Abstract
As an extracellular vesicle, exosomes play an important role in intercellular information transmission, delivering cargos of the parent cell, such as RNA, DNA, proteins, and lipids, activating different signaling pathways in the target cell and regulating inflammation, angiogenesis, and tumor progression. In particular, exosomes secreted by tumor cells can change the function of surrounding cells, creating a microenvironment conducive to tumor growth and metastasis. For example, after macrophages phagocytose exosomes and accept their cargos, they activate macrophage polarization-related signaling pathways and polarize macrophages into M1 or M2 types to exert antitumor or protumor functions. Currently, the study of exosomes affecting the polarization of macrophages has attracted increasing attention. Therefore, this paper reviews relevant studies in this field to better understand the mechanism of exosome-induced macrophage polarization and provide evidence for exploring novel targets for tumor therapy and new diagnostic markers in the future.
Keywords: Tumor, Exosomes, Macrophage polarization, Signaling pathways
1. Introduction
Exosomes are extracellular vesicles with a diameter of approximately 30–150 nm enclosed by a bilayer lipid membrane [1]. They play a role in the transmission of information between cells [2]. Exosomes contain transmembrane and membrane anchoring proteins that can fuse with the target cell membrane, enhancing endocytosis of the target cell and promoting exosome cargos delivered directly to the cytoplasm of target cells, such as mRNA, miRNA, lncRNA, circRNA, DNA fragments, proteins and lipids [[3], [4], [5], [6]]. miRNAs, lncRNAs, and circRNAs are noncoding RNAs that are transcribed but not translated into proteins and function to alter gene expression at the transcriptional, translational and posttranslational levels [7]. Noncoding RNAs can regulate tumor glycolysis, amino acid metabolism and lipid metabolism reprogramming to influence tumor outcomes, such as promoting cancer cell growth, invasion, metastasis, angiogenesis and drug resistance [8]. These cargos can lead to the development of tumors by activating specific signaling pathways [9]. Although exosomes can be secreted by a variety of cells, exosomes secreted by tumor cells are involved in the exchange of substances and information between tumor cells and surrounding cells [10,11]. For example, upon communicating with immune cells [12], they inhibit their immune responses, promoting tumor growth and even creating a premetastatic niche conducive to tumor metastasis [13]. In addition, a hypoxic environment can increase the release of exosomes and promote tumor progression [14,15].
The tumor microenvironment (TME) is where tumor cells communicate with surrounding cells such as immune cells and the extracellular matrix [16]. As a type of immune cell, macrophages can exert immunomodulatory effects through phagocytosis, exogenous antigen presentation, and secretion of cytokines and growth factors. Notably, macrophages can be polarized into two subtypes, antitumor classically activated M1 phenotype and pro-tumor alternatively activated M2 phenotype [17]. Among them, M2 macrophages can secrete chemokines, including CCL2, CCL17 and CCL22, to promote tumor progression [18]. These two subtypes coexist in the tumor microenvironment and produce a corresponding effect when one type is predominant. The polarized phenotype can be identified by macrophage markers [19].
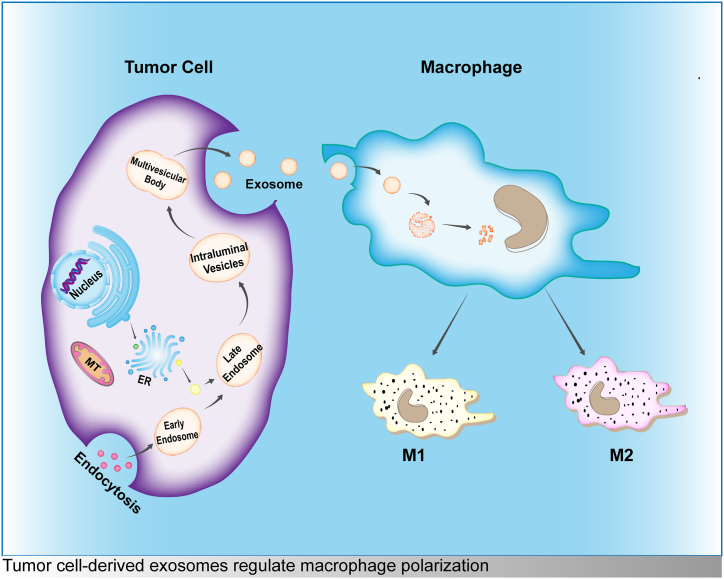
In recent years, with the deepening understanding of the TME, an increasing number of studies focus on the tumor microenvironment and tumor therapy [[20], [21], [22], [23]]. It has been found that the interaction between tumor cells and macrophages can have an impact on the development of primary tumors. Macrophages that take up exosomes secreted by tumor cells can be polarized into M1/M2 phenotypes (Fig. 1) [[24], [25], [26]]. This process is mostly mediated by various molecules in exosomes, which regulate target gene expression and affect tumor progression. This review summarizes the mechanism of exosome-induced polarization of macrophages and provides evidence for exploring new targets for tumor diagnosis and treatment.
Fig. 1.
Tumor cell-derived exosomes regulate macrophage polarization.
2. Signal pathways
2.1. PI3k/AKT
The PI3K/AKT pathway is involved in regulating cell survival and proliferation. Activated PI3Ks cause AKT recruitment by phosphorylating PIP2 (phosphatidylinositol-4,5-bisphosphate) to produce PIP3 (phosphatidylinositol-3,4,5-triphosphate), and activation of this signaling pathway plays an important role in tumorigenesis [27]. The following studies also suggest their involvement in the regulation of macrophage polarization.
PTEN (phosphate and tension homology deleted on chromosome ten) is a negative regulator of the PI3Kγ signaling pathway [28], which is involved in macrophage polarization [29]. PTEN mutations lead to the accumulation of PIP3 and PIP2, activating the PI3K signaling pathway [27]. PTEN downregulation can be mediated by miR-301a-3p, activating the PI3Kg/mTOR pathway [30]. miR-301a-3p expression is upregulated in pancreatic cancer cells and cell-derived exosomes in hypoxic environments, inducing macrophage M2 polarization and promoting the migration and epithelial cell mesenchymal transformation (EMT) of pancreatic cancer cells. Mice treated with exosomes derived from hypoxic pancreatic cancer cells containing miR-301a-3p had more pronounced lung metastatic nodules. Moreover, miR-301a-3p expression was reported to be negatively correlated with TNM staging and prognosis.
miR-934 is expressed in colorectal cancer (CRC) tissue, serum, cells, and cell-derived exosomes [31] and is positively correlated with colorectal cancer liver metastases (CRLMs) and adverse prognosis. miR-934 can induce macrophage M2 polarization under the regulation of the RNA-binding protein hnRNPA2B1 [32] and downregulate PTEN genes, activating the PI3K/AKT signaling pathway [33]. Polarized macrophages enhance the invasion and liver metastasis of weakly metastatic CRC cells. Liver metastases are associated with miR-934-mediated activation of the CXCL13/CXCR5 axis. The chemokine CXCL13 and its receptor CXCR5 bind to activate the NF-κB pathway to amplify the inflammatory response in tumor cells [34], promoting p65 phosphorylation. Inhibition of NF-κB/p65 signaling reduces the expression of miR-934, indicating that miR-934 first activates the PTEN/PI3K/AKT polarization pathway to promote macrophage differentiation to the protumor phenotype and then accelerates colorectal cancer metastasis via the CXCL13/CXCR5/NF-κB signaling pathway.
Activation of the PI3K/AKT pathway [35] enhances STAT3 expression and promotes macrophage polarization [36]. As a miR-21-5p target gene, increased miR-21-5p expression inhibits the level of PTEN and activates the PI3K/Akt/STAT6 signaling pathway to promote M2 polarization [37]. Then, modulating EMT-related genes activates TGF-β signaling pathways to regulate the migration and invasion of esophageal cancer. miR-21-5p has been shown to be highly expressed in esophageal cancer cells and plasma-derived exosomes [38] and is closely related to high-risk factors for esophageal cancer and macrophage polarization, affecting the progression of esophageal squamous cell carcinoma (ESCC) [39]. Coculture of exosome-carried miR-21-5p with macrophages increased the expression of M2 macrophage markers. However, inhibition of miR-21-5p expression in M2 macrophages led to increased expression of M1 macrophage markers [38]. This indicates that there is a positive feedback mechanism between M2 macrophage polarization and esophageal cancer cell EMT in tandem through tumor cell-derived exosomes. In addition, miR-21 is also closely related to bladder cancer progression [40]. It is highly expressed in exosomes secreted by bladder cancer cells, inducing macrophage M2 polarization, thereby enhancing the migration and proliferation of cancer cells [41]. Similar to esophageal cancer, miR-21 in exosomes secreted by bladder cancer cells induces M2 polarization by downregulating PTEN and enhancing PI3K/AKT-induced STAT3 signaling activity. Similar to miR-21, miR-1231-5p and miR-92b-3p [42] also activate the PI3K/AKT/STAT3/6 pathway by downregulating PTEN, inducing macrophages to polarize to immunosuppressive types. However, the exosome inhibitor GW4869 can reverse this process and inhibit bladder tumor progression.
Programmed cell death 4 (PDCD4) is involved in the PI3K γ/Akt/mTOR signaling pathway regulating cell activities [43]. As a target gene for miR-106b, PDCD4 also participates in the regulation of macrophage polarization [44]. miR-106b, which is highly expressed in CRC cell exosomes, induces M2 polarization and promotes CRC metastasis by strengthening tumor cell EMT [45]. The expression levels of p-PI3Kγ, p-AKT, and p-mTOR in miR-106b-treated macrophages were increased, while the expression levels of PDCD4 were attenuated. In accordance with in vitro experiments, miR-106b in CRC exosomes induces M2 polarization by downregulating PDCD4 expression, activating the PI3K γ/Akt/mTOR pathway [44]. EMT of CRC cells is maintained in a positive feedback manner, enhancing the migration and invasion ability of cells. As expected, miR-106b is highly expressed in CRC tissue and plasma exosomes, which is inversely connected with overall survival time and favorably correlated with tumor fraction.
eIF4A3 (Eukaryotic initiation Factor 4A-III) regulates the expression of circFARSA in non-small cell lung cancer (NSCLC) tissues, cells, and cell-derived exosomes [46]. High expression of eIF4A3 can lead to downregulation of PTEN, activation of the PI3K/AKT signaling pathway, and induction of macrophage polarization to the M2 type, thus promoting NSCLC cell migration and EMT [46].
Circ-0001142 can be delivered to macrophages by breast cancer cell-derived exosomes and activate the PI3K/AKT pathway to regulate the M2 polarization of macrophages through competitive binding with miR-361-3p [47]. Circ-0001142 also promotes the expression of the miR-361-3p target gene PIK3CB, which could be reversed by miR-361-3p overexpression. Meanwhile, PI3K/AKT is an autophagy-related pathway [47]. Circ-0001142 can inhibit autophagy, and the change in autophagy level also affects the polarization of macrophages. Polarized macrophages can enhance the proliferation, migration, invasion, and EMT of breast cancer cells and promote liver metastasis in breast cancer mice. Therefore, the circ-0001142/miR-361-3p/PIK3CB axis in breast cancer exosomes plays an important role in inducing macrophage polarization and interrupting autophagy in tumor cells [47]. Additionally, endoplasmic reticulum stress can increase the secretion of breast cancer cell exosomes [48].
2.2. STAT3
STAT (signal transducer and activator of transcription) proteins are a family of cytoplasmic transcription factors containing STAT1-6. Among them, the STAT3 signaling pathway can be activated by phosphorylation of Janus kinases (JAKs) and cytokines and is involved in cell proliferation and cancer development [49]. Additionally, it is a common pathway for the induction of macrophage polarization.
The STAT3 signaling pathway is associated with macrophage polarization to promote tumor development [50,51] and is activated by SOCS3 [52]. miR-222-3p inhibits SOCS3 expression by binding to its 3′-UTR [53]. miR-222-3p packaged in epithelial ovarian cancer (EOC) cell-derived exosomes can be delivered to macrophages, inducing M2 polarization and enhancing the migration and invasion of EOC cells. The tumor volume of mice treated with polarized macrophages increased significantly, as well as the positive staining of CD206, CD31, and LYVE-1 in tumor tissue slices, indicating that EOC cell-derived exosomes can promote tumor vascular and lymph node regeneration. Increased phosphorylated STAT3 expression and decreased SOCS3 levels in EOC-derived exosome-treated macrophages can be observed [53]. The studies above indicate that miR-222-3p induces macrophage M2 polarization by downregulating SOCS3 and activating the STAT3 signaling pathway, accelerating EOC progression. Other studies have shown that [54] the expression levels of miR-21-3p, miR-125b-5p, and miR-181d-5p in EOC cell-derived exosomes in hypoxic environments were higher than those in the normal oxygen environment. Moreover, they can promote M2 polarization, EOC cell proliferation, and migration by regulating SOCS4/5 gene expression and activating STAT3 signaling.
High expression of miR-29b-3p in oral squamous cell carcinoma (OSCC) affects the occurrence and development of this cancer [55]. Its family member miR-29a-3p is highly expressed in OSCC cell exosomes [56], promoting tumor progression by altering macrophage polarization [57]. There is a targeted regulatory relationship between miR-29-3p and SOCS1, while the STAT6 pathway associated with macrophage polarization [58] is affected by SOCS1 [59]. In miR-29a-3p exosome-treated macrophages, the expression of M2 markers and p-STAT6 increased, followed by enhanced proliferation and invasion of OSCC cells. Arguably, OSCC cell-derived exosomes downregulated SOCS1 expression by transmitting miR-23a-3p to macrophages, activating the STAT6 signaling pathway to induce macrophage M2 polarization and promote OSCC progression.
M2 macrophage markers and polarization-related signaling pathway STAT3 phosphorylation [60] are increased after macrophages obtain exosomes from hepatocyte and hepatocellular carcinoma (HCC) cells [61,62]. This process is accompanied by increased expression of CD3+ T-cell-inhibiting receptors. Moreover, the lytic activity and expansion ability of T cells to HCC cells is reduced. HCC-derived exosomes generate immunosuppressive activity by educating macrophages to induce T-cell failure. Previous studies have shown that miRNAs can affect tumor development by altering macrophage polarization [[63], [64], [65], [66], [67]]. Similarly, miRNAs in HCC cell-derived exosomes can be involved in the development of HCC [[68], [69], [70], [71], [72]]. For example, the expression of M2 markers, M2 polarization-related transcription Factors C/EBPβ, and p-STAT3 in miR-146-5p-treated macrophages was elevated. This may be related to exosomes that contain miR-146-5p-mediated immunosuppression. The miR-146-5p promoter can bind to the transcription factor SALL4 (spalt-like transcription Factor 4), which is regulated by STAT3 and is highly expressed in HCC [73]. Silencing SALL4 decreases the number of M2 macrophages in mouse serum and the expression level of miR-146-5p in exosomes. As a result, T-cell inhibitory receptors and tumor volume are reduced. miR-146-5p is delivered to macrophages via exosomes and binds targetively to SALL4 to activate the STAT3 pathway, leading to M2 polarization and T-cell depletion and accelerating HCC progression [73].
Phosphorylation and activation of STAT3 can often be observed in macrophages in the TME, and activating STAT3 signaling could increase the secretion of anti-inflammatory cytokines to promote tumor progression. Blocking STAT3 signaling increases the expression of proinflammatory cytokines and exerts an antitumor effect [74,75]. When the common inflammatory cytokine IL-6 signal is activated, the IL-6 receptor gp130 [76] activates JAK tyrosine kinase and STAT3 [77] to promote tumor growth. IL-6 is also expressed in macrophages [78]. Related studies have shown that breast cancer cell-derived exosomes can reduce T-cell proliferation and NK cytotoxicity, thus facilitating tumor metastasis [79]. Exosome-educated macrophages can improve the expression levels of gp130, phosphorylated STAT3, and IL6 [80]. This is due to the transfer of gp130 to macrophages with exosomes, activating the gp130-STAT3 signal and promoting the secretion of IL-6. In addition, gp130 induces a transition to a pro-tumor type of macrophage and increases their survival, which can be reversed with the use of the gp130 inhibitor SC144 [81]. Breast cancer cell-derived exosome gp130 plays a key role in the tumor microenvironment by activating the IL-6/STAT3 pathway in macrophages to tilt macrophages toward the pro-cancer phenotype. Another study found that in a hypoxic environment, IL-6 and miR-155-3p in glioma (GBM) cells can induce polarization of M2 macrophages by activating STAT3 and CAMP-responsive element-binding protein 3 (CREB3), becoming autophagy initiators [82] and reactivating the STAT3 pathway [83]. Similar to breast cancer, IL-6 and miR-155-3p are both expressed in glioma cell-derived exosomes [84] and can be delivered to macrophages through exosomes to promote the development of gliomas. The use of the STAT3 inhibitor S3I-201 inhibits IL-6 expression, blocking macrophage autophagy, suggesting that IL-6 triggers macrophage autophagy by activating the STAT3 pathway. miR-155-3p-triggered autophagy cannot be blocked by S3I-201 but instead participates in IL-6-induced autophagy by lowering the CREB3 regulator (CREBRF).
The target gene telomere repeats binding Factor 2 interacting protein (TERF2IP) of miR-1246 is involved in the regulation of the NF-κB and STAT3 signaling pathways [[85], [86], [87]]. Activating STAT3 and inhibiting NF-κb can induce polarization of M2 macrophages [88]. However, miR-1246 is expressed in exosomes derived from hypoxic glioma cells (H-GDEs). Hypoxia can affect the content of glioma-derived exosome (GDE) cargo, enhancing the expansion and immunosuppressive functions of myeloid-origin inhibitory cells (MDSCs) [89,90]. miR-1246 is also enriched in cerebrospinal fluid (CSF) in preoperative patients with malignant glioma (GBM) and significantly reduced in postoperative CSF. Elevated expression of miR-1246 in excised GBM tissue is accompanied by increased expression of M2 macrophage markers. H-GDE-treated macrophages induce M2 polarization and promote tumor cell proliferation, invasion, and metastasis. Finally, H-GDE-derived miR-1246 induces M2 polarization to promote the malignant process of GBMs.
miR-19b-3p has carcinogenic effects [91]. LncRNA-LINC00273 can promote cancer cell metastasis [92]. They have a synergistic effect in promoting the progression of lung adenocarcinoma (LUAD) [93]. Exosomes secreted by LUAD cells can induce macrophage M2 polarization to promote LUAD cell invasion and migration. This process is associated with PTPRD, a molecule upstream of the STAT3 pathway associated with M2 polarization. miR-19b-3p can bind to PTPRD (protein tyrosine phosphatase, receptor type D) and be highly expressed in exosomes. Knocking down miR-19b-3p highly increased PTPRD expression and significantly decreased the proportion of M2 macrophages. Polarized macrophages can also secrete exosomes, increase miR-19b-3p levels in LUAD cell exosomes, activate YAP signaling, and promote cell migration and invasion [93]. In addition, STAT3 can regulate the expression of LINC00273 in macrophages. LINC00273 is upregulated in miR-19b-3p-induced macrophage-derived exosomes, but expression levels in LUAD cells are not affected by miR-19b-3p. This indicates that LINC00273 is transferred to LUAD cells mainly through exosomes secreted by macrophages. After being transferred to LUAD cells, LINC00273 induces LATS2 ubiquitination by recruiting the ubiquitin ligase NEDD4, downregulating TEAD4, regulating the transcription of RBMX, activating the Hippo/YAP pathways, and promoting miR-19b-3p packaging into LUAD cell-derived exosomes. Inhibition of miR-19b-3p and LINC00273 can reduce the occurrence of LUAD [93]. Overall, miR-19b-3p in LUAD cell exosomes can induce Linc00273 transcription and M2 macrophage polarization, which in turn will be secreted by exosomes. LINC00273 is delivered to LUAD cells, facilitating the packaging of miR-19b-3p into exosomes secreted by LUAD cells and promoting the progression of LUAD [93].
Leptin can promote tumor progression by regulating the STAT3 pathway [94]. Meanwhile, leptin is highly expressed in exosomes derived from gallbladder carcinoma (GBC) cells and can be internalized by macrophages to activate the STAT3 signaling pathway and induce M2 polarization [95]. M2 macrophages can further enhance the proliferation and migration of GBC cells. Interference with leptin expression or inhibition of STAT3 phosphorylation could reverse these effects.
In addition to M2 polarization, tumor-derived exosomes can also induce macrophage M1 polarization. Protein tyrosine phosphatase receptor type O (PTPRO) inhibits breast cancer progression by dephosphorylation, leading to ERBB2 signal inactivation and endosomal internalization of ERBB2 [96]. Breast cancer cell-derived exosomes loaded with PTPRO significantly increased the proportion of M1 macrophages in the TME and reduced breast cancer cell invasion and migration [97]. PTPRO knocks down cell-derived exosomes and has the opposite effect. In addition, STAT3/STAT6 phosphorylation is inactivated by PTPRO dephosphorylation. In summary, PTPRO in tumor cell-derived exosomes can promote macrophage differentiation into an M1-like phenotype by inactivating STAT signaling to inhibit breast cancer progression.
2.3. NF-κb
NF-κB is a transcription factor involved in inflammation and carcinogenesis and belongs to the Rel family of proteins, including RelA/p65, RelB, c-Rel, p50 and p52, which are regulated and activated by IKKα and IKKβ [98]. NF-κb plays an important role in cancer pathogenesis; for example, the NFκβ1 signaling pathway is associated with colorectal carcinogenesis and with the radiosensitizing effect of ursolic acid, and miR-9/NFKB1 plays an important role in radiotherapy resistance in colorectal cancer [99,100]. Gastric cancer cell exosomes can mediate mesenchymal stem cell activation [101], induce macrophage pro-tumor polarization and enhance the proliferation and migration ability of gastric cancer cells [102]. In this process, the phosphorylation of the classical signaling element NF-κb increased, and the expression of proinflammatory factors was also elevated, indicating that gastric cancer cell-derived exosomes promoted the migration and invasion of gastric cancer cells by activating the NF-κb pathway to induce macrophage polarization [103].
2.4. MAPK
MAPK contains three signaling pathways, ERK, JNK and p38. Among them, the ERK kinase family is divided into ERK1/2, which can be activated by growth factor signaling, and when this pathway is deranged, cell proliferation increases and promotes tumorigenesis [104]. Recently, it has also been shown to be involved in macrophage polarization.
CKLF-like Marvell transmembrane domain 6 (CMTM6), which is overexpressed in a variety of tumors [105], regulates the expression of PD-L1 in tumor cells and inhibits the antitumor immune response [106]. Macrophages block the antitumor immune response [107]. In particular, M2 macrophages positively correlate with a poor prognosis for early OSCC [108]. The ERK1/2 signaling pathway is involved in the regulation of macrophage polarization [109]. Studies have shown that CMTM6 is highly expressed in OSCC tissues and is positively correlated with T staging, pathological grading, and lymph node metastasis [110]. In CMTM6 high-expression OSCC tissue, the expression of CD163 and PD-L1 was increased. This suggests that CMTM6 may be associated with M2 macrophage infiltration, PD-L1 expression, and poor prognosis. Deficiency of CMTM6 in OSCC cell-derived exosomes impairs the proliferation, migration, and invasion of OSCC cells. The level of PD-L1 expression, ERK1/2 phosphorylation, and M2 polarization ability also decreased. These results confirm that OSCC cells secrete CMTM6 in exosomes by activating the ERK1/2 signaling pathway to induce macrophage M2 polarization and participate in tumor malignant progression [110].
miR-770 and its target gene, MAP3K1, are involved in regulating the development of NSCLC [111]. miR-770 is expressed at low levels in exosomes secreted by NSCLC cells. Increasing its expression can induce apoptosis of NSCLC cells, inhibit the polarization of M2 macrophages, and reduce the migration, invasion, and EMT ability of NSCLC cells. Upon adding miR-770 agonists to exosome-treated macrophages, the levels of MAP3K1, p-JNK, and p-ERK1/2 were significantly reduced. Mice with high miR-770 expression had smaller tumor volumes and increased tumor tissue apoptosis. Hence, it can be assumed that miR-770 in exosomes derived from NSCLC inhibits macrophage M2 polarization by modulating MAP3K1 to activate the MAPK signaling pathway, inhibiting NSCLC tumorigenesis [111].
2.5. CXCR
LncRNA TUC339 is abundant in exosomes derived from liver cancer cells and can promote the proliferation of hepatocarcinoma [112]. Knocking down TUC339 can lead to increased macrophage secretion of the proinflammatory cytokines IL-1β and TNF-α, strengthened phagocytosis and decreased viability [113]. TUC339 expression is higher in M2 macrophages than in the M1 type. After repolarizing M2 to M1 macrophages, TUC339 expression decreased. Interfering with TUC339 expression partially inhibits M2 polarization. Finally, microarray analysis showed that lncRNA TUC339-induced M2 polarization is associated with cytokine receptor signaling pathways and CXCR chemokine receptor binding pathways [113].
Based on the above studies, it can be concluded that the currently known pathways for inducing macrophage polarization by tumor cell-derived exosomes include PI3k/AKT, STAT3, NF-κb, MAPK, and CXCR. Activation of these pathways can cause macrophages to exhibit either pro-cancer phenotypes or antitumor phenotypes. The phenotype varies depending on which molecules are encapsulated in exosomes. All signaling pathways are summarized in Table 1 and Fig. 2.
Table 1.
Macrophage polarization related pathways, target genes and exosome contents summarized in this review.
| Author | Year | Tumor | M1/M2 | Exosome cargos | Target genes | Pathways |
|---|---|---|---|---|---|---|
| Su et al. [136] | 2016 | PDAC | M2 | miR-155, miR-125b | NA | NA |
| Wang et al. [30] | 2018 | M2 | miR-301a-3p | PTEN | PI3K/AKT/mTOR | |
| Linton et al. [154] | 2018 | M2 | Arachidonic acid | NA | NA | |
| Li et al. [113] | 2018 | HCC | M2 | LncRNA TUC339 | CXCR | CRSa |
| Yin et al. [60] | 2019 | M2 | miR-146-5p | SALL4 | STAT3 | |
| Wang et al. [145] | 2021 | M2 | circ-0074854 | NA | NA | |
| Wang et al. [143] | 2022 | M2 | LncRNA HMMR-AS1, miR-147a | ARID3A | NA | |
| Liang et al. [114] | 2019 | CRC | M2 | LncRNA RPPH1 | TUBB3 | NA |
| Zhao et al. [31] | 2020 | M2 | miR-934 | PTEN, CXCL13, CXCR5 | PI3K/AKT, NFκB/p65 | |
| Yang et al. [44] | 2021 | M2 | miR-106b | PDCD4 | PI3Kγ/Akt/mTOR | |
| Lu et al. [116] | 2020 | EC | M2 | circ-0048117 | TLR4 | NA |
| Song et al. [38] | 2021 | M2 | miR-21-5p | PTEN | PI3K/AKT/STAT6 | |
| Wu et al. [102] | 2016 | GC | M1 | NA | NA | NFκB |
| Xin et al. [144] | 2021 | M2 | LncRNA HCG18, miR-875-3p | KLF4 | NA | |
| Ham et al. [80] | 2018 | BC | M2 | Gp130 | IL-6 | STAT3 |
| Moradi et al. [141] | 2020 | M1 | miR-130, miR-33 | NA | NA | |
| Moradi et al. [142] | 2021 | M1 | miR-130 | NA | NA | |
| Xun et al. [124] | 2021 | M2 | miR-138-5p | KDM6B | NA | |
| Dong et al. [97] | 2021 | M1 | PTPRO | NA | STAT3/STAT6 | |
| Lu et al. [47] | 2022 | M2 | circ-0001142 | PIK3CB | PI3K/AKT | |
| Ying et al. [53] | 2016 | EOC | M2 | miR-222-3p | SOCS3 | SOCS3/STAT3 |
| Chen et al. [137] | 2017 | M2 | miR-940 | NA | NA | |
| Chen et al. [54] | 2018 | M2 | miR-21-3p, miR-125b-5p, miR-181d-5p | SOCS4/5 | SOCS/STAT3 | |
| Xiao et al. [138] | 2020 | EC | M2 | miR-21 | NA | NA |
| Qian et al. [88] | 2019 | Glioma | M2 | miR-1246 | TERF2IP | NF-κB, STAT3 |
| Xu et al. [84] | 2021 | M2 | miR-155-3p | IL-6, CREB3 | STAT3 | |
| Tong et al. [129] | 2020 | HNSCC | M2 | miR-9 | PPARδ | NA |
| Cai et al. [57] | 2019 | OSCC | M2 | miR-29a-3p | SOCS1 | SOCS1/STAT6 |
| Pang et al. [110] | 2020 | M2 | CMTM6 | NA | ERK1/2 | |
| Ono et al. [146] | 2020 | M2 | CDC37, HSP90α, HSP90β | NA | NA | |
| Chen et al. [46] | 2021 | LC | M2 | circ-FARSA | PTEN | PI3K/AKT |
| Liu et al. [111] | 2021 | M2 | miR-770 | MAP3K1 | MAPK | |
| Chen et al. [93] | 2021 | M2 | miR-19b-3p, LINC00273 | PTPRD, NEDD4, RBMX, TEAD4 | STAT3, Hippo/YAP | |
| Cheng et al. [153] | 2021 | Osteosarcoma | M2 | Tim-3 | NA | NA |
| Lin et al. [41] | 2019 | Bladder cancer | M2 | miR-21 | PTEN | PI3K/AKT/STAT3 |
| Jiang et al. [42] | 2021 | M2 | miR-92b-3p, miR-1231-5p | PTEN | AKT/STAT3/6 | |
| Zhao et al. [95] | 2021 | GBC | M2 | Leptin | NA | STAT3 |
CRS: Cytokine receptor signaling; NA: NONE.
Fig. 2.
Schematic diagram of macrophage polarization induced by tumor cell-derived exosomes.
3. Target genes
3.1. TUBB3 (β-III Tubulin)
LncRNA RPPH1 was obtained by next-generation sequencing from tissues in patients with CRC liver metastases, and its interaction protein TUBB3 can promote CRC progression [114]. RPPH1 expression was positively correlated with CRC TNM staging and metastasis, and the overall survival and disease-free survival of patients with highly expressed RPPH1 were shorter. RPPH1 is abundant in CRC cells, cell-secreted exosomes, and patient plasma-derived exosomes [115]. In addition, RPPH1 can induce M2 polarization of macrophages. In vitro, mice injected with exosome-treated macrophages had larger tumor volumes, more metastatic nodules, and more circulating tumor cells while shortening survival time. Therefore, CRC cell-derived exosomal lncRNA RPPH1 can enhance the migration, proliferation, and EMT of CRC cells. Interfering with TUBB3 expression can reverse the above effects.
3.2. TLR4 (Toll-like receptor 4)
CircRNA-hsa-circ-0048117 is found in exosomes secreted by ESCC cells in hypoxic environments [116]. Circ-0048117 can bind to ARID3A, promoting macrophage maturation and differentiation [117] and accelerating the malignant progression of esophageal cancer [118,119]. Treating macrophages with exosomes containing circ-0048117 induces M2 polarization. As a competitive RNA for circ-0048117, silencing miR-140 can increase the number of M2 macrophages, and inhibition of circ-0048117 counteracts this effect [116]. This process is regulated by the target gene TLR4 of miR-140. Combined with blood samples and clinicopathological data of ESCC patients and healthy populations, the plasma content of circ-0048117 in ESCC patients increased, which was positively correlated with T stage, N stage, and TNM stage. Circ-0048117 in ESCC-derived exosomes under hypoxic conditions acts as a miR-140 sponge, competing with TLR4 to bind miR-140, upregulating TLR4 expression and promoting macrophage M2 polarization [116].
3.3. KDM6B (Lysine demethylase 6B)
Recent studies have suggested that histone-modified enzyme-mediated epigenetic remodeling is associated with phenotypic regulation of tumor-associated macrophages [120]. For instance, the substrate containing histone H3 (H3K27me3/2) of triglycine and diglycine residue 27 of KDM6B may participate in positively regulating the transcription of genes required by macrophages [121]. In addition, LPS and IL4 can induce KDM6B to adjust the activation of macrophages [122,123]. miR-138-5p in breast cancer cell-derived exosomes inhibits the expression of KDM6B and induces M2 polarization in macrophages [124]. Overexpression of KDM6B can specifically stimulate the activity of promoters encoding M1-related genes and reduce the level of H3K27me3. These results revealed that breast cancer cell-derived exosomal miR-138-5p promotes macrophage M2-type polarization by inhibiting KDM6B expression, an effect related to H3K27me3 levels on M1-associated transcriptional promoters.
3.4. PPARδ (Peroxisome proliferation-activated receptor δ)
Unlike HPV-negative (HPV-) head and neck squamous cell carcinoma (HNSCC), HPV-positive (HPV+) HNSCC is more sensitive to radiation therapy [125]. The HPV + HNSCC TME contains more M1 macrophages, and the HPV- HNSCC TME contains more M2 macrophages [126]. In addition, miR-9 is closely related to HPV [127]. Exosomes can deliver miRNAs to immune cells [128]. Interestingly, miR-9 is more expressed in HPV + HNSCC cell-derived exosomes than in HPV-HNSCC [129], and exosome-treated macrophages are predominantly polarized to the M1 phenotype. Correlation analysis between the expression of M1 macrophage-specific markers and HNSCC radiosensitivity showed that M1 macrophages were positively correlated with HNSCC cell radiosensitivity. The miR-9 target gene PPARδ is involved in this process. miR-9 in exosomes secreted by HPV + HNSCC cells promotes macrophage M1 polarization by inhibiting PPARδ, increasing the sensitivity of HPV + HNSCC patients to radiotherapy.
All four genes are involved in inducing macrophage polarization, but it is still unknown which signaling pathways contribute to this effect, and further research is still needed. All target genes are summarized in Table 1.
4. Others
4.1. Non-coding RNAs
4.1.1. MiRNA
miR-155 plays an important role in hematopoiesis, inflammation, tumors, and immunity [130,131]. Knocking down miR-155 induces macrophages to trigger M2/Th2 responses [132]. miRNA-125b expression is upregulated in M1 macrophages, which improves antigen presentation and increases T-cell activity to kill tumors [133] by inhibiting the effect of interferon regulator-4 [134]. miR-155 and miR-125b are specifically delivered into pancreatic cancer cells via hyaluronic acid with polyethyleneimine and polyethylene glycol (HA-PEI/HA-PEG) nanocarriers [135]. The expression of both miRNAs in their secreted exosomes is also upregulated, thus inducing macrophage reprogramming to exert antitumor invasion and metastasis [136]. Moreover, miR-940 [137] and miRNA-21 [138] contained in EOC and EC cell-derived exosomes also induced M2 polarization. In addition, miR-130 and miR-33 have an impact on macrophage M1 polarization [139,140]. They can be transferred from exosomes derived from breast cancer cells to macrophages, enhance the phagocytic ability of macrophages, induce transformation from the M2 type to the M1 type, reduce the migration and invasion of breast cancer cells, and inhibit the development of breast cancer [141,142].
4.1.2. LncRNA
Exosomal lncRNA HMMR-AS1 mediates macrophage polarization by regulating the expression of ARID3A (AT rich interactive domain 3A) through competitive adsorption of miR-147a under the action of hypoxia-inducible factor HIF-1 to promote HCC cell proliferation, EMT and tumor growth [143]. Moreover, the hypoxic environment can increase the secretion of exosomes from HCC cells. Similarly, lncRNA HCG18 from gastric cancer cell-derived exosomes increased the expression of KLF4 (Krüppel-like Factor 4) and promoted M2 polarization by decreasing miR-875-3p in macrophages [144].
4.1.3. circRNA
hsa_circ_00074854 expression is increased in HCC cell lines and their secreted exosomes [145]. Knocking down the expression of circ_00074854 can inhibit the migration, invasion, EMT, and tumor growth of HCC cells by interacting with human antigen R (HuR) and inhibiting the polarization of M2 macrophages. In vitro experiments simultaneously confirmed that tumor growth in mice treated with exosomes at low levels of circ_00074854 was impaired. circ-0074854 in HCC cell-derived exosomes can control macrophage polarization by binding to HuR, affecting liver cancer development.
4.2. Proteins
Highly metastatic OSCC cells secrete extracellular vesicles (EVs) rich in HSP90 (heat shock protein 90) α, HSP90 β, CD326, and CD9. Double knockdown of HSP90 and HSP90β significantly reduces the survival time of highly metastatic OSCC cells [146]. CDC37 (cell division control 37) plays a key role in stabilizing the HSP90 client protein [147] and has been linked to cancer progression [148]. Knockdown of CDC37 reduces the release of proteins in EVs, such as CD9 [149]. EVs secreted by highly metastatic OSCC are rich in HSP90α and HSP90β and synergistically promote cancer cell migration [150]. Triple knockdown of CDC37/HSP90α/β reduces the survival of highly metastatic OSCC cells, and there is a compensatory response in the CDC37/HSP90 system. Double or triple knockdown decreased the levels of the three proteins in metastatic OSCC cells, and triple knockdown more effectively reduced the EMT characteristics of metastatic OSCC cells. In addition, triple knockdown also reduces the release of EV proteins, Gluc-EVs and intracellular protein stabilization [151]. Above all, triple knockdown of CDC37/HSP90α/β can diminish the release of OSCC cell EVs, reduce the EMT initiation activity of metastatic OSCC cell EVs, and inhibit M2 polarization, thereby weakening the migration and invasion function of OSCC cells. Triple knockdown also impairs EV delivery activity, which is necessary for communication between cancer cells and surrounding cells in the TME.
T-cell immunoglobulins and mucin domain-3 (Tim-3) are significantly elevated in osteosarcoma, promoting the occurrence and development of osteosarcoma [152]. Tim-3 expression in human osteosarcoma cell-derived exosomes is also elevated and induces macrophage M2 polarization [153]. M2 macrophages containing Tim-3 significantly promote osteosarcoma cell migration, invasion, and EMT. Knocking down the expression of Tim-3 in macrophages suppressed tumor growth and lung metastasis. Osteosarcoma-derived exosomes induce M2 polarization by regulating the expression of Tim-3, promoting tumor metastasis.
4.3. Lipids
Pancreatic cancer AsPC-1 cells have a high content of phospholipid esterified arachidonic acid (AA) and the highest rate of fusion with macrophages [154]. AA is a lipid that affects the fusion of exosomes with cell membranes [155]. After the removal of AA using recombinant human phospholipase A2, the fusion rate was significantly reduced, indicating that lipids in AsPC-1 cell-derived exosomes can affect exosome-macrophage fusion. After fusion, the expression of the M1 marker and immunomodulatory protein PD-L1 in macrophages did not change significantly, but the expression of the M2 marker was upregulated. Simultaneously, a variety of cytokines, chemokines, and biologically active factors (PGE2, IL-1β, VEGF, MCP-1, MMP-9, TNFα, IL-6) increased, indicating that AsPC-1-derived exosomes induce macrophages to polarize to the pro-tumor M2 type and promote the occurrence and development of pancreatic cancer.
In summary, except for breast cancer cell-derived exosomes, miR-130 and miR-33, which induce M1 polarization to exert antitumor effects, the rest of the exosome cargos induce M2 polarization to promote tumor progression. However, the specific mechanism has not been clarified, and its downstream signaling pathways can be further explored in the future. All exosome cargos are summarized in Table 1.
5. Conclusion
With the progress of continuing research, tumor treatment has gradually developed to individualization and precision in recent years. For instance, nanotechnology has been applied to the diagnosis and treatment of tumors. The use of artificial nanomaterials can more accurately transport drugs to target organs, increase drug concentrations, and reduce toxic side effects on normal tissues. However, as an exogenous material, there are still certain shortcomings, such as loss of targeting ability and rapid clearance after completion of administration. Exosomes secreted by a variety of cells in the human body are similar to an endogenous nanomaterial, which plays the role of interacting between cells, transmitting proteins, DNA, RNA, and lipids to adjacent cells. Moreover, they participate in the process of inflammation regulation, angiogenesis and tumorigenesis. Exosomes secreted by tumor cells can also change the function of surrounding cells by creating a microenvironment conducive to tumor growth and metastasis. Macrophages in the microenvironment, for example, can be polarized into two different phenotypes, antitumor growth M1 type or pro-tumor growth M2 type, by phagocytosis of tumor cells secreting exosomes. If one could determine which component in the exosome affects macrophage polarization, it might be possible to restore or enhance the antitumor function of macrophages by changing the expression of this component. To this end, this review summarizes recent studies in this field and finds that tumor cell secretory exosomes can influence tumor growth, metastasis, and drug resistance by activating multiple classical signaling pathways that induce polarization in macrophages. For example, esophageal squamous cell carcinoma can induce M2 polarization through the PI3k/AKT pathway to promote tumor angiogenesis [156], renal clear cell carcinoma can promote M2 polarization through STAT3 to enhance tumor proliferation [157], NF-κ b pathway activation induces M2 polarization to promote pancreatic cancer progression [158], M2 polarization accelerates gastric cancer liver metastasis by inducing angiogenesis and promoting intrahepatic metastatic microhabitat formation associated with ERK pathway activation [159], and MAPK pathway activation of M2 polarization can lead to temozolomide resistance in glioblastoma [160]. Currently, drugs that block activation of such pathways for antitumor purposes have been extensively studied, such as PI3K inhibitors in combination with endocrine therapy to prolong progression-free survival in hormone receptor-positive patients with advanced breast cancer [161]; mTOR inhibitors to improve the prognosis of hepatocellular carcinoma treated with transplantation, especially in patients with high postoperative AFP [162]; STAT3 inhibitors to improve overall survival in pSTAT3-positive patients with progressive colorectal cancer [163]; selective blockade of upstream and downstream targets of the NF-κB pathway, such as TNF-α, to stabilize renal cancer progression [164]; and pediatric low-grade gliomas and plexiform neurofibromas to benefit from MAPK/ERK targeted therapy [165]. There have been recent studies on targeted therapies targeting molecules carried by exosomes. For example, through engineered exosomes loaded with STAT6 antisense oligonucleotides, STAT6 was selectively silenced in M2 macrophages to reprogram them to M1, reshape the tumor microenvironment to an antitumor state, and significantly inhibit tumor growth in colorectal and hepatocellular carcinoma models [166]. This method uses exosomes to deliver targeted drugs to macrophages, altering their polarization without depleting the entire macrophage population. Taking this as an inspiration, future targeted therapies could be aimed at regulating the expression of upstream molecules related to the polarization pathway carried by exosomes, but there are still many questions to be explored. Examples include exploring more signaling pathways by which tumor cell-derived exosomes regulate macrophage polarization, extracting tumor cell-derived exosomes more precisely without the influence of secreted exosomes from other cells, determining whether tumor-derived exosomes can enter the blood circulation and affect distant organs, and determining whether existing antitumor drugs can exert antineoplasm effects by changing the way exosomes affect the polarization of macrophages. In summary, this paper aims to provide new ideas for tumor therapy by summarizing the mechanism of tumor-derived exosome-induced macrophage polarization.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by National Natural Science Foundation Of China {81572408}, Natural Science Foundation of Jiangsu Province {BE2015712} and The Program of Medical Innovation Team and Leading Medical Talents in Jiangsu Province {2017ZXKJQW09}.
Data availability statement
This study is a review article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhao X., Ren Y., Lu Z. Potential diagnostic and therapeutic roles of exosomes in pancreatic cancer. Biochim. Biophys. Acta Rev. Canc. 2020;1874(2) doi: 10.1016/j.bbcan.2020.188414. [DOI] [PubMed] [Google Scholar]
- 2.Wang W., Lotze M.T. Good things come in small packages: exosomes, immunity and cancer. Cancer Gene Ther. 2014;21(4):139–141. doi: 10.1038/cgt.2014.14. [DOI] [PubMed] [Google Scholar]
- 3.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowal J., Tkach M., Théry C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Qu L., Ding J., Chen C., Wu Z.J., Liu B., Gao Y., et al. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29(5):653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Xie L., Chen J., Ren X., Zhang M., Thuaksuban N., Nuntanaranont T., et al. Alteration of circRNA and lncRNA expression profile in exosomes derived from periodontal ligament stem cells undergoing osteogenic differentiation. Arch. Oral Biol. 2021;121 doi: 10.1016/j.archoralbio.2020.104984. [DOI] [PubMed] [Google Scholar]
- 7.Bhan A., Soleimani M., Mandal S.S. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):3965–3981. doi: 10.1158/0008-5472.Can-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin X., Wu Z., Hu H., Luo M.L., Song E. Non-coding RNAs rewire cancer metabolism networks. Semin. Cancer Biol. 2021;75:116–126. doi: 10.1016/j.semcancer.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Li M., Guo H., Wang Q., Chen K., Marko K., Tian X., et al. Pancreatic stellate cells derived exosomal miR-5703 promotes pancreatic cancer by downregulating CMTM4 and activating PI3K/Akt pathway. Cancer Lett. 2020;490:20–30. doi: 10.1016/j.canlet.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Han L., Xu J., Xu Q., Zhang B., Lam E.W., Sun Y. Extracellular vesicles in the tumor microenvironment: therapeutic resistance, clinical biomarkers, and targeting strategies. Med. Res. Rev. 2017;37(6):1318–1349. doi: 10.1002/med.21453. [DOI] [PubMed] [Google Scholar]
- 11.Becker A., Thakur B.K., Weiss J.M., Kim H.S., Peinado H., Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao J., Zhang M., Xie F., Lou J., Zhou X., Zhang L., et al. Exosomes in head and neck cancer: roles, mechanisms and applications. Cancer Lett. 2020;494:7–16. doi: 10.1016/j.canlet.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Wortzel I., Dror S., Kenific C.M., Lyden D. Exosome-mediated metastasis: communication from a distance. Dev. Cell. 2019;49(3):347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Wahlgren J., De L.K.T., Brisslert M., Vaziri Sani F., Telemo E., Sunnerhagen P., et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40(17) doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King H.W., Michael M.Z., Gleadle J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarrilli G., Businello G., Dieci M.V., Paccagnella S., Carraro V., Cappellesso R., et al. The tumor microenvironment of primitive and metastatic breast cancer: implications for novel therapeutic strategies. Int. J. Mol. Sci. 2020;21(21) doi: 10.3390/ijms21218102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122(3):787–795. doi: 10.1172/jci59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantovani A., Biswas S.K., Galdiero M.R., Sica A., Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013;229(2):176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 19.Boutilier A.J., Elsawa S.F. Macrophage polarization states in the tumor microenvironment. Int. J. Mol. Sci. 2021;22(13) doi: 10.3390/ijms22136995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rihawi K., Ricci A.D., Rizzo A., Brocchi S., Marasco G., Pastore L.V., et al. Tumor-associated macrophages and inflammatory microenvironment in gastric cancer: novel translational implications. Int. J. Mol. Sci. 2021;22(8) doi: 10.3390/ijms22083805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carloni R., Rizzo A., Ricci A.D., Federico A.D., De Luca R., Guven D.C., et al. Targeting tumor microenvironment for cholangiocarcinoma: opportunities for precision medicine. Trans. Oncol. 2022;25 doi: 10.1016/j.tranon.2022.101514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Federico A., Rizzo A., Carloni R., De Giglio A., Bruno R., Ricci D., et al. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expet Opin. Invest. Drugs. 2022;31(4):361–369. doi: 10.1080/13543784.2022.2009455. [DOI] [PubMed] [Google Scholar]
- 23.Rizzo A., Cusmai A., Gadaleta-Caldarola G., Palmiotti G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expet Rev. Gastroenterol. Hepatol. 2022;16(4):333–339. doi: 10.1080/17474124.2022.2064273. [DOI] [PubMed] [Google Scholar]
- 24.Sceneay J., Smyth M.J., Möller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013;32(3–4):449–464. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 25.Piao Y.J., Kim H.S., Hwang E.H., Woo J., Zhang M., Moon W.K. Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis. Oncotarget. 2018;9(7):7398–7410. doi: 10.18632/oncotarget.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pritchard A., Tousif S., Wang Y., Hough K., Khan S., Strenkowski J., et al. Lung tumor cell-derived exosomes promote M2 macrophage polarization. Cells. 2020;9(5) doi: 10.3390/cells9051303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ediriweera M.K., Tennekoon K.H., Samarakoon S.R. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: biological and therapeutic significance. Semin. Cancer Biol. 2019;59:147–160. doi: 10.1016/j.semcancer.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Pfeifer M., Grau M., Lenze D., Wenzel S.S., Wolf A., Wollert-Wulf B., et al. PTEN loss defines a PI3K/AKT pathway-dependent germinal center subtype of diffuse large B-cell lymphoma. Proc. Natl. Acad. Sci. U.S.A. 2013;110(30):12420–12425. doi: 10.1073/pnas.1305656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu J., Xie L., Liu C., Zhang Q., Sun S. PTEN/PI3k/AKT regulates macrophage polarization in emphysematous mice. Scand. J. Immunol. 2017;85(6):395–405. doi: 10.1111/sji.12545. [DOI] [PubMed] [Google Scholar]
- 30.Wang X., Luo G., Zhang K., Cao J., Huang C., Jiang T., et al. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer metastasis. Cancer Res. 2018;78(16):4586–4598. doi: 10.1158/0008-5472.Can-17-3841. [DOI] [PubMed] [Google Scholar]
- 31.Zhao S., Mi Y., Guan B., Zheng B., Wei P., Gu Y., et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 2020;13(1):156. doi: 10.1186/s13045-020-00991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villarroya-Beltri C., Gutiérrez-Vázquez C., Sánchez-Cabo F., Pérez-Hernández D., Vázquez J., Martin-Cofreces N., et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cantley L.C., Neel B.G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. U.S.A. 1999;96(8):4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kazanietz M.G., Durando M., Cooke M. CXCL13 and its receptor CXCR5 in cancer: inflammation, immune response, and beyond. Front. Endocrinol. 2019;10:471. doi: 10.3389/fendo.2019.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tacke R.S., Tosello-Trampont A., Nguyen V., Mullins D.W., Hahn Y.S. Extracellular hepatitis C virus core protein activates STAT3 in human monocytes/macrophages/dendritic cells via an IL-6 autocrine pathway. J. Biol. Chem. 2011;286(12):10847–10855. doi: 10.1074/jbc.M110.217653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sica A., Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 2007;117(5):1155–1166. doi: 10.1172/jci31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng Y., Tian Y., Xia J., Wu X., Yang Y., Li X., et al. The role of PTEN in regulation of hepatic macrophages activation and function in progression and reversal of liver fibrosis. Toxicol. Appl. Pharmacol. 2017;317:51–62. doi: 10.1016/j.taap.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Song J., Yang P., Li X., Zhu X., Liu M., Duan X., et al. Esophageal cancer-derived extracellular vesicle miR-21-5p contributes to EMT of ESCC cells by disorganizing macrophage polarization. Cancers. 2021;13(16) doi: 10.3390/cancers13164122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao J., Liu R., Yin L., Pu Y. Expression profiling of exosomal miRNAs derived from human esophageal cancer cells by Solexa high-throughput sequencing. Int. J. Mol. Sci. 2014;15(9):15530–15551. doi: 10.3390/ijms150915530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohno R., Uozaki H., Kikuchi Y., Kumagai A., Aso T., Watanabe M., et al. Both cancerous miR-21 and stromal miR-21 in urothelial carcinoma are related to tumour progression. Histopathology. 2016;69(6):993–999. doi: 10.1111/his.13032. [DOI] [PubMed] [Google Scholar]
- 41.Lin F., Yin H.B., Li X.Y., Zhu G.M., He W.Y., Gou X. Bladder cancer cellsecreted exosomal miR21 activates the PI3K/AKT pathway in macrophages to promote cancer progression. Int. J. Oncol. 2020;56(1):151–164. doi: 10.3892/ijo.2019.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Z., Zhang Y., Zhang Y., Jia Z., Zhang Z., Yang J. Cancer derived exosomes induce macrophages immunosuppressive polarization to promote bladder cancer progression. Cell Commun. Signal. 2021;19(1):93. doi: 10.1186/s12964-021-00768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhen Y., Fang W., Zhao M., Luo R., Liu Y., Fu Q., et al. miR-374a-CCND1-pPI3K/AKT-c-JUN feedback loop modulated by PDCD4 suppresses cell growth, metastasis, and sensitizes nasopharyngeal carcinoma to cisplatin. Oncogene. 2017;36(2):275–285. doi: 10.1038/onc.2016.201. [DOI] [PubMed] [Google Scholar]
- 44.Yang C., Dou R., Wei C., Liu K., Shi D., Zhang C., et al. Tumor-derived exosomal microRNA-106b-5p activates EMT-cancer cell and M2-subtype TAM interaction to facilitate CRC metastasis. Mol. Ther. 2021;29(6):2088–2107. doi: 10.1016/j.ymthe.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei C., Yang C., Wang S., Shi D., Zhang C., Lin X., et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer. 2019;18(1):64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen T., Liu Y., Li C., Xu C., Ding C., Chen J., et al. Tumor-derived exosomal circFARSA mediates M2 macrophage polarization via the PTEN/PI3K/AKT pathway to promote non-small cell lung cancer metastasis. Canc. Treat Res. Commun. 2021;28 doi: 10.1016/j.ctarc.2021.100412. [DOI] [PubMed] [Google Scholar]
- 47.Lu C., Shi W., Hu W., Zhao Y., Zhao X., Dong F., et al. Endoplasmic reticulum stress promotes breast cancer cells to release exosomes circ_0001142 and induces M2 polarization of macrophages to regulate tumor progression. Pharmacol. Res. 2022;177 doi: 10.1016/j.phrs.2022.106098. [DOI] [PubMed] [Google Scholar]
- 48.Yao X., Tu Y., Xu Y., Guo Y., Yao F., Zhang X. Endoplasmic reticulum stress-induced exosomal miR-27a-3p promotes immune escape in breast cancer via regulating PD-L1 expression in macrophages. J. Cell Mol. Med. 2020;24(17):9560–9573. doi: 10.1111/jcmm.15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou S., Tong Q., Liu B., Huang W., Tian Y., Fu X. Targeting STAT3 in cancer immunotherapy. Mol. Cancer. 2020;19(1):145. doi: 10.1186/s12943-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gironella M., Calvo C., Fernández A., Closa D., Iovanna J.L., Rosello-Catafau J., et al. Reg3β deficiency impairs pancreatic tumor growth by skewing macrophage polarization. Cancer Res. 2013;73(18):5682–5694. doi: 10.1158/0008-5472.Can-12-3057. [DOI] [PubMed] [Google Scholar]
- 51.Yuan F., Fu X., Shi H., Chen G., Dong P., Zhang W. Induction of murine macrophage M2 polarization by cigarette smoke extract via the JAK2/STAT3 pathway. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dimitriou I.D., Clemenza L., Scotter A.J., Chen G., Guerra F.M., Rottapel R. Putting out the fire: coordinated suppression of the innate and adaptive immune systems by SOCS1 and SOCS3 proteins. Immunol. Rev. 2008;224:265–283. doi: 10.1111/j.1600-065X.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- 53.Ying X., Wu Q., Wu X., Zhu Q., Wang X., Jiang L., et al. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget. 2016;7(28):43076–43087. doi: 10.18632/oncotarget.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X., Zhou J., Li X., Wang X., Lin Y., Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018;435:80–91. doi: 10.1016/j.canlet.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Manikandan M., Deva Magendhra Rao A.K., Arunkumar G., Manickavasagam M., Rajkumar K.S., Rajaraman R., et al. Oral squamous cell carcinoma: microRNA expression profiling and integrative analyses for elucidation of tumourigenesis mechanism. Mol. Cancer. 2016;15:28. doi: 10.1186/s12943-016-0512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hulsmans M., Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc. Res. 2013;100(1):7–18. doi: 10.1093/cvr/cvt161. [DOI] [PubMed] [Google Scholar]
- 57.Cai J., Qiao B., Gao N., Lin N., He W. Oral squamous cell carcinoma-derived exosomes promote M2 subtype macrophage polarization mediated by exosome-enclosed miR-29a-3p. Am. J. Physiol. Cell Physiol. 2019;316(5):C731–C740. doi: 10.1152/ajpcell.00366.2018. [DOI] [PubMed] [Google Scholar]
- 58.Tariq M., Zhang J.Q., Liang G.K., He Q.J., Ding L., Yang B. Gefitinib inhibits M2-like polarization of tumor-associated macrophages in Lewis lung cancer by targeting the STAT6 signaling pathway. Acta Pharmacol. Sin. 2017;38(11):1501–1511. doi: 10.1038/aps.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ritz O., Guiter C., Dorsch K., Dusanter-Fourt I., Wegener S., Jouault H., et al. STAT6 activity is regulated by SOCS-1 and modulates BCL-XL expression in primary mediastinal B-cell lymphoma. Leukemia. 2008;22(11):2106–2110. doi: 10.1038/leu.2008.85. [DOI] [PubMed] [Google Scholar]
- 60.Yin C., Han Q., Xu D., Zheng B., Zhao X., Zhang J. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. OncoImmunology. 2019;8(7) doi: 10.1080/2162402X.2019.1601479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solís-Martínez R., Cancino-Marentes M., Hernández-Flores G., Ortiz-Lazareno P., Mandujano-Álvarez G., Cruz-Gálvez C., et al. Regulation of immunophenotype modulation of monocytes-macrophages from M1 into M2 by prostate cancer cell-culture supernatant via transcription factor STAT3. Immunol. Lett. 2018;196:140–148. doi: 10.1016/j.imlet.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 62.Kwon Y.C., Meyer K., Peng G., Chatterjee S., Hoft D.F., Ray R. Hepatitis C virus E2 envelope glycoprotein induces an immunoregulatory phenotype in macrophages. Hepatology. 2019;69(5):1873–1884. doi: 10.1002/hep.29843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.An Y., Yang Q. MiR-21 modulates the polarization of macrophages and increases the effects of M2 macrophages on promoting the chemoresistance of ovarian cancer. Life Sci. 2020;242 doi: 10.1016/j.lfs.2019.117162. [DOI] [PubMed] [Google Scholar]
- 64.Xi Q., Zhang J., Yang G., Zhang L., Chen Y., Wang C., et al. Restoration of miR-340 controls pancreatic cancer cell CD47 expression to promote macrophage phagocytosis and enhance antitumor immunity. J. Immunother. Canc. 2020;8(1) doi: 10.1136/jitc-2019-000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L., Hu Y.Y., Zhao J.L., Huang F., Liang S.Q., Dong L., et al. Targeted delivery of miR-99b reprograms tumor-associated macrophage phenotype leading to tumor regression. J. Immunother. Canc. 2020;8(2) doi: 10.1136/jitc-2019-000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sánchez-González I., Bobien A., Molnar C., Schmid S., Strotbek M., Boerries M., et al. miR-149 suppresses breast cancer metastasis by blocking paracrine interactions with macrophages. Cancer Res. 2020;80(6):1330–1341. doi: 10.1158/0008-5472.Can-19-1934. [DOI] [PubMed] [Google Scholar]
- 67.Weng Y.S., Tseng H.Y., Chen Y.A., Shen P.C., Al Haq A.T., Chen L.M., et al. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol. Cancer. 2019;18(1):42. doi: 10.1186/s12943-019-0988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun X., Zhang J., Hou Z., Han Q., Zhang C., Tian Z. miR-146a is directly regulated by STAT3 in human hepatocellular carcinoma cells and involved in anti-tumor immune suppression. Cell Cycle. 2015;14(2):243–252. doi: 10.4161/15384101.2014.977112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang B., Majumder S., Nuovo G., Kutay H., Volinia S., Patel T., et al. Role of microRNA-155 at early stages of hepatocarcinogenesis induced by choline-deficient and amino acid-defined diet in C57BL/6 mice. Hepatology. 2009;50(4):1152–1161. doi: 10.1002/hep.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shimizu S., Takehara T., Hikita H., Kodama T., Miyagi T., Hosui A., et al. The let-7 family of microRNAs inhibits Bcl-xL expression and potentiates sorafenib-induced apoptosis in human hepatocellular carcinoma. J. Hepatol. 2010;52(5):698–704. doi: 10.1016/j.jhep.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 71.Zhu K., Pan Q., Zhang X., Kong L.Q., Fan J., Dai Z., et al. MiR-146a enhances angiogenic activity of endothelial cells in hepatocellular carcinoma by promoting PDGFRA expression. Carcinogenesis. 2013;34(9):2071–2079. doi: 10.1093/carcin/bgt160. [DOI] [PubMed] [Google Scholar]
- 72.Kota J., Chivukula R.R., O'Donnell K.A., Wentzel E.A., Montgomery C.L., Hwang H.W., et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun C., Lan P., Han Q., Huang M., Zhang Z., Xu G., et al. Oncofetal gene SALL4 reactivation by hepatitis B virus counteracts miR-200c in PD-L1-induced T cell exhaustion. Nat. Commun. 2018;9(1):1241. doi: 10.1038/s41467-018-03584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang T., Niu G., Kortylewski M., Burdelya L., Shain K., Zhang S., et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat. Med. 2004;10(1):48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 75.Yu H., Kortylewski M., Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007;7(1):41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 76.Skiniotis G., Boulanger M.J., Garcia K.C., Walz T. Signaling conformations of the tall cytokine receptor gp130 when in complex with IL-6 and IL-6 receptor. Nat. Struct. Mol. Biol. 2005;12(6):545–551. doi: 10.1038/nsmb941. [DOI] [PubMed] [Google Scholar]
- 77.Heinrich P.C., Behrmann I., Haan S., Hermanns H.M., Müller-Newen G., Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003;374(Pt 1):1–20. doi: 10.1042/bj20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roca H., Varsos Z.S., Sud S., Craig M.J., Ying C., Pienta K.J. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J. Biol. Chem. 2009;284(49):34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wen S.W., Sceneay J., Lima L.G., Wong C.S., Becker M., Krumeich S., et al. The biodistribution and immune suppressive effects of breast cancer-derived exosomes. Cancer Res. 2016;76(23):6816–6827. doi: 10.1158/0008-5472.Can-16-0868. [DOI] [PubMed] [Google Scholar]
- 80.Ham S., Lima L.G., Chai E.P.Z., Muller A., Lobb R.J., Krumeich S., et al. Breast cancer-derived exosomes alter macrophage polarization via gp130/STAT3 signaling. Front. Immunol. 2018;9:871. doi: 10.3389/fimmu.2018.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu S., Neamati N. gp130: a promising drug target for cancer therapy. Expert Opin. Ther. Targets. 2013;17(11):1303–1328. doi: 10.1517/14728222.2013.830105. [DOI] [PubMed] [Google Scholar]
- 82.Xue H., Yuan G., Guo X., Liu Q., Zhang J., Gao X., et al. A novel tumor-promoting mechanism of IL6 and the therapeutic efficacy of tocilizumab: hypoxia-induced IL6 is a potent autophagy initiator in glioblastoma via the p-STAT3-MIR155-3p-CREBRF pathway. Autophagy. 2016;12(7):1129–1152. doi: 10.1080/15548627.2016.1178446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu K., Zhao E., Ilyas G., Lalazar G., Lin Y., Haseeb M., et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy. 2015;11(2):271–284. doi: 10.1080/15548627.2015.1009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu J., Zhang J., Zhang Z., Gao Z., Qi Y., Qiu W., et al. Hypoxic glioma-derived exosomes promote M2-like macrophage polarization by enhancing autophagy induction. Cell Death Dis. 2021;12(4):373. doi: 10.1038/s41419-021-03664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cai Y., Sukhova G.K., Wong H.K., Xu A., Tergaonkar V., Vanhoutte P.M., et al. Rap1 induces cytokine production in pro-inflammatory macrophages through NFκB signaling and is highly expressed in human atherosclerotic lesions. Cell Cycle. 2015;14(22):3580–3592. doi: 10.1080/15384101.2015.1100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qi L., Yu H., Zhang Y., Zhao D., Lv P., Zhong Y., et al. IL-10 secreted by M2 macrophage promoted tumorigenesis through interaction with JAK2 in glioma. Oncotarget. 2016;7(44):71673–71685. doi: 10.18632/oncotarget.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Beule N., De Veirman K., Maes K., De Bruyne E., Menu E., Breckpot K., et al. Tumour-associated macrophage-mediated survival of myeloma cells through STAT3 activation. J. Pathol. 2017;241(4):534–546. doi: 10.1002/path.4860. [DOI] [PubMed] [Google Scholar]
- 88.Qian M., Wang S., Guo X., Wang J., Zhang Z., Qiu W., et al. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-kappaB pathways. Oncogene. 2020;39(2):428–442. doi: 10.1038/s41388-019-0996-y. [DOI] [PubMed] [Google Scholar]
- 89.Guo X., Qiu W., Liu Q., Qian M., Wang S., Zhang Z., et al. Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways. Oncogene. 2018;37(31):4239–4259. doi: 10.1038/s41388-018-0261-9. [DOI] [PubMed] [Google Scholar]
- 90.Guo X., Qiu W., Wang J., Liu Q., Qian M., Wang S., et al. Glioma exosomes mediate the expansion and function of myeloid-derived suppressor cells through microRNA-29a/Hbp1 and microRNA-92a/Prkar1a pathways. Int. J. Cancer. 2019;144(12):3111–3126. doi: 10.1002/ijc.32052. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y., Li H., Lu H., Qin Y. Circular RNA SMARCA5 inhibits the proliferation, migration, and invasion of non-small cell lung cancer by miR-19b-3p/HOXA9 axis. OncoTargets Ther. 2019;12:7055–7065. doi: 10.2147/ott.S216320. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Sarkar A., Rahaman A., Biswas I., Mukherjee G., Chatterjee S., Bhattacharjee S., et al. TGFβ mediated LINC00273 upregulation sponges mir200a-3p and promotes invasion and metastasis by activating ZEB1. J. Cell. Physiol. 2020;235(10):7159–7172. doi: 10.1002/jcp.29614. [DOI] [PubMed] [Google Scholar]
- 93.Chen J., Zhang K., Zhi Y., Wu Y., Chen B., Bai J., et al. Tumor-derived exosomal miR-19b-3p facilitates M2 macrophage polarization and exosomal LINC00273 secretion to promote lung adenocarcinoma metastasis via Hippo pathway. Clin. Transl. Med. 2021;11(9):e478. doi: 10.1002/ctm2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gorrab A., Pagano A., Ayed K., Chebil M., Derouiche A., Kovacic H., et al. Leptin promotes prostate cancer proliferation and migration by stimulating STAT3 pathway. Nutr. Cancer. 2021;73(7):1217–1227. doi: 10.1080/01635581.2020.1792946. [DOI] [PubMed] [Google Scholar]
- 95.Zhao S., Liu Y., He L., Li Y., Lin K., Kang Q., et al. Gallbladder cancer cell-derived exosome-mediated transfer of leptin promotes cell invasion and migration by modulating STAT3-mediated M2 macrophage polarization. Anal. Cell Pathol. 2022;2022 doi: 10.1155/2022/9994906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dong H., Ma L., Gan J., Lin W., Chen C., Yao Z., et al. PTPRO represses ERBB2-driven breast oncogenesis by dephosphorylation and endosomal internalization of ERBB2. Oncogene. 2017;36(3):410–422. doi: 10.1038/onc.2016.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dong H., Xie C., Jiang Y., Li K., Lin Y., Pang X., et al. Tumor-derived exosomal protein tyrosine phosphatase receptor type O polarizes macrophage to suppress breast tumor cell invasion and migration. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.703537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barnabei L., Laplantine E., Mbongo W., Rieux-Laucat F., Weil R. NF-κB: at the borders of autoimmunity and inflammation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.716469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Afshar S., Sedighi Pashaki A., Najafi R., Nikzad S., Amini R., Shabab N., et al. Cross-resistance of acquired radioresistant colorectal cancer cell line to gefitinib and regorafenib. Iran. J. Med. Sci. 2020;45(1):50–58. doi: 10.30476/ijms.2019.44972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hasan Abdali M., Afshar S., Sedighi Pashaki A., Dastan D., Gholami M.H., Mahmoudi R., et al. Investigating the effect of radiosensitizer for ursolic acid and kamolonol acetate on HCT-116 cell line. Bioorg. Med. Chem. 2020;28(1) doi: 10.1016/j.bmc.2019.115152. [DOI] [PubMed] [Google Scholar]
- 101.Gu J., Qian H., Shen L., Zhang X., Zhu W., Huang L., et al. Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-β/Smad pathway. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu L., Zhang X., Zhang B., Shi H., Yuan X., Sun Y., et al. Exosomes derived from gastric cancer cells activate NF-kappaB pathway in macrophages to promote cancer progression. Tum. Biol. 2016;37(9):12169–12180. doi: 10.1007/s13277-016-5071-5. [DOI] [PubMed] [Google Scholar]
- 103.Baig M.S., Zaichick S.V., Mao M., de Abreu A.L., Bakhshi F.R., Hart P.C., et al. NOS1-derived nitric oxide promotes NF-κB transcriptional activity through inhibition of suppressor of cytokine signaling-1. J. Exp. Med. 2015;212(10):1725–1738. doi: 10.1084/jem.20140654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moon H., Ro S.W. MAPK/ERK signaling pathway in hepatocellular carcinoma. Cancers. 2021;13(12) doi: 10.3390/cancers13123026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mezzadra R., Sun C., Jae L.T., Gomez-Eerland R., de Vries E., Wu W., et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature. 2017;549(7670):106–110. doi: 10.1038/nature23669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burr M.L., Sparbier C.E., Chan Y.C., Williamson J.C., Woods K., Beavis P.A., et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature. 2017;549(7670):101–105. doi: 10.1038/nature23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singh Y., Pawar V.K., Meher J.G., Raval K., Kumar A., Shrivastava R., et al. Targeting tumor associated macrophages (TAMs) via nanocarriers. J. Contr. Release. 2017;254:92–106. doi: 10.1016/j.jconrel.2017.03.395. [DOI] [PubMed] [Google Scholar]
- 108.Weber M., Iliopoulos C., Moebius P., Büttner-Herold M., Amann K., Ries J., et al. Prognostic significance of macrophage polarization in early stage oral squamous cell carcinomas. Oral Oncol. 2016;52:75–84. doi: 10.1016/j.oraloncology.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 109.Mu X., Shi W., Xu Y., Xu C., Zhao T., Geng B., et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle. 2018;17(4):428–438. doi: 10.1080/15384101.2018.1444305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pang X., Wang S.S., Zhang M., Jiang J., Fan H.Y., Wu J.S., et al. OSCC cell-secreted exosomal CMTM6 induced M2-like macrophages polarization via ERK1/2 signaling pathway. Cancer Immunol. Immunother. 2021;70(4):1015–1029. doi: 10.1007/s00262-020-02741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu J., Luo R., Wang J., Luan X., Wu D., Chen H., et al. Tumor cell-derived exosomal miR-770 inhibits M2 macrophage polarization via targeting MAP3K1 to inhibit the invasion of non-small cell lung cancer cells. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.679658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kogure T., Yan I.K., Lin W.L., Patel T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Gen. Canc. 2013;4(7–8):261–272. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li X., Lei Y., Wu M., Li N. Regulation of macrophage activation and polarization by HCC-derived exosomal lncRNA TUC339. Int. J. Mol. Sci. 2018;19(10) doi: 10.3390/ijms19102958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liang Z.X., Liu H.S., Wang F.W., Xiong L., Zhou C., Hu T., et al. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Dis. 2019;10(11):829. doi: 10.1038/s41419-019-2077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li S., Li Y., Chen B., Zhao J., Yu S., Tang Y., et al. exoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018;46(D1):D106–d112. doi: 10.1093/nar/gkx891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu Q., Wang X., Zhu J., Fei X., Chen H., Li C. Hypoxic tumor-derived exosomal Circ0048117 facilitates M2 macrophage polarization acting as miR-140 sponge in esophageal squamous cell carcinoma. OncoTargets Ther. 2020;13:11883–11897. doi: 10.2147/OTT.S284192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ratliff M.L., Garton J., Garman L., Barron M.D., Georgescu C., White K.A., et al. ARID3a gene profiles are strongly associated with human interferon alpha production. J. Autoimmun. 2019;96:158–167. doi: 10.1016/j.jaut.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Y., Xu Y., Li Z., Zhu Y., Wen S., Wang M., et al. Identification of the key transcription factors in esophageal squamous cell carcinoma. J. Thorac. Dis. 2018;10(1):148–161. doi: 10.21037/jtd.2017.12.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma J., Zhan Y., Xu Z., Li Y., Luo A., Ding F., et al. ZEB1 induced miR-99b/let-7e/miR-125a cluster promotes invasion and metastasis in esophageal squamous cell carcinoma. Cancer Lett. 2017;398:37–45. doi: 10.1016/j.canlet.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 120.Larionova I., Kazakova E., Patysheva M., Kzhyshkowska J. Transcriptional, epigenetic and metabolic programming of tumor-associated macrophages. Cancers. 2020;12(6) doi: 10.3390/cancers12061411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ishii M., Wen H., Corsa C.A., Liu T., Coelho A.L., Allen R.M., et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114(15):3244–3254. doi: 10.1182/blood-2009-04-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Santa F., Narang V., Yap Z.H., Tusi B.K., Burgold T., Austenaa L., et al. Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J. 2009;28(21):3341–3352. doi: 10.1038/emboj.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yıldırım-Buharalıoğlu G., Bond M., Sala-Newby G.B., Hindmarch C.C., Newby A.C. Regulation of epigenetic modifiers, including KDM6B, by interferon-γ and interleukin-4 in human macrophages. Front. Immunol. 2017;8:92. doi: 10.3389/fimmu.2017.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xun J., Du L., Gao R., Shen L., Wang D., Kang L., et al. Cancer-derived exosomal miR-138-5p modulates polarization of tumor-associated macrophages through inhibition of KDM6B. Theranostics. 2021;11(14):6847–6859. doi: 10.7150/thno.51864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mirghani H., Amen F., Tao Y., Deutsch E., Levy A. Increased radiosensitivity of HPV-positive head and neck cancers: molecular basis and therapeutic perspectives. Cancer Treat Rev. 2015;41(10):844–852. doi: 10.1016/j.ctrv.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 126.Chen X., Yan B., Lou H., Shen Z., Tong F., Zhai A., et al. Immunological network analysis in HPV associated head and neck squamous cancer and implications for disease prognosis. Mol. Immunol. 2018;96:28–36. doi: 10.1016/j.molimm.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 127.Arora H., Qureshi R., Jin S., Park A.K., Park W.Y. miR-9 and let-7g enhance the sensitivity to ionizing radiation by suppression of NFκB1. Exp. Mol. Med. 2011;43(5):298–304. doi: 10.3858/emm.2011.43.5.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Record M., Carayon K., Poirot M., Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta. 2014;1841(1):108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 129.Tong F., Mao X., Zhang S., Xie H., Yan B., Wang B., et al. HPV + HNSCC-derived exosomal miR-9 induces macrophage M1 polarization and increases tumor radiosensitivity. Cancer Lett. 2020;478:34–44. doi: 10.1016/j.canlet.2020.02.037. [DOI] [PubMed] [Google Scholar]
- 130.Strachan D.C., Ruffell B., Oei Y., Bissell M.J., Coussens L.M., Pryer N., et al. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8(+) T cells. OncoImmunology. 2013;2(12) doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Faraoni I., Antonetti F.R., Cardone J., Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochim. Biophys. Acta. 2009;1792(6):497–505. doi: 10.1016/j.bbadis.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 132.Zonari E., Pucci F., Saini M., Mazzieri R., Politi L.S., Gentner B., et al. A role for miR-155 in enabling tumor-infiltrating innate immune cells to mount effective antitumor responses in mice. Blood. 2013;122(2):243–252. doi: 10.1182/blood-2012-08-449306. [DOI] [PubMed] [Google Scholar]
- 133.Graff J.W., Dickson A.M., Clay G., McCaffrey A.P., Wilson M.E. Identifying functional microRNAs in macrophages with polarized phenotypes. J. Biol. Chem. 2012;287(26):21816–21825. doi: 10.1074/jbc.M111.327031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chaudhuri A.A., So A.Y., Sinha N., Gibson W.S., Taganov K.D., O'Connell R.M., et al. MicroRNA-125b potentiates macrophage activation. J. Immunol. 2011;187(10):5062–5068. doi: 10.4049/jimmunol.1102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ganesh S., Iyer A.K., Morrissey D.V., Amiji M.M. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34(13):3489–3502. doi: 10.1016/j.biomaterials.2013.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Su M.J., Aldawsari H., Amiji M. Pancreatic cancer cell exosome-mediated macrophage reprogramming and the role of MicroRNAs 155 and 125b2 transfection using nanoparticle delivery systems. Sci. Rep. 2016;6 doi: 10.1038/srep30110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen X., Ying X., Wang X., Wu X., Zhu Q., Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol. Rep. 2017;38(1):522–528. doi: 10.3892/or.2017.5697. [DOI] [PubMed] [Google Scholar]
- 138.Xiao L., He Y., Peng F., Yang J., Yuan C. Endometrial cancer cells promote M2-like macrophage polarization by delivering exosomal miRNA-21 under hypoxia condition. J. Immun. Res. 2020;2020 doi: 10.1155/2020/9731049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ahluwalia P.K., Pandey R.K., Sehajpal P.K., Prajapati V.K. Perturbed microRNA expression by Mycobacterium tuberculosis promotes macrophage polarization leading to pro-survival foam cell. Front. Immunol. 2017;8:107. doi: 10.3389/fimmu.2017.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang J., Zhao W.S., Xu L., Wang X., Li X.L., Yang X.C. Endothelium-specific endothelin-1 expression promotes pro-inflammatory macrophage activation by regulating miR-33/NR4A axis. Exp. Cell Res. 2021;399(1) doi: 10.1016/j.yexcr.2020.112443. [DOI] [PubMed] [Google Scholar]
- 141.Moradi-Chaleshtori M., Bandehpour M., Soudi S., Mohammadi-Yeganeh S., Hashemi S.M. In vitro and in vivo evaluation of anti-tumoral effect of M1 phenotype induction in macrophages by miR-130 and miR-33 containing exosomes. Cancer Immunol. Immunother. 2021;70(5):1323–1339. doi: 10.1007/s00262-020-02762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moradi-Chaleshtori M., Shojaei S., Mohammadi-Yeganeh S., Hashemi S.M. Transfer of miRNA in tumor-derived exosomes suppresses breast tumor cell invasion and migration by inducing M1 polarization in macrophages. Life Sci. 2021;282 doi: 10.1016/j.lfs.2021.119800. [DOI] [PubMed] [Google Scholar]
- 143.Wang X., Zhou Y., Dong K., Zhang H., Gong J., Wang S. Exosomal lncRNA HMMR-AS1 mediates macrophage polarization through miR-147a/ARID3A axis under hypoxia and affects the progression of hepatocellular carcinoma. Environ. Toxicol. 2022 doi: 10.1002/tox.23489. [DOI] [PubMed] [Google Scholar]
- 144.Xin L., Wu Y., Liu C., Zeng F., Wang J.L., Wu D.Z., et al. Exosome-mediated transfer of lncRNA HCG18 promotes M2 macrophage polarization in gastric cancer. Mol. Immunol. 2021;140:196–205. doi: 10.1016/j.molimm.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 145.Wang Y., Gao R., Li J., Tang S., Li S., Tong Q., et al. Downregulation of hsa_circ_0074854 suppresses the migration and invasion in hepatocellular carcinoma via interacting with HuR and via suppressing exosomes-mediated macrophage M2 polarization. Int. J. Nanomed. 2021;16:2803–2818. doi: 10.2147/IJN.S284560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ono K., Eguchi T., Sogawa C., Calderwood S.K., Futagawa J., Kasai T., et al. HSP-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. J. Cell. Biochem. 2018;119(9):7350–7362. doi: 10.1002/jcb.27039. [DOI] [PubMed] [Google Scholar]
- 147.Calderwood S.K. Cdc37 as a co-chaperone to Hsp90. Subcell. Biochem. 2015;78:103–112. doi: 10.1007/978-3-319-11731-7_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Calderwood S.K. Molecular cochaperones: tumor growth and cancer treatment. Sci. Tech. Rep. 2013;2013 doi: 10.1155/2013/217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Eguchi T., Sogawa C., Ono K., Matsumoto M., Tran M.T., Okusha Y., et al. Cell stress induced stressome release including damaged membrane vesicles and extracellular HSP90 by prostate cancer cells. Cells. 2020;9(3) doi: 10.3390/cells9030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ono K., Sogawa C., Kawai H., Tran M.T., Taha E.A., Lu Y., et al. Triple knockdown of CDC37, HSP90-alpha and HSP90-beta diminishes extracellular vesicles-driven malignancy events and macrophage M2 polarization in oral cancer. J. Extracell. Vesicles. 2020;9(1) doi: 10.1080/20013078.2020.1769373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lai C.P., Mardini O., Ericsson M., Prabhakar S., Maguire C., Chen J.W., et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8(1):483–494. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pu F., Chen F., Zhang Z., Qing X., Lin H., Zhao L., et al. TIM-3 expression and its association with overall survival in primary osteosarcoma. Oncol. Lett. 2019;18(5):5294–5300. doi: 10.3892/ol.2019.10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cheng Z., Wang L., Wu C., Huang L., Ruan Y., Xue W. Tumor-derived exosomes induced M2 macrophage polarization and promoted the metastasis of osteosarcoma cells through tim-3. Arch. Med. Res. 2021;52(2):200–210. doi: 10.1016/j.arcmed.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 154.Linton S.S., Abraham T., Liao J., Clawson G.A., Butler P.J., Fox T., et al. Tumor-promoting effects of pancreatic cancer cell exosomes on THP-1-derived macrophages. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0206759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chattopadhyay S., Sun P., Wang P., Abonyo B., Cross N.L., Liu L. Fusion of lamellar body with plasma membrane is driven by the dual action of annexin II tetramer and arachidonic acid. J. Biol. Chem. 2003;278(41):39675–39683. doi: 10.1074/jbc.M212594200. [DOI] [PubMed] [Google Scholar]
- 156.Shou Y., Wang X., Chen C., Liang Y., Yang C., Xiao Q., et al. Exosomal miR-301a-3p from esophageal squamous cell carcinoma cells promotes angiogenesis by inducing M2 polarization of macrophages via the PTEN/PI3K/AKT signaling pathway. Cancer Cell Int. 2022;22(1):153. doi: 10.1186/s12935-022-02570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shen T., Miao S., Zhou Y., Yi X., Xue S., Du B., et al. Exosomal AP000439.2 from clear cell renal cell carcinoma induces M2 macrophage polarization to promote tumor progression through activation of STAT3. Cell Commun. Signal. 2022;20(1):152. doi: 10.1186/s12964-022-00957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.He Z., Wang J., Zhu C., Xu J., Chen P., Jiang X., et al. Exosome-derived FGD5-AS1 promotes tumor-associated macrophage M2 polarization-mediated pancreatic cancer cell proliferation and metastasis. Cancer Lett. 2022;548 doi: 10.1016/j.canlet.2022.215751. [DOI] [PubMed] [Google Scholar]
- 159.Qiu S., Xie L., Lu C., Gu C., Xia Y., Lv J., et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J. Exp. Clin. Cancer Res. 2022;41(1):296. doi: 10.1186/s13046-022-02499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li Z., Meng X., Wu P., Zha C., Han B., Li L., et al. Glioblastoma cell-derived lncRNA-containing exosomes induce microglia to produce complement C5, promoting chemotherapy resistance. Cancer Immunol. Res. 2021;9(12):1383–1399. doi: 10.1158/2326-6066.Cir-21-0258. [DOI] [PubMed] [Google Scholar]
- 161.Baselga J., Im S.A., Iwata H., Cortés J., De Laurentiis M., Jiang Z., et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(7):904–916. doi: 10.1016/s1470-2045(17)30376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schnitzbauer A.A., Filmann N., Adam R., Bachellier P., Bechstein W.O., Becker T., et al. mTOR inhibition is most beneficial after liver transplantation for hepatocellular carcinoma in patients with active tumors. Ann. Surg. 2020;272(5):855–862. doi: 10.1097/sla.0000000000004280. [DOI] [PubMed] [Google Scholar]
- 163.Jonker D.J., Nott L., Yoshino T., Gill S., Shapiro J., Ohtsu A., et al. Napabucasin versus placebo in refractory advanced colorectal cancer: a randomised phase 3 trial. Lanc. Gastroent. Hepat. 2018;3(4):263–270. doi: 10.1016/s2468-1253(18)30009-8. [DOI] [PubMed] [Google Scholar]
- 164.Harrison M.L., Obermueller E., Maisey N.R., Hoare S., Edmonds K., Li N.F., et al. Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J. Clin. Oncol. 2007;25(29):4542–4549. doi: 10.1200/jco.2007.11.2136. [DOI] [PubMed] [Google Scholar]
- 165.Perreault S., Larouche V., Tabori U., Hawkin C., Lippé S., Ellezam B., et al. A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer. 2019;19(1):1250. doi: 10.1186/s12885-019-6442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kamerkar S., Leng C., Burenkova O., Jang S.C., McCoy C., Zhang K., et al. Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity. Sci. Adv. 2022;8(7) doi: 10.1126/sciadv.abj7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study is a review article.