Abstract
While plasma biomarkers for Alzheimer’s disease (AD) are increasingly being evaluated for clinical diagnosis and prognosis, few population-based autopsy studies have evaluated their utility in the context of predicting neuropathological changes. Our goal was to investigate the utility of clinically available plasma markers in predicting Braak staging, neuritic plaque score, Thal phase, and overall AD neuropathological change (ADNC).We utilized a population-based prospective study of 350 participants with autopsy and antemortem plasma biomarker testing using clinically available antibody assay (Quanterix) consisting of Aβ42/40 ratio, p-tau181, GFAP, and NfL. We utilized a variable selection procedure in cross-validated (CV) logistic regression models to identify the best set of plasma predictors along with demographic variables, and a subset of neuropsychological tests comprising the Mayo Clinic Preclinical Alzheimer Cognitive Composite (Mayo-PACC). ADNC was best predicted with plasma GFAP, NfL, p-tau181 biomarkers along with APOE ε4 carrier status and Mayo-PACC cognitive score (CV AUC = 0.798). Braak staging was best predicted using plasma GFAP, p-tau181, and cognitive scores (CV AUC = 0.774). Neuritic plaque score was best predicted using plasma Aβ42/40 ratio, p-tau181, GFAP, and NfL biomarkers (CV AUC = 0.770). Thal phase was best predicted using GFAP, NfL, p-tau181, APOE ε4 carrier status and Mayo-PACC cognitive score (CV AUC = 0.754). We found that GFAP and p-tau provided non-overlapping information on both neuritic plaque and Braak stage scores whereas Aβ42/40 and NfL were mainly useful for prediction of neuritic plaque scores. Separating participants by cognitive status improved predictive performance, particularly when plasma biomarkers were included. Plasma biomarkers can differentially inform about overall ADNC pathology, Braak staging, and neuritic plaque score when combined with demographics and cognitive variables and have significant utility for earlier detection of AD.
Keywords: Plasma biomarkers, Neuropathology, Alzheimer’s disease, Personalized medicine
Introduction
Alzheimer’s disease (AD) biomarkers in the blood and cerebrospinal fluid (CSF) have been identified as important potential clinical tools for disease diagnosis and prognosis [2, 12]. Plasma biomarkers are more cost-effective than positron emission tomography (PET) imaging and less invasive than CSF biomarkers [6, 9, 16, 18, 21–23, 33, 42]. Plasma biomarkers have already shown high performance in the discrimination of those with clinical mild cognitive impairment (MCI) or dementia from cognitively unimpaired individuals and have been shown to be comparable markers to CSF benchmarks of tau- and amyloid-PET in the detection of AD neuropathological change (ADNC) as evidenced by neurodegeneration as well as amyloid-β and tau deposition [7, 8, 11, 22, 24, 32, 33, 35, 36, 40, 43]. Such plasma biomarkers include phosphorylated tau protein 181 (p-tau 181), p-tau 217, p-tau 231, total tau (t-tau), neurofilament light chain (NfL), amyloid-β 42/40 ratio (Aβ42/40), and glial fibrillary acidic protein (GFAP). The existing literature has focused on comparing cognitively unimpaired individuals, individuals with AD, MCI, or dementia, and individuals with other dementias using subsets of these biomarkers to predict either clinical dementia [5, 8, 16, 22, 32, 36, 40, 42], ADNC via surrogates such as CSF markers and/or PET positivity [7, 22, 24, 33, 35, 42, 43], or confirmed neuropathological change [29, 31]. Thus far, few studies have shown the neuropathologic correlates of plasma biomarkers, and these studies are limited by sample sizes of convenience samples.
The measurement of amyloid, tau, and neurodegeneration (as proposed by the A/T/N framework) [14] aids in estimating the contribution of AD neuropathological burden to clinical symptoms [13]. An accurate and early diagnosis of AD can benefit patients and caregivers, not only for prognostication but also for clinical trial candidacy and emerging therapies. There are limited data comparing a panel of plasma biomarkers with the goal of predicting AD neuropathology in a population-based sample. Moreover, interpretation of plasma biomarkers cannot be done in isolation. As part of the clinical assessment, it is necessary to consider the role of additional demographic and clinical information such as sex, education, apolipoprotein E (APOE) ɛ4 allele, and cognitive testing scores in the prediction of ADNC. Lastly, understanding the effect of plasma biomarkers on specific components of ADNC such as Braak staging or neuritic plaques can provide valuable insight in risk-stratifying the probability of underlying ADNC.
In this work, our goal was to predict elevated brain amyloid-β and tau pathology at autopsy from a panel of plasma AD biomarkers in a population-based sample. To this end, we set out to determine which specific plasma biomarkers are important to consider from a clinically available SIMOA Quanterix antibody assay consisting of Aβ42/40 ratio, p-tau181, GFAP, and NfL for prediction of ADNC when combined with cognitive and demographic information would best predict AD pathology at autopsy. In this work, we focused on prediction of Braak staging, neuritic plaque score, Thal amyloid phase, and overall ADNC values as indices of AD neuropathological change.
Methods
Participants
All plasma samples were collected from participants in the Mayo Clinic Study of Aging (MCSA) at Mayo Clinic Rochester. The MCSA is a population-based prospective study of residents of Olmsted County, Minnesota. MCSA visits include a physician exam, cognitive testing, and a blood draw on the same day. Inclusion criteria for this study were MCSA participants who had available antemortem plasma biomarkers within 4 years of death followed by autopsy available. Clinical diagnoses of MCI and dementia were determined by the consensus of a committee composed of a physician, a study coordinator, and a neuropsychologist employing existing criteria [1, 34]. The Institutional Review Boards of Mayo Clinic and Olmsted Medical Center approved this study. All participants provided written informed consent.
Plasma assays
EDTA-plasma samples were collected from participants after an overnight fast. Samples were centrifuged, and 500 μL of plasma was aliquoted into polypropylene tubes and stored at − 80 °C until testing. Plasma Aβ 1–40, Aβ 1–42, GFAP, and NfL were measured using the Simoa® Neurology 4-Plex E Advantage kit (N4PE, item #103,670). Plasma phospho-Tau 181 (pTau-181) was measured the Simoa® pTau-181 Advantage V2 kit (item #103,714). Both kits were used per manufacturer’s instructions and ran on a Quanterix HD-X analyzer (Quanterix, Lexington, MA, United States). Briefly, after thawing and mixing, plasma samples were centrifuged 5 min × 4000 g. Samples were diluted 1:4 using the instrument’s onboard dilution protocol and tested in singlet. A seven-point calibration curve and sample concentrations were determined on the Simoa® HD-X Analyzer software using a weighting factor of 1/y2 and a 4-parameter logistic curve fitting algorithm for p-tau-181. The N4PE test used eight-point calibration curves with 1/y2 weighting; a 4-parameter logistic fitting algorithm was used for NfL and GFAP, while a 5-parameter logistic fitting algorithm was used for Aβ 1–40 and Aβ 1–42. Two levels of quality control material were run in duplicate with each batch following the assay calibrators. Inter-assay imprecision for the quality control material (expressed as % coefficient of variation) were as follows: Aβ 1–40, 5% and 3% at approximate concentrations of 16 and 117 pg/mL; Aβ 1–42, 4% and 7% at approximate concentrations of 5.5 and 31 pg/mL; GFAP, 7% and 7% at approximate concentrations of 181 and 3702 pg/mL; NfL, 12% and 14% at approximate concentrations of 21 and 432 pg/mL; pTau-181, 6% and 5% at approximate concentrations of 3.7 and 119 pg/mL.
Neuropathological assessments
Neuropathologic sampling followed recommendations of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) [25]. Immunohistochemistry was performed on 5 μm thick formalin-fixed and paraffin-embedded tissue sections. To evaluate AD neuropathologic change (ADNC), brain sections were immunostained on a Thermo Fisher Lab Vision 480S autostainer using 3,3-diaminobenzidine (DAB) as the chromogen, with primary antibodies being amyloid-β (mouse monoclonal (6F/3D), DAKO M0872) and phospho-tau (mouse monoclonal, AT8; Thermo Fisher MN1020).
AD neuropathologic change was assessed according to National Institute on Aging Alzheimer’s Association (NIA-AA) criteria [13, 27], including (A) Thal amyloid phase for the distribution of amyloid-β plaques [41], (B) Braak tangle stage [4], and (C) CERAD neuritic plaque score [25]. To evaluate the probability of greater amyloid-β and tau deposition, Thal amyloid phase and Braak tangle stage were recategorized into 4-point scales (absent, low, intermediate, high). The ABC scores were subsequently combined into a 4-point scale. A score of 2 or more was considered consistent with the presence of AD neuropathologic change.
Each neuropathologic scale was binarized into a “high” and “low” neuropathology category to facilitate logistic regression modeling between plasma biomarker levels and the presence of underlying pathology. Braak staging I–III were placed in the “low” category and IV-VI were categorized as “high”. For neuritic plaque, gradings of “none” or “sparse” were given the “low” category and “moderate” or “frequent” placed in the “high” category. Thal phases of 0–2 were categorized as “low” and phases 3–5 were categorized as “high.” Lastly, ADNC was binarized into “low” if there was “none” or “low” evidence of neuropathologic change, whereas “intermediate” or “high” ADNC was binarized as “high” category.
Cognitive measures
The Mayo Preclinical Alzheimer’s Cognitive Composite (Mayo-PACC) is comprised of Auditory Verbal Learning Test (AVLT) sum of trials 1–5 + 6 + delay, Trails B, and animal fluency, and is comparable to other composites as previously described [39]. Study-specific z-scores were derived for all component PACC measures administered as part of the MCSA study visit at which the plasma was drawn using the cognitively unimpaired group at baseline as the normative reference. A priori selection of Mayo-PACC component variables prioritized parsimony and measures readily available in clinical practice.
Statistical analyses
The statistical analysis consisted of multiple components. Basic descriptive statistics were computed for the variables utilized in this study. Simple comparisons of the plasma markers by ADNC, Braak stage, Thal phase, and neuritic plaques score were carried out using Wilcoxon rank-sum tests and were accompanied by box plots for a visual representation. Plasma biomarker values were log transformed prior to all statistical modeling except for the Aβ42/40 ratio. To determine which plasma markers best predicted the neuropathologic variables, a selection procedure was used where the best set of predictors were identified for a given outcome from the selection pool. This selection procedure was run on the overall sample, CU only, and MCI/dementia only. Four different sets of variables were considered for each outcome: only plasma markers, plasma markers plus demographics (age, sex, APOE ε4 status, and years from plasma collection to death), plasma markers plus demographics plus Mayo-PACC, and demographics plus Mayo-PACC without any plasma biomarkers. For selection, logistic regression models were run on the entire data set and the model was selected for each outcome and predictor pool that minimized the Bayesian Information Criterion (BIC). This procedure was carried out via the ‘bestglm’ package in R [20]. Univariate logistic regression models were also run utilizing each of the plasma markers, covariates, and Mayo-PACC individually as predictors of the neuropathological variables. Area under the curve (AUC) values were computed for all models. Dichotomizing neuropathological scales into positive and negative allows us to estimate AUC which provides a single metric of prediction accuracy and is also synonymous to positivity determination done for AD biomarkers. Since variable selection procedures and models run on the data may overfit the data (providing overly optimistic results), a tenfold cross-validation (CV) procedure on the variable selection and logistic regression process was used to obtain CV AUCs and corresponding confidence intervals to accompany the variables selected and corresponding raw AUC values from the full data set. This tenfold CV was also run on the univariate models. To obtain p-values for comparing pairs of tenfold cross-validated AUCs, for each of the folds, a difference in AUCs was computed, and then, a one-sample t-test was run on the set of 10 AUC differences. This procedure was run to compare the univariate models against each other and the multiple variable models against each other for each of the neuropathology outcomes. Two-sample t-tests were used to obtain p-values for comparing models run on the CU group to those run on the MCI/dementia group. For a few models run on the MCI/dementia group, a ninefold CV was run due to data sparsity. Subgroup analysis was performed by excluding participants with chronic kidney disease (CKD) to explore the effect of metabolic medical comorbidities in the predictive performance of plasma biomarkers. The diagnosis of CKD was determined using the published set of ICD-9 and ICD-10 billing codes from the electronic health record as well as codes from the Hospital Adaptation of the International Classification of Diseases (HICDA) [38]. As an additional observation to the role of plasma biomarkers and neuropathology, we performed an ordinal logistic regression including all demographic variables, Mayo-PACC cognitive scores, and plasma biomarkers. Of note, when keeping the neuropathology scales at the most granular level available, the data were too sparse to perform the ordinal regression analysis. Consequently, we combined the neuropathology scales in the following manner: For Braak, we follow the ADNC B-score categorization into scores of 0–2, 3–4, and 5–6. Here, category 0 was grouped with 1–2 due to data sparsity. For Thal, we followed the ADNC A-score categorization: 0, 1–2, 3, and 4–5. For neuritic plaque, we used the standard four categories of None, Sparse, Moderate, and Frequent. Similarly, we used the standard ADNC categories of None, Low, Intermediate, and High. The odds ratios from these models can be interpreted as the odds of being in any set of higher categories relative to the odds of being in any set of lower categories (i.e., Braak 0–2 vs. 3–4 and 5–6 or Braak 0–2 and 3–4 vs. 5–6. For statistical tests, an alpha level of 0.05 was used. All data preparation and analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participant demographics
The characteristics of the 350 participants are shown in Table 1. The mean and standard deviation (SD) age at the visit when plasma was drawn was 85.9 (6.46) years and 141 (40.3%) of the participants were women. The mean (SD) time to death from plasma biomarker measurement to autopsy was 1.53 (1.01) years. There were 197 (56.3%) cognitively unimpaired participants, 93 (26.6%) participants with MCI, and 56 (16.0%) participants with dementia at the clinical assessment when the blood sample was collection. The average time from plasma collection to death was 1.53 ± 1.01 years.
Table 1.
Participant demographics, cognitive scores, and neuropathology scales
| N = 350 | |
|---|---|
|
| |
| Demographics | |
| Age at Visit (years) | 85.9 (6.4) |
| Female | 141 (40.3%) |
| Time-to-Death (years) | 1.53 (1.0) |
| Education (years) | 14.7 (3.2) |
| APOE ε4 carrier status | 95 (27.1%) |
| Body mass index | 27.8 (5.9) |
| Time from plasma biomarker collection to death (years) | 1.53 (1.01) |
| Cognitive status | |
| Mayo-PACC Cognitive Score | −0.56 (1.0) |
| Cognitively unimpaired | 197 (56.3%) |
| Mild cognitive impairment | 93 (26.6%) |
| Dementia | 56 (16.0%) |
| Other | 3 (0.9%) |
| Plasma biomarkers | |
| Aβ42/40 ratio | 0.06 (0.01) |
| GFAP (pg/mL) | 208.1 (113.1) |
| NfL (pg/mL) | 62.0 (42.6) |
| P-tau181 (pg/mL) | 3.96 (2.7) |
| Braak Tangle Stages | |
| 0–III | 211 (61.9%) |
| IV–VI | 130 (38.1%) |
| Neuritic Plaque Score | |
| None/sparse | 193 (55.5%) |
| Moderate/frequent | 155 (44.5%) |
| Thal amyloid phase | |
| Thal phase 0–2 | 133 (48.2%) |
| Thal phase 3–5 | 143 (51.8%) |
| ADNC | |
| None/low | 176 (55.7%) |
| Intermediate/high | 140 (44.3%) |
Mean (SD) listed for continuous variables and count (%) for the categorical variables
Univariate prediction of elevated Alzheimer’s disease neuropathology scales
Univariate prediction of elevated ADNC, Braak stage, neuritic plaque score, or Thal phase using demographic variables, cognitive scores, or plasma biomarkers is shown in Table 2. Among all plasma biomarkers, GFAP had the best individual prediction of Braak staging and neuritic plaque with CV AUC of 0.712 [0.644, 0.781] and 0.702 [0.642, 0.781], respectively. The best individual predictor of ADNC and Thal phase was p-tau181 with CV AUC of 0.680 [0.614, 0.746] and 0.659 [0.578, 0.740], respectively. While individual demographic information such as age, sex, APOE ε4 status, and time from plasma collection to death showed slightly lower performance than plasma biomarkers, Mayo-PACC cognitive scores performed comparatively to individual plasma biomarker levels with CV AUC of 0.711 [0.607,0.815] for prediction of Braak stage, CV AUC of 0.638 [0.577, 0.698] for prediction of neuritic plaques, CV AUC of 0.667 [0.567, 0.767] for prediction of Thal phase, and had the best overall performance, better than any plasma biomarker, for prediction of ADNC with CV AUC of 0.703 [0.629, 0.777].
Table 2.
Univariate predictions of elevated Alzheimer’s disease neuropathology scores using demographic information, cognitive score, and plasma biomarkers
| Elevated ADNC | Elevated Braak tangle stage | Elevated neuritic plaque score | Elevated Thal amyloid phase | |
|---|---|---|---|---|
|
| ||||
| Age at Visit | 0.598 [0.519, 0.677] | 0.616 [0.549, 0.684] | 0.601 [0.558, 0.645] | 0.604 [0.529, 0.678] |
| Female | 0.481 [0.417, 0.546] | 0.572 [0.514, 0.630] | 0.556 [0.501, 0.610] | 0.522 [0.467, 0.577] |
| APOE ε4 carrier | 0.613 [0.574, 0.652] | 0.596 [0.529, 0.663] | 0.598 [0.538, 0.657] | 0.639 [0.586, 0.691] |
| Time from plasma collection to death | 0.537 [0.487, 0.587] | 0.577 [0.525, 0.629] | 0.563 [0.497, 0.629] | 0.610 [0.564, 655] |
| Mayo-PACC | 0.703 [0.629, 0.777] | 0.711 [0.607, 0.815] | 0.638 [0.577, 0.698] | 0.654 [0.580, 0.727] |
| Aβ42/40 Ratio | 0.619 [0.534, 0.705] | 0.639 [0.571, 0.709] | 0.639 [0.557, 0.720] | 0.639 [0.543, 0.736] |
| GFAP | 0.671 [0.586, 0.756] | 0.712 [0.644, 0.781] | 0.702 [0.642, 0.762] | 0.648 [0.577, 0.720] |
| NfL | 0.588 [0.524, 0.652] | 0.556 [0.498, 0.613] | 0.612 [0.547, 0.676] | 0.510 [0.440, 0.579] |
| P-tau181 | 0.680 [0.614, 0.746] | 0.673 [0.607, 0.815] | 0.669 [0.610,0.727] | 0.659 [0.578, 0.740] |
Values listed indicate tenfold cross-validated area under the curve (CV AUC) for a receiver-operating characteristic curve with 95% confidence intervals. Highest value in each pathology scale is shown in bold
ADNC Alzheimer’s disease neuropathologic change; PACC preclinical Alzheimer’s cognitive composite; GFAP glial fibrillary acidic protein; NfL neurofilament light; p-tau181 phosphorylated tau 181
Groupwise comparisons of plasma biomarker levels are shown in Figs. 1, 2, 3, and 4. Participants with elevated ADNC had a significantly lower Aβ42/40 ratio (median = 0.059 vs 0.065, p < 0.001), whereas both GFAP (218.86 vs 151.57, p < 0.001) and p-tau181 (3.85 vs 2.48, p < 0.001) were elevated in those with higher ADNC. NfL levels did not differ between ADNC groups (53.28 vs 51.68, p = 0.443). A similar pattern was observed with Braak stages, such that participants with high Braak stages had a lower Aβ42/40 ratio (0.061 vs 0.065, p = 0.009), higher GFAP (222.37 vs 151.06, p < 0.001) and p-tau181 (3.68 vs 2.68, p < 0.001) levels, and no difference in NfL levels (52.02 vs 50.44, p = 0.068). Again, neuritic plaque scores were associated with a lower Aβ42/40 ratio (0.060 vs 0.066, p < 0.001), higher GFAP (220.67 vs 151.06, p < 0.001) and p-tau181 (3.63 vs 2.63, p < 0.001) levels, and no difference in NfL levels (50.02 vs 53.06, p = 0.734). Similarly, elevated Thal phase was associated with a lower Aβ42/40 ratio (0.060 vs 0.068, p < 0.001), higher GFAP (215.15 vs 163.40, p < 0.001), and higher p-tau181 (3.78 vs 2.62, p < 0.001) but there was no difference in NfL levels (55.15 vs 52.11, p = 0.553).
Fig. 1.
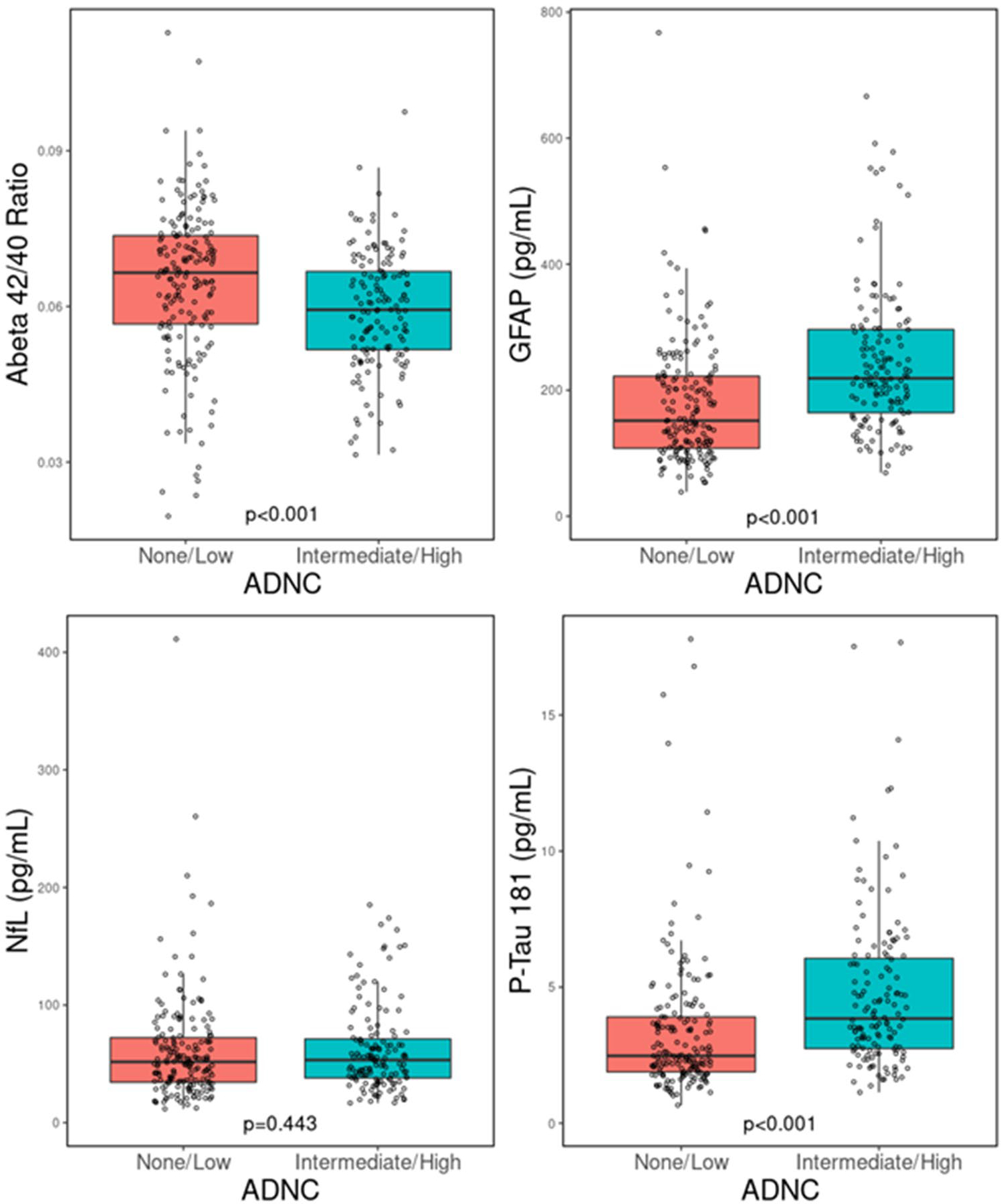
Comparison of plasma biomarker concentrations for Aβ42/40 ratio, GFAP, NfL, and p-tau181 when stratified by high and low ADNC groups. ADNC Alzheimer’s disease neuropathologic change; Aβ42/40 ratio: amyloid-β 42/40 ratio; NfL neurofilament light; p-tau181 phosphorylated tau 181
Fig. 2.
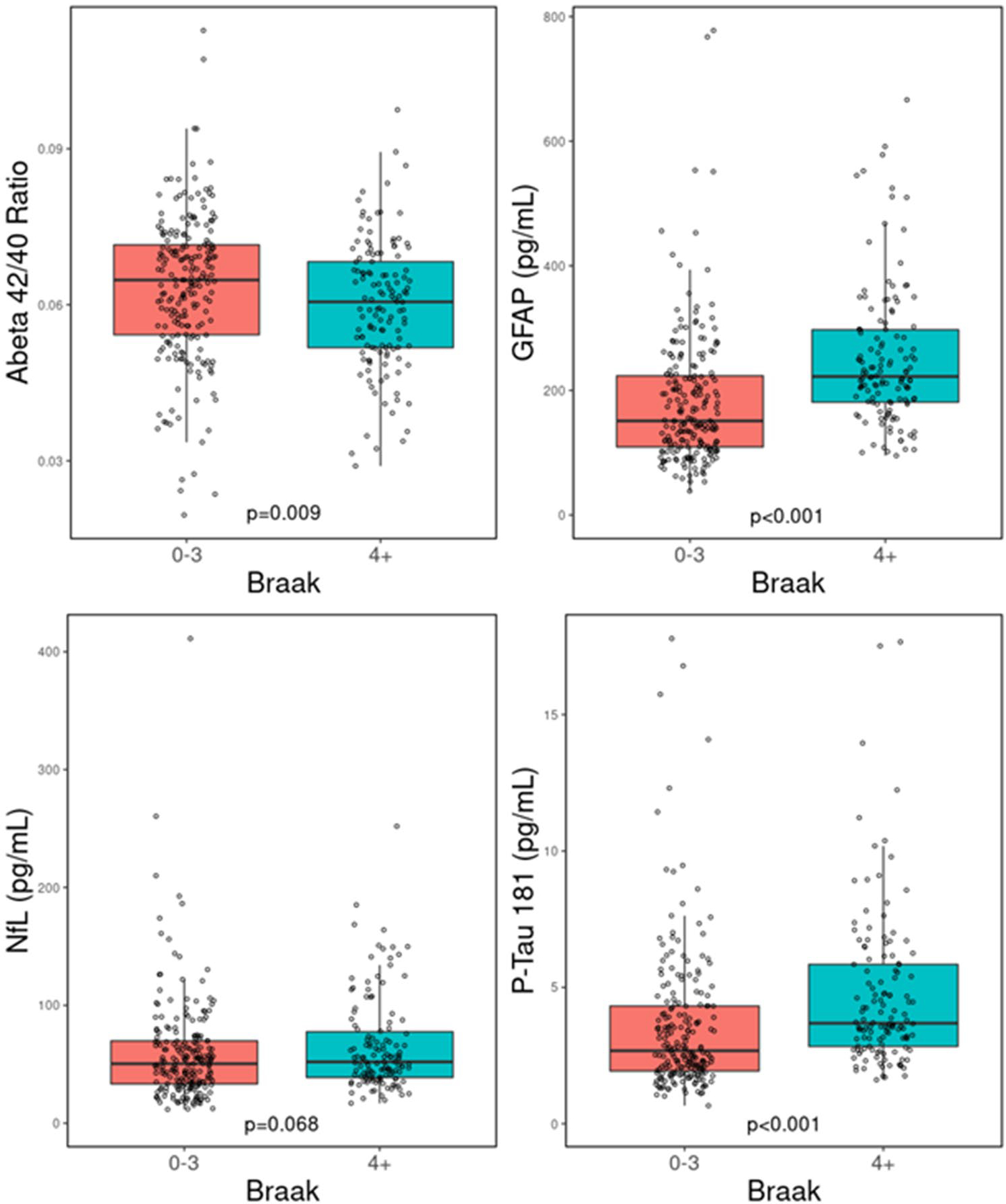
Comparison of plasma biomarker concentrations for Aβ42/40 ratio, GFAP, NfL, and p-tau181 when stratified by high and low Braak stage. Aβ42/40 ratio amyloid-β 42/40 ratio; NfL neurofilament Light; p-tau181 phosphorylated tau 181
Fig. 3.

Comparison of plasma biomarker concentrations for Aβ42/40 ratio, GFAP, NfL, and p-tau181 when stratified by high and low neuritic plaque burden. Aβ42/40 ratio amyloid-β 42/40 ratio; NfL neurofilament light; p-tau181 phosphorylated tau 181
Fig. 4.

Comparison of plasma biomarker concentrations for Aβ42/40 ratio, GFAP, NfL, and p-tau181 when stratified by high and low Thal amyloid phase. Aβ42/40 ratio amyloid-β 42/40 ratio; NfL neurofilament light; p-tau181 phosphorylated tau 181
Selection of plasma and demographic variables for multivariate prediction of Alzheimer’s disease neuropathology scales
Multivariate prediction performance of all four neuropathology scales is shown in Fig. 5. The prediction performance of each variable pool and variables selected are summarized in Table 3. The highest predictive performance among all neuropathology scales was seen with ADNC prediction. The best prediction variable pool for elevated ADNC was the inclusion of plasma biomarkers plus demographic variables plus cognitive scores with a CV AUC [CI] of 0.798 [0.753, 0.843]. Final variables selected in this model included all plasma variables except Aβ42/40 ratio, APOE ε4 carrier status, and Mayo-PACC. Without the Mayo-PACC scores in the variable pool, CV AUC was lowered to 0.777 [0.714, 0.841] with age at visit replacing cognitive scores and Aβ42/40 ratio entering the model. Plasma biomarkers alone had a performance of 0.734 [0.623, 0.846] whereas a variable pool that included all demographics and cognitive scores, but no plasma biomarkers achieved the worst performance with CV AUC of 0.681 [0.565, 0.797] using all variables. Groupwise comparisons in predictive performance between variable pools showed no statistical difference despite a trend towards better performance in the largest variable pool.
Fig. 5.
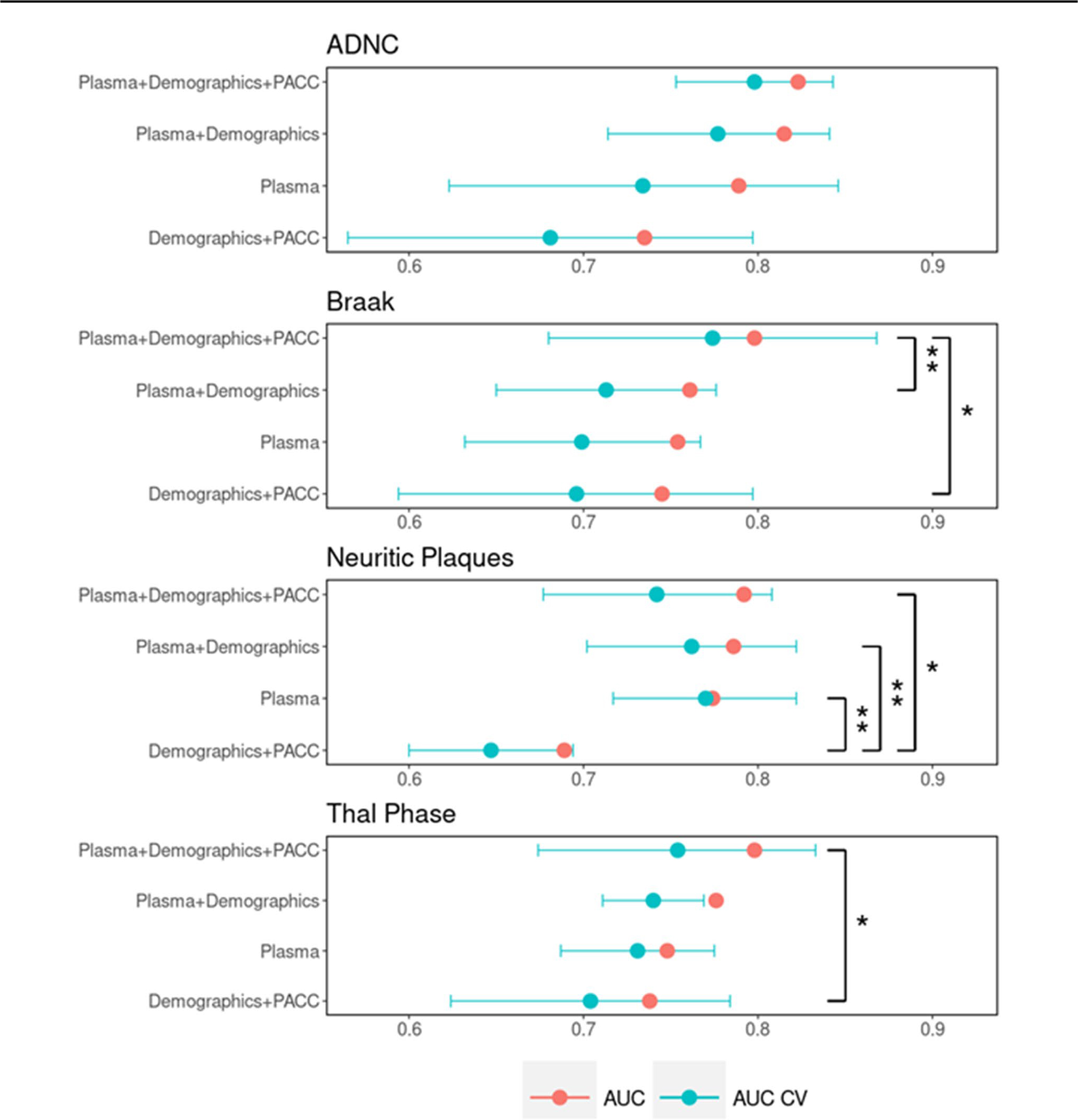
Comparison of predictive performance of neuropathologic scales with different pools of variables available. Plasma was the best model selected from just the plasma markers, Plasma + Demographics was the best model selected when including plasma markers plus covariates in the election pool, and Plasma + Demographics + PACC was the best model selected when additionally including PACC in the selection pool. * indicates p < 0.05, ** indicates p < 0.01
Table 3.
Selection of plasma and demographic variables for multivariate prediction of Alzheimer’s disease neuropathology scores
| Elevated ADNC | Elevated Braak tangle stage | Elevated neuritic plaque score | elevated thal amyloid phase | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Variables selected | AUC [CI] | Variables selected | AUC [CI] | Variables selected | AUC [CI] | Variables selected | AUC [CI] | |
|
| ||||||||
| Plasma biomarkers | Aβ42/40 Ratio | 0.734 [0.623, 0.846] | GFAP | 0.699 [0.632, 0.767] | Aβ42/40 Ratio | 0.770 [0.717, 0.822] | Aβ42/40 Ratio | 0.731 [0.687, 0.775] |
| GFAP | NfL | GFAP | GFAP | |||||
| NfL | P-tau181 | NfL | NfL | |||||
| P-tau181 | P-tau181 | P-tau181 | ||||||
| Plasma biomarkers + demographics | Aβ42/40 Ratio | 0.777 [0.714, 0.841] | GFAP | 0.713 [0.650, 0.776] | Aβ42/40 Ratio | 0.761 [0.702, 0.822] | GFAP | 0.740 [0.711, 0.769] |
| GFAP | P-tau181 | GFAP | NfL | |||||
| NfL | APOE ε4 carrier | NfL | P-tau181 | |||||
| P-tau181 | P-tau181 | APOE ε4 carrier | ||||||
| APOE ε4 carrier | APOE ε4 carrier | |||||||
| Age at Visit | ||||||||
| Demographics + cognitive scores | APOE ε4 carrier | 0.681 [0.565, 0.797] | APOE ε4 carrier | 0.696 [0.594, 0.797] | APOE ε4 carrier | 0.647 [0.600, 0.694] | APOE ε4 carrier | 0.704 [0.624, 0.784] |
| Age at Visit | Age at Visit | Age at Visit | Mayo-PACC | |||||
| Mayo-PACC | Mayo -PACC | Mayo-PACC | ||||||
| Plasma biomarkers + demographics + cognitive scores | GFAP | 0.798 [0.753, 0.843] | GFAP | 0.774 [0.680, 0.868] | Aβ42/40 Ratio | 0.742 [0.677, 0.808] | GFAP | 0.754 [0.674, 0.833] |
| NfL | P-tau181 | GFAP | NfL | |||||
| P-tau181 | Mayo-PACC | NfL | P-tau181 | |||||
| APOE ε4 carrier | P-tau181 | APOE ε4 carrier | ||||||
| Mayo-PACC | Mayo-PACC | Mayo-PACC | ||||||
Values listed indicate a tenfold cross-validation average of the area under the curve (AUC) for a receiver-operating characteristic curve with 95% confidence intervals
ADNC Alzheimer’s disease neuropathologic change; PACC Preclinical Alzheimer’s cognitive composite; GFAP Glial fibrillary acidic protein; NfL neurofilament light; p-tau181 phosphorylated tau 181; CI Confidence interval
Braak stage was best predicted with the plasma biomarkers plus demographics plus cognitive scores and the inclusion of GFAP, p-tau181, and Mayo-PACC scores, as predictors achieved a CV AUC [CI] of 0.774 [0.680, 0.868]. With plasma biomarkers alone, GFAP, p-tau181, and NfL were selected from the plasma biomarker pool with CV AUC [CI] of 0.699 [0.632, 0.767]. The plasma biomarker plus demographics model achieved slightly better performance in which NfL was removed and APOE ε4 carrier status was included to achieve a CV AUC [CI] of 0.713 [0.650, 0.776]. A variable pool without plasma biomarkers, i.e., demographics and cognitive scores performed similarly with CV AUC of 0.696 [0.594, 0.797] using all variables available. Groupwise comparisons between variable pools showed that prediction with the full variable pool was statistically higher than demographics plus Mayo-PACC (p = 0.02) and plasma plus demographics (p = 0.08) but not plasma alone (p = 0.055).
Neuritic plaque scores were best predicted with the plasma biomarker pool, achieving a CV AUC [CI] of 0.770 [0.717, 0.822] with all four plasma biomarkers being selected. The pool of plasma biomarkers and demographics achieved similar performance with CV AUC [CI] of 0.761 [0.702, 0.822] in which all plasma biomarkers were selected with the additional inclusion of APOE ε4 carrier status. The complete variable pool consisting of plasma biomarkers plus demographics plus cognitive scores achieved slightly lower performance with CV AUC of 0.742 [0.677, 0.808] where all plasma biomarkers were selected and Mayo-PACC replaced APOE ε4 carrier status. Again, the lowest overall predictive performance was observed in the variable pool that excluded plasma biomarkers with CV AUC of 0.647 [0.600, 0.694] using all demographics and cognitive scores available. Groupwise comparisons in predictive performance between variable pools showed demographics plus Mayo-PACC performance was statistically lower against all other groups with plasma biomarkers including plasma alone (p = 0.008), plasma plus demographics (p = 0.009) and plasma plus demographics plus Mayo-PACC (p = 0.011). There was no significant difference between two pools where both included plasma biomarkers.
A high Thal amyloid phase was best predicted using the complete variable pool of plasma biomarkers, demographics, and cognitive scores with a CV AUC of 0.754 [0.674, 0.833]. Variables selected included GFAP, NfL, p-tau181, APOE ε4 carrier status, and Mayo-PACC cognitive scores. The variable pool consisting of only plasma biomarkers achieved a CV AUC of 0.731 [0.687, 0.775] with selection of all plasma biomarkers as predictors. Inclusion of demographics showed a slightly better performance with CV AUC of 0.740 [0.711, 0.769] replacing Aβ42/40 ratio with APOE ε4 carrier status. The variable pool that included only demographics and cognitive scores had the lowest performance in the prediction of elevated Thal phase with a CV AUC of 0.704 [0.624, 0.784] using APOE ε4 carrier status and Mayo-PACC scores. Groupwise comparisons between variable pools showed a statistical increase in predictive performance between the complete variable pool and the pool with demographics plus Mayo-PACC (p = 0.023).
Regression coefficients and odds ratio of the ordinal logistic regression are shown in the Supplemental Table 2 (online resource). Overall, GFAP, NfL, p-tau181, Mayo-PACC score, and APOE ε4 carrier status were all significant predictors of increasing degree of neuropathology across all four metrics (p < 0.05). Aβ42/40 ratio was a significant predictor of ADNC (p < 0.01), neuritic plaque score (p < 0.01), and Thal phase (p < 0.01) but not Braak stage (p = 0.13). Age at time of visit was a significant predictor of ADNC (p < 0.01), Braak stage (p = 0.036), and Thal phase (p = 0.026) but not neuritic plaque score (p = 0.070). Female sex was not a significant predictor of increasing burden across any of the neuropathology metrics.
Subgroup analysis in participants stratified by cognitive status
A subgroup analysis was performed to test differences in predictive performance between participants who were cognitively unimpaired (CU) and those with cognitive impairment (CI) consisting of MCI or dementia (Supplemental Table 1, online resource). CI participants were more likely to be APOE ε4 carriers (p = 0.028, Pearson Chi-squared test) and have worse Mayo-PACC cognitive scores (p < 0.001, Kruskal–Wallis rank-sum test) but no other differences in demographics. CI participants has significantly higher neuropathology scores across all four metrics (p < 0.01, Pearson Chi-squared test). With respect to plasma biomarkers, CI participants had significantly higher levels of GFAP (p < 0.001, Kruskal–Wallis rank-sum test) but not NfL, p-tau181, or Aβ42/40 ratio.
In univariate analyses (Supplemental Table 5, online resource), separation by cognitive status resulted in a general improvement in prediction performance in p-tau181 across all neuropathology metrics with CV AUC for ADNC, Braak staging, Neuritic plaque, and Thal phase of 0.755 (p = 0.009), 0.745 (p = 0.213), 0.725 (p = 0.006), and 0.848 (p < 0.001) amongst those with MCI or dementia versus 0.625, 0.663, 0.637, 0.651, respectively, for those CU. There was no significant difference between subgroups among Aβ42/40 ratio, GFAP, or NfL, although there was a trend towards improvement of prediction of ADNC with GFAP, Neuritic plauqe with GFAP and Aβ42/40 ratio, and Thal phase with GFAP and Aβ42/40 ratio. Demographic variables and cognitive scores performed similarly in both subgroups (Table 4).
Table 4.
Selection of plasma, demographic, and cognitive score variables for multivariate prediction of Alzheimer’s disease neuropathology scores on participants stratified into cognitively unimpaired (CU) versus mild cognitive impairment or dementia (MCI/D)
| Elevated ADNC | Elevated Braak Tangle Stage | Elevated Neuritic Plaque Score | Elevated Thal Amyloid Phase | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||||||||
| CU | MCI/D | p value | CU | MCI/D | p value | CU | MCI/D | p value | CU | MCI/D | p value | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Variables Selected | AUC [CI] | Variables Selected | AUC [CI] | Variables Selected | AUC [CI] | Variables Selected | AUC [CI] | Variables Selected | AUC [CI] | Variables Selected | AUC [CI] | Variables Selected | AUC [CI] | Variables Selected | AUC [CI] | |||||
|
| ||||||||||||||||||||
| Plasma Biomarkers | GFAP | 0.636 [0.527, 0.744] | Aβ42/40 Ratio | 0.811 [0.748, 0.874] | 0.007 | GFAP | 0.741 [0.626, 0.856] | GFAP | 0.723 [0.601, 0.845] | 0.812 | GFAP | 0.687 [0.597, 0.778] | Aβ42/40 Ratio | 0.797 [0.726, 0.868] | 0.045 | Intercept Only | 0.557 [0.471, 0.643] | Aβ42/40 Ratio | 0.783 [0.653, 0.913] | 0.005 |
| GFAP | P-tau181 | GFAP | GFAP | |||||||||||||||||
| NfL | NfL | NfL | ||||||||||||||||||
| P-tau181 | P-tau181 | P-tau181 | ||||||||||||||||||
| Plasma Biomarkers + Demographics | APOEε4 carrier | 0.694 [0.605, 0.783] | Aβ42/40 Ratio | 0.813 [0.751, 0.875] | 0.024 | GFAP | 0.636 [0.586, 0.686] | GFAP | 0.733 [0.634, 0.831] | 0.069 | GFAP | 0.630 [0.550, 0.711] | Aβ42/40 Ratio | 0.797 [0.726, 0.868] | 0.003 | APOEε4 carrier | 0.683 [0.575, 0.790] | GFAP | 0.789 [0.683, 0.895] | 0.128 |
| Age at Visit | GFAP | APOEε4 carrier | P-tau181 | APOEε4 carrier | GFAP | Age at Visit | P-tau181 | |||||||||||||
| NfL | NfL | APOEε4 carrier | ||||||||||||||||||
| P-tau181 | P-tau181 | |||||||||||||||||||
| Demographics + Cognitive Scores | APOEε4 carrier | 0.700 [0.626, 0.774] | Intercept Only | 0.513 [0.472, 0.554] | <0.001 | Age at Visit | 0.638 [0.577, 0.699] | Mayo-PACC | 0.623 [0.520, 0.726] | 0.778 | APOEε4 carrier | 0.602 [0.536, 0.667] | Intercept Only | 0.494 [0.455, 0.533] | 0.006 | APOEε4 carrier | 0.686 [0.573, 0.800] | APOEε4 carrier | 0.698 [0.609, 0.787] | 0.858 |
| Age at Visit | Age at Visit | Age at Visit | ||||||||||||||||||
| Plasma Biomarkers + Demographics + Cognitive Scores | GFAP | 0.659 [0.601, 0.717] | GFAP | 0.851 [0.736, 0.966] | 0.005 | GFAP | 0.677 [0.594, 0.760] | GFAP | 0.725 [0.606, 0.843] | 0.466 | GFAP | 0.666 [0.564, 0.769] | Aβ42/40 Ratio | 0.681 [0.587, 0.776] | 0.808 | APOEε4 carrier | 0.648 [0.555, 0.742] | GFAP | 0.796 [0.700, 0.892] | 0.022 |
| APOEε4 carrier | NfL | Mayo-PACC | P-tau181 | GFAP | Age at Visit | P-tau181 | ||||||||||||||
| Mayo-PACC | P-tau181 | Female | NfL | APOEε4 carrier | ||||||||||||||||
| Mayo-PACC | P-tau181 | |||||||||||||||||||
p-values in bold indicate a statistically significant difference in predictive performance between the CU and MCI/Dementia subgroups (p < 0.05)
Values listed indicate a tenfold cross-validation average of the area under the curve (AUC) for a receiver-operating characteristic curve with 95% confidence intervals
ADNC Alzheimer’s disease neuropathologic change; pCC preclinical Alzheimer’s cognitive composite; GFAP glial fibrillary acidic protein; NfL neurofilament light; p-tau181 phosphorylated tau 181; CI confidence interval
In the multivariate analyses, there was a general improved predictive performance in the MCI/dementia subgroup across all four neuropathology scales when plasma biomarkers were included in the available variable pool (Table 4). In the case of ADNC, the best prediction performance was seen with the complete variable pool in participants with MCI/dementia vs cognitively unimpaired (CV AUC 0.851 vs 0.659, p = 0.005), whereas cognitively unimpaired individuals showed best performance in the demographics plus Mayo-PACC pool (CV AUC of 0.700 vs 0.513 in the MCI/dementia subgroup, p < 0.001). Variables chosen by the MCI/dementia subgroup included GFAP, NfL, and p-tau181 whereas the best prediction of ADNC in cognitively unimpaired individuals included variables of APOE ε4 carrier status and age at visit but did not include plasma biomarkers. Groupwise comparisons between variable pools showed a statistical improvement in prediction of ADNC when plasma biomarkers were included in the MCI/dementia subgroup (p < 0.001) but not in the cognitively unimpaired group.
Braak stage prediction was best in the variable pool of plasma plus demographics CV AUC 0.733 [0.634, 831] in the MCI/dementia subgroup with GFAP and p-tau181 as variables selected and the plasma biomarkers pool for the cognitively unimpaired group CV AUC 0.741 [0.626, 0.856] using GFAP alone. Despite a trend towards improvement, there was no statistically significant difference between subgroups or across variable pools.
The best neuritic plaque score prediction was achieved in the MCI/dementia subgroup which was predicted using the plasma biomarkers plus demographics (CV AUC 0.797 vs 0.630, p = 0.003) using all plasma biomarkers in the MCI/dementia subgroup versus GFAP and APOE ε4 carrier status in the cognitively unimpaired subgroup. Without plasma biomarkers, prediction was significantly better in the cognitively unimpaired group (CV AUC 0.602 vs 0.494, p = 0.006). There were no groupwise differences between variable pools in the cognitively unimpaired subgroup, but there was an improvement in prediction when including plasma biomarkers in the MCI/dementia subgroup (p < 0.001).
The best prediction of Thal amyloid phase was observed in the MCI/dementia subgroup using the complete variable pool with a CV AUC of 0.796 using GFAP, p-tau181, and APOE ε4 carrier status vs a CV AUC of 0.648 in the cognitively unimpaired, using APOE ε4 carrier status and age at visit (p < 0.022). The best performance in the cognitively unimpaired was in the demographics and cognitive scores variable pool using APOE ε4 carrier status and age at visit, with similar performance in the MCI/ dementia subgroup using APOE ε4 carrier status alone (CV AUC 0.686 vs 0.698, p = 0.858). Groupwise comparisons showed no difference in performance between variable pools in either subgroup.
Subgroup analysis in participants without chronic kidney disease
A subgroup analysis was performed to test differences in predictive performance between participants with and without chronic kidney disease (CKD). There was no groupwise statistical difference in demographic variables, cognitive status, or any neuropathologic scale (Supplemental Table 1 online resource). Participants with CKD had significantly higher plasma biomarkers concentrations of GFAP, NfL, and p-tau181 (p < 0.001, Kruskal–Wallis rank-sum test) but not Aβ42/40 ratio. Limiting the subjects to those without chronic kidney disease (CKD) showed slight improvement in performance in some univariate prediction models (see Supplemental Table 3 online resource).
In the multivariate models (Supplemental Table 4 online resource), prediction of ADNC had similar CV AUC across all variable pools when those with CKD were excluded, with the best performance in both groups seen in the full variable pool (CV AUC 0.798 with CKD participants vs 0.776 without). Braak stage prediction model performance increased slightly with plasma alone and plasma plus demographics cohort but decreased again when cognitive scores were included additionally. Again, the best performance in both cohorts was seen in the full variable pool with CV AUC of 0.774 when including participants with CKD compared to 0.766 when CKD participants were excluded, with the same variables selected in both groups. Neuritic plaque prediction showed a slight decrease in prediction performance across all variable pools that included plasma biomarkers. Both cohorts showed the best prediction with only the plasma biomarker pool (CV AUC 0.770 vs 0.764) by selecting all biomarkers. Again, Thal phase prediction performed similarly in both groups with a slight increase in performance with the best CV AUC in the plasma plus demographics variable pool of 0.751 in those without CKD vs 0.740 in the full cohort. The statistically significant differences seen when including plasma markers in prediction of neuritic plaque and Thal phase in the full cohort were no longer observed in the cohort that excluded CKD.
Discussion
This study investigated the utility of plasma biomarkers in the prediction of ADNC and its components of amyloid and tau pathology. The goal of this work was to provide clinical insights into the use of plasma biomarkers in the diagnosis and prognosis of AD pathology by identifying specific plasma biomarkers for accurate prediction of amyloid and tau neuropathology status along with demographics and cognitive scores. A key finding of this work was that the highest predictive accuracy of AD neuropathology scales can be achieved with a combination of plasma biomarkers suggesting that it is best to combine the non-overlapping information from the four plasma biomarkers in comparison to a single plasma marker. We also found that inclusion of contextual patient information specifically demographics and cognitive testing scores along with plasma biomarkers can further improve prediction of AD neuropathology scales. In addition, plasma biomarkers greatly improve prediction of underlying neuropathologic change in individuals with preexisting cognitive impairment such as MCI or dementia.
With the recent growth in plasma markers—GFAP[6, 33, 44], p-tau181 [16, 18, 19, 37, 42], p-tau 217[31, 42], and p-tau231[3, 37] as possible tools for screening and diagnosis, a comprehensive evaluation of the accuracy with which clinically available plasma markers can predict amyloid, tau, and overall ADNC neuropathology scales is important. Further, the evaluation of the plasma markers along with participant information, i.e., cognitive scores and demographics in a population-based sample as done here allowed us to evaluate how these plasma markers can be utilized clinically.
Non-overlapping information from GFAP and p-tau181 for prediction of amyloid and tau pathology
Our findings support that plasma GFAP and p-tau181 may contain the most independent and complementary information among the four marker measures as both were chosen across all variable prediction pools. A study by Winder et al. [44] showed positive associations between increased GFAP levels and ADNC, neuritic plaque, Thal phase, and Braak stage score in a sample of 90 participants, but was underpowered to show significant differences. The current study extends this observation to show that GFAP may be a key plasma biomarker necessary for prediction of Braak staging and a key element for the prediction of neuritic plaque and overall ADNC. These findings are supported by the ordinal regression results, which show increasing levels of these two plasma biomarkers with higher neuropathologic burden across all four scales. While P-tau181 alone has been shown to be a good independent predictor of high ADNC [5, 19, 37] and considered as a biomarker for amyloidosis[16, 18, 22, 26, 30]; our analyses highlighted that GFAP and p-tau181 provide non-overlapping information and are likely capturing slightly different pathological processes and are both important for the prediction of amyloid and tau pathologies.
Aβ42/40 and NfL prediction of neuritic plaque scores
Conversely, Aβ42/40 ratio was uniformly selected in the prediction of elevated neuritic plaque and in some models of ADNC and Thal phase, possibly having a stronger association with amyloid pathology in comparison with tau pathology. This observation is further supported by our ordinal regression results, where both Aβ42/40 ratio and NfL showed significant decreasing levels with higher neuropathologic burden across most neuropathology scales, with the exception of Aβ42/40 ratio associations with Braak staging. Aβ42/40 ratio has been previously shown to be an important biomarker of AD neuropathology [5, 44]. NfL was selected in all variable pools for ADNC, neuritic plaque, and Thal phase again suggesting a stronger association with amyloid over tau deposition. Several studies have shown a weak association between NfL and AD neuropathologic change [5, 9, 44]. The broad response of NfL observed in this work may be attributed to the plasma samples being collected near death, thus reflecting biomarker sensitivity to any pathology causing neuronal injury due to multiple comorbidities instead of specific pathology such as AD.
Demographics and cognitive scores improve prediction accuracy of plasma markers
The addition of demographic information improved predictive performance overall, with key variables being APOE ε4 and age at visit. Time between plasma collection and death was not selected in any of the multivariate models for any neuropathological scale, suggesting that the signal from this variable may already be captured in other demographic or biomarker variables. Interestingly, inclusion of Mayo-PACC cognitive scores, replaced age at visit but not APOE ε4 carrier status for the prediction of ADNC, likely due to high collinearity between cognitive scores and aging. However, adding Mayo-PACC to Braak stage and neuritic plaque prediction models replaced APOE ε4 as a necessary variable, suggesting that Mayo-PACC may be capturing the early neuropathologic changes due to age and APOE ε4 status [39]. APOE ε4 status was a constant demographic predictor of Thal phase regardless of inclusion of Mayo-PACC. There were three instances where contextual demographic information excluded plasma variables: (1) The inclusion of Mayo-PACC for prediction of ADNC excluded Aβ42/40 ratio for better performance; (2) the inclusion of APOE ε4 carrier status for prediction of Braak staging excluded NfL from the chosen predictors; and (3) inclusion of APOE ε4 carrier status excluded Aβ42/40 ratio for prediction of Thal phase. This suggests that the signal captured by the demographic variables easily obtained during clinical visits has overlapping information with plasma biomarkers because demographic variables are often reasonable surrogates of various disease processes. The inclusion of years from plasma collection to death as an available variable was not chosen in any of the models, suggesting it did not provide any additional predictive information to neuropathology scales. However, most of these samples were collected within 2 years of death and the relevance of this marker may change as the time to death increases.
Prediction with plasma biomarkers in cognitively unimpaired versus cognitively impaired
The subgroup analysis that stratified participants by cognitively unimpaired versus MCI/dementia showed an overall improvement in predictive performance in the MCI/dementia subgroup when using plasma biomarkers in both the univariate and multivariate analyses, again reflecting the important role of plasma biomarkers as noninvasive measures of neurodegeneration in the central nervous system. In terms of variable selection, GFAP was a common predictor across both cognitive subgroups, but more plasma biomarkers, p-tau181 in particular, were used in the MCI/dementia subgroup for better predictive performance. This suggests that GFAP may represent a general marker of neurodegeneration, whereas other markers like p-tau181 may better reflect the underlying presence of amyloid and tau pathology. Interestingly, no plasma biomarkers were chosen as important for prediction of ADNC or Thal phase among cognitively unimpaired individuals from a pool of plasma plus demographics variable pool. Demographic variables and cognitive scores were important predictors of underlying neuropathologic changes in the cognitively unimpaired group whereas addition of plasma biomarkers showed a consistent improvement in predictive performance in the MCI/dementia subgroup.
Study limitations and future directions
This study presents results of analysis on the largest existing cohort of participants with data available on multiple plasma biomarkers and neuropathologic evaluation. However, there are some limitations. Namely, our top predictive performance was that of ADNC with plasma biomarkers, demographics, and Mayo-PACC with AUC of 0.821 when comparing intermediate/high versus low/none ADNC. This is lower compared to other studies, which have shown AUC of 0.93 when comparing a high ADNC group versus a mixed intermediate/low/none group [5]. Smirnov et al. achieved similar performance to our work using p-tau181in a large cohort to predict high ADNC, but the added value of combining plasma biomarkers was not explored [37]. This performance may be due to the sample composition in these studies, which is reflective of an unselected population in comparison to clinic sample of AD dementia participants and controls seen in other studies. Though the AUC is lower, it is likely reflective of variability seen in the population. The intermediate ADNC category encompasses more variability in the amyloid and tau spectrum, while still showing evidence of neuropathologic change. This group may benefit most from early diagnosis for prognostication and treatment strategies. However, it is important to note that all plasma samples in this work were collected on average 1.5 years prior to death. Although the findings illustrate the biological relationships of the plasma biomarkers and neuropathology, the terminal nature of the participants probably overstates the associations for those persons with longer survivals.
The subgroup analysis of participants without CKD showed an overall trend towards lower prediction performance of neuropathologic change and loss of significance in the variable pool that includes plasma biomarkers. The lower prediction performance seen here are likely due to multiple medical comorbidities that are present in heterogenous participants from a population-based study. CKD and other associated metabolic comorbidities such as cardiovascular disease likely introduce variability in plasma biomarker level measurements and associated with neurodegeneration, making it more difficult to reliably predict underlying neuropathologic changes due to amyloid or tau [10, 28].
Another limitation is the assay used for plasma biomarkers and its scope. Prior head-to-head comparisons have shown that Quanterix p-tau181 and Aβ42/40 ratio may be on the lower end diagnostic performance [15, 17]. Additionally, evidence has shown utility of biomarkers such as p-tau217 and p-tau231, which may further improve prediction of underlying neuropathologic changes [3, 24, 42]. Other platforms such as mass spectrometry may provide more sensitive measures than immunoassays. However, the assay we used is a clinically available assay which increases the clinical relevance of our work. A limitation of this study is that no separate cohort with neuropathology and plasma biomarkers acquired under a different platform assay was included in this study. Such a cohort could confirm the findings described in this work and enhance generalizability. Lastly, collecting demographic, cognitive, and plasma biomarker data from a large population-based study presents several challenges and limitations. For instance, not all participants had complete data across all data pools, particularly when it came to Mayo-PACC scores. Consequently, the sample size decreased as the variable pool increased, which may explain why different variables were selected for the prediction of Braak staging and ADNC from the biomarker only pool (N = 348) compared to the biomarker plus demographics plus cognitive scores pool (N = 262) or why performance decreased in larger variable pools when the same variables were selected.
Overall, this work showed that a combination of plasma biomarker assays and individualized patient information can predict neuropathologic change with high accuracy. Being able to establish the presence of amyloid and tau positivity with a clinical visit and a blood draw will significantly lower the threshold for early detection of AD, which will have an important impact on clinical diagnosis and prognosis and inclusion for participants in emerging amyloid therapies.
Supplementary Material
Acknowledgements
We thank all the study participants and staff in the Mayo Clinic Study of Aging, Mayo Alzheimer’s Disease Research Center, and Aging Dementia Imaging Research laboratory at the Mayo Clinic for making this study possible. This work was supported by grants from National Institutes of Health (NIH) (U01 AG006786, R01 AG034676, RF1 AG069052). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Declarations
Conflict of interest The authors do not have any pertinent conflicts of interest relevant to this study.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00401-023-02594-w.
Data availability
Deidentified data and analysis software can be made available upon request.
References
- 1.American Psychiatric Association D, Association AP (2013) Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association, Washington [Google Scholar]
- 2.Ashton NJ, Janelidze S, Mattsson-Carlgren N, Binette AP, Strandberg O, Brum WS et al. (2022) Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nat Med. 10.1038/s41591-022-02074-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashton NJ, Pascoal TA, Karikari TK, Benedet AL, Lantero-Rodriguez J, Brinkmalm G et al. (2021) Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol 141:709–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brickman AM, Manly JJ, Honig LS, Sanchez D, Reyes-Dumeyer D, Lantigua RA et al. (2021) Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement 17:1353–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cicognola C, Janelidze S, Hertze J, Zetterberg H, Blennow K, Mattsson-Carlgren N et al. (2021) Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimer’s Res Ther 13:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark C, Lewczuk P, Kornhuber J, Richiardi J, Maréchal B, Karikari TK et al. (2021) Plasma neurofilament light and phosphorylated tau 181 as biomarkers of Alzheimer’s disease pathology and clinical disease progression. Alzheimer’s Res Ther 13:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullen NC, Leuzy A, Janelidze S, Palmqvist S, Svenningsson AL, Stomrud E et al. (2021) Plasma biomarkers of Alzheimer’s disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat Commun 12:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graff-Radford J, Mielke MM, Hofrenning EI, Kouri N, Lesnick TG, Moloney CM et al. (2022) Association of plasma biomarkers of amyloid and neurodegeneration with cerebrovascular disease and Alzheimer’s disease. Neurobiol Aging 119:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graff-Radford J, Mielke MM, Hofrenning EI, Kouri N, Lesnick T, Moloney CM et al. (2022) Plasma biomarkers of amyloid and neurodegeneration predictive of neuropathologic scales of cerebrovascular disease. Alzheimers Dement 18:e067350 [Google Scholar]
- 11.Grothe MJ, Moscoso A, Ashton NJ, Karikari TK, Lantero-Rodriguez J, Snellman A et al. (2021) Associations of fully automated CSF and novel plasma biomarkers with Alzheimer disease neuropathology at autopsy. Neurology 97:e1229–1242. 10.1212/WNL.0000000000012513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansson O, Edelmayer RM, Boxer AL, Carrillo MC, Mielke MM, Rabinovici GD et al. (2022) The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dement. 10.1002/alz.070020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC et al. (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack CR, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB et al. (2016) A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87:539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janelidze S, Bali D, Ashton NJ, Barthélemy NR, Vanbrabant J, Stoops E et al. (2022) Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer’s disease. Brain. 10.1093/brain/awac333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE et al. (2020) Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med 26:379–386 [DOI] [PubMed] [Google Scholar]
- 17.Janelidze S, Teunissen CE, Zetterberg H, Allué JA, Sarasa L, Eichenlaub U et al. (2021) Head-to-head comparison of 8 plasma amyloid-β 42/40 assays in Alzheimer disease. JAMA Neurol 78:1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL et al. (2020) Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 19:422–433 [DOI] [PubMed] [Google Scholar]
- 19.Lantero Rodriguez J, Karikari TK, Suárez-Calvet M, Troakes C, King A, Emersic A et al. (2020) Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol 140:267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLeod A, Xu C (2010) bestglm: Best subset GLM. CRAN http://cran.nexr.com/web/packages/bestglm/vignettes/bestglm.pdf [Google Scholar]
- 21.Mielke MM, Hagen CE, Wennberg AM, Airey DC, Savica R, Knopman DS et al. (2017) Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the mayo clinic study on aging. JAMA Neurol 74:1073–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ et al. (2018) Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau-and amyloid-positron emission tomography. Alzheimers Dement 14:989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mielke MM, Syrjanen JA, Blennow K, Zetterberg H, Vemuri P, Skoog I et al. (2019) Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology 93:e252–e260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milà-Alomà M, Ashton NJ, Shekari M, Salvadó G, OrtizRomero P, Montoliu-Gaya L et al. (2022) Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer’s disease. Nat Med 29:1787–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirra SS, Heyman A, McKeel D, Sumi S, Crain BJ, Brownlee L et al. (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–479 [DOI] [PubMed] [Google Scholar]
- 26.Moloney CM, Labuzan SA, Crook JE, Siddiqui H, Castanedes-Casey M, Lachner C et al. (2022) Phosphorylated tau sites that are elevated in Alzheimer’s disease fluid biomarkers are visualized in early neurofibrillary tangle maturity levels in the post mortem brain. Alzheimers Dement 19:1029–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW et al. (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray AM, Vemuri P (2022) Kidney Disease and Brain Health: Current Knowledge and Next Steps. Am J Kidney Dis. 10.1053/j.ajkd.2022.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray ME, Moloney CM, Kouri N, Syrjanen JA, Matchett BJ, Rothberg DM et al. (2022) Global neuropathologic severity of Alzheimer’s disease and locus coeruleus vulnerability influences plasma phosphorylated tau levels. Mol Neurodegener 17:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oberstein TJ, Schmidt MA, Florvaag A, Haas A-L, Siegmann E-M, Olm P et al. (2022) Amyloid-β levels and cognitive trajectories in non-demented pTau181-positive subjects without amyloidopathy. Brain 145:4032–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E et al. (2020) Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 324:772–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmqvist S, Stomrud E, Cullen N, Janelidze S, Manuilova E, Jethwa A et al. (2022) An accurate fully automated panel of plasma biomarkers for Alzheimer’s disease. Alzheimers Dement. 10.1002/alz.12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira JB, Janelidze S, Smith R, Mattsson-Carlgren N, Palmqvist S, Teunissen CE et al. (2021) Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer’s disease. Brain 144:3505–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256:183–194 [DOI] [PubMed] [Google Scholar]
- 35.Shen XN, Li JQ, Wang HF, Li HQ, Huang YY, Yang YX et al. (2020) Plasma amyloid, tau, and neurodegeneration biomarker profiles predict Alzheimer’s disease pathology and clinical progression in older adults without dementia. Alzheimer’s Dement 12:e12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simrén J, Leuzy A, Karikari TK, Hye A, Benedet AL, Lantero-Rodriguez J et al. (2021) The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer’s disease. Alzheimers Dement 17:1145–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smirnov DS, Ashton NJ, Blennow K, Zetterberg H, Simren J, Lantero-Rodriguez J et al. (2022) Plasma biomarkers for Alzheimer’s disease in relation to neuropathology and cognitive change. Acta Neuropathol 143:487–503. 10.1007/s00401-022-02408-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.St Sauver JL, Chamberlain AM, Bobo WV, Boyd CM, Rutten LJF, Jacobson DJ et al. (2021) Implementing the US Department of Health and Human Services definition of multimorbidity: a comparison between billing codes and medical record review in a population-based sample of persons 40–84 years old. BMJ Open 11:e042870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stricker NH, Twohy EL, Albertson SM, Karstens AJ, Kremers WK, Machulda MM et al. (2022) Mayo-PACC: A parsimonious preclinical Alzheimer’s disease cognitive composite comprised of public-domain measures to facilitate clinical translation. Alzheimers Dement. 10.1002/alz.12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Syrjanen JA, Campbell MR, Algeciras-Schimnich A, Vemuri P, Graff-Radford J, Machulda MM et al. (2022) Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement 18:1128–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thal DR, Rüb U, Orantes M, Braak H (2002) Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800 [DOI] [PubMed] [Google Scholar]
- 42.Thijssen EH, La Joie R, Strom A, Fonseca C, Iaccarino L, Wolf A et al. (2021) Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol 20:739–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tosun D, Veitch D, Aisen P, Jack CR Jr, Jagust WJ, Petersen RC et al. (2021) Detection of β-amyloid positivity in Alzheimer’s Disease Neuroimaging Initiative participants with demographics, cognition MRI and plasma biomarkers. Brain Commun 3:008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winder Z, Sudduth TL, Anderson S, Patel E, Neltner J, Martin BJ et al. (2022) Examining the association between blood-based biomarkers and human post mortem neuropathology in the University of Kentucky Alzheimer’s Disease Research Center autopsy cohort. Alzheimers Dement. 10.1002/alz.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data and analysis software can be made available upon request.


