Abstract
Background:
Penile size is considered a symbol of manhood and is a subjective problem for men, especially those with small penis syndrome. Penile augmentation was introduced to correct penile size problems from a medical, psychological, or esthetic point of view. Hyaluronic acid (HA) and polylactic acid (PLA) are two types of augmentation agents that are popularly used today. However, no systematic studies and meta-analyses have compared these two modalities as penile augmentation agents. This study aimed to analyze the efficacy and safety of penile filler injections with HA compared to PLA.
Methods:
This study was based on the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines. Articles examining the differences in efficacy and adverse events of the administration of HA and PLA in patients undergoing penile augmentation were systematically reviewed from the PubMed, Proquest, Web of Science, and Scopus databases. An odds ratio with a 95% CI was applied to measure the study outcome. The analysis was performed with RevMan 5.4 software. The risk of bias for each study was evaluated using the Risk of Bias v2 instrument from Cochrane. This research protocol is registered in the International Prospective Register of Systematic Reviews (PROSPERO) registry.
Result:
Four articles consisting of 283 research subjects were included in this study. The meta-analysis for penile girth enhancement after penile augmentation found significant results in the HA group compared to the PLA group (P=0.01). There was no difference in the level of satisfaction with penile appearance 4 weeks after penile augmentation in the HA group compared to the PLA group (P=0.79). HA was significantly superior in sexual satisfaction 12 weeks postpenile augmentation (P=0.0004). There was no difference in the incidence of pain after penile augmentation in the HA group compared to the PLA group (P=0.33). In the postaugmentation penile inflammation, there was no difference (P=0.98) in the HA group compared to the PLA group.
Conclusion:
There are differences in the efficacy of penile augmentation with the superiority of HA in increasing penile diameter and postaugmentation sexual satisfaction compared to PLA. There was no difference in the incidence of complications between using HA and PLA.
Keyword: hyaluronic acid, polylactic acid, penile augmentation, penile filler
Introduction
HIGHLIGHTS
This systematic review and meta-analysis compared the efficacy and safety of hyaluronic acid and polylactic acid for penile enlargement.
The results showed that hyaluronic acid increased penile diameter more and had better patient satisfaction than polylactic acid.
The meta-analysis also found that hyaluronic acid and polylactic acid semi-permanent penile augmentation is safe and effective. Still, more research is needed to determine its long-term physical and psychological effects before it can be recommended.
We concluded that hyaluronic acid is superior to polylactic acid because it offers more benefits to the patient.
Penile girth enhancement (PGE) has been introduced in the treatment of patients with sexual dysfunction and anatomic abnormalities such as penile curvature1,2. Medical and psychological issues can be addressed with several penile augmentation methods. Small penis syndrome (SPS) is excessive anxiety about a penis that is smaller than normal for adult men, despite a normal clinical examination3. If initial SPS interventions fail, penile augmentation may be considered3,4.
Hyaluronic acid was FDA-approved as a filler in 2003. Since 2004, hyaluronic acid penile enlargement for premature ejaculation has gained popularity, especially in Asia1. Hyaluronic acid is biocompatible and lasts longer than other fillers. Hyaluronic acid has been shown to enlarge peniles in recent studies5,6. Common fillers include polylactic acid. Polylactic acid biostimulates fibroblast proliferation and neo-collogenesis, unlike hyaluronic acid. Polylactic acid induces dermal fibroplasia and foreign body inflammation to augment tissue7. Hyaluronic acid and polylactic acid enhance penile girth for 18 months without side effects6,8.
While surgical PGE procedures are still limited, demand is rising9. Cosmetic goals require safe, effective, and minimally invasive methods. PGE is based on the patient’s needs, so it is important to compare the clinical outcomes of different fillers. Cosmetic goals require safe, effective, and minimally invasive methods. PGE is based on the patient’s needs, so it is important to compare clinical outcomes of different fillers. This systematic review and meta-analysis compared the efficacy and safety of hyaluronic acid and polylactic acid for penile enlargement. We found no PROSPERO-registered studies or meta-analyses on PGE with hyaluronic acid or polylactic acid, so we designed this study to compare their efficacy and side effects.
Methods
Study design
This review followed the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA)10. The protocol of this review has been registered in the International Prospective Register of Systematic Reviews (PROSPERO), maintained by the National Institute for Health Research (NIHR), and contains information about this study (Identification number CRD42023188174). Since the information was accessible to the general public, institutional review board (IRB) approval was not necessary. We also self-evaluate the quality of our systematic review using AMSTAR 2 criteria11.
Systematic search strategy
A systematic search using medical subject heading (MeSH) terms with the Boolean operator was performed in PubMed, Scopus, ScienceDirect, and ProQuest databases for studies published up to March 2023. The primary keywords used in the searching process were as follows: small penis syndrome, penile augmentation, penile enhancement, hyaluronic acid, polylactic acid, HA, and PLA.
Data extraction
Three independent examiners collected article information, including authors, publication date, study location, and sample size. Along with penile augmentation, baseline characteristics like mean age, filler type, average volume of injection, injection material, outcome, and evaluation were extracted. These include PGE, VAS-improved penile appearance, sexual satisfaction, pain events, and penile inflammation. If data extraction disagrees, the third examiner will be consulted.
Statistical analysis
Postoperative follow-up revealed study endpoints. The SMD and 95% CI were used to calculate the SD from the mean for continuous variables. The odds ratio (OR) was used to estimate the outcome of the interest difference between groups. Heterogeneity analysis is generated as the index of I2, of which the value >50% along with P<0.05 was judged to be significant. Thus, a random-effects model will analyze the pooled outcome. Fixed-effects analysis is justified by low heterogeneity. This systematic review and meta-analysis uses Revman 5.4.1 by the Cochrane Collaboration for statistics.
Assessment of study quality
Cochrane risk of bias (RoB) 2.0 will evaluate randomized controlled trials (RCTs) for the randomization process, deviations from the intended intervention, missing outcome data, measurement of the outcome, and selection of the reported result.
Results
Systematic search result
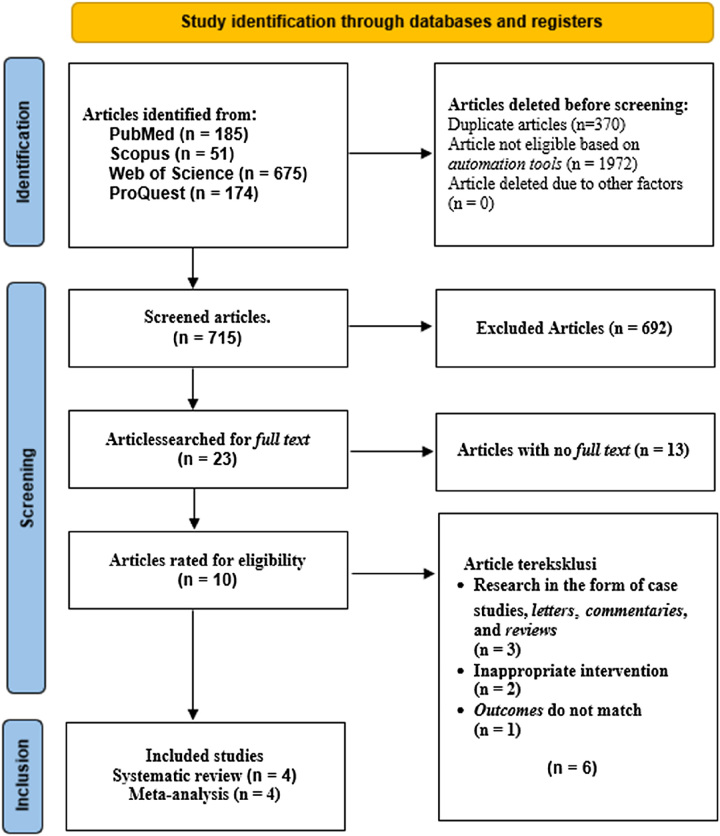
The PRISMA flow diagram (Fig. 1) shows article searching and selection. Four online databases yielded 1085 initial articles for the systematic study. Table 1 shows search engine results. Three hundred and seventy duplicate articles were removed from the acquisition during screening. Twenty-three studies were extractable and candidates for eligibility evaluation after the primary screening, resulting in ten studies that were thoroughly evaluated according to study criteria. Qualitative synthesis and meta-analysis included four studies. The flowchart shows the PRISMA-guided systematic literature search.
Figure 1.
Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) flow diagram.
Table 1.
Baseline characteristics of the research population
| References | Country | Design | Follow-up (weeks) | Age | n | Group | Types of fillers (trade name) | Mean vol. injection, ml (mean±SD) | Injection materials | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Yang et al.5 2019a | Korea | RCT | 48 | 20–65 | 36 | Intervention | Hyaluronic acid (Chaeum Shape) | 19.14±1.4 | 20 mg/ml HA cross-linked | Penile girth, satisfaction rate appearance, sexual satisfaction level, side effects |
| 34 | Control | Polylactic acid (PowerFill) | 20.59±1.28 | 10 g/3 ml microparticles PLA | ||||||
| Yang et al.22 2019b | Korea | RCT | 24 | 19–65 | 37 | Intervention | Hyaluronic acid (Neuramis Deep) | 20.8±1.5 | 20 mg/ml HA cross-linked | Penile girth, satisfaction rate appearance, sexual satisfaction level, side effects |
| 35 | Control | Polylactic acid (PowerFill) | 21.8±1.7 | 10 g/3 ml microparticles PLA | ||||||
| Yang et al.6 2020 | Korea | RCT | 72 | 20–66 | 33 | Intervention | Hyaluronic acid (Hyafilia Impact) | 16.4±2.7 | 20 mg HA cross-linked+3 mg lidocaine/ml | Penile girth, satisfaction rate appearance, sexual satisfaction level, side effects |
| 34 | Control | Polylactic acid (PowerFill) | 17.7±2.3 | 10 g/3 ml microparticles PLA | ||||||
| Ahn et al.17 | Korea | RCT | 24 | 20–65 | 32 | Intervention | Hyaluronic acid (Doublofill) | 15–22 | 23 mg HA cross-linked+3 mg lidocaine/2 ml | Penile girth, sexual satisfaction rate, side effects |
| 32 | Control | Polylactic acid (PowerFill) | 15–22 | 10 g/3 ml microparticles PLA |
PLA, polylactic acid.
Characteristics of the pooled studies
All four inclusion studies were RCTs with 283 participants. Tables 1 and 2 present baseline characteristics and extracted study data. Four (2019–2021) studies had 24– 72-week follow-ups. Each study included 19–66-year-olds. Two clinical trial protocol groups exist. A different product with 20 mg/ml cross-linked hyaluronic acid filler is used. One study found 23 mg cross-linked HA and 3 mg lidocaine/2 ml. The four studies used the 10 g/3 ml PLA microparticle injection material’s polylactic acid filler. The two intervention groups had 16.4–20.8 ml injection volumes. Each study examined penile girth, satisfaction with penile appearance, sexual satisfaction, and postenlargement side effects like penile pain and swelling.
Table 2.
Outcome profile inclusion study systematic review and meta-analysis
| References | Types of fillers (trade name) | Increase in penile diameter, mm (mean±SD) | Improved penile appearance satisfaction, VAS score* (mean±SD) | Increased sexual satisfaction, VAS score* (mean±SD) | Pain events (n) | Penile inflammatory events (n) |
|---|---|---|---|---|---|---|
| Yang et al.5 2019a | Hyaluronic acid (Chaeum Shape) | 20.6±10.9 | 1,59±1.13 | 1.13±1.24 | 0 | 0 |
| Polylactic acid (PowerFill) | 14.6±10.4 | 1.5±1.26 | 0.88±1.56 | 1 | 1 | |
| Yang et al.22 2019b | Hyaluronic acid (Neuramis Deep) | 21±10 | 1.7±1.7 | 1.1±1 | 0 | 1 |
| Polylactic acid (PowerFill) | 16±9 | 1.7±1.4 | 0.4±1.1 | 2 | 0 | |
| Yang et al.6 2020 | Hyaluronic acid (Hyafilia Impact) | 19.1±14.9 | 1.2±1.1 | 1.1±0.9 | 2 | 1 |
| Polylactic acid (PowerFill) | 19.5±10.8 | 1.4±1 | 0.9±1.2 | 1 | 1 | |
| Ahn et al.17 | Hyaluronic acid (Doublofill) | 22.7±12.6 | TD | 1.16±1.07 | 0 | 2 |
| Polylactic acid (PowerFill) | 20.2±8.73 | TD | 0.42±0.89 | 1 | 2 |
VAS Score: Visual Analogue Scale Score
Assessment of study quality and risk of study bias
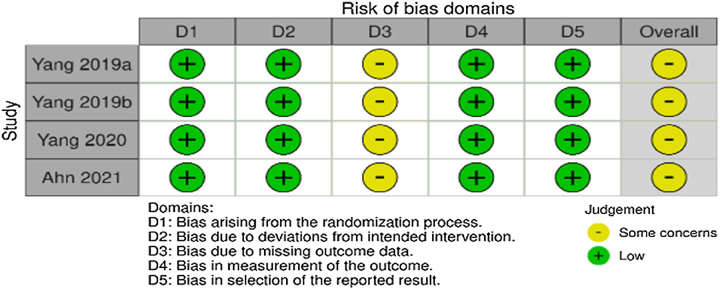
This systematic review and meta-analysis included RCT clinical trials, so the Cochrane RoB v2 instrument could assess research bias. Five domains were assessed sequentially: participant selection bias (randomization), deviation from intervention protocols, unreported or incomplete study data, means of measuring outcomes, and likelihood of selectively reported outcomes. The final score was determined according to the algorithm described by Higgins et al.12, showed that the three RCTs conducted by Yang et al. and research by Ahn et al. RoB toward the third domain. Protocol deviations, personal choices, withdrawal of consent, and loss of follow-up caused withdrawals in every study. Figure 2 summarizes assessment results.
Figure 2.
Evaluation of the risk of bias with the Cochrane risk of bias tool v2.
Meta-analysis of PGE postaugmentation with HA versus PLA fillers
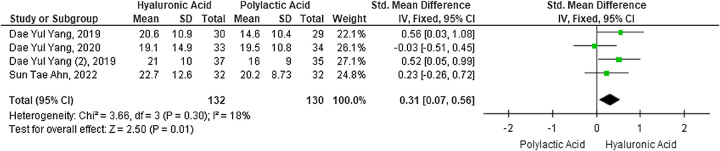
The forest plot compares the mean penile girth increase in the two study groups. HA increased penile diameter more than PLA in four studies with 262 participants (SMD 0.31; 95% CI: 0.07–0.65; P=0.01). Due to low study heterogeneity, the forest plot (Fig. 3) used the fixed-effects model (I2=18%).
Figure 3.
Forest plot of penile girth enhancement after penile augmentation with hyaluronic acid versus polylactic acid fillers.
Meta-analysis of satisfaction with penile appearance 4 weeks after penile augmentation with HA versus PLA fillers
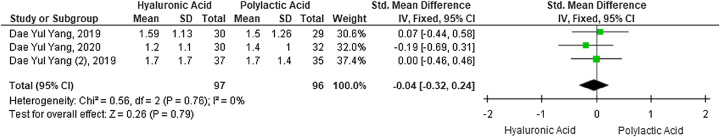
Contrary to penile enlargement results, a meta-analysis on a subjective scale of satisfaction with penile appearance 4 weeks after penile augmentation showed no difference between the HA and PLA intervention groups (SMD −0.04; 95% CI: −0.32to 0.24; P=0.79) involving three studies and 193 participants (Fig. 4). These outcomes were analyzed using the fixed-effects model due to low heterogeneity between studies with an I2 of 0%.
Figure 4.
Forest plot of satisfaction with penile appearance 4 weeks after penile augmentation with hyaluronic acid versus polylactic acid fillers.
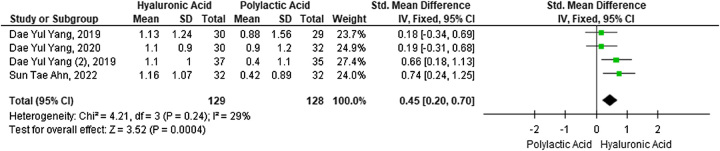
Meta-analysis of levels of sexual satisfaction 12 weeks postpenile augmentation with HA versus PLA fillers
The next major outcome was the difference in sexual satisfaction between the two study groups 12 weeks after the augmentation. A meta-analysis of four studies with 257 participants found a significant difference in sexual satisfaction between the two intervention groups (SMD 0.45; 95% CI: 0.20–0.70; P=0.0004), HA filler outperforms PLA. Forest plots (Fig. 5) used the fixed-effects model due to low study heterogeneity (I2=29%).
Figure 5.
Forest plot of sexual satisfaction level 12 weeks after penile augmentation with hyaluronic acid versus polylactic acid filler.
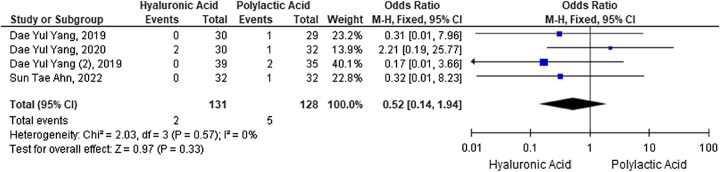
Meta-analysis of postaugmentation pain incidence with HA fillers versus PLA
The OR from each study’s dichotomous data was used to estimate pain side effects. HA injection did not increase pain compared to PLA (OR 0.52) (95% CI: 0.14–1.94; P=0.33). Analysis of heterogeneity between studies resulted in an index of I2=0%, so study heterogeneity was not significant. The forest plot shown in Figure 6 uses the fixed effect model based on the heterogeneity test results of the four studies.
Figure 6.
Forest plot of the incidence of postaugmentation pain with hyaluronic acid versus polylactic acid fillers.
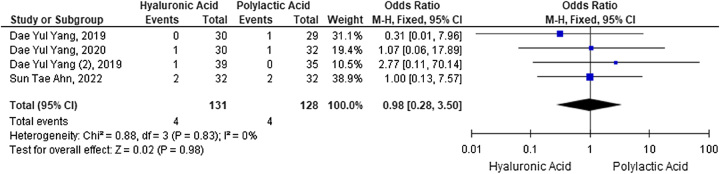
Meta-analysis of postaugmentation inflammatory incidence with HA versus PLA fillers
The incidence of postaugmentation penile inflammation was analyzed as an OR using dichotomous data. The results of the meta-analysis showed no difference in the incidence of penile inflammation as indicated by an OR of 0.98 (95% CI: 2.80–3.50; P=0.98). Analysis of heterogeneity between studies resulted in an index of I2=0%, so it can be said that heterogeneity between studies was not significant. A meta-analysis was carried out using the fixed effect model based on the heterogeneity test results. A collection of meta-analyses of the adverse effects of penile inflammation are shown in the forest plots in Figure 7.
Figure 7.
Forest plot of inflammatory incidence after penile augmentation with hyaluronic acid versus polylactic acid fillers.
Discussions
For years, men have sought penile enlargement surgeries for medical and nonmedical reasons13. SPS patients, who may have unrealistic penile size and appearance expectations, must be treated carefully3. Hyaluronic acid and polylactic acid fillers are now preferred for penile augmentation over autologous fat, silicone, and polymethylmethacrylate due to biomaterials advancements that improve results and reduce patient downtime13,14. Several isolated and comparative studies have shown both fillers to be safe and effective6,8,15,16.
Four RCT clinical trials from South Korea met this study’s methodological and outcome requirements, according to a comprehensive literature search. These studies have 24 to 72-week follow-ups. Hyaluronic acid and polylactic acid metabolism stabilizes 24 weeks after injection13,17. Due to the rapid development of esthetic surgery and the higher proportion of men who undergo esthetic surgery in South Korea, more studies on penile augmentation are likely to be discovered there18. Four inclusion studies used monophasic and biphasic HA products with comparable doses but different cross-linking structures. High-cross-linked HA increases filler viscosity and cohesiveness, resulting in a longer-lasting augmentative effect. This decreases HA biocompatibility, increasing the risk of adverse effects like local responses, discomfort, edema, and granulomas9,19. After biphasic and monophasic HA injection, the dermis has a large pool of HA in the lower dermis and no HA in the upper and middle reticulate dermis, according to histological studies. Monophasic products better distribute HA particles across the dermis, but in large clusters20.
The pooled meta-analysis showed that hyaluronic acid increased penis diameter more than polylactic acid for penile girth enlargement. In a recent prospective study by Zhang et al.21, which used HA gel injection with similar material and average injection volume (21.5±3.7 ml) and followed patients for 1-year postinjection, penis diameter, and length increased significantly in both flaccid and erectile phases. One of the four RCTs in this meta-analysis showed comparable clinical efficacy between HA and PLA with an average maximum addition size of 2.5 cm and 2.3 cm, respectively6. HA is naturally absorbed into the bloodstream faster than PLA, which is synthetic14. The uniformly distributed HA gel’s hydrophilic strength increases volume and weight, preventing the penis corpus from contracting, especially when flaccid21.
Penile appearance satisfaction both fillers were similar 4 weeks postaugmentation. The four inclusion studies show that HA and PLA fillers are significantly more satisfying than baseline without penile augmentation. Filler increases the penile diameter. HA and PLA fillers improve penile esthetics after augmentation5,6,22,23.
After segmentation, the hyaluronic acid filler group had greater sexual pleasure. A review of nonsurgical penile augmentation techniques found that HA increases satisfaction24. In the study by Ahn et al., some patients had a reduction in ejaculation-related symptoms, which is intriguing. Several research have demonstrated the efficiency of penile glans augmentation using HA fillers; nevertheless, the effects of ejaculation on penile augmentation with HA fillers remain unknown. Hyaluronic acid-based penile fillers infiltrate the buck and dartos fascias. Hyaluronic acid fillers can increase the threshold of penile dorsalis nervus receptors by blocking tactile stimuli. Obviously, this can boost patient’s pleasure with their sexual performance if they have HA fillers23. SPS patients have normal libidos, and therapies reduce their psychological suffering25,26. Two studies added lidocaine to HA fillers. Smith et al. found that lidocaine-containing HA fillers significantly reduced pain during and after injection without changing side effects. Safe and comfortable penile augmentation may improve patient satisfaction27.
Hyaluronic acid and polylactic acid fillers cause similar postaugmentation penile discomfort and inflammation. HA had 1.5% penile discomfort and inflammation, while PLA had 3.9 and 3%. Another study found penile injection with HA or PLA was statistically safe and most side effects resolved spontaneously28. Zhang et al. found two cases of penile edema and one case of subcutaneous hemorrhage after HA and PLA penile injections with a 1-year follow-up. Both cases improved spontaneously within 4 weeks21.
The study shows that hyaluronic acid and polylactic acid semi-permanent penile augmentation is safe and effective. The researcher’s view is consistent with previous systematic studies that found penile augmentation can be an option for patients, but clinically this modality is controversial, so more research is needed to determine its long-term physical and psychological effects before it can be recommended14,24.
HA fillers can cause severe infections, including fatal sepsis, after penile augmentation, as shown in Table 3. Abscesses and sepsis can result from filler biofilms29–31. Biofilms protect bacteria from antibiotic bactericides but harm cultures. Immunosuppression, trauma, or iatrogenic manipulation can activate latent biofilm microorganisms. Postaugmentation sexual activity spreads bacteria from the penile, pubic hair, and vagina. Sexual activity should wait at least 1 month after augmentation to heal the filler injection wound28,31,32. Rare side effects may be late-onset due to the limited number of documented cases and short follow-up period. Superfluous preputium in uncircumcised patients can alter negative effects. Standard injection protocols and aseptic preoperative preparations reduce complications28.
Table 3.
Case reports of postaugmentation complications of the penile
This study’s limitations include the small sample size of fewer than 10 studies, which precludes publication bias analysis. There are differences in cross-linking methods and monophasic/biphasic types of hyaluronic acid preparations used, although the injection dose and volume are comparable. The length of follow-up in inclusion studies is still variable and may not be sufficient to describe the true durability of the outcome. Because there is no group division based on dose titration, the optimal recommended dose of injectable filler is inconclusive. Currently, there is no standardized method for measuring postaugmentation satisfaction. In the study of inclusion, it was also discovered that three of the four studies had the same first author, allowing for bias. In addition, this systematic review and meta-analysis require additional studies involving multicenter participants from different countries and researchers in order to enhance external validation.
Conclusion
Our systematic review and meta-analysis found that HA filler improves penile girth and sexual satisfaction 12 weeks after penile augmentation compared to PA. HA filler improved penile girth and sexual satisfaction in this study.
Ethical approval
Ethical approval is not required in this type of study.
Consent
Informed consent is not required in this type of study.
Sources of funding
The authors received no financial support for this work.
Author contribution
A.K., M.R.S., and J.R.: concept; A.K., M.R.S., M.A.S., and S.W.: design; M.A.S. and S.W.: supervision; A.K., M.R.S., M.A.S., and S.W.: resources; A.K., M.R.S., M.A.S., and S.W.: materials; A.K., M.R.S., M.A.S., and S.W.: data collection and/or processing; A.K., M.R.S., M.A.S., and S.W.: analysis and/or interpretation; A.K., M.R.S., M.A.S., and S.W.: literature search; A.K., M.R.S., M.A.S., and S.W.: writing manuscript; A.K., M.R.S., M.A.S., and S.W.: critical review.
Conflicts of interest disclosure
The authors declare that there is no competing interest.
Research registration unique identifying number (UIN)
Name of the registry: not applicable.
Unique identifying number or registration ID: not applicable.
Hyperlink to your specific registration (must be publicly accessible and will be checked): not applicable.
Guarantor
Prof Soetojo Wirjopranoto.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Soetojo Wirjopranoto, upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
None.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 22 July 2023
Contributor Information
Ahmad Kusumaputra, Email: ahmad.kusumaputra@gmail.com.
Muhammad R. Setiawan, Email: setiawanrifki12@gmail.com.
Mohammad A. Soebadi, Email: yodisoebadi@gmail.com.
Soetojo Wirjopranoto, Email: s.tojowirjopranoto@yahoo.com.
References
- 1. Abdallah H, Abdelnasser T, Hosny H, et al. Treatment of premature ejaculation by glans penis augmentation using hyaluronic acid gel: a pilot study. Andrologia 2011;44:650–653. [DOI] [PubMed] [Google Scholar]
- 2. Chung E. Penile reconstructive surgery in peyronie disease: challenges in restoring normal penis size, shape, and function. World J Mens Health 2020;38:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wylie KR, Eardley I. Penile size and the ‘small penis syndrome’. BJU Int 2007;99:1449–1455. [DOI] [PubMed] [Google Scholar]
- 4. Ghanem H, Glina S, Assalian P, et al. Position paper: management of men complaining of a small penis despite an actually normal size. J Sex Med 2013;10:294–303. [DOI] [PubMed] [Google Scholar]
- 5. Yang DY, Ko K, Lee SH, et al. A comparison of the efficacy and safety between hyaluronic acid and polylactic acid filler injection in penile augmentation: a multicenter, patient/evaluator-blinded, randomized trial. J Sex Med 2019;16:577–585. [DOI] [PubMed] [Google Scholar]
- 6. Yang DY, Jeong HC, Ko K, et al. Comparison of clinical outcomes between hyaluronic and polylactic acid filler injections for penile augmentation in men reporting a small penis: a multicenter, patient-blinded/evaluator-blinded, non-inferiority, randomized comparative trial with 18 months of follow-up. J Clin Med 2020;9:1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hyun MY, Lee Y, No YA, et al. Efficacy and safety of injection with poly-L-lactic acid compared with hyaluronic acid for correction of nasolabial fold: a randomized, evaluator-blinded, comparative study. Clin Exp Dermatol 2014;40:129–135. [DOI] [PubMed] [Google Scholar]
- 8. Kwak T, Oh M, Kim J, et al. The effects of penile girth enhancement using injectable hyaluronic acid gel, a filler. J Sex Med 2010;8:3407–3413. [DOI] [PubMed] [Google Scholar]
- 9. Goldberg DJ. Breakthroughs in US dermal fillers for facial soft-tissue augmentation. J Cosmet Laser Ther 2009;11:240–247. [DOI] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 11. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Online) 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park NC, Kim SW, Moon DG. Penile Augmentation. Springer; 2016. [Google Scholar]
- 14. Xing MH, Hou SW, Raheem OA. Aesthetic penile augmentation procedures: a comprehensive and current perspective. Curr Urol Rep 2022;23:355–361. [DOI] [PubMed] [Google Scholar]
- 15. Yang DY, Ko K, Lee SH, et al. Efficacy and safety of a newly developed polylactic acid microsphere as an injectable bulking agent for penile augmentation: 18-months follow-up. Int J Impot Res 2017;29:136–141. [DOI] [PubMed] [Google Scholar]
- 16. Yang DY, Ko K, Lee SH, et al. Efficacy and safety of newly developed cross-linked dextran gel injection for glans penis augmentation with a novel technique. Asian J Androl 2018;20:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahn ST, Shim JS, Bae WJ, et al. Efficacy and safety of penile girth enhancement using hyaluronic acid filler and the clinical impact on ejaculation: a multi-center, patient/ evaluator-blinded, randomized active-controlled trial. World J Mens Health 2021;39:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holliday R, Elfving-Hwang J. Gender, globalization and aesthetic surgery in South Korea. Body Soc 2012;18:58–81. [Google Scholar]
- 19. Herrmann JL, Hoffmann RK, Ward CE, et al. Biochemistry, physiology, and tissue interactions of contemporary biodegradable injectable dermal fillers. Dermatol Surg 2018;44:S19–S31. [DOI] [PubMed] [Google Scholar]
- 20. Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg 2011;37:637–643. [DOI] [PubMed] [Google Scholar]
- 21. Zhang CL, Quan Y, Li H, et al. Penile augmentation with injectable hyaluronic acid gel: an alternative choice for small penis syndrome. Asian J Androl 2022;24:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang DY, Jeong HC, Ahn ST, et al. A comparison between hyaluronic acid and polylactic acid filler injections for temporary penile augmentation in patients with small penis syndrome: a multicenter, patient/evaluator-blind, comparative, randomized trial. J Sex Med 2019;17:133–141. [DOI] [PubMed] [Google Scholar]
- 23. Ahn ST, Shim JS, Bae WJ, et al. Efficacy and safety of penile girth enhancement using hyaluronic acid filler and the clinical impact on ejaculation: a multi-center, patient/evaluator-blinded, randomized active-controlled trial. World J Mens Health 2022;40:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salloum A, Bazzi N, Haber R. Nonsurgical methods for penile augmentation: a systematic review. Dermatol Surg 2021;47:e81–e85. [DOI] [PubMed] [Google Scholar]
- 25. Veale D, Miles S, Read J, et al. Sexual functioning and behavior of men with body dysmorphic disorder concerning penis size compared with men anxious about penis size and with controls: a cohort study. Sex Med 2015;3:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang DY, Jeong HC, Ahn ST, et al. A comparison between hyaluronic acid and polylactic acid filler injections for temporary penile augmentation in patients with small penis syndrome: a multicenter, patient/evaluator-blind, comparative, randomized trial. J Sex Med 2020;17:133–141. [DOI] [PubMed] [Google Scholar]
- 27. Smith L, Cockerham K. Hyaluronic acid dermal fillers: can adjunctive lidocaine improve patient satisfaction without decreasing efficacy or duration. Patient Prefer Adherence 2011;5:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quan Y, Gao ZR, Dai X, et al. Complications and management of penile augmentation with hyaluronic acid injection. Asian J Androl 2021;23:392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al-Maghlouth AK, Alwesali S, Faqeeh A, et al. Late onset penile abscess after 4 years from hyaluronic acid injection. A rare case report. Urol Case Rep 2021;37:101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baird Bryce A, Robertson N, Broderick Gregory A. Penile girth injection complications: a case report. Sex Med 2021;9:100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khor NWM, Dhar A, Cameron-Strange A. The perils of penile enhancement: case report of a fulminant penile infection. BMC Urol 2021;21:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marusza W, Olszanski R, Sierdzinski J, et al. Treatment of late bacterial infections resulting from soft-tissue filler injections. Infect Drug Resist 2019;12:469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Soetojo Wirjopranoto, upon reasonable request.