Abstract
Even though water is the most essential nutrient for poultry production, adequate data on individual water intake in broiler chickens and its relationship with other traits of economic importance is scant. Water is provided to chickens in an unrestricted manner in spite of being a finite resource. Climate change continues to affect water sources and efficient bird use of water is long overdue. Understanding the biological basis of water intake is essential for sustainability of the poultry industry. Individual water and feed intake, and growth data was collected on 520 commercial broilers aged 14 to 42 days. We introduced the concepts of water conversion ratio (WCR) and residual water intake (RWI) as parameters that can be used to assess water intake efficiency. Water conversion ratio was defined as the amount of water consumed per unit of body weight gain, and RWI was defined as the difference between the actual water intake (WI) of a given bird and the expected WI by an average bird from the population with the same metabolic body weight, feed intake (FI) and body weight gain (BWG). The correlation between WI and FI was positive (r=0.77; P<0.0001), and the correlation between WI and BWG was positive (r=0.80; P<0.0001). Based on the distribution of RWI, the bottom 5 birds (LRWI) and the top 5 birds (HRWI) for RWI were selected for mRNA expression differences. The average broiler consumed about 7.8 L (± 1L) of water from 14 to 42 days of age. The mRNA expression of arginine vasopressin (AVP) antidiuretic hormone, calcium sensing receptor (CasR), sodium channel epithelial 1 subunit alpha (SCNN1A) and SCNN1D in the hypothalamus was upregulated in the LRWI group compared to the HRWI group. Similarly, kidney aquaporins (AQP) 2, 3, and 4 were upregulated in the LRWI group compared with the HRWI group. Given that water was provided ad libitum, the up-regulation of AVP and AQP gene mRNA expressions seem to indicate that the LRWI birds were more efficient in water reabsorption in the kidney compared to their HRWI counterparts. Increased water reabsorption will reduce the amount of water consumed to attain hydration. The water reabsorption potential was reflected in the excreta moisture levels as the LRWI birds had significantly lower excreta moisture than the HRWI birds. Excreta moisture level require further studies and could be considered as a potential proxy trait for water intake.
Key words: water intake, residual water intake, water conversion ratio, arginine vasopressin, aquaporins
INTRODUCTION
The 2020 World Population Data Sheet indicates that world population is projected to increase from 8 billion in 2022 to 9.9 billion by 2050. This will represent an increase of more than 25% over a thirty-year period (IISD, 2020). Changing patterns of resource use by humans and food consumption have profoundly impacted the earth's biosphere (Bennett, et al., 2018). Bennett et al. (2018) showed that the broiler chicken is a good indicator that can be used to track this change in resource use by humans. Understanding the nature of change (in resource use and consumption) is essential for sustainability. The production of broiler meat has paralleled the rising global human population. The United States has seen a major switch in consumption across the three major meat categories. In 1999, beef consumption was greater than chicken or pork. By 2020, beef consumption had declined from 44 kg per capita in 1999 to 38 Kg in 2020. Pork consumption has remained stable. Chicken consumption increased from 34 Kg in 1999 to 44 Kg per capita in 2021. During this short period of time, the number of broilers produced increased from 8.12 billion in 1999 to 9.18 billion in 2019 (NCC, 2022).
The increase in per capita consumption of broiler meat and the concomitant increase in the number of chickens raised would have significant impact on resource use (feed, water, energy, etc.). The ever-increasing number of broilers produced needs to be addressed in the context of finite natural resources and sustainability (FAO, 2022). The desire to improve sustainability in poultry production has long been recognized and through genetic selection, feed conversion ratio (FCR) has improved from 2.0 in 1990 to 1.79 in 2020 (NCC, 2022). The lion's share of the efforts to maintain sustainability in broiler meat production has been devoted to reducing the amount of feed per unit of body weight gain. However, feed and water intake and potentially their use efficiencies are closely linked. Chickens consume about 1.6 to 2.0 times as much water as feed, and if water intake is limited, then feed intake declines (Alltech, 2017).
Even though water is a finite resource, it is provided ad libitum to chickens. According to the US Geological Survey, only about 4% of the 332.5 million cubic miles of water on the earth consists of fresh water (Jamshidi, 2021). Climate change is disrupting weather patterns, leading to extreme climatic events that have significantly impacted fresh water supply, even in the United States (NPR, 2021). This can significantly influence the amount of broiler meat produced to meet the global protein challenge for a fast-growing global population.
Water is the most essential nutrient for life (Jequier and Constant, 2010), yet it is often ignored and under researched (Rush, 2013). Efficient water use by broiler chickens would significantly improve production efficiency and sustainability, and will help save fresh water for other societal uses. Despite the essential role water plays in broiler meat production, critical data pertaining to the biological basis of water intake, its interrelationships with feed efficiency and other traits of economic importance is scant.
Our objective was to study the quantitative parameters of water intake in commercial broilers and to elucidate the conceptual molecular basis for the differences in residual water intake among efficient and inefficient water using chickens.
MATERIALS AND METHODS
All experiments in this study were performed under the Animal Use Proposal (AUP) number A2021 07-003-Y1-A0 approved by the Animal Care and Use Committee (IACUC) of the University of Georgia
Animal Population
Two batches of 360 commercial day-old chicks (Ross 708) each were sexed, wing-banded and raised in colony cages (3.1 m x 1.2 m) for 14 days. The chicks were managed based on husbandry recommendations from Aviagen, Inc. At 14 days of age, birds were randomly assigned to individual cages (L=30.48 cm, W=45.72 cm, H=60.96 cm) with each cage fitted with its own feeder and drinker (a container with a nipple). Equal number of males and females were placed. The room temperature and relative humidity were maintained at 20-22°C and 60-65% RH, respectively from 14-42 days. Feed and water intake were measured by the differences in feeder and drinker weights at days 14, 21, 28, 35 and 42. Birds were fed on the recommended grower diet from 14 to 28 days, and finisher diet from 28 to 42 days. All the diets contained 0.2% titanium dioxide. Water and feed were supplied to each bird on an ad libitum basis. Records from individuals that did not feed and/or drink were removed. Also, partial records from birds that died before day 42 were removed. Individual weekly water, body weight and feed intake of 520 birds were used for the analysis. Chickens were humanely euthanized at day 42.
Parameters for Assessing Water Intake Efficiency
Water use efficiency (WUE) is a term introduced over 100 years ago by Briggs and Shanz (1913) demonstrating the relationship between plant productivity and water use. The parameter WUE was defined as a measure of the amount of biomass produced per unit of water used. A review by Basso and Ritchie (2018) demonstrated that maize (Zea mays L.) productivity increased with no change in water use rate and resulted in increased WUE. In animal production, a similar parameter to measure water use efficiency does not exist. In poultry, WUE could be defined similarly to the “gain:feed” as the ratio between body weight gain and water intake.
| (1) |
The inverse of WUE is defined as the water conversion ratio (WCR).
| (2) |
Bichet (2018) indicated that the response to drinking and feeding is bidirectional, yet asymmetric. This suggests that WCR will also be affected by variation in feed intake (FI) and maintenance requirement, a major contributing factor to FI but the relationship is not linear. WUE and WCR, as ratio traits are likely not to be normally distributed. The non-normality of a ratio trait increases with magnitude of the coefficient of variation of the denominator (Atchley, et al., 1976). The relative response due to selection on a ratio are not equal for the numerator and denominator trait (Essl, 1989). Also, the ratio of 1/2 and 2/4 are considered equal by genetic selection, in which case penalizes larger birds.
In order to account for the variation in maintenance requirement, feed intake, and growth rate, we are introducing the concept of residual water intake (RWI) which is an equivalent of residual feed intake (Koch, et al., 1963). RWI is defined as the difference between the water intake (WI) of a bird and the expected WI by an average bird from the population with the same metabolic body weight (MBW), feed intake (FI) and body weight gain (BWG). It is expected that the estimating components will be phenotypically independent of RWI as a result of the distributing properties of the regression procedure (Netter, et al., 2004).
RWI will be calculated as:
| (3) |
where is an intercept, are estimated regression coefficients on respectively. We predicted RWI of each bird using the cumulated data from 14-42 days by PROC REG (SAS, 2000).
Excreta Moisture
At 35 days of age we used FI, WI and BWG data from 28-35 days to determine RWI. Based on the distribution of RWI, we collected 20 g of fresh excreta in triplicates from the top 20 (10 males and 10 females) (HRWI) and bottom 20 (10 males and 10 females) (LRWI) birds. The excreta samples were dried in an oven at 105°C for 3 hours. The difference in weight between the wet and dry excreta was used to calculate the percent moisture content.
Tissue Sampling, RNA Extraction and Real-Time Polymerase Chain Reaction
Using the distribution of RWI, five males with the lowest RWI (average=-1,196 mL) designated low RWI (LRWI) group and 5 males with the highest RWI (average=+1,485 mL) designated as high RWI (HRWI) group were selected and humanely euthanized by cervical dislocation at day 42. The hypothalamus and kidney were sampled, snap frozen in liquid nitrogen and stored at -86°C for later use. Total RNA was extracted from the tissues using Trizol reagents (Invitrogen, Carlsbad, CA) and purified with RNeasy kits (Qiagen, CA) according to the manufacturer's protocol. The concentration of the RNA was determined with UV absorbance and the OD260/280 ratios for all samples were >1.9.
Two micrograms of total RNA were transcribed with cDNA reversed transcription kits according to the manufacturer's protocol (Applied Biosystems, Carlsbad, CA) using a Gradient Mastercycler (Eppendorf, NY) adjusted for repeated cycles of 10 minutes at 25 °C, 120 minutes at 37 °C, 5 minutes at 85 °C, and a final cycle at 4 °C. Each cDNA sample was measured on a NanoDrop 2000 spectrophotometer and hereafter diluted to 20 ng/µL. Each RT-qPCR reaction consisted of 2µL of 20 ng/µL cDNA, 0.6 µL of each forward and reverse primer (10 µM) (Table 1), 6.8 µL of nuclease-free water, and 10 µL of PowerUp SYBRTM Green Master Mix (Applied Biosystems, Carlsbad, CA). Each reaction was run in triplicate.
Table 1.
List of gene symbol, accession number, product size and primer sequences used in qPCR.
| Gene Symbol | Accession Number | Product Size (bp) | Primer Sequence | |
|---|---|---|---|---|
| AQP1 | NM_001039453 | 90 | FWD | 5′ AAGTGAGATTGAAGAGCAGTAG 3′ |
| REV | 5′ GAACAGCCACAGGAACAA 3′ | |||
| AQP2 | NM_001292072 | 76 | FWD | 5′ GTTACACCGGTTGCTCTATG 3′ |
| REV | 5′ CCAATGGTCACTGAAGTCTC 3′ | |||
| AQP3 | XM_424500 | 103 | FWD | 5′ CTGGTATCTTTGCCACCTAC 3′ |
| REV | 5′ GATAGCCAAGACACAAACAATC 3′ | |||
| AQP4 | NM_001317827 | 98 | FWD | 5′ CGCTCGCAGCAGCAGTAA 3′ |
| (Orlowski et al., 2017) | REV | 5′ ATGCTACCATGATGCTCTCACACT 3′ | ||
| AVP | NM_205185 | 124 | FWD | 5′ TGTGGACCTGGGAACAG 3′ |
| REV | 5′ AGGGTGAAGGCATGTAGT 3′ | |||
| CaSR | XM_416491 | 107 | FWD | 5′ CCTGAGGATTACTGGTCTAATG 3′ |
| REV | 5′ GCACAGCAAAGAGAGTTAAAG 3′ | |||
| KCNJ1 | XM_004947967.5 | 118 | FWD | 5′ GAAGCTAAAGCCAAAGAGAAGAAAG 3′ |
| REV | 5′ CAGGAAGGTGCAGTCTGATAAA 3′ | |||
| KCNMA1 | NM_204224.2 | 95 | FWD | 5′ GTGCCGACAGACTTGATCTT 3′ |
| REV | 5′ GAATAGGAGGAATGGGAGGAATG 3′ | |||
| SCNN1A | NM_205145 | 105 | FWD | 5′ CCTCAACCTCAACCTAAACTC 3′ |
| REV | 5′ CAGTTCGTCCAGCTTCTTC 3′ | |||
| SCNN1B | XM_046900880 | 96 | FWD | 5′ GAATCCTCATCAACACCTACC 3′ |
| REV | 5′ GCATTGCAGACTGTGACT 3′ | |||
| SCNN1D | XM_040689036 | 94 | FWD | 5′ CAAGAGAGGAGGAAGAGAGAA 3′ |
| REV | 5′ GTGGATGGTGGTGTTCTTAC 3′ | |||
Real time qPCR reactions were carried out using the StepOnePlus (Applied Biosystems, Carlsbad, CA). with settings for 50 °C for 120s, 95 °C for 120s, and 40 cycles of 95°C for 15s, 55°C for 15s, and 72°C for 60s, followed by a melt curve stage. The Ct values at the endpoint and the melting temperature curve for each endpoint were measured. The Ct values of the genes of interest were normalized against the Ct values of the β-actin gene (endogenous control), and the fold change of the LRWI was calculated relative to the HRWI group. The relative mRNA expression was calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001) for arginine vasopressin (AVP) antidiuretic hormone, calcium sensing receptor (CasR), sodium channel epithelial 1 subunit alpha (SCNN1A), SCNN1B and SCNN1D in the hypothalamus, and aquaporin (AQP)-1, AQP2, AQP3, AQP4, CasR, potassium inwardly rectifying channel subfamily J (KCNJ1), potassium calcium-activated channel subfamily (KCNMA1), SCNN1A and SCNN1B in the kidney (Table 1). These genes were chosen for the current study because they are intricately associated with the physiology and mechanisms of water intake. Statistical difference in gene expression between groups was determined using PROC GLM (SAS, 2000). A p_value ≤ 0.05 was used to declare statistical significance.
RESULTS
A summary description of the data collected for BWG, FI, FCR, WCR and W:F ratio is presented in Table 2. As expected, the variability of BWG, FI and WI increased with age. Similarly, water intake increased with age; however, the relative increase during days 35- 42 was smaller compared to when birds were younger (<35 days of age). The average broiler consumed about 7.8 L of water from 14 to 42 days of age. Whereas FCR increased with age, WCR appeared to be constant from 14-21, 21-28, 28-35, and 35-42 days of age.
Table 2.
Growth, feed and water related traits of commercial broiler chickens.
| Age (days) |
|||||
|---|---|---|---|---|---|
| Trait1 | 14-21 | 21-28 | 28-35 | 35-42 | 14-42 |
| BWG (g) | 381 ± 68 | 586 ± 82 | 671 ± 112 | 683 ± 142 | 2,322 ± 308 |
| FI (g) | 488 ± 71 | 759 ± 95 | 1,009 ± 144 | 1,127 ± 194 | 3,385 ± 436 |
| WI (g) | 1,284 ± 249 | 1,836 ± 275 | 2,233 ± 355 | 2,508 ± 461 | 7,860 ± 1,063 |
| FCR (g/g) | 1.29 ± 0.12 | 1.31 ± 0.15 | 1.52 ± 0.14 | 1.67 ± 0.20 | 1.46 ± 0.06 |
| WCR (g/g) | 3.41 ± 0.54 | 3.17 ± 0.51 | 3.37 ± 0.51 | 3.66 ± 0.21 | 3.39 ± 0.32 |
| Water:Feed | 2.64 ± 0.42 | 2.43 ± 0.32 | 2.22 ± 0.27 | 2.24 ± 0.27 | 2.33 ± 0.22 |
BWG: Body weight gain; FI: Feed intake; WI: Water intake; FCR: Feed conversion ratio; WCR: Water conversion ratio.
Phenotypic Correlations
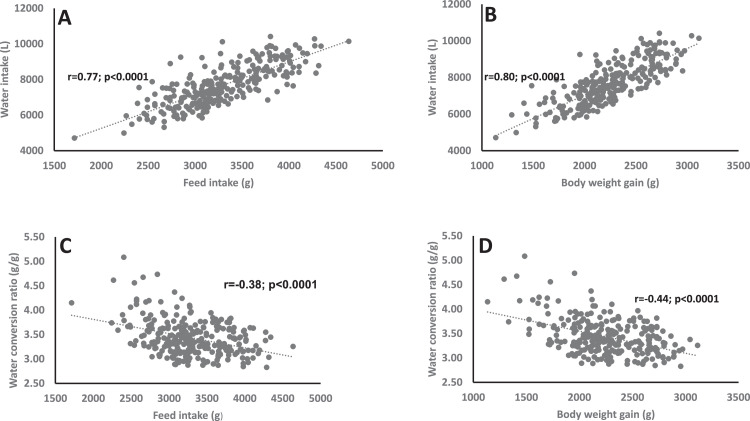
The phenotypic correlations among WI, FI, BWG, and WCR are presented in Figure 1. The correlation between WI and FI was positive (r=0.77; P<0.0001), and the correlation between WI and BWG was positive (r=0.80; P<0.0001). However, the correlation between WCR and FI was negative (r=-0.38; P<0.0001). The correlation between WCR and BWG was also negative (r=-0.44; P<0.0001). The correlation between FCR and water intake (r=-0.31; P<0.0001), and that between FI and BWG (r=0.94; P<0.0001) are presented in Supplementary Figure 1.
Figure 1.
Relationship between (A) feed intake water intake; (B) Body weight gain and water intake; (C) Feed intake and conversion ratio; (D) Body weight gain and water conversion ratio.
Residual Water Intake
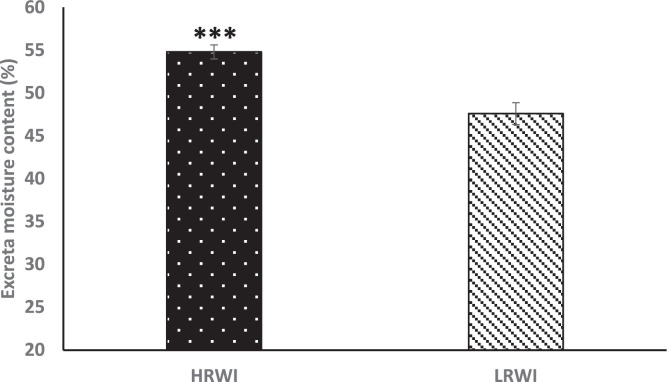
Based on the distribution of RWI, we selected the top and bottom 50 birds to further dissect the trait. The bottom 25 and top 25 birds were designated as low (LRWI) and high residual water intake (HRWI) groups, respectively. Similarly, the bottom 25 and top 25 birds based on water conversion ratio were assigned to low (LWCR) and high water conversion ratio (HWCR) groups, respectively (Table 3). Whereas, there were no differences in MBW, BWG, FI and FCR between the LRWI and HRWI groups (P>0.05), there were significant differences (P<0.05) in WI, WCR and W:F ratio between the RWI groups. There was 2.2 L difference in WI between the LRWI and HRWI groups (P<0.05). On the other hand, there were differences in MBW, BWG and W:F ratio (P<0.05) between the WCR groups. There was a 2 L difference (P<0.05) between the LWCR and HWCR groups. We ascertained the excreta moisture content of the LRWI and HRWI groups. The results from Figure 2 show that the LRWI group had lower (P<0.05) excreta moisture compared to the HRWI group.
Table 3.
Trait characteristics of low (LRWI) and high (HRWI) residual water intake (RWI) and low (WCR) and high (WCR) water conversion ratio (WCR).
| Trait1 | LRWI | HRWI | Pr>F | LWCR | HWCR | Pr>F |
|---|---|---|---|---|---|---|
| MBW (g) | 83.16 ± 5.91 | 82.93 ± 6.72 | 0.9000 | 80.64 ± 7.08 | 82.73 ± 6.74 | 0.2898 |
| BWG (g) | 2,336 ± 266 | 2,328 ± 313 | 0.9230 | 2,456 ± 269 | 2,132 ± 284 | 0.0001 |
| FI (g) | 3,383 ± 394 | 3,394 ± 443 | 0.9308 | 3,523 ± 412 | 3,097 ± 417 | 0.0007 |
| WI (g) | 6,892 ± 665 | 9,141 ± 819 | <0.0001 | 7,221 ± 765 | 8,514 ± 1009 | <0.0001 |
| FCR (g/g) | 1.45 ± 0.06 | 1.46 ± 0.06 | 0.5343 | 1.43 ± 0.04 | 1.45 ± 06 | 0.2092 |
| WCR (g/g) | 2.96 ± 0.08 | 3.96 ± 0.28 | <0.0001 | 2.94 ± 0.05 | 4.01 ± 0.24 | <0.0001 |
| RWI | −1,008 ± 121 | 1,259 ± 342 | <0.0001 | −966 ± 181 | 1,159 ± 437 | <0.0001 |
| W:F | 2.04 ± 0.07 | 2.71 ± 0.22 | <0.0001 | 2.05 ± 0.07 | 2.76 ± 0.19 | <0.0001 |
MBW: metabolic body weight; BWG: body weight gain; FI: feed intake; FCR: feed conversion ratio; W:F: water to feed ratio.
Figure 2.
Excreta moisture content of low residual water intake (LRWI) and high residual water intake (HRWI) groups. (***P<0.001).
Gene Expression
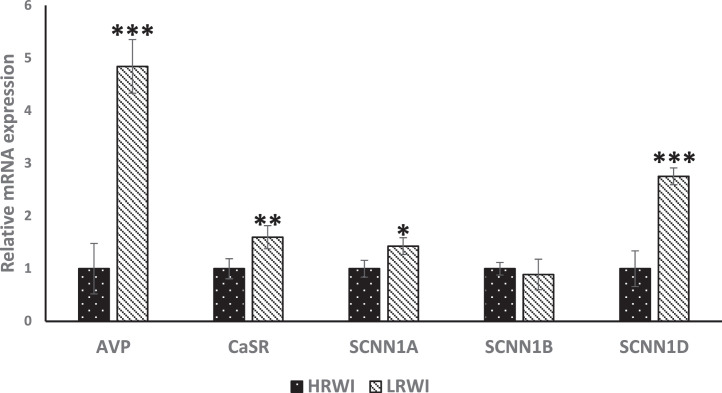
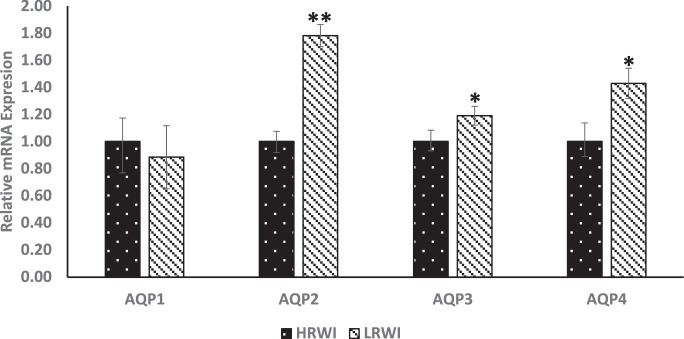
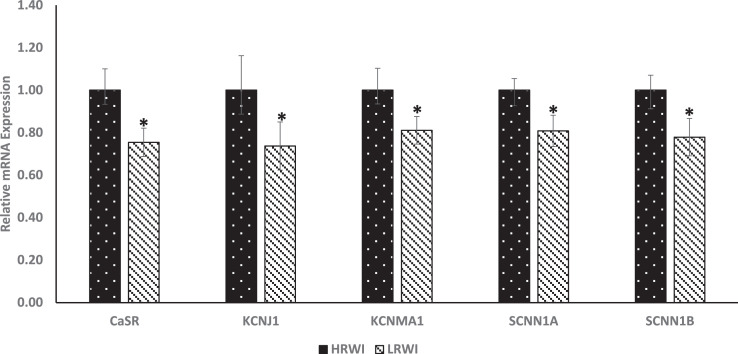
Genes encoding arginine vasopressin (AVP) antidiuretic hormone, calcium sensing receptor (CasR), sodium channel epithelial 1 subunit alpha (SCNN1A), SCNN1B and SCNN1D were evaluated for their relative expressions in the hypothalamus (Figure 3). The AVP gene was upwardly expressed (P<0.05) in the LRWI group when compared with the HRWI group. The calcium sensing gene, CaSR, and epithelial sodium channel genes, SCNN1A and SCNN1D genes were upwardly expressed (P<0.05) in the LRWI group compared to the HRWI group. There were no significant differences in expression for SCNN1B. The mRNA expression differences of the kidney AQP1-4 genes between the LRWI and HRWI groups are shown in Figure 4. The AQP2, 3 and 4 genes were upwardly expressed in the LRWI group compared to the HRWI counterpart. However, there were no significant expression differences for AQP1 between the two groups. Contrary to the direction of mRNA expression in the hypothalamus, the gene expressions of CasR, KCNJ1, SCNN1A and SCNN1B were downwardly expressed (P<0.05) in the kidneys of the LRWI group when compared with the HRWI group (Figure 5).
Figure 3.
Effect of low residual water intake (LRWI) and high residual water intake (HRWI) on mRNA expression of vasopressin (AVP), calcium signaling receptor (CaSR) and sodium channel epithelial channel 1 (SCNN1A), SCNN1B and SCNN1D in the hypothalamus. (*P<0.05, **P<0.01, ***P<0.001).
Figure 4.
Effect of low residual water intake (LRWI) and high residual water intake (HRWI) on mRNA expression of aquaporin in the kidney (AQP) 1, AQP2, AQP3 and AQP4. (*P<0.05, **P<0.01).
Figure 5.
Effect of low residual water intake (LRWI) and high residual water intake (HRWI) on mRNA expression of calcium signaling receptor (CaSR), potassium inwardly rectifying channel subfamily J (KCNJ1), potassium calcium-activated channel subfamily (KCNMA1) sodium channel epithelial channel 1 (SCNN1A) and SCNN1B in the kidney. (*P<0.05).
DISCUSSION
Individual water and feed intakes are arduous to measure because of the associated logistics and labor. However, such measurements provide essential data to understand the biology of these traits and to develop management and breeding decision tools. Water intake increased with age; however, the relative increase during the week of 35 to 42 days of age was smaller when compared to the previous days. This was consistent with flock data presented by Williams et al. (2013). The average broiler chicken consumed about 7.8 L of water from 14 to 42 days. This is higher than the cumulative water consumption reported by Williams et al. (2013) on a 2010/2011 flock. Broiler chickens are under constant improvement for growth. As such, chickens in 2022 grow faster than chickens in 2011, and growth rate is positively correlated with water intake. The standard deviation of WI from 14 to 42 days of age in the commercial broiler population used in the current study was 1.1 liters with a coefficient of variation of around 14 indicating a substantial variation in water intake between birds.
Water intake is positively correlated with growth, and since market weight has been increasing since 1925 (NCC, 2022), it should be expected that water intake in the current broiler populations will be greater than that of previous years. The current study also shows that the correlation (r=0.77) between water and feed intake is positive. This positive correlation could in part be due to the continuous increase in growth of the modern broiler.
Meat is approximately 70-75% water and 20% protein by weight (Deltoro, et al., 1988; Heinz and Hautzinger, 2007; Nethery, et al., 2022). The water content in meat is directly related to the water-holding capacity as well as water-binding capacity. Therefore, to maintain this 3.5 -3.75 water to protein ratio, water intake will automatically increase with increased growth. Morel (2014) showed that the relationship between water deposition rate and protein deposition rate was positive. This is corroborated by the negative correlation between FCR and water intake in the current study (Supplementary Figure 1). Earlier, Morel (2014) had demonstrated a negative relationship between FCR and water deposition in broilers. Subsequently, broilers with low WCR gained more weight and consumed less water than broilers with high WCR. The average water to feed ratio from day 14-42 was 2.33. However, Williams et al. (2013) reported that in 2010/2011 commercial flocks, the water to feed ratio declined exponentially from about 3.0 to 1.7 from 10 to 42 days. It should be pointed out that, the data from Williams et al. (2013) is an aggregate of flock data which could be affected by many factors, and unlike the current data which was collected under the same environmental conditions (ambient temperature, humidity, diet, etc.).
Dynamics of Water Conversion Ratio and Residual Water Intake
Water conversion ratio is a ratio trait similar to FCR. The statistical implications of the limitations of using ratio have long been recognized (Pearson, 1897). Kendall and Stuart (1963) reported that ratios of random normal variables are distributed as Cauchy distribution characterized by undefined moments (e.g., mean, variance). The non-normality of ratio traits increases with the increase in the coefficient of variation (Atchley, et al., 1976; Sutherland, 1965). Similar to FCR, we showed that WCR is correlated with its components (water intake and body weight gain) and as such, improvements in WCR will be confounded with both WI and BWG. However, the linear approximation of the ratios of the two traits is effective in genetic improvement (Lin, 1980; Gunsett, 1984; Essl, 1989; Famula, 1990; Zetouni, et al., 2017). Based on these results, improvement in water use efficiency could be achieved through a linear index of water intake and body weight gain rather than WCR.
The limitations in the use of FCR led to the introduction of residual feed intake (Koch, et al., 1963). Herein, we used similar reasoning to derive residual water intake (RWI). Residual water intake predicted from equation 3 relies on fixed effect model producing average partial regression coefficients. However, the use of mixed or random model (Aggrey and Rekaya, 2013; Rekaya and Aggrey, 2015) will allow for individual variations in the partial regression coefficients, and in pedigreed populations, the genetic correlations among the partial regression coefficients can be used to simultaneously improve WI, FI and BWG.
Using birds in the extremes of the distributions of RWI and WCR, it is clear that water intake can be delineated from feed intake (Table 2). After correcting for feed intake and growth, the LRWI chickens consumed about 1 liter less than the average, and the HRWI group consumed over 1 liter more than their average counterparts did. It should be pointed out that there were no differences in metabolic body weight, FI and BWG between the LRWI and the HRWI groups (Table 2). Therefore, these two RWI subpopulations can be used to elucidate the cellular and molecular mechanisms that underlie the differences in their water intake.
The data in Table 2 shows that there were significant differences in BWG and FI between the LWCR and HWCR groups. Moisture is approximately 10% of the "air-dry" diet on weight basis. The LWCR group consumed more feed than the HWCR group, which implies that, they have also consumed more water from feed, which is not accounted for. This further exacerbates the bias in using WCR to assess water intake efficiency. Thus, molecular studies using WCR as a primary trait would have confounding effects due to growth and FI differences. The biology of water intake in poultry is in its infancy stage and as such both RWI and WCR should be studied for thorough understanding of the mechanisms that underlie both traits and how that knowledge could be used to improve water intake and use in broiler chickens. Nevertheless, herein, we show RWI to be the optimal parameter to use.
Molecular Variation in Residual Water Intake
The biological models of water intake are based on an animal's response to thirst or salt consumption (Andersson, et al., 1982; Oka, et al., 2015; Zimmerman, et al., 2017). However, under the current study, both the LRWI and HRWI birds were provided ad libitum water. Furthermore, they were reared under the same environmental conditions using identical feed and water inputs. Thus, differences in water use efficiency between these two groups of birds is likely to be at least partially under genetic control. Arginine vasopressin is a key player in water homeostasis (Guelinckx, et al., 2016). Changes in blood Na+ concentration can be due to changes in volume and/or osmolality (Zimmerman, et al., 2019). Volume deficit or excess indicates a change in the amount of fluid in the extracellular space that generally does not change the salt to water ratio. Sodium ion related osmolality pertains to changes in either Na+ or water, thus leading to changes in the salt to water ratio (Castañeda-Bueno, et al., 2012). In the current study, the calcium sensing receptor gene, CasR and sodium channel genes SCNN1A and SCNN1D were upwardly expressed in the hypothalamus of the LRWI group compared to the HRWI group. Genetic differences in the expression of CasR and SCNN genes in the LRWI group rather than changes in the osmolality may be responsible for the high upward expression of the AVP gene in the LRWI group compared to its HRWI counterpart. The differences in water intake of the two groups may reflect genetic differences rather than a response to hyperosmolality as both groups were provided with ad libitum supply of water from the same source. The AVP gene encodes for antidiuretic hormone. When released, it acts on the kidney to reabsorb more water. There was a 4.8-fold difference in the hypothalamic AVP mRNA expression between the LRWI and HRWI groups. Kim et al. (1993) showed that there can be a non-osmolalitic stimulation of AVP gene expression in cirrhotic rats when compared to their controls. The differences in AVP gene expression between LRWI and HRWI groups may lead to different water reabsorption capacity in the kidney. AVP increases water reabsorption in the kidney by increasing the mRNA expression of AQP2 channels thereby increasing water reabsorption capacity in the collecting duct (Guelinckx, et al., 2016).
Aquaporins, also called water channels, are integral membrane proteins that form pores in cell membranes and are mainly involved in transmembrane diffusion of water and small solutes in a bidirectional manner (Preston and Agre, 1991; Benga, 2004). AQP2-4 genes were upwardly expressed in the LRWI group. Using immunochemistry tools, AQP1 has been identified in all tubule types in the renal medulla, glomerular podocytes and both proximal and distal tubules of the renal cortex in the kidney, and within the mucosa of all four regions of the lower intestine (caeca, proximal rectum, distal rectum and coprodeum) as reported by Casotti et al. (2007). AQP1 is involved in water movement in the kidney and water conservation (Casotti, et al., 2007; Yang, et al., 2021). Perhaps there are no differences in upstream or other factors that control AQP1 between the two groups. Similar to mammals, the avian AQP2 is present at the apical membrane of the medullary and cortical collecting duct (Nishimura and Fan, 2003), and its synthesis, secretion and activity is regulated by AVP (Goldstein, 2006). Nishimura and Yang (2013) showed that in Japanese quail, both AQP2 mRNA and protein expression are increased by increased AVP. Notably, both AVP and AQP2 were upwardly expressed in the LRWI group compared to the HRWI group. In rats, AQP2 has been shown to play a significant role in urine concentration (Sasaki and Noda, 2007). The avian kidneys have looped nephrons that drive countercurrent urine concentration by using sodium recycling and AVP-regulated AQP2 (Nishimura and Yang, 2013). Therefore, it is possible that water reabsorption in the kidney was more efficient in the LRWI group than the HRWI group. The AQP3 gene was also upwardly expressed in the LRWI compared with the HRWI group. Both AQP3 and AQP4 are abundant in the basolateral plasma membranes and are potential exit pathways from the cells for water entering through AQP2. AQP3 is expressed along the connecting tubule, cortical and outer medullary collecting duct (Ecelbarger, et al., 1995). On the other hand, AQP4 is mainly present in the inner medulla; however, it is also expressed in the more proximal segment (Ecelbarger, et al., 1995). Terris et al. (1996) demonstrated that AQP3 expression is also regulated by changes in vasopressin levels. On the contrary, Terris et al. (1996) showed that long-term vasopressin infusion did not affect AQP4 expression in rats.. An AQP3 knockout exhibited marked urinary concentrating defect with severe polyuria (Ma, et al., 2000). Thus, both AQP2 and AQP3 are essential for urinary concentration. Further, mice lacking AQP4 showed a mild urinary concentration defect (Ma, et al., 1997). Chou et al. (1998) suggested that AQP4 may be responsible for a large proportion of the basolateral membrane water movement. However, Yang and Nishimura (2021) suggested that AQP4 may not have water channel activity. From the current study, AQP2 and AQP3 water handling activities in the kidneys and AQP2 activity in the collecting duct of the LRWI group may putatively be efficient than that of the HRWI group.
The downward expression of CasR, KCNJ1, KCNMA1, SCNN1A and SCNN1B in the LRWI group compared to the HRWI may be related to differential mineral resorption or excretion. However, since calcium, sodium and potassium levels in the excreta were not measured in the two groups, the relative degree of mineral retention in the two groups cannot be ascertained. The differential water and sodium reabsorptions in the two groups of chickens require further investigation. Nevertheless, Dunson and Buss (1968) speculated that excessive drinking might be the secondary result of deficiencies in hypothalamic AVP synthesis or decrease in the ability of organs like the kidney, cloaca or intestines to respond to AVP release with antidiuretic response.
It is expected that urine concentration due to high water reabsorption in the kidney may be reflected in the excreta moisture level. Indeed, the excreta moisture level in the LRWI group was significantly lower than that of the HRWI group. The excreta moisture level could also have significant implications on the ammonia levels and litter moisture content. High litter moisture content could increase the incidence of breast blisters, skin burns, scabby areas, bruising, condemnations and downgrades. Birds that retain more water and have lower RWI may not only assist in reducing water use, but also in improving production costs in the poultry industry. The relationship between water intake, water intake efficiency and excreta moisture should be explored further. Collecting water intake on individual birds is arduous and logistically challenging. Therefore, excreta moisture should be explored as a potential proxy trait for water intake. In a layer study where manure wetness was converted into a subjective score, it was reported that manure score was heritable across different strains (0.14-0.36) (Icken and Presinger, 2010). Zhu et al. (2020) have also reported a heritability of 0.25 to 0.32 for chicken dropping moisture in a Rhode Island Red population. They further identified potential genomic regions associated with manure moisture.
The current study shows that water consumption from 14 to 42 days has a variability of one liter (a range of ∼2 liters). It is estimated that 9.2 billion broilers consuming around 71 billion liters of water are produced in the US annually. If water efficiency is improved by 2 liters, 18.4 billion liters of water will be saved annually in the US from broiler production alone. To put this into perspective, this amount of water will be enough to satisfy the yearly domestic needs in water of around 225,000 individuals in the US (228 liters/day per person) (Scavetta, 2022), 370,000 individuals in Spain (136 liters/day per person) (Fernández, 2022) and over 1.1 million individuals in Africa (47 liters/day per person) (FAO, 2022).
CONCLUSION
The current study has demonstrated the existence of a large variability in water intake in commercial broilers. This variability can be assessed using water conversion efficiency and residual water intake. Water conversion efficiency is confounded with feed intake and growth, whereas residual water intake is not confounded, at least at the phenotypic level. Expression of the AVP gene in the hypothalamus was upregulated in low residual water intake birds compared with their high residual water intake counterparts. There was a concomitant increase in the mRNA expression of AQP2-4 in the water efficient group compared with the inefficient group suggesting an increased water reabsorption capacity in the kidney and collecting duct of the water intake in the efficient birds. This was reflected in the significant difference in excreta moisture levels of the two groups of birds. Data collection on individual water intake is difficult, therefore, excreta moisture level could be considered as a potential proxy trait.
ACKNOWLEDGMENTS
This article is derived from the Subject Data funded in part by the National Academy of Sciences through Sub-award 2000012480 and partly by the Egyptian Scientific Fund STDF EG-US Cycle 20-Project ID 45897 and USAID. Any opinions, findings, conclusions, or recommendations expressed in such article are those of the authors alone, and do not necessarily reflect the views of STDF-EG, USAID or NAS.
DISCLOSURES
No conflict of interest is declared.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2023.102973.
Appendix. Supplementary materials
Supplementary 1. Relationship between (A) feed conversion ratio and water intake; (B) Feed intake and body weight gain
REFERENCES
- Aggrey S.E., Rekaya R. Dissection of Koch's residual feed intake: implications for selection. Poult. Sci. 2013;92:2600–2605. doi: 10.3382/ps.2013-03302. [DOI] [PubMed] [Google Scholar]
- Alltech 2017. https://www.alltech.com/blog/water-most-basic-yet-overlooked-element-poultry-nutrition/. Accessed: March 14, 2023.
- Andersson B., Leksell L.G., Rundgren M. Regulation of water intake. Annual Rev. of Nutr. 1982;2:73–89. doi: 10.1146/annurev.nu.02.070182.000445. [DOI] [PubMed] [Google Scholar]
- Atchley W.R., Gaskins C.T., Anderson D. Statistical properties of ratios. I. Empirical results. Sys. Zool. 1976;25:137–148. [Google Scholar]
- Basso B., Ritchie J.T. Evapotranspiration in high-yielding maize and under increased vapor pressure deficit in the US Midwest. Agric. and Env. Lett. 2018;3 [Google Scholar]
- Benga G. The first water channel protein (later called aquaporin 1) was first discovered in Cluj-Napoca, Romania. Rom. J. Physiol. 2004;41:3–20. [PubMed] [Google Scholar]
- Bennett C.E., Thomas R., Williams M., Zalasiewicz J., Edgeworth M., Miller H., Coles B., Foster A., Burton E.J., Marume U. The broiler chicken as a signal of a human reconfigured biosphere. R. Soc. Open Sci. 2018;5 doi: 10.1098/rsos.180325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichet D.G. Vasopressin and the regulation of thirst. Ann. Nutr. Metab. 2018;72(Suppl. 2):3–7. doi: 10.1159/000488233. [DOI] [PubMed] [Google Scholar]
- Briggs, L. J., and H. L. Shantz. 1913. The Water Requirement of Plants: Investigations in the Great Plains in 1910 and 1911. I. US Department of Agriculture, Bulletin (United States. Bureau of Plant Industry), no. 284. Bureau of Plant Industry. 10.5962/bhl.title.119192. [DOI]
- Casotti G., Waldron T., Misquith G., Powers D., Slusher L. Expression and localization of an aquaporin-1 homologue in the avian kidney and lower intestinal tract. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007;147:355–362. doi: 10.1016/j.cbpa.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Castañeda-Bueno M., Arroyo J.P., Gamba G. Independent regulation of Na+ and K+ balance by the kidney. Med. Prin. Prac. 2012;21:101–114. doi: 10.1159/000332580. [DOI] [PubMed] [Google Scholar]
- Chou C.L., Ma T., Yang B., Knepper M.A., Verkman A.S. Fourfold reduction of water permeability in inner medullary collecting duct of aquaporin-4 knockout mice. Am. J. Physiol. 1998;274:C549–C554. doi: 10.1152/ajpcell.1998.274.2.C549. [DOI] [PubMed] [Google Scholar]
- Deltoro J., López A.M., Camacho J. Seasonal effects on the patterns of deposition of water, fat and protein in rabbit meat. Meat Sci. 1988;23:87–97. doi: 10.1016/0309-1740(88)90017-4. [DOI] [PubMed] [Google Scholar]
- Dunson W.A., Buss E.G. Abnormal water balance in a mutant strain of chickens. Science. 1968;161:167–169. doi: 10.1126/science.161.3837.167. [DOI] [PubMed] [Google Scholar]
- Ecelbarger C.A., Terris J., Frindt G., Echevarria M., Marples D., Nielsen S., Knepper M.A. Aquaporin-3 water channel localization and regulation in rat kidney. Am. J. Physiol. 1995;269:F663–F672. doi: 10.1152/ajprenal.1995.269.5.F663. [DOI] [PubMed] [Google Scholar]
- Essl A. Selection for a ratio of two traits: results of a simulation study. J. Anim. Breed. Genet. 1989;106:81–88. [Google Scholar]
- Famula T.R. The equivalence of two linear methods for the improvement of traits expressed as ratios. Theor. and Appl. Genet. 1990;79:853–856. doi: 10.1007/BF00224256. [DOI] [PubMed] [Google Scholar]
- FAO 2022. https://www.fao.org/livestock-environment/en/. Accessed: March 14, 2023.
- Fernández, L. 2022. https://www.statista.com/statistics/801686/per-capita-daily-water-consumption-in-spain/#:∼:text=Spain%20was%20one%20of%20the,liters%20per%20person%20that%20year Accessed: March 14, 2023.
- Goldstein D.L. Regulation of the avian kidney by arginine vasotocin. Gen. Comp. Endocrinol. 2006;147:78–84. doi: 10.1016/j.ygcen.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Guelinckx I., Vecchio M., Perrier E.T., Lemetais G. Fluid intake and vasopressin: connecting the dots. Ann. Nutr. Metab. 2016;68(Suppl. 2):6–11. doi: 10.1159/000446198. [DOI] [PubMed] [Google Scholar]
- Gunsett F.C. Linear index selection to improve traits defined as ratios. J. Anim. Sci. 1984;59:1185–1193. [Google Scholar]
- Heinz G., Hautzinger P. FAO Regional Office for Asia and the Pacific Publication; 2007. Meat processing technology for small- to medium-scale producers. 2007/20 (ai407e.pdf) (Accessed: March 14, 2023) [Google Scholar]
- Icken W., Preisenger R. Selection of laying hens for improved consistency of excreta. Lohman Info. 2010;45:14–17. [Google Scholar]
- IISD 2020. https://sdg.iisd.org/news/world-population-to-reach-9-9-billion-by-2050/. Accessed: March 14, 2023 2022.
- Jamshidi, N. 2021. https://solsarin.com/how-percentage-of-water-in-earth/. Accessed: March 14, 2023.
- Jequier E., Constant F. Water as an essential nutrient: the physiological basis of hydration. Eur. J Clin. Nutr. 2010;64:115–123. doi: 10.1038/ejcn.2009.111. [DOI] [PubMed] [Google Scholar]
- Kendall M.G., Stuart A. Second Edition. Hafner Publishing; New York: 1963. The Advanced Theory of Statistics. Volume 1. Distribution Theory. [Google Scholar]
- Kim J.K., Summer S.N., Howard R.L., Schrier R.W. Vasopressin gene expression in rats with experimental cirrhosis. Hepatology. 1993;17:143–147. [PubMed] [Google Scholar]
- Koch R.M., Swiger L.A., Chambers D., Gregory K.E. Efficiency of feed use in beef cattle. J. Anim. Sci. 1963;22:486–494. [Google Scholar]
- Lin C.Y. Relative efficiency of selection methods for improvement of feed efficiency. J. Dairy Sci. 1980;63:491–494. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;4:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma T., Yang B., Gillespie A., Carlson E.J., Epstein C.J., Verkman A.S. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J. Clin. Invest. 1997;100:957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Song Y., Yang B., Gillespie A., Carlson E.J., Epstein C.J., Verkman A.S. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc. Natl. Acad. Sci. USA. 2000;97:4386–4391. doi: 10.1073/pnas.080499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel P.C.H. Proceedings of the Fifth International Broiler Nutritionists’ Conference. 2014. Maximizing protein deposition of modern broilers; pp. 230–239. PgISBN 978-0-473-24924-3. [Google Scholar]
- NCC 2022. https://www.nationalchickencouncil.org/statistic/per-capita-consumption-poultry/. Accessed:March 14, 2023.
- Nethery T.N., Boler D.D., Harsh B.N., Dilger A.C. Relationship between Inherent Cooking Rate and Warner-Bratzler Shear Force of Pork Chops Cooked to Two Degrees of Doneness. Foods. 2022;11:131. doi: 10.3390/foods11010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netter J., Wasserman W., Kutner M. McGraw-Hill; New York: 2004. Applied Linear Statistical Model. [Google Scholar]
- Nishimura H., Yang Y. Aquaporins in avian kidneys: function and perspectives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R1201–R1214. doi: 10.1152/ajpregu.00177.2013. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Fan Z. Regulation of water movement across vertebrate renal tubules. Comp. Biochem. Physiol. Part A Mol Integr Physiol. 2003;136:479–498. doi: 10.1016/s1095-6433(03)00162-4. [DOI] [PubMed] [Google Scholar]
- NPR, 2021.https://www.npr.org/2021/06/09/1003424717/the-drought-in-the-western-u-s-is-getting-bad-climate-change-is-making-it-worse Accessed: March 14, 2023.
- Oka Y., Ye M., Zuker C.S. Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature. 2015;520:349–352. doi: 10.1038/nature14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski S., Flees J., Anthony N., Dridi S. Differential expression of water channel- and noncoding RNA biogenesis-related genes in three lines of chickens under a short-term water restriction. Poult. Sci. 2017;96:4172–4181. doi: 10.3382/ps/pex263. [DOI] [PubMed] [Google Scholar]
- Pearson K. Mathematical contributions to the theory of evolution-on a form of spurious correlation which may arise when indices are used in the measurement of organs. Proc. Roy. Soc. (London) 1897;60:489–498. [Google Scholar]
- Preston G.M., Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc. Natl. Acad. Sci. USA. 1991;88:11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekaya R., Aggrey S.E. Genetic properties of residual feed intakes for maintenance and growth and the implications of error measurement. J. Anim. Sci. 2015;93:944–948. doi: 10.2527/jas.2014-8061. [DOI] [PubMed] [Google Scholar]
- Rush E.C. Water: neglected, unappreciated and under researched. Eur. J. Clin. Nutr. 2013;67:492–495. doi: 10.1038/ejcn.2013.11. [DOI] [PubMed] [Google Scholar]
- SAS . SAS Institute; Cary: 2000. SAS User's Guide. Version 12. [Google Scholar]
- Sasaki S., Noda Y. Aquaporin-2 protein dynamics within the cell. Curr. Opin. Nephrol. Hypertens. 2007;16:348–352. doi: 10.1097/MNH.0b013e32818b27bf. [DOI] [PubMed] [Google Scholar]
- Scavetta, A. 2022. Average Water Usage in the United States.https://www.aquasana.com/info/average-water-usage-in-the-united-states-pd.html. Accessed: March 14, 2023.
- Sutherland T.M. The correlation between feed efficiency and rate of gain, a ratio and its denominator. Biometrics. 1965;21:739–749. [PubMed] [Google Scholar]
- Terris J., Ecelbarger C.A., Nielsen S., Knepper M.A. Long-term regulation of four renal aquaporins in rats. Am. J. Physiol. 1996;271:F414–F422. doi: 10.1152/ajprenal.1996.271.2.F414. [DOI] [PubMed] [Google Scholar]
- Williams C., Tabler G., Watkins S.E. Comparison of broiler flock daily water consumption and water-to-feed ratios for flocks grown in 1991, 2000–2001, and 2010–2011. J. Appl. Poult. Res. 2013;22:934–941. [Google Scholar]
- Yang Y., Nishimura H. Bird aquaporins: molecular machinery for urine concentration. Biochim. Biophys. Acta Biomembr. 2021;1863 doi: 10.1016/j.bbamem.2021.183688. [DOI] [PubMed] [Google Scholar]
- Zetouni L., Henryon M., Kargo M., Lassen J. Direct multitrait selection realizes the highest genetic response for ratio traits1. J. Anim. Sci. 2017;95:1921–1925. doi: 10.2527/jas.2016.1324. [DOI] [PubMed] [Google Scholar]
- Zhu T., Zhang T.Y., Wen J., Zhao X., Chen Y., Jia Y., Wang L., Lv X., Yang W., Guan Z., Ning Z., Qu L. The genetic architecture of the chickens dropping moisture by genetic parameter estimation and Genome-Wide Association. Front. Genet. 2020;11:806. doi: 10.3389/fgene.2020.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman C.A., Leib D.E., Knight Z.A. Neural circuits underlying thirst and fluid homeostasis. Nat. Rev. Neurosci. 2017;18:459–469. doi: 10.1038/nrn.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman C.A., Huey E.L., Ahn J.S., Beutler L.R., Tan C.L., Kosar S., Bai L., Chen Y., Corpuz T.V., Madisen L., Zeng H., Knight Z.A. A gut-to-brain signal of fluid osmolarity controls thirst satiation. Nature. 2019;568:98–102. doi: 10.1038/s41586-019-1066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary 1. Relationship between (A) feed conversion ratio and water intake; (B) Feed intake and body weight gain