Summary
Hepatic encephalopathy (HE) is a complication of cirrhosis characterised by neuropsychiatric and motor dysfunction. Microbiota-host interactions play an important role in HE pathogenesis. Therapies targeting microbial community composition and function have been explored for the treatment of HE. Prebiotics, probiotics and faecal microbiota transplant (FMT) have been used with the aim of increasing the abundance of potentially beneficial taxa, while antibiotics have been used to decrease the abundance of potentially harmful taxa. Other microbiome therapeutics, including postbiotics and absorbents, have been used to target microbial products. Microbiome-targeted therapies for HE have had some success, notably lactulose and rifaximin, with probiotics and FMT also showing promise. However, there remain several challenges to the effective application of microbiome therapeutics in HE, including the resilience of the microbiome to sustainable change and unpredictable clinical outcomes from microbiota alterations. Future work in this space should focus on rigorous trial design, microbiome therapy selection, and a personalised approach to HE.
Keywords: lactulose, cirrhosis, rifaximin, fecal transplant, ammonia, probiotic
Introduction
Hepatic encephalopathy (HE) is a complication of cirrhosis characterised by neuropsychiatric and motor dysfunction. Manifestations can range from subtle (minimal HE) to severe (overt HE) and even coma. HE is associated with considerable patient and caregiver burden, decreased quality of life, and poor survival.1–3 HE therapeutics represent one of the clearest unmet needs in cirrhosis care, where at least 50% of patients on current optimal therapy have breakthrough episodes.
Emerging data closely link HE pathophysiology to the gut microbiome. The role of microbiota in HE therapeutics has undergone a revolution, shaped by several landmark trials and major advances in microbiology.4–12 Novel sequencing and analytic methods have enabled the field to move from simply cataloguing the gut ecosystem to understanding the complex interactions between gut microbiota members, and how they react to environmental factors and to therapies. In parallel, we now have results from new investigations on microbiome-targeted therapies for other gastrointestinal and neurological diseases, which influences our understanding of the potential for microbiome therapies in HE.
In this review, we will discuss the limitations of current therapies for HE and the potential for novel microbiome therapies to improve outcomes.
Pathogenesis of hepatic encephalopathy and potential therapeutic targets
In health, the host and microbiota are connected by a shared physical space (the gut), but also by a shared metabolism.13–15 There are numerous examples of the host supplying microbiota with life-sustaining nutrients, and separately the microbiota providing key metabolism services to the host. Key elements of protein, lipid, and carbohydrate metabolism are symbiotic between host and microbiota. One important example is that bacteria ferment non-digestible polysaccharides, from the host diet, and produce short-chain fatty acids (SCFAs). In turn, SCFAs are an essential energy source for host colonic epithelium – they increase intestinal epithelial production of tight junction proteins and mucin, both of which contribute to barrier function.16–18 Intestinal bacteria also play a central role in bile acid metabolism and separately in developing host epithelial immune responses, both of which have been associated with intestinal barrier function. Bacterial products are not universally beneficial to the host, a notable example being lipopolysaccharide (LPS). However, bacterial products are often not absorbed into the systemic circulation due to a robust intestinal barrier. Ammonia, a product of bacterial urea and protein metabolism, is detoxified via the urea cycle in the healthy liver, keeping circulating ammonia levels low.
In cirrhosis and HE, the shared metabolism between the host and microbiota is altered (Fig. 1). SCFA-producing species, Anaerostipes caccae, Bacteroides eggerthii, and Clostridial species are depleted in patients with HE, who appear to have lower intestinal SCFA levels.19 Intestinal bile acid concentrations are reduced in cirrhosis, as is microbiota-induced bile acid metabolism.20–24 Finally, intestinal immune function is altered in advanced cirrhosis.25 These changes to the hostmicrobiota relationship influence intestinal barrier function and permeability, enhancing the translocation of neurotoxic factors. Investigations at the intersection of neurology and microbiology have identified several pathways that link microbiota to neuropsychiatric disease.26 Altered bile acid signalling may impact blood-brain-barrier permeability and neuroinflammation.27 In HE, ammonia was implicated early on as one such molecule with neurotoxic effects. In cirrhosis, portosystemic shunting and impaired hepatic ammonia metabolism leads to increased serum ammonia levels, with additional contributions from renal and muscle sources.28 Ammonia is able to cross the blood-brain-barrier and enter astrocytes where it is converted to glutamine which acts as an osmole, causing astrocyte swelling, oxidative stress, cellular dysfunction and ultimately neurological deficits.29 Streptococcus salivarius has been identified as a gut bacterial species that produces ammonia and is more abundant in patients with minimal HE than those without.30 Overt HE episodes are characterised by dramatic changes to gut microbiota composition, alongside changes in microbiota-mediated ammonia metabolism.31 Systemic inflammation has also been established as a major contributing factor to HE pathogenesis,32,33 synergising with ammonia to enhance neurotoxicity via increased blood-brain-barrier permeability and cerebral oxidative stress.34 Despite advances in recent decades, there are likely additional unidentified mediators influencing the relationship between the microbiota, intestinal barrier, and brain in HE.
Fig. 1. The pathogenic mechanisms of hepatic encephalopathy.
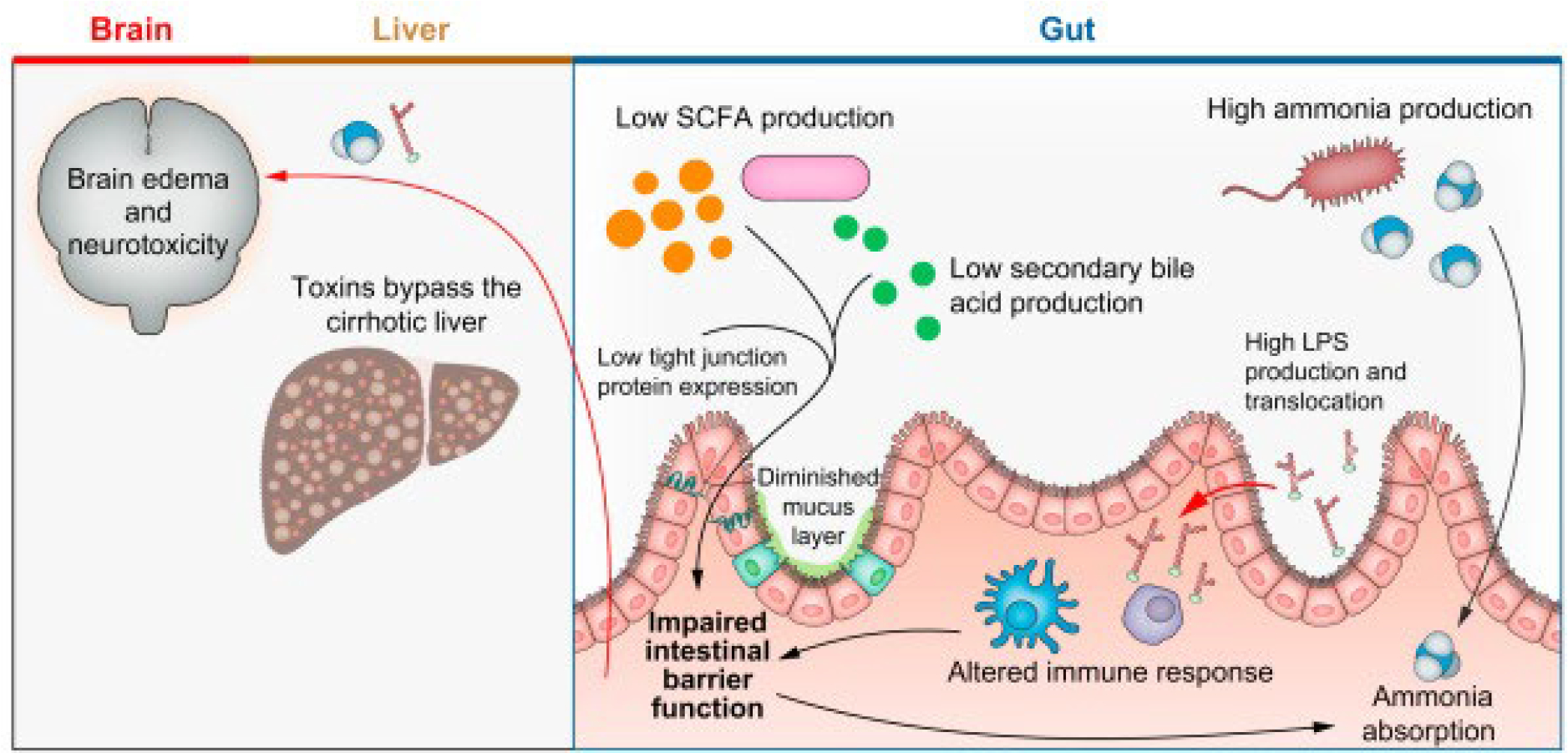
Changes to SCFAs, secondary bile acid, tight junction protein, and mucus production contribute to increased intestinal permeability. Intestinal bacterial products, including ammonia and LPS, are able to traverse the epithelial membrane and bypass the liver due to hepatic dysfunction and portosystemic shunting. They enter systemic circulation and reach the brain, where ammonia enters astrocytes and leads to neurotoxicity. LPS, lipopolysaccharide; SCFAs, short-chain fatty acids.
Key point.
Alterations in shared hostmicrobiota metabolism, intestinal permeability, and host immune response play a role in HE pathogenesis.
Given the role of microbiota-host interactions in the pathogenesis of HE, therapies targeting microbial community composition and function have been explored. A variety of approaches have been investigated, including directly targeting the microbiota – either by increasing the abundance of beneficial taxa or decreasing harmful taxa (Table 1). Other approaches have targeted the products of microbiota. Yet unstudied, future therapies may directly target host intestinal barrier function and immune regulation. Precision medicine may be on the horizon for HE, wherein therapies target specific host or microbiota deficits in a patient-specific fashion.
Table 1.
Potential benefits and limitations of microbiome therapies for hepatic encephalopathy.
| Therapy | Definition | Potential benefits | Clinical efficacy | Clinical limitations |
|---|---|---|---|---|
|
| ||||
| Target microbes: Increase potentially beneficial taxa | ||||
| Prebiotic (e.g. lactulose) | Substrates selectively utilised by host microorganisms, conferring a health benefit | •Increase beneficial taxa abundance, short-chain fatty acids•Inhibit pathogen growth (via lowering luminal pH)•Stimulate mucus-producing goblet cells•Improve tight junctions | Two large meta-analyses show lactulose reverses minimal HE and prevents overt HE | Lactulose has undesirable side effects and prevents <50% of recurrent overt HE |
| Probiotic | Living microorganisms that, when administered in adequate amounts, confer a health benefit | •Produce antimicrobial proteins, short-chain fatty acids, secondary bile acids•Inhibit pathogen growth (via lowering luminal pH and decreasing available nutrients)•Regulate immune response | Low to moderate quality evidence show probiotics improve HE symptoms, reverse minimal HE, and lead to fewer overt HE episodes | Clinical data is low to moderate quality; many trials have high risk of bias; different strains used in each trial |
| Engineered probiotics | Genetically modified bacteria | •Varies by genetic modification | SYNB1020 reduces blood ammonia in mice | First trial in cirrhosis failed |
| Faecal microbiota transplant | Transfer of processed stool from healthy donor to recipient | •Decrease ammonia production, intestinal permeability•Increase short-chain fatty acids, secondary bile acids, tight junction proteins, antimicrobial proteins | Small single-centre trials suggest FMT improves cognition and reduces overt HE episodes | Limited data; supply and implementation challenges; inherent risk of uncharacterised components |
| Synbiotic | Probiotic and prebiotic in 1 therapy | •Combined benefits of prebiotics and postbiotics | Limited supportive data | No evidence of synergistic benefit |
|
| ||||
| Target microbes: Decrease potentially harmful taxa | ||||
| Antibiotics (e.g. rifaximin) | Treatment with anti-bacterial effects; target and effects vary by antibiotic | •Decrease pathogen abundance, gut inflammation, endotoxin•Increase long-chain fatty acids, beneficial taxa, tight junction proteins | High quality data that rifaximin reduces risk of overt HE; also improves cognition in MHE | High rate of overt HE recurrence despite rifaximin; other antibiotics have limiting side effects |
| Bacteriophages | Viruses that target bacteria | •Eliminate specific pathogenic bacteria | No clinical efficacy data | No clinical efficacy data |
|
| ||||
| Target products of microbes | ||||
| Postbiotic | Bioactive products of beneficial taxa | •Increase tight junction proteins, mucin production•Regulate immune response | No clinical efficacy data | No clinical efficacy data |
| Absorbents | Synthetic molecules that absorb undesirable substances | •Decrease serum ammonia, endotoxin, cytokines | AST-120 and Yaq-001 with positive biological effects in rat model | AST-120 without clinical benefit in humans; Yaq-001 human data not available yet |
HE, hepatic encephalopathy; FMT, faecal microbiota transplant; MHE, minimal hepatic encephalopathy.
Microbe-targeted therapies: Increase potentially beneficial taxa
Prebiotics
Prebiotics are substrates selectively utilised by host microorganisms that confer a health benefit.35 Prebiotics are most often non-absorbable carbohydrates, able to be fermented by luminal bacteria.13 Fermentation of prebiotics leads to increased abundance of beneficial taxa that can utilise these substrates, produce SCFAs, and reduce pH in the intestinal lumen.36 Increased biomass of beneficial taxa reduces available nutrients for invading microbial pathogens.13 The reduced pH caused by prebiotics also inhibits pathogen growth. In vitro and human studies showed that prebiotics improve intestinal barrier function by stimulating mucus-producing goblet cells, augmenting tight junction assembly, and mitigating inflammation.37–40 Lactulose, a non-absorbable disaccharide and a prebiotic, is the primary therapy for HE. While the literature on its actions is not uniform, it appears that lactulose’s benefits in HE are mediated through changes in intestinal microbes, likely a result of its pH-lowering effect and positive pressure on beneficial taxa.
Lactulose is an effective treatment for HE. A Cochrane review including 38 randomised controlled trials of non-absorbable disaccharides demonstrated their beneficial effect on HE (risk ratio 0.58; 95% CI 0.50–0.69).7 A more recent network meta-analysis of 25 trials found that when comparing lactulose, rifaximin, probiotics, and L-ornithine L-aspartate (an ammonia-lowering agent) for the treatment of minimal HE, lactulose was the only agent able to meet all 3 endpoints: reverse minimal HE, prevent overt HE, and improve quality of life.9 However, many patients experience breakthrough overt HE episodes while on lactulose.41
Early culture-based studies demonstrated that lactulose promotes the growth of the beneficial taxa Lactobacillus and Bifidobacteria.42–44 Lactulose fermentation by these beneficial taxa requires increased bacterial amino acid synthesis using ammonia as the substrate, leading to reduced luminal ammonia concentrations.45–48 Increased amino acid synthesis with lactulose does not occur in germ-free rats, supporting the role of bacteria in this process.49 Lactulose transits through the small intestine largely unchanged and is fermented by colonic bacteria,28,50 leading to SCFA production and subsequent colonic acidification.44 In patients with minimal HE, lactulose decreases bacterial DNA in the serum and improves neurocognitive test scores, presumably through changes to bacterial composition and improved intestinal permeability – the latter of which may be a result of increased SCFA production.51 In the setting of colonic acidification from SCFAs, ammonia production from gram-negative bacteria decreases, likely reflecting diminished metabolic activity as well as growth inhibition of those bacteria.45 A recent multicentre study of lactulose for minimal HE found no significant change in microbial composition (using 16S rRNA sequencing); however, those with a clinical response experienced a significant decrease in certain Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria relative to non-responders.52 Lactulose may reduce serum ammonia through additional mechanisms, including trapping ammonium ions in the colon.16,25,53 Novel prebiotics such as synthetic glycans, which are in development, appear to be more potent than lactulose in lowering ammonia production; further studies will be required to determine their efficacy in treating HE.54
Probiotics
Probiotics are living microorganisms that, when administered in adequate amounts, confer a health benefit on the host.55 For decades there has been interest in using probiotics to treat HE, ranging from yogurts to probiotic powders and encapsulated probiotic strains.4,56–58 There are 3 evidence-based mechanisms by which probiotics may improve HE: improving intestinal barrier function, immune modulation, and decreasing portal hypertension. First, probiotics in HE may enhance tight junction protein production or integrity, thus improving intestinal barrier function and reducing translocation of bacterial products into the systemic circulation. In a murine model of colitis, VSL#3 (a combination of 8 bacterial strains) and separately Escherichia coli (E. Coli) Nissle 1917 prevented an increase in intestinal permeability by maintaining tight junction expression and suppressing apoptosis.59,60 Probiotics in rodent models of alcohol-related liver disease and non-alcoholic steatohepatitis were also found to reduce serum LPS levels and to increase tight junction expression.61–63 Furthermore, a randomised trial of Lactobacillus GG in patients with minimal HE reduced serum LPS.4 Second, probiotics interact with the host intestinal epithelium, and have an established role in immune modulation.13,64 Systemic inflammation enhances the cerebral effect of ammonia and exacerbates HE symptoms.32,33 One trial of VSL#3 in patients with recent overt HE found that the probiotic reduced serum cytokines (tumour necrosis factor [TNF]-α, interleukin (IL)-1β, and IL-6 levels) in the subgroup (24%) of patients who completed 24 weeks of follow-up.8 Part of the immune regulation provided by probiotics may be specific to neutrophil function. Chronic neutrophil activation may lead to neutrophil exhaustion in cirrhosis, leaving patients vulnerable to infection and poor survival. Infection is a very common precipitant of HE. Supplementation with a probiotic mixture of Bifidobacterium, Lactobacillus, and Lactococcus strains in patients with cirrhosis compared to placebo led to increased neutrophil production of reactive oxygen species, thus restoring neutrophil function58 – a benefit also seen in studies of Lactobacillus casei Shirota as well as with a probiotic mixture of Bifidobacterium, Lactobacillus, and Streptococcus.10,65 Third, probiotics may reduce portal hypertension. One study found that VSL#3 decreased serum and hepatic vein TNF-α levels in patients with large oesophageal varices, and led to an additional reduction in hepatic venous pressure gradient beyond propranolol monotherapy.66 Probiotics additionally produce organic acids, antimicrobial compounds, and bile salt hydrolases – mechanisms that should be explored in future studies of probiotics for HE.13
Two recent large meta-analyses found that probiotics improve HE symptoms, reverse minimal HE, lead to fewer episodes of overt HE, and lower ammonia levels, though evidence is low to moderate quality as all but 2 trials are at high risk of bias.9,11 In addition, probiotics do not impact patient quality of life or mortality, and were not superior to lactulose for all outcomes. Clinical trial data on probiotics for HE is difficult to compare because each trial uses different probiotic strains.67 Furthermore, the low level of colony-forming units in most commercial probiotic formulations limits optimism that probiotics are sufficient to overtake the resident microbial community structure of cirrhosis and HE.16
Key point.
Potential targets for HE microbiome therapeutics include microbiota abundance, microbial products, intestinal barrier function, and host immune responses.
Bacterial genomes can be manipulated by modern genetic tools for therapeutic purposes.68 SYNB1020 is an E. coli Nissle 1917 strain genetically engineered to convert ammonia to L-arginine.69 The engineered probiotic successfully reduced ammonia levels in preclinical studies and was well tolerated in a phase I study. However, according to reporting on ClinicalTrials.gov, SYNB1020 did not lower blood ammonia levels in patients with cirrhosis. This should not be taken as a failure of all engineered probiotics for HE; other engineered probiotics could impact different aspects of HE pathogenesis, including immune regulation or intestinal barrier integrity.
Faecal microbiota transplant
Faecal microbiota transplant (FMT) is the transfer of processed stool from a healthy donor to a recipient. Three small trials have investigated FMT for the treatment of HE, with early evidence suggesting a potential clinical benefit.5,6,70 All 3 trials enrolled patients with a history of overt HE on lactulose therapy and, in many cases, rifaximin. The first trial randomised patients to standard of care vs. broad-spectrum antibiotics followed by a single FMT enema, and the second trial by the same study team (Bajaj et al.) randomised patients to 1 day of oral FMT capsules vs. placebo capsules.5,6 The FMT in both trials was derived from the same donor. The primary outcome of these 2 trials evaluated the safety of FMT and found no safety concerns, aside from a reversible and small increase in model for end-stage liver disease (MELD) score after broad-spectrum antibiotics. The patients who received antibiotics and an FMT enema improved on 2 validated cognitive tests and had fewer episodes of overt HE during long-term follow-up compared to the standard of care arm.5,71 Antibiotics were given prior to FMT to decrease host bacterial burden and to enable FMT colonisation,72–75 and were not given to the control group, limiting the ability to interpret the relative contributions of antibiotics and FMT to cognitive improvement. Lack of blinding also introduces observer bias. In the second trial by Bajaj et al., the patients who received oral FMT capsules improved in one cognitive test (EncephalApp, a version of the Stroop test) but not in another cognitive test (psychometric HE score).6 There was no difference in overt HE episodes during follow-up of 5 months. The third pilot trial is our open-label trial of 5 doses of oral FMT capsules over 3 weeks. Preliminary results suggest improvement in the psychometric HE score but not in EncephalApp 1 week after completing 5 days of oral FMT capsules (administered over 3 weeks).70 In this trial, FMT transmitted extended-spectrum beta-lactamase (ESBL)-producing E. coli to 1 recipient, despite following FDA-approved donor screening protocols.76 Overall, the studies evaluating FMT for HE are small and designed to evaluate safety, not efficacy. Clinical efficacy outcomes were mixed, though there were some promising signals. A single dose of FMT from 1 donor was used in the 2 published trials, and it is possible that benefit may vary by donor and additional doses of FMT may be necessary.77 Larger trials powered to detect clinical improvement in HE using different FMT dosing strategies are needed.
There are several potential mechanisms by which FMT may impact the pathogenesis of HE: SCFA production, changes to microbiome community structure, bile acid metabolism, and reduced ammonia production (Fig. 2). Results of the Bajaj et al. FMT enema trial are difficult to interpret because of antibiotic use only in the FMT arm: changes in microbial diversity and taxa abundance changed primarily with antibiotic exposure, and returned to pre-antibiotic levels after FMT.5 However, there was a clear increase in Ruminococcaceae abundance from baseline to post-FMT. Ruminococcaceae was heavily enriched in the donor, and is known to produce SCFAs, which in turn impacts intestinal barrier function – a possible mechanism by which these FMT enemas improved clinical outcomes.5 However, this increase in Ruminococcaceae disappeared over long-term follow-up, despite ongoing improvement in HE clinical outcomes.71 In an elegant mouse study, the bacteria themselves and not a sterile supernatant led to cognitive improvement after FMT, suggesting that the benefits of FMT in HE are not simply a result of bacterial products but also the influence of the bacteria themselves, perhaps on microbiome community composition and function.78 In the second trial by Bajaj et al. of FMT for HE, oral FMT capsules did not change alpha diversity in stool samples, but did increase diversity of the duodenal mucosal microbiome, suggesting an impact in the proximal bowel.6 There was also an increase in duodenal Ruminococcaceae and Bifidobacteriacceae (generally beneficial taxa) and a decrease in Veillonellaceae after FMT capsules, though duodenal samples were not studied in the control group so it is unknown if these changes may be due to natural fluctuation. In conjunction with these microbial changes, patients who received FMT had an increase in duodenal antimicrobial protein DefA5, increase in tight junction protein E-cadherin (CDH) expression, and a decrease in IL-6 expression. LPS-binding protein also decreased after FMT, suggesting that oral FMT capsules may change duodenal microbial community structure, influencing several aspects of small intestinal barrier function, and decreasing translocation of bacterial products.6 Correlation network analysis showed that certain taxa were linked with improved immunomodulatory milieu and cognitive test performance. Those taxa have also been associated with decreased inflammation and strengthened intestinal barrier function in patients with and without liver disease.79 Post hoc analysis also revealed an increase in secondary bile acids after FMT for HE.79 Patients with cirrhosis have a diminished ability to produce secondary bile acids, likely due to a relative reduction in Clostridial species. Secondary bile acids are associated with protection from pathogenic organisms and impact intestinal barrier function. Therefore, FMT may exert some of its therapeutic effect by influencing bile acid metabolism and, as a result, intestinal barrier function. Finally, the PROFIT trial, a placebo-controlled trial of jejunal FMT delivery in patients with cirrhosis (not all had prior HE) reported that FMT was associated with a reduction in serum ammonia levels through as yet unknown mechanisms.80,81 Thus, another potential mechanism by which FMT could help patients with HE is by ameliorating hyperammonaemia.
Fig. 2. Faecal microbiota transplant for hepatic encephalopathy: From bench to bedside.
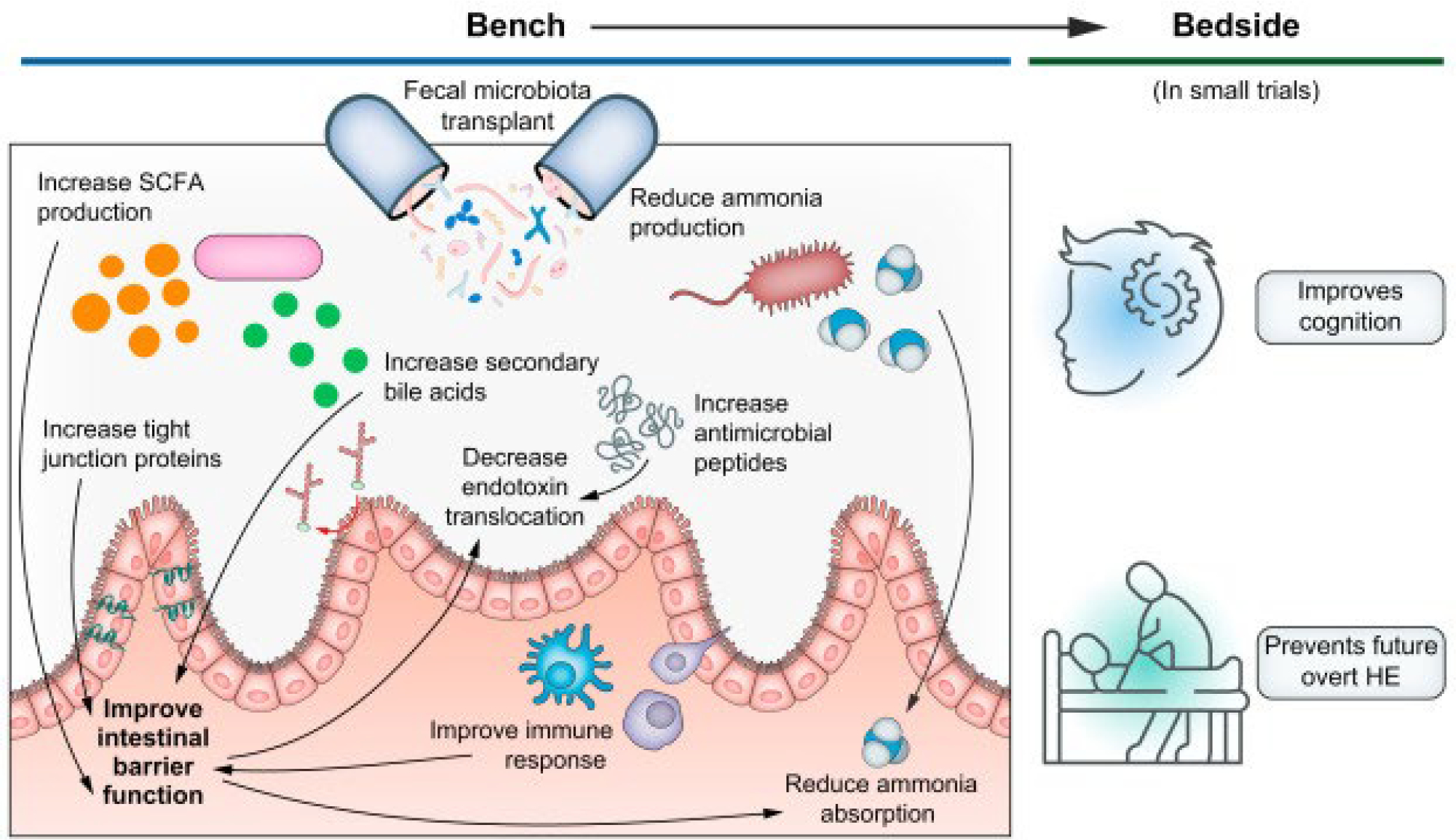
Faecal microbiota transplant has multiple potential therapeutic mechanisms in HE, including several which improve intestinal barrier function: increasing SCFA, secondary bile acid, tight junction protein, and antimicrobial peptide production. By changing microbial community structure, ammonia and endotoxin production and translocation decreases. At the bedside, several small trials have suggested improved cognition and fewer overt HE episodes with faecal microbiota transplant. HE, hepatic encephalopathy; SCFA, short-chain fatty acid.
Synbiotics
Synbiotics are probiotics and prebiotics combined into a single therapy. The hope for such combined products is that prebiotics will enhance the efficacy of probiotics, though evidence of synergism is lacking.37 One small single-centre trial showed cognitive benefit and decreased serum ammonia with a synbiotic (Bifidobacterium longum and fructo-oligosaccharide).82 In a trial of patients with minimal HE, both a synbiotic and a prebiotic alone reversed minimal HE in half of participants, with the symbiotic demonstrating no clear superiority over the prebiotic alone.83 Patients who received the synbiotic or prebiotic alone developed acidified faecal contents, decreased venous ammonia levels, serum LPS levels, and E. coli faecal abundance. The clinical benefits of synbiotics compared to prebiotics and probiotics used alone remains to be confirmed.
Microbe-targeted therapy: Decrease potentially harmful taxa
Antibiotics
Antibiotics have been proposed to treat HE, as a method to deplete intestinal taxa that produce neurotoxins (namely ammonia), increase intestinal permeability, and diminish host systemic immune responses. Certain antibiotics may selectively suppress harmful taxa, while allowing potentially beneficial taxa to survive and even proliferate. Rifaximin is approved in the United States and Europe to reduce the risk of recurrent overt HE. Several randomised controlled trials have found that rifaximin markedly reduces the risk of recurrent overt HE.41,84 Rifaximin also improves cognition and quality of life for patients with minimal HE.85,86 While the clinical benefits of rifaximin are undisputed, its mechanisms of action in HE are less clear and likely multifactorial.
Rifaximin inhibits bacterial RNA synthesis and has broad-spectrum antimicrobial activity, notably for pathogenic bacteria like enterotoxigenic E. coli, Shigella, and Salmonella.87,88 However, several small studies in patients with minimal HE suggest that rifaximin exerts little influence on microbial community composition.85,89–91 Since these studies used 16S rRNA amplicon sequencing methods, the effect of rifaximin on changes to subtaxa like species or strains may have been missed. Some effects from microbiome therapies are species and even strain specific.92 There are several reasons to suspect that rifaximin’s benefit in HE may be a result of relevant microbial composition changes. First, rifaximin changes the ratio of secondary to primary bile acids, which has implications for microbiota composition.89 Second, rifaximin has been shown in other populations to change microbial community abundance and structure. In a visceral hyperalgesia rat model, rifaximin decreased the total small bowel bacterial burden, increased Lactobacillus species, and decreased small bowel inflammation and permeability.93 In irritable bowel syndrome and Crohn’s colitis, rifaximin increased the abundance of Bifidobacterium and Faecalibacterium prausnitzii, known beneficial taxa, and increased production of SCFAs.94,95 While family- and genus-level changes have not been detected with rifaximin treatment for HE, species- and strain-level changes have yet to be explored.
Key point.
Lactulose and rifaximin influence intestinal microbiota and have been used with success in HE, with early promise for probiotics and faecal microbiota transplant.
Rifaximin significantly lowers serum LPS levels in humans and animal models, which may be the result of changes in microbiome composition (i.e. less LPS produced) or a result of decreased LPS translocation across the intestinal barrier.85,89 Rifaximin decreases cytokine expression and intestinal inflammation, and simultaneously increases tight junction protein expression – all of which contribute to barrier function.89,96–98 Rifaximin reduces adherence of bacteria to the gut wall and decreases bacterial virulence, both of which are potential mechanisms by which it reduces translocation.99–101 Rifaximin also influences bacterial metabolism. Bajaj and colleagues found that 8 weeks of treatment with rifaximin led to an increase in bacterial carbohydrate and lipid metabolism, resulting in an increase in patients’ serum long-chain and unsaturated fatty acid levels.85 Some of these fatty acids have been shown to increase in the brain with probiotic supplementation and are capable of improving cognitive processes like learning and memory.92 Finally, data are mixed with regards to rifaximin’s impact on ammonia levels;89,101–103 however, one notable study of germ-free mice found a bacteria-independent mechanism by which rifaximin could reduce intestinal ammonia production: via intestinal glutaminase.89 Overall, available evidence suggests that rifaximin may enhance intestinal barrier function, ameliorate microbiome-induced inflammatory dysregulation, decrease translocation of bacterial products, and influence gut bacterial metabolism in a way that may improve cognitive function.
To date, no antibiotics have demonstrated superior or even comparable efficacy and safety to rifaximin for HE.104–108 Rifaximin’s minimal systemic absorption accounts for its excellent tolerability and safety profile.109–111 With regards to efficacy, rifaximin may selectively deplete harmful taxa while allowing beneficial taxa to survive and increase metabolic activities compared to other antibiotics tested for HE.
Bacteriophages
Bacteriophages are viruses which specifically target bacteria. The potential impact of bacteriophages in hepatology was recently highlighted when a bacteriophage was used to target and eliminate cytolysin-producing Enterococcus faecalis, a species playing a key pathogenic role in alcohol-associated hepatitis.112 Bajaj and colleagues have recently demonstrated that bacteriophage abundance varies by MELD score, HE status, and HE treatment status, though bacterial composition seemed more relevant to clinical outcomes than bacteriophage composition.113 In particular, phages for Streptococcal species seemed the most influenced by disease severity and rifaximin therapy, which is notable given that many of those species are urease-producing and therefore ammonia-generating.
Therapy targeting microbial products
Postbiotics
Postbiotics is a term used to describe bioactive products of beneficial bacteria. SCFAs are the main postbiotics of interest in HE. SCFAs are produced by bacterial fermentation of non-digestible polysaccharides; they are an essential energy source for the colonic epithelium and contribute to barrier function.17 They also prime the intestinal epithelium to respond to bacterial products, induce tolerance to commensals, and regulate immune responses.25,114–116
Advanced liver disease and HE are associated with reduced intestinal SCFA levels.19 In a study of hepatic vein and peripheral blood sampled during transjugular intrahepatic portosystemic shunt placement, a moderate inverse correlation between butyrate and MELD score in both blood sources was observed. Stool SCFA content is also inversely related to MELD score.19 SCFAs have not been directly tested as a treatment for HE. The most common way to experimentally increase SCFAs in the intestinal lumen is by encouraging growth of bacterial species that produce SCFAs, usually with prebiotics. Not all SCFA types and delivery modalities have the same or even desirable effects.17,18,117–121 For example, butyrogenic bacteria are associated with steroid-refractory graft-versus-host disease, possibly through butyrate-induced inhibition of colonic stem cells.122 Further research is needed to better understand which SCFAs are beneficial for HE and the best administration strategy.
Absorbents
For the last decade there has been interest in ingestible devices which can absorb undesirable substances in the gut lumen, thus limiting their intestinal absorption.53 AST-120 is one such device: a carbon bead with pores small enough to bind microscopic molecules, including ammonia. AST-120 was able to decrease serum ammonia concentrations and reduce brain oedema in a rat model; however, it did not produce clinical benefit in patients with HE.123,124 Yaq-001, another absorbent carbon bead, is able to absorb larger molecules including LPS. It appears that Yaq-001 had a myriad of effects in a rat model: reduced LPS levels, markers of liver and systemic inflammation, portal hypertension, and HE, and altered the microbiome.53 Unfortunately, the phase I trial of Yaq-001 had to be halted due to the COVID-19 pandemic and results are not yet available.125 Despite these challenges, there is still optimism around methods that remove potentially toxic bacterial products in the gut lumen.
Challenges
One major obstacle to the effective use of microbiome-targeted therapies for HE is the resilience of the human adult microbiome. The human microbiome is constantly exposed to external challenges including the diet, medications, and numerous host factors, but it has an incredible ability to restore its equilibrium after perturbation – even if that microbial community structure is associated with disease.126 It is possible that the disease state (in this case, cirrhosis) exerts constant pressure on the microbiome, promoting overgrowth of potentially harmful taxa while limiting colonisation of beneficial taxa. In contrast to Clostridioides difficile (C. difficile) colitis, microbiome therapies for HE will likely need to be administered as recurrent courses or continuously instead of as a single short course.
Another related challenge in using microbiome-based therapeutics is that the gut-liver ecosystem is saturated with connections.126 Every functional pathway in the gut and liver involves numerous interconnected components such that if one element changes, there are likely many compensatory mechanisms. Thus, an impact on one microbiome component can have unpredicted ripple effects or no effect at all.
One challenge with antibiotics as a therapy for HE is the potential to promote multi-drug resistance. The prevalence of multi-drug resistant bacteria has grown considerably in patients with cirrhosis in the last decade, from 29% of infections to 38% in a large European cohort.127 In a study of 77 patients with cirrhosis starting prophylaxis for spontaneous bacterial peritonitis, nearly 50% carried multi-drug resistant organisms prior to antibiotic initiation, and at 180 days of prophylaxis this prevalence increased to 74%.128 Rifaximin, the best validated antibiotic for HE, does not appear to induce bacterial resistance and actually has bactericidal activity against many multi-drug resistant bacteria.91,129 However, as we continue to use antibiotics to treat HE, we must increasingly keep in mind the problem of resistance and balance benefit-to-harm.
FMT is associated with its own specific set of challenges (Fig. 3). The central limitation of FMT is an inherent lack of certainty about what is administered. FMT originates from the stool of a healthy individual, and as such there will be changes over time within the same donor and across donors.130 Despite extensive testing outlined by FDA-approved protocols, FMT has recently led to infections by ESBL-producing E. coli, Shigatoxin-producing E. coli, and enteropathogenic E. coli.76,131,132 In all cases, donor stool was extensively screened for pathogens and in some cases for the ultimate infectious culprit, thus highlighting the challenges in identifying pathogens in FMT material. In at least one of these cases, the antimicrobial resistance patterns differed between the FMT E. coli and the recipient’s E. coli, despite genetic testing confirming they were identical strains.76 The presence of divergent antibiograms with clonal bacteria raises the question of whether prior FMT studies have underestimated the risk of infection related to FMT. While FMT has been reported to be safe in numerous studies, there remains some risk of infection.133 In the COVID-19 era, potentially infectious SARS-CoV-2 is present in the stool of infected individuals134 and may be transmitted from asymptomatic carriers. While potential donors could be screened by symptoms and nasopharyngeal swab, those measures do not have perfect sensitivity and no stool-based SARS-CoV-2 test has been approved for clinical use.135,136 Furthermore, some studies suggest that virus shedding in stool may outlast virus detection in nasopharyngeal swabs.137 As the list of required donor tests appropriately grows in length, the practical limitations of FMT also grow. Prior to COVID-19 and some recent additions to testing, the cost of finding a suitable FMT donor was estimated at $15,190.138 There are other logistical challenges with using FMT to treat HE including identification of appropriate donors, standardisation of the product, and sustainability of supply. There remain significant unanswered scientific questions as well. Each trial of FMT for HE or cirrhosis has used a different route, quantity and timing of FMT administration. At this time, we do not know the best FMT dosing regimen or whether patients will require repeat dosing. In addition, the ideal donor for FMT has not been defined. Studies outside of C. difficile infection, in ulcerative colitis for example, have found that only 1 in 6 donors achieve desired clinical endpoints.139,140 Given the uncertainty around the mechanism of FMT in treating HE, it is hard to determine criteria for the ideal donor. Optimal donor selection may require analysis of microbiota function and not just composition. Finally, most FMT is aerobically prepared, and thus anaerobes are not administered. Anaerobically prepared FMT was found to induce remission in patients with ulcerative colitis, after several failed trials with aerobically prepared FMT, suggesting a potential therapeutic benefit of the anaerobic components.141 Many probiotics, such as Faecalibacterium prausnitzi, are lost with aerobic stool processing and preserved with anaerobic processing.142 Therefore, there may be benefits to anaerobically prepared FMT in HE, beyond what has been found with aerobic preparations.
Fig. 3. The strengths, challenges, weaknesses, and future directions of faecal microbiota transplant for hepatic encephalopathy.
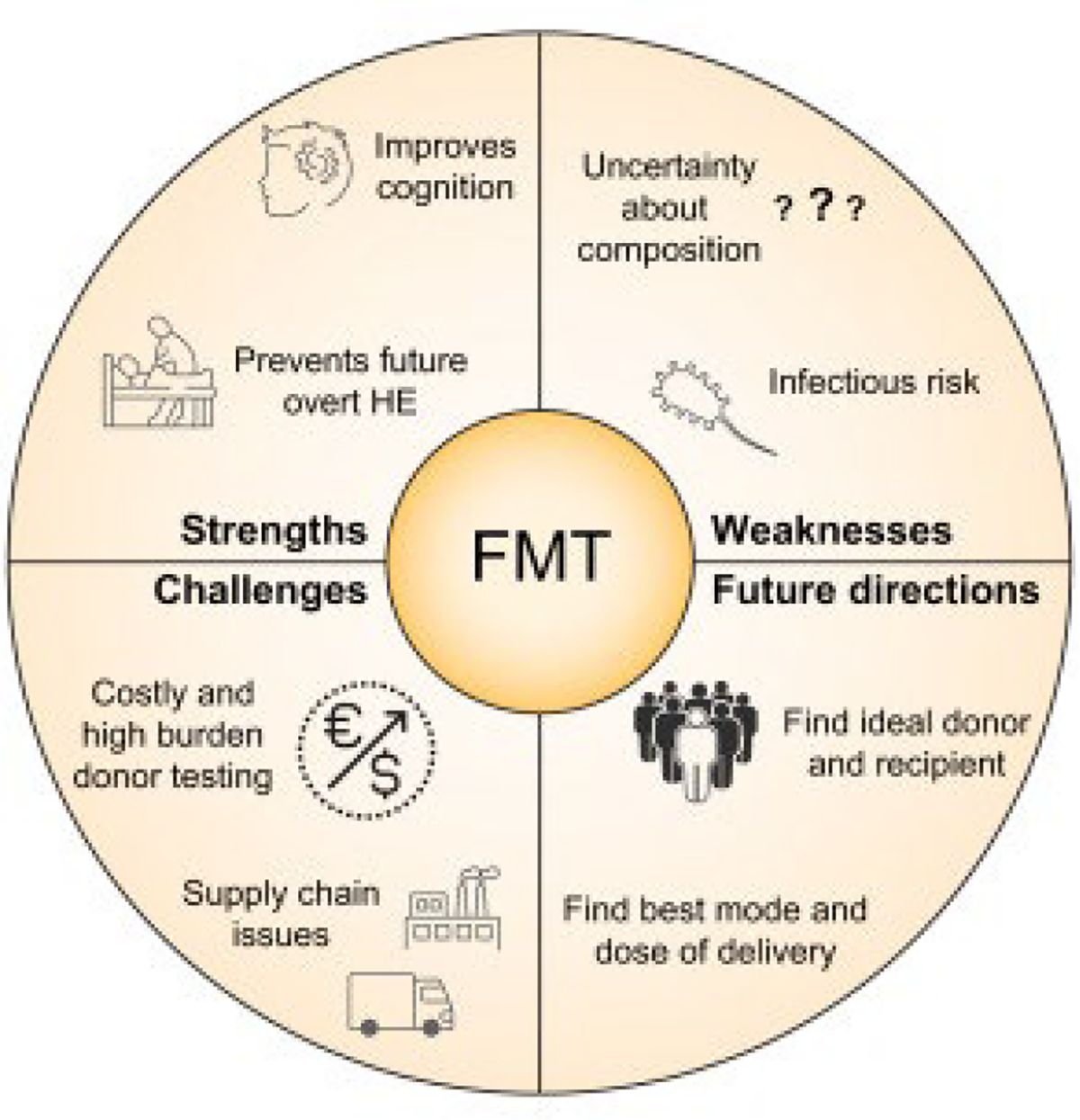
FMT, faecal microbiota transplant; HE, hepatic encephalopathy.
Key point.
Future research on microbiome-targeted therapies for HE should focus on patient and primary outcome selection, microbiome therapy selection, and a personalised therapeutic approach based on baseline enterotype and other patient factors.
Future directions
Microbiome-targeted therapies for HE have had some success, namely lactulose and rifaximin, with early promise for probiotics and FMT. Despite available therapies, many patients with HE suffer from persistent symptoms and many are unable to tolerate lactulose. Future work in this space should focus on trial design, microbiome therapy selection, and a personalised approach to HE (Table 2).
Table 2.
Future directions of microbiome-targeted therapies for hepatic encephalopathy.
| Future directions | |
|---|---|
|
| |
| Trial design | Patient selection: target specific groups in need of more effective and/or better tolerated intervention (e.g. at high risk of developing recurrent overt HE, or those who do not tolerate lactulose) Primary outcome selection: clinically important primary outcomes such as overt HE, cognitive function, quality of life, or other patient-reported outcomes (microbiome changes may be included as secondary outcome) Translational component: explore mechanism within clinical trials High rigour: minimise bias through blinding, randomisation ± risk stratification; meet enrolment target to achieve adequate power for trial |
| Microbiome therapy selection | Target gut segment: match route of administration to optimal gut segment (upper vs. lower intestine) for that mechanism Living biotherapeutics: select probiotic consortium with biological actions with potential to reverse HE, determine optimal dose and duration, assess need for antibiotic priming to ensure grafting Faecal microbiota transplant: determine characteristics of ideal donor, optimal dose regimen and preparation |
| Personalised approach | Patient enterotype: determine baseline microbiome characteristics to match appropriate microbiome therapy Biomarkers of response: identify other biomarkers predictive of response to microbiome therapies |
HE, hepatic encephalopathy.
Future trials should be designed to target the highest-need populations as well as focus on clinically relevant primary outcomes. Trials should be designed with a translational component, to allow for gaps in our mechanistic knowledge to be closed. Practices of rigorous and reproducible research should be applied, including those which minimise bias and achieve sufficient power to assess clinical efficacy. Microbiome therapy selection is also critical. Microbiome therapies have varying impact throughout the intestines and colon. For example, an FMT enema impacts the distal colon while orally administered FMT capsules have a more dispersed effect. Further studies are needed to identify which segment of the bowel has the most impaired barrier function in cirrhosis and is most responsible for HE, as well as the impact of microbiome therapies on intestinal permeability.143 Additional studies on FMT including larger cohorts are needed to determine efficacy, ideal dosing regimen, favourable donor characteristics, benefit of anaerobic preparation, duration of benefit, and predictors of response. Living biotherapeutic products with a larger biomass than traditional commercial probiotics and with known metabolic effects (similar to FMT) are being studied in C. difficile infection and inflammatory bowel disease. The therapies that produce metabolites known to be deficient in patients with HE, such as SCFAs and secondary bile acids, should be trialled for HE. Finally, the efficacy of each microbiome therapy may depend on a patient’s existing microbiome community, or enterotype. A personalised approach to microbiome therapy, based on baseline community structure and function, may yield the most clinical success.
Supplementary Material
Financial support
Patricia P. Bloom receives funding from the American College of Gastroenterology (ACG Junior Faculty Award) and the American Association for the Study of Liver Diseases (AASLD Advanced Hepatology Award). VBY was funded by NIH Award AI124255. EBT is funded by NIDDK K23 DK117055.
Abbreviations
- C. difficile
Clostridioides difficile
- E. coli
Escherichia coli
- ESBL
extended-spectrum beta-lactamase
- FMT
faecal microbiota transplant
- HE
hepatic encephalopathy
- LPS
lipopolysaccharide
- MELD
model for end-stage liver disease
- SCFA(s)
short-chain fatty acid(s).
Footnotes
Conflict of interest
Patricia P. Bloom serves as a consultant for Synlogic. ASL: no conflict. VBY serves as a consultant to Vedanta, Bio-K+ International and Pantheryx. Elliot Tapper has served as consultant for Axcella, Kaleido, Mallinckrodt, Novo Nordisk, Allergan, and Takeida. He has received unrestricted grants from Valeant and Gilead.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.08.004.
References
- [1].Bajaj JS, O’Leary JG, Tandon P, Wong F, Garcia-Tsao G, Kamath PS, et al. Hepatic encephalopathy is associated with mortality in patients with cirrhosis independent of other extrahepatic organ failures. Clin Gastroenterol Hepatol 2017;15(4):565–574.e4. [DOI] [PubMed] [Google Scholar]
- [2].Rabiee A, Ximenes RO, Nikayin S, Hickner A, Juthani P, Rosen RH, et al. Factors associated with health-related quality of life in patients with cirrhosis: a systematic review. Liver Int 2020. [DOI] [PubMed] [Google Scholar]
- [3].Tapper EB, Aberasturi D, Zhao Z, Hsu CY, Parikh ND. Outcomes after hepatic encephalopathy in population-based cohorts of patients with cirrhosis. Aliment Pharmacol Ther 2020;51(12):1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, Sterling RK, et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther 2014;39(10):1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology (Baltimore, Md) 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bajaj JS, Salzman NH, Acharya C, Sterling RK, White MB, Gavis EA, et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo-controlled trial. Hepatology (Baltimore, Md) 2019;70(5):1690–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev 2016;2016(5):Cd003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology 2014;147(6). 1327–1337.e3. [DOI] [PubMed] [Google Scholar]
- [9].Dhiman RK, Thumburu KK, Verma N, Chopra M, Rathi S, Dutta U, et al. Comparative efficacy of treatment options for minimal hepatic encephalopathy: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol 2020;18(4). 800–812.e25. [DOI] [PubMed] [Google Scholar]
- [10].Román E, Nieto JC, Gely C, Vidal S, Pozuelo M, Poca M, et al. Effect of a multistrain probiotic on cognitive function and risk of falls in patients with cirrhosis: a randomized trial. Hepatol Commun 2019;3(5):632–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dalal R, McGee RG, Riordan SM, Webster AC. Probiotics for people with hepatic encephalopathy. Cochrane database Syst Rev 2017;2:CD008716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhao LN, Yu T, Lan SY, Hou JT, Zhang ZZ, Wang SS, et al. Probiotics can improve the clinical outcomes of hepatic encephalopathy: an update meta-analysis. Clin Res Hepatol Gastroenterol 2015;39(6):674–682. [DOI] [PubMed] [Google Scholar]
- [13].Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol 2019;16(10):605–616. [DOI] [PubMed] [Google Scholar]
- [14].Visconti A, Le Roy CI, Rosa F, Rossi N, Martin TC, Mohney RP, et al. Interplay between the human gut microbiome and host metabolism. Nat Commun 2019;10(1):4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Asnicar F, Berry SE, Valdes AM, Nguyen LH, Piccinno G, Drew DA, et al. Microbiome connections with host metabolism and habitual diet from 1, 098 deeply phenotyped individuals. Nat Med 2021;27(2):321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Woodhouse CA, Patel VC, Singanayagam A, Shawcross DL. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther 2018;47(2):192–202. [DOI] [PubMed] [Google Scholar]
- [17].Brahe LK, Astrup A, Larsen LH. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes Rev 2013;14(12):950–959. [DOI] [PubMed] [Google Scholar]
- [18].Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 2008;27(2):104–119. [DOI] [PubMed] [Google Scholar]
- [19].Bloom PP, Luévano JM Jr, Miller KJ, Chung RT. Deep stool microbiome analysis in cirrhosis reveals an association between short-chain fatty acids and hepatic encephalopathy. Ann Hepatol 2021;25:100333. [DOI] [PubMed] [Google Scholar]
- [20].Acharya C, Bajaj JS. Chronic liver diseases and the microbiome-translating our knowledge of gut microbiota to management of chronic liver disease. Gastroenterology 2021;160(2):556–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Úbeda M, Lario M, Muñoz L, Borrero MJ, Rodríguez-Serrano M, Sánchez-Díaz AM, et al. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J Hepatol 2016;64(5):1049–1057. [DOI] [PubMed] [Google Scholar]
- [22].Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A 2006;103(10):3920–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011;60(4):463–472. [DOI] [PubMed] [Google Scholar]
- [24].Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011;141(5):1773–1781. [DOI] [PubMed] [Google Scholar]
- [25].Tranah TH, Edwards LA, Schnabl B, Shawcross DL. Targeting the gut-liver-immune axis to treat cirrhosis. Gut 2020. [DOI] [PubMed] [Google Scholar]
- [26].Skolnick SD, Greig NH. Microbes and monoamines: potential neuropsychiatric consequences of dysbiosis. Trends Neurosci 2019;42(3):151–163. [DOI] [PubMed] [Google Scholar]
- [27].DeMorrow S Bile acids in hepatic encephalopathy. J Clin Exp Hepatol 2019;9(1):117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Levitt MD, Levitt DG. Use of quantitative modelling to elucidate the roles of the liver, gut, kidney, and muscle in ammonia homeostasis and how lactulose and rifaximin alter this homeostasis. Int J Gen Med 2019;12:367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jaffe A, Lim JK, Jakab SS. Pathophysiology of hepatic encephalopathy. Clin Liver Dis 2020;24(2):175–188. [DOI] [PubMed] [Google Scholar]
- [30].Zhang Z, Zhai H, Geng J, Yu R, Ren H, Fan H, et al. Large-scale survey of gut microbiota associated with MHE via 16S rRNA-based pyrosequencing. Am J Gastroenterol 2013;108(10):1601–1611. [DOI] [PubMed] [Google Scholar]
- [31].Sung CM, Lin YF, Chen KF, Ke HM, Huang HY, Gong YN, et al. Predicting clinical outcomes of cirrhosis patients with hepatic encephalopathy from the fecal microbiome. Cell Mol Gastroenterol Hepatol 2019;8(2). 301–18.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shawcross DL, Davies NA, Williams R, Jalan R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol 2004;40(2):247–254. [DOI] [PubMed] [Google Scholar]
- [33].Shawcross DL, Sharifi Y, Canavan JB, Yeoman AD, Abeles RD, Taylor NJ, et al. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol 2011;54(4):640–649. [DOI] [PubMed] [Google Scholar]
- [34].Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol 2015;5(Suppl 1):S7–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 2017;14(8):491–502. [DOI] [PubMed] [Google Scholar]
- [36].Vulevic J, Juric A, Walton GE, Claus SP, Tzortzis G, Toward RE, et al. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br J Nutr 2015;114(4):586–595. [DOI] [PubMed] [Google Scholar]
- [37].Krumbeck JA, Rasmussen HE, Hutkins RW, Clarke J, Shawron K, Keshavarzian A, et al. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018;6(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bhatia S, Prabhu PN, Benefiel AC, Miller MJ, Chow J, Davis SR, et al. Galacto-oligosaccharides may directly enhance intestinal barrier function through the modulation of goblet cells. Mol Nutr Food Res 2015;59(3):566–573. [DOI] [PubMed] [Google Scholar]
- [39].Akbari P, Fink-Gremmels J, Willems R, Difilippo E, Schols HA, Schoterman MHC, et al. Characterizing microbiota-independent effects of oligosaccharides on intestinal epithelial cells: insight into the role of structure and size : structure-activity relationships of non-digestible oligosaccharides. Eur J Nutr 2017;56(5):1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011;53(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, et al. Rifaximin treatment in hepatic encephalopathy. N Eng J Med 2010;362(12):1071–1081. [DOI] [PubMed] [Google Scholar]
- [42].Patterson JA, Burkholder KM. Application of prebiotics and probiotics in poultry production. Poult Sci 2003;82(4):627–631. [DOI] [PubMed] [Google Scholar]
- [43].Cho JH, Kim IH. Effects of lactulose supplementation on performance, blood profiles, excreta microbial shedding of Lactobacillus and Escherichia coli, relative organ weight and excreta noxious gas contents in broilers. J Anim Physiol Anim Nutr (Berl) 2014;98(3):424–430. [DOI] [PubMed] [Google Scholar]
- [44].Salminen S, Salminen E. Lactulose, lactic acid bacteria, intestinal microecology and mucosal protection. Scand J Gastroenterol Suppl 1997;222:45–48. [DOI] [PubMed] [Google Scholar]
- [45].Vince AJ, Burridge SM. Ammonia production by intestinal bacteria: the effects of lactose, lactulose and glucose. J Med Microbiol 1980;13(2):177–191. [DOI] [PubMed] [Google Scholar]
- [46].Vince AJ, McNeil NI, Wager JD, Wrong OM. The effect of lactulose, pectin, arabinogalactan and cellulose on the production of organic acids and metabolism of ammonia by intestinal bacteria in a faecal incubation system. Br J Nutr 1990;63(1):17–26. [DOI] [PubMed] [Google Scholar]
- [47].Agostini L, Down PF, Murison J, Wrong OM. Faecal ammonia and pH during lactulose administration in man: comparison with other cathartics. Gut 1972;13(11):859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Weber FL Jr. The effect of lactulose on urea metabolism and nitrogen excretion in cirrhotic patients. Gastroenterology 1979;77(3):518–523. [PubMed] [Google Scholar]
- [49].Bird SP, Hewitt D, Ratcliffe B, Gurr MI. Effects of lactulose and lactitol on protein digestion and metabolism in conventional and germ free animal models: relevance of the results to their use in the treatment of portosystemic encephalopathy. Gut 1990;31(12):1403–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Avery GS, Davies EF, Brogden RN. Lactulose: a review of its therapeutic and pharmacological properties with particular reference to ammonia metabolism and its mode of action of portal systemic encephalopathy. Drugs 1972;4(1):7–48. [DOI] [PubMed] [Google Scholar]
- [51].Moratalla A, Ampuero J, Bellot P, Gallego-Durán R, Zapater P, Roger M, et al. Lactulose reduces bacterial DNA translocation, which worsens neurocognitive shape in cirrhotic patients with minimal hepatic encephalopathy. Liver Int 2017;37(2):212–223. [DOI] [PubMed] [Google Scholar]
- [52].Wang JY, Bajaj JS, Wang JB, Shang J, Zhou XM, Guo XL, et al. Lactulose improves cognition, quality of life, and gut microbiota in minimal hepatic encephalopathy: a multicenter, randomized controlled trial. J Dig Dis 2019;20(10):547–556. [DOI] [PubMed] [Google Scholar]
- [53].Wiest R, Albillos A, Trauner M, Bajaj J, Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol 2017;67:1084–1103. [DOI] [PubMed] [Google Scholar]
- [54].Bajaj J, Miller K, Tan J, Lawrence J, Mittleman R, Meehan B. Microbiome metabolic therapies reduce microbiota-associated ammonia in ex vivo fecal samples from healthy subjects and patients with minimal hepatic encephalopathy and demonstrate improved tolerability over lactulose in a clinical study. In: The digital international liver Congress™; 2020. [Google Scholar]
- [55].Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11(8):506–514. [DOI] [PubMed] [Google Scholar]
- [56].Bajaj JS, Saeian K, Christensen KM, Hafeezullah M, Varma RR, Franco J, et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol 2008;103(7):1707–1715. [DOI] [PubMed] [Google Scholar]
- [57].Sharma K, Pant S, Misra S, Dwivedi M, Misra A, Narang S, et al. Effect of rifaximin, probiotics, and l-ornithine l-aspartate on minimal hepatic encephalopathy: a randomized controlled trial. Saudi J Gastroenterol 2014;20(4):225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Horvath A, Leber B, Schmerboeck B, Tawdrous M, Zettel G, Hartl A, et al. Randomised clinical trial: the effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment Pharmacol Ther 2016;44(9):926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 2009;296(5):G1140–G1149. [DOI] [PubMed] [Google Scholar]
- [60].Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PloS One 2007;2(12):e1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cano PG, Santacruz A, Trejo FM, Sanz Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity (Silver Spring) 2013;21(11):2310–2321. [DOI] [PubMed] [Google Scholar]
- [62].Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med 1994;205(3):243–247. [DOI] [PubMed] [Google Scholar]
- [63].Chen RC, Xu LM, Du SJ, Huang SS, Wu H, Dong JJ, et al. Lactobacillus rhamnosus GG supernatant promotes intestinal barrier function, balances Treg and TH17 cells and ameliorates hepatic injury in a mouse model of chronic-binge alcohol feeding. Toxicol Lett 2016;241:103–110. [DOI] [PubMed] [Google Scholar]
- [64].Roselli M, Finamore A, Nuccitelli S, Carnevali P, Brigidi P, Vitali B, et al. Prevention of TNBS-induced colitis by different Lactobacillus and Bifidobacterium strains is associated with an expansion of gammadeltaT and regulatory T cells of intestinal intraepithelial lymphocytes. Inflamm Bowel Dis 2009;15(10):1526–1536. [DOI] [PubMed] [Google Scholar]
- [65].Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol 2008;48(6):945–951. [DOI] [PubMed] [Google Scholar]
- [66].Gupta N, Kumar A, Sharma P, Garg V, Sharma BC, Sarin SK. Effects of the adjunctive probiotic VSL#3 on portal haemodynamics in patients with cirrhosis and large varices: a randomized trial. Liver Int 2013;33(8):1148–1157. [DOI] [PubMed] [Google Scholar]
- [67].Khoruts A, Hoffmann DE, Britton RA. Probiotics: promise, evidence, and hope. Gastroenterology 2020;159(2):409–413. [DOI] [PubMed] [Google Scholar]
- [68].Ainsworth C. Therapeutic microbes to tackle disease. Nature. England 2020. p. S20–s2. [DOI] [PubMed] [Google Scholar]
- [69].Kurtz CB, Millet YA, Puurunen MK, Perreault M, Charbonneau MR, Isabella VM, et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci Transl Med 2019;11(475). [DOI] [PubMed] [Google Scholar]
- [70].Bloom P, Donlan J, Torres Soto M, Bloom J, Scherrer A, Xavier R, et al. Oral FMT capsules improve cognition in patients with persistent hepatic encephalopathy. American Association for the Study of Liver Diseases; 2019. [Google Scholar]
- [71].Bajaj JS, Fagan A, Gavis EA, Kassam Z, Sikaroodi M, Gillevet PM. Long-term outcomes of fecal microbiota transplantation in patients with cirrhosis. Gastroenterology 2019;156(6). 1921–3.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. Plos Biol 2008;6(11):e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lankelma JM, Belzer C, Hoogendijk AJ, de Vos AF, de Vos WM, van der Poll T, et al. Antibiotic-induced gut microbiota disruption decreases TNF-α release by mononuclear cells in healthy adults. Clin Transl Gastroenterol 2016;7(8):e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Eng J Med 2013;368(5):407–415. [DOI] [PubMed] [Google Scholar]
- [75].Ishikawa D, Sasaki T, Osada T, Kuwahara-Arai K, Haga K, Shibuya T, et al. Changes in intestinal microbiota following combination therapy with fecal microbial transplantation and antibiotics for ulcerative colitis. Inflamm Bowel Dis 2017;23(1):116–125. [DOI] [PubMed] [Google Scholar]
- [76].DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med 2019. [DOI] [PubMed] [Google Scholar]
- [77].Pringle PL, Soto MT, Chung RT, Hohmann E. Patients with cirrhosis require more fecal microbiota capsules to cure refractory and recurrent Clostridium difficile infections. Clin Gastroenterol Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- [78].Liu R, Kang JD, Sartor RB, Sikaroodi M, Fagan A, Gavis EA, et al. Neuroinflammation in murine cirrhosis is dependent on the gut microbiome and is attenuated by fecal transplant. Hepatology 2020;71(2):611–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bajaj JS, Salzman N, Acharya C, Takei H, Kakiyama G, Fagan A, et al. Microbial functional change is linked with clinical outcomes after capsular fecal transplant in cirrhosis. JCI Insight 2019;4(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Woodhouse CA, Patel VC, Goldenberg S, Sanchez-Fueyo A, China L, O’Brien A, et al. PROFIT, a PROspective, randomised placebo controlled feasibility trial of Faecal mIcrobiota Transplantation in cirrhosis: study protocol for a single-blinded trial. BMJ Open 2019;9(2):e023518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Woodhouse C, Edwards L, Mullish B, Kronsten V, Tranah T, Zamalloa A, et al. Results of the PROFIT trial, a PROspective randomised placebo-controlled feasibility trial of Faecal mIcrobiota Transplantation in advanced cirrhosis. EASL; 2020. [Google Scholar]
- [82].Malaguarnera M, Greco F, Barone G, Gargante MP, Toscano MA. Bifidobacterium longum with fructo-oligosaccharide (FOS) treatment in minimal hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Dig Dis Sci 2007;52(11):3259–3265. [DOI] [PubMed] [Google Scholar]
- [83].Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 2004;39(5):1441–1449. [DOI] [PubMed] [Google Scholar]
- [84].Kimer N, Krag A, Møller S, Bendtsen F, Gluud LL. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther 2014;40(2):123–132. [DOI] [PubMed] [Google Scholar]
- [85].Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PloS one 2013;8(4):e60042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sidhu SS, Goyal O, Mishra BP, Sood A, Chhina RS, Soni RK. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial). Am J Gastroenterol 2011;106(2):307–316. [DOI] [PubMed] [Google Scholar]
- [87].DuPont HL, Jiang ZD, Ericsson CD, Adachi JA, Mathewson JJ, DuPont MW, et al. Rifaximin versus ciprofloxacin for the treatment of traveler’s diarrhea: a randomized, double-blind clinical trial. Clin Infect Dis 2001;33(11):1807–1815. [DOI] [PubMed] [Google Scholar]
- [88].DuPont HL. Biologic properties and clinical uses of rifaximin. Expert Opin Pharmacother 2011;12(2):293–302. [DOI] [PubMed] [Google Scholar]
- [89].Kang DJ, Kakiyama G, Betrapally NS, Herzog J, Nittono H, Hylemon PB, et al. Rifaximin exerts beneficial effects independent of its ability to alter microbiota composition. Clin Transl Gastroenterol 2016;7(8):e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Schulz C, Schütte K, Vilchez-Vargas R, Vasapolli R, Malfertheiner P. Long-term effect of rifaximin with and without lactulose on the active bacterial assemblages in the proximal small bowel and faeces in patients with minimal hepatic encephalopathy. Dig Dis 2019;37(2):161–169. [DOI] [PubMed] [Google Scholar]
- [91].Caraceni P, Vargas V, Solà E, Alessandria C, de Wit K, Trebicka J, et al. The use of rifaximin in patients with cirrhosis. Hepatology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wall R, Marques TM, O’Sullivan O, Ross RP, Shanahan F, Quigley EM, et al. Contrasting effects of Bifidobacterium breve NCIMB 702258 and Bifidobacterium breve DPC 6330 on the composition of murine brain fatty acids and gut microbiota. Am J Clin Nutr 2012;95(5):1278–1287. [DOI] [PubMed] [Google Scholar]
- [93].Xu D, Gao J, Gillilland M 3rd, Wu X, Song I, Kao JY, et al. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 2014;146(2). 484–496.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Maccaferri S, Vitali B, Klinder A, Kolida S, Ndagijimana M, Laghi L, et al. Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother 2010;65(12):2556–2565. [DOI] [PubMed] [Google Scholar]
- [95].Soldi S, Vasileiadis S, Uggeri F, Campanale M, Morelli L, Fogli MV, et al. Modulation of the gut microbiota composition by rifaximin in non-constipated irritable bowel syndrome patients: a molecular approach. Clin Exp Gastroenterol 2015;8:309–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Fiorucci S, Distrutti E, Mencarelli A, Barbanti M, Palazzini E, Morelli A. Inhibition of intestinal bacterial translocation with rifaximin modulates lamina propria monocytic cells reactivity and protects against inflammation in a rodent model of colitis. Digestion 2002;66(4):246–256. [DOI] [PubMed] [Google Scholar]
- [97].Acosta A, Camilleri M, Shin A, Linker Nord S, O’Neill J, Gray AV, et al. Effects of rifaximin on transit, permeability, fecal microbiome, and organic acid excretion in irritable bowel syndrome. Clin Transl Gastroenterol 2016;7(5):e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Peleman C, Camilleri M. Rifaximin, microbiota biology, and hepatic encephalopathy. Clin Transl Gastroenterol 2016:e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Jiang ZD, Ke S, Dupont HL. Rifaximin-induced alteration of virulence of diarrhoea-producing Escherichia coli and Shigella sonnei. Int J Antimicrob Agents 2010;35(3):278–281. [DOI] [PubMed] [Google Scholar]
- [100].Brown EL, Xue Q, Jiang ZD, Xu Y, Dupont HL. Pretreatment of epithelial cells with rifaximin alters bacterial attachment and internalization profiles. Antimicrob Agents Chemother 2010;54(1):388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ricci A, Coppo E, Barbieri R, Debbia EA, Marchese A. The effect of subinhibitory concentrations of rifaximin on urease production and on other virulence factors expressed by Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa and Staphylococcus aureus. J Chemother 2017;29(2):67–73. [DOI] [PubMed] [Google Scholar]
- [102].Mas A, Rodés J, Sunyer L, Rodrigo L, Planas R, Vargas V, et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol 2003;38(1):51–58. [DOI] [PubMed] [Google Scholar]
- [103].Pedretti G, Calzetti C, Missale G, Fiaccadori F. Rifaximin versus neomycin on hyperammoniemia in chronic portal systemic encephalopathy of cirrhotics. A double-blind, randomized trial. Ital J Gastroenterol 1991;23(4):175–178. [PubMed] [Google Scholar]
- [104].Strauss E, Tramote R, Silva EP, Caly WR, Honain NZ, Maffei RA, et al. Double-blind randomized clinical trial comparing neomycin and placebo in the treatment of exogenous hepatic encephalopathy. Hepatogastroenterology 1992;39(6):542–545. [PubMed] [Google Scholar]
- [105].Garcovich M, Zocco MA, Roccarina D, Ponziani FR, Gasbarrini A. Prevention and treatment of hepatic encephalopathy: focusing on gut microbiota. World J Gastroenterol 2012;18(46):6693–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Phongsamran PV, Kim JW, Cupo Abbott J, Rosenblatt A. Pharmacotherapy for hepatic encephalopathy. Drugs 2010;70(9):1131–1148. [DOI] [PubMed] [Google Scholar]
- [107].Als-Nielsen B, Gluud LL, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane database Syst Rev 2004;2(2):CD003044. [DOI] [PubMed] [Google Scholar]
- [108].Abd-Elsalam S, El-Kalla F, Elwan N, Badawi R, Hawash N, Soliman S, et al. A randomized controlled trial comparing nitazoxanide plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. J Clin Gastroenterol 2019;53(3):226–230. [DOI] [PubMed] [Google Scholar]
- [109].Venturini AP. Pharmacokinetics of L/105, a new rifamycin, in rats and dogs, after oral administration. Chemotherapy 1983;29(1):1–3. [DOI] [PubMed] [Google Scholar]
- [110].Jiang ZD, DuPont HL. Rifaximin: in vitro and in vivo antibacterial activity–a review. Chemotherapy 2005;51(Suppl 1):67–72. [DOI] [PubMed] [Google Scholar]
- [111].Descombe JJ, Dubourg D, Picard M, Palazzini E. Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int J Clin Pharmacol Res 1994;14(2):51–56. [PubMed] [Google Scholar]
- [112].Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019;575(7783):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Bajaj JS, Sikaroodi M, Shamsaddini A, Henseler Z, Santiago-Rodriguez T, Acharya C, et al. Interaction of bacterial metagenome and virome in patients with cirrhosis and hepatic encephalopathy. Gut 2020. [DOI] [PubMed] [Google Scholar]
- [114].Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016;7(3):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 2015;8(1):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Tsilingiri K, Barbosa T, Penna G, Caprioli F, Sonzogni A, Viale G, et al. Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut 2012;61(7):1007–1015. [DOI] [PubMed] [Google Scholar]
- [117].Beards E, Tuohy K, Gibson G. Bacterial, SCFA and gas profiles of a range of food ingredients following in vitro fermentation by human colonic microbiota. Anaerobe 2010;16(4):420–425. [DOI] [PubMed] [Google Scholar]
- [118].Mortensen PB, Clausen MR. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol Suppl 1996;216:132–148. [DOI] [PubMed] [Google Scholar]
- [119].Abbasi A, Hajipour N, Hasannezhad P, Baghbanzadeh A, Aghebati-Maleki L. Potential in vivo delivery routes of postbiotics. Crit Rev Food Sci Nutr 2020:1–39. [DOI] [PubMed] [Google Scholar]
- [120].Lührs H, Gerke T, Müller JG, Melcher R, Schauber J, Boxberge F, et al. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol 2002;37(4):458–466. [DOI] [PubMed] [Google Scholar]
- [121].Roda A, Simoni P, Magliulo M, Nanni P, Baraldini M, Roda G, et al. A new oral formulation for the release of sodium butyrate in the ileo-cecal region and colon. World J Gastroenterol 2007;13(7):1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Golob JL, DeMeules MM, Loeffelholz T, Quinn ZZ, Dame MK, Silvestri SS, et al. Butyrogenic bacteria after acute graft-versus-host disease (GVHD) are associated with the development of steroid-refractory GVHD. Blood Adv 2019;3(19):2866–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Bosoi CR, Parent-Robitaille C, Anderson K, Tremblay M, Rose CF. AST-120 (spherical carbon adsorbent) lowers ammonia levels and attenuates brain edema in bile duct-ligated rats. Hepatology 2011;53(6):1995–2002. [DOI] [PubMed] [Google Scholar]
- [124].Zacharias HD, Zacharias AP, Gluud LL, Morgan MY. Pharmacotherapies that specifically target ammonia for the prevention and treatment of hepatic encephalopathy in adults with cirrhosis. Cochrane Database Syst Rev 2019;6(6):Cd012334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].R J. Safety and Tolerability of Yaq-001 in Patients with Cirrhosis. ClinicalTrials.gov; [cited 2020]. [Google Scholar]
- [126].Fassarella M, Blaak EE, Penders J, Nauta A, Smidt H, Zoetendal EG. Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut 2020. [DOI] [PubMed] [Google Scholar]
- [127].Fernández J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol 2019;70(3):398–411. [DOI] [PubMed] [Google Scholar]
- [128].Mücke MM, Mayer A, Kessel J, Mücke VT, Bon D, Schwarzkopf K, et al. Quinolone and multidrug resistance predicts failure of antibiotic prophylaxis of spontaneous bacterial peritonitis. Clin Infect Dis 2020;70(9):1916–1924. [DOI] [PubMed] [Google Scholar]
- [129].Ramos JM, Vidal I, Bellot P, Gómez-Hurtado I, Zapater P, Such J. Comparison of the in vitro susceptibility of rifaximin versus norfloxacin against multidrug resistant bacteria in a hospital setting. A proof-of-concept study for use in advanced cirrhosis. Gut. England 2016:182–183. [DOI] [PubMed] [Google Scholar]
- [130].Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med 2018;24(4):392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Zellmer C, Sater MRA, Huntley MH, Osman M, Olesen SW, Ramakrishna B. Shiga toxin-producing Escherichia coli transmission via fecal microbiota transplant. Clin Infect Dis 2020. [DOI] [PubMed] [Google Scholar]
- [132].Administration UFaD. Safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse events likely due to transmission of pathogenic organisms. 2020. Available from: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse-events-likely.
- [133].Marcella C, Cui B, Kelly CR, Ianiro G, Cammarota G, Zhang F. Systematic review: the global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol Ther 2021;53(1):33–42. [DOI] [PubMed] [Google Scholar]
- [134].Zuo T, Liu Q, Zhang F, Lui GC, Tso EY, Yeoh YK, et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 2021;70(2):276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Ianiro G, Mullish BH, Kelly CR, Kassam Z, Kuijper EJ, Ng SC, et al. Reorganisation of faecal microbiota transplant services during the COVID-19 pandemic. Gut 2020;69(9):1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Control CfD. Frequently asked questions for Coronavirus for laboratories. 2021. [01/11/2021]; Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/faqs.html#Specimen-Types.
- [137].Kipkorir V, Cheruiyot I, Ngure B, Misiani M, Munguti J. Prolonged SARS-CoV-2 RNA detection in anal/rectal swabs and stool specimens in COVID-19 patients after negative conversion in nasopharyngeal RT-PCR test. J Med Virol 2020:2328–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Craven LJ, Nair Parvathy S, Tat-Ko J, Burton JP, Silverman MS. Extended screening costs associated with selecting donors for fecal microbiota transplantation for treatment of metabolic syndrome-associated diseases. Open Forum Infect Dis 2017;4(4):ofx243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Olesen SW, Gurry T, Alm EJ. Designing fecal microbiota transplant trials that account for differences in donor stool efficacy. Stat Methods Med Res 2017. 962280216688502. [DOI] [PubMed] [Google Scholar]
- [140].Olesen SW. Fecal microbiota transplantation “donor effects” are not clinically relevant for Clostridioides difficile infection. Gastroenterology 2020. [DOI] [PubMed] [Google Scholar]
- [141].Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. Jama 2019;321(2):156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Chu ND, Smith MB, Perrotta AR, Kassam Z, Alm EJ. Profiling living bacteria informs preparation of fecal microbiota transplantations. PloS One 2017;12(1):e0170922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wang L, Llorente C, Hartmann P, Yang AM, Chen P, Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods 2015;421:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


