Abstract
Investigation into the role of cells with respect to extracellular matrix (ECM) remodeling is still in its infancy. Particularly, ECM degradation is an indispensable process during the recovery from fibrosis. Cells with ECM degradation ability due to the secretion of various matrix metalloproteinases (MMPs) have emerged as novel contributors to the treatment of fibrotic diseases. In this review, we focus on the ECM degradation ability of cells associated with the repertoire of MMPs that facilitate the attenuation of fibrosis through the inhibition of ECM deposition. Besides, innovative approaches to engineering and characterizing cells with degradation ability, as well as elucidating the mechanism of the ECM degradation, are also illustrated. Studies conducted to date on the use of cell-based degradation for therapeutic purposes to combat fibrosis are summarized. Finally, we discuss the therapeutic potential of cells with high degradation ability, hoping to bridge the gap between benchside research and bedside applications in treating fibrotic diseases.
Keywords: Fibrosis, Extracellular matrix, Degradation, Matrix metalloproteinases
Background
Fibrosis originates from an excessive deposition of the ECM caused by different types of tissue injuries, thus leading to organ dysfunction (Panizo et al. 2021). During the healing and regeneration process, fibroblasts are activated and differentiated into myofibroblasts, which then secrete ECM components to repair the tissue (Krafts 2010). The process of regeneration is accompanied by the restructuring of the ECM, primarily through the action of proteases that break down the ECM. Upon completion of regeneration, activated fibroblasts either undergo apoptosis (Micallef et al. 2012) or return to a quiescent state (Henderson et al. 2020). However, in fibrosis, fibroblasts remain activated and an abundance of ECM is produced. An imbalance between ECM synthesis and degradation leads to collagen deposition (Zhang and Shaw 2013), while the shifting of the balance between MMPs and tissue inhibitors of metalloproteinases (TIMPs) further exacerbates the process of fibrosis (Arthur 2003). MMP-mediated ECM degradation is immensely associated with cell migration, cell invasion (Cheng et al. 2010) and tissue remodeling (Green and Lund 2005). Briefly, bioactive ECM fragments generated from proteolytic degradation of the ECM promote the regulation of inflammation and tissue regeneration during the process of fibrosis (de Castro Bras and Frangogiannis 2020).
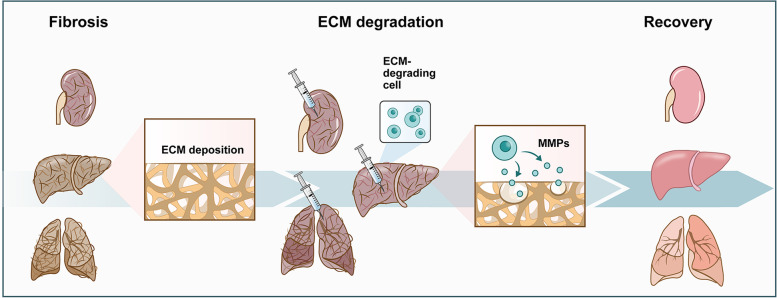
However, previous studies have paid inadequate attention to the ability of cells to degrade the ECM, which emerges as a potent property in the treatment of fibrotic diseases in the future. In this review, we introduce the potential cells with strong degradation abilities and prospect their applications in the treatment of fibrosis (Fig. 1).
Fig. 1.
Schematic of ECM-degrading cell therapy applied to fibrosis. During fibrosis, the ECM is excessively secreted and deposited. Delivery of ECM-degrading cells to fibrotic organs or tissues can degrade the deposited ECM mainly by secreting MMPs, which are expected to reduce excessive fibrosis
ECM degradation
Proteases in ECM degradation
MMPs and their inhibitors
Proteases are comprised of serine protease, cysteine protease, aspartic protease and metalloproteinase, among which MMPs play a major role in the degradation of the ECM. To date, 28 members of the MMP family have been found in vertebrates, and at least 23 members have been detected in humans (Xi and Khalil 2017). The structure of MMPs from N-terminal to C-terminal generally includes (1) a hydrophobic signal peptide; (2) an N-terminal propeptide, which is cleaved by exogenous enzymes to activate the MMP zymogen; (3) a catalytic (CAT) domain with a zinc ion binding site that can catalyze the hydrolysis of peptide bonds; (4) a linker (hinge) region; and (5) a C-terminal hemopexin-like (HPX) domain, which recognizes its substrates. In addition, transmembrane-type MMPs contain a transmembrane (TM) domain and a cytoplasmic tail that regulates intracellular trafficking and activity, while membrane-type MMPs (MT-MMPs) are anchored to the cell membrane by glycosylphosphatidylinositol (Sagi and Gaffney 2015).
According to substrate specificities, MMPs can be divided into collagenases, gelatinases, stromelysins, and matrilysins. Collagenases (e.g., collagenase 1 [MMP1], collagenase 2, neutrophil collagenase [MMP8], collagenase 3 [MMP13], and MT1-MMP) are dominant enzymes that degrade collagen fibers and unwind the triple helix. Meanwhile, gelatinases (e.g., gelatinase A [MMP2], gelatinase B [MMP9]) insert three fibronectin type II-like (FN2) repeats in the CAT domain to degrade gelatin (i.e., a product of the partial hydrolysis of collagen) and type IV collagen. Stromelysins (e.g., MMP3, MMP10, and MMP13) degrade a multitude of ECM components such as proteoglycans, laminin, fibronectin, gelatin, type IV and type IX collagen, while matrilysins (e.g., MMP7 and MMP26) degrade gelatin, fibronectin and type IV collagen but not the triple helical collagen (Sagi and Gaffney 2015). In addition, MMPs can also catalyze various non-ECM proteins. For instance, MMP7 catalyzes the hydrolysis of cytokines, growth factors, and receptors (Li et al. 2002).
The activity of MMPs is tightly regulated at the transcriptional level and by the activation of precursor zymogens and TIMPs. TIMPs bind to the CAT sites of MMPs in a substrate-like manner, thus inhibiting the degradation of substrates. Four homologous TIMPs (e.g., TIMP1, TIMP2, TIMP3, and TIMP4) can inhibit a variety of MMPs with divergent inhibitory effects. For example, the ability of TIMP1 and TIMP2 to bind to MMP3 is ten times stronger than their binding to MMP10 (Batra et al. 2012).
Plasmin
Plasmin is a proteolytic enzyme that specifically degrades fibrin in the body and is critical for the dissolution of clots. Plasminogen is the inactive precursor of plasmin that can be activated by two plasminogen activator (PA) systems: the tissue plasminogen activator (tPA) and the urokinase plasminogen activator (uPA) (Mahmood et al. 2018). Besides fibrin, plasmin has been reported to degrade several basement membrane proteins, including laminin and fibronectin, thus demonstrating its ability to degrade the ECM (Nakagami et al. 2000; Uemura et al. 2005). In addition, plasmin activates a variety of MMP zymogens, including MMP1, MMP2, MMP3, MMP9, MMP13, and MMP14, indicating that plasmin can indirectly degrade the ECM by activating MMPs (Deryugina and Quigley 2016).
Cathepsins
Cathepsins contain 15 members, which are located in the lysosome or secreted extracellularly. They can be classified by catalytic site residues as serine cathepsins (cathepsins A and G), cysteine cathepsins (cathepsins B, C, F, H, K, L, O, S, V, X, and W), and aspartate cathepsins (cathepsins D and E) (Kryczka and Boncela 2017). Some members of the cathepsin family have been found to degrade the ECM proteins. For instance, cysteines B, X, S, L, and H, which can catalyze type I collagen, type IV collagen, fibronectin, and laminin, are involved in ECM degradation in podosomes formed by fibroblasts (Tu et al. 2008) and macrophages (Jevnikar et al. 2012). Besides, cathepsin K is highly expressed in osteoclasts and shows potent collagenase activity, which plays an important role in cancer and bone resorption (Qian et al. 2022). Elastin, the main ECM fiber component that provides tissue elasticity, is known to be cleaved by cathepsins K, S, and V (Yoo et al. 2022). Moreover, cathepsin L has been found to activate pro-urokinase-type plasminogen activator (pro-uPA) (Tagirasa and Yoo 2022).
Mechanism of ECM degradation
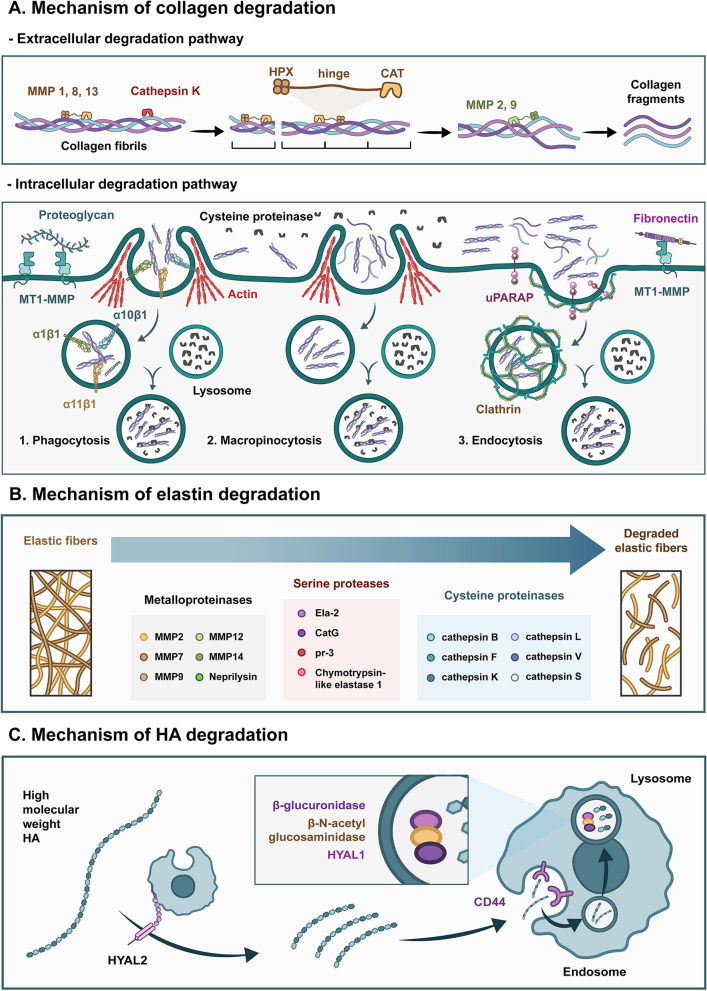
Collagen is degraded through extracellular and intracellular pathways (Sprangers and Everts 2019). In the extracellular pathway, cells secrete various collagenases including MMP1, MMP8, and MMP13, to recognize and bind to specific sites on collagen fibers through the HPX-like domain and guide the CAT domain with a zinc ion toward the cleavage site of collagen (Bertini et al. 2012). Subsequently, MMPs will unwind the triple helix structure of the collagen fibrils and cleave the intact collagen fibrils (i.e., types I, II and III collagen) at specific sites, releasing one-fourth and three-fourths lengths of the fragments (Chung et al. 2004). The CAT domain of collagenase can cleave non-collagen matrices in the presence of the HPX-like domain on MMPs, while cathepsin K, without the HPX-like domain, can degrade intact fibrillar collagens, mainly types I and II collagens. It first binds to the glycosaminoglycan (GAG) covering the collagen fibers and cleaves at the ends of the collagen and multiple sites of the triple helices (Aguda et al. 2014). Collagen fragments are then further degraded by cell-secreted gelatinases (i.e., MMP2 and MMP9) and other nonspecific proteases. The intracellular pathway comprises phagocytosis, micropinocytosis, and endocytosis. Phagocytosis acts directly on relatively intact collagen fibers, while non-collagen proteins such as fibronectin and proteoglycans that cover the surface of the collagen fibers are recognized and bound by the β1-integrin family (α1β1, α10β1, α11β1) on the membrane surface. Subsequently, actin-rich pseudopods engulf partial collagen fibers, and MT1-MMP on the membrane surface cleaves and internalizes them (Lee et al. 2007; Takaki et al. 2006). Finally, collagen is degraded by intracellular lysosomal cysteine proteases (Arora et al. 2000). Actin-mediated macropinocytosis involves the uptake of large numbers of soluble molecules, including collagen fragments, which are eventually degraded by intracellular lysosomal cysteine proteases. Macropinocytosis is the major route of collagen internalization, performing a relatively rapid and non-selective degradation of tissue. Endocytosis also acts on collagen fragments, where collagen sites bind to uPARAP/Endo180 receptors on the cell surface, followed by the formation of clathrin-mediated vesicles (Madsen et al. 2012). After fusion with lysosomes, collagen fragments are further degraded by cysteine proteases. While the extracellular degradation pathway often occurs rapidly in the pathological environment, the intracellular degradation pathway occurs relatively slowly in the physiological environment. Collectively, the extracellular and intracellular degradation pathways work in concert with each other to remodel and repair tissues concomitantly (Fig. 2A).
Fig. 2.
Mechanism of ECM degradation. A Mechanism of collagen degradation. Extracellular collagen degradation pathway: The triple helix structure of the collagen fibrils is unwound by collagenases (including MMP1, MMP8, MMP13, cathepsin K, etc.). Collagen fibrils are cleaved by these collagenases and then further degraded by gelatinases (i.e., MMP2 and MMP9). Intracellular collagen degradation pathway: During phagocytosis, uptake of collagen fibrils is mediated by the β1-integrin family and the actin-rich pseudopods. Macropinocytosis involves the uptake of collagen fragments mediated by actin. In endocytosis, collagen is recognized by the uPARAP/Endo180 receptors and subsequently taken up by clathrin-mediated vesicles. The ingested collagen is further degraded by cysteine proteases in the lysosomes. B Mechanism of elastin degradation. Elastin can be degraded by metalloproteinases (MMP2, MMP7, MMP9, MMP12, MMP14 and neprilysin), serine proteinases (Ela-2, CatG, PR-3 and Chymotrypsin-like elastase 1), and cysteine proteinases (cathepsin B, F, K, L, V and S). C Mechanism of HA degradation. HA with high molecular weight is first degraded into fragments of approximately 20 kDa by HYAL2, which is then recognized by CD44 and internalized. Finally, the fragment is completely degraded by β-glucuronidase, β-N-acetyl glucosaminidase, and HYAL1 in the lysosome
Insoluble elastin can be cleaved by elastases, which have been identified in the families of metalloproteinases, serine proteinases, and cysteine proteinases (Heinz 2020). Metalloproteinases with elastolytic activity include MMP2 (Diaz-Canestro et al. 2022), MMP7, MMP9, MMP12 (Mora Huertas et al. 2018), MMP14 (Miekus et al. 2019), and neprilysin (Mora Huertas et al. 2018). Four serine proteases are efficient elastases: neutrophil elastase (Ela-2), cathepsin G (CatG), protease-3 (pr-3) (Heinz et al. 2012) and Chymotrypsin-like elastase 1 (Joshi et al. 2018). Ela-2, pr-3, and CatG have been shown to completely degrade tropoelastin (Maurice et al. 2013). Six cysteine proteases have been reported to degrade elastin: cathepsin B, F (Yasuda et al. 2004), K (Panwar et al. 2020), L (Biniossek et al. 2011), V, and S (Panwar et al. 2020). The cleavage sites of elastases are mainly hydrophobic, such as Pro, Gly, Ile, Val, and Leu, or aromatic residues, such as Phe and Tyr, with some differences between elastases (Heinz 2020). For example, MMP7 showed a strong preference for Leu at P1’ (Heinz et al. 2011), while cathepsin K showed a higher affinity for Gly (Panwar et al. 2020) (Fig. 2B).
Hyaluronic acid (HA) is degraded under physiological conditions by members of the hyaluronidase (HYAL) family. Six hyaluronidases have been identified in humans, including HYAL1-4, PH-20, and HYALP1 (Papakonstantinou et al. 2012). HYAL 1 and 2 are the main hyaluronic enzymes involved in the catabolism of HA (Kaul et al. 2021). HYAL2, a glycosylphosphatidylinositol (GPI)-anchored membrane protein, hydrolyzes HA with high molecular weight and produces HA fragments of approximately 20 kDa. These fragments are then further degraded into small oligosaccharides by PH-20 (Harada and Takahashi 2007). With the recognition of HA receptors (such as CD44), HA fragments are ingested into cells and further degraded into low-molecular-weight oligosaccharides by β-glucuronidase, β-N-acetyl glucosaminidase, and HYAL1 in the lysosome (Csoka et al. 2001). Besides HYAL, hyaladherin KIAA1199 (Yoshida et al. 2013) and the transmembrane protein TMEM2 (Yamaguchi et al. 2019) show catalytic activities to degrade HA as well. Additionally, HA can be degraded through free-radical mechanisms without enzymes in the presence of molecular oxygen and reducing agents such as ascorbic acid, and cupric or ferric ions (Andre and Villain 2017) (Fig. 2C).
Cells with degradation ability
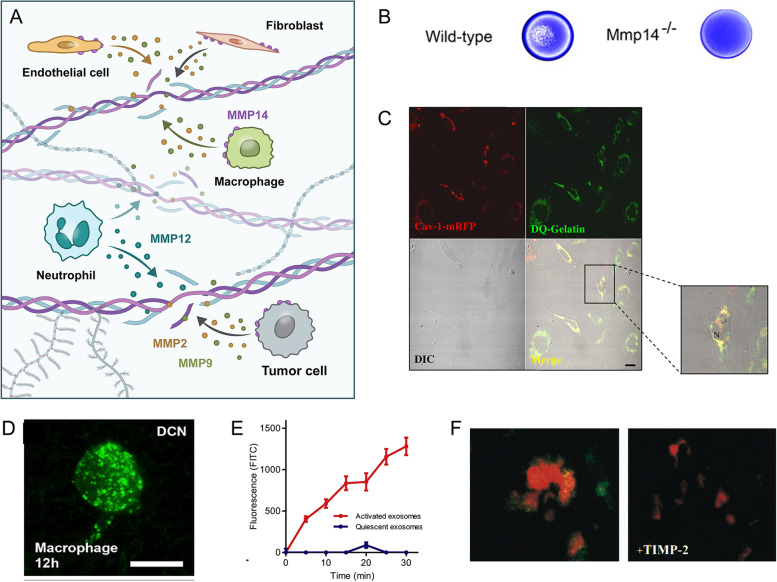
A myriad of cells can degrade the ECM, mainly including fibroblasts, endothelial cells, macrophages, neutrophils and tumor cells (Xi and Khalil 2017).
For fibroblasts, transforming growth factor (TGF)-β1 or tumor necrosis factor (TNF)-α induction can enhance the expression of MMP2 and MMP9 in fibroblasts (Kobayashi et al. 2003), while the endogenous MMP2 and MMP9 contribute to the survival and proliferation of rheumatoid arthritis (RA) synovial fibroblasts (Xue et al. 2014). Farideh Sabeh et al. found that MMP14 (MT1-MMP), but not soluble MMPs, was necessary for fibroblast invasion (Sabeh et al. 2009). Besides, MMP14 also played an important role in maintaining the homeostasis of the skin ECM, given that the knockout of MMP14 in mouse dermal fibroblasts resulted in impaired resolution of skin fibrosis (Zigrino et al. 2016). Wenyue Zhang et al. showed that fibroblast-derived MMP2 and MT1-MMP, which were involved in cervical squamous cell carcinoma (HNSCC), were critical for tumor growth and invasion (Zhang et al. 2006). Cathepsin K is synthesized by synovial fibroblasts that may be involved in collagen degradation in the bone tissue (Silva et al. 2020).
For endothelial cells, ECM remodeling is important for angiogenesis and endothelial cell tube formation. Dora Cavallo-Medved et al. observed that human umbilical vein endothelial cells (HUVECs) performed degradation functions by secreting active cathepsin B during an in vitro angiogenesis experiment through live cell imaging technology, accompanied by high expression of MMP2, MMP14, pro-urokinase (Pro-uPA) and urokinase-type plasminogen activator receptor (uPAR) (Cavallo-Medved et al. 2009). MT1-MMP, MMP2, and MMP9 expressed by endothelial cells are involved in the process of sprouting and angiogenesis (van Hinsbergh and Koolwijk 2008). Besides, they cooperate to degrade the basement membrane (i.e., type-IV collagen) and facilitate the migration of endothelial cells. Compared with MMP2 and MMP9, MT1-MMP on the surface of the cell membrane is the key nexus for endothelial cell invasion and migration (Chun et al. 2004). Studies showed that the knockout of Mmp14 in endothelial cells in vivo affected melanoma growth and metastasis (Kümper et al. 2022). Vascular endothelial cells play a significant role in regulating the degradation of ECM by plasmin, which is essential for the development of angiogenesis (Wileman et al. 2000).
Macrophages and neutrophils are vital regulators in tissue remodeling. Briefly, the adhesion of macrophages is closely related to the protein degradation of the ECM in vitro. Macrophages-derived MT1-MMPs are critical for ECM degradation, while their surface localization is associated with macrophage migration (Linder and Scita 2015). Particularly, researchers have envisaged the effects of MMP-producing macrophages on ECM remodeling. They found that M2 macrophages and CX3CR1-positive macrophages can internalize and remove dermal collagen, thus allowing the ECM to be remodeled by fibroblasts (Madsen et al. 2013). Besides, the study investigated the behavior of macrophages on hydrogels with different densities. In dense collagen hydrogels, fibroblasts first secreted MMPs to degrade the surrounding ECM, while macrophages migrated along the path of fibroblasts and secreted MMPs continuously. In the loose collagen hydrogel network, fibroblasts initially pulled the collagen fibers into a straight line, followed by the migration of macrophages along the collagen fibers (Ford et al. 2019). Furthermore, macrophages have a critical role in the process of liver fibrosis. In the initiation and progression stages, Ly6Chi monocyte-derived macrophages produce TGF-β and platelet derived growth factor (PDGF), which stimulate fibroblasts differentiation into myofibroblasts and enhance ECM deposition. However, during the liver fibrosis resolution stage, their function shifts (Tacke and Zimmermann 2014). Moreover, CD11Bhi F4/80int Ly6Clo macrophages predominate at maximal fibrosis resolution with high expression of MMP9 and MMP12 (Ramachandran et al. 2012). As the co-culture of MSCs and macrophages could intensify the expression of MMPs in macrophages, the combination therapy is expected to promote the proliferation of hepatocytes and impede the process of liver fibrosis (Watanabe et al. 2019). In anti-LOXL2-treated mice, reparative monocyte-derived macrophages (MoMFs) secreted MMP14, which degraded the dense ECM (Klepfish et al. 2020). Ardi V et al. found that neutrophils infiltrated into the site of the primary tumor by releasing pre-stored MMP9, thereby promoting tumor angiogenesis (Deryugina et al. 2014). In addition, the exosomes from neutrophils could degrade normal collagen in the lungs by neutrophil elastase, laying a foundation for the construction of an in vitro model of chronic obstructive pulmonary disease (COPD) (Genschmer et al. 2019).
Tumor cells are found to secrete MMPs, which assist in breaking down the dense ECM and facilitate their infiltration into other organs. Tumor cells cleave type I collagen and fibrin through MT1-MMP to invade surrounding tissues, while some other soluble MMPs have minimal effect on the degradation of the ECM (Holmbeck et al. 2003). MT1-MMP not only participates in matrix remodeling, but also activates soluble MMPs (e.g., MMP2) (van Hinsbergh and Koolwijk 2008), clearing the complement components C3b and C4b to help tumors escape from the immune system (Rozanov et al. 2004) (Fig. 3).
Fig. 3.
Potential cell candidates with high ECM degradation ability. A Schematic of cells with degradation ability, including fibroblasts, endothelial cells, macrophages, neutrophils and tumor cells. B Collagen degradation ability of primary murine skin fibroblasts isolated from wild-type or Mmp14−/− mice was detected after they were cultured on type I collagen gels by Coomassie Blue staining (Sabeh et al. 2009). C Collagenolytic activity of human umbilical vein endothelial cells (HUVECs). HUVECs transfected with cav-1-mRFP were grown on a reconstituted basement membrane containing fluorescein conjugated-collagen IV. Confocal images were taken of cav-1-mRFP (red), substrate degradation products (green) and live cells (DIC) (Cavallo-Medved et al. 2009). Scale bars, 20 μm. D Endocytosis of fluorescein conjugated-collagen (green) by RAW 264.7 macrophages (Ford et al. 2019). Scale bars, 20 μm. E Collagenolytic activity of exosomes from activated or quiescent polymorphonuclear leukocytes was measured by culturing them with FITC-labeled type I collagen (Genschmer et al. 2019). F Collagen degradation ability of squamous carcinoma cells (SCC1) cultured with or without TIMP2. SCC1 cells (red) were labeled with propidium iodide and degraded collagen (green) was stained with mAb HUI77 (Hotary et al. 2003). B–F is reused with permission from Elsevier
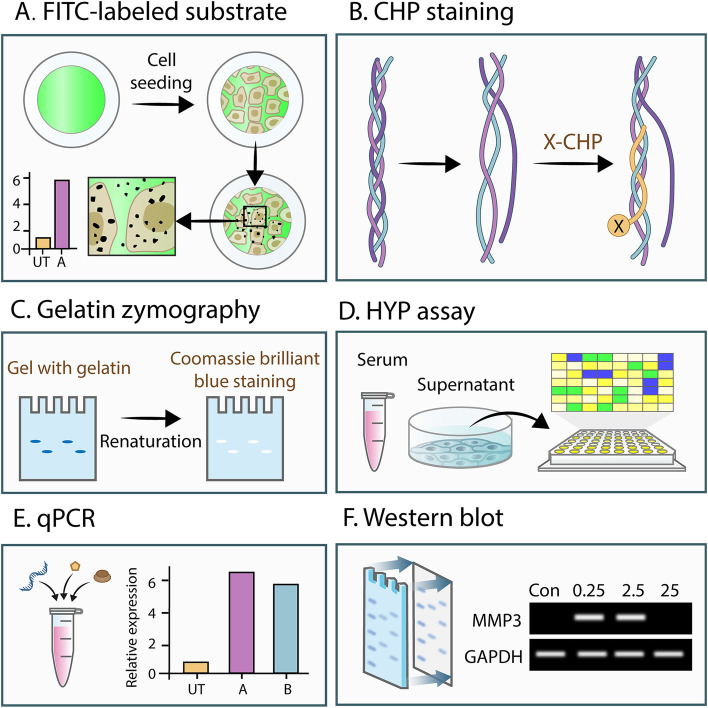
Methods to characterize ECM degradation
Collagen Hybridizing Peptide (CHP) is a synthetic peptide equivalent to the collagen. It can bind specifically to denatured collagen in the form of hydrogen bonding, but is unable to bind intact collagen fibers both in vitro and in vivo. Therefore, when fluorescent-dye conjugated CHP is incubated with denatured collagen, areas of ECM degradation could be observed and quantified (Hwang et al. 2017; Li et al. 2012; Zitnay et al. 2017). Given that multiple cancer cell lines could degrade ECM at focal adhesions associated with MT1-MMP, this method can be employed to study the ECM-degrading ability of tumor cells by analyzing the irregular and dark pattern of degradation (Wang and McNiven 2012). However, the aforementioned methods are image-based and intended to analyze the ECM-degrading ability of cells qualitatively. Despite these image-based methods, the ECM-degrading ability of cells can be examined at the gene level by detecting the expression of MMP genes through qPCR (Christina et al. 2021; Moammeri et al. 2022; Popov et al. 2006). However, a high expression of MMP genes does not necessarily depict a high expression of proteins. Apart from gene detection, the ECM-degrading ability of cells can be detected through protein aspects. Hydroxyproline (HYP), which is abundant in collagen, is considered a good indicator for quantifying the levels of ECM degradation of cells (Langrock and Hoffmann 2019; Zhao et al. 2022). However, HYP is not the end product of collagen degradation and is inadequate to directly describe the ECM-degrading ability of cells (Islam et al. 2016). Besides, western blot can also quantify the expression of a certain MMP from the protein level (Kim et al. 2022; Tang et al. 2021). Nevertheless, the high expression of MMPs does not indicate a high activity of them. Gelatin zymography was used to demonstrate the activation of tumor-secreted proMMP9 by the MT1-MMP/MMP2 axis, thus hydrolyzing the surrounding ECM (Li et al. 2017; Lopez Lobato et al. 2022; Rajkumar and Mariswamy 2021). However, this method is limited to the detection of MMP2 and MMP9, and is insufficient for detecting other MMPs. In addition, despite its inefficiency in performing high-throughput screening, the commercially available collagenase kit can also be used to detect elastase on the surface of activated neutrophil-derived exosomes (Genschmer et al. 2019). Therefore, a high-throughput, inexpensive and universal method for the quantitative analysis of collagen degradation has been underwhelming thus far (Fig. 4).
Fig. 4.
Methods to characterize ECM degradation ability of cells. A FITC-labeled substrate. The substrates are pre-labeled with fluorescent molecules (such as FITC) and then co-cultured with cells. Detection of areas without fluorescence can indirectly characterize the degradation ability of cells. B CHP staining. CHP can specifically bind denatured collagen and the degraded collagen can be stained with CHP conjugated to fluorescent molecules. C Gelatin zymography. Protein samples prepared from cells are separated by electrophoresis in a gel containing gelatin, and then MMPs in the samples degrade the gelatin at their respective sites, which are characterized by Coomassie Brilliant Blue staining. D HYP assay. The major proteins containing HYP are collagens. The HYP content of the supernatant, serum, or matrix co-cultured with cells can reflect the degree of degradation of the ECM. E qPCR. Real-time quantitative PCR (qPCR) can detect the expression of ECM-degrading related enzymes at the mRNA level, indicating the potential of cells to degrade the ECM. F Western blot. Using the specific combination of antigen and antibody, western blot can relatively qualitatively reflect the expression of ECM-degrading related enzymes at the protein level
ECM-degrading cells in anti-fibrosis treatment
Deposited ECM is often overlooked in traditional fibrosis therapies. Existing studies usually focus on promoting the proliferation of tissue cells or inhibiting the activation of fibroblasts. The obstruction of tissue repair by deposited ECM needs to be taken into account in anti-fibrosis therapy. ECM-degrading cells have become therapeutics against the deposited ECM, releasing proteases (MMPs, etc.) continuously to degrade the ECM after reaching the fibrotic organ and promote the recovery of fibrosis.
In existing studies on anti-fibrosis treatment related to ECM-degrading cells, the delivered cells express proteases or inhibit the imbalance of MMPs and TIMPs in fibrotic organs (Table 1). In particular, mesenchymal stem cells (MSCs) have been reported for therapeutic applications in a variety of fibrotic organs. Sing Wan Wong et al. found that a soft matrix enhanced MMP production in TNF-α-stimulated MSCs, and an alginate-RGD gel-coated MSCs promoted normal ECM remodeling in the bleomycin-induced lung injury model (Wong et al. 2022). The combination of MSCs and serelaxin enhanced the activity of MMP2 in the obstructed kidney and alleviated established renal fibrosis in mice with unilateral ureteral obstruction (Huuskes et al. 2015). Additionally, human bone marrow MSCs could express MMPs in the fibrotic liver of rats induced by carbon tetrachloride (CCl4) and significantly decreased fibrosis (Huuskes et al. 2015). Human adipose MSCs could increase the MMP1 / TIMP1 ratio in tissues and efficiently reduced fibrosis in hypochlorite (HOCl) -induced systemic sclerosis (Maria et al. 2016). In cardiac fibroblasts cultured with MSC-conditioned medium in vitro, the expressions of α-smooth muscle actin (α-SMA) and TIMP2 were downregulated while the expression of MT1-MMP and the activities of MMP2 and MMP9 were increased. MSC transplantation correspondingly reduced cardiac ventricular fibrosis after myocardial infarction induced by coronary artery ligation (Mias et al. 2009).
Table 1.
Studies on anti-fibrosis treatment related to ECM-degrading cells
| Cell | Treatment | Mechanism | Application | Dose | Animal model | Injection method | Ref |
|---|---|---|---|---|---|---|---|
| Liver sinusoidal endothelial cells | Treatment with PMA and accutase | Increased expression of MMP1, MMP2, MMP9, etc | Advanced liver fibrosis | 4 × 105 | 6-week-old nude mice, CCl4 induced liver fibrosis | Intrasplenic injection | Zhao et al. 2022 |
| MSCs | Treatment with TNF-α | Increased expression of Mmp13 | Pulmonary fibrosis | 1 × 105 | 8 to 12-week-old C57BL/6 J mice, bleomycin-induced lung injury | Intratracheal injection | Wong et al. 2022 |
| Bone MSCs | Overexpression of Smad7 | Increased expression of MMP1 | Liver cirrhosis | (3~5) × 106 | 6-week-old Wistar rats, CCl4 induced liver fibrosis | Intrahepatic injection | Su et al. 2020 |
| Clonal mesenchymal stem cells | - | Increased expression of MMP2 and MMP9 | Liver fibrosis | 3 × 107 | 6-week-old Wistar rats, CCl4 induced liver fibrosis | Intrasplenic injection | Hardjo et al. 2009 |
| MSCs | Combination therapy with serelaxin | Increased expression of MMP2 in kidney | Renal fibrosis | 1 × 106 | Male C57BL/6 J mice, unilateral ureteral obstruction induced renal fibrosis | Renal vein injection | Huuskes et al. 2015 |
| Bone marrow mesenchymal stromal cells | - | Increased expression of MMP13 in M2 macrophage | Liver fibrosis | 5 × 105 | 10-week-old C57BL/6 J male mice, CCl4 induced liver fibrosis | Tail vein injection | Luo et al. 2019 |
| Human amnion epithelial cells | - | Downregulation of TIMP1, 2, 3, 4 in lung | Pulmonary fibrosis | 1 × 106 | 8-week-old SCID mice, bleomycin induced pulmonary fibrosis | Tail vein injection | Moodley et al. 2010 |
| Adipose-derived stem cells | Increased expression of MMP9 in liver | Liver fibrosis | 1 × 106 | 8-week-old male Wistar rats, TAA induced liver fibrosis | Intrahepatic injection | Harn et al. 2012 | |
| Kupffer cells | - | Increased expression of MMP9 | Liver fibrosis | 2 × 106 | C57BL/6 mice, TAA induced liver fibrosis | Intravenous injection | Feng et al. 2018 |
| MSCs | Overexpression of hepatocyte growth factor (HGF) | Increased expression of MMP9, 13, 14 and uPA, decreased expression of TIMP1 in liver | Liver fibrosis | 1 × 107 | 5-week-old Sprague–Dawley (SD) male rats, DMN induced liver fibrosis | Intrasplenic injection | Kim et al. 2014 |
| Bone marrow-derived liver stem cells | Overexpression of uPA | Activation of pro-MMP3 by uPA, which activates MMP2 and MMP9, then degrading collagen | Liver fibrosis | 2 × 106 | Female F344 rats, CCl4 induced liver fibrosis | Tail vein injection | Sun et al. 2008 |
| Bone marrow-derived MSCs | Treatment with IC-2 | Increased expression of MMP1, MMP2, and MMP14 | Liver fibrosis | - | 7–9-week-old BALB/c-nu/nu male mice, CCl4 induced liver fibrosis | Intrahepatic transplantation | Itaba et al. 2019 |
| Bone marrow‑derived MSCs | Transfection with survivin | Increased expression of MMP9 in lung | Pulmonary fibrosis | 1 × 106 | 6–8-week-old C57BL/6 male mice, bleomycin induced pulmonary fibrosis | Caudal vein injection | Zhou et al. 2015 |
| Induced pluripotent stem cells | - | Inhibition of imbalance in the expression ratios of MMP2/TIMP2 and MMP9/TIMP1 | Pulmonary fibrosis | 2 × 106 | C57BL/6 male mice, bleomycin-induced pulmonary fibrosis | Intravenous injection | Zhou et al. 2016 |
| Bone marrow MSCs | - | Increased expression of MMPs | Liver fibrosis | 1 × 106 | Adult male Wistar Kyoto (WKY) rats, CCl4 induced liver fibrosis | Portal vein injection | Zhao et al. 2012 |
| Endometrial regenerative cells | - | Increased expression of MMP9 | Pulmonary fibrosis | 1 × 106 | 6–8-week-old C57BL/6 female mice, bleomycin-induced pulmonary fibrosis | Tail vein injection | Zhao et al. 2018 |
| Bone-marrow-derived macrophages | Treatment with lipopolysaccharide (M1-polarized) | Increased expression of MMP2, 9, and 13 in recruited Ly6Clo macrophages | Liver fibrosis | 1 × 106 | 8-week-old C57BL/6 male mice, CCl4 induced liver fibrosis | Tail vein injection | Ma et al. 2017 |
| Bone marrow cells | - | Increased expression of MMP13 and MMP9 in some of bone marrow-derived cells and liver resident cells | Liver fibrosis | 5 × 106 | 6-week-old C57BL/6 mice, CCl4 induced liver fibrosis | Intravenous injection | Higashiyama et al. 2007 |
| Bone marrow-derived MSCs | - | Increased expression of MMP2 in liver | Liver fibrosis | 3 × 106 | Male Wistar rats, BDL induced liver fibrosis | Tail vein injection | Mohamed et al. 2016 |
| Chorionic plate-derived MSCs | - | Increased expression of MMP9 in liver | Liver fibrosis | 2 × 106 | 6-week-old male Sprague–Dawley rats, CCl4 induced liver fibrosis | Intrahepatic transplantation | Lee et al. 2010 |
| Adipose-derived MSCs | - | Enhanced ratio of Mmp1/Timp1 in skin and lung tissues | Systemic sclerosis | 2.5 × 105 | C57BL/6 mice, hypochlorite (HOCl) induced systemic sclerosis | Tail vein injection | Maria et al. 2016 |
| Bone marrow-derived MSCs | - | Enhanced ratio of MMP/TIMP production by cardiac fibroblasts | Cardiac ventricular fibrosis | 3 × 106 | Lewis congenic rats, interventricular artery ligation induced myocardial infarction | Intramyocardial injection | Mias et al. 2009 |
At present, there is a lack of research on anti-fibrosis treatment through cell-mediated ECM degradation. Most notably, Zhao et al. found that liver sinusoidal endothelial cells stimulated by accutase and phorbol myristate acetate (PMA) showed significant ECM degradation and relieved CCl4-induced advanced liver fibrosis in mice (Zhao et al. 2022). In addition, stimulated HUVECs also showed the ability to degrade the ECM and treat liver fibrosis, demonstrating their potential for clinical application.
Conclusions and perspectives
Conclusion
The degradation of the ECM is essential for the reversal of fibrosis, and a variety of proteases are involved in this process. Several types of cells can degrade ECM through extracellular or intracellular pathways in certain physiological states. The ECM-degrading ability of cells can be characterized by detecting the expression of related enzymes or analyzing co-cultured substrates. ECM-degrading cells are expected to degrade the deposited ECM in fibrotic organs to treat fibrosis, and the feasibility of this therapeutic strategy has been demonstrated in several studies.
Potential causes of limiting the clinical application of ECM-degrading cells in anti-fibrosis treatment
By secreting MMPs, ECM-degrading cells can degrade the ECM, potentially providing a therapeutic benefit to fibrotic diseases. Nevertheless, multiple factors impede the application of ECM-degrading cells in clinical settings for anti-fibrosis treatment.
First, when liver fibrosis progresses to cirrhosis, the normal structure of the liver is severely disrupted with excessive fibronectin and collagens I, III, V, and VI deposited in the fibrotic septa (Iredale et al. 2013). Additionally, the biophysical properties of the ECM are changed by cross-linking reactions. Most collagen cross-linking is mediated by lysyl oxidases (LOX), transglutaminases (TGase), and advanced glycosylation end products (AGEs), which increase the difficulty of degrading the ECM that cannot be easily broken down (Kong et al. 2021; Lyu et al. 2023). ECM-degrading cells may not possess the capability to break down the cross-linked and multi-component ECM in vivo. Therefore, we should devise an efficient screening system to detect the ECM-degrading potential of cells in vitro, taking the bionics of the ECM components and the degree of cross-linking into account.
Second, previous studies demonstrated that ECM-degrading liver sinusoidal endothelial cells possessed a strong ECM degradation ability for liver fibrosis (Zhao et al. 2022); however, there is insufficient evidence of ECM-degrading cells with similar capability for other fibrotic diseases, such as lung fibrosis, skin fibrosis, and myocardial fibrosis. In the future, we can use single-cell sequencing data from fibrotic and normal organs to determine the cell types with high expression of ECM-degrading proteases; these cells may serve as potential treatments for fibrosis.
Third, the safety of cell therapies in clinical application is an unavoidable issue, as allogeneic cells may cause immune responses in patients. Besides safety, precise delivery of therapeutic cells to the liver fibrotic areas in vivo should be taken into account to preclude damages caused to other organs. How to avoid degradation in healthy tissues and excessive degradation in fibrotic organs needs to be considered. In addition, for organs with weak regeneration capabilities, it is uncertain whether the structure and function of fibrotic tissues can be restored after ECM degradation.
Fourth, human-derived cell therapies may be clinically viable; yet, the majority of studies have only utilized nude mice to assess the efficacy of anti-fibrosis treatments with ECM-degrading cells (Cao et al. 2017; Nakamura et al. 2016; Nakamura et al. 2012; Woo et al. 2012). To further explore the effects of ECM-degrading cells, immunocompetent mice and primates should be employed in the future. Moreover, multiple causative models of fibrosis should be taken into account.
Fifth, a pro-inflammatory microenvironment, an imbalance of MMPs and TIMPs, and necrosis of certain cells can all be causes of fibrotic diseases (Tan et al. 2021). ECM-degrading cells may primarily be responsible for degrading the ECM that has been deposited. Currently, increasing clinical trials based on cell therapies, especially MSC therapy, for treating a myriad of diseases have achieved unprecedented breakthroughs. MSCs can be utilized to regulate the immune microenvironment and further augment the proliferation of particular cells (Nakamura et al. 2012). In the future, we can develop a combination of ECM-degrading cells and MSC therapies to facilitate organ regeneration while simultaneously degrading scars.
ECM-degrading cells derived vesicles
Despite the cells, cell-derived vesicles could release MMPs to promote tumor invasion (Turturici et al. 2014). Therefore, there is the possibility that cell-derived vesicles can degrade the ECM through the secretion and delivery of metalloproteinases to degrade the deposited ECM in the fibrotic area, thus promoting the recovery of fibrosis. Compared with ECM-degrading cell therapy, ECM-degrading vesicle therapy will have numerous advantages. Briefly, cell-derived vesicles are less immunogenic and more persistent in the circulatory system (Kazemi and Sobhania 2018). Furthermore, cell-derived vesicles are smaller in size, which will benefit their infiltration into the densely structured fibrotic tissue to perform degradation functions. However, at present, due to a lack of cell-derived vesicles with high degradability and low vesicle yields (Yamashita et al. 2018), the clinical application of cell-derived vesicles for fibrosis treatment has been limited.
First, it is challenging and arduous to screen cells with high degradation ability due to the low throughput and cost-ineffective methods as mentioned above. Therefore, it is a prerequisite to develop a platform for high-throughput and quantitative detection of the cellular degradation capacity.
Besides, during the production of cell-derived vesicles, traditional methods for isolating native extracellular vesicles are used, including ultracentrifugation, size- exclusion chromatography, and immunocapture. However, regardless of the isolation methods, the amount of vesicle protein obtained was low and insufficient for downstream applications (Coumans et al. 2017). In recent years, the method of culturing cells on a large-scale bioreactor, collecting the supernatant, and isolating extracellular vesicles has gained much interest. This method greatly liberates manpower and is cost-effective. However, the complicated methods of application have hindered their utilization, indicating that the culture device requires constant adjustment of the environmental parameters promptly to prevent high shear stress which may cause cell death (Colao et al. 2018). Therefore, great strides have been made by scientists to produce engineered vesicles which are reassembled by cell membranes. Using a liposome extrusion preparation device, a study sequentially passed the U937 cells and Raw264.7 cells through filter membranes with pore sizes of 10 μm, 5 μm, and 1 μm. Then, these cells were broken into nanovesicles, which have physicochemical and biological properties similar to those of exosomes (Jang et al. 2013). However, this method relies on manpower, which is relatively uncontrollable. Therefore, the scientists hope that automated extrusion control can be performed through microfluidics. Yong Song Gho et al. proposed a novel and efficient method for ESCs to enter narrow, hydrophilic microchannels, rupture their membranes, and generate artificial nanovesicles. These nanovesicles contained mRNA, intracellular proteins, and plasma membrane proteins, and they had a similar shape to exosomes secreted by cells (Jo et al. 2014). However, studies of producing vesicles using microchannels are limited to ESCs, and the confined dimension of the channel (5 μm) results in poor vesicle morphology and uneven particle size. In conclusion, the discovery of the ECM-degrading ability of various cells has paved the way for the development of effective new treatments for fibrotic diseases. The development of a universal preparation process suitable for a variety of cell types is necessary to engender an easy-to-operate, controllable preparation process to obtain nanovesicles with good quality and high yield.
Acknowledgements
This work was supported by National Science Foundation for Distinguished Young Scholars (82125018)
Abbreviations
- AGE
Advanced glycosylation end products
- CAT
Catalytic
- CatG
Cathepsin G
- CCl4
Carbon tetrachloride
- CHP
Collagen Hybridizing peptide
- COPD
Chronic obstructive pulmonary disease
- ECM
Extracellular matrix
- Ela-2
Neutrophil elastase
- GAG
Glycosaminoglycan
- GPI
Glycosylphosphatidylinositol
- HA
Hyaluronic acid
- HNSCC
Cervical squamous cell carcinoma
- HOCl
Hypochlorite
- HPX
Hemopexin
- HUVEC
Human umbilical vein endothelial cell
- HYAL
Hyaluronidase
- HYP
Hydroxyproline
- LOX
Lysyl oxidases
- MMP
Matrix metalloproteinase
- MoMF
Monocyte-derived macrophage
- MSC
Mesenchymal stem cell
- MT-MMP
Membrane-type matrix metalloproteinase
- PA
Plasminogen activator
- PDGF
Platelet derived growth factor
- PMA
Phorbol myristate acetate
- pr-3
Protease-3
- pro-uPA
Pro-urokinase-type plasminogen activator
- qPCR
Real-time quantitative PCR
- SCC1
Squamous carcinoma cell
- SMA
Smooth muscle actin
- TGase
Transglutaminases
- TGF
Transforming growth factor
- TIMP
Tissue inhibitors of metalloproteinase
- TM
Transmembrane
- TNF
Tumor necrosis factor
- tPA
Tissue plasminogen activator
- uPA
Urokinase plasminogen activator
- uPAR
Urokinase-type plasminogen activator receptor
Authors’ contributions
P.Z. and T.S. contributed equally to this work. P.Z. outlined and organized the review. P.Z. and T.S. searched all related literature and drafted the manuscript. C.L. helped edit the manuscript. Y.D. provided insightful comments and revised the manuscript. K.L. prepared the schematics. Y.D. is the principal investigator of the supporting grants. All the authors read and approved the final manuscript.
Funding
This work is financially supported by the National Natural Science Foundation of China (82125018).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Yanan Du is a member of the Editorial Board for Cell Regeneration. He was not involved in the journal’s review of, or decisions related to, this manuscript. All authors declare that they have no competing interests.
Footnotes
Peng Zhao and Tian Sun contributed equally to this work.
References
- Aguda AH, Panwar P, Du X, Nguyen NT, Brayer GD, Bromme D. Structural basis of collagen fiber degradation by cathepsin K. Proc Natl Acad Sci U S A. 2014;111(49):17474–17479. doi: 10.1073/pnas.1414126111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre P, Villain F. Free radical scavenging properties of mannitol and its role as a constituent of hyaluronic acid fillers: a literature review. Int J Cosmetic Sci. 2017;39(4):355–360. doi: 10.1111/ics.12386. [DOI] [PubMed] [Google Scholar]
- Arora PD, Manolson MF, Downey GP, Sodek J, McCulloch CA. A novel model system for characterization of phagosomal maturation, acidification, and intracellular collagen degradation in fibroblasts. J Biol Chem. 2000;275(45):35432–35441. doi: 10.1074/jbc.M003221200. [DOI] [PubMed] [Google Scholar]
- Arthur M. Role of tissue inhibitors of metalloproteinases (TIMPs) in the progression and regression of liver fibrosis. Extracellular Matrix Liver. 2003:347–59. 10.1016/B978-012525251-5/50020-8.
- Batra J, Robinson J, Soares AS, Fields AP, Radisky DC, Radisky ES. Matrix metalloproteinase-10 (MMP-10) interaction with tissue inhibitors of metalloproteinases TIMP-1 and TIMP-2: binding studies and crystal structure. J Biol Chem. 2012;287(19):15935–15946. doi: 10.1074/jbc.M112.341156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini I, Fragai M, Luchinat C, Melikian M, Toccafondi M, Lauer JL, et al. Structural basis for matrix metalloproteinase 1-catalyzed collagenolysis. J Am Chem Soc. 2012;134(4):2100–2110. doi: 10.1021/ja208338j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biniossek ML, Nagler DK, Becker-Pauly C, Schilling O. Proteomic Identification of protease cleavage sites characterizes prime and non-prime specificity of cysteine cathepsins B, L, and S (vol 10, pg 5363, 2011) J Proteome Res. 2011;10(12):5577. doi: 10.1021/pr201080h. [DOI] [PubMed] [Google Scholar]
- Cao ZW, Ye TH, Sun Y, Ji GL, Shido K, Chen YT, et al. Targeting the vascular and perivascular niches as a regenerative therapy for lung and liver fibrosis. Sci Transl Med. 2017;9(405):eaai8710. doi: 10.1126/scitranslmed.aai8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo-Medved D, Rudy D, Blum G, Bogyo M, Caglic D, Sloane BF. Live-cell imaging demonstrates extracellular matrix degradation in association with active cathepsin B in caveolae of endothelial cells during tube formation. Exp Cell Res. 2009;315(7):1234–1246. doi: 10.1016/j.yexcr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Lu SH, Cui Y. ECRG2 regulates ECM degradation and uPAR/FPRL1 pathway contributing cell invasion/migration. Cancer Lett. 2010;290(1):87–95. doi: 10.1016/j.canlet.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Christina VS, Sundaram RL, Sivamurugan V, Kumar DT, Mohanapriya CD, Shailaja VL, et al. Inhibition of MMP2-PEX by a novel ester of dihydroxy cinnamic and linoleic acid from the seagrass Cymodocea serrulata. Sci Rep. 2021;11(1):11451. doi: 10.1038/s41598-021-90845-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, et al. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167(4):757–767. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung L, Dinakarpandian D, Yoshida N, Lauer-Fields JL, Fields GB, Visse R, et al. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J. 2004;23(15):3020–3030. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing exosomes: a promising therapeutic platform. Trends Mol Med. 2018;24(3):242–256. doi: 10.1016/j.molmed.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, et al. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120(10):1632–1648. doi: 10.1161/CIRCRESAHA.117.309417. [DOI] [PubMed] [Google Scholar]
- Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20(8):499–508. doi: 10.1016/s0945-053x(01)00172-x. [DOI] [PubMed] [Google Scholar]
- de Castro Bras LE, Frangogiannis NG. Extracellular matrix-derived peptides in tissue remodeling and fibrosis. Matrix Biol. 2020;91–92:176–187. doi: 10.1016/j.matbio.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryugina EI, Quigley JP. Cell surface remodeling by plasmin: a new function for an old enzyme. J Biomed Biotechnol. 2016;2012:564259. doi: 10.1155/2012/564259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryugina EI, Zajac E, Juncker-Jensen A, Kupriyanova TA, Welter L, Quigley JP. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia. 2014;16(10):771–788. doi: 10.1016/j.neo.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Canestro C, Puspitasari YM, Liberale L, Guzik TJ, Flammer AJ, Bonetti NR, et al. MMP-2 knockdown blunts age-dependent carotid stiffness by decreasing elastin degradation and augmenting eNOS activation. Cardiovasc Res. 2022;118(10):2385–2396. doi: 10.1093/cvr/cvab300. [DOI] [PubMed] [Google Scholar]
- Feng M, Ding J, Wang M, Zhang J, Zhu XH, Guan WX. Kupffer-derived matrix metalloproteinase-9 contributes to liver fibrosis resolution. Int J Biol Sci. 2018;14(9):1033–1040. doi: 10.7150/ijbs.25589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AJ, Orbach SM, Rajagopalan P. Fibroblasts stimulate macrophage migration in interconnected extracellular matrices through tunnel formation and fiber alignment. Biomaterials. 2019;209:88–102. doi: 10.1016/j.biomaterials.2019.03.044. [DOI] [PubMed] [Google Scholar]
- Genschmer KR, Russell DW, Lal C, Szul T, Bratcher PE, Noerager BD, et al. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell. 2019;176(1–2):113–26 e15. doi: 10.1016/j.cell.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KA, Lund LR. ECM degrading proteases and tissue remodelling in the mammary gland. BioEssays. 2005;27(9):894–903. doi: 10.1002/bies.20281. [DOI] [PubMed] [Google Scholar]
- Harada H, Takahashi M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J Biol Chem. 2007;282(8):5597–5607. doi: 10.1074/jbc.M608358200. [DOI] [PubMed] [Google Scholar]
- Hardjo M, Miyazaki M, Sakaguchi M, Masaka T, Ibrahim S, Kataoka K, et al. Suppression of carbon tetrachloride-induced liver fibrosis by transplantation of a clonal mesenchymal stem cell line derived from rat bone marrow. Cell Transplant. 2009;18(1):89–99. doi: 10.3727/096368909788237140. [DOI] [PubMed] [Google Scholar]
- Harn HJ, Lin SZ, Hung SH, Subeq YM, Li YS, Syu WS, et al. Adipose-derived stem cells can abrogate chemical-induced liver fibrosis and facilitate recovery of liver function. Cell Transplant. 2012;21(12):2753–2764. doi: 10.3727/096368912x652959. [DOI] [PubMed] [Google Scholar]
- Heinz A. Elastases and elastokines: elastin degradation and its significance in health and disease. Crit Rev Biochem Mol. 2020;55(3):252–273. doi: 10.1080/10409238.2020.1768208. [DOI] [PubMed] [Google Scholar]
- Heinz A, Taddese S, Sippl W, Neubert RHH, Schmelzer CEH. Insights into the degradation of human elastin by matrilysin-1. Biochimie. 2011;93(2):187–194. doi: 10.1016/j.biochi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jung MC, Jahreis G, Rusciani A, Duca L, Debelle L, et al. The action of neutrophil serine proteases on elastin and its precursor. Biochimie. 2012;94(1):192–202. doi: 10.1016/j.biochi.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature. 2020;587(7835):555–566. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45(1):213–222. doi: 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, et al. MT1-mmp: a collagenase essential for tumor cell invasive growth. Cancer Cell. 2003 doi: 10.1016/S1535-6108(03)00196-X. [DOI] [PubMed] [Google Scholar]
- Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114(1):33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- Huuskes BM, Wise AF, Cox AJ, Lim EX, Payne NL, Kelly DJ, et al. Combination therapy of mesenchymal stem cells and serelaxin effectively attenuates renal fibrosis in obstructive nephropathy. Faseb J. 2015;29(2):540–553. doi: 10.1096/fj.14-254789. [DOI] [PubMed] [Google Scholar]
- Hwang J, Huang Y, Burwell TJ, Peterson NC, Connor J, Weiss SJ, et al. In situ imaging of tissue remodeling with collagen hybridizing peptides. ACS Nano. 2017;11(10):9825–9835. doi: 10.1021/acsnano.7b03150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale JP, Thompson A, Henderson NC. Extracellular matrix degradation in liver fibrosis: biochemistry and regulation. Biochim Biophys Acta. 2013;1832(7):876–883. doi: 10.1016/j.bbadis.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Islam MS, Khunkar SJ, Nakashima S, Sadr A, Nikaido T, Tagami J. Comparative study of demineralized collagen degradation determined by hydroxyproline assay and microscopic depth measurement. J Dent. 2016;47:94–97. doi: 10.1016/j.jdent.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Itaba N, Kono Y, Watanabe K, Yokobata T, Oka H, Osaki M, et al. Reversal of established liver fibrosis by IC-2-engineered mesenchymal stem cell sheets. Sci Rep. 2019;9(1):6841. doi: 10.1038/s41598-019-43298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- Jevnikar Z, Mirkovic B, Fonovic UP, Zidar N, Svajger U, Kos J. Three-dimensional invasion of macrophages is mediated by cysteine cathepsins in protrusive podosomes. Eur J Immunol. 2012;42(12):3429–3441. doi: 10.1002/eji.201242610. [DOI] [PubMed] [Google Scholar]
- Jo W, Jeong D, Kim J, Cho S, Jang SC, Han C, et al. Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers. Lab Chip. 2014;14(7):1261–1269. doi: 10.1039/c3lc50993a. [DOI] [PubMed] [Google Scholar]
- Joshi R, Heinz A, Fan Q, Guo S, Monia B, Schmelzer CEH, et al. Role for Cela1 in postnatal lung remodeling and alpha-1 antitrypsin-deficient emphysema. Am J Respir Cell Mol Biol. 2018;59(2):167–178. doi: 10.1165/rcmb.2017-0361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul A, Short WD, Wang X, Keswani SG. Hyaluronidases in human diseases. Int J Mol Sci. 2021;22(6):320. doi: 10.3390/ijms22063204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi M, Sobhania Z. Exosomes, microvesicles as diagnosis, therapeutic and drug delivery tools. Int Pharm Acta. 2018;1(1):100–101. doi: 10.22037/ipa.v1i1.19968. [DOI] [Google Scholar]
- Kim MD, Kim SS, Cha HY, Jang SH, Chang DY, Kim W, et al. Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp Mol Med. 2014;46:e110. doi: 10.1038/emm.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NY, Jung YY, Yang MH, Um JY, Sethi G, Ahn KS. Isoimperatorin down-regulates epithelial mesenchymal transition through modulating NF-Kappa B signaling and CXCR4 expression in colorectal and hepatocellular carcinoma cells. Cell Signal. 2022;99:110433. doi: 10.1016/j.cellsig.2022.110433. [DOI] [PubMed] [Google Scholar]
- Klepfish M, Gross T, Vugman M, Afratis NA, Sagi I. LOXL2 inhibition paves the way for macrophage-mediated collagen degradation in liver fibrosis. Front Immunol. 2020;11:480. doi: 10.3389/fimmu.2020.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Hattori S, Shinkai H. Matrix metalloproteinases-2 and -9 are secreted from human fibroblasts. Acta Derm Venereol. 2003;83(2):105–107. doi: 10.1080/00015550310007436. [DOI] [PubMed] [Google Scholar]
- Kong WY, Lyu C, Liao HG, Du YN. Collagen crosslinking: effect on structure, mechanics and fibrosis progression. Biomed Mater. 2021;16(6):062005. doi: 10.1088/1748-605X/ac2b79. [DOI] [PubMed] [Google Scholar]
- Krafts KP. Tissue repair: the hidden drama. Organogenesis. 2010;6(4):225–233. doi: 10.4161/org.6.4.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczka J, Boncela J. Proteases revisited: roles and therapeutic implications in fibrosis. Mediators Inflamm. 2017;2017:2570154. doi: 10.1155/2017/2570154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümper M, Hessenthaler S, Zamek J, Niland S, Pach E, Mauch C, et al. Loss of endothelial cell matrix metalloproteinase 14 reduces melanoma growth and metastasis by increasing tumor vessel stability. J Invest Dermatol. 2022;142(7):1923–33.e5. doi: 10.1016/j.jid.2021.12.016. [DOI] [PubMed] [Google Scholar]
- Langrock T, Hoffmann R. Analysis of hydroxyproline in collagen hydrolysates. New York: Springer; 2019. pp. 47–56. [DOI] [PubMed] [Google Scholar]
- Lee H, Sodek KL, Hwang Q, Brown TJ, Ringuette M, Sodek J. Phagocytosis of collagen by fibroblasts and invasive cancer cells is mediated by MT1-MMP. Biochem Soc T. 2007;35:704–706. doi: 10.1042/BST0350704. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Jung J, Na KH, Moon JS, Lee HJ, Kim JH, et al. Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl4-injured liver: potential application to the treatment of hepatic diseases. J Cell Biochem. 2010;111(6):1453–1463. doi: 10.1002/jcb.22873. [DOI] [PubMed] [Google Scholar]
- Li QL, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111(5):635–646. doi: 10.1016/S0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- Li Y, Foss CA, Summerfield DD, Doyle JJ, Torok CM, Dietz HC, et al. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc Natl Acad Sci U S A. 2012;109(37):14767–14772. doi: 10.1073/pnas.1209721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Takino T, Endo Y, Sato H. Activation of MMP-9 by membrane type-1 MMP/MMP-2 axis stimulates tumor metastasis. Cancer Sci. 2017;108(3):347–353. doi: 10.1111/cas.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S, Scita G. RABGTPases in MT1-MMP trafficking and cell invasion: physiology versus pathology. Small GTPases. 2015;6(3):145–152. doi: 10.4161/21541248.2014.985484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Lobato AL, Garrido MLA, Mesa HGA, Sampieri CL, Lozano VHS. Quantification of the presence of enzymes in gelatin zymography using the Gini index. J Bioinform Comput Biol. 2022;20(6):2250025. doi: 10.1142/S0219720022500251. [DOI] [PubMed] [Google Scholar]
- Luo XY, Meng XJ, Cao DC, Wang W, Zhou K, Li L, et al. Transplantation of bone marrow mesenchymal stromal cells attenuates liver fibrosis in mice by regulating macrophage subtypes. Stem Cell Res Ther. 2019;10:16. doi: 10.1186/s13287-018-1122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu C, Kong WY, Liu ZQ, Wang SH, Zhao P, Liang KN, et al. Advanced glycation end-products as mediators of the aberrant crosslinking of extracellular matrix in scarred liver tissue. Nat Biomed Eng. 2023 doi: 10.1038/s41551-023-01019-z. [DOI] [PubMed] [Google Scholar]
- Ma PF, Gao CC, Yi J, Zhao JL, Liang SQ, Zhao Y, et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice. J Hepatol. 2017;67(4):770–779. doi: 10.1016/j.jhep.2017.05.022. [DOI] [PubMed] [Google Scholar]
- Madsen DH, Jurgensen HJ, Ingvarsen S, Melander MC, Vainer B, Egerod KL, et al. Endocytic collagen degradation: a novel mechanism involved in protection against liver fibrosis. J Pathol. 2012;227(1):94–105. doi: 10.1002/path.3981. [DOI] [PubMed] [Google Scholar]
- Madsen DH, Leonard D, Masedunskas A, Moyer A, Jurgensen HJ, Peters DE, et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol. 2013;202(6):951–966. doi: 10.1083/jcb.201301081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood N, Mihalcioiu C, Rabbani SA. Multifaceted role of the urokinase-type plasminogen activator (uPA) and its receptor (uPAR): diagnostic, prognostic, and therapeutic applications. Front Oncol. 2018;8:24. doi: 10.3389/fonc.2018.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria ATJ, Toupet K, Maumus M, Fonteneau G, Le Quellec A, Jorgensen C, et al. Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. J Autoimmun. 2016;70:31–39. doi: 10.1016/j.jaut.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Maurice P, Blaise S, Gayral S, Debelle L, Laffargue M, Hornebeck W, et al. Elastin fragmentation and atherosclerosis progression: the elastokine concept. Trends Cardiovasc Med. 2013;23(6):211–221. doi: 10.1016/j.tcm.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas MH, et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27(11):2734–2743. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- Micallef L, Vedrenne N, Billet F, Coulomb B, Darby IA, Desmouliere A. The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S5. doi: 10.1186/1755-1536-5-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miekus N, Luise C, Sippl W, Baczek T, Schmelzer CEH, Heinz A. MMP-14 degrades tropoelastin and elastin. Biochimie. 2019;165:32–39. doi: 10.1016/j.biochi.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Moammeri A, Abbaspour K, Zafarian A, Jamshidifar E, Motasadizadeh H, Dabbagh Moghaddam F, et al. pH-responsive, adorned nanoniosomes for codelivery of cisplatin and epirubicin: synergistic treatment of breast cancer. ACS Appl Bio Mater. 2022;5(2):675–690. doi: 10.1021/acsabm.1c01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed HE, Elswefy SE, Rashed LA, Younis NN, Shaheen MA, Ghanim AMH. Bone marrow-derived mesenchymal stem cells effectively regenerate fibrotic liver in bile duct ligation rat model. Exp Biol Med. 2016;241(6):581–591. doi: 10.1177/1535370215627219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley Y, Ilancheran S, Samuel C, Vaghjiani V, Atienza D, Williams ED, et al. Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. Am J Resp Crit Care. 2010;182(5):643–651. doi: 10.1164/rccm.201001-0014OC. [DOI] [PubMed] [Google Scholar]
- Mora Huertas AC, Schmelzer CEH, Luise C, Sippl W, Pietzsch M, Hoehenwarter W, et al. Degradation of tropoelastin and skin elastin by neprilysin. Biochimie. 2018;146:73–78. doi: 10.1016/j.biochi.2017.11.018. [DOI] [PubMed] [Google Scholar]
- Nakagami Y, Abe K, Nishiyama N, Matsuki N. Laminin degradation by plasmin regulates long-term potentiation. J Neurosci. 2000;20(5):2003–2010. doi: 10.1523/JNEUROSCI.20-05-02003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Tsutsumi V, Torimura T, Naitou M, Iwamoto H, Masuda H, et al. Human peripheral blood CD34-positive cells enhance therapeutic regeneration of chronically injured liver in nude rats. J Cell Physiol. 2012;227(4):1538–1552. doi: 10.1002/jcp.22873. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Koga H, Iwamoto H, Tsutsumi V, Imamura Y, Naitou M, et al. Ex vivo expansion of circulating CD34(+) cells enhances the regenerative effect on rat liver cirrhosis. Mol Ther Methods Clin Dev. 2016;3:16025. doi: 10.1038/mtm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizo S, Martinez-Arias L, Alonso-Montes C, Cannata P, Martin-Carro B, Fernandez-Martin JL, et al. Fibrosis in chronic kidney disease: pathogenesis and consequences. Int J Mol Sci. 2021;22(1):408. doi: 10.3390/ijms22010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar P, Hedtke T, Heinz A, Andrault PM, Hoehenwarter W, Granville DJ, et al. Expression of elastolytic cathepsins in human skin and their involvement in age-dependent elastin degradation. Biochim Biophys Acta Gen Subj. 2020;1864(5):129544. doi: 10.1016/j.bbagen.2020.129544. [DOI] [PubMed] [Google Scholar]
- Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4(3):253–258. doi: 10.4161/derm.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov Y, Patsenker E, Bauer M, Niedobitek E, Schulze-Krebs A, Schuppan D. Halofuginone induces matrix metalloproteinases in rat hepatic stellate cells via activation of p38 and NFkappaB. J Biol Chem. 2006;281(22):15090–15098. doi: 10.1074/jbc.M600030200. [DOI] [PubMed] [Google Scholar]
- Qian D, He L, Zhang Q, Li W, Tang D, Wu C, et al. Cathepsin K: a versatile potential biomarker and therapeutic target for various cancers. Curr Oncol. 2022;29(8):5963–5987. doi: 10.3390/curroncol29080471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar DS, Mariswamy AB. Comparative evaluation of Emblica officinalis as an etchant and an MMP inhibitor with orthophosphoric acid and chlorhexidine on the microshear bond strength of composite resin: an ex vivo study. Restor Dent Endod. 2021;46(3):e36. doi: 10.5395/rde.2021.46.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. P Natl Acad Sci USA. 2012;109(46):E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanov DV, Savinov AY, Golubkov VS, Postnova TI, Remacle A, Tomlinson S, et al. Cellular membrane type-1 matrix metalloproteinase (MT1-MMP) cleaves C3b, an essential component of the complement system. J Biol Chem. 2004;279(45):46551–46557. doi: 10.1074/jbc.m405284200. [DOI] [PubMed] [Google Scholar]
- Sabeh F, Li XY, Saunders TL, Rowe RG, Weiss SJ. Secreted versus membrane-anchored collagenases: relative roles in fibroblast-dependent collagenolysis and invasion. J Biol Chem. 2009;284(34):23001–23011. doi: 10.1074/jbc.M109.002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi I, Gaffney JP. Matrix Metalloproteinases: From Structure to Function. In: Sagi I, Gaffney J, editors. Matrix Metalloproteinase Biology. John Wiley & Sons, Incorporated: United States; 2015. pp. 1–22. [Google Scholar]
- Silva TL, Bartolomeu AD, de Jesus HCR, de Oliveira KT, Fernandes JB, Bromme D, et al. New synthetic quinolines as cathepsin K inhibitors. J Brazil Chem Soc. 2020;31(8):1605–1613. doi: 10.21577/0103-5053.20200046. [DOI] [Google Scholar]
- Sprangers S, Everts V. Molecular pathways of cell-mediated degradation of fibrillar collagen. Matrix Biol. 2019;75–76:190–200. doi: 10.1016/j.matbio.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Su DN, Wu SP, Xu SZ. Mesenchymal stem cell-based Smad7 gene therapy for experimental liver cirrhosis. Stem Cell Res Ther. 2020;11(1):395. doi: 10.1186/s13287-020-01911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Li DG, Chen YW, Chen YW, Wang BC, Sun QL, et al. Transplantation of urokinase-type plasminogen activator gene-modified bone marrow-derived liver stem cells reduces liver fibrosis in rats. J Gene Med. 2008;10(8):855–866. doi: 10.1002/jgm.1206. [DOI] [PubMed] [Google Scholar]
- Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60(5):1090–1096. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- Tagirasa R, Yoo E. Role of serine proteases at the tumor-stroma interface. Front Immunol. 2022;13:832418. doi: 10.3389/fimmu.2022.832418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki T, Kobayashi M, Okubo K, Takahashi N, Okamatsu Y, Mochizuki S, et al. Interferon-gamma inhibits collagen phagocytosis in human fibroblasts by inducing subcortical actin assembly and reducing ability of beta1 integrin to bind to collagen. Inflamm Res. 2006;55(12):534–542. doi: 10.1007/s00011-006-5088-0. [DOI] [PubMed] [Google Scholar]
- Tan Z, Sun HB, Xue TX, Gan CL, Liu HY, Xie YT, et al. Liver fibrosis: therapeutic targets and advances in drug therapy. Front Cell Dev Biol. 2021;9:730176. doi: 10.3389/fcell.2021.730176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Tang T, Gao G, Wei Q, Sun K, Huang W. Bone marrow mesenchymal stem cell-derived exosomes inhibit chondrocyte apoptosis and the expression of MMPs by regulating Drp1-mediated mitophagy. Acta Histochem. 2021;123(8):151796. doi: 10.1016/j.acthis.2021.151796. [DOI] [PubMed] [Google Scholar]
- Tu C, Ortega-Cava CF, Chen G, Fernandes ND, Cavallo-Medved D, Sloane BF, et al. Lysosomal cathepsin B participates in the podosome-mediated extracellular matrix degradation and invasion via secreted lysosomes in v-Src fibroblasts. Cancer Res. 2008;68(22):9147–9156. doi: 10.1158/0008-5472.CAN-07-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol-Cell Ph. 2014;306(7):C621–C633. doi: 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- Uemura A, Nakamura M, Kachi S, Nishizawa Y, Asami T, Miyake Y, et al. Effect of plasmin on laminin and fibronectin during plasmin-assisted vitrectomy. Arch Ophthalmol. 2005;123(2):209–213. doi: 10.1001/archopht.123.2.209. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res. 2008;78(2):203–212. doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol. 2012;196(3):375–385. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Tsuchiya A, Seino S, Kawata Y, Kojima Y, Ikarashi S, et al. Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice. Stem Cells Transl Med. 2019;8(3):271–284. doi: 10.1002/sctm.18-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman SM, Booth NA, Moore N, Redmill B, Forrester JV, Knott RM. Regulation of plasminogen activation by TGF-beta in cultured human retinal endothelial cells. Br J Ophthalmol. 2000;84(4):417–422. doi: 10.1136/bjo.84.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SW, Tamatam CR, Cho IS, Toth PT, Bargi R, Belvitch P, et al. Inhibition of aberrant tissue remodelling by mesenchymal stromal cells singly coated with soft gels presenting defined chemomechanical cues. Nat Biomed Eng. 2022;6(1):54. doi: 10.1038/s41551-021-00740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo DH, Kim SK, Lim HJ, Heo J, Park HS, Kang GY, et al. Direct and indirect contribution of human embryonic stem cell-derived hepatocyte-like cells to liver repair in mice. Gastroenterology. 2012;142(3):602–611. doi: 10.1053/j.gastro.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Xi W, Khalil RA. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharmacol. 2017 doi: 10.1016/bs.apha.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, McKelvey K, Shen K, Minhas N, March L, Park SY, et al. Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology (oxford) 2014;53(12):2270–2279. doi: 10.1093/rheumatology/keu254. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Yamamoto H, Tobisawa Y, Irie F. TMEM2: a missing link in hyaluronan catabolism identified? Matrix Biol. 2019;78–79:139–146. doi: 10.1016/j.matbio.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Takahashi Y, Takakura Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull. 2018;41(6):835–842. doi: 10.1248/bpb.b18-00133. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Li Z, Greenbaum D, Bogyo M, Weber E, Bromme D, Cathepsin V. a novel and potent elastolytic activity expressed in activated macrophages. J Biol Chem. 2004;279(35):36761–36770. doi: 10.1074/jbc.M403986200. [DOI] [PubMed] [Google Scholar]
- Yoo Y, Choi E, Kim Y, Cha Y, Um E, Kim Y, et al. Therapeutic potential of targeting cathepsin S in pulmonary fibrosis. Biomed Pharmacother. 2022;145:112245. doi: 10.1016/j.biopha.2021.112245. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Nagaoka A, Kusaka-Kikushima A, Tobiishi M, Kawabata K, Sayo T, et al. KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc Natl Acad Sci U S A. 2013;110(14):5612–5617. doi: 10.1073/pnas.1215432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SS, Shaw RM. Multilayered regulation of cardiac ion channels. Biochim Biophys Acta. 2013;1833(4):876–885. doi: 10.1016/j.bbamcr.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Matrisian LM, Holmbeck K, Vick CC, Rosenthal EL. Fibroblast-derived MT1-MMP promotes tumor progression in vitro and in vivo. BMC Cancer. 2006;6:52. doi: 10.1186/1471-2407-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Feng Z, Hu B, Chi X, Jiao S. Ex vivo-expanded bone marrow mesenchymal stem cells facilitate recovery from chemically induced acute liver damage. Hepatogastroenterology. 2012;59(120):2389–2394. doi: 10.5754/hge12288. [DOI] [PubMed] [Google Scholar]
- Zhao YM, Lan X, Wang Y, Xu XX, Lu SZ, Li X, et al. Human endometrial regenerative cells attenuate bleomycin-induced pulmonary fibrosis in mice. Stem Cells Int. 2018;2018:3475137. doi: 10.1155/2018/3175137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Sun T, Lyu C, Liang KN, Niu YD, Zhang YY, et al. Scar-degrading endothelial cells as a treatment for advanced liver fibrosis. Adv Sci. 2022 doi: 10.1002/advs.202203315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou MI, Chen DL, Jiang T, Feng YM, Han XL. Effects of bone marrow-derived mesenchymal stem cells transfected with survivin on pulmonary fibrosis in mice. Exp Ther Med. 2015;10(5):1857–1864. doi: 10.3892/etm.2015.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, He Z, Gao Y, Zheng R, Zhang X, Zhao L, et al. Induced pluripotent stem cells inhibit bleomycin-induced pulmonary fibrosis in mice through suppressing TGF-beta1/Smad-mediated epithelial to mesenchymal transition. Front Pharmacol. 2016;7:430. doi: 10.3389/fphar.2016.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigrino P, Brinckmann J, Niehoff A, et al. Fibroblast-derived MMP-14 regulates collagen homeostasis in adult skin. J Invest Dermatol. 2016;136(8):1575–1583. doi: 10.1016/j.jid.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitnay JL, Li Y, Qin Z, San BH, Depalle B, Reese SP, et al. Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nat Commun. 2017;8:14913. doi: 10.1038/ncomms14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.