Abstract
Adult neural stem cells are neurogenesis progenitor cells that play an important role in neurogenesis. Therefore, neural regeneration may be a promising target for treatment of many neurological illnesses. The regenerative capacity of adult neural stem cells can be characterized by two states: quiescent and active. Quiescent adult neural stem cells are more stable and guarantee the quantity and quality of the adult neural stem cell pool. Active adult neural stem cells are characterized by rapid proliferation and differentiation into neurons which allow for integration into neural circuits. This review focuses on differences between quiescent and active adult neural stem cells in nutrition metabolism and protein homeostasis. Furthermore, we discuss the physiological significance and underlying advantages of these differences. Due to the limited number of adult neural stem cells studies, we referred to studies of embryonic adult neural stem cells or non-mammalian adult neural stem cells to evaluate specific mechanisms.
Keywords: adult neurogenesis, cell metabolic pathway, cellular proliferation, neural stem cell niches, neural stem cells, neuronal differentiation, nutrient sensing pathway, proteostasis
Introduction
Studies have shown that the brain retains neuroregenerative capacity after mammals, such as rodents (Altman and Das, 1965) and primates (Kaplan and Hinds, 1977), reach adulthood. However, the neuroregenerative capacity of the adult human brain remains unclear, with some studies showing negligible levels of adult neurogenesis (Sorrells et al., 2018). The general belief is that two main areas maintain neurogenesis: the subventricular zone (SVZ) of the lateral ventricle (Morshead et al., 1994; Dillen et al., 2020) and the subgranular zone (SGZ) of the dentate gyrus (Bonaguidi et al., 2012; Berg et al., 2018). Discovery of a complex microenvironment and crucial roles played by neuronal stem cell (NSC) development has led to coining of the term “neurogenic niche.” Recent animal studies have shown neurogenesis in other brain regions such as in tanycytes in the hypothalamus (Rodríguez et al., 2019), the striatum (Parent et al., 1995), and the cerebral cortex (Ge et al., 2020; Figure 1).
Figure 1.
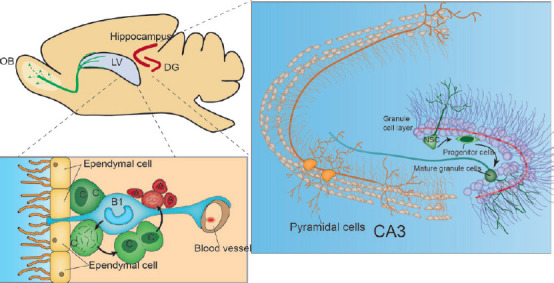
In rodent brains, two main areas maintain neurogenesis: the SVZ of the LV.
In the subventricular zone (SVZ), nascent neurons migrate long distances to the olfactory bulb (OB) along the dorsal migratory stream (depicted with green lines) and the subgranular zone (SGZ) of the dentate gyrus (DG). Nascent neurons then migrate short distances to form the granule cell layer of mossy fiber pathways and synaptic connections with Cornu Ammonis region 3 (CA3) neurons (DG and CA3 depicted with red lines). In the SVZ, neural stem cells (NSCs) are termed B-type cells, a subpopulation of quiescent cells with an astrocyte-like appearance. Morphologically, B cells have a long base protrusion terminating in blood vessels and an apex that terminates on the surface of the ventricle. Then, B cells differentiate into transport amplifying cells, called C cells, and later into neuroblasts, which are also called A cells. In the SGZ, NSC cells are called type 1 cells, which are similar to B cells of the SVZ. Type 1 cells differentiate into intermediate progenitors, which are called type 2 cells. Type 2 cells give rise to type 3 cells or neuroblasts (not shown), which form mature cells in contact with pyramidal cells. Created with Adobe illustrator. LV: Lateral ventricle; SVZ: subventricular zone.
In the SVZ and SGZ, NSCs have different names, forms, and surroundings (McMillan et al., 2022; Malik et al., 2023). In the SVZ, NSCs are called B-type cells and are astrocyte-like in appearance. NSCs in the SVZ express GFAP, Nestin, and Sox2 (Doetsch et al., 1997). Morphologically, a B cell presents with a long base with a protrusion terminating in a blood vessel and an apex that terminates on the surface of the ventricle, where ependymal cells surround the apices of these B cells to form a pinwheel-like tissue morphology (Mirzadeh et al., 2008). A study showed that direct interaction between B cells and vascular endothelial cells promoted B cell quiescence through ephrinB2 and Jagged1 (Ottone et al., 2014). In the SGZ, NSCs are called type 1 cells and are similar to B cells in the SVZ in that they present with astrocyte-like electrophysiological properties and express GFAP, Nestin, and Sox2 (Filippov et al., 2003). Morphologically, type 1 cell bodies are located in the SGZ, but type 1 cell protrusions stick out of the granular layer and make contact with the molecular layer while maintaining permanent contact with blood vessels (Kumar et al., 2019; Ribeiro and Xapelli, 2021). Through direct contact with blood vessels, NSCs can monitor changes in blood flow signals, thus monitoring delivery of nutrients and oxygen, which are raw materials for NSC metabolism (Andreotti et al., 2019). The perception of NSCs to the surrounding metabolic conditions may be inextricably linked with the metabolic changes from qNSCs to aNSCs. At the same time, in the face of the environment’s changing conditions, how NSC maintains a stable ecological niche seems to be an important topic. In this review, metabolism and protein homeostasis differences between qNSCs and aNSCs are discussed.
Database Search Strategy
In this narrative review, we included studies that discussed factors affecting adult NSC regeneration and differentiation. The majority of articles referenced (~90% of all references) were written in the English language and were full-text articles published between January 2005 and June 2022. The studies were primarily conducted on mammals such as rodents and humans. The authors searched the PubMed database to identify relevant publications using the following criteria: adult to avoid studies focused on embryonic neurogenesis. Then, the following terms were searched: (1) neurogenesis, (2) neural regeneration, (3) NSCs. Representative articles were screened studies related to metabolism and protein homeostasis, then relevant references were used to clarify each aspect, i.e., nutrient-sensing and metabolic pathways and protein homeostasis.
Nutrient-Sensing and Metabolic Pathways
Nutrient-sensing pathways
Nutrients and oxygen are the two extrinsic elements of metabolism, and monitoring of these determines the behavior of NSCs. In naive cognition cells have better proliferative potential in a nutrient-rich environment. Cells have a series of nutrient sensing pathways to detect nutrition in the environment. The most well-characterized nutrient sensing pathway is the insulin/insulin-like growth factor 1 (IGF1)/forkhead box protein O (FOXO) pathway (Tia et al., 2018). In this pathway, the presence of insulin represents nutritional sufficiency and insulin-stimulated cells activate Akt and other protein kinases, resulting in phosphorylation and inactivation of FOXO. FOXO then translocates to the cytoplasm for degradation via ubiquitination (Greer and Brunet, 2005). Nuclear FOXO mediates transcription of various target genes, such as those associated with G1 arrest, G2 arrest, DNA repair, antioxidative stress, cell differentiation, and other effects in stem cells (Paik et al., 2009).
In NSCs, FOXO regulation of the expression of these genes has been demonstrated in many studies (Du and Zheng, 2021; Tay et al., 2021). Some studies (McLaughlin and Broihier, 2018; Ludikhuize and Rodríguez Colman, 2021) showed that FOXO inhibited NSC proliferation and turnover, resulting in NSC quiescence. Although FOXO deficiency initially increased NSC proliferation, sustained ventriculomegaly and SVZ thinning were observed in response to prolonged FOXO deficiency in the adult brain. These long-term effects were associated with loss of the NSC pool and increased oxidative stress. Therefore, inhibition of the insulin/IGF1-FOXO pathway participates in NSC pool maintenance. Interestingly, patients with Alzheimer’s disease (AD) had persistent ventriculomegaly and SVZ thinning, and studies have suggested that AD development is associated with reduced hippocampal neurogenesis (Mu and Gage, 2011). The amyloid precursor protein (APP) intracellular domain (AICD) is a byproduct of APP metabolism. Elevated AICD results in AD, possibly through interaction with FOXO3a (Figure 2). In addition, FOXO3a overexpression leads to a marked reduction in NSC proliferation and differentiation (Jiang et al., 2020) and regulates phosphatase and tensin homolog (PTEN)-induced kinase 1 (Pink-1) expression (Goiran et al., 2018). Pink-1 is associated with mitochondrial dynamics (Poole et al., 2008) and mitochondria-induced autophagy (Vives-Bauza et al., 2010). AICD activates FOXO3a, and FOXO3a inhibits AICD (Jiang et al., 2020) which is similar to the homeostatic loop in which blood glucose regulates insulin and glucagon levels and the daily rhythm of REM-ON and REM-OFF neuron activity. Furthermore, other factors may alter the FOXO pathway in neurons in individuals with AD.
Figure 2.
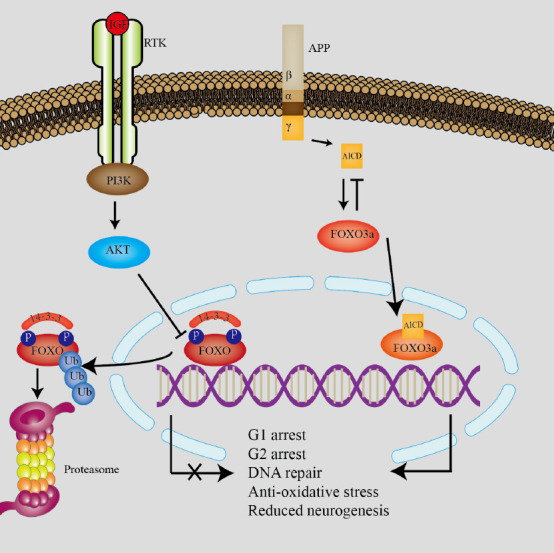
The classic insulin/IGF1/FOXO pathway and its association with APP.
The left signaling pathway shows the classic insulin/IGF1 (insulin-like growth factor 1)/FOXO (forkhead box protein O) pathway. In this pathway, insulin-stimulated cells activate Akt. Akt then phosphorylates and inactivates FOXO, resulting in translocation to the cytoplasm for degradation by ubiquitination. When FOXO is in the nucleus it mediates transcription of various target genes such as G1 arrest, G2 arrest, DNA repair, anti-oxidative stress, cell differentiation, and other effects in stem cells. The right signaling pathway shows that amyloid precursor protein (APP) may be involved in development of Alzheimer’s disease. The amyloid APP intracellular domain (AICD) is a byproduct of APP metabolism, produced by sequential proteolytic cleavage by α/β and γ-secretases. AICD activates FOXO3a resulting in reduced neurogenesis. Reduced neurogenesis may be a mechanisms of brain shrinkage in AD. Created with Adobe illustrator. FOXO: Forkhead box protein O; IGF1: insulin-like growth factor 1; PI3K: phosphoinositide 3-kinase; RTK: receptor tyrosine kinases.
Studies have shown that activation of the IGF1 signaling pathway is necessary to increase the number of functional neurons to counteract FOXO3a-induced decreases in neuronal differentiation in the olfactory bulb (OB) (Hurtado-Chong et al., 2009) and in the hippocampus (Nieto-Estévez et al., 2016). However, high levels of IGF do not maintain the NSC pool because neuronal differentiation exhausts the NSC pool, possibly because proliferation and differentiation may be contradictory cellular processes. Lack of IGF-1 typically results in decreased NSC proliferation. However, in the mouse hippocampus IGF-1 deficiency resulted in larger neurospheres (Nieto-Estévez et al., 2016), possibly because IGF-1 deficiency resulted in progenitor cell misplacement and morphological abnormalities in the dentate gyrus (DG), blocking neuron differentiation and forcing NSC self-renewal. To address this paradox, the effects of cyclical fasting and feeding were analyzed, and cyclical IGF-1 changes were found to increase hippocampal neurogenesis and cognitive performance in mice (Brandhorst et al., 2015).
Sensing oxygen
Hypoxia-inducible factor-1 (HIF-1) is an important molecular oxygen sensor. HIF-1 regulates glycolytic metabolism, and increased HIF-1 expression leads to enhanced glycolysis and decreased OXPHOS, which is critical for induction of pluripotency of stem cells (Semenza, 2012). Under normoxic conditions, HIF-1α rapidly degrades via the ubiquitin protease pathway. However, under hypoxic conditions degradation is blocked, and HIF-1α accumulates and associates with HIF1β, resulting in decreased association with target genes and reduced transcription (Huang et al., 1996). Increase in HIF-1α expression results in increased self-renewal of stem cells. Symptoms of Von Hippel-Lindau disease, characterized by systemic cysts and tumors with high malignant potential, supports this concept (Bader and Hsu, 2012).
In NSCs HIF activation facilitates conversion of qNSCs to aNSCs. In a rat model of cerebral ischemia, hypoxia resulting from vascular disruption in the damaged area increased HIF-1α expression, resulting in neurogenesis (Liu et al., 2014). Remote limb ischemia postconditioning has been shown to improve cerebral ischemia/reperfusion injury and may be associated with increased HIF-1α expression (Zong et al., 2015). The effects of HIF-1α on nerve regeneration through the Wnt/β-catenin signaling pathway have been well-characterized. Hypoxia results in increased NSC proliferation and increased mRNA and protein expression of HIF-1α, β-catenin, and cyclin D1. Knockdown of HIF-1α results in decreased expression of β-catenin (Mazumdar et al., 2010; Qi et al., 2017). Interestingly, HIF-1α modulates mitochondrial dynamics and reactive oxygen species (ROS) production during neural differentiation of induced pluripotent stem cells. When mitochondria are excessively fragmented, insufficient mitochondrial function results in decreased mitochondrial capacity to regulate solute Ca2+ levels, resulting in increased solute Ca2+ levels and upregulation of CaMKII activity and noncanonical proteasome degradation of β-Catenin protein (Zhong et al., 2019). A recent study showed that expression of HIF-1α inhibited mitofusin 2 (MFN2) expression (Cui et al., 2021), resulting in disruption of mitochondrial dynamics due to inhibition of MFN activity and defects in neuronal differentiation. Therefore, HIF-1α may affect NSC differentiation through β-Catenin signaling mediated by MFN2 (Cui et al., 2021). While the Wnt/β-catenin pathway is important for NSC renewal and the differentiation of embryonic stem cells (ESCs) and induced pluripotent stem cells, it may be dispensable for adult NSC homeostasis and activation. Activated or quiescent NSCs showed similar levels of Wnt/β-catenin signaling activity. Knockout of Wnt/β-catenin signaling did not alter the activation state of NSCs in vitro. Notch and BMP signaling is elevated in quiescent NSCs (Austin et al., 2021). Although endogenous β-catenin signaling may not be associated with NSC niche cues, exogenous Wnt signaling plays a significant role in NSC fate (Heppt et al., 2020).
Inflammatory signaling is regulated by HIF-1α under cerebral hypoxic conditions (Amin et al., 2021). Inflammation plays an important role in neural regeneration, especially in the hippocampal region (Monje et al., 2003). Neuroinflammation-mediated neurogenesis is a distinct pathological feature of Alzheimer’s disease (Sung et al., 2020) and depression (Borsini et al., 2020). In most tissues HIF is overexpressed in response to increased oxygen consumption and through NF-KB-mediated tissue inflammation, resulting in immune cell survival and activation (Elks et al., 2011). Under normoxic conditions proinflammatory signaling molecules such as IL-1β (Jung et al., 2003) can increase HIF-1α protein expression. Expression of HIF results in a positive feedback loop, leading to pathogenic bacteria death and impaired cell clearance. These processes were explained in detail in a recent review (Kiani et al., 2021). However, some studies on hippocampal nervous tissue hypoxia (Xing and Lu, 2016; Lee et al., 2022) reported the opposite results. Stabilization of HIF-1α for 24 hours after transient total ischemia attenuated rat hippocampal IL-6R and TNFR1 activity, and reduced caspase-3 protein expression after ischemia/reperfusion injury (Xing and Lu, 2016).
Glucose metabolism
Metabolism is closely related to energy supply. Stem cells exhibit a unique metabolic pattern, and stem cells that differentiate and mature into adult cells undergo unique metabolic transitions (Ludikhuize and Rodríguez Colman, 2021). Stem cells in the quiescent state under anaerobic conditions use glycolysis as their main energy source, activated stem cells exhibit enhanced oxidative phosphorylation (Suda et al., 2011), and adult NSCs are no exception (Feng and Liu, 2017). The adult aNSCs prefer oxidative phosphorylation but qNSCs prefer glycolysis Recent studies have shown that hypoxic metabolism regulates proliferation of stem cells (Antebi et al., 2018; Wobma et al., 2018; Xing et al., 2018) and that glycolysis (Khacho and Slack, 2017; Sun et al., 2018) and glutamine catabolism (Zhou et al., 2019) contribute to survival and reproduction of stem cells during hypoxic metabolism. Although glycolytic breakdown produces less ATP than does oxidative phosphorylation (OXPHOS), it is kinetically faster. Furthermore, glycolysis and glutamine breakdown products are critical components of signaling pathways that support cell division (Lunt and Vander Heiden, 2011). For example, ten-eleven translocation methylcytosine dioxygenase 1 (TET1) catalyzes conversion of the modified DNA base 5-methylcytosine to 5-hydroxymethylcytosine via oxidation of 5-methylcytosine in an iron- and α-ketoglutarate (α-KG)-dependent manner. In addition, TET1 demethylates pluripotency genes, resulting in increased transcriptional activity. Glutaminase catalyzes conversion of glutamine to glutamate, which is then converted to α-KG by glutamate dehydrogenase or transaminases (PSAT/GOT/GPT) (Tambay et al., 2021), resulting in increased catalytic activity of TET1. Glycolysis and glutamine fuel the hexosamine biosynthesis pathway (Hanover et al., 2010). In embryonic stem cells the hexosamine biosynthesis pathway produces O-linked β-N-acetylglucosamine (O-GlcNAc) through O-GlcNAc transferase, resulting in O-GlcNAcylation-mediated increases in TET1 activity (Vella et al., 2013; Figure 3). In addition, anaerobic metabolism enables stem cells to maintain low ROS production. Stem cells first undergo anaerobic metabolism, characterized by low oxygen consumption rates and immature mitochondria with low mitochondrial DNA (mtDNA) density (Facucho-Oliveira and St John, 2009; Rehman, 2010). However, although mitochondrial OXPHOS is the main driver of ROS generation, the repair system through which oxidative mtDNA damage is mitigated is less efficient than the nuclear DNA repair system (de Souza-Pinto et al., 2008). Therefore, decreased ROS production via anaerobic metabolism in stem cells allows for more efficient maintenance of nuclear DNA and mtDNA genome integrity. This phenomenon was first observed during early research on yeast. The yeast cell cycle is accompanied by periodic changes in cellular oxygen consumption. In the S phase during DNA synthesis, oxygen consumption is low, resulting in protection of single-stranded DNA, which is more vulnerable to oxidative damage during replication (Tu et al., 2005; Tu and McKnight, 2007). Simultaneous anaerobic metabolism enhances defense of stem cells against ROS. First, glycolysis and pentose phosphate pathways increase the production of NADPH, which is a key cofactor for maintaining the reduced forms of thioredoxin and glutathione, which are important ROS scavengers (Perales-Clemente et al., 2014). Second, glutamine is required to maintain high levels of the intracellular antioxidant glutathione. Increased glutamine in stem cells promotes scavenging of ROS. Levels of ROS affect signaling proteins such as the pluripotency gene octamer-binding transcription factor 4 (OCT4), which maintains cell stemness. Oxidation induces spontaneous degradation of OCT4, resulting in cell differentiation, and glutathione produced by glutamine protects OCT4 from oxidative degradation (Marsboom et al., 2016).
Figure 3.
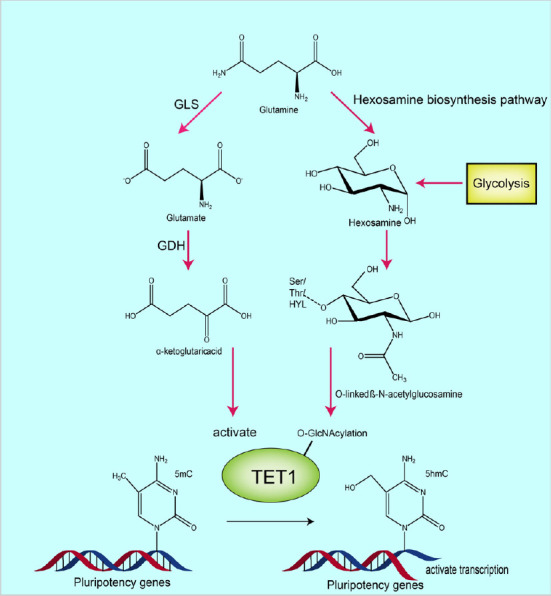
The underlying mechanisms by which glutamine metabolism and glycolysis promote TET1 catalysis under hypoxic conditions.
The process on the left shows that glutamine is converted to glutamate by glutaminase, then further converted to α-ketoglutarate (α-KG) by glutamate dehydrogenase or transaminases (PSAT/GOT/GPT). Catalytic activity of TET1 is then increased by α-KG. The process on the right shows that glycolysis and glutamine can fuel the hexosamine biosynthesis pathway, the product of which is O-linked β-N-acetylglucosamine (O-GlcNAc), which is produced by O-GlcNAc transferase-mediated O-GlcNAcylation of TET1. This product positively regulates TET1 activity. Created with Adobe illustrator. GDH: Glutamate dehydrogenase; GLS: glutaminase; TET1: ten-eleven translocation methylcytosine dioxygenase 1.
Mitochondrial dynamics
Redox status is important in regulation of stem cell differentiation (Ogasawara and Zhang, 2009) and mitochondrial dynamics (Khacho et al., 2016). In embryonic NSCs, mitochondria have different morphologies at different stages. Mitochondria in qNSCs exhibit an elongated morphology, whereas mitochondria in aNSCs are rounder and either more spherical or more tubular (Beckervordersandforth et al., 2017). As NSCs differentiate their mitochondria fragment. Mitochondria then undergo fusion once fully differentiated as neurons.
Changes in mitochondrial morphology may regulate differentiation of NSCs. Suppression of Drp1, which mediates mitochondrial fission, resulted in fused mitochondria and NSC self-renewal (Iwata et al., 2020). Some studies have shown that small, fragmented mitochondria have higher electron transport chain and OXPHOS activity and higher mitochondrial membrane potentials (Beckervordersandforth et al., 2017) resulting in higher ROS levels. Although fused mitochondria in self-renewing NSCs can function normally when these cells are forced to undergo OXPHOS due to lack of energy, proton leakage caused by UCP2 and IF1 inhibits OXPHOS (Khacho et al., 2016).
Fragmented mitochondria produce higher levels of ROS, and ROS inhibit self-renewal by upregulating nuclear transcription factor 2 and downregulating Notch signaling, resulting in differentiation (Khacho et al., 2016). In addition, Sirtuin1 (Iwata et al., 2020), a protein deacetylase/mono-ADP ribosyltransferase, plays a role in NSC differentiation. Sirtuin1 activity depends on nicotinamide adenine dinucleotide (NAD+). Therefore, sirtuins are associated with regulation of cellular metabolic state (Schwer and Verdin, 2008). Increased ETC activity leads to increased NAD+/NADH ratio, resulting in activation of Sirtuin1. Sirtuin1 is recruited by BCL6 to promote neural differentiation through epigenetic inhibition of the Notch signaling target gene Hes (Tiberi et al., 2012; Iwata et al., 2020). The Notch signaling pathway promotes NSC self-renewal. A recent study has shown that increased Notch signaling pathway activity increased NSC self-renewal via symmetrical division of hippocampal NSCs in mice with intracerebral hemorrhage (Chen et al., 2021).
However, mitochondrial fission only affects NSC differentiation selection. When NSCs differentiate into neurons, intact mitochondrial function is essential. Knockout or mutation of the Mfn2 gene, which mediates fragmented mitochondrial fusion, causes mitochondrial metabolism and defects in signaling networks, neurogenesis, and synapse formation, causing various neurological deficits (Fang et al., 2016). The AMPK-PGC-1a-NRF (Zhang et al., 2018) and the SIRT1-PGC-1a axes (Gomes et al., 2013) play important roles in mitochondrial biogenesis. Increased ROS may result in increased mitochondrial activity and increased OXPHOS in mature neurons. Mitochondrial fission is thought to be a possible mechanism of mitochondrial biogenesis, and increased mitochondrial fission produces many fragmented mitochondria. Mitochondrial fragmentation is an important step in elimination of damaged mitochondria and generation of new mitochondria to meet increased energy demands (Youle and van der Bliek, 2012).
Lipid metabolism
Lipid metabolism plays an important role in neurogenesis. Whether NSCs are in the quiescent or activated states in the hippocampus is dependent on FA metabolism. Carnitine palmitoyltransferase-1a-dependent FA oxidation (FAO) activity is high in quiescent NSCs (Knobloch et al., 2017). Inhibition of FAO leads to increased differentiation and decreased self-renewal of NSCs, resulting in a reduced pool of NSCs. This decrease in the pool of NSCs is associated with various neuropsychiatric disorders such as autism spectrum disorder. For example, carnitine has different types of carnitine depending on the length of the carbon atom, and the different proportions of their different types of carnitine in autism spectrum disorder compared with healthy individuals may be one of the reasons for the decrease in FAO flux, and appropriate carnitine supplementation improves symptoms (Barone et al., 2018).
Increased FA synthase-mediated de novo lipid synthesis in activated NSCs promotes nerve regeneration (Knobloch et al., 2013). This process may be associated with SPOT14, which is negatively regulated via de novo lipogenesis. Synthesis of medium and long chain FA controls NSC proliferation, and increased levels of these fatty acids are associated with low proliferation rates in NSCs (Knobloch et al., 2013, 2014). Exercise promotes neurogenesis in the hippocampus, which aids in cognition. Exercise is also associated with increased FA synthase expression and increased mobilization of SPOT14-positive qNSCs (Knobloch et al., 2014). Additional evidence has suggested that FA synthesis plays an important role in neurogenesis. Thyroid hormone-induced liver protein, also known as SPOT14, is encoded by the THRSP gene. Overexpression of SPOT14 in the prefrontal cortex (Dela Peña et al., 2015) and striatum (Custodio et al., 2018) may be associated with attention deficits. For example, attention-deficit/hyperactivity disorder symptoms have been shown to be highly correlated with long-chain polyunsaturated FA (LCPUFA) deficiency (Janssen and Kiliaan, 2014). As previously stated, SPOT14 negatively regulates synthesis of medium and long chain FA. Although biosynthesis of LCPUFAs such as docosahexaenoic acid mainly depends on the diet and requires the liver, a small percentage of LCPUFAs is synthesized in the brain. These fatty acids are mainly synthesized in glial cells, but may also be synthesized by NSCs with a glial cell phenotype (Gharami et al., 2015), as long-chain FAs do not easily cross the blood-brain barrier. Malonyl-CoA is the common modulator of both FAO and FA synthesis. Malonyl-CoA is a raw material for FA synthesis. In FAO, malonyl-CoA may inhibit the enzymatic activity of carnitine palmitoyltransferase-1 (CPT1) through malonylation of CPT1 (Fadó et al., 2021). GPT1 accelerates transport of long-chain FAs from the cytoplasm into mitochondria where they undergo β-oxidation. Therefore, malonyl-CoA levels may modulate FA synthesis and FAO. Levels of malonyl-CoA determine NSC fate through regulation of lipid metabolism.
Fatty acid metabolism is partially reflected by the respiratory quotient (RQ). Human premature infants have brain RQs 10% lower than those in adults (Gluck, 1962), which reflects elevated FAO in premature infants, possibly related to highly proliferative NSCs. During sleep and under anesthesia, the human brain RQ is less than 1, and metabolic studies of sleep have shown that synthesis and decomposition of brain lipids were both greater during sleep than during wakefulness (Aalling et al., 2018), which may indicate that sleep is associated with increased self-renewal and neurogenesis of NSCs. Recent studies have shown that freely moving mice exhibit greater NSC proliferation during the day or in the presence of light, which may be due to melatonin-mediated effects on NSC calcium dynamics and reduced NSC proliferation (Gengatharan et al., 2021). Specifically, in stem cells, high cytoplasmic Ca2+ levels trigger proliferation, while Ca2+ oscillations maintain stem cell quiescence, which is important in the intestines where stem cells sense external metabolic and mechanical changes (He et al., 2018). Increasing studies have shown that Ca2+ level plays an important role in NSC sensing of cerebrospinal fluid flow. Under high flow conditions, ENaC ion channels in NSCs transport sodium into cells. Sodium influx induces membrane depolarization, which stimulates calcium exchangers and channels, leading to increased cytoplasmic calcium and NSC proliferation (Petrik et al., 2018).
Protein homeostasis
Proteostasis is important for maintenance of long-term NSC self-renewal and differentiation. Loss of proteostasis leads to protein aggregation, which is associated with many neurodegenerative diseases such as AD, Parkinson’s disease, and prion diseases (Aguzzi and O’Connor, 2010). Although mainstream research has concluded that neurodegenerative diseases are primarily associated with dysfunction and apoptosis of differentiated neurons, the important role played by the loss of homeostasis and the NSC pool during NSC ageing cannot be ignored. For example, decreased expression of the molecular chaperone TRiC in NSPCs resulted in a senescence phenotype, whereas decreased expression of small Hsps (sHsps) in differentiated neurons did not lead to senescence (Vonk et al., 2020). This finding suggested that NSPCs may be particularly sensitive to loss of protein homeostasis. Loss of protein homeostasis results in accumulation of protein aggregates, which disrupt the ability of NSCs to cease proliferation and prevent nerve regeneration (Morrow et al., 2020). Sequestration of protein aggregates via the autophagy-lysosomal pathway, the proteasome pathway, or chaperones promotes protein homeostasis.
When protein homeostasis is compromised, accumulated misfolded proteins form inclusion bodies in cells to protect themselves from toxic proteins such as JUNQ and IPOD (Ogrodnik et al., 2014; Radwan et al., 2017). In a recent study vimentin cages were shown to be important for NSC activation. Knockout of vimentin resulted in marked downregulation of NSC proliferation, rendering these cells incapable of exiting quiescence (Morrow et al., 2020). The vimentin cage mediates asymmetric division resulting in the formation of JUNQ perinuclear inclusion bodies. When a cell divides into two, the cell that inherits these inclusion bodies maintains a low-division phenotype while the other cell maintains a high division rate to ensure the population dominance (Bento et al., 2016). Moreover, vimentin also localizes the proteasome to protein aggregates to promote protein homeostasis (Morrow et al., 2020).
The autophagy-lysosomal pathway connects many nutrient sensing-related pathways. For example, mTORC1 inhibition and AMP-activated protein kinase (AMPK) activation occur in response to nutrient deprivation (Saikia and Joseph, 2021). A recent study showed that FOXO3 regulates protein homeostasis in adult neural stem cells through autophagy, which may be one mechanism by which FOXO inhibits proliferation of NSCs in response to nutrient sensing (Audesse et al., 2019). Interestingly, the transition between activation and quiescence of NSCs was also recently found to depend on the autophagy-lysosomal pathway as indicated by higher lysosomal activity in qNSCs than that in aNSCs. Furthermore, this transition was associated with EGFR (epidermal growth factor receptor) degradation and activation of TFEB (transcription factor EB) (Kobayashi et al., 2019), which is a key gene in lysosomal biogenesis (Ballabio and Bonifacino, 2020). In addition, this pathway favors qNSCs and clearance of damaged organelles and aggregates to ensure niche stability. This pathway may also be associated with nutrient level regulation, and lack of energy may induce neural quiescence (Zhao et al., 2016). This is partly reflected in degradation of growth factors such as EFGR.
The proteasome pathway is a cellular clearance mechanism for maintaining intracellular protein homeostasis. Inhibition of the proteasome pathway by MG132, a proteasome inhibitor, leads to impaired NPC proliferation and differentiation (Kim and Kim, 2020). However, one study showed that MG132 treatment resulted in an increased proportion of cells that expressed BDNF expression, which led to neurogenesis and may have been related to the high apoptosis rate induced by MG132 (Mekala et al., 2020). Another study showed that MG132 administration significantly increased intracellular ROS levels and decreased the mitochondrial membrane potential, whereas the proteasome activator 18α-GA induced the opposite effect (Kim and Kim, 2020). These findings were consistent with our assertion that ROS promote differentiation of NSCs into neurons, which suggests that inhibition of the proteasome may cause ROS to force NSCs to undergo apoptosis or differentiation to ensure niche stability.
Chaperones direct polypeptides into a folded state or facilitate protein quality control through refolding, degradation, and sequestration. A variety of chaperones are expressed in NSCs, and sHsps, TRiC, and HspB5 have been recently found to be significantly differentially expressed in NSPCs and their differentiated progeny. The expression of TRiC is high in NSPCs prior to differentiation and the expression of HspB5 is high after differentiation. The activity of TRiC is highly dependent on ATP, which maintains the solubility of TRiC and prevents its aggregation. In contrast, HspB5 activity, which promotes misfolded protein spatial isolation, is independent of ATP (Vonk et al., 2020). Undifferentiated NSPCs consume more energy during proteostasis than during other functions to prevent the aggregate formation that diminishes niche stability. This high consumption rate may be related to increased ATP kinetics caused by high glycolysis rate.
Limitations
This review is subject to several limitations. First, since neurogenesis is a broad subject, this paper does not explore the relationship between each factor in neurogenesis, and does not explore the relationship between astrocytes and microglia. Second, most of the cited studies included animal and cell experiments. Lack of clinical data limits the generalizability of the findings in the referenced studies to humans. Third, the articles were limited to English-language publications or translations. Therefore, relevant international data could be lacking.
Conclusions
In this review, we summarized studies focused on the effects of energy metabolism and protein homeostasis on the transition from quiescent to activated NSCs (Table 1). For qNSCs, the focus was on maintaining quantitative and qualitative stability, whereas for aNSCs, the focus was on rapid proliferation and differentiation because of more aggressive metabolism. In energy metabolism qNSCs maintain activation of FOXO, resulting in slowed cell cycle, improve DNA inspection and repair, and also increased resistance to oxidative stress. In contrast, aNSCs move FOXO out of the nucleus to accelerate the cell cycle. Furthermore, qNSCs use glycolysis as the main mode of metabolism, resulting in reduced OXPHOS-generated ROS. In contrast, aNSCs prefer OXPHOS as an energy source to promote rapid proliferation. The mitochondria in qNSCs inhibit OXPHOS through proton leakage and show an elongated fused morphology. In contrast, mitochondria in aNSCs have higher OXPHOS activity and show a smaller and more fragmented morphology. Increased hypoxia-induced HIF-1 expression results in enhanced NSC proliferation, which seems to contradict the preference of qNSCs for hypoxic glycolysis. A possible explanation is that qNSCs may undergo anaerobic metabolism independent of environmental pressures. With regard to lipid metabolism, qNSCs prefer FA for FAO, while aNSCs prefer lipid synthesis via FA synthase to meet proliferation needs. In protein homeostasis,during the division of NSCs, the one that retains protein aggregates is gradually activated and divides and differentiates into mature functional cells, while the other will retain quiescent and stemness. In addition, qNSCs exhibit higher lysosomal activity, and the lack of a proteasome forces qNSCs to exit quiescence. With regard to chaperone proteins, NSCs use energy-consuming but more powerful TriC for aggregate removal prior to differentiation and non-energy-consuming HspB5 for aggregate isolation after differentiation. Although the difference lies in the differentiation process, it also shows that the NSC chooses a more expensive strategy in ensuring its own homeostasis.
Table 1.
The role of nutrient-sensing and metabolic pathways/protein homeostasis in NSCs from quiescence to activity
| Role | qNSCs | aNSCs |
|---|---|---|
| Nutrient-sensing and metabolic pathways | ||
| Nutrient-sensing pathways | Activation of FOXO | Move FOXO out of the nucleus |
| Oxygen sensing | Stable HIF-1α | Enhanced HIF-1α |
| Glucose metabolism | Glycolysis and glutamine catabolism | Oxidative phosphorylation |
| Mitochondrial dynamics | Elongated fused morphology | Smaller and more fragmented morphology |
| Lipid metabolism | Prefer FA oxidation | Prefer lipid synthesis |
| Protein homeostasis | ||
| Autophagy-lysosomal pathway | Higher lysosomal activity | Stable lysosomal activity |
| Proteasome pathway | More sensitive | Less sensitive |
| Chaperones | Energy-consuming TriC | Non-energy-consuming HspB5 |
aNSCs: Active NSCs; FOXO: forkhead box protein O; HIF: hypoxia-inducible factor; NSCs: neural stem cells; qNSCs: quiescent NSCs.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 82171336 (to XX).
Conflicts of interest: There are no potential conflicts of interest among the authors.
Data availability statement: Not applicable.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Aalling NN, Nedergaard M, DiNuzzo M. Cerebral metabolic changes during sleep. Curr Neurol Neurosci Rep. (2018);18:57. doi: 10.1007/s11910-018-0868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguzzi A, O'Connor T. Protein aggregation diseases:pathogenicity and therapeutic perspectives. Nat Rev Drug Discov. (2010);9:237–348. doi: 10.1038/nrd3050. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. (1965);124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 4.Amin N, Chen S, Ren Q, Tan X, Botchway BOA, Hu Z, Chen F, Ye S, Du X, Chen Z, Fang M. Hypoxia inducible factor-1αattenuates ischemic brain damage by modulating inflammatory response and glial activity. Cells. (2021);10:1359. doi: 10.3390/cells10061359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreotti JP, Silva WN, Costa AC, Picoli CC, Bitencourt FCO, Coimbra-Campos LMC, Resende RR, Magno LAV, Romano-Silva MA, Mintz A, Birbrair A. Neural stem cell niche heterogeneity. Semin Cell Dev Biol. (2019);95:42–53. doi: 10.1016/j.semcdb.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antebi B, Rodriguez LA, Walker KP, Asher AM, Kamucheka RM, Alvarado L, Mohammadipoor A, Cancio LC. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res Ther. (2018);9:265. doi: 10.1186/s13287-018-1007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Audesse AJ, Dhakal S, Hassell L-A, Gardell Z, Nemtsova Y, Webb AE. FOXO3 directly regulates an autophagy network to functionally regulate proteostasis in adult neural stem cells. PLoS Genet. (2019);15:e1008097. doi: 10.1371/journal.pgen.1008097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austin SHL, Gabarró-Solanas R, Rigo P, Paun O, Harris L, Guillemot F, Urbán N. Wnt/β-catenin signalling is dispensable for adult neural stem cell homeostasis and activation. Development. (2021);148:dev199629. doi: 10.1242/dev.199629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bader HL, Hsu T. Systemic VHL gene functions and the VHL disease. FEBS Lett. (2012);586:1562–1569. doi: 10.1016/j.febslet.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballabio A, Bonifacino JS. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat Rev Mol Cell Biol. (2020);21:101–118. doi: 10.1038/s41580-019-0185-4. [DOI] [PubMed] [Google Scholar]
- 11.Barone R, Alaimo S, Messina M, Pulvirenti A, Bastin J, Ferro A, Frye RE, Rizzo R. A subset of patients with autism spectrum disorders show a distinctive metabolic profile by dried blood spot analyses. Front Psychiatry. (2018);9:636. doi: 10.3389/fpsyt.2018.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckervordersandforth R, Ebert B, Schäffner I, Moss J, Fiebig C, Shin J, Moore DL, Ghosh L, Trinchero MF, Stockburger C, Friedland K, Steib K, von Wittgenstein J, Keiner S, Redecker C, Hölter SM, Xiang W, Wurst W, Jagasia R, Schinder AF, et al. Role of mitochondrial metabolism in the control of early lineage progression and aging phenotypes in adult hippocampal neurogenesis. Neuron. (2017);93:1518. doi: 10.1016/j.neuron.2017.03.008. Erratum for:Neuron. 2017; 93:560-573.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bento CF, Renna M, Ghislat G, Puri C, Ashkenazi A, Vicinanza M, Menzies FM, Rubinsztein DC. Mammalian autophagy:how does it work? Annu Rev Biochem. (2016);85:685–713. doi: 10.1146/annurev-biochem-060815-014556. [DOI] [PubMed] [Google Scholar]
- 14.Berg DA, Bond AM, Ming GL, Song H. Radial glial cells in the adult dentate gyrus: what are they and where do they come from? F1000Research. (2018);7:277. doi: 10.12688/f1000research.12684.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonaguidi MA, Song J, Ming GL, Song H. A unifying hypothesis on mammalian neural stem cell properties in the adult hippocampus. Curr Opin Neurobiol. (2012);22:754–761. doi: 10.1016/j.conb.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borsini A, Di Benedetto MG, Giacobbe J, Pariante CM. Pro- and anti-inflammatory properties of interleukin in vitro:relevance for major depression and human hippocampal neurogenesis. Int J Neuropsychopharmacol. (2020);23:738–750. doi: 10.1093/ijnp/pyaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, Di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. (2015);22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Yuan XY, Zhang X. Intracerebral hemorrhage influences hippocampal neurogenesis and neurological function recovery via Notch1 signaling. Neuroreport. (2021);32:489–497. doi: 10.1097/WNR.0000000000001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui P, Zhang P, Yuan L, Wang L, Guo X, Cui G, Zhang Y, Li M, Zhang X, Li X, Yin Y, Yu Z. HIF-1αaffects the neural stem cell differentiation of human induced pluripotent stem cells via MFN2-mediated Wnt/β-Catenin signaling. Front Cell Dev Biol. (2021);9:671704. doi: 10.3389/fcell.2021.671704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Custodio RJP, Botanas CJ, de la Peña JB, Dela Peña IJ, Kim M, Sayson LV, Abiero A, Ryoo ZY, Kim BN, Kim HJ, Cheong JH. Overexpression of the thyroid hormone-responsive (THRSP) gene in the striatum leads to the development of inattentive-like phenotype in mice. Neuroscience. (2018);390:141–150. doi: 10.1016/j.neuroscience.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 21.de Souza-Pinto NC, Wilson DM 3rd, Stevnsner TV, Bohr VA. Mitochondrial DNA, base excision repair and neurodegeneration. DNA Repair (Amst) (2008);7:1098–1109. doi: 10.1016/j.dnarep.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dela Peña I, Bang M, Lee J, de la Peña JB, Kim BN, Han DH, Noh M, Shin CY, Cheong JH. Common prefrontal cortical gene expression profiles between adolescent SHR/NCrl and WKY/NCrl rats which showed inattention behavior. Behav Brain Res. (2015);291:268–276. doi: 10.1016/j.bbr.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Dillen Y, Kemps H, Gervois P, Wolfs E, Bronckaers A. Adult neurogenesis in the subventricular zone and its regulation after ischemic stroke:implications for therapeutic approaches. Transl Stroke Res. (2020);11:60–79. doi: 10.1007/s12975-019-00717-8. [DOI] [PubMed] [Google Scholar]
- 24.Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. (1997);17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du S, Zheng H. Role of FoxO transcription factors in aging and age-related metabolic and neurodegenerative diseases. Cell Biosci. (2021);11:188. doi: 10.1186/s13578-021-00700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elks PM, van Eeden FJ, Dixon G, Wang X, Reyes-Aldasoro CC, Ingham PW, Whyte MKB, Walmsley SR, Renshaw SA. Activation of hypoxia-inducible factor-1α(Hif-1α) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. (2011);118:712–722. doi: 10.1182/blood-2010-12-324186. [DOI] [PubMed] [Google Scholar]
- 27.Facucho-Oliveira JM, St John JC. The relationship between pluripotency and mitochondrial DNA proliferation during early embryo development and embryonic stem cell differentiation. Stem Cell Rev Rep. (2009);5:140–158. doi: 10.1007/s12015-009-9058-0. [DOI] [PubMed] [Google Scholar]
- 28.Fadó R, Rodríguez-Rodríguez R, Casals N. The return of malonyl-CoA to the brain:Cognition and other stories. Prog Lipid Res. (2021);81:101071. doi: 10.1016/j.plipres.2020.101071. [DOI] [PubMed] [Google Scholar]
- 29.Fang D, Yan S, Yu Q, Chen D, Yan SS. Mfn2 is required for mitochondrial development and synapse formation in human induced pluripotent stem cells/hiPSC derived cortical neurons. Sci Rep. (2016);6:31462. doi: 10.1038/srep31462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng W, Liu HK. Revealing the hidden powers that fuel adult neurogenesis. Cell Stem Cell. (2017);20:154–156. doi: 10.1016/j.stem.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. (2003);23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 32.Ge LJ, Yang FH, Li W, Wang T, Lin Y, Feng J, Chen NH, Jiang M, Wang JH, Hu XT, Chen G. In vivo neuroregeneration to treat ischemic stroke through NeuroD1 AAV-based gene therapy in adult non-human primates. Front Cell Dev Biol. (2020);8:590008. doi: 10.3389/fcell.2020.590008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gengatharan A, Malvaut S, Marymonchyk A, Ghareghani M, Snapyan M, Fischer-Sternjak J, Ninkovic J, Götz M, Saghatelyan A. Adult neural stem cell activation in mice is regulated by the day/night cycle and intracellular calcium dynamics. Cell. (2021);184:709–722.e713. doi: 10.1016/j.cell.2020.12.026. [DOI] [PubMed] [Google Scholar]
- 34.Gharami K, Das M, Das S. Essential role of docosahexaenoic acid towards development of a smarter brain. Neurochem Int. (2015);89:51–62. doi: 10.1016/j.neuint.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Gluck L. Somatic stability in the newly born. Yale J Biol Med. (1962);34:630–631. [Google Scholar]
- 36.Goiran T, Duplan E, Chami M, Bourgeois A, El Manaa W, Rouland L, Dunys J, Lauritzen I, You H, Stambolic V, Biféri MG, Barkats M, Pimplikar SW, Sergeant N, Colin M, Morais VA, Pardossi-Piquard R, Checler F, Alves da Costa C. β-Amyloid precursor protein intracellular domain controls mitochondrial function by modulating phosphatase and tensin homolog-induced kinase 1 transcription in cells and in Alzheimer mice models. Biol Psychiatry. (2018);83:416–427. doi: 10.1016/j.biopsych.2017.04.011. Erratum in:Biol Psychiatry. 2023; 93: 291-292. [DOI] [PubMed] [Google Scholar]
- 37.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. (2013);155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. (2005);24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 39.Hanover JA, Krause MW, Love DC. The hexosamine signaling pathway:O-GlcNAc cycling in feast or famine. Biochim Biophys Acta. (2010);1800:80–95. doi: 10.1016/j.bbagen.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He L, Si G, Huang J, Samuel ADT, Perrimon N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature. (2018);555:103–106. doi: 10.1038/nature25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heppt J, Wittmann MT, Schäffner I, Billmann C, Zhang J, Vogt-Weisenhorn D, Prakash N, Wurst W, Taketo MM, Lie DC. β-catenin signaling modulates the tempo of dendritic growth of adult-born hippocampal neurons. EMBO J. (2020);39:e104472. doi: 10.15252/embj.2020104472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. (1996);271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 43.Hurtado-Chong A, Yusta-Boyo MJ, Vergaño-Vera E, Bulfone A, de Pablo F, Vicario-Abejón C. IGF-I promotes neuronal migration and positioning in the olfactory bulb and the exit of neuroblasts from the subventricular zone. Eur J Neurosci. (2009);30:742–755. doi: 10.1111/j.1460-9568.2009.06870.x. [DOI] [PubMed] [Google Scholar]
- 44.Iwata R, Casimir P, Vanderhaeghen P. Mitochondrial dynamics in postmitotic cells regulate neurogenesis. Science. (2020);369:858–862. doi: 10.1126/science.aba9760. [DOI] [PubMed] [Google Scholar]
- 45.Janssen CI, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence:the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res. (2014);53:1–17. doi: 10.1016/j.plipres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Jiang M, Vanan S, Tu HT, Zhang W, Zhang ZW, Chia SY, Jang SE, Zeng XX, Yu WP, Xu J, Guo KH, Zeng L. Amyloid precursor protein intracellular domain-dependent regulation of FOXO3a inhibits adult hippocampal neurogenesis. Neurobiol Aging. (2020);95:250–263. doi: 10.1016/j.neurobiolaging.2020.07.031. [DOI] [PubMed] [Google Scholar]
- 47.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1βmediated up-regulation of HIF-lαvia an NFkB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. (2003);17:1–22. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan MS, Hinds JW. Neurogenesis in the adult rat:electron microscopic analysis of light radioautographs. Science. (1977);197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 49.Khacho M, Slack RS. Mitochondrial activity in the regulation of stem cell self-renewal and differentiation. Curr Opin Cell Biol. (2017);49:1–8. doi: 10.1016/j.ceb.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Khacho M, Clark A, Svoboda DS, Azzi J, MacLaurin JG, Meghaizel C, Sesaki H, Lagace DC, Germain M, Harper ME, Park DS, Slack RS. Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional program. Cell Stem Cell. (2016);19:232–247. doi: 10.1016/j.stem.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 51.Kiani AA, Elyasi H, Ghoreyshi S, Nouri N, Safarzadeh A, Nafari A. Study on hypoxia-inducible factor and its roles in immune system. Immunol Med. (2021);44:223–236. doi: 10.1080/25785826.2021.1910187. [DOI] [PubMed] [Google Scholar]
- 52.Kim YM, Kim HJ. Proteasome inhibitor MG132 is toxic and inhibits the proliferation of rat neural stem cells but increases BDNF expression to protect neurons. Biomolecules. (2020);10:1507. doi: 10.3390/biom10111507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knobloch M, von Schoultz C, Zurkirchen L, Braun SM, Vidmar M, Jessberger S. SPOT14-positive neural stem/progenitor cells in the hippocampus respond dynamically to neurogenic regulators. Stem Cell Reports. (2014);3:735–742. doi: 10.1016/j.stemcr.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knobloch M, Pilz GA, Ghesquière B, Kovacs WJ, Wegleiter T, Moore DL, Hruzova M, Zamboni N, Carmeliet P, Jessberger S. A fatty acid oxidation-dependent metabolic shift regulates adult neural stem cell activity. Cell Rep. (2017);20:2144–2155. doi: 10.1016/j.celrep.2017.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knobloch M, Braun SM, Zurkirchen L, von Schoultz C, Zamboni N, Araúzo-Bravo MJ, Kovacs WJ, Karalay O, Suter U, Machado RA, Roccio M, Lutolf MP, Semenkovich CF, Jessberger S. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. (2013);493:226–230. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobayashi T, Piao W, Takamura T, Kori H, Miyachi H, Kitano S, Iwamoto Y, Yamada M, Imayoshi I, Shioda S, Ballabio A, Kageyama R. Enhanced lysosomal degradation maintains the quiescent state of neural stem cells. Nat Commun. (2019);10:5446. doi: 10.1038/s41467-019-13203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar A, Pareek V, Faiq MA, Ghosh SK, Kumari C. Adult neurogenesis in humans:a review of basic concepts, history, current research, and clinical implications. Innov Clin Neurosci. (2019);16:30–37. [PMC free article] [PubMed] [Google Scholar]
- 58.Lee TK, Kim DW, Sim H, Lee JC, Kim HI, Shin MC, Cho JH, Park JH, Lee CH, Won MH, Ahn JH. Hyperthermia accelerates neuronal loss differently between the hippocampal CA1 and CA2/3 through different HIF-1αexpression after transient ischemia in gerbils. Int J Mol Med. (2022);49:55. doi: 10.3892/ijmm.2022.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu BN, Han BX, Liu F. Neuroprotective effect of pAkt and HIF-1 αon ischemia rats. Asian Pac J Trop Med. (2014);7:221–225. doi: 10.1016/S1995-7645(14)60025-0. [DOI] [PubMed] [Google Scholar]
- 60.Ludikhuize MC, Rodríguez Colman MJ. Metabolic regulation of stem cells and differentiation:a forkhead box O transcription factor perspective. Antioxid Redox Signal. (2021);34:1004–1024. doi: 10.1089/ars.2020.8126. [DOI] [PubMed] [Google Scholar]
- 61.Lunt SY, Vander Heiden MG. Aerobic glycolysis:meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. (2011);27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 62.Malik SC, Chu YH, Schachtrup C. Pointing fingers at blood contact:mechanisms of subventricular zone neural stem cell differentiation. Neural Regen Res. (2023);18:137–138. doi: 10.4103/1673-5374.338998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marsboom G, Zhang GF, Pohl-Avila N, Zhang Y, Yuan Y, Kang H, Hao B, Brunengraber H, Malik AB, Rehman J. Glutamine metabolism regulates the pluripotency transcription factor OCT4. Cell Rep. (2016);16:323–332. doi: 10.1016/j.celrep.2016.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mazumdar J, O'Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC. O2 regulates stem cells through Wnt/β-catenin signalling. Nat Cell Biol. (2010);12:1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLaughlin CN, Broihier HT. Keeping neurons young and foxy:FoxOs promote neuronal plasticity. Trends Genet. (2018);34:65–78. doi: 10.1016/j.tig.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McMillan N, Kirschen GW, Desai S, Xia E, Tsirka SE, Aguirre A. ADAM10 facilitates rapid neural stem cell cycling and proper positioning within the subventricular zone niche via JAMC/RAP1Gap signaling. Neural Regen Res. (2022);17:2472–2483. doi: 10.4103/1673-5374.339007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mekala NK, Sasikumar S, Akula KK, Parekh Y, Rao CM, Bokara KK. HspB5 protects mouse neural stem/progenitor cells from paraquat toxicity. Am J Stem Cells. (2020);9:68–77. [PMC free article] [PubMed] [Google Scholar]
- 68.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. (2008);3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. (2003);302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 70.Morrow CS, Porter TJ, Xu N, Arndt ZP, Ako-Asare K, Heo HJ, Thompson EAN, Moore DL. Vimentin coordinates protein turnover at the aggresome during neural stem cell quiescence exit. Cell Stem Cell. (2020);26:558–568.e559. doi: 10.1016/j.stem.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain:a relatively quiescent subpopulation of subependymal cells. Neuron. (1994);13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 72.Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer's disease. Mol Neurodegener. (2011);6:85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nieto-Estévez V, Oueslati-Morales CO, Li L, Pickel J, Morales AV, Vicario-Abejón C. Brain insulin-like growth factor-i directs the transition from stem cells to mature neurons during postnatal/adult hippocampal neurogenesis. Stem Cells. (2016);34:2194–2209. doi: 10.1002/stem.2397. [DOI] [PubMed] [Google Scholar]
- 74.Ogasawara MA, Zhang H. Redox regulation and its emerging roles in stem cells and stem-like cancer cells. Antioxid Redox Signal. (2009);11:1107–1122. doi: 10.1089/ars.2008.2308. [DOI] [PubMed] [Google Scholar]
- 75.Ogrodnik M, Salmonowicz H, Brown R, Turkowska J, Średniawa W, Pattabiraman S, Amen T, Abraham AC, Eichler N, Lyakhovetsky R, Kaganovich D. Dynamic JUNQ inclusion bodies are asymmetrically inherited in mammalian cell lines through the asymmetric partitioning of vimentin. Proc Natl Acad Sci U S A. (2014);111:8049–8054. doi: 10.1073/pnas.1324035111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, Adams RH, Parrinello S. Direct cell–cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol. (2014);16:1045–1056. doi: 10.1038/ncb3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, Chae SS, Zheng H, Ying H, Mahoney J, Hiller D, Jiang S, Protopopov A, Wong WH, Chin L, Ligon KL, DePinho RA. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. (2009);5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parent A, Cicchetti F, Beach TG. Calretinin-immunoreactive neurons in the human striatum. Brain Res. (1995);674:347–351. doi: 10.1016/0006-8993(95)00124-9. [DOI] [PubMed] [Google Scholar]
- 79.Perales-Clemente E, Folmes CD, Terzic A. Metabolic regulation of redox status in stem cells. Antioxid Redox Signal. (2014);21:1648–1659. doi: 10.1089/ars.2014.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petrik D, Myoga MH, Grade S, Gerkau NJ, Pusch M, Rose CR, Grothe B, Götz M. Epithelial sodium channel regulates adult neural stem cell proliferation in a flow-dependent manner. Cell Stem Cell. (2018);22:865–878.e868. doi: 10.1016/j.stem.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 81.Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. (2008);105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qi C, Zhang J, Chen X, Wan J, Wang J, Zhang P, Liu Y. Hypoxia stimulates neural stem cell proliferation by increasing HIF-1αexpression and activating Wnt/β-catenin signaling. Cell Mol Biol (Noisy-le-grand) (2017);63:12–19. doi: 10.14715/cmb/2017.63.7.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Radwan M, Wood RJ, Sui X, Hatters DM. When proteostasis goes bad:Protein aggregation in the cell. IUBMB Life. (2017);69:49–54. doi: 10.1002/iub.1597. [DOI] [PubMed] [Google Scholar]
- 84.Rehman J. Empowering self-renewal and differentiation:the role of mitochondria in stem cells. J Mol Med (Berl) (2010);88:981–986. doi: 10.1007/s00109-010-0678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ribeiro FF, Xapelli S. An Overview of Adult Neurogenesis. In: Calzà L, Aloe L, Giardino L, editors. Recent advances in NGF and related molecules:the continuum of the NGF “Saga”. Cham: Springer International Publishing; (2021). pp. 77–94. [Google Scholar]
- 86.Rodríguez E, Guerra M, Peruzzo B, Blázquez JL. Tanycytes:A rich morphological history to underpin future molecular and physiological investigations. J Neuroendocrinol. (2019);31:e12690. doi: 10.1111/jne.12690. [DOI] [PubMed] [Google Scholar]
- 87.Saikia R, Joseph J. AMPK:a key regulator of energy stress and calcium-induced autophagy. J Mol Med (Berl) (2021);99:1539–1551. doi: 10.1007/s00109-021-02125-8. [DOI] [PubMed] [Google Scholar]
- 88.Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. (2008);7:104–112. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 89.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. (2012);148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. (2018);555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suda T, Takubo K, Semenza Gregg L. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. (2011);9:298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 92.Sun Z, Zhu M, Lv P, Cheng L, Wang Q, Tian P, Yan Z, Wen B. The long noncoding RNA Lncenc1 maintains naive states of mouse ESCs by promoting the glycolysis pathway. Stem Cell Reports. (2018);11:741–755. doi: 10.1016/j.stemcr.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sung PS, Lin PY, Liu CH, Su HC, Tsai KJ. Neuroinflammation and neurogenesis in Alzheimer's disease and potential therapeutic approaches. Int J Mol Sci. (2020);21:701. doi: 10.3390/ijms21030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tambay V, Raymond VA, Bilodeau M. MYC rules:leading glutamine metabolism toward a distinct cancer cell phenotype. Cancers (Basel) (2021);13:4484. doi: 10.3390/cancers13174484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tay EXY, Chia K, Ong DST. Epigenetic plasticity and redox regulation of neural stem cell state and fate. Free Radic Biol Med. (2021);170:116–130. doi: 10.1016/j.freeradbiomed.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 96.Tia N, Singh AK, Pandey P, Azad CS, Chaudhary P, Gambhir IS. Role of Forkhead Box O (FOXO) transcription factor in aging and diseases. Gene. (2018);648:97–105. doi: 10.1016/j.gene.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 97.Tiberi L, van den Ameele J, Dimidschstein J, Piccirilli J, Gall D, Herpoel A, Bilheu A, Bonnefont J, Iacovino M, Kyba M, Bouschet T, Vanderhaeghen P. BCL6 controls neurogenesis through Sirt1-dependent epigenetic repression of selective Notch targets. Nat Neurosci. (2012);15:1627–1635. doi: 10.1038/nn.3264. [DOI] [PubMed] [Google Scholar]
- 98.Tu BP, McKnight SL. The yeast metabolic cycle:insights into the life of a eukaryotic cell. Cold Spring Harb Symp Quant Biol. (2007);72:339–343. doi: 10.1101/sqb.2007.72.019. [DOI] [PubMed] [Google Scholar]
- 99.Tu BP, Kudlicki A, Rowicka M, McKnight SL. Logic of the yeast metabolic cycle:temporal compartmentalization of cellular processes. Science. (2005);310:1152–1158. doi: 10.1126/science.1120499. [DOI] [PubMed] [Google Scholar]
- 100.Vella P, Scelfo A, Jammula S, Chiacchiera F, Williams K, Cuomo A, Roberto A, Christensen J, Bonaldi T, Helin K, Pasini D. Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells. Mol Cell. (2013);49:645–656. doi: 10.1016/j.molcel.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 101.Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrané J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. (2010);107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vonk WIM, Rainbolt TK, Dolan PT, Webb AE, Brunet A, Frydman J. Differentiation drives widespread rewiring of the neural stem cell chaperone network. Mol Cell. (2020);78:329–345.e329. doi: 10.1016/j.molcel.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wobma HM, Tamargo MA, Goeta S, Brown LM, Duran-Struuck R, Vunjak-Novakovic G. The influence of hypoxia and IFN-γon the proteome and metabolome of therapeutic mesenchymal stem cells. Biomaterials. (2018);167:226–234. doi: 10.1016/j.biomaterials.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xing J, Lu J. HIF-1αactivation attenuates IL-6 and TNF-αpathways in hippocampus of rats following transient global ischemia. Cell Physiol Biochem. (2016);39:511–520. doi: 10.1159/000445643. [DOI] [PubMed] [Google Scholar]
- 105.Xing J, Ying Y, Mao C, Liu Y, Wang T, Zhao Q, Zhang X, Yan F, Zhang H. Hypoxia induces senescence of bone marrow mesenchymal stem cells via altered gut microbiota. Nat Commun. (2018);9:2020. doi: 10.1038/s41467-018-04453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. (2012);337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang H, Liu B, Li T, Zhu Y, Luo G, Jiang Y, Tang F, Jian Z, Xiao Y. AMPK activation serves a critical role in mitochondria quality control via modulating mitophagy in the heart under chronic hypoxia. Int J Mol Med. (2018);41:69–76. doi: 10.3892/ijmm.2017.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao Y, Liu X, He Z, Niu X, Shi W, Ding JM, Zhang L, Yuan T, Li A, Yang W, Lu L. Essential role of proteasomes in maintaining self-renewal in neural progenitor cells. Sci Rep. (2016);6:19752. doi: 10.1038/srep19752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhong X, Cui P, Cai Y, Wang L, He X, Long P, Lu K, Yan R, Zhang Y, Pan X, Zhao X, Li W, Zhang H, Zhou Q, Gao P. Mitochondrial dynamics is critical for the full pluripotency and embryonic developmental potential of pluripotent stem cells. Cell Metab. (2019);29:979–992.e974. doi: 10.1016/j.cmet.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 110.Zhou T, Yang Y, Chen Q, Xie L. Glutamine metabolism is essential for stemness of bone marrow mesenchymal stem cells and bone homeostasis. Stem Cells Int. (2019);2019:8928934. doi: 10.1155/2019/8928934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zong Y, Jiang L, Zhang M, Zhou F, Qi W, Li S, Yang H, Zou Y, Xia Q, Zhou X, Hu X, Wang T. Limb remote ischemic postconditioning protects cerebral ischemia from injury associated with expression of HIF-1αin rats. BMC Neurosci. (2015);16:97. doi: 10.1186/s12868-015-0235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


