Abstract
How do socioeconomic disparities shape brain health and disease? Ibáñez et al. discuss the need for further research into how wealth and socioeconomic status affect biological models of dementia, highlighting the biological ripple effects of socioeconomic inequalities and the importance of globally inclusive brain health research.
How do socioeconomic disparities shape brain health and disease? We address current scientific shortcomings, highlight the biological ripple effects of socioeconomic inequalities, and propose more globally inclusive brain health research.
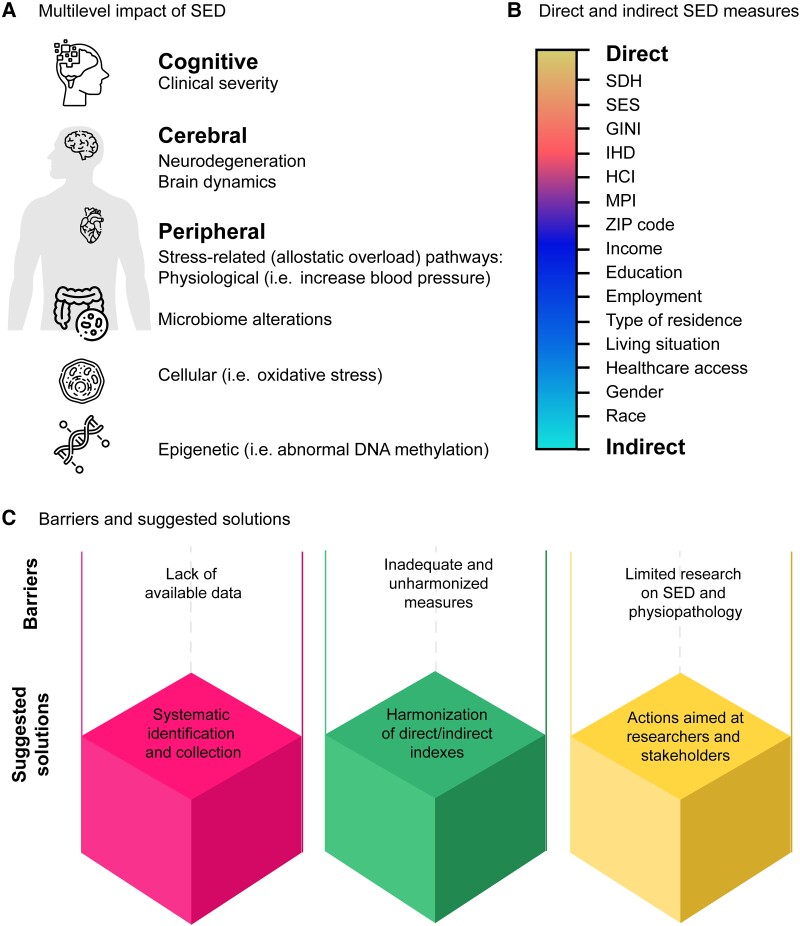
Socioeconomic disparities (SED) significantly impact brain health and contribute to the development of dementia.1,2 The consequences of SED are evident across various aspects of brain health, such as clinical severity, biomarkers, neurodegeneration, altered brain dynamics, heightened proteinopathy and allostatic overload1–6 (Fig. 1A). Two fundamental approaches to examining SED at the individual level involve the social determinants of health (SDH) and socioeconomic status (SES). SDH, defined by the WHO as non-medical factors influencing health outcomes, encompasses various aspects of daily living SED. On the other hand, SES refers to an individual or group’s standing within the socioeconomic spectrum, determined by factors like income, education and occupation. Also, country-level measurements of SED capture aggregated data and indices related to brain health outcomes.7 Thus, their disparate measures include income inequality, poverty rates, educational attainment and employment (Fig. 1B).
Figure 1.
SED and brain health. An overview of the multilevel impact of socioeconomic disparities (SED) on brain health. (A) Multilevel impact of SED and their effects on various aspects of brain health, including cognitive (clinical severity, daily-life functioning and cognitive dysfunction), cerebral (brain dynamics and neurodegeneration), and peripheral factors (inflammatory and immunological dysregulation, microbiome alterations, stress-related pathways, physiological dysregulations, cellular alterations and epigenetic modifications). (B) Direct and indirect measures of SED. Differentiation between direct and indirect measures of SED, with direct measures including social determinants of health (SDH) and socioeconomic status (SES), and indirect measures encompassing factors such as postcode (ZIP code), income, education, race, gender, employment, type of residence, living situation and healthcare access. (C) Barriers and suggested solutions. Identification of barriers and suggested solutions for addressing challenges in future research aimed at developing equitable global approaches to brain health that consider the impact of SED. HCI = human capital index; IHD = index of human development; MPI = multidimensional poverty measures.
Critical scientific flaws currently hinder our understanding of the integration between socioeconomic and biological factors in brain health and disease. Despite the acknowledged role of SED in brain health and dementia, most research across various biological domains adopts universal models,1–4 overlooking the distinct impact of socioeconomic environments (Santamaria-Garcia et al., accepted for publication). Likewise, most research in this field has predominantly originated from high-income countries like the USA and European countries. Current scientific findings fail to sufficiently address the pressing need to assess regional diversity and provide tailored evidence for under-represented samples concerning SED across multiple areas, including genetics, epigenetics, environmental factors, brain-phenotype associations and risk factors for ageing and dementia.2–6
Data deficiency, granularity limitations and disconnection with biomedical research
The development of diversity-oriented global approaches to brain health and SED faces three main critical gaps (Fig. 1C).
A first and crucial gap is the systematic lack of available evidence concerning SED beyond measures of educational level. Most publicly accessible large datasets on brain health and dementia (Table 1 and Supplementary Table 1) do not provide information on SED, and most of these datasets come from high-income settings. Similarly, large-scale volunteer databanks in ageing have biased participant demographics such as white ethnicity, high-education biases, and a lack of detailed and variable information on socioeconomic metrics. This hinders a comprehensive understanding of SED’s impact on brain health and dementia.
Table 1.
SED assessments in representative online datasets
| Dataset | Type of information provided | Direct SED measure | Indirect SED measures | Data origin |
|---|---|---|---|---|
| Alzheimer’s Disease Neuroimaging Initiative (ADNI) http://adni.loni.usc.edu |
Clinical, genetic, neuroimaging, biospecimen | NAI | Demographics: age, education, gender, race, ethnicity, occupation, type of residence | USA, Canada |
| National Alzheimer’s Coordinating Center (NACC) database https://naccdata.org |
Clinical, neuropathology, neuroimaging, fluid biomarker, genotypic and genomic data | NAI | Demographics: age, education, sex, race, ethnicity, type of residence, living situation, postcode | USA |
| Rush Alzheimer’s Disease Center (RADC) datasets https://www.radc.rush.edu |
Clinical, neuroimaging, fluid biomarkers, health and lifestyle factors, genetics, economics, medical history, sleep | SES: income, income (age 40), parental education level (maternal education, paternal education), subjective SES | Demographics: age, education, sex, race, ethnicity, marital status Income, income (age 40). Cognitive resources | USA |
| Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL) https://aibl.csiro.au |
Clinical, neuroimaging, fluid biomarkers, health and lifestyle factors | NAI | NAI | Australia |
| Parkinson’s Progression Markers Initiative (PPMI) https://www.ppmi-info.org |
Clinical, physical and neurological examination Neuroimaging, biomarkers, genetic data |
NAI | Demographics: age, education, gender, race, ethnicity | USA, Canada, Spain, Luxembourg, Greece, Austria, Germany, UK, Netherlands, Italy, Israel, Nigeria |
| Cambridge Centre for Ageing and Neuroscience (Cam-CAN) http://www.cam-can.org |
Clinical, neuroimaging physiological measures | NAI | Demographics: age, education, gender, ethnicity, marital status, accommodation, employment, income, birthplace, and English language history | UK |
| National Institute on Aging Genetics of Alzheimer’s Disease Data Storage Site (NIAGADS) https://www.niagads.org |
Genetic data | NAI | NAI | USA |
| UK Biobank https://www.ukbiobank.ac.uk |
Clinical, neuroimaging, biomarkers, early life and reproductive factors, genetic data, lifestyle, geographical measures | Index of multiple deprivation: access to service; community safe, crime, education, health, housing, income, living and physical environment scores. | Demographics: age, education, sex, ethnicity. Employment, employment history. Household (gas, heating, n people, own or rent, vehicles). Type of residence. Private healthcare. Geographical measures: greenspace and coastal proximity. Home locations. Residential air and noise pollution | UK |
| Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) http://enigma.ini.usc.edu |
Medical, genetic, neuroimaging data | NAI | NAI | NAI |
| Human Connectome Project (HCP) https://www.humanconnectome.org |
Clinical, lifestyle data, neuroimaging | NAI | Demographics: age, sex | USA |
See Supplementary Table 1 for further details. NAI = non-available information; SED = socioeconomic disparities; SES = socioeconomic status.
Second, there is inadequate granularity in assessing SED. Broad demographic categories such as income or SES do not adequately account for other SED factors. Furthermore, disparate measures are not harmonized or systematically compared. For instance, country-level indices (e.g. World Bank country classifications, Gini, index of human development, human capital index, multidimensional poverty measures, etc.) are not harmonized. The absence of convergent definitions increases discrepancies and inconsistencies in research findings. Although SDH measures appear to partially overcome this barrier by providing individual-level data on different dimensions, their current assessments in brain health research are extremely limited in scope and application.
Similarly, there is a lack of convergent measures, dimensions and hierarchies of categories for SDH. For example, the specific measures and inclusion of economic and occupational status, social and community context, neighbourhood and physical environment, health behaviours, and healthcare access and quality are not harmonized nor systematically assessed. SES research presents similar controversies. It is unclear which specific factor or combination best represents SES, including income, education, occupation and wealth. SES is not a static construct, and individuals can experience upward or downward mobility over their lifetime. Finally, other indirect measures of SED (e.g. ZIP/postcode) are rarely included in standard protocols or are differently operationalized (e.g. area of deprivation, different geographical segregation indices). Including these measures require de-identification, and this is not frequently assessed. Thus, various SED measures involve interrelated but not integrated dimensions, encompassing inadequate granularity, lack of harmonization, inconsistencies in indicators, and under-utilization of indirect measurements.
Previous gaps contribute to the third gap, which involves limited research on SED interactions with biological biomarkers and pathophysiological pathways. Research on SED has been primarily confined to sociological studies and health economics. Only a few approaches assess SED in domains such as biomarkers or pathophysiological processes,2,4,5 leaving significant gaps in our understanding of the biological processes underlying these disparities. The socioeconomic-related exposome4 has considerable effects on various biological processes, including inflammation, oxidative stress, microbiome, stress-related pathways, allostatic overload and epigenetics. However, these domains have been sporadically assessed, and consequently, direct actionable recommendations for research and policymakers are scarce.
A call for increasing socioeconomic diversity and actionable initiatives
Urgent initiatives aimed at bridging the three main gaps are required. Datasets must include relevant SED variables beyond the classical assessment of formal education. For instance, parental education (paternal/maternal years of formal education), income (household income from all sources), employment (current status, lifetime job), birthplace (city, country), home location (neighbourhood, city, country, de-identified postcode), household (access to drinking water, gas, electricity, number of people in the house, social isolation, type of residence, ownership or rent, number of vehicles), and healthcare access should be included.8 Systematic harmonization and normalization procedures3 are critically needed to assess comparability in multicentric research. Using heterogeneity and dimensionality reduction techniques can help identify reduced and shared critical indicators. Access to this harmonized information can help develop global comparative measures and improve granularity across SED indices.
Second, future studies should thoroughly target the relationships between SED (with harmonized and granular measures) and biological markers. For instance, country-level disparities assess with meta-regressions7 and SDH/SES individual predictors2,3 can provide important insights across levels, including metabolic processes, plasma biomarkers, epigenetic influences, neurocognitive measures and clinical assessments.8,9 Global initiatives for brain health research should be extended, considering the heterogeneity from under-represented low-income and middle-income countries.
Establishing a global consortium dedicated to collecting, harmonizing and standardizing SED measures and systematic data in brain health research could significantly address current gaps in this field. Recent brain research collaboration models have proven successful in this regard. Projects such as the Lifepath research consortium 2020 started to investigate the effects of SED on biological ageing in cohorts from Europe, USA and Australia. Also, the new WHO Intersectoral Global Action Plan on Epilepsy and other Neurological Disorders 2022–2031 may help to overcome the lack of evidence between SED and brain health in low- and middle-income countries. Programs like the WHO Mental Health Gap Action Programme (mhGAP) or the National Institutes of Health’s (NIH) Fogarty International Center could devote specific efforts to support research on global SED impact on brain health. Current calls for diversity in global approaches to dementia research should also encompass a comprehensive inclusion of SED.
Here, we have considered the importance of understanding the complex interactions between socioeconomic factors and brain health by providing basic recommendations for overcoming the identified gaps. Such an in-depth framework should be developed at different levels, from risk factors to biomarkers, disease heterogeneity and pharmacological and non-pharmacological interventions. A multilevel, systematic shift towards diversity through the lens of SED might be relevant for developing more effective, inclusive and tailored research, interventions and therapies for brain-related disorders.8
Supplementary Material
Biography
Agustín Ibáñez is the director of the Latin American Brain Health Institute (BrainLat) and group leader of the Predictive Brain Health Modelling group at Trinity College Dublin. He founded and co-directs the Multi-Partner Consortium to Expand Dementia Research in Latin America. Agustina Legaz is a neuroscientist, currently a post-doctoral fellow at BrainLat and a professor at the University of San Andrés. Manuel Ruiz-Adame, a psychologist and health economist, is a professor at the University of Granada and a visiting research fellow at Trinity College Dublin.
Contributor Information
Agustín Ibáñez, Global Brain Health Institute (GBHI), University of California San Francisco (UCSF), San Francisco, CA 94158, USA; Trinity College Dublin, The University of Dublin, Dublin 2, Ireland; Latin American Brain Health (BrainLat), Universidad Adolfo Ibáñez, Santiago, Chile.
Agustina Legaz, Latin American Brain Health (BrainLat), Universidad Adolfo Ibáñez, Santiago, Chile; Cognitive Neuroscience Center (CNC), and CONICET, Universidad de San Andrés, C1116, Buenos Aires, Argentina.
Manuel Ruiz-Adame, Department of Applied Economics, University of Granada, Campus of Melilla, 52004 Melilla, Spain.
Funding
A.I. is supported by grants from CONICET; ANID/FONDECYT Regular (1210195 and 1210176 and 1220995); ANID/FONDAP/15150012; ANID/PIA/ANILLOS ACT210096; FONDEF ID20I10152, ID22I10029; ANID/FONDAP 15150012; Takeda CW2680521 and the MULTI-PARTNER CONSORTIUM TO EXPAND DEMENTIA RESEARCH IN LATIN AMERICA [ReDLat, supported by Fogarty International Center (FIC), National Institutes of Health, National Institutes of Aging (R01 AG057234, R01 AG075775, R01 AG21051, CARDS-NIH), Alzheimer’s Association (SG-20-725707), Rainwater Charitable Foundation—The Bluefield project to cure FTD, and Global Brain Health Institute)]. The contents of this publication are solely the responsibility of the authors and do not represent the official views of these Institutions.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Walsh S, Merrick R, Brayne C. The relevance of social and commercial determinants for neurological health. Lancet Neurol. 2022;21:1151–1160. [DOI] [PubMed] [Google Scholar]
- 2. Resende EPF, Llibre Guerra JJ, Miller BL. Health and socioeconomic inequities as contributors to brain health. JAMA Neurol. 2019;76:633–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greene AS, Shen X, Noble S, et al. Brain-phenotype models fail for individuals who defy sample stereotypes. Nature. 2022;609:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vermeulen R, Schymanski EL, Barabási AL, Miller GW. The exposome and health: Where chemistry meets biology. Science. 2020;367:392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Felice FG, Gonçalves RA, Ferreira ST. Impaired insulin signalling and allostatic load in Alzheimer disease. Nat Rev Neurosci. 2022;23:215–230. [DOI] [PubMed] [Google Scholar]
- 6. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zugman A, Alliende LM, Medel V, et al. Country-level gender inequality is associated with structural differences in the brains of women and men. Proc Natl Acad Sci U S A. 2023;120:e2218782120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weissman DG, Hatzenbuehler ML, Cikara M, Barch DM, McLaughlin KA. State-level macro-economic factors moderate the association of low income with brain structure and mental health in US children. Nat Commun. 2023;14:2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ibanez A, Zimmer E. Time to synergize mental health with brain health. Nat Mental Health. 2023;1:441–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.