Abstract
Psoriatic arthritis (PsA) is a complex, multiform and chronic inflammatory disease characterised by the association of arthritis and psoriasis combined with other related conditions and comorbidities. Treatment of PsA has rapidly evolved by the introduction of new biological drugs and small molecules which allow to achieve disease remission or low disease activity in most of the patients. However, unmet treatment needs still persist for those patients with persistent disease activity or symptoms, impaired function, reduced quality of life or comorbidities. In this context, non-pharmacological approaches, including diet modifications, an adequate sleep quality and physical activity could provide additional benefits. In recent years, diet modifications, improvement of sleep quality and physical activity became an area of interest for researchers and some studies showed how a holistic non-pharmacological approach may ameliorate the quality of life of patients with PsA.
The aim of this manuscript was to review the current evidence on the intriguing link and potential effects of diet, sleep and exercise in PsA patients. In particular, we reviewed the literature focusing on the possible benefits of a holistic approach to PsA patients considering lifestyle modifications.
Keywords: psoriatic arthritis; treatment; rehabilitation; outcome assessment, health care
WHAT IS ALREADY KNOWN ON THIS TOPIC
Psoriatic arthritis (PsA) is a multiform and chronic inflammatory disease associated with other related conditions and comorbidities in which unmet treatment needs still persist.
WHAT THIS STUDY ADDS
Diet modifications, improvement of sleep quality and physical activity became an area of interest for researchers for the non-pharmacological approach to PsA and, in general to inflammatory arthritis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Recent researches showed that some diet regimes, physical activity and potentially, a good sleep quality, may be effective in PsA patients and may be implemented in the overall management of PsA.
Introduction
Psoriatic arthritis (PsA) is a complex inflammatory disease in which different cutaneous, musculoskeletal, extra-articular manifestations and comorbidities run together to define the clinical phenotype.1 The hallmark of articular manifestations is the presence of arthritis (peripheral and/or axial), dactylitis and enthesitis, with the possible association with extra-articular manifestations and comorbidities, which should lead the treating physician to a ‘holistic’ approach of these patients.2 In the past years, PsA treatment has rapidly evolved with the possibility to achieve remission or low disease activity in up to two-thirds of patients with PsA by using novel approaches, mainly based on the use of biological and target synthetic disease-modifying antirheumatic drugs.3
However, unmet needs still persist for the ‘difficult-to-treat’ patients in which disease activity or related symptoms are still present despite the use of several treatment approaches or for those with impaired function, reduced quality of life or comorbidities.4 In a multifaceted disease such as PsA, many patients may have residual disease activity in one or more domains, which could be an important issue not only for physicians, but mainly for patients’ quality of life.5 6 Less specific symptoms such as fatigue and chronic pain can persist after successful interventions in a complex pattern, thereby complicating the goal of achieving a state of regained health for the PsA patient. Hence, pain, function, fatigue and health-related quality of life are key components in the comprehensive management of PsA (included in OMERACT-endorsed PsA core domain set) and it should be considered by all treating physicians.7
In this context, beyond pharmacological treatment strategy, other approaches may be useful in PsA patients. In particular, general aspect of well-being, including physical activity, exercise, diet regimens and sleep quality are rarely discussed during routine clinical visits and may be relevant for the management to psoriatic disease.8 These elements may also be useful to reduce the cardiovascular risk, improve the metabolic profile and, more in general, to improve patient’s quality of life. Of note, lifestyle modifications can also be useful to improve patient’s quality of life in those with well-controlled disease.
The aim of this manuscript is to review the current evidence on the intriguing link and potential effects of factors such as diet, sleep and exercise in the overall management of PsA patients.
Methods
We searched PubMed and the Cochrane library for articles and reviews in the English language published between1 January 1990 and 30 October 2022. Search terms were “psoriatic arthritis”, “diet”, “diet supplements” “nutrition”, “psoriatic disease”, “sleep disturbance”, “rehabilitation”, “exercise”, “physical therapy”, “physical activities”, “comorbidities” and limited the search to human, clinical studies, clinical trials, review and meta-analyses. We followed the guidelines for writing a narrative review previously published. We also added other papers suggested by co-authors, in order to improve the quality of the review.
Diet, sleep and physical exercise: general aspects
Diet, exercise and sleep are three pillars of a healthy life. While improving just one of these lifestyle factors can help people in reaching longer lives by reducing cardiovascular risk, onset of malignancy and improving quality of life, several recent studies have suggested that improving all three may be a better way to improve physical and mental health.9
The WHO (https://www.who.int/news-room/fact-sheets/detail/healthy-diet) states that all adults should aim for a healthy, balanced diet containing fruits, vegetables, nuts and whole grains, and limited free sugar, fat and salt. Furthermore, physical activity is fundamental to obtain good performance and to prevent cardiovascular disease and obesity. These messages should be applied also for patients suffering from rheumatic disease including PsA. Although, in these diseases, there is a lack of consensus regarding which lifestyle modifications can meaningfully modify disease activity, general aspects such as pain and fatigue, or disease progression. Recently a European Alliance of Associations for Rheumatology (EULAR) task force provided recommendations covering a range of lifestyle behaviours in which, overall, diet modification and exercise can be beneficial for many health outcomes.8
Nutritional and diet aspects in PsA
PsA is frequently associated with different comorbidities.2 Evidence demonstrated an increased prevalence of cardiovascular diseases, mainly linked to chronic inflammation. Risk factors for cardiovascular diseases (hypertension, diabetes mellitus, hyperlipidaemia and obesity) are also increased in PsA when compared with those affected by other inflammatory joint diseases and psoriasis alone. Finally, metabolic syndrome (MetS) is quite common in PsA and represents a challenge for the management of patients.2
Adiposity and higher body mass index (BMI) were also associated with an increased risk of developing PsA and several lines of evidence suggest that adipose tissue has a central role in the pathogenesis of PsA through the induction of a low-level inflammatory state, in which adipokines and other cytokines produced by adipose cells are dysregulated.10 In particular, overweight and obesity are known risk factors for the developing of PsA.11
Of note, in a recent study on 90 189 incident cases of psoriasis (42% male, mean age 51 years), of whom 1409 had a subsequent PsA diagnosis, increased BMI (over 25 kg/m2) was significantly associated with an increased risk of developing PsA compared with normal weight. Furthermore, reducing BMI over a 10-year period was associated with a reduction in the risk of developing PsA compared with BMI remaining constant over the same period.12 This is an interesting indirect piece of evidence about the role of factors (including diet and physical exercise) which could be used as primary prevention for PsA.
Together with the evidence of a reduction of risk of PsA in overweight individuals, exercise may provide benefits for those patients with MetS and psoriasis.
Effectiveness of specific diet regimens in PsA
Different studies were published on the effect of some diet regimens on disease activity, pain, function and quality of life in patients with inflammatory arthritis and osteoarthritis. Most of the studies are focused on Rheumatoid Arthritis (RA) but some reports are available for patients with PsA and, in general, for spondyloarthritis (SpA).12
The rationale to use specific diet regimens such us hypocaloric diet, Mediterranean diet and ketogenic diet is based on the potential anti-inflammatory effect, with a reduction of expression of cytokines such us IL-1 and Il-6, as seen in animal models of inflammatory arthritis. Furthermore, anti-inflammatory capacities of Mediterranean diet are attributed not only to its favourable lipid profile, with oleic acid being its major fraction (55%–83%), but also to its minor compounds, with the phenols oleuropein and hydroxytyrosol, which are present in many compounds.13 Indirect evidence coming from in vitro studies on inflammatory disease and suggest that 3 mM oleuropein induces reduced production of TNF-α, IL-1β, IL-17 and COX-2 in cells of patients with ulcerative colitis.14
In PsA, Di Minno et al previously showed that a condition of minimal disease activity was more often achieved by PsA patients starting anti-TNF subjected to hypocaloric diet regimen than PsA patients in free diet regimen (HR 1.85, 95% CI 1.019 to 3.345, p=0.043). The study also showed that, regardless of the type of diet, after 6 months of treatment with TNFα blockers, ≥5% of weight loss was a predictor of the achievement of minimal disease activity.15
An interesting paper was recently published on the effect of very low energy diet on disease activity in PsA. In this study, obese PsA patients with BMI>33 kg/m2 were asked to follow a very low energy diet with a daily intake of 640 kcal, including recommended doses of vitamins, minerals and other essential nutrients. The diet consisted of four daily portions of powder dissolved in cold or hot water and consumed as shakes or soups. At 6 months, significant improvement of disease activity in joints, entheses and skin was observed. Furthermore, the percentage of patients achieving the minimal disease activity increased from 29% (baseline) to 54% (6 months follow-up). The diet protocol was safe, well tolerated and had a significant impact even on articular function and quality of life, demonstrating how a diet change may be important in the management of PsA patients with obesity.16
Caso et al recently showed, in a cohort of 211 PsA patients, how higher levels of disease activity, as measured by disease activity score for PsA, correlates with low adherence to Mediterranean diet, indirectly suggesting that following Mediterranean diet may be beneficial on disease activity.17
A recent systematic review was published on the effectiveness of low inflammatory diet on adults with arthritis. Authors examined seven studies with different interventions (from Mediterranean diet to fish oil and strawberry powder integration). In comparison to a usual diet, very low-quality evidence suggested that a low-inflammatory diet was associated with more weight loss, lower inflammation, improved physical function measures (only for patients with RA) and reduced joint pain. However, the heterogeneity of study intervention and the poor quality of evidence and the absence of trials in PsA do not permit to come up with strong conclusions.18
No formal studies have been conducted on the effects of ketogenic diet in PsA. This particular diet regimens may have an effect on systemic inflammation by reducing insulin production, by the increasing in beta-hydroxybutyrate and glucagon production. This peculiar effect of ketogenic diet may further lead to a decrease of several proinflammatory cytokines and weight loss, with potential beneficial effects. Of note, it has been showed that beta-hydroxybutyrate also induces the production of IL-10, resulting in an inhibitory effect on Th17 cells that are the main effector cells in the pathogenesis of psoriatic disease.19
Some interesting information is coming from a study on PsA patients during the Ramadan fasting.20 In this work, authors demonstrated that intermittent fasting improved the clinical manifestations of PsA, including PsA disease activity scores, enthesitis and dactylitis. Furthermore, the patients’ improvement was independent of changes in the patients’ weight.20
Ketogenic diet might be associated with improvement of cardiovascular risk factors, mainly driven by weight loss but further studies are needed to evaluate the long-term effects on cardiovascular outcomes and also to assess which is the optimal macronutrients composition.
Dietary supplementation and prebiotic in PsA
Prebiotics are foods (typically high-fibre foods) that regulate human microflora. Prebiotics are used with the intention of improving the balance of these micro-organisms. The role of diet supplementation with prebiotics or other molecules in terms of their potential effects on disease activity, was further evaluated: Kristensen et al demonstrated, in a randomised double-blind, placebo-controlled trial, that PsA patients receiving n-3 polyunsaturated fatty acids (PUFAs) showed a significant reduction in non-steroidal anti-inflammatory drug and paracetamol use compared with controls after 24 months. Moreover, a trend to a reduction of disease activity in terms of tender joints, enthesitis and skin involvement was also observed, although not significantly in comparison to placebo. Interestingly, the use of PUFAs significantly reduced the serum levels of leukotriene B4, which is a known mediator of inflammation.21 The same authors also showed a significant decrease of hearth rate in PUFAs supplemented patients compared with controls, although blood pressure, pulse wave velocity and central blood pressure did not change after supplementation with PUFAs.22
Lassus et al performed an interventional uncontrolled study on 80 plaque psoriasis (PsO) patients, of which 34 had PsA, with a medication containing 1.122 mg/day eicosapentaenoic acidethyl-ester (EPA) and 756 mg/day docosahexaenoic acid-ethyl-ester in capsules.23 This study showed a statistically significant reduction of severity of the psoriasis, measured by the Psoriasis Area and Severity Index (PASI) (PASI change: 3.56–1.98 after 8 weeks). In PsA, improvement was recorded by the percentages of patients reporting severe/moderate/mild/no pain, observing that the proportion of patients complaining of severe pain decreased after treatment.23 Veale et al, in 1994, examined in a small trial the potential benefits of Efamol marine, a compound of evening primrose oil and fish oil (rich in omega-3 and omega-6 fatty acids), in 38 patients with PsA.24 They found no effect on parameters of skin or joint disease activity, nor in the NSAIDs supply for PsA patients. However, they noted a decrease in serum leukotrienes and tromboxanes, consistent with an anti-inflammatory effect. The authors speculated, therefore, that the lower dose of EPA in their compound (240 mg daily) compared with previous studies could be the cause for the lack of clinical effect.24
Probiotic supplements in PsA
Probiotics are defined as a foods or supplements that contain live microorganisms intended to maintain or improve the normal bacterial microflora in the body. The vast majority of evidence on the use of probiotics coming from inflammatory bowel disease and RA studies, in which some data suggest a possible role in reducing disease activity and even disability in both conditions.25 One randomised double-blind study was performed in psoriasis patients and evaluated a combined probiotic mixture of Bifidobacterium longum, Bifidobacterium lactis and Lactobacillus rhamnosus. At 12-week follow-up, 66.7% of patients in the probiotic group and 41.9% in the placebo group showed a reduction in PASI of up to 75% (p<0.05). A clinically relevant difference was observed in Physician Global Assessment index: 48.9% in the probiotic group achieved a score of 0 or 1 (representative of remission), compared with 30.2% in the placebo group.26 Other studies using Streptococcus salivarius K-12 for 24 months27 seem to confirm these data.
In PsA, a small open-label pilot study in 10 patients with stable disease showed a reduction of disease activity index and faecal level of calprotectine and zonulin after receiving 3 g of probiotic containing corn starch, maltodextrin, fructo-oligosaccharide P6, inulin P2, vegetable protein and bacterial strains of Lactobacillus and Bifidobacterium. However, the effect of supplementation was not long-lasting.28
On the other hand, Grinnell et al did not find statistical differences in health outcomes after probiotic use in a cohort of PsA patients attending The National Databank for Rheumatic Diseases.29
Furthermore, Jenks et al performed a monocentric double-blind randomised controlled trial that investigated the effect of a 12-week course of oral probiotics on disease activity, fatigue, quality of life and intestinal symptoms in SpA patients. All patients had active disease; the majority was classified as ankylosing spondylitis (49/63). The primary outcome was BASFI, but various other patient-reported outcomes were assessed at 12 weeks. No significant differences between the placebo and probiotics arms were observed. Laboratory assessment (C reactive protein and faecal calprotectin) displayed no changes as well.30
Data on the possible effect of probiotics are, therefore, still contradictory since the lack of well performed randomised placebo-controlled trials with sufficient sample in this condition.
Finally, dietary recommendations for adults with psoriasis and PsA were recently published from the National Psoriasis Foundation: based on the review of literature, the authors stated that dietary interventions, when implemented, should be used in conjunction with standard medical therapies for both PsA and psoriasis. In particular, hypocaloric diet in overweight and obese patients should be implemented. Select foods, nutrients and dietary patterns may affect psoriasis but the evidence is of low quality. For patients with PsA, a week recommendation of vitamin D supplementation was proposed.31
In conclusion, different studies may suggest that the use of Mediterranean diet, low-calories intake diet and reducing body weight, an intermittent fasting and supplementation of some vitamin such as vitamin D may be associated with a reduction of disease activity and disease symptoms and to a reduction of the oxidative stressors that may be associated with disease severity.
Sleep disturbance in PsA and possible benefits of sleep quality
According to the National Sleep Foundation, most adults need at least 7–9 hours of sleep.32 Sleep deprivation increases the risk of health conditions like diabetes, heart disease and stroke.
Without enough sleep, people tend to overeat and choose unhealthy foods. Sleep deprivation affects the body’s release of ghrelin and leptin, two neurotransmitters that tell our brain when to consume calories. People who are sleep deprived are more drawn towards high-calories foods. Chronic sleep loss has been linked to having a larger waist circumference, and an increased risk of obesity.33
It has been showed that acute total sleep deprivation significantly increased cortisol levels. Moreover, circadian misalignment significantly increased plasma levels of C reactive protein and of both pro-inflammatory (TNF-α) and anti-inflammatory (IL-10) cytokines in healthy subjects.34 Sleep deprivation and loss of sleep also result in an activation of the immune system, and, in inflammatory chronic disease such as inflammatory bowel disease, sleep disturbance has been associated with an increase in proinflammatory cytokines such as IL-6, IL-17 and IL-23.35
Sleep disturbance is an important feature of PsA and may be present even in patient with only psoriasis. In a previous survey, 49.5% out of 420 PsA patients indicated psoriasis interfered with sleep at least once per month.36 Moreover 11.3% of survey respondents showed sleep impairment for more than half the month.
In the literature, sleep impairment has been reported to be more severe in PsA patients than in patients with only skin involvement; in fact, in a Nordic survey, sleep disturbances were reported by 16% of patients with psoriasis but by 45% of patients with PsA.37 Furthermore, musculoskeletal involvement in PsA, mainly expressed as pain, was independently associated with increased sleep disturbances and fatigue.38
In another study, Wong et al reported that the prevalence of poor sleep quality was 84%, 69% and 50% in PsA, psoriasis and controls, respectively. Total Pittsburgh Sleep Quality Index (PSQI) was higher in both patients with PsA and patients with psoriasis alone compared with controls (p<0.01) and higher in patients with PsA compared with patients with psoriasis (p<0.0001). EQ-5D anxiety component, EQ-5D final and FACIT-fatigue were independently associated with worse PSQI in patients with psoriasis and those with PsA. Actively inflamed (tender or swollen) joints are independently associated with worse PSQI in patients with PsA, with poor sleep associated with active joint inflammation.39
Although the evidence in literature potentially showed a link between sleep disturbance/quality and disease activity in different chronic inflammatory conditions, very few studies reported the effect of sleep disturbance on disease activity in patients with PsA. A recent prospective study was published on the effect of jet-lag in patients with both psoriasis and PsA. Of the 70 psoriasis patients enrolled 20 had PsA. Authors showed that, after a flight crossing a minimum of 2 time zones and jet-lag (defined as insomnia or sleepiness, general malaise, diurnal dysfunction and/or somatic symptoms 1–2 days after the trip) a significant worsening in different parameters such as Dermatology Life Quality Index and disease activity on Visual Analogue Scale was found.40
Moreover, a previous report suggests that risk of developing psoriasis is associated to night working shifts and poor sleep quality and it has been supposed that it could be partly due to disrupted circadian rhythm, decreased melatonin synthesis, vitamin D deficiency and behavioural risk factors.41 However, no formal studies on the association of sleep disturbance with the risk of developing PsA were published.
Physical activity and PsA
Physical activity and exercise are probably the most studied factors in PsA.
In a recent survey (HUNT study), conducted in Norway, lower levels of physical activity were associated with a slightly higher risk to develop PsA than the highest physical activity level, with physical activity performed at high levels that demonstrated to modify the risk of developing PsA in overweight/obese individuals.42 A narrative review outlined the role of physical activity, physical therapy and exercise in PsA, tacking in consideration general principle of exercise and showing potential capacity to decrease musculoskeletal pain.43
Exercise for the management of inflammatory arthritis is recommended in clinical guidelines to manage symptoms, to reduce disability and for the prevention and treatment of comorbidities. In this context, the EULAR recently published recommendations for physical activity, specifically focused on people with inflammatory arthritis, including PsA.44 In particular, four domains (cardiorespiratory fitness, muscle strength, flexibility and neuromotor performance) were considered to be applicable to people with inflammatory arthritis, including PsA.44 More important, physical activity showed to be feasible and safe.
Other International associations, such as the Canadian Spondylitis Association (https://spondylitis.ca/wp-content/uploads/2018/10/2018-PSA), recommend, for PsA, physical exercise (≥150 min of moderate intensity aerobic physical activity/week) muscle strengthening exercise, stretching exercise, heat or cold applications and kinesiotherapy to maintain range of motion.
To reinforce this concept, a recent systematic review was published on the possible effect of cardiorespiratory and strength exercise on disease activity in patients with inflammatory diseases. It shows high to moderate quality evidence for a small beneficial effect on disease activity scores and joint damage, including beneficial effects on erythrocyte sedimentation rate and on articular symptoms.45
Another recent systematic review focused on the role of physical activity in PsA.46 In particular, Thomsen et al42 assessed the impact of high-intensity interval training (HIIT) on disease activity and disease perception in patients with PsA. Using a standardised exercise protocol, the authors showed that fatigue improved significantly and there was no worsening in most of the outcome variables during the study follow-up, suggesting the safety of exercise programmes. However, no clear benefits of the HIIT were demonstrated on disease activity and pain.43 46
In the same review, it was also reported the efficacy of a resistance exercise programme for upper limbs, lower limbs and trunk, evaluated in a randomised controlled trial in patients with PsA.46
The study showed that resistance exercises were able to improve functional capacity and quality of life and to reduce axial disease activity, with beneficial effect on pain. However, these improvements were not coupled to the improvement of muscle strength and, unfortunately, authors did not find a significant reduction of peripheral disease activity measured by the Disease Activity Score on 28 joints.43 46 Finally, the effects of strength and aerobic exercise on disease activity in both patients with PsA and RA was also reviewed in the same article.46 Biking, walking or swimming seemed to reduce articular symptoms and sedimentation rate at 6 months of follow-up, although the results were not statistically significant.
Axial disease in PsA (axPsA) showed some similarities and differences with ankylosing spondylitis and is an interesting field of active research. Although few studies are available on the evidences for the effectiveness of exercise programmes in the management of axPsA, evidence mainly coming from axial SpA, in which the cornerstone of non-pharmacological management is regular exercise and, in a broader view, this concept could also be valid for the axPsA. Physical exercise in axSpA has been shown to reduce disease activity, pain and stiffness and improve physical functioning, chest expansion, spinal mobility and cardiorespiratory performance in patients. It also has the potential to reduce depressive symptoms.47
In axSpA, exercise a key component of disease management as suggested by the recent ASAS/EULAR recommendations.48 This evidence may be also applied to axPsA patients, in which the burden of disease is similar.
In this context, beyond the pharmacological treatment, exercise programmes may play an important role in lowering not only the cardiovascular risk in PsA, in which subclinical atherosclerosis may be present, but even the risk to develop the disease. In addition, a recent review showed how exercise programmes and dietary improves waist circumference, blood pressure, triglycerides and fasting glucose in subjects with MetS. Furthermore, a recent study showed how weight loss could be associated with the improvement of disease activity in PsA.49 Thus, as stated by the EULAR recommendations, physical activity is useful not only to reduce the burden of disease but even to treat cardiometabolic comorbidities.
An intriguing topic in PsA is the effect of exercise in inducing mechanical stress on joint and enthesis, potentially leading to a worsening of disease.50 However, this aspect was indirectly studied only in few works and revealed conflicting data.
Conclusion
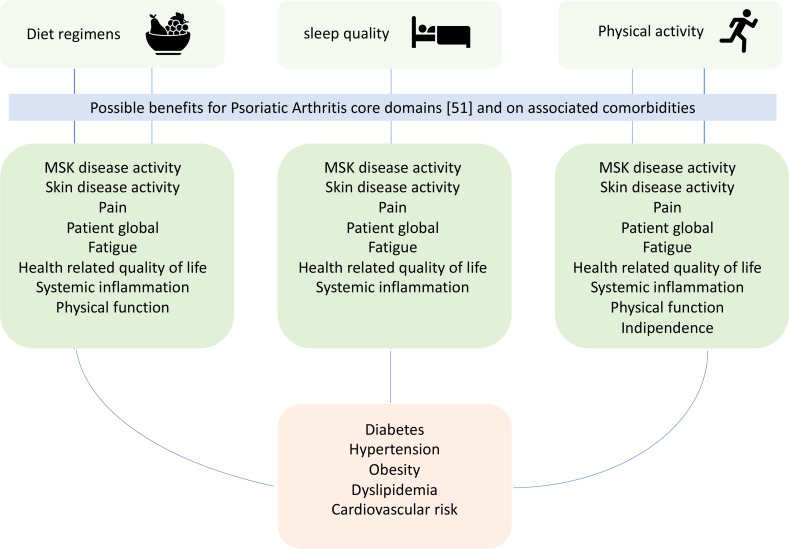
Increasing evidence is now available on the potential beneficial effects of physical activity and change in diet regimen in PsA patients and different studies suggest poor sleep quality potentially leading to worse outcome in this disease. Potentially, these three pillars of healthy life may ameliorate almost all domains important to be measured in PsA, endorsed by the Outcome Measures in Rheumatology51 (figure 1). In particular, as discussed above, physical activity may reduce both articular and extra-articular signs and symptoms and even systemic inflammation. Furthermore, it could improve pain and fatigue with a positive impact on articular function and quality of life. For what concern diet changes, some studies showed an impact on both musculo-skeletal and skin disease activity, leading to an improvement of function and quality of life and further studies may increase our knowledge on the potential link between sleep quality, disease activity, systemic inflammation, pain and fatigue in PsA. Furthermore, all these three elements have a fundamental and known role in the management of PsA comorbidities. However, data from literature are still scarce and high-quality evidences coming from randomised clinical trials with rigorous methodology should be performed in order to evaluate the impact of non-pharmacological interventions in this multifaceted disease. Nevertheless, patients with PsA with impaired function and quality of life or with comorbidities such as obesity, cardiovascular diseases or cardiovascular risk factors may have further benefits from diet changes and for structured physical activity as suggested by recommendations recently published by international societies. Finally, these approaches may represent a ‘triple jump’ for the optimal management in our outpatient clinics and also at patient level by increasing patient’s awareness and education.
Figure 1.
Possible impact of diet regimens, physical activity and sleep quality on patients with psoriatic arthritis.
Footnotes
Contributors: All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: Leuven Research and Development, the technology transfer office of KU Leuven, has received consultancy and speaker fees and research grants on behalf of R.J.L. from Abbvie, Boehringer-Ingelheim, Amgen (formerly Celgene), Eli-Lilly, Galapagos, Janssen, Fresenius Kabi, MSD, Novartis, Pfizer, Biosplice Therapeutics (formerly Samumed), UCB and Viatris.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Lubrano E, Scriffignano S, Perrotta FM. Psoriatic arthritis, Psoriatic disease, or Psoriatic syndrome J Rheumatol 2019;46:1428–30. 10.3899/jrheum.190054 [DOI] [PubMed] [Google Scholar]
- 2.Lubrano E, Scriffignano S, Perrotta FM. Multimorbidity and Comorbidity in Psoriatic arthritis - a perspective. Expert Review of Clinical Immunology 2020;16:963–72. 10.1080/1744666X.2021.1825941 [DOI] [PubMed] [Google Scholar]
- 3.Hagège B, Tan E, Gayraud M, et al. Remission and low disease activity in Psoriatic arthritis publications: a systematic literature review with meta-analysis. Rheumatology (Oxford) 2020;59:1818–25. 10.1093/rheumatology/keaa030 [DOI] [PubMed] [Google Scholar]
- 4.Perrotta FM, Scriffignano S, Ciccia F, et al. “Clinical characteristics of potential "difficult-to-treat" patients with Psoriatic arthritis: A retrospective analysis of a longitudinal cohort”. Rheumatol Ther 2022;9:1193–201. 10.1007/s40744-022-00461-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lubrano E, Scriffignano S, Perrotta FM. Residual disease activity and associated factors in Psoriatic arthritis. J Rheumatol 2020;47:1490–5. 10.3899/jrheum.190679 [DOI] [PubMed] [Google Scholar]
- 6.Coates LC, de Wit M, Buchanan-Hughes A, et al. Residual disease associated with suboptimal treatment response in patients with Psoriatic arthritis: A systematic review of real-world evidence. Rheumatol Ther 2022;9:803–21. 10.1007/s40744-022-00443-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogdie A, de Wit M, Callis Duffin K, et al. Defining outcome measures for Psoriatic arthritis: A report from the GRAPPA-OMERACT working group. J Rheumatol 2017;44:697–700. 10.3899/jrheum.170150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gwinnutt JM, Wieczorek M, Balanescu A, et al. 2021 EULAR recommendations regarding lifestyle behaviours and work participation to prevent progression of rheumatic and musculoskeletal diseases. Ann Rheum Dis 2023;82:48–56. 10.1136/annrheumdis-2021-222020 [DOI] [PubMed] [Google Scholar]
- 9.Briguglio M, Vitale JA, Galentino R, et al. Healthy eating, physical activity, and sleep hygiene (HEPAS) as the winning Triad for sustaining physical and mental health in patients at risk for or with neuropsychiatric disorders: considerations for clinical practice. Neuropsychiatr Dis Treat 2020;16:55–70. 10.2147/NDT.S229206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toussirot E, Aubin F, Dumoulin G. Relationships between Adipose tissue and psoriasis, with or without arthritis. Front Immunol 2014;5:368. 10.3389/fimmu.2014.00368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green A, Shaddick G, Charlton R, et al. Modifiable risk factors and the development of Psoriatic arthritis in people with psoriasis. Br J Dermatol 2020;182:714–20. 10.1111/bjd.18227 Available: https://onlinelibrary.wiley.com/toc/13652133/182/3 [DOI] [PubMed] [Google Scholar]
- 12.Ortolan A, Felicetti M, Lorenzin M, et al. The impact of diet on disease activity in Spondyloarthritis: A systematic literature review. Joint Bone Spine 2023;90:105476. 10.1016/j.jbspin.2022.105476 [DOI] [PubMed] [Google Scholar]
- 13.Katsimbri P, Korakas E, Kountouri A, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and Psoriatic arthritis phenotype. Antioxidants (Basel) 2021;10:157. 10.3390/antiox10020157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larussa T, Oliverio M, Suraci E, et al. Oleuropein decreases Cyclooxygenase-2 and Interleukin-17 expression and attenuates inflammatory damage in Colonic samples from ulcerative colitis patients. Nutrients 2017;9:391. 10.3390/nu9040391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Minno MND, Peluso R, Iervolino S, et al. Weight loss and achievement of minimal disease activity in patients with Psoriatic arthritis starting treatment with tumour necrosis factor alpha blockers. Ann Rheum Dis 2014;73:1157–62. 10.1136/annrheumdis-2012-202812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klingberg E, Bilberg A, Björkman S, et al. Weight loss improves disease activity in patients with Psoriatic arthritis and obesity: an Interventional study. Arthritis Res Ther 2019;21:17. 10.1186/s13075-019-1810-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caso F, Navarini L, Carubbi F, et al. Mediterranean diet and Psoriatic arthritis activity: a multicenter cross-sectional study. Rheumatol Int 2020;40:951–8. 10.1007/s00296-019-04458-7 [DOI] [PubMed] [Google Scholar]
- 18.Genel F, Kale M, Pavlovic N, et al. Health effects of a low-inflammatory diet in adults with arthritis: a systematic review and meta-analysis. J Nutr Sci 2020;9:e37. 10.1017/jns.2020.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciaffi J, Mitselman D, Mancarella L, et al. The effect of Ketogenic diet on inflammatory arthritis and cardiovascular health in rheumatic conditions: A mini review. Front Med (Lausanne) 2021;8:792846. 10.3389/fmed.2021.792846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adawi M, Damiani G, Bragazzi NL, et al. The impact of intermittent fasting (Ramadan fasting) on Psoriatic arthritis disease activity, Enthesitis, and Dactylitis: a Multicentre study. Nutrients 2019;11:601. 10.3390/nu11030601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristensen S, Schmidt EB, Schlemmer A, et al. Beneficial effect of N-3 polyunsaturated fatty acids on inflammation and analgesic use in Psoriatic arthritis: a randomized, double blind, placebo-controlled trial. Scand J Rheumatol 2018;47:27–36. 10.1080/03009742.2017.1287304 [DOI] [PubMed] [Google Scholar]
- 22.Kristensen S, Schmidt EB, Schlemmer A, et al. The effect of Marine N-3 polyunsaturated fatty acids on cardiac autonomic and hemodynamic function in patients with Psoriatic arthritis: a randomised, double-blind, placebo-controlled trial. Lipids Health Dis 2016;15:216. 10.1186/s12944-016-0382-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassus A, Dahlgren AL, Halpern MJ, et al. Effects of dietary supplementation with polyunsaturated ethyl ester lipids (Angiosan) in patients with psoriasis and Psoriatic arthritis. J Int Med Res 1990;18:68–73. 10.1177/030006059001800109 [DOI] [PubMed] [Google Scholar]
- 24.Veale DJ, Torley HI, Richards IM, et al. A double-blind placebo-controlled trial of Efamol marine on skin and joint symptoms of Psoriatic arthritis. Br J Rheumatol 1994;33:954–8. 10.1093/rheumatology/33.10.954 [DOI] [PubMed] [Google Scholar]
- 25.Jadhav P, Jiang Y, Jarr K, et al. Efficacy of dietary supplements in inflammatory bowel disease and related autoimmune diseases. Nutrients 2020;12:2156. 10.3390/nu12072156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navarro-López V, Martínez-Andrés A, Ramírez-Boscá A, et al. Efficacy and safety of oral administration of a mixture of Probiotic strains in patients with psoriasis: A randomized controlled clinical trial. Acta Derm Venereol 2019;99:1078–84. 10.2340/00015555-3305 [DOI] [PubMed] [Google Scholar]
- 27.Zangrilli A, Diluvio L, Di Stadio A, et al. Improvement of psoriasis using oral Probiotic Streptococcus Salivarius K-12: a case–control 24-month longitudinal study. Probiotics & Antimicro Prot 2022;14:573–8. 10.1007/s12602-022-09937-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haidmayer A, Bosch P, Lackner A, et al. Effects of Probiotic strains on disease activity and Enteric permeability in Psoriatic arthritis-A pilot open-label study. Nutrients 2020;12:2337. 10.3390/nu12082337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grinnell M, Ogdie A, Wipfler K, et al. Probiotic use and Psoriatic arthritis disease activity. ACR Open Rheumatol 2020;2:330–4. 10.1002/acr2.11143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenks K, Stebbings S, Burton J, et al. Probiotic therapy for the treatment of Spondyloarthritis: a randomized controlled trial. J Rheumatol 2010;37:2118–25. 10.3899/jrheum.100193 [DOI] [PubMed] [Google Scholar]
- 31.Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or Psoriatic arthritis from the medical board of the National psoriasis foundation: A systematic review. JAMA Dermatol 2018;154:934–50. 10.1001/jamadermatol.2018.1412 [DOI] [PubMed] [Google Scholar]
- 32.Hirshkowitz M, Whiton K, Albert SM, et al. National sleep foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 2015;1:40–3. 10.1016/j.sleh.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 33.Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care 2011;14:402–12. 10.1097/MCO.0b013e3283479109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright KP, Drake AL, Frey DJ, et al. Influence of sleep deprivation and circadian misalignment on Cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun 2015;47:24–34. 10.1016/j.bbi.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sobolewska-Włodarczyk A, Włodarczyk M, Talar M, et al. The Association of the quality of sleep with proinflammatory cytokine profile in inflammatory bowel disease patients. Pharmacol Rep 2021;73:1660–9. 10.1007/s43440-021-00333-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Callis Duffin K, Wong B, Horn EJ, et al. Psoriatic arthritis is a strong Predictor of sleep interference in patients with psoriasis. J Am Acad Dermatol 2009;60:604–8. 10.1016/j.jaad.2008.10.059 [DOI] [PubMed] [Google Scholar]
- 37.Duvetorp A, Østergaard M, Skov L, et al. Quality of life and contact with Healthcare systems among patients with psoriasis and Psoriatic arthritis: results from the Nordic patient survey of psoriasis and Psoriatic. Arch Dermatol Res 2019;311:351–60. 10.1007/s00403-019-01906-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haugeberg G, Hoff M, Kavanaugh A, et al. Psoriatic arthritis: exploring the occurrence of sleep disturbances, fatigue, and depression and their correlates. Arthritis Res Ther 2020;22:198. 10.1186/s13075-020-02294-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong ITY, Chandran V, Li S, et al. Sleep disturbance in Psoriatic disease: prevalence and associated factors. J Rheumatol 2017;44:1369–74. 10.3899/jrheum.161330 [DOI] [PubMed] [Google Scholar]
- 40.Damiani G, Bragazzi NL, Garbarino S, et al. Psoriatic and Psoriatic arthritis patients with and without jet-lag: does it matter for disease severity scores? insights and implications from a pilot, prospective study. Chronobiol Int 2019;36:1733–40. 10.1080/07420528.2019.1678629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol 2013;133:565–7. 10.1038/jid.2012.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomsen RS, Nilsen TIL, Haugeberg G, et al. Adiposity and physical activity as risk factors for developing Psoriatic arthritis. longitudinal data from the HUNT study. Arthritis Care Res (Hoboken) 2021;73:432–41. 10.1002/acr.24121 [DOI] [PubMed] [Google Scholar]
- 43.Perrotta FM, Scriffignano S, Benfaremo D, et al. New insights in physical therapy and rehabilitation in Psoriatic arthritis: A review. Rheumatol Ther 2021;8:639–49. 10.1007/s40744-021-00298-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rausch Osthoff A-K, Niedermann K, Braun J, et al. EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis 2018;77:1251–60. 10.1136/annrheumdis-2018-213585 [DOI] [PubMed] [Google Scholar]
- 45.Sveaas SH, Smedslund G, Hagen KB, et al. Effect of cardiorespiratory and strength exercises on disease activity in patients with inflammatory rheumatic diseases: a systematic review and meta-analysis. Br J Sports Med 2017;51:1065–72. 10.1136/bjsports-2016-097149 [DOI] [PubMed] [Google Scholar]
- 46.Kessler J, Chouk M, Ruban T, et al. Psoriatic arthritis and physical activity: a systematic review. Clin Rheumatol 2021;40:4379–89. 10.1007/s10067-021-05739-y [DOI] [PubMed] [Google Scholar]
- 47.Millner JR, Barron JS, Beinke KM, et al. Exercise for Ankylosing Spondylitis: an evidence-based consensus statement. Semin Arthritis Rheum 2016;45:411–27. 10.1016/j.semarthrit.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 48.Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial Spondyloarthritis: 2022 update. Ann Rheum Dis 2023;82:19–34. 10.1136/ard-2022-223296 [DOI] [PubMed] [Google Scholar]
- 49.Klingberg E, Björkman S, Eliasson B, et al. Weight loss is associated with sustained improvement of disease activity and cardiovascular risk factors in patients with Psoriatic arthritis and obesity: a prospective intervention study with two years of follow-up. Arthritis Res Ther 2020;22:254. 10.1186/s13075-020-02350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perrotta FM, Lories R, Lubrano E. To move or not to move: the paradoxical effect of physical exercise in axial Spondyloarthritis. RMD Open 2021;7:e001480. 10.1136/rmdopen-2020-001480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orbai A-M, de Wit M, Mease PJ, et al. Updating the Psoriatic arthritis (PSA) core domain set: A report from the PSA workshop at OMERACT 2016. J Rheumatol 2017;44:1522–8. 10.3899/jrheum.160904 [DOI] [PMC free article] [PubMed] [Google Scholar]