Abstract
Ovarian reserve is essential for fertility and influences healthy aging in women. Advanced maternal age correlates with the progressive loss of both the quantity and quality of oocytes. The molecular mechanisms and various contributing factors underlying ovarian aging have been uncovered. In this review, we highlight some of critical factors that impact oocyte quantity and quality during aging. Germ cell and follicle reserve at birth determines reproductive lifespan and timing the menopause in female mammals. Accelerated diminishing ovarian reserve leads to premature ovarian aging or insufficiency. Poor oocyte quality with increasing age could result from chromosomal cohesion deterioration and misaligned chromosomes, telomere shortening, DNA damage and associated genetic mutations, oxidative stress, mitochondrial dysfunction and epigenetic alteration. We also discuss the intervention strategies to delay ovarian aging. Both the efficacy of senotherapies by antioxidants against reproductive aging and mitochondrial therapy are discussed. Functional oocytes and ovarioids could be rejuvenated from pluripotent stem cells or somatic cells. We propose directions for future interventions. As couples increasingly begin delaying parenthood in life worldwide, understanding the molecular mechanisms during female reproductive aging and potential intervention strategies could benefit women in making earlier choices about their reproductive health.
Keywords: mitochondrial therapy, oocyte quality, oocyte quantity, ovarian aging, ovarioids, senotherapy
Introduction
Life expectancy of human beings has greatly increased from 45 to 85 years over the past 150 years [1], but the age at natural menopause (ANM) has remained around the age of 50 years [2]. According to cohort studies, the decline in female fertility begins around the late 20s to early 30s and accelerates after the age of 35 years, especially among nulliparous women [3], [4], [5], [6], thus, fecundity decreases as age increases. Ovarian aging can be part of normal biological aging, where genetic as well as environmental factors influence its onset and determine the age of menopause [7], [8], [9], [10]. The genetic integrity of oocytes decreased with advancing age [11] and almost half of women are infertile by the age of 40 years (Figure 1) [6, 12, 13]. More women are choosing to delay childbearing to more advanced age worldwide, resulting in more frequent use of assisted reproductive technology (ART) [14, 15]. Also, there is only an approximately 6.5% chance of achieving pregnancy with each mature oocyte thawed from the preservation of oocytes or ovarian tissue, which decreases with age [16, 17]. Therefore, prevention of age-associated infertility becomes extremely important to the woman as well as to the family and society.
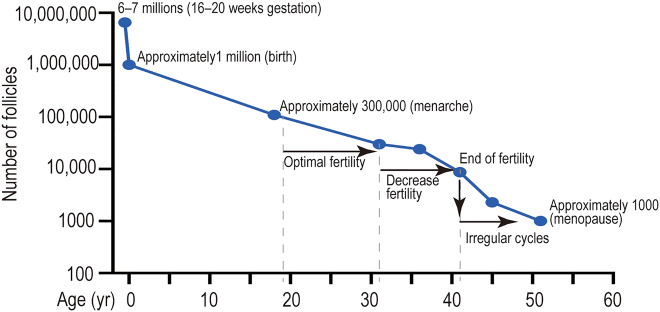
Figure 1:
The dynamics of human follicular development and reserve during fetal development and in adult. Oocyte and follicle reserve decline in the ovary during aging. Follicular quantity reaches maximum at 16/20 weeks of fetal development [18, 19]. Approximately one million viable follicles remain at birth, the mechanism of primordial follicles loss remains to be fully determined [20]. At the time of menarche, women have 300,000 to 400,000 follicles, which are recruited cyclically until menopause (approximately 1000 follicles) [21]. yr, year.
In addition to reproductive functions, the ovarian also plays an important role in maintaining female hormone secretion. Fertility loss during female aging is associated with increasing basal follicle stimulating hormone (FSH) and decreasing anti-Mullerian hormone (AMH) concentrations, together with compromised oocyte quality, presumably due to increased oxidative stress (OS) and DNA damage, as well as reduced metabolic and meiotic competences [22, 23]. Excessive serum FSH also acts directly on hippocampal and cortical neurons to accelerate amyloid-β and tau deposition and impair cognition in female mice displaying features of Alzheimer’s disease [24]. Estrogen is produced in the ovaries by the follicles that house the oocytes. Physiological functional endocrine endows women their feminine characteristics and reproductive abilities, and is also key for maintaining healthy lifespan including heart health, bone density and mood. With a depleting ovarian reserve, both physiological fluctuation of estrogen and progesterone production will decline unpredictably and, eventually, halt [25]. Ovarian aging and disrupted endocrine function increases risk of reproductive aging-associated chronic diseases such as heart disease, incontinence, and osteoporosis.
Multiple factors, including non-germ-line stem cells in adulthood female mammals, chromosomal, genetic, mitochondrial, reactive oxygen species (ROS) and environmental factors, impact the quantity and quality of oocytes in the ovarian reserve (Figure 2). Elucidating the molecular mechanisms underlying the decline in ovarian reserve and oocyte function would facilitate achieving targeted intervention in postponing ovarian aging and improving oocyte quality. Although achievements have been gained in diagnostic and intervention field, once the ovarian reserve has declined markedly, few treatments other than ART are effective in treating infertility. Understanding of gonadal development and ovarian physiology may allow reconstituting the ovarioids to provide an alternative source of gametes for reproduction and rebuilding physiological endocrine function. Thus, there is requirement for developing better diagnostic tools and improving treatments in the fields of reproductive medicine. We here broadly review molecular mechanisms of ovarian aging, recent developed intervention strategies in the reproductive field, and prospective directions.
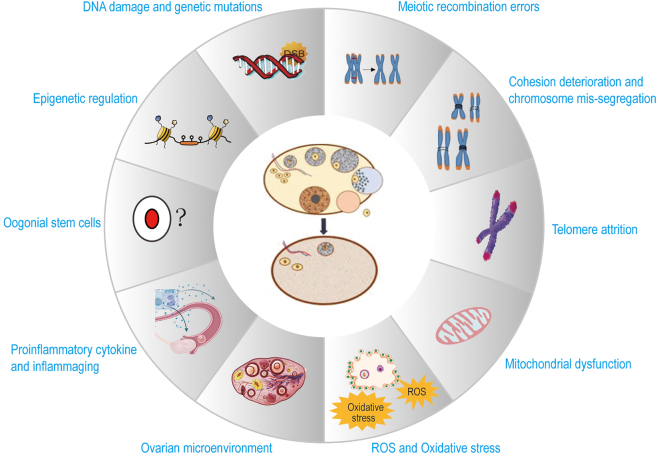
Figure 2:
Current understanding of molecular mechanisms underlying ovarian aging. A multitude of physiological factors and molecular changes that can diminish the quantity and quality of ovarian reserve lead to ovarian aging. Most factors are explained further under subheadings in the text. DSBs, double-strand breaks; ROS, reactive oxygen species.
Main mechanisms of ovarian aging
Oocyte quantity and ovarian aging
Oocyte quantity
Women are born with a limited number of oocytes. During the fifth week of human pregnancy, female fetal ovaries contain 500 to 1300 primordial germ cells (PGCs) that undergo mitosis to generate approximately 6–7 million germ cells at the 20th gestation week [18]. Following the completion of mitosis, female germ cells undergo prophase of meiosis I, beginning at the time of E12.5 to E13.5 in mice and approximately E10 weeks to E11 weeks in human [19], exhibiting typical stages including leptotene, zygotene, pachytene and diplotene stage when the meiocytes arrest. This phase may last for months for mouse oocytes or years for human oocytes. The meiotic prophase resumes the diakinesis phase when the puberty is reached. The meiotic germ cells form cysts and generate primordial follicles that each oocyte is surrounded by a single layer of granulosa cells. Only 1–2 million viable follicles remain at birth, the mechanism of primordial follicles loss remains to be fully determined [20]. The number of primordial follicles is down to approximately 400,000 at puberty, of which about 350 oocytes ultimately are ovulated during reproductive life [21]. Follicle depletion is associated with reduced production of the hormones estrogen and inhibin by the ovary, disrupting the hypothalamic-pituitary-gonadal hormonal axis and eventually leading to menopause [26]. When reaching ANM at the mean age of about 51 years old, fewer than 1000 primordial follicles are left, which no longer sustain ovulation [27]. Mathematical models show that ovarian reserve depletion during the fertile years appears to accelerate with age, mainly because of increased oocyte atresia [18]. Therefore, ANM is determined by the non-renewable functional ovarian reserve, which is established during fetal development and continuously depleted until ovarian senescence. ANM can vary significantly between individuals depending on genetic and environmental factors. Of the non-genetic risk factors, cigarette smoking is the main factor proven to accelerate the age of menopause by about three years [28], limiting reproductive potential. Primary ovarian insufficiency (POI), is determined by exhaustion of functional ovarian reserve in the individuals suffered certain genetic disorders, autoimmune diseases or after chemo- or radiotherapy for cancer treatment, leading to infertility before the age of 40 years [29]. Moreover, maternal age reduces pregnancy outcomes [30]. Therefore, follicle depletion serves as a timer for the major landmarks of female reproduction and ovarian aging.
Dispute over germ-line stem cells in female mammals
Stem cells are featured with the capacity of self-renewal as well as lineage differentiation. Germ-line stem cells share common features of self-renewal and differentiation specifically into meiotic cells or meiocytes that undergo homologous pairing and recombination. Germ-line stem cells are present and functional in many non-mammalian organisms and male mammals. Traditionally, they have been thought not to exist in female mammals. However, this traditional view had been challenged according to the expression of germ-cell markers by extra-follicular cells, arguing that female mammals possess oogonial stem cells (OSCs) that replenish their supply of oocytes in a physiological context. It was reported that ovarian surface epithelium of adult mice contained OSCs that sustained postnatal follicular renewal [31]. Moreover, using anti-Ddx4 antibody coupled to fluorescence activated cell sorting, up to 1000 Ddx4-positive cells were isolated per young adult mouse ovary [32]. The Vasa/Mvh/Ddx4 positive germ cells existed in the postnatal and adult ovaries were claimed as germline stem cells [31, 33]. It remains to be tested whether these Vasa/Mvh/Ddx4 positive germ cells isolated from adult ovaries can undergo self-renewal as well as are capable of differentiation into meiotic cells featured with homologous pairing shown by synaptonemal complex protein 1/synaptonemal complex protein 3 (SCP1/SCP3) filaments and recombination (e.g. presence of specific MutL homolog 1 [MLH1] foci). Interestingly, by labeling the Oct4-expressing small germ cells and tracing their fates for up to 4 months in mouse ovary, Guo et al. observed that Oct4+Dazl−, but not Oct4+Dazl+ or Oct4+Ddx4+ cells, contain a population of germ stem cells in extra-follicular ovarian cells [34]. It remains to be investigated whether the marked cells can undergo meiotic homologous pairing and recombination and differentiate into oocytes. Thus far, existence of typical meiotic cells at prophase I stage that exhibit homologous pairing and recombination in the adult ovaries under physiological conditions has not been rigorously demonstrated.
Additionally, it was reported that bone marrow transplantation restored oocyte production in wild-type mice sterilized by chemotherapy, as well as in ataxia telangiectasia-mutated gene-deficient mice, which were otherwise incapable of making oocytes, and the bone marrow was considered to be a potential source of germ cells that could sustain oocyte production in adulthood [35]. However, Eggan et al. [36] established transplantation and parabiotic mouse models to assess the capacity of circulating bone marrow cells to generate ovulated oocytes, both in the steady state and after induced damage and showed that ovulated oocytes in adult mice derive from non-circulating germ cells [36].
The straightforward way to detect whether OSCs exist in vivo in the adult is to identify the extra-follicular ovarian cells that express the germ-line stem cell markers, which are expressed prior to differentiation into meiocytes in fetal ovaries. Interferon-induced transmembrane protein 3 (IFITM3) is expressed in the PGCs [37]. Using the anti-IFITM3 antibody, we isolated cells with germ-line characteristics from E12.5 embryos, which contain mitotically active germ cells, but not from adult mice ovaries [38]. Moreover, Ddx4, Dppa3 and POU class 5 homeobox 1 (POU5F1) were detected by immunoblotting in ovarian cells isolated using anti-IFITM3 from E12.5 female mouse embryos, which contained mitotically active germ cells. However, only Dppa3 was detected in the IFITM3-positive cells that were isolated from the adult ovaries [38]. These results are consistent with the traditional view that no OSCs remain in the adult ovary.
The germ-line cells also can be marked in the unperturbed ovary and traced their fate. Lei and Spradling generated CAG-CreER/Esr1TM;ROSA-EGFP mouse model [39], and Zhang et al. generated Sohlh1-CreER T2 ;R26R mouse model [40], the brief tamoxifen-based treatment strategy was taken to mark a subset of follicles. The marked primordial follicles were subsequently lost at the same rate as the unmarked follicles. Instead of monitoring the loss of marked follicles, Zhang et al. generated tamoxifen-induced Forkhead box L2 (Foxl2)-CreER T2 ;mT/mG mouse model to search for evidence that new follicles are occurred [40]. Foxl2 is expressed in granulosa cells but not in the pre-granulosa cells of the ovarian epithelium [41]. The authors triggered a switch from tdTomato to green fluorescent protein (GFP) expression in cells expressing Foxl2 during the brief tamoxifen treatment. Thus, if new follicles were assembled, their granulosa cells would be red. Yet, all ovaries at 2 weeks and at 2, 6, and 12 months contain only follicles with green fluorescent somatic cells. This implies that adult mice do not generate new follicles. Furthermore, Zhang et al. discovered that no adult oogenesis occurred after ablation of all oocytes in Gdf9-Cre;iDTR ovaries [40].
Another key criterion for identifying the presence of a newborn egg or neo-oogenesis is whether the germ cell undergo normal meiosis, where homologous chromosome pairing and recombination occur [42]. During meiosis, homologous chromosome pairing and recombination take place in the prophase of meiosis I, and the appearance of synaptonemal complexes provides the structural basis for the recombination between chromosomes. SCP3 is the key to the formation of synaptonemal complex, and the joint detection of the distribution of proteins such as SCP1, SCP3 and MLH1 can be used as an important marker to measure the normality of homologous chromosome synapsis and homologous recombination. In primate monkeys, the number of eggs decreases markedly with age. In monkey ovaries of different ages (3–18 years old), no meiotic cells were found to undergo SCP1/SCP3 homologous chromosome pairing and homologous recombination (MLH1 junctions), which indicated that no new eggs occurred in adult ovaries [43]. Also, neo-oogenesis and meiocytes were not found in adult human ovaries [44]. Thus far, no studies have reported neo-oogenesis and meiocytes in mice.
A more complete map of cell types in the human ovarian cortex was characterized by single-cell RNA-seq recently [45]. The important finding in the study was to determine that DDX4-positive cells, isolated by an DDX4 antibody used in previous study [32, 46], from ovarian cortex are perivascular cells but not oogonial stem cells. This result was verified using a different analysis, including immunostaining validation, which showed DDX4 antibody localization at perivascular sites in human ovarian tissue. This result provides strong evidence opposing the controversial studies arguing for the existence of oogonial stem cells in mammalian adult ovaries [47]. By careful comparison of the published results and analysis of the data, Hainaut and Clarke conclude that the weight of evidence strongly supports the traditional interpretation that germ-line stem cells do not exist post-natally in female mammals [48]. This concept is consistent with the fact that the continuously depleted non-renewable ovarian reserve with age is the key limiting factor in ovarian aging.
Oocyte quality and ovarian aging
Oocyte quality
In addition to the diminishing follicle reserve, oocyte quality also is declined with increasing maternal age. At birth, the finite ovarian reserve comprises non-growing primordial follicles arrested in the dictyate stage and do not complete their first meiotic division before puberty is reached. Some oocytes and their surrounding cells remain arrest stage for 40 years or more. ROS causes OS and damage to cells, which, over time, contributes to structural and functional damages that are distinctive for cellular senescence and aging [49]. An increase in ROS levels in ovaries has been suggested to play a key role in the pathogenesis of age-related infertility in female mammals [50, 51]. The gradual deterioration in oocyte quality begins at least after the age of 31 years. This coincides with a decrease in oocyte quantity and partly explains the decline in fertility long before the onset of menopause, along with the abnormal endocrine profile found in reproductively older women [52], [53], [54]. On cellular levels, the oocyte aging phenomenon are mainly manifested by cohesin dysfunction [55], crossover maturation inefficiency (CMI) [56], chromosome misalignments and meiotic spindle disruption [57], telomere attrition [58], DNA damage [59] and mitochondrial dysfunction [60]. CMI is a major contributing factor to increase the incidence of both chromosome mis-segregation, especially with increased age. The loss of oocyte quality is believed to be due to an increase in meiotic nondisjunction, resulting in aneuploidies and unsuccessful pregnancy outcomes at higher female ages [61, 62].
Main molecular mechanisms for the deterioration of oocyte quality
(1). Chromosomal cohesion deterioration
Aneuploidy in eggs is a leading cause of infertility, miscarriage and congenital syndromes. Aneuploidy and maternal aging follow a U-shaped relationship [11]. More than 20% of eggs are aneuploidy from young women 20–32 years of age, however, women in both very young (< 20 years) and advanced age group (> 35 years) can be greatly more at risk of generating aneuploid eggs than women 20–32 years of age. Notably, aneuploidy occurs in more than 50% of eggs from women aged 35 years and above [11, 63]. Data from mice suggest that cohesion levels on chromosomes could determine the U-shaped curve [64], [65], [66], [67], [68]. Sister chromatids are bound together by many ring-like protein structures called cohesion complexes that are installed during DNA replication [66]. Cohesion complexes are essential for the correct segregation of chromosomes during the divisions of meiosis in each ovulation cycle of adult female mammals. Importantly, evidence from mouse oocytes indicated that chromosomes might initially be overloaded with cohesin to ensure that adequate cohesin levels are maintained for the entire reproductive life span. This overloading may cause higher rates of chromosome mis-segregation during the first cohorts of oocytes to be ovulated in young women [11]. With increasing female age, chromosomes cohesion in oocytes naturally deteriorates, leading to premature chromosome separation [69], [70], [71]. Therefore, in the 20s and early 30s, cohesin levels might be at an ideal intermediate level, which is not too high to prevent chromosomes from segregating at anaphase but also not too low to maintain the integrity of the chromosomes. In contrast, cohesin in old oocytes are susceptible to removal by separase, a cysteine protease that cleaves the cohesin subunit REC8 and then destroy the cohesin ring. Importantly, separase is restricted from cleaving most cohesin that holds sister chromatids together as these links need to be maintained for sister chromatid alignment and separation during the second meiotic division. This function is provided by Shugoshin-like 2 (SGO2) in complex with PP2A that locally dephosphorylate REC8 [72, 73]. Notably, SGO2 expression decreases in oocytes with advancing age [74, 75]. Depletion of SGO2 in mouse oocytes causes loss of centromeric cohesion during anaphase I [75]. Decreased cohesion results in a greater probability of chromosome mis-segregation, premature chromatid separation, and aneuploid [69, 70, 76].
(2). Telomere shortening
Telomeres are composed of repetitive nucleotide sequences that form a “cap structure” which functions to maintain the integrity of chromosomes. Telomere length is known to directly correlated with both life expectancy and reproductive life span [77], [78], [79]. Comparison of telomere lengths in the leukocytes of women revealed that the average telomere length in the extended fertility group is significantly longer than in the normal fertility group [80, 81]. Telomeres are shorter in the leukocytes of postmenopausal women than those of a similar age but still menstruating [82]. Moreover, women with longer telomeres entered menopause up to 3 years later than in those with shorter telomeres [82]. These results suggest that menopause may occur after telomere shortening reaches a specific length. Moreover, patients with polycystic ovary syndrome with low telomerase activity and short telomeres in granulosa cells, which support oocyte maturation, show an earlier onset of infertility [83].
A significant cause of telomere attrition in oocytes is the accumulation of ROS [84]. Telomere length is pivotal for accurate chromosomal alignment as well as adequate function of the spindle assembly checkpoint during meiosis, both of which prevent aneuploidy in embryos [79]. Shorter telomere is associated with higher rates of aneuploidy among infertile women undergoing in vitro fertilization [58, 85, 86]. Short telomeres impede germ cell specification by upregulating mitogen-activated protein kinase (MAPK) and transforming growth factor beta (TGF-β) signaling [87]. Therefore, oocytes and early embryos with telomere attrition may be an indicator of improper chromosome segregation, leading to aneuploidy, moreover, telomere shortening has been suggested to be involved in embryonic fragmentation and developmental arrest [79, 88].
Loss of telomerase activity also leads to telomere shortening in ovaries. Telomerase activity presents in adult granulosa cells, but almost unmeasurable in mature oocytes [89, 90]. In mouse models, telomerase components (TERT and Terc) and telomere-associated proteins (TRF1, TRF2, and POT1a) have been shown to gradually decrease in the ovary from young to aged group as well as in the follicles from primordial to antral stages and their oocytes and granulosa cells, contributing to telomere shortening [90]. In women at 37 years old or less, a lack of telomerase activity or aberrant telomere homeostasis in human granulosa is associated with occult ovarian insufficiency [91]. Shortened telomere length and diminished telomerase activity were shown to be associated with biochemical POI [81, 92, 93]. Additionally, a woman with dyskeratosis congenita, a telomeropathy associated with a reduced ovarian reserve, responded poorly to hormonal treatment before in vitro fertilization and her oocytes contained critically short telomeres [94]. Therefore, telomere length and telomerase activity have a great impact on ovarian aging, and a mathematic model of telomere shortening in leukocytes could potentially be used to evaluate the reproductive aging status or ovarian aging.
(3). DNA damage response associated genetic mutations
The ANM varies between individuals. Genetic factors play an important role in ANM. Ovarian oocyte reserve is required to generate eggs and to response to gonadotropic hormones. POI is determined by exhaustion of follicles in the ovaries, which leads to infertility prior to the age of 40s. It is characterized by a strong familial and heterogeneous. Over the last years, several cohort studies have elucidated a likely oligogenic inheritance of POI [95], [96], [97], [98], [99], 107 genes related to POI etiology were identified. Of the 107 genes, the most genes linked to syndromic POI are mainly implicated in meiosis/DNA repair and metabolism function. Additionally, the majority of genes associated with nonsyndromic POI are also mainly implicated in meiosis/DNA repair pathways [59, 100]. A recent genome-wide association study of approximately 200,000 women of European ancestry identified 290 genetic determinants of ovarian aging [10], highlighting a much broader involvement of DNA damage response (DDR) and of metabolic signaling networks such as the phosphatidylinositol 3-kinase pathway in the regulation of ovarian aging. Intact DNA repair mechanisms are essential to maintain metabolic balance and delay aging phenotypes [101, 102]. Cellular mechanisms that repair DNA damage become less effective during aging. In oocytes, deceased efficacy of DNA repair mechanisms could result in poor quality, apoptosis, and ultimately menopause, infertility and miscarriage [10, 103]. Activation of the DDR during meiosis can result in the elimination of oocytes with unrepaired meiotic DNA double-strand breaks (DSBs) above a threshold level via the TRP53 and TAp63 pathways in mice [104, 105]. DNA damage or the failure to mount DNA damage response promotes cell death, resulting in depleted oocyte reserve that induces POF.
Breast cancer-associated 1 (BRCA1) and BRCA2, with functions in homologous recombination repair, are associated with depleted oocyte reserve [106]. Both BRCA1 and BRCA2 are involved in the repair of ataxia telangiectasia mutated (ATM) and RAD3-related (ATR) protein kinase-mediated DNA DSBs in oocyte [107, 108]. The expression of ATM in ovaries and oocytes is dramatically reduced after mid-thirties, which is in line with the lower fecundity at the women from aged 35 years and above. BRCA1 expression declines around a decade earlier [107]. In Brca1-deficient mice, reproductive capacity is impaired, primordial follicle counts are lower, and DSBs are increased in remaining follicles with age relative to wild-type mice [107]. AMH is one of the best predictors known to evaluate ovarian reserve [109]. Women with BRCA mutations, particularly of the BRCA1 gene, have lower serum AMH levels, consistent with diminished ovarian reserve [107, 110], [111], [112], [113], [114], [115]. Also, women with BRCA mutations appear to experience menopause at earlier age [116], [117], [118]. Furthermore, women carrying loss-of-function variants in BRCA1 and BRCA2 reported ANM 2.63 years earlier and 1.54 years earlier [10]. In general, women with BRCA mutations have lower ovarian reserve and experience earlier menopause.
Another component essential to the repair of DSBs is the cell cycle checkpoint kinase 2 (CHK2) [10, 119]. Women carrying loss-of-function variants in CHK2 manifested ANM 3.49 years later [10]. CHK2 is a downstream effector of the ATM kinase that responds primarily to DSBs, and can also be activated by the ATR kinase that responds primarily to single stranded DNA [86, 120]. CHK2 has important functions in checkpoint signaling during the progression of the cell cycle in mitosis and meiosis [10, 104, 105, 119, 121]. CHK2 is essential for culling mouse oocytes bearing unrepaired meiotic or induced DNA DSBs. Both meiotically programmed and induced DSBs detected by CHK2 trigger the CHK2-dependent activation of TRP53 and TRP63 pathway to eliminate the defective oocytes [119].
(4). ROS and OS
ROS are the by-products of mitochondrial oxidative phosphorylation and include superoxide, hydroxyl, and hydrogen peroxide. ROS react with the surrounding DNA, proteins and lipids, leading to mutations and macromolecular damage. Under physiological conditions, the production and neutralization of ROS are balanced. Early oocytes have an apparent lack of ROS and balance essential mitochondrial activity by suppressing complex I [122].
Excessive ROS generation overwhelms the cellular anti-oxidant defenses, resulting in OS, mitochondrial and nuclear DNA damage, and premature aging of ovaries [123]. In mice, long-term OS caused by ROS has been associated with decreased follicle and oocyte quality without affecting oocyte quantity [124]. Ovaries of ozone-exposed mice show high levels of ROS as well as the decreased fertility [124]. Mice fed with the antioxidant N-acetyl-L-cysteine (NAC) have better quality oocytes and longer reproductive lifespan [125]. Moreover, NAC treatment also maintains oocyte telomere length and telomerase activity [125].
Humans and non-human primates have similar age-related ovarian alterations. Wang et al. [126] used single-cell RNA-seq analysis and found the down-regulation of antioxidant genes, including GPX1, GSR, GPX4 and PON1, in aged early-stage monkey oocytes, and increased apoptosis plus decreased reductase activity-related genes, including IDH1, PRDX4 and NDUFB10, in aged monkey granulosa cells, suggesting oxidative damage as an essential factor in ovarian aging. Furthermore, human granulosa cells isolated from the follicular fluid also exhibited aging-associated down-regulation of IDH1, PRDX4 and NDUFB10 [126], indicating that these genes can be biomarkers and targets for diagnosis and treatment of human age-related ovarian diseases, such as POI, and decreased ovarian reserve. In a study of physiologic aging in C57BL/6 wild type mice, ovarian peroxiredoxin 3 and thioredoxin 2 mRNA expression decreased significantly with age [127]. In human, high levels of OS within the ovary, follicular fluid, granulosa, and cumulus cells correlate with follicular atresia and poor oocyte quality, as well as decreased oocyte fertilization, embryo development and fertility [128]. Collectively, oxidative damage caused by ROS impacts reproductive potential by decreasing the quality of ovarian follicles and oocytes [129].
Increasing evidences supports the notion that lifestyle modification to minimize OS preconception can improve female fertility. Caloric restriction (CR) is known to delay the aging caused functional decline in tissue throughout the body [130], [131], [132]. CR has also been found to extend reproductive lifespan in female mice [133, 134]. Obesity is being considered as a state of OS and is known to reduce the live birth [135, 136]. Maternal obesity induces the defective telomeres in oocytes and embryos [137]. Of note, Children of mothers who had the metabolic syndrome (or obesity) in pregnancy had significant shorter telomeres than of normal [138, 139]. Additionally, several environmental factors such as cigarette smoke [140, 141], alcohol [142], bisphenol A [143, 144], exposure to chemicals and radiation have been linked OS with ovarian aging. However, a healthy balanced diet and regular exercise can reduce excess OS and correlates positively with clinical pregnancy rate in in vitro fertilization (IVF) [145], [146], [147], [148].
(5). Mitochondrial dysfunction
Mitochondria in mammalian oocytes are spherical with a few cristae surrounding the electron-dense matrix [149, 150]. Mitochondria function requires coordination of both mitochondrial and nuclear genomes. Normally, mitochondria in the oocyte are generated during oogenesis, and then production ceases at the stage of the mature oocyte [151]. Mitochondria play a central role in follicular atresia and could be the main target of the ooplasmic factors determining oocyte quality adversely affected by aging. The aging effects on oocyte mitochondria have been seen through morphological and functional abnormalities. Mitochondrial swelling and vacuolization are associated with reduced adenosine-triphosphate (ATP) and mitochondrial DNA (mtDNA) content in aged mouse and hamster oocytes [152, 153]. Mitochondria are highly dynamic organelles responding to cellular stress through changes in overall mass, interconnectedness, and sub-cellular localization [154, 155]. The multiple mechanisms through which mitochondria drive ovarian aging include mitochondrial dysfunction, impaired mitochondrial dynamics (biogenesis, fusion and fission, mitophagy, apoptosis, mitocytosis [156], and spatial dynamics), accumulation of mtDNA mutations, altered membrane potential, and defective electron transport chain function. mtDNA mutations exacerbate female reproductive aging by impairing nicotinamide adenine dinucleotide (NADH/NAD+) redox [157], and increasing ovarian NAD+ levels improve mitochondrial functions and reverse ovarian aging [158]. Mitochondrial biogenesis is required for cell growth to produce increased metabolites and energy and defects in biogenesis are frequently lethal to cells and organisms. Mitochondrial biogenesis might be initiated by the small, round-shaped vesicles in the nuclei, followed by their translocation into the cytoplasm and protein import to achieve their complete construction and biological functions [159], [160], [161]. Mitochondrial biogenesis depends upon the activity of a hierarchy of nuclear factors, including peroxisome proliferator activator receptor alpha (PPARα), PPARγ, nuclear respiratory factor-1 and -2, estrogen related receptor. All of these transcription factors are critical dependent for their activity on PPARγ coactivator 1 alpha (PGC-1α). Nutrient supply and energy balance in the cell modulates PGC-1α activity at both the transcriptional and post-translational level [162]. Deceased mitochondrial biogenesis, as indicated by lower mtDNA content, is routinely observed during POI, diminished ovarian reserve (DOR), and physiological ovarian aging. Compared with those of normal ovarian reserve, PGC-1α expression is lower in cumulus cells and accompanied by decreased mtDNA content in cumulus cells and oocytes in women with DOR [163].
Re-modeling of the mitochondrial network in cells is mechanically regulated by key dynamin-related fusion and fission gene products. Mitochondrial fusion is promoted by homotypic/heterotypic interaction of the Mitofusin 1 (Mfn1) and Mitofusion 2 (Mfn2) at the outer mitochondrial membrane (OMM) of adjacent mitochondria and by Opa-1 at the inner mitochondria membrane [154, 164]. Mitochondrial fission is promoted by the GTPase activity of the dynamin-related protein (DRP1) that is recruited to the OMM where it forms ring-like oligomers that pinch off mitochondria into small fragmented mitochondria in response to stresses [164]. Fusion and fission defects have significant implications in ovarian aging. Oocyte-specific deletion of Mfn1 in mice leads to female sterility associated with the defective folliculogenesis and impaired oocyte quality [165, 166]. Targeted deletion of Mfn2 in oocytes results in mitochondrial dysfunction and female subfertility associated with impaired oocyte maturation and follicle development [167]. Oocyte-specific depletion of Drp1 leads to decreased oocyte quality due to maturation defects [168, 169]. Also, fission-deficient oocytes due to the oocyte-specific depletion of Drp1 exhibit a high frequency of failure in peri- and post-implantation development [170].
Mitochondrial mass in cells is also regulated by the changes in mitophagy and mitocytosis, and these processes are tightly regulated in response to cellular stress. Phosphoglycerate translocase 5 (PGAM5), located in the mitochondrial membrane, is associated with mitophagy, necroptosis and apoptosis. PGAM5 activates DRP1 through dephosphorylation and induces mitochondrial fracture and necrosis [171]. Moreover, highly expressed PGAM5 is directly related to a significant reduction in cellular ATP production [172].
(6). Epigenetic influences on ovarian aging
Mature oocyte DNA has higher methylation levels, but with age, the levels of DNA methyltransferases (DNMTs) decrease in oocytes and DNA demethylases (TETs) increased, hence overall DNA methylation levels decreased [173]. Combined analysis of single oocyte transcriptomes and DNA methylomes in young and old mice showed that changes in cellular transcription of aged oocytes result from differential gene methylation [174]. Tet1-deficiency in mouse model downregulates the premature ovarian failure (POF) related gene Fmr1, reduces the number of follicles, causes POF, and thus reduces reproductive performance [175]. Tet2 deletion increases DNA damage and impairs pathways such as the spindle checkpoint and the actin cytoskeleton, thereby reducing oocyte quality and reproductive performance [176].
During the maturation of oocytes, histone modifications show dynamic changes, and in the germinal vesicle (GV) phase, H4K12 and H4K16 are acetylated. Deacetylation by deacetylase occurs during MI and MII of oocytes [177]. With increasing age, the acetylation levels of H4K12 and H4K16 in aged GV oocytes decrease, but the deacetylation of H4K12 is impaired in MII oocytes. Excessive histone acetylation in MII oocytes can lead to increased aneuploidy and death of post-fertilization embryos [178], [179], [180]. Histone methylation modifications however are relatively stable during oocyte maturation [181]. Yet, the level of histone H3K4 trimethylation (H3K4me3) is high in fully grown oocytes derived from young mammalian females but decreased during aging due to the decreased expression of epigenetic factors responsible for H3K4me3 accumulation [182]. Oocyte-specific knockout of the gene encoding CxxC-finger protein 1 (CXXC1), a DNA-binding subunit of SETD1 methyltransferase, causes ooplasm changes associated with accelerated aging and impaired maternal mRNA translation and degradation [182]. Therefore, a network of CXXC1-maintained H3K4me3, in association with mRNA decay competence, sets a timer for oocyte deterioration and plays a role in oocyte aging in both mouse and human oocytes.
(7). The ovarian microenvironment affects ovarian aging
Ovarian tissue consists of two parts: the cortex and the medulla. The medulla is composed of connective tissue, fibrous tissue, and blood vessels. The ovarian microenvironment is composed of different types of cells within the ovary, and communication between oocytes and ovarian microenvironment is mediated by direct contact with surrounding cells, the extracellular matrix, and signaling molecules, including hormones, growth factors, and metabolites [183]. Therefore, the ovarian microenvironment can affect the quality of oocytes, and even accelerates the oocyte aging and leads to diseases such as infertility. Bidirectional communication between the oocyte and its associated somatic cells plays a key role in fertility and embryogenesis. Cumulus cells provide essential nutrients for oocyte maturation through different paracrine signaling pathways. In addition, cumulus cells are also involved in the regulation of oocyte aging [151]. For example, cumulus cells accelerate oocyte senescence by activating the Fas/FasL pathway [184, 185]. Ceramides can also be transferred from cumulus cells to oocytes through gap junctions, increasing age-related oocyte apoptosis [186, 187]. Altered homeostasis between synthesis and degradation of extracellular matrix (ECM) components affects tissue structure and function, and excessive accumulation of ECM contributes to tissue fibrosis. In addition to organs such as heart, lung, and kidney, fibrosis is also present in ovarian tissue, and fibrosis within the mouse ovarian stroma increases with reproductive age, so fibrosis is an early marker of ovarian stroma aging [188]. Ovarian fibrosis is reversible, and antifibrosis drugs (pirfenidone and BGP-15) is reported to eliminate fibrotic collagen and restore ovulation in reproductively old and obese mice, in association with dampened M2 macrophage polarization and up-regulated MMP13 protease [189]. On the other hand, the temporary suppression of angiogenesis by axitinib administration pauses ovarian development and keeps the ovarian reserve in the long term, leading to postponed ovarian senescence and an extension of the female reproductive life span [190].
miRNAs are small non-coding RNAs that bind to target messenger RNA (mRNA) to inhibit their expression. miRNAs, either freely present or enclosed in vesicles (exosomes) in human follicular fluid [191], are also involved in the regulation of oocyte senescence. For example, miR-16-5p, miR214-3p, and miR-449a are down-regulated and miR-125b, miR-155-5p, and miR-372 up-regulated in the follicular fluid of older women, affecting vesicular release, oocyte maturation, and response to stress response [192]. Circular RNAs (circRNAs) are a unique class of endogenous RNAs that can be used as potential diagnostic and prognostic biomarkers for many diseases. Abnormal expression of circRNAs is found in aging ovaries, which are related to the metabolic processes, regulatory secretory pathways, redox processes, and steroid hormone biosynthesis associated with ovarian aging [193]. Specifically, the expression of circRNA_103827 and circRNA_104816 is increased in aged human granulosa cells, and the high expression is closely associated with decreased ovarian reserve and poor reproductive outcomes in women [194]. Therefore, the ovarian microenvironment modulates the process of ovarian aging.
Ovarian aging intervention strategy
To date, there are no clinically feasible techniques to either maintain or reverse ovarian dysfunction associated with advanced age [195]. However, important advances have been made in the field of senotherapy during the last few years, although the majority of the studies are performed in mouse models, these pre-clinical tests may show potential in improving female fertility.
Efficacy of antioxidants on reproductive aging
Even though there are no clinically practical less invasive therapeutic measures to reverse ovarian dysfunction associated with advanced age, several drugs are performed in organismal models. Important advances have been made in the field of senotherapy to extend the reproductive lifespan in female mammals [195, 196]. Oxidative damage is a crucial factor in ovarian functional decline with age, therefore, antioxidants, such as resveratrol, nicotinamide mononucleotide (NMN), NAC, melatonin and coenzyme Q10 (CoQ10), may prevent oxidative damage and delay ovarian aging.
Resveratrol (3,5,4′-trihydroxystilbene) is a polyphenolic compound found in the skin of red grapes with antioxidant, anti-inflammatory, cardioprotective and anti-neoplastic properties [197]. Resveratrol improves mitochondrial function and protects against metabolic disease by activating longevity-related protein Sirtuin 1 (SIRT1) and PGC-1a in different type of cells [198], [199], [200]. Several studies have established that resveratrol regulates animal reproduction. Resveratrol enhances maturation and quality of aged oocytes [201], and is an effective cryo-protectant with antioxidant and anti-apoptotic effects [202]. Excitingly, long-term-oral administration of resveratrol protects against the reduction of fertility with reproductive aging in mice by improving healthy follicle number, telomere length and telomerase activity, as well as oocyte quantity and quality [203]. Methylglyoxal affects the mouse oocyte quality by resulting in excessive ROS production, aberrant mitochondrial distribution and high level lipid peroxidation. Resveratrol protects the oocytes from methylglyoxal-induced cytotoxicity and this is mainly through the correction of the abnormity of cellular ROS metabolism [204]. Moreover, resveratrol significantly increases the weights of POF mice and their ovaries as well as the number of follicles, while decreasing the atresia rate of follicles [205]. Therefore, resveratrol has the potential in intervening the diminished ovarian reserve and function through its suppression of OS. But resveratrol also has adverse effects on implantation and endometrial decidualization [206]. Effects of resveratrol are time and dosage dependent. While low dose of resveratrol promotes oocyte quality and ovarian function, the high dose leads to embryo apoptosis [203]. Given that doses ≥ 1.0 g may produce side effects, including headache, dizziness, nausea, diarrhea, and liver dysfunction [207, 208], the optimal frequency, dosage for resveratrol treatment needs to be further explored.
NMN is an intermediate of the metabolic cofactor nicotinamide adenine dinucleotide (NAD+/NADH), and decreased with advanced age. NAD+ is a very important metabolic redox cofactor and enzyme substrate that mediates a variety of biological processes including cell death, aging, gene expression, neuro-inflammation and DNA repair [209]. As revealed by many recent studies, deficiency of NAD+ can be compensated by the NMN supplementation that successfully prevent age-associated conditions from metabolic and neurodegenerative disease to cancer [210]. In aged mice ovaries, NMN supplementation improves the oocyte quality as evidenced by meiotic competency, increases ovulation and fertilisation capability through restoring NAD+ levels [211]. Furthermore, NMN reinstates mitochondrial functions of aged oocytes to mitigate accumulation of both DNA damage and ROS. Moreover, long-term treatment of NMN improves age-related diminished ovary reserve through enhancing the mitophagy level of granulosa cells in mice [212]. To date, a number of NMN commercial products have been produced by various pharmaceutical, biotechnology and health food companies. The quantity of NMN in available products vary from 50 to 500 mg/capsule, whereas some consumers take two 150 mg capsules per day [213, 214]. Given NMN treatment (400 mg/kg) obstructed the exercise-induced benefits of a mouse model of diet-induced obesity such as reduced hepatic triglyceride accumulation, glucose stimulated insulin secretion from islets and glucose tolerance [215], the recommended safe dose levels for long term administration requires rigorous scientific preclinical, clinical and toxicological testing.
Melatonin (5-methoxy-N-acetyltryptamine) is an endogenous hormone that is primarily released by the pineal gland to modulate many important physiological reactions [216]. Additionally, melatonin can be synthesized in the follicular granulosa cells and oocytes. Melatonin and its metabolites efficiently decrease OS levels by directly scavenging ROS as well as enhancing antioxidant activity and modulation of mitochondrial and inflammatory activities [217], [218], [219]. Melatonin administration induced high melatonin concentration in the follicular fluid microenvironment improves oocyte quantity and quality in reproductive old females [220, 221]. Insufficient amounts of melatonin in follicular fluid are highly correlated with advanced maternal age-related meiotic defects [222, 223]. Melatonin can increase telomere length, upregulate the glutathione peroxidase expression and SIRT pathways, and decrease inflammation [224, 225]. Moreover, melatonin improves the quality of maternally aged oocytes by maintaining intercellular communication and antioxidant metabolite supply [221].
Additionally, other small compounds could be used for delaying ovarian aging and improving fertility. For example, α-Ketoglutarate can delay ovarian aging and telomere shortening in mice by inhibiting metabolism and mammalian target of rapamycin (mTOR) pathway [226]. Some natural antioxidants such as quercetin and curcumin can also protect the ovaries [129]. More compounds are proving beneficial in improving oocyte and ovarian functions.
Mitochondrial therapy
Aged oocytes have significantly reduced amounts of mitochondria, leading to lower fertilization rates and poor embryonic development. Therefore, search for better sources of mitochondria improvement by means of mitochondrial nutrient therapy or mitochondrial-transfer therapy to combat the stress caused by aging are among the most challenging approaches.
CoQ10 is an essential component for transporting electrons in the mitochondrial respiratory chain to produce cellular energy [227, 228]. Ubiquinol, the reduced form of CoQ10, acts as an antioxidant in cellular metabolism via inhibition of lipid peroxidation, protein, and DNA oxidation [229], [230], [231]. CoQ10 promotes repair of dysfunctional oocyte mitochondria and reduces the ovarian expression of 8-hydroxydeoxyguanosine [232]. CoQ10 synthesis decreases in the oocyte with age, coinciding with the decline in oocyte quality and general fertility [233]. CoQ10 supplementation in an aged animal model delayed depletion of ovarian reserve, restored oocyte mitochondrial gene expression, and improved mitochondrial activity. But CoQ10 supplementation had no impact on ovarian reserve or oocyte quality of young females in which mitochondrial function is intact, implying the effect of CoQ10 to specifically target age-associated mitochondrial dysfunction [233]. A randomized double-blind study conducted on a small group of IVF-ICSI (in vitro fertilization-intracytoplasmic sperm injection) women (35–43 years of age) found the rate of oocyte aneuploidy to be 46.5% with CoQ10 treatment (600 mg) vs. 62.8% in the control group. Clinical pregnancy rate was 33% for the CoQ10 group and 26.7% for the control group, neither of these results was statistically significant owing to the small scale of the study [234]. Another randomized controlled trial on low-prognosis young women with decreased ovarian reserve demonstrated pretreatment with CoQ10 to be effective in improving the ovarian response to stimulation and embryo quality during IVF-ICSI cycles [235]. These results are encouraging for the treatment of age-associated infertility using CoQ10, although further work is needed to determine the overall effect on pregnancy complications and live birth rates as well as the optimal timing and dosage of CoQ10 supplementation.
Due to the relevance of aging in infertility, mitochondrial enrichment has been proposed as a potential therapy option in infertile patients to improve oocyte quality. Various techniques have been attempted to use heterologous or autologous sources of mitochondria to rejuvenate and improve oocyte health by introducing new sources of energy [236, 237]. In the heterologous approach, mitochondrial enrichment can be performed by relocating a healthy cytoplasm into the patient’s oocyte (partial cytoplasm transfer) or replacing the compromised cytoplasm with a competent one by means of nuclear transfer technology (total cytoplasm transfer). In 1982, Ooplasmic transfer (OT) in mice laid the foundations of oocyte rejuvenation treatments [238]. OT involves transferring a cytoplasm portion from a donor’s oocyte to the patient’s oocyte to introduce potentially beneficial components that might restore oocyte viability [239]. In 1997, Cohen and colleagues announced the first human pregnancy after OT [240]. Following this achievement, this method has been successfully used in low-prognosis patients [239, 241, 242]. But this approach entails many ethical and safety concerns that mainly arise from the uncertain degree of mitochondrial heteroplasmy deriving from it. Mitochondrial heteroplasmy in the offspring and its unknown consequences have led to alternative strategies being proposed to improve oocyte quality.
Germinal vesicle, spindle, pronuclear, polar body and blastomere transfer constitute different ways of relocating the genetic material from a patient’s compromised oocyte or zygote to a healthy cytoplasm. GV transfer consists of relocating the GV from the compromised oocyte to an enucleated healthy oocyte at the same developmental stage by electrofusion, subsequently matured in vitro to the MII stage [243]. The GV transfer in humans was performed by Zhang’s group and Takeuchi respectively [244, 245], but no live birth and healthy offspring have been described in humans yet, compared to what has been found in animal studies [246, 247].
Using germinal vesicle nuclear transfer, Liu and colleagues found that both nuclear and cytoplasmic factors contributed to the meiotic defects of the old oocytes of Senescence-accelerated mice and that the nuclear compartment played the predominant role in the etiology of aging-related meiotic defects [248]. To data, the main limitations of GV transfer include: high mitochondrial aggregation around the GV and the maturation process needed from the GV to the MII stage [244, 249]. Nuclear genetic materials assemble in a spindle structure at the metaphase of the second meiosis [236]. Given spindle structure removal is a common procedure employed in cloning [250], spindle transfer is less invasive than GV transfer, as condensed chromosomes can be easily aspirated with a smaller enucleation pipette and a minimal amount of cytoplasm due to its location at the periphery of the oocyte [243]. Tachibana and colleagues demonstrated that monkey reconstructed oocytes following spindle transfer were capable of supporting normal fertilization, embryo development and produced healthy offspring [251]. However, they failed to translate these successful results to humans [252]. In 2017, the first human live birth derived from oocyte spindle transfer to prevent Leigh syndrome. It was reported that transfer of the embryo resulted in a pregnancy with delivery of a boy with neonatal mtDNA mutation load of 2.36%–9.23% in his tested tissues [253].
Yet, the heterologous approach entails many ethical and safety concerns that mainly arise from the uncertain degree of mitochondrial heteroplasmy deriving from it. Therefore, the autologous approach is proposed a suitable potential tool to improve oocyte quality by overcoming the heteroplasmy issue. Mitochondrial autologous sources include immature oocytes [254], granulosa cells [255], germline stem cells [256], and adipose-derived stem cells [257]. However, the autologous germline mitochondrial energy transfer (AUGMENT) has not yielded as many beneficial outcomes as initially expected [256]. The triple-blind, single-center, randomized, controlled experimental pilot study was conducted by Labarta and colleagues at IVI-RMA Valencia (Spain) from October 2015 to June 2017. A total of 57 poor-prognosis patients with previous IVF failures and well-documented poor embryo quality were included in this study, and an ovarian cortex biopsy was performed to isolate egg precursor cells to obtain their mitochondria. Sibling MIIs were randomly allocated to AUGMENT (experimental) or intracytoplasmic sperm injection (Control). In AUGMENT, mitochondrial suspension was injected along with the sperm. Eventually, AUGMENT did not seem to improve prognosis in this population [256]. Together, it seems that these autologous techniques do not improve clinical outcomes in human infertile patients presently. Search for better sources of mitochondria and organelle improvement by means of gene editing/engineering or culture media additives to combat the stress caused by aging are among the most challenging approaches.
Fertility preservation by ovarioids rejuvenated from pluripotent stem cells
Given the limited ovarian reserve at birth and no self-renewal germ cells in the adulthood, any intervention by anti-aging compounds or mimics of antioxidation cannot extend the reproductive lifespan for longer term. Reconstitution of ovarian functions emerge as a prosper strategy for extending reproductive lifespan in future. In early studies, transplantation of young ovaries restored cardio-protective influence and increased life span in post-reproductive-aged mice [258, 259]. Reconstruction of ovarian and reproductive endocrine functions is a new strategy for fertility preservation, which is of great significance for fertility protection and reducing reproductive aging-related chronic diseases. PGCs, which give rise to both oocytes and the spermatozoa, are specified at around E7.25 in the mouse. Spermatogonia and oogonia differentiate from PGCs and initiate meiosis after E12.5–13.5 via signaling from the somatic genital ridge [260, 261]. PGCs-like cells (PGCLCs) have been successfully induced from pluripotent stem cells (PSCs) [262, 263]. PGCs and PGCLCs complete meiosis in vitro and generate functional gametes that produce live offspring [264, 265]. Importantly, functional oocytes can be developed in the recipients following transplantation of PGCs aggregated with E12.5 fetal somatic pre-granulosa cells into kidney capsule, ovarian bursa or intra-ovarian injection [266], [267], [268], [269]. Transplantation of PGCs aggregated with gonadal somatic cells effectively restores folliculogenesis and endocrine function (Figure 3A). However, the majority of PGCs enter meiosis and undergo folliculogenesis soon after transplantation, and proliferative PGCs and meiocytes disappear about six weeks following transplantation [270]. Encouragingly, mTOR inhibition by INK128 improves and extends the reconstituted ovarian and endocrine functions in reproductive aging and premature aging mice [271].
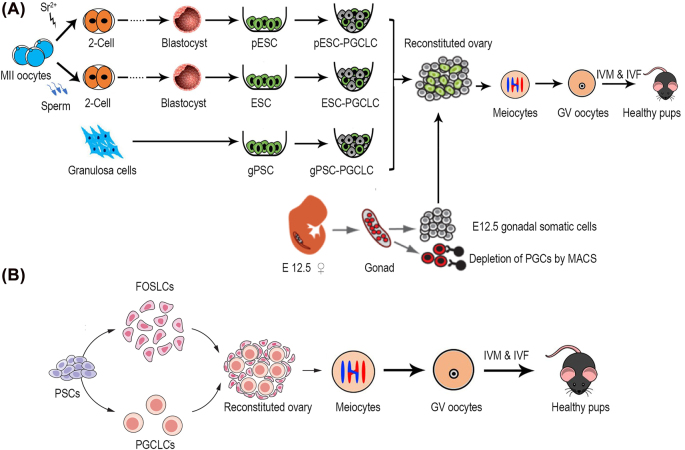
Figure 3:
Current strategies for reconstitution of follicle structures. (A) Scheme for PGCLC induction from PSCs and ovary reconstitution. PGCLCs aggregated with gonadal somatic cells effectively restores folliculogenesis and endocrine function. (B) Scheme for reconstitution of both FOSLCs and PGCLCs from PSCs. Oocytes in the reconstituted environment gave rise to offspring after fertilization. PGCLCs, primordial germ cells-like cells; PSCs, pluripotent stem cells; FOSLCs, fetal ovarian somatic cells-like cells; ESC, embryonic stem cell; pESC, parthenogenetic embryonic stem cell; gPSC, granulosa cells-derived germline competent pluripotent stem cell; Sr, strontium; GV, germinal vesicle; IVM, in vitro maturation; IVF, in vitro fertilization.
Follicular granulosa cells isolated from adult mouse ovaries can be robustly induced to generate germline-competent pluripotent stem cells (gPSCs) by a purely chemical approach, with additional Rock inhibition and critical reprogramming facilitated by crotonic sodium or acid. These gPSCs acquired high germline competency and could consistently be directed to differentiate into PGCLCs and form functional oocytes that produce fertile mice (Figure 3A) [272]. Also, parthenogenetic embryonic stem cells are able to differentiate into PGCLCs and form oocytes following in vivo transplantation into kidney capsule that produce fertile pups and reconstitute ovarian endocrine function (Figure 3A) [273]. Recently, culture conditions were developed to recreate the stepwise differentiation process from pluripotent cells to fetal ovarian somatic cell-like cells (FOSLCs). When FOSLCs were aggregated with PGCLCs derived from embryonic stem cells, the PGCLCs entered meiosis to generate functional oocytes capable of fertilization and development to live offspring (Figure 3B) [274]. This methodology opens the possibility for application in fertility preservation because it does not require embryonic gonads. Taken together, we can conclude that germ cells could be generated from mouse somatic cells without transfection of transcription factors [272, 275]. Human oogonia also could be generated from PSCs in culture [276, 277].
Conclusion and perspectives
Women increasingly delay childbearing later in their life worldwide [14, 15]. Though cryogenically stored eggs or ovarian tissues avoid exposure to the biological and environmental factors that compromise the quality of oocytes in the ovary, these procedures together with ART are costly and carry an approximately only 6.5% chance of achieving pregnancy with each mature oocyte thawed from the preservation of oocytes or ovarian tissue, which further decreases with age. Moreover, after ANM, ovaries are doomed to lose the function in maintaining female hormone secretion, leading to increased risk for certain chronic diseases associated with age. Thus, a better understanding of oocyte biology is essential to develop the strategies to prevent the decline in fertility as maternal age advances. This review provides a broad survey and insight into mechanisms of ovarian aging.
Recent studies have provided a deeper understanding of the mechanisms of ovarian aging and the major factors that impact it including non-OSCs in adulthood female mammals, chromosomal cohesion deterioration, telomere shortening, DNA damage and DDR-associated genetic mutations, mitochondrial dysfunction, ROS and OS, epigenetic alteration and aging ovarian microenvironment. These factors influence both the quantity and quality of the oocytes. A range of promising strategies have been developed to prolong fertility in women. Limiting exposure to oxidative damage may help preserve more oocytes with high quality within the ovaries during aging. Antioxidants, such as resveratrol, NMN, NAC, melatonin and CoQ10, may prevent oxidative damage and delay oocyte aging. If successful, delaying reproductive aging with the use of antioxidants could help lower the costs of infertility treatment when using ART. Currently, the main limitation for use the antioxidants in human reproductive aging is to establish the correct time, the optimal frequency and dosage, as well as the possible side effect of long-term administration. In future, it is possible to realize fertility preservation or reconstruct the ovarian function by ovarioids rejuvenated from pluripotent stem cells, however, this methodology may have the challenges with ethical and technical concerns to overcome.
Footnotes
Research funding: This work was funded by the National Natural Science Foundation of China (31970667; 82230052; 91749129; 32030033) and China National Key R&D Program (2018YFC1003004; 2018YFA0107002).
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Informed consent: Informed consent was obtained from all individuals included in this review.
Ethical approval: The local Institutional Review Board deemed this manuscript exempt from review.
Contributor Information
Zhengmao Zhu, Email: zhuzhengmao@nankai.edu.cn.
Lin Liu, Email: liulin@nankai.edu.cn.
References
- 1.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–208. doi: 10.1016/s0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inter LST. Variations in reproductive events across life: a pooled analysis of data from 505 147 women across 10 countries. Hum Reprod. 2019;34:881–93. doi: 10.1093/humrep/dez015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howe G, Westhoff C, Vessey M, Yeates D. Effects of age, cigarette smoking, and other factors on fertility: findings in a large prospective study. Br Med J (Clin Res Ed) 1985;290:1697–700. doi: 10.1136/bmj.290.6483.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothman KJ, Wise LA, Sorensen HT, Riis AH, Mikkelsen EM, Hatch EE. Volitional determinants and age-related decline in fecundability: a general population prospective cohort study in Denmark. Fertil Steril. 2013;99:1958–64. doi: 10.1016/j.fertnstert.2013.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eijkemans MJ, van Poppel F, Habbema DF, Smith KR, Leridon H, te Velde ER. Too old to have children? Lessons from natural fertility populations. Hum Reprod. 2014;29:1304–12. doi: 10.1093/humrep/deu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Z, Zheng D, Wu H, Li R, Xu S, Kang Y, et al. Epidemiology of infertility in China: a population-based study. BJOG. 2018;125:432–41. doi: 10.1111/1471-0528.14966. [DOI] [PubMed] [Google Scholar]
- 7.Moslehi N, Mirmiran P, Tehrani FR, Azizi F. Current evidence on associations of nutritional factors with ovarian reserve and timing of menopause: a systematic review. Adv Nutr. 2017;8:597–612. doi: 10.3945/an.116.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow ET, Mahalingaiah S. Cosmetics use and age at menopause: is there a connection? Fertil Steril. 2016;106:978–90. doi: 10.1016/j.fertnstert.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appt SE, Chen H, Goode AK, Hoyer PB, Clarkson TB, Adams MR, et al. The effect of diet and cardiovascular risk on ovarian aging in cynomolgus monkeys (Macaca fascicularis) Menopause. 2010;17:741–8. doi: 10.1097/gme.0b013e3181d20cd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruth KS, Day FR, Hussain J, Martinez-Marchal A, Aiken CE, Azad A, et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature. 2021;596:393–7. doi: 10.1038/s41586-021-03779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruhn JR, Zielinska AP, Shukla V, Blanshard R, Capalbo A, Cimadomo D, et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science. 2019;365:1466–9. doi: 10.1126/science.aav7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tehrani FR, Firouzi F, Behboudi-Gandevani S. Investigating the clinical utility of the anti-mullerian hormone testing for the prediction of age at menopause and assessment of functional ovarian reserve: a practical approach and recent updates. Aging Dis. 2022;13:458–67. doi: 10.14336/AD.2021.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambalk CB, van Disseldorp J, de Koning CH, Broekmans FJ. Testing ovarian reserve to predict age at menopause. Maturitas. 2009;63:280–91. doi: 10.1016/j.maturitas.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Andersen CY, Mamsen LS, Kristensen SG. Fertility preservation: freezing of ovarian tissue and clinical opportunities. Reproduction. 2019;158:F27–34. doi: 10.1530/rep-18-0635. [DOI] [PubMed] [Google Scholar]
- 15.Qiao J, Wang Y, Li X, Jiang F, Zhang Y, Ma J, et al. A lancet commission on 70 years of women’s reproductive, maternal, newborn, child, and adolescent health in China. Lancet. 2021;397:2497–536. doi: 10.1016/s0140-6736(20)32708-2. [DOI] [PubMed] [Google Scholar]
- 16.Gook DA, Edgar DH. Cryopreservation of female reproductive potential. Best Pract Res Clin Obstet Gynaecol. 2019;55:23–36. doi: 10.1016/j.bpobgyn.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update. 2016;22:440–9. doi: 10.1093/humupd/dmw007. [DOI] [PubMed] [Google Scholar]
- 18.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–6. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Dong J, Yan L, Yong J, Liu X, Hu Y, et al. Single-cell RNA-seq analysis maps development of human germline cells and gonadal niche interactions. Cell Stem Cell. 2017;20:891–2. doi: 10.1016/j.stem.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Markström E, Svensson E, Shao R, Svanberg B, Billig H. Survival factors regulating ovarian apoptosis – dependence on follicle differentiation. Reproduction. 2002;123:23–30. doi: 10.1530/rep.0.1230023. [DOI] [PubMed] [Google Scholar]
- 21.van Noord-Zaadstra BM, Looman CW, Alsbach H, Habbema JD, te Velde ER, Karbaat J. Delaying childbearing: effect of age on fecundity and outcome of pregnancy. Bmj. 1991;302:1361–5. doi: 10.1136/bmj.302.6789.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buratini J, Dellaqua TT, Dal Canto M, La Marca A, Carone D, Mignini Renzini M, et al. The putative roles of FSH and AMH in the regulation of oocyte developmental competence: from fertility prognosis to mechanisms underlying age-related subfertility. Hum Reprod Update. 2022;28:232–54. doi: 10.1093/humupd/dmab044. [DOI] [PubMed] [Google Scholar]
- 23.Casarini L, Crepieux P. Molecular mechanisms of action of FSH. Front Endocrinol. 2019;10:305. doi: 10.3389/fendo.2019.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong J, Kang SS, Wang Z, Liu X, Kuo TC, Korkmaz F, et al. FSH blockade improves cognition in mice with alzheimer’s disease. Nature. 2022;603:470–6. doi: 10.1038/s41586-022-04463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genazzani AR, Pluchino N, Luisi S, Luisi M. Estrogen, cognition and female ageing. Hum Reprod Update. 2007;13:175–87. doi: 10.1093/humupd/dml042. [DOI] [PubMed] [Google Scholar]
- 26.Honour JW. Biochemistry of the menopause. Ann Clin Biochem. 2018;55:18–33. doi: 10.1177/0004563217739930. [DOI] [PubMed] [Google Scholar]
- 27.Kok HS, van Asselt KM, van der Schouw YT, Peeters PH, Wijmenga C. Genetic studies to identify genes underlying menopausal age. Hum Reprod Update. 2005;11:483–93. doi: 10.1093/humupd/dmi024. [DOI] [PubMed] [Google Scholar]
- 28.Hayatbakhsh MR, Clavarino A, Williams GM, Sina M, Najman JM. Cigarette smoking and age of menopause: a large prospective study. Maturitas. 2012;72:346–52. doi: 10.1016/j.maturitas.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Jin M, Yu Y, Huang H. An update on primary ovarian insufficiency. Sci China Life Sci. 2012;55:677–86. doi: 10.1007/s11427-012-4355-2. [DOI] [PubMed] [Google Scholar]
- 30.Yan J, Wu K, Tang R, Ding L, Chen ZJ. Effect of maternal age on the outcomes of in vitro fertilization and embryo transfer (IVF-ET) Sci China Life Sci. 2012;55:694–8. doi: 10.1007/s11427-012-4357-0. [DOI] [PubMed] [Google Scholar]
- 31.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–50. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 32.White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–21. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–6. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 34.Guo K, Li CH, Wang XY, He DJ, Zheng P. Germ stem cells are active in postnatal mouse ovary under physiological conditions. Mol Hum Reprod. 2016;22:316–28. doi: 10.1093/molehr/gaw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–15. doi: 10.1016/j.cell.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 36.Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. 2006;441:1109–14. doi: 10.1038/nature04929. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka SS, Nagamatsu G, Tokitake Y, Kasa M, Tam PP, Matsui Y. Regulation of expression of mouse interferon-induced transmembrane protein like gene-3, Ifitm3 (mil-1, fragilis), in germ cells. Dev Dynam. 2004;230:651–9. doi: 10.1002/dvdy.20085. [DOI] [PubMed] [Google Scholar]
- 38.Sheng X, Tian C, Liu L, Wang L, Ye X, Li J, et al. Characterization of oogonia stem cells in mice by Fragilis. Protein Cell. 2019;10:825–31. doi: 10.1007/s13238-019-00654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei L, Spradling AC. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc Natl Acad Sci U S A. 2013;110:8585–90. doi: 10.1073/pnas.1306189110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Liu L, Li X, Busayavalasa K, Shen Y, Hovatta O, et al. Life-long in vivo cell-lineage tracing shows that no oogenesis originates from putative germline stem cells in adult mice. Proc Natl Acad Sci U S A. 2014;111:17983–8. doi: 10.1073/pnas.1421047111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niu W, Spradling AC. Two distinct pathways of pregranulosa cell differentiation support follicle formation in the mouse ovary. Proc Natl Acad Sci U S A. 2020;117:20015–26. doi: 10.1073/pnas.2005570117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Handel MA, Eppig JJ, Schimenti JC. Applying “gold standards” to in-vitro-derived germ cells. Cell. 2014;157:1257–61. doi: 10.1016/j.cell.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan J, Zhang D, Wang L, Liu M, Mao J, Yin Y, et al. No evidence for neo-oogenesis may link to ovarian senescence in adult monkey. Stem Cell. 2013;31:2538–50. doi: 10.1002/stem.1480. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Wu C, Lyu Q, Yang D, Albertini DF, Keefe DL, et al. Germline stem cells and neo-oogenesis in the adult human ovary. Dev Biol. 2007;306:112–20. doi: 10.1016/j.ydbio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun. 2020;11:1147. doi: 10.1038/s41467-020-14936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woods DC, Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc. 2013;8:966–88. doi: 10.1038/nprot.2013.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Yang R, Yin C, Kee K. Studying human reproductive biology through single-cell analysis and in vitro differentiation of stem cells into germ cell-like cells. Hum Reprod Update. 2020;26:670–88. doi: 10.1093/humupd/dmaa021. [DOI] [PubMed] [Google Scholar]
- 48.Hainaut M, Clarke HJ. Germ cells of the mammalian female: a limited or renewable resource? Biol Reprod. 2021;105:774–88. doi: 10.1093/biolre/ioab115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pomatto LCD, Davies KJA. Adaptive homeostasis and the free radical theory of ageing. Free Radic Biol Med. 2018;124:420–30. doi: 10.1016/j.freeradbiomed.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasaki H, Hamatani T, Kamijo S, Iwai M, Kobanawa M, Ogawa S, et al. Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front Endocrinol. 2019;10:811. doi: 10.3389/fendo.2019.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–93. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 53.Santos M, Cordts EB, Bianco B, Barbosa CP, Christofolini DM. Oocyte quality in patients with increased FSH levels. JBRA Assist Reprod. 2015;19:227–9. doi: 10.5935/1518-0557.20150044. [DOI] [PubMed] [Google Scholar]
- 54.Li Q, Geng X, Zheng W, Tang J, Xu B, Shi Q. Current understanding of ovarian aging. Sci China Life Sci. 2012;55:659–69. doi: 10.1007/s11427-012-4352-5. [DOI] [PubMed] [Google Scholar]
- 55.Handyside AH, Montag M, Magli MC, Repping S, Harper J, Schmutzler A, et al. Multiple meiotic errors caused by predivision of chromatids in women of advanced maternal age undergoing in vitro fertilisation. Eur J Hum Genet. 2012;20:742–7. doi: 10.1038/ejhg.2011.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S, Hassold T, Hunt P, White MA, Zickler D, Kleckner N, et al. Inefficient crossover maturation underlies elevated aneuploidy in human female meiosis. Cell. 2017;168:977–89.e17. doi: 10.1016/j.cell.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu L, Blasco MA, Keefe DL. Requirement of functional telomeres for metaphase chromosome alignments and integrity of meiotic spindles. EMBO Rep. 2002;3:230–4. doi: 10.1093/embo-reports/kvf055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Treff NR, Su J, Taylor D, Scott RT. Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011;7:e1002161. doi: 10.1371/journal.pgen.1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franca MM, Mendonca BB. Genetics of ovarian insufficiency and defects of folliculogenesis. Best Pract Res Clin Endocrinol Metabol. 2022;36:101594. doi: 10.1016/j.beem.2021.101594. [DOI] [PubMed] [Google Scholar]
- 60.Chiang JL, Shukla P, Pagidas K, Ahmed NS, Karri S, Gunn DD, et al. Mitochondria in ovarian aging and reproductive longevity. Ageing Res Rev. 2020;63:101168. doi: 10.1016/j.arr.2020.101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217–22. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- 62.Huber S, Fieder M. Evidence for a maximum “shelf-life” of oocytes in mammals suggests that human menopause may be an implication of meiotic arrest. Sci Rep. 2018;8:14099. doi: 10.1038/s41598-018-32502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tyc KM, McCoy RC, Schindler K, Xing J. Mathematical modeling of human oocyte aneuploidy. Proc Natl Acad Sci U S A. 2020;117:10455–64. doi: 10.1073/pnas.1912853117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brooker AS, Berkowitz KM. The roles of cohesins in mitosis, meiosis, and human health and disease. Methods Mol Biol. 2014;1170:229–66. doi: 10.1007/978-1-4939-0888-2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burkhardt S, Borsos M, Szydlowska A, Godwin J, Williams SA, Cohen PE, et al. Chromosome cohesion established by rec8-cohesin in fetal oocytes is maintained without detectable turnover in oocytes arrested for months in mice. Curr Biol. 2016;26:678–85. doi: 10.1016/j.cub.2015.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haering CH, Farcas AM, Arumugam P, Metson J, Nasmyth K. The cohesin ring concatenates sister DNA molecules. Nature. 2008;454:297–301. doi: 10.1038/nature07098. [DOI] [PubMed] [Google Scholar]
- 67.Tachibana-Konwalski K, Godwin J, van der Weyden L, Champion L, Kudo NR, Adams DJ, et al. Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev. 2010;24:2505–16. doi: 10.1101/gad.605910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Charalambous C, Webster A, Schuh M. Aneuploidy in mammalian oocytes and the impact of maternal ageing. Nat Rev Mol Cell Biol. 2022 doi: 10.1038/s41580-022-00517-3. [DOI] [PubMed] [Google Scholar]
- 69.Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol. 2010;20:1522–8. doi: 10.1016/j.cub.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiang T, Schultz RM, Lampson MA. Meiotic origins of maternal age-related aneuploidy. Biol Reprod. 2012;86:1–7. doi: 10.1095/biolreprod.111.094367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu L, Keefe DL. Defective cohesin is associated with age-dependent misaligned chromosomes in oocytes. Reprod Biomed Online. 2008;16:103–12. doi: 10.1016/s1472-6483(10)60562-7. [DOI] [PubMed] [Google Scholar]
- 72.Marston AL. Shugoshins: tension-sensitive pericentromeric adaptors safeguarding chromosome segregation. Mol Cell Biol. 2015;35:634–48. doi: 10.1128/mcb.01176-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keating L, Touati SA, Wassmann K. A PP2A-B56-centered view on metaphase-to-anaphase transition in mouse oocyte meiosis I. Cells. 2020;9:390–405. doi: 10.3390/cells9020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol. 2010;20:1511–21. doi: 10.1016/j.cub.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 75.Rattani A, Wolna M, Ploquin M, Helmhart W, Morrone S, Mayer B, et al. Sgol2 provides a regulatory platform that coordinates essential cell cycle processes during meiosis I in oocytes. Elife. 2013;2:e01133. doi: 10.7554/elife.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holton RA, Harris AM, Mukerji B, Singh T, Dia F, Berkowitz KM. CHTF18 ensures the quantity and quality of the ovarian reserve. Biol Reprod. 2020;103:24–35. doi: 10.1093/biolre/ioaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chakravarti D, LaBella KA, DePinho RA. Telomeres: history, health, and hallmarks of aging. Cell. 2021;184:306–22. doi: 10.1016/j.cell.2020.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keefe DL, Liu L, Marquard K. Telomeres and aging-related meiotic dysfunction in women. Cell Mol Life Sci. 2007;64:139–43. doi: 10.1007/s00018-006-6466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalmbach KH, Fontes Antunes DM, Dracxler RC, Knier TW, Seth-Smith ML, Wang F, et al. Telomeres and human reproduction. Fertil Steril. 2013;99:23–9. doi: 10.1016/j.fertnstert.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Michaeli J, Smoom R, Serruya N, El Ayoubi H, Rotshenker-Olshinka K, Srebnik N, et al. Leukocyte telomere length correlates with extended female fertility. Cells. 2022;11:513–23. doi: 10.3390/cells11030513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu X, Chen X, Zhang X, Liu Y, Wang Z, Wang P, et al. Impaired telomere length and telomerase activity in peripheral blood leukocytes and granulosa cells in patients with biochemical primary ovarian insufficiency. Hum Reprod. 2017;32:201–7. doi: 10.1093/humrep/dew283. [DOI] [PubMed] [Google Scholar]
- 82.Gray KE, Schiff MA, Fitzpatrick AL, Kimura M, Aviv A, Starr JR. Leukocyte telomere length and age at menopause. Epidemiology. 2014;25:139–46. doi: 10.1097/ede.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y, Deng B, Ouyang N, Yuan P, Zheng L, Wang W. Telomere length is short in PCOS and oral contraceptive does not affect the telomerase activity in granulosa cells of patients with PCOS. J Assist Reprod Genet. 2017;34:849–59. doi: 10.1007/s10815-017-0929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamada-Fukunaga T, Yamada M, Hamatani T, Chikazawa N, Ogawa S, Akutsu H, et al. Age-associated telomere shortening in mouse oocytes. Reprod Biol Endocrinol. 2013;11:108. doi: 10.1186/1477-7827-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hanson BM, Tao X, Zhan Y, Kim JG, Klimczak AM, Herlihy NS, et al. Shorter telomere length of white blood cells is associated with higher rates of aneuploidy among infertile women undergoing in vitro fertilization. Fertil Steril. 2021;115:957–65. doi: 10.1016/j.fertnstert.2020.09.164. [DOI] [PubMed] [Google Scholar]
- 86.Yu TN, Cheng EH, Tsai HN, Lin PY, Chen CH, Huang CC, et al. Assessment of telomere length and mitochondrial DNA copy number in granulosa cells as predictors of aneuploidy rate in young patients. J Clin Med. 2022;11:1824–35. doi: 10.3390/jcm11071824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian C, Heng D, Zhao N, Liu L, Sheng X, Chen J, et al. Short telomeres impede germ cell specification by upregulating MAPK and TGFbeta signaling. Sci China Life Sci. 2022 doi: 10.1007/s11427-022-2151-0. [DOI] [PubMed] [Google Scholar]
- 88.Keefe DL. Telomeres and genomic instability during early development. Eur J Med Genet. 2020;63:103638. doi: 10.1016/j.ejmg.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 89.Kosebent EG, Uysal F, Ozturk S. Telomere length and telomerase activity during folliculogenesis in mammals. J Reprod Dev. 2018;64:477–84. doi: 10.1262/jrd.2018-076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kosebent EG, Uysal F, Ozturk S. The altered expression of telomerase components and telomere-linked proteins may associate with ovarian aging in mouse. Exp Gerontol. 2020;138:110975. doi: 10.1016/j.exger.2020.110975. [DOI] [PubMed] [Google Scholar]
- 91.Butts S, Riethman H, Ratcliffe S, Shaunik A, Coutifaris C, Barnhart K. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J Clin Endocrinol Metab. 2009;94:4835–43. doi: 10.1210/jc.2008-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Derevyanko A, Skowronska A, Skowronski MT, Kordowitzki P. The interplay between telomeres, mitochondria, and chronic stress exposure in the aging egg. Cells. 2022;11:2612–7. doi: 10.3390/cells11162612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rossiello F, Jurk D, Passos JF, d’Adda di Fagagna F. Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol. 2022;24:135–47. doi: 10.1038/s41556-022-00842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robinson LG, Pimentel R, Wang F, Kramer YG, Gonullu DC, Agarwal S, et al. Impaired reproductive function and fertility preservation in a woman with a dyskeratosis congenita. J Assist Reprod Genet. 2020;37:1221–5. doi: 10.1007/s10815-020-01758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Franca MM, Funari MFA, Lerario AM, Santos MG, Nishi MY, Domenice S, et al. Screening of targeted panel genes in Brazilian patients with primary ovarian insufficiency. PLoS One. 2020;15:e0240795. doi: 10.1371/journal.pone.0240795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patino LC, Beau I, Carlosama C, Buitrago JC, Gonzalez R, Suarez CF, et al. New mutations in non-syndromic primary ovarian insufficiency patients identified via whole-exome sequencing. Hum Reprod. 2017;32:1512–20. doi: 10.1093/humrep/dex089. [DOI] [PubMed] [Google Scholar]
- 97.Bouilly J, Beau I, Barraud S, Bernard V, Azibi K, Fagart J, et al. Identification of multiple gene mutations accounts for a new genetic architecture of primary ovarian insufficiency. J Clin Endocrinol Metab. 2016;101:4541–50. doi: 10.1210/jc.2016-2152. [DOI] [PubMed] [Google Scholar]
- 98.Tucker EJ, Grover SR, Robevska G, van den Bergen J, Hanna C, Sinclair AH. Identification of variants in pleiotropic genes causing “isolated” premature ovarian insufficiency: implications for medical practice. Eur J Hum Genet. 2018;26:1319–28. doi: 10.1038/s41431-018-0140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Venturella R, De Vivo V, Carlea A, D’Alessandro P, Saccone G, Arduino B, et al. The genetics of non-syndromic primary ovarian insufficiency: a systematic review. Int J Fertil Steril. 2019;13:161–8. doi: 10.22074/ijfs.2019.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stolk L, Perry JR, Chasman DI, He C, Mangino M, Sulem P, et al. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat Genet. 2012;44:260–8. doi: 10.1038/ng.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vermeij WP, Dolle ME, Reiling E, Jaarsma D, Payan-Gomez C, Bombardieri CR, et al. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016;537:427–31. doi: 10.1038/nature19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Milanese C, Bombardieri CR, Sepe S, Barnhoorn S, Payan-Gomez C, Caruso D, et al. DNA damage and transcription stress cause ATP-mediated redesign of metabolism and potentiation of anti-oxidant buffering. Nat Commun. 2019;10:4887. doi: 10.1038/s41467-019-12640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laven JSE, Visser JA, Uitterlinden AG, Vermeij WP, Hoeijmakers JHJ. Menopause: genome stability as new paradigm. Maturitas. 2016;92:15–23. doi: 10.1016/j.maturitas.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 104.Rinaldi VD, Bolcun-Filas E, Kogo H, Kurahashi H, Schimenti JC. The DNA damage checkpoint eliminates mouse oocytes with chromosome synapsis failure. Mol Cell. 2017;67:1026–36.e2. doi: 10.1016/j.molcel.2017.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rinaldi VD, Bloom JC, Schimenti JC. Oocyte elimination through DNA damage signaling from CHK1/CHK2 to p53 and p63. Genetics. 2020;215:373–8. doi: 10.1534/genetics.120.303182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Q, Engebrecht J. BRCA1 and BRCA2 tumor suppressor function in meiosis. Front Cell Dev Biol. 2021;9:668309. doi: 10.3389/fcell.2021.668309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5:172ra21. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. 2017;102:3839–47. doi: 10.1210/jc.2017-00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–71. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 110.Michaelson-Cohen R, Mor P, Srebnik N, Beller U, Levy-Lahad E, Eldar-Geva T. BRCA mutation carriers do not have compromised ovarian reserve. Int J Gynecol Cancer. 2014;24:233–7. doi: 10.1097/igc.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 111.Wang ET, Pisarska MD, Bresee C, Chen YD, Lester J, Afshar Y, et al. BRCA1 germline mutations may be associated with reduced ovarian reserve. Fertil Steril. 2014;102:1723–8. doi: 10.1016/j.fertnstert.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giordano S, Garrett-Mayer E, Mittal N, Smith K, Shulman L, Passaglia C, et al. Association of BRCA1 mutations with impaired ovarian reserve: connection between infertility and breast/ovarian cancer risk. J Adolesc Young Adult Oncol. 2016;5:337–43. doi: 10.1089/jayao.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Tilborg TC, Derks-Smeets IA, Bos AM, Oosterwijk JC, van Golde RJ, de Die-Smulders CE, et al. Serum AMH levels in healthy women from BRCA1/2 mutated families: are they reduced? Hum Reprod. 2016;31:2651–9. doi: 10.1093/humrep/dew242. [DOI] [PubMed] [Google Scholar]
- 114.Lambertini M, Goldrat O, Ferreira AR, Dechene J, Azim HA, Desir J, et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol. 2018;29:237–43. doi: 10.1093/annonc/mdx639. [DOI] [PubMed] [Google Scholar]
- 115.Gunnala V, Fields J, Irani M, D’Angelo D, Xu K, Schattman G, et al. BRCA carriers have similar reproductive potential at baseline to noncarriers: comparisons in cancer and cancer-free cohorts undergoing fertility preservation. Fertil Steril. 2019;111:363–71. doi: 10.1016/j.fertnstert.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 116.Rzepka-Gorska I, Tarnowski B, Chudecka-Glaz A, Gorski B, Zielinska D, Toloczko-Grabarek A. Premature menopause in patients with BRCA1 gene mutation. Breast Cancer Res Treat. 2006;100:59–63. doi: 10.1007/s10549-006-9220-1. [DOI] [PubMed] [Google Scholar]
- 117.Finch A, Valentini A, Greenblatt E, Lynch HT, Ghadirian P, Armel S, et al. Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertil Steril. 2013;99:1724–8. doi: 10.1016/j.fertnstert.2013.01.109. [DOI] [PubMed] [Google Scholar]
- 118.Lin WT, Beattie M, Chen LM, Oktay K, Crawford SL, Gold EB, et al. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California. Cancer. 2013;119:1652–9. doi: 10.1002/cncr.27952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science. 2014;343:533–6. doi: 10.1126/science.1247671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang XQ, Redpath JL, Fan ST, Stanbridge EJ. ATR dependent activation of Chk2. J Cell Physiol. 2006;208:613–9. doi: 10.1002/jcp.20700. [DOI] [PubMed] [Google Scholar]
- 121.Tuppi M, Kehrloesser S, Coutandin DW, Rossi V, Luh LM, Strubel A, et al. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat Struct Mol Biol. 2018;25:261–9. doi: 10.1038/s41594-018-0035-7. [DOI] [PubMed] [Google Scholar]
- 122.Rodriguez-Nuevo A, Torres-Sanchez A, Duran JM, De Guirior C, Martinez-Zamora MA, Boke E. Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature. 2022;607:756–61. doi: 10.1038/s41586-022-04979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rizzo A, Roscino MT, Binetti F, Sciorsci RL. Roles of reactive oxygen species in female reproduction. Reprod Domest Anim. 2012;47:344–52. doi: 10.1111/j.1439-0531.2011.01891.x. [DOI] [PubMed] [Google Scholar]
- 124.Shi L, Zhang J, Lai Z, Tian Y, Fang L, Wu M, et al. Long-term moderate oxidative stress decreased ovarian reproductive function by reducing follicle quality and progesterone production. PLoS One. 2016;11:e0162194. doi: 10.1371/journal.pone.0162194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu J, Liu M, Ye X, Liu K, Huang J, Wang L, et al. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC) Hum Reprod. 2012;27:1411–20. doi: 10.1093/humrep/des019. [DOI] [PubMed] [Google Scholar]
- 126.Wang S, Zheng Y, Li J, Yu Y, Zhang W, Song M, et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell. 2020;180:585–600.e19. doi: 10.1016/j.cell.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 127.Lim J, Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod. 2011;84:775–82. doi: 10.1095/biolreprod.110.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Avila J, Gonzalez-Fernandez R, Rotoli D, Hernandez J, Palumbo A. Oxidative stress in granulosa-lutein cells from in vitro fertilization patients. Reprod Sci. 2016;23:1656–61. doi: 10.1177/1933719116674077. [DOI] [PubMed] [Google Scholar]
- 129.Yang L, Chen Y, Liu Y, Xing Y, Miao C, Zhao Y, et al. The role of oxidative stress and natural antioxidants in ovarian aging. Front Pharmacol. 2020;11:617843. doi: 10.3389/fphar.2020.617843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Longo VD, Anderson RM. Nutrition, longevity and disease: from molecular mechanisms to interventions. Cell. 2022;185:1455–70. doi: 10.1016/j.cell.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ma S, Sun S, Geng L, Song M, Wang W, Ye Y, et al. Caloric restriction reprograms the single-cell transcriptional landscape of Rattus norvegicus aging. Cell. 2020;180:984–1001.e22. doi: 10.1016/j.cell.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 132.Lopez-Otin C, Galluzzi L, Freije JMP, Madeo F, Kroemer G. Metabolic control of longevity. Cell. 2016;166:802–21. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 133.Schneider A, Saccon TD, Garcia DN, Zanini BM, Isola JVV, Hense JD, et al. The interconnections between somatic and ovarian aging in murine models. J Gerontol A Biol Sci Med Sci. 2021;76:1579–86. doi: 10.1093/gerona/glaa258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Isola JVV, Zanini BM, Hense JD, Alvarado-Rincon JA, Garcia DN, Pereira GC, et al. Mild calorie restriction, but not 17alpha-estradiol, extends ovarian reserve and fertility in female mice. Exp Gerontol. 2022;159:111669. doi: 10.1016/j.exger.2021.111669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7:e330–41. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 136.Luke B, Brown MB, Missmer SA, Bukulmez O, Leach R, Stern JE, et al. The effect of increasing obesity on the response to and outcome of assisted reproductive technology: a national study. Fertil Steril. 2011;96:820–5. doi: 10.1016/j.fertnstert.2011.07.1100. [DOI] [PubMed] [Google Scholar]
- 137.Ge J, Li C, Sun H, Xin Y, Zhu S, Liu Y, et al. Telomere dysfunction in oocytes and embryos from obese mice. Front Cell Dev Biol. 2021;9:617225. doi: 10.3389/fcell.2021.617225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Clemente DBP, Maitre L, Bustamante M, Chatzi L, Roumeliotaki T, Fossati S, et al. Obesity is associated with shorter telomeres in 8 year-old children. Sci Rep. 2019;9:18739. doi: 10.1038/s41598-019-55283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martens DS, Plusquin M, Gyselaers W, De Vivo I, Nawrot TS. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 2016;14:148. doi: 10.1186/s12916-016-0689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Paszkowski T, Clarke RN, Hornstein MD. Smoking induces oxidative stress inside the graafian follicle. Hum Reprod. 2002;17:921–5. doi: 10.1093/humrep/17.4.921. [DOI] [PubMed] [Google Scholar]
- 141.de Ziegler D, Santulli P, Seroka A, Decanter C, Meldrum DR, Chapron C. In women, the reproductive harm of toxins such as tobacco smoke is reversible in 6 months: basis for the “olive tree” hypothesis. Fertil Steril. 2013;100:927–8. doi: 10.1016/j.fertnstert.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 142.Schrieks IC, van den Berg R, Sierksma A, Beulens JW, Vaes WH, Hendriks HF. Effect of red wine consumption on biomarkers of oxidative stress. Alcohol Alcohol. 2013;48:153–9. doi: 10.1093/alcalc/ags086. [DOI] [PubMed] [Google Scholar]
- 143.Wu HJ, Liu C, Duan WX, Xu SC, He MD, Chen CH, et al. Melatonin ameliorates bisphenol A-induced DNA damage in the germ cells of adult male rats. Mutat Res. 2013;752:57–67. doi: 10.1016/j.mrgentox.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 144.Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, et al. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect. 2012;120:978–83. doi: 10.1289/ehp.1104307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10:668–80. doi: 10.1016/s2213-8587(22)00163-2. [DOI] [PubMed] [Google Scholar]
- 146.Lim SS, Hutchison SK, Van Ryswyk E, Norman RJ, Teede HJ, Moran LJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2019;3:CD007506. doi: 10.1002/14651858.CD007506.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meldrum DR, Casper RF, Diez-Juan A, Simon C, Domar AD, Frydman R. Aging and the environment affect gamete and embryo potential: can we intervene? Fertil Steril. 2016;105:548–59. doi: 10.1016/j.fertnstert.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 148.Bedaiwy MA, Elnashar SA, Goldberg JM, Sharma R, Mascha EJ, Arrigain S, et al. Effect of follicular fluid oxidative stress parameters on intracytoplasmic sperm injection outcome. Gynecol Endocrinol. 2012;28:51–5. doi: 10.3109/09513590.2011.579652. [DOI] [PubMed] [Google Scholar]
- 149.Motta PM, Nottola SA, Makabe S, Heyn R. Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod. 2000;15(2 Suppl):129–47. doi: 10.1093/humrep/15.suppl_2.129. [DOI] [PubMed] [Google Scholar]
- 150.Dumollard R, Duchen M, Carroll J. The role of mitochondrial function in the oocyte and embryo. Curr Top Dev Biol. 2007;77:21–49. doi: 10.1016/S0070-2153(06)77002-8. [DOI] [PubMed] [Google Scholar]
- 151.May-Panloup P, Boucret L, Chao de la Barca JM, Desquiret-Dumas V, Ferre-L’Hotellier V, Moriniere C, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update. 2016;22:725–43. doi: 10.1093/humupd/dmw028. [DOI] [PubMed] [Google Scholar]
- 152.Muller-Hocker J, Schafer S, Weis S, Munscher C, Strowitzki T. Morphological-cytochemical and molecular genetic analyses of mitochondria in isolated human oocytes in the reproductive age. Mol Hum Reprod. 1996;2:951–8. doi: 10.1093/molehr/2.12.951. [DOI] [PubMed] [Google Scholar]
- 153.Simsek-Duran F, Li F, Ford W, Swanson RJ, Jones HW, Castora FJ. Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS One. 2013;8:e64955. doi: 10.1371/journal.pone.0064955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–52. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 155.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–59. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jiao H, Jiang D, Hu X, Du W, Ji L, Yang Y, et al. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell. 2021;184:2896–910 e13. doi: 10.1016/j.cell.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 157.Yang L, Lin X, Tang H, Fan Y, Zeng S, Jia L, et al. Mitochondrial DNA mutation exacerbates female reproductive aging via impairment of the NADH/NAD(+) redox. Aging Cell. 2020;19:e13206. doi: 10.1111/acel.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang Q, Cong L, Wang Y, Luo X, Li H, Wang H, et al. Increasing ovarian NAD(+) levels improve mitochondrial functions and reverse ovarian aging. Free Radic Biol Med. 2020;156:1–10. doi: 10.1016/j.freeradbiomed.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 159.Prigione A, Ruiz-Perez MV, Bukowiecki R, Adjaye J. Metabolic restructuring and cell fate conversion. Cell Mol Life Sci. 2015;72:1759–77. doi: 10.1007/s00018-015-1834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Folmes CD, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 2012;11:596–606. doi: 10.1016/j.stem.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hoffman DP, Shtengel G, Xu CS, Campbell KR, Freeman M, Wang L, et al. Correlative three-dimensional super-resolution and block-face electron microscopy of whole vitreously frozen cells. Science. 2020;367:265–76. doi: 10.1126/science.aaz5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dominy JE, Lee Y, Gerhart-Hines Z, Puigserver P. Nutrient-dependent regulation of PGC-1alpha’s acetylation state and metabolic function through the enzymatic activities of Sirt1/GCN5. Biochim Biophys Acta. 2010;1804:1676–83. doi: 10.1016/j.bbapap.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boucret L, de la Barca JMC, Moriniere C, Desquiret V, Ferre-L’Hotellier V, Descamps P, et al. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum Reprod. 2015;30:1653–64. doi: 10.1093/humrep/dev114. [DOI] [PubMed] [Google Scholar]
- 164.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–9. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 165.Zhang M, Bener MB, Jiang Z, Wang T, Esencan E, Scott R, et al. Mitofusin 1 is required for female fertility and to maintain ovarian follicular reserve. Cell Death Dis. 2019;10:560. doi: 10.1038/s41419-019-1799-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hou X, Zhu S, Zhang H, Li C, Qiu D, Ge J, et al. Mitofusin1 in oocyte is essential for female fertility. Redox Biol. 2019;21:101110. doi: 10.1016/j.redox.2019.101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang M, Bener MB, Jiang Z, Wang T, Esencan E, Scott R, et al. Mitofusin 2 plays a role in oocyte and follicle development, and is required to maintain ovarian follicular reserve during reproductive aging. Aging (Albany NY) 2019;11:3919–38. doi: 10.18632/aging.102024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang H, Pan Z, Ju J, Xing C, Li X, Shan M, et al. DRP1 deficiency induces mitochondrial dysfunction and oxidative stress-mediated apoptosis during porcine oocyte maturation. J Anim Sci Biotechnol. 2020;11:77. doi: 10.1186/s40104-020-00489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Udagawa O, Ishihara T, Maeda M, Matsunaga Y, Tsukamoto S, Kawano N, et al. Mitochondrial fission factor Drp1 maintains oocyte quality via dynamic rearrangement of multiple organelles. Curr Biol. 2014;24:2451–8. doi: 10.1016/j.cub.2014.08.060. [DOI] [PubMed] [Google Scholar]
- 170.Adhikari D, Lee IW, Al-Zubaidi U, Liu J, Zhang QH, Yuen WS, et al. Depletion of oocyte dynamin-related protein 1 shows maternal-effect abnormalities in embryonic development. Sci Adv. 2022;8:eabl8070. doi: 10.1126/sciadv.abl8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yu B, Ma J, Li J, Wang D, Wang Z, Wang S. Mitochondrial phosphatase PGAM5 modulates cellular senescence by regulating mitochondrial dynamics. Nat Commun. 2020;11:2549. doi: 10.1038/s41467-020-16312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li CJ, Lin LT, Tsai HW, Wen ZH, Tsui KH. Phosphoglycerate mutase family member 5 maintains oocyte quality via mitochondrial dynamic rearrangement during aging. Aging Cell. 2022;21:e13546. doi: 10.1111/acel.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yue MX, Fu XW, Zhou GB, Hou YP, Du M, Wang L, et al. Abnormal DNA methylation in oocytes could be associated with a decrease in reproductive potential in old mice. J Assist Reprod Genet. 2012;29:643–50. doi: 10.1007/s10815-012-9780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Castillo-Fernandez J, Herrera-Puerta E, Demond H, Clark SJ, Hanna CW, Hemberger M, et al. Increased transcriptome variation and localised DNA methylation changes in oocytes from aged mice revealed by parallel single-cell analysis. Aging Cell. 2020;19:e13278. doi: 10.1111/acel.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu L, Wang H, Xu GL, Liu L. Tet1 deficiency leads to premature ovarian failure. Front Cell Dev Biol. 2021;9:644135. doi: 10.3389/fcell.2021.644135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang H, Liu L, Gou M, Huang G, Tian C, Yang J, et al. Roles of Tet2 in meiosis, fertility and reproductive aging. Protein Cell. 2021;12:578–85. doi: 10.1007/s13238-020-00805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kageyama S, Liu H, Kaneko N, Ooga M, Nagata M, Aoki F. Alterations in epigenetic modifications during oocyte growth in mice. Reproduction. 2007;133:85–94. doi: 10.1530/rep-06-0025. [DOI] [PubMed] [Google Scholar]
- 178.Manosalva I, Gonzalez A. Aging alters histone H4 acetylation and CDC2A in mouse germinal vesicle stage oocytes. Biol Reprod. 2009;81:1164–71. doi: 10.1095/biolreprod.109.078386. [DOI] [PubMed] [Google Scholar]
- 179.van den Berg IM, Eleveld C, van der Hoeven M, Birnie E, Steegers EA, Galjaard RJ, et al. Defective deacetylation of histone 4 K12 in human oocytes is associated with advanced maternal age and chromosome misalignment. Hum Reprod. 2011;26:1181–90. doi: 10.1093/humrep/der030. [DOI] [PubMed] [Google Scholar]
- 180.Chamani IJ, Keefe DL. Epigenetics and female reproductive aging. Front Endocrinol. 2019;10:473. doi: 10.3389/fendo.2019.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Qiao J, Chen Y, Yan LY, Yan J, Liu P, Sun QY. Changes in histone methylation during human oocyte maturation and IVF- or ICSI-derived embryo development. Fertil Steril. 2010;93:1628–36. doi: 10.1016/j.fertnstert.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 182.Wu YW, Li S, Zheng W, Li YC, Chen L, Zhou Y, et al. Dynamic mRNA degradome analyses indicate a role of histone H3K4 trimethylation in association with meiosis-coupled mRNA decay in oocyte aging. Nat Commun. 2022;13:3191. doi: 10.1038/s41467-022-31224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ahmed TA, Ahmed SM, El-Gammal Z, Shouman S, Ahmed A, Mansour R, et al. Oocyte aging: the role of cellular and environmental factors and impact on female fertility. Adv Exp Med Biol. 2020;1247:109–23. doi: 10.1007/5584_2019_456. [DOI] [PubMed] [Google Scholar]
- 184.Zhu J, Zhang J, Li H, Wang TY, Zhang CX, Luo MJ, et al. Cumulus cells accelerate oocyte aging by releasing soluble fas ligand in mice. Sci Rep. 2015;5:8683. doi: 10.1038/srep08683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhu J, Lin FH, Zhang J, Lin J, Li H, Li YW, et al. The signaling pathways by which the Fas/FasL system accelerates oocyte aging. Aging (Albany NY) 2016;8:291–303. doi: 10.18632/aging.100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Perez GI, Jurisicova A, Matikainen T, Moriyama T, Kim MR, Takai Y, et al. A central role for ceramide in the age-related acceleration of apoptosis in the female germline. Faseb J. 2005;19:860–2. doi: 10.1096/fj.04-2903fje. [DOI] [PubMed] [Google Scholar]
- 187.Kujjo LL, Perez GI. Ceramide and mitochondrial function in aging oocytes: joggling a new hypothesis and old players. Reproduction. 2012;143:1–10. doi: 10.1530/rep-11-0350. [DOI] [PubMed] [Google Scholar]
- 188.Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, Pritchard MT, et al. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction. 2016;152:245–60. doi: 10.1530/rep-16-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Umehara T, Winstanley YE, Andreas E, Morimoto A, Williams EJ, Smith KM, et al. Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary. Sci Adv. 2022;8:eabn4564. doi: 10.1126/sciadv.abn4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xu X, Mu L, Li L, Liang J, Zhang S, Jia L, et al. Imaging and tracing the pattern of adult ovarian angiogenesis implies a strategy against female reproductive aging. Sci Adv. 2022;8:eabi8683. doi: 10.1126/sciadv.abi8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, et al. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013;98:3068–79. doi: 10.1210/jc.2013-1715. [DOI] [PubMed] [Google Scholar]
- 192.Battaglia R, Musumeci P, Ragusa M, Barbagallo D, Scalia M, Zimbone M, et al. Ovarian aging increases small extracellular vesicle CD81(+) release in human follicular fluid and influences miRNA profiles. Aging (Albany NY) 2020;12:12324–41. doi: 10.18632/aging.103441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cai H, Li Y, Li H, Niringiyumukiza JD, Zhang M, Chen L, et al. Identification and characterization of human ovary-derived circular RNAs and their potential roles in ovarian aging. Aging (Albany NY) 2018;10:2511–34. doi: 10.18632/aging.101565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cheng J, Huang J, Yuan S, Zhou S, Yan W, Shen W, et al. Circular RNA expression profiling of human granulosa cells during maternal aging reveals novel transcripts associated with assisted reproductive technology outcomes. PLoS One. 2017;12:e0177888. doi: 10.1371/journal.pone.0177888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Secomandi L, Borghesan M, Velarde M, Demaria M. The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions. Hum Reprod Update. 2022;28:172–89. doi: 10.1093/humupd/dmab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bertoldo MJ, Listijono DR, Ho WJ, Riepsamen AH, Goss DM, Richani D, et al. NAD(+) repletion rescues female fertility during reproductive aging. Cell Rep. 2020;30:1670–81.e7. doi: 10.1016/j.celrep.2020.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhou DD, Luo M, Huang SY, Saimaiti A, Shang A, Gan RY, et al. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid Med Cell Longev. 2021;2021:9932218. doi: 10.1155/2021/9932218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 199.Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM, et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metabol. 2013;18:533–45. doi: 10.1016/j.cmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–9. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sugiyama M, Kawahara-Miki R, Kawana H, Shirasuna K, Kuwayama T, Iwata H. Resveratrol-induced mitochondrial synthesis and autophagy in oocytes derived from early antral follicles of aged cows. J Reprod Dev. 2015;61:251–9. doi: 10.1262/jrd.2015-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Comizzoli P, Wildt DE, Pukazhenthi BS. In vitro compaction of germinal vesicle chromatin is beneficial to survival of vitrified cat oocytes. Reprod Domest Anim. 2009;44(2 Suppl):269–74. doi: 10.1111/j.1439-0531.2009.01372.x. [DOI] [PubMed] [Google Scholar]
- 203.Liu M, Yin Y, Ye X, Zeng M, Zhao Q, Keefe DL, et al. Resveratrol protects against age-associated infertility in mice. Hum Reprod. 2013;28:707–17. doi: 10.1093/humrep/des437. [DOI] [PubMed] [Google Scholar]
- 204.Liu Y, He XQ, Huang X, Ding L, Xu L, Shen YT, et al. Resveratrol protects mouse oocytes from methylglyoxal-induced oxidative damage. PLoS One. 2013;8:e77960. doi: 10.1371/journal.pone.0077960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jiang Y, Zhang Z, Cha L, Li L, Zhu D, Fang Z, et al. Resveratrol plays a protective role against premature ovarian failure and prompts female germline stem cell survival. Int J Mol Sci. 2019;2020:3605. doi: 10.3390/ijms20143605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ochiai A, Kuroda K, Ozaki R, Ikemoto Y, Murakami K, Muter J, et al. Resveratrol inhibits decidualization by accelerating downregulation of the CRABP2-RAR pathway in differentiating human endometrial stromal cells. Cell Death Dis. 2019;10:276. doi: 10.1038/s41419-019-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–11. doi: 10.1158/0008-5472.can-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chow HH, Garland LL, Heckman-Stoddard BM, Hsu CH, Butler VD, Cordova CA, et al. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: effects on systemic sex steroid hormones. J Transl Med. 2014;12:223. doi: 10.1186/s12967-014-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, et al. NAD(+) supplementation normalizes key alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci U S A. 2018;115:E1876–85. doi: 10.1073/pnas.1718819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Okabe K, Yaku K, Tobe K, Nakagawa T. Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci. 2019;26:34. doi: 10.1186/s12929-019-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Miao Y, Cui Z, Gao Q, Rui R, Xiong B. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Rep. 2020;32:107987. doi: 10.1016/j.celrep.2020.107987. [DOI] [PubMed] [Google Scholar]
- 212.Huang P, Zhou Y, Tang W, Ren C, Jiang A, Wang X, et al. Long-term treatment of nicotinamide mononucleotide improved age-related diminished ovary reserve through enhancing the mitophagy level of granulosa cells in mice. J Nutr Biochem. 2022;101:108911. doi: 10.1016/j.jnutbio.2021.108911. [DOI] [PubMed] [Google Scholar]
- 213.Yoshino M, Yoshino J, Kayser BD, Patti GJ, Franczyk MP, Mills KF, et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021;372:1224–9. doi: 10.1126/science.abe9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Brenner C. Comment on “Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women”. Science. 2021;373:eabj1696. doi: 10.1126/science.abj1696. [DOI] [PubMed] [Google Scholar]
- 215.Yu J, Laybutt DR, Kim LJ, Quek LE, Wu LE, Morris MJ, et al. Exercise-induced benefits on glucose handling in a model of diet-induced obesity are reduced by concurrent nicotinamide mononucleotide. Am J Physiol Endocrinol Metab. 2021;321:E176–89. doi: 10.1152/ajpendo.00446.2020. [DOI] [PubMed] [Google Scholar]
- 216.Benitez-King G. Melatonin as a cytoskeletal modulator: implications for cell physiology and disease. J Pineal Res. 2006;40:1–9. doi: 10.1111/j.1600-079x.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 217.Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J Pineal Res. 2013;54:245–57. doi: 10.1111/jpi.12010. [DOI] [PubMed] [Google Scholar]
- 218.Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253–78. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 219.Li XQ, Wang Y, Yang SJ, Liu Y, Ma X, Liu L, et al. Melatonin protects against maternal diabetes-associated meiotic defects by maintaining mitochondrial function. Free Radic Biol Med. 2022;188:386–94. doi: 10.1016/j.freeradbiomed.2022.06.243. [DOI] [PubMed] [Google Scholar]
- 220.Song C, Peng W, Yin S, Zhao J, Fu B, Zhang J, et al. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci Rep. 2016;6:35165. doi: 10.1038/srep35165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang H, Li C, Wen D, Li R, Lu S, Xu R, et al. Melatonin improves the quality of maternally aged oocytes by maintaining intercellular communication and antioxidant metabolite supply. Redox Biol. 2022;49:102215. doi: 10.1016/j.redox.2021.102215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang M, Lu Y, Chen Y, Zhang Y, Xiong B. Insufficiency of melatonin in follicular fluid is a reversible cause for advanced maternal age-related aneuploidy in oocytes. Redox Biol. 2020;28:101327. doi: 10.1016/j.redox.2019.101327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yang C, Liu Q, Chen Y, Wang X, Ran Z, Fang F, et al. Melatonin delays ovarian aging in mice by slowing down the exhaustion of ovarian reserve. Commun Biol. 2021;4:534. doi: 10.1038/s42003-021-02042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang J, Chen Q, Du D, Wu T, Wen J, Wu M, et al. Can ovarian aging be delayed by pharmacological strategies? Aging (Albany NY) 2019;11:817–32. doi: 10.18632/aging.101784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang L, Zhang Z, Wang J, Lv D, Zhu T, Wang F, et al. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via MT1/AMPK pathway. J Pineal Res. 2019;66:e12550. doi: 10.1111/jpi.12550. [DOI] [PubMed] [Google Scholar]
- 226.Zhang Z, He C, Gao Y, Zhang L, Song Y, Zhu T, et al. alpha-ketoglutarate delays age-related fertility decline in mammals. Aging Cell. 2021;20:e13291. doi: 10.1111/acel.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wang Y, Hekimi S. Understanding ubiquinone. Trends Cell Biol. 2016;26:367–78. doi: 10.1016/j.tcb.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 228.Murphy MP, Chouchani ET. Why succinate? Physiological regulation by a mitochondrial coenzyme Q sentinel. Nat Chem Biol. 2022;18:461–9. doi: 10.1038/s41589-022-01004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Santos-Ocaña C, Do TQ, Padilla S, Navas P, Clarke CF. Uptake of exogenous coenzyme Q and transport to mitochondria is required for bc1 complex stability in yeast coq mutants. J Biol Chem. 2002;277:10973–81. doi: 10.1074/jbc.m112222200. [DOI] [PubMed] [Google Scholar]
- 230.Villalba JM, Navas P. Plasma membrane redox system in the control of stress-induced apoptosis. Antioxidants Redox Signal. 2000;2:213–30. doi: 10.1089/ars.2000.2.2-213. [DOI] [PubMed] [Google Scholar]
- 231.Kashka RH, Zavareh S, Lashkarbolouki T. Augmenting effect of vitrification on lipid peroxidation in mouse preantral follicle during cultivation: modulation by coenzyme Q(10) Syst Biol Reprod Med. 2016;62:404–14. doi: 10.1080/19396368.2016.1235236. [DOI] [PubMed] [Google Scholar]
- 232.Özcan P, Fıçıcıoğlu C, Kizilkale O, Yesiladali M, Tok OE, Ozkan F, et al. Can coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage? J Assist Reprod Genet. 2016;33:1223–30. doi: 10.1007/s10815-016-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14:887–95. doi: 10.1111/acel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bentov Y, Hannam T, Jurisicova A, Esfandiari N, Casper RF. Coenzyme Q10 supplementation and oocyte aneuploidy in women undergoing IVF-ICSI treatment. Clin Med Insights Reprod Health. 2014;8:31–6. doi: 10.4137/cmrh.s14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Xu Y, Nisenblat V, Lu C, Li R, Qiao J, Zhen X, et al. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod Biol Endocrinol. 2018;16:29. doi: 10.1186/s12958-018-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Labarta E, de Los Santos MJ, Escribá MJ, Pellicer A, Herraiz S. Mitochondria as a tool for oocyte rejuvenation. Fertil Steril. 2019;111:219–26. doi: 10.1016/j.fertnstert.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 237.Rodríguez-Varela C, Herraiz S, Labarta E. Mitochondrial enrichment in infertile patients: a review of different mitochondrial replacement therapies. Ther Adv Reprod Health. 2021;15:26334941211023544. doi: 10.1177/26334941211023544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Muggleton-Harris A, Whittingham DG, Wilson L. Cytoplasmic control of preimplantation development in vitro in the mouse. Nature. 1982;299:460–2. doi: 10.1038/299460a0. [DOI] [PubMed] [Google Scholar]
- 239.Cohen J, Scott R, Alikani M, Schimmel T, Munné S, Levron J, et al. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4:269–80. doi: 10.1093/molehr/4.3.269. [DOI] [PubMed] [Google Scholar]
- 240.Cohen J, Scott R, Schimmel T, Levron J, Willadsen S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet. 1997;350:186–7. doi: 10.1016/s0140-6736(05)62353-7. [DOI] [PubMed] [Google Scholar]
- 241.Huang CC, Cheng TC, Chang HH, Chang CC, Chen CI, Liu J, et al. Birth after the injection of sperm and the cytoplasm of tripronucleate zygotes into metaphase II oocytes in patients with repeated implantation failure after assisted fertilization procedures. Fertil Steril. 1999;72:702–6. doi: 10.1016/s0015-0282(99)00309-x. [DOI] [PubMed] [Google Scholar]
- 242.Dale B, Wilding M, Botta G, Rasile M, Marino M, Di Matteo L, et al. Pregnancy after cytoplasmic transfer in a couple suffering from idiopathic infertility: case report. Hum Reprod. 2001;16:1469–72. doi: 10.1093/humrep/16.7.1469. [DOI] [PubMed] [Google Scholar]
- 243.Cree L, Loi P. Mitochondrial replacement: from basic research to assisted reproductive technology portfolio tool-technicalities and possible risks. Mol Hum Reprod. 2015;21:3–10. doi: 10.1093/molehr/gau082. [DOI] [PubMed] [Google Scholar]
- 244.Zhang J, Wang CW, Krey L, Liu H, Meng L, Blaszczyk A, et al. In vitro maturation of human preovulatory oocytes reconstructed by germinal vesicle transfer. Fertil Steril. 1999;71:726–31. doi: 10.1016/s0015-0282(98)00549-4. [DOI] [PubMed] [Google Scholar]
- 245.Takeuchi T, Rosenwaks Z, Palermo GD. A successful model to assess embryo development after transplantation of prophase nuclei. Hum Reprod. 2004;19:975–81. doi: 10.1093/humrep/deh149. [DOI] [PubMed] [Google Scholar]
- 246.Liu H, Wang CW, Grifo JA, Krey LC, Zhang J. Reconstruction of mouse oocytes by germinal vesicle transfer: maturity of host oocyte cytoplasm determines meiosis. Hum Reprod. 1999;14:2357–61. doi: 10.1093/humrep/14.9.2357. [DOI] [PubMed] [Google Scholar]
- 247.Li GP, Chen DY, Lian L, Sun QY, Wang MK, Liu JL, et al. Viable rabbits derived from reconstructed oocytes by germinal vesicle transfer after intracytoplasmic sperm injection (ICSI) Mol Reprod Dev. 2001;58:180–5. doi: 10.1002/1098-2795(200102)58:2<180::aid-mrd7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 248.Liu L, Keefe DL. Nuclear origin of aging-associated meiotic defects in senescence-accelerated mice. Biol Reprod. 2004;71:1724–9. doi: 10.1095/biolreprod.104.028985. [DOI] [PubMed] [Google Scholar]
- 249.Sathananthan AH, Trounson AO. Mitochondrial morphology during preimplantational human embryogenesis. Hum Reprod. 2000;15(2 Suppl):148–59. doi: 10.1093/humrep/15.suppl_2.148. [DOI] [PubMed] [Google Scholar]
- 250.Iuso D, Czernik M, Zacchini F, Ptak G, Loi P. A simplified approach for oocyte enucleation in mammalian cloning. Cell Reprogr. 2013;15:490–4. doi: 10.1089/cell.2013.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Tachibana M, Sparman M, Sritanaudomchai H, Ma H, Clepper L, Woodward J, et al. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–72. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tachibana M, Amato P, Sparman M, Woodward J, Sanchis DM, Ma H, et al. Towards germline gene therapy of inherited mitochondrial diseases. Nature. 2013;493:627–31. doi: 10.1038/nature11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhang J, Liu H, Luo S, Lu Z, Chávez-Badiola A, Liu Z, et al. Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reprod Biomed Online. 2017;34:361–8. doi: 10.1016/j.rbmo.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 254.Kristensen SG, Pors SE, Andersen CY. Improving oocyte quality by transfer of autologous mitochondria from fully grown oocytes. Hum Reprod. 2017;32:725–32. doi: 10.1093/humrep/dex043. [DOI] [PubMed] [Google Scholar]
- 255.Kong LH, Liu Z, Li H, Zhu L, Chen SM, Chen SL, et al. Mitochondria transfer from self-granular cells to improve embryos’ quality. Zhonghua Fu Chan Ke Za Zhi. 2004;39:105–7. [PubMed] [Google Scholar]
- 256.Labarta E, de Los Santos MJ, Herraiz S, Escribá MJ, Marzal A, Buigues A, et al. Autologous mitochondrial transfer as a complementary technique to intracytoplasmic sperm injection to improve embryo quality in patients undergoing in vitro fertilization-a randomized pilot study. Fertil Steril. 2019;111:86–96. doi: 10.1016/j.fertnstert.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 257.Wang ZB, Hao JX, Meng TG, Guo L, Dong MZ, Fan LH, et al. Transfer of autologous mitochondria from adipose tissue-derived stem cells rescues oocyte quality and infertility in aged mice. Aging (Albany NY) 2017;9:2480–8. doi: 10.18632/aging.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mason JB, Cargill SL, Anderson GB, Carey JR. Transplantation of young ovaries to old mice increased life span in transplant recipients. J Gerontol A Biol Sci Med Sci. 2009;64:1207–11. doi: 10.1093/gerona/glp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mason JB, Cargill SL, Griffey SM, Reader JR, Anderson GB, Carey JR. Transplantation of young ovaries restored cardioprotective influence in postreproductive-aged mice. Aging Cell. 2011;10:448–56. doi: 10.1111/j.1474-9726.2011.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Saitou M, Miyauchi H. Gametogenesis from pluripotent stem cells. Cell Stem Cell. 2016;18:721–35. doi: 10.1016/j.stem.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 261.Lin Y, Gill ME, Koubova J, Page DC. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science. 2008;322:1685–7. doi: 10.1126/science.1166340. [DOI] [PubMed] [Google Scholar]
- 262.Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338:971–5. doi: 10.1126/science.1226889. [DOI] [PubMed] [Google Scholar]
- 263.Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146:519–32. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 264.Zhou Q, Wang M, Yuan Y, Wang X, Fu R, Wan H, et al. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell. 2016;18:330–40. doi: 10.1016/j.stem.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 265.Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539:299–303. doi: 10.1038/nature20104. [DOI] [PubMed] [Google Scholar]
- 266.Qing T, Liu H, Wei W, Ye X, Shen W, Zhang D, et al. Mature oocytes derived from purified mouse fetal germ cells. Hum Reprod. 2008;23:54–61. doi: 10.1093/humrep/dem334. [DOI] [PubMed] [Google Scholar]
- 267.Hayama T, Yamaguchi T, Kato-Itoh M, Hamanaka S, Kawarai M, Sanbo M, et al. Generation of mouse functional oocytes in rat by xeno-ectopic transplantation of primordial germ cells. Biol Reprod. 2014;91:89. doi: 10.1095/biolreprod.114.121640. [DOI] [PubMed] [Google Scholar]
- 268.Matoba S, Ogura A. Generation of functional oocytes and spermatids from fetal primordial germ cells after ectopic transplantation in adult mice. Biol Reprod. 2011;84:631–8. doi: 10.1095/biolreprod.110.087122. [DOI] [PubMed] [Google Scholar]
- 269.Chen B, Zhang L, Tang J, Feng X, Feng Y, Liang G, et al. Recovery of functional oocytes from cultured premeiotic germ cells after kidney capsule transplantation. Stem Cell Dev. 2013;22:567–80. doi: 10.1089/scd.2012.0436. [DOI] [PubMed] [Google Scholar]
- 270.Zeng M, Sheng X, Keefe DL, Liu L. Reconstitution of ovarian function following transplantation of primordial germ cells. Sci Rep. 2017;7:1427. doi: 10.1038/s41598-017-01648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Heng D, Sheng X, Tian C, Li J, Liu L, Gou M, et al. Mtor inhibition by INK128 extends functions of the ovary reconstituted from germline stem cells in aging and premature aging mice. Aging Cell. 2021;20:e13304. doi: 10.1111/acel.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tian C, Liu L, Ye X, Fu H, Sheng X, Wang L, et al. Functional oocytes derived from granulosa cells. Cell Rep. 2019;29:4256–67.e9. doi: 10.1016/j.celrep.2019.11.080. [DOI] [PubMed] [Google Scholar]
- 273.Tian C, Liu L, Zeng M, Sheng X, Heng D, Wang L, et al. Generation of developmentally competent oocytes and fertile mice from parthenogenetic embryonic stem cells. Protein Cell. 2021;12:947–64. doi: 10.1007/s13238-021-00865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Yoshino T, Suzuki T, Nagamatsu G, Yabukami H, Ikegaya M, Kishima M, et al. Generation of ovarian follicles from mouse pluripotent stem cells. Science. 2021;373:298–305. doi: 10.1126/science.abe0237. [DOI] [PubMed] [Google Scholar]
- 275.Oqani RK, So S, Lee Y, Ko JJ, Kang E. Artificial oocyte: development and potential application. Cells. 2022;11:1135–49. doi: 10.3390/cells11071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Yamashiro C, Sasaki K, Yabuta Y, Kojima Y, Nakamura T, Okamoto I, et al. Generation of human oogonia from induced pluripotent stem cells in vitro. Science. 2018;362:356–60. doi: 10.1126/science.aat1674. [DOI] [PubMed] [Google Scholar]
- 277.Yamashiro C, Sasaki K, Yokobayashi S, Kojima Y, Saitou M. Generation of human oogonia from induced pluripotent stem cells in culture. Nat Protoc. 2020;15:1560–83. doi: 10.1038/s41596-020-0297-5. [DOI] [PubMed] [Google Scholar]