; Hassanzadeh 2018; Karadag 2019; Mohammadpourhodki 2021; Muz 2017; Varaei 2020) compared aromatherapy, including lavender essence (Ahmady 2019; Bagheri‐Nesami 2016; Karadag 2019; Hassanzadeh 2018; Mohammadpourhodki 2021; Varaei 2020) or sweet orange and lavender oil (Muz 2017) to placebo or standard care in people receiving HD, during a median follow‐up of 0.9 months. Aromatherapy was delivered as massage aromatherapy (Mohammadpourhodki 2021; Varaei 2020), while all other studies delivered aromatherapy as inhalation. The certainty of the evidence was low to very low (Table 2).
Fatigue
Compared to placebo or standard care, aromatherapy may improve fatigue (Analysis 15.1 (7 studies, 542 participants): FSS, RFS, PFS or BFI score; SMD ‐1.23, 95% CI ‐1.96 to ‐0.50; I2 = 93%, low certainty evidence). Substantial heterogeneity was observed between the studies.
15.1. Analysis.

Comparison 15: Aromatherapy versus placebo or standard care, Outcome 1: Fatigue
Change in fatigue
Compared to placebo or standard care, aromatherapy may improve change in fatigue, but the evidence is very uncertain (Analysis 15.2 (1 study, 60 participants): FSS score; MD 6.86, 95% CI 4.76 to 8.96; very low certainty evidence).
15.2. Analysis.

Comparison 15: Aromatherapy versus placebo or standard care, Outcome 2: Change in fatigue
Vitality
Compared to placebo or standard care, aromatherapy had uncertain effects on vitality (Analysis 15.3 (1 study, 105 participants): FSS score; MD 0.07, 95% CI ‐6.89 to 7.03; very low certainty evidence).
15.3. Analysis.

Comparison 15: Aromatherapy versus placebo or standard care, Outcome 3: Vitality
Death (any cause)
Compared to placebo or standard care, aromatherapy had uncertain effects on death (any cause) (Analysis 15.4: 6 studies 473 participants), as no events were reported in the eligible studies.
15.4. Analysis.

Comparison 15: Aromatherapy versus placebo or standard care, Outcome 4: Death (any cause)
Cardiovascular death
Compared to placebo or standard care, aromatherapy had uncertain effects on cardiovascular death (Analysis 15.5: 6 studies, 473 participants), as no events were reported in the eligible studies.
15.5. Analysis.

Comparison 15: Aromatherapy versus placebo or standard care, Outcome 5: Cardiovascular death
Quality of life (overall)
Compared to placebo or standard care, aromatherapy may improve the overall QoL, but the evidence is very uncertain (Analysis 15.6 (1 study, 105 participants): SF‐36 score; MD 16.20, 95% CI 9.16 to 23.24; very low certainty evidence).
15.6. Analysis.

Comparison 15: Aromatherapy versus placebo or standard care, Outcome 6: Quality of life (overall)
Global sleep quality
Compared to placebo or standard care, aromatherapy may improve global sleep quality, but the evidence is very uncertain (Analysis 15.7 (1 study, 62 participants): PSQI score; MD ‐10.96, 95% CI ‐12.47 to ‐9.45; very low certainty evidence).
15.7. Analysis.

Comparison 15: Aromatherapy versus placebo or standard care, Outcome 7: Global sleep quality
Change in global sleep quality
Compared to placebo or standard care, aromatherapy may increase change in global sleep quality, but the evidence is very uncertain (Analysis 15.8 (1 study, 62 participants): PSQI score; MD 11.59, 95% CI 10.21 to 12.97; very low certainty evidence).
15.8. Analysis.

Comparison 15: Aromatherapy versus placebo or standard care, Outcome 8: Change in global sleep quality
Sleep disturbance
Compared to placebo or standard care, aromatherapy may reduce sleep disturbance, but the evidence is very uncertain (Analysis 15.9 (1 study, 62 participants): PSQI score; MD ‐0.91, 95% CI ‐1.14 to ‐0.68; very low certainty evidence).
15.9. Analysis.

Comparison 15: Aromatherapy versus placebo or standard care, Outcome 9: Sleep disturbance
Change in sleep disturbance
Compared to placebo or standard care, aromatherapy may improve change in sleep disturbance, but the evidence is very uncertain (Analysis 15.10 (1 study, 62 participants): PSQI score; MD 0.90, 95% CI 0.75 to 1.05; very low certainty evidence).
15.10. Analysis.

Comparison 15: Aromatherapy versus placebo or standard care, Outcome 10: Change in sleep disturbance
No other primary or secondary outcomes were reported.
Aromatherapy versus another type of aromatherapy
Balouchi 2016 compared two different aromatherapy techniques (lavender versus orange extract) in people undergoing HD during a follow‐up of 0.5 months. The certainty of the evidence was very low.
Fatigue
Compared to orange extract, lavender extract had uncertain effects on fatigue (Analysis 16.1 (1 study, 30 participants): MFIS score; MD ‐2.00, 95% CI ‐6.92 to 2.92; very low certainty evidence)
16.1. Analysis.

Comparison 16: Aromatherapy (lavender extract) versus aromatherapy (orange extract), Outcome 1: Fatigue
No other primary or secondary outcomes were reported.
Aromatherapy versus relaxation
Hassanzadeh 2018 compared aromatherapy (lavender essence) to relaxation techniques in people undergoing HD during a follow‐up of 0.9 months. The certainty of the evidence was very low.
Fatigue
Compared to relaxation, aromatherapy may reduce fatigue, but the evidence is very uncertain (Analysis 17.1 (1 study, 70 participants): BFI score; MD ‐1.48, 95% CI ‐1.92 to ‐1.04; very low certainty evidence).
17.1. Analysis.

Comparison 17: Aromatherapy versus relaxation, Outcome 1: Fatigue
No other primary or secondary outcomes were reported.
Massage versus no intervention
Seven studies (Cecen 2021; Habibzadeh 2020: Lazarus 2020; Ozdemir 2013; Roshanravan 2016; Shahdadi 2016; Unal 2016) compared massage, including slow‐stroke back massage (Shahdadi 2016), slow‐stroke back massage or foot reflexology (Unal 2016), foot reflexology (Ozdemir 2013; Roshanravan 2016; Unal 2016), foot massage with chamomile oil, almond oil or no oils (Habibzadeh 2020), and olive oil massage (Lazarus 2020), to no intervention in people receiving HD, during a median follow‐up of 0.9 months. The certainty of the evidence was low or very low (Table 3).
Fatigue
Compared to no intervention, massage may improve fatigue (Analysis 18.1 (7 studies, 657 participants): FSS, RFS, PFS or VAS score; SMD ‐1.06, 95% CI ‐1.47, ‐0.65; I2 = 81%, low certainty evidence). Substantial heterogeneity was observed between the studies.
18.1. Analysis.

Comparison 18: Massage versus no intervention, Outcome 1: Fatigue
Change in fatigue
Compared to no intervention, massage may reduce the change in fatigue, but the evidence is very uncertain (Analysis 18.2 (1 study, 120 participants): FSS score; MD ‐0.91, 95% CI ‐1.40 to ‐0.42; very low certainty evidence).
18.2. Analysis.

Comparison 18: Massage versus no intervention, Outcome 2: Change in fatigue
Number of participants with severe fatigue
Compared to no intervention, massage may reduce the number of participants with severe fatigue (Analysis 18.3 (1 study, 200 participants): RR 0.15, 95% CI 0.09 to 0.27, low certainty evidence).
18.3. Analysis.

Comparison 18: Massage versus no intervention, Outcome 3: Number with severe fatigue
Energy
Compared to no intervention, massage may increase energy (Analysis 18.4 (2 studies, 152 participants): VAS score; MD 4.87, 95% CI 1.69 to 8.06, I2 = 59%; low certainty evidence). Moderate heterogeneity was reported between studies.
18.4. Analysis.

Comparison 18: Massage versus no intervention, Outcome 4: Energy
Death (any cause)
Compared to no intervention, massage had uncertain effects on death (any cause) (Analysis 18.5 (3 studies, 404 participants): RR 1.53, 95% CI 0.06 to 36.31; very low certainty evidence).
18.5. Analysis.

Comparison 18: Massage versus no intervention, Outcome 5: Death (any cause)
Cardiovascular death
Compared to no intervention, massage had uncertain effects on cardiovascular death (Analysis 18.6: 2 studies, 320 participants), as no events were reported in the eligible studies.
18.6. Analysis.

Comparison 18: Massage versus no intervention, Outcome 6: Cardiovascular death
Quality of life (overall)
Compared to no intervention, massage had uncertain effects on the overall QoL (Analysis 18.7 (1 study, 120 participants): KDQOL‐SF score; MD 3.27, 95% CI ‐1.82 to 8.36; very low certainty evidence).
18.7. Analysis.

Comparison 18: Massage versus no intervention, Outcome 7: Quality of life (overall)
Change in quality of life (overall)
Compared to no intervention, massage may increase change in the overall QoL, but the evidence is very uncertain (Analysis 18.8 (1 study, 120 participants): KDQOL‐SF score; MD 2.54, 95% CI 2.06 to 3.02; very low certainty evidence).
18.8. Analysis.

Comparison 18: Massage versus no intervention, Outcome 8: Change in quality of life
Sleep (overall)
Compared to no intervention, massage may improve sleep (overall), but the evidence is very uncertain (Analysis 18.9 (1 study, 70 participants): PSQI score; MD ‐6.34, 95% CI ‐7.42 to ‐5.26; very low certainty evidence).
18.9. Analysis.

Comparison 18: Massage versus no intervention, Outcome 9: Sleep (overall)
No other primary or secondary outcomes were reported.
Massage versus sham massage
Roshanravan 2016 compared massage to sham massage in people receiving HD during a follow‐up of 0.9 months. The certainty of the evidence was very low.
Fatigue
Compared to sham massage, massage may slightly reduce fatigue, but the evidence is very uncertain (Analysis 19.1 (1 study, 51 participants): PFS score; MD ‐0.63, 95% CI ‐1.22 to ‐0.04; very low certainty evidence).
19.1. Analysis.

Comparison 19: Massage versus sham massage, Outcome 1: Fatigue
No other primary or secondary outcomes were reported.
Sham massage versus no intervention
Roshanravan 2016 compared sham massage to no intervention in people receiving HD during a follow‐up of 0.9 months. The certainty of the evidence was very low.
Fatigue
Compared to no intervention, sham massage may slightly reduce fatigue, but the evidence is very uncertain (Analysis 20.1 (1 study, 52 participants): PFS score; MD ‐0.76, 95% CI ‐1.23 to ‐0.29; very low certainty evidence).
20.1. Analysis.

Comparison 20: Sham massage versus no intervention, Outcome 1: Fatigue
No other primary or secondary outcomes were reported.
Massage versus another type of massage
Two studies (Habibzadeh 2020; Unal 2016) compared massage to another type of massage in people receiving HD during a median follow‐up of 1.5 months. Unal 2016 compared foot reflexology to back massage, while Habibzadeh 2020 compared foot massage with chamomile oil or almond oil to massage without oil. The certainty of the evidence was low or very low.
Fatigue
Compared to back massage or massage without oil, foot reflexology, chamomile, or almond oil may slightly reduce fatigue (Analysis 21.1 (2 studies, 160 participants): VAS or FSS score; MD ‐0.77, 95% CI ‐1.10 to ‐0.43; low certainty evidence).
21.1. Analysis.

Comparison 21: Massage versus massage, Outcome 1: Fatigue
Change in fatigue
Compared to back massage, foot reflexology may slightly reduce the change in fatigue, but the evidence is very uncertain (Analysis 21.2 (1 study, 90 participants): FSS score; MD ‐0.50, 95% CI ‐0.95 to ‐0.05; very low certainty evidence).
21.2. Analysis.

Comparison 21: Massage versus massage, Outcome 2: Change in fatigue
Energy
Compared to back massage, foot reflexology may increase energy, but the evidence is very uncertain (Analysis 21.3 (1 study, 70 participants): VAS score; MD 4.54, 95% CI 1.28 to 7.80; very low certainty evidence).
21.3. Analysis.

Comparison 21: Massage versus massage, Outcome 3: Energy
Death (any cause)
Compared to massage without oil, foot massage with chamomile or almond oil had uncertain effects on death (any cause) (Analysis 21.4: 1 study, 90 participants), as no events were reported.
21.4. Analysis.

Comparison 21: Massage versus massage, Outcome 4: All‐cause death
Cardiovascular death
Compared to massage without oil, foot massage with chamomile or almond oil had uncertain effects on cardiovascular death (Analysis 21.5: 1 study, 90 participants), as no events were reported.
21.5. Analysis.

Comparison 21: Massage versus massage, Outcome 5: Cardiovascular death
Quality of life (overall)
Compared to massage without oil, foot massage with chamomile or almond oil may increase the overall QoL, but the evidence is very uncertain (Analysis 21.6 (1 study, 90 participants): KDQOL‐SF score; MD 4.60, 95% CI 0.74 to 8.46; very low certainty evidence).
21.6. Analysis.

Comparison 21: Massage versus massage, Outcome 6: Quality of life (overall)
Change in quality of life (overall)
Compared to massage without oil, foot massage with chamomile or almond oil may increase change in the overall QoL, but the evidence is very uncertain (Analysis 21.7 (1 study, 90 participants): KDQOL‐SF score; MD 1.87, 95% CI 1.30 to 2.44; very low certainty evidence).
21.7. Analysis.

Comparison 21: Massage versus massage, Outcome 7: Change in quality of life
Sleep (overall)
Compared to back massage, foot reflexology may improve sleep (overall), but the evidence is very uncertain (Analysis 21.8 (1 study, 70 participants): PSQI score; MD ‐2.80, 95% CI ‐3.87 to ‐1.73; very low certainty evidence).
21.8. Analysis.

Comparison 21: Massage versus massage, Outcome 8: Sleep (overall)
No other primary or secondary outcomes were reported.
Erythropoietin stimulating agents versus placebo
Two studies (Canadian EPO 1990; Lillevang 1990) compared ESA to placebo in people receiving HD during a median follow‐up of 3.9 months. The certainty of the evidence was very low.
Fatigue
Compared to placebo, ESA had uncertain effects on fatigue (Analysis 22.1 (1 study, 99 participants): KDQ score; MD 0.70, 95% CI 0.26 to 1.14; very low certainty evidence).
22.1. Analysis.

Comparison 22: Erythropoietin stimulating agents versus placebo, Outcome 1: Fatigue
Weakness
Compared to placebo, ESA had uncertain effects on weakness (Analysis 22.2 (1 study, 99 participants): KDQ score; MD 1.00, 95% CI 0.29 to 1.71; very low certainty evidence).
22.2. Analysis.

Comparison 22: Erythropoietin stimulating agents versus placebo, Outcome 2: Weakness
Energy
Compared to placebo, ESA had uncertain effects on energy (Analysis 22.3 (1 study, 99 participants): KDQ score; MD 0.40, 95% CI ‐0.43 to 1.23; very low certainty evidence).
22.3. Analysis.

Comparison 22: Erythropoietin stimulating agents versus placebo, Outcome 3: Energy
Death (any cause)
Compared to placebo, ESA had uncertain effects on death (any cause) (Analysis 22.4 (2 studies, 137 participants); RR 0.17, 95% CI 0.01 to 4.15; very low certainty evidence).
22.4. Analysis.

Comparison 22: Erythropoietin stimulating agents versus placebo, Outcome 4: Death (any cause)
Cardiovascular death
Compared to placebo, ESA had uncertain effects on cardiovascular death (Analysis 22.5: 1 study, 19 participants), as no events were reported.
22.5. Analysis.

Comparison 22: Erythropoietin stimulating agents versus placebo, Outcome 5: Cardiovascular death
Depression
Compared to placebo, ESA had uncertain effects on depression (Analysis 22.6 (1 study, 99 participants): KDQ score; MD 0.20, 95% CI ‐0.35 to 0.75; very low certainty evidence).
22.6. Analysis.

Comparison 22: Erythropoietin stimulating agents versus placebo, Outcome 6: Depression
Clotting of vascular access
Compared to placebo, ESA had uncertain effects on clotting of vascular access (Analysis 22.7 (1 study, 118 participants): RR 5.64, 95% CI 0.75 to 42.16; very low certainty evidence).
22.7. Analysis.

Comparison 22: Erythropoietin stimulating agents versus placebo, Outcome 7: Clotting of vascular access
No other primary or secondary outcomes were reported.
Normal haemoglobin target with erythropoietin stimulating agents (ESA) versus subnormal or high haemoglobin target with or without ESA
Three studies (Foley 2000; Linde 2001; Parfrey 2005) compared ESA (normal Hb target) versus subnormal or high Hb target with or without ESA. Two studies (Foley 2000; Parfrey 2005) compared EPO alpha to achieve a target Hb of 9.5 to 10.5 g/dL (normal Hb target group) or 13.0 to 14.0 g/dL (high Hb target group) in people receiving HD. Linde 2001 compared EPO alpha to achieve a normal Hb target with a subnormal Hb target with or without ESA in people receiving HD and PD during a median follow‐up of 14.3 months. The certainty of the evidence was low or very low.
Fatigue
Compared to a high Hb target with ESA, a normal Hb target had uncertain effects on fatigue (Analysis 23.1 (1 study, 582 participants): FACIT‐F score; MD ‐3.30, 95% CI ‐7.32 to 0.72; very low certainty evidence).
23.1. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 1: Fatigue
Change in fatigue
Compared to a high Hb target with ESA, a normal Hb target had uncertain effects on change in fatigue (Analysis 23.2 (1 study, 582 participants): FACIT‐F score; MD ‐2.21, 95% CI ‐4.98 to 0.56; very low certainty evidence).
23.2. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 2: Change in fatigue
Vitality
Compared to a high Hb target with ESA, a normal Hb target had uncertain effects on vitality (Analysis 23.3 (1 study, 564 participants): FACIT‐F score; MD ‐2.90, 95% CI ‐7.06 to 1.26; very low certainty evidence).
23.3. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 3: Vitality
Change in vitality
Compared to a high Hb target with ESA, a normal Hb target may reduce change in vitality, but the evidence is very uncertain (Analysis 23.4 (1 study, 564 participants): FACIT‐F score; MD ‐3.52, 95% CI ‐6.51 to ‐0.53; very low certainty evidence).
23.4. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 4: Change in vitality
Death (any cause)
Compared to a high Hb target with ESA or a sub‐optimal Hb target with or without ESA, a normal Hb target had uncertain effects on death (any cause) (Analysis 23.5 (3 studies, 1085 participants): RR 1.05, 95% CI 0.71 to 1.56; I2 = 0%; very low certainty evidence) in people receiving HD and PD.
23.5. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 5: Death (any cause)
Cardiovascular events
Compared to sub‐optimal Hb target with or without ESA, a normal HB target had uncertain effects on cardiovascular events (Analysis 23.6 (1 study, 344 participants): RR 1.30, 95% CI 0.68 to 2.48; very low certainty evidence) in people receiving HD and PD.
23.6. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 6: Cardiovascular death
Cardiovascular events
Compared to a high Hb target with ESA, a normal Hb target had uncertain effects on cardiovascular events (Analysis 23.7 (1 study, 146 participants): RR 1.00, 95% CI 0.44 to 2.26; very low certainty evidence).
23.7. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 7: Cardiovascular events (angina pectoris, myocardial infarction, pulmonary oedema or cardiac failure)
Arteriovenous access thrombosis
Compared to a high Hb target with ESA, a normal Hb target had uncertain effects on arteriovenous access thrombosis membrane (Analysis 23.8 (1 study, 146 participants): RR 1.67, 95% CI 0.64 to 4.35; very low certainty evidence).
23.8. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 8: Arteriovenous access thrombosis
Hypertension
Compared to a high Hb target with ESA, a normal Hb target may have little or no effect on hypertension (Analysis 23.9 (1 study, 596 participants): RR 0.90, 95% CI 0.74 to 1.11, low certainty evidence).
23.9. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 9: Hypertension
Myocardial infarction
Compared to a high Hb target with ESA, a normal Hb target had uncertain effects on myocardial infarction (Analysis 23.10 (1 study, 596 participants): RR 0.56, 95% CI 0.17 to 1.91; very low certainty evidence).
23.10. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 10: Myocardial infarction
Congestive heart failure
Compared to a high Hb target with ESA, a normal Hb target had uncertain effects on congestive heart failure (Analysis 23.11 (1 study, 596 participants): RR 1.08, 95% CI 0.48 to 2.40; very low certainty evidence).
23.11. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 11: Congestive heart failure
Permanent catheter thrombosis
Compared to a high Hb target with ESA, a normal Hb target had uncertain effects on permanent catheter thrombosis (Analysis 23.12 (1 study, 596 participants): RR 1.11, 95% CI 0.43 to 2.84; very low certainty evidence).
23.12. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 12: Permanent catheter thrombosis
Arteriovenous graft loss
Compared to a high Hb target with ESA, a normal Hb target had uncertain effects on arteriovenous graft loss (Analysis 23.13 (1 study, 596 participants): RR 0.99, 95% CI 0.40 to 2.45; very low certainty evidence).
23.13. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 13: Arterious graft loss
Arteriovenous fistula thrombosis
Compared to a high Hb target with ESA, a normal Hb target had uncertain effects on arteriovenous fistula thrombosis (Analysis 23.14 (1 study, 596 participants): RR 0.79, 95% CI 0.53 to 1.19; very low certainty evidence).
23.14. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 14: Arterious fistula thrombosis
Arteriovenous fistula loss
Compared to a high Hb target with ESA, a normal Hb target had uncertain effects on arteriovenous fistula loss (Analysis 23.15 (1 study, 596 participants): RR 0.89, 95% CI 0.54 to 1.46; very low certainty evidence).
23.15. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 15: Arterious fistula loss
Permanent catheter loss
Compared to a high Hb target with ESA, a normal Hb target had uncertain effects on permanent catheter loss (Analysis 23.16 (1 study, 596 participants): RR 0.85, 95% CI 0.29 to 2.49; very low certainty evidence).
23.16. Analysis.

Comparison 23: Erythropoietin stimulating agents: normal versus high haemoglobin target, Outcome 16: Permanent catheter loss
No other primary or secondary outcomes were reported.
Frequent versus conventional haemodialysis
Two studies (FHN DAILY 2007; FHN NOCTURNAL 2007) compared frequent HD (six times/week) with conventional HD (three times/week) in people receiving HD during a median follow‐up of 12 months. The certainty of the evidence was very low.
Death (any cause)
Compared to conventional HD, frequent HD had uncertain effects on death (any cause) (Analysis 24.1 (2 studies, 332 participants): RR 0.66, 95% CI 0.25 to 1.74; I2 = 0%; very low certainty evidence).
24.1. Analysis.

Comparison 24: Frequent versus conventional haemodialysis, Outcome 1: Death (any cause)
Cardiovascular events
Compared to conventional HD, frequent HD had uncertain effects on cardiovascular events (Analysis 24.2 (1 study, 245 participants): RR 0.19, 95% CI 0.01 to 3.96; very low certainty evidence).
24.2. Analysis.

Comparison 24: Frequent versus conventional haemodialysis, Outcome 2: Cardiovascular death
Depression
Compared to conventional HD, frequent HD had uncertain effects on depression (Analysis 24.3 (1 study, 189 participants): BDI score; MD ‐1.80, 95% CI ‐4.45 to 0.85; very low certainty evidence).
24.3. Analysis.

Comparison 24: Frequent versus conventional haemodialysis, Outcome 3: Depression
Vascular access outcomes
Compared to conventional HD, frequent HD may increase the number of vascular access outcomes, but the evidence is very uncertain (Analysis 24.4 (2 studies, 332 participants): RR 1.53, 95% CI 1.13 to 2.07; I2 = 0%; very low certainty evidence).
24.4. Analysis.

Comparison 24: Frequent versus conventional haemodialysis, Outcome 4: Vascular access outcomes (repair, loss, or access‐related hospitalisation)
Access loss
Compared to conventional HD, frequent HD had uncertain effects on access loss (Analysis 24.5 (2 studies, 332 participants): RR 1.21, 95% CI 0.72 to 2.03; I2 = 0%; very low certainty evidence).
24.5. Analysis.

Comparison 24: Frequent versus conventional haemodialysis, Outcome 5: Access loss
Access stenosis
Compared to conventional HD, frequent HD had uncertain effects on access stenosis (Analysis 24.6 (2 studies, 332 participants): RR 1.10, 95% CI 0.37 to 3.25; I2 = 0%; very low certainty evidence).
24.6. Analysis.

Comparison 24: Frequent versus conventional haemodialysis, Outcome 6: Access stenosis
Access thrombosis
Compared to conventional HD, frequent HD had uncertain effects on access thrombosis (Analysis 24.7 (2 studies, 332 participants): RR 1.53, 95% CI 0.28 to 8.51; I2 = 28%; very low certainty evidence).
24.7. Analysis.

Comparison 24: Frequent versus conventional haemodialysis, Outcome 7: Access thrombosis
No other primary or secondary outcomes were reported.
Home versus pre‐dialysis blood pressure monitoring
BOLD 2020 compared home BP monitoring to pre‐dialysis BP monitoring in people receiving HD during a follow‐up of 4 months. The certainty of the evidence was very low.
Number of participants reporting fatigue
Compared to pre‐dialysis BP monitoring, home BP monitoring had uncertain effects on the number of participants reporting fatigue (Analysis 25.1 (1 study, 50 participants): RR 0.94, 95% CI 0.61 to 1.45; very low certainty evidence).
25.1. Analysis.

Comparison 25: Home versus pre‐dialysis blood pressure monitoring, Outcome 1: Number reporting fatigue
Death (any cause)
Compared to pre‐dialysis BP monitoring, home BP monitoring had uncertain effects on death (any cause) (Analysis 25.2: 1 study, 50 participants), as no events were reported.
25.2. Analysis.

Comparison 25: Home versus pre‐dialysis blood pressure monitoring, Outcome 2: Death (any cause)
Cardiovascular death
Compared to pre‐dialysis BP monitoring, home BP monitoring had uncertain effects on cardiovascular death (Analysis 25.3: 1 study, 50 participants), as no events were reported.
25.3. Analysis.

Comparison 25: Home versus pre‐dialysis blood pressure monitoring, Outcome 3: Cardiovascular death
No other primary or secondary outcomes were reported.
Blood flow rate reduction versus standard care
Duggal 2019 compared blood flow rate reduction to standard care in people undergoing HD during a follow‐up of 0.9 months. The certainty of the evidence was very low.
Death (any cause)
Compared to standard care, blood flow rate reduction had uncertain effects on death (any cause) (Analysis 26.1: 1 study, 102 participants), as no events were reported.
26.1. Analysis.

Comparison 26: Blood flow rate reduction versus standard care, Outcome 1: Death (any cause)
Cardiovascular death
Compared to standard care, blood flow rate reduction had uncertain effects on cardiovascular death (Analysis 26.2: 1 study, 102 participants), as no events were reported.
26.2. Analysis.

Comparison 26: Blood flow rate reduction versus standard care, Outcome 2: Cardiovascular death
No other primary or secondary outcomes were reported.
Serotonin reuptake inhibitor versus placebo
ASSertID 2015 compared serotonin reuptake inhibitor (sertraline) to placebo in people receiving HD during a follow‐up of 6 months. The certainty of the evidence was very low.
Death (any cause)
Compared to placebo, serotonin reuptake inhibitor had uncertain effects on death (any cause) (Analysis 27.1 (1 study, 30 participants): RR 3.00, 95% CI 0.13 to 68.26; very low certainty evidence).
27.1. Analysis.

Comparison 27: Serotonin reuptake inhibitor versus placebo, Outcome 1: Death (any cause)
Cardiovascular events
Compared to placebo, serotonin reuptake inhibitor had uncertain effects on cardiovascular events (Analysis 27.2 (1 study, 30 participants): RR 3.00, 95% CI 0.13 to 68.26; very low certainty evidence).
27.2. Analysis.

Comparison 27: Serotonin reuptake inhibitor versus placebo, Outcome 2: Cardiovascular death
Depression
Compared to placebo, serotonin reuptake inhibitor had uncertain effects on depression (Analysis 27.3 (1 study, 21 participants): BDI score; MD ‐0.60, 95% CI ‐5.48 to 4.28; very low certainty evidence).
27.3. Analysis.

Comparison 27: Serotonin reuptake inhibitor versus placebo, Outcome 3: Depression
No other primary or secondary outcomes were reported.
Beta‐blockers versus angiotensin‐converting enzyme inhibitors
HDPAL 2014 compared beta‐blockers (atenolol) to ACEi (lisinopril) in people receiving HD during a follow‐up of 12 months. The certainty of the evidence was very low.
Change in energy/fatigue
Compared to ACEi, beta‐blockers may increase change in energy/fatigue, but the evidence is very uncertain (Analysis 28.1 (1 study, 87 participants): KDQOL score; MD 4.00, 95% CI 2.79 to 5.21; very low certainty evidence).
28.1. Analysis.

Comparison 28: Beta‐blockers versus angiotensin‐converting enzyme inhibitors, Outcome 1: Change in energy/fatigue
Change in overall health
Compared to ACEi, beta‐blockers may reduce change in overall health, but the evidence is very uncertain (Analysis 28.2 (1 study, 83 participants): KDQOL score; MD ‐2.20, 95% CI ‐3.55 to ‐0.85; very low certainty evidence).
28.2. Analysis.

Comparison 28: Beta‐blockers versus angiotensin‐converting enzyme inhibitors, Outcome 2: Change in overall health (QoL)
Change in general health
Compared to ACEi, beta‐blockers may increase change in general health, but the evidence is very uncertain (Analysis 28.3 (1 study, 88 participants): KDQOL score; MD 6.20, 95% CI 5.04 to 7.36; very low certainty evidence).
28.3. Analysis.

Comparison 28: Beta‐blockers versus angiotensin‐converting enzyme inhibitors, Outcome 3: Change in general health (QoL)
Death (any cause)
Compared to ACEi, beta‐blockers had uncertain effects on death (any cause) (Analysis 28.4 (1 study, 200 participants): RR 1.00, 95% CI 0.26 to 3.89; very low certainty evidence).
28.4. Analysis.

Comparison 28: Beta‐blockers versus angiotensin‐converting enzyme inhibitors, Outcome 4: Death (any cause)
Cardiovascular death
Compared to ACEi, beta‐blockers had uncertain effects on cardiovascular death (Analysis 28.5 (1 study, 200 participants): RR 0.67, 95% CI 0.11 to 3.90; very low certainty evidence).
28.5. Analysis.

Comparison 28: Beta‐blockers versus angiotensin‐converting enzyme inhibitors, Outcome 5: Cardiovascular death
Cardiovascular events
Compared to ACEi, beta‐blockers may reduce cardiovascular events, but the evidence is very uncertain (Analysis 28.6 (1 study, 200 participants): RR 0.57, 95% CI 0.33 to 0.99, very low certainty evidence).
28.6. Analysis.

Comparison 28: Beta‐blockers versus angiotensin‐converting enzyme inhibitors, Outcome 6: Cardiovascular events
Access‐related events
Compared to ACEi, beta‐blockers had uncertain effects on access‐related events (Analysis 28.7 (1 study, 200 participants): RR 0.89, 95% CI 0.49 to 1.62; very low certainty evidence).
28.7. Analysis.

Comparison 28: Beta‐blockers versus angiotensin‐converting enzyme inhibitors, Outcome 7: Access‐related events
Change in sleep quality
Compared to ACEi, beta‐blockers may reduce change in sleep quality, but the evidence is very uncertain (Analysis 28.8 (1 study, 87 participants): KDQOL score; MD ‐1.50, 95% CI ‐2.63 to ‐0.37; very low certainty evidence).
28.8. Analysis.

Comparison 28: Beta‐blockers versus angiotensin‐converting enzyme inhibitors, Outcome 8: Change in sleep quality
No other primary or secondary outcomes were reported.
Anabolic steroids versus placebo
Two studies (Johansen 1999; Johansen 2006) compared anabolic steroids (nandrolone decanoate) to placebo in people receiving HD or PD during a median follow‐up of 4.4 months. The certainty of the evidence was very low.
Fatigue
Compared to placebo, anabolic steroids had uncertain effects on fatigue (Analysis 29.1 (2 studies, 52 participants): POMS‐F score; MD 1.24, 95% CI ‐3.66 to 6.13; I2 = 76%; very low certainty evidence) in people receiving HD or PD. Moderate heterogeneity was observed between the studies.
29.1. Analysis.

Comparison 29: Anabolic steroids versus placebo, Outcome 1: Fatigue
Change in fatigue
Compared to placebo, anabolic steroids had uncertain effects on change in fatigue (Analysis 29.2 (1 study, 33 participants): POMS‐F score; MD 2.00, 95% CI ‐1.74 to 5.74; very low certainty evidence) in people receiving HD.
29.2. Analysis.

Comparison 29: Anabolic steroids versus placebo, Outcome 2: Change in fatigue
Death (any cause)
Compared to placebo, anabolic steroids had uncertain effects on death (any cause) (Analysis 29.3 (2 studies, 68 participants): RR 0.35, 95% CI 0.04 to 3.23; I2 = 0%, very low certainty evidence) in people receiving HD or PD.
29.3. Analysis.

Comparison 29: Anabolic steroids versus placebo, Outcome 3: Death (any cause)
No other primary or secondary outcomes were reported.
Anabolic steroids versus exercise
Johansen 2006 compared anabolic steroids (nandrolone decanoate) to exercise in people receiving HD during a follow‐up of 2.7 months. The certainty of the evidence was very low.
Fatigue
Compared to exercise, anabolic steroids had uncertain effects on fatigue (Analysis 30.1 (1 study, 35 participants): POMS‐F score; MD 3.00, 95% CI ‐0.02 to 6.02; very low certainty evidence).
30.1. Analysis.

Comparison 30: Anabolic steroids versus exercise, Outcome 1: Fatigue
Change in fatigue
Compared to exercise, anabolic steroids may increase change in fatigue, but the evidence is very uncertain (Analysis 30.2 (1 study, 35 participants): POMS‐F score; MD 4.30, 95% CI 1.38 to 7.22; very low certainty evidence).
30.2. Analysis.

Comparison 30: Anabolic steroids versus exercise, Outcome 2: Change in fatigue
Death (any cause)
Compared to exercise, anabolic steroids had uncertain effects on death (any cause) (Analysis 30.3: 1 study, 39 participants), as no events were reported.
30.3. Analysis.

Comparison 30: Anabolic steroids versus exercise, Outcome 3: Death (any cause)
Cardiovascular death
Compared to exercise, anabolic steroids had uncertain effects on cardiovascular death (Analysis 30.4: 1 study, 39 participants), as no events were reported.
30.4. Analysis.

Comparison 30: Anabolic steroids versus exercise, Outcome 4: Cardiovascular death
No other primary or secondary outcomes were reported.
Anabolic steroids alone versus anabolic steroids plus exercise
Johansen 2006 compared anabolic steroids (nandrolone decanoate) alone to anabolic steroids (nandrolone decanoate) plus exercise in people receiving HD during a follow‐up of 2.7 months. The certainty of the evidence was very low.
Fatigue
Compared to anabolic steroids plus exercise, anabolic steroids alone may increase fatigue, but the evidence is very uncertain (Analysis 31.1 (1 study, 32 participants): POMS‐F score; MD 4.60, 95% CI 1.06 to 8.14; very low certainty evidence).
31.1. Analysis.

Comparison 31: Anabolic steroids alone versus anabolic steroids + exercise, Outcome 1: Fatigue
Change in fatigue
Compared to anabolic steroids plus exercise, anabolic steroids alone may increase change in fatigue, but the evidence is very uncertain (Analysis 31.2 (1 study, 32 participants): POMS‐F score; MD 4.00, 95% CI 1.34 to 6.66; very low certainty evidence).
31.2. Analysis.

Comparison 31: Anabolic steroids alone versus anabolic steroids + exercise, Outcome 2: Change in fatigue
Death (any cause)
Compared to anabolic steroids plus exercise, anabolic steroids alone had uncertain effects on death (any cause) (Analysis 31.3 (1 study, 39 participants): RR 0.35, 95% CI 0.02 to 8.10; very low certainty evidence).
31.3. Analysis.

Comparison 31: Anabolic steroids alone versus anabolic steroids + exercise, Outcome 3: Death (any cause)
No other primary or secondary outcomes were reported.
Anabolic steroids plus exercise versus placebo
Johansen 2006 compared anabolic steroids (nandrolone decanoate) plus exercise to placebo in people receiving HD during a follow‐up of 2.7 months. The certainty of the evidence was very low.
Fatigue
Compared to placebo, anabolic steroids plus exercise had uncertain effects on fatigue (Analysis 32.1 (1 study, 33 participants): POMS‐F score; MD ‐1.00, 95% CI ‐4.26 to 2.26; very low certainty evidence).
32.1. Analysis.

Comparison 32: Anabolic steroids + exercise versus placebo, Outcome 1: Fatigue
Change in fatigue
Compared to placebo, anabolic steroids plus exercise had uncertain effects on change in fatigue (Analysis 32.2 (1 study, 33 participants): POMS‐F score; MD ‐2.00, 95% CI ‐5.98 to 1.98; very low certainty evidence).
32.2. Analysis.

Comparison 32: Anabolic steroids + exercise versus placebo, Outcome 2: Change in fatigue
Death (any cause)
Compared to placebo, anabolic steroids plus exercise had uncertain effects on death (any cause) (Analysis 32.3 (1 study, 40 participants): RR 1.00, 95% CI 0.07 to 14.90; very low certainty evidence).
32.3. Analysis.

Comparison 32: Anabolic steroids + exercise versus placebo, Outcome 3: Death (any cause)
No other primary or secondary outcomes were reported.
Anabolic steroids plus exercise versus exercise alone
Johansen 2006 compared anabolic steroids (nandrolone decanoate) plus exercise to exercise alone in people receiving HD during a follow‐up of 2.7 months. The certainty of the evidence was very low.
Fatigue
Compared to exercise alone, anabolic steroids plus exercise had uncertain effects on fatigue (Analysis 33.1 (1 study, 35 participants): POMS‐F score; MD ‐1.60, 95% CI ‐4.85 to 1.65; very low certainty evidence).
33.1. Analysis.

Comparison 33: Anabolic steroids + exercise versus exercise alone, Outcome 1: Fatigue
Change in fatigue
Compared to exercise alone, anabolic steroids plus exercise had uncertain effects on change in fatigue (Analysis 33.2 (1 study, 35 participants): POMS‐F score; MD 0.30, 95% CI ‐2.91 to 3.51; very low certainty evidence).
33.2. Analysis.

Comparison 33: Anabolic steroids + exercise versus exercise alone, Outcome 2: Change in fatigue
Death (any cause)
Compared to exercise alone, anabolic steroids plus exercise had uncertain effects on death (any cause) (Analysis 33.3 (1 study, 40 participants): RR 3.00, 95% CI 0.13 to 69.52; very low certainty evidence).
33.3. Analysis.

Comparison 33: Anabolic steroids + exercise versus exercise alone, Outcome 3: Death (any cause)
No other primary or secondary outcomes were reported.
Glucose dialysate versus another type of glucose dialysate
Raimann 2010 compared dialysates with 200 mg/dL of glucose (glucose‐enriched dialysate) with 100 mg/dL of glucose in patients receiving HD during a follow‐up of 0.7 months. The certainty of the evidence was very low.
Death (any cause)
Compared to 100 mg/dL glucose dialysate, glucose‐enriched dialysate had uncertain effects on death (any cause) (Analysis 34.1: 1 study, 29 participants), as no events were reported.
34.1. Analysis.

Comparison 34: Glucose dialysate versus another glucose dialysate, Outcome 1: Death (any cause)
Cardiovascular events
Compared to 100 mg/dL glucose dialysate, glucose‐enriched dialysate had uncertain effects on cardiovascular events (Analysis 34.2: 1 study, 29 participants), as no events were reported.
34.2. Analysis.

Comparison 34: Glucose dialysate versus another glucose dialysate, Outcome 2: Cardiovascular death
No other primary or secondary outcomes were reported.
Acupressure versus placebo or control
Seven studies (Bicer 2022; Cho 2004; Lin 2011; Sabouhi 2013; Su 2009; Tsay 2004a; Tsay 2004b) compared acupressure, including far‐infrared rays (Lin 2011; Su 2009), acupressure without a specific definition (Cho 2004; Sabouhi 2013; Tsay 2004a), acupressure with an electrostimulation device (Bicer 2022), and acupressure or TEAS (Tsay 2004b) to placebo or control in people receiving HD, during a median follow‐up of one month. Control included different types of intervention, according to the authors' definition (e.g. no intervention, standard care, heat path therapy). The certainty of the evidence was low or very low (Table 4).
Fatigue
Compared to placebo or control, acupressure may reduce fatigue (Analysis 35.1 (6 studies, 459 participants): PFS, revised PFS, or FI score; SMD ‐0.64, 95% CI ‐1.03 to ‐0.25; I2 = 75%, low certainty evidence). Moderate heterogeneity was observed between the studies.
35.1. Analysis.

Comparison 35: Acupressure versus placebo or control, Outcome 1: Fatigue
Change in fatigue
Compared to no intervention, acupressure may reduce change in fatigue, but the evidence is very uncertain (Analysis 35.2 (1 study, 64 participants): PFS score; MD ‐2.15, 95% CI ‐2.56 to ‐1.73; very low certainty evidence).
35.2. Analysis.

Comparison 35: Acupressure versus placebo or control, Outcome 2: Change in fatigue
Fatigue in the last week
Compared to no intervention, acupressure had uncertain effects on fatigue in the last week (Analysis 35.3 (1 study, 61 participants): BFI score; MD ‐0.09, 95% CI ‐1.27 to 1.09; very low certainty evidence).
35.3. Analysis.

Comparison 35: Acupressure versus placebo or control, Outcome 3: Fatigue in the last week
Fatigue strength rate
Compared to no intervention, acupressure had uncertain effects on fatigue strength rate (Analysis 35.4 (1 study, 61 participants): BFI score; MD ‐0.97, 95% CI ‐6.28 to 4.34; very low certainty evidence).
35.4. Analysis.

Comparison 35: Acupressure versus placebo or control, Outcome 4: Fatigue strength rate
Usual level of fatigue during the past 24 hours
Compared to no intervention, acupressure had uncertain effects on the usual level of fatigue during the past 24 hours (Analysis 35.5 (1 study, 61 participants): BFI score; MD ‐0.26, 95% CI ‐5.53 to 5.01; very low certainty evidence).
35.5. Analysis.

Comparison 35: Acupressure versus placebo or control, Outcome 5: Usual level of fatigue during past 24 hours
The worst level of fatigue during the past 24 hours
Compared to no intervention, acupressure had uncertain effects on the worst level of fatigue during the past 24 hours (Analysis 35.6 (1 study, 61 participants): BFI score; MD ‐0.24, 95% CI ‐5.60 to 5.12; very low certainty evidence).
35.6. Analysis.

Comparison 35: Acupressure versus placebo or control, Outcome 6: Worst level of fatigue during past 24 hours
Death (any cause)
Compared to placebo or control, acupressure had uncertain effects on death (any cause) (Analysis 35.7: 2 studies, 169 participants), as no events were reported in the eligible studies.
35.7. Analysis.

Comparison 35: Acupressure versus placebo or control, Outcome 7: Death (any cause)
Cardiovascular death
Compared to placebo or control, acupressure had uncertain effects on cardiovascular death (Analysis 35.8: 2 studies, 169 participants), as no events were reported in the eligible studies.
35.8. Analysis.

Comparison 35: Acupressure versus placebo or control, Outcome 8: Cardiovascular death
Quality of life (overall)
Compared to heat pad therapy, acupressure had uncertain effects on the overall QoL (Analysis 35.9 (1 study, 61 participants): World Health Organization Quality of Life‐Brief Form (WHOQOL‐BREF) score; MD ‐0.08, 95% CI ‐0.63 to 0.47; very low certainty evidence).
35.9. Analysis.

Comparison 35: Acupressure versus placebo or control, Outcome 9: Quality of life (overall)
Depression
Compared to control, acupressure may reduce depression (Analysis 35.10 (3 studies, 199 participants): BDI score; MD ‐4.10, 95% CI ‐6.73 to ‐1.47; I2 = 0%, low certainty evidence).
35.10. Analysis.

Comparison 35: Acupressure versus placebo or control, Outcome 10: Depression
Mood
Compared to no intervention, acupressure had uncertain effects on mood (Analysis 35.11 (1 study, 61 participants): BFI score; MD ‐0.07, 95% CI ‐6.75 to 6.61; very low certainty evidence).
35.11. Analysis.

Comparison 35: Acupressure versus placebo or control, Outcome 11: Mood
Sleep quality
Compared to usual care, acupressure had uncertain effects on sleep quality (Analysis 35.12 (2 studies, 141 participants): PSQI score; MD ‐1.17, 95% CI ‐2.59 to 0.24; I2 = 5%, low certainty evidence).
35.12. Analysis.

Comparison 35: Acupressure versus placebo or control, Outcome 12: Sleep quality
No other primary or secondary outcomes were reported.
Acupressure versus sham acupressure
Two studies (Sabouhi 2013; Tsay 2004a) compared acupressure with sham acupressure in people receiving HD during a median follow‐up of 0.9 months. The certainty of the evidence was very low.
Fatigue
Compared to sham acupressure, acupressure had uncertain effects on fatigue (Analysis 36.1 (2 studies, 134 participants): PFS score; MD ‐0.71, 95% CI ‐1.95 to 0.52; I2 = 87%, low certainty evidence). Substantial heterogeneity was observed between the studies.
36.1. Analysis.

Comparison 36: Acupressure versus sham acupressure, Outcome 1: Fatigue
Change in fatigue
Compared to sham acupressure, acupressure may reduce change in fatigue, but the evidence is very uncertain (Analysis 36.2 (1 study, 64 participants): PFS score; MD ‐1.59, 95% CI ‐2.00 to ‐1.17; very low certainty evidence).
36.2. Analysis.

Comparison 36: Acupressure versus sham acupressure, Outcome 2: Change in fatigue
Death (any cause)
Compared to sham acupressure, acupressure had uncertain effects on death (any cause) (Analysis 36.3: 1 study, 32 participants), as no events were reported.
36.3. Analysis.

Comparison 36: Acupressure versus sham acupressure, Outcome 3: Death (any cause)
Cardiovascular death
Compared to sham acupressure, acupressure had uncertain effects on cardiovascular death (Analysis 36.4: 1 study, 32 participants), as no events were reported.
36.4. Analysis.

Comparison 36: Acupressure versus sham acupressure, Outcome 4: Cardiovascular death
Depression
Compared to sham acupressure, acupressure had uncertain effects on depression (Analysis 36.5 (1 study, 70 participants): BDI score; MD 2.17, 95% CI ‐2.93 to 7.27; very low certainty evidence).
36.5. Analysis.

Comparison 36: Acupressure versus sham acupressure, Outcome 5: Depression
Sleep quality
Compared to sham acupressure, acupressure had uncertain effects on sleep quality (Analysis 36.6 (1 study, 70 participants): PSQI score; MD 1.72, 95% CI ‐0.40 to 3.84; very low certainty evidence).
36.6. Analysis.

Comparison 36: Acupressure versus sham acupressure, Outcome 6: Sleep quality
No other secondary outcomes were reported.
Sham acupressure versus standard care
Two studies (Sabouhi 2013; Tsay 2004a) compared sham acupressure to standard care in people receiving HD during a median follow‐up of 0.9 months. The certainty of the evidence was very low.
Fatigue
Compared to standard care, sham acupressure may slightly reduce fatigue, but the evidence is very uncertain (Analysis 37.1 (2 studies, 135 participants): PFS score; MD ‐0.62, 95% CI ‐1.19, ‐0.05; I2 = 44%; very low certainty evidence). Moderate heterogeneity was observed between the studies.
37.1. Analysis.

Comparison 37: Sham acupressure versus standard care, Outcome 1: Fatigue
Change in fatigue
Compared to standard care, sham acupressure may slightly reduce change in fatigue, but the evidence is very uncertain (Analysis 37.2 (1 study, 64 participants): PFS score; MD ‐0.56, 95% CI ‐0.83 to ‐0.29; very low certainty evidence).
37.2. Analysis.

Comparison 37: Sham acupressure versus standard care, Outcome 2: Change in fatigue
Depression
Compared to standard care, sham acupressure had uncertain effects on depression (Analysis 37.3 (1 study, 71 participants): BDI score; MD ‐3.41, 95% CI ‐8.71 to 1.89; very low certainty evidence).
37.3. Analysis.

Comparison 37: Sham acupressure versus standard care, Outcome 3: Depression
Sleep quality
Compared to standard care, sham acupressure may reduce sleep quality, but the evidence is very uncertain (Analysis 37.4 (1 study, 71 participants): PSQI score; MD ‐2.22, 95% CI ‐4.11 to ‐0.33; very low certainty evidence).
37.4. Analysis.

Comparison 37: Sham acupressure versus standard care, Outcome 4: Sleep quality
No other primary or secondary outcomes were reported.
Acupressure versus another type of acupressure
Tsay 2004b compared acupressure to another type of acupressure (TEAS) in people receiving HD during a follow‐up of 1 month. The certainty of the evidence was very low.
Fatigue
Compared to TEAS, acupressure had uncertain effects on fatigue (Analysis 38.1 (1 study, 71 participants): PFS score; MD ‐0.09, 95% CI ‐0.84 to 0.66; very low certainty evidence).
38.1. Analysis.

Comparison 38: Acupressure versus transcutaneous electrical acupoint stimulation, Outcome 1: Fatigue
Death (any cause)
Compared to TEAS, acupressure had uncertain effects on death (any cause) (Analysis 38.2: 1 study, 72 participants), as no events were reported.
38.2. Analysis.

Comparison 38: Acupressure versus transcutaneous electrical acupoint stimulation, Outcome 2: Death (any cause)
Cardiovascular death
Compared to TEAS, acupressure had uncertain effects on cardiovascular death (Analysis 38.3: 1 study, 72 participants), as no events were reported.
38.3. Analysis.

Comparison 38: Acupressure versus transcutaneous electrical acupoint stimulation, Outcome 3: Cardiovascular death
Depression
Compared to TEAS, acupressure had uncertain effects on depression (Analysis 38.4 (1 study, 71 participants): BDI score; MD 0.90, 95% CI ‐2.92 to 4.72; very low certainty evidence).
38.4. Analysis.

Comparison 38: Acupressure versus transcutaneous electrical acupoint stimulation, Outcome 4: Depression
Sleep quality
Compared to TEAS, acupressure had uncertain effects on sleep quality (Analysis 38.5 (1 study, 71 participants): PSQI score; MD 1.48, 95% CI ‐0.51 to 3.47; very low certainty evidence).
38.5. Analysis.

Comparison 38: Acupressure versus transcutaneous electrical acupoint stimulation, Outcome 5: Sleep quality
No other primary or secondary outcomes were reported.
Light therapy versus no intervention
Schardong 2021 compared light therapy (photo‐biomodulation therapy) to no intervention in people receiving HD during a follow‐up of 1.8 months. The certainty of the evidence was very low.
Death (any cause)
Compared to no intervention, light therapy had uncertain effects on death (any cause) (Analysis 39.1: 1 study, 33 participants), as no events were reported.
39.1. Analysis.

Comparison 39: Light versus no intervention, Outcome 1: Death (any cause)
Cardiovascular death
Compared to no intervention, light therapy had uncertain effects on cardiovascular death (Analysis 39.2: 1 study, 33 participants), as no events were reported.
39.2. Analysis.

Comparison 39: Light versus no intervention, Outcome 2: Cadiovascular death
Quality of life (overall)
Compared to no intervention, light therapy had uncertain effects on the overall QoL (Analysis 39.3 (1 study, 28 participants): Euro‐Qol 5‐dimensions (EQ‐5D) health questionnaire; MD 0.05, 95% CI ‐0.05 to 0.16; very low certainty evidence).
39.3. Analysis.

Comparison 39: Light versus no intervention, Outcome 3: Quality of life (overall)
No other primary or secondary outcomes were reported.
Subgroup analyses
Subgroup analyses did not provide substantively different results or were not possible due to few data and studies.
Sensitivity analyses
Sensitivity analyses did not provide substantively different results or were not possible due to few data and studies.
Discussion
Summary of main results
We identified 94 studies (8191 randomised participants) evaluating interventions for fatigue in people with CKD requiring dialysis, including people receiving HD or PD. No studies were carried out in children. Risks of bias in the included studies were often unclear or high, leading to GRADE rated at low or very low certainty evidence.
Exercise, aromatherapy, massage and acupressure may improve fatigue compared to placebo, standard care or no intervention. A wide range of heterogenous interventions and fatigue‐related outcomes were reported for exercise, aromatherapy, massage and acupressure, preventing us from pooling and analysing the data.
Due to the paucity of studies, the effects of other pharmacological and other non‐pharmacological interventions on fatigue, including non‐physiological neutral amino acids, relaxation with or without music therapy, exercise with nandrolone, nutritional supplementation, CBT, ESAs, frequent HD sections, home BP monitoring, blood flow rate reduction, serotonin reuptake inhibitor, beta‐blockers, anabolic steroids, glucose‐enriched dialysate, or light therapy, were very uncertain.
The effects of pharmacological and non‐pharmacological treatments on death, cardiovascular diseases, vascular access, QoL, depression, anxiety, hypertension or diabetes were sparse. No studies assessed tiredness, exhaustion or asthenia. Adverse events were rarely and inconsistently reported. Meta‐analysis was not possible for the majority of the outcomes for these compared treatments due to single studies available for clinical outcomes.
Overall completeness and applicability of evidence
In this review, we identified 94 studies comparing a broad range of interventions for fatigue in people receiving dialysis. Currently, evidence from existing studies is of low or very low certainty and is therefore not available to inform clinical care or policy. The majority of the included studies were performed in people on HD. No studies were conducted in children.
Most studies compared an intervention for fatigue with a placebo or control, and clinically important outcome data were rarely reported. A description of the interventions has been reported in Appendix 4. The majority of studies had a small sample size with a short duration, had methodological limitations, cross‐over or quasi‐RCT design, or were primarily designed to evaluate surrogate measures of effect. No outcome data were available for tiredness, exhaustion, or asthenia. Adverse events related to treatment were not systematically reported (see Appendix 5).
Future studies on interventions for treating fatigue in people undergoing HD and PD should evaluate outcomes as prioritised by patients, caregivers and health professionals (SONG‐HD; SONG‐PD) to better inform clinical practice and decision‐making.
Quality of the evidence
We used the standard risk of bias domains within the Cochrane tool together with GRADE methodology (GRADE 2008) to assess the certainty of study evidence. Since the certainty of evidence was low or very low for all outcomes, future studies might provide different results.
Some studies were at high or unclear risks of bias for most of the risk of bias domains assessment. The majority of studies did not report adequate blinding, attrition or selective reporting, and some received some funding from pharmaceutical companies. Relevant clinical outcomes were rarely available for many of the included studies.
Fatigue has been measured using different tools, and a high heterogeneity in the fatigue‐related outcomes definition has been provided by authors, preventing our capability to pool the data. The variability in the reporting methods of some outcomes hamper data synthesis by meta‐analysis. The limited number of studies prevented the exploration of other potential sources of heterogeneity in the analyses. Subgroup and sensitivity analysis could not be done to explore heterogeneity owing to insufficient data. Due to the limited number of studies, the assessment of adverse events was not possible. All studies reported SD or SE as an estimate of variance, and some of them provided data in descriptive or figure format only.
Potential biases in the review process
This review was carried out using standard Cochrane methods. A highly sensitive search of the Cochrane Kidney Transplant specialised register was undertaken in October 2022, without language restriction and including grey literature. Each step was completed independently by at least two authors, including the selection of studies, data management, and risk of bias assessment to minimise the risks of misclassification and adjudication of evidence. Authors were contacted to collect further data as possible. Many studies did not report key outcomes in a format available for meta‐analysis.
Potential biases identified in our review included:
The limited number of studies was a constraint on our ability to assess for potential reporting bias and selective outcome reporting
Fatigue was assessed using a broad range of measures and definitions
Poor quality studies could not be excluded due to the small number of included studies
Heterogeneity between treatment interventions was precluded due to the small number of data observations
The effects of interventions for fatigue on longer‐term outcomes were uncertain, and the treatment endpoints were principally surrogate outcomes (e.g. laboratory parameters)
A large number of comparisons were identified that prevented pooling and meta‐analysis of the data. In addition, the definitions of both the intervention and control groups were quite heterogeneous among the included studies
Some outcomes reported zero events, referred to a single study or both
Adverse events were rarely and inconsistently reported
Formal assessment for publication bias through visualisation of asymmetry in funnel plots could not be performed due to the limited number of studies available
Agreements and disagreements with other studies or reviews
We believe this is the first large and comprehensive review that included both pharmacological and non‐pharmacological interventions for fatigue in people receiving dialysis. However, some studies have examined the efficacy of either pharmacological or non‐pharmacological interventions for fatigue in this population, but the number of meta‐analyses published is still limited.
Astroth 2013 performed a systematic review of non‐pharmaceutical interventions for fatigue in adults receiving HD. The data showed that non‐pharmacological interventions (including infrared rays, exercise and acupressure) reduced fatigue in this setting. The main differences with our review included that Astroth 2013 excluded patients undergoing PD, children and non‐English papers.
Picariello 2017 carried out a systematic review and meta‐analysis to evaluate the efficacy of social‐psychological interventions for the management of fatigue in CKD. Sixteen RCTs (1536 participants) were included. Out of the 16 studies, only six reported social‐psychological interventions improved fatigue in this setting, and data were not meta‐analysed. However, they included adults with CKD stages 3‐5, including people requiring KRT (HD, PD and kidney transplant recipients).
Melo 2020 conducted a systematic review of the effects of acupressure in CKD on QoL, sleep and fatigue. Only three out of nine studies (270 participants) focused on fatigue, showing a positive effect of this intervention on fatigue. However, they evaluated RCTs, including any CKD stages and excluded studies classified with a level of evidence lower than three by the Jadad scores. GRADE assessment was not performed.
Song 2018 conducted a systematic review and meta‐analysis on the effects of exercise training compared to routine care in adult patients receiving HD. The treatment was not specifically provided for managing fatigue, but fatigue was reported as an outcome in three included studies (139 participants). Exercise training improved fatigue in HD. The main differences with our review were related to the inclusion and exclusion criteria, analyses were performed using a fixed model, and there was no information regarding the GRADE approach was provided.
Bouya 2018 performed a systematic review to assess the effect of aromatherapy on a broad range of complications of HD, including fatigue. Although the authors included 22 studies, only four addressed fatigue in this setting. Two out of four studies reported that lavender essence aromatherapy reduced fatigue in HD. Compared to our review, Bouya 2018 included both RCTs and observational studies and used the Jadad scale to assess the studies. GRADE assessment was not performed.
Johansen 2012 carried out a systematic review of the impact of ESAs on fatigue in adults receiving dialysis. This review included both RCTs and observational studies. Non‐English papers were excluded. Although ESAs showed improvement in fatigue, the main differences with our review were related to the inclusion and exclusion criteria, which prevented our ability to compare their findings with our data.
Authors' conclusions
Implications for practice.
Exercise, aromatherapy, massage and acupressure may improve fatigue compared to placebo, standard care or no intervention. Pharmacological and other non‐pharmacological interventions had uncertain effects on fatigue or fatigue‐related outcomes in people receiving dialysis. There is no evidence to inform decision‐making in children. Evidence is largely lacking in PD. Adverse events were rarely and inconsistently reported.
Implications for research.
Future well‐designed and adequately powered RCTs should be conducted to assess the benefits and harms of treatments to increase our confidence in the interventions for fatigue in people receiving HD or PD.
Further research is likely to change the estimated effects of interventions for fatigue and fatigue‐related outcomes in people receiving dialysis. Evaluation of cost‐effectiveness for interventions for fatigue would assist decision‐making by policy‐makers and health care providers in this setting.
History
Protocol first published: Issue 8, 2018
Acknowledgements
We would like to thank the Cochrane Kidney and Transplant editorial team and the referees for their comments and feedback during the preparation of this protocol.
We thank Filip Ericsson and Vahid Reisi for helping us in translation of foreign papers.
We would also like to thank the peer reviewers for their comments and feedback.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Appendix 3. Outcome definitions
| Outcome | Definition |
| ADL: Activities of Daily Living | Eight questions: they range from 0, meaning they have no difficulty, to 2, which means they can not do it even with help |
| Asthenia | Scored as slight if fatigue appeared at less than 60 sec of exercise A and at less than 30 ascents and descents during exercise B, intense at less than 15 sec of exercise A and at less than 10 ascents and descents of exercise B. Moderate degree of asthenia was between the two extremes |
| BAI: Beck Anxiety Inventory | 21 questions about how the subject has been feeling in the last week expressed as common symptoms of anxiety. Each question has the same set of four possible answer choices (0 (never) and 3 (critically)). The total score ranges from 0 to 63 points, with higher scores meaning more anxiety |
| BDI: Beck Depression Inventory | 21‐question multiple‐choice self‐report inventory for measuring the severity of depression. The total score ranges from 0 to 63 points, with higher total scores indicating more severe depressive symptoms |
| BFI: Brief Fatigue Inventory | Checklist with 10 questions so that the first question asks if the respondents had felt fatigue over the last week. Other questions ask about level of fatigue felt by the respondent at the time, normal and highest level of fatigue over the past 24 h, and the effects of fatigue on their general activity, mood, ability to walk, communicate with others, and enjoying life. The questions are designed based on an 11‐point scale (0‐10) so that 'zero' is the best possible condition and 10 is the worst. Eventually, total fatigue level of the patient is calculated as the total score of the questions 2‐10 (9 questions) divided by nine |
| Bouchard's PAL | Activities are categorized into 9 levels, with 1 as the least intense (0.26 kcal/kg/15 min) and 9 as the highest intensity (1.96 kcal/kg/15 min |
| CES‐D: Center for Epidemiologic Studies Depression Scale | Scores ≥ 16 indicate clinically meaningful symptoms |
| COPM: Canadian Occupational Performance Measure | Individuals asked to rate, on a 10‐point Likert scale, his/her performance in each of three self‐selected priority activities of everyday living. Higher scores out of 10 indicate better performance/satisfaction with performance |
| Cramps | Frequency, severity, site, and duration of the cramps were recorded and scored as slight when they lasted less than 5 mm; moderate 5 to 10 mm; intense more than 10 mm |
| ENRICH questionnaire | One of these 10 items assessed sexual satisfaction. The total score was the sum of positive and negative items and ranged from 10 to 50 |
| EQ‐5D: EuroQol‐5 dimension health questionnaire | Number 1 indicates the best state of health (perfect health) and 0 the worst state of health (death) |
| Fatigue Management Questionnaire | Individuals asked to rate various aspects of their fatigue management (e.g. overall impact on life participation; satisfaction; self‐efficacy), out of 10, on 5‐point Likert‐scale questions. Scores are then summed and averaged for each of two subscales (Performance Subscale and Satisfaction Subscale), with higher scores out of 10 indicating better fatigue management) |
| Fatigue score | HD patients fatigue scale developed by Chung and Kao: fatigue was measured on a five‐point rating scale inquiring about 25 essential symptoms of fatigue, with 5 indicating the most fatigue and 1 the least |
| FI: Fatigue Index questionnaire | Each domain rated from 1 to 5, recorded hourly during the entire study period on a fatigue intensity form as follows: 0, none; 1, mild (noticeable but without effect); 2, moderate (felt sluggish); 3, severe (required rest); or 4, overwhelming (slept). The maximal fatigue score recorded within 6 hours after dialysis or at similar time periods on non‐dialysis days (baseline) was used to rate the level of fatigue for the period in question |
| FSS: Fatigue Severity Score | Nine questions, which questions 1–4 and 6 focus on the quality of fatigue, questions 5–7 and 9 are about physical and mental fatigue and their effects on the social life of individuals, and question 8 measures the severity of fatigue. The score range for each question is between 1 and 7, with a score of 1 for absolute disagreement and a score of 7 for absolute agreement. The total score range of the questionnaire is between 7 and 63, so a score of 36 or higher is an indication of fatigue. Hence, higher scores are indicative of higher fatigue |
| GAD: Generalized Anxiety Disorder | Brief 7‐item self‐report scale on the basis of Diagnostic and Statistical Manual of Mental Disorders‐IV criteria for generalized anxiety disorder, with items scored from 0 (not at all) to 3 (nearly every day) |
| HADS: Hospital Anxiety and Depression Scale | 14‐item self‐report screening scale that comprises 7 items for each of the Anxiety and Depression subscales. The questionnaire assesses symptoms over the preceding week. Each item is scored on a 4‐point Likert scale, giving maximum subscale scores of 21 for depression and anxiety |
| HFS: Haemodialysis Fatigue scale | 26 items; it used a 4‐point scoring, from rarely or never happening) to often happening (3). A higher score means worse fatigue |
| HSS: Haemodialysis Stressor Scale | 5‐point Likert‐type scale (always: 5,mostly: 4, sometimes: 3, rarely: 2, and never). The Physiological Hemodialysis Stressor subscale score ranges between 6 and 30, and the Psychosocial Hemodialysis Stressor subscale score varies between 23 and 115. The total HSS score can range from 29 to 145. The higher the scores, the higher the perceived stress levels are |
| Health Utilities Index | This is an interval scale that can vary in theory between 0 (death) and 1 (perfect health) |
| IFS: Iowa Fatigue Scale | Eleven questions determined the level of fatigue (four questions were in cognitive aspects, a pair of questions were about physical fatigue, three questions were about energy rate and pair of questions were about work output). Fatigue score range was from 11 to 55. Score indicated the minimum fatigue rate, and 55 was maximum rate |
| IPOS‐Renal: Integrated Palliative Outcome Scale‐Renal | All symptoms cores are reported on a 0 to 4 scale (0=not at all, 1=slightly, 2=moderately, 3=severely, 4=overwhelmingly bothered) and indicate the effect of the symptom on the respondent over the past week |
| ItchyQoL: QoL questionnaire fo patients with pruritus | Consists of 27 questions. The answers to each question consist of five levels: never, rarely, sometimes, often, and always, which are scored from 1 to 5, respectively |
| KDQ: Kidney Disease Questionnaire | Follows a 7‐point Likert‐type scale (7 = no problem, 1 = a severe problem) with higher scores indicating better health‐related quality of life. A clinically meaningful difference in KDQ score was a 0.5 point change, and a mean change of 1.0 represented a large clinical change |
| KDQOL‐SF: Kidney Disease Quality of Life‐Short Form | 43 items related to the quality of life in relation to kidney patients, with 36 items related to general health. Specific dimensions of the questionnaire include: symptoms and the list of problems (12 items), the effect of kidney disease (8 items), the burden of kidney disease (4 items), job performance (2 items), cognitive function (3 items), the quality of social relationships (3 items), sexual function (2 items), sleep (4 items), social support (2 items), medical) staff support (2 items), and general health status (1 item). 22 Different questions have different answer options. As to scoring, each question is scored in a scale ranging from 0 (worst health) to 100 (best health) |
| LEVIL: London Evaluation of Illness | Subject responses were rated from 0 (worst symptoms) to 100 (no symptoms |
| MFI‐20: Multidimensional Fatigue Inventory | Each dimension includes four items, and responses are score based on a 5‐point Likert scale from strongly agree to strongly disagree. Higher scores indicate greater fatigue. Total score of each dimension ranges between 4 and 20, and the total fatigue score ranges between 20 and 100. Scores of 20‐41 indicate mild fatigue, scores of 48‐74 indicate moderate fatigue, and scores of 75‐100 indicate severe fatigue |
| MFIS: Modified Fatigue Impact Scale | A 21‐item Likert‐based scale that assesses the effects of fatigue on physical, cognitive and psychosocial functioning. Scores are summed to produce an overall score out of 84, with higher scores indicating worse fatigue impact |
| PHQ‐9: Patient Health Questionnaire‐9 | Brief 9‐item self‐report scale on the basis of the Diagnostic and Statistical Manual of Mental Disorders‐IV criteria for major depressive disorder, in which each item is scored from 0 (not at all) to 3 (nearly every day) |
| PFS: Piper Fatigue Scale | Includes a total of 27 items and evaluates subjective perception of the patients on fatigue under four subscales. Responses for each item were scored between 0‐10 points. The total fatigue score was obtained by summing the points of 22 items, then dividing the sum into the number of items. High scores signify a high level of perceived fatigue |
| PROMIS‐Fatique Short Form | Seven items about energy or exhaustion |
| PSQI: Pittsburgh Sleep Quality Index | Scale comprised 18 items and 7 component scores. Every component was evaluated from 0 to 3. The total of these component points yielded the total score of the scale, which ranged from 0 to 21. A high score (5 or above) indicated poor sleep quality. Sleep quality classified as good (0–4) and poor (5–21) |
| RNLI: Reintegration to Normal Living Index | Assesses the degree to which individuals who have experienced traumatic or incapacitating illness achieve reintegration into normal activities, using 11 declarative statements each accompanied by a 10‐point visual analogue scale. Scores are then added to produce an overall score out of 110, with higher scores indicating better reintegration to normal living |
| SF‐12: 12‐item Short Form Health Survey | Higher Mental Component Scores and Physical Component scores indicate better HRQoL |
| SF‐36: 36‐item Short Form Health Survey | Eight subscales include physical function, role limitation due to physical problems, social function, role limitation due to emotional problems, mental health, fitness/fatigue, pain, and understanding of general health. By calculating the scores obtained from the subscales, 2 main scale scores are obtained; physical and mental scales. Each subscale score ranges from 0 to 100. The physical and mental scale scores are also between 0 and 100. Zero indicates the worst and 100 indicates the best health condition |
| SMMT: Standardized Mini Mental Test | Covers five main areas and consists of 11 items, takes approximately 10 min to complete. The highest score obtainable from the SMMT is 30. In the SMMT, a score of 24–30 points is considered normal, 20–23 is considered to indicate light/mild dementia, 10–19 to indicate intermediate/mid‐stage dementia, and 0–9 to indicate advanced dementia |
| SNAG: Simplified Nutritional Appetite Questionnaire | Maximum score of 20 and a score < 14 indicates poor appetite |
| SONG‐HDF: Standardised Outcomes in Nephrology‐Haemodialysis Fatigue | Assesses the severity of fatigue, and its impact on daily living, in people on maintenance haemodialysis using 3 Likert‐style questions. Scores are summed to produce a total score out of 9, with higher scores indicating worse fatigue |
| STAI / STAI‐Y1: State‐Trait Anxiety Inventory | Composed of 20 items concerning state anxiety. 4‐point Likert scale: 1 = “not at all”; 2 = “a little”; 3 = “enough”; and 4 = “very much.” The final score is obtained by sum of the responses to the individual items and can vary from a minimum of 20 to a maximum of 80. A higher score indicates a greater level of anxiety in the subject) with scores ≥ 40 indicating elevated anxiety |
| Symptoms related to orthostatic hypotension questionnaire | Assessed using a 4‐point rating scale; severe (daily activities were greatly disturbed by the symptom), moderate (daily activities were disturbed by symptoms), mild (patients were aware of the symptoms, but daily activities were not disturbed), and asymptomatic (there was no symptom at all and patients were not bothered by any symptoms). The improvement for each symptom or the global improvement rating was assessed using a 6‐point rating scale (marked improvement (4 or higher), moderate improvement (3 or 2 and if patients have no new symptoms), slight improvement (2 or 1 and if patients have no new symptoms), no changes (±1, 0), aggravation (‐2 or less, or if patients develop new symptoms), asymptomatic (if patients have no new symptoms) |
| VAS: Visual Analogue Scale | Numbers were placed at equal intervals on a horizontal line. The presence of the worst value was rated the highest point (e.g. 10 on a 10‐point scale) Example: 1‐3 mild; 4‐6 moderate; 7‐10 severe |
| WHOQOL‐BREF: WHO Quality of Life ‐ brief form | 26 items; it used a 5‐point Likert scale. Items 3, 4 and 26 are scored in reverse. A higher score represents better quality of life |
| World Health Adverse Reactions Terminology |
Haemorrhage: epistaxis, gastric ulcer haemorrhagic, gastrointestinal haemorrhage, haematoma, haematuria, haemoptysis, nose haemorrhage, rectal haemorrhage, haemothorax, oral haemorrhage, peptic ulcer haemorrhagic, vaginal haemorrhage, and cystitis haemorrhagic Infection: fever, herpes zoster, infection, bacterial infection, fungal infection, influenza‐like symptoms, peritonitis, pneumonia, sinusitis, and tooth caries Vascular access problems: arteriovenous fistula loss or thrombosis, device‐related complications, permanent dialysis catheter loss, and thrombosis Surgical intervention Anaemia and related symptoms: anaemia, asthenia, fatigue, and malaise Cardiovascular: blood pressure fluctuation, cardiac failure, chest pain, coronary artery disorder, dizziness, hypertension, hypotension, myocardial infarction, non‐site‐specific vascular disorder, palpitations, pericarditis, peripheral gangrene, pulmonary oedema, and vascular disorder Respiratory: coughing, cyanosis, dyspnoea, and atrial fibrillation Gastrointestinal: abdominal pain, anorexia, ascites, ulcerative colitis, diarrhoea, gastric ulcer, hepatic cirrhosis, intestinal obstruction, nausea, oesophagitis, and vomiting Musculoskeletal: arthralgia, arthritis, arthropathy, back pain, bone disorder, fall, fracture pathologic, injury, leg pain, myalgia, skeletal pain, and ankylosing spondylitis Skin: folliculitis, pruritus, purpura, rash, skin disorder, and skin ulceration Neurologic: cerebellar infarction, cerebral atrophy, cerebrovascular disorder, coma, confusion, gait abnormal, headache, hearing decreased, insomnia, ischial neuralgia, somnolence, and abnormal vision Miscellaneous: acidosis, allergic reaction, anxiety, aggravated diabetes mellitus, dysuria, hydronephrosis, hyperkalaemia, hyperparathyroidism, hypoglycaemia, nail disorder, non‐site‐specific embolism, thrombosis, oedema, generalized oedema, peripheral oedema, pain, renal cyst, thrombocytopenia, thrombosis, transplant rejection, Wegener’s granulomatosis, weight decrease |
Appendix 4. TIDieR framework of interventions descriptions for included studies
| Study ID | Intervention | Control | Aim | What | How | Who, where, when | Tailoring/modification | How well: planned | How well: actual |
| Ahmady 2019 | Aromatherapy | Control | To assess the effects of aromatherapy on fatigue in HD | Participants received aromatherapy with lavender, aromatherapy with orange essential oil or were assigned to the control group | Five drops of each essence were poured on a cotton ball and pinned to the patient's collar for 30 min. In the control group, five drops of distilled water were used | 14 interventions were provided both in the hospital and at home | ‐ | Patients were trained to perform the interventions at home, and a reminder was sent to them by the first author every morning at 8 o'clock via text messages | All participants completed the study |
| Akizawa 2002 | L‐DOPS (400 or 200 mg) | Placebo | To assess the effect of L‐DOPS on post‐dialysis orthostatic hypotension in HD | Different doses of L‐DOPS were compared to placebo | L‐DOPS was administered 30 min before the start of HD | The treatment was provided in the clinic for 4 weeks | ‐ | ‐ | 141/149 participants completed the study |
| Amini 2016 |
1) Relaxation 2) Exercise |
Control | To investigate the effect of aerobic exercise or PMR on anxiety, fatigue, and sleep in HD | PMR group received a CD; aerobic exercises were performed at a certain time of the day | The PMR group used the CD and contract and relax the muscles. The aerobic exercise group did predetermined exercise | Both interventions were performed daily for 60 days. PMR was performed at home before going to sleep, exercises were performed in the clinic with the researcher, for 8 weeks | The defective performance of the patients was corrected | A checklist of the exercises was delivered. The researcher supervised and followed up through telephone call or in person | ‐ |
| ASCEND 2016 | Sertraline | CBT | To evaluate the efficacy of CBT versus sertraline for treating depression in HD | CBT or sertraline therapy | The CBT group scheduled for 10 sessions of 60 min. Sertraline started with 25 mg/day during the first week and increased to 50 mg/day in the second week. The goal of the titration phase was to achieve a dose of 200 mg | The session were conducted over 12 weeks, and were conducted face‐to‐face by trained therapists during HD | The sertraline group had dosage titrated every 2 weeks for the first 6 weeks and then maintained for 6 weeks in accordance with measurement‐based care | ‐ | 120/120 participants completed the study |
| ASSertID 2015 | Sertraline | Placebo | To test MFI questionnaire in HD patients with depression | Sertraline or placebo was administered | ‐ | Research psychiatrist assessed all patients for 6 months | ‐ | ‐ | ‐ |
| BA16285 2007 | CERA once/week | CERA once every 2 weeks | To determine the optimal dose and tolerability for IV CERA in HD patients with CKD | Participants taken IV CERA, administered at 3 different doses (0.25 µg/150 IU, 0.4 µg/150 IU, or 0.6 µg/150 IU) and were switched to once/week or once every 2 weeks | ‐ | The follow‐up was 12 months | After the first 6 weeks, dose adjustments were allowed every 3 weeks in the once/week group, and every 4 weeks in the once every 2 weeks group. Dose adjustments were also permitted for safety at any point during the study | ‐ | 53/91 participants completed the study |
| Babamohammadi 2006 | Home‐care educational program | Control | To assess the effects of a confined program of home‐care on the health status in HD | Educational program on kidneys, HD, fistula care, diet and daily consumption of drugs was performed | Home‐care contained four visits/month (1 session/week before day of the HD schedule) | Researchers and nurses supported the educational sessions (1/week) for 1 month | ‐ | Researchers answered to patient and family questions, reviewed of before session and provided final evaluation plan | ‐ |
| Bagheri‐Nesami 2016 | Aromatherapy | Control | To examine the efficacy of lavender essential oil for the alleviation of fatigue in HD | The intervention group inhaled lavender essence 5% | A cotton ball soaked in 3 drops of essential oil was attached and patients were asked to breathe slowly | The intervention group inhaled lavender essence 5% for 10 min, 3 times/week for 4 weeks in the clinic | ‐ | ‐ | 59/60 participants completed the study |
| Balouchi 2016 | Aromatherapy | Aromatherapy | To examine the effects of inhaling lavender and orange extracts in HD | Patients inhaled either lavender or orange extract | Patients were instructed to pour a drop of essential oil on a gauze and pin it to their shirt and rest the night after dialysis | The intervention was performed 3 times/week for 2 weeks at home | ‐ | ‐ | ‐ |
| Barre 1988 | Low dialysate sodium | High dialysate sodium | To assess if higher dialysate sodium increase, thirst, hypertension, weight gain and oedema in HD | Dialysate (145, 150 or 155 mEq/L) of sodium was performed to all patients | ‐ | Intervention was performed in the clinic for 1 month period each time (overall 2 months of the same treatment) | ‐ | ‐ | ‐ |
| Bellinghieri 1983 | L‐carnitine | Placebo | To evaluate the effect of L‐carnitine on serum and muscle carnitine levels in HD | L‐carnitine (2 g/day orally) was divided in two administrations | ‐ | ‐ | ‐ | ‐ | ‐ |
| Bicer 2022 | Acupressure | Placebo | To determine the effect of acupressure on blood pressure, headache, and fatigue level in HD | Participants were randomised to acupressure or placebo | An electrostimulation device was attached to the Neiguan acupuncture point. In the placebo group, the device was attached on the wrist to the same acupuncture point but without battery | 12‐session body acupressure, performed by electrostimulation device operated during each dialysis session 3 times/week for 1 month | ‐ | The researcher participated in an “Acupressure and Aromatherapy Course,” including a 24‐hour theoretical and applied training in this skill | 135/150 participants completed the study |
| Biniaz 2015 | Nutritional supplementation | Placebo | To assess the effects of vitamin C on fatigue in HD | Participants were randomised to vitamin C or placebo | The intervention group received vitamin C. The control group, placebo saline was injected | 250 mg of vitamin C was injected intravenously immediately at the end of each HD session 3 times/week for 8 weeks | ‐ | ‐ | 57/62 participants completed the study |
| BOLD 2020 | Home SBP | Pre‐dialysis SBP | To assess the effect of home SBP or pre‐dialysis SBP in HD | Participants were randomised to home SBP or pre‐dialysis SBP | In the home BP arm participants measured their BPs twice/day. Participants were trained by research staff on proper techniques for home BP measurement. In the other arm SBP readings were taken immediately prior to the start of each HD treatment | Participants were instructed by research staff to take their home BP the day after the dialysis session. Participants were asked to only take 2 BP readings over a 2‐week period to not be burdensome. In the other arm, the staff took readings over 2 weeks (6 readings) | ‐ | They received in‐person visits at their HD sessions or phone calls by the local study team at least weekly to remind them to take their home BPs. Participants shared the readings with the study team using text messaging, phone call, in‐person or paper log | 49/50 participants completed the study. However ITT was performed |
| Brass 2001 | L‐carnitine | Placebo | To assess if L‐carnitine increases plasmatic carnitine, maximal exercise capacity, and improve QoL in HD (2 RCTs) |
Study A: L‐carnitine 20 mg/kg Study B: L‐carnitine 10, 20, 40 mg/kg |
‐ | Both RCTs were performed after dialysis for 24 weeks | ‐ | ‐ | 56/60 participants completed the study A 127/133 participants completed the study B |
| Canadian EPO 1990 | EPO alfa | Placebo | To ascertain the impact of EPO treatment on anaemia symptoms in HD | Patients in the treatment groups received IV EPO alfa | EPO was injected IV as a 10 mL bolus at the end of each session of dialysis (3 times/week), for 6 months in the clinic | ‐ | The dose was subsequently adjusted to achieve the target Hb concentration | Standard encouragement was given during both exercise tests | 99/118 participants completed the study |
| Cecen 2021 | Massage | Control | To examine the effect of hand massage and foot massage on fatigue in HD | Patients were randomised to hand or foot massage or control | The patients in the massage groups used liquid vaseline using repeated patting and kneading movements. The control group did not received the intervention | Massage groups received the intervention 3 times/week for 4 weeks. The control group continued to receive HD and nursing care | ‐ | ‐ | 82/84 participants completed the study |
| Chang 2010 | Exercise | Control | To assess the effect of leg ergometry exercise on fatigue and physical activity in HD | The ergometer was placed on the bed for patients to pedal while supine for dialysis | Warm‐up by stepping for 5 min. The first exercise session was for 10 min, the second for 20 min and then for 30 min | The leg ergometry exercise was performed in the bed within the first hour of each HD session for 30 min for 8 weeks, in the clinic | Patients were permitted to rest or request to train at a lower intensity if they were stressed | ‐ | 71/90 participants completed the study |
| Chen 2008a | CBT | Education | To assess the effectiveness of CBT, evaluating changes in sleep quality and inflammatory cytokines in PD | The intervention group received CBT and sleep hygiene education, whereas the control group received only sleep hygiene education | The intervention group received 4 CBT sessions. The sleep‐focused intervention involved the cognitive, sleep, stimulus control, relaxation, and educational components | A psychiatrist performed 4 x 1‐hour‐weekly treatment sessions of CBT for 4 weeks in the clinic | ‐ | ‐ | All participants were included into the analyses |
| Chen 2011a | CBT | Education | To validate the efficacy of CBT on sleep, fatigue, depression, anxiety, inflammation and oxidative stress in HD | The intervention group received 30 min of CBT and sleep hygiene education. The control group received sleep problem consultations | CBT included a psychiatrist‐oriented, video‐assisted CBT program, and group discussion and education | Two psychiatrists performed the intervention 3 times/week for a 6‐week period in the clinic, and gave consultations to the control group at least once/week | Control group received consultations from psychiatrists as long as the participants needed during the trial | ‐ | 72/80 participants completed the study |
| Cho 2004 | Acupressure | Control | To assess the difference in fatigue and depression between acupressure therapy or usual care in HD | The intervention consisted in pressing and rubbing the fingers pads for 5 sec and then releasing for 1 sec | Every acupoint was pressed for 3 min for a total of 12 min, and then the two lower limbs were massaged for 3 min | The researcher performed the intervention for 12 min 3 days/week for 4 months, in the clinic | ‐ | The precision of acupoints was confirmed if subjects' treatment area felt sore, numb, heavy, distended and/or warm during the massage. 2 experts, who confirmed the 100% accuracy and agreement, evaluated the accuracy of acupoint | 58/62 participants completed the study |
| Chow 2010 | Nurse‐led case management programme | Control | To examine the effectiveness of a nurse‐led case management programme in improving the QoL in PD | Intervention group received a discharge planning protocol and a telephone follow‐up. Control group received routine discharge care | The discharge planning included participation of patients in discussing the discharge plan and an assessment of the patient’s physical, social, cognitive and emotional needs | Nurse managers contacted patients by telephone weekly for six consecutive weeks (20‐30 min of call), when the patients was outside the clinic | Patients could contact the case manager as needed should they require further assistance, or could call the 24‐hour hotline service if the case manager was not available at any time | The content of the call was guided by the protocol. The nurse checked and reinforced the patient’s behaviours in achieving the objectives, identifying new and potential complications | 85/100 participants completed the study |
| Dashti‐Khavidaki 2013 | Pharmaceutical care | Control | To assess the impact of pharmaceutical care on HRQoL in HD | Intervention group received pharmaceutical care, control group received standard care | Patients were educated about their disease, medications lifestyle modification, and their nutrition | Patients in the case group were visited weekly by clinical pharmacist, for 6 months | The pharmacist interviewed patients and his/her caregiver to evaluate patient’s medication adherence | Two booklets regarding correct drug administration and nutrition for HD were given to the patients | 60/92 participants completed the study |
| Duggal 2019 | Blood flow rate reduced | Control | To assess the effects of blood flow rate reduced in HD | Participants were randomised to blood flow rate reduced or usual care | Subjects in the intervention arm had their blood flow rate reduced by 100 mL/min or to a minimum blood flow rate of 300 mL/min, whichever was higher. Patients in the control arm continued usual care | Intervention was provided for 4 weeks in the clinic | ‐ | ‐ | 86/102 participants completed the study |
| Eroglu 2022 | Relaxation + music | Control | To investigate the effects of the BRT combined with music therapy on fatigue, anxiety, and depression levels in HD | Participants were randomised to relaxation + music therapy or no treatment | Small groups of 10‐12 subjects performed the intervention. The PI demonstrated deep breathing techniques step by step. Then, the PI opened the music piece and gave BRT comments in a slightly lower voice. The control group received regular care | The PI delivered a training booklet in the intervention group. The intervention was performed in the clinic twice a week for 8 weeks (20 min each) | ‐ | The PI was trained with the BRT protocol and in music therapy | 61/62 participants completed the study |
| Fatigue‐HD 2019 | Education | Control | To assess the effects of PEP programme on fatigue in HD | Participants randomised to the treatment arm completed the tailored, 7–9 weeks PEP programme. The control arm reviewed info on the website | The PEP programme is a 2‐part intervention that teaches participants how to use energy management strategies to improve participation in three self‐selected life activities. The control arm reviewed general information about kidney disease management from the Kidney School online learning modules with a trained study coordinator | The intervention group performed a web‐supported 7–9 weeks energy management programme in the clinic. | The intervention was a tailored programme. | Study coordinators received in‐person training from a trained therapist prior to administering the intervention. Study coordinators monitored and encouraged participant adherence to the treatment protocol | 22/30 participants completed the study |
| Fatouros 2010 | L‐carnitine | Placebo | To examine the effect of L‐carnitine supplementation on exercise performance and blood redox status in HD | L‐carnitine or placebo was administered intravenously after each dialysis session | ‐ | Intervention was administered 3 times/week for 8 week in the clinic | ‐ | ‐ | ‐ |
| FHN DAILY 2007 | HD 6 times/week | Control | To conduct randomised controlled clinical trials in daily HD | Intervention group conducted 6 times/week HD, compared with conventional 3 times/week HD | ‐ | The interventions were performed in the clinic (3 or 6 times/week) for 12 months | ‐ | ‐ | Numbers of participants analysed varied in base of the outcome |
| FHN NOCTURNAL 2007 | Nocturnal HD 6 times/week | Control | To conduct randomised controlled clinical trials in nocturnal HD | Intervention group conducted 6 times/week nocturnal HD, compared with conventional 3 times/week nocturnal HD | ‐ | The interventions were performed in the clinic (3 or 6 times/week) for 12 months | ‐ | ‐ | Numbers of participants analysed varied in base of the outcome |
| Figueiredo 2018 |
1) Exercise: inspiratory muscle training (IMT) 2) Exercise: aerobic training (AT) |
Exercise (combination therapy) | To assess the effect of IMT, AT or both in HD | Patients were randomised to ITM, AT or combination therapy | The IMT group performed 3 sets of 15 deep inspirations at the equipment mouthpiece and rested for 60 sec. The AT was performed by cycle ergometer (5‐min warm‐up, 30 min of cycling, and a 5 min cooling‐down period). In the combination therapy sessions, IMT was performed immediately before AT and, in the AT group, the participants performed sets of inspirations with IMT devices, but without resistance to inspiration |
All interventions were intradialytic, and they were performed during the first 2 hours of dialysis, 3 times/week for 8 weeks or 24 sessions | ‐ | ITM: MIP was reevaluated every 6 sessions for load adjustment AT: During exercise, patients were asked every 5 min about the fatigue score, and the cycle ergometer load was adjusted to achieve a fatigue score between 3‐5 points in the modified Borg Scale |
31/37 participants completed the study |
| Foley 2000 | EPO alpha (Hb target 9.5 to 10.6 g/dL) | EPO alpha (Hb target 13 to 14 g/dL) | To assess the effects of a normal Hb target in HD patients who are at an earlier phase of their cardiac disease | Patients were randomised to receive epoetin alpha either to reach low or high target Hb | ‐ | The intervention was performed in the clinic for 48 weeks | When the Hb level was below target levels, the epoetin dose was increased by 25%; when the Hb was above target levels, the epoetin dose was decreased by 25% | ‐ | 134/146 participants completed the study |
| Fukuda 2015 | Nutritional supplementation | Placebo | To examine the effects of nutritional supplementation on fatigue, QoL, and immune dysfunction in HD | Patients received active treatment or placebo | One bottle of “AMP01” or placebo was administered | Treatments were administered after each dialysis session (3 times/week) for 12 weeks | ‐ | ‐ | 172/202 participants completed the study |
| Grigoriou 2021 | Exercise | Control | To investigate whether a single bout of hybrid intradialytic exercise affects left‐ventricular function in HD | All participants completed two different HD trials on 2 different days, separated by 1 week: (1) standard HD and (2) HD including a single bout of hybrid intradialytic exercise (aerobic and resistance) | Hybrid intradialytic training included the usual intradialytic cycling followed by resistance training using elastic bands and dumbbells | Patients were instructed to cycle between 50 and 55 rpm for 45 min in the clinic | ‐ | ‐ | 21/22 participants completed the study |
| Habibzadeh 2020 | Massage | Control | To explore the impact of foot massage with chamomile oil and almond oil on the severity of fatigue and QoL in HD | Participants were randomised to massage with chamomile oil, almond oil, no oils or no treatment by the trained researcher | The foot massage was performed on the thenar and thumb by briefly pressing as rotationally, from the heel to the toes, with 3 mL of oil In the control group, there was no intervention and the participants were only monitored |
All massages were performed for 20 min, 3 times/week for 8 weeks | ‐ | The trained researcher, who learned foot massage techniques from a traditional medicine practitioner and received a certificate of foot massage at a recognized Iranian Traditional Medicine Association | All participants completed the study |
| Hadadian 2016 | Acupressure | Sham acupressure | To evaluate the effects of TEAS on fatigue in HD | TEAS group treated by acupuncture in real points Sham group procedure was performed on false points |
The sham TEAS treatment followed the same protocol as the TEAS treatment except for the positioning of the points electro‐stimulation | The intervention was limited to 5 min of TEAS (50 sec/acupoint) 6 acupoints bilaterally for 10 sessions, 2‐3 times/week for 5 weeks in the clinic | ‐ | Three acupoints were selected for TEAS treatment after consultation with acupuncturists. The device guideline and its instruction brochure were provided | 56/60 participants completed the study |
| Hadadian 2018 | Relaxation | Control | To determine the effect of progressive muscle relaxation technique on fatigue in HD | Participants were randomised to progressive muscle relaxation technique or no treatment | A CD containing the first and second steps was placed on the test group. After being assured of the person's learning, he was asked to do 2 relaxation sessions/day, according to the program set at
home, so that one should be aware of the frequency of relaxation before bedtime. In the control group, no intervention was performed |
Each relaxation step lasts about 15 min. The entire test group performed relaxation exercises for 30 days at home according to the schedule | ‐ | The researcher also regularly monitored the process of doing work by attending a dialysis session and telephone follow‐up of patients at home. Also, the researcher's telephone number was provided to patients to resolve the patient's ambiguity | 65 participants were randomised but the number of patients analysed were not clearly stated |
| Hasankhani 2013 | Massage | Control | To study the effect of back massage on fatigue in HD | The intervention group received back massage by slow‐stroke method. The control group received usual care | The patients in the intervention group were sited and small rotational movements with the thumb on the neck was performed | The intervention was provided 3 times/week, on dialysis, for 10 min, within 4 weeks | ‐ | ‐ | ‐ |
| Hassanzadeh 2018 |
1) Relaxation 2) Aromatherapy |
Control | To assess the effects of relaxation, aromatherapy compared to control on fatigue in HD | Participants were randomised to Benson muscle relaxation techniques, 5% lavender essential oil or standard care alone | Two drops of 5% lavender essential oil inoculated in sweet almond oil was added on a cotton ball and pinned to the subjects' collar In the Benson relaxation techniques group the intervention was applied in the dialysis ward and at home for 15‐20 min twice/day for 4 weeks by themselves The control group only received regular healthcare actions |
The patients were trained how to perform the intervention procedure in individual interventions groups in 3, 20‐min sessions, before, during and after the HD. This was followed in the dialysis ward and at home twice/day for 4 weeks | In the lavender essential oil group, the intervention was carried out by the patients in the morning after waking and before bed at night. For those that did not perform the intervention in the morning, it was performed during dialysis after the patient's condition was stabilised | The audio file and training pamphlet of relaxation and aromatherapy methods also were given to the patients for better learning at home. Authors followed up patients in HD wards directly and in their home by phone | ‐ |
| HDPAL 2014 | Atenolol | Lisinopril | To develop a tool to evaluate symptoms and examine the relationship between the change in symptoms with BP control in HD | Patients received atenolol or lisinopril | ‐ | Both treatments were administered 3 times/week after dialysis for 12 months | If BP control was not possible felodipine or amlodipine 10 mg (once/day) was added, followed by other antihypertensive therapies in the following order: doxazosin, minoxidil and guanfacine. If ambulatory BP was ≥ 155 mm Hg SBP or ≥ 9 5 mm Hg DBP patients, the maximum dose of the drug was used: for lisinopril 40 mg or for atenolol 100 mg | ‐ | 133/200 participants completed the questionnaire that reported fatigue |
| Huang 2021 | Exercise | Control | To assess the effects of exercise on fatigue in HD | Participants were randomised to breathing‐based leg exercises during HD or not intervention | The breathing‐based leg exercises program comprised abdominal breathing and low intensity leg exercise, including leg lifts, quadriceps femoris contraction and knee flexion. The control group performed standard care | The intervention lasted for 15 min at one time, 3 times/week for 12 weeks in the clinic by researchers | ‐ | A video was delivered until the exercise could be performed correctly. The safety of the program was evaluate considering oxyhaemoglobin saturation | 83/86 participants completed the study |
| Jalalian 2015 | Aromatherapy | Aromatherapy | To examine the effects of inhaling lavender and orange extracts in HD | Patients inhaled either lavender or orange extract | 2 drops of lavender essence with fresh orange was poured on a 2x2 gauze and pinned to the patients' collar | Subjects breathed normally for 15‐20 min, 3 times/week for 8 weeks | ‐ | ‐ | ‐ |
| Johansen 1999 | Nandrolone decanoate | Placebo | To assess the effect of nandrolone decanoate on lean body mass, functional status, and QoL in HD and PD | Patients received nandrolone decanoate or placebo | ‐ | Nandrolone decanoate or placebo was administered by intramuscular injection once a week for 6 months by the staff, in the clinic | Monthly liver function test were checked. Dose was also reduced for signs of virilization | ‐ | 23/29 participants completed all the measurements |
| Johansen 2006 |
1) Nandrolone decanoate with exercise 2) Nandrolone decanoate without exercise |
1) Placebo with exercise 2) Placebo without exercise |
To compare changes in LBM, muscle size and strength, physical performance, and self‐reported functioning in HD | Participants were randomised to nandrolone with or without exercise training, and placebo with or without exercise training | Training started with two sets of 10 repetitions. Patients were sited and performed 5 maximal leg extension repetitions at 90 degrees and 15 repetitions at 120 degrees | Exercise was performed by nurses under the supervision of study personnel 3 times/week, for 12 weeks, in the clinic | When patients could perform three sets with correct technique, the weight was increased | Investigators received a package with 12 vials of study drug or placebo and a card with exercise group assignment from the pharmacy after each participant was assigned | 68/79 participants completed the study |
| Kaplin Serin 2020 | Relaxation | No intervention | To compare changes in LBM, muscle size and strength, physical performance, and self‐reported functioning in HD | Participants were randomised to nandrolone with or without exercise training, and placebo with or without exercise training | Training started with two sets of 10 repetitions. Patients were sited and performed 5 maximal leg extension repetitions at 90 degrees and 15 repetitions at 120 degrees | Exercise was performed by nurses under the supervision of study personnel 3 times/week, for 12 weeks, in the clinic | When patients could perform three sets with correct technique, the weight was increased | Investigators received a package with 12 vials of study drug or placebo and a card with exercise group assignment from the pharmacy after each participant was assigned | 68/79 participants completed the study. |
| Karadag 2019 | Aromatherapy | Control | To assess the effect of aromatherapy on fatigue and anxiety in HD | Participants were randomised to lavender oil or no intervention | The patients inhaled 2% lavender oil before HD. 2 drops of lavender oil were dropped on a 2 × 2 cm gauze dressing, placed on the chest area of the patients’ clothes, for a duration of 20 min, with direction to patients to breathe normally | The intervention group inhaled lavender oil during the dialysis for 30 days (2 or 3 times/week). No application was made to the control group. | ‐ | ‐ | All participants completed the study |
| Konstadinidou‐ND 2002 | Exercise | Control | To compare the effects of 3 modes of exercise training on cardiorespiratory fitness in HD. | Patients were randomised to receive exercise during non‐HD days, during HD, at home or conduct usual lifestyle | Group A followed exercise on the non‐HD days (10 min warm‐up, 30 min exercise). Group B followed exercise during HD (5 min warm‐up, active cycling, 5 min cool‐down). Group C followed an unsupervised exercise at home, using a cycle‐ergometer and followed instructions. All exercises were performed for 60 min | The treatments were performed in the clinic or outside the clinic under the supervision of a sports physician and the 2 physical education teachers, 3 times/week for 6 months | The intensity of exercise was prescribed on an individual basis | The patients were divided into subgroups to keep a high frequency of patient–therapist contact. Doctors kept close contact with patients who performed exercise at home, visiting them monthly and answering questions | 48/58 participants completed the study. |
| Krase 2022 | EPO | Placebo | To assess the effect of EPO on fatigue in HD | Participants were randomised to EPO or placebo | ‐ | ‐ | ‐ | ‐ | ‐ |
| Lazarus 2020 | Massage | Control | To determine the effect of olive oil massage therapy on fatigue in HD | Participants were randomised to olive oil massage or no treatment | The massages were all performed manually and used the classic techniques of effleurage and kneading with constant touch and pressure. The control group continues to receive routine care | The intervention group were given a lower back and lower leg massage using olive oil at the beginning, and after every hour, of their HD using olive oil for a period of 8 weeks | ‐ | ‐ | All participants completed the study |
| Leski 1979 | Dialysate containing glucose | Dialysate without glucose | To evaluate the effect of a glucose‐enriched dialysate in HD | 12 dialysis sessions sequentially were performed used either a dialysate containing glucose 400 mg/100 mL or a dialysate without glucose | ‐ | The intervention was performed by medical staff in the clinic | ‐ | ‐ | ‐ |
| Li 2014b | Nurse led telephone support | Control | To test the effectiveness of post‐discharge nurse‐led telephone support in PD | Control group received routine discharge care; intervention group received nurse led telephone support | Control group received doctor support, a telephone hotline service, self‐help printed materials. Intervention group received a discharge planning protocol and a post‐discharge nurse‐led telephone support. After discharge, nurse called patients | intervention was performed at home for 6 weeks | An individualized education program was conducted by the nurse prior to discharge to consolidate learning experiences and clarify misconceptions | The content of each telephone call was guided by the protocol and the specific problems identified. The telephone conversations were audio taped to ensure consistency of the interventions | 135/160 participants completed the study |
| Lillevang 1990 | EPO | Placebo | To investigate HD patients’ own perception of their quality of life, before and after EPO‐treatment | Patients were randomised to EPO or placebo | ‐ | The intervention was provided at the end of HD in the clinic from the medical staff | ‐ | ‐ | 18/19 participants completed the study |
| Lin 2011 | Acupressure | Control | The aim of this study is to evaluate the effects of far‐infrared (FIR) rays on the meridian in HD. | The intervention group received acupressure treatment: the control group received no intervention | The acupoint was kept in place by a piece, and fixed onto the four acupoints. The patients in the experimental group were trained to administer this FIR acupoint treatment on every point | FIR irradiation on each acupoint for 30 min, thrice weekly by the patient | ‐ | An explanatory note was provided. To minimize participants’ misunderstanding of the BFI‐T, the data were collected via interview and they could ask questions | All participants completed the study |
| Linde 2001 | EPO alpha to achieve normal‐HB target | Subnormal‐HB target with or without ESA | To examine if normalization of Hb with EPO alfa improves QoL and is safe in pre‐dialysis, HD and PD | Participants were randomised to EPO alfa to reach normal Hb of 135–160 g/L or subnormal HB of 90–120 g/L with or without EPO alfa | ‐ | The intervention was performed by the clinical staff in the clinic for 48‐76 weeks | ‐ | ‐ | 210/416 participants completed the study |
| Mohajeranirad 2021 | Nutritional supplementation | Placebo | To assess the effects of psudoplicatum capsules on pruritus, fatigue, quality of life in HD | Participants were randomised to psudoplicatum capsules or placebo | Patients in the intervention group were given 250mg H. psudoplicatum and patients in the control group were given 250 mg placebo | The intervention was performed for 6 weeks, 3 times/day | ‐ | ‐ | 50/54 participants completed the study |
| Mohamed 2013 | Higher dialysate glucose concentration | Standard dialysate glucose concentration | To assess quality of life among HD patients randomised to two different dialysate glucose concentration baths | Higher (11 mmol/L) or standard dialysate glucose concentration baths (5.5 mmol/L) were provided | ‐ | Treatments were performed for 12 weeks in the clinic | ‐ | ‐ | All participants completed the study |
| Mohamed 2014 | Education | Control | To evaluate the effectiveness of an educational intervention on fatigue in HD | The intervention provided instruction to enhance the patient's knowledge about CKD, coping, nutrition and exercises. Controls received instruction | 4 interventional session of 30 ‐ 45 minutes with lectures, discussions, booklet and demonstration. The control group received the usual care recommended by the nephrologists' in relation with healthy lifestyle | The intervention consisted in 4 sessions over 2 weeks | ‐ | ‐ | ‐ |
| Mohammadpourhodki 2021 | Aromatherapy (massage) | Placebo | To evaluate the effect of aromatherapy on quality of life in HD | The intervention groups received aromatherapy massage with lavender essential oil or Citrus Aurantium essential oil for 4 weeks. For the control group, only foot massage was performed | Effleurage massage method was conducted using approximately 10 to 15 mL of 1.5% of oil | The intervention was performed 3 times/week by trained nurses one hour after the beginning of the HD in the clinic (20 min/session) | Aromatherapy massage was performed by trained female and male nurses for female and male patients, respectively | ‐ | All participants completed the study |
| Motedayen 2014 | Exercise | Control | To investigate the effect of intradialytic physical and mental exercises on fatigue in HD | The experimental group participated in a intradialytic training program | Each session began whit positive thinking. Then the patients were encouraged to do stretching and flexibility movements in the muscles and taking a deep breath with soft music | The intervention was performed twice/week for 2 months (20 min), by a senior expert in the clinic | Each patient was initially questioned about their limitations to design a personalised exercise program. The exercises would be stopped in case of problems | ‐ | 66/75 participants completed the study |
| Muz 2017 | Aromatherapy | Control | To determine the effect of aromatherapy practiced by inhalation on the sleep quality and fatigue in HD | Sweet orange and lavender oil inhalation was performed. | Lavender and sweet orange oils were dropped to a gauze bandage, which was placed 5 cm away from under the nose and patients smelled for 2 min. Education about aromatherapy was provided | The researcher trained patients. The patients performed intervention before sleeping every day for a month | Aromatherapy was prepared by the research with the aid of an expert. A message was sent to patient's phone daily to remind to apply aromatherapy. Issues were solved | 62/80 participants completed the study | |
| Ozdemir 2013 | Acupressure | Control | To evaluate the effect of reflexology on fatigue, pain and cramp in HD | The intervention group received foot reflexology treatment | Reflexology was applied 15 min for each foot. Relaxing techniques at the beginning at the end of the session was performed. Feet were positioned at the chest level of the researchers. | Reflexology application was performed by a researcher for 1 week in 3 sessions (30 min each), in the clinic | Pressure force was adjusted according to the patient’s physical appearance and age | ‐ | ‐ |
| Parfrey 2005 | EPO alpha (Hb target 9.5 to 11.5 g/dL) | EPO alpha (Hb target 13.5 to 14.5 g/dL) | To compare the impact of higher versus lower Hb targets on fatigue and QoL in HD | Participants were randomised to receive EPO alfa to reach low target (9.5 to 11.5 g/dL) or high target (13.5 to 14.5 g/dL) Hb | After random treatment assignment, patients assigned to the low target remained on their pre‐study epoetin dose. Patients with the higher target received a 25% dose escalation, or an initial dose of 150 units/Kg/week if naive to epoetin | In both groups, when haemoglobin levels deviated from target, epoetin doses were changed by 25% of the previous dose or 25 units per kilogram. | ‐ | ‐ | 324/596 participants completed the study |
| PEDAL 2020 | Exercise | Control | To assess the effect of exercise on QoL in HD | Participants were randomised to exercise or no treatment | The intervention consisted of using a modified cycle ergometer to perform aerobic exercise in a semirecumbent position. Twice/week, after the aerobic cycling exercise, participants completed lower extremity muscular conditioning exercises | The intervention was performed, 3 times per week during the first 2 hours of HD. | The prescribed individualized training intensity was derived from a peak aerobic capacity (VO2peak) assessment. New exercise intensity ranges were established at the 3‐month follow‐up assessment point | ‐ | 234/335 participants who did the baseline visit completed the study |
| Pellizzaro 2013 | Exercise | Control | To study respiratory and peripheral muscle training, and changes in functional, biochemical, and inflammatory parameters in HD | The respiratory training program consisted of training the inspiratory muscles, while the peripheral muscle program trained the knee extensor muscles | The RMT group performed three sets of 15 inspirations at the equipment mouthpiece and rested for 60 sec. The PMT group performed 3 sets of 15 knee extension repetitions, resting for 60 sec in between. The control group did not perform any intervention | The training was performed for 10 weeks (30 sessions) in the sitting position, in the clinic | The exercise load was changed throughout the training according to 50% of PImax or according to 1MR found at 30 days. | In order to estimate the optimal distance to be walked, walked distance prediction formulas were used according to gender | 39/45 participants completed the study |
| Picariello 2018 | CBT | Control | To evaluate the feasibility and acceptability of the CBT for fatigue in HD | CBT versus waiting‐list control arm | The CBT targets individuals fatigue thoughts, emotions, and behaviours by identifying and managing unhelpful thoughts in relation to fatigue. The control group received usual renal care and a manual | The CBT was performed by a therapist (3‐5 sessions: first and last sessions face‐to‐face for 1‐hour, remaining over the phone for 30 min) | Tailored CBT‐based self‐management intervention | ‐ | 18/24 participants completed the study |
| Raimann 2010 | Dialysate with low‐dose glucose | Dialysate with high‐dose glucose | To investigate fatigue using 100 mg/dL versus 200 mg/dL dialysate glucose in HD | Participants were randomised either to 100 or 200 mg/dL dialysate glucose | ‐ | The intervention was provided from 3 weeks in the clinic | ‐ | ‐ | 29/29 participants completed the study |
| Reilly‐Spong 2015 | Meditation | Control | To assess if yoga improve HD and PD patients to cope with pain and distress following transplant surgery | Patient received yoga exercise training and psychosocial support or only psychosocial support | Yoga poses and homework were performed in the intervention group. Support performed six one‐hour teleconferences | A certified yoga teacher led all sessions performed the exercise for 8 weeks in the clinic and at home | ‐ | Each weekly teleconference included discussions. Telephone conference calls were selected to reduce travel time | Not reported only for patient in HD and PD |
| Roshanravan 2016 | Acupressure |
1) Control 2) Placebo |
To assess the effect of foot reflexology on fatigue in HD | Participants were randomised to foot reflexology, placebo or control group | The patients in intervention group received foot reflexology for 20 min, and simple foot reflexology without pressing certain parts of the foot was done in placebo group. The patients in control group received only routine care | The researcher and a female co‐researcher performed the reflexology in the clinic, 3 times/week and for 4 weeks. | The duration of reflective massage depends on patients age and some other factors and varies from 5 to 30 min | ‐ | 78/81 participants completed the study |
| Sabouhi 2013 | Acupressure |
1) Control 2) Placebo |
To investigate the effectiveness of acupressure on fatigue in HD | Intervention and placebo groups received acupressure or sham acupressure. Control group received usual care | This intervention was carried out in both legs, hands, and the waist. | Researchers provided 6 acupoints with massage for 20 min/day, 3 days/week for 4 weeks, in the clinic. | ‐ | Determination of acupoints was made based on the second supervisor's guidance on the acupoints standard location | ‐ |
| Sajadi 2016 | Cold dialysis | Warm dialysis | The purpose of this study was to explore the effect of cold dialysis on fatigue in HD | Patients received 3 sessions of HD with a 37°C or 35.5°C solution | ‐ | The intervention was performed 3 times/week for 1 week from the medical staff in the clinic | ‐ | The weighing scale for patients, dialysis machines, and barometer were calibrated by a technician to assured precision | ‐ |
| Salehi 2020 | Exercise | Control | To assess the effect of exercise on fatigue in HD | Patients were randomised to bike exercise or no treatment | The intervention was performed using the electric exercise bike. The researcher placed the bike on the bed, fixed the patient’s feet to the pedals using adhesive straps | The exercise program was conducted twice a week for 12 weeks during HD (20 min). | If the participant had a blood pressure of 180/110 mmHg and higher, systolic pressure lower than 90 mm Hg, chest pain, shortness of breath, or high body temperature (> 37.8 C) before or during dialysis, the exercise would be discontinued | Participants were instructed on how to exercise and verbal encouragement was provided to them during exercise | 37/54 participants completed the study |
| Sang 1997 | Steady dialysate sodium |
1) Linear sodium ramping 2) Stepwise sodium ramping |
Patients were randomised to steady, linear or stepwise ramping sodium in HD | Steady (140 mEq/L), linear (from 155 mEq/L to 140 mEq/L) and stepwise ramping sodium (155 mEq/L for 3 hours and 140 mEq/L for the last hour of dialysis) were performed | ‐ | All patients underwent 6 weeks of experimental treatment in the clinic, performed by the staff | ‐ | Stopping HD or changing the protocol was considered as protocol failure | 23/29 participants completed the study |
| Schardong 2021 | Laser | Control | To evaluate the chronic effect of photo‐biomodulation (PBM) on the functional capacity in HD | Participants were randomised to PBM or standard care | The control group did not receive any physical therapy intervention. PBM was applied at 6 points demarcated in the quadriceps and 2 points in the gastrocnemius muscle |
The intervention group received 24 sessions of PBM during HD | ‐ | ‐ | 28/33 participants completed the study |
| Schmitz 2016 | Citrate dialysate | Standard citrate | To investigate the effect of citrate dialysate in patients on different dialysis modalities | Patients were randomised to citrate dialysate or standard citrate | ‐ | The treatment was provided in the clinic from the staff for 4 weeks | ‐ | ‐ | 92/95 were included into the analysis. All participants completed the analysis |
| Semeniuk 2000 | Nutritional supplements | Placebo | To investigate the effect of L‐carnitine on fatigue in HD | Patients were randomised to L‐carnitine or placebo | ‐ | Patients were randomised to L‐carnitine or placebo for 12 weeks and then they were crossed‐over | ‐ | ‐ | 10/12 participants completed the study |
| Shahdadi 2016 | Massage | Control | To assess the effect of slow stroke back massage on fatigue in HD | Patients were randomised to slow stroke back massage or control group | Massage was performed in sitting position. Movements is per formed several times | 2 sessions/week (6 in total) was performed by a nurse for 10 min, for 3 weeks in the clinic | ‐ | ‐ | All participants completed the study |
| Singer 2010 | Nutritional supplementation | Placebo | To determine the effect of ascorbate on cardiovascular stability in dialysis | Patients were randomised to ascorbic acid or placebo | ‐ | The intervention was performed for 3 months | ‐ | ‐ | Not clearly reported for people in HD and PD |
| Singh 2003 | Dialyser | Dialyser | To ascertain the effect of membrane on TNF‐alfa and fatigue in HD | Patients were randomised to polysulfone or cuprophan membrane | ‐ | The intervention was performed for 3 weeks in the clinic | ‐ | ‐ | Not clearly reported at the end of the first phase |
| Singh 2008a | Iron replacement product | Placebo | To assess the safety of ferumoxytol in HD | Patients were randomised to ferumoxytol or placebo | Ferumoxytol or placebo on day 0 was administered as a rapid IV push over 17 sec. | The intervention was performed for 1 week in the clinic | ‐ | ‐ | Not clearly reported at the end of the first phase |
| Sklar 1998 | Dialyzer | Dialyzer | To compare low flux polysulfone and cuprophan membrane on cytokine and intradialytic symptoms in HD | Patients were randomised to poly methylmethacrylate or cuprophan membrane | ‐ | The intervention was performed for 1 week in the clinic by medical staff and investigators | ‐ | ‐ | Not clearly reported at the end of the first phase |
| Sklar 1999 | Dialysis procedures | Sham dialysis procedures | To assess the fatigue response to isolated aspects of the dialysis procedure | Patients were randomised to hypernatremic HD, routine dialysis, isolated ultrafiltration, isolated diffusion, sham procedures with isolated membrane, and sham procedures without recirculation exposure to a dialysis membrane | Hypernatremia HD was performed with 150‐155‐mEq/L sodium bath, routine dialysis with 135‐140‐mEq/L sodium bath. No further information was reported for other dialysis procedures | The intervention was performed in the clinic, 2 cycles each | Patients receiving treatments without ultrafiltration who complained dyspnoea and/or excessive weight gain were switched to regular HD | Patients were seen at the completion of their treatments and called at home the next day by the investigators | Not clearly reported at the end of the first phase |
| SOCIABLE 2017 | Education | Control | To assess the effect of SOCIABLE services in HD | SOCIABLE services gave emphasis on supporting the social function and the physical and everyday living function | SOCIABLE services support function among older adults with ESKD. The occupational therapist taught energy conservation techniques and supplied assistive devices so the individual could get dressed without fatigue | SOCIABLE services involve a nurse, and occupational therapist and a handyman. Participants will receive 10 home visits plus minor home repairs and assistive devices over a 4‐month period of time | ‐ | The nurse wrote a letter to the primary care provider and nephrologist summarizing the participant’s goal achievements | 9/12 participants completed the study |
| Soliman 2015 | Exercise | Control | To determine the impact of Intradialytic exercise on fatigue in HD | Participants were randomised to Range of Motion (ROM) exercise or no treatment | ROM exercises performed to all joint of upper and lower limb excluded body part connected to dialysis machine and paid attention to other limb involved in exercise to avoid disconnection | ROM exercise was prescribed for 15 min/day, 3 times/week, during HD | ROM exercises lasted for 15 min, in the first 2 hours of dialysis according to patients tolerance and stopped next 2 hours of HD | Pre‐demonstration and post‐description of the exercise technique, the patients demonstrated in 3 training sessions and received the booklet | 30/40 participants completed the study |
| Su 2009 | Acupressure | Heat | To determine the impact of far infrared ray stimulation treatment in HD | The intervention group performed far infrared ray stimulation on acupoints, the control group performed heat pad therapy | In both groups were applied acupoints or heat | Each participant received three 30 min intervals of either acupressure treatment or heat at 40◦C/week for 12 weeks in the clinic | ‐ | ‐ | 61/69 participants completed the study |
| Suzuki 2018 | Exercise | Control | To evaluate the effects of intradialytic electrical muscle stimulation (EMS) in HD | Participants were randomised to EMS or no treatment | Silicone‐rubber electrode bands, 5.5 cm in width, were wrapped around the waist. An anode was set at the distal femurs and a cathode at the waist and ankles to stimulate the gluteal and upper‐ and lower‐leg muscle groups | EMS training of the lower extremities was performed within the first 2 hours of the HD session. The training was conducted 3 times/week for 8 weeks using a handheld muscle stimulator | For each training session, the stimulus intensity was individually adjusted by a rehabilitation physician to the highest level attainable, not exceeding the patients' perceived discomfort | ‐ | 26/29 participants completed the study |
| SWIFT 2020 | Education | Control | To assess if regular symptom monitoring with feedback in HD can improve QoL | Symptom monitoring WIth Feedback Trial (SWIFT) versus usual care | Participants in the intervention arm will complete the IPOS‐Renal at baseline up to 12 months | IPOS‐Renal results will be emailed to the centre nurse unit manager or delegate, and the participant’s treating nephrologist | If a participant does not have their own email address, they can nominate the address of a family member or close friend | ‐ | ‐ |
| Thomas 2017 | Meditation | Control | To determine the feasibility, tolerability and enrolment rates and to examine whether the intervention reduced depression and anxiety in HD | The intervention group received individual chairside meditation intervention. The control group received treatment as usual in the HD setting | The intervention consisted of meditative practices (body scan, guided meditation, silent meditation, gentle arm movements). Before and after each session patients performed a 1–2 min to explore their experience | The expert interventionists provided the intervention for 8 weeks, 10–15 min, 3 times/week in the clinic. Patients were pushed to practice at home | The intervention was practiced in alternating fashion, on the basis of patient preference. | Interventionists received qualitative subjective comments from participants, and asked for overall feedback after each session. | 32/41 participants completed the study |
| Tsai 2016 | Acupressure | Sham acupressure | The evaluate the efficacy and safety of herbal acupoint therapy for intradialytic hypotension in HD | Patients were randomised to acupressure or sham acupressure | The patches were applied before the HD. The patches were placed on 3 points and each acupoint was covered with gauze for 4 hours | Four hours of treatment was administered for 3 times/week for 4 weeks, in the clinic | ‐ | Participants were supervised by nurses to prevent them from touching the patches during each session | 27/32 participants completed the study |
| Tsay 2004a | Acupressure |
1) Sham acupressure 2) Control |
The purpose of the study is to investigate the effectiveness of acupressure on fatigue in HD | Patients were randomised to receive acupressure (plus usual care), sham acupressure (plus usual care) or usual care alone | Acupressure group received acupressure massage (3 min of massage to relax and 12 min of acupoints), the placebo group received a massage at locations with no acupoints. Control group received usual care only | The researcher and her assistants provided massage 3 times/week for 4 weeks, 15 min each, in the clinic | ‐ | The precision of the acupoint was confirmed if subjects felt sore during the massage. Two experts, evaluated the accuracy of acupoints selection for this study | All participants were included into the analyses |
| Tsay 2004b |
1) Acupressure 2) TEAS |
Control | To test the effectiveness of acupressure and TEAS on fatigue, sleep and depression in HD | Patients were randomised to receive acupressure, TEAS (using paired skin electrodes) or routine unit care | Patients in the acupressure and TEAS groups received treatment, whereas patients in the control group only received routine unit care. Subjects in the treatment groups were instructed not to massage any acupoints | The researcher and her assistants provided acupressure and TEAS for 15 min of treatment 3 times/week for 1 month, in the clinic | ‐ | The precision of the acupoint was confirmed if subjects felt sore during the massage. Two experts, evaluated the accuracy of acupoints selection for this study | 106/108 participants completed the study |
| Unal 2016 | Massage | Control | To examine the effectiveness of foot reflexology and back massage on sleep and fatigue in HD | Patients were randomised to foot reflexology, the back massage or control group | The foot reflexology group placed in either sitting or lying position and begins with relaxation exercises. In the back massage group patients were lying down. In both groups, 3 to 5 drops of baby oil were applied | A researcher provided the interventions, twice/week, 30 min each, for 4 weeks in the clinic | ‐ | ‐ | 105/110 participants completed the study |
| Varaei 2020 |
1) Aromatherapy (inhalation) 2) Aromatherapy (massage) |
Control | To assess the effect of aromatherapy on fatigue in HD | The three groups of this study were lavender and sweet orange inhalation aromatherapy group, lavender and sweet orange aromatherapy massage group, and a control group | One drop of lavender and one drop of sweet orange essential oils were poured on a 2 × 2 cm gauze and it was attached to the shirt collar of each eligible patient for 20 min. The patient was asked to breathe gently. Patients in the control group received neither inhalation aromatherapy nor aromatherapy massage | This intervention was implemented for all patients in the inhalation aromatherapy group 3 times/week for 8 consecutive weeks. | A massage therapist (a female therapist for female patients and a male one for male patients) stood at the bottom of the patient’s bed and held patient’s foot in her/his own hands | ‐ | All participants completed the study |
| VENOUS 2020 | Anti‐thrombotic polymethyl‐methacrylate | Placebo | To examine the effects of anti‐thrombotic polymethyl‐methacrylate on nutritional status in dialysis | Patients were randomised to anti‐thrombotic polymethyl‐methacrylate or placebo | ‐ | ‐ | ‐ | ‐ | 25/54 participants completed the study |
| Vishnevskii 2014 | Transcutaneous Electrical Muscle Stimulation | Control | To evaluate the Transcutaneous Electrical Muscle Stimulation capability in improvement of the efficiency and physical ability in HD | Patients were randomised to intervention or control group | The intervention group received muscle stimulation of the lower extremities (3 times each session for 30 min). The control group remained on previous dialysis regimen | The intervention group received the treatment during HD sessions for 4 weeks, 3 times/week | ‐ | ‐ | ‐ |
| Yurtkuran 2007 | Exercise (yoga) | Control | To evaluate the effects of a yoga‐based exercise program on pain, fatigue, sleep, and biochemical markers in HD. | The intervention group performed yoga exercises, the control group did not attend the yoga class | The exercises were done in the standing, sitting and lying positions. The rhythm consisted of 6‐sec expiration and stretching/4‐sec inspiration and relaxing; 10 repetitions were done for every movement. Every session ended with relaxation | Yoga‐based exercises were by an instructor for 30 min/day twice a week for 3 months, in the clinic. Both groups performed exercises at home for 10 minutes | Modifications of various postures were based on participant abilities/tolerance | Each patient in the yoga group was provided with an illustrated booklet explaining the poses. The home‐based exercises was explained by a physiotherapist. We kept contact with all patients to answer questions | 37/40 participants completed the study |
| Footnotes: BP: blood pressure; BRT: Benson relaxation technique; CBT: cognitive‐behavioural therapy; CERA: Continuous Erythropoietin Receptor Activator; CKD: chronic kidney disease; DBP: diastolic blood pressure; EPO alfa: epoetin alfa; Hb: haemoglobin; HD: haemodialysis; HRQoL: health‐related quality of life; IV: intravenous; L‐DOPS: L‐threo‐3,4‐dihydroxyphenylserine; PD: peritoneal dialysis; PEP: Personal Energy Planning; PMR: progressive muscle relaxation; QoL: quality of life; RCT: randomised controlled trial; SBP: systolic blood pressure; TEAS: Transcutaneous Electrical Acupoint Stimulation | |||||||||
Appendix 5. Studies reporting adverse events
| Study ID | Intervention | Control | Adverse events in the intervention arm | Adverse events in the control arm | Comments |
| Akizawa 2002 | L‐DOPS (400 or 200 mg) | Placebo | Overall, 5/100 patients in the treatment group reported headache, increased blood pressure, urinary retention, facial hot flushed, bad feeling, drug eruption, but number of patients who reported each adverse event was not reported | Feeling irritated (1), insomnia (1) | Quote: "Adverse events occurred in 3/51 patients in the 400 mg group (5.9%; i.e.,headache, increased blood pressure, urinary retention), 2/49 patients in the 200 mg group (4.1%; i.e., headache, increased blood pressure, facial hot flushed, bad feeling, drug eruption), and 1/49 patients in the placebo group (2.0%; i.e., feeling irritated, insomnia)." |
| ASCEND 2016 | Sertraline | CBT | Death 0/60, hospitalisation/other 9/60, major bleeding 1/60, cardiac 3/60, gastrointestinal 1/60, infection 2/60, other 2/60 | Death 2/60, hospitalisation/other 14/60, major bleeding 2/60, cardiac 4/60, gastrointestinal 1/60, infection 2/60, other 8/60 | Quote: "Serious adverse events occurred in both treatment groups: 13 in 11 patients in the CBT group and 18 in 14 patients in the sertraline group. Nonserious adverse events were more frequent in the sertraline (56 events in 25 patients) than the CBT (17 events in 12 patients) group." |
| ASSertID 2015 | Sertraline | Placebo | Adverse events and/or serious adverse events (9/15); cardiovascular death (1/15) | Adverse events and/or serious adverse events (9/15); cardiovascular death (0/15) | Quote: "Eighteen patients experienced adverse events (24) and/ or SAEs (13), nine in each randomized group. Infections (8) and nausea (4) were the most commonly reported adverse events. With regard to the SAEs, there was one death that was possibly related to the study medication as mentioned above, six SAEs that were unlikely to be related, and six SAEs that were not related to the study medication." "In the sertraline group, there were six dropouts within the first 2 months. One patient died of cardiac arrest having taken one tablet. Three patients withdrew because of adverse events (one after 3 days with nausea, another after 12 days with headaches and dizziness, and the third due to insomnia after 23 days). The fifth patient withdrew because of concern about side effects, having taken no study medication. The sixth patient was admitted for a prolonged hospital stay with leg ulcers shortly after randomisation and subsequently withdrawn without having taken any study medication. At 3 months, a seventh patient withdrew because of sweating and palpitations. In the placebo group, one patient withdrew after the baseline interview because of concern about taking additional medication, and a second decided against continuing after 3 months." |
| BA16285 2007 | C.E.R.A. (once/week) | C.E.R.A. (once every 2 weeks) | Adverse events were reported for all study participants without distinction between groups (fatigue, anaemia, headache, vomiting, dizziness, diarrhoea, upper respiratory tract infection, nausea, dyspnoea, kidney transplantation, chest pain, muscle cramp or spasm, pyrexia, constipation, pruritus, rectal cancer, accelerated hypertension, cerebrovascular accident, death, acute myocardial infarction and multiple organ failure, chronic renal failure) | Adverse events were reported for all study participants without distinction between groups (fatigue, anaemia, headache, vomiting, dizziness, diarrhoea, upper respiratory tract infection, nausea, dyspnoea, kidney transplantation, chest pain, muscle cramp or spasm, pyrexia, constipation, pruritus, rectal cancer, accelerated hypertension, cerebrovascular accident, death, acute myocardial infarction and multiple organ failure, chronic renal failure) | Quote: "During the core study period, 4 AEs led to premature withdrawal (worsening anaemia [2 patients in group A and 1 in group C] and kidney transplant [1 in group A]). All cases of anaemia were considered to be related to study medication, whereas the kidney transplant was not. There were 5 withdrawals because of AEs in the extension period (pruritus, chest pain, rectal cancer, accelerated hypertension, and cerebrovascular accident [1 patient each]). In addition, dialysis was discontinued in 1 patient (at the request of her family), who was subsequently withdrawn from the study. The investigator classified this as an AE of chronic renal failure. All AEs leading to withdrawal in the extension period were considered to be unrelated to the study medication with the exception of accelerated hypertension (1 patient), which was classified as an SAE. Nineteen patients experienced an SAE during the core period, and 22, during the extension period. Two patients died during the extension period of the study (acute myocardial infarction and multiple organ failure); these deaths were deemed as SAEs. Neither death was considered related to treatment." |
| Barre 1988 | Low dialysate sodium | High dialysate sodium | In general, adverse events were reported for all study participants without clear distinction between groups (fatigue, thirst, cramps, back pain, stomach‐ache, irritability, nausea, vomiting, headache, weakness, restlessness, itchiness, or any other symptoms) | In general, adverse events were reported for all study participants without clear distinction between groups (fatigue, thirst, cramps, back pain, stomach‐ache, irritability, nausea, vomiting, headache, weakness, restlessness, itchiness, or any other symptoms) | Quote: "One patients accounted for 52% of symptoms during dialysis and 58% of symptoms between dialyses. As noted, thirst was significantly less frequent with a sodium dialysate of 150 mEq/L, whereas headache was more frequent with the same dialysate. Fatigue during dialysis was more frequent with sodium dialysate of 145 mEq/L, whereas other symptoms, including cramps, back pain, stomachache, and irritability, were less frequent with a sodium dialysate of 155 mEq/L. Symptoms between dialyses, including thirst and headache, were more frequent with dialysate sodium of 155 mEq/L but were only present in two patients." |
| Bicer 2022 | Acupressure | Placebo | 70.1% of the patients in the intervention group experienced hypotension | 0.9% of the patients in the placebo group experienced hypotension | Quote:" The procedure‐related side effects did not develop in all the patients included in the study and no patients felt unwell during or after the procedure." |
| BOLD 2020 | Home SBP | Pre‐dialysis SBP | Post‐dialysis SBP<90 mmHg 2/25, post‐dialysis SBP>200 mmHg 3/25, syncope 1/25, fall 3/25, flash pulmonary oedema 0/25, cramping 13/25, dizziness 10/25, light‐headedness 14/25, hypotension 21/25. | Post‐dialysis SBP<90 mmHg 0/25, post‐dialysis SBP>200 mmHg 2/25, syncope 1/25, fall 6/25, flash pulmonary oedema 0/25, cramping 18/25, dizziness 14/25, light‐headedness 12/25, hypotension 18/25. | Quote: "The proportion of dialysis treatments with either excessively low or high pre or post dialysis SBP was small and similar in the two treatment groups. The rates of syncope, falls and flash pulmonary edema were also comparable between treatment groups." |
| Brass 2001 | L‐carnitine | Placebo | In general, adverse events were reported for all study participants without clear distinction between groups (flu syndrome, injection‐site reaction, pain, pharyngitis, headache, hypertension). The intervention group reported serious adverse events (data included study A and B): body injection site reaction (4/130), infection (2/130), chest pain (3/130), abdominal pain (2/130), fever (1/130), accidental injury (1/130), neck pain (1/130), tachycardia (3/130), atrial fibrillation (1/130), hypertension (1/130), hypotension (1/130), aortic stenosis (1/130), colitis (1/130), vomiting (2/130), parathyroid disease (1/130), hyperkalaemia (2/130), hypervolaemia (1/130), lung oedema (1/130), pneumonia (1/130), skin carcinoma (1/130), amblyopia (1/130), urogenital kidney failure (8/130) |
In general, adverse events were reported for all study participants without clear distinction between groups (flu syndrome, injection‐site reaction, pain, pharyngitis, headache, hypertension). The control group reported serious adverse events (data included study A and B): body injection site reaction (6/63), infection (4/63), chest pain (0/63), abdominal pain (0/63), fever (0/63), accidental injury (0/63), neck pain (0/63), tachycardia (0/63), atrial fibrillation (0/63), hypertension (0/63), hypotension (0/63), aortic stenosis (0/63), colitis (0/63), vomiting (0/63), parathyroid disease (0/63), hyperkalaemia (0/63), hypervolaemia (0/63), lung oedema (0/63), pneumonia (0/63), skin carcinoma (0/63), amblyopia (0/63), urogenital kidney failure (3/63) |
Quote: "The most commonly reported adverse events were flu syndrome, injection‐site reaction, pain, pharyngitis, headache, and hypertension and showed no difference in frequency between L‐carnitine and placebo. Several serious adverse events occurred during the course of the study, with no differences between active and placebo groups. No serious adverse event was believed by the investigators to be certainly or probably drug related and they were consistent with the population's underlying disease and maintenance haemodialysis treatment." Table 7 reported "events that occurred only in placebo groups were not listed". |
| Canadian EPO 1990 | EPO alfa | Placebo | Adverse events for both intervention groups were reported: seizure (2/78), clotting of vascular access (11/78), clotting of tubing in dialysis machine (8/78), pain in chest (13/78), epistaxis or haemorrhage (10/78), abnormal sense of taste (11/78), headache (26/78), redness of eyes (5/78), flu‐like symptoms (18/78), aches in bone or muscle (20/78). | Adverse events in the control group were reported: seizure (1/40), clotting of vascular access (1/40), clotting of tubing in dialysis machine (4/40), pain in chest (6/40), epistaxis or haemorrhage (7/40), abnormal sense of taste (6/40), headache (19/40), redness of eyes (0/40), flu‐like symptoms (12/40), aches in bone or muscle (9/40). | Table V reported in the Canadian EPO 1990. |
| Chang 2010 | Exercise | Control | Adverse events were not reported in the intervention group | All adverse events were not reported in the control group. However, authors reported that a muscle/joint pain (1/35) led to early termination | Quote: "There were three early terminations due to a Borg score of 15 (1), muscle/joint pain (1), and unsteady pedal speed (1). All occurred among the sedentary subjects." |
| Chen 2008a | CBT | Education | One participant with underlying stable major depression experienced a minor episode, but it was not reported in which treatment group he was allocated. 1/13 participants in the intervention group experienced morning dyspnoea |
One participant with underlying stable major depression experienced a minor episode, but it was not reported in which treatment group he was allocated. Other adverse events were not reported in the control group |
Quote: "One participant with underlying stable major depression experienced a minor episode because of cessation of antidepressant therapy. One participant in the CBT group experienced 1 episode of morning dyspnoea after a large meal during the last week of this 4‐week trial." |
| Chen 2011a | CBT | Education | There were no adverse events in the intervention group | There were no adverse events in the control group | Quote: "No adverse events were reported during the intervention." |
| Eroglu 2022 | Relaxation + music | Control | No information was reported in detail | No information was reported in detail | Quote: "Moreover, no participants dropped out owing to unexpected adverse effects of BRT combined with music therapy." |
| Fatouros 2010 | L‐carnitine | Placebo | No adverse events were reported in the intervention group | No adverse events were reported in the control group | Quote: "No adverse clinical effect related to L‐carnitine supplementation was reported." |
| FHN DAILY 2007 | Frequent HD | Conventional HD | Death 5/125, all hospitalisation 109/125, all interventions related to vascular access 95/125, hypokalaemia 13/125, hyperphosphataemia 15/125 | Death 9/120, all hospitalisation 114/120, all interventions related to vascular access 65/120, hypokalaemia 6/120, hyperphosphataemia 9/120 | Quote from FHN trial 2010: "Adverse events were reported in table 4." |
| Foley 2000 | EPO alpha (Hb target 9.5‐10.6 g/dL) | EPO alpha (Hb target 13‐14 g/dL) | Arteriovenous access thrombosis and cardiac events were reported in the low target Hb group but the number of patients was not reported. During the study period 3/73 participants died in the low target Hb group | Arteriovenous access thrombosis and cardiac events were reported in the high target Hb group but the number of patients was not reported. During the study period 4/73 participants died in the high target Hb group | Quote: "The comparative incidence of arteriovenous access thrombosis, cardiac events, and death." |
| Fukuda 2015 | Nutritional supplementation | Placebo | Adverse events in the intervention group were reported: increased blood pressure (1/103), dizziness (1/103), insomnia (1/103), nausea (1/103), diarrhoea (2/103), sudden hearing loss (1/103). One participant in each group had cramp in the lower leg | Adverse events in the control group were reported: increased glucose level (1/99), felt sick (2/99), stomach discomfort (1/99), hospitalisation (2/99). One participant in each group had cramp in the lower leg |
Quote: "In the nutritional drink group, one participant reported increased blood pressure, one complained of dizziness, one complained of insomnia, one reported nausea, and two had diarrhoea. One participant in each group had cramp in the lower leg. In the placebo group, one participant reported increased glucose level, two felt sick, and one complained of stomach discomfort. One participant developed sudden hearing loss and was prescribed any vitamins. Two participants were hospitalised in the placebo group. The safety monitoring board confirmed no serious adverse events, and hospitalisation was determined relating to the study intervention." |
| Grigoriou 2021 | Exercise | Control | Not reported in sufficient detail | Not reported in sufficient detail | Quote: "All participants completed both scenarios without any adverse effects or significant complaints." |
| HDPAL 2014 | Atenolol | Lisinopril | A questionnaire assessed the following adverse events: fatigue or tiredness, chest pain, abdominal pain, cold hands or feet, dizziness on standing, muscle cramps, diarrhoea, nausea, vomiting, dry cough, upper respiratory infection or common cold, shortness of breath, headaches, persistent dizziness, numbness in hands or feet, decreased sex drive, decreased ability to have sex, drowsiness or sleepiness, depression or feeling sad and nightmares. However, data were not reported considering the treatment assigned (for only 133 patients who completed the questionnaire). Adverse events in the intervention group were reported: overall serious adverse events (58/100), all‐cause hospitalisation (37/100), infections (24/100), access‐related (17/100), central nervous system (3/100), cancer‐related complications (2/100), cardiovascular event (16/100), angina (0/100), arrhythmia (2/100), cardiac arrest (0/100), congestive heart failure (5/100), myocardial infarction (2/100), peripheral vascular disease (1/100), revascularization (3/100), stroke (2/100), valve replacement surgery (1/100), cardiovascular death (2/100), non‐cardiovascular death (2/100), fractures (7/100), parathyroidectomy (3/100), biliary‐related (1/100), bowel‐related (3/100), falls (6/100), gastrointestinal bleeding (2/100), hypertensive crisis (3/100), hyperglycaemia (1/100), hyperkalaemia (3/100), hypoglycaemia (2/100), hypotension with hospitalisation (6/100), miscellaneous (12/100) |
A questionnaire assessed the following adverse events: fatigue or tiredness, chest pain, abdominal pain, cold hands or feet, dizziness on standing, muscle cramps, diarrhoea, nausea, vomiting, dry cough, upper respiratory infection or common cold, shortness of breath, headaches, persistent dizziness, numbness in hands or feet, decreased sex drive, decreased ability to have sex, drowsiness or sleepiness, depression or feeling sad and nightmares. However, data were not reported considering the treatment assigned (for only 133 patients who completed the questionnaire). Adverse events in the control group were reported: overall serious adverse events (70/100), all‐cause hospitalisation (59/100), infections (20/100), access‐related (19/100), central nervous system (3/100), cancer‐related complications (2/100), cardiovascular event (28/100), angina (2/100), arrhythmia (3/100), cardiac arrest (2/100), congestive heart failure (10/100), myocardial infarction (3/100), peripheral vascular disease (5/100), revascularization (4/100), stroke (2/100), valve replacement surgery (1/100), cardiovascular death (3/100), non‐cardiovascular death (1/100), fractures (1/100), parathyroidectomy (1/100), biliary‐related (2/100), bowel‐related (5/100), falls (3/100), gastrointestinal bleeding (5/100), hypertensive crisis (10/100), hyperglycaemia (3/100), hyperkalaemia (10/100), hypoglycaemia (4/100), hypotension with hospitalisation (5/100), miscellaneous (18/100) |
Quote from Agarwal 2016: "The symptoms were as follows: fatigue or tiredness, chest pain, abdominal pain, cold hands or feet, dizziness on standing, muscle cramps, diarrhoea, nausea, vomiting, dry cough, upper respiratory infection or common cold, shortness of breath, headaches, persistent dizziness, numbness in hands or feet, decreased sex drive, decreased ability to have sex, drowsiness or sleepiness, depression or feeling sad and nightmares." Quote from Agarwal 2014;"Table 3 shows the serious adverse events between groups over the course of the trial." |
| Johansen 1999 | Nandrolone decanoate | Placebo | Adverse events in the intervention group were reported: hematoma (1/14), reduction in testicular size (1/14), amenorrhoea (1/14), acne (1/14), hypertension (3/14) | Adverse events in the control group were reported: hematoma (1/15), skin rash (2/15), hypertension (3/15) | Quote:"Reason for inability to undergo treadmill included coronary artery disease (7 subjects), severe hypertension (2 subjects), hospitalisation (3 subjects), study drop‐out (2 subjects), valvular heart disease, amputation, arthritis, abdominal hernia, and diabetic foot ulcer (1 subject). [...] The study was generally well tolerated, but minor adverse events occurred. Two subject (one in each arm) developed hematoma. One nandrolone recipient complained of a reduction in testicular size that resolved with dose reduction.two men (both in the placebo group) complained of skin rash. Of the 3 women who received nandrolone, 2 required dose reduction for amenorrhoea and acne, respectively. [...] Six subjects (3 in each group) required increase in antihypertensive medication dosages." |
| Johansen 2006 | Nandrolone decanoate with or without exercise | Placebo with or without exercise | Adverse events in both intervention groups were reported: interference with sexual function (1/39) | Adverse events in the both control groups were reported: death (1/40), not feeling well (1/40), abdominal pain (1/40), itchy reaction (1/40) | Quote: "Those who received placebo discontinued because of an itchy reaction at the injection site, a nonspecific feeling that the drug was having adverse effects, abdominal pain and liver function test abnormalities, and discovery of a history of prostate cancer. Those who received nandrolone discontinued because of interference with sexual function (after five doses) and fear of possible adverse effects (after three doses)." |
| Konstadinidou‐ND 2002 | Exercise | Control | Adverse events in both intervention groups were reported: death (1/36) | Adverse events in the control group were reported: death (1/12) | Quote:"However, during the study 5 patients from Group A, 1 from Group B, 2 from C voluntarily withdrew, while 1 patient from Group B and 1 from D died of causes unrelated to exercise." |
| Krase 2022 | Exercise | Control | No detailed information was reported | No detailed information was reported | Quote: "Exercise was well tolerated by all patients, and no adverse reactions were reported." |
| Leski 1979 | Dialysate containing glucose | Dialysate without glucose | The study assessed fatigue, headache and leg cramps using a questionnaire: number of patients who reported these adverse events after dialysis was not reported. Hypotension was recorded but the author did not report information neither on the intervention group allocation nor on the number of cases | The study assessed fatigue, headache and leg cramps using a questionnaire: number of patients who reported these adverse events after dialysis was not reported. Hypotension was recorded but the author did not report information neither on the intervention group allocation nor on the number of cases | Quote: "Headache diminished in frequency during the sessions after the sessions. Fatigue during dialysis was not significantly altered, however post‐dialysis fatigue dropped significantly. The episodes of hypotension decreased in number, but not significantly. The same was the case for cramps." |
| Li 2014b | Nurse led telephone support | Control | As reported in figure 1, death was reported in the intervention group but the number of patients was not reported. No data were clearly reported for oedema, peritonitis, catheter infections, exit‐site condition in the intervention group |
As reported in figure 1, death was reported in the control group but the number of patients was not reported. No data were clearly reported for oedema, peritonitis, catheter infections, exit‐site condition in the control group |
Quote: "The presence of edema, existence of peritonitis, catheter infections, exit‐site condition and weight gain were observed as the complication control of the participants within 42 days (6 weeks) and 84 days (12 weeks) post‐discharge." |
| Lillevang 1990 | EPO | Placebo | Adverse events in the intervention group were reported: skeletal pain (1/9), abdominal pain (1/10) | Adverse events in the control group were reported: bodily distress (1/9), leg cramps (1/10) | Quote:"During the study period, the following adverse effects was registered (defined as new complaints from the patients, independent of the patients perception of relationship with the treatment): in the treatment group, there was one case of skeletal pain and one case of abdominal pain; in the placebo group, there was one case of “bodily distress” and one case of leg cramps." |
| Linde 2001 | EPO alpha to achieve normal‐Hb target | Subnormal‐Hb target with or without ESA | Overall, in the intervention group there were the following adverse events: death (25/180), adverse events (29/180). Specifically:
5 participants had sepsis Data related to predialysis patient were not reported because they were out of our scope |
Overall, in the control group there were the following adverse events: death (26/164), adverse events (14/164). Specifically:
7 participants had sepsis Data related to predialysis patient were not reported because they were out of our scope |
Quote from Furuland 2003: "In a multivariate logistic regression analysis of SAE the number of patients with at least one SAE was 110 and 97 in the N‐Hb and S‐Hb groups, respectively. [...] Five patients in the N‐Hb group and seven in the S‐Hb group had sepsis." |
| Mohammadpourhodki 2021 | Aromatherapy | Placebo | None known | None known | Quote: "None of patients included in the intervention groups reported side effects or local or general complications." |
| Muz 2017 | Aromatherapy | Control | As reported in figure 1, participants in the intervention group reported: nausea and vomiting (1/41), increase of blood pressure (2/41). | No adverse events were reported for the control group in figure 1. | No relevant quotations were reported |
| Parfrey 2005 | ESA (normal Hb target) | ESA (high Hb target) | Adverse events in the intervention group 1 were reported: any (281/300), hypertension (110/300), hypotension (105/300), platelet/bleeding/clotting arteriovenous fistula thrombosis (23/300), hematoma (36/300), arteriovenous fistula loss (26/300), vomiting (52/300), diarrhoea (53/300), nausea (53/300), abdominal pain (46/300), upper respiratory tract infection (69/300), dyspnoea (42/300), cough (36/300), pharyngitis (31/300), myalgia (85/300), skeletal pain (64/300), arthralgia (43/300), headache (64/300), dizziness (40/300), skin disorder (39/300), pruritus (33/300), infection (32/300), urinary tract infection (27/300), hyperparathyroidism (30/300), pain (47/300), back pain (40/300), fever (42/300), influenza (37/300), device complication (27/300), surgery (39/300), arteriovenous fistula thrombosis (36/300), non‐site‐specific embolism thrombosis (12/300), permanent catheter thrombosis (9/300), cerebrovascular disorder (4/300), peripheral ischaemia (7/300), angina pectoris (8/300), myocardial infarction (4/300), chest pain (7/300), permanent catheter loss (6/300), arteriovenous fistula loss (27/300), arteriovenous graft loss (9/300), tachycardia (15/300), palpitations (9/300), atrial fibrillation (7/300), bradycardia (5/300), pulmonary oedema (16/300), cardiac failure (6/300), pulmonary oedema or heart failure (19/300), death (20/300) | Adverse events in the intervention group 2 were reported: any (284/296), hypertension (120/296), hypotension (85/296), platelet/bleeding/clotting arteriovenous fistula thrombosis (30/296), hematoma (45/296), arteriovenous fistula loss (30/296), vomiting (54/296), diarrhoea (50/296), nausea (47/296), abdominal pain (45/296), upper respiratory tract infection (72/296), dyspnoea (35/296), cough (35/296), pharyngitis (29/296), myalgia (81/296), skeletal pain (39/296), arthralgia (36/296), headache (86/296), dizziness (16/296), skin disorder (41/296), pruritus (23/296), infection (34/296), urinary tract infection (29/296), hyperparathyroidism (19/296), pain (41/296), back pain (35/296), fever (30/296), influenza (30/296), device complication (42/296), surgery (20/296), arteriovenous fistula thrombosis (45/296), non‐site‐specific embolism thrombosis (14/296), permanent catheter thrombosis (8/296), cerebrovascular disorder (12/296), peripheral ischaemia (8/296), angina pectoris (9/296), myocardial infarction (7/296), chest pain (4/296), permanent catheter loss (7/296), arteriovenous fistula loss (30/296), arteriovenous graft loss (9/296), tachycardia (22/296), palpitations (6/296), atrial fibrillation (6/296), bradycardia (7/296), pulmonary oedema (9/296), cardiac failure (2/296), pulmonary oedema or heart failure (11/296), death (13/296) | Quote from Parfrey 2005: "Treatment‐emergent adverse events that occurred in at least 10% of patients; vascular, access loss, and cardiac events that occurred in at least 2% of patients; and death in lower and higher target groups. " |
| PEDAL 2020 | Exercise | Control | Greenwood 2021 reported: serious adverse events 69/175, blood and lymphatic system disorders 2/175, cardiac disorders 9/175, congenital disorders 0/175, gastrointestinal disorders 10/175, hepatobiliary disorders 2/175, infections and infestations 29/175, injury 15/175, metabolism and nutritional disorders 13/175, musculoskeletal and connective tissue disorders 3/175, cancer 1/175, nervous system disorders 5/175, psychiatric disorders 3/175, renal and urinary disorders 0/175, respiratory disorders 10/175, reproductive disorders 1/175, skin and subcutaneous tissue disorders 1/175, surgical procedures 24/175, vascular disorders 4/175 | Greenwood 2021 reported: serious adverse events 56/160, blood and lymphatic system disorders 0/160, cardiac disorders 6/160, congenital disorders 1/160, gastrointestinal disorders 4/160, hepatobiliary disorders 1/160, infections and infestations 18/160, injury 12/160, metabolism and nutritional disorders 4/160, musculoskeletal and connective tissue disorders 1/160, cancer 0/160, nervous system disorders 3/160, psychiatric disorders 1/160, renal and urinary disorders 1/160, respiratory disorders 3/160, reproductive disorders 1/160, skin and subcutaneous tissue disorders 0/160, surgical procedures 13/160, vascular disorders 6/160 | Quote from Greenwood 2021: "The number of patients with harms (serious adverse events) was similar in the intervention group (n = 69) and control group (n = 56)." |
| Picariello 2018 | CBT | Control | Admission to hospital 1/18 | Admission to hospital 2/7 | Quote: "No trial adverse events occurred." |
| Reilly‐Spong 2015 | Meditation | Control | There were no adverse events in the intervention group | There were no adverse events in the control group | Quote from Reilly‐Spong 2015: "No adverse effects related to the interventions were reported." |
| Salehi 2020 | Exercise | Control | None known | None known | Quote: "None of the patients suffered from such complications and all participated without interruption." |
| Sang 1997 | Steady dialysate sodium |
Linear sodium ramping Stepwise sodium ramping |
Adverse events (hypotension, cramps, fatigue, thirsty and total symptoms) were recorded as score in all treatment groups. Other adverse events were not clearly stated | Adverse events (fatigue, thirsty and total symptoms) were recorded as score in all treatment groups. Other adverse events were not clearly stated | Quote: "The number of symptomatic or asymptomatic hypotensive episodes and the time of occurrence of a hypotensive episode were recorded. [...] It was noted when the patient complained of angina, cramps, nausea, or headaches, or vomited. [...] Thirst, fatigue, dizziness and total symptoms were also recorded." |
| Schardong 2021 | Laser | Control | Not reported in sufficient detail | Not reported in sufficient detail | Quote: "Regarding the safety of this therapy, no changes were observed in patients’ vital signs and adverse effects during laser applications, as well as in the interval between them." |
| Schmitz 2016 | Citrate dialysate | Standard citrate | Adverse events (including death) were not clearly reported per group in the first phase on the study period | Adverse events (including death) were not clearly reported per group in the first phase on the study period | Quote: "The events such as cramps and hypotension were more frequent with citrate dialysate. [...] The most common adverse events during standard dialysate use were infections and vascular disorders. During the citrate dialysate phase, the most frequent events were general disorders like fatigue, followed by infections and musculoskeletal disorders, e.g. muscle spasm or pain." |
| Semeniuk 2000 | Nutritional supplementation | Placebo | Not reported in sufficient detail | Not reported in sufficient detail | Some adverse events were reported (gastrointestinal and cardiovascular adverse events, hypotension). However, no data were clearly reported for the first phase of the cross‐over study. |
| Singer 2010 | Vitamin C | Placebo | Adverse events in the intervention group were reported: acute coronary syndrome (0/37). Other adverse events (including commence dialysis, bacteraemia and dialysis access thrombosis) were not clearly stated |
Adverse events in the control group were reported: acute coronary syndrome (0/38). Other adverse events (including commence dialysis, bacteraemia and dialysis access thrombosis) were not clearly stated |
Quote: "During the study, there were no episodes of acute coronary syndrome, and two subjects commenced dialysis, both to the PD modality. There were too few bacteraemia (two in ascorbate group and one in placebo group) and dialysis access thromboses (one in ascorbate group) to analyse differences between the groups." |
| Singh 2003 | Dialyser | Dialyser | Adverse events were not clearly reported per group in the first phase on the study period | Adverse events were not clearly reported per group in the first phase on the study period | Quote: "Among clinical symptoms nausea was the most common symptom, which occurred in 62% sessions on cuprophan, and 54% on polysulfone membrane, the difference was not significant. Vomiting, chest pain, fever, chills, and breathlessness occurred significantly more during dialysis with cuprophan membrane as compared with polysulfone. Cramps, back pain, itching, restlessness, post dialysis fatigue, and hypotension did not significantly differ." |
| Singh 2008a | Iron replacement product | Placebo | Adverse events (including death) were not clearly reported per group in the first phase on the study period | Adverse events (including death) were not clearly reported per group in the first phase on the study period | No relevant quotations were reported. |
| Sklar 1999 | Dialysis procedures | Sham dialysis procedures | Adverse events (including death) were not clearly reported per group in the first phase on the study period | Adverse events (including death) were not clearly reported per group in the first phase on the study period | No relevant quotations were reported. |
| Suzuki 2018 | Exercise | Control | Cramps 1/13, muscle pain 3/13 | Cramps 0/13, muscle pain 0/13 | Quote: "In the EMS group, leg cramps occurred in one patient during EMS but rapidly faded without treatment. Muscle pain was reported by three patients after EMS but spontaneously healed within a few days." |
| Thomas 2017 | Meditation | Control | There were no adverse events in the intervention group | There were no adverse events in the control group | Quote: "No adverse events were observed." |
| Tsai 2016 | Acupressure | Sham acupressure | Adverse events in the intervention group were reported: localized erythema (2/14), pruritus (2/14), infection (0/14). There were no serious adverse events in the intervention group |
There were neither adverse events nor serious adverse events in the control group | Quote: "No serious adverse events were reported. In the intervention group, we observed localized erythema below the non‐woven adhesive plaster after early treatment in two patients, who withdrew during the study due to an intolerable pruritus reaction. No patient was found to have an infection. No adverse events were reported for patients in the sham group." |
| Yurtkuran 2007 | Exercise | Control | There were no adverse events in the intervention group | There were no adverse events in the control group | Quote: "No side‐effects were seen". |
| Footnotes: L‐DOPS: L‐threo‐3,4‐dihydroxyphenylserine; C.E.R.A.: Continuous Erythropoietin Receptor Activator; EPO alfa: epoetin alfa; CBT: cognitive‐behavioral therapy | |||||
Data and analyses
Comparison 1. Non‐physiological neutral amino acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Number with improvement of fatigue | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4 Number with aggravation of fatigue | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5 Death (any cause) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 Haemodialysis | 3 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.6 Cardiovascular death | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 Haemodialysis | 2 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.7 Quality of life (overall) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.7.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8 Change in quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.9 Depresssion | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.9.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.10 Change in depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.10.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.11 Hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.11.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Relaxation versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Fatigue | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 Haemodialysis | 3 | 234 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.51 [‐2.28, ‐0.73] |
| 2.2 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2.1 HD | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5 Sleep quality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Relaxation versus exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.2 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3 Sleep quality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Relaxation + music versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.2 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.2.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. Meditation versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2 Death (any cause) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.2.1 Haemodialysis | 2 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.3 Cardiovascular death | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.3.1 Haemodialysis | 2 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.4 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.5 Change in depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.5.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.6 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.6.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.7 Change in anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.7.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.8 Sleep disturbance | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.8.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 6. Exercise versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Fatigue | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1.1 Haemodialysis | 4 | 217 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐2.04, ‐0.31] |
| 6.2 Number reporting fatigue | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.2.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.3 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.3.1 HD | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.4 General fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.4.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.5 Physical fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.5.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.6 Mental fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.6.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.7 Number with moderate fatigue | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.7.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.8 Number with severe fatigue | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.8.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.9 Vitality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.9.1 HD | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.10 Energy/fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.10.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.11 Death (any cause) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.11.1 Haemodialysis | 8 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.76] |
| 6.12 Cardiovascular death | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.12.1 Haemodialysis | 5 | 587 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.10, 3.62] |
| 6.13 Quality of life (overall) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.13.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.14 General health | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.14.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.15 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.15.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.16 Cardiovascular events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.16.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. Exercise with nandrolone versus control with nandrolone placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.3 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.3.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 8. Exercise (inspiratory muscle training) versus exercise (aerobic training).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 9. Single versus combined exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 10. Education versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Fatigue | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1.1 Haemodialysis | 2 | 117 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.97, 0.52] |
| 10.2 Remission of fatigue symptoms | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.2.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.3 Medium fatigue symptoms | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.3.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.4 Severe fatigue symptoms | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.4.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.5 Weakness | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.5.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.6 Energy/fatigue | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.6.1 Peritoneal dialysis | 2 | 220 | Mean Difference (IV, Random, 95% CI) | 4.50 [‐0.55, 9.54] |
| 10.7 Death (any cause) | 5 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.25, 3.57] |
| 10.7.1 Peritoneal dialysis | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 71.92] |
| 10.7.2 Haemodialysis | 4 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.08, 4.74] |
| 10.8 Cardiovascular death | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.8.1 Haemodialysis | 2 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 10.9 Quality of life (overall) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.9.1 Peritoneal dialysis | 2 | 220 | Mean Difference (IV, Random, 95% CI) | 1.86 [‐2.96, 6.69] |
| 10.10 Sleep (overall) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.10.1 Peritoneal dialysis | 2 | 220 | Mean Difference (IV, Random, 95% CI) | 7.46 [2.04, 12.87] |
Comparison 11. Nutritional supplements versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Fatigue | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1.1 Haemodialysis | 2 | 230 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐1.16, 0.50] |
| 11.2 Vitality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.3 General health | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.3.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.4 Death (any cause) | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.4.1 Haemodialysis | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.4.2 Peritoneal dialysis | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.5 Cardiovascular death | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.5.1 Haemodialysis | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.5.2 Peritoneal dialysis | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.6 Sleep problems | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.6.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 12. Cognitive behavioural therapy versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.2 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.2.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.3 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.3.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.4 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.4.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.5 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.5.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.6 Sleep quality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.6.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 13. Cognitive behavioural therapy versus education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.2 Number with decline in fatigue | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.2.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.3 Death (any cause) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.3.1 Haemodialysis | 2 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 13.4 Cardiovascular death | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.4.1 Haemodialysis | 2 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 13.5 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.5.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.6 Number with decline in depression | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.6.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.7 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.7.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.8 Number with decline in anxiety | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.8.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.9 Sleep (overall) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.9.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 14. Cognitive behavioural therapy versus serotonin reuptake inhibitor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14.1.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 15. Aromatherapy versus placebo or standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Fatigue | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1.1 Haemodialysis | 7 | 542 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐1.96, ‐0.50] |
| 15.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.3 Vitality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.3.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.4 Death (any cause) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.4.1 Haemodialysis | 6 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 15.5 Cardiovascular death | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.5.1 Haemodialysis | 6 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 15.6 Quality of life (overall) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.6.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.7 Global sleep quality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.7.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.8 Change in global sleep quality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.8.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.9 Sleep disturbance | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.9.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.10 Change in sleep disturbance | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.10.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 16. Aromatherapy (lavender extract) versus aromatherapy (orange extract).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 17. Aromatherapy versus relaxation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 18. Massage versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Fatigue | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1.1 Haemodialysis | 7 | 657 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.47, ‐0.65] |
| 18.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18.3 Number with severe fatigue | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 18.3.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 18.4 Energy | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.4.1 Haemodialysis | 2 | 152 | Mean Difference (IV, Random, 95% CI) | 4.87 [1.69, 8.06] |
| 18.5 Death (any cause) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.5.1 Haemodialysis | 3 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.06, 36.31] |
| 18.6 Cardiovascular death | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.6.1 Haemodialysis | 2 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 18.7 Quality of life (overall) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18.7.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18.8 Change in quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18.8.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18.9 Sleep (overall) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18.9.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 19. Massage versus sham massage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 19.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 20. Sham massage versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 20.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 21. Massage versus massage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 21.1 Fatigue | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1.1 Haemodialysis | 2 | 160 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.10, ‐0.43] |
| 21.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21.2.1 HD | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21.3 Energy | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21.3.1 HD | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21.4 All‐cause death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 21.4.1 HD | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 21.5 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 21.5.1 HD | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 21.6 Quality of life (overall) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21.6.1 HD | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21.7 Change in quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21.7.1 HD | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21.8 Sleep (overall) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21.8.1 HD | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 22. Erythropoietin stimulating agents versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 22.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22.2 Weakness | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22.3 Energy | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22.3.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22.4 Death (any cause) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.4.1 Haemodialysis | 2 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 4.15] |
| 22.5 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 22.5.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 22.6 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22.6.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22.7 Clotting of vascular access | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 22.7.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 23. Erythropoietin stimulating agents: normal versus high haemoglobin target.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 23.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.2.1 Haemoglobin | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.3 Vitality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.3.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.4 Change in vitality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.4.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.5 Death (any cause) | 3 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.71, 1.56] |
| 23.5.1 Haemodialysis | 3 | 1035 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.73, 1.68] |
| 23.5.2 Peritoneal dialysis | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.17, 2.17] |
| 23.6 Cardiovascular death | 1 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.68, 2.48] |
| 23.6.1 Haemodialysis | 1 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.75, 3.26] |
| 23.6.2 Peritoneal dialysis | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.19, 2.74] |
| 23.7 Cardiovascular events (angina pectoris, myocardial infarction, pulmonary oedema or cardiac failure) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.7.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.8 Arteriovenous access thrombosis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.8.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.9 Hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.9.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.10 Myocardial infarction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.10.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.11 Congestive heart failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.11.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.12 Permanent catheter thrombosis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.12.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.13 Arterious graft loss | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.13.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.14 Arterious fistula thrombosis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.14.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.15 Arterious fistula loss | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.15.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.16 Permanent catheter loss | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.16.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 24. Frequent versus conventional haemodialysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 24.1 Death (any cause) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.1.1 Haemodialysis | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.25, 1.74] |
| 24.2 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 24.2.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 24.3 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 24.3.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 24.4 Vascular access outcomes (repair, loss, or access‐related hospitalisation) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.4.1 Haemodialysis | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.13, 2.07] |
| 24.5 Access loss | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.5.1 Haemodialysis | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.72, 2.03] |
| 24.6 Access stenosis | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.6.1 Haemodialysis | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.37, 3.25] |
| 24.7 Access thrombosis | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.7.1 Haemodialysis | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.28, 8.51] |
Comparison 25. Home versus pre‐dialysis blood pressure monitoring.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 25.1 Number reporting fatigue | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 25.1.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 25.2 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 25.2.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 25.3 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 25.3.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 26. Blood flow rate reduction versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 26.1 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 26.1.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 26.2 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 26.2.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 27. Serotonin reuptake inhibitor versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 27.1 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 27.1.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 27.2 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 27.2.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 27.3 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 27.3.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 28. Beta‐blockers versus angiotensin‐converting enzyme inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 28.1 Change in energy/fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 28.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 28.2 Change in overall health (QoL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 28.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 28.3 Change in general health (QoL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 28.3.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 28.4 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 28.4.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 28.5 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 28.5.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 28.6 Cardiovascular events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 28.6.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 28.7 Access‐related events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 28.7.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 28.8 Change in sleep quality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 28.8.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 29. Anabolic steroids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 29.1 Fatigue | 2 | 52 | Mean Difference (IV, Random, 95% CI) | 1.24 [‐3.66, 6.13] |
| 29.1.1 Haemodialysis and peritoneal dialysis | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐5.19, 2.39] |
| 29.1.2 Haemodialysis | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 3.60 [0.58, 6.62] |
| 29.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 29.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 29.3 Death (any cause) | 2 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.23] |
| 29.3.1 Haemodialysis and peritoneal dialysis | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.07] |
| 29.3.2 Haemodialysis | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.02, 8.10] |
Comparison 30. Anabolic steroids versus exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 30.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 30.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 30.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 30.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 30.3 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 30.3.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 30.4 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 30.4.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 31. Anabolic steroids alone versus anabolic steroids + exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 31.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 31.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 31.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 31.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 31.3 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 31.3.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 32. Anabolic steroids + exercise versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 32.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 32.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 32.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 32.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 32.3 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 32.3.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 33. Anabolic steroids + exercise versus exercise alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 33.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 33.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 33.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 33.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 33.3 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 33.3.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 34. Glucose dialysate versus another glucose dialysate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 34.1 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 34.1.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 34.2 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 34.2.1 HD | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 35. Acupressure versus placebo or control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 35.1 Fatigue | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 35.1.1 Haemodialysis | 6 | 459 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.03, ‐0.25] |
| 35.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.3 Fatigue in the last week | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.3.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.4 Fatigue strength rate | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.4.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.5 Usual level of fatigue during past 24 hours | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.5.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.6 Worst level of fatigue during past 24 hours | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.6.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.7 Death (any cause) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 35.7.1 Haemodialysis | 2 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 35.8 Cardiovascular death | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 35.8.1 Haemodialysis | 2 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 35.9 Quality of life (overall) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.9.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.10 Depression | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 35.10.1 Haemodialysis | 3 | 199 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐6.73, ‐1.47] |
| 35.11 Mood | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.11.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.12 Sleep quality | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 35.12.1 Haemodialysis | 2 | 141 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐2.59, 0.24] |
Comparison 36. Acupressure versus sham acupressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 36.1 Fatigue | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 36.1.1 Haemodialysis | 2 | 134 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.95, 0.52] |
| 36.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 36.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 36.3 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 36.3.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 36.4 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 36.4.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 36.5 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 36.5.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 36.6 Sleep quality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 36.6.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 37. Sham acupressure versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 37.1 Fatigue | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 37.1.1 Haemodialysis | 2 | 135 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.19, ‐0.05] |
| 37.2 Change in fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 37.2.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 37.3 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 37.3.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 37.4 Sleep quality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 37.4.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 38. Acupressure versus transcutaneous electrical acupoint stimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 38.1 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 38.1.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 38.2 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 38.2.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 38.3 Cardiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 38.3.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 38.4 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 38.4.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 38.5 Sleep quality | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 38.5.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 39. Light versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 39.1 Death (any cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 39.1.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 39.2 Cadiovascular death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 39.2.1 Haemodialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 39.3 Quality of life (overall) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 39.3.1 Haemodialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmady 2019.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random block of numbers. Block randomisation was conducted as follows: the group of aromatherapy with lavender essential oil was given the code “A,” the group of aromatherapy with orange essential oil was given the code “B,” and the group of distilled water was given the code “C.” Then, six blocks of three were formed: ABC, ACB, BAC, BCA, CAB, and CBA. In order to select the groups, block BAC was randomly selected. Thus, on the first day (which was Saturday), 30 subjects were assigned to the group of aromatherapy with orange essential oil. On Sunday, 30 subjects were assigned to lavender essential oil group and finally on Monday, another 30 subjects were assigned to the control group." Comment: random numbers are considered at low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "The names of subjects in each group were registered in the coming days. The statistical adviser of the study (second author) was responsible for determining the blocks, and the subjects were allocated into the study groups by the first author." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "There was no possibility of blinding subjects for the type of the assigned group." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. No other outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. No lost to follow‐up were reported |
| Selective reporting (reporting bias) | High risk | Protocol was published. It was reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue data were cumulated for 2 RCTs, all time points were not reported. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding did not influence the data analysis and conflicts of interest were not reported. No other source of bias were apparent |
Akizawa 2002.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind design." Comment: Although the author reported that the study used a double‐blind design, information about blinding of participants and investigators was not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Eight subjective symptoms related to orthostatic hypotension (fatigability, malaise/weakness, physical disturbing on standing up, dizziness on standing up, bad feeling, sleep disorder) were monitored through doctor's questions, based on notebooks kept by the patients. The severity of each symptom was separately assessed using a 4‐point rating scale, i.e. severe (daily activities were greatly disturbed by the symptom), moderate (daily activities were disturbed by symptoms), mild (patients were aware of the symptoms, but daily activities were not disturbed), and asymptomatic (there was no symptom at all and patients were not bothered by any symptoms)." Comment: Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 149 patients, 5 were excluded from efficacy assessment due to missing blood pressure data, and 3 were also excluded because L‐DOPS therapy was discontinued within 2 weeks of the trial. A total of 141 patients (400 mg group 48 patients, 200 mg group 46 patients, and placebo group 47 patients) were thus subjected to efficacy assessment." Comment: 48/51 participants in intervention group 1 (400 mg L‐DOPS), 46/49 participants in intervention group 2 (200 mg L‐DOPS) and 47/49 participants in the control group (placebo) completed the study (> 5% lost to follow‐up, whit differences between groups). In addition, some analyses were reported on a lower number of participants |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan was not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | High risk | There was no evidence of different baseline characteristics or different non‐randomised co‐interventions between groups. Funding (pharmaceutical company) could influence the data analysis, and conflicts of interest were not reported. |
Amini 2016.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "In this double‐blind clinical trial." Comment: Although author reported that the study used a double‐blind design, information about blinding of participants and investigators were not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Questionnaires of anxiety, sleep quality, and fatigue were completed by participants before and after the interventions." Comment: Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Other subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study was not clearly stated. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Protocol was approved by the Ethics Committee of the Shahrekord University of Medical Sciences (not clear if it was published). Fatigue was reported in accordance with a pre‐specified analysis plan, using multiple eligible outcome measurements (scales, time points). All outcomes that should be reported (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding and conflicts of interest were not reported |
ASCEND 2016.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomizations were performed through a Web portal by using computer‐generated permuted blocks of various sizes." Comment: Computer‐generated is considered as low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomizations were performed through a Web portal by using computer‐generated permuted blocks of various sizes." Comment: Web portal is considered as low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Open‐label." Comment: An open‐label study is considered at high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "An independent group charged with monitoring the safety of patients in the ASCEND trial, and the scientific integrity of the trial (unblinded)." Quote: "Randomized participants undergo blinded serial assessment of depressive symptoms every 6 weeks using the clinician‐rated 16‐item Quick Inventory of Depression Symptomatology (QIDS‐C16) administered by research personnel blinded to intervention arm, via a Computer Assisted Telephone Interview (CATI)." Comment: Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used. It was not stated whether outcomes were assessed without knowledge of treatment allocation (it was stated that a blinded interviewer assessed the QIDS‐C16, but no information was reported for the assessment of the fatigue questionnaire), and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Other subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from Merhotra 2019: "Of the 636 patients with a BDI‐II score of 15 or above, 310 (49%) consented to screening and 184 were randomly assigned to the engagement (n = 92) or control (n = 92) group. Of these participants, 120 were randomly assigned to the CBT (n = 60) or sertraline (n = 60) group, 20 who declined treatment within or outside the study enrolled in the observation group, and 44 withdrew from the study." Comment: Although some participants withdrew, all were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Information about the protocol and the statistical analysis plan was reported. Multiple eligible outcome measurements (scales and time points) were assessed as pre‐specified in the study protocol. Fatigue at the end of treatment was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were reported |
| Other bias | High risk | Quote: "The funding organizations had no input in the analysis or interpretation of the data, the drafting of the manuscript, or the decision to submit the manuscript for publication." Comment: Similar baseline characteristics between groups were reported. Funding was unlikely to influence the data analysis and reporting. However, conflicts of interest were reported |
ASSertID 2015.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from Friedli 2015 (protocol): "Randomisation will take place in blocks using pre‐prepared codes for each centre. These will be incorporated into a protected web based randomisation programme prepared by Norwich CTU." Comment: Sequence generation methods seemed to use a computer. No data were available to assess the possible imbalance between groups |
| Allocation concealment (selection bias) | Low risk | Quote from Friedli 2015 (protocol): "Randomisation will take place in blocks using pre‐prepared codes for each centre. These will be incorporated into a protected web based randomisation programme prepared by Norwich CTU. Only the research psychiatrist will have authorised access to the online randomisation programme. Following randomisation the relevant pharmacy will be informed of the allocation (treatment A or B) by automatically generated email. The pharmacist will be blind to the allocation. The CTU will hold the patient‐specific allocation data on a secure server. The CI and PI at each centre will have access to this data file only via a special log‐in should the need arise to un‐blind. No user identifiable data will stored in the randomisation database. Web traffic will be encrypted using standard secure sockets layer technology." Comment: A web‐based system is considered as low risk of bias. No data were available to assess the possible imbalance between groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from Friedli 2017: "The patients, dispensing pharmacies, study psychiatrist, research nurses, all clinicians, trial manager, and study statistician were blind to the allocation of the study medication." Comment: A double‐blind study is considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from Chilcot 2017: "Fatigue was assessed using the MFI‐20." Comment: fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Twenty‐one (70%) patients completed the trial: eight (53%) in the sertraline group and 13 (87%) in the placebo group (P=0.05). In the sertraline group, there were six dropouts within the first 2 months. One patient died of cardiac arrest having taken one tablet. Three patients withdrew because of adverse events (one after 3 days with nausea, another after 12 days with headaches and dizziness, and the third due to insomnia after 23 days). The fifth patient withdrew because of concern about side effects, having taken no study medication. The sixth patient was admitted for a prolonged hospital stay with leg ulcers shortly after randomisation and subsequently withdrawn without having taken any study medication. At 3 months, a seventh patient withdrew because of sweating and palpitations. In the placebo group, one patient withdrew after the baseline interview because of concern about taking additional medication, and a second decided against continuing after 3 months. The number of dropouts due to adverse or severe adverse events was greater in the sertraline group." Comment: overall, 21/30 participants completed the study (>5% lost to follow‐up, differences between groups). Some reasons for discontinuations could be related to the treatment assigned |
| Selective reporting (reporting bias) | High risk | Protocol was published. Trial registration number reported that fatigue should be assessed using MFI‐20 and SF‐36 energy/fatigue sub scale, but data were reported only using MFI‐20. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | Similar baseline characteristics between groups were reported. Funding was unlikely to influence the data analysis and reporting and authors had no conflicts of interest |
BA16285 2007.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This randomised, open‐label, dose‐finding study was conducted at 14 study centres across the United States." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This randomised, open‐label, dose‐finding study was conducted at 14 study centres across the United States." Comment: An open‐label study is considered as a high risk of bias. Participants experienced side effects that participants and/or investigators could know to be specific for one of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Adverse events (including fatigue) were recorded in the patients' case‐report forms by the investigators throughout the study." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, fatigue was assessed as an adverse events and it was not stated whether it was assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant/investigators beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "A total of 91 patients entered the core period (mean age, 58 years; 66% male). Fifty‐three patients continued into the extension period; 22 patients withdrew during this period (6 because of adverse e events, and 16 for other reasons). [...] Ten patients were withdrawn from the core treatment period. Four of these patients withdrew due to adverse events and 6, for other reasons (treatment refusal (2) and insufficient therapeutic response, kidney transplant, inadvertent concomitant administration of epoetin alfa, and anaemia not related to CRD (1 patient each)). All of these patients were included in the intention‐to‐treat (ITT) analysis and were also included in the per protocol (PP) analysis if they met the criteria for the latter. Twelve patients were separately excluded from the PP analysis, for a total PP population of 79 patients (28, 24, and 27 patients in groups A, B, and C, respectively). Fifty‐three patients were entered into the extension phase of the study, 27 in the QW group and 26 in the Q2W group. Six patients withdrew because of adverse events, and 16, for other reasons (kidney transplant (4), site closure or transfer (4), treatment refusal (4), insufficient therapeutic response (2), elevated parathyroid hormone concentration (1), and positive pregnancy test (1))." Comment: Although the authors stated that the analysis were performed according to ITT and PP, Figure 2 showed that not all participants completed the study. Reasons for discontinuations were reported |
| Selective reporting (reporting bias) | High risk | Protocol was approved by the local ethics committees of the institutions taking part into the study (not clear if it was published). Statistical analysis plan was not available. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was reported in a format that was not extractable per group. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | High risk | Baseline characteristics between groups were not reported. Funding was not reported but authors had conflicts of interest |
Babamohammadi 2006.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "37 patients afflicted by chronic renal insufficiency were chosen and put into two categories randomly," Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Demographic data questionnaire and health assessment form and rating scale (designed by researchers) were used to collect the data." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It was not clear if outcome data were provided for all patients. it It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan was not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was not reported and authors had no conflicts of interest |
Bagheri‐Nesami 2016.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The sample was randomly allocated in two groups using the Excel RANDBETWEEN function." Comment: Sequence generation was performed using Excel RANDBETWEEN. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Fatigue was measured using the Fatigue Severity Scale in both groups for a total of three times (before the intervention, and after the last intervention in the second and fourth weeks) by only one researcher who was blind to the treatment allocation." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was stated that the interviewer was blinded to the intervention. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Only one patient from the experimental group was excluded because of an infection, resulting in 29 patients in the experimental group and 30 patients in the routine care group." Comment: 29/30 participants in the intervention group and 30/30 participants in the control group completed the study (<5% lost to follow‐up). Reasons for discontinuations seemed to be not related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Protocol was provided. Fatigue was reported in accordance with a pre‐specified analysis plan, using multiple eligible outcome measurements (scales, time points). Fatigue at the end of treatment was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was not reported and authors had no conflicts of interest |
Balouchi 2016.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Data were collected using a demographic questionnaire and the Multi‐dimensional Fatigue Inventory (MFI‐20)." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study was not clearly stated for the first phase. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis (cross‐over study: data related to the first period were not reported). All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | No data were available to assess the possible imbalance between groups. Funding was unlikely to influence the data analysis and conflicts of interest were not reported |
Barre 1988.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Intervention group 3
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Dialysis was performed in random sequence with dialysate sodium of 145, 150, or 155 mEq/L for 2 months at a time." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The customise coded dialysis concentrates were provided by Erika (Rockleigh, Nj)." Comment: The sponsor performed the allocation. Not sure if they were unaware of treatment assigned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A double blind prospective study was carried out in five stable men on chronic haemodialysis." Comment: Although author reported that the study used a double‐blind design, information about blinding of participants and investigators were not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Each patient completed a questionnaire for each dialysis and was asked to report symptoms during and between dialysis. These included thirst, nausea, vomiting, headache, weakness, restless, fatigue, itchiness, crams, or any other symptoms." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, fatigue was assessed as an adverse event and it was not stated whether it was assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study was not clearly stated for the first phase. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis (cross‐over study: data related to the first period were not reported). All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | High risk | No data were available to assess the possible imbalance between groups. Funding was likely to influence data analyses and interpretation and conflicts of interest were not reported |
Bellinghieri 1983.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind study." Comment: Although the author reported that the study used a double‐blind design, information about blinding of participants and investigators were not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Objective examination of asthenia consisted in making the patient flex the knees with the trunk in upright position for different intervals (exercise A) and walk repeatedly up band down three steps (exercise B). Asthenia was scored as slight if fatigue appeared at less than 60 sec of exercise A and at less than 30 ascents and descents during exercise B, intense at less than 15 sec of exercise A and at less than 10 ascents and descents of exercise B. Moderate degree of asthenia was between the two extremes. The exercises were performed immediately after and between haemodialysis. In the latter case the patients did the exercises at home and recorded the results." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. All outcomes that should be addressed (fatigue, cardiovascular disease, death and vascular access) were not reported. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study was not clearly stated for the first phase. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis (cross‐over study: data related to the first period were not reported). All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | High risk | No data were available to assess the possible imbalance between groups. Funding (pharmaceutical company) could influenced the data analysis and conflicts of interest were not reported |
Bicer 2022.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Cointerventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The patient data relating to the questionnaire, VAS pain (measurement of pain level), VAS fatigue, and Piper fatigue scale at the first follow‐up (the first interview before acupressure) were collected by the researcher." Comment: Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "A total of 150 patients, meeting the inclusion criteria, were reached during the study. Five patients in the intervention group did not agree to participate in the study. Two of these patients experienced local pruritus in the area the device was applied, one patient developed a fistula problem, and two patients left the city during the follow‐up. Additionally, three patients in the placebo group did not want to continue the study since two of these patients were receiving treatment in a hospital out of the city due to coronary angiography. Therefore, the study was completed with 135 patients." Comment: 135/150 participants completed the study (> 5% loss to follow‐up. Some reasons for discontinuation were provided, and some were related to the intervention |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan was not reported. It was reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue data at all time points were reported. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding did not influence the data analysis and conflicts of interest were not reported. No other source of bias were apparent |
Biniaz 2015.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The samples were randomly distributed by a lottery method into two equal groups (simple random sampling)." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blinded." Comment: Although author reported that the study used a double‐blind design, information about blinding of participants and investigators were not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Only 57 patients completed the study (30 persons in the intervention and 27 persons in the control group)." Comment: 57/60 participants completed the study (> 5% loss to follow‐up). Reasons for discontinuation were not provided and it was not clear if there was a difference between the two groups |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue was measured all time points. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding did not influence the data analysis and conflicts of interest were not reported. No other source of bias were apparent |
BOLD 2020.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Cointerventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised using 1: 1 block randomisation, stratified by site." "Randomization was done by a computer algorithm, in random size blocks (e.g. 2, 4, or 6) stratified by recruitment site." Comment: a sequence computer generation is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This was a non‐blinded 4‐month, parallel group randomised controlled trial." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Analysis followed the intent to treat principle." "Forty‐nine of the 50 enrolled participants (98%) completed the study successfully (Figure 1). The sole participant who withdrew (from the pre‐dialysis SBP treatment group) did so when she unexpectedly received a deceased donor kidney transplant." Comment: 25/25 participants in intervention group 1 (home SBP) and 24/25 participants in intervention group 2 (pre‐dialysis SBP) completed the study. There were differences between intervention groups (> 5% loss to follow‐up). Reasons for discontinuations were provided, and they did not seem to be related to the treatment arm. However, ITT analysis was performed |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan was reported. Fatigue was not reported using multiple eligible outcome measurements (scales and time points). It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding did not influenced the data analysis and conflicts of interest were not reported. No other source of bias were apparent |
Brass 2001.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Study A Intervention group
Control group
Study B Intervention group 1
Intervention group 2
Intervention group 3
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Two placebo‐controlled, double‐blinded, randomised studies of carnitine supplementation were performed." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Two placebo‐controlled, double‐blinded, randomised studies of carnitine supplementation were performed." Comment: Although author reported that the study used a double‐blind design, information about blinding of participants and investigators were not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The Kidney Disease Questionnaire (KDQ) is a validated questionnaire for measuring quality of life in patients with ESRD. It was administered in English or Spanish by trained interviewers on non dialysis days. [...] A standardized chemistry panel was assessed during screening, at baseline, and after 12 and 24 weeks of treatment." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Study A randomised 60 patients, 30 patients on each study arm. Four patients (2 patients from each group) were excluded from the intention‐to‐treat population because they withdrew before and post baseline exercise tests (2 patients received renal transplants, 1 patient relocated, and 1 patient withdrew after developing elevated serum transaminase levels). Within the intention‐to‐treat population (n = 56), 7 patients (1 patient, placebo; 6 patients, L‐carnitine) withdrew before completing the 24‐week protocol. Three patients received renal transplants, 1 patient withdrew consent, 1 patient became pregnant, 1 patient was unable to perform the exercise test, and 1 patient withdrew from the study after a serious adverse event unrelated to study drug. Study B randomised 133 patients. Six patients (all administered carnitine) did not have post baseline exercise assessments and were thus excluded from the intention‐to‐treat population (2 patients received renal transplants, 1 patient withdrew from the study, 1 patient experienced worsening of arthralgia, 1 patient died, and 1 patient withdrew because of ECG changes). Within the intention‐to‐treat population (n = 127), 9 patients (2 patients, placebo; 3 patients, 10 mg/kg of L‐carnitine; 2 patients, 20 mg/kg of L‐carnitine; 2 patients, 40 mg/kg of L‐carnitine) failed to complete the full 24‐week study. One patient had exercise‐related problems, 1 patient was unable to exercise because of carpal tunnel syndrome, 4 patients received renal transplants, 1 patient withdrew because of back spasms, I patient refused the study drug because of abdominal pain, and 1 patient withdrew because of ECG changes." Comment: 11/60 in the study A did not complete the study (> 5% lost to follow‐up, with differences between groups). Reasons for discontinuations seemed to be not related to the treatment allocation. 15/133 in the study B did not complete the study (>5% lost to follow‐up, with differences between groups). Reasons for discontinuations seemed to be not related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue data were cumulated for 2 RCTs, all time points were not reported. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | High risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding (pharmaceutical company) could influenced the data analysis and conflicts of interest were not reported |
Canadian EPO 1990.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
intervention group 1
Intervention group 2
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from Canadian EPO 1990: "Patients were stratified by hospital and randomised in blocks to receive placebo; erythropoietin at a dose adjusted to maintain the haemoglobin concentration at 95‐110 g/l (low erythropoietin group); or erythropoietin at a dose adjusted to maintain the haemoglobin concentration at 115‐130 g/l (high erythropoietin group)." Comment: sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote from Canadian EPO 1990: "Patients were stratified by hospital." Comment: method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from Canadian EPO 1990: "To ensure that the study was double blind we established two teams of staff at each study centre. The unblinded team consisted of a doctor, a pharmacist, and a data clerk and was responsible for adjusting the dose of erythropoietin, prescribing iron supplements or transfusions, and sending haematological data to the coordinating centre. The blinded team consisted of nurses in the dialysis unit and our study group and all doctors in the dialysis unit other than those in the unblinded team; this team carried out routine clinical care and recorded adverse reactions and other clinical events but did not have access to the results of haematological tests or know the dose of erythropoietin or placebo that each patient was receiving." Comment: a clear explanation of the double‐blind study was provided |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from Canadian EPO 1990: "The nurses in the study group administered tests to assess quality of life and exercise capacity." Quote from Keown 2010: "Health‐related quality of life was measured by the Kidney Disease Questionnaire (KDQ) and Sickness Impact Profile (SIP) between the placebo group and the combined Epoetin alfa‐treated group." Comment: the outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used (although nurses were part of the blinded team) it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from Canadian EPO 1990: "Nineteen patients were withdrawn during the study: eight in the placebo group (because of transplantation (five), non‐compliance (one), reaction to transfusion (one), seizure and death (one)); six in the low erythropoietin group (transplantation (two), hypertension (one), hypertension and seizure (one), subarachnoid haemorrhage and seizure (one), pregnancy (one)); and five in the high erythropoietin group (transplantation (three), hypertension (two)). Six patients were withdrawn before the follow‐up at two months, and the 13 others were withdrawn before the follow‐up at four months. The patient who became pregnant continued to receive erythropoietin but had a spontaneous miscarriage at 11‐ 1 2 weeks' gestation." Quote from Muirhead 2008: "Analysis was conducted using ITT." Comment: 11/78 in the two intervention groups and 8/40 in the placebo group did not completed the study. However, ITT analyses was performed |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | High risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding (pharmaceutical company) could influenced the data analysis and authors had conflicts of interest |
Cecen 2021.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Since the study was a quasi‐experimental trial, no random element was used in generating the allocation sequence or the sequence was predictable |
| Allocation concealment (selection bias) | High risk | Method of allocation concealment was not reported in sufficient detail to permit judgement. However, since the study was a quasi‐experimental trial, there was a reason to suspect that the enrolling investigator or the participant had knowledge of the forthcoming allocation. No imbalance between intervention groups was apparent |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant/investigators beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The patients involved in the preliminary application were included in the number of samples and new patients who met the criteria in each group of 28 patients were included in the groups by computerized randomisation and a total of 84 patients was reached. Since one patient from the hand massage group left the dialysis canter temporarily after the fifth massage session, and one patient from the foot massage group died after being taken to the intensive care prior to the fourth session, one patient from each group was excluded from the research. As a result, a total of 82 patients, including 27 patients in each of the hand massage and foot massage groups, and 28 patients in the control group, formed the sample of the research." Comment: 54/56 participants in the intervention groups and 28/28 in the control group completed the study (< 5% loss to follow‐up). Differences between subgroups were reported. Reasons for discontinuation were reported |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan was not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest. The study seemed to be free from other source of bias |
Chang 2010.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "This was a quasi‐experimental clinical trial in a medical centre with two haemodialyses units managed by the same medical and nursing team. The patients were assigned randomly to either unit. The experimental group was recruited from one unit and the control group from another, and participants were pair‐matched based on age and gender." Comment: Since the study was a quasi‐experimental trial, no random element was used in generating the allocation sequence or the sequence was predictable |
| Allocation concealment (selection bias) | High risk | Method of allocation concealment was not reported in sufficient detail to permit judgement. However, since the study was a quasi‐experimental trial, there was a reason to suspect that the enrolling investigator or the participant had knowledge of the forthcoming allocation. No imbalance between intervention groups was apparent |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Subjects were interviewed by a research assistant to fill‐out the fatigue scale and Bouchard’s PAL on enrolment, during the fourth week and the eighth week of their haemodialysis visits. The research nurse is not a staff working in these haemodialysis units. She collected data independently and did not participate in patient care." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant/investigators beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed. It was not stated if the interviewer was blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "From August to November 2008, there were 44 and 46 subjects in each unit who met the criteria and were invited to participate. Fourteen refused in the beginning as they were unwilling to participate. Five subjects dropped‐out in later stages for various reasons (Figure 1). Thirty‐six subjects (80%) in the experimental group and 35 patients (76%) in the control group completed the study." Comment: 36/44 participants in the intervention group and 35/46 participants in the control group completed the study (> 5% lost to follow‐up with differences between groups). Reasons for discontinuations were not reported |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest. The study seemed to be free from other source of bias |
Chen 2008a.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomly assigned participants by using computer generated randomised numbers with an allocation ratio of 1:1; to either the CBT group (13) or the control group (13). No stratification or blocking factors were used." Comment: Computer generated randomised numbers is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The sequence was concealed until the interventions were assigned. [...] The generation of allocation sequence and assignment of participants was performed by the project director." Comment: It was not stated if the enrolling investigator (project director) had knowledge of the forthcoming allocation. No imbalance between intervention groups was apparent |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This pilot study did not use a double‐blind design, and participants were informed of their allocation sequence by telephone." Comment: An open‐label study is considered as high risk of bias. Interventions were different and participants and/or investigators were aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Fatigue was assessed using a questionnaire. The 2 measurements were completed before and after the 4‐week trial by all participants in both groups." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote:"Two participants in the control group withdrew after randomisation for personal considerations (1 person lived too far from the hospital, and the other needed to work in the night‐time during the trial." Comment: Although 2/13 participants withdrawal from the control group, Figure 1 and Table 4 showed that all patients were included in the analysis |
| Selective reporting (reporting bias) | High risk | Protocol was provided. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest. The study seemed to be free from other source of bias |
Chen 2011a.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomised participants by computer‐generated random numbers with an allocation ratio of 1:1; that is, either to the CBT group or to the control group." Comment: Computer‐generated randomised numbers is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The generation of allocation sequence and assignment of participants was performed by the project director." Comment: It was not stated if the enrolling investigator (project director) had knowledge of the forthcoming allocation. No imbalance between intervention groups was apparent |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This study was an open‐labelled design. Participants were informed of their allocation sequence by the nursing staff, and the sequence was concealed until the interventions were assigned." Comment: An open‐blinded study is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Fatigue was assessed using a questionnaire. The four measurements were completed before and after the 6‐week trial by all of the participants in both groups." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "After randomisation, three participants in the CBT group and five participants in the control group refused to participate and withdrew their informed consent because of personal considerations. Therefore, a total of 72 subjects (37 in the CBT group and 35 in the control group) participated." Comment: 37/40 participants in the intervention group and 35/40 participants in the control group completed the study (> 5% lost to follow‐up, with differences between groups). Reasons for discontinuations seemed to be not related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest. The study seemed to be free from other source of bias |
Cho 2004.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classificaiton
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were then assigned randomly to either experimental or the control group. [...] There were no differences in demographic data between the groups (p > 0.05). However, a significant difference in age (p < 0.05) was found between groups." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | It was not stated if the enrolling investigator (project director) had knowledge of the forthcoming allocation. Although there were some differences between groups, these differences did not suggest a problem with the randomisation process |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, other subjective outcomes were reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "62 cases were recruited to this study and 3 cases in the experimental and 1 case in the control group dropped out. [...] The reasons for dropping out were relocation or being transferred to other dialysis centre." Comment: 28/31 participants in the intervention group and 30/31 participants in the control group completed the study (> 5% lost to follow‐up, with differences between group). Reasons for discontinuations seemed to be not related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan was not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | Although there were some differences between groups, there was no substantial evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding and conflicts of interest were not reported |
Chow 2010.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was a randomised controlled trial with a pre‐test and post‐test." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The data were collected in 2005 at three time intervals using a structured self‐report questionnaire. [...] Data collection was through face‐to‐face interview." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The 100 patients who joined the study were randomly assigned to either the study or control group. There were 50 patients in each of the treatment arms. At week 12, 43 of the 50 study patients and 42 of the 50 controls had completed the follow‐up questionnaires. A total of 85 patients completed the protocol and were included in the analysis (Figure 1)." Comment: 43/50 participants in the intervention group and 42/50 participants in the control group completed the study (> 5% loss to follow‐up). Reasons for discontinuations seemed to be not related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest. The study seemed to be free from other source of bias |
Dashti‐Khavidaki 2013.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "SF‐36 was completed by patients and was read for patients who were unable to read." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of these 92 patients, 45 and 47 patients assigned to the case and control groups respectively. Twenty‐six patients in the case group and 34 subjects in the control group completed the study." Comment: 26/45 participants in the intervention group and 34/47 participants in the control group completed the study (> 5% lost to follow‐up, with differences between group). Reasons for discontinuations seemed to be not related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest. The study seemed to be free from other source of bias |
Duggal 2019.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised in a 1:1 manner to intervention or control arms using a computer‐generated sequence of randomly permuted blocks." Comment: Comupter generation is considered at low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "The random allocation sequence was generated by statisticians who were not involved in the survey process." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Single‐blinded." "Patients were blinded to group assignment." Comment: A single blinded study is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "There were 102 patients enrolled in the study. A total of 86 (84.3%) of those subjects completed the study. Of
those in the control group, 42 (84.0%) completed the study, and 44 (84.6%) of those in the intervention group
completed the study. Causes of discontinuation are noted." Comment: 44/52 participants in the intervention group and 42/50 participants in the control group completed the study (> 5% lost to follow‐up). There were differences between groups and reasons for discontinuation were provided |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were reported. Regarding fatigue, it was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis. No other source of bias were apparent |
Eroglu 2022.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A blinded study could not be conducted as per the limitations of blinding for non‐pharmacological tests." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The second investigator randomly assigned all the participants to the intervention group (n = 31) and the control group (n = 31) in a 1:1 ratio using a random number table. After the allocation of all participants, 1 patient in the intervention group did not want to participate due to psychological/familial issues. Finally, the study was completed with a total of 61 patients, 30 in the intervention group and 31 in the control group." Comment: 30/31 participants in the intervention group and 31/31 participants in the control group completed the study (< 5% loss to follow‐up with slight differences between groups). Reasons for discontinuation were provided and they were not related to the intervention |
| Selective reporting (reporting bias) | High risk | Protocol was published. Fatigue was reported (Unruh 2013) in accordance with a pre‐specified analysis plan, using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest. No other source of bias were apparent |
Fatigue‐HD 2019.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised using a computer‐generated random number sequence according to permuted blocked randomisation, stratified by dialysis unit." Comment: A computer‐generated random number sequence is considered as low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "We concealed allocation by having a research manager not otherwise involved with the study, provide treatment allocation to study coordinators over the phone." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants were blinded to treatment allocation. It was not feasible to blind study coordinators, given the extensive training they received to learn to administer the intervention compared with the control." Comment: A single blind study is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from the study protocol: "As the proposed study is small and its risks to participants are low, a Data and Safety Monitoring Board is not needed." Comment: Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/15 participants in the intervention group and 14/15 participants in the control group completed the study (> 5% lost to follow‐up). There were differences between treatment groups. Reasons for discontinuation were provided |
| Selective reporting (reporting bias) | High risk | Protocol was published. Fatigue was reported in accordance with a pre‐specified analysis plan, using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest. No other source of bias were apparent |
Fatouros 2010.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional classification
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Twelve haemodialysis patients received either L‐carnitine (20 mg/kg‐1 IV) or placebo in a double‐blind, placebo‐controlled, counterbalanced, and cross‐over design for 8 weeks." Comment: Although the author reported that the study used a double‐blind design, information about blinding of participants and investigators were not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "In their second visit, subjects returned their diet recall forms and underwent a progressive diagnostic test to exhaustion (GXT) on a stationary cycle ergometer to evaluate their peak oxygen consumption (VO2 peak) while blood was collected before and immediately after testing." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was not reported in sufficient detail to permit judgment in the first phase of the study |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan was not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis (cross‐over study: data related to the first period were not reported). All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | No data were available to assess the possible imbalance between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest |
FHN DAILY 2007.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from Suri 2007: "Eligible subjects are then randomly assigned 1:1 to the frequent haemodialysis intervention or control arms, by a central, web‐based program. Randomization is stratified by clinical centre and diabetic status, using permuted blocks." Comment: A web‐based program is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Quote from FHN Trial Group 2010: "Randomization was stratified according to clinical centre and diabetes status, with the use of randomly permuted blocks. Although treatment assignments could not be concealed, between group comparisons of the outcomes were concealed from the investigators throughout the course of the trial." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from Kuella 2013: "Unblinded intervention." Comment: An open‐label study is considered as high risk of bias. Possible deviations from the intended intervention that arose from the trial context were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from Suri 2013: "A vascular access outcomes committee blinded to group allocation reviewed all access events to determine whether the event met the definition of repair or loss. [...] An independent outcomes committee blinded to group allocation reviewed these forms, discharge summaries, and supplementary chart information to determine whether each death or hospitalisation was access related or non–access related." Quote from Suri 2014: "Patients also completed several questionnaires that were centrally administered by telephone before randomisation and 4 (F4) and 12 months (F12) after randomisation." Quote from Ornt 2013: "An independent data and Safety Monitoring Board reviewed safety data and interim results." Comment: An independent Data Safety Monitoring Board assessed the outcomes.The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was stated that outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may not have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall, attrition seemed to be > 5% of loss to follow‐up with some difference between groups. Attrition was not reported in sufficient detail to permit judgment in the first phase of the study. Tamura 2010 reported that 239 participants were randomised but there were no data on the missing participants |
| Selective reporting (reporting bias) | Low risk | Protocol was published. Fatigue was reported (Unruh 2013) in accordance with a pre‐specified analysis plan, using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Founding were unlikely to influence the data analysis and authors had no conflicts of interest |
FHN NOCTURNAL 2007.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from Suri 2007: "Eligible subjects are then randomly assigned 1:1 to the frequent HD intervention or control arms, by a central, web‐based program. Randomization is stratified by clinical centre and diabetic status, using permuted blocks." Comment: A web‐based program is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Quote from FHN Trial Group 2010: "Randomization was stratified according to clinical centre and diabetes status, with the use of randomly permuted blocks. Although treatment assignments could not be concealed, between group comparisons of the outcomes were concealed from the investigators throughout the course of the trial." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from Kuella 2013: "Unblinded intervention." Comment: An open‐label study is considered as high risk of bias. Possible deviations from the intended intervention that arose from the trial context were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from Suri 2013: "A vascular access outcomes committee blinded to group allocation reviewed all access events to determine whether the event met the definition of repair or loss. [...] An independent outcomes committee blinded to group allocation reviewed these forms, discharge summaries, and supplementary chart information to determine whether each death or hospitalisation was access related or non–access related." Quote from Suri 2014: "Patients also completed several questionnaires that were centrally administered by telephone before randomisation and 4 (F4) and 12 months (F12) after randomisation." Quote from Ornt 2013: "An independent data and Safety Monitoring Board reviewed safety data and interim results." Comment: An independent Data Safety Monitoring Board assessed the outcomes.The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was stated that outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may not have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall, attrition seemed to be >5% of lost to follow‐up with some difference between groups. Attrition was not reported in sufficient detail to permit judgment in the first phase of the study. Tamura 2010 reported that 84 participants were randomised but there were no data on the missing participants |
| Selective reporting (reporting bias) | Low risk | Protocol was published. Fatigue was reported (Unruh 2013) in accordance with a pre‐specified analysis plan, using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Fundings were unlikely to influence the data analysis and authors had no conflicts of interest |
Figueiredo 2018.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Intervention group 3
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed using individual allocation codes placed within opaque, sealed envelopes by a person having no contact with the participants." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant/investigators beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. It was not stated if the monitoring group was blinded to the treatment assigned. However, objective and subjective outcomes were assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Intention‐to‐treat." Comment: 10/11 participants in the intervention group 1 (IMT), 10/13 participants in the intervention group 2 (at), and 11/13 participants in the intervention group3 3 (combination) completed the study. However ITT was performed. |
| Selective reporting (reporting bias) | Low risk | Information about the protocol and the statistical analysis plan were reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were reported, but fatigue was not extractable. |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had conflicts of interests. No other source of bias were apparent. |
Foley 2000.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from Foley 2000: "This was a 48‐week, open‐label, randomised, controlled trial." Comment: An open‐label study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "A study monitoring group (R.N.F., P.S.P., and J.M.) at the coordinating centre in St. John’s met weekly to review each patient’s haemoglobin level, epoetin dose, iron saturation, and blood pressure level." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting (bot sure if the committee assessed also fatigue). Participant/investigators beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. It was not stated if the monitoring group was blinded to the treatment assigned. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Follow‐up studies were unavailable in 12 patients, 5 in the low target and 7 in the high target group. The reasons included transplantation (3), death (3), withdrawal of consent (3), Ischaemic heart disease (1) and other causes (1)." Comment: 68/73 participants in the intervention group 1 (epoetin alpha to achieve HB of 9.5‐10.5 g/dL) and 66/73 participants in the intervention group 2 (epoetin alpha to achieve Hb of 13‐14 g/dL) completed the study (> 5% lost to follow‐up, with differences between groups). Some reasons for discontinuations appeared to be related with the intervention. However, analyses were performed in 45 and 49 participants, respectively |
| Selective reporting (reporting bias) | Low risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were reported, but fatigue was not extractable |
| Other bias | High risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding (pharmaceutical company) could influenced the data analysis and authors had conflicts of interests |
Fukuda 2015.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation by means of a computer‐generated random number table (1:1) was to either the nutritional drink, or matching placebo in accordance with the minimization method with three factors (sex, age, each of four dialysis centre); one drink was taken by patients after each dialysis session under the supervision of a nurse." Comment: Computer generation is considered as low‐risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Originally assigned code numbers were kept in closed envelopes within the coordinating centre." Comment: It was not reported if envelopes were numbered and opaque. No imbalance between intervention groups was apparent |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients and attending physicians were blinded to the treatment. [...] All study investigators, medical staff, statistician and participants were blinded to the randomisation procedure and treatment assignments." Comment: A double‐blind study was considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The safety of the intervention and scientific integrity of the study were supervised by an independent data and safety monitoring board located at the Center for Drug & Food Clinical Evaluation, Osaka City University Hospital, Osaka, Japan (coordinating centre)." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. It was not clearly stated if the independent data and safety monitoring was blinded to the treatment assigned. However, subjective and objective outcomes were reported. It was not stated if the independent data safety and monitoring board was blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "172 patients (86 in each group) completed the study. [...] One participant withdrew consent before the randomisation and a total of 202 patients [Inoue Hospital, Suita, Japan (n = 72); Ohno Memorial Hospital, Osaka, Japan (n = 54); Okada Clinic, Osaka, Japan (n = 31); Shirasagi Hospital, Osaka, Japan (n = 46)] were included in the trial and were randomly assigned to one of the two treatment arms. Of the 202 participants, six in the nutritional drink group and two in the placebo group did not receive allocation. Four participants in the nutritional group and two in the placebo group did not receive allocation because they withdrew consent. Two participants in the nutritional drink group did not receive allocation because of hospitalisation or changing the time of dialysis from afternoon to morning. Ten participants in each group discontinued intervention (in the nutritional group, 4 withdrew consent and 6 experienced adverse effects; in the placebo group, 1 withdrew consent, 1 was hospitalised, 5 experienced adverse effects, 2 changed the time of dialysis from afternoon to morning, and 1 had unknown reasons). Finally, 68 patients in Inoue Hospital, 43 in Ohno Memorial Hospital, 24 in Okada Clinic, and 39 in Shirasagi hospital completed the intervention. One patient was excluded from the final analysis because of changing the hospital visit date from a weekday to the weekend." Comment: Figure 1 reported that no patients were lost to follow‐up. However, 87/103 participants in the intervention group and 86/99 participants in the control group were analysed. 0/103 participants in the intervention group and 1/99 participants in the control group were excluded from the analyses (ITT) |
| Selective reporting (reporting bias) | High risk | Protocol was published. Fatigue was reported in accordance with a pre‐specified analysis plan, using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was not involved in the design, execution, analysis, or reporting of the results of this study. The study seemed to be free from other source of bias |
Grigoriou 2021.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
|
|
| Interventions | Intervention classificationn
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The order of the two scenarios was randomly applied in all patients using a computer random number generator." Comment: A computer random number generator is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It was not clear if outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Fatigue was not clearly reported. However, other subjective outcome were reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall 21/22 participants completed the study. No information were reported to assess differences between groups |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan was reported. Fatigue was not clearly assessed and data were not reported in a format that was extractable for meta‐analysis (cross‐over study). All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | Baseline characteristics were not clearly reported. Funding was unlikely to influence the data analysis |
Habibzadeh 2020.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Intervention group 3
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly allocated into four groups (three intervention and one control group) by the first researcher. Numbers 1 through 120 were written on a small paper and placed in a basket; the participants were asked to take a number from the basket and classified based on this number (1 to 30 in the control group, 31 to 60 in the “Foot massage with chamomile oil group”, 61 to 90 in the “Foot massage with almond oil group” and 91 to the last in the “Foot massage without oil group”)." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Both participants and researcher were blind to participant allocation; however, due to noticeable differences in the oils used in foot massage, it was not possible to blind the researcher who performed the foot massage intervention and participants." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It was not clear if fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, other subjective outcome were reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Protocol was published. Fatigue was reported in accordance with a pre‐specified analysis plan, using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was not involved in the design, execution, analysis, or reporting of the results of this study. The study seemed to be free from other source of bias |
Hadadian 2016.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Fifty six patients who had undergone haemodialysis and meeting the inclusion criteria, were divided into two groups by simple random sampling." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This study was done as a single‐blind clinical trial. [...] TEAS group treated by acupuncture in real points, while, in the TEAS‐Sham patients, based on the acupuncture expert opinion, the procedure was implemented for them in the false points, so that the patients were not aware of their grouping and blinded about it." Comment: A single‐blind study is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The questionnaires were filled up by the researcher before and after 10th session of intervention." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant/investigators beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. It was not stated if the interviewer was blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "In this study 95 patients were screened, 72 patients met the inclusion criteria and 60 patients agreed and consented to the study. Four patients were excluded over the intervention: 2 in the TEAS group and 2 in the sham group. Finally, 56 cases including 28 cases in the TEAS group and 28 cases in the Sham group completed the research." Comment: 28/30 participants in the intervention group and 28/30 participants in the control group completed the study (> 5% lost to follow‐up, without differences between groups). Reasons for discontinuations were not reported |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis |
Hadadian 2018.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It was not clear how fatigue was assessed, although an appropriate measure was used. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant/investigators beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was not clearly reported. Fatigue was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | Baseline characteristics were not clearly reported. Funding was unlikely to influence the data analysis |
Hasankhani 2013.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Samples were selected at random." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The control group in the past four weeks, they received usual care and they were not aware from massage therapy by the intervention group." Comment: Not reported if investigators and all participants were aware on the treatment assigned. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study was not clearly stated. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | No data were available to assess the possible imbalance between groups. Funding was unlikely to influence the data analysis and reporting but conflicts of interest were not reported |
Hassanzadeh 2018.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were allocated into three groups randomly by lottery based on the days of week done. The two hospital‐based research environments were divided based on morning and afternoon shifts and even and odd days. Then, every shift, the hospital and day were assigned randomly to one of the groups: A (relaxation techniques), B (aromatherapy), or C (control group). At first, each group was assigned a number and drew, in that order, another set of numbers to determine their lottery drawing order." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. It was not clearly stated if the independent data and safety monitoring was blinded to the treatment assigned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were reported. Fatigue was reported in accordance with a pre‐specified analysis plan, using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the design, execution, analysis, or reporting of the results of this study and authors had no conflicts of interest. The study seemed to be free from other source of bias |
HDPAL 2014.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from Georgianos 2015: "Randomization was performed using a random permuted block design, and computer‐generated random sequence was used for allocation concealment." Comment: A computer‐generated random sequence is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Low risk | Quote from Agarwal 2014: "Subjects were randomised in a 1:1 ratio to either atenolol or lisinopril using concealed opaque envelopes, using a random permuted block design. A permuted block design was chosen to avoid imbalance in assignment to the study drugs over time. Random sequence was generated by a statistician using a computer program and study technicians opened these envelopes after confirming eligibility with the principal investigator." Comment: There was no reason to suspect that the statistician had knowledge of the forthcoming allocation. However, It was not reported if envelopes were numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from Agarwal 2014: "The Hypertension in Hemodialysis Patients Treated with Atenolol or Lisinopril (HDPAL) was a randomised, open‐label, parallel group, active control, single‐centre trial that compared the safety and efficacy of ACE‐inhibitor‐based therapy with β‐ blocker‐based treatment, each administered three times weekly after dialysis." Comment: An open‐label study is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from Agarwal 2016: "To accurately capture the adverse effects of atenolol and lisinopril in the HDPAL trial, we used a structured questionnaire." Quote from Agarwal 2014: "An independent data and safety monitoring board reviewed the safety data and the study progress on an annual basis." Comment: An independent data and safety monitoring board reviewed outcomes. The outcomes were assessed with an appropriate measure, without differences between groups (fatigue was reported as an adverse event). It was not stated if the independent data and safety monitoring was blinded to the treatment assigned. However, objective and subjective outcomes were assessed. It was not stated if the interviewer was blinded to the treatment allocation to evaluate fatigue |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from supplementary Figure 1 in Agaewal 2014: "Reasons for removal by PI: Four subjects refused to perform home blood pressure monitoring repeatedly, one had pain with home BP measurements and one was excluded following a stroke. Reasons for withdrawal of consent was as follows. Atenolol: not feeling well, light‐headed, BP too low, changed mind, wanted original medications, worry about BP, study medication made the subject sick Lisinopril: dizziness, headaches, high BP, fear of stroke, tired of taking BP, tired of participating (n= 4), no reason offered (n= 3), refused home BP monitoring, did not want study medication (n=2), wanted to go back on metoprolol." Comment: As reported in Figure 1, 58/100 in the intervention group 1 (Atenolol) and 46/100 in the intervention group 2 (Lisinopril) completed the study (> 5% lost to follow‐up, with differences between groups). Some reasons for discontinuations appeared to be related with the intervention |
| Selective reporting (reporting bias) | Low risk | Protocol was published. Fatigue was reported in accordance with a pre‐specified analysis plan, using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were reported. Fatigue was reported but not extractable |
| Other bias | High risk | Quote from Agarwal 2014: "We terminated the trial on the unanimous recommendation of the independent data safety monitoring board which found a clear signal for cardiovascular safety on an annual monitoring meeting after complete randomisation. At their annual meeting, the committee also noted that the lisinopril group experienced an increase in the following: all‐cause serious adverse events, all‐cause hospitalisation rates, hypertension and hyperkalaemia." Comment: There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors did not report conflicts of interest. However, the study was terminated early |
Huang 2021.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly ordered permuted blocks of four were computer generated." Comment: Computer generator is considered as low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed offsite by a research team. To prevent possible bias, the study researchers involved in the recruitment process and intervention did not conduct randomisation. Resulting in the code names in order were placed in prepared, sealed, opaque envelopes with a series of numbers, which were later drawn for group assignment by one study researcher." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Blinding participants of their group assignments were not feasible." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, other subjective outcome were reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 40/43 participants in the intervention group and 43/43 participants in the control group completed the study (> 5% loss to follow‐up). There were differences between treatment groups. Reason for discontinuation were provided |
| Selective reporting (reporting bias) | High risk | Protocol was published. Fatigue was reported using multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Information related to fatigue were not reported in sufficient detail to permit judgment. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and there were no conflicts of interest. No other source of bias were apparent |
Jalalian 2015.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Data were collected using demographic questionnaire, Rhoten Fatigue Scale and Kidney Disease Quality of Life Short Form (KDQOLSF)." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, other subjective outcome were reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study was not clearly stated. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Information related to fatigue were not reported in sufficient detail to permit judgment. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | No data were available to assess the possible imbalance between groups. Funding and conflicts of interest were not reported |
Johansen 1999.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote; "Randomisation was computer‐generated in block of 4." Comment: Computer‐generation is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Low risk | Quote: "Assignments were made sequentially by a research pharmacist who dispensed medication but was not otherwise involved in the study." Quote: "Esternal research pharmacist seemed to ensure allocation concealment. No imbalance between intervention groups was apparent." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Dialysis staff, patients, and investigators were blinded through the study to treatment assigned." Comment: A double‐blind trial is considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Quality of life was assessed by and instrument administered by personal interview." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "25 subjects completed the 6‐month protocol and 23 of these (12 in the nandrolone group and 11 in the placebo group) had all measurements made. Two subjects completed the study but were unable to have final measurements taken because of medical instability. Three subjects were withdrawn from the placebo group because of elevated transaminase, hematoma at the study drug injection site, ans sudden death. One subject in the nandrolone group was withdrawn after developing angina." Comment: 12/14 participants in the intervention group and 11/15 participants in the control group completed and reported all measurements of the study (> 5% lost to follow‐up, with differences between groups). Reasons for discontinuations seemed to be related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Protocol was approved by the Committee on Human Research at the University of California. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and conflicts of interest were not reported |
Johansen 2006.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Control group 1
Control group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned to treatment groups in a 1:1:1:1 manner by the research pharmacist using variable block sizes, which were not known to investigators until the completion of the study." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Low risk | Quote: "Nandrolone decanoate and a placebo that was identical in appearance to the active drug were prepared and supplied to the research pharmacy by Organon, Inc. (Roseland, NJ)." Quote: "Participants were randomly assigned to treatment groups in a 1:1:1:1 manner by the research pharmacist using variable block sizes, which were not known to investigators until the completion of the study." Comment: Esternal research pharmacist seemed to ensure allocation concealment. No imbalance between intervention groups was apparent |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Interventions included double‐blinded weekly nandrolone decanoate (100 mg for women; 200 mg for men) or placebo injections." Comment: Although author reported that the study used a double‐blind design, information about blinding of participants and investigators were not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Eighty haemodialysis patients were enrolled in the study, and 79 were randomly assigned. [...] Sixty‐eight patients completed the study. Reasons for non completion are shown in Figure 1. Six participants discontinued study drug (four who were receiving placebo and two who were receiving nandrolone) before the end of the treatment period, only two of whom discontinued all study participation. Therefore, results for the four patients who discontinued study drug but were still available for follow‐up measures are included in analyses. Those who received placebo discontinued because of an itchy reaction at the injection site, a nonspecific feeling that the drug was having adverse effects, abdominal pain and liver function test abnormalities, and discovery of a history of prostate cancer. Those who received nandrolone discontinued because of interference with sexual function (after five doses) and fear of possible adverse effects (after three doses)." Comment: 16/19 participants in the intervention group 1 (nandrolone), 16/20 participants in the intervention group 2 (nandrolone + lower extremity resistance exercise training), 17/20 participants in the control group 1 (placebo) and 19/20 participants in the control group 2 (placebo + lower extremity resistance exercise training) completed and reported all measurements of the study (> 5% lost to follow‐up, with differences between groups). Reasons for discontinuations seemed to be related to the treatment allocation. |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | High risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Pharmaceutical company who provided the drugs could influenced the data analysis and authors did not report conflicts of interest |
Kaplin Serin 2020.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The data were collected by the researchers through face‐to‐face interviews with the patients." Comment: Fatigue was assessed with an appropriate measure, without differences between groups. Objective measures were used. However, objective and subjective outcomes were assessed. It was not stated if the interviewer was blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there were no lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis |
Karadag 2019.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included into the analysis. There were no lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was not reported |
Konstadinidou‐ND 2002.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Intervention group 3
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Treadmill exercise test to fatigue endpoints. [...] To measure lactic acid, blood samples were taken from the right ear before and 4 min after the end of the exercise test. Lactic acid measurement was carried out in a photometer." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. Objective measures were used. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "However, during the study 5 patients from Group A, 1 from Group B, 2 from C voluntarily withdrew, while 1 patient from Group B and 1 from D died of causes unrelated to exercise. Finally, 48 patients on HD completed the entire study. [...] Group A had a higher dropout rate (23.8%) and the reasons were lack of time, transportation difficulties and medical reasons unrelated to exercise. The dropout rate in both Groups B and C was 16.7% and the reason for withdrawal was an acute illness." Comment: 16/21 participants in intervention group 1 (supervised aerobic training), 10/12 participants in intervention group 2 (supervised exercise program), 10/12 participants in intervention group 3 (unsupervised moderate exercise) and 12/13 participants in the control group (usual lifestyle) completed the study (> 5% lost to follow‐up, with differences between groups). Reasons for discontinuations seemed to be not related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported. |
| Other bias | Unclear risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding and conflicts of interest were not reported |
Krase 2022.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients enrolled by a research assistant assigned into the study while the order that the patients assigned to the first scenario was randomly using a computer random number generator." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients enrolled by a research assistant assigned into the study while the order that the patients assigned to the first scenario was randomly using a computer random number generator." Comment: Although it was not clear if research assistant was aware of treatment allocation, the use of computer seemed to prevent bias in allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Fatigue was not clearly reported. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21/24 participants in the intervention group and 23/24 participants in the control group completed the study (> 5% lost to follow‐up). There were differences between groups. Reasons for discontinuation were provided |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence data analysis and interpretation. No other source of bias were apparent |
Lazarus 2020.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "In a randomised double blind placebo controlled study." Comment: Although the study was reported as a double blind study, it was not reported if participants and investigators were blinded to the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. There was no lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported with multiple eligible outcome measurements (scales and time points). It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | Baseline characteristics were not clearly reported. There were neither funding nor conflict of interests |
Leski 1979.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Each patient was interrogated in a standardized fashion by the same person (Th. N.) during each dialysis concerning the preceding one. [...] The questionnaire was evaluated on a three‐point system, 0, +, + +, headache during and after dialysis, fatigue and leg cramps." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study was not clearly stated. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | No data were available to assess the possible imbalance between groups. Funding and conflicts of interest were not reported |
Li 2014b.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were assigned to the study or control group using fifty sets of computer‐generated random numbers." Comment: Computer generation is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The 160 patients who joined the study were randomly assigned to either the study or control group. There were 80 patients in each of the treatment arms. At week 12, 69 of the 80 (86.3%) study patients and 66 of the 80 (82.5%) controls had completed the follow‐up questionnaires. A total of 135 patients completed the protocol and were included in the analysis (Figure 1)." Comment: 69/80 participants in the intervention group and 66/80 participants in the control group completed the study (> 5% lost to follow‐up, without differences between groups). Reasons for discontinuations seemed to be not related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest. The study seemed to be free from other source of bias |
Lillevang 1990.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The design of the study was a double blinded, placebo‐controlled study with a duration of eights weeks." Comment: Although author reported that the study used a double‐blind design, information about blinding of participants and investigators were not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "In order to investigate the effect of the treatment methods on the patients’ quality of life, a structured interview was performed before and after the study, where the interviewer (the same person for all patients), based upon the patients answers given, calculated a score for the most common complaints that can be seen among haemodialysis patients. [...] Neither the patient, nor the interviewer, saw the results from week 0 during the week 8 interview." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "19 adult haemodialysis patients in stable phase. The study was sent to and accepted by the regional ethical research committee. One patient chose to not want to participate. [...] All patients in the EPO‐group completed their study. In the placebo group, three patients had to be excluded due to need of blood transfusion at week 3 (2) and week 5 (1)." Comment: 9/9 participants in the intervention group and 7/10 participants in the control group completed the study (> 5% lost to follow‐up, with differences between groups) |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | No sufficient data were available to assess the possible imbalance between groups. Funding and conflicts of interest were not reported |
Lin 2011.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions: not reported |
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Prior to the intervention process, the selected patients were randomly divided by computer into two groups." Comment: The study was a quasi‐experimental study. Computer generation is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | High risk | Method of allocation concealment was not reported in sufficient detail to permit judgement. However the study used a quasi‐experimental design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "To minimize participants’ misunderstanding of the Brief Fatigue Inventory‐Taiwan Form (BFI‐T), the data were collected mainly via a face‐to‐face survey interview. The participants were allowed to ask any questions about the study at any stage." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant/investigators beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Other outcomes were objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and were included into the analyses |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding and conflicts of interest were not reported |
Linde 2001.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from Furuland 2003: "This was a multicenter, randomised, open‐label trial in patients with renal anaemia." Comment: An open‐label study is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from Furuland 2003: "Thrombovascular events and vascular access thrombosis were recorded and categorized centrally by one coordinator based on a WHO classification." Comment: Some outcomes were recorded centrally (not sure that it was valid also for fatigue). The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | As reported in table 2, overall 73/180 participants in the intervention group and 83/164 participants in the control group completed the study (> 5% lost to follow‐up, with differences between groups). Some reasons for discontinuations (adverse events) seemed to be related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding and conflicts of interest were not reported |
Mohajeranirad 2021.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "In a randomised double blind placebo controlled study." Comment: Although the study was reported as a double blind study, it was not reported if participants and investigators were blinded to the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "54 participants were selected and randomly assigned into two groups: intervention and placebo. During the study, four patients dropped out of the interventional and placebo group due to personal reasons. Finally, 50 patients [interventional (n=25) and placebo (n=25)] completed the trial and included in the analysis." Comment: 25/27 participants in the intervention group and 25/27 participants in the control group completed the study (>5% lost to follow‐up). Reasons for discontinuation were not reported |
| Selective reporting (reporting bias) | High risk | Protocol was reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence data analysis and interpretation. No other source of bias were apparent |
Mohamed 2013.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants were randomised in an open‐label fashion." Comment: An open‐label study was considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "One patient withdrew in the third week from the higher DGC group." Comment: The number of patients who completed the study was not clearly stated. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Outcomes information were not reported in sufficient detail to permit judgment. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | No data were available to assess the possible imbalance between groups. Funding and conflicts of interest were not reported |
Mohamed 2014.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The patient assessment sheet was filled by the researcher through personal interview." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant/investigators beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed. It was not stated if the interviewer was blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Table 3 reported that all participants completed the study. However, it was not clearly stated if some participants discontinued |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding and conflicts of interest were not reported |
Mohammadpourhodki 2021.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Block randomisation." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Not blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant/investigators beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. There were no lost to‐follow‐up |
| Selective reporting (reporting bias) | High risk | Protocol was published. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. There was no source of funding or conflict of interests. No other source of bias were apparent |
Motedayen 2014.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The Fatigue Severity Scale (FSS) questionnaire was completed by the subjects prior to the study and at the end of the first and the second months." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Other objective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Initially, 75 patients were assigned to the experimental and control groups; nine patients were excluded from the study because of death, transplantation, transportation from the health centre, or refusing to do the exercises regularly due to fatigue, boredom, and sleeplessness on the night before dialysis. Therefore, the findings of the study were extracted from the information of two 33‐patient groups." Comment: Overall, 66/75 participants completed the study (>5% lost to follow‐up; possible differences between groups were not reported). Reasons for discontinuations seemed to be not related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and conflicts of interest was not reported |
Muz 2017.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Treatment group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random selection of samples was performed." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Visual Analogue Scale (VAS) score, Piper fatigue scale, and Pittsburgh Sleep Quality Index (PSQI) were determined via face‐to‐face interview and patient documents. In the first week (the first follow‐up), second week (second follow‐up), and third week (third follow‐up), Visual Analogue Scale (VAS) score and Piper fatigue scale were obtained by the researcher." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant/investigators beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Figure 1 reported the number of participants who did not complete the follow‐up. 27/41 participants in the intervention group and 35/39 participants in the control group completed the study (> 5% lost to follow‐up, with differences between groups) |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest. The study seemed to be free from other source of bias |
Ozdemir 2013.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by MedCalc software to give equal chance to each intervention group." Comment: Computer‐generation is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The data of the intervention and control groups were collected by using the questionnaire, Piper Fatigue Scale and Visual Analogue Scale (VAS)." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study was not clearly stated. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding and conflicts of interest were not reported |
Parfrey 2005.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from Foley 2008: "The study was centrally coordinated from St. John’s, Canada for Canadian patients and Manchester, England for European patients. Randomization was performed at the coordinating centres with an interactive voice randomisation telephone system using permuted blocks stratified by concurrent epoetin use and sex." Comment: The interactive voice system is likely to be a computer. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Low risk | Quote from Foley 2008: "The study was centrally coordinated from St. John’s, Canada for Canadian patients and Manchester, England for European patients. Randomization was performed at the coordinating centres with an interactive voice randomisation telephone system using permuted blocks stratified by concurrent epoetin use and sex." Comment: An interactive voice system is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from Foley 2009: "Patients and attending physicians were masked to treatment assignment. [...] Local investigators and the dialysis unit were also masked to treatment assignment." Quote from Parfley 2005: "A randomised, double‐blind design was used with patients and outcome assessors but not treating physicians, who were blinded to assigned haemoglobin target." Comment: A double‐blind trial is considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from Foley 2009: "Quality of life was assessed using the KDQoL questionnaire, with prespecified outcomes being Energy/Fatigue scores, and Quality of Social Interaction Scores." Quote from Foley 2009: "Independent Data Monitoring Committee Members." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting (not sure that the Independent Data Monitoring Committee Members assessed fatigue). Participant/investigators beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. It was not stated if the independent data monitoring was blinded to the treatment assigned. However, objective and subjective outcomes were assessed. It was not stated if the interviewer was blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from Parfley 2005: "324 (54%) patients remained in the study for 96 weeks, 160 (54%) in the higher and 164 (55%) in the lower target groups. The reasons for study exit— renal transplantation (n 133, 67 in the higher and 66 in the lower target group), adverse events (n 76, 39 and 37), patient choice (n 28, 9 and 19), loss to follow‐up (n 2, 1 and 1), and other (n 36, 21 and 15)—were similar in the two target groups." Comment: 164/300 participants in intervention group 1 (epoetin alfa to reach low target haemoglobin) and 160/296 participants in intervention group 2 (epoetin alfa to reach high target Hb) completed the study (> 5% lost to follow‐up, without differences between groups). Reasons for discontinuations (adverse events) seemed to be related to the treatment allocation. However, all outcomes have been reported on the ITT population |
| Selective reporting (reporting bias) | Low risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were reported |
| Other bias | High risk | Quote from Foley 2009: "Baseline characteristics were similar except for the older age of high target subjects (52.2 versus 49.4 years)." Comment: There was no substantial evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding (pharmaceutical company) could influence the data analysis and authors reported conflicts of interest |
PEDAL 2020.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from Greenword 2020: "Randomization was conducted via a centrally controlled web based randomisation system, run by the Glasgow Clinical Trials Unit (GCTU). To ensure balanced assignment across critical variables, a minimization algorithm was employed, taking into account baseline age, gender and diabetes status." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from Greenword 2020: " It was impossible to blind the ‘treating’ physiotherapy assistants or the participants, and thus the study implemented a blinded outcome assessment and analysis." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from Greenword 2020: "This was a prospective, pragmatic multicenter RCT with blinded outcome assessment." Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from Greenword 2021: "In total, the trial recruited 379 participants. A total of 335 participants attended a baseline study visit: 175 participants who were randomised to the exercise intervention and 160 participants who were randomised to usual care. Participants were informed of group allocation only after completing all baseline assessments. Fifty‐nine patients allocated to the exercise intervention and 60 participants allocated to usual care did not complete the 6‐month assessment. In total, seven participants died during the study: three participants from the intervention group and four participants from the usual‐care group. In the intervention group, 40 participants were withdrawn and 16 did not attend for the final 6‐month assessment. In the usual‐care group, 15 participants were withdrawn and 14 participants did not attend the 6‐month assessment." Comment: 116/175 participants in the intervention group and 127/160 participants in the control group (no intervention) completed the study (> 5% lost to follow‐up, with differences between groups). Reasons for discontinuations were reported |
| Selective reporting (reporting bias) | Low risk | Protocol was published. Fatigue was reported in accordance with a pre‐specified analysis plan, using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. However, objective and subjective outcomes were assessed. All outcomes that should be addressed (fatigue, CVD, and death) were reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest. The study seemed to be free from other source of bias |
Pellizzaro 2013.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was made by dividing the subjects into three blocks of 15 each, five in each group." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 45 patients initially included, six did not complete the study protocol due to non‐compliance (n = 5) or death (n = 1) and were not included in the analysis." Comment: 11/15 participants in the intervention group 1 (respiratory muscle training), 14/15 participants in the intervention group 2 (peripheral muscle training), and 14/15 participants in the control group (no treatment) completed the study (> 5% lost to follow‐up, with differences between groups). Reasons for discontinuations seemed to be not related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Protocol was published. Fatigue was reported in accordance with a pre‐specified analysis plan, using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. However, objective and subjective outcomes were assessed. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest. The study seemed to be free from other source of bias |
Picariello 2018.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was stratified by centre and randomly varying block sizes were used to maintain balance of numbers in each arm across the period of recruitment while maintaining allocation concealment. King’s College London’s Independent Randomisation Service was used. Because the randomisation sequence was automated in real time, the allocation sequence was concealed from researchers." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was stratified by centre and randomly varying block sizes were used to maintain balance of numbers in each arm across the period of recruitment while maintaining allocation concealment. King’s College London’s Independent Randomisation Service was used. Because the randomisation sequence was automated in real time, the allocation sequence was concealed from researchers." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The nature of the trial meant participants were unblinded to their allocations." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Follow‐up measures were completed independently by participants via post. An independent researcher, who was not involved in the intervention development or delivery, assisted seven participants with the completion of the follow‐up measures. The statistician (SN) remained blind to treatment allocation until after the analyses were conducted." Comment: Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed. It was not stated if the interviewer was blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Eighteen participants completed the follow‐up measures at T1." Comment: 11/12 participants in the intervention group and 7/12 participants in the control group completed the study (> 5% lost to follow‐up, with differences between groups). Reasons for discontinuations were not reported |
| Selective reporting (reporting bias) | High risk | Information about the protocol were reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of intervention was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | Quote: "The authors alone are responsible for the content and writing of the article." Comment: There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and reporting and authors had no conflicts of interest |
Raimann 2010.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Chronic haemodialysis patients participated in this randomised, single masked, controlled crossover trial. [...] Throughout the entire study, patients were masked to dialysate glucose levels" Comment: A single‐blind study is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. No patients were loss to follow‐up |
| Selective reporting (reporting bias) | High risk | The study protocol was approved by the Institutional Review Board of Beth Israel Medical Center. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis (cross‐over study: data related to the first period were not reported). All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | No data were available to assess the possible imbalance between groups. There was no funding and the authors did not have conflicts of interest |
Reilly‐Spong 2015.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from Reilly‐Sponge 2015: "Randomisation schedules were computer‐generated using SAS, and designed using small randomly permuted blocks to promote balance within strata across treatment arms." Comment: Computer‐generated is considered as low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote from Reilly‐Sponge 2015: "The randomisation schedule was generated by the study statistician who was masked with respect to variables other than stratification variables." Comment: The statistician should ensure concealment and it was assessed as low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from Gross 2017: "We conducted a randomised, active‐controlled, open‐label trial to test whether a Mindfulness‐based Stress Reduction (MBSR) program delivered in a novel workshop‐teleconference format would reduce symptoms and improve health‐related quality of life in patients awaiting kidney transplantation." Comment: An open‐label study is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from Gross 2017: "Participants completed self‐report questionnaires at baseline, post‐intervention, and after 6‐months." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study with GFR < 15 mL/min/1.73 m2 was not clearly stated. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and reporting and authors had no conflicts of interest |
Roshanravan 2016.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group 1
Control group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Patients filled the questionnaire when their dialysis has been completed and have been disconnected from the dialysis machine." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26/27 participants in the intervention group (foot reflexology), 25/27 participants in the control group 1 (sham) and 27/27 participants in the control group 2 (no treatment) completed the study (> 5% lost to follow‐up, with differences between groups). Reasons for discontinuations were not reported |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and conflicts of interest were not reported |
Sabouhi 2013.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group 1
Control group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After random subjects’ allocation through minimization method, 32 subjects were assigned to each group of the study, placebo and control." Comment: Minimization method is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported in sufficient detail to permit judgment. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study was not clearly stated. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and reporting and authors had no conflicts of interest. The study seemed to be free from other sources of bias |
Sajadi 2016.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The participants were allocated into 2 groups through simple random sampling method." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "In a double‐blinded cross‐over clinical trial, 46 participants were recruited from a haemodialysis unit in Iran." Comment: Although author reported that the study used a double‐blind design, information about blinding of participants and investigators were not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "A self‐reported questionnaire was used to collect data. [...] The researcher read and completed it for illiterate patients." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Other objective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study was not clearly stated for the first period. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis (cross‐over study: data related to the first period were not clearly reported). All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | Baseline characteristics between groups were not reported in sufficient detail. Funding was unlikely to influence the data analysis and reporting and authors had no conflicts of interest |
Salehi 2020.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, subjective and objective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20/27 participants in the intervention group and 17/27 participants in the control group completed the study (> 5% lost to follow‐up). There were differences between groups. Reasons for discontinuation were reported |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were reported. Fatigue was assessed using multiple eligible outcome measurements (scales and time points). Fatigue at the end of treatment was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. There was no source of funding or conflict of interests. No other source of bias were apparent |
Sang 1997.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Intervention group 3
Cointerventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The patients were blinded as to what sodium concentration was used in the dialysate. [...] There were eight protocol violations; two occurred during standard haemodialysis, one during linear ramping, and five during stepwise ramping. Data for the eight haemodialysis sessions were excluded in the analysis." Comment: Authors reported that patients were blinded. However, interventions were different and investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Six of the 29 patients did not complete the protocol and were not included in the analysis, apart from the reason for discontinuation. [...] Six patients stopped their treatments because of thirst. When they stopped, they were evenly distributed with two in each protocol." Comment: Overall, 23/29 participants completed the study (> 5% lost to follow‐up, difference between groups could not be assessed). Reasons for discontinuations seemed to be related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | Baseline characteristics between groups were not reported. Funding and conflicts of interest were not reported |
Schardong 2021.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization occurred through the www.random.org website." |
| Allocation concealment (selection bias) | Low risk | Quote: "The sequence of numbers was generated by a researcher “blinded” to the study, and it was kept confidential until the beginning of the intervention to guarantee the concealment of the allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All analyses were conducted by a researcher blind to the study procedures (randomisation, evaluations, and intervention)." Comment: Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, subjective and objective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Thirty‐six patients with CKF on HD were evaluated for eligibility and possible admission into the study. Twenty‐eight met the inclusion criteria and finalized the protocol." Comment: 14/17 participants in the intervention group and 14/16 participants in the control group completed the study (> 5% lost to follow‐up). There were differences between groups. Reasons for discontinuation were reported |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were reported. Fatigue was assessed using multiple eligible outcome measurements (scales and time points). Fatigue at the end of treatment was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence data analysis and interpretation. No other source of bias were apparent |
Schmitz 2016.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each patient was treated for 4 weeks with standard dialysate (standard phase) and 4 weeks with citrate dialysate (citrate phase) in the sequence determined by the computer‐generated randomisation scheme. A centralized fax randomisation in a 1:1 ratio with stratification for centre and dialysis modality was carried out." Comment: A computer‐generated randomisation scheme is considered as low risk of bias. No data were available to assess the possible imbalance between groups |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used (fatigue was assessed as an adverse event), it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 95 patients enrolled (HDF post‐dilution: 44, HDF pre‐dilution: 26, HD: 25), 7 terminated the study prematurely for reasons not associated with the study protocol, e.g. kidney transplantation or death due to an exacerbation of concomitant diseases. Three of them were completely excluded from the analysis because they were withdrawn before the first study treatment, so the full analysis set (FAS) constituted of 92 patients." Comment: Overall, 92/95 participants were reported in the analysis. However, Figure 1 showed that only 48/95 participants were assessed per protocol analysis. Tables 2, 3 and 5 reported data for 90 participants in the standard dialysate phase |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis (cross‐over study: data related to the first period were not clearly reported). All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | High risk | Baseline characteristics between groups were not reported. Funding (pharmaceutical company) could influence the data analysis and some authors had conflicts of interest |
Semeniuk 2000.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised using a table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind." Comment: Although author reported that the study used a double‐blind design, information about blinding of participants and investigators were not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue was assessed with an appropriate measure, without differences between groups. However, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported in sufficient detail at the end of the first phase to perform adjudication |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. It was not reported if fatigue was assessed using multiple eligible outcome measurements (scales and time points) were pre‐specified for the first period of the study. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis (cross‐over study: data related to the first period were not clearly reported). All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | High risk | Baseline characteristics between groups were not reported. Funding (pharmaceutical company) could influence the data analysis and interpretation |
Shahdadi 2016.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly divided into two groups." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The data collecting tool was included Individual demography and fatigue severity questionnaire." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants completed the study. However, it was not stated if some patients discontinued |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding and conflicts of interest were not reported |
Singer 2010.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was by computer‐generated random number with subjects stratified according to diabetic status and by the need for maintenance dialysis treatment." Comment: A computer‐generated randomisation scheme is considered as low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Study drugs were compounded and packaged by an external pharmacy." Comment: External pharmacy performed the allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study design was a prospective, single‐centre, double‐blind, randomised, placebo‐controlled trial. [...] Both the subjects and investigators were blinded as to allocation until after the final subject had completed the study, and all follow‐up data had been collected." Comment: A double‐blind trial is considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote; "The Kidney Dialysis Quality of Life‐Short Form (KDQOL‐SF) symptom and cognitive sub scales were administered either face to face or by telephone by a single research assistant." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Baseline ascorbate levels were not available in three subjects. This was due to mishandling of the samples in two subjects and one subject refusing venesection. A further one subject withdrew from the study after randomisation and collection of an ascorbate level, but before completing other baseline data. Data for all subjects were included until their exit from the study." Comment: As reported in Figure 1, 48/49 participants in the intervention group and 48/51 participants in the control group completed the follow‐up period. 49/49 participants in the intervention group and 49/51 participants in the control group completed the analysis. However, data on participants undergoing dialysis were not reported in sufficient detail to permit judgment |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors did not have conflicts of interest |
Singh 2003.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and investigators/participants could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | For fatigue, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study was not clearly stated. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis (cross‐over study: data related to the first period were not clearly reported). All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | Baseline characteristics between groups were not reported. Funding and conflicts of interest were not reported |
Singh 2008a.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned in a 1:1 ratio (simple block randomisation) to either ferumoxytol or placebo by using a telephone‐based system (ClinPhone Interactive Voice Response System, East Windsor, NJ)." Comment: The interactive voice systems could be considered as a computer |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned in a 1:1 ratio (simple block randomisation) to either ferumoxytol or placebo by using a telephone‐based system (ClinPhone Interactive Voice Response System, East Windsor, NJ)." Comment: Interactive system voice is considering as low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, investigators, and study coordinators were blinded, with the exception of 1 individual at each site designated the Test Article Administrator, who administered study treatments." Comment: A double‐blind trial is considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The blinded investigators and study coordinators, but not the Test Article Administrator, were involved in the assessment and attribution of adverse events. [...] All laboratory tests were performed at a central laboratory. [...] Relatedness of AEs to treatment was determined by the blinded site investigators. [...] Direct questioning of study patients regarding adverse events." Comment: Blinded investigators and study coordinators were involved in the assessment and attribution of adverse events (including fatigue) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study was not clearly stated. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis (cross‐over study: data related to the first period were not clearly reported). All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | High risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Government funding was unlikely to influence the data analysis but the pharmaceutical company could influence the data analysis and authors had conflicts of interest |
Sklar 1998.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were blinded with respect to the type of membrane used during all dialysis treatments throughout the study." Comment: Not reported if investigators were blind. However, interventions were different and investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Levels of post dialysis fatigue were determined by analysis of 6‐hour logs of sleep and perception of fatigue recorded by patients after each of these dialysis treatments. At the completion of the study, the patients submitted their log sheets to one of the investigators." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Other objective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Five patients were not included in the data analysis because they were individuals who destabilized medically (2) or submitted incomplete log sheets (3)." Comment: Overall, 16/21 participants completed the study (> 5% lost to follow‐up, difference between groups could not be assessed). Reasons for discontinuations seemed to be not related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis (cross‐over study: data related to the first period were not clearly reported). All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | Baseline characteristics between groups were not reported. Funding was unlikely to influence the data analysis and conflicts of interest were not reported |
Sklar 1999.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristic
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Intervention group 3
Intervention group 4
Control group 1
Control group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The order of procedures was determined from a random numbers table." Comment: Random numbers table is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The order of procedures was determined from a random numbers table and performed in single‐blinded fashion, with weight scales and dialysis machines hidden from the view of the patients." Comment: A single‐blind study is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Each patient recorded hourly fatigue scores during the entire study period on a fatigue intensity." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 17 patients entered onto the study, 5 patients dropped out early: 2 patients could not tolerate the dietary restrictions and 3 patients required surgical procedures, of which 1 patient died of complications. The remaining 12 patients were able to complete at least one of each type of treatment over the two cycles, with only 2 patients unable to undergo all procedures in the second cycle; 1 patient could not undergo the sham procedures because of progressive intolerance to fluid restrictions and 1 patient developed exacerbation of chronic obstructive lung disease and could not tolerate isolated diffusion and recirculation." Comment: Overall, 12/17 participants completed the study (> 5% lost to follow‐up, difference between groups could not be assessed). Some reasons for discontinuations seemed to be related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis (cross‐over study: data related to the first period were not clearly reported). All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | Baseline characteristics between groups were not reported. Funding was unlikely to influence the data analysis and conflicts of interests were not reported |
SOCIABLE 2017.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants |
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Cointerventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement. No imbalance between intervention groups was apparent |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from the study suggested by authors: "Single blind study". Comment: A single blind is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from the study suggested by authors: "Our outcome assessor was masked to randomisation assignment." Comment: Fatigue was not clearly reported, although the therapy helped people in addressing fatigue during their activities. However, subjective measures were used. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/12 participants completed the study (> 5% loss to follow‐up). Reasons were not provided |
| Selective reporting (reporting bias) | High risk | Information about the protocol were reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding did not influence the data analysis and authors did not have conflicts of interest |
Soliman 2015.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement. No imbalance between intervention groups was apparent |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and investigators/participants could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Fatigue was assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "40 met the inclusion criteria and agreed to participate within the proposed study, 10 patients were excluded from the study due to death, transplantation or refusing to try to do exercise regularly due to fatigue. Of those, 30 patients completed the study, 18 in experimental group and twelve in control group." Comment: 18/23 participants in the intervention group and 12/17 participants in the control group completed the study (> 5% lost to follow‐up). There were differences between groups. Reasons for discontinuation were provided |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was not reported |
Su 2009.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention clasification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomised sample of 69 patients block in 4 was originally chosen." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and investigators/participants could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "During the study, 3 patients from the experimental group and 5 from the control group left for undisclosed reasons. Hence, the final count was 31 patients for Far infrared ray therapy and 30 patients for heat pad therapy." Comment: 31/34 participants in the intervention group and 30/35 participants in the control group completed the study (> 5% lost to follow‐up, with difference between group). Reasons for discontinuations were reported |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, death and vascular access) were not reported |
| Other bias | Unclear risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding and conflicts of interest were not reported |
Suzuki 2018.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 29 HD patients were eligible for inclusion in the study and were randomly assigned to either the EMS or the control (no training) group by simple random allocation
(drawing lots)." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This was a prospective, open‐label, randomised controlled trial." Comment: An open‐label study is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes (including vitality) were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The EMS group included 14 men and 1 woman, while the control group included 13 men and 1 woman. Thirteen (86.7%) participants in the EMS group completed EMS training. The reasons for failure to complete training were hospitalisation before intervention (n51) and dropout due to discomfort of the wet electrode bands (n51). Likewise, 13 (92.9%) participants in the control group completed the protocol. One participant in the control group withdrew consent to join the study. The final analyses included 13 patients in each group." Comment: 13/15 participants in the intervention group and 13/14 participants in the control group completed the study (> 5% lost to follow‐up, with difference between group). Reasons for discontinuations were reported |
| Selective reporting (reporting bias) | High risk | Information about the protocol were reported. Vitality was reported using multiple eligible outcome measurements (scales, time points). Vitality was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors did not have conflicts of interest. No other source of bias were apparent |
SWIFT 2020.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation takes place, on a state‐by‐state basis using the method of minimisation as they agree to participate. The randomisation was stratified based upon location (state), metropolitan or regional, private or public unit or prior or current use of the IPOS‐Renal questionnaire for symptom monitoring within each centre and cluster size." |
| Allocation concealment (selection bias) | Low risk | Quote: "The trial statistician concealed until the site initiation visit. Access to the allocations is limited to the CI (RLM), the trial statistician (CB), the CTC trial operations coordinator (PW) and ANZDATA Registry Manager (Ms. Kylie Hurst) to minimise risk of inadvertently influencing sites or prematurely revealing allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Blinding to allocation is not possible within clusters due to the nature of the intervention; however, all staff compiling and analysing outcome data will be blinded to allocation." Comment: Interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Blinding to allocation is not possible within clusters due to the nature of the intervention; however, all staff compiling and analysing outcome data will be blinded to allocation." Comment: Fatigue was assessed with an appropriate measure. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Other subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported in sufficient detail to perform adjudication |
| Selective reporting (reporting bias) | High risk | Information about the protocol were reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | Quote: "The study sponsor and the study founders (Australian NHMRC, Kidney Health Australia), did not have any role or ultimate authority in study design; collection, management, analysis, interpretation of data, writing of the report, or the decision to submit the report for publication." Comment: Baseline characteristics were not reported. Funding did not influence the data analysis and authors did not have conflicts of interest |
Thomas 2017.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The interventionists randomised the participant codes to the intervention group or the control group, using a simple 1:1 computer‐generated sequence." Comment: A computer‐generated sequence is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and investigators/participants could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Participants completed questionnaires with an independent assessor who then assigned each of them an anonymous code.The interventionists, who were not involved in the recruitment process and patient assessment, randomised the participant codes to the intervention group or the control group, using a simple 1:1 computer‐generated sequence." [...] "This study was a randomised, controlled, assessor‐blinded trial conducted in an urban haemodialysis unit. Both the assessor and the statistical associate were blinded to randomisation allocation." Comment: Fatigue was assessed as an adverse event. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Other subjective outcomes were assessed. Not sure if the outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of missed sessions, 55% were due to logistic issues (switches in the location or time of assigned haemodialysis shifts) and 45% were due to refusals (most common reasons given were “too tired” or “too ill” on the given day). Five patients dropped out early in treatment (<2 sessions) for “feeling too medically ill” (n=1), “feeling already improved” (n=1), and “lack of interest” (n=3). One patient stopped after five sessions when they were transferred to home peritoneal dialysis therapy." Comment: 17/21 participants in the intervention group and 15/20 participants in the control group participants completed the study (> 5% lost to follow‐up, with difference between groups). Reasons for discontinuations seemed to be not related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Fatigue at the end of treatment was not reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | High risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding (pharmaceutical company) could influence the data analysis and authors did not have conflicts of interest |
Tsai 2016.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from Tsai 2016: "Participants were randomly and equally allocated to either the herbal acupoint therapy (HAT) or placebo group by computer‐generated randomisation." Quote from Tsai 2016 protocol: "Randomisation will be generated by a computerised random number function in Microsoft Excel, and the patients, programme assessors and statisticians will be unaware of the group to which they have been assigned. A block randomisation procedure (based on age, comorbidities such as cardiovascular disease and diabetes mellitus) will be employed to ensure that group allocation is equal and that the characteristics of the trial participants are similar." Comment: A computer‐generated sequence with random numbers is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from Tsai 2016: "All patients, program assessors, outcome assessors, and statisticians were blind to the group allocations until the end of the clinical trial." Comment: A double blind study is considered as low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from Tsai 2016: "Patient subjective assessments of the degree of fatigue and recovery time from fatigue after dialysis in both groups. [...] All patients, program assessors, outcome assessors, and statisticians were blind to the group allocations until the end of the clinical trial." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was stated that outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment that may have influenced reporting. However, objective and subjective outcomes were assessed. Overall the outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from Tsai 2016: "In all, 27 patients (84%) completed the entire study. [...] These patients were randomly divided into a group receiving HAT therapy (18 patients) and a group receiving sham‐HAT therapy (14 patients), and 5 patients (15.6%) dropped out before week 2. The remainder of the patients provided complete data at follow‐up." Comment: As reported in Figure 1, 14/18 participants in the intervention group and 13/14 participants in the control group participants completed the study (> 5% lost to follow‐up, with difference between groups). Reasons for discontinuations seemed to be not related to the treatment allocation (discontinuation for disease progression and withdrawal in the intervention group and withdrawal in the control group |
| Selective reporting (reporting bias) | High risk | Protocol was published and approved by the Institutional Review Board of Chang Gung Memorial Hospital. Fatigue was reported in accordance with a pre‐specified analysis plan, using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors did not have conflicts of interest. The study seemed to be free from other sources of bias |
Tsay 2004a.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group 1
Control group 2
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This prospective, randomised controlled trial with a pre‐test, post‐test design was carried out over a 6‐month period." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Other subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants completed the study. However, it was not stated if some participants discontinued |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and conflicts of interest was not reported |
Tsay 2004b.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was a randomised controlled trial." Comment: Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Pre‐dialysis fatigue was assessed routinely by asking patients to rate their perception of fatigue using a rating of 0 to 10, 0 indicating no fatigue and 10 indicating severe fatigue." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Other subjective outcomes were reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "108 patients agreed and consented to the study. One hundred and six patients completed the study. Two patients were dropped over the 1‐month intervention: 1 in the acupressure group and 1 in the control group. One patient was lost for medical reasons, while the other patient relocated." Comment: 35/36 participants in the intervention group 1 (acupressure), 36/36 participants in the intervention group 2 (Transcutaneous Electrical Acupoint Stimulation) and 35/36 participants in the control group (routine unit care) completed the study (< 5% lost to follow‐up, without difference between groups). Reasons for discontinuations seemed to be not related to the treatment allocation |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and conflicts of interest was not reported |
Unal 2016.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The Visual Analogue Scale (VAS) for Fatigue and the Pittsburg Sleep Quality Index (PSQI) were administered to the patients as a pretest immediately before they were taken to haemodialysis." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Other subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "From the 110 patients, a total of 105 patients (35 patients per group) reached the end of the study, with one patient in the foot reflexology group and two patients in the back massage group having withdrawn from the study, and two patients in the control group having left the dialysis centre." Comment: 35/36 participants in the intervention group 1 (foot reflexology), 35/37 participants in the intervention group 2 (back massage) and 35/37 participants in the control group (control) completed the study (> 5% lost to follow‐up, with difference between groups) |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Low risk | There was no evidence of difference in the baseline characteristics, or different non‐randomised co‐interventions between groups. Funding was unlikely to influence the data analysis and authors had no conflicts of interest. The study seemed to be free from other sources of bias |
Varaei 2020.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group 1
Intervention group 2
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This was a three‐group single‐blind randomised controlled trial." Comment: A single blind study is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The biostatistician who analysed the study data was blind to the interventions." Comment: Fatigue assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Other subjective outcomes were assessed. However, the outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and there was no lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Information about the protocol were reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | Baseline characteristics were not clearly reported. Funding was unlikely to influence the data analysis and authors had no conflicts of interest |
VENOUS 2020.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It was unclear if fatigue was assessed with an appropriate measure. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. Other subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "11 patients in the NF group and 10 patients in the PS group were dropped out from the study. The reasons of the discontinuation were hypoalbuminaemia (1), increased bete‐2 microglobulin, social reasons (2), dead (1), unknown reason (5) in NF group, and modality change (2), unknown reason (8). Finally, 15 patients in NF and 14 patients terminated the study, however, 2 patients with the data deficit in each group were excluded." |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported in a format that was not extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | No data were available to assess the possible imbalance between groups. Funding and conflicts of interest were not reported |
Vishnevskii 2014.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, interventions were different and participants and/or investigators could be aware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. The outcomes were assessed with an appropriate measure, without differences between groups. It was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients who completed the study was not clearly stated. It was unclear if there was evidence that the results were not biased by missing outcome data |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. It was not reported if multiple eligible outcome measurements (scales and time points) were pre‐specified. It was unclear if the reported approach to analysing this outcome was pre‐specified or influenced by the results. Outcomes information were not reported in sufficient detail to permit judgment. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | No data were available to assess the possible imbalance between groups. Funding and conflicts of interest were not reported |
Yurtkuran 2007.
| Study characteristics | ||
| Methods | Study design
Study dates
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention classification
Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Addtional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In the single‐blind study, simple randomisation was done by a physician using a computer‐generated table of random numbers, and 40 participants were allocated to two groups." Comment: A computer‐generated table of random numbers is considered as low risk of bias. No imbalance between intervention groups was apparent |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The procedure was concealed from the evaluating physician." Comment: Method of allocation concealment was not reported in sufficient detail to permit judgement. No imbalance between intervention groups was apparent |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "In the single‐blind study, simple randomisation was done by a physician using a computer‐generated table of random numbers, and 40 participants were allocated to two groups." Comment: A single‐blind study is considered as high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Clinical and laboratory variables were evaluated in the intervention and control groups. The physician who did the examination was blind to the allocation." Comment: The outcomes were assessed with an appropriate measure, without differences between groups. However, subjective measures were used, it was not stated whether outcomes were assessed without knowledge of treatment allocation, and knowledge of treatment assignment may have influenced reporting. Participant/investigators beliefs about the superiority/inferiority of either intervention could have influenced their assessment of the outcome, but there was no evidence that this was likely. However, objective and subjective outcomes were assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Three of the 40 patients who met the inclusion criteria were dropped, as they missed three sessions in a 3‐month‐period and adhered poorly to the exercise instructions. Thus, 19 patients in the exercise group and 18 patients in the control group were left." Comment: 19/20 participants in the intervention group and 18/20 participants in the control group completed the study (> 5% lost to follow‐up, with differences between groups). Reasons for discontinuations were not reported |
| Selective reporting (reporting bias) | High risk | Information about the protocol and the statistical analysis plan were not reported. Fatigue was reported using multiple eligible outcome measurements (scales, time points). Fatigue was reported in a format that was extractable for meta‐analysis. All outcomes that should be addressed (fatigue, cardiovascular disease, and death) were not reported |
| Other bias | Unclear risk | There was no evidence of different baseline characteristics, or different non‐randomised co‐interventions between groups. Funding and conflicts of interest were not reported |
6MWT: 6‐minute walk test; ACTH: adrenocorticotropic hormone; ADL: activity of daily living; AKI: acute kidney injury; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; BFI: Brief Fatigue Inventory; BMI: body mass index; BP: blood pressure; BUN: blood urea nitrogen; CBT: Cognitive Behavioural Therapy; CERA: Continuous Erythropoietin Receptor Activator; CES‐D: Center for Epidemiologic Studies Depression Scale; CFQ: Chalder Fatigue Questionnaire; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CrCl: creatinine clearance; CRP: C‐reactive protein; CVC: central venous catheter; CVD: cardiovascular disease; COPM: DBP: diastolic blood pressure; DM: diabetes mellitus; DSM: Diagnostic and Statistical Manual of Mental Disorders; ECG: electrocardiogram; eGFR: estimated glomerular filtration rate; EPO: erythropoietin; EQ‐5D (‐5L): Euro‐Qol 5‐dimensions (5‐level); EAS: erythropoiesis‐stimulating agents; ESKD: end‐stage kidney disease; FACIT: Functional Assessment of Chronic Illness Therapy; FIBSER: Frequency, Intensity and Burden of Side Effects Rating Scale; FSS: Fatigue Severity Scale; GAD‐7: Generalized Anxiety Disorder; GI: gastrointestinal; GOT: glutamic oxaloacetic transaminase; GPT: glutamic pyruvic transaminase; HADS: Hospital Anxiety and Depression Scale; Hb: haemoglobin; HCT: haematocrit; HD: haemodialysis; HDL: high‐density lipoprotein; HFS: haemodialysis fatique scale; HIV: human immunodeficiency virus; HRQoL: Health‐related quality of life; IFS: Iowa Fatigue Scale; IL: interleukin; IM: intramuscular injection; IPOS‐Renal: Integrated Palliative Outcome Scale‐Renal; IQR: interquartile range; ItchyQoL: QoL questionnaire fo patients with pruritus; ITT: intention to treat; IV: intravenous; KDOQI: Kidney Disease Outcomes Quality Initiative; KDQ: kidney disease questionnaire; KDQoL(SF): Kidney Disease Quality of life (Short Form); Kt/V: dialyser urea clearance adequacy; L‐DOPS: L‐threo‐3,4‐dihydroxyphenylserine; LDH: lactate dehydrogenase;; LEVIL: London Evaluation of Illness; LDL‐ low‐density lipoprotein; LV: left ventricular; M/F: male/female; MAAS: Mindful Attention Awareness Scale; MADSR: Montgomery–Asberg Depression Rating Scale; MAP: mean arterial pressure; MBSR: Mindfulness Based Stress Reduction; MFI‐20: Multidimensional fatigue inventory; MFIS: Modified Fatigue Impact Scale; MI: myocardial infarction; MINI: Mini International Neuropsychiatric Interview; Mini‐Cog: mini cognitive; MIP: maximal inspiratory pressure; MRI: magnetic resonance imaging; NRS: numerical rating scale; NYHA: New York Heart Association; OSA: obstructive sleep apnea; PAL: physical activity log; PD: peritoneal dialysis; PEP: Personal Energy Planning; PFS: Piper Fatigue Scale; PHQ‐9: Patient Health Questionnaire‐9; PSQI: Pittsburgh Sleep Quality Index; PTH: parathyroid hormone; QIDS‐16: Quick Inventory of Depression Symptomatology; QoL: quality of life; RCT: randomised controlled trial; rHuEPO: recombinant human erythropoietin; RNLI: Reintegration to Normal Living Index; SBP: systolic blood pressure; SC: subcutaneous; SCr: serum creatinine; SD: standard deviation; SF‐8: 8‐item Short Form Health Survey; SF‐12: 12‐item Short Form Health Survey; SF‐36: 36‐Item Short Form Health Survey; SIP: sickness impact profile; SMMT: Standardized Mini Mental Test; SNAG: Simplified Nutritional Appetite Questionnaire; SNRI: serotonin‐norepinephrine reuptake inhibitor; SONG: Standardised Outcomes in Nephrology; SSRI: selective serotonin reuptake inhibitor; STAI: State‐Trait Anxiety Inventory; TEAS: Trans Cutaneous Electrical Acupoint Stimulation; TSAT: transferrin saturation; UR: ultrafiltration rate; URR: urea reduction ratio; VAS: visual analogue scale; WHOQOL‐BREF: WHO quality of life ‐ brief form
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| CHAIR 2015 | Fatigue was not a primary or secondary outcome (chair stand exercise versus passive stretch exercise) |
| Churchill 1987 | Fatigue was not a primary or secondary outcome (dialysis reuse versus single use) |
| Dashti‐Khavidaki 2011 | Fatigue was not a primary or secondary outcome (clonazepam versus zolpidem) |
| Eglence 2013 | Not RCT: all participants in the intervention group came from a Turkish HD centre, and all participants in the control group came from another Turkish HD centre |
| Gram 1998 | Fatigue was not a primary or secondary outcome (growth hormone versus placebo) |
| Heshmati Far 2015 | Fatigue was not a primary or secondary outcome (Benson relaxation technique versus control) |
| Heshmatifar 2015 | Fatigue was not a primary or secondary outcome (Benson relaxation technique versus usual care) |
| Laupacis 1992 | Not RCT |
| Macagnan 2019 | Fatigue was not a primary or secondary outcome (photo‐biomodulation therapy versus placebo) |
| Nakamoto 2008 | Fatigue was not a primary or secondary outcome (Juzen‐taiho‐to (TJ‐48) versus placebo) |
| Sharp 2005 | Fatigue was not a primary or secondary outcome (immediate CBT versus deferred‐treatment) |
| Shimizu 1983 | Fatigue was not a primary or secondary outcome (high sodium + bicarbonate concentrate group versus high sodium + acetate concentrate group versus low sodium + bicarbonate concentrate group versus low sodium + acetate concentrate) |
| Siami 1991 | Fatigue was not a primary or secondary outcome (IV L‐carnitine versus placebo) |
| Tawney 2000 | Fatigue was not a primary or secondary outcome (physical rehabilitation program versus usual care) |
| TREAT 2005 | Wrong population: CKD patients who not required dialysis |
| Tsai 2015 | Fatigue was not a primary or secondary outcome (nurse‐led breathing training program versus waiting list) |
CBT: cognitive behavioural therapy; CKD: chronic kidney disease; HD: haemodialysis; IV: intravenous; RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
NCT00440869.
| Methods |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Notes | Additional information
|
DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; HD: haemodialysis; IV: intravenous; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12617000420347.
| Study name | Evaluating the effectiveness of practicing yoga during haemodialysis for fatigue in patients with end stage kidney disease |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group
Control group
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Starting date | July 2017 |
| Contact information | Kylie Barr Phone: +61 409 992 262 Email: k.barr@westernsydney.edu.au |
| Notes | ClinicalTrials.gov Identifier: ACTRN12617000420347. Funding: none. Recruitment status: completed |
ACTRN12618000724279.
| Study name | Evaluation of the effectiveness of home‐base physical training in patients undergoing haemodialysis |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group
Control group
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Starting date | August 2015 |
| Contact information | Katarzyna Chojak‐Fijalka Phone: +48 683 11 24 Email: katarzyna.chojak@awf.krakow.pl |
| Notes | ClinicalTrials.gov Identifier: ACTRN12618000724279. Funding: University of Physical Education in Cracow. Recruitment status: completed |
ACTRN12620000408987.
| Study name | Structured exercise prograM to reduce Fatigue In patients receiving dialysis: a preference‐stratified adaptive Trial (M‐FIT) |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group 1
Intervention group 2
Intervention group 3
Control group
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Starting date | March 2020 |
| Contact information | Allison Jaure Phone: +61 2 9845 1467 Email: allison.jaure@sydney.edu.au |
| Notes | ClinicalTrials.gov Identifier: ACTRN12620000408987. Funding: Australian Department of Health. Recruitment status: Not yet recruiting |
Burrai 2019a.
| Study name | Effects of virtual reality in patients undergoing dialysis study protocol |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group
Control group
|
| Outcomes | Planned outcomes
|
| Starting date | Not reported |
| Contact information | Francesco Burrai Phone: not reported Email: francesco.burrai@atssardegna.it |
| Notes | ClinicalTrials.gov Identifier: not reported. Funding: not reported. Recruitment status: not reported |
Cardoso 2019.
| Study name | Effects of continuous moderate exercise with partial blood flow restriction during hemodialysis: A protocol for a randomized clinical trial |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group 1
Intervention group 2
Control group
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Starting date | Not reported |
| Contact information | Rodrigo Kohn Cardoso Phone: not reported Email: rafaelorcy@gmail.com |
| Notes | ClinicalTrials.gov Identifier: RBR‐8T2P2M. Funding: not reported. Conflict of interests: none. Recruitment status: not reported |
CONVINCE 2020.
| Study name | Benefits and harms of high‐dose haemodiafiltration versus high‐flux haemodialysis: the comparison of high‐dose haemodiafiltration with high‐flux haemodialysis (CONVINCE) trial protocol |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group 1
Intervention group 2
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Starting date | Not reported |
| Contact information | Peter J Blankestijn Phone: not reported Email: P.J.Blankestijn@umcutrecht.nl |
| Notes | Netherlands National Trial Register (NTR 7138). Funding: European Union's Horizon 2020 research and innovation programme under grant agreement No 754803. Recruitment status: recruiting |
CTRI/2018/02/012021.
| Study name | The effectiveness of Intradialytic exercise on fatigue and quality of sleep among patients undergoing hemodialysis |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group
Control group
|
| Outcomes | Planned outcomes
|
| Starting date | Not reported. The trial was posted on 2018 |
| Contact information | PD Rai Phone: not reported Email: not reported |
| Notes | Only RIS.txt file was available to extract data. ClinicalTrials.gov Identifier: CTRI/2018/02/012021. Funding: not reported. Recruitment status: not reported |
Hamad 2021.
| Study name | Effect of plantar electrical nerve stimulation during routine hemodialysis process on the daily physical activity in adults with diabetes and end stage renal disease‐a randomized double blinded controlled trial |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group
Control group
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Starting date | Not reported |
| Contact information | Mishra, R. K. Phone: not reported Email: not reported |
| Notes | ClinicalTrials.gov Identifier: not reported. Funding: not reported. Fatigue was not clearly reported and this study would be evaluated in the following update |
NCT01620580.
| Study name | Symptom management program for hemodialysis patients |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group
Control group
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Starting date | September 2011 |
| Contact information | Francess V Danquah Phone: not reported Email: not reported |
| Notes | ClinicalTrials.gov Identifier: NCT01620580. Funding: The University of Texas Health Science Center, Houston. Recruitment status: completed |
NCT02361268.
| Study name | End‐Stage Renal Disease Intra‐dialysis Lifestyle Education study (END‐IDLE) |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group 1
Intervention group 2
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Starting date | July 2015 |
| Contact information | Gurjeet S Birdee Phone: not reported Email: not reported |
| Notes | ClinicalTrials.gov Identifier: NCT02361268. Funding: Vanderbilt University Medical Center and National Center for Complementary and Integrative Health (NCCIH). Recruitment status: completed |
Quintiliano 2019.
| Study name | Transcranial direct current stimulation in management of pain, mood, functionality, and quality of life in patients undergoing hemodialysis: a study protocol for a double‐blind controlled randomized trial |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group 1
Intervention group 2
Control group
Co‐interventions Not reported |
| Outcomes | Planned outcomes
|
| Starting date | Not reported. Trial was registered on 2018 |
| Contact information | Artur Quintiliano Phone: not reported Email: artur_bezerra@hotmail.com |
| Notes | Brazilian Clinical Trials Registry/Registro Brasileiro de Ensaios Clínicos (ensaiosclinicos.gov.br), 1111–1216‐0137. Funding: funded by the authors. Recruitment status: not reported |
Sharma 2022.
| Study name | Energy conservation education intervention for people with end‐stage kidney disease receiving haemodialysis (EVEREST): protocol for a cluster randomised control trial |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group
Control group
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Starting date | Not reported |
| Contact information | Sita Sharma Phone: not reported Email: sita.sharma@griffithuni.edu.au |
| Notes | Trials registration number NCT04360408. Funding: none. Recruitment status: not reported |
SLEEP‐HD 2021.
| Study name | Tailoring of cognitive behavior therapy for insomnia for patients with kidney failure undergoing hemodialysis: The sleep‐HD study |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group 1
Intervention group 2
Placebo group
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Starting date | Not reported |
| Contact information | McCurry, S. Phone: not reported Email: not reported |
| Notes | Trials registration number not reported. Funding: not reported. Recruitment status: not reported |
TACcare 2018.
| Study name | Rationale and design of technology assisted stepped collaborative care intervention to improve patient‐centered outcomes in hemodialysis patients (TACcare trial) |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group
Control group
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Starting date | February 2018 |
| Contact information | Manisha Jhamb Phone: 412‐647‐7062 Email: jhambm@upmc.edu |
| Notes | Trial Registeration Numbrer: NCT03440853. Funding: University of Pittsburgh. Recruitment status: recruiting |
van der Borg 2016.
| Study name | Protocol of a mixed method, randomized controlled study to assess the efficacy of a psychosocial intervention to reduce fatigue in patients with End‐Stage Renal Disease (ESRD) |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group
Control group
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Starting date | Not reported. Trial was registered on August 2015 |
| Contact information | Wieke E. van der Borg Phone: not reported Email: not reported |
| Notes | The Netherlands National Trial Register (NTR): NTR5366. Funding: Dutch Kidney Foundation. Recruitment status: not reported |
van der Veen 2021.
| Study name | A clinical approach of intradialytic creatine supplementation in dialysis‐dependent CKD patients: a rationale and study design |
| Methods | Study design
|
| Participants | Study characteristics
|
| Interventions | Intervention group 1
Intervention group 2
Intervention group 3
Intervention group 4
Placebo group
Co‐interventions
|
| Outcomes | Planned outcomes
|
| Starting date | Not reported |
| Contact information | Van Der Veen, Y. Phone: not reported Email: not reported |
| Notes | Trial Registeration Numbrer: not reported. Funding: not reported. Recruitment status: not reported |
6‐minute walking test; AIDS: acquired immunodeficiency syndrome; ALP: alkaline phosphatase; BDI: Beck Depression Inventory; BFI: Brief Fatigue Inventory; BIA: Bioelectrical Impedance Analysis; BMI: body mass index; BP: blood pressure; BPI: Brief Pain Inventory; CBT: Cognitive‐behavior therapy; CEQ: Credibility Expectancy Questionnaire; CFQ: Chalder Fatigue Questionnair; CIS: Checklist Individual Strength; CKD: chronic kidney disease; CRP: C‐reactive protein; DBP: diastolic blood pressure; ESA: erythropoiesis stimulating agents; ESKD: end‐stage kidney disease; FACIT: Functional Assessment of Chronic Illness Therapy; HADS: Hospital Anxiety and Depression Scale; Hb: haemoglobin; HCT: haematocrit; HDF: haemodiafiltration; HRQoL: health‐related quality of life; IPAQ: International Physical Activity Questionnaire; KDQoL‐SF: Kidney Disease and Quality of Life Short form; Kt/V: dialyser urea clearance adequacy; MAQ: Medication Adherence Questionnaire; PD: peritoneal dialysis; PHQ‐9: Patient Health Questionnaire‐9; PSQI: Pittsburgh Sleep Quality Index; PTH: parathyroid hormone; QoL: quality of life; RBC: red blood cells; RCT: randomised controlled trial; SBP: systolic blood pressure; SF‐36: 36‐Item Short Form Health Survey; SONG‐HD: Standard Outcomes in Nephrology‐Haemodialysis; TSAT: transferrin saturation; VAS: visual analogue scale
Differences between protocol and review
We have clarified our objectives as follows: "This review aims to evaluate the effects of any pharmacological and non‐pharmacological interventions on fatigue in people with chronic kidney disease requiring dialysis, such as haemodialysis and peritoneal dialysis, including any setting (e.g. dialysis performed in the clinic or at home) and frequency."
We have clarified the inclusion criteria of the population of interest as follows: "Patients of any age with ESKD on any form of dialysis. The dialysis treatment could be performed both in the clinic and at home. Any frequency of the dialysis treatment was included."
We have clarified our interventions as follows: "We considered any intervention affecting levels of self‐reported fatigue in patients on dialysis." In addition, we have added hypoxia‐inducible factors in the type of pharmacological interventions.
We have added sleep and mood to the secondary outcomes.
Contributions of authors
Draft the protocol: Angela Ju, Allison Jaure, Valeria Saglimbene, Mark Unruh, Jonathan Craig, Giovanni Strippoli
Study selection: Patrizia Natale, Angela Ju, Valeria Saglimbene
Extract data from studies: Patrizia Natale, Valeria Saglimbene
Enter data into RevMan: Patrizia Natale
Carry out the analysis: Patrizia Natale, Allison Jaure
Interpret the analysis: Patrizia Natale, Angela Ju, Giovanni Strippoli, Jonathan Craig, Valeria Saglibene, Mark Unruh, Giovanni Stallone, Allison Jaure
Draft the final review: Patrizia Natale, Allison Jaure
Disagreement resolution: Allison Jaure, Jonathan Craig, Giovanni Strippoli
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
Patrizia Natale: no relevant interests were disclosed
Angela Ju: no relevant interests were disclosed
Giovanni FM Strippoli: no relevant interests were disclosed
Jonathan C Craig: no relevant interests were disclosed
Valeria M Saglimbene: no relevant interests were disclosed
Mark L Unruh: no relevant interests were disclosed
Giovanni Stallone: no relevant interests were disclosed
Allison Jaure: no relevant interests were disclosed
New
References
References to studies included in this review
Ahmady 2019 {published data only}
- Ahmady S, Rezaei M, Khatony A. Comparing effects of aromatherapy with lavender essential oil and orange essential oil on fatigue of hemodialysis patients: A randomized trial. Complementary Therapies in Clinical Practice 2019;36:64-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Akizawa 2002 {published data only}
- Akizawa T, Koshikawa S, Iida N, Marumo F, Akiba T, Kawaguchi Y, et al. Clinical effects of L-threo-3,4-dihydroxyphenylserine on orthostatic hypotension in hemodialysis patients. Nephron 2002;90(4):384-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Akizawa T, Koshikawa S, Iida Y, Marumo F, Kawaguchi Y, Shirai D, et al. Clinical effect of l-threo-3,4-dihydroxyprenylserine (DOPS) on orthostatic hypotension in haemodialysis patients. DOPS Study Group [abstract]. Nephrology Dialysis Transplantation 1996;11(6):A218. [CENTRAL: CN-00261299] [Google Scholar]
Amini 2016 {published data only}
- Amini E, Goudarzi I, Masoudi R, Ahmadi A, Momeni A. Effect of progressive muscle relaxation and aerobic exercise on anxiety, sleep quality, and fatigue in patients with chronic renal failure undergoing hemodialysis. International Journal of Pharmaceutical & Clinical Research 2016;8(12):1634-9. [EMBASE: 614181031] [Google Scholar]
ASCEND 2016 {published data only}
- Cukor D, Rue T, Unruh ML, Heagerty PJ, Cohen SD, Dember LM, et al. Patient treatment adherence in the ASCEND trial for depression in patients undergoing maintenance hemodialysis [abstract no: FR-PO429]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):549. [EMBASE: 633768241] [Google Scholar]
- Hedayati SS, Daniel DM, Cohen S, Comstock B, Cukor D, Diaz-Linhart Y, et al. Rationale and design of a trial of sertraline vs. cognitive behavioral therapy for end-stage renal disease patients with depression (ASCEND). Contemporary Clinical Trials 2016;47:1-11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra R, Cukor D, Unruh M, Rue T, Heagerty P, Cohen SD, et al. Comparative efficacy of therapies for treatment of depression for patients undergoing maintenance hemodialysis: a randomized clinical trial. Annals of Internal Medicine 2019;170(6):369-79. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mehrotra R, Cukor D, Unruh ML, Rue T, Heagerty PJ, Cohen SD, et al. Comparative efficacy of therapies for depression for patients undergoing hemodialysis [abstract no: FR-OR148]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):B3. [Google Scholar]
ASSertID 2015 {published data only}06146268
- Chilcot J, Almond MK, Guirguis A, Friedli K, Day C, Davenport A, et al. Self-reported depression symptoms in haemodialysis patients: Bi-factor structures of two common measures and their association with clinical factors. General Hospital Psychiatry 2018;54:31-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chilcot J, Guirguis A, Friedli K, Almond M, Davenport A, Day C, et al. Measuring fatigue using the multidimensional fatigue inventory-20: a questionable factor structure in haemodialysis patients. Nephron 2017;136(2):121-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chilcot J, Guirguis A, Friedli K, Almond M, Day C, Da Silva-Gane M, et al. Depression symptoms in haemodialysis patients predict all-cause mortality but not kidney transplantation: a cause-specific outcome analysis. Annals of Behavioral Medicine 2018;52(1):1-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedli K, Almond M, Day C, Chilcot J, da Silva Gane M, Davenport A, et al. A study of sertraline in dialysis (ASSertID): a protocol for a pilot randomised controlled trial of drug treatment for depression in patients undergoing haemodialysis. BMC Nephrology 2015;16:172. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedli K, Guirguis A, Almond M, Day C, Chilcot J, Da Silva-Gane M, et al. Sertraline versus placebo in patients with major depressive disorder undergoing hemodialysis: a randomized, controlled feasibility trial. Clinical Journal of the American Society of Nephrology: CJASN 2017;12(2):280-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirguis A, Almond MK, Chilcot J, Davenport A, Day C, Fineberg N, et al. The ASSertID study:feasibility randomised controlled trial of drug treatment for depression in patients on hemodialysis [abstract no: SA-PO1118]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):B10. [Google Scholar]
BA16285 2007 {published data only}
- Besarab A, Beyer U, Dougherty FC. Long-term intravenous CERA (Continuous Erythropoietin Receptor Activator) maintains hemoglobin concentrations in hemodialysis patients [abstract no: W-PO40127]. Nephrology 2005;10(Suppl 1):A312-3. [CENTRAL: CN-00747326] [Google Scholar]
- Besarab A, Salifu MO, Lunde NM, Bansal V, Fishbane S, Dougherty FC, et al. Efficacy and tolerability of intravenous continuous erythropoietin receptor activator: a 19-week, phase II, multicenter, randomized, open-label, dose-finding study with a 12-month extension phase in patients with chronic renal disease. Clinical Therapeutics 2007;29(4):626-39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dougherty FC, Beyer U. No changes in blood pressure in dialysis patients after 12 months of treatment with IV/SC CERA (Continuous Erythropoietin Receptor Activator) [abstract no: W-PO40131]. Nephrology 2005;10(Suppl 1):A313-4. [CENTRAL: CN-00602138] [Google Scholar]
- Dougherty FC, Beyer U. Safety and tolerability profile of continuous erythropoietin receptor activator (CERA) with extended dosing intervals in patients with chronic kidney disease on dialysis [abstract no: W-PO40130]. Nephrology 2005;10(Suppl 1):A313. [CENTRAL: CN-00602137] [Google Scholar]
- Dougherty FC, Loghman-Adham M, Schultze N, Beyer U. Adequate hemoglobin levels are maintained with continuous erythropoietin receptor activator (CERA) in dialysis patients regardless of gender, age, race and diabetic status [abstract no: MP206]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v269. [CENTRAL: CN-00602143] [Google Scholar]
- Dutka P, Tilocca P, BA16285 and BA16286 Study Groups. CERA (Continuous Erythropoietin Receptor Activator) maintains stable hemoglobin concentrations in dialysis patients irrespective of gender, age, race or diabetic status [abstract]. Nephrology Nursing Journal 2006;33(2):138. [CENTRAL: CN-00602144] [Google Scholar]
- Salifu M, Villa G, Dougherty FC. Adequate hemoglobin levels are maintained with continuous erythropoietin receptor activator (CERA) in dialysis patients with different ranges of iron status and pre-existing conditions [abstract no: 138]. American Journal of Kidney Diseases 2006;47(4):A53. [CENTRAL: CN-00602141] [Google Scholar]
Babamohammadi 2006 {published data only}
- Babamohammadi H, Khalili H. Effect of a confined program of home-care on the health status of patients recieving hemodialysis. Acta Medica Iranica 2006;44(1):28-32. [EMBASE: 46995829] [Google Scholar]
Bagheri‐Nesami 2016 {published data only}
- Bagheri-Nesami M, Shorofi SA, Nikkhah A, Espahbodi F, Ghaderi Koolaee FS. The effects of aromatherapy with lavender essential oil on fatigue levels in haemodialysis patients: a randomized clinical trial. Complementary Therapies in Clinical Practice 2016 Feb;22:33-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Balouchi 2016 {published data only}
- Balouchi A, Masinaeinezhad N, Abdallahimohammad A, Firouzkouhi MR, Sepehri Z. Comparison of effects of orange and lavender extract on fatigue in hemodialysis patients. Der Pharmacia Lettre 2016;8(8):50-4. [EMBASE: 610743015] [Google Scholar]
Barre 1988 {published data only}
- Barre PE, Brunelle G, Gascon-Barre M. A randomized double blind trial of dialysate sodiums of 145 mEq/L, 150 mEq/L, and 155 mEq/L. ASAIO Transactions 1988;34(3):338-41. [MEDLINE: ] [PubMed] [Google Scholar]
Bellinghieri 1983 {published data only}
- Bellinghieri G, Savica V, Mallamace A, Di Stefano C, Consolo F, Spagnoli LG, et al. Correlation between increased serum and tissue L-carnitine levels and improved muscle symptoms in hemodialyzed patients. American Journal of Clinical Nutrition 1983;38(4):523-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bicer 2022 {published data only}
- Bicer S, Tasci S. The effect of body acupressure on blood pressure and fatigue levels in individuals suffering from hypotension during hemodialysis: a randomized controlled trial. Alternative Therapies in Health & Medicine 2022;28(2):6-16. [PMID: ] [PubMed] [Google Scholar]
Biniaz 2015 {published data only}
- Biniaz V, Tayebi A, Ebadi A, Sadeghi S, Einollahi B. Effect of vitamin C supplementation on marital satisfaction in patients undergoing hemodialysis: a randomized, double-blind and placebo-controlled trial. Saudi Journal of Kidney Diseases & Transplantation 2015;26(3):468-76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
BOLD 2020 {published data only}
- Bansal N, Glidden DV, Mehrotra R, Townsend RR, Cohen J, Linke L, et al. Treating home versus predialysis blood pressure among in-center hemodialysis patients: a pilot randomized trial. American Journal of Kidney Diseases 2021;77(1):12-22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal N, Glidden DV, Mehrotra R, Townsend RR, Larson HL, Linke L, et al. A pilot trial targeting home vs. pre-dialysis BP in hemodialysis (HD) patients [abstract no: FR-PO1032]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):710. [EMBASE: 633770522] [Google Scholar]
Brass 2001 {published data only}
- Brass EP, Adler S, Sietsema KE, Hiatt WR, Orlando AM, Amato A, et al. Intravenous L-carnitine increases plasma carnitine, reduces fatigue, and may preserve exercise capacity in hemodialysis patients. American Journal of Kidney Diseases 2001;37(5):1018-28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Canadian EPO 1990 {published data only}
- Anonymous. Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. Canadian Erythropoietin Study Group. BMJ 1990;300(6724):573-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Effect of recombinant human erythropoietin therapy on blood pressure in hemodialysis patients. Canadian Erythropoietin Study Group. American Journal of Nephrology 1991;11(1):23-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Canadian Erythropoietin Study Group. The clinical effects and side-effects of recombinant human erythropoietin (EPO) in anaemic patients on chronic hemodialysis [abstract]. Kidney International 1990;37(1):278. [CENTRAL: CN-00583134] [Google Scholar]
- Canadian Erythropoietin Study Group. The effect of recombinant human erythropoietin (EPO) upon quality of life and exercise capacity of anemic patients on chronic hemodialysis [abstract]. Kidney International 1990;37(1):278. [CENTRAL: CN-00583135] [Google Scholar]
- Donnelly S, Posen G, Ali M. Oral iron absorption in hemodialysis (HD), patients treated with erythropoietin (EPO) [abstract]. Kidney International 1990;37(1):293. [CENTRAL: CN-00747347] [Google Scholar]
- Donnelly SM, Ali MA, Churchill DN. Bioavailability of iron in hemodialysis patients treated with erythropoietin: evidence for the inhibitory role of aluminum. American Journal of Kidney Diseases 1990;16(5):447-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Keown PA, Churchill DN, Poulin-Costello M, Lei L, Gantotti S, Agodoa I, et al. Dialysis patients treated with Epoetin alfa show improved anemia symptoms: a new analysis of the Canadian Erythropoietin Study Group trial. Hemodialysis International 2010;14(2):168-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Keown PA. Quality of life in end-stage renal disease patients during recombinant human erythropoietin therapy. The Canadian Erythropoietin Study Group. Contributions to Nephrology 1991;88:81-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Keown PA, Canadian Erythropoietin Study Group. The effect of recombinant human erythropoietin (EPO) upon quality of life (QL) and functional capacity (FC) of anemic patients on chronic hemodialysis [abstract]. Kidney International 1989;35(1):195. [CENTRAL: CN-00583136] [Google Scholar]
- Laupacis A, Wong C, Churchill D. The use of generic and specific quality-of-life measures in hemodialysis patients treated with erythropoietin. The Canadian Erythropoietin Study Group. Controlled Clinical Trials 1991;12(4 Suppl):168-79S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Laupacis A. A randomized double-blind study of recombinant human erythropoietin in anaemic hemodialysis patients. Canadian Erythropoietin Study Group. Transplantation Proceedings 1991;23(2):1825-6. [MEDLINE: ] [PubMed] [Google Scholar]
- Laupacis A. Changes in quality of life and functional capacity in hemodialysis patients treated with recombinant human erythropoietin. The Canadian Erythropoietin Study Group. Seminars in Nephrology 1990;10(2 Suppl 1):11-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Muirhead N, Keown P, Churchill DN, Lei L, Gitlin M, Mayne TJ. An intent-to-treat (ITT) analysis of anemia symptoms in the Canadian Erythropoietin Study Group (CESG) [abstract no: PUB537]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):932A. [CENTRAL: CN-00790629] [Google Scholar]
- Muirhead N, Keown P, Gitlin M, Mayne TJ, Churchill DN. A reanalysis of the Canadian erythropoietin study group (CESG) patient-reported outcomes (PRO) trial [abstract no: 177]. American Journal of Kidney Diseases 2008;51(4):A72. [CENTRAL: CN-00790972] [Google Scholar]
- Muirhead N, Keown P, Lei L, Gitlin M, Mayne TJ, Churchill D. The relationship between achieved hemoglobin (HB) & exercise tolerance [abstract no: 161]. American Journal of Kidney Diseases 2008;51(4):A68. [CENTRAL: CN-00796608] [Google Scholar]
- Muirhead N, Keown PA, Churchill DN, Poulin-Costello M, Gantotti S, Lei L, et al. Dialysis patients treated with Epoetin alpha show improved exercise tolerance and physical function: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodialysis International 2011;15(1):87-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Muirhead N, Laupacis A, Wong C. Erythropoietin for anaemia in haemodialysis patients: results of a maintenance study (the Canadian Erythropoietin Study Group). Nephrology Dialysis Transplantation 1992;7(8):811-6. [MEDLINE: ] [PubMed] [Google Scholar]
Cecen 2021 {published data only}
- Cecen S, Lafci D. The effect of hand and foot massage on fatigue in hemodialysis patients: a randomized controlled trial. Complementary Therapies in Clinical Practice 2021;43:101344. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chang 2010 {published data only}
- Chang Y, Cheng SY, Lin M, Gau FY, Chao YF. The effectiveness of intradialytic leg ergometry exercise for improving sedentary life style and fatigue among patients with chronic kidney disease: a randomized clinical trial. International Journal of Nursing Studies 2010;47(11):1383-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2008a {published data only}
- Chen HY, Chiang CK, Wang HH, Hung KY, Lee YJ, Peng YS, et al. Cognitive-behavioral therapy for sleep disturbance in patients undergoing peritoneal dialysis: a pilot randomized controlled trial. American Journal of Kidney Diseases 2008;52(2):314-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2011a {published data only}
- Chen HY, Cheng IC, Pan YJ, Chiu YL, Hsu SP, Pai MF, et al. Cognitive-behavioral therapy for sleep disturbance decreases inflammatory cytokines and oxidative stress in hemodialysis patients. Kidney International 2011;80(4):415-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cho 2004 {published data only}
- Cho YC, Tsay SL. The effect of acupressure with massage on fatigue and depression in patients with end-stage renal disease. Journal of Nursing Research 2004;12(1):51-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chow 2010 {published data only}
- Chow SK, Wong FK. Health-related quality of life in patients undergoing peritoneal dialysis: effects of a nurse-led case management programme. Journal of Advanced Nursing 2010;66(8):1780-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wong FK, Chow SK, Chan TM. Evaluation of a nurse-led disease management programme for chronic kidney disease: a randomized controlled trial. International Journal of Nursing Studies 2010;47(3):268-78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dashti‐Khavidaki 2013 {published data only}
- Dashti-Khavidaki S, Sharif Z, Khalili H, Badri S, Alimadadi A, Ahmadi F, et al. The use of pharmaceutical care to improve health-related quality of life in hemodialysis patients in Iran. International Journal of Clinical Pharmacy 2013;35(2):260-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Duggal 2019 {published data only}
- Duggal V, Abra GE, Reiterman M, Hussein WF, Schiller B. Preliminary analysis of the effect of blood flow rate reduction on post-dialysis fatigue [abstract no: TH-PO313]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):194-5. [EMBASE: 633737000] [Google Scholar]
- Duggal V, Hussein WF, Reiterman M, Sun SJ, Abra GE, Schiller B. The effect of blood flow rate on dialysis recovery time in patients undergoing maintenance hemodialysis: a prospective, parallel-group, randomized controlled trial. Hemodialysis International 2019;23(2):223-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eroglu 2022 {published data only}
- Eroglu H, Gok Metin Z. Benson relaxation technique combined with music therapy for fatigue, anxiety, and depression in hemodialysis patients: a randomized controlled trial. Holistic Nursing Practice 2022;36(3):139-48. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fatigue‐HD 2019 {published data only}
- Farragher J, Davis J, Polatajko H, Elliott M, Ravani P, Manns B, et al. Exploring the life participation experiences of people on chronic hemodialysis who participated in an energy management education program [abstract no: POS-572]. Kidney International Reports 2021;6(4 Suppl):S252. [EMBASE: 2011684286] [Google Scholar]
- Farragher JF, Ravani P, Manns B, Elliott M, Thomas C, Donald M, et al. A pilot randomised controlled trial of an energy management programme for adults on maintenance haemodialysis: the fatigue-HD study. BMJ Open 2022;12(2):e051475. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farragher JF, Ravani P, Manns BJ, Elliott MJ, Thomas CM, Donald M, et al. A pilot randomized control trial of an energy management program for adults on chronic hemodialysis with fatigue: The FATIGUE-HD study [abstract no: PO1077]. Journal of the American Society of Nephrology 2020;31(Abstract Suppl):366. [EMBASE: 633704070] [Google Scholar]
- Farragher JF, Thomas C, Ravani P, Manns B, Elliott MJ, Hemmelgarn BR. Protocol for a pilot randomised controlled trial of an educational programme for adults on chronic haemodialysis with fatigue (Fatigue-HD). BMJ Open 2019;9(7):e030333. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fatouros 2010 {published data only}
- Fatouros IG, Douroudos I, Panagoutsos S, Pasadakis P, Nikolaidis MG, Chatzinikolaou A, et al. Effects of L-carnitine on oxidative stress responses in patients with renal disease. Medicine & Science in Sports & Exercise 2010;42(10):1809-18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Frequent Hemodialysis Network (FHN) Trial Group. The Frequent Hemodialysis Network multicenter randomized trial of in-center daily hemodialysis [abstract no: F-PO009]. Journal of the American Society of Nephrology 2006;17(Abstracts):338A. [Google Scholar]
FHN DAILY 2007 {published data only}
- Beck GJ, Chertow GM, Eggers PW, Greene T, Larive B, Levin NW, et al. Influence of methodology on left ventricular mass measurement by cardiac magnetic resonance in the Frequent Hemodialysis Network (FHN) nocturnal trial [abstract no: SA-PO437]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):726A. [Google Scholar]
- Chan CT, Beck GJ, Chertow GM, Daugirdas JT, Greene TH, Kotanko P, et al. Effects on ventricular volumes by frequent hemodialysis: results from the Frequent Hemodialysis Network (FHN) trials [abstract no: SA-OR391]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):94A. [Google Scholar]
- Chan CT, Chertow GM, Daugirdas JT, Greene T, Kotanko P, Larive B, et al. Effects of daily hemodialysis on heart rate variability: results from the Frequent Hemodialysis Network (FHN) Daily trial [abstract no: SA-OR038]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):74A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Chertow GM, Daugirdas JT, Greene TH, Kotanko P, Larive B, et al. Effects of daily hemodialysis on heart rate variability: results from the frequent hemodialysis network (FHN) daily trial. Nephrology Dialysis Transplantation 2014;29(1):168-78. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, et al. Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials. Circulation. Cardiovascular Imaging 2012;5(2):251-61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, et al. Effects of frequent hemodialysis on ventricular volumes and left ventricular remodeling. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(12):2106-16. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Kaysen GA, Beck GJ, Li M, Lo JC, Rocco MV, et al. Changes in biomarker profile and left ventricular hypertrophy regression: results from Frequent Hemodialysis Network Trials [abstract no: FR-OR048]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):49-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Kaysen GA, Beck GJ, Li M, Lo JC, Rocco MV, et al. The effect of frequent hemodialysis on matrix metalloproteinases, their tissue inhibitors, and FGF23: Implications for blood pressure and left ventricular mass modification in the Frequent Hemodialysis Network trials. Hemodialysis International 2020;24(2):162-74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chan CT, Kaysen GA, Beck GJ, Rocco MV, Kliger AS. The effect of frequent hemodialysis on phosphate and fibroblast growth factor 23: results from the Frequent Hemodialysis Network trials [abstract no: TH-PO347]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):204. [EMBASE: 633736427] [Google Scholar]
- Chan CT, Levin NW, Chertow GM, Larive B, Schulman G, Kotanko P. Determinants of cardiac autonomic dysfunction in ESRD. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(10):1821-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Rocco MV, Chertow GM, Kaysen GA, Levin NW, Larive B, et al. The role of cardiomyocyte apoptosis in the pathogenesis of left ventricular hypertrophy (LVH): results from the Frequent Hemodialysis Network (FHN) [abstract no: TH-OR143]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):34A. [Google Scholar]
- Chertow GM, Levin NW, Beck GJ, Daugirdas JT, Eggers PW, Kliger AS, et al. Long-term effects of frequent in-center hemodialysis. Journal of the American Society of Nephrology 2016;27(6):1830-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugirdas JT, Chertow GM, Larive B, Pierratos A, Greene T, Ayus JC, et al. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. Journal of the American Society of Nephrology 2012;23(4):727-38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugirdas JT, Chertow GM, Pierratos A, Ayus JC, Greene TH, Miller BW, et al. Effects of frequent hemodialysis on measures of chronic kidney disease mineral and bone disorder (CKD-MBD): the Frequent Hemodialysis Network Daily and Nocturnal Trials [abstract no: FR-PO1210]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):392A. [Google Scholar]
- Daugirdas JT, Greene T, Rocco MV, Kaysen GA, Depner TA, Levin NW, et al. Effect of frequent hemodialysis on residual kidney function. Kidney International 2013;83(5):949-58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- FHN Trial Group, Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, et al. In-center hemodialysis six times per week versus three times per week [Erratum in: N Engl J Med. 2011 Jan 6;364(1):93]. New England Journal of Medicine 2010;363(24):2287-300. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frequent Hemodialysis Network (FHN) Trial Group. Progress of the frequent hemodialysis network randomized trial of in-center daily hemodialysis [abstract no: 61]. American Journal of Kidney Diseases 2007;49(4):A40. [CENTRAL: CN-00601916] [Google Scholar]
- Frequent Hemodialysis Network (FHN) Trial Group. The Frequent Hemodialysis Network multicenter randomized trial of in-center daily hemodialysis [abstract no: FPO009]. Journal of the American Society of Nephrology 2006;17(Abstracts):338A. [Google Scholar]
- Garg AX, Suri RS, Eggers P, Finkelstein FO, Greene T, Kimmel PL, et al. Patients receiving frequent hemodialysis have better health-related quality of life compared to patients receiving conventional hemodialysis. Kidney international 2017;91(3):746-54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene T, Daugirdas J, Depner T, Gotch F, Kuhlmann M, Kotanko P, et al. Clearance and volume removal values for target dialysis prescriptions in the Frequent Hemodialysis Network (FHN) trials [abstract no: F-PO003]. Journal of the American Society of Nephrology 2006;17(Abstracts):337A. [CENTRAL: CN-00602073] [Google Scholar]
- Greene T, Daugirdas JT, Chertow GM, Levin NW, Rocco MV, Beck GJ, et al. Bayesian interpretation of the estimated treatment effects on mortality in the daily and nocturnal Frequent Hemodialysis Network (FHN) [abstract no: FR-PO329]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):439A. [Google Scholar]
- Greene T, Daugirdas JT, Depner TA, Gotch F, Kuhlman M. Solute clearances and fluid removal in the frequent hemodialysis network trials. American Journal of Kidney Diseases 2009;53(5):835-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hall YN, Larive B, Painter P, Kaysen GA, Lindsay RM, Nissenson AR, et al. Effects of six versus three times per week hemodialysis on physical performance, health, and functioning Frequent Hemodialysis Network (FHN) randomized trials. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(5):782-94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall YN, Larive B, Painter PL, Kaysen GA, Lindsay RM, Nissenson AR, et al. Effects of six versus three times per week hemodialysis on physical performance, health and functioning: Frequent Hemodialysis Network (FHN) trials [abstract no: FR-OR275]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):66A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SS, Kaysen GA, Levin NW, Kliger AS, Beck GJ, Rocco MV, et al. The effect of increased frequency of hemodialysis on serum cystatin C and beta2-microglobulin concentrations: a secondary analysis of the Frequent Hemodialysis Network (FHN) trial. Hemodialysis International 2019;23(3):297-305. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jhamb M, Tamura MK, Gassman J, Garg AX, Lindsay RM, Suri RS, et al. Design and rationale of health-related quality of life and patient-reported outcomes assessment in the Frequent Hemodialysis Network trials. Blood Purification 2011;31(1-3):151-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RM, Herzog CA, Larive B, Subacius H, Nearing BD, Verrier R, et al. T-wave alternans, heart rate turbulence, and ventricular ectopy in standard versus daily hemodialysis: results from the FHN Daily Trial. Annals of Noninvasive Electrocardiology 2016;21(6):566-71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen GA, Greene T, Larive B, Mehta RL, Lindsay RM, Depner TA, et al. The effect of frequent hemodialysis on nutrition and body composition: Frequent Hemodialysis Network Trial. Kidney International 2012;82(1):90-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen GA, Larive B, Painter P, Craig A, Lindsay RM, Rocco MV, et al. Baseline physical performance, health, and functioning of participants in the Frequent Hemodialysis Network (FHN) trial. American Journal of Kidney Diseases 2011;57(1):101-12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Unruh ML, Larive B, Eggers PW, Garg AX, Gassman JJ, et al. The impact of frequent hemodialysis on sleep quality: Frequent Hemodialysis Network (FHN) trials [abstract no: FR-PO376]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):458A. [Google Scholar]
- Kliger AS, Chertow GM, Levin NW, Beck GJ, Daugirdas JT, Eggers PW, et al. Long-term effects of frequent in-center hemodialysis: FHN daily trial [abstract no: MO037]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii37-38. [EMBASE: 71491555] [Google Scholar]
- Kotanko P, Garg AX, Depner T, Pierratos A, Chan CT, Levin NW, et al. Effects of frequent hemodialysis on blood pressure: Results from the randomized frequent hemodialysis network trials. Hemodialysis International 2015;19(3):386-401. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotanko P, Stokes JB, Garg AX, Depner TA, Chan CT, Pierratos A, et al. Temporal evolution of systolic and diastolic blood pressure in the Frequent Hemodialysis Network (FHN) Trials [abstract no: FR-PO1622]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):490A. [Google Scholar]
- Kotanko P, Stokes JB, Garg AX, Depner TA, Pierratos A, Chan CT, et al. Intradialytic hypotension in 6x weekly in-center hemodialysis: results from the randomized Frequent Hemodialysis Network Daily Trial [abstract no: FR-PO373]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):457A. [Google Scholar]
- Kurella Tamura M, Larive B, Unruh ML, Stokes JB, Nissenson A, Mehta RL, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network Trials. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(8):1429-38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurella Tamura M, Unruh ML, Nissenson AR, Larive B, Eggers PW, Gassman J, et al. Effect of more frequent hemodialysis on cognitive function in the frequent hemodialysis network trials. American Journal of Kidney Diseases 2013;61(2):228-37. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfino A, Beck GJ, Li M, Lo JC, Kaysen GA, for the FHN Investigators. Association between change in serum bicarbonate and change in thyroid hormone levels in patients receiving conventional or more frequent maintenance haemodialysis. Nephrology 2019;24(1):81-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornt DB, Kliger AS, Suri R, Rashid MA, Rastogi A, Tamura MK, et al. The impact of frequent in-center versus conventional hemodialysis on anemia: the frequent hemodialysis network trial [abstract no: FR-PO1567]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):476A. [Google Scholar]
- Ornt DB, Larive B, Rastogi A, Rashid M, Daugirdas JT, Hernandez A, et al. Impact of frequent hemodialysis on anemia management: results from the Frequent Hemodialysis Network (FHN) Trials. Nephrology Dialysis Transplantation 2013;28(7):1888-98. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimann JG, Abbas SR, Liu L, Larive B, Beck G, Kotanko P, et al. The effect of increased frequency of hemodialysis on vitamin C concentrations: an ancillary study of the randomized Frequent Hemodialysis Network (FHN) daily trial. BMC Nephrology 2019;20(1):179. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimann JG, Abbas SR, Liu L, Larive B, Liu Y, Beck GJ, et al. The effects of more frequent hemodialysis (HD) on plasma vitamin C concentration: an ancillary study of the frequent hemodialysis network (FHN) daily trial. [abstract no: SA-PO162]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):720. [EMBASE: 633702139] [Google Scholar]
- Raimann JG, Abbas SR, Liu L, Zhu F, Larive B, Kotanko P, et al. Agreement of single- and multi-frequency bioimpedance measurements in hemodialysis patients: an ancillary study of the Frequent Hemodialysis Network Daily Trial. Nephron 2014;128(1-2):115-26. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimann JG, Chan CT, Daugirdas JT, Depner T, Gotch FA, Greene T, et al. The effect of increased frequency of hemodialysis on volume-related outcomes: a secondary analysis of the Frequent Hemodialysis Network Trials. Blood Purification 2016;41(4):277-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Raimann JG, Chan CT, Daugirdas JT, Depner TA, Gotch FA, Greene T, et al. Low predialysis serum sodium modifies the effect of hemodialysis (HD) frequency on left ventricular mass: the frequent hemodialysis network (FHN) trial [abstract no: TH-OR081]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):20A. [Google Scholar]
- Raimann JG, Daugirdas JT, Gotch FA, Greene T, Kaysen GA, Kliger AS, et al. Treatment effect of frequent hemodialysis (HD) on interdialytic weight gain (IDWG) and extracelluar volume (ECV) and their relation to changes (^) in left ventricular mass (LVM): Frequent Hemodialysis Network (FHN) Daily Trial [abstract no: FR-PO375]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):458A. [Google Scholar]
- Rocco MV, Larive B, Eggers PW, Beck GJ, Chertow GM, Levin NW, et al. Baseline characteristics of participants in the Frequent Hemodialysis Network (FHN) daily and nocturnal trials. American Journal of Kidney Diseases 2011;57(1):90-100. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyeva O, Gorodetskaya I, Champagne J, Sherer S, Carter M. Recruitment successes and challenges in the frequent hemodialysis network (FHN) daily trial [abstract no: SU-FC058]. Journal of the American Society of Nephrology 2007;18(Abstracts):79A. [Google Scholar]
- Sergeyeva O, Gorodetskaya I, Ramos R, Figuracion E, Ulloa D, Riley J, et al. Challenges to enrollment in a randomized clinical trial (RCT) of frequent in-center hemodialysis (HD): NIDDK Frequent Hemodialysis Network (FHN) trial [abstract no: SA-PO2462]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):675A. [Google Scholar]
- Sergeyeva O, Gorodetskaya I, Ramos R, Schiller BM, Larive B, Raimann JG, et al. Challenges to enrollment and randomization of the Frequent Hemodialysis Network (FHN) Daily Trial. Journal of Nephrology 2012;25(3):302-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sirich TL, Fong K, Larive B, Beck GJ, Chertow GM, Levin NW, et al. Limited reduction in uremic solute concentrations with increased dialysis frequency and time in the Frequent Hemodialysis Network Daily Trial. Kidney International 2017;91(5):1186-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stokes JB. Consequences of frequent hemodialysis: comparison to conventional hemodialysis and transplantation. Transactions of the American Clinical & Climatological Association 2011;122:124-36. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Suri R, Larive B, Sherer SG, Gassman JJ, Ornt DB, Rocco MV, et al. Risk of vascular access events in the FHN Daily trial [abstract no: SA-OR451]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):108A. [Google Scholar]
- Suri RS, Garg AX, Chertow GM, Levin NW, Rocco MV, Greene T, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney International 2007;71(4):349-59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suri RS, Larive B, Hall Y, Kimmel PL, Kliger AS, Levin N, et al. Effects of frequent hemodialysis on perceived caregiver burden in the Frequent Hemodialysis Network trials. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(5):936-42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri RS, Larive B, Sherer S, Eggers P, Gassman J, James SH, et al. Risk of vascular access complications with frequent hemodialysis. Journal of the American Society of Nephrology 2013;24(3):498-505. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh ML, Larive B, Chertow GM, Eggers PW, Garg AX, Gassman J, et al. Effects of 6-times-weekly versus 3-times-weekly hemodialysis on depressive symptoms and self-reported mental health: Frequent Hemodialysis Network (FHN) Trials. American Journal of Kidney Diseases 2013;61(5):748-58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh ML, Larive B, Chertow GM, Eggers PW, Garg AX, Gassman JJ, et al. Effects of six versus three times per week hemodialysis on depressive affect on mental health: Frequent Hemodialysis Network (FHN) trials [abstract no: TH-PO623]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):255A. [Google Scholar]
- Unruh ML, Larive B, Eggers PW, Garg AX, Gassman JJ, Finkelstein FO, et al. The effect of frequent hemodialysis on self-reported sleep quality: Frequent Hemodialysis Network Trials. Nephrology Dialysis Transplantation 2016;31(6):984-91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhandl E, Collins A. Post-dialysis recovery time in the Frequent Hemodialysis Network [abstract]. Hemodialysis International 2017;21(1):A25. [EMBASE: 614510715] [Google Scholar]
FHN NOCTURNAL 2007 {published data only}
- Beck GJ, Chertow GM, Eggers PW, Greene T, Larive B, Levin NW, et al. Influence of methodology on left ventricular mass measurement by cardiac magnetic resonance in the Frequent Hemodialysis Network (FHN) nocturnal trial [abstract no: SA-PO437]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):726A. [Google Scholar]
- Chan CT, Beck GJ, Chertow GM, Daugirdas JT, Greene TH, Kotanko P, et al. Effects on ventricular volumes by frequent hemodialysis: results from the Frequent Hemodialysis Network (FHN) trials [abstract no: SA-OR391]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):94A. [Google Scholar]
- Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, et al. Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials. Circulation. Cardiovascular Imaging 2012;5(2):251-61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Kaysen GA, Beck GJ, Li M, Lo JC, Rocco MV, et al. Changes in biomarker profile and left ventricular hypertrophy regression: results from Frequent Hemodialysis Network Trials [abstract no: FR-OR048]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):49-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Kaysen GA, Beck GJ, Rocco MV, Kliger AS. The effect of frequent hemodialysis on phosphate and fibroblast growth factor 23: results from the Frequent Hemodialysis Network trials [abstract no: TH-PO347]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):204. [Google Scholar]
- Chan CT, Levin NW, Chertow GM, Larive B, Schulman G, Kotanko P, et al. Determinants of cardiac autonomic dysfunction in ESRD. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(10):1821-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugirdas JT, Chertow GM, Larive B, Pierratos A, Greene T, Ayus JC, et al. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. Journal of the American Society of Nephrology 2012;23(4):727-38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugirdas JT, Chertow GM, Pierratos A, Ayus JC, Greene TH, Miller BW, et al. Effects of frequent hemodialysis on measures of chronic kidney disease mineral and bone disorder (CKD-MBD): the Frequent Hemodialysis Network Daily and Nocturnal Trials [abstract no: FR-PO1210]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):392A. [Google Scholar]
- Daugirdas JT, Greene T, Rocco MV, Kaysen GA, Depner TA, Levin NW, et al. Effect of frequent hemodialysis on residual kidney function. Kidney International 2013;83(5):949-58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- FHN Trial Group, Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, et al. In-center hemodialysis six times per week versus three times per week [Erratum in: N Engl J Med. 2011 Jan 6;364(1):93]. New England Journal of Medicine 2010;363(24):2287-300. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frequent Hemodialysis Network (FHN) Trial Group. Progress of the frequent hemodialysis network randomized trial of in-center daily hemodialysis [abstract no: 61]. American Journal of Kidney Diseases 2007;49(4):A40. [CENTRAL: CN-00601916] [Google Scholar]
- Frequent Hemodialysis Network (FHN) Trial Group. The Frequent Hemodialysis Network randomized trial of home nocturnal hemodialysis [abstract no: F-PO010]. Journal of the American Society of Nephrology 2006;17(Abstracts):338A. [CENTRAL: CN-00602072] [Google Scholar]
- Frequent Hemodialysis Network (FHN) Trial Group. The Frequent Hemodialysis Network randomized trial of home nocturnal hemodialysis: change of trial design [abstract no: 62]. American Journal of Kidney Diseases 2007;49(4):A40. [CENTRAL: CN-00601917] [Google Scholar]
- Gassman JJ, Rocco M, Beck G, Larive B, Sherer S, Kliger A, et al. Changing a protocol early in a trial's vanguard phase: the Frequent Hemodialysis Network Home Nocturnal Hemodialysis Trial [abstract no: P71]. Clinical Trials 2007;4(4):421. [CENTRAL: CN-00782507] [Google Scholar]
- Greene T, Daugirdas J, Depner T, Gotch F, Kuhlmann M, Kotanko P, et al. Clearance and volume removal values for target dialysis prescriptions in the Frequent Hemodialysis Network (FHN) trials [abstract no: F-PO003]. Journal of the American Society of Nephrology 2006;17(Abstracts):337A. [CENTRAL: CN-00602073] [Google Scholar]
- Greene T, Daugirdas JT, Chertow GM, Levin NW, Rocco MV, Beck GJ, et al. Bayesian interpretation of the estimated treatment effects on mortality in the daily and nocturnal Frequent Hemodialysis Network (FHN) [abstract no: FR-PO329]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):439A. [Google Scholar]
- Greene T, Daugirdas JT, Depner TA, Gotch F, Kuhlman M. Solute clearances and fluid removal in the frequent hemodialysis network trials. American Journal of Kidney Diseases 2009;53(5):835-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hall YN, Larive B, Painter P, Kaysen GA, Lindsay RM, Nissenson AR, et al. Effects of six versus three times per week hemodialysis on physical performance, health, and functioning Frequent Hemodialysis Network (FHN) Randomized Trials. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(5):782-94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall YN, Larive B, Painter PL, Kaysen GA, Lindsay RM, Nissenson AR, et al. Effects of six versus three times per week hemodialysis on physical performance, health and functioning: Frequent Hemodialysis Network (FHN) trials [abstract no: FR-OR275]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):66A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SS, Kaysen GA, Levin NW, Kliger AS, Beck GJ, Rocco MV, et al. The effect of increased frequency of hemodialysis on serum cystatin C and beta2-microglobulin concentrations: a secondary analysis of the Frequent Hemodialysis Network (FHN) trial. Hemodialysis International 2019;23(3):297-305. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jhamb M, Tamura MK, Gassman J, Garg AX, Lindsay RM, Suri RS, et al. Design and rationale of health-related quality of life and patient-reported outcomes assessment in the Frequent Hemodialysis Network trials. Blood Purification 2011;31(1-3):151-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen GA, Greene T, Larive B, Mehta RL, Lindsay RM, Depner TA, et al. The effect of frequent hemodialysis on nutrition and body composition: Frequent Hemodialysis Network Trial. Kidney International 2012;82(1):90-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen GA, Larive B, Painter P, Craig A, Lindsay RM, Rocco MV, et al. Baseline physical performance, health, and functioning of participants in the Frequent Hemodialysis Network (FHN) trial. American Journal of Kidney Diseases 2011;57(1):101-12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Unruh ML, Larive B, Eggers PW, Garg AX, Gassman JJ, et al. The impact of frequent hemodialysis on sleep quality: Frequent Hemodialysis Network (FHN) trials [abstract no: FR-PO376]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):458A. [Google Scholar]
- Kotanko P, Garg AX, Depner T, Pierratos A, Chan CT, Levin NW, et al. Effects of frequent hemodialysis on blood pressure: results from the randomized frequent hemodialysis network trials. Hemodialysis International 2015;19(3):386-401. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotanko P, Stokes JB, Garg AX, Depner TA, Chan CT, Pierratos A, et al. Temporal evolution of systolic and diastolic blood pressure in the Frequent Hemodialysis Network (FHN) Trials [abstract no: FR-PO1622]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):490A. [Google Scholar]
- Kurella Tamura M, Larive B, Unruh ML, Stokes JB, Nissenson A, Mehta RL, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network Trials. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(8):1429-38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurella Tamura M, Unruh ML, Nissenson AR, Larive B, Eggers PW, Gassman J, et al. Effect of more frequent hemodialysis on cognitive function in the frequent hemodialysis network trials. American Journal of Kidney Diseases 2013;61(2):228-37. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfino A, Beck GJ, Li M, Lo JC, Kaysen GA. Association between change in serum bicarbonate and change in thyroid hormone levels in patients receiving conventional or more frequent maintenance haemodialysis. Nephrology 2019;24(1):81-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornt DB, Larive B, Rastogi A, Rashid M, Daugirdas JT, Hernandez A, et al. Impact of frequent hemodialysis on anemia management: results from the Frequent Hemodialysis Network (FHN) Trials. Nephrology Dialysis Transplantation 2013;28(7):1888-98. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipkin M, Brett L, Stokes J, Suri R, Lockridge R, Rocco M, et al. Barriers to recruitment for home hemodialysis [abstract no: SA-PO2464]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):676A. [Google Scholar]
- Pipkin M, Eggers PW, Larive B, Rocco MV, Stokes JB, Suri RS, et al. Recruitment and training for home hemodialysis: experience and lessons from the nocturnal dialysis trial. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(9):1614-20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimann JG, Chan CT, Daugirdas JT, Depner T, Gotch FA, Greene T, et al. The effect of increased frequency of hemodialysis on volume-related outcomes: a secondary analysis of the Frequent Hemodialysis Network Trials. Blood Purification 2016;41(4):277-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Raimann JG, Chan CT, Daugirdas JT, Depner TA, Gotch FA, Greene T, et al. Low predialysis serum sodium modifies the effect of hemodialysis (HD) frequency on left ventricular mass: the frequent hemodialysis network (FHN) trial [abstract no: TH-OR081]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):20A. [Google Scholar]
- Rocco M, Daugirdas J, Greene T, Lockridge R, Chan C, Pierratos A, et al. Mortality during extended follow-up in the Frequent Hemodialysis Network Nocturnal Trial [abstract no: MO035]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii37. [EMBASE: 71491553] [Google Scholar]
- Rocco MV, Daugirdas JT, Greene T, Lockridge RS, Chan C, Pierratos A, et al. Long-term effects of frequent nocturnal hemodialysis on mortality: The Frequent Hemodialysis Network (FHN) Nocturnal Trial. American Journal of Kidney Diseases 2015;66(3):459-68. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco MV, Daugirdas JT, Greene T, Lockridge RS, Pierratos A, Larive B, et al. Effects of randomization to frequent nocturnal hemodialysis on long-term mortality: Frequent Hemodialysis Network Nocturnal Trial [abstract no: FR-PO345]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):443A. [Google Scholar]
- Rocco MV, Kliger A, Beck G, Lockridge R, Eggers P. Baseline results from the Frequent Hemodialysis Network Nocturnal trial [abstract no: TH-PO348]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):193A. [Google Scholar]
- Rocco MV, Larive B, Eggers PW, Beck GJ, Chertow GM, Levin NW, et al. Baseline characteristics of participants in the Frequent Hemodialysis Network (FHN) daily and nocturnal trials. American Journal of Kidney Diseases 2011;57(1):90-100. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco MV, Lockridge J, Beck GJ, Eggers PW, Gassman JJ, Greene T, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney International 2011;80(10):1080-91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes JB. Consequences of frequent hemodialysis: comparison to conventional hemodialysis and transplantation. Transactions of the American Clinical & Climatological Association 2011;122:124-36. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Suri RS, Garg AX, Chertow GM, Levin NW, Rocco MV, Greene T, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney International 2007;71(4):349-59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suri RS, Larive B, Hall Y, Kimmel PL, Kliger AS, Levin N, et al. Effects of frequent hemodialysis on perceived caregiver burden in the Frequent Hemodialysis Network trials. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(5):936-42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri RS, Larive B, Sherer S, Eggers P, Gassman J, James SH, et al. Risk of vascular access complications with frequent hemodialysis. Journal of the American Society of Nephrology 2013;24(3):498-505. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh ML, Larive B, Chertow GM, Eggers PW, Garg AX, Gassman J, et al. Effects of 6-times-weekly versus 3-times-weekly hemodialysis on depressive symptoms and self-reported mental health: Frequent Hemodialysis Network (FHN) Trials. American Journal of Kidney Diseases 2013;61(5):748-58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh ML, Larive B, Chertow GM, Eggers PW, Garg AX, Gassman JJ, et al. Effects of six versus three times per week hemodialysis on depressive affect on mental health: Frequent Hemodialysis Network (FHN) trials [abstract no: TH-PO623]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):255A. [Google Scholar]
- Unruh ML, Larive B, Eggers PW, Garg AX, Gassman JJ, Finkelstein FO, et al. The effect of frequent hemodialysis on self-reported sleep quality: Frequent Hemodialysis Network Trials. Nephrology Dialysis Transplantation 2016;31(6):984-91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhandl E, Collins A. Post-dialysis recovery time in the Frequent Hemodialysis Network [abstract]. Hemodialysis International 2017;21(1):A25. [EMBASE: 614510715] [Google Scholar]
Figueiredo 2018 {published data only}
- Figueiredo PH, Lima MM, Costa HS, Martins JB, Flecha OD, Goncalves PF, et al. Effects of the inspiratory muscle training and aerobic training on respiratory and functional parameters, inflammatory biomarkers, redox status and quality of life in hemodialysis patients: a randomized clinical trial. PLoS ONE [Electronic Resource] 2018;13(7):e0200727. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foley 2000 {published data only}
- Foley RN, Parfrey PS, Morgan J, Barre P, Campbell P, Cartier P, et al. A randomized controlled trial of complete vs partial correction of anemia in hemodialysis patients with asymptomatic concentric lV hypertrophy or lV dilation [abstract no: A1064]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):208A. [Google Scholar]
- Foley RN, Parfrey PS, Morgan J, Barre P, Campbell P, Cartier P, et al. Diastolic dysfunction in hemodialysis patients: the Canadian Normalization of Hemoglobin Study Group [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):261A. [CENTRAL: CN-00550674] [Google Scholar]
- Foley RN, Parfrey PS, Morgan J, Barre PE, Campbell P, Cartier P, et al. Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney International 2000;58(3):1325-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Foley RN, Parfrey PS, Morgan J, Barre PE, Campbell P, Cartier P, et al. Hemoglobin levels and hospitalization in hemodialysis patients without symptomatic cardiac disease [abstract no: SA-PO818]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):432A. [CENTRAL: CN-00445362] [Google Scholar]
- Wells GA, Coyne D, Lee KM, Foley RN, Parfrey PS, et al. Quality of life effects of normalization of hemoglobin in asymptomatic hemodialysis patients [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):230A. [CENTRAL: CN-00448336] [Google Scholar]
Fukuda 2015 {published data only}
- Fukuda S, Koyama H, Fujii H, Hirayama Y, Tabata T, Okamura M, et al. Effect of nutritional support on reactivation of fatigue-related human herpers virus 6/7 reactivation in hemodialysis patients: a randomized double-blind placebo-controlled study [abstract no: F-PO1486]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):453A. [Google Scholar]
- Fukuda S, Koyama H, Kondo K, Fujii H, Hirayama Y, Tabata T, et al. Effects of nutritional supplementation on fatigue, and autonomic and immune dysfunction in patients with end-stage renal disease: a randomized, double-blind, placebo-controlled, multicenter trial. PLoS ONE [Electronic Resource] 2015;10(3):e0119578. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Fukuda S, Fujii H, Hirayama Y, Tabata T, Okamura M, et al. Effects of nutritional support on fatigue and autonomic dysfunction in patients with end-stage renal diseases: a randomized double-blind placebo-controlled study [abstract no: F-PO1511]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):458A. [Google Scholar]
Grigoriou 2021 {published data only}
- Grigoriou SS, Giannaki CD, George K, Karatzaferi C, Zigoulis P, Eleftheriadis T, et al. A single bout of hybrid intradialytic exercise did not affect left-ventricular function in exercise-naive dialysis patients: a randomized, cross-over trial. International Urology & Nephrology 2021;53(1):771–84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Habibzadeh 2020 {published data only}
- Habibzadeh H, Wosoi Dalavan O, Alilu L, Wardle J, Khalkhali H, Nozad A. Effects of foot massage on severity of fatigue and quality of life in hemodialysis patients: a randomized controlled trial. International Journal of Community Based Nursing & Midwifery 2020;8(2):92-102. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadadian 2016 {published data only}
- Hadadian F, Sohrabi N, Farokhpayam M, Farokhpayam H, Towhidi F, Fayazi S, et al. The effects of transcutaneous electrical acupoint stimulation (TEAS) on fatigue in haemodialysis patients. Journal of Clinical and Diagnostic Research: JCDR 2016;10(9):YC01-4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadadian 2018 {published data only}
- Hadadian F, Jalalvandi F, Karimi S, Abdi A, Salari N, Ghobadi A. Studying the effect of progressive muscle relaxation technique on fatigue in hemodialysis patients-Kermanshah-Iran. Annals of Tropical Medicine & Public Health 2018;11(1):8-12. [EMBASE: 630312180] [Google Scholar]
Hasankhani 2013 {published data only}
- Hasankhani H, Ghaderi F, Lakdizaji S, Nahamin M. The effect of the slow-stroke back massage on fatigue of dialyzed patients. International Research Journal of Applied & Basic Science 2013;4(10):3004-8. [Google Scholar]
Hassanzadeh 2018 {published data only}
- Hassanzadeh M, Kiani F, Bouya S, Zarei M. Comparing the effects of relaxation technique and inhalation aromatherapy on fatigue in patients undergoing hemodialysis. Complementary Therapies in Clinical Practice 2018;31:210-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HDPAL 2014 {published data only}
- Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrology Dialysis Transplantation 2014;29(3):672-81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R, Sinha AD, Pappas MK, Abraham TN. Hypertension in hemodialysis patients treated with atenolol or lisinopril (HDPAL): a randomized controlled trial [abstract no: HI-OR04]. Journal of the American Society of Nephrology 2013;24(Abstracts):1B. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R. Treating hypertension in hemodialysis improves symptoms seemingly unrelated to volume excess. Nephrology Dialysis Transplantation 2016;31(1):142-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgianos PI, Agarwal R. Aortic stiffness, ambulatory blood pressure, and predictors of response to antihypertensive therapy in hemodialysis. American Journal of Kidney Diseases 2015;66(2):305-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Georgianos PI, Agarwal R. Effect of lisinopril and atenolol on aortic stiffness in patients on hemodialysis. Clinical Journal of the American Society of Nephrology: CJASN 2015;10(4):639-45. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgianos PI, Agarwal R. Relative importance of aortic stiffness and volume as predictors of treatment-induced improvement in left ventricular mass index in dialysis. PLoS ONE [Electronic Resource] 2015;10(9):e0135457. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2021 {published data only}
- Huang HY, Hung KS, Yeh ML, Chou HL, Yeh AL, Liao TY. Breathing-based leg exercises during hemodialysis improve quality of life: a randomized controlled trial. Clinical Rehabilitation 2020;35(8):1175-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jalalian 2015 {published data only}
- Jalalian Z, Varayi S, Nejad MS. Effects of aromatherapy on fatigue and quality of life in patients undergoing hemodialysis [abstract]. Avicenna Journal of Phytomedicine 2015;5(Suppl 1):66-7. [EMBASE: 72156754] [Google Scholar]
Johansen 1999 {published data only}
- Johansen KL, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients on dialysis: a randomized, placebo-controlled trial [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):212A. [CENTRAL: CN-00445936] [Google Scholar]
- Johansen KL, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial. JAMA 1999;281(14):1275-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johansen 2006 {published data only}
- Johansen KL, Painter PL, Gordon P, Doyle J, Sakkas GK. Effects of resistance exercise training and anabolic steroid treatment among hemodialysis patients: results of the NEXT study [abstract no: SU-PO382]. Journal of the American Society of Nephrology 2004;15(Oct):617A. [CENTRAL: CN-00550563] [Google Scholar]
- Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. Journal of the American Society of Nephrology 2006;17(8):2307-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kaplin Serin 2020 {published data only}
- Kaplan Serin E, Ovayolu N, Ovayolu O. The effect of progressive relaxation exercises on pain, fatigue, and quality of life in dialysis patients. Holistic Nursing Practice 2020;34(2):121-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Karadag 2019 {published data only}
- Karadag E, Samancioglu Baglama S. The effect of aromatherapy on fatigue and anxiety in patients undergoing hemodialysis treatment: a randomized controlled study. Holistic Nursing Practice 2019;33(4):222-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Konstadinidou‐ND 2002 {published data only}
- Konstantinidou E, Koukouvou G, Kouidi E, Deligiannis A, Tourkantonis A. Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. Journal of Rehabilitation Medicine 2002;34(1):40-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krase 2022 {published data only}
- Krase AA, Terzis G, Giannaki CD, Stasinaki AN, Wilkinson TJ, Smith AC, et al. Seven months of aerobic intradialytic exercise training can prevent muscle loss in haemodialysis patients: an ultrasonography study. International Urology & Nephrology 2022;54(2):447-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lazarus 2020 {published data only}
- Lazarus ER, Deva Amirtharaj A, Jacob D, Chandrababu R, Isac C. The effects of an olive-oil massage on hemodialysis patients suffering from fatigue at a hemodialysis unit in southern India - a randomized controlled trial. Journal of Complementary & Integrative Medicine 2020;18(2):397-403. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leski 1979 {published data only}
- Leski M, Niethammer T, Wyss T. Glucose-enriched dialysate and tolerance to maintenance hemodialysis. Nephron 1979;24(6):271-3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2014b {published data only}
- Li J, Wang H, Xie H, Mei G, Cai W, Ye J, et al. Effects of post-discharge nurse-led telephone supportive care for patients with chronic kidney disease undergoing peritoneal dialysis in China: a randomized controlled trial. Peritoneal Dialysis International 2014;34(3):278-88. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lillevang 1990 {published data only}
- Lillevang ST, Pedersen FB. Quality of life of hemodialysis patients before and after erythropoietin therapy. A double-blind, randomized, placebo controlled study [Haemodialysepatienters livskvalitet for og efter erytropoietinbehandling. En dobbeltblind, randomiseret, placebokontrolleret undersogelse]. Ugeskrift for Laeger 1990;152(41):2999-3002. [MEDLINE: ] [PubMed] [Google Scholar]
Lin 2011 {published data only}
- Lin CH, Lee LS, Su LH, Huang TC, Liu CF. Thermal therapy in dialysis patients - a randomized trial. American Journal of Chinese Medicine 2011;39(5):839-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Linde 2001 {published data only}
- Danielson BG, Furuland H, Ahlmen J, Christensson A, Linde T, Strombom U. Scandinavian study of normalizing hemoglobin with Rhu-EPO in end stage renal failure [abstract no: A0822]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):160A. [CENTRAL: CN-00550642] [Google Scholar]
- Furuland H, Linde T, Ahlmen J, Christensson A, Strombom U, Danielson BG. A randomized controlled trial of haemoglobin normalization with epoetin alfa in pre-dialysis and dialysis patients. Nephrology Dialysis Transplantation 2003;18(2):353-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Furuland H, Linde T, Danielson BG. Cardiac function in patients with end-stage renal disease after normalization of hemoglobin with erythropoietin (EPO) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):337A. [CENTRAL: CN-00445402] [Google Scholar]
- Furuland H, Linde T, Danielson BG. Dialysis adequacy after normalization of hemoglobin with erythropoietin (EPO) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):296A. [CENTRAL: CN-00445403] [Google Scholar]
- Furuland H, Linde T, Danielson BG. Physical exercise capacity in patients with end-stage renal disease after normalizaton of hemoglobin with erythropoietin (EPO) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):337A. [CENTRAL: CN-00445404] [Google Scholar]
- Furuland H, Linde T, Sandhagen B, Andren B, Wikstrom B, Danielson BG. Hemorheological and hemodynamic changes in predialysis patients after normalization of hemoglobin with epoetin-alpha. Scandinavian Journal of Urology & Nephrology 2005;39(5):399-404. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Furuland H, Linde T, Wikstrom B, Danielson BG. Reduced hemodialysis adequacy after hemoglobin normalization with epoetin. Journal of Nephrology 2005;18(1):80-5. [MEDLINE: ] [PubMed] [Google Scholar]
- Linde T, Ekberg H, Forslund T, Furuland H, Holdaas H, Nyberg G, et al. The use of pretransplant erythropoietin to normalize hemoglobin levels has no deleterious effects on renal transplantation outcome. Transplantation 2001;71(1):79-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Linde T, Wahlberg J, Furuland H, Danielson BG. Results of renal transplantation in patients randomized to EPO treatment aimed to reach a subnormal or normal Hb [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):684A. [CENTRAL: CN-00446408] [Google Scholar]
- Strombom U, Ahlmen J, Danielsson B. The Scandinavian erythropoietin study: effects on quality of life of normalizing hemoglobin levels in uremic patients [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):269A. [Google Scholar]
Mohajeranirad 2021 {published data only}
- Mohajeranirad M, Saeidi N, Kamali Nejad M, Almasi-Hashiani A, Salehi M, Latifi SA. Effects of Helichrysum psudoplicatum supplementation on pruritus intensity, fatigue, quality of life and anorexia in hemodialysis patients: a randomized, double-blind placebo-controlled trial. Hormone Molecular Biology & Clinical Investigation 2021;43(2):211-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mohamed 2013 {published data only}
- Mohamed A, Alam JH, Barre P, Vasilevsky M, Beauchemin R, Iqbal S. A randomized controlled trial to examine the effect of dialysate glucose concentration on quality of life among hemodialysis patients with type 2 diabetes mellitus [abstract]. Hemodialysis International 2013;17(1):167. [EMBASE: 71022215] [Google Scholar]
Mohamed 2014 {published data only}
- Mohamed SA. The effectiveness of an educational intervention on fatigue in hemodialysis patients: a randomized controlled trial. Journal of Nursing & Health Science 2014;3(4):40-50. [CENTRAL: CN-01657905] [Google Scholar]
Mohammadpourhodki 2021 {published data only}
- Mohammadpourhodki R, Sadeghnezhad H, Ebrahimi H, Basirinezhad MH, Maleki M, Bossola M. The effect of aromatherapy massage with lavender and citrus aurantium essential oil on quality of life of patients on chronic hemodialysis: a parallel randomized clinical trial study. Journal of Pain & Symptom Management 2021;61(3):456-63.e1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Motedayen 2014 {published data only}
- Motedayen Z, Nehrir B, Tayebi A, Ebadi A, Einollahi B. The effect of the physical and mental exercises during hemodialysis on fatigue: a controlled clinical trial. Nephrourology Monthly 2014;6(4):e14686. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muz 2017 {published data only}
- Muz G, Tasci S. Effect of aromatherapy via inhalation on the sleep quality and fatigue level in people undergoing hemodialysis. Applied Nursing Research 2017;37:28-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ozdemir 2013 {published data only}
- Ozdemir G, Ovayolu N, Ovayolu O. The effect of reflexology applied on haemodialysis patients with fatigue, pain and cramps. International Journal of Nursing Practice 2013;19(3):265-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parfrey 2005 {published data only}
- Foley RN, Curtis BM, Parfrey PS. Erythropoietin therapy, hemoglobin targets, and quality of life in healthy hemodialysis patients: a randomized trial. Clinical Journal of the American Society of Nephrology: CJASN 2009;4(4):726-33. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley RN, Curtis BM, Parfrey PS. Hemoglobin targets and blood transfusions in hemodialysis patients without symptomatic cardiac disease receiving erythropoietin therapy. Clinical Journal of the American Society of Nephrology: CJASN 2008;3(6):1669-75. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley RN, Curtis BM, Parfrey PS. Hemoglobin targets, blood transfusions and quality of life in hemodialysis patients without symptomatic cardiac disease [abstract no: SA-PO2745]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):730A. [CENTRAL: CN-00756852] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley RN, Curtis BM, Randell EW, Parfrey PS. Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(5):805-13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley RN, Parfrey PS, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D, et al. The effect of higher haemoglobin levels on left ventricular cavity volume in patients starting haemodialysis: a blinded, randomised, controlled trial in 596 patients without symptomatic cardiac disease [abstract]. In: 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15-18; Lisbon, Portugal. 2004:217.
- Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D, et al. Double-blind comparison of full and partial anemia correction with erythropoietin in incident hemodialysis patients without symptomatic heart disease [abstract no: PUB002]. Journal of the American Society of Nephrology 2004;15(Oct):762A. [CENTRAL: CN-00583759] [DOI] [PubMed] [Google Scholar]
- Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D, et al. The effect of higher haemoglobin levels on quality of life in patients starting haemodialysis: a blinded, randomised, controlled trial in 596 patients without symptomatic cardiac disease [abstract]. In: 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15-18; Lisbon, Portugal. 2004:229. [CENTRAL: CN-00509402]
- Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. Journal of the American Society of Nephrology 2005;16(7):2180-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Williams CE, Curtis BM, Randell EW, Foley RN, Parfrey PS. Cardiac biomarkers and health-related quality of life in new hemodialysis patients without symptomatic cardiac disease. Canadian Journal of Kidney Health & Disease 2014;1:16. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PEDAL 2020 {published data only}
- Greenwood SA, Koufaki P, Macdonald J, Bhandari S, Burton J, Dasgupta I, et al. The PrEscription of intraDialytic exercise to improve quAlity of Life in patients with chronic kidney disease trial: study design and baseline data for a multicentre randomized controlled trial. Clinical Kidney Journal 2021;14(5):1345–55. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood SA, Koufaki P, Macdonald JH, Bhandari S, Burton JO, Dasgupta I, et al. Randomized trial - PrEscription of intraDialytic exercise to improve quAlity of Life in patients receiving hemodialysis. KI Reports 2021;6(8):2159-70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood SA, Koufaki P, Macdonald JH, Bulley C, Bhandari S, Burton JO, et al. Exercise programme to improve quality of life for patients with end-stage kidney disease receiving haemodialysis: the PEDAL RCT. Health Technology Assessment 2021;25(40):1-52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pellizzaro 2013 {published data only}
- Pellizzaro CO, Thome FS, Veronese FV. Effect of peripheral and respiratory muscle training on the functional capacity of hemodialysis patients. Renal Failure 2013;35(2):189-97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Picariello 2018 {published data only}91238019
- Picariello F, Moss-Morris R, Macdougall IC, Norton S, Da Silva-Gane M, Farrington K, et al. Cognitive-behavioural therapy (CBT) for renal fatigue (BReF): a feasibility randomised-controlled trial of CBT for the management of fatigue in haemodialysis (HD) patients. BMJ Open 2018;8(3):e020842. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picariello F, Moss-Morris R, Norton S, Macdougall IC, Da Silva-Gane M, Farrington K, et al. Feasibility trial of cognitive behavioral therapy for fatigue in hemodialysis (BReF Intervention). Journal of Pain & Symptom Management 2021;61(6):1234-46.e5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raimann 2010 {published data only}
- Ferrario M, Raimann JG, Thijssen S, Signorini MG, Kruse A, Diaz-Buxo JA, et al. Effects of dialysate glucose concentration on heart rate variability in chronic hemodialysis patients: Results of a prospective randomized trial. Kidney & Blood Pressure Research 2011;34(5):334-43. [EMBASE: 21613795] [DOI] [PubMed] [Google Scholar]
- Ferrario M, Signorini MG, Raimann J, Kruse A, Thijssen S, Kotanko P, et al. The effects of dialysate dextrose concentration of the autonomic control in diabetic and non-diabetic populations [abstract no: M541]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009.
- Kruse A, Raimann J, Kuntsevich V, Dabel P, Arthur B, Thijssen S, et al. Arrhythmias in hemodialysis patients: a comparison between 100 and 200 mg/dl dialysate dextrose concentration [abstract no: SU606]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009.
- Raimann J, Kruse A, Kuntsevich V, Dabel P, Thijssen S, Kotanko P, et al. Prospective randomized study of two levels of dialysate dextrose concentration: 100 versus 200 mg/dL [abstract no: SU603]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009.
- Raimann J, Kruse A, Kuntsevich V, Winchester J, Diaz-Buxo J, Kotanko P, et al. Fatigue in chronic hemodialysis patients -results from a prospective randomized cross-over trial of 200 mg/dL and 100 mg/dL dialysate dextrose concentrations [abstract no: TH-PO339]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):190A. [Google Scholar]
- Raimann JG, Kruse A, Kuntsevich V, Thijssen S, Diaz-Buxo J, Levin NW, et al. Effects of dialysate glucose on blood pressure in chronic hemodialysis patients [abstract no: Sa603]. NDT Plus 2010;3(Suppl 3):iii245. [EMBASE: 70484069] [Google Scholar]
- Raimann JG, Kruse A, Thijssen S, Kuntsevich V, Dabel P, Bachar M, et al. Metabolic effects of dialyzate glucose in chronic hemodialysis: results from a prospective, randomized crossover trial. Nephrology Dialysis Transplantation 2012;27(4):1559-68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Raimann JG, Kruse A, Thijssen S, Kuntsevich V, Diaz-Buxo JA, Levin NW, et al. Fatigue in hemodialysis patients with and without diabetes: results from a randomized controlled trial of two glucose-containing dialysates. Diabetes Care 2010;33(9):e121. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reilly‐Spong 2015 {published data only}
- Gross CR, Reilly-Spong M, Park T, Zhao R, Gurvich O. Randomized clinical trial of telephone-adapted mindfulness training on anxiety, symptom distress, and quality of life in patients with progressive renal disease [abstract]. Journal of Alternative & Complementary Medicine 2016;22(6):A77. [EMBASE: 611808428] [Google Scholar]
- Gross CR, Reilly-Spong M, Park T, Zhao R, Gurvich OV, Ibrahim HN. Telephone-adapted Mindfulness-based Stress Reduction (tMBSR) for patients awaiting kidney transplantation. Contemporary Clinical Trials 2017;57:37-43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly-Spong M, Reibel D, Pearson T, Koppa P, Gross CR. Telephone-adapted mindfulness-based stress reduction (tMBSR) for patients awaiting kidney transplantation: Trial design, rationale and feasibility. Contemporary Clinical Trials 2015;42:169-84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roshanravan 2016 {published data only}
- Roshanravan M, Jouybari L, Bahrami-Taghanaki H, Vakili MA, Sanagoo A, Amini Z. Effect of foot reflexology on fatigue in patients undergoing hemodialysis: a sham-controlled randomized trial. Journal of Mazandaran University of Medical Sciences 2016;26(137):32-41. [EMBASE: 610668018] [Google Scholar]
Sabouhi 2013 {published data only}
- Sabouhi F, Kalani L, Valiani M, Mortazavi M, Bemanian M. Effect of acupressure on fatigue in patients on hemodialysis. Iranian Journal of Nursing & Midwifery Research 2013;18(6):429-34. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Sajadi 2016 {published data only}
- Sajadi M, Gholami Z, Hekmatpour D, Soltani P, Haghverdi F. Cold dialysis solution for hemodialysis patients with fatigue: a cross-over study [Erratum in: Iran J Kidney Dis. 2016 Nov;10 (6):419 PMID: 27904003]. Iranian Journal of Kidney Diseases 2016;10(5):319-24. [PMID: ] [PubMed] [Google Scholar]
Salehi 2020 {published data only}
- Salehi F, Dehghan M, Mangolian Shahrbabaki P, Ebadzadeh MR. Effectiveness of exercise on fatigue in hemodialysis patients: a randomized controlled trial. BMC Sports Science, Medicine & Rehabilitation 2020;12:19. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sang 1997 {published data only}
- Sang GL, Kovithavongs C, Ulan R, Kjellstrand CM. Sodium ramping in hemodialysis: a study of beneficial and adverse effects. American Journal of Kidney Diseases 1997;29(5):669-77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schardong 2021 {published data only}
- Schardong J, Falster M, Sisto IR, Barbosa AP, Normann TC, Souza KS, et al. Photobiomodulation therapy increases functional capacity of patients with chronic kidney failure: randomized controlled trial. Lasers in Medical Science 2021;36(1):119-29. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schmitz 2016 {published data only}
- Schmitz M, Loke O, Fach B, Kalb K, Heering PJ, Meinke D, et al. Effects of citrate dialysate in chronic dialysis: a multicentre randomized crossover study. Nephrology Dialysis Transplantation 2016;31(8):1327-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Semeniuk 2000 {published data only}
- Semeniuk J, Shalansky KF, Taylor N, Jastrzebski J, Cameron EC. Evaluation of the effect of intravenous l-carnitine on quality of life in chronic hemodialysis patients. Clinical Nephrology 2000;54(6):470-7. [MEDLINE: ] [PubMed] [Google Scholar]
Shahdadi 2016 {published data only}
- Shahdadi H, Hodki RM, Abadi AA, Sheikh A, Moghadasi A. The effect of slow stroke back massage on fatigue in patients undergoing hemodialysis: a randomized clinical trial. International Journal of Pharmacy & Technology 2016;8(3):16016-23. [EMBASE: 612952307] [Google Scholar]
Singer 2010 {published data only}
- Singer R. Vitamin C supplementation in kidney failure: effect on uraemic symptoms [abstract no: 066]. Nephrology 2010;15(Suppl 4):44. [CENTRAL: 70467070] [DOI] [PubMed] [Google Scholar]
- Singer RF. Vitamin C supplementation in kidney failure: effect on uraemic symptoms. Nephrology Dialysis Transplantation 2011;26(2):614-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singh 2003 {published data only}
- Singh NP, Banal R, Thakur A, Kohli R, Bansal RC, Agarwal SK. Effect of membrane composition on cytokine production and clinical symptoms during hemodialysis: a crossover study. Renal Failure 2003;25(3):419-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singh 2008a {published data only}
- Singh A, Hertel J, Bernardo M, Baptista J, Kausz A, Brenner L, et al. A double-blind, placebo-controlled, randomized phase III study of the safety of ferumoxytol as a new intravenous iron replacement therapy [abstract no: 29]. American Journal of Kidney Diseases 2007;49(4):A32. [CENTRAL: CN-00716121] [Google Scholar]
- Singh A, Patel T, Hertel J, Bernardo M, Kausz A, Brenner L. Safety of ferumoxytol in patients with anemia and CKD. American Journal of Kidney Diseases 2008;52(5):907-15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sklar 1998 {published data only}
- Sklar AH, Beezhold DH, Dreisbach AW, Hendrickson T, Riesenberg LA. Effect of a biocompatible membrane on cytokines and postdialysis fatigue [abstract no: P1343]. Nephrology 1997;3(Suppl 1):S408. [Google Scholar]
- Sklar AH, Beezhold DH, Newman N, Hendrickson T, Dreisbach AW. Postdialysis fatigue: lack of effect of a biocompatible membrane. American Journal of Kidney Diseases 1998;31(6):1007-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sklar 1999 {published data only}
- Sklar A, Newman N, Scott R, Semenyuk L, Schultz J, Fiacco V. Identification of factors responsible for postdialysis fatigue. American Journal of Kidney Diseases 1999;34(3):464-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SOCIABLE 2017 {published data only}
- Crews DC, Delaney AM, Walker JL, Cudjoe TK, Evelyn-Gustave A, Roth J, et al. Improving physical function and social networks of older adults with ESRD: development and testing of seniors optimizing community integration to advance better living with ESRD (SOCIABLE) [abstract no: FR-PO924]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):643. [EMBASE: 633700279] [Google Scholar]
- Crews DC, Delaney AM, Walker Taylor JL, Cudjoe TK, Nkimbeng M, Roberts L, et al. Pilot intervention addressing social support and functioning of low socioeconomic status older adults with ESRD: The Seniors Optimizing Community Integration to Advance Better Living with ESRD (SOCIABLE) Study. Kidney Medicine 2019;1(1):13-20. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soliman 2015 {published data only}
- Soliman HM. Effect of intradialytic exercise on fatigue, electrolytes level and blood pressure in hemodialysis patients: a randomized controlled trial. Journal of Nursing Education & Practice 2015;5(11):16-28. [Google Scholar]
Su 2009 {published data only}
- Su LH, Wu KD, Lee LS, Wang H, Liu CF. Effects of far infrared acupoint stimulation on autonomic activity and quality of life in hemodialysis patients. American Journal of Chinese Medicine 2009;37(2):215-26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Suzuki 2018 {published data only}
- Morton RL. The symptom monitoring with feedback trial (swift): A novel registry-based cluster randomised controlled trial among Australian and New Zealand adults with end-stage kidney disease managed on haemodialysis [abstract]. Nephrology 2018;23(Suppl 3):82-3. [EMBASE: 623841124] [Google Scholar]
- Suzuki T, Ikeda M, Minami M, Matayoshi Y, Nakao M, Nakamura T, et al. Beneficial effect of intradialytic electrical muscle stimulation in hemodialysis patients: a randomized controlled trial. Artificial Organs 2018;42(9):899-910. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SWIFT 2020 {published data only}
- Duncanson E, Bennett PN, Viecelli A, Dansie K, Handke W, Tong A, et al. Feasibility and acceptability of e-PROMs data capture and feedback among patients receiving haemodialysis in the Symptom monitoring WIth Feedback Trial (SWIFT) pilot: protocol for a qualitative study in Australia. BMJ Open 2020;10(11):e039014. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenham L, Bennett PN, Dansie K, Viecelli AK, Jesudason S, Mister R, et al. The Symptom Monitoring with Feedback Trial (SWIFT): protocol for a registry-based cluster randomised controlled trial in haemodialysis. Trials [Electronic Resource] 2022;23(1):419. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton R. Study protocol for the symptom monitoring with feedback trial (SWIFT): a novel registry-based cluster randomised trial among adults with end-stage kidney disease managed on haemodialysis [abstract no: SAT-037]. Kidney International Reports 2019;4(7 Suppl):S19. [EMBASE: 2002179660] [Google Scholar]
- Morton RL, Dansie K, Bennett PN, Duncanson E, Viecelli AK, Jesudason S, et al. Feasibility and acceptability of symptom monitoring with feedback trial (SWIFT) for adults on hemodialysis: a pilot ANZDATA registry-based cluster randomized trial [abstract no: PO1080]. Journal of the American Society of Nephrology 2020;31(Abstract Suppl):367. [EMBASE: 633704095] [Google Scholar]
- Viecelli A, Dansie K, McDonald S, Jesudason S, Duncanson E, Bennett P, et al. Symptom monitoring with feedback trial (SWIFT) pilot to explore the feasibility and acceptability of electronic patient reported outcome measures (e-proms) data capture and feedback [abstract no: MO037]. Nephrology Dialysis Transplantation 2020;35(Suppl 3):iii137. [EMBASE: 633422179] [Google Scholar]
Thomas 2017 {published data only}
- Thomas Z, Novak M, Platas SG, Gautier M, Holgin AP, Fox R, et al. Brief mindfulness meditation for depression and anxiety symptoms in patients undergoing hemodialysis: a pilot feasibility study. Clinical Journal of The American Society of Nephrology: CJASN 2017;12(12):2008-15. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 2016 {published data only}
- Tsai MY, Su YJ, Ng HY, Chen SY, Huang YC, Wu CH, et al. Study protocol for a single-blind, placebo-controlled randomised trial of Tianjiu effects in patients with intradialytic hypotension. BMJ Open 2016;6(3):e009976. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MY, Wu CH, Huang YC, Chen SY, Ng HY, Su YJ, et al. Treatment of intradialytic hypotension with an herbal acupoint therapy in hemodialysis patients: a randomized pilot study. Complementary Therapies in Medicine 2018;38:67-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsay 2004a {published data only}
- Tsay SL. Acupressure and fatigue in patients with end-stage renal disease-a randomized controlled trial. International Journal of Nursing Studies 2004;41(1):99-106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsay 2004b {published data only}
- Tsay SL, Cho YC, Chen ML. Acupressure and transcutaneous electrical acupoint stimulation in improving fatigue, sleep quality and depression in hemodialysis patients. American Journal of Chinese Medicine 2004;32(3):407-16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Unal 2016 {published data only}
- Unal KS, Balci Akpinar R. The effect of foot reflexology and back massage on hemodialysis patients' fatigue and sleep quality. Complementary Therapies in Clinical Practice 2016;24:139-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Varaei 2020 {published data only}
- Varaei S, Jalalian Z, Yekani Nejad MS, Shamsizadeh M. Comparison the effects of inhalation and massage aromatherapy with lavender and sweet orange on fatigue in hemodialysis patients: a randomized clinical trial. Journal of Complementary & Integrative Medicine 2020;18(1):193-200. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
VENOUS 2020 {published data only}
- Masakne I. Verification of nutrition maintaining effects by anti-thrombotic PMMA membrane (VENUS Study) [abstract no: SAT-256]. Kidney International Reports 2020;5(3 Suppl):S108-9. [EMBASE: 2005255703] [Google Scholar]
Vishnevskii 2014 {published data only}
- Vishnevskii KA, Rumyantsev AS, Zemchenkov AY, Smirnov AV. Improvement of the physical ability and hemodialysis efficiency due to transcutaneous electrical muscle stimulation of lower extremities [abstract no: SP506]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii240-1. [EMBASE: 71492133] [Google Scholar]
Yurtkuran 2007 {published data only}
- Yurtkuran M, Alp A, Yurtkuran M, Dilek K. A modified yoga-based exercise program in hemodialysis patients: a randomized controlled study. Complementary Therapies in Medicine 2007;15(3):164-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
CHAIR 2015 {published data only}
- Matsufuji S, Shoji T, Yano Y, Tsujimoto Y, Kishimoto H, Tabata T, et al. Effect of chair stand exercise on activity of daily living: a randomized controlled trial in hemodialysis patients. Journal of Renal Nutrition 2015;25(1):17-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Churchill 1987 {published data only}
- Churchill DN, Taylor DW, Sackett DL, Shimizu AG. Reuse of dialyzers: a multiple cross-over trial with random allocation to treatment order [abstract]. Kidney International 1987;31(1):230. [CENTRAL: CN-00550365] [Google Scholar]
- Churchill DN, Taylor DW, Shimizu AG, Beecroft ML, Singer J, Barnes CC, et al. Dialyzer re-use--a multiple crossover study with random allocation to order of treatment. Nephron 1988;50(4):325-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dashti‐Khavidaki 2011 {published data only}
- Dashti-Khavidaki S, Chamani N, Khalili H, Hajhossein TA, Ahmadi F, Lessan-Pezeshki M, et al. Comparing effects of clonazepam and zolpidem on sleep quality of patients on maintenance hemodialysis. Iranian journal of Kidney Diseases 2011;5(6):404-9. [MEDLINE: ] [PubMed] [Google Scholar]
Eglence 2013 {published data only}
- Eglence R, Karatas N, Tasci S. The effect of acupressure on the level of fatigue in hemodialysis patients. Alternative Therapies in Health & Medicine 2013;19(6):23-31. [MEDLINE: ] [PubMed] [Google Scholar]
Gram 1998 {published data only}
- Gram J, Hansen TB, Jensen PB, Christensen JH, Ladefoged S, Pedersen FB. The effect of recombinant human growth hormone treatment on bone and mineral metabolism in haemodialysis patients. Nephrology Dialysis Transplantation 1998;13(6):1529-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hansen TB, Gram J, Jensen PB, Kristiansen JH, Ekelund B, Christiansen JS, et al. Influence of growth hormone on whole body and regional soft tissue composition in adult patients on hemodialysis. A double-blind, randomized, placebo-controlled study. Clinical Nephrology 2000;53(2):99-107. [MEDLINE: ] [PubMed] [Google Scholar]
- Jensen PB, Ekelund B, Nielsen FT, Baumbach L, Pedersen FB, Oxhoj H. Changes in cardiac muscle mass and function in hemodialysis patients during growth hormone treatment. Clinical Nephrology 2000;53(1):25-32. [MEDLINE: ] [PubMed] [Google Scholar]
- Jensen PB, Hansen TB, Frystyk J, Ladefoged SD, Pedersen FB, Christiansen JS. Growth hormone, insulin-like growth factors and their binding proteins in adult hemodialysis patients treated with recombinant human growth hormone. Clinical Nephrology 1999;52(2):103-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Jensen PB, Hansen TB, Oxhoj H, Froberg K, Ekelund B, Nielsen FT, et al. What are the clinical benefits of correcting the catabolic state in haemodialysis patients? British Journal of Clinical Practice Supplement 1996;85:47-51. [MEDLINE: ] [PubMed] [Google Scholar]
Heshmatifar 2015 {published data only}
- Heshmatifar N, Sadeghi H, Mahdavi A, Shegarf Nakhaie MR, Rakhshani MH. The effect of Benson relaxation technique on depression in patients undergoing hemodialysis. Journal of Babol University of Medical Sciences 2015;17(8):34-40. [EMBASE: 605531696] [Google Scholar]
Heshmati Far 2015 {published data only}
- Heshmati Far N, Salari M, Rakhshani MH, Borzoee F, Sahebka M. The effects of Benson relaxation technique on activities of daily living in hemodialysis patients; a single-blind, randomized, parallel-group, controlled trial study. Complementary Therapies in Clinical Practice 2020;39:101133. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laupacis 1992 {published data only}
- Laupacis A, Muirhead N, Keown P, Wong C. A disease-specific questionnaire for assessing quality of life in patients on haemodialysis [Erratum in: Nephron 1992;61(2):248]. Nephron 1992;60(3):302-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Macagnan 2019 {published data only}
- Macagnan FE, Baroni BM, Cristofoli EZ, Godoy M, Schardong J, Plentz RD. Acute effect of photobiomodulation therapy on handgrip strength of chronic kidney disease patients during hemodialysis. Lasers in Medical Science 2019;34(4):835-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakamoto 2008 {published data only}
- Nakamoto H, Mimura T, Honda N. Orally administrated Juzen-taiho-to/TJ-48 ameliorates erythropoietin (rHuEPO)-resistant anemia in patients on hemodialysis. Hemodialysis International 2008;12 Suppl 2:S9-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sharp 2005 {published data only}
- Sharp J, Wild MR, Gumley AI, Deighan CJ. A cognitive behavioral group approach to enhance adherence to hemodialysis fluid restrictions: a randomized controlled trial. American Journal of Kidney Diseases 2005;45(6):1046-57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shimizu 1983 {published data only}
- Shimizu AG, Taylor DW, Sackett DL, Smith EK, Barnes CC, Hoda P, et al. Reducing patient morbidity from high-efficiency hemodialysis: a double-blind crossover trial. Transactions - American Society for Artificial Internal Organs 1983;29:666-8. [MEDLINE: ] [PubMed] [Google Scholar]
Siami 1991 {published data only}
- Siami G, Clinton ME, Mrak R, Griffis J, Stone W. Evaluation of the effect of intravenous L-carnitine therapy on function, structure and fatty acid metabolism of skeletal muscle in patients receiving chronic hemodialysis. Nephron 1991;57(3):306-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tawney 2000 {published data only}
- Tawney KW, Tawney PJ, Hladik G, Hogan SL, Falk RJ, Weaver C, et al. The life readiness program: a physical rehabilitation program for patients on hemodialysis. American Journal of Kidney Diseases 2000;36(3):581-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tawney KW, Tawney PJ, Hladik GA, Hogan SL, Moore DT, Falk RJ. The life readiness program: a rehabilitation program for end-stage renal disease patients on hemodialysis [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):270A. [CENTRAL: CN-00583258] [DOI] [PubMed] [Google Scholar]
TREAT 2005 {published data only}
- Bello NA, Lewis EF, Desai AS, Anand IS, Krum H, McMurray JJ, et al. Increased risk of stroke with darbepoetin alfa in anaemic heart failure patients with diabetes and chronic kidney disease. European Journal of Heart Failure 2015;17(11):1201-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charytan DM, Lewis EF, Desai AS, Weinrauch LA, Ivanovich P, Toto RD, et al. Cause of death in patients with diabetic CKD enrolled in the trial to reduce cardiovascular events with Aranesp therapy (TREAT). American Journal of Kidney Diseases 2015;66(3):429-40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charytan DM, Solomon SD, Ivanovich P, Remuzzi G, Cooper ME, McGill JB, et al. ESRD after heart failure, myocardial infarction, or stroke in type 2 diabetic patients with CKD. American Journal of Kidney Diseases 2017;70(4):522-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Desai AS, Toto R, Jarolim P, Uno H, Eckardt KU, Kewalramani R, et al. Association between cardiac biomarkers and the development of ESRD in patients with type 2 diabetes mellitus, anemia, and CKD. American Journal of Kidney Diseases 2011;58(5):717-28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lewis EF, Claggett B, Parfrey PS, Burdmann EA, McMurray JJ, Solomon SD, et al. Race and ethnicity influences on cardiovascular and renal events in patients with diabetes mellitus. American Heart Journal 2015;170(2):322-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lewis EF, Pfeffer MA, Feng A, Uno H, McMurray JJ, Toto R, et al. Darbepoetin alfa impact on health status in diabetes patients with kidney disease: a randomized trial. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(4):845-55. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli F. Iron treatment and the TREAT trial. NDT Plus 2011;4(Suppl 1):i3-5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Causland FR, Claggett B, Burdmann EA, Eckardt KU, Kewalramani R, Levey AS, et al. C-reactive protein and risk of ESRD: results from the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT). American Journal of Kidney Diseases 2016;68(6):873-81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray JJ, Uno H, Jarolim P, Desai AS, Zeeuw D, Eckardt KU, et al. Predictors of fatal and nonfatal cardiovascular events in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia: an analysis of the Trial to Reduce cardiovascular Events with Aranesp (darbepoetin-alfa) Therapy (TREAT). American Heart Journal 2011;162(4):748-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mix TC, Brenner RM, Cooper ME, Zeeuw D, Ivanovich P, Levey AS, et al. Rationale--Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT): evolving the management of cardiovascular risk in patients with chronic kidney disease. American Heart Journal 2005;149(3):408-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Burdmann EA, Chen C, Cooper ME, Zeeuw D, Eckardt K, et al. Trial to reduce cardiovascular events with Aranesp therapy [abstract no: 114]. Circulation 2010;120(21):2154-5. [EMBASE: 70089295] [Google Scholar]
- Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, Zeeuw D, Eckardt KU, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. New England Journal of Medicine 2009;361(21):2019-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, Zeeuw D, Eckardt KU, et al. Baseline characteristics in the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT). American Journal of Kidney Diseases 2009;54(1):59-69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pfeffer MA. An ongoing study of anemia correction in chronic kidney disease. New England Journal of Medicine 2007;356(9):959-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sabe MA, Claggett B, Burdmann EA, Desai AS, Ivanovich P, Kewalramani R, et al. Coronary artery disease is a predictor of progression to dialysis in patients with chronic kidney disease, type 2 diabetes mellitus, and anemia: an analysis of the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT). Journal of the American Heart Association 2016;5(4):e002850. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skali H, Lin J, Pfeffer MA, Chen CY, Cooper ME, McMurray JJ, et al. Hemoglobin stability in patients with anemia, CKD, and type 2 diabetes: an analysis of the TREAT (Trial to Reduce Cardiovascular Events With Aranesp Therapy) placebo arm. American Journal of Kidney Diseases 2013;61(2):238-46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Skali H, Parving HH, Parfrey PS, Burdmann EA, Lewis EF, Ivanovich P, et al. Stroke in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia treated with Darbepoetin Alfa: the trial to reduce cardiovascular events with Aranesp therapy (TREAT) experience. Circulation 2011;124(25):2903-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. New England Journal of Medicine 2010;363(12):1146-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Theilade S, Claggett B, Hansen TW, Skali H, Lewis EF, Solomon SD, et al. Pulse pressure is not an independent predictor of outcome in type 2 diabetes patients with chronic kidney disease and anemia--the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT). Journal of Human Hypertension 2016;30(1):46-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thomas MC, Cooper ME, Rossing K, Parving HH. Anaemia in diabetes: Is there a rationale to TREAT? Diabetologia 2006;49(6):1151-7. [EMBASE: 2006226725] [DOI] [PubMed] [Google Scholar]
- Toto R, Ivanovich P, Levey A, Parfrey PS, Pereira BJG, Remuzzi G, et al. Trial to Reduce cardiovascular Events with Aranesp® (darbepoetin alfa) Therapy (TREAT) [abstract no: SU-PO239]. Journal of the American Society of Nephrology 2004;15(Oct):584A. [CENTRAL: CN-00550766] [Google Scholar]
- Weinrauch LA, D'Elia JA, Finn P, Lewis EF, Desai AS, Claggett BL, et al. Strategies for glucose control in a study population with diabetes, renal disease and anemia (TREAT study). Diabetes Research & Clinical Practice 2016;113:143-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsai 2015 {published data only}
- Tsai SH, Wang MY, Miao NF, Chian PC, Chen TH, Tsai PS. CE: original research: The efficacy of a nurse-led breathing training program in reducing depressive symptoms in patients on hemodialysis: a randomized controlled trial. American Journal of Nursing 2015;115(4):24-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00440869 {published data only}
- Johansen KL. Effects of n-acetylcysteine on muscle fatigue in hemodialysis (NAC) [Effects of N-acetylcysteine on muscle fatigue in ESRD]. clinicaltrials.gov/ct2/show/NCT00440869 received 27 February 2007.
References to ongoing studies
ACTRN12617000420347 {published data only}
- Barr K. Evaluating the effectiveness of practicing yoga during haemodialysis for fatigue in patients with end stage kidney disease [Evaluating the effectiveness of intradialytic yoga for fatigue in patients with end stage kidney disease receiving haemodialysis: a mixed methods approach]. www.anzctr.org.au (first received 17 March 2017).
ACTRN12618000724279 {published data only}
- Chojak-Fijalka K. Evaluation of the effectiveness of home-based physical training in patients undergoing haemodialysis [The effects of home-based physical training in patients undergoing haemodialysis on physical functioning, body composition, quality of life, fatigue and selected properties of blood]. www.anzctr.org.au (first received 18 March 2018).
ACTRN12620000408987 {published data only}
- Tong A. Structured exercise prograM to reduce Fatigue In patients receiving dialysis: a preference-stratified adaptive Trial (M-FIT). www.anzctr.org.au 2020.
Burrai 2019a {published data only}
- Burrai F, Othman S, Brioni E, Micheluzzi V, Luppi M, Apuzzo L, et al. Effects of virtual reality in patients undergoing dialysis: study protocol. Holistic Nursing Practice 2019;33(6):327-37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cardoso 2019 {published data only}
- Cardoso RK, Araujo AM, Orcy RB, Bohlke M, Oses JP, Del Vecchio FB, et al. Effects of continuous moderate exercise with partial blood flow restriction during hemodialysis: a protocol for a randomized clinical trial. MethodsX 2019;6:190-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CONVINCE 2020 {published data only}
- Blankestijn PJ, Fischer KI, Barth C, Cromm K, Canaud B, Davenport A, et al. Benefits and harms of high-dose haemodiafiltration versus high-flux haemodialysis: the comparison of high-dose haemodiafiltration with high-flux haemodialysis (CONVINCE) trial protocol. BMJ Open 2020;10(2):e033228. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Blankestijn PJ, Cromm K, Bots M, Barth CM, Canaud B, et al. Assessment of generic and dialysis-related fatigue: identification of individual trajectories and investigation of ecological validity [abstract no: 3200]. Quality of Life Research 2020;29(Suppl 1):S180. [EMBASE: 634412125] [Google Scholar]
- Fischer KI, Blankestijn P, Cromm K, Bots M, Barth CM, Canaud B, et al. Assessing the patient's perspective in end-stage kidney disease - a domain-guided approach applied in the CONVINCE trial [abstract]. In: International Society for Quality of Life Research (ISOQOL); 26th Annual Conference; 2019 Oct 20-23, San Diego, CA. 2019. [https://www.ucl.ac.uk/convince-hemodiafiltration-dialysis-study/sites/convince-hemodiafiltration-dialysis-study/files/assessing_the_patients_perspective_in_end-stage_kidney_disease_04102019.pdf]
- Fischer KI, Blankestijn PJ, Cromm K, Canaud BJ, Barth CM, Hegbrant JB, et al. A new approach to assess patient-reported outcomes of patients with ESKD in an international randomized clinical trial [abstract no: PUB117]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):1104. [EMBASE: 633769665] [Google Scholar]
CTRI/2018/02/012021 {published data only}
- Raj PD, Bai RS. Exercise during dialysis for reducing tiredness and improving the sleep for patients undergoing hemodialysis [The effectiveness of intradialytic exercise on fatigue and quality of sleep among patients undergoing hemodialysis]. www.ctri.nic.in (first received 21 February 2018).
Hamad 2021 {published data only}
- Hamad AI, Mishra RK, Ibrahim RA, Mathew M, Mohamed MY, Ateya HM, et al. Effectiveness of intradialytic plantar electrical nerve stimulation during hemodialysis to improve the gait in adults with diabetes and renal failure: A randomized double-blinded controlled trial [abstract no: PO0773]. Journal of the American Society of Nephrology 2021;32:271. [EMBASE: 636328282] [Google Scholar]
- Mishra RK, Al-Ali F, Hamad A, Ibrahim R, Mathew M, Najafi B. Effect of plantar electrical nerve stimulation during routine hemodialysis process on the daily physical activity in adults with diabetes and endstage renal disease-a randomized doubleblinded controlled trial [abstract no: MO623). Nephrology Dialysis Transplantation 2021;36(Suppl 1):i367. [PMID: 635918437] [Google Scholar]
NCT01620580 {published data only}
- Danquah FV. Symptom management program for hemodialysis patients. www.clinicaltrials.gov/ct2/show/NCT01620580 (first received 24 October 2014).
NCT02361268 {published data only}
- Birdee GS. End-stage renal disease intra-dialysis lifestyle education study (END-IDLE). www.clinicalTrials.gov/show/NCT02361268 (first received 29 January 2015).
Quintiliano 2019 {published data only}
- Quintiliano A, Oehmen T, Kirsztajn GM, Pegado R. Transcranial direct current stimulation in management of pain, mood, functionality, and quality of life in patients undergoing hemodialysis: a study protocol for a double-blind controlled randomized trial. Trials [Electronic Resource] 2019;20(1):805. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2022 {published data only}
- Sharma S, Alexander KE, Green T, Wu MW, Bonner A. Energy conservation education intervention for people with end-stage kidney disease receiving haemodialysis (EVEREST): protocol for a cluster randomised control trial. BMJ Open 2022;12(2):e056544. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
SLEEP‐HD 2021 {published data only}
- McCurry S, Cukor D, Clark C, Brady N, Rue T, Unruh M, et al. Tailoring of cognitive behavior therapy for insomnia for patients with kidney failure undergoing hemodialysis: the sleep-HD study [abstract no: 359]. Sleep 2021;44(Suppl 2):A143. [EMBASE: 635915012] [Google Scholar]
TACcare 2018 {published data only}
- Devaraj SM, Yabes J, Roumelioti ME, Steel JL, Erickson SJ, Unruh ML, et al. Prevalence and demographic correlates of pain, depression, fatigue, and readiness to seek treatment for these symptoms in hemodialysis patients [abstract no: PO0834]. Journal of the American Society of Nephrology 2021;32:289. [EMBASE: 636330313] [Google Scholar]
- Roumelioti ME, Steel JL, Yabes J, Vowles KE, Vodovotz Y, Beach S, et al. Rationale and design of technology assisted stepped collaborative care intervention to improve patient-centered outcomes in hemodialysis patients (TACcare trial). Contemporary Clinical Trials 2018;73:81-91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Borg 2016 {published data only}
- Borg WE, Schipper K, Abma TA. Protocol of a mixed method, randomized controlled study to assess the efficacy of a psychosocial intervention to reduce fatigue in patients with end-stage renal disease (ESRD). BMC Nephrology 2016;17(1):73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Veen 2021 {published data only}
- Veen Y, Post A, Kremer D, Westerhuis R, Franssen CF, Wallimann T, et al. A clinical approach of intradialytic creatine supplementation in dialysis-dependent CKD patients: a rationale and study design [abstract no: PO0858]. Journal of the American Society of Nephrology 2021;32:295. [EMBASE: 636331594] [Google Scholar]
Additional references
Artom 2014
- Artom M, Moss-Morris R, Caskey F, Chilcot J. Fatigue in advanced kidney disease. Kidney International 2014;86(3):497-505. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Astroth 2013
- Astroth KS, Russell CL, Welch JL. Non-pharmaceutical fatigue interventions in adults receiving hemodialysis: a systematic review. Nephrology Nursing Journal 2013;40(5):407-27. [MEDLINE: ] [PubMed] [Google Scholar]
Bergstrom 2000
- Bergström J, Lindholm B, Lacson E Jr, Owen W Jr, Lowrie EG, Glassock RJ, et al. What are the causes and consequences of the chronic inflammatory state in chronic dialysis patients? Seminars in Dialysis 2000;13(3):163-75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bossola 2020
- Bossola M, Di Stasio E, Monteburini T, Parodi E, Ippoliti F, Bonomini M, et al. Intensity, duration, and frequency of post-dialysis fatigue in patients on chronic haemodialysis. Journal of Renal Care 2020;46(2):115-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bouya 2018
- Bouya S, Ahmadidarehsima S, Badakhsh M, Balouchi A, Koochakzai M. Effect of aromatherapy interventions on hemodialysis complications: a systematic review. Complementary Therapies in Clinical Practice 2018;32:130-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chang 2001
- Chang WK, Hung KY, Huang JW, Wu KD, Tsai TJ. Chronic fatigue in long-term peritoneal dialysis patients. American Journal of Nephrology 2001;21(6):479-85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2008
- Chen HY, Chiang CK, Wang HH, Hung KY, Lee YJ, Peng YS, et al. Cognitive-behavioral therapy for sleep disturbance in patients undergoing peritoneal dialysis: a pilot randomized controlled trial. American Journal of Kidney Diseases 2008;52(2):314-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chertow 2021
- Chertow GM, Pergola PE, Farag YM, Agarwa lR, Arnold S, Bako G, et al. Vadadustat in patients with anemia and non dialysis-dependent CKD. New England Journal of Medicine 2021;384(17):1589-600. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chiaranai 2016
- Chiaranai C. The lived experience of patients receiving hemodialysis treatment for end-stage renal disease: a qualitative study. Journal of Nursing Research 2016;24(2):101-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davey 2019
- Davey CH, Webel AR, Sehgal AR, Voss JG, Huml A. Fatigue in individuals with end stage renal disease. Nephrology Nursing Journal 2019;46(5):497-508. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Debnath 2021
- Debnath S, Rueda R, Bansal S, Kasinath BS, Sharma K, Lorenzo C. Fatigue characteristics on dialysis and non-dialysis days in patients with chronic kidney failure on maintenance hemodialysis. BMC Nephrology 2021;22(1):112. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eglence 2013
- Eglence R, Karatas, N, Tasci S. The effect of acupressure on the level of fatigue in hemodialysis patients. Alternative Therapies in Health & Medicine 2013;19(6):23-31. [MEDLINE: ] [PubMed] [Google Scholar]
Evangelidis 2017
- Evangelidis N, Tong A, Manns B, Hemmelgarn B, Wheeler DC, Tugwell P, et al. Developing a set of core outcomes for trials in hemodialysis: an international Delphi survey. American Journal of Kidney Diseases 2017;70(4):464-75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foley 2009
- Foley RN, Curtis BM, Parfrey PS. Erythropoietin therapy, hemoglobin targets, and quality of life in healthy hemodialysis patients: a randomized trial. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(4):726-33. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Habibzadeh 2020
- Habibzadeh H, Wosoi Dalavan O, Alilu L, Wardle J, Khalkhali H, Nozad A. Effects of foot massage on severity of fatigue and quality of life in hemodialysis patients: a randomized controlled trial. International Journal of Community Based Nursing & Midwifery 2020;8(2):92-102. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, PageMJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Horigan 2013
- Horigan AE, Schneider SM, Docherty S, Barroso J. The experience and self-management of fatigue in patients on hemodialysis. Nephrology Nursing Journal 2013;40(2):113-22. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Jhamb 2008
- Jhamb M, Weisbord SD, Steel JL, Unruh M. Fatigue in patients receiving maintenance dialysis: a review of definitions, measures, and contributing factors. American Journal of Kidney Diseases 2008;52(2):353-65. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johansen 2012
- Johansen KL, Finkelstein FO, Revicki DA, Evans C, Wan S, Gitlin M, et al. Systematic review of the impact of erythropoiesis-stimulating agents on fatigue in dialysis patients. Nephrology Dialysis Transplantation 2012;27(6):2418-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ju 2018a
- Ju A, Unruh M, Davison S, Dapueto J, Dew MA, Fluck R, et al. Establishing a core outcome measure for fatigue in patients on hemodialysis: a Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) Consensus Workshop Report. American Journal of Kidney Diseases 2018;72(1):104-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ju 2018b
- Ju A, Unruh ML, Davison SN, Dapueto J, Dew MA, Fluck R, et al. Patient-reported outcome measures for fatigue in patients on hemodialysis: a systematic review. American Journal of Kidney Diseases 2018;71(3):327-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ju 2021
- Ju A, Scholes-Robertson N, Johnson DW, Cho Y, Zwieten A, Manera K, et al. Patient-led identification and prioritization of exercise interventions for fatigue on dialysis: a workshop report. Clinical Kidney Journal 2021;14(3):831–9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karadag 2019
- Karadag E, Samancioglu Baglama S. The effect of aromatherapy on fatigue and anxiety in patients undergoing hemodialysis treatment: a randomized controlled study. Holistic Nursing Practice 2019;33(4):222-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 1991
- Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Research 1991;36(3):291–8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liangos 2010
- Liangos O, Jaber BL. Chapter 24 - Acute complications associated with hemodialysis. In: Himmelfarb J, Sayegh MH, editors(s). Chronic Kidney Disease, Dialysis and Transplantation. 3rd edition. JB Saunders, 2010. [ISBN: 9781437709872] [Google Scholar]
Manera 2019
- Manera KE, Johnson DW, Craig JC, Shen JI, Ruiz L, Wang AY, et al. Patient and caregiver priorities for outcomes in peritoneal dialysis: multinational nominal group technique study. Clinical Journal of The American Society of Nephrology: CJASN 2019;14(1):74-83. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maruyama 2021
- Maruyama Y, Nakayama M, Ueda A, Miyazaki M, Yokoo T. Comparisons of fatigue between dialysis modalities: a cross-sectional study. PLoS ONE [Electronic Resource] 2021;16(2):e0246890. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Melo 2020
- Melo GA, Aguiar LL, Silva RA, Pereira FG, Silva FL, Caetano JA. Effects of acupuncture in patients with chronic kidney disease: a systematic review. Revista Brasileira de Enfermagem 2020;73(4):e20180784. [PMID: ] [DOI] [PubMed] [Google Scholar]
Monaro 2014
- Monaro S, Stewart G, Gullick J. A 'lost life': coming to terms with haemodialysis. Journal of Clinical Nursing 2014;23(21-22):3262-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ossareh 2003
- Ossareh S, Roozbeh J, Krishnan M, Liakopoulos V, Bargman JM, Oreopoulos DG. Fatigue in chronic peritoneal dialysis patients [Erratum in: Int Urol Nephrol. 2004;36(3):477]. International Urology & Nephrology 2003;35(4):535-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Picariello 2017
- Picariello F, Hudson JL, Moss-Morris R, Macdougall IC, Chilcot J. Examining the efficacy of social-psychological interventions for the management of fatigue in end-stage kidney disease (ESKD): a systematic review with meta-analysis. Health Psychology Review 2017;11(2):197-216. [PMID: ] [DOI] [PubMed] [Google Scholar]
Picariello 2017a
- Picariello F, Moss-Morris R, Macdougall IC, Chilcot J. The role of psychological factors in fatigue among end-stage kidney disease patients: a critical review. Clinical Kidney Journal 2017;10(1):79–88. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2007
- Rao M, Wong C, Kanetsky P, Girndt M, Stenvinkel P, Reilly M, et al. Cytokine gene polymorphism and progression of renal and cardiovascular diseases. Kidney International 2007;72(5):549-56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ross 2003
- Ross SD, Fahrbach K, Frame D, Scheye R, Connelly JE, Glaspy J. The effect of anemia treatment on selected health-related quality-of-life domains: a systematic review. Clinical Therapeutics 2003;25(6):1786-805. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salehi 2020
- Salehi F, Dehghan M, Mangolian Shahrbabaki P, Ebadzadeh MR. Effectiveness of exercise on fatigue in hemodialysis patients: a randomized controlled trial. BMC Sports Science, Medicine & Rehabilitation 2020;12:19. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schreiber 2005
- Schreiber B. Levocarnitine and dialysis: a review. Nutrition in Clinical Practice 2005;20(2):218-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2022a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Schünemann 2022b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, ChandlerJ, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Song 2018
- Song YY, Hu RJ, Diao YS, Chen L, Jian XI. Effects of exercise training on restless legs syndrome, depression, sleep quality, and fatigue among hemodialysis patients: a systematic review and meta-analysis. Journal of Pain & Symptom Management 2018;55(4):1184-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
SONG‐HD
- Standardised Outcomes in Nephrology - Haemodialysis. https://songinitiative.org/projects/song-hd/ (date accessed 17 July 2023).
SONG‐PD
- Standardised Outcomes in Nephrology - Peritoneal Dialysis. https://songinitiative.org/projects/song-pd/ (date accessed 17 July 2023).
Tian 2020
- Tian C, Zhang B, Liang W, Yang Q, Xiong Q, Jin Q, et al. Fatigue in peritoneal dialysis patients and an exploration of contributing factors: a cross-sectional study. Journal of Pain & Symptom Management 2020;59(5):1074-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tong 2017
- Tong A, Manns B, Hemmelgarn B, Wheeler DC, Evangelidis N, Tugwell P, et al. Establishing core outcome domains in hemodialysis: report of the Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) consensus workshop. American Journal of Kidney Diseases 2017;69(1):97-107. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Unruh 2020
- Unruh M, Cukor D, Rue T, Abad K, Roumelioti ME, McCurry SM, et al. Sleep-HD trial: short and long-term effectiveness of existing insomnia therapies for patients undergoing hemodialysis. BMC Nephrology 2020;21(1):443. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Urquhart‐Secord 2016
- Urquhart-Secord R, Craig JC, Hemmelgarn B, Tam-Tham H, Manns B, Howell M, et al. Patient and caregiver priorities for outcomes in hemodialysis: an international nominal group technique study. American Journal of Kidney Diseases 2016;68(3):444-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Sandwijk 2019
- Sandwijk MS, Al Arashi D, de Hare FM, Torren JM, Kersten MJ, Bijlsma JA, et al. Fatigue, anxiety, depression and quality of life in kidney transplant recipients, haemodialysis patients, patients with a haematological malignancy and healthy controls. Nephrology Dialysis Transplantation 2019;34(5):833–8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yngman‐Uhlin 2010
- Yngman-Uhlin P, Friedrichsen M, Gustavsson M, Fernstrom A, Edell-Gustaffson U. Circling around in tiredness: perspectives of patients on peritoneal dialysis. Nephrology Nursing Journal 2010;37(4):407-13. [MEDLINE: ] [PubMed] [Google Scholar]
Yurtkuran 2007
- Yurtkuran M, Alp A, Yurtkuran M, Dilek K. A modified yoga-based exercise program in hemodialysis patients: a randomized controlled study. Complementary Therapies in Medicine 2007;15(3):164-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ju 2018
- Ju A, Strippoli GF, Craig JC, Tong A, Saglimbene VM, Unruh ML. Interventions for fatigue in people with chronic kidney disease requiring dialysis. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD013074. [DOI: 10.1002/14651858.CD013074] [DOI] [PMC free article] [PubMed] [Google Scholar]


