Summary
Despite universal recommendations for COVID-19 mRNA vaccination in pregnancy, uptake has been lower than desired. There have been limited studies of the direct impact of COVID-19 mRNA vaccine exposure in human placental tissue. Using a primary human placental explants model, we investigated the uptake of two common mRNA vaccines (BNT162b2 Pfizer-BioNTech or mRNA-1273 Moderna), and whether exposure altered villous cytokine responses. Explants derived from second or third trimester chorionic villi were incubated with vaccines at supraphysiologic concentrations and analyzed at two time points. We observed minimal uptake of mRNA vaccines in placental explants by in situ hybridization and quantitative RT-PCR. No specific or global cytokine response was elicited by either of the mRNA vaccines in multiplexed immunoassays. Our results suggest that the human placenta does not readily absorb the COVID-19 mRNA vaccines nor generate a significant inflammatory response after exposure.
Subject areas: Biological sciences, Immunology, Immune response, Microbiology
Graphical abstract
Highlights
-
•
The human placental explants do not readily absorb the COVID-19 mRNA vaccines
-
•
Human placental chorionic villi are resistant to mRNA vaccine uptake
-
•
Minimal inflammation is detected in human chorionic villi after mRNA vaccine exposure
-
•
COVID-19 mRNA vaccines are minimally active in the human placenta
Biological sciences; Immunology; Immune response; Microbiology
Introduction
Despite strong recommendations for vaccination against COVID-19 in pregnancy by all major public health agencies and professional societies,1,2 vaccination rates have been low in this vulnerable population.3 Concerns include the exclusion of pregnant individuals from randomized clinical trials4,5 and the lack of studies examining the direct impact of COVID-19 mRNA vaccines on the placenta.
The placenta acts as a semi-protective barrier against certain pathogens, environmental chemicals, and pollutants; however, many compounds, small-molecules, viral agents, etc., are known to cross the placenta to varying degrees.6 COVID-19 mRNA vaccines deliver the mRNA encoding SARS-CoV-2 spike protein encapsulated by lipid nanoparticles.7 While cellular uptake is necessary to induce systemic immunity, whether this lipid-mRNA complex can transfer across the placental barrier remains unknown.
Even in the absence of transplacental transmission, exposures may stimulate placental inflammatory responses. The human placenta has the capacity to express all known cytokines, thought to be mainly produced by immunogenic fetal cell populations, e.g., fetal macrophages (Hofbauer cells), syncytiotrophoblasts, and cytotrophoblasts.8 However, in general, pregnancy is a tolerogenic condition and the placental immune environment tends to be shifted toward an anti- vs. pro-inflammatory state. Aberrant inflammatory responses to drugs or microorganisms have been associated with adverse pregnancy outcomes such as fetal growth restriction, preterm birth, and preeclampsia.9 Imbalances in cytokine levels may also negatively impact fetal brain development, increasing the risk of long-term neurodevelopmental disorders such as hyperactivity, autism spectrum disorders, and schizophrenia.10,11,12,13
The exogenous mRNA vaccine is immunostimulatory by design and can be recognized by a variety of cell surface receptors and endosomal sensors such as Toll-like receptors 7 (TLR7) and TLR8, resulting in potent immune activation and production of type I interferons and other inflammatory cytokines and chemokines.7,14 Intradermal injection of the mRNA vaccine produces both local and systemic inflammation,15 as evidenced by mild to moderate pain at the injection site as well as fever, headache, and chills within 7 days after injection. Endogenous placental responses to direct mRNA vaccine exposures are unknown.
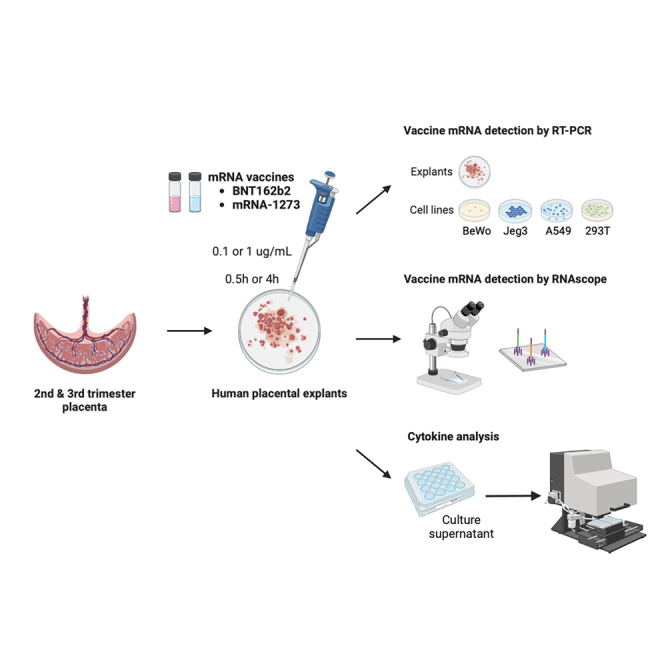
In this study, we investigated the capacity of mRNA vaccine uptake in human placental tissue after direct exposures in vitro using a primary human placental explants model in comparison with common human placental/non-placental cell lines. Additionally, using the human placental explants model, we evaluated whether the presence of the mRNA vaccine alters the release of cytokines in placental inflammatory response.
Results
mRNA vaccine uptake after in vitro exposures in human placental explants and trophoblast cell lines
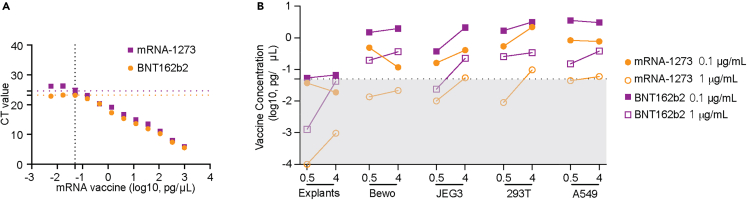
Human placental explants from second and third trimester placentas and four human cell lines—two of placental origin—BeWo placental choriocarcinoma cells and JEG-3 choriocarcinoma cells, and two other widely used cell lines that have similar epithelial morphology and adherent monolayer, A549 lung carcinoma cells and 293T embryonic kidney cells, were incubated with either mRNA-1273 (Moderna) or BNT162b2 (Pfizer) vaccines at two concentrations (0.1 μg/mL or 1 μg/mL) or durations (0.5 and 4 h). Total RNA was isolated from explant tissue and/or cell pellets and analyzed by RT-PCR against vaccine-specific spike mRNA sequence as previously described.16 Standard curves were generated with known concentrations of the two mRNA vaccines, mRNA-1273 and BNT162b2, and cycle threshold (CT) values corresponding to > 0.05 pg/μL, the estimated lower limit of detection for both vaccines, were reported as positively detected (mRNA-1237; CT < 23.2; BNT162b2; CT < 24.6; Figure 1A). In general, vaccine mRNA was minimally or not detected in human placental explants exposed to either of the mRNA vaccines with either 0.1 μg/mL or 1 μg/mL exposures. In contrast, both vaccines were detected in BeWo, JEG-3, 392T, and A549 cell lines at varying amounts in a dose- and time-dependent manner (Figure 1B). For example, in JEG-3 exposed to 0.1 μg/mL of mRNA-1273, we did not detect the mRNA vaccine at 0.5 h (mean < 0.05 pg/mL), however, by 4 h it was found at 0.223 pg/uL. In JEG-3 exposed to 1 μg/mL, we detected a mean concentration of 0.37 pg/μL at 0.5 h, and these levels increased to 2.116 pg/μL at 4 h. In general, higher levels (average 3.3-fold) of mRNA-1273 were detected in all four cell lines than those exposed to BNT162b2 at equivalent concentrations and exposure durations. Exceptions were noted for BeWo (1 μg/mL BNT162B2) and A549 (1 μg/mL mRNA-1273) in which detectable vaccine levels at 4 h versus 0.5 h. In general, our results suggest that there is a concentration and time (i.e., exposure duration) dependence on the amount of vaccine mRNA detected.
Figure 1.
Detection of vaccine-derived spike protein mRNA in human placental explants and four human cell lines using qRT-PCR
(A) Vaccine messenger RNA (mRNA) standard curves. Standard curves for mRNA-1273 and BNT162b2 vaccines were generated to calculate vaccine-derived spike mRNA concentrations in human placental explants and cells. The limit of detection (LOD) level was set according to the standard curve (0.05 pg/μL, mRNA-1237 CT = 23.2, BNT162b2 CT = 24.6).
(B) Vaccine mRNA detected in human placental explants and four different types of cell lines exposed to mRNA-1273 or BNT162B2 at 0.1 μg/mL or 1 μg/mL for 0.5 h or 4 h. Results were calculated by extrapolating CT values based on the derived standard curves for each mRNA vaccine. Black dotted line represents the LOD level of 0.05 pg/μL. Gray box represents the area under LOD. All data represent 2 independent experiments. CT, Cycle threshold.
Detection of mRNA COVID-19 vaccines by RNAscope in situ hybridization (ISH) in human placental explants
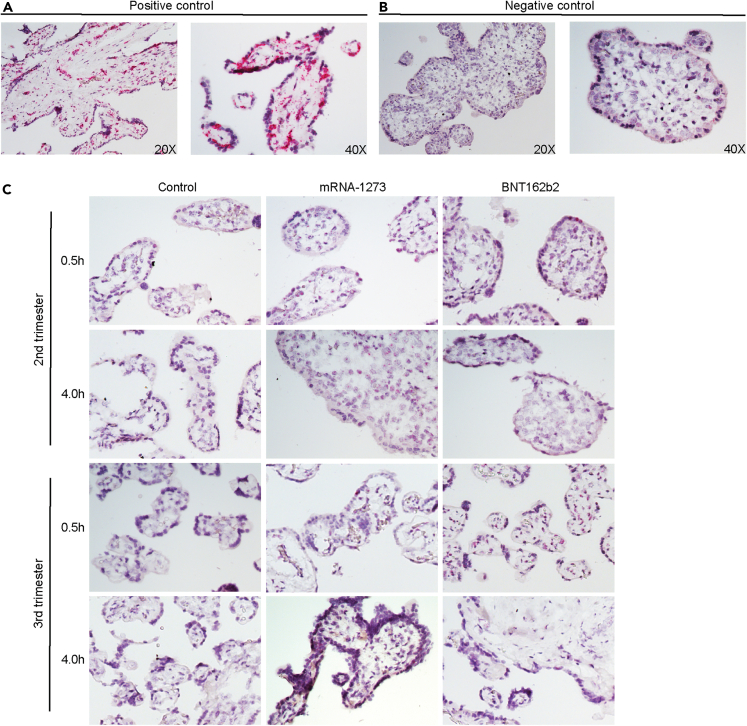
To further analyze the degree of uptake and tissue localization of the mRNA vaccines into human placental explants, we used RNAscope-based ISH assay—a method proposed to be more sensitive than qRT-PCR17—to detect the vaccine-related mRNA. Explants cultured from second trimester or third trimester placentas were incubated with mRNA-1273 or BNT162b2 vaccines, at 0.1 μg/mL or 1 μg/mL for 0.5 or 4 h, respectively. Explants cultured in optimized medium without mRNA vaccines were used as a control group. Peptidylprolyl isomerase B (PPIB, positive control) and DapB (negative control) probes were used to interpret the results (Figures 2A and 2B). At 0.5 or 4 h, in explants exposed to either of the two vaccines, we did not find a positive signal corresponding to the detection of either mRNA-1273 or BNT162b2 in the placental explants (n = 2; Figure 2C).
Figure 2.
Lack of detection of COVID mRNA vaccine via in situ hybridization in human placental explants
Chorionic villi explants derived from second (n = 2) or third trimester (n = 2) human placentas were incubated with 0.1 μg/mL (not shown) or 1 μg/mL mRNA-1237 or BNT162B2 vaccines. After 0.5 h or 4 h, tissues were fixed, paraffin-embedded, sectioned and probed for mRNA vaccine using RNAscope in situ hybridization.
(A and B) Positive and (B) negative controls for RNAscope detection of mRNA vaccine. Peptidylprolyl isomerase B (PPIB) was used as a positive control. Pink dots corresponding to PPIB mRNA can be observed within the chorionic villi at 20X and 40X. DapB was used as a negative control. No signal was detected at 20 or 40X.
(C) In situ detection of mRNA vaccine in vaccine-exposed explants. No signal was evident in explants incubated with either vaccine at any of the two time points.
mRNA vaccines stimulation of cytokine expression in human placental explants
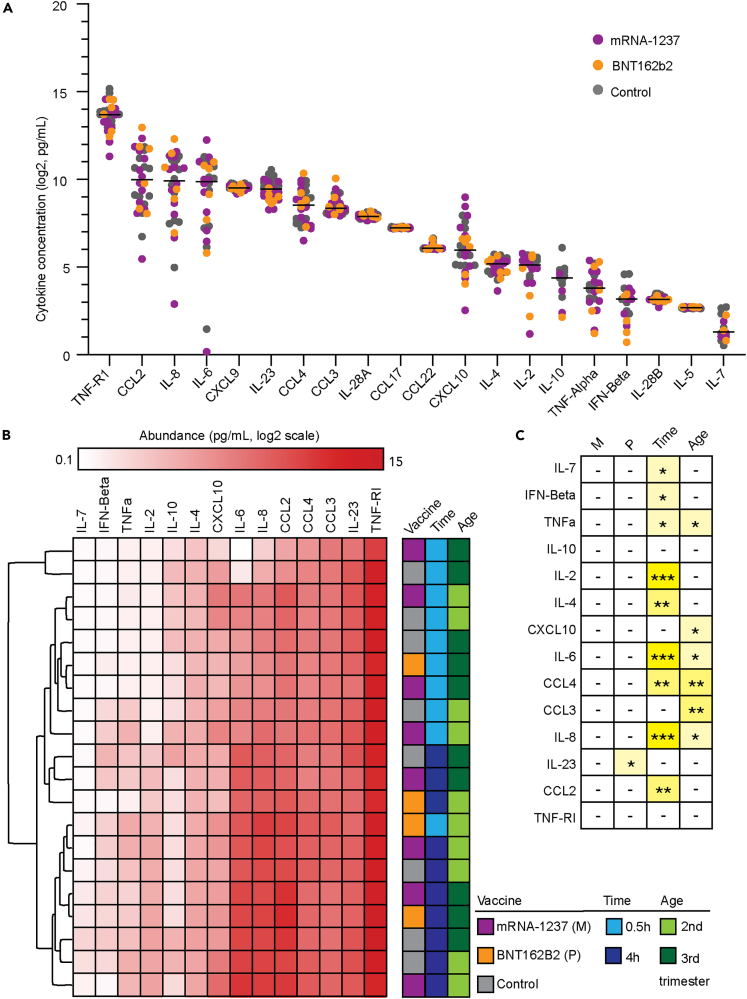
Even in the absence of significant uptake into placental tissue, exogenous exposures may induce cytokine secretion that could have an adverse impact on the placenta/fetus.18 Thus, we utilized a Luminex assay to quantify 24 unique cytokines in the conditioned media of second trimester and third trimester explants treated with either of the two mRNA vaccines at 0.1 μg/mL or 1 μg/mL for 0.5 or 4 h. Standards for each cytokine were used to interpolate concentrations in the conditioned media. Of the 24 cytokines tested, four (IL-1β, IL-17A, IL-12p70, IFN-γ) were not detected in any samples in the interpolable range. Levels of different cytokines varied widely; IL-7 had the lowest level (median 2.48 pg/mL) while TNF-R1 had the highest level (median 13258.41 pg/mL) (Figure 3A). Levels of cytokines (IL-5, IL-28A, IL-28B, CXCL9, CCL17, and CCL22) with low variance (< 15%) were excluded from downstream analyses to reduce detection of false positives.
Figure 3.
Expression of inflammatory mediators in conditioned media of human placental explants incubated with COVID mRNA vaccine
Chorionic villi explants derived from second or third trimester human placenta (n = 6) were incubated with 0.1 μg/mL or 1 μg/mL mRNA-1237 (n = 4) or BNT162B2 (n = 2) vaccines for 0.5 h or 4 h. Conditioned media were collected and levels of 20 unique cytokines were measured using the Luminex multiplex assay.
(A) Distribution in abundance of cytokines in all samples. Black bars represent median levels in cytokine abundance.
(B) Hierarchical clustering of samples by levels of cytokines. We excluded cytokines of low variance from this analysis (n = 6 cytokines). In general, sample profiles clustered by culture time (0.5 vs. 4 h).
(C) Significance of factors in cytokine expression evaluated using multiple linear regression models. Asterisks represent significance cutoffs: p < 0.05 (∗); p < 0.005 (∗∗); and p < 0.0005 (∗∗∗). Abbreviations listed for factors assessed in our analysis: mRNA-1237 (M), BNT162B2 (P) vaccines, culture time (Time), or trimester (Age). See also Figure S1.
We assessed the remaining 14 cytokines in the context of vaccine exposure, exposure duration or gestational age of the explants. We applied unsupervised hierarchical clustering analysis to determine if global patterns in cytokine profiles segregated by one of the three variables (Figure 3B). Notably, the majority of samples clustered by exposure duration, indicating that this factor was most influential in affecting cytokine levels. In general, patterns in cytokine levels were not associated with either of the two vaccines in comparison with the control or gestational age.
In the next step of our analysis, we implemented multiple linear regression models to assess whether specific cytokines were associated with exposure to one of the two vaccines while adjusting for culture duration. In general, significant changes in cytokine levels were not observed with mRNA-1273 exposure (Figure 3C). These observations were similar with BNT162b2 exposure, where overall we did not see cytokine induction to be associated with vaccine exposure vs. control, with the exception of IL-23 which was modestly decreased at the two time points (∼−0.2 pg/mL; p = 0.03. Figure S1). Of note, several cytokines were associated with culture duration or gestational age irrespective of vaccine exposure. IL-2, IL-4, IL-6, IL-8, CCL2, and CCL4) increased in abundance in the conditioned media over culture time. Specific cytokines seem to be released higher into the culture medium in second vs. third trimester explants such as IL-8, CCL3, CCL4, TNF-α, CXCL10, and IL-6 (Figure 3C). In general, vaccine exposures did not considerably modify cytokine abundance in vaccine-exposed vs. control groups.
Discussion
To our knowledge, this is the first study to directly investigate the effects of COVID-19 mRNA vaccines in a human placental explant model of the placental barrier. We report minimal uptake of two commonly used COVID-19 mRNA vaccines. Additionally, direct exposure of explants to COVID-19 mRNA vaccines did not profoundly affect cytokine release, suggesting that human placental chorionic villi are resistant to mRNA vaccine penetration and interactions that lead to changes in inflammatory status.
We implemented two approaches to detect mRNA vaccine uptake: qRT-PCR which is the gold standard for mRNA detection and quantification, and RNAscope ISH which provides additional information regarding the localization of the mRNA vaccine within the tissue architecture of explants. Following a 30 min or 4 h duration, vaccine mRNA was detected in monolayer trophoblast cell lines BeWo and JEG-3, which lack the cell-to-cell interactions of different cell types in tissue, and also may not perfectly recapitulate the syncytiotrophoblast barrier (Figure 1C). In contrast, there was very limited (or none at all) detectable vaccine mRNA in the human placental explants. As a secondary measure, we applied RNAscope ISH and did not observe vaccine mRNA in the chorionic villi, including syncytiotrophoblast, intravillous cytotrophoblasts or other cells that comprise the villous stroma. This suggests that the placental architecture and inherent cellular relationships serve as an effective barrier, potentially blocking transfer of circulating mRNA vaccine across the placental syncytium. The syncytiotrophoblast layer is considered a robust physical and immunologic barrier that prevents hematogenous transmission of both virus and nonviral pathogens.19,20,21 In our study, we did not detect either mRNA vaccine in the syncytiotrophoblast layer (after 0.5 or 4 h of exposure). The lipid nanoparticle delivery system is designed to facilitate membrane fusion and intracellular delivery of the vaccine.22,23 It is unknown how the placental tissue resists lipid nanoparticle uptake, despite direct exposure of fresh vaccine in the culture media. Different lipid nanoparticle composition has been recently shown to impact the degree of placental uptake.24 We would anticipate that in vivo, the uptake of mRNA vaccines into the placenta would be less due to many other factors that likely contribute to vaccine degradation. In summary, our study suggests limited uptake of the mRNA vaccine in chorionic villi.
Several cytokines were identified to increase in the conditioned media in a time-dependent manner irrespective of vaccine treatment or gestational age (Figure 3C). Therefore, we implemented a statistical approach to account for temporal differences to determine if specific cytokines were evoked by the mRNA vaccines in human placental explants compared to the no-vaccine control group. In general, the presence of either mRNA vaccine did not lead to significant perturbations in the release of cytokines into the media. These results suggest that the mRNA vaccines are not significantly immunogenic to the placenta. Thus, we provide evidence that the fetus is unlikely to experience a placental or fetal immune response to the vaccine itself which may allay persistent concerns about vaccine effects on the fetus. This is in contrast to COVID-19 infection, which is known to alter placental and fetal immune status, even in mild infections.25
The human placenta is complex and unique in its anatomy and function, as such, there are limitations in the ability of animal models to replicate human placentation and pregnancy.26 Human placental explants models maintain the natural structure and cellular organization of chorionic tissue that cell lines lack, permitting the investigation of tissue responses in vitro.27 In this study, we incubated human placental explants with the vaccine for up to several hours, with the goal of exaggerating the impact of vaccines on the chorionic villus and associated cell populations. We chose selected supraphysiologic concentrations (0.1 or 1 μg/mL) based on estimated local circulating levels of mRNA vaccines in mice vaccinated for influenza, which reported maximum concentrations of mRNA vaccines detected in the spleen and liver to be 0.087 μg/mL and 0.047 μg/mL, respectively.28 Local concentrations at the maternal-fetal placental barrier are likely to be lower than the testing concentrations used in this study. Under physiological conditions, vaccine concentrations are low in peripheral blood following tissue absorption, hematologic circulation, and natural degradation, which is further decreased by the physiologic hemodilution of pregnancy and increased circulatory rate.
Another advantage of our human placental explant model is the ability to examine the tissue responses at different gestational ages, as the placenta matures to support the different developmental stages of the fetus. Different patterns of cytokine secretion were observed between placentas from different trimesters (Figure 3C). For example, there were generally higher levels of IL-8, CCL3, CCL4, TNF-α, CXCL10, and IL-6 secreted from second trimester placental explants compared with those from third trimester. However, future studies may address this question with a larger sample size due to the potential dynamics in sensitivity to inflammatory stimuli across pregnancy.
Limitations of the study
Our study is not without limitations. First, we tested the effect of short-term exposure of the mRNA vaccine with the goal of identifying the initial cell and tissue responses in in vitro and ex vivo experiments. The durability of active mRNA vaccine in vivo is not known as multiple RNase enzymes contribute to degradation.29 One study of patients with chronic hepatitis C detected trace amounts of mRNA vaccine sequences have been detected in blood up to 28 days in 10% of patients,30 and another study of 16 healthy individuals detected vaccine mRNA at 14 days after inoculation.31 Prior work from our group did not detect any trace of vaccine mRNA or spike protein in 20 pregnant women at 2 to 75 (mean 32 days) after their second dose.32
Further studies are needed to evaluate the durability of mRNA vaccines in pregnancy, as the increased metabolic state may alter vaccine pharmacokinetics. Another limitation is the lack of testing on first trimester placental samples due to the lack of availability of tissue from this gestational age. However, since most vaccines are administered after the first trimester in pregnancy (usually due to patient preference), our findings are directly applicable to the majority of pregnant patients receiving vaccination.
In conclusion, our results provide further support to published clinical research that COVID-19 vaccination during pregnancy was not associated with increased pregnancy complications or adverse neonatal outcomes.33,34 There have been no adverse histopathologic findings in placentas isolated from vaccinated pregnancies.35,36 Thus, our results provide additional reassurance regarding the safety of COVID-19 mRNA vaccines in pregnancy, which have been shown to protect both the mother and the baby against COVID-19 and associated complications.37,38,39,40,41
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Human placental tissue | University of California, San Francisco (UCSF) | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Ham’s F-12K (Kaighn’s) Medium | Gibco | Cat#21127022 |
| Dulbecco’s modified Eagle’s medium | Gibco | Cat#21068028 |
| Fetal bovine serum | Sigma-Aldrich | Cat#F0926 |
| Penicillin-streptomycin | Gibco | Cat#15140122 |
| Gentamicin | Gibco | Cat#15750060 |
| DPBS | Gibco | Cat#14190250 |
| DME/H-21 | Gibco | Cat#11995065 |
| Glutamine plus | Atlanta Biologicals | Cat#B90210 |
| Nutridoma | Roche | Cat#11011375001 |
| Sodium pyruvate | Gibco | Cat#11360070 |
| HEPES buffer | Gibco | Cat#15630080 |
| SYBR™ Green qPCR Master Mix | Applied Biosystems | Cat#43-676-59 |
| 10% Neutral Buffered Formalin | RPI | Cat#F10800–500.0 |
| Ethanol 200PRF | Decon Labs | Cat#V1001 |
| Critical commercial assays | ||
| Human Luminex Premixed Multi-Analyte Kit | Bio-Techne | Cat#LXSAHM |
| RNeasy Micro Kit | Qiagen | Cat#74004 |
| qScript cDNA Synthesis Kit | Quantabio | Cat#95047-100 |
| RNAscope® 2.5 HD Reagent Kit - RED | ACD | Cat#322350 |
| Experimental models: Cell lines | ||
| BeWo choriocarcinoma cells | ATCC | CCL-98 |
| JEG-3 choriocarcinoma cells | ATCC | HTB-36 |
| A549 lung carcinoma cells | ATCC | CCL-185 |
| 293T cells | ATCC | CRL-3216 |
| Oligonucleotides | ||
| BNT162b2 vaccine | Pfizer-BioNTech | N/A |
| mRNA-1273 vaccine | Moderna | N/A |
| RNAscope Probe -V-nCoV2019-S | ACD | Cat# 848561 |
| RNAscope Positive Control Probe -Hs-PPIB | ACD | Cat# 313901 |
| RNAscope Negative Control Probe - DapB | ACD | Cat# 310043 |
| Forward primer for COVID mRNA vaccines 5′-AACGCCACCAACGTGGTCATC -3′ | Golan et al.16 | N/A |
| Reverse primer for COVID mRNA vaccines 5′-GTTGTTGGCGCTGCTGTACAC -3′ | Golan et al.16 | N/A |
| Software and algorithms | ||
| GraphPad Prism 9 | GraphPad | https://www.graphpad.com/ |
| R studio 2022.12.0 + 353 (R 4.2.2) | RStudio, Inc. | https://www.rstudio.com/ |
| LAS Science Microscope Software | Leica | https://www.leica-microsystems.com |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Stephanie L. Gaw (Stephanie.Gaw@ucsf.edu).
Materials availability
This study did not generate new unique reagents or materials.
Experimental model and study participant details
Culture of cell lines
BeWo placental choriocarcinoma cells were cultured in F-12K medium (Gibco, Waltham, MA). JEG-3 choriocarcinoma cells, A549 lung carcinoma cells and 293T embryonic kidney cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco). All media were supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and 1% penicillin-streptomycin (Invitrogen, Waltham, MA). Twenty-four hours prior to exposure to mRNA vaccines, cells were seeded at a density of 3 × 105 cells per well in a six-well plate and cultured at 37°C and 5% CO2 in a humidified incubator. On the next day, after discarding the old media and washing the cells with DPBS (Gibco), complete culture media with BNT162b2 or mRNA-1273 at the concentration of 0.1 μg/mL or 1 μg/mL were separately added to each well. After incubation (30 min or 4 h), media was removed and cells were washed with DPBS twice and then lysed in RTL (Qiagen, Hilden, Germany).
Tissue specimens
The study was approved by the institutional review board of the University of California, San Francisco. Second trimester placentas without evidence of pathology were obtained from consenting female participants following termination of pregnancy. Third trimester placentas from uncomplicated pregnancies were obtained following late preterm or term (36–39 weeks gestational age) planned cesarean deliveries. Indications for early delivery included history of classical cesarean section, history of uterine surgery, or placenta previa. Written consent was obtained from all participants. Placental biopsies measuring 3 × 3 cm were collected, washed in Cytowash (see below) and stored on ice prior to proceeding with explant dissection.
Placental explant cultures
Placental biopsies were washed in Cytowash (consisting of DME/H-21 (Gibco), 2.5% fetal bovine serum (HyClone), 1% glutamine plus (Atlanta Biologicals, Minneapolis, MN), 1% penicillin/streptomycin (Invitrogen), and 1% gentamicin (Gibco)42 and dissected into 1.0 cm2 chorionic villi explants. A single villus was placed in one well of a 24-well plate containing 0.5 mL of prewarmed cell free media [DME/H-21, 2% Nutridoma (Roche, Basel, Switzerland), 1% sodium pyruvate (Sigma-Aldrich, St. Louis, MO), 1% HEPES buffer (Invitrogen), 1% glutamate plus (Atlanta Biologicals), and 1% penicillin/streptomycin (Invitrogen)] with 10% fetal bovine serum and incubated at 37°C in 5% CO2/95% O2 for 30 min. Vaccine mixtures for explant exposure were then prepared such that final vaccine concentrations were: BNT162b2 and mRNA-1273 vaccine 0.1 μg/mL and 1 μg/mL, as well as control medium. Testing concentrations were based on estimates of localized circulating levels of mRNA vaccines in organs of mice vaccinated for influenza.28 Explants were exposed for 0.5 or 4 h and incubated at 37°C, after which 200 μl of supernatant were collected and stored at -4°C for cytokine analysis. Exposed explants were washed in PBS and then stored at −80°C until RNA extraction, or formalin-fixed for 24 h at room temperature, then washed in 70% ethanol and stored at -4°C until undergoing paraffin embedding.
Methods details
RNA extraction and real-time PCR (RT-PCR)
Total RNA was extracted from cells and villi using the RNeasy Micro Kit (Qiagen) RNA quantity and quality were measured by a NanoDrop spectrophotometer. Complementary DNA (cDNA) synthesis was performed using qScript cDNA Synthesis Kit (Quantabio, Beverly, MA), followed by qPCR using SYBR Green qPCR Master Mix with QuantaStudio 6 Flex system (Applied Biosystems, Waltham, MA). Each sample was run in duplicates. Primer sequences for both BNT162b2 and mRNA-1273 were as follows: AACGCCACCAACGTGGTCATC (forward, 5′ to 3′) and GTTGTTGGCGCTGCTGTACAC (reverse, 5′ to 3′).16 Vaccine concentrations in the cells and villi were determined by interpolation on the standard curve made from known concentrations of BNT162b2 and mRNA-1273 vaccines (Figure 1A).
RNAscope
The antisense probe targeting the spike region (21,631-23,303) of SARS-CoV-2 Wuhan-Hu-1 strain (GenBank accession number NC_045512.2) were purchased (Advanced Cell Diagnostics [ACD], Newark, CA). Sequence alignment of the probe and the mRNA vaccine sequence showed 73–79% identity. RNAscope 2.5 Assay was preformed according to the manufacturer’s protocol. Briefly, following formalin-fixed paraffin-embedded sample preparation and pretreatment, gene-specific probe pairs were hybridized to target mRNA. Probe targeting the spike region were hybridized to a cascade of signal amplification molecules, culminating in binding of HRP- or AP- labeled probes. Fast Red substrate was added to detect target RNA in tissue. Visualization of target RNA was preformed using standard bright field microscope and images were captured with Leica DFC495 digital camera (Leica, Hesse, Germany). Peptidylprolyl isomerase B (PPIB, positive control) and DapB (negative control) probes were used as the positive and negative controls.
Cytokine assay
Concentrations of 24 cytokines, IFN-γ, IFN-β, TNF-α, IL-1β, IL-10, IL-17A, IL-2, IL-4, IL-5, IL-6, IL-7, CXCL8/IL-8, IL12p70, IL-23, TNF-RI, CCL17/TARC, CCL2/MCP-1, CCL22/MDC, CCL3/MIP-1α, CCL4/MIP-1b, CXCL9/MIG, CXCL10/IP-10, IL-28A or IL-28B, were measured using the custom human premixed multiplex magnetic Luminex assay (Bio-Techne, Minneapolis, MN) according to the manufacturer’s protocol. Briefly, supernatant from placental explant cultures treated with vaccines were mixed with microparticle cocktail and added to each well of a 96-well plate. Plate was incubated at room temperature on a shaker at 800 rpm for 2 h, followed by 1h incubation with biotin-antibody and 30-min incubation with streptavidin-PE. Plate was washed with wash buffer 3 times after each incubation. Then wash buffer was add to wells for resuspending the microparticle and plate was read on a Luminex MAGPIX CCD Imager. All samples were run in duplicate, and standards and blanks were applied to each plate. Median fluorescence intensity (MFI) of each cytokine was corrected by subtracting background and interpolated to the standard curve.
Quantification and statistical analysis
Statistical analyses were performed using GraphPad Prism 9.1.0 (GraphPad, San Diego, CA). We used Mann-Whitney test or Kruskal-Wallis test to determine the statistical significance between vaccine-treated groups and control groups. We implemented multiple regression models to determine the significance of these variables on levels of specific cytokines: 1) BNT162b2 and culture duration; 2) mRNA-1273 and culture duration; or 3) culture duration and gestational age. We applied unsupervised hierarchical clustering of cytokine levels using average linkage and Euclidean distance to determine whether global patterns associated with culture duration, vaccine exposure, or gestational age.43 p-values <0.05 were interpreted as significant.
Acknowledgments
We thank Kenneth Scott (University of California, San Francisco, UCSF Health Pharmacy) and Hannah J. Jang, PhD, RN, PHN, CNL (University of California, San Francisco, UCSF School of Nursing and the Center for Nursing Excellence and Innovation) for providing unused vaccine for this study.
Funding: These studies were supported by the National Institutes of Health (NIAID K23AI127886 to M.P.; NIAID K08AI141728 to S.L.G.; and the National Center for Advancing Translational Sciences through UCSF-CTSI Grant Number #UL1 TR001872-06 to V.J.G.) and the Marino Family Foundation (to M.P.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Author contributions
V.G., J.R., and S.G. conceived and designed the study. V.G., L.L., S.B., M.P., and J.R. conducted the experiments. L.L., M.P., J.R., and S.G. analyzed the data. V.G. and L.L. wrote the first draft of the manuscript. J.R. and S.G. supervised the experiments. All authors provided critical editing of the manuscript.
Declaration of interests
The authors declare no conflict of interest.
Inclusion and diversity
We worked to ensure diversity in experimental samples through the selection of the cell lines. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper self-identifies as a gender minority in their field of research. One or more of the authors of this paper received support from a program designed to increase minority representation in their field of research.
Published: August 7, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107549.
Contributor Information
Joshua F. Robinson, Email: joshua.robinson@ucsf.edu.
Stephanie L. Gaw, Email: stephanie.gaw@ucsf.edu.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon.
References
- 1.Prevention, C.f.D.C.A CDC COVID-19 Study Shows mRNA Vaccines Reduce Risk of Infection by 91 Percent for Fully Vaccinated People. https://www.cdc.gov/media/releases/2021/p0607-mrna-reduce-risks.html
- 2.Prevention, C.f.D.C.a COVID-19 Vaccines While Pregnant or Breastfeeding. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html
- 3.Prevention, C.f.D.C.a COVID-19 vaccination among pregnant people aged 18-49 years overall, by race/ethnicity, and date reported to CDC - Vaccine Safety Datalink. https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women
- 4.Smith D.D., Pippen J.L., Adesomo A.A., Rood K.M., Landon M.B., Costantine M.M. Exclusion of Pregnant Women from Clinical Trials during the Coronavirus Disease 2019 Pandemic: A Review of International Registries. Am. J. Perinatol. 2020;37:792–799. doi: 10.1055/s-0040-1712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dashraath P., Nielsen-Saines K., Madhi S.A., Baud D. COVID-19 vaccines and neglected pregnancy. Lancet. 2020;396:e22. doi: 10.1016/S0140-6736(20)31822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tetro N., Moushaev S., Rubinchik-Stern M., Eyal S. The Placental Barrier: the Gate and the Fate in Drug Distribution. Pharm. Res. (N. Y.) 2018;35:71. doi: 10.1007/s11095-017-2286-0. [DOI] [PubMed] [Google Scholar]
- 7.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naidu S.A.G., Clemens R.A., Pressman P., Zaigham M., Kadkhoda K., Davies K.J.A., Naidu A.S. COVID-19 during Pregnancy and Postpartum. J. Diet. Suppl. 2022;19:115–142. doi: 10.1080/19390211.2020.1834049. [DOI] [PubMed] [Google Scholar]
- 9.Peltier M.R. Immunology of term and preterm labor. Reprod. Biol. Endocrinol. 2003;1:122. doi: 10.1186/1477-7827-1-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi G.B., Yim Y.S., Wong H., Kim S., Kim H., Kim S.V., Hoeffer C.A., Littman D.R., Huh J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yockey L.J., Iwasaki A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity. 2018;49:397–412. doi: 10.1016/j.immuni.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zerbo O., Qian Y., Yoshida C., Grether J.K., Van de Water J., Croen L.A. Maternal Infection During Pregnancy and Autism Spectrum Disorders. J. Autism Dev. Disord. 2015;45:4015–4025. doi: 10.1007/s10803-013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bronson S.L., Bale T.L. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 2014;155:2635–2646. doi: 10.1210/en.2014-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards D.K., Jasny E., Yoon H., Horscroft N., Schanen B., Geter T., Fotin-Mleczek M., Petsch B., Wittman V. Adjuvant effects of a sequence-engineered mRNA vaccine: translational profiling demonstrates similar human and murine innate response. J. Transl. Med. 2017;15:1. doi: 10.1186/s12967-016-1111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowalczyk A., Doener F., Zanzinger K., Noth J., Baumhof P., Fotin-Mleczek M., Heidenreich R. Self-adjuvanted mRNA vaccines induce local innate immune responses that lead to a potent and boostable adaptive immunity. Vaccine. 2016;34:3882–3893. doi: 10.1016/j.vaccine.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 16.Golan Y., Prahl M., Cassidy A., Lin C.Y., Ahituv N., Flaherman V.J., Gaw S.L. Evaluation of Messenger RNA From COVID-19 BTN162b2 and mRNA-1273 Vaccines in Human Milk. JAMA Pediatr. 2021;175:1069–1071. doi: 10.1001/jamapediatrics.2021.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts K., Bayraktar O.A. Automation of Multiplexed RNAscope Single-Molecule Fluorescent In Situ Hybridization and Immunohistochemistry for Spatial Tissue Mapping. Methods Mol. Biol. 2020;2148:229–244. doi: 10.1007/978-1-0716-0623-0_15. [DOI] [PubMed] [Google Scholar]
- 18.Cribiù F.M., Erra R., Pugni L., Rubio-Perez C., Alonso L., Simonetti S., Croci G.A., Serna G., Ronchi A., Pietrasanta C., et al. Severe SARS-CoV-2 placenta infection can impact neonatal outcome in the absence of vertical transmission. J. Clin. Invest. 2021;131 doi: 10.1172/jci145427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidima W.B. Syncytiotrophoblast Functions and Fetal Growth Restriction during Placental Malaria: Updates and Implication for Future Interventions. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/451735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid A.J., Vermont S.J., Cotton J.A., Harris D., Hill-Cawthorne G.A., Könen-Waisman S., Latham S.M., Mourier T., Norton R., Quail M.A., et al. Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora N., Sadovsky Y., Dermody T.S., Coyne C.B. Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe. 2017;21:561–567. doi: 10.1016/j.chom.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suga K., Tanabe T., Tomita H., Shimanouchi T., Umakoshi H. Conformational change of single-stranded RNAs induced by liposome binding. Nucleic Acids Res. 2011;39:8891–8900. doi: 10.1093/nar/gkr568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsay K.E., Bhosle S.M., Zurla C., Beyersdorf J., Rogers K.A., Vanover D., Xiao P., Araínga M., Shirreff L.M., Pitard B., et al. Visualization of early events in mRNA vaccine delivery in non-human primates via PET-CT and near-infrared imaging. Nat. Biomed. Eng. 2019;3:371–380. doi: 10.1038/s41551-019-0378-3. [DOI] [PubMed] [Google Scholar]
- 24.Young R.E., Nelson K.M., Hofbauer S.I., Vijayakumar T., Alameh M.G., Weissman D., Papachristou C., Gleghorn J.P., Riley R.S. Lipid Nanoparticle Composition Drives mRNA Delivery to the Placenta. bioRxiv. 2022 doi: 10.1101/2022.12.22.521490. Preprint at. [DOI] [Google Scholar]
- 25.Garcia-Flores V., Romero R., Xu Y., Theis K.R., Arenas-Hernandez M., Miller D., Peyvandipour A., Bhatti G., Galaz J., Gershater M., et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat. Commun. 2022;13:320. doi: 10.1038/s41467-021-27745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grigsby P.L. Animal Models to Study Placental Development and Function throughout Normal and Dysfunctional Human Pregnancy. Semin. Reprod. Med. 2016;34:11–16. doi: 10.1055/s-0035-1570031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller R.K., Genbacev O., Turner M.A., Aplin J.D., Caniggia I., Huppertz B. Human placental explants in culture: approaches and assessments. Placenta. 2005;26:439–448. doi: 10.1016/j.placenta.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Bahl K., Senn J.J., Yuzhakov O., Bulychev A., Brito L.A., Hassett K.J., Laska M.E., Smith M., Almarsson Ö., Thompson J., et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. 2017;25:1316–1327. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uddin M.N., Roni M.A. Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccines. 2021;9:1033. doi: 10.3390/vaccines9091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castruita J.A.S., Schneider U.V., Mollerup S., Leineweber T.D., Weis N., Bukh J., Pedersen M.S., Westh H. SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination. Apmis. 2023;131:128–132. doi: 10.1111/apm.13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fertig T.E., Chitoiu L., Marta D.S., Ionescu V.-S., Cismasiu V.B., Radu E., Angheluta G., Dobre M., Serbanescu A., Hinescu M.E., Gherghiceanu M. Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination. Biomedicines. 2022;10:1538. doi: 10.3390/biomedicines10071538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prahl M., Golan Y., Cassidy A.G., Matsui Y., Li L., Alvarenga B., Chen H., Jigmeddagva U., Lin C.Y., Gonzalez V.J., et al. Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and infancy. Nat. Commun. 2022;13:4422. doi: 10.1038/s41467-022-32188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theiler R.N., Wick M., Mehta R., Weaver A.L., Virk A., Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am. J. Obstet. Gynecol. MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L., Marquez P.L., Olson C.K., Liu R., Chang K.T., et al. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shanes E.D., Otero S., Mithal L.B., Mupanomunda C.A., Miller E.S., Goldstein J.A. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccination in Pregnancy: Measures of Immunity and Placental Histopathology. Obstet. Gynecol. 2021;138:281–283. doi: 10.1097/aog.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smithgall M.C., Murphy E.A., Schatz-Siemers N., Matrai C., Tu J., Baergen R.N., Yang Y.J. Placental pathology in women vaccinated and unvaccinated against SARS-CoV-2. Am. J. Obstet. Gynecol. 2022;227:782–784. doi: 10.1016/j.ajog.2022.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beharier O., Plitman Mayo R., Raz T., Nahum Sacks K., Schreiber L., Suissa-Cohen Y., Chen R., Gomez-Tolub R., Hadar E., Gabbay-Benziv R., et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Invest. 2021;131 doi: 10.1172/jci150319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kugelman N., Nahshon C., Shaked-Mishan P., Cohen N., Sher M.L., Gruber M., Marom I., Zolotarevsky A., Lavie O., Damti A., et al. Maternal and Neonatal SARS-CoV-2 Immunoglobulin G Antibody Levels at Delivery After Receipt of the BNT162b2 Messenger RNA COVID-19 Vaccine During the Second Trimester of Pregnancy. JAMA Pediatr. 2022;176:290–295. doi: 10.1001/jamapediatrics.2021.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halasa N.B., Olson S.M., Staat M.A., Newhams M.M., Price A.M., Boom J.A., Sahni L.C., Cameron M.A., Pannaraj P.S., Bline K.E., et al. Effectiveness of Maternal Vaccination with mRNA COVID-19 Vaccine During Pregnancy Against COVID-19-Associated Hospitalization in Infants Aged <6 Months - 17 States, July 2021-January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:264–270. doi: 10.15585/mmwr.mm7107e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halasa N.B., Olson S.M., Staat M.A., Newhams M.M., Price A.M., Pannaraj P.S., Boom J.A., Sahni L.C., Chiotos K., Cameron M.A., et al. Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. N. Engl. J. Med. 2022;387:109–119. doi: 10.1056/NEJMoa2204399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsui Y., Li L., Prahl M., Cassidy A.G., Ozarslan N., Golan Y., Gonzalez V.J., Lin C.Y., Jigmeddagva U., Chidboy M.A., et al. Neutralizing antibody activity against SARS-CoV-2 variants in gestational age–matched mother-infant dyads after infection or vaccination. JCI Insight. 2022;7 doi: 10.1172/jci.insight.157354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunkapiller N.M., Fisher S.J. Chapter 12. Placental remodeling of the uterine vasculature. Methods Enzymol. 2008;445:281–302. doi: 10.1016/s0076-6879(08)03012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saeed A.I., Sharov V., White J., Li J., Liang W., Bhagabati N., Braisted J., Klapa M., Currier T., Thiagarajan M., et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon.