Abstract
Background
Bacterial vaginosis (BV) is a common vaginal dysbiosis that often recurs following first-line antibiotics. We investigated if vaginal microbiota composition was associated with BV recurrence.
Methods
We analyzed samples and data from 121 women who participated in 3 published trials evaluating novel interventions for improving BV cure, including concurrent antibiotic treatment of regular sexual partners (RSPs). Women diagnosed with BV received first-line antibiotics and self-collected vaginal swabs pretreatment and the day after finishing antibiotics (immediately posttreatment). 16S rRNA gene sequencing was performed on vaginal samples. Logistic regression explored associations between BV recurrence and features of the vaginal microbiota pre- and posttreatment.
Results
Sixteen women (13% [95% confidence interval {CI}, 8%–21%]) experienced BV recurrence within 1 month of treatment. Women with an untreated RSP were more likely to experience recurrence than women with no RSP (P = .008) or an RSP who received treatment (P = .011). A higher abundance of Prevotella pretreatment (adjusted odds ratio [AOR], 1.35 [95% CI, 1.05–1.91]) and Gardnerella immediately posttreatment (AOR, 1.23 [95% CI, 1.03–1.49]) were associated with increased odds of BV recurrence.
Conclusions
Having specific Prevotella spp prior to recommended treatment and persistence of Gardnerella immediately posttreatment may contribute to the high rates of BV recurrence. Interventions that target these taxa are likely required to achieve sustained BV cure.
Keywords: bacterial vaginosis, Gardnerella, Prevotella, regular sexual partner, vaginal microbiota
Specific Prevotella and Gardnerella species may contribute to the high rates of treatment failure following recommended antibiotic therapy for bacterial vaginosis. Likely mechanisms of treatment failure include persistence of a multispecies biofilm and reinfection from an untreated sexual partner.
Bacterial vaginosis (BV) is a common vaginal condition that affects approximately 30% of reproductive-aged women globally [1]. While the etiology of BV has not been established, the vaginal microbiota of women with BV is typically deficient in optimal Lactobacillus spp (eg, Lactobacillus crispatus), with high diversity and high abundance of anaerobes [2]. Recommended treatment for BV involves antibiotic therapy with metronidazole or clindamycin, both of which provide broad coverage of anaerobes. One-month cure posttreatment is approximately 70%–85% [3]; however, up to 50% of women experience BV recurrence within 12 weeks following antibiotics [4]. BV recurrences are distressing for women [5] and result in repeat presentations to clinical services and repeated antibiotic use. Furthermore, the sequelae associated with BV carry significant implications for a woman's reproductive and sexual health and impose a large economic burden [1].
There has been little progress made toward improving long-term BV cure, partly because the mechanisms driving BV recurrence are multifactorial and incompletely understood [6]. Prior studies exploring the association between microbial signatures of the vaginal microbiota (ie, diversity metrics, community types, and individual taxa) and BV treatment outcomes [7–14] have produced mixed results regarding the contribution of bacterial diversity to treatment failure. Interestingly, a number of studies have reported a positive association between BV recurrence and high relative and absolute abundance of Gardnerella (or individual Gardnerella spp) pretreatment [10–12]. Furthermore, treatment success has been associated with significant reductions in Gardnerella spp posttreatment [7, 11]. Together these data highlight the importance of Gardnerella spp to BV pathogenesis [15]. However, prior studies have been limited by small numbers, and few have adjusted microbiota findings for confounding factors that may influence an individual's likelihood of recurrence [11, 13], such as posttreatment sexual practices [6].
To overcome limitations of past studies and to provide insight into the microbial drivers of BV recurrence, we aimed to determine if the vaginal microbiota composition immediately before or immediately after treatment was associated with BV recurrence within 1 month of recommended treatment, after adjustment for confounding factors.
METHODS
Study Populations and Study Procedures
We conducted a secondary analysis of vaginal microbiota data and accompanying clinical and behavioral data collected from women who participated in 3 clinical trials conducted at the Melbourne Sexual Health Centre (MSHC), Australia, that evaluated new interventions for improving BV cure: Strategies to prevent BV (SToPBV), StepUp-1, and StepUp-2. The parent trials have been described elsewhere [16–18]. In brief, SToPBV was an open-label randomized controlled trial of combined oral contraceptive pill use following first-line antibiotic treatment to prevent BV recurrence [16]. StepUp-1 and StepUp-2 were open-label single-arm pilot studies that assessed the acceptability and tolerability of concurrent male partner treatment for BV and its impact on the genital microbiota of couples [17, 18]. The Supplementary Material details the interventions assessed in the parent studies.
Across the 3 studies, women presenting to MSHC were eligible to participate if they were diagnosed with BV (defined as ≥3 Amsel criteria and Nugent score [NS] = 4–10) and received first-line treatment (oral metronidazole 400 mg twice daily for 7 days, or if contraindicated, clindamycin 2% vaginal cream applied intravaginally nocte for 7 nights or metronidazole 0.75% gel applied intravaginally nocte for 5 nights). Additional study-specific eligibility criteria were applied [16–18]. Of note, women were eligible for SToPBV if they were not currently using hormonal contraception and had no combined oral contraceptive pill–related contraindications. Women were eligible for the StepUp studies if they had a male regular sexual partner (RSP) of ≥2 months who was willing to receive treatment and had no additional sexual partners.
Women self-collected a high-vaginal swab for microbiota characterization and completed questionnaires concerning demographic, clinical, and behavioral information prior to commencing antibiotics at day 0 (termed pretreatment) and the day after finishing antibiotics at day 8 (termed immediately posttreatment). Posttreatment, women were followed longitudinally, with the follow-up duration differing between studies: Women were followed for 6 months postenrollment in SToPBV, 1 month in StepUp-1, and 3 months in StepUp-2. However, the current study only used samples collected at day 0 and day 8 and metadata collected up to 1 month postenrollment (Supplementary Figure 1). At 1 month postenrollment, women completed a questionnaire and self-collected a vaginal smear at home that was assessed for BV using NS criteria. Women who had NS = 7–10 on a home smear or who experienced symptoms were encouraged to attend MSHC for BV assessment using combined Amsel and NS criteria.
Ethical approval was obtained from the Human Research and Ethics Committee of the Alfred Hospital, Melbourne, Australia [16–18]. All participants provided informed written consent.
Study Outcome
The study outcome for the current study was BV recurrence within 1 month of enrollment (recurrence cases) or reaching 1 month without recurrence (cure cases). Recurrence was defined as ≥3 Amsel criteria and NS = 4–10. If a woman was unable to return to clinic, a NS = 7–10 on a home smear was accepted as a BV diagnosis. We investigated the association between BV recurrence and bacterial diversity and composition of the vaginal microbiota at 2 timepoints: (i) pretreatment at day 0 and (ii) immediately posttreatment at day 8. Women were eligible for inclusion if they provided a day 0 and/or day 8 microbiota sample and reached the study endpoint. If a woman provided only a day 0 or a day 8 sample they could still be included in the study, but they only contributed to the analysis at 1 timepoint. Of the 95 women recruited to SToPBV and 73 women recruited to the StepUp studies, 121 women (72 from SToPBV, 49 from StepUp) were eligible for inclusion in the current study.
Microbiota Characterization
Sample storage, DNA extraction, amplification, and sequencing methodology are described in detail in the original publications [17–19]. Laboratory methods applied to SToPBV and StepUp-1 samples were identical, whereas aspects of the methodology for StepUp-2 (including DNA extraction and library preparation methods) differed. The vaginal microbiota was characterized by 16S ribosomal RNA (rRNA) gene sequencing of the V3–V4 regions using the Illumina MiSeq platform. DADA2 v1.20.0 was used for quality filtering, inferring amplicon sequence variants, chimera identification, merging of paired end reads and taxonomic assignment, as described previously [18]. Laboratory and bioinformatics methods are described in the Supplementary Material.
Statistical Analysis
We compared demographic, contraceptive, and behavioral characteristics between the 2 study populations (SToPBV and StepUp), and between recurrence and cure cases. χ2, Fisher exact, and Wilcoxon rank-sum tests were used where appropriate.
Diversity and ordination analyses were performed using vegan software v2.6.2. Nonmetric multidimensional scaling (NMDS) and analysis of similarity (ANOSIM), utilizing the Bray–Curtis metric, were used to visualize and test for differences in the pretreatment and immediate posttreatment composition of the vaginal microbiota between recurrence and cure cases. α-diversity was calculated using the Shannon diversity index. Bray–Curtis distances calculated between paired pretreatment and posttreatment samples provided a measure of compositional change of the vaginal microbiota following treatment. We used logistic regression models to examine the association between BV recurrence and (i) α-diversity of the pre- and posttreatment vaginal microbiota, as well as (ii) compositional change posttreatment. Models were adjusted for laboratory method to account for potential confounding due to differences in the methods used for SToPBV and StepUp-1 versus StepUp-2 (see Supplementary Material). Models were also adjusted for sexual partner type and their treatment status (no RSP vs treated RSP vs untreated RSP), as this characteristic was found to differ between recurrence and cure cases (see Results). We did not adjust models for study (SToPBV/StepUp-1/StepUp-2), as this variable was highly correlated with sexual partner type and treatment status (Cramer V = 0.7071).
Analysis of compositions of microbiomes with bias correction (ANCOM-BC) version 1.6.2 [20] was used to identify differentially abundant taxa between recurrence and cure cases pre- and immediately posttreatment. ANCOM-BC analyses were adjusted for laboratory method and taxa that were present in ≤10% of samples were excluded. Bacterial taxa identified as differentially abundant by ANCOM-BC with a P value <.05 were explored further using logistic regression. Logistic regression was used to examine the association between BV recurrence and the centered log-ratio (CLR)–transformed abundance of each taxon, adjusting for laboratory method and partner type/treatment status.
Finally, using an approach similar to Lee et al [9], we explored the relationship between BV recurrence and the ratio of nonoptimal to optimal bacteria present in the vagina pre- and posttreatment. The following ratios were examined: (i) total BV-associated bacteria to Lactobacillus spp (BVAB:Lactobacillus), (ii) Gardnerella to Lactobacillus iners (Gardnerella:L iners) and (iii) total pathobionts to Lactobacillus spp (pathobionts:Lactobacillus). Pathobionts are organisms that may become inflammatory and pathogenic under specific conditions, and high pretreatment pathobiont load has been associated with metronidazole treatment failure [12]. Supplementary Table 1 lists the bacterial taxa included in each group. We calculated ratios using log10-transformed relative abundance data and evaluated the association between BV recurrence and bacterial ratios using logistic regression models, adjusted as above.
RStudio version 2022.02.2 software running R version 4.2.0 was used for analyses.
RESULTS
Participant Characteristics and Factors Associated With BV Recurrence
Table 1 summarizes baseline characteristics and sexual practices of the 121 women included in analyses. The pooled cohort of women had a median age of 28 years (interquartile range, 25–32 years). Approximately half of participants were born in Australia or New Zealand (56/121 [46%]), 24% were born in Europe (most commonly the United Kingdom and Ireland), and 22% were born in Asia (most commonly China, Hong Kong, Taiwan, and Southeast Asia). At baseline, 87 women (72%) reported having an RSP (most of whom were male [83/87]), 26 (21%) reported hormonal contraceptive use, and 13 (11%) had an intrauterine device in situ. Most women (89/121 [74%]) had a history of BV. Some characteristics differed between the StepUp and SToPBV cohorts (ie, having an RSP at baseline, baseline contraceptive practices, and posttreatment sexual practices; Table 1), which was primarily due to differences in the eligibility criteria applied in each study.
Table 1.
Demographic, Contraceptive, and Behavioral Characteristics of the Study Population (N = 121)
| Characteristic | Total (N = 121) |
StepUpa (n = 49) |
SToPBV (n = 72) |
P Valueb |
|---|---|---|---|---|
| Characteristics reported at baseline | ||||
| Age, y, median (IQR) | 28 (25–32) | 29 (26–33) | 28 (24–30) | .064 |
| Region of birth | ||||
| Australia/New Zealand | 56 (46) | 25 (51) | 31 (43) | .960 |
| Asia | 27 (22) | 11 (22) | 16 (22) | |
| Europe | 29 (24) | 10 (20) | 19 (26) | |
| Middle East or Africa | 3 (2) | 1 (2) | 2 (3) | |
| North America | 3 (2) | 1 (2) | 2 (3) | |
| South America | 3 (2) | 1 (2) | 2 (3) | |
| Past history of BV | ||||
| No | 32 (26) | 9 (18) | 23 (32) | .096 |
| Yes | 89 (74) | 40 (82) | 49 (68) | |
| Any hormonal contraceptive use | ||||
| No | 95 (79) | 23 (47) | 72 (100) | <.001 |
| Yes | 26 (21) | 26 (53)c | 0 (0) | |
| Any IUD use | ||||
| No | 108 (89) | 36 (73) | 72 (100) | <.001 |
| Yes | 13 (11) | 13 (27)d | 0 (0) | |
| Any smoking | ||||
| No | 75 (63) | 38 (79) | 37 (51) | .002 |
| Yes | 45 (38) | 10 (21) | 35 (49) | |
| Lifetime No. of male partners, median (IQR) | 15 (7–25) | 15 (7–30) | 15 (7–23) | .547 |
| Lifetime No. of female partners, median (IQR) | 0 (0–1) | 0 (0–0) | 0 (0–2) | .328 |
| Current RSP | ||||
| No | 34 (28) | 0 (0) | 34 (47) | <.001 |
| Yese | 87 (72) | 49 (100) | 38 (53) | |
| Characteristics between day 0 and study endpoint | ||||
| Any hormonal contraceptive use | ||||
| No | 64 (53) | 23 (47) | 41 (57) | .279 |
| Yes | 57 (47) | 26 (53) | 31 (43) | |
| Any smoking | ||||
| No | 76 (63) | 38 (79) | 38 (53) | .003 |
| Yes | 44 (37) | 10 (21) | 34 (47) | |
| Any penile–vaginal sex | ||||
| No | 29 (24) | 2 (4) | 27 (37.5) | <.001 |
| Yes | 92 (76) | 47 (96) | 45 (62.5) | |
| Condom use for penile–vaginal sex | ||||
| Always/no penile–vaginal sex | 44 (36) | 2 (4) | 42 (58) | <.001 |
| Not always | 77 (64) | 47 (96) | 30 (42) | |
| Any receptive oral sex | ||||
| No | 47 (39) | 11 (22) | 36 (50) | .002 |
| Yes | 74 (61) | 38 (78) | 36 (50) | |
| Any anal sex | ||||
| No | 105 (87) | 41 (84) | 64 (89) | .406 |
| Yes | 16 (13) | 8 (16) | 8 (11) | |
| Any sex with RSP | ||||
| No | 47 (39) | 3 (6) | 44 (61) | <.001 |
| Yes | 74 (61) | 46 (94) | 28 (39) | |
| Any new sexual partner | ||||
| No | 98 (81) | 47 (96) | 51 (71) | <.001 |
| Yes | 23 (19) | 2 (4) | 21 (29) | |
| Gender of sexual partner(s) during follow-up | ||||
| Male | 91 (91) | 48 (98) | 43 (84) | .051 |
| Female | 4 (4) | 0 | 4 (8) | |
| Male and female | 1 (2) | 1 (2) | 4 (8) | |
Data are presented as No. (%) unless otherwise indicated. Missing data from up to 9 women for some variables. P values <.05 are bolded to indicate statistically significant associations.
Abbreviations: BV, bacterial vaginosis; IQR, interquartile range; IUD, intrauterine device; RSP, regular sexual partner; SToPBV, Strategies to prevent BV.
Women from StepUp-1 and StepUp-2 were combined as the eligibility criteria applied in these studies were identical and participant characteristics did not differ between the 2 studies.
The χ2 or Fisher exact test was used for categorical data, and Wilcoxon signed-rank test was used for continuous variables to examine differences between StepUp and SToPBV participants.
At baseline, 13 women reported current oral contraceptive use, 6 reported using a levonorgestrel IUD, 6 reported using a contraceptive implant, and 1 reported using a hormonal injection (Depo-Provera).
At baseline, 6 women reported using a levonorgestrel IUD and 7 women reported using a copper IUD.
Four women in SToPBV reported a female RSP at baseline; all other women reported a male RSP.
Of the 121 women, 16 (13% [95% confidence interval {CI}, 8%–21%]) experienced BV recurrence. Only 1 characteristic differed significantly between recurrence and cure cases (Table 2). Women who reported sex with an untreated RSP during follow-up were significantly more likely to experience BV recurrence compared to women who had no RSP (9/28 [32%] vs 3/44 [7%], P = .008) and women who had an RSP treated through StepUp-1/2 (9/28 [32%] vs 4/49 [8%], P = .011). Interestingly, there was no difference in the proportion of women who experienced recurrence between those with a treated RSP and those with no RSP (4/49 vs 3/44, P = 1.000). No other characteristics, including treatment prescribed, treatment adherence, or history of BV, differed significantly between recurrence and cure cases.
Table 2.
Baseline and Longitudinal Characteristics by Bacterial Vaginosis Recurrence Status (n = 121)
| Characteristic | Cure (n = 105) | Recurrence (n = 16) | P Valuea |
|---|---|---|---|
| Study | |||
| StepUp studies | 45 (43) | 4 (25) | .274 |
| SToPBV | 60 (57) | 12 (75) | |
| Past history of BV | |||
| No | 30 (29) | 2 (12) | .232 |
| Yes | 75 (71) | 14 (88) | |
| Antibiotic prescribed | |||
| Oral metronidazole | 92 (88) | 14 (87.5) | 1.000 |
| Intravaginal clindamycin | 12 (11) | 2 (12.5) | |
| Intravaginal metronidazole | 1 (1) | 0 (0) | |
| 100% treatment adherenceb | |||
| No | 10 (10) | 2 (12) | .662 |
| Yes | 94 (90) | 14 (88) | |
| Characteristics between day 0 and study endpoint | |||
| Any hormonal contraceptive usec | |||
| No | 55 (52) | 9 (56) | .796 |
| Yes | 50 (48) | 7 (44) | |
| Any IUD used | |||
| No | 94 (90) | 13 (81) | .396 |
| Yes | 11 (10) | 3 (19) | |
| Any douching | |||
| No | 94 (90) | 15 (94) | 1.000 |
| Yes | 11 (10) | 1 (6) | |
| Any smoking | |||
| No | 68 (65) | 8 (50) | .271 |
| Yes | 36 (35) | 8 (50) | |
| Any penile–vaginal sex | |||
| No | 28 (27) | 1 (6) | .114 |
| Yes | 77 (73) | 15 (94) | |
| Condom use for penile–vaginal sex | |||
| Always/no penile–vaginal sex | 38 (36) | 6 (38) | 1.000 |
| Not always | 67 (64) | 10 (62) | |
| Any receptive oral sex | |||
| No | 42 (40) | 5 (31) | .590 |
| Yes | 63 (60) | 11 (69) | |
| Any anal sex | |||
| No | 93 (89) | 12 (75) | .225 |
| Yes | 12 (11) | 4 (25) | |
| Any sex with an RSP | |||
| No | 44 (42) | 3 (19) | .100 |
| Yes | 61 (58) | 13 (81) | |
| RSP treatment details | |||
| No RSP | 41 (39) | 3 (19) | .007 |
| Treated RSP | 45 (43) | 4 (25) | |
| Untreated RSP | 19 (18) | 9 (56) | |
| Any new sexual partner | |||
| No | 85 (81) | 13 (81) | 1.000 |
| Yes | 20 (19) | 3 (19) | |
Data are presented as No. (%) unless otherwise indicated. P values <.05 are bolded to indicate statistically significant associations.
Abbreviations: BV, bacterial vaginosis; IUD, intrauterine device; RSP, regular sexual partner; SToPBV, Strategies to prevent BV.
The χ2 or Fisher exact test was used for categorical data and Wilcoxon signed-rank test was used for continuous variables.
Antibiotics prescribed included oral metronidazole (n = 106), intravaginal clindamycin (n = 14), and intravaginal metronidazole (n = 1). One woman did not report adherence data.
Between day 0 and month 1, 44 women reported current oral contraceptive use, 6 reported using a levonorgestrel IUD, 6 reported using a contraceptive implant, and 1 reported using a hormonal injection (Depo-Provera).
Between day 0 and month 1, 6 women reported using a levonorgestrel IUD and 8 women reported using a copper IUD. Of the 3 women with an IUD in situ who experienced recurrence, 2 reported using a copper IUD and 1 reported using a levonorgestrel IUD.
Overall Composition and Diversity of the Vaginal Microbiota of Women Who Experienced BV Recurrence and Women Who Did Not
Two hundred thirty specimens were included in analyses (Supplementary Figure 2); 109 women provided both day 0 and day 8 samples, 5 provided a day 0 sample only, and 7 provided a day 8 sample only. As expected, pretreatment specimens were predominantly high in diversity with high relative abundance of BV-associated bacteria including Gardnerella spp, Prevotella spp, Fannyhessea vaginae (previously Atopobium vaginae), and Megasphaera spp, with low relative abundance of lactobacilli. Immediately posttreatment, most specimens were low in diversity and dominated by L iners.
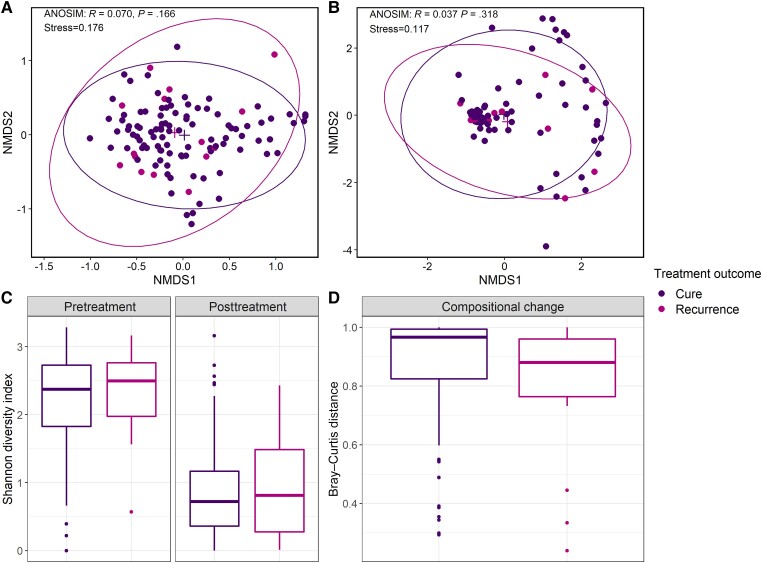
NMDS and ANOSIM showed no difference in the overall composition of the vaginal microbiota between recurrence and cure cases either pretreatment at day 0 (ANOSIM: R = 0.070, P = .166; Figure 1A) or immediately posttreatment at day 8 (ANOSIM: R = 0.037, P = .318; Figure 1B). Similarly, the α-diversity of the vaginal microbiota did not differ pretreatment (adjusted odds ratio [AOR], 1.99 [95% CI, .77–6.71], P = .203) or posttreatment (AOR, 1.37 [95% CI, .60–2.97], P = .435; Table 3, Figure 1C) between recurrence and cure cases. Furthermore, there was no difference in the degree of compositional change of the vaginal microbiota following treatment between recurrence and cure cases (AOR, 0.21 [95% CI, .02–3.01], P = .235; Table 3, Figure 1D).
Figure 1.
Diversity metrics of the vaginal microbiota of women who experienced bacterial vaginosis recurrence and those that remained cured at 1 month postenrollment. Nonmetric multidimensional scaling plots of the overall composition of the vaginal microbiota pretreatment (A) and immediately posttreatment (B). Box plots showing the bacterial diversity (measured using the Shannon diversity index) of the vaginal microbiota of cure and recurrence cases pretreatment and immediately posttreatment (C). Box plots displaying differences in the compositional change of the vaginal microbiota following treatment (measured using Bray–Curtis distances calculated between paired pretreatment and immediate posttreatment samples) between cure and recurrence cases (D). Abbreviations: ANOSIM, analysis of similarity; NMDS, nonmetric multidimensional scaling.
Table 3.
Associations Between Bacterial Vaginosis Recurrence and Characteristics of the Vaginal Microbiota Pretreatment and Immediately Posttreatment
| Characteristic | Cure, Median (IQR)a | Recurrence, Median (IQR)a | AOR (95% CI)b | P Value |
|---|---|---|---|---|
| Compositional change (Bray–Curtis)c |
0.97 (0.82–0.99) | 0.88 (0.76–0.96) | 0.22 (.02–2.96) | .357 |
| Pretreatment (day 0) | ||||
| α-diversity (Shannon diversity) |
2.37 (1.83–2.73) | 2.49 (1.98–2.76) | 1.99 (.77–6.71) | .203 |
| Gardnerella spp | 7.07 (6.44–7.66) | 6.69 (6.44–7.09) | 1.15 (.80–1.86) | .514 |
| Prevotella sppd | 4.99 (2.44–6.03) | 5.92 (5.52–6.54) | 1.35 (1.05–1.91) | .041 |
| Lactobacillus crispatus | −0.86 (−1.22 to −0.51) | −0.88 (−1.13 to −0.44) | 0.89 (.52–1.25) | .576 |
| Lactobacillus fornicalis | −0.87 (−1.23 to −0.56) | −0.88 (−1.13 to −0.44) | 0.78 (.33–1.25) | .418 |
| Immediately posttreatment (day 8) | ||||
| α-diversity (Shannon diversity) |
0.72 (0.36–1.17) | 0.81 (0.28–1.49) | 1.37 (.60–2.97) | .435 |
| Gardnerella spp | 2.33 (−0.17 to 5.18) | 5.41 (1.18–7.83) | 1.23 (1.03–1.49) | .026 |
| Prevotella sppd | −0.22 (−0.36 to −0.11) | −0.20 (−0.38 to −0.13) | 1.00 (.72–1.31) | .983 |
| Lactobacillus crispatus | −0.22 (−0.48 to −0.11) | −0.30 (−0.52 to −0.16) | 0.71 (.29–1.03) | .228 |
| Lactobacillus fornicalis | −0.18 (−0.39 to −0.08) | −0.30 (−0.52 to −0.16) | 0.67 (.30–1.03) | .168 |
P values <.05 are bolded to indicate statistically significant associations.
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; IQR, interquartile range.
Values for individual bacterial taxa represent the centered log-ratio transformed abundance.
Regression models were adjusted for partner type (treated regular sexual partner [RSP], untreated RSP, no RSP) and laboratory method.
Compositional change was measured using Bray–Curtis distances calculated between paired pretreatment and immediate posttreatment samples, where a value of 0 indicates no compositional change following treatment and a value closer to 1 indicates substantial change following treatment. Only women who provided both day 0 and day 8 samples (n = 109) were included in this analysis.
Represents Prevotella amplicon sequence variants that were not able to be classified at the species level.
Individual Bacterial Taxa Associated With BV Recurrence
ANCOM-BC was used to identify bacterial taxa that were differentially abundant pre- and posttreatment between recurrence and cure cases. Pretreatment, the mean abundance of Prevotella spp (representing Prevotella amplicon sequence variants that were not able to be classified at the species level) was significantly higher in women who experienced recurrence compared to those who were cured at 1 month posttreatment (log fold change [LFC] = 1.44, standard error [SE] = 0.56, P = .011).
Immediately posttreatment, the mean abundance of Gardnerella was increased in recurrence versus cure cases (LFC = 1.90, SE = 0.86, P = .028). Conversely, the mean abundance of both L crispatus and Lactobacillus fornicalis was decreased in women who experienced recurrence compared to those who did not (L crispatus LFC = −1.55, SE = 0.40, P = .0001; L fornicalis LFC = −1.19, SE = 0.34, P = .0006). There was no significant difference in the abundance of L iners between recurrence and cure cases at day 0 (LFC = 0.08, SE = 0.71, P > .05) or day 8 (LFC = −1.60, SE = 1.02, P > .05). ANCOM-BC findings were mostly consistent with findings from a second differential abundance analysis method (Supplementary Material). Supplementary Table 2 presents the prevalence and relative abundance of taxa identified as differentially abundant by ANCOM-BC. Of note, L crispatus was present in low relative abundance both pretreatment and immediately posttreatment in most participants, except for 8 cure cases who had a L crispatus relative abundance >50% at day 8.
In logistic regression analyses adjusted for partner type/treatment status and laboratory method, a high CLR-transformed abundance of Prevotella spp pretreatment (AOR, 1.35 [95% CI, 1.05–1.91], P = .041; Table 3) and a high CLR-transformed abundance of Gardnerella immediately posttreatment (AOR, 1.23 [95% CI, 1.03–1.49], P = .026; Table 3) were both associated with increased odds of BV recurrence. No other taxa were associated with BV recurrence.
Bacterial Ratios Associated With BV Recurrence
Finally, we explored the relationship between BV recurrence and the ratio of nonoptimal to optimal bacteria present in the vaginal microbiota pretreatment and immediately posttreatment. Pretreatment, the ratios of BVAB:Lactobacillus and Gardnerella:L iners were not associated with BV recurrence (Table 4). Immediately posttreatment however, higher levels of total BVAB relative to Lactobacillus spp and higher levels of Gardnerella relative to L iners were both associated with increased odds of BV recurrence (AOR, 1.38 [95% CI, 1.06–1.81], P = .017 and AOR, 1.44 [95% CI, 1.09–1.95], P = .012; Table 4). The ratio of pathobionts:Lactobacillus was not associated with recurrence at either timepoint.
Table 4.
Associations Between Bacterial Vaginosis Recurrence and the Ratio of Nonoptimal to Optimal Bacteria Present in the Vaginal Microbiota Pretreatment and Immediately Posttreatment
| Ratio | Cure, Median (IQR)a | Recurrence, Median (IQR)a | AOR (95% CI)b | P Value |
|---|---|---|---|---|
| Pretreatment (day 0) | ||||
| BVABc:Lactobacillus | 1.40 (0.61–2.25) | 1.53 (0.95–2.08) | 1.17 (.78–1.81) | .445 |
| Gardnerella:L iners | 0.97 (0.16–1.97) | 0.95 (0.46–1.43) | 1.15 (.77–1.74) | .511 |
| Pathobiontsd:Lactobacillus | −1.35 (−2.22 to −0.23) | −1.55 (−1.80 to −0.83) | 0.99 (.61–1.56) | .976 |
| Immediately posttreatment (day 8) | ||||
| BVABc:Lactobacillus | −2.15 (−2.76 to −0.38) | −0.28 (−1.63 to 0.31) | 1.38 (1.06–1.81) | .017 |
| Gardnerella:L iners | −2.01 (−3.71 to −0.42) | −0.43 (−1.90 to 0.22) | 1.44 (1.09–1.95) | .012 |
| Pathobiontsd:Lactobacillus | −2.63 (−3.02 to −1.43) | −2.28 (−2.54 to −0.99) | 1.14 (.87–1.45) | .314 |
P values <.05 are bolded to indicate statistically significant associations.
Abbreviations: AOR, adjusted odds ratio; BVAB, bacterial vaginosis–associated bacteria; CI, confidence interval; IQR, interquartile range; L iners, Lactobacillus iners.
Ratios were calculated using log10-transformed relative abundance data. A positive ratio reflects a higher abundance of nonoptimal bacteria (BVAB or pathobionts) relative to optimal bacteria (Lactobacillus spp or L iners). A negative ratio reflects a lower abundance of nonoptimal bacteria relative to optimal bacteria.
Logistic regression models were adjusted for partner type (treated regular sexual partner [RSP], untreated RSP, no RSP) and laboratory method.
BVAB: Gardnerella, Dialister, Prevotella, Finegoldia, Atopobium, Fannyhessea, Peptoniphilus, Aerococcus, Sneathia, Megasphaera, Peptostreptococcus, Porphyromonas, Prevotella, Mobiluncus, Peptococcus, Fusobacterium, Saccharibacteria (BVAB TM7), BVAB1-3.
Pathobionts: Campylobacter, Enterobacter, Enterobacteriaceae, Enterococcus, Escherichia/Shigella, Haemophilus, Klebsiella, Morganella, Neisseria, Proteus, Staphylococcus, Streptococcus.
DISCUSSION
In this study, we identified specific vaginal taxa that were associated with BV recurrence within 1 month of treatment. Specifically, we found that a higher abundance of Prevotella spp immediately prior to treatment and of Gardnerella spp immediately following treatment were both associated with increased odds of BV recurrence. Additionally, women who experienced recurrence had increased levels of BV-associated bacteria (primarily representing Gardnerella) relative to Lactobacillus spp (primarily representing L iners) present in their vaginal microbiota immediately posttreatment compared to women who did not experience recurrence. Neither bacterial diversity nor the degree of compositional change following treatment were associated with BV recurrence. These data provide insight into key taxa that may play a role in treatment failure following recommended BV therapy.
Prevotella and Gardnerella are commonly recovered from the vaginal microbiome of women with BV, and species from both genera possess characteristics that are important for BV initiation and persistence. In vitro, Prevotella bivia has been shown to produce ammonia and amino acids which enhance the growth of other BV-associated bacteria including Gardnerella spp and Peptostreptococcus spp [21, 22]. Gardnerella in turn produces amino acids, which stimulates growth of P bivia [21]. In addition, both Gardnerella spp and Prevotella spp produce sialidases [23, 24], and there is evidence that Prevotella may be a dominant source of vaginal sialidase. One early study found that sialidase activity in women with BV was attributable to Prevotella spp in a majority of cases [25], and another reported that among women with molecular BV, Prevotella was a key taxon that was enriched in the presence of cervicovaginal sialidase activity [26]. Sialidases degrade the cervicovaginal mucus [27], which assists bacterial adherence to vaginal epithelium and formation of biofilms [28]. Dense polymicrobial biofilms are a characteristic feature of BV [29] and likely contribute to treatment failure and BV recurrence [30]; not only do they act as a barrier to reduce antibiotic penetration, but quorum sensing between organisms present in a biofilm can result in activation of resistance mechanisms including expression of efflux pumps [31]. Indeed, BV biofilms have been shown to persist following metronidazole treatment [32]. Gardnerella spp typically dominate BV biofilms [29, 33]. However, other organisms including F vaginae are known to co-occur [29, 33], and both F vaginae and P bivia can integrate into established Gardnerella biofilms in vitro and may contribute to ongoing maintenance of the biofilm [34, 35]. Therefore, it is possible that our observed association between BV recurrence and high pretreatment abundance of Prevotella spp reflects the presence of a highly developed multispecies biofilm that is refractory to treatment.
The observed association between recurrence and high abundance of Gardnerella spp and high BVAB:Lactobacillus ratio immediately posttreatment may also suggest treatment failure due to the presence of a dense biofilm, or alternatively resistance to first-line therapy, which has been documented for metronidazole [36–39]. Our finding that high Gardnerella abundance posttreatment may contribute to recurrence is supported by a previous 16S rRNA gene sequencing study of 41 women that found that sustained cure was associated with lower relative abundance of Gardnerella posttreatment [10]. A quantitative polymerase chain reaction (qPCR)–based analysis of the same samples showed that persistently high levels of Gardnerella genomospecies GS07 pre- and posttreatment predicted treatment failure [11]. Furthermore, an analysis of 33 women found that sustained cure at 1 month posttreatment was associated with a marked decrease in Gardnerella vaginalis absolute abundance (quantified using qPCR) following intravaginal metronidazole [7]. Collectively, these data suggest that posttreatment persistence of Gardnerella spp contributes to treatment failure. Metronidazole has limited ability to disrupt established Gardnerella biofilms [40], and in vitro studies have found that the minimum inhibitory concentration for both metronidazole and clindamycin is higher for biofilm-forming G vaginalis isolates compared to planktonic isolates [41, 42]. Furthermore, 2 recent studies both reported that while metronidazole and clindamycin had some in vitro activity against single-species Gardnerella biofilms, neither antibiotic significantly altered the composition of multispecies biofilms [42, 43]. Together these data suggest that antibiofilm agents may be needed to effectively treat BV [30], particularly in the setting of persistent infections. It is also possible that the high abundance of Gardnerella immediately posttreatment is a marker of other factor(s) (eg, presence of an intrauterine device, smoking, or douching) or perhaps other organisms that are driving disease recurrence.
Prevotella spp are also commonly detected in the cutaneous penile and male urethral microbiota [44–46], and prior studies conducted by our group and others have reported the relative abundance of specific Prevotella spp to be moderately to strongly correlated between the vaginal and male genital microbiota of couples [17, 18, 47]. Therefore, an alternative hypothesis for our findings is that the high abundance of Prevotella spp pretreatment may also reflect women who are at increased risk of recurrence due to ongoing exposure to an untreated RSP. There is now substantial microbiological and epidemiological evidence that supports a role for sexual transmission in both the acquisition and recurrence of BV [6]. Notably, a recent study demonstrated that detection of several BV-associated bacteria in the penile urethra (including Prevotella amnii and G vaginalis) was contingent upon prior penile–vaginal sex, and that these organisms could persist in the urethra >60 days after exposure [46]. In our study, women who reported sex with an untreated RSP during follow-up experienced BV recurrence more commonly compared to women with no RSP or women who had an RSP who received partner treatment. Of note, the proportion of women with a treated RSP who experienced recurrence (8%) was comparable to women with no RSP (7%). These findings suggest that combined oral and topical male partner treatment reduces BV recurrence at 1 month postenrollment to the level of a woman without an RSP. A multisite randomized controlled trial evaluating this intervention is ongoing (ACTRN12619000196145) and will provide the necessary data on its efficacy for preventing BV recurrence over a 3-month follow-up period.
This study analyzed pooled samples and data from 3 well-characterized cohorts of women undergoing BV treatment. Study strengths included the availability of detailed metadata including sexual practices, contraceptive practices, and treatment adherence, allowing us to investigate the relationship between these factors and treatment outcome, and adjust analyses for potential confounders. Furthermore, we investigated several features of the vaginal microbiota, providing a comprehensive analysis. Additionally, the sample size of our study size was larger than prior studies that have investigated the role of the vaginal microbiota in BV treatment outcome [7–14, 48].
There are limitations to this study. The relatively small number of women who experienced BV recurrence may have influenced our ability to identify associations between diversity metrics and BV recurrence. Sequencing short fragments of the 16S rRNA gene provides limited resolution beyond the genus level, and we were unable to identify the specific species (and strains) of Gardnerella and Prevotella that may contribute to treatment failure. Furthermore, 16S rRNA gene sequencing generates relative abundance data, which may not accurately reflect the absolute abundance of individual bacteria [49, 50]. A quantitative method would provide additional insight into the contribution of key taxa to posttreatment recurrence. Finally, the laboratory methods were not identical between the studies; however, we adjusted for this in regression analyses.
Our findings suggest that key taxa may contribute to the high rates of treatment failure following recommended BV therapy. Future studies should prioritize understanding the role of specific Prevotella and Gardnerella strains in BV recurrence, as well as their concordance between sexual partners. Additionally, in vivo studies investigating the role of Prevotella spp in biofilm formation and persistence are needed, as much of the work in this space is in vitro. Importantly, our results suggest there are 2 dominant factors driving posttreatment recurrence: persistence of disease due to presence of a multispecies biofilm and/or antibiotic resistance, and reinfection from an untreated sexual partner. Ultimately, interventions that target these factors are needed to achieve sustained BV cure.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Erica L Plummer, Central Clinical School, Monash University, Melbourne, Victoria, Australia; Melbourne Sexual Health Centre, Alfred Hospital, Carlton, Victoria, Australia.
Amelia M Sfameni, Central Clinical School, Monash University, Melbourne, Victoria, Australia; Melbourne Sexual Health Centre, Alfred Hospital, Carlton, Victoria, Australia.
Lenka A Vodstrcil, Central Clinical School, Monash University, Melbourne, Victoria, Australia; Melbourne Sexual Health Centre, Alfred Hospital, Carlton, Victoria, Australia; Melbourne School of Population and Global Health, University of Melbourne, Parkville, Victoria, Australia.
Jennifer A Danielewski, Molecular Microbiology, Murdoch Children's Research Institute, Parkville, Victoria, Australia; Women's Centre for Infectious Diseases, The Royal Women's Hospital, Parkville, Victoria, Australia; Department of Obstetrics and Gynaecology, University of Melbourne, Parkville, Victoria, Australia.
Gerald L Murray, Molecular Microbiology, Murdoch Children's Research Institute, Parkville, Victoria, Australia; Women's Centre for Infectious Diseases, The Royal Women's Hospital, Parkville, Victoria, Australia; Department of Obstetrics and Gynaecology, University of Melbourne, Parkville, Victoria, Australia.
Glenda Fehler, Melbourne Sexual Health Centre, Alfred Hospital, Carlton, Victoria, Australia.
Christopher K Fairley, Central Clinical School, Monash University, Melbourne, Victoria, Australia; Melbourne Sexual Health Centre, Alfred Hospital, Carlton, Victoria, Australia.
Suzanne M Garland, Molecular Microbiology, Murdoch Children's Research Institute, Parkville, Victoria, Australia; Women's Centre for Infectious Diseases, The Royal Women's Hospital, Parkville, Victoria, Australia; Department of Obstetrics and Gynaecology, University of Melbourne, Parkville, Victoria, Australia.
Eric P F Chow, Central Clinical School, Monash University, Melbourne, Victoria, Australia; Melbourne Sexual Health Centre, Alfred Hospital, Carlton, Victoria, Australia; Melbourne School of Population and Global Health, University of Melbourne, Parkville, Victoria, Australia.
Jane S Hocking, Melbourne School of Population and Global Health, University of Melbourne, Parkville, Victoria, Australia.
Catriona S Bradshaw, Central Clinical School, Monash University, Melbourne, Victoria, Australia; Melbourne Sexual Health Centre, Alfred Hospital, Carlton, Victoria, Australia; Melbourne School of Population and Global Health, University of Melbourne, Parkville, Victoria, Australia.
Notes
Author contributions. E. L. P., L. A. V., and C. S. B. conceived and designed this secondary analysis. E. L. P. analyzed the sequencing data. A. M. S., with statistical support from E. L. P. and L. A. V., performed statistical analyses. J. A. D. and G. L. M. performed and supervised the laboratory work. L. A. V. and C. S. B. conceived of the original trials from which the specimens were derived. L. A. V., C. S. B., E. L. P., C. K. F., J. A. D., G. L. M., and J. S. H. contributed to study design and/or implementation of 1 or more of the original trials. L. A. V., C. K. F., S. M. G., and C. S. B. acquired funding for the microbiota analyses. G. F. performed Nugent scoring in the parent trials. E. L. P., A. M. S., L. A. V., and C. S. B. wrote the original draft. All authors critically reviewed and edited the manuscript. All authors approved the final version of the manuscript.
Acknowledgments. We thank Michelle Doyle, Colette McGuinness, Marti Kaiser, Karen Worthington, Mieken Grant, Lucy Williamson, Genevieve Lilley, Susan Peterson, and Melbourne Sexual Health Centre clinicians for their contributions to the original parent trials to which participants were recruited. We thank Larissa Ratten for contributions to the laboratory work related to the SToPBV study. We also thank the study participants.
Data availability. The raw sequencing data are publicly available in the National Center for Biotechnology Information Sequence Read Archive (accession numbers PRJNA592384, PRJNA398590, and PRJNA735440).
Financial support. The original trials were supported by grants from Central Clinical School, Monash University (Near Miss) and Alfred Health (Alfred Research Trusts Seeding Grant) awarded to C. S. B., L. A. V., C. K. F., and S. M. G. SToPBV was additionally supported by an Early Career Researcher grant from the University of Melbourne to L. A. V. This work was also supported by the Australian National Health and Medical Research Council (NHMRC) (program grant number 1071269 awarded to C. K. F. and S. M. G.). C. S. B., C. K. F., and S. M. G. are supported by the NHMRC (Leadership Investigator grant numbers 1173361, 1172900, and 1197951, respectively) and E. P. F. C. by an NHMRC Emerging Leadership Investigator grant (grant number 1172873). J. S. H. is supported by a NHMRC Senior Research Fellowship (award number 1136117). Funding to pay the Open Access publication charges for this article was provided by Monash University.
References
- 1. Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis 2019; 46:304–11. [DOI] [PubMed] [Google Scholar]
- 2. McKinnon LR, Achilles SL, Bradshaw CS, et al. The evolving facets of bacterial vaginosis: implications for HIV transmission. AIDS Res Hum Retrovir 2019; 35:219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev 2009; 3:CD006055. [DOI] [PubMed] [Google Scholar]
- 4. Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006; 193:1478–86. [DOI] [PubMed] [Google Scholar]
- 5. Bilardi JE, Walker S, Temple-Smith M, et al. The burden of bacterial vaginosis: women's experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS One 2013; 8:e74378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vodstrcil LA, Muzny CA, Plummer EL, Sobel JD, Bradshaw CS. Bacterial vaginosis: drivers of recurrence and challenges and opportunities in partner treatment. BMC Med 2021; 19:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armstrong E, Hemmerling A, Joag V, et al. Treatment success following standard antibiotic treatment for bacterial vaginosis is not associated with pre-treatment genital immune or microbial parameters. Open Forum Infect Dis 2023; 10:ofad007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gustin AT, Thurman AR, Chandra N, et al. Recurrent bacterial vaginosis following metronidazole treatment is associated with microbiota richness at diagnosis. Am J Obstet Gynecol 2022; 226:225.e1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee CY, Cheu RK, Lemke MM, et al. Quantitative modeling predicts mechanistic links between pre-treatment microbiome composition and metronidazole efficacy in bacterial vaginosis. Nat Commun 2020; 11:6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mollin A, Katta M, Sobel JD, Akins RA. Association of key species of vaginal bacteria of recurrent bacterial vaginosis patients before and after oral metronidazole therapy with short- and long-term clinical outcomes. PLoS One 2022; 17:e0272012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turner E, Sobel JD, Akins RA. Prognosis of recurrent bacterial vaginosis based on longitudinal changes in abundance of Lactobacillus and specific species of Gardnerella. PLoS One 2021; 16:e0256445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verwijs MC, Agaba SK, Darby AC, van de Wijgert J. Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure. Am J Obstet Gynecol 2020; 222:157.e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang B, Xiao BB, Shang CG, et al. Molecular analysis of the relationship between specific vaginal bacteria and bacterial vaginosis metronidazole therapy failure. Eur J Clin Microbiol Infect Dis 2014; 33:1749–56. [DOI] [PubMed] [Google Scholar]
- 14. Xiao B, Wu C, Song W, et al. Association analysis on recurrence of bacterial vaginosis revealed microbes and clinical variables important for treatment outcome. Front Cell Infect Microbiol 2019; 9:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muzny CA, Taylor CM, Swords WE, et al. An updated conceptual model on the pathogenesis of bacterial vaginosis. J Infect Dis 2019; 220:1399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vodstrcil LA, Plummer ME, Fairley CK, et al. Combined oral contraceptive pill-exposure alone does not reduce the risk of bacterial vaginosis recurrence in a pilot randomised controlled trial. Sci Rep 2019; 9:3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plummer EL, Vodstrcil LA, Danielewski JA, et al. Combined oral and topical antimicrobial therapy for male partners of women with bacterial vaginosis: acceptability, tolerability and impact on the genital microbiota of couples—a pilot study. PLoS One 2018; 13:e0190199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Plummer EL, Vodstrcil LA, Doyle M, et al. A prospective, open-label pilot study of concurrent male partner treatment for bacterial vaginosis. mBio 2021; 12:e0232321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ratten LK, Plummer EL, Murray GL, et al. Sex is associated with the persistence of non-optimal vaginal microbiota following treatment for bacterial vaginosis: a prospective cohort study. BJOG 2021; 128:756–67. [DOI] [PubMed] [Google Scholar]
- 20. Lin H, Peddada SD. Analysis of compositions of microbiomes with bias correction. Nat Commun 2020; 11:3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pybus V, Onderdonk AB. Evidence for a commensal, symbiotic relationship between Gardnerella vaginalis and Prevotella bivia involving ammonia: potential significance for bacterial vaginosis. J Infect Dis 1997; 175:406–13. [DOI] [PubMed] [Google Scholar]
- 22. Pybus V, Onderdonk AB. A commensal symbiosis between Prevotella bivia and Peptostreptococcus anaerobius involves amino acids: potential significance to the pathogenesis of bacterial vaginosis. FEMS Immunol Med Microbiol 1998; 22:317–27. [DOI] [PubMed] [Google Scholar]
- 23. Gilbert NM, Lewis WG, Li G, Sojka DK, Lubin JB, Lewis AL. Gardnerella vaginalis and Prevotella bivia trigger distinct and overlapping phenotypes in a mouse model of bacterial vaginosis. J Infect Dis 2019; 220:1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santiago GL, Deschaght P, El Aila N, et al. Gardnerella vaginalis comprises three distinct genotypes of which only two produce sialidase. Am J Obstet Gynecol 2011; 204:450.e1–7. [DOI] [PubMed] [Google Scholar]
- 25. Briselden AM, Moncla BJ, Stevens CE, Hillier SL. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis–associated microflora. J Clin Microbiol 1992; 30:663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferreira CST, Marconi C, Parada C, Ravel J, da Silva MG. Sialidase activity in the cervicovaginal fluid is associated with changes in bacterial components of Lactobacillus-deprived microbiota. Front Cell Infect Microbiol 2022; 11:813520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewis WG, Robinson LS, Gilbert NM, Perry JC, Lewis AL. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J Biol Chem 2013; 288:12067–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hardy L, Jespers V, Van den Bulck M, et al. The presence of the putative Gardnerella vaginalis sialidase A gene in vaginal specimens is associated with bacterial vaginosis biofilm. PLoS One 2017; 12:e0172522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Swidsinski A, Mendling W, Loening-Baucke V, et al. Adherent biofilms in bacterial vaginosis. Obstet Gynecol 2005; 106:1013–23. [DOI] [PubMed] [Google Scholar]
- 30. Muzny CA, Schwebke JR. Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin Infect Dis 2015; 61:601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharma D, Misba L, Khan AU. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control 2019; 8:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol 2008; 198:97.e1–6. [DOI] [PubMed] [Google Scholar]
- 33. Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PLoS One 2015; 10:e0136658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Castro J, Machado D, Cerca N. Unveiling the role of Gardnerella vaginalis in polymicrobial bacterial vaginosis biofilms: the impact of other vaginal pathogens living as neighbors. ISME J 2019; 13:1306–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castro J, Rosca AS, Muzny CA, Cerca N. Atopobium vaginae and Prevotella bivia are able to incorporate and influence gene expression in a pre-formed Gardnerella vaginalis biofilm. Pathogens 2021; 10:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kharsany AB, Hoosen AA, Van den Ende J. Antimicrobial susceptibilities of Gardnerella vaginalis. Antimicrob Agents Chemother 1993; 37:2733–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Souza DM K, Diniz CG, Filho DS, et al. Antimicrobial susceptibility and vaginolysin in Gardnerella vaginalis from healthy and bacterial vaginosis diagnosed women. J Infect Dev Ctries 2016; 10:913–9. [DOI] [PubMed] [Google Scholar]
- 38. Nagaraja P. Antibiotic resistance of Gardnerella vaginalis in recurrent bacterial vaginosis. Indian J Med Microbiol 2008; 26:155–7. [DOI] [PubMed] [Google Scholar]
- 39. Landlinger C, Oberbauer V, Podpera Tisakova L, et al. Preclinical data on the Gardnerella-specific endolysin PM-477 indicate its potential to improve the treatment of bacterial vaginosis through enhanced biofilm removal and avoidance of resistance. Antimicrob Agents Chemother 2022; 66:e0231921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gottschick C, Szafranski SP, Kunze B, et al. Screening of compounds against Gardnerella vaginalis biofilms. PLoS One 2016; 11:e0154086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li T, Zhang Z, Wang F, et al. Antimicrobial susceptibility testing of metronidazole and clindamycin against Gardnerella vaginalis in planktonic and biofilm formation. Can J Infect Dis Med Microbiol 2020; 2020:1361825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johnston W, Ware A, Kuiters WF, et al. In vitro bacterial vaginosis biofilm community manipulation using endolysin therapy. Biofilm 2023; 5:100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosca AS, Castro J, Sousa LGV, Franca A, Vaneechoutte M, Cerca N. In vitro interactions within a biofilm containing three species found in bacterial vaginosis (BV) support the higher antimicrobial tolerance associated with BV recurrence. J Antimicrob Chemother 2022; 77:2183–90. [DOI] [PubMed] [Google Scholar]
- 44. Mehta SD, Zhao D, Green SJ, et al. The microbiome composition of a man's penis predicts incident bacterial vaginosis in his female sex partner with high accuracy. Front Cell Infect Microbiol 2020; 10:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu CM, Hungate BA, Tobian AA, et al. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. mBio 2015; 6:e00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toh E, Xing Y, Gao X, et al. Sexual behavior shapes male genitourinary microbiome composition. Cell Rep Med 2023; 4:100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zozaya M, Ferris MJ, Siren JD, et al. Bacterial communities in penile skin, male urethra, and vaginas of heterosexual couples with and without bacterial vaginosis. Microbiome 2016; 4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zwittink RD, van den Munckhof EHA, Leverstein-van Hall MA, et al. The vaginal microbiota in the course of bacterial vaginosis treatment. Eur J Clin Microbiol Infect Dis 2021; 40:651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boshier FA T, Srinivasan S, Lopez A, et al. Complementing 16S rRNA gene amplicon sequencing with total bacterial load to infer absolute species concentrations in the vaginal microbiome. mSystems 2020; 5:e00777-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Armstrong E, Hemmerling A, Miller S, et al. Metronidazole treatment rapidly reduces genital inflammation through effects on bacterial vaginosis-associated bacteria rather than lactobacilli. J Clin Invest 2022; 132:e152930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.