Abstract
Purpose:
Cemiplimab is approved for the treatment of locally advanced basal cell carcinomas (BCC), although with mitigated results. We sought to interrogate the cellular and molecular transcriptional reprogramming underlying BCC resistance to immunotherapy.
Experimental Design:
Here, we combined spatial and single-cell transcriptomics to deconvolute the spatial heterogeneity of the tumor microenvironment in regard with response to immunotherapy, in a cohort of both naïve and resistant BCCs.
Results:
We identified subsets of intermingled cancer-associated fibroblasts (CAF) and macrophages contributing the most to CD8 T-cell exclusion and immunosuppression. Within this spatially resolved peritumoral immunosuppressive niche, CAFs and adjacent macrophages were found to display Activin A–mediated transcriptional reprogramming towards extracellular matrix remodeling, suggesting active participation to CD8 T-cell exclusion. In independent datasets of human skin cancers, Activin A–conditioned CAFs and macrophages were associated with resistance to immune checkpoint inhibitors (ICI).
Conclusions:
Altogether, our data identify the cellular and molecular plasticity of tumor microenvironment (TME) and the pivotal role of Activin A in polarizing the TME towards immune suppression and ICI resistance.
Translational Relevance.
Improving response to immunotherapy requires a better understanding of how tumors regulate their immune landscape. Until recently, technical limitations hampered the global deciphering of the various cellular components and their complex interactions within the peritumoral niche. Here we integrate single-cell RNA sequencing (RNA-seq) with spatially resolved RNA-seq to decipher the complex spatial organization and transcriptional reprogramming of the immune peritumoral landscape. We identify an Activin A–mediated concurrent polarization of carcinoma-associated fibroblasts and macrophages towards extracellular matrix remodeling, contributing to the immunosuppressive peritumoral niche. In independent single-cell RNA-seq cohorts, we highlight the association of the identified immunosuppressive niche with resistance to immune checkpoint inhibitors (ICI). Overall, by deconvoluting the heterogeneity of the tumor microenvironment in regard with immune polarization, our data identify major cellular and molecular crosstalks leading to CD8 T-cell exclusion and unresponsiveness to ICI. They open new perspectives for the development of therapeutic strategies to overcome resistance to immunotherapy.
Introduction
Immune checkpoint inhibitors (ICI) have greatly improved the survival of patients with advanced stages of various tumor types (1–6). However, a significant proportion of patients still do not respond, emphasizing the need for a deeper understanding of the reciprocal interactions between tumors and the immune system. For efficient killing, checkpoint immunotherapy must promote effective infiltration of tumor-specific cytotoxic CD8 T cells, a phenomenon that can be impeded by various resistance mechanisms. Tumor oncogenes/oncoproteins in relevant oncogenic pathways may affect CD8 T-cell infiltration (7), or PD-L1 signaling (8–11). Both genetic and epigenetic mechanisms regulate HLA expression and thereby neoantigen processing and presentation (12–14), while neoantigen expression itself may be diminished (15). Alternatively, ICI resistance may implicate dysfunctional T cells, either because of T-cell exhaustion (16, 17), absent or incomplete T-cell costimulation (18), dysfunctional IFNγ signaling by T cells (19), or impaired generation of memory T cells that promote durable responses (20, 21). In addition, tumor–T-cell interactions are modeled by the architecture of the tumor microenvironment (TME), which encompasses a number of bone marrow–derived immune cells, endothelial cells, pericytes, fibroblasts, and extracellular matrix components (22). As a result of complex and heterogeneous cellular interactions within the TME, tumors are polarized towards either a “immune-hot” phenotype, characterized by high numbers of intratumoral cytotoxic CD8 T cells, and responsiveness to ICI, or “immune-cold” and “immune-excluded” phenotypes, which typically display low or no intratumoral cytotoxic CD8 T cells and resistance to ICI (23, 24). Further understanding of this phenomenon of immune privilege may provide biomarkers to predict responsiveness to ICI and novel targets to optimize efficacy of ICI using combinatorial therapies (24).
Basal cell carcinomas (BCC) are the most frequent type of cancer in immunocompetent patients. Given their very high tumor mutational burden (TMB; ref. 25), one would expect excellent response to ICI. On the contrary, BCCs display low cytotoxic T-cell infiltration, and only partial response to ICI, which appears to be unrelated to TMB and MHC class I expression (26). These findings suggest that BCCs may escape the immune attack by immunosuppressive reprogramming of the TME. Interestingly, BCCs display a wide spectrum of histopathological TME organization, reflecting plasticity at the transcriptional level (27–29). Deciphering the peritumoral heterogeneity with respect to the immune polarization offers a unique possibility to understand the mechanisms of TME-mediated ICI resistance.
Here, by using spatial and single-cell RNA sequencing (scRNA-seq) in human BCCs, we deconvoluted for cell type composition the transcriptomes of spatially resolved immunoreactive and immunosuppressive TME, associated with ICI response or resistance respectively. By doing so, we identified an immunosuppressive niche devoid of CD8 cytotoxic T cells, enriched in cancer-associated fibroblasts (CAF) and macrophages concurrently rewired towards extracellular matrix (ECM) remodeling and shared by multiple skin cancer types. Activin A was found to be a pivotal mediator of this immunosuppressive TME polarization and a marker for ICI resistance in advanced skin cancers.
Materials and Methods
Human samples
Studies were approved by the institutional review board of Lausanne University Hospital CHUV, and the local ethics committee, in accordance with the Helsinki Declaration (CER-VD 2020–02204). Written informed consent was obtained from each patient. Histological diagnoses were obtained from an independent dermatopathologist. Comprehensive information on age, diagnosis, tumor localization, previous treatment, and immunosuppression are listed in Supplementary Fig. S1A. Patient ICI response was classified according to the RECIST guidelines (30) into responder and nonresponders (Supplementary Tables S6 and S7).
GeoMx digital spatial profiling analysis
Previously obtained digital count conversion (DCC) files were imported into the GeoMx DSP instrument for QC and data analyses using GeoMx DSP analysis suite version 2.2.0.111 (Nanostring; RRID:SCR_023424). As previously described (27), a minimum of 10,000 reads were required for each sample. Probes were checked for outlier status by implementing a global Grubb outlier test with alpha set to 0.01. The counts for all remaining probes for a given target were then collapsed into a single metric by taking the geometric mean of probe counts. For each sample, an RNA-probe-pool–specific negative probe normalization factor was generated on the basis of the geometric mean of negative probes in each pool. To ensure good data quality, we calculated the 75th percentile of the gene counts (i.e., geometric mean across all non-outlier probes for a given gene) for each AOI, and normalized to the geometric mean of the 75th percentile across all AOIs to give the upper quartile (Q3) normalization factors for each AOI. The distribution of these Q3 normalization factors were then checked for outliers. Differentially expressed genes (DEG) and principal component analysis (PCA) analyses were performed on the GeoMx DSP instrument software.
scRNA-seq processing and analysis
FASTQ raw sequencing data were processed as previously described (27) using the count function of the Cell Ranger pipeline (v.5.0.1, 10x Genomics; https://support.10xgenomics.com/single-cell-gene-expression/software/downloads/5.0; RRID:SCR_017344). Briefly, the count function allowed us to demultiplex sequencing reads to individual cells, to align the reads to the human GRCh38 genome reference (refdata-gex-GRCh38–2020-A, https://support.10xgenomics.com/single-cell-gene-expression/software/release-notes/build#GRCh38_2020A), and to generate filtered matrices of gene counts by cell barcodes. Filtered gene by cell barcode counts matrices were imported into R (v. 4.2.1, https://www.R-project.org/) for further analyses using the Seurat (31) package (v. 4.2.1; RRID:SCR_007322). We first filtered the cells to only retain cells that had between 500 and 6,000 detected genes, less than 20% of reads mapping to mitochondrial genes, and less than 20% of reads mapping to dissociation protocol-related genes (32). The single cells of each sample were first processed separately: raw counts were normalized using the NormalizeData function using the “LogNormalize” method and a scale factor of 10,000. The top 2,000 variable genes were selected using the FindVariableFeatures function with the “vst” selection method. For visualization purposes, the five samples were integrated by using the FindIntegrationAnchors and IntegrateData functions with default parameters. Dimensionality reduction was performed by first scaling and centering the integrated data, running a PCA, followed by a Uniform Manifold Approximation and Projection (UMAP) dimensional reduction on 25 principal components. Finally, cells were clustered using the sheared nearest neighbor (SNN) modularity optimization based clustering algorithm implemented in the FindNeighbors and FindClusters functions, with 25 principal components and a resolution parameter of 0.8. The expression level of canonical marker genes (33–35) was used to identify the biological cell types present in each cluster of the total population.
Cell clusters expressing unexpected marker combinations (e.g., KRT14 and CD3E) were considered as doublets and manually removed. To assess the degree of sample mixing performed by the dataset integration, the Local Inverse Simpson's Index (LISI) score was calculated using the published code (36) and the lisi (v.1.0) package.
When subclustering, the same analysis method as described above was applied, including dimensionality reduction on 25 principal components and clustering, with resolution parameters of 0.5, 1.9, and 0.6 for Fibroblasts, Immune cells, and Myeloid cells, respectively. Subclusters with abnormal distribution of detected genes or abnormal distribution of mitochondrial/ribosomal gene percentages were excluded for further analysis. Published datasets were analyzed following the authors'instructions, using our own subtype classification (e.g., Tumor cells, Melanomas, Epithelial cells, Fibroblasts, Endothelial cells, Pericytes, Melanocytes, T cells, Myeloid cells, and B cells).
Overrepresentation analysis of Gene Ontology biological process gene sets (37) was performed using the gseGO function implemented in the gprofiler2 (v.0.2.1; RRID:SCR_018190) package.
Using the AddModuleScore function implemented in the Seurat package, we calculated the average expression per cell of genes belonging to several gene signatures. This function calculates the average expression of the genes in a signature, subtracted by the average expression of control genes that are selected at random among genes with similar expression level as the genes included in the signature of interest.
Trajectory and pseudotime analysis
Pseudotime calculations were performed as previously described (27) using the R package Monocle 3 (v.1.2.9; RRID:SCR_018685; ref. 38). We first imported the Myeloid cells Seurat object and adapted it to obtain a Monocle3 object using the new_cell_data_set function. Then, we calculated 100 principal components using the preprocess_cds function of Monocle 3 package. Next, a UMAP was generated with the reduce_dimension (default parameters), and cells were clustered using the cluster_cells function, with a resolution parameter of 0.009. Then, UMAP embeddings and clustering at resolution 0.6 previously calculated using Seurat were transferred to the Monocle3 object. Myeloid cells were subjected to trajectory analysis using the learn_graph function with default parameters except for use_partition = FALSE. A pseudotime value was assigned to each cell using the order_cells function, selecting MC1 as root state. Branchpoint variations in expression are not indicated in graphs showing gene expression along the pseudotemporal trajectory.
Secretome analysis
The average expressions were first calculated for each Myeloid cell cluster using the AverageExpression function of the R package Seurat (v.4.2.1; RRID:SCR_007322). A heatmap showing the average expression of the human secretome genes (39) was then generated. This heatmap was subsequently reordered using generateHeatmap function of the ClustVis package (v.0.0.0.9000; RRID:SCR_017133), with default parameters, except no clustering for clustDistCols.
For the single myeloid cell secretome, a matrix of the human secretome genes expressed by each myeloid cell was first created. A heatmap showing the average expression of the human secretome genes in each myeloid cell was generated using the pheatmap package (v.1.0.12; RRID:SCR_016418), with “correlation” as clustering_distance_rows and clustering_distance_cols parameters, “average” as clustering_method parameters, and 40 row clusters.
Immunofluorescence and FISH codetection
Formalin-fixed, paraffin-embedded (FFPE) skin blocks were cut and 5-μm sections were mounted onto Superfrost Plus microscope slides. FFPE skin sections were heated 10 minutes at 60°C. Slides were deparaffinized in 2 xylene baths of 3 minutes, then rehydrated in an ethanol gradient from 100% ethanol (EtOH; 2 baths of 3 minutes), followed by 95% EtOH (3 minutes) and 70% EtOH (3 minutes).
For immunofluorescence (IF) only, slides were washed in Phosphate Buffered Saline 1× (PBS: Bichsel, 100 0 324). Antigen retrieval was done in Retriever with EDTA pH9 buffer at 100°C for 20 minutes, followed by cooling for 20 minutes at room temperature. Slides were then washed three times for 5 minutes in PBS 1×. Each section was permeabilized with Triton-X 100 0.012% in PBS for 30 seconds. After washes in PBS 1×, costaining with primary antibodies anti-FAP (Abcam, ab207178, clone EPR20021, RRID:AB_2864720) at 1:100 and anti-CD8 (DAKO, M7103, clone C8/144B, RRID:AB_2075537) at 1:50 or anti-FAP and anti-CD163 (Diagnostic BioSystems, Mob460–05, clone 10D6, RRID:AB_1792051) at 1:100 in Antibody Diluent DAKO REAL (DAKO, S2022) was performed for 2 hours at room temperature. Slides were then washed and stained with secondary antibodies goat F(ab')2 fragment IgG (H+L) anti-mouse Alexa Fluor 488 antibody (Life Technologies, A11017, RRID:AB_143160) and chicken IgG (H+L) anti-rabbit Alexa Fluor 594 antibody (Life Technologies, A21442, RRID:AB_2535860) diluted 1:500 in Antibody Diluent for 1 hour at room temperature. Slides were then washed twice for 5 minutes in PBS 1× and finally mounted with mounting medium with DAPI-Aqueous Fluoroshield (Abcam, ab104139). Images were acquired with Panoramic 250 slide scanner and processed using CaseViewer 2.4 (RRID:SCR_017654) and (Fiji Is Just) ImageJ (RRID:SCR_003070) softwares.
For IF and FISH codetection, slides were then washed in distilled water. RNAscope Hydrogen Peroxide (ACD, 322335) was added to the tissue sections and slides were incubated for 10 minutes at room temperature. Slides were then washed for 5 minutes in distilled water followed by 5 minutes in PBS 1×. Antigen retrieval was done in 1× Co-detection Target Retrieval buffer (ACD, 323163) at 100°C for 15 minutes. Slides were then directly washed twice 1 minute in distilled water and 2 minutes in PBS 1× with 0.1% Tween-20 (Sigma, P1379–500 mL; PBS-T). Each tissue section was then covered with the primary antibody solution [anti-CD163, RRID:AB_1792051 and anti-FAP, RRID:AB_2864720 diluted 1:50 in Co-Detection Antibody Diluent (ACD, 323160)] and incubated overnight at 4°C in a humidified box. Following primary antibody incubation, slides were washed twice in PBS-T for 2 minutes. Slides were then fixed for 30 minutes in Buffered Zinc Formalin (Thermo Scientific, 5701ZF) at room temperature. After fixation, slides were washed four times in PBS-T for 2 minutes. Protease treatment, probe hybridization, amplifications, and stainings were performed with the RNAscope Multiplex Fluorescent Reagent Kit v2 Assay Kit (ACD, 323100) according to the manufacturer's instructions. The following RNAscope probes were used: Hs-RNASE1-C3 (556551-C3), Hs-NLRP3 (478021), Hs-INHBA-C2 (415111-C2), Hs-LAIR1-CDS (427171). Opal 570 Reagent (AKOYA Bioscience, OP-001003) and Opal 650 Reagent (AKOYA Bioscience, OP-001005) were used for fluorescence visualization. After the final in situ hybridization horseradish peroxidase (HRP)-blocker step, each tissue section was covered with the secondary antibody dilution (goat anti-mouse Alexa Fluor 488, RRID:AB_143160 and chicken anti-rabbit Alexa Fluor 594, RRID:AB_2535860 antibodies diluted 1:500 in Co-Detection Antibody Diluent) for 30 minutes at room temperature. Slides were then washed twice for 2 minutes in PBS-T. Finally, slides were mounted with mounting medium with DAPI-Aqueous Fluoroshield. The slides were acquired with Panoramic 250 slide scanner and processed using CaseViewer 2.4 (RRID:SCR_017654) and (Fiji Is Just) ImageJ (RRID:SCR_003070) softwares.
Cell culture
To induce macrophage differentiation, 4 × 10e5 low-passage Thp1, a human monocytic leukemia cell line (CSL), were seeded onto 12-well plate in RPMI GlutaMAX (Invitrogen) supplemented with 10% FBS (VWR) and 1% penicillin/streptomycin (Sigma-Aldrich), and treated with 162 nmol/L Phorbol 12-Myristate 13-Acetate (Sigma-Aldrich) and 20 ng/mL IL4 (StemCell), according to the previously published protocol (40). Follistatin (FST, Peprotech) was used at 50 ng/mL. Thp1 cells were maintained at 37°C in humidified conditions with 5% CO2 and were confirmed as negative for Mycoplasma prior to use in assays.
RNA isolation and RT-qPCR
Cells were collected in TRIZol (Life Technologies) according to the manufacturer's instructions. RNAs (1 μg) were reverse-transcribed with a qScript cDNA SuperMix (VWR) according to the manufacturer's instructions. cDNA quantifications were performed with TaqMan Fast Master Mix (Life Technologies). No template control and no reverse transcriptase enzyme sample were used as negative controls. TaqMan Gene Expression Assay (Thermo Fisher) used are: C3 (Hs00163811_m1), CXCL12 (Hs03676656_mH), INHBA (Hs01081598_m1), LAIR1 (Hs00253790_m1), SPP1 (Hs00959010_m1). Gene expression was normalized to GAPDH housekeeping gene (Hs02786624_g1) expression. In all experiments, control treatments were set at one.
Statistical analyses
Data represent results from three or more independent biological samples, unless otherwise specified. Statistical comparisons were performed using unpaired two-sided Student t test or Mann–Whitney U test, according to the variances. Correlation analyses were calculated by Spearman correlation test. The software used for statistical analyses are GraphPad Prism (version 9.1; RRID:SCR_002798) and GeoMx DSP analysis suite (version 2.2.0.111, NanoString; RRID:SCR_023424). P values are mentioned in the figures. A normal distribution was observed for all data. No technical replication was performed. No statistical method was used to predetermine sample size. No samples were excluded from the analyses. The experiments were not randomized.
Data availability
The scRNA-seq data used in this study are available in the Gene Expression Omnibus database (RRID:SCR_005012) under accession codes GSE181907 (27), GSE144239 (33), GSE115978 (41), GSE123813 (42), and GSE120575 (43). The DSP data used in this study are available in the Gene Expression Omnibus database (RRID:SCR_005012) under accession code GSE210648 (27). The following databases and datasets were used in this study: GRCh38 human reference genome reference (refdata-gex-GRCh38–2020-A, https://support.10xgenomics.com/single-cell-gene-expression/software/release-notes/build#GRCh38_2020A) and GeoMx Cancer Transcriptome Atlas (https://www.nanostring.com/products/geomx-digital-spatial-profiler/geomx-rna-assays/geomx-cancer-transcriptome- atlas/). All processed data are available from the corresponding author upon request.
Results
Spatial transcriptomics reveals immune heterogeneity of TME in human BCCs
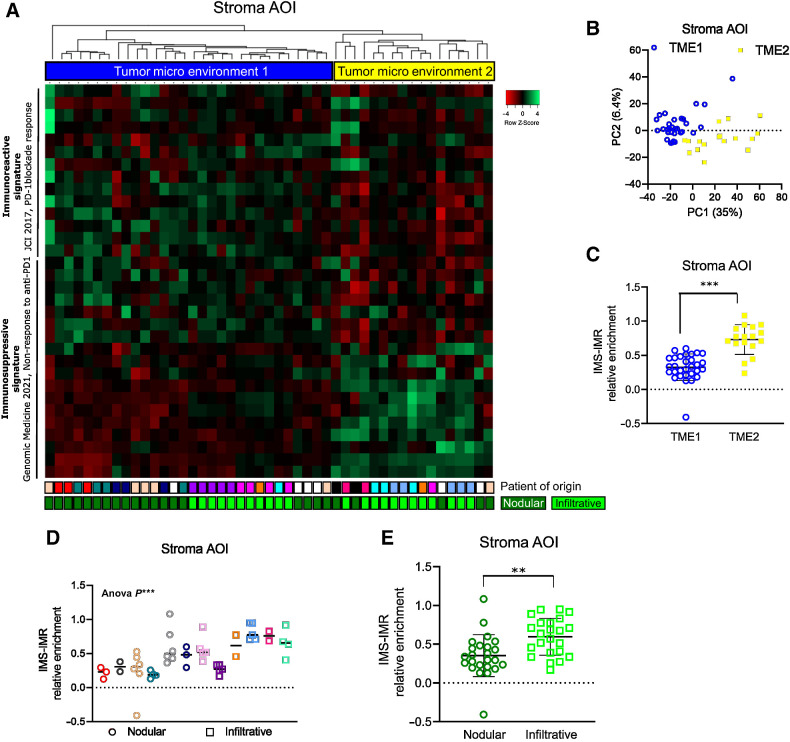
To determine how the TME defines tumor immunogenicity and response to ICI, we first assessed the immune profile of spatially-resolved TME, taking advantage of the inter- and intratumor heterogeneity of BCCs. To do so, we analyzed spatial transcriptomics previously obtained using digital spatial profiling (DSP) technology from GeoMx (NanoString; refs. 27, 44) on 47 peritumoral stromas from 12 naïve BCCs (Supplementary Fig. S1A). Peritumoral stroma areas of interest (AOI) were selected based on panCK negativity (panCKneg; Supplementary Fig. S1B), and individually sequenced using the cancer transcriptome atlas library (CTA; 1,812 genes). Individual peritumoral stroma AOIs were analyzed for the presence of either an immunoreactive signature associated with response to PD-1 blockade (IMR; ref. 45) or an immunosuppressive signature associated with non-response to PD-1 blockade (IMS; Supplementary Table S1; ref. 46). Remarkably, unsupervised clustering identified two major TME gene expression profiles, named Tumor MicroEnvironment 1 (TME1) and Tumor MicroEnvironment 2 (TME2; Fig. 1A). TME1 and TME2 profiles consistently segregated on a PCA plot (Fig. 1B), while the number of counts per AOI was not affected by the TME profile (Supplementary Fig. S1C) and TME1 and TME2 showed similar infiltration by hematopoietic CD45+ cells (Supplementary Fig. S1D). Overall, TME2 displayed a higher degree of immunosuppressive features when compared with TME1 (Fig. 1C; Supplementary Fig. S1E). To confirm these data, we used four additional immunoreactive signatures associated with increased survival and response to ICI (47–50), and one additional immunosuppressive signature, associated with reduced survival (Supplementary Table S1; ref. 50). TME1 was consistently associated with the immunoreactive signatures, whereas TME2 displayed immunosuppressive signature enrichment associated with poor clinical outcome (Supplementary Fig. S1F). When comparing the histologic tumor phenotypes, we observed that infiltrative BCCs were significantly associated with immunosuppressive TME features compared with nodular BCCs (Fig. 1D and E). Overall, we identify preexisting immune heterogeneity in the TME of naïve BCCs, with immunosuppressive features preferentially found in infiltrative BCCs.
Figure 1.
Spatial transcriptomics reveals immune heterogeneity of TME in human BCCs. A, Heatmap of the unsupervised clustering of peritumoral AOIs from human BCC samples, processed by spatial transcriptomics and analyzed for IMR and IMS signatures, associated to PD-1 blockade response. B, Unsupervised PCA plot of the spatial transcriptomics analysis on all stroma AOIs. C, Average expression level of the difference between the IMS and IMR signature scores in the Tumor MicroEnvironment 1 (TME1) and Tumor MicroEnvironment 2 (TME2) AOIs. D, Average expression level of the difference between the IMS and IMR signature scores in the individual nodular and infiltrative BCC samples. E, Average expression level of the difference between the IMS and IMR signature scores in the pooled nodular and infiltrative BCC samples. Horizontal bars in C, D, and E indicate the mean ± SD. P values in C and E were calculated by unpaired two-sided Student t test. P value in D was calculated using Anova test. **, P < 0.01; ***, P < 0.001.
Integrated spatial and single-cell transcriptomics identify the cell types composing TME with distinct immune profiles
To deconvolute the cellular composition of TMEs associated with distinct immune profiles, we coupled spatial transcriptomics with scRNA-seq data. To do so, we first established specific spatial signatures reflecting the respective TME1 and TME2 spatially resolved peritumoral niches by identifying the DEGs. A total of 310 genes were differentially expressed between TME2 and TME1 (Supplementary Fig. S2A and S2B). To avoid RNA cross-contamination of stroma AOIs by adjacent tumor cells, we filtered the DEGs, excluding genes with higher expression in adjacent tumor AOIs, or ubiquitously/not expressed in KRT14neg stromal clusters on scRNA-seq data. After filtering, we obtained a spatial TME1 signature consisting of 114 genes (STME1) and a spatial TME2 signature consisting of 118 genes (STME2; Supplementary Fig. S2B; Supplementary Table S2). As expected, the spatial TME signatures were enriched in the Stroma AOIs when compared with Tumor AOIs (Supplementary Fig. S2C), and when applied to Stroma AOIs, segregated TME1 from TME2 AOIs (Supplementary Fig. S2D). Importantly, individual STME1 and STME2 signatures showed highly significant and specific enrichment in TME1 and TME2 AOIs, respectively (Supplementary Fig. S2E). Consistently with the distinct immune profiles of TME1 and TME2 AOIs, Gene Ontology (GO) term analysis on STME1 and STME2 signatures reflected immune response and ECM/adhesion respectively (Supplementary Fig. S2F).
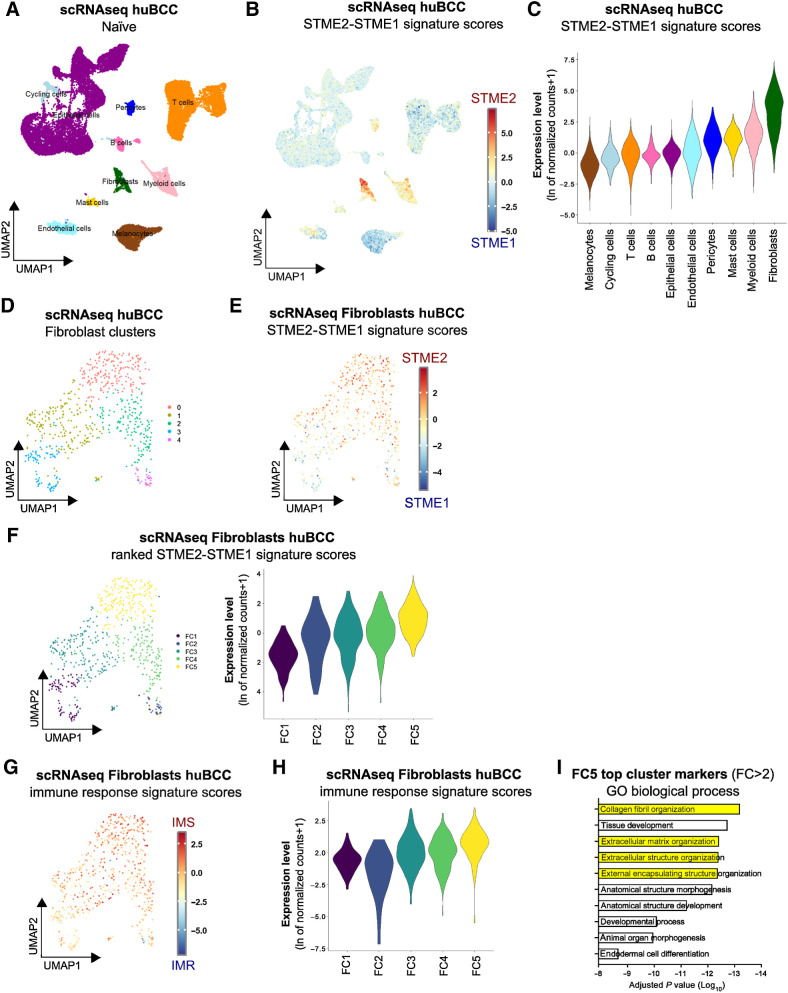
Having established specific spatial signatures, we proceeded to the deconvolution of the spatial signatures using scRNA-seq data previously obtained from 5 immunocompetent patients with naïve BCCs (27), and shown to contain all expected stroma cell types (Fig. 2A; Supplementary Fig. S3A). Using this dataset, STME2 and STME1 signatures were analyzed in the individual cells (Fig. 2B; Supplementary Fig. S3B and S3C). As expected from the spatial discrimination of TME1 and TME2 compartments, we observed a negative correlation between spatial STME1 and STME2 enrichments in stromal cell clusters (Supplementary Fig. S3D). Clusters were then ranked based on their mean STME2 over STME1 signature scores (Fig. 2C). The highest STME2/STME1 scores were found in the Fibroblast and Myeloid clusters, while the lowest scores were found in the Melanocyte and T-cell clusters. For consistency, we applied a similar approach using the published immunoreactive signature associated with PD-1 blockade response and the immunosuppressive signature associated with nonresponse to PD-1 blockade (Supplementary Fig. S3E and S3F; Supplementary Table S1). When computing the difference between signature scores for individual cells, we confirmed the highest immunosuppressive signature score in the Fibroblast and Myeloid cell clusters, and the lowest in the T-cell cluster (Supplementary Fig. S3G). At the single-cell level, STME2/STME1 and published immunosuppressive/immunoreactive signature scores showed significant positive correlation (Supplementary Fig. S3H), indicating that STME2 and STME1 signatures captured the prominent cell states regulating immune response in BCC. Altogether, by integrating spatial with single-cell transcriptomics, we identify the distinct cellular composition of T cell–enriched immunoreactive and Fibroblast/Myeloid cell–enriched immunosuppressive peritumoral niches.
Figure 2.
Immunosuppressive peritumoral niches are predominantly composed of ECM-remodeling CAFs. A, UMAP plot of 28,810 single cells integrated from five infiltrative BCCs colored according to 10 distinct cell types that were annotated using canonical cell type markers. B, Color scale overlaid UMAP plot of the average expression level of the difference between spatial Tumor MicroEnvironment 2 (STME2) and spatial Tumor MicroEnvironment 1 (STME1) signature scores in the human BCC samples. C, Violin plots of the average expression level of the difference between STME2 and STME1 signature scores in the human BCC samples, colored according to the clustering used in A. D, UMAP plot of the unsupervised subclustering of the Fibroblast population. E, Color scale overlaid UMAP plot of the average expression level of the difference between STME2 and STME1 signature scores in the Fibroblast population. F, Unsupervised clusters ranked according to the average expression level of the difference between STME2 and STME1 signature scores in the Fibroblast population, represented as a UMAP plot (left) and as violin plots (right). G, Color scale overlaid UMAP plot of the average expression level of the difference between IMS and IMR signature scores in the Fibroblast population. H, Violin plot of the average expression level of the difference between IMS and IMR signature scores in the Fibroblast population, per ranked cluster. I, Bar plot showing the GO biological processes enriched in the top cluster marker genes (fold change > 2) in the Fibroblast FC5 cluster.
Immunosuppressive peritumoral niches are predominantly composed of ECM-remodeling CAFs
To further assess the mechanisms of immune regulation at the peritumoral rim, we next assessed the transcriptional program of fibroblasts within immunosuppressive regions. To do so, fibroblasts were subclustered into five clusters with satisfactory integration (Fig. 2D; Supplementary Fig. S3I; Supplementary Table S3). We then computed the difference between STME2 and STME1 signature scores in each individual cell (Fig. 2E; Supplementary Fig. S3J and S3K) and Fibroblast clusters were ranked based on their STME2/STME1 signature scores from lowest (FC1) to highest (FC5; Fig. 2F). As expected, when using published immunoreactive (IMR) and immunosuppressive (IMS) signatures, FC5 showed preferential immunosuppressive features (Fig. 2G and H; Supplementary Fig. S3L and S3M; Supplementary Table S3). In particular, FC5 fibroblasts displayed marked ECM-remodeling features (Fig. 2I). These findings are in line with the CAF subset that we previously described at the edges of infiltrative BCCs (27) and confirmed by the preferential expression of COL1A2, POSTN, FAP, and ACTA2 (Supplementary Fig. S3N; ref. 51). Squamous cell carcinoma (SCC) and melanoma are the next most common skin cancers and both display partial response to ICI (52, 20). Remarkably, STME2/STME1 deconvolution in scRNA-seq cohorts of SCC (33) and melanoma (41) identified a similar immunosuppressive niche enriched for FC5-like, ECM-remodeling CAF (Supplementary Fig. S4A–S4J). Altogether, our data identify CAFs transcriptionally reprogrammed towards ECM remodeling as key components of the immunosuppressive niche in skin cancer.
Immunosuppressive niches associated with ECM-remodeling CAFs are infiltrated with CD163+ macrophages but devoid of CD8 T cells
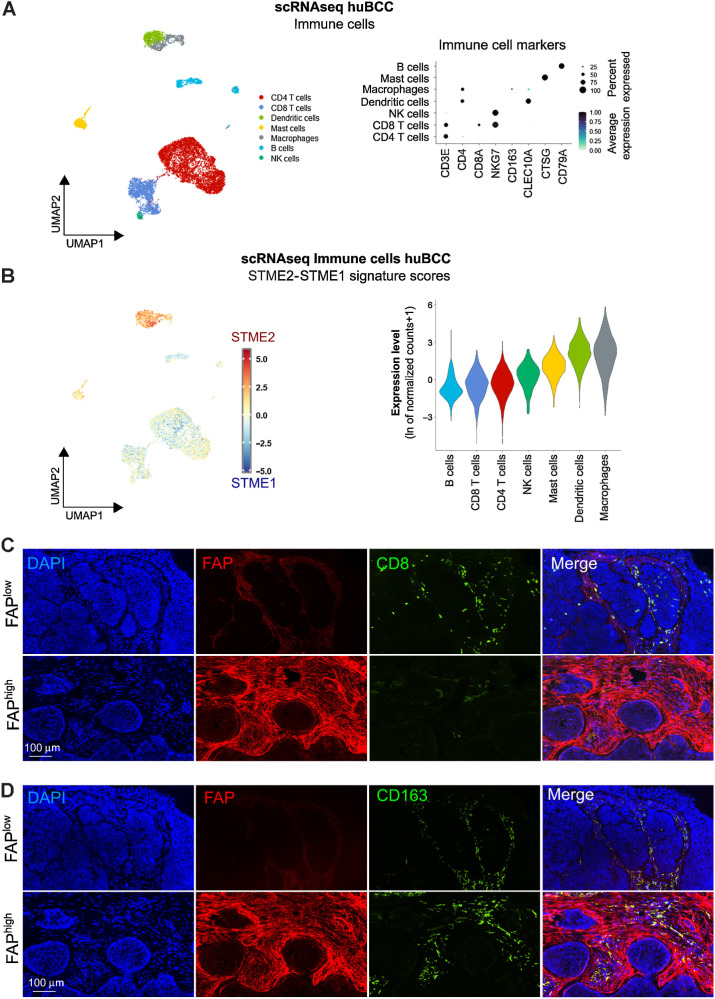
Having identified ECM-remodeling CAFs as being a key determinant of immunosuppressive TME polarization, we wondered how they would affect infiltration by immune cells. We thus subclustered the previously identified immune cell populations into CD4 and CD8 T cells, Natural Killer (NK) cells, B cells, Macrophages, Dendritic cells and Mast cells, as defined by canonical gene markers (Fig. 3A). Enrichments for STME2/STME1 as well as published immunosuppressive/immunoreactive signatures were found to be low in B cells and CD8 T cells (Fig. 3B; Supplementary Fig. S5A), consistent with the immunostimulatory role of these immune cells (Supplementary Fig. S5B and S5C). To investigate whether immunosuppressive TME areas enriched for ECM-remodeling CAFs would be devoid of CD8 T cells, we thus performed costainings for FAP (an ECM-remodeling CAF marker; Supplementary Fig. S3N) and CD8 and found significant depletion of CD8 T cells in FAP+ cell–rich peritumoral regions (Fig. 3C; Supplementary Fig. S5D). Notably, CD8 T cell–depleted peritumoral regions were preferentially found within infiltrative BCCs. On the other hand, unlike CD8 T cells, macrophages displayed high STME2/STME1 and immunosuppressive/immunoreactive signature scores (Fig. 3B; Supplementary Fig. S5A–S5C). Consistently, costaining with FAP showed CD163+ macrophages infiltrated within regions enriched in ECM-remodeling CAFs (Fig. 3D; Supplementary Fig. S5E).
Figure 3.
Immunosuppressive niches associated with ECM-remodeling CAFs are infiltrated with CD163+ macrophages but devoid of CD8 T cells. A, Subclustering of the Immune cells shown as a UMAP plot (left) and a dot plot of the average expression level of the immune marker genes (right). B, Average expression level of the difference between STME2 and STME1 signature scores in the Immune cells represented as a color scale overlaid UMAP plot (left) and violin plots (right). C, Representative images of a CD8 (green) and FAP (red) costaining on human BCC samples. Nuclei are stained with DAPI (blue). D, Representative images of a CD163 (green) and FAP (red) costaining on human BCC samples. Nuclei are stained with DAPI (blue). Bar scales in C and D indicate 100 μm.
Infiltrating macrophages within immunosuppressive peritumoral niches undergo Activin A–mediated transcriptional reprogramming
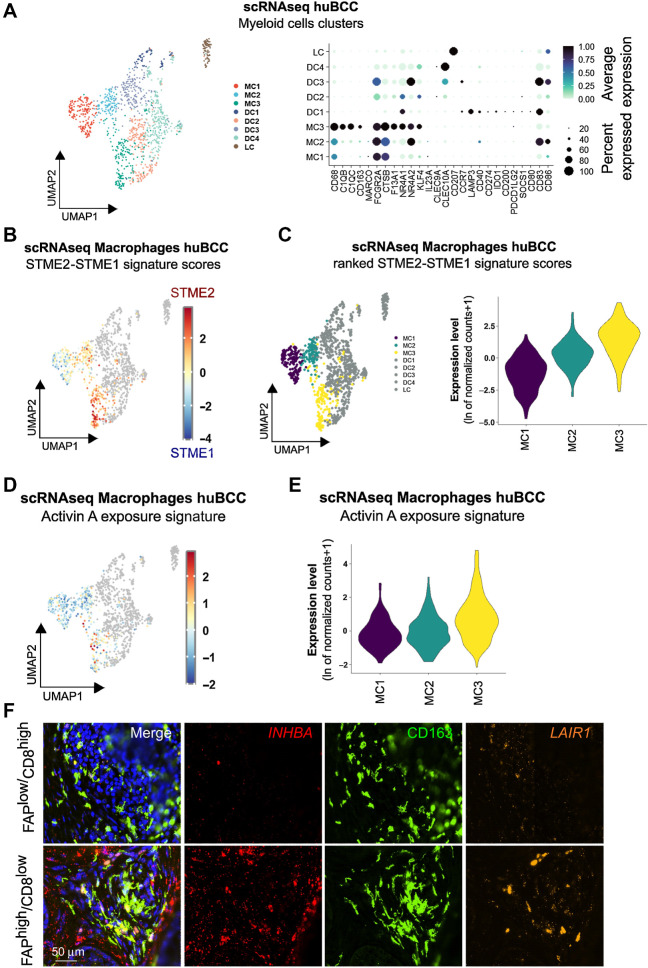
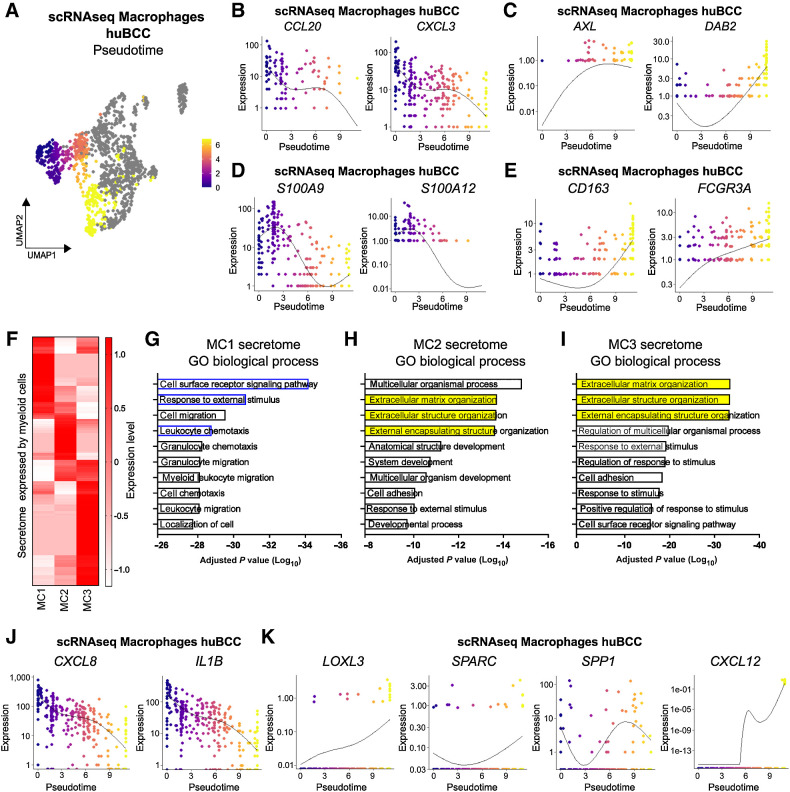
The presence of macrophages in both CAF-enriched and CAF-depleted peritumoral regions raises the question whether macrophages are differentially polarized. To address the question, we subdivided Myeloid cells into 8 unsupervised clusters, which corresponded to 3 Macrophage clusters, 4 Dendritic cell clusters, and 1 Langerhans cell cluster according to canonical gene markers and published signatures (Fig. 4A; Supplementary Fig. S6A–S6C; Supplementary Table S4; refs. 53–55). Macrophages were analyzed for the STME2/STME1 signature score (Fig. 4B; Supplementary Fig. S6D) and ranked from MC1 to MC3 (Fig. 4C). We then stained a BCC tumor harboring both TME1-like immunoreactive areas annotated as FAPlow/CD8high and TME2-like immunosuppressive areas annotated as FAPhigh/CD8low (Supplementary Fig. S7A), for MC1 and MC3 CD163+ macrophages using the MC1 top marker gene NLRP3 and the MC3 top marker gene RNASE1 (Supplementary Fig. S7B). While NLRP3+ MC1 macrophages were found ubiquitously, RNASE1+ MC3 macrophages were entrapped within densely packed FAP+ CAFs, in TME2-like immunosuppressive areas (Supplementary Fig. S7A–S7C).
Figure 4.
Infiltrating macrophages within immunosuppressive peritumoral niches undergo Activin A–mediated transcriptional reprogramming. A, Subclustering of the Myeloid cells shown as a UMAP plot (left) and a dot plot of the average expression level of the myeloid marker genes (right). B, Color scale overlaid UMAP plot of the average expression level of the difference between STME2 and STME1 signature scores in the Macrophage population. C, Clusters ranked according to the average expression level of the difference between STME2 and STME1 signature scores in the Macrophage population, represented as a UMAP plot (left) and as violin plots (right). D, Color scale overlaid UMAP plot of the average expression level of the genes present in the Activin A exposure signature in the Macrophage population. E, Violin plots of the clusters ranked according to the average expression level of the genes present in the Activin A exposure signature in the Macrophage population. F, Representative images of a CD163 (green) and DAPI (blue) costaining with INHBA (red) and LAIR1 (orange) RNA FISH probes on a human BCC within previously identified FAPlow/CD8high versus FAPhigh/CD8low areas. Scale bar, 50 μm.
We and others have previously reported that Activin A is a key inducer of FAP+ ECM-remodeling CAFs (27, 56) and a driver of immunosuppression through CD8 T-cell exclusion (57). Consistently, we observed enrichment of Activin A exposure signature in immunosuppressive, FC5 Fibroblasts in BCC (Supplementary Fig. S8A), and related Fibroblast clusters in SCC and melanoma (Supplementary Fig. S8B and S8C). To investigate the role of Activin A in polarizing macrophages towards the MC3 gene expression profile, we took advantage of a mouse skin cancer model overexpressing inhibin βA (INHBA, encoding for Activin A) in tumor cells, from which macrophages were previously collected and processed for RNA-seq (58). Remarkably, Activin A–conditioned macrophages showed significant and stronger polarization towards MC3 profile, compared with MC1 or MC2 profiles (Supplementary Fig. S8D). Because Activin A typically signals through ubiquitously-expressed ACVRII/ACVRIB (ALK4) heterodimers (56, 59), and ALK4 was found to be expressed in Myeloid cell clusters (Supplementary Fig. S8E), we next asked whether Activin A directly signals on macrophages. To test this hypothesis, we used human Thp1-derived macrophages which express Activin A (INHBA) and the MC3 markers SPP1, C3, and LAIR1. In this in vitro model, the secreted activin antagonist Follistatin (FST) significantly reduced SPP1, C3, and LAIR1 MC3 marker expression (Supplementary Fig. S8F). This suggests that, at least in part, MC3 polarization depends on the direct effect of secreted Activin A on macrophages. Consistently, the macrophage Activin A exposure signature (58) showed progressive enrichment from MC1 to MC3 macrophages in BCCs (Fig. 4D and E; Supplementary Fig. S8G). We thus stained annotated FAPlow/CD8high (TME1-like) and FAPhigh/CD8low (TME2-like) areas (Supplementary Fig. S7A) for the fibroblast Activin A exposure gene INHBA and the macrophage Activin A exposure gene LAIR1 using RNA FISH (Supplementary Fig. S8H) and demonstrated colocalization of Activin A–conditioned CAFs and macrophages in FAP+ CAF-enriched, CD8 T cell–depleted immunosuppressive TME2-like areas (Fig. 4F). Altogether, these findings indicate that macrophage MC3 polarization is dependent on Activin A in the immunosuppressive TME of BCCs. As suggested by the previous finding of shared FC5-like, Activin A–responsive CAFs among various skin cancer types (Supplementary Fig. S4), we found similar Activin A–conditioned, MC3-like macrophages in the immunosuppressive niches of human SCCs and melanomas (Supplementary Fig. S9).
Immunosuppressive peritumoral niches are predominantly composed of macrophages with ECM-remodeling features
Given the inversed enrichment STME1 and STME2 and the gradual enrichment of Activin A exposure signature from MC1 to MC3 (Supplementary Fig. S6D; Fig. 4E), we wondered whether macrophage heterogeneity may reflect diverse intratumoral differentiation. We thus took advantage of the pseudotime analysis, which allows ordering of the macrophages according to their transcriptional profile (38). We found a pseudotemporal trajectory starting from the TME1-like MC1 cluster, progressing to MC2 and ending in the TME2-like MC3 cluster (Fig. 5A), as illustrated by the progressive decrease in expression of STME1 signature genes (Fig. 5B) and the concomitant increase in expression of the STME2 signature genes (Fig. 5C). Importantly, we observed the progressive decrease in macrophage progenitor markers (like S100A9 and S100A12; Fig. 5D; refs. 54, 55) and progressive increase in macrophage differentiation markers (like CD163 and FCGR3A; Fig. 5E; refs. 54, 60). To assess the biological impact of the macrophage differentiation process within CAF-enriched immunosuppressive areas, we assessed the secretome of macrophage subclusters (39). Unsupervised clustering of secretome gene highlighted three gene sets, differentially expressed in MC1, MC2, and MC3 respectively (Fig. 5F; Supplementary Fig. S10A and S10B; Supplementary Table S5). GO term analysis identified a progressive switch from an inflammatory towards an ECM-remodeling profile (Fig. 5G–I), illustrated by the high expression of secreted inflammatory chemokines/cytokines like CXCL8 and IL1B (60, 61) at the MC1 stage and the reversed high expression of ECM-remodeling genes like LOXL3 (62), SPARC (63), and SPP1 (64) at the MC3 stage (Fig. 5J and K). SPP1, which encodes for osteopontin, was previously reported to prevent ICI response through profibrotic interactions with ECM-remodeling CAFs and physical exclusion of CD8 T cells (65, 66). Interestingly, we found that SPP1 expression in Thp1-derived macrophages was dependent on Activin A signaling (Supplementary Fig. S7F). To further investigate the potential effect of Activin A–polarized macrophages on CD8 T-cell depletion, we next inferred from pseudotime and secretome analyses the various genes driving macrophage MC3 polarization and overlapped the identified genes with a series of known T-cell chemokines and modulators (Supplementary Fig. S10C; ref. 67). Interestingly, the known CD8 T-cell chemoattractants CXCL9, CXCL10, and CXCL11 did not show significant correlation with MC3 polarization. In contrast, CXCL12, a well-established potent chemorepulsant for CD8 T cells (68–70), showed the highest pseudotime autocorrelation and very specific association with MC3 macrophage cluster (Supplementary Fig. S10C; Fig. 5K). Remarkably, as for SPP1, we found that CXCL12 expression in Thp1-derived macrophages was dependent on Activin A signaling (Supplementary Fig. S10D).
Figure 5.
Immunosuppressive peritumoral niches are predominantly composed of macrophages with ECM-remodeling features. A, Pseudotemporal trajectory across the Macrophage population starting in TME1-enriched cluster (MC1). B, Expression level of CCL20 and CXCL3 along the Macrophage pseudotemporal trajectory. C, Expression level of AXL and DAB2 along the Macrophage pseudotemporal trajectory. D, Expression level of S100A9 and S100A12 along the Macrophage pseudotemporal trajectory. E, Expression level of CD163 and FCGR3A along the Macrophage pseudotemporal trajectory. F, Heatmap of the expression level of secretome genes in Macrophage clusters. G, Bar plot showing the GO biological processes enriched in the secretome genes expressed in the MC1 cluster. H, Bar plot showing the GO biological processes enriched in the secretome genes expressed in the MC2 cluster. I, Bar plot showing the GO biological processes enriched in the secretome genes expressed in the MC3 cluster. J, Expression level of CXCL8 and IL1B along the Macrophage pseudotemporal trajectory. K, Expression level of LOXL3, SPARC, SPP1, and CXCL12 along the Macrophage pseudotemporal trajectory.
Altogether, we identify Activin A as a pivotal regulator of the spatial organization and immune polarization of the TME. Importantly, macrophages entrapped within immunosuppressive CAFs are rewired toward an ECM-remodeling secreted repertoire, participating in CD8 T-cell exclusion.
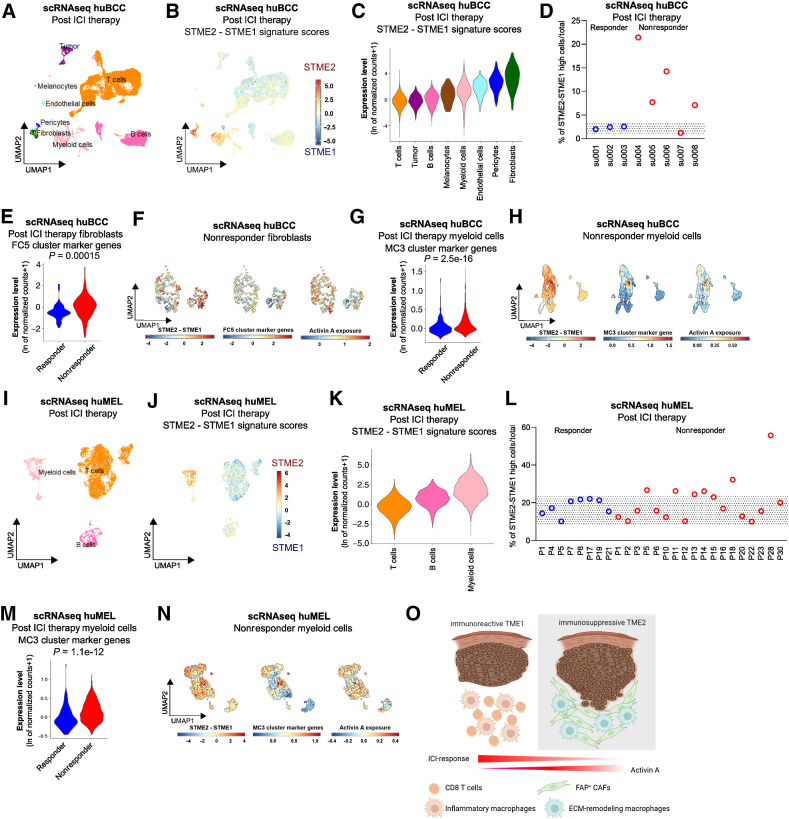
Immunosuppressive Activin A–conditioned CAFs and macrophages are biomarkers of ICI resistance
We identified intermingled ECM-remodeling CAFs and macrophages as potential drivers of immunosuppression in naïve BCCs. We thus hypothesized that these cell subsets would determine ICI resistance in BCCs. To address the question, we analyzed scRNA-seq data of ICI-treated BCCs annotated for response to therapy according to RECIST criteria (Fig. 6A; Supplementary Fig. S11A; ref. 42). We then computed the STME2/STME1 signature scores for individual cells (Fig. 6B; Supplementary Fig. S11B and S11C) and ranked the clusters based on the scores (Fig. 6C). Consistent with our observations in naïve BCCs, we found the highest STME2/STME1 signature scores in the Fibroblast and Myeloid cell clusters, while the lowest score was found in the T-cell cluster (Fig. 6C). Remarkably, 4 of 5 nonresponders displayed a high signature score, whereas none of the responders (n = 3) presented significant score levels (Fig. 6D). This reflected a higher proportion of FC5-like CAFs and MC3-like Myeloid cells among nonresponders (Fig. 6E–G; Supplementary Fig. S11D and S11E). Consistently with our previous observations, FC5-like Fibroblasts found within nonresponders displayed Activin A–mediated, immunosuppressive features, as suggested by the overlapping enrichment of STEM2/STEM1 signatures score, FC5 cluster marker genes, and Activin A exposure signature (Fig. 6F). Similarly, MC3-like Myeloid cells found within nonresponders displayed Activin A–mediated, immunosuppressive features, as suggested by the overlapping enrichment of STEM2/STEM1 signatures score, MC3 cluster marker genes (including SPP1 and CXCL12), and Activin A exposure signature (Fig. 6H; Supplementary Fig. S11F). To further assess the relevance of the identified immunosuppressive niche to ICI therapy, we next analyzed scRNA-seq of CD45+ immune cells obtained from melanoma annotated for response to immunotherapy according to RECIST criteria (Fig. 6I; ref. 43). When computing the STME2/STME1 signature scores for individual cells, we found the highest STME2/STME1 signature scores in the Myeloid cell clusters, while the lowest score was found in the T-cell cluster (Fig. 6J and K). Remarkably, 6 of 18 nonresponders displayed a higher STME2/STME1 signature score compared with responders (Fig. 6L), reflecting an enrichment for MC3-like myeloid cells (Fig. 6M). Consistently with the previous observations, MC3-like Myeloid cells found within nonresponders displayed Activin A–mediated immunosuppressive features, as suggested by the overlapping enrichment of STEM2/STEM1 signatures scores, MC3 cluster marker genes, and Activin A exposure signature (Fig. 6N). Altogether, these findings show that Activin A–conditioned CAFs and macrophages are specific and sensitive biomarkers for ICI resistance.
Figure 6.
Immunosuppressive Activin A–conditioned CAFs and macrophages are biomarkers for ICI resistance. A, UMAP plot of 25,400 single cells integrated from eight BCCs treated with ICI and colored according to eight distinct cell types that were annotated using canonical cell type markers. B, Color scale overlaid UMAP plot of the average expression level of the difference between STME2 and STME1 signature scores in the BCC populations. C, Violin plots of the average expression level of the difference between STME2 and STME1 signature scores in the posttreatment BCC populations, colored according to the clustering used in A. D, Scatter dot plot showing the percentage of cells having a high (>2) average expression level of the difference between STME2 and STME1 signature scores compared to the total number of cells in the posttreatment BCCs, shown for individual patient. E, Violin plots of the average expression level of the FC5 cluster marker genes in the Fibroblasts found in the responder (blue) and nonresponder (red) BCCs, respectively. F, UMAP plot of average expression level of the difference between STME2 and STME1 signature scores (left), FC5 cluster marker genes (middle), and Activin A exposure signature (right) in the Fibroblasts found in the nonresponder BCCs. G, Violin plots of the average expression level of the MC3 cluster marker genes in the Myeloid cells found in the responder (blue) and nonresponder (red) BCCs, respectively. H, UMAP plot of average expression level of the difference between STME2 and STME1 signature scores (left), MC3 cluster marker genes (middle), and Activin A exposure signature (right) in the Myeloid cells found in nonresponder BCCs. I, UMAP plot of 10,362 CD45+ immune single cells intagrated from 29 melanoma treated with ICI and colored according to three distinct cell types that were annotated using canonical cell type markers. J, Color scale overlaid UMAP plot of the average expression level of the difference between STME2 and STME1 signature scores in the melanoma-derived immune populations. K, Violin plots of the average expression level of the difference between STME2 and STME1 signature scores in the posttreatment melanoma-derived immune populations, colored according to the clustering used in I. L, Scatter dot plot showing the percentage of immune cells having a high (>1) average expression level of the difference between STME2 and STME1 signature scores compared with the total number of cells in the posttreatment melanoma-derived immune cells, shown for individual patient. M, Violin plots of the average expression level of the MC3 cluster marker genes in the Myeloid cells found in the responder (blue) and nonresponder (red) melanomas, respectively. N, UMAP plot of average expression level of the difference between STME2 and STME1 signature scores (left), MC3 cluster marker genes (middle), and Activin A exposure signature (right) in the Myeloid cells found in nonresponder melanomas. O, Summary scheme depicting the cellular and molecular deconvolution of TME with different immune profile and prognostic response to ICI. The gray rectangles in D and L highlight the range of enrichment found in responder BCCs and melanomas, respectively. (O, Created with BioRender.com.)
Overall, using integrated spatial and scRNA-seq, we deciphered the cellular subtypes and their transcriptional reprogramming within spatially resolved peritumoral niches. In particular, we characterized an immunosuppressive TME with CD8 T-cell depletion and interconnected CAFs and macrophages harboring Activin A–driven ECM-remodeling features (Fig. 6O). We show that Activin A–conditioned CAFs and macrophages are enriched in ICI-resistant tumors, where they may serve as immune escape biomarkers and therapeutic targets to overcome resistance.
Discussion
Here, we show that CD8 T-cell exclusion by a remodeled microenvironment critically affects tumor immune surveillance in BCC. By combining spatial and single-cell transcriptomics, we deconvolute and spatially resolve the heterogeneous immune polarization within BCC. We identify Activin A–responsive profibrotic CAFs as major contributors to the immunosuppressive peritumoral niches. In their proximity, Activin A–responsive tumor-associated macrophages are rewired towards an ECM-remodeling phenotype. Remarkably, the identified intermingled CAFs and macrophages are shared by skin SCCs and melanomas and preferentially found in ICI-resistant tumors, suggesting that therapeutic modulation of their ECM-remodeling properties may render tumor responsive to immunotherapy.
CAFs harbor significant heterogeneity and plasticity (71). We and others previously reported that ECM-remodeling CAFs have protumorigenic properties by promoting tumor migration (27, 29, 71). Here, we show that ECM-remodeling CAFs also confer immunosuppressive properties to the peritumoral niche. By secreting a high amount of stiff ECM proteins, CAFs promote a dense fibrotic stroma in the peritumoral areas, which prevents T-cell migration towards tumors (72–75). The dual protumorigenic and immunosuppressive function of ECM-remodeling CAFs illustrates how tumors hijack tissue repair processes to favor progression. Dense ECM deposition (fibronectin, collagen) is typically found during wound healing, where reprogramming of fibroblasts towards scar-forming myofibroblasts drives keratinocyte proliferation and differentiation, and at the same time, dampens the immune response (76). In tumors, desmoplastic CAFs are associated with poor survival as a result of invasion and metastasis (77, 78), as well as reduced response to immunotherapy (74, 79, 80). We now show that locally aggressive, infiltrative BCCs are significantly associated with immunosuppression and non-responsiveness to ICI. In that sense, CAF polarization towards ECM remodeling, by coupling protumorigenic and immune escape properties, appears as a strategical target to overcome tumor progression.
Interestingly, we also identified immunosuppressive macrophages with Activin A–mediated transcriptional re-wiring towards ECM remodeling. Activin A is known to be a chemoattractant, potentially participating in both migration and survival of macrophages within the peritumoral stroma of various cancer types (58, 81–83). Macrophages, including tumor-associated macrophages, are however highly plastic (84), and it is still debated how Activin A participates to macrophage polarization. While initial findings reported that Activin A contributes to proinflammatory antitumoral phenotype of circulating macrophages (85), Activin A–exposed tissue macrophages were recently shown to display tumor-promoting properties (58). In line with their protumorigenic properties (86), we report here that Activin A–conditioned macrophages are associated with CD8 T-cell depletion in peritumoral areas. Indeed, Activin A–polarized macrophages express SPP1, previously reported to support profibrotic CAFs and thereby promoting the physical barrier between CD8 T cells and tumor cells (66). In addition, Activin A–polarized macrophages may also lose proinflammatory signals like CXCL8 (87) and IL1B (88) and thereby contribute to CD8 T-cell exclusion. In melanoma, Activin A–mediated depletion of CD8 T cells was recently shown to be mediated by reduced CXCL9/10 production in myeloid cells (57), both critical chemokines for CD8 T-cell recruitment into tumors (89–91). Here, we observe Activin A–dependent expression of CXCL12, a well-established CD8 T-cell chemorepulsant, whose inhibition restores response to PD-1 blockade (68–70).
While further studies will be required to elucidate how the concurrent Activin A–mediated rewiring of CAFs and macrophages regulates CD8 T-cell exclusion, our recent data indicate that Activin A is a pivotal remodeler of the peritumoral stroma supporting tumor migration (27) and escape to immune surveillance. These findings are in line with previously reported association of Activin A with poor prognosis in various cancer types, including skin cancer (56, 92–94), and correlation with ICI resistance in melanoma (57, 86). Consistently, we observe specific enrichment of Activin A–responsive CAFs and macrophages in the TME of ICI-resistant BCCs and melanomas. It further supports that Activin A signaling may serve as an immune escape biomarker and a promising therapeutic target to break the fibrotic immunosuppressive TME and render tumor responsive to immunotherapy (95).
Altogether, our data show how tissue repair mechanisms are repurposed by the tumor to overcome immune surveillance. We found that Activin A is a major driver of the transcriptional reprogramming of CAFs and macrophages within immunosuppressive peritumoral niches, leading to CD8 T-cell exclusion and unresponsiveness to ICI. Our findings support the development of Activin A–targeting strategies to avoid progression and resistance in skin cancer in the context of immunotherapy.
Supplementary Material
Supplementary Figure S1: Patient information, quality controls and immune characterization of TME1 and TME2.
Supplementary Figure S2: Establishment of spatial transcriptional signatures for TMEs with distinct immune profiles.
Supplementary Figure S3: Immunosuppressive TME2 are enriched in ECM-remodeling CAFs.
Supplementary Figure S4: Immunosuppressive, ECM-remodeling CAFs are found in both human SCC and melanomas.
Supplementary Figure S5: TME2 is enriched in immunosuppressive macrophages, but devoid of immunoreactive CD8 T cells.
Supplementary Figure S6: Macrophages harbor different profiles in TME1 and TME2.
Supplementary Figure S7: ECM-remodeling CAFs and macrophages co-localize in CD8 T cells-depleted areas.
Supplementary Figure S8: Activin A exposure in Fibroblasts and Macrophages clusters.
Supplementary Figure S9: Immunosuppressive, Activin A-exposed macrophages are found in both human SCC and melanomas.
Supplementary Figure S10: Unsupervised clustering of secretome gene expression reflects Macrophage subpopulations.
Supplementary Figure S11: ICI-resistant BCCs harbor higher levels of TME2-like CAFs and myeloid cells.
Supplementary Table S1. Published immunoreactive and immunosuppressive gene signatures.
Supplementary Table S2. Spatial TME1 (STME1) and TME2 (STME2) gene signatures.
Supplementary Table S3. Cluster marker genes of the Fibroblast populations.
Supplementary Table S4. Cluster marker genes of the Myeloid cell populations.
Supplementary Table S5. MC1, MC2 and MC3 secretome genesets.
Supplementary Table S6. Clinical response annotation of the scRNAseq dataset obtained from BCCs treated with ICI.
Supplementary Table S7. Clinical response annotation of the scRNAseq dataset obtained from melanomas treated with ICI.
Acknowledgments
This work was supported by a SNSF fellowship PZ00P3–185926 (F. Kuonen), a UNIL-CHUV fellowship (F. Kuonen), and the PROMEDICA Stiftung (F. Kuonen). M. Gilliet is member of the SKINTEGRITY.CH collaborative research consortium.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
No discosures were reported.
Authors' Contributions
C. Pich-Bavastro: Conceptualization, data curation, formal analysis, writing–original draft. L. Yerly: Data curation. J. Di Domizio: Resources. S. Tissot-Renaud: Data curation. M. Gilliet: Resources. F. Kuonen: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, writing–original draft.
References
- 1. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- 3. Ascierto PA, Long GV, Robert C, Brady B, Dutriaux C, Di Giacomo AM, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol 2019;5:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:1480–92. [DOI] [PubMed] [Google Scholar]
- 5. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non–small cell lung cancer treated with nivolumab. JAMA Oncol 2019;5:1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn M-J, et al. Five-year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol 2019;37:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Massi D, Romano E, Rulli E, Merelli B, Nassini R, De Logu F, et al. Baseline β-catenin, programmed death-ligand 1 expression and tumour-infiltrating lymphocytes predict response and poor prognosis in BRAF inhibitor-treated melanoma patients. Eur J Cancer 2017;78:70–81. [DOI] [PubMed] [Google Scholar]
- 8. Hanna A, Metge BJ, Bailey SK, Chen D, Chandrashekar DS, Varambally S, et al. Inhibition of Hedgehog signaling reprograms the dysfunctional immune microenvironment in breast cancer. OncoImmunology 2019;8:1548241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coelho MA, de Carné Trécesson S, Rana S, Zecchin D, Moore C, Molina-Arcas M, et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity 2017;47:1083–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang T-H, et al. The immune landscape of cancer. Immunity 2018;48:812–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Héninger E, Krueger TEG, Lang JM. Augmenting antitumor immune responses with epigenetic modifying agents. Front Immunol 2015;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garrido F. MHC/HLA class I loss in cancer cells. In: Garrido F, editor. MHC Cl- loss cancer immune escape. Cham: Springer International Publishing; 2019. p.15–78. [Google Scholar]
- 13. Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer 2021;21:298–312. [DOI] [PubMed] [Google Scholar]
- 14. Ye Q, Shen Y, Wang X, Yang J, Miao F, Shen C, et al. Hypermethylation of HLA class I gene is associated with HLA class I down-regulation in human gastric cancer. Tissue Antigens 2010;75:30–9. [DOI] [PubMed] [Google Scholar]
- 15. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329–60. [DOI] [PubMed] [Google Scholar]
- 16. Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017;545:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 2016;537:417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schietinger A, Philip M, Krisnawan VE, Chiu EY, Delrow JJ, Basom RS, et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity 2016;45:389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 2016;375:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 2016;165:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 2019;20:326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal 2020;18:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sackstein R, Schatton T, Barthel SR. T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy. Lab Invest 2017;97:669–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015;348:74–80. [DOI] [PubMed] [Google Scholar]
- 25. Bonilla X, Parmentier L, King B, Bezrukov F, Kaya G, Zoete V, et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet 2016;48:398–406. [DOI] [PubMed] [Google Scholar]
- 26. Stratigos AJ, Sekulic A, Peris K, Bechter O, Prey S, Kaatz M, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol 2021;22:848–57. [DOI] [PubMed] [Google Scholar]
- 27. Yerly L, Pich-Bavastro C, Di Domizio J, Wyss T, Tissot-Renaud S, Cangkrama M, et al. Integrated multi-omics reveals cellular and molecular interactions governing the invasive niche of basal cell carcinoma. Nat Commun 2022;13:4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Villani R, Murigneux V, Alexis J, Sim S-L, Wagels M, Saunders N, et al. Subtype-specific analyses reveal infiltrative basal cell carcinomas are highly interactive with their environment. J Invest Dermatol 2021;141:2380–90. [DOI] [PubMed] [Google Scholar]
- 29. Kuonen F, Surbeck I, Sarin KY, Dontenwill M, Rüegg C, Gilliet M, et al. TGFβ, fibronectin and integrin α5β1 promote invasion in basal cell carcinoma. J Invest Dermatol 2018;138:2432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 31. Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, et al. Integrated analysis of multimodal single-cell data. Cell 2021;184:3573–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van den Brink SC, Sage F, Vértesy Á, Spanjaard B, Peterson-Maduro J, Baron CS, et al. Single-cell sequencing reveals dissociation-induced gene expression in tissue subpopulations. Nat Methods 2017;14:935–6. [DOI] [PubMed] [Google Scholar]
- 33. Ji AL, Rubin AJ, Thrane K, Jiang S, Reynolds DL, Meyers RM, et al. Multimodal analysis of composition and spatial architecture in human squamous cell carcinoma. Cell 2020;182:497–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solé-Boldo L, Raddatz G, Schütz S, Mallm J-P, Rippe K, Lonsdorf AS, et al. Single-cell transcriptomes of the human skin reveal age-related loss of fibroblast priming. Commun Biol 2020;3:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dwyer DF, Barrett NA, Austen KF. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat Immunol 2016;17:878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat Methods 2019;16:1289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. Nat Genet 2000;25:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cao J, Spielmann M, Qiu X, Huang X, Ibrahim DM, Hill AJ, et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 2019;566:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uhlén M, Karlsson MJ, Hober A, Svensson A-S, Scheffel J, Kotol D, et al. The human secretome. Sci Signal 2019;12:eaaz0274. [DOI] [PubMed] [Google Scholar]
- 40. Genin M, Clement F, Fattaccioli A, Raes M, Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015;15:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jerby-Arnon L, Shah P, Cuoco MS, Rodman C, Su M-J, Melms JC, et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell 2018;175:984–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med 2019;25:1251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 2018;175:998–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Merritt CR, Ong GT, Church SE, Barker K, Danaher P, Geiss G, et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat Biotechnol 2020;38:586–99. [DOI] [PubMed] [Google Scholar]
- 45. Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adolphe C, Xue A, Fard AT, Genovesi LA, Yang J, Wainwright BJ. Genetic and functional interaction network analysis reveals global enrichment of regulatory T cell genes influencing basal cell carcinoma susceptibility. Genome Med 2021;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mysona DP, Tran L, Bai S, Dos Santos B, Ghamande S, Chan J, et al. Tumor-intrinsic and -extrinsic (immune) gene signatures robustly predict overall survival and treatment response in high grade serous ovarian cancer patients. Am J Cancer Res 2021;11:181–99. [PMC free article] [PubMed] [Google Scholar]
- 48. Givechian KB, Wnuk K, Garner C, Benz S, Garban H, Rabizadeh S, et al. Identification of an immune gene expression signature associated with favorable clinical features in Treg-enriched patient tumor samples. NPJ Genomic Med 2018;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang H, Li S, Wang Q, Jin Z, Shao W, Gao Y, et al. Tumor immunological phenotype signature-based high-throughput screening for the discovery of combination immunotherapy compounds. Sci Adv 2021;7:eabd7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang L, Yang H, Dorn P, Berezowska S, Blank F, Wotzkow C, et al. Peritumoral CD90+CD73+ cells possess immunosuppressive features in human non-small cell lung cancer. EBioMedicine 2021;73:103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luo H, Xia X, Huang L-B, An H, Cao M, Kim GD, et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat Commun 2022;13:6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 2018;379:341–51. [DOI] [PubMed] [Google Scholar]
- 53. Reynolds G, Vegh P, Fletcher J, Poyner EFM, Stephenson E, Goh I, et al. Developmental cell programs are co-opted in inflammatory skin disease. Science 2021;371:eaba6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O'Brien SA, et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 2020;181:442–59. [DOI] [PubMed] [Google Scholar]
- 55. Fawkner-Corbett D, Antanaviciute A, Parikh K, Jagielowicz M, Gerós AS, Gupta T, et al. Spatiotemporal analysis of human intestinal development at single-cell resolution. Cell 2021;184:810–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cangkrama M, Wietecha M, Mathis N, Okumura R, Ferrarese L, Al-Nuaimi D, et al. A paracrine activin A–mDia2 axis promotes squamous carcinogenesis via fibroblast reprogramming. EMBO Mol Med 2020;12:e11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pinjusic K, Dubey OA, Egorova O, Nassiri S, Meylan E, Faget J, et al. Activin-A impairs CD8 T cell-mediated immunity and immune checkpoint therapy response in melanoma. J Immunother Cancer 2022;10:e004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Antsiferova M, Piwko-Czuchra A, Cangkrama M, Wietecha M, Sahin D, Birkner K, et al. Activin promotes skin carcinogenesis by attraction and reprogramming of macrophages. EMBO Mol Med 2017;9:27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tsuchida K, Nakatani M, Hitachi K, Uezumi A, Sunada Y, Ageta H, et al. Activin signaling as an emerging target for therapeutic interventions. Cell Commun Signal 2009;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Orekhov AN, Orekhova VA, Nikiforov NG, Myasoedova VA, Grechko AV, Romanenko EB, et al. Monocyte differentiation and macrophage polarization. Vessel Plus2019;3:10. [Google Scholar]
- 61. Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol 2021;21:485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang N, Cao D-F, Yin X-X, Zhou H-H, Mao X-Y. Lysyl oxidases: emerging biomarkers and therapeutic targets for various diseases. Biomed Pharmacother 2020;131:110791. [DOI] [PubMed] [Google Scholar]
- 63. Hu J, Ma Y, Ma J, Chen S, Zhang X, Guo S, et al. Macrophage-derived SPARC attenuates M2-mediated pro-tumour phenotypes. J Cancer 2020;11:2981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kariya Y, Kariya Y. Osteopontin in cancer: mechanisms and therapeutic targets. Int J Transl Med 2022;2:419–47. [Google Scholar]
- 65. Zheng Y, Hao S, Xiang C, Han Y, Shang Y, Zhen Q, et al. The correlation between SPP1 and immune escape of EGFR mutant lung adenocarcinoma was explored by bioinformatics analysis. Front Oncol 2021;11:592854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Qi J, Sun H, Zhang Y, Wang Z, Xun Z, Li Z, et al. Single-cell and spatial analysis reveal interaction of FAP+ fibroblasts and SPP1+ macrophages in colorectal cancer. Nat Commun 2022;13:1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang Y, Guan X, Jiang P. Cytokine and chemokine signals of T-cell exclusion in tumors. Front Immunol 2020;11:594609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Poznansky MC, Olszak IT, Foxall R, Evans RH, Luster AD, Scadden DT. Active movement of T cells away from a chemokine. Nat Med 2000;6:543–8. [DOI] [PubMed] [Google Scholar]
- 69. Zboralski D, Hoehlig K, Eulberg D, Frömming A, Vater A. Increasing tumor-infiltrating T cells through inhibition of CXCL12 with NOX-A12 synergizes with PD-1 blockade. Cancer Immunol Res 2017;5:950–6. [DOI] [PubMed] [Google Scholar]
- 70. Vianello F, Papeta N, Chen T, Kraft P, White N, Hart WK, et al. Murine B16 melanomas expressing high levels of the chemokine stromal-derived factor-1/CXCL12 induce tumor-specific T cell chemorepulsion and escape from immune control. J Immunol 2006;176:2902–14. [DOI] [PubMed] [Google Scholar]
- 71. Ping Q, Yan R, Cheng X, Wang W, Zhong Y, Hou Z, et al. Cancer-associated fibroblasts: overview, progress, challenges, and directions. Cancer Gene Ther 2021;28:984–99. [DOI] [PubMed] [Google Scholar]
- 72. Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean M-C, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest 2012;122:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hartmann N, Giese NA, Giese T, Poschke I, Offringa R, Werner J, et al. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin Cancer Res 2014;20:3422–33. [DOI] [PubMed] [Google Scholar]
- 74. Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 2016;22:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kaur A, Ecker BL, Douglass SM, Kugel CH, Webster MR, Almeida FV, et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov 2019;9:64–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Larouche J, Sheoran S, Maruyama K, Martino MM. Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv Wound Care 2018;7:209–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Eble JA, Niland S. The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis 2019;36:171–98. [DOI] [PubMed] [Google Scholar]
- 78. Venning FA, Wullkopf L, Erler JT. Targeting ECM disrupts cancer progression. Front Oncol 2015;5:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen IX, Chauhan VP, Posada J, Ng MR, Wu MW, Adstamongkonkul P, et al. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc Natl Acad Sci U S A 2019;116:4558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schmiechen ZC, Stromnes IM. Mechanisms governing immunotherapy resistance in pancreatic ductal adenocarcinoma. Front Immunol 2021;11:613815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Held-Feindt J, Hattermann K, Müerköster SS, Wedderkopp H, Knerlich-Lukoschus F, Ungefroren H, et al. CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs). Exp Cell Res 2010;316:1553–66. [DOI] [PubMed] [Google Scholar]
- 82. Zheng J, Yang M, Shao J, Miao Y, Han J, Du J. Chemokine receptor CX3CR1 contributes to macrophage survival in tumor metastasis. Mol Cancer 2013;12:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schmall A, Al-tamari HM, Herold S, Kampschulte M, Weigert A, Wietelmann A, et al. Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer. Am J Respir Crit Care Med 2015;191:437–47. [DOI] [PubMed] [Google Scholar]
- 84. Pittet MJ, Michielin O, Migliorini D. Clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol 2022;19:402–21. [DOI] [PubMed] [Google Scholar]
- 85. Sierra-Filardi E, Puig-Kröger A, Blanco FJ, Nieto C, Bragado R, Palomero MI, et al. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood 2011;117:5092–101. [DOI] [PubMed] [Google Scholar]
- 86. Donovan P, Dubey OA, Kallioinen S, Rogers KW, Muehlethaler K, Müller P, et al. Paracrine Activin-A signaling promotes melanoma growth and metastasis through immune evasion. J Invest Dermatol 2017;137:2578–87. [DOI] [PubMed] [Google Scholar]
- 87. Hess C, Means TK, Autissier P, Woodberry T, Altfeld M, Addo MM, et al. IL-8 responsiveness defines a subset of CD8 T cells poised to kill. Blood 2004;104:3463–71. [DOI] [PubMed] [Google Scholar]
- 88. Ben-Sasson SZ, Hogg A, Hu-Li J, Wingfield P, Chen X, Crank M, et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med 2013;210:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 2017;31:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. House IG, Savas P, Lai J, Chen AXY, Oliver AJ, Teo ZL, et al. Macrophage-derived CXCL9 and CXCL10 are required for antitumor immune responses following immune checkpoint blockade. Clin Cancer Res 2020;26:487–504. [DOI] [PubMed] [Google Scholar]
- 91. Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity 2019;50:1317–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Seder CW, Hartojo W, Lin L, Silvers AL, Wang Z, Thomas DG, et al. Upregulated INHBA expression may promote cell proliferation and is associated with poor survival in lung adenocarcinoma. Neoplasia N Y N 2009;11:388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chang K-P, Kao H-K, Liang Y, Cheng M-H, Chang Y-L, Liu S-C, et al. Overexpression of Activin A in oral squamous cell carcinoma: association with poor prognosis and tumor progression. Ann Surg Oncol 2010;17:1945–56. [DOI] [PubMed] [Google Scholar]
- 94. Bashir M, Damineni S, Mukherjee G, Kondaiah P. Activin-A signaling promotes epithelial–mesenchymal transition, invasion, and metastatic growth of breast cancer. NPJ Breast Cancer 2015;1:15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ries A, Schelch K, Falch D, Pany L, Hoda MA, Grusch M. Activin A: an emerging target for improving cancer treatment? Expert Opin Ther Targets 2020;24:985–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Patient information, quality controls and immune characterization of TME1 and TME2.
Supplementary Figure S2: Establishment of spatial transcriptional signatures for TMEs with distinct immune profiles.
Supplementary Figure S3: Immunosuppressive TME2 are enriched in ECM-remodeling CAFs.
Supplementary Figure S4: Immunosuppressive, ECM-remodeling CAFs are found in both human SCC and melanomas.
Supplementary Figure S5: TME2 is enriched in immunosuppressive macrophages, but devoid of immunoreactive CD8 T cells.
Supplementary Figure S6: Macrophages harbor different profiles in TME1 and TME2.
Supplementary Figure S7: ECM-remodeling CAFs and macrophages co-localize in CD8 T cells-depleted areas.
Supplementary Figure S8: Activin A exposure in Fibroblasts and Macrophages clusters.
Supplementary Figure S9: Immunosuppressive, Activin A-exposed macrophages are found in both human SCC and melanomas.
Supplementary Figure S10: Unsupervised clustering of secretome gene expression reflects Macrophage subpopulations.
Supplementary Figure S11: ICI-resistant BCCs harbor higher levels of TME2-like CAFs and myeloid cells.
Supplementary Table S1. Published immunoreactive and immunosuppressive gene signatures.
Supplementary Table S2. Spatial TME1 (STME1) and TME2 (STME2) gene signatures.
Supplementary Table S3. Cluster marker genes of the Fibroblast populations.
Supplementary Table S4. Cluster marker genes of the Myeloid cell populations.
Supplementary Table S5. MC1, MC2 and MC3 secretome genesets.
Supplementary Table S6. Clinical response annotation of the scRNAseq dataset obtained from BCCs treated with ICI.
Supplementary Table S7. Clinical response annotation of the scRNAseq dataset obtained from melanomas treated with ICI.
Data Availability Statement
The scRNA-seq data used in this study are available in the Gene Expression Omnibus database (RRID:SCR_005012) under accession codes GSE181907 (27), GSE144239 (33), GSE115978 (41), GSE123813 (42), and GSE120575 (43). The DSP data used in this study are available in the Gene Expression Omnibus database (RRID:SCR_005012) under accession code GSE210648 (27). The following databases and datasets were used in this study: GRCh38 human reference genome reference (refdata-gex-GRCh38–2020-A, https://support.10xgenomics.com/single-cell-gene-expression/software/release-notes/build#GRCh38_2020A) and GeoMx Cancer Transcriptome Atlas (https://www.nanostring.com/products/geomx-digital-spatial-profiler/geomx-rna-assays/geomx-cancer-transcriptome- atlas/). All processed data are available from the corresponding author upon request.