ABSTRACT
Systemic hypertension is the most common medical comorbidity affecting the adult population globally, with multiple associated outcomes including cerebrovascular diseases, cardiovascular diseases, vascular calcification, chronic kidney disease, metabolic syndrome and mortality. Despite advancements in the therapeutic field approximately one in every five adult patients with hypertension is classified as having treatment-resistant hypertension, indicating the need for studies to provide better understanding of the underlying pathophysiology and the need for more therapeutic targets. Recent pre-clinical studies have demonstrated the role of the innate and adaptive immune system including various cell types and cytokines in the pathophysiology of hypertension. Moreover, pre-clinical studies have indicated the potential beneficial effects of immunosuppressant medications in the control of hypertension. Nevertheless, it is unclear whether such pathophysiological mechanisms and therapeutic alternatives are applicable to human subjects, while this area of research is undoubtedly a rapidly growing field.
Keywords: autoimmune disease, hypertension, immune system, immunosuppression, therapeutics
INTRODUCTION
Hypertension affects more than one-quarterof the global adult population while approximately 3.5 billion adults have non-optimal systolic blood pressure measurements [1, 2]. Almost 54% of strokes and 47% of ischemic heart diseases are direct consequences of hypertension according to the estimates of the World Health Organization. In addition, hypertension has been linked to 9.4 million annual deaths and 212 million loss of health life-years annually, accounting for the 8.5% of the total loss [3, 4]. Despite its considerable disease burden the diagnosis of hypertension is not straightforward, especially with masked hypertension and white-coat hypertension. Ambulatory blood pressure monitoring or home blood pressure monitoring gain crucial importance following the demonstration of statistically significant association between the lack of nighttime blood pressure dipping and/or nighttime hypertension and cardiovascular disease risk by the Japan Ambulatory Blood Pressure Monitoring Prospective clinical trial conducted in 6359 asymptomatic participants with at least one cardiovascular disease risk factor over a follow-up period of 4.5 years [5].
Primary (essential) hypertension, responsible for nearly 95% of the hypertension burden in adults, has a complicated underlying pathophysiology, with many mechanisms still not fully known [6]. Its pathogenesis is complex and related to aberrancies in metabolic parameters, genes and possibly immunity [6, 7]. The two major over-activated and extensively studied physiological systems in the pathophysiology of hypertension are the sympathetic nervous system and the renin–angiotensin–aldosterone system (RAAS), both of which are therapeutic targets via beta-adrenergic blockers, alpha-adrenergic blockers, renin inhibitors, angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), aldosterone synthase inhibitors and mineralocorticoid receptor antagonists [8]. In addition to these established pathophysiological mechanisms, recent studies have shown a potential relationship between immune system dysfunction and high blood pressure, therefore suggesting an important role of aberrant immune function and autoimmunity in the etiopathogenesis of hypertension [9]. In this review, our aim is to describe the potential pathophysiological and therapeutic role of immune mechanisms, a novel aspect of hypertension research in arterial hypertension.
GENERAL BACKGROUND
The innate immune system includes various cell types including monocytes/macrophages, neutrophils, dendritic cells, natural killer (NK) cells, basophils and eosinophils, anatomical barriers such as skin, gastrointestinal or genitourinary mucosa, and certain chemicals such as cytokines, complement proteins and chemokines [10]. On the other hand, the adaptive immune system includes T-lymphocytes, B-lymphocytes, plasma cells and various subtypes of cytokines and complement proteins. Antigen-presenting cells including monocytes, macrophages and dendritic cells play crucial role in the link between innate and adaptive immune responses via the recognition of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) by their pathogen recognition receptors (PRRs) such as Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) and retinoic acid–inducible gene I–like helicases [11]. NK cells are crucial component of the innate immune response via the recognition and differentiation of self-antigen versus non-self-antigen, which is important in the anti-tumor or anti-viral response which is mediated via NK cell–mediated cytotoxicity [12]. Use of anti-NK antibodies inhibiting the functions of NK cells has shown to be protective against vascular dysfunction on pre-clinical studies [13].
Although the primary mechanism leading to the activation of innate and/or adaptive immune response in hypertension remains unclear, multiple hypothetical insults including reactive oxygen species (ROS), increased sympathetic output, over-activity of spleen and ROS, and changes in the gut microbiota have been proposed [14–16]. Dendritic cells have been proposed as the source of DAMPs in hypertension following the studies demonstrating the formation of ROS such as highly reactive gamma-ketoaldehydes via NADPH oxidase [17]. The recognition of DAMPs or PAMPs via various TLRs lead to the activation of different downstream signaling pathways while major downstream pathways include interferon regulatory factor (IRF)-3, nuclear factor kappa light chain enhancer of activated B cells (NF-κB) or mitogen-activated protein kinase (MAPK) [18].
A study in which peripheral monocytes have been obtained from 43 non-diabetic hypertensive patients and 16 non-diabetic normotensive patients has demonstrated that monocytes from hypertensive patients have higher levels of TLR-4 mRNA while intensive anti-hypertensive treatment results in statistically significant decline in the levels of TLR-4 and TLR-2 messenger RNA (mRNA) [19]. Moreover, mouse models genetically modified to lack the TLR-4 gene have been shown to demonstrate attenuated blood pressure elevation in response to either angiotensin II or NG-nitro-l-arginine methyl ester (L-NAME; an inhibitor of nitric oxide), infusions [20, 21]. Additionally, antibody-mediated blockage of TLR-4 leads to attenuated blood pressure elevation in DOCA-salt salt (deoxycorticosterone acetate + 0.9% NaCl) rats and spontaneously hypertensive rats but not in angiotensin II–infused rats unless TLR-4 is antagonized at periventricular nuclei [22–24]. Furthermore, recent studies have demonstrated that inhibition of TLR-9 has been linked to decline in angiotensin II–induced hypertension [25, 26]. Other types of TLRs, besides TLR-2/4/9, have rarely been investigated in models of hypertension except for pre-eclampsia and eclampsia [27]. The most commonly investigated NLR in the context of hypertension is NLRP3, whose polymorphism has been linked to both systolic and diastolic hypertension [28]. NLRP3 knockout mouse models have not developed hypertension in response to two kidneys–one clip (2K1C) or DOCA-salt treatment environments [29]. Despite rarely being studied, other subtypes of NLRs have been associated with blood pressure alterations such as NOD1-associated hypotension, and decline in vasoconstriction in response to contractile stimuli and NOD2-associated hypertension [30, 31]. The major importance of such studies is the identification of multiple TLRs and NLRs as potential therapeutic targets for clinical arterial hypertension. Furthermore, the gut microbiota is known to regulate the host's immunity, cell proliferation and metabolism, and to play a role in different cardiovascular and kidney diseases [15, 16, 32, 33]. The gut microbiota was shown to modulate blood pressure via short-chain fatty acids [34]. A study conducted in total of 196 participants, including 41 healthy controls, 56 individuals with pre-hypertension and 99 individuals with primary hypertension, showed that fecal transplantation from hypertensive patients to germ-free mice increased blood pressure [35]. Thus, gut microbiota dysbiosis can increase blood pressure, and future studies are needed to better understand this association.
INNATE IMMUNE SYSTEM IN HYPERTENSION
The innate immune system is a diverse network of mucosal barriers, various cell types, and chemicals including chemokines, cytokines and complement proteins. The role of the innate immune system in the pathophysiology of hypertension is a relatively new area of research, with the identification of promising therapeutic targets. Neutrophils are the most abundant white blood cell type in the circulation while macrophages are the most common type of immune cell in various tissue. A study conducted in a mouse model demonstrated that monocytes and neutrophils are involved in the pathogenesis of angiotensin II–induced hypertension and endothelial dysfunction [36]. Selective ablation of lysozyme M–positive myelomonocytic cells via low-dose diphtheria toxin resulting in low circulating levels and low tissue infiltration attenuated angiotensin II–induced blood pressure elevation, formation of ROS and vascular smooth muscle cell dysfunction [36]. Another mouse model with functionally deficient osteopetrotic mice illustrates lower blood pressure measurements in response to angiotensin II infusion [37]. Moreover, hyperosmotic environment in response to either high salt intake or hyperglycemia leads to increased infiltration of renal interstitium via macrophages, conversion to pro-inflammatory phenotype and tissue damage due to the secretion of pro-inflammatory cytokines [38, 39]. Elevated levels of circulating monocytes have been demonstrated on multiple pre-clinical studies on angiotensin II or aldosterone-induced hypertension models [40–44]. A similar pattern has been observed in spontaneously hypertensive rats or models of salt-sensitive or deoxycorticosterone acetate–induced hypertension [45–50].
Only a few studies have been conducted in human subjects. According to a study in which peripheral monocytes were isolated from 22 hypertensive and 24 normotensive participants, monocytes from hypertensive individuals were shown to secrete higher levels of tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) in response to either stimulation by angiotensin II which may be attenuated via incubation with losartan and lipopolysaccharides [51]. Another study conducted in 76 hypertensive and 146 normotensive male participants illustrated similar outcomes, while additionally monocytes derived from hypertensive individuals were found to be less susceptible to glucocorticoids [52]. A prospective clinical trial conducted in 42 patients with treatment-resistant hypertension demonstrated that renal denervation therapy leads to statistically significant decline in blood pressure during a 6-month follow-up period, along with a decline in monocyte activation and levels of pro-inflammatory cytokines such as TNF-α, IL-12, IL-1β and plasma monocyte chemoattractant protein-1 (MCP-1) levels [53].
An observational study conducted in over 9383 initially normotensive participants with normal white blood cell count over a 40-year follow-up period demonstrated that elevated neutrophil count is associated with hypertension risk after adjustment for multiple confounding factors [54]. Even though the elevated number of neutrophils and functional indicators such as myeloperoxidase activity have been recorded in animal models of hypertension, the causal link is missing since adoptive transfer of neutrophils fails to improve blood pressure measurements [36, 55, 56]. Moreover, depletion of CD11c-positive dendritic cells via diphtheria toxin results in the prevention of hypertension in response to either high-salt diet or angiotensin II infusion [57]. Another key aspect of dendritic cells in the pathogenesis of hypertension is the salt-sensing serum/glucocorticoid kinase1 (SGK1), while mouse models lacking SGK1 illustrate lower levels of dendritic cell activation or blood pressure elevation in response to a high-salt diet [55, 58]. The role of NK cells in hypertension is not well understood, with only a few pre-clinical studies investigating the association [59, 60].
Nevertheless, the data regarding the role of eosinophils and basophils in the pathogenesis of hypertension is limited. Even though observational studies have indicated a potential link between elevated peripheral eosinophil count and coronary artery disease or pulmonary artery hypertension, the exact relationship is yet to be demonstrated [61, 62]. Mouse models lacking eosinophils demonstrate higher mean arterial blood pressure and glucose intolerance, while such modifications are reversible with the reconstitution of eosinophils indicating the crucial role of eosinophils in the regulation of normal blood pressure [63, 64]. To the best of our knowledge, no pre-clinical or clinical study has yet evaluated the role of basophils in the pathogenesis of hypertension.
ADAPTIVE IMMUNE SYSTEM IN HYPERTENSION
The adaptive immune system is a complex network of multiple cell types and chemicals including cytokines and complement proteins with varying degree of relationship with the innate immune system. The pathophysiological role of the adaptive immune system in hypertension is a developing area of research. The role of T-lymphocytes in the pathogenesis of hypertension has been illustrated by mouse models of double-hit rag1 mutation leading to lack of both T- and B-lymphocytes in which infiltration of perivascular tissue by T-cells, expression of intercellular adhesion molecule (ICAM)-1, secretion of pro-inflammatory cytokines such as TNF-α and endothelial cell–dependent vasodilation are all suppressed in response to angiotensin II [65]. Moreover, adoptive transfer of T-cells leads to restoration of all of the mentioned functions while adoptive transfer of B-cells does not lead to any improvement [65]. Moreover, mutation of the exon 1 of rag1 gene in Dahl salt-sensitive rats led to decline in blood pressure elevations in response to a high-salt diet while pathological specimens demonstrate decline in renal infiltration by T-lymphocytes [66]. Another mouse model with scid mutation illustrates blunted hypertensive response to angiotensin II stimuli along with reduced cardiac and/or renal remodeling in response to such stimuli [67]. Nevertheless, multiple types of T-lymphocytes with distinct physiological functions are present in both animals and humans.
Even though one might expect the CD4+ T-helper cell type to play a central role in the pathophysiology of T-cell-mediated hypertension, data from animal models suggest otherwise. Adoptive transfer of CD4+ T-cells to the rag1 double-hit mouse model did not result in the restoration of angiotensin II–induced hypertension, while adoptive transfer of CD8+ T-cells resulted in full restoration [65]. A similar finding has also been established in another mouse model study [68]. Another crucial T-cell type in the pathogenesis of arterial hypertension is the T-regulatory cells. Adoptive transfer of T-cells from the Scurfy mouse model lacking T-regulatory cells due to mutation at the Foxp3 gene to the double-hit rag1 mouse model results in exaggerated hypertensive response to stimuli of angiotensin II, implicating the potential protective role of T-regulatory cells against hypertension [69]. Administration of T-regulatory cells as a single dose or once weekly dose leads to improvement in cardiac hypertrophy, endothelial vasodilatation, formation of ROS and pro-inflammatory signals without any improvement in blood pressure measurements while administration of T-regulatory cells at sustained high doses leads to improvements at blood pressure [70–72]. Furthermore, oxidative stress can modify self-antigens and cause them to behave as novel antigens referred to as neo-antigens. Neo-antigens activate T-cells and sensitize them against specific antigens which results in infiltration and inflammation in the vasculature and kidney. Furthermore, elevated blood pressure can possibly generate neo-antigens by barotrauma [73]. The role of B-lymphocytes has largely been uninvestigated in hypertension research while early studies indicated a potential role in the pathogenesis and identified them as a potential therapeutic target via administration of anti-CD20 antibodies [74]. However, there is clear need for future studies investigating the role of B-lymphocytes in the pathogenesis of hypertension.
CYTOKINES IN HYPERTENSION
A number of different cytokines are reported to be a factor in the pathophysiology of hypertension.
Interleukin-1
IL-1β levels have been suggested to be elevated in patients with essential hypertension [75, 76]. IL-1 has been shown to contribute to the inflammatory and remodeling processes in the vasculature as well as to affect the functionality and phenotype of the vascular smooth muscle cell in hypertension [77]. The two IL-1 isoforms were reported to be highly expressed in the renal tissues in cases of hypertension caused by the increased activity of RAAS in murine models [43, 78]. IL-1 has also been reported to promote diuresis and natriuresis through prostaglandin generation in the kidney tissues [79–84]. The prevention of the maturation of myeloid cells in the kidney to Ly6C+ Ly6G− macrophages responsible for producing nitric oxide, a suppressor of Na-K-Cl cotransporter 2 (NKCC2), occurred following the activation of IL-1 receptor [78]. A deficient or blocked IL-1 receptor was shown to restrict the rise in blood pressure by lessening the amount of reabsorbed sodium through the NKCC2 co-transporter in mice [78]. Additional studies in mouse models reported that inhibiting IL-1 lowered angiotensin II–related hypertension as well as kidney damage, showing a potential role of this blockade in the treatment of hypertension [85].
The role of IL-1 has also been investigated in humans. In a meta-analysis of cohort studies assessing the relationship between inflammatory markers and the development of hypertension, elevated C-reactive protein (CRP), interleukin-6 (IL-6) and high-sensitive CRP (hs-CRP) were significantly related with a risk of the development of hypertension [86]. However, IL-1β was not associated with a significant risk [86]. Furthermore, two clinical trials have evaluated the effects of IL-1 blockade on blood pressure. In a secondary analysis of CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcomes Study), IL-1β, inhibited by canakinumab, decreased cardiac and vascular events; however, this was not linked to alterations in incident hypertension and blood pressure [87, 88]. In a separate trial, anakinra, an IL-1 receptor antagonist, resulted in the fall of blood pressure and peripheral vascular resistance in subjects with obesity and led to a rise in angiotensin-(1–7) levels, a molecule responsible for vasodilation [89].
Interleukin-6
IL-6 has also been thought to contribute to the pathophysiology of hypertension, with a number of studies reporting a correlation with IL-6 levels in the plasma and elevated blood pressure [90–93]. One study assessed the role of IL-6 when exposed prenatally on the development of hypertension; the authors suggested that IL-6 introduction before birth could result in hypertension via alterations in RAAS as well as fluid and electrolyte balance, particularly in female rats [94]. Furthermore, the vasculature is responsible for the release of IL-6 following angiotensin II exposure [95–99]. The prevention and attenuation of angiotensin II hypertension, to which IL-6 is thought be a contributor, has been shown to be possible by blocking IL-6 in wild-type mice or by IL-6 knockout mouse models [90, 95, 100, 101]. Similarly, IL-6 when inhibited by IL-6 neutralizing antibody was reported to mitigate hypertension and related injury to the kidneys in Dahl salt-sensitive rats [46].
The involvement of IL-6 in high blood pressure has also been studied in humans. One study suggested that IL-6 and TNF-α were potentially risk factors for an elevated blood pressure in healthy individuals [102]. Furthermore, a hypomethylated IL-6 gene was associated with an elevated risk of hypertension and the variations in methylated promoter regions were related to alcohol consumption, gender and being a smoker [103]. As previously mentioned, a meta-analysis showed a significant relationship between high IL-6 levels and hypertension development [86]. The use of anti-hypertensive drugs has also affected IL-6 levels. One study reported a fall in IL-6 and TNF-α levels after anti-hypertensive medications in elderly females [104]. In a separate study, spironolactone led to a decrease in IL-6 and interferon-γ (IFN-γ) levels in subjects with diabetes and treatment-resistant hypertension [105]. These findings suggest a link between IL-6 and hypertension; future studies are needed to better understand this association.
Interleukin-8
A few studies have explored the role of interleukin-8 (IL-8) in hypertension. One study showed a higher calcium-dependent potassium efflux in hypertensive individuals, and IL-8 as well as ICAM-1 heightened this efflux in erythrocytes of individuals with hypertension in a significant manner [106]. In a different study, elevated degrees of IL-8 and MCP-1 were shown in the vitreous of subjects with retinopathy due to diabetes and hypertension [107]. Further studies are required to investigate the association between elevated blood pressure findings and IL-8.
Transforming growth factor-β
Transforming growth factor-β (TGF-β) plays an important role in the homeostasis of the body, cellular growth, tolerance against self-tissues and differentiation [108–110]. An overexpression of the mRNA and protein of TGF-β1, the principle TGF-β isoform responsible for immune function and having mainly inhibitory roles, is observed in individuals with primary hypertension [110–115]. TGF-β1 generation may be explained by elevated blood pressure values in itself but also by a higher shear stress of fluids as well as by increased angiotensin II levels [115]. Polymorphisms on Arg25 in the gene of this cytokine have shown an association with hypertension [115].
TGF-β has also been suggested to contribute to the development of fibrotic interstitium and tubules as well as glomerulosclerosis in the kidneys with angiotensin II inducing TGF-β production in renal tissues [116]. This interaction has been found to be important in several conditions such as cyclosporin nephrotoxicity, diabetic kidney disease, obstruction of a single ureter and different hypertension models, contributing to these diseases becoming progressive [116]. Furthermore, a higher level of TGF-β1 is seen in the bloodstream of individuals who have concurrently a hypertrophied left ventricle, hypertension and microalbuminuria [115]. A decrease in TGF-β1 levels was observed in these subjects after they received ARB and ACEi [115]. In a separate study, TGF-β1 was increased in the serum and was related to albumin in the urine in patients with primary hypertension [117]. The administration of valsartan and/or benazepril resulted in a mitigation of TGF-β1 in the bloodstream and microalbuminuria, with the best effect achieved after the combination of both drugs [117]. Moreover, a different study showed that in diabetic and hypertensive individuals with an increased albumin excretion, losartan decreased TGF-β release in the urine [118]. In a double-blind, randomized clinical trial, losartan was shown to lower blood pressure and proteinuria together with a fall in TGF-β release in the urine among non-diabetic kidney disease patients exhibiting proteinuria [119].
These studies show that TGF-β is an important cytokine in hypertensive patients and could possibly function as a surrogate of kidney injury in hypertensive patients. Moreover, ACEi and ARB can benefit in decreasing TGF-β levels in the serum and urine. Further studies are needed to understand the role and utility of targeting TGF-β in hypertensive patients as well as investigate the association between the duration of TGF-β elevation and the development of hypertension.
Tumor necrosis factor-α
A variety of different cells are responsible for the generation of TNF-α such as dendritic cells, macrophages, lymphocytes and neutrophils [79]. TNF-α has been suggested as a mediator of end-organ damage according to hypertensive disease models [79]. Interestingly, TNF-α can lead to both rises and falls in blood pressure, with considerably TNF-α resulting in a reduction while modestly elevated levels increase blood pressure and NaCl preservation in the body [120]. The injuring results of TNF-α could potentially be caused by lymphocyte-based effects during angiotensin II–induced hypertension, meanwhile a lack of renal damage, even with increased levels of TNF-α, can potentially be explained by a rise in sodium release in the setting of RAAS activity [79].
Antagonizing TNF-α was associated with a mitigation of hypertension in the majority of disease models; however, conflicting results have also been reported. In one study, etanercept, an inhibitory agent of TNF-α, was shown to mitigate hypertension as well as the generation of the epithelial sodium channel a–subunit in a chronic kidney disease model in mice lacking angiotensin II type 1 receptor–associated protein (ATRAP), a molecule involved in internalizing the angiotensin II receptor type 1 and controlling the signaling surrounding this receptor [121]. Another study reported that the prevention of angiotensin II–related hypertension was possible following the application of etanercept [65]. Meanwhile, a separate study reported no effect of etanercept on hypertension but a decrease in kidney injury in DOCA-salt hypertensive rats [122].
The role of TNF-α on blood pressure has also been studied in humans. An elevated blood pressure was suggested to increase oxidative stress as well as to lead to a significant rise in TNF-α and malondialdehyde in a study on 60 individuals [123]. Similarly, TNF levels in the bloodstream and urine were reported to be significantly elevated in patients with hypertension compared with healthy controls in a different study [124]. In addition, TNF-α and IL-6 were considered to be potential risk factors for an elevated blood pressure in individuals who were apparently healthy, and increased levels of these two cytokines were suggested to be predictors of a dysfunctional endothelium in the coronary arteries in subjects with hypertension [102, 125]. Conflicting results regarding the impact of the inhibition of TNF-α on blood pressure was also seen in research conducted in human subjects. One study assessing the effect of infliximab, a drug inhibiting TNF-α, on blood pressure in 16 rheumatoid arthritis patients showed that infliximab decreased the 24-h systolic blood pressure, morning blood pressure and daytime blood pressure in a significant manner [126]. However, in a meta-analysis evaluating the impact of administering TNF-α inhibitors on hypertension in patients with rheumatoid arthritis consisting of 6321 rheumatoid arthritis patients derived from 11 trials, the results were significant regarding the effect of TNF-α inhibitors elevating the risk of hypertension development [127]. As seen from these studies, future research is warranted to better understand the role of TNF-α and its inhibition on blood pressure values. More detailed reviews regarding the role of TNF-α have recently been conducted by Crorkin et al. and Lamb et al. [79, 128].
THE EFFECT OF SODIUM ON THE IMMUNE SYSTEM
A high dietary sodium intake has been linked to hypertension in numerous studies [129]. Although several mechanisms have been proposed, the pathophysiology of sodium-induced hypertension is not fully understood. Growing evidence suggests that high sodium not only causes hemodynamic alterations but also modulates the immune system [130]. The salt-driven proinflammatory response causes immune cell activation, vascular endothelial dysfunction and cytokine secretion, typical of hypertension [131].
Several animal studies have demonstrated overexpression of leukocyte adhesion molecules, including ICAM-1, MCP-1, vascular cell adhesion protein 1 (VCAM-1) and macrophage adhesion ligand-1 [132, 133]. Overexpression of these leukocyte adhesion molecules is associated with local inflammation, which drives endothelial dysfunction [134]. Moreover, leukocyte adhesion molecule expression increased before hypertension developed [132]. Increased leukocyte infiltration may not be solely due to increased expression of these leukocyte adhesion markers. High perfusion pressure itself also promotes T-cell infiltration in the kidney [135].
A high-salt diet increases IL-6, one of the most prominent cytokines related to elevated blood pressure. A high-salt diet increased IL-6 in the kidney of Dahl salt-sensitive rats [136]. In angiotensin II–induced hypertension and deoxycorticosterone acetate-salt hypertension models, high sodium intake increases IL-17 expression via an SGK1-dependent pathway [137]. Increased IL-17 expression enhances sodium reabsorption by renal sodium transporters [138]. Increased sodium concentration elevates IL-2 expression, thus enhancing T-cell proliferation [139].
High sodium concentration also has direct effects on immune cells, promoting inflammation [130]. Increased sodium concentration impairs T-regulatory cell function with increased IFN-γ secretion [140]. Sodium also modulates macrophages. High sodium levels were found to stimulate proinflammatory M1 macrophages while suppressing anti-inflammatory M2 macrophages in a study with lipopolysaccharide-induced lung injury [141]. Increased intracellular sodium concentration in dendritic cells was found to secrete more IL-1β and stimulate T-cells to secrete IFN-γ and IL-17A [142]. Thus, these suggest a role of sodium on the immune system, and further studies are needed to understand how dietary salt intake and immune response link to hypertension and cardiovascular illnesses.
RENAL INFLAMMATION IN HYPERTENSION
Pressure natriuresis, a concept referring to increased renal salt excretion in response to elevation in blood pressure leading to the homeostasis of blood pressure, is commonly impaired in hypertensive subjects [143]. Moreover, kidney tissue is involved in the regulation of blood pressure homeostasis via multiple mechanisms including the secretion of renin and control of RAAS, alterations at renal ultrafiltration, and secretion of pro-inflammatory and anti-inflammatory cytokines. In spontaneously hypertensive rats models, renal infiltration via immune system cells occurs prior to the development of clinical hypertension while impairment of such homeostasis mechanisms plays crucial role [144]. Moreover, the degree of infiltration by immune cells is highly correlated with the degree of systolic blood pressure measurements, indicating the clinical importance of such infiltration and potentially highlighting the importance of reversal of such infiltration in the treatment of hypertension [145]. Furthermore, the central role of IL-17 secreted from T-helper cells has been proposed following the preclinical study in double-hit IL-17 mouse models lacking vascular dysfunction or hypertension following angiotensin II infusion [138]. IL-17 leads to upregulation of glucocorticoid regulated kinase-1 at renal proximal tubules which causes upregulation of renal sodium–hydrogen exchanger-3 [138].
Additionally, renal inflammatory response has led to disruption of pressure natriuresis in response to deterioration of dopamine D1 receptor functions, loss of peritubular capillaries and increased concentration of intrarenal angiotensin II [146–149]. Furthermore, impaired production of renal angiotensin-converting enzyme leading to variable intrarenal angiotensin II concentration which is required for the control of glomerular filtration rate, and tubular sodium transport is another mechanism linking renal inflammation to hypertension via the impairment of the pressure-natriuresis model [150, 151]. Studies on animal models clearly demonstrate the central role of renal inflammation in the pathogenesis of hypertension while it is unclear whether renal inflammation is the triggering event leading to clinical hypertension or just a contributing factor to the development of hypertension.
VASCULAR INFLAMMATION IN HYPERTENSION
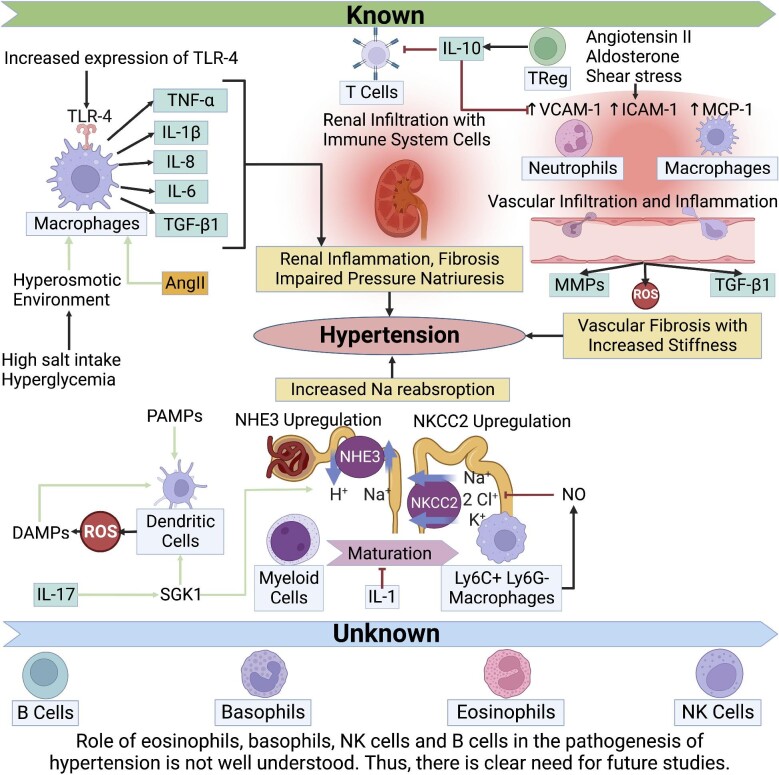
Multiple mediators including angiotensin II, aldosterone, shear stress and various cytokines contribute to the development of vascular inflammation via secretion of VCAM-1, ICAM-1 and MCP-1, while the end result includes over-production of ROS via myeloperoxidase enzyme of infiltrating neutrophils and/or NADH/NADPH oxidase enzyme of infiltrating monocytes/macrophages [152]. Such a response leads to upregulation of matrix metalloproteinases, secretion of pro-inflammatory and pro-fibrotic cytokines such as TGF-β, and further infiltration of vascular wall via immune system cells, thereby creating a vicious cycle [152]. The importance of monocyte/macrophages is demonstrated via a preclinical study conducted in a transgenic mouse model with monocyte/macrophage depletion which showed a decline in the formation of superoxide radical, attenuation of angiotensin II–induced hypertension and vascular dysfunction [36]. Such a condition is reversible via adoptive transfer of monocyte/macrophages, while it is not by the adoptive transfer of NADPH oxidase mutant monocytes [36]. Another major pathophysiological role of macrophages in vascular inflammation includes secretion of pro-fibrotic cytokines such as TGF-β and extracellular matrix remodeling via matrix metalloproteinases leading to vascular stiffness [74]. On the other hand, T-regulatory cells appear to have a protective role against vascular inflammation, as evident from double-hit IL-10 mouse models demonstrating higher mean arterial blood pressure measurement that is reversible via adoptive transfer of T-regulatory cells [153]. T-regulatory cells appear to inhibit monocyte/macrophage infiltration of vascular wall, as well as the inhibition of reactive oxygen species formation in response to angiotensin II or aldosterone stimuli [153]. The role of the immune system in the pathogenesis of hypertension is shown in Fig. 1.
Figure 1:
The role of the immune system in the pathogenesis of hypertension. AngII: Angiotensin 2; MMPs: matrix metalloproteinases; NHE3: sodium/hydrogen exchanger isoform 3; NO: nitric oxide; TReg: T regulatory cells.
THE PREVALENCE AND RISK FACTORS OF HYPERTENSION IN AUTOIMMUNE DISEASES
Cardiovascular diseases have a high prevalence and are the main causes of death in individuals with autoimmune diseases, while hypertension is an important risk factor for the development of cardiac and vascular diseases and is also highly common in this patient population [154–157]. One study consisting of 28 208 rheumatoid arthritis patients reported hypertension to have an elevated prevalence of 31% in the patient group in comparison with the public at 23% [157, 158]. In a separate observational cross-sectional study with 622 patients among which 39.2% had rheumatoid arthritis, 14.5% had osteoarthritis, 10.8% had seronegative spondyloarthropathies, 10.5% had systemic lupus erythematosus and 8.1% had psoriatic arthritis, 54.5% and 72.4% of the study group had hypertension according to the 2018 ESC/ESH and 2017 ACC/AHA guidelines, respectively [159]. Hypertension prevalence was 84.8% and 69.6% in patients with osteoarthritis, 80% and 57.8% in the psoriatic arthritis group, 75.2% and 60.9% in individuals with rheumatoid arthritis, and 63.5% and 35.7% in subjects with lupus according to the ACC/AHA and ESC/ESH guidelines, respectively [159]. Furthermore, other studies have reported a high prevalence of hypertension in individuals with scleroderma particularly those having kidney disease, and in subjects with lupus [157, 160–163].
A recent systematic review evaluated the risk factors of hypertension in individuals with rheumatoid arthritis from 14 studies and showed that regular exercise and methotrexate were protective against, whereas leflunomide, cyclooxygenase-2 inhibitors, prednisone were associated with heightened risk of, high blood pressure [164]. Disease-modifying anti-rheumatic drug use showed conflicting results [164]. In case of systemic lupus erythematosus, body mass index and smoking have been related to an elevated risk of hypertension development and worse outcomes; in addition genetic, environmental, metabolic and sex-specific factors have all contributed to a risk of high blood pressure in patients with lupus [156, 157, 165–167].
These findings suggest a high prevalence of hypertension in autoimmune conditions as well as different factors elevating the risk of having a high blood pressure. Clinicians should be aware of these conditions and manage patients accordingly.
ROLE OF IMMUNOSUPPRESSIVE AGENTS AS ANTI-HYPERTENSIVE MEDICATION
The role of various immunosuppressive agents in the treatment of hypertension has been investigated across multiple animal models (Table 1). According to a study conducted in spontaneously hypertensive rats chronic immunosuppression with cyclophosphamide results in statistically significant decline in systolic blood pressure to a mean of 158 mmHg compared with a mean of 174 mmHg in the control group, with effects recognizable after 2 weeks of therapy [168]. Another study investigating the effects of cyclophosphamide therapy on blood pressure conducted in genetically hypertensive rats of Lyon strain illustrated a statistically significant decline in blood pressure to a mean of 135 mmHg compared with a mean of 175 mmHg in the control group, while no significant difference was recorded in genetically normotensive subjects [169]. In a separate study on spontaneously hypertensive rats, treatment with mycophenolate mofetil resulted in a decline in blood pressure measurements correlated with the decline in renal infiltration by macrophages and lymphocytes, and renal and urinary markers of oxidative stress such as malondialdehyde in a statistically significant manner [145]. Comparable effects of mycophenolate mofetil treatment on blood pressure have been recorded in a study conducted in Dahl salt-sensitive rats [170]. Similarly, decline in renal tissue pro-inflammatory cells and markers of oxidative stress have been recorded via administration of pyrrolidine dithiocarbamate, which is an inhibitor of the pro-inflammatory NF‐κB signaling pathway, leading to a decline in systolic blood pressure to a mean of 127 mmHg compared with a mean of 198 mmHg [171]. Additionally, a study conducted in fawn-hooded hypertensive rats illustrates that treatment with pyrrolidine dithiocarbamate leads to a statistically significant decline in systolic blood pressure to a mean of 128 mmHg compared with a mean of 159 mmHg in the control group [172]. Administration of tacrolimus, a calcineurin inhibitor, to Dahl salt-sensitive rats resulted in a decline in systolic blood pressure to a mean of 150 mmHg compared with a mean of 170 mmHg along with a decline in urinary albumin and thiobarbituric acid–reactive substances excretion and renal infiltration via pro-inflammatory cells and NADPH oxidase [173]. Another calcineurin inhibitor referred to as cyclosporine A results in comparable outcomes in terms of blood pressure control [174]. Moreover, anti-oxidant-enriched diet and melatonin therapy result in a decline in blood pressure measurements in spontaneously hypertensive rat subjects via suppression of oxidative stress and renal tissue infiltration by pro-inflammatory cells [175, 176].
Table 1:
The role of various immunosuppressive agents in the treatment of hypertension has been investigated across multiple animal models.
| Treatment | Experimental model | Effect on blood pressure |
|---|---|---|
| Cyclophosphamide | Spontaneously hypertensive rats [168] | Statistically significant decline in SBP to a mean of 158 mmHg compared with a mean of 174 mmHg in the control group, while effects are recognizable after two weeks of therapy. |
| Cyclophosphamide | Genetically hypertensive rats of Lyon strain [169] | Statistically significant decline in blood pressure to a mean of 135 mmHg compared with a mean of 175 mmHg in the control group, while no significant difference has been recorded in genetically normotensive subjects. |
| Cyclosporine A | dTGR harboring human renin and angiotensinogen genes [174] | Statistically significant decline in 24-h urinary albumin excretion, perivascular monocyte/macrophage infiltration in the kidney and decline in SBP to a mean of 150 mmHg compared with a mean of 190 mmHg observed in cyclosporine A–treated dTGR rats. |
| Etanercept | C57BL/6 mice and RAG-1-deficient mice [65] | Statistically significant decline in SBP to a mean of 130 mmHg compared with a mean of 150 mmHg with decrease in aortic superoxide formation observed in etanercept treated C57BL/6 mice. Etanercept also decreased aortic superoxide formation in RAG-1-deficient mice after adoptive transfer of T-cells. |
| Mycophenolate mofetil | Spontaneously hypertensive rats [145] | Statistically significant decline in blood pressures correlated with the decline in renal infiltration by macrophages and lymphocytes, renal and urinary markers of oxidative stress such as malondialdehyde. |
| Mycophenolate mofetil | Dahl salt-sensitive rats [170] | Statistically significant decline in mean arterial pressure (158 mmHg compared with 174 mmHg in the control group), protein and albumin excretion rate in mycophenolate administered rats, while creatinine levels and body weight were not different between the groups. |
| Pyrrolidine dithiocarbamate | Spontaneously hypertensive rats [171] | Statistically significant decline in urinary protein excretion, renal tissue pro-inflammatory cells and markers of oxidative stress, decline in SBP to a mean of 127 mmHg compared with a mean of 198 mmHg was recorded in pyrrolidine dithiocarbamate–treated rats. |
| Pyrrolidine dithiocarbamate | Fawn-hooded hypertensive rats [172] | Statistically significant decline in urinary protein excretion, glomerulosclerosis and SBP by an average of 25 mmHg observed in pyrrolidine dithiocarbamate–treated fawn-hooded hypertensive rats. |
| Tacrolimus | Dahl salt-sensitive rats [173] | Statistically significant decline in SBP to a mean of 150 mmHg compared with a mean of 170 mmHg, along with a decline in urinary albumin and thiobarbituric acid–reactive substances excretion and renal infiltration via pro-inflammatory cells, and NADPH oxidase was recorded. |
dTGR: double transgenic rats; SBP: systolic blood pressure.
Furthermore, animal models have demonstrated the beneficial effects of anti-TNF-α therapy, namely etanercept, anti-IL-6 therapy, anti-CD20 antibody and anti-TGF-β antibody, on blood pressure control [136, 177–181]. Neonatal thymectomy has also shown to be beneficial in the later development of hypertension with lower maximum systolic blood pressure measurement according to a study conducted in genetically hypertensive rats of Lyon strain [169]. Other hypothetical models for blood pressure control include the deletion of CD247 or mutation at RAG1 or SH2B3 genes [182, 66]. Despite growing literature on immunosuppressive agents in the treatment of hypertension on animal models, there is lack of clinical trials conducted in human subjects. Therefore, it is unclear whether such consistent findings of improved blood pressure with various immunosuppressive medications is applicable to humans. There is a clear need for clinical trials investigating the efficiency of such therapeutic approaches on patients with treatment-resistant hypertension.
ANTI-HYPERTENSIVE MEDICATIONS AS IMMUNOMODULATORS
There are multiple classes of anti-hypertensive medications that are currently being utilized in the management of hypertension while the latest studies indicate that their anti-hypertensive properties may include varying degrees of immunomodulatory effects.
RAAS blockers
ACEi/ARBs are among the most commonly utilized anti-hypertensive medications globally, along with their use in congestive heart failure, ischemic cardiovascular diseases and diabetic nephropathy. The immune-modulating effects of ACEi have been highly investigated in multiple models. In mouse models of lupus nephritis–associated hypertension, early initiation of ACEi therapy may delay or reverse the degree of kidney involvement and proteinuria which may be attributable to the decline in T-helper cell polarization towards T-helper-2 type, decline in cytokine production such as IL-4 and decline in the production of TGF-β [183]. Also, ACEi therapy may lead to impairment ofm antigen presentation via major histocompatibility complex I/II [184]. On the other hand, high-dose telmisartan therapy, an ARB agent, leads to inhibition of NLRP3 inflammasome in dose-dependent manner and may result in improvement in vascular function [185]. Other potential beneficial effects of ARB therapy may include suppression of T-cell activity and suppression of serum IL-6 levels [186, 187]. The immunomodulatory effects of mineralocorticoid receptor antagonists such as spironolactone are quite different from other RAAS blockers such as ACEi or ARB and include upregulation of T-regulatory cells, decline in T-helper-17 response, decline in IL-17 production and inhibition of leucocyte migration towards endothelium [188, 189].
Diuretics
Thiazide and loop diuretics are the most commonly prescribed diuretic-type anti-hypertensive medications while their effects on the immune system are unclear, with conflicting results from different studies. A prospective trial conducted in 110 elderly female subjects with multiple medical comorbidities demonstrated that diuretic therapy leads to a statistically significant decline in serum IL-6 and TNF‐α levels without any considerable change in IFN-γ levels, whereas a study conducted in Dahl salt-sensitive rats demonstrated no change in oxidative stress or inflammation with thiazide therapy despite effective blood pressure control [104, 190]. Another clinical trial conducted in 88 hypertensive patients receiving either perindopril, hydrochlorothiazide or indapamide demonstrated that despite decline in blood pressure measurements and improvements in flow-mediated dilatation, thiazide therapy leads to elevation of immunoglobulin M–type anti-ApoB-D peptide antibodies without any change in immunoglobulin G–type antibodies [191]. Therefore, there is a clear need for future studies investigating the immune properties of diuretic therapy in order to enlighten such uncertainty.
Calcium channel blockers
Calcium channel blockers are commonly utilized in order to obtain target blood pressure, however their immunomodulatory properties are currently under-studied. Amlodipine therapy has been shown to lead to the downregulation of NADPH oxidase and VCAM-1 in the aortic wall and inhibition of the NF‐κB pathway, thereby indicating that the potential anti-hypertensive potential of amlodipine therapy may not be limited to its vasodilatory properties [192].
CONCLUSION
Despite being responsible for almost 95% of hypertensive cases, the pathophysiology of primary hypertension is still unknown. Recent studies have suggested a potential role of the immune system in the etiopathogenesis of hypertension [9]. In this review, we provided the potential pathophysiological and therapeutic roles of the immune mechanisms in hypertension. Future studies are needed to better understand the current knowns, and shine a light on the unknowns, of the role of immunity in hypertension (Table 2).
Table 2:
The knowns and unknowns of the role of the immune system in hypertension.
| Knowns | Unknowns |
|---|---|
| TLR-4 gene–deficient mouse models have exhibited less blood pressure rise in reaction to angiotensin II or L-NAME infusions. Blocking TLR-9 is associated with a decrease in angiotensin II–induced hypertension. | Although early studies suggest a possible role in the pathophysiology and identification as a potential therapeutic target by injection of anti-CD20 antibodies, the significance of B-lymphocytes has largely been uninvestigated in research on hypertension. Further research on the function of B-lymphocytes in the pathophysiology of hypertension is highly necessary. |
| Numerous pre-clinical investigations using angiotensin II- or aldosterone-induced hypertension models have shown increased circulating monocytes. Rats that develop hypertension spontaneously or in salt-sensitive or deoxycorticosterone acetate–induced hypertension models have shown a similar trend. | There is little information on how eosinophils and basophils contribute to the etiology of hypertension. Observational studies have suggested a possible connection between coronary artery disease or pulmonary artery hypertension and a higher peripheral eosinophil count, although the precise relationship has not yet been proven. |
| Hs-CRP, IL-6 and elevated CRP were all substantially associated with an increased risk of developing hypertension. IL-1β, however, was not linked to a serious risk. | Although studies using on animal models clearly show the major involvement of renal inflammation in the pathogenesis of hypertension, it is not apparent whether renal inflammation is the triggering event that results in clinical hypertension or only a contributing factor to the onset of hypertension. |
| In people with primary hypertension, mRNA and protein of TGF-β1 are found to be overexpressed. | Further research is necessary to fully understand the impact of TNF- and its inhibition on blood pressure readings because there are conflicting results in the literature. |
| High blood pressure was hypothesized to enhance TNF and oxidative stress significantly. According to a different study, patients with hypertension had considerably higher TNF levels in their blood and urine than healthy controls. | Further studies are needed to understand how dietary salt intake and immune response are linked to hypertension and cardiovascular illnesses. |
| Immune system cells infiltrate the kidneys of spontaneously hypertensive rats before clinical hypertension manifests itself. Furthermore, there is a strong correlation between the level of SBP measurements and immune cell infiltration. | |
| A preclinical investigation using a transgenic mouse model with monocyte/macrophage depletion revealed a decrease in superoxide radical generation, a reduction in angiotensin II–induced hypertension and a reduction in vascular dysfunction. | |
| The release of pro-fibrotic cytokines like TGF and the remodeling of the extracellular matrix by matrix metalloproteinases, which cause vascular stiffness, are two crucial pathophysiological roles of macrophages in vascular inflammation. | |
| T-regulatory cells could prevent vascular inflammation. These cells seem to prevent angiotensin II- or aldosterone-induced ROS production and monocyte/macrophage infiltration of the vascular wall. | |
| T-regulatory cell administration as a single dose or once weekly dose improves cardiac hypertrophy, endothelial vasodilatation, the production of ROS and pro-inflammatory signals, but has no effect on blood pressure readings. In contrast, administering T-regulatory cells at sustained high doses improves blood pressure. | |
| High dietary sodium intake not only causes hemodynamic alterations but also modulates the immune system by immune cell activation, vascular endothelial dysfunction and cytokine secretion, eventually leading inflammation and hypertension. |
SBP: systolic blood pressure.
Contributor Information
Sidar Copur, Department of Medicine, Koc University School of Medicine, Istanbul, Turkey.
Ibrahim B Peltek, Department of Medicine, Koc University School of Medicine, Istanbul, Turkey.
Ali Mutlu, Department of Medicine, Koc University School of Medicine, Istanbul, Turkey.
Cem Tanriover, Department of Medicine, Koc University School of Medicine, Istanbul, Turkey.
Mehmet Kanbay, Department of Medicine, Section of Nephrology, Koc University School of Medicine, Istanbul, Turkey.
FUNDING
This study was not funded by any grant.
AUTHORS’ CONTRIBUTIONS
Conception or design of the work: M.K., C.T. and S.C. Literature search and screening: C.T., I.B.P., A.M. and S.C. Drafted the manuscript: C.T. and S.C. Generation of figures and tables: A.M. and I.B.P. Reviewed the manuscript for intellectual content: M.K. All authors approved the final version of the manuscript.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research.
CONFLICT OF INTEREST STATEMENT
M.K. is member of the CKJ editorial board. All other authors declare that they have no conflict of interest.
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by any of the authors. The figure was crafted using biorender.com.
REFERENCES
- 1. Forouzanfar MH, Liu P, Roth GAet al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA 2017;317:165–82. 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 2. Oparil S, Acelajado MC, Bakris GLet al. Hypertension. Nat Rev Dis Primers 2018;4:18014. 10.1038/nrdp.2018.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lawes CM, Vander Hoorn S, Rodgers A.. Global burden of blood-pressure-related disease, 2001. Lancet North Am Ed 2008;371:1513–8. 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 4. GBD 2015 Risk Factors Collaborators . Global, regional , and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet North Am Ed 2016;388:1659–724. 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kario K, Hoshide S, Mizuno Het al. Nighttime blood pressure phenotype and cardiovascular prognosis: practitioner-based nationwide JAMP study. Circulation 2020;142:1810–20. 10.1161/CIRCULATIONAHA.120.049730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kanbay M, Demiray A, Afsar Bet al. Role of Klotho in the development of essential hypertension. Hypertension 2021;77:740–50. 10.1161/HYPERTENSIONAHA.120.16635. [DOI] [PubMed] [Google Scholar]
- 7. Eren OC, Ortiz A, Afsar Bet al. Multilayered interplay between fructose and salt in development of hypertension. Hypertension 2019;73:265–72. 10.1161/HYPERTENSIONAHA.118.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller ED. The role of the renin-angiotensin-aldosterone system in circulatory control and hypertension. Br J Anaesth 1981;53:711–8. 10.1093/bja/53.7.711. [DOI] [PubMed] [Google Scholar]
- 9. Solak Y, Afsar B, Vaziri NDet al. Hypertension as an autoimmune and inflammatory disease. Hypertens Res 2016;39:567–73. 10.1038/hr.2016.35. [DOI] [PubMed] [Google Scholar]
- 10. Yatim KM, Lakkis FG.. A brief journey through the immune system. Clin J Am Soc Nephrol 2015;10:1274–81. 10.2215/CJN.10031014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mikolajczyk TP, Guzik TJ.. Adaptive immunity in hypertension. Curr Hypertens Rep 2019;21:68. 10.1007/s11906-019-0971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paul S, Lal G.. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol 2017;8:1124. 10.3389/fimmu.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kossmann S, Schwenk M, Hausding Met al. Angiotensin II-induced vascular dysfunction depends on interferon-γ-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler Thromb Vasc Biol 2013;33:1313–9. 10.1161/ATVBAHA.113.301437. [DOI] [PubMed] [Google Scholar]
- 14. Navaneethabalakrishnan S, Smith HL, Arenaz CMet al. Update on immune mechanisms in hypertension. Am J Hypertens 2022;35:842–51. 10.1093/ajh/hpac077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanbay M, Onal EM, Afsar Bet al. The crosstalk of gut microbiota and chronic kidney disease: role of inflammation, proteinuria, hypertension, and diabetes mellitus. Int Urol Nephrol 2018;50:1453–66. 10.1007/s11255-018-1873-2. [DOI] [PubMed] [Google Scholar]
- 16. Onal EM, Afsar B, Covic Aet al. Gut microbiota and inflammation in chronic kidney disease and their roles in the development of cardiovascular disease. Hypertens Res 2019;42:123–40. 10.1038/s41440-018-0144-z. [DOI] [PubMed] [Google Scholar]
- 17. Kirabo A, Fontana V, de Faria APet al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 2014;124:4642–56. 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawasaki T, Kawai T.. Toll-like receptor signaling pathways. Front Immunol 2014;5:461. 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marketou ME, Kontaraki JE, Zacharis EAet al. TLR2 and TLR4 gene expression in peripheral monocytes in nondiabetic hypertensive patients: the effect of intensive blood pressure-lowering. J Clin Hypertens (Greenwich) 2012;14:330–5. 10.1111/j.1751-7176.2012.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dange RB, Peng H, Feng Yet al. Mice lacking the gene for Toll like receptor-4 (TLR4) had an attenuated blood pressure response to Angiotensin II infusion. Wiley Online Library, 2013. https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fasebj.27.1_supplement.696.4. [Google Scholar]
- 21. Sollinger D, Eißler R, Lorenz Set al. Damage-associated molecular pattern activated Toll-like receptor 4 signalling modulates blood pressure in L-NAME-induced hypertension. Cardiovasc Res 2014;101:464–72. 10.1093/cvr/cvt265. [DOI] [PubMed] [Google Scholar]
- 22. Dange RB, Agarwal D, Masson GSet al. Central blockade of TLR4 improves cardiac function and attenuates myocardial inflammation in angiotensin II-induced hypertension. Cardiovasc Res 2014;103:17–27. 10.1093/cvr/cvu067. [DOI] [PubMed] [Google Scholar]
- 23. Bomfim GF, Dos Santos RA, Oliveira MAet al. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond) 2012;122:535–43. 10.1042/CS20110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nunes KP, Bomfim GF, Toque HAet al. Toll-like receptor 4 (TLR4) impairs nitric oxide contributing to Angiotensin II-induced cavernosal dysfunction. Life Sci 2017;191:219–26. 10.1016/j.lfs.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 25. Ishikawa T, Abe K, Takana-Ishikawa Met al. Chronic inhibition of toll-like receptor 9 ameliorates pulmonary hypertension in rats. JAHA 2021;10:e019247. 10.1161/JAHA.120.019247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pushpakumar S, Ren L, Kundu Set al. Toll-like receptor 4 deficiency reduces oxidative stress and macrophage mediated inflammation in hypertensive kidney. Sci Rep 2017;7:6349. 10.1038/s41598-017-06484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bomfim GF, Cau SBA, Bruno ASet al. Hypertension: a new treatment for an old disease? Targeting the immune system. Br J Pharmacol 2019;176:2028–48. 10.1111/bph.14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kunnas T, Määttä K, Nikkari ST.. NLR family pyrin domain containing 3 (NLRP3) inflammasome gene polymorphism rs7512998 (C>T) predicts aging-related increase of blood pressure, the TAMRISK study. Immun Ageing 2015;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Q, So A, Nussberger Jet al. Impact of NLRP3 inflammasome on the development of hypertension and renal and cardiac hypertrophy in 2K1C and DOCA/salt mice. Kidney Res Clin Pract 2012;31:A83. [Google Scholar]
- 30. Cartwright N, Murch O, McMaster SKet al. Selective NOD1 agonists cause shock and organ injury/dysfunction in vivo. Am J Respir Crit Care Med 2007;175:595–603. 10.1164/rccm.200608-1103OC. [DOI] [PubMed] [Google Scholar]
- 31. Gu CC, Hunt SC, Kardia Set al. An investigation of genome-wide associations of hypertension with microsatellite markers in the family blood pressure program (FBPP). Hum Genet 2007;121:577–90. 10.1007/s00439-007-0349-8. [DOI] [PubMed] [Google Scholar]
- 32. Rooks MG, Garrett WS.. Gut microbiota, metabolites and host immunity. Nat Rev Immunol 2016;16:341–52. 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tanriover C, Ucku D, Basile Cet al. On the importance of the interplay of residual renal function with clinical outcomes in end-stage kidney disease. J Nephrol 2022;35:2191–204. 10.1007/s40620-022-01388-9. [DOI] [PubMed] [Google Scholar]
- 34. Jose PA, Raj D.. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens 2015;24:403–9. 10.1097/MNH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li J, Zhao F, Wang Yet al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017;5:14. 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wenzel P, Knorr M, Kossmann Set al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 2011;124:1370–81. 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 37. De Ciuceis C, Amiri F, Brassard Pet al. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol 2005;25:2106–13. 10.1161/01.ATV.0000181743.28028.57. [DOI] [PubMed] [Google Scholar]
- 38. Fehrenbach DJ, Mattson DL.. Inflammatory macrophages in the kidney contribute to salt-sensitive hypertension. Am J Physiol Renal Physiol 2020;318:F544–8. 10.1152/ajprenal.00454.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kanbay M, Aslan G, Afsar Bet al. Acute effects of salt on blood pressure are mediated by serum osmolality. J Clin Hypertens 2018;20:1447–54. 10.1111/jch.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martín-Fernández B, Rubio-Navarro A, Cortegano Iet al. Aldosterone induces renal fibrosis and inflammatory M1-macrophage subtype via mineralocorticoid receptor in rats. PLoS One 2016;11:e0145946. 10.1371/journal.pone.0145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mervaala EM, Müller DN, Park JKet al. Monocyte infiltration and adhesion molecules in a rat model of high human renin hypertension. Hypertension 1999;33:389–95. 10.1161/01.HYP.33.1.389. [DOI] [PubMed] [Google Scholar]
- 42. Ishibashi M, Hiasa K, Zhao Qet al. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res 2004;94:1203–10. 10.1161/01.RES.0000126924.23467.A3. [DOI] [PubMed] [Google Scholar]
- 43. Crowley SD, Song YS, Sprung Get al. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 2010;55:99–108. 10.1161/HYPERTENSIONAHA.109.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang L, Wang A, Hao Yet al. Macrophage depletion lowered blood pressure and attenuated hypertensive renal injury and fibrosis. Front Physiol 2018;9:473. 10.3389/fphys.2018.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hartner A, Porst M, Gauer Set al. Glomerular osteopontin expression and macrophage infiltration in glomerulosclerosis of DOCA-salt rats. Am J Kidney Dis 2001;38:153–64. 10.1053/ajkd.2001.25209. [DOI] [PubMed] [Google Scholar]
- 46. Harwani SC, Ratcliff J, Sutterwala FSet al. Nicotine mediates CD161a+ renal macrophage infiltration and premature hypertension in the spontaneously hypertensive rat. Circ Res 2016;119:1101–15. 10.1161/CIRCRESAHA.116.309402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wenstedt EF, Verberk SG, Kroon Jet al. Salt increases monocyte CCR2 expression and inflammatory responses in humans. JCI Insight 2019;4:e130508. 10.1172/jci.insight.130508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Asagami T, Reaven GM, Tsao PS.. Enhanced monocyte adherence to thoracic aortae from rats with two forms of experimental hypertension. Am J Hypertens 1999;12:890–3. 10.1016/S0895-7061(99)00072-2. [DOI] [PubMed] [Google Scholar]
- 49. Clozel M, Kuhn H, Hefti Fet al. Endothelial dysfunction and subendothelial monocyte macrophages in hypertension. Effect of angiotensin converting enzyme inhibition. Hypertension 1991;18:132–41. 10.1161/01.HYP.18.2.132. [DOI] [PubMed] [Google Scholar]
- 50. Schmid-Schönbein GW, Seiffge D, DeLano FAet al. Leukocyte counts and activation in spontaneously hypertensive and normotensive rats. Hypertension 1991;17:323–30. 10.1161/01.HYP.17.3.323. [DOI] [PubMed] [Google Scholar]
- 51. Dörffel Y, Lätsch C, Stuhlmüller Bet al. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension 1999;34:113–7. 10.1161/01.HYP.34.1.113. [DOI] [PubMed] [Google Scholar]
- 52. Wirtz PH, von Känel R, Frey Ket al. Glucocorticoid sensitivity of circulating monocytes in essential hypertension. Am J Hypertens 2004;17:489–94. 10.1016/j.amjhyper.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 53. Zaldivia MT, Rivera J, Hering Det al. Renal denervation reduces monocyte activation and monocyte-platelet aggregate formation: an anti-inflammatory effect relevant for cardiovascular risk. Hypertension 2017;69:323–31. 10.1161/HYPERTENSIONAHA.116.08373. [DOI] [PubMed] [Google Scholar]
- 54. Tatsukawa Y, Hsu WL, Yamada Met al. White blood cell count, especially neutrophil count, as a predictor of hypertension in a Japanese population. Hypertens Res 2008;31:1391–7. 10.1291/hypres.31.1391. [DOI] [PubMed] [Google Scholar]
- 55. Lu X, Rudemiller NP, Privratsky JRet al. Classical dendritic cells mediate hypertension by promoting renal oxidative stress and fluid retention. Hypertension 2020;75:131–8. 10.1161/HYPERTENSIONAHA.119.13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang R, Inagawa H, Kazumura Ket al. Evaluation of a hypertensive rat model using peripheral blood neutrophil activity, phagocytic activity and oxidized LDL evaluation. Anticancer Res 2018;38:4289–94. 10.21873/anticanres.12726. [DOI] [PubMed] [Google Scholar]
- 57. Hevia D, Araos P, Prado Cet al. Myeloid CD11c(+) antigen-presenting cells ablation prevents hypertension in response to Angiotensin II plus high-salt diet. Hypertension 2018;71:709–18. 10.1161/HYPERTENSIONAHA.117.10145. [DOI] [PubMed] [Google Scholar]
- 58. Van Beusecum JP, Barbaro NR, McDowell Zet al. High salt activates CD11c(+) antigen-presenting cells via SGK (serum glucocorticoid kinase) 1 to promote renal inflammation and salt-sensitive hypertension. Hypertension 2019;74:555–63. 10.1161/HYPERTENSIONAHA.119.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kossmann S, Schwenk M, Hausding Met al. Angiotensin II–induced vascular dysfunction depends on interferon-γ–driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler Thromb Vasc Biol 2013;33:1313–9. 10.1161/ATVBAHA.113.301437. [DOI] [PubMed] [Google Scholar]
- 60. Taherzadeh Z, VanBavel E, de Vos Jet al. Strain-dependent susceptibility for hypertension in mice resides in the natural killer gene complex. Am J Physiol Heart Circ Physiol 2010;298:H1273–82. 10.1152/ajpheart.00508.2009. [DOI] [PubMed] [Google Scholar]
- 61. Weng M, Baron DM, Bloch KDet al. Eosinophils are necessary for pulmonary arterial remodeling in a mouse model of eosinophilic inflammation-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2011;301:L927–36. 10.1152/ajplung.00049.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tanaka M, Fukui M, Tomiyasu Ket al. Eosinophil count is positively correlated with coronary artery calcification. Hypertens Res 2012;35:325–8. 10.1038/hr.2011.191. [DOI] [PubMed] [Google Scholar]
- 63. Withers SB, Forman R, Meza-Perez Set al. Eosinophils are key regulators of perivascular adipose tissue and vascular functionality. Sci Rep 2017;7:44571. 10.1038/srep44571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu D, Molofsky AB, Liang HEet al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011;332:243–7. 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Guzik TJ, Hoch NE, Brown KAet al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007;204:2449–60. 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mattson DL, Lund H, Guo Cet al. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 2013;304:R407–14. 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Crowley SD, Song YS, Lin EEet al. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 2010;298:R1089–97. 10.1152/ajpregu.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Trott DW, Thabet SR, Kirabo Aet al. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 2014;64:1108–15. 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mian MO, Barhoumi T, Briet Met al. Deficiency of T-regulatory cells exaggerates angiotensin II-induced microvascular injury by enhancing immune responses. J Hypertens 2016;34:97–108. 10.1097/HJH.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 70. Matrougui K, Abd Elmageed Z, Kassan Met al. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol 2011;178:434–41. 10.1016/j.ajpath.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kvakan H, Kleinewietfeld M, Qadri Fet al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 2009;119:2904–12. 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 72. Kasal DA, Barhoumi T, Li MWet al. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 2012;59:324–30. 10.1161/HYPERTENSIONAHA.111.181123. [DOI] [PubMed] [Google Scholar]
- 73. Trott DW, Harrison DG.. The immune system in hypertension. Adv Physiol Educ 2014;38:20–4. 10.1152/advan.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chan CT, Sobey CG, Lieu Met al. Obligatory role for B cells in the development of Angiotensin II-dependent hypertension. Hypertension 2015;66:1023–33. 10.1161/HYPERTENSIONAHA.115.05779. [DOI] [PubMed] [Google Scholar]
- 75. Dalekos GN, Elisaf MS, Papagalanis Net al. Elevated interleukin-1 beta in the circulation of patients with essential hypertension before any drug therapy: a pilot study. Eur J Clin Invest 1996;26:936–9. 10.1111/j.1365-2362.1996.tb02141.x. [DOI] [PubMed] [Google Scholar]
- 76. Dalekos GN, Elisaf M, Bairaktari Eet al. Increased serum levels of interleukin-1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med 1997;129:300–8. 10.1016/S0022-2143(97)90178-5. [DOI] [PubMed] [Google Scholar]
- 77. Melton E, Qiu H.. Interleukin-1β in multifactorial hypertension: inflammation, vascular smooth muscle cell and extracellular matrix remodeling, and non-coding RNA regulation. Int J Mol Sci 2021;22:8639. 10.3390/ijms22168639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang J, Rudemiller NP, Patel MBet al. Interleukin-1 receptor activation potentiates salt reabsorption in Angiotensin II-induced hypertension via the NKCC2 co-transporter in the nephron. Cell Metab 2016;23:360–8. 10.1016/j.cmet.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Crorkin P, Hao S, Ferreri NR.. Responses to Ang II (Angiotensin II), salt intake, and lipopolysaccharide reveal the diverse actions of TNF-alpha (tumor necrosis factor-alpha) on blood pressure and renal function. Hypertension 2022;79:2656–70. 10.1161/HYPERTENSIONAHA.122.19464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Beasley D, Dinarello CA, Cannon JG.. Interleukin-1 induces natriuresis in conscious rats: role of renal prostaglandins. Kidney Int 1988;33:1059–65. 10.1038/ki.1988.111. [DOI] [PubMed] [Google Scholar]
- 81. Kohan DE, Schreiner GF.. Interleukin 1 modulation of renal epithelial glucose and amino acid transport. Am J Physiol 1988;254:F879–86. [DOI] [PubMed] [Google Scholar]
- 82. Boesen EI. Chronic elevation of IL-1beta induces diuresis via a cyclooxygenase 2-mediated mechanism. Am J Physiol Renal Physiol 2013;305:F189–98. 10.1152/ajprenal.00075.2013. [DOI] [PubMed] [Google Scholar]
- 83. Kohan DE, Merli CA, Simon EE.. Micropuncture localization of the natriuretic effect of interleukin 1. Am J Physiol 1989;256:F810–13. [DOI] [PubMed] [Google Scholar]
- 84. Kohan DE. Interleukin-1 regulation of prostaglandin E2 synthesis by the papillary collecting duct. J Lab Clin Med 1989;114:717–23. [PubMed] [Google Scholar]
- 85. Akita K, Isoda K, Ohtomo Fet al. Blocking of interleukin-1 suppresses angiotensin II-induced renal injury. Clin Sci (Lond) 2021;135:2035–48. 10.1042/CS20201406. [DOI] [PubMed] [Google Scholar]
- 86. Jayedi A, Rahimi K, Bautista LEet al. Inflammation markers and risk of developing hypertension: a meta-analysis of cohort studies. Heart 2019;105:686–92. 10.1136/heartjnl-2018-314216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rothman AM, MacFadyen J, Thuren Tet al. Effects of interleukin-1beta inhibition on blood pressure, incident hypertension, and residual inflammatory risk: a secondary analysis of CANTOS. Hypertension 2020;75:477–82. 10.1161/HYPERTENSIONAHA.119.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ridker PM, Everett BM, Thuren Tet al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 89. Urwyler SA, Ebrahimi F, Burkard Tet al. IL (interleukin)-1 receptor antagonist increases Ang (Angiotensin [1-7]) and decreases blood pressure in obese individuals. Hypertension 2020;75:1455–63. 10.1161/HYPERTENSIONAHA.119.13982. [DOI] [PubMed] [Google Scholar]
- 90. Lee DL, Sturgis LC, Labazi Het al. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol 2006;290:H935–40. 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 91. Chae CU, Lee RT, Rifai Net al. Blood pressure and inflammation in apparently healthy men. Hypertension 2001;38:399–403. 10.1161/01.HYP.38.3.399. [DOI] [PubMed] [Google Scholar]
- 92. Ridker PM, Rifai N, Stampfer MJet al. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000;101:1767–72. 10.1161/01.CIR.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 93. Chamarthi B, Williams GH, Ricchiuti Vet al. Inflammation and hypertension: the interplay of interleukin-6, dietary sodium, and the renin-angiotensin system in humans. Am J Hypertens 2011;24:1143–8. 10.1038/ajh.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Samuelsson AM, Alexanderson C, Molne Jet al. Prenatal exposure to interleukin-6 results in hypertension and alterations in the renin-angiotensin system of the rat. J Physiol 2006;575:855–67. 10.1113/jphysiol.2006.111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Brands MW, Banes-Berceli AK, Inscho EWet al. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension 2010;56:879–84. 10.1161/HYPERTENSIONAHA.110.158071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kranzhofer R, Schmidt J, Pfeiffer CAet al. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol1999;19:1623–9. 10.1161/01.ATV.19.7.1623. [DOI] [PubMed] [Google Scholar]
- 97. Funakoshi Y, Ichiki T, Ito Ket al. Induction of interleukin-6 expression by angiotensin II in rat vascular smooth muscle cells. Hypertension 1999;34:118–25. 10.1161/01.HYP.34.1.118. [DOI] [PubMed] [Google Scholar]
- 98. Han Y, Runge MS, Brasier AR.. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kappa B transcription factors. Circ Res 1999;84:695–703. 10.1161/01.RES.84.6.695. [DOI] [PubMed] [Google Scholar]
- 99. Moriyama T, Fujibayashi M, Fujiwara Yet al. Angiotensin II stimulates interleukin-6 release from cultured mouse mesangial cells. J Am Soc Nephrol 1995;6:95–101. 10.1681/ASN.V6195. [DOI] [PubMed] [Google Scholar]
- 100. Lee DL, Leite R, Fleming Cet al. Hypertensive response to acute stress is attenuated in interleukin-6 knockout mice. Hypertension 2004;44:259–63. 10.1161/01.HYP.0000139913.56461.fb. [DOI] [PubMed] [Google Scholar]
- 101. Coles B, Fielding CA, Rose-John Set al. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am J Pathol 2007;171:315–25. 10.2353/ajpath.2007.061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bautista LE, Vera LM, Arenas IAet al. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens 2005;19:149–54. 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 103. Mao SQ, Sun JH, Gu TLet al. Hypomethylation of interleukin-6 (IL-6) gene increases the risk of essential hypertension: a matched case-control study. J Hum Hypertens 2017;31:530–6. 10.1038/jhh.2017.7. [DOI] [PubMed] [Google Scholar]
- 104. Toledo JO, Moraes CF, Souza VCet al. Tailored antihypertensive drug therapy prescribed to older women attenuates circulating levels of interleukin-6 and tumor necrosis factor-α. Clin Interv Aging 2015;10:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Thangaraj SS, Oxlund CS, Fonseca MPDet al. The mineralocorticoid receptor blocker spironolactone lowers plasma interferon-gamma and interleukin-6 in patients with type 2 diabetes and treatment-resistant hypertension. J Hypertens 2022;40:153–62. 10.1097/HJH.0000000000002990. [DOI] [PubMed] [Google Scholar]
- 106. Buemi M, Marino D, Floccari Fet al. Effect of interleukin 8 and ICAM-1 on calcium-dependent outflow of K+ in erythrocytes from subjects with essential hypertension. Curr Med Res Opin 2004;20:19–24. 10.1185/030079903125002720. [DOI] [PubMed] [Google Scholar]
- 107. Wakabayashi Y, Usui Y, Okunuki Yet al. Increases of vitreous monocyte chemotactic protein 1 and interleukin 8 levels in patients with concurrent hypertension and diabetic retinopathy. Retina 2011;31:1951–7. 10.1097/IAE.0b013e31820d3cee. [DOI] [PubMed] [Google Scholar]
- 108. Letterio JJ, Roberts AB.. Regulation of immune responses by TGF-beta. Annu Rev Immunol 1998;16:137–61. 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 109. Li MO, Flavell RA.. TGF-beta: a master of all T cell trades. Cell 2008;134:392–404. 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kim HS, Kim HY.. Hypertensive effects of transforming growth factor-beta1 in vascular smooth muscles cells from spontaneously hypertensive rats are mediated by sulfatase 2. Cytokine 2022;150:155754. 10.1016/j.cyto.2021.155754. [DOI] [PubMed] [Google Scholar]
- 111. Zhang Q, Liu H, Yang J.. Regulation of TGF-beta1 on PI3KC3 and its role in hypertension-induced vascular injuries. Exp Ther Med 2019;17:1717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fu S, Li YL, Wu YTet al. Icariside II attenuates myocardial fibrosis by inhibiting nuclear factor-kappaB and the TGF-beta1/Smad2 signalling pathway in spontaneously hypertensive rats. Biomed Pharmacother 2018;100:64–71. 10.1016/j.biopha.2018.01.138. [DOI] [PubMed] [Google Scholar]
- 113. Laviades C, Varo N, Diez J.. Transforming growth factor beta in hypertensives with cardiorenal damage. Hypertension 2000;36:517–22. 10.1161/01.HYP.36.4.517. [DOI] [PubMed] [Google Scholar]
- 114. Li B, Khanna A, Sharma Vet al. TGF-beta1 DNA polymorphisms, protein levels, and blood pressure. Hypertension 1999;33:271–5. 10.1161/01.HYP.33.1.271. [DOI] [PubMed] [Google Scholar]
- 115. Lijnen PJ, Petrov VV, Fagard RH.. Association between transforming growth factor-beta and hypertension. Am J Hypertens 2003;16:604–11. 10.1016/S0895-7061(03)00847-1. [DOI] [PubMed] [Google Scholar]
- 116. Wolf G. Link between angiotensin II and TGF-beta in the kidney. Miner Electrolyte Metab 1998;24:174–80. 10.1159/000057367. [DOI] [PubMed] [Google Scholar]
- 117. Zhu S, Liu Y, Wang Let al. Transforming growth factor-beta1 is associated with kidney damage in patients with essential hypertension: renoprotective effect of ACE inhibitor and/or angiotensin II receptor blocker. Nephrol Dial Transplant 2008;23:2841–6. 10.1093/ndt/gfn159. [DOI] [PubMed] [Google Scholar]
- 118. Houlihan CA, Akdeniz A, Tsalamandris Cet al. Urinary transforming growth factor-beta excretion in patients with hypertension, type 2 diabetes, and elevated albumin excretion rate: effects of angiotensin receptor blockade and sodium restriction. Diabetes Care 2002;25:1072–7. 10.2337/diacare.25.6.1072. [DOI] [PubMed] [Google Scholar]
- 119. Praga M, Andrade CF, Luno Jet al. Antiproteinuric efficacy of losartan in comparison with amlodipine in non-diabetic proteinuric renal diseases: a double-blind, randomized clinical trial. Nephrol Dial Transplant 2003;18:1806–13. 10.1093/ndt/gfg284. [DOI] [PubMed] [Google Scholar]
- 120. Ramseyer VD, Garvin JL.. Tumor necrosis factor-alpha: regulation of renal function and blood pressure. Am J Physiol Renal Physiol 2013;304:F1231–42. 10.1152/ajprenal.00557.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kobayashi R, Wakui H, Azushima Ket al. An angiotensin II type 1 receptor binding molecule has a critical role in hypertension in a chronic kidney disease model. Kidney Int 2017;91:1115–25. 10.1016/j.kint.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 122. Elmarakby AA, Quigley JE, Imig JDet al. TNF-alpha inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol 2008;294:R76–83. 10.1152/ajpregu.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Verma MK, Jaiswal A, Sharma Pet al. Oxidative stress and biomarker of TNF-alpha, MDA and FRAP in hypertension. JMedLife 2019;12:253–9. 10.25122/jml-2019-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Puszkarska A, Niklas A, Gluszek Jet al. The concentration of tumor necrosis factor in the blood serum and in the urine and selected early organ damages in patients with primary systemic arterial hypertension. Medicine (Baltimore) 2019;98:e15773. 10.1097/MD.0000000000015773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Naya M, Tsukamoto T, Morita Ket al. Plasma interleukin-6 and tumor necrosis factor-alpha can predict coronary endothelial dysfunction in hypertensive patients. Hypertens Res 2007;30:541–8. 10.1291/hypres.30.541. [DOI] [PubMed] [Google Scholar]
- 126. Yoshida S, Takeuchi T, Kotani Tet al. Infliximab, a TNF-alpha inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens 2014;28:165–9. 10.1038/jhh.2013.80. [DOI] [PubMed] [Google Scholar]
- 127. Zhao Q, Hong D, Zhang Yet al. Association between anti-TNF therapy for rheumatoid arthritis and hypertension: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e731. 10.1097/MD.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lamb FS, Choi H, Miller MRet al. TNFalpha and reactive oxygen signaling in vascular smooth muscle cells in hypertension and atherosclerosis. Am J Hypertens 2020;33:902–13. 10.1093/ajh/hpaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ma Y, He FJ, MacGregor GA.. High salt intake: independent risk factor for obesity? Hypertension 2015;66:843–9. 10.1161/HYPERTENSIONAHA.115.05948. [DOI] [PubMed] [Google Scholar]
- 130. Afsar B, Kuwabara M, Ortiz Aet al. Salt intake and immunity. Hypertension 2018;72:19–23. 10.1161/HYPERTENSIONAHA.118.11128. [DOI] [PubMed] [Google Scholar]
- 131. Rucker AJ, Rudemiller NP, Crowley SD. Salt, hypertension, and immunity. Annu Rev Physiol 2018;80:283–307. 10.1146/annurev-physiol-021317-121134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Takahashi H, Nakagawa S, Wu Yet al. A high-salt diet enhances leukocyte adhesion in association with kidney injury in young Dahl salt-sensitive rats. Hypertens Res 2017;40:912–20. 10.1038/hr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Dmitrieva NI, Burg MB.. Secretion of von Willebrand factor by endothelial cells links sodium to hypercoagulability and thrombosis. Proc Natl Acad Sci USA 2014;111:6485–90. 10.1073/pnas.1404809111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Krieglstein CF, Granger DN.. Adhesion molecules and their role in vascular disease. Am J Hypertens 2001;14:S44–54. 10.1016/S0895-7061(01)02069-6. [DOI] [PubMed] [Google Scholar]
- 135. Evans LC, Petrova G, Kurth Tet al. Increased perfusion pressure drives renal T-cell infiltration in the Dahl salt-sensitive rat. Hypertension 2017;70:543–51. 10.1161/HYPERTENSIONAHA.117.09208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hashmat S, Rudemiller N, Lund Het al. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 2016;311:F555–61. 10.1152/ajprenal.00594.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Norlander AE, Saleh MA, Pandey AKet al. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight 2017;2:e92801. 10.1172/jci.insight.92801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Norlander AE, Saleh MA, Kamat NVet al. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension 2016;68:167–74. 10.1161/HYPERTENSIONAHA.116.07493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Junger WG, Hoyt DB, Hamreus Met al. Hypertonic saline activates protein tyrosine kinases and mitogen-activated protein kinase p38 in T-cells. J Trauma 1997;42:437–45;discussion 443–5. 10.1097/00005373-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 140. Hernandez AL, Kitz A, Wu Cet al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest 2015;125:4212–22. 10.1172/JCI81151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhang WC, Zheng XJ, Du LJet al. High salt primes a specific activation state of macrophages, M(Na). Cell Res 2015;25:893–910. 10.1038/cr.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Barbaro NR, Foss JD, Kryshtal DOet al. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep 2017;21:1009–20. 10.1016/j.celrep.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Guyton AC, Coleman TG, Cowley AVet al. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 1972;52:584–94. 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 144. Rodríguez-Iturbe B, Quiroz Y, Ferrebuz Aet al. Evolution of renal interstitial inflammation and NF-kappaB activation in spontaneously hypertensive rats. Am J Nephrol 2004;24:587–94. 10.1159/000082313. [DOI] [PubMed] [Google Scholar]
- 145. Rodríguez-Iturbe B, Quiroz Y, Nava Met al. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol 2002;282:F191–201. 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 146. Franco M, Martínez F, Quiroz Yet al. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 2007;293:R251–6. 10.1152/ajpregu.00645.2006. [DOI] [PubMed] [Google Scholar]
- 147. Franco M, Tapia E, Bautista Ret al. Impaired pressure natriuresis resulting in salt-sensitive hypertension is caused by tubulointerstitial immune cell infiltration in the kidney. Am J Physiol Renal Physiol 2013;304:F982–90. 10.1152/ajprenal.00463.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Johnson RJ, Schreiner GF.. Hypothesis: the role of acquired tubulointerstitial disease in the pathogenesis of salt-dependent hypertension. Kidney Int 1997;52:1169–79. 10.1038/ki.1997.442. [DOI] [PubMed] [Google Scholar]
- 149. Asghar M, Chugh G, Lokhandwala MF.. Inflammation compromises renal dopamine D1 receptor function in rats. Am J Physiol Renal Physiol 2009;297:F1543–9. 10.1152/ajprenal.00366.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Gonzalez-Villalobos RA, Janjoulia T, Fletcher NKet al. The absence of intrarenal ACE protects against hypertension. J Clin Invest 2013;123:2011–23. 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Giani JF, Bernstein KE, Janjulia Tet al. Salt sensitivity in response to renal injury requires renal angiotensin-converting enzyme. Hypertension 2015;66:534–42. 10.1161/HYPERTENSIONAHA.115.05320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhang RM, McNerney KP, Riek AEet al. Immunity and hypertension. Acta Physiol 2021;231:e13487. 10.1111/apha.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lima VV, Zemse SM, Chiao CWet al. Interleukin-10 limits increased blood pressure and vascular RhoA/Rho-kinase signaling in angiotensin II-infused mice. Life Sci 2016;145:137–43. 10.1016/j.lfs.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 154. Giannelou M, Mavragani CP.. Cardiovascular disease in systemic lupus erythematosus: a comprehensive update. J Autoimmun 2017;82:1–12. 10.1016/j.jaut.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 155. Panoulas VF, Douglas KM, Milionis HJet al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1477–82. 10.1093/rheumatology/kem169. [DOI] [PubMed] [Google Scholar]
- 156. Neimann AL, Shin DB, Wang Xet al. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 2006;55:829–35. 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 157. Wolf VL, Ryan MJ.. Autoimmune disease-associated hypertension. Curr Hypertens Rep 2019;21:10. 10.1007/s11906-019-0914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Han C, Robinson DW, Hackett MVet al. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 2006;33:2167–72. [PubMed] [Google Scholar]
- 159. Anyfanti P, Gkaliagkousi E, Triantafyllou Aet al. Hypertension in rheumatic diseases: prevalence, awareness, treatment, and control rates according to current hypertension guidelines. J Hum Hypertens 2021;35:419–27. 10.1038/s41371-020-0348-y. [DOI] [PubMed] [Google Scholar]
- 160. Al-Herz A, Ensworth S, Shojania Ket al. Cardiovascular risk factor screening in systemic lupus erythematosus. J Rheumatol 2003;30:493–6. [PubMed] [Google Scholar]
- 161. Sabio JM, Vargas-Hitos JA, Navarrete-Navarrete Net al. Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol 2011;38:1026–32. 10.3899/jrheum.101132. [DOI] [PubMed] [Google Scholar]
- 162. Shaharir SS, Mustafar R, Mohd Ret al. Persistent hypertension in lupus nephritis and the associated risk factors. Clin Rheumatol 2015;34:93–7. 10.1007/s10067-014-2802-0. [DOI] [PubMed] [Google Scholar]
- 163. Shanmugam VK, Steen VD.. Renal manifestations in scleroderma: evidence for subclinical renal disease as a marker of vasculopathy. Int J Rheumatol 2010;2010:1–8. 10.1155/2010/538589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hadwen B, Stranges S, Barra L.. Risk factors for hypertension in rheumatoid arthritis patients-a systematic review. Autoimmun Rev 2021;20:102786. 10.1016/j.autrev.2021.102786. [DOI] [PubMed] [Google Scholar]
- 165. Barbhaiya M, Costenbader KH.. Environmental exposures and the development of systemic lupus erythematosus. Curr Opin Rheumatol 2016;28:497–505. 10.1097/BOR.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Teh P, Zakhary B, Sandhu VK.. The impact of obesity on SLE disease activity: findings from the Southern California Lupus Registry (SCOLR). Clin Rheumatol 2019;38:597–600. 10.1007/s10067-018-4336-3. [DOI] [PubMed] [Google Scholar]
- 167. Speyer CB, Costenbader KH.. Cigarette smoking and the pathogenesis of systemic lupus erythematosus. Expert Rev Clin Immunol 2018;14:481–7. 10.1080/1744666X.2018.1473035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Khraibi AA, Norman RA, Dzielak DJ. Chronic immunosuppression attenuates hypertension in Okamoto spontaneously hypertensive rats. Am J Physiol 1984;247:H722–6. [DOI] [PubMed] [Google Scholar]
- 169. Bataillard A, Vincent M, Sassard Jet al. Antihypertensive effect of an immunosuppressive agent, cyclophosphamide, in genetically hypertensive rats of the Lyon strain. Int J Immunopharmacol 1989;11:377–84. 10.1016/0192-0561(89)90084-2. [DOI] [PubMed] [Google Scholar]
- 170. Mattson DL, James L, Berdan EAet al. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 2006;48:149–56. 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 171. Rodríguez-Iturbe B, Ferrebuz A, Vanegas Vet al. Early and sustained inhibition of nuclear factor-kappaB prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther 2005;315:51–7. 10.1124/jpet.105.088062. [DOI] [PubMed] [Google Scholar]
- 172. Koeners MP, Wesseling S, Sánchez Met al. Perinatal inhibition of NF-kappaB has long-term antihypertensive and renoprotective effects in fawn-hooded hypertensive rats. Am J Hypertens 2016;29:123–31. 10.1093/ajh/hpv065. [DOI] [PubMed] [Google Scholar]
- 173. De Miguel C, Guo C, Lund Het al. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 2011;300:F734–42. 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Mervaala E, Müller DN, Park JKet al. Cyclosporin A protects against angiotensin II-induced end-organ damage in double transgenic rats harboring human renin and angiotensinogen genes. Hypertension 2000;35:360–6. 10.1161/01.HYP.35.1.360. [DOI] [PubMed] [Google Scholar]
- 175. Nava M, Quiroz Y, Vaziri Net al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol 2003;284:F447–54. 10.1152/ajprenal.00264.2002. [DOI] [PubMed] [Google Scholar]
- 176. Rodriguez-Iturbe B, Zhan CD, Quiroz Yet al. Antioxidant-rich diet relieves hypertension and reduces renal immune infiltration in spontaneously hypertensive rats. Hypertension 2003;41:341–6. 10.1161/01.HYP.0000052833.20759.64. [DOI] [PubMed] [Google Scholar]
- 177. Huang B, Cheng Y, Usa Ket al. Renal tumor necrosis factor α contributes to hypertension in Dahl salt-sensitive rats. Sci Rep 2016;6:21960. 10.1038/srep21960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Venegas-Pont M, Manigrasso MB, Grifoni SCet al. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 2010;56:643–9. 10.1161/HYPERTENSIONAHA.110.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Mathis KW, Wallace K, Flynn ERet al. Preventing autoimmunity protects against the development of hypertension and renal injury. Hypertension 2014;64:792–800. 10.1161/HYPERTENSIONAHA.114.04006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Murphy SR, Dahly-Vernon AJ, Dunn KMet al. Renoprotective effects of anti-TGF-β antibody and antihypertensive therapies in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 2012;303:R57–69. 10.1152/ajpregu.00263.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Dahly AJ, Hoagland KM, Flasch AKet al. Antihypertensive effects of chronic anti-TGF-beta antibody therapy in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 2002;283:R757–67. 10.1152/ajpregu.00098.2002. [DOI] [PubMed] [Google Scholar]
- 182. Rudemiller NP, Lund H, Priestley JRet al. Mutation of SH2B3 (LNK), a genome-wide association study candidate for hypertension, attenuates Dahl salt-sensitive hypertension via inflammatory modulation. Hypertension 2015;65:1111–7. 10.1161/HYPERTENSIONAHA.114.04736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. De Albuquerque DA, Saxena V, Adams DEet al. An ACE inhibitor reduces Th2 cytokines and TGF-beta1 and TGF-beta2 isoforms in murine lupus nephritis. Kidney Int 2004;65:846–59. 10.1111/j.1523-1755.2004.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Bernstein KE, Khan Z, Giani JFet al. Angiotensin-converting enzyme in innate and adaptive immunity. Nat Rev Nephrol 2018;14:325–36. 10.1038/nrneph.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Liu W, Yamashita T, Kurata Tet al. Protective effect of telmisartan on neurovascular unit and inflammasome in stroke-resistant spontaneously hypertensive rats. Neurol Res 2015;37:491–501. 10.1179/1743132815Y.0000000002. [DOI] [PubMed] [Google Scholar]
- 186. Sepehri Z, Masoumi M, Ebrahimi Net al. Losartan and captopril lead to upregulation of TGF-β, and downregulation of IL-6 in coronary artery disease and hypertension. PLoS One 2016;11:e0168312. 10.1371/journal.pone.0168312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Sonmez A, Kisa U, Uckaya Get al. Effects of losartan treatment on T-cell activities and plasma leptin concentrations in primary hypertension. J Renin Angiotensin Aldosterone Syst 2001;2:112–6. 10.3317/jraas.2001.011. [DOI] [PubMed] [Google Scholar]
- 188. Hofbauer R, Frass M, Pasching Eet al. Furosemide and spironolactone reduce transmigration of leukocytes through endothelial cell monolayers. J Toxicol Environ Health A 2002;65:685–93. 10.1080/15287390252900386. [DOI] [PubMed] [Google Scholar]
- 189. Amador CA, Barrientos V, Peña Jet al. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 2014;63:797–803. 10.1161/HYPERTENSIONAHA.113.02883. [DOI] [PubMed] [Google Scholar]
- 190. Zhou MS, Schulman IH, Jaimes EAet al. Thiazide diuretics, endothelial function, and vascular oxidative stress. J Hypertens 2008;26:494–500. 10.1097/HJH.0b013e3282f3e39d. [DOI] [PubMed] [Google Scholar]
- 191. Fonseca HA, Fonseca FA, Lins LCet al. Antihypertensive therapy increases natural immunity response in hypertensive patients. Life Sci 2015;143:124–30. 10.1016/j.lfs.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 192. Toba H, Nakagawa Y, Miki Set al. Calcium channel blockades exhibit anti-inflammatory and antioxidative effects by augmentation of endothelial nitric oxide synthase and the inhibition of angiotensin converting enzyme in the N(G)-nitro-L-arginine methyl ester-induced hypertensive rat aorta: vasoprotective effects beyond the blood pressure-lowering effects of amlodipine and manidipine. Hypertens Res 2005;28:689–700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.