Abstract
Depression is a common mental disorder characterized by heterogeneous cognitive and behavioral symptoms. The emerging research paradigm of functional connectomics has provided a quantitative theoretical framework and analytic tools for parsing variations in the organization and function of brain networks in depression. In this review, we first discuss recent progress in depression-associated functional connectome variations. We then discuss treatment-specific brain network outcomes in depression and propose a hypothetical model highlighting the advantages and uniqueness of each treatment in relation to the modulation of specific brain network connectivity and symptoms of depression. Finally, we look to the future promise of combining multiple treatment types in clinical practice, using multisite datasets and multimodal neuroimaging approaches, and identifying biological depression subtypes.
Keywords: depression, antidepressant treatments, resting-state networks, functional magnetic resonance imaging, connectome, therapy-specific network connectivity
Depression and therapies: a brain network perspective
Depression is a prevalent and disabling psychiatric disorder characterized by persistently depressed mood as well as a host of other heterogeneous symptoms, such as loss of pleasure or interest, feelings of guilt and worthlessness, changes in appetite and weight, tiredness and lack of energy, sleep disturbances, and impairments in cognitive function such as attention and memory [1]. In addition, depression is the leading cause of persistent impairment in well-being and quality of life and is associated with significant morbidity and mortality [2].
A variety of treatments are available in clinical practice, such as pharmacotherapy [3], psychotherapy [4], neuromodulation [5], and sleep deprivation [6]. Typically, depressed patients are treated with pharmacotherapy using various antidepressant medications or different types of psychotherapy, such as the most frequently used cognitive behavioral therapy (CBT). However, approximately 30% of depressed patients do not respond to pharmacotherapy or psychotherapy [7]. To improve the treatment response (see Glossary) and clinical remission rates, alternative treatment options are used for patients with treatment-resistant depression (TRD [8]) when the full course of pharmacotherapy or psychotherapy is completed without sufficient improvement. Specifically, neuromodulation approaches, such as transcranial magnetic stimulation (TMS) [9], electroconvulsive therapy (ECT) [10], and deep brain stimulation (DBS) [11,12], have been used to improve depression symptoms by modulating neuronal activity in the brain.
Critically, when combined with imaging, treatments can help uncover the causal circuit-based mechanisms of symptoms improvement [13]. Approaches that elicit fast-acting antidepressant effects such as ketamine and sleep deprivation are especially appealing, as they offer experimentally tractable opportunities to investigate how fast brain changes could induce rapid remission from depression [6,14]. However, these interventions are not yet optimal due to low response/remission rates and high relapse rates [3,4,6,9,11,15], and there is an urgent need to identify the neurobiological mechanisms of depression and effective therapies, as this holds promise for the development of novel interventions and the identification of novel personalized treatment targets.
Understanding the functional connectivity and topological organization of the human brain, commonly referred to as the functional connectome, is crucial for comprehending normal brain functioning [16] and the neurobiological mechanisms of brain disorders [17–20]. Depression, in particular, is increasingly being recognized as a brain network disorder [21–24]. Consequently, there is a growing tendency to conceptualize antidepressant treatments as network therapies that improve depressive symptoms by modulating the functional connectivity of multiple brain subnetworks (for review, see [13,25–27]).
In this article, we first briefly describe the key concepts and organization of human functional brain networks. In the next two sections, we review the existing empirical data on functional brain network abnormalities and related depressive symptoms in untreated depressed individuals. We then discuss treatment-specific functional network changes in depression. In particular, we highlight the advantages and uniqueness of each treatment in relation to the modulation of specific network connectivity and dimensional symptoms of depression. Next, we propose a hypothetical three-dimensional network model that provides a conceptual framework for understanding the relationships between brain network connectivity changes, specific depressive symptoms, and different treatment effects in depression. Finally, we outline the prospects of the combination of multiple treatment types in clinical practice with the use of multisite datasets and multimodal neuroimaging approaches, and of the identification of biological depression subtypes for addressing disease heterogeneity and developing novel symptom-specific and connectome-guided therapeutic targets. For information on how we identified relevant studies, see Supplementary Materials.
Human functional brain networks
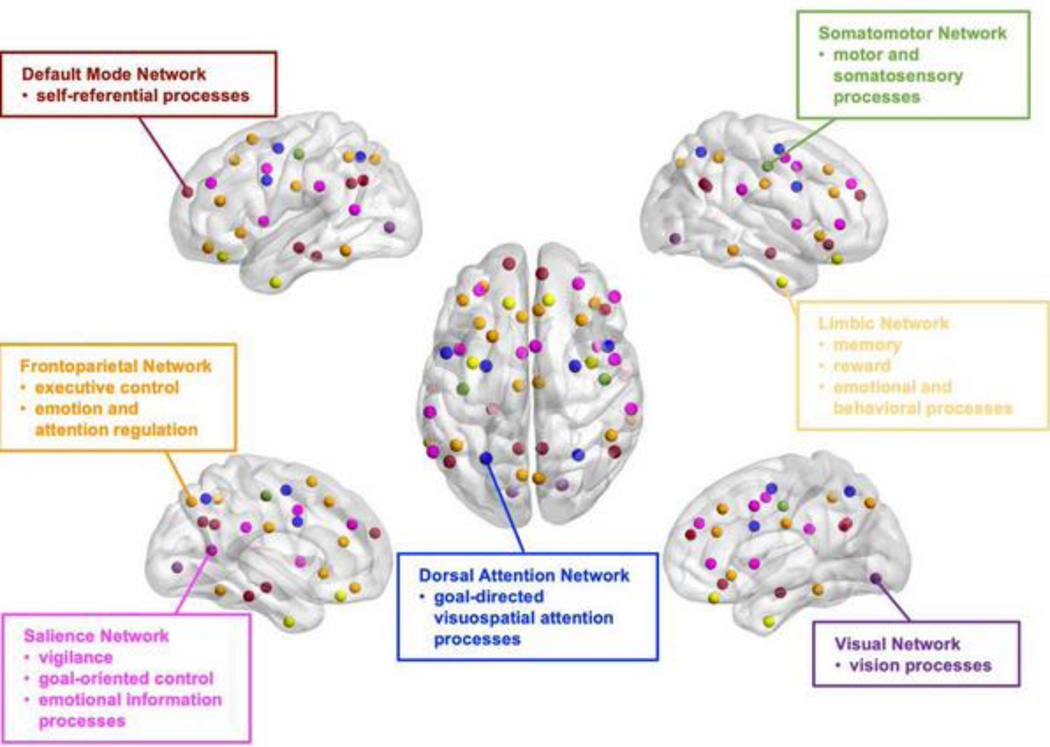
The human brain is a complex network (i.e., connectome) consisting of subnetworks and hubs (highly connected brain regions) [16,18,28]. In this review, we focus on functional brain network studies identified using functional magnetic resonance imaging (fMRI) data. Resting-state fMRI (rs-fMRI) studies have identified multiple subnetworks, also called resting-state networks (RSNs; see Box 1 and Figure 1), including the default mode network (DMN), frontoparietal network (FPN), salience network (SAN, also termed as cingulo-opercular network (CON) [29] or ventral attention network (VAN) [30]), limbic network (LIM), dorsal attention network (DAN), somatomotor network (SMN), and visual network (VIS). Each of these RSNs plays an important role in a specific cognitive and/or behavioral domain [31], and their architectures are crucial for maintaining brain function. Importantly, these RSNs are linked by hubs, such as the precuneus, angular gyrus, and ventromedial prefrontal cortex (vmPFC) in the DMN [32] and posterior middle frontal gyrus (pMFG) in the FPN [33]. These hubs facilitate efficient communication and information integration from multiple RSNs [34]. Functional network connectivity estimated from resting-state or task-related fMRI data has shown better behavioral prediction performance than anatomical and diffusion MRI (dMRI) measures, regardless of regression model or behavioral measure [35]. This highlights the significance of investigating functional network abnormalities in the context of depression.
Box 1. Resting-state networks.
The DMN includes mPFC, PCC, precuneus, IPL, and middle temporal lobe [36]. This task-negative network plays an important role in self-referential processes such as evaluating the salience of internal and external cues, remembering the past and planning the future.
The FPN [29], also termed as executive control network [191], is comprised of the dlPFC, OFC, MFG, SFG, rostral ACC, and other brain regions that are active during goal-directed tasks [31]. This task-positive network is implicated in executive control, cognitive reappraisal, and top-down regulation of attentional and emotional processing. It has been suggested that the FPN may contain two separate subnetworks: one connected to the DMN and involved in the regulation of introspective processes; another connected to the DAN and involved in the regulation of visuospatial perceptual attention [192].
There is an anatomical overlap between the limbic network (LIM) identified by fMRI studies and the limbic system defined by the anatomical boundary between the cerebral hemispheres and the brainstem. Specifically, subcortical regions, such as the amygdala, hippocampus, striatum, thalamus, and insula, are central components of the LIM and are involved in memory, reward, and emotional and behavioral processes. [193].
The SAN, consisting of dACC, AI and striatum, is involved in detecting and orienting attention toward salient stimuli, and switching between internal and external thinking [20,37]. In contrast to the FPN, which initiates and adjusts control on a trail-by-trial basis, the SAN provides stable goal-directed control over tasks [194]. The SAN has also been termed as CON [29] or VAN [30]. A recent review study [195] recommended the use of a universal taxonomy to name RSNs, where SAN, CON and VAN were named as ‘midcingulo-insular network’.
There are two sensory orienting attention systems, the VAN (or SAN) and DAN [196]. The VAN is involved in directing attention to stimuli that suddenly appears rather than towards stimuli that are currently the focus of the task at hand. The DAN includes the posterior intraparietal sulcus, frontal eye fields, middle temporal region, ventral premotor cortex, pre- and postcentral gyrus. The DAN is prominently involved in goal-directed and voluntary orienting of visuospatial attention [30].
FMRI and multimodal MRI studies have consistently identified several sensory networks [197,198], such as the SMN, auditory network (AUD) and VIS. The SMN primarily includes the somatosensory (e.g., postcentral gyrus) and motor (e.g., precentral gyrus) regions and extends to the supplementary motor areas. This network plays a major role in the execution of movements of the contralateral side of the body. The AUD is a part of the temporal lobe that processes auditory information. The VIS located in the occipital lobe consists of regions of visual cortex that processes visual information.
Figure 1. Cognition related resting-state networks (RSNs).
Each RSN has been suggested to play an important role in specific cognitive and/or behavioral domain(s): DMN, self-referential processes; LIM, memory, reward, emotional and behavioral processes; FPN, executive control, emotion and attention regulation; SAN, vigilance, goal-oriented control, and emotional information processes; DAN, goal-directed visuospatial attention processes; SMN, motor and somatosensory processes; VIS, vision processes (for details, see Box 1). The color-coded nodes depicts seven RSNs defined by the Yeo parcellation [208]. RSNs, resting-state networks; DMN, default mode network; LIM, limbic network; FPN, frontoparietal network; SAN, salience network; DAN, dorsal attention network; SMN, somatomotor network; VIS, visual network. The brain mapping for RSNs were plotted by BrainNet Viewer.
Human functional brain network abnormalities in untreated depression
Many fMRI studies have reported abnormal functional network connectivity in multiple RSNs, including the DMN, FPN, SAN, and LIM, in individuals with unipolar or bipolar depressive disorder compared with controls. These RSNs are involved in a variety of cognitive functioning (Figure 1), such as self-referential thinking (e.g., DMN) [36], executive control (e.g., FPN) [20], detection and integration of emotionally significant internal and external stimuli (e.g., SAN) [37], and emotion and memory processing (e.g., LIM) [38,39]. In particular, depressed individuals have shown hypoconnectivity (reduced FC) within the FPN [40,41] and SAN [42,43]. However, a few studies have reported hyperconnectivity (increased FC) in the FPN [44] and SAN [40] in depressed individuals compared with controls. Depressed individuals have also shown hyperconnectivity in the DMN [40,45– 47] and LIM [48]. However, a few studies have reported hypoconnectivity [49] or mixed results [44,50] of the DMN. Some studies have also observed hyperconnectivity within the VIS [40], and hypoconnectivity within the DAN [40,42], SAN [40], and SMN [42] in depression, although these findings are less frequently reported than those for the DMN, FPN, SAN, and LIM.
Inconsistent results are often attributed to genetic, environmental, and clinical heterogeneity of the samples and to variability in analytic methods. The use of multisite data [51–53] could help produce converging and generalizable findings by improving statistical power. Until we are able to identify a unified analytic framework, reliable findings are likely to be generated when tested with different approaches (e.g., brain atlases [51,54], connectivity [51,55], and network [56] metrics, preprocessing methods [57], etc.).
Regarding between-network connectivity, depressed individuals have shown hypoconnectivity between the LIM and some other RSNs, such as the FPN [58,59], DMN [41,60], and SAN [61], hypoconnectivity between the DMN and FPN [58], and hypoconnectivity between the FPN and SAN [43,44]. A few studies have also reported hyperconnectivity between the DMN and FPN [40–42], hyper-[40,46] or hypoconnectivity [42] between the DMN and SAN, and hyper- [40] or hypoconnectivity [41,42] between the FPN and DAN.
Recent research of connectomics using graph-theoretical tools has also revealed disrupted topological organization of brain networks in depression [23], including disturbed global integrity (quantified by clustering coefficient [62], shortest path length [62–64] and global efficiency [63,64]), modular structure [65], and regional nodal connectivity (characterized by nodal degree [64], betweenness centrality [63,64,66], and local efficiency [63,64]).
Overall, depression has been associated with altered topological organization and functional connectivity of RSNs primarily in the DMN, FPN, SAN, and LIM.
Symptom-specific functional brain network abnormalities in untreated depression
Depressed patients often have heterogeneous symptoms as described above. As specific depressive symptoms might differ in the underlying neurobiology, symptom-specific connectome studies are a promising tool to identify biomarkers [67]. Recent studies have reported a set of symptom-specific functional network abnormalities in depression [68]. Specifically, hyper- [50,69–72] or hypoconnectivity [73] within the DMN was associated with increased rumination. Hyperconnectivity within the DMN was also associated with increased pessimism [74]. Several studies found that patients with higher levels of depressive mood and anxiety showed hypoconnectivity within the SAN [e.g., ventral striatum (VS)-caudate/midcingulate] [75] and between the FPN [e.g., dorsolateral prefrontal cortex (dlPFC)] and SAN [e.g., anterior insula (AI)] [76]. Depressed patients with greater anhedonia showed hyperconnectivity within the SAN (e.g., VS-caudate/midcingulate) [75], DMN [e.g., vmPFCdorsomedial prefrontal cortex (dmPFC)], VIS [77], and between the DMN (e.g., vmPFC) and right FPN (e.g., right dlPFC), as well as hypoconnectivity between the FPN [e.g., MFG and dorsal anterior cingulate cortex (dACC)] and SAN (e.g., striatum) [78,79], between the DMN (e.g., vmPFC) and LIM (e.g., ventral tegmental area/striatum), and between the DMN (e.g., vmPFC) and left FPN (e.g., left dlPFC) [80]. In addition, some studies found that patients with higher levels of somatic symptom severity showed lower neural activity in the SAN (e.g., dorsal mid-insula deactivation) [81] and hypoconnectivity between the FPN [e.g., orbitofrontal cortex (OFC)] and SAN (e.g., AI) [82]. Moreover, greater neuroticism in depressed patients was associated with higher within-network connectivity in the SAN, FPN, and SMN, as well as between-network connectivity of SAN-FPN, SAN-SMN, and DMN-LIM [83]. Suicidality was associated with higher connectivity between the FPN [e.g., middle/superior frontal gyrus (MFG/SFG)] and SAN (e.g., VS) [75]. Low flexibility in FPN may be involved in anxiety syndrome while hypoflexibility in DMN may be indicative of hysteresis [84]. In addition, abnormalities in network connectivity have also been found to be associated with depression severity [64,75], illness duration [42,63], number and length of episodes [43,52], and traumatic childhood experiences [40,85].
Taken together, different depressive symptoms were related to the connectivity of different RSNs, such as rumination in relation to DMN connectivity, depressive mood in relation to FPN-involved connectivity, anxiety in relation to SAN- and FPN-involved connectivity, and anhedonia in relation to SAN-, DMN-, VIS-, and FPN-involved connectivity.
Therapy-specific functional brain network changes in depression
As summarized above, depression is associated with impaired functional connectivity in multiple brain networks related to different depression symptoms. Although the therapeutic implications of these findings remain unclear, one possible implication could be that antidepressant interventions improve depressive symptoms by selectively modulating pathological connectivity patterns. Such an explanation has gained crucial support from recent neuroimaging studies (see key studies listed in Table 1; for a complete list, see Table S1 in the Supplementary Materials). In the following sections, we will first discuss treatment-induced connectivity changes and then targeted interventions for depression.
Table 1.
Treatment-induced network connectivity changes
| Pharmacotherapy | Psychotherapy | Sleep Deprivation | Electroconvulsive Therapy | Transcranial Magnetic Stimulation |
Deep Brain Stimulation | |
|---|---|---|---|---|---|---|
| DMN | ↓ DMN [48,93,94] | ↓ DMN [137] | ↓ DMN [142] | |||
| FPN | ↑ FPN-SAN [43,91,92]; ↑ FPN-DMN [99] |
↑ FPN [102] | ↑ FPN-posterior DMN [58,114,115]; ↓ FPN-anterior DMN [96,116] |
↓ FPN-DMN [117,136,137] |
↑ FPN [142] | |
| SAN | ↓ SAN-cerebellum [95]; ↑ SAN-DMN [99] |
↓ SAN [101] | ↓ SAN [143]; ↑ SAN-DMN [143] |
|||
| LIM | ↓ LIM [48]; ↑ LIM-FPN/SAN [48,59,60,86–90] |
↑ LIM-FPN/DMN [61,104,204–207] |
↑ LIM-SAN [113] |
↑ LIM-DMN [138,139] |
↓ LIM [142]; ↑ LIM- DMN/FPN/SAN [144,145] |
|
| DAN | ↑ DAN [142] | |||||
| SMN | ↑ SMN [95]; ↓ SMN-FPN/SAN [43] |
↓ SMN-SAN [143] |
||||
| VIS | ↑ VIS [96]; ↑ VIS-FPN [96]; ↓ VIS-posterior DMN [96] |
Treatment-induced connectivity changes
Antidepressant medications.
Antidepressants (e.g., SSRIs, SNRIs, and ketamine; Box 2) improve the performance of depressive individuals through modulating FC primarily within and between the FPN, DMN, SAN, and LIM networks (Table 1). Multiple fMRI studies, both during active tasks and in a resting state, have consistently indicated that antidepressants can aid in the restoration of emotional regulation and promote a positive mindset by reducing self-referential thinking. Of note, medication-induced reduction of LIM connectivity (e.g., amygdala, hippocampus, medial thalamus, and nucleus accumbens) [48] and increase of connectivity between FPN/SAN and LIM (e.g., between PFC/ACC and amygdala, hippocampus, medial thalamus, and pallidostriatum) [48,59,60,86–90] might reflect antidepressants’ role in the suppression of bottom-up emotional hyperarousal and the normalization of abnormal top-down cortical control over subcortical emotional processes in depressed patients.
Box 2. Pharmacotherapy.
Pharmacotherapy primarily involves several typical and atypical antidepressants. Typical antidepressants generally include selective serotonin reuptake inhibitors (SSRIs, such as sertraline, fluoxetine, paroxetine, citalopram, and escitalopram), serotonin and norepinephrine reuptake inhibitors (SNRIs, such as venlafaxine, duloxetine, agomelatine, and mirtazapine), tricyclic antidepressants (TCAs), noradrenergic and specific serotonergic antidepressants (NaSSA), and serotonin antagonist and reuptake inhibitors (SARIs). Atypical antidepressants include ketamine, bupropion, amisulpride, among others. Typical antidepressants such as selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs) are usually used as first-line medications for depression [199]. SSRIs exert action by inhibiting the reuptake of serotonin back into the nerve cells that released them, which increases serotonin activity in the brain. Similarly, SNRIs work by blocking the reuptake of serotonin and norepinephrine, thereby increasing the levels of active neurotransmitters. It usually takes at least two to three weeks for SSRIs/SNRIs to achieve symptom relief and three to six months for clinical remission. Among depressed individuals who take SSRIs/SNRIs, approximately 40%−60% of them respond to a single course of treatment, and the remission rate is typically in the range of 30%−49% [3].
Intriguingly, a single subanesthetic dose of ketamine, an ionotropic glutamatergic N-methyl-D-aspartate (NMDA) receptor antagonist, produced a rapid antidepressant effect lasting up to 7 days in depression with a response rate of 52.6% and remission rate of 30% [14]. While many antidepressants act on monoamine neurotransmitters (e.g., serotonin, norepinephrine, or dopamine), ketamine triggers the production of glutamate, the most prominent neurotransmitter of the brain. Glutamate receptors are found throughout the brain and spinal cord in neurons and glia.
It is of high interest for future studies to explore if different antidepressants share a common or specific mechanism in modulating brain network changes. For instance, as discussed above, different antidepressants modulate neural transport by increasing or decreasing the levels of specific neurotransmitters in the brain. Of note, recent studies [200] demonstrate that density distributions of receptors across multiple neurotransmitter systems are related to the structural and functional organization of connectomes and disease vulnerability of multiple psychiatric disorders, including major depression. Therefore, it would be interesting for future studies to explore the spatial relationships between functional network connectivity patterns and the receptor densities of specific neurotransmitters targeted by different antidepressants.
Several event-related and resting-state fMRI studies have also reported medication-elicited FC increases in frontostriatal reward circuitry (e.g., between ventrolateral prefrontal cortex/ACC and caudate/putamen) [43,91,92], which indicates that the increased connectivity between the FPN and SAN, which leads to the restoration of reward processing, is a crucial factor that contributes to the clinical improvement seen with antidepressant medications. Antidepressants have also been found to modulate the heightened DMN connectivity in depression, and this could be interpreted as a restoration of the capacity to appropriately regulate self-referential activities [48,93,94]. The effects of antidepressants were also associated with FC changes of other RSNs (e.g., DAN, SMN, VIS, and cerebellum-SAN) [43,95–97] or baseline functional connectome characteristics (e.g., connectome gradient, connectome fingerprint, functional connectivity strength) [53,83,98]. For instance, a recent connectome study [99] found reduced DMN connectivity and increased connectivity between the DMN and FPN and between the DMN and SAN one day after applying psilocybin therapy.
Psychotherapy.
As the most frequently used psychotherapy, CBT may be used to improve specific symptoms by modulating brain networks regarding emotional attention control (e.g., the VAN/SAN) and cognitive control (e.g., the FPN) [100]. A longitudinal rs-fMRI study [101] reported symptom-specific connectivity changes in the SAN following 12 weeks of CBT in patients with MDD and posttraumatic stress disorder (PTSD). Specifically, this study found that after 12 weeks of CBT treatment, improvement of depressive symptoms, but not anxious arousal symptoms, was associated with decreased within- and between-network connectivity in the SAN among both MDD and PTSD patients. A task-based CBT-fMRI study [102] confirmed the symptom-specific transdiagnostic brain-symptom association––abnormal task-induced (emotional conflict task) brain activities in the FPN regions (e.g., dlPFC) were related to improved depressive symptoms in both MDD and PTSD after CBT. These findings of common symptom-specific transdiagnostic network changes supports the use of the NIMH Research Domain Criteria (RDoC; [103]) for classifying mental disorders based on behavioral dimensions and neurobiological measures.
The efficacy of psychotherapy has also been shown to associate with pre-treatment connectivity between the LIM and FPN/DMN. In an rs-fMRI study [104] of MDD individuals treated with either CBT or antidepressant medication (escitalopram or duloxetine), the summed pre-treatment FC between the subcallosal cingulate cortex (SCC, a component of the LIM) and left ventrolateral prefrontal cortex (vlPFC)/AI, vmPFC, and dorsal midbrain were positively connected in CBT remitters and negative in CBT failures, whereas the inverse was true for antidepressant medication remitters and failures. Moreover, the correlations between the summed FC and percent change in depression severity across all MDD individuals were significant for both treatments, though the strength of the negative correlation was stronger among CBT-treated individuals than the strength of the positive correlation among medication-treated individuals. Findings from this study suggest that abnormal LIM-FPN/DMN connectivity related to emotion dysregulation in MDD individuals might be an important predictor of outcomes to differing forms of antidepressant treatment, with higher FC prior to treatment predicting better response to CBT but poorer response to pharmacotherapy.
Sleep deprivation.
Sleep deprivation (SD; [105]) is an effective and rapid antidepressant treatment, which offers immediate relief from depression in 40%−60% of depressed individuals with a single night of total or partial SD [6,106] (Box 3). SD has not been widely used in clinical settings, partially because symptom improvement due to SD is usually short-lived (depressive symptoms relapse after recovery sleep) [106]. A few studies focusing on healthy individuals have shown the effects of SD on brain network connectivity changes [105,107,108] and neurocognitive deficits for example episodic memory deficits due to one night of total SD (TSD) [107]. However, neuroimaging studies of SD in depression are still rare, and it remains unclear how SD could improve depression symptoms by modulating brain network connectivity.
Box 3. Psychotherapy and sleep deprivation.
Several different types of psychotherapies, such as cognitive-behavioral therapy (CBT), interpersonal psychotherapy (IPT) and problem-solving therapy (PST), are recommended as first-line treatments for depression [4]. As one of the most commonly used psychotherapies, CBT focuses on identifying cognitive distortions that lead to persistent depressive mood and utilizes emotional regulation skills to change the irrational thoughts. IPT is a structured and brief intervention addressing social issues that maintain depression. PST imparts problem solving skills aimed to increase the individual’s mastery of the environment and reduce the experience of adversity. As a second-line treatment, behavioral activation therapy for depression (BATD) is developed to define problematic behaviors that maintain depression, thereby ameliorating depressive symptoms (anhedonia symptoms in particular) by promoting rewarding activities and inhibiting avoidance behaviors. Psychotherapies usually require weekly 45- to 90-minite sessions over 10 to 20 weeks. Overall, these psychotherapies have a response rate ranging from 37% to 46%, and a remission rate ranging from 26% to 34% [4].
Sleep Deprivation (SD) has been proven to be a fast-acting (immediate response after a night of sleep loss) and effective treatment for depression with an overall response rate of 45%−50% [6]. SD has not been widely used in clinical practice partly due to its temporary effect on depressive symptoms (e.g., clinical improvement in mood decreases as soon as individuals resume sleeping) [106]. However, the rapidity of onset and offset of clinical improvement is an advantage, as it allows for an ABA study design for investigation of underlying mechanisms of action with greater certainty of causality.
The dorsal nexus (DN), first identified by Sheline et al. [109], is a region in bilateral dorsal medial prefrontal cortex with increased connectivity to DMN, FPN, and affective network (e.g., LIM) in MDD, since implicated in adolescents at risk for depression [110] and in the mechanism of action of ketamine [111]. An rs-fMRI study [112] of partial SD in healthy participants found increased FC of the DN with dlPFC areas and reduced FC of the posterior cingulate cortex (PCC) with ACC. It was indicated that this shift from affective to cognitive network contributions to the DMN could be beneficial in depressed individuals who suffer from excessive ACC and/or impaired dlPFC function. These findings suggest DN-dlPFC connectivity change as a potential explanation of the rapid antidepressant treatment response to SD.
A recent study used TSD as a probe along with rs-fMRI to examine associations between mood changes and changes of the amygdala- and DN-involved connectivity after one night of TSD in both patients with MDD and healthy individuals [113]. This study found that TSD enhanced both amygdala-ACC and DN-dlPFC connectivity in healthy individuals. Amygdala-ACC connectivity increased significantly after TSD in depressed patients with mood improvement but not in depressed patients without mood improvement. Moreover, the enhanced amygdala-ACC connectivity was associated with antidepressant effects of TSD in depressed patients and better mood in healthy individuals. These findings support the key role of the amygdala-ACC circuit in mood regulation in both depressed and healthy populations and suggest that rapid antidepressant treatment may target the enhancement of amygdala-ACC connectivity.
Electroconvulsive therapy.
ECT might alleviate depressive symptoms through modulating FC primarily between the FPN and differing components of the DMN. Several studies have suggested increased FPN-posterior DMN connectivity following ECT being a potential biomarker of recovery from a depressive episode. In particular, two rs-fMRI studies [58,114] reported ECT-elicited FC increases between FPN (e.g., vlPFC, anterolateral PFC, MFG) and posterior DMN (e.g., PCC) at both network and nodal levels, and their associations with depression improvement in MDD patients compared to controls [114]. In line with these findings, an rs-fMRI study in late-life TRD showed normalized hypoconnectivity between posterior DMN and FPN (e.g., left dlPFC) after ECT [115], with greater FC increases in ECT remitters but not non-remitters.
There is also evidence that ECT decreased FC between the FPN (e.g., dlPFC) and anterior DMN (e.g., medial frontal cortex and ACC) in depressed patients from an event-related [116] and a resting-state [96] fMRI study, with FC decreases accompanied by significant symptom reductions. Findings from these studies suggest that FC between the FPN and different subnetworks of the DMN might contribute to diverse treatment effects of ECT.
Targeting intervention
Transcranial magnetic stimulation.
TMS is a noninvasive neuromodulation technique that uses magnetic fields to modulate neuronal activity in a specific brain area (Box 4). However, rTMS can effectively relieve symptoms of many but not all (30%−40%) depressed patients, possibly because of the heterogeneity of individual symptoms and brain network organization. For example, there is converging evidence [117–119] that the most effective TMS target is located at the left dlPFC, which shows strong anti-correlation with the subgenual anterior cingulate cortex (sgACC). However, the exact location and connectivity pattern of the left dlPFC varies across individuals, resulting in variability in treatment response. Therefore, identifying personalized rTMS targets may play a key role in optimally treating depressed patients (for review, see [13,120]). Indeed, two fMRI-TMS studies [121,122] have reported consistent association between the proximity to personalized targets estimated by individualized functional connectivity and clinical response to rTMS treatment in MDD patients. Of note, this association disappeared when group-average rTMS targets were used instead, indicating that personalized rTMS targets are perhaps more useful than group-average targets in predicting clinical outcomes. Of note, previous studies [123–126] reported that reliable individual-level functional network connectivity estimated by single-echo fMRI may require a large amount of data collection (e.g., 5 hours of rs-fMRI data), which is challenging for clinical studies. A recent study [127] developed a novel personalized TMS targeting approach by leveraging individualized fMRI-based functional connectome and cortical folding patterns to identify optimized TMS coil placement. This study showed the feasibility of the clinical application of personalized TMS targeting by using 30 minutes of multi-echo fMRI data for each patient [127].
Box 4. Neuromodulation: TMS.
Transcranial magnetic stimulation (TMS) is a noninvasive technique that uses a rapidly alternating magnetic field to stimulate specific cortical areas of the brain, thereby inducing electrical currents and action potentials in underlying cortical tissue [25]. The biological mechanism of rTMS in treating depression is thought to involve both short-term and long-term changes in synaptic plasticity, which is the ability of neurons to modify their connections in response to experience. In the short-term, rTMS can increase or decrease the excitability of the neurons in the stimulated area, depending on the frequency and intensity of the stimulation. For example, repeated pulses at high frequencies (e.g., 20–50 Hz) are thought to excite cortical activities whereas low-frequency stimulation (e.g., 1 Hz) suppress activity. In the long-term, rTMS is often thought to induce neuroplastic changes in the brain that may be related to the therapeutic effects of the treatment. These changes may include the modulation of neurotransmitter systems (such as dopamine, serotonin, and glutamate), the alteration of synaptic connectivity, and the promotion of neurogenesis (the formation of new neurons). However, the precise mechanisms underlying these effects are still being investigated. TMS changes communication between a cortical stimulation site and any remote target or set of targets, impacting a distributed network of brain regions [13]. Among brain regions that show depression-related abnormalities, the left dlPFC and the sgACC have been most frequently selected as targets of focal brain stimulation [25,117–119]. Other targets include dmPFC [128] and OFC [130], which might affect different neural networks. TMS is typically used for the treatment of medication-resistant depression, and it is usually administered 4 or 5 times weekly for 4 to 6 weeks. The response rate of TMS is typically in the range of 29%−46% and remission rate in the range of 18%−31% [9].
Personalized rTMS could be a promising approach for treating specific depression symptoms by targeting specific brain networks [128–130] (for review, see [25,26]). For example, one study [129] found that dysphoric symptoms, such as sadness, anhedonia, and suicidal thoughts, responded best to stimulation of the circuit involving anterolateral dlPFC sites with anticorrelated FC to the sgACC, while anxiety and somatic symptoms, such as insomnia, decreased libido, and irritability, responded best to stimulation of a different circuit involving FC of posterior dlPFC sites with mPFC. Another study [131] developed a novel approach to identify personalized, symptom-specific TMS targets. Specifically, this study combined each individual’s whole brain resting-state functional connectivity, depression symptom scores, and electric-field modelling to predict symptom changes in anxious misery patients [132]. A generalized research protocol [133] for conducting electric field-optimized, fMRI-based network connectivity-guided, and subject-specific TMS targeting has been proposed. This line of research highlighted the importance of identifying symptom-specific brain networks, which might yield new targets for different symptoms, patient subgroups, or treatment personalization [25]. Although brain network-based personalized rTMS is promising, its clinical efficacy needs to be validated by randomized clinical trials.
Of note, many brain network studies [25] have shown that rTMS may not only stimulate the targeted region (e.g., dlPFC), but also a network (e.g., FPN) of regions functionally connected to the target. In addition, single-pulse TMS studies [134,135] have also demonstrated target engagement with networks. These findings shift the focus of rTMS studies from single brain regions to large-scale brain networks for improving spatial TMS targeting for depression.
Targeting dlPFC, findings from rs-fMRI studies converged on a common decrease of dlPFC-sgACC connectivity (e.g., DMN-FPN) relating to therapeutic efficacy of TMS [117,136,137]. Targeting dmPFC, better treatment outcomes were associated with increased FC between dmPFC and thalamic/striatal emotion-related regions (e.g., DMN-LIM) [138,139]. Baseline hyper- [80,137,138,140] or hypoconnectivity [118,119,121,122] between sgACC and multiple areas of the FPN was predictive of greater clinical improvements after TMS. These findings suggest that the action of TMS to the dlPFC might relate to remote suppression of neural activity in the sgACC, whereas TMS to the dmPFC might relate to improved executive control over emotional functions.
Deep brain stimulation.
DBS might improve depressive patients’ clinical pathology through modulating FC within and between the FPN, DAN, DMN, and LIM [141]. In support, a recent multimodal study (rs-fMRI combined with structural MRI and/or head CT) leveraging 14 independent datasets [142] found that DBS and TMS stimulation sites that modulate depression were connected to a brain circuit involving the FPN and DAN while anticorrelated with the DMN and LIM, which may represent a refined neurostimulation target for depression.
In a recent rs-fMRI study [143], DBS at the SCC site suppressed activity in corticolimbic regions in patients with MDD, bipolar disorder, or anorexia nervosa. Moreover, SCC-DBS modulated corticolimbic FC through strengthening FC of the three seed regions [e.g., dACC, PCC, and precuneus] with each other, FC of dACC with rostral ACC, mPFC, and frontal pole, FC of PCC with IFG, motor cortical areas, and subcortical structures, and FC of precuneus with insular-opercular cortex, temporal areas, and inferior parietal lobule (IPL). SCC-DBS was also associated with decreased FC between right and left dACC and between right dACC and motor cortical areas. Another rs-fMRI study [144] from the same research group reported increased habenula (a region in the LIM) connectivity with several prefrontal and corticolimbic regions (e.g., mPFC, dlPFC, ACC, PCC) following SCC-DBS in a sub-cohort of patients. Together, SCC-DBS appears to regulate depressive mood by increasing FC between the LIM and DMN/FPN/SAN and between the SAN and DMN, while decreasing FC within the SAN and between the SAN and SMN.
Besides fMRI, functional connectomics constructed from electrophysiology data [17], in particular intracranial EEG (iEEG), have been used to identify DBS targets. One recent study [145] implemented a novel biomarker-driven closed-loop DBS therapy in a female, who was not responsive to different types of treatment, such as antidepressant medications, ECT, and TMS. Specifically, the authors stimulated and measured neural activity from ten iEEG electrodes implanted in the OFC, amygdala, hippocampus, ventral capsule (VC)/VS, and sgACC [12]. They identified several state-dependent symptom-specific DBS targets and built a directed connectome by estimating effective connectivity between neural signals recorded from the brain areas mentioned above. The right VC/VS in this directed connectome had the highest weighted outdegree and therefore was identified as the DBS target. As a result, when stimulating the VC/VS site, there was a strong evoked response in the amygdala [e.g., increased VC/VS-amygdala connectivity]. The authors used DTI data to identify structural connectivity between the amygdala and VC/VS, providing the evidence that the effective connectivity between the two brain areas was supported by their underlying structural backbone (i.e., axonal fiber tracts; please also see another study [146]). Moreover, decreased amygdala gamma power was related to depression symptom improvement, which could be modulated by the VC/VS stimulation. Therefore, the identified VC/VS-amygdala circuit might serve as a promising personalized symptom-specific biomarker, which was translated into a closed-loop therapy in this study. Findings of this study highlight the importance of integrating multimodal neuroimaging techniques (i.e., dMRI and iEEG) in accurately developing network-guided, symptom-specific personalized DBS targeting.
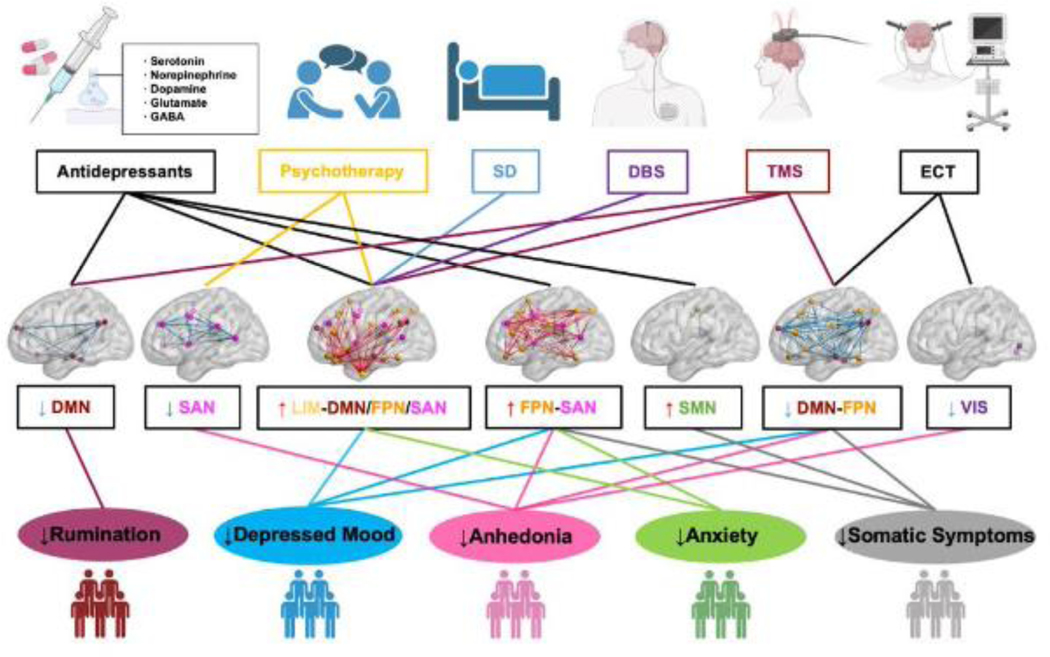
A hypothetical three-dimensional network-symptom-therapy model
Inspired by the triple network model by Menon [20], we now propose a three-dimensional network model offering a conceptual framework for understanding the relationships between specific depression symptoms and connectivity changes in functional subnetworks (or RSNs), as well as the effects of different therapies (Figure 2). Briefly, Menon’s triple network model of psychopathology posits that abnormal functional organization of the SAN, FPN, and DMN and their dynamic cross-network interactions underlie a wide range of psychopathologies, including major depression. Thus, the triple network model is essentially a two-dimensional model, relating brain network changes in three RSNs (network dimension) to multiple psychopathologies (cognitive dysfunction dimension). In this review, we focus on MDD and expand the triple network model by adding a therapy dimension, highlighting the common or distinct effects of different therapies on symptom-specific brain network changes. In addition, as the field evolves, besides the three brain subnetworks (SAN, FPN and DMN) included in the triple network model, recent studies have found additional brain subnetworks as described above, such as the DAN, LIM, and sensory networks (SMN and VIS), that demonstrate abnormal network connectivity in patients with MDD compared with controls. Therefore, we broaden the network dimension from three networks to seven networks.
Figure 2. Hypothetical model of symptom-specific, network-guided treatments.
The model was created by a summary of key studies (Table 1) from an emerging literature describing associations between six treatments, brain network abnormalities involving seven RSNs, and five depressive symptoms. Abnormal functional connectivity has been found in multiple RSNs in depressed patients with heterogeneous symptoms. The abnormal connectivity shown in each RSN tend to be associated with its cognitive domain-related symptoms of depression. Of note, different types of treatments are likely to modulate the abnormal connectivity within and/or between common or distinct cognitive-related RSNs, which might improve specific subtypes of depression symptoms, such as rumination, depressed mood, anhedonia, anxiety, somatic symptoms, etc. Specifically, the levels of rumination in depressed patients could be reduced by modulating DMN connectivity using antidepressants and TMS. Antidepressants, TMS and ECT could modulate FPN- and SMN-involved connectivity and reduce the levels of somatic symptoms. All the six treatments could be used to reduce the levels of depressed mood by modulating the connectivity between the FPN and other RSNs, except the two sensory networks, SMN and VIS. Changes of SAN-, FPN-, and VIS-involved connectivity induced by different treatment types (except SD and DBS) are related to the reduction of anhedonia levels. Multiple treatments (except ECT) are used to modulate the FPN- and SAN-involved between network connectivity, which can decrease the levels of anxiety. SD, sleep deprivation; DBS, deep brain stimulation; TMS, transcranial magnetic stimulation; ECT, electroconvulsive therapy. The illustrations of treatments and symptom subtypes were adapted from BioRender.com.
The main objective of our hypothetical network model is to provide a theoretical framework for hypothesis testing that relates functional connectome changes with specific symptoms and therapies in major depression, which might ultimately facilitate the development of symptom-specific, network-guided treatment targets. For example, studies have found that abnormal rumination-related DMN hyperconnectivity could be reduced by antidepressants [68,72,93,94] and TMS [137] (Figure 2). However, patient with recurrent depression showed abnormal DMN hypoconnectivity after using antidepressants [49], suggesting that further decreasing DMN connectivity to abnormal levels might re-induce depressive symptoms. Future studies could use network control tools together with antidepressants or TMS to optimally control the DMN connectivity and therefore maintain the benefits of treatments [147,148]. In the same spirit, other treatments, such as psychotherapy, SD, ECT and DBS, could be used to modulate specific cognitive-related RSNs (Table 1) and improve corresponding depression symptom subtypes, such as depressed mood, anhedonia, anxiety, and somatic symptoms (Figure 2). It should be noted that while we have utilized the existing data to reinforce our model, we acknowledge that certain suggested associations are in the form of hypotheses, rather than established facts. Of course, this framework would need to be validated through empirical research, not only in human studies, but also in animal models, to ensure that it accurately explains the pathophysiology of depression and provides a basis for developing effective treatments.
Concluding remarks and future perspectives
This review has taken a network perspective toward understanding the mechanisms of depression and antidepressant effects to facilitate the development of network-guided personalized targets. Using modern neuroimaging tools and connectome models, depression has been conceptualized as a network disorder with abnormal hypoconnectivity and hyperconnectivity within and between multiple RSNs. Antidepressant treatments modulate specific connectivity among these RSNs that lead to clinical improvement in specific dimensions of depression symptoms. Encouragingly, the modulated network connectivity appears to track with clinical improvement following treatment. Therefore, these RSNs might be convergent substrates for both depression and antidepressant effects, supporting a growing belief that network-level effects might be important in understanding depression pathophysiology and therapeutic mechanisms [142,149,150] and highlights the therapeutic potential of targeted brain network modulation.
Importantly, different therapies tend to modulate symptom-related connectivity changes of distinct RSNs (Figure 1), suggesting different brain-to-behavior effects of these therapies. For example, pharmacotherapy in general appears to modulate FC in distributed brain networks. In contrast, TMS and DBS tend to modulate FC in more specific RSNs. One explanation may come from the different neurobiological mechanisms of different therapies (see Boxes 2–4). For example, neurotransmitter receptors are spread across different brain networks and are thought to respond to pharmacotherapy and others, whereas focal brain stimulation therapies like rTMS and DBS by necessity target a specific region of a brain network. Of note, the effects of rTMS and DBS are not restricted to the stimulated region but will propagate to specific downstream parts of the network. However, due to the lack of studies directly comparing the effects of different therapies (e.g., pharmacotherapy vs. rTMS) to functional brain network changes, we could not exclude the possibility that the different effects of different therapies to network changes might be due to heterogeneity of the studies, such as genetic, environmental, and clinical heterogeneity of the individuals. Nonetheless, we argue that reliable symptom-specific, network-guided targets are likely to be identified in individuals with more homogenous genotypes and phenotypes than have hitherto been analyzed. Therefore, to control for the issue of heterogeneity, homogeneous sampling approaches could be considered in future comparable studies [151].
The findings regarding therapy-specific changes of brain subnetworks and related symptoms support the speculation that patients with multiple depression symptoms may benefit from a combination of multiple symptom-specific treatments by simultaneously modulating connectivity in multiple RSNs. A growing literature has shown the efficacy of combining multiple treatments in sustaining clinical gains. For example, augmentation of citalopram with methylphenidate led to higher remission rates compared to either drug alone [152,153]. A combination of treatment types (e.g., antidepressants and psychotherapy) improved remission rates and reduced relapse and recurrence rates of depression [154,155]. SD in patients taking antidepressants is associated with lower rates of relapse [106]. Neuroimaging and connectome techniques could help identify combined treatment plans with symptom-specific and network-guided targeting for depression, eventually facilitating personalized treatment(s) for each individual patient.
Most brain–phenotype modelling studies have assumed that a single brain–phenotype relationship would generalize across all individuals, but models do not work equally well in all participants, especially in participants who defy sample stereotypes [156]. Recent studies [157,158] have uncovered limitations in generalizing population-specific models to other socio-demographic groups. Moreover, individual functional network topology differences [123,126,159,160] have been frequently reported and likely also contribute to difficulty matching networks across studies [161]. Also, there are inconsistent overlaps between what is measured by “depression” scales across studies that may add additional noise [162]. In addition, the results’ inconsistency across studies might be explained by the different sources of variation captured by individual-level and group-level analyses. In particular, individual pathology may get lost in aggregated statistical estimates in group level analysis. For example, one study [163] found that conclusions drawn from aggregated data may be inaccurate, as the variance in individuals is up to four times larger than in group. Therefore, the sample-by-sample group differences in the discussed studies could be real but are weighted by the brain/symptom state of the most abnormal patients in the group at that timepoint. Such failures in group-to-individual generalization have been noted as a particular challenge in research on humans [164]. Future studies focused on developing individualized targets will be promising to overcome the limitations of group-level analysis.
There are likely to be real effects in the neuroimaging literature but there are certainly false positives that cannot be refuted until the use of better-characterized samples (e.g., deep phenotyping [165]) is combined with the use of broader-scale statistical approaches that are less focused on localizing single brain areas [166]. In addition, the test-retest reliability of resting-state connectivity measurements can be improved using multi-echo fMRI [167], which will facilitate longitudinal precision mapping of functional brain networks in clinical populations. Furthermore, recent studies have developed several computational models, such as normative modelling [168] and functional random forest [169], for overcoming the comorbidity and heterogeneity problems [170]. Finally, as noted above, MDD pathology is a multifactorial process that depends on the interactions between clinical and cognitive dysfunction, connectome changes, and genetics. Multivariate analytical approaches [171], such as canonical correlation analysis, partial least squares, and structural equation modeling, could be promising tools for integrating the multidimensional data and studying their relationships, although they might require large sample size for ensuring sufficient power and reliability [172,173].
To increase statistical power and generalizability of results, future studies should leverage large-scale multisite datasets, such as the UK Biobank [174], the Enhancing Neuroimaging Genetics Through Meta-Analysis (ENIGMA) consortium [175], and the Adolescent Brain Cognitive Development (ABCD) study [176]. Meanwhile, statistical harmonization methods should be used to minimize confounding effects of site-related differences due to different scanner manufacturers, imaging acquisition protocols, and subject recruitment criteria. For example, the site effects in FC measurements could be successfully removed using the widely used ComBat harmonization approach [51,52] and other methods [177,178].
Moving forward, given the heterogeneity of depression, it is unlikely that a single neuroimaging marker can capture all the important features of depression. Multimodal neuroimaging techniques, such as structural, functional, and dMRI, provide complementary but also redundant information in the estimation of individualized connectivity maps. The recently developed multilayer biophysical network models [179,180] can be used to integrate functional and structural network connectivity patterns and study interactions between different network configurations, which may provide more reliable markers to inform diagnostics and identify individualized treatment targets. Moreover, to address the clinical heterogeneity of depression, biological depression subtypes could be identified by using symptom- and treatment-specific large-scale brain network connectivity patterns [27,80,181]. However, current depression biotypes are not yet optimally reproducible. MDD has been classified into different subtypes based on the presence or absence of specific symptoms, age of onset, severity, and other clinical features. It should be noted that reliable biotypes of depression can only be identified if we are able to find distinct biological (e.g., brain network features) and clinical features in major depression. However, due to the biological and clinical heterogeneity of the disease [169,182], it remains a challenge to identify reliable biotypes of depression. In addition, genotypes and environmental factors may also play important roles in the identification of biotypes of major depression [183]. Therefore, we argue that reliable biotypes of major depression are likely to be identified in individuals with more homogenous genotypes and phenotypes than have hitherto been analyzed. Future neuroimaging studies should leverage knowledge of connectomics [16,184], high-quality [185] and large sample-sized neuroimaging data [172,186], and data dimensionality reduction methods [187], as well as proper statistical or machine-learning techniques [188,189] to identify replicable and interpretable connectome-based depression biotypes, which can ultimately be applied in clinical settings [190].
To conclude, emerging evidence suggests that depressive symptoms are likely to be related to abnormal functional network dysconnectivity of multiple brain subnetworks and different therapies might improve specific symptoms through modulating functional connectivity of specific brain subnetworks. The proposed hypothetical three-dimensional network model offers a conceptual framework to synthesize a wide range of studies that examine how the effects of various therapies are related to brain connectivity changes across multiple functional subnetworks, as well as specific symptoms of depression. We hope this review and hypothetical network model could facilitate the development of personalized symptom-specific and connectome-guided therapeutic targets.
Supplementary Material
Box 5. Neuromodulation: ECT, DBS, and VNS.
Electroconvulsive therapy (ECT) is also a noninvasive medical treatment which remains the most effective approach for medication-resistant depression. ECT causes a small amount of electric current to pass through the electrodes to the brain, intentionally triggering a brief seizure that usually lasts less than 60 seconds while the patient is under anesthesia. The ECT stimulus was delivered by electrodes placed either bilaterally or unilaterally at the frontal and/or temporal lobe. ECT is generally administered 2 or 3 times a week over a course of 3 to 4 weeks. The response rate is better for ECT (50%−80%) than for other currently available treatments [10], and the remission rate of ECT generally ranges from 48% to 65% [15].
Deep brain stimulation (DBS) is an invasive neuromodulation technique that employs surgically implanted electrodes into deep neural targets to deliver low-voltage electrical pulses to a specific brain region to modulate brain activity [12]. Several target sites for stimulation have been proposed for the treatment of refractory depression, such as the subcallosal cingulate (SCC), VC/VS, and medial forebrain bundle (MFB). It usually takes 4 to 6 months for the DBS to work. The response and remission rates were 56% (ranging from 43% to 69%) and 35% (ranging from 27% to 44%), respectively [11].
Vagus nerve stimulation (VNS; [201,202]) is also a U.S. Food and Drug Administration (FDA) approved neuromodulation treatment for chronic depression that remains unresponsive to at least two different types of antidepressants. Currently, due to limited available fMRI-based connectome studies conducted in conjunction with VNS, here we provide a brief overview of the biological mechanism of VNS for completeness. Briefly, VNS uses a device to stimulate the vagus nerve (a long nerve that extends from the brainstem through the neck and down into the abdomen) with electrical impulses. The device sends electrical signals to the vagus nerve, which then travels to the brain, where it can affect mood, appetite, and other physiological processes. To our knowledge, there is only one fMRI study [203] reported that transcutaneous VNS could improve depressive symptoms by modulating DMN-involved functional connectivity.
Outstanding questions.
How can we develop new brain stimulation techniques and machine learning tools to accurately map symptoms onto brain networks? It remains unclear if different depression symptoms are associated with specific connectivity changes of different brain networks.
How do different treatments (i.e., TMS vs. medications) interact and in which way they could collaboratively treat patients with multiple symptoms? What would be a feasible temporal ordering to apply different treatments for patients with heterogeneous symptoms?
How can we accurately extract and integrate unique neural information from each neuroimaging modality? Different neuroimaging approaches could provide complementary but also redundant neuronal information.
How can we identify replicable and interpretable connectome-based, symptom- and treatment-specific depression biotypes? Current depression biological subtypes identified by neuroimaging-based machine learning models are often not generalizable.
Which spatial and temporal scales of the connectome are most valuable in identifying connectome-guided and symptom-specific targeting? Currently, human connectome analysis, structural or functional, are conducted at different spatial and temporal scales, which leads to high variability and low reproducibility of findings.
Highlights.
Depressed patients often have cognitive and behavioral deficits, characterized by heterogeneous symptoms, the neurobiological mechanism of which remains unknown.
There are different treatment types for depression. However, the response and remission rates remain low, and the neurobiological basis of treatment effects is unclear.
Functional connectomics has provided useful tools to characterize depression as a brain network disorder and guide treatment decision-making.
Recent work suggests that multi-dimensional depression symptoms are linked with abnormalities of functional connectivity and network organization in different brain networks. Each treatment type tends to improve specific symptoms by modulating specific networks, suggesting the need of combining different treatments.
Identifying network-guided, symptom-specific personalized treatment targets is the key for addressing heterogeneity in depression and treatment response.
We proposed a hypothetical model offering a theoretical framework for hypothesis testing that relates functional connectome changes with specific symptoms and therapies in major depression.
Acknowledgements
The preparation of this review was supported in part by Alzheimer’s Association Research Fellowship Program (AARF-22-722571) and by U.S. National Institutes of Mental Health grants: U01 MH109991, R01MH111886, RF1MH116920, and K01MH121777. The authors report no conflicts of interest.
Glossary
- Clinical remission
a HAMD score ≤7 or a MADRS score ≤10 for two consecutive weeks
- Connectome
a comprehensive map of neural connections in the brain
- Connectomics
the production and study of connectomes. It may range in scale from a detailed map of the full set of neurons and synapses within part or all the nervous system of an organism to a macro scale description of the functional and structural connectivity between all cortical areas and subcortical structures
- Research Domain Criteria (RDoC)
an initiative being developed to refine psychiatric nosology. It is a dimensional approach to nosology that characterizes individuals across continuous traits
- Topology
the quantification of features of a graph in the context of space defined by the graph itself, without respect to any physical embedding
- Treatment response
a >50% reduction in score of the Hamilton Rating Scale for Depression (HAMD) or Montgomery Asberg Depression Rating Scale (MADRS) between the initial and follow-up assessments
- Treatment-resistant depression
non-responsiveness to at least two adequate trials with different classes of antidepressants with adequate dosage, duration (a minimum of 6 weeks for each trial), and compliance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Malhi GS and Mann JJ (2018) Depression. Lancet 392, 2299–2312 [DOI] [PubMed] [Google Scholar]
- 2.Chesney E. et al. (2014) Risks of all-cause and suicide mortality in mental disorders: A meta-review. World Psychiatry 13, 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rush AJ et al. (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry 163, 1905–1917 [DOI] [PubMed] [Google Scholar]
- 4.Cuijpers P. et al. (2021) The effects of psychotherapies for depression on response, remission, reliable change, and deterioration: A meta-analysis. Acta Psychiatr. Scand 144, 288–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marangell LB et al. (2007) Neurostimulation therapies in depression: a review of new modalities. Acta Psychiatr. Scand 116, 174–181 [DOI] [PubMed] [Google Scholar]
- 6.Boland EM et al. (2017) Meta-analysis of the antidepressant effects of acute sleep depri vation. J. Clin. Psychiatry 78, e1020–e1034 [DOI] [PubMed] [Google Scholar]
- 7.Otte C. et al. (2016) Major depressive disorder. Nat. Rev. Dis. Prim 2, 16065 [DOI] [PubMed] [Google Scholar]
- 8.Fava M. (2003) Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry 53, 649–659 [DOI] [PubMed] [Google Scholar]
- 9.Berlim MT et al. (2014) Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol. Med 44, 225–239 [DOI] [PubMed] [Google Scholar]
- 10.Rhee TG et al. (2022) Efficacy and Safety of Ketamine vs Electroconvulsive Therapy Among Patients With Major Depressive Episode: A Systematic Review and Meta-analysis. JAMA Psychiatry DOI: 10.1001/jamapsychiatry.2022.3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y. et al. Deep Brain Stimulation in Treatment-Resistant Depression: A Systematic Review and Meta-Analysis on Efficacy and Safety., Frontiers in neuroscience, 15. (2021), 655412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayberg HS et al. (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660 [DOI] [PubMed] [Google Scholar]
- 13.Oathes DJ et al. (2021) Combining transcranial magnetic stimulation with functional magnetic resonance imaging for probing and modulating neural circuits relevant to affective disorders. Wiley Interdiscip. Rev. Cogn. Sci 12, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newport DJ et al. (2015) Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am. J. Psychiatry 172, 950–966 [DOI] [PubMed] [Google Scholar]
- 15.Heijnen WT et al. (2010) Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J. Clin. Psychopharmacol 30, 616–619 [DOI] [PubMed] [Google Scholar]
- 16.Bullmore E. and Sporns O. (2009) Complex brain networks : graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci 10, 186–198 [DOI] [PubMed] [Google Scholar]
- 17.Stam CJ (2014) Modern network science of neurological disorders. Nat. Rev. Neurosci 15, 683–695 [DOI] [PubMed] [Google Scholar]
- 18.Fornito A. et al. (2015) The connectomics of brain disorders. Nat. Rev. Neurosci 16, 159–172 [DOI] [PubMed] [Google Scholar]
- 19.van den Heuvel MP and Sporns O. (2019) A cross-disorder connectome landscape of brain dysconnectivity. Nat. Rev. Neurosci 20, 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menon V. (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci 15, 483–506 [DOI] [PubMed] [Google Scholar]
- 21.Spellman T. and Liston C. (2020) Toward circuit mechanisms of pathophysiology in depression. Am. J. Psychiatry 177, 381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips ML et al. (2015) Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: Neuroimaging approaches. Am. J. Psychiatry 172, 124–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong Q. and He Y. (2015) Depression, neuroimaging and connectomics: A selective overview. Biol. Psychiatry 77, 223–235 [DOI] [PubMed] [Google Scholar]
- 24.Brakowski J. et al. (2017) Resting state brain network function in major depression – Depression symptomatology, antidepressant treatment effects, future research. J. Psychiatr. Res 92, 147–159 [DOI] [PubMed] [Google Scholar]
- 25.Cash RFH et al. (2021) Using Brain Imaging to Improve Spatial Targeting of Transcranial Magnetic Stimulation for Depression. Biol. Psychiatry 90, 689–700 [DOI] [PubMed] [Google Scholar]
- 26.Fox MD (2018) Mapping Symptoms to Brain Networks with the Human Connectome. N. Engl. J. Med 379, 2237–2245 [DOI] [PubMed] [Google Scholar]
- 27.Dunlop K. et al. (2019) Intrinsic Brain Network Biomarkers of Antidepressant Response: a Review. Curr. Psychiatry Rep 21, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu M. et al. (2021) The human connectome in Alzheimer disease — relationship to biomarkers and genetics. Nat. Rev. Neurol 17, 545–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dosenbach NUF et al. (2007) Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci 104, 11073–11078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox MD et al. (2006) Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U. S. A 103, 10046–10051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox MD and Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci 8, 700–711 [DOI] [PubMed] [Google Scholar]
- 32.Gordon EM et al. (2018) Three Distinct Sets of Connector Hubs Integrate Human Brain Function. Cell Rep. 24, 1687–1695.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z. et al. (2022) Meta-connectomic analysis maps consistent, reproducible, and transcriptionally relevant functional connectome hubs in the human brain. Commun. Biol 5, 1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sporns O. (2013) Network attributes for segregation and integration in the human brain. Curr. Opin. Neurobiol 23, 162–171 [DOI] [PubMed] [Google Scholar]
- 35.Ooi LQR et al. (2022) Comparison of individualized behavioral predictions across anatomical, diffusion and functional connectivity MRI. Neuroimage 263, 119636 [DOI] [PubMed] [Google Scholar]
- 36.Raichle ME (2015) The brain’s default mode network. Annu. Rev. Neurosci 38, 433–447 [DOI] [PubMed] [Google Scholar]
- 37.Seeley WW (2019) The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci 39, 9878–9882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Admon R. and Pizzagalli DA (2015) Dysfunctional reward processing in depression. Curr. Opin. Psychol 4, 114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kupfer DJ et al. (2012) Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Lancet 379, 1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu M. et al. (2019) Childhood trauma history is linked to abnormal brain connectivity in major depression. Proc. Natl. Acad. Sci 116, 8582–8590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaiser RH et al. (2015) Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sacchet MD et al. (2016) Large-scale hypoconnectivity between resting-state functional networks in unmedicated adolescent major depressive disorder. Neuropsychopharmacology 41, 2951–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J. et al. (2021) The neuroprogressive nature of major depressive disorder: evidence from an intrinsic connectome analysis. Transl. Psychiatry 11, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sha Z. et al. (2019) Common Dysfunction of Large-Scale Neurocognitive Networks Across Psychiatric Disorders. Biol. Psychiatry 85, 379–388 [DOI] [PubMed] [Google Scholar]
- 45.Greicius MD et al. (2007) Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol. Psychiatry 62, 429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheline YI et al. (2009) The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U. S. A 106, 1942–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scalabrini A. et al. (2020) All roads lead to the default-mode network-global source of DMN abnormalities in major depressive disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 45, 2058–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siegel JS et al. (2021) Prolonged ketamine infusion modulates limbic connectivity and induces sustained remission of treatment-resistant depression. Psychopharmacology (Berl). 238, 1157–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan CG et al. (2019) Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc. Natl. Acad. Sci 116, 9078–9083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu X. et al. (2012) Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol. Psychiatry 71, 611–617 [DOI] [PubMed] [Google Scholar]
- 51.Yu M. et al. (2018) Statistical harmonization corrects site effects in functional connectivity measurements from multi-site fMRI data. Hum. Brain Mapp 39, 4213–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia M. et al. (2019) Reproducibility of functional brain alterations in major depressive disorder: Evidence from a multisite resting-state functional MRI study with 1,434 individuals. Neuroimage 189, 700–714 [DOI] [PubMed] [Google Scholar]
- 53.Xia M. et al. (2022) Connectome gradient dysfunction in major depression and its association with gene expression profiles and treatment outcomes. Mol. Psychiatry 27, 1384–1393 [DOI] [PubMed] [Google Scholar]
- 54.Bryce NV et al. (2021) Brain parcellation selection: An overlooked decision point with meaningful effects on individual differences in resting-state functional connectivity. Neuroimage 243, 118487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahadevan AS et al. (2021) Evaluating the sensitivity of functional connectivity measures to motion artifact in resting-state fMRI data. Neuroimage 241, 118408 [DOI] [PubMed] [Google Scholar]
- 56.Deuker L. et al. (2009) Reproducibility of graph metrics of human brain functional networks. Neuroimage 47, 1460–1468 [DOI] [PubMed] [Google Scholar]
- 57.Botvinik-Nezer R. et al. (2020) Variability in the analysis of a single neuroimaging dataset by many teams. Nature 582, 84–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J. et al. (2020) Electroconvulsive therapy modulates functional interactions between submodules of the emotion regulation network in major depressive disorder. Transl. Psychiatry 10, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vasavada MM et al. (2021) Effects of Serial Ketamine Infusions on Corticolimbic Functional Connectivity in Major Depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6, 735–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y. et al. (2021) Functional impairment-based segmentation of anterior cingulate cortex in depression and its relationship with treatment effects. Hum. Brain Mapp 42, 4035–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Straub J. et al. (2017) Successful group psychotherapy of depression in adolescents alters fronto-limbic resting-state connectivity. J. Affect. Disord 209, 135–139 [DOI] [PubMed] [Google Scholar]
- 62.Jin C. et al. (2011) A preliminary study of the dysregulation of the resting networks in first-episode medication-naive adolescent depression. Neurosci. Lett 503, 105–109 [DOI] [PubMed] [Google Scholar]
- 63.Meng C. et al. (2014) Aberrant topology of striatum’s connectivity is associated with the number of episodes in depression. Brain 137, 598–609 [DOI] [PubMed] [Google Scholar]
- 64.Zhang J. et al. (2011) Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol. Psychiatry 70, 334–342 [DOI] [PubMed] [Google Scholar]
- 65.Lord A. et al. (2012) Changes in community structure of resting state functional connectivity in unipolar depression. PLoS One 7, e41282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bohr IJ et al. (2013) Resting-state functional connectivity in late-life depression: Higher global connectivity and more long distance connections. Front. Psychiatry 3, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fried EI and Nesse RM (2015) Depression sum-scores don’t add up: why analyzing specific depression symptoms is essential. BMC Med. 13, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li BJ et al. (2018) A brain network model for depression: From symptom understanding to disease intervention. CNS Neurosci. Ther 24, 1004–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berman MG et al. (2011) Depression, rumination and the default network. Soc. Cogn. Affect. Neurosci 6, 548–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamilton JP et al. (2015) Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol. Psychiatry 78, 224–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou H-X et al. (2020) Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. Neuroimage 206, 116287 [DOI] [PubMed] [Google Scholar]
- 72.Dutta A. et al. (2019) Regional default mode network connectivity in major depressive disorder: modulation by acute intravenous citalopram. Transl. Psychiatry 9, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Connolly CG et al. (2013) Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol. Psychiatry 74, 898–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alexopoulos GS et al. (2012) Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord 139, 56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quevedo K. et al. (2017) Ventral Striatum Functional Connectivity during Rewards and Losses and Symptomatology in Depressed Patients. Biol. Psychol 123, 62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan H. et al. (2020) Connectivity between the anterior insula and dorsolateral prefrontal cortex links early symptom improvement to treatment response. J. Affect. Disord 260, 490–497 [DOI] [PubMed] [Google Scholar]
- 77.Geller WN et al. (2021) Specificity of anhedonic alterations in resting-state network connectivity and structure: A transdiagnostic approach. Psychiatry Res. Neuroimaging 317, 111349 [DOI] [PubMed] [Google Scholar]
- 78.Heller AS et al. (2009) Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc. Natl. Acad. Sci 106, 22445–22450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Admon R. et al. (2015) Dissociable cortico-striatal connectivity abnormalities in major depression in response to monetary gains and penalties. Psychol. Med 45, 121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Downar J. et al. (2014) Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol. Psychiatry 76, 176–185 [DOI] [PubMed] [Google Scholar]
- 81.Avery JA et al. (2014) Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol. Psychiatry 76, 258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang T. et al. (2021) Abnormal connectivity of anterior-insular subdivisions and relationship with somatic symptom in depressive patients. Brain Imaging Behav. 15, 1760–1768 [DOI] [PubMed] [Google Scholar]
- 83.Braund TA et al. (2021) Intrinsic Functional Connectomes Characterize Neuroticism in Major Depressive Disorder and Predict Antidepressant Treatment Outcomes. Biol. Psychiatry Cogn. Neurosci. Neuroimaging DOI: 10.1016/j.bpsc.2021.07.010 [DOI] [PubMed] [Google Scholar]
- 84.Tian S. et al. (2020) Antidepressants normalize brain flexibility associated with multidimensional symptoms in major depressive patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 100, 109866 [DOI] [PubMed] [Google Scholar]
- 85.Teicher MH et al. (2016) The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci 17, 652–666 [DOI] [PubMed] [Google Scholar]
- 86.Anand A. et al. (2005) Antidepressant effect on connectivity of the mood-regulating circuit: An fMRI study. Neuropsychopharmacology 30, 1334–1344 [DOI] [PubMed] [Google Scholar]
- 87.Anand A. et al. (2007) Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: An fMRI study. J. Neuropsychiatry Clin. Neurosci 19, 274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen CH et al. (2008) Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology 33, 1909–1918 [DOI] [PubMed] [Google Scholar]
- 89.Altinay M. et al. (2016) Quetiapine Extended Release Open-Label Treatment Associated Changes in Amygdala Activation and Connectivity in Anxious Depression: An fMRI Study. J. Clin. Psychopharmacol 36, 562–571 [DOI] [PubMed] [Google Scholar]
- 90.Wang L. et al. (2015) The effects of antidepressant treatment on resting-state functional brain networks in patients with major depressive disorder. Hum. Brain Mapp 36, 768–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mkrtchian A. et al. (2021) Ketamine modulates fronto-striatal circuitry in depressed and healthy individuals. Mol. Psychiatry 26, 3292–3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fischer AS et al. (2021) Intrinsic reward circuit connectivity profiles underlying symptom and quality of life outcomes following antidepressant medication: a report from the iSPOT-D trial. Neuropsychopharmacology 46, 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li B. et al. (2013) A treatment-resistant default mode subnetwork in major depression. Biol. Psychiatry 74, 48–54 [DOI] [PubMed] [Google Scholar]
- 94.Andreescu C. et al. (2013) Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. 214, 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sahib AK et al. (2020) Modulation of the functional connectome in major depressive disorder by ketamine therapy. Psychol. Med 3, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moreno-Ortega M. et al. (2019) Resting state functional connectivity predictors of treatment response to electroconvulsive therapy in depression. Sci. Rep 9, 5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Korgaonkar MS et al. (2020) Intrinsic connectomes are a predictive biomarker of remission in major depressive disorder. Mol. Psychiatry 25, 1537–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abdallah CG et al. (2021) A robust and reproducible connectome fingerprint of ketamine is highly associated with the connectomic signature of antidepressants. Neuropsychopharmacology 46, 478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Daws RE et al. (2022) Increased global integration in the brain after psilocybin therapy for depression. Nat. Med 28, 844–851 [DOI] [PubMed] [Google Scholar]
- 100.Leaver AM et al. (2022) Parsing the Network Mechanisms of Electroconvulsive Therapy. Biol. Psychiatry 92, 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang Z. et al. (2018) Network changes associated with transdiagnostic depressive symptom improvement following cognitive behavioral therapy in MDD and PTSD. Mol. Psychiatry 23, 2314–2323 [DOI] [PubMed] [Google Scholar]
- 102.Yang Z. et al. (2018) Cognitive Behavioral Therapy Is Associated With Enhanced Cognitive Control Network Activity in Major Depression and Posttraumatic Stress Disorder. Biol. psychiatry. Cogn. Neurosci. neuroimaging 3, 311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cuthbert BN (2020) The role of RDoC in future classification of mental disorders. Dialogues Clin. Neurosci 22, 81–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dunlop BW et al. (2017) Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. Am. J. Psychiatry 174, 533–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krause AJ et al. (2017) The sleep-deprived human brain. Nat. Rev. Neurosci 18, 404–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu JC and Bunney WE (1990) The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am. J. Psychiatry 147, 14–21 [DOI] [PubMed] [Google Scholar]
- 107.Chai Y. et al. (2020) Two nights of recovery sleep restores hippocampal connectivity but not episodic memory after total sleep deprivation. Sci. Rep 10, 8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fang Z. et al. (2015) Altered salience network connectivity predicts macronutrient intake after sleep deprivation. Sci. Rep 5, 8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sheline YI et al. (2010) Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci 107, 11020–11025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gabbay V. et al. (2013) Striatum-based circuitry of adolescent depression and anhedonia. J. Am. Acad. Child Adolesc. Psychiatry 52, 628–41.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scheidegger M. et al. (2012) Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One 7, e44799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bosch OG et al. (2013) Sleep deprivation increases dorsal nexus connectivity to the dorsolateral prefrontal cortex in humans. Proc. Natl. Acad. Sci 110, 19597–19602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chai Y. et al. (2023) Enhanced amygdala connectivity predicts negative mood changes in both healthy and depressive individuals after sleep deprivation. Proc. Natl. Acad. Sci (In Revis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang J. et al. (2018) Functional reorganization of intra- and internetwork connectivity in major depressive disorder after electroconvulsive therapy. Hum. Brain Mapp 39, 1403–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abbott CC et al. (2013) Electroconvulsive therapy response in major depressive disorder: A pilot functional network connectivity resting state fMRI investigation. Front. Psychiatry 4, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Perrin JS et al. (2012) Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc. Natl. Acad. Sci 109, 5464–5468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fox MD et al. (2012) Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry 72, 595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weigand A. et al. (2018) Prospective Validation That Subgenual Connectivity Predicts Antidepressant Efficacy of Transcranial Magnetic Stimulation Sites. Biol. Psychiatry 84, 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cash RFH et al. (2019) Subgenual Functional Connectivity Predicts Antidepressant Treatment Response to Transcranial Magnetic Stimulation: Independent Validation and Evaluation of Personalization. Biol. Psychiatry 86, e5–e7 [DOI] [PubMed] [Google Scholar]
- 120.Klooster DCW et al. (2022) Personalizing Repetitive Transcranial Magnetic Stimulation Parameters for Depression Treatment Using Multimodal Neuroimaging. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 7, 536–545 [DOI] [PubMed] [Google Scholar]
- 121.Siddiqi SH et al. (2021) Identification of Personalized Transcranial Magnetic Stimulation Targets Based on Subgenual Cingulate Connectivity: An Independent Replication. Biol. Psychiatry 90, e55–e56 [DOI] [PubMed] [Google Scholar]
- 122.Cash RFH et al. (2021) Functional Magnetic Resonance Imaging–Guided Personalization of Transcranial Magnetic Stimulation Treatment for Depression. JAMA Psychiatry 78, 337–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gordon EM et al. (2017) Precision Functional Mapping of Individual Human Brains. Neuron 95, 791–807.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Laumann TO et al. (2015) Functional System and Areal Organization of a Highly Sampled Individual Human Brain. Neuron 87, 657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Greene DJ et al. (2020) Integrative and Network-Specific Connectivity of the Basal Ganglia and Thalamus Defined in Individuals. Neuron 105, 742–758.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gratton C. et al. (2018) Functional Brain Networks Are Dominated by Stable Group and Individual Factors, Not Cognitive or Daily Variation. Neuron 98, 439–452.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lynch CJ et al. (2022) Automated optimization of TMS coil placement for personalized functional network engagement. Neuron 110, 3263–3277.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Downar J. and Daskalakis ZJ (2013) New targets for rTMS in depression: a review of convergent evidence. Brain Stimul. 6, 231–240 [DOI] [PubMed] [Google Scholar]
- 129.Siddiqi SH et al. (2020) Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am. J. Psychiatry 177, 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Downar J. (2019) Orbitofrontal Cortex: A “Non-rewarding” New Treatment Target in Depression? Curr. Biol 29, R59–R62 [DOI] [PubMed] [Google Scholar]
- 131.Balderston NL et al. (2022) Proof of concept study to develop a novel connectivity-based electric-field modelling approach for individualized targeting of transcranial magnetic stimulation treatment. Neuropsychopharmacology 47, 588–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Seok D. et al. (2020) Dimensional connectomics of anxious misery, a human connectome study related to human disease: Overview of protocol and data quality. NeuroImage Clin. 28, 102489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Balderston NL et al. (2020) A generalized workflow for conducting electric field–optimized, fMRI-guided, transcranial magnetic stimulation. Nat. Protoc 15, 3595–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oathes DJ et al. (2021) Resting fMRI-guided TMS results in subcortical and brain network modulation indexed by interleaved TMS/fMRI. Exp. Brain Res 239, 1165–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen AC et al. (2013) Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc. Natl. Acad. Sci 110, 19944–19949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Taylor SF et al. (2018) Changes in brain connectivity during a sham-controlled, transcranial magnetic stimulation trial for depression. J. Affect. Disord 232, 143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liston C. et al. (2014) Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol. Psychiatry 76, 517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Salomons TV et al. (2014) Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology 39, 488–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Eshel N. et al. (2020) Global connectivity and local excitability changes underlie antidepressant effects of repetitive transcranial magnetic stimulation. Neuropsychopharmacology 45, 1018–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baeken C. et al. (2017) Subgenual Anterior Cingulate–Medial Orbitofrontal Functional Connectivity in Medication-Resistant Major Depression: A Neurobiological Marker for Accelerated Intermittent Theta Burst Stimulation Treatment? Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 556–565 [DOI] [PubMed] [Google Scholar]
- 141.Horn A. and Fox MD (2020) Opportunities of connectomic neuromodulation. Neuroimage 221, 117180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Siddiqi SH et al. (2021) Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat. Hum. Behav 5, 1707–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Elias GJB et al. (2021) 3T MRI of rapid brain activity changes driven by subcallosal cingulate deep brain stimulation. Brain 7, awab447 [DOI] [PubMed] [Google Scholar]
- 144.Elias GJB et al. (2022) Habenular Involvement in Response to Subcallosal Cingulate Deep Brain Stimulation for Depression. Front. Psychiatry 13, 12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scangos KW et al. (2021) Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat. Med 27, 1696–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Riva-Posse P. et al. (2018) A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol. Psychiatry 23, 843–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Medaglia JD et al. (2018) Network Controllability in the Inferior Frontal Gyrus Relates to Controlled Language Variability and Susceptibility to TMS. J. Neurosci 38, 6399LP–6410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Parkes L. et al. (2021) Network Controllability in Transmodal Cortex Predicts Positive Psychosis Spectrum Symptoms. Biol. Psychiatry 90, 409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lozano AM and Lipsman N. (2013) Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 77, 406–424 [DOI] [PubMed] [Google Scholar]
- 150.Dayan E. et al. (2013) Noninvasive brain stimulation: from physiology to network dynamics and back. Nat. Neurosci 16, 838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Palinkas LA et al. (2015) Purposeful Sampling for Qualitative Data Collection and Analysis in Mixed Method Implementation Research. Adm. Policy Ment. Heal. Ment. Heal. Serv. Res 42, 533–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dew MA et al. (2007) Recovery from major depression in older adults receiving augmentation of antidepressant pharmacotherapy. Am. J. Psychiatry 164, 892–899 [DOI] [PubMed] [Google Scholar]
- 153.Lavretsky H. et al. (2015) Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am. J. Psychiatry 172, 561–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Reynolds CF 3rd et al. (2006) Maintenance treatment of major depression in old age. N. Engl. J. Med 354, 1130–1138 [DOI] [PubMed] [Google Scholar]
- 155.Dunlop BW (2016) Evidence-Based Applications of Combination Psychotherapy and Pharmacotherapy for Depression. Focus (Am. Psychiatr. Publ) 14, 156–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Greene AS et al. (2022) Brain–phenotype models fail for individuals who defy sample stereotypes. Nature 609, 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dhamala E. et al. (2023) One Size Does Not Fit All: Methodological Considerations for Brain-Based Predictive Modeling in Psychiatry. Biol. Psychiatry 93, 717–728 [DOI] [PubMed] [Google Scholar]
- 158.Li J. et al. (2022) Cross-ethnicity/race generalization failure of behavioral prediction from resting-state functional connectivity. Sci. Adv 8, eabj1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sylvester CM et al. (2020) Individual-specific functional connectivity of the amygdala: A substrate for precision psychiatry. Proc. Natl. Acad. Sci 117, 3808–3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Seitzman BA et al. (2019) Trait-like variants in human functional brain networks. Proc. Natl. Acad. Sci 116, 22851–22861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Braga RM and Buckner RL (2017) Parallel Interdigitated Distributed Networks within the Individual Estimated by Intrinsic Functional Connectivity. Neuron 95, 457–471.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fried EI et al. (2022) Revisiting the theoretical and methodological foundations of depression measurement. Nat. Rev. Psychol 1, 358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fisher AJ et al. (2018) Lack of group-to-individual generalizability is a threat to human subjects research. Proc. Natl. Acad. Sci 115, E6106–E6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Etkin A. (2019) A Reckoning and Research Agenda for Neuroimaging in Psychiatry. Am. J. Psychiatry 176, 507–511 [DOI] [PubMed] [Google Scholar]
- 165.Gratton C. et al. (2020) Defining Individual-Specific Functional Neuroanatomy for Precision Psychiatry. Biol. Psychiatry 88, 28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Noble S. et al. (2022) Improving power in functional magnetic resonance imaging by moving beyond cluster-level inference. Proc. Natl. Acad. Sci. U. S. A 119, e2203020119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lynch CJ et al. (2020) Rapid Precision Functional Mapping of Individuals Using MultiEcho fMRI. Cell Rep. 33, 108540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marquand AF et al. (2016) Understanding Heterogeneity in Clinical Cohorts Using Normative Models: Beyond Case-Control Studies. Biol. Psychiatry 80, 552–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Feczko E. et al. (2019) The Heterogeneity Problem: Approaches to Identify Psychiatric Subtypes. Trends Cogn. Sci 23, 584–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Feczko E. and Fair DA (2020) Methods and Challenges for Assessing Heterogeneity. Biol. Psychiatry 88, 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McIntosh AR and Mišić B. (2013) Multivariate statistical analyses for neuroimaging data. Annu. Rev. Psychol 64, 499–525 [DOI] [PubMed] [Google Scholar]
- 172.Marek S. et al. (2022) Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Spisak T. et al. Multivariate BWAS can be replicable with moderate sample sizes., Nature, 615. Mar-(2023), E4–E7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bycroft C. et al. (2018) The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thompson PM et al. (2014) The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 8, 153–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Casey BJ et al. (2018) The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci 32, 43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.An L. et al. (2022) Goal-specific brain MRI harmonization. Neuroimage 263, 119570 [DOI] [PubMed] [Google Scholar]
- 178.Yamashita A. et al. (2019) Harmonization of resting-state functional MRI data across multiple imaging sites via the separation of site differences into sampling bias and measurement bias, 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yu M. et al. (2017) Selective impairment of hippocampus and posterior hub areas in Alzheimer’s disease: An MEG-based multiplex network study. Brain 140, 1466–1485 [DOI] [PubMed] [Google Scholar]
- 180.Gosak M. et al. (2018) Network science of biological systems at different scales: A review. Phys. Life Rev 24, 118–135 [DOI] [PubMed] [Google Scholar]
- 181.Williams LM (2016) Precision psychiatry: A neural circuit taxonomy for depression and anxiety. The Lancet Psychiatry 3, 472–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lynch CJ et al. (2020) Causes and Consequences of Diagnostic Heterogeneity in Depression: Paths to Discovering Novel Biological Depression Subtypes. Biol. Psychiatry 88, 83–94 [DOI] [PubMed] [Google Scholar]
- 183.Flint J. and Kendler KS (2014) The genetics of major depression. Neuron 81, 484–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Siddiqi SH et al. (2022) Causal mapping of human brain function. Nat. Rev. Neurosci 23, 361–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bijsterbosch J. et al. (2020) Challenges and future directions for representations of functional brain organization. Nat. Neurosci 23, 1484–1495 [DOI] [PubMed] [Google Scholar]
- 186.Smith SM and Nichols TE (2018) Statistical Challenges in “Big Data” Human Neuroimaging. Neuron 97, 263–268 [DOI] [PubMed] [Google Scholar]
- 187.Cunningham JP and Yu BM (2014) Dimensionality reduction for large-scale neural recordings. Nat. Neurosci 17, 1500–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen J. et al. (2022) Leveraging Machine Learning for Gaining Neurobiological and Nosological Insights in Psychiatric Research. Biol. Psychiatry DOI: 10.1016/j.biopsych.2022.07.025 [DOI] [PubMed] [Google Scholar]
- 189.Dinsdale NK et al. (2022) Challenges for machine learning in clinical translation of big data imaging studies. Neuron DOI: 10.1016/j.neuron.2022.09.012 [DOI] [PubMed] [Google Scholar]
- 190.Brucar LR et al. (2023) Current Approaches in Computational Psychiatry for the Data-Driven Identification of Brain-Based Subtypes. Biol. Psychiatry 93, 704–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Seeley WW et al. (2007) Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci 27, 2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dixon ML et al. (2018) Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc. Natl. Acad. Sci 115, E1598–E1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Price JL and Drevets WC (2012) Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci 16, 61–71 [DOI] [PubMed] [Google Scholar]
- 194.Dosenbach NUF et al. (2008) A dual-networks architecture of top-down control. Trends Cogn. Sci 12, 99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Uddin LQ et al. (2019) Towards a Universal Taxonomy of Macro-scale Functional Human Brain Networks. Brain Topogr. 32, 926–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Petersen SE and Posner MI (2012) The attention system of the human brain: 20 years after. Annu. Rev. Neurosci 35, 73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Glasser MF et al. (2016) A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Power JD et al. (2011) Functional Network Organization of the Human Brain. Neuron 72, 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Davidson JRT (2010) Major depressive disorder treatment guidelines in America and Europe. J. Clin. Psychiatry 71 Suppl E, e04 [DOI] [PubMed] [Google Scholar]
- 200.Hansen JY et al. (2022) Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. Nat. Neurosci 25, 1569–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Austelle CW et al. (2022) A Comprehensive Review of Vagus Nerve Stimulation for Depression. Neuromodulation 25, 309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Carreno FR and Frazer A. (2017) Vagal Nerve Stimulation for Treatment-Resistant Depression. Neurotherapeutics 14, 716–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fang J. et al. (2016) Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biol. Psychiatry 79, 266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chattopadhyay S. et al. (2017) Cognitive Behavioral Therapy Lowers Elevated Functional Connectivity in Depressed Adolescents. EBioMedicine 17, 216–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shou H. et al. (2017) Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. NeuroImage Clin. 14, 464–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lv X. et al. (2021) Effects of TIP treatment on brain network topology of frontolimbic circuit in first-episode, treatment-naïve major depressive disorder. J. Affect. Disord 279, 122–130 [DOI] [PubMed] [Google Scholar]
- 207.Pantazatos SP et al. (2020) Depression-related anterior cingulate prefrontal resting state connectivity normalizes following cognitive behavioral therapy. Eur. Psychiatry 63, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yeo BTT et al. (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol 106, 1125–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.