Abstract
Maple syrup is a naturally sweet product consumed directly or introduced in the preparation of various maple-derived food products. Several studies have described the chemical isolation and identification of maple syrup compounds, with some presenting pharmacological properties. However, a detailed review on maple syrup nutritional properties has not been undertaken. This review presents detailed information about the nutritional, organoleptic, and pharmacological properties of maple syrup. Studies carried out on animal models and a limited number of human models emphasize the potential benefits of maple syrup as a substitute for refined sugars, indicating that it could contribute to improved metabolic health when used in moderation. However, further medical and nutritional health studies based on human health assessments are needed to better understand the mechanisms of action of the various components of maple syrup and its potential therapeutic properties to demonstrate a stronger justification for its consumption relative to refined sugars. In addition, we compare maple syrup and common sweeteners to provide a further critical perspective on the potential nutritional and health benefits of maple syrup.
Keywords: sweetener, Pharmacological potential, Functional properties, Organoleptic properties, Nutritional value
1. Introduction
Overconsumption of refined sugar is currently considered one of the main reasons for the global rise in obesity rates, type 2 diabetes, and other metabolic disorders [1]. To attenuate this trend, maple syrup is frequently presented as an alternative to refined sugar, together with honey and agave, natural sweeteners that could be nutritionally effective if introduced as part of a reduced-sugar diet [1]. According to the United States Department of Agriculture, sugar has a glycemic index of 65, whereas maple syrup has a glycemic index of 54. From a health standpoint, natural sweeteners are preferred due to their higher nutritional value resulting from a higher concentration of compounds deemed to be healthy, which compensate for most of the negative refined sugar effects [2].
Maple syrup is appreciated in many countries by a broad range of consumers due to its taste and alleged nutrition and health benefits. It is prepared by boiling and concentrating the maple sap that is collected from the sugar maple tree (Acer saccharum) in late winter or early spring [3,4]. In 2022, approximately 17.4 million gallons (ca 65.8 million liters) of maple syrup were produced in Canada and 5.3 million gallons (ca 20.2 million liters) in the United States (Statistics Canada, USDA, 2022). Maple syrup contains a wide range of chemical compounds, many of which present potential health benefits but some of which are recognized as contributing to health issues. Included among the elements and nutrients potentially conferring health benefits are macronutrients (primarily sucrose), and phytochemicals (primarily phenolics) [5]. There is growing interest in studying the bioactivity of maple syrup's phenolic compound composition owing to the antioxidant properties of many phenolics. Maple syrup also contains minerals (K, Ca, Na, Mg, Fe, Mn, Zn, Al, etc.), organic acids (malic acid, fumaric acid, etc.), amino acids (arginine, threonine, proline, etc.), phytohormones (phaseic and abscisic acids and their metabolites), and vitamins (niacin, thiamine, riboflavin, etc.) which enhance its nutritional value [ [[6], [7], [8], [9], [10]]]. Further, other compounds could present a positive influence on human health through variousbiological properties (e.g., anti-inflammatory, anticancer, antidiabetic, anti-aging, anti-alzheimer, antimutagenic, and anti-oxidative), noting that such properties have been principally evidenced only in vitro, often on isolated compounds, and rarely using maple syrup as a whole component [ [[11], [12], [13], [14], [15]]].
In contrast, carbohydrates are the main dietary component of maple syrup and it must be recognized that the potential health benefits as noted above, may be partially offset by the link between carbohydrate consumption and diseases such as diabetes and obesity. In light of these contrasting attributes, there is growing interest in better characterizing the bioactive properties of maple syrup with respect to the overall implications for human health. Although several previous studies have focused on the nutritional importance of maple syrup by studying its pharmacological properties, to date, a review synthesizing the current state of knowledge on the nutraceutical characteristics of maple syrup has not been performed. The goal of this review, the first on this subject, is to provide a detailed report on the nutritional, organoleptic, and physiological roles of maple syrup compounds to inform about the use of maple syrup in the human diet and to serve as a resource that will help to guide future studies on assessing such properties in maple syrup.
2. Search procedure
We conducted a Google Scholar search to identify references containing information on the nutritional, organoleptic, and physiological roles of maple syrup. We used single or combinatorial searches on keywords such as “maple syrup”, “nutrition”, “nutritional potentials”, “pharmacological and sensory properties”, “functional properties”, “health benefits”, “bioavailability”, etc. For each identified paper, we then reviewed the references therein to determine if additional relevant references could be identified. The initial search yielded 105 papers, from which 95 were selected for consideration in the present review.
3. Nutritional and functional properties of maple syrup
Recent trends in the search for improved dietary nutrition have principally focused on the nutritional potential of natural foods, with current recommendations for healthy diets highlighting the need for greater ingestion of natural foods containing antioxidants, vitamins, nutrients and elements rather than foods in which these specific nutrients are supplemented exogenously [16].
Honey and plant-based foods, such as maple syrup or agave nectar, are available as natural alternatives to refined sugars. Unrefined sweeteners generally have higher levels of antioxidants, similar to the disparity between whole and refined grain products [17]. This review principally discusses the nutritional and functional potential of maple syrup.
3.1. Nutritional potentials
In the human diet, maple syrup is considered a source of carbohydrates instead of refined sugars. In addition, maple syrup has been deemed a good source of vitamins (Table 1), and minerals [8] necessary for the human body for biochemical reactions such as the synthesis of enzymes or hormones. Minerals are also involved in vital processes such as bone, muscle, heart, and brain maintenance and function. Potassium and Ca are the most abundant elements of maple syrup with concentrations between 541 and 4031 ppm, and 278–2800 ppm, respectively [ [[18], [19], [20]]]. Regarding other elements and vitamins, a 60 mL (1/4 cup) serving of maple syrup provides 100% of the recommended daily value of Mn, 34% of riboflavin (vitamin B2), 11% of Zn, 6% of Ca and lesser amounts of K, and Mg [9,21]. According to Canadian and U.S. FDA, portion provides 217 calories [8,22], almost 10% of the average energy needs of the human body, which is between 2100 and 2700 kcal per day for females and males, respectively [23].
Table 1.
Elemental composition of maple syrup: Sugars, ester, organic acid, and vitamins..
| Identification | Molecular formula | Content% | Ref | |
|---|---|---|---|---|
| Sugars | ||||
| Sucrose | C₁₂H₂₂O₁₁ | 51.7–99 | [19,20] | |
| Glucose | C₆H₁₂O₆ | 0.43–9.6 | [19,20] | |
| Fructose | C6H12O6 | 0.30–3.9 | [19,20] | |
| Oligosaccharides | C37H62N2O29 | nd-0.02 | [18,20] | |
| Quebrachitol | C7H14O6 | nd-0.15 | [[18], [19], [20]] | |
| Maplebiose1 | C12H20O11 | – | [24] | |
| Inulin | C6nH10n+2O5n+1 | – | [25] | |
| Nystose | C24H42O21 | – | [26] | |
| 1-Kestose | C18H32O16 | – | [26] | |
| Neokestose | C18H32O16 | – | [26] | |
| Vitamins | ||||
| Thiamine | C12H17N4OS+ | nd- 1.3х10−4 | [20] | |
| Niacin | C₆H₅NO₂ | 1.6х10−4- 1х10−3 | [20] | |
| Riboflavin (B2) | C₁₇H₂₀N₄O₆ | 4.6 х10−6- 6 х10−4 | [20] | |
| Folic Acid | C19H19N7O6 | – | [20] | |
| Biotin | C10H16N2O3S | – | [20] | |
| Vitamin A | C20H30O | – | [20] | |
| Pyridoxine (B6) | C₈H₁₁NO₃ | – | [20] | |
| Organic acid | ||||
| Malic acid | C4H6O5 | 0.22–0.33 | [20] | |
| Fumaric acid | C4H4O4 | 0.10–0.01 | [20] | |
| Succinic acid | C4H6O4 | 0.001–0.001 | [20] | |
| Citric acid | C₆H₈O₇ | 0.02–0.01 | [20] | |
| Acetic acid | C2H4O2 | 0.012–0.013 | [20] | |
| Lactic acid | C3H6O3 | – | [27] | |
| Levulinic acid | C5H8O3 | – | [27] | |
| Aliphatic acids | C5H8O3 | – | [27] | |
| Oxalic acid | C2H2O4 | – | [27] | |
| Hexanoic acid | C6H12O2 | – | [10] | |
| n-Hexanoic acid | C6H12O2 | – | [28] | |
| 2-Ethylhexanoic acid | C8H16O2 | – | [28] | |
| n-Nonanoic acid | C9H18O2 | – | [28] | |
| Ester compound | ||||
| Trimethylgallic acid methyl ester | C11H14O5 | – | [29] | |
As with any sweetener, natural or artificial, overconsumption of maple syrup could lead to obesity problems. However, some studies have shown that controlled consumption of maple syrup, as an alternative sweetener for inclusion in the diet, could have health benefits. Most of the studies performed to support this claim have been done using animal models, making it difficult to extrapolate definitively to human health. Notwithstanding, the protective effects of maple syrup ingestion on liver function have been evidenced in rats [30]. In that study, one group of rats were fed a 20% maple syrup diet while a second group was fed a 20% sugar mix syrup diet, each for 11 days. The maple syrup group showed significantly lower values of hepatic function biomarkers compared to the sugar syrup group. The study also found that the expressions of genes for serine/threonine dehydratase and histidine ammonia lyase, which are linked to ammonia formation, were reduced in the liver of the maple syrup group compared to the sugar only group. Excessive formation of free ammonia in the body results in a harmful increase of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels [31]. If not treated or eliminated, ammonia builds up in the liver, which can lead to oxidative stress and tissue damage. Assuming similar biochemical processes in humans, these results suggest a potential beneficial effect of maple syrup in the diet to protect liver function compared to sugar only [31]. Subsequently, the effect of polyphenol-rich maple syrup extract (MSE) on type 2-diabetes mice (model KK-Ay, a cross between diabetic KK and lethal yellow (Ay) mice) was investigated [32]. After 43 days of administration of a 0.1% MSE diet, the serum alanine aminotransferase and aspartate aminotransferase levels of mice decreased, and lipogenesis and lipolysis hepatic genes were regulated. The study confirmed that MSE intake can mitigate liver injury and prevent lipid accumulation in type 2-diabetes mouse livers. Such an effect could also exist in humans, but studies are needed to compare responses in mice treated with MES versus natural maple syrup before we can be confidence aboout this extrapolation.
Nagai et al. [33] investigated whether maple syrup could be an appropriate sweetener for type 2 diabetes patients using Otsuka Long-Evans Tokushima Fatty (OLETF) rats as a model. In the Nagai et al. study [33], the authors compared the effect of ingestion of maple syrup or sucrose on plasma glucose (PG) levels in OLETF rats. They showed a significant increase in PG in both 30-and-60-week-old rats administered sucrose compared to those administered maple syrup. The study also showed that oral administration of sucrose or maple syrup resulted in increased insulin levels in 30-week-old OLETF rats, while no increase in insulin levels was noted in 60-week-old OLETF rats. Hence, maple syrup consumption at moderate levels might help protect against type 2 diabetes.
Polyphenols are also preponderant components in maple syrup composition [34,35]. The role of polyphenols in the nutritional properties of maple syrup has therefore been suggested and intensively investigated. As previously stated, most studies were performed on polyphenol-rich maple syrup extracts and not raw maple syrup even though observed results have been extrapolated to maple syrup.
Regarding additional nutritional properties of the sugar fraction of maple syrup, maplebiose1, a functional oligosaccharide consisting of glucose and fructose moieties with the C-6 of glucose substituted by the C-2 of fructose, has been identified in maple syrup [24]. In rats, maplebiose1 inhibits the hydrolysis of maltose by α-(1–4) glucosidase (IC50: 1.72 mmol/L) and the release of fructose from sucrose by invertase (IC50: 1.17 mmol/L). These results indicate that maplebiose1 may represent a useful molecule for inclusion in diets of people afflicted by diabetes mellitus and hence, maple syrup could be of major nutritional importance for diabetics as a substitute for processed sugar.
The polyphenol-rich maple syrup extract is a commercially available standardized maple extract prepared by filtration of maple syrup on Amberlite XAD-16 H P using deionized water as the mobile phase, then washing of the resin to collect the initially adsorbed fraction using alcohol. Final evaporation yields the polyphenol-rich extract. MSX is a polyphenol-rich extract that contains 1.61% w/w gallic acid equivalents of polyphenols whereas MSHX is enriched in polyphenols and contains 15.02% w/w gallic acid equivalents.
The study of the nutritional properties of MSXH carried out on mice fed a low- and high-fat diet supplemented with MSXH, 0.02% or 0.05% for 4 weeks, evidenced that the intake of MSXH was efficient against the metabolic changes resulting from high-fat feeding [35]. To further clarify the underlying mechanism of the physiological response to maple syrup and its impacts, the effect of MSXH on the lipid metabolism in obese diabetic mice has been examined [36]. The study showed that the intake of 0.05% MSXH for 42 days did not affect chronic hyperglycemia, but it reduced the LDL cholesterol level in blood and accelerated fatty ascid degradation in the liver. Cholesterol and ketone bodies are produced from acetyl-CoA. Thus, the authors suggested that MSXH intake boosted ketone body production from acetyl-CoA rather than cholesterol synthesis, resulting in reduced LDL cholesterol levels in blood.
The above-mentioned studies performed on animal models emphasize potential maple syrup benefits as a substitute compared to refined sugars. Indeed, even though maple syrup is rich in sugar, the current data suggest that it is more than just a sugar source and maple syrup intake can improve metabolic health when used as. However, further studies on human models are required to develop a stronger justification for the consumption of maple syrup as a nutritionally advantageous food/sweetener.
3.2. Is maple syrup more nutritious than other common sweeteners?
St-Pierre et al. [37] compared the metabolic response in rats following ingestion of maple syrup or brown rice syrup, blue agave syrup, corn syrup, and natural honey. Compared to other sweeteners, maple syrup is particularly rich in the phytohormone abscisic acid and some of its derivatives, as well as polyphenolic lignans, both of which could directly act on adipose and muscle cells to stimulate the transmission of glucose transporters to the surface of cells, therefore stimulating insulin-independent glucose uptake and metabolism in these cells [38]. Therefore, maple syrup would produce a significantly lower peak and global responses of insulin, glucose, gastric inhibitory polypeptide (GIP), and amylin compared to brown rice syrup and corn syrup. Agave syrup yielded similar responses to maple syrup, while honey intake caused a higher peak response for amylin, insulin, and GIP and a similar response to glucose compared to maple syrup. In rats, therefore, maple syrup integration in the diet, as a replacement for refined sugars, has been shown to yield some health benefits.
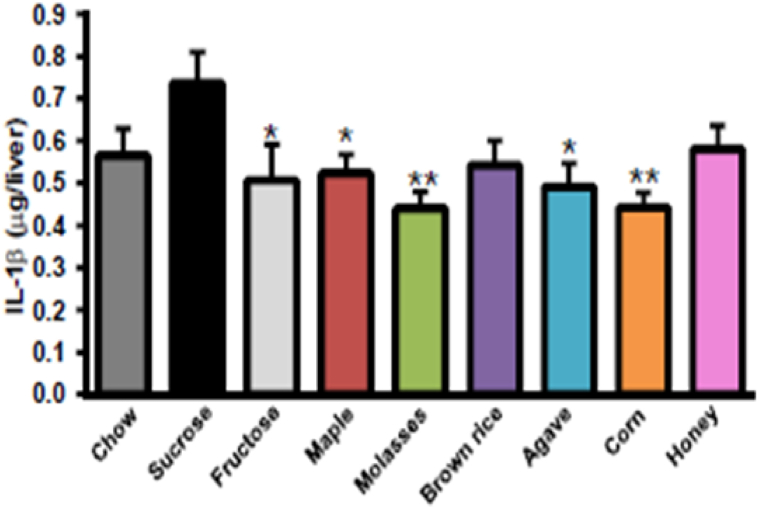
Maple syrup total sugar is 60.5 g/100 g, a value lower than that of agave syrup (68 g/100 g), honey (82.1), molasses (74.7), high fructose corn syrup (75.7), and sucrose (99.8) [39]. Investigation on the metabolic responses of diet-induced Wistar obese rats to chronic intake of refined sugars compared to natural sweeteners (maple syrup, brown rice syrup, blue agave syrup, corn syrup, and natural honey) as as well as fructose indicated that maple, agave, and corn syrups reduce hepatic IL-1β levels compared to the refined sugars (Fig. 1) [40]. Ingestion of maple, agave, and corn syrups could therefore be a less detrimental alternative compared to refined sugars in the context of obesity in rats. However, in the case of high-fructose corn syrup, which is prevalent in many food products, this must be weighed against its demonstrated contribution to diabetes. Immoderate intake of HFCS drinks without a proper diet is one of the reasons why T2D leads to non-obese impaired glucose tolerance (IGT) due to an insulin-secretion defect [41].
Fig. 1.
Natural sweeteners (maple syrup, brown rice syrup, blue agave syrup, corn syrup, and natural honey) as well as fructose alleviated hepatic IL-1β levels compared to refined sugars [40].
Trace amounts of organic acids, free amino acids, protein, minerals, and phenolic compounds present in maple syrup suggest potential health benefits in maple syrup compared to sucrose, which is devoid of these nutritional molecules [33]. Indeed, based on an abscisic acid-supplemented diet performed on mice, Guri et al. [42] reported that abscisic acid ingestion decreased the concentration of fasting blood glucose and improved glucose tolerance.
Comparison of the effect of oral administration of maple syrup and sucrose by rats on plasma glucose concentrations showed that the concentration of plasma glucose was significantly lower in rats ingesting maple syrup than in rats fed with sucrose, without having any impact on the concentration of insulin [24].
Limited nutritious properties of maple products have also been investigated in humans. Based on ratings of registered exertion at every 30 min during 120 min of steady-state exercise, sports drinks containing maple syrup were decribed as “well appreciated” during prolonged exercise and seem to be an applicable alternative to more common carbohydrate sources [43].
Recently, Sato et al. [26] reported that in addition to sucrose, fructose, and glucose, maple syrup also contains fructo-oligosaccharide (FOS) such as nystose, 1-kestose, and neokestose (Table 1), which have only been found in a few natural foods.
Nutritional properties of maple syrup are likely to be similar to that of other natural sweeteners. However, comparative clinical studies between maple syrup and common sweeteners in humans are missing and needed to gain a clearer understanding of the nutritional and health benefits of maple syrup compared to other natural syrups.
3.3. Pharmacological properties of maple syrup
3.3.1. Maple syrup polyphenols: antioxidant and anti-inflammatory activity
3.3.1.1. Antioxidant activity
An antioxidant is a material that at low levels mitigates or prevents oxidation by scavenging free radicals that otherwise interact with and damage cell membranes. Antioxidant compounds work through hydrogen atom transfer, single electron transfer, and the ability to chelate transition metals [44]. In recent decades, interest in antioxidants derived from natural food products has increased significantly in the nutritional field and maple syrup, a natural source of antioxidant compounds, has received much attention.
The antioxidant activity of maple syrup largely reflects the activity of two groups of compounds: glycosylated phenolics and aglycones. The relative contribution of each to the antioxidant properties of maple syrup depends on when sap is collected during the harvesting season. Glycosylated phenolic compounds of maple syrup exhibit greatest activity early in the sap harvesting season, while the best antioxidant activity of aglycone compounds was observed at the end of the season (Table 2) [15].
Table 2.
List of the pharmacologically active compounds isolated from maple syrup extracts.
| Comp | Name | MS-BuOH | MS-EtOAc | MS-MeOH | Ref |
|---|---|---|---|---|---|
| Antioxidant | |||||
| 1 | Gallic acid | + | [45] | ||
| 2 | 1-Ogalloyl- β-d-glucose | + | [45] | ||
| 3 | γ -Resorcylic acid | + | [45] | ||
| 4 | p-coumaric acid | + | [45] | ||
| 5 | 4-Methoxycinnamic acid | + | [45] | ||
| 6 | Caffeic acid | + | [45] | ||
| 7 | Sinapic acid | + | [45] | ||
| 8 | Chlorogenic acid | + | [45] | ||
| 9 | Quercetin 3-O-β-D-glucoside | + | [45] | ||
| 10 | Kaempferol 3- O-β-d-galactoside | + | [45] | ||
| 11 | (+)-Catechin | + | [45] | ||
| 12 | Quercetin 3-O-β-l-rhamnoside | + | [45] | ||
| 13 | Quercetin 3-O-rhamnoglucoside | + | [45] | ||
| Anti-inflammatory | |||||
| 14 | (E)-3,3′-Dimethoxy-4,4′-dihydroxystilbene | + | [46] | ||
| 15 | 4-Acetylcatechol | + | [46] | ||
| 16 | Tyrosol | + | [46] | ||
| 17 | Protocatechuic acid | + | [46] | ||
| 18 | Gallic acid | [47] | |||
| 19 | 1,2-Dihydroxybenzene | [47] | |||
| 20 | 3,4-Dihydroxybenzaldehyde | [47] | |||
| 21 | Syringaldehyde | [47] | |||
| 22 | Vanillin | [47] | |||
| 23 | 3-Hydroxybenzoic acid | [47] | |||
| 24 | Quebecol | + | [48] | ||
| 25 | 2,3-Dihydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-1-propanone | + | [4] | ||
| 26 | (Threo, erythro)-1-[4-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydrox ymethyl)ethoxy]-3,5-dimethoxyphenyl]-1,2,3-propanetriol | + | [4] | ||
| 27 | 3-Hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)propan-1-one | + | [4] | ||
| 28 | [3-[4-[(6-Deoxy-α-l-mannopyranosyl)oxy]-3-methoxyphenyl]methyl]-5-(3,4-dimethoxyphenyl)-dihydro-3-hydroxy-4-(hydroxymethyl)-2(3H)-furanone | + | [4] | ||
| 29 | Syringaresinol | + | [4] | ||
| 30 | (Threo, erythro)-1-[4-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)ethoxy]-3,5-dimethoxyphenyl]-1,2,3-propanetriol | + | [4] | ||
| Antiproliferative | |||||
| 18 | Gallic acid | + | + | + | [49] |
| 31 | C-Veratroylglycol | + | + | + | [49] |
| 32 | 1,2-Benzenediol (catechol) | + | + | [49] | |
| 33 | Syringaldehyde | + | + | [49] | |
| 34 | Catechaldehyde | + | + | [49] | |
| Comp | Name | MS-BuOH | MS-EtOAc | MS-MeOH | Ref |
| 35 | Scopoletin | + | + | + | [49] |
| 36 | Secoisolariciresinol | + | + | + | [49] |
| 37 | 4-Acetylcatechol | + | + | [49] | |
| 38 | Quebecol | + | [49] | ||
| 39 | Fraxetin | + | + | [49] | |
| 40 | E)-Coniferyl alcohol (coniferol) | + | + | [49] | |
| 41 | 1-(2,3,4-Trihydroxy-5-methylphenyl)-ethanone | + | + | [49] | |
| 42 | 1-[4-[(1R,2R)-2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)ethoxy]-3-methoxyphenyl]-1,2,3-propanetriol | + | + | [49] | |
| 43 | Syringic acid | + | + | + | [49] |
| 44 | 1-(4-Hydroxy-3-methoxyphenyl)-2-[4-(3-hydroxypropyl)-2-methoxyphenoxy]-propane-1,3-diol (guaiacylglycerol-b-O-40-dihydroconiferyl alcohol) |
+ | + | + | [49] |
| 45 | Syringenin | + | + | [49] | |
| 46 | Vanillin | + | + | + | [49] |
| 47 | Ferulic acid | + | [49] | ||
| 48 | Tyrosol | + | + | [49] | |
| 49 | Ginnalin A | [50] | |||
| Anti-aging | |||||
| 50 | 1,2-Benzenediol | + | [51] | ||
| 51 | 3,4,5-trihydroxybenzoic acid | + | [51] | ||
| 52 | 3,4-dihydroxybenzaldehyde | + | [51] | ||
| 53 | 3,4-dihydroxybenzaldehyde | + | [51] | ||
| 54 | Erythro-guaiacylglycerol-β-O-4′-dihydroconiferyl alcohol | + | [4] | ||
| 55 | [3-[4-[(6-Deoxy-α-l-mannopyranosyl)oxy]-3-methoxyphenyl]methyl]-5-(3,4-dimethoxyphenyl)-dihydro-3-hydroxy-4-(hydroxymethyl)-2(3H)-furanone | [4] | |||
| 56 | Secoisolariciresinol | + | [4] | ||
| 57 | Icariside E4 | + | [4] | ||
| 58 | Sakuraresinol | + | [4] | ||
| 59 | Dehydroconiferyl alcohol | + | [4] | ||
| 60 | Syringaresinol | + | [4] | ||
| 61 | 2-[4-[2,3-Dihydro-3-(hydroxymethyl)-5-(3-hydroxypropyl)-7-methoxy-2-benzofuranyl]-2,6-dimethoxyphenoxy]-1-(4-hydroxy-3-methoxyphenyl)-1,3-propanediol | + | [4] | ||
| 62 | Buddlenol E | + | [4] | ||
| Anti-diabetic | |||||
| 63 | Abscisic acid | [52] | |||
In another study, comparison of the antioxidant activity of maple syrup to that of refined sugar and other sweetners using the ferric-reducing ability of the plasma (FRAP) assay indicated that maple syrup displays a FRAP of 0.2–0.7 mmol/100 g, a value similar to that of honey and brown sugar [17]. Similarly, Legault et al. [53] showed that the antioxidant activity of maple syrup is similar to that of strawberry and orange syrups.
Abou-Zaid et al. [45] also identified several compounds known to possess strong antioxidant activity from the ethyl acetate–soluble fractions of maple syrup samples (Table 2).
Li and Seeram in 2010 and 2011a [29,54] analyzed successively the butanol extract (MS-BuOH) and ethyl acetate extract (MS-EtOAc) of maple syrup (Table 3). The antioxidant activity of the isolated extracts was evaluated using the DPPH radical scavenging assay. The antioxidant activity of MS-EtOAc and the pure isolates were 75.5 μg/mL and 68–3000 μM, respectively, values comparable to that of vitamin C and superior to that of butylhydroxytoluene.
Table 3.
Antioxidant activities of chemical compounds isolated from maple syrup showing 50% inhibitory concentrations (IC50) in the diphenylpicrylhydrazyl (DPPH) radical scavenging assay.
| Compd | Identification | IC50(μM) | Ref |
|---|---|---|---|
| (MS-BuOH) | |||
| 1 | Lyoniresinol | 101.5 ± 5.9 | [29] |
| 2 | Secoisolariciresinol | 147.9 ± 3.6 | [29] |
| 3 | Dehydroconiferyl alcohol | 1040.9 ± 103 | [29] |
| 4 | 5′-methoxy-dehydroconiferyl alcohol | 136.7 ± 3.9 | [29] |
| 5 | Guaiacylglycerol-β-O-4′-coniferyl alcohol | 943.5 ± 21.9 | [29] |
| 6 | Guaiacylglycerol-β-O-4′-dihydroconiferyl alcohol | 1335.9 ± 47.6 | [29] |
| 7 | 3-[4-[(6-deoxy-R-l-mannopyranosyl)oxy]-3-methoxyphenyl]methyl]-5-(3,4-dimethoxyphenyl)-dihydro-3-hydroxy-4-(hydroxymethyl)-2(3H)-furanone | 679.3 ± 45.6 | [29] |
| 8 | Scopoletin | 68.2 ± 31.2 | [29] |
| 9 | Fraxetin | 46.5 ± 3.6 | [29] |
| 10 | (E)-3,3′-dimethoxy-4,4′-dihydroxystilbene | >2600 | [29] |
| 11 | 2-Hydroxy-3′,4′-dihydroxyacetophenone | 51.8 ± 8.1 | [29] |
| 12 | 1-(2,3,4-Trihydroxy-5-methylphenyl)ethanone | 31.3 ± 0.6 | [29] |
| 13 | Catechaldehyde | 35.5 ± 3.7 | [29] |
| 14 | Vanillin | >2600 | [29] |
| 15 | Syringaldehyde | 357.1 | [29] |
| 16 | Gallic acid | 20.3 ± 0.3 | [29] |
| 17 | Syringic acid | 191.85 ± 20.99 | [29] |
| 18 | (E)-coniferol | 115 | [29] |
| 19 | C-veratroylglycol | 641 ± 1 0.6 | [29] |
| 20 | Catechol | 89.5 ± 2.7 | [29] |
| (MS-EtOAc) | |||
| 21 | 5-(-(3″,4″-Dimethoxyphenyl)-3-hydroxy-3-(4′-hydroxy-3′-methoxybenzyl)-4-(hydroxymethyl)dihydrofuran-2-one | 946.37 ± 58.5 | [54] |
| 22 | (Erythro, erythro)-1-[4-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)ethoxy]-3,5-dimethoxyphenyl]-1,2,3-propanetriol | 1540.91 ± 0.5 | [54] |
| 23 | (Erythro, threo)-1-[4-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)ethoxy]-3,5-dimethoxyphenyl]-1,2,3-propanetriol | 925 ± 179.0 | [54] |
| 24 | Erythro-1-(4-hydroxy-3-methoxyphenyl)-2-[4-(3-hydroxypropyl)-2,6-dimethoxyphenoxy]-1,3-propanediol | 740.20 ± 3.4 | [54] |
| 25 | 2-[4-[2,3-Dihydro-3-(hydroxymethyl)-5-(3-hydroxypropyl)-7-methoxy-2-benzofuranyl]-2,6-dimethoxyphenoxy]-1-(4-hydroxy-3-methoxyphenyl)-1,3-propanediol | 655.29 ± 14.4 | [54] |
| 26 | Acernikol | 478.95 ± 42.1 | [54] |
| 27 | Leptolepisol D | 578.49 ± 1.3 | [54] |
| Compd | Identification | IC50(μM) | Ref |
| 28 | Buddlenol E | 422.94 ± 2.4 | [54] |
| 29 | (1S,2R)-2-[2,6-Dimethoxy-4-[(1S,3aR,4S,6aR)-tetrahydro-4-(4-hydroxy-3,5-dimethoxyphenyl)-1H,3H-furo [3,4-c]furan-1-yl]phenoxy]-1- (4-hydroxy-3 methoxyphenyl)-1,3-propanediol | 207.93 ± 41.3 | [54] |
| 30 | Syringaresinol | 68.90 ± 5.7 | [54] |
| 31 | Isolariciresinol | 694.44 ± 110.2 | [54] |
| 32 | Icariside E4 | 1810.28 ± 265.6 | [54] |
| 33 | Sakuraresinol | 2876.44 ± 44.0 | [54] |
| 34 | 1,2-Diguaiacyl-1,3-propanediol | 703.12 ± 141.4 | [54] |
| 35 | 2,3-Dihydroxy-1-(3,4-dihydroxyphenyl)-1-propanone | 111.78 ± 5.1 | [54] |
| 36 | 2,3-Dihydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-1-propanone | 258.40 ± 33.8 | [54] |
| 37 | 3-Hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)propan-1-one | 321.53 ± 31.9 | [54] |
| 38 | 4-Acetylcatechol | 138.16 ± 28.2 | [54] |
| 39 | 3′,4′,5′-Trihydroxyacetophenone | 10125 + 1668.0 | [54] |
| 40 | 3,4-Dihydroxy-2-methylbenzaldehyde | 254.17 ± 32.5 | [54] |
| 41 | Protocatechuic acid | 97.83 ± 24.0 | [54] |
| 42 | Tyrosol | 163.93 ± 15.2 | [54] |
| 43 | Isofraxidin | 813.81 ± 37.7 | [54] |
| 44 | 4-hydroxycatechol | 139.42 ± 13.3 | [54] |
| 45 | phaseic acid | 903.57 | [54] |
| 46 | MSX-1 | 97.6 ± 4.73 | [14] |
| 47 | MSX-2 | 102.4 ± 7.25 | [14] |
Maple sap and syrup extracts significantly inhibited lipopolysaccharide-induced NO overproduction in RAW 264.7 murine macrophages [53]. The activity of the maple syrup extract was higher than that of raw maple sap with a mean of about 75% NO inhibition per 25 μg/mL of extract (0.18 mL of pure maple syrup), indicating that the conversion of maple sap into syrup elevates NO inhibition activity.
Zhang et al. [14] carried out biological studies in vitro and in vivo to assess the safety (animal toxicity) of two syrup extracts (MSX-1 and -2) derived from two different maple syrup samples classified based on their elevated light transmission. At doses of up to 1000 mg/kg/day no sign of explicit toxicity was observed in rats. Ma et al. [55] investigated the effects of a chemically standardized MSX on oxidative stress induced by H2O2. The results showed that MSX reduced H2O2-induced oxidative stress in a type of microglial cell derived from C57/BL6 murine (BV-2 cells). At a concentration of 100 μg/mL, MSX decreased H2O2-induced reactive oxygen species (ROS) production by 16.1%. The reduction of ROS levels mitigates microglial activation, an important cellular response in neurodegenerative diseases pathogenesis [55], and related neuronal disorders [56].
In vitro studies carried out on normal/non-tumorigenic human colon CCD-18Co cells by Liu et al. [13] evidenced that MSX at a concentration of 61.7 μg/mL scanvenges 50% of free radicals and decreases free radical generation by 20% during the glycation process. In a concentration-dependent manner, MSX restricted free radical production by 9.2–86.8% at concentrations ranging from 8 to 500 μg/mL, respectively.
Using human keratinocytes (HaCaT cells), Sheng et al. [57] reported that MSX (at 50 μg/mL) (ca. 400 μM) showed protective effects against H2O2- and methylglyoxal (MGO)-associated cytotoxicity through increasing cell viability by 21.5 and 25.9%, respectively. MSX decreased H2O2- and MGO-induced reactive oxygen species (ROS) production by 69.4 and 56.6%, respectively. MSHX also reduced MGO-caused DNA damage by 47.5%.
3.3.1.2. Anti-inflammatory activity
As was the case for antioxidant activity, the anti-inflammatory properties of maple syrup are often largely attributed to the occurrence of a variety of phenolic compounds [13].
Nahar et al. [46] evaluated the anti-inflammatory effects of MS-EtOAc and several purified phenolic constituents of maple syrup (Table 2) using a LPS-stimulated RAW 264.7 murine macrophage cell model. At 10, 50 and 100 μg/mL concentrations, MS-EtOAc reduced nitric oxide (NO) production by 28.8, 84.9 and 94.3%, respectively), and prostaglandin-E2 (PGE2) production by 3.4% and 85.2%, respectively. The inhibition of observed NO was a direct result of decreased gene expression through suppression of NF-κB transcriptional activation and nitric oxide synthase (iNOS) protein. MS-EtOAc also organized the expression of protein and the mRNA of cyclooxygenase-2. Among the evaluated phenolic compounds, (E)-3,3′-dimethoxy-4,4′-dihydroxystilbene was most efficient, reducing NO levels by 92.5% and PGE2 levels by 89.5% at 50 μM. 4-acetylcatechol, tyrosol, and protocatechuic acid decreased PGE2 levels by 95%, 72.6%, and 55.3%, respectively (at 50 μM). Since the authors of the Nehar et al. study [46] used an extract of maple syrup, it is possible that the potential anti-inflammatory activity of MS-EtOAc is due to its unique combination of compounds and not due to a single purified phenolic constituent.
Zhang et al. [14] reported that maple syrup extracts MSX-1 and MSX-2 showed anti-inflammatory effects in RAW 264.7 macrophages. Both MS−EtOAc extracts significantly inhibited nitrite levels in cell media. The MSX-1 significantly decreased nitrite production by 25.1% at 50 μg/mL and 67.1% at 100 μg/mL. Also, the MSX-2 reduced nitrite levels by 24.8% and 41.3%, at concentrations of 50 and 100 μg/mL, respectively.
Kamei et al. [34] evaluated the effect of maple syrup extract (MSX, containing 1.61% polyphenols) on hepatic gene expression of mice fed a high-fat diet (HFD). The results revealed changes in gene expression related to lipid metabolism and the immune reply in MSX-fed mice. The study indicated that MSX ingestion relieves hepatic inflammation induced by the consumption of HFDs.
Evaluation of the effects of MSX on the production of lipopolysaccharide (LPS)-stimulated inflammatory markers in murine BV-2 microglial cells showed that MSX (100 μg/mL) reduced the production of lipopolysaccharide (LPS)-stimulated inflammatory markers by 22.1, 19.9, 74.8, and 87.6% in nitric oxide species (NOS), interleukin-6 (IL-6), prostaglandin E2 (PGE2), and tumor necrosis factorα (TNFα) levels, respectively [55]. Shortly after, Liu et al. [4] reported the investigation of twenty phenolic compounds isolated from MS-EtOAc against LPS-induced inflammation in BV-2 microglia in vitro. Several compounds isolated from maple syrup significantly reduced NO levels (Table 2).
Quebecol is a polyphenolic compound isolated from maple syrup [58]. Quebecol has anti-inflammatory properties, as indicated by a significant inhibitory effect on the lipopolysaccharide (LPS)-induced Nuclear Factor Kappa B (NF-κB) activation at 100 mM, a concentration that presented no cytotoxicity [48]. Quebecol prevents the excretion of pro-inflammatory, interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) by LPS-catalyzed macrophages at the highest non-cytotoxic concentration.
It is undeniable that maple syrup is rich in phenolic compounds that exhibit antioxidant and anti-inflammatory activity in vitro. The antioxidant capacity of maple syrup is sometimes claimed to participate in its nutritional value. However, many of these studies are based on maple syrup extracts and the quantities of maple syrup in normal food applications and products that would need to be consumed daily by humans to obtain this beneficial effect are still unknown, as are the negative effects that such consumption could present. Consequently, more studies are necessary to demonstrate the health benefits of the phenolic portion of maple syrup via an antioxidative process in humans. The same reasoning can be applied in relation to the anti-inflammatory activity of maple syrup. Furthermore, a growing number of studies have conlcuded that at normal food consumption levels, the antioxidant activity of polyphenols does not translate into any significant physiological activity.
3.3.2. Antiproliferative activity
The antiproliferative activity of maple syrup extracts (50% inhibitory concentration = 42 ± 6 μg/mL) and of pure maple syrup using various human cell lines has been investigated [53]. The study indicated that ethyl acetate extracts of maple syrup inhibited the growth of prostate (74%), lung (63%), breast (45%) and colorectal (37%) cancer cells in vitro.
Darker maple syrups tend to have more useful antiproliferative traits and might be applied in evolving functional foods and value-added products [59]. González-Sarrías et al. [49] evaluated the effectiveness of purified phenolic-enriched compounds from MS-EtOAc, MS-BuOH, and methanol (MS-MeOH) extracts from amber and dark maple syrup (Table 2) against human tumourigenic (HT-29, HCT-116, and CaCo-2) and non-tumourigenic (CCD-18Co) colon cells. MS-EtOAc, MS-BuOH, and MS-MeOH extracts inhibited proliferation of the HCT-116, Caco-2 and HT-29 colon cancer cell lines and were more effective against the tumourigenic versus non-tumourigenic colon cells. The antiproliferative activities of phenolic compounds (18 and 31–48 in Table 2) of dark maple syrup were higher than those of amber maple syrup (∼3-fold in MS-BuOH extract, and ∼1.5-fold in the MS-MeOH and MS-EtOAc extracts). Similar to the observation of the antiproliferative impacts of the extracts, the study revealed that HCT116 cells were the most sensitive among the cell lines to maple syrup compounds. The antiproliferative activities of compounds were classified based on their IC50 values against HCT-116 cells. Compounds 18, 31–37 (Table 2) showed the greatest antiproliferative effects against colon cancer cells (IC50 = 42–67 μM). Compounds 43–47 exhibited moderate active (IC50 = 75–85 μM), while compounds 43–48 (Table 2) showed the lowest activity against colon cancer cells (IC50 = 94–110 μM). These results showed that maple syrup extracts did not motivate apoptosis of the colon cancer cells but induced cell cycle arrest that was also related to a reduction in the levels of cyclins A and D1.
The antiproliferative impacts of ginnalins A–C, gallotannin compounds found in maple syrup, on breast (MCF-7) tumourigenic, colon (HCT-116), and non-tumorigenic colon (CCD-18Co) cells were evaluated by González-Sarrías et al. [60]. Ginnalins A–C were twofold more efficacious against tumorigenic than non-tumorigenic cells. At 50 μM concentrations, ginnalin A (84%, HCT-116; 49%, MCF-7) was more efficacious than ginnalins B and C (50%, HCT-116; 30%, MCF-7). Ginnalin stopped cell cycle (in the S- and G2/M-phases) and reduced D1 protein and cyclins A levels. The study indicated that ginnalin A-C might have potential cancer chemopreventive properties mediated through cell cycle arrest.
Yamamoto et al. [61] evaluated the effect of maple syrup on cell proliferation, migration and invasion of human colorectal cancer (CRC) cells to assess the potential of maple syrup as a suitable phytomedicine for cancer. The results showed that CRC cells treated with maple syrup exhibited significantly lower growth rates than cells treated with sucrose. In addition, administration of maple syrup to CRC cells resulted in inhibition of cell invasion, whereas there was no impact on cell migration. While the study did not identify specific chemicals repsonsible for the changes observed, these results indicate that maple syrup might inhibit cell proliferation and invasion through repression of the AKT signaling pathway. Thus, the authors suggested that it is possible to consider maple syrup as a suitable phytomedicine for CRC cells, with fewer adverse impacts than traditional chemotherapy.
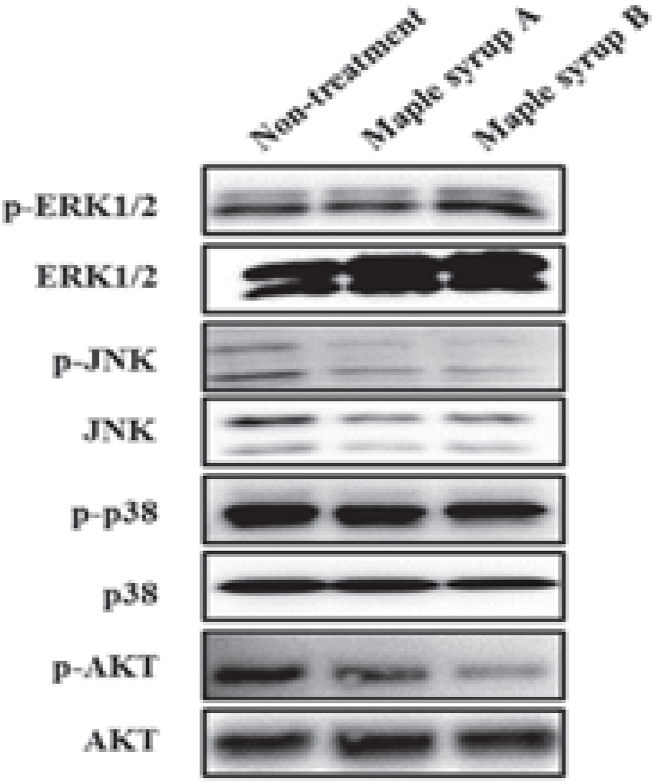
Yamamoto et al. [62] evaluated the effect of two different grades of maple syrup (sightly golden and very dark brown) on human gastrointestinal cancer cell reproduction and examined whether dark-color maple syrup could be used as a phytomedicine for gastrointestinal cancer treatment. The results showed that the ingestion of dark-color maple syrup significantly inhibited gastrointestinal cancer cell growth compared with non-treated cancer cells. Furthermore, ingestion of dark-color maple syrup clearly inhibited protein kinase B (AKT) phosphorylation in all tested gastrointestinal cancer cell lines compared with non-treated and treated cells with gold-color maple syrup (Fig. 2). In brief, dark-color maple syrup may prevent cell proliferation through repression of AKT activation and, hence, might be suitable as a phytomedicine for gastrointestinal cancer treatment.
Fig. 2.
Effects of maple syrup on the mitogen-associated protein kinase and AKT signaling pathways of gastrointestinal cancer cell lines AKT, protein kinase B [62].
Hepatocellular carcinoma is one of the main reasons for cancer deaths worldwide [12]. Özden et al. [50] investigated the apoptotic impact of Ginnalin A in a human hepatocellular carcinoma cell line (Hep3B). IC50 of Ginnalin A was calculated to be 155 μM in Hep3B cells for 72h. A significant increase in gene expression including the CASP3, CASP8, CASP9, CYCS and P53 genes was also observed suggesting that Ginnalin A might be further evaluated as an anticancer agent in hepatocellular carcinoma.
3.3.3. Anti-diabetic activity
Diabetes mellitus is a metabolic and chronic disease that is associated with high blood sugar and fat, carbohydrate, and protein metabolism disorders resulting from flaws in insulin excretion, insulin action, or both [63]. Food and other natural sources have been a useful source of natural drugs for treating patients with diabetes mellitus. Although it may seem counter-intuitive that a natural, sugar-based product possesses anti-diabetic properties, several studies have shown that maple syrup contains various phenolic compounds and extracts that act as α-glucosidase, α-amylase, and plasma glucose inhibitors, key elements in the formation of diabetes, and could be developed into drug candidates in the future [14,64].
Apostolidis et al. [64] evaluated the ability of MS-EtOAc and MS-BuOH extracts to inhibit carbohydrate hydrolyzing enzymes relevant to type 2 diabetes management. The MS-BuOH extract showed higher inhibitory activity than the MS-EtOAc extract (82%, IC50 = 68.38 and 67%, IC50 = 107.9 μg phenolics, respectively). MS-BuOH and MS-EtOAc extracts were further examined for repression of porcine α-amylase and rat α-glucosidase enzymes which aid in the digestion of carbohydrates and starches to glucose for intestinal adsorption. MS-BuOH displayed higher rat α-glucosidase and porcine α-amylase inhibitory activities (69%, IC50 = 135 and 103 μg phenolics, respectively) than MS-EtOAC extract (8%, IC50 > 187 μg phenolics in both assays). These studies suggest that phenolic-based maple syrup extracts might be suitable for treatment of type 2 diabetes, with the MS-BuOH phenolic-enriched extract having the greatest bioactivity.
Zhang et al. [14] investigated the potential of the MS-EtOAc to regulate glucose consumption by HepG2 rats’ cells in vitro, when applied in conjunction with metformin, a clinical drug well known as an AMP-dependent protein kinase (AMPK) activator. The results showed that MSX-1 and MSX-2 significantly reduced glucose concentrations in the cell supernatants. The MSX-1 treatment, at 50 and 100 μg/mL, reduced media glucose levels by almost 16%. Similarly, the glucose levels in the media with 50 and 100 μg/mL MSX-2 treatment decreased by 16% and 18%, respectively. Metformin, at 1 mM concentration, also significantly reduced glucose levels by 23% compared with the control in the HepG2 cell culture media.
Maple syrup is particularly rich in abscisic acid. This acid presents a strong defense against diabetes and metabolic syndrome because it promotes the excretion of insulin from pancreatic cells and boosts fat cells' sensitivity to insulin [52].
Nagai et al. [65] investigated the efficiency of maple syrup in the prevention of type 2 diabetes using OLETF rats fed with maple syrup or sucrose samples. No statistically significant differences were found in insulin levels, but a lowering in plasma glucose, and reduction of glucose absorption in the small intestine, after oral ingestion of maple syrup compared to the sucrose-only administration was observed. The results indicate that maple syrup might partially prevent the absorption of glucose from the small intestine and inhibit the enhancement of PG in OLETF rats.
Maple syrup could provide a natural defense in controlling diabetes and other metabolic syndromes. While diabetics must exercise caution when consuming natural or refined sugars, the above studies suggest that maple syrup and its extracts could offer health benefits that are not present in refined sugars.
3.3.4. Anti-ageing activity
Aaron et al. [51] evaluated the effect of maple syrup on TDP-43 proteotoxicity in Caenorhabditis elegans pattern of amyotrophic lateral sclerosis (ALS). ALS is a neurodegenerative disease giving rise to death of motor neurons. Proteotoxicity caused by TDP-43 protein is an important side effect of ALS pathogenesis, with TDP-43 being the main component of the aggregates present in patients. Maple syrup contains several active molecules that offer neuroprotective activity. At 4% maple syrup, gaps or breaks along the GABAergic neurons, considered as markers of neurodegeneration, were attenuated in worms cultivated on petri plates treated with maple syrup (53% of untreated animals, and 25% of animals treated with 4% maple syrup showed signficant gaps or breaks in the neurons). The study showed that maple syrup reduced many age-dependent phenotypes through attenuation of TDP-43A315T in C. elegans motor neurons which depend on the FOXO transcription factor DAF-16 to be efficient and maintain neuronal integrity during aging.
Several compounds (54–62 in Table 2) presenting neuroprotective effects were isolated from MS-EtOAc [4]. Nine of the isolated phenolic compounds displayed statistically significant effects (p< 0.01) in extending the lifespan of C. elegans.
Using biochemical and biophysical methods, the anti-glycation properties of MSX were evaluated by Liu et al. [13]. The results showed that the MSX extract (500 μg/mL) decreased the formation of advanced glycation end products (AGEs) by 40% in the bovine serum albumin (BSA)-fructose assay and by 30% in the BSA-MGO assay. MSX also prevented the formation of crosslinks that typically appeared in the late phase of glycation and maintained the structure of BSA during glycation. The study showed that maple syrup has protective effects against the formation of AGEs.
Due to the potent concentration of antioxidants and anti-aging compounds found in maple syrup, its ingestion has the potential to play a positive role in protecting skin and enhancing complexion. However, more studies are necessary to assess the skin protection potential of maple syrup, particularly in relation to normal dietary consumption levels.
3.3.5. Anti alzheimer activity
Alzheimer's disease (AD) is a type of neurodegenerative dementia resulting from a brain disorder that slowly destroys memory and affects behavior. Several natural compounds have been claimed to be of interest in different preclinical and clinical studies in the treatment of AD [66].
The effect of MSX on Alzheimer's disease-associated neuroinflammation was evaluated by Rose et al. [11]. Mice) with Alzheimer's disease (AD; 3xTg-AD model) were orally fed with 100 and 200 mg/kg/day of MSX for 30 days. The results showed that MSX reduced expression of several inflammatory proteins, including the stimulation of interferon genes TMEM173, suppression of cytokine signaling-6 (SOCS6), and AD risk-related protein ‘triggering receptor expressed on myeloid cells-2’ (TREM2). However, this reduction did not synchronize with a decrease in lipid peroxidation or pathogenic amyloid generation.
Hawco et al. [67] evaluated the effect of MS-EtOAc on the aggregation of β-amyloid (Aβ) and tau peptides, the two pathological hallmarks of AD. The results showed that MS-EtOAc inhibited Aβ aggregation by 47% at 30 μg/mL and prevented the aggregation of tau peptides by 41% at a concentration of 30 μg/mL. Overall, the study showed that MS-EtOAc reduced oligomerization and aggregation of both β-amyloid (Aβ) and tau peptides in vitro.
Ma et al. [55] investigated the effects of a standardized phenolic-enriched maple syrup extract on β-amyloid aggregation. Based on biophysical results, MSX was shown to decrease amyloid β1−42 peptide (Aβ1−42) fibrillation in a concentration dependent manner (50–500 μg/mL) with similar efficacy and neuroprotection as resveratrol at its highest test concentration (63.5% at 500 μg/mL vs. 77.3% at 50 μg/mL). Cellular proteins that drape incorrectly and clump with each other, pile and evolve into the plaque can lead to Alzheimer's disease [68]. β-Amyloid is the main composition of the amyloid plaques present in the brains of people with AD.
Maple syrup may also prevent the misfolding and accumulation of two kinds of proteins, β-Amyloid and tau peptide, which have been associated with AD. This property gives maple syrup pharmacological importance in the diet. However, the underlying mechanisms and effectiveness of maple syrup against AD have still to be discovered.
3.3.6. Antimutagenic activity
Many chemicals act as mutagens, leading to mutations in celullar DNA. Anti-mutagens are compounds or factors that mitigate or prevent the mutagenicity of a substance. This intervention may be in the form of preventing the transformation of a non-mutagenic compound into a mutagen. Much research has been conducted to characterize the antimutagenic activity of plant extracts and identify new and effective antimutagenic prevention factors [69]. Maple syrup contains several compounds that exhibit anti-mutagenic activity.
Thériault et al. [15] investigated the antimutagenic effects of glycosylated and aglycone phenolic compounds extracted from maple syrup samples collected at different time points during the maple syrup season as potential inhibitors of SOS induction by chemical factors in Salmonella typhimurium TA1535/pSK1002 which included the fusion gene umuC-lacZ. The study evaluated induction of the SOS gene (umuC) expression by measuring accumulated-galactosidase activity using a modified Umu test. The results showed that the optimum antimutagenic efficacy of the glycosylated and aglycone metabolites of phenolic compounds occurred approximately at the beginning of the season with antimutagenicity percentages of 14.05% and 42.33%, respectively.
At the present time, the detection and exploration of antimutagenic chemical compounds is of great importance because of the unwanted consequences of an increased rate of mutations and the related possible risks of human cancer (Kaur & Saini, 2000) [70]. Maple syrup contains chemical compounds such as glycosylated and aglycone phenolic compounds that have positive antimutagenic roles.
3.3.7. Anti-microbial activity
Maisuria et al. [47] investigated the antimicrobial activities MSHX. They showed antimicrobial activity and effectiveness against Gram-negative clinical strains of Escherichia coli, Pseudomonas aeruginosa PAO1, P. aeruginosa PA14, and Proteus mirabilis. Among the phenolic components of MSHX, gallic acid, 1,2-dihydroxybenzene (catechol) (at 1.25 mg ml−1 for both E. coli strain CFT073 and P. mirabilis HI4320), 3,4-dihydroxybenzaldehyde (catechaldehyde) (at 1.25 mg ml−1 for all four strains), syringaldehyde, vanillin, and 3-hydroxybenzoic acid (at 2.5 mg ml−1) for P. aeruginosa (PAO1 and PA14) displayed strong synergy with antibiotics (ciprofloxacin, and carbenicillin) and also with other phenolic constituents of MSHX against bacterial growth. At sublethal concentrations, MSHX and catechol effectively decreased biofilm formation of pathogenic bacteria and augmented the sensitivity of bacterial biofilms to antibiotics. MSHX increased the permeability of the outer membrane of all bacterial strains and efficiently inhibited efflux pump activity. Moreover, MSHX significantly suppressed the genes conferring multiple-drug resistance as well as genes associated with adhesion, motility, virulence formation, and biofilm formation.
n summary, maple syrup contains a wide range of chemical compounds that possess various pharmacological attributes such as antioxidant, anti-inflammatory, anticancer, antiaging, anti-alzheimer, and antimutagenic activities, as well as decreasing blood glucose levels. Given these pharmacological properties, it is not surprising that maple syrup is often located in the health and nutrition section of supermarkets and in health food stores. In this review, we have highlighted the many pharmacological activities of chemicals in maple syrup. Unfortunately, because almost all the studies have been performed on animal models or isolated cells, and often focus on extracts rather than pure maple syrup, the health benefits of maple syrup when used in typical dietary quantities is unknown and it is not yet possible to indicate a daily amount to consume to fully enjoy the health benefits of maple.
4. Effect of maple syrup production processes on its nutritional value and component bioactivity
Maple syrup is produced by boiling sap of the sugar maple (Acer saccharum) at 104 °C to evaporate water and concentrate the content of sugar to 66% or 66–67 brix, which results in a thick, sweet syrup [53,60]. Maple syrup has different grades (range from light to dark) that differ in color, composition, and flavor. Maple sap collected early in the season delivers a syrup whose color is light, while the syrup made later, when the weather is warmer, is dark [62]. Compared to light color maple syrup, dark maple syrup contains different bioactive compounds, such as polyphenols [15,29]. The antimutagenic and antioxidant activities of phenolic compounds extracted from maple syrup is high early in the sap harvesting season [15] whereas darker maple syrup is more active than clear maple syrup for inhibition NO production in RAW264.7 [53]. This suggests that some colored oxidized compounds could be accountable in part for the greater activity. The MS-BuOH extract showed higher inhibitory activity to the enzymes hydrolyzing carbohydrates, rat α-glucosidase and porcine α-amylase inhibitory activities compared to MS-EtOAC extract [64]. The antiproliferative activity of dark maple syrup was higher than that of amber maple syrup [49] and darker maple syrup showed inhibitory impacts on the cell growth of colorectal cancer and invasion [61]. In another study, Yamamoto et al. [62] reported that dark color maple syrup inhibited the cell growth of gastrointestinal cancer compared to untreated cancer cells, and it also had more effect than gold-color maple syrup.
Evaluation of the variation in antioxidant activity of 5 maple syrup grades (extralight, light, medium, amber, and dark) by the hydrophilic oxygen radical absorbance capacity method has been performed [71]. The values of hydrophilic oxygen radical absorbance capacity differed widely based on the coloration degree. Dark maple syrups demonstrated stronger antioxidant activity and contained reducing sugars (glucose and fructose) compared to other maple syrup grades in the following order: extra-light (576 ± 185 μmol) < light (673 ± 143 μmol) < medium (820 ± 126 μmol) < amber (1136 ± 182 μmol) < dark (1502 ± 310 μmol). Brown pigments (melanoidins) resulting from condensation of amines and reducing groups may contribute greatly to the maple syrup antioxidant activity. Takano in 2016 [72] notified that the concentrations of iron, calcium, glucose, and fructose tended to be higher, whereas sucrose concentration tended to be lower in darker maple syrup compared to lighter syrup. In another study, Yamamoto et al. [62] reported that dark-color maple syrup yielded greater inhibition of the cell growth of gastrointestinal cancer compared to gold-color maple syrup. Bhatta et al. [73] investigated the effect of drying processes on characteristics of polyphenol-enriched maple sugar powders (MSP). The study exhibited that drying processes caused a reduction in total phenolic content and antioxidant capacity of MSP.
Maple syrup production processes cause remarkable changes in maple chemistry, either through the formation of new chemicals or the concentration of existing chemicals, which in turn affect the bioactivity of its components. In brief, production processes have real effects on the reproducibility of the nutritional and health benefits of maple syrup.
5. Questions about the nutritional effect of maple syrup: bioavailability of phenolic compounds
Phenolic compounds constitute one of the main categories of secondary metabolites in plants [74]. Even though there is an increasing interest in phenolic compounds, and their assumed role in the protection of various chronic diseases, the bioavailability and absorption of phenolic compounds in the human body are still controversial [74], as is their role to manage obesity [75]. Therefore, extensive investigations of the behavior, absorption, and metabolism of phenolics in the gastrointestinal tract still need to be carried out. Several studies have shown that numerous factors may affect the absorption and bioavailability of phenolics in humans. Scalbert and Williamson in 2000 [76] indicated that partition coefficients play an effective role in describing the absorption of phenolic compounds that do not contain organic acid substitutes or sugars in their structure. The stomach does not absorb hydrophilic compounds. Other studies confirmed that partition coefficients have an effective role in the absorption of hydrophobic phenolics that contain ester and organic acid linked substitutions or sugar in their structure, while hydrophilic phenolics that have similar structure are deteriorated by the produced esterases from the microflora in the colon [ [[76], [77], [78], [79], [80]]]. Sugar molecules play an efficient role in the absorption of phenolics. The phenolics that are substituted with sugar molecules such as galactose, glucose or xylose are absorbed in the small intestine by the lactase phlorizin hydrolase/cytosolic β-glucosidase [ [76,[81], [82], [83], [84], [85]]] whereas isoflavone aglycones are absorbed by the stomach, and glycosides are absorbed by the duodenum. Some acylated flavonoids such as (−)-epigallocatechin and (−)-epicatechin are absorbed by the body without hydrolysis and deconjugation [ [76,83,86]]. Karakaya in 2010 [74] indicated that rhamnose-containing phenolics cannot be absorbed by the small intestine and are degraded by rhamnosidases produced from the colonic microflora.
Suárez et al. [87] indicated that the absorption or transportation of phenolics is greatly dependent on the person. Minatel et al. [88] reported that the resulting modifications by different processing methods in phenolics have an effective influence on the bioavailability of phenolic compounds. For example, during food processing, molecular modifications that occur in phenols are catalyzed, the absorption of these compounds are triggered by both non-enzymatic and enzymatic reactions, and thus these compounds may be subject to conjugation reactions that may increase or reduce their bioavailability.
Phenolic compounds of maple syrup are one of the main classes of chemicals behind its healthy properties. Therefore, the ingestion of maple syrup could increase the daily intake of beneficial phenolic compounds. Nonetheless, to better understand the potential health benefits of phenolics in maple syrup, there is a crucial need to determine the exact bioavailability and uptake of phenolics in the digestive tract following ingestion of maple syrup. As maple syrup is rich in sugar molecules, esters, and organic acids (Table 1), these molecules could impact the bioavailability of phenolics in humans. In addition, the processing method of maple syrup differs from other syrups, and it might have an effective role in determining the bioavailability of phenolic compounds as evidenced by Minatel et al. [88].
6. Sensory properties of maple syrup
Maple syrup has a unique flavor that distinguishes it from other syrups and sucrose. The flavor of maple syrup is influenced by multiple factors such as chemical change in the composition of the sugar, production location, time of the season, production methods, filtering, and packaging conditions, as well as environmental conditions. The flavor of maple syrup depends on the syrup grade. It can range from a very light, sweet, but otherwise nearly tasteless substance, to a very dark, bitter, burnt taste [9,89].
Maple syrup constituents such as organic acids, free amino acids, protein, and phenolic compounds not only contribute to its potential health benefits compared to sucrose, but also participate in its sensory profile [33].
Belford et al. [89] reported that 5′‐inosine monophosphate is a compound that plays an important role in the taste characteristics of maple syrup. Sair and Sne in 1939 [90] notified that enolic viscous oil found in maple syrup is one of the major elements responsible for the distinct maple syrup odor. Vanillin, syringaldehyde, and dihydroconiferyl alcohol contribute to the flavor of maple syrup [91,92]. Filipic et al. [93] reported that acetol, acetoin, ethyl vanillate, syringoyl methyl ketone, and methylcyclopentenolone contribute significantly to the flavor properties of maple syrup. Filipic et al. [27] reported several flavour-related compounds detected in maple syrup including oxalic, fumaric and malic acids that have also been linked to the flavor characteristics of maple syrup (Table 4). Kallio in 1988 [28] identified additional compounds that contribute to the flavor of maple syrup (Table 4). Alli et al. and Sabik et al. [94,95], reported the presence of several pyrazine derivatives that play a significant role in contributing to the flavor characteristics of maple syrup (Table 4).
Table 4.
List of the organoleptic active compounds isolated from maple syrup extracts.
| Comp | Name | MS-BuOH | MS-EtOAc | MS-MeOH | Chloroform | Dichloromethane | Ref |
|---|---|---|---|---|---|---|---|
| Taste compounds | |||||||
| 1 | 5′‐Inosine monophosphate | [89] | |||||
| Odor compounds | |||||||
| 2 | Enolic viscous | + | [90] | ||||
| 3 | Vanillin | + | [91] | ||||
| 4 | Syringaldehyde | + | [91] | ||||
| Flavor compounds | |||||||
| 5 | Vanillin | + | [92] | ||||
| 6 | Syringaldehyde | + | [92] | ||||
| 7 | Dihydroconiferyl alcohol | + | [92] | ||||
| 8 | Acetol | + | [93] | ||||
| 9 | Acetoin | + | [93] | ||||
| 10 | Ethyl vanillate | + | [93] | ||||
| 11 | Syringoyl methyl ketone | + | [93] | ||||
| 12 | Methylcyclopentenolone | + | [93] | ||||
| 13 | Acetovanillone | + | [93] | ||||
| 14 | Guaiacyl acetone | + | [27] | ||||
| 15 | Vanilloyl methyl ketone | + | [27] | ||||
| 16 | Furfural | + | [27] | ||||
| 17 | Hydroxymethylfurfural | + | [27] | ||||
| 18 | Lactic acid | + | [27] | ||||
| 19 | Levulinic acid | + | [27] | ||||
| 20 | C5 to C9 Aliphatic acids | + | [27] | ||||
| 21 | Oxalic acids | + | [27] | ||||
| 22 | Fumaric acids | + | [27] | ||||
| 23 | Malic acids | + | [27] | ||||
| 24 | 2-Ethyl-l-hexanol | + | [28] | ||||
| 25 | 2-Hydroxymethylcyclopenten-2-en-ol | + | [28] | ||||
| 26 | 2-Furanmethanol | + | [28] | ||||
| 27 | Three organic acids (2-ethyl-l-hexanoic acid | + | [28] | ||||
| 28 | n-Hexanoic acid | + | [28] | ||||
| 29 | n-Nonanoic acid | + | [28] | ||||
| 30 | 2-Hydroxy-3-methyl-2-cyclopenten-l-one | + | [28] | ||||
| 31 | 2-Methyl-2-cyclopenten- 1-one | + | [28] | ||||
| 32 | 2-Methyl-2,5-cyclohexadien-l,4-dione | + | [28] | ||||
| 33 | 2,3-Dihydro-3,5-dihydroxy-6-methyl(4H)-pyran-4-one | + | [28] | ||||
| 34 | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone | + | [28] | ||||
| 35 | 3-Methyl-3-buten-2-one | + | [28] | ||||
| 36 | 3-Methyl-2,5-furandione | + | [28] | ||||
| 37 | Methylpyrazine | + | [28] | ||||
| 38 | 2,3-Dimethylpyrazine | + | [94,95] | ||||
| 39 | 2,5-Dimethylpyrazine | + | [94,95] | ||||
| 40 | 2,6-Dimethylpyrazine | + | [94,95] | ||||
| 41 | Ethylpyrazine | + | [94] | ||||
| 42 | 2-Ethyl-6-methylpyrazine | + | [94] | ||||
| 43 | Trimethylpyrazine | + | [94] | ||||
| 44 | 2-Methylpyrazine | [94] | |||||
| 45 | 2-Ethylpyrazine | [94] | |||||
| 46 | 2-Ethyl-5-methylpyrazine | [95] | |||||
| 47 | 2-Ethyl-3-methylpyrazine | [95] | |||||
| 48 | 2-Methoxy-3-(1-methylethyl)pyrazine | [95] | |||||
| 49 | 2-Ethyl-3,5-dimethylpyrazine | [95] | |||||
| 50 | 2,3-Diethylpyrazine | [95] | |||||
| 51 | Tetramethylpyrazine | [95] | |||||
| 52 | 2,3-Diethyl-5-methylpyrazine | [95] | |||||
| 53 | 2-Ethenyl-5-methylpyrazine | [95] | |||||
| 54 | 2-Ethenyl-5-methylpyrazine | [95] | |||||
| 55 | 2-Acetyl-3-ethylpyrazine | [95] | |||||
Maple syrup contains a combination of several chemical compounds that play a significant role in the overall sensory characteristics of maple syrup and give maple syrup a special character and distinguish it from other syrups.
7. Conclusion and future perspective
Maple syrup is a naturally sweet syrup consumed throughout the world and is characterized by its unique flavor, taste, and smell. The source of these sensory characteristics, particularly its flavor, lies in its chemical composition which may be affected by the processing method which, in turn, might play an important role in determining the bioavailability of phenolic compounds. Presently, overconsumption of sugar results in a dramatic increase of diseases as diabetes or cardiovascular diseases. However, consumers demand sugary foods, so a healthy source of sweet tasting foods is therefore actively sought. Maple syrup is frequently presented as a natural and harmless sweetener that can replace refined sugars, but its nutritional properties are far from being exactly defined. Though several studies have reported the pharmacological potential of maple syrup, more studies and clinical data are needed to provide further evidence supporting the pharmacological effectiveness and nutritional benefit of maple syrup in humans. Considerable interest in developing novel mechanisms of action for isolation and extraction of these known/new compounds that might also be useful in clinical applications is necessary since there is great potential to get new chemical scaffolds for medications with improved properties from maple syrup. More studies are needed to better understand how much maple syrup could be ingested, as part of a regular diet, to promote these pharmacological properties without triggering obesity or weight-related disorders.
Funding
None
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
The data that has been used is confidential.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This project is jointly supported from the University of Guelph and the Institute of International Education's Scholar Rescue Fund (IIE-SRF).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19216.
Contributor Information
Faez Mohammed, Email: faez@uoguelph.ca, faez89@hotmail.fr.
Paul Sibley, Email: psibley@uoguelph.ca.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Mora M.R., Dando R. The sensory properties and metabolic impact of natural and synthetic sweeteners. Compr. Rev. Food Sci. Food Saf. 2021;20:1554–1583. doi: 10.1111/1541-4337.1270. [DOI] [PubMed] [Google Scholar]
- 2.Arshad S., Rehman T., Saif S., Shahid M., Rajoka R., Modassar M., Ranjha A.N., et al. Replacement of refined sugar by natural sweeteners: focus on potential health benefits. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammed F., Warland J., Guillaume D. A comprehensive review on analytical techniques to detect adulteration of maple syrup. Microchem. J. 2021;164 doi: 10.1016/j.microc.2021.105969. [DOI] [Google Scholar]
- 4.Liu Y., Rose K.N., DaSilva N.A., Johnson S.L., Seeram N.P. Isolation, identification, and biological evaluation of phenolic compounds from the traditional north american confectionery, maple sugar. J. Agric. Food Chem. 2017;65:4289–4295. doi: 10.1021/acs.jafc.7b01969. [DOI] [PubMed] [Google Scholar]
- 5.Mohammed F., Sibley P., Guillaume D., Abdulwali N. Chemical composition and mineralogical residence of maple syrup: a comprehensive review. Food Chem. 2021;374 doi: 10.1016/j.foodchem.2021.131817. https://doi:10.1016/j.foodchem.2021.131817 [DOI] [PubMed] [Google Scholar]
- 6.Saraiva A., Carrascosa C., Ramos F., Raheem D., Lopes M., Raposo A. Maple syrup: chemical analysis and nutritional profile, health impacts, safety and quality control, and food industry applications. Int. J. Environ. Res. Publ. Health. 2022;19 doi: 10.3390/ijerph192013684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gad H.A., Ramadan M.F., Farag M.A. Authentication and quality control determination of maple syrup: a comprehensive review. J. Food Compos. Anal. 2021;100 doi: 10.1016/j.jfca.2021.103901. [DOI] [Google Scholar]
- 8.Nimalaratne C., Blackburn J., Lada R.R. A comparative physicochemical analysis of maple (Acer saccharum Marsh.) syrup produced in North America with special emphasis on seasonal changes in Nova Scotia maple syrup composition. J. Food Compos. Anal. 2020;92 doi: 10.1016/j.jfca.2020.103573. [DOI] [Google Scholar]
- 9.Perkins T.D., van den Berg A.K. Chapter 4 maple syrup—production, composition, chemistry, and sensory characteristics. Adv. Food Nutr. Res. 2009;56:101–143. doi: 10.1016/S1043-4526(08)00604-9. [DOI] [PubMed] [Google Scholar]
- 10.Ball D.W. The chemical composition of maple syrup. J. Chem. Educ. 2007;84:1647–1650. doi: 10.1021/ed084p1647. [DOI] [Google Scholar]
- 11.Rose K.N., Barlock B.J., DaSilva N.A., Johnson S.L., Chang L.C., et al. Anti-neuroinflammatory effects of a food-grade phenolic-enriched maple syrup extract in a mouse model of Alzheimer's disease. Nutr. Neurosci. 2019;24:710–719. doi: 10.1080/1028415X.2019.1672009. [DOI] [PubMed] [Google Scholar]
- 12.Rawla P., Sunkara T., Muralidharan P., Raj J.P. Update in global trends and aetiology of hepatocellular carcinoma. Contemp. Oncol. 2018;22:141–150. doi: 10.5114/wo.2018.78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W., Wei Z., Ma H., Liu A.C.Y., Sun J., DaSilva N.A., et al. Anti-glycation and anti-oxidative effects of a phenolic-enriched maple syrup extract and its protective effects on normal human colon cells. Food Funct. 2017;8:757–766. doi: 10.1039/C6FO01360K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y., Yuan T., Li L., Nahar P., Slitt A., Seeram N.P. Chemical compositional, biological, and safety studies of a novel maple syrup derived extract for nutraceutical applications. J. Agric. Food Chem. 2014;62:6687–6698. doi: 10.1021/jf501924y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thériault M., Caillet S., Kermasha S., Lacroix M. Antioxidant, antiradical and antimutagenic activities of phenolic compounds present in maple products. Food Chem. 2006;98(3):490–501. doi: 10.1016/j.foodchem.2005.05.079. [DOI] [Google Scholar]
- 16.Freeland-Graves J.H., Nitzke S. Position of the academy of nutrition and dietetics: total diet approach to healthy eating. J. Acad. Nutr. Diet. 2013;113:307–317. doi: 10.1016/j.jand.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Phillips K., Carlsen M., Blomhoff R. Total antioxidant content of alternatives to refined sugar. J. Am. Diet Assoc. 2009;109:64–71. doi: 10.1016/j.jada.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Morselli M.F. Nutritional value of pure maple syrup. Nat. Maple Syrup Dig. 1975;14:12. [Google Scholar]
- 19.Stuckel J.G., Low N.H. The chemical composition of 80 pure maple syrup samples produced in North America. Food Res. Int. 1996;29:373–379. doi: 10.1016/0963-9969(96)00000-2. [DOI] [Google Scholar]
- 20.Perkins T.D., Morselli M.F., van den Berg A.K., Wilmot T.R. In: ‘‘North American Maple Syrup ProducersManual,’’. Heiligmann R.B., Koelling M.R., Perkins T.D., editors. Ohio State University; 2006. Maple chemistry and quality. Appendix 2. (Ohio State University Extension Bulletin 856). [Google Scholar]
- 21.Heinsohn R.J., Cimballa J. Taylor & Francis, Technology & Engineering; 2003. Indoor Air Quality Engineering: Environmental Health and Control of Indoor Pollutants; p. 920. [Google Scholar]
- 22.David Julian McClements D.J., Grossmann L. The science of plant-based foods: constructing next-generation meat, fish, milk, and egg analogs. Compr. Rev. Food Sci. Food Saf. 2021;20:4049–4100. doi: 10.1111/1541-4337.12771. [DOI] [PubMed] [Google Scholar]
- 23.Mathias D. Fit and Healthy from 1 to 100 with Nutrition and Exercise. Springer; Berlin, Heidelberg: 2022. Energy consumption III—performance output. [DOI] [Google Scholar]
- 24.Sato K., Yamamoto T., Mitamura K/Taga A. Identification of a novel oligosaccharide in maple syrup as a potential alternative saccharide for diabetes mellitus patients. Int. J. Mol. Sci. 2019;20:1–13. doi: 10.3390/ijms20205041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun J., Ma H., Seeram N.P., Rowley D.C. Detection of inulin, a prebiotic polysaccharide, in maple syrup. J. Agric. Food Chem. 2016;64:7142–7147. doi: 10.1021/acs.jafc.6b03139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato K., Yamamoto T., Mitamura K., Taga A Separation of fructosyl oligosaccharides in maple syrup by using charged aerosol detection. Foods. 2021;10:3160. doi: 10.3390/foods10123160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filipic V.J., Underwood J.C., Dooley C.J. Trace components of the flavor fraction of maple syrup. J. Food Sci. 1969;34:105–110. doi: 10.1111/j.1365-2621.1969.tb00897.x. [DOI] [Google Scholar]
- 28.Kallio H. In: Comparison and Characteristics of Aroma Compounds from Maple and Birch Syrup. Proceedings, 5th International Flavor Conference. Charalambos G., editor. Elsevier; Amsterdam: 1988. pp. 241–248. [Google Scholar]
- 29.Li L., Seeram N.P. Maple syrup phytochemicals include lignans, coumarins, a stilbene, and other previously unreported antioxidant phenolic compounds. J. Agric. Food Chem. 2010;58:11673–11679. doi: 10.1021/jf1033398. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe Y., Kamei A., Shinozaki F., Ishijima T., Iida K., Nakai Y., Arai S., Abe K. Ingested maple syrup evokes a possible liver-protecting effect physiologic and genomic investigations with rats. Biosci. Biotechnol. Biochem. 2011;75:2408–2410. doi: 10.1271/bbb.110532. [DOI] [PubMed] [Google Scholar]
- 31.Subash S., Subramanian P. Effect of morin on the levels of circulatory liver markers and redox status in experimental chronic hyperammonaemic rats. Singap. Med. J. 2008;49:650–655. PMID: 18756351. [PubMed] [Google Scholar]
- 32.Toyoda T., Iida K., Ishijima T., Abea K., Okadaa S., Nakaia Y. A maple syrup extract alleviates liver injury in type 2 diabetic model mice. Nutr. Res. 2020;73:97–101. doi: 10.1016/j.nutres.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Nagai N., Ito Y., Taga A. Comparison of the enhancement of plasma glucose levels in type 2 diabetes Otsuka Long-Evans Tokushima Fatty rats by oral administration of sucrose or maple syrup. J. Oleo Sci. 2013;62:737–743. doi: 10.5650/jos.62.737. [DOI] [PubMed] [Google Scholar]
- 34.Kamei A., Watanabe Y., Shinozaki F., Yasuoka A., Kondo T., Ishijima T., Toyoda T., Arai S., Abe K. Administration of a maple syrup extract to mitigate their hepatic inflammation induced by a high-fat diet: a transcriptome analysis. Biosci., Biotechnol., Biochem. 2015;79:1893–1897. doi: 10.1080/09168451.2015.1042833. [DOI] [PubMed] [Google Scholar]
- 35.Kamei A., Watanabe Y., Shinozaki F., Yasuoka A., Shimada K., et al. Quantitative deviating effects of maple syrup extract supplementation on the hepatic gene expression of mice fed a high-fat diet. Mol. Nutr. Food Res. 2017;61:1–56. doi: 10.1002/mnfr.201600477. [DOI] [PubMed] [Google Scholar]
- 36.Toyoda T., Kamei A., Ishijima T., Abe K., Okada S. A maple syrup extract alters lipid metabolism in obese type 2 diabetic model mice. Nutr. Metabol. 2019;16:1–8. doi: 10.1186/s12986-019-0403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.St-Pierre P., Pilon G., Dumais V., Dion C., Dubois M.J., Dubé P., Desjardins Y., Aarette A. Comparative analysis of maple syrup to other natural sweeteners and evaluation of their metabolic responses in healthy rats. J. Funct.Foods. 2014;11:460–471. doi: 10.1016/j.jff.2014.10.001. [DOI] [Google Scholar]
- 38.Bruzzone S., Ameri P., Briatore L., Mannino E., Basile G., Andraghetti G., et al. The plant hormone abscisic acid increases in human plasma after hyperglycemia and stimulates glucose consumption by adipocytes and myoblasts. Faseb. J. 2012;26:1251–1260. doi: 10.1096/fj.11-190140. [DOI] [PubMed] [Google Scholar]
- 39.Edwards C.H., Rossi M., Corpe C.P., Peter J., Butterworth P.J., Ellis P.R. The role of sugars and sweeteners in food, diet and health: alternatives for the future. Trends Food Sci. Technol. 2016;56:158–166. doi: 10.1016/j.tifs.2016.07.008. [DOI] [Google Scholar]
- 40.Valle M., St-Pierre P., Pilon G., Marette A. Differential effects of chronic ingestion of refined sugars versus natural sweeteners on insulin resistance and hepatic steatosis in a rat model of diet-induced obesity. Nutrients. 2020;12:2292. doi: 10.3390/nu12082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hattori H., Hanai Y., Oshima Y., Kataoka H., Eto N. Excessive intake of high-fructose corn syrup drinks induces impaired glucose tolerance. Biomedicines. 2021;9:541. doi: 10.3390/biomedicines9050541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guri A.J., Hontecillas R., Si H., Liu D., Bassaganya-Riera J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin. Nutr. 2007;26:107–116. doi: 10.1016/j.clnu.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Lavoie L., Tremblay J. Ingestion of maple-based and other carbohydrate sports drinks: effect on sensory perceptions during prolonged exercise. Sports Nutr. Rev. J. 2020;17:2–6. doi: 10.1186/s12970-020-00384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernández-Carlos B. Antioxidant compounds and their antioxidant mechanism. Intechopen. 2019 doi: 10.5772/intechopen.85270. [DOI] [Google Scholar]
- 45.Abou-Zaid M.M., Nozzolillo C., Tonon A., Coppens M., Lombardo D.A. High-performance liquid chromatography characterization and identification of antioxidant polyphenols in maple syrup. Pharmaceut. Biol. 2008;46:117–125. doi: 10.1080/13880200701735031. [DOI] [Google Scholar]
- 46.Nahar P.P., Driscoll M.V., Li L., Slitt A.L., Seeram N.P. Phenolic mediated anti-inflammatory properties of a maple syrup extract in RAW 264.7 murine macrophages. J. Funct.Foods. 2014;6:126–136. doi: 10.1016/j.jff.2013.09.026. [DOI] [Google Scholar]
- 47.Maisuria V.B., Hosseinidoust Z., Tufenkji N. Polyphenolic extract from maple syrup potentiates antibiotic susceptibility and reduces biofilm formation of pathogenic bacteria. Appl. Environ. Microbiol. 2015;8:3782–3792. doi: 10.1128/AEM.00239-15. https://10.1128/AEM.01341-20 10.1128/AEM.00239-15 and correction Appl Environ Microbiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardinal S., Azelmat J., Grenier D., Voyer N. Anti-inflammatory properties of quebecol and its derivatives. Bioorg. Med. Chem. Lett. 2016;26:440–444. doi: 10.1016/j.bmcl.2015.11.096. [DOI] [PubMed] [Google Scholar]
- 49.González-Sarrías A., Li L., Seeram N.P. Anticancer effects of maple syrup phenolics and extracts on proliferation, apoptosis, and cell cycle arrest of human colon cells. J. Funct.Foods. 2012;4:185–196. doi: 10.1016/j.jff.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Özden P., Avcı E., Vural H. Survey of the apoptotic effect of ginnalin A on Hep3b human hepatocellular carcinoma cell line. Proceedings. 2017;1:1064. doi: 10.3390/proceedings1101064. [DOI] [Google Scholar]
- 51.Aaron C., Beaudry G., Parker J.A., Therrien M. Maple syrup decreases TDP-43 proteotoxicity in a Caenorhabditis elegans model of amyotrophic lateral sclerosis (ALS) J. Agric. Food Chem. 2016;64:3338–3344. doi: 10.1021/acs.jafc.5b05432. [DOI] [PubMed] [Google Scholar]
- 52.Bruzzone S., Bodrato N., Usai C., Guida L., Moreschi I., Nano R., et al. Abscisic acid is an endogenous stimulator of insulin release from human pancreatic islets with cyclic ADP ribose as second messenger. J. Biol. Chem. 2008;283:32188–32197. doi: 10.1074/jbc.M802603200. [DOI] [PubMed] [Google Scholar]
- 53.Legault J., Girard-Lalancette K., Grenon C., Dussault C., Pichette A. Antioxidant activity, inhibition of nitricoxide overproduction, and in vitro antiproliferative effect of maple sap and syrup from Acer saccharum. J. Med. Food. 2010;13:460–468. doi: 10.1089/jmf.2009.0029. [DOI] [PubMed] [Google Scholar]
- 54.Li L., Seeram N.P. Further investigation into maple syrup yields 3 new lignans, a new phenylpropanoid, and 26 other phytochemicals. J. Agric. Food Chem. 2011;59:7708–7716. doi: 10.1021/jf2011613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma H., DaSilva N.A., Liu W., Nahar P.P., Wei Z., et al. Effects of a standardized phenolic-enriched maple syrup extract on β-amyloid aggregation, neuroinflammation in microglial and neuronal cells, and β-amyloid induced neurotoxicity in Caenorhabditis elegans. Neurochem. Res. 2016;41:2836–2847. doi: 10.1007/s11064-016-1998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong L.M., Sun L., Guo J.Z., Zhang W., He Y., Song R., Wang W.M., Xiao C.J., Lu D. Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PLoS One. 2012;7:1–12. doi: 10.1371/journal.pone.0032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheng J., Liu C., Petrovas S., Wan Y., Chen H.D., Seeram N.P., Ma H. Phenolic-enriched maple syrup extract protects human keratinocytes against hydrogen peroxide and methylglyoxal induced cytotoxicity. Dermatol. Ther. 2020;33:1–6. doi: 10.1111/dth.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li L., Seeram N.P. Quebecol, a novel phenolic compound isolated from Canadian maple syrup. J. Funct.Foods. 2011;3:125–128. doi: 10.1016/j.jff.2011.02.004. [DOI] [Google Scholar]
- 59.Singh A.S., Jones A.M.P., Saxena P.K. Variation and correlation of properties in different grades of maple syrup. Plant Foods Hum. Nutr. 2014;69:50–56. doi: 10.1007/s11130-013-0401-x. [DOI] [PubMed] [Google Scholar]
- 60.González-Sarrías A., Ma H., Edmonds M.E., Seeram N.P. Maple polyphenols, ginnalins A–C, induce S- and G2/M-cell cycle arrest in colon and breast cancer cells mediated by decreasing cyclins A and D1 levels. Food Chem. 2013;136:15 636–642. doi: 10.1016/j.foodchem.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto T., Uemura K., Moriyama K., Mitamura K., Taga A. Inhibitory effect of maple syrup on the cell growth and invasion of human colorectal cancer cells. Oncol. Rep. 2015;33:1579–1584. doi: 10.3892/or.2015.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamamoto T., Sato K., Kubota Y., Mitamura K., Taga A Effect of dark-colored maple syrup on cell proliferation of human gastrointestinal cancer cell. Biomedical Reports. 2017;7:6–10. doi: 10.3892/br.2017.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.N Tran, Pham B., Le L. Bioactive compounds in anti-diabetic plants: from herbal medicine to modern drug discovery. Biology. 2020;9:1–32. doi: 10.3390/biology9090252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Apostolidis E., Li L., Lee C., Seeram N.P. In vitro evaluation of phenolic-enriched maple syrup extracts for inhibition of carbohydrate hydrolyzing enzymes relevant to type 2 diabetes management. J. Funct.Foods. 2011;3:100–106. doi: 10.1016/j.jff.2011.03.003. [DOI] [Google Scholar]
- 65.Nagai N., Yamamoto T., Tanabe W., Ito Y., Kurabuchi S., Mitamura K., Taga A. Changes in plasma glucose in Otsuka Long-Evans Tokushima fatty rats after oral administration of maple syrup. J. Oleo Sci. 2015;64:331–335. doi: 10.5650/jos.ess14075. [DOI] [PubMed] [Google Scholar]
- 66.Andrade S., Ramalho M.J., Loureiro J.A., Pereira MdoC. Natural compounds for alzheimer's disease therapy: a systematic review of preclinical and clinical studies. Int. J. Mol. Sci. 2019;20:1–41. doi: 10.3390/ijms20092313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hawco C.L., Wang Y., Taylor M., Weaver D.F. A maple syrup extract prevents β-amyloid aggregation. Can. J. Neurol. Sci. 2016;43:198–201. doi: 10.1017/cjn.2015.270. [DOI] [PubMed] [Google Scholar]
- 68.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Makhafola T.J., Elgorashi E.E., McGaw L.J., Verschaeve L., Eloff J.N. The correlation between antimutagenic activity and total phenolic content of extracts of 31 plant species with high antioxidant activity. BMC Compl. Alternative Med. 2016;16:1–13. doi: 10.1186/s12906-016-1437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaur I.P., Saini A. Sesamol exhibits antimutagenic activity against oxygen species mediated mutagenicity. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000;470:71–76. doi: 10.1016/S1383-5718(00)00096-6. [DOI] [PubMed] [Google Scholar]
- 71.Unno T. Antioxidant activity of different grades of maple syrup as determined by the hydrophilic oxygen radical absorbance capacity method. Food Sci. Technol. Res. 2015;21 doi: 10.3136/fstr.21.495. 495_498. [DOI] [Google Scholar]
- 72.Takano H. Investigation of chemical and physical properties of Southwestern Wisconsin maple syrup. Journal of Student Research. 2016:97–107. http://digital.library.wisc.edu/1793/52713 [Google Scholar]
- 73.Bhatta S., Ratti C., Stevanovic T. Impact of drying processes on properties of polyphenol-enriched maple sugar powders. Food Sci. Technol. Res. 2019;21:495–498. doi: 10.1111/jfpe.13239. [DOI] [Google Scholar]
- 74.Karakaya S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2010;44:453–464. doi: 10.1080/10408690490886683. [DOI] [PubMed] [Google Scholar]
- 75.Bruno R.S., Neilson A.P., Lambert J.D., Moustaid-Moussa N. Polyphenols, obesity, and cardiometabolic health. JNB (J. Nutr. Biochem.) 2021;89 doi: 10.1016/j.jnutbio.2020.108565. [DOI] [PubMed] [Google Scholar]
- 76.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. The Journal of Nutrition. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 77.Rechner A.R., Spencer J., P.E, Kuhnle G., U Hahn, Rice-Evans C.A. Novel biomarkers of the metabolism of caffeic acid derivatives in vivo. Free Radic. Biol. Med. 2001;30:1213–1222. doi: 10.1016/S0891-5849(01)00506-8. [DOI] [PubMed] [Google Scholar]
- 78.Olthof M.R., Hollman P.C.H., Katan M.B. Chlorogenic acid and caffeic acid are absorbed in humans. The Journal of Nutrition. 2001;131:66–71. doi: 10.1093/jn/131.1.66. [DOI] [PubMed] [Google Scholar]
- 79.Rondini L., Peyrat-Maillard M.N., Marsset-Baglieri A., Berset C. Sulfated ferulic acid is the main in vivo metabolite found after short-term ingestion of free ferulic acid in rats. J. Agric. Food Chem. 2002;50:3037–3041. doi: 10.1021/jf011295i. [DOI] [PubMed] [Google Scholar]
- 80.Adam A., Crespy V., Levrat-Verny M.A., Leenhardt F., Leuillet M., Demign′e C., R′em′esy C. The bioavailability of ferulic acid is governed primarily by the food matrix rather than its metabolism in intestine and liver in rats. The Journal of Nutrition. 2002;132:1962–1968. doi: 10.1093/jn/132.7.1962. [DOI] [PubMed] [Google Scholar]
- 81.Hollman P.C.H., van Trijp J.M.P., Buysman M.N.C.P., v.d, Gaag M.S., Mengelers M.J.B., de Vries J.H.M., Katan M.B. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1997;418:152–156. doi: 10.1016/S0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- 82.Gee J.M., DuPont S., Day A.J., Plumb G.W., Williamson G., Johnson I.T. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. The Journal of Nutrition. 2000;130:2765–2771. doi: 10.1093/jn/130.11.2765. [DOI] [PubMed] [Google Scholar]
- 83.Hollman P.C.H., Arts I., Flavonols C.W. flavones, and flavanols Nature, occurrence, and dietary burden. J. Sci. Food Agric. 2000;80:1081–1093. [Google Scholar]
- 84.Hollman P.C.H. Evidence for health benefits of plant phenols: local or systemic effects. J. Sci. Food Agric. 2001;81:842–852. doi: 10.1002/(SICI)1097-0010. 20000515)80:7. [DOI] [Google Scholar]
- 85.Murota K., Shimizu S., Miyamoto S., Izumi T., Obata A., Kikuchi M., Terao J. Unique uptake and transport of isoflavone aglycones by human intestinal Caco-2 cells: comparison of isoflavones and flavonoids. The Journal of Nutrition. 2002;132:1956–1961. doi: 10.1093/jn/132.7.1956. [DOI] [PubMed] [Google Scholar]
- 86.Manach C., Texier O., Morand C., Crespy V., Regerat F., D′emign′e C., R′em′esy C Comparison of the bioavailability of quercetin and catechin in rats. Free Radic. Biol. Med. 1999;27:1259–1266. doi: 10.1016/S0891-5849(99)00159-8. [DOI] [PubMed] [Google Scholar]
- 87.Suárez M., Valls R.M., Romero M.P., Macià A., Fernández S., Giralt M., Solà R., Motilva M.J. Bioavailability of phenols from a phenol-enriched olive oil. Br. J. Nutr. 2011;106:1691–1701. doi: 10.1017/S0007114511002200. [DOI] [PubMed] [Google Scholar]
- 88.Minatel I.O., Borges C.V., Ferreira M.I., Gomez H.A.G., Chen C.Y.O., Lima G.P.P. IntechOpen Book Series; 2016. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. [DOI] [Google Scholar]
- 89.Belford A.L., Lindsay R.C., Ridley S.C. Contributions of selected flavor compounds to the sensory properties of maple syrup. J. Sensory Stud. 1991;6:101–118. doi: 10.1111/j.1745-459X.1991.tb00506.x. [DOI] [Google Scholar]
- 90.Sair L., Sne J.F. Fractionation of the chloroform extract of maple syrup. Can. J. Res. 1939;17:281–289. doi: 10.1139/cjr39b-039. [DOI] [Google Scholar]
- 91.Underwood J.C., Willits C.O., Lento H.G., Maple Sirup X.V.I. Isolation and identification of compounds contributing to the flavor of maple sirup. J. Food Sci. 1961;26:288–290. doi: 10.1111/j.1365-2621.1961.tb01656.x. [DOI] [Google Scholar]
- 92.Underwood J.C., Filipic V.J. Gas chromatographic identification of components in maple sirup flavor extract. J. Assoc. Off. Agric. Chem. 1963;46:334–337. doi: 10.1093/jaoac/46.2.334. [DOI] [Google Scholar]
- 93.Filipic V.J., Underwood J.C., Willits C.O. The identification of methylcyclopentenolone and other compounds in maple sirup flavor extract. J. Food Sci. 1965;30:1008–1015. doi: 10.1111/j.1365-2621.1965.tb01878.x. [DOI] [Google Scholar]
- 94.Alli I., Bourque J., Metusin R., Liang R., Yaylayan V. Identification of pyrazines in maple syrup. J. Agric. Food Chem. 1990;38:1242–1244. doi: 10.1021/jf00095a019. [DOI] [Google Scholar]
- 95.Sabik H., Fortin J., Martin N. Identification of pyrazine derivatives in atypical maple syrup using headspace solid-phase microextraction with gaschromatography–mass spectrometry. Food Chem. 2012;133:1006–1010. doi: 10.1016/j.foodchem.2011.07.132. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.