Abstract
Knee osteoarthritis (KOA) is a chronic joint bone disease characterized by inflammatory destruction and hyperplasia of bone. Its main clinical symptoms are joint mobility difficulties and pain, severe cases can lead to limb paralysis, which poses major pressure to the quality of life and mental health of patients, but also brings serious economic burden to society. The occurrence and development of KOA is influenced by many factors, including systemic factors and local factors. The joint biomechanical changes caused by aging, trauma and obesity, abnormal bone metabolism caused by metabolic syndrome, the effects of cytokines and related enzymes, genetic and biochemical abnormalities caused by plasma adiponectin, etc. all directly or indirectly lead to the occurrence of KOA. However, there is little literature that systematically and comprehensively integrates macro‐ and microscopic KOA pathogenesis. Therefore, it is necessary to comprehensively and systematically summarize the pathogenesis of KOA in order to provide a better theoretical basis for clinical treatment.
Keywords: Bone Metabolism, Cytokines, Inflammation, Knee Osteoarthritis, Long Non‐Coding RNA
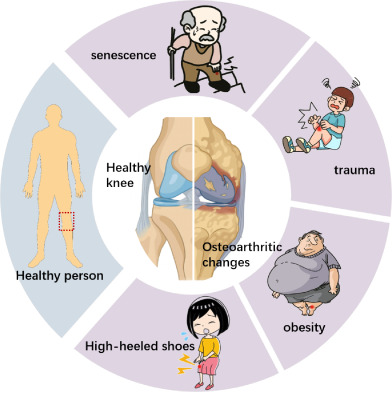
Macroscopic factors affecting the occurrence and development of knee osteoarthritis.
Introduction
Knee osteoarthritis (KOA) is the most common joint inflammatory lesion in orthopaedics. The progressive degeneration and loss of articular cartilage is its main feature, accompanied by the changes of joint structure and function. Meniscus, periarticular ligaments, subchondral bone alterations, and synovium are further pathological signs. 1 The main clinical symptoms of KOA are limb mobility difficulties, pain and swelling around the joint, which is more obvious after morning, and can lead to paralysis in the later stage. 2 Because of the rapid development of the economy, which leads to a rise in life expectancy and body mass index (BMI), the prevalence of KOA patients has increased dramatically and become younger since the 21st century. Currently, KOA accounts for 85% of osteoarthritis worldwide, and the incidence of symptomatic KOA is 8.1%. 3 Studies have shown that the prevalence of KOA is slightly higher in women than in men, and is mainly in people over 50 years old. 4 Inflammation is the most common cause of joint pain and disability in patients, and few effective treatments has been found to delay the progression of KOA. To alleviate symptoms and enhance physical performance, the majority of patients will thus undergo complete joint replacement; nevertheless, it is costly, painful, and takes a long time to heal. 5 Therefore, understanding the pathogenesis of KOA is essential for formulating and targeting effective interventions to appropriate joint sites and injuries. There are many publications on the pathogenesis of KOA, but there is little literature that systematically and thoroughly combines macro‐ and microscopic KOA pathogenesis, according to a study of domestic and foreign literature. Therefore, it is necessary to comprehensively and systematically summarize the pathogenesis of KOA in order to provide a better theoretical basis for clinical treatment.
The combination of several factors, which results from the interaction of systemic elements and local factors, is what drives KOA's growth. We discovered that the occurrence and progression of this debilitating and progressive disease are primarily related to the biomechanical changes of joints brought on by aging, trauma, knee joint dislocation, obesity, abnormal bone metabolism brought on by metabolic syndrome, the effects of cytokines and related enzymes, genetic factors, plasma adiponectin, etc.(Figure 1). 6 , 7 Briefly, the pathogenesis of KOA can be summarized into two categories: mechanical factors and biological factors. Mechanical elements include changes in the biomechanics, that is the breakdown of the balancing system of the mechanical conduction of lower limb strength at the knee joint, which contribute to KOA. The imbalance of proliferation and apoptosis of chondrocytes in the knee joint is an appearance of biological factors, which are aberrant biological alterations of chondrocytes brought on by a variety of causes.
Fig. 1.
Macroscopic factors affecting the occurrence and development of knee osteoarthritis.
Biomechanical Change
The knee joint's primary roles are weight bearing and load transfer, participation in lower limb motion, and force generation for lower leg activities. The internal osseous stabilization system of the knee joint is composed of the femoral head, internal and external meniscus, tibia head, fibula and patella, joint capsule and accessory ligament structure, so as to coordinate the support of body mass and maintain mechanical balance. 8 When the mechanical balance is broken, the distribution of forces within the knee joint is affected, resulting in decompensation of the knee joint and its surrounding tissues, accelerating the degenerative changes of the articular cartilage. 9 In recent years, numerous studies have demonstrated that aging, trauma, knee dislocation, obesity, increased joint weight bearing, and decreased joint stability all play a significant role in the development of KOA. These factors also cause and affect one another, creating a vicious cycle that results in the development of KOA. 10
Age is considered to be the most prominent risk factor for KOA development. 11 The aging of the body leads to degeneration of joint tissue, from subchondral bone dilatation, myelopathy, meniscus tear and compression, to cartilage defects, and a series of structural changes occur in the joint. It may eventually lead to advanced cartilage loss and osteoarthritic changes. 12 The meniscus, ligaments, periarticular muscles, and joint capsule may also have a role in the development of osteoarthritis, according to a number of different pieces of data. Patients with KOA even have inflammatory cells in their sub‐patellar fat pads, which can contribute to discomfort in the anterior KOA region. 13 KOA tends to cause bone enlargement and swelling, especially in larger joints. Bone swelling occurs due to pathological changes such as soft tissue edema, blood circulation obstruction, chondrocyte damage, increased bone density, and cystic changes during osteoarthritis. Together, these pathological changes trigger bone remodeling, leading to adverse outcomes such as marginal osteophytes, joint subluxation, joint capsule thickening, synovial hyperplasia, and synovial effusion. 14 The presence of inflammation in the course of KOA, particularly IL‐1a and TNF‐a, induces a high expression of genes associated with senescence and thus exacerbates the aging process. Thus, the changes in bone structure associated with aging lead to reduced range of active and passive movement in patients. 15 In addition, articular cartilage is a durable tissue that can withstand repeated stress created by daily physical activity. However, it is still prone to damage of cartilage and subchondral bone after trauma. Repetitive exercise‐induced joint injury, especially squatting and kneeling in the elderly, is a common risk factor for KOA. Occupations that squatted or knelt for >2 h a day were associated with a significant two‐fold increased risk of moderate to severe KOA. 16 Periarticular muscles play an important role in maintaining joint stability, and the structure and function of skeletal muscles are related to the development and progression of KOA. 17 The decline of muscle strength in the elderly and knee arthritis has a pathological effect on each other. As aging muscles gradually atrophy and the strength of knee muscles declines, the system functions of wrapping, binding, and maintaining balance decline, which will lead to the reduction of the stability of knee joints, uneven distribution of joint load, and the loss of cartilage due to metabolic imbalance caused by local cartilage stress exceeding its physiological load. 18 With the progression of KOA, pain, joint adverse symptoms aggravated, reduced activity, further affecting the biomechanical properties of muscle.
Obesity is considered to be another important risk factor for KOA progression. There was a linear relationship between BMI and severity of KOA. 19 Obesity can directly increase the mechanical pressure of the knee joint, 20 aggravate cartilage damage, and cause abnormal bone metabolism and remodeling response, resulting in uneven stress distribution of the tibial plateau and increased knee joint load. 21 The chance of developing or worsening osteoarthritis later on can be increased by increased strain on the knee, which can result in stress fractures and anterior cruciate ligament rupture. 22 In obese individuals, the incidence of cartilage damage and permanent disintegration is higher than that of articular chondrogenesis. However, obesity is also the most modifiable risk factor among the many risk factors affecting KOA. 23 Studies have shown that weight loss or removal of the infrapatellar fat pad can improve OA severity and reduce mechanical load around the knee joint. 24 In addition, the metabolic and inflammatory changes associated with obesity are also important factors affecting the progression of KOA, and the specific mechanisms are described below.
Another significant factor in knee joint abnormalities is abnormal gait. Women are twice as likely as males to develop KOA. 25 It is believed that heel height may be a potential factor contributing to the higher incidence of osteoarthritis in women because studies have shown that heel height significantly influences knee kinematics and dynamics during walking and that walking in high heels increases knee extension torque, which increases knee joint load. 26 Furthermore, increased trunk flexion in patients with KOA is associated with elevated knee flexor activity and medial co‐contraction. 27 Gait adjustment was most effective in reducing the knee adduction and also increased joint contraction, thus reducing the impact on the net load of the knee joint. 28
In conclusion, age, trauma, obesity, increased joint loading, and reduced joint stability all play a role in the development of KOA. These factors also lead to and influence each other, creating a vicious circle that leads to the development of KOA. At the same time, these macroscopic factors are also causative factors leading to changes in the microscopic environment, in particular, changes in non‐exogenous factors such as age and obesity that are associated with changes in the body itself.
Metabolic Syndrome and Bone Metabolism
In recent years, the association between metabolic syndrome and KOA has aroused many scholars' interests in the field of osteoarthritis. The metabolic syndrome consists mainly of hypertension, hyperglycemia, dyslipidemia, and central obesity, all associated with the pathology and risk of progression of KOA. 29 Articular cartilage in particular is vulnerable to the harmful effects of elevated glucose and lipids.
Hypertension is one of the most common comorbidities of OA. A cross‐sectional analysis of 254 patients with KOA showed that 63% of KOA patients had a history of hypertension, 30 which can physically and biochemical disrupt joint homeostasis. The mechanism of hypertension affecting OA mainly includes the following two aspects: hypertension increases intraosseous pressure and causes hypoxia, which in turn triggers the remodeling of the junction of subchondral bone and osteochondral. 31 Elevated blood pressure may lead to increased RANKL/OPG ratio, IL‐6 and M‐CSF expression, all of which promote osteoclast formation and enhanced activity, thus promoting osteoclast differentiation and bone resorption. 32 On the other hand, there is growing evidence that vascular dysfunction is closely related to the pathogenesis of KOA. In patients with hypertension, the state of equilibrium between the vascular regulators produced by endothelial cells is disrupted, leading to changes in vascular tone, manifested by a decrease in peripheral blood flow, leading to ischemia and cell death, which negatively affects the subchondral bone. 33 Hypertension can also lead to arteriosclerosis, which directly affects subchondral bone ischemia, resulting in changes in joint cell metabolism. High concentrations of pro‐inflammatory peroxidase lipids detected in plasma of patients with metabolic syndrome accelerate degradation of periarticular matrix, directly affecting articular cartilage. 34
Both type 2 diabetes and KOA are common chronic diseases in the older population, and clinical observations and studies have found a significantly increased risk of developing KOA in patients with type 2 diabetes. 35 Hyperglycemia and insulin resistance are important factors inducing KOA in patients with type 2 diabetes. Oxidative stress caused by chronic hyperglycemia can lead to excessive production of pro‐inflammatory cytokines and advanced glycation end products (AGEs) and insulin resistance in joint tissues, which may lead to local and systemic low‐grade chronic inflammation, causing damage to cell matrix, subchondral bone, and chondrocyte. 36 , 37 For articular chondrocytes, glucose is not only an important source of energy, but also an important precursor of glycosaminoglycan synthesis. However, due to reduced collagen synthesis and increased proteoglycan catabolism, excessive glucose can lead to abnormal cell metabolism and morphological changes. Hyperglycemia is directly related to the accumulation of AGEs, which can lead to lipid peroxidation and cell membrane damage by stimulating extracellular matrix (ECM) degradation and oxidative stress. In addition, AGEs can promote the development of diabetic peripheral neuropathy, thereby accelerating KOA. 38
Lipid abnormalities may also promote the pathological process of KOA. The disruption of high‐density lipoprotein (HDL) metabolic pathway and the elevation of free fatty acid levels can aggravate adipose tissue inflammation by activating macrophages through TLR‐2/4, leading to imbalance in cartilage remodeling and destruction of bone homeostasis. 39 By analyzing the serum of 41 patients with KOA, it was found that hypertriglyceridemia was closely related to cartilage anabolism and catabolism. Hypertriglyceridemia had a significant independent effect on cartilage metabolism and could make cartilage homeostasis change to degeneration. 40
BMI‐related central obesity is another important component of the metabolic syndrome, and up to two‐thirds of elderly obese people are affected by KOA. 41 Obese people may have considerable overload and injury to their weight‐bearing joints due to weight increase brought on by elevated BMI. Obesity can bring mechanical overload to weight‐bearing joints, such as knee joints, hip joints, etc., resulting in wear, injury and microtrauma, uneven stress on the joint surface, joint dysfunction, further leading to cartilage loss, osteophyte formation, and osteoarthritis. 42 In addition, metabolic problems can also be brought on by high BMI. KOA is closely correlated with abnormal adipocyte production of leptin and adiponectin in adipose tissue. Leptin is one of the most important factors secreted by adipose tissue and plays an important role in the pathogenesis of KOA. Jiang et al. 43 found that leptin induced CD14/TLR4 activation through JAK2‐STAT3 signaling pathway to promote KOA. In addition, Wang et al. 44 found elevated levels of TNF‐α, IL‐1, and IL‐6 in synovial fluid, synovium, subchondral bone, and cartilage in KOA patients, and adipose tissue is considered to be a major source of these pro‐inflammatory cytokines. TNF‐α, IL‐1, and IL‐6 can induce the production of other cytokines, such as matrix metalloproteinases (MMPs) and prostaglandins, and inhibit the synthesis of proteoglycans and type II collagen. It can be seen that obesity plays an important role in the bone metabolism of KOA.
In summary, KOA is closely related to the molecular mechanisms of oxidative stress, inflammatory response, vascular endothelial injury, metabolic abnormalities, obesity, hypertension, and lipid metabolism dysfunction. Those pathogeneses may extend to multiple organs of the body, and actively control the metabolic syndrome. Systemic treatment may help delay the progress of KOA, and the improvement of KOA activity disorder may also reduce the metabolic syndrome.
Cytokines and Related Proteases
Cytokines and Chemokines
During the illness process, chemicals influence the creation of cells, cytokines, and other inflammatory substances and enzymes in joints via intracellular signal transduction pathways. The tissue secretions of cartilage, synovium, infrapatellar fat pad, and meniscus in knee joint have many cytokines, chemokines, and growth factors. These factors activate MAPK, AKT, NFКB, and other signaling pathways in chondrocytes, release a variety of inflammatory mediators, accelerate chondrocytes dedifferentiation, and increase chondrocytes fibrosis. It has been observed in KOA subjects that the levels of IL‐7, IL‐12, interferon (IFN)‐γ, IL‐10, and IL‐13 in synovial fluid are associated with knee pain during KOA progression. 45 Among the many cytokines associated with KOA, IL‐1β, TNF‐α, and IL‐6 are considered to be the most critical pro‐inflammatory factors that promote the progression of KOA 46 (Figure 2).
Fig. 2.
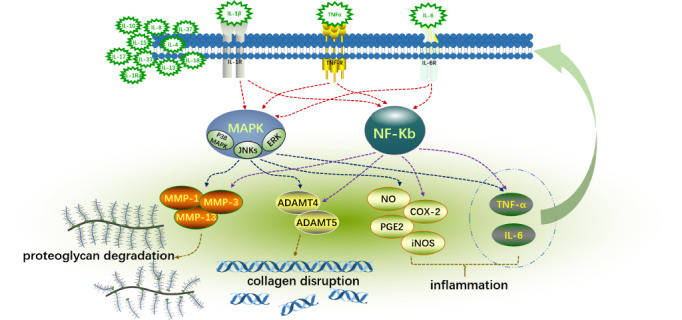
The role of proinflammatory factors in the pathogenesis of knee osteoarthritis schematic diagram. IL‐1β, TNF‐α, IL‐6, and other pro‐inflammatory factors bind to their corresponding receptors, activate NF‐kB and MAPK signaling pathways. By promoting the expression of ADAMT‐4, ADAMT‐5, MMP1, MMP3, MMP13, and other enzymes, it inhibits the synthesis of proteoglycans and collagen resulting in ECM degradation. In addition, the same signaling pathway can increase the expression of inflammatory mediators such as COX‐2, PGE‐2, NO, iNOS, and induce inflammatory factors such as IL‐6 and TNF‐α to aggravate the inflammatory response.
IL‐1β is a member of the IL‐1 family and achieves its role by binding to the corresponding receptor (IL‐1RI). 47 IL‐1β is mainly involved in induced catabolic events (such as cartilage degradation) through mitogen‐activated protein kinase (MAPK) signaling, 48 which is the most important process in KOA (Figure 3). MAPK is mainly composed of extracellular signal‐regulated kinases (ERK), c‐Jun N‐terminal kinases (JNKs) and p38 MAPK. 23 IL‐1β can induce the production of collagenases and aggresomes through these signaling pathways of MAPK, and inhibit the synthesis of proteoglycans and collagen, leading to ECM degradation. In addition, it can also stimulate the secretion of IL‐6, and other pro‐inflammatory cytokines through three signaling pathways of MAPK, 49 and induce the gene expression or enhanced activity of COX‐2 and iNOS in chondrocytes to stimulate nitric oxide (NO), prostaglandin E2 (PGE2), and other inflammatory mediators. NF‐κB is another important signaling pathway involved in the activation of IL‐1β. Its participation in the process of KOA is similar to MAPK, which can not only induce the release of MMP1 and MMP13, but also decrease the disengagement of inflammatory factors such as IL‐6. 50 , 51 It can also produce inflammatory mediators such as NO and PGE2 through the PI3K/AKT signaling pathway (Figure 4). 52
Fig. 3.
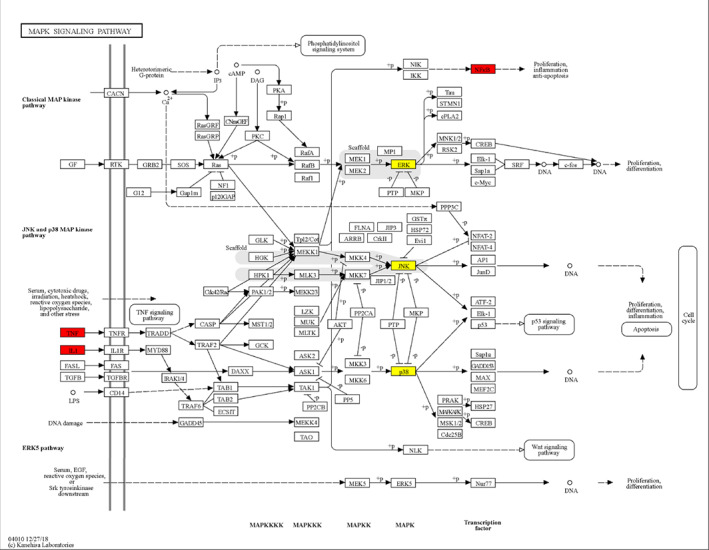
Diagram of MAPK signaling pathway in knee osteoarthritis. The pathway diagram shows that factors such as IL‐1 and TNF‐a can be used as initiators of MAPK signaling pathway to mediate signal expression and participate in catabolic events such as cartilage degradation.
Fig. 4.
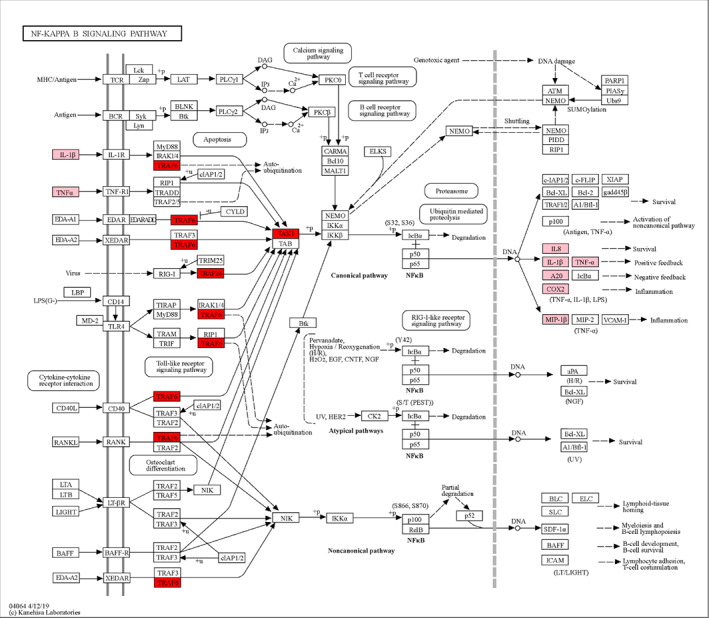
Diagram of NF‐kB signaling pathway in knee osteoarthritis. The pathway diagram showed that IL‐1β, TNF‐α, and other factors could mediate signal expression as initiators of NF‐kB signaling pathway. TRAF6 and TAK1 are NF‐κB pathway hub factors, which ultimately lead to the expression of inflammatory factors such as IL‐8, IL‐1β, TNF‐α, A20, COX‐2, and MIP‐1β, leading to increased inflammation and ECM degradation.
TNF‐α is also an effective pro‐inflammatory cytokine and plays an important role in the process of KOA. TNF‐α is a protein that shows cytotoxic activity and causes certain types of necrosis to subside. It is part of the tumor necrosis factor superfamily and was discovered by Carswell et al. in 1975. 53 Tumor necrosis factor receptor 1 (TNRF‐1) can be activated by two forms of TNF‐α, but TNRF‐1 activity has a greater effect on local cartilage tissue loss. 54 TNF‐α, like IL‐1β, can induce the degradation of ECM by inducing collagenase and aggrecanases (including MMP‐1, MMP‐3, MMP‐13, and ADAMTS‐4). 55 In addition, TNF‐α increases the synthesis of IL‐6, IL‐8, RANTES, and VEGF. 56
One significant inflammatory cytokine is IL‐6, which is mostly generated by chondrocytes, osteoblasts, and synovial cells in KOA patients. 57 IL‐6 activates JAK/STAT, MAPK, and PI3K signaling pathways by binding to two receptors: binding protein IL‐6R(gp80, CD126) and signal transduction protein gp130(CD130). 58 , 59 By activating the above signaling pathways, it regulates the production of enzymes such as TIMP, MMP, and ADAMTS, thereby affecting the synthesis of type II collagen and proteoglycans. Therefore, IL‐6 plays a dominant role in anti‐inflammatory and pro‐inflammatory effects, leading to the progression of osteoarthritis. 56
Chemokines, also known as chemotactic cytokines, are small molecular proteins that induce the chemotaxis of a variety of cells. They exert biological functions mainly by interacting with corresponding chemokine receptors and glycosaminoglycans on the surface of endothelial cells. It has been found to play an important role in the development of KOA. 60 Chemokine CCL2, a monocyte chemoattractant, works by binding to the CCR2 receptor. A significant increase in CCL2 was found in synovial fluid of patients with KOA and knee injury. 61 , 62 Overexpression of CCL2 increases the expression of MMP3 and MMP13, leading to the loss of proteoglycans and cartilage degradation. Blocking the CCL2 signaling pathway significantly attenuated macrophage accumulation, synovitis, and cartilage damage in the OA mouse model. In addition, CCL3, CCL4, and CCL5 in CC family chemokines have also been found to be important chemokines in the development of KOA. The increase of CCL3, CCL4, and CCL5 can be detected in plasma and synovial fluid of KOA patients. 63
KOA patients stimulated by proinflammatory factors such as lipopolysaccharide (LPS) and TNF‐αsecrete‐related chemokines (CXCL6, CXCL10 and CXCL16) and growth factors such as angiopoietin‐like protein 1(ANGPTL1), fibroblast growth factor 5 (FGF5)and insulin‐like growth factor 2 (IGF2). 64 In vitro studies have shown that human synovial fibroblasts (HFLS) can stimulate bone regeneration by activating the PI3K/AKT signaling pathway in mesenchymal stem cells, leading to the formation of osteophytes. 65 These cytokines and chemokines in the catabolic cascade amplification cartilage matrix decomposition further enhance the destruction of articular cartilage cells, causing the induction of catabolic pathways, inhibition of matrix synthesis, and promotion of apoptosis.
Proteinase
Matrix metalloproteinases (MMPs), a class of ECM‐eroding enzymes that are expressed in joint tissues, are thought to be a key factor in the degeneration of joints in patients. MMP‐1, MMP‐3, MMP‐9, and MMP‐13 in the MMPs family are considered to be the core substances that promote the degradation of cartilage matrix and are the main promoters of the occurrence and aggravation of KOA. 66 Jarecki et al. evaluated the correlation between serum and synovial fluid MMPs and clinical stage in obese women with KOA. The results showed that the levels of MMP‐3, MMP‐9, and pro‐MMP‐13 were higher in the later stage of KOA. 67 MMP‐13 is the most expressed in KOA, 68 which has the same function as other MMPs (such as MMP‐1, MMP‐3), that is, the destruction of collagen network. In MMPs, MMP‐1 is mainly expressed by synovial cells, and MMP‐13 is mainly expressed by chondrocytes. Both MMPs can degrade collagen, but MMP‐13 can also degrade proteoglycan molecules and polymeric proteins, suggesting that MMP‐13 has a dual role in matrix degradation. 69 Although MMP‐13 can degrade many collagens, even proteoglycan and other proteins, the main target of MMP‐13 is type II collagen, which is the most common type of collagen in cartilage structure. 68 With the change of ECM, the matrix around the cell is also degraded by the increase of protease activation. 70 The production of cartilage degradation products and the secretion of damage‐associated molecular patterns (DAMPs) further increase the release of pro‐inflammatory mediators such as IL‐1β, TNF‐α, etc. These cytokines can trigger the increase of MMPs expression and inhibit MMPs enzyme inhibitors, reducing ECM synthesis. They also affect the proliferation of adjacent synovium and induce inflammation, ultimately aggravating the decomposition of cartilage. 3
Long Non‐coding RNA(LncRNA)
LncRNA is an RNA molecule with a length of more than 200 nucleotides and is a by‐product of RNA polymerase II transcription. LncRNA does not encode proteins and was previously considered to have no biological function. With the development of bioinformatics and high‐throughput sequencing technology, more and more studies have reported that lncRNA plays a key role in gene regulation and epigenetic modification. 71 It also participates in cell proliferation, apoptosis, differentiation, and other biological processes, and affects the occurrence and prognosis of the disease. 72 In recent years, numerous lncRNA have been shown to be differently expressed in distinct pathogenic stages of osteoarthritis. Liu et al. found 418 up‐regulated lncRNA and 347 down‐regulated lncRNA in KOA patients. Enrichment analysis found that DE lncRNA was mainly enriched in biological processes, molecular functions, and signaling pathways related to inflammation and bone formation. 73 Even some lncRNA are considered to be biomarkers that distinguish osteoarthritis from healthy people and disease progression. They found that eight lncRNA had the value of predicting KOA risk, and six candidate lncRNA could independently predict KOA risk. 73 In addition, Chen et al. conducted a comprehensive network analysis of lncRNA‐related ceRNA and found eight lncRNA molecular biomarkers associated with the progression of KOA. 74 Zhao et al. found that exosomal lncRNA PCGEM1 may be a powerful indicator for distinguishing early and late OA. 75 In addition, lncRNA CRNDE and LINC00152 were identified as key lncRNA for age‐related degeneration of articular cartilage. 76 More and more studies have confirmed that lncRNA is involved in ECM degradation, chondrocyte apoptosis, inflammatory response, autophagy, and other processes. Table 1 summarizes the lncRNA involved in the development of KOA. A large number of studies have found that the intervention of specific LncRNA has an inhibitory effect on the development of osteoarthritis. For example, Achyranthes bidentata polysaccharide (ABP) can slow down the degradation of ECM by regulating the expression of lncRNA GAS5. 77 It can also inhibit thapsigargin (TG)‐induced chondrocyte endoplasmic reticulum stress (ERS) 78 through the lncRNA NEAT1/miR‐377‐3p axis to protect osteoarthritis. In conclusion, lncRNA plays an important role in regulating gene expression, maintaining cartilage and synovial cell phenotype, and stabilizing the intra‐articular environment. 79 It is expected to become a new way for the diagnosis and treatment of KOA.
Table 1.
Aberrant expression of lncRNAs and their function in the development of knee osteoarthritis
| Gene name | Expression | Related factor | Function | Tissue and cell sources |
|---|---|---|---|---|
| LncRNA SNHG1 | Down | PI3K/Akt/mTOR | ECM degradation↑ Inflammation↑ Apoptosis↑ | Rat chondrocyte |
| LncRNA MEG3 | Down | miR‐93/TGFBR2 |
Chondrocyte proliferation↓ Apoptosis↑ ECM degradation↑ |
Rat knee cartilage tissue |
| LncRNA MEG3 | Down | miR‐16/SMAD7 |
Chondrocytes proliferation↑ Apoptosis↓ |
Rat knee cartilage tissue |
| LncRNA MEG3 | Down | MEG3/miR‐361‐5p/FOXO1 |
Chondrocyte proliferation↓ Apoptosis↑ Cartilage matrix degradation↑ |
Human knee cartilage tissue |
| LncRNA ANRIL | Up | miR‐122‐5p/DUSP4 | Synovial cell proliferation↑ | Human knee cartilage tissue, Normal synoviocytes (NS) and osteoarthritis synoviocytes (OAS) |
| LncRNA HOTTIP | Up | miR‐663a/FRK |
Chondrocyte proliferation↑ Chondrocyte apoptosis↓ |
Human knee cartilage tissue |
| LncRNA FAS‐AS1 | Up | MMP1, MMP13, COL2A1 |
Chondrocyte proliferation↓ Apoptosis↑ ECM degradation↑ |
Human knee cartilage tissue |
| LncRNA THUMPD3‐AS1 | Down |
p38MAPK NF‐kB p65 |
Apoptosis↑ Inflammation↓ | Human knee cartilage tissue, Human cartilage C28/I2 cells |
| LncRNA HOTAIR | Up | miR‐1277‐5p/SGTB | Chondrocyte apoptosis↑ Inflammation↑ | Human chondrocyte cell line CHON‐001 |
| LncRNA HOTAIR | Up | Wnt/β‐catenin |
Synovial Inflammation↑ Synovial cell proliferation↑ Synoviocyte cell apoptosis↓ |
Synovial tissue of rat knee joint |
| LncRNA‐CIR | Up | Activated autophagy | ECM degradation↑ | Human articular cartilage tissue |
| LncRNA CIR | Up | miR‐27/MMP13 | ECM degradation↑ | Human knee cartilage tissue |
| LncRNA‐CIR | Up | miR‐130a/Bim |
ROS generation↑ Inflammatory mediators↑ Chondrocyte apoptosis↑ |
Human knee cartilage tissue |
| LncRNA NKILA | Down | miR‐145/SP1/NFκB |
Inflammation↑ Chondrocyte proliferation↓ Apoptosis↑ |
Human articular cartilage tissue |
| LncRNA SNHG5 | Down | miR‐26a/SOX2 | Chondrocyte proliferation↓ | Human knee cartilage tissue |
| LncRNA SNHG5 | Down | miR‐10a‐5p/H3F3B | Chondrocyte apoptosis↑ | Human knee cartilage tissue |
| LncRNA‐H19 | Up | miR‐140‐5p | Chondrocyte apoptosis | Human knee cartilage tissue |
| LncRNA H19 | Up | miR‐130a | apoptosis↑ Inflammation↑ | Human cartilage C28/I2 cells and human renal epithelial cells 293T |
| LncRNA TM1‐3P | Up | miR‐144‐3p/ONECUT2 | ECM degradation↑ | Rat knee cartilage tissue |
| LncRNA TUG1 | Up | miR‐195/MMP‐13 | ECM degradation↑ | Human knee cartilage tissue |
| LncRNA CASC2 | Up | IL‐17 |
Chondrocyte proliferation↓ Chondrocyte apoptosis↑ |
Human plasma and synovial fluid |
| LncRNA CASC19 | Up | miR‐152‐3p/DDX6 | Chondrocyte apoptosis↑ Proinflammatory cytokine↑ | Human knee cartilage tissue, Human cartilage C28/I2 cells |
| LncRNA MIAT | Up | miR‐488‐3p/SOX11 | Apoptosis↑ Inflammation↑ | Human knee cartilage tissue, Human cartilage C28/I2 cells |
| LncRNA SNHG14 | Up |
miR‐124‐3p/FSTL‐1/NLRP3 TLR4/NF‐κB |
Chondrocyte apoptosis↑ Inflammatory mediator↑ |
Human articular cartilage tissue, Rat knee articular cartilage tissue |
| LncRNA GAS5 | Down |
KLF2/NF‐Κb KLF2/Notch pathways |
Inflammation↑ | Murine chondrocytic ATDC5 cell line |
| LncRNA GAS5 | Down | mir‐146a/Smad4 | Chondrocyte apoptosis↑ | Human knee and hip cartilage tissue |
| LncRNA LEMD1‐AS1 | Down | miR‐944/PGAP1 |
Chondrocyte apoptosis↑ Chondrocyte proliferation↓ Inflammation↑ |
Human knee cartilage tissue |
| LncRNA ZFAS1 | Down | Wnt3a |
Chondrocyte proliferation↑ Apoptosis↓ Extracellular matrix↓ |
Human knee cartilage tissue |
| LncRNA SNHG16 | Up | miR‐373‐3p |
Apoptosis↑ Inflammation↑ |
Human chondrocyte cell line CHON‐001, Human joint tissue |
| Linc00341 | Down | YAF2 | Chondrocyte apoptosis↑ | Human knee cartilage tissue |
| LncRNA MIR22HG | Up | miR‐9‐3p/ADAMTS5 | Chondrocyte apoptosis↑ ECM degradation↑ | Human knee cartilage tissue |
| LncRNA SNHG7 | Down | miR‐214‐5p/PPARGC1B |
Chondrocyte viability↓ Cartilage apoptosis↑ Inflammation↑ |
Human knee cartilage tissue, Mouse knee cartilage tissue |
| LncRNA SNHG7 | Down | miR‐34a‐5p/SYVN1 |
Cell proliferation↓ Apoptosis↑ Autophagy reaction↑ |
Human knee cartilage tissue |
| LncRNA SNHG9 | Down | miR‐34a | Chondrocyte apoptosis↓ | Synovial fluid of human knee joint and hip joint |
| LncRNA CTBP1‐AS2 | Up | miR‐130a | Chondrocyte proliferation↓ | Synovial fluid of human knee joint and hip joint |
| LncRNA LOXL1‐AS1 | Up | miR‐423‐5p/KDM5C |
Chondrocyte proliferation↑ Inflammation↑ |
Human knee and hip cartilage tissue |
| LncRNA MEG8 | Down | PI3K/AKT |
Cell proliferation↓ Apoptosis↑ Inflammation↑ |
Human knee cartilage tissue, Human cartilage C28/I2 cells |
| LncRNA DANCR | Up | miR‐577/SphK2 | Apoptosis↑ | Human knee cartilage tissue |
| LncRNA‐MSR | Up | TMSB4 | ECM degradation↑ | Human knee cartilage tissue |
| LncRNA MINCR | Down | miR‐146a‐5p/BMPR2 |
Cell proliferation↓ Apoptosis↑ ECM degradation↑ |
Human knee cartilage tissue |
| LncRNA‐ROR | Down |
HIFla p53 |
Chondrocyte viability↓ Autophagy reaction↓ Apoptosis↑ |
Human knee cartilage tissue |
| LncRNA‐ATB | Down |
miR‐223/MyD88/NF‐Κb miR‐223/p38MAPK |
Inflammation↑ | Murine chondrocytic ATDC5 cell line |
| LncRNA RP11‐445H22.4 | Up | mir‐301a/cxcr4 |
Apoptosis↑ Inflammation↑ |
Human cartilage ATDC5 cells |
| LncRNA FOXD2‐AS1 | Up | miR‐206/CCND1 | Chondrocyte proliferation↑ | Human knee cartilage tissue, Human cartilage C28/I2 cells |
| LncRNA FOXD2‐AS1 | Up | miR‐27a‐3p/TLR4 |
Chondrocyte proliferation↑ Inflammation↑ ECM degradation↑ |
Human articular cartilage tissue, Human cartilage C28/I2 cells |
| LncRNA OIP5‐AS1 | Down | miR‐29b‐3p/PGRN |
Apoptosis↑ Inflammation↑ |
Human knee cartilage tissue, Human chondrocyte cell line CHON‐001, Murine chondrocytic ATDC5 cell line and human embryonic kidney cell line HEK293 |
| LncRNA PVT1 | Up | miR‐149 |
ECM degradation↑ Inflammation↑ |
Human knee cartilage tissue |
| LncRNA PVT1 | Up | miR‐140 | ECM degradation↑ | Human knee cartilage tissue |
| LncRNA PVT1 | Up | miR‐488‐3p | Chondrocyte apoptosis↑ | Human knee cartilage tissue |
| LncRNA SNHG15 | Down | mir‐7/KLF4 |
ECM degradation↑ Chondrocyte formation↓ |
Human knee cartilage tissue |
| LncRNA NEAT1 | Up | miR‐16‐5p | Chondrocyte proliferation↑ | Human knee cartilage tissue |
| LncRNA NEAT1 | Up | miR‐543/PLA2G4A |
Chondrocyte proliferation↓ Apoptosis↑ |
Human knee cartilage tissue |
| LncRNA NEAT1 | Up | miR‐181c/OPN | Synovial cell proliferation↑ | Human synovium tissues |
| LncRNA RMRP | Up | miR‐206/CDK9 |
Chondrocyte proliferation↓ Apoptosis↑ |
Human knee cartilage tissue |
| LncRNA MCM3AP‐AS1 | Up | miR‐142‐3p/HMGB1 | Chondrocyte apoptosis↑ | Synovial fluid of human knee joint and hip joint |
| LncRNA MALAT1 | Up | miR‐150‐5p/AKT3 |
Cell proliferation↑ Apoptosis↓ ECM degradation↓ |
Human knee cartilage tissue |
| LncRNA MALAT1 | Up | miR‐146a‐PI3K/Akt/mTOR | Apoptosis↓ | Rat knee cartilage tissue, Human knee cartilage tissue |
| LncRNA MALAT1 | Up | MALAT1/miR‐145 |
Chondrocyte viability↓ ECM degradation↑ |
Human articular cartilage tissue |
| LncRNA—p21 | Up | miR‐451 |
Cell viability↓ Chondrocyte apoptosis↑ |
Human knee cartilage tissue |
| LncRNA CHRF | Up | miR‐146a/NFκB, miR‐146a/JAK1/STAT3 |
Cell viability↓ Apoptosis↑ Inflammation factors↑ |
ATDC5 cells |
| LncRNA PACER | Down | lncRNA HOTAIR | Chondrocyte apoptosis↑ | Human discomfort plasma, Human chondrocyte cell line CHON‐001 |
| LncRNA LUADT1 | Down | miR‐34a/SIRT1 | Chondrocyte apoptosis↑ | Synovial fluid of human knee joint and hip joint |
| LncRNA LOC101928134 | Up | IFNA1/JAK/STAT |
Synovial hyperplasia↑ Cartilage destruction↑ |
Synovial tissue of rat knee joint |
| LncRNA AFAP1‐AS1 | Up | miR‐512‐3p/MMP‐13 |
Chondrocyte proliferation↑ Extracellular matrix↑ |
Human knee cartilage tissue, Mouse articular cartilage tissue |
| LncRNA PART‐1 | Down | miR‐590‐3p/TGFBR2/Smad3 |
Cell viability↓ Chondrocyte apoptosis↑ |
Human knee cartilage tissue, The immortalized human chondrocytes cell lines C20/A4 |
| LncRNA CAIF | Down | miR‐1246 |
Chondrocyte apoptosis↑ IL‐6↑ |
Synovial fluid of human joint tissue, Human chondrocyte cell line CHON‐001 |
| LINC00623 | Down | miR‐101/HRAS/MAPK |
ECM degradation↑ Chondrocyte apoptosis↑ |
Human knee cartilage tissue |
| LncRNA UFC1 | Down | miR‐34a |
Chondrocyte proliferation↓ Apoptosis↑ |
Human knee cartilage tissue |
Conclusion
Systemic and local elements combine to create KOA in the development process. In terms of the macrocosm, it is mostly related to changes in joint biomechanics brought on by aging, trauma, knee dislocation, and obesity. The micro‐level is brought on by the metabolic syndrome, aberrant bone metabolism, cytokines and associated enzymes, genetic mutations, and alterations in plasma adiponectin. There are no rigid borders between macro and micro; rather, they interact and affect one another. Consequently, a pathological process from the macro to the micro system develops. People will find it easier to diagnose and treat KOA effectively if they completely comprehend its pathophysiology.
Author Contributions
The concept of the manuscript was devised by Xin Du, Zi‐yu Liu and Xing‐xing Tao. Yong‐liang Mei performed the overall literature searches. Xin Du and Zi‐yu Liu were in charge of writing. Tables and figures were designed by Xin Du, Chao Song, Da‐qian Zhou, Kang Cheng and Si‐long Gao. Xiao‐min Zhang, Chao Song and Hou‐yin Shi discussed the content of the article and gave suggestions.
Conflict of Interest Statement
Each author certifies that he or she has no conflict of interest in connection with the submitted article.
Acknowledgement
This work was supported by Key R & D Projects of Sichuan Science and Technology Department (Grant number: 2022YFS0609) and Luzhou Science and Technology Project (Grant number: 2022‐SYF‐88).
Xin Du, Zi‐yu Liu these authors contributed to the work equally and should be regarded as co‐first authors.
Chao Song, Xiao‐min Zhang these authors contributed to the work equally and should be regarded as co‐corresponding authors.
References
- 1. Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104(2):293–311. [DOI] [PubMed] [Google Scholar]
- 2. Martel‐Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. [DOI] [PubMed] [Google Scholar]
- 3. Hunter DJ, Bierma‐Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–59. [DOI] [PubMed] [Google Scholar]
- 4. Hana S, Aicha BT, Selim D, Ines M, Rawdha T. Clinical and radiographic features of knee osteoarthritis of elderly patients. Curr Rheumatol Rev. 2018;14(2):181–7. [DOI] [PubMed] [Google Scholar]
- 5. Willinger ML, Heimroth J, Sodhi N, Garbarino LJ, Gold PA, Rasquinha V, et al. Management of Refractory Pain after Total Joint Replacement. Curr Pain Headache Rep. 2021;25(6):42. [DOI] [PubMed] [Google Scholar]
- 6. Batushansky A, Zhu S, Komaravolu RK, South S, Mehta‐D'souza P, Griffin TM, et al. An initiative of osteoarthritis and cartilage. Obesity and metabolic factors in OA. Osteoarthr Cartil. 2022;30(4):501–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomas S, Browne H, Mobasheri A, Rayman MP. What is the evidence for a role for diet and nutrition in osteoarthritis? Rheumatology. 2018;57(suppl_4):iv61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith PN, Refshauge KM, Scarvell JM. Development of the concepts of knee kinematics11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated. Arch Phys Med Rehabil. 2003;84(12):1895–902. [DOI] [PubMed] [Google Scholar]
- 9. Chen Z, Yan F, Lu Y. The function of mechanical loading on chondrogenesis. Front Biosci (Landmark Ed). 2016;21(6):1222–32. [DOI] [PubMed] [Google Scholar]
- 10. Novakov VB, Novakova ON, Churnosov MI. Risk factors and molecular entities of the etiopathogenesis of the knee osteoarthritis (literature review). Genij Ortopedii. 2021;27(1):112–20. [Google Scholar]
- 11. Shane Anderson A, Loeser RF. Why is osteoarthritis an age‐related disease? Best Pract Res Clin Rheumatol. 2010;24(1):15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bolduc JA, Collins JA, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clockaerts S, Bastiaansen‐Jenniskens YM, Runhaar J, Van Osch GJ, Van Offel JF, Verhaar JA, et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthr Cartil. 2010;18(7):876–82. [DOI] [PubMed] [Google Scholar]
- 14. Chu L, Liu X, He Z, Han X, Yan M, Qu X, et al. Articular cartilage degradation and aberrant subchondral bone remodeling in patients with osteoarthritis and osteoporosis. J Bone Miner Res. 2020;35(3):505–15. [DOI] [PubMed] [Google Scholar]
- 15. Dai Q‐p, Qiu M‐l, Shao PH, et al. Clinical observation on treatment of 60 cases of osteoarthritis of knee joint by electroacupuncture. Journal of Acupuncture&Tuina. Science. 2003;300:1730–4.12805543 [Google Scholar]
- 16. Zhang Y, Hunter DJ, Nevitt MC, Xu L, Niu J, Lui LY, et al. Association of squatting with increased prevalence of radiographic tibiofemoral knee osteoarthritis: the Beijing osteoarthritis study. Arthritis Rheum. 2004;50(4):1187–92. [DOI] [PubMed] [Google Scholar]
- 17. Krishnasamy P, Hall M, Robbins SR. The role of skeletal muscle in the pathophysiology and management of knee osteoarthritis. Rheumatology (Oxford). 2018;57(suppl_4):iv22–33. [DOI] [PubMed] [Google Scholar]
- 18. Patsika G, Kellis E, Kofotolis N, Salonikidis K, Amiridis IG. Synergetic and antagonist muscle strength and activity in women with knee osteoarthritis. J Geriatr Phys Ther. 2014;37(1):17–23. [DOI] [PubMed] [Google Scholar]
- 19. Efendi R, Wahab Z, Setiawan MR. Central and peripheral obesity on severity knee osteoarthritis. 2021.
- 20. Kulkarni K, Karssiens T, Kumar V, Pandit H. Obesity and osteoarthritis. Maturitas. 2016;89:22–8. [DOI] [PubMed] [Google Scholar]
- 21. Kim N, Browning RC, Lerner ZF. The effects of pediatric obesity on patellofemoral joint contact force during walking. Gait Posture. 2019;73:209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Silva M, Dechichi P, Limirio P. Impact of childhood obesity on bone metabolism. Pediatr Endocrinol Rev. 2020;17(4):308–16. [DOI] [PubMed] [Google Scholar]
- 23. Wang X, Li F, Fan C, Wang C, Ruan H. Effects and relationship of ERK1 and ERK2 in interleukin‐1β‐induced alterations in MMP3, MMP13, type II collagen and aggrecan expression in human chondrocytes. Int J Mol Med. 2011;27(4):583–9. [DOI] [PubMed] [Google Scholar]
- 24. Van Beeck A, Clockaerts S, Somville J, Van Heeswijk JH, Van Glabbeek F, Bos PK, et al. Does infrapatellar fat pad resection in total knee arthroplasty impair clinical outcome? A Systematic review. Knee. 2013;20(4):226–31. [DOI] [PubMed] [Google Scholar]
- 25. Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42(1):1–9. v. [DOI] [PubMed] [Google Scholar]
- 26. Lythgo N, Craze M, Selva Raj I, Lim Y. Increased shoe heel height generates greater peak knee extension moments than fast walking speeds. 6th international conference on the development of biomedical engineering in Vietnam (BME6). Berlin: IFMBE Proceedings; 2018. p. 167–70. [Google Scholar]
- 27. Preece SJ, Alghamdi W. Increased trunk flexion may underlie elevated knee flexor activity in people with knee osteoarthritis. Knee. 2021;33:216–25. [DOI] [PubMed] [Google Scholar]
- 28. Booij MJ, Richards R, Harlaar J, van den Noort JC. Effect of walking with a modified gait on activation patterns of the knee spanning muscles in people with medial knee osteoarthritis. Knee. 2020;27(1):198–206. [DOI] [PubMed] [Google Scholar]
- 29. Lee BJ, Yang S, Kwon S, Choi KH, Kim W. Association between metabolic syndrome and knee osteoarthritis: a cross‐sectional nationwide survey study. J Rehabil Med. 2019;51(6):464–70. [DOI] [PubMed] [Google Scholar]
- 30. Calvet J, Orellana C, Larrosa M, Navarro N, Chillaron JJ, Pedro‐Botet J, et al. High prevalence of cardiovascular co‐morbidities in patients with symptomatic knee or hand osteoarthritis. Scand J Rheumatol. 2016;45(1):41–4. [DOI] [PubMed] [Google Scholar]
- 31. Ching K, Houard X, Berenbaum F, Wen C. Hypertension meets osteoarthritis — revisiting the vascular aetiology hypothesis. Nat Rev Rheumatol. 2021;17(9):533–49. [DOI] [PubMed] [Google Scholar]
- 32. Tiyasatkulkovit W, Promruk W, Rojviriya C, Pakawanit P, Chaimongkolnukul K, Kengkoom K, et al. Impairment of bone microstructure and upregulation of osteoclastogenic markers in spontaneously hypertensive rats. Sci Rep. 2019;9(1):12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ashmeik W, Joseph GB, Nevitt MC, Lane NE, McCulloch CE, Link TM. Association of blood pressure with knee cartilage composition and structural knee abnormalities: data from the osteoarthritis initiative. Skeletal Radiol. 2020;49(9):1359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berenbaum F, Wallace IJ, Lieberman DE, Felson DT. Modern‐day environmental factors in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2018;14(11):674–81. [DOI] [PubMed] [Google Scholar]
- 35. Duclos M. Osteoarthritis, obesity and type 2 diabetes: the weight of waist circumference. Ann Phys Rehabil Med. 2016;59(3):157–60. [DOI] [PubMed] [Google Scholar]
- 36. Veronese N, Cooper C, Reginster JY, Hochberg M, Branco J, Bruyere O, et al. Type 2 diabetes mellitus and osteoarthritis. Semin Arthritis Rheum. 2019;49(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berenbaum F. Diabetes‐induced osteoarthritis: from a new paradigm to a new phenotype. Postgrad Med J. 2012;88(1038):240–2. [DOI] [PubMed] [Google Scholar]
- 38. Cannata F, Vadala G, Ambrosio L, Napoli N, Papalia R, Denaro V, et al. Osteoarthritis and type 2 diabetes: from pathogenetic factors to therapeutic intervention. Diabetes Metab Res Rev. 2020;36(3):e3254. [DOI] [PubMed] [Google Scholar]
- 39. Kulkarni P, Martson A, Vidya R, Chitnavis S, Harsulkar A. Pathophysiological landscape of osteoarthritis. Adv Clin Chem. 2021;100:37–90. [DOI] [PubMed] [Google Scholar]
- 40. Onkarappa RS, Chauhan DK, Saikia B, Karim A, Kanojia RK. Metabolic syndrome and its effects on cartilage degeneration vs regeneration: a pilot study using osteoarthritis biomarkers. Indian J Orthop. 2020;54(Suppl 1):20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59(9):1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Felson DT, Niu J, Neogi T, Goggins J, Nevitt MC, Roemer F, et al. Synovitis and the risk of knee osteoarthritis: the MOST study. Osteoarthr Cartil. 2016;24(3):458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jiang M, He J, Sun Y, Dong X, Yao J, Gu H, et al. Leptin induced TLR4 expression via the JAK2‐STAT3 pathway in obesity‐related osteoarthritis. Oxid Med Cell Longev. 2021;2021:7385160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang T, He C. Pro‐inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. [DOI] [PubMed] [Google Scholar]
- 45. Nees TA, Rosshirt N, Zhang JA, Reiner T, Sorbi R, Tripel E, et al. Synovial cytokines significantly correlate with osteoarthritis‐related knee pain and disability: inflammatory mediators of potential clinical relevance. J Clin Med. 2019;8(9):1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koyama T, Uchida K, Fukushima K, Ohashi Y, Uchiyama K, Inoue G, et al. Elevated levels of TNF‐α, IL‐1β and IL‐6 in the synovial tissue of patients with labral tear: a comparative study with hip osteoarthritis. BMC Musculoskelet Disord. 2021;22(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fields JK, Gunther S, Sundberg EJ. Structural basis of IL‐1 family cytokine signaling. Front Immunol. 2019;10:1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro‐Watanabe M. KEGG for taxonomy‐based analysis of pathways and genomes. Nucleic Acids Res. 2023;51(D1):D587–d92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jenei‐Lanzl Z, Meurer A, Zaucke F. Interleukin‐1beta signaling in osteoarthritis – chondrocytes in focus. Cell Signal. 2019;53:212–23. [DOI] [PubMed] [Google Scholar]
- 50. Zhong HM, Ding QH, Chen WP, Luo RB. Vorinostat, a HDAC inhibitor, showed anti‐osteoarthritic activities through inhibition of iNOS and MMP expression, p38 and ERK phosphorylation and blocking NF‐kappaB nuclear translocation. Int Immunopharmacol. 2013;17(2):329–35. [DOI] [PubMed] [Google Scholar]
- 51. Fan Z, Yang H, Bau B, Soder S, Aigner T. Role of mitogen‐activated protein kinases and NFkappaB on IL‐1beta‐induced effects on collagen type II, MMP‐1 and 13 mRNA expression in normal articular human chondrocytes. Rheumatol Int. 2006;26(10):900–3. [DOI] [PubMed] [Google Scholar]
- 52. Xue EX, Lin JP, Zhang Y, Sheng SR, Liu HX, Zhou YL, et al. Pterostilbene inhibits inflammation and ROS production in chondrocytes by activating Nrf2 pathway. Oncotarget. 2017;8(26):41988–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27(1):19–26. [DOI] [PubMed] [Google Scholar]
- 54. Westacott CI, Barakat AF, Wood L, Perry MJ, Neison P, Bisbinas I, et al. Tumor necrosis factor alpha can contribute to focal loss of cartilage in osteoarthritis. Osteoarthr Cartil. 2000;8(3):213–21. [DOI] [PubMed] [Google Scholar]
- 55. Xue J, Wang J, Liu Q, Luo A. Tumor necrosis factor‐alpha induces ADAMTS‐4 expression in human osteoarthritis chondrocytes. Mol Med Rep. 2013;8(6):1755–60. [DOI] [PubMed] [Google Scholar]
- 56. Molnar V, Matisic V, Kodvanj I, Bjelica R, Jelec Z, Hudetz D, et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci. 2021;22(17):9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, et al. Interleukins, from 1 to 37, and interferon‐gamma: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127(3):701–721.e70. [DOI] [PubMed] [Google Scholar]
- 58. Rose‐John S. Interleukin‐6 family cytokines. Cold Spring Harb Perspect Biol. 2018;10(2):a028415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baran P, Hansen S, Waetzig GH, Akbarzadeh M, Lamertz L, Huber HJ, et al. The balance of interleukin (IL)‐6, IL‐6.Soluble IL‐6 receptor (sIL‐6R), and IL‐6.sIL‐6R.sgp130 complexes allows simultaneous classic and trans‐signaling. J Biol Chem. 2018;293(18):6762–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scanzello CR. Chemokines and inflammation in osteoarthritis: insights from patients and animal models. J Orthop Res. 2017;35(4):735–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Monibi F, Roller BL, Stoker A, Garner B, Bal S, Cook JL. Identification of synovial fluid biomarkers for knee osteoarthritis and correlation with radiographic assessment. J Knee Surg. 2016;29(3):242–7. [DOI] [PubMed] [Google Scholar]
- 62. Watt FE, Paterson E, Freidin A, Kenny M, Judge A, Saklatvala J, et al. Acute molecular changes in synovial fluid following human knee injury: association with early clinical outcomes. Arthritis Rheumatol. 2016;68(9):2129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhao XY, Yang ZB, Zhang ZJ, Zhang ZQ, Kang Y, Huang GX, et al. CCL3 serves as a potential plasma biomarker in knee degeneration (osteoarthritis). Osteoarthr Cartil. 2015;23(8):1405–11. [DOI] [PubMed] [Google Scholar]
- 64. Chwastek J, Kedziora M, Borczyk M, Korostynski M, Starowicz K. Inflammation‐driven secretion potential is upregulated in osteoarthritic fibroblast‐like Synoviocytes. Int J Mol Sci. 2022;23(19):11817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen L, Jiang W, Huang J, He BC, Zuo GW, Zhang W, et al. Insulin‐like growth factor 2 (IGF‐2) potentiates BMP‐9‐induced osteogenic differentiation and bone formation. J Bone Miner Res. 2010;25(11):2447–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Plsikova Matejova J, Spakova T, Harvanova D, Lacko M, Filip V, Sepitka R, et al. A preliminary study of combined detection of COMP, TIMP‐1, and MMP‐3 in synovial fluid: potential indicators of osteoarthritis progression. Cartilage. 2021;13(2_suppl):1421S–30S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jarecki J, Malecka‐Masalska T, Kosior‐Jarecka E, Widuchowski W, Krasowski P, Gutbier M, et al. Concentration of selected Metalloproteinases and Osteocalcin in the serum and synovial fluid of obese women with advanced knee osteoarthritis. Int J Environ Res Public Health. 2022;19(6):3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hu Q, Ecker M. Overview of MMP‐13 as a promising target for the treatment of osteoarthritis. Int J Mol Sci. 2021;22(4):1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu S, Deng Z, Chen K, Jian S, Zhou F, Yang Y, et al. Cartilage tissue engineering: from proinflammatory and anti‐inflammatory cytokines to osteoarthritis treatments (review). Mol Med Rep. 2022;25(3):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jang S, Lee K, Ju JH. Recent updates of diagnosis, pathophysiology, and treatment on osteoarthritis of the knee. Int J Mol Sci. 2021;22(5):2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gaballah HH, Gaber RA, Elrashidy MA, Elshahat DA, Hablus MA, Ebeid AM. Expression of long non‐coding RNA CCHE1 in colorectal carcinoma: correlations with clinicopathological features and ERK/COX‐2 pathway. Mol Biol Rep. 2019;46(1):657–67. [DOI] [PubMed] [Google Scholar]
- 72. Wang JB, Jin Y, Wu P, Liu Y, Zhao WJ, Chen JF, et al. Tumor suppressor PLZF regulated by lncRNA ANRIL suppresses proliferation and epithelial mesenchymal transformation of gastric cancer cells. Oncol Rep. 2019;41(2):1007–18. [DOI] [PubMed] [Google Scholar]
- 73. Liu X, Li M, Zhang B, Zhang N, Feng Q. A landscape of circulating long non‐coding RNA (lncRNA) expression profile and the predictive value of candidate lncRNAs for disease risk of knee osteoarthritis. J Clin Lab Anal. 2020;34(9):e23423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74. Chen Y, Lin Y, Bai Y, Cheng D, Bi Z. A long noncoding RNA (lncRNA)‐associated competing endogenous RNA (ceRNA) network identifies eight lncRNA biomarkers in patients with osteoarthritis of the knee. Med Sci Monit. 2019;25:2058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhao Y, Xu J. Synovial fluid‐derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. 2018;42(12):2865–72. [DOI] [PubMed] [Google Scholar]
- 76. Hu P, Sun F, Ran J, Wu L. Identify CRNDE and LINC00152 as the key lncRNAs in age‐related degeneration of articular cartilage through comprehensive and integrative analysis. PeerJ. 2019;7:e7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fu C, Qiu Z, Huang Y, Mei Y, Lin Q, Zeng J, et al. Protective role of Achyranthes bidentata polysaccharides against chondrocyte extracellular matrix degeneration through lncRNA GAS5 in osteoarthritis. Exp Ther Med. 2022;24(2):532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fu C, Qiu Z, Huang Y, Lin Q, Jin L, Tu H, et al. Achyranthes bidentata polysaccharides alleviate endoplasmic reticulum stress in osteoarthritis via lncRNA NEAT1/miR‐377‐3p pathway. Biomed Pharmacother. 2022;154:113551. [DOI] [PubMed] [Google Scholar]
- 79. He CP, Jiang XC, Chen C, Zhang HB, Cao WD, Wu Q, et al. The function of lncRNAs in the pathogenesis of osteoarthritis. Bone Joint Res. 2021;10(2):122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


