Abstract
Cutaneous metastases from urothelial carcinoma (UC) are very rare and indicate advanced disease with a poor prognosis. A 63-year-old female patient with a history of urothelial bladder carcinoma, treated 2 months prior with radical cystectomy and adjuvant gemcitabine and cisplatin (GC) therapy, presented a skin lesion localized in the lower third of the right leg. Punch biopsy revealed carcinomatous metastasis whose urothelial origin was confirmed by immunohistochemical analysis. 18-FDG PET-CT showed the spread of metastases to the lung and left ischium. Our review focuses on the time between surgery and skin metastasis, localization, and prognosis after metastasis diagnosis. In many cases, skin metastases occur within one year of initial UC surgery and in most cases occur on the abdominal wall. Local wide excision of metastasis should be considered in selected cases; however, chemotherapy remains the main treatment.
Keywords: bladder, cancer, metastasis, urothelial, cutaneous, cutaneous metastasis, urothelial bladder carcinoma, systematic literature review
Introduction
Urothelial carcinoma (UC), also known as transitional cell carcinoma (TCC), represents the predominant histological type of all bladder cancers, accounting for approximately 90%. It is the ninth most common cancer in the world (1, 2).
The 5-year survival rate for urothelial carcinoma of the bladder is 77%. While the 5-year survival rate is 96% for “in situ” diagnosed cases, the survival rate falls down to only 4.6% for metastatic cancer (5% of all diagnosed cases) (3).
The most common metastases sites of UC include the lymph node, bone, liver, and lung (4), while UBC metastasis to the skin is a rare occurrence (5).
We present a rare case of urothelial bladder carcinoma with cutaneous metastases localized in the lower third of the right leg, rare because of its nodular clinical form.
The purpose of this study is to identify the mean time within which skin metastasis can occur after surgery for urothelial carcinoma, the most frequent location of skin metastasis, and survival from the diagnosis of skin metastasis. The uncommon occurrence and poor prognosis of cutaneous metastases in UC lead to unclear management and treatment protocols for these patients.
Methods and case report
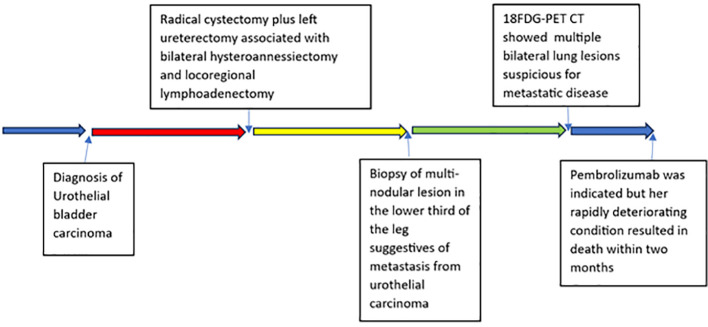
A 63-year-old woman treated at Policlinico Umberto I – Rome in 2020 is described. Consensus-based clinical case report (CARE) guidelines were applied to present the case report (6). The patient provided written informed consent for the publication. A timeline with relevant data is shown in Figure 1 .
Figure 1.
Timeline of the patient’s clinical course with major clinical events.
A systematic review of the online database MEDLINE/PubMed, using the search algorithm [(urothelial OR transitional OR bladder) AND metastasis AND (carcinoma OR cancer) AND (skin OR cutaneous), year > 2000], was carried out. The references in each identified article were reviewed to find additional published case reports.
The identified articles were further processed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA checklist) (7) in order to identify original case reports of cutaneous metastasis of UC after surgery in the last 22 years.
Case report
A 63-year-old woman presented with a 2–3 month history of hematuria and anemia. Her chronic illness included hypertension and chronic renal failure. Her ECOG performance score was 1. Ultrasonography showed left hydronephrosis. An abdominal-pelvis CT scan ( Figure 2 ) and multiparametric magnetic resonance imaging (mpMRI) for bladder cancer showed a VI-RADS score of 5 and tumoral lesions in the trigone of the bladder extending up to the left ureteral ostium, with no lymphadenopathies detected. Clinicians performed transurethral resection of the bladder tumor (TURBT) as the treatment. Pathological diagnosis of the TURBTS showed high grade muscle-invasive urothelial carcinoma. A total body CT was obtained for radiological staging, and this did not show metastatic disease. The patient underwent a radical cystectomy and left ureterectomy associated with bilateral hysteroannessiectomy and locoregional lymphoadenectomy.
Figure 2.

Contrast-enhanced computed tomography scan of the pelvis showing lesions in trigone of bladder extending up to the left ureteral ostium.
Postoperative pathological results revealed urothelial carcinoma pT4a pN1 (1/36), R0. Due to the creatinine value (1.5 mg/dl, eGFR <50 mL/min) and comorbidities, CARBOplatin instead of cisplatin-based chemotherapy was administered. Gemcitabine (1000 mg/m2) was administered on day 1 and day 8 and CARBOplatin (AUC5) on day 1 of a 21-day cycle for 4 cycles.
Two months after radical cystectomy, a multi-nodular lesion associated with skin rash in the lower third of the leg appeared ( Figure 3 ). The lesion was not painful. A skin biopsy of the leg lesion was performed, showing poorly differentiated carcinomatous proliferation with extensive infiltration from atypical pleomorphic cells with round nuclei and a moderate amount of cytoplasm ( Figure 3 ). The following immunohistochemical markers were performed: CK7, CK20, and GATA3. Positivity was suggestive of metastasis from urothelial carcinoma. Furthermore, 18FDG-PET CT showed multiple bilateral lung lesions, suspicious for metastatic disease. Immunotherapy with Pembrolizumab was performed; however, her rapidly deteriorating condition resulted in death within 2 months of diagnosis.
Figure 3.
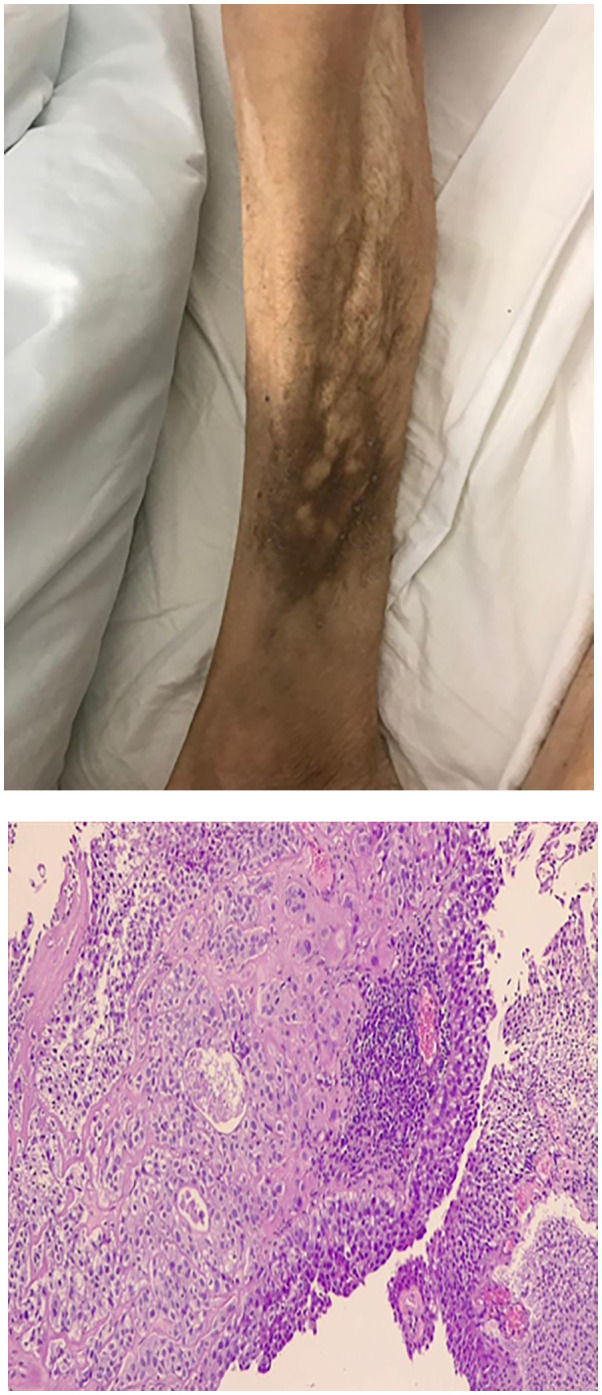
Enlarged lower third of the leg. Clinical appearance of multi-nodular lesion, biopsy showed cutaneous metastasis urothelial carcinoma. Microscopic view of biopsy specimen shows subcutaneous infiltration of urothelial cell carcinoma.
Review of the literature
A systematic review of the online database MEDLINE/PubMed yielded 49 articles. Twenty-seven articles not exactly matching the aim of the study (patients who did not undergo surgical treatment of a primary tumor; non transitional cell carcinoma histological type; metastatic disease at first diagnosis of UC) were excluded.
The remaining articles consisted of 22 original case reports of cutaneous metastases of urothelial carcinoma in patients who have undergone surgery. The main characteristics of the published cases are summarized in Table 1 .
Table 1.
Characteristics of published case reports of cutaneous metastasis from urothelial carcinoma in patients who have undergone surgery for a primary tumor in the last 22 years.
| Year | Study | Site | Sex Age |
Surgical Treatment +\- Systemic Treatment | Time after surgery; Treatment | Survival time from diagnosis of cutaneous metastases |
|---|---|---|---|---|---|---|
| 2022 | Cutaneous metastasis of bladder urothelial carcinoma; a rare conditions (8) | Abdomen | Female 80 |
Cystectomy plus Urethrectomy. Adjuvant Immunotherapy |
2 months | – |
| 2021 | Urothelial carcinoma of the bladder with cutaneous metastases after robot-assisted radical cystectomy. Case report (9) | Left chest | Male 70 |
Neoadjuvant chemotherapy and Robot-Assisted laparoscopic radical cystoprostatectomy. | 2 months; immunotherapy | Alive after 5 months |
| 2020 | A patient with history of bladder cancer presenting with an axillary mass: A rare case of supradiaphragmatic cutaneous bladder cancer metastasis (10) | Axillary metastasis | Male 71 |
Cystoprostatectomy | 5 months | – |
| 2019 | Multiple cutaneous metastasis of synchronous urothelial carcinoma of the bladder and the renal pelvis: a case report (11) | Hypochondriac, the back and the cervical region | Male 61 |
radical cystectomy and a left ureteronephrectomy ➔ adjuvant chemotherapy | 12 months | 1 month |
| 2017 | Metastatic urothelial carcinoma presenting as carcinoma erysipeloides (12) | Bilateral inguinal and suprapubic painful rash | Male 61 |
Cystoprostatectomy | 3 years; chemotherapy |
1 month |
| 2016 | Transitional bladder cell carcinoma spreading to the skin (13). | Multiple subcutaneous chest lesions | Male 74 |
cysto-prostatectomy + adjuvant chemotherapy | 4 months | – |
| 2015 | Metastatic transitional cell carcinoma presenting with skin metastasis (14) | Right hypogastric region | Female 60 |
Nephroureterectomy | 1 year; metastasectomy, chemotherapy |
3 months |
| 2015 | Cutaneous metastasis of transitional cell carcinoma of the urinary bladder eight years after the primary: a case report (15). | Left lower limb and anterior thigh | Male 81 |
Cystoprostatectomy | 8 years; Radiotherapy |
6 months |
| 2015 | Cutaneous metastasis of micropapillary urothelial carcinoma (16). | Sovrapubic area | Male 59 |
Radical nephroureerectomy + chemotherapy | 2 months | – |
| 2014 | Malignant priapism secondary to isolated penile metastasis from a renal pelvic carcinoma (17). | Penile metastasis | Male 69 |
Radical resection of the renal pelvic carcinoma | 2 months | – |
| 2014 | Choroidal and cutaneous metastasis from urothelial carcinoma of the bladder after radical cystectomy: a case report and literature review (18). | Head and lower abdomen | Male 48 |
Radical cystectomy | 17 months | 5 months |
| 2014 | Cutaneous metastasis of transitional cell carcinoma of the urinary bladder: Cytological aspect (19) | Subscapular, umbilical region and right shoulder | Male 51 |
transurethral resection of bladder tumor | 10 months | – |
| 2012 | Cutaneous and subcutaneous metastases from bladder carcinoma (20). | Sovrapubic region | Male 60 |
Cystoprostatectomy | 1 months; Chemotherapy | – |
| 2011 | Brain and skin metastasis from urothelial carcinoma of the bladder (21) | Abdominal skin | Male 60 |
Radical cistectomy | 10 years; Chemotherapy |
Several days |
| 2011 | Cutaneous metastasis of transitional cell bladder carcinoma: a rare presentation and literature review (22). | Lower abdomen | Male 68 |
Radical cystectomy and bilateral ureterostomy | 14 months | 2 months |
| 2010 | Superficially invasive transitional cell carcinoma of the bladder associated with distant cutaneous metastases (23) | Mid upper back | Male 69 |
Resected transurethrally | 3 months | Several weeks |
| 2006 | Skin metastasis of ‘nested type’ of urothelial carcinoma of the urinary bladder (24). | Neck | Male 68 |
Radical cystoprostatovesiculectomy ➔ chemotherapy |
3 years | – |
| 2004 | Penile metastasis from primary transitional cell carcinoma of the renal pelvis: first manifestation of systemic spread (25). | Penile metastasis | Male 76 |
Nephroureterectomy | 8 years | – |
| 2004 | Twenty-three years of disease-free survival following cutaneous metastasis from a primary bladder transitional cell carcinoma (26). | Skin over the left iliac fossa | Male 51 |
Cystoprostatourethrectomy | 3 months; Wide local excision |
Alive 23 years later |
| 2003 | Extensive cutaneous metastasis of transitional cell carcinoma of the bladder (27) | Trunk | Male 78 |
Transurethral resection | 6 months | 10 months |
| 2003 | Transitional cell carcinoma metastasis to arm skin from the renal pelvis (28). | Bilateral arm nodules | Female 68 |
Right nephroureterectomy | 18 months | 1 month |
| 2000 | Metastasis to the male breast from carcinoma of the urinary bladder (29). | Breast | Male 69 |
Transurethral resection | 2 months | – |
Discussion
Skin metastases from solid primary tumors are rare. The most frequent primary tumors metastasizing to the skin are subject to the gender of the patient and are most common for breast (69%) followed by colon (9%), lung (4%), and ovary (4%) tumors in women and lung (24%) followed by colon (19%) and head and neck malignancy (12%) in men (30).
Mueller et al. (31), in a 2004 review, identify 2,369 cases of dermatologic metastases arising from 81,618 primary solid malignancies. Cutaneous metastases from primary urologic malignancies of the bladder, prostate, or kidney were noted in 116 (1.1%) of 10,417 cases. Of these, 63 cases (3.4%) of metastatic renal cell carcinoma from 1,877 cases were noted, and 38 cases (0.84%) of cutaneous metastatic transitional cell carcinoma (TCC) from 4,516 cases have been reported.
The most common location of metastasis of transitional cell carcinoma disease are known to be the bones, lungs, and the liver (32). However, skin metastasis of transitional cell carcinoma of the bladder is very rare, representing only 0.84% of all cutaneous metastases (19).
There are four recognized mechanisms that can explain a tumor spreading to the skin: hematogenous spread, lymphatics spread, direct invasion, and iatrogenic implantation during surgical treatment (33).
The clinical appearance of skin metastases from UC should mimic many other common dermatologic illnesses (34); therefore, correct diagnosis requires a lesion biopsy with histological examination to establish the urothelial beginning of these skin lesions (28). Metastatic lesions do not often preserve histologic similarities to the primary lesion; in these cases, immunohistochemical examination is mandatory for correct diagnosis and management. Nevertheless, in one case report, a fine needle aspiration biopsy was carried out in order to establish definitive diagnosis (35).
In our patient, skin metastasis affected the lower limb 2 months after the initial diagnosis of UC. Eleven cases found in the literature presented metastases localized on the abdominal wall, while two involved the penis. Seven cases involved chest skin, of which two presented abdominal cutaneous lesions at the same time. Only one case reported axillary skin metastasis. One case showed cutaneous metastases to the lower limb, one to the upper arm and one involved the skin of the neck region. Therefore, in the majority of cases, cutaneous metastases develop in the locoregional skin, spreading through the lymphatic channels (36, 37) or via iatrogenic implantation in surgery (14, 38)
Our patient was a 63-year-old woman; the cases reported in the literature showed a median age of 69 years in female patients. Most cases (19/22) were men with a median age of 65.
The systematic review showed that in 11 cases, the metastases occurred within 6 months of surgery, of which five emerged during adjuvant chemotherapy. Four cases presented skin metastases between 6 and 12 months after surgery. In seven cases, skin metastases were found more than 1 year after surgery, of which two cases were administered adjuvant chemotherapy.
Our patient expired within two months of the diagnosis of UC cutaneous metastasis. Only 12 cases (12/22) reported the evolution from the diagnosis of cutaneous spread from UC. Eleven cases showed a poor prognosis, with a median survival of less than 12 months. One case described a patient alive 23 years after diagnosis of a skin lesion treated with wide surgical excision.
The strength of this article lies in the fact that it is very well documented, describing the patient’s clinical history point-by-point and allowing the reader to have a full understanding of the text, thus hoping that they will get the maximum benefit from this manuscript. However, one must remember the very nature of the article which, as a case report, has obvious limitations, despite a review of the literature.
Conclusion
Cutaneous dissemination from UC is an extremely rare form of presentation and is a sign of systemic involvement linked to a poor prognosis. Punch biopsy of the lesion should be performed for pathological examination in order to prevent misdiagnosis. Poor outcomes could be attributed to the aggressive nature of the disease, limited treatment options, and rarity of the disease process. Chemotherapy remains the main treatment of choice and metastasectomy should be considered in single-site metastasis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
Creators of the work: PI, SI, and CDI; Research: GD’O, PG, and LI; For the English translation: SL and MC; Reviewers: AP, RG, and SS. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer FP declared a shared affiliation with the authors PI, LI, SL, GD’O, PG, RG, AP, SS, MC, and CDI to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today (2018). Lyon, France: International Agency for Research on Cancer. Available at: https://gco.iarc.fr/ (Accessed 10 January 2020). [Google Scholar]
- 2. Miyazaki J, Nishiyama H. Epidemiology of urothelial carcinoma. Int J Urol (2017) 24(10):730–4. doi: 10.1111/iju.13376 [DOI] [PubMed] [Google Scholar]
- 3. Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci (Basel). (2020) 8(1):15. doi: 10.3390/medsci8010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babaian RJ, Johnson DE, Llamas L, Ayala AG. Metastases from transitional cell carcinoma of urinary bladder. Urology (1980) 16:142–4. doi: 10.1016/0090-4295(80)90067-9 [DOI] [PubMed] [Google Scholar]
- 5. Teyateeti P, Ungtrakul T. Retrospective review of cutaneous metastasis among 11,418 patients with solid Malignancy: A tertiary cancer center experience. Med (Baltimore). (2021) 100(29):e26737. doi: 10.1097/MD.0000000000026737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med (2013) 2:38–43. doi: 10.7453/gahmj.2013.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (2009) 339:b2535. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Öztürk H, Yurtsever S, Özer A, Arslan Ç, Pehlivan FS, Tekeli A. Cutaneous metastasis of bladder urothelial carcinoma; A rare conditions. Urol Case Rep (2021) 41:101955. doi: 10.1016/j.eucr.2021.101955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yajima S, Nakanishi Y, Wantanabe R, Matsumoto S, Tanabe K, Masuda H. Urothelial carcinoma of the bladder with cutaneous metastases after robot-assisted radical cystectomy. Case Rep Urol Case Rep (2021) 38:101709. doi: 10.1016/j.eucr.2021.101709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhakta PS, Harris EA, Connolly MM. A patient with history of bladder cancer presenting with an axillary mass: A rare case of supradiaphragmatic cutaneous bladder cancer metastasis. Urol Case Rep (2020) 31:101157. doi: 10.1016/j.eucr.2020.101157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghalleb M, Ayadi MA, Slim S, Zemni I, Doghri R, Ben Hassouna J, et al. Multiple cutaneous metastasis of synchronous urothelial carcinoma of the bladder and the renal pelvis: a case report. J Med Case Rep (2019) 13(1):34. doi: 10.1186/s13256-019-1997-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol (2017) 44(6):513–5. doi: 10.1111/cup.12953 [DOI] [PubMed] [Google Scholar]
- 13. Kerkeni W, Ayari Y, Charfi L, Bouzouita A, Ayed H, Cherif M, et al. Transitional bladder cell carcinoma spreading to the skin. Urol Case Rep (2017) 11:17–8. doi: 10.1016/j.eucr.2016.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Açıkgöz O, Ölçücüoğlu E, Kasap Y, Yığman M, Güneş ZE, Gazel E. Metastatic transitional cell carcinoma presenting with skin metastasis. Int J Crit Illn Inj Sci (2015) 5(1):53–5. doi: 10.4103/2229-5151.152346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lees AN. Cutaneous metastasis of transitional cell carcinoma of the urinary bladder eight years after the primary: a case report. J Med Case Rep (2015) 9:102. doi: 10.1186/s13256-015-0585-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Truong H, Parsons TM, Trabulsi EJ. Cutaneous metastasis of micropapillary urothelial carcinoma. Urology (2015) 85(2):e7–8. doi: 10.1016/j.urology.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 17. Liu S, Zeng F, Qi L, Jiang S, Tan P, Zu X, et al. Malignant priapism secondary to isolated penile metastasis from a renal pelvic carcinoma. Can Urol Assoc J (2014) 8(7-8):E558–60. doi: 10.5489/cuaj.1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mitsui Y, Arichi N, Inoue K, Hiraki M, Nakamura S, Hiraoka T, et al. Choroidal and cutaneous metastasis from urothelial carcinoma of the bladder after radical cystectomy: a case report and literature review. Case Rep Urol (2014) 2014:491541. doi: 10.1155/2014/491541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Narayana MA, Patnayak R, Rukmangadha N, Chowhan AK, Kottu R, Phaneendra BV. Cutaneous metastasis of transitional cell carcinoma of the urinary bladder: Cytological aspect. J Cytol (2014) 31(1):50–2. doi: 10.4103/0970-9371.130707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dirican A, Küçükzeybek Y, Somalι I, Erten C, Demir L, Can A, et al. Cutaneous and subcutaneous metastases from bladder carcinoma. Contemp Oncol (Pozn). (2012) 16(5):451–2. doi: 10.5114/wo.2012.31779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chung JH, Lee JY, Pyo JY, Oh YH, Lee SW, Moon HS, et al. Brain and skin metastasis from urothelial carcinoma of the bladder. Korean J Urol (2013) 54(1):66–8. doi: 10.4111/kju.2013.54.1.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salemis NS, Gakis C, Zografidis A, Gourgiotis S. Cutaneous metastasis of transitional cell bladder carcinoma: a rare presentation and literature review. J Cancer Res Ther (2011) 7(2):217–9. doi: 10.4103/0973-1482.82940 [DOI] [PubMed] [Google Scholar]
- 23. Swick BL, Gordon JR. Superficially invasive transitional cell carcinoma of the bladder associated with distant cutaneous metastases. J Cutan Pathol (2010) 37(12):1245–50. doi: 10.1111/j.1600-0560.2009.01471.x [DOI] [PubMed] [Google Scholar]
- 24. Zwenzner EM, Kaatz M, Ziemer M. Skin metastasis of ‘nested type’ of urothelial carcinoma of the urinary bladder. J Cutan Pathol (2006) 33(11):754–5. doi: 10.1111/j.1600-0560.2006.00503.x [DOI] [PubMed] [Google Scholar]
- 25. Pomara G, Pastina I, Simone M, Casale P, Marchetti G, Francesca F. Penile metastasis from primary transitional cell carcinoma of the renal pelvis: first manifestation of systemic spread. BMC Cancer (2004) 4:90. doi: 10.1186/1471-2407-4-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gowardhan B, Mathers ME, Feggetter JG. Twenty-three years of disease-free survival following cutaneous metastasis from a primary bladder transitional cell carcinoma. Int J Urol (2004) 11(11):1031–2. doi: 10.1111/j.1442-2042.2004.00939.x [DOI] [PubMed] [Google Scholar]
- 27. Akman Y, Cam K, Kavak A, Alper M. Extensive cutaneous metastasis of transitional cell carcinoma of the bladder. Int J Urol (2003) 10(2):103–4. doi: 10.1046/j.1442-2042.2003.00571.x [DOI] [PubMed] [Google Scholar]
- 28. Lin CY, Lee CT, Huang JS, Chang LC. Transitional cell carcinoma metastasis to arm skin from the renal pelvis. Chang Gung Med J (2003) 26(7):525–9. [PubMed] [Google Scholar]
- 29. Cappabianca S, Grassi R, D’Alessandro P, Del Vecchio A, Maioli A, Donofrio V. Metastasis to the male breast from carcinoma of the urinary bladder. Br J Radiol (2000) 73(876):1326–8. doi: 10.1259/bjr.73.876.11205680 [DOI] [PubMed] [Google Scholar]
- 30. Brownstein MH, Helwig EB. Metastatic tumors of the skin. Cancer (1972) 29:1298–307. doi: [DOI] [PubMed] [Google Scholar]
- 31. Mueller TJ, Wu H, Greenberg RE, Hudes G, Topham N, Lessin SR, et al. Cutaneous metastases from genitourinary Malignancies. Urology (2004) 63(6):1021–6. doi: 10.1016/j.urology.2004.01.014 [DOI] [PubMed] [Google Scholar]
- 32. Elston DM, Tuthill RJ, Pierson J, Marden JD, Bergfeld WF. Carcinoma erysipelatoides resulting from genitourinary cancer. J Am Acad Dermatol (1996) 35:993–5. doi: 10.1016/S0190-9622(96)90131-0 [DOI] [PubMed] [Google Scholar]
- 33. Scott LS, Head MA, Mack WS. Cutaneous metastases from tumours of the bladder, urethra, and penis. Br J Urol (1954) 26:387–400. doi: 10.1111/j.1464-410X.1954.tb04922.x [DOI] [PubMed] [Google Scholar]
- 34. Persechino F, Franceschini C, Cota C, Frascione P, Ardigò M. A new single red nodule on the abdomen of a wOman with history of endometrial carcinoma: Noninvasive evaluation and histologic correlation. JAAD Case Rep (2018) 4(9):941–3. doi: 10.1016/j.jdcr.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumar PV, Salimi B, Musallaye A, Tadayyon A. Subcutaneous metastasis from transitional cell carcinoma of the bladder diagnosed by fine needle aspiration biopsy. A Case Rep Acta Cytol (2000) 44:657–60. doi: 10.1159/000328543 [DOI] [PubMed] [Google Scholar]
- 36. Saito S. Solitary cutaneous metastasis of superficial bladder cancer. Urol Int (1998) 61:126. doi: 10.1159/000030304 [DOI] [PubMed] [Google Scholar]
- 37. Hollander A, Grots IA. Oculocutaneous metastases from carcinoma of the urinary bladder. Case Rep Rev lit Arch Dermatol (1968) 97:678. doi: 10.1001/archderm.1968.01610120068010 [DOI] [PubMed] [Google Scholar]
- 38. Miyamoto T, Ikehara A, Araki M, Akaeda T, Mihara M. Cutaneous metastatic carcinoma of the penis: suspected metastasis implantation from a bladder tumor. J Urol (2000) 163(5):1519. doi: 10.1097/00005392-200005000-00030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.