Abstract
Genome editing of hematopoietic stem cells (HSCs) represents a therapeutic option for a number of hematological genetic diseases, as HSCs have the potential for self-renewal and differentiation into all blood cell lineages. This review presents advances of genome editing in HSCs utilizing adenovirus vectors as delivery vehicles. We focus on capsid-modified, helper-dependent adenovirus vectors that are devoid of all viral genes and therefore exhibit an improved safety profile. We discuss HSC genome engineering for several inherited disorders and infectious diseases including hemoglobinopathies, Fanconi anemia, hemophilia, and HIV-1 infection by ex vivo and in vivo editing in transgenic mice, nonhuman primates, as well as in human CD34+ cells. Mechanisms of therapeutic gene transfer including episomal expression of designer nucleases and base editors, transposase-mediated random integration, and targeted homology-directed repair triggered integration into selected genomic safe harbor loci are also reviewed.
Keywords: adenovirus vector, base editors, CRISPR/Cas9, genome editing, HSCs, in vivo transduction, targeted integration
A major focus of gene therapy research has been genome editing of hematopoietic stem cells (HSCs), owing to that HSCs are the only cell type within the blood system possessing the ability of both self-renewal and multilineage differentiation. For congenital hematological disorders, the correction of a defect gene in HSCs implies that all progeny cells would carry the engineered traits and that a one-time treatment could provide a lifelong therapeutic and even curative remedy. In addition, efforts are also being dedicated to use HSC gene therapy for cancer and HIV-1 infection, based on the modification of host factors required for virus infection, directly targeting essential viral genes, or modulation of immune responses toward tumor destruction.
Efficient delivery of therapeutic transgene is a prerequisite for successful gene therapy. When gene therapy was conceptualized in the early 1970s, mammalian viruses were proposed as an effective vehicle to deliver a gene ‘drug’ [1,2]. In the ensuing nearly four decades, viral vectors have been intensively investigated and broadly used in gene transfer applications. A majority of the ongoing clinical trials are based on gene delivery using viral vectors including γ-retrovirus, lentivirus, adeno-associated virus, and adenovirus-derived vectors.
γ-retroviral vectors (γ-RVs), derived from murine leukemia virus, are one of the earliest vectors developed [3]. Based on ex vivo γ-RV transduction of CD34+ cells, several clinical studies for severe combined immunodeficiency disease (SCID) due to deficiency of the enzyme adenosine deaminase (ADA) and X-linked SCID have observed substantial overall improvements in clinical and immunological features in most of the patients [4-6]. However, as a result of insertional oncogenesis by γ-RV integration, leukemia was observed in five out of 20 X-SCID patients, which lead to one patient’s death [7,8]. The nature of vector insertion near oncogenes largely tempered further clinical use of early generations of γ-RV.
Lentiviral vectors (LVs) and recombinant adeno-associated viral vectors (rAAVs) are vigorously pursued in current HSC gene therapy. HIV-1-based LVs were pioneered in the second half of 1990s [9]. Compared to γ-RV, LV exhibited more efficient transduction of nondividing primitive HSCs and a safer integration profile [10]. Over recent years, LV-based HSC gene therapy has delivered promising results in patients with β-globin disorders [11,12]. However, the preferential insertion into active genes and local hotspots, and the observation of clonal expansion due to transactivation of HMGA2 in a treated β-thalassemia patient warrant concern over genotoxicity of early generation of LVs [13,14]. Additionally, current vesicular stomatitis virus G protein pseudotyped LVs are not suitable for in vivo systemic delivery because of neutralization by the human serum complement system [15]. In contrast, the largely episomal and nonpathogenic features of rAAVs have attracted much attention in the past two decades. Exceptional achievements using rAAVs have been made in both preclinical studies and human trials outside the HSC gene therapy field; for example, by in vivo delivery of rAAVs expressing Factor IX, more than fifty hemophilia B patients have been treated and most of the subjects have demonstrated efficient and relatively durable FIX activity [16]. For HSC genome editing, a recent study reported up to 90% homology-directed repair (HDR) by combining electroporation with CRISPR/Cas9 ribonucleoproteins (RNP) and transduction with a rAAV6 vector containing a donor transgene cassette [17]. Nevertheless, the < 5 kb packaging capacity, broadly detected preexisting immunity, and high cost for large-scale vector manufacturing present limitations to rAAV application.
The application of newer generation of adenoviral vectors (AdVs) may address the above limitations in HSC genome engineering and expand our treatment armamentarium for human diseases. In this review, we introduce the properties of a new generation of AdVs, that is, helper-dependent adenovirus vectors (HDAd) with HSC tropism, discuss various strategies for transient or stable transgene expression by HDAd, and then review recent advances in HSC gene editing for several representative blood disorders. Development of in vivo transduction is highlighted owing to its efficacy, technical simplicity, and potential for clinical application.
General concept of adenovirus vectors
Adenovirus was first isolated in the early 1950s [18], long before the discovery of other commonly adapted viral gene transfer vectors. When in vivo gene transfer was first attempted in the late 1980s for α1 antitrypsin deficiency, extensive knowledge had already been accumulated about first-generation AdVs (Group C serotypes 5 and 2 with E1 and/or E3 deletion). Their highly efficient transduction of a wide variety of dividing and quiescent cells, relative ease of preparation and purification, and well-characterized viral genome sequence and biology made AdV the vehicle of choice, leading to the first report of efficient in vivo gene transfer [19]. In the following decade, numerous studies have demonstrated the strong transduction efficiency of AdVs in multiple disease models. However, it was soon recognized that the first generation of AdVs is strongly immunogenic [20]. Local administration of AdVs elicits severe inflammatory responses and adaptive immune reactions, resulting in T cell-mediated clearance of transduced cells. In addition, adaptive antiviral immunity hindered repeated administration. Systemic delivery caused liver toxicity and triggered acute innate immune responses, which can be fatal at high vector doses [21]. On the other hand, the strong immunogenicity of AdVs can be harnessed as a beneficial property for oncolytic therapy [22,23].
Receptors and transduction
Virus tropism is largely dictated by a high-affinity attachment receptor. For human adenovirus, there are over 57 serotypes (Ad1–Ad57) with seven Groups (A–G). The serotype is defined historically by their characteristic neutralizing ability, and currently by DNA homology. Most adenovirus serotypes, including Group C Ad5, use the coxsackie adenovirus receptor (CAR) as a primary attachment receptor to target cells [24]. CAR is expressed in a broad range of tissues, rendering Ad5-based vectors a broad targeting spectrum. However, human HSCs express only low levels of CAR, making them refractory to infection by most adenoviruses [25].
Species B adenovirus tropism to human CD34+ cells
Group B viruses do not utilize CAR as a primary attachment receptor, and this influences their tissue tropism and subsequent disease pathogenesis. We and others have reported that group B adenoviruses are capable of using the complement regulatory protein CD46 as a primary attachment receptor [26,27]. CD46, also named membrane cofactor protein (MCP), is a type I transmembrane glycoprotein expressed on all nucleated human cells. It is described as a pathogen magnet [28], because CD46 also serves as a receptor for several other human pathogens, including laboratory strains of measles virus [29], human herpes virus 6 [30], pathogenic Neisseria [31], and Streptococcus pyogenes [32,33]. In humans, there are four major isoforms of CD46 (BC1, BC2, C1, and C2), depending on the alternative splicing of a region encoding an extracellular domain and the choice between one or two cytoplasmic tails, Cyt1 and Cyt2 [34]. CD46 expression is greatly up-regulated in malignant tumor cells [35-37]. Importantly, human HSCs express high level of CD46, rendering them highly susceptible to transduction by group B adenoviruses [25,38].
Three generations of adenovirus vectors
Adenoviral vectors are generated by replacing viral genes with foreign transgenes to be delivered to a wide variety of both dividing and nondividing cells, as well as mediating high levels of transgene expression. First-generation AdVs were constructed by deleting the early genes E1 and E3. However, at high multiplicities of infection, the E1 region becomes dispensable for replication. This may be related to the expression of specific host gene products with E1A-like activity, or to the proliferative state of cells. Leaky viral DNA replication is followed by low-level viral late protein production [39]. Accumulation of adenoviral cytotoxic late gene products following gene transfer causes direct cytopathic effects on the transduced cells and triggers host cellular immune response. These adverse effects can be aggravated by emergence of E1-containing (E1+), replication-competent adenovirus during the production of first-generation viruses in 293 cells.
These problems are diminished in second-generation adenovirus vectors, that is, vectors in which additional essential genes, E2 and E4, are deleted. However, it proved difficult to maintain cell lines that would efficiently transcomplement the deleted viral proteins.
Third generation of AdVs is devoid of all coding viral genes. Only inverted terminal repeats (ITRs) and the packaging signal are retained. The production of these vectors requires a helper virus to provide all structural proteins in trans; therefore, the vectors are also called HDAds or ‘gutless’ vectors. Cloning techniques such as Gibson assembly and recombineering have greatly simplified the construction of corresponding HDAd plasmids [40]. Furthermore, the properties of CRISPR/Cpf1 are favorable for engineering large DNA constructs including pHDAd genomes [41].
Helper-dependent adenovirus vectors
Helper-dependent adenovirus vector possess a number of properties that make them suitable tools for HSC genome editing.
Large packaging capacity
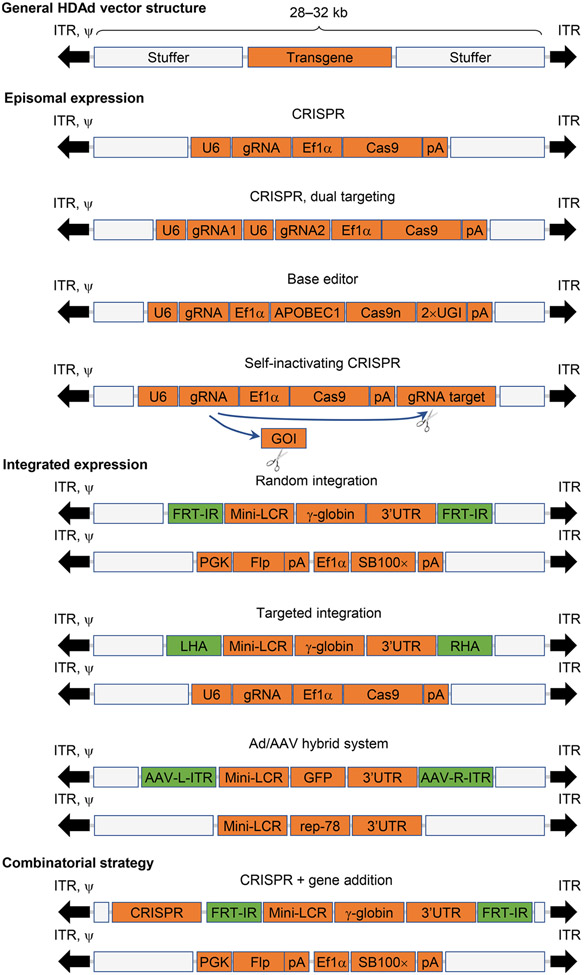
In theory, HDAds can accommodate transgene cassettes of up to 36 kb. For this reason, they are also called high-capacity AdV (HCAd) [42]. To facilitate vector purification and separation from the helper virus by cesium chloride ultracentrifugation, the insert size is usually kept within 28–32 kb. This payload is remarkably large, in sharp contrast to the 7 and 5 kb maximum carrying capacity of LVs and rAAVs, respectively [43]. The insert capacity of LVs and rAAVs limits their capability of incorporating large transgenes such as the most widely used CRISPR system (clustered regularly interspaced short palindromic repeats; ~ 5 kb with the premise of using minimally essential promoters and transcription elements), base editors (BEs; ~ 8 kb), and combinatorial systems (Fig. 1). With the utilization of HDAds, these cassettes as well as most of therapeutic genes can be readily accommodated. For example, we generated an HDAd vector that contained about 27kb of the human globin locus control region (LCR) [44]. The insert capacity is also relevant for using HDAd as donor vectors. It is thought that the efficiency of homologous recombination directly correlates with the length of the homology region. This has also been demonstrated in gene targeting studies with HDAd vectors [45-47].
Fig. 1.
Examples for genome engineering strategies using HDAd vectors. HDAd vectors do not contain any viral coding genes. Stuffer DNA sequences are usually included to make up the total genome size of 28–32 kb. The episomal nature of HDAds can be exploited for transient expression of nucleases (e.g., CRISPR/Cas9) and BEs (e.g., CBEs). Simultaneous editing of two target sites can be achieved by incorporating two guide RNAs (gRNA). An example of a SIN CRISPR vector is shown to demonstrate a strategy for controlling CRISPR duration. For integrating vectors using SB100× transposase, two vectors are co-delivered. One expresses the transgene (e.g., human γ-globin driven by β-globin LCR/promoter) flanked by two FRT-IRs. The other vector expresses SB100× transposase and flippase. For HDR-mediated targeted integration, one donor template is flanked by two homology regions neighboring targeted genomic sites. The second vector is used to express CRISPR for creating DSBs. Ad/AAV hybrid vectors mediate targeted integration into AAVS1 locus. Two vectors are used to express transgene flanked by AAV ITRs and AAV Rep78, respectively. Episomal expression can be combined with integrated expression. As an example, cloning of CRISPR outside of the two FRT-IRs leads to transient expression of the nuclease system while concomitantly allowing the γ-globin cassettes bracketed by two FRT-IRs for SB100×-mediated integration. 3′UTR, 3′ end UTR; APOBEC1, APOBEC1 cytidine deaminase; Cas9n, Cas9 nickase; EF1α, human elongation factor-1 α promoter; GOI, gene of interest; LCR, β-globin LCR; LHA, left HA; pA, polyA signal; RHA, right HA; U6, U6 promoter; UGI, uracil-DNA glycosylase inhibitor; ψ, packaging signal sequence.
Relatively low cost for vector manufacturing
A major obstacle for a widespread clinical application of gene therapy is the cost associated with clinical grade vector production. This is particularly the case for the production of rAAVs [48] and lentivirus vectors [49], which require, for each new vector preparation, large-scale plasmid transfection or more recently, transduction with baculovirus vectors that express corresponding viral proteins. In contrast, once a HDAd vector is generated and characterized, it can be used as a seed stock for further amplification.
The virus is produced at large scale in a 2-L spinner culture that routinely yields > 1 × 1013 viral particles per spinner (sufficient to treat a 10 kg patient) [50]. The relatively low costs for producing high yields of HDAd vectors are particularly relevant for in vivo gene therapy. Based on our experience, the costs for laboratory-grade HDAd vector are about $2000 per 1 × 1013 viral genomes. For intravenous injection, this would correspond to a cost of $10–20 per 25 g mouse. In comparison, for in vivo delivery of rAAV, 0.5–1 × 1013 viral genomes per adult mouse are routinely used, which would be a cost in the range of $500–$1000 per mouse (J. Chamberlain, personal communication). Ads including HDAds can be lyophilized and are stable at 4 °C [51].
Episomal expression
Adenoviral serotypes currently used as gene transfer vectors do not integrate into the host genome. Shortly after infection, the double-stranded viral DNA together with core proteins and the covalently linked terminal protein (TP) are translocated to the nucleus. TP binding confers stability of viral genomes, supporting long-term expression of the transgene, especially in nondividing cells. The episomal nature is a preferable feature for delivering designer nucleases such as CRISPR/Cas9 whose expression is only needed short-term to create the double-strand DNA breaks. We and others have produced HDAds expressing various nucleases [52-54]. In this context, it is noteworthy that the duration of Cas9 expression can be controlled by timed expression of anti-Cas9 inhibitors [55]. A recent report demonstrated a self-inactivating (SIN) CRISPR system by incorporating anti-Cas9 inhibitors in the same HDAd vector expressing CRISPR/Cas9 [56].
Integrated HDAd vector forms
Hematopoietic stem cell gene therapy requires that the gene modification is passed on to all progeny cells either through a permanent gene editing event or through gene addition after chromosomal integration. The latter can be achieved by various integration mechanisms, such as the Sleeping Beauty (SB) 100× transposase and HDR-mediated targeted integration. These approaches will be described in more detail in the following sections.
Absence of viral gene expression
Helper-dependent AdV do not contain any viral genes. Cytotoxicity associated with leaky viral gene expression seen with first-generation Ad vectors is therefore absent [57]. However, it should be mentioned that deletion of viral genes does not abrogate transcription-independent acute toxicity associated with virus uptake which will be discussed in the next section.
HDAd5/35++ with enhanced HSC tropism
Ad5 vectors use CAR for attachment to target cells. Group B adenoviruses utilize either desmoglein 2 (DSG2) [58] or CD46 [27] as primary receptors. While on HSCs CAR expression is only marginal, DSG2 and CD46 are uniformly expressed [59-62]. As consequence of this, HSCs can be readily transduced by members of the group B adenoviruses targeting either DSG2 [63] or CD46 [25], including Ad35, Ad11, Ad50, and Ad3. In order to be able to harness both, the stem cell transduction potential of these serotypes and the well-understood Ad5 vector system, fiber chimeric adenovirus vectors have been developed. These vectors are based on Ad5 and contain either just the fiber knob or the fiber knob and shaft of other serotypes. Examples include Ad5/3 [64], Ad5/35 [25], Ad5/11 [65], and Ad5/50 [66] fiber chimeric vectors. Studies done by us and others demonstrated that fiber chimeric Ad5/3 and Ad5/35 vectors can efficiently transduce HSCs of both humans and nonhuman primates (NHPs) [63,67]. Specifically, Ad5/35 vectors have been widely used for gene transfer into human HSCs [25,59,68-70] and human lymphocytes, the main target for HIV-1 [71-77]. Ad5/35 vectors also have been and are currently being employed in clinical trials to treat viral infections in immunocompromised individuals [76,78].
HDAd5/35++ denotes helper-dependent chimeric Ad5/35 vectors containing a mutant Ad35 fiber knob that was obtained by library screening. This mutant displayed over 25-fold higher affinity to CD46 and allowed for more efficient cell transduction at lower multiplicity of infection [79]. Of significance to innate acute toxicity is that upon systemic delivery, HDAd5/35++ showed much less liver sequestration and hepatocyte transduction than HDAd5 [38]. Although the definitive mechanism remains to be elucidated, this is probably due to the strong affinity of HDAd5/35++ to CD46 and a blockade of the interaction between Ad5 hexon and coagulation factor X (FX). The hexon–FX interaction is considered to mediate hepatocyte uptake of the virus [80-82].
Animal models for HDAd5/35++-mediated HSC gene therapy
Of all mammals, only NHPs express CD46 in a pattern similar to humans [83]. The only notable difference is that human erythrocytes lack CD46, while it is expressed on erythrocytes from NHPs. In previous studies, we showed that both baboons and macaques are adequate models for safety and efficacy studies with Ad35 fiber containing vectors or recombinant proteins [84-86].
In contrast to humans, where CD46 is expressed on all nucleated cells, CD46 is expressed only in the testis of mice. For further safety and efficacy studies, we therefore used human CD46 transgenic mice (strain MCP-8B) that express CD46 in a human-like pattern [87].
Another model is humanized mice. We transplanted human CD34 cells into sublethally irradiated, immunodeficient NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac (NOG) mice. Average engraftment rates at 6 weeks after transplantation were 25% based on huCD45 cells in the blood. Of note, all human cells express the HDAd5/35 receptor hCD46. Disadvantages of this model include an abnormal bone marrow structure and hematopoiesis [88].
Genome engineering strategies using HDAds
A variety of strategies have been adopted for genome engineering in HSCs. We will mainly discuss transient expression of nucleases and BEs, approaches to control transgene duration, as well as stimulation of transgene integration through transposases, homology-dependent repair (HDR), and hybrid vectors. A diagram is included to show these examples (Fig. 1).
Transient/episomal expression
Site-specific DNA breaks mediated by endonucleases
A major tool for genome engineering is engineered nucleases that generate double-stranded DNA breaks (DSBs) in preselected genomic sites (for a review, see [89]). The DSBs trigger endogenous cellular repair mechanism, that is, nonhomologous end joining (NHEJ), creating micro insertions or deletions (Indels) and, as a consequence, perturbing gene function. In the presence of a donor template, homology-dependent repair may occur, resulting in controlled modifications. There are now several different nuclease platforms available, with the most widely used ones being zinc finger nuclease (ZFN) [90], transcription activator-like effector nuclease (TALEN) [91], and CRISPR/Cas9 ([92,93]). By constructing a tandem array of various artificially engineered modules, ZFN and TALEN can target virtually any DNA sequence [94,95]. CRISPR/Cas9, derived from the CRISPR/Cas system of S. pyogenes, is composed of a human codon-optimized Cas9 protein and a single-guide RNA (sgRNA). It can be programmed to target any genomic locus followed by a 5′-protospacer adjacent motif sequence of NGG, with the specificity determined by the sgRNA containing a 20-nt guide sequence complementary to the genome locus of interest [92,93]. Nucleases are employed to knockout genes, correct frame shift mutations, delete or rearrange chromosomal regions, or knock-in wild-type or mutated cDNA into the endogenous or a heterologous site. Moreover, a variety of Cas9 derivatives and orthologs have been developed, for instance, CRISPRa [96], Cpf1 [97], saCas9 [98], and Cas13 [99] to name a few. Owning to the simplicity of manipulation and versatility, these robust tools allow widespread applications [100,101]. A CCR5-ZFN expressed by an Ad5/35 vector from Sangamo Therapeutics is currently tested clinically in HIV/AIDS patients [102]. In addition to our work with ZFNs, TALENs, and CRISPR/Cas9 expressed from HDAd5/35 vectors [52,53,55,103], many other research groups have demonstrated successful genomic editing using Ad vectors, including HDAd, expressing CRISPR/Cas9 [104-109]. Tsukamoto et al. reported the production of a panel of first generation of Ad vectors containing CRISPR/Cpf1 system [110,111].
High expression of endonucleases is considered to be harmful to the Ad producer cells and can lead to genomic rearrangements within the Ad genome or to low vector yields. A number of approaches have been developed to address this problem including the use of conditionally active promoters and downregulation of nuclease expression by shRNA [111]. We have exploited microRNA regulation to suppress ZFN and CRISPR/Cas9 expression in 293 and 116 cells [52]. In the context of HDAd production, we have also incorporated anti-CRISPR peptides into the helper vector [55,103]. Ng et al. expressed anti-CRISPR peptides in 116 cells [56].
Single nucleotide mutations by base editors
Base editors comprise a catalytically disabled nuclease, such as Cas9 nickase, fused to a nucleobase deaminase enzyme and, in some cases, a DNA glycosylase inhibitor. Currently, there are two categories, cytidine BEs (CBEs) and adenine BEs (ABEs) [112-114]. They are capable of installing precise nucleotide mutations at targeted genomic loci without creating DSBs. Nuclease-induced DSBs are found to be detrimental to host cells by causing unwanted large fragment deletion [115] and p53-dependent DNA damage responses [116,117]. This warrants concern particularly when editing fragile primitive stem cells. It is estimated that ~ 60% of pathogenic point mutations can be reversed by current BEs [118]. Current BE platforms appear to exert significant off-target activity at the RNA level [119,120]. With the rapid development of novel applications [121,122] and new BE variants [123,124], the amount of targetable mutations is projected to expand dramatically.
Adenoviral vectors are advantageous for delivery of BEs, with regard to the ~ 8 kb size for an all-in-one BE construct. We have successfully generated HDAd5/35++ vectors carrying CBE- or ABE-targeting critical motifs regulating γ-globin reactivation [125]. Naturally occurring loss-of-function mutations in the angiopoietin-like 3 (ANGPTL3) gene are associated with reduced blood triglycerides and low-density lipoprotein cholesterol. Chadwick et al. [126] obtained on average 35% on-target editing after intravenous injection of an Ad5 vector-bearing BE components targeting ANGPTL3 into mice. Another study performed by the same research group targeted the Pcsk9 gene using a similar in vivo method in mice [127]. Ad-BE-Pcsk9 installed 24% base conversion in the liver, leading to > 50% reduction of plasma PCSK9 protein level. By employing the latest versions of BEs with improved activity [128,129], rather than the suboptimal BE3 in their study, it is conceivable that higher editing efficacy is likely to be achieved. The efficacy of in vivo HSC base editing remains to be investigated.
Controlling transgene duration
Adenoviral vectors predominantly persist as episomal DNA [130]. Various mechanisms can be utilized to either shorten or extend the episomal transgene expression of Ad vectors [131-133]. More extensive E3 deletion in first generation of Ad vectors was associated with weaker immune responses and, consequently, contributed to extended transgene expression [134]. For pursuing prolonged episomal expression, HDAds are of significant advantage because of the absence of adaptive immunity against viral gene products [135]. It is known that the binding of TPs with ITRs is a major player in the stability of episomal DNA and its retention during mitosis. It is also found that the inclusion of retroviral LTRs in Ad vectors helped the virus persist [136]. Another interesting study presented by Gil et al. [137] showed that the introduction of self-replicating elements of Epstein-Barr virus into HDAd genome resulted in extended and nonintegrating expression.
With regard to delivery of nucleases and BEs, of more importance is to limit the duration of their expression in target cells. Extended presence of the editing machinery is considered to be detrimental to the cells because it can increase chances of off-target effects. This is particularly relevant for HSC gene editing. It is generally thought that once editing is completed, the editing machinery should be eliminated, the faster the better. This is called a ‘hit-and-run’ strategy [138,139]. As to controlling duration, electroporation of nuclease mRNA may be the best way. However, this approach is associated with significant cytotoxicity particularly for HSCs and, more importantly, not applicable for in vivo genome engineering. The naturally existing CRISPR inhibitors have shown potential to regulate the duration of CRISPR/Cas9 activity. CRISPR systems protect bacteria against invading bacteriophages. In response to this, phages have evolved counteracting peptides that bind to and inactivate Cas proteins as they search for foreign nucleic acid [140]. Among the reported peptides, AcrIIA2 and AcrIIA4 showed high anti-Cas9 potency [141]. We found that by timed delivery of a second HDAd5/35++ vector expressing AcrIIA2 and AcrIIA4, the Cas9 cleavage activity was not compromised in both primary cell lines and human CD34+ cells, demonstrating that a short-term expression is sufficient for CRISPR/Cas9 to perform editing. Importantly, the controlled Cas9 expression significantly preserved the engraftment capability of edited CD34+ cells in humanized NSG (NOD/Shi-scid/IL-2Rγnul1) mouse model [107]. Development of SIN and all-in-one CRISPR systems represents another promising strategy. This approach requires robust suppression of nuclease expression during vector production. By exploiting miRNA regulation to degrade Cas9 mRNA and anti-Cas9 peptides to inhibit Cas9 protein activity, Philip Ng’s group demonstrated the successful production of such HDAd vectors [56].
Integrated expression
Random integration mediated by transposase
In contrast to the intrinsic transient episomal expression, the incorporation of integrating mechanisms can lead to long-term transgene expression. For gene therapy of many diseases, the stable transgene expression is a prerequisite to confer sustainable treatment benefits. Transposon systems have emerged as promising tools for stable gene transfer. Ongoing clinical trials have employed them for therapeutic gene integration [142]. SB [143], piggyBac (PB) [144], and Tol2 [145] are among the most widely utilized transposon systems [146]. These systems usually require co-delivery of a donor transposon vector containing the gene of interests flanked by transposon DNA elements (i.e., inverted repeats/direct repeats, IR/DRs) and another vector expressing the transposase. Yant et al. [147] showed for the first time that systemic delivery of HDAd vectors carrying a SB system with a human factor IX transgene resulted in integration and persistent factor IX expression in mice. Since circular DNA is required for efficient transposition by the SB system, a Flp-FRT recombination system was also incorporated into the SB gene-containing vector [147]. Through molecular evolution, a new generation of hyperactive SB transposase (SB100×) exerted 100-fold enhancement in efficiency and strong activity in human CD34+ cells without affecting their multipotency [148]. Notably, 70% of the integrations occurred in intergenic regions. No preferential integration into genes was observed, in contrary to the integration pattern mediated by RVs and LVs. SB100× appeared to be superior to the PB system that exhibited a preference for integrations in regions surrounding transcriptional start sites and within long terminal repeat elements [149]. We have demonstrated the efficacy of SB100×-mediated integration into HSCs by HDAd5/35++ vectors in several disease models, including β-hemoglobinopathies, hemophilia A, and Fanconi anemia (FA) [150,151]. More studies have reported gene transfer by Ad vectors expressing other transposon systems, such as PB [152], T2TP [153], and bacteriophage-derived integrase PhiC31 [154]. Delivery of these transposons/integrases to HSCs by HDAd vectors remains to be fully explored.
Targeted integration into preselected sites
The semi-random integration pattern mediated by γ-RVs and LVs entails a risk of perturbing the expression of neighboring genes, including cancer-related genes. Theoretically, the random integration profile mediated by SB100× transposase and the lack of a preference for integration into active genes and promoters should be safer but concerns about genotoxicity remain. In addition, the integration into heterochromain associated sites may lead to gene silencing [44,155]. Therefore, a major effort in the field is aimed toward targeted transgene integration into preselected sites. A number of ‘safe harbors’ for targeted integration into the human genome have been suggested, for example, AAVS1 and CCR5 loci [156].
Targeted integration via homologous recombination without site-specific DNA breaks (DSBs) is inefficient [46]. It has been shown that DSBs stimulate HDR pathways and gene addition through homologous donor templates [157-161]. Hence, nucleases are commonly used to create DSBs, accompanied by the delivery of the donor cassette. However, the HDR pathway is still much less efficient that the NHEJ repair mechanism, particularly in quiescent stem cells [162,163]. Recently, with the remarkable advancement in genome editing techniques [17,164], the frequency of HDR has been improved dramatically. For ex vivo gene transfer into human CD34+ HSCs, zinc-finger nuclease mRNA and rAAV6-mediated donor template delivery resulted in ~ 15% targeted integration into the AAVS1 locus [165]. In another study that employed RNP electroporation to express CRISPR/Cas9 combined with rAAV6 transduction to deliver the donor template, the frequency of site-specific integration to correct the HBB sickle mutation was on average 25% [166]. These high-level HSC gene editing and HDR events are achieved through optimization of experimental parameters, such as modifications of sgRNA, time, and dose of AAV6 transduction. Of interest is to compare the performance of AdV with rAAV6 as the donor template vector. One study found that rAAV6 was more efficient than the first generation of Ad5/35 as a donor vehicle [167]. Recently, we demonstrated the utility of a HDAd5/35++ as a donor vector in mediating high frequency of HDR- and AAVS1-targeted integration [103]. In an in vivo HSC gene transfer study with AAVS1-transgenic mice, we have obtained efficient gene marking in peripheral blood cells with 80% of responsive rate. Of particular importance was that the gene expression level was significantly higher than that of SB100×-mediated random integration, which is most likely the result of integration into AAVS1, a locus with open chromatin [103]. Notably, HDAd5/35++ represents a potential good candidate for the delivery of large donor cassettes exceeding the capacity of rAAV6.
Other integrating hybrid vectors
In addition to the above-discussed Ad/transposase hybrid systems, other hybrid vectors have also been exploited for integrated expression of transgenes delivered by Ad vectors, such as AdV/γ-RV [168,169], AdV/foamy viral vectors [170,171], and Ad/AAV [172,173] (For a comprehensive review, see [174]). In essence, the integration mechanism relies on the incorporated viral elements from other viruses. As an example, we have previously reported on an HDAd5/35.AAV hybrid vector expressing a GFP reporter gene driven by the β-globin LCR. The LCR-GFP cassette with a length of 27kb was flanked by AAV ITRs. Integrated stable expression was observed in human CD34+ cells [44]. By co-infection with another HDAd5/35 vector expressing Rep78, the AAV gene responsible for AAV integration into the AAVS1 locus, targeted integration region was shown [175].
In vivo HSC transduction
Current HSC gene therapy involves harvesting hematopoietic stem/progenitor cells (HSPCs) from the patient, genetically engineering HSPCs ex vivo, and reinfusion back to the same patient following myeloablative conditioning. This procedure is not without risks. Commonly encountered challenges include insufficient collection of HSPCs by leukapheresis or bone marrow aspiration, genotoxicity and limitations of the viral vectors, loss of HSC multipotency during ex vivo manipulation, inadequate number of modified cells to confer successful engraftment, and morbidity and mortality associated with myeloablation. Moreover, the technical complexity and high cost of current ex vivo HSC gene therapy are barriers to a widespread application for common diseases. As an example, Strimvelis, an HSC gene therapy for ADA-SCID that has been approved for marketing in the European Union in 2016, comes at a price of close to $700 000 per treatment, and the regimen is only available at a single center in Europe, making access to it difficult even within the developed countries of Europe.
Our group recently developed an in vivo HSC transduction approach that offers the potential to address the aforementioned obstacles in the ex vivo procedure [38]. In our approach, the HSCs residing in the bone marrow niche were mobilized into the peripheral circulation through administration of granulocyte colonystimulating factor and the CXCR4 antagonist, Plerixafor/AMD3100. At the peak of mobilization (i.e., when HSPCs in the circulation reached the highest level), an integrating HDAd5/35++ vectors expressing a GFP reporter together with a SB100x/Flpe-expressing vector were injected intravenously. HSPCs transduced in peripheral blood homed back to bone marrow shortly after transduction. At week 12 post-transduction, 16.5% of total colony-forming cells stably expressed the reporter gene. This level of gene marking was maintained in HSPCs and lineage cells of secondary transplanted mice, confirming genetic modification of HSCs with multilineage, long-term repopulating capability [38]. However, it is noteworthy that the high level of gene marking in bone marrow HSCs was not reflected in peripheral blood mononuclear cells (PBMCs) of the primary mice. This was likely in part due to a lack of a proliferative stimulus for the modified HSCs. To overcome this drawback, we therefore combined the in vivo transduction with an in vivo selection mechanism by incorporating a mutant O6-methylguanine–DNA methyltransferase (mgmtP140K) gene that confers resistance to O6-BG/BCNU (O6-Benzylguanine/Carmustine) [176-178]. After in vivo transduction and selection with three low doses of O6-BG/BCNU administered intraperitoneally with interval of 2 weeks, transgene marking in PBMCs was increased to > 50% [176]. Importantly, the approach has been tested by delivering several therapeutic genes, for instance, γ-globin, and with optimized procedures we routinely obtained more than 80% gene marking in HSCs and peripheral blood cells [53,103,179].
The in vivo HSC transduction approach has a number of preferable features. First, the ability of cost-efficient production of high yields of Ad vectors is desirable for in vivo transduction, which requires large amounts of vectors. Second, HDAd5/35++ vectors have a ~ 32 kb insert capacity, and importantly, high tropism to HSCs that uniformly express the HDAd5/35++ receptor, CD46, at high levels. Third, mobilized HSPCs are transduced with HDAd5/35++ vectors in the periphery, and home back to the bone marrow shortly after transduction. No ex vivo manipulation of HSCs and no myeloablation are needed. Fourth, the integrating HDAd5/35++ vectors are designed for persistent transgene expression. The SB100×-mediated integration shows a good safety profile without preferential insertion into exons. Notably, the selection mechanism can be combined with transient expression simply by inserting the genes outside of the two IR/DRs elements. Fifth, because of the fiber modification to HDAd5/35++ and dexamethasone administration prior to transduction, the procedure of systemic delivery is well tolerated by the mobilized animals reflected by low transduction of liver cells, no elevation of transaminases and pro-inflammatory cytokines. Ongoing studies in NHPs have confirmed the safety and efficacy of the in vivo transduction/selection approach.
A large fraction of humans has neutralizing serum antibodies directed against Ad5 capsid proteins, which will block in vivo transduction with Ad5/35 vectors, that is, vectors that contain Ad5 capsids proteins and chimeric Ad35 fibers. An alternative would be vectors derived from Ad35. (a) Ad35 is one of the rarest of the 57 known human serotypes, with a seroprevalence of < 7% [180-184]; (b) Ad35 is less immunogenic than Ad5 [185], which is, in part, due to attenuation of T-cell activation by the Ad35 fiber knob [186-188]; (c) after intravenous injection, there is only minimal transduction (only detectable by PCR) of tissues, including the liver, in human CD46 transgenic (hCD46tg) mice [189] and NHPs [190]. Work on HDAd35 vectors is ongoing in several laboratories including ours.
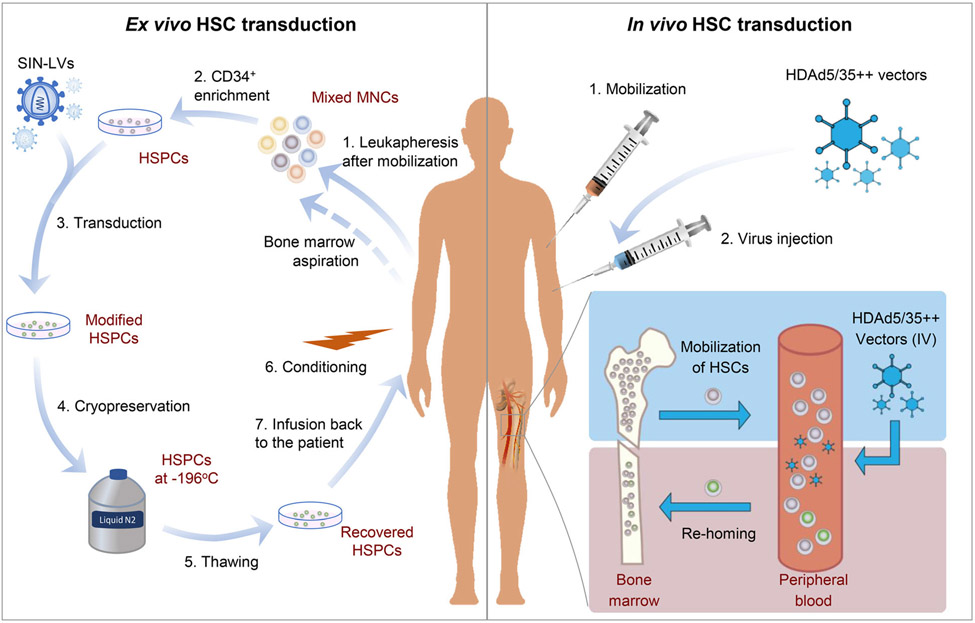
Figure 2 shows typical procedure for ex vivo and in vivo HSC gene transfer. Others have also reported in vivo HSC transduction approaches using nonviral [191] or viral vectors [192,193]. Readers are recommended to refer to our recent review for more examples [194].
Fig. 2.
Ex vivo VS in vivo HSC transduction. Ex vivo HSC gene transfer is started with harvesting white MNCs in blood by leukapheresis after mobilization. Alternatively, bone marrow cells can be collected by aspiration. HSPCs are usually enriched from total MNCs by selection for CD34+ cells. The HSPCs are then be transduced using viral vectors (usually SIN-LVs) carrying transgenes. Modified cells are cryopreserved for shipment or if multiple harvesting/transductions are needed for accumulating more cells. Subsequently, the modified HSPCs are thawed, recovered, and infused back to the same patient following myeloablative conditioning. For in vivo HSC transduction, viral vectors (e.g., HDAd5/35++ vectors) expressing transgenes are administered intravenously following mobilization of HSCs. HSCs egressed from bone marrow are transduced in blood. Shortly following transduction, the modified cells home back to bone marrow and persist long-term.
Progress in gene editing for hematological disorders
β-hemoglobinopathies
β-hemoglobinopathies are a group of genetic disorders with abnormal production of β-globin chain of hemoglobin—mainly including β-thalassemia and sickle cell disease (SCD). β-thalassemia is caused by diverse mutations resulting in various severity including thalassemia intermedia (β+) and major (β0). SCD results from a single nucleotide transversion (A–T) in the sixth codon of the β-globin gene HBB leading to Glu6-Val mutation. SCD is named based on the characteristic phenotype of red blood cells: a crescent or sickle shape caused by polymerization of the pathogenic β-globin (βS) upon deoxygenation. Currently, treatments, including blood transfusion and hydroxyurea, are largely supportive. The only curative approach relies on allogenic HSC transplantation, which is not available to most patients due to lack of matching donors.
Gene therapy using autologous HSC transplantation has achieved encouraging progress. Current advancements in clinical trials mainly come from gene addition strategies. They are based on LV-mediated gene transfer of the wild-type human β-globin gene, the fetal γ-globin gene, or β-globin mutants with antisickling mutations. Specific vector examples that are under clinical trials include BB305 (βA-T87Q, NCT02140554) [195], sGbG (γ-globin, NCT02186418) [196,197], βAS3-FB (βA-AS3, NCT02247843), GLOBE (βA, NCT02453477) [198], and TNS9.3.55.A1 (βA, NCT01639690) [199]. Among them, BB305 (trade name: Zynteglo) has received conditional approval from European Medicines Agency (EMA) [200]. Overall, therapeutic benefits have been observed in most of the reported patients after treatment, with non-β0/β0 patients showing more significant improvement [11-13,195,201,202]. Nevertheless, there are challenging aspects of LV design because of the requirement to achieve erythroid-specific and high-level globin expression while avoiding genotoxicity and not compromising viral titer. Although all these above vectors are delivered by SIN-LVs that should have an improved safety profile [203], the risk of insertional mutagenesis cannot be ruled out. A clonal dominance was observed in the first β-thalassemia patient who became transfusion independent after treatment with HPV569-transduced HSPCs (HPV569 is an early generation of BB305) [13]. This command caution and calls for longer follow-up to better evaluate the vector safety profile.
AdVs have become an efficient vehicle for HSC gene transfer since the development of vectors derived from group B adenoviruses [25]. We recently generated an integrating HDAd5/35++ vector system for γ-globin gene addition [204]. The γ-globin was driven by a 5 kb β-globin LCR/promoter. An mgmtP140K gene under an elongation factor 1 α promoter (EF1α) was included for selection. The 12 kb transgene with regulatory elements was flanked by two FRT-IR/DRs sequences at each side. Upon co-transduction of lineage-negative cells from CD46 transgenic mice with another vector expressing a SB transposase and Flpe followed by transplantation into irradiated C57BL/6 mice (ex vivo transduction setting), γ-globin expression was detected in 80% of peripheral erythrocytes without selection. The percentage of γ-globin-positive erythrocytes increased to nearly 100% after selection. The γ-globin level was 10–20% of adult mouse globin. This ex vivo transduction efficacy was recapitulated in human CD34+ cells transplanted into NSG mice. Importantly, this vector system also worked efficiently in in vivo transduction settings. In a thalassemia CD46+/+/Hbbth-3 mouse model, we detected similar levels of γ-globin and a near complete phenotypic correction. Genome-wide integration analyses revealed a random pattern without a preference for genes [179], in agreement with previous reports [148,205]. To achieve higher levels of γ-globin expression, we established a targeted integration system with two vectors [103]. One contained a CRISPR/Cas9 specific for human AAVS1 locus. The other provided a donor template that carried the same 12 kb γ-globin transgene cassette flanked by two homology arms (HAs). The two HAs, amplified from human AAVS1 locus surrounding the CRISPR cutting sites, were further flanked by two CRISPR cutting sites to facilitate the release of donor template. In a human AAVS1 transgenic mice model, 80% responsive rate was obtained after transduction with the two vectors and in vivo selection. Of particular importance is that the γ-globin level reached on average 24% of adult mouse globin, significantly higher than levels achieved with SB100×-mediated random integration [103]. Integration site analyses revealed a predominant targeted integration into the intended AAVS1 locus. Another finding is that longer HAs appeared to increase the efficacy of targeted integration. It remains to be investigated whether further extension of the homology regions is beneficial. As an alternative target locus, one of the α-globin loci can be considered because an excess amount of α-globin production in β-thalassemia and SCD patients causes ineffective erythropoiesis and hemolysis [206].
Gene editing has also generated enormous enthusiasm for the treatment of hemoglobinopathies. The main objective is to reactivate the endogenous γ-globin that is silenced shortly after birth and capable of functioning as an alternative to β-globin, or to directly reverse the sickle mutation. Through genome-wide association study, BCL11A was found to be a master suppressor responsible for γ-globin dormancy [207,208]. Knockout of the BCL11A gene is not a viable option due to its indispensable roles in development. However, reports demonstrated that partially knockdown of BCL11A by targeting some critical motifs in its erythroid enhancer led to efficient de-repression of γ-globin while not affecting animal viability [209,210]. Strategies based on shRNA- and CRISPR-mediated down-modulation of BCL11A have entered clinical stage (NCT03282656 and NCT03655678). Using an HDAd5/35++ vector, significant γ-globin reactivation was observed by CRISPR/Cas9 targeting the critical GATAA motif in the BCL11A enhancer [55,211]. Alternative strategies include the disruption of binding sites of repressors within the HBG promoter region [212-214], the repair of the sickle mutation [215], and the forced modification of chromatin structure [216]. These strategies would be benefited considerably from recent advancement of gene editing efficiency. Latest reports have shown more than 90% disruption of the BCL11A GATAA motif in HSCs by electroporation of Cas9 RNPs [164]. When combining with rAAV6 transduction to deliver a donor template, over 20% HDR-mediated repair of the sickle mutation has been demonstrated [166]. As an alternative, Romero et al. [167] reported that RNP electroporation can be combined with Ad5/35 transduction for ex vivo gene editing of human HSCs to correct the sickle HBB mutation. Li et al. [217] demonstrated efficient repair of the sickle mutation in induced pluripotent cells using an HDAd5/35 vector. We constructed an HDAd5/35++ vector-expressing CRISPR/Cas9 specific for the BCL11A binding site in HBG promoter. Transduction of human CD34+ cells with this vector resulted in markedly increased production of γ-globin. In a β-YAC/CD46 transgenic mice model, in vivo transduction resulted in ~ 33% target site cleavage and ~ 15% γ-globin production over mouse β-major globin [53]. The degree of phenotypic correction of β-thalassemia or SCD is closely correlated with the level of γ-globin expression. We therefore combined the two mechanisms, that is, CRISPR/Cas9-triggered reactivation of endogenous γ-globin and SB100× transposase-mediated γ-globin gene addition, into one vector and obtained additive levels of γ-globin (~ 40% over mouse β-major globin) [218]. One additional advantage of this strategy is the shortened duration of CRISPR/Cas9 expression as a result of disruption of viral genome by transposition (Fig. 1). In addition to the CRISPR system, emerging base editing technologies are being pursued to precisely install single nucleotide mutations controlling fetal globin de-repression [125].
Fanconi anemia
Fanconi anemia is caused by mutations in any of the 22 identified FANC genes: FANCA–FANCJ, and FANCL–FANCW. FA pathway genes play critical roles in repair of damaged DNA—specifically, DNA interstrand cross-links. Thus, pathogenic mutations in FA genes result in chromosomal instability. FA patients are clinically manifested by congenital impairment, bone marrow failure, and cancer occurrence. Among these genes, FANCA mutations are responsible for over 60% of all cases of FA [219-221]. The same as β-thalassemia and SCD, allogenic transplantation with healthy donor HSCs is the only curative therapy.
Gene therapy for FA has been investigated for more than two decades. Early studies mainly used γ-RVs and led to no persistent therapeutic benefits [222,223], partly due to the inefficiency of γ-RVs to transduce primitive HSCs. With the advancement of LV vectorology, some important progress has been made. Juan Bueren’s research team from Spain recently published data from their clinical trial (NCT03157804) [224]. Four patients with FANCA mutations received transplantation with autologous CD34+ cells that were transduced ex vivo with a LV expressing a functional FANCA under a phosphoglycerate kinase (PGK) promoter. No conditioning was conducted prior to engraftment. Progressive repopulation of the bone marrow cells from gene-corrected HSCs was observed over a period of up to 30 months following treatment. Although the results are encouraging, continued surveillance will corroborate the integration safety profile and successful transduction of multipotent HSCs.
Besides LV-mediated gene transfer, other vectors are being investigated, such as foamy viral vectors [225], and measles virus envelope pseudotyped LVs [226]. HDAd5/35++ vectors also represent a promising strategy—particularly of relevance for in vivo HSC transduction. In addition to the above-described advantages of the HDAd5/35++ vector platform, several lines of evidence related to the nature and pathophysiology of FA further highlight the therapeutic potential of in vivo HSC gene transfer: (a) HSCs of FA patients are very fragile—making ex vivo manipulation more challenging; (b) generally, it is difficult to harvest large amounts of CD34+ cells from FA patients, and transplantation with insufficient number of gene-modified cells tends to result in reduced or even failure of engraftment; (c) due to proliferative advantage of gene-corrected HSCs over mutant HSCs, a few in vivo corrected primitive cells are likely to progressively reconstitute the blood system by overgrowing defect cells (supported by the evidence from natural gene therapy in monozygotic twins [227]). Our previous study demonstrated that in vivo transduction led to ~ 20% of gene-marked colony-forming unit (CFU)-forming cells in mice bone marrow [38]; therefore, selection may not be necessarily needed. Our HDAd5/35++ FancA gene therapy vector contained the human FancA gene under control of the PGK promoter as well as an EF1α-promoter-GFP gene expression cassette. Integration was mediated by SB100x. To generate a FancA model for in vivo HSC transduction with HDAd5/35++ vectors, we crossed CD46 transgenic mice with FancA−/− mice. We found that GSCF/AMD3100 treatment of hCD46+/−/FancA−/− mice resulted in efficient HSC mobilization, that is, a > 10-fold increase in peripheral blood LSK cells 1 h after AMD3100, at which time animals were injected with the HDAd-FancA/GFP vector. Six weeks after HDAd injection, 120 mg/kg of the DNA damaging drug cyclophosphamide (CY) was given to determine the presence of any gene-modified FA cells, which should be more resistant to CY exposure than uncorrected FancA cells. Before CY (week 5) FancA mRNA was not detectable by qRT-PCR in PBMCs. One week after CY (week 7), the level of FancA mRNA was ~ 0.4% of the house-keeping gene mRPL10. The percentage of GFP-positive PBMCs before CY (week 5) was, on average, 0.25%. One week after CY, it increased to 0.8% and was then stable at a marking rate of ~ 15%. Bone marrow mononuclear cells (MNCs) harvested from mice 1 week after CY treatment, displayed a significantly greater resistance to the DNA cross-linker mitomycin C in progenitor CFU assays (> 3-fold more colonies than in control animals) indicating a partial phenotypic correction of FA. Two weeks after CY, hematological parameters were fully recovered and a second injection of CY (60 mg·kg−1) was given. This preliminary study indicates that in vivo HSC gene therapy of FancA is possible without in vivo selection with O6BG/BCNU.
Hemophilia
Hemophilia is an inherited bleeding disorder resulting from a deficiency of blood coagulation factors. Genomic mutations in the X-linked factor VIII and IX coding genes (F8 and F9) cause hemophilia A and B, respectively. Severe forms of hemophilia are characterized by spontaneous bleeding episodes. Current standard treatment requires repeated infusions of recombinant FVIII and FIX factors that are very costly (~ $400 000 per year) and accompanied by complications.
Gene therapy for hemophilia—by providing a functional copy or by correcting mutations of the deficient F8 or F9 genes—may lead to sustained benefits. A number of features collectively make hemophilia an ideal target for gene therapy: the monogenic etiology, maintained clotting activity when synthesized by various cell types, low factor activity needed for clinical benefits (5% of normal activity), and simple approaches for activity measurement, etc. These features also have contributed to human trial achievements. After treatment, a majority of patients exhibited improvement in ongoing clinical trials, which are all based on liver-targeted rAAV vectors. Three of these trials sponsored by different companies have reached Phase III [two for hemophilia B (NCT03861273 and NCT03569891); one for hemophilia A (NCT03370913)] [16]. The results are undoubtedly encouraging. However, the widespread application of existing rAAV-based approach could face several obstacles: (a) mostly episomal nature of rAAV genomes affecting durability of efficacy, specifically in children with actively dividing hepatocytes (patients under 18 years old are excluded by most of current gene therapy trials); (b) the high cost of rAAV vector production, estimated to be > $1 M per patient; (c) the limited packaging capacity of rAAV which cannot accommodate large transcriptionally regulatory elements (viral titer drops precipitously with transgenes > 5 kb); and (d) the pre-existing immunity to AAV capsid leads to loss of efficacy and circumvents repeated administration (subjects with pre-existing antibodies to the used rAAV serotype are not eligible for current clinical trials) [228]. Hence, the search for alternative approaches could address these limitations and expand our treatment options.
Helper-dependent AdVs represent an effective delivery vehicle. For Ad5-based HDAd vectors, they retain extremely high tropism to hepatocytes while exhibiting much reduced immunogenicity. A series of studies have demonstrated that HDAd vectors mediated longterm therapeutic FVIII or FIX expression in transgenic mice, hemophilia dogs, and NHPs [229-234]. In addition, optimization of HDAd vector design, such as development of chimeric vectors with eliminated uptake by Kupffer cells [235], could further improve the transduction efficacy and safety profile. For integrated expression, SB transposase was observed to result in persistent hemostatic correction of canine hemophilia B with negligible toxicity [236]. Targeted integration of a corrective F9 gene into the ROSA26 safe harbor locus by AdV-delivered CRISPR/Cas9, and a donor template has led to phenotypic correction in a murine model with juvenile hemophilia B [106]. Efficient targeted integration of a F9 gene was also demonstrated by using a single HDAd vector simultaneously carrying CRISPR/Cas9 and a donor cassette [105]. Instead of liver-directed gene transfer, HSC-directed F8 gene transfer has been investigated and showed advantages in tackling pre-existing immunity [237]. We recently reported on an approach for HSC-derived, erythroid-specific F8 production based on HDAd5/35++ vectors [151]. It has been shown that the abundance and systemic distribution of erythroid cells can be harnessed for high-level production of therapeutic proteins with induced immune tolerance [238]. Using a vector carrying a GFP reporter under β-globin LCR/promoter together with the SB100× vector for targeted integration, we first showed that in vivo transduction led to sustained erythroid cell-restricted GFP expression, confirming the lineage specificity of LCR promoter. We then constructed a therapy vector by replacing GFP with ET3, a bioengineered human factor VIII [239]. After in vivo transduction, we detected stable supraphysiological levels (~ 300%) of ET3 in plasma. A phenotypic correction of bleeding was observed after in vivo HSC transduction of hCD46+/+/F8−/− hemophilia A mice in spite of high plasma anti-ET3 antibody titers. This suggests that ET3 levels were high enough to provide sufficient noninhibited ET3 systemically and/or locally (in blood clots) to control bleeding. The high-level ET3 production in erythroid cells did not affect erythropoiesis. Importantly, a phenotypic correction of bleeding was observed after tested in a hemophilia A mouse model. In addition to its relevance for hemophilia A gene therapy, our approach has implications for the therapy of other inherited or acquired diseases that require high levels of therapeutic proteins in the blood circulation.
HIV-1/AIDS
HIV infection is the etiological cause of AIDS. Since the first reported case of AIDS [240], more than 38 years have elapsed. It is estimated that there were 38 million people worldwide living with HIV-1/AIDS by the end of 2018 [241]. Although the introduction of effective antiretroviral therapy (ART) has resulted in massive reductions in HIV-1 incidence and substantially improved the life quality of HIV-1 patients, HIV-1 infection remains incurable to the general population, and no vaccine is currently available.
Gene therapy has been explored as a potential treatment option for HIV-1/AIDS. One of the strategies is to target the predominant coreceptor CCR5, which is required for HIV-1 entry into cells. It is inspired by the observation that some Caucasian individuals carrying an inactive mutant of CCR5 (CCR5Δ32) are resistant to HIV-1 infection [242], and ostensibly, this mutation does not affect their health status. Various approaches have been investigated, such as shRNA and nucleases. Perez et al. have demonstrated that an Ad5/35 vector-encoding ZFN.CCR5 efficiently knocked out CCR5 expression in up to 50% of human primary CD4+ T cells, the most important HIV-1 target in vivo. As expected, genetic disruption of CCR5 provided robust and heritable protection against HIV-1 infection [76]. In a clinical trial based on this approach, a survival advantage for CCR5-modified cells over unmodified cells and reduced viral DNA were found [243]. Using a similar vector, we delivered CRISPR/Cas9 to target CCR5 and achieved near complete inhibition of HIV-1 infection in edited primary CD4+ T cells [244]. Robert Doms’s group adopted two Ad5/35 vectors expressing ZFNs to simultaneously target CCR5 and CXCR4, the other HIV-1 coreceptor. This strategy conferred the cells with resistance to infection by both R5- and X4-tropic viruses, and ablation of the two receptors did not affect cell proliferation and engraftment of the modified CD4+ T cells [245]. All these studies were based on first-generation AdVs. It would be interesting to test the efficiency of HDAd5/35++ vectors.
In addition to differentiated T cells, HSC-directed CCR5 gene editing has also been investigated. It is largely inspired by the case of the ‘Berlin Patient’ [246], who has become free of viremia for more than 12 years since he received two rounds of HSC transplantations with donor cells homozygous for CCR5Δ32. He is considered the first HIV-1 patient being functionally cured. Early this year, Gupta el al. reported a second case, the ‘London Patient’, who received a similar transplantation with CCR5Δ32 HSPCs but using a milder conditioning regime [247]. This subject has been negative for HIV-1 traces for over 2 years. A research team from China has attempted to reproduce these two cases by creating CCR5-null CD34+ cells using CRISPR/Cas9 [248]. The edited cells were later transplanted into a patient with both HIV-1 infection and acute lymphoblastic leukemia. While the level of CCR5-negative T cells derived from edited CD34+ cells was modest (around 5%), the patient achieved remission for both HIV-1 infection and leukemia for over 19 months after the procedure, and the investigators did not observe gene editing-related side effects. It is likely that the remission was achieved by several effects in combination, such as myeloablation, donor cell-mediated immune clearance, and CCR5 gene editing. Despite of this progress, most of other allogenic transplantations attempting to re-create the case of Berlin patient did not succeed, partly due to graft versus host diseases (GvHD) and viral tropism shift as a result of CCR5 mutation [249]. This calls for further optimization of the approach with less invasive interventions.
Another strategy is to target the proviral DNA integrated into the host genome. Although ART could suppress viremia to a level below the detection limit by current approaches, viral rebound from latent viral reservoir eventually appeared in most patients after discontinuation of treatment. A general ideal is to make two DSBs by nucleases in proviral DNA (e.g., in the LTRs), leading to the removal of the viral genome [250]. Alternatively, targeting conserved and critical viral genes has been found to disable the provirus [251]. Nevertheless, this strategy faces profound challenges, including the diversity of viral quasi-species, the fast mutating nature of HIV-1, the transcriptionally inactive latency, the establishment of latent infection in long-lived cell subsets (such as resident macrophages in various tissues), and the lack of ideal animal models.
With these challenges, multipronged combinatorial strategies likely hold more promise. This, in some way, resembles the principle of ART that uses a cocktail of medications blocking different steps of HIV-1 replication. A number of combinations have been investigated, such as targeted integration of an antiviral inhibitor-encoding gene into CCR5 locus [252], simultaneous targeting the entry receptor and viral essential genes [253], and combining CRISPR/Cas9-mediated excision of provirus with ART [254]. The capacity of HDAd5/35++ vectors is advantageous with regard to accommodating multiple antiviral mechanisms, and at the same time incorporating a selection mechanism to transduced cells. Notably, in vivo HSC engineering is likely of significance regarding its capability to remodel the immune system with HIV-resistant cells while avoiding GvHD-related complications.
Conclusions and perspectives
Adenoviral vectors are the most widely used vector in clinical trials. By the end of 2018, there have been 569 out of 2930 gene therapy clinical trials that used AdVs, mainly in anticancer studies (Gene Therapy Clinical Trials Worldwide by The Journal of Gene Medicine). The development of HDAds substantially reduced the immunogenicity and improved the safety profile of AdVs as a gene delivery vehicle. Chimeric HDAd5/35++ vectors possess ~ 32 kb payload capacity that can accommodate most therapeutic genes and gene editing tools. Importantly, they can efficiently mediate gene transfer into HSCs with an episomal nature while formulable for integrated expression. The addition of AdVs into our toolkit has provided more gene transfer options that may be beyond the capability of other vectors.
Hematopoietic stem cell gene therapy could provide a curative treatment for a number of blood diseases. The conventional approach is based on ex vivo HSC gene transfer and has achieved encouraging results. Two of the six FDA- or EMA-approved gene therapy products are carried out by ex vivo HSC transduction—Strimvelis for ADA-SCID; and Zynteglo conditionally approved by EMA for β-thalassemia. However, the high cost and side effects limit the patient accessibility of ex vivo HSC gene therapy, particularly in resource limited regions where most hematological diseases prevail. In contrast, in vivo HSC transduction is highlighted by its relatively low cost and technical simplicity. It could be provided as an outpatient treatment. Its efficacy has been demonstrated in several murine disease models, including β-thalassemia and hemophilia A [151,179], and its safety is currently tested in NHPs. With more gene therapy products on the horizon (such as hemophilia), the application of in vivo HSC transduction could extrapolate genetic treatments to a larger patient population. Further improvements of in vivo HSC gene therapy with HDAd vectors on the road to clinical application include more effective mobilization protocols (particularly for some patients, such as SCD patients), complete elimination of innate responses upon intravenous injection, more advanced capsid modifications that circumvent pre-existing anti-Ad immunity, improved selection regimens [240,255], as well as new methods for purification of clinical grade HDAd vectors.
Acknowledgements
We thank Jeff Chamberlain (University of Washington) for providing valuable information on rAAV production. We acknowledge the following funding sources: NIH R01HL128288, R01HL141781 and a grant from the Bill and Melinda Gates Foundation.
Abbreviations
- ABEs
adenine BEs
- Ad
adenovirus
- ADA
adenosine deaminase
- AdV
adenoviral vector
- ANGPTL3
angiopoietin-like 3
- ART
antiretroviral therapy
- BEs
base editors
- CAR
coxsackie adenovirus receptor
- CBEs
cytidine BEs
- CFU
colony-forming unit
- CRISPR
clustered regularly interspaced short palindromic repeats
- CY
cyclophosphamide
- DRs
direct repeats
- DSBs
double-stranded DNA breaks
- DSG2
desmoglein 2
- EF1α
elongation factor 1 α promoter
- EMA
European Medicines Agency
- FA
fanconi anemia
- FIX
Factor IX
- FVIII
Factor VIII
- FX
factor X
- GvHD
graft versus host diseases
- HAs
homology arms
- HDAd
helper-dependent adenovirus vectors
- HDAd5/35++
helper-dependent chimeric Ad5/35 vectors containing a mutant Ad35 fiber knob
- HDR
homology-directed repair
- HSC
hematopoietic stem cell
- HSPCs
hematopoietic stem/progenitor cells
- IRs
inverted repeats
- ITRs
inverted terminal repeats
- LCR
locus control region
- LV
lentiviral vector
- MCP
membrane cofactor protein
- mgmt
O6-methylguanine–DNA methyltransferase
- NHEJ
nonhomologous end joining
- NHPs
nonhuman primates
- O6-BG
O6-Benzylguanine
- PB
piggyBac
- PBMCs
peripheral blood mononuclear cells
- PGK
phosphoglycerate kinase
- rAAVs
recombinant adeno-associated viral vectors
- RNP
ribonucleoproteins
- SB
sleeping beauty
- SB100x
hyperactive SB transposase with100-fold enhanced activity
- SCD
sickle cell disease
- SCID
severe combined immunodeficiency disease
- sgRNA
single-guide RNA
- SIN
self-inactivating
- TALEN
transcription activator-like effector nuclease
- TP
terminal protein
- ZFN
zinc finger nuclease
- γ-RVs
γ-retroviral vectors
References
- 1.Aposhian HV (1970) The use of DNA for gene therapy–the need, experimental approach, and implications. Perspect Biol Med 14, 98–108. [DOI] [PubMed] [Google Scholar]
- 2.Friedmann T and Roblin R (1972) Gene therapy for human genetic disease? Science 175, 949–955. [DOI] [PubMed] [Google Scholar]
- 3.Mann R, Mulligan RC and Baltimore D (1983) Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell 33, 153–159. [DOI] [PubMed] [Google Scholar]
- 4.Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, Scaramuzza S, Andolfi G, Mirolo M, Brigida I et al. (2009) Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med 360, 447–458. [DOI] [PubMed] [Google Scholar]
- 5.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL et al. (2000) Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288, 669–672. [DOI] [PubMed] [Google Scholar]
- 6.Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J, Brouns G, Schmidt M, Von Kalle C, Barington T et al. (2004) Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet (London, England) 364, 2181–2187. [DOI] [PubMed] [Google Scholar]
- 7.Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, Brugman MH, Pike-Overzet K, Chatters SJ, de Ridder D et al. (2008) Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Investig 118, 3143–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K et al. (2008) Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Investig 118, 3132–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM and Trono D (1996) In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267. [DOI] [PubMed] [Google Scholar]
- 10.Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, Sergi Sergi L, Benedicenti F, Ambrosi A, Di Serio C et al. (2006) Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol 24, 687–696. [DOI] [PubMed] [Google Scholar]
- 11.Thompson AA, Walters MC, Kwiatkowski J, Rasko JEJ, Ribeil JA, Hongeng S, Magrin E, Schiller GJ, Payen E, Semeraro M et al. (2018) Gene therapy in patients with transfusion-dependent beta-thalassemia. N Engl J Med 378, 1479–1493. [DOI] [PubMed] [Google Scholar]
- 12.Ribeil JA, Hacein-Bey-Abina S, Payen E, Magnani A, Semeraro M, Magrin E, Caccavelli L, Neven B, Bourget P, El Nemer W et al. (2017) Gene therapy in a patient with sickle cell disease. N Engl J Med 376, 848–855. [DOI] [PubMed] [Google Scholar]
- 13.Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, Down J, Denaro M, Brady T, Westerman K et al. (2010) Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 467, 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroder AR, Shinn P, Chen H, Berry C, Ecker JR and Bushman F (2002) HIV-1 integration in the human genome favors active genes and local hotspots. Cell 110, 521–529. [DOI] [PubMed] [Google Scholar]
- 15.DePolo NJ, Reed JD, Sheridan PL, Townsend K, Sauter SL, Jolly DJ and Dubensky TW Jr (2000) VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol Ther 2, 218–222. [DOI] [PubMed] [Google Scholar]
- 16.Peyvandi F and Garagiola I (2019) Clinical advances in gene therapy updates on clinical trials of gene therapy in haemophilia. Haemophilia 25, 738–746. [DOI] [PubMed] [Google Scholar]
- 17.Martin RM, Ikeda K, Cromer MK, Uchida N, Nishimura T, Romano R, Tong AJ, Lemgart VT, Camarena J, Pavel-Dinu M et al. (2019) Highly efficient and marker-free genome editing of human pluripotent stem cells by CRISPR-Cas9 RNP and AAV6 donor-mediated homologous recombination. Cell Stem Cell 24, 821–828.e5. [DOI] [PubMed] [Google Scholar]
- 18.Rowe WP, Huebner RJ, Gilmore LK, Parrott RH and Ward TG (1953) Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med 84, 570–573. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeld M, Siegfried W, Yoshimura K, Yoneyama K, Fukayama M, Stier L, Paakko P, Gilardi P, Stratford-Perricaudet L, Perricaudet M et al. (1991) Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science 252, 431–434. [DOI] [PubMed] [Google Scholar]
- 20.Crystal RG (2014) Adenovirus: the first effective in vivo gene delivery vector. Hum Gene Ther 25, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson JM (2009) Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol Genet Metab 96, 151–157. [DOI] [PubMed] [Google Scholar]
- 22.Russell SJ and Peng KW (2017) Oncolytic virotherapy: a contest between apples and oranges. Mol Ther 25, 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosengart TK, Lee LY, Patel SR, Sanborn TA, Parikh M, Bergman GW, Hachamovitch R, Szulc M, Kligfield PD, Okin PM et al. (1999) Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation 100, 468–474. [DOI] [PubMed] [Google Scholar]
- 24.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL and Finberg RW (1997) Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275, 1320–1323. [DOI] [PubMed] [Google Scholar]
- 25.Shayakhmetov DM, Papayannopoulou T, Stamatoyannopoulos G and Lieber A (2000) Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. J Virol 74, 2567–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segerman A, Atkinson JP, Marttila M, Dennerquist V, Wadell G and Arnberg N (2003) Adenovirus type 11 uses CD46 as a cellular receptor. J Virol 77, 9183–9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaggar A, Shayakhmetov DM and Lieber A (2003) CD46 is a cellular receptor for group B adenoviruses. Nat Med 9, 1408–1412. [DOI] [PubMed] [Google Scholar]
- 28.Cattaneo R (2004) Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as Pathogens’ magnet. J Virol 78, 4385–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorig RE, Marcil A, Chopra A and Richardson CD (1993) The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75, 295–305. [DOI] [PubMed] [Google Scholar]
- 30.Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA and Lusso P (1999) CD46 is a cellular receptor for human herpesvirus 6. Cell 99, 817–827. [DOI] [PubMed] [Google Scholar]
- 31.Kallstrom H, Liszewski MK, Atkinson JP and Jonsson AB (1997) Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol 25, 639–647. [DOI] [PubMed] [Google Scholar]
- 32.Johansson L, Rytkonen A, Bergman P, Albiger B, Kallstrom H, Hokfelt T, Agerberth B, Cattaneo R and Jonsson AB (2003) CD46 in meningococcal disease. Science 301, 373–375. [DOI] [PubMed] [Google Scholar]
- 33.Okada N, Liszewski MK, Atkinson JP and Caparon M (1995) Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc Natl Acad Sci USA 92, 2489–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riley-Vargas RC, Gill DB, Kemper C, Liszewski MK and Atkinson JP (2004) CD46: expanding beyond complement regulation. Trends Immunol 25, 496–503. [DOI] [PubMed] [Google Scholar]
- 35.Anderson BD, Nakamura T, Russell SJ and Peng K-W (2004) High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Can Res 64, 4919–4926. [DOI] [PubMed] [Google Scholar]
- 36.Su Y, Liu Y, Behrens CR, Bidlingmaier S, Lee N-K, Aggarwal R, Sherbenou DW, Burlingame AL, Hann BC, Simko JP et al. (2018) Targeting CD46 for both adenocarcinoma and neuroendocrine prostate cancer. JCI Insight 3(17), e121497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong HT, Timm MM, Greipp PR, Witzig TE, Dispenzieri A, Russell SJ and Peng KW (2006) Oncolytic measles virus targets high CD46 expression on multiple myeloma cells. Exp Hematol 34, 713–720. [DOI] [PubMed] [Google Scholar]
- 38.Richter M, Saydaminova K, Yumul R, Krishnan R, Liu J, Nagy EE, Singh M, Izsvak Z, Cattaneo R, Uckert W et al. (2016) In vivo transduction of primitive mobilized hematopoietic stem cells after intravenous injection of integrating adenovirus vectors. Blood 128, 2206–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lieber A, He CY, Kirillova I and Kay MA (1996) Recombinant adenoviruses with large deletions generated by Cre-mediated excision exhibit different biological properties compared with first-generation vectors in vitro and in vivo. J Virol 70, 8944–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muck-Hausl M, Solanki M, Zhang W, Ruzsics Z and Ehrhardt A (2015) Ad 2.0: a novel recombineering platform for high-throughput generation of tailored adenoviruses. Nucleic Acids Res 43, e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lei C, Li SY, Liu JK, Zheng X, Zhao GP and Wang J (2017) The CCTL (Cpf1-assisted cutting and Taq DNA ligase-assisted ligation) method for efficient editing of large DNA constructs in vitro. Nucleic Acids Res 45, e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreppel F (2014) Production of high-capacity adenovirus vectors. Methods Mol Biol 1089, 211–229. [DOI] [PubMed] [Google Scholar]
- 43.Grieger JC and Samulski RJ (2005) Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol 79, 9933–9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Shayakhmetov DM, Leege T, Harkey M, Li Q, Papayannopoulou T, Stamatoyannopolous G and Lieber A (2005) A capsid-modified helper-dependent adenovirus vector containing the beta-globin locus control region displays a nonrandom integration pattern and allows stable, erythroid-specific gene expression. J Virol 79, 10999–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balamotis MA, Huang K and Mitani K (2004) Efficient delivery and stable gene expression in a hematopoietic cell line using a chimeric serotype 35 fiber pseudotyped helper-dependent adenoviral vector. Virology 324, 229–237. [DOI] [PubMed] [Google Scholar]
- 46.Ohbayashi F, Balamotis MA, Kishimoto A, Aizawa E, Diaz A, Hasty P, Graham FL, Caskey CT and Mitani K (2005) Correction of chromosomal mutation and random integration in embryonic stem cells with helper-dependent adenoviral vectors. Proc Natl Acad Sci USA 102, 13628–13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki K, Mitsui K, Aizawa E, Hasegawa K, Kawase E, Yamagishi T, Shimizu Y, Suemori H, Nakatsuji N and Mitani K (2008) Highly efficient transient gene expression and gene targeting in primate embryonic stem cells with helper-dependent adenoviral vectors. Proc Natl Acad Sci USA 105, 13781–13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song L, Li X, Jayandharan GR, Wang Y, Aslanidi GV, Ling C, Zhong L, Gao G, Yoder MC, Tan M et al. (2013) High-efficiency transduction of primary human hematopoietic stem cells and erythroid lineage-restricted expression by optimized AAV6 serotype vectors in vitro and in a murine xenograft model in vivo. PLoS ONE 8, e58757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Til NP and Wagemaker G (2014) Lentiviral gene transduction of mouse and human hematopoietic stem cells. Methods Mol Biol 1185, 311–319. [DOI] [PubMed] [Google Scholar]
- 50.Palmer D and Ng P (2009) Methods for the production of helper-dependent adenoviral vectors. In Methods in Molecular Biology (Le Doux JM, ed), pp. 33–53. Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 51.Croyle MA, Cheng X and Wilson JM (2001) Development of formulations that enhance physical stability of viral vectors for gene therapy. Gene Ther 8, 1281–1290. [DOI] [PubMed] [Google Scholar]
- 52.Saydaminova K, Ye X, Wang H, Richter M, Ho M, Chen H, Xu N, Kim JS, Papapetrou E, Holmes MC et al. (2015) Efficient genome editing in hematopoietic stem cells with helper-dependent Ad5/35 vectors expressing site-specific endonucleases under microRNA regulation. Mol Ther Methods Clin Dev 1, 14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li C, Psatha N, Sova P, Gil S, Wang H, Kim J, Kulkarni C, Valensisi C, Hawkins RD, Stamatoyannopoulos G et al. (2018) Reactivation of gamma-globin in adult beta-YAC mice after ex vivo and in vivo hematopoietic stem cell genome editing. Blood 131, 2915–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki K, Yu C, Qu J, Li M, Yao X, Yuan T, Goebl A, Tang S, Ren R, Aizawa E et al. (2014) Targeted gene correction minimally impacts whole-genome mutational load in human-disease-specific induced pluripotent stem cell clones. Cell Stem Cell 15, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li C, Psatha N, Gil S, Wang H, Papayannopoulou T and Lieber A (2018) HDAd5/35(++) adenovirus vector expressing anti-CRISPR peptides decreases CRISPR/Cas9 toxicity in human hematopoietic stem cells. Mol Ther Methods Clin Dev 9, 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmer DJ, Turner DL and Ng P (2019) Production of CRISPR/Cas9-mediated self-cleaving helper-dependent adenoviruses. Mol Ther Methods Clin Dev 13, 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brunetti-Pierri N and Ng P (2008) Progress and prospects: gene therapy for genetic diseases with helper-dependent adenoviral vectors. Gene Ther 15, 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H, Li ZY, Liu Y, Persson J, Beyer I, Moller T, Koyuncu D, Drescher MR, Strauss R, Zhang XB et al. (2011) Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med 17, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nilsson M, Karlsson S and Fan X (2004) Functionally distinct subpopulations of cord blood CD34+ cells are transduced by adenoviral vectors with serotype 5 or 35 tropism. Mol Ther 9, 377–388. [DOI] [PubMed] [Google Scholar]
- 60.Mizuguchi H, Sasaki T, Kawabata K, Sakurai F and Hayakawa T (2005) Fiber-modified adenovirus vectors mediate efficient gene transfer into undifferentiated and adipogenic-differentiated human mesenchymal stem cells. Biochem Biophys Res Commun 332, 1101–1106. [DOI] [PubMed] [Google Scholar]
- 61.Manchester M, Smith KA, Eto DS, Perkin HB and Torbett BE (2002) Targeting and hematopoietic suppression of human CD34+ cells by measles virus. J Virol 76, 6636–6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Beyer I, Persson J, Song H, Li Z, Richter M, Cao H, van Rensburg R, Yao X, Hudkins K et al. (2012) A new human DSG2-transgenic mouse model for studying the tropism and pathology of human adenoviruses. J Virol 86, 6286–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuve S, Wang H, Ware C, Liu Y, Gaggar A, Bernt K, Shayakhmetov D, Li Z, Strauss R, Stone D et al. (2006) A new group B adenovirus receptor is expressed at high levels on human stem and tumor cells. J Virol 80, 12109–12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krasnykh VN, Mikheeva GV, Douglas JT and Curiel DT (1996) Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol 70, 6839–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stecher H, Shayakhmetov DM, Stamatoyannopoulos G and Lieber A (2001) A capsid-modified adenovirus vector devoid of all viral genes: assessment of transduction and toxicity in human hematopoietic cells. Mol Ther 4, 36–44. [DOI] [PubMed] [Google Scholar]
- 66.Holkers M, Cathomen T and Goncalves MA (2014) Construction and characterization of adenoviral vectors for the delivery of TALENs into human cells. Methods 69, 179–187. [DOI] [PubMed] [Google Scholar]
- 67.van Rensburg R, Beyer I, Yao XY, Wang H, Denisenko O, Li ZY, Russell DW, Miller DG, Gregory P, Holmes M et al. (2013) Chromatin structure of two genomic sites for targeted transgene integration in induced pluripotent stem cells and hematopoietic stem cells. Gene Ther 20, 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yotnda P, Onishi H, Heslop HE, Shayakhmetov D, Lieber A, Brenner M and Davis A (2001) Efficient infection of primitive hematopoietic stem cells by modified adenovirus. Gene Ther 8, 930–937. [DOI] [PubMed] [Google Scholar]
- 69.Lu ZZ, Ni F, Hu ZB, Wang L, Wang H, Zhang QW, Huang WR, Wu CT and Wang LS (2006) Efficient gene transfer into hematopoietic cells by a retargeting adenoviral vector system with a chimeric fiber of adenovirus serotype 5 and 11p. Exp Hematol 34, 1171–1182. [DOI] [PubMed] [Google Scholar]
- 70.Li L, Krymskaya L, Wang J, Henley J, Rao A, Cao LF, Tran CA, Torres-Coronado M, Gardner A, Gonzalez N et al. (2013) Genomic editing of the HIV-1 coreceptor CCR5 in adult hematopoietic stem and progenitor cells using zinc finger nucleases. Mol Ther 21, 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Z, Ahonen M, Hamalainen H, Bergelson JM, Kahari VM and Lahesmaa R (2002) High-efficiency gene transfer to primary T lymphocytes by recombinant adenovirus vectors. J Immunol Methods 260, 79–89. [DOI] [PubMed] [Google Scholar]
- 72.Cho HI, Kim HJ, Oh ST and Kim TG (2003) In vitro induction of carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes by dendritic cells transduced with recombinant adenoviruses. Vaccine 22, 224–236. [DOI] [PubMed] [Google Scholar]
- 73.Hurez V, Hautton RD, Oliver J, Matthews RJ and Weaver CK (2002) Gene delivery into primary T cells: overview and characterization of a transgenic model for efficient adenoviral transduction. Immunol Res 26, 131–141. [DOI] [PubMed] [Google Scholar]
- 74.Jung D, Neron S, Drouin M and Jacques A (2005) Efficient gene transfer into normal human B lymphocytes with the chimeric adenoviral vector Ad5/F35. J Immunol Methods 304, 78–87. [DOI] [PubMed] [Google Scholar]
- 75.Leen AM, Sili U, Savoldo B, Jewell AM, Piedra PA, Brenner MK and Rooney CM (2004) Fiber-modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood 103, 1011–1019. [DOI] [PubMed] [Google Scholar]
- 76.Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, Wang N, Lee G, Bartsevich VV, Lee YL et al. (2008) Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol 26, 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schroers R, Hildebrandt Y, Hasenkamp J, Glass B, Lieber A, Wulf G and Piesche M (2004) Gene transfer into human T lymphocytes and natural killer cells by Ad5/F35 chimeric adenoviral vectors. Exp Hematol 32, 536–546. [DOI] [PubMed] [Google Scholar]
- 78.Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, Carrum G, Krance RA, Chang CC, Molldrem JJ et al. (2006) Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med 12, 1160–1166. [DOI] [PubMed] [Google Scholar]
- 79.Wang H, Liu Y, Li Z, Tuve S, Stone D, Kalyushniy O, Shayakhmetov D, Verlinde CL, Stehle T, McVey J et al. (2008) In vitro and in vivo properties of adenovirus vectors with increased affinity to CD46. J Virol 82, 10567–10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL and Shayakhmetov DM (2008) Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci USA 105, 5483–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doronin K, Flatt JW, Di Paolo NC, Khare R, Kalyuzhniy O, Acchione M, Sumida JP, Ohto U, Shimizu T, Akashi-Takamura S et al. (2012) Coagulation factor X activates innate immunity to human species C adenovirus. Science 338, 795–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L et al. (2008) Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132, 397–409. [DOI] [PubMed] [Google Scholar]
- 83.Hsu EC, Dorig RE, Sarangi F, Marcil A, Iorio C and Richardson CD (1997) Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J Virol 71, 6144–6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beyer I, Cao H, Persson J, Wang H, Liu Y, Yumul R, Li Z, Woodle D, Manger R, Gough M et al. (2013) Transient removal of CD46 is safe and increases B-cell depletion by rituximab in CD46 transgenic mice and macaques. Mol Ther 21, 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ni S, Bernt K, Gaggar A, Li ZY, Kiem HP and Lieber A (2005) Evaluation of biodistribution and safety of adenovirus vectors containing group B fibers after intravenous injection into baboons. Hum Gene Ther 16, 664–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Richter M, Yumul R, Saydaminova K, Wang H, Gough M, Baldessari A, Cattaneo R, Lee F, Wang CH, Jang H et al. (2016) Preclinical safety, pharmacokinetics, pharmacodynamics, and biodistribution studies with Ad35K++ protein: a novel rituximab cotherapeutic. Mol Ther Methods Clin Dev 5, 16013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kemper C, Leung M, Stephensen CB, Pinkert CA, Liszewski MK, Cattaneo R and Atkinson JP (2001) Membrane cofactor protein (MCP; CD46) expression in transgenic mice. Clin Exp Immunol 124, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kallinikou K, Anjos-Afonso F, Blundell MP, Ings SJ, Watts MJ, Thrasher AJ, Linch DC, Bonnet D and Yong KL (2012) Engraftment defect of cytokine-cultured adult human mobilized CD34(+) cells is related to reduced adhesion to bone marrow niche elements. Br J Haematol 158, 778–787. [DOI] [PubMed] [Google Scholar]
- 89.Boissel S, Jarjour J, Astrakhan A, Adey A, Gouble A, Duchateau P, Shendure J, Stoddard BL, Certo MT, Baker D et al. (2014) megaTALs: a rare-cleaving nuclease architecture for therapeutic genome engineering. Nucleic Acids Res 42, 2591–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pavletich NP and Pabo CO (1991) Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science 252, 809–817. [DOI] [PubMed] [Google Scholar]
- 91.Mak AN, Bradley P, Cernadas RA, Bogdanove AJ and Stoddard BL (2012) The crystal structure of TAL effector PthXo1 bound to its DNA target. Science 335, 716–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA et al. (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE and Church GM (2013) RNA-guided human genome engineering via Cas9. Science 339, 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A and Bonas U (2009) Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326, 1509–1512. [DOI] [PubMed] [Google Scholar]
- 95.Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD and Holmes MC (2005) Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 435, 646–651. [DOI] [PubMed] [Google Scholar]
- 96.Cheng AW, Wang H, Yang H, Shi L, Katz Y, Theunissen TW, Rangarajan S, Shivalila CS, Dadon DB and Jaenisch R (2013) Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res 23, 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zetsche B, Heidenreich M, Mohanraju P, Fedorova I, Kneppers J, DeGennaro EM, Winblad N, Choudhury SR, Abudayyeh OO, Gootenberg JS et al. (2017) Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat Biotechnol 35, 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS et al. (2015) In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J and Zhang F (2017) RNA editing with CRISPR-Cas13. Science 358, 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Knott GJ and Doudna JA (2018) CRISPR-Cas guides the future of genetic engineering. Science 361, 866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pickar-Oliver A and Gersbach CA (2019) The next generation of CRISPR–Cas technologies and applications. Nat Rev Mol Cell Biol 20, 490–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G et al. (2014) Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 370, 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li C, Mishra AS, Gil S, Wang M, Georgakopoulou A, Papayannopoulou T, Hawkins RD and Lieber A (2019) Targeted integration and high-level transgene expression in AAVS1 transgenic mice after in vivo HSC transduction with HDAd5/35++ vectors. Mol Ther. 10.1016/j.ymthe.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee J, Ma J and Lee K (2019) Direct delivery of adenoviral CRISPR/Cas9 vector into the blastoderm for generation of targeted gene knockout in quail. Proc Natl Acad Sci USA 116, 13288–13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao J, Bergmann T, Zhang W, Schiwon M, Ehrke-Schulz E and Ehrhardt A (2019) Viral vector-based delivery of CRISPR/Cas9 and donor DNA for homology-directed repair in an in vitro model for canine hemophilia B. Mol Ther Nucleic Acids 14, 364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stephens CJ, Lauron EJ, Kashentseva E, Lu ZH, Yokoyama WM and Curiel DT (2019) Long-term correction of hemophilia B using adenoviral delivery of CRISPR/Cas9. J Control Release 298, 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li C, Psatha N, Gil S, Wang H, Papayannopoulou T and Lieber A (2018) HDAd5/35++ adenovirus vector expressing anti-CRISPR peptides decreases CRISPR/Cas9 toxicity in human hematopoietic stem cells. Mol Ther Methods Clin Dev 9, 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ehrke-Schulz E, Schiwon M, Leitner T, David S, Bergmann T, Liu J and Ehrhardt A (2017) CRISPR/Cas9 delivery with one single adenoviral vector devoid of all viral genes. Sci Rep 7, 17113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Voets O, Tielen F, Elstak E, Benschop J, Grimbergen M, Stallen J, Janssen R, van Marle A and Essrich C (2017) Highly efficient gene inactivation by adenoviral CRISPR/Cas9 in human primary cells. PLoS ONE 12, e0182974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shimizu K, Sakurai F, Tomita K, Nagamoto Y, Nakamura S-I, Katayama K, Tachibana M, Kawabata K and Mizuguchi H (2014) Suppression of leaky expression of adenovirus genes by insertion of microRNA-targeted sequences in the replication-incompetent adenovirus vector genome. Mol Ther Methods Clin Dev 1, 14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsukamoto T, Sakai E, Nishimae F, Sakurai F and Mizuguchi H (2019) Efficient generation of adenovirus vectors carrying the Clustered regularly interspaced short palindromic repeat (CRISPR)-CRISPR associated proteins (Cas)12a system by suppressing Cas12a expression in packaging cells. J Biotechnol 304, 1–9. [DOI] [PubMed] [Google Scholar]
- 112.Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI and Liu DR (2017) Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature 551, 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Komor AC, Kim YB, Packer MS, Zuris JA and Liu DR (2016) Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nishida K, Arazoe T, Yachie N, Banno S, Kakimoto M, Tabata M, Mochizuki M, Miyabe A, Araki M, Hara KY et al. (2016) Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 353, aaf8729. [DOI] [PubMed] [Google Scholar]
- 115.Kosicki M, Tomberg K and Bradley A (2018) Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol 36, 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haapaniemi E, Botla S, Persson J, Schmierer B and Taipale J (2018) CRISPR–Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med 24, 927–930. [DOI] [PubMed] [Google Scholar]
- 117.Ihry RJ, Worringer KA, Salick MR, Frias E, Ho D, Theriault K, Kommineni S, Chen J, Sondey M, Ye C et al. (2018) p53 inhibits CRISPR–Cas9 engineering in human pluripotent stem cells. Nat Med 24, 939–946. [DOI] [PubMed] [Google Scholar]
- 118.Rees HA and Liu DR (2018) Base editing: precision chemistry on the genome and transcriptome of living cells. Nat Rev Genet 19, 770–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grunewald J, Zhou R, Garcia SP, Iyer S, Lareau CA, Aryee MJ and Joung JK (2019) Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature 569, 433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grunewald J, Zhou R, Iyer S, Lareau CA, Garcia SP, Aryee MJ and Joung JK (2019) CRISPR DNA base editors with reduced RNA off-target and self-editing activities. Nat Biotechnol 37, 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kuscu C, Parlak M, Tufan T, Yang J, Szlachta K, Wei X, Mammadov R and Adli M (2017) CRISPR-STOP: gene silencing through base-editing-induced nonsense mutations. Nat Methods 14, 710–712. [DOI] [PubMed] [Google Scholar]
- 122.Gapinske M, Luu A, Winter J, Woods WS, Kostan KA, Shiva N, Song JS and Perez-Pinera P (2018) CRISPR-SKIP: programmable gene splicing with single base editors. Genome Biol 19, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang TP, Zhao KT, Miller SM, Gaudelli NM, Oakes BL, Fellmann C, Savage DF and Liu DR (2019) Circularly permuted and PAM-modified Cas9 variants broaden the targeting scope of base editors. Nat Biotechnol 37, 626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grünewald J, Zhou R, Iyer S, Lareau CA, Garcia SP, Aryee MJ and Joung JK (2019) CRISPR DNA base editors with reduced RNA off-target and self-editing activities. Nat Biotechnol 37, 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Georgakopoulou A, Li C, Wang H, Yannaki E, Anagnostopoulos A, Humbert O, Kiem H-P and Lieber A (2019) Comparison of DNA Break-Inducing and Base-Editing CRISPR/CAS9 Approaches for Fetal Globin Re-Activation after HDAd5/35++ Transduction of Human and Monkey CD34+ Cells. Paper presented at the 2019 Annual ASCGT Meeting, abstract #602. [Google Scholar]
- 126.Chadwick AC, Evitt NH, Lv W and Musunuru K (2018) Reduced blood lipid levels with in vivo CRISPR-Cas9 base editing of ANGPTL3. Circulation 137, 975–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chadwick AC, Wang X and Musunuru K (2017) In vivo base editing of PCSK9 (proprotein convertase subtilisin/kexin type 9) as a therapeutic alternative to genome editing. Arterioscler Thromb Vasc Biol 37, 1741–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zafra MP, Schatoff EM, Katti A, Foronda M, Breinig M, Schweitzer AY, Simon A, Han T, Goswami S and Montgomery E (2018) Optimized base editors enable efficient editing in cells, organoids and mice. Nat Biotechnol 36, 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koblan LW, Doman JL, Wilson C, Levy JM, Tay T, Newby GA, Maianti JP, Raguram A and Liu DR (2018) Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat Biotechnol 36, 843–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stephen SL, Sivanandam VG and Kochanek S (2008) Homologous and heterologous recombination between adenovirus vector DNA and chromosomal DNA. J Gene Med 10, 1176–1189. [DOI] [PubMed] [Google Scholar]
- 131.Ehrhardt A, Xu H and Kay MA (2003) Episomal persistence of recombinant adenoviral vector genomes during the cell cycle in vivo. J Virol 77, 7689–7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ehrhardt A, Haase R, Schepers A, Deutsch MJ, Lipps HJ and Baiker A (2008) Episomal vectors for gene therapy. Curr Gene Ther 8, 147–161. [DOI] [PubMed] [Google Scholar]
- 133.Appaiahgari MB and Vrati S (2015) Adenoviruses as gene/vaccine delivery vectors: promises and pitfalls. Expert Opin Biol Ther 15, 337–351. [DOI] [PubMed] [Google Scholar]
- 134.Zheng C, Cotrim AP, Nikolov N, Mineshiba F, Swaim W and Baum BJ (2012) A novel hybrid adenoretroviral vector with more extensive E3 deletion extends transgene expression in submandibular glands. Hum Gene Ther Methods 23, 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rauschhuber C, Noske N and Ehrhardt A (2012) New insights into stability of recombinant adenovirus vector genomes in mammalian cells. Eur J Cell Biol 91, 2–9. [DOI] [PubMed] [Google Scholar]
- 136.Zheng C, Vitolo JM, Zhang W, Mineshiba F, Chiorini JA and Baum BJ (2008) Extended transgene expression from a nonintegrating adenoviral vector containing retroviral elements. Mol Ther 16, 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gil JS, Gallaher SD and Berk AJ (2010) Delivery of an EBV episome by a self-circularizing helper-dependent adenovirus: long-term transgene expression in immunocompetent mice. Gene Ther 17, 1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lu B, Javidi-Parsijani P, Makani V, Mehraein-Ghomi F, Sarhan WM, Sun D, Yoo KW, Atala ZP, Lyu P and Atala A (2019) Delivering SaCas9 mRNA by lentivirus-like bionanoparticles for transient expression and efficient genome editing. Nucleic Acids Res 47, e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Amabile A, Migliara A, Capasso P, Biffi M, Cittaro D, Naldini L and Lombardo A (2016) Inheritable silencing of endogenous genes by hit-and-run targeted epigenetic editing. Cell 167, 219–232.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pawluk A, Davidson AR and Maxwell KL (2018) Anti-CRISPR: discovery, mechanism and function. Nat Rev Microbiol 16, 12–17. [DOI] [PubMed] [Google Scholar]
- 141.Rauch BJ, Silvis MR, Hultquist JF, Waters CS, McGregor MJ, Krogan NJ and Bondy-Denomy J (2017) Inhibition of CRISPR-Cas9 with bacteriophage proteins. Cell 168, 150–158.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kebriaei P, Singh H, Huls MH, Figliola MJ, Bassett R, Olivares S, Jena B, Dawson MJ, Kumaresan PR, Su S et al. (2016) Phase I trials using sleeping beauty to generate CD19-specific CAR T cells. J Clin Invest 126, 3363–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ivics Z, Hackett PB, Plasterk RH and Izsvak Z (1997) Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 91, 501–510. [DOI] [PubMed] [Google Scholar]
- 144.Ding S, Wu X, Li G, Han M, Zhuang Y and Xu T (2005) Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 122, 473–483. [DOI] [PubMed] [Google Scholar]
- 145.Koga A, Suzuki M, Inagaki H, Bessho Y and Hori H (1996) Transposable element in fish. Nature 383, 30. [DOI] [PubMed] [Google Scholar]
- 146.Tipanee J, VandenDriessche T and Chuah MK (2017) Transposons: moving forward from preclinical studies to clinical trials. Hum Gene Ther 28, 1087–1104. [DOI] [PubMed] [Google Scholar]
- 147.Yant SR, Ehrhardt A, Mikkelsen JG, Meuse L, Pham T and Kay MA (2002) Transposition from a gutless adeno-transposon vector stabilizes transgene expression in vivo. Nat Biotechnol 20, 999–1005. [DOI] [PubMed] [Google Scholar]
- 148.Mates L, Chuah MK, Belay E, Jerchow B, Manoj N, Acosta-Sanchez A, Grzela DP, Schmitt A, Becker K, Matrai J et al. (2009) Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat Genet 41, 753–761. [DOI] [PubMed] [Google Scholar]
- 149.Wilson MH, Coates CJ and George AL (2007) PiggyBac transposon-mediated gene transfer in human cells. Mol Ther 15, 139–145. [DOI] [PubMed] [Google Scholar]
- 150.Wang H, Georgakopoulou A, Psatha N, Li C, Capsali C, Samal HB, Anagnostopoulos A, Ehrhardt A, Izsvak Z, Papayannopoulou T et al. (2019) In vivo hematopoietic stem cell gene therapy ameliorates murine thalassemia intermedia. J Clin Invest 129, 598–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang H, Liu Z, Li C, Gil S, Papayannopoulou T, Doering CB and Lieber A (2019) High-level protein production in erythroid cells derived from in vivo transduced hematopoietic stem cells. Blood Adv 3, 2883–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cooney AL, Singh BK, Loza LM, Thornell IM, Hippee CE, Powers LS, Ostedgaard LS, Meyerholz DK, Wohlford-Lenane C, Stoltz DA et al. (2018) Widespread airway distribution and short-term phenotypic correction of cystic fibrosis pigs following aerosol delivery of piggyBac/adenovirus. Nucleic Acids Res 46, 9591–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Suzuki JI, Dezawa M and Kitada M (2017) Prolonged but non-permanent expression of a transgene in ependymal cells of adult rats using an adenovirus-mediated transposon gene transfer system. Brain Res 1675, 20–27. [DOI] [PubMed] [Google Scholar]
- 154.Ehrhardt A, Yant SR, Giering JC, Xu H, Engler JA and Kay MA (2007) Somatic integration from an adenoviral hybrid vector into a hot spot in mouse liver results in persistent transgene expression levels in vivo. Mol Ther 15, 146–156. [DOI] [PubMed] [Google Scholar]
- 155.Knight S, Zhang F, Mueller-Kuller U, Bokhoven M, Gupta A, Broughton T, Sha S, Antoniou MN, Brendel C, Grez M et al. (2012) Safer, silencing-resistant lentiviral vectors: optimization of the ubiquitous chromatin-opening element through elimination of aberrant splicing. J Virol 86, 9088–9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Papapetrou EP, Lee G, Malani N, Setty M, Riviere I, Tirunagari LM, Kadota K, Roth SL, Giardina P, Viale A et al. (2011) Genomic safe harbors permit high beta-globin transgene expression in thalassemia induced pluripotent stem cells. Nat Biotechnol 29, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cristea S, Freyvert Y, Santiago Y, Holmes MC, Urnov FD, Gregory PD and Cost GJ (2013) In vivo cleavage of transgene donors promotes nuclease-mediated targeted integration. Biotechnol Bioeng 110, 871–880. [DOI] [PubMed] [Google Scholar]
- 158.Wang J, Friedman G, Doyon Y, Wang NS, Li CJ, Miller JC, Hua KL, Yan JJ, Babiarz JE, Gregory PD et al. (2012) Targeted gene addition to a predetermined site in the human genome using a ZFN-based nicking enzyme. Genome Res 22, 1316–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carbery ID, Ji D, Harrington A, Brown V, Weinstein EJ, Liaw L and Cui X (2010) Targeted genome modification in mice using zinc-finger nucleases. Genetics 186, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wefers B, Meyer M, Ortiz O, Hrabe de Angelis M, Hansen J, Wurst W and Kuhn R (2013) Direct production of mouse disease models by embryo microinjection of TALENs and oligodeoxynucleotides. Proc Natl Acad Sci USA 110, 3782–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zou J, Maeder ML, Mali P, Pruett-Miller SM, Thibodeau-Beganny S, Chou BK, Chen G, Ye Z, Park IH, Daley GQ et al. (2009) Gene targeting of a disease-related gene in human induced pluripotent stem and embryonic stem cells. Cell Stem Cell 5, 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Iyama T and Wilson DM 3rd (2013) DNA repair mechanisms in dividing and non-dividing cells. DNA Repair 12, 620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lin S, Staahl BT, Alla RK and Doudna JA (2014) Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLife 3, e04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu Y, Zeng J, Roscoe BP, Liu P, Yao Q, Lazzarotto CR, Clement K, Cole MA, Luk K, Baricordi C et al. (2019) Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat Med 25, 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.De Ravin SS, Reik A, Liu PQ, Li L, Wu X, Su L, Raley C, Theobald N, Choi U, Song AH et al. (2016) Targeted gene addition in human CD34(+) hematopoietic cells for correction of X-linked chronic granulomatous disease. Nat Biotechnol. 34, 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Park SH, Lee CM, Dever DP, Davis TH, Camarena J, Srifa W, Zhang Y, Paikari A, Chang AK, Porteus MH et al. (2019) Highly efficient editing of the β-globin gene in patient-derived hematopoietic stem and progenitor cells to treat sickle cell disease. Nucleic Acids Res 47, 7955–7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Romero Z, Lomova A, Said S, Miggelbrink A, Kuo CY, Campo-Fernandez B, Hoban MD, Masiuk KE, Clark DN, Long J et al. (2019) Editing the sickle cell disease mutation in human hematopoietic stem cells: comparison of endonucleases and homologous donor templates. Mol Ther 27, 1389–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bilbao G, Feng M, Rancourt C, Jackson WH Jr and Curiel DT (1997) Adenoviral/retroviral vector chimeras: a novel strategy to achieve high-efficiency stable transduction in vivo. FASEB J 11, 624–634. [DOI] [PubMed] [Google Scholar]
- 169.Kubo S, Haga K, Tamamoto A, Palmer DJ, Ng P, Okamura H and Kasahara N (2011) Adenovirus-retrovirus hybrid vectors achieve highly enhanced tumor transduction and antitumor efficacy in vivo. Mol Ther 19, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Heinkelein M, Pietschmann T, Jarmy G, Dressler M, Imrich H, Thurow J, Lindemann D, Bock M, Moebes A, Roy J et al. (2000) Efficient intracellular retrotransposition of an exogenous primate retrovirus genome. EMBO J 19, 3436–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Picard-Maureau M, Kreppel F, Lindemann D, Juretzek T, Herchenroder O, Rethwilm A, Kochanek S and Heinkelein M (2004) Foamy virus–adenovirus hybrid vectors. Gene Ther 11, 722–728. [DOI] [PubMed] [Google Scholar]
- 172.Goncalves MA, van Nierop GP, Tijssen MR, Lefesvre P, Knaan-Shanzer S, van der Velde I, van Bekkum DW, Valerio D and de Vries AA (2005) Transfer of the full-length dystrophin-coding sequence into muscle cells by a dual high-capacity hybrid viral vector with site-specific integration ability. J Virol 79, 3146–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Recchia A, Parks RJ, Lamartina S, Toniatti C, Pieroni L, Palombo F, Ciliberto G, Graham FL, Cortese R, La Monica N et al. (1999) Site-specific integration mediated by a hybrid adenovirus/adeno-associated virus vector. Proc Natl Acad Sci USA 96, 2615–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Huang S and Kamihira M (2013) Development of hybrid viral vectors for gene therapy. Biotechnol Adv 31, 208–223. [DOI] [PubMed] [Google Scholar]
- 175.Wang H and Lieber A (2006) A helper-dependent capsid-modified adenovirus vector expressing adeno-associated virus rep78 mediates site-specific integration of a 27-kilobase transgene cassette. J Virol 80, 11699–11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang H, Richter M, Psatha N, Li C, Kim J, Liu J, Ehrhardt A, Nilsson SK, Cao B, Palmer D et al. (2018) A Combined in vivo HSC transduction/selection approach results in efficient and stable gene expression in peripheral blood cells in mice. Mol Ther Methods Clin Dev 8, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Neff T, Horn PA, Peterson LJ, Thomasson BM, Thompson J, Williams DA, Schmidt M, Georges GE, von Kalle C and Kiem HP (2003) Methylguanine methyltransferase-mediated in vivo selection and chemoprotection of allogeneic stem cells in a large-animal model. J Clin Invest 112, 1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Beard BC, Trobridge GD, Ironside C, McCune JS, Adair JE and Kiem HP (2010) Efficient and stable MGMT-mediated selection of long-term repopulating stem cells in nonhuman primates. J Clin Invest 120, 2345–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang H, Georgakopoulou A, Psatha N, Li C, Capsali C, Samal HB, Anagnostopoulos A, Ehrhardt A, Izsvak Z, Papayannopoulou T et al. (2018) In vivo hematopoietic stem cell gene therapy ameliorates murine thalassemia intermedia. J Clin Investig 129, 598–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, Kostense S, Penders G, Helmus N, Koudstaal W et al. (2003) Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol 77, 8263–8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR et al. (2007) Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 81, 4654–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kostense S, Koudstaal W, Sprangers M, Weverling GJ, Penders G, Helmus N, Vogels R, Bakker M, Berkhout B, Havenga M et al. (2004) Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. Aids 18, 1213–1216. [DOI] [PubMed] [Google Scholar]
- 183.Flomenberg PR, Chen M, Munk G and Horwitz MS (1987) Molecular epidemiology of adenovirus type 35 infections in immunocompromised hosts. J Infect Dis 155, 1127–1134. [DOI] [PubMed] [Google Scholar]
- 184.Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, Clark S, Ng’ang’a D, Brandariz KL, Abbink P et al. (2011) International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 29, 5203–5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Johnson MJ, Petrovas C, Yamamoto T, Lindsay RW, Lore K, Gall JG, Gostick E, Lefebvre F, Cameron MJ, Price DA et al. (2012) Type I IFN induced by adenovirus serotypes 28 and 35 has multiple effects on T cell immunogenicity. J Immunol 188, 6109–6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Adams WC, Berenson RJ, Karlsson Hedestam GB, Lieber A, Koup RA and Lore K (2012) Attenuation of CD4+ T cell function by human adenovirus type-35 is mediated by the knob protein. J Gen Virol 93(Pt 6), 1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Adams WC, Gujer C, McInerney G, Gall JG, Petrovas C, Karlsson Hedestam GB, Koup RA and Lore K (2011) Adenovirus type-35 vectors block human CD4+ T-cell activation via CD46 ligation. Proc Natl Acad Sci USA 108, 7499–7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shoji M, Yoshizaki S, Mizuguchi H, Okuda K and Shimada M (2012) Immunogenic comparison of chimeric adenovirus 5/35 vector carrying optimized human immunodeficiency virus clade C genes and various promoters. PLoS ONE 7, e30302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sakurai F, Kawabata K, Koizumi N, Inoue N, Okabe M, Yamaguchi T, Hayakawa T and Mizuguchi H (2006) Adenovirus serotype 35 vector-mediated transduction into human CD46-transgenic mice. Gene Ther 13, 1118–1126. [DOI] [PubMed] [Google Scholar]
- 190.Sakurai F, Nakamura S, Akitomo K, Shibata H, Terao K, Kawabata K, Hayakawa T and Mizuguchi H (2008) Transduction properties of adenovirus serotype 35 vectors after intravenous administration into nonhuman primates. Mol Ther 16, 726–733. [DOI] [PubMed] [Google Scholar]
- 191.Bahal R, Ali McNeer N, Quijano E, Liu Y, Sulkowski P, Turchick A, Lu YC, Bhunia DC, Manna A, Greiner DL et al. (2016) In vivo correction of anaemia in beta-thalassemic mice by gammaPNA-mediated gene editing with nanoparticle delivery. Nat Commun 7, 13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Burtner CR and Beard BC (2014) Intravenous injection of a foamy virus vector to correct canine SCID-X1. Blood 123, 3578–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang CX, Sather BD, Wang X, Adair J, Khan I, Singh S, Lang S, Adams A, Curinga G, Kiem HP et al. (2014) Rapamycin relieves lentiviral vector transduction resistance in human and mouse hematopoietic stem cells. Blood 124, 913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Richter M, Stone D, Miao C, Humbert O, Kiem H-P, Papayannopoulou T and Lieber A (2017) In vivo hematopoietic stem cell transduction. Hematol Oncol Clin North Am 31, 771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tisdale JF, Kanter J, Mapara MY, Kwiatkowski JL, Krishnamurti L, Schmidt M, Miller AL, Pierciey FJ, Shi W, Ribeil J-A et al. (2018) Current results of lentiglobin gene therapy in patients with severe sickle cell disease treated under a refined protocol in the phase 1 Hgb-206 study. Blood 132, 1026. [Google Scholar]
- 196.Malik P, Grimley M, Quinn CT, Shova A, Courtney L, Lutzko C, Kalfa TA, Niss O, Mehta PA, Chandra S et al. (2018) Gene therapy for sickle cell anemia using a modified gamma globin lentivirus vector and reduced intensity conditioning transplant shows promising correction of the disease phenotype. Blood 132, 1021. [Google Scholar]
- 197.Perumbeti A, Higashimoto T, Urbinati F, Franco R, Meiselman HJ, Witte D and Malik P (2009) A novel human gamma-globin gene vector for genetic correction of sickle cell anemia in a humanized sickle mouse model: critical determinants for successful correction. Blood 114, 1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Marktel S, Cicalese MP, Giglio F, Scaramuzza S, Calbi V, Casiraghi M, Ciotti F, Lidonnici MR, Rossi C, Masera N et al. (2017) Gene therapy for beta thalassemia: preliminary results from the PHASE I/II tiget-bthal trial of autologous hematopoietic stem cells genetically modified with GLOBE lentiviral vector. Blood 130, 355. [Google Scholar]
- 199.Boulad F, Wang X, Qu J, Taylor C, Ferro L, Karponi G, Bartido S, Giardina P, Heller G, Prockop SE et al. (2014) Safe mobilization of CD34+ cells in adults with beta-thalassemia and validation of effective globin gene transfer for clinical investigation. Blood 123, 1483–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.High KA and Roncarolo MG (2019) Gene therapy. N Engl J Med 381, 455–464. [DOI] [PubMed] [Google Scholar]
- 201.Ghiaccio V, Chappell M, Rivella S and Breda L (2019) Gene therapy for beta-hemoglobinopathies: milestones, new therapies and challenges. Mol Diagn Ther 23, 173–186. [DOI] [PubMed] [Google Scholar]
- 202.Marktel S, Scaramuzza S, Cicalese MP, Giglio F, Galimberti S, Lidonnici MR, Calbi V, Assanelli A, Bernardo ME, Rossi C et al. (2019) Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-dependent ss-thalassemia. Nat Med 25, 234–241. [DOI] [PubMed] [Google Scholar]
- 203.Cavazzana M, Antoniani C and Miccio A (2017) Gene therapy for β-hemoglobinopathies. Mol Ther 25, 1142–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li C, Psatha N, Wang H, Singh M, Samal HB, Zhang W, Ehrhardt A, Izsvak Z, Papayannopoulou T and Lieber A (2018) Integrating HDAd5/35++ vectors as a new platform for HSC Gene therapy of hemoglobinopathies. Mol Ther Methods Clin Dev 9, 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang W, Muck-Hausl M, Wang J, Sun C, Gebbing M, Miskey C, Ivics Z, Izsvak Z and Ehrhardt A (2013) Integration profile and safety of an adenovirus hybrid-vector utilizing hyperactive sleeping beauty transposase for somatic integration. PLoS ONE 8, e75344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mettananda S, Fisher CA, Hay D, Badat M, Quek L, Clark K, Hublitz P, Downes D, Kerry J, Gosden M et al. (2017) Editing an a-globin enhancer in primary human hematopoietic stem cells as a treatment for β-thalassemia. Nat Commun 8, 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sankaran VG, Menne TF, Xu J, Akie TE, Lettre G, Van Handel B, Mikkola HK, Hirschhorn JN, Cantor AB and Orkin SH (2008) Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 322, 1839–1842. [DOI] [PubMed] [Google Scholar]
- 208.Menzel S, Garner C, Gut I, Matsuda F, Yamaguchi M, Heath S, Foglio M, Zelenika D, Boland A, Rooks H et al. (2007) A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat Genet 39, 1197. [DOI] [PubMed] [Google Scholar]
- 209.Canver MC, Smith EC, Sher F, Pinello L, Sanjana NE, Shalem O, Chen DD, Schupp PG, Vinjamur DS, Garcia SP et al. (2015) BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature 527, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Orkin SH and Bauer DE (2019) Emerging genetic therapy for sickle cell disease. Annu Rev Med 70, 257–271. [DOI] [PubMed] [Google Scholar]
- 211.Psatha N, Li C, Wang H, Dalas D, Stamatoyannopoulos G, Papayannopoulou T and Lieber A (2017) Introduction of two simultaneous mutations by genome editing greatly enhances the accumulation of the endogenous fetal hemoglobin in human normal erythroid cells. Blood 130, 947. [Google Scholar]
- 212.Liu N, Hargreaves VV, Zhu Q, Kurland JV, Hong J, Kim W, Sher F, Macias-Trevino C, Rogers JM, Kurita R et al. (2018) Direct promoter repression by BCL11A controls the fetal to adult hemoglobin switch. Cell 173, 430–442.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Martyn GE, Wienert B, Yang L, Shah M, Norton LJ, Burdach J, Kurita R, Nakamura Y, Pearson RCM, Funnell APW et al. (2018) Natural regulatory mutations elevate the fetal globin gene via disruption of BCL11A or ZBTB7A binding. Nat Genet 50, 498–503. [DOI] [PubMed] [Google Scholar]
- 214.Traxler EA, Yao Y, Wang YD, Woodard KJ, Kurita R, Nakamura Y, Hughes JR, Hardison RC, Blobel GA, Li C et al. (2016) A genome-editing strategy to treat beta-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat Med 22, 987–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dever DP, Bak RO, Reinisch A, Camarena J, Washington G, Nicolas CE, Pavel-Dinu M, Saxena N, Wilkens AB, Mantri S et al. (2016) CRISPR/Cas9 beta-globin gene targeting in human haematopoietic stem cells. Nature 539, 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Deng W, Rupon Jeremy W, Krivega I, Breda L, Motta I, Jahn Kristen S, Reik A, Gregory Philip D, Rivella S, Dean A et al. (2014) Reactivation of developmentally silenced globin genes by forced chromatin looping. Cell 158, 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li M, Suzuki K, Qu J, Saini P, Dubova I, Yi F, Lee J, Sancho-Martinez I, Liu GH and Izpisua Belmonte JC (2011) Efficient correction of hemoglobinopathy-causing mutations by homologous recombination in integration-free patient iPSCs. Cell Res 21, 1740–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li C, Wang H, Gil S and Lieber A (2019) Combination of CRISPR/CAS9 -Triggered Re-Activation of Endogenous Fetal Globin and SB100X-Transposase-Mediated Gamma-Globin Gene Addition for In Vivo HSC Gene Therapy of Hemoglobinopathies. Paper presented at the 2019 Annual ASCGT Meeting, abstract #593. [Google Scholar]
- 219.Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, De Die-Smulders C, Persky N, Grompe M, Joenje H, Pals G et al. (2002) Biallelic inactivation of BRCA2 in Fanconi anemia. Science 297, 606–609. [DOI] [PubMed] [Google Scholar]
- 220.Sumpter R Jr, Sirasanagandla S, Fernandez AF, Wei Y, Dong X, Franco L, Zou Z, Marchal C, Lee MY, Clapp DW et al. (2016) Fanconi anemia proteins function in mitophagy and immunity. Cell 165, 867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Garaycoechea JI and Patel KJ (2014) Why does the bone marrow fail in Fanconi anemia? Blood 123, 26–34. [DOI] [PubMed] [Google Scholar]
- 222.Liu JM, Kim S, Read EJ, Futaki M, Dokal I, Carter CS, Leitman SF, Pensiero M, Young NS and Walsh CE (1999) Engraftment of hematopoietic progenitor cells transduced with the Fanconi anemia group C gene (FANCC). Hum Gene Ther 10, 2337–2346. [DOI] [PubMed] [Google Scholar]
- 223.Kelly PF, Radtke S, von Kalle C, Balcik B, Bohn K, Mueller R, Schuesler T, Haren M, Reeves L, Cancelas JA et al. (2007) Stem cell collection and gene transfer in Fanconi anemia. Mol Ther 15, 211–219. [DOI] [PubMed] [Google Scholar]
- 224.Río P, Navarro S, Wang W, Sánchez-Domínguez R, Pujol RM, Segovia JC, Bogliolo M, Merino E, Wu N, Salgado R et al. (2019) Successful engraftment of gene-corrected hematopoietic stem cells in non-conditioned patients with Fanconi anemia. Nat Med 25, 1396–1401. [DOI] [PubMed] [Google Scholar]
- 225.Si Y, Pulliam AC, Linka Y, Ciccone S, Leurs C, Yuan J, Eckermann O, Fruehauf S, Mooney S, Hanenberg H et al. (2008) Overnight transduction with foamyviral vectors restores the long-term repopulating activity of Fancc−/− stem cells. Blood 112, 4458–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Levy C, Amirache F, Girard-Gagnepain A, Frecha C, Roman-Rodriguez FJ, Bernadin O, Costa C, Negre D, Gutierrez-Guerrero A, Vranckx LS et al. (2017) Measles virus envelope pseudotyped lentiviral vectors transduce quiescent human HSCs at an efficiency without precedent. Blood Adv 1, 2088–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mankad A, Taniguchi T, Cox B, Akkari Y, Rathbun RK, Lucas L, Bagby G, Olson S, D’Andrea A and Grompe M (2006) Natural gene therapy in monozygotic twins with Fanconi anemia. Blood 107, 3084–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Calcedo R, Vandenberghe LH, Gao G, Lin J and Wilson JM (2009) Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 199, 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hu C, Cela RG, Suzuki M, Lee B and Lipshutz GS (2011) Neonatal helper-dependent adenoviral vector gene therapy mediates correction of hemophilia A and tolerance to human factor VIII. Proc Natl Acad Sci USA 108, 2082–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Brunetti-Pierri N, Liou A, Patel P, Palmer D, Grove N, Finegold M, Piccolo P, Donnachie E, Rice K, Beaudet A et al. (2012) Balloon catheter delivery of helper-dependent adenoviral vector results in sustained, therapeutic hFIX expression in rhesus macaques. Mol Ther 20, 1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Brunetti-Pierri N and Ng P (2013) Adenoviral vectors for hemophilia gene therapy. J Genet Syndr Gene Ther 2, 017. [PMC free article] [PubMed] [Google Scholar]
- 232.Mc CW, Seiler MP, Bertin TK, Ubhayakar K, Palmer DJ, Ng P, Nichols TC and Lee B (2006) Helper-dependent adenoviral gene therapy mediates long-term correction of the clotting defect in the canine hemophilia A model. J Thromb Haemost 4, 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Reddy PS, Sakhuja K, Ganesh S, Yang L, Kayda D, Brann T, Pattison S, Golightly D, Idamakanti N, Pinkstaff A et al. (2002) Sustained human factor VIII expression in hemophilia A mice following systemic delivery of a gutless adenoviral vector. Mol Ther 5, 63–73. [DOI] [PubMed] [Google Scholar]
- 234.Ehrhardt A, Xu H, Dillow AM, Bellinger DA, Nichols TC and Kay MA (2003) A gene-deleted adenoviral vector results in phenotypic correction of canine hemophilia B without liver toxicity or thrombocytopenia. Blood 102, 2403–2411. [DOI] [PubMed] [Google Scholar]
- 235.Khare R, May SM, Vetrini F, Weaver EA, Palmer D, Rosewell A, Grove N, Ng P and Barry MA (2011) Generation of a Kupffer cell-evading adenovirus for systemic and liver-directed gene transfer. Mol Ther 19, 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hausl MA, Zhang W, Muther N, Rauschhuber C, Franck HG, Merricks EP, Nichols TC, Kay MA and Ehrhardt A (2010) Hyperactive sleeping beauty transposase enables persistent phenotypic correction in mice and a canine model for hemophilia B. Mol Ther 18, 1896–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lytle AM, Brown HC, Paik NY, Knight KA, Wright JF, Spencer HT and Doering CB (2016) Effects of FVIII immunity on hepatocyte and hematopoietic stem cell-directed gene therapy of murine hemophilia A. Mol Ther Methods Clin Dev 3, 15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chang AH, Stephan MT and Sadelain M (2006) Stem cell-derived erythroid cells mediate long-term systemic protein delivery. Nat Biotechnol 24, 1017–1021. [DOI] [PubMed] [Google Scholar]
- 239.Brown HC, Wright JF, Zhou S, Lytle AM, Shields JE, Spencer HT and Doering CB (2014) Bioengineered coagulation factor VIII enables long-term correction of murine hemophilia A following liver-directed adeno-associated viral vector delivery. Mol Ther Methods Clin Dev 1, 14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.CDC-USA, (1981) Pneumocystis pneumonia - Los Angeles. Morb Mortal Wkly Rep 30, 1–3. [PubMed] [Google Scholar]
- 241.WHO (2019) Number of people (all ages) living with HIV estimates by WHO region in. https://www.who.int/gho/hiv/epidemic_status/cases_all/en/
- 242.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C et al. (1996) Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382, 722–725. [DOI] [PubMed] [Google Scholar]
- 243.Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G et al. (2014) Gene editing of CCR5 in autologous CD4+ T cells of persons infected with HIV. N Engl J Med 370, 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Li C, Guan X, Du T, Jin W, Wu B, Liu Y, Wang P, Hu B, Griffin GE, Shattock RJ et al. (2015) Inhibition of HIV-1 infection of primary CD4+ T-cells by gene editing of CCR5 using adenovirus-delivered CRISPR/Cas9. J Gen Virol 96, 2381–2393. [DOI] [PubMed] [Google Scholar]
- 245.Didigu CA, Wilen CB, Wang J, Duong J, Secreto AJ, Danet-Desnoyers GA, Riley JL, Gregory PD, June CH, Holmes MC et al. (2014) Simultaneous zinc-finger nuclease editing of the HIV coreceptors ccr5 and cxcr4 protects CD4+ T cells from HIV-1 infection. Blood 123, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O et al. (2009) Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 360, 692–692. [DOI] [PubMed] [Google Scholar]
- 247.Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M, Martinez-Picado J, Nijhuis M, Wensing AMJ, Lee H et al. (2019) HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature 568, 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Xu L, Wang J, Liu Y, Xie L, Su B, Mou D, Wang L, Liu T, Wang X, Zhang B et al. (2019) CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N Engl J Med 381, 1240–1247. [DOI] [PubMed] [Google Scholar]
- 249.Kordelas L, Verheyen J, Beelen DW, Horn PA, Heinold A, Kaiser R, Trenschel R, Schadendorf D, Dittmer U and Esser S (2014) Shift of HIV tropism in stem-cell transplantation with CCR5 Delta32 mutation. N Engl J Med 371, 880–882. [DOI] [PubMed] [Google Scholar]
- 250.Bella R, Kaminski R, Mancuso P, Young W-B, Chen C, Sariyer R, Fischer T, Amini S, Ferrante P, Jacobson JM et al. (2018) Removal of HIV DNA by CRISPR from patient blood engrafts in humanized mice. Mol Ther Nucleic Acids 12, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ophinni Y, Inoue M, Kotaki T and Kameoka M (2018) CRISPR/Cas9 system targeting regulatory genes of HIV-1 inhibits viral replication in infected T-cell cultures. Sci Rep 8, 7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sather BD, Romano Ibarra GS, Sommer K, Curinga G, Hale M, Khan IF, Singh S, Song Y, Gwiazda K, Sahni J et al. (2015) Efficient modification of CCR5 in primary human hematopoietic cells using a megaTAL nuclease and AAV donor template. Sci Transl Med 7, 307ra156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Spanevello F, Calistri A, Del Vecchio C, Mantelli B, Frasson C, Basso G, Palu G, Cavazzana M and Parolin C (2016) Development of lentiviral vectors simultaneously expressing multiple siRNAs Against CCR5, vif and tat/rev genes for an HIV-1 gene therapy approach. Mol Ther Nucleic Acids 5, e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dash PK, Kaminski R, Bella R, Su H, Mathews S, Ahooyi TM, Chen C, Mancuso P, Sariyer R, Ferrante P et al. (2019) Sequential LASER ART and CRISPR treatments eliminate HIV-1 in a subset of infected humanized mice. Nat Commun 10, 2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Czechowicz A, Palchaudhuri R, Scheck A, Hu Y, Hoggatt J, Saez B, Pang WW, Mansour MK, Tate TA, Chan YY et al. (2019) Selective hematopoietic stem cell ablation using CD117-antibody-drug-conjugates enables safe and effective transplantation with immunity preservation. Nat Commun 10, 617. [DOI] [PMC free article] [PubMed] [Google Scholar]