Key Points
Question
Do patients with melanoma have different fecal microbiota profiles than individuals without melanoma, and does the fecal microbiome differ based on disease stage?
Findings
In this case-control study of 228 participants, patients with melanoma had a different structure of microbiome, with lower abundance of multiple beneficial commensals, compared with control participants. The gut microbiota of patients with early-stage melanoma was characterized by higher alpha diversity and a distinct microbiome structure compared with those with late-stage melanoma.
Meaning
These findings suggest that gut dysbiosis may be a targetable component of melanomagenesis and disease progression, but further prospective investigation is needed to validate these findings.
This case-control study compares differences in the fecal microbiota—including overall diversity, composition, and putative function—between control participants and patients with melanoma and between patients with early- and late-stage melanoma.
Abstract
Importance
The gut microbiome modulates the immune system and responses to immunotherapy in patients with late-stage melanoma. It is unknown whether fecal microbiota profiles differ between healthy individuals and patients with melanoma or if microbiota profiles differ among patients with different stages of melanoma. Defining gut microbiota profiles in individuals without melanoma and those with early-stage and late-stage melanoma may reveal features associated with disease progression.
Objective
To characterize and compare gut microbiota profiles between healthy volunteers and patients with melanoma and between patients with early-stage and late-stage melanoma.
Design, Setting, and Participants
This single-site case-control study took place at an academic comprehensive cancer center. Fecal samples were collected from systemic treatment−naive patients with stage I to IV melanoma from June 1, 2015, to January 31, 2019, and from healthy volunteers from June 1, 2021, to January 31, 2022. Patients were followed up for disease recurrence until November 30, 2021.
Main Outcomes and Measures
Fecal microbiota was profiled by 16S ribosomal RNA sequencing. Clinical and pathologic characteristics, treatment, and disease recurrence were extracted from electronic medical records. Fecal microbiome diversity, taxonomic profiles and inferred functional profiles were compared between groups.
Results
A total of 228 participants were enrolled (126 men [55.3%]; median age, 59 [range, 21-90] years), including 49 volunteers without melanoma, 38 patients with early-stage melanoma (29 with stage I or melanoma in situ and 9 with stage II), and 141 with late-stage melanoma (66 with stage III and 75 with stage IV). Community differences were observed between patients with melanoma and volunteers. Patients with melanoma had a higher relative abundance of Fusobacterium compared with controls on univariate analysis (0.19% vs 0.003%; P < .001), but this association was attenuated when adjusted for covariates (log2 fold change of 5.18 vs controls; P = .09). Microbiomes were distinct between patients with early-stage and late-stage melanoma. Early-stage melanoma had a higher alpha diversity (Inverse Simpson Index 14.6 [IQR, 9.8-23.0] vs 10.8 [IQR, 7.2-16.8]; P = .003), and a higher abundance of the genus Roseburia on univariate analysis (2.4% vs 1.2%; P < .001) though statistical significance was lost with covariate adjustment (log2 fold change of 0.86 vs controls; P = .13). Multiple functional pathways were differentially enriched between groups. No associations were observed between the microbial taxa and disease recurrence in patients with stage III melanoma treated with adjuvant immunotherapy.
Conclusions and Relevance
The findings of this case-control study suggest that fecal microbiota profiles were significantly different among patients with melanoma and controls and between patients with early-stage and late-stage melanoma. Prospective investigations of the gut microbiome and changes that occur with disease progression may identify future microbial targets for intervention.
Introduction
The gut microbiome represents the largest collection of microbial communities within the human body and has become increasingly recognized for its potential effect on clinical cancer outcomes.1,2,3 Emerging evidence suggests that the gut microbiome may influence cancer initiation, disease progression, drug toxicity, and treatment response.3,4,5,6,7,8,9 In melanoma, an association between the presence of specific microbes in the gut and response to immunotherapy has been demonstrated in both preclinical models3,5,10 and patient cohorts with metastatic melanoma,3,4,5,6,7,9,11,12,13 but limited data exist on the microbiome composition in patients with localized or early-stage melanoma or if differences exist compared with individuals without melanoma.
Immunotherapy has meaningfully improved survival for a subset of patients with melanoma; however, many patients do not respond to or are unable to receive immune checkpoint inhibitor (ICI) therapy. To overcome this ICI resistance, ongoing research seeks to understand how modulating the gut microbiome can optimize immunotherapy response.2,14,15,16 Previous studies2,3,11 demonstrated that patients with metastatic melanoma who respond to immunotherapy have a higher abundance of certain microbial taxa (eg, Faecalibacterium) compared with nonresponders. Other groups have validated this finding but also identified other commensal bacteria associated with response to immunotherapy in metastatic melanoma, all of which have been shown in other populations to play critical roles in gut barrier maintenance.3,4,5,6,7,9,11,12,13 McQuade and colleagues1,2 have demonstrated that other patient factors associated with the microbiome, including body mass index (BMI), sex, dietary fiber intake, and use of probiotic supplements, may modulate ICI response and outcomes in patients with metastatic melanoma. Further mechanistic studies17,18,19 have demonstrated that microbiome-derived metabolites, such as short-chain fatty acids, can enhance the immune response to cancer cells or limit tumor growth. Early fecal microbiota transplant studies have shown promising signals in overcoming ICI response in metastatic melanoma,15,16 and ongoing diet intervention studies are aimed at microbiome modulation in this population.20,21 Despite these advances in the populations with metastatic disease, it is unclear whether microbiome modulation strategies may benefit patients with early-stage melanoma.
When considering the role of the microbiome in the melanoma continuum, dysbiosis has been noted in the skin microbiome, but the role of the gut microbiome has been relatively underexplored in melanoma initiation and progression.22,23,24,25 In this study, we compared differences in the fecal microbiota, including overall diversity, composition, and putative function, between healthy volunteers (n = 49) and patients with melanoma (n = 179). We then compared the microbiomes of patients with localized, early-stage melanoma (defined as melanoma in situ [stage 0], stage I, or stage II) and metastatic late-stage melanoma (defined as stage III or stage IV)26,27 and explored the baseline gut microbiota for associations with clinical outcomes in patients with stage III melanoma treated with adjuvant immunotherapy.
Methods
Study Participants
This case-control study received ethics approval from the MD Anderson Cancer Center Institutional Review Board. Written informed consent was obtained from all participants. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
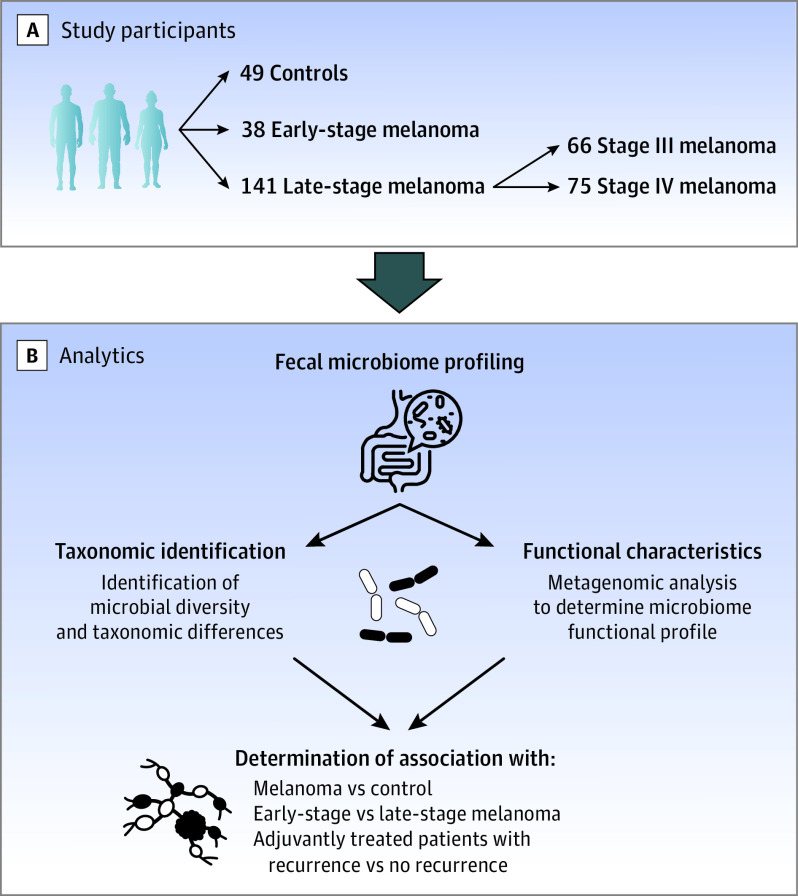
Patients with melanoma were identified from a large prospective cohort study of the fecal microbiome in melanoma (n = 438) at The University of Texas MD Anderson Cancer Center in Houston from June 1, 2015, to January 31, 2019 (Figure 1).2 Fecal samples were collected from systemic treatment−naive patients with stage I to IV melanoma from June 1, 2015, to January 31, 2019, and from healthy volunteers from June 1, 2021, to January 31, 2022. We included all patients from this cohort with early-stage (stage 0, I, or II) melanoma (n = 38) and matched these patients 1:2 with patients with late-stage melanoma (stage III or IV) (n = 141) based on age, sex, BMI, and race and ethnicity using hierarchical clustering with simple matching. Exclusion criteria included being younger than 18 years and having a history of melanoma treatment (eg, ICI, targeted therapy) prior to stool sample collection. Patient demographic characteristics (age, sex, and patient-reported race and ethnicity) and clinical and pathological data on melanoma diagnosis, staging, systemic treatment, concomitant medications used (proton pump inhibitors [PPIs], histamine H2-receptor antagonists, corticosteroids, hydroxymethyl glutaryl coenzyme A reductase inhibitors [statins], and antibiotics) were extracted from electronic medical records. Disease recurrence was determined by board-certified oncologists based on the results of staging studies performed as standard of care. Patients were followed up for disease outcomes (recurrence and death) from any cause through November 30, 2021.
Figure 1. Study Design to Assess Gut Microbiome Among Patients With Melanoma and Controls.
A, Study participants were enrolled from MD Anderson Cancer Center from a large prospective longitudinal study of the gut microbiome in melanoma, and controls were volunteers within the MD Anderson Cancer Center employee pool. B, Multiple analytic studies were performed on baseline stool samples, including taxonomic identification and functional characterization, which were used to determine association with various disease stages.
Control volunteers were employees of the same academic cancer center where patients with melanoma were evaluated and treated (Figure 1). Inclusion criteria were being 18 years or older and consenting to undergo fecal microbiome profiling prior to enrollment in a flu vaccine study.28
Specimen Collection and 16S Ribosomal RNA Gene Sequencing
Fecal specimens for the cohort with melanoma and the control volunteer studies were handled and analyzed with the same methods. Each participant was provided with a microbiome collection kit for fecal samples (OMNIgene GUT; DNA Genotek) for fecal sample collection. One baseline sample was collected for each patient (ie, prior to any systemic treatment for melanoma). Fecal samples were obtained in the outpatient setting and delivered for analysis via mail or clinic drop-off. After receipt, all samples were stored at −80°C prior to DNA extraction and 16S ribosomal RNA (rRNA) gene sequencing. The samples were amplified by polymerase chain reaction with primers 515F-806R that target the V4 region of the 16S SSU rRNA and sequenced using a commercially available platform (MiSeq; Illumina Inc) as previously described.29,30 To characterize the fecal microbiota profiles, measures of interest included the relative abundance of various microbial taxa, alpha diversity (variability within a single sample), and beta diversity (variability between different samples within a group). The bioinformatics software package PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States), version 2.4.2 (Python 3.8 package, Python programming language), was used to estimate the putative function of the microbes present.
Statistical Analysis
Descriptive statistics were used to characterize the clinical and pathologic parameters of the patient cohorts. Median and range were determined for continuous variables; frequency and percentage were used for categorical variables. For the adjuvant-treated patients with stage III melanoma, patients were categorized by recurrence (yes or no). The Wilcoxon rank-sum test was used to compare continuous variables with unknown distributions. The Fisher exact test was used to compare categorical variables.
Alpha diversity (ie, diversity within an individual sample) was assessed using the Inverse Simpson Index31 and then compared between groups (melanoma vs control, early-stage vs late-stage melanoma) using analysis of variance (ANOVA) with adjustment for potential confounding variables (age, sex, BMI, and concomitant medication). Race and ethnicity were included as a covariate in the analysis for patients with melanoma vs controls, as there was a significant difference in the racial diversity of our controls compared with our patients with melanoma. Race and ethnicity were not included in the covariate analysis between groups with melanoma, as the 2 groups were predominantly White. Beta diversity between different samples within each group was compared with Bray-Curtis distances as a measure of dissimilarity, visualized as 2-dimensional ordination plots from principal coordinate analysis. Permutational multivariate ANOVA (PERMANOVA) with Bray-Curtis distances were used to assess statistical significance between groups. Covariates identified as significant by PERMANOVA were then controlled for in differential abundance analysis using DESeq2, version 1.436.0 (R Project for Statistical Computing),32 which was used as a tool to identify differentially abundant genera between groups.33,34 We next focused on candidate taxa, which had previously been associated with response to ICI in a meta-analysis of previously published cohorts of patients with melanoma.12 Differential abundance of the candidate taxa was compared between groups using DESeq2 with adjustment for covariates identified as significant by PERMANOVA (eTable 1 in Supplement 1). Correction for multiple hypothesis testing was performed using false discovery rate adjustments (by Benjamini Hochberg-procedure) with a 2-sided P < .05 deemed statistically significant. Microbiome Multivariable Associations with Linear Models (MaAsLin2),35 version 1.12.0, used a negative binomial after data were normalized with the transfer matrix method to define the effect size of metabolic pathway associations between groups, after adjustment for covariates (age, BMI, sex, and medications). A 2-sided P < .05 was deemed statistically significant.
Microbiome data analyses were facilitated using the R package phyloseq,36 version 1.40.0 (R Project for Statistical Computing). Statistical analysis was performed using JMP Pro, version 15.0 (SAS Institute Inc), and R, version 4.2.1 (R Project for Statistical Computing).37
Results
Patient Characteristics
Relevant demographic and clinical characteristics of the 228 participants in the cohort (102 women [44.7%] and 126 men [55.3%]; median age, 59 [range, 21-90] years) are listed in the Table. The cohort with melanoma (n = 179) was predominantly White (177 [98.9%] vs 2 [1.1%] Asian) while the control cohort (n = 49) included 7 Asian individuals (14.3%), 27 White individuals (55.1%), and 15 individuals of unknown race or ethnicity (30.6%). Compared with patients with melanoma, controls were more likely to be women and younger, to have a lower BMI, and to be less likely to report use of PPIs and statins. Importantly, when systemic medication use in the 30 days prior to sample collection was assessed, no controls reported taking antibiotics or corticosteroids, whereas 65 patients with melanoma (36.3%) had recent antibiotic exposure and 12 (6.7%) had recent corticosteroid exposure. Within the melanoma cohort, the late-stage cohort was matched 2:1 to the early-stage cohort based on age, sex, BMI, and race and ethnicity. No statistically significant differences were noted in the rates of PPIs, metformin, or statin use between the melanoma groups, but significantly more patients with late-stage melanoma had antibiotic exposure (61 of 141 [43.3%] vs 4 of 38 [10.5%]; P < .001). More patients with late-stage melanoma had corticosteroid exposure within the last 30 days, but this did not reach statistical significance (12 of 141 [8.5%] vs 0; P = .07).
Table. Demographic and Clinical Characteristics of Patients With Melanoma and Controlsa.
| Characteristic | Controls (n = 49) | All patients with melanoma (n = 179) | P valueb | Patients with early-stage melanoma (n = 38) | Patients with late-stage melanoma (n = 141) | P valuec |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age, median (range), y | 40 (24-66) | 64 (21-90) | <.001 | 65 (23-82) | 64 (21-90) | .96 |
| Sex | ||||||
| Women | 34 (69.4) | 68 (38.0) | <.001 | 17 (44.7) | 51 (36.2) | .35 |
| Men | 15 (30.6) | 111 (62.0) | 21 (55.3) | 90 (63.8) | ||
| BMI, median (range) | 25.1 (16.2-45.4) | 28.6 (19.3-50.2)d | .002 | 28.0 (19.3-38.8) | 28.8 (19.6-50.2)d | .20 |
| Race and ethnicity | ||||||
| Asian | 7 (14.3) | 2 (1.1) | <.001 | 0 | 2 (1.4) | .99 |
| White | 27 (55.1) | 177 (98.9) | 38 (100) | 139 (98.6) | ||
| Missing | 15 (30.6) | 0 | 0 | 0 | ||
| Clinical | ||||||
| Medications used within 30 d | ||||||
| Antibiotics | 0 | 65 (36.3) | <.001 | 4 (10.5) | 61 (43.3) | <.001 |
| PPIs or H2-receptor antagonists | 3 (6.1) | 43 (24.0) | .01 | 6 (15.8) | 37 (26.2) | .21 |
| Metformin | 1 (2.0) | 15 (8.4) | .20 | 2 (5.3) | 13 (9.2) | .74 |
| Statins | 4 (8.2) | 58 (32.4) | <.001 | 12 (31.6) | 46 (32.6) | .99 |
| Corticosteroids | 0 | 12 (6.7) | .07 | 0 | 12 (8.5) | .07 |
| Stage | ||||||
| In situ | NA | 7 (3.9) | NA | 7 (18.4) | NA | <.001 |
| I | NA | 22 (12.3) | 22 (57.9) | NA | ||
| II | NA | 9 (5.0) | 9 (23.7) | NA | ||
| III | NA | 66 (36.9) | NA | 66 (46.8) | ||
| IV | NA | 75 (41.9) | NA | 75 (53.2) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); H2, histamine; NA, not applicable; PPI, proton pump inhibitor.
Unless otherwise indicated, data are expressed as No. (%) of participants.
Compares characteristics between controls and all patients with melanoma.
Compares characteristics between patients with early-stage and late-stage melanoma.
Missing BMI value for 1 patient with late-stage melanoma.
Microbiome Features of Patients With Melanoma vs Healthy Controls
We first compared microbiome features between patients with melanoma and control volunteers. We examined microbial diversity within samples (alpha diversity) using the Inverse Simpson Index, which describes the mean proportional abundance of identified taxa in a weighted fashion,38 and found the alpha diversity to be numerically higher in the controls though not statistically significant (median, 16.7 [IQR, 11.0-23.1] vs 11.4 [IQR, 7.4-18.1]; P = .06, ANOVA adjusted for covariates) (Figure 2A). We next examined beta diversity variability between different samples within a group. We found that controls, compared with patients with melanoma, formed distinct or distant communities (R2 = 0.0148; P < .001) (Figure 2B). We next sought to determine the differential abundance of particular taxa within each group using DESeq2 analysis to further interrogate differences observed in community structure, controlled for age and BMI. We found that the diversity differences were primarily driven by the members of the bacterial phyla Actinomycetota (Bifidobacterium, Collinsella, and Adlercreutzia) and Firmicutes (Eubacterium hallii and Streptococcus), all of which were more abundant in controls than patients with melanoma as a whole (Figure 2C).
Figure 2. Gut Microbial Diversity Between Patients With Melanoma and Controls.
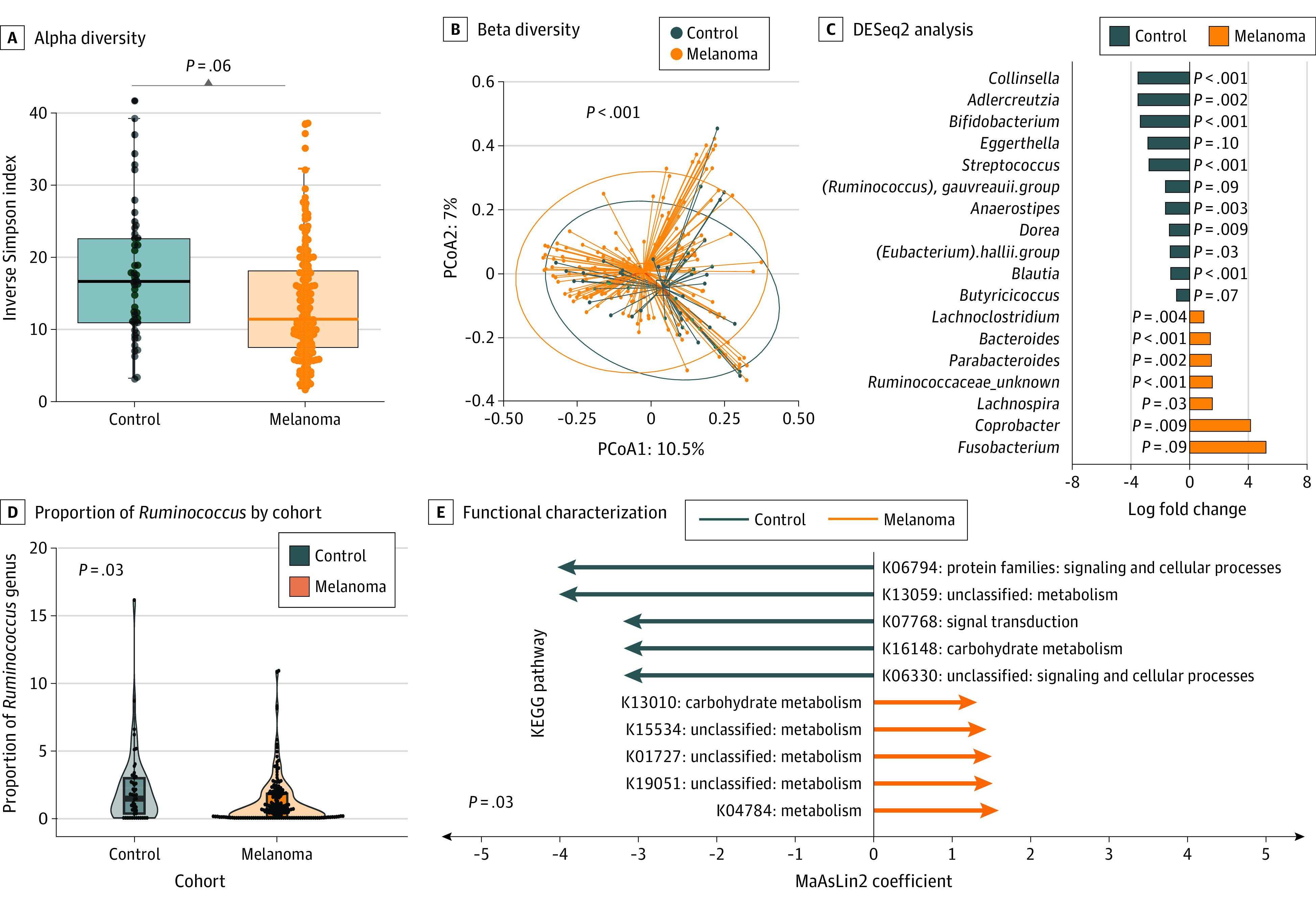
A, Alpha diversity was greater in controls than their melanoma counterparts (P = .06, analysis of variance [ANOVA] with covariates). Boxes represent the median Inverse Simpson Index and IQR; whiskers extend from minimum to maximum values up to 1.5 times above or below the IQR. B, Beta diversity was significantly different in controls compared with patients with melanoma (R2 = 0.0148; P <.001, permutational multivariate ANOVA using Bray-Curtis distance, diagnosis, age, BMI, race and ethnicity, and medications). Dots indicate individual samples within a 95% CI circle based on the t-distribution to represent the Euclidean distance from the centroid. PCoA1 indicates principal component analysis axis 1; PCoA2, principal component analysis axis 2. C, DESeq2 found diversity differences primarily driven by bacterial phyla Actinomycetota and Firmicutes (log fold change SE < 3; P < .10). D, The proportion of the genus Ruminococcus was significantly greater in controls than melanoma patients (adjusted P = .03). E, Functional characterization with PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) demonstrated numerous differentially enriched pathways between the 2 groups. KEGG indicates Kyoto Encyclopedia of Genes and Genomes; MaAsLin2, microbiome multivariable associations with linear models.
Interestingly, the bacterial genus Fusobacterium, which has been implicated in colorectal cancer carcinogenesis and gut inflammation,39,40,41,42 was enriched in our cohort with melanoma compared with controls in univariate analysis (0.19% vs 0.003%; P < .001), but this association was attenuated with adjustment for medications and other factors (log2 fold change of 5.18 vs controls; P = .09). Based on prior findings in this cohort implicating Ruminococcus in treatment response among patients with late-stage melanoma,3 we performed a targeted analysis and found that the prevalence and abundance of Ruminococcus was significantly higher in controls relative to our patients with melanoma (1.7% vs 1.4%; P = .03) (Figure 2E). To interrogate the metabolic functional potential of the microbiota present in these groups, we performed analyses with PICRUSt to putatively estimate the functional gene content based on the 16S data sets. Multiple functional pathways were differentially enriched between patients with melanoma and controls (Figure 2D).
Patients With Early-Stage vs Late-Stage Melanoma
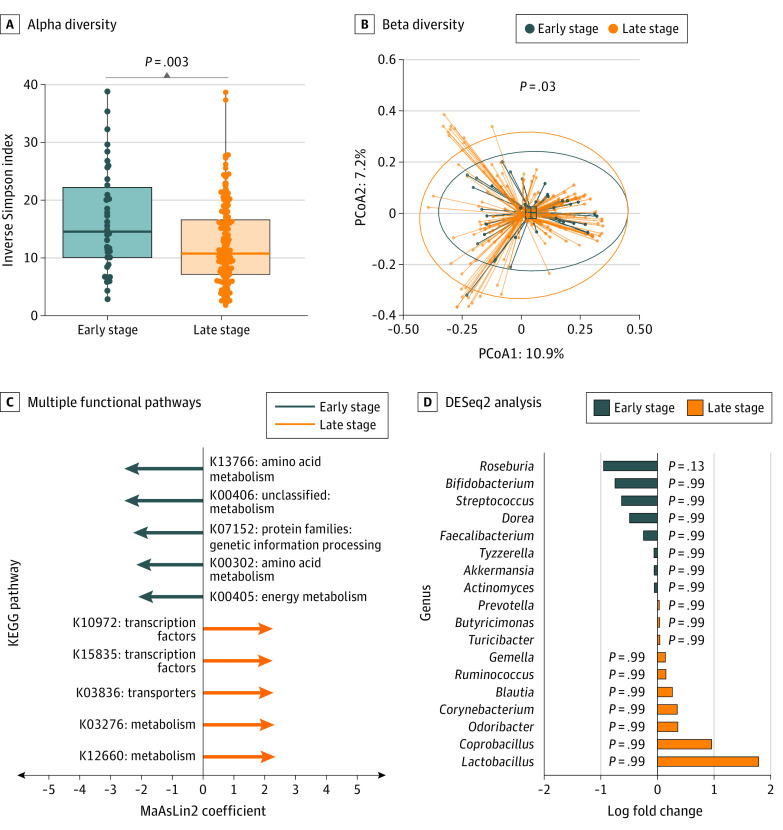
We next compared microbiome features in patients with early-stage melanoma with those of a matched set of systemic therapy–naive patients with late-stage melanoma. We observed significantly higher alpha diversity (median, 14.6 [IQR, 9.8-23.0] vs 10.8 [IQR, 7.2-16.8]; P = .003, adjusted for covariates) (Figure 3A) among patients with early-stage compared with late-stage melanoma, as well as differences in beta diversity (R2 = 0.0085; P = .03, adjusted for covariates with PERMANOVA) (Figure 3B). Putative functional pathway analysis obtained with PICRUSt indicated differential enrichment in multiple pathways between the 2 groups (Figure 3C). We then performed a targeted analysis of bacterial taxa previously associated with ICI response in prior patient cohorts with metastatic melanoma and also explored potential new candidates via DESeq2.43 We found a greater abundance of the bacterial genus Roseburia within the early-stage (2.4% vs 1.2%; P < .001) compared with the late-stage group in univariate analysis, though this was attenuated with covariate adjustment (log2 fold change of 0.86 vs controls; P = .13) (Figure 3D).
Figure 3. Gut Microbial Diversity Among Patients With Early-Stage vs Late-Stage Melanoma .
A, Gut microbiome profiling showed significantly greater alpha diversity (P = .003, analysis of variance [ANOVA] with covariates) in patients with early-stage melanoma. Boxes represent the median Inverse Simpson Index and IQR; whiskers extend from minimum to maximum values up to 1.5 times above or below the IQR. B, Beta diversity analysis showed a significant difference (P = .03, permutational multivariate ANOVA controlled for covariates). Dots indicate individual samples within a 95% CI circle based on the t-distribution to represent the Euclidean distance from the centroid. PCoA1 indicates principal component analysis axis 1; PCoA2, principal component analysis axis 2. C, Multiple functional pathways were found to be differentially enriched between early-stage and late-stage melanoma samples. MaAsLin2 indicates microbiome multivariable associations with linear models. D, DESeq2 analysis of known bacterial genera that affect response to immunotherapy was performed, with Roseburia demonstrating a nonsignificant higher proportion in patients with early-stage melanoma (P = .13, false discovery rate applied after filtering for selected genera). KEGG indicates Kyoto Encyclopedia of Genes and Genomes.
Microbiome and Disease Recurrence
Finally, we investigated baseline microbiome features (prior to treatment) among patients with stage III disease who were treated with adjuvant therapy and followed up for disease recurrence. Of these 66 patients, 20 experienced recurrence (30.3%) during follow-up (eTable 2 and eFigure, A, in Supplement 1). We observed higher alpha diversity in patients with recurrence (median, 15.1 [IQR, 7.1-19.3] vs 11.4 [IQR, 8.1-17.9]; P = .008) (eFigure, B, in Supplement 1) but with no difference in beta diversity (R2 = 0.016; P = .32) (eFigure, C, in Supplement 1). A targeted analysis of candidate taxa identified no significant differences in bacterial taxa abundance by outcome (eFigure, D, in Supplement 1).
Discussion
In this case-control study, we examined fecal microbiota profiles of healthy volunteers and systemic treatment–naive patients with melanoma and then further compared patients with either clinically localized (early-stage) vs metastatic (late-stage) melanoma to assess differences in microbiome diversity and composition. Alpha diversity was not statistically significantly different in patients with melanoma compared with controls but was lower in patients with late-stage compared with early-stage melanoma. Our group and others have demonstrated that gut microbiota diversity is associated with improved outcomes in patients with metastatic melanoma receiving immunotherapy.1,2,3,9,15 Lower diversity of the gut microbiome is often considered to be a dysbiotic state and has been associated with multiple disease states, including malignant neoplasms.44,45,46 Indeed, observed community-level differences, coupled with lower abundance of well-characterized gut commensals in patients with melanoma, such as Roseburia and Ruminococcus, suggest compromised balance or dysbiosis within the gut microbiome across melanoma initiation and progression. This hypothesis warrants continued follow-up in this and other prospective longitudinal studies following up patients with early-stage melanoma with serial microbiome collections over years in the presence or absence of recurrence or development of distant metastatic disease.
Interestingly, we identified the genus Fusobacterium to be differentially enriched among patients with melanoma compared with controls on initial univariate analysis. Fusobacterium has been previously implicated in colorectal cancer,40 upper aerodigestive squamous cell carcinoma,47,48,49 and breast cancer50 tumorigenesis and progression by activating the Wnt pathway and inhibiting antitumoral immune responses.51,52,53,54,55 In melanoma, Fusobacterium has been found to be enriched in porcine melanoma tumor tissue as well as in the fecal microbiomes of pigs with melanoma.22 Fusobacterium has also been implicated in inflammatory diseases both within the gut and beyond, inducing both gut and systemic inflammation,42 important features that also affect immunotherapy response and toxicity.43,56 Presence of this oral bacteria in the stool is generally an indicator of breakdown of the oral-gut niche, impaired gut-barrier integrity, and early dysbiosis.57,58 Future studies should examine the association between Fusobacterium in both the skin and gut with tumor progression and treatment response and toxicity.
While our study demonstrated enrichment of potentially pathogenic Fusobacterium in our melanoma cohort, volunteer control profiles were characterized by the bacterial phyla Actinomycetota and Firmicutes, both of which are involved in gut homeostasis. This includes the genus Bifidobacterium (in Actinomycetota) that plays a key role in maintaining the intestinal mucosal barrier59 and Ruminococcus (in Firmicutes) and which was previously demonstrated to be enriched in immunotherapy responders.3 These bacterial taxa appear to be associated with a balanced and diverse gut microbiome and are active targets of therapeutic investigation. In a preclinical model, oral administration of Bifidobacterium alone improved local tumor control to the same degree as anti–programmed cell death ligand 1 (PD-L1) monotherapy; when combined with anti–PD-L1 treatment, synergistic response was demonstrated.60 Both Bifidobacterium and dietary fiber maintain the quality of microbiota-mediated colonic mucus, a protective layer between the gut microbiome and intestinal epithelium.61 In aggregate, these differences in gut microbiota structure and composition between patients with melanoma and controls suggest that the patients have a relative dysbiosis, though this needs to be confirmed in large case-control studies matched for critical differences in patient demographics and exposures.
When comparing imputed pathways between the microbiomes of the cohorts with early-stage and late-stage melanoma, the late-stage melanoma cohort demonstrated enrichment of pathways related to bacterial cell wall components and carbohydrate metabolism, while the early-stage group showed an enrichment of pathways related to energy harvest and cell growth. At a taxonomic level, Roseburia appeared to be depleted in patients with late-stage compared with early-stage melanoma. Notably, Roseburia has been associated with immunotherapy response and is one of the most abundant butyrate-producing anaerobic bacteria in the human gut.5,12,62,63 Butyrate is a critical metabolite for gut barrier homeostasis and has been shown to epigenetically enhance CD8+ T-cell responses in vitro and in vivo,64 and supplementation with Roseburia decreases colorectal cancer burden in preclinical models.65
We did not identify any significant differences in baseline microbiome taxa features among our patients with stage III melanoma when stratified by disease recurrence aside from increased alpha diversity in those with recurrence. In this limited cohort, we did not observe associations between outcome and clinicopathological factors (eTable 1 in Supplement 1), which may be due to the limited sample size or limited events within the follow-up period. The association between the microbiome and recurrence-free survival with adjuvant immunotherapy should be investigated in larger, adequately powered cohorts before further conclusions can be drawn.
Limitations
This study has limitations. While we were able to enroll a large number of patients with late-stage melanoma within this study, the limited sample size of other subcohorts among our participants with early-stage melanoma and controls precluded thorough comparisons among these groups, particularly with adjustment for medications and other confounding factors. Residual (unmeasured) confounding is a clear limitation in this study and many observational microbiome studies,2,4,5,7,8 as we are unable to control for diet, lifestyle, and medication use within each of our cohorts. Although our patients with early-stage compared with late-stage melanoma were matched on key factors and were recruited from the same clinical area, this was not possible in our control cohort. Microbiome specimens from our control cohort were recruited from the same institution and handled and analyzed in the same way, but despite this, our nonmelanoma cohort was inherently different from our melanoma cohort, and therefore our findings must be framed in this context. Furthermore, the limited sample size and follow-up of our adjuvant cohort challenged our ability to explore associations of the gut microbiome in patients with stage III melanoma followed up for recurrence.
Conclusions
In this case-control study, gut microbiome profiles varied between systemic treatment–naive patients with melanoma of different disease stages and controls. Between these groups, we observed differences in candidate taxa implicated in other melanoma cohorts, including Roseburia, Bifidobacterium, Ruminoccocus, and Fusobacterium.5,10,62,63 These findings suggest that gut dysbiosis may be linked to melanomagenesis and disease progression. Further investigation is needed to confirm these findings and determine whether modifying the gut microbiome could influence melanoma development and progression.
eTable 1. Permutational Multivariate Analysis of Variance (PERMANOVA) Results for All Relevant Covariates per Cohorts Tested
eTable 2. Characteristics of Patients With Melanoma Who Received Adjuvant Treatment After Excision
eFigure. Comparison of the Gut Microbiome of Patients With Stage III With Disease Recurrence Following Adjuvant Immune Checkpoint Inhibition
Data Sharing Statement
References
- 1.McQuade JL, Daniel CR, Helmink BA, Wargo JA. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019;20(2):e77-e91. doi: 10.1016/S1470-2045(18)30952-5 [DOI] [PubMed] [Google Scholar]
- 2.Spencer CN, McQuade JL, Gopalakrishnan V, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632-1640. doi: 10.1126/science.aaz7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97-103. doi: 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frankel AE, Coughlin LA, Kim J, et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia. 2017;19(10):848-855. doi: 10.1016/j.neo.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104-108. doi: 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368-1379. doi: 10.1093/annonc/mdx108 [DOI] [PubMed] [Google Scholar]
- 7.Peters BA, Wilson M, Moran U, et al. Relating the gut metagenome and metatranscriptome to immunotherapy responses in melanoma patients. Genome Med. 2019;11(1):61. doi: 10.1186/s13073-019-0672-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riquelme E, Zhang Y, Zhang L, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178(4):795-806.e12. doi: 10.1016/j.cell.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018;15(6):382-396. doi: 10.1038/s41571-018-0006-2 [DOI] [PubMed] [Google Scholar]
- 10.Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33(4):570-580. doi: 10.1016/j.ccell.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews MC, Duong CPM, Gopalakrishnan V, et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med. 2021;27(8):1432-1441. doi: 10.1038/s41591-021-01406-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KA, Thomas AM, Bolte LA, et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat Med. 2022;28(3):535-544. doi: 10.1038/s41591-022-01695-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson RC, Shanahan ER, Batten M, et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat Med. 2022;28(11):2344-2352. doi: 10.1038/s41591-022-01965-2 [DOI] [PubMed] [Google Scholar]
- 14.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350-1355. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baruch EN, Youngster I, Ben-Betzalel G, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602-609. doi: 10.1126/science.abb5920 [DOI] [PubMed] [Google Scholar]
- 16.Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science. 2021;371(6529):595-602. doi: 10.1126/science.abf3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nomura M, Nagatomo R, Doi K, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open. 2020;3(4):e202895. doi: 10.1001/jamanetworkopen.2020.2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luu M, Riester Z, Baldrich A, et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat Commun. 2021;12(1):4077. doi: 10.1038/s41467-021-24331-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Tinoco R, Elmén L, et al. Gut microbiota dependent anti-tumor immunity restricts melanoma growth in Rnf5−/− mice. Nat Commun. 2019;10(1):1492. doi: 10.1038/s41467-019-09525-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Effect of diet on the immune system in patients with stage III-IV melanoma receiving immunotherapy, DIET study (DIET). ClinicalTrials.gov identifier: NCT04645680. Updated April 6, 2023. Accessed July 31, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT04645680
- 21.The effect of diet and exercise on immunotherapy and the microbiome (EDEN). ClinicalTrials.gov identifier: NCT04866810. Updated July 13, 2023. Accessed July 31, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT04866810
- 22.Mekadim C, Skalnikova HK, Cizkova J, et al. Dysbiosis of skin microbiome and gut microbiome in melanoma progression. BMC Microbiol. 2022;22(1):63. doi: 10.1186/s12866-022-02458-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16(3):143-155. doi: 10.1038/nrmicro.2017.157 [DOI] [PubMed] [Google Scholar]
- 24.Camara NOS. Do the gut and skin microbiomes share thoughts on melanoma development? Br J Dermatol. 2022;186(1):12-13. doi: 10.1111/bjd.20811 [DOI] [PubMed] [Google Scholar]
- 25.Vitali F, Colucci R, Di Paola M, et al. Early melanoma invasivity correlates with gut fungal and bacterial profiles. Br J Dermatol. 2022;186(1):106-116. doi: 10.1111/bjd.20626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gershenwald JE, Scolyer RA, Hess KR, et al. ; for members of the American Joint Committee on Cancer Melanoma Expert Panel and the International Melanoma Database and Discovery Platform . Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472-492. doi: 10.3322/caac.21409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gershenwald JE Sr, Scolyer RA, Hess KR, Thompson JF. Melanoma of the Skin. Springer Cham; 2017. doi: 10.1007/978-3-319-40618-3_47 [DOI] [Google Scholar]
- 28.Association between gut microbiome and dietary determinants and vaccine response. ClinicalTrials.gov identifier: NCT05239403. Updated February 14, 2023. Accessed March 7, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT05239403
- 29.Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621-1624. doi: 10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minich JJ, Zhu Q, Janssen S, et al. KatharoSeq enables high-throughput microbiome analysis from low-biomass samples. mSystems. 2018;3(3):e00218-e00217. doi: 10.1128/mSystems.00218-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537-7541. doi: 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Differential gene expression analysis based on the negative binomial distribution. February 22, 2021. Accessed January 5, 2023. https://rdrr.io/bioc/DESeq2/
- 33.Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr. 1957;27(4):325-349. doi: 10.2307/1942268 [DOI] [Google Scholar]
- 34.Lin H, Peddada SD. Analysis of compositions of microbiomes with bias correction. Nat Commun. 2020;11(1):3514. doi: 10.1038/s41467-020-17041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mallick H, Rahnavard A, McIver LJ, et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput Biol. 2021;17(11):e1009442. doi: 10.1371/journal.pcbi.1009442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.phyloseq: handling and analysis of high-throughput microbiome census data. November 8, 2020. Accessed December 18, 2022. https://rdrr.io/bioc/phyloseq/
- 37.R Project for Statistical Computing . Index of/src/base/R-4. October 31, 2022. Accessed January 5, 2023. https://cran.r-project.org/src/base/R-4/
- 38.Simpson EH. Measurement of diversity. Nature. 1949;163(4148):688. doi: 10.1038/163688a0 [DOI] [Google Scholar]
- 39.Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65(12):1973-1980. doi: 10.1136/gutjnl-2015-310101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(3):548-563.e16. doi: 10.1016/j.cell.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mima K, Sukawa Y, Nishihara R, et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015;1(5):653-661. doi: 10.1001/jamaoncol.2015.1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bashir A, Miskeen AY, Hazari YM, Asrafuzzaman S, Fazili KM. Fusobacterium nucleatum, inflammation, and immunity: the fire within human gut. Tumour Biol. 2016;37(3):2805-2810. doi: 10.1007/s13277-015-4724-0 [DOI] [PubMed] [Google Scholar]
- 43.McCulloch JA, Davar D, Rodrigues RR, et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti–PD-1. Nat Med. 2022;28(3):545-556. doi: 10.1038/s41591-022-01698-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yonekura S, Terrisse S, Alves Costa Silva C, et al. Cancer induces a stress ileopathy depending on β-adrenergic receptors and promoting dysbiosis that contributes to carcinogenesis. Cancer Discov. 2022;12(4):1128-1151. doi: 10.1158/2159-8290.CD-21-0999 [DOI] [PubMed] [Google Scholar]
- 45.Huttenhower C, Gevers D, Knight R, et al. ; Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207-214. doi: 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin J, Li R, Raes J, et al. ; MetaHIT Consortium . A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59-65. doi: 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neuzillet C, Marchais M, Vacher S, et al. Prognostic value of intratumoral Fusobacterium nucleatum and association with immune-related gene expression in oral squamous cell carcinoma patients. Sci Rep. 2021;11(1):7870. doi: 10.1038/s41598-021-86816-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang CY, Yeh YM, Yu HY, et al. Oral microbiota community dynamics associated with oral squamous cell carcinoma staging. Front Microbiol. 2018;9:862. doi: 10.3389/fmicb.2018.00862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujiwara N, Kitamura N, Yoshida K, Yamamoto T, Ozaki K, Kudo Y. Involvement of Fusobacterium species in oral cancer progression: a literature review including other types of cancer. Int J Mol Sci. 2020;21(17):6207. doi: 10.3390/ijms21176207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parhi L, Alon-Maimon T, Sol A, et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat Commun. 2020;11(1):3259. doi: 10.1038/s41467-020-16967-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292-298. doi: 10.1101/gr.126573.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299-306. doi: 10.1101/gr.126516.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li YY, Ge QX, Cao J, et al. Association of Fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World J Gastroenterol. 2016;22(11):3227-3233. doi: 10.3748/wjg.v22.i11.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195-206. doi: 10.1016/j.chom.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abed J, Emgård JE, Zamir G, et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe. 2016;20(2):215-225. doi: 10.1016/j.chom.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brennan CA, Garrett WS. Fusobacterium nucleatum: symbiont, opportunist and oncobacterium. Nat Rev Microbiol. 2019;17(3):156-166. doi: 10.1038/s41579-018-0129-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The HC, Florez de Sessions P, Jie S, et al. Assessing gut microbiota perturbations during the early phase of infectious diarrhea in Vietnamese children. Gut Microbes. 2018;9(1):38-54. doi: 10.1080/19490976.2017.1361093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pop M, Walker AW, Paulson J, et al. Diarrhea in young children from low-income countries leads to large-scale alterations in intestinal microbiota composition. Genome Biol. 2014;15(6):R76. doi: 10.1186/gb-2014-15-6-r76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinzone MR, Celesia BM, Di Rosa M, Cacopardo B, Nunnari G. Microbial translocation in chronic liver diseases. Int J Microbiol. 2012;2012:694629. doi: 10.1155/2012/694629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084-1089. doi: 10.1126/science.aac4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schroeder BO, Birchenough GMH, Ståhlman M, et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe. 2018;23(1):27-40.e7. doi: 10.1016/j.chom.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duncan SH, Holtrop G, Lobley GE, Calder AG, Stewart CS, Flint HJ. Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr. 2004;91(6):915-923. doi: 10.1079/BJN20041150 [DOI] [PubMed] [Google Scholar]
- 63.Hold GL, Schwiertz A, Aminov RI, Blaut M, Flint HJ. Oligonucleotide probes that detect quantitatively significant groups of butyrate-producing bacteria in human feces. Appl Environ Microbiol. 2003;69(7):4320-4324. doi: 10.1128/AEM.69.7.4320-4324.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He Y, Fu L, Li Y, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 2021;33(5):988-1000.e7. doi: 10.1016/j.cmet.2021.03.002 [DOI] [PubMed] [Google Scholar]
- 65.Montalban-Arques A, Katkeviciute E, Busenhart P, et al. Commensal Clostridiales strains mediate effective anti-cancer immune response against solid tumors. Cell Host Microbe. 2021;29(10):1573-1588.e7. doi: 10.1016/j.chom.2021.08.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Permutational Multivariate Analysis of Variance (PERMANOVA) Results for All Relevant Covariates per Cohorts Tested
eTable 2. Characteristics of Patients With Melanoma Who Received Adjuvant Treatment After Excision
eFigure. Comparison of the Gut Microbiome of Patients With Stage III With Disease Recurrence Following Adjuvant Immune Checkpoint Inhibition
Data Sharing Statement