Abstract
Both early (ELA) and recent life adversity (RLA) have been linked with chronic pain conditions and persistent alterations of neuroendocrine and inflammatory responses. Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is a chronic urologic disorder characterized by bladder and/or pelvic pain, and excessive urinary frequency and/or urgency. IC/BPS has been associated with high levels of ELA as well as a distinct inflammatory signature. However, associations between ELA and RLA with inflammatory mechanisms in IC/BPS that might underlie the link between adversity and symptoms have not been examined. Here we investigated ELA and RLA in women with IC/BPS as potential risk factors for inflammatory processes and hypothalamic-pituitary-adrenal (HPA) abnormalities using data from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. Women with IC/BPS and healthy controls (n=154 and 32, respectively) completed surveys, collected salivary cortisol at awakening and bedtime for 3 days, and gave a blood sample which was analyzed for 7 LPS-stimulated cytokines and chemokines (IL-6, TNFα, IL-1β MIP1α, MCP1, IL-8, and IL-10). Two cytokine/chemokine composites were identified using principal components analysis. Patients with greater exposure to RLA or cumulative ELA and RLA of at least moderate severity showed elevated levels of a composite of all cytokines, adjusting for age, body mass index, and study site. Furthermore, there was a trending relationship between ELA and the pro-inflammatory composite score. Nocturnal cortisol and cortisol slope were not associated with ELA, RLA, or inflammation. The present findings support the importance of adverse events in IC/BPS via a biological mechanism and suggest that ELA and RLA should be assessed as risk factors for inflammation as part of a clinical workup for IC/BPS.
Keywords: Early life adversity, recent life adversity, chronic stress, interstitial cystitis, inflammation, IC/BPS, pain, HPA axis, cortisol
1. INTRODUCTION
Early life adversity (ELA) has been associated with multiple chronic pain conditions and ongoing alterations of neuroendocrine and inflammatory responses that persist into adulthood (Baumeister et al., 2016; Finegood and Miller, 2022; Nusslock and Miller, 2016; Schrepf et al., 2018). Recent life adversity (RLA) and ongoing chronic stressors have also been associated with neuroendocrine and inflammatory alterations (Kiecolt-Glaser et al., 1998; Sun et al., 2021; Tursich et al., 2014), chronic pain (Hannibal and Bishop, 2014; Timmers et al., 2019) and other disease syndromes (Slavich, 2016). Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is a chronic debilitating urologic disorder primarily diagnosed in women, characterized by bladder and/or pelvic pain, excessive urinary frequency, and/or urgency (Clemens et al., 2019). IC/BPS affects approximately 1.08% (CI: 0.03, 2.13) of the adult US female population (Anger et al., 2022) and thus is a prevalent healthcare issue (Konkle et al., 2012). Previous findings from the NIDDK-funded Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network indicated that study participants with IC/BPS have higher reported levels of ELA than healthy controls (Gupta et al., 2019; Schrepf et al., 2018). Additionally, in IC/BPS, higher levels of ELA were related to worse symptom levels and prognosis, including complex chronic pain, less likelihood of improvement in pain over time, worse overall functioning, greater sensitivity to local but not distal pain, and alterations in brain connectivity (Gupta et al., 2019; Pierce et al, 2023.; Schrepf et al., 2018).
ELA has been shown to induce a host of changes that ultimately impact the development of the organism’s neurobiological stress response and shape the development of immune and inflammatory responses, particularly in monocytes (Finegood and Miller, 2022; Nusslock and Miller, 2016). Stress has a priming effect on monocytes in the brain such that subsequent encounters with a pathogen will induce an enhanced inflammatory response (Frank et al., 2007; Frank et al., 2011). ELA is also thought to amplify crosstalk between threat-related neural circuitries and peripheral inflammation resulting in chronic low-grade inflammation (Nusslock and Miller, 2016). Additionally, ELA has been associated with reductions in the number of glucocorticoid receptors (GR), thus dampening the ability of GRs to respond to cortisol and regulate inflammation, resulting in elevated cortisol levels in response to stress along with chronic HPA dysregulation (Liu and Nusslock, 2018). RLA events have also been associated with immune and neuroendocrine alterations including elevations in circulating catecholamines and glucocorticoids (Chen and Miller, 2012; Hänsel et al., 2010; Karlamangla et al., 2019), decreases in glucocorticoid sensitivity (Cohen et al., 2012), and changes in inflammatory biology (Hänsel et al., 2010; Sun et al., 2021). The cumulative contribution of ELA and RLA has also been recognized (Hostinar et al, 2015). Taken together, these pathways point to persistent dysregulations in the stress response, heightened inflammation and impaired glucocorticoid inflammatory control from both ELA and RLA, along with potentially enhanced inflammatory responsivity when someone with ELA also encounters a subsequent life stressor.
We have previously reported that patients with IC/BPS have a distinctive inflammatory physiology, including a greater ex-vivo inflammatory responsivity to stimulation by Toll-like Receptor-2 (TLR-2) ligands than healthy controls, and greater Toll-like Receptor-4 (TLR-4) responsivity to stimulation with lipopolysaccharide (LPS) (Schrepf et al., 2014). This inflammatory sensitivity in IC/BPS is associated with more widespread pain symptoms (Schrepf et al., 2014), and less improvement in genitourinary symptoms and urinary pain over time (Schrepf et al., 2016). The TLR-4 response particularly has shown relationships with the presence/degree of comorbid pain in both MAPP Network cohort studies, representing one of the first replicable biological markers of disease patterns in IC/BPS (Schrepf et al., 2015; Schrepf et al., 2022).
Compared to healthy controls, female IC/BPS patients also have higher levels of nocturnal cortisol and flatter cortisol slopes, indicative of a blunted diurnal cortisol rhythm and suggestive of poorer inflammatory control (Schrepf et al., 2014). Higher nocturnal cortisol was associated with greater pain in women with IC/BPS (Lutgendorf et al., 2002) and flatter cortisol slopes were associated with less improvement in pain over time (Schrepf et al., 2016). Additionally, a synergistic relationship between cortisol, inflammation, and pain was observed, such that female IC/BPS patients with the highest levels of inflammation combined with the flattest cortisol slopes had the highest level of painful symptoms (Schrepf et al., 2014).
Associations between ELA and RLA with inflammatory mechanisms that might be responsible for the link between adversity and symptoms shown in the literature have not been examined in IC/BPS. Following our recent publication showing relationships between widespread pain, chronic overlapping pain conditions, and TLR-4 inflammatory responses (Schrepf et al., 2022), we focus here on the relationship between life adversity and these TLR-4 stimulated inflammatory markers. We examined whether ELA and/or RLA in women with IC/BPS serve as potential risk factors for enhanced inflammatory responsivity and HPA abnormalities. We examined history of ELA and RLA in a population of women presenting with IC/BPS in conjunction with a panel of TLR-4 ex-vivo stimulated inflammatory cytokines and diurnal cortisol levels to determine whether a link between adversity, inflammation, and cortisol regulation would be observed. The relationship between trauma and ex-vivo inflammatory responses in healthy control women was also examined for comparison. Further delineation of these underlying processes is important for understanding risk factors for inflammation, symptoms, and prognosis and for developing targeted clinical interventions.
2. METHODS
2.1. MAPP Network Study and Participant Characteristics
Patients and healthy controls were recruited as part of the second phase study of the multi-site MAPP Research Network (MAPP II; ClinicalTrials.gov Identifier: NCT02514265-MAPP Research Network: The Trans-MAPP Symptom Patterns Study [SPS]) that examined symptoms, clinical variables, and psychosocial factors prospectively and longitudinally (Clemens et al., 2020). A detailed description of the MAPP protocol is available (Clemens et al., 2020). The protocol was approved by the institutional review boards of all participating sites. At the week 4 “baseline” phenotyping visit, we evaluated a subset of female IC/BPS patients (n=155) and healthy female controls (n=32). These patients fulfilled inclusion criteria to participate in the SPS protocol (described in Clemens et al., 2020) and had completed collection of blood for assessment of inflammatory markers; most had also completed collection of salivettes for measurement of diurnal cortisol. Funding limitations precluded analysis of all collected samples in MAPP II. For one IC/BPS participant, inflammatory markers were not viable, leaving a final sample of 154 patients. We have previously reported that there were no clinical or demographic differences between the biomarker sample and the rest of the MAPP SPS baseline cohort (Schrepf et al., 2022).
2.2. Early and Recent Life Adversity
Early and Recent Life Adversity were assessed using the Childhood Traumatic Events Scale (CTES) and the Recent Traumatic Events Scale (RTES) (Pennebaker and Susman, 1988). Respondents are asked to indicate whether any of 6 types of adversity occurred before age 17 (death of friend or family, parental divorce or separation, traumatic sexual experience, victim of violence, extremely ill or injured, and other major upheaval), age at the time of the event, whether they confided in anyone, and subjective experience of how traumatic it was from 1 (not at all) to 7 (extremely traumatic). Recent life adversity (within the last 3 years) was assessed by asking whether an event occurred, how traumatic it was, and extent of confiding in others. Categories of recent adversity include death of friend or family member, divorce or separation or major upheaval in intimate relationship, traumatic sexual experience, victim of violence, extremely ill or injured, major change in work, and other major upheaval. There is no standardized scoring for the CTES. Traumatic events have been examined using either number of different types of trauma events or severity of events, or both. (e.g., Schrepf et al., 2018; Hostinar et al, 2015). Because of the literature on the effects of repeated traumatic events on inflammation and on neural circuitry (Frank et al., 2010; Frank et al., 2011; Nusslock and Miller, 2016) and findings indicating effects of cumulative trauma at moderate to severe levels (Steine et al, 2017) we implemented a measure that included both number and severity. We focused on number of events rated as at least moderately traumatic as our primary independent variable, reasoning that an event needed to be rated as at least somewhat traumatic to have an impact on the relevant biological systems. For both ELA and RLA, number of experiences rated ≥ 4 (somewhat traumatic) were summed to create an index of adverse events of at least moderate severity for each domain (ELA or RLA) separately, and then total number of ELA and RLA rated ≥ 4 were summed to give a measure of cumulative adversity. The exposure variables used in primary analyses were 1) number ELA of at least moderate severity, 2) number RLA of at least moderate severity, and 3) combined number of ELA and RLA of at least moderate severity.
For parsimony these are referred to as ELA, RLA, and cumulative adversity. To further support our findings, we conducted supplemental analyses examining 1) total number of traumatic experiences of any intensity and 2) total severity of trauma at each timepoint. Severity was calculated as previously reported (Schrepf, 2018) by summing the severity ratings for each category of trauma at each timepoint (early, recent, and cumulative). These analyses are reported in the supplement and are designated as ELA, RLA, and cumulative number of traumas of any severity, and ELA severity, RLA severity, and cumulative severity.
2.3. Inflammatory markers
The methods used to collect and analyze stimulated samples have been described in detail elsewhere (Schrepf et al., 2022). In brief, whole blood was collected and stimulated using the TruCulture system (Myriad RBM), an ex-vivo stimulation system. Vacutainers preloaded with LPS were kept frozen at −20° C until use. Whole blood was drawn directly into the tube and incubated at 37° for 24 hours. Following incubation, the supernatant was isolated and stored at −80° C and later analyzed in batches by the MAPP Tissue Analysis and Technology Core (TATC) at the University of Colorado Anschutz Medical Campus Pathology Shared Resource. For analysis, the supernatant was thawed and analyzed for seven cytokines using Luminex® Xmap technology with R&D systems high performance assays. The cytokines included monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1-α (MIP1α), tumor necrosis factor -α (TNF-α), and interleukin (IL)-1β, IL- 6, IL-8, IL-10 (Schrepf et al., 2022). Intra-assay coefficients of variability were as follows: IL-10: 3.0%−3.9%; IL-1β: 4.7%−5.4%; IL-6: 6.0%−10.9%; IL-8: 3.1%−13.0%; IFNγ: 3.0%−12.9%; MCP-1: 11.3%−4.3%; MIP-1α: 2.4%-1.6%; TNF-α: 5.5%−7.4%. Inter-assay coefficients of variability were as follows: IL-10: 4.5%-8.2%; IL-1β: 5.0%−7.0%; IL-6: 5.9%−12.5%; IL-8: 4.2%−19.2%; IFNγ: 4.9%−10.6%; MCP-1: 8.8%−15.0%; MIP-1α: 1.6%−2.4%; TNF-α: 4.0%−17.1%. These specific cytokines and chemokines represent various components of the inflammatory process that are part of the cellular response to LPS stimulation and are upregulated by NFκ-B, including pro-inflammatory cytokines (i.e. TNF-α, IL-1β, IL-6), chemotactic (MCP-1, MIP-1α, IL-8) and regulatory (IL-10) (Lu et al., 2008).
To derive a composite measure of TLR-4 inflammation, we performed a principal components analysis based on the correlation matrix between the seven transformed (Box-Cox [Box and Cox, 1964]) cytokine/chemokine values, retaining components with an eigenvalue greater than one (Grice and Harris, 1998). Factor scores for the resulting components were then extracted by the regression method.
2.4. Cortisol collection
Participants collected salivary cortisol using salivettes (Sarstedt) at two timepoints (upon wakening: 4–9 am, and at bedtime: 8pm-12pm) for three consecutive days following the baseline visit. To maintain homogeneity, samples collected outside this time frame were excluded from analyses. Use of an individual’s own waking and bedtimes in sample collection has been shown to better approximate diurnal cortisol rhythms than mandating a scheduled time of collection for all participant (Kraemer et al., 2006). Participants were instructed to avoid eating, exercise, or caffeine during the 30 minutes prior to sample collection. Sample collection time was self-reported, a technique that has been shown to be reliable in previous studies (Kraemer et al., 2006). Salivettes were collected from each site, sent to the MAPP TATC, and analyzed in one batch by chemiluminescence immunoassay (IBL, Hamburg, Germany) at the Technical University of Dresden. Inter-assay and intra-assay coefficients of variance are less than 10% and the lower detection limit is 0.41 nmol/L.
2.5. Statistical Analyses
Analyses of Variance (ANOVAs) and Chi squared analyses were used to compare demographic and trauma information of IC/BPS patients and healthy controls. Mann-Whitney U tests were used to compare LPS-stimulated cytokines and cortisol measures between IC/BPS patients and controls. Mixed-effects linear models were used to analyze the relationship between the trauma variables and TLR-4 mediated inflammation in IC/BPS patients and in healthy controls. The PCA factor scores were used as dependent variables in models with ELA, RLA, and cumulative adversity scores as the independent variables. For cortisol analyses, regressions of the cortisol variables with ELA, RLA, and cumulative adversity, and with the two inflammatory composites were conducted. Age and body mass index (BMI) are known to be associated with inflammatory and cortisol dynamics (Frasca et al., 2017; Purnell et al., 2004; Yiallouris et al., 2019) and so were included as a priori covariates in all regressions. A random intercept term was included for site of collection in all analyses. Supplemental analyses examining the independent variables of total trauma severity (early, recent, cumulative) and total number of traumas of any severity (early, recent, both) were conducted and are reported in Supplemental Tables 1 and 2.
3. RESULTS
3.1. Demographic characteristics and adversity
As seen in Table 1, the mean age of women with IC/BPS was 43.4 years (range 18–78 years) and the majority (93.5%) were Non-Hispanic and White (89%). Over 70% of the sample was college educated. There were no significant differences between women with IC/BPS and healthy controls on demographic variables, with the exception of a significantly greater proportion of non-white women (34.3%) in the control participants compared to the IC/BPS participants (11%). Women with IC/BPS on average had been exposed to 1.63 ELA events of at least moderate severity and 1.37 RLA events of at least moderate severity, with a cumulative exposure to a mean of 3 events of at least moderate severity (cumulative adversity). Overall, as reported previously (Gupta et al., 2019; Schrepf et al., 2018) healthy controls had significantly less trauma exposure than women with IC/BPS (all p values < 0.019). For example, the total trauma severity (ELA and RLA combined) in controls (9.53 ± 6.39) was approximately half that reported by women with IC/BPS 18.63 (± 8.56). Additionally, as seen in Table 2, levels of TLR4 immunoreactivity did not differ significantly between patients and controls (all p values > .05).
Table 1.
Demographic, Clinical, and Adversity Variables in Women with IC/BPS and Healthy Controls
| Variable | IC/BPS (n=154 | Healthy Controls (n=32) | p value |
|---|---|---|---|
|
| |||
| Age: years, Mean (SD) | 43.4 (15.38) | 40.86 (14.99) | P = 0.39 |
| Body Mass Index: Mean (SD) | 26.97 (5.63) | 28.28 (5.99) | P = 0.24 |
| Employment % , (n=154, 32) | % (n) | % (n) | P = 0.17 |
| Employed | 66.23 (102) | 87.5 (28) | |
| Unemployed | 11.68 (18) | 6.25 (2) | |
| On Disability | 13.64 (21) | 3.12 (1) | |
| Retired | 3.90 (6) | 3.12 (1) | |
| Full Time Homemaker | 4.55 (7) | 0 (0) | |
| Family Income Level/$ % , (n=154, 32) | P =0.100 | ||
| < $10,000 | 5.84 (9) | 3.12 (1) | |
| $10,001-$25,000 | 10.39 (16) | 3.12 (1) | |
| $25,001-$50,000 | 18.83 (29) | 31.25 (10) | |
| $50,001-$100,000 | 31.17 (48) | 37.5 (12) | |
| >$100,000 | 20.13 (31) | 21.88 (7) | |
| Prefer not to answer | 13.64 (21) | 3.12 (1) | |
| Education Level % , (n =154, 32) | P =0.177 | ||
| High-School | 5.84 (9) | 3.12 (1) | |
| Some College | 20.78 (32) | 18.75 (6) | |
| College Degree | 50.00 (77) | 40.63 (13) | |
| Graduate Degree | 23.38 (36) | 37.5 (12) | |
| Ethnicity %, (n=153, 32) | P =0.183 | ||
| Hispanic | 5.84 (9) | 12.5 (4) | |
| Non-Hispanic | 93.51 (144) | 87.5 (28) | |
| Race % , (n) | P = 0.012 | ||
| White | 88.96 (137) | 65.63 (21) | |
| Black | 6.49 (10) | 21.88 (7) | |
| Native American | 0.65 (1) | 0 (0) | |
| Asian | 1.30 (2) | 6.25 (2) | |
| Multi-Racial | 2.60 (4) | 6.25 (2) | |
| Number of ELA of ≥ Moderate Severity Mean, (SD) | 1.63 (140) | 1.00 (113) | P = 0.018 |
| Number RLA of ≥ Moderate Severity Mean, (SD) | 1.37 (114) | 0.56 (0.67) | P < .001 |
| Cumulative Number ELA and RLA of Moderate Severity, Mean, (SD) | 3.00 (2.00) | 1.56 (1.43) | P < .001 |
| Total ELA Severity Mean, (SD) | 9.89 (8.56) | 5.81 (6.39) | P = 0.012 |
| Total RLA Severity Mean, (SD) | 8.74 (6.80) | 3.72 (3.92) | P < .001 |
| Total Both Severity Mean, (SD) | 18.63 (12.07) | 9.53 (8.13) | P < .001 |
| Number any severity ELA | 1.90 (142) | 1.22 (126) | P <=.015 |
| Mean, (SD) | |||
| Number any severity RLA | 1.92 (1.21) | 1.06 (1.00) | P< .001 |
| Mean, (SD) | |||
| Cumulative Number ELA and RLA of any severity Mean, (SD) | 3.82 (2.10) | 2.29 (1.75) | P < .001 |
Table 2.
LPS-stimulated cytokine and Cortisol values in female participants with IC/BPS and female healthy community participants. There were no differences in levels of stimulated analytes or cortisol measures between groups by Mann-Whitney U tests (all p> .05).
| IC/PBS (n=154) | Healthy Controls (N=32) | |||
|---|---|---|---|---|
| Cytokine (Stimulated: pg/ML) | Median | 25th – 75th Percentile | Median | 25th – 75th Percentile |
| Monocyte chemoattractant protein-1 (MCP-1) | 1524.65 | 985.18 – 2313.23 | 1520.90 | 1059.18–2143.93 |
| Macrophage inflammatory protein 1-alpha (MIP1α) |
44443.55 | 33012.63 – 67268.13 | 60742.80 | 35883.1077066.73 |
| Interleukin-1 beta (IL-1β) | 8311.30 | 4157.50 – 12244.05 | 7949.60 | 3419.35–11966.80 |
| Interleukin-6 (IL-6) | 19244.70 | 13294.00 – 24990.30 | 20156.00 | 14892.5023882.25 |
| Interleukin-8 (IL-8) | 12451.10 | 8926.20 – 19456.20 | 11846.30 | 8661.50–17428.55 |
| Interleukin-10 (IL-10) | 64.80 | 35.35 – 94.40 | 58.31 | 24.85–82.90 |
| Tumor necrosis factor-alpha (TNF-α) | 3910.10 | 2540.40 – 5480.30 | 4002.90 | 2778.70–5949.90 |
| Cortisol | ||||
| Morning mean nmol/L (n=103) | 7.55 | 5.11 – 9.86 | 7.75 | 5.22–9.98 |
| Nocturnal nmol/L (n=118) | 0.92 | .64 −1.55 | 1.11 | .80–2.17 |
| Slope (n=97) | −0.148 | −.18 - −0.09 | −0.123 | −0.185- −0.057 |
Note: All values are raw values.
pg/mL= picograms/liter nmol/L= nanamole/liter
3.2. Relationship between trauma and inflammation
Descriptive statistics for inflammatory markers and cortisol levels are shown in Table 2. The PCA analysis identified two components of the inflammatory markers. The first had high loadings on all seven cytokines and explained 61% of the variance in the cytokine values. This was used as the “TLR-4 global inflammatory cytokine composite score” for further analyses. A second factor, which showed a strong positive loading for IL-10 and strong negative loadings for IL-1β, IL-6 and TNF-α explained an additional 19% of the variance and was used in subsequent analyses as an additional outcome. It is described as the “TLR-4 anti-inflammatory/regulatory/chemotactic composite score”.
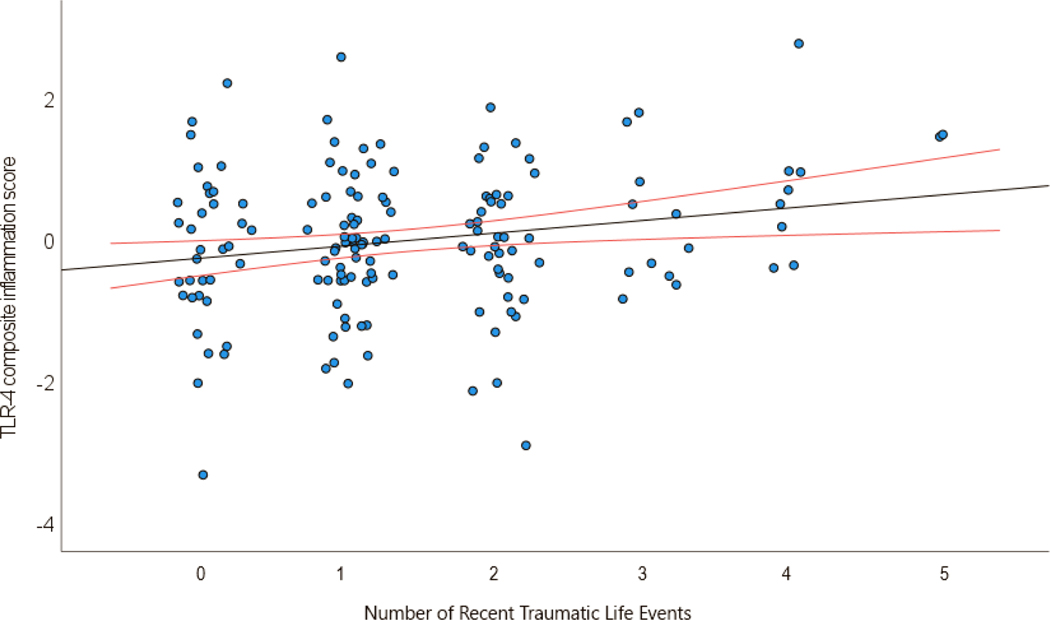
In models controlling for age, BMI, and site of collection, a greater number of ELA events of moderate or greater severity showed a trending relationship to higher levels of the TLR-4 inflammatory cytokine composite score (p = 0.067). In parallel models there was a statistically significant relationship between a greater number of RLA events of moderate or greater severity and a higher inflammatory cytokine composite score (p = 0.036), and between cumulative adversity and a higher inflammatory cytokine composite score (p = 0.013). (See Table 3A for model parameters and Fig. 1).
Table 3A.
Relationships between number of Trauma Exposures of moderate or greater severity and the TLR-4 global inflammatory cytokine composite score.
| Est | SE | DF | t value | P | 95% Cl lower limit | 95% Cl upper limit | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| (Intercept) | −0.146 | 0.458 | 75.66 | −0.320 | 0.750 | −1.028 | 0.738 |
| Age | 0.000 | 0.005 | 147.40 | −0.086 | 0.932 | −0.010 | 0.009 |
| BMI | −0.003 | 0.014 | 147.70 | −0.190 | 0.849 | −0.030 | 0.025 |
| ELA events | 0.102 | 0.055 | 147.90 | 1.847 | 0.067 | −0.007 | 0.209 |
| (Intercept) | −0.259 | 0.458 | 87.79 | −0.565 | 0.574 | −1.142 | 0.628 |
| Age | −0.001 | 0.005 | 147.90 | −0.156 | 0.876 | −0.011 | 0.009 |
| BMI | 0.002 | 0.014 | 147.40 | 0.130 | 0.897 | −0.025 | 0.029 |
| RLA events | 0.140 | 0.066 | 146.60 | 2.121 | 0.036 | 0.012 | 0.270 |
| (Intercept) | −0.273 | 0.458 | 80.69 | −0.597 | 0.552 | −1.155 | 0.612 |
| Age | 0.000 | 0.005 | 147.50 | −0.025 | 0.980 | −0.010 | 0.010 |
| BMI | −0.003 | 0.014 | 147.30 | −0.186 | 0.853 | −0.030 | 0.025 |
| Combined ELA/RLA events | 0.095 | 0.038 | 146.20 | 2.507 | 0.013 | 0.021 | 0.168 |
Note: Degrees-of-freedom estimated using Satterthwalte approximation in fitted Mixed effects models.
Figure 1.
Relationship of TLR-4 composite inflammation score (derived using principal components analysis) with number of recent traumatic events. Red lines represent 95% confidence intervals.
In analogous models with the TLR-4 anti-inflammatory/regulatory/chemotactic score as the dependent variable, there was no statistically significant relationship between ELA and the chemotactic/regulator score (p=0.107). However, both RLA (p=0.029) and cumulative adversity (p=0.018) were positively associated with the anti-inflammatory/regulatory/chemotactic score. (See Table 3B).
Table 3B.
Parameter estimates for the relationship between Trauma Exposures of moderate or greater severity and the TLR-4 anti-inflammatory/regulatory/chemotactie composite score.
| Est | SE | DF | t value | P | 95% Cl lower limit | 95% Q upper limit | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| (Intercept) | −1.180 | 0.439 | 122.17 | −2.685 | 0.008 | −2.063 | −0.336 |
| Age | 0.004 | 0.005 | 149.52 | 0.753 | 0.453 | −0.006 | 0.014 |
| BMI | 0.033 | 0.014 | 148.90 | 2.305 | 0.023 | 0.005 | 0.061 |
| ELA events | 0.091 | 0.056 | 149.10 | 1.623 | 0.107 | −0.017 | 0.203 |
| (Intercept) | −1.300 | 0.446 | 115.97 | −2.913 | 0.004 | −2.183 | −0.443 |
| Age | 0.004 | 0.005 | 149.23 | 0.691 | 0.490 | −0.006 | 0.014 |
| BMI | 0.037 | 0.014 | 147.91 | 2.617 | 0.010 | 0.009 | 0.064 |
| RLA events | 0.147 | 0.067 | 147.18 | 2.199 | 0.029 | 0.016 | 0.278 |
| (Intercept) | −1.308 | 0.441 | 122.33 | −2.965 | 0.004 | −2.193 | −0.460 |
| Age | 0.004 | 0.005 | 149.50 | 0.830 | 0.408 | −0.006 | 0.015 |
| BMI | 0.033 | 0.014 | 148.27 | 2.315 | 0.022 | 0.005 | 0.060 |
| Combined ELA/RLA events | 0.093 | 0.039 | 147.07 | 2.401 | 0.018 | 0.018 | 0.169 |
Note: Degrees-of-freedom estimated using Satterthwaite approximation in fitted Mixed effects models.
3.3. Supplemental Analyses
3.3.1. Total number of traumas of any severity and inflammation.
To ascertain whether total number of adverse events regardless of severity was a predictor of inflammation, supplemental analyses were performed. For ELA, number of traumas of any severity was not associated with either the inflammatory composite score or the anti-inflammatory/regulatory score (p values > 0.10). For RLA, there was no relationship between total number of traumas and the pro-inflammatory score (p > 0.10), but total number of traumas trended towards a positive association with the TLR-4 regulatory score (p=0.056). Moreover, a greater number of cumulative (ELA plus RLA) traumas of any severity predicted significantly higher TLR-4 inflammatory cytokine composite scores (p=0.043) and TLR-4 anti-inflammatory/regulatory chemotactic scores (p=0.034). (Table S1A and S1B).
3.3.2. Trauma severity and inflammation.
The association between trauma severity and inflammation was similar but even more pronounced. Specifically, supplemental analyses demonstrated that greater RLA severity (p=0.04) and greater combined severity of ELA and RLA trauma (p=0.015) were related to significantly higher levels of both the TLR-4 inflammatory cytokine composite score and the TLR-4 regulatory score (RLA severity: p=0.021; combined ELA and RLA severity: p=0.028). ELA severity trended in the same direction for the inflammatory composite (p=0.07) but was not significant and was not significantly related to the regulatory composite. (Tables S2A and S2B).
3.4. Cortisol, Adversity, and Inflammation
There were no significant associations between exposure to ELA, RLA, or cumulative adversity of at least moderate severity and either cortisol slope or night cortisol, adjusting for age, BMI, and site of collection (all p values > 0.13). Supplemental analyses indicated that there were no significant associations between total number of trauma exposures of any severity and either cortisol slope (all p values > 0.31) or night cortisol (all p values > 0.65) at any timepoint. Additionally, there were no significant associations between severity of trauma exposure and cortisol slope (all p values > 0.20) or night cortisol (all p values > 0.62) at any timepoint. Additionally, there was no relationship between cortisol measures and either the global inflammatory cytokine composite score (p> 0.74) or the anti-inflammatory/regulatory/chemotactic composite score (p > 0.058), adjusting for age, BMI, and site.
3.4. Trauma and Inflammation in Healthy Controls
In healthy controls, there was no relationship between trauma exposure at any timepoint (early, recent, cumulative) and either inflammatory composite, adjusting for covariates as above. This was true for the primary analyses examining number of traumas of at least moderate severity (inflammatory composite: all p values > 0.83; regulatory composite: all p> 0.25), as well as for supplemental analyses examining total number of traumas of any severity, and total trauma severity (all supplemental p values > 0.31). There were too few healthy controls with complete cortisol data to reliably calculate relationships between trauma and cortisol variables.
4. DISCUSSION
The key results of this study were that among women with IC/BPS, those with higher levels of recent life adversity (RLA) of at least moderate intensity showed elevated levels of a global TLR-4 inflammatory cytokine composite, adjusting for age, BMI, and site. Additionally, greater cumulative adversity (levels of ELA and RLA of at least moderate intensity combined) was also related to elevated levels of the inflammatory cytokine composite, suggesting a cumulative effect of life stressors. There was an analogous trending association between extent of early life events (ELA) and higher levels of this inflammatory composite. Similar relationships were observed between life adversity and the anti-inflammatory/regulatory/chemotactic composite, whereby higher levels of RLA or of cumulative adversity were associated with significantly higher levels of this second composite variable, but ELA was not. Supplemental analyses largely confirmed the associations seen in our primary analyses, with severity of RLA and cumulative severity both showing relationships with composite inflammatory measures. There were also broadly similar relationships between inflammatory composites and trauma of any severity. Levels of nocturnal cortisol and cortisol slope were not associated with either ELA or RLA or their combination, or with inflammation. These relationships were also not observed in healthy controls.
Findings are consistent with data on elevated inflammation accompanying RLA and repeated trauma (Hänsel et al., 2010; Sun et al., 2021) as well as with research documenting relationships between ELA and higher levels of inflammation in adulthood (Baumeister et al., 2016; Miller et al., 2011; Nusslock and Miller, 2016). Interestingly, the present findings ascribed a stronger role for recent and cumulative life adversity in relation to inflammation than to ELA in the context of women with IC/BPS. These findings are consistent with the known effects of psychological stress in increasing the expression of NFκB in immune cells, thereby leading to an increase in the level of pro-inflammatory cytokines (Gu et al., 2012; Hänsel et al., 2010). In animal models, social defeat, used as a proxy for traumatic victimization, has been associated with mobilization of pro-inflammatory monocytes into peripheral circulation (Engler et al., 2005; Wohleb et al., 2012); these cells induce an exaggerated inflammatory cytokine response coupled with a blunted sensitivity to inhibitory glucocorticoid signaling, resulting in an amplified inflammatory response (Liu and Nusslock, 2018; Miller et al., 2011). Both acute and chronic stress have been shown to sensitize neuroinflammatory responsivity to subsequent immune challenges (Frank et al., 2010; Wohleb et al., 2012) and chronic stress has been shown to induce reprogramming of the monocyte transcriptome, which primes myeloid cells to be hyperresponsive to stimulation with ligands such as TLR4 (Barrett et al., 2021). Additionally, ELA is known to modify threat- and reward- related neural circuitry to increase responsivity to subsequent behavioral stressors (Finegood and Miller, 2022; Nusslock and Miller, 2016; Uchida et al., 2010). This may explain not only the associations of RLA with inflammation, but the elevations seen in women who had experienced both ELA and RLA. It is possible that ELA may have provided an initial stimulus to modulate the neuroinflammatory system, but that a second stimulus in adulthood from a chronic stressor, recent life event, or recent urinary tract infection, served to stimulate an amplified or sustained inflammatory response in patients with cumulative adversity.
The chemokines and regulatory cytokines in the second composite are NFκB responsive, as well as stress responsive (Cohen et al., 2011; Madrigal et al., 2010; Marsland et al., 2017; Shahzad et al., 2010) and thus serve as additional indicators of stress-induced responsivity. Although IL-10 serves as a regulatory anti-inflammatory cytokine (Steen et al., 2020), its levels are also increased by stress (Curtin et al., 2009; Marsland et al., 2017) and by IL-6 (Steensberg et al., 2003), and at the time-scale of the stimulation protocol (24 hours) is likely reflective of the response to pro-inflammatory processes.
The role of these cytokines in relation to nociplastic pain in IC/BPS is important, as pro-inflammatory cytokines, mediated by microglial and neural activity in the brain and spinal cord, are known to exacerbate pain and hyperalgesia in both animal and human studies (Qi et al., 2016; Zhao et al., 2019). The relationship between pro-inflammatory cytokines and hyperalgesia has been documented for conditions like IBS and chronic prostatitis (Alexander et al., 1998; Choghakhori et al., 2017) and an inflammatory cytokine profile has been previously implicated in IC/BPS pain in previous work from our group (Schrepf et al., 2015; Schrepf et al., 2014; Schrepf et al., 2022). The current findings extend this previous work by showing a potential mechanism underlying inflammation.
Levels of TLR-4 immunoreactivity did not differ between patients and controls, mirroring the same findings in the MAPP Epidemiology and Phenotyping Study (EPS), where only TLR-2 reactivity differentiated patients from controls (Schrepf et al., 2015, BBI). As we have now shown in both the MAPP EPS and SPS, TLR-4 immunoreactivity appears to be elevated in patients with chronic overlapping pain conditions and/or widespread pain when compared to patients with localized pain (Schrepf et al., 2015, BBI; Schrepf et al., 2022, PAIN). These findings suggest that TLR-4 immunoreactivity tracks with the development of nociplastic pain symptoms in IC/BPS patients. The fact that there was no relationship in healthy controls between trauma variables and TLR-4 immunoreactivity suggests that the relationship between trauma and elevated TLR-4 response effect is likely limited to patients. However, the relatively low levels of trauma in controls and the small sample size of controls leave this to be determined definitively.
The lack of relationship between ELA or RLA and levels of diurnal cortisol was surprising in light of previous findings of dysregulated cortisol secretion (Karlamangla et al., 2019) and/or glucocorticoid resistance (Liu and Nusslock, 2018; Nusslock and Miller, 2016; Ouellet-Morin et al., 2011; Slopen et al., 2013) related to ELA, RLA (Cohen et al., 2012) and IC/BPS (Lutgendorf et al., 2002; Schrepf et al., 2014). Since this study only assessed diurnal cortisol rhythms and not parameters such as glucocorticoid sensitivity, HPA recovery from stress, or the response of the glucocorticoid system to acute stress, it is quite possible that we may not have captured alterations in glucocorticoid signaling accompanying life stress that would have been observed through other types of assessments.
4.1. Limitations
Assessment of the glucocorticoid system was limited by what could be readily completed and replicated in a multisite setting, which precluded stress reactivity protocols or assessment of glucocorticoid sensitivity which may have yielded more sensitive and dynamic assessments of relevant aspects of the glucocorticoid system. There is no standard scoring of the CTES, potentially limiting the reliability of our scoring. Our primary adversity variable was a combination of number and severity, however the robustness of our findings is supported by supplemental analyses examining both number of adverse events of any severity and total severity at any timepoint (ELA, RLA, cumulative). Assessments of adversity were limited, and subject to retrospective recall and subjective evaluations of severity.
4.2. Clinical Implications
This study establishes a relationship between RLA and cumulative adversity and inflammation in women with IC/BPS and notes a similar but less strong association between ELA and inflammation. These findings strengthen the importance of adverse events in IC/BPS through a biological mechanism. While there is extensive literature on ELA, inflammation, and pain in other contexts, the present findings indicate that levels of current and chronic life stress should be assessed along with ELA as potential risk factors for inflammation, widespread pain, and potentially poorer prognosis as part of a clinical workup for IC/BPS. Future research should examine potential benefits for these patients from anti-inflammatory therapy and therapy targeting central mechanisms.
Supplementary Material
Highlights.
This study establishes a relationship between recent life adversity, cumulative adversity, and inflammation in women with interstitial cystitis/bladder pain syndrome (IC/BPS)
A similar but less strong association was noted between early life adversity and inflammation.
Cortisol slopes and nocturnal cortisol levels were not associated with early or recent life adversity.
Recent and chronic life stress should be assessed along with early life adversity as potential risk factors for inflammation, widespread pain, and potentially poorer prognosis as part of a clinical workup for IC/BPS.
Acknowledgements:
We are grateful to all the patients who participated in this research and to Mary Eno for her extensive assistance in collecting this data.
Funding:
Funding for the MAPP Research Network was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) [DK082315 (Andriole, G; Lai, H), DK082316 (Landis, J), DK082325 (Buchwald, D), DK082333 (Lucia, M), DK082342 (Klumpp, D; Schaeffer A), DK082344 (Kreder, K), DK082345 (Clauw, D; Clemens, JQ), DK082370 (Mayer, E; Rodriguez L), DK103227 (Moses, M), DK103260 (Anger, J; Freeman, M), DK103271 (Nickel, J)].
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alexander RB, Ponniah S, Hasday J, & Hebel JR (1998). Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology, 52(5), 744–749. 10.1016/s0090-4295(98)00390-2 [DOI] [PubMed] [Google Scholar]
- Anger JT, Dallas KB, Bresee C, De Hoedt AM, Barbour KE, Hoggatt KJ, Goodman MT, Kim J, & Freedland SJ (2022). National prevalence of IC/BPS in women and men utilizing veterans health administration data. Frontiers of Pain Research (Lausanne), 3, 925834. 10.3389/fpain.2022.925834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett TJ, Corr EM, van Solingen C, Schlamp F, Brown EJ, Koelwyn GJ, Lee AH, Shanley LC, Spruill TM, Bozal F, de Jong A, Newman AAC, Drenkova K, Silvestro M, Ramkhelawon B, Reynolds HR, Hochman JS, Nahrendorf M, Swirski FK, … Moore KJ. (2021). Chronic stress primes innate immune responses in mice and humans. Cell Reports, 36(10), 109595. 10.1016/j.celrep.2021.109595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, & Mondelli V. (2016). Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Molecular Psychiatry, 21(5), 642–649. 10.1038/mp.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box GEP, & Cox DR (1964). An analysis of transformations. Journal of the Royal Statistical Society, Series B, 26, 211–252. [Google Scholar]
- Chen E, & Miller GE (2013). Socioeconomic Status and Health: Mediating and Moderating Factors. Annual Review of Clinical Psychology, 9, 713–749. [DOI] [PubMed] [Google Scholar]
- Choghakhori R, Abbasnezhad A, Hasanvand A, & Amani R. (2017). Inflammatory cytokines and oxidative stress biomarkers in irritable bowel syndrome: Association with digestive symptoms and quality of life. Cytokine, 93, 34–43. 10.1016/j.cyto.2017.05.005 [DOI] [PubMed] [Google Scholar]
- Clemens JQ, Kutch JJ, Mayer EA, Naliboff BD, Rodriguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Clauw DJ, Harte SE, Schrepf AD, Williams DA, Andriole GL, Lai HH, Buchwald D, Lucia MS, van Bokhoven A, Mackey S, Moldwin RM, Pontari MA, … Landis JR. (2020). The Multidisciplinary Approach to The Study of Chronic Pelvic Pain (MAPP) Research Network*: Design and implementation of the Symptom Patterns Study (SPS). Neurourology and Urodynamics, 39(6), 1803–1814. 10.1002/nau.24423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens JQ, Mullins C, Ackerman AL, Bavendam T, van Bokhoven A, Ellingson BM, Harte SE, Kutch JJ, Lai HH, Martucci KT, Moldwin R, Naliboff BD, Pontari MA, Sutcliffe S, & Landis JR (2019). Urologic chronic pelvic pain syndrome: insights from the MAPP Research Network. Nature Reviews Urology, 16(3), 187–200. 10.1038/s41585-018-0135-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Meir T, Klein E, Volpin G, Assaf M, & Pollack S. (2011). Cytokine levels as potential biomarkers for predicting the development of posttraumatic stress symptoms in casualties of accidents. International Journal of Psychiatry in Medicine, 42(2), 117–131. 10.2190/PM.42.2.b [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, & Turner RB (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences, U S A, 109 (16), 5995–5999. 10.1073/pnas.1118355109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin NM, Mills KH, & Connor TJ (2009). Psychological stress increases expression of IL-10 and its homolog IL-19 via beta-adrenoceptor activation: reversal by the anxiolytic chlordiazepoxide. Brain Behavior and Immunity, 23(3), 371–379. 10.1016/j.bbi.2008.12.010 [DOI] [PubMed] [Google Scholar]
- Engler H, Engler A, Bailey MT, & Sheridan JF (2005). Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. Journal of Neuroimmunology, 163(1–2), 110–119. 10.1016/j.jneuroim.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Finegood ED, & Miller GE (2022). Childhood violence exposure, inflammation, and cardiometabolic health. Current Topics in Behavioral Neuroscience, 54, 439–459. 10.1007/7854_2021_283 [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, & Maier SF (2007). Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain, Behavior, and Immunity, 21(1), 47–59. [DOI] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, & Maier SF (2010). Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behavior and Immunity, 24(1), 19–30. 10.1016/j.bbi.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, & Maier SF (2011). Stress-and glucocorticoid-induced priming of neuroinflammatory responses: potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain, Behavior, and Immunity, 25, S21–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB, & Paganelli R. (2017). Aging, Obesity, and Inflammatory Age-Related Diseases. Frontiers of Immunology, 8, 1745. 10.3389/fimmu.2017.01745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice JW, & Harris RJ (1998). A comparison of regression and loading weights for the computation of factor scores. . Multivariate Behavioral Research, 33, 221–247. [DOI] [PubMed] [Google Scholar]
- Gu HF, Tang CK, & Yang YZ (2012). Psychological stress, immune response, and atherosclerosis. Atherosclerosis, 223(1), 69–77. 10.1016/j.atherosclerosis.2012.01.021 [DOI] [PubMed] [Google Scholar]
- Gupta A, Bhatt RR, Naliboff BD, Kutch JJ, Labus JS, Vora PP, Alaverdyan M, Schrepf A, Lutgendorf S, Mayer EA, & Network MR (2019). Impact of early adverse life events and sex on functional brain networks in patients with urological chronic pelvic pain syndrome (UCPPS): A MAPP Research Network study. PLoS One, 14(6), e0217610. 10.1371/journal.pone.0217610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal KE, & Bishop MD (2014). Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Physical Therapy, 94(12), 1816–1825. 10.2522/ptj.20130597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänsel A, Hong S, Cámara RJ, & Von Kaenel R. (2010). Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neuroscience & Biobehavioral Reviews, 35(1), 115–121. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Lachman ME, Mroczek DK, Seeman TE, Miller GE (2015). Additive Contributions of Childhood Adversity and Recent Stressors to Inflammation at Midlife: Findings from the MIDUS Study. Developmental Psychology, 51(11), 1630–1644. 10.1037/dev0000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Merkin SS, Almeida DM, Friedman EM, Mogle JA, & Seeman TE (2019). Early-Life Adversity and Dysregulation of Adult Diurnal Cortisol Rhythm. Journals of Gerontology B Psychological Sciences and Social Sciences, 74(1), 160–169. 10.1093/geronb/gby097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Cacioppo JT, & Malarkey WB (1998). Marital stress: immunologic, neuroendocrine, and autonomic correlates. Annals of the New York Academy of Sciences, 840, 656–663. 10.1111/j.1749-6632.1998.tb09604.x [DOI] [PubMed] [Google Scholar]
- Konkle KS, Berry SH, Elliott MN, Hilton L, Suttorp MJ, Clauw DJ, & Clemens JQ (2012). Comparison of an interstitial cystitis/bladder pain syndrome clinical cohort with symptomatic community women from the RAND Interstitial Cystitis Epidemiology study. Journal of Urology, 187(2), 508–512. 10.1016/j.juro.2011.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Giese-Davis J, Yutsis M, Neri E, Gallagher-Thompson D, Taylor B, & Spiegel D. (2006). Design decisions to optimize reliability of daytime cortisol slopes in an older population. American Journal of Geriatric Psychiatry, 14(4), 325–333. [DOI] [PubMed] [Google Scholar]
- Liu PZ, & Nusslock R. (2018). How Stress Gets Under the Skin: Early Life Adversity and Glucocorticoid Receptor Epigenetic Regulation. Current Genomics, 19(8), 653–664. 10.2174/1389202919666171228164350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Yeh W, & Ohashi P. (2008). LPS/TLR4 signal transduction pathway. Cytokine, 42(2), 145–151. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Kreder KJ, Rothrock NE, Hoffman A, Kirschbaum C, Sternberg EM, Zimmerman MB, & Ratliff TL (2002). Diurnal cortisol variations and symptoms in patients with interstitial cystitis. Journal of Urology, 167(3), 1338–1343. [PubMed] [Google Scholar]
- Madrigal JL, Garcia-Bueno B, Hinojosa AE, Polak P, Feinstein DL, & Leza JC (2010). Regulation of MCP-1 production in brain by stress and noradrenaline-modulating drugs. Journal of Neurochemistry, 113(2), 543–551. 10.1111/j.1471-4159.2010.06623.x [DOI] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, & John-Henderson NA (2017). The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behavior and Immunity, 64, 208–219. 10.1016/j.bbi.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137(6), 959–997. 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Miller GE (2016). Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biological Psychiatry, 80(1), 23–32. 10.1016/j.biopsych.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, Caspi A, Moffitt TE, & Arseneault L. (2011). Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biological Psychiatry, 70(11), 1016–1023. 10.1016/j.biopsych.2011.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennebaker JW, & Susman JR (1988). Disclosure of traumas and psychosomatic processes. Social Science and Medicine, 26, 327–332. [DOI] [PubMed] [Google Scholar]
- Pierce J, Harte SE, Afari N, Bradley CS, Griffith JW, Kim J, Lutgendorf SK, Naliboff BD, Rodriguez LV, Taple BJ, Williams D, Harris RE, Schrepf A, & the MAPP Research Network. (2023).Mediators of the Association Between Childhood Trauma and Pain Sensitivity in Adulthood: A MAPP Research Network Symptom Patterns Study Analysis. Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, & Samuels MH (2004). Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. Journal of Clinical Endocrinology and Metabolism, 89(1), 281–287. 10.1210/jc.2003-030440 [DOI] [PubMed] [Google Scholar]
- Qi J, Chen C, Meng QX, Wu Y, Wu H, & Zhao TB (2016). Crosstalk between Activated Microglia and Neurons in the Spinal Dorsal Horn Contributes to Stress-induced Hyperalgesia. Scientific Reports, 6, 39442. 10.1038/srep39442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepf A, Bradley CS, O’Donnell M, Luo Y, Harte SE, Kreder K, & Lutgendorf S. (2015). Toll-like receptor 4 and comorbid pain in Interstitial Cystitis/Bladder Pain Syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behavior and Immunity, 49, 66–74. 10.1016/j.bbi.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepf A, Kaplan C, Harris RE, Williams DA, Clauw DJ, As-Sanie S, Till S, Clemens JQ, Rodriguez LV, Van Bokhoven A, Landis R, Gallop R, Bradley C, Naliboff B, Pontari M, O’Donnell M, Luo Y, Kreder K, Lutgendorf SK, & Harte SE (2022). Stimulated whole blood cytokine/chemokine responses are associated with Interstitial Cystitis/Bladder Pain Syndrome phenotypes and features of nociplastic pain: A MAPP research network study. PAIN, doi: 10.1097/j.pain.0000000000002813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepf A, Naliboff B, Williams DA, Stephens-Shields AJ, Landis JR, Gupta A, Mayer E, Rodriguez LV, Lai H, Luo Y, Bradley C, Kreder K, & Lutgendorf SK (2018). Adverse Childhood Experiences and Symptoms of Urologic Chronic Pelvic Pain Syndrome: A Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network Study. Annals of Behavioral Medicine, 52(10), 865–877. 10.1093/abm/kax060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepf A, O’Donnell MA, Luo Y, Bradley CS, Kreder KJ, & Lutgendorf SK (2016). Inflammation and Symptom Change in Interstitial Cystitis or Bladder Pain Syndrome: A Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network Study. Urology, 90, 56–61. 10.1016/j.urology.2015.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepf A, O’Donnell M, Luo Y, Bradley C, Kreder K, Lutgendorf S, & Netand the Multidisciplinary Apoproach to the Study of Chronic Pelvic Pain (MAPP) Research Network, (2014). Inflammation and inflammatory control in interstitial cystitis/bladder pain syndrome: Associations with painful symptoms. PAIN, 155(9), 1755–1761. Inflammation and inflammatory control in interstitial cystitis/bladder pain syndrome: Associations with painful symptoms. 10.1016/j.pain.2014.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad MM, Arevalo JM, Armaiz-Pena GN, Lu C, Stone RL, Moreno-Smith M, Nishimura M, Lee JW, Jennings NB, Bottsford-Miller J, Vivas-Mejia P, Lutgendorf SK, Lopez-Berestein G, Bar-Eli M, Cole SW, & Sood AK (2010). Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. Journal of Biological Chemistry, 285(46), 35462–35470. 10.1074/jbc.M110.109579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM (2016). Life Stress and Health: A Review of Conceptual Issues and Recent Findings. Teaching of Psychology, 43(4), 346–355. 10.1177/0098628316662768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, & Koenen KC (2013). Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology, 38(2), 188–200. 10.1016/j.psyneuen.2012.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen EH, Wang X, Balaji S, Butte MJ, Bollyky PL, & Keswani SG (2020). The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Advances in Wound Care (New Rochelle), 9(4), 184–198. 10.1089/wound.2019.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Fischer CP, Keller C, Moeller K, & Pedersen KB (2003). IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. American Journal of Physiology-Endocrinology and Metabolism, 285(2), E433–E437. 10.1152/ajpendo.00074.2003 [DOI] [PubMed] [Google Scholar]
- Steine IM, Winje D, Krystal JH, Bjorvatn B, Milde AM, Grønli J, Nordhus IH, & Pallesen S. (2017). Cumulative childhood maltreatment and its dose-response relation with adult symptomatology: Findings in a sample of adult survivors of sexual abuse. Child Abuse Negl, 65, 99–111. 10.1016/j.chiabu.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Sun Y, Qu Y, & Zhu J. (2021). The Relationship Between Inflammation and Post-traumatic Stress Disorder. Frontiers in Psychiatry, 12, 707543, doi: 10.3389/fpsyt.2021.707543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers I, Quaedflieg C, Hsu C, Heathcote LC, Rovnaghi CR, & Simons LE (2019). The interaction between stress and chronic pain through the lens of threat learning. Neuroscience and Biobehavioral Reviews, 107, 641–655. 10.1016/j.neubiorev.2019.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursich M, Neufeld RW, Frewen PA, Harricharan S, Kibler JL, Rhind SG, & Lanius RA (2014). Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Translational Psychiatry, 4(7), e413. 10.1038/tp.2014.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Yamagata H, McEwen BS, & Watanabe Y. (2010). Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. Journal of Neuroscience, 30(45), 15007–15018. 10.1523/jneurosci.1436-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, & Godbout JP (2012). Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology, 37(9), 1491–1505. 10.1016/j.psyneuen.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiallouris A, Tsioutis C, Agapidaki E, Zafeiri M, Agouridis AP, Ntourakis D, & Johnson EO (2019). Adrenal Aging and Its Implications on Stress Responsiveness in Humans. Frontiers of Endocrinology (Lausanne), 10, 54. 10.3389/fendo.2019.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Gao X, Wang A, Wang Y, Du Y, Li L, Li M, Li C, Jin X, & Zhao M. (2019). Depression comorbid with hyperalgesia: Different roles of neuroinflammation induced by chronic stress and hypercortisolism. Journal of Affective Disorders, 256, 117–124. 10.1016/j.jad.2019.05.065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.